KR20160093012A - 암 치료를 위한 체크포인트 억제제 및 치료제의 배합물 - Google Patents
암 치료를 위한 체크포인트 억제제 및 치료제의 배합물 Download PDFInfo
- Publication number
- KR20160093012A KR20160093012A KR1020167014867A KR20167014867A KR20160093012A KR 20160093012 A KR20160093012 A KR 20160093012A KR 1020167014867 A KR1020167014867 A KR 1020167014867A KR 20167014867 A KR20167014867 A KR 20167014867A KR 20160093012 A KR20160093012 A KR 20160093012A
- Authority
- KR
- South Korea
- Prior art keywords
- cancer
- cell
- tumor
- checkpoint inhibitor
- cells
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Withdrawn
Links
- 206010028980 Neoplasm Diseases 0.000 title claims abstract description 283
- 229940076838 Immune checkpoint inhibitor Drugs 0.000 title claims abstract description 187
- 239000012274 immune-checkpoint protein inhibitor Substances 0.000 title claims abstract description 187
- 201000011510 cancer Diseases 0.000 title claims abstract description 123
- 239000003814 drug Substances 0.000 title claims abstract description 70
- 238000000034 method Methods 0.000 claims abstract description 114
- 229940124597 therapeutic agent Drugs 0.000 claims abstract description 61
- 238000002648 combination therapy Methods 0.000 claims abstract description 23
- 210000001744 T-lymphocyte Anatomy 0.000 claims description 152
- 210000004027 cell Anatomy 0.000 claims description 121
- 238000011282 treatment Methods 0.000 claims description 62
- 229940029030 dendritic cell vaccine Drugs 0.000 claims description 60
- 239000000427 antigen Substances 0.000 claims description 59
- 102000036639 antigens Human genes 0.000 claims description 59
- 108091007433 antigens Proteins 0.000 claims description 59
- 229960005486 vaccine Drugs 0.000 claims description 53
- 230000000735 allogeneic effect Effects 0.000 claims description 44
- 102100039498 Cytotoxic T-lymphocyte protein 4 Human genes 0.000 claims description 43
- 238000002659 cell therapy Methods 0.000 claims description 42
- 210000000987 immune system Anatomy 0.000 claims description 38
- 239000000203 mixture Substances 0.000 claims description 35
- 239000003446 ligand Substances 0.000 claims description 32
- 108010021064 CTLA-4 Antigen Proteins 0.000 claims description 30
- 102000015696 Interleukins Human genes 0.000 claims description 27
- 108010063738 Interleukins Proteins 0.000 claims description 27
- 102100034458 Hepatitis A virus cellular receptor 2 Human genes 0.000 claims description 22
- 102100024213 Programmed cell death 1 ligand 2 Human genes 0.000 claims description 22
- 230000006023 anti-tumor response Effects 0.000 claims description 22
- 206010039491 Sarcoma Diseases 0.000 claims description 20
- 108010002586 Interleukin-7 Proteins 0.000 claims description 19
- 102000000704 Interleukin-7 Human genes 0.000 claims description 19
- 239000003112 inhibitor Substances 0.000 claims description 19
- 201000001441 melanoma Diseases 0.000 claims description 19
- 102100024263 CD160 antigen Human genes 0.000 claims description 18
- 101000761938 Homo sapiens CD160 antigen Proteins 0.000 claims description 18
- 230000000694 effects Effects 0.000 claims description 18
- 206010033128 Ovarian cancer Diseases 0.000 claims description 17
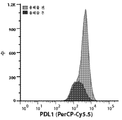
- 102100024216 Programmed cell death 1 ligand 1 Human genes 0.000 claims description 17
- 208000002154 non-small cell lung carcinoma Diseases 0.000 claims description 17
- 102100029822 B- and T-lymphocyte attenuator Human genes 0.000 claims description 16
- 102100038078 CD276 antigen Human genes 0.000 claims description 16
- 101710185679 CD276 antigen Proteins 0.000 claims description 16
- 101000864344 Homo sapiens B- and T-lymphocyte attenuator Proteins 0.000 claims description 16
- 101001117317 Homo sapiens Programmed cell death 1 ligand 1 Proteins 0.000 claims description 16
- 101000666896 Homo sapiens V-type immunoglobulin domain-containing suppressor of T-cell activation Proteins 0.000 claims description 16
- 102000017578 LAG3 Human genes 0.000 claims description 16
- 206010061535 Ovarian neoplasm Diseases 0.000 claims description 16
- 108010079206 V-Set Domain-Containing T-Cell Activation Inhibitor 1 Proteins 0.000 claims description 16
- 102100038929 V-set domain-containing T-cell activation inhibitor 1 Human genes 0.000 claims description 16
- 102100038282 V-type immunoglobulin domain-containing suppressor of T-cell activation Human genes 0.000 claims description 16
- 230000000977 initiatory effect Effects 0.000 claims description 16
- 101150051188 Adora2a gene Proteins 0.000 claims description 15
- 206010006187 Breast cancer Diseases 0.000 claims description 15
- 208000026310 Breast neoplasm Diseases 0.000 claims description 15
- 102100031351 Galectin-9 Human genes 0.000 claims description 15
- 101100229077 Gallus gallus GAL9 gene Proteins 0.000 claims description 15
- 101001068133 Homo sapiens Hepatitis A virus cellular receptor 2 Proteins 0.000 claims description 15
- 101001137987 Homo sapiens Lymphocyte activation gene 3 protein Proteins 0.000 claims description 15
- 101001117312 Homo sapiens Programmed cell death 1 ligand 2 Proteins 0.000 claims description 15
- 101000611936 Homo sapiens Programmed cell death protein 1 Proteins 0.000 claims description 15
- 101000777277 Homo sapiens Serine/threonine-protein kinase Chk2 Proteins 0.000 claims description 15
- 102000002698 KIR Receptors Human genes 0.000 claims description 15
- 108010043610 KIR Receptors Proteins 0.000 claims description 15
- 108010061593 Member 14 Tumor Necrosis Factor Receptors Proteins 0.000 claims description 15
- 102100031075 Serine/threonine-protein kinase Chk2 Human genes 0.000 claims description 15
- 102100028785 Tumor necrosis factor receptor superfamily member 14 Human genes 0.000 claims description 15
- 230000000259 anti-tumor effect Effects 0.000 claims description 15
- 230000002708 enhancing effect Effects 0.000 claims description 15
- IJJVMEJXYNJXOJ-UHFFFAOYSA-N fluquinconazole Chemical compound C=1C=C(Cl)C=C(Cl)C=1N1C(=O)C2=CC(F)=CC=C2N=C1N1C=NC=N1 IJJVMEJXYNJXOJ-UHFFFAOYSA-N 0.000 claims description 15
- 101000834898 Homo sapiens Alpha-synuclein Proteins 0.000 claims description 14
- 101000652359 Homo sapiens Spermatogenesis-associated protein 2 Proteins 0.000 claims description 14
- 208000006265 Renal cell carcinoma Diseases 0.000 claims description 14
- 206010073071 hepatocellular carcinoma Diseases 0.000 claims description 14
- 231100000844 hepatocellular carcinoma Toxicity 0.000 claims description 14
- 210000004881 tumor cell Anatomy 0.000 claims description 14
- 102000000588 Interleukin-2 Human genes 0.000 claims description 13
- 208000021712 Soft tissue sarcoma Diseases 0.000 claims description 13
- 206010009944 Colon cancer Diseases 0.000 claims description 12
- 108010002350 Interleukin-2 Proteins 0.000 claims description 12
- 102100040678 Programmed cell death protein 1 Human genes 0.000 claims description 12
- 206010060862 Prostate cancer Diseases 0.000 claims description 12
- 208000000236 Prostatic Neoplasms Diseases 0.000 claims description 12
- 208000005718 Stomach Neoplasms Diseases 0.000 claims description 12
- 108020001507 fusion proteins Proteins 0.000 claims description 12
- 102000037865 fusion proteins Human genes 0.000 claims description 12
- 206010017758 gastric cancer Diseases 0.000 claims description 12
- 208000032839 leukemia Diseases 0.000 claims description 12
- 201000011549 stomach cancer Diseases 0.000 claims description 12
- 101100463133 Caenorhabditis elegans pdl-1 gene Proteins 0.000 claims description 11
- 101000777293 Homo sapiens Serine/threonine-protein kinase Chk1 Proteins 0.000 claims description 11
- 102000003812 Interleukin-15 Human genes 0.000 claims description 11
- 108090000172 Interleukin-15 Proteins 0.000 claims description 11
- 102100031081 Serine/threonine-protein kinase Chk1 Human genes 0.000 claims description 11
- 230000003308 immunostimulating effect Effects 0.000 claims description 11
- 230000005764 inhibitory process Effects 0.000 claims description 11
- 230000005855 radiation Effects 0.000 claims description 11
- 150000003384 small molecules Chemical class 0.000 claims description 11
- 206010055113 Breast cancer metastatic Diseases 0.000 claims description 10
- 229940045513 CTLA4 antagonist Drugs 0.000 claims description 10
- 206010052358 Colorectal cancer metastatic Diseases 0.000 claims description 10
- 229940088597 hormone Drugs 0.000 claims description 10
- 239000005556 hormone Substances 0.000 claims description 10
- 206010061902 Pancreatic neoplasm Diseases 0.000 claims description 9
- 206010035226 Plasma cell myeloma Diseases 0.000 claims description 9
- 208000000102 Squamous Cell Carcinoma of Head and Neck Diseases 0.000 claims description 9
- 201000000459 head and neck squamous cell carcinoma Diseases 0.000 claims description 9
- 229960001438 immunostimulant agent Drugs 0.000 claims description 9
- 239000003022 immunostimulating agent Substances 0.000 claims description 9
- 208000015486 malignant pancreatic neoplasm Diseases 0.000 claims description 9
- 201000002528 pancreatic cancer Diseases 0.000 claims description 9
- 208000008443 pancreatic carcinoma Diseases 0.000 claims description 9
- 230000004614 tumor growth Effects 0.000 claims description 9
- 208000031261 Acute myeloid leukaemia Diseases 0.000 claims description 8
- 208000001333 Colorectal Neoplasms Diseases 0.000 claims description 8
- 208000000461 Esophageal Neoplasms Diseases 0.000 claims description 8
- 206010058467 Lung neoplasm malignant Diseases 0.000 claims description 8
- 208000034578 Multiple myelomas Diseases 0.000 claims description 8
- 208000033776 Myeloid Acute Leukemia Diseases 0.000 claims description 8
- 201000004101 esophageal cancer Diseases 0.000 claims description 8
- 201000005202 lung cancer Diseases 0.000 claims description 8
- 208000020816 lung neoplasm Diseases 0.000 claims description 8
- 208000032791 BCR-ABL1 positive chronic myelogenous leukemia Diseases 0.000 claims description 7
- 206010030155 Oesophageal carcinoma Diseases 0.000 claims description 7
- 206010041067 Small cell lung cancer Diseases 0.000 claims description 7
- 208000035250 cutaneous malignant susceptibility to 1 melanoma Diseases 0.000 claims description 7
- 208000005017 glioblastoma Diseases 0.000 claims description 7
- 201000010536 head and neck cancer Diseases 0.000 claims description 7
- 208000014829 head and neck neoplasm Diseases 0.000 claims description 7
- 230000003211 malignant effect Effects 0.000 claims description 7
- 230000002265 prevention Effects 0.000 claims description 7
- 208000000587 small cell lung carcinoma Diseases 0.000 claims description 7
- 238000002626 targeted therapy Methods 0.000 claims description 7
- 230000001988 toxicity Effects 0.000 claims description 7
- 231100000419 toxicity Toxicity 0.000 claims description 7
- 201000009030 Carcinoma Diseases 0.000 claims description 6
- 208000010833 Chronic myeloid leukaemia Diseases 0.000 claims description 6
- 208000002030 Merkel cell carcinoma Diseases 0.000 claims description 6
- 201000003793 Myelodysplastic syndrome Diseases 0.000 claims description 6
- 208000033761 Myelogenous Chronic BCR-ABL Positive Leukemia Diseases 0.000 claims description 6
- 206010029266 Neuroendocrine carcinoma of the skin Diseases 0.000 claims description 6
- 208000008385 Urogenital Neoplasms Diseases 0.000 claims description 6
- 208000017763 cutaneous neuroendocrine carcinoma Diseases 0.000 claims description 6
- 230000000683 nonmetastatic effect Effects 0.000 claims description 6
- 208000037964 urogenital cancer Diseases 0.000 claims description 6
- 206010006007 bone sarcoma Diseases 0.000 claims description 5
- 230000030833 cell death Effects 0.000 claims description 5
- 230000006872 improvement Effects 0.000 claims description 5
- 239000008194 pharmaceutical composition Substances 0.000 claims description 5
- 208000029742 colonic neoplasm Diseases 0.000 claims description 4
- 208000006178 malignant mesothelioma Diseases 0.000 claims description 4
- 208000024893 Acute lymphoblastic leukemia Diseases 0.000 claims description 3
- 208000014697 Acute lymphocytic leukaemia Diseases 0.000 claims description 3
- 206010061218 Inflammation Diseases 0.000 claims description 3
- 208000006664 Precursor Cell Lymphoblastic Leukemia-Lymphoma Diseases 0.000 claims description 3
- 206010054094 Tumour necrosis Diseases 0.000 claims description 3
- 230000008602 contraction Effects 0.000 claims description 3
- 230000012010 growth Effects 0.000 claims description 3
- 230000006698 induction Effects 0.000 claims description 3
- 230000004054 inflammatory process Effects 0.000 claims description 3
- 101710089372 Programmed cell death protein 1 Proteins 0.000 claims description 2
- 230000001464 adherent effect Effects 0.000 claims description 2
- 230000004547 gene signature Effects 0.000 claims description 2
- 102100026882 Alpha-synuclein Human genes 0.000 claims 4
- 230000000973 chemotherapeutic effect Effects 0.000 claims 2
- 201000010255 female reproductive organ cancer Diseases 0.000 claims 2
- 206010004446 Benign prostatic hyperplasia Diseases 0.000 claims 1
- 208000002250 Hematologic Neoplasms Diseases 0.000 claims 1
- 208000004403 Prostatic Hyperplasia Diseases 0.000 claims 1
- 230000003394 haemopoietic effect Effects 0.000 claims 1
- 230000009826 neoplastic cell growth Effects 0.000 claims 1
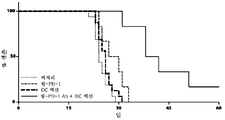
- 230000004044 response Effects 0.000 abstract description 32
- 230000009286 beneficial effect Effects 0.000 abstract description 4
- 210000004443 dendritic cell Anatomy 0.000 description 99
- 230000028993 immune response Effects 0.000 description 44
- 241000699670 Mus sp. Species 0.000 description 37
- -1 CGEN- Proteins 0.000 description 32
- 206010018338 Glioma Diseases 0.000 description 32
- 229940022511 therapeutic cancer vaccine Drugs 0.000 description 32
- 208000032612 Glial tumor Diseases 0.000 description 30
- 230000005975 antitumor immune response Effects 0.000 description 25
- 230000002401 inhibitory effect Effects 0.000 description 20
- 210000004698 lymphocyte Anatomy 0.000 description 20
- 108090000623 proteins and genes Proteins 0.000 description 18
- 102000004169 proteins and genes Human genes 0.000 description 17
- 230000004083 survival effect Effects 0.000 description 16
- 238000002255 vaccination Methods 0.000 description 16
- 230000008901 benefit Effects 0.000 description 15
- 208000029729 tumor suppressor gene on chromosome 11 Diseases 0.000 description 15
- 229940022399 cancer vaccine Drugs 0.000 description 14
- 238000009566 cancer vaccine Methods 0.000 description 14
- 230000037361 pathway Effects 0.000 description 14
- 101000889276 Homo sapiens Cytotoxic T-lymphocyte protein 4 Proteins 0.000 description 13
- 238000001727 in vivo Methods 0.000 description 13
- 210000003491 skin Anatomy 0.000 description 13
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 description 12
- 238000002560 therapeutic procedure Methods 0.000 description 12
- 102000004127 Cytokines Human genes 0.000 description 11
- 108090000695 Cytokines Proteins 0.000 description 11
- 230000001154 acute effect Effects 0.000 description 11
- 210000003719 b-lymphocyte Anatomy 0.000 description 11
- 239000003795 chemical substances by application Substances 0.000 description 11
- 239000012634 fragment Substances 0.000 description 11
- 230000001939 inductive effect Effects 0.000 description 11
- 239000002243 precursor Substances 0.000 description 11
- 108020004414 DNA Proteins 0.000 description 10
- 206010025323 Lymphomas Diseases 0.000 description 10
- 210000002865 immune cell Anatomy 0.000 description 10
- 206010061289 metastatic neoplasm Diseases 0.000 description 10
- 230000035935 pregnancy Effects 0.000 description 10
- 239000000126 substance Substances 0.000 description 10
- 208000003174 Brain Neoplasms Diseases 0.000 description 9
- 101000914514 Homo sapiens T-cell-specific surface glycoprotein CD28 Proteins 0.000 description 9
- 241001529936 Murinae Species 0.000 description 9
- 102100027213 T-cell-specific surface glycoprotein CD28 Human genes 0.000 description 9
- 210000001185 bone marrow Anatomy 0.000 description 9
- 229940030156 cell vaccine Drugs 0.000 description 9
- 230000001394 metastastic effect Effects 0.000 description 9
- 238000002600 positron emission tomography Methods 0.000 description 9
- 230000002829 reductive effect Effects 0.000 description 9
- 210000000952 spleen Anatomy 0.000 description 9
- 210000001519 tissue Anatomy 0.000 description 9
- 101000914484 Homo sapiens T-lymphocyte activation antigen CD80 Proteins 0.000 description 8
- 108700030875 Programmed Cell Death 1 Ligand 2 Proteins 0.000 description 8
- 102100027222 T-lymphocyte activation antigen CD80 Human genes 0.000 description 8
- 239000002246 antineoplastic agent Substances 0.000 description 8
- 230000000903 blocking effect Effects 0.000 description 8
- 210000004369 blood Anatomy 0.000 description 8
- 239000008280 blood Substances 0.000 description 8
- 230000001684 chronic effect Effects 0.000 description 8
- 210000001151 cytotoxic T lymphocyte Anatomy 0.000 description 8
- 229940127089 cytotoxic agent Drugs 0.000 description 8
- 230000007423 decrease Effects 0.000 description 8
- 229940079593 drug Drugs 0.000 description 8
- 238000009472 formulation Methods 0.000 description 8
- 230000001965 increasing effect Effects 0.000 description 8
- 230000003993 interaction Effects 0.000 description 8
- 210000001165 lymph node Anatomy 0.000 description 8
- 210000000822 natural killer cell Anatomy 0.000 description 8
- 101710083479 Hepatitis A virus cellular receptor 2 homolog Proteins 0.000 description 7
- 101100407308 Mus musculus Pdcd1lg2 gene Proteins 0.000 description 7
- 229940126547 T-cell immunoglobulin mucin-3 Drugs 0.000 description 7
- 230000004913 activation Effects 0.000 description 7
- 239000000090 biomarker Substances 0.000 description 7
- 210000002798 bone marrow cell Anatomy 0.000 description 7
- 239000000306 component Substances 0.000 description 7
- 201000010099 disease Diseases 0.000 description 7
- 230000006870 function Effects 0.000 description 7
- 238000002347 injection Methods 0.000 description 7
- 239000007924 injection Substances 0.000 description 7
- 238000007917 intracranial administration Methods 0.000 description 7
- 239000000463 material Substances 0.000 description 7
- 108090000765 processed proteins & peptides Proteins 0.000 description 7
- 230000035755 proliferation Effects 0.000 description 7
- 102000005962 receptors Human genes 0.000 description 7
- 108020003175 receptors Proteins 0.000 description 7
- 230000001105 regulatory effect Effects 0.000 description 7
- 206010005003 Bladder cancer Diseases 0.000 description 6
- 241000699666 Mus <mouse, genus> Species 0.000 description 6
- 208000007097 Urinary Bladder Neoplasms Diseases 0.000 description 6
- 239000003124 biologic agent Substances 0.000 description 6
- 230000003915 cell function Effects 0.000 description 6
- 230000010261 cell growth Effects 0.000 description 6
- 238000004519 manufacturing process Methods 0.000 description 6
- 230000001225 therapeutic effect Effects 0.000 description 6
- 201000005112 urinary bladder cancer Diseases 0.000 description 6
- 239000013598 vector Substances 0.000 description 6
- 208000023275 Autoimmune disease Diseases 0.000 description 5
- 206010008342 Cervix carcinoma Diseases 0.000 description 5
- 206010014733 Endometrial cancer Diseases 0.000 description 5
- 206010014759 Endometrial neoplasm Diseases 0.000 description 5
- 101001057504 Homo sapiens Interferon-stimulated gene 20 kDa protein Proteins 0.000 description 5
- 101001055144 Homo sapiens Interleukin-2 receptor subunit alpha Proteins 0.000 description 5
- 102100027268 Interferon-stimulated gene 20 kDa protein Human genes 0.000 description 5
- NWIBSHFKIJFRCO-WUDYKRTCSA-N Mytomycin Chemical compound C1N2C(C(C(C)=C(N)C3=O)=O)=C3[C@@H](COC(N)=O)[C@@]2(OC)[C@@H]2[C@H]1N2 NWIBSHFKIJFRCO-WUDYKRTCSA-N 0.000 description 5
- 208000034176 Neoplasms, Germ Cell and Embryonal Diseases 0.000 description 5
- 208000006105 Uterine Cervical Neoplasms Diseases 0.000 description 5
- 241000700605 Viruses Species 0.000 description 5
- 201000010881 cervical cancer Diseases 0.000 description 5
- 238000002512 chemotherapy Methods 0.000 description 5
- 208000035475 disorder Diseases 0.000 description 5
- 210000003128 head Anatomy 0.000 description 5
- 238000003384 imaging method Methods 0.000 description 5
- 238000009169 immunotherapy Methods 0.000 description 5
- 229940047122 interleukins Drugs 0.000 description 5
- 230000000527 lymphocytic effect Effects 0.000 description 5
- 239000006166 lysate Substances 0.000 description 5
- 230000007246 mechanism Effects 0.000 description 5
- 230000015654 memory Effects 0.000 description 5
- 210000001616 monocyte Anatomy 0.000 description 5
- 239000002953 phosphate buffered saline Substances 0.000 description 5
- 102000004196 processed proteins & peptides Human genes 0.000 description 5
- 150000003839 salts Chemical class 0.000 description 5
- 208000024891 symptom Diseases 0.000 description 5
- 238000012360 testing method Methods 0.000 description 5
- 238000002054 transplantation Methods 0.000 description 5
- 108700028369 Alleles Proteins 0.000 description 4
- 206010003571 Astrocytoma Diseases 0.000 description 4
- IJGRMHOSHXDMSA-UHFFFAOYSA-N Atomic nitrogen Chemical compound N#N IJGRMHOSHXDMSA-UHFFFAOYSA-N 0.000 description 4
- 108010041986 DNA Vaccines Proteins 0.000 description 4
- 229940021995 DNA vaccine Drugs 0.000 description 4
- AOJJSUZBOXZQNB-TZSSRYMLSA-N Doxorubicin Chemical compound O([C@H]1C[C@@](O)(CC=2C(O)=C3C(=O)C=4C=CC=C(C=4C(=O)C3=C(O)C=21)OC)C(=O)CO)[C@H]1C[C@H](N)[C@H](O)[C@H](C)O1 AOJJSUZBOXZQNB-TZSSRYMLSA-N 0.000 description 4
- GHASVSINZRGABV-UHFFFAOYSA-N Fluorouracil Chemical compound FC1=CNC(=O)NC1=O GHASVSINZRGABV-UHFFFAOYSA-N 0.000 description 4
- 208000017604 Hodgkin disease Diseases 0.000 description 4
- 208000021519 Hodgkin lymphoma Diseases 0.000 description 4
- 208000010747 Hodgkins lymphoma Diseases 0.000 description 4
- 102000004388 Interleukin-4 Human genes 0.000 description 4
- 108090000978 Interleukin-4 Proteins 0.000 description 4
- 208000028018 Lymphocytic leukaemia Diseases 0.000 description 4
- 241001465754 Metazoa Species 0.000 description 4
- ZDZOTLJHXYCWBA-VCVYQWHSSA-N N-debenzoyl-N-(tert-butoxycarbonyl)-10-deacetyltaxol Chemical compound O([C@H]1[C@H]2[C@@](C([C@H](O)C3=C(C)[C@@H](OC(=O)[C@H](O)[C@@H](NC(=O)OC(C)(C)C)C=4C=CC=CC=4)C[C@]1(O)C3(C)C)=O)(C)[C@@H](O)C[C@H]1OC[C@]12OC(=O)C)C(=O)C1=CC=CC=C1 ZDZOTLJHXYCWBA-VCVYQWHSSA-N 0.000 description 4
- 208000015914 Non-Hodgkin lymphomas Diseases 0.000 description 4
- 229930012538 Paclitaxel Natural products 0.000 description 4
- 230000005867 T cell response Effects 0.000 description 4
- 238000009825 accumulation Methods 0.000 description 4
- 239000002253 acid Substances 0.000 description 4
- 210000004556 brain Anatomy 0.000 description 4
- 231100000433 cytotoxic Toxicity 0.000 description 4
- 230000001472 cytotoxic effect Effects 0.000 description 4
- 229960002949 fluorouracil Drugs 0.000 description 4
- 230000001976 improved effect Effects 0.000 description 4
- 238000000338 in vitro Methods 0.000 description 4
- 230000000670 limiting effect Effects 0.000 description 4
- 201000007270 liver cancer Diseases 0.000 description 4
- 208000003747 lymphoid leukemia Diseases 0.000 description 4
- 210000002540 macrophage Anatomy 0.000 description 4
- 230000004048 modification Effects 0.000 description 4
- 238000012986 modification Methods 0.000 description 4
- 229960001592 paclitaxel Drugs 0.000 description 4
- 238000012545 processing Methods 0.000 description 4
- 239000000523 sample Substances 0.000 description 4
- 230000011664 signaling Effects 0.000 description 4
- UCSJYZPVAKXKNQ-HZYVHMACSA-N streptomycin Chemical compound CN[C@H]1[C@H](O)[C@@H](O)[C@H](CO)O[C@H]1O[C@@H]1[C@](C=O)(O)[C@H](C)O[C@H]1O[C@@H]1[C@@H](NC(N)=N)[C@H](O)[C@@H](NC(N)=N)[C@H](O)[C@H]1O UCSJYZPVAKXKNQ-HZYVHMACSA-N 0.000 description 4
- RCINICONZNJXQF-MZXODVADSA-N taxol Chemical compound O([C@@H]1[C@@]2(C[C@@H](C(C)=C(C2(C)C)[C@H](C([C@]2(C)[C@@H](O)C[C@H]3OC[C@]3([C@H]21)OC(C)=O)=O)OC(=O)C)OC(=O)[C@H](O)[C@@H](NC(=O)C=1C=CC=CC=1)C=1C=CC=CC=1)O)C(=O)C1=CC=CC=C1 RCINICONZNJXQF-MZXODVADSA-N 0.000 description 4
- 229960000575 trastuzumab Drugs 0.000 description 4
- STQGQHZAVUOBTE-UHFFFAOYSA-N 7-Cyan-hept-2t-en-4,6-diinsaeure Natural products C1=2C(O)=C3C(=O)C=4C(OC)=CC=CC=4C(=O)C3=C(O)C=2CC(O)(C(C)=O)CC1OC1CC(N)C(O)C(C)O1 STQGQHZAVUOBTE-UHFFFAOYSA-N 0.000 description 3
- QTBSBXVTEAMEQO-UHFFFAOYSA-N Acetic acid Chemical compound CC(O)=O QTBSBXVTEAMEQO-UHFFFAOYSA-N 0.000 description 3
- 102000019034 Chemokines Human genes 0.000 description 3
- 108010012236 Chemokines Proteins 0.000 description 3
- CMSMOCZEIVJLDB-UHFFFAOYSA-N Cyclophosphamide Chemical compound ClCCN(CCCl)P1(=O)NCCCO1 CMSMOCZEIVJLDB-UHFFFAOYSA-N 0.000 description 3
- UHDGCWIWMRVCDJ-CCXZUQQUSA-N Cytarabine Chemical compound O=C1N=C(N)C=CN1[C@H]1[C@@H](O)[C@H](O)[C@@H](CO)O1 UHDGCWIWMRVCDJ-CCXZUQQUSA-N 0.000 description 3
- 239000006144 Dulbecco’s modified Eagle's medium Substances 0.000 description 3
- 102100039620 Granulocyte-macrophage colony-stimulating factor Human genes 0.000 description 3
- 208000031886 HIV Infections Diseases 0.000 description 3
- 102000037982 Immune checkpoint proteins Human genes 0.000 description 3
- 108091008036 Immune checkpoint proteins Proteins 0.000 description 3
- 108060003951 Immunoglobulin Proteins 0.000 description 3
- 108010002386 Interleukin-3 Proteins 0.000 description 3
- 102100039064 Interleukin-3 Human genes 0.000 description 3
- FBOZXECLQNJBKD-ZDUSSCGKSA-N L-methotrexate Chemical compound C=1N=C2N=C(N)N=C(N)C2=NC=1CN(C)C1=CC=C(C(=O)N[C@@H](CCC(O)=O)C(O)=O)C=C1 FBOZXECLQNJBKD-ZDUSSCGKSA-N 0.000 description 3
- 208000003445 Mouth Neoplasms Diseases 0.000 description 3
- FOCVUCIESVLUNU-UHFFFAOYSA-N Thiotepa Chemical compound C1CN1P(N1CC1)(=S)N1CC1 FOCVUCIESVLUNU-UHFFFAOYSA-N 0.000 description 3
- 206010064390 Tumour invasion Diseases 0.000 description 3
- 239000002671 adjuvant Substances 0.000 description 3
- 230000001093 anti-cancer Effects 0.000 description 3
- 210000000612 antigen-presenting cell Anatomy 0.000 description 3
- 238000003556 assay Methods 0.000 description 3
- 229940037642 autologous vaccine Drugs 0.000 description 3
- 229960000397 bevacizumab Drugs 0.000 description 3
- 229960000074 biopharmaceutical Drugs 0.000 description 3
- 230000009400 cancer invasion Effects 0.000 description 3
- 208000002458 carcinoid tumor Diseases 0.000 description 3
- 230000001413 cellular effect Effects 0.000 description 3
- 238000006243 chemical reaction Methods 0.000 description 3
- 150000001875 compounds Chemical class 0.000 description 3
- STQGQHZAVUOBTE-VGBVRHCVSA-N daunorubicin Chemical compound O([C@H]1C[C@@](O)(CC=2C(O)=C3C(=O)C=4C=CC=C(C=4C(=O)C3=C(O)C=21)OC)C(C)=O)[C@H]1C[C@H](N)[C@H](O)[C@H](C)O1 STQGQHZAVUOBTE-VGBVRHCVSA-N 0.000 description 3
- 238000011161 development Methods 0.000 description 3
- 230000018109 developmental process Effects 0.000 description 3
- 230000004069 differentiation Effects 0.000 description 3
- 229960003668 docetaxel Drugs 0.000 description 3
- VJJPUSNTGOMMGY-MRVIYFEKSA-N etoposide Chemical compound COC1=C(O)C(OC)=CC([C@@H]2C3=CC=4OCOC=4C=C3[C@@H](O[C@H]3[C@@H]([C@@H](O)[C@@H]4O[C@H](C)OC[C@H]4O3)O)[C@@H]3[C@@H]2C(OC3)=O)=C1 VJJPUSNTGOMMGY-MRVIYFEKSA-N 0.000 description 3
- 229960005420 etoposide Drugs 0.000 description 3
- 235000012907 honey Nutrition 0.000 description 3
- 230000002267 hypothalamic effect Effects 0.000 description 3
- 102000018358 immunoglobulin Human genes 0.000 description 3
- 230000008595 infiltration Effects 0.000 description 3
- 238000001764 infiltration Methods 0.000 description 3
- 210000004153 islets of langerhan Anatomy 0.000 description 3
- 210000003734 kidney Anatomy 0.000 description 3
- 210000002751 lymph Anatomy 0.000 description 3
- 239000011159 matrix material Substances 0.000 description 3
- 239000002609 medium Substances 0.000 description 3
- GLVAUDGFNGKCSF-UHFFFAOYSA-N mercaptopurine Chemical compound S=C1NC=NC2=C1NC=N2 GLVAUDGFNGKCSF-UHFFFAOYSA-N 0.000 description 3
- 229960000485 methotrexate Drugs 0.000 description 3
- 229960004857 mitomycin Drugs 0.000 description 3
- 210000005087 mononuclear cell Anatomy 0.000 description 3
- 210000004498 neuroglial cell Anatomy 0.000 description 3
- 201000008968 osteosarcoma Diseases 0.000 description 3
- 230000001575 pathological effect Effects 0.000 description 3
- 229920001184 polypeptide Polymers 0.000 description 3
- 210000003289 regulatory T cell Anatomy 0.000 description 3
- 201000000849 skin cancer Diseases 0.000 description 3
- 239000000243 solution Substances 0.000 description 3
- 210000001541 thymus gland Anatomy 0.000 description 3
- 206010044412 transitional cell carcinoma Diseases 0.000 description 3
- 239000000107 tumor biomarker Substances 0.000 description 3
- 230000003827 upregulation Effects 0.000 description 3
- 210000000626 ureter Anatomy 0.000 description 3
- 210000000239 visual pathway Anatomy 0.000 description 3
- 230000004400 visual pathway Effects 0.000 description 3
- FPVKHBSQESCIEP-UHFFFAOYSA-N (8S)-3-(2-deoxy-beta-D-erythro-pentofuranosyl)-3,6,7,8-tetrahydroimidazo[4,5-d][1,3]diazepin-8-ol Natural products C1C(O)C(CO)OC1N1C(NC=NCC2O)=C2N=C1 FPVKHBSQESCIEP-UHFFFAOYSA-N 0.000 description 2
- WYWHKKSPHMUBEB-UHFFFAOYSA-N 6-Mercaptoguanine Natural products N1C(N)=NC(=S)C2=C1N=CN2 WYWHKKSPHMUBEB-UHFFFAOYSA-N 0.000 description 2
- 208000030507 AIDS Diseases 0.000 description 2
- 206010060971 Astrocytoma malignant Diseases 0.000 description 2
- 108010006654 Bleomycin Proteins 0.000 description 2
- 206010006143 Brain stem glioma Diseases 0.000 description 2
- 101150050673 CHK1 gene Proteins 0.000 description 2
- DLGOEMSEDOSKAD-UHFFFAOYSA-N Carmustine Chemical compound ClCCNC(=O)N(N=O)CCCl DLGOEMSEDOSKAD-UHFFFAOYSA-N 0.000 description 2
- 206010007953 Central nervous system lymphoma Diseases 0.000 description 2
- 108010071942 Colony-Stimulating Factors Proteins 0.000 description 2
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 description 2
- 208000021309 Germ cell tumor Diseases 0.000 description 2
- 108010017213 Granulocyte-Macrophage Colony-Stimulating Factor Proteins 0.000 description 2
- 241001326189 Gyrodactylus prostae Species 0.000 description 2
- 208000037357 HIV infectious disease Diseases 0.000 description 2
- 101000946889 Homo sapiens Monocyte differentiation antigen CD14 Proteins 0.000 description 2
- VSNHCAURESNICA-UHFFFAOYSA-N Hydroxyurea Chemical compound NC(=O)NO VSNHCAURESNICA-UHFFFAOYSA-N 0.000 description 2
- 102000037978 Immune checkpoint receptors Human genes 0.000 description 2
- 108091008028 Immune checkpoint receptors Proteins 0.000 description 2
- 206010062016 Immunosuppression Diseases 0.000 description 2
- 102000014150 Interferons Human genes 0.000 description 2
- 108010050904 Interferons Proteins 0.000 description 2
- 108050003558 Interleukin-17 Proteins 0.000 description 2
- 102000013691 Interleukin-17 Human genes 0.000 description 2
- 206010061252 Intraocular melanoma Diseases 0.000 description 2
- 208000031671 Large B-Cell Diffuse Lymphoma Diseases 0.000 description 2
- 206010023825 Laryngeal cancer Diseases 0.000 description 2
- KDXKERNSBIXSRK-UHFFFAOYSA-N Lysine Natural products NCCCCC(N)C(O)=O KDXKERNSBIXSRK-UHFFFAOYSA-N 0.000 description 2
- 239000004472 Lysine Substances 0.000 description 2
- 208000006644 Malignant Fibrous Histiocytoma Diseases 0.000 description 2
- 206010027480 Metastatic malignant melanoma Diseases 0.000 description 2
- 102100035877 Monocyte differentiation antigen CD14 Human genes 0.000 description 2
- 241000699660 Mus musculus Species 0.000 description 2
- 201000007224 Myeloproliferative neoplasm Diseases 0.000 description 2
- 208000010505 Nose Neoplasms Diseases 0.000 description 2
- 238000012879 PET imaging Methods 0.000 description 2
- 229930182555 Penicillin Natural products 0.000 description 2
- JGSARLDLIJGVTE-MBNYWOFBSA-N Penicillin G Chemical compound N([C@H]1[C@H]2SC([C@@H](N2C1=O)C(O)=O)(C)C)C(=O)CC1=CC=CC=C1 JGSARLDLIJGVTE-MBNYWOFBSA-N 0.000 description 2
- 201000000582 Retinoblastoma Diseases 0.000 description 2
- 239000006146 Roswell Park Memorial Institute medium Substances 0.000 description 2
- 208000004337 Salivary Gland Neoplasms Diseases 0.000 description 2
- 206010061934 Salivary gland cancer Diseases 0.000 description 2
- 208000000453 Skin Neoplasms Diseases 0.000 description 2
- 230000006044 T cell activation Effects 0.000 description 2
- 206010042971 T-cell lymphoma Diseases 0.000 description 2
- 208000027585 T-cell non-Hodgkin lymphoma Diseases 0.000 description 2
- NKANXQFJJICGDU-QPLCGJKRSA-N Tamoxifen Chemical compound C=1C=CC=CC=1C(/CC)=C(C=1C=CC(OCCN(C)C)=CC=1)/C1=CC=CC=C1 NKANXQFJJICGDU-QPLCGJKRSA-N 0.000 description 2
- 208000024770 Thyroid neoplasm Diseases 0.000 description 2
- 208000015778 Undifferentiated pleomorphic sarcoma Diseases 0.000 description 2
- 201000005969 Uveal melanoma Diseases 0.000 description 2
- 108091008605 VEGF receptors Proteins 0.000 description 2
- 102000009484 Vascular Endothelial Growth Factor Receptors Human genes 0.000 description 2
- JXLYSJRDGCGARV-WWYNWVTFSA-N Vinblastine Natural products O=C(O[C@H]1[C@](O)(C(=O)OC)[C@@H]2N(C)c3c(cc(c(OC)c3)[C@]3(C(=O)OC)c4[nH]c5c(c4CCN4C[C@](O)(CC)C[C@H](C3)C4)cccc5)[C@@]32[C@H]2[C@@]1(CC)C=CCN2CC3)C JXLYSJRDGCGARV-WWYNWVTFSA-N 0.000 description 2
- 208000036142 Viral infection Diseases 0.000 description 2
- 239000013543 active substance Substances 0.000 description 2
- 208000020990 adrenal cortex carcinoma Diseases 0.000 description 2
- 208000007128 adrenocortical carcinoma Diseases 0.000 description 2
- 239000000556 agonist Substances 0.000 description 2
- 229940100198 alkylating agent Drugs 0.000 description 2
- 239000002168 alkylating agent Substances 0.000 description 2
- 230000003110 anti-inflammatory effect Effects 0.000 description 2
- 230000005809 anti-tumor immunity Effects 0.000 description 2
- 238000009175 antibody therapy Methods 0.000 description 2
- 239000012298 atmosphere Substances 0.000 description 2
- VSRXQHXAPYXROS-UHFFFAOYSA-N azanide;cyclobutane-1,1-dicarboxylic acid;platinum(2+) Chemical compound [NH2-].[NH2-].[Pt+2].OC(=O)C1(C(O)=O)CCC1 VSRXQHXAPYXROS-UHFFFAOYSA-N 0.000 description 2
- 229960001561 bleomycin Drugs 0.000 description 2
- OYVAGSVQBOHSSS-UAPAGMARSA-O bleomycin A2 Chemical compound N([C@H](C(=O)N[C@H](C)[C@@H](O)[C@H](C)C(=O)N[C@@H]([C@H](O)C)C(=O)NCCC=1SC=C(N=1)C=1SC=C(N=1)C(=O)NCCC[S+](C)C)[C@@H](O[C@H]1[C@H]([C@@H](O)[C@H](O)[C@H](CO)O1)O[C@@H]1[C@H]([C@@H](OC(N)=O)[C@H](O)[C@@H](CO)O1)O)C=1N=CNC=1)C(=O)C1=NC([C@H](CC(N)=O)NC[C@H](N)C(N)=O)=NC(N)=C1C OYVAGSVQBOHSSS-UAPAGMARSA-O 0.000 description 2
- 210000005013 brain tissue Anatomy 0.000 description 2
- 238000009395 breeding Methods 0.000 description 2
- 230000001488 breeding effect Effects 0.000 description 2
- 238000004364 calculation method Methods 0.000 description 2
- 229960004562 carboplatin Drugs 0.000 description 2
- 229960005243 carmustine Drugs 0.000 description 2
- 239000006285 cell suspension Substances 0.000 description 2
- 210000003169 central nervous system Anatomy 0.000 description 2
- 201000007335 cerebellar astrocytoma Diseases 0.000 description 2
- 208000030239 cerebral astrocytoma Diseases 0.000 description 2
- 229960005395 cetuximab Drugs 0.000 description 2
- 229960004630 chlorambucil Drugs 0.000 description 2
- JCKYGMPEJWAADB-UHFFFAOYSA-N chlorambucil Chemical compound OC(=O)CCCC1=CC=C(N(CCCl)CCCl)C=C1 JCKYGMPEJWAADB-UHFFFAOYSA-N 0.000 description 2
- 208000006990 cholangiocarcinoma Diseases 0.000 description 2
- DQLATGHUWYMOKM-UHFFFAOYSA-L cisplatin Chemical compound N[Pt](N)(Cl)Cl DQLATGHUWYMOKM-UHFFFAOYSA-L 0.000 description 2
- 229960004316 cisplatin Drugs 0.000 description 2
- 239000000470 constituent Substances 0.000 description 2
- 229960004397 cyclophosphamide Drugs 0.000 description 2
- 229960000684 cytarabine Drugs 0.000 description 2
- 229960000975 daunorubicin Drugs 0.000 description 2
- 230000034994 death Effects 0.000 description 2
- 231100000517 death Toxicity 0.000 description 2
- 230000007850 degeneration Effects 0.000 description 2
- 230000003111 delayed effect Effects 0.000 description 2
- 230000001419 dependent effect Effects 0.000 description 2
- 206010012818 diffuse large B-cell lymphoma Diseases 0.000 description 2
- POULHZVOKOAJMA-UHFFFAOYSA-N dodecanoic acid Chemical compound CCCCCCCCCCCC(O)=O POULHZVOKOAJMA-UHFFFAOYSA-N 0.000 description 2
- 229960004679 doxorubicin Drugs 0.000 description 2
- 229950009791 durvalumab Drugs 0.000 description 2
- 230000007717 exclusion Effects 0.000 description 2
- OVBPIULPVIDEAO-LBPRGKRZSA-N folic acid Chemical compound C=1N=C2NC(N)=NC(=O)C2=NC=1CNC1=CC=C(C(=O)N[C@@H](CCC(O)=O)C(O)=O)C=C1 OVBPIULPVIDEAO-LBPRGKRZSA-N 0.000 description 2
- 230000004927 fusion Effects 0.000 description 2
- CHPZKNULDCNCBW-UHFFFAOYSA-N gallium nitrate Chemical compound [Ga+3].[O-][N+]([O-])=O.[O-][N+]([O-])=O.[O-][N+]([O-])=O CHPZKNULDCNCBW-UHFFFAOYSA-N 0.000 description 2
- 238000001415 gene therapy Methods 0.000 description 2
- 230000002068 genetic effect Effects 0.000 description 2
- 102000054766 genetic haplotypes Human genes 0.000 description 2
- 239000003102 growth factor Substances 0.000 description 2
- 210000002768 hair cell Anatomy 0.000 description 2
- 210000003958 hematopoietic stem cell Anatomy 0.000 description 2
- 208000029824 high grade glioma Diseases 0.000 description 2
- 208000033519 human immunodeficiency virus infectious disease Diseases 0.000 description 2
- 229960001330 hydroxycarbamide Drugs 0.000 description 2
- 206010020718 hyperplasia Diseases 0.000 description 2
- 229960001101 ifosfamide Drugs 0.000 description 2
- HOMGKSMUEGBAAB-UHFFFAOYSA-N ifosfamide Chemical compound ClCCNP1(=O)OCCCN1CCCl HOMGKSMUEGBAAB-UHFFFAOYSA-N 0.000 description 2
- 230000036737 immune function Effects 0.000 description 2
- 238000003364 immunohistochemistry Methods 0.000 description 2
- 230000001506 immunosuppresive effect Effects 0.000 description 2
- 239000003018 immunosuppressive agent Substances 0.000 description 2
- 238000002513 implantation Methods 0.000 description 2
- 238000001802 infusion Methods 0.000 description 2
- UWKQSNNFCGGAFS-XIFFEERXSA-N irinotecan Chemical compound C1=C2C(CC)=C3CN(C(C4=C([C@@](C(=O)OC4)(O)CC)C=4)=O)C=4C3=NC2=CC=C1OC(=O)N(CC1)CCC1N1CCCCC1 UWKQSNNFCGGAFS-XIFFEERXSA-N 0.000 description 2
- 206010023841 laryngeal neoplasm Diseases 0.000 description 2
- 230000002045 lasting effect Effects 0.000 description 2
- 208000012987 lip and oral cavity carcinoma Diseases 0.000 description 2
- 239000007788 liquid Substances 0.000 description 2
- 208000014018 liver neoplasm Diseases 0.000 description 2
- 230000007774 longterm Effects 0.000 description 2
- 238000002595 magnetic resonance imaging Methods 0.000 description 2
- 208000030883 malignant astrocytoma Diseases 0.000 description 2
- 201000011614 malignant glioma Diseases 0.000 description 2
- 230000035800 maturation Effects 0.000 description 2
- 230000001404 mediated effect Effects 0.000 description 2
- 229960001924 melphalan Drugs 0.000 description 2
- SGDBTWWWUNNDEQ-LBPRGKRZSA-N melphalan Chemical compound OC(=O)[C@@H](N)CC1=CC=C(N(CCCl)CCCl)C=C1 SGDBTWWWUNNDEQ-LBPRGKRZSA-N 0.000 description 2
- 229960001428 mercaptopurine Drugs 0.000 description 2
- 208000021039 metastatic melanoma Diseases 0.000 description 2
- 229960001156 mitoxantrone Drugs 0.000 description 2
- KKZJGLLVHKMTCM-UHFFFAOYSA-N mitoxantrone Chemical compound O=C1C2=C(O)C=CC(O)=C2C(=O)C2=C1C(NCCNCCO)=CC=C2NCCNCCO KKZJGLLVHKMTCM-UHFFFAOYSA-N 0.000 description 2
- 208000025113 myeloid leukemia Diseases 0.000 description 2
- 210000003643 myeloid progenitor cell Anatomy 0.000 description 2
- 208000037830 nasal cancer Diseases 0.000 description 2
- 229960001420 nimustine Drugs 0.000 description 2
- VFEDRRNHLBGPNN-UHFFFAOYSA-N nimustine Chemical compound CC1=NC=C(CNC(=O)N(CCCl)N=O)C(N)=N1 VFEDRRNHLBGPNN-UHFFFAOYSA-N 0.000 description 2
- 229910052757 nitrogen Inorganic materials 0.000 description 2
- 229960003301 nivolumab Drugs 0.000 description 2
- 102000039446 nucleic acids Human genes 0.000 description 2
- 108020004707 nucleic acids Proteins 0.000 description 2
- 150000007523 nucleic acids Chemical class 0.000 description 2
- 235000015097 nutrients Nutrition 0.000 description 2
- 201000002575 ocular melanoma Diseases 0.000 description 2
- 210000001328 optic nerve Anatomy 0.000 description 2
- 210000001672 ovary Anatomy 0.000 description 2
- 230000036961 partial effect Effects 0.000 description 2
- 229940049954 penicillin Drugs 0.000 description 2
- 229960002340 pentostatin Drugs 0.000 description 2
- FPVKHBSQESCIEP-JQCXWYLXSA-N pentostatin Chemical compound C1[C@H](O)[C@@H](CO)O[C@H]1N1C(N=CNC[C@H]2O)=C2N=C1 FPVKHBSQESCIEP-JQCXWYLXSA-N 0.000 description 2
- 238000009520 phase I clinical trial Methods 0.000 description 2
- 238000009521 phase II clinical trial Methods 0.000 description 2
- 208000010626 plasma cell neoplasm Diseases 0.000 description 2
- BASFCYQUMIYNBI-UHFFFAOYSA-N platinum Chemical compound [Pt] BASFCYQUMIYNBI-UHFFFAOYSA-N 0.000 description 2
- 208000016800 primary central nervous system lymphoma Diseases 0.000 description 2
- 230000002062 proliferating effect Effects 0.000 description 2
- RXWNCPJZOCPEPQ-NVWDDTSBSA-N puromycin Chemical compound C1=CC(OC)=CC=C1C[C@H](N)C(=O)N[C@H]1[C@@H](O)[C@H](N2C3=NC=NC(=C3N=C2)N(C)C)O[C@@H]1CO RXWNCPJZOCPEPQ-NVWDDTSBSA-N 0.000 description 2
- 238000001959 radiotherapy Methods 0.000 description 2
- 229960003876 ranibizumab Drugs 0.000 description 2
- 230000010076 replication Effects 0.000 description 2
- 238000002271 resection Methods 0.000 description 2
- 201000009410 rhabdomyosarcoma Diseases 0.000 description 2
- 229960004641 rituximab Drugs 0.000 description 2
- 238000009097 single-agent therapy Methods 0.000 description 2
- 208000037968 sinus cancer Diseases 0.000 description 2
- 210000004872 soft tissue Anatomy 0.000 description 2
- 241000894007 species Species 0.000 description 2
- 238000010186 staining Methods 0.000 description 2
- 230000004936 stimulating effect Effects 0.000 description 2
- 230000000638 stimulation Effects 0.000 description 2
- 229960005322 streptomycin Drugs 0.000 description 2
- 229960001052 streptozocin Drugs 0.000 description 2
- ZSJLQEPLLKMAKR-GKHCUFPYSA-N streptozocin Chemical compound O=NN(C)C(=O)N[C@H]1[C@@H](O)O[C@H](CO)[C@@H](O)[C@@H]1O ZSJLQEPLLKMAKR-GKHCUFPYSA-N 0.000 description 2
- 150000003467 sulfuric acid derivatives Chemical class 0.000 description 2
- 239000006228 supernatant Substances 0.000 description 2
- 238000001356 surgical procedure Methods 0.000 description 2
- 230000002459 sustained effect Effects 0.000 description 2
- 229940021747 therapeutic vaccine Drugs 0.000 description 2
- 229960001196 thiotepa Drugs 0.000 description 2
- 208000008732 thymoma Diseases 0.000 description 2
- 201000002510 thyroid cancer Diseases 0.000 description 2
- MNRILEROXIRVNJ-UHFFFAOYSA-N tioguanine Chemical compound N1C(N)=NC(=S)C2=NC=N[C]21 MNRILEROXIRVNJ-UHFFFAOYSA-N 0.000 description 2
- 229960003087 tioguanine Drugs 0.000 description 2
- 238000011830 transgenic mouse model Methods 0.000 description 2
- 229940124676 vascular endothelial growth factor receptor Drugs 0.000 description 2
- 230000002861 ventricular Effects 0.000 description 2
- 229960003048 vinblastine Drugs 0.000 description 2
- JXLYSJRDGCGARV-XQKSVPLYSA-N vincaleukoblastine Chemical compound C([C@@H](C[C@]1(C(=O)OC)C=2C(=CC3=C([C@]45[C@H]([C@@]([C@H](OC(C)=O)[C@]6(CC)C=CCN([C@H]56)CC4)(O)C(=O)OC)N3C)C=2)OC)C[C@@](C2)(O)CC)N2CCC2=C1NC1=CC=CC=C21 JXLYSJRDGCGARV-XQKSVPLYSA-N 0.000 description 2
- 229960004528 vincristine Drugs 0.000 description 2
- OGWKCGZFUXNPDA-XQKSVPLYSA-N vincristine Chemical compound C([N@]1C[C@@H](C[C@]2(C(=O)OC)C=3C(=CC4=C([C@]56[C@H]([C@@]([C@H](OC(C)=O)[C@]7(CC)C=CCN([C@H]67)CC5)(O)C(=O)OC)N4C=O)C=3)OC)C[C@@](C1)(O)CC)CC1=C2NC2=CC=CC=C12 OGWKCGZFUXNPDA-XQKSVPLYSA-N 0.000 description 2
- OGWKCGZFUXNPDA-UHFFFAOYSA-N vincristine Natural products C1C(CC)(O)CC(CC2(C(=O)OC)C=3C(=CC4=C(C56C(C(C(OC(C)=O)C7(CC)C=CCN(C67)CC5)(O)C(=O)OC)N4C=O)C=3)OC)CN1CCC1=C2NC2=CC=CC=C12 OGWKCGZFUXNPDA-UHFFFAOYSA-N 0.000 description 2
- 230000009385 viral infection Effects 0.000 description 2
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 2
- CGMTUJFWROPELF-YPAAEMCBSA-N (3E,5S)-5-[(2S)-butan-2-yl]-3-(1-hydroxyethylidene)pyrrolidine-2,4-dione Chemical compound CC[C@H](C)[C@@H]1NC(=O)\C(=C(/C)O)C1=O CGMTUJFWROPELF-YPAAEMCBSA-N 0.000 description 1
- FDKXTQMXEQVLRF-ZHACJKMWSA-N (E)-dacarbazine Chemical compound CN(C)\N=N\c1[nH]cnc1C(N)=O FDKXTQMXEQVLRF-ZHACJKMWSA-N 0.000 description 1
- LKJPYSCBVHEWIU-KRWDZBQOSA-N (R)-bicalutamide Chemical compound C([C@@](O)(C)C(=O)NC=1C=C(C(C#N)=CC=1)C(F)(F)F)S(=O)(=O)C1=CC=C(F)C=C1 LKJPYSCBVHEWIU-KRWDZBQOSA-N 0.000 description 1
- DSSYKIVIOFKYAU-XCBNKYQSSA-N (R)-camphor Chemical compound C1C[C@@]2(C)C(=O)C[C@@H]1C2(C)C DSSYKIVIOFKYAU-XCBNKYQSSA-N 0.000 description 1
- AGNGYMCLFWQVGX-AGFFZDDWSA-N (e)-1-[(2s)-2-amino-2-carboxyethoxy]-2-diazonioethenolate Chemical compound OC(=O)[C@@H](N)CO\C([O-])=C\[N+]#N AGNGYMCLFWQVGX-AGFFZDDWSA-N 0.000 description 1
- GZCWLCBFPRFLKL-UHFFFAOYSA-N 1-prop-2-ynoxypropan-2-ol Chemical compound CC(O)COCC#C GZCWLCBFPRFLKL-UHFFFAOYSA-N 0.000 description 1
- BTOTXLJHDSNXMW-POYBYMJQSA-N 2,3-dideoxyuridine Chemical compound O1[C@H](CO)CC[C@@H]1N1C(=O)NC(=O)C=C1 BTOTXLJHDSNXMW-POYBYMJQSA-N 0.000 description 1
- BOMZMNZEXMAQQW-UHFFFAOYSA-N 2,5,11-trimethyl-6h-pyrido[4,3-b]carbazol-2-ium-9-ol;acetate Chemical compound CC([O-])=O.C[N+]1=CC=C2C(C)=C(NC=3C4=CC(O)=CC=3)C4=C(C)C2=C1 BOMZMNZEXMAQQW-UHFFFAOYSA-N 0.000 description 1
- QCXJFISCRQIYID-IAEPZHFASA-N 2-amino-1-n-[(3s,6s,7r,10s,16s)-3-[(2s)-butan-2-yl]-7,11,14-trimethyl-2,5,9,12,15-pentaoxo-10-propan-2-yl-8-oxa-1,4,11,14-tetrazabicyclo[14.3.0]nonadecan-6-yl]-4,6-dimethyl-3-oxo-9-n-[(3s,6s,7r,10s,16s)-7,11,14-trimethyl-2,5,9,12,15-pentaoxo-3,10-di(propa Chemical compound C[C@H]1OC(=O)[C@H](C(C)C)N(C)C(=O)CN(C)C(=O)[C@@H]2CCCN2C(=O)[C@H](C(C)C)NC(=O)[C@H]1NC(=O)C1=C(N=C2C(C(=O)N[C@@H]3C(=O)N[C@H](C(N4CCC[C@H]4C(=O)N(C)CC(=O)N(C)[C@@H](C(C)C)C(=O)O[C@@H]3C)=O)[C@@H](C)CC)=C(N)C(=O)C(C)=C2O2)C2=C(C)C=C1 QCXJFISCRQIYID-IAEPZHFASA-N 0.000 description 1
- PWMYMKOUNYTVQN-UHFFFAOYSA-N 3-(8,8-diethyl-2-aza-8-germaspiro[4.5]decan-2-yl)-n,n-dimethylpropan-1-amine Chemical compound C1C[Ge](CC)(CC)CCC11CN(CCCN(C)C)CC1 PWMYMKOUNYTVQN-UHFFFAOYSA-N 0.000 description 1
- AOJJSUZBOXZQNB-VTZDEGQISA-N 4'-epidoxorubicin Chemical compound O([C@H]1C[C@@](O)(CC=2C(O)=C3C(=O)C=4C=CC=C(C=4C(=O)C3=C(O)C=21)OC)C(=O)CO)[C@H]1C[C@H](N)[C@@H](O)[C@H](C)O1 AOJJSUZBOXZQNB-VTZDEGQISA-N 0.000 description 1
- DODQJNMQWMSYGS-QPLCGJKRSA-N 4-[(z)-1-[4-[2-(dimethylamino)ethoxy]phenyl]-1-phenylbut-1-en-2-yl]phenol Chemical compound C=1C=C(O)C=CC=1C(/CC)=C(C=1C=CC(OCCN(C)C)=CC=1)/C1=CC=CC=C1 DODQJNMQWMSYGS-QPLCGJKRSA-N 0.000 description 1
- TVZGACDUOSZQKY-LBPRGKRZSA-N 4-aminofolic acid Chemical compound C1=NC2=NC(N)=NC(N)=C2N=C1CNC1=CC=C(C(=O)N[C@@H](CCC(O)=O)C(O)=O)C=C1 TVZGACDUOSZQKY-LBPRGKRZSA-N 0.000 description 1
- IDPUKCWIGUEADI-UHFFFAOYSA-N 5-[bis(2-chloroethyl)amino]uracil Chemical compound ClCCN(CCCl)C1=CNC(=O)NC1=O IDPUKCWIGUEADI-UHFFFAOYSA-N 0.000 description 1
- NMUSYJAQQFHJEW-KVTDHHQDSA-N 5-azacytidine Chemical compound O=C1N=C(N)N=CN1[C@H]1[C@H](O)[C@H](O)[C@@H](CO)O1 NMUSYJAQQFHJEW-KVTDHHQDSA-N 0.000 description 1
- WYXSYVWAUAUWLD-SHUUEZRQSA-N 6-azauridine Chemical compound O[C@@H]1[C@H](O)[C@@H](CO)O[C@H]1N1C(=O)NC(=O)C=N1 WYXSYVWAUAUWLD-SHUUEZRQSA-N 0.000 description 1
- 229960005538 6-diazo-5-oxo-L-norleucine Drugs 0.000 description 1
- YCWQAMGASJSUIP-YFKPBYRVSA-N 6-diazo-5-oxo-L-norleucine Chemical compound OC(=O)[C@@H](N)CCC(=O)C=[N+]=[N-] YCWQAMGASJSUIP-YFKPBYRVSA-N 0.000 description 1
- ZGXJTSGNIOSYLO-UHFFFAOYSA-N 88755TAZ87 Chemical compound NCC(=O)CCC(O)=O ZGXJTSGNIOSYLO-UHFFFAOYSA-N 0.000 description 1
- SHGAZHPCJJPHSC-ZVCIMWCZSA-N 9-cis-retinoic acid Chemical compound OC(=O)/C=C(\C)/C=C/C=C(/C)\C=C\C1=C(C)CCCC1(C)C SHGAZHPCJJPHSC-ZVCIMWCZSA-N 0.000 description 1
- HDZZVAMISRMYHH-UHFFFAOYSA-N 9beta-Ribofuranosyl-7-deazaadenin Natural products C1=CC=2C(N)=NC=NC=2N1C1OC(CO)C(O)C1O HDZZVAMISRMYHH-UHFFFAOYSA-N 0.000 description 1
- 208000002008 AIDS-Related Lymphoma Diseases 0.000 description 1
- 102000013563 Acid Phosphatase Human genes 0.000 description 1
- 108010051457 Acid Phosphatase Proteins 0.000 description 1
- CEIZFXOZIQNICU-UHFFFAOYSA-N Alternaria alternata Crofton-weed toxin Natural products CCC(C)C1NC(=O)C(C(C)=O)=C1O CEIZFXOZIQNICU-UHFFFAOYSA-N 0.000 description 1
- 206010061424 Anal cancer Diseases 0.000 description 1
- 208000007860 Anus Neoplasms Diseases 0.000 description 1
- 229940122815 Aromatase inhibitor Drugs 0.000 description 1
- CIWBSHSKHKDKBQ-JLAZNSOCSA-N Ascorbic acid Chemical compound OC[C@H](O)[C@H]1OC(=O)C(O)=C1O CIWBSHSKHKDKBQ-JLAZNSOCSA-N 0.000 description 1
- NOWKCMXCCJGMRR-UHFFFAOYSA-N Aziridine Chemical compound C1CN1 NOWKCMXCCJGMRR-UHFFFAOYSA-N 0.000 description 1
- 108091008875 B cell receptors Proteins 0.000 description 1
- 208000010839 B-cell chronic lymphocytic leukemia Diseases 0.000 description 1
- 108010074708 B7-H1 Antigen Proteins 0.000 description 1
- 102000008096 B7-H1 Antigen Human genes 0.000 description 1
- 229940125431 BRAF inhibitor Drugs 0.000 description 1
- 241000894006 Bacteria Species 0.000 description 1
- VGGGPCQERPFHOB-MCIONIFRSA-N Bestatin Chemical compound CC(C)C[C@H](C(O)=O)NC(=O)[C@@H](O)[C@H](N)CC1=CC=CC=C1 VGGGPCQERPFHOB-MCIONIFRSA-N 0.000 description 1
- 206010005949 Bone cancer Diseases 0.000 description 1
- 208000018084 Bone neoplasm Diseases 0.000 description 1
- 238000009010 Bradford assay Methods 0.000 description 1
- 102000010910 CD28 Antigens Human genes 0.000 description 1
- 108010062433 CD28 Antigens Proteins 0.000 description 1
- 239000012275 CTLA-4 inhibitor Substances 0.000 description 1
- KLWPJMFMVPTNCC-UHFFFAOYSA-N Camptothecin Natural products CCC1(O)C(=O)OCC2=C1C=C3C4Nc5ccccc5C=C4CN3C2=O KLWPJMFMVPTNCC-UHFFFAOYSA-N 0.000 description 1
- 241000282472 Canis lupus familiaris Species 0.000 description 1
- GAGWJHPBXLXJQN-UORFTKCHSA-N Capecitabine Chemical compound C1=C(F)C(NC(=O)OCCCCC)=NC(=O)N1[C@H]1[C@H](O)[C@H](O)[C@@H](C)O1 GAGWJHPBXLXJQN-UORFTKCHSA-N 0.000 description 1
- GAGWJHPBXLXJQN-UHFFFAOYSA-N Capecitabine Natural products C1=C(F)C(NC(=O)OCCCCC)=NC(=O)N1C1C(O)C(O)C(C)O1 GAGWJHPBXLXJQN-UHFFFAOYSA-N 0.000 description 1
- 208000005623 Carcinogenesis Diseases 0.000 description 1
- 206010007275 Carcinoid tumour Diseases 0.000 description 1
- 102000014914 Carrier Proteins Human genes 0.000 description 1
- 102000000844 Cell Surface Receptors Human genes 0.000 description 1
- 108010001857 Cell Surface Receptors Proteins 0.000 description 1
- 206010057248 Cell death Diseases 0.000 description 1
- JWBOIMRXGHLCPP-UHFFFAOYSA-N Chloditan Chemical compound C=1C=CC=C(Cl)C=1C(C(Cl)Cl)C1=CC=C(Cl)C=C1 JWBOIMRXGHLCPP-UHFFFAOYSA-N 0.000 description 1
- MKQWTWSXVILIKJ-LXGUWJNJSA-N Chlorozotocin Chemical compound OC[C@@H](O)[C@@H](O)[C@H](O)[C@H](C=O)NC(=O)N(N=O)CCCl MKQWTWSXVILIKJ-LXGUWJNJSA-N 0.000 description 1
- VYZAMTAEIAYCRO-UHFFFAOYSA-N Chromium Chemical compound [Cr] VYZAMTAEIAYCRO-UHFFFAOYSA-N 0.000 description 1
- 241000723346 Cinnamomum camphora Species 0.000 description 1
- 102000029816 Collagenase Human genes 0.000 description 1
- 108060005980 Collagenase Proteins 0.000 description 1
- 102000007644 Colony-Stimulating Factors Human genes 0.000 description 1
- 241000195493 Cryptophyta Species 0.000 description 1
- 239000012625 DNA intercalator Substances 0.000 description 1
- 229940123780 DNA topoisomerase I inhibitor Drugs 0.000 description 1
- 229940124087 DNA topoisomerase II inhibitor Drugs 0.000 description 1
- 108010092160 Dactinomycin Proteins 0.000 description 1
- ZBNZXTGUTAYRHI-UHFFFAOYSA-N Dasatinib Chemical compound C=1C(N2CCN(CCO)CC2)=NC(C)=NC=1NC(S1)=NC=C1C(=O)NC1=C(C)C=CC=C1Cl ZBNZXTGUTAYRHI-UHFFFAOYSA-N 0.000 description 1
- WEAHRLBPCANXCN-UHFFFAOYSA-N Daunomycin Natural products CCC1(O)CC(OC2CC(N)C(O)C(C)O2)c3cc4C(=O)c5c(OC)cccc5C(=O)c4c(O)c3C1 WEAHRLBPCANXCN-UHFFFAOYSA-N 0.000 description 1
- 102000016911 Deoxyribonucleases Human genes 0.000 description 1
- 108010053770 Deoxyribonucleases Proteins 0.000 description 1
- BWGNESOTFCXPMA-UHFFFAOYSA-N Dihydrogen disulfide Chemical compound SS BWGNESOTFCXPMA-UHFFFAOYSA-N 0.000 description 1
- 102000001301 EGF receptor Human genes 0.000 description 1
- 108060006698 EGF receptor Proteins 0.000 description 1
- SAMRUMKYXPVKPA-VFKOLLTISA-N Enocitabine Chemical compound O=C1N=C(NC(=O)CCCCCCCCCCCCCCCCCCCCC)C=CN1[C@H]1[C@@H](O)[C@H](O)[C@@H](CO)O1 SAMRUMKYXPVKPA-VFKOLLTISA-N 0.000 description 1
- 102000004190 Enzymes Human genes 0.000 description 1
- 108090000790 Enzymes Proteins 0.000 description 1
- HTIJFSOGRVMCQR-UHFFFAOYSA-N Epirubicin Natural products COc1cccc2C(=O)c3c(O)c4CC(O)(CC(OC5CC(N)C(=O)C(C)O5)c4c(O)c3C(=O)c12)C(=O)CO HTIJFSOGRVMCQR-UHFFFAOYSA-N 0.000 description 1
- OBMLHUPNRURLOK-XGRAFVIBSA-N Epitiostanol Chemical compound C1[C@@H]2S[C@@H]2C[C@]2(C)[C@H]3CC[C@](C)([C@H](CC4)O)[C@@H]4[C@@H]3CC[C@H]21 OBMLHUPNRURLOK-XGRAFVIBSA-N 0.000 description 1
- HKVAMNSJSFKALM-GKUWKFKPSA-N Everolimus Chemical compound C1C[C@@H](OCCO)[C@H](OC)C[C@@H]1C[C@@H](C)[C@H]1OC(=O)[C@@H]2CCCCN2C(=O)C(=O)[C@](O)(O2)[C@H](C)CC[C@H]2C[C@H](OC)/C(C)=C/C=C/C=C/[C@@H](C)C[C@@H](C)C(=O)[C@H](OC)[C@H](O)/C(C)=C/[C@@H](C)C(=O)C1 HKVAMNSJSFKALM-GKUWKFKPSA-N 0.000 description 1
- 208000012468 Ewing sarcoma/peripheral primitive neuroectodermal tumor Diseases 0.000 description 1
- 208000001382 Experimental Melanoma Diseases 0.000 description 1
- 241000282326 Felis catus Species 0.000 description 1
- 208000022072 Gallbladder Neoplasms Diseases 0.000 description 1
- 241000287828 Gallus gallus Species 0.000 description 1
- 201000003741 Gastrointestinal carcinoma Diseases 0.000 description 1
- WQZGKKKJIJFFOK-GASJEMHNSA-N Glucose Chemical compound OC[C@H]1OC(O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-GASJEMHNSA-N 0.000 description 1
- BLCLNMBMMGCOAS-URPVMXJPSA-N Goserelin Chemical compound C([C@@H](C(=O)N[C@H](COC(C)(C)C)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N1[C@@H](CCC1)C(=O)NNC(N)=O)NC(=O)[C@H](CO)NC(=O)[C@H](CC=1C2=CC=CC=C2NC=1)NC(=O)[C@H](CC=1NC=NC=1)NC(=O)[C@H]1NC(=O)CC1)C1=CC=C(O)C=C1 BLCLNMBMMGCOAS-URPVMXJPSA-N 0.000 description 1
- 108010069236 Goserelin Proteins 0.000 description 1
- 108010017080 Granulocyte Colony-Stimulating Factor Proteins 0.000 description 1
- 102000004269 Granulocyte Colony-Stimulating Factor Human genes 0.000 description 1
- 102000006354 HLA-DR Antigens Human genes 0.000 description 1
- 108010058597 HLA-DR Antigens Proteins 0.000 description 1
- 101001021491 Homo sapiens HERV-H LTR-associating protein 2 Proteins 0.000 description 1
- 101001002657 Homo sapiens Interleukin-2 Proteins 0.000 description 1
- 101001012157 Homo sapiens Receptor tyrosine-protein kinase erbB-2 Proteins 0.000 description 1
- 101000984753 Homo sapiens Serine/threonine-protein kinase B-raf Proteins 0.000 description 1
- 206010062904 Hormone-refractory prostate cancer Diseases 0.000 description 1
- 206010020649 Hyperkeratosis Diseases 0.000 description 1
- 102000001284 I-kappa-B kinase Human genes 0.000 description 1
- 108060006678 I-kappa-B kinase Proteins 0.000 description 1
- 102100034980 ICOS ligand Human genes 0.000 description 1
- 101710093458 ICOS ligand Proteins 0.000 description 1
- MPBVHIBUJCELCL-UHFFFAOYSA-N Ibandronate Chemical compound CCCCCN(C)CCC(O)(P(O)(O)=O)P(O)(O)=O MPBVHIBUJCELCL-UHFFFAOYSA-N 0.000 description 1
- 206010061598 Immunodeficiency Diseases 0.000 description 1
- 102000037984 Inhibitory immune checkpoint proteins Human genes 0.000 description 1
- 108091008026 Inhibitory immune checkpoint proteins Proteins 0.000 description 1
- 102100022297 Integrin alpha-X Human genes 0.000 description 1
- 108010002352 Interleukin-1 Proteins 0.000 description 1
- 102000000589 Interleukin-1 Human genes 0.000 description 1
- 102000003815 Interleukin-11 Human genes 0.000 description 1
- 108090000177 Interleukin-11 Proteins 0.000 description 1
- 108010017535 Interleukin-15 Receptors Proteins 0.000 description 1
- 102000004556 Interleukin-15 Receptors Human genes 0.000 description 1
- 108010038453 Interleukin-2 Receptors Proteins 0.000 description 1
- 102100030704 Interleukin-21 Human genes 0.000 description 1
- 102000017761 Interleukin-33 Human genes 0.000 description 1
- 108010067003 Interleukin-33 Proteins 0.000 description 1
- 108010002616 Interleukin-5 Proteins 0.000 description 1
- 102000000743 Interleukin-5 Human genes 0.000 description 1
- 108090001005 Interleukin-6 Proteins 0.000 description 1
- 102000004889 Interleukin-6 Human genes 0.000 description 1
- PIWKPBJCKXDKJR-UHFFFAOYSA-N Isoflurane Chemical compound FC(F)OC(Cl)C(F)(F)F PIWKPBJCKXDKJR-UHFFFAOYSA-N 0.000 description 1
- 208000007766 Kaposi sarcoma Diseases 0.000 description 1
- YQEZLKZALYSWHR-UHFFFAOYSA-N Ketamine Chemical compound C=1C=CC=C(Cl)C=1C1(NC)CCCCC1=O YQEZLKZALYSWHR-UHFFFAOYSA-N 0.000 description 1
- 208000008839 Kidney Neoplasms Diseases 0.000 description 1
- ONIBWKKTOPOVIA-BYPYZUCNSA-N L-Proline Chemical compound OC(=O)[C@@H]1CCCN1 ONIBWKKTOPOVIA-BYPYZUCNSA-N 0.000 description 1
- FEWJPZIEWOKRBE-JCYAYHJZSA-L L-tartrate(2-) Chemical compound [O-]C(=O)[C@H](O)[C@@H](O)C([O-])=O FEWJPZIEWOKRBE-JCYAYHJZSA-L 0.000 description 1
- 239000005517 L01XE01 - Imatinib Substances 0.000 description 1
- 239000005411 L01XE02 - Gefitinib Substances 0.000 description 1
- 239000005551 L01XE03 - Erlotinib Substances 0.000 description 1
- 239000002147 L01XE04 - Sunitinib Substances 0.000 description 1
- 239000002067 L01XE06 - Dasatinib Substances 0.000 description 1
- 239000002136 L01XE07 - Lapatinib Substances 0.000 description 1
- 239000002118 L01XE12 - Vandetanib Substances 0.000 description 1
- 239000002144 L01XE18 - Ruxolitinib Substances 0.000 description 1
- 101150030213 Lag3 gene Proteins 0.000 description 1
- 239000005639 Lauric acid Substances 0.000 description 1
- 229920001491 Lentinan Polymers 0.000 description 1
- 108010000817 Leuprolide Proteins 0.000 description 1
- 206010061523 Lip and/or oral cavity cancer Diseases 0.000 description 1
- GQYIWUVLTXOXAJ-UHFFFAOYSA-N Lomustine Chemical compound ClCCN(N=O)C(=O)NC1CCCCC1 GQYIWUVLTXOXAJ-UHFFFAOYSA-N 0.000 description 1
- 208000031422 Lymphocytic Chronic B-Cell Leukemia Diseases 0.000 description 1
- 206010025312 Lymphoma AIDS related Diseases 0.000 description 1
- 208000004059 Male Breast Neoplasms Diseases 0.000 description 1
- 206010025557 Malignant fibrous histiocytoma of bone Diseases 0.000 description 1
- 208000032271 Malignant tumor of penis Diseases 0.000 description 1
- 241000124008 Mammalia Species 0.000 description 1
- 102000018697 Membrane Proteins Human genes 0.000 description 1
- 108010052285 Membrane Proteins Proteins 0.000 description 1
- 206010027201 Meningitis aseptic Diseases 0.000 description 1
- 206010027406 Mesothelioma Diseases 0.000 description 1
- 206010063569 Metastatic squamous cell carcinoma Diseases 0.000 description 1
- 229930192392 Mitomycin Natural products 0.000 description 1
- 208000014767 Myeloproliferative disease Diseases 0.000 description 1
- OVBPIULPVIDEAO-UHFFFAOYSA-N N-Pteroyl-L-glutaminsaeure Natural products C=1N=C2NC(N)=NC(=O)C2=NC=1CNC1=CC=C(C(=O)NC(CCC(O)=O)C(O)=O)C=C1 OVBPIULPVIDEAO-UHFFFAOYSA-N 0.000 description 1
- 206010028851 Necrosis Diseases 0.000 description 1
- 206010029260 Neuroblastoma Diseases 0.000 description 1
- 201000004404 Neurofibroma Diseases 0.000 description 1
- SYNHCENRCUAUNM-UHFFFAOYSA-N Nitrogen mustard N-oxide hydrochloride Chemical compound Cl.ClCC[N+]([O-])(C)CCCl SYNHCENRCUAUNM-UHFFFAOYSA-N 0.000 description 1
- 206010033268 Ovarian low malignant potential tumour Diseases 0.000 description 1
- 239000012270 PD-1 inhibitor Substances 0.000 description 1
- 239000012668 PD-1-inhibitor Substances 0.000 description 1
- 239000012271 PD-L1 inhibitor Substances 0.000 description 1
- SHGAZHPCJJPHSC-UHFFFAOYSA-N Panrexin Chemical compound OC(=O)C=C(C)C=CC=C(C)C=CC1=C(C)CCCC1(C)C SHGAZHPCJJPHSC-UHFFFAOYSA-N 0.000 description 1
- 206010033701 Papillary thyroid cancer Diseases 0.000 description 1
- 208000002471 Penile Neoplasms Diseases 0.000 description 1
- 206010034299 Penile cancer Diseases 0.000 description 1
- 108091000080 Phosphotransferase Proteins 0.000 description 1
- 208000007913 Pituitary Neoplasms Diseases 0.000 description 1
- 241000206607 Porphyra umbilicalis Species 0.000 description 1
- 241000288906 Primates Species 0.000 description 1
- 102000043850 Programmed Cell Death 1 Ligand 2 Human genes 0.000 description 1
- 101710094000 Programmed cell death 1 ligand 1 Proteins 0.000 description 1
- ONIBWKKTOPOVIA-UHFFFAOYSA-N Proline Natural products OC(=O)C1CCCN1 ONIBWKKTOPOVIA-UHFFFAOYSA-N 0.000 description 1
- XBDQKXXYIPTUBI-UHFFFAOYSA-M Propionate Chemical compound CCC([O-])=O XBDQKXXYIPTUBI-UHFFFAOYSA-M 0.000 description 1
- 229940079156 Proteasome inhibitor Drugs 0.000 description 1
- 201000008183 Pulmonary blastoma Diseases 0.000 description 1
- 206010037596 Pyelonephritis Diseases 0.000 description 1
- 108091030071 RNAI Proteins 0.000 description 1
- AHHFEZNOXOZZQA-ZEBDFXRSSA-N Ranimustine Chemical compound CO[C@H]1O[C@H](CNC(=O)N(CCCl)N=O)[C@@H](O)[C@H](O)[C@H]1O AHHFEZNOXOZZQA-ZEBDFXRSSA-N 0.000 description 1
- 241000700159 Rattus Species 0.000 description 1
- 102100030086 Receptor tyrosine-protein kinase erbB-2 Human genes 0.000 description 1
- 208000015634 Rectal Neoplasms Diseases 0.000 description 1
- 206010038389 Renal cancer Diseases 0.000 description 1
- 241001338644 Retinia Species 0.000 description 1
- 102100027103 Serine/threonine-protein kinase B-raf Human genes 0.000 description 1
- 208000009359 Sezary Syndrome Diseases 0.000 description 1
- 208000021388 Sezary disease Diseases 0.000 description 1
- 230000024932 T cell mediated immunity Effects 0.000 description 1
- 230000006052 T cell proliferation Effects 0.000 description 1
- 108091008874 T cell receptors Proteins 0.000 description 1
- 102000016266 T-Cell Antigen Receptors Human genes 0.000 description 1
- NAVMQTYZDKMPEU-UHFFFAOYSA-N Targretin Chemical compound CC1=CC(C(CCC2(C)C)(C)C)=C2C=C1C(=C)C1=CC=C(C(O)=O)C=C1 NAVMQTYZDKMPEU-UHFFFAOYSA-N 0.000 description 1
- CGMTUJFWROPELF-UHFFFAOYSA-N Tenuazonic acid Natural products CCC(C)C1NC(=O)C(=C(C)/O)C1=O CGMTUJFWROPELF-UHFFFAOYSA-N 0.000 description 1
- 208000024313 Testicular Neoplasms Diseases 0.000 description 1
- 206010057644 Testis cancer Diseases 0.000 description 1
- 210000000447 Th1 cell Anatomy 0.000 description 1
- 201000009365 Thymic carcinoma Diseases 0.000 description 1
- 239000000365 Topoisomerase I Inhibitor Substances 0.000 description 1
- 239000000317 Topoisomerase II Inhibitor Substances 0.000 description 1
- UMILHIMHKXVDGH-UHFFFAOYSA-N Triethylene glycol diglycidyl ether Chemical compound C1OC1COCCOCCOCCOCC1CO1 UMILHIMHKXVDGH-UHFFFAOYSA-N 0.000 description 1
- FYAMXEPQQLNQDM-UHFFFAOYSA-N Tris(1-aziridinyl)phosphine oxide Chemical compound C1CN1P(N1CC1)(=O)N1CC1 FYAMXEPQQLNQDM-UHFFFAOYSA-N 0.000 description 1
- 229920004890 Triton X-100 Polymers 0.000 description 1
- 239000013504 Triton X-100 Substances 0.000 description 1
- GLNADSQYFUSGOU-GPTZEZBUSA-J Trypan blue Chemical compound [Na+].[Na+].[Na+].[Na+].C1=C(S([O-])(=O)=O)C=C2C=C(S([O-])(=O)=O)C(/N=N/C3=CC=C(C=C3C)C=3C=C(C(=CC=3)\N=N\C=3C(=CC4=CC(=CC(N)=C4C=3O)S([O-])(=O)=O)S([O-])(=O)=O)C)=C(O)C2=C1N GLNADSQYFUSGOU-GPTZEZBUSA-J 0.000 description 1
- 108060008682 Tumor Necrosis Factor Proteins 0.000 description 1
- 102000000852 Tumor Necrosis Factor-alpha Human genes 0.000 description 1
- 206010046431 Urethral cancer Diseases 0.000 description 1
- 206010046458 Urethral neoplasms Diseases 0.000 description 1
- 206010047741 Vulval cancer Diseases 0.000 description 1
- 208000004354 Vulvar Neoplasms Diseases 0.000 description 1
- 208000033559 Waldenström macroglobulinemia Diseases 0.000 description 1
- HCHKCACWOHOZIP-UHFFFAOYSA-N Zinc Chemical compound [Zn] HCHKCACWOHOZIP-UHFFFAOYSA-N 0.000 description 1
- IHGLINDYFMDHJG-UHFFFAOYSA-N [2-(4-methoxyphenyl)-3,4-dihydronaphthalen-1-yl]-[4-(2-pyrrolidin-1-ylethoxy)phenyl]methanone Chemical compound C1=CC(OC)=CC=C1C(CCC1=CC=CC=C11)=C1C(=O)C(C=C1)=CC=C1OCCN1CCCC1 IHGLINDYFMDHJG-UHFFFAOYSA-N 0.000 description 1
- 238000010521 absorption reaction Methods 0.000 description 1
- 150000007513 acids Chemical class 0.000 description 1
- 229930183665 actinomycin Natural products 0.000 description 1
- 230000003213 activating effect Effects 0.000 description 1
- 230000003044 adaptive effect Effects 0.000 description 1
- 230000004721 adaptive immunity Effects 0.000 description 1
- 238000011374 additional therapy Methods 0.000 description 1
- 210000004404 adrenal cortex Anatomy 0.000 description 1
- 208000037844 advanced solid tumor Diseases 0.000 description 1
- 229960000548 alemtuzumab Drugs 0.000 description 1
- 229960001445 alitretinoin Drugs 0.000 description 1
- 230000002009 allergenic effect Effects 0.000 description 1
- 125000003275 alpha amino acid group Chemical group 0.000 description 1
- 150000001413 amino acids Chemical group 0.000 description 1
- 229960003437 aminoglutethimide Drugs 0.000 description 1
- ROBVIMPUHSLWNV-UHFFFAOYSA-N aminoglutethimide Chemical compound C=1C=C(N)C=CC=1C1(CC)CCC(=O)NC1=O ROBVIMPUHSLWNV-UHFFFAOYSA-N 0.000 description 1
- 229960002749 aminolevulinic acid Drugs 0.000 description 1
- 229960003896 aminopterin Drugs 0.000 description 1
- 229960001220 amsacrine Drugs 0.000 description 1
- XCPGHVQEEXUHNC-UHFFFAOYSA-N amsacrine Chemical compound COC1=CC(NS(C)(=O)=O)=CC=C1NC1=C(C=CC=C2)C2=NC2=CC=CC=C12 XCPGHVQEEXUHNC-UHFFFAOYSA-N 0.000 description 1
- 238000004458 analytical method Methods 0.000 description 1
- 239000003098 androgen Substances 0.000 description 1
- 229940030486 androgens Drugs 0.000 description 1
- 239000003242 anti bacterial agent Substances 0.000 description 1
- 230000002280 anti-androgenic effect Effects 0.000 description 1
- 230000003388 anti-hormonal effect Effects 0.000 description 1
- 230000000340 anti-metabolite Effects 0.000 description 1
- 230000000692 anti-sense effect Effects 0.000 description 1
- 239000000051 antiandrogen Substances 0.000 description 1
- 229940030495 antiandrogen sex hormone and modulator of the genital system Drugs 0.000 description 1
- 229940088710 antibiotic agent Drugs 0.000 description 1
- 229940124691 antibody therapeutics Drugs 0.000 description 1
- 230000010056 antibody-dependent cellular cytotoxicity Effects 0.000 description 1
- 229940100197 antimetabolite Drugs 0.000 description 1
- 239000002256 antimetabolite Substances 0.000 description 1
- 229940045687 antimetabolites folic acid analogs Drugs 0.000 description 1
- 229940045988 antineoplastic drug protein kinase inhibitors Drugs 0.000 description 1
- 201000011165 anus cancer Diseases 0.000 description 1
- 238000003782 apoptosis assay Methods 0.000 description 1
- 230000006907 apoptotic process Effects 0.000 description 1
- 150000008209 arabinosides Chemical class 0.000 description 1
- SCJNCDSAIRBRIA-DOFZRALJSA-N arachidonyl-2'-chloroethylamide Chemical compound CCCCC\C=C/C\C=C/C\C=C/C\C=C/CCCC(=O)NCCCl SCJNCDSAIRBRIA-DOFZRALJSA-N 0.000 description 1
- 239000003886 aromatase inhibitor Substances 0.000 description 1
- 230000010455 autoregulation Effects 0.000 description 1
- 229940120638 avastin Drugs 0.000 description 1
- 229960002756 azacitidine Drugs 0.000 description 1
- 229950011321 azaserine Drugs 0.000 description 1
- 150000001541 aziridines Chemical class 0.000 description 1
- 230000001580 bacterial effect Effects 0.000 description 1
- 229960001212 bacterial vaccine Drugs 0.000 description 1
- 229960002938 bexarotene Drugs 0.000 description 1
- 229960000997 bicalutamide Drugs 0.000 description 1
- 108091008324 binding proteins Proteins 0.000 description 1
- 239000003139 biocide Substances 0.000 description 1
- 239000012620 biological material Substances 0.000 description 1
- 230000031018 biological processes and functions Effects 0.000 description 1
- 230000015572 biosynthetic process Effects 0.000 description 1
- 238000010352 biotechnological method Methods 0.000 description 1
- 230000017531 blood circulation Effects 0.000 description 1
- 239000012503 blood component Substances 0.000 description 1
- 210000004204 blood vessel Anatomy 0.000 description 1
- 210000000988 bone and bone Anatomy 0.000 description 1
- 208000012172 borderline epithelial tumor of ovary Diseases 0.000 description 1
- 229960001467 bortezomib Drugs 0.000 description 1
- GXJABQQUPOEUTA-RDJZCZTQSA-N bortezomib Chemical compound C([C@@H](C(=O)N[C@@H](CC(C)C)B(O)O)NC(=O)C=1N=CC=NC=1)C1=CC=CC=C1 GXJABQQUPOEUTA-RDJZCZTQSA-N 0.000 description 1
- 210000000133 brain stem Anatomy 0.000 description 1
- 201000002143 bronchus adenoma Diseases 0.000 description 1
- 239000000872 buffer Substances 0.000 description 1
- 239000006172 buffering agent Substances 0.000 description 1
- 108700002839 cactinomycin Proteins 0.000 description 1
- 229950009908 cactinomycin Drugs 0.000 description 1
- 229930195731 calicheamicin Natural products 0.000 description 1
- HXCHCVDVKSCDHU-LULTVBGHSA-N calicheamicin Chemical compound C1[C@H](OC)[C@@H](NCC)CO[C@H]1O[C@H]1[C@H](O[C@@H]2C\3=C(NC(=O)OC)C(=O)C[C@](C/3=C/CSSSC)(O)C#C\C=C/C#C2)O[C@H](C)[C@@H](NO[C@@H]2O[C@H](C)[C@@H](SC(=O)C=3C(=C(OC)C(O[C@H]4[C@@H]([C@H](OC)[C@@H](O)[C@H](C)O4)O)=C(I)C=3C)OC)[C@@H](O)C2)[C@@H]1O HXCHCVDVKSCDHU-LULTVBGHSA-N 0.000 description 1
- 229960000846 camphor Drugs 0.000 description 1
- 229930008380 camphor Natural products 0.000 description 1
- 229940127093 camptothecin Drugs 0.000 description 1
- VSJKWCGYPAHWDS-FQEVSTJZSA-N camptothecin Chemical compound C1=CC=C2C=C(CN3C4=CC5=C(C3=O)COC(=O)[C@]5(O)CC)C4=NC2=C1 VSJKWCGYPAHWDS-FQEVSTJZSA-N 0.000 description 1
- 230000036952 cancer formation Effects 0.000 description 1
- 230000005907 cancer growth Effects 0.000 description 1
- 239000012830 cancer therapeutic Substances 0.000 description 1
- 229960004117 capecitabine Drugs 0.000 description 1
- 229910002091 carbon monoxide Inorganic materials 0.000 description 1
- 231100000504 carcinogenesis Toxicity 0.000 description 1
- 108010047060 carzinophilin Proteins 0.000 description 1
- 230000020411 cell activation Effects 0.000 description 1
- 230000022534 cell killing Effects 0.000 description 1
- 230000004663 cell proliferation Effects 0.000 description 1
- 238000001516 cell proliferation assay Methods 0.000 description 1
- 230000008614 cellular interaction Effects 0.000 description 1
- 230000036755 cellular response Effects 0.000 description 1
- 210000001638 cerebellum Anatomy 0.000 description 1
- 230000002490 cerebral effect Effects 0.000 description 1
- 235000013330 chicken meat Nutrition 0.000 description 1
- 208000011654 childhood malignant neoplasm Diseases 0.000 description 1
- 229960001480 chlorozotocin Drugs 0.000 description 1
- 229910052804 chromium Inorganic materials 0.000 description 1
- 239000011651 chromium Substances 0.000 description 1
- ZYVSOIYQKUDENJ-WKSBCEQHSA-N chromomycin A3 Chemical compound O([C@@H]1C[C@@H](O[C@H](C)[C@@H]1OC(C)=O)OC=1C=C2C=C3C[C@H]([C@@H](C(=O)C3=C(O)C2=C(O)C=1C)O[C@@H]1O[C@H](C)[C@@H](O)[C@H](O[C@@H]2O[C@H](C)[C@@H](O)[C@H](O[C@@H]3O[C@@H](C)[C@H](OC(C)=O)[C@@](C)(O)C3)C2)C1)[C@H](OC)C(=O)[C@@H](O)[C@@H](C)O)[C@@H]1C[C@@H](O)[C@@H](OC)[C@@H](C)O1 ZYVSOIYQKUDENJ-WKSBCEQHSA-N 0.000 description 1
- 208000032852 chronic lymphocytic leukemia Diseases 0.000 description 1
- 239000004927 clay Substances 0.000 description 1
- 229960002424 collagenase Drugs 0.000 description 1
- 229940047120 colony stimulating factors Drugs 0.000 description 1
- 230000004154 complement system Effects 0.000 description 1
- 239000002872 contrast media Substances 0.000 description 1
- 230000001276 controlling effect Effects 0.000 description 1
- 238000011254 conventional chemotherapy Methods 0.000 description 1
- 238000000315 cryotherapy Methods 0.000 description 1
- 230000009089 cytolysis Effects 0.000 description 1
- 230000003013 cytotoxicity Effects 0.000 description 1
- 231100000135 cytotoxicity Toxicity 0.000 description 1
- 238000002784 cytotoxicity assay Methods 0.000 description 1
- 231100000263 cytotoxicity test Toxicity 0.000 description 1
- 229960002806 daclizumab Drugs 0.000 description 1
- 229960002448 dasatinib Drugs 0.000 description 1
- 230000003247 decreasing effect Effects 0.000 description 1
- 230000007812 deficiency Effects 0.000 description 1
- 230000004041 dendritic cell maturation Effects 0.000 description 1
- 229960001251 denosumab Drugs 0.000 description 1
- WVYXNIXAMZOZFK-UHFFFAOYSA-N diaziquone Chemical compound O=C1C(NC(=O)OCC)=C(N2CC2)C(=O)C(NC(=O)OCC)=C1N1CC1 WVYXNIXAMZOZFK-UHFFFAOYSA-N 0.000 description 1
- 229950002389 diaziquone Drugs 0.000 description 1
- 125000001028 difluoromethyl group Chemical group [H]C(F)(F)* 0.000 description 1
- 239000003085 diluting agent Substances 0.000 description 1
- LOKCTEFSRHRXRJ-UHFFFAOYSA-I dipotassium trisodium dihydrogen phosphate hydrogen phosphate dichloride Chemical compound P(=O)(O)(O)[O-].[K+].P(=O)(O)([O-])[O-].[Na+].[Na+].[Cl-].[K+].[Cl-].[Na+] LOKCTEFSRHRXRJ-UHFFFAOYSA-I 0.000 description 1
- 238000004090 dissolution Methods 0.000 description 1
- VSJKWCGYPAHWDS-UHFFFAOYSA-N dl-camptothecin Natural products C1=CC=C2C=C(CN3C4=CC5=C(C3=O)COC(=O)C5(O)CC)C4=NC2=C1 VSJKWCGYPAHWDS-UHFFFAOYSA-N 0.000 description 1
- 239000003534 dna topoisomerase inhibitor Substances 0.000 description 1
- AOARVGJNIOXRPG-UHFFFAOYSA-L dodecyl-[3-[dodecyl(dimethyl)azaniumyl]-2-hydroxypropyl]-dimethylazanium;dibromide Chemical compound [Br-].[Br-].CCCCCCCCCCCC[N+](C)(C)CC(O)C[N+](C)(C)CCCCCCCCCCCC AOARVGJNIOXRPG-UHFFFAOYSA-L 0.000 description 1
- ZWAOHEXOSAUJHY-ZIYNGMLESA-N doxifluridine Chemical compound O[C@@H]1[C@H](O)[C@@H](C)O[C@H]1N1C(=O)NC(=O)C(F)=C1 ZWAOHEXOSAUJHY-ZIYNGMLESA-N 0.000 description 1
- 229950005454 doxifluridine Drugs 0.000 description 1
- 238000001647 drug administration Methods 0.000 description 1
- 239000003937 drug carrier Substances 0.000 description 1
- 238000007876 drug discovery Methods 0.000 description 1
- 230000004064 dysfunction Effects 0.000 description 1
- 238000002592 echocardiography Methods 0.000 description 1
- 239000012636 effector Substances 0.000 description 1
- 230000008030 elimination Effects 0.000 description 1
- 238000003379 elimination reaction Methods 0.000 description 1
- 229950000549 elliptinium acetate Drugs 0.000 description 1
- 210000002889 endothelial cell Anatomy 0.000 description 1
- 238000005516 engineering process Methods 0.000 description 1
- 229950011487 enocitabine Drugs 0.000 description 1
- 229940088598 enzyme Drugs 0.000 description 1
- 210000002615 epidermis Anatomy 0.000 description 1
- 229960001904 epirubicin Drugs 0.000 description 1
- 210000000981 epithelium Anatomy 0.000 description 1
- 229950002973 epitiostanol Drugs 0.000 description 1
- 229960001433 erlotinib Drugs 0.000 description 1
- AAKJLRGGTJKAMG-UHFFFAOYSA-N erlotinib Chemical compound C=12C=C(OCCOC)C(OCCOC)=CC2=NC=NC=1NC1=CC=CC(C#C)=C1 AAKJLRGGTJKAMG-UHFFFAOYSA-N 0.000 description 1
- 229950002017 esorubicin Drugs 0.000 description 1
- ITSGNOIFAJAQHJ-BMFNZSJVSA-N esorubicin Chemical compound O([C@H]1C[C@@](O)(CC=2C(O)=C3C(=O)C=4C=CC=C(C=4C(=O)C3=C(O)C=21)OC)C(=O)CO)[C@H]1C[C@H](N)C[C@H](C)O1 ITSGNOIFAJAQHJ-BMFNZSJVSA-N 0.000 description 1
- 150000002148 esters Chemical class 0.000 description 1
- 229960001842 estramustine Drugs 0.000 description 1
- FRPJXPJMRWBBIH-RBRWEJTLSA-N estramustine Chemical compound ClCCN(CCCl)C(=O)OC1=CC=C2[C@H]3CC[C@](C)([C@H](CC4)O)[C@@H]4[C@@H]3CCC2=C1 FRPJXPJMRWBBIH-RBRWEJTLSA-N 0.000 description 1
- 229960005237 etoglucid Drugs 0.000 description 1
- 238000011156 evaluation Methods 0.000 description 1
- 229960005167 everolimus Drugs 0.000 description 1
- 210000004700 fetal blood Anatomy 0.000 description 1
- 239000000945 filler Substances 0.000 description 1
- 239000000834 fixative Substances 0.000 description 1
- 238000000684 flow cytometry Methods 0.000 description 1
- 229960000390 fludarabine Drugs 0.000 description 1
- GIUYCYHIANZCFB-FJFJXFQQSA-N fludarabine phosphate Chemical compound C1=NC=2C(N)=NC(F)=NC=2N1[C@@H]1O[C@H](COP(O)(O)=O)[C@@H](O)[C@@H]1O GIUYCYHIANZCFB-FJFJXFQQSA-N 0.000 description 1
- 238000001943 fluorescence-activated cell sorting Methods 0.000 description 1
- 239000007850 fluorescent dye Substances 0.000 description 1
- 229960002074 flutamide Drugs 0.000 description 1
- MKXKFYHWDHIYRV-UHFFFAOYSA-N flutamide Chemical compound CC(C)C(=O)NC1=CC=C([N+]([O-])=O)C(C(F)(F)F)=C1 MKXKFYHWDHIYRV-UHFFFAOYSA-N 0.000 description 1
- 229960000304 folic acid Drugs 0.000 description 1
- 235000019152 folic acid Nutrition 0.000 description 1
- 239000011724 folic acid Substances 0.000 description 1
- 150000002224 folic acids Chemical class 0.000 description 1
- 239000012737 fresh medium Substances 0.000 description 1
- 201000010175 gallbladder cancer Diseases 0.000 description 1
- 229940044658 gallium nitrate Drugs 0.000 description 1
- 230000002496 gastric effect Effects 0.000 description 1
- 208000017211 gastric neuroendocrine tumor G1 Diseases 0.000 description 1
- 229960002584 gefitinib Drugs 0.000 description 1
- XGALLCVXEZPNRQ-UHFFFAOYSA-N gefitinib Chemical compound C=12C=C(OCCCN3CCOCC3)C(OC)=CC2=NC=NC=1NC1=CC=C(F)C(Cl)=C1 XGALLCVXEZPNRQ-UHFFFAOYSA-N 0.000 description 1
- 229960005277 gemcitabine Drugs 0.000 description 1
- SDUQYLNIPVEERB-QPPQHZFASA-N gemcitabine Chemical compound O=C1N=C(N)C=CN1[C@H]1C(F)(F)[C@H](O)[C@@H](CO)O1 SDUQYLNIPVEERB-QPPQHZFASA-N 0.000 description 1
- 229960000578 gemtuzumab Drugs 0.000 description 1
- 230000009368 gene silencing by RNA Effects 0.000 description 1
- 210000004907 gland Anatomy 0.000 description 1
- 239000011521 glass Substances 0.000 description 1
- 229960002913 goserelin Drugs 0.000 description 1
- 239000001963 growth medium Substances 0.000 description 1
- 231100001261 hazardous Toxicity 0.000 description 1
- 230000036541 health Effects 0.000 description 1
- 210000002216 heart Anatomy 0.000 description 1
- 229940022353 herceptin Drugs 0.000 description 1
- 230000013632 homeostatic process Effects 0.000 description 1
- 230000003054 hormonal effect Effects 0.000 description 1
- 238000001794 hormone therapy Methods 0.000 description 1
- 102000048362 human PDCD1 Human genes 0.000 description 1
- 230000002390 hyperplastic effect Effects 0.000 description 1
- 230000003463 hyperproliferative effect Effects 0.000 description 1
- 210000003026 hypopharynx Anatomy 0.000 description 1
- 210000003016 hypothalamus Anatomy 0.000 description 1
- 229940015872 ibandronate Drugs 0.000 description 1
- 229960002411 imatinib Drugs 0.000 description 1
- KTUFNOKKBVMGRW-UHFFFAOYSA-N imatinib Chemical compound C1CN(C)CCN1CC1=CC=C(C(=O)NC=2C=C(NC=3N=C(C=CN=3)C=3C=NC=CC=3)C(C)=CC=2)C=C1 KTUFNOKKBVMGRW-UHFFFAOYSA-N 0.000 description 1
- 229960003685 imatinib mesylate Drugs 0.000 description 1
- YLMAHDNUQAMNNX-UHFFFAOYSA-N imatinib methanesulfonate Chemical compound CS(O)(=O)=O.C1CN(C)CCN1CC1=CC=C(C(=O)NC=2C=C(NC=3N=C(C=CN=3)C=3C=NC=CC=3)C(C)=CC=2)C=C1 YLMAHDNUQAMNNX-UHFFFAOYSA-N 0.000 description 1
- RAXXELZNTBOGNW-UHFFFAOYSA-N imidazole Substances C1=CNC=N1 RAXXELZNTBOGNW-UHFFFAOYSA-N 0.000 description 1
- 230000008004 immune attack Effects 0.000 description 1
- 230000037451 immune surveillance Effects 0.000 description 1
- 230000000899 immune system response Effects 0.000 description 1
- 230000006058 immune tolerance Effects 0.000 description 1
- 238000011532 immunohistochemical staining Methods 0.000 description 1
- 230000006054 immunological memory Effects 0.000 description 1
- 229960003444 immunosuppressant agent Drugs 0.000 description 1
- 230000001861 immunosuppressant effect Effects 0.000 description 1
- 229940125721 immunosuppressive agent Drugs 0.000 description 1
- 238000002650 immunosuppressive therapy Methods 0.000 description 1
- 239000007943 implant Substances 0.000 description 1
- 238000011534 incubation Methods 0.000 description 1
- 208000015181 infectious disease Diseases 0.000 description 1
- 229960000598 infliximab Drugs 0.000 description 1
- 108091008042 inhibitory receptors Proteins 0.000 description 1
- 210000005007 innate immune system Anatomy 0.000 description 1
- 238000003780 insertion Methods 0.000 description 1
- 230000037431 insertion Effects 0.000 description 1
- 239000000138 intercalating agent Substances 0.000 description 1
- 229940079322 interferon Drugs 0.000 description 1
- 229940047124 interferons Drugs 0.000 description 1
- 230000004073 interleukin-2 production Effects 0.000 description 1
- 108010074108 interleukin-21 Proteins 0.000 description 1
- 229940028885 interleukin-4 Drugs 0.000 description 1
- 210000002570 interstitial cell Anatomy 0.000 description 1
- 201000002313 intestinal cancer Diseases 0.000 description 1
- 230000003834 intracellular effect Effects 0.000 description 1
- 238000007912 intraperitoneal administration Methods 0.000 description 1
- 230000002601 intratumoral effect Effects 0.000 description 1
- 238000001990 intravenous administration Methods 0.000 description 1
- 229960005386 ipilimumab Drugs 0.000 description 1
- 229960004768 irinotecan Drugs 0.000 description 1
- 229960002725 isoflurane Drugs 0.000 description 1
- 229960003299 ketamine Drugs 0.000 description 1
- 201000010982 kidney cancer Diseases 0.000 description 1
- 210000003292 kidney cell Anatomy 0.000 description 1
- 230000002147 killing effect Effects 0.000 description 1
- 210000001821 langerhans cell Anatomy 0.000 description 1
- 229960004891 lapatinib Drugs 0.000 description 1
- BCFGMOOMADDAQU-UHFFFAOYSA-N lapatinib Chemical compound O1C(CNCCS(=O)(=O)C)=CC=C1C1=CC=C(N=CN=C2NC=3C=C(Cl)C(OCC=4C=C(F)C=CC=4)=CC=3)C2=C1 BCFGMOOMADDAQU-UHFFFAOYSA-N 0.000 description 1
- 229940115286 lentinan Drugs 0.000 description 1
- 210000000265 leukocyte Anatomy 0.000 description 1
- GFIJNRVAKGFPGQ-LIJARHBVSA-N leuprolide Chemical compound CCNC(=O)[C@@H]1CCCN1C(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](CC(C)C)NC(=O)[C@@H](NC(=O)[C@H](CO)NC(=O)[C@H](CC=1C2=CC=CC=C2NC=1)NC(=O)[C@H](CC=1N=CNC=1)NC(=O)[C@H]1NC(=O)CC1)CC1=CC=C(O)C=C1 GFIJNRVAKGFPGQ-LIJARHBVSA-N 0.000 description 1
- 229960004338 leuprorelin Drugs 0.000 description 1
- 229960002247 lomustine Drugs 0.000 description 1
- 229940076783 lucentis Drugs 0.000 description 1
- 210000004072 lung Anatomy 0.000 description 1
- 210000001365 lymphatic vessel Anatomy 0.000 description 1
- 208000001419 lymphocytic choriomeningitis Diseases 0.000 description 1
- 210000003563 lymphoid tissue Anatomy 0.000 description 1
- 229940124302 mTOR inhibitor Drugs 0.000 description 1
- 201000000564 macroglobulinemia Diseases 0.000 description 1
- 201000003175 male breast cancer Diseases 0.000 description 1
- 208000010907 male breast carcinoma Diseases 0.000 description 1
- 230000036210 malignancy Effects 0.000 description 1
- 239000003628 mammalian target of rapamycin inhibitor Substances 0.000 description 1
- VXYRWKSIAWIQMG-UHFFFAOYSA-K manganese(2+) N-[2-(sulfidocarbothioylamino)ethyl]carbamodithioate triphenylstannyl acetate Chemical compound [Mn++].[S-]C(=S)NCCNC([S-])=S.CC(=O)O[Sn](c1ccccc1)(c1ccccc1)c1ccccc1 VXYRWKSIAWIQMG-UHFFFAOYSA-K 0.000 description 1
- 230000010534 mechanism of action Effects 0.000 description 1
- 229960004961 mechlorethamine Drugs 0.000 description 1
- HAWPXGHAZFHHAD-UHFFFAOYSA-N mechlorethamine Chemical compound ClCCN(C)CCCl HAWPXGHAZFHHAD-UHFFFAOYSA-N 0.000 description 1
- 210000000716 merkel cell Anatomy 0.000 description 1
- 108020004999 messenger RNA Proteins 0.000 description 1
- 239000002207 metabolite Substances 0.000 description 1
- 208000011645 metastatic carcinoma Diseases 0.000 description 1
- HPNSFSBZBAHARI-UHFFFAOYSA-N micophenolic acid Natural products OC1=C(CC=C(C)CCC(O)=O)C(OC)=C(C)C2=C1C(=O)OC2 HPNSFSBZBAHARI-UHFFFAOYSA-N 0.000 description 1
- 244000005700 microbiome Species 0.000 description 1
- 230000000116 mitigating effect Effects 0.000 description 1
- 229960003539 mitoguazone Drugs 0.000 description 1
- MXWHMTNPTTVWDM-NXOFHUPFSA-N mitoguazone Chemical compound NC(N)=N\N=C(/C)\C=N\N=C(N)N MXWHMTNPTTVWDM-NXOFHUPFSA-N 0.000 description 1
- 229960000350 mitotane Drugs 0.000 description 1
- 238000010172 mouse model Methods 0.000 description 1
- 206010051747 multiple endocrine neoplasia Diseases 0.000 description 1
- 229960000951 mycophenolic acid Drugs 0.000 description 1
- HPNSFSBZBAHARI-RUDMXATFSA-N mycophenolic acid Chemical compound OC1=C(C\C=C(/C)CCC(O)=O)C(OC)=C(C)C2=C1C(=O)OC2 HPNSFSBZBAHARI-RUDMXATFSA-N 0.000 description 1
- 201000005962 mycosis fungoides Diseases 0.000 description 1
- 201000000050 myeloid neoplasm Diseases 0.000 description 1
- 230000017074 necrotic cell death Effects 0.000 description 1
- 230000017128 negative regulation of NF-kappaB transcription factor activity Effects 0.000 description 1
- QZGIWPZCWHMVQL-UIYAJPBUSA-N neocarzinostatin chromophore Chemical compound O1[C@H](C)[C@H](O)[C@H](O)[C@@H](NC)[C@H]1O[C@@H]1C/2=C/C#C[C@H]3O[C@@]3([C@@H]3OC(=O)OC3)C#CC\2=C[C@H]1OC(=O)C1=C(O)C=CC2=C(C)C=C(OC)C=C12 QZGIWPZCWHMVQL-UIYAJPBUSA-N 0.000 description 1
- 229960002653 nilutamide Drugs 0.000 description 1
- XWXYUMMDTVBTOU-UHFFFAOYSA-N nilutamide Chemical compound O=C1C(C)(C)NC(=O)N1C1=CC=C([N+]([O-])=O)C(C(F)(F)F)=C1 XWXYUMMDTVBTOU-UHFFFAOYSA-N 0.000 description 1
- OSTGTTZJOCZWJG-UHFFFAOYSA-N nitrosourea Chemical compound NC(=O)N=NO OSTGTTZJOCZWJG-UHFFFAOYSA-N 0.000 description 1
- 239000002777 nucleoside Substances 0.000 description 1
- 150000003833 nucleoside derivatives Chemical class 0.000 description 1
- 229960000470 omalizumab Drugs 0.000 description 1
- 238000011275 oncology therapy Methods 0.000 description 1
- 208000022982 optic pathway glioma Diseases 0.000 description 1
- 150000002894 organic compounds Chemical class 0.000 description 1
- 230000008520 organization Effects 0.000 description 1
- 229940047091 other immunostimulants in atc Drugs 0.000 description 1
- 208000021284 ovarian germ cell tumor Diseases 0.000 description 1
- 229960001756 oxaliplatin Drugs 0.000 description 1
- DWAFYCQODLXJNR-BNTLRKBRSA-L oxaliplatin Chemical compound O1C(=O)C(=O)O[Pt]11N[C@@H]2CCCC[C@H]2N1 DWAFYCQODLXJNR-BNTLRKBRSA-L 0.000 description 1
- 201000002530 pancreatic endocrine carcinoma Diseases 0.000 description 1
- 229940096763 panretin Drugs 0.000 description 1
- 239000012188 paraffin wax Substances 0.000 description 1
- 244000052769 pathogen Species 0.000 description 1
- 229940121655 pd-1 inhibitor Drugs 0.000 description 1
- 229940121656 pd-l1 inhibitor Drugs 0.000 description 1
- 229960002621 pembrolizumab Drugs 0.000 description 1
- 230000010412 perfusion Effects 0.000 description 1
- 210000005259 peripheral blood Anatomy 0.000 description 1
- 239000011886 peripheral blood Substances 0.000 description 1
- JGWRKYUXBBNENE-UHFFFAOYSA-N pexidartinib Chemical compound C1=NC(C(F)(F)F)=CC=C1CNC(N=C1)=CC=C1CC1=CNC2=NC=C(Cl)C=C12 JGWRKYUXBBNENE-UHFFFAOYSA-N 0.000 description 1
- 239000000825 pharmaceutical preparation Substances 0.000 description 1
- 229940127557 pharmaceutical product Drugs 0.000 description 1
- 102000020233 phosphotransferase Human genes 0.000 description 1
- 230000001766 physiological effect Effects 0.000 description 1
- 230000035790 physiological processes and functions Effects 0.000 description 1
- 230000006461 physiological response Effects 0.000 description 1
- 210000004560 pineal gland Anatomy 0.000 description 1
- 208000010916 pituitary tumor Diseases 0.000 description 1
- 229920003023 plastic Polymers 0.000 description 1
- 239000004033 plastic Substances 0.000 description 1
- 238000007747 plating Methods 0.000 description 1
- 229910052697 platinum Inorganic materials 0.000 description 1
- 229920000642 polymer Polymers 0.000 description 1
- 108091033319 polynucleotide Proteins 0.000 description 1
- 102000040430 polynucleotide Human genes 0.000 description 1
- 239000002157 polynucleotide Substances 0.000 description 1
- 210000003240 portal vein Anatomy 0.000 description 1
- 238000002360 preparation method Methods 0.000 description 1
- 239000013630 prepared media Substances 0.000 description 1
- 230000037452 priming Effects 0.000 description 1
- 210000001948 pro-b lymphocyte Anatomy 0.000 description 1
- CPTBDICYNRMXFX-UHFFFAOYSA-N procarbazine Chemical compound CNNCC1=CC=C(C(=O)NC(C)C)C=C1 CPTBDICYNRMXFX-UHFFFAOYSA-N 0.000 description 1
- 229960000624 procarbazine Drugs 0.000 description 1
- 239000000047 product Substances 0.000 description 1
- 238000004393 prognosis Methods 0.000 description 1
- 230000005522 programmed cell death Effects 0.000 description 1
- 230000000770 proinflammatory effect Effects 0.000 description 1
- 230000002035 prolonged effect Effects 0.000 description 1
- 210000002307 prostate Anatomy 0.000 description 1
- 239000003207 proteasome inhibitor Substances 0.000 description 1
- 239000003909 protein kinase inhibitor Substances 0.000 description 1
- 229940034080 provenge Drugs 0.000 description 1
- WOLQREOUPKZMEX-UHFFFAOYSA-N pteroyltriglutamic acid Chemical compound C=1N=C2NC(N)=NC(=O)C2=NC=1CNC1=CC=C(C(=O)NC(CCC(=O)NC(CCC(=O)NC(CCC(O)=O)C(O)=O)C(O)=O)C(O)=O)C=C1 WOLQREOUPKZMEX-UHFFFAOYSA-N 0.000 description 1
- 210000001147 pulmonary artery Anatomy 0.000 description 1
- 210000003492 pulmonary vein Anatomy 0.000 description 1
- 230000000541 pulsatile effect Effects 0.000 description 1
- 150000003212 purines Chemical class 0.000 description 1
- 229950010131 puromycin Drugs 0.000 description 1
- 150000003230 pyrimidines Chemical class 0.000 description 1
- 150000003254 radicals Chemical class 0.000 description 1
- 229960004622 raloxifene Drugs 0.000 description 1
- GZUITABIAKMVPG-UHFFFAOYSA-N raloxifene Chemical compound C1=CC(O)=CC=C1C1=C(C(=O)C=2C=CC(OCCN3CCCCC3)=CC=2)C2=CC=C(O)C=C2S1 GZUITABIAKMVPG-UHFFFAOYSA-N 0.000 description 1
- 229960002185 ranimustine Drugs 0.000 description 1
- ZAHRKKWIAAJSAO-UHFFFAOYSA-N rapamycin Natural products COCC(O)C(=C/C(C)C(=O)CC(OC(=O)C1CCCCN1C(=O)C(=O)C2(O)OC(CC(OC)C(=CC=CC=CC(C)CC(C)C(=O)C)C)CCC2C)C(C)CC3CCC(O)C(C3)OC)C ZAHRKKWIAAJSAO-UHFFFAOYSA-N 0.000 description 1
- 230000009257 reactivity Effects 0.000 description 1
- 210000003370 receptor cell Anatomy 0.000 description 1
- 229960000160 recombinant therapeutic protein Drugs 0.000 description 1
- 206010038038 rectal cancer Diseases 0.000 description 1
- 201000001275 rectum cancer Diseases 0.000 description 1
- 230000009467 reduction Effects 0.000 description 1
- 208000019465 refractory cytopenia of childhood Diseases 0.000 description 1
- 229940116176 remicade Drugs 0.000 description 1
- 238000011160 research Methods 0.000 description 1
- 230000029058 respiratory gaseous exchange Effects 0.000 description 1
- SHGAZHPCJJPHSC-YCNIQYBTSA-N retinoic acid group Chemical class C\C(=C/C(=O)O)\C=C\C=C(\C=C\C1=C(CCCC1(C)C)C)/C SHGAZHPCJJPHSC-YCNIQYBTSA-N 0.000 description 1
- 238000012552 review Methods 0.000 description 1
- 239000011435 rock Substances 0.000 description 1
- VHXNKPBCCMUMSW-FQEVSTJZSA-N rubitecan Chemical compound C1=CC([N+]([O-])=O)=C2C=C(CN3C4=CC5=C(C3=O)COC(=O)[C@]5(O)CC)C4=NC2=C1 VHXNKPBCCMUMSW-FQEVSTJZSA-N 0.000 description 1
- 229960000215 ruxolitinib Drugs 0.000 description 1
- HFNKQEVNSGCOJV-OAHLLOKOSA-N ruxolitinib Chemical compound C1([C@@H](CC#N)N2N=CC(=C2)C=2C=3C=CNC=3N=CN=2)CCCC1 HFNKQEVNSGCOJV-OAHLLOKOSA-N 0.000 description 1
- 210000004761 scalp Anatomy 0.000 description 1
- 230000028327 secretion Effects 0.000 description 1
- 229960000714 sipuleucel-t Drugs 0.000 description 1
- 229960002930 sirolimus Drugs 0.000 description 1
- QFJCIRLUMZQUOT-HPLJOQBZSA-N sirolimus Chemical compound C1C[C@@H](O)[C@H](OC)C[C@@H]1C[C@@H](C)[C@H]1OC(=O)[C@@H]2CCCCN2C(=O)C(=O)[C@](O)(O2)[C@H](C)CC[C@H]2C[C@H](OC)/C(C)=C/C=C/C=C/[C@@H](C)C[C@@H](C)C(=O)[C@H](OC)[C@H](O)/C(C)=C/[C@@H](C)C(=O)C1 QFJCIRLUMZQUOT-HPLJOQBZSA-N 0.000 description 1
- 201000008261 skin carcinoma Diseases 0.000 description 1
- 210000003625 skull Anatomy 0.000 description 1
- 239000007787 solid Substances 0.000 description 1
- 210000001082 somatic cell Anatomy 0.000 description 1
- 229950006315 spirogermanium Drugs 0.000 description 1
- 206010041823 squamous cell carcinoma Diseases 0.000 description 1
- 210000000130 stem cell Anatomy 0.000 description 1
- 238000011476 stem cell transplantation Methods 0.000 description 1
- 235000000346 sugar Nutrition 0.000 description 1
- 150000008163 sugars Chemical class 0.000 description 1
- 229960001796 sunitinib Drugs 0.000 description 1
- WINHZLLDWRZWRT-ATVHPVEESA-N sunitinib Chemical compound CCN(CC)CCNC(=O)C1=C(C)NC(\C=C/2C3=CC(F)=CC=C3NC\2=O)=C1C WINHZLLDWRZWRT-ATVHPVEESA-N 0.000 description 1
- 239000013589 supplement Substances 0.000 description 1
- 230000000153 supplemental effect Effects 0.000 description 1
- 239000000725 suspension Substances 0.000 description 1
- 238000013268 sustained release Methods 0.000 description 1
- 239000012730 sustained-release form Substances 0.000 description 1
- 229940036185 synagis Drugs 0.000 description 1
- 238000003786 synthesis reaction Methods 0.000 description 1
- 238000007910 systemic administration Methods 0.000 description 1
- 229960001603 tamoxifen Drugs 0.000 description 1
- 229940095064 tartrate Drugs 0.000 description 1
- 229940063683 taxotere Drugs 0.000 description 1
- 201000003120 testicular cancer Diseases 0.000 description 1
- 210000001550 testis Anatomy 0.000 description 1
- BPEWUONYVDABNZ-DZBHQSCQSA-N testolactone Chemical compound O=C1C=C[C@]2(C)[C@H]3CC[C@](C)(OC(=O)CC4)[C@@H]4[C@@H]3CCC2=C1 BPEWUONYVDABNZ-DZBHQSCQSA-N 0.000 description 1
- 229960005353 testolactone Drugs 0.000 description 1
- 230000004797 therapeutic response Effects 0.000 description 1
- 208000030045 thyroid gland papillary carcinoma Diseases 0.000 description 1
- YFTWHEBLORWGNI-UHFFFAOYSA-N tiamiprine Chemical compound CN1C=NC([N+]([O-])=O)=C1SC1=NC(N)=NC2=C1NC=N2 YFTWHEBLORWGNI-UHFFFAOYSA-N 0.000 description 1
- 229950011457 tiamiprine Drugs 0.000 description 1
- 229940044693 topoisomerase inhibitor Drugs 0.000 description 1
- 229960000303 topotecan Drugs 0.000 description 1
- UCFGDBYHRUNTLO-QHCPKHFHSA-N topotecan Chemical compound C1=C(O)C(CN(C)C)=C2C=C(CN3C4=CC5=C(C3=O)COC(=O)[C@]5(O)CC)C4=NC2=C1 UCFGDBYHRUNTLO-QHCPKHFHSA-N 0.000 description 1
- XFCLJVABOIYOMF-QPLCGJKRSA-N toremifene Chemical compound C1=CC(OCCN(C)C)=CC=C1C(\C=1C=CC=CC=1)=C(\CCCl)C1=CC=CC=C1 XFCLJVABOIYOMF-QPLCGJKRSA-N 0.000 description 1
- 229960005026 toremifene Drugs 0.000 description 1
- 238000012546 transfer Methods 0.000 description 1
- 230000001131 transforming effect Effects 0.000 description 1
- 230000009261 transgenic effect Effects 0.000 description 1
- 230000032258 transport Effects 0.000 description 1
- 238000011269 treatment regimen Methods 0.000 description 1
- 229950007217 tremelimumab Drugs 0.000 description 1
- IUCJMVBFZDHPDX-UHFFFAOYSA-N tretamine Chemical compound C1CN1C1=NC(N2CC2)=NC(N2CC2)=N1 IUCJMVBFZDHPDX-UHFFFAOYSA-N 0.000 description 1
- 229950001353 tretamine Drugs 0.000 description 1
- 229960001670 trilostane Drugs 0.000 description 1
- KVJXBPDAXMEYOA-CXANFOAXSA-N trilostane Chemical compound OC1=C(C#N)C[C@]2(C)[C@H]3CC[C@](C)([C@H](CC4)O)[C@@H]4[C@@H]3CC[C@@]32O[C@@H]31 KVJXBPDAXMEYOA-CXANFOAXSA-N 0.000 description 1
- 229950000212 trioxifene Drugs 0.000 description 1
- 208000029387 trophoblastic neoplasm Diseases 0.000 description 1
- HDZZVAMISRMYHH-LITAXDCLSA-N tubercidin Chemical compound C1=CC=2C(N)=NC=NC=2N1[C@@H]1O[C@@H](CO)[C@H](O)[C@H]1O HDZZVAMISRMYHH-LITAXDCLSA-N 0.000 description 1
- 229940030325 tumor cell vaccine Drugs 0.000 description 1
- 230000005909 tumor killing Effects 0.000 description 1
- 210000003171 tumor-infiltrating lymphocyte Anatomy 0.000 description 1
- 239000005483 tyrosine kinase inhibitor Substances 0.000 description 1
- 229950009811 ubenimex Drugs 0.000 description 1
- 208000018417 undifferentiated high grade pleomorphic sarcoma of bone Diseases 0.000 description 1
- 229960001055 uracil mustard Drugs 0.000 description 1
- 208000037965 uterine sarcoma Diseases 0.000 description 1
- 206010046885 vaginal cancer Diseases 0.000 description 1
- 208000013139 vaginal neoplasm Diseases 0.000 description 1
- 229960000241 vandetanib Drugs 0.000 description 1
- UHTHHESEBZOYNR-UHFFFAOYSA-N vandetanib Chemical compound COC1=CC(C(/N=CN2)=N/C=3C(=CC(Br)=CC=3)F)=C2C=C1OCC1CCN(C)CC1 UHTHHESEBZOYNR-UHFFFAOYSA-N 0.000 description 1
- 230000002792 vascular Effects 0.000 description 1
- 229940126580 vector vaccine Drugs 0.000 description 1
- 210000005135 veiled cell Anatomy 0.000 description 1
- GBABOYUKABKIAF-GHYRFKGUSA-N vinorelbine Chemical compound C1N(CC=2C3=CC=CC=C3NC=22)CC(CC)=C[C@H]1C[C@]2(C(=O)OC)C1=CC([C@]23[C@H]([C@]([C@H](OC(C)=O)[C@]4(CC)C=CCN([C@H]34)CC2)(O)C(=O)OC)N2C)=C2C=C1OC GBABOYUKABKIAF-GHYRFKGUSA-N 0.000 description 1
- 229960002066 vinorelbine Drugs 0.000 description 1
- 229960004854 viral vaccine Drugs 0.000 description 1
- 230000003612 virological effect Effects 0.000 description 1
- 201000005102 vulva cancer Diseases 0.000 description 1
- BPICBUSOMSTKRF-UHFFFAOYSA-N xylazine Chemical compound CC1=CC=CC(C)=C1NC1=NCCCS1 BPICBUSOMSTKRF-UHFFFAOYSA-N 0.000 description 1
- 229960001600 xylazine Drugs 0.000 description 1
- 210000005253 yeast cell Anatomy 0.000 description 1
- 229940055760 yervoy Drugs 0.000 description 1
- 239000011701 zinc Substances 0.000 description 1
- 229910052725 zinc Inorganic materials 0.000 description 1
- 229950009268 zinostatin Drugs 0.000 description 1
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F7/00—Heating or cooling appliances for medical or therapeutic treatment of the human body
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K35/00—Medicinal preparations containing materials or reaction products thereof with undetermined constitution
- A61K35/12—Materials from mammals; Compositions comprising non-specified tissues or cells; Compositions comprising non-embryonic stem cells; Genetically modified cells
- A61K35/14—Blood; Artificial blood
- A61K35/15—Cells of the myeloid line, e.g. granulocytes, basophils, eosinophils, neutrophils, leucocytes, monocytes, macrophages or mast cells; Myeloid precursor cells; Antigen-presenting cells, e.g. dendritic cells
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K35/00—Medicinal preparations containing materials or reaction products thereof with undetermined constitution
- A61K35/12—Materials from mammals; Compositions comprising non-specified tissues or cells; Compositions comprising non-embryonic stem cells; Genetically modified cells
- A61K35/14—Blood; Artificial blood
- A61K35/17—Lymphocytes; B-cells; T-cells; Natural killer cells; Interferon-activated or cytokine-activated lymphocytes
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K38/00—Medicinal preparations containing peptides
- A61K38/16—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- A61K38/17—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans
- A61K38/19—Cytokines; Lymphokines; Interferons
- A61K38/20—Interleukins [IL]
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K38/00—Medicinal preparations containing peptides
- A61K38/16—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- A61K38/17—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans
- A61K38/19—Cytokines; Lymphokines; Interferons
- A61K38/20—Interleukins [IL]
- A61K38/2046—IL-7
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K38/00—Medicinal preparations containing peptides
- A61K38/16—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- A61K38/17—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans
- A61K38/19—Cytokines; Lymphokines; Interferons
- A61K38/20—Interleukins [IL]
- A61K38/2086—IL-13 to IL-16
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K39/0005—Vertebrate antigens
- A61K39/0011—Cancer antigens
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K39/39—Medicinal preparations containing antigens or antibodies characterised by the immunostimulating additives, e.g. chemical adjuvants
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K39/395—Antibodies; Immunoglobulins; Immune serum, e.g. antilymphocytic serum
- A61K39/39533—Antibodies; Immunoglobulins; Immune serum, e.g. antilymphocytic serum against materials from animals
- A61K39/3955—Antibodies; Immunoglobulins; Immune serum, e.g. antilymphocytic serum against materials from animals against proteinaceous materials, e.g. enzymes, hormones, lymphokines
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K39/395—Antibodies; Immunoglobulins; Immune serum, e.g. antilymphocytic serum
- A61K39/39533—Antibodies; Immunoglobulins; Immune serum, e.g. antilymphocytic serum against materials from animals
- A61K39/39558—Antibodies; Immunoglobulins; Immune serum, e.g. antilymphocytic serum against materials from animals against tumor tissues, cells, antigens
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K40/00—Cellular immunotherapy
- A61K40/10—Cellular immunotherapy characterised by the cell type used
- A61K40/19—Dendritic cells
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K40/00—Cellular immunotherapy
- A61K40/20—Cellular immunotherapy characterised by the effect or the function of the cells
- A61K40/24—Antigen-presenting cells [APC]
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K40/00—Cellular immunotherapy
- A61K40/40—Cellular immunotherapy characterised by antigens that are targeted or presented by cells of the immune system
- A61K40/41—Vertebrate antigens
- A61K40/42—Cancer antigens
- A61K40/428—Undefined tumor antigens, e.g. tumor lysate or antigens targeted by cells isolated from tumor
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K45/00—Medicinal preparations containing active ingredients not provided for in groups A61K31/00 - A61K41/00
- A61K45/06—Mixtures of active ingredients without chemical characterisation, e.g. antiphlogistics and cardiaca
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61N—ELECTROTHERAPY; MAGNETOTHERAPY; RADIATION THERAPY; ULTRASOUND THERAPY
- A61N5/00—Radiation therapy
- A61N5/10—X-ray therapy; Gamma-ray therapy; Particle-irradiation therapy
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
- A61P35/02—Antineoplastic agents specific for leukemia
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P37/00—Drugs for immunological or allergic disorders
- A61P37/02—Immunomodulators
- A61P37/04—Immunostimulants
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P43/00—Drugs for specific purposes, not provided for in groups A61P1/00-A61P41/00
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/18—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans
- C07K16/28—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants
- C07K16/2803—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants against the immunoglobulin superfamily
- C07K16/2818—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants against the immunoglobulin superfamily against CD28 or CD152
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K2039/505—Medicinal preparations containing antigens or antibodies comprising antibodies
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K2039/51—Medicinal preparations containing antigens or antibodies comprising whole cells, viruses or DNA/RNA
- A61K2039/515—Animal cells
- A61K2039/5154—Antigen presenting cells [APCs], e.g. dendritic cells or macrophages
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K2039/545—Medicinal preparations containing antigens or antibodies characterised by the dose, timing or administration schedule
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K2039/555—Medicinal preparations containing antigens or antibodies characterised by a specific combination antigen/adjuvant
- A61K2039/55511—Organic adjuvants
- A61K2039/55516—Proteins; Peptides
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K2039/555—Medicinal preparations containing antigens or antibodies characterised by a specific combination antigen/adjuvant
- A61K2039/55511—Organic adjuvants
- A61K2039/55522—Cytokines; Lymphokines; Interferons
- A61K2039/55527—Interleukins
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K2239/00—Indexing codes associated with cellular immunotherapy of group A61K40/00
- A61K2239/31—Indexing codes associated with cellular immunotherapy of group A61K40/00 characterized by the route of administration
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K2239/00—Indexing codes associated with cellular immunotherapy of group A61K40/00
- A61K2239/38—Indexing codes associated with cellular immunotherapy of group A61K40/00 characterised by the dose, timing or administration schedule
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K2239/00—Indexing codes associated with cellular immunotherapy of group A61K40/00
- A61K2239/46—Indexing codes associated with cellular immunotherapy of group A61K40/00 characterised by the cancer treated
- A61K2239/47—Brain; Nervous system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K2300/00—Mixtures or combinations of active ingredients, wherein at least one active ingredient is fully defined in groups A61K31/00 - A61K41/00
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Chemical & Material Sciences (AREA)
- General Health & Medical Sciences (AREA)
- Veterinary Medicine (AREA)
- Animal Behavior & Ethology (AREA)
- Public Health (AREA)
- Immunology (AREA)
- Medicinal Chemistry (AREA)
- Epidemiology (AREA)
- Pharmacology & Pharmacy (AREA)
- Engineering & Computer Science (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Organic Chemistry (AREA)
- Biomedical Technology (AREA)
- Zoology (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- General Chemical & Material Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Mycology (AREA)
- Microbiology (AREA)
- Hematology (AREA)
- Gastroenterology & Hepatology (AREA)
- Cell Biology (AREA)
- Oncology (AREA)
- Virology (AREA)
- Developmental Biology & Embryology (AREA)
- Biotechnology (AREA)
- Molecular Biology (AREA)
- Biochemistry (AREA)
- Biophysics (AREA)
- Genetics & Genomics (AREA)
- Radiology & Medical Imaging (AREA)
- Heart & Thoracic Surgery (AREA)
- Vascular Medicine (AREA)
- Endocrinology (AREA)
- Pathology (AREA)
- Medicines That Contain Protein Lipid Enzymes And Other Medicines (AREA)
- Medicines Containing Material From Animals Or Micro-Organisms (AREA)
Applications Claiming Priority (5)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US201361900309P | 2013-11-05 | 2013-11-05 | |
| US201361900355P | 2013-11-05 | 2013-11-05 | |
| US61/900,309 | 2013-11-05 | ||
| US61/900,355 | 2013-11-05 | ||
| PCT/US2014/064133 WO2015069770A1 (en) | 2013-11-05 | 2014-11-05 | Combinations of checkpoint inhibitors and therapeutics to treat cancer |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| KR20160093012A true KR20160093012A (ko) | 2016-08-05 |
Family
ID=53042035
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020167014867A Withdrawn KR20160093012A (ko) | 2013-11-05 | 2014-11-05 | 암 치료를 위한 체크포인트 억제제 및 치료제의 배합물 |
Country Status (15)
| Country | Link |
|---|---|
| US (4) | US20150202291A1 (enExample) |
| EP (2) | EP4461372A3 (enExample) |
| JP (1) | JP2016540042A (enExample) |
| KR (1) | KR20160093012A (enExample) |
| CN (1) | CN105828834A (enExample) |
| AU (1) | AU2014346852A1 (enExample) |
| BR (1) | BR112016010224A2 (enExample) |
| CA (1) | CA2929407A1 (enExample) |
| EA (1) | EA201690912A1 (enExample) |
| ES (1) | ES2991853T3 (enExample) |
| HK (1) | HK1232119A1 (enExample) |
| IL (3) | IL318946A (enExample) |
| MX (2) | MX2016005925A (enExample) |
| PH (1) | PH12016500841A1 (enExample) |
| WO (1) | WO2015069770A1 (enExample) |
Cited By (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| KR20210022669A (ko) * | 2018-06-19 | 2021-03-03 | 사이토베이션 에이에스 | 펩타이드를 사용하는 병용 요법 |
| US11229668B2 (en) | 2017-02-07 | 2022-01-25 | Nantcell, Inc. | Maximizing T-cell memory and compositions and methods therefor |
Families Citing this family (189)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2010077634A1 (en) | 2008-12-09 | 2010-07-08 | Genentech, Inc. | Anti-pd-l1 antibodies and their use to enhance t-cell function |
| US9675561B2 (en) | 2011-04-28 | 2017-06-13 | President And Fellows Of Harvard College | Injectable cryogel vaccine devices and methods of use thereof |
| PE20160953A1 (es) | 2013-12-12 | 2016-09-26 | Shanghai hengrui pharmaceutical co ltd | Anticuerpo pd-1, fragmento de union al antigeno de este y uso medico de este |
| CA2974651A1 (en) | 2014-01-24 | 2015-07-30 | Children's Hospital Of Eastern Ontario Research Institute Inc. | Smc combination therapy for the treatment of cancer |
| US10682400B2 (en) * | 2014-04-30 | 2020-06-16 | President And Fellows Of Harvard College | Combination vaccine devices and methods of killing cancer cells |
| JO3663B1 (ar) | 2014-08-19 | 2020-08-27 | Merck Sharp & Dohme | الأجسام المضادة لمضاد lag3 وأجزاء ربط الأنتيجين |
| JP2018504446A (ja) * | 2015-02-09 | 2018-02-15 | シンタ ファーマシューティカルズ コーポレーション | がんを処置するためのhsp90阻害剤およびpd−1阻害剤の組み合わせ治療 |
| CN112263677A (zh) * | 2015-02-26 | 2021-01-26 | 默克专利股份公司 | 用于治疗癌症的pd-1/pd-l1抑制剂 |
| SG10202008673WA (en) | 2015-03-06 | 2020-10-29 | Beyondspring Pharmaceuticals Inc | Method of treating cancer associated with a ras mutation |
| EP3268012A4 (en) * | 2015-03-12 | 2018-10-31 | Health Research, Inc. | Enrichment of cd16+ monocytes to improve dendritic cell vaccine quality |
| WO2016145578A1 (en) | 2015-03-13 | 2016-09-22 | Syz Cell Therapy Co. | Methods of cancer treatment using activated t cells |
| MX2017011644A (es) | 2015-03-13 | 2017-12-04 | Cytomx Therapeutics Inc | Anticuerpos anti-pdl1, anticuerpos anti-pdl1 activables y metodos de uso de los mismos. |
| AU2016259020B2 (en) * | 2015-05-07 | 2021-12-09 | Baylor College Of Medicine | Dendritic cell immunotherapy |
| TWI716405B (zh) | 2015-05-07 | 2021-01-21 | 美商艾吉納斯公司 | 抗ox40抗體及其使用方法 |
| US10314854B2 (en) * | 2015-05-15 | 2019-06-11 | University Of Iowa Foundation | Methods for treating tumors in situ including intratumor injection of cytotoxic particles and immune checkpoint blockade therapy |
| BR112017025166A2 (en) | 2015-05-28 | 2018-07-31 | Kite Pharma, Inc. | Methods of Conditioning Patients for T-Cell Therapy |
| KR20230148387A (ko) * | 2015-05-28 | 2023-10-24 | 카이트 파마 인코포레이티드 | T 세포 요법을 위한 진단 방법 |
| CN107849144B (zh) | 2015-05-29 | 2021-09-17 | 艾吉纳斯公司 | 抗-ctla-4抗体及其使用方法 |
| ES2987696T3 (es) * | 2015-05-29 | 2024-11-15 | Dynavax Tech Corp | Agonista polinucleotídico del receptor de tipo toll 9 para el tratamiento del cáncer de pulmón |
| KR102661603B1 (ko) * | 2015-06-01 | 2024-04-29 | 더 유니버서티 오브 시카고 | 공생 미생물총의 조작에 의한 암의 치료 |
| MY193229A (en) | 2015-06-16 | 2022-09-26 | Merck Patent GmbH | Pd-l1 antagonist combination treatments |
| BR112017028530A2 (pt) * | 2015-07-02 | 2018-08-28 | Celgene Corp | terapia combinada para tratamento de cânceres hematológicos e tumores sólidos |
| EP3322732A2 (en) | 2015-07-13 | 2018-05-23 | Cytomx Therapeutics Inc. | Anti-pd-1 antibodies, activatable anti-pd-1 antibodies, and methods of use thereof |
| CN109516981B (zh) | 2015-07-13 | 2019-10-22 | 大连万春布林医药有限公司 | 普那布林组合物 |
| CA2986705A1 (en) * | 2015-07-16 | 2017-01-19 | Biokine Therapeutics Ltd. | A cxcr4 inhibitor and a pdi antagonist for use in treating cancer |
| HK1248115A1 (zh) * | 2015-07-16 | 2018-10-12 | Bioxcel Therapeutics, Inc. | 一种使用免疫调节治疗癌症的新颖方法 |
| US10842763B2 (en) | 2015-07-31 | 2020-11-24 | The Johns Hopkins University | Methods for cancer and immunotherapy using prodrugs of glutamine analogs |
| WO2017023787A1 (en) | 2015-07-31 | 2017-02-09 | The Johns Hopkins University | Methods and compositions for treating metabolic reprogramming disorders |
| MX384906B (es) | 2015-07-31 | 2025-03-14 | Univ Johns Hopkins | Profármacos de análogos de glutamina. |
| US10036751B2 (en) * | 2015-08-10 | 2018-07-31 | China Medical University Hospital | Method of evaluating glioblastoma multiforme patient applicable to immunotherapy treatment based on dendritic cell tumor vaccines and method of prognosticating survival rate in glioblastoma mulztiforme patient after treatment |
| HK1257840A1 (zh) | 2015-09-01 | 2019-11-01 | Agenus Inc. | 抗-pd-1抗体及其使用方法 |
| US10894044B2 (en) | 2015-09-16 | 2021-01-19 | Board Of Regents, The University Of Texas System | Combination of topoisomerase-I inhibitors with immunotherapy in the treatment of cancer |
| US20180296561A1 (en) * | 2015-10-07 | 2018-10-18 | The University Of North Carolina At Chapel Hill | The Methods For Treatment Of Tumors |
| IL258521B2 (en) | 2015-10-08 | 2024-01-01 | Macrogenics Inc | Combination therapy for the treatment of cancer |
| JP6850290B2 (ja) * | 2015-10-15 | 2021-03-31 | デューク ユニバーシティー | 併用療法 |
| KR20180086195A (ko) * | 2015-10-21 | 2018-07-30 | 테크리슨 리미티드 | 면역 매개 암 치료를 위한 조성물 및 방법 |
| WO2017068349A1 (en) * | 2015-10-23 | 2017-04-27 | E-Therapeutics Plc | Cannabinoid for use in immunotherapy |
| SI3370768T1 (sl) * | 2015-11-03 | 2022-04-29 | Janssen Biotech, Inc. | Protitelesa, ki se specifično vežejo na PD-1, in njihove uporabe |
| WO2017079520A1 (en) * | 2015-11-04 | 2017-05-11 | Duke University | Combination therapy of immunotoxin and checkpoint inhibitor |
| EP3370755B1 (en) * | 2015-11-06 | 2021-09-29 | Regents of the University of Minnesota | Activation of resident memory t cells for cancer immunotherapy |
| CN108884159A (zh) * | 2015-11-07 | 2018-11-23 | 茂体外尔公司 | 用于癌症治疗的包含肿瘤抑制基因治疗和免疫检查点阻断的组合物 |
| CN116327902A (zh) * | 2015-11-20 | 2023-06-27 | 纪念斯隆凯特林癌症中心 | 用于治疗癌症的方法和组合物 |
| EP4015537A1 (en) * | 2015-12-01 | 2022-06-22 | GlaxoSmithKline Intellectual Property Development Limited | Combination treatments and uses and methods thereof |
| EP3383430A4 (en) | 2015-12-02 | 2019-12-18 | Agenus Inc. | ANTIBODIES AND METHOD FOR USE THEREOF |
| SG11201804263PA (en) | 2015-12-04 | 2018-06-28 | Seattle Genetics Inc | Cancer treatment using 2-deoxy-2-fluoro-l-fucose in combination with a checkpoint inhibitor |
| EP3389652B1 (en) | 2015-12-14 | 2022-09-28 | X4 Pharmaceuticals, Inc. | Methods for treating cancer |
| US10953003B2 (en) | 2015-12-14 | 2021-03-23 | X4 Pharmaceuticals, Inc. | Methods for treating cancer |
| MX2018007406A (es) | 2015-12-16 | 2018-08-15 | Merck Sharp & Dohme | Anticuerpos anti-lag3 y fragmentos de enlace al antigeno. |
| ES2935834T3 (es) | 2015-12-22 | 2023-03-10 | X4 Pharmaceuticals Inc | Métodos para tratar enfermedad de inmunodeficiencia |
| WO2017120589A1 (en) * | 2016-01-08 | 2017-07-13 | Washington University | Compositions comprising chemerin and methods of use thereof |
| WO2017119499A1 (en) * | 2016-01-08 | 2017-07-13 | Momotaro-Gene Inc. | A combination therapy using REIC/Dkk-3 gene and a checkpoint inhibitor |
| US20190022129A1 (en) * | 2016-01-08 | 2019-01-24 | Biothera, Inc. | Beta-glucan immunotherapies affecting the immune microenvironment |
| WO2017123643A1 (en) * | 2016-01-11 | 2017-07-20 | Flagship Pioneering, Inc. | Methods and compositions for modulating thymic function |
| US20190030023A1 (en) * | 2016-01-22 | 2019-01-31 | X4 Pharmaceuticals, Inc. | Methods for treating cancer |
| GB201601868D0 (en) * | 2016-02-02 | 2016-03-16 | Lytix Biopharma As | Methods |
| US10912748B2 (en) | 2016-02-08 | 2021-02-09 | Beyondspring Pharmaceuticals, Inc. | Compositions containing tucaresol or its analogs |
| US10335461B2 (en) * | 2016-02-15 | 2019-07-02 | Trizell Limited | Interferon therapy |
| EP3419645B1 (en) | 2016-02-23 | 2020-09-02 | BioLineRx Ltd. | Method of selecting a treatment regimen in acute myeloid leukemia (aml) |
| US10358496B2 (en) * | 2016-03-01 | 2019-07-23 | Ralf Kleef | Low dose immune checkpoint blockade in metastatic cancer |
| GB201604213D0 (en) * | 2016-03-11 | 2016-04-27 | Proximagen Ltd | Drug combination and its use in therapy |
| EP3426299A4 (en) * | 2016-03-11 | 2019-10-16 | University of Louisville Research Foundation, Inc. | METHOD AND COMPOSITIONS FOR TREATMENT OF TUMORS |
| JP2019510041A (ja) * | 2016-03-30 | 2019-04-11 | マイクロバイオ カンパニー, リミテッド | 免疫チェックポイントモジュレーターおよび共生微生物叢による発酵産物の併用がん療法 |
| CA3019394A1 (en) | 2016-04-08 | 2017-10-12 | X4 Pharmaceuticals, Inc. | Methods for treating cancer |
| CA3021645A1 (en) | 2016-04-29 | 2017-11-02 | Icahn School Of Medicine At Mount Sinai | Targeting the innate immune system to induce long-term tolerance and to resolve macrophage accumulation in atherosclerosis |
| WO2017203362A1 (en) * | 2016-05-25 | 2017-11-30 | The Council Of The Queensland Institute Of Medical Research | Immune checkpoint inhibitors and cytotoxic t cells for the treatment of cancer |
| MA45123A (fr) | 2016-05-27 | 2019-04-10 | Agenus Inc | Anticorps anti-tim-3 et leurs méthodes d'utilisation |
| KR20240091084A (ko) | 2016-06-06 | 2024-06-21 | 비욘드스프링 파마수티컬스, 인코포레이티드. | 호중구감소증을 줄이는 조성물 및 방법 |
| WO2017212021A1 (en) * | 2016-06-10 | 2017-12-14 | INSERM (Institut National de la Santé et de la Recherche Médicale) | Methods and pharmaceutical compositions for the treatment of cancer |
| CN109562106B (zh) | 2016-06-21 | 2023-03-21 | X4 制药有限公司 | Cxcr4抑制剂及其用途 |
| CN109640988A (zh) | 2016-06-21 | 2019-04-16 | X4 制药有限公司 | Cxcr4抑制剂及其用途 |
| WO2017223243A1 (en) | 2016-06-21 | 2017-12-28 | X4 Pharmaceuticals, Inc. | Cxcr4 inhibitors and uses thereof |
| AU2017297506A1 (en) | 2016-07-14 | 2019-02-21 | Bristol-Myers Squibb Company | Antibodies against TIM3 and uses thereof |
| WO2018014260A1 (en) * | 2016-07-20 | 2018-01-25 | Nanjing Legend Biotech Co., Ltd. | Multispecific antigen binding proteins and methods of use thereof |
| EP3493842A4 (en) | 2016-08-02 | 2020-07-29 | President and Fellows of Harvard College | BIOMATERIALS TO MODULATE IMMUNE RESPONSES |
| US11875877B2 (en) | 2016-08-25 | 2024-01-16 | Nantomics, Llc | Immunotherapy markers and uses therefor |
| CN109906088A (zh) | 2016-08-26 | 2019-06-18 | 奥野哲治 | 微血管血流减少剂及其应用 |
| JP6956091B2 (ja) * | 2016-08-26 | 2021-10-27 | 哲治 奥野 | 微小ナノ化薬剤およびその利用 |
| WO2018053242A1 (en) | 2016-09-15 | 2018-03-22 | Idera Pharmaceuticals, Inc. | Immune modulation with tlr9 agonists for cancer treatment |
| EP3515486A4 (en) * | 2016-09-26 | 2020-05-20 | Advantagene, Inc. | METHOD FOR TREATING TIM-3 INCREASE |
| KR20240042177A (ko) * | 2016-10-05 | 2024-04-01 | 유니버시티 오브 센트럴 플로리다 리서치 파운데이션, 인코포레이티드 | Nk 세포 및 항-pdl1 암 요법과 관련된 방법 및 조성물 |
| EP3522923A1 (en) | 2016-10-06 | 2019-08-14 | Pfizer Inc | Dosing regimen of avelumab for the treatment of cancer |
| US20200163988A1 (en) * | 2016-10-07 | 2020-05-28 | Secarna Pharmaceuticals Gmbh & Co. Kg | Immunosuppression-Reverting Oligonucleotides Inhibiting the Expression of IDO |
| CA3037380A1 (en) | 2016-10-11 | 2018-04-19 | Agenus Inc. | Anti-lag-3 antibodies and methods of use thereof |
| WO2018068201A1 (en) | 2016-10-11 | 2018-04-19 | Nanjing Legend Biotech Co., Ltd. | Single-domain antibodies and variants thereof against ctla-4 |
| US20200038509A1 (en) * | 2016-10-14 | 2020-02-06 | Baylor College Of Medicine | Radiofrequency field hyperthermia and solid tumor immunomodulation |
| WO2018075447A1 (en) * | 2016-10-19 | 2018-04-26 | The Trustees Of Columbia University In The City Of New York | Combination of braf inhibitor, talimogene laherparepvec, and immune checkpoint inhibitor for use in the treatment cancer (melanoma) |
| MA46770A (fr) | 2016-11-09 | 2019-09-18 | Agenus Inc | Anticorps anti-ox40, anticorps anti-gitr, et leurs procédés d'utilisation |
| FI3551660T3 (fi) | 2016-12-07 | 2023-12-11 | Agenus Inc | Anti-ctla-4-vasta-aineita ja niiden käyttömenetelmiä |
| EP3551225A1 (en) | 2016-12-07 | 2019-10-16 | Agenus Inc. | Antibodies and methods of use thereof |
| CN118718020A (zh) * | 2016-12-09 | 2024-10-01 | 基因药物株式会社 | 含有表达细胞外基质的降解因子的重组腺病毒的抗癌组合物 |
| KR20190134591A (ko) * | 2016-12-15 | 2019-12-04 | 프로젠 피지500 시리즈 피티와이 리미티드 | 조성물 및 그의 용도 |
| CA3122548A1 (en) | 2016-12-23 | 2018-06-28 | Keio University | Compositions and methods for the induction of cd8+ t-cells |
| JP2020503363A (ja) | 2017-01-06 | 2020-01-30 | ビヨンドスプリング ファーマシューティカルズ,インコーポレイテッド | チューブリン結合化合物およびその治療的使用 |
| JP7244422B2 (ja) | 2017-01-11 | 2023-03-22 | ブリストル-マイヤーズ スクイブ カンパニー | Psgl-1アンタゴニスト及びその使用 |
| IL268305B2 (en) | 2017-02-01 | 2024-08-01 | Beyondspring Pharmaceuticals Inc | Plinabulin in combination with one or more g-csf drug for use in the therapeutic treatment of docetaxel-induced |
| US20200017586A1 (en) * | 2017-02-07 | 2020-01-16 | Memorial Sloan Kettering Cancer Center | Use of immune checkpoint modulators in combination with antigen-specific t cells in adoptive immunotherapy |
| WO2018147291A1 (en) * | 2017-02-07 | 2018-08-16 | Saitama Medical University | Immunological biomarker for predicting clinical effect of cancer immunotherapy |
| US11815435B2 (en) | 2017-02-24 | 2023-11-14 | Hibercell, Inc. | Beta glucan immunopharmacodynamics |
| EP3366703B1 (en) * | 2017-02-28 | 2019-04-03 | Ralf Kleef | Immune checkpoint therapy with hyperthermia |
| IL268919B2 (en) * | 2017-03-03 | 2023-10-01 | Treos Bio Zrt | A personalized immunogenic peptide identification platform |
| JP7148151B2 (ja) * | 2017-03-08 | 2022-10-05 | イェール ユニバーシティー | 抗レナラーゼ抗体および抗pd-1抗体を用いる、がんを処置するための組成物および方法 |
| SG11201907848YA (en) | 2017-03-14 | 2019-09-27 | Five Prime Therapeutics Inc | Antibodies binding to vista at acidic ph |
| CN107082812B (zh) | 2017-03-29 | 2018-11-13 | 上海科医联创生物科技有限公司 | 一种恢复衰竭性免疫细胞功能的融合蛋白及其应用 |
| CN108728440A (zh) * | 2017-04-25 | 2018-11-02 | 上海吉倍生物技术有限公司 | Ctla-4基因的用途及相关药物 |
| UY37695A (es) * | 2017-04-28 | 2018-11-30 | Novartis Ag | Compuesto dinucleótido cíclico bis 2’-5’-rr-(3’f-a)(3’f-a) y usos del mismo |
| GB2562721A (en) * | 2017-05-16 | 2018-11-28 | Fastbase Solutions Ltd | Kits, methods and their uses for detecting cell-cell interactions in a sample |
| EP3630838A1 (en) * | 2017-06-01 | 2020-04-08 | CytomX Therapeutics, Inc. | Activatable anti-pdl1 antibodies, and methods of use thereof |
| WO2019011879A1 (en) * | 2017-07-09 | 2019-01-17 | Rainer Henning | THERAPEUTIC AGENT FOR THE TREATMENT OF CAPILLARY LEAK SYNDROME |
| EP3658914A1 (en) * | 2017-07-28 | 2020-06-03 | Bristol-Myers Squibb Company | Predictive peripheral blood biomarker for checkpoint inhibitors |
| US12370256B2 (en) | 2017-08-03 | 2025-07-29 | Regents Of The University Of Minnesota | Activation of resident memory T cells for the treatment of cancer |
| WO2019036438A1 (en) * | 2017-08-14 | 2019-02-21 | Phosphorex, Inc. | ADENOSINE RECEPTOR ANTAGONIST MICROPARTICLE FORMULATIONS FOR THE TREATMENT OF CANCER |
| UA128472C2 (uk) | 2017-08-25 | 2024-07-24 | Файв Прайм Терапеутікс Інк. | B7-h4 антитіла і методи їх використання |
| JP7236385B2 (ja) * | 2017-09-26 | 2023-03-09 | 中外製薬株式会社 | 医薬組成物 |
| WO2019070850A1 (en) | 2017-10-03 | 2019-04-11 | Crititech, Inc. | LOCAL ADMINISTRATION OF ANINEOPLASTIC PARTICLES IN COMBINATION WITH SYSTEMIC DELIVERY OF IMMUNOTHERAPEUTIC AGENTS FOR THE TREATMENT OF CANCER |
| EA202090746A1 (ru) | 2017-11-03 | 2020-08-17 | Ориджен Дискавери Текнолоджис Лимитед | Двойные ингибиторы путей tim-3 и pd-1 |
| CN111386128A (zh) | 2017-11-06 | 2020-07-07 | 奥瑞基尼探索技术有限公司 | 用于免疫调节的联合疗法 |
| CA3082830A1 (en) * | 2017-11-21 | 2019-05-31 | Icahn School Of Medicine At Mount Sinai | Promoting trained immunity with therapeutic nanobiologic compositions |
| WO2019122941A1 (en) * | 2017-12-21 | 2019-06-27 | Debiopharm International Sa | Combination anti cancer therapy with an iap antagonist and an anti pd-1 molecule |
| CN117050184A (zh) | 2017-12-28 | 2023-11-14 | 南京传奇生物科技有限公司 | 针对tigit的单域抗体和其变体 |
| WO2019137384A1 (en) * | 2018-01-09 | 2019-07-18 | Microbio Co., Ltd. | Method of activating tumor-infiltrating lymphocytes (tils) |
| JP7366908B2 (ja) | 2018-01-15 | 2023-10-23 | ナンジン レジェンド バイオテック カンパニー,リミテッド | Pd-1に対する単一ドメイン抗体及びその変異体 |
| US11786523B2 (en) | 2018-01-24 | 2023-10-17 | Beyondspring Pharmaceuticals, Inc. | Composition and method for reducing thrombocytopenia |
| MA51673B1 (fr) | 2018-01-26 | 2025-10-31 | Exelixis, Inc. | Composés pour le traitement des troubles dépendants de kinases |
| AU2019212800B2 (en) | 2018-01-26 | 2024-05-23 | Exelixis, Inc. | Compounds for the treatment of kinase-dependent disorders |
| SG11202006945PA (en) | 2018-01-26 | 2020-08-28 | Exelixis Inc | Compounds for the treatment of kinase-dependent disorders |
| US20220033490A1 (en) * | 2018-01-31 | 2022-02-03 | Flagship Pioneering Innovations V, Inc. | Methods and compositions for treating cancer using chrna6 inhibitors |
| WO2019149884A1 (en) * | 2018-02-02 | 2019-08-08 | Murray & Poole Enterprises, Ltd | Use of colchicine to inhibit tumor growth and metastases |
| CN111886333A (zh) * | 2018-02-09 | 2020-11-03 | 学校法人庆应义塾 | 诱导cd8+t细胞的组合物和方法 |
| BR112020016986A2 (pt) | 2018-02-21 | 2021-03-02 | Five Prime Therapeutics, Inc. | formulações de anticorpo contra b7-h4 |
| RU2756899C1 (ru) | 2018-02-28 | 2021-10-06 | ЭйПи БАЙОСАЙЕНСИЗ, ИНК. | Бифункциональные белки, комбинирующие блокаду контрольной точки, для таргетной терапии |
| CA3091801A1 (en) | 2018-03-02 | 2019-09-06 | Five Prime Therapeutics, Inc. | B7-h4 antibodies and methods of use thereof |
| US20210379024A1 (en) | 2018-03-14 | 2021-12-09 | Aurigene Discovery Technologies Limited | Method of modulating tigit and pd-1 signalling pathways using 1,2,4-oxadiazole compounds |
| AU2019322487B2 (en) | 2018-03-19 | 2024-04-18 | Multivir Inc. | Methods and compositions comprising tumor suppressor gene therapy and CD122/CD132 agonists for the treatment of cancer |
| MX2020009786A (es) | 2018-03-21 | 2020-10-12 | Five Prime Therapeutics Inc | Anticuerpos de union a supresor de la activacion de celulas t que contiene el dominio variable de inmunoglobulina (vista) a ph acido. |
| WO2019183924A1 (en) | 2018-03-30 | 2019-10-03 | Syz Cell Therapy Co. | Improved multiple antigen specific cell therapy methods |
| WO2019195759A1 (en) * | 2018-04-05 | 2019-10-10 | Mayo Foundation For Medical Education And Research | Materials and methods for treating cancer |
| WO2019195753A1 (en) | 2018-04-05 | 2019-10-10 | Tolero Pharmaceuticals, Inc. | Axl kinase inhibitors and use of the same |
| TWI787500B (zh) | 2018-04-23 | 2022-12-21 | 美商南特細胞公司 | 新抗原表位疫苗及免疫刺激組合物及方法 |
| US11564980B2 (en) | 2018-04-23 | 2023-01-31 | Nantcell, Inc. | Tumor treatment method with an individualized peptide vaccine |
| EP3566718A1 (en) | 2018-05-07 | 2019-11-13 | Universitätsmedizin der Johannes Gutenberg-Universität Mainz | A pharmazeutical combination (treg depleting agent, checkpoint inhibitor, tlr9 agonist) for use in the treatment of cancer |
| CN112203688A (zh) * | 2018-05-31 | 2021-01-08 | 小野药品工业株式会社 | 用于确定免疫检查点抑制剂的有效性的生物标志物 |
| IL278740B2 (en) * | 2018-06-01 | 2024-01-01 | Eisai R&D Man Co Ltd | Methods for using splicing modulators |
| CN112292126A (zh) * | 2018-06-01 | 2021-01-29 | 塔弗达治疗有限公司 | 联合疗法 |
| EP3813803A1 (en) * | 2018-06-08 | 2021-05-05 | Harrow IP, LLC | Pyrimethamine-based pharmaceutical compositions and methods for fabricating thereof |
| US11266615B2 (en) | 2018-06-08 | 2022-03-08 | Harrow Ip, Llc | Pyrimethamine-based pharmaceutical compositions and methods for fabricating thereof |
| JP7329860B2 (ja) | 2018-06-15 | 2023-08-21 | ボード オブ レジェンツ,ザ ユニバーシティ オブ テキサス システム | S-エクオールを用いた乳癌の治療及び予防方法 |
| AU2019285641A1 (en) | 2018-06-15 | 2021-04-08 | The Board Of Regents Of The University Of Texas System | Methods of treating and preventing melanoma with s-equol |
| US12091462B2 (en) | 2018-07-11 | 2024-09-17 | Five Prime Therapeutics, Inc. | Antibodies binding to vista at acidic pH |
| US20210170005A1 (en) * | 2018-08-15 | 2021-06-10 | University Of Florida Research Foundation, Inc. | Methods of sensitizing tumors to treatment with immune checkpoint inhibitors |
| WO2020047319A1 (en) * | 2018-08-29 | 2020-03-05 | Shattuck Labs, Inc. | Combination therapies comprising sirp alpha-based chimeric proteins |
| US10548889B1 (en) | 2018-08-31 | 2020-02-04 | X4 Pharmaceuticals, Inc. | Compositions of CXCR4 inhibitors and methods of preparation and use |
| WO2020061129A1 (en) | 2018-09-19 | 2020-03-26 | President And Fellows Of Harvard College | Compositions and methods for labeling and modulation of cells in vitro and in vivo |
| BR112021005540A2 (pt) * | 2018-09-24 | 2021-06-29 | Biovaxys Llc | vacinas autólogas bi-haptenizadas e seus usos |
| WO2020091944A1 (en) * | 2018-10-31 | 2020-05-07 | Nantomics, Llc | Genomic and immune infiltration differences between msi and mss gi tumors |
| WO2020092839A1 (en) | 2018-10-31 | 2020-05-07 | Mayo Foundation For Medical Education And Research | Methods and materials for treating cancer |
| EP3873540A4 (en) | 2018-10-31 | 2022-07-27 | Mayo Foundation for Medical Education and Research | METHODS AND MATERIALS FOR THE TREATMENT OF CANCER |
| WO2020092589A1 (en) * | 2018-10-31 | 2020-05-07 | Nantomics, Llc | Immune checkpoint therapeutic methods |
| JP2022507495A (ja) * | 2018-11-16 | 2022-01-18 | アーキュール・インコーポレイテッド | 癌治療のための医薬の組合せ |
| EP3893925A4 (en) * | 2018-12-13 | 2023-01-18 | Rhode Island Hospital | INHIBITING THE GROWTH AND DEVELOPMENT OF ASPH-EXPRESSING TUMORS |
| CA3123338A1 (en) * | 2018-12-21 | 2020-06-25 | Ose Immunotherapeutics | Bifunctional anti-pd-1/il-7 molecule |
| TWI852977B (zh) | 2019-01-10 | 2024-08-21 | 美商健生生物科技公司 | 前列腺新抗原及其用途 |
| EP3921851B1 (en) | 2019-02-06 | 2023-10-18 | Cornell University | Darc expression as prognosticator of immunotherapy outcomes |
| JP2022519727A (ja) * | 2019-02-08 | 2022-03-24 | エイチ リー モフィット キャンサー センター アンド リサーチ インスティテュート インコーポレイテッド | Sirt2除去型キメラt細胞 |
| JP7606225B2 (ja) | 2019-02-20 | 2024-12-25 | 学校法人 埼玉医科大学 | 放射線治療による抗腫瘍免疫効果を評価する末梢血バイオマーカー |
| EP3930719B1 (en) * | 2019-02-27 | 2025-08-20 | Takeda Pharmaceutical Company Limited | Administration of sumo-activating enzyme inhibitor and checkpoint inhibitors |
| CN109771445B (zh) * | 2019-03-20 | 2022-08-05 | 青岛东海药业有限公司 | 酪酸梭菌在制备诱导抗肿瘤免疫及免疫检查点抑制剂增敏制剂中的应用 |
| CN111751545A (zh) * | 2019-03-28 | 2020-10-09 | 中国科学院上海药物研究所 | 一种筛选pd-l1/pd-1检测点抑制剂的方法 |
| MX2021011654A (es) * | 2019-04-03 | 2022-02-21 | Targimmune Therapeutics Ag | Inmunoterapia para el tratamiento del cancer. |
| EP3721899A1 (en) * | 2019-04-08 | 2020-10-14 | China Medical University | Combination therapies comprising dendritic cells-based vaccine and immune checkpoint inhibitor |
| WO2020215037A1 (en) | 2019-04-18 | 2020-10-22 | The Regents Of The University Of Michigan | Combination with checkpoint inhibitors to treat cancer |
| WO2020246846A1 (ko) * | 2019-06-05 | 2020-12-10 | 연세대학교 산학협력단 | Tox에 기초한 면역 항암 요법에 대한 치료 반응 예측 방법 |
| US12308095B1 (en) | 2019-06-11 | 2025-05-20 | Nantbio, Inc. | Prediction of computational pathway circuits |
| AU2020298454A1 (en) * | 2019-06-30 | 2022-01-27 | Memorial Sloan Kettering Cancer Center | Methods and compositions for treatment of pancreatic cancer |
| US11364291B1 (en) | 2019-07-18 | 2022-06-21 | Nantcell, Inc. | Bacillus Calmette-Guerin (BCG) and antigen presenting cells for treatment of bladder cancer |
| WO2021034774A1 (en) * | 2019-08-16 | 2021-02-25 | H. Lee Moffitt Cancer Center And Research Institute, Inc. | Fucosylation and immune modulation in cancer |
| TWI776276B (zh) * | 2019-11-13 | 2022-09-01 | 中國醫藥大學 | 異種組織細胞組合物治療癌症之用途 |
| EP4061405A1 (en) | 2019-11-18 | 2022-09-28 | Janssen Biotech, Inc. | Vaccines based on mutant calr and jak2 and their uses |
| MX2022008216A (es) * | 2020-01-02 | 2022-08-04 | Merck Sharp & Dohme Llc | Tratamiento de cancer en combinacion usando un antagonista de pd-1, un antagonista de ilt4 y lenvatinib o sales del mismo. |
| JP2023515675A (ja) * | 2020-03-05 | 2023-04-13 | メルク・シャープ・アンド・ドーム・エルエルシー | Pd-1アンタゴニスト、ctla4アンタゴニストおよびレンバチニブまたはその薬学的に許容される塩の組合せを用いる癌を治療する方法 |
| EP4117662A4 (en) | 2020-03-10 | 2024-04-03 | X4 Pharmaceuticals, Inc. | METHODS OF TREATING NEUTROPENIA |
| EP4140495A4 (en) | 2020-03-18 | 2024-05-01 | GI Innovation, Inc. | Pharmaceutical composition for cancer treatment comprising fusion protein including il-2 protein and cd80 protein and anticancer drug |
| EP4175664A2 (en) | 2020-07-06 | 2023-05-10 | Janssen Biotech, Inc. | Prostate neoantigens and their uses |
| CN117355298A (zh) | 2021-03-19 | 2024-01-05 | 生物治疗探索股份有限公司 | 用于调节训练免疫的化合物及其使用方法 |
| JP2024513505A (ja) | 2021-04-09 | 2024-03-25 | ビヨンドスプリング ファーマシューティカルズ,インコーポレイテッド | 腫瘍を治療するための組成物及び方法 |
| JP7018531B1 (ja) | 2021-04-30 | 2022-02-10 | 潤 齋藤 | Axl阻害剤 |
| JP2024545147A (ja) * | 2021-12-10 | 2024-12-05 | ▲應▼世生物科技(南京)有限公司 | 腫瘍治療の医薬組合せ及び使用 |
| JP2025087939A (ja) * | 2022-04-13 | 2025-06-11 | アステラス製薬株式会社 | がん治療におけるpd-1シグナル阻害剤との組み合わせによる抗tspan8-抗cd3二重特異性抗体の使用 |
| WO2023203561A1 (en) * | 2022-04-19 | 2023-10-26 | Enlivex Therapeutics Rdo Ltd | Apoptotic cell - check point inhibitor combination therapy |
| CN117050177B (zh) * | 2023-08-25 | 2024-03-08 | 遵义北科融汇生命科技有限公司 | 血液分离的免疫细胞联合药物治疗癌症的用途 |
Family Cites Families (22)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5892019A (en) | 1987-07-15 | 1999-04-06 | The United States Of America, As Represented By The Department Of Health And Human Services | Production of a single-gene-encoded immunoglobulin |
| US5851795A (en) | 1991-06-27 | 1998-12-22 | Bristol-Myers Squibb Company | Soluble CTLA4 molecules and uses thereof |
| US6051227A (en) | 1995-07-25 | 2000-04-18 | The Regents Of The University Of California, Office Of Technology Transfer | Blockade of T lymphocyte down-regulation associated with CTLA-4 signaling |
| US5811097A (en) | 1995-07-25 | 1998-09-22 | The Regents Of The University Of California | Blockade of T lymphocyte down-regulation associated with CTLA-4 signaling |
| US5855887A (en) | 1995-07-25 | 1999-01-05 | The Regents Of The University Of California | Blockade of lymphocyte down-regulation associated with CTLA-4 signaling |
| WO1998042752A1 (en) | 1997-03-21 | 1998-10-01 | Brigham And Women's Hospital Inc. | Immunotherapeutic ctla-4 binding peptides |
| WO2000037504A2 (en) | 1998-12-23 | 2000-06-29 | Pfizer Inc. | Human monoclonal antibodies to ctla-4 |
| US7109003B2 (en) | 1998-12-23 | 2006-09-19 | Abgenix, Inc. | Methods for expressing and recovering human monoclonal antibodies to CTLA-4 |
| EE05627B1 (et) | 1998-12-23 | 2013-02-15 | Pfizer Inc. | CTLA-4 vastased inimese monoklonaalsed antikehad |
| US7605238B2 (en) | 1999-08-24 | 2009-10-20 | Medarex, Inc. | Human CTLA-4 antibodies and their uses |
| EP1212422B1 (en) | 1999-08-24 | 2007-02-21 | Medarex, Inc. | Human ctla-4 antibodies and their uses |
| JP2003520828A (ja) | 2000-01-27 | 2003-07-08 | ジェネティクス インスティテュート,エルエルシー | Ctla4(cd152)に対する抗体、これを含む結合体、およびその使用 |
| NZ536420A (en) * | 2002-04-12 | 2008-04-30 | Medarex Inc | Methods of treatment using CTLA-4 antibodies |
| WO2004035607A2 (en) | 2002-10-17 | 2004-04-29 | Genmab A/S | Human monoclonal antibodies against cd20 |
| WO2004053072A2 (en) | 2002-12-06 | 2004-06-24 | Northwest Biotherapeutics, Inc. | Administration of dendritic cells partially matured in vitro for the treatment of tumors |
| GB0406598D0 (en) * | 2004-03-24 | 2004-04-28 | Univ Leicester | Vaccine |
| CN109485727A (zh) * | 2005-05-09 | 2019-03-19 | 小野药品工业株式会社 | 程序性死亡-1(pd-1)的人单克隆抗体及使用抗pd-1抗体来治疗癌症的方法 |
| NZ600758A (en) * | 2007-06-18 | 2013-09-27 | Merck Sharp & Dohme | Antibodies to human programmed death receptor pd-1 |
| GB0920258D0 (en) * | 2009-11-19 | 2010-01-06 | Alligator Bioscience Ab | New medical agents and use thereof |
| US9220776B2 (en) * | 2011-03-31 | 2015-12-29 | Merck Sharp & Dohme Corp. | Stable formulations of antibodies to human programmed death receptor PD-1 and related treatments |
| EP2723381A4 (en) * | 2011-06-21 | 2015-03-18 | Univ Johns Hopkins | FOCUSED RADIATION FOR THE REINFORCEMENT OF IMMUNE TREATMENTS AGAINST NEOPLASMS |
| US9856320B2 (en) * | 2012-05-15 | 2018-01-02 | Bristol-Myers Squibb Company | Cancer immunotherapy by disrupting PD-1/PD-L1 signaling |
-
2014
- 2014-11-05 KR KR1020167014867A patent/KR20160093012A/ko not_active Withdrawn
- 2014-11-05 EA EA201690912A patent/EA201690912A1/ru unknown
- 2014-11-05 EP EP24190410.1A patent/EP4461372A3/en active Pending
- 2014-11-05 ES ES14859634T patent/ES2991853T3/es active Active
- 2014-11-05 BR BR112016010224-0A patent/BR112016010224A2/pt not_active Application Discontinuation
- 2014-11-05 US US14/534,158 patent/US20150202291A1/en not_active Abandoned
- 2014-11-05 CA CA2929407A patent/CA2929407A1/en active Pending
- 2014-11-05 JP JP2016552469A patent/JP2016540042A/ja active Pending
- 2014-11-05 CN CN201480070071.7A patent/CN105828834A/zh active Pending
- 2014-11-05 EP EP14859634.9A patent/EP3065772B1/en active Active
- 2014-11-05 IL IL318946A patent/IL318946A/en unknown
- 2014-11-05 AU AU2014346852A patent/AU2014346852A1/en not_active Abandoned
- 2014-11-05 US US14/533,879 patent/US20150273033A1/en not_active Abandoned
- 2014-11-05 WO PCT/US2014/064133 patent/WO2015069770A1/en not_active Ceased
- 2014-11-05 MX MX2016005925A patent/MX2016005925A/es unknown
- 2014-11-05 IL IL292510A patent/IL292510A/en unknown
- 2014-11-05 HK HK17102406.5A patent/HK1232119A1/zh unknown
-
2016
- 2016-05-01 IL IL245337A patent/IL245337B/en unknown
- 2016-05-04 MX MX2022006726A patent/MX2022006726A/es unknown
- 2016-05-05 PH PH12016500841A patent/PH12016500841A1/en unknown
-
2023
- 2023-10-18 US US18/489,222 patent/US20240358807A1/en active Pending
-
2024
- 2024-03-25 US US18/615,955 patent/US20240382572A1/en active Pending
Cited By (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US11229668B2 (en) | 2017-02-07 | 2022-01-25 | Nantcell, Inc. | Maximizing T-cell memory and compositions and methods therefor |
| US12491201B2 (en) | 2017-02-07 | 2025-12-09 | Immunitybio, Inc. | Maximizing T-cell memory and compositions and methods therefor |
| KR20210022669A (ko) * | 2018-06-19 | 2021-03-03 | 사이토베이션 에이에스 | 펩타이드를 사용하는 병용 요법 |
Also Published As
| Publication number | Publication date |
|---|---|
| US20150273033A1 (en) | 2015-10-01 |
| MX2016005925A (es) | 2016-11-28 |
| EP3065772A4 (en) | 2017-09-13 |
| WO2015069770A1 (en) | 2015-05-14 |
| IL292510A (en) | 2022-06-01 |
| HK1232119A1 (zh) | 2018-01-05 |
| CA2929407A1 (en) | 2015-05-14 |
| EP4461372A3 (en) | 2025-01-22 |
| CN105828834A (zh) | 2016-08-03 |
| MX2022006726A (es) | 2022-06-09 |
| ES2991853T3 (es) | 2024-12-05 |
| IL245337A0 (en) | 2016-06-30 |
| US20150202291A1 (en) | 2015-07-23 |
| EA201690912A1 (ru) | 2016-10-31 |
| JP2016540042A (ja) | 2016-12-22 |
| EP4461372A2 (en) | 2024-11-13 |
| EP3065772B1 (en) | 2024-07-24 |
| EP3065772A1 (en) | 2016-09-14 |
| IL245337B (en) | 2022-05-01 |
| AU2014346852A1 (en) | 2016-06-16 |
| BR112016010224A2 (pt) | 2018-05-02 |
| US20240358807A1 (en) | 2024-10-31 |
| IL318946A (en) | 2025-04-01 |
| US20240382572A1 (en) | 2024-11-21 |
| PH12016500841A1 (en) | 2016-07-04 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US20240382572A1 (en) | Combinations of checkpoint inhibitors and therapeutics to treat cancer | |
| JP6936221B2 (ja) | Cd80細胞外ドメインポリペプチドと、がん治療でのそれらの使用 | |
| JP6879996B2 (ja) | 悪性腫瘍の治療方法 | |
| EP2766035B1 (en) | Combination medicament comprising il-12 and an agent for blockade of t-cell inhibitory molecules for tumour therapy | |
| Skak et al. | Interleukin 21: combination strategies for cancer therapy | |
| EP3057990B1 (en) | Compositions comprising a combination of a vegf antagonist and an anti-ctla-4 antibody | |
| KR20170072928A (ko) | 조합물 | |
| JP2015528514A (ja) | 癌の処置における特異的免疫療法を向上する方法 | |
| US11986538B2 (en) | Immunoswitch nanoparticles for reprogrammed T cell responses | |
| Mivehchi et al. | Microenvironment-based immunotherapy in oral cancer: a comprehensive review | |
| CN109937051A (zh) | 治疗tim-3升高的方法 | |
| JP2021523098A (ja) | T細胞による認識を強化するよう抗原性を調節する方法 | |
| JP7407452B2 (ja) | がんの治療及び/又は予防のための医薬 | |
| Brito | Cancer Immunotherapy: Current Landscape and Future Directions | |
| Brito | CANCER IMMUNOTHERAPY | |
| WO2024102874A1 (en) | Fenofibrate improves t-cell therapies | |
| US20220047701A1 (en) | Combination of her2/neu antibody with heme for treating cancer | |
| WO2023203561A1 (en) | Apoptotic cell - check point inhibitor combination therapy | |
| EP4149512A1 (en) | Methods for treating glioblastoma | |
| NZ750663A (en) | Compositions and methods for cancer immunotherapy | |
| NZ750663B2 (en) | Compositions and methods for cancer immunotherapy | |
| HK1227888A1 (en) | Compositions comprising a combination of a vegf antagonist and an anti-ctla-4 antibody | |
| HK1227888B (en) | Compositions comprising a combination of a vegf antagonist and an anti-ctla-4 antibody |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PA0105 | International application |
Patent event date: 20160603 Patent event code: PA01051R01D Comment text: International Patent Application |
|
| PG1501 | Laying open of application | ||
| PC1203 | Withdrawal of no request for examination | ||
| WITN | Application deemed withdrawn, e.g. because no request for examination was filed or no examination fee was paid |