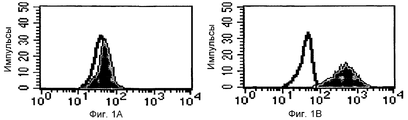
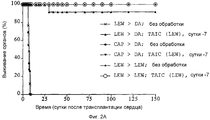
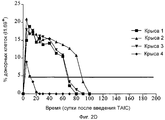
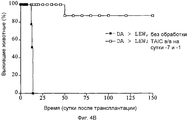
RU2370535C2 - Способ получения индуцирующих воспринимаемость трансплантата клеток моноцитарного происхождения, способ получения фармацевтической композиции для подавления реакций отторжения трансплантата, индуцирующие воспринимаемость трансплантата клетки моноцитарного происхождения, клеточный препарат для индукции воспринимаемости трансплантата, фармацевтическая композиция для подавления реакций отторжения трансплантата, применение индуцирующих воспринимаемость трансплантата клеток (варианты), способ получения и/или размножения регуляторных т-лимфоцитов, гибридомная клеточная линия, антитело и применение антитела - Google Patents
Способ получения индуцирующих воспринимаемость трансплантата клеток моноцитарного происхождения, способ получения фармацевтической композиции для подавления реакций отторжения трансплантата, индуцирующие воспринимаемость трансплантата клетки моноцитарного происхождения, клеточный препарат для индукции воспринимаемости трансплантата, фармацевтическая композиция для подавления реакций отторжения трансплантата, применение индуцирующих воспринимаемость трансплантата клеток (варианты), способ получения и/или размножения регуляторных т-лимфоцитов, гибридомная клеточная линия, антитело и применение антитела Download PDFInfo
- Publication number
- RU2370535C2 RU2370535C2 RU2005103627/13A RU2005103627A RU2370535C2 RU 2370535 C2 RU2370535 C2 RU 2370535C2 RU 2005103627/13 A RU2005103627/13 A RU 2005103627/13A RU 2005103627 A RU2005103627 A RU 2005103627A RU 2370535 C2 RU2370535 C2 RU 2370535C2
- Authority
- RU
- Russia
- Prior art keywords
- cells
- transplant
- inducing
- taic
- cell
- Prior art date
Links
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K39/0005—Vertebrate antigens
- A61K39/001—Preparations to induce tolerance to non-self, e.g. prior to transplantation
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K40/00—Cellular immunotherapy
- A61K40/10—Cellular immunotherapy characterised by the cell type used
- A61K40/17—Monocytes; Macrophages
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K40/00—Cellular immunotherapy
- A61K40/20—Cellular immunotherapy characterised by the effect or the function of the cells
- A61K40/22—Immunosuppressive or immunotolerising
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K40/00—Cellular immunotherapy
- A61K40/20—Cellular immunotherapy characterised by the effect or the function of the cells
- A61K40/24—Antigen-presenting cells [APC]
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K40/00—Cellular immunotherapy
- A61K40/40—Cellular immunotherapy characterised by antigens that are targeted or presented by cells of the immune system
- A61K40/41—Vertebrate antigens
- A61K40/418—Antigens related to induction of tolerance to non-self
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P37/00—Drugs for immunological or allergic disorders
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P37/00—Drugs for immunological or allergic disorders
- A61P37/02—Immunomodulators
- A61P37/06—Immunosuppressants, e.g. drugs for graft rejection
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/18—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans
- C07K16/28—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/18—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans
- C07K16/28—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants
- C07K16/2803—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants against the immunoglobulin superfamily
- C07K16/2833—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants against the immunoglobulin superfamily against MHC-molecules, e.g. HLA-molecules
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N5/00—Undifferentiated human, animal or plant cells, e.g. cell lines; Tissues; Cultivation or maintenance thereof; Culture media therefor
- C12N5/06—Animal cells or tissues; Human cells or tissues
- C12N5/0602—Vertebrate cells
- C12N5/0634—Cells from the blood or the immune system
- C12N5/0636—T lymphocytes
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N5/00—Undifferentiated human, animal or plant cells, e.g. cell lines; Tissues; Cultivation or maintenance thereof; Culture media therefor
- C12N5/06—Animal cells or tissues; Human cells or tissues
- C12N5/0602—Vertebrate cells
- C12N5/0634—Cells from the blood or the immune system
- C12N5/0645—Macrophages, e.g. Kuepfer cells in the liver; Monocytes
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K35/00—Medicinal preparations containing materials or reaction products thereof with undetermined constitution
- A61K35/12—Materials from mammals; Compositions comprising non-specified tissues or cells; Compositions comprising non-embryonic stem cells; Genetically modified cells
- A61K2035/122—Materials from mammals; Compositions comprising non-specified tissues or cells; Compositions comprising non-embryonic stem cells; Genetically modified cells for inducing tolerance or supression of immune responses
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K35/00—Medicinal preparations containing materials or reaction products thereof with undetermined constitution
- A61K35/12—Materials from mammals; Compositions comprising non-specified tissues or cells; Compositions comprising non-embryonic stem cells; Genetically modified cells
- A61K2035/124—Materials from mammals; Compositions comprising non-specified tissues or cells; Compositions comprising non-embryonic stem cells; Genetically modified cells the cells being hematopoietic, bone marrow derived or blood cells
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2501/00—Active agents used in cell culture processes, e.g. differentation
- C12N2501/20—Cytokines; Chemokines
- C12N2501/22—Colony stimulating factors (G-CSF, GM-CSF)
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2501/00—Active agents used in cell culture processes, e.g. differentation
- C12N2501/20—Cytokines; Chemokines
- C12N2501/24—Interferons [IFN]
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2502/00—Coculture with; Conditioned medium produced by
- C12N2502/11—Coculture with; Conditioned medium produced by blood or immune system cells
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Immunology (AREA)
- Chemical & Material Sciences (AREA)
- General Health & Medical Sciences (AREA)
- Engineering & Computer Science (AREA)
- Organic Chemistry (AREA)
- Biomedical Technology (AREA)
- Veterinary Medicine (AREA)
- Genetics & Genomics (AREA)
- Animal Behavior & Ethology (AREA)
- Public Health (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Epidemiology (AREA)
- Zoology (AREA)
- Wood Science & Technology (AREA)
- Medicinal Chemistry (AREA)
- Biotechnology (AREA)
- Biochemistry (AREA)
- Microbiology (AREA)
- Cell Biology (AREA)
- Biophysics (AREA)
- Pharmacology & Pharmacy (AREA)
- General Engineering & Computer Science (AREA)
- Molecular Biology (AREA)
- Hematology (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Transplantation (AREA)
- Gastroenterology & Hepatology (AREA)
- Chemical Kinetics & Catalysis (AREA)
- General Chemical & Material Sciences (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Mycology (AREA)
- Micro-Organisms Or Cultivation Processes Thereof (AREA)
- Medicines Containing Material From Animals Or Micro-Organisms (AREA)
- Preparation Of Compounds By Using Micro-Organisms (AREA)
- Peptides Or Proteins (AREA)
- Measuring Or Testing Involving Enzymes Or Micro-Organisms (AREA)
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| DE10231655.4 | 2002-07-12 | ||
| DE10231655A DE10231655A1 (de) | 2002-07-12 | 2002-07-12 | Transplantat-Akzeptanz induzierende Zellen monozytären Ursprungs, sowie deren Herstellung und Verwendung |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| RU2005103627A RU2005103627A (ru) | 2005-08-27 |
| RU2370535C2 true RU2370535C2 (ru) | 2009-10-20 |
Family
ID=30009939
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| RU2005103627/13A RU2370535C2 (ru) | 2002-07-12 | 2003-07-11 | Способ получения индуцирующих воспринимаемость трансплантата клеток моноцитарного происхождения, способ получения фармацевтической композиции для подавления реакций отторжения трансплантата, индуцирующие воспринимаемость трансплантата клетки моноцитарного происхождения, клеточный препарат для индукции воспринимаемости трансплантата, фармацевтическая композиция для подавления реакций отторжения трансплантата, применение индуцирующих воспринимаемость трансплантата клеток (варианты), способ получения и/или размножения регуляторных т-лимфоцитов, гибридомная клеточная линия, антитело и применение антитела |
Country Status (18)
| Country | Link |
|---|---|
| US (1) | US20060286670A1 (enExample) |
| EP (2) | EP1557462B1 (enExample) |
| JP (1) | JP2005532803A (enExample) |
| CN (1) | CN100523176C (enExample) |
| AT (2) | ATE390480T1 (enExample) |
| AU (1) | AU2003250939B2 (enExample) |
| BR (1) | BR0312693A (enExample) |
| CA (1) | CA2492383A1 (enExample) |
| DE (3) | DE10231655A1 (enExample) |
| DK (2) | DK1557462T3 (enExample) |
| ES (3) | ES2304647T3 (enExample) |
| IL (3) | IL166021A0 (enExample) |
| NO (1) | NO20050719L (enExample) |
| PT (2) | PT1557462E (enExample) |
| RU (1) | RU2370535C2 (enExample) |
| SI (3) | SI1492869T1 (enExample) |
| WO (1) | WO2004007701A1 (enExample) |
| ZA (2) | ZA200501054B (enExample) |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| RU2508924C1 (ru) * | 2013-02-21 | 2014-03-10 | Государственное бюджетное учреждение здравоохранения Московской области "Московский областной научно-исследовательский клинический институт им. М.Ф. Владимирского" | Способ профилактики и лечения отторжения почечного трансплантата |
Families Citing this family (26)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP1644484B1 (en) * | 2003-07-11 | 2008-09-17 | Blasticon Biotechnologische Forschung GmbH | Autologous self-tolerance inducing cells of monocytic origin and their use in pharmaceutical proparations |
| DE10362002B4 (de) | 2003-06-23 | 2006-10-12 | Fraunhofer-Gesellschaft zur Förderung der angewandten Forschung e.V. | Adulte pluripotente Stammzellen |
| EP1928479B1 (en) * | 2005-08-24 | 2016-06-08 | Yeda Research And Development Co., Ltd. | Universal donor-derived tolerogenic cells for inducing non-syngeneic transplantation tolerance |
| WO2007043641A1 (ja) | 2005-10-14 | 2007-04-19 | Fukuoka University | 膵島移植における移植膵島障害抑制剤 |
| BRPI0617664B8 (pt) | 2005-10-21 | 2021-05-25 | Chugai Pharmaceutical Co Ltd | uso de um anticorpo que reconhece a il-6 para a produção de uma composição farmacêutica para tratar o enfarte do miocárdio ou suprimir a remodelagem ventricular esquerda depois do enfarte do miocárdio |
| AR057582A1 (es) | 2005-11-15 | 2007-12-05 | Nat Hospital Organization | Agentes para suprimir la induccion de linfocitos t citotoxicos |
| WO2007116962A1 (ja) | 2006-04-07 | 2007-10-18 | Osaka University | 筋再生促進剤 |
| CN101646459B (zh) | 2007-01-23 | 2014-02-12 | 国立大学法人信州大学 | 慢性排斥反应抑制剂 |
| JP5544290B2 (ja) | 2008-06-05 | 2014-07-09 | 独立行政法人国立がん研究センター | 神経浸潤抑制剤 |
| ES2615861T3 (es) * | 2008-10-30 | 2017-06-08 | Yeda Research And Development Company Ltd. | Células T de memoria central anti-terceros, métodos de producción de las mismas y uso de las mismas en trasplante y tratamiento de enfermedades |
| JP5436905B2 (ja) * | 2009-03-26 | 2014-03-05 | テルモ株式会社 | シート状細胞培養物の製造方法 |
| JP5667357B2 (ja) * | 2009-11-30 | 2015-02-12 | テルモ株式会社 | シート状細胞培養物の製造方法 |
| CN101812428A (zh) * | 2010-04-26 | 2010-08-25 | 西北农林科技大学 | 一种乳腺上皮细胞的组织块种植方法 |
| HUE060454T2 (hu) | 2010-05-28 | 2023-03-28 | Chugai Pharmaceutical Co Ltd | Daganatellenes T-sejt-választ fokozó szer |
| DE102011004335A1 (de) | 2011-02-17 | 2012-08-23 | Thomas Grammel | Verfahren zur Herstellung eines Vakzins |
| JP5727665B2 (ja) * | 2014-12-11 | 2015-06-03 | テルモ株式会社 | シート状細胞培養物の製造方法 |
| JP5893786B2 (ja) * | 2015-04-02 | 2016-03-23 | テルモ株式会社 | シート状細胞培養物の製造方法 |
| KR102769850B1 (ko) * | 2015-07-03 | 2025-02-20 | 인스티튜트 내셔날 드 라 싼테 에 드 라 리셰르셰 메디칼르 | 조절 t 세포를 수득하는 방법 및 이의 용도 |
| JP6903866B2 (ja) * | 2016-01-27 | 2021-07-14 | 国立大学法人 熊本大学 | 血液由来単球の増殖誘導方法 |
| JP6140322B2 (ja) * | 2016-02-24 | 2017-05-31 | テルモ株式会社 | シート状細胞培養物の製造方法 |
| JP6490738B2 (ja) * | 2017-04-28 | 2019-03-27 | テルモ株式会社 | シート状細胞培養物の製造方法 |
| JP7185884B2 (ja) | 2017-05-02 | 2022-12-08 | 国立研究開発法人国立精神・神経医療研究センター | Il-6及び好中球の関連する疾患の治療効果の予測及び判定方法 |
| EP3698808B1 (en) | 2017-10-20 | 2025-01-01 | Hyogo College Of Medicine | Anti-il-6 receptor antibody-containing medicinal composition for preventing post-surgical adhesion |
| JP6657452B2 (ja) * | 2019-02-27 | 2020-03-04 | テルモ株式会社 | シート状細胞培養物の製造方法 |
| JP7002497B2 (ja) * | 2019-06-20 | 2022-01-20 | テルモ株式会社 | シート状細胞培養物の製造方法 |
| JP6872051B2 (ja) * | 2020-02-05 | 2021-05-19 | テルモ株式会社 | シート状細胞培養物の製造方法 |
Citations (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| RU2169009C2 (ru) * | 1994-04-25 | 2001-06-20 | Трастиз Оф Дартмут Колледж | Способы индуцирования т-клеточной толерантности к тканевому или органному трансплантату |
Family Cites Families (10)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5766920A (en) * | 1982-08-11 | 1998-06-16 | Cellcor, Inc. | Ex vivo activation of immune cells |
| GB9502022D0 (en) * | 1995-02-02 | 1995-03-22 | Abuljadayel Ilham M S | A method for preparing lymphohaematopoietic progenitor cells |
| US9068164B1 (en) * | 1995-02-02 | 2015-06-30 | Tristem Trading (Cyprus) Limited | Method of preparing an undifferentiated cell |
| US7410773B2 (en) * | 1995-02-02 | 2008-08-12 | Ghazi Jaswinder Dhoot | Method of preparing an undifferentiated cell |
| US7112440B2 (en) * | 1995-02-02 | 2006-09-26 | Ghazi Jaswinder Dhoot | Method of increasing the relative number of CD45 low cells in a cell population |
| ES2201177T3 (es) * | 1995-03-08 | 2004-03-16 | The Scripps Research Institute | Sistema de presentacion de antigenos y activacion de celulas-t. |
| WO1998053048A1 (en) * | 1997-05-21 | 1998-11-26 | The Government Of The United States Of America As Represented By The Secretary Of The Department Of Health And Human Services | Methods and compositions for making dendritic cells from expanded populations of monocytes and for activating t cells |
| NL1017973C2 (nl) * | 2000-05-10 | 2002-11-08 | Tristem Trading Cyprus Ltd | Inrichting. |
| AU2002224851A1 (en) * | 2000-11-14 | 2002-05-27 | Universite Libre De Bruxelles | Generation and use of dendritic cells |
| TWI288779B (en) * | 2002-03-28 | 2007-10-21 | Blasticon Biotech Forschung | Dedifferentiated, programmable stem cells of monocytic origin, and their production and use |
-
2002
- 2002-07-12 DE DE10231655A patent/DE10231655A1/de not_active Ceased
-
2003
- 2003-07-11 US US10/520,931 patent/US20060286670A1/en not_active Abandoned
- 2003-07-11 AU AU2003250939A patent/AU2003250939B2/en not_active Ceased
- 2003-07-11 EP EP05009317A patent/EP1557462B1/en not_active Expired - Lifetime
- 2003-07-11 AT AT05009317T patent/ATE390480T1/de not_active IP Right Cessation
- 2003-07-11 BR BR0312693-5A patent/BR0312693A/pt not_active IP Right Cessation
- 2003-07-11 SI SI200330146T patent/SI1492869T1/sl unknown
- 2003-07-11 ES ES05009317T patent/ES2304647T3/es not_active Expired - Lifetime
- 2003-07-11 CA CA002492383A patent/CA2492383A1/en not_active Abandoned
- 2003-07-11 DE DE60302231T patent/DE60302231T2/de not_active Expired - Fee Related
- 2003-07-11 PT PT05009317T patent/PT1557462E/pt unknown
- 2003-07-11 SI SI200331240T patent/SI1557462T1/sl unknown
- 2003-07-11 RU RU2005103627/13A patent/RU2370535C2/ru not_active IP Right Cessation
- 2003-07-11 ES ES03763823T patent/ES2252696T3/es not_active Expired - Lifetime
- 2003-07-11 AT AT03763823T patent/ATE309331T1/de not_active IP Right Cessation
- 2003-07-11 WO PCT/EP2003/007551 patent/WO2004007701A1/en not_active Ceased
- 2003-07-11 DE DE60320009T patent/DE60320009T2/de not_active Expired - Fee Related
- 2003-07-11 EP EP03763823A patent/EP1492869B9/en not_active Expired - Lifetime
- 2003-07-11 IL IL16602103A patent/IL166021A0/xx unknown
- 2003-07-11 DK DK05009317T patent/DK1557462T3/da active
- 2003-07-11 CN CNB038215748A patent/CN100523176C/zh not_active Expired - Fee Related
- 2003-07-11 DK DK03763823T patent/DK1492869T3/da active
- 2003-07-11 JP JP2004520603A patent/JP2005532803A/ja active Pending
-
2004
- 2004-01-09 SI SI200430965T patent/SI1644484T1/sl unknown
- 2004-01-09 ES ES04701001T patent/ES2310711T3/es not_active Expired - Lifetime
- 2004-01-09 PT PT04701001T patent/PT1644484E/pt unknown
- 2004-12-28 IL IL166021A patent/IL166021A/en not_active IP Right Cessation
-
2005
- 2005-02-04 ZA ZA200501054A patent/ZA200501054B/xx unknown
- 2005-02-10 NO NO20050719A patent/NO20050719L/no not_active Application Discontinuation
- 2005-12-27 IL IL172854A patent/IL172854A0/en unknown
-
2006
- 2006-02-07 ZA ZA200601074A patent/ZA200601074B/en unknown
Patent Citations (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| RU2169009C2 (ru) * | 1994-04-25 | 2001-06-20 | Трастиз Оф Дартмут Колледж | Способы индуцирования т-клеточной толерантности к тканевому или органному трансплантату |
Non-Patent Citations (1)
| Title |
|---|
| BUCKLE A.M. et al., Colony-stimulating factors and interferon-gamma differentially affect cell surface molecules shared by monocytes and neutrophils, Clin. Exp.Immunol., 1990, v.81, n.2, p.339-345. TANAKA J. et al., The role of accessory cells in allogeneic peripheral blood stem cell transplantation, Int. J. Hematol., 1999, v.69, n.2, p.70-74. * |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| RU2508924C1 (ru) * | 2013-02-21 | 2014-03-10 | Государственное бюджетное учреждение здравоохранения Московской области "Московский областной научно-исследовательский клинический институт им. М.Ф. Владимирского" | Способ профилактики и лечения отторжения почечного трансплантата |
Also Published As
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| RU2370535C2 (ru) | Способ получения индуцирующих воспринимаемость трансплантата клеток моноцитарного происхождения, способ получения фармацевтической композиции для подавления реакций отторжения трансплантата, индуцирующие воспринимаемость трансплантата клетки моноцитарного происхождения, клеточный препарат для индукции воспринимаемости трансплантата, фармацевтическая композиция для подавления реакций отторжения трансплантата, применение индуцирующих воспринимаемость трансплантата клеток (варианты), способ получения и/или размножения регуляторных т-лимфоцитов, гибридомная клеточная линия, антитело и применение антитела | |
| JP6089004B2 (ja) | 免疫調節活性を有する細胞集団、単離方法および使用 | |
| JP2002502823A (ja) | 移植における補刺激遮断および混合キメラ現象 | |
| US11701392B2 (en) | Compositions for establishing mixed chimerism and methods of manufacture thereof | |
| CA2486759C (en) | Universal chimera bank | |
| RU2346041C2 (ru) | Аутологичные клетки моноцитарного происхождения, индуцирующие аутотолерантность, и их применение в фармацевтических препаратах | |
| Scherer et al. | Immunologic considerations for therapeutic strategies utilizing allogeneic hepatocytes: hepatocyte-expressed membrane-bound major histocompatibility complex class I antigen sensitizes while soluble antigen suppresses the immune response in rats | |
| WO2013076245A1 (en) | Allogeneic immune response control | |
| KR20050018998A (ko) | 단구성 기원의 이식 조직편 허용 유도성 세포, 및 이의생성 방법 및 용도 | |
| HK1071586B (en) | Transplant acceptance inducing cells of monocytic origin and their preparation and use | |
| JP2009521459A (ja) | 肝機能障害治療において肝前駆細胞を用いる方法 | |
| HK1088926B (en) | Autologous self-tolerance inducing cells of monocytic origin and their use in pharmaceutical proparations | |
| ISCHEMIC | 0005 REDUCED DOSE-ADAPTED MMF EXPOSURE UNDER PROTON PUMP INHIBITOR CO-MEDICATION IN STABLE HEART TRANSPLANT RECIPIENTS | |
| TW201907949A (zh) | 西瑞香素於預防組織或器官移植排斥或是移植物抗宿主疾病之用途 | |
| MX2008008333A (en) | Method of using hepatic progenitors in treating liver dysfunction |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| MM4A | The patent is invalid due to non-payment of fees |
Effective date: 20100712 |