JP7175397B2 - リチウムイオン電池及び装置 - Google Patents
リチウムイオン電池及び装置 Download PDFInfo
- Publication number
- JP7175397B2 JP7175397B2 JP2021533826A JP2021533826A JP7175397B2 JP 7175397 B2 JP7175397 B2 JP 7175397B2 JP 2021533826 A JP2021533826 A JP 2021533826A JP 2021533826 A JP2021533826 A JP 2021533826A JP 7175397 B2 JP7175397 B2 JP 7175397B2
- Authority
- JP
- Japan
- Prior art keywords
- substituted
- unsubstituted
- ion battery
- additive
- lithium ion
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Active
Links
Images
Classifications
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M10/00—Secondary cells; Manufacture thereof
- H01M10/42—Methods or arrangements for servicing or maintenance of secondary cells or secondary half-cells
- H01M10/4235—Safety or regulating additives or arrangements in electrodes, separators or electrolyte
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D239/00—Heterocyclic compounds containing 1,3-diazine or hydrogenated 1,3-diazine rings
- C07D239/02—Heterocyclic compounds containing 1,3-diazine or hydrogenated 1,3-diazine rings not condensed with other rings
- C07D239/04—Heterocyclic compounds containing 1,3-diazine or hydrogenated 1,3-diazine rings not condensed with other rings having no double bonds between ring members or between ring members and non-ring members
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D241/00—Heterocyclic compounds containing 1,4-diazine or hydrogenated 1,4-diazine rings
- C07D241/02—Heterocyclic compounds containing 1,4-diazine or hydrogenated 1,4-diazine rings not condensed with other rings
- C07D241/04—Heterocyclic compounds containing 1,4-diazine or hydrogenated 1,4-diazine rings not condensed with other rings having no double bonds between ring members or between ring members and non-ring members
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D251/00—Heterocyclic compounds containing 1,3,5-triazine rings
- C07D251/02—Heterocyclic compounds containing 1,3,5-triazine rings not condensed with other rings
- C07D251/04—Heterocyclic compounds containing 1,3,5-triazine rings not condensed with other rings having no double bonds between ring members or between ring members and non-ring members
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M10/00—Secondary cells; Manufacture thereof
- H01M10/05—Accumulators with non-aqueous electrolyte
- H01M10/052—Li-accumulators
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M10/00—Secondary cells; Manufacture thereof
- H01M10/05—Accumulators with non-aqueous electrolyte
- H01M10/052—Li-accumulators
- H01M10/0525—Rocking-chair batteries, i.e. batteries with lithium insertion or intercalation in both electrodes; Lithium-ion batteries
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M10/00—Secondary cells; Manufacture thereof
- H01M10/05—Accumulators with non-aqueous electrolyte
- H01M10/056—Accumulators with non-aqueous electrolyte characterised by the materials used as electrolytes, e.g. mixed inorganic/organic electrolytes
- H01M10/0564—Accumulators with non-aqueous electrolyte characterised by the materials used as electrolytes, e.g. mixed inorganic/organic electrolytes the electrolyte being constituted of organic materials only
- H01M10/0566—Liquid materials
- H01M10/0567—Liquid materials characterised by the additives
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M4/00—Electrodes
- H01M4/02—Electrodes composed of, or comprising, active material
- H01M4/36—Selection of substances as active materials, active masses, active liquids
- H01M4/48—Selection of substances as active materials, active masses, active liquids of inorganic oxides or hydroxides
- H01M4/50—Selection of substances as active materials, active masses, active liquids of inorganic oxides or hydroxides of manganese
- H01M4/505—Selection of substances as active materials, active masses, active liquids of inorganic oxides or hydroxides of manganese of mixed oxides or hydroxides containing manganese for inserting or intercalating light metals, e.g. LiMn2O4 or LiMn2OxFy
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M4/00—Electrodes
- H01M4/02—Electrodes composed of, or comprising, active material
- H01M4/36—Selection of substances as active materials, active masses, active liquids
- H01M4/48—Selection of substances as active materials, active masses, active liquids of inorganic oxides or hydroxides
- H01M4/52—Selection of substances as active materials, active masses, active liquids of inorganic oxides or hydroxides of nickel, cobalt or iron
- H01M4/525—Selection of substances as active materials, active masses, active liquids of inorganic oxides or hydroxides of nickel, cobalt or iron of mixed oxides or hydroxides containing iron, cobalt or nickel for inserting or intercalating light metals, e.g. LiNiO2, LiCoO2 or LiCoOxFy
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M4/00—Electrodes
- H01M4/02—Electrodes composed of, or comprising, active material
- H01M2004/026—Electrodes composed of, or comprising, active material characterised by the polarity
- H01M2004/028—Positive electrodes
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M2300/00—Electrolytes
- H01M2300/0017—Non-aqueous electrolytes
- H01M2300/0025—Organic electrolyte
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M4/00—Electrodes
- H01M4/02—Electrodes composed of, or comprising, active material
- H01M4/36—Selection of substances as active materials, active masses, active liquids
- H01M4/362—Composites
- H01M4/364—Composites as mixtures
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M4/00—Electrodes
- H01M4/02—Electrodes composed of, or comprising, active material
- H01M4/36—Selection of substances as active materials, active masses, active liquids
- H01M4/38—Selection of substances as active materials, active masses, active liquids of elements or alloys
- H01M4/386—Silicon or alloys based on silicon
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M4/00—Electrodes
- H01M4/02—Electrodes composed of, or comprising, active material
- H01M4/36—Selection of substances as active materials, active masses, active liquids
- H01M4/48—Selection of substances as active materials, active masses, active liquids of inorganic oxides or hydroxides
- H01M4/483—Selection of substances as active materials, active masses, active liquids of inorganic oxides or hydroxides for non-aqueous cells
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M4/00—Electrodes
- H01M4/02—Electrodes composed of, or comprising, active material
- H01M4/36—Selection of substances as active materials, active masses, active liquids
- H01M4/48—Selection of substances as active materials, active masses, active liquids of inorganic oxides or hydroxides
- H01M4/485—Selection of substances as active materials, active masses, active liquids of inorganic oxides or hydroxides of mixed oxides or hydroxides for inserting or intercalating light metals, e.g. LiTi2O4 or LiTi2OxFy
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M4/00—Electrodes
- H01M4/02—Electrodes composed of, or comprising, active material
- H01M4/36—Selection of substances as active materials, active masses, active liquids
- H01M4/58—Selection of substances as active materials, active masses, active liquids of inorganic compounds other than oxides or hydroxides, e.g. sulfides, selenides, tellurides, halogenides or LiCoFy; of polyanionic structures, e.g. phosphates, silicates or borates
- H01M4/583—Carbonaceous material, e.g. graphite-intercalation compounds or CFx
- H01M4/587—Carbonaceous material, e.g. graphite-intercalation compounds or CFx for inserting or intercalating light metals
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y02—TECHNOLOGIES OR APPLICATIONS FOR MITIGATION OR ADAPTATION AGAINST CLIMATE CHANGE
- Y02E—REDUCTION OF GREENHOUSE GAS [GHG] EMISSIONS, RELATED TO ENERGY GENERATION, TRANSMISSION OR DISTRIBUTION
- Y02E60/00—Enabling technologies; Technologies with a potential or indirect contribution to GHG emissions mitigation
- Y02E60/10—Energy storage using batteries
Landscapes
- Chemical & Material Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Electrochemistry (AREA)
- General Chemical & Material Sciences (AREA)
- Engineering & Computer Science (AREA)
- Manufacturing & Machinery (AREA)
- Inorganic Chemistry (AREA)
- Organic Chemistry (AREA)
- Physics & Mathematics (AREA)
- Condensed Matter Physics & Semiconductors (AREA)
- General Physics & Mathematics (AREA)
- Materials Engineering (AREA)
- Secondary Cells (AREA)
- Battery Electrode And Active Subsutance (AREA)
- Composite Materials (AREA)
Description
20min~60min内に、原料P-1へ30%~40%濃度のP-2水溶液を滴下して迅速に攪拌し、滴下が完了した後、迅速に15h~30h攪拌し、70℃~90℃の油浴で3h~5h還流及び攪拌して、無色の発煙粘稠液体中間生成物I-1-1を取得する。続いて、K2CO3、KI、無水アセトニトリルを添加し、迅速に攪拌して固液混合相を形成し、40℃~60℃下で原料P-3を迅速に添加し、続いて10h~20h攪拌した後室温までに冷却し、分離及び精製を行って、式I-1で表される化合物を取得する。
無水炭酸ナトリウム、原料P-4及び原料P-3を無水エタノールで混合し、2h~5h反応及び攪拌する。熱エタノールで数回洗浄することにより粗生成物を取得し、再結晶により式I-2で表される化合物を取得する。
無水炭酸ナトリウム、原料P-5及び原料P-3を無水エタノールで混合し、2h~5h反応及び攪拌する。熱エタノール数回洗浄することにより粗生成物を取得し、再結晶により式I-3で表される化合物を取得する。
2 上部ハウジング、
3 下部ハウジング、
4 電池モジュール、
5 リチウムイオン電池。
Claims (15)
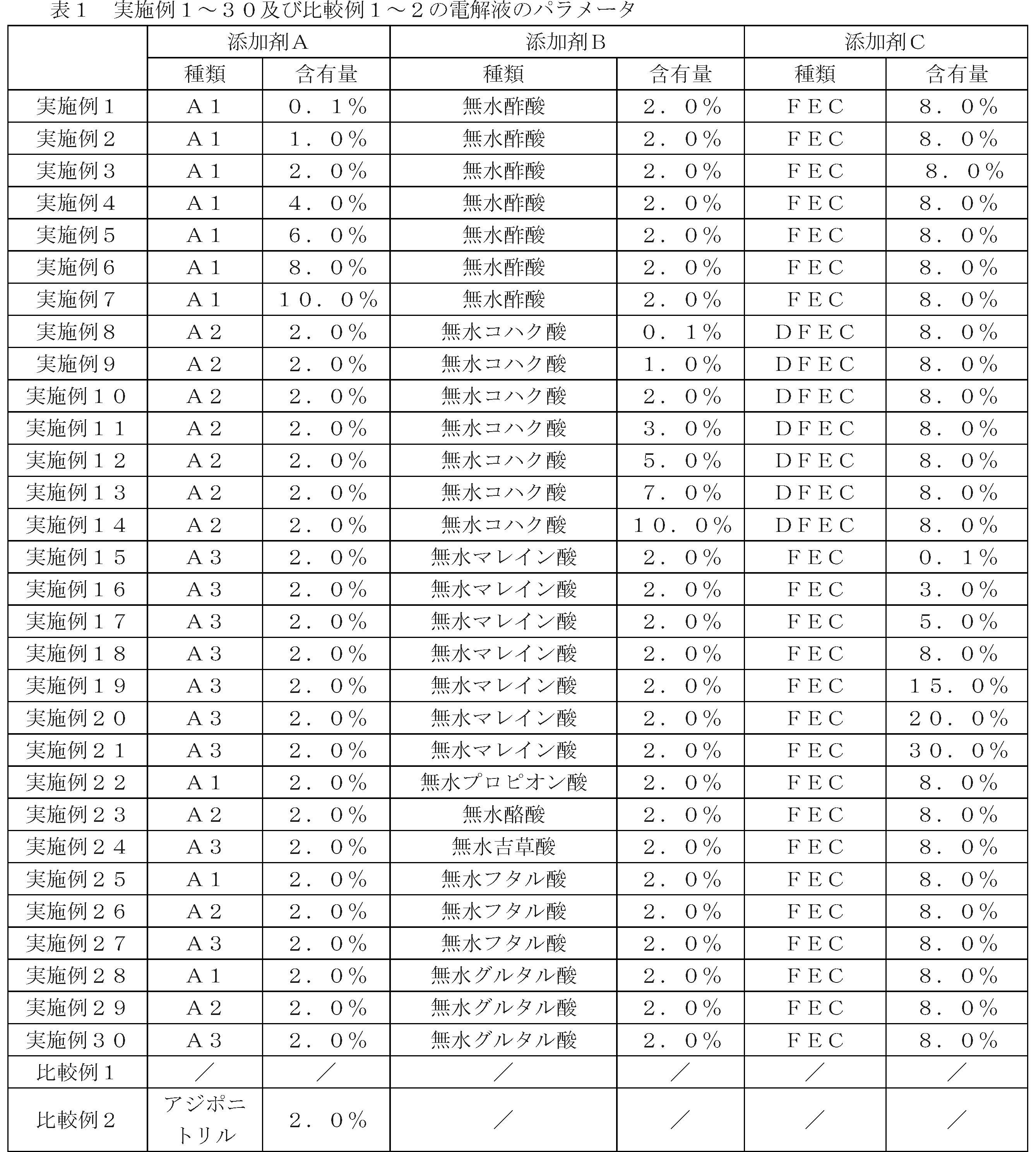
- 正極シート、負極シート及びセパレータを備える電極アセンブリと電解液とを含むリチウムイオン電池であって、
前記正極シートにおける正極活物質は、Lix1Coy1M1-y1O2-z1Qz1を含み、0.5≦x1≦1.2、0.8≦y1<1.0、0≦z1≦0.1であり、Mは、Al、Ti、Zr、Y、Mgから選択される一種類又は複数種類であり、Qは、F、Cl、Sから選択される一種類又は複数種類であり、
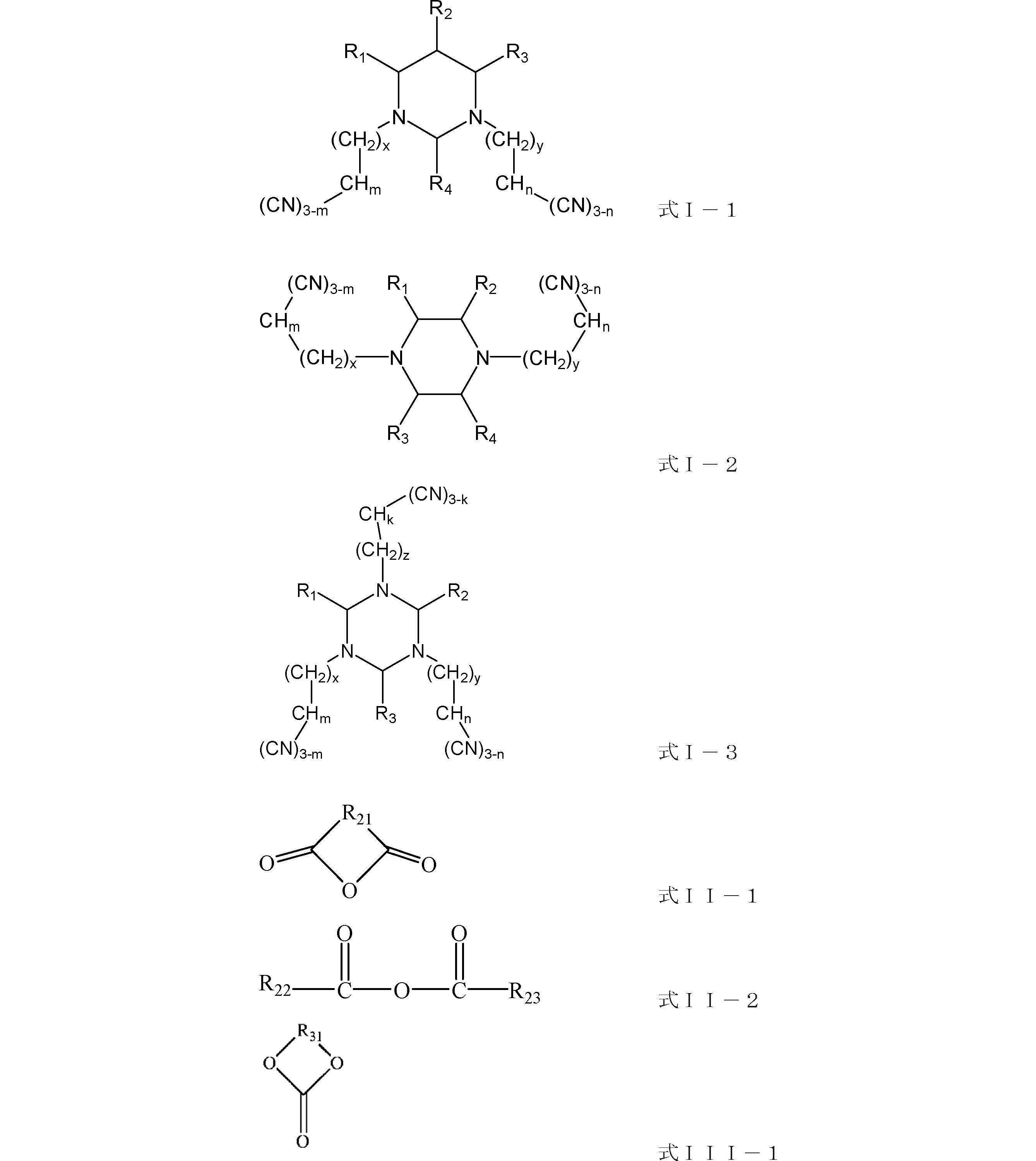
前記電解液には、添加剤A、添加剤B及び添加剤Cが含有され、前記添加剤Aは、式I-1、式I-2、式I-3で表される化合物から選択される一種類又は複数種類であり、前記添加剤Bは、式II-1、式II-2で表される化合物から選択される一種類又は複数種類であり、前記添加剤Cは、式III-1で表される化合物から選択される一種類又は複数種類であり、
上記の式I-1、式I-2、式I-3において、R1、R2、R3、R4は、それぞれ別々に、水素原子、ハロゲン原子、置換又は未置換のC1~C12アルキル、置換又は未置換のC1~C12アルコキシ、置換又は未置換のC1~C12アミノ、置換又は未置換のC2~C12アルケニル、置換又は未置換のC2~C12アルキニル、置換又は未置換のC6~C26アリール、置換又は未置換のC2~C12複素環基から選択され、ここで、置換基は、ハロゲン原子、ニトリル、C1~C6アルキル、C2~C6アルケニル、C1~C6アルコキシから選択される一種類又は複数種類であり、x、y、zは、それぞれ別々に、0~8から選択される整数であり、m、n、kは、それぞれ別々に、0~2から選択される整数であり、
上記の式II-1において、R21は、置換又は未置換のC2~C12アルキル、置換又は未置換のC2~C12アルコキシ、置換又は未置換のC2~C12アミノ、置換又は未置換のC2~C12アルケニル、置換又は未置換のC2~C12アルキニル、置換又は未置換のC6~C26アリール、置換又は未置換のC2~C12複素環基から選択され、ここで、置換基は、ハロゲン原子、ニトリル、C1~C6アルキル、C2~C6アルケニル、C1~C6アルコキシから選択される一種類又は複数種類であり、
上記の式II-2において、R22、R23は、それぞれ別々に、水素原子、置換又は未置換のC1~C12アルキル、置換又は未置換のC1~C12アルコキシ、置換又は未置換のC1~C12アミノ、置換又は未置換のC2~C12アルケニル、置換又は未置換のC2~C12アルキニル、置換又は未置換のC6~C26アリール、置換又は未置換のC2~C12複素環基から選択され、ここで、置換基は、ハロゲン原子、ニトリル、C1~C6アルキル、C2~C6アルケニル、C1~C6アルコキシから選択される一種類又は複数種類であり、
上記の式III-1において、R31は、ハロゲン置換のC1~C6アルキレン、ハロゲン置換のC2~C6アルケニレンから選択される、
ことを特徴とするリチウムイオン電池。 - 上記の式I-1、式I-2、式I-3において、R1、R2、R3及びR4は、それぞれ別々に、水素原子、ハロゲン原子、置換又は未置換のC1~C3直鎖又は分岐鎖アルキル、置換又は未置換のC5~C7環状アルキル、置換又は未置換のC1~C3アルコキシ、置換又は未置換のC1~C3アミノ、置換又は未置換のC2~C3アルケニル、置換又は未置換のC2~C3アルキニル、置換又は未置換のC6~C8アリール、置換又は未置換のC2~C7複素環基から選択され、ここで、置換基は、ハロゲン原子から選択され、
x、y、zは、それぞれ別々に、0、1又は2から選択され、
m、n、kは、それぞれ別々に、1又は2から選択され、
R21は、置換又は未置換のC2~C3直鎖又は分岐鎖アルキル、置換又は未置換のC5~C7環状アルキル、置換又は未置換のC2~C3アルコキシ、置換又は未置換のC2~C3アミノ、置換又は未置換のC2~C3アルケニル、置換又は未置換のC2~C3アルキニル、置換又は未置換のC6~C8アリール、置換又は未置換のC2~C7複素環基から選択され、ここで、置換基は、ハロゲン原子から選択される一種類又は複数種類であり、
R22、R23は、それぞれ別々に、置換又は未置換のC2~C3直鎖又は分岐鎖アルキル、置換又は未置換のC5~C7環状アルキル、置換又は未置換のC2~C3アルコキシ、置換又は未置換のC2~C3アミノ、置換又は未置換のC2~C3アルケニル、置換又は未置換のC2~C3アルキニル、置換又は未置換のC6~C8アリール、置換又は未置換のC2~C7複素環基から選択され、ここで、置換基は、ハロゲン原子から選択される一種類又は複数種類であり、
R31は、ハロゲン置換のC2~C4アルキレン、ハロゲン置換のC2~C4アルケニレンから選択される、
ことを特徴とする請求項1に記載のリチウムイオン電池。 - 上記の式I-1において、R1、R3は、いずれも水素原子であり、
上記の式I-2において、R1、R2、R3、R4における少なくとも二個が水素原子であり、
上記の式I-3において、R1、R2、R3における少なくとも二個が水素原子である、
ことを特徴とする請求項1に記載のリチウムイオン電池。 - 上記の式I-1において、R 1 、R 3 、R 4 は、いずれも水素原子であり、
上記の式I-2において、R 1 、R 2 、R 3 、R 4 における少なくとも三個が水素原子である、
ことを特徴とする請求項3に記載のリチウムイオン電池。 - 前記添加剤Bは、無水コハク酸、無水グルタル酸、無水マレイン酸、無水フタル酸、無水酢酸、無水プロピオン酸、無水酪酸及び無水吉草酸から選択される一種類又は複数種類である、
ことを特徴とする請求項1に記載のリチウムイオン電池。 - 前記添加剤Cは、フルオロエチレンカーボネート、フルオロプロピレンカーボネート、トリフルオロプロピレンカーボネート、トランス又はシス-4,5-ジフルオロ-1,3-ジオキソラン-2-オンから選択される一種類又は複数種類である、
ことを特徴とする請求項1に記載のリチウムイオン電池。 - 前記電解液における前記添加剤Aの質量百分率は、0.1%~10%であり、
前記電解液における前記添加剤Bの質量百分率は、0.1%~10%であり、
前記電解液における前記添加剤Cの質量百分率は、0.1%~30%であり、
ことを特徴とする請求項1に記載のリチウムイオン電池。 - 前記電解液における前記添加剤Aの質量百分率は、0.1~6%であり、
前記電解液における前記添加剤Bの質量百分率は、0.1%~5%であり、
前記電解液における前記添加剤Cの質量百分率は、5%~15%である、
ことを特徴とする請求項8に記載のリチウムイオン電池。 - 前記電解液における前記添加剤Aの質量百分率は、0.1%~3.5%である、
ことを特徴とする請求項8又は9に記載のリチウムイオン電池。 - 前記電解液の導電率は、4mS/cm~12mS/cmである、
ことを特徴とする請求項1に記載のリチウムイオン電池。 - 前記負極シートにおける負極活物質は、Si、SiOx2、Si/C複合材料、Si合金のうちの一種類又は複数種類を含み、0<x2≦2である、
ことを特徴とする請求項1に記載のリチウムイオン電池。 - 前記リチウムイオン電池の充電カットオフ電圧は、4.2V以上であり、
ことを特徴とする請求項1に記載のリチウムイオン電池。 - 前記リチウムイオン電池の充電カットオフ電圧は、4.35V以上である、
ことを特徴とする請求項13に記載のリチウムイオン電池。 - 請求項1乃至10のいずれか1項に記載のリチウムイオン電池を備える、
ことを特徴とする装置。
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN201811537017.5 | 2018-12-14 | ||
| CN201811537017.5A CN111326728B (zh) | 2018-12-14 | 2018-12-14 | 锂离子电池 |
| PCT/CN2019/125323 WO2020119805A1 (zh) | 2018-12-14 | 2019-12-13 | 锂离子电池及装置 |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| JP2022514239A JP2022514239A (ja) | 2022-02-10 |
| JP7175397B2 true JP7175397B2 (ja) | 2022-11-18 |
Family
ID=71076233
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2021533826A Active JP7175397B2 (ja) | 2018-12-14 | 2019-12-13 | リチウムイオン電池及び装置 |
Country Status (7)
| Country | Link |
|---|---|
| US (1) | US10938068B2 (ja) |
| EP (1) | EP3787089B1 (ja) |
| JP (1) | JP7175397B2 (ja) |
| KR (1) | KR102490008B1 (ja) |
| CN (1) | CN111326728B (ja) |
| HU (1) | HUE065148T2 (ja) |
| WO (1) | WO2020119805A1 (ja) |
Families Citing this family (15)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN109216765B (zh) * | 2017-07-05 | 2020-05-05 | 宁德时代新能源科技股份有限公司 | 一种电解液及电化学装置 |
| CN111326733B (zh) * | 2018-12-14 | 2021-05-04 | 宁德时代新能源科技股份有限公司 | 锂离子电池 |
| CA3191127A1 (en) * | 2020-08-27 | 2022-03-03 | NOHMs Technologies, Inc. | Epoxy modified additives for lithium ion batteries |
| CN113066975B (zh) * | 2021-03-25 | 2022-06-17 | 珠海市赛纬电子材料股份有限公司 | 锂离子电池 |
| WO2022198667A1 (zh) * | 2021-03-26 | 2022-09-29 | 宁德新能源科技有限公司 | 一种正极极片、包含该正极极片的电化学装置和电子装置 |
| CN113851613B (zh) * | 2021-11-02 | 2023-05-05 | 惠州亿纬锂能股份有限公司 | 一种具有人工sei膜的硅碳负极材料及其制备方法与应用 |
| CN114552008B (zh) * | 2022-02-21 | 2025-01-10 | 宁德新能源科技有限公司 | 电化学装置及电子装置 |
| KR20230129746A (ko) * | 2022-03-02 | 2023-09-11 | 주식회사 엘지에너지솔루션 | 비수계 전해액 조성물 및 이를 포함하는 리튬 이차전지 |
| CN114678592B (zh) * | 2022-05-30 | 2022-09-13 | 深圳澳睿新能源科技有限公司 | 含氰基环状胺化合物的非水电解液、锂离子电池及其应用 |
| WO2023240628A1 (zh) * | 2022-06-17 | 2023-12-21 | 宁德时代新能源科技股份有限公司 | 一种添加剂及其制备方法和用途、正极极片及其制备方法 |
| CN115275346A (zh) * | 2022-08-22 | 2022-11-01 | 株洲万氟化工科技有限公司 | 一种基于无机酸酐的电解液添加剂 |
| CN119998950A (zh) * | 2023-05-16 | 2025-05-13 | 宁德时代新能源科技股份有限公司 | 电池单体、包含其的电池和用电装置 |
| CA3208448A1 (fr) * | 2023-08-04 | 2025-06-19 | Hydro-Quebec | Composes trappeurs de sulfure d'hydrogene, compositions les comprenant et leurs utilisations |
| CN119852538B (zh) * | 2024-04-02 | 2025-10-21 | 宁德时代新能源科技股份有限公司 | 二次电池和用电装置 |
| CN118040058B (zh) * | 2024-04-10 | 2024-07-19 | 广州天赐高新材料股份有限公司 | 电解液添加剂、电解液和电池 |
Citations (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2001223008A (ja) | 1999-12-02 | 2001-08-17 | Honjo Chemical Corp | リチウムイオン二次電池、そのための正極活物質及びその製造方法 |
| JP2011119097A (ja) | 2009-12-02 | 2011-06-16 | Sony Corp | 非水電解質電池 |
| CN103078140A (zh) | 2013-02-03 | 2013-05-01 | 宁德新能源科技有限公司 | 锂离子二次电池及其电解液 |
| JP2016536776A (ja) | 2014-09-29 | 2016-11-24 | シェンヂェン キヤプケム テクノロジー 力ンパニー リミテッドShenzhen Capchem Technology Co.,Ltd. | 高電圧リチウムイオン電池の電解液及び高電圧リチウムイオン電池 |
| US20180013168A1 (en) | 2015-03-31 | 2018-01-11 | Lg Chem, Ltd. | Non-aqueous electrolyte and lithium secondary battery comprising same |
Family Cites Families (15)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPH11111332A (ja) * | 1997-09-30 | 1999-04-23 | Sanyo Electric Co Ltd | 非水電解質電池 |
| JP4815660B2 (ja) * | 2000-06-16 | 2011-11-16 | 三菱化学株式会社 | 非水電解液及び非水電解液二次電池 |
| EP1317013B1 (en) * | 2001-07-10 | 2017-03-15 | Mitsubishi Chemical Corporation | Non-aqueous electrolyte and secondary cell using the same |
| JP4271448B2 (ja) * | 2003-01-16 | 2009-06-03 | パナソニック株式会社 | 非水電解質二次電池用正極活物質 |
| JP2012104439A (ja) | 2010-11-12 | 2012-05-31 | Mitsubishi Chemicals Corp | 非水系電解液及びそれを用いた非水系電解液二次電池 |
| CN103022556B (zh) * | 2013-01-05 | 2015-06-03 | 宁德新能源科技有限公司 | 锂离子电池及其电解液 |
| CN110010882A (zh) * | 2013-02-27 | 2019-07-12 | 三菱化学株式会社 | 非水电解液及使用该非水电解液的非水电解质电池 |
| KR102161266B1 (ko) * | 2013-08-30 | 2020-09-29 | 삼성전자주식회사 | 리튬 이차 전지용 전해질 및 이를 포함하는 리튬 이차 전지 |
| CN103618081A (zh) * | 2013-11-22 | 2014-03-05 | 南通瑞翔新材料有限公司 | 一种高电压高容量锂离子电池正极材料及其制备方法 |
| CN105633460B (zh) * | 2014-11-10 | 2019-01-11 | 宁德时代新能源科技股份有限公司 | 锂离子二次电池电解液及锂离子二次电池 |
| CN106356561B (zh) * | 2015-07-13 | 2019-07-30 | 宁德时代新能源科技股份有限公司 | 防过充电解液及锂离子电池 |
| KR102446364B1 (ko) * | 2015-09-03 | 2022-09-21 | 삼성에스디아이 주식회사 | 리튬 이차 전지용 전해질 및 이를 포함하는 리튬 이차 전지 |
| CN105655639B (zh) * | 2016-01-11 | 2018-10-12 | 东莞新能源科技有限公司 | 电解液以及包括该电解液的锂离子电池 |
| CN109148950B (zh) * | 2017-06-15 | 2020-10-02 | 宁德时代新能源科技股份有限公司 | 一种电解液及电池 |
| CN110391460B (zh) | 2018-04-20 | 2021-02-02 | 宁德时代新能源科技股份有限公司 | 电解液及电池 |
-
2018
- 2018-12-14 CN CN201811537017.5A patent/CN111326728B/zh active Active
-
2019
- 2019-12-13 KR KR1020217020056A patent/KR102490008B1/ko active Active
- 2019-12-13 US US17/043,683 patent/US10938068B2/en active Active
- 2019-12-13 JP JP2021533826A patent/JP7175397B2/ja active Active
- 2019-12-13 WO PCT/CN2019/125323 patent/WO2020119805A1/zh not_active Ceased
- 2019-12-13 EP EP19897379.4A patent/EP3787089B1/en active Active
- 2019-12-13 HU HUE19897379A patent/HUE065148T2/hu unknown
Patent Citations (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2001223008A (ja) | 1999-12-02 | 2001-08-17 | Honjo Chemical Corp | リチウムイオン二次電池、そのための正極活物質及びその製造方法 |
| JP2011119097A (ja) | 2009-12-02 | 2011-06-16 | Sony Corp | 非水電解質電池 |
| CN103078140A (zh) | 2013-02-03 | 2013-05-01 | 宁德新能源科技有限公司 | 锂离子二次电池及其电解液 |
| JP2016536776A (ja) | 2014-09-29 | 2016-11-24 | シェンヂェン キヤプケム テクノロジー 力ンパニー リミテッドShenzhen Capchem Technology Co.,Ltd. | 高電圧リチウムイオン電池の電解液及び高電圧リチウムイオン電池 |
| US20180013168A1 (en) | 2015-03-31 | 2018-01-11 | Lg Chem, Ltd. | Non-aqueous electrolyte and lithium secondary battery comprising same |
Also Published As
| Publication number | Publication date |
|---|---|
| EP3787089A1 (en) | 2021-03-03 |
| EP3787089A4 (en) | 2021-07-28 |
| WO2020119805A1 (zh) | 2020-06-18 |
| HUE065148T2 (hu) | 2024-05-28 |
| US20210036368A1 (en) | 2021-02-04 |
| CN111326728A (zh) | 2020-06-23 |
| US10938068B2 (en) | 2021-03-02 |
| KR102490008B1 (ko) | 2023-01-17 |
| CN111326728B (zh) | 2021-09-21 |
| EP3787089B1 (en) | 2023-11-22 |
| JP2022514239A (ja) | 2022-02-10 |
| KR20210094633A (ko) | 2021-07-29 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP7175397B2 (ja) | リチウムイオン電池及び装置 | |
| US11695157B2 (en) | Lithium-ion battery and apparatus | |
| EP3783723B1 (en) | Lithium-ion battery and apparatus | |
| US11177508B2 (en) | Lithium-ion battery and apparatus | |
| WO2020119807A1 (zh) | 锂离子电池及装置 | |
| EP3796449B1 (en) | Lithium-ion battery and device comprising the same | |
| US12183922B2 (en) | Lithium-ion battery and apparatus | |
| CN111326718B (zh) | 锂离子电池 | |
| CN119786723A (zh) | 一种电解液、电池、电池组及用电设备 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20210618 |
|
| A977 | Report on retrieval |
Free format text: JAPANESE INTERMEDIATE CODE: A971007 Effective date: 20220413 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20220418 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20220617 |
|
| TRDD | Decision of grant or rejection written | ||
| A01 | Written decision to grant a patent or to grant a registration (utility model) |
Free format text: JAPANESE INTERMEDIATE CODE: A01 Effective date: 20221025 |
|
| A61 | First payment of annual fees (during grant procedure) |
Free format text: JAPANESE INTERMEDIATE CODE: A61 Effective date: 20221108 |
|
| R150 | Certificate of patent or registration of utility model |
Ref document number: 7175397 Country of ref document: JP Free format text: JAPANESE INTERMEDIATE CODE: R150 |
|
| S111 | Request for change of ownership or part of ownership |
Free format text: JAPANESE INTERMEDIATE CODE: R313113 |
|
| R350 | Written notification of registration of transfer |
Free format text: JAPANESE INTERMEDIATE CODE: R350 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |