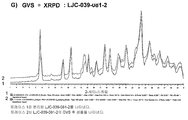
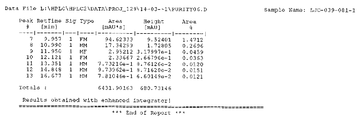
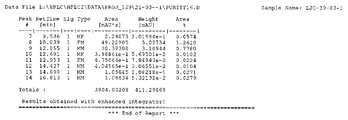
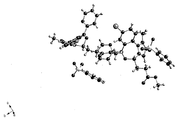
KR20150081370A - 속효형 벤조디아제핀 염 및 이의 중합체 형태 - Google Patents
속효형 벤조디아제핀 염 및 이의 중합체 형태 Download PDFInfo
- Publication number
- KR20150081370A KR20150081370A KR1020157016814A KR20157016814A KR20150081370A KR 20150081370 A KR20150081370 A KR 20150081370A KR 1020157016814 A KR1020157016814 A KR 1020157016814A KR 20157016814 A KR20157016814 A KR 20157016814A KR 20150081370 A KR20150081370 A KR 20150081370A
- Authority
- KR
- South Korea
- Prior art keywords
- salt
- formula
- compound
- type
- subject
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Ceased
Links
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D401/00—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom
- C07D401/02—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom containing two hetero rings
- C07D401/04—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom containing two hetero rings directly linked by a ring-member-to-ring-member bond
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/435—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with one nitrogen as the only ring hetero atom
- A61K31/44—Non condensed pyridines; Hydrogenated derivatives thereof
- A61K31/4427—Non condensed pyridines; Hydrogenated derivatives thereof containing further heterocyclic ring systems
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/55—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having seven-membered rings, e.g. azelastine, pentylenetetrazole
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/55—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having seven-membered rings, e.g. azelastine, pentylenetetrazole
- A61K31/551—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having seven-membered rings, e.g. azelastine, pentylenetetrazole having two nitrogen atoms, e.g. dilazep
- A61K31/5513—1,4-Benzodiazepines, e.g. diazepam or clozapine
- A61K31/5517—1,4-Benzodiazepines, e.g. diazepam or clozapine condensed with five-membered rings having nitrogen as a ring hetero atom, e.g. imidazobenzodiazepines, triazolam
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/0012—Galenical forms characterised by the site of application
- A61K9/0019—Injectable compositions; Intramuscular, intravenous, arterial, subcutaneous administration; Compositions to be administered through the skin in an invasive manner
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P21/00—Drugs for disorders of the muscular or neuromuscular system
- A61P21/02—Muscle relaxants, e.g. for tetanus or cramps
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
- A61P25/08—Antiepileptics; Anticonvulsants
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
- A61P25/18—Antipsychotics, i.e. neuroleptics; Drugs for mania or schizophrenia
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
- A61P25/20—Hypnotics; Sedatives
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
- A61P25/22—Anxiolytics
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C309/00—Sulfonic acids; Halides, esters, or anhydrides thereof
- C07C309/01—Sulfonic acids
- C07C309/28—Sulfonic acids having sulfo groups bound to carbon atoms of six-membered aromatic rings of a carbon skeleton
- C07C309/29—Sulfonic acids having sulfo groups bound to carbon atoms of six-membered aromatic rings of a carbon skeleton of non-condensed six-membered aromatic rings
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D487/00—Heterocyclic compounds containing nitrogen atoms as the only ring hetero atoms in the condensed system, not provided for by groups C07D451/00 - C07D477/00
- C07D487/02—Heterocyclic compounds containing nitrogen atoms as the only ring hetero atoms in the condensed system, not provided for by groups C07D451/00 - C07D477/00 in which the condensed system contains two hetero rings
- C07D487/04—Ortho-condensed systems
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07B—GENERAL METHODS OF ORGANIC CHEMISTRY; APPARATUS THEREFOR
- C07B2200/00—Indexing scheme relating to specific properties of organic compounds
- C07B2200/07—Optical isomers
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07B—GENERAL METHODS OF ORGANIC CHEMISTRY; APPARATUS THEREFOR
- C07B2200/00—Indexing scheme relating to specific properties of organic compounds
- C07B2200/13—Crystalline forms, e.g. polymorphs
Landscapes
- Health & Medical Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Pharmacology & Pharmacy (AREA)
- Veterinary Medicine (AREA)
- Public Health (AREA)
- General Health & Medical Sciences (AREA)
- Animal Behavior & Ethology (AREA)
- Medicinal Chemistry (AREA)
- Life Sciences & Earth Sciences (AREA)
- Engineering & Computer Science (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Chemical Kinetics & Catalysis (AREA)
- General Chemical & Material Sciences (AREA)
- Neurology (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Neurosurgery (AREA)
- Biomedical Technology (AREA)
- Epidemiology (AREA)
- Pain & Pain Management (AREA)
- Orthopedic Medicine & Surgery (AREA)
- Physical Education & Sports Medicine (AREA)
- Dermatology (AREA)
- Psychiatry (AREA)
- Anesthesiology (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
- Nitrogen Condensed Heterocyclic Rings (AREA)
- Plural Heterocyclic Compounds (AREA)
- Organic Low-Molecular-Weight Compounds And Preparation Thereof (AREA)
- Developing Agents For Electrophotography (AREA)
- Steroid Compounds (AREA)
Applications Claiming Priority (5)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| GB0613692A GB0613692D0 (en) | 2006-07-10 | 2006-07-10 | Benzodiazepine salts |
| GB0613694A GB0613694D0 (en) | 2006-07-10 | 2006-07-10 | Benzodiazepine salts |
| GB0613694.9 | 2006-07-10 | ||
| GB0613692.3 | 2006-07-10 | ||
| PCT/GB2007/002565 WO2008007071A1 (en) | 2006-07-10 | 2007-07-10 | Short-acting benzodiazepine salts and their polymorphic forms |
Related Parent Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020097002747A Division KR20090045233A (ko) | 2006-07-10 | 2007-07-10 | 속효형 벤조디아제핀 염 및 이의 중합체 형태 |
Related Child Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020177021356A Division KR20170091770A (ko) | 2006-07-10 | 2007-07-10 | 속효형 벤조디아제핀 염 및 이의 중합체 형태 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| KR20150081370A true KR20150081370A (ko) | 2015-07-13 |
Family
ID=38535381
Family Applications (3)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020157016814A Ceased KR20150081370A (ko) | 2006-07-10 | 2007-07-10 | 속효형 벤조디아제핀 염 및 이의 중합체 형태 |
| KR1020177021356A Ceased KR20170091770A (ko) | 2006-07-10 | 2007-07-10 | 속효형 벤조디아제핀 염 및 이의 중합체 형태 |
| KR1020097002747A Ceased KR20090045233A (ko) | 2006-07-10 | 2007-07-10 | 속효형 벤조디아제핀 염 및 이의 중합체 형태 |
Family Applications After (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020177021356A Ceased KR20170091770A (ko) | 2006-07-10 | 2007-07-10 | 속효형 벤조디아제핀 염 및 이의 중합체 형태 |
| KR1020097002747A Ceased KR20090045233A (ko) | 2006-07-10 | 2007-07-10 | 속효형 벤조디아제핀 염 및 이의 중합체 형태 |
Country Status (17)
| Country | Link |
|---|---|
| US (7) | US9193730B2 (enExample) |
| EP (1) | EP2081921B1 (enExample) |
| JP (2) | JP5433413B2 (enExample) |
| KR (3) | KR20150081370A (enExample) |
| CN (3) | CN103288834B (enExample) |
| AT (1) | ATE480532T1 (enExample) |
| AU (1) | AU2007274054B2 (enExample) |
| BR (1) | BRPI0714886B8 (enExample) |
| CA (1) | CA2657347C (enExample) |
| DE (1) | DE602007009128D1 (enExample) |
| DK (1) | DK2081921T3 (enExample) |
| MX (1) | MX2009000404A (enExample) |
| PL (1) | PL2081921T3 (enExample) |
| PT (1) | PT2081921E (enExample) |
| RU (1) | RU2470935C2 (enExample) |
| SI (1) | SI2081921T1 (enExample) |
| WO (1) | WO2008007071A1 (enExample) |
Families Citing this family (22)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| GB0613693D0 (en) | 2006-07-10 | 2006-08-16 | Cenes Ltd | Benzodiazepine salts (3) |
| DK2081921T3 (da) | 2006-07-10 | 2011-01-03 | Paion Uk Ltd | Korttidsvirkende benzodiazepinsalte og deres polymorfe former |
| EP2305647A1 (en) * | 2009-09-18 | 2011-04-06 | PAION UK Limited | Process for preparing 3-[(4S)-8-bromo-1-methyl-6-(2-pyridinyl)-4 H-imidazo[1,2-a][1,4]benzodiazepine-4-yl] propionic acid methyl ester or the benzene sulfonate salt thereof, and compounds useful in that process |
| EP2450039A1 (en) | 2010-11-08 | 2012-05-09 | PAION UK Ltd. | Dosing regimen for sedation with CNS 7056 (Remimazolam) |
| PT2852389T (pt) | 2012-05-22 | 2017-12-13 | Paion Uk Ltd | Composições que compreendem benzodiazepinas de ação rápida |
| BR112015004477A8 (pt) * | 2012-08-31 | 2019-08-27 | Paion Uk Ltd | uso de composto na preparação de um sedativo e composição farmacêutica |
| AR094963A1 (es) | 2013-03-04 | 2015-09-09 | Ono Pharmaceutical Co | Reacción de oxidación excelente en el índice de conversión |
| PL3162804T3 (pl) * | 2014-07-23 | 2020-04-30 | Jiangsu Nhwaluokang Pharmaceutical Research And Development Co., Ltd. | Nowa pochodna benzodiazepiny i jej zastosowanie |
| US20170081954A1 (en) * | 2015-09-23 | 2017-03-23 | Tesco Corporation | Pipe joint location detection system and method |
| CN110511224A (zh) * | 2016-04-08 | 2019-11-29 | 四川科伦药物研究院有限公司 | 苯并二氮杂*衍生物的盐及其晶体形式、制备方法和用途 |
| WO2017178663A1 (en) * | 2016-04-14 | 2017-10-19 | Paion Uk Limited | Orally inhaled and nasal benzodiazepines |
| CN108503644B (zh) * | 2016-12-09 | 2019-06-14 | 成都倍特药业有限公司 | 一种苯并二氮杂*衍生物的氢溴酸盐及其制备方法和用途 |
| EP3580219B1 (en) | 2017-02-09 | 2022-04-13 | Assia Chemical Industries Ltd. | Process for the preparation of remimazolam and solid state forms of remimazolam salts |
| CN108948018B (zh) * | 2017-05-17 | 2021-03-30 | 四川科伦博泰生物医药股份有限公司 | 苯并二氮*衍生物及其盐和相关晶体形式、制备方法和用途 |
| US11708368B2 (en) | 2018-02-13 | 2023-07-25 | Jiangsu Nhwaluokang Pharmaceutical Research And Development Co., Ltd. | Crystal form of ethyl (S)-3-(8-bromo-1-methyl-6-(pyridin-2-yl)-4H-benzo[f]imidazo[1,2-a][1,4]diazepin-4-yl)propanoate hydrochloride |
| CN110398514B (zh) * | 2018-04-24 | 2023-04-28 | 恒天纤维集团有限公司 | 一种快速判断纤维素胶液中金属杂质含量的方法 |
| ES2803099B2 (es) | 2019-07-22 | 2021-11-08 | Moehs Iberica Sl | Procedimiento de obtención de besilato de remimazolam amorfo |
| CN114478535B (zh) * | 2020-10-23 | 2024-02-09 | 成都苑东生物制药股份有限公司 | 一种苯磺酸瑞马唑仑ii晶型的制备方法 |
| WO2023037237A1 (en) * | 2021-09-09 | 2023-03-16 | Wavelength Enterprises Ltd | Process for the preparation of remimazolam |
| EP4504731A1 (en) | 2022-04-08 | 2025-02-12 | Fresenius Kabi Oncology Ltd | An improved process for the preparation of remimazolam |
| CN115448927A (zh) * | 2022-10-20 | 2022-12-09 | 重庆瑞泊莱医药科技有限公司 | 一种氢溴酸依匹斯汀晶型ii及其制备方法 |
| WO2025052259A1 (en) | 2023-09-05 | 2025-03-13 | Reena Patel | Ready to use compositions of remimazolam |
Family Cites Families (69)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US3520877A (en) | 1967-10-02 | 1970-07-21 | Hoffmann La Roche | 3-carbonyl amino acetic acid ethyl ester substituted benzodiazepines |
| BR6915069D0 (pt) | 1969-02-28 | 1973-08-16 | Upjohn Co | Compostos organicos e processo |
| DK124481B (da) | 1969-09-01 | 1972-10-23 | Takeda Chemical Industries Ltd | Fremgangsmåde til fremstilling af 1,4-benzodiazepinderivater samt deres 4-N-oxyder og salte. |
| DE2156472A1 (de) | 1970-11-23 | 1972-05-31 | Ciba-Geigy Ag, Basel (Schweiz) | Verfahren zur Herstellung von neuen Diazepinderivaten |
| US3933794A (en) | 1971-08-04 | 1976-01-20 | The Upjohn Company | 2-(2-Alkynylamino)-3H-1,4-benzodiazepines |
| FR2183716A1 (en) | 1972-05-05 | 1973-12-21 | Centre Etd Ind Pharma | Substd-6-phenyl-4h-imidazo (1,2-a)-1,4-benzo diazepines - - tranquillisers anxiolytics,sedatives and muscle-relaxants |
| IE41197B1 (en) | 1973-05-29 | 1979-11-07 | Squibb & Sons Inc | 4h-s-triazolo (4,3-a)(1,5)benzodiazepin-5-ones |
| CH608234A5 (en) | 1974-08-30 | 1978-12-29 | Crc Ricerca Chim | Process for the preparation of lithium salts of 1,4-benzodiazepines |
| DE2900017A1 (de) | 1978-01-10 | 1979-07-12 | Clin Midy | 3-alkoxycarbonyl-benzodiazepin- derivate, verfahren zu ihrer herstellung und sie enthaltende arzneimittel |
| MC1528A1 (fr) | 1982-07-21 | 1984-04-13 | Hoffmann La Roche | Imidazobenzodiazepines |
| IT1165588B (it) | 1983-03-23 | 1987-04-22 | Medosan Ind Biochimi | 1,4 benzodiazepine terapeuticamente attive |
| US4820834A (en) | 1984-06-26 | 1989-04-11 | Merck & Co., Inc. | Benzodiazepine analogs |
| US4724237A (en) | 1984-06-26 | 1988-02-09 | Merck & Co., Inc. | 2-substituted-aminomethyl-1,4-benzodiazepines |
| CA1332410C (en) | 1984-06-26 | 1994-10-11 | Roger M. Freidinger | Benzodiazepine analogs |
| US4755508A (en) | 1984-06-26 | 1988-07-05 | Merck & Co., Inc. | Benzodiazepine analogs and use as antogonists of gastrin and cholecystokinin |
| DE3435973A1 (de) | 1984-10-01 | 1986-04-10 | Boehringer Ingelheim KG, 6507 Ingelheim | Diazepine enthaltende pharmazeutische zusammensetzungen mit paf-antagonistischer wirkung |
| ES2084577T3 (es) | 1986-10-24 | 1996-05-16 | Abbott Lab | Ensayo, trazadores, inmunogenos y anticuerpos para benzodiazepinas. |
| WO1989010127A1 (en) | 1988-04-29 | 1989-11-02 | Baker Cummins Pharmaceuticals, Inc. | Method of safely providing anesthesia or conscious sedation |
| US5019583A (en) | 1989-02-15 | 1991-05-28 | Glaxo Inc. | N-phenyl-N-(4-piperidinyl)amides useful as analgesics |
| JPH0374328A (ja) | 1989-08-04 | 1991-03-28 | Warner Lambert Co | 薬学的活性を有する中枢コレシストキニン拮抗剤 |
| DE122009000062I2 (de) | 1989-10-20 | 2011-01-13 | Otsuka Pharma Co Ltd | Benzoheterozyklische verbindungen |
| CA2032427A1 (en) | 1989-12-18 | 1991-06-19 | Mark G. Bock | Benzodiazepines analogs |
| IL96613A0 (en) | 1989-12-18 | 1991-09-16 | Merck & Co Inc | Pharmaceutical compositions containing benzodiazepine analogs |
| US5324726A (en) | 1989-12-18 | 1994-06-28 | Merck & Co., Inc. | Benzodiazepine analogs |
| US5220017A (en) | 1991-04-10 | 1993-06-15 | Merck & Co., Inc. | Cholecystokinin antagonists |
| US5185331A (en) | 1991-05-14 | 1993-02-09 | Merck & Co., Inc. | Triazolobenzodiazepines |
| EP0523845A3 (en) | 1991-06-14 | 1993-11-18 | Merck & Co Inc | New benzodiazepine analogs |
| GB9117852D0 (en) | 1991-08-19 | 1991-10-09 | Merck Sharp & Dohme | Therapeutic agents |
| CA2120939A1 (en) | 1991-10-11 | 1993-04-15 | Tetsuya Tahara | Therapeutic agent for osteoporosis and diazepine compound |
| AU678503B2 (en) | 1993-09-24 | 1997-05-29 | Takeda Chemical Industries Ltd. | Condensed heterocyclic compounds and their use as squalene synthetase inhibitors |
| CA2143246C (en) | 1994-03-16 | 2000-08-22 | Thierry Godel | Imidazodiazepines |
| US5834464A (en) | 1994-11-18 | 1998-11-10 | Merck & Co., Inc. | Imidazolinobenzodiazepines |
| BR9606823A (pt) | 1995-01-06 | 1997-12-30 | Hoffmann La Roche | Hidroximetil-imidazoazepinas e seus èsteres |
| WO1996023790A1 (de) | 1995-02-02 | 1996-08-08 | F. Hoffmann-La Roche Ag | Acetylimidazobenzodiazepine |
| UA57734C2 (uk) | 1996-05-07 | 2003-07-15 | Пфайзер Інк. | Комплекси включення арилгетероциклічних солей |
| JP2000510155A (ja) | 1996-06-28 | 2000-08-08 | メルク エンド カンパニー インコーポレーテッド | 医薬製剤 |
| US6635632B1 (en) | 1996-12-23 | 2003-10-21 | Athena Neurosciences, Inc. | Cycloalkyl, lactam, lactone and related compounds, pharmaceutical compositions comprising same, and methods for inhibiting β-amyloid peptide release and/or its synthesis by use of such compounds |
| EP0881235A1 (fr) | 1997-05-26 | 1998-12-02 | Societe De Conseils De Recherches Et D'applications Scientifiques (S.C.R.A.S.) | Nouveaux ylures de phosphore, leur préparation et leurs utilisations notamment en tant que bases fortes faiblement nucléophiles |
| US6440959B1 (en) | 1999-04-21 | 2002-08-27 | Hoffman-La Roche Inc. | Pyrazolobenzodiazepines |
| GB9911152D0 (en) * | 1999-05-14 | 1999-07-14 | Glaxo Group Ltd | Short-acting benzodiazepines |
| US7160880B1 (en) | 1999-05-14 | 2007-01-09 | Cenes Limited | Short-acting benzodiazepines |
| US7806886B2 (en) | 1999-06-03 | 2010-10-05 | Medtronic Minimed, Inc. | Apparatus and method for controlling insulin infusion with state variable feedback |
| EP1161949A1 (en) | 2000-06-09 | 2001-12-12 | Warner-Lambert Company | Use od diazepinoindoles for the treatment of chronic obstructive pulmonary disease |
| IL161134A0 (en) | 2002-02-28 | 2004-08-31 | Japan Tobacco Inc | Ester compound and medical use thereof |
| HU226410B1 (en) * | 2003-04-22 | 2008-11-28 | Egis Gyogyszergyar Nyilvanosan | Novel polymorphous forms of olanzapine hydrochlorides, process for producing them, use thereof and pharmaceutical compositions containing them |
| IN2012DN03921A (enExample) | 2004-02-11 | 2015-09-04 | Amylin Pharmaceuticals Inc | |
| US7687736B2 (en) | 2004-04-29 | 2010-03-30 | Smart Technologies Ulc | Tensioned touch panel and method of making same |
| SI21850A (sl) * | 2004-07-28 | 2006-02-28 | Krka, Tovarna Zdravil, D.D., Novo Mesto | Soli olanzapina in njihova pretvorba v prosto bazo olanzapina |
| RU2007117741A (ru) | 2004-10-13 | 2008-11-20 | Мерк энд Ко., Инк. (US) | Антагонисты рецептора cgrp |
| WO2006078554A2 (en) | 2005-01-18 | 2006-07-27 | Merck & Co., Inc. | Cgrp receptor antagonists |
| MX2007009915A (es) | 2005-02-15 | 2007-11-06 | Elan Pharma Int Ltd | Formulaciones en aerosol e inyectables de benzodiazepina nanoparticulada. |
| US20070040501A1 (en) | 2005-08-18 | 2007-02-22 | Aitken Bruce G | Method for inhibiting oxygen and moisture degradation of a device and the resulting device |
| GB0613692D0 (en) | 2006-07-10 | 2006-08-16 | Cenes Ltd | Benzodiazepine salts |
| DK2081921T3 (da) | 2006-07-10 | 2011-01-03 | Paion Uk Ltd | Korttidsvirkende benzodiazepinsalte og deres polymorfe former |
| GB0613693D0 (en) | 2006-07-10 | 2006-08-16 | Cenes Ltd | Benzodiazepine salts (3) |
| WO2008147815A1 (en) | 2007-05-22 | 2008-12-04 | Chemocentryx, Inc. | 3-(imidazolyl)-pyrazolo[3,4-b]pyridines |
| WO2009145323A1 (ja) | 2008-05-30 | 2009-12-03 | 日産化学工業株式会社 | 多環式化合物を用いるアルコールの酸化方法 |
| JP2012505843A (ja) | 2008-10-16 | 2012-03-08 | パイオン ユーケー リミテッド | 術後疼痛管理のための極性オピオイドの投与スキーム |
| US8865886B2 (en) | 2009-03-30 | 2014-10-21 | Nippon Paper Industries Co., Ltd. | Method for recovery/reuse of N-oxyl compound |
| EP2305647A1 (en) | 2009-09-18 | 2011-04-06 | PAION UK Limited | Process for preparing 3-[(4S)-8-bromo-1-methyl-6-(2-pyridinyl)-4 H-imidazo[1,2-a][1,4]benzodiazepine-4-yl] propionic acid methyl ester or the benzene sulfonate salt thereof, and compounds useful in that process |
| DK2496582T3 (en) | 2009-11-05 | 2016-03-21 | Glaxosmithkline Llc | Benzodiazepine-BROMDOMAeNEINHIBITOR. |
| JP5635275B2 (ja) | 2010-01-28 | 2014-12-03 | 日清紡ホールディングス株式会社 | アルコールの酸化方法 |
| EP2450039A1 (en) | 2010-11-08 | 2012-05-09 | PAION UK Ltd. | Dosing regimen for sedation with CNS 7056 (Remimazolam) |
| CN102964349A (zh) | 2011-08-31 | 2013-03-13 | 江苏恒瑞医药股份有限公司 | 苯并二氮杂*衍生物的托西酸盐及其多晶型、它们的制备方法和用途 |
| JP5585597B2 (ja) | 2012-01-27 | 2014-09-10 | 株式会社デンソー | 車輪位置検出装置およびそれを備えたタイヤ空気圧検出装置 |
| PT2852389T (pt) | 2012-05-22 | 2017-12-13 | Paion Uk Ltd | Composições que compreendem benzodiazepinas de ação rápida |
| BR112015004477A8 (pt) | 2012-08-31 | 2019-08-27 | Paion Uk Ltd | uso de composto na preparação de um sedativo e composição farmacêutica |
| CN107659350A (zh) | 2012-09-28 | 2018-02-02 | 华为技术有限公司 | 信道状态信息进程处理方法、网络设备和用户设备 |
| AR094963A1 (es) | 2013-03-04 | 2015-09-09 | Ono Pharmaceutical Co | Reacción de oxidación excelente en el índice de conversión |
-
2007
- 2007-07-10 DK DK07733504.0T patent/DK2081921T3/da active
- 2007-07-10 KR KR1020157016814A patent/KR20150081370A/ko not_active Ceased
- 2007-07-10 WO PCT/GB2007/002565 patent/WO2008007071A1/en not_active Ceased
- 2007-07-10 PL PL07733504T patent/PL2081921T3/pl unknown
- 2007-07-10 US US12/373,472 patent/US9193730B2/en active Active
- 2007-07-10 JP JP2009518952A patent/JP5433413B2/ja active Active
- 2007-07-10 CN CN201310166860.8A patent/CN103288834B/zh active Active
- 2007-07-10 AT AT07733504T patent/ATE480532T1/de active
- 2007-07-10 KR KR1020177021356A patent/KR20170091770A/ko not_active Ceased
- 2007-07-10 MX MX2009000404A patent/MX2009000404A/es active IP Right Grant
- 2007-07-10 AU AU2007274054A patent/AU2007274054B2/en active Active
- 2007-07-10 RU RU2009104311/04A patent/RU2470935C2/ru active
- 2007-07-10 PT PT07733504T patent/PT2081921E/pt unknown
- 2007-07-10 EP EP07733504A patent/EP2081921B1/en active Active
- 2007-07-10 SI SI200730415T patent/SI2081921T1/sl unknown
- 2007-07-10 KR KR1020097002747A patent/KR20090045233A/ko not_active Ceased
- 2007-07-10 CN CN201410147078.6A patent/CN104059071B/zh active Active
- 2007-07-10 CA CA2657347A patent/CA2657347C/en active Active
- 2007-07-10 DE DE602007009128T patent/DE602007009128D1/de active Active
- 2007-07-10 BR BRPI0714886A patent/BRPI0714886B8/pt active IP Right Grant
- 2007-07-10 CN CN201410147318.2A patent/CN104059072B/zh active Active
-
2012
- 2012-10-25 JP JP2012235328A patent/JP5785923B2/ja active Active
-
2015
- 2015-11-23 US US14/948,889 patent/US9777007B2/en active Active
-
2017
- 2017-09-13 US US15/703,945 patent/US9914738B2/en active Active
-
2018
- 2018-02-28 US US15/908,081 patent/US10472365B2/en active Active
-
2019
- 2019-10-10 US US16/598,876 patent/US10961250B2/en active Active
-
2021
- 2021-02-18 US US17/178,346 patent/US12365684B2/en active Active
-
2025
- 2025-06-20 US US19/244,560 patent/US20250382299A1/en active Pending
Also Published As
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| KR20150081370A (ko) | 속효형 벤조디아제핀 염 및 이의 중합체 형태 | |
| CN101501019B (zh) | 短效苯并二氮杂*盐及其多晶型 | |
| US8642588B2 (en) | Short-acting benzodiazepine salts and their polymorphic forms | |
| HK1189383B (en) | Short-acting benzodiazepine salts and their polymorphic forms | |
| HK1202113B (en) | Short-acting benzodiazepine salts and their polymorphic forms | |
| HK1202114B (en) | Short-acting benzodiazepine salts and their polymorphic forms | |
| HK1137739B (en) | Short-acting benzodiazepine salts and their polymorphic forms |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A107 | Divisional application of patent | ||
| PA0104 | Divisional application for international application |
St.27 status event code: A-0-1-A10-A17-div-PA0104 St.27 status event code: A-0-1-A10-A16-div-PA0104 |
|
| PG1501 | Laying open of application |
St.27 status event code: A-1-1-Q10-Q12-nap-PG1501 |
|
| A201 | Request for examination | ||
| PA0201 | Request for examination |
St.27 status event code: A-1-2-D10-D11-exm-PA0201 |
|
| E902 | Notification of reason for refusal | ||
| PE0902 | Notice of grounds for rejection |
St.27 status event code: A-1-2-D10-D21-exm-PE0902 |
|
| T11-X000 | Administrative time limit extension requested |
St.27 status event code: U-3-3-T10-T11-oth-X000 |
|
| T11-X000 | Administrative time limit extension requested |
St.27 status event code: U-3-3-T10-T11-oth-X000 |
|
| T13-X000 | Administrative time limit extension granted |
St.27 status event code: U-3-3-T10-T13-oth-X000 |
|
| T11-X000 | Administrative time limit extension requested |
St.27 status event code: U-3-3-T10-T11-oth-X000 |
|
| T13-X000 | Administrative time limit extension granted |
St.27 status event code: U-3-3-T10-T13-oth-X000 |
|
| T11-X000 | Administrative time limit extension requested |
St.27 status event code: U-3-3-T10-T11-oth-X000 |
|
| T13-X000 | Administrative time limit extension granted |
St.27 status event code: U-3-3-T10-T13-oth-X000 |
|
| T11-X000 | Administrative time limit extension requested |
St.27 status event code: U-3-3-T10-T11-oth-X000 |
|
| T13-X000 | Administrative time limit extension granted |
St.27 status event code: U-3-3-T10-T13-oth-X000 |
|
| T11-X000 | Administrative time limit extension requested |
St.27 status event code: U-3-3-T10-T11-oth-X000 |
|
| T13-X000 | Administrative time limit extension granted |
St.27 status event code: U-3-3-T10-T13-oth-X000 |
|
| T11-X000 | Administrative time limit extension requested |
St.27 status event code: U-3-3-T10-T11-oth-X000 |
|
| T13-X000 | Administrative time limit extension granted |
St.27 status event code: U-3-3-T10-T13-oth-X000 |
|
| T11-X000 | Administrative time limit extension requested |
St.27 status event code: U-3-3-T10-T11-oth-X000 |
|
| T13-X000 | Administrative time limit extension granted |
St.27 status event code: U-3-3-T10-T13-oth-X000 |
|
| T11-X000 | Administrative time limit extension requested |
St.27 status event code: U-3-3-T10-T11-oth-X000 |
|
| T13-X000 | Administrative time limit extension granted |
St.27 status event code: U-3-3-T10-T13-oth-X000 |
|
| T11-X000 | Administrative time limit extension requested |
St.27 status event code: U-3-3-T10-T11-oth-X000 |
|
| T13-X000 | Administrative time limit extension granted |
St.27 status event code: U-3-3-T10-T13-oth-X000 |
|
| T11-X000 | Administrative time limit extension requested |
St.27 status event code: U-3-3-T10-T11-oth-X000 |
|
| T13-X000 | Administrative time limit extension granted |
St.27 status event code: U-3-3-T10-T13-oth-X000 |
|
| T11-X000 | Administrative time limit extension requested |
St.27 status event code: U-3-3-T10-T11-oth-X000 |
|
| T13-X000 | Administrative time limit extension granted |
St.27 status event code: U-3-3-T10-T13-oth-X000 |
|
| T11-X000 | Administrative time limit extension requested |
St.27 status event code: U-3-3-T10-T11-oth-X000 |
|
| T13-X000 | Administrative time limit extension granted |
St.27 status event code: U-3-3-T10-T13-oth-X000 |
|
| T11-X000 | Administrative time limit extension requested |
St.27 status event code: U-3-3-T10-T11-oth-X000 |
|
| T13-X000 | Administrative time limit extension granted |
St.27 status event code: U-3-3-T10-T13-oth-X000 |
|
| AMND | Amendment | ||
| E13-X000 | Pre-grant limitation requested |
St.27 status event code: A-2-3-E10-E13-lim-X000 |
|
| P11-X000 | Amendment of application requested |
St.27 status event code: A-2-2-P10-P11-nap-X000 |
|
| P13-X000 | Application amended |
St.27 status event code: A-2-2-P10-P13-nap-X000 |
|
| E601 | Decision to refuse application | ||
| PE0601 | Decision on rejection of patent |
St.27 status event code: N-2-6-B10-B15-exm-PE0601 |
|
| T11-X000 | Administrative time limit extension requested |
St.27 status event code: U-3-3-T10-T11-oth-X000 |
|
| T13-X000 | Administrative time limit extension granted |
St.27 status event code: U-3-3-T10-T13-oth-X000 |
|
| AMND | Amendment | ||
| E13-X000 | Pre-grant limitation requested |
St.27 status event code: A-2-3-E10-E13-lim-X000 |
|
| J201 | Request for trial against refusal decision | ||
| P11-X000 | Amendment of application requested |
St.27 status event code: A-2-2-P10-P11-nap-X000 |
|
| P13-X000 | Application amended |
St.27 status event code: A-2-2-P10-P13-nap-X000 |
|
| PJ0201 | Trial against decision of rejection |
St.27 status event code: A-3-3-V10-V11-apl-PJ0201 |
|
| PA0104 | Divisional application for international application |
St.27 status event code: A-0-1-A10-A18-div-PA0104 St.27 status event code: A-0-1-A10-A16-div-PA0104 |
|
| PB0901 | Examination by re-examination before a trial |
St.27 status event code: A-6-3-E10-E12-rex-PB0901 |
|
| B601 | Maintenance of original decision after re-examination before a trial | ||
| PB0601 | Maintenance of original decision after re-examination before a trial |
St.27 status event code: N-3-6-B10-B17-rex-PB0601 |
|
| J121 | Written withdrawal of request for trial | ||
| PC1202 | Submission of document of withdrawal before decision of registration |
St.27 status event code: N-1-6-B10-B11-nap-PC1202 |
|
| PJ1201 | Withdrawal of trial |
St.27 status event code: A-3-3-V10-V13-apl-PJ1201 |
|
| WITB | Written withdrawal of application | ||
| R18-X000 | Changes to party contact information recorded |
St.27 status event code: A-3-3-R10-R18-oth-X000 |
|
| P22-X000 | Classification modified |
St.27 status event code: A-2-2-P10-P22-nap-X000 |
|
| R18-X000 | Changes to party contact information recorded |
St.27 status event code: A-3-3-R10-R18-oth-X000 |