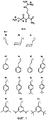RU2548905C2 - Терапевтические пептиды - Google Patents
Терапевтические пептиды Download PDFInfo
- Publication number
- RU2548905C2 RU2548905C2 RU2012121264/04A RU2012121264A RU2548905C2 RU 2548905 C2 RU2548905 C2 RU 2548905C2 RU 2012121264/04 A RU2012121264/04 A RU 2012121264/04A RU 2012121264 A RU2012121264 A RU 2012121264A RU 2548905 C2 RU2548905 C2 RU 2548905C2
- Authority
- RU
- Russia
- Prior art keywords
- amino acid
- peptide
- peptidomimetic
- groups
- group
- Prior art date
Links
- 108090000765 processed proteins & peptides Proteins 0.000 title claims abstract description 82
- 102000004196 processed proteins & peptides Human genes 0.000 title description 27
- 230000001225 therapeutic effect Effects 0.000 title description 3
- 150000001413 amino acids Chemical class 0.000 claims abstract description 75
- 125000004122 cyclic group Chemical group 0.000 claims abstract description 45
- 125000004435 hydrogen atom Chemical group [H]* 0.000 claims abstract description 31
- 150000001576 beta-amino acids Chemical class 0.000 claims abstract description 26
- 239000000126 substance Substances 0.000 claims abstract description 3
- 150000003862 amino acid derivatives Chemical class 0.000 claims abstract 7
- -1 cationic amino acid Chemical class 0.000 claims description 60
- 239000000816 peptidomimetic Substances 0.000 claims description 59
- 125000001424 substituent group Chemical group 0.000 claims description 47
- 238000000034 method Methods 0.000 claims description 39
- 230000000845 anti-microbial effect Effects 0.000 claims description 32
- 229910052799 carbon Inorganic materials 0.000 claims description 19
- 125000004432 carbon atom Chemical group C* 0.000 claims description 17
- 208000015181 infectious disease Diseases 0.000 claims description 17
- 229910052757 nitrogen Inorganic materials 0.000 claims description 16
- 125000004429 atom Chemical group 0.000 claims description 13
- 230000000813 microbial effect Effects 0.000 claims description 13
- 229910052760 oxygen Inorganic materials 0.000 claims description 8
- 229910052717 sulfur Inorganic materials 0.000 claims description 6
- KDXKERNSBIXSRK-UHFFFAOYSA-N Lysine Natural products NCCCCC(N)C(O)=O KDXKERNSBIXSRK-UHFFFAOYSA-N 0.000 claims description 5
- 125000000113 cyclohexyl group Chemical group [H]C1([H])C([H])([H])C([H])([H])C([H])(*)C([H])([H])C1([H])[H] 0.000 claims description 5
- 239000000546 pharmaceutical excipient Substances 0.000 claims description 5
- 125000001997 phenyl group Chemical group [H]C1=C([H])C([H])=C(*)C([H])=C1[H] 0.000 claims description 5
- 239000004475 Arginine Substances 0.000 claims description 4
- 239000004472 Lysine Substances 0.000 claims description 4
- ODKSFYDXXFIFQN-UHFFFAOYSA-N arginine Natural products OC(=O)C(N)CCCNC(N)=N ODKSFYDXXFIFQN-UHFFFAOYSA-N 0.000 claims description 4
- 239000008194 pharmaceutical composition Substances 0.000 claims description 2
- 239000003085 diluting agent Substances 0.000 claims 1
- 239000003814 drug Substances 0.000 abstract description 21
- 230000000694 effects Effects 0.000 abstract description 7
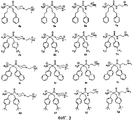
- 235000001014 amino acid Nutrition 0.000 description 81
- 150000001875 compounds Chemical class 0.000 description 58
- 210000003743 erythrocyte Anatomy 0.000 description 45
- GOJUJUVQIVIZAV-UHFFFAOYSA-N 2-amino-4,6-dichloropyrimidine-5-carbaldehyde Chemical group NC1=NC(Cl)=C(C=O)C(Cl)=N1 GOJUJUVQIVIZAV-UHFFFAOYSA-N 0.000 description 36
- 230000001093 anti-cancer Effects 0.000 description 32
- 230000015572 biosynthetic process Effects 0.000 description 30
- OKKJLVBELUTLKV-UHFFFAOYSA-N Methanol Chemical compound OC OKKJLVBELUTLKV-UHFFFAOYSA-N 0.000 description 27
- 238000003786 synthesis reaction Methods 0.000 description 27
- XEKOWRVHYACXOJ-UHFFFAOYSA-N Ethyl acetate Chemical compound CCOC(C)=O XEKOWRVHYACXOJ-UHFFFAOYSA-N 0.000 description 24
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 22
- 241000588724 Escherichia coli Species 0.000 description 20
- 210000004027 cell Anatomy 0.000 description 20
- 239000000203 mixture Substances 0.000 description 18
- 231100000419 toxicity Toxicity 0.000 description 18
- 230000001988 toxicity Effects 0.000 description 18
- 229940079593 drug Drugs 0.000 description 17
- 210000004899 c-terminal region Anatomy 0.000 description 16
- 239000000243 solution Substances 0.000 description 15
- 206010028980 Neoplasm Diseases 0.000 description 14
- 239000011347 resin Substances 0.000 description 14
- 229920005989 resin Polymers 0.000 description 14
- 238000012360 testing method Methods 0.000 description 13
- QTBSBXVTEAMEQO-UHFFFAOYSA-N Acetic acid Chemical compound CC(O)=O QTBSBXVTEAMEQO-UHFFFAOYSA-N 0.000 description 12
- JGFZNNIVVJXRND-UHFFFAOYSA-N N,N-Diisopropylethylamine (DIPEA) Chemical compound CCN(C(C)C)C(C)C JGFZNNIVVJXRND-UHFFFAOYSA-N 0.000 description 12
- 238000010521 absorption reaction Methods 0.000 description 12
- 239000011541 reaction mixture Substances 0.000 description 12
- 210000004881 tumor cell Anatomy 0.000 description 11
- 125000000022 2-aminoethyl group Chemical group [H]C([*])([H])C([H])([H])N([H])[H] 0.000 description 10
- RJQXTJLFIWVMTO-TYNCELHUSA-N Methicillin Chemical compound COC1=CC=CC(OC)=C1C(=O)N[C@@H]1C(=O)N2[C@@H](C(O)=O)C(C)(C)S[C@@H]21 RJQXTJLFIWVMTO-TYNCELHUSA-N 0.000 description 10
- WQDUMFSSJAZKTM-UHFFFAOYSA-N Sodium methoxide Chemical compound [Na+].[O-]C WQDUMFSSJAZKTM-UHFFFAOYSA-N 0.000 description 10
- 241000191967 Staphylococcus aureus Species 0.000 description 10
- DTQVDTLACAAQTR-UHFFFAOYSA-N Trifluoroacetic acid Chemical compound OC(=O)C(F)(F)F DTQVDTLACAAQTR-UHFFFAOYSA-N 0.000 description 10
- 230000001461 cytolytic effect Effects 0.000 description 10
- 229960003085 meticillin Drugs 0.000 description 10
- IJGRMHOSHXDMSA-UHFFFAOYSA-N Atomic nitrogen Chemical compound N#N IJGRMHOSHXDMSA-UHFFFAOYSA-N 0.000 description 9
- 230000001580 bacterial effect Effects 0.000 description 9
- 230000002949 hemolytic effect Effects 0.000 description 9
- 239000000047 product Substances 0.000 description 9
- RYHBNJHYFVUHQT-UHFFFAOYSA-N 1,4-Dioxane Chemical compound C1COCCO1 RYHBNJHYFVUHQT-UHFFFAOYSA-N 0.000 description 8
- RTZKZFJDLAIYFH-UHFFFAOYSA-N Diethyl ether Chemical compound CCOCC RTZKZFJDLAIYFH-UHFFFAOYSA-N 0.000 description 8
- WMFOQBRAJBCJND-UHFFFAOYSA-M Lithium hydroxide Chemical compound [Li+].[OH-] WMFOQBRAJBCJND-UHFFFAOYSA-M 0.000 description 8
- 239000004599 antimicrobial Substances 0.000 description 8
- ALBYIUDWACNRRB-UHFFFAOYSA-N hexanamide Chemical compound CCCCCC(N)=O ALBYIUDWACNRRB-UHFFFAOYSA-N 0.000 description 8
- 239000012074 organic phase Substances 0.000 description 8
- 241000894006 Bacteria Species 0.000 description 7
- 239000002246 antineoplastic agent Substances 0.000 description 7
- 238000006243 chemical reaction Methods 0.000 description 7
- LOKCTEFSRHRXRJ-UHFFFAOYSA-I dipotassium trisodium dihydrogen phosphate hydrogen phosphate dichloride Chemical compound P(=O)(O)(O)[O-].[K+].P(=O)(O)([O-])[O-].[Na+].[Na+].[Cl-].[K+].[Cl-].[Na+] LOKCTEFSRHRXRJ-UHFFFAOYSA-I 0.000 description 7
- 239000002953 phosphate buffered saline Substances 0.000 description 7
- 238000002360 preparation method Methods 0.000 description 7
- 238000000746 purification Methods 0.000 description 7
- 241000282412 Homo Species 0.000 description 6
- GLUUGHFHXGJENI-UHFFFAOYSA-N Piperazine Chemical compound C1CNCCN1 GLUUGHFHXGJENI-UHFFFAOYSA-N 0.000 description 6
- 230000000844 anti-bacterial effect Effects 0.000 description 6
- 230000004071 biological effect Effects 0.000 description 6
- 125000003178 carboxy group Chemical group [H]OC(*)=O 0.000 description 6
- 238000004519 manufacturing process Methods 0.000 description 6
- ANGDWNBGPBMQHW-UHFFFAOYSA-N methyl cyanoacetate Chemical compound COC(=O)CC#N ANGDWNBGPBMQHW-UHFFFAOYSA-N 0.000 description 6
- 125000004433 nitrogen atom Chemical group N* 0.000 description 6
- 230000002829 reductive effect Effects 0.000 description 6
- 238000004007 reversed phase HPLC Methods 0.000 description 6
- 238000012216 screening Methods 0.000 description 6
- 208000035143 Bacterial infection Diseases 0.000 description 5
- 108010016626 Dipeptides Proteins 0.000 description 5
- 108090000790 Enzymes Proteins 0.000 description 5
- 102000004190 Enzymes Human genes 0.000 description 5
- 206010027476 Metastases Diseases 0.000 description 5
- 241000191963 Staphylococcus epidermidis Species 0.000 description 5
- 150000001408 amides Chemical class 0.000 description 5
- 150000001412 amines Chemical class 0.000 description 5
- 125000003277 amino group Chemical group 0.000 description 5
- 208000022362 bacterial infectious disease Diseases 0.000 description 5
- 201000011510 cancer Diseases 0.000 description 5
- 229940088598 enzyme Drugs 0.000 description 5
- 125000006239 protecting group Chemical group 0.000 description 5
- 238000010992 reflux Methods 0.000 description 5
- 150000003839 salts Chemical class 0.000 description 5
- HOKSRCHGQCOMHM-CYBMUJFWSA-N (2R)-2-[3-(benzylamino)propanoylamino]-5-(diaminomethylideneamino)pentanoic acid Chemical compound C1(=CC=CC=C1)CNCCC(=O)N[C@H](CCCNC(N)=N)C(=O)O HOKSRCHGQCOMHM-CYBMUJFWSA-N 0.000 description 4
- 125000003088 (fluoren-9-ylmethoxy)carbonyl group Chemical group 0.000 description 4
- KBPLFHHGFOOTCA-UHFFFAOYSA-N 1-Octanol Chemical compound CCCCCCCCO KBPLFHHGFOOTCA-UHFFFAOYSA-N 0.000 description 4
- KCXVZYZYPLLWCC-UHFFFAOYSA-N EDTA Chemical compound OC(=O)CN(CC(O)=O)CCN(CC(O)=O)CC(O)=O KCXVZYZYPLLWCC-UHFFFAOYSA-N 0.000 description 4
- NQRYJNQNLNOLGT-UHFFFAOYSA-N Piperidine Chemical compound C1CCNCC1 NQRYJNQNLNOLGT-UHFFFAOYSA-N 0.000 description 4
- 230000009471 action Effects 0.000 description 4
- 108010027597 alpha-chymotrypsin Proteins 0.000 description 4
- 230000000259 anti-tumor effect Effects 0.000 description 4
- 229940041181 antineoplastic drug Drugs 0.000 description 4
- AGEZXYOZHKGVCM-UHFFFAOYSA-N benzyl bromide Chemical compound BrCC1=CC=CC=C1 AGEZXYOZHKGVCM-UHFFFAOYSA-N 0.000 description 4
- 210000004369 blood Anatomy 0.000 description 4
- 239000008280 blood Substances 0.000 description 4
- 238000004364 calculation method Methods 0.000 description 4
- 125000002091 cationic group Chemical group 0.000 description 4
- 239000012043 crude product Substances 0.000 description 4
- 238000009472 formulation Methods 0.000 description 4
- 239000012634 fragment Substances 0.000 description 4
- 238000004128 high performance liquid chromatography Methods 0.000 description 4
- 230000009401 metastasis Effects 0.000 description 4
- 239000013642 negative control Substances 0.000 description 4
- 150000002825 nitriles Chemical class 0.000 description 4
- 230000035699 permeability Effects 0.000 description 4
- 239000013641 positive control Substances 0.000 description 4
- 230000002265 prevention Effects 0.000 description 4
- QLNJFJADRCOGBJ-UHFFFAOYSA-N propionamide Chemical compound CCC(N)=O QLNJFJADRCOGBJ-UHFFFAOYSA-N 0.000 description 4
- 230000009467 reduction Effects 0.000 description 4
- 210000002966 serum Anatomy 0.000 description 4
- 239000002904 solvent Substances 0.000 description 4
- 238000006467 substitution reaction Methods 0.000 description 4
- 125000003352 4-tert-butyl benzyl group Chemical group [H]C1=C([H])C(=C([H])C([H])=C1C([H])([H])*)C(C([H])([H])[H])(C([H])([H])[H])C([H])([H])[H] 0.000 description 3
- TVEXGJYMHHTVKP-UHFFFAOYSA-N 6-oxabicyclo[3.2.1]oct-3-en-7-one Chemical compound C1C2C(=O)OC1C=CC2 TVEXGJYMHHTVKP-UHFFFAOYSA-N 0.000 description 3
- WEVYAHXRMPXWCK-UHFFFAOYSA-N Acetonitrile Chemical compound CC#N WEVYAHXRMPXWCK-UHFFFAOYSA-N 0.000 description 3
- 108700042778 Antimicrobial Peptides Proteins 0.000 description 3
- 102000044503 Antimicrobial Peptides Human genes 0.000 description 3
- IAZDPXIOMUYVGZ-UHFFFAOYSA-N Dimethylsulphoxide Chemical compound CS(C)=O IAZDPXIOMUYVGZ-UHFFFAOYSA-N 0.000 description 3
- ODKSFYDXXFIFQN-BYPYZUCNSA-N L-arginine Chemical compound OC(=O)[C@@H](N)CCCN=C(N)N ODKSFYDXXFIFQN-BYPYZUCNSA-N 0.000 description 3
- KDXKERNSBIXSRK-YFKPBYRVSA-N L-lysine Chemical compound NCCCC[C@H](N)C(O)=O KDXKERNSBIXSRK-YFKPBYRVSA-N 0.000 description 3
- 241000589517 Pseudomonas aeruginosa Species 0.000 description 3
- 108010059993 Vancomycin Proteins 0.000 description 3
- 206010052428 Wound Diseases 0.000 description 3
- 208000027418 Wounds and injury Diseases 0.000 description 3
- 230000004913 activation Effects 0.000 description 3
- 238000013019 agitation Methods 0.000 description 3
- 125000003368 amide group Chemical group 0.000 description 3
- 125000004103 aminoalkyl group Chemical group 0.000 description 3
- 125000004202 aminomethyl group Chemical group [H]N([H])C([H])([H])* 0.000 description 3
- 239000007864 aqueous solution Substances 0.000 description 3
- QVGXLLKOCUKJST-UHFFFAOYSA-N atomic oxygen Chemical compound [O] QVGXLLKOCUKJST-UHFFFAOYSA-N 0.000 description 3
- 125000001797 benzyl group Chemical group [H]C1=C([H])C([H])=C(C([H])=C1[H])C([H])([H])* 0.000 description 3
- 239000003153 chemical reaction reagent Substances 0.000 description 3
- 230000001684 chronic effect Effects 0.000 description 3
- KRKNYBCHXYNGOX-UHFFFAOYSA-N citric acid Chemical compound OC(=O)CC(O)(C(O)=O)CC(O)=O KRKNYBCHXYNGOX-UHFFFAOYSA-N 0.000 description 3
- 231100000433 cytotoxic Toxicity 0.000 description 3
- 230000001472 cytotoxic effect Effects 0.000 description 3
- 230000034994 death Effects 0.000 description 3
- 231100000517 death Toxicity 0.000 description 3
- 238000011161 development Methods 0.000 description 3
- 238000007865 diluting Methods 0.000 description 3
- 150000002148 esters Chemical class 0.000 description 3
- 230000006870 function Effects 0.000 description 3
- 230000009931 harmful effect Effects 0.000 description 3
- 238000005534 hematocrit Methods 0.000 description 3
- 239000001257 hydrogen Substances 0.000 description 3
- 229910052739 hydrogen Inorganic materials 0.000 description 3
- 230000002401 inhibitory effect Effects 0.000 description 3
- 230000002601 intratumoral effect Effects 0.000 description 3
- 230000010534 mechanism of action Effects 0.000 description 3
- 239000012528 membrane Substances 0.000 description 3
- 238000007069 methylation reaction Methods 0.000 description 3
- 210000000056 organ Anatomy 0.000 description 3
- 239000001301 oxygen Substances 0.000 description 3
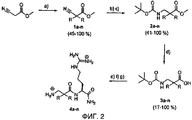
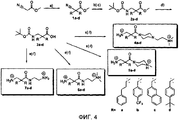
- 238000010647 peptide synthesis reaction Methods 0.000 description 3
- 239000006228 supernatant Substances 0.000 description 3
- 125000000999 tert-butyl group Chemical group [H]C([H])([H])C(*)(C([H])([H])[H])C([H])([H])[H] 0.000 description 3
- 229940126585 therapeutic drug Drugs 0.000 description 3
- 150000003573 thiols Chemical class 0.000 description 3
- MYPYJXKWCTUITO-LYRMYLQWSA-N vancomycin Chemical compound O([C@@H]1[C@@H](O)[C@H](O)[C@@H](CO)O[C@H]1OC1=C2C=C3C=C1OC1=CC=C(C=C1Cl)[C@@H](O)[C@H](C(N[C@@H](CC(N)=O)C(=O)N[C@H]3C(=O)N[C@H]1C(=O)N[C@H](C(N[C@@H](C3=CC(O)=CC(O)=C3C=3C(O)=CC=C1C=3)C(O)=O)=O)[C@H](O)C1=CC=C(C(=C1)Cl)O2)=O)NC(=O)[C@@H](CC(C)C)NC)[C@H]1C[C@](C)(N)[C@H](O)[C@H](C)O1 MYPYJXKWCTUITO-LYRMYLQWSA-N 0.000 description 3
- 229960003165 vancomycin Drugs 0.000 description 3
- MYPYJXKWCTUITO-UHFFFAOYSA-N vancomycin Natural products O1C(C(=C2)Cl)=CC=C2C(O)C(C(NC(C2=CC(O)=CC(O)=C2C=2C(O)=CC=C3C=2)C(O)=O)=O)NC(=O)C3NC(=O)C2NC(=O)C(CC(N)=O)NC(=O)C(NC(=O)C(CC(C)C)NC)C(O)C(C=C3Cl)=CC=C3OC3=CC2=CC1=C3OC1OC(CO)C(O)C(O)C1OC1CC(C)(N)C(O)C(C)O1 MYPYJXKWCTUITO-UHFFFAOYSA-N 0.000 description 3
- 239000003643 water by type Substances 0.000 description 3
- ADOHASQZJSJZBT-SANMLTNESA-N (2s)-2-(9h-fluoren-9-ylmethoxycarbonylamino)-3-[1-[(2-methylpropan-2-yl)oxycarbonyl]indol-3-yl]propanoic acid Chemical compound C12=CC=CC=C2N(C(=O)OC(C)(C)C)C=C1C[C@@H](C(O)=O)NC(=O)OCC1C2=CC=CC=C2C2=CC=CC=C21 ADOHASQZJSJZBT-SANMLTNESA-N 0.000 description 2
- 0 *c1ccc(CC(Cc2ccc(*)cc2)(CNC([C@@](Cc2c[n]c3ccccc23)NC(CNC(CN)=O)=O)=O)C(C2(CCC(NC(CCCCN)C(C3N(CC(N)=O)C3)=O)=O)NC2)=O)cc1 Chemical compound *c1ccc(CC(Cc2ccc(*)cc2)(CNC([C@@](Cc2c[n]c3ccccc23)NC(CNC(CN)=O)=O)=O)C(C2(CCC(NC(CCCCN)C(C3N(CC(N)=O)C3)=O)=O)NC2)=O)cc1 0.000 description 2
- 125000004042 4-aminobutyl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])C([H])([H])N([H])[H] 0.000 description 2
- XKRFYHLGVUSROY-UHFFFAOYSA-N Argon Chemical compound [Ar] XKRFYHLGVUSROY-UHFFFAOYSA-N 0.000 description 2
- 108010023063 Bacto-peptone Proteins 0.000 description 2
- 201000009030 Carcinoma Diseases 0.000 description 2
- ODKSFYDXXFIFQN-SCSAIBSYSA-N D-arginine Chemical compound OC(=O)[C@H](N)CCCNC(N)=N ODKSFYDXXFIFQN-SCSAIBSYSA-N 0.000 description 2
- 229930028154 D-arginine Natural products 0.000 description 2
- UFHFLCQGNIYNRP-UHFFFAOYSA-N Hydrogen Chemical compound [H][H] UFHFLCQGNIYNRP-UHFFFAOYSA-N 0.000 description 2
- AHLPHDHHMVZTML-BYPYZUCNSA-N L-Ornithine Chemical compound NCCC[C@H](N)C(O)=O AHLPHDHHMVZTML-BYPYZUCNSA-N 0.000 description 2
- 229930064664 L-arginine Natural products 0.000 description 2
- 235000014852 L-arginine Nutrition 0.000 description 2
- ODKSFYDXXFIFQN-BYPYZUCNSA-P L-argininium(2+) Chemical compound NC(=[NH2+])NCCC[C@H]([NH3+])C(O)=O ODKSFYDXXFIFQN-BYPYZUCNSA-P 0.000 description 2
- HNDVDQJCIGZPNO-YFKPBYRVSA-N L-histidine Chemical compound OC(=O)[C@@H](N)CC1=CN=CN1 HNDVDQJCIGZPNO-YFKPBYRVSA-N 0.000 description 2
- 241001465754 Metazoa Species 0.000 description 2
- 208000003445 Mouth Neoplasms Diseases 0.000 description 2
- 206010029260 Neuroblastoma Diseases 0.000 description 2
- AHLPHDHHMVZTML-UHFFFAOYSA-N Orn-delta-NH2 Natural products NCCCC(N)C(O)=O AHLPHDHHMVZTML-UHFFFAOYSA-N 0.000 description 2
- UTJLXEIPEHZYQJ-UHFFFAOYSA-N Ornithine Natural products OC(=O)C(C)CCCN UTJLXEIPEHZYQJ-UHFFFAOYSA-N 0.000 description 2
- FAPWRFPIFSIZLT-UHFFFAOYSA-M Sodium chloride Chemical compound [Na+].[Cl-] FAPWRFPIFSIZLT-UHFFFAOYSA-M 0.000 description 2
- 229920004890 Triton X-100 Polymers 0.000 description 2
- 239000013504 Triton X-100 Substances 0.000 description 2
- 239000004480 active ingredient Substances 0.000 description 2
- 208000009956 adenocarcinoma Diseases 0.000 description 2
- 150000001335 aliphatic alkanes Chemical class 0.000 description 2
- 238000004458 analytical method Methods 0.000 description 2
- 235000009697 arginine Nutrition 0.000 description 2
- 125000003118 aryl group Chemical group 0.000 description 2
- 230000008901 benefit Effects 0.000 description 2
- 230000000975 bioactive effect Effects 0.000 description 2
- UORVGPXVDQYIDP-UHFFFAOYSA-N borane Chemical compound B UORVGPXVDQYIDP-UHFFFAOYSA-N 0.000 description 2
- 239000012267 brine Substances 0.000 description 2
- 230000015556 catabolic process Effects 0.000 description 2
- 230000006037 cell lysis Effects 0.000 description 2
- 239000003795 chemical substances by application Substances 0.000 description 2
- 210000001072 colon Anatomy 0.000 description 2
- 230000008878 coupling Effects 0.000 description 2
- 238000010168 coupling process Methods 0.000 description 2
- 238000005859 coupling reaction Methods 0.000 description 2
- 238000006731 degradation reaction Methods 0.000 description 2
- 238000009826 distribution Methods 0.000 description 2
- 125000000524 functional group Chemical group 0.000 description 2
- HNDVDQJCIGZPNO-UHFFFAOYSA-N histidine Natural products OC(=O)C(N)CC1=CN=CN1 HNDVDQJCIGZPNO-UHFFFAOYSA-N 0.000 description 2
- 239000000852 hydrogen donor Substances 0.000 description 2
- 238000011534 incubation Methods 0.000 description 2
- 125000000814 indol-3-yl group Chemical group [H]C1=C([H])C([H])=C2N([H])C([H])=C([*])C2=C1[H] 0.000 description 2
- 238000002347 injection Methods 0.000 description 2
- 239000007924 injection Substances 0.000 description 2
- 238000001990 intravenous administration Methods 0.000 description 2
- 208000012987 lip and oral cavity carcinoma Diseases 0.000 description 2
- 210000004072 lung Anatomy 0.000 description 2
- 238000001819 mass spectrum Methods 0.000 description 2
- 201000001441 melanoma Diseases 0.000 description 2
- 150000004702 methyl esters Chemical class 0.000 description 2
- HGASFNYMVGEKTF-UHFFFAOYSA-N octan-1-ol;hydrate Chemical compound O.CCCCCCCCO HGASFNYMVGEKTF-UHFFFAOYSA-N 0.000 description 2
- 229960003104 ornithine Drugs 0.000 description 2
- 210000001672 ovary Anatomy 0.000 description 2
- 230000010412 perfusion Effects 0.000 description 2
- 230000003389 potentiating effect Effects 0.000 description 2
- 235000018102 proteins Nutrition 0.000 description 2
- 108090000623 proteins and genes Proteins 0.000 description 2
- 102000004169 proteins and genes Human genes 0.000 description 2
- 238000011084 recovery Methods 0.000 description 2
- 238000000926 separation method Methods 0.000 description 2
- HPALAKNZSZLMCH-UHFFFAOYSA-M sodium;chloride;hydrate Chemical compound O.[Na+].[Cl-] HPALAKNZSZLMCH-UHFFFAOYSA-M 0.000 description 2
- 239000007790 solid phase Substances 0.000 description 2
- 239000000725 suspension Substances 0.000 description 2
- 230000002194 synthesizing effect Effects 0.000 description 2
- 231100001274 therapeutic index Toxicity 0.000 description 2
- GMKMEZVLHJARHF-UHFFFAOYSA-N (2R,6R)-form-2.6-Diaminoheptanedioic acid Natural products OC(=O)C(N)CCCC(N)C(O)=O GMKMEZVLHJARHF-UHFFFAOYSA-N 0.000 description 1
- NMDDZEVVQDPECF-LURJTMIESA-N (2s)-2,7-diaminoheptanoic acid Chemical compound NCCCCC[C@H](N)C(O)=O NMDDZEVVQDPECF-LURJTMIESA-N 0.000 description 1
- UMRUUWFGLGNQLI-QFIPXVFZSA-N (2s)-2-(9h-fluoren-9-ylmethoxycarbonylamino)-6-[(2-methylpropan-2-yl)oxycarbonylamino]hexanoic acid Chemical compound C1=CC=C2C(COC(=O)N[C@@H](CCCCNC(=O)OC(C)(C)C)C(O)=O)C3=CC=CC=C3C2=C1 UMRUUWFGLGNQLI-QFIPXVFZSA-N 0.000 description 1
- VHDOAFBNVURINJ-QMMMGPOBSA-N (2s)-5-amino-2-(dimethylamino)-2-methylpentanoic acid Chemical compound CN(C)[C@](C)(C(O)=O)CCCN VHDOAFBNVURINJ-QMMMGPOBSA-N 0.000 description 1
- PVOAHINGSUIXLS-UHFFFAOYSA-N 1-Methylpiperazine Chemical class CN1CCNCC1 PVOAHINGSUIXLS-UHFFFAOYSA-N 0.000 description 1
- PJUPKRYGDFTMTM-UHFFFAOYSA-N 1-hydroxybenzotriazole;hydrate Chemical compound O.C1=CC=C2N(O)N=NC2=C1 PJUPKRYGDFTMTM-UHFFFAOYSA-N 0.000 description 1
- BLCJBICVQSYOIF-UHFFFAOYSA-N 2,2-diaminobutanoic acid Chemical compound CCC(N)(N)C(O)=O BLCJBICVQSYOIF-UHFFFAOYSA-N 0.000 description 1
- SKWCZPYWFRTSDD-UHFFFAOYSA-N 2,3-bis(azaniumyl)propanoate;chloride Chemical compound Cl.NCC(N)C(O)=O SKWCZPYWFRTSDD-UHFFFAOYSA-N 0.000 description 1
- QSKDCDXNVDSXTJ-UHFFFAOYSA-N 2-(aminomethyl)-1-(4-methylpiperazin-1-yl)-3-[4-(trifluoromethyl)phenyl]-2-[[4-(trifluoromethyl)phenyl]methyl]propan-1-one Chemical compound C1CN(C)CCN1C(=O)C(CN)(CC=1C=CC(=CC=1)C(F)(F)F)CC1=CC=C(C(F)(F)F)C=C1 QSKDCDXNVDSXTJ-UHFFFAOYSA-N 0.000 description 1
- OEQPLOLNYDYFAD-UHFFFAOYSA-N 2-(aminomethyl)-1-(4-methylpiperazin-1-yl)-3-naphthalen-2-yl-2-(naphthalen-2-ylmethyl)propan-1-one Chemical compound C1CN(C)CCN1C(=O)C(CN)(CC=1C=C2C=CC=CC2=CC=1)CC1=CC=C(C=CC=C2)C2=C1 OEQPLOLNYDYFAD-UHFFFAOYSA-N 0.000 description 1
- SZKYSLMCLAOCLE-UHFFFAOYSA-N 2-(aminomethyl)-1-(4-methylpiperazin-1-yl)-5-phenyl-2-(3-phenylpropyl)pentan-1-one Chemical compound C1CN(C)CCN1C(=O)C(CN)(CCCC=1C=CC=CC=1)CCCC1=CC=CC=C1 SZKYSLMCLAOCLE-UHFFFAOYSA-N 0.000 description 1
- SXIXYNYGGGBIBP-UHFFFAOYSA-N 2-(aminomethyl)-1-[4-[2-(dimethylamino)ethyl]piperazin-1-yl]-3-(3,5-dimethylphenyl)-2-[(3,5-dimethylphenyl)methyl]propan-1-one Chemical compound C1CN(CCN(C)C)CCN1C(=O)C(CN)(CC=1C=C(C)C=C(C)C=1)CC1=CC(C)=CC(C)=C1 SXIXYNYGGGBIBP-UHFFFAOYSA-N 0.000 description 1
- YQMSMHNKRGSCAM-UHFFFAOYSA-N 2-(aminomethyl)-1-[4-[2-(dimethylamino)ethyl]piperazin-1-yl]-3-[4-(trifluoromethyl)phenyl]-2-[[4-(trifluoromethyl)phenyl]methyl]propan-1-one Chemical compound C1CN(CCN(C)C)CCN1C(=O)C(CN)(CC=1C=CC(=CC=1)C(F)(F)F)CC1=CC=C(C(F)(F)F)C=C1 YQMSMHNKRGSCAM-UHFFFAOYSA-N 0.000 description 1
- QPBRWBZMCPNEFM-UHFFFAOYSA-N 2-(aminomethyl)-1-[4-[2-(dimethylamino)ethyl]piperazin-1-yl]-3-naphthalen-2-yl-2-(naphthalen-2-ylmethyl)propan-1-one Chemical compound C1CN(CCN(C)C)CCN1C(=O)C(CN)(CC=1C=C2C=CC=CC2=CC=1)CC1=CC=C(C=CC=C2)C2=C1 QPBRWBZMCPNEFM-UHFFFAOYSA-N 0.000 description 1
- IHDMGHIWRBSLNB-UHFFFAOYSA-N 2-(aminomethyl)-3-(3,5-dimethylphenyl)-2-[(3,5-dimethylphenyl)methyl]-1-(4-methylpiperazin-1-yl)propan-1-one Chemical compound C1CN(C)CCN1C(=O)C(CN)(CC=1C=C(C)C=C(C)C=1)CC1=CC(C)=CC(C)=C1 IHDMGHIWRBSLNB-UHFFFAOYSA-N 0.000 description 1
- FXTFNKKEUSXQQA-UHFFFAOYSA-N 2-(aminomethyl)-3-(4-tert-butylphenyl)-2-[(4-tert-butylphenyl)methyl]-1-(4-methylpiperazin-1-yl)propan-1-one Chemical compound C1CN(C)CCN1C(=O)C(CN)(CC=1C=CC(=CC=1)C(C)(C)C)CC1=CC=C(C(C)(C)C)C=C1 FXTFNKKEUSXQQA-UHFFFAOYSA-N 0.000 description 1
- GSZCLKHYJBMCHT-UHFFFAOYSA-N 2-(aminomethyl)-n-[2-(dimethylamino)ethyl]-5-phenyl-2-(3-phenylpropyl)pentanamide Chemical compound C=1C=CC=CC=1CCCC(CN)(C(=O)NCCN(C)C)CCCC1=CC=CC=C1 GSZCLKHYJBMCHT-UHFFFAOYSA-N 0.000 description 1
- FFDDLJYKJQGSPW-UHFFFAOYSA-N 2-[4-[2-hydroxy-3-(propan-2-ylamino)propoxy]phenyl]acetamide;hydrochloride Chemical compound Cl.CC(C)NCC(O)COC1=CC=C(CC(N)=O)C=C1 FFDDLJYKJQGSPW-UHFFFAOYSA-N 0.000 description 1
- QKNYBSVHEMOAJP-UHFFFAOYSA-N 2-amino-2-(hydroxymethyl)propane-1,3-diol;hydron;chloride Chemical compound Cl.OCC(N)(CO)CO QKNYBSVHEMOAJP-UHFFFAOYSA-N 0.000 description 1
- JHFFYTDZGNWIEI-UHFFFAOYSA-N 2-ethyl-N,N-dimethylpiperazin-1-amine Chemical class CN(N1C(CNCC1)CC)C JHFFYTDZGNWIEI-UHFFFAOYSA-N 0.000 description 1
- FJKROLUGYXJWQN-UHFFFAOYSA-N 4-hydroxybenzoic acid Chemical class OC(=O)C1=CC=C(O)C=C1 FJKROLUGYXJWQN-UHFFFAOYSA-N 0.000 description 1
- 125000006181 4-methyl benzyl group Chemical group [H]C1=C([H])C(=C([H])C([H])=C1C([H])([H])[H])C([H])([H])* 0.000 description 1
- TYMLOMAKGOJONV-UHFFFAOYSA-N 4-nitroaniline Chemical compound NC1=CC=C([N+]([O-])=O)C=C1 TYMLOMAKGOJONV-UHFFFAOYSA-N 0.000 description 1
- ZCYVEMRRCGMTRW-UHFFFAOYSA-N 7553-56-2 Chemical compound [I] ZCYVEMRRCGMTRW-UHFFFAOYSA-N 0.000 description 1
- 229920001817 Agar Polymers 0.000 description 1
- 101800002011 Amphipathic peptide Proteins 0.000 description 1
- 108091006334 Anaphylatoxin receptors Proteins 0.000 description 1
- 102000014133 Antimicrobial Cationic Peptides Human genes 0.000 description 1
- 108010050820 Antimicrobial Cationic Peptides Proteins 0.000 description 1
- 241000203069 Archaea Species 0.000 description 1
- 208000011691 Burkitt lymphomas Diseases 0.000 description 1
- AHSHQVINSCWCMT-UHFFFAOYSA-N C1=CC=C(C=C1)CCC(CC(CN)C2=CC=CC=C2)C(=O)NCCN Chemical compound C1=CC=C(C=C1)CCC(CC(CN)C2=CC=CC=C2)C(=O)NCCN AHSHQVINSCWCMT-UHFFFAOYSA-N 0.000 description 1
- ALZRNKYUESVGGJ-UHFFFAOYSA-N CC(C)(C)c1ccc(CC(Cc2ccc(C(C)(C)C)cc2)(CN)C(NCCN(C)C)=O)cc1 Chemical compound CC(C)(C)c1ccc(CC(Cc2ccc(C(C)(C)C)cc2)(CN)C(NCCN(C)C)=O)cc1 ALZRNKYUESVGGJ-UHFFFAOYSA-N 0.000 description 1
- GOVXKUCVZUROAN-UHFFFAOYSA-N CCc1c[nH]c2ccccc12 Chemical compound CCc1c[nH]c2ccccc12 GOVXKUCVZUROAN-UHFFFAOYSA-N 0.000 description 1
- ZFRKQXVRDFCRJG-UHFFFAOYSA-N Cc1c[nH]c2ccccc12 Chemical compound Cc1c[nH]c2ccccc12 ZFRKQXVRDFCRJG-UHFFFAOYSA-N 0.000 description 1
- ZAMOUSCENKQFHK-UHFFFAOYSA-N Chlorine atom Chemical compound [Cl] ZAMOUSCENKQFHK-UHFFFAOYSA-N 0.000 description 1
- 208000017667 Chronic Disease Diseases 0.000 description 1
- 206010052360 Colorectal adenocarcinoma Diseases 0.000 description 1
- 102000004127 Cytokines Human genes 0.000 description 1
- 108090000695 Cytokines Proteins 0.000 description 1
- PIICEJLVQHRZGT-UHFFFAOYSA-N Ethylenediamine Chemical class NCCN PIICEJLVQHRZGT-UHFFFAOYSA-N 0.000 description 1
- 241000192125 Firmicutes Species 0.000 description 1
- PXGOKWXKJXAPGV-UHFFFAOYSA-N Fluorine Chemical compound FF PXGOKWXKJXAPGV-UHFFFAOYSA-N 0.000 description 1
- 241000233866 Fungi Species 0.000 description 1
- 230000005526 G1 to G0 transition Effects 0.000 description 1
- CEAZRRDELHUEMR-URQXQFDESA-N Gentamicin Chemical compound O1[C@H](C(C)NC)CC[C@@H](N)[C@H]1O[C@H]1[C@H](O)[C@@H](O[C@@H]2[C@@H]([C@@H](NC)[C@@](C)(O)CO2)O)[C@H](N)C[C@@H]1N CEAZRRDELHUEMR-URQXQFDESA-N 0.000 description 1
- 229930182566 Gentamicin Natural products 0.000 description 1
- 206010018910 Haemolysis Diseases 0.000 description 1
- 102000001554 Hemoglobins Human genes 0.000 description 1
- 108010054147 Hemoglobins Proteins 0.000 description 1
- HTTJABKRGRZYRN-UHFFFAOYSA-N Heparin Chemical compound OC1C(NC(=O)C)C(O)OC(COS(O)(=O)=O)C1OC1C(OS(O)(=O)=O)C(O)C(OC2C(C(OS(O)(=O)=O)C(OC3C(C(O)C(O)C(O3)C(O)=O)OS(O)(=O)=O)C(CO)O2)NS(O)(=O)=O)C(C(O)=O)O1 HTTJABKRGRZYRN-UHFFFAOYSA-N 0.000 description 1
- WRYCSMQKUKOKBP-UHFFFAOYSA-N Imidazolidine Chemical compound C1CNCN1 WRYCSMQKUKOKBP-UHFFFAOYSA-N 0.000 description 1
- QUOGESRFPZDMMT-UHFFFAOYSA-N L-Homoarginine Natural products OC(=O)C(N)CCCCNC(N)=N QUOGESRFPZDMMT-UHFFFAOYSA-N 0.000 description 1
- ULEBESPCVWBNIF-BYPYZUCNSA-N L-arginine amide Chemical group NC(=O)[C@@H](N)CCCNC(N)=N ULEBESPCVWBNIF-BYPYZUCNSA-N 0.000 description 1
- QUOGESRFPZDMMT-YFKPBYRVSA-N L-homoarginine Chemical compound OC(=O)[C@@H](N)CCCCNC(N)=N QUOGESRFPZDMMT-YFKPBYRVSA-N 0.000 description 1
- QIVBCDIJIAJPQS-VIFPVBQESA-N L-tryptophane Chemical compound C1=CC=C2C(C[C@H](N)C(O)=O)=CNC2=C1 QIVBCDIJIAJPQS-VIFPVBQESA-N 0.000 description 1
- OUYCCCASQSFEME-QMMMGPOBSA-N L-tyrosine Chemical compound OC(=O)[C@@H](N)CC1=CC=C(O)C=C1 OUYCCCASQSFEME-QMMMGPOBSA-N 0.000 description 1
- 206010023856 Laryngeal squamous cell carcinoma Diseases 0.000 description 1
- 206010025323 Lymphomas Diseases 0.000 description 1
- 238000007126 N-alkylation reaction Methods 0.000 description 1
- IEKVOZMMICOSTB-AYFHWJSNSA-N NCCCC[C@@H](C(C1N(CC(N)=O)C1)=O)NC(CCC1(C(C(Cc2cc3ccccc3cc2)(Cc2cc(cccc3)c3cc2)CNC([C@@H](Cc2c[nH]c3c2cccc3)NC(CNC(CN)=O)=O)=O)=O)NC1)=O Chemical compound NCCCC[C@@H](C(C1N(CC(N)=O)C1)=O)NC(CCC1(C(C(Cc2cc3ccccc3cc2)(Cc2cc(cccc3)c3cc2)CNC([C@@H](Cc2c[nH]c3c2cccc3)NC(CNC(CN)=O)=O)=O)=O)NC1)=O IEKVOZMMICOSTB-AYFHWJSNSA-N 0.000 description 1
- 108010043958 Peptoids Proteins 0.000 description 1
- 239000004793 Polystyrene Substances 0.000 description 1
- 206010060862 Prostate cancer Diseases 0.000 description 1
- VYPSYNLAJGMNEJ-UHFFFAOYSA-N Silicium dioxide Chemical compound O=[Si]=O VYPSYNLAJGMNEJ-UHFFFAOYSA-N 0.000 description 1
- NINIDFKCEFEMDL-UHFFFAOYSA-N Sulfur Chemical compound [S] NINIDFKCEFEMDL-UHFFFAOYSA-N 0.000 description 1
- QIVBCDIJIAJPQS-UHFFFAOYSA-N Tryptophan Natural products C1=CC=C2C(CC(N)C(O)=O)=CNC2=C1 QIVBCDIJIAJPQS-UHFFFAOYSA-N 0.000 description 1
- 238000010306 acid treatment Methods 0.000 description 1
- 239000008272 agar Substances 0.000 description 1
- 125000001931 aliphatic group Chemical group 0.000 description 1
- 150000001336 alkenes Chemical class 0.000 description 1
- 125000000278 alkyl amino alkyl group Chemical group 0.000 description 1
- 125000000217 alkyl group Chemical group 0.000 description 1
- 125000006242 amine protecting group Chemical group 0.000 description 1
- 229940124350 antibacterial drug Drugs 0.000 description 1
- 239000003429 antifungal agent Substances 0.000 description 1
- 239000000427 antigen Substances 0.000 description 1
- 108091007433 antigens Proteins 0.000 description 1
- 102000036639 antigens Human genes 0.000 description 1
- GHPGOEFPKIHBNM-UHFFFAOYSA-N antimony(3+);oxygen(2-) Chemical compound [O-2].[O-2].[O-2].[Sb+3].[Sb+3] GHPGOEFPKIHBNM-UHFFFAOYSA-N 0.000 description 1
- 238000013459 approach Methods 0.000 description 1
- 229910052786 argon Inorganic materials 0.000 description 1
- 150000007980 azole derivatives Chemical class 0.000 description 1
- 210000003719 b-lymphocyte Anatomy 0.000 description 1
- 230000003385 bacteriostatic effect Effects 0.000 description 1
- 230000004888 barrier function Effects 0.000 description 1
- 125000001584 benzyloxycarbonyl group Chemical group C(=O)(OCC1=CC=CC=C1)* 0.000 description 1
- 230000003115 biocidal effect Effects 0.000 description 1
- 230000002051 biphasic effect Effects 0.000 description 1
- 230000000903 blocking effect Effects 0.000 description 1
- 229910000085 borane Inorganic materials 0.000 description 1
- 210000004556 brain Anatomy 0.000 description 1
- 239000000872 buffer Substances 0.000 description 1
- 244000309464 bull Species 0.000 description 1
- 229940022399 cancer vaccine Drugs 0.000 description 1
- 239000002775 capsule Substances 0.000 description 1
- 238000001460 carbon-13 nuclear magnetic resonance spectrum Methods 0.000 description 1
- 125000002915 carbonyl group Chemical group [*:2]C([*:1])=O 0.000 description 1
- 239000000969 carrier Substances 0.000 description 1
- 150000001768 cations Chemical class 0.000 description 1
- 230000030833 cell death Effects 0.000 description 1
- 210000000170 cell membrane Anatomy 0.000 description 1
- 238000005119 centrifugation Methods 0.000 description 1
- 239000003638 chemical reducing agent Substances 0.000 description 1
- 239000000460 chlorine Substances 0.000 description 1
- 229910052801 chlorine Inorganic materials 0.000 description 1
- DQLATGHUWYMOKM-UHFFFAOYSA-L cisplatin Chemical compound N[Pt](N)(Cl)Cl DQLATGHUWYMOKM-UHFFFAOYSA-L 0.000 description 1
- 229960004316 cisplatin Drugs 0.000 description 1
- 238000003776 cleavage reaction Methods 0.000 description 1
- 229940125898 compound 5 Drugs 0.000 description 1
- 230000008094 contradictory effect Effects 0.000 description 1
- 229940127089 cytotoxic agent Drugs 0.000 description 1
- 230000003247 decreasing effect Effects 0.000 description 1
- 238000010511 deprotection reaction Methods 0.000 description 1
- 238000013461 design Methods 0.000 description 1
- 239000000645 desinfectant Substances 0.000 description 1
- 230000001687 destabilization Effects 0.000 description 1
- 230000001066 destructive effect Effects 0.000 description 1
- 230000029087 digestion Effects 0.000 description 1
- 201000010099 disease Diseases 0.000 description 1
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 description 1
- 238000009510 drug design Methods 0.000 description 1
- 238000009509 drug development Methods 0.000 description 1
- 239000000839 emulsion Substances 0.000 description 1
- 230000003511 endothelial effect Effects 0.000 description 1
- 230000007515 enzymatic degradation Effects 0.000 description 1
- 230000006862 enzymatic digestion Effects 0.000 description 1
- 125000001033 ether group Chemical group 0.000 description 1
- AEOCXXJPGCBFJA-UHFFFAOYSA-N ethionamide Chemical compound CCC1=CC(C(N)=S)=CC=N1 AEOCXXJPGCBFJA-UHFFFAOYSA-N 0.000 description 1
- 238000011156 evaluation Methods 0.000 description 1
- 230000001747 exhibiting effect Effects 0.000 description 1
- 238000002474 experimental method Methods 0.000 description 1
- 238000000605 extraction Methods 0.000 description 1
- 210000002950 fibroblast Anatomy 0.000 description 1
- 229910052731 fluorine Inorganic materials 0.000 description 1
- 239000011737 fluorine Substances 0.000 description 1
- 125000001153 fluoro group Chemical group F* 0.000 description 1
- 125000004216 fluoromethyl group Chemical group [H]C([H])(F)* 0.000 description 1
- 229960002518 gentamicin Drugs 0.000 description 1
- 210000004907 gland Anatomy 0.000 description 1
- 208000005017 glioblastoma Diseases 0.000 description 1
- 239000003102 growth factor Substances 0.000 description 1
- ZRALSGWEFCBTJO-UHFFFAOYSA-N guanidine group Chemical group NC(=N)N ZRALSGWEFCBTJO-UHFFFAOYSA-N 0.000 description 1
- 229910052736 halogen Inorganic materials 0.000 description 1
- 150000002367 halogens Chemical class 0.000 description 1
- 230000036541 health Effects 0.000 description 1
- 230000008588 hemolysis Effects 0.000 description 1
- 229960002897 heparin Drugs 0.000 description 1
- 229920000669 heparin Polymers 0.000 description 1
- 125000005842 heteroatom Chemical group 0.000 description 1
- 125000000623 heterocyclic group Chemical group 0.000 description 1
- 150000004678 hydrides Chemical class 0.000 description 1
- 150000002431 hydrogen Chemical class 0.000 description 1
- 230000007062 hydrolysis Effects 0.000 description 1
- 238000006460 hydrolysis reaction Methods 0.000 description 1
- 230000003301 hydrolyzing effect Effects 0.000 description 1
- 230000002209 hydrophobic effect Effects 0.000 description 1
- 125000002887 hydroxy group Chemical group [H]O* 0.000 description 1
- 230000001900 immune effect Effects 0.000 description 1
- 210000000987 immune system Anatomy 0.000 description 1
- 230000036039 immunity Effects 0.000 description 1
- 239000002955 immunomodulating agent Substances 0.000 description 1
- 229940121354 immunomodulator Drugs 0.000 description 1
- 230000006872 improvement Effects 0.000 description 1
- 238000000338 in vitro Methods 0.000 description 1
- 238000001727 in vivo Methods 0.000 description 1
- 238000010348 incorporation Methods 0.000 description 1
- 208000027866 inflammatory disease Diseases 0.000 description 1
- 238000001802 infusion Methods 0.000 description 1
- 229910052740 iodine Inorganic materials 0.000 description 1
- 239000011630 iodine Substances 0.000 description 1
- 210000003734 kidney Anatomy 0.000 description 1
- 150000002605 large molecules Chemical class 0.000 description 1
- 239000010985 leather Substances 0.000 description 1
- 208000032839 leukemia Diseases 0.000 description 1
- 239000003446 ligand Substances 0.000 description 1
- 125000003473 lipid group Chemical group 0.000 description 1
- 239000002502 liposome Substances 0.000 description 1
- 239000007788 liquid Substances 0.000 description 1
- 210000004185 liver Anatomy 0.000 description 1
- 244000144972 livestock Species 0.000 description 1
- 238000011068 loading method Methods 0.000 description 1
- 235000018977 lysine Nutrition 0.000 description 1
- 230000002101 lytic effect Effects 0.000 description 1
- 229920002521 macromolecule Polymers 0.000 description 1
- 230000014759 maintenance of location Effects 0.000 description 1
- 210000005075 mammary gland Anatomy 0.000 description 1
- 238000004949 mass spectrometry Methods 0.000 description 1
- 239000000463 material Substances 0.000 description 1
- 230000007246 mechanism Effects 0.000 description 1
- GMKMEZVLHJARHF-SYDPRGILSA-N meso-2,6-diaminopimelic acid Chemical compound [O-]C(=O)[C@@H]([NH3+])CCC[C@@H]([NH3+])C([O-])=O GMKMEZVLHJARHF-SYDPRGILSA-N 0.000 description 1
- WDWDWGRYHDPSDS-UHFFFAOYSA-N methanimine Chemical compound N=C WDWDWGRYHDPSDS-UHFFFAOYSA-N 0.000 description 1
- 125000000956 methoxy group Chemical group [H]C([H])([H])O* 0.000 description 1
- 230000011987 methylation Effects 0.000 description 1
- 235000013336 milk Nutrition 0.000 description 1
- 239000008267 milk Substances 0.000 description 1
- 210000004080 milk Anatomy 0.000 description 1
- 238000002156 mixing Methods 0.000 description 1
- 230000004048 modification Effects 0.000 description 1
- 238000012986 modification Methods 0.000 description 1
- 210000000214 mouth Anatomy 0.000 description 1
- GKUKXYDPDYIORP-UHFFFAOYSA-N n-(2-aminoethyl)-2-(aminomethyl)-2-propylpentanamide Chemical compound CCCC(CN)(CCC)C(=O)NCCN GKUKXYDPDYIORP-UHFFFAOYSA-N 0.000 description 1
- SMKGPOBRXVODMK-UHFFFAOYSA-N n-(2-aminoethyl)-2-(aminomethyl)-4-cyclohexyl-2-(2-cyclohexylethyl)butanamide Chemical compound C1CCCCC1CCC(CN)(C(=O)NCCN)CCC1CCCCC1 SMKGPOBRXVODMK-UHFFFAOYSA-N 0.000 description 1
- JVOZATDVXUSPKC-UHFFFAOYSA-N n-(2-aminoethyl)-2-(aminomethyl)-5-phenyl-2-(3-phenylpropyl)pentanamide Chemical compound C=1C=CC=CC=1CCCC(CN)(C(=O)NCCN)CCCC1=CC=CC=C1 JVOZATDVXUSPKC-UHFFFAOYSA-N 0.000 description 1
- SOGZMDYBQZTXIJ-UHFFFAOYSA-N n-(cyclohexylmethyl)propanamide Chemical compound CCC(=O)NCC1CCCCC1 SOGZMDYBQZTXIJ-UHFFFAOYSA-N 0.000 description 1
- VRBPVCCMUXYKQU-UHFFFAOYSA-N n-[(3,5-dimethoxyphenyl)methyl]propanamide Chemical compound CCC(=O)NCC1=CC(OC)=CC(OC)=C1 VRBPVCCMUXYKQU-UHFFFAOYSA-N 0.000 description 1
- DWUKPZRHBMSEBP-UHFFFAOYSA-N n-[(3,5-dimethylphenyl)methyl]propanamide Chemical compound CCC(=O)NCC1=CC(C)=CC(C)=C1 DWUKPZRHBMSEBP-UHFFFAOYSA-N 0.000 description 1
- OSTGXTASNHUYFX-UHFFFAOYSA-N n-[(3,5-ditert-butylphenyl)methyl]propanamide Chemical compound CCC(=O)NCC1=CC(C(C)(C)C)=CC(C(C)(C)C)=C1 OSTGXTASNHUYFX-UHFFFAOYSA-N 0.000 description 1
- VOUJNJOXQSJIKB-UHFFFAOYSA-N n-[(4-propan-2-ylphenyl)methyl]propanamide Chemical compound CCC(=O)NCC1=CC=C(C(C)C)C=C1 VOUJNJOXQSJIKB-UHFFFAOYSA-N 0.000 description 1
- WJSVLIMLQVTJDD-UHFFFAOYSA-N n-[(4-tert-butylphenyl)methyl]propanamide Chemical compound CCC(=O)NCC1=CC=C(C(C)(C)C)C=C1 WJSVLIMLQVTJDD-UHFFFAOYSA-N 0.000 description 1
- RFBIIXCJFYHQCT-UHFFFAOYSA-N n-[[4-(trifluoromethyl)phenyl]methyl]propanamide Chemical compound CCC(=O)NCC1=CC=C(C(F)(F)F)C=C1 RFBIIXCJFYHQCT-UHFFFAOYSA-N 0.000 description 1
- 125000001624 naphthyl group Chemical group 0.000 description 1
- 239000007922 nasal spray Substances 0.000 description 1
- 239000002547 new drug Substances 0.000 description 1
- QJGQUHMNIGDVPM-UHFFFAOYSA-N nitrogen group Chemical group [N] QJGQUHMNIGDVPM-UHFFFAOYSA-N 0.000 description 1
- 230000008520 organization Effects 0.000 description 1
- 239000007800 oxidant agent Substances 0.000 description 1
- 125000006503 p-nitrobenzyl group Chemical group [H]C1=C([H])C(=C([H])C([H])=C1[N+]([O-])=O)C([H])([H])* 0.000 description 1
- 210000000496 pancreas Anatomy 0.000 description 1
- 238000005192 partition Methods 0.000 description 1
- HGBOYTHUEUWSSQ-UHFFFAOYSA-N pentanal Chemical compound CCCCC=O HGBOYTHUEUWSSQ-UHFFFAOYSA-N 0.000 description 1
- 229940124531 pharmaceutical excipient Drugs 0.000 description 1
- 230000000144 pharmacologic effect Effects 0.000 description 1
- 239000012071 phase Substances 0.000 description 1
- 150000003904 phospholipids Chemical class 0.000 description 1
- 210000002381 plasma Anatomy 0.000 description 1
- 238000007747 plating Methods 0.000 description 1
- 229920000232 polyglycine polymer Polymers 0.000 description 1
- 229920002223 polystyrene Polymers 0.000 description 1
- 239000000843 powder Substances 0.000 description 1
- 239000002243 precursor Substances 0.000 description 1
- 239000003755 preservative agent Substances 0.000 description 1
- 125000001749 primary amide group Chemical group 0.000 description 1
- 125000002924 primary amino group Chemical group [H]N([H])* 0.000 description 1
- 230000008569 process Effects 0.000 description 1
- 230000002035 prolonged effect Effects 0.000 description 1
- UFUASNAHBMBJIX-UHFFFAOYSA-N propan-1-one Chemical compound CC[C]=O UFUASNAHBMBJIX-UHFFFAOYSA-N 0.000 description 1
- 230000000069 prophylactic effect Effects 0.000 description 1
- 210000002307 prostate Anatomy 0.000 description 1
- 201000001514 prostate carcinoma Diseases 0.000 description 1
- 230000001681 protective effect Effects 0.000 description 1
- 230000002797 proteolythic effect Effects 0.000 description 1
- 238000000425 proton nuclear magnetic resonance spectrum Methods 0.000 description 1
- USPWKWBDZOARPV-UHFFFAOYSA-N pyrazolidine Chemical compound C1CNNC1 USPWKWBDZOARPV-UHFFFAOYSA-N 0.000 description 1
- 108020003175 receptors Proteins 0.000 description 1
- 230000000717 retained effect Effects 0.000 description 1
- 230000007017 scission Effects 0.000 description 1
- 125000003156 secondary amide group Chemical group 0.000 description 1
- 208000011581 secondary neoplasm Diseases 0.000 description 1
- 210000003491 skin Anatomy 0.000 description 1
- 239000011734 sodium Substances 0.000 description 1
- 239000011780 sodium chloride Substances 0.000 description 1
- 206010041823 squamous cell carcinoma Diseases 0.000 description 1
- 238000013112 stability test Methods 0.000 description 1
- 239000003381 stabilizer Substances 0.000 description 1
- 239000011550 stock solution Substances 0.000 description 1
- 239000000758 substrate Substances 0.000 description 1
- 125000005420 sulfonamido group Chemical group S(=O)(=O)(N*)* 0.000 description 1
- 239000011593 sulfur Substances 0.000 description 1
- 230000001629 suppression Effects 0.000 description 1
- 230000008961 swelling Effects 0.000 description 1
- 208000024891 symptom Diseases 0.000 description 1
- 238000001308 synthesis method Methods 0.000 description 1
- 239000003826 tablet Substances 0.000 description 1
- 229940124597 therapeutic agent Drugs 0.000 description 1
- 238000002560 therapeutic procedure Methods 0.000 description 1
- 150000003568 thioethers Chemical class 0.000 description 1
- 230000000699 topical effect Effects 0.000 description 1
- 125000002221 trityl group Chemical group [H]C1=C([H])C([H])=C([H])C([H])=C1C([*])(C1=C(C(=C(C(=C1[H])[H])[H])[H])[H])C1=C([H])C([H])=C([H])C([H])=C1[H] 0.000 description 1
- 125000000430 tryptophan group Chemical group [H]N([H])C(C(=O)O*)C([H])([H])C1=C([H])N([H])C2=C([H])C([H])=C([H])C([H])=C12 0.000 description 1
- OUYCCCASQSFEME-UHFFFAOYSA-N tyrosine Natural products OC(=O)C(N)CC1=CC=C(O)C=C1 OUYCCCASQSFEME-UHFFFAOYSA-N 0.000 description 1
- 210000003606 umbilical vein Anatomy 0.000 description 1
- 125000000391 vinyl group Chemical group [H]C([*])=C([H])[H] 0.000 description 1
- 229920002554 vinyl polymer Polymers 0.000 description 1
- 238000005406 washing Methods 0.000 description 1
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K5/00—Peptides containing up to four amino acids in a fully defined sequence; Derivatives thereof
- C07K5/02—Peptides containing up to four amino acids in a fully defined sequence; Derivatives thereof containing at least one abnormal peptide link
- C07K5/0202—Peptides containing up to four amino acids in a fully defined sequence; Derivatives thereof containing at least one abnormal peptide link containing the structure -NH-X-X-C(=0)-, X being an optionally substituted carbon atom or a heteroatom, e.g. beta-amino acids
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K5/00—Peptides containing up to four amino acids in a fully defined sequence; Derivatives thereof
- C07K5/02—Peptides containing up to four amino acids in a fully defined sequence; Derivatives thereof containing at least one abnormal peptide link
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K38/00—Medicinal preparations containing peptides
- A61K38/04—Peptides having up to 20 amino acids in a fully defined sequence; Derivatives thereof
- A61K38/07—Tetrapeptides
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K38/00—Medicinal preparations containing peptides
- A61K38/04—Peptides having up to 20 amino acids in a fully defined sequence; Derivatives thereof
- A61K38/08—Peptides having 5 to 11 amino acids
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P31/00—Antiinfectives, i.e. antibiotics, antiseptics, chemotherapeutics
- A61P31/04—Antibacterial agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
- A61P35/04—Antineoplastic agents specific for metastasis
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P43/00—Drugs for specific purposes, not provided for in groups A61P1/00-A61P41/00
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C237/00—Carboxylic acid amides, the carbon skeleton of the acid part being further substituted by amino groups
- C07C237/02—Carboxylic acid amides, the carbon skeleton of the acid part being further substituted by amino groups having the carbon atoms of the carboxamide groups bound to acyclic carbon atoms of the carbon skeleton
- C07C237/20—Carboxylic acid amides, the carbon skeleton of the acid part being further substituted by amino groups having the carbon atoms of the carboxamide groups bound to acyclic carbon atoms of the carbon skeleton the carbon skeleton containing six-membered aromatic rings
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D209/00—Heterocyclic compounds containing five-membered rings, condensed with other rings, with one nitrogen atom as the only ring hetero atom
- C07D209/02—Heterocyclic compounds containing five-membered rings, condensed with other rings, with one nitrogen atom as the only ring hetero atom condensed with one carbocyclic ring
- C07D209/04—Indoles; Hydrogenated indoles
- C07D209/10—Indoles; Hydrogenated indoles with substituted hydrocarbon radicals attached to carbon atoms of the hetero ring
- C07D209/18—Radicals substituted by carbon atoms having three bonds to hetero atoms with at the most one bond to halogen, e.g. ester or nitrile radicals
- C07D209/20—Radicals substituted by carbon atoms having three bonds to hetero atoms with at the most one bond to halogen, e.g. ester or nitrile radicals substituted additionally by nitrogen atoms, e.g. tryptophane
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D237/00—Heterocyclic compounds containing 1,2-diazine or hydrogenated 1,2-diazine rings
- C07D237/02—Heterocyclic compounds containing 1,2-diazine or hydrogenated 1,2-diazine rings not condensed with other rings
- C07D237/06—Heterocyclic compounds containing 1,2-diazine or hydrogenated 1,2-diazine rings not condensed with other rings having three double bonds between ring members or between ring members and non-ring members
- C07D237/10—Heterocyclic compounds containing 1,2-diazine or hydrogenated 1,2-diazine rings not condensed with other rings having three double bonds between ring members or between ring members and non-ring members with hetero atoms or with carbon atoms having three bonds to hetero atoms with at the most one bond to halogen, e.g. ester or nitrile radicals, directly attached to ring carbon atoms
- C07D237/20—Nitrogen atoms
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D279/00—Heterocyclic compounds containing six-membered rings having one nitrogen atom and one sulfur atom as the only ring hetero atoms
- C07D279/10—1,4-Thiazines; Hydrogenated 1,4-thiazines
- C07D279/14—1,4-Thiazines; Hydrogenated 1,4-thiazines condensed with carbocyclic rings or ring systems
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D295/00—Heterocyclic compounds containing polymethylene-imine rings with at least five ring members, 3-azabicyclo [3.2.2] nonane, piperazine, morpholine or thiomorpholine rings, having only hydrogen atoms directly attached to the ring carbon atoms
- C07D295/16—Heterocyclic compounds containing polymethylene-imine rings with at least five ring members, 3-azabicyclo [3.2.2] nonane, piperazine, morpholine or thiomorpholine rings, having only hydrogen atoms directly attached to the ring carbon atoms acylated on ring nitrogen atoms
- C07D295/18—Heterocyclic compounds containing polymethylene-imine rings with at least five ring members, 3-azabicyclo [3.2.2] nonane, piperazine, morpholine or thiomorpholine rings, having only hydrogen atoms directly attached to the ring carbon atoms acylated on ring nitrogen atoms by radicals derived from carboxylic acids, or sulfur or nitrogen analogues thereof
- C07D295/182—Radicals derived from carboxylic acids
- C07D295/185—Radicals derived from carboxylic acids from aliphatic carboxylic acids
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D295/00—Heterocyclic compounds containing polymethylene-imine rings with at least five ring members, 3-azabicyclo [3.2.2] nonane, piperazine, morpholine or thiomorpholine rings, having only hydrogen atoms directly attached to the ring carbon atoms
- C07D295/16—Heterocyclic compounds containing polymethylene-imine rings with at least five ring members, 3-azabicyclo [3.2.2] nonane, piperazine, morpholine or thiomorpholine rings, having only hydrogen atoms directly attached to the ring carbon atoms acylated on ring nitrogen atoms
- C07D295/18—Heterocyclic compounds containing polymethylene-imine rings with at least five ring members, 3-azabicyclo [3.2.2] nonane, piperazine, morpholine or thiomorpholine rings, having only hydrogen atoms directly attached to the ring carbon atoms acylated on ring nitrogen atoms by radicals derived from carboxylic acids, or sulfur or nitrogen analogues thereof
- C07D295/182—Radicals derived from carboxylic acids
- C07D295/192—Radicals derived from carboxylic acids from aromatic carboxylic acids
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K7/00—Peptides having 5 to 20 amino acids in a fully defined sequence; Derivatives thereof
- C07K7/02—Linear peptides containing at least one abnormal peptide link
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K38/00—Medicinal preparations containing peptides
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C2601/00—Systems containing only non-condensed rings
- C07C2601/12—Systems containing only non-condensed rings with a six-membered ring
- C07C2601/14—The ring being saturated
Landscapes
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- General Health & Medical Sciences (AREA)
- Medicinal Chemistry (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Veterinary Medicine (AREA)
- Pharmacology & Pharmacy (AREA)
- Animal Behavior & Ethology (AREA)
- Public Health (AREA)
- Biochemistry (AREA)
- Molecular Biology (AREA)
- Genetics & Genomics (AREA)
- Biophysics (AREA)
- General Chemical & Material Sciences (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Engineering & Computer Science (AREA)
- Gastroenterology & Hepatology (AREA)
- Oncology (AREA)
- Immunology (AREA)
- Epidemiology (AREA)
- Crystallography & Structural Chemistry (AREA)
- Communicable Diseases (AREA)
- Medicines That Contain Protein Lipid Enzymes And Other Medicines (AREA)
- Peptides Or Proteins (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
- Acyclic And Carbocyclic Compounds In Medicinal Compositions (AREA)
- Organic Low-Molecular-Weight Compounds And Preparation Thereof (AREA)
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| GB0919194.1 | 2009-11-02 | ||
| GBGB0919194.1A GB0919194D0 (en) | 2009-11-02 | 2009-11-02 | Compounds |
| PCT/GB2010/002024 WO2011051692A1 (en) | 2009-11-02 | 2010-11-02 | Therapeutic peptides |
Related Child Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| RU2015111706A Division RU2676713C2 (ru) | 2009-11-02 | 2010-11-02 | Терапевтические пептиды |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| RU2012121264A RU2012121264A (ru) | 2013-12-10 |
| RU2548905C2 true RU2548905C2 (ru) | 2015-04-20 |
Family
ID=41435032
Family Applications (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| RU2012121264/04A RU2548905C2 (ru) | 2009-11-02 | 2010-11-02 | Терапевтические пептиды |
| RU2015111706A RU2676713C2 (ru) | 2009-11-02 | 2010-11-02 | Терапевтические пептиды |
Family Applications After (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| RU2015111706A RU2676713C2 (ru) | 2009-11-02 | 2010-11-02 | Терапевтические пептиды |
Country Status (15)
| Country | Link |
|---|---|
| US (1) | US8809280B2 (enExample) |
| EP (1) | EP2496596B1 (enExample) |
| JP (1) | JP5810090B2 (enExample) |
| KR (1) | KR101730680B1 (enExample) |
| CN (1) | CN102762586B (enExample) |
| AU (1) | AU2010311186B2 (enExample) |
| BR (1) | BR112012010333A2 (enExample) |
| CA (1) | CA2777749C (enExample) |
| DK (1) | DK2496596T3 (enExample) |
| ES (1) | ES2621188T3 (enExample) |
| GB (1) | GB0919194D0 (enExample) |
| NZ (1) | NZ599791A (enExample) |
| PL (1) | PL2496596T3 (enExample) |
| RU (2) | RU2548905C2 (enExample) |
| WO (1) | WO2011051692A1 (enExample) |
Families Citing this family (16)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP3974563A1 (en) | 2011-12-28 | 2022-03-30 | Chugai Seiyaku Kabushiki Kaisha | Cyclic peptides |
| JP7020910B2 (ja) | 2015-03-13 | 2022-02-16 | 中外製薬株式会社 | 改変アミノアシルtRNA合成酵素およびその用途 |
| GB201601868D0 (en) | 2016-02-02 | 2016-03-16 | Lytix Biopharma As | Methods |
| CN109071600A (zh) * | 2016-04-14 | 2018-12-21 | 道健康生活医药株式会社 | 淀粉样蛋白球体(aspd)结合抑制肽以及评价和筛选方法 |
| WO2018143145A1 (ja) | 2017-01-31 | 2018-08-09 | 中外製薬株式会社 | 無細胞翻訳系におけるペプチドの合成方法 |
| GB201705255D0 (en) * | 2017-03-31 | 2017-05-17 | Univ I Tromsø - Norges Arktiske Univ | Bioactive cyclic compounds |
| WO2018188761A1 (en) | 2017-04-13 | 2018-10-18 | Lytix Biopharma As | Method of reducing population size of tregs and/or mdscs |
| US11542299B2 (en) | 2017-06-09 | 2023-01-03 | Chugai Seiyaku Kabushiki Kaisha | Method for synthesizing peptide containing N-substituted amino acid |
| JP7411414B2 (ja) | 2017-12-15 | 2024-01-11 | 中外製薬株式会社 | ペプチドの製造方法、及び塩基の処理方法 |
| KR20250119653A (ko) | 2018-11-07 | 2025-08-07 | 추가이 세이야쿠 가부시키가이샤 | O-치환 세린 유도체의 제조 방법 |
| EP3889164A4 (en) | 2018-11-30 | 2022-11-02 | Chugai Seiyaku Kabushiki Kaisha | METHOD FOR DEPROTECTION AND METHOD FOR RESIN REMOVAL IN A SOLID PHASE REACTION OF A PEPTIDE COMPOUND OR AN AMIDE COMPOUND, AND METHOD FOR PRODUCTION OF A PEPTIDE COMPOUND |
| JP7472101B2 (ja) | 2019-03-15 | 2024-04-22 | 中外製薬株式会社 | 芳香族アミノ酸誘導体の製造方法 |
| CN110294793B (zh) * | 2019-06-24 | 2023-10-27 | 合肥科生景肽生物科技有限公司 | Sumo荧光探针及其制备方法 |
| JP6880352B1 (ja) | 2019-11-07 | 2021-06-02 | 中外製薬株式会社 | Kras阻害作用を有する環状ペプチド化合物 |
| CN114790151B (zh) * | 2022-02-14 | 2023-03-31 | 湖南省湘中制药有限公司 | 一种2-氰基-2-丙戊酸甲酯的复合催化制备方法 |
| KR20250122523A (ko) | 2022-12-20 | 2025-08-13 | 리틱스 바이오파마 에이에스 | 종양 용해성 펩티드 및 키토산을 포함하는 조성물 |
Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO1992012168A1 (en) * | 1990-12-27 | 1992-07-23 | Abbott Laboratories | Modified hexa- and heptapeptide anaphylatoxin receptor ligands |
| DE102006018080A1 (de) * | 2006-04-13 | 2007-10-18 | Aicuris Gmbh & Co. Kg | Lysobactinamide |
| EP2105433A1 (en) * | 2008-03-18 | 2009-09-30 | Technische Universität Dresden | Modular scaffold for the design of specific molecules for the use as peptidomimetics and inhibitors of protein interaction |
| RU2468033C2 (ru) * | 2006-07-10 | 2012-11-27 | Пба3 БиоМед ГмбХ | Антибактериальные пептиды |
Family Cites Families (13)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4342705A (en) | 1979-11-23 | 1982-08-03 | Monsanto Company | Methylene thioethers |
| AU565221B2 (en) | 1982-06-03 | 1987-09-10 | Montedison S.P.A. | 2-(1,2,4-triazol-1-yl) propanone derivatives |
| IT1161927B (it) | 1983-03-31 | 1987-03-18 | Montedison Spa | Furano-derivati ad attivita' fungicida |
| DK523288A (da) | 1987-10-06 | 1989-04-07 | Hoffmann La Roche | Aminosyrederivater |
| CA1328333C (en) | 1988-03-04 | 1994-04-05 | Quirico Branca | Amino acid derivatives |
| CA2088195A1 (en) | 1990-08-31 | 1992-03-01 | David C. Horwell | Cholecystokinin antagonists, their preparation and therapeutic use |
| US5593967A (en) | 1990-08-31 | 1997-01-14 | Warner-Lambert Company | Cholecystokinin antagonists, their preparation and therapeutic use |
| US5885782A (en) | 1994-09-13 | 1999-03-23 | Nce Pharmaceuticals, Inc. | Synthetic antibiotics |
| CZ297544B6 (cs) | 1996-01-02 | 2007-02-07 | Aventis Pharmaceuticals Inc. | Substituované N-[(aminoiminomethyl)-fenyl]propylamidy nebo N-[(aminomethyl)fenyl]propylamidy |
| US6323227B1 (en) | 1996-01-02 | 2001-11-27 | Aventis Pharmaceuticals Products Inc. | Substituted N-[(aminoiminomethyl or aminomethyl)phenyl]propyl amides |
| US6080767A (en) | 1996-01-02 | 2000-06-27 | Aventis Pharmaceuticals Products Inc. | Substituted n-[(aminoiminomethyl or aminomethyl)phenyl]propyl amides |
| JP3734907B2 (ja) | 1996-12-19 | 2006-01-11 | 富士写真フイルム株式会社 | 現像処理方法 |
| GB0724951D0 (en) * | 2007-12-20 | 2008-01-30 | Lytix Biopharma As | Compounds |
-
2009
- 2009-11-02 GB GBGB0919194.1A patent/GB0919194D0/en not_active Ceased
-
2010
- 2010-11-02 WO PCT/GB2010/002024 patent/WO2011051692A1/en not_active Ceased
- 2010-11-02 BR BR112012010333A patent/BR112012010333A2/pt active IP Right Grant
- 2010-11-02 NZ NZ599791A patent/NZ599791A/en unknown
- 2010-11-02 US US13/505,625 patent/US8809280B2/en active Active
- 2010-11-02 CA CA2777749A patent/CA2777749C/en active Active
- 2010-11-02 JP JP2012535924A patent/JP5810090B2/ja active Active
- 2010-11-02 RU RU2012121264/04A patent/RU2548905C2/ru active
- 2010-11-02 AU AU2010311186A patent/AU2010311186B2/en active Active
- 2010-11-02 EP EP10784830.1A patent/EP2496596B1/en active Active
- 2010-11-02 DK DK10784830.1T patent/DK2496596T3/en active
- 2010-11-02 RU RU2015111706A patent/RU2676713C2/ru active
- 2010-11-02 PL PL10784830T patent/PL2496596T3/pl unknown
- 2010-11-02 KR KR1020127014266A patent/KR101730680B1/ko active Active
- 2010-11-02 CN CN201080049560.6A patent/CN102762586B/zh active Active
- 2010-11-02 ES ES10784830.1T patent/ES2621188T3/es active Active
Patent Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO1992012168A1 (en) * | 1990-12-27 | 1992-07-23 | Abbott Laboratories | Modified hexa- and heptapeptide anaphylatoxin receptor ligands |
| DE102006018080A1 (de) * | 2006-04-13 | 2007-10-18 | Aicuris Gmbh & Co. Kg | Lysobactinamide |
| RU2468033C2 (ru) * | 2006-07-10 | 2012-11-27 | Пба3 БиоМед ГмбХ | Антибактериальные пептиды |
| EP2105433A1 (en) * | 2008-03-18 | 2009-09-30 | Technische Universität Dresden | Modular scaffold for the design of specific molecules for the use as peptidomimetics and inhibitors of protein interaction |
Non-Patent Citations (1)
| Title |
|---|
| STROEM M B ET AL: "The pharmacophore of short cationic antibacterial peptides", JOURNAL OF MEDICINAL CHEMISTRY, AMERICAN CHEMICAL SOCIETY, 2003, vol. 46, no. 9, pages 1567-1570 . HANSEN TERKEL ET AL: "Antimicrobial activity of small beta-peptidomimetics based on the pharmacophore model of short cationic antimicrobial peptides.", JOURNAL OF MEDICINAL CHEMISTRY,2010, vol. 53, no. 2, pages 595-606 * |
Also Published As
| Publication number | Publication date |
|---|---|
| DK2496596T3 (en) | 2017-04-03 |
| EP2496596A1 (en) | 2012-09-12 |
| JP2013509389A (ja) | 2013-03-14 |
| CA2777749C (en) | 2019-08-06 |
| ES2621188T3 (es) | 2017-07-03 |
| GB0919194D0 (en) | 2009-12-16 |
| JP5810090B2 (ja) | 2015-11-11 |
| RU2015111706A3 (enExample) | 2018-10-29 |
| US20130035296A1 (en) | 2013-02-07 |
| KR20120104986A (ko) | 2012-09-24 |
| BR112012010333A2 (pt) | 2016-03-29 |
| AU2010311186B2 (en) | 2015-09-24 |
| RU2676713C2 (ru) | 2019-01-10 |
| RU2012121264A (ru) | 2013-12-10 |
| WO2011051692A1 (en) | 2011-05-05 |
| CN102762586A (zh) | 2012-10-31 |
| CN102762586B (zh) | 2016-10-05 |
| PL2496596T3 (pl) | 2017-08-31 |
| AU2010311186A1 (en) | 2012-05-31 |
| US8809280B2 (en) | 2014-08-19 |
| KR101730680B1 (ko) | 2017-04-26 |
| NZ599791A (en) | 2014-10-31 |
| RU2015111706A (ru) | 2015-11-10 |
| EP2496596B1 (en) | 2017-01-11 |
| CA2777749A1 (en) | 2011-05-05 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| RU2548905C2 (ru) | Терапевтические пептиды | |
| US9096642B2 (en) | Therapeutic compounds for immunomodulation | |
| US20210299211A1 (en) | Peptidomimetic macrocycles and formulations thereof | |
| US9102710B2 (en) | Cyclic peptides binding CXCR4 receptor and relative medical and diagnostic uses | |
| CN111615517B (zh) | Yeats抑制剂及其使用方法 | |
| JP2002523517A (ja) | 生物活性ペプチド | |
| TW570929B (en) | Cyclic adhesion inhibitors | |
| CN103304631A (zh) | 具有抗肿瘤活性的九肽 | |
| JP2016065018A (ja) | Cxcr7結合剤およびcxcr7結合剤を含有する医薬組成物 | |
| EA009294B1 (ru) | Антагонисты в1 рецептора брадикинина (варианты), фармацевтическая композиция, лекарственное средство, способ лечения заболеваний (варианты) | |
| JP2017081917A (ja) | 新規nk3受容体アゴニスト | |
| US20230372551A1 (en) | Radiolabeled ligands for targeted pet/spect imaging and methods of their use | |
| HUP0401728A2 (hu) | P-szelektinhez szelektíven kötődő peptidszerű vegyületek | |
| WO2019061812A1 (zh) | 氨基酸骨架类新型cxcr4拮抗剂及其制备与生物医学应用 | |
| JP2024504074A (ja) | 抗感染性二環式ペプチドリガンド | |
| RU2834861C1 (ru) | Пептиды с противовирусной активностью | |
| JP5924593B2 (ja) | 抗癌剤の活性増強剤 | |
| NL2025303B1 (en) | Coronavirus targeting drugs | |
| KR20250122523A (ko) | 종양 용해성 펩티드 및 키토산을 포함하는 조성물 | |
| WO2025085675A1 (en) | Conjugates, compositions and methods for treating rsv infection | |
| WO2023085402A1 (ja) | 白血球の細胞外トラップ形成阻害活性を有するペプチド | |
| EP3071238A1 (en) | Conjugate comprising folic acid and indole-3-carbinol for medical use | |
| JP2014001237A (ja) | ペプチド及び関連化合物の経皮送達システム |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| HE9A | Changing address for correspondence with an applicant |