JP2015525781A - 免疫応答の調節 - Google Patents
免疫応答の調節 Download PDFInfo
- Publication number
- JP2015525781A JP2015525781A JP2015525500A JP2015525500A JP2015525781A JP 2015525781 A JP2015525781 A JP 2015525781A JP 2015525500 A JP2015525500 A JP 2015525500A JP 2015525500 A JP2015525500 A JP 2015525500A JP 2015525781 A JP2015525781 A JP 2015525781A
- Authority
- JP
- Japan
- Prior art keywords
- ceacam1
- tim3
- interaction
- agent
- cells
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Pending
Links
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K7/00—Peptides having 5 to 20 amino acids in a fully defined sequence; Derivatives thereof
- C07K7/04—Linear peptides containing only normal peptide links
- C07K7/08—Linear peptides containing only normal peptide links having 12 to 20 amino acids
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K38/00—Medicinal preparations containing peptides
- A61K38/04—Peptides having up to 20 amino acids in a fully defined sequence; Derivatives thereof
- A61K38/10—Peptides having 12 to 20 amino acids
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K39/395—Antibodies; Immunoglobulins; Immune serum, e.g. antilymphocytic serum
- A61K39/39533—Antibodies; Immunoglobulins; Immune serum, e.g. antilymphocytic serum against materials from animals
- A61K39/3955—Antibodies; Immunoglobulins; Immune serum, e.g. antilymphocytic serum against materials from animals against proteinaceous materials, e.g. enzymes, hormones, lymphokines
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P37/00—Drugs for immunological or allergic disorders
- A61P37/02—Immunomodulators
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P37/00—Drugs for immunological or allergic disorders
- A61P37/02—Immunomodulators
- A61P37/04—Immunostimulants
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K14/00—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- C07K14/435—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans
- C07K14/705—Receptors; Cell surface antigens; Cell surface determinants
- C07K14/70503—Immunoglobulin superfamily
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/18—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans
- C07K16/28—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants
- C07K16/2803—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants against the immunoglobulin superfamily
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/18—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans
- C07K16/28—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants
- C07K16/30—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants from tumour cells
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K2039/505—Medicinal preparations containing antigens or antibodies comprising antibodies
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K2039/505—Medicinal preparations containing antigens or antibodies comprising antibodies
- A61K2039/507—Comprising a combination of two or more separate antibodies
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/30—Immunoglobulins specific features characterized by aspects of specificity or valency
- C07K2317/31—Immunoglobulins specific features characterized by aspects of specificity or valency multispecific
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/70—Immunoglobulins specific features characterized by effect upon binding to a cell or to an antigen
- C07K2317/73—Inducing cell death, e.g. apoptosis, necrosis or inhibition of cell proliferation
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2319/00—Fusion polypeptide
- C07K2319/30—Non-immunoglobulin-derived peptide or protein having an immunoglobulin constant or Fc region, or a fragment thereof, attached thereto
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y02—TECHNOLOGIES OR APPLICATIONS FOR MITIGATION OR ADAPTATION AGAINST CLIMATE CHANGE
- Y02A—TECHNOLOGIES FOR ADAPTATION TO CLIMATE CHANGE
- Y02A50/00—TECHNOLOGIES FOR ADAPTATION TO CLIMATE CHANGE in human health protection, e.g. against extreme weather
- Y02A50/30—Against vector-borne diseases, e.g. mosquito-borne, fly-borne, tick-borne or waterborne diseases whose impact is exacerbated by climate change
Landscapes
- Health & Medical Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Immunology (AREA)
- Life Sciences & Earth Sciences (AREA)
- Organic Chemistry (AREA)
- Medicinal Chemistry (AREA)
- General Health & Medical Sciences (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Veterinary Medicine (AREA)
- Pharmacology & Pharmacy (AREA)
- Animal Behavior & Ethology (AREA)
- Public Health (AREA)
- Genetics & Genomics (AREA)
- Molecular Biology (AREA)
- Biochemistry (AREA)
- Biophysics (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Engineering & Computer Science (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- General Chemical & Material Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Gastroenterology & Hepatology (AREA)
- Epidemiology (AREA)
- Cell Biology (AREA)
- Endocrinology (AREA)
- Microbiology (AREA)
- Mycology (AREA)
- Toxicology (AREA)
- Zoology (AREA)
- Medicines Containing Antibodies Or Antigens For Use As Internal Diagnostic Agents (AREA)
- Peptides Or Proteins (AREA)
- Medicines That Contain Protein Lipid Enzymes And Other Medicines (AREA)
- Micro-Organisms Or Cultivation Processes Thereof (AREA)
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US201261677596P | 2012-07-31 | 2012-07-31 | |
| US61/677,596 | 2012-07-31 | ||
| PCT/US2013/052612 WO2014022332A1 (en) | 2012-07-31 | 2013-07-30 | Modulation of the immune response |
Related Child Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2019047201A Division JP7096186B2 (ja) | 2012-07-31 | 2019-03-14 | 免疫応答の調節 |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| JP2015525781A true JP2015525781A (ja) | 2015-09-07 |
| JP2015525781A5 JP2015525781A5 (enExample) | 2016-09-23 |
Family
ID=50028463
Family Applications (3)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2015525500A Pending JP2015525781A (ja) | 2012-07-31 | 2013-07-30 | 免疫応答の調節 |
| JP2019047201A Active JP7096186B2 (ja) | 2012-07-31 | 2019-03-14 | 免疫応答の調節 |
| JP2020161836A Pending JP2020200351A (ja) | 2012-07-31 | 2020-09-28 | 免疫応答の調節 |
Family Applications After (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2019047201A Active JP7096186B2 (ja) | 2012-07-31 | 2019-03-14 | 免疫応答の調節 |
| JP2020161836A Pending JP2020200351A (ja) | 2012-07-31 | 2020-09-28 | 免疫応答の調節 |
Country Status (5)
| Country | Link |
|---|---|
| US (2) | US10513540B2 (enExample) |
| EP (2) | EP2879709B1 (enExample) |
| JP (3) | JP2015525781A (enExample) |
| CA (1) | CA2916638C (enExample) |
| WO (1) | WO2014022332A1 (enExample) |
Families Citing this family (102)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP3049442A4 (en) | 2013-09-26 | 2017-06-28 | Costim Pharmaceuticals Inc. | Methods for treating hematologic cancers |
| EP3763387B1 (en) | 2013-11-25 | 2024-03-27 | FameWave Ltd | Compositions comprising anti-ceacam1 and anti-pd antibodies for cancer therapy |
| US10640569B2 (en) | 2013-12-19 | 2020-05-05 | Novartis Ag | Human mesothelin chimeric antigen receptors and uses thereof |
| JOP20200094A1 (ar) | 2014-01-24 | 2017-06-16 | Dana Farber Cancer Inst Inc | جزيئات جسم مضاد لـ pd-1 واستخداماتها |
| JOP20200096A1 (ar) | 2014-01-31 | 2017-06-16 | Children’S Medical Center Corp | جزيئات جسم مضاد لـ tim-3 واستخداماتها |
| KR20230097209A (ko) | 2014-03-12 | 2023-06-30 | 예다 리서치 앤드 디벨럽먼트 캄파니 리미티드 | Cns의 질환 및 손상을 치료하기 위한 전신적 조절 t 세포 수준 또는 활성의 감소 |
| US10519237B2 (en) | 2014-03-12 | 2019-12-31 | Yeda Research And Development Co. Ltd | Reducing systemic regulatory T cell levels or activity for treatment of disease and injury of the CNS |
| US10618963B2 (en) | 2014-03-12 | 2020-04-14 | Yeda Research And Development Co. Ltd | Reducing systemic regulatory T cell levels or activity for treatment of disease and injury of the CNS |
| US20150259420A1 (en) | 2014-03-14 | 2015-09-17 | Novartis Ag | Antibody molecules to lag-3 and uses thereof |
| ES2939760T3 (es) | 2014-03-15 | 2023-04-26 | Novartis Ag | Tratamiento de cáncer utilizando un receptor quimérico para antígenos |
| HUE054588T2 (hu) | 2014-04-07 | 2021-09-28 | Novartis Ag | Rák kezelése CD19 elleni, kiméra antigénreceptor alkalmazásával |
| WO2016014530A1 (en) | 2014-07-21 | 2016-01-28 | Novartis Ag | Combinations of low, immune enhancing. doses of mtor inhibitors and cars |
| US9777061B2 (en) | 2014-07-21 | 2017-10-03 | Novartis Ag | Treatment of cancer using a CD33 chimeric antigen receptor |
| US11542488B2 (en) | 2014-07-21 | 2023-01-03 | Novartis Ag | Sortase synthesized chimeric antigen receptors |
| EP3174546B1 (en) | 2014-07-31 | 2019-10-30 | Novartis AG | Subset-optimized chimeric antigen receptor-containing t-cells |
| JP6919118B2 (ja) | 2014-08-14 | 2021-08-18 | ノバルティス アーゲー | GFRα−4キメラ抗原受容体を用いる癌の治療 |
| PL3183268T3 (pl) | 2014-08-19 | 2020-09-07 | Novartis Ag | Chimeryczny receptor antygenowy (CAR) anty-CD123 do zastosowania w leczeniu nowotworu złośliwego |
| US20170281624A1 (en) | 2014-09-13 | 2017-10-05 | Novartis Ag | Combination therapies of alk inhibitors |
| WO2016044605A1 (en) | 2014-09-17 | 2016-03-24 | Beatty, Gregory | Targeting cytotoxic cells with chimeric receptors for adoptive immunotherapy |
| EP3662903A3 (en) | 2014-10-03 | 2020-10-14 | Novartis AG | Combination therapies |
| WO2016057705A1 (en) | 2014-10-08 | 2016-04-14 | Novartis Ag | Biomarkers predictive of therapeutic responsiveness to chimeric antigen receptor therapy and uses thereof |
| ES2952717T3 (es) | 2014-10-14 | 2023-11-03 | Novartis Ag | Moléculas de anticuerpos contra PD-L1 y usos de las mismas |
| SG11201703403TA (en) | 2014-10-27 | 2017-05-30 | Agency Science Tech & Res | Anti-tim-3 antibodies |
| GB201419094D0 (en) | 2014-10-27 | 2014-12-10 | Agency Science Tech & Res | Anti-TIM-3-antibodies |
| US20180334490A1 (en) | 2014-12-03 | 2018-11-22 | Qilong H. Wu | Methods for b cell preconditioning in car therapy |
| WO2016100882A1 (en) | 2014-12-19 | 2016-06-23 | Novartis Ag | Combination therapies |
| WO2016120331A1 (de) * | 2015-01-28 | 2016-08-04 | Karl Sebastian Lang | Agonistische anti-cd66cd66 antikörper für die antiviralen therapie |
| US11161907B2 (en) | 2015-02-02 | 2021-11-02 | Novartis Ag | Car-expressing cells against multiple tumor antigens and uses thereof |
| US20180140602A1 (en) | 2015-04-07 | 2018-05-24 | Novartis Ag | Combination of chimeric antigen receptor therapy and amino pyrimidine derivatives |
| ES2948133T3 (es) | 2015-04-17 | 2023-08-31 | Novartis Ag | Métodos para mejorar la eficacia y expansión de células que expresan un receptor de antígeno quimérico |
| US12128069B2 (en) | 2015-04-23 | 2024-10-29 | The Trustees Of The University Of Pennsylvania | Treatment of cancer using chimeric antigen receptor and protein kinase a blocker |
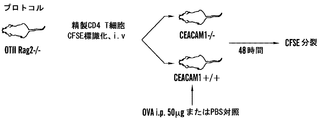
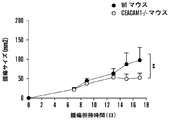
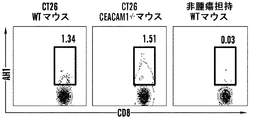
| US20180153986A1 (en) * | 2015-04-24 | 2018-06-07 | The Brigham And Women's Hospital, Inc. | Interactions between ceacam and tim family members |
| US20180298095A1 (en) * | 2015-04-24 | 2018-10-18 | The Brigham And Women's Hospital, Inc. | Interactions between ceacam and tim family members |
| EP3744340A3 (en) | 2015-07-16 | 2021-03-03 | Biokine Therapeutics Ltd. | Compositions and methods for treating cancer |
| TWI859112B (zh) | 2015-07-21 | 2024-10-21 | 瑞士商諾華公司 | 改良免疫細胞之功效及擴展之方法 |
| EP3878465A1 (en) | 2015-07-29 | 2021-09-15 | Novartis AG | Combination therapies comprising antibody molecules to tim-3 |
| PT3317301T (pt) | 2015-07-29 | 2021-07-09 | Novartis Ag | Terapias de associação compreendendo moléculas de anticorpo contra lag-3 |
| JP6878405B2 (ja) | 2015-07-29 | 2021-05-26 | ノバルティス アーゲー | Pd−1に対する抗体分子を含む組み合わせ治療 |
| JP6905163B2 (ja) | 2015-09-03 | 2021-07-21 | ザ トラスティーズ オブ ザ ユニバーシティ オブ ペンシルバニア | サイトカイン放出症候群を予測するバイオマーカー |
| EP4046655A1 (en) | 2015-11-03 | 2022-08-24 | Janssen Biotech, Inc. | Antibodies specifically binding pd-1 and their uses |
| JP2019503349A (ja) | 2015-12-17 | 2019-02-07 | ノバルティス アーゲー | Pd−1に対する抗体分子およびその使用 |
| US11433136B2 (en) | 2015-12-18 | 2022-09-06 | The General Hospital Corporation | Polyacetal polymers, conjugates, particles and uses thereof |
| CA3008102A1 (en) | 2015-12-18 | 2017-06-22 | Novartis Ag | Antibodies targeting cd32b and methods of use thereof |
| CN109069597A (zh) | 2015-12-22 | 2018-12-21 | 诺华股份有限公司 | 间皮素嵌合抗原受体(car)和抗pd-l1抗体抑制剂联用于抗癌治疗 |
| EP3423482A1 (en) | 2016-03-04 | 2019-01-09 | Novartis AG | Cells expressing multiple chimeric antigen receptor (car) molecules and uses therefore |
| KR102366813B1 (ko) | 2016-05-27 | 2022-02-24 | 아게누스 인코포레이티드 | 항-tim-3 항체 및 이의 사용 방법 |
| RU2769282C2 (ru) | 2016-06-20 | 2022-03-30 | Кимаб Лимитед | Анти-PD-L1 и IL-2 цитокины |
| PE20190418A1 (es) | 2016-07-14 | 2019-03-19 | Bristol Myers Squibb Co | Anticuerpos contra proteina 3 que contiene el dominio de mucina e inmunoglobulina de linfocitos t (tim3) y sus usos |
| WO2018017708A1 (en) | 2016-07-20 | 2018-01-25 | University Of Utah Research Foundation | Cd229 car t cells and methods of use thereof |
| EP3523331A1 (en) | 2016-10-07 | 2019-08-14 | Novartis AG | Chimeric antigen receptors for the treatment of cancer |
| MX2019006285A (es) | 2016-12-03 | 2019-12-16 | Juno Therapeutics Inc | Metodos para modulacion de celulas cart-t. |
| WO2018106738A1 (en) | 2016-12-05 | 2018-06-14 | Massachusetts Institute Of Technology | Brush-arm star polymers, conjugates and particles, and uses thereof |
| WO2018201056A1 (en) | 2017-04-28 | 2018-11-01 | Novartis Ag | Cells expressing a bcma-targeting chimeric antigen receptor, and combination therapy with a gamma secretase inhibitor |
| WO2018201051A1 (en) | 2017-04-28 | 2018-11-01 | Novartis Ag | Bcma-targeting agent, and combination therapy with a gamma secretase inhibitor |
| CN111225675B (zh) | 2017-06-02 | 2024-05-03 | 朱诺治疗学股份有限公司 | 使用过继细胞疗法治疗的制品和方法 |
| WO2018229715A1 (en) | 2017-06-16 | 2018-12-20 | Novartis Ag | Compositions comprising anti-cd32b antibodies and methods of use thereof |
| JP2020526194A (ja) | 2017-06-29 | 2020-08-31 | ジュノー セラピューティクス インコーポレイテッド | 免疫療法薬と関連する毒性を評価するためのマウスモデル |
| EP3668876B1 (en) | 2017-08-18 | 2024-01-24 | Cothera Bioscience, Inc. | Polymorphic form of tg02 |
| US20210040205A1 (en) | 2017-10-25 | 2021-02-11 | Novartis Ag | Antibodies targeting cd32b and methods of use thereof |
| US11066475B2 (en) | 2017-11-01 | 2021-07-20 | Juno Therapeutics, Inc. | Chimeric antigen receptors specific for B-cell maturation antigen and encoding polynucleotides |
| AU2018358067A1 (en) | 2017-11-01 | 2020-05-07 | Juno Therapeutics, Inc. | Antibodies and chimeric antigen receptors specific for B-cell maturation antigen |
| WO2019089858A2 (en) | 2017-11-01 | 2019-05-09 | Juno Therapeutics, Inc. | Methods of assessing or monitoring a response to a cell therapy |
| KR20200110745A (ko) | 2017-12-15 | 2020-09-25 | 주노 쎄러퓨티크스 인코퍼레이티드 | 항 - cct5 결합 분자 및 이의 사용 방법 |
| US12398209B2 (en) | 2018-01-22 | 2025-08-26 | Janssen Biotech, Inc. | Methods of treating cancers with antagonistic anti-PD-1 antibodies |
| WO2019152743A1 (en) | 2018-01-31 | 2019-08-08 | Celgene Corporation | Combination therapy using adoptive cell therapy and checkpoint inhibitor |
| US20210047405A1 (en) | 2018-04-27 | 2021-02-18 | Novartis Ag | Car t cell therapies with enhanced efficacy |
| WO2019213282A1 (en) | 2018-05-01 | 2019-11-07 | Novartis Ag | Biomarkers for evaluating car-t cells to predict clinical outcome |
| CA3100724A1 (en) | 2018-06-13 | 2019-12-19 | Novartis Ag | B-cell maturation antigen protein (bcma) chimeric antigen receptors and uses thereof |
| WO2020069405A1 (en) | 2018-09-28 | 2020-04-02 | Novartis Ag | Cd22 chimeric antigen receptor (car) therapies |
| EP3856782A1 (en) | 2018-09-28 | 2021-08-04 | Novartis AG | Cd19 chimeric antigen receptor (car) and cd22 car combination therapies |
| BR112021008289A2 (pt) | 2018-11-01 | 2021-10-26 | Juno Therapeutics, Inc. | Métodos para tratamento utilizando receptores de antígeno quimérico específico para antígeno de maturação de célula b |
| TW202021981A (zh) | 2018-11-01 | 2020-06-16 | 美商奇諾治療有限公司 | G蛋白偶合受體c類第5群成員d(gprc5d)特異性嵌合抗原受體 |
| JP2022513062A (ja) | 2018-11-16 | 2022-02-07 | ジュノー セラピューティクス インコーポレイテッド | B細胞悪性腫瘍を処置するために、操作されたt細胞を投薬する方法 |
| CN113710256A (zh) | 2018-11-30 | 2021-11-26 | 朱诺治疗学股份有限公司 | 使用过继细胞疗法的治疗方法 |
| MX2021009087A (es) | 2019-01-29 | 2021-09-08 | Juno Therapeutics Inc | Anticuerpos y receptores quimericos de antigenos especificos para receptor 1 huerfano tipo receptor tirosina-cinasa (ror1). |
| MX2021011753A (es) | 2019-03-26 | 2022-01-31 | Univ Michigan Regents | Degradadores de moleculas peque?as de stat3. |
| US20220185831A1 (en) | 2019-03-29 | 2022-06-16 | The Regents Of The University Of Michigan | Stat3 protein degraders |
| EP3725370A1 (en) | 2019-04-19 | 2020-10-21 | ImmunoBrain Checkpoint, Inc. | Modified anti-pd-l1 antibodies and methods and uses for treating a neurodegenerative disease |
| JP2022536728A (ja) | 2019-06-12 | 2022-08-18 | ヴァンダービルト ユニバーシティー | アミノ酸輸送阻害剤としてのジベンジルアミン類 |
| CA3141405A1 (en) | 2019-06-12 | 2020-12-17 | H. Charles Manning | Amino acid transport inhibitors and the uses thereof |
| JP2022538284A (ja) | 2019-06-27 | 2022-09-01 | ザ ジョージ ワシントン ユニバーシティ, ア コングレッショナリー チャータード ノット-フォー-プロフィット コーポレイション | Hdac6活性化マクロファージ、その組成物および使用 |
| KR102911673B1 (ko) | 2019-07-16 | 2026-01-13 | 더 리젠츠 오브 더 유니버시티 오브 미시간 | Eed 억제제로서의 이미다조피리미딘 및 이의 용도 |
| AU2020336381A1 (en) | 2019-08-27 | 2022-03-03 | The Regents Of The University Of Michigan | Cereblon E3 ligase inhibitors |
| JP2022548775A (ja) | 2019-09-19 | 2022-11-21 | ザ リージェンツ オブ ザ ユニヴァシティ オブ ミシガン | スピロ環式アンドロゲン受容体タンパク質分解剤 |
| CN114746119A (zh) * | 2019-09-27 | 2022-07-12 | 詹森生物科技公司 | 抗-ceacam抗体及其用途 |
| AU2020394441B2 (en) | 2019-11-26 | 2025-11-13 | Novartis Ag | CD19 and CD22 chimeric antigen receptors and uses thereof |
| CN111533785B (zh) * | 2020-02-11 | 2022-03-08 | 北京市肿瘤防治研究所 | 靶向免疫检查点tim3结合肽及其应用 |
| US12522623B2 (en) | 2020-03-26 | 2026-01-13 | Regents Of The University Of Michigan | Small molecule STAT protein degraders |
| WO2021207689A2 (en) | 2020-04-10 | 2021-10-14 | Juno Therapeutics, Inc. | Methods and uses related to cell therapy engineered with a chimeric antigen receptor targeting b-cell maturation antigen |
| PL4153597T3 (pl) | 2020-05-19 | 2026-01-26 | Pharmacosmos Holding A/S | Związki hamujące kinazę zależną od cykliny do leczenia zaburzeń medycznych |
| US20230257365A1 (en) | 2020-07-10 | 2023-08-17 | The Regents Of The University Of Michigan | Small molecule androgen receptor protein degraders |
| US20230233690A1 (en) | 2020-07-10 | 2023-07-27 | The Regents Of The University Of Michigan | Androgen receptor protein degraders |
| JP7787164B2 (ja) | 2020-09-23 | 2025-12-16 | アキリオン ファーマシューティカルズ, インコーポレーテッド | 補体媒介性障害の治療のための医薬化合物 |
| EP4221842A4 (en) | 2020-10-02 | 2024-11-20 | Dracen Pharmaceuticals, Inc. | LYOPHILIZED COMPOSITION CONTAINING (S)-ISOPROPYL 2-((S)-2-ACETAMIDO-3-(1H-INDOL-3-YL)PROPANAMIDO)-6-DIAZO-5-OXOHEXANOATE FOR SUBCUTANEOUS ADMINISTRATION AND ITS USE |
| US20240166647A1 (en) | 2021-03-03 | 2024-05-23 | The Regents Of The University Of Michigan | Cereblon Ligands |
| US20240190874A1 (en) | 2021-03-03 | 2024-06-13 | The Regents Of The University Of Michigan | Small molecule degraders of androgen receptor |
| WO2022254337A1 (en) | 2021-06-01 | 2022-12-08 | Novartis Ag | Cd19 and cd22 chimeric antigen receptors and uses thereof |
| US20250381272A1 (en) | 2022-06-22 | 2025-12-18 | Juno Therapeutics, Inc. | Treatment methods for second line therapy of cd19-targeted car t cells |
| JP2025525937A (ja) | 2022-08-05 | 2025-08-07 | ジュノー セラピューティクス インコーポレイテッド | Gprc5dおよびbcmaに特異的なキメラ抗原受容体 |
| CN120712102A (zh) | 2022-12-13 | 2025-09-26 | 朱诺治疗学股份有限公司 | 对baff-r和cd19具特异性的嵌合抗原受体及其方法和用途 |
| WO2024165403A1 (en) | 2023-02-06 | 2024-08-15 | Philogen S.P.A. | Anti-cea antibodies |
| WO2025151487A2 (en) | 2024-01-08 | 2025-07-17 | Regents Of The University Of Michigan | Small-molecule inhibitors of adar1 |
Citations (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2009502129A (ja) * | 2005-07-22 | 2009-01-29 | ピエール ファーブル メディカモン | 新規の抗igf−ir抗体及びその使用 |
Family Cites Families (40)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US3270960A (en) | 1964-09-11 | 1966-09-06 | Sperry Rand Corp | Fluid sensor |
| US3536809A (en) | 1969-02-17 | 1970-10-27 | Alza Corp | Medication method |
| US3598123A (en) | 1969-04-01 | 1971-08-10 | Alza Corp | Bandage for administering drugs |
| US3773919A (en) | 1969-10-23 | 1973-11-20 | Du Pont | Polylactide-drug mixtures |
| US3845770A (en) | 1972-06-05 | 1974-11-05 | Alza Corp | Osmatic dispensing device for releasing beneficial agent |
| US3916899A (en) | 1973-04-25 | 1975-11-04 | Alza Corp | Osmotic dispensing device with maximum and minimum sizes for the passageway |
| US4008719A (en) | 1976-02-02 | 1977-02-22 | Alza Corporation | Osmotic system having laminar arrangement for programming delivery of active agent |
| US4747825A (en) | 1984-06-29 | 1988-05-31 | Ferring Laboratories, Inc. | Apparatus and methodology for pulsed administration of growth promoting agents |
| IE58110B1 (en) | 1984-10-30 | 1993-07-14 | Elan Corp Plc | Controlled release powder and process for its preparation |
| US4948592A (en) | 1986-05-09 | 1990-08-14 | Alza Corporation | Pulsed drug delivery |
| US4723958A (en) | 1986-05-23 | 1988-02-09 | Merck & Co., Inc. | Pulsatile drug delivery system |
| US4965251A (en) | 1987-04-03 | 1990-10-23 | The Board Of Regents Of The University Of Washington | Pulse treatment of hemoglobinopathies with erythropoietin |
| US5073543A (en) | 1988-07-21 | 1991-12-17 | G. D. Searle & Co. | Controlled release formulations of trophic factors in ganglioside-lipsome vehicle |
| IT1229203B (it) | 1989-03-22 | 1991-07-25 | Bioresearch Spa | Impiego di acido 5 metiltetraidrofolico, di acido 5 formiltetraidrofolico e dei loro sali farmaceuticamente accettabili per la preparazione di composizioni farmaceutiche in forma a rilascio controllato attive nella terapia dei disturbi mentali organici e composizioni farmaceutiche relative. |
| DE3920358A1 (de) | 1989-06-22 | 1991-01-17 | Behringwerke Ag | Bispezifische und oligospezifische, mono- und oligovalente antikoerperkonstrukte, ihre herstellung und verwendung |
| US5120548A (en) | 1989-11-07 | 1992-06-09 | Merck & Co., Inc. | Swelling modulated polymeric drug delivery device |
| US5733566A (en) | 1990-05-15 | 1998-03-31 | Alkermes Controlled Therapeutics Inc. Ii | Controlled release of antiparasitic agents in animals |
| ATE297465T1 (de) | 1991-11-25 | 2005-06-15 | Enzon Inc | Verfahren zur herstellung von multivalenten antigenbindenden proteinen |
| US5580578A (en) | 1992-01-27 | 1996-12-03 | Euro-Celtique, S.A. | Controlled release formulations coated with aqueous dispersions of acrylic polymers |
| US5403590A (en) | 1992-12-21 | 1995-04-04 | New England Deaconess Hospital Corporation | Method of pulsatile drug infusion |
| US5591767A (en) | 1993-01-25 | 1997-01-07 | Pharmetrix Corporation | Liquid reservoir transdermal patch for the administration of ketorolac |
| IT1270594B (it) | 1994-07-07 | 1997-05-07 | Recordati Chem Pharm | Composizione farmaceutica a rilascio controllato di moguisteina in sospensione liquida |
| US5641870A (en) | 1995-04-20 | 1997-06-24 | Genentech, Inc. | Low pH hydrophobic interaction chromatography for antibody purification |
| US6365185B1 (en) | 1998-03-26 | 2002-04-02 | University Of Cincinnati | Self-destructing, controlled release peroral drug delivery system |
| DE69925909T2 (de) | 1998-04-15 | 2006-05-11 | Brigham & Women's Hospital, Inc., Boston | T-zell inhibierende rezeptorzusammensetzungen sowie deren verwendung |
| SE0002835D0 (sv) * | 2000-08-07 | 2000-08-07 | Karolinska Innovations Ab | Method and kit for production of monoclonal antibodies |
| US7470428B2 (en) | 2002-01-30 | 2008-12-30 | The Brigham And Women's Hospital, Inc. | Compositions and methods related to TIM-3, a Th1-specific cell surface molecule |
| US20040047858A1 (en) | 2002-09-11 | 2004-03-11 | Blumberg Richard S. | Therapeutic anti-BGP(C-CAM1) antibodies and uses thereof |
| EP1674480A4 (en) | 2003-09-04 | 2007-06-20 | Riken | ANTIBODY RECOGNIZING THE REGIONAL SECTION CONTROLLING TGF-BETA ACTIVATION |
| CN1902227A (zh) * | 2003-10-03 | 2007-01-24 | 布赖汉姆妇女医院 | Tim-3配体及其方法 |
| WO2005058358A2 (en) | 2003-12-17 | 2005-06-30 | Nalan Utku | Use of agents derived from ceacam1 for the treatment of inflammatory diseases |
| WO2007063424A2 (en) * | 2005-06-09 | 2007-06-07 | Gal Markel | The modulation of immunity and ceacam1 activity |
| EP2081961A2 (en) | 2006-11-15 | 2009-07-29 | The Brigham and Women's Hospital, Inc. | Therapeutic uses of tim-3 modulators |
| US9416165B2 (en) * | 2007-10-26 | 2016-08-16 | The Regents Of The University Of California | Methods of inhibiting viral replication and improving T cell function employing soluble Tim-3 inhibitors |
| ES2571235T3 (es) * | 2009-04-10 | 2016-05-24 | Kyowa Hakko Kirin Co Ltd | Procedimiento para el tratamiento de un tumor sanguíneo que utiliza el anticuerpo anti-TIM-3 |
| US8598322B2 (en) * | 2009-04-30 | 2013-12-03 | Tel Hashomer Medical Research Infrastucture and Services Ltd. | Anti CEACAM1 antibodies and methods of using same |
| WO2011155607A1 (ja) * | 2010-06-11 | 2011-12-15 | 協和発酵キリン株式会社 | 抗tim-3抗体 |
| US9163087B2 (en) * | 2010-06-18 | 2015-10-20 | The Brigham And Women's Hospital, Inc. | Bi-specific antibodies against TIM-3 and PD-1 for immunotherapy in chronic immune conditions |
| DE102010024636B4 (de) * | 2010-06-22 | 2024-04-18 | Universität Duisburg-Essen | Antikörper, insbesondere für die Diagnostik |
| US9556271B2 (en) | 2011-12-01 | 2017-01-31 | The Brigham And Women's Hospital, Inc. | Anti-CEACAM1 recombinant antibodies for cancer therapy |
-
2013
- 2013-07-30 EP EP13826181.3A patent/EP2879709B1/en active Active
- 2013-07-30 CA CA2916638A patent/CA2916638C/en active Active
- 2013-07-30 JP JP2015525500A patent/JP2015525781A/ja active Pending
- 2013-07-30 WO PCT/US2013/052612 patent/WO2014022332A1/en not_active Ceased
- 2013-07-30 US US14/418,726 patent/US10513540B2/en active Active
- 2013-07-30 EP EP20150590.6A patent/EP3698809A1/en active Pending
-
2019
- 2019-03-14 JP JP2019047201A patent/JP7096186B2/ja active Active
- 2019-11-06 US US16/676,036 patent/US20200095284A1/en not_active Abandoned
-
2020
- 2020-09-28 JP JP2020161836A patent/JP2020200351A/ja active Pending
Patent Citations (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2009502129A (ja) * | 2005-07-22 | 2009-01-29 | ピエール ファーブル メディカモン | 新規の抗igf−ir抗体及びその使用 |
Non-Patent Citations (2)
| Title |
|---|
| NGIOW, S.F. ET AL.: ""Prospects for TIM3-Targeted Antitumor Immunotherapy"", CANCER RES., vol. 71, no. 21, JPN6017018697, 1 November 2011 (2011-11-01), pages 6567 - 6571, ISSN: 0003564040 * |
| SAPOZNIK, S. ET AL.: ""Novel Anti-Melanoma Immunotherapies: Disarming Tumor Escape Mechanisms"", CLIN. DEV. IMMUNOL., vol. Vol.2012,Article ID 818214, JPN6017018698, 23 April 2012 (2012-04-23), pages 1 - 9, ISSN: 0003564041 * |
Also Published As
| Publication number | Publication date |
|---|---|
| CA2916638C (en) | 2021-01-12 |
| WO2014022332A1 (en) | 2014-02-06 |
| CA2916638A1 (en) | 2014-02-06 |
| JP2020200351A (ja) | 2020-12-17 |
| JP7096186B2 (ja) | 2022-07-05 |
| US20200095284A1 (en) | 2020-03-26 |
| HK1211240A1 (en) | 2016-05-20 |
| EP3698809A1 (en) | 2020-08-26 |
| EP2879709A1 (en) | 2015-06-10 |
| US20150225457A1 (en) | 2015-08-13 |
| EP2879709B1 (en) | 2020-01-08 |
| US10513540B2 (en) | 2019-12-24 |
| JP2019112445A (ja) | 2019-07-11 |
| EP2879709A4 (en) | 2016-03-23 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP7096186B2 (ja) | 免疫応答の調節 | |
| JP7467527B2 (ja) | 慢性免疫病に対する免疫治療のためのtim-3およびpd-1に対する二重特異性抗体 | |
| JP6356134B2 (ja) | 免疫応答の増強 | |
| US20240228653A1 (en) | PSGL-1 Antagonists and Uses Thereof | |
| JP2018508483A (ja) | 抗lapモノクローナル抗体による癌の処置 | |
| JP2020038216A (ja) | 免疫応答を調節するための組成物および方法 | |
| CN108064166A (zh) | Slamf1拮抗剂及其用途 | |
| US20180153986A1 (en) | Interactions between ceacam and tim family members | |
| US20180298095A1 (en) | Interactions between ceacam and tim family members | |
| HK1211240B (en) | Modulation of the immune response |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20150407 |
|
| RD04 | Notification of resignation of power of attorney |
Free format text: JAPANESE INTERMEDIATE CODE: A7424 Effective date: 20150610 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20160801 |
|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20160801 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20170525 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20170825 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20171127 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20180402 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20180622 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20180830 |
|
| A02 | Decision of refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A02 Effective date: 20181115 |