JP6700263B2 - エロビキシバットの結晶変態 - Google Patents
エロビキシバットの結晶変態 Download PDFInfo
- Publication number
- JP6700263B2 JP6700263B2 JP2017519487A JP2017519487A JP6700263B2 JP 6700263 B2 JP6700263 B2 JP 6700263B2 JP 2017519487 A JP2017519487 A JP 2017519487A JP 2017519487 A JP2017519487 A JP 2017519487A JP 6700263 B2 JP6700263 B2 JP 6700263B2
- Authority
- JP
- Japan
- Prior art keywords
- crystalline
- liver
- erobixivat
- positions
- peaks
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Active
Links
- 239000013078 crystal Substances 0.000 title description 60
- 230000009466 transformation Effects 0.000 title description 6
- 238000000634 powder X-ray diffraction Methods 0.000 claims description 67
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 claims description 34
- 150000004683 dihydrates Chemical class 0.000 claims description 29
- 201000010099 disease Diseases 0.000 claims description 21
- 238000011282 treatment Methods 0.000 claims description 18
- 206010010774 Constipation Diseases 0.000 claims description 12
- 208000035475 disorder Diseases 0.000 claims description 12
- 208000024891 symptom Diseases 0.000 claims description 11
- 208000019423 liver disease Diseases 0.000 claims description 10
- 208000008338 non-alcoholic fatty liver disease Diseases 0.000 claims description 10
- 206010008635 Cholestasis Diseases 0.000 claims description 9
- 206010019708 Hepatic steatosis Diseases 0.000 claims description 8
- 239000003613 bile acid Substances 0.000 claims description 8
- 230000007870 cholestasis Effects 0.000 claims description 8
- 231100000359 cholestasis Toxicity 0.000 claims description 8
- 150000001875 compounds Chemical class 0.000 claims description 8
- 210000004185 liver Anatomy 0.000 claims description 8
- HSINOMROUCMIEA-FGVHQWLLSA-N (2s,4r)-4-[(3r,5s,6r,7r,8s,9s,10s,13r,14s,17r)-6-ethyl-3,7-dihydroxy-10,13-dimethyl-2,3,4,5,6,7,8,9,11,12,14,15,16,17-tetradecahydro-1h-cyclopenta[a]phenanthren-17-yl]-2-methylpentanoic acid Chemical compound C([C@@]12C)C[C@@H](O)C[C@H]1[C@@H](CC)[C@@H](O)[C@@H]1[C@@H]2CC[C@]2(C)[C@@H]([C@H](C)C[C@H](C)C(O)=O)CC[C@H]21 HSINOMROUCMIEA-FGVHQWLLSA-N 0.000 claims description 7
- 239000008194 pharmaceutical composition Substances 0.000 claims description 7
- 206010023126 Jaundice Diseases 0.000 claims description 6
- 230000001684 chronic effect Effects 0.000 claims description 6
- 208000019425 cirrhosis of liver Diseases 0.000 claims description 6
- 206010053219 non-alcoholic steatohepatitis Diseases 0.000 claims description 6
- 230000015572 biosynthetic process Effects 0.000 claims description 5
- 239000000126 substance Substances 0.000 claims description 5
- 208000001072 type 2 diabetes mellitus Diseases 0.000 claims description 5
- 208000008439 Biliary Liver Cirrhosis Diseases 0.000 claims description 4
- 208000033222 Biliary cirrhosis primary Diseases 0.000 claims description 4
- 208000004930 Fatty Liver Diseases 0.000 claims description 4
- 229940125922 IBAT inhibitor Drugs 0.000 claims description 4
- 208000017170 Lipid metabolism disease Diseases 0.000 claims description 4
- 208000012654 Primary biliary cholangitis Diseases 0.000 claims description 4
- 201000002150 Progressive familial intrahepatic cholestasis Diseases 0.000 claims description 4
- 208000003251 Pruritus Diseases 0.000 claims description 4
- 150000008064 anhydrides Chemical class 0.000 claims description 4
- 239000003814 drug Substances 0.000 claims description 4
- 208000010706 fatty liver disease Diseases 0.000 claims description 4
- 208000002551 irritable bowel syndrome Diseases 0.000 claims description 4
- 230000035935 pregnancy Effects 0.000 claims description 4
- 231100000240 steatosis hepatitis Toxicity 0.000 claims description 4
- 208000032928 Dyslipidaemia Diseases 0.000 claims description 3
- 206010028980 Neoplasm Diseases 0.000 claims description 3
- 206010061902 Pancreatic neoplasm Diseases 0.000 claims description 3
- 239000003085 diluting agent Substances 0.000 claims description 3
- 238000003786 synthesis reaction Methods 0.000 claims description 3
- 201000011374 Alagille syndrome Diseases 0.000 claims description 2
- 206010003827 Autoimmune hepatitis Diseases 0.000 claims description 2
- 208000008964 Chemical and Drug Induced Liver Injury Diseases 0.000 claims description 2
- 201000003883 Cystic fibrosis Diseases 0.000 claims description 2
- 206010012735 Diarrhoea Diseases 0.000 claims description 2
- 206010072268 Drug-induced liver injury Diseases 0.000 claims description 2
- WQZGKKKJIJFFOK-GASJEMHNSA-N Glucose Natural products OC[C@H]1OC(O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-GASJEMHNSA-N 0.000 claims description 2
- 208000027761 Hepatic autoimmune disease Diseases 0.000 claims description 2
- 206010019728 Hepatitis alcoholic Diseases 0.000 claims description 2
- 206010019799 Hepatitis viral Diseases 0.000 claims description 2
- 208000035150 Hypercholesterolemia Diseases 0.000 claims description 2
- 208000031226 Hyperlipidaemia Diseases 0.000 claims description 2
- 208000001019 Inborn Errors Metabolism Diseases 0.000 claims description 2
- 206010022489 Insulin Resistance Diseases 0.000 claims description 2
- 206010065973 Iron Overload Diseases 0.000 claims description 2
- 208000001145 Metabolic Syndrome Diseases 0.000 claims description 2
- 208000008589 Obesity Diseases 0.000 claims description 2
- 206010033645 Pancreatitis Diseases 0.000 claims description 2
- 208000017855 Progressive familial intrahepatic cholestasis type 1 Diseases 0.000 claims description 2
- 201000004525 Zellweger Syndrome Diseases 0.000 claims description 2
- 201000000690 abdominal obesity-metabolic syndrome Diseases 0.000 claims description 2
- 238000005299 abrasion Methods 0.000 claims description 2
- 208000002353 alcoholic hepatitis Diseases 0.000 claims description 2
- 210000003445 biliary tract Anatomy 0.000 claims description 2
- 201000011510 cancer Diseases 0.000 claims description 2
- 230000002490 cerebral effect Effects 0.000 claims description 2
- 201000001883 cholelithiasis Diseases 0.000 claims description 2
- 229940079593 drug Drugs 0.000 claims description 2
- 201000008865 drug-induced hepatitis Diseases 0.000 claims description 2
- 208000009866 extrahepatic cholestasis Diseases 0.000 claims description 2
- 208000001130 gallstones Diseases 0.000 claims description 2
- 239000008103 glucose Substances 0.000 claims description 2
- 208000006454 hepatitis Diseases 0.000 claims description 2
- 231100000283 hepatitis Toxicity 0.000 claims description 2
- 208000016245 inborn errors of metabolism Diseases 0.000 claims description 2
- 208000015978 inherited metabolic disease Diseases 0.000 claims description 2
- 208000001024 intrahepatic cholestasis Diseases 0.000 claims description 2
- 201000007270 liver cancer Diseases 0.000 claims description 2
- 208000014018 liver neoplasm Diseases 0.000 claims description 2
- 230000009826 neoplastic cell growth Effects 0.000 claims description 2
- 235000020824 obesity Nutrition 0.000 claims description 2
- 201000002528 pancreatic cancer Diseases 0.000 claims description 2
- 208000007232 portal hypertension Diseases 0.000 claims description 2
- 230000000750 progressive effect Effects 0.000 claims description 2
- 201000002162 progressive familial intrahepatic cholestasis 1 Diseases 0.000 claims description 2
- 208000010157 sclerosing cholangitis Diseases 0.000 claims description 2
- 208000011580 syndromic disease Diseases 0.000 claims description 2
- 210000002435 tendon Anatomy 0.000 claims description 2
- 230000001225 therapeutic effect Effects 0.000 claims description 2
- 201000001862 viral hepatitis Diseases 0.000 claims description 2
- 239000000178 monomer Substances 0.000 claims 6
- 125000002057 carboxymethyl group Chemical group [H]OC(=O)C([H])([H])[*] 0.000 claims 5
- 230000002265 prevention Effects 0.000 claims 3
- 230000007547 defect Effects 0.000 claims 2
- 206010016654 Fibrosis Diseases 0.000 claims 1
- 206010067584 Type 1 diabetes mellitus Diseases 0.000 claims 1
- 230000007882 cirrhosis Effects 0.000 claims 1
- 230000007872 intrahepatic cholestasis Effects 0.000 claims 1
- 230000004048 modification Effects 0.000 description 74
- 238000012986 modification Methods 0.000 description 74
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 41
- 239000000523 sample Substances 0.000 description 34
- XEKOWRVHYACXOJ-UHFFFAOYSA-N Ethyl acetate Chemical compound CCOC(C)=O XEKOWRVHYACXOJ-UHFFFAOYSA-N 0.000 description 18
- 239000002002 slurry Substances 0.000 description 16
- 239000012453 solvate Substances 0.000 description 13
- WEVYAHXRMPXWCK-UHFFFAOYSA-N Acetonitrile Chemical compound CC#N WEVYAHXRMPXWCK-UHFFFAOYSA-N 0.000 description 12
- 238000002360 preparation method Methods 0.000 description 12
- 230000005855 radiation Effects 0.000 description 12
- 230000008859 change Effects 0.000 description 10
- 239000000203 mixture Substances 0.000 description 10
- 238000004458 analytical method Methods 0.000 description 9
- 239000007787 solid Substances 0.000 description 9
- 150000004682 monohydrates Chemical group 0.000 description 8
- 239000000843 powder Substances 0.000 description 8
- 238000001179 sorption measurement Methods 0.000 description 8
- IMNFDUFMRHMDMM-UHFFFAOYSA-N N-Heptane Chemical compound CCCCCCC IMNFDUFMRHMDMM-UHFFFAOYSA-N 0.000 description 7
- 238000003795 desorption Methods 0.000 description 7
- 239000002904 solvent Substances 0.000 description 7
- 238000012360 testing method Methods 0.000 description 7
- CSCPPACGZOOCGX-UHFFFAOYSA-N Acetone Chemical compound CC(C)=O CSCPPACGZOOCGX-UHFFFAOYSA-N 0.000 description 6
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 description 6
- KFZMGEQAYNKOFK-UHFFFAOYSA-N Isopropanol Chemical compound CC(C)O KFZMGEQAYNKOFK-UHFFFAOYSA-N 0.000 description 6
- 238000003756 stirring Methods 0.000 description 6
- NTIZESTWPVYFNL-UHFFFAOYSA-N Methyl isobutyl ketone Chemical compound CC(C)CC(C)=O NTIZESTWPVYFNL-UHFFFAOYSA-N 0.000 description 5
- UIHCLUNTQKBZGK-UHFFFAOYSA-N Methyl isobutyl ketone Natural products CCC(C)C(C)=O UIHCLUNTQKBZGK-UHFFFAOYSA-N 0.000 description 5
- XUIMIQQOPSSXEZ-UHFFFAOYSA-N Silicon Chemical compound [Si] XUIMIQQOPSSXEZ-UHFFFAOYSA-N 0.000 description 5
- 238000002425 crystallisation Methods 0.000 description 5
- 230000008025 crystallization Effects 0.000 description 5
- 238000009472 formulation Methods 0.000 description 5
- 238000011321 prophylaxis Methods 0.000 description 5
- 229910052710 silicon Inorganic materials 0.000 description 5
- 239000010703 silicon Substances 0.000 description 5
- 241001465754 Metazoa Species 0.000 description 4
- 239000004480 active ingredient Substances 0.000 description 4
- 238000000034 method Methods 0.000 description 4
- 239000012071 phase Substances 0.000 description 4
- OKKJLVBELUTLKV-UHFFFAOYSA-N Methanol Chemical compound OC OKKJLVBELUTLKV-UHFFFAOYSA-N 0.000 description 3
- VYPSYNLAJGMNEJ-UHFFFAOYSA-N Silicium dioxide Chemical compound O=[Si]=O VYPSYNLAJGMNEJ-UHFFFAOYSA-N 0.000 description 3
- PNEYBMLMFCGWSK-UHFFFAOYSA-N aluminium oxide Inorganic materials [O-2].[O-2].[O-2].[Al+3].[Al+3] PNEYBMLMFCGWSK-UHFFFAOYSA-N 0.000 description 3
- 230000008901 benefit Effects 0.000 description 3
- 108091022863 bile acid binding Proteins 0.000 description 3
- 102000030904 bile acid binding Human genes 0.000 description 3
- 230000007812 deficiency Effects 0.000 description 3
- 238000005259 measurement Methods 0.000 description 3
- 230000007246 mechanism Effects 0.000 description 3
- 239000013557 residual solvent Substances 0.000 description 3
- 230000004044 response Effects 0.000 description 3
- 230000002441 reversible effect Effects 0.000 description 3
- 239000012047 saturated solution Substances 0.000 description 3
- 239000007790 solid phase Substances 0.000 description 3
- 239000011800 void material Substances 0.000 description 3
- DHMQDGOQFOQNFH-UHFFFAOYSA-N Glycine Natural products NCC(O)=O DHMQDGOQFOQNFH-UHFFFAOYSA-N 0.000 description 2
- 239000004471 Glycine Substances 0.000 description 2
- FAPWRFPIFSIZLT-UHFFFAOYSA-M Sodium chloride Chemical compound [Na+].[Cl-] FAPWRFPIFSIZLT-UHFFFAOYSA-M 0.000 description 2
- GWEVSGVZZGPLCZ-UHFFFAOYSA-N Titan oxide Chemical compound O=[Ti]=O GWEVSGVZZGPLCZ-UHFFFAOYSA-N 0.000 description 2
- MCMNRKCIXSYSNV-UHFFFAOYSA-N Zirconium dioxide Chemical compound O=[Zr]=O MCMNRKCIXSYSNV-UHFFFAOYSA-N 0.000 description 2
- 239000013543 active substance Substances 0.000 description 2
- OSGAYBCDTDRGGQ-UHFFFAOYSA-L calcium sulfate Chemical compound [Ca+2].[O-]S([O-])(=O)=O OSGAYBCDTDRGGQ-UHFFFAOYSA-L 0.000 description 2
- 125000003917 carbamoyl group Chemical group [H]N([H])C(*)=O 0.000 description 2
- 239000003795 chemical substances by application Substances 0.000 description 2
- 230000000694 effects Effects 0.000 description 2
- XFLQIRAKKLNXRQ-UUWRZZSWSA-N elobixibat Chemical compound C12=CC(SC)=C(OCC(=O)N[C@@H](C(=O)NCC(O)=O)C=3C=CC=CC=3)C=C2S(=O)(=O)CC(CCCC)(CCCC)CN1C1=CC=CC=C1 XFLQIRAKKLNXRQ-UUWRZZSWSA-N 0.000 description 2
- 229950000820 elobixibat Drugs 0.000 description 2
- 108010007192 elobixibat Proteins 0.000 description 2
- 239000003112 inhibitor Substances 0.000 description 2
- 238000004519 manufacturing process Methods 0.000 description 2
- 229910052751 metal Inorganic materials 0.000 description 2
- 239000002184 metal Substances 0.000 description 2
- 125000002496 methyl group Chemical group [H]C([H])([H])* 0.000 description 2
- 238000002156 mixing Methods 0.000 description 2
- 150000002894 organic compounds Chemical class 0.000 description 2
- 230000036961 partial effect Effects 0.000 description 2
- 230000003449 preventive effect Effects 0.000 description 2
- 125000002924 primary amino group Chemical group [H]N([H])* 0.000 description 2
- 108090000765 processed proteins & peptides Proteins 0.000 description 2
- 238000000746 purification Methods 0.000 description 2
- 229920006395 saturated elastomer Polymers 0.000 description 2
- 239000000741 silica gel Substances 0.000 description 2
- 229910002027 silica gel Inorganic materials 0.000 description 2
- 230000007704 transition Effects 0.000 description 2
- ZILVNHNSYBNLSZ-UHFFFAOYSA-N 2-(diaminomethylideneamino)guanidine Chemical compound NC(N)=NNC(N)=N ZILVNHNSYBNLSZ-UHFFFAOYSA-N 0.000 description 1
- IGRCWJPBLWGNPX-UHFFFAOYSA-N 3-(2-chlorophenyl)-n-(4-chlorophenyl)-n,5-dimethyl-1,2-oxazole-4-carboxamide Chemical compound C=1C=C(Cl)C=CC=1N(C)C(=O)C1=C(C)ON=C1C1=CC=CC=C1Cl IGRCWJPBLWGNPX-UHFFFAOYSA-N 0.000 description 1
- 229910018072 Al 2 O 3 Inorganic materials 0.000 description 1
- USFZMSVCRYTOJT-UHFFFAOYSA-N Ammonium acetate Chemical compound N.CC(O)=O USFZMSVCRYTOJT-UHFFFAOYSA-N 0.000 description 1
- 239000005695 Ammonium acetate Substances 0.000 description 1
- IJGRMHOSHXDMSA-UHFFFAOYSA-N Atomic nitrogen Chemical compound N#N IJGRMHOSHXDMSA-UHFFFAOYSA-N 0.000 description 1
- BVKZGUZCCUSVTD-UHFFFAOYSA-M Bicarbonate Chemical compound OC([O-])=O BVKZGUZCCUSVTD-UHFFFAOYSA-M 0.000 description 1
- UXVMQQNJUSDDNG-UHFFFAOYSA-L Calcium chloride Chemical compound [Cl-].[Cl-].[Ca+2] UXVMQQNJUSDDNG-UHFFFAOYSA-L 0.000 description 1
- 206010053684 Cerebrohepatorenal syndrome Diseases 0.000 description 1
- 229940124213 Dipeptidyl peptidase 4 (DPP IV) inhibitor Drugs 0.000 description 1
- 102000000820 Enterotoxin Receptors Human genes 0.000 description 1
- 108010001687 Enterotoxin Receptors Proteins 0.000 description 1
- 240000001973 Ficus microcarpa Species 0.000 description 1
- 102100025353 G-protein coupled bile acid receptor 1 Human genes 0.000 description 1
- 101000857733 Homo sapiens G-protein coupled bile acid receptor 1 Proteins 0.000 description 1
- DGAQECJNVWCQMB-PUAWFVPOSA-M Ilexoside XXIX Chemical compound C[C@@H]1CC[C@@]2(CC[C@@]3(C(=CC[C@H]4[C@]3(CC[C@@H]5[C@@]4(CC[C@@H](C5(C)C)OS(=O)(=O)[O-])C)C)[C@@H]2[C@]1(C)O)C)C(=O)O[C@H]6[C@@H]([C@H]([C@@H]([C@H](O6)CO)O)O)O.[Na+] DGAQECJNVWCQMB-PUAWFVPOSA-M 0.000 description 1
- 229940123993 Incretin mimetic Drugs 0.000 description 1
- 229940126033 PPAR agonist Drugs 0.000 description 1
- 229940123934 Reductase inhibitor Drugs 0.000 description 1
- PMZURENOXWZQFD-UHFFFAOYSA-L Sodium Sulfate Chemical compound [Na+].[Na+].[O-]S([O-])(=O)=O PMZURENOXWZQFD-UHFFFAOYSA-L 0.000 description 1
- 208000036813 Zellweger spectrum disease Diseases 0.000 description 1
- 230000002378 acidificating effect Effects 0.000 description 1
- 239000008186 active pharmaceutical agent Substances 0.000 description 1
- 239000000556 agonist Substances 0.000 description 1
- 229940043376 ammonium acetate Drugs 0.000 description 1
- 235000019257 ammonium acetate Nutrition 0.000 description 1
- 239000011324 bead Substances 0.000 description 1
- 230000009286 beneficial effect Effects 0.000 description 1
- 210000000941 bile Anatomy 0.000 description 1
- 239000011230 binding agent Substances 0.000 description 1
- 239000001110 calcium chloride Substances 0.000 description 1
- 229910001628 calcium chloride Inorganic materials 0.000 description 1
- 239000001506 calcium phosphate Substances 0.000 description 1
- 229910000389 calcium phosphate Inorganic materials 0.000 description 1
- 235000011010 calcium phosphates Nutrition 0.000 description 1
- 239000002775 capsule Substances 0.000 description 1
- 238000001816 cooling Methods 0.000 description 1
- 229910052593 corundum Inorganic materials 0.000 description 1
- 239000010431 corundum Substances 0.000 description 1
- 239000002178 crystalline material Substances 0.000 description 1
- 230000003247 decreasing effect Effects 0.000 description 1
- 239000002274 desiccant Substances 0.000 description 1
- 206010012601 diabetes mellitus Diseases 0.000 description 1
- 238000010586 diagram Methods 0.000 description 1
- GUJOJGAPFQRJSV-UHFFFAOYSA-N dialuminum;dioxosilane;oxygen(2-);hydrate Chemical compound O.[O-2].[O-2].[O-2].[Al+3].[Al+3].O=[Si]=O.O=[Si]=O.O=[Si]=O.O=[Si]=O GUJOJGAPFQRJSV-UHFFFAOYSA-N 0.000 description 1
- 238000002050 diffraction method Methods 0.000 description 1
- 229910001873 dinitrogen Inorganic materials 0.000 description 1
- 239000003603 dipeptidyl peptidase IV inhibitor Substances 0.000 description 1
- 238000004090 dissolution Methods 0.000 description 1
- 239000002552 dosage form Substances 0.000 description 1
- 230000005611 electricity Effects 0.000 description 1
- 239000003480 eluent Substances 0.000 description 1
- 239000003623 enhancer Substances 0.000 description 1
- 238000001704 evaporation Methods 0.000 description 1
- 238000002474 experimental method Methods 0.000 description 1
- 210000002603 extrahepatic bile duct Anatomy 0.000 description 1
- 230000004129 fatty acid metabolism Effects 0.000 description 1
- 238000004108 freeze drying Methods 0.000 description 1
- 210000000232 gallbladder Anatomy 0.000 description 1
- 210000001035 gastrointestinal tract Anatomy 0.000 description 1
- 239000000499 gel Substances 0.000 description 1
- 230000002068 genetic effect Effects 0.000 description 1
- 230000002440 hepatic effect Effects 0.000 description 1
- 150000004677 hydrates Chemical class 0.000 description 1
- 230000003301 hydrolyzing effect Effects 0.000 description 1
- 230000002209 hydrophobic effect Effects 0.000 description 1
- 230000002401 inhibitory effect Effects 0.000 description 1
- 230000000968 intestinal effect Effects 0.000 description 1
- 210000003228 intrahepatic bile duct Anatomy 0.000 description 1
- 238000012886 linear function Methods 0.000 description 1
- 239000000463 material Substances 0.000 description 1
- 239000002808 molecular sieve Substances 0.000 description 1
- 229910052901 montmorillonite Inorganic materials 0.000 description 1
- 239000004570 mortar (masonry) Substances 0.000 description 1
- 210000000056 organ Anatomy 0.000 description 1
- 230000001991 pathophysiological effect Effects 0.000 description 1
- 239000002307 peroxisome proliferator activated receptor agonist Substances 0.000 description 1
- 230000000144 pharmacologic effect Effects 0.000 description 1
- 210000003240 portal vein Anatomy 0.000 description 1
- 238000001556 precipitation Methods 0.000 description 1
- 238000002953 preparative HPLC Methods 0.000 description 1
- 230000009103 reabsorption Effects 0.000 description 1
- 239000011541 reaction mixture Substances 0.000 description 1
- 229940075993 receptor modulator Drugs 0.000 description 1
- 238000001953 recrystallisation Methods 0.000 description 1
- 230000002829 reductive effect Effects 0.000 description 1
- 238000011160 research Methods 0.000 description 1
- 150000003839 salts Chemical class 0.000 description 1
- 238000000926 separation method Methods 0.000 description 1
- 239000000952 serotonin receptor agonist Substances 0.000 description 1
- 210000002966 serum Anatomy 0.000 description 1
- 229910052708 sodium Inorganic materials 0.000 description 1
- 239000011734 sodium Substances 0.000 description 1
- URGAHOPLAPQHLN-UHFFFAOYSA-N sodium aluminosilicate Chemical compound [Na+].[Al+3].[O-][Si]([O-])=O.[O-][Si]([O-])=O URGAHOPLAPQHLN-UHFFFAOYSA-N 0.000 description 1
- 239000011780 sodium chloride Substances 0.000 description 1
- FQENQNTWSFEDLI-UHFFFAOYSA-J sodium diphosphate Chemical compound [Na+].[Na+].[Na+].[Na+].[O-]P([O-])(=O)OP([O-])([O-])=O FQENQNTWSFEDLI-UHFFFAOYSA-J 0.000 description 1
- 239000001488 sodium phosphate Substances 0.000 description 1
- 229910000162 sodium phosphate Inorganic materials 0.000 description 1
- 229940048086 sodium pyrophosphate Drugs 0.000 description 1
- 229910052938 sodium sulfate Inorganic materials 0.000 description 1
- 235000011152 sodium sulphate Nutrition 0.000 description 1
- 239000011343 solid material Substances 0.000 description 1
- 239000000243 solution Substances 0.000 description 1
- 238000002336 sorption--desorption measurement Methods 0.000 description 1
- 230000002269 spontaneous effect Effects 0.000 description 1
- 239000007858 starting material Substances 0.000 description 1
- 230000003068 static effect Effects 0.000 description 1
- 238000003860 storage Methods 0.000 description 1
- 239000000758 substrate Substances 0.000 description 1
- 238000004441 surface measurement Methods 0.000 description 1
- -1 tert-butoxyl ester Chemical class 0.000 description 1
- 235000019818 tetrasodium diphosphate Nutrition 0.000 description 1
- 239000001577 tetrasodium phosphonato phosphate Substances 0.000 description 1
- 238000002560 therapeutic procedure Methods 0.000 description 1
- 238000000844 transformation Methods 0.000 description 1
- QORWJWZARLRLPR-UHFFFAOYSA-H tricalcium bis(phosphate) Chemical compound [Ca+2].[Ca+2].[Ca+2].[O-]P([O-])([O-])=O.[O-]P([O-])([O-])=O QORWJWZARLRLPR-UHFFFAOYSA-H 0.000 description 1
- RYFMWSXOAZQYPI-UHFFFAOYSA-K trisodium phosphate Chemical compound [Na+].[Na+].[Na+].[O-]P([O-])([O-])=O RYFMWSXOAZQYPI-UHFFFAOYSA-K 0.000 description 1
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D281/00—Heterocyclic compounds containing rings of more than six members having one nitrogen atom and one sulfur atom as the only ring hetero atoms
- C07D281/02—Seven-membered rings
- C07D281/04—Seven-membered rings having the hetero atoms in positions 1 and 4
- C07D281/08—Seven-membered rings having the hetero atoms in positions 1 and 4 condensed with carbocyclic rings or ring systems
- C07D281/10—Seven-membered rings having the hetero atoms in positions 1 and 4 condensed with carbocyclic rings or ring systems condensed with one six-membered ring
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/55—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having seven-membered rings, e.g. azelastine, pentylenetetrazole
- A61K31/554—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having seven-membered rings, e.g. azelastine, pentylenetetrazole having at least one nitrogen and one sulfur as ring hetero atoms, e.g. clothiapine, diltiazem
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P1/00—Drugs for disorders of the alimentary tract or the digestive system
- A61P1/10—Laxatives
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P1/00—Drugs for disorders of the alimentary tract or the digestive system
- A61P1/12—Antidiarrhoeals
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P1/00—Drugs for disorders of the alimentary tract or the digestive system
- A61P1/16—Drugs for disorders of the alimentary tract or the digestive system for liver or gallbladder disorders, e.g. hepatoprotective agents, cholagogues, litholytics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P29/00—Non-central analgesic, antipyretic or antiinflammatory agents, e.g. antirheumatic agents; Non-steroidal antiinflammatory drugs [NSAID]
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P3/00—Drugs for disorders of the metabolism
- A61P3/04—Anorexiants; Antiobesity agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P3/00—Drugs for disorders of the metabolism
- A61P3/06—Antihyperlipidemics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P3/00—Drugs for disorders of the metabolism
- A61P3/08—Drugs for disorders of the metabolism for glucose homeostasis
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P3/00—Drugs for disorders of the metabolism
- A61P3/08—Drugs for disorders of the metabolism for glucose homeostasis
- A61P3/10—Drugs for disorders of the metabolism for glucose homeostasis for hyperglycaemia, e.g. antidiabetics
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07B—GENERAL METHODS OF ORGANIC CHEMISTRY; APPARATUS THEREFOR
- C07B2200/00—Indexing scheme relating to specific properties of organic compounds
- C07B2200/13—Crystalline forms, e.g. polymorphs
Landscapes
- Health & Medical Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Veterinary Medicine (AREA)
- Public Health (AREA)
- General Health & Medical Sciences (AREA)
- Animal Behavior & Ethology (AREA)
- Life Sciences & Earth Sciences (AREA)
- Pharmacology & Pharmacy (AREA)
- Medicinal Chemistry (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- General Chemical & Material Sciences (AREA)
- Diabetes (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Engineering & Computer Science (AREA)
- Obesity (AREA)
- Hematology (AREA)
- Endocrinology (AREA)
- Emergency Medicine (AREA)
- Epidemiology (AREA)
- Pain & Pain Management (AREA)
- Rheumatology (AREA)
- Child & Adolescent Psychology (AREA)
- Gastroenterology & Hepatology (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
- Medicines That Contain Protein Lipid Enzymes And Other Medicines (AREA)
- Peptides Or Proteins (AREA)
Description
「結晶変態」なる用語は、有機化合物の結晶性固相を指す。結晶変態は、溶媒和物又は非溶媒和物のいずれであってもよい。
X線粉末回折(XRPD)分析
必要に応じて、めのう乳鉢中、乾燥試料を軽く粉砕した後、試料ホルダーに塗り広げた。スラリー試料を湿潤状態で試料ホルダーに添加し、湿潤状態と乾燥状態の両方で分析した。X´celerator又はPIXcel検出器を装備したPANalytical X´Pert Pro回折計を使用し、カットシリコン型ゼロバックグラウンドホルダー(ZBH)又は多孔質アルミナフィルター型試料ホルダーにおいてXRPDデータを収集した。試料は、分析中、回転させ、Cu線を使用した。下記の実験設定を使用した。
管の電圧及び電流: 40kV、50mA
波長アルファ1(CuKα1): 1.5406Å
波長アルファ2(CuKα2): 1.5444Å
波長アルファ1と波長アルファ2との平均(CuKα): 1.5418Å
開始角度[2θ]: 1〜4°
終了角度[2θ]: 30〜40°
分析時間: 50秒(「1分走査」)、125秒(「2分走査」)、192秒(「3分走査」)、397秒(「6分走査」)、780秒(「13分走査」)、1020秒(「17分走査」)、4560秒(「1時間走査」)
およそ2〜16mgの試料を金属製受器に量り取り、次いで、試料から静電気を放出させた。次いで金属製受器を、Surface Measurements System Ltd製のDVS Advantage機器内で位置決めした。試料に、2回の逐次的な収着-脱着サイクルを施した。各サイクルは、0%〜95%〜0%までの相対湿度(%RH)で運転した。1回のサイクルは21の工程から構成されるが、0%RHから90%RHまでの間の工程については、10%のRH毎に実施した。各段階で、以下の平衡基準を使用した:dm/dt、5分以上<0.002%であり、各ステージにおける最小および最大時間はそれぞれ10分および360分であった。
形態A一水和物は、2014年4月25日に出願された国際出願番号第PCT/EP2014/058432号に記載の方法に従って調製することができる。なお、前記国際出願では、結晶変態IVと称されている。簡潔に述べると、形態Aは、100mgのエロビキシバットの乾燥固体材料を2mLのエタノールと、密閉容器中で1週間混合することによって調製可能である。次いで、固形物を単離し、エタノールが乾燥するまで高温で真空中、乾燥させる。最後に、前記固体は、高度に結晶質の形態A一水和物が形成されるまで、常温で湿った空気に晒される。
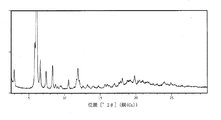
102mgのエロビキシバット形態A一水和物を、60℃で10mL試験管中、0.5mLのメチルイソブチルケトン(MIBK)に溶解した。次いで、N-ヘプタンを0.1mL分量で2時間かけて添加した。0.6mLのn-ヘプタンを添加した時点で、沈殿が生じた。次いで、濃い白色スラリーが形成されるまで、容器を4日間撹拌した。容器を21〜22℃に冷却し、次いでスラリーのサンプルをパスツールピペットで取り出し、カットシリコン型ゼロバックグラウンドホルダ(ZBH)に試料採取した。湿ったサンプルをXRPDで分析したところ、形態G MIBK溶媒和物からなることが示された(図8)。
441mgのエロビキシバット形態A一水和物、0.6mLの酢酸エチルおよび0.6mLのn-ヘプタンを10mL試験管に添加した。磁気撹拌子を加え、容器を閉じ、次いで62℃で2日間撹拌した。このスラリーを熱(60℃)多孔質アルミナXRPD基質にサンプリングし、XRPDで分析したところ、形態H酢酸エチル溶媒和物からなることが示された(図9)。
111mgのエロビキシバット形態A一水和物を10mL試験管に秤量した。磁気撹拌フリアおよび2.0mLのアセトン:水50:50%v/v混合物を添加した。試験管を閉じて21〜22℃で磁気撹拌する前に、手動撹拌し、数分間、激しく振盪したところ、ゲル状物質を生じた。2日後、白色スラリーの試料をパスツールピペットで取り出し、カットシリコン型ゼロバックグラウンドホルダ(ZBH)に試料採取した。湿った試料をXRPDで分析したところ、形態E二水和物からなることが示された(図7)。
95mgのエロビキシバット形態A一水和物を10mL試験管に秤量した。磁気撹拌フリアと2.0mLの酢酸エチルを加えた。これによりスラリーが生成し、これを21〜22℃で磁気撹拌した。2日後、白色スラリーの試料をパスツールピペットで取り出し、カットシリコン型ゼロバックグラウンドホルダ(ZBH)に試料採取した。湿った試料をXRPDで分析したところ、形態D溶媒和物からなることが示された(図12)。XRPD試料を21〜22℃および30%相対湿度で3日間保存し、次いでXRPDで再分析したところ、形態C無水物を含有するものであった(図4)。
99mgのエロビキシバット形態A一水和物を10mL試験管に秤量した。磁気撹拌フリアおよびアセトニトリル2.0mLを添加した。固体は溶解したが、再び急速に沈殿し、スラリーを形成した。試験管を封じ、21〜22℃で磁気撹拌した。2日後、白色スラリーの試料をパスツールピペットで取り出し、カットシリコン型ゼロバックグラウンドホルダ(ZBH)に試料採取した。湿った試料をXRPDで分析したところ、形態Mアセトニトリル溶媒和物からなることが示された(図13)。
形態L無水物の平坦な試料を、その上から水滴を加えることによって湿らせた。固体の疎水性のために、水滴は固体に非常にゆっくりと取りこまれた。水が試料に取り込まれたとき、XRPDで分析したところ、形態N二水和物からなるものであった(図6)。
30mgのエロビキシバットを1.0mLの試験管に秤量した。磁気撹拌フリアおよび0.5mLの2-プロパノール:水の50:50%v/v混合物を添加した。試験管を閉じ、5℃で磁気撹拌した。1週間後、白色スラリーの試料をパスツールピペットで採取し、多孔性コランダム試料ホルダーに試料採取した。湿った試料をXRPDで分析したところ、形態N二水和物のそれと一致した。
本願に記載されているエロビキシバットの固体状態挙動の概要は、図15に示される。
Claims (12)
- 6.1±0.2及び5.9±0.2の°2θ位置にピークを有する、CuKα1線によって得たXRPDパターンを有する、エロビキシバットの結晶性無水物。
- 12.4±0.2及び5.8±0.2の°2θ位置にピークを有する、CuKα1線によって得たXRPDパターンを有する、エロビキシバットの結晶性無水物。
- 6.4±0.2及び12.7±0.2の°2θ位置にピークを有する、CuKα1線によって得たXRPDパターンを有する、エロビキシバットの結晶性無水物。
- 6.1±0.2及び12.0±0.4の°2θ位置にピークを有する、CuKα1線によって得たXRPDパターンを有する、エロビキシバットの結晶性二水和物。
- 6.1±0.2、8.0±0.2、及び12.0±0.2の°2θ位置にピークを有する、CuKα1線によって得たXRPDパターンを有する、エロビキシバットの結晶性二水和物。
- 6.1±0.2、12.1±0.2、及び20.9±0.2の°2θ位置にピークを有する、CuKα1線によって得たXRPDパターンを有する、エロビキシバットの結晶性二水和物。
- 治療用の請求項1から6のいずれか一項に記載のエロビキシバットの結晶性無水物または結晶性二水和物。
- 医薬として許容される希釈剤又は担体とともに、請求項1から6のいずれか一項に記載のエロビキシバットの結晶性無水物または結晶性二水和物を含む、医薬組成物。
- 結晶性無水物または結晶性二水和物の化学的純度が>99%である、請求項8に記載の医薬組成物。
- 高コレステロール血症、脂質異常症、代謝症候群、肥満、脂肪酸代謝の障害、グルコース利用率障害、インスリン抵抗性が関与する障害、1型糖尿病及び2型糖尿病、肝臓疾患、IBAT阻害剤化合物を含む治療中の下痢、慢性便秘を含めた便秘、例えば、慢性便秘及び便秘型過敏性腸症候群(IBS-C)を含めた機能的便秘からなる群より選択される疾患の処置又は予防のための、請求項1から6のいずれか一項に記載のエロビキシバットの結晶性無水物または結晶性二水和物。
- 肝実質、肝臓の先天性代謝異常、バイラー症候群、脳腱黄色腫瘍症等の胆汁酸(BA)合成に関する一次性欠損症、ツェルウェガー症候群等の二次性欠損症、新生児肝炎、嚢胞性線維症(肝臓における発現)、ALGS(アラジール症候群)、進行性家族性肝内胆汁うっ滞(PFIC)、自己免疫性肝炎、原発性胆汁性肝硬変(PBC)、肝線維症、非アルコール性脂肪肝疾患、NAFLD/NASH、門脈圧亢進症、薬物に起因した黄疸又は妊娠中の黄疸等の一般的な胆汁うっ滞、遺伝型の胆汁うっ滞、例えばPFIC1等の肝内胆汁うっ滞及び肝外胆汁うっ滞、原発性硬化性胆管炎(PSC)、胆石及び総胆管結石症、胆管系の閉塞を引き起こす悪性腫瘍、胆汁うっ滞/黄疸に起因した症状(擦過傷、そう痒症)、膵炎、進行性胆汁うっ滞に帰結する慢性自己免疫性肝臓疾患、胆汁うっ滞性肝臓疾患に関するそう痒症、並びに、高脂質症の疾患に付随する疾患状態からなる群より選択される疾患の処置又は予防のための、請求項1から6のいずれか一項に記載のエロビキシバットの結晶性無水物または結晶性二水和物。
- 肝臓障害及び肝臓障害に関連した疾患、脂肪肝、肝脂肪症、非アルコール性脂肪性肝炎(NASH)、アルコール性肝炎、急性脂肪肝、妊娠による脂肪肝、薬物によって誘導される肝炎、鉄過剰症障害、肝線維症、肝硬変、肝癌、ウイルス性肝炎、並びに、肝臓、胆道及び膵臓の腫瘍及び新生物に関連した問題からなる群より選択される疾患の処置又は予防のための、請求項1から6のいずれか一項に記載のエロビキシバットの結晶性無水物または結晶性二水和物。
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| EP14190290.8 | 2014-10-24 | ||
| EP14190290.8A EP3012252A1 (en) | 2014-10-24 | 2014-10-24 | Crystal modifications of elobixibat |
| PCT/EP2015/074573 WO2016062848A1 (en) | 2014-10-24 | 2015-10-23 | Crystal modifications of elobixibat |
Publications (3)
| Publication Number | Publication Date |
|---|---|
| JP2017537061A JP2017537061A (ja) | 2017-12-14 |
| JP2017537061A5 JP2017537061A5 (ja) | 2018-10-18 |
| JP6700263B2 true JP6700263B2 (ja) | 2020-05-27 |
Family
ID=51786891
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2017519487A Active JP6700263B2 (ja) | 2014-10-24 | 2015-10-23 | エロビキシバットの結晶変態 |
Country Status (10)
| Country | Link |
|---|---|
| US (2) | US10183920B2 (ja) |
| EP (3) | EP3012252A1 (ja) |
| JP (1) | JP6700263B2 (ja) |
| KR (1) | KR102498539B1 (ja) |
| CN (2) | CN112375044A (ja) |
| AU (1) | AU2015334883B2 (ja) |
| CA (1) | CA2959760C (ja) |
| ES (1) | ES2874573T3 (ja) |
| RU (1) | RU2017114109A (ja) |
| WO (1) | WO2016062848A1 (ja) |
Families Citing this family (30)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| RS60901B1 (sr) | 2010-11-08 | 2020-11-30 | Albireo Ab | Ibat inhibitori za lečenje oboljenja jetre |
| CA2952406A1 (en) | 2014-06-25 | 2015-12-30 | Ea Pharma Co., Ltd. | Solid formulation and method for preventing or reducing coloration thereof |
| US10786529B2 (en) | 2016-02-09 | 2020-09-29 | Albireo Ab | Oral cholestyramine formulation and use thereof |
| US10441604B2 (en) | 2016-02-09 | 2019-10-15 | Albireo Ab | Cholestyramine pellets and methods for preparation thereof |
| US10441605B2 (en) | 2016-02-09 | 2019-10-15 | Albireo Ab | Oral cholestyramine formulation and use thereof |
| KR101844184B1 (ko) * | 2017-07-21 | 2018-04-02 | 씨제이헬스케어 주식회사 | 아미노알킬벤조티아제핀 유도체의 용도 |
| CA3071285A1 (en) | 2017-08-09 | 2019-02-14 | Albireo Ab | Cholestyramine granules, oral cholestyramine formulations and use thereof |
| WO2019172834A1 (en) * | 2018-03-09 | 2019-09-12 | Elobix Ab | Process for the preparation of elobixibat |
| US10428109B1 (en) | 2018-03-09 | 2019-10-01 | Elobix Ab | Process for the preparation of 1,5-benzothiazepine compounds |
| JP7391048B2 (ja) | 2018-06-05 | 2023-12-04 | アルビレオ・アクチボラグ | ベンゾチア(ジ)アゼピン化合物及び胆汁酸モジュレータとしてのそれらの使用 |
| US10793534B2 (en) | 2018-06-05 | 2020-10-06 | Albireo Ab | Benzothia(di)azepine compounds and their use as bile acid modulators |
| CA3100691A1 (en) | 2018-06-20 | 2019-12-26 | Albireo Ab | Crystal modifications of odevixibat |
| US11801226B2 (en) | 2018-06-20 | 2023-10-31 | Albireo Ab | Pharmaceutical formulation of odevixibat |
| US10722457B2 (en) | 2018-08-09 | 2020-07-28 | Albireo Ab | Oral cholestyramine formulation and use thereof |
| US11007142B2 (en) | 2018-08-09 | 2021-05-18 | Albireo Ab | Oral cholestyramine formulation and use thereof |
| US11549878B2 (en) | 2018-08-09 | 2023-01-10 | Albireo Ab | In vitro method for determining the adsorbing capacity of an insoluble adsorbant |
| US10941127B2 (en) | 2019-02-06 | 2021-03-09 | Albireo Ab | Benzothiadiazepine compounds and their use as bile acid modulators |
| US10975045B2 (en) | 2019-02-06 | 2021-04-13 | Aibireo AB | Benzothiazepine compounds and their use as bile acid modulators |
| EP4029860A4 (en) * | 2019-09-09 | 2023-08-23 | Elobix Ab | PROCESS FOR PREPARING A 1,5-BENZOTHIAZEPINE COMPOUND |
| CA3158181A1 (en) | 2019-12-04 | 2021-06-10 | Albireo Ab | Benzothia(di)azepine compounds and their use as bile acid modulators |
| EP4069247A1 (en) | 2019-12-04 | 2022-10-12 | Albireo AB | Benzothiadiazepine compounds and their use as bile acid modulators |
| US11014898B1 (en) | 2020-12-04 | 2021-05-25 | Albireo Ab | Benzothiazepine compounds and their use as bile acid modulators |
| JP2023504645A (ja) | 2019-12-04 | 2023-02-06 | アルビレオ・アクチボラグ | ベンゾチア(ジ)アゼピン化合物及び胆汁酸モジュレータとしてのそれらの使用 |
| CN114786772B (zh) | 2019-12-04 | 2024-04-09 | 阿尔比里奥公司 | 苯并硫杂(二)氮杂环庚三烯化合物及其作为胆汁酸调节剂的用途 |
| JP2023537285A (ja) | 2020-08-03 | 2023-08-31 | アルビレオ・アクチボラグ | ベンゾチア(ジ)アゼピン化合物及び胆汁酸モジュレータとしてのそれらの使用 |
| WO2022101379A1 (en) | 2020-11-12 | 2022-05-19 | Albireo Ab | Odevixibat for treating progressive familial intrahepatic cholestasis (pfic) |
| EP4243831A1 (en) | 2020-11-12 | 2023-09-20 | Albireo AB | Odevixibat for treating progressive familial intrahepatic cholestasis (pfic) |
| JP2024500309A (ja) | 2020-12-04 | 2024-01-09 | アルビレオ エービー | ベンゾチア(ジ)アゼピン化合物および胆汁酸モジュレータとしてのその使用 |
| WO2024027013A1 (zh) * | 2022-08-02 | 2024-02-08 | 上海皓元医药股份有限公司 | 一种依洛西巴特晶型ii及其制备方法 |
| US20240173333A1 (en) | 2022-11-03 | 2024-05-30 | Albireo Ab | Treating Alagille Syndrome (ALGS) |
Family Cites Families (115)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US3539380A (en) | 1968-01-08 | 1970-11-10 | Upjohn Co | Methylcellulose and polyalkylene glycol coating of solid medicinal dosage forms |
| GB1530201A (en) | 1976-04-14 | 1978-10-25 | Pfizer Ltd | Process for the preparation of aminoglycoside antibiotics and intermediates therefor |
| GB1566609A (en) | 1977-03-10 | 1980-05-08 | Reckitt & Colmann Prod Ltd | Pharmaceutical compositions containing cholestyramine and alginic acid |
| DE3066728D1 (en) | 1979-04-30 | 1984-03-29 | Max Planck Gesellschaft | Tyrosine derivatives, process for their production and their use in the synthesis of peptides |
| US4900757A (en) | 1988-12-08 | 1990-02-13 | Merrell Dow Pharmaceuticals Inc. | Hypocholesterolemic and antiatherosclerotic uses of bix(3,5-di-tertiary-butyl-4-hydroxyphenylthio)methane |
| IL95574A (en) | 1989-09-09 | 1994-11-11 | Knoll Ag | Colstiramine preparation |
| DE3930168A1 (de) | 1989-09-09 | 1991-03-14 | Knoll Ag | Verbesserte verabreichungsform fuer colestyramin |
| FI106800B (fi) | 1990-12-06 | 2001-04-12 | Hoechst Ag | Menetelmä uusien terapeuttisesti käyttökelpoisten dimeeristen sappihappojohdannaisten valmistamiseksi |
| EP0549967B1 (de) | 1991-12-20 | 1996-03-13 | Hoechst Aktiengesellschaft | Polymere und Oligomere von Gallensäurederivaten, Verfahren zu ihrer Herstellung und ihre Verwendung als Arzneimittel |
| JPH05186357A (ja) | 1991-12-31 | 1993-07-27 | Shigeo Ochi | 飲食物消化分解産物吸収抑制手段 |
| GB9203347D0 (en) | 1992-02-17 | 1992-04-01 | Wellcome Found | Hypolipidaemic compounds |
| IT1257793B (it) | 1992-05-18 | 1996-02-13 | Composizione farmaceutica a base di acidi biliari in microgranuli a rilascio controllato | |
| ES2111092T3 (es) | 1992-06-12 | 1998-03-01 | Hoechst Ag | Derivados de acidos biliares, procedimiento para su preparacion y utilizacion de estos compuestos como medicamentos. |
| US5350584A (en) | 1992-06-26 | 1994-09-27 | Merck & Co., Inc. | Spheronization process using charged resins |
| IL108634A0 (en) | 1993-02-15 | 1994-05-30 | Wellcome Found | Hypolipidaemic heterocyclic compounds, their prepatation and pharmaceutical compositions containing them |
| IL108633A (en) | 1993-02-15 | 1998-07-15 | Wellcome Found | History of Benzothiazepine Hypolipidemic Preparation and Pharmaceutical Preparations Containing Them |
| DE69425453T2 (de) | 1993-04-23 | 2001-04-12 | Novartis Ag, Basel | Wirkstoffabgabevorrichtung mit gesteuerter Freigabe |
| TW289021B (ja) | 1993-05-08 | 1996-10-21 | Hoechst Ag | |
| EP0624593A3 (de) | 1993-05-08 | 1995-06-07 | Hoechst Ag | Gallensäurederivate, Verfahren zu ihrer Herstellung und Verwendung dieser Verbindungen als Arzneimittel. |
| TW289020B (ja) | 1993-05-08 | 1996-10-21 | Hoechst Sktiengesellschaft | |
| TW289757B (ja) | 1993-05-08 | 1996-11-01 | Hoechst Ag | |
| ZA956647B (en) | 1994-08-10 | 1997-02-10 | Wellcome Found | Hypolipidaemic compounds. |
| DK0781278T3 (da) | 1994-09-13 | 2001-04-17 | Monsanto Co | Nye benzothiepiner, som har aktivitet som inhibitorer af ileal galdsyretransport og taurocholatoptagelse |
| US6642268B2 (en) | 1994-09-13 | 2003-11-04 | G.D. Searle & Co. | Combination therapy employing ileal bile acid transport inhibiting benzothipines and HMG Co-A reductase inhibitors |
| US6262277B1 (en) | 1994-09-13 | 2001-07-17 | G.D. Searle And Company | Intermediates and processes for the preparation of benzothiepines having activity as inhibitors of ileal bile acid transport and taurocholate uptake |
| US5994391A (en) | 1994-09-13 | 1999-11-30 | G.D. Searle And Company | Benzothiepines having activity as inhibitors of ileal bile acid transport and taurocholate uptake |
| GB9423172D0 (en) | 1994-11-17 | 1995-01-04 | Wellcom Foundation The Limited | Hypolipidemic benzothiazepines |
| US5811388A (en) | 1995-06-07 | 1998-09-22 | Cibus Pharmaceutical, Inc. | Delivery of drugs to the lower GI tract |
| AU724620B2 (en) | 1996-01-16 | 2000-09-28 | Sokol, Ronald J Dr. | Use of antioxidant agents to treat cholestatic liver disease |
| ATE303378T1 (de) | 1996-03-11 | 2005-09-15 | Searle Llc | Benzothiepinen mit wirkung als inhibitoren des ileumgallensäuretransports und der taurocholate- aufnahme |
| ATE197842T1 (de) | 1996-07-24 | 2000-12-15 | Zumtobel Staff Gmbh | Adapter für ein haltemittel, welches zum befestigen einer einbauleuchte in einer einbauöffnung bestimmt ist, oder haltemittel oder einbauleuchte mit einem solchen adapter |
| DE19633268A1 (de) | 1996-08-19 | 1998-02-26 | Hoechst Ag | Polymere Gallensäure-Resorptionsinhibitoren mit gleichzeitiger Gallensäure-Adsorberwirkung |
| GB9704208D0 (en) | 1997-02-28 | 1997-04-16 | Glaxo Group Ltd | Chemical compounds |
| EP0971744A2 (en) | 1997-03-11 | 2000-01-19 | G.D. SEARLE & CO. | COMBINATION THERAPY EMPLOYING ILEAL BILE ACID TRANSPORT INHIBITING BENZOTHIEPINES AND HMG Co-A REDUCTASE INHIBITORS |
| PT864582E (pt) | 1997-03-14 | 2003-10-31 | Aventis Pharma Gmbh | 1,4-benzotiazepina-1,1-dioxidos hipolipidemicos |
| US6635280B2 (en) | 1997-06-06 | 2003-10-21 | Depomed, Inc. | Extending the duration of drug release within the stomach during the fed mode |
| AUPO763197A0 (en) | 1997-06-30 | 1997-07-24 | Sigma Pharmaceuticals Pty Ltd | Health supplement |
| US6369220B1 (en) | 1997-12-19 | 2002-04-09 | Jinglin (James T.) Li | Method of preparing enantiomerically-enriched tetrahydrobenzothiepine oxides |
| GB9800428D0 (en) | 1998-01-10 | 1998-03-04 | Glaxo Group Ltd | Chemical compounds |
| EP1073650A1 (en) | 1998-04-24 | 2001-02-07 | Fujisawa Pharmaceutical Co., Ltd. | Guanidine derivatives |
| DE19825804C2 (de) | 1998-06-10 | 2000-08-24 | Aventis Pharma Gmbh | 1,4-Benzothiepin-1,1-dioxidderivate, Verfahren zu deren Herstellung und diese Verbindungen enthaltende Arzneimittel |
| US6221897B1 (en) | 1998-06-10 | 2001-04-24 | Aventis Pharma Deutschland Gmbh | Benzothiepine 1,1-dioxide derivatives, a process for their preparation, pharmaceuticals comprising these compounds, and their use |
| AU5194999A (en) | 1998-08-07 | 2000-02-28 | Takeda Chemical Industries Ltd. | Benzothiepin derivatives, process for the preparation of the same and uses thereof |
| EP1140187B1 (en) | 1998-12-23 | 2003-09-03 | G.D. Searle LLC. | Combinations of an ibat inhibitor and a mtp inhibitor for cardiovascular indications |
| IL143938A0 (en) | 1998-12-23 | 2002-04-21 | Searle Llc | Combinations of ileal bile acid transport inhibitors and nicotinic acid derivatives for cardiovascular indications |
| JP2002533414A (ja) | 1998-12-23 | 2002-10-08 | ジー.ディー.サール エルエルシー | 心臓血管に適用するための回腸胆汁酸輸送阻害剤および胆汁酸隔離剤の組み合わせ |
| ES2200587T3 (es) | 1998-12-23 | 2004-03-01 | G.D. Searle Llc | Combinaciones de inhibidors del transporte de acidos biliares del ileon e inhibidores de la proteina de transferencia de colesteril ester para indicaciones cardiovasculares. |
| DE69907960T2 (de) | 1998-12-23 | 2004-02-26 | G.D. Searle Llc, Chicago | Kombinationen von ileumgallensäuretransports inhibitoren und fibronsäure derivaten für kardiovaskuläre indikationen |
| CN1195748C (zh) | 1999-02-12 | 2005-04-06 | G.D.瑟尔有限公司 | 具有作为回肠胆汁酸转运和牛磺胆酸摄取抑制剂活性的新的1,2-苯并硫氮杂 |
| DE19916108C1 (de) | 1999-04-09 | 2001-01-11 | Aventis Pharma Gmbh | Mit Zuckerresten substituierte 1,4-Benzothiazepin-1,1-dioxidderivate, Verfahren zu deren Herstellung und deren Verwendung |
| SE9901387D0 (sv) | 1999-04-19 | 1999-04-19 | Astra Ab | New pharmaceutical foromaulations |
| US6287609B1 (en) | 1999-06-09 | 2001-09-11 | Wisconsin Alumni Research Foundation | Unfermented gel fraction from psyllium seed husks |
| AU784722B2 (en) | 2000-02-18 | 2006-06-01 | Merck & Co., Inc. | Aryloxyacetic acids for diabetes and lipid disorders |
| SE0000772D0 (sv) | 2000-03-08 | 2000-03-08 | Astrazeneca Ab | Chemical compounds |
| EP1286984A2 (en) | 2000-03-10 | 2003-03-05 | Pharmacia Corporation | Method for the preparation of tetrahydrobenzothiepines |
| WO2001068096A2 (en) | 2000-03-10 | 2001-09-20 | Pharmacia Corporation | Combination therapy for the prophylaxis and treatment of hyperlipidemic conditions and disorders |
| TWI241195B (en) | 2000-04-10 | 2005-10-11 | Shionogi & Co | Preventive agent for bile acidic diarrhea |
| US20020183307A1 (en) | 2000-07-26 | 2002-12-05 | Tremont Samuel J. | Novel 1,4-benzothiazephine and 1,5-benzothiazepine compounds as inhibitors of apical sodium co-dependent bile acid transport and taurocholate uptake |
| PL204443B1 (pl) | 2000-07-28 | 2010-01-29 | Hoffmann La Roche | Kompozycja farmaceutyczna zawierająca inhibitor lipazy, sposób wytwarzania kompozycji farmaceutycznej, zestaw do leczenia otyłości, zastosowanie kompozycji farmaceutycznej oraz zastosowanie orlistatu i substancji wiążącej kwasy żółciowe |
| FR2812886B1 (fr) | 2000-08-08 | 2002-11-08 | Assist Publ Hopitaux De Paris | Depistage d'un nouveau syndrome hepatique et ses applications |
| SE0003766D0 (sv) | 2000-10-18 | 2000-10-18 | Astrazeneca Ab | Novel formulation |
| EG26979A (en) | 2000-12-21 | 2015-03-01 | Astrazeneca Ab | Chemical compounds |
| WO2002053548A1 (en) | 2000-12-27 | 2002-07-11 | Banyu Pharmaceutical Co.,Ltd. | Benzothiazepine derivatives |
| US6506921B1 (en) | 2001-06-29 | 2003-01-14 | Virginia Tech Intellectual Properties, Inc. | Amine compounds and curable compositions derived therefrom |
| GB0121337D0 (en) | 2001-09-04 | 2001-10-24 | Astrazeneca Ab | Chemical compounds |
| GB0121622D0 (en) | 2001-09-07 | 2001-10-31 | Astrazeneca Ab | Chemical compounds |
| GB0121621D0 (en) | 2001-09-07 | 2001-10-31 | Astrazeneca Ab | Chemical compounds |
| PL216023B1 (pl) | 2001-09-08 | 2014-02-28 | Astrazeneca Ab | Pochodna benzotiadiazepiny, kompozycja farmaceutyczna zawierajaca pochodna benzotiadiazepiny oraz zastosowania pochodnej benzotiadiazepiny |
| BR0212495A (pt) | 2001-09-12 | 2004-08-24 | Searle Llc | Método para a preparação de tetraidrobenzotiepinas cristalinas |
| GB0314079D0 (en) | 2003-06-18 | 2003-07-23 | Astrazeneca Ab | Therapeutic agents |
| GB0229931D0 (en) | 2002-12-21 | 2003-01-29 | Astrazeneca Ab | Therapeutic agents |
| SE0104334D0 (sv) | 2001-12-19 | 2001-12-19 | Astrazeneca Ab | Therapeutic agents |
| JP2005518408A (ja) | 2001-12-29 | 2005-06-23 | ノボ ノルディスク アクティーゼルスカブ | 異常脂肪血症を治療するための、glp−1化合物と他の薬物との組み合わせ使用 |
| GB0201850D0 (en) | 2002-01-26 | 2002-03-13 | Astrazeneca Ab | Therapeutic treatment |
| GB0209467D0 (en) | 2002-04-25 | 2002-06-05 | Astrazeneca Ab | Chemical compounds |
| GB0213669D0 (en) | 2002-06-14 | 2002-07-24 | Astrazeneca Ab | Chemical compounds |
| GB0216321D0 (en) | 2002-07-13 | 2002-08-21 | Astrazeneca Ab | Therapeutic treatment |
| CA2497345C (en) | 2002-08-28 | 2008-10-14 | Asahi Kasei Pharma Corporation | Novel quaternary ammonium compounds |
| US7312208B2 (en) | 2002-08-28 | 2007-12-25 | Asahi Kasei Pharma Corporation | Quaternary ammonium compounds |
| GB0304194D0 (en) | 2003-02-25 | 2003-03-26 | Astrazeneca Ab | Chemical compounds |
| WO2004108067A2 (en) | 2003-04-03 | 2004-12-16 | Sun Pharmaceutical Industries Limited | Programmed drug delivery system |
| GB0307918D0 (en) | 2003-04-05 | 2003-05-14 | Astrazeneca Ab | Therapeutic use |
| CN1930137B (zh) | 2004-02-27 | 2011-07-20 | 旭化成制药株式会社 | 苯并硫氮杂卓和苯并虑平化合物 |
| TW200533336A (en) | 2004-03-02 | 2005-10-16 | Fujisawa Pharmaceutical Co | Concomitant drugs |
| EP1593671A1 (en) | 2004-03-05 | 2005-11-09 | Graffinity Pharmaceuticals AG | DPP-IV inhibitors |
| TWI415635B (zh) | 2004-05-28 | 2013-11-21 | 必治妥施貴寶公司 | 加衣錠片調製物及製備彼之方法 |
| JP4896480B2 (ja) | 2004-10-01 | 2012-03-14 | 第一三共ヘルスケア株式会社 | 陰イオン交換樹脂の粒子状組成物 |
| EP1877373A2 (en) | 2005-05-05 | 2008-01-16 | Microbia, Inc. | Biphenylazetidinone cholesterol absorption inhibitors |
| DE102005033099A1 (de) | 2005-07-15 | 2007-01-18 | Sanofi-Aventis Deutschland Gmbh | Neues 1,4-Benzothiazepin-1,1-Dioxidderivat mit verbesserten Eigenschaften, Verfahren zu dessen Herstellung, diese Verbindung enthaltende Arzneimittel und dessen Verwendung |
| DE102005033100B3 (de) | 2005-07-15 | 2007-01-25 | Sanofi-Aventis Deutschland Gmbh | Neues 1,4-Benzothiazepin-1,1-Dioxidderivat mit verbesserten Eigenschaften, diese Verbindung enthaltene Arzneimittel und Verfahren zu deren Herstellung |
| AU2006292377B2 (en) | 2005-09-20 | 2011-03-03 | Emisphere Technologies, Inc. | Use of a DPP-IV inhibitor to reduce hypoglycemic events |
| US20100286122A1 (en) | 2006-04-10 | 2010-11-11 | Kevin Belyk | CGRP Antagonist Salt |
| GB0607534D0 (en) | 2006-04-13 | 2006-05-24 | Univ London Pharmacy | Colonic drug delivery formulation |
| WO2008058631A1 (de) | 2006-11-14 | 2008-05-22 | Sanofi-Aventis Deutschland Gmbh | Neue 1,4-benzothiepin-1,1-dioxidderivate mit verbesserten eigenschaften, verfahren zu deren herstellung, diese verbindungen enthaltende arzneimittel und deren verwendung |
| DE102006053637B4 (de) | 2006-11-14 | 2011-06-30 | Sanofi-Aventis Deutschland GmbH, 65929 | Neue mit Fluor substituierte 1,4-Benzothiepin-1,1-Dioxidderivate, diese Verbindungen enthaltende Arzneimittel und deren Verwendung |
| DE102006053635B4 (de) | 2006-11-14 | 2011-06-30 | Sanofi-Aventis Deutschland GmbH, 65929 | Neue mit Benzylresten substituierte 1,4-Benzothiepin-1,1-Dioxidderivate, diese Verbindungen enthaltende Arzneimittel und deren Verwendung |
| WO2009040818A1 (en) | 2007-09-25 | 2009-04-02 | Solubest Ltd | Compositions comprising lipophilic active compounds and method for their preparation |
| CA2735962A1 (en) | 2008-09-02 | 2010-04-15 | Usv Limited | Crosslinked polymers |
| US9339480B2 (en) | 2008-11-26 | 2016-05-17 | Satiogen Pharmaceuticals, Inc. | Bile acid recycling inhibitors for treatment of obesity and diabetes |
| JO3131B1 (ar) | 2010-04-27 | 2017-09-20 | Glaxosmithkline Llc | مركبات كيميائية |
| WO2011150286A2 (en) | 2010-05-26 | 2011-12-01 | Satiogen Pharmaceuticals,Inc. | Bile acid recycling inhibitors and satiogens for treatment of diabetes, obesity, and inflammatory gastrointestinal conditions |
| MX345040B (es) | 2010-11-08 | 2017-01-16 | Albireo Ab | Una combinacion farmaceutica que comprende un inhibidor de ibat y un aglutinante de acido biliar. |
| US20120114588A1 (en) | 2010-11-08 | 2012-05-10 | Albireo Ab | Ibat inhibitors for treatment of metabolic disorders and related conditions |
| RS60901B1 (sr) | 2010-11-08 | 2020-11-30 | Albireo Ab | Ibat inhibitori za lečenje oboljenja jetre |
| CA2815941A1 (en) | 2010-11-08 | 2012-05-18 | Albireo Ab | Ibat inhibitors for treatment of metabolic disorders and related conditions |
| CN104023727B (zh) | 2011-10-28 | 2017-04-05 | 鲁美纳医药公司 | 用于治疗小儿胆汁淤积性肝病的胆汁酸再循环抑制剂 |
| CN104023718B (zh) | 2011-10-28 | 2017-04-05 | 鲁美纳医药公司 | 用于治疗高胆血症和胆汁淤积性肝病的胆汁酸再循环抑制剂 |
| JO3301B1 (ar) * | 2013-04-26 | 2018-09-16 | Albireo Ab | تعديلات بلورية على إيلوبيكسيبات |
| WO2015193788A1 (en) | 2014-06-16 | 2015-12-23 | Valpharma International S.P.A. | Formulation for oral administration containing mesalazine |
| CA2952406A1 (en) | 2014-06-25 | 2015-12-30 | Ea Pharma Co., Ltd. | Solid formulation and method for preventing or reducing coloration thereof |
| CA2952405A1 (en) | 2014-06-25 | 2015-12-30 | Ea Pharma Co., Ltd. | Solid formulation and method for stabilizing the same |
| US9684018B2 (en) | 2014-11-19 | 2017-06-20 | Texas Instruments Incorporated | Current sense circuit that operates over a wide range of currents |
| US10441604B2 (en) | 2016-02-09 | 2019-10-15 | Albireo Ab | Cholestyramine pellets and methods for preparation thereof |
| US10786529B2 (en) | 2016-02-09 | 2020-09-29 | Albireo Ab | Oral cholestyramine formulation and use thereof |
| US10441605B2 (en) | 2016-02-09 | 2019-10-15 | Albireo Ab | Oral cholestyramine formulation and use thereof |
-
2014
- 2014-10-24 EP EP14190290.8A patent/EP3012252A1/en not_active Withdrawn
-
2015
- 2015-10-23 JP JP2017519487A patent/JP6700263B2/ja active Active
- 2015-10-23 RU RU2017114109A patent/RU2017114109A/ru unknown
- 2015-10-23 CN CN202011101686.5A patent/CN112375044A/zh active Pending
- 2015-10-23 US US15/519,808 patent/US10183920B2/en active Active
- 2015-10-23 CN CN201580055918.9A patent/CN107001301A/zh active Pending
- 2015-10-23 AU AU2015334883A patent/AU2015334883B2/en active Active
- 2015-10-23 CA CA2959760A patent/CA2959760C/en active Active
- 2015-10-23 EP EP21167339.7A patent/EP3904344A1/en not_active Withdrawn
- 2015-10-23 KR KR1020177012754A patent/KR102498539B1/ko active IP Right Grant
- 2015-10-23 WO PCT/EP2015/074573 patent/WO2016062848A1/en active Application Filing
- 2015-10-23 EP EP15784385.5A patent/EP3209649B1/en active Active
- 2015-10-23 ES ES15784385T patent/ES2874573T3/es active Active
-
2018
- 2018-12-27 US US16/234,364 patent/US10519120B2/en active Active
Also Published As
| Publication number | Publication date |
|---|---|
| EP3209649A1 (en) | 2017-08-30 |
| US20170240516A1 (en) | 2017-08-24 |
| KR102498539B1 (ko) | 2023-02-09 |
| CA2959760C (en) | 2023-10-10 |
| CN107001301A (zh) | 2017-08-01 |
| WO2016062848A1 (en) | 2016-04-28 |
| EP3012252A1 (en) | 2016-04-27 |
| EP3209649B1 (en) | 2021-04-21 |
| AU2015334883A1 (en) | 2017-03-23 |
| EP3904344A1 (en) | 2021-11-03 |
| CN112375044A (zh) | 2021-02-19 |
| KR20170072908A (ko) | 2017-06-27 |
| RU2017114109A3 (ja) | 2019-04-30 |
| RU2017114109A (ru) | 2018-11-26 |
| CA2959760A1 (en) | 2016-04-28 |
| US10183920B2 (en) | 2019-01-22 |
| JP2017537061A (ja) | 2017-12-14 |
| US20190177286A1 (en) | 2019-06-13 |
| AU2015334883B2 (en) | 2019-10-24 |
| US10519120B2 (en) | 2019-12-31 |
| ES2874573T3 (es) | 2021-11-05 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP6700263B2 (ja) | エロビキシバットの結晶変態 | |
| RU2666368C2 (ru) | Кристаллическая модификация | |
| CZ303389B6 (cs) | Sul valsartanu a farmaceutický prípravek obsahující tuto sul | |
| US8410288B2 (en) | Polymorphs of Saxagliptin hydrochloride and processes for preparing them | |
| JP5042861B2 (ja) | 結晶性1H−イミダゾ[4,5−b]ピリジン−5−アミン,7−[5−[(シクロヘキシルメチルアミノ)−メチル]−1H−インドール−2−イル]−2−メチル,硫酸塩(1:1),三水和物およびその医薬的使用 | |
| CZ20023651A3 (cs) | Stabilizované přípravky 6-hydroxy-3-(4-[2-(piperidin-1-yl)-ethoxy]fenoxy)-2-(4-methoxyfenyl)benzo[b]thiofen a jejich sole | |
| WO2024121431A1 (en) | Cocrystals of odevixibat | |
| EA042192B1 (ru) | Фумарат (r)-3-(1-(2,3-дихлор-4-(пиразин-2-ил)фенил)-2,2,2-трифторэтил)-1-метил-1-(1-метилпиперидин-4-ил)мочевины, способы его получения и применения |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20180903 |
|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20180903 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20190819 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20191115 |
|
| TRDD | Decision of grant or rejection written | ||
| A01 | Written decision to grant a patent or to grant a registration (utility model) |
Free format text: JAPANESE INTERMEDIATE CODE: A01 Effective date: 20200406 |
|
| A61 | First payment of annual fees (during grant procedure) |
Free format text: JAPANESE INTERMEDIATE CODE: A61 Effective date: 20200430 |
|
| R150 | Certificate of patent or registration of utility model |
Ref document number: 6700263 Country of ref document: JP Free format text: JAPANESE INTERMEDIATE CODE: R150 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |