JP6564512B2 - 有機ホスフェートを含有する液状タンパク質製剤 - Google Patents
有機ホスフェートを含有する液状タンパク質製剤 Download PDFInfo
- Publication number
- JP6564512B2 JP6564512B2 JP2018183794A JP2018183794A JP6564512B2 JP 6564512 B2 JP6564512 B2 JP 6564512B2 JP 2018183794 A JP2018183794 A JP 2018183794A JP 2018183794 A JP2018183794 A JP 2018183794A JP 6564512 B2 JP6564512 B2 JP 6564512B2
- Authority
- JP
- Japan
- Prior art keywords
- viscosity
- protein
- liquid pharmaceutical
- pharmaceutical formulation
- formulation
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Active
Links
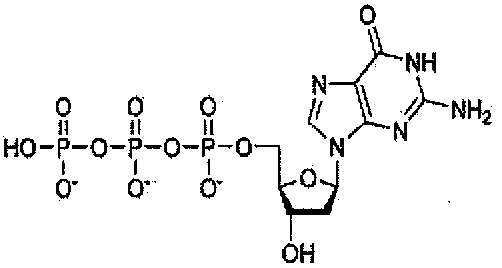
- IVOMOUWHDPKRLL-DGPXGRDGSA-N Nc1c2nc[n]([C@@H](C3O)O[C@H](CO4)C3OP4(O)=O)c2ncn1 Chemical compound Nc1c2nc[n]([C@@H](C3O)O[C@H](CO4)C3OP4(O)=O)c2ncn1 IVOMOUWHDPKRLL-DGPXGRDGSA-N 0.000 description 1
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/06—Organic compounds, e.g. natural or synthetic hydrocarbons, polyolefins, mineral oil, petrolatum or ozokerite
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61G—TRANSPORT, PERSONAL CONVEYANCES, OR ACCOMMODATION SPECIALLY ADAPTED FOR PATIENTS OR DISABLED PERSONS; OPERATING TABLES OR CHAIRS; CHAIRS FOR DENTISTRY; FUNERAL DEVICES
- A61G3/00—Ambulance aspects of vehicles; Vehicles with special provisions for transporting patients or disabled persons, or their personal conveyances, e.g. for facilitating access of, or for loading, wheelchairs
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K38/00—Medicinal preparations containing peptides
- A61K38/16—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- A61K38/43—Enzymes; Proenzymes; Derivatives thereof
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K39/395—Antibodies; Immunoglobulins; Immune serum, e.g. antilymphocytic serum
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K39/395—Antibodies; Immunoglobulins; Immune serum, e.g. antilymphocytic serum
- A61K39/39533—Antibodies; Immunoglobulins; Immune serum, e.g. antilymphocytic serum against materials from animals
- A61K39/3955—Antibodies; Immunoglobulins; Immune serum, e.g. antilymphocytic serum against materials from animals against proteinaceous materials, e.g. enzymes, hormones, lymphokines
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/06—Organic compounds, e.g. natural or synthetic hydrocarbons, polyolefins, mineral oil, petrolatum or ozokerite
- A61K47/08—Organic compounds, e.g. natural or synthetic hydrocarbons, polyolefins, mineral oil, petrolatum or ozokerite containing oxygen, e.g. ethers, acetals, ketones, quinones, aldehydes, peroxides
- A61K47/12—Carboxylic acids; Salts or anhydrides thereof
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/06—Organic compounds, e.g. natural or synthetic hydrocarbons, polyolefins, mineral oil, petrolatum or ozokerite
- A61K47/20—Organic compounds, e.g. natural or synthetic hydrocarbons, polyolefins, mineral oil, petrolatum or ozokerite containing sulfur, e.g. dimethyl sulfoxide [DMSO], docusate, sodium lauryl sulfate or aminosulfonic acids
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/06—Organic compounds, e.g. natural or synthetic hydrocarbons, polyolefins, mineral oil, petrolatum or ozokerite
- A61K47/22—Heterocyclic compounds, e.g. ascorbic acid, tocopherol or pyrrolidones
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/06—Organic compounds, e.g. natural or synthetic hydrocarbons, polyolefins, mineral oil, petrolatum or ozokerite
- A61K47/24—Organic compounds, e.g. natural or synthetic hydrocarbons, polyolefins, mineral oil, petrolatum or ozokerite containing atoms other than carbon, hydrogen, oxygen, halogen, nitrogen or sulfur, e.g. cyclomethicone or phospholipids
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/0012—Galenical forms characterised by the site of application
- A61K9/0019—Injectable compositions; Intramuscular, intravenous, arterial, subcutaneous administration; Compositions to be administered through the skin in an invasive manner
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/08—Solutions
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/14—Particulate form, e.g. powders, Processes for size reducing of pure drugs or the resulting products, Pure drug nanoparticles
- A61K9/19—Particulate form, e.g. powders, Processes for size reducing of pure drugs or the resulting products, Pure drug nanoparticles lyophilised, i.e. freeze-dried, solutions or dispersions
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P29/00—Non-central analgesic, antipyretic or antiinflammatory agents, e.g. antirheumatic agents; Non-steroidal antiinflammatory drugs [NSAID]
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P31/00—Antiinfectives, i.e. antibiotics, antiseptics, chemotherapeutics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P37/00—Drugs for immunological or allergic disorders
- A61P37/02—Immunomodulators
- A61P37/06—Immunosuppressants, e.g. drugs for graft rejection
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P43/00—Drugs for specific purposes, not provided for in groups A61P1/00-A61P41/00
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P7/00—Drugs for disorders of the blood or the extracellular fluid
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K1/00—General methods for the preparation of peptides, i.e. processes for the organic chemical preparation of peptides or proteins of any length
- C07K1/14—Extraction; Separation; Purification
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/18—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans
- C07K16/22—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against growth factors ; against growth regulators
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/18—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans
- C07K16/24—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against cytokines, lymphokines or interferons
- C07K16/241—Tumor Necrosis Factors
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/18—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans
- C07K16/28—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants
- C07K16/2839—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants against the integrin superfamily
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/18—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans
- C07K16/28—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants
- C07K16/2863—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants against receptors for growth factors, growth regulators
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/18—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans
- C07K16/28—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants
- C07K16/2887—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants against CD20
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/18—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans
- C07K16/32—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against translation products of oncogenes
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K2039/505—Medicinal preparations containing antigens or antibodies comprising antibodies
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K38/00—Medicinal preparations containing peptides
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y02—TECHNOLOGIES OR APPLICATIONS FOR MITIGATION OR ADAPTATION AGAINST CLIMATE CHANGE
- Y02P—CLIMATE CHANGE MITIGATION TECHNOLOGIES IN THE PRODUCTION OR PROCESSING OF GOODS
- Y02P20/00—Technologies relating to chemical industry
- Y02P20/50—Improvements relating to the production of bulk chemicals
- Y02P20/54—Improvements relating to the production of bulk chemicals using solvents, e.g. supercritical solvents or ionic liquids
Landscapes
- Health & Medical Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Medicinal Chemistry (AREA)
- General Health & Medical Sciences (AREA)
- Organic Chemistry (AREA)
- Public Health (AREA)
- Animal Behavior & Ethology (AREA)
- Veterinary Medicine (AREA)
- Pharmacology & Pharmacy (AREA)
- Immunology (AREA)
- Engineering & Computer Science (AREA)
- Epidemiology (AREA)
- General Chemical & Material Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Molecular Biology (AREA)
- Biophysics (AREA)
- Biochemistry (AREA)
- Genetics & Genomics (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Oil, Petroleum & Natural Gas (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Oncology (AREA)
- Microbiology (AREA)
- Mycology (AREA)
- Dermatology (AREA)
- Endocrinology (AREA)
- Gastroenterology & Hepatology (AREA)
- Diabetes (AREA)
- Analytical Chemistry (AREA)
- Pain & Pain Management (AREA)
- Communicable Diseases (AREA)
- Hematology (AREA)
- Biomedical Technology (AREA)
- Neurosurgery (AREA)
- Transplantation (AREA)
- Neurology (AREA)
- Rheumatology (AREA)
- Medicinal Preparation (AREA)
Description
本願は、2014年7月29日出願の「Low-Viscosity Protein Formulations Containing Hydrophobic Salts」との表題の米国仮出願第62/030,521号;2014年7月18日出願の「Low-Viscosity Protein Formulations Containing GRAS Viscosity-Reducing Agents」との表題の米国仮出願第62/026,497号;2014年6月5日出願の「Low-Viscosity Protein Formulations Containing Ionic Liquids」との表題の米国仮出願第62/008,050号;2014年5月2日出願の「Low-Viscosity Protein Formulations Containing Organophosphates」との表題の米国仮出願第61/988,005号;2014年2月28日出願の「Concentrated, Low-Viscosity Infliximab Formulations」との表題の米国仮出願第61/946,436号;2014年2月21日出願の「Concentrated, Low-Viscosity, High-Molecular-Weight-Protein Formulations」との表題の米国仮出願第61/943,197号;2014年2月14日出願の「Concentrated, Low-Viscosity High-Molecular-Weight Protein Formulations」との表題の米国仮出願第61/940,227号、および2013年9月11日出願の「Concentrated, Low-Viscosity, High-Molecular-Weight Protein Formulations」との表題の米国仮出願第61,876,621号の優先権を主張し、かつその利益を主張する。これらの出願の開示は、本明細書に参考として明白に援用される。
本発明は、一般に、モノクローナル抗体などのタンパク質の注射可能な医薬製剤、ならびにその作製方法および使用方法の分野におけるものである。
モノクローナル抗体(mAb)は、がん、感染症、炎症および自己免疫疾患などの様々なヒト疾患を処置するための、重要なタンパク質をベースとする治療薬である。20種を超えるmAb製品が、米国食品医薬品局(FDA)によって承認されており、臨床試験において現在、評価されているバイオ医薬品の約20%が、mAbである(Daughertyら、Adv. Drug Deliv. Rev. 58巻:686〜706頁、2006年、およびBussら、Curr. Opinion in Pharmacol. 12巻:615〜622頁、2012年)。
(項目1)
(i)1種または複数のタンパク質;
(ii)1種または複数の粘度低下有機ホスフェート;および
(iii)薬学的に許容される溶媒;
を含む、注射用液状医薬製剤であって、
該タンパク質が、注射に適した体積で溶媒および有機ホスフェートと組み合わされる場合、該製剤が、円錐平板粘度計を使用して測定すると、25℃において絶対粘度約1cP〜約50cPを有しており、該製剤の該絶対粘度が、該有機ホスフェートの代わりに等量のリン酸ナトリウムを含む他は同じ該製剤の該絶対粘度よりも低く、
各場合において、該絶対粘度が外挿したゼロせん断粘度である、
注射用医薬製剤。
(項目2)
前記タンパク質(複数可)が、約70kDa〜100kDaまでの間、約100kDa〜約250kDa、または約250kDa〜約500kDaの間の分子量を有する高分子量タンパク質である、項目1に記載の製剤。
(項目3)
前記タンパク質が、約120kDa〜約250kDaの分子量を有する、項目1および2のいずれか一項に記載の製剤。
(項目4)
前記タンパク質の少なくとも1種が、酵素、抗体もしくは抗体断片、融合タンパク質、またはPEG化タンパク質である、項目1から3のいずれかに記載の製剤。
(項目5)
前記タンパク質(複数可)が、1mLあたり約100mg〜約2,000mg(mg/mL)、任意選択で約150mg/mLより多い合わせた量で存在する、項目1から4のいずれか一項に記載の製剤。
(項目6)
少なくとも2種の異なるタンパク質を含み、好ましくは、該タンパク質の両方が、少なくとも約50kDaの分子量を有する、項目1から5のいずれか一項に記載の製剤。
(項目7)
有機ホスフェートを添加する前の、同じ前記タンパク質濃度における初期絶対粘度が、約60cPを超える、約80cPを超える、または約100cPを超える、項目1から6のいずれか一項に記載の製剤。
(項目8)
液状の前記製剤が、約5.0〜約8.0の間のpHを有する水性である、項目1から7のいずれか一項に記載の製剤。
(項目9)
約0.01M〜約1.0Mの濃度で存在する前記有機ホスフェートを含む、項目1から8のいずれか一項に記載の製剤。
(項目10)
0.30M未満、または0.15M未満の量で存在する前記有機ホスフェートを含む、項目1から9のいずれか一項に記載の製剤。
(項目11)
皮下または筋肉注射用の1種または複数の薬学的に許容される賦形剤であって、糖または糖アルコール、緩衝剤、保存剤、担体、酸化防止剤、キレート剤、天然または合成ポリマー、凍結保護剤、凍結乾燥保護剤、界面活性剤、増量剤および安定化剤からなる群から選択される、1種または複数の薬学的に許容される賦形剤を含む、項目1から10のいずれか一項に記載の製剤。
(項目12)
前記賦形剤の1種または複数が、ポリソルベート、ポロキサマー188、ラウリル硫酸ナトリウム、マンニトールおよびソルビトールなどの糖アルコール、ポリ(エチレングリコール)、グリセロール、プロピレングリコールおよびポリ(ビニルアルコール)からなる群から選択されるポリオールからなる群から選択される、項目11に記載の製剤。
(項目13)
前記界面活性剤が、約10mg/mL未満の量で存在する、項目11に記載の製剤。
(項目14)
約2mg/mL〜約900mg/mLの量で存在しているポリオールを含む、項目12に記載の製剤。
(項目15)
前記絶対粘度が、25℃において、約5cP〜約50cPである、項目1から14のいずれか一項に記載の製剤。
(項目16)
前記絶対粘度が、前記有機ホスフェートをほぼ同じ濃度の適切な緩衝剤に置き換えた以外同一条件下で測定した場合、該有機ホスフェートを含まない製剤の該絶対粘度よりも少なくとも約30%低い、項目1から15のいずれか一項に記載の製剤。
(項目17)
前記絶対粘度が、前記有機ホスフェートをほぼ同じ濃度の適切な緩衝剤に置き換えた以外同一条件下で測定した場合、該有機ホスフェートを含まない製剤の該絶対粘度よりも少なくとも約2倍または4倍低い、項目1から16のいずれか一項に記載の製剤。
(項目18)
単位用量バイアル、容器またはプレフィルドシリンジ中の、項目1から17のいずれか一項に記載の製剤。
(項目19)
前記タンパク質、有機ホスフェートおよび/または賦形剤が乾燥形態にあり、好ましくは凍結乾燥されている、項目18に記載の製剤。
(項目20)
有機ホスフェート、タンパク質および溶媒を組み合わせた場合の前記製剤の体積が、SC注射の場合、約1.5mL未満であり、IM注射の場合、約3mL未満である、項目1から19のいずれかに記載の製剤。
(項目21)
ヒト血清に等張である、項目1から20のいずれか一項に記載の製剤。
(項目22)
それを必要とするヒトに投与される条件下で、レオロジー的に、本質的にニュートン液体として挙動する、項目1から21のいずれか一項に記載の製剤。
(項目23)
静脈内注入により投与される同じ用量の前記タンパク質と比べて、治療有効な投与量を実現する、項目1から22のいずれか一項に記載の製剤。
(項目24)
前記有機ホスフェートが、皮下注射または筋肉内注射により投与される場合、毒性または注射部位の刺激の臨床的に有意な徴候を引き起こさない濃度で存在する、項目1から23のいずれか一項に記載の製剤。
(項目25)
前記製剤の前記絶対粘度が、円錐平板粘度計を使用して測定される場合、少なくとも約0.5s−1のせん断速度で測定される、項目1から24のいずれかに記載の製剤。
(項目26)
前記製剤の前記絶対粘度が、マイクロ流体粘度計を使用して測定した場合、少なくとも約1.0s−1のせん断速度で、測定される、項目1から24のいずれか一項に記載の製剤。
(項目27)
項目1から26のいずれか一項に記載の製剤の、皮下注射または筋肉内注射を含む、治療有効量のタンパク質を投与する方法。
(項目28)
前記皮下注射または筋肉内注射が、加熱シリンジ、自己混合式シリンジ、オートインジェクター、プレフィルドシリンジ、およびそれらの組合せからなる群から選択されるシリンジを用いて行われる、項目27に記載の方法。
(項目29)
前記シリンジが、加熱シリンジであり、前記製剤が、25℃〜40℃の間の温度で投与される、項目28に記載の方法。
(項目30)
前記製剤が、ドレイズ評点システムを使用して評価した場合、3未満の一次刺激インデックスを惹起する、項目27から29のいずれかに記載の方法。
(項目31)
射出力が、前記有機ホスフェートを含まないがそれ以外は同じ製剤を同じ様式で投与した場合の射出力よりも、少なくとも10%または20%小さい、項目27から30のいずれか一項に記載の方法。
(項目32)
前記注射が、直径がゲージ27〜31の間の針を使用して施行され、かつ該ゲージ27の針を使用した場合、前記射出力が30N未満である、項目27から31のいずれか一項に記載の方法。
(項目33)
項目1から26のいずれかに記載のタンパク質、溶媒および有機ホスフェートを合わせるステップを含む、医薬製剤を調製する方法。
(項目34)
前記製剤が、プレフィルドシリンジまたはカートリッジ内にある、項目33に記載の方法。
(項目35)
項目1、または7から10のいずれかに記載の有機ホスフェートの有効量を、タンパク質溶液に加えて、該タンパク質溶液の前記粘度を低下させることを含む、タンパク質の精製を容易にする方法。
(項目36)
前記タンパク質−有機ホスフェート溶液が、限外ろ過/ダイアフィルトレーション、タンジェンシャルフローろ過、遠心濃縮、および透析からなる群から選択される方法を使用して精製または濃縮される、項目35に記載の方法。
発明の概要
タンパク質の、低粘度で低体積の濃縮液状医薬製剤を開発した。こうした製剤は、長時間の静脈内注入によるよりもむしろ、皮下(SC)または筋肉内(IM)注射によって、迅速かつ便利に投与することができる。これらの製剤は、mAbなどの低分子量および/または高分子量タンパク質、および有機ホスフェートを含む。代表的な有機ホスフェートには、チアミンピロリン酸(TPP)、アデノシン三リン酸(ATP)、デオキシアデノシン三リン酸(dATP)、デオキシグアノシン三リン酸(dGTP)、デオキシチミジン三リン酸(dTTP)、デオキシシチジン三リン酸(dCTP)、環状アデノシン一リン酸(cAMP)、ニコチンアミドアデニンジヌクレオチドリン酸(NADP+)およびピリドキサールリン酸、ならびにそれらの塩が、好ましくは約0.01M〜約0.50Mの間、最も好ましくは約0.05M〜約0.25Mの間の濃度で含まれる。
本明細書において一般に使用される、用語「タンパク質」とは、ペプチド結合により互いに連結されて、その鎖長が少なくとも検出可能な三次元構造を生じるのに十分な、ポリペプチドを形成する、アミノ酸のポリマーを指す。約100kDaを超える分子量(kDaで表され、ここで、「Da」は「ダルトン」を表し、1kDa=1,000Daである)を有する、タンパク質は、「高分子量タンパク質」と称することができる一方、約100kDa未満の分子量を有するタンパク質は、「低分子量タンパク質」と称することができる。用語「低分子量タンパク質」は、タンパク質と見なすのに必要な少なくとも三次元の構造という要件を欠く、小さなペプチドを除外する。タンパク質の分子量は、質量分析法(例えば、ESI、MALDI)または公知のアミノ酸配列およびグリコシル化からの計算を含むがこれらに限定されない、当業者に公知の標準方法を使用して決定することができる。タンパク質は、天然に存在するかまたは天然に存在しないもの、合成によるものまたは半合成によるものとすることができる。
有機ホスフェートは、バイオシミラーAVASTIN(登録商標)の濃縮水溶液の粘度を低下させる
バイオシミラーAVASTIN(登録商標)の水溶液の粘度低下は、有機ホスフェートの濃度に依存する
有機ホスフェートは、治療関連性のあるモノクローナル抗体の多くの粘度を低下させる。
Claims (25)
- (i)約150mg/ml〜約250mg/mlの抗体;
(ii)チアミンピロリン酸(TPP);および
(iii)薬学的に許容される溶媒;
を含む、注射用液状医薬製剤であって、
該液状医薬製剤は、注射に適した体積にある場合、円錐平板粘度計またはマイクロ流体粘度計を使用して測定すると、25℃において約1cP〜約100cPの絶対粘度を有しており、該液状医薬製剤の該絶対粘度が、該抗体および該薬学的に許容される溶媒を含むが、該TPPを含まない対照組成物の絶対粘度よりも低く、
該絶対粘度が外挿したゼロせん断粘度である、
注射用液状医薬製剤。 - 前記抗体がモノクローナル抗体である、請求項1に記載の液状医薬製剤。
- 前記抗体が、約120kDa〜約250kDaの分子量を有する、請求項1または2に記載の液状医薬製剤。
- 約158mg/ml〜約216mg/mlの前記抗体を含む、請求項1から3のいずれか一項に記載の液状医薬製剤。
- 前記薬学的に許容される溶媒が、水性である、請求項1から4のいずれか一項に記載の液状医薬製剤。
- 前記TPPが、約0.10M〜約0.25Mの濃度で存在する、請求項1から5のいずれか一項に記載の液状医薬製剤。
- 1種または複数の薬学的に許容される賦形剤であって、糖、糖アルコール、緩衝剤、保存剤、担体、酸化防止剤、キレート剤、天然ポリマー、合成ポリマー、凍結保護剤、凍結乾燥保護剤、界面活性剤、増量剤、安定化剤またはこれらの組合せを含む、1種または複数の薬学的に許容される賦形剤をさらに含む、請求項1から6のいずれか一項に記載の液状医薬製剤。
- 前記1種または複数の薬学的に許容される賦形剤が、ポリソルベート、ポロキサマー188、ラウリル硫酸ナトリウム、ポリオール、ポリ(エチレングリコール)、グリセロール、プロピレングリコールまたはポリ(ビニルアルコール)である、請求項7に記載の液状医薬製剤。
- 前記糖アルコールがソルビトールまたはマンニトールである、請求項7に記載の液状医薬製剤。
- 単位用量バイアル、複数回用量バイアル、カートリッジ、またはプレフィルドシリンジ中の、請求項1から9のいずれか一項に記載の液状医薬製剤。
- ヒト血清に等張である、請求項1から10のいずれか一項に記載の液状医薬製剤。
- 前記絶対粘度が、円錐平板粘度計を使用して測定される場合、少なくとも約0.5s−1のせん断速度で測定される、請求項1から11のいずれか一項に記載の液状医薬製剤。
- 前記絶対粘度が、マイクロ流体粘度計を使用して測定した場合、少なくとも約1.0s−1のせん断速度で、測定される、請求項1から11のいずれか一項に記載の液状医薬製剤。
- 前記液状医薬製剤が、凍結乾燥組成物から再構成される、請求項1から13のいずれか一項に記載の液状医薬製剤。
- 前記液状医薬製剤が対象に投与され、ここで該投与が皮下注射または筋肉内注射を含むことを特徴とする、請求項1から14のいずれか一項に記載の液状医薬製剤。
- 前記注射が、シリンジを用いて行われる、請求項15に記載の液状医薬製剤。
- 前記シリンジが、加熱シリンジ、自己混合式シリンジ、オートインジェクター、プレフィルドシリンジ、またはそれらの組合せである、請求項16に記載の液状医薬製剤。
- 前記シリンジが、加熱シリンジであり、前記液状医薬製剤が、25℃〜40℃の間の温度を有する、請求項16または17に記載の液状医薬製剤。
- 前記液状医薬製剤が、ドレイズ評点システムを使用して評価した場合、3未満の一次刺激インデックスを惹起する、請求項15から18のいずれかに記載の液状医薬製剤。
- 前記液状医薬製剤が、前記抗体および前記薬学的に許容される溶媒を含むが前記TPPを含まない液状医薬製剤の射出力よりも、少なくとも10%小さい射出力によって注射される、請求項15から19のいずれか一項に記載の液状医薬製剤。
- 前記液状医薬製剤が、前記抗体および前記薬学的に許容される溶媒を含むが前記TPPを含まない液状医薬製剤の射出力よりも、少なくとも20%小さい射出力によって注射される、請求項15から19のいずれか一項に記載の液状医薬製剤。
- 前記注射が、直径がゲージ27〜31の間の針を使用して行われ、かつ該ゲージ27の針を使用した場合、前記射出力が30N未満である、請求項15から21のいずれか一項に記載の液状医薬製剤。
- 前記抗体、前記薬学的に許容される溶媒および前記TPPを合わせるステップを含む、請求項1から14のいずれか一項に記載の液状医薬製剤を調製する方法。
- (i)抗体;
(ii)TPP;および
(iii)薬学的に許容される賦形剤
を含む、凍結乾燥組成物。 - 再構成されると、前記抗体が少なくとも100mg/mlの濃度を有する、請求項24に記載の凍結乾燥組成物。
Applications Claiming Priority (16)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US201361876621P | 2013-09-11 | 2013-09-11 | |
| US61/876,621 | 2013-09-11 | ||
| US201461940227P | 2014-02-14 | 2014-02-14 | |
| US61/940,227 | 2014-02-14 | ||
| US201461943197P | 2014-02-21 | 2014-02-21 | |
| US61/943,197 | 2014-02-21 | ||
| US201461946436P | 2014-02-28 | 2014-02-28 | |
| US61/946,436 | 2014-02-28 | ||
| US201461988005P | 2014-05-02 | 2014-05-02 | |
| US61/988,005 | 2014-05-02 | ||
| US201462008050P | 2014-06-05 | 2014-06-05 | |
| US62/008,050 | 2014-06-05 | ||
| US201462026497P | 2014-07-18 | 2014-07-18 | |
| US62/026,497 | 2014-07-18 | ||
| US201462030521P | 2014-07-29 | 2014-07-29 | |
| US62/030,521 | 2014-07-29 |
Related Parent Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2016542105A Division JP6412575B2 (ja) | 2013-09-11 | 2014-09-11 | 有機ホスフェートを含有する液状タンパク質製剤 |
Related Child Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2019137552A Division JP6840797B2 (ja) | 2013-09-11 | 2019-07-26 | 有機ホスフェートを含有する液状タンパク質製剤 |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| JP2018199735A JP2018199735A (ja) | 2018-12-20 |
| JP6564512B2 true JP6564512B2 (ja) | 2019-08-21 |
Family
ID=51626611
Family Applications (12)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2016542116A Active JP6515105B2 (ja) | 2013-09-11 | 2014-09-11 | イオン性液体を含有する注射用液状タンパク質製剤およびその使用 |
| JP2016542105A Active JP6412575B2 (ja) | 2013-09-11 | 2014-09-11 | 有機ホスフェートを含有する液状タンパク質製剤 |
| JP2016542118A Active JP6463581B2 (ja) | 2013-09-11 | 2014-09-11 | 粘度低下剤を含む液状タンパク質製剤 |
| JP2016542103A Active JP6469113B2 (ja) | 2013-09-11 | 2014-09-11 | 水溶性有機色素を含有する液状タンパク質製剤 |
| JP2018183794A Active JP6564512B2 (ja) | 2013-09-11 | 2018-09-28 | 有機ホスフェートを含有する液状タンパク質製剤 |
| JP2018248768A Active JP6774484B2 (ja) | 2013-09-11 | 2018-12-29 | 粘度低下剤を含む液状タンパク質製剤 |
| JP2019031843A Active JP6768858B2 (ja) | 2013-09-11 | 2019-02-25 | イオン性液体を含有する液状タンパク質製剤 |
| JP2019108536A Active JP6941137B2 (ja) | 2013-09-11 | 2019-06-11 | イオン性液体を含有する液状タンパク質製剤 |
| JP2019137552A Active JP6840797B2 (ja) | 2013-09-11 | 2019-07-26 | 有機ホスフェートを含有する液状タンパク質製剤 |
| JP2020167521A Active JP7214697B2 (ja) | 2013-09-11 | 2020-10-02 | 粘度低下剤を含む液状タンパク質製剤 |
| JP2022200135A Active JP7583455B2 (ja) | 2013-09-11 | 2022-12-15 | 粘度低下剤を含む液状タンパク質製剤 |
| JP2024188410A Pending JP2025010224A (ja) | 2013-09-11 | 2024-10-25 | 粘度低下剤を含む液状タンパク質製剤 |
Family Applications Before (4)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2016542116A Active JP6515105B2 (ja) | 2013-09-11 | 2014-09-11 | イオン性液体を含有する注射用液状タンパク質製剤およびその使用 |
| JP2016542105A Active JP6412575B2 (ja) | 2013-09-11 | 2014-09-11 | 有機ホスフェートを含有する液状タンパク質製剤 |
| JP2016542118A Active JP6463581B2 (ja) | 2013-09-11 | 2014-09-11 | 粘度低下剤を含む液状タンパク質製剤 |
| JP2016542103A Active JP6469113B2 (ja) | 2013-09-11 | 2014-09-11 | 水溶性有機色素を含有する液状タンパク質製剤 |
Family Applications After (7)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2018248768A Active JP6774484B2 (ja) | 2013-09-11 | 2018-12-29 | 粘度低下剤を含む液状タンパク質製剤 |
| JP2019031843A Active JP6768858B2 (ja) | 2013-09-11 | 2019-02-25 | イオン性液体を含有する液状タンパク質製剤 |
| JP2019108536A Active JP6941137B2 (ja) | 2013-09-11 | 2019-06-11 | イオン性液体を含有する液状タンパク質製剤 |
| JP2019137552A Active JP6840797B2 (ja) | 2013-09-11 | 2019-07-26 | 有機ホスフェートを含有する液状タンパク質製剤 |
| JP2020167521A Active JP7214697B2 (ja) | 2013-09-11 | 2020-10-02 | 粘度低下剤を含む液状タンパク質製剤 |
| JP2022200135A Active JP7583455B2 (ja) | 2013-09-11 | 2022-12-15 | 粘度低下剤を含む液状タンパク質製剤 |
| JP2024188410A Pending JP2025010224A (ja) | 2013-09-11 | 2024-10-25 | 粘度低下剤を含む液状タンパク質製剤 |
Country Status (16)
| Country | Link |
|---|---|
| US (13) | US9913905B2 (ja) |
| EP (8) | EP3043772B1 (ja) |
| JP (12) | JP6515105B2 (ja) |
| KR (5) | KR102295633B1 (ja) |
| CN (7) | CN105848636B (ja) |
| AU (10) | AU2014318637B2 (ja) |
| CA (4) | CA2923843C (ja) |
| ES (5) | ES2959451T3 (ja) |
| HK (1) | HK1226309A1 (ja) |
| IL (5) | IL312865B2 (ja) |
| MX (6) | MX382917B (ja) |
| RU (2) | RU2710542C2 (ja) |
| SG (6) | SG10201913950XA (ja) |
| SI (4) | SI3043774T1 (ja) |
| WO (4) | WO2015038811A2 (ja) |
| ZA (5) | ZA201601968B (ja) |
Families Citing this family (73)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| MY177062A (en) | 2010-03-12 | 2020-09-03 | Debiopharm Int Sa | Cd37-binding molecules and immunoconjugates thereof |
| EA201391342A1 (ru) | 2011-04-01 | 2014-11-28 | Иммьюноджен, Инк. | Cd37-связывающие молекулы и их иммуноконъюгаты |
| US20140348719A1 (en) * | 2013-03-14 | 2014-11-27 | Bureau Veritas | Pharmaceutical compound stabilizing filter compositions and methods of making and using same |
| IL312865B2 (en) | 2013-09-11 | 2025-06-01 | Eagle Biologics Inc | Liquid protein formulations containing viscosity-lowering agents |
| FI3083686T4 (fi) * | 2013-12-17 | 2023-05-09 | Menetelmiä syöpien hoitamiseksi käyttäen pd-1-akselia sitovia antagonisteja ja taksaaneja | |
| CA2938884C (en) | 2014-02-07 | 2024-02-13 | eXIthera Pharmaceuticals Inc. | Substituted azetidine compounds and their use as factor xia or kallikrein inhibitors |
| US11357857B2 (en) | 2014-06-20 | 2022-06-14 | Comera Life Sciences, Inc. | Excipient compounds for protein processing |
| US10478498B2 (en) | 2014-06-20 | 2019-11-19 | Reform Biologics, Llc | Excipient compounds for biopolymer formulations |
| US20160074515A1 (en) * | 2014-06-20 | 2016-03-17 | Reform Biologics, Llc | Viscosity-reducing excipient compounds for protein formulations |
| CA2962768C (en) | 2014-10-01 | 2023-10-10 | Alyssa M. Larson | Polysaccharide and nucleic acid formulations containing viscosity-lowering agents |
| EA201792300A1 (ru) * | 2015-06-08 | 2018-09-28 | Дебиофарм Интернэшнл, С.А. | Комбинации иммуноконъюгата к cd37 и антитела к cd20 |
| IL256080B2 (en) | 2015-06-17 | 2025-06-01 | Genentech Inc | Methods of treating locally advanced or metastatic breast cancers using pd-1 axis binding antagonists and taxanes |
| TW202440903A (zh) * | 2015-08-04 | 2024-10-16 | 美商再生元醫藥公司 | 補充牛磺酸之細胞培養基及用法(一) |
| HUE061985T2 (hu) * | 2015-08-19 | 2023-09-28 | Astrazeneca Ab | Stabil IFNAR1-elleni készítmény |
| KR102766022B1 (ko) | 2015-08-28 | 2025-02-10 | 데비오팜 인터네셔날 에스 에이 | Cd37 의 검출을 위한 항체 및 검정 |
| WO2017049205A2 (en) * | 2015-09-18 | 2017-03-23 | Amunix Operating Inc. | Growth hormone formulation |
| WO2017055966A1 (en) * | 2015-10-01 | 2017-04-06 | Pfizer Inc. | Low viscosity antibody compositions |
| WO2017070501A1 (en) | 2015-10-23 | 2017-04-27 | Reform Biologics, Llc | Excipient compounds for biopolymer formulations |
| WO2017075173A2 (en) * | 2015-10-30 | 2017-05-04 | Genentech, Inc. | Anti-factor d antibodies and conjugates |
| US20210206834A1 (en) * | 2015-12-03 | 2021-07-08 | Mor Research Applications Ltd. | Compositions and methods for treatment of ocular diseases |
| CN109152831A (zh) * | 2015-12-29 | 2019-01-04 | 安口生物公司 | 贝伐珠单抗的缓冲制剂 |
| CN105699555B (zh) * | 2016-03-24 | 2018-09-11 | 中国标准化研究院 | 一种丙烯基乙基愈创木酚的液相色谱制备方法 |
| AU2017257505B2 (en) * | 2016-04-25 | 2020-05-14 | Medimmune, Llc | Compositions comprising coformulation of anti-PD-L1 and Anti-CTLA-4 antibodies |
| MX2018013523A (es) * | 2016-05-05 | 2019-06-10 | Univ Pennsylvania | Anticuerpos monoclonales de adn dirigidos a il-6 y cd126. |
| WO2017214332A1 (en) * | 2016-06-07 | 2017-12-14 | National Technology & Engineering Solutions Of Sandia, Llc | Conversion of sugars to ionic liquids |
| WO2018035470A1 (en) * | 2016-08-18 | 2018-02-22 | Regeneron Pharmaceuticals, Inc. | Assay for determining potential to self-association of a protein using concentration-dependent self-interaction nanoparticle spectroscopy |
| US11382976B1 (en) | 2016-10-04 | 2022-07-12 | United States Of America As Represented By The Secretary Of The Air Force | Ultra-stable protein ionic liquids |
| US11274137B1 (en) | 2016-10-04 | 2022-03-15 | United States Of America As Represented By The Secretary Of The Air Force | Ultra-stable protein ionic liquids |
| US10463733B1 (en) | 2016-10-04 | 2019-11-05 | United States of America as represented by the Secetary of the Air Force | Ultra-stable protein ionic liquids |
| US11385201B1 (en) | 2019-09-30 | 2022-07-12 | United States Of America As Represented By The Secretary Of The Air Force | Ultra-stable protein ionic liquids |
| US11274289B1 (en) | 2016-10-04 | 2022-03-15 | United States Of America As Represented By The Secretary Of The Air Force | Ultra-stable protein ionic liquids |
| US11274163B1 (en) | 2016-10-04 | 2022-03-15 | United States Of America As Represented By The Secretary Of The Air Force | Ultra-stable protein ionic liquids |
| AU2017347838B2 (en) * | 2016-10-27 | 2023-04-27 | Eisai R&D Management Co., Ltd. | Composition comprising an anti-Αβ protofibril antibody and a beta-secretase BACE1 inhibitor for the treatment of Alzheimer's disease |
| CN116407644A (zh) * | 2016-11-02 | 2023-07-11 | 波拉里集团 | 聚乙二醇化精氨酸脱亚氨酶的调配物 |
| EP3535297B1 (en) | 2016-11-02 | 2022-08-10 | Debiopharm International, S.A. | Methods for improving anti-cd37 immunoconjugate therapy |
| KR102572453B1 (ko) * | 2017-02-16 | 2023-08-29 | 코메라 라이프 사이언시스, 인코포레이티드 | 단백질 가공 처리를 위한 부형제 화합물 |
| JOP20190204A1 (ar) | 2017-03-08 | 2019-09-05 | Actelion Pharmaceuticals Ltd | تركيبة صيدلانية تشتمل على سيليكسيباغ |
| US10238600B2 (en) | 2017-04-13 | 2019-03-26 | Richard C. Fuisz | Package, system and methods for custody and control of drugs, and method and composition for making an oral soluble film, containing at least one active agent |
| US9901545B1 (en) | 2017-04-13 | 2018-02-27 | Richard C. Fuisz | Method and composition for making an oral soluble film, containing at least one active agent |
| WO2018193471A1 (en) * | 2017-04-18 | 2018-10-25 | Dr. Reddy's Laboratories Limited | Stable liquid pharmaceutical composition |
| WO2018200533A1 (en) * | 2017-04-28 | 2018-11-01 | Amgen Inc. | Excipients to reduce the viscosity of antibody formulations and formulation compositions |
| JOP20190260A1 (ar) | 2017-05-02 | 2019-10-31 | Merck Sharp & Dohme | صيغ ثابتة لأجسام مضادة لمستقبل الموت المبرمج 1 (pd-1) وطرق استخدامها |
| US11845798B2 (en) | 2017-05-02 | 2023-12-19 | Merck Sharp & Dohme Llc | Formulations of anti-LAG3 antibodies and co-formulations of anti-LAG3 antibodies and anti-PD-1 antibodies |
| WO2018211517A1 (en) | 2017-05-16 | 2018-11-22 | Bhami's Research Laboratory, Pvt. Ltd. | High concentration protein formulations with reduced viscosity |
| US20200148757A1 (en) * | 2017-06-30 | 2020-05-14 | Korea Advanced Institute Of Science And Technology | Conjugate of vegf-grab protein and drug, and use thereof |
| JP2020531521A (ja) * | 2017-08-22 | 2020-11-05 | バイオジェン・エムエイ・インコーポレイテッドBiogen MA Inc. | 抗アルファ(v)ベータ(6)抗体を含有する医薬組成物及び投薬計画 |
| CA3074565A1 (en) | 2017-09-05 | 2019-03-14 | Merck Sharp & Dohme Corp. | Compounds for reducing the viscosity of biological formulations |
| SG11202003835PA (en) * | 2017-11-07 | 2020-05-28 | Alphacore Pharma Llc | Methods of treating and protecting against cardiac disease, cardiovascular disease and related conditions and symptoms |
| EP3709974A4 (en) | 2017-11-17 | 2021-08-04 | President And Fellows Of Harvard College | IONIC LIQUIDS FOR INTERNAL ADMINISTRATION |
| CN108096185A (zh) * | 2017-12-20 | 2018-06-01 | 珠海冀百康生物科技有限公司 | 一种速效胰岛素制剂及其制备方法 |
| KR102493469B1 (ko) * | 2018-03-07 | 2023-02-07 | 코메라 라이프 사이언시스, 인코포레이티드 | 단백질 제형을 위한 부형제 화합물 |
| EP3928765A1 (en) * | 2018-04-16 | 2021-12-29 | Merck Patent GmbH | Viscosity reduction of highly concentrated protein formulations |
| CN108671229B (zh) * | 2018-05-08 | 2022-03-25 | 华博生物医药技术(上海)有限公司 | 一种重组人血管内皮生长因子受体-抗体融合蛋白的药物组合制剂 |
| JP2021525735A (ja) | 2018-05-30 | 2021-09-27 | デビオファーム インターナショナル, エス. アー. | 抗cd37免疫コンジュゲート投薬レジメン |
| EP3824904A4 (en) * | 2018-07-19 | 2021-08-25 | Celltrion, Inc. | STABLE LIQUID PHARMACEUTICAL PREPARATION |
| AU2019362605A1 (en) * | 2018-10-19 | 2021-04-29 | F. Hoffmann-La Roche Ag | Microdosing |
| KR20210089215A (ko) | 2018-11-07 | 2021-07-15 | 머크 샤프 앤드 돔 코포레이션 | 항-lag3 항체 및 항-pd-1 항체의 공동-제제 |
| CA3118306A1 (en) * | 2018-11-21 | 2020-05-28 | Regeneron Pharmaceuticals, Inc. | High concentration protein formulation |
| US11058770B1 (en) * | 2019-01-24 | 2021-07-13 | United States Of America As Represented By The Secretary Of The Air Force | Ultrastable antibody ionic liquids |
| KR102735988B1 (ko) | 2019-02-18 | 2024-12-03 | 일라이 릴리 앤드 캄파니 | 치료 항체 제제 |
| TW202106711A (zh) * | 2019-04-24 | 2021-02-16 | 美商健生生物科技公司 | 抗體調配物 |
| EP3771463A1 (en) | 2019-07-29 | 2021-02-03 | Ludwig-Maximilians-Universität München | Stabilizing therapeutic proteins with piperazin- or morpholine-containing zwitterionic buffering substances |
| PH12022550238A1 (en) * | 2019-09-17 | 2023-01-04 | Merck Patent Gmbh | Camphorsulfonic acid and combinations thereof with cationic excipients as viscosity reducing agents in high concentrated protein formulations |
| US11684594B2 (en) | 2020-05-12 | 2023-06-27 | President And Fellows Of Harvard College | Antifungal prophylaxis for cornea |
| JP2023533704A (ja) * | 2020-07-13 | 2023-08-04 | メルク パテント ゲゼルシャフト ミット ベシュレンクテル ハフツング | 高濃度タンパク質製剤用の粘度低減賦形剤及びそれらの組み合わせ |
| JP2023539699A (ja) * | 2020-09-01 | 2023-09-15 | アイ2オー セラピューティクス,インコーポレーテッド | 糖尿病を治療するためのイオン性液体製剤 |
| EP4294368A4 (en) | 2021-02-19 | 2024-11-06 | Nova Thin Film Pharmaceuticals LLC | METHOD AND SYSTEM FOR PRODUCING ORAL SOLUBLE FILMS, ORAL SOLUBLE FILMS COMPOSITIONS, ORAL SOLUBLE FILMS PRODUCED THEREWITH AND METHODS OF USE THEREOF |
| CN113249358B (zh) * | 2021-05-14 | 2022-10-14 | 天津大学 | 一种利用离子液体调控溶菌酶晶型的方法 |
| US20240277840A1 (en) * | 2021-07-01 | 2024-08-22 | Upkara, Inc. | Superconcentrated formulations of bioactive agents |
| US20240350366A1 (en) * | 2021-08-25 | 2024-10-24 | Guido Knobel | Device for producing a pharmaceutical, dietary supplement or cosmetic |
| IL312672A (en) | 2021-11-10 | 2024-07-01 | I2O Therapeutics Inc | Ionic liquid compositions |
| CN115350330B (zh) * | 2022-09-01 | 2023-10-20 | 北京化工大学 | 一种负电性小分子调控的表面在蛋白差异性黏附上的应用 |
| KR20250152101A (ko) | 2023-03-13 | 2025-10-22 | 하이델베르크 파마 리서치 게엠베하 | 암 치료에의 사용을 위한 피하 투여 항체-약물 접합체 |
Family Cites Families (183)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US190082A (en) | 1877-04-24 | Improvement in apparatus for cooling liquids | ||
| GB750373A (en) | 1953-01-15 | 1956-06-13 | Pfizer & Co C | Improvements in or relating to injectable suspension of penicillin salts |
| DK128416B (da) | 1964-08-13 | 1974-04-29 | Ciba Geigy Ag | Analogifremgangsmåde til fremstilling af adrenocorticotropt aktive nonadecapeptidamider eller syreadditionssalte eller kompleksforbindelser deraf. |
| BE757195A (fr) | 1969-10-07 | 1971-03-16 | Century Disposable Devices | Seringue servant a injecter un melange fraichement prepare de poudre etde liquide |
| JPS5270014A (en) | 1975-12-05 | 1977-06-10 | Senju Pharma Co | Stabilization of pyrido*3*22a*phenoxadine compounds |
| US4171698A (en) | 1977-08-15 | 1979-10-23 | Abbott Laboratories | Prefilled two-compartment syringe |
| US4816567A (en) | 1983-04-08 | 1989-03-28 | Genentech, Inc. | Recombinant immunoglobin preparations |
| US5168062A (en) | 1985-01-30 | 1992-12-01 | University Of Iowa Research Foundation | Transfer vectors and microorganisms containing human cytomegalovirus immediate-early promoter-regulatory DNA sequence |
| JP2528107B2 (ja) | 1985-03-15 | 1996-08-28 | サマ−トン,ジエ−ムス | ポリヌクレオチド測定試薬と方法 |
| US5217866A (en) | 1985-03-15 | 1993-06-08 | Anti-Gene Development Group | Polynucleotide assay reagent and method |
| US5034506A (en) | 1985-03-15 | 1991-07-23 | Anti-Gene Development Group | Uncharged morpholino-based polymers having achiral intersubunit linkages |
| US5166315A (en) | 1989-12-20 | 1992-11-24 | Anti-Gene Development Group | Sequence-specific binding polymers for duplex nucleic acids |
| US5521063A (en) | 1985-03-15 | 1996-05-28 | Antivirals Inc. | Polynucleotide reagent containing chiral subunits and methods of use |
| US5506337A (en) | 1985-03-15 | 1996-04-09 | Antivirals Inc. | Morpholino-subunit combinatorial library and method |
| AU4128089A (en) | 1988-09-15 | 1990-03-22 | Rorer International (Overseas) Inc. | Monoclonal antibodies specific to human epidermal growth factor receptor and therapeutic methods employing same |
| US5001000A (en) | 1988-09-26 | 1991-03-19 | E. I. Du Pont De Nemours And Company | Process for forming a composite structure of thermoplastic polymer and sheet molding compound |
| US5114703A (en) | 1989-05-30 | 1992-05-19 | Alliance Pharmaceutical Corp. | Percutaneous lymphography using particulate fluorocarbon emulsions |
| US5730978A (en) | 1989-09-01 | 1998-03-24 | Fred Hutchinson Cancer Research Center | Inhibition of lymphocyte adherence with α4β1-specific antibodies |
| US6033665A (en) | 1989-09-27 | 2000-03-07 | Elan Pharmaceuticals, Inc. | Compositions and methods for modulating leukocyte adhesion to brain endothelial cells |
| JPH03190823A (ja) | 1989-12-21 | 1991-08-20 | Snow Brand Milk Prod Co Ltd | エリスロポエチン皮下又は筋肉投与剤 |
| US5698195A (en) | 1991-03-18 | 1997-12-16 | New York University Medical Center | Methods of treating rheumatoid arthritis using chimeric anti-TNF antibodies |
| US5714331A (en) | 1991-05-24 | 1998-02-03 | Buchardt, Deceased; Ole | Peptide nucleic acids having enhanced binding affinity, sequence specificity and solubility |
| US5539082A (en) | 1993-04-26 | 1996-07-23 | Nielsen; Peter E. | Peptide nucleic acids |
| CA2103059C (en) | 1991-06-14 | 2005-03-22 | Paul J. Carter | Method for making humanized antibodies |
| WO1994004679A1 (en) | 1991-06-14 | 1994-03-03 | Genentech, Inc. | Method for making humanized antibodies |
| US5436150A (en) | 1992-04-03 | 1995-07-25 | The Johns Hopkins University | Functional domains in flavobacterium okeanokoities (foki) restriction endonuclease |
| US5356802A (en) | 1992-04-03 | 1994-10-18 | The Johns Hopkins University | Functional domains in flavobacterium okeanokoites (FokI) restriction endonuclease |
| US5487994A (en) | 1992-04-03 | 1996-01-30 | The Johns Hopkins University | Insertion and deletion mutants of FokI restriction endonuclease |
| US5736137A (en) | 1992-11-13 | 1998-04-07 | Idec Pharmaceuticals Corporation | Therapeutic application of chimeric and radiolabeled antibodies to human B lymphocyte restricted differentiation antigen for treatment of B cell lymphoma |
| NZ261259A (en) | 1993-01-12 | 1996-12-20 | Biogen Inc | Humanised recombinant anti-vla4 antibody and diagnostic compositions and medicaments |
| US5334162A (en) | 1993-03-15 | 1994-08-02 | Eli Lilly And Company | Cartridge assembly for a lyophilized compound forming a disposable portion of an injector pen and method for same |
| US5744155A (en) | 1993-08-13 | 1998-04-28 | Friedman; Doron | Bioadhesive emulsion preparations for enhanced drug delivery |
| US5527675A (en) | 1993-08-20 | 1996-06-18 | Millipore Corporation | Method for degradation and sequencing of polymers which sequentially eliminate terminal residues |
| DE4331012A1 (de) | 1993-09-13 | 1995-03-16 | Bayer Ag | Nukleinsäuren-bindende Oligomere mit N-Verzweigung für Therapie und Diagnostik |
| DE4344824C1 (de) | 1993-12-28 | 1995-08-31 | Immuno Ag | Hochkonzentriertes Immunglobulin-Präparat und Verfahren zu seiner Herstellung |
| US5840299A (en) | 1994-01-25 | 1998-11-24 | Athena Neurosciences, Inc. | Humanized antibodies against leukocyte adhesion molecule VLA-4 |
| GB9418092D0 (en) | 1994-09-08 | 1994-10-26 | Red Cross Found Cent Lab Blood | Organic compounds |
| JP3524974B2 (ja) | 1994-12-26 | 2004-05-10 | ブラッコ インターナショナル ビーヴィ | プレフィルドシリンジおよびその包装体 |
| US5569193A (en) | 1995-03-22 | 1996-10-29 | Abbott Laboratories | Syringe system accommodating separately storable prefilled containers for two constituents |
| US5779668A (en) | 1995-03-29 | 1998-07-14 | Abbott Laboratories | Syringe barrel for lyophilization, reconstitution and administration |
| US5641870A (en) | 1995-04-20 | 1997-06-24 | Genentech, Inc. | Low pH hydrophobic interaction chromatography for antibody purification |
| US6267958B1 (en) | 1995-07-27 | 2001-07-31 | Genentech, Inc. | Protein formulation |
| US6685940B2 (en) | 1995-07-27 | 2004-02-03 | Genentech, Inc. | Protein formulation |
| DE19617369A1 (de) | 1996-04-30 | 1997-11-06 | Immuno Ag | Lagerstabile Fibrinogen-Präparate |
| US6184037B1 (en) | 1996-05-17 | 2001-02-06 | Genemedicine, Inc. | Chitosan related compositions and methods for delivery of nucleic acids and oligonucleotides into a cell |
| GB9610992D0 (en) | 1996-05-24 | 1996-07-31 | Glaxo Group Ltd | Concentrated antibody preparation |
| US5819998A (en) | 1996-09-30 | 1998-10-13 | Everything Kids | Knapsack with simulated basketball court |
| IN184589B (ja) | 1996-10-16 | 2000-09-09 | Alza Corp | |
| US6065645A (en) | 1997-04-01 | 2000-05-23 | Discus Dental Impressions, Inc. | Double-barreled syringe with detachable locking mixing tip |
| US5819988A (en) | 1997-04-01 | 1998-10-13 | Sawhney; Ravi K. | Double-barreled syringe with detachable locking mixing tip |
| US6235883B1 (en) | 1997-05-05 | 2001-05-22 | Abgenix, Inc. | Human monoclonal antibodies to epidermal growth factor receptor |
| US5786571A (en) | 1997-05-09 | 1998-07-28 | Lexmark International, Inc. | Wrapped temperature sensing assembly |
| US6171586B1 (en) | 1997-06-13 | 2001-01-09 | Genentech, Inc. | Antibody formulation |
| EP1029548B1 (en) * | 1997-10-15 | 2007-04-04 | Asahi Kasei Pharma Corporation | Method for keeping the quality of aqueous parenteral solution of thrombomodulin in storage and distribution |
| EP1043586B1 (en) | 1997-10-22 | 2007-03-21 | Chugai Seiyaku Kabushiki Kaisha | METHOD OF SCREENING TGF-beta INHIBITORY SUBSTANCES |
| US7919109B2 (en) | 1999-02-08 | 2011-04-05 | Intarcia Therapeutics, Inc. | Stable non-aqueous single phase viscous vehicles and formulations utilizing such vehicles |
| FR2794649B1 (fr) | 1999-06-11 | 2003-04-11 | Solutions | Biomateriau a base d'un derive de dextrane insolubilise et d'un facteur de croissance, son procede de preparation et ses applications |
| AUPQ132599A0 (en) | 1999-07-01 | 1999-07-22 | Commonwealth Scientific And Industrial Research Organisation | Nasogastric enteral formulations |
| US7030097B1 (en) | 1999-07-14 | 2006-04-18 | Cornell Research Foundation, Inc. | Controlled nucleic acid delivery systems |
| EP1072323B1 (de) | 1999-07-29 | 2003-09-10 | Wilhelm A. Keller | Kartuschen-Austraggerät mit Antrieb für dynamischen Mischer |
| ATE464062T1 (de) | 1999-10-04 | 2010-04-15 | Novartis Vaccines & Diagnostic | Stabilisierte flüssige pharmazeutische zusammensetzung enthaltend tfpi |
| US6394314B1 (en) | 1999-10-12 | 2002-05-28 | Discus Dental Impressions, Inc. | Double-barreled syringe with detachable locking mixing tip |
| US6443612B1 (en) | 1999-12-02 | 2002-09-03 | Wilhelm A. Keller | Dynamic mixer |
| ES2332402T5 (es) | 2000-10-12 | 2018-05-14 | Genentech, Inc. | Formulaciones de proteína concentradas de viscosidad reducida |
| US8703126B2 (en) | 2000-10-12 | 2014-04-22 | Genentech, Inc. | Reduced-viscosity concentrated protein formulations |
| US7931614B2 (en) | 2000-11-30 | 2011-04-26 | Valeritas, Inc. | Injection systems |
| US20040259247A1 (en) | 2000-12-01 | 2004-12-23 | Thomas Tuschl | Rna interference mediating small rna molecules |
| CA2446127A1 (en) | 2001-05-16 | 2002-11-21 | Biomarin Pharmaceutical Inc. | Destruction of prions using vibriolysin or variants thereof |
| GB0113179D0 (en) | 2001-05-31 | 2001-07-25 | Novartis Ag | Organic compounds |
| US7758890B2 (en) | 2001-06-23 | 2010-07-20 | Lyotropic Therapeutics, Inc. | Treatment using dantrolene |
| JP4317010B2 (ja) | 2001-07-25 | 2009-08-19 | ピーディーエル バイオファーマ,インコーポレイティド | IgG抗体の安定な凍結乾燥医薬製剤 |
| CA2454079A1 (en) | 2001-08-02 | 2003-02-13 | 3M Innovative Properties Company | Glass-ceramics |
| JP4167820B2 (ja) | 2001-10-19 | 2008-10-22 | アルインコ株式会社 | デッキプレート走行台車 |
| GB0212405D0 (en) * | 2002-05-29 | 2002-07-10 | Insignion Holdings Ltd | Composition and its therapeutic use |
| IL166418A0 (en) * | 2002-07-31 | 2006-01-15 | Alza Corp | Injectable depot compositions and uses thereof |
| US9415102B2 (en) | 2002-09-06 | 2016-08-16 | Alexion Pharmaceuticals, Inc. | High concentration formulations of anti-C5 antibodies |
| US20040191243A1 (en) | 2002-12-13 | 2004-09-30 | Bei Chen | System and method for stabilizing antibodies with histidine |
| ES2564103T3 (es) | 2003-03-05 | 2016-03-17 | Halozyme, Inc. | Glicoproteína hialuronidasa soluble (sHASEGP), proceso para prepararla, usos y composiciones farmacéuticas que la comprenden |
| JP2006522133A (ja) | 2003-03-31 | 2006-09-28 | アルザ・コーポレーション | 非水性単一相の媒体、及び、そのような媒体を利用するフォーミュレーション |
| HRP20050934B1 (hr) | 2003-04-04 | 2014-09-26 | Genentech, Inc. | Formulacije s visokom koncentracijom antitijela i proteina |
| US20050158303A1 (en) | 2003-04-04 | 2005-07-21 | Genentech, Inc. | Methods of treating IgE-mediated disorders comprising the administration of high concentration anti-IgE antibody formulations |
| WO2005030119A2 (en) | 2003-04-11 | 2005-04-07 | Allergan, Inc. | Botulinum toxin a peptides and methods of predicting and reducing immunoresistance to botulinum toxin therapy |
| EP1616004A1 (en) * | 2003-04-15 | 2006-01-18 | Ista Pharmaceuticals, Inc | Process for isolating and purifing ovine hyaluronidase |
| US20040247672A1 (en) | 2003-05-16 | 2004-12-09 | Alkermes Controlled Therapeutics, Inc. | Injectable sustained release compositions |
| US20070184084A1 (en) | 2003-05-30 | 2007-08-09 | Guohua Chen | Implantable elastomeric caprolactone depot compositions and uses thereof |
| JP5275566B2 (ja) | 2003-06-18 | 2013-08-28 | ザ スクリプス リサーチ インスティチュート | 反応性非天然アミノ酸遺伝コード付加 |
| MXPA05014171A (es) | 2003-06-23 | 2007-02-21 | Sanofi Pasteur Inc | Metodo de inmunizacion contra neisseria meningitidis serogrupos a y c. |
| CN1206344C (zh) | 2003-07-28 | 2005-06-15 | 中国海洋大学 | 一种用细菌生产岩藻聚糖硫酸酯酶的方法 |
| GB0327723D0 (en) | 2003-09-15 | 2003-12-31 | Vectura Ltd | Pharmaceutical compositions |
| WO2005094785A2 (en) | 2003-09-17 | 2005-10-13 | Chiasma, Ltd. | Compositions capable of facilitating penetration across a biological barrier |
| EP1532983A1 (en) | 2003-11-18 | 2005-05-25 | ZLB Bioplasma AG | Immunoglobulin preparations having increased stability |
| GB0403938D0 (en) | 2004-02-21 | 2004-03-24 | West Pharm Serv Drug Res Ltd | Chitosan containing solution |
| JP5160887B2 (ja) | 2004-06-21 | 2013-03-13 | メダレックス インコーポレイテッド | インターフェロンアルファレセプター1抗体及びその使用法 |
| ATE469191T1 (de) * | 2004-11-25 | 2010-06-15 | Chr Hansen As | Verfahren zur herstellung eines karminsaürelackes |
| US20060142234A1 (en) | 2004-12-23 | 2006-06-29 | Guohua Chen | Injectable non-aqueous suspension |
| US20060141040A1 (en) | 2004-12-23 | 2006-06-29 | Guohua Chen | Injectable non-aqueous suspension |
| JP2008528638A (ja) * | 2005-01-28 | 2008-07-31 | ワイス | ポリペプチドの安定化液体処方 |
| JP5713534B2 (ja) | 2005-10-07 | 2015-05-07 | ザ ユニバーシティ オブ アラバマ | 多機能イオン性液体組成物 |
| CN100416002C (zh) | 2005-10-26 | 2008-09-03 | 曲新华 | 污水再生利用装置 |
| KR101378194B1 (ko) | 2005-12-20 | 2014-03-27 | 브리스톨-마이어스 스큅 컴퍼니 | 안정한 단백질 제제 |
| US9309316B2 (en) | 2005-12-20 | 2016-04-12 | Bristol-Myers Squibb Company | Stable subcutaneous protein formulations and uses thereof |
| WO2007076062A2 (en) * | 2005-12-21 | 2007-07-05 | Wyeth | Protein formulations with reduced viscosity and uses thereof |
| US9084777B2 (en) | 2005-12-28 | 2015-07-21 | Chugai Seiyaku Kabushiki Kaisha | Stabilized antibody-containing formulations |
| CN1823768A (zh) | 2006-01-09 | 2006-08-30 | 徐新盛 | 西咪替丁冻干组合物 |
| WO2007092772A2 (en) | 2006-02-03 | 2007-08-16 | Medimmune, Inc. | Protein formulations |
| DE102006005094A1 (de) | 2006-02-04 | 2007-08-09 | Degussa Gmbh | Titandioxid und Polycarboxylatether enthaltende Dispersion |
| ITMI20061030A1 (it) | 2006-05-26 | 2007-11-27 | Altergon Sa | Nuova composizione comprendente glicosamminoglicani a viscosita' controllata e uso di tale composizione nella terapuia della cistite cronica |
| CU23526B6 (es) * | 2006-10-03 | 2010-05-19 | Ct Ingenieria Genetica Biotech | Método para la restauración morfofuncional de nervios periféricos en la neuropatía diabética |
| WO2008092084A2 (en) | 2007-01-26 | 2008-07-31 | Centocor, Inc. | Injectable non-aqueous suspension with high concentration of therapeutic agent |
| CA2688415C (en) | 2007-05-31 | 2015-11-10 | Anterios, Inc. | Nucleic acid nanoparticles and uses therefor |
| KR101502305B1 (ko) | 2007-07-20 | 2015-03-13 | 어플라이드 머티어리얼스, 인코포레이티드 | 플라즈마 처리 장치 내의 rf 구동된 전극으로 가스를 전달하기 위한 rf 초크 |
| WO2009015367A2 (en) | 2007-07-25 | 2009-01-29 | Arizona Board Of Regents For And On Behalf Of Arizona State University | Stabilizing proteins using ionic liquids |
| CA2696049A1 (en) | 2007-08-17 | 2009-02-26 | Amgen Inc. | Formulations of antibodies and fc-fusion molecules using polycations |
| ES2750254T3 (es) | 2007-09-27 | 2020-03-25 | Amgen Inc | Formulaciones farmacéuticas |
| EP2586794A3 (en) | 2007-10-16 | 2013-07-17 | Pharmacyclics, Inc. | Manufacture, Compositions and Uses of Coagulationfactor VIIA Modulator |
| EP3381445B1 (en) | 2007-11-15 | 2023-10-25 | Amgen Inc. | Aqueous formulation of antibody stablised by antioxidants for parenteral administration |
| TWI543768B (zh) * | 2007-11-30 | 2016-08-01 | 艾伯維生物技術有限責任公司 | 蛋白質調配物及製造其之方法 |
| CA2710418A1 (en) | 2007-12-21 | 2009-07-09 | Lyotropic Therapeutics, Inc. | Stabilized formulations of peptides and proteins |
| PE20091174A1 (es) | 2007-12-27 | 2009-08-03 | Chugai Pharmaceutical Co Ltd | Formulacion liquida con contenido de alta concentracion de anticuerpo |
| US20120251615A1 (en) * | 2008-01-18 | 2012-10-04 | Horst Kief | Agent for intra-articular injection |
| WO2009120684A1 (en) * | 2008-03-25 | 2009-10-01 | Medimmune, Llc | Antibody formulation |
| WO2009137471A2 (en) | 2008-05-05 | 2009-11-12 | University Of Miami | Azo dye related small molecule modulators of protein-protein interactions |
| EP2123307A1 (en) | 2008-05-20 | 2009-11-25 | Hexal Ag | Method for reducing leachables and extractables in syringes |
| EP2376522A4 (en) | 2008-11-16 | 2013-12-25 | Univ Texas | HIGHLY CONCENTRATED LOW VISCOSITY SUSPENSIONS |
| TWI342781B (en) | 2008-12-01 | 2011-06-01 | Univ China Medical | Blood sugar-modulating polypeptides |
| SG174258A1 (en) | 2009-03-06 | 2011-10-28 | Genentech Inc | Antibody formulation |
| WO2010129469A1 (en) * | 2009-05-04 | 2010-11-11 | Abbott Biotechnology Ltd. | Stable high protein concentration formulations of human anti-tnf-alpha-antibodies |
| WO2010132047A1 (en) | 2009-05-14 | 2010-11-18 | Rensselaer Polytechnic Institute | Guanosine/gmp gels and uses thereof |
| WO2010134345A1 (ja) | 2009-05-20 | 2010-11-25 | 積水メディカル株式会社 | 血液凝固時間延長剤 |
| EP2475376B1 (en) | 2009-06-17 | 2016-03-30 | BioMarin Pharmaceutical Inc. | Formulations for lysosomal enzymes |
| US20120121580A1 (en) * | 2009-07-28 | 2012-05-17 | Merck Sharp & Dohme Corp. | Methods for producing high concentration lyophilized pharmaceutical formulations |
| US9345661B2 (en) * | 2009-07-31 | 2016-05-24 | Genentech, Inc. | Subcutaneous anti-HER2 antibody formulations and uses thereof |
| SG178226A1 (en) | 2009-08-04 | 2012-03-29 | Genentech Inc | Concentrated polypeptide formulations with reduced viscosity |
| AR078161A1 (es) * | 2009-09-11 | 2011-10-19 | Hoffmann La Roche | Formulaciones farmaceuticas muy concentradas de un anticuerpo anti cd20. uso de la formulacion. metodo de tratamiento. |
| CA2774053C (en) | 2009-09-17 | 2015-04-28 | Baxter Healthcare, S.A. | Stable co-formulation of hyaluronidase and immunoglobulin, and methods of use thereof |
| EP2491123B1 (en) | 2009-10-20 | 2018-04-18 | The Regents of The University of California | Single molecule nucleic acid nanoparticles |
| UY32979A (es) | 2009-10-28 | 2011-02-28 | Abbott Lab | Inmunoglobulinas con dominio variable dual y usos de las mismas |
| JP5896471B2 (ja) | 2009-11-20 | 2016-03-30 | バイオコン リミテッドBiocon Limited | 抗体製剤 |
| WO2011069037A2 (en) * | 2009-12-03 | 2011-06-09 | The University Of North Carolina At Charlotte | Stabilization and storage of biological pharmaceutical compositions |
| CN106834320B (zh) | 2009-12-10 | 2021-05-25 | 明尼苏达大学董事会 | Tal效应子介导的dna修饰 |
| ES2522935T3 (es) | 2010-01-08 | 2014-11-19 | Chanel Parfums Beauté | Uso de al menos un extracto de flores de Camellia japonica alba plena para hidratar la piel |
| DK2531218T3 (en) | 2010-02-04 | 2019-04-01 | Csl Behring Ag | immunoglobulin |
| EP2538973A2 (en) | 2010-02-26 | 2013-01-02 | Novo Nordisk A/S | Stable antibody containing compositions |
| EP2542221A4 (en) | 2010-03-01 | 2013-10-23 | Cytodyn Inc | CONCENTRATED PROTEIN FORMULATIONS AND USES THEREOF |
| US20130171128A1 (en) | 2010-03-02 | 2013-07-04 | Amgen Inc. | Reducing viscosity of pharmaceutical formulations |
| US20110223208A1 (en) * | 2010-03-09 | 2011-09-15 | Beth Hill | Non-Aqueous High Concentration Reduced Viscosity Suspension Formulations |
| US9072668B2 (en) | 2010-03-09 | 2015-07-07 | Janssen Biotech, Inc. | Non-aqueous high concentration reduced viscosity suspension formulations of antibodies |
| RU2012144017A (ru) * | 2010-03-17 | 2014-04-27 | Эбботт Рисерч Б.В. | Композиции антител против фактора роста нервов (ngf) |
| WO2011121560A2 (en) | 2010-03-31 | 2011-10-06 | Universite De Geneve | Stabilized antibody preparations and uses thereof |
| CN101851267A (zh) | 2010-04-22 | 2010-10-06 | 江南大学 | 一种抗体保护剂及其应用 |
| RU2012151500A (ru) * | 2010-05-03 | 2014-06-10 | Дженентек, Инк. | Композиции и способы, пригодные для снижения вязкости белковосодержащих составов |
| SMT202000095T1 (it) * | 2010-05-14 | 2020-03-13 | Amgen Inc | Formulazioni di anticorpi anti-sclerostina ad alta concentrazione |
| GB201012179D0 (en) | 2010-07-20 | 2010-09-01 | Future Injection Technologies | Injection device |
| JP5919606B2 (ja) * | 2010-11-11 | 2016-05-18 | アッヴィ バイオテクノロジー リミテッド | 改良型高濃度抗tnfアルファ抗体液体製剤 |
| US8936827B2 (en) | 2011-02-25 | 2015-01-20 | Abbott Cardiovascular Systems Inc. | Methods of loading a hollow stent with a drug or drug formulation |
| EP3058952A1 (en) | 2011-04-07 | 2016-08-24 | Glaxosmithkline LLC | Formulations with reduced viscosity |
| US20140044727A1 (en) | 2011-04-07 | 2014-02-13 | Glaxosmithkline Llc | Formulations with reduced viscosity |
| CN103619368B (zh) | 2011-05-12 | 2017-02-08 | 拜耳医药保健有限公司 | 具有用于控制注射程序的不同系统的流体注射系统 |
| RU2485133C2 (ru) * | 2011-05-30 | 2013-06-20 | Закрытое Акционерное Общество "Фарм-Синтез" | Белково-полипептидный комплекс, обладающий тканеспецифическим регенеративно-репаративным и омолаживающим действием на кожную ткань, способ его получения и фармацевтическая композиция на его основе |
| CN102349895B (zh) | 2011-09-28 | 2013-04-10 | 海南良方医药有限公司 | 一种注射用西咪替丁组合物及其制备方法 |
| WO2013063277A1 (en) | 2011-10-25 | 2013-05-02 | Corning Incorporated | Delamination resistant pharmaceutical glass containers containing active pharmaceutical ingredients |
| CA2853823C (en) | 2011-10-28 | 2016-12-20 | Integritybio Inc. | Protein formulations containing amino acids |
| WO2013096835A1 (en) * | 2011-12-23 | 2013-06-27 | Abbvie Inc. | Stable protein formulations |
| WO2013096791A1 (en) | 2011-12-23 | 2013-06-27 | Genentech, Inc. | Process for making high concentration protein formulations |
| AU2013211824B2 (en) | 2012-01-27 | 2017-06-01 | Gliknik Inc. | Fusion proteins comprising IgG2 hinge domains |
| WO2013123114A2 (en) * | 2012-02-16 | 2013-08-22 | Santarus, Inc. | Antibody formulations |
| US9574193B2 (en) | 2012-05-17 | 2017-02-21 | Ionis Pharmaceuticals, Inc. | Methods and compositions for modulating apolipoprotein (a) expression |
| WO2013173789A2 (en) | 2012-05-17 | 2013-11-21 | Isis Pharmaceuticals, Inc. | Antisense oligonucleotide compositions |
| CA2873646C (en) | 2012-05-18 | 2022-04-26 | Genentech, Inc. | High-concentration monoclonal antibody formulations |
| RS59199B1 (sr) | 2012-05-25 | 2019-10-31 | Univ California | Metode i jedinjenja za rnk-upravljanu ciljanu dnk modifikaciju i za rnk- upravljanu modulaciju transkripta |
| AU2013265255B2 (en) | 2012-05-25 | 2018-03-29 | Novartis Ag | Aqueous pharmaceutical composition containing a biologic therapeutic agent and guanidine or a guanidine derivative and an injection including the composition |
| EA201590061A1 (ru) | 2012-06-21 | 2015-05-29 | Юсб Фарма С.А. | Фармацевтическая композиция |
| EP3494997B1 (en) | 2012-07-25 | 2019-09-18 | The Broad Institute, Inc. | Inducible dna binding proteins and genome perturbation tools and applications thereof |
| CN104704001B (zh) | 2012-08-09 | 2019-02-12 | 罗切格利卡特公司 | Asgpr抗体及其用途 |
| FR2994390B1 (fr) | 2012-08-10 | 2014-08-15 | Adocia | Procede d'abaissement de la viscosite de solutions de proteines a concentration elevee |
| US9592297B2 (en) | 2012-08-31 | 2017-03-14 | Bayer Healthcare Llc | Antibody and protein formulations |
| FR2995214B1 (fr) | 2012-09-10 | 2014-11-21 | Adocia | Solution a viscosite reduite de proteine a concentration elevee |
| WO2014160083A1 (en) | 2013-03-13 | 2014-10-02 | Applied Cardiovascular Solutions, Llc. | Methods, compositions, and devices for the occlusion of cavities and passageways |
| US9669242B2 (en) | 2013-07-01 | 2017-06-06 | L'oreal | Compositions containing at least two phenolic compounds, a lipid-soluble antioxidant and at least one hydrotrope for cosmetic use |
| IL312865B2 (en) * | 2013-09-11 | 2025-06-01 | Eagle Biologics Inc | Liquid protein formulations containing viscosity-lowering agents |
| US20160074515A1 (en) | 2014-06-20 | 2016-03-17 | Reform Biologics, Llc | Viscosity-reducing excipient compounds for protein formulations |
| CA2962768C (en) * | 2014-10-01 | 2023-10-10 | Alyssa M. Larson | Polysaccharide and nucleic acid formulations containing viscosity-lowering agents |
| US10812184B1 (en) | 2019-04-03 | 2020-10-20 | Board Of Trustees Of The University Of Alabama, For And On Behalf Of The University Of Alabama In Huntsville | Signal analysis systems and methods |
-
2014
- 2014-09-10 IL IL312865A patent/IL312865B2/en unknown
- 2014-09-11 JP JP2016542116A patent/JP6515105B2/ja active Active
- 2014-09-11 CN CN201480061378.0A patent/CN105848636B/zh active Active
- 2014-09-11 EP EP14781338.0A patent/EP3043772B1/en active Active
- 2014-09-11 CN CN201480061505.7A patent/CN105722501B/zh active Active
- 2014-09-11 EP EP23183441.7A patent/EP4272763A3/en active Pending
- 2014-09-11 CN CN201480061377.6A patent/CN105705139B/zh active Active
- 2014-09-11 CA CA2923843A patent/CA2923843C/en active Active
- 2014-09-11 ES ES14781338T patent/ES2959451T3/es active Active
- 2014-09-11 WO PCT/US2014/055245 patent/WO2015038811A2/en not_active Ceased
- 2014-09-11 AU AU2014318637A patent/AU2014318637B2/en active Active
- 2014-09-11 AU AU2014318725A patent/AU2014318725B2/en active Active
- 2014-09-11 JP JP2016542105A patent/JP6412575B2/ja active Active
- 2014-09-11 RU RU2016113283A patent/RU2710542C2/ru active
- 2014-09-11 RU RU2016113385A patent/RU2675824C2/ru active
- 2014-09-11 IL IL318339A patent/IL318339A/en unknown
- 2014-09-11 AU AU2014318691A patent/AU2014318691B2/en active Active
- 2014-09-11 EP EP14776954.1A patent/EP3043775B1/en active Active
- 2014-09-11 SI SI201431701T patent/SI3043774T1/sl unknown
- 2014-09-11 KR KR1020167009556A patent/KR102295633B1/ko active Active
- 2014-09-11 US US14/483,896 patent/US9913905B2/en active Active
- 2014-09-11 EP EP20205250.2A patent/EP3791862B1/en active Active
- 2014-09-11 MX MX2016003182A patent/MX382917B/es unknown
- 2014-09-11 SI SI201432065T patent/SI3043776T1/sl unknown
- 2014-09-11 SG SG10201913950XA patent/SG10201913950XA/en unknown
- 2014-09-11 US US14/484,053 patent/US9833513B2/en active Active
- 2014-09-11 MX MX2016003183A patent/MX386756B/es unknown
- 2014-09-11 ES ES20205250T patent/ES2980815T3/es active Active
- 2014-09-11 JP JP2016542118A patent/JP6463581B2/ja active Active
- 2014-09-11 EP EP24178356.2A patent/EP4403187A3/en active Pending
- 2014-09-11 SI SI201432039T patent/SI3043772T1/sl unknown
- 2014-09-11 SI SI201431702T patent/SI3043775T1/sl unknown
- 2014-09-11 WO PCT/US2014/055203 patent/WO2015038777A1/en not_active Ceased
- 2014-09-11 CN CN201910735461.6A patent/CN110559435B/zh active Active
- 2014-09-11 IL IL275388A patent/IL275388B2/en unknown
- 2014-09-11 EP EP20199033.0A patent/EP3808338A1/en active Pending
- 2014-09-11 JP JP2016542103A patent/JP6469113B2/ja active Active
- 2014-09-11 ES ES14776954T patent/ES2837629T3/es active Active
- 2014-09-11 US US14/483,880 patent/US10179172B2/en active Active
- 2014-09-11 US US14/484,160 patent/US9925263B2/en active Active
- 2014-09-11 ES ES14776953T patent/ES2841134T3/es active Active
- 2014-09-11 WO PCT/US2014/055254 patent/WO2015038818A2/en not_active Ceased
- 2014-09-11 KR KR1020217026965A patent/KR102435648B1/ko active Active
- 2014-09-11 CN CN202010059409.6A patent/CN111202711B/zh active Active
- 2014-09-11 KR KR1020167009551A patent/KR102289825B1/ko active Active
- 2014-09-11 KR KR1020227028657A patent/KR102651018B1/ko active Active
- 2014-09-11 HK HK16114708.6A patent/HK1226309A1/zh unknown
- 2014-09-11 CA CA2923859A patent/CA2923859C/en active Active
- 2014-09-11 CA CA2924069A patent/CA2924069C/en active Active
- 2014-09-11 AU AU2014318696A patent/AU2014318696B2/en active Active
- 2014-09-11 KR KR1020247009336A patent/KR102772684B1/ko active Active
- 2014-09-11 SG SG11201601728YA patent/SG11201601728YA/en unknown
- 2014-09-11 EP EP14781337.2A patent/EP3043776B1/en active Active
- 2014-09-11 ES ES14781337T patent/ES2972527T3/es active Active
- 2014-09-11 CN CN201910681927.9A patent/CN110496099B/zh active Active
- 2014-09-11 SG SG10201913952TA patent/SG10201913952TA/en unknown
- 2014-09-11 SG SG11201601730TA patent/SG11201601730TA/en unknown
- 2014-09-11 CN CN201480061504.2A patent/CN105722500B/zh active Active
- 2014-09-11 SG SG10201806539XA patent/SG10201806539XA/en unknown
- 2014-09-11 EP EP14776953.3A patent/EP3043774B1/en active Active
- 2014-09-11 CA CA2923844A patent/CA2923844C/en active Active
- 2014-09-11 WO PCT/US2014/055210 patent/WO2015038782A1/en not_active Ceased
- 2014-09-11 SG SG10201809401XA patent/SG10201809401XA/en unknown
-
2016
- 2016-03-03 IL IL244430A patent/IL244430B/en active IP Right Grant
- 2016-03-03 IL IL244433A patent/IL244433B/en active IP Right Grant
- 2016-03-10 MX MX2021012048A patent/MX2021012048A/es unknown
- 2016-03-10 MX MX2021012049A patent/MX2021012049A/es unknown
- 2016-03-10 MX MX2021005907A patent/MX2021005907A/es unknown
- 2016-03-10 MX MX2021012050A patent/MX2021012050A/es unknown
- 2016-03-22 ZA ZA2016/01968A patent/ZA201601968B/en unknown
-
2017
- 2017-11-10 US US15/809,602 patent/US10821183B2/en active Active
-
2018
- 2018-02-06 US US15/890,044 patent/US10821184B2/en active Active
- 2018-02-23 US US15/903,523 patent/US10646571B2/en active Active
- 2018-02-23 US US15/903,635 patent/US10849977B2/en active Active
- 2018-03-12 ZA ZA2018/01669A patent/ZA201801669B/en unknown
- 2018-04-03 ZA ZA2018/02108A patent/ZA201802108B/en unknown
- 2018-09-28 JP JP2018183794A patent/JP6564512B2/ja active Active
- 2018-12-29 JP JP2018248768A patent/JP6774484B2/ja active Active
-
2019
- 2019-02-25 JP JP2019031843A patent/JP6768858B2/ja active Active
- 2019-06-11 JP JP2019108536A patent/JP6941137B2/ja active Active
- 2019-07-26 JP JP2019137552A patent/JP6840797B2/ja active Active
-
2020
- 2020-03-30 AU AU2020202240A patent/AU2020202240B2/en active Active
- 2020-04-06 AU AU2020202408A patent/AU2020202408B2/en active Active
- 2020-04-06 AU AU2020202407A patent/AU2020202407B2/en active Active
- 2020-10-02 JP JP2020167521A patent/JP7214697B2/ja active Active
- 2020-10-09 US US17/067,058 patent/US11819550B2/en active Active
- 2020-10-19 US US17/073,618 patent/US11986526B2/en active Active
- 2020-11-23 US US17/101,063 patent/US20210085792A1/en not_active Abandoned
-
2021
- 2021-04-30 ZA ZA2021/02906A patent/ZA202102906B/en unknown
-
2022
- 2022-12-15 JP JP2022200135A patent/JP7583455B2/ja active Active
-
2023
- 2023-02-17 AU AU2023200941A patent/AU2023200941A1/en active Pending
- 2023-03-15 ZA ZA2023/03593A patent/ZA202303593B/en unknown
- 2023-09-16 AU AU2023229619A patent/AU2023229619A1/en active Pending
- 2023-10-20 AU AU2023251546A patent/AU2023251546A1/en active Pending
-
2024
- 2024-02-09 US US18/438,199 patent/US20240181063A1/en active Pending
- 2024-04-15 US US18/635,538 patent/US20240285766A1/en active Pending
- 2024-10-25 JP JP2024188410A patent/JP2025010224A/ja active Pending
Also Published As
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP6564512B2 (ja) | 有機ホスフェートを含有する液状タンパク質製剤 | |
| HK40101422A (en) | Liquid protein formulations containing organophosphates | |
| HK40030539B (en) | Liquid protein formulations containing organophosphates | |
| HK40030539A (en) | Liquid protein formulations containing organophosphates | |
| HK40018131A (en) | Liquid protein formulations containing ionic liquids | |
| HK1225979B (zh) | 包含有机磷酸酯的液体蛋白质制剂 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20180928 |
|
| TRDD | Decision of grant or rejection written | ||
| A01 | Written decision to grant a patent or to grant a registration (utility model) |
Free format text: JAPANESE INTERMEDIATE CODE: A01 Effective date: 20190626 |
|
| A61 | First payment of annual fees (during grant procedure) |
Free format text: JAPANESE INTERMEDIATE CODE: A61 Effective date: 20190726 |
|
| R150 | Certificate of patent or registration of utility model |
Ref document number: 6564512 Country of ref document: JP Free format text: JAPANESE INTERMEDIATE CODE: R150 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |