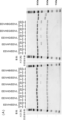
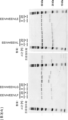
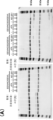
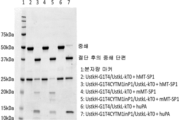
KR20230073346A - 리간드 결합 활성을 조정 가능한 리간드 결합 분자 - Google Patents
리간드 결합 활성을 조정 가능한 리간드 결합 분자 Download PDFInfo
- Publication number
- KR20230073346A KR20230073346A KR1020237015916A KR20237015916A KR20230073346A KR 20230073346 A KR20230073346 A KR 20230073346A KR 1020237015916 A KR1020237015916 A KR 1020237015916A KR 20237015916 A KR20237015916 A KR 20237015916A KR 20230073346 A KR20230073346 A KR 20230073346A
- Authority
- KR
- South Korea
- Prior art keywords
- ligand
- antibody
- seq
- binding molecule
- protease
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Ceased
Links
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/18—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans
- C07K16/24—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against cytokines, lymphokines or interferons
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P1/00—Drugs for disorders of the alimentary tract or the digestive system
- A61P1/04—Drugs for disorders of the alimentary tract or the digestive system for ulcers, gastritis or reflux esophagitis, e.g. antacids, inhibitors of acid secretion, mucosal protectants
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/50—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates
- A61K47/51—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent
- A61K47/68—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent the modifying agent being an antibody, an immunoglobulin or a fragment thereof, e.g. an Fc-fragment
- A61K47/6801—Drug-antibody or immunoglobulin conjugates defined by the pharmacologically or therapeutically active agent
- A61K47/6803—Drugs conjugated to an antibody or immunoglobulin, e.g. cisplatin-antibody conjugates
- A61K47/6811—Drugs conjugated to an antibody or immunoglobulin, e.g. cisplatin-antibody conjugates the drug being a protein or peptide, e.g. transferrin or bleomycin
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/50—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates
- A61K47/51—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent
- A61K47/68—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent the modifying agent being an antibody, an immunoglobulin or a fragment thereof, e.g. an Fc-fragment
- A61K47/6801—Drug-antibody or immunoglobulin conjugates defined by the pharmacologically or therapeutically active agent
- A61K47/6803—Drugs conjugated to an antibody or immunoglobulin, e.g. cisplatin-antibody conjugates
- A61K47/6811—Drugs conjugated to an antibody or immunoglobulin, e.g. cisplatin-antibody conjugates the drug being a protein or peptide, e.g. transferrin or bleomycin
- A61K47/6813—Drugs conjugated to an antibody or immunoglobulin, e.g. cisplatin-antibody conjugates the drug being a protein or peptide, e.g. transferrin or bleomycin the drug being a peptidic cytokine, e.g. an interleukin or interferon
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P1/00—Drugs for disorders of the alimentary tract or the digestive system
- A61P1/16—Drugs for disorders of the alimentary tract or the digestive system for liver or gallbladder disorders, e.g. hepatoprotective agents, cholagogues, litholytics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P11/00—Drugs for disorders of the respiratory system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P11/00—Drugs for disorders of the respiratory system
- A61P11/06—Antiasthmatics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P13/00—Drugs for disorders of the urinary system
- A61P13/12—Drugs for disorders of the urinary system of the kidneys
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P17/00—Drugs for dermatological disorders
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P19/00—Drugs for skeletal disorders
- A61P19/02—Drugs for skeletal disorders for joint disorders, e.g. arthritis, arthrosis
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P29/00—Non-central analgesic, antipyretic or antiinflammatory agents, e.g. antirheumatic agents; Non-steroidal antiinflammatory drugs [NSAID]
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P3/00—Drugs for disorders of the metabolism
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P37/00—Drugs for immunological or allergic disorders
- A61P37/02—Immunomodulators
- A61P37/06—Immunosuppressants, e.g. drugs for graft rejection
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P37/00—Drugs for immunological or allergic disorders
- A61P37/08—Antiallergic agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P9/00—Drugs for disorders of the cardiovascular system
- A61P9/04—Inotropic agents, i.e. stimulants of cardiac contraction; Drugs for heart failure
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P9/00—Drugs for disorders of the cardiovascular system
- A61P9/10—Drugs for disorders of the cardiovascular system for treating ischaemic or atherosclerotic diseases, e.g. antianginal drugs, coronary vasodilators, drugs for myocardial infarction, retinopathy, cerebrovascula insufficiency, renal arteriosclerosis
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K14/00—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- C07K14/435—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans
- C07K14/52—Cytokines; Lymphokines; Interferons
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K14/00—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- C07K14/435—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans
- C07K14/52—Cytokines; Lymphokines; Interferons
- C07K14/521—Chemokines
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K14/00—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- C07K14/435—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans
- C07K14/705—Receptors; Cell surface antigens; Cell surface determinants
- C07K14/70503—Immunoglobulin superfamily
- C07K14/70521—CD28, CD152
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/18—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans
- C07K16/24—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against cytokines, lymphokines or interferons
- C07K16/244—Interleukins [IL]
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/18—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans
- C07K16/28—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants
- C07K16/2803—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants against the immunoglobulin superfamily
- C07K16/2818—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants against the immunoglobulin superfamily against CD28 or CD152
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/18—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans
- C07K16/28—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants
- C07K16/2866—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants against receptors for cytokines, lymphokines, interferons
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/18—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans
- C07K16/28—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants
- C07K16/30—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants from tumour cells
-
- C—CHEMISTRY; METALLURGY
- C40—COMBINATORIAL TECHNOLOGY
- C40B—COMBINATORIAL CHEMISTRY; LIBRARIES, e.g. CHEMICAL LIBRARIES
- C40B40/00—Libraries per se, e.g. arrays, mixtures
- C40B40/04—Libraries containing only organic compounds
- C40B40/10—Libraries containing peptides or polypeptides, or derivatives thereof
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K2039/505—Medicinal preparations containing antigens or antibodies comprising antibodies
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/70—Immunoglobulins specific features characterized by effect upon binding to a cell or to an antigen
- C07K2317/76—Antagonist effect on antigen, e.g. neutralization or inhibition of binding
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/90—Immunoglobulins specific features characterized by (pharmaco)kinetic aspects or by stability of the immunoglobulin
- C07K2317/92—Affinity (KD), association rate (Ka), dissociation rate (Kd) or EC50 value
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2319/00—Fusion polypeptide
- C07K2319/50—Fusion polypeptide containing protease site
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N15/00—Mutation or genetic engineering; DNA or RNA concerning genetic engineering, vectors, e.g. plasmids, or their isolation, preparation or purification; Use of hosts therefor
- C12N15/09—Recombinant DNA-technology
Landscapes
- Health & Medical Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Life Sciences & Earth Sciences (AREA)
- Medicinal Chemistry (AREA)
- General Health & Medical Sciences (AREA)
- Engineering & Computer Science (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Immunology (AREA)
- Animal Behavior & Ethology (AREA)
- Public Health (AREA)
- Pharmacology & Pharmacy (AREA)
- Veterinary Medicine (AREA)
- Chemical Kinetics & Catalysis (AREA)
- General Chemical & Material Sciences (AREA)
- Molecular Biology (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Biochemistry (AREA)
- Genetics & Genomics (AREA)
- Biophysics (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Gastroenterology & Hepatology (AREA)
- Zoology (AREA)
- Pulmonology (AREA)
- Toxicology (AREA)
- Cardiology (AREA)
- Cell Biology (AREA)
- Epidemiology (AREA)
- Rheumatology (AREA)
- Heart & Thoracic Surgery (AREA)
- Urology & Nephrology (AREA)
- Orthopedic Medicine & Surgery (AREA)
- Wood Science & Technology (AREA)
- Physical Education & Sports Medicine (AREA)
- Dermatology (AREA)
- Pain & Pain Management (AREA)
- Obesity (AREA)
- Biomedical Technology (AREA)
- Biotechnology (AREA)
- General Engineering & Computer Science (AREA)
Applications Claiming Priority (4)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JPJP-P-2016-229882 | 2016-11-28 | ||
| JP2016229882 | 2016-11-28 | ||
| KR1020197017817A KR102533814B1 (ko) | 2016-11-28 | 2017-11-28 | 리간드 결합 활성을 조정 가능한 리간드 결합 분자 |
| PCT/JP2017/042570 WO2018097308A1 (ja) | 2016-11-28 | 2017-11-28 | リガンド結合活性が調整可能なリガンド結合分子 |
Related Parent Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020197017817A Division KR102533814B1 (ko) | 2016-11-28 | 2017-11-28 | 리간드 결합 활성을 조정 가능한 리간드 결합 분자 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| KR20230073346A true KR20230073346A (ko) | 2023-05-25 |
Family
ID=62195875
Family Applications (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020197017817A Active KR102533814B1 (ko) | 2016-11-28 | 2017-11-28 | 리간드 결합 활성을 조정 가능한 리간드 결합 분자 |
| KR1020237015916A Ceased KR20230073346A (ko) | 2016-11-28 | 2017-11-28 | 리간드 결합 활성을 조정 가능한 리간드 결합 분자 |
Family Applications Before (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020197017817A Active KR102533814B1 (ko) | 2016-11-28 | 2017-11-28 | 리간드 결합 활성을 조정 가능한 리간드 결합 분자 |
Country Status (12)
| Country | Link |
|---|---|
| US (2) | US12060654B2 (enExample) |
| EP (1) | EP3546480A4 (enExample) |
| JP (3) | JP7626577B2 (enExample) |
| KR (2) | KR102533814B1 (enExample) |
| CN (1) | CN110214151A (enExample) |
| AU (1) | AU2017364818C1 (enExample) |
| BR (1) | BR112019008265A2 (enExample) |
| CA (1) | CA3039316A1 (enExample) |
| IL (1) | IL266827B2 (enExample) |
| MX (3) | MX2019005947A (enExample) |
| TW (2) | TWI776827B (enExample) |
| WO (1) | WO2018097308A1 (enExample) |
Families Citing this family (20)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| KR102533814B1 (ko) | 2016-11-28 | 2023-05-19 | 추가이 세이야쿠 가부시키가이샤 | 리간드 결합 활성을 조정 가능한 리간드 결합 분자 |
| KR20240018673A (ko) | 2016-11-28 | 2024-02-13 | 추가이 세이야쿠 가부시키가이샤 | 항원 결합 도메인 및 운반 부분을 포함하는 폴리펩티드 |
| TWI818934B (zh) | 2017-11-28 | 2023-10-21 | 日商中外製藥股份有限公司 | 可調整配體結合活性的配體結合分子 |
| JP7482630B2 (ja) | 2017-11-28 | 2024-05-14 | 中外製薬株式会社 | 抗原結合ドメインおよび運搬部分を含むポリペプチド |
| EP3794022A1 (en) * | 2018-05-14 | 2021-03-24 | Werewolf Therapeutics, Inc. | Activatable cytokine polypeptides and methods of use thereof |
| JP7414736B2 (ja) | 2018-05-30 | 2024-01-16 | 中外製薬株式会社 | アグリカン結合ドメインおよび運搬部分を含むポリペプチド |
| US20210253672A1 (en) * | 2018-05-30 | 2021-08-19 | Chugai Seiyaku Kabushiki Kaisha | Ligand-binding molecule containing single domain antibody |
| JP2021530243A (ja) * | 2018-07-25 | 2021-11-11 | アスクジーン・ファーマ・インコーポレイテッドAskGene Pharma, Inc. | 新規il−21プロドラッグおよびそれを使用する方法 |
| US20220048966A1 (en) * | 2018-09-28 | 2022-02-17 | Pierre Fabre Medicament | New immunocytokines for the treatment of cancer |
| CN120022352A (zh) | 2018-10-22 | 2025-05-23 | 联邦高等教育系统匹兹堡大学 | Cxcr3的可切割激活剂及其使用方法 |
| WO2020116498A1 (ja) * | 2018-12-04 | 2020-06-11 | 中外製薬株式会社 | Cxcr3リガンド |
| CN113905757A (zh) | 2019-06-05 | 2022-01-07 | 中外制药株式会社 | 抗体切割位点结合分子 |
| WO2020246567A1 (ja) * | 2019-06-05 | 2020-12-10 | 中外製薬株式会社 | プロテアーゼ基質、及びプロテアーゼ切断配列を含むポリペプチド |
| WO2020252264A1 (en) | 2019-06-12 | 2020-12-17 | AskGene Pharma, Inc. | Novel il-15 prodrugs and methods of use thereof |
| EP4021927A1 (en) * | 2019-08-29 | 2022-07-06 | Antagonis Biotherapeutics GmbH | T-cell mobilizing cxcl10 mutant with increased glycosaminoglycan binding affinity |
| WO2021149697A1 (en) * | 2020-01-20 | 2021-07-29 | Chugai Seiyaku Kabushiki Kaisha | Ligand-binding fusion proteins |
| EP3889183A1 (en) * | 2020-04-01 | 2021-10-06 | Pierre Fabre Medicament | A protein complex comprising an immunocytokine |
| EP4373862A1 (en) * | 2021-07-19 | 2024-05-29 | Chugai Seiyaku Kabushiki Kaisha | Protease-mediated target specific cytokine delivery using fusion polypeptide |
| WO2023120643A1 (ja) | 2021-12-23 | 2023-06-29 | 中外製薬株式会社 | Cxcr3発現細胞遊走活性が増強したcxcr3リガンド。 |
| CN120112547A (zh) | 2022-11-16 | 2025-06-06 | 瑞泽恩制药公司 | 包含膜结合il-12和蛋白酶可裂解接头的嵌合蛋白 |
Family Cites Families (109)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4816567A (en) | 1983-04-08 | 1989-03-28 | Genentech, Inc. | Recombinant immunoglobin preparations |
| GB8607679D0 (en) | 1986-03-27 | 1986-04-30 | Winter G P | Recombinant dna product |
| GB9015198D0 (en) | 1990-07-10 | 1990-08-29 | Brien Caroline J O | Binding substance |
| DK0585287T3 (da) | 1990-07-10 | 2000-04-17 | Cambridge Antibody Tech | Fremgangsmåde til fremstilling af specifikke bindingsparelementer |
| AU8507191A (en) | 1990-08-29 | 1992-03-30 | Genpharm International, Inc. | Transgenic non-human animals capable of producing heterologous antibodies |
| EP0605522B1 (en) | 1991-09-23 | 1999-06-23 | Medical Research Council | Methods for the production of humanized antibodies |
| ES2341666T3 (es) | 1991-12-02 | 2010-06-24 | Medimmune Limited | Produccion de autoanticuerpos de repertorios de segmentos de anticue rpos expresados en la superficie de fagos. |
| EP0746609A4 (en) | 1991-12-17 | 1997-12-17 | Genpharm Int | NON-HUMAN TRANSGENIC ANIMALS CAPABLE OF PRODUCING HETEROLOGOUS ANTIBODIES |
| DE69333823T2 (de) | 1992-03-24 | 2006-05-04 | Cambridge Antibody Technology Ltd., Melbourn | Verfahren zur herstellung von gliedern von spezifischen bindungspaaren |
| NZ255101A (en) | 1992-07-24 | 1997-08-22 | Cell Genesys Inc | A yeast artificial chromosome (yac) vector containing an hprt minigene expressible in murine stem cells and genetically modified rodent therefor |
| JPH08509612A (ja) | 1993-04-26 | 1996-10-15 | ジェンファーム インターナショナル インコーポレイテッド | 異種抗体を産生することができるトランスジェニック非ヒト動物 |
| GB9313509D0 (en) | 1993-06-30 | 1993-08-11 | Medical Res Council | Chemisynthetic libraries |
| FR2707189B1 (fr) | 1993-07-09 | 1995-10-13 | Gradient Ass | Procédé de traitement de résidus de combustion et installation de mise en Óoeuvre dudit procédé. |
| JPH09506508A (ja) | 1993-12-03 | 1997-06-30 | メディカル リサーチ カウンシル | 組換え結合タンパク質およびペプチド |
| HU221385B1 (en) | 1994-07-13 | 2002-09-28 | Chugai Pharmaceutical Co Ltd | Reconstituted human antibody against human interleukin-8 |
| EP0822830B1 (en) | 1995-04-27 | 2008-04-02 | Amgen Fremont Inc. | Human anti-IL-8 antibodies, derived from immunized xenomice |
| EP0823941A4 (en) | 1995-04-28 | 2001-09-19 | Abgenix Inc | HUMAN ANTIBODIES DERIVED FROM IMMUNIZED XENO MOUSES |
| WO2006069036A2 (en) | 2004-12-21 | 2006-06-29 | Centocor, Inc. | Anti-il-12 antibodies, epitopes, compositions, methods and uses |
| JP5291279B2 (ja) | 2000-09-08 | 2013-09-18 | ウニヴェルジテート・チューリッヒ | 反復モジュールを含む反復タンパク質の集合体 |
| JP2004526419A (ja) | 2000-10-16 | 2004-09-02 | フィロス インク. | 抗体模倣物および他の結合タンパク質のためのタンパク質骨格 |
| US20030157561A1 (en) | 2001-11-19 | 2003-08-21 | Kolkman Joost A. | Combinatorial libraries of monomer domains |
| PT2208784E (pt) | 2001-06-22 | 2013-04-03 | Chugai Pharmaceutical Co Ltd | Inibidores da proliferação celular contendo um anticorpo anti-glipicano 3 |
| WO2003029462A1 (en) | 2001-09-27 | 2003-04-10 | Pieris Proteolab Ag | Muteins of human neutrophil gelatinase-associated lipocalin and related proteins |
| CN1665932B (zh) | 2002-04-30 | 2010-12-15 | 株式会社载体研究所 | 具有修饰的蛋白酶依赖向性的载体 |
| AU2003242024A1 (en) | 2002-06-05 | 2003-12-22 | Chugai Seiyaku Kabushiki Kaisha | Method of constructing antibody |
| AU2003265866A1 (en) | 2002-09-03 | 2004-03-29 | Vit Lauermann | Targeted release |
| AU2002328429A1 (en) | 2002-09-04 | 2004-03-29 | Chugai Seiyaku Kabushiki Kaisha | CONSTRUCTION OF ANTIBODY USING MRL/lpr MOUSE |
| AU2004284090A1 (en) | 2003-10-24 | 2005-05-06 | Avidia, Inc. | LDL receptor class A and EGF domain monomers and multimers |
| JP2005168328A (ja) | 2003-12-08 | 2005-06-30 | Hokkaido Univ | シグナル伝達活性を有する細胞質蛋白質の活性制御方法 |
| US20050191293A1 (en) | 2003-12-10 | 2005-09-01 | Shrikant Deshpande | IP-10 antibodies and their uses |
| WO2005110453A2 (en) | 2004-04-12 | 2005-11-24 | Catalyst Biosciences, Inc. | Cleavage of vegf and vegf receptor by wildtype and mutant mt-sp1 |
| EP2216046B1 (en) | 2004-07-09 | 2014-03-12 | Chugai Seiyaku Kabushiki Kaisha | Anti-glypican 3 antibody |
| EP2418278A3 (en) | 2005-05-09 | 2012-07-04 | Ono Pharmaceutical Co., Ltd. | Human monoclonal antibodies to programmed death 1(PD-1) and methods for treating cancer using anti-PD-1 antibodies alone or in combination with other immunotherapeutics |
| US7666817B2 (en) | 2005-08-31 | 2010-02-23 | The Regents Of The University Of California | Cellular libraries of peptide sequences (CLiPS) and methods of using the same |
| JP2009519011A (ja) | 2005-12-01 | 2009-05-14 | ドマンティス リミテッド | インターロイキン1受容体1型に結合する非競合ドメイン抗体フォーマット |
| EA200801166A1 (ru) | 2005-12-01 | 2008-12-30 | Домантис Лимитед | Форматы конкурентного доменного антитела, которые связываются с рецептором интерлейкина 1 первого типа |
| TWI417301B (zh) | 2006-02-21 | 2013-12-01 | Wyeth Corp | 對抗人類介白素-22(il-22)之抗體及其用途 |
| TWI369402B (en) | 2006-07-05 | 2012-08-01 | Catalyst Biosciences Inc | Protease screening methods and proteases identified thereby |
| WO2008016854A2 (en) | 2006-08-02 | 2008-02-07 | The Uab Research Foundation | Methods and compositions related to soluble monoclonal variable lymphocyte receptors of defined antigen specificity |
| AU2007285695B2 (en) | 2006-08-18 | 2012-05-24 | Ablynx N.V. | Amino acid sequences directed against IL-6R and polypeptides comprising the same for the treatment of diseases and disorders associated with IL-6-mediated signalling |
| EP2139919A2 (en) | 2007-02-28 | 2010-01-06 | Novimmune Sa | Human anti-ip-10 antibodies and uses thereof |
| CA2688434A1 (en) | 2007-06-06 | 2008-12-11 | Domantis Limited | Polypeptides, antibody variable domains and antagonists |
| KR101799337B1 (ko) | 2007-06-21 | 2017-12-20 | 마크로제닉스, 인크. | 공유결합형 디아바디 및 이것의 사용 |
| ES2628395T3 (es) | 2007-08-15 | 2017-08-02 | Bayer Pharma Aktiengesellschaft | Anticuerpo regulado por proteasa |
| CA3128656A1 (en) | 2007-08-22 | 2009-02-26 | The Regents Of The University Of California | Activatable binding polypeptides and methods of identification and use thereof |
| AU2016213702C1 (en) | 2007-08-22 | 2018-11-29 | Cytomx Therapeutics, Inc. | Activatable binding polypeptides and methods of identification and use thereof |
| CN102482347B (zh) | 2009-01-12 | 2017-04-26 | 希托马克斯医疗有限责任公司 | 修饰抗体组合物及其制备和使用方法 |
| MX2011010681A (es) | 2009-04-10 | 2012-01-20 | Ablynx Nv | Secuencias mejoradas de aminoacidos dirigidas contra il-6r y polipeptidos que comprenden el mismo para el tratamiento de enfermedades y trastornos relacionados con il-6r. |
| JP2011026294A (ja) | 2009-06-26 | 2011-02-10 | Canon Inc | 化合物 |
| ES2534085T3 (es) | 2009-08-17 | 2015-04-17 | Roche Glycart Ag | Inmunoconjugados dirigidos |
| US8734774B2 (en) | 2010-04-02 | 2014-05-27 | University Of Rochester | Protease activated cytokines |
| US9193791B2 (en) | 2010-08-03 | 2015-11-24 | City Of Hope | Development of masked therapeutic antibodies to limit off-target effects |
| RU2013110876A (ru) | 2010-08-24 | 2014-09-27 | Рош Гликарт Аг | Активируемые биспецифические антитела |
| WO2012028697A1 (en) | 2010-09-01 | 2012-03-08 | Eth Zürich, Institute Of Molecular Biology And Biophysics | Affinity purification system based on donor strand complementation |
| JP6130307B2 (ja) | 2011-03-17 | 2017-05-17 | ザ ユニバーシティ オブ バーミンガム | 再指向性免疫療法 |
| CN107936121B (zh) | 2011-05-16 | 2022-01-14 | 埃泰美德(香港)有限公司 | 多特异性fab融合蛋白及其使用方法 |
| TW201326209A (zh) | 2011-09-30 | 2013-07-01 | Chugai Pharmaceutical Co Ltd | 具有促進抗原清除之FcRn結合域的治療性抗原結合分子 |
| GB201203442D0 (en) | 2012-02-28 | 2012-04-11 | Univ Birmingham | Immunotherapeutic molecules and uses |
| JP6283017B2 (ja) | 2012-03-30 | 2018-02-21 | バイエル・ヘルスケア・エルエルシーBayer HealthCare LLC | プロテアーゼ制御抗体 |
| ES2670668T3 (es) | 2012-04-06 | 2018-05-31 | Omeros Corporation | Composiciones y métodos para inhibir la MASP-1 y/o la MASP-3 para el tratamiento de la hemoglobinuria nocturna paroxísmica |
| JP6178846B2 (ja) | 2012-05-22 | 2017-08-09 | ライフ テクノロジーズ エーエス | 組み換え抗体組成物およびその使用方法 |
| EP2863948B1 (en) | 2012-06-22 | 2018-10-24 | Cytomx Therapeutics Inc. | Anti-jagged 1/jagged 2 cross-reactive antibodies, activatable anti-jagged antibodies and methods of use thereof |
| WO2014052462A2 (en) | 2012-09-25 | 2014-04-03 | Cytomx Therapeutics, Inc. | Activatable antibodies that bind interleukin-6 receptor and methods of use thereof |
| JP6453208B2 (ja) | 2013-02-15 | 2019-01-16 | 国立大学法人京都工芸繊維大学 | 抗体のリフォールディング方法、リフォールディングされた抗体の製造方法、リフォールディングされた抗体、及びこれらの利用 |
| US20150087810A1 (en) | 2013-09-25 | 2015-03-26 | Cytomx Therapeutics, Inc. | Matrix Metalloproteinase Substrates And Other Cleavable Moieties And Methods Of Use Thereof |
| US9540440B2 (en) | 2013-10-30 | 2017-01-10 | Cytomx Therapeutics, Inc. | Activatable antibodies that bind epidermal growth factor receptor and methods of use thereof |
| WO2015089283A1 (en) | 2013-12-11 | 2015-06-18 | Cytomx Therapeutics, Inc. | Antibodies that bind activatable antibodies and methods of use thereof |
| BR112016017649B1 (pt) | 2014-01-31 | 2024-01-30 | Cytomx Therapeutics, Inc | Polipeptídeo isolado compreendendo substratos de matriptase e de ativador do plasminogênio u e outras porções cliváveis, composição farmacêutica compreendendo o dito polipeptídeo, bem como métodos para produzir e fabricar um polipeptídeo isolado compreendendo uma porção clivável e uso da composição farmacêutica e quantidade terapeuticamente eficaz da dita composição no tratamento de um distúrbio ou doença |
| MX2016010174A (es) | 2014-02-06 | 2016-11-15 | Hoffmann La Roche | Proteinas de fusion de interleucina-10 y usos de las mismas. |
| WO2016014974A2 (en) | 2014-07-25 | 2016-01-28 | Cytomx Therapeutics, Inc. | Anti-cd3 antibodies, activatable anti-cd3 antibodies, multispecific anti-cd3 antibodies, multispecific activatable anti-cd3 antibodies, and methods of using the same |
| GB201413357D0 (en) | 2014-07-28 | 2014-09-10 | Philogen Spa | Antibodies for treatment and diagnosis |
| JP2017529853A (ja) | 2014-09-25 | 2017-10-12 | アムジエン・インコーポレーテツド | プロテアーゼにより活性化可能な二重特異性タンパク質 |
| CA2964968A1 (en) | 2014-11-11 | 2016-05-19 | Amunix Operating Inc. | Targeted xten conjugate compositions and methods of making same |
| MA41374A (fr) | 2015-01-20 | 2017-11-28 | Cytomx Therapeutics Inc | Substrats clivables par métalloprotéase matricielle et clivables par sérine protéase et procédés d'utilisation de ceux-ci |
| WO2016149201A2 (en) | 2015-03-13 | 2016-09-22 | Cytomx Therapeutics, Inc. | Anti-pdl1 antibodies, activatable anti-pdl1 antibodies, and methods of use thereof |
| EP3778640A1 (en) | 2015-05-01 | 2021-02-17 | Genentech, Inc. | Masked anti-cd3 antibodies and methods of use |
| CN107849133B (zh) | 2015-05-04 | 2022-08-23 | 西托姆克斯治疗公司 | 抗cd166抗体、可活化抗cd166抗体及其使用方法 |
| US10233244B2 (en) | 2015-05-04 | 2019-03-19 | Cytomx Therapeutics, Inc. | Anti-ITGA3 antibodies, activatable anti-ITGA3 antibodies, and methods of use thereof |
| SG10201913762QA (en) | 2015-05-04 | 2020-03-30 | Cytomx Therapeutics Inc | Anti-cd71 antibodies, activatable anti-cd71 antibodies, and methods of use thereof |
| US11400157B2 (en) | 2015-05-13 | 2022-08-02 | Chugai Seiyaku Kabushiki Kaisha | Multiple antigen binding molecular fusion, pharmaceutical composition, method for identifying linear epitope, and method for preparing multiple antigen binding molecular fusion |
| WO2017025698A1 (en) | 2015-08-11 | 2017-02-16 | Queen Mary University Of London | Bispecific, cleavable antibodies |
| BR112018016281A2 (pt) | 2016-03-22 | 2019-01-02 | F. Hoffmann-La Roche Ag | molécula biespecífica ativadora de célula t ativável por protease, polipeptídeo idiotipo-específico, composição farmacêutica, usos da molécula biespecífica e método de tratamento de uma doença em um indivíduo |
| JP7461741B2 (ja) | 2016-06-20 | 2024-04-04 | カイマブ・リミテッド | 抗pd-l1およびil-2サイトカイン |
| CA3042679A1 (en) | 2016-11-03 | 2018-05-11 | Bristol-Myers Squibb Company | Activatable anti-ctla-4 antibodies and uses thereof |
| KR102533814B1 (ko) | 2016-11-28 | 2023-05-19 | 추가이 세이야쿠 가부시키가이샤 | 리간드 결합 활성을 조정 가능한 리간드 결합 분자 |
| KR20240018673A (ko) | 2016-11-28 | 2024-02-13 | 추가이 세이야쿠 가부시키가이샤 | 항원 결합 도메인 및 운반 부분을 포함하는 폴리펩티드 |
| CA3046432A1 (en) | 2016-12-13 | 2018-06-21 | Astellas Pharma Inc. | Anti-human cd73 antibody |
| AU2018277310B2 (en) | 2017-06-02 | 2024-07-11 | Ablynx Nv | Aggrecan binding immunoglobulins |
| JP7249961B2 (ja) | 2017-06-02 | 2023-03-31 | メルク パテント ゲゼルシャフト ミット ベシュレンクテル ハフツング | Adamts5、mmp13およびアグリカンに結合するポリペプチド |
| AU2018297248A1 (en) | 2017-07-03 | 2020-02-20 | Torque Therapeutics, Inc. | Fusion molecules targeting immune regulatory cells and uses thereof |
| US20190038935A1 (en) | 2017-08-07 | 2019-02-07 | Timofey Ignatyev | Exercise game and apparatus employing color-changing base locations |
| CN107602706B (zh) | 2017-10-16 | 2020-12-04 | 湖北大学 | 一种切割效率增强的hrv 3c蛋白酶底物突变体及其应用 |
| TWI818934B (zh) | 2017-11-28 | 2023-10-21 | 日商中外製藥股份有限公司 | 可調整配體結合活性的配體結合分子 |
| JP7482630B2 (ja) | 2017-11-28 | 2024-05-14 | 中外製薬株式会社 | 抗原結合ドメインおよび運搬部分を含むポリペプチド |
| KR102020131B1 (ko) | 2017-12-29 | 2019-09-09 | 박성원 | 광경화성 조성물 및 이를 이용하여 제조된 성형품 |
| CA3115461A1 (en) | 2018-03-09 | 2019-09-12 | AskGene Pharma, Inc. | Novel cytokine prodrugs |
| EP3794022A1 (en) | 2018-05-14 | 2021-03-24 | Werewolf Therapeutics, Inc. | Activatable cytokine polypeptides and methods of use thereof |
| JP7460609B2 (ja) | 2018-05-14 | 2024-04-02 | ウェアウルフ セラピューティクス, インコーポレイテッド | 活性化可能なインターロイキン-2ポリペプチド及びその使用方法 |
| JP7414736B2 (ja) | 2018-05-30 | 2024-01-16 | 中外製薬株式会社 | アグリカン結合ドメインおよび運搬部分を含むポリペプチド |
| EP3802831A4 (en) | 2018-05-30 | 2022-07-27 | Chugai Seiyaku Kabushiki Kaisha | Polypeptide comprising il-1r1 binding domain and carrying moiety |
| US20210253672A1 (en) | 2018-05-30 | 2021-08-19 | Chugai Seiyaku Kabushiki Kaisha | Ligand-binding molecule containing single domain antibody |
| US20210355219A1 (en) | 2018-09-21 | 2021-11-18 | Harpoon Therapeutics, Inc. | Conditionally activated target-binding molecules |
| EP3856764A4 (en) | 2018-09-27 | 2022-11-02 | Xilio Development, Inc. | MASKED CYTOKINE POLYPEPTIDES |
| SG11202103192RA (en) | 2018-10-03 | 2021-04-29 | Xencor Inc | Il-12 heterodimeric fc-fusion proteins |
| WO2020246567A1 (ja) | 2019-06-05 | 2020-12-10 | 中外製薬株式会社 | プロテアーゼ基質、及びプロテアーゼ切断配列を含むポリペプチド |
| CN113905757A (zh) | 2019-06-05 | 2022-01-07 | 中外制药株式会社 | 抗体切割位点结合分子 |
| BR112022001320A2 (pt) | 2019-07-25 | 2022-04-12 | Univ Chicago | Composições e métodos compreendendo agentes terapêuticos ativados por protease |
| WO2021149697A1 (en) | 2020-01-20 | 2021-07-29 | Chugai Seiyaku Kabushiki Kaisha | Ligand-binding fusion proteins |
| EP4373862A1 (en) | 2021-07-19 | 2024-05-29 | Chugai Seiyaku Kabushiki Kaisha | Protease-mediated target specific cytokine delivery using fusion polypeptide |
-
2017
- 2017-11-28 KR KR1020197017817A patent/KR102533814B1/ko active Active
- 2017-11-28 TW TW106141341A patent/TWI776827B/zh active
- 2017-11-28 MX MX2019005947A patent/MX2019005947A/es unknown
- 2017-11-28 AU AU2017364818A patent/AU2017364818C1/en active Active
- 2017-11-28 US US16/463,218 patent/US12060654B2/en active Active
- 2017-11-28 CA CA3039316A patent/CA3039316A1/en active Pending
- 2017-11-28 CN CN201780084377.1A patent/CN110214151A/zh active Pending
- 2017-11-28 KR KR1020237015916A patent/KR20230073346A/ko not_active Ceased
- 2017-11-28 JP JP2018553014A patent/JP7626577B2/ja active Active
- 2017-11-28 IL IL266827A patent/IL266827B2/en unknown
- 2017-11-28 WO PCT/JP2017/042570 patent/WO2018097308A1/ja not_active Ceased
- 2017-11-28 TW TW111105054A patent/TW202235438A/zh unknown
- 2017-11-28 BR BR112019008265A patent/BR112019008265A2/pt unknown
- 2017-11-28 EP EP17872925.7A patent/EP3546480A4/en active Pending
-
2019
- 2019-05-21 MX MX2024003513A patent/MX2024003513A/es unknown
- 2019-05-21 MX MX2024003516A patent/MX2024003516A/es unknown
-
2023
- 2023-03-13 JP JP2023038624A patent/JP7671794B2/ja active Active
-
2024
- 2024-05-06 US US18/656,351 patent/US20250034756A1/en active Pending
- 2024-12-24 JP JP2024226961A patent/JP2025041812A/ja active Pending
Non-Patent Citations (9)
| Title |
|---|
| Antigen specificity can be irrelevant to immunocytokine efficacy and biodistribution. Tzeng A, Kwan BH, Opel CF, Navaratna T, Wittrup KD. Proc Natl Acad Sci U S A. 2015 Mar 17;112(11):3320-5. |
| Cancer Immunol Immunother. 2006 Dec;55(12):1590-600. Epub 2006 Apr 25. Target-selective activation of a TNF prodrug by urokinase-type plasminogen activator (uPA) mediated proteolytic processing at the cell surface. Gerspach J1, Nemeth J, Munkel S, Wajant H, Pfizenmaier K. |
| Cyclophosphamide and tucotuzumab (huKS-IL2) following first-line chemotherapy in responding patients with extensive-disease small-cell lung cancer. Gladkov O, Ramlau R, Serwatowski P, Milanowski J, Tomeczko J, Komarnitsky PB, Kramer D, Krzakowski MJ. Anticancer Drugs. 2015 Nov;26(10):1061-8. |
| Defining the Pharmacodynamic Profile and Therapeutic Index of NHS-IL12 Immunocytokine in Dogs with Malignant Melanoma. Paoloni M, Mazcko C, Selting K, Lana S, Barber L, Phillips J, Skorupski K, Vail D, Wilson H, Biller B, Avery A, Kiupel M, LeBlanc A, Bernhardt A, Brunkhorst B, Tighe R, Khanna C. PLoS One. 2015 Jun 19;10(6):e0129954. |
| Immunology. 2011 Jun;133(2):206-20. doi: 10.1111/j. 1365-2567. 2011.03428.x. Epub 2011 Mar 23. Development of an attenuated interleukin-2 fusion protein that can be activated by tumour-expressed proteases. Puskas J1, Skrombolas D, Sedlacek A, Lord E, Sullivan M, Frelinger J. |
| Isolated limb perfusion with the tumor-targeting human monoclonal antibody-cytokine fusion protein L19-TNF plus melphalan and mild hyperthermia in patients with locally advanced extremity melanoma. Papadia F, Basso V, Patuzzo R, Maurichi A, Di Florio A, Zardi L, Ventura E, Gonzalez-Iglesias R, Lovato V, Giovannoni L, Tasciotti A, Neri D, Santinami M, Menssen HD, De Cian F. J Surg Oncol. 2013 Feb;107(2):173-9. |
| Monoclonal antibodies: versatile platforms for cancer immunotherapy. Weiner LM, Surana R, Wang S., Nat. Rev. Immunol. (2010) 10 (5), 317-327 |
| Monoclonal antibody successes in the clinic. Janice M Reichert, Clark J Rosensweig, Laura B Faden & Matthew C Dewitz, Nat. Biotechnol. (2005) 23, 1073-1078 |
| The therapeutic antibodies market to 2008. Pavlou AK, Belsey MJ., Eur. J. Pharm. Biopharm. (2005) 59 (3), 389-396 |
Also Published As
| Publication number | Publication date |
|---|---|
| US12060654B2 (en) | 2024-08-13 |
| RU2019117223A (ru) | 2020-12-28 |
| US20250034756A1 (en) | 2025-01-30 |
| EP3546480A1 (en) | 2019-10-02 |
| JP2023075256A (ja) | 2023-05-30 |
| TWI776827B (zh) | 2022-09-11 |
| TW202235438A (zh) | 2022-09-16 |
| KR102533814B1 (ko) | 2023-05-19 |
| US20200207846A1 (en) | 2020-07-02 |
| MX2024003513A (es) | 2024-04-08 |
| RU2019117223A3 (enExample) | 2021-02-18 |
| IL266827A (en) | 2019-08-29 |
| WO2018097308A1 (ja) | 2018-05-31 |
| JP7671794B2 (ja) | 2025-05-02 |
| JP2025041812A (ja) | 2025-03-26 |
| BR112019008265A2 (pt) | 2019-07-09 |
| MX2024003516A (es) | 2024-04-01 |
| KR20190087525A (ko) | 2019-07-24 |
| CA3039316A1 (en) | 2018-05-31 |
| JPWO2018097308A1 (ja) | 2019-10-17 |
| IL266827B2 (en) | 2025-04-01 |
| JP7626577B2 (ja) | 2025-02-07 |
| AU2017364818A1 (en) | 2019-05-23 |
| AU2017364818C1 (en) | 2025-05-15 |
| AU2017364818B2 (en) | 2024-11-21 |
| TW201833136A (zh) | 2018-09-16 |
| EP3546480A4 (en) | 2020-07-29 |
| CN110214151A (zh) | 2019-09-06 |
| IL266827B1 (en) | 2024-12-01 |
| MX2019005947A (es) | 2019-08-26 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP7671794B2 (ja) | リガンド結合活性が調整可能なリガンド結合分子 | |
| JP7751606B2 (ja) | リガンド結合活性が調整可能なリガンド結合分子 | |
| WO2021149697A1 (en) | Ligand-binding fusion proteins | |
| JP2024056935A (ja) | 単ドメイン抗体含有リガンド結合分子 | |
| RU2803067C2 (ru) | Лигандсвязывающая молекула с регулируемой лигандсвязывающей активностью | |
| RU2852787C2 (ru) | Лигандсвязывающая молекула, обладающая регулируемой лигандсвязывающей активностью | |
| HK40007165A (en) | Ligand-binding molecule having adjustable ligand binding activity | |
| HK40029817A (en) | Ligand-binding molecule having adjustable ligand-binding activity |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A107 | Divisional application of patent | ||
| PA0104 | Divisional application for international application |
Comment text: Divisional Application for International Patent Patent event code: PA01041R01D Patent event date: 20230511 Application number text: 1020197017817 Filing date: 20190620 |
|
| PG1501 | Laying open of application | ||
| E902 | Notification of reason for refusal | ||
| PE0902 | Notice of grounds for rejection |
Comment text: Notification of reason for refusal Patent event date: 20230728 Patent event code: PE09021S01D |
|
| PE0601 | Decision on rejection of patent |
Patent event date: 20240626 Comment text: Decision to Refuse Application Patent event code: PE06012S01D |
|
| AMND | Amendment | ||
| PX0901 | Re-examination |
Patent event code: PX09012R01I Patent event date: 20241125 Comment text: Amendment to Specification, etc. |
|
| PX0601 | Decision of rejection after re-examination |
Comment text: Decision to Refuse Application Patent event code: PX06014S01D Patent event date: 20241227 Comment text: Decision to Refuse Application Patent event code: PX06011S01I Patent event date: 20241227 |
|
| X601 | Decision of rejection after re-examination | ||
| J201 | Request for trial against refusal decision | ||
| PJ0201 | Trial against decision of rejection |
Patent event date: 20250529 Comment text: Request for Trial against Decision on Refusal Patent event code: PJ02012R01D Appeal kind category: Appeal against decision to decline refusal Appeal identifier: 2025101000969 Request date: 20250529 |