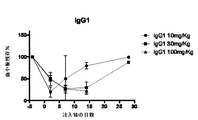
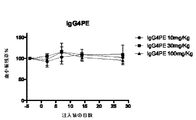
JP2016507555A - 血小板非減少性かつ赤血球非減少性cd47抗体及びその使用方法 - Google Patents
血小板非減少性かつ赤血球非減少性cd47抗体及びその使用方法 Download PDFInfo
- Publication number
- JP2016507555A JP2016507555A JP2015556930A JP2015556930A JP2016507555A JP 2016507555 A JP2016507555 A JP 2016507555A JP 2015556930 A JP2015556930 A JP 2015556930A JP 2015556930 A JP2015556930 A JP 2015556930A JP 2016507555 A JP2016507555 A JP 2016507555A
- Authority
- JP
- Japan
- Prior art keywords
- antibody
- seq
- antibodies
- human
- active fragment
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Pending
Links
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/18—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans
- C07K16/28—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants
- C07K16/2896—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants against molecules with a "CD"-designation, not provided for elsewhere
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K39/395—Antibodies; Immunoglobulins; Immune serum, e.g. antilymphocytic serum
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
- A61P35/02—Antineoplastic agents specific for leukemia
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P43/00—Drugs for specific purposes, not provided for in groups A61P1/00-A61P41/00
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K2039/505—Medicinal preparations containing antigens or antibodies comprising antibodies
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/20—Immunoglobulins specific features characterized by taxonomic origin
- C07K2317/24—Immunoglobulins specific features characterized by taxonomic origin containing regions, domains or residues from different species, e.g. chimeric, humanized or veneered
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/30—Immunoglobulins specific features characterized by aspects of specificity or valency
- C07K2317/34—Identification of a linear epitope shorter than 20 amino acid residues or of a conformational epitope defined by amino acid residues
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/70—Immunoglobulins specific features characterized by effect upon binding to a cell or to an antigen
- C07K2317/71—Decreased effector function due to an Fc-modification
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/70—Immunoglobulins specific features characterized by effect upon binding to a cell or to an antigen
- C07K2317/76—Antagonist effect on antigen, e.g. neutralization or inhibition of binding
Landscapes
- Health & Medical Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Medicinal Chemistry (AREA)
- Life Sciences & Earth Sciences (AREA)
- Organic Chemistry (AREA)
- General Health & Medical Sciences (AREA)
- Immunology (AREA)
- Pharmacology & Pharmacy (AREA)
- Animal Behavior & Ethology (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
- General Chemical & Material Sciences (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Engineering & Computer Science (AREA)
- Genetics & Genomics (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Molecular Biology (AREA)
- Biochemistry (AREA)
- Biophysics (AREA)
- Microbiology (AREA)
- Mycology (AREA)
- Epidemiology (AREA)
- Hematology (AREA)
- Oncology (AREA)
- Peptides Or Proteins (AREA)
- Medicines Containing Antibodies Or Antigens For Use As Internal Diagnostic Agents (AREA)
- Preparation Of Compounds By Using Micro-Organisms (AREA)
- Medicines That Contain Protein Lipid Enzymes And Other Medicines (AREA)
- Investigating Or Analysing Biological Materials (AREA)
Applications Claiming Priority (7)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| USPCT/US2013/024995 | 2013-02-06 | ||
| PCT/US2013/024995 WO2013119714A1 (en) | 2012-02-06 | 2013-02-06 | Cd47 antibodies and methods of use thereof |
| US13/761,087 | 2013-02-06 | ||
| US13/761,087 US9045541B2 (en) | 2012-02-06 | 2013-02-06 | CD47 antibodies and methods of use thereof |
| US201361815219P | 2013-04-23 | 2013-04-23 | |
| US61/815,219 | 2013-04-23 | ||
| PCT/US2013/053818 WO2014123580A1 (en) | 2013-02-06 | 2013-08-06 | Non-platelet depleting and non-red blood cell depleting cd47 antibodies and methods of use thereof |
Related Child Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2018146460A Division JP6726238B2 (ja) | 2013-02-06 | 2018-08-03 | 血小板非減少性かつ赤血球非減少性cd47抗体及びその使用方法 |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| JP2016507555A true JP2016507555A (ja) | 2016-03-10 |
| JP2016507555A5 JP2016507555A5 (enExample) | 2016-10-06 |
Family
ID=51300031
Family Applications (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2015556930A Pending JP2016507555A (ja) | 2013-02-06 | 2013-08-06 | 血小板非減少性かつ赤血球非減少性cd47抗体及びその使用方法 |
| JP2018146460A Active JP6726238B2 (ja) | 2013-02-06 | 2018-08-03 | 血小板非減少性かつ赤血球非減少性cd47抗体及びその使用方法 |
Family Applications After (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2018146460A Active JP6726238B2 (ja) | 2013-02-06 | 2018-08-03 | 血小板非減少性かつ赤血球非減少性cd47抗体及びその使用方法 |
Country Status (19)
| Country | Link |
|---|---|
| EP (2) | EP4137518A1 (enExample) |
| JP (2) | JP2016507555A (enExample) |
| KR (2) | KR102276974B1 (enExample) |
| CN (1) | CN105101997B (enExample) |
| AU (2) | AU2013377886B2 (enExample) |
| BR (1) | BR112015018851A2 (enExample) |
| CA (1) | CA2900468C (enExample) |
| CL (1) | CL2015002203A1 (enExample) |
| EA (1) | EA036963B1 (enExample) |
| ES (1) | ES2944477T3 (enExample) |
| IL (2) | IL240390B (enExample) |
| MX (2) | MX2015010115A (enExample) |
| NI (1) | NI201500103A (enExample) |
| NZ (1) | NZ710695A (enExample) |
| PE (1) | PE20151408A1 (enExample) |
| PH (1) | PH12015501729A1 (enExample) |
| SG (2) | SG10201706383XA (enExample) |
| WO (1) | WO2014123580A1 (enExample) |
| ZA (2) | ZA201505745B (enExample) |
Cited By (11)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2019515899A (ja) * | 2016-04-15 | 2019-06-13 | ザ ボード オブ トラスティーズ オブ ザ レランド スタンフォード ジュニア ユニバーシティー | がん処置における抗cd47薬の治療有効用量を決定及び達成する方法 |
| JP2020511481A (ja) * | 2017-03-22 | 2020-04-16 | アーチ オンコロジー, インコーポレイテッドArch Oncology, Inc. | 固形及び血液癌の治療のための併用療法 |
| JP2020536488A (ja) * | 2017-08-02 | 2020-12-17 | フェインズ セラピューティクス,インコーポレーテッド | 抗cd47抗体及びその使用 |
| JP2021502324A (ja) * | 2017-11-10 | 2021-01-28 | アイ−マブ バイオファーマ ユーエス リミテッド | Cd47抗体およびサイトカインを含む融合タンパク質 |
| JP2021527110A (ja) * | 2018-06-11 | 2021-10-11 | 康▲諾▼▲亞▼生物医葯科技(成都)有限公司Chengdu Conmed Biosciences Co., Ltd | CD47とSIRPaの相互作用を遮断できる抗体及びその応用 |
| JP2021535743A (ja) * | 2018-08-31 | 2021-12-23 | 南京聖和薬業股▲ふん▼有限公司Nanjing Sanhome Pharmaceutical Co., Ltd. | 抗cd47抗体及びその応用 |
| JP2023052145A (ja) * | 2017-03-27 | 2023-04-11 | セルジーン コーポレイション | 免疫原性の低下のための方法及び組成物 |
| JP2023083424A (ja) * | 2017-06-21 | 2023-06-15 | ザ ボード オブ トラスティーズ オブ ザ レランド スタンフォード ジュニア ユニバーシティー | 血液悪性腫瘍に対するcd47標的化治療のための投与パラメータ |
| US11692035B2 (en) | 2016-10-21 | 2023-07-04 | Arch Oncology, Inc. | Therapeutic CD47 antibodies |
| US12091467B2 (en) | 2016-10-20 | 2024-09-17 | I-Mab Biopharma Us Limited | CD47 monoclonal antibodies and uses thereof |
| JP7591917B2 (ja) | 2015-08-07 | 2024-11-29 | エーエルエックス オンコロジー インコーポレイテッド | Sirp-アルファドメインまたはそのバリアントを有する構築物 |
Families Citing this family (53)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN104271757B (zh) | 2012-02-06 | 2020-06-09 | 印希彼有限公司 | Cd47抗体及其使用方法 |
| US9221908B2 (en) | 2012-12-12 | 2015-12-29 | Vasculox, Inc. | Therapeutic CD47 antibodies |
| CN105102479B (zh) | 2012-12-12 | 2019-06-28 | 瓦斯库劳克斯有限公司 | 治疗性cd47抗体 |
| EP3575326B8 (en) | 2012-12-17 | 2022-05-25 | Trillium Therapeutics ULC | Treatment of cd47+ disease cells with sirp alpha-fc fusions |
| AU2014238105B2 (en) * | 2013-03-15 | 2018-11-15 | The Board Of Trustees Of The Leland Stanford Junior University | Methods for achieving therapeutically effective doses of anti-CD47 agents |
| EP3012271A1 (en) * | 2014-10-24 | 2016-04-27 | Effimune | Method and compositions for inducing differentiation of myeloid derived suppressor cell to treat cancer and infectious diseases |
| US10864268B2 (en) * | 2014-11-18 | 2020-12-15 | Janssen Pharmaceutica Nv | CD47 antibodies, methods, and uses |
| EA037654B1 (ru) * | 2014-12-30 | 2021-04-27 | Селджин Корпорейшн | Анти-cd47-антитела и их применения |
| US20170151281A1 (en) | 2015-02-19 | 2017-06-01 | Batu Biologics, Inc. | Chimeric antigen receptor dendritic cell (car-dc) for treatment of cancer |
| US10035855B2 (en) * | 2015-03-04 | 2018-07-31 | Sorrento Therapeutics, Inc. | Antibody therapeutics that bind CD47 |
| CN108348589B (zh) | 2015-09-18 | 2022-09-23 | 安驰肿瘤公司 | 治疗性cd47抗体 |
| AU2016326423A1 (en) | 2015-09-21 | 2018-04-26 | Erasmus University Medical Center | Anti-CD47 antibodies and methods of use |
| SI3402820T1 (sl) * | 2016-01-11 | 2021-04-30 | Forty Seven, Inc. | Humanizirana, mišja ali himerna monoklonska protitelesa proti-CD47 |
| EP4438626A3 (en) | 2016-04-14 | 2024-12-04 | OSE Immunotherapeutics | New anti-sirpa antibodies and their therapeutic applications |
| WO2017184553A1 (en) * | 2016-04-18 | 2017-10-26 | Baylor College Of Medicine | Cancer gene therapy targeting cd47 |
| CN106084052B (zh) * | 2016-06-17 | 2019-12-27 | 长春金赛药业股份有限公司 | 抗cd47单克隆抗体及其应用 |
| CN106117354B (zh) * | 2016-06-24 | 2020-01-14 | 安徽未名细胞治疗有限公司 | 一种全人源抗CD47的全分子IgG抗体及其应用 |
| WO2018009499A1 (en) | 2016-07-06 | 2018-01-11 | Celgene Corporation | Antibodies with low immunogenicity and uses thereof |
| US10277886B2 (en) | 2016-07-19 | 2019-04-30 | Gopro, Inc. | Mapping of spherical image data into rectangular faces for transport and decoding across networks |
| CN107955071B (zh) * | 2016-10-18 | 2021-03-26 | 上海赛远生物科技有限公司 | 人源抗人cd47抗体及其编码基因与应用 |
| EP3532497B1 (en) | 2016-10-26 | 2024-07-24 | The Board of Trustees of the Leland Stanford Junior University | Modified immunoglobulin hinge regions to reduce hemagglutination |
| US11446315B2 (en) | 2016-11-03 | 2022-09-20 | Pf Argentum Ip Holdings Llc | Enhancement of CD47 blockade therapy by proteasome inhibitors |
| CA3042583A1 (en) | 2016-11-03 | 2018-05-11 | Trillium Therapeutics Inc. | Improvements in cd47 blockade therapy by hdac inhibitors |
| WO2018089508A2 (en) | 2016-11-08 | 2018-05-17 | Ablexis, Llc | Anti-cd47 antibodies |
| CN108779179B (zh) * | 2016-11-28 | 2022-02-08 | 江苏恒瑞医药股份有限公司 | Cd47抗体、其抗原结合片段及其医药用途 |
| KR20240039236A (ko) | 2016-12-09 | 2024-03-26 | 알렉터 엘엘씨 | 항-sirp-알파 항체 및 그의 사용 방법 |
| AU2018213718B2 (en) | 2017-01-26 | 2022-08-25 | Zlip Holding Limited | CD47 antigen binding unit and uses thereof |
| CN109422811A (zh) | 2017-08-29 | 2019-03-05 | 信达生物制药(苏州)有限公司 | 抗cd47抗体及其用途 |
| EA039662B1 (ru) | 2017-10-03 | 2022-02-24 | Закрытое Акционерное Общество "Биокад" | Антитела, специфичные к cd47 и pd-l1 |
| EP3706775A4 (en) | 2017-11-06 | 2021-09-01 | Trillium Therapeutics Inc. | BLOCKING OF CD47 ASSOCIATED WITH RADIATION THERAPY |
| WO2019091449A1 (zh) * | 2017-11-10 | 2019-05-16 | 江苏恒瑞医药股份有限公司 | Cd96抗体、其抗原结合片段及医药用途 |
| WO2019157432A1 (en) | 2018-02-12 | 2019-08-15 | Forty Seven, Inc. | Anti-cancer regimen using anti-cd47 and anti-cd20 antibodies |
| CN110305212A (zh) | 2018-03-27 | 2019-10-08 | 信达生物制药(苏州)有限公司 | 抗cd47抗体及其用途 |
| KR20210015872A (ko) | 2018-05-25 | 2021-02-10 | 알렉터 엘엘씨 | 항-sirpa 항체 및 그의 사용 방법 |
| JP7557920B2 (ja) | 2018-09-04 | 2024-09-30 | ファイザー・インク | 疾患治療のためのparp阻害と組み合わせたcd47遮断 |
| KR20210098427A (ko) * | 2018-10-31 | 2021-08-10 | 아이-맵 바이오파마 유에스 리미티드 | 신규한 cd47 항체 및 이의 사용방법 |
| TW202104260A (zh) * | 2019-04-05 | 2021-02-01 | 美商西建公司 | 腫瘤選擇性結合cd47之抗體之工程 |
| CN114206912B (zh) | 2019-06-07 | 2025-02-11 | Alx肿瘤生物技术公司 | 用于在血清学测定中减少结合cd47的药物的干扰的方法和试剂 |
| US10980836B1 (en) | 2019-12-11 | 2021-04-20 | Myeloid Therapeutics, Inc. | Therapeutic cell compositions and methods of manufacturing and use thereof |
| CN113461817B (zh) * | 2020-03-31 | 2025-04-18 | 苏州泽璟生物制药股份有限公司 | 一种抗人cd47抗体及其抗原结合片段、制备方法和应用 |
| TWI869582B (zh) * | 2020-04-10 | 2025-01-11 | 大陸商和記黃埔醫藥(上海)有限公司 | 抗cd47抗體及其用途 |
| CN116171284A (zh) * | 2020-04-24 | 2023-05-26 | 维尔托索宾科公司 | 用于治疗cd47相关疾病的双特异性抗体 |
| JP7789304B2 (ja) * | 2020-07-31 | 2025-12-22 | バイオ-テラ ソリュ-ションズ,エルティーディー. | Cd47抗体及びその応用 |
| JP2023549140A (ja) | 2020-11-04 | 2023-11-22 | マイエロイド・セラピューティクス,インコーポレーテッド | 操作されたキメラ融合タンパク質組成物およびその使用方法 |
| US20220196651A1 (en) | 2020-12-06 | 2022-06-23 | ALX Oncology Inc. | Multimers for reducing the interference of drugs that bind cd47 in serological assays |
| IL303928A (en) * | 2020-12-23 | 2023-08-01 | Guangdong Fapon Biopharma Inc | Antibody targeting CD47 and its application |
| WO2022148412A1 (zh) * | 2021-01-08 | 2022-07-14 | 北京韩美药品有限公司 | 特异性结合cd47的抗体及其抗原结合片段 |
| AU2022237618A1 (en) | 2021-03-17 | 2023-10-12 | Create Medicines, Inc. | Engineered chimeric fusion protein compositions and methods of use thereof |
| CA3217814A1 (en) | 2021-04-27 | 2022-11-03 | Pfizer Inc. | Enhancement of cd47 blockade therapy with dhfr inhibitors |
| WO2022241029A1 (en) | 2021-05-11 | 2022-11-17 | Myeloid Therapeutics, Inc. | Methods and compositions for genomic integration |
| WO2023073580A1 (en) | 2021-10-29 | 2023-05-04 | Pfizer Inc. | Enhancement of cd47 blockade with taxanes for cd47+ cancer therapy |
| US20250367255A1 (en) | 2021-11-08 | 2025-12-04 | Pfizer Inc. | Enhancement Of CD47 Blockade Therapy With Anti-VEGF Agents |
| CN118221812A (zh) * | 2022-12-14 | 2024-06-21 | 上海迈石生物技术有限公司 | 一种靶向cd47的单克隆抗体及其应用 |
Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2002512776A (ja) * | 1998-04-28 | 2002-05-08 | スミスクライン・ビーチャム・コーポレイション | 免疫原性の低下したモノクローナル抗体 |
| JPWO2001066737A1 (ja) * | 2000-03-10 | 2003-07-02 | 中外製薬株式会社 | アポトーシスを誘起するポリペプチド |
| JP4637749B2 (ja) * | 2003-11-11 | 2011-02-23 | 中外製薬株式会社 | ヒト化抗cd47抗体 |
| JP2015508072A (ja) * | 2012-02-06 | 2015-03-16 | インヒブルクス エルエルシーInhibrx Llc | Cd47抗体及びその使用方法 |
Family Cites Families (81)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US3773919A (en) | 1969-10-23 | 1973-11-20 | Du Pont | Polylactide-drug mixtures |
| FR2413974A1 (fr) | 1978-01-06 | 1979-08-03 | David Bernard | Sechoir pour feuilles imprimees par serigraphie |
| US4485045A (en) | 1981-07-06 | 1984-11-27 | Research Corporation | Synthetic phosphatidyl cholines useful in forming liposomes |
| US4522811A (en) | 1982-07-08 | 1985-06-11 | Syntex (U.S.A.) Inc. | Serial injection of muramyldipeptides and liposomes enhances the anti-infective activity of muramyldipeptides |
| US4816567A (en) | 1983-04-08 | 1989-03-28 | Genentech, Inc. | Recombinant immunoglobin preparations |
| US4544545A (en) | 1983-06-20 | 1985-10-01 | Trustees University Of Massachusetts | Liposomes containing modified cholesterol for organ targeting |
| US4681581A (en) | 1983-12-05 | 1987-07-21 | Coates Fredrica V | Adjustable size diaper and folding method therefor |
| US4683195A (en) | 1986-01-30 | 1987-07-28 | Cetus Corporation | Process for amplifying, detecting, and/or-cloning nucleic acid sequences |
| US4683202A (en) | 1985-03-28 | 1987-07-28 | Cetus Corporation | Process for amplifying nucleic acid sequences |
| US5776093A (en) | 1985-07-05 | 1998-07-07 | Immunomedics, Inc. | Method for imaging and treating organs and tissues |
| US5101827A (en) | 1985-07-05 | 1992-04-07 | Immunomedics, Inc. | Lymphographic and organ imaging method and kit |
| US4735210A (en) | 1985-07-05 | 1988-04-05 | Immunomedics, Inc. | Lymphographic and organ imaging method and kit |
| US4676980A (en) | 1985-09-23 | 1987-06-30 | The United States Of America As Represented By The Secretary Of The Department Of Health And Human Services | Target specific cross-linked heteroantibodies |
| EP0279862B1 (en) | 1986-08-28 | 1993-11-03 | Teijin Limited | Cytocidal antibody complex and process for its preparation |
| US4946778A (en) | 1987-09-21 | 1990-08-07 | Genex Corporation | Single polypeptide chain binding molecules |
| US5648471A (en) | 1987-12-03 | 1997-07-15 | Centocor, Inc. | One vial method for labeling antibodies with Technetium-99m |
| US5057604A (en) | 1988-08-03 | 1991-10-15 | Washington University | Novel monoclonal antibodies |
| US5223409A (en) | 1988-09-02 | 1993-06-29 | Protein Engineering Corp. | Directed evolution of novel binding proteins |
| GB8823869D0 (en) | 1988-10-12 | 1988-11-16 | Medical Res Council | Production of antibodies |
| US5175384A (en) | 1988-12-05 | 1992-12-29 | Genpharm International | Transgenic mice depleted in mature t-cells and methods for making transgenic mice |
| US5530101A (en) | 1988-12-28 | 1996-06-25 | Protein Design Labs, Inc. | Humanized immunoglobulins |
| EP0479909B1 (en) | 1989-06-29 | 1996-10-30 | Medarex, Inc. | Bispecific reagents for aids therapy |
| EP0486622B1 (en) | 1989-08-09 | 1998-11-04 | Rhomed, Incorporated | Direct radiolabeling of antibodies and other proteins with technetium or rhenium |
| US5013556A (en) | 1989-10-20 | 1991-05-07 | Liposome Technology, Inc. | Liposomes with enhanced circulation time |
| US6150584A (en) | 1990-01-12 | 2000-11-21 | Abgenix, Inc. | Human antibodies derived from immunized xenomice |
| DK0710719T3 (da) | 1990-01-12 | 2007-07-09 | Amgen Fremont Inc | Frembringelse af xenogene antistoffer |
| US6673986B1 (en) | 1990-01-12 | 2004-01-06 | Abgenix, Inc. | Generation of xenogeneic antibodies |
| US6075181A (en) | 1990-01-12 | 2000-06-13 | Abgenix, Inc. | Human antibodies derived from immunized xenomice |
| US5151510A (en) | 1990-04-20 | 1992-09-29 | Applied Biosystems, Inc. | Method of synethesizing sulfurized oligonucleotide analogs |
| GB9015198D0 (en) | 1990-07-10 | 1990-08-29 | Brien Caroline J O | Binding substance |
| US5165424A (en) | 1990-08-09 | 1992-11-24 | Silverman Harvey N | Method and system for whitening teeth |
| US5789650A (en) | 1990-08-29 | 1998-08-04 | Genpharm International, Inc. | Transgenic non-human animals for producing heterologous antibodies |
| WO1993012227A1 (en) | 1991-12-17 | 1993-06-24 | Genpharm International, Inc. | Transgenic non-human animals capable of producing heterologous antibodies |
| DE69127627T2 (de) | 1990-08-29 | 1998-02-19 | Genpharm Int | Produktion und Nützung nicht-menschliche transgentiere zur Produktion heterologe Antikörper |
| CA2090473A1 (en) | 1990-08-29 | 1992-03-01 | Robert M. Kay | Homologous recombinatin in mammalian cells |
| US6300129B1 (en) | 1990-08-29 | 2001-10-09 | Genpharm International | Transgenic non-human animals for producing heterologous antibodies |
| US5814318A (en) | 1990-08-29 | 1998-09-29 | Genpharm International Inc. | Transgenic non-human animals for producing heterologous antibodies |
| US5661016A (en) | 1990-08-29 | 1997-08-26 | Genpharm International Inc. | Transgenic non-human animals capable of producing heterologous antibodies of various isotypes |
| US5874299A (en) | 1990-08-29 | 1999-02-23 | Genpharm International, Inc. | Transgenic non-human animals capable of producing heterologous antibodies |
| US5770429A (en) | 1990-08-29 | 1998-06-23 | Genpharm International, Inc. | Transgenic non-human animals capable of producing heterologous antibodies |
| US5545806A (en) | 1990-08-29 | 1996-08-13 | Genpharm International, Inc. | Ransgenic non-human animals for producing heterologous antibodies |
| US5625126A (en) | 1990-08-29 | 1997-04-29 | Genpharm International, Inc. | Transgenic non-human animals for producing heterologous antibodies |
| US6255458B1 (en) | 1990-08-29 | 2001-07-03 | Genpharm International | High affinity human antibodies and human antibodies against digoxin |
| US5877397A (en) | 1990-08-29 | 1999-03-02 | Genpharm International Inc. | Transgenic non-human animals capable of producing heterologous antibodies of various isotypes |
| US5633425A (en) | 1990-08-29 | 1997-05-27 | Genpharm International, Inc. | Transgenic non-human animals capable of producing heterologous antibodies |
| US5194594A (en) | 1990-09-07 | 1993-03-16 | Techniclone, Inc. | Modified antibodies |
| CA2102511A1 (en) | 1991-05-14 | 1992-11-15 | Paul J. Higgins | Heteroconjugate antibodies for treatment of hiv infection |
| WO1992022670A1 (en) | 1991-06-12 | 1992-12-23 | Genpharm International, Inc. | Early detection of transgenic embryos |
| AU2235992A (en) | 1991-06-14 | 1993-01-12 | Genpharm International, Inc. | Transgenic immunodeficient non-human animals |
| WO1993004169A1 (en) | 1991-08-20 | 1993-03-04 | Genpharm International, Inc. | Gene targeting in animal cells using isogenic dna constructs |
| WO1993008829A1 (en) | 1991-11-04 | 1993-05-13 | The Regents Of The University Of California | Compositions that mediate killing of hiv-infected cells |
| ATE408012T1 (de) | 1991-12-02 | 2008-09-15 | Medical Res Council | Herstellung von autoantikörpern auf phagenoberflächen ausgehend von antikörpersegmentbibliotheken |
| US5777085A (en) | 1991-12-20 | 1998-07-07 | Protein Design Labs, Inc. | Humanized antibodies reactive with GPIIB/IIIA |
| WO1993016043A1 (en) | 1992-02-18 | 1993-08-19 | Otsuka Kagaku Kabushiki Kaisha | β-LACTAM COMPOUND AND CEPHEM COMPOUND, AND PRODUCTION THEREOF |
| US5233409A (en) | 1992-02-25 | 1993-08-03 | Schwab Karl W | Color analysis of organic constituents in sedimentary rocks for thermal maturity |
| US5714350A (en) | 1992-03-09 | 1998-02-03 | Protein Design Labs, Inc. | Increasing antibody affinity by altering glycosylation in the immunoglobulin variable region |
| US5733743A (en) | 1992-03-24 | 1998-03-31 | Cambridge Antibody Technology Limited | Methods for producing members of specific binding pairs |
| AU4541093A (en) | 1992-06-18 | 1994-01-24 | Genpharm International, Inc. | Methods for producing transgenic non-human animals harboring a yeast artificial chromosome |
| NZ255101A (en) | 1992-07-24 | 1997-08-22 | Cell Genesys Inc | A yeast artificial chromosome (yac) vector containing an hprt minigene expressible in murine stem cells and genetically modified rodent therefor |
| NZ258392A (en) | 1992-11-13 | 1997-09-22 | Idec Pharma Corp | Chimeric and radiolabelled antibodies to the b lymphocyte cellsurface antigen bp35 (cd-20) and their use in the treatment of b cell lymphona |
| AU6819494A (en) | 1993-04-26 | 1994-11-21 | Genpharm International, Inc. | Transgenic non-human animals capable of producing heterologous antibodies |
| ATE196313T1 (de) | 1993-06-04 | 2000-09-15 | Us Health | Verfahren zur behandlung von kaposi-sarcoma mit antisense-oligonukleotide |
| US5625825A (en) | 1993-10-21 | 1997-04-29 | Lsi Logic Corporation | Random number generating apparatus for an interface unit of a carrier sense with multiple access and collision detect (CSMA/CD) ethernet data network |
| AU1925195A (en) | 1994-02-22 | 1995-09-04 | Dana-Farber Cancer Institute | Nucleic acid delivery system, method of synthesis and uses thereof |
| US5643763A (en) | 1994-11-04 | 1997-07-01 | Genpharm International, Inc. | Method for making recombinant yeast artificial chromosomes by minimizing diploid doubling during mating |
| US5731168A (en) | 1995-03-01 | 1998-03-24 | Genentech, Inc. | Method for making heteromultimeric polypeptides |
| US5703057A (en) | 1995-04-07 | 1997-12-30 | Board Of Regents The University Of Texas System | Expression library immunization |
| WO1996034096A1 (en) | 1995-04-28 | 1996-10-31 | Abgenix, Inc. | Human antibodies derived from immunized xenomice |
| WO1997007671A1 (en) | 1995-08-29 | 1997-03-06 | Kirin Beer Kabushiki Kaisha | Chimeric animal and method for constructing the same |
| US6025130A (en) | 1996-04-04 | 2000-02-15 | Mercator Genetics, Inc. | Hereditary hemochromatosis gene |
| ATE549918T1 (de) | 1996-12-03 | 2012-04-15 | Amgen Fremont Inc | Menschliche antikörper, die ausdrücklich menschliches tnf alpha binden |
| US6833268B1 (en) | 1999-06-10 | 2004-12-21 | Abgenix, Inc. | Transgenic animals for producing specific isotypes of human antibodies via non-cognate switch regions |
| TWI241345B (en) * | 2000-03-10 | 2005-10-11 | Chugai Pharmaceutical Co Ltd | Apoptosis inducing polypeptide |
| US7282556B2 (en) * | 2001-05-15 | 2007-10-16 | Emory University | Polynucleotides and polypeptides relating to the modulation of SIRPα-CD47 |
| EP1575509B1 (en) * | 2002-05-10 | 2011-10-26 | Purdue Research Foundation | Epha2 agonistic monoclonal antibodies and methods of use thereof |
| GB2468232B (en) * | 2007-11-30 | 2012-10-24 | Glaxo Group Ltd | Antigen-bindng constructs |
| US8420784B2 (en) | 2008-05-27 | 2013-04-16 | Kyowa Hakko Kirin Co., Ltd. | Interleukin 10 receptor, (IL-10R) antibodies |
| AR073060A1 (es) * | 2008-08-14 | 2010-10-13 | Arana Therapeutic Ltd | Anticuerpos anti-il-12/il-23 |
| HUE031778T2 (en) * | 2010-05-14 | 2017-07-28 | Univ Leland Stanford Junior | Humanized and chimeric monoclonal antibodies against CD47 |
| AU2012234335B2 (en) * | 2011-03-29 | 2016-09-01 | Roche Glycart Ag | Antibody Fc variants |
| US9111624B2 (en) | 2013-03-22 | 2015-08-18 | Katsuyuki Fujita | Semiconductor memory device |
-
2013
- 2013-08-06 EA EA201500818A patent/EA036963B1/ru not_active IP Right Cessation
- 2013-08-06 KR KR1020207030070A patent/KR102276974B1/ko active Active
- 2013-08-06 AU AU2013377886A patent/AU2013377886B2/en active Active
- 2013-08-06 SG SG10201706383XA patent/SG10201706383XA/en unknown
- 2013-08-06 BR BR112015018851A patent/BR112015018851A2/pt not_active Application Discontinuation
- 2013-08-06 WO PCT/US2013/053818 patent/WO2014123580A1/en not_active Ceased
- 2013-08-06 PE PE2015001628A patent/PE20151408A1/es active IP Right Grant
- 2013-08-06 CA CA2900468A patent/CA2900468C/en active Active
- 2013-08-06 EP EP22185286.6A patent/EP4137518A1/en active Pending
- 2013-08-06 KR KR1020157024103A patent/KR102170196B1/ko active Active
- 2013-08-06 CN CN201380075409.3A patent/CN105101997B/zh active Active
- 2013-08-06 NZ NZ710695A patent/NZ710695A/en unknown
- 2013-08-06 EP EP13874383.6A patent/EP2953643B1/en active Active
- 2013-08-06 JP JP2015556930A patent/JP2016507555A/ja active Pending
- 2013-08-06 ES ES13874383T patent/ES2944477T3/es active Active
- 2013-08-06 MX MX2015010115A patent/MX2015010115A/es unknown
- 2013-08-06 SG SG11201506132PA patent/SG11201506132PA/en unknown
-
2015
- 2015-08-04 MX MX2021008851A patent/MX2021008851A/es unknown
- 2015-08-06 PH PH12015501729A patent/PH12015501729A1/en unknown
- 2015-08-06 CL CL2015002203A patent/CL2015002203A1/es unknown
- 2015-08-06 IL IL240390A patent/IL240390B/en active IP Right Grant
- 2015-08-06 NI NI201500103A patent/NI201500103A/es unknown
- 2015-08-11 ZA ZA2015/05745A patent/ZA201505745B/en unknown
-
2018
- 2018-08-03 JP JP2018146460A patent/JP6726238B2/ja active Active
-
2019
- 2019-02-11 AU AU2019200942A patent/AU2019200942B2/en active Active
- 2019-08-12 ZA ZA2019/05292A patent/ZA201905292B/en unknown
-
2020
- 2020-05-10 IL IL274566A patent/IL274566B/en unknown
Patent Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2002512776A (ja) * | 1998-04-28 | 2002-05-08 | スミスクライン・ビーチャム・コーポレイション | 免疫原性の低下したモノクローナル抗体 |
| JPWO2001066737A1 (ja) * | 2000-03-10 | 2003-07-02 | 中外製薬株式会社 | アポトーシスを誘起するポリペプチド |
| JP4637749B2 (ja) * | 2003-11-11 | 2011-02-23 | 中外製薬株式会社 | ヒト化抗cd47抗体 |
| JP2015508072A (ja) * | 2012-02-06 | 2015-03-16 | インヒブルクス エルエルシーInhibrx Llc | Cd47抗体及びその使用方法 |
Cited By (20)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP7591917B2 (ja) | 2015-08-07 | 2024-11-29 | エーエルエックス オンコロジー インコーポレイテッド | Sirp-アルファドメインまたはそのバリアントを有する構築物 |
| JP7532009B2 (ja) | 2016-04-15 | 2024-08-13 | ザ ボード オブ トラスティーズ オブ ザ レランド スタンフォード ジュニア ユニバーシティー | がん処置における抗cd47薬の治療有効用量を決定及び達成する方法 |
| JP2019515899A (ja) * | 2016-04-15 | 2019-06-13 | ザ ボード オブ トラスティーズ オブ ザ レランド スタンフォード ジュニア ユニバーシティー | がん処置における抗cd47薬の治療有効用量を決定及び達成する方法 |
| US11472878B2 (en) | 2016-04-15 | 2022-10-18 | The Board Of Trustees Of The Leland Stanford Junior University | Methods for determining and achieving therapeutically effective doses of anti-CD47 agents in treatment of cancer |
| US12091467B2 (en) | 2016-10-20 | 2024-09-17 | I-Mab Biopharma Us Limited | CD47 monoclonal antibodies and uses thereof |
| US11692035B2 (en) | 2016-10-21 | 2023-07-04 | Arch Oncology, Inc. | Therapeutic CD47 antibodies |
| JP7170331B2 (ja) | 2017-03-22 | 2022-11-14 | アーチ オンコロジー,インコーポレイテッド | 固形及び血液癌の治療のための併用療法 |
| JP2020511481A (ja) * | 2017-03-22 | 2020-04-16 | アーチ オンコロジー, インコーポレイテッドArch Oncology, Inc. | 固形及び血液癌の治療のための併用療法 |
| JP2023052145A (ja) * | 2017-03-27 | 2023-04-11 | セルジーン コーポレイション | 免疫原性の低下のための方法及び組成物 |
| JP2023083424A (ja) * | 2017-06-21 | 2023-06-15 | ザ ボード オブ トラスティーズ オブ ザ レランド スタンフォード ジュニア ユニバーシティー | 血液悪性腫瘍に対するcd47標的化治療のための投与パラメータ |
| JP7262440B2 (ja) | 2017-08-02 | 2023-04-21 | フェインズ セラピューティクス,インコーポレーテッド | 抗cd47抗体及びその使用 |
| JP2020536488A (ja) * | 2017-08-02 | 2020-12-17 | フェインズ セラピューティクス,インコーポレーテッド | 抗cd47抗体及びその使用 |
| JP7084045B2 (ja) | 2017-11-10 | 2022-06-14 | アイ-マブ バイオファーマ ユーエス リミテッド | Cd47抗体およびサイトカインを含む融合タンパク質 |
| JP2021502324A (ja) * | 2017-11-10 | 2021-01-28 | アイ−マブ バイオファーマ ユーエス リミテッド | Cd47抗体およびサイトカインを含む融合タンパク質 |
| JP7143452B2 (ja) | 2018-06-11 | 2022-09-28 | 康▲諾▼▲亞▼生物医葯科技(成都)有限公司 | CD47とSIRPaの相互作用を遮断できる抗体及びその応用 |
| JP2021527110A (ja) * | 2018-06-11 | 2021-10-11 | 康▲諾▼▲亞▼生物医葯科技(成都)有限公司Chengdu Conmed Biosciences Co., Ltd | CD47とSIRPaの相互作用を遮断できる抗体及びその応用 |
| US11820820B2 (en) | 2018-06-11 | 2023-11-21 | Chengdu Conmed Biosciences Co., Ltd. | Antibody capable of blocking CD47-SIRPa interaction and application thereof |
| JP2021535743A (ja) * | 2018-08-31 | 2021-12-23 | 南京聖和薬業股▲ふん▼有限公司Nanjing Sanhome Pharmaceutical Co., Ltd. | 抗cd47抗体及びその応用 |
| JP7583707B2 (ja) | 2018-08-31 | 2024-11-14 | 南京聖和薬業股▲ふん▼有限公司 | 抗cd47抗体及びその応用 |
| US11987627B2 (en) | 2018-08-31 | 2024-05-21 | Nanjing Sanhome Pharmaceutical Co., Ltd. | Anti-CD47 antibody and application thereof |
Also Published As
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP6726238B2 (ja) | 血小板非減少性かつ赤血球非減少性cd47抗体及びその使用方法 | |
| US20240166744A1 (en) | Non-platelet depleting and non-red blood cell depleting cd47 antibodies and methods of use thereof | |
| JP6273212B2 (ja) | Cd47抗体及びその使用方法 | |
| US20210371522A1 (en) | Cd47 antibodies and methods of use thereof | |
| HK40088423A (en) | Non-platelet depleting and non-red blood cell depleting cd47 antibodies and methods of use thereof | |
| HK40018837A (en) | Cd47 antibodies and methods of use thereof | |
| HK1218863B (en) | Non-platelet depleting and non-red blood cell depleting cd47 antibodies and methods of use thereof | |
| HK1205195B (en) | Cd47 antibodies and methods of use thereof |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20151007 |
|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20160805 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20160818 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20161012 |
|
| A977 | Report on retrieval |
Free format text: JAPANESE INTERMEDIATE CODE: A971007 Effective date: 20170614 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20170620 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20170919 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20171220 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20180316 |
|
| A02 | Decision of refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A02 Effective date: 20180403 |