KR20130119945A - 3-히드록시프로피온산 생산을 위한 조성물 및 방법 - Google Patents
3-히드록시프로피온산 생산을 위한 조성물 및 방법 Download PDFInfo
- Publication number
- KR20130119945A KR20130119945A KR1020137015983A KR20137015983A KR20130119945A KR 20130119945 A KR20130119945 A KR 20130119945A KR 1020137015983 A KR1020137015983 A KR 1020137015983A KR 20137015983 A KR20137015983 A KR 20137015983A KR 20130119945 A KR20130119945 A KR 20130119945A
- Authority
- KR
- South Korea
- Prior art keywords
- gene
- seq
- genetically modified
- exogenous
- modified yeast
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Ceased
Links
- 238000000034 method Methods 0.000 title claims description 67
- ALRHLSYJTWAHJZ-UHFFFAOYSA-N 3-hydroxypropionic acid Chemical compound OCCC(O)=O ALRHLSYJTWAHJZ-UHFFFAOYSA-N 0.000 title description 589
- 238000004519 manufacturing process Methods 0.000 title description 42
- 239000000203 mixture Substances 0.000 title description 13
- 210000004027 cell Anatomy 0.000 claims abstract description 180
- 210000005253 yeast cell Anatomy 0.000 claims abstract description 153
- 230000037361 pathway Effects 0.000 claims abstract description 146
- 238000000855 fermentation Methods 0.000 claims abstract description 135
- 230000004151 fermentation Effects 0.000 claims abstract description 135
- ISFBDLBZPVCEKD-ONNFQVAWSA-N chembl123303 Chemical compound N\C(S)=N\N=C\C1=NC=CC=C1O ISFBDLBZPVCEKD-ONNFQVAWSA-N 0.000 claims abstract 13
- 108090000623 proteins and genes Proteins 0.000 claims description 465
- 229920001184 polypeptide Polymers 0.000 claims description 96
- 108090000765 processed proteins & peptides Proteins 0.000 claims description 96
- 102000004196 processed proteins & peptides Human genes 0.000 claims description 96
- 239000002773 nucleotide Substances 0.000 claims description 84
- 125000003729 nucleotide group Chemical group 0.000 claims description 84
- PEDCQBHIVMGVHV-UHFFFAOYSA-N Glycerine Chemical compound OCC(O)CO PEDCQBHIVMGVHV-UHFFFAOYSA-N 0.000 claims description 54
- WQZGKKKJIJFFOK-GASJEMHNSA-N Glucose Natural products OC[C@H]1OC(O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-GASJEMHNSA-N 0.000 claims description 41
- 238000012217 deletion Methods 0.000 claims description 41
- 230000037430 deletion Effects 0.000 claims description 41
- 239000008103 glucose Substances 0.000 claims description 37
- 101150096049 pyc gene Proteins 0.000 claims description 37
- 230000001105 regulatory effect Effects 0.000 claims description 37
- JVTAAEKCZFNVCJ-UHFFFAOYSA-N lactic acid Chemical compound CC(O)C(O)=O JVTAAEKCZFNVCJ-UHFFFAOYSA-N 0.000 claims description 36
- 108091028043 Nucleic acid sequence Proteins 0.000 claims description 34
- 101150023977 Baat gene Proteins 0.000 claims description 27
- 230000035772 mutation Effects 0.000 claims description 26
- 101150006589 adc gene Proteins 0.000 claims description 22
- 108010093796 4-hydroxybutyrate dehydrogenase Proteins 0.000 claims description 21
- 241000894007 species Species 0.000 claims description 21
- 239000004310 lactic acid Substances 0.000 claims description 18
- 235000014655 lactic acid Nutrition 0.000 claims description 18
- 101150023641 ppc gene Proteins 0.000 claims description 17
- 229910052799 carbon Inorganic materials 0.000 claims description 16
- OKTJSMMVPCPJKN-UHFFFAOYSA-N Carbon Chemical compound [C] OKTJSMMVPCPJKN-UHFFFAOYSA-N 0.000 claims description 15
- 101150037054 aat gene Proteins 0.000 claims description 12
- 101150116670 gabT gene Proteins 0.000 claims description 12
- 241000235070 Saccharomyces Species 0.000 claims description 9
- SRBFZHDQGSBBOR-IOVATXLUSA-N D-xylopyranose Chemical compound O[C@@H]1COC(O)[C@H](O)[C@H]1O SRBFZHDQGSBBOR-IOVATXLUSA-N 0.000 claims description 8
- 108090000472 Phosphoenolpyruvate carboxykinase (ATP) Proteins 0.000 claims description 7
- PYMYPHUHKUWMLA-UHFFFAOYSA-N arabinose Natural products OCC(O)C(O)C(O)C=O PYMYPHUHKUWMLA-UHFFFAOYSA-N 0.000 claims description 7
- SRBFZHDQGSBBOR-UHFFFAOYSA-N beta-D-Pyranose-Lyxose Natural products OC1COC(O)C(O)C1O SRBFZHDQGSBBOR-UHFFFAOYSA-N 0.000 claims description 7
- 238000012239 gene modification Methods 0.000 claims description 7
- 230000005017 genetic modification Effects 0.000 claims description 7
- 235000013617 genetically modified food Nutrition 0.000 claims description 7
- 101150035384 HIBADH gene Proteins 0.000 claims description 6
- 241000222120 Candida <Saccharomycetales> Species 0.000 claims description 5
- 229930091371 Fructose Natural products 0.000 claims description 4
- 239000005715 Fructose Substances 0.000 claims description 4
- RFSUNEUAIZKAJO-ARQDHWQXSA-N Fructose Chemical compound OC[C@H]1O[C@](O)(CO)[C@@H](O)[C@@H]1O RFSUNEUAIZKAJO-ARQDHWQXSA-N 0.000 claims description 4
- 101100229074 Gallus gallus GAL6 gene Proteins 0.000 claims description 4
- 101150046686 LAP3 gene Proteins 0.000 claims description 4
- 238000012258 culturing Methods 0.000 claims description 4
- 241000235649 Kluyveromyces Species 0.000 claims description 3
- 241001580565 Peachia Species 0.000 claims description 3
- 229930006000 Sucrose Natural products 0.000 claims description 3
- CZMRCDWAGMRECN-UGDNZRGBSA-N Sucrose Chemical compound O[C@H]1[C@H](O)[C@@H](CO)O[C@@]1(CO)O[C@@H]1[C@H](O)[C@@H](O)[C@H](O)[C@@H](CO)O1 CZMRCDWAGMRECN-UGDNZRGBSA-N 0.000 claims description 3
- 241000235006 Torulaspora Species 0.000 claims description 3
- PYMYPHUHKUWMLA-WDCZJNDASA-N arabinose Chemical compound OC[C@@H](O)[C@@H](O)[C@H](O)C=O PYMYPHUHKUWMLA-WDCZJNDASA-N 0.000 claims description 3
- 239000005720 sucrose Substances 0.000 claims description 3
- 239000001913 cellulose Substances 0.000 claims description 2
- 229920002678 cellulose Polymers 0.000 claims description 2
- 125000003275 alpha amino acid group Chemical group 0.000 claims 6
- 102100027950 Bile acid-CoA:amino acid N-acyltransferase Human genes 0.000 claims 1
- 101000697858 Homo sapiens Bile acid-CoA:amino acid N-acyltransferase Proteins 0.000 claims 1
- 244000309464 bull Species 0.000 claims 1
- GVVPGTZRZFNKDS-JXMROGBWSA-N geranyl diphosphate Chemical compound CC(C)=CCC\C(C)=C\CO[P@](O)(=O)OP(O)(O)=O GVVPGTZRZFNKDS-JXMROGBWSA-N 0.000 claims 1
- 125000002791 glucosyl group Chemical group C1([C@H](O)[C@@H](O)[C@H](O)[C@H](O1)CO)* 0.000 claims 1
- 150000001413 amino acids Chemical class 0.000 description 184
- 238000006243 chemical reaction Methods 0.000 description 180
- 239000012634 fragment Substances 0.000 description 176
- 239000000047 product Substances 0.000 description 117
- 230000000694 effects Effects 0.000 description 116
- 240000004808 Saccharomyces cerevisiae Species 0.000 description 113
- 235000014680 Saccharomyces cerevisiae Nutrition 0.000 description 103
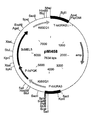
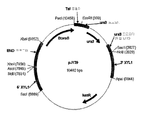
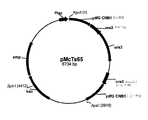
- 239000013612 plasmid Substances 0.000 description 102
- 101150050575 URA3 gene Proteins 0.000 description 87
- UCMIRNVEIXFBKS-UHFFFAOYSA-N beta-alanine Chemical compound NCCC(O)=O UCMIRNVEIXFBKS-UHFFFAOYSA-N 0.000 description 82
- 102000004190 Enzymes Human genes 0.000 description 76
- 108090000790 Enzymes Proteins 0.000 description 76
- 239000000499 gel Substances 0.000 description 72
- 101100246753 Halobacterium salinarum (strain ATCC 700922 / JCM 11081 / NRC-1) pyrF gene Proteins 0.000 description 71
- 108010011939 Pyruvate Decarboxylase Proteins 0.000 description 70
- 239000003550 marker Substances 0.000 description 64
- KHPXUQMNIQBQEV-UHFFFAOYSA-N oxaloacetic acid Chemical compound OC(=O)CC(=O)C(O)=O KHPXUQMNIQBQEV-UHFFFAOYSA-N 0.000 description 64
- 239000013598 vector Substances 0.000 description 64
- 108020004414 DNA Proteins 0.000 description 61
- IAZDPXIOMUYVGZ-UHFFFAOYSA-N Dimethylsulphoxide Chemical compound CS(C)=O IAZDPXIOMUYVGZ-UHFFFAOYSA-N 0.000 description 60
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 description 60
- LCTONWCANYUPML-UHFFFAOYSA-M Pyruvate Chemical compound CC(=O)C([O-])=O LCTONWCANYUPML-UHFFFAOYSA-M 0.000 description 54
- 230000014509 gene expression Effects 0.000 description 51
- 239000002609 medium Substances 0.000 description 51
- 230000010354 integration Effects 0.000 description 50
- OAKURXIZZOAYBC-UHFFFAOYSA-N 3-oxopropanoic acid Chemical class OC(=O)CC=O OAKURXIZZOAYBC-UHFFFAOYSA-N 0.000 description 48
- 230000001580 bacterial effect Effects 0.000 description 46
- 239000000872 buffer Substances 0.000 description 46
- 230000012010 growth Effects 0.000 description 45
- 238000003780 insertion Methods 0.000 description 44
- 230000037431 insertion Effects 0.000 description 44
- 229940000635 beta-alanine Drugs 0.000 description 41
- 108030001569 3-hydroxypropionate dehydrogenases Proteins 0.000 description 39
- WQZGKKKJIJFFOK-VFUOTHLCSA-N beta-D-glucose Chemical compound OC[C@H]1O[C@@H](O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-VFUOTHLCSA-N 0.000 description 39
- 238000000246 agarose gel electrophoresis Methods 0.000 description 38
- DTBNBXWJWCWCIK-UHFFFAOYSA-K phosphonatoenolpyruvate Chemical compound [O-]C(=O)C(=C)OP([O-])([O-])=O DTBNBXWJWCWCIK-UHFFFAOYSA-K 0.000 description 38
- 229930029653 phosphoenolpyruvate Natural products 0.000 description 37
- 108091026890 Coding region Proteins 0.000 description 33
- 108010014303 DNA-directed DNA polymerase Proteins 0.000 description 33
- 102000016928 DNA-directed DNA polymerase Human genes 0.000 description 33
- 239000007795 chemical reaction product Substances 0.000 description 31
- 238000000605 extraction Methods 0.000 description 31
- RGWHQCVHVJXOKC-SHYZEUOFSA-J dCTP(4-) Chemical compound O=C1N=C(N)C=CN1[C@@H]1O[C@H](COP([O-])(=O)OP([O-])(=O)OP([O-])([O-])=O)[C@@H](O)C1 RGWHQCVHVJXOKC-SHYZEUOFSA-J 0.000 description 27
- HAAZLUGHYHWQIW-KVQBGUIXSA-N dGTP Chemical compound C1=NC=2C(=O)NC(N)=NC=2N1[C@H]1C[C@H](O)[C@@H](COP(O)(=O)OP(O)(=O)OP(O)(O)=O)O1 HAAZLUGHYHWQIW-KVQBGUIXSA-N 0.000 description 27
- NHVNXKFIZYSCEB-XLPZGREQSA-N dTTP Chemical compound O=C1NC(=O)C(C)=CN1[C@@H]1O[C@H](COP(O)(=O)OP(O)(=O)OP(O)(O)=O)[C@@H](O)C1 NHVNXKFIZYSCEB-XLPZGREQSA-N 0.000 description 27
- 108091000041 Phosphoenolpyruvate Carboxylase Proteins 0.000 description 26
- 108010002447 aspartate-alpha-decarboxylase Proteins 0.000 description 26
- SUYVUBYJARFZHO-RRKCRQDMSA-N dATP Chemical compound C1=NC=2C(N)=NC=NC=2N1[C@H]1C[C@H](O)[C@@H](COP(O)(=O)OP(O)(=O)OP(O)(O)=O)O1 SUYVUBYJARFZHO-RRKCRQDMSA-N 0.000 description 26
- SUYVUBYJARFZHO-UHFFFAOYSA-N dATP Natural products C1=NC=2C(N)=NC=NC=2N1C1CC(O)C(COP(O)(=O)OP(O)(=O)OP(O)(O)=O)O1 SUYVUBYJARFZHO-UHFFFAOYSA-N 0.000 description 26
- 239000000243 solution Substances 0.000 description 26
- 238000005382 thermal cycling Methods 0.000 description 26
- 108020004530 Transaldolase Proteins 0.000 description 25
- 102100028601 Transaldolase Human genes 0.000 description 25
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 25
- -1 BAAT Proteins 0.000 description 24
- 108010053763 Pyruvate Carboxylase Proteins 0.000 description 24
- OSBLTNPMIGYQGY-UHFFFAOYSA-N 2-amino-2-(hydroxymethyl)propane-1,3-diol;2-[2-[bis(carboxymethyl)amino]ethyl-(carboxymethyl)amino]acetic acid;boric acid Chemical compound OB(O)O.OCC(N)(CO)CO.OC(=O)CN(CC(O)=O)CCN(CC(O)=O)CC(O)=O OSBLTNPMIGYQGY-UHFFFAOYSA-N 0.000 description 23
- 102100031408 Acidic amino acid decarboxylase GADL1 Human genes 0.000 description 23
- 241000894006 Bacteria Species 0.000 description 23
- 241000235645 Pichia kudriavzevii Species 0.000 description 23
- 239000008051 TBE buffer Substances 0.000 description 23
- ZSLZBFCDCINBPY-ZSJPKINUSA-N acetyl-CoA Chemical compound O[C@@H]1[C@H](OP(O)(O)=O)[C@@H](COP(O)(=O)OP(O)(=O)OCC(C)(C)[C@@H](O)C(=O)NCCC(=O)NCCSC(=O)C)O[C@H]1N1C2=NC=NC(N)=C2N=C1 ZSLZBFCDCINBPY-ZSJPKINUSA-N 0.000 description 23
- 108020003281 3-hydroxyisobutyrate dehydrogenase Proteins 0.000 description 22
- 208000004160 Rasmussen subacute encephalitis Diseases 0.000 description 22
- 230000029087 digestion Effects 0.000 description 22
- 108020002663 Aldehyde Dehydrogenase Proteins 0.000 description 21
- 101710088194 Dehydrogenase Proteins 0.000 description 21
- 102100039895 Pyruvate carboxylase, mitochondrial Human genes 0.000 description 21
- 239000002253 acid Substances 0.000 description 21
- 102000004169 proteins and genes Human genes 0.000 description 21
- 238000012216 screening Methods 0.000 description 21
- 102000006027 3-hydroxyisobutyrate dehydrogenase Human genes 0.000 description 20
- 108090000489 Carboxy-Lyases Proteins 0.000 description 20
- 108090001042 Hydro-Lyases Proteins 0.000 description 20
- 238000004458 analytical method Methods 0.000 description 20
- 238000011144 upstream manufacturing Methods 0.000 description 20
- 108010003415 Aspartate Aminotransferases Proteins 0.000 description 19
- 241000588724 Escherichia coli Species 0.000 description 19
- 239000000543 intermediate Substances 0.000 description 19
- CKLJMWTZIZZHCS-REOHCLBHSA-N L-aspartic acid Chemical compound OC(=O)[C@@H](N)CC(O)=O CKLJMWTZIZZHCS-REOHCLBHSA-N 0.000 description 18
- 229940009098 aspartate Drugs 0.000 description 18
- 108010008386 malonyl-Coa reductase Proteins 0.000 description 18
- 235000018102 proteins Nutrition 0.000 description 18
- SEHFUALWMUWDKS-UHFFFAOYSA-N 5-fluoroorotic acid Chemical compound OC(=O)C=1NC(=O)NC(=O)C=1F SEHFUALWMUWDKS-UHFFFAOYSA-N 0.000 description 17
- 102000004625 Aspartate Aminotransferases Human genes 0.000 description 17
- 108090000364 Ligases Proteins 0.000 description 17
- 101150031265 PCK gene Proteins 0.000 description 17
- 238000003556 assay Methods 0.000 description 17
- 108010016219 Acetyl-CoA carboxylase Proteins 0.000 description 16
- 102000005369 Aldehyde Dehydrogenase Human genes 0.000 description 16
- 108010018763 Biotin carboxylase Proteins 0.000 description 16
- KCXVZYZYPLLWCC-UHFFFAOYSA-N EDTA Chemical compound OC(=O)CN(CC(O)=O)CCN(CC(O)=O)CC(O)=O KCXVZYZYPLLWCC-UHFFFAOYSA-N 0.000 description 16
- 230000003321 amplification Effects 0.000 description 16
- 238000003199 nucleic acid amplification method Methods 0.000 description 16
- 150000007524 organic acids Chemical class 0.000 description 16
- 108010072869 indolepyruvate decarboxylase Proteins 0.000 description 15
- 101150034686 PDC gene Proteins 0.000 description 14
- 239000000284 extract Substances 0.000 description 14
- 239000008188 pellet Substances 0.000 description 14
- 239000000523 sample Substances 0.000 description 14
- 235000000346 sugar Nutrition 0.000 description 14
- 102000004031 Carboxy-Lyases Human genes 0.000 description 13
- QNAYBMKLOCPYGJ-REOHCLBHSA-N L-alanine Chemical compound C[C@H](N)C(O)=O QNAYBMKLOCPYGJ-REOHCLBHSA-N 0.000 description 13
- 102000014701 Transketolase Human genes 0.000 description 13
- 108010043652 Transketolase Proteins 0.000 description 13
- 235000004279 alanine Nutrition 0.000 description 13
- 230000002068 genetic effect Effects 0.000 description 13
- 229930027945 nicotinamide-adenine dinucleotide Natural products 0.000 description 13
- 108010077268 3-hydroxyisobutyryl-CoA hydrolase Proteins 0.000 description 12
- 101710094518 4-aminobutyrate aminotransferase Proteins 0.000 description 12
- 102100035923 4-aminobutyrate aminotransferase, mitochondrial Human genes 0.000 description 12
- IJGRMHOSHXDMSA-UHFFFAOYSA-N Atomic nitrogen Chemical compound N#N IJGRMHOSHXDMSA-UHFFFAOYSA-N 0.000 description 12
- 241000196324 Embryophyta Species 0.000 description 12
- 241000233866 Fungi Species 0.000 description 12
- KWYUFKZDYYNOTN-UHFFFAOYSA-M Potassium hydroxide Chemical compound [OH-].[K+] KWYUFKZDYYNOTN-UHFFFAOYSA-M 0.000 description 12
- HEMHJVSKTPXQMS-UHFFFAOYSA-M Sodium hydroxide Chemical compound [OH-].[Na+] HEMHJVSKTPXQMS-UHFFFAOYSA-M 0.000 description 12
- 239000008049 TAE buffer Substances 0.000 description 12
- 108090000992 Transferases Proteins 0.000 description 12
- HGEVZDLYZYVYHD-UHFFFAOYSA-N acetic acid;2-amino-2-(hydroxymethyl)propane-1,3-diol;2-[2-[bis(carboxymethyl)amino]ethyl-(carboxymethyl)amino]acetic acid Chemical compound CC(O)=O.OCC(N)(CO)CO.OC(=O)CN(CC(O)=O)CCN(CC(O)=O)CC(O)=O HGEVZDLYZYVYHD-UHFFFAOYSA-N 0.000 description 12
- POODSGUMUCVRTR-IEXPHMLFSA-N acryloyl-CoA Chemical compound O[C@@H]1[C@H](OP(O)(O)=O)[C@@H](COP(O)(=O)OP(O)(=O)OCC(C)(C)[C@@H](O)C(=O)NCCC(=O)NCCSC(=O)C=C)O[C@H]1N1C2=NC=NC(N)=C2N=C1 POODSGUMUCVRTR-IEXPHMLFSA-N 0.000 description 12
- 229940024606 amino acid Drugs 0.000 description 12
- 235000001014 amino acid Nutrition 0.000 description 12
- 239000008280 blood Substances 0.000 description 12
- 210000004369 blood Anatomy 0.000 description 12
- BJEPYKJPYRNKOW-UHFFFAOYSA-N malic acid Chemical compound OC(=O)C(O)CC(O)=O BJEPYKJPYRNKOW-UHFFFAOYSA-N 0.000 description 12
- BDAGIHXWWSANSR-UHFFFAOYSA-N methanoic acid Natural products OC=O BDAGIHXWWSANSR-UHFFFAOYSA-N 0.000 description 12
- BASFCYQUMIYNBI-UHFFFAOYSA-N platinum Chemical compound [Pt] BASFCYQUMIYNBI-UHFFFAOYSA-N 0.000 description 12
- 108010031025 Alanine Dehydrogenase Proteins 0.000 description 11
- 102100036431 Calcineurin subunit B type 1 Human genes 0.000 description 11
- 108700039691 Genetic Promoter Regions Proteins 0.000 description 11
- 101000714321 Homo sapiens Calcineurin subunit B type 1 Proteins 0.000 description 11
- 108090000604 Hydrolases Proteins 0.000 description 11
- 108010007014 alanine 2,3-aminomutase Proteins 0.000 description 11
- RUWSXZUPLIXLGD-IEXPHMLFSA-N beta-alanyl-CoA Chemical compound O[C@@H]1[C@H](OP(O)(O)=O)[C@@H](COP(O)(=O)OP(O)(=O)OCC(C)(C)[C@@H](O)C(=O)NCCC(=O)NCCSC(=O)CCN)O[C@H]1N1C2=NC=NC(N)=C2N=C1 RUWSXZUPLIXLGD-IEXPHMLFSA-N 0.000 description 11
- 235000013601 eggs Nutrition 0.000 description 11
- XIXADJRWDQXREU-UHFFFAOYSA-M lithium acetate Chemical compound [Li+].CC([O-])=O XIXADJRWDQXREU-UHFFFAOYSA-M 0.000 description 11
- 101150076071 panD gene Proteins 0.000 description 11
- 239000011535 reaction buffer Substances 0.000 description 11
- AKXKFZDCRYJKTF-UHFFFAOYSA-N 3-Hydroxypropionaldehyde Chemical compound OCCC=O AKXKFZDCRYJKTF-UHFFFAOYSA-N 0.000 description 10
- 102000000452 Acetyl-CoA carboxylase Human genes 0.000 description 10
- 241000238631 Hexapoda Species 0.000 description 10
- 108700023483 L-lactate dehydrogenases Proteins 0.000 description 10
- JVTAAEKCZFNVCJ-UHFFFAOYSA-M Lactate Chemical compound CC(O)C([O-])=O JVTAAEKCZFNVCJ-UHFFFAOYSA-M 0.000 description 10
- 102000003960 Ligases Human genes 0.000 description 10
- LTYOQGRJFJAKNA-KKIMTKSISA-N Malonyl CoA Natural products S(C(=O)CC(=O)O)CCNC(=O)CCNC(=O)[C@@H](O)C(CO[P@](=O)(O[P@](=O)(OC[C@H]1[C@@H](OP(=O)(O)O)[C@@H](O)[C@@H](n2c3ncnc(N)c3nc2)O1)O)O)(C)C LTYOQGRJFJAKNA-KKIMTKSISA-N 0.000 description 10
- 238000010367 cloning Methods 0.000 description 10
- 229940049920 malate Drugs 0.000 description 10
- LTYOQGRJFJAKNA-DVVLENMVSA-N malonyl-CoA Chemical compound O[C@@H]1[C@H](OP(O)(O)=O)[C@@H](COP(O)(=O)OP(O)(=O)OCC(C)(C)[C@@H](O)C(=O)NCCC(=O)NCCSC(=O)CC(O)=O)O[C@H]1N1C2=NC=NC(N)=C2N=C1 LTYOQGRJFJAKNA-DVVLENMVSA-N 0.000 description 10
- 230000001737 promoting effect Effects 0.000 description 10
- 239000000126 substance Substances 0.000 description 10
- 108010082126 Alanine transaminase Proteins 0.000 description 9
- 102000016912 Aldehyde Reductase Human genes 0.000 description 9
- 108010053754 Aldehyde reductase Proteins 0.000 description 9
- 108030006993 Beta-alanyl-CoA ammonia-lyases Proteins 0.000 description 9
- 108010058076 D-xylulose reductase Proteins 0.000 description 9
- 239000003109 Disodium ethylene diamine tetraacetate Substances 0.000 description 9
- 102000004157 Hydrolases Human genes 0.000 description 9
- 102000003855 L-lactate dehydrogenase Human genes 0.000 description 9
- 108090000854 Oxidoreductases Proteins 0.000 description 9
- 102100026974 Sorbitol dehydrogenase Human genes 0.000 description 9
- 239000007983 Tris buffer Substances 0.000 description 9
- 235000019301 disodium ethylene diamine tetraacetate Nutrition 0.000 description 9
- 238000005516 engineering process Methods 0.000 description 9
- 229940093915 gynecological organic acid Drugs 0.000 description 9
- 238000002744 homologous recombination Methods 0.000 description 9
- 230000006801 homologous recombination Effects 0.000 description 9
- 101150108412 kgd gene Proteins 0.000 description 9
- 108010067653 lactate dehydratase Proteins 0.000 description 9
- VZCYOOQTPOCHFL-UPHRSURJSA-N maleic acid Chemical compound OC(=O)\C=C/C(O)=O VZCYOOQTPOCHFL-UPHRSURJSA-N 0.000 description 9
- 235000005985 organic acids Nutrition 0.000 description 9
- 108010025593 phenylalanine (histidine) aminotransferase Proteins 0.000 description 9
- 230000008569 process Effects 0.000 description 9
- 230000002829 reductive effect Effects 0.000 description 9
- 239000006228 supernatant Substances 0.000 description 9
- 238000012360 testing method Methods 0.000 description 9
- VZCYOOQTPOCHFL-UHFFFAOYSA-N trans-butenedioic acid Natural products OC(=O)C=CC(O)=O VZCYOOQTPOCHFL-UHFFFAOYSA-N 0.000 description 9
- LENZDBCJOHFCAS-UHFFFAOYSA-N tris Chemical compound OCC(N)(CO)CO LENZDBCJOHFCAS-UHFFFAOYSA-N 0.000 description 9
- 108010021680 2-oxoglutarate decarboxylase Proteins 0.000 description 8
- IKHGUXGNUITLKF-UHFFFAOYSA-N Acetaldehyde Chemical compound CC=O IKHGUXGNUITLKF-UHFFFAOYSA-N 0.000 description 8
- 229920000936 Agarose Polymers 0.000 description 8
- 108010005694 Aspartate 4-decarboxylase Proteins 0.000 description 8
- 108010071778 Benzoylformate decarboxylase Proteins 0.000 description 8
- 102000004867 Hydro-Lyases Human genes 0.000 description 8
- FAPWRFPIFSIZLT-UHFFFAOYSA-M Sodium chloride Chemical compound [Na+].[Cl-] FAPWRFPIFSIZLT-UHFFFAOYSA-M 0.000 description 8
- 108090000340 Transaminases Proteins 0.000 description 8
- ISAKRJDGNUQOIC-UHFFFAOYSA-N Uracil Chemical compound O=C1C=CNC(=O)N1 ISAKRJDGNUQOIC-UHFFFAOYSA-N 0.000 description 8
- 238000010790 dilution Methods 0.000 description 8
- 239000012895 dilution Substances 0.000 description 8
- 238000011534 incubation Methods 0.000 description 8
- VIWKEBOLLIEAIL-FBMOWMAESA-N lactoyl-CoA Chemical compound O[C@@H]1[C@H](OP(O)(O)=O)[C@@H](COP(O)(=O)OP(O)(=O)OCC(C)(C)[C@@H](O)C(=O)NCCC(=O)NCCSC(=O)C(O)C)O[C@H]1N1C2=NC=NC(N)=C2N=C1 VIWKEBOLLIEAIL-FBMOWMAESA-N 0.000 description 8
- BOPGDPNILDQYTO-NNYOXOHSSA-N nicotinamide-adenine dinucleotide Chemical compound C1=CCC(C(=O)N)=CN1[C@H]1[C@H](O)[C@H](O)[C@@H](COP(O)(=O)OP(O)(=O)OC[C@@H]2[C@H]([C@@H](O)[C@@H](O2)N2C3=NC=NC(N)=C3N=C2)O)O1 BOPGDPNILDQYTO-NNYOXOHSSA-N 0.000 description 8
- 239000011541 reaction mixture Substances 0.000 description 8
- KPGXRSRHYNQIFN-UHFFFAOYSA-L 2-oxoglutarate(2-) Chemical compound [O-]C(=O)CCC(=O)C([O-])=O KPGXRSRHYNQIFN-UHFFFAOYSA-L 0.000 description 7
- 239000002028 Biomass Substances 0.000 description 7
- 102000012410 DNA Ligases Human genes 0.000 description 7
- 108010061982 DNA Ligases Proteins 0.000 description 7
- 108010025885 Glycerol dehydratase Proteins 0.000 description 7
- JVTAAEKCZFNVCJ-REOHCLBHSA-N L-lactic acid Chemical compound C[C@H](O)C(O)=O JVTAAEKCZFNVCJ-REOHCLBHSA-N 0.000 description 7
- 229910013594 LiOAc Inorganic materials 0.000 description 7
- 108010026217 Malate Dehydrogenase Proteins 0.000 description 7
- 101150050255 PDC1 gene Proteins 0.000 description 7
- 102000011755 Phosphoglycerate Kinase Human genes 0.000 description 7
- 101001099217 Thermotoga maritima (strain ATCC 43589 / DSM 3109 / JCM 10099 / NBRC 100826 / MSB8) Triosephosphate isomerase Proteins 0.000 description 7
- 102000003929 Transaminases Human genes 0.000 description 7
- 102000004357 Transferases Human genes 0.000 description 7
- 230000002378 acidificating effect Effects 0.000 description 7
- 230000004913 activation Effects 0.000 description 7
- QVGXLLKOCUKJST-UHFFFAOYSA-N atomic oxygen Chemical compound [O] QVGXLLKOCUKJST-UHFFFAOYSA-N 0.000 description 7
- 239000006227 byproduct Substances 0.000 description 7
- 229910052737 gold Inorganic materials 0.000 description 7
- 239000010931 gold Substances 0.000 description 7
- 239000001301 oxygen Substances 0.000 description 7
- 229910052760 oxygen Inorganic materials 0.000 description 7
- 108091008146 restriction endonucleases Proteins 0.000 description 7
- 230000002441 reversible effect Effects 0.000 description 7
- 230000009466 transformation Effects 0.000 description 7
- YBJHBAHKTGYVGT-ZKWXMUAHSA-N (+)-Biotin Chemical compound N1C(=O)N[C@@H]2[C@H](CCCCC(=O)O)SC[C@@H]21 YBJHBAHKTGYVGT-ZKWXMUAHSA-N 0.000 description 6
- SMZOUWXMTYCWNB-UHFFFAOYSA-N 2-(2-methoxy-5-methylphenyl)ethanamine Chemical compound COC1=CC=C(C)C=C1CCN SMZOUWXMTYCWNB-UHFFFAOYSA-N 0.000 description 6
- NIXOWILDQLNWCW-UHFFFAOYSA-N 2-Propenoic acid Natural products OC(=O)C=C NIXOWILDQLNWCW-UHFFFAOYSA-N 0.000 description 6
- 102100034767 3-hydroxyisobutyryl-CoA hydrolase, mitochondrial Human genes 0.000 description 6
- OSWFIVFLDKOXQC-UHFFFAOYSA-N 4-(3-methoxyphenyl)aniline Chemical compound COC1=CC=CC(C=2C=CC(N)=CC=2)=C1 OSWFIVFLDKOXQC-UHFFFAOYSA-N 0.000 description 6
- 101100351264 Candida albicans (strain SC5314 / ATCC MYA-2876) PDC11 gene Proteins 0.000 description 6
- 238000001712 DNA sequencing Methods 0.000 description 6
- 101710088791 Elongation factor 2 Proteins 0.000 description 6
- 108010041921 Glycerolphosphate Dehydrogenase Proteins 0.000 description 6
- 102000000587 Glycerolphosphate Dehydrogenase Human genes 0.000 description 6
- 108030004530 Malonate-semialdehyde dehydrogenases Proteins 0.000 description 6
- OKKJLVBELUTLKV-UHFFFAOYSA-N Methanol Chemical compound OC OKKJLVBELUTLKV-UHFFFAOYSA-N 0.000 description 6
- 102000004316 Oxidoreductases Human genes 0.000 description 6
- 108010022181 Phosphopyruvate Hydratase Proteins 0.000 description 6
- 102000012288 Phosphopyruvate Hydratase Human genes 0.000 description 6
- 108091081024 Start codon Proteins 0.000 description 6
- 101710205823 Translation elongation factor 2 Proteins 0.000 description 6
- 101150004098 UGA1 gene Proteins 0.000 description 6
- XJLXINKUBYWONI-DQQFMEOOSA-N [[(2r,3r,4r,5r)-5-(6-aminopurin-9-yl)-3-hydroxy-4-phosphonooxyoxolan-2-yl]methoxy-hydroxyphosphoryl] [(2s,3r,4s,5s)-5-(3-carbamoylpyridin-1-ium-1-yl)-3,4-dihydroxyoxolan-2-yl]methyl phosphate Chemical compound NC(=O)C1=CC=C[N+]([C@@H]2[C@H]([C@@H](O)[C@H](COP([O-])(=O)OP(O)(=O)OC[C@@H]3[C@H]([C@@H](OP(O)(O)=O)[C@@H](O3)N3C4=NC=NC(N)=C4N=C3)O)O2)O)=C1 XJLXINKUBYWONI-DQQFMEOOSA-N 0.000 description 6
- 239000011543 agarose gel Substances 0.000 description 6
- 238000005119 centrifugation Methods 0.000 description 6
- 238000002474 experimental method Methods 0.000 description 6
- 235000019253 formic acid Nutrition 0.000 description 6
- 230000006870 function Effects 0.000 description 6
- 230000034659 glycolysis Effects 0.000 description 6
- 108010046473 iso-2-cytochrome C Proteins 0.000 description 6
- 230000004048 modification Effects 0.000 description 6
- 238000012986 modification Methods 0.000 description 6
- 238000002703 mutagenesis Methods 0.000 description 6
- 231100000350 mutagenesis Toxicity 0.000 description 6
- 229910052757 nitrogen Inorganic materials 0.000 description 6
- 229910052697 platinum Inorganic materials 0.000 description 6
- 238000000746 purification Methods 0.000 description 6
- 230000006798 recombination Effects 0.000 description 6
- 238000005215 recombination Methods 0.000 description 6
- KDYFGRWQOYBRFD-UHFFFAOYSA-N succinic acid Chemical compound OC(=O)CCC(O)=O KDYFGRWQOYBRFD-UHFFFAOYSA-N 0.000 description 6
- 229940088594 vitamin Drugs 0.000 description 6
- 235000013343 vitamin Nutrition 0.000 description 6
- 239000011782 vitamin Substances 0.000 description 6
- 229930003231 vitamin Natural products 0.000 description 6
- 101150090985 ydfG gene Proteins 0.000 description 6
- 239000007222 ypd medium Substances 0.000 description 6
- FWMNVWWHGCHHJJ-SKKKGAJSSA-N 4-amino-1-[(2r)-6-amino-2-[[(2r)-2-[[(2r)-2-[[(2r)-2-amino-3-phenylpropanoyl]amino]-3-phenylpropanoyl]amino]-4-methylpentanoyl]amino]hexanoyl]piperidine-4-carboxylic acid Chemical compound C([C@H](C(=O)N[C@H](CC(C)C)C(=O)N[C@H](CCCCN)C(=O)N1CCC(N)(CC1)C(O)=O)NC(=O)[C@H](N)CC=1C=CC=CC=1)C1=CC=CC=C1 FWMNVWWHGCHHJJ-SKKKGAJSSA-N 0.000 description 5
- 108010017192 4-hydroxy-4-methyl-2-oxoglutarate aldolase Proteins 0.000 description 5
- QTBSBXVTEAMEQO-UHFFFAOYSA-M Acetate Chemical compound CC([O-])=O QTBSBXVTEAMEQO-UHFFFAOYSA-M 0.000 description 5
- 102100029589 Acylpyruvase FAHD1, mitochondrial Human genes 0.000 description 5
- 229920001817 Agar Polymers 0.000 description 5
- 102100036475 Alanine aminotransferase 1 Human genes 0.000 description 5
- 108010021809 Alcohol dehydrogenase Proteins 0.000 description 5
- 241000283690 Bos taurus Species 0.000 description 5
- 101000705592 Cucumis melo Phytoene synthase, chloroplastic Proteins 0.000 description 5
- 101710162684 Glyceraldehyde-3-phosphate dehydrogenase 3 Proteins 0.000 description 5
- 102000013460 Malate Dehydrogenase Human genes 0.000 description 5
- 101100242684 Mesorhizobium japonicum (strain LMG 29417 / CECT 9101 / MAFF 303099) panD1 gene Proteins 0.000 description 5
- 108700026244 Open Reading Frames Proteins 0.000 description 5
- 101150019587 PDH gene Proteins 0.000 description 5
- 108020001027 Ribosomal DNA Proteins 0.000 description 5
- 239000008272 agar Substances 0.000 description 5
- 238000013459 approach Methods 0.000 description 5
- 229940041514 candida albicans extract Drugs 0.000 description 5
- 239000003153 chemical reaction reagent Substances 0.000 description 5
- 230000002538 fungal effect Effects 0.000 description 5
- 230000004927 fusion Effects 0.000 description 5
- 230000000670 limiting effect Effects 0.000 description 5
- 230000037353 metabolic pathway Effects 0.000 description 5
- 244000005700 microbiome Species 0.000 description 5
- 238000002156 mixing Methods 0.000 description 5
- 235000019796 monopotassium phosphate Nutrition 0.000 description 5
- 239000002002 slurry Substances 0.000 description 5
- 238000002415 sodium dodecyl sulfate polyacrylamide gel electrophoresis Methods 0.000 description 5
- 239000008223 sterile water Substances 0.000 description 5
- 239000000758 substrate Substances 0.000 description 5
- 150000008163 sugars Chemical class 0.000 description 5
- 239000012138 yeast extract Substances 0.000 description 5
- QGZKDVFQNNGYKY-UHFFFAOYSA-N Ammonia Chemical compound N QGZKDVFQNNGYKY-UHFFFAOYSA-N 0.000 description 4
- CURLTUGMZLYLDI-UHFFFAOYSA-N Carbon dioxide Chemical compound O=C=O CURLTUGMZLYLDI-UHFFFAOYSA-N 0.000 description 4
- HEDRZPFGACZZDS-UHFFFAOYSA-N Chloroform Chemical compound ClC(Cl)Cl HEDRZPFGACZZDS-UHFFFAOYSA-N 0.000 description 4
- 102100031181 Glyceraldehyde-3-phosphate dehydrogenase Human genes 0.000 description 4
- 101710171812 Glycerol-3-phosphate phosphatase Proteins 0.000 description 4
- 102100038261 Glycerol-3-phosphate phosphatase Human genes 0.000 description 4
- WHUUTDBJXJRKMK-VKHMYHEASA-N L-glutamic acid Chemical compound OC(=O)[C@@H](N)CCC(O)=O WHUUTDBJXJRKMK-VKHMYHEASA-N 0.000 description 4
- 108091000076 Lysine 2,3-aminomutase Proteins 0.000 description 4
- 239000001888 Peptone Substances 0.000 description 4
- 108010080698 Peptones Proteins 0.000 description 4
- ISWSIDIOOBJBQZ-UHFFFAOYSA-N Phenol Chemical compound OC1=CC=CC=C1 ISWSIDIOOBJBQZ-UHFFFAOYSA-N 0.000 description 4
- CDBYLPFSWZWCQE-UHFFFAOYSA-L Sodium Carbonate Chemical compound [Na+].[Na+].[O-]C([O-])=O CDBYLPFSWZWCQE-UHFFFAOYSA-L 0.000 description 4
- QAOWNCQODCNURD-UHFFFAOYSA-N Sulfuric acid Chemical compound OS(O)(=O)=O QAOWNCQODCNURD-UHFFFAOYSA-N 0.000 description 4
- 241001441724 Tetraodontidae Species 0.000 description 4
- 244000288561 Torulaspora delbrueckii Species 0.000 description 4
- XSQUKJJJFZCRTK-UHFFFAOYSA-N Urea Chemical compound NC(N)=O XSQUKJJJFZCRTK-UHFFFAOYSA-N 0.000 description 4
- DRTQHJPVMGBUCF-XVFCMESISA-N Uridine Chemical compound O[C@@H]1[C@H](O)[C@@H](CO)O[C@H]1N1C(=O)NC(=O)C=C1 DRTQHJPVMGBUCF-XVFCMESISA-N 0.000 description 4
- 101150098232 YMR226C gene Proteins 0.000 description 4
- BFNBIHQBYMNNAN-UHFFFAOYSA-N ammonium sulfate Chemical compound N.N.OS(O)(=O)=O BFNBIHQBYMNNAN-UHFFFAOYSA-N 0.000 description 4
- 229910052921 ammonium sulfate Inorganic materials 0.000 description 4
- 235000011130 ammonium sulphate Nutrition 0.000 description 4
- 230000008901 benefit Effects 0.000 description 4
- 229910002092 carbon dioxide Inorganic materials 0.000 description 4
- 230000001413 cellular effect Effects 0.000 description 4
- 239000013599 cloning vector Substances 0.000 description 4
- 239000008121 dextrose Substances 0.000 description 4
- RXKJFZQQPQGTFL-UHFFFAOYSA-N dihydroxyacetone Chemical compound OCC(=O)CO RXKJFZQQPQGTFL-UHFFFAOYSA-N 0.000 description 4
- GNGACRATGGDKBX-UHFFFAOYSA-N dihydroxyacetone phosphate Chemical compound OCC(=O)COP(O)(O)=O GNGACRATGGDKBX-UHFFFAOYSA-N 0.000 description 4
- 238000001914 filtration Methods 0.000 description 4
- 229930182830 galactose Natural products 0.000 description 4
- 239000006481 glucose medium Substances 0.000 description 4
- 239000007788 liquid Substances 0.000 description 4
- 239000012092 media component Substances 0.000 description 4
- 101150043391 mmsB gene Proteins 0.000 description 4
- 235000016709 nutrition Nutrition 0.000 description 4
- 235000019319 peptone Nutrition 0.000 description 4
- 238000011084 recovery Methods 0.000 description 4
- 230000004044 response Effects 0.000 description 4
- 150000003839 salts Chemical class 0.000 description 4
- 239000011780 sodium chloride Substances 0.000 description 4
- 239000011573 trace mineral Substances 0.000 description 4
- 235000013619 trace mineral Nutrition 0.000 description 4
- 229940035893 uracil Drugs 0.000 description 4
- 150000003722 vitamin derivatives Chemical class 0.000 description 4
- OPIFSICVWOWJMJ-YGEXGZRRSA-N 5-bromo-4-chloro-3-indolyl alpha-D-galactoside Chemical compound O[C@@H]1[C@@H](O)[C@@H](O)[C@@H](CO)O[C@@H]1OC1=CNC2=CC=C(Br)C(Cl)=C12 OPIFSICVWOWJMJ-YGEXGZRRSA-N 0.000 description 3
- ROWKJAVDOGWPAT-UHFFFAOYSA-N Acetoin Chemical compound CC(O)C(C)=O ROWKJAVDOGWPAT-UHFFFAOYSA-N 0.000 description 3
- 241000722954 Anaerobiospirillum succiniciproducens Species 0.000 description 3
- 241001244729 Apalis Species 0.000 description 3
- 241000194108 Bacillus licheniformis Species 0.000 description 3
- 108020004705 Codon Proteins 0.000 description 3
- WSFSSNUMVMOOMR-UHFFFAOYSA-N Formaldehyde Chemical compound O=C WSFSSNUMVMOOMR-UHFFFAOYSA-N 0.000 description 3
- 101150038242 GAL10 gene Proteins 0.000 description 3
- 101150022730 MEL5 gene Proteins 0.000 description 3
- OFOBLEOULBTSOW-UHFFFAOYSA-L Malonate Chemical compound [O-]C(=O)CC([O-])=O OFOBLEOULBTSOW-UHFFFAOYSA-L 0.000 description 3
- 108090000301 Membrane transport proteins Proteins 0.000 description 3
- 229910019142 PO4 Inorganic materials 0.000 description 3
- 241000521553 Pichia fermentans Species 0.000 description 3
- 229920002562 Polyethylene Glycol 3350 Polymers 0.000 description 3
- 238000010521 absorption reaction Methods 0.000 description 3
- 101150008263 accD gene Proteins 0.000 description 3
- 108010084631 acetolactate decarboxylase Proteins 0.000 description 3
- 101150019439 aldB gene Proteins 0.000 description 3
- 230000015572 biosynthetic process Effects 0.000 description 3
- 229960002685 biotin Drugs 0.000 description 3
- 235000020958 biotin Nutrition 0.000 description 3
- 239000011616 biotin Substances 0.000 description 3
- 239000003054 catalyst Substances 0.000 description 3
- 230000008859 change Effects 0.000 description 3
- 238000012411 cloning technique Methods 0.000 description 3
- 150000001875 compounds Chemical class 0.000 description 3
- 210000000805 cytoplasm Anatomy 0.000 description 3
- 238000006114 decarboxylation reaction Methods 0.000 description 3
- 230000007812 deficiency Effects 0.000 description 3
- 239000007857 degradation product Substances 0.000 description 3
- 230000001419 dependent effect Effects 0.000 description 3
- LOKCTEFSRHRXRJ-UHFFFAOYSA-I dipotassium trisodium dihydrogen phosphate hydrogen phosphate dichloride Chemical compound P(=O)(O)(O)[O-].[K+].P(=O)(O)([O-])[O-].[Na+].[Na+].[Cl-].[K+].[Cl-].[Na+] LOKCTEFSRHRXRJ-UHFFFAOYSA-I 0.000 description 3
- 239000012153 distilled water Substances 0.000 description 3
- 238000001952 enzyme assay Methods 0.000 description 3
- 238000001976 enzyme digestion Methods 0.000 description 3
- 239000007789 gas Substances 0.000 description 3
- 230000002496 gastric effect Effects 0.000 description 3
- 229930195712 glutamate Natural products 0.000 description 3
- 208000037584 hereditary sensory and autonomic neuropathy Diseases 0.000 description 3
- 238000004128 high performance liquid chromatography Methods 0.000 description 3
- WRUGWIBCXHJTDG-UHFFFAOYSA-L magnesium sulfate heptahydrate Chemical compound O.O.O.O.O.O.O.[Mg+2].[O-]S([O-])(=O)=O WRUGWIBCXHJTDG-UHFFFAOYSA-L 0.000 description 3
- 229940061634 magnesium sulfate heptahydrate Drugs 0.000 description 3
- 230000004060 metabolic process Effects 0.000 description 3
- 229940111688 monobasic potassium phosphate Drugs 0.000 description 3
- 239000010452 phosphate Substances 0.000 description 3
- 239000008363 phosphate buffer Substances 0.000 description 3
- 239000002953 phosphate buffered saline Substances 0.000 description 3
- ZWLUXSQADUDCSB-UHFFFAOYSA-N phthalaldehyde Chemical compound O=CC1=CC=CC=C1C=O ZWLUXSQADUDCSB-UHFFFAOYSA-N 0.000 description 3
- GNSKLFRGEWLPPA-UHFFFAOYSA-M potassium dihydrogen phosphate Chemical compound [K+].OP(O)([O-])=O GNSKLFRGEWLPPA-UHFFFAOYSA-M 0.000 description 3
- NLKNQRATVPKPDG-UHFFFAOYSA-M potassium iodide Chemical compound [K+].[I-] NLKNQRATVPKPDG-UHFFFAOYSA-M 0.000 description 3
- 239000001965 potato dextrose agar Substances 0.000 description 3
- 230000002028 premature Effects 0.000 description 3
- 238000002360 preparation method Methods 0.000 description 3
- 230000009467 reduction Effects 0.000 description 3
- 238000006722 reduction reaction Methods 0.000 description 3
- 238000000926 separation method Methods 0.000 description 3
- AWUCVROLDVIAJX-GSVOUGTGSA-N sn-glycerol 3-phosphate Chemical compound OC[C@@H](O)COP(O)(O)=O AWUCVROLDVIAJX-GSVOUGTGSA-N 0.000 description 3
- 239000001384 succinic acid Substances 0.000 description 3
- 230000008685 targeting Effects 0.000 description 3
- 238000012546 transfer Methods 0.000 description 3
- 238000013519 translation Methods 0.000 description 3
- DNIAPMSPPWPWGF-VKHMYHEASA-N (+)-propylene glycol Chemical compound C[C@H](O)CO DNIAPMSPPWPWGF-VKHMYHEASA-N 0.000 description 2
- BJEPYKJPYRNKOW-REOHCLBHSA-N (S)-malic acid Chemical compound OC(=O)[C@@H](O)CC(O)=O BJEPYKJPYRNKOW-REOHCLBHSA-N 0.000 description 2
- YPFDHNVEDLHUCE-UHFFFAOYSA-N 1,3-propanediol Substances OCCCO YPFDHNVEDLHUCE-UHFFFAOYSA-N 0.000 description 2
- 229940035437 1,3-propanediol Drugs 0.000 description 2
- VVJYUAYZJAKGRQ-UHFFFAOYSA-N 1-[4,5-dihydroxy-6-(hydroxymethyl)oxan-2-yl]-5-methylpyrimidine-2,4-dione Chemical compound O=C1NC(=O)C(C)=CN1C1OC(CO)C(O)C(O)C1 VVJYUAYZJAKGRQ-UHFFFAOYSA-N 0.000 description 2
- SXGZJKUKBWWHRA-UHFFFAOYSA-N 2-(N-morpholiniumyl)ethanesulfonate Chemical compound [O-]S(=O)(=O)CC[NH+]1CCOCC1 SXGZJKUKBWWHRA-UHFFFAOYSA-N 0.000 description 2
- KPGXRSRHYNQIFN-UHFFFAOYSA-N 2-oxoglutaric acid Chemical compound OC(=O)CCC(=O)C(O)=O KPGXRSRHYNQIFN-UHFFFAOYSA-N 0.000 description 2
- 108020005065 3' Flanking Region Proteins 0.000 description 2
- RSTKLPZEZYGQPY-UHFFFAOYSA-N 3-(indol-3-yl)pyruvic acid Chemical compound C1=CC=C2C(CC(=O)C(=O)O)=CNC2=C1 RSTKLPZEZYGQPY-UHFFFAOYSA-N 0.000 description 2
- ALYNCZNDIQEVRV-UHFFFAOYSA-N 4-aminobenzoic acid Chemical compound NC1=CC=C(C(O)=O)C=C1 ALYNCZNDIQEVRV-UHFFFAOYSA-N 0.000 description 2
- SJZRECIVHVDYJC-UHFFFAOYSA-M 4-hydroxybutyrate Chemical compound OCCCC([O-])=O SJZRECIVHVDYJC-UHFFFAOYSA-M 0.000 description 2
- OCKGFTQIICXDQW-ZEQRLZLVSA-N 5-[(1r)-1-hydroxy-2-[4-[(2r)-2-hydroxy-2-(4-methyl-1-oxo-3h-2-benzofuran-5-yl)ethyl]piperazin-1-yl]ethyl]-4-methyl-3h-2-benzofuran-1-one Chemical compound C1=C2C(=O)OCC2=C(C)C([C@@H](O)CN2CCN(CC2)C[C@H](O)C2=CC=C3C(=O)OCC3=C2C)=C1 OCKGFTQIICXDQW-ZEQRLZLVSA-N 0.000 description 2
- 101150051977 AAT2 gene Proteins 0.000 description 2
- 241000023308 Acca Species 0.000 description 2
- QTBSBXVTEAMEQO-UHFFFAOYSA-N Acetic acid Chemical compound CC(O)=O QTBSBXVTEAMEQO-UHFFFAOYSA-N 0.000 description 2
- 108010085238 Actins Proteins 0.000 description 2
- 241000194110 Bacillus sp. (in: Bacteria) Species 0.000 description 2
- VTYYLEPIZMXCLO-UHFFFAOYSA-L Calcium carbonate Chemical compound [Ca+2].[O-]C([O-])=O VTYYLEPIZMXCLO-UHFFFAOYSA-L 0.000 description 2
- UXVMQQNJUSDDNG-UHFFFAOYSA-L Calcium chloride Chemical compound [Cl-].[Cl-].[Ca+2] UXVMQQNJUSDDNG-UHFFFAOYSA-L 0.000 description 2
- 102100030497 Cytochrome c Human genes 0.000 description 2
- 108010017826 DNA Polymerase I Proteins 0.000 description 2
- 102000004594 DNA Polymerase I Human genes 0.000 description 2
- 108700036404 EC 1.1.1.298 Proteins 0.000 description 2
- YQYJSBFKSSDGFO-UHFFFAOYSA-N Epihygromycin Natural products OC1C(O)C(C(=O)C)OC1OC(C(=C1)O)=CC=C1C=C(C)C(=O)NC1C(O)C(O)C2OCOC2C1O YQYJSBFKSSDGFO-UHFFFAOYSA-N 0.000 description 2
- 102100024637 Galectin-10 Human genes 0.000 description 2
- 101150019065 HBD gene Proteins 0.000 description 2
- 241000235644 Issatchenkia Species 0.000 description 2
- TWRXJAOTZQYOKJ-UHFFFAOYSA-L Magnesium chloride Chemical compound [Mg+2].[Cl-].[Cl-] TWRXJAOTZQYOKJ-UHFFFAOYSA-L 0.000 description 2
- CSNNHWWHGAXBCP-UHFFFAOYSA-L Magnesium sulfate Chemical compound [Mg+2].[O-][S+2]([O-])([O-])[O-] CSNNHWWHGAXBCP-UHFFFAOYSA-L 0.000 description 2
- OFOBLEOULBTSOW-UHFFFAOYSA-N Malonic acid Chemical compound OC(=O)CC(O)=O OFOBLEOULBTSOW-UHFFFAOYSA-N 0.000 description 2
- 241000589195 Mesorhizobium loti Species 0.000 description 2
- 241001302042 Methanothermobacter thermautotrophicus Species 0.000 description 2
- PVNIIMVLHYAWGP-UHFFFAOYSA-N Niacin Chemical compound OC(=O)C1=CC=CN=C1 PVNIIMVLHYAWGP-UHFFFAOYSA-N 0.000 description 2
- 108010077524 Peptide Elongation Factor 1 Proteins 0.000 description 2
- 108090000608 Phosphoric Monoester Hydrolases Proteins 0.000 description 2
- 102000004160 Phosphoric Monoester Hydrolases Human genes 0.000 description 2
- 241000521509 Pichia deserticola Species 0.000 description 2
- 241000235062 Pichia membranifaciens Species 0.000 description 2
- DBMJMQXJHONAFJ-UHFFFAOYSA-M Sodium laurylsulphate Chemical compound [Na+].CCCCCCCCCCCCOS([O-])(=O)=O DBMJMQXJHONAFJ-UHFFFAOYSA-M 0.000 description 2
- 240000000878 Spathiostemon javensis Species 0.000 description 2
- 241001468227 Streptomyces avermitilis Species 0.000 description 2
- 108700005078 Synthetic Genes Proteins 0.000 description 2
- 102100037116 Transcription elongation factor 1 homolog Human genes 0.000 description 2
- 239000007984 Tris EDTA buffer Substances 0.000 description 2
- 241000235015 Yarrowia lipolytica Species 0.000 description 2
- MCMNRKCIXSYSNV-UHFFFAOYSA-N Zirconium dioxide Chemical compound O=[Zr]=O MCMNRKCIXSYSNV-UHFFFAOYSA-N 0.000 description 2
- 101150069122 aam gene Proteins 0.000 description 2
- 101150046124 accA gene Proteins 0.000 description 2
- 101150013885 accB gene Proteins 0.000 description 2
- 101150070497 accC gene Proteins 0.000 description 2
- 150000007513 acids Chemical class 0.000 description 2
- 150000001299 aldehydes Chemical class 0.000 description 2
- PPQRONHOSHZGFQ-LMVFSUKVSA-N aldehydo-D-ribose 5-phosphate Chemical compound OP(=O)(O)OC[C@@H](O)[C@@H](O)[C@@H](O)C=O PPQRONHOSHZGFQ-LMVFSUKVSA-N 0.000 description 2
- 229910021529 ammonia Inorganic materials 0.000 description 2
- 238000000137 annealing Methods 0.000 description 2
- 101150005925 aspC gene Proteins 0.000 description 2
- 101150036080 at gene Proteins 0.000 description 2
- 239000011324 bead Substances 0.000 description 2
- HUMNYLRZRPPJDN-UHFFFAOYSA-N benzaldehyde Chemical compound O=CC1=CC=CC=C1 HUMNYLRZRPPJDN-UHFFFAOYSA-N 0.000 description 2
- DRTQHJPVMGBUCF-PSQAKQOGSA-N beta-L-uridine Natural products O[C@H]1[C@@H](O)[C@H](CO)O[C@@H]1N1C(=O)NC(=O)C=C1 DRTQHJPVMGBUCF-PSQAKQOGSA-N 0.000 description 2
- KGBXLFKZBHKPEV-UHFFFAOYSA-N boric acid Chemical compound OB(O)O KGBXLFKZBHKPEV-UHFFFAOYSA-N 0.000 description 2
- 239000004327 boric acid Substances 0.000 description 2
- CDQSJQSWAWPGKG-UHFFFAOYSA-N butane-1,1-diol Chemical compound CCCC(O)O CDQSJQSWAWPGKG-UHFFFAOYSA-N 0.000 description 2
- 239000001110 calcium chloride Substances 0.000 description 2
- 229910001628 calcium chloride Inorganic materials 0.000 description 2
- 239000004202 carbamide Substances 0.000 description 2
- 239000001569 carbon dioxide Substances 0.000 description 2
- 125000002915 carbonyl group Chemical group [*:2]C([*:1])=O 0.000 description 2
- 239000006285 cell suspension Substances 0.000 description 2
- 239000003795 chemical substances by application Substances 0.000 description 2
- 238000013461 design Methods 0.000 description 2
- 229940120503 dihydroxyacetone Drugs 0.000 description 2
- XBDQKXXYIPTUBI-UHFFFAOYSA-N dimethylselenoniopropionate Natural products CCC(O)=O XBDQKXXYIPTUBI-UHFFFAOYSA-N 0.000 description 2
- 150000002009 diols Chemical class 0.000 description 2
- 238000004821 distillation Methods 0.000 description 2
- 230000002255 enzymatic effect Effects 0.000 description 2
- 230000001747 exhibiting effect Effects 0.000 description 2
- 238000013401 experimental design Methods 0.000 description 2
- 239000013604 expression vector Substances 0.000 description 2
- 238000010353 genetic engineering Methods 0.000 description 2
- 238000012268 genome sequencing Methods 0.000 description 2
- PCHJSUWPFVWCPO-UHFFFAOYSA-N gold Chemical compound [Au] PCHJSUWPFVWCPO-UHFFFAOYSA-N 0.000 description 2
- 239000001963 growth medium Substances 0.000 description 2
- 229910052500 inorganic mineral Inorganic materials 0.000 description 2
- 150000002500 ions Chemical group 0.000 description 2
- PHTQWCKDNZKARW-UHFFFAOYSA-N isoamylol Chemical compound CC(C)CCO PHTQWCKDNZKARW-UHFFFAOYSA-N 0.000 description 2
- 229930027917 kanamycin Natural products 0.000 description 2
- 229960000318 kanamycin Drugs 0.000 description 2
- SBUJHOSQTJFQJX-NOAMYHISSA-N kanamycin Chemical compound O[C@@H]1[C@@H](O)[C@H](O)[C@@H](CN)O[C@@H]1O[C@H]1[C@H](O)[C@@H](O[C@@H]2[C@@H]([C@@H](N)[C@H](O)[C@@H](CO)O2)O)[C@H](N)C[C@@H]1N SBUJHOSQTJFQJX-NOAMYHISSA-N 0.000 description 2
- 229930182823 kanamycin A Natural products 0.000 description 2
- 150000004715 keto acids Chemical class 0.000 description 2
- 230000002147 killing effect Effects 0.000 description 2
- 101150104734 ldh gene Proteins 0.000 description 2
- 239000001630 malic acid Substances 0.000 description 2
- 235000011090 malic acid Nutrition 0.000 description 2
- 239000012528 membrane Substances 0.000 description 2
- 229910052751 metal Inorganic materials 0.000 description 2
- 239000002184 metal Substances 0.000 description 2
- 235000010755 mineral Nutrition 0.000 description 2
- 239000011707 mineral Substances 0.000 description 2
- 229910000402 monopotassium phosphate Inorganic materials 0.000 description 2
- 230000007935 neutral effect Effects 0.000 description 2
- 230000003472 neutralizing effect Effects 0.000 description 2
- 235000015097 nutrients Nutrition 0.000 description 2
- 238000010979 pH adjustment Methods 0.000 description 2
- 230000036961 partial effect Effects 0.000 description 2
- 101150086832 pdhC gene Proteins 0.000 description 2
- NBIIXXVUZAFLBC-UHFFFAOYSA-K phosphate Chemical compound [O-]P([O-])([O-])=O NBIIXXVUZAFLBC-UHFFFAOYSA-K 0.000 description 2
- PJNZPQUBCPKICU-UHFFFAOYSA-N phosphoric acid;potassium Chemical compound [K].OP(O)(O)=O PJNZPQUBCPKICU-UHFFFAOYSA-N 0.000 description 2
- 229920001223 polyethylene glycol Polymers 0.000 description 2
- 229920000166 polytrimethylene carbonate Polymers 0.000 description 2
- BWHMMNNQKKPAPP-UHFFFAOYSA-L potassium carbonate Chemical compound [K+].[K+].[O-]C([O-])=O BWHMMNNQKKPAPP-UHFFFAOYSA-L 0.000 description 2
- 239000012264 purified product Substances 0.000 description 2
- NGVDGCNFYWLIFO-UHFFFAOYSA-N pyridoxal 5'-phosphate Chemical compound CC1=NC=C(COP(O)(O)=O)C(C=O)=C1O NGVDGCNFYWLIFO-UHFFFAOYSA-N 0.000 description 2
- 235000007682 pyridoxal 5'-phosphate Nutrition 0.000 description 2
- 239000011589 pyridoxal 5'-phosphate Substances 0.000 description 2
- 238000011002 quantification Methods 0.000 description 2
- 238000010791 quenching Methods 0.000 description 2
- 230000000171 quenching effect Effects 0.000 description 2
- 102000037983 regulatory factors Human genes 0.000 description 2
- 108091008025 regulatory factors Proteins 0.000 description 2
- 239000012266 salt solution Substances 0.000 description 2
- 238000011218 seed culture Methods 0.000 description 2
- 239000006152 selective media Substances 0.000 description 2
- 238000012163 sequencing technique Methods 0.000 description 2
- 229910000029 sodium carbonate Inorganic materials 0.000 description 2
- 238000003756 stirring Methods 0.000 description 2
- 239000011550 stock solution Substances 0.000 description 2
- 238000013518 transcription Methods 0.000 description 2
- 230000035897 transcription Effects 0.000 description 2
- 230000001131 transforming effect Effects 0.000 description 2
- DRTQHJPVMGBUCF-UHFFFAOYSA-N uracil arabinoside Natural products OC1C(O)C(CO)OC1N1C(=O)NC(=O)C=C1 DRTQHJPVMGBUCF-UHFFFAOYSA-N 0.000 description 2
- 229940045145 uridine Drugs 0.000 description 2
- 108700026220 vif Genes Proteins 0.000 description 2
- 108091032973 (ribonucleotides)n+m Proteins 0.000 description 1
- OWEGMIWEEQEYGQ-UHFFFAOYSA-N 100676-05-9 Natural products OC1C(O)C(O)C(CO)OC1OCC1C(O)C(O)C(O)C(OC2C(OC(O)C(O)C2O)CO)O1 OWEGMIWEEQEYGQ-UHFFFAOYSA-N 0.000 description 1
- NMDWGEGFJUBKLB-UHFFFAOYSA-M 2-acetyllactate Chemical compound CC(=O)C(C)(O)C([O-])=O NMDWGEGFJUBKLB-UHFFFAOYSA-M 0.000 description 1
- QKNYBSVHEMOAJP-UHFFFAOYSA-N 2-amino-2-(hydroxymethyl)propane-1,3-diol;hydron;chloride Chemical compound Cl.OCC(N)(CO)CO QKNYBSVHEMOAJP-UHFFFAOYSA-N 0.000 description 1
- VOKUMXABRRXHAR-UHFFFAOYSA-N 2-methyl-3-oxopropanoic acid Chemical compound O=CC(C)C(O)=O VOKUMXABRRXHAR-UHFFFAOYSA-N 0.000 description 1
- KVZLHPXEUGJPAH-UHFFFAOYSA-N 2-oxidanylpropanoic acid Chemical class CC(O)C(O)=O.CC(O)C(O)=O KVZLHPXEUGJPAH-UHFFFAOYSA-N 0.000 description 1
- FECNVDHIIJYRIA-UHFFFAOYSA-N 2-oxopentanedioic acid Chemical compound OC(=O)CCC(=O)C(O)=O.OC(=O)CCC(=O)C(O)=O FECNVDHIIJYRIA-UHFFFAOYSA-N 0.000 description 1
- WWEOGFZEFHPUAM-MIZDRFBCSA-N 3-hydroxy-2-methylpropanoyl-CoA Chemical compound O[C@@H]1[C@H](OP(O)(O)=O)[C@@H](COP(O)(=O)OP(O)(=O)OCC(C)(C)[C@@H](O)C(=O)NCCC(=O)NCCSC(=O)C(CO)C)O[C@H]1N1C2=NC=NC(N)=C2N=C1 WWEOGFZEFHPUAM-MIZDRFBCSA-N 0.000 description 1
- ALRHLSYJTWAHJZ-UHFFFAOYSA-M 3-hydroxypropionate Chemical compound OCCC([O-])=O ALRHLSYJTWAHJZ-UHFFFAOYSA-M 0.000 description 1
- 108030005924 3-hydroxypropionyl-CoA dehydratases Proteins 0.000 description 1
- DBTMGCOVALSLOR-UHFFFAOYSA-N 32-alpha-galactosyl-3-alpha-galactosyl-galactose Natural products OC1C(O)C(O)C(CO)OC1OC1C(O)C(OC2C(C(CO)OC(O)C2O)O)OC(CO)C1O DBTMGCOVALSLOR-UHFFFAOYSA-N 0.000 description 1
- MEUAVGJWGDPTLF-UHFFFAOYSA-N 4-(5-benzenesulfonylamino-1-methyl-1h-benzoimidazol-2-ylmethyl)-benzamidine Chemical compound N=1C2=CC(NS(=O)(=O)C=3C=CC=CC=3)=CC=C2N(C)C=1CC1=CC=C(C(N)=N)C=C1 MEUAVGJWGDPTLF-UHFFFAOYSA-N 0.000 description 1
- 108010060511 4-Aminobutyrate Transaminase Proteins 0.000 description 1
- 108030000921 4-aminobutyrate-2-oxoglutarate transaminases Proteins 0.000 description 1
- SJZRECIVHVDYJC-UHFFFAOYSA-N 4-hydroxybutyric acid Chemical compound OCCCC(O)=O SJZRECIVHVDYJC-UHFFFAOYSA-N 0.000 description 1
- 108020005029 5' Flanking Region Proteins 0.000 description 1
- XTWYTFMLZFPYCI-KQYNXXCUSA-N 5'-adenylphosphoric acid Chemical compound C1=NC=2C(N)=NC=NC=2N1[C@@H]1O[C@H](COP(O)(=O)OP(O)(O)=O)[C@@H](O)[C@H]1O XTWYTFMLZFPYCI-KQYNXXCUSA-N 0.000 description 1
- ACNXXUCYLYKAPB-UHFFFAOYSA-N 5-bromo-6-pyrrolidin-1-yl-1h-pyrimidine-2,4-dione Chemical compound OC1=NC(O)=C(Br)C(N2CCCC2)=N1 ACNXXUCYLYKAPB-UHFFFAOYSA-N 0.000 description 1
- 101150058703 ACC1 gene Proteins 0.000 description 1
- 241000589212 Acetobacter pasteurianus Species 0.000 description 1
- HRPVXLWXLXDGHG-UHFFFAOYSA-N Acrylamide Chemical compound NC(=O)C=C HRPVXLWXLXDGHG-UHFFFAOYSA-N 0.000 description 1
- 102000007469 Actins Human genes 0.000 description 1
- ZKHQWZAMYRWXGA-KQYNXXCUSA-N Adenosine triphosphate Chemical compound C1=NC=2C(N)=NC=NC=2N1[C@@H]1O[C@H](COP(O)(=O)OP(O)(=O)OP(O)(O)=O)[C@@H](O)[C@H]1O ZKHQWZAMYRWXGA-KQYNXXCUSA-N 0.000 description 1
- 241000588813 Alcaligenes faecalis Species 0.000 description 1
- 244000300657 Alchornea rugosa Species 0.000 description 1
- 102000007698 Alcohol dehydrogenase Human genes 0.000 description 1
- 108020004774 Alkaline Phosphatase Proteins 0.000 description 1
- 102000002260 Alkaline Phosphatase Human genes 0.000 description 1
- 108700028369 Alleles Proteins 0.000 description 1
- ATRRKUHOCOJYRX-UHFFFAOYSA-N Ammonium bicarbonate Chemical compound [NH4+].OC([O-])=O ATRRKUHOCOJYRX-UHFFFAOYSA-N 0.000 description 1
- VHUUQVKOLVNVRT-UHFFFAOYSA-N Ammonium hydroxide Chemical compound [NH4+].[OH-] VHUUQVKOLVNVRT-UHFFFAOYSA-N 0.000 description 1
- 241001136167 Anaerotignum propionicum Species 0.000 description 1
- 241000235349 Ascomycota Species 0.000 description 1
- 108030003579 Aspartate 1-decarboxylases Proteins 0.000 description 1
- 102100034193 Aspartate aminotransferase, mitochondrial Human genes 0.000 description 1
- 108030002081 Aspartate transaminases Proteins 0.000 description 1
- 241000972773 Aulopiformes Species 0.000 description 1
- 102000016614 Autophagy-Related Protein 5 Human genes 0.000 description 1
- 108010092776 Autophagy-Related Protein 5 Proteins 0.000 description 1
- 241000193830 Bacillus <bacterium> Species 0.000 description 1
- 235000014469 Bacillus subtilis Nutrition 0.000 description 1
- 101100378010 Bacillus subtilis (strain 168) accC1 gene Proteins 0.000 description 1
- 101100322122 Bacillus subtilis (strain 168) accC2 gene Proteins 0.000 description 1
- 108010006654 Bleomycin Proteins 0.000 description 1
- 108091003079 Bovine Serum Albumin Proteins 0.000 description 1
- 241000589174 Bradyrhizobium japonicum Species 0.000 description 1
- 108030003513 Branched-chain-2-oxoacid decarboxylases Proteins 0.000 description 1
- 241000193764 Brevibacillus brevis Species 0.000 description 1
- 101150087048 CYB2 gene Proteins 0.000 description 1
- 101100480861 Caldanaerobacter subterraneus subsp. tengcongensis (strain DSM 15242 / JCM 11007 / NBRC 100824 / MB4) tdh gene Proteins 0.000 description 1
- 241001640117 Callaeum Species 0.000 description 1
- 101100447466 Candida albicans (strain WO-1) TDH1 gene Proteins 0.000 description 1
- 241000228092 Candida entomophila Species 0.000 description 1
- BVKZGUZCCUSVTD-UHFFFAOYSA-L Carbonate Chemical compound [O-]C([O-])=O BVKZGUZCCUSVTD-UHFFFAOYSA-L 0.000 description 1
- 240000001817 Cereus hexagonus Species 0.000 description 1
- 241000588919 Citrobacter freundii Species 0.000 description 1
- 241000186570 Clostridium kluyveri Species 0.000 description 1
- 241000193468 Clostridium perfringens Species 0.000 description 1
- 241000186226 Corynebacterium glutamicum Species 0.000 description 1
- 241000252867 Cupriavidus metallidurans Species 0.000 description 1
- RBNPOMFGQQGHHO-UWTATZPHSA-N D-glyceric acid Chemical compound OC[C@@H](O)C(O)=O RBNPOMFGQQGHHO-UWTATZPHSA-N 0.000 description 1
- RXVWSYJTUUKTEA-UHFFFAOYSA-N D-maltotriose Natural products OC1C(O)C(OC(C(O)CO)C(O)C(O)C=O)OC(CO)C1OC1C(O)C(O)C(O)C(CO)O1 RXVWSYJTUUKTEA-UHFFFAOYSA-N 0.000 description 1
- 102000053602 DNA Human genes 0.000 description 1
- 101100499356 Dictyostelium discoideum lpd gene Proteins 0.000 description 1
- 101710090144 Dihydrolipoyl dehydrogenase Proteins 0.000 description 1
- 101150053890 EDA gene Proteins 0.000 description 1
- 102000010911 Enzyme Precursors Human genes 0.000 description 1
- 108010062466 Enzyme Precursors Proteins 0.000 description 1
- 241000190844 Erythrobacter Species 0.000 description 1
- 101100243945 Fusarium vanettenii PDAT9 gene Proteins 0.000 description 1
- 241000605986 Fusobacterium nucleatum Species 0.000 description 1
- 101150079046 GAL6 gene Proteins 0.000 description 1
- 101150064643 GPD gene Proteins 0.000 description 1
- 108700007698 Genetic Terminator Regions Proteins 0.000 description 1
- 102000057621 Glycerol kinases Human genes 0.000 description 1
- 108700016170 Glycerol kinases Proteins 0.000 description 1
- 108010015895 Glycerone kinase Proteins 0.000 description 1
- XKMLYUALXHKNFT-UUOKFMHZSA-N Guanosine-5'-triphosphate Chemical compound C1=2NC(N)=NC(=O)C=2N=CN1[C@@H]1O[C@H](COP(O)(=O)OP(O)(=O)OP(O)(O)=O)[C@@H](O)[C@H]1O XKMLYUALXHKNFT-UUOKFMHZSA-N 0.000 description 1
- 101100135734 Haloferax mediterranei (strain ATCC 33500 / DSM 1411 / JCM 8866 / NBRC 14739 / NCIMB 2177 / R-4) pccB gene Proteins 0.000 description 1
- 229920002488 Hemicellulose Polymers 0.000 description 1
- 241000282414 Homo sapiens Species 0.000 description 1
- 101000579123 Homo sapiens Phosphoglycerate kinase 1 Proteins 0.000 description 1
- 108090001007 Interleukin-8 Proteins 0.000 description 1
- 108091092195 Intron Proteins 0.000 description 1
- 108090000769 Isomerases Proteins 0.000 description 1
- 102000004195 Isomerases Human genes 0.000 description 1
- 241000171379 Kazachstania bulderi Species 0.000 description 1
- 241000512931 Kazachstania humilis Species 0.000 description 1
- 241000588915 Klebsiella aerogenes Species 0.000 description 1
- 241001138401 Kluyveromyces lactis Species 0.000 description 1
- 241001480034 Kodamaea ohmeri Species 0.000 description 1
- 229930195714 L-glutamate Natural products 0.000 description 1
- ROHFNLRQFUQHCH-YFKPBYRVSA-N L-leucine Chemical compound CC(C)C[C@H](N)C(O)=O ROHFNLRQFUQHCH-YFKPBYRVSA-N 0.000 description 1
- QIVBCDIJIAJPQS-VIFPVBQESA-N L-tryptophane Chemical compound C1=CC=C2C(C[C@H](N)C(O)=O)=CNC2=C1 QIVBCDIJIAJPQS-VIFPVBQESA-N 0.000 description 1
- 101150007280 LEU2 gene Proteins 0.000 description 1
- 241000235087 Lachancea kluyveri Species 0.000 description 1
- ROHFNLRQFUQHCH-UHFFFAOYSA-N Leucine Natural products CC(C)CC(N)C(O)=O ROHFNLRQFUQHCH-UHFFFAOYSA-N 0.000 description 1
- 102100024032 Linker for activation of T-cells family member 1 Human genes 0.000 description 1
- KDXKERNSBIXSRK-UHFFFAOYSA-N Lysine Natural products NCCCCC(N)C(O)=O KDXKERNSBIXSRK-UHFFFAOYSA-N 0.000 description 1
- GUBGYTABKSRVRQ-PICCSMPSSA-N Maltose Natural products O[C@@H]1[C@@H](O)[C@H](O)[C@@H](CO)O[C@@H]1O[C@@H]1[C@@H](CO)OC(O)[C@H](O)[C@H]1O GUBGYTABKSRVRQ-PICCSMPSSA-N 0.000 description 1
- 229910021380 Manganese Chloride Inorganic materials 0.000 description 1
- GLFNIEUTAYBVOC-UHFFFAOYSA-L Manganese chloride Chemical compound Cl[Mn]Cl GLFNIEUTAYBVOC-UHFFFAOYSA-L 0.000 description 1
- 241000604448 Megasphaera elsdenii Species 0.000 description 1
- 102000003939 Membrane transport proteins Human genes 0.000 description 1
- 241000157876 Metallosphaera sedula Species 0.000 description 1
- 101150020500 Msed_0709 gene Proteins 0.000 description 1
- 101100381816 Mycobacterium tuberculosis (strain ATCC 25618 / H37Rv) bkdC gene Proteins 0.000 description 1
- 102100037214 Orotidine 5'-phosphate decarboxylase Human genes 0.000 description 1
- 108010055012 Orotidine-5'-phosphate decarboxylase Proteins 0.000 description 1
- 101150026476 PAO1 gene Proteins 0.000 description 1
- 238000009004 PCR Kit Methods 0.000 description 1
- 208000012204 PDA1 Diseases 0.000 description 1
- KJWZYMMLVHIVSU-IYCNHOCDSA-N PGK1 Chemical compound CCCCC[C@H](O)\C=C\[C@@H]1[C@@H](CCCCCCC(O)=O)C(=O)CC1=O KJWZYMMLVHIVSU-IYCNHOCDSA-N 0.000 description 1
- 240000003388 Parartocarpus venenosus Species 0.000 description 1
- 241000228143 Penicillium Species 0.000 description 1
- 108090000645 Phosphoenolpyruvate carboxykinase (GTP) Proteins 0.000 description 1
- 102100028251 Phosphoglycerate kinase 1 Human genes 0.000 description 1
- 241000948155 Phytophthora sojae Species 0.000 description 1
- 241000235648 Pichia Species 0.000 description 1
- 206010035664 Pneumonia Diseases 0.000 description 1
- 241000186334 Propionibacterium freudenreichii subsp. shermanii Species 0.000 description 1
- 229940124158 Protease/peptidase inhibitor Drugs 0.000 description 1
- 241000589540 Pseudomonas fluorescens Species 0.000 description 1
- 241000589776 Pseudomonas putida Species 0.000 description 1
- 241000589614 Pseudomonas stutzeri Species 0.000 description 1
- 238000012181 QIAquick gel extraction kit Methods 0.000 description 1
- 102000018120 Recombinases Human genes 0.000 description 1
- 108010091086 Recombinases Proteins 0.000 description 1
- 241001148115 Rhizobium etli Species 0.000 description 1
- 244000281247 Ribes rubrum Species 0.000 description 1
- 108091006232 SLC7A5 Proteins 0.000 description 1
- 241000235072 Saccharomyces bayanus Species 0.000 description 1
- 101100013868 Saccharomyces cerevisiae (strain ATCC 204508 / S288c) UGA1 gene Proteins 0.000 description 1
- 244000253911 Saccharomyces fragilis Species 0.000 description 1
- 241001138501 Salmonella enterica Species 0.000 description 1
- 241000235346 Schizosaccharomyces Species 0.000 description 1
- 241000589196 Sinorhizobium meliloti Species 0.000 description 1
- 101100272606 Streptoalloteichus hindustanus ble gene Proteins 0.000 description 1
- 244000057717 Streptococcus lactis Species 0.000 description 1
- 241000194020 Streptococcus thermophilus Species 0.000 description 1
- 241001454746 Streptomyces niveus Species 0.000 description 1
- 241000160715 Sulfolobus tokodaii Species 0.000 description 1
- 101100009687 Synechocystis sp. (strain PCC 6803 / Kazusa) lpdA gene Proteins 0.000 description 1
- 108010006785 Taq Polymerase Proteins 0.000 description 1
- 108020005038 Terminator Codon Proteins 0.000 description 1
- 229920004890 Triton X-100 Polymers 0.000 description 1
- 239000013504 Triton X-100 Substances 0.000 description 1
- QIVBCDIJIAJPQS-UHFFFAOYSA-N Tryptophan Natural products C1=CC=C2C(CC(N)C(O)=O)=CNC2=C1 QIVBCDIJIAJPQS-UHFFFAOYSA-N 0.000 description 1
- 108010084455 Zeocin Proteins 0.000 description 1
- 241000235017 Zygosaccharomyces Species 0.000 description 1
- 241000400042 Zygosaccharomyces kombuchaensis Species 0.000 description 1
- 241001655839 Zygosaccharomyces lentus Species 0.000 description 1
- 241000588902 Zymomonas mobilis Species 0.000 description 1
- 241000192381 [Candida] diddensiae Species 0.000 description 1
- 241000509461 [Candida] ethanolica Species 0.000 description 1
- 241000228120 [Candida] vanderwaltii Species 0.000 description 1
- DTPIAWSSVGTUPF-UHFFFAOYSA-N [Mo].[Na].[Na] Chemical compound [Mo].[Na].[Na] DTPIAWSSVGTUPF-UHFFFAOYSA-N 0.000 description 1
- 101150015189 aceE gene Proteins 0.000 description 1
- 101150077561 aceF gene Proteins 0.000 description 1
- 229960000583 acetic acid Drugs 0.000 description 1
- 229940100228 acetyl coenzyme a Drugs 0.000 description 1
- 239000003377 acid catalyst Substances 0.000 description 1
- 239000002535 acidifier Substances 0.000 description 1
- 150000001252 acrylic acid derivatives Chemical class 0.000 description 1
- 101150067366 adh gene Proteins 0.000 description 1
- 239000000853 adhesive Substances 0.000 description 1
- 230000001070 adhesive effect Effects 0.000 description 1
- 238000005273 aeration Methods 0.000 description 1
- 238000013019 agitation Methods 0.000 description 1
- 101150055425 aldh gene Proteins 0.000 description 1
- 150000004716 alpha keto acids Chemical class 0.000 description 1
- 102000005840 alpha-Galactosidase Human genes 0.000 description 1
- 108010030291 alpha-Galactosidase Proteins 0.000 description 1
- 101150072110 alt2 gene Proteins 0.000 description 1
- 229960004050 aminobenzoic acid Drugs 0.000 description 1
- 229940126575 aminoglycoside Drugs 0.000 description 1
- 229940126574 aminoglycoside antibiotic Drugs 0.000 description 1
- 239000002647 aminoglycoside antibiotic agent Substances 0.000 description 1
- 239000001099 ammonium carbonate Substances 0.000 description 1
- 235000012501 ammonium carbonate Nutrition 0.000 description 1
- 239000000908 ammonium hydroxide Substances 0.000 description 1
- 150000003863 ammonium salts Chemical class 0.000 description 1
- AVKUERGKIZMTKX-NJBDSQKTSA-N ampicillin Chemical compound C1([C@@H](N)C(=O)N[C@H]2[C@H]3SC([C@@H](N3C2=O)C(O)=O)(C)C)=CC=CC=C1 AVKUERGKIZMTKX-NJBDSQKTSA-N 0.000 description 1
- 229960000723 ampicillin Drugs 0.000 description 1
- 239000003242 anti bacterial agent Substances 0.000 description 1
- 230000000692 anti-sense effect Effects 0.000 description 1
- 229940088710 antibiotic agent Drugs 0.000 description 1
- 230000011681 asexual reproduction Effects 0.000 description 1
- 238000013465 asexual reproduction Methods 0.000 description 1
- 239000012131 assay buffer Substances 0.000 description 1
- 230000001651 autotrophic effect Effects 0.000 description 1
- 230000009286 beneficial effect Effects 0.000 description 1
- GUBGYTABKSRVRQ-QUYVBRFLSA-N beta-maltose Chemical compound OC[C@H]1O[C@H](O[C@H]2[C@H](O)[C@@H](O)[C@H](O)O[C@@H]2CO)[C@H](O)[C@@H](O)[C@@H]1O GUBGYTABKSRVRQ-QUYVBRFLSA-N 0.000 description 1
- 230000001588 bifunctional effect Effects 0.000 description 1
- 239000011942 biocatalyst Substances 0.000 description 1
- 230000033228 biological regulation Effects 0.000 description 1
- 230000006696 biosynthetic metabolic pathway Effects 0.000 description 1
- 101150037483 bkdA gene Proteins 0.000 description 1
- 101150103637 bkdB gene Proteins 0.000 description 1
- 229960001561 bleomycin Drugs 0.000 description 1
- OYVAGSVQBOHSSS-UAPAGMARSA-O bleomycin A2 Chemical compound N([C@H](C(=O)N[C@H](C)[C@@H](O)[C@H](C)C(=O)N[C@@H]([C@H](O)C)C(=O)NCCC=1SC=C(N=1)C=1SC=C(N=1)C(=O)NCCC[S+](C)C)[C@@H](O[C@H]1[C@H]([C@@H](O)[C@H](O)[C@H](CO)O1)O[C@@H]1[C@H]([C@@H](OC(N)=O)[C@H](O)[C@@H](CO)O1)O)C=1N=CNC=1)C(=O)C1=NC([C@H](CC(N)=O)NC[C@H](N)C(N)=O)=NC(N)=C1C OYVAGSVQBOHSSS-UAPAGMARSA-O 0.000 description 1
- 238000009835 boiling Methods 0.000 description 1
- 229940098773 bovine serum albumin Drugs 0.000 description 1
- 101150015366 budA gene Proteins 0.000 description 1
- FAPWYRCQGJNNSJ-UBKPKTQASA-L calcium D-pantothenic acid Chemical compound [Ca+2].OCC(C)(C)[C@@H](O)C(=O)NCCC([O-])=O.OCC(C)(C)[C@@H](O)C(=O)NCCC([O-])=O FAPWYRCQGJNNSJ-UBKPKTQASA-L 0.000 description 1
- 229910000019 calcium carbonate Inorganic materials 0.000 description 1
- AXCZMVOFGPJBDE-UHFFFAOYSA-L calcium dihydroxide Chemical compound [OH-].[OH-].[Ca+2] AXCZMVOFGPJBDE-UHFFFAOYSA-L 0.000 description 1
- 239000000920 calcium hydroxide Substances 0.000 description 1
- 229910001861 calcium hydroxide Inorganic materials 0.000 description 1
- 229960002079 calcium pantothenate Drugs 0.000 description 1
- 159000000007 calcium salts Chemical class 0.000 description 1
- 230000025938 carbohydrate utilization Effects 0.000 description 1
- 150000001732 carboxylic acid derivatives Chemical class 0.000 description 1
- 230000000747 cardiac effect Effects 0.000 description 1
- 230000015556 catabolic process Effects 0.000 description 1
- 230000003197 catalytic effect Effects 0.000 description 1
- MLYYVTUWGNIJIB-BXKDBHETSA-N cefazolin Chemical compound S1C(C)=NN=C1SCC1=C(C(O)=O)N2C(=O)[C@@H](NC(=O)CN3N=NN=C3)[C@H]2SC1 MLYYVTUWGNIJIB-BXKDBHETSA-N 0.000 description 1
- 238000004113 cell culture Methods 0.000 description 1
- 230000030833 cell death Effects 0.000 description 1
- 230000024245 cell differentiation Effects 0.000 description 1
- 230000006037 cell lysis Effects 0.000 description 1
- 230000010307 cell transformation Effects 0.000 description 1
- 210000000349 chromosome Anatomy 0.000 description 1
- GFHNAMRJFCEERV-UHFFFAOYSA-L cobalt chloride hexahydrate Chemical compound O.O.O.O.O.O.[Cl-].[Cl-].[Co+2] GFHNAMRJFCEERV-UHFFFAOYSA-L 0.000 description 1
- 239000002299 complementary DNA Substances 0.000 description 1
- 238000001816 cooling Methods 0.000 description 1
- JZCCFEFSEZPSOG-UHFFFAOYSA-L copper(II) sulfate pentahydrate Chemical compound O.O.O.O.O.[Cu+2].[O-]S([O-])(=O)=O JZCCFEFSEZPSOG-UHFFFAOYSA-L 0.000 description 1
- 230000009089 cytolysis Effects 0.000 description 1
- 238000000354 decomposition reaction Methods 0.000 description 1
- 230000003247 decreasing effect Effects 0.000 description 1
- 230000002950 deficient Effects 0.000 description 1
- 238000006731 degradation reaction Methods 0.000 description 1
- 238000006297 dehydration reaction Methods 0.000 description 1
- 238000006356 dehydrogenation reaction Methods 0.000 description 1
- 238000001514 detection method Methods 0.000 description 1
- 239000001177 diphosphate Substances 0.000 description 1
- XPPKVPWEQAFLFU-UHFFFAOYSA-J diphosphate(4-) Chemical compound [O-]P([O-])(=O)OP([O-])([O-])=O XPPKVPWEQAFLFU-UHFFFAOYSA-J 0.000 description 1
- 235000011180 diphosphates Nutrition 0.000 description 1
- 238000004090 dissolution Methods 0.000 description 1
- 101150107471 dpgB gene Proteins 0.000 description 1
- 230000005014 ectopic expression Effects 0.000 description 1
- 238000004520 electroporation Methods 0.000 description 1
- 229940096118 ella Drugs 0.000 description 1
- 238000005265 energy consumption Methods 0.000 description 1
- 239000003797 essential amino acid Substances 0.000 description 1
- 235000020776 essential amino acid Nutrition 0.000 description 1
- RIUKRCNLZYDWHS-UHFFFAOYSA-N ethane;methanesulfonic acid Chemical compound CC.CS(O)(=O)=O RIUKRCNLZYDWHS-UHFFFAOYSA-N 0.000 description 1
- 238000011156 evaluation Methods 0.000 description 1
- 230000010429 evolutionary process Effects 0.000 description 1
- 230000005284 excitation Effects 0.000 description 1
- 239000012526 feed medium Substances 0.000 description 1
- 238000012262 fermentative production Methods 0.000 description 1
- 238000011049 filling Methods 0.000 description 1
- 239000000834 fixative Substances 0.000 description 1
- 108010008221 formate C-acetyltransferase Proteins 0.000 description 1
- 125000000524 functional group Chemical group 0.000 description 1
- DBTMGCOVALSLOR-AXAHEAMVSA-N galactotriose Natural products OC[C@@H]1O[C@@H](O[C@@H]2[C@@H](O)[C@H](CO)O[C@@H](O[C@H]3[C@@H](O)[C@H](O)O[C@@H](CO)[C@@H]3O)[C@@H]2O)[C@H](O)[C@H](O)[C@H]1O DBTMGCOVALSLOR-AXAHEAMVSA-N 0.000 description 1
- BTCSSZJGUNDROE-UHFFFAOYSA-N gamma-aminobutyric acid Chemical compound NCCCC(O)=O BTCSSZJGUNDROE-UHFFFAOYSA-N 0.000 description 1
- 238000012224 gene deletion Methods 0.000 description 1
- 238000003209 gene knockout Methods 0.000 description 1
- 239000012362 glacial acetic acid Substances 0.000 description 1
- 239000011521 glass Substances 0.000 description 1
- 230000004190 glucose uptake Effects 0.000 description 1
- 108010032776 glycerol-1-phosphatase Proteins 0.000 description 1
- 239000005431 greenhouse gas Substances 0.000 description 1
- 239000010440 gypsum Substances 0.000 description 1
- 229910052602 gypsum Inorganic materials 0.000 description 1
- 238000010438 heat treatment Methods 0.000 description 1
- 150000002402 hexoses Chemical class 0.000 description 1
- 238000009396 hybridization Methods 0.000 description 1
- 230000007062 hydrolysis Effects 0.000 description 1
- 238000006460 hydrolysis reaction Methods 0.000 description 1
- GFAZHVHNLUBROE-UHFFFAOYSA-N hydroxymethyl propionaldehyde Natural products CCC(=O)CO GFAZHVHNLUBROE-UHFFFAOYSA-N 0.000 description 1
- 238000000338 in vitro Methods 0.000 description 1
- 238000001727 in vivo Methods 0.000 description 1
- WHOOUMGHGSPMGR-UHFFFAOYSA-N indol-3-ylacetaldehyde Chemical compound C1=CC=C2C(CC=O)=CNC2=C1 WHOOUMGHGSPMGR-UHFFFAOYSA-N 0.000 description 1
- 208000015181 infectious disease Diseases 0.000 description 1
- 239000004615 ingredient Substances 0.000 description 1
- 239000003112 inhibitor Substances 0.000 description 1
- 230000005764 inhibitory process Effects 0.000 description 1
- 238000002347 injection Methods 0.000 description 1
- 239000007924 injection Substances 0.000 description 1
- 239000002054 inoculum Substances 0.000 description 1
- 229910052816 inorganic phosphate Inorganic materials 0.000 description 1
- 229960000367 inositol Drugs 0.000 description 1
- CDAISMWEOUEBRE-GPIVLXJGSA-N inositol Chemical compound O[C@H]1[C@H](O)[C@@H](O)[C@H](O)[C@H](O)[C@@H]1O CDAISMWEOUEBRE-GPIVLXJGSA-N 0.000 description 1
- 238000005342 ion exchange Methods 0.000 description 1
- SURQXAFEQWPFPV-UHFFFAOYSA-L iron(2+) sulfate heptahydrate Chemical compound O.O.O.O.O.O.O.[Fe+2].[O-]S([O-])(=O)=O SURQXAFEQWPFPV-UHFFFAOYSA-L 0.000 description 1
- FBJQEBRMDXPWNX-FYHZSNTMSA-N isomaltotriose Chemical compound O[C@@H]1[C@@H](O)[C@H](O)[C@@H](CO)O[C@@H]1OC[C@@H]1[C@@H](O)[C@H](O)[C@@H](O)[C@@H](OC[C@@H]2[C@H]([C@H](O)[C@@H](O)C(O)O2)O)O1 FBJQEBRMDXPWNX-FYHZSNTMSA-N 0.000 description 1
- 125000001449 isopropyl group Chemical group [H]C([H])([H])C([H])(*)C([H])([H])[H] 0.000 description 1
- 229940116871 l-lactate Drugs 0.000 description 1
- 238000009630 liquid culture Methods 0.000 description 1
- 238000000622 liquid--liquid extraction Methods 0.000 description 1
- 231100000053 low toxicity Toxicity 0.000 description 1
- 239000006166 lysate Substances 0.000 description 1
- 239000012139 lysis buffer Substances 0.000 description 1
- 108010056929 lyticase Proteins 0.000 description 1
- 229910001629 magnesium chloride Inorganic materials 0.000 description 1
- 229910052943 magnesium sulfate Inorganic materials 0.000 description 1
- 235000019341 magnesium sulphate Nutrition 0.000 description 1
- 238000012423 maintenance Methods 0.000 description 1
- LTYOQGRJFJAKNA-VFLPNFFSSA-N malonyl-coa Chemical compound O[C@@H]1[C@H](OP(O)(O)=O)[C@@H](COP(O)(=O)OP(O)(=O)OCC(C)(C)C(O)C(=O)NCCC(=O)NCCSC(=O)CC(O)=O)O[C@H]1N1C2=NC=NC(N)=C2N=C1 LTYOQGRJFJAKNA-VFLPNFFSSA-N 0.000 description 1
- 239000011565 manganese chloride Substances 0.000 description 1
- 235000002867 manganese chloride Nutrition 0.000 description 1
- 229940099607 manganese chloride Drugs 0.000 description 1
- FYGDTMLNYKFZSV-UHFFFAOYSA-N mannotriose Natural products OC1C(O)C(O)C(CO)OC1OC1C(CO)OC(OC2C(OC(O)C(O)C2O)CO)C(O)C1O FYGDTMLNYKFZSV-UHFFFAOYSA-N 0.000 description 1
- 239000000463 material Substances 0.000 description 1
- 230000002503 metabolic effect Effects 0.000 description 1
- 210000003470 mitochondria Anatomy 0.000 description 1
- 239000011259 mixed solution Substances 0.000 description 1
- 150000004682 monohydrates Chemical class 0.000 description 1
- 239000000178 monomer Substances 0.000 description 1
- 231100000219 mutagenic Toxicity 0.000 description 1
- 230000003505 mutagenic effect Effects 0.000 description 1
- 239000013642 negative control Substances 0.000 description 1
- 238000006386 neutralization reaction Methods 0.000 description 1
- 229960003512 nicotinic acid Drugs 0.000 description 1
- 235000001968 nicotinic acid Nutrition 0.000 description 1
- 239000011664 nicotinic acid Substances 0.000 description 1
- 229910017464 nitrogen compound Inorganic materials 0.000 description 1
- 150000002830 nitrogen compounds Chemical class 0.000 description 1
- 235000021048 nutrient requirements Nutrition 0.000 description 1
- 230000035764 nutrition Effects 0.000 description 1
- 235000003715 nutritional status Nutrition 0.000 description 1
- 150000002894 organic compounds Chemical class 0.000 description 1
- 230000002018 overexpression Effects 0.000 description 1
- 230000003647 oxidation Effects 0.000 description 1
- 238000007254 oxidation reaction Methods 0.000 description 1
- 230000036284 oxygen consumption Effects 0.000 description 1
- 238000012856 packing Methods 0.000 description 1
- QNGNSVIICDLXHT-UHFFFAOYSA-N para-ethylbenzaldehyde Natural products CCC1=CC=C(C=O)C=C1 QNGNSVIICDLXHT-UHFFFAOYSA-N 0.000 description 1
- 101150102492 pda1 gene Proteins 0.000 description 1
- 101150008465 pdb1 gene Proteins 0.000 description 1
- 101150084945 pdhA gene Proteins 0.000 description 1
- 101150028177 pdhB gene Proteins 0.000 description 1
- 101150015354 pdhD gene Proteins 0.000 description 1
- 239000000137 peptide hydrolase inhibitor Substances 0.000 description 1
- 230000000737 periodic effect Effects 0.000 description 1
- 239000003348 petrochemical agent Substances 0.000 description 1
- 239000003209 petroleum derivative Substances 0.000 description 1
- FAQJJMHZNSSFSM-UHFFFAOYSA-N phenylglyoxylic acid Chemical compound OC(=O)C(=O)C1=CC=CC=C1 FAQJJMHZNSSFSM-UHFFFAOYSA-N 0.000 description 1
- CWCMIVBLVUHDHK-ZSNHEYEWSA-N phleomycin D1 Chemical compound N([C@H](C(=O)N[C@H](C)[C@@H](O)[C@H](C)C(=O)N[C@@H]([C@H](O)C)C(=O)NCCC=1SC[C@@H](N=1)C=1SC=C(N=1)C(=O)NCCCCNC(N)=N)[C@@H](O[C@H]1[C@H]([C@@H](O)[C@H](O)[C@H](CO)O1)O[C@@H]1[C@H]([C@@H](OC(N)=O)[C@H](O)[C@@H](CO)O1)O)C=1N=CNC=1)C(=O)C1=NC([C@H](CC(N)=O)NC[C@H](N)C(N)=O)=NC(N)=C1C CWCMIVBLVUHDHK-ZSNHEYEWSA-N 0.000 description 1
- 238000007747 plating Methods 0.000 description 1
- 229920000728 polyester Polymers 0.000 description 1
- 229920000642 polymer Polymers 0.000 description 1
- 229910000027 potassium carbonate Inorganic materials 0.000 description 1
- 238000004886 process control Methods 0.000 description 1
- ULWHHBHJGPPBCO-UHFFFAOYSA-N propane-1,1-diol Chemical compound CCC(O)O ULWHHBHJGPPBCO-UHFFFAOYSA-N 0.000 description 1
- 235000019260 propionic acid Nutrition 0.000 description 1
- QQONPFPTGQHPMA-UHFFFAOYSA-N propylene Natural products CC=C QQONPFPTGQHPMA-UHFFFAOYSA-N 0.000 description 1
- 125000004805 propylene group Chemical group [H]C([H])([H])C([H])([*:1])C([H])([H])[*:2] 0.000 description 1
- 238000002731 protein assay Methods 0.000 description 1
- 239000012460 protein solution Substances 0.000 description 1
- 230000002797 proteolythic effect Effects 0.000 description 1
- ZUFQODAHGAHPFQ-UHFFFAOYSA-N pyridoxine hydrochloride Chemical compound Cl.CC1=NC=C(CO)C(CO)=C1O ZUFQODAHGAHPFQ-UHFFFAOYSA-N 0.000 description 1
- 229960004172 pyridoxine hydrochloride Drugs 0.000 description 1
- 235000019171 pyridoxine hydrochloride Nutrition 0.000 description 1
- 239000011764 pyridoxine hydrochloride Substances 0.000 description 1
- IUVKMZGDUIUOCP-BTNSXGMBSA-N quinbolone Chemical compound O([C@H]1CC[C@H]2[C@H]3[C@@H]([C@]4(C=CC(=O)C=C4CC3)C)CC[C@@]21C)C1=CCCC1 IUVKMZGDUIUOCP-BTNSXGMBSA-N 0.000 description 1
- 239000000376 reactant Substances 0.000 description 1
- 238000006268 reductive amination reaction Methods 0.000 description 1
- 230000008929 regeneration Effects 0.000 description 1
- 238000011069 regeneration method Methods 0.000 description 1
- 230000009711 regulatory function Effects 0.000 description 1
- 230000008439 repair process Effects 0.000 description 1
- 238000011160 research Methods 0.000 description 1
- 235000019515 salmon Nutrition 0.000 description 1
- 239000012723 sample buffer Substances 0.000 description 1
- 238000005070 sampling Methods 0.000 description 1
- CDAISMWEOUEBRE-UHFFFAOYSA-N scyllo-inosotol Natural products OC1C(O)C(O)C(O)C(O)C1O CDAISMWEOUEBRE-UHFFFAOYSA-N 0.000 description 1
- 239000011734 sodium Substances 0.000 description 1
- AKHNMLFCWUSKQB-UHFFFAOYSA-L sodium thiosulfate Chemical compound [Na+].[Na+].[O-]S([O-])(=O)=S AKHNMLFCWUSKQB-UHFFFAOYSA-L 0.000 description 1
- 235000019345 sodium thiosulphate Nutrition 0.000 description 1
- 238000000638 solvent extraction Methods 0.000 description 1
- 238000001228 spectrum Methods 0.000 description 1
- 230000002269 spontaneous effect Effects 0.000 description 1
- 239000012192 staining solution Substances 0.000 description 1
- 238000010561 standard procedure Methods 0.000 description 1
- 238000003860 storage Methods 0.000 description 1
- KDYFGRWQOYBRFD-UHFFFAOYSA-L succinate(2-) Chemical compound [O-]C(=O)CCC([O-])=O KDYFGRWQOYBRFD-UHFFFAOYSA-L 0.000 description 1
- UIUJIQZEACWQSV-UHFFFAOYSA-N succinic semialdehyde Chemical compound OC(=O)CCC=O UIUJIQZEACWQSV-UHFFFAOYSA-N 0.000 description 1
- 229920000247 superabsorbent polymer Polymers 0.000 description 1
- 239000004583 superabsorbent polymers (SAPs) Substances 0.000 description 1
- 239000013589 supplement Substances 0.000 description 1
- 230000009469 supplementation Effects 0.000 description 1
- 230000001629 suppression Effects 0.000 description 1
- 230000004083 survival effect Effects 0.000 description 1
- 101150088047 tdh3 gene Proteins 0.000 description 1
- DPJRMOMPQZCRJU-UHFFFAOYSA-M thiamine hydrochloride Chemical compound Cl.[Cl-].CC1=C(CCO)SC=[N+]1CC1=CN=C(C)N=C1N DPJRMOMPQZCRJU-UHFFFAOYSA-M 0.000 description 1
- 229960000344 thiamine hydrochloride Drugs 0.000 description 1
- 235000019190 thiamine hydrochloride Nutrition 0.000 description 1
- 239000011747 thiamine hydrochloride Substances 0.000 description 1
- 231100000167 toxic agent Toxicity 0.000 description 1
- 239000003053 toxin Substances 0.000 description 1
- 231100000765 toxin Toxicity 0.000 description 1
- 108700012359 toxins Proteins 0.000 description 1
- 230000002103 transcriptional effect Effects 0.000 description 1
- 230000007704 transition Effects 0.000 description 1
- 230000014621 translational initiation Effects 0.000 description 1
- 101150035767 trp gene Proteins 0.000 description 1
- 239000012137 tryptone Substances 0.000 description 1
- 201000008827 tuberculosis Diseases 0.000 description 1
- OOLLAFOLCSJHRE-ZHAKMVSLSA-N ulipristal acetate Chemical compound C1=CC(N(C)C)=CC=C1[C@@H]1C2=C3CCC(=O)C=C3CC[C@H]2[C@H](CC[C@]2(OC(C)=O)C(C)=O)[C@]2(C)C1 OOLLAFOLCSJHRE-ZHAKMVSLSA-N 0.000 description 1
- 238000009281 ultraviolet germicidal irradiation Methods 0.000 description 1
- 241001515965 unidentified phage Species 0.000 description 1
- 229920001221 xylan Polymers 0.000 description 1
- 150000004823 xylans Chemical class 0.000 description 1
- RZLVQBNCHSJZPX-UHFFFAOYSA-L zinc sulfate heptahydrate Chemical compound O.O.O.O.O.O.O.[Zn+2].[O-]S([O-])(=O)=O RZLVQBNCHSJZPX-UHFFFAOYSA-L 0.000 description 1
- FYGDTMLNYKFZSV-BYLHFPJWSA-N β-1,4-galactotrioside Chemical compound O[C@@H]1[C@@H](O)[C@H](O)[C@@H](CO)O[C@H]1O[C@@H]1[C@H](CO)O[C@@H](O[C@@H]2[C@@H](O[C@@H](O)[C@H](O)[C@H]2O)CO)[C@H](O)[C@H]1O FYGDTMLNYKFZSV-BYLHFPJWSA-N 0.000 description 1
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N15/00—Mutation or genetic engineering; DNA or RNA concerning genetic engineering, vectors, e.g. plasmids, or their isolation, preparation or purification; Use of hosts therefor
- C12N15/09—Recombinant DNA-technology
- C12N15/11—DNA or RNA fragments; Modified forms thereof; Non-coding nucleic acids having a biological activity
- C12N15/52—Genes encoding for enzymes or proenzymes
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N1/00—Microorganisms, e.g. protozoa; Compositions thereof; Processes of propagating, maintaining or preserving microorganisms or compositions thereof; Processes of preparing or isolating a composition containing a microorganism; Culture media therefor
- C12N1/14—Fungi; Culture media therefor
- C12N1/16—Yeasts; Culture media therefor
- C12N1/18—Baker's yeast; Brewer's yeast
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N15/00—Mutation or genetic engineering; DNA or RNA concerning genetic engineering, vectors, e.g. plasmids, or their isolation, preparation or purification; Use of hosts therefor
- C12N15/09—Recombinant DNA-technology
- C12N15/63—Introduction of foreign genetic material using vectors; Vectors; Use of hosts therefor; Regulation of expression
- C12N15/79—Vectors or expression systems specially adapted for eukaryotic hosts
- C12N15/80—Vectors or expression systems specially adapted for eukaryotic hosts for fungi
- C12N15/81—Vectors or expression systems specially adapted for eukaryotic hosts for fungi for yeasts
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N15/00—Mutation or genetic engineering; DNA or RNA concerning genetic engineering, vectors, e.g. plasmids, or their isolation, preparation or purification; Use of hosts therefor
- C12N15/09—Recombinant DNA-technology
- C12N15/63—Introduction of foreign genetic material using vectors; Vectors; Use of hosts therefor; Regulation of expression
- C12N15/79—Vectors or expression systems specially adapted for eukaryotic hosts
- C12N15/80—Vectors or expression systems specially adapted for eukaryotic hosts for fungi
- C12N15/81—Vectors or expression systems specially adapted for eukaryotic hosts for fungi for yeasts
- C12N15/815—Vectors or expression systems specially adapted for eukaryotic hosts for fungi for yeasts for yeasts other than Saccharomyces
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N9/00—Enzymes; Proenzymes; Compositions thereof; Processes for preparing, activating, inhibiting, separating or purifying enzymes
- C12N9/0004—Oxidoreductases (1.)
- C12N9/0006—Oxidoreductases (1.) acting on CH-OH groups as donors (1.1)
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N9/00—Enzymes; Proenzymes; Compositions thereof; Processes for preparing, activating, inhibiting, separating or purifying enzymes
- C12N9/10—Transferases (2.)
- C12N9/1096—Transferases (2.) transferring nitrogenous groups (2.6)
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N9/00—Enzymes; Proenzymes; Compositions thereof; Processes for preparing, activating, inhibiting, separating or purifying enzymes
- C12N9/88—Lyases (4.)
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N9/00—Enzymes; Proenzymes; Compositions thereof; Processes for preparing, activating, inhibiting, separating or purifying enzymes
- C12N9/93—Ligases (6)
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12P—FERMENTATION OR ENZYME-USING PROCESSES TO SYNTHESISE A DESIRED CHEMICAL COMPOUND OR COMPOSITION OR TO SEPARATE OPTICAL ISOMERS FROM A RACEMIC MIXTURE
- C12P7/00—Preparation of oxygen-containing organic compounds
- C12P7/40—Preparation of oxygen-containing organic compounds containing a carboxyl group including Peroxycarboxylic acids
- C12P7/42—Hydroxy-carboxylic acids
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12P—FERMENTATION OR ENZYME-USING PROCESSES TO SYNTHESISE A DESIRED CHEMICAL COMPOUND OR COMPOSITION OR TO SEPARATE OPTICAL ISOMERS FROM A RACEMIC MIXTURE
- C12P7/00—Preparation of oxygen-containing organic compounds
- C12P7/40—Preparation of oxygen-containing organic compounds containing a carboxyl group including Peroxycarboxylic acids
- C12P7/52—Propionic acid; Butyric acids
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12Y—ENZYMES
- C12Y101/00—Oxidoreductases acting on the CH-OH group of donors (1.1)
- C12Y101/01—Oxidoreductases acting on the CH-OH group of donors (1.1) with NAD+ or NADP+ as acceptor (1.1.1)
- C12Y101/01059—3-Hydroxypropionate dehydrogenase (1.1.1.59)
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12Y—ENZYMES
- C12Y206/00—Transferases transferring nitrogenous groups (2.6)
- C12Y206/01—Transaminases (2.6.1)
- C12Y206/01018—Beta-alanine-pyruvate transaminase (2.6.1.18)
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12Y—ENZYMES
- C12Y206/00—Transferases transferring nitrogenous groups (2.6)
- C12Y206/01—Transaminases (2.6.1)
- C12Y206/01019—4-Aminobutyrate—2-oxoglutarate transaminase (2.6.1.19)
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12Y—ENZYMES
- C12Y401/00—Carbon-carbon lyases (4.1)
- C12Y401/01—Carboxy-lyases (4.1.1)
- C12Y401/01031—Phosphoenolpyruvate carboxylase (4.1.1.31)
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12Y—ENZYMES
- C12Y206/00—Transferases transferring nitrogenous groups (2.6)
- C12Y206/01—Transaminases (2.6.1)
- C12Y206/01001—Aspartate transaminase (2.6.1.1), i.e. aspartate-aminotransferase
Landscapes
- Life Sciences & Earth Sciences (AREA)
- Health & Medical Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Engineering & Computer Science (AREA)
- Genetics & Genomics (AREA)
- Organic Chemistry (AREA)
- Wood Science & Technology (AREA)
- Zoology (AREA)
- Bioinformatics & Cheminformatics (AREA)
- General Engineering & Computer Science (AREA)
- Biotechnology (AREA)
- Biomedical Technology (AREA)
- General Health & Medical Sciences (AREA)
- Biochemistry (AREA)
- Microbiology (AREA)
- Mycology (AREA)
- Molecular Biology (AREA)
- Medicinal Chemistry (AREA)
- Biophysics (AREA)
- Physics & Mathematics (AREA)
- Plant Pathology (AREA)
- Chemical Kinetics & Catalysis (AREA)
- General Chemical & Material Sciences (AREA)
- Botany (AREA)
- Tropical Medicine & Parasitology (AREA)
- Virology (AREA)
- Micro-Organisms Or Cultivation Processes Thereof (AREA)
- Preparation Of Compounds By Using Micro-Organisms (AREA)
Applications Claiming Priority (5)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US41619910P | 2010-11-22 | 2010-11-22 | |
| US61/416,199 | 2010-11-22 | ||
| US201161535181P | 2011-09-15 | 2011-09-15 | |
| US61/535,181 | 2011-09-15 | ||
| PCT/US2011/061717 WO2012074818A2 (en) | 2010-11-22 | 2011-11-21 | Compositions and methods for 3-hydroxypropionic acid production |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| KR20130119945A true KR20130119945A (ko) | 2013-11-01 |
Family
ID=45099210
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020137015983A Ceased KR20130119945A (ko) | 2010-11-22 | 2011-11-21 | 3-히드록시프로피온산 생산을 위한 조성물 및 방법 |
Country Status (11)
| Country | Link |
|---|---|
| US (7) | US9090918B2 (enExample) |
| EP (2) | EP2643450A2 (enExample) |
| JP (1) | JP6061862B2 (enExample) |
| KR (1) | KR20130119945A (enExample) |
| CN (2) | CN103502432A (enExample) |
| AU (1) | AU2011336923B2 (enExample) |
| BR (1) | BR112013012630B1 (enExample) |
| CA (1) | CA2818499A1 (enExample) |
| MX (1) | MX2013005654A (enExample) |
| WO (1) | WO2012074818A2 (enExample) |
| ZA (1) | ZA201303544B (enExample) |
Cited By (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| KR20190096229A (ko) * | 2018-02-08 | 2019-08-19 | 주식회사 엘지화학 | 고농도 3-하이드록시프로피온산 수용액의 제조 방법 |
| KR20220061044A (ko) * | 2020-11-05 | 2022-05-12 | 주식회사 엘지화학 | 3-하이드록시프로피온산의 생산 방법 |
| WO2025165198A1 (ko) * | 2024-01-31 | 2025-08-07 | 주식회사 엘지화학 | 3-하이드록시프로피온산의 생산 방법 |
Families Citing this family (89)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP2001992B1 (en) | 2006-03-13 | 2016-07-20 | Cargill Inc. | Yeast cells having disrupted pathway from dihydroxyacetone phosphate to glycerol |
| CA2807561C (en) | 2010-08-06 | 2022-04-12 | Mascoma Corporation | Production of malonyl-coa derived products via anaerobic pathways |
| CA2885934C (en) * | 2010-11-22 | 2018-05-01 | Cargill, Incorporated | Compositions and methods for increased ethanol titer from biomass |
| MX2013005654A (es) | 2010-11-22 | 2013-07-17 | Novozymes Inc | Composiciones y metodos para la produccion de acido 3-hidroxipropionico. |
| CA2840525A1 (en) * | 2011-06-28 | 2013-01-03 | Brookhaven Science Associates, Llc | Modified plants with increased oil content |
| IN2014CN02136A (enExample) * | 2011-08-24 | 2015-05-29 | Novozymes Inc | |
| EP2751261B1 (en) | 2011-09-30 | 2018-08-29 | Novozymes, Inc. | Dehydrogenase variants and polynucleotides encoding same |
| CN118086086A (zh) * | 2012-03-06 | 2024-05-28 | 利戈斯股份有限公司 | 用于产生丙二酸的重组宿主细胞 |
| KR102098849B1 (ko) * | 2012-05-30 | 2020-04-09 | 란자테크 뉴질랜드 리미티드 | 재조합 미생물 및 이의 용도 |
| BR112015002940A2 (pt) | 2012-08-10 | 2018-04-24 | Opx Biotechnologies Inc | microorganismos e métodos para a produção de ácidos graxos e produtos derivados de ácidos graxos. |
| JP2015531237A (ja) * | 2012-10-11 | 2015-11-02 | テクニカル・ユニヴァーシティ・オブ・デンマーク | 遺伝子組換え酵母 |
| JP2015536669A (ja) * | 2012-11-30 | 2015-12-24 | ノボザイムス,インコーポレイティド | 組換え酵母による3−ヒドロキシプロピオン酸の生産 |
| WO2014160050A1 (en) * | 2013-03-14 | 2014-10-02 | Butamax Advanced Biofuels Llc | Glycerol 3- phosphate dehydrogenase for butanol production |
| JP2016518821A (ja) | 2013-03-15 | 2016-06-30 | カーギル・インコーポレイテッド | 3−ヒドロキシプロピオン酸の回収 |
| US9447438B2 (en) | 2013-03-15 | 2016-09-20 | Cargill, Incorporated | Acetyl-coA carboxylases |
| US20150044746A1 (en) * | 2013-03-15 | 2015-02-12 | Opx Biotechnologies, Inc. | Method of enhanced bioproduction |
| EP3008178A1 (en) | 2013-06-14 | 2016-04-20 | Technical University of Denmark | Microbial production of 3-hydroxypropionic acid |
| EP3008179A1 (en) | 2013-06-14 | 2016-04-20 | Technical University of Denmark | 3hp tolerance |
| WO2015010103A2 (en) | 2013-07-19 | 2015-01-22 | Opx Biotechnologies, Inc. | Microorganisms and methods for the production of fatty acids and fatty acid derived products |
| US11408013B2 (en) | 2013-07-19 | 2022-08-09 | Cargill, Incorporated | Microorganisms and methods for the production of fatty acids and fatty acid derived products |
| US9556443B2 (en) | 2013-07-19 | 2017-01-31 | Samsung Electronics Co., Ltd. | Yeast cell with inactivated NADH dehydrogenase and method of producing lactate using the yeast cell |
| US9845484B2 (en) | 2013-07-31 | 2017-12-19 | Novozymes A/S | 3-hydroxypropionic acid production by recombinant yeasts expressing an insect aspartate 1-decarboxylase |
| WO2015031504A1 (en) * | 2013-08-27 | 2015-03-05 | The Regents Of The University Of California | RECOMBINANT PATHWAY AND ORGANISMS FOR MALONYL-CoA SYNTHESIS |
| WO2015036278A1 (de) | 2013-09-12 | 2015-03-19 | Basf Se | Verfahren zur dehydratisierung von 3-hydroxypropionsäure zu acrylsäure |
| WO2015036273A1 (de) | 2013-09-12 | 2015-03-19 | Basf Se | Verfahren zur herstellung von acrylsäure |
| US10252970B2 (en) | 2013-09-12 | 2019-04-09 | Basf Se | Method of dehydrating 3-hydroxypropionic acid to form acrylic acid |
| JP6499587B2 (ja) | 2013-11-22 | 2019-04-10 | Jmtcエンザイム株式会社 | 形質転換体およびその製造方法、ならびに乳酸の製造方法 |
| JP6488666B2 (ja) * | 2013-11-22 | 2019-03-27 | Agc株式会社 | クローニングベクター、発現ベクターおよび形質転換体の製造方法 |
| KR101555867B1 (ko) | 2014-04-18 | 2015-10-01 | 부산대학교 산학협력단 | 3-하이드록시프로피온산을 분해하지 않는 슈도모나스 데니트리피칸스 결실 변이균주 및 이를 이용한 3-하이드록시프로피온산의 생산방법 |
| WO2015200545A1 (en) * | 2014-06-26 | 2015-12-30 | Lygos, Inc. | Recombinant host cells for the production of malonate |
| WO2016026763A1 (en) | 2014-08-18 | 2016-02-25 | Basf Se | Process for preparing acrylic acid using a heterogeneous alumina catalyst |
| JP6786477B2 (ja) | 2014-08-29 | 2020-11-18 | エスケー イノベーション カンパニー リミテッドSk Innovation Co.,Ltd. | 3−hpを生産することができる組換え酵母およびこれを利用した3−hpの製造方法 |
| EP2993228B1 (en) | 2014-09-02 | 2019-10-09 | Cargill, Incorporated | Production of fatty acid esters |
| US10214754B2 (en) | 2014-10-10 | 2019-02-26 | Jmtc Enzyme Corporation | Transformant and its production process, and method for producing lactic acid |
| US20170362613A1 (en) * | 2014-12-19 | 2017-12-21 | Novozymes A/S | Recombinant Host Cells For The Production Of 3-Hydroxypropionic Acid |
| US20180273915A1 (en) * | 2014-12-19 | 2018-09-27 | Novozymes A/S | Recombinant Host Cells For The Production Of 3-Hydroxypropionic Acid |
| WO2016135020A1 (de) | 2015-02-24 | 2016-09-01 | Basf Se | Verfahren zur kontinuierlichen dehydratisierung von 3-hydroxypropionsäure zu acrylsäure |
| WO2016138303A1 (en) | 2015-02-27 | 2016-09-01 | Novozymes A/S | Mutant host cells for the production of 3-hydroxypropionic acid |
| WO2016162175A1 (de) | 2015-04-07 | 2016-10-13 | Basf Se | Verfahren zur dehydratisierung von 3-hydroxypropionsäure zu acrylsäure |
| WO2016200239A1 (ko) * | 2015-06-11 | 2016-12-15 | 부산대학교 산학협력단 | 3-하이드록시프로피온산에 의해 발현이 유도되는 프로모터 시스템 및 이를 이용한 3-하이드록시프로피온산의 생물학적 생산방법 |
| EP3321364B1 (en) | 2015-06-11 | 2022-12-28 | Noroo IC Co., Ltd. | Promoter system inducing expression by 3-hydroxypropionic acid and method for biological production of 3-hydroxypropionic acid using same |
| EP3325608B1 (en) * | 2015-07-21 | 2021-11-24 | The Governing Council of the University of Toronto | Methods and microorganisms for the production of 1,3-butanediol |
| WO2017035270A1 (en) | 2015-08-24 | 2017-03-02 | Novozymes A/S | Beta-alanine aminotransferases for the production of 3-hydroxypropionic acid |
| WO2017083683A1 (en) * | 2015-11-12 | 2017-05-18 | Lygos, Inc. | Recombinant host cells and methods for the anaerobic production of l-aspartate and beta-alanine |
| US20180258437A1 (en) * | 2015-11-12 | 2018-09-13 | Lygos, Inc. | Recombinant host cells and methods for the production of l-aspartate and beta-alanine |
| DE102016114555A1 (de) | 2016-08-05 | 2018-02-08 | Thyssenkrupp Ag | Prozess zur Herstellung von Acrylsäure aus einer wässrigen 3-Hydroxypropanol-Lösung |
| US20190345522A1 (en) | 2016-12-06 | 2019-11-14 | Novozymes A/S | Improved Processes For Production Of Ethanol From Xylose-Containing Cellulosic Substrates Using Engineered Yeast Strains |
| CN110494566A (zh) | 2017-02-02 | 2019-11-22 | 嘉吉公司 | 产生c6-c10脂肪酸衍生物的经遗传修饰的细胞 |
| US11091753B2 (en) | 2017-05-31 | 2021-08-17 | Novozymes A/S | Xylose fermenting yeast strains and processes thereof for ethanol production |
| CA3064042A1 (en) | 2017-06-02 | 2018-12-06 | Novozymes A/S | Improved yeast for ethanol production |
| CN110997619B (zh) | 2017-08-17 | 2023-06-27 | 巴斯夫欧洲公司 | 连续制备丙烯酸正丁酯或丙烯酸异丁酯的方法 |
| US11834484B2 (en) | 2017-08-29 | 2023-12-05 | Novozymes A/S | Bakers's yeast expressing anti-staling/freshness amylases |
| AU2019213033A1 (en) | 2018-01-29 | 2020-09-17 | Microbiogen Pty. Ltd. | Microorganisms with improved nitrogen utilization for ethanol production |
| BR112020024347A2 (pt) | 2018-05-31 | 2021-02-23 | Novozymes A/S | processos para aumentar o crescimento e a produtividade de levedura |
| CA3143683A1 (en) * | 2018-06-25 | 2020-01-02 | Lygos, Inc. | Recombinant host cells and methods for the production of aspartic acid and s-alanine |
| BR112021001282A2 (pt) | 2018-07-25 | 2021-04-27 | Novozymes A/S | levedura expressando enzimas para produção de etanol |
| CA3114783A1 (en) | 2018-10-08 | 2020-04-16 | Novozymes A/S | Enzyme-expressing yeast for ethanol production |
| JP7370587B2 (ja) * | 2018-10-30 | 2023-10-30 | GreenEarthInstitute株式会社 | 有機化合物の製造方法およびコリネ型細菌 |
| EP3877525A2 (en) | 2018-12-18 | 2021-09-15 | Braskem S.A. | Co-production pathway for 3-hp and acetyl-coa derivatives from malonate semialdehyde |
| CN109852605A (zh) * | 2019-01-16 | 2019-06-07 | 徐州工程学院 | 一种诱变筛选高产3-羟基丙酸菌株的方法 |
| CA3143381A1 (en) | 2019-07-26 | 2021-02-04 | Novozymes A/S | Microorganisms with improved nitrogen transport for ethanol production |
| CN112390855B (zh) * | 2019-07-31 | 2022-04-29 | 上海交通大学医学院附属仁济医院 | Pretide-146a在制备缓解或治疗自身免疫性疾病的药物中的应用 |
| MX2022000831A (es) | 2019-08-05 | 2022-02-10 | Novozymes As | Mezclas de enzimas y procesos para producir un ingrediente alimenticio de alto contenido proteico a partir de un subproducto de la vinaza entera. |
| BR112021026477A2 (pt) | 2019-08-06 | 2022-02-08 | Novozymes As | Célula hospedeira recombinante, métodos de produção de um produto de fermentação a partir de um material contendo amido ou contendo celulose, de produção do polipeptídeo maduro, de produção de um derivado de uma célula hospedeira recombinante e de produção de etanol, construto de ácido nucleico ou vetor de expressão, composição, e, uso de uma célula hospedeira recombinante |
| MX2022002834A (es) | 2019-09-16 | 2022-04-06 | Novozymes As | Polipeptidos con actividad beta-glucanasa y polinucleotidos que los codifican. |
| EP4073089A1 (en) | 2019-12-10 | 2022-10-19 | Novozymes A/S | Microorganism for improved pentose fermentation |
| EP4103709A2 (en) | 2020-02-10 | 2022-12-21 | Novozymes A/S | Polypeptides having alpha-amylase activity and polynucleotides encoding same |
| CA3188324A1 (en) | 2020-06-29 | 2022-01-06 | Basf Se | Device for carrying out material exchange processes |
| EP4190907A4 (en) * | 2020-07-31 | 2023-12-06 | LG Chem, Ltd. | PROCESS FOR PREPARING 3-HYDROXYPROPIONIC ACID IN TWO STEP |
| CN111985041B (zh) * | 2020-09-17 | 2021-03-30 | 青岛理工大学 | 一种加固边坡挡墙高度确定方法及加固边坡挡墙 |
| EP4222167A1 (en) | 2020-09-30 | 2023-08-09 | Nobell Foods, Inc. | Recombinant milk proteins and food compositions comprising the same |
| KR20230076829A (ko) * | 2020-09-30 | 2023-05-31 | 게노미엄, 아이엔씨. | 바이오아크롤레인 및 바이오아크릴산을 생산하는 발효 공정 |
| US10894812B1 (en) | 2020-09-30 | 2021-01-19 | Alpine Roads, Inc. | Recombinant milk proteins |
| US10947552B1 (en) | 2020-09-30 | 2021-03-16 | Alpine Roads, Inc. | Recombinant fusion proteins for producing milk proteins in plants |
| EP4237555A1 (en) | 2020-11-02 | 2023-09-06 | Novozymes A/S | Glucoamylase variants and polynucleotides encoding same |
| CN116323929A (zh) * | 2020-11-05 | 2023-06-23 | 株式会社Lg化学 | 具有从葡萄糖生产3-羟基丙酸能力的微生物及其用途 |
| AU2022218205A1 (en) | 2021-02-08 | 2023-05-25 | Lanzatech, Inc. | Recombinant microorganisms and uses therefor |
| AU2022288057A1 (en) | 2021-06-07 | 2023-12-21 | Novozymes A/S | Engineered microorganism for improved ethanol fermentation |
| FR3125042B1 (fr) | 2021-07-09 | 2024-04-12 | Snf Sa | Procédé d’obtention d’alkyl(méth)acrylamide substitué biosourcé |
| FR3125048B1 (fr) | 2021-07-09 | 2024-04-19 | Snf Sa | Polymère biosourcé à biodégradabilité améliorée |
| FR3125040B1 (fr) | 2021-07-09 | 2024-04-26 | Snf Sa | Procédé d’obtention de bio-monomère à partir de diméthylaminoethanol d’origine renouvelable |
| WO2023168244A1 (en) | 2022-03-03 | 2023-09-07 | Cargill, Incorporated | Genetically modified yeast and fermentation processes for the production of 3-hydroxypropionate |
| WO2023168233A1 (en) | 2022-03-03 | 2023-09-07 | Cargill, Incorporated | Genetically modified yeast and fermentation processes for the production of 3-hydroxypropionate |
| CN115595328A (zh) * | 2022-03-07 | 2023-01-13 | 中国科学院微生物研究所(Cn) | 天冬氨酸脱羧酶在发酵生产维生素b5中的应用 |
| CN116024184B (zh) * | 2022-07-26 | 2024-03-15 | 南京科技职业学院 | 醛脱氢酶突变体及其在制备5-羟甲基-2-呋喃甲酸中的应用 |
| EP4638768A2 (en) | 2022-12-19 | 2025-10-29 | Novozymes A/S | Processes for producing fermentation products using fiber-degrading enzymes with engineered yeast |
| WO2024226289A1 (en) | 2023-04-26 | 2024-10-31 | Cargill, Incorporated | Methods for producing propionic acid |
| WO2024258820A2 (en) | 2023-06-13 | 2024-12-19 | Novozymes A/S | Processes for producing fermentation products using engineered yeast expressing a beta-xylosidase |
| WO2025058922A1 (en) | 2023-09-15 | 2025-03-20 | Cargill, Incorporated | Rising film evaporator clean-in-place method and system |
Family Cites Families (67)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| IL39710A (en) | 1972-06-19 | 1975-04-25 | Imi Inst For Res & Dev | Recovery of acids from aqueous solutions by solvent extraction |
| US4771001A (en) | 1986-03-27 | 1988-09-13 | Neurex Corp. | Production of lactic acid by continuous fermentation using an inexpensive raw material and a simplified method of lactic acid purification |
| US5210296A (en) | 1990-11-19 | 1993-05-11 | E. I. Du Pont De Nemours And Company | Recovery of lactate esters and lactic acid from fermentation broth |
| US5132456A (en) | 1991-05-07 | 1992-07-21 | The Regents Of The University Of California | Sorption of carboxylic acid from carboxylic salt solutions at PHS close to or above the pKa of the acid, with regeneration with an aqueous solution of ammonia or low-molecular-weight alkylamine |
| US5420304A (en) | 1992-03-19 | 1995-05-30 | Biopak Technology, Ltd. | Method to produce cyclic esters |
| AT398982B (de) | 1993-02-18 | 1995-02-27 | Vogelbusch Gmbh | Verfahren zur abtrennung und reinigung von milchsäure |
| US5510526A (en) | 1993-06-29 | 1996-04-23 | Cargill, Incorporated | Lactic acid production, separation and/or recovery process |
| IL109003A (en) | 1994-03-16 | 1999-09-22 | Yissum Res Dev Co | Process and extractant composition for extracting water-soluble carboxylic and mineral acids |
| IT1294728B1 (it) | 1997-09-12 | 1999-04-12 | Biopolo S C A R L | Ceppi di lievito per la riproduzione di acido lattico |
| WO1999053035A1 (en) | 1998-04-13 | 1999-10-21 | The University Of Georgia Research Foundation, Inc. | Pyruvate carboxylase overexpression for enhanced production of oxaloacetate-derived biochemicals in microbial cells |
| DE69938105T2 (de) * | 1998-08-04 | 2009-01-29 | Metabolix, Inc., Cambridge | Herstellung von polyhydroxyalkanoaten aus polyolen |
| CA2374482C (en) | 1999-05-21 | 2012-09-18 | Cargill Dow Llc | Methods and materials for the synthesis of organic products |
| WO2001016346A1 (en) | 1999-08-30 | 2001-03-08 | Wisconsin Alumni Research Foundation | Production of 3-hydroxypropionic acid in recombinant organisms |
| US6852517B1 (en) | 1999-08-30 | 2005-02-08 | Wisconsin Alumni Research Foundation | Production of 3-hydroxypropionic acid in recombinant organisms |
| JP4098455B2 (ja) | 2000-03-10 | 2008-06-11 | 株式会社日立製作所 | マーク画像中の電子透かし情報の参照方法及び計算機 |
| JP4490628B2 (ja) | 2000-11-20 | 2010-06-30 | カーギル インコーポレイテッド | 3−ヒドロキシプロピオン酸および他の有機化合物 |
| US7109010B2 (en) | 2000-11-22 | 2006-09-19 | Nature Works Llc | Methods and materials for the synthesis of organic products |
| CA2474152A1 (en) | 2001-11-23 | 2003-06-19 | Cargill Dow Llc | Methods and materials for the production of organic products in cells of candida species |
| DE60327804D1 (de) | 2002-01-18 | 2009-07-09 | Novozymes As | Alanin-2,3-aminomutase |
| JP2005521718A (ja) | 2002-03-25 | 2005-07-21 | カーギル,インコーポレイティド | β−ヒドロキシカルボン酸の誘導体の製造方法 |
| WO2003102200A2 (en) | 2002-05-30 | 2003-12-11 | Cargill Dow Llc | Fermentation process using specific oxygen uptake rates as a process control |
| US7126034B2 (en) | 2002-11-01 | 2006-10-24 | Cargill, Incorporated | Process for preparation of 1,3-propanediol |
| CA2516913A1 (en) | 2003-02-24 | 2004-09-10 | Cargill, Incorporated | Process for preparing 3-hydroxycarboxylic acids |
| BRPI0411925A (pt) | 2003-06-26 | 2006-08-15 | Cargill Inc | processo para separação e recuperação de ácido 3-hidroxipropiÈnico e ácido acrìlico |
| CN100422131C (zh) | 2003-06-26 | 2008-10-01 | 嘉吉有限公司 | 分离和回收3-羟基丙酸和丙烯酸的方法 |
| US7326557B2 (en) | 2003-11-14 | 2008-02-05 | Rice University | Increasing intracellular NADPH availability in E. coli |
| DK1706457T3 (da) | 2003-12-04 | 2012-05-21 | Novozymes As | Fremstilling af 3-hydroxypropionsyre ved anvendelse af beta-alanin/pyruvataminotransferase |
| WO2006022664A2 (en) | 2004-07-30 | 2006-03-02 | Cargill Incorporated | Alanine 2, 3 aminomutases |
| US7987056B2 (en) | 2004-09-20 | 2011-07-26 | The Regents Of The University Of Colorado, A Body Corporate | Mixed-library parallel gene mapping quantitative micro-array technique for genome-wide identification of trait conferring genes |
| MX2007004980A (es) | 2004-10-25 | 2007-06-14 | Codexis Inc | Alanina 2,3-aminomutasas mejoradas, y polinucleotidos relacionados. |
| DK1760156T3 (da) | 2005-08-10 | 2013-03-18 | Univ Florida | Materialer og fremgangsmåder til effektiv mælkesyrefremstilling |
| US7846688B2 (en) | 2005-08-15 | 2010-12-07 | The Regents Of The University Of Colorado | Broad host range vectors for shotgun and expression library cloning in Gram negative bacteria |
| DE102005048818A1 (de) | 2005-10-10 | 2007-04-12 | Degussa Ag | Mikrobiologische Herstellung von 3-Hydroxypropionsäure |
| EP2001992B1 (en) | 2006-03-13 | 2016-07-20 | Cargill Inc. | Yeast cells having disrupted pathway from dihydroxyacetone phosphate to glycerol |
| WO2007130745A1 (en) | 2006-03-15 | 2007-11-15 | Regents Of The University Of Colorado | Enhanced alcohol tolerant microorganism and methods of use thereof |
| US20080103060A1 (en) | 2006-08-08 | 2008-05-01 | Regents Of The University Of Colorado | Compositions and methods for chemical reporter vectors |
| BRPI0716212A2 (pt) * | 2006-08-30 | 2013-10-15 | Cargill Inc | Beta-alanina/alfa-cetoglutarato aminotransferase para produção de ácido 3-hidroxipropiônico |
| US8938765B2 (en) | 2006-12-22 | 2015-01-20 | Time Warner Cable Enterprises Llc | Methods, apparatus and user interface for providing content on demand |
| AU2008206403A1 (en) | 2007-01-12 | 2008-07-24 | The Regents Of The University Of Colorado, A Body Corporate | Compositions and methods for enhancing tolerance for the production of organic chemicals produced by microorganisms |
| US8673601B2 (en) | 2007-01-22 | 2014-03-18 | Genomatica, Inc. | Methods and organisms for growth-coupled production of 3-hydroxypropionic acid |
| US8048624B1 (en) | 2007-12-04 | 2011-11-01 | Opx Biotechnologies, Inc. | Compositions and methods for 3-hydroxypropionate bio-production from biomass |
| BRPI0821230A2 (pt) | 2007-12-19 | 2019-09-24 | Novozymes As | "constructo de ácido nucleico, cédula microbiana hospedeira recombinante, métodos para priduzir um polipeptídeo tendo atividade intensificadora celulolítica, para produzir um mutante de uma cédula precursora, para inibir a expressão de um polipeptídeo com atividade de intensificação celulolítica em uma célula, para produzir uma proteína, para degradar ou converter um material celulósico, e, para fermentar um material celulósico" |
| WO2009089457A1 (en) | 2008-01-11 | 2009-07-16 | Novozymes A/S | Methods for producing 3-hydroxypropionic acid and compounds thereof |
| EP2313491A4 (en) | 2008-07-08 | 2011-12-07 | Opx Biotechnologies Inc | METHODS, COMPOSITIONS AND SYSTEMS FOR BIOSYNTHETIC BIOPRODUCTION OF 1,4-BUTANEDIOL |
| US20100021978A1 (en) * | 2008-07-23 | 2010-01-28 | Genomatica, Inc. | Methods and organisms for production of 3-hydroxypropionic acid |
| US20110244575A1 (en) * | 2008-07-23 | 2011-10-06 | Lipscomb Tanya E W | Methods, systems and compositions for increased microorganism tolerance to and production of 3-hydroxypropionic acid (3-hp) |
| US20110281314A1 (en) | 2008-08-04 | 2011-11-17 | Lynch Michael D | Methods, systems and compositions related to microbial bio-production of butanol and/or isobutanol |
| EP2326709A4 (en) * | 2008-09-15 | 2013-01-23 | Opx Biotechnologies Inc | METHODS, SYSTEMS AND COMPOSITIONS RELATED TO REDUCED MICROBIAL PRODUCTION OF 3-HYDROXYPROPIONIC ACID (3-HP) IN ALDEHYDMETABOLITE |
| US8518684B2 (en) | 2008-11-18 | 2013-08-27 | Novozymes, Inc. | Methods and compositions for degrading cellulosic material |
| US20110144377A1 (en) | 2009-06-16 | 2011-06-16 | E. I. Du Pont Nemours And Company | Process for the biological production of 3-hydroxypropionic acid with high yield |
| WO2011038364A1 (en) | 2009-09-27 | 2011-03-31 | Opx Biotechnologies, Inc. | Method for producing 3-hydroxypropionic acid and other products |
| US8809027B1 (en) | 2009-09-27 | 2014-08-19 | Opx Biotechnologies, Inc. | Genetically modified organisms for increased microbial production of 3-hydroxypropionic acid involving an oxaloacetate alpha-decarboxylase |
| EP2491122A1 (en) | 2009-10-23 | 2012-08-29 | Novozymes Inc. | Cellobiohydrolase variants and polynucleotides encoding same |
| BR112012006847A2 (pt) | 2009-10-29 | 2015-09-08 | Novozymes As | polipeptídeo polinucleotídeo, célula hospedeira recombinante, métodos para produzir o polipeptídeo para produzir um mutante de uma célula parental, para inibir a expressão de um polipeptídeo, para produzir uma proteína, para degradar ou converter um material celulósico, para produzir um produto de fermentação, para produzir um produto de fermentação e pra fermentar um material celulósico, planta transgênica, parte da planta ou célula da planta, molécula de rna inibitória de filamento duplo, e, composição. |
| WO2011063363A2 (en) | 2009-11-20 | 2011-05-26 | Opx Biotechnologies, Inc. | Production of an organic acid and/or related chemicals |
| CA2788045A1 (en) | 2010-01-27 | 2011-08-04 | Opx Biotechnologies, Inc. | Microorganism production of high-valve chemical products, and related compositions, methods and systems |
| US20130052702A1 (en) | 2010-03-30 | 2013-02-28 | Novozymes North America, Inc. | Methods for Enhancing By-Products From Fermentation Processes |
| MX2013001540A (es) | 2010-08-12 | 2013-04-24 | Novozymes Inc | Composiciones que comprende un polipeptido que tiene actividad de incremento celulolititico y un compuesto de quinona, y uso de las mismas. |
| MX2013005654A (es) * | 2010-11-22 | 2013-07-17 | Novozymes Inc | Composiciones y metodos para la produccion de acido 3-hidroxipropionico. |
| US20140121118A1 (en) | 2010-11-23 | 2014-05-01 | Opx Biotechnologies, Inc. | Methods, systems and compositions regarding multiplex construction protein amino-acid substitutions and identification of sequence-activity relationships, to provide gene replacement such as with tagged mutant genes, such as via efficient homologous recombination |
| EP2864282A4 (en) | 2012-06-20 | 2016-01-20 | Opx Biotechnologies Inc | CLEANING OF 3-HYDROXYPROPIONIC ACID FROM RAW CELLBOUILLON AND DEHYDRATION TO ACRYLIC ACID |
| US20130345470A1 (en) | 2012-06-20 | 2013-12-26 | Opx Biotechnologies, Inc. | Purification of 3-Hydroxypropionic Acid from Crude Cell Broth and Production of Acrylamide |
| JP2016518821A (ja) | 2013-03-15 | 2016-06-30 | カーギル・インコーポレイテッド | 3−ヒドロキシプロピオン酸の回収 |
| US9447438B2 (en) | 2013-03-15 | 2016-09-20 | Cargill, Incorporated | Acetyl-coA carboxylases |
| WO2014145343A1 (en) | 2013-03-15 | 2014-09-18 | Opx Biotechnologies, Inc. | Bioproduction of chemicals |
| US20150044746A1 (en) | 2013-03-15 | 2015-02-12 | Opx Biotechnologies, Inc. | Method of enhanced bioproduction |
| EP2976141A4 (en) | 2013-03-15 | 2016-10-05 | Cargill Inc | FLASHING FOR PRODUCTION CLEANING AND RECOVERY |
-
2011
- 2011-11-21 MX MX2013005654A patent/MX2013005654A/es unknown
- 2011-11-21 WO PCT/US2011/061717 patent/WO2012074818A2/en not_active Ceased
- 2011-11-21 EP EP11791715.3A patent/EP2643450A2/en not_active Withdrawn
- 2011-11-21 US US13/301,556 patent/US9090918B2/en active Active
- 2011-11-21 CN CN201180065738.0A patent/CN103502432A/zh active Pending
- 2011-11-21 KR KR1020137015983A patent/KR20130119945A/ko not_active Ceased
- 2011-11-21 AU AU2011336923A patent/AU2011336923B2/en active Active
- 2011-11-21 EP EP17190408.9A patent/EP3287519B1/en active Active
- 2011-11-21 CN CN201711043999.8A patent/CN107828671B/zh active Active
- 2011-11-21 JP JP2013540104A patent/JP6061862B2/ja active Active
- 2011-11-21 CA CA2818499A patent/CA2818499A1/en not_active Abandoned
- 2011-11-21 BR BR112013012630-2A patent/BR112013012630B1/pt active IP Right Grant
-
2013
- 2013-05-15 ZA ZA2013/03544A patent/ZA201303544B/en unknown
- 2013-11-06 US US14/073,441 patent/US20140065681A1/en not_active Abandoned
-
2016
- 2016-03-03 US US15/060,036 patent/US9777280B2/en active Active
-
2017
- 2017-08-28 US US15/688,436 patent/US10260072B2/en active Active
-
2019
- 2019-04-15 US US16/384,150 patent/US10633664B2/en active Active
-
2020
- 2020-04-23 US US16/857,119 patent/US11118187B2/en active Active
-
2021
- 2021-09-13 US US17/473,058 patent/US12054721B2/en active Active
Cited By (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| KR20190096229A (ko) * | 2018-02-08 | 2019-08-19 | 주식회사 엘지화학 | 고농도 3-하이드록시프로피온산 수용액의 제조 방법 |
| KR20220061044A (ko) * | 2020-11-05 | 2022-05-12 | 주식회사 엘지화학 | 3-하이드록시프로피온산의 생산 방법 |
| WO2025165198A1 (ko) * | 2024-01-31 | 2025-08-07 | 주식회사 엘지화학 | 3-하이드록시프로피온산의 생산 방법 |
Also Published As
| Publication number | Publication date |
|---|---|
| US20190249181A1 (en) | 2019-08-15 |
| BR112013012630B1 (pt) | 2021-09-08 |
| AU2011336923B2 (en) | 2017-02-16 |
| US20160177317A1 (en) | 2016-06-23 |
| MX2013005654A (es) | 2013-07-17 |
| US10633664B2 (en) | 2020-04-28 |
| AU2011336923A1 (en) | 2013-05-30 |
| US20220064654A1 (en) | 2022-03-03 |
| US11118187B2 (en) | 2021-09-14 |
| JP6061862B2 (ja) | 2017-01-18 |
| EP3287519B1 (en) | 2020-03-18 |
| JP2013542747A (ja) | 2013-11-28 |
| US9090918B2 (en) | 2015-07-28 |
| ZA201303544B (en) | 2014-11-26 |
| BR112013012630A2 (pt) | 2020-09-24 |
| US20180044684A1 (en) | 2018-02-15 |
| CN103502432A (zh) | 2014-01-08 |
| EP2643450A2 (en) | 2013-10-02 |
| US10260072B2 (en) | 2019-04-16 |
| EP3287519A1 (en) | 2018-02-28 |
| CA2818499A1 (en) | 2012-06-07 |
| US12054721B2 (en) | 2024-08-06 |
| WO2012074818A2 (en) | 2012-06-07 |
| US20120135481A1 (en) | 2012-05-31 |
| CN107828671A (zh) | 2018-03-23 |
| US20200291408A1 (en) | 2020-09-17 |
| US20140065681A1 (en) | 2014-03-06 |
| US9777280B2 (en) | 2017-10-03 |
| CN107828671B (zh) | 2022-03-08 |
| WO2012074818A3 (en) | 2012-09-27 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| CN107828671B (zh) | 用于生产3-羟基丙酸的组合物和方法 | |
| US9845484B2 (en) | 3-hydroxypropionic acid production by recombinant yeasts expressing an insect aspartate 1-decarboxylase | |
| CN107406821B (zh) | 用于生产3-羟基丙酸的突变宿主细胞 | |
| CN105209626B (zh) | 通过重组酵母生产3-羟基丙酸 | |
| CN110869488A (zh) | 增强型代谢物生产酵母 | |
| US20230295671A1 (en) | Enzymes and methods for production of malonic acid and derivatives thereof | |
| US20230272440A1 (en) | Enzymes and methods for production of malonic acid and derivatives thereof | |
| CN110914434A (zh) | 苏氨酸生产酵母 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PA0105 | International application |
Patent event date: 20130620 Patent event code: PA01051R01D Comment text: International Patent Application |
|
| PG1501 | Laying open of application | ||
| A201 | Request for examination | ||
| PA0201 | Request for examination |
Patent event code: PA02012R01D Patent event date: 20160818 Comment text: Request for Examination of Application |
|
| E902 | Notification of reason for refusal | ||
| PE0902 | Notice of grounds for rejection |
Comment text: Notification of reason for refusal Patent event date: 20171029 Patent event code: PE09021S01D |
|
| E601 | Decision to refuse application | ||
| PE0601 | Decision on rejection of patent |
Patent event date: 20180517 Comment text: Decision to Refuse Application Patent event code: PE06012S01D Patent event date: 20171029 Comment text: Notification of reason for refusal Patent event code: PE06011S01I |