JP2008520739A - 長鎖多価不飽和脂肪酸由来のオキシリピンならびにその作製および使用方法 - Google Patents
長鎖多価不飽和脂肪酸由来のオキシリピンならびにその作製および使用方法 Download PDFInfo
- Publication number
- JP2008520739A JP2008520739A JP2007543444A JP2007543444A JP2008520739A JP 2008520739 A JP2008520739 A JP 2008520739A JP 2007543444 A JP2007543444 A JP 2007543444A JP 2007543444 A JP2007543444 A JP 2007543444A JP 2008520739 A JP2008520739 A JP 2008520739A
- Authority
- JP
- Japan
- Prior art keywords
- dpan
- dihydroxy
- oil
- lcpufa
- hydroxy
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Pending
Links
- 238000000034 method Methods 0.000 title claims abstract description 194
- 235000020978 long-chain polyunsaturated fatty acids Nutrition 0.000 title claims description 270
- 238000002360 preparation method Methods 0.000 title description 3
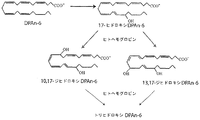
- YUFFSWGQGVEMMI-JLNKQSITSA-N (7Z,10Z,13Z,16Z,19Z)-docosapentaenoic acid Chemical compound CC\C=C/C\C=C/C\C=C/C\C=C/C\C=C/CCCCCC(O)=O YUFFSWGQGVEMMI-JLNKQSITSA-N 0.000 claims abstract description 268
- 239000003921 oil Substances 0.000 claims abstract description 244
- 150000002026 docosanoids Chemical class 0.000 claims abstract description 206
- 235000021294 Docosapentaenoic acid Nutrition 0.000 claims abstract description 154
- 239000000203 mixture Substances 0.000 claims abstract description 147
- 235000021292 Docosatetraenoic acid Nutrition 0.000 claims abstract description 122
- FPRKGXIOSIUDSE-SYACGTDESA-N (2z,4z,6z,8z)-docosa-2,4,6,8-tetraenoic acid Chemical compound CCCCCCCCCCCCC\C=C/C=C\C=C/C=C\C(O)=O FPRKGXIOSIUDSE-SYACGTDESA-N 0.000 claims abstract description 119
- 150000001875 compounds Chemical class 0.000 claims abstract description 98
- 238000004519 manufacturing process Methods 0.000 claims abstract description 73
- -1 C22 fatty acids Chemical class 0.000 claims abstract description 50
- 235000020777 polyunsaturated fatty acids Nutrition 0.000 claims abstract description 38
- 239000000758 substrate Substances 0.000 claims abstract description 28
- 235000016709 nutrition Nutrition 0.000 claims abstract description 21
- 239000002253 acid Substances 0.000 claims abstract description 14
- 230000001225 therapeutic effect Effects 0.000 claims abstract description 14
- 239000002537 cosmetic Substances 0.000 claims abstract description 13
- MBMBGCFOFBJSGT-KUBAVDMBSA-N all-cis-docosa-4,7,10,13,16,19-hexaenoic acid Chemical compound CC\C=C/C\C=C/C\C=C/C\C=C/C\C=C/C\C=C/CCC(O)=O MBMBGCFOFBJSGT-KUBAVDMBSA-N 0.000 claims description 347
- 235000019198 oils Nutrition 0.000 claims description 243
- MZQXAWAWDWCIKG-SPSBLGDNSA-N Avenoleic acid Chemical class CCC[C@@H](O)C\C=C/C\C=C/CCCCCCCC(O)=O MZQXAWAWDWCIKG-SPSBLGDNSA-N 0.000 claims description 240
- 235000020669 docosahexaenoic acid Nutrition 0.000 claims description 178
- 229940090949 docosahexaenoic acid Drugs 0.000 claims description 176
- KFINXCASWPGHEW-UHFFFAOYSA-N (9S*,10R*,11R*,12Z,15Z)-9,10,11-trihydroxyoctadeca-12,15-dienoic acid Natural products CCC=CCC=CC(O)C(O)C(O)CCCCCCCC(O)=O KFINXCASWPGHEW-UHFFFAOYSA-N 0.000 claims description 155
- 102000004190 Enzymes Human genes 0.000 claims description 79
- 108090000790 Enzymes Proteins 0.000 claims description 79
- 241000196324 Embryophyta Species 0.000 claims description 68
- 230000004054 inflammatory process Effects 0.000 claims description 53
- 206010061218 Inflammation Diseases 0.000 claims description 50
- JAZBEHYOTPTENJ-JLNKQSITSA-N all-cis-5,8,11,14,17-icosapentaenoic acid Chemical compound CC\C=C/C\C=C/C\C=C/C\C=C/C\C=C/CCCC(O)=O JAZBEHYOTPTENJ-JLNKQSITSA-N 0.000 claims description 49
- 235000020673 eicosapentaenoic acid Nutrition 0.000 claims description 48
- 238000006243 chemical reaction Methods 0.000 claims description 43
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 claims description 36
- 244000005700 microbiome Species 0.000 claims description 36
- 150000003839 salts Chemical class 0.000 claims description 36
- 201000010099 disease Diseases 0.000 claims description 33
- 239000003795 chemical substances by application Substances 0.000 claims description 29
- 108090000128 Lipoxygenases Proteins 0.000 claims description 26
- 102000003820 Lipoxygenases Human genes 0.000 claims description 26
- 102000009515 Arachidonate 15-Lipoxygenase Human genes 0.000 claims description 25
- 108010048907 Arachidonate 15-lipoxygenase Proteins 0.000 claims description 25
- 241001465754 Metazoa Species 0.000 claims description 24
- 208000024891 symptom Diseases 0.000 claims description 23
- 102000004005 Prostaglandin-endoperoxide synthases Human genes 0.000 claims description 22
- 108090000459 Prostaglandin-endoperoxide synthases Proteins 0.000 claims description 22
- 230000001580 bacterial effect Effects 0.000 claims description 22
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 claims description 21
- 230000008569 process Effects 0.000 claims description 20
- 108010015742 Cytochrome P-450 Enzyme System Proteins 0.000 claims description 19
- 102000002004 Cytochrome P-450 Enzyme System Human genes 0.000 claims description 19
- 230000004770 neurodegeneration Effects 0.000 claims description 19
- 230000003389 potentiating effect Effects 0.000 claims description 19
- 238000000746 purification Methods 0.000 claims description 19
- 239000000126 substance Substances 0.000 claims description 19
- 230000037361 pathway Effects 0.000 claims description 18
- 239000004593 Epoxy Substances 0.000 claims description 17
- 108060008682 Tumor Necrosis Factor Proteins 0.000 claims description 17
- 102000000852 Tumor Necrosis Factor-alpha Human genes 0.000 claims description 17
- 229910052799 carbon Inorganic materials 0.000 claims description 16
- 238000000605 extraction Methods 0.000 claims description 16
- 108010093579 Arachidonate 5-lipoxygenase Proteins 0.000 claims description 14
- 239000003814 drug Substances 0.000 claims description 14
- 235000021588 free fatty acids Nutrition 0.000 claims description 14
- 235000015112 vegetable and seed oil Nutrition 0.000 claims description 14
- BSYNRYMUTXBXSQ-UHFFFAOYSA-N Aspirin Chemical compound CC(=O)OC1=CC=CC=C1C(O)=O BSYNRYMUTXBXSQ-UHFFFAOYSA-N 0.000 claims description 13
- 229960001138 acetylsalicylic acid Drugs 0.000 claims description 13
- 210000002540 macrophage Anatomy 0.000 claims description 13
- 150000003904 phospholipids Chemical class 0.000 claims description 13
- 235000020660 omega-3 fatty acid Nutrition 0.000 claims description 12
- 102000001554 Hemoglobins Human genes 0.000 claims description 11
- 108010054147 Hemoglobins Proteins 0.000 claims description 11
- 230000002255 enzymatic effect Effects 0.000 claims description 10
- 102000011730 Arachidonate 12-Lipoxygenase Human genes 0.000 claims description 9
- 108010076676 Arachidonate 12-lipoxygenase Proteins 0.000 claims description 9
- 108010037462 Cyclooxygenase 2 Proteins 0.000 claims description 9
- 102000010907 Cyclooxygenase 2 Human genes 0.000 claims description 9
- 229940079593 drug Drugs 0.000 claims description 9
- 229960005135 eicosapentaenoic acid Drugs 0.000 claims description 9
- 238000001914 filtration Methods 0.000 claims description 9
- 235000020667 long-chain omega-3 fatty acid Nutrition 0.000 claims description 9
- 239000008158 vegetable oil Substances 0.000 claims description 9
- 241000251468 Actinopterygii Species 0.000 claims description 8
- 210000001744 T-lymphocyte Anatomy 0.000 claims description 8
- 238000001816 cooling Methods 0.000 claims description 8
- JAZBEHYOTPTENJ-UHFFFAOYSA-N eicosapentaenoic acid Natural products CCC=CCC=CCC=CCC=CCC=CCCCC(O)=O JAZBEHYOTPTENJ-UHFFFAOYSA-N 0.000 claims description 8
- 230000000813 microbial effect Effects 0.000 claims description 8
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 claims description 8
- 241000894006 Bacteria Species 0.000 claims description 7
- 239000003963 antioxidant agent Substances 0.000 claims description 7
- 238000004061 bleaching Methods 0.000 claims description 7
- 229940012843 omega-3 fatty acid Drugs 0.000 claims description 7
- 239000003960 organic solvent Substances 0.000 claims description 7
- 239000010775 animal oil Substances 0.000 claims description 6
- 230000003399 chemotactic effect Effects 0.000 claims description 6
- FJNCXZZQNBKEJT-UHFFFAOYSA-N 8beta-hydroxymarrubiin Natural products O1C(=O)C2(C)CCCC3(C)C2C1CC(C)(O)C3(O)CCC=1C=COC=1 FJNCXZZQNBKEJT-UHFFFAOYSA-N 0.000 claims description 5
- 101000668058 Infectious salmon anemia virus (isolate Atlantic salmon/Norway/810/9/99) RNA-directed RNA polymerase catalytic subunit Proteins 0.000 claims description 5
- 230000003078 antioxidant effect Effects 0.000 claims description 5
- 230000001877 deodorizing effect Effects 0.000 claims description 5
- 239000000839 emulsion Substances 0.000 claims description 5
- 239000004090 neuroprotective agent Substances 0.000 claims description 5
- 210000000440 neutrophil Anatomy 0.000 claims description 5
- 150000003626 triacylglycerols Chemical class 0.000 claims description 5
- 229940121710 HMGCoA reductase inhibitor Drugs 0.000 claims description 4
- 102100033075 Prostacyclin synthase Human genes 0.000 claims description 4
- 241000592344 Spermatophyta Species 0.000 claims description 4
- 241001467333 Thraustochytriaceae Species 0.000 claims description 4
- 238000012258 culturing Methods 0.000 claims description 4
- 239000002471 hydroxymethylglutaryl coenzyme A reductase inhibitor Substances 0.000 claims description 4
- 229940021182 non-steroidal anti-inflammatory drug Drugs 0.000 claims description 4
- 230000000087 stabilizing effect Effects 0.000 claims description 4
- 102100024902 Cytochrome P450 4F2 Human genes 0.000 claims description 3
- 101000909122 Homo sapiens Cytochrome P450 4F2 Proteins 0.000 claims description 3
- 238000004587 chromatography analysis Methods 0.000 claims description 3
- 238000004332 deodorization Methods 0.000 claims description 3
- 150000004702 methyl esters Chemical class 0.000 claims description 3
- 238000000926 separation method Methods 0.000 claims description 3
- 230000002194 synthesizing effect Effects 0.000 claims description 3
- DVSZKTAMJJTWFG-SKCDLICFSA-N (2e,4e,6e,8e,10e,12e)-docosa-2,4,6,8,10,12-hexaenoic acid Chemical compound CCCCCCCCC\C=C\C=C\C=C\C=C\C=C\C=C\C(O)=O DVSZKTAMJJTWFG-SKCDLICFSA-N 0.000 claims description 2
- LOGFVTREOLYCPF-KXNHARMFSA-N (2s,3r)-2-[[(2r)-1-[(2s)-2,6-diaminohexanoyl]pyrrolidine-2-carbonyl]amino]-3-hydroxybutanoic acid Chemical compound C[C@@H](O)[C@@H](C(O)=O)NC(=O)[C@H]1CCCN1C(=O)[C@@H](N)CCCCN LOGFVTREOLYCPF-KXNHARMFSA-N 0.000 claims description 2
- MZOFCQQQCNRIBI-VMXHOPILSA-N (3s)-4-[[(2s)-1-[[(2s)-1-[[(1s)-1-carboxy-2-hydroxyethyl]amino]-4-methyl-1-oxopentan-2-yl]amino]-5-(diaminomethylideneamino)-1-oxopentan-2-yl]amino]-3-[[2-[[(2s)-2,6-diaminohexanoyl]amino]acetyl]amino]-4-oxobutanoic acid Chemical compound OC[C@@H](C(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](CC(O)=O)NC(=O)CNC(=O)[C@@H](N)CCCCN MZOFCQQQCNRIBI-VMXHOPILSA-N 0.000 claims description 2
- GZJLLYHBALOKEX-UHFFFAOYSA-N 6-Ketone, O18-Me-Ussuriedine Natural products CC=CCC=CCC=CCC=CCC=CCC=CCCCC(O)=O GZJLLYHBALOKEX-UHFFFAOYSA-N 0.000 claims description 2
- 108010000561 Cytochrome P-450 CYP2C8 Proteins 0.000 claims description 2
- 102100029359 Cytochrome P450 2C8 Human genes 0.000 claims description 2
- 102100027567 Cytochrome P450 4A11 Human genes 0.000 claims description 2
- 102100027419 Cytochrome P450 4B1 Human genes 0.000 claims description 2
- 102100024916 Cytochrome P450 4F11 Human genes 0.000 claims description 2
- 102100024918 Cytochrome P450 4F12 Human genes 0.000 claims description 2
- 102100024901 Cytochrome P450 4F3 Human genes 0.000 claims description 2
- 101000725111 Homo sapiens Cytochrome P450 4A11 Proteins 0.000 claims description 2
- 101000909111 Homo sapiens Cytochrome P450 4F11 Proteins 0.000 claims description 2
- 101000909108 Homo sapiens Cytochrome P450 4F12 Proteins 0.000 claims description 2
- 101000909121 Homo sapiens Cytochrome P450 4F3 Proteins 0.000 claims description 2
- 101000653005 Homo sapiens Thromboxane-A synthase Proteins 0.000 claims description 2
- 102000003777 Interleukin-1 beta Human genes 0.000 claims description 2
- 108090000193 Interleukin-1 beta Proteins 0.000 claims description 2
- 101710179550 Prostacyclin synthase Proteins 0.000 claims description 2
- 102100030973 Thromboxane-A synthase Human genes 0.000 claims description 2
- 238000006701 autoxidation reaction Methods 0.000 claims description 2
- 230000031018 biological processes and functions Effects 0.000 claims description 2
- 108010018719 cytochrome P-450 CYP4B1 Proteins 0.000 claims description 2
- KAUVQQXNCKESLC-UHFFFAOYSA-N docosahexaenoic acid (DHA) Natural products COC(=O)C(C)NOCC1=CC=CC=C1 KAUVQQXNCKESLC-UHFFFAOYSA-N 0.000 claims description 2
- 239000003937 drug carrier Substances 0.000 claims description 2
- 125000001495 ethyl group Chemical group [H]C([H])([H])C([H])([H])* 0.000 claims description 2
- 238000002955 isolation Methods 0.000 claims description 2
- 230000005012 migration Effects 0.000 claims description 2
- 238000013508 migration Methods 0.000 claims description 2
- 108010064377 prostacyclin synthetase Proteins 0.000 claims description 2
- 102100029437 Serine/threonine-protein kinase A-Raf Human genes 0.000 claims 3
- 239000000041 non-steroidal anti-inflammatory agent Substances 0.000 claims 2
- 102000001381 Arachidonate 5-Lipoxygenase Human genes 0.000 claims 1
- CYQFCXCEBYINGO-IAGOWNOFSA-N delta1-THC Chemical compound C1=C(C)CC[C@H]2C(C)(C)OC3=CC(CCCCC)=CC(O)=C3[C@@H]21 CYQFCXCEBYINGO-IAGOWNOFSA-N 0.000 claims 1
- 230000003110 anti-inflammatory effect Effects 0.000 abstract description 45
- 235000014113 dietary fatty acids Nutrition 0.000 abstract description 31
- 229930195729 fatty acid Natural products 0.000 abstract description 31
- 239000000194 fatty acid Substances 0.000 abstract description 31
- 150000007513 acids Chemical class 0.000 abstract description 4
- 230000002555 anti-neurodegenerative effect Effects 0.000 abstract 1
- 239000000047 product Substances 0.000 description 78
- 229940088598 enzyme Drugs 0.000 description 64
- YZXBAPSDXZZRGB-DOFZRALJSA-N arachidonic acid Chemical compound CCCCC\C=C/C\C=C/C\C=C/C\C=C/CCCC(O)=O YZXBAPSDXZZRGB-DOFZRALJSA-N 0.000 description 39
- 238000009472 formulation Methods 0.000 description 32
- 125000002887 hydroxy group Chemical group [H]O* 0.000 description 30
- 230000000694 effects Effects 0.000 description 27
- 241001491670 Labyrinthula Species 0.000 description 26
- 210000004027 cell Anatomy 0.000 description 26
- 150000004665 fatty acids Chemical class 0.000 description 25
- 235000021342 arachidonic acid Nutrition 0.000 description 20
- 229940114079 arachidonic acid Drugs 0.000 description 20
- 239000007795 chemical reaction product Substances 0.000 description 20
- 239000012634 fragment Substances 0.000 description 20
- 108090000623 proteins and genes Proteins 0.000 description 19
- 235000013305 food Nutrition 0.000 description 18
- 230000015572 biosynthetic process Effects 0.000 description 16
- 230000000770 proinflammatory effect Effects 0.000 description 16
- 102100022364 Polyunsaturated fatty acid 5-lipoxygenase Human genes 0.000 description 15
- 238000003786 synthesis reaction Methods 0.000 description 15
- 241000195493 Cryptophyta Species 0.000 description 14
- 102000004127 Cytokines Human genes 0.000 description 14
- 108090000695 Cytokines Proteins 0.000 description 14
- 150000001793 charged compounds Chemical class 0.000 description 14
- 235000015872 dietary supplement Nutrition 0.000 description 14
- OKTJSMMVPCPJKN-UHFFFAOYSA-N Carbon Chemical compound [C] OKTJSMMVPCPJKN-UHFFFAOYSA-N 0.000 description 12
- 210000004712 air sac Anatomy 0.000 description 12
- 150000001993 dienes Chemical class 0.000 description 12
- 150000002066 eicosanoids Chemical class 0.000 description 12
- 150000002148 esters Chemical class 0.000 description 12
- VLKZOEOYAKHREP-UHFFFAOYSA-N n-Hexane Chemical compound CCCCCC VLKZOEOYAKHREP-UHFFFAOYSA-N 0.000 description 12
- 241000894007 species Species 0.000 description 12
- 241001138694 Botryochytrium radiatum Species 0.000 description 11
- 230000009467 reduction Effects 0.000 description 11
- 229940124599 anti-inflammatory drug Drugs 0.000 description 10
- 230000008901 benefit Effects 0.000 description 10
- 230000001965 increasing effect Effects 0.000 description 10
- CGIGDMFJXJATDK-UHFFFAOYSA-N indomethacin Chemical compound CC1=C(CC(O)=O)C2=CC(OC)=CC=C2N1C(=O)C1=CC=C(Cl)C=C1 CGIGDMFJXJATDK-UHFFFAOYSA-N 0.000 description 10
- 230000028327 secretion Effects 0.000 description 10
- 125000001424 substituent group Chemical group 0.000 description 10
- 150000003278 haem Chemical class 0.000 description 9
- 239000000543 intermediate Substances 0.000 description 9
- 230000000324 neuroprotective effect Effects 0.000 description 9
- QTBSBXVTEAMEQO-UHFFFAOYSA-N Acetic acid Chemical compound CC(O)=O QTBSBXVTEAMEQO-UHFFFAOYSA-N 0.000 description 8
- 241000233671 Schizochytrium Species 0.000 description 8
- 235000013361 beverage Nutrition 0.000 description 8
- LOKCTEFSRHRXRJ-UHFFFAOYSA-I dipotassium trisodium dihydrogen phosphate hydrogen phosphate dichloride Chemical compound P(=O)(O)(O)[O-].[K+].P(=O)(O)([O-])[O-].[Na+].[Na+].[Cl-].[K+].[Cl-].[Na+] LOKCTEFSRHRXRJ-UHFFFAOYSA-I 0.000 description 8
- DQRSJOZDBPWTCH-UHFFFAOYSA-N docosa-1,3,5-triene Chemical compound CCCCCCCCCCCCCCCCC=CC=CC=C DQRSJOZDBPWTCH-UHFFFAOYSA-N 0.000 description 8
- 150000002632 lipids Chemical class 0.000 description 8
- 239000002953 phosphate buffered saline Substances 0.000 description 8
- 238000001228 spectrum Methods 0.000 description 8
- 230000009466 transformation Effects 0.000 description 8
- 238000002211 ultraviolet spectrum Methods 0.000 description 8
- 241001467308 Labyrinthuloides Species 0.000 description 7
- 229930184725 Lipoxin Natural products 0.000 description 7
- 241000233675 Thraustochytrium Species 0.000 description 7
- 230000036541 health Effects 0.000 description 7
- 238000002347 injection Methods 0.000 description 7
- 239000007924 injection Substances 0.000 description 7
- 150000002639 lipoxins Chemical class 0.000 description 7
- 239000002207 metabolite Substances 0.000 description 7
- 238000012545 processing Methods 0.000 description 7
- 235000018102 proteins Nutrition 0.000 description 7
- 102000004169 proteins and genes Human genes 0.000 description 7
- 230000002829 reductive effect Effects 0.000 description 7
- 238000010183 spectrum analysis Methods 0.000 description 7
- 238000006467 substitution reaction Methods 0.000 description 7
- 238000004885 tandem mass spectrometry Methods 0.000 description 7
- 239000002028 Biomass Substances 0.000 description 6
- 241001474374 Blennius Species 0.000 description 6
- RTZKZFJDLAIYFH-UHFFFAOYSA-N Diethyl ether Chemical compound CCOCC RTZKZFJDLAIYFH-UHFFFAOYSA-N 0.000 description 6
- 206010030113 Oedema Diseases 0.000 description 6
- 108010030975 Polyketide Synthases Proteins 0.000 description 6
- 102100031950 Polyunsaturated fatty acid lipoxygenase ALOX15 Human genes 0.000 description 6
- 101710164073 Polyunsaturated fatty acid lipoxygenase ALOX15 Proteins 0.000 description 6
- 125000004429 atom Chemical group 0.000 description 6
- 230000009286 beneficial effect Effects 0.000 description 6
- 239000011203 carbon fibre reinforced carbon Substances 0.000 description 6
- 230000003247 decreasing effect Effects 0.000 description 6
- 238000006911 enzymatic reaction Methods 0.000 description 6
- 238000000338 in vitro Methods 0.000 description 6
- 238000001819 mass spectrum Methods 0.000 description 6
- 239000003826 tablet Substances 0.000 description 6
- 210000001519 tissue Anatomy 0.000 description 6
- 241000699666 Mus <mouse, genus> Species 0.000 description 5
- 235000001014 amino acid Nutrition 0.000 description 5
- 150000001413 amino acids Chemical class 0.000 description 5
- 239000002260 anti-inflammatory agent Substances 0.000 description 5
- 230000001028 anti-proliverative effect Effects 0.000 description 5
- 230000012292 cell migration Effects 0.000 description 5
- 239000006071 cream Substances 0.000 description 5
- 235000013365 dairy product Nutrition 0.000 description 5
- 238000001212 derivatisation Methods 0.000 description 5
- 230000002708 enhancing effect Effects 0.000 description 5
- 210000000416 exudates and transudate Anatomy 0.000 description 5
- 210000001508 eye Anatomy 0.000 description 5
- 235000021323 fish oil Nutrition 0.000 description 5
- 235000013350 formula milk Nutrition 0.000 description 5
- 125000000524 functional group Chemical group 0.000 description 5
- 238000001727 in vivo Methods 0.000 description 5
- 229960000905 indomethacin Drugs 0.000 description 5
- 239000007788 liquid Substances 0.000 description 5
- 210000004698 lymphocyte Anatomy 0.000 description 5
- 239000006014 omega-3 oil Substances 0.000 description 5
- 238000007254 oxidation reaction Methods 0.000 description 5
- 229940002612 prodrug Drugs 0.000 description 5
- 239000000651 prodrug Substances 0.000 description 5
- 239000000243 solution Substances 0.000 description 5
- 230000008961 swelling Effects 0.000 description 5
- SWTYBBUBEPPYCX-VIIQGJSXSA-N (4Z,7Z,10Z,13Z,15E,19Z)-17-hydroxydocosahexaenoic acid Chemical compound CC\C=C/CC(O)\C=C\C=C/C\C=C/C\C=C/C\C=C/CCC(O)=O SWTYBBUBEPPYCX-VIIQGJSXSA-N 0.000 description 4
- IJGRMHOSHXDMSA-UHFFFAOYSA-N Atomic nitrogen Chemical compound N#N IJGRMHOSHXDMSA-UHFFFAOYSA-N 0.000 description 4
- CURLTUGMZLYLDI-UHFFFAOYSA-N Carbon dioxide Chemical compound O=C=O CURLTUGMZLYLDI-UHFFFAOYSA-N 0.000 description 4
- 241000233866 Fungi Species 0.000 description 4
- 244000068988 Glycine max Species 0.000 description 4
- 235000010469 Glycine max Nutrition 0.000 description 4
- NTYJJOPFIAHURM-UHFFFAOYSA-N Histamine Chemical compound NCCC1=CN=CN1 NTYJJOPFIAHURM-UHFFFAOYSA-N 0.000 description 4
- 108010074328 Interferon-gamma Proteins 0.000 description 4
- 241001491708 Macrocystis Species 0.000 description 4
- MUBZPKHOEPUJKR-UHFFFAOYSA-N Oxalic acid Chemical compound OC(=O)C(O)=O MUBZPKHOEPUJKR-UHFFFAOYSA-N 0.000 description 4
- 102000004020 Oxygenases Human genes 0.000 description 4
- 108090000417 Oxygenases Proteins 0.000 description 4
- 230000000975 bioactive effect Effects 0.000 description 4
- 230000037396 body weight Effects 0.000 description 4
- 230000016396 cytokine production Effects 0.000 description 4
- 238000000132 electrospray ionisation Methods 0.000 description 4
- 238000010353 genetic engineering Methods 0.000 description 4
- 208000027866 inflammatory disease Diseases 0.000 description 4
- 230000002757 inflammatory effect Effects 0.000 description 4
- 150000002617 leukotrienes Chemical class 0.000 description 4
- 125000001570 methylene group Chemical group [H]C([H])([*:1])[*:2] 0.000 description 4
- 230000000626 neurodegenerative effect Effects 0.000 description 4
- 235000015097 nutrients Nutrition 0.000 description 4
- 230000035764 nutrition Effects 0.000 description 4
- 150000007524 organic acids Chemical class 0.000 description 4
- 230000003647 oxidation Effects 0.000 description 4
- 239000012071 phase Substances 0.000 description 4
- 239000002243 precursor Substances 0.000 description 4
- 150000003180 prostaglandins Chemical class 0.000 description 4
- 230000002633 protecting effect Effects 0.000 description 4
- 239000007858 starting material Substances 0.000 description 4
- 229940124597 therapeutic agent Drugs 0.000 description 4
- VZCYOOQTPOCHFL-UHFFFAOYSA-N trans-butenedioic acid Natural products OC(=O)C=CC(O)=O VZCYOOQTPOCHFL-UHFFFAOYSA-N 0.000 description 4
- CSCPPACGZOOCGX-UHFFFAOYSA-N Acetone Chemical compound CC(C)=O CSCPPACGZOOCGX-UHFFFAOYSA-N 0.000 description 3
- WEVYAHXRMPXWCK-UHFFFAOYSA-N Acetonitrile Chemical compound CC#N WEVYAHXRMPXWCK-UHFFFAOYSA-N 0.000 description 3
- 208000024827 Alzheimer disease Diseases 0.000 description 3
- 101800004538 Bradykinin Proteins 0.000 description 3
- 108010055167 CD59 Antigens Proteins 0.000 description 3
- 102100022002 CD59 glycoprotein Human genes 0.000 description 3
- 102000000013 Chemokine CCL3 Human genes 0.000 description 3
- 241000199914 Dinophyceae Species 0.000 description 3
- 241001462977 Elina Species 0.000 description 3
- XEKOWRVHYACXOJ-UHFFFAOYSA-N Ethyl acetate Chemical compound CCOC(C)=O XEKOWRVHYACXOJ-UHFFFAOYSA-N 0.000 description 3
- VZCYOOQTPOCHFL-OWOJBTEDSA-N Fumaric acid Chemical compound OC(=O)\C=C\C(O)=O VZCYOOQTPOCHFL-OWOJBTEDSA-N 0.000 description 3
- QXZGBUJJYSLZLT-UHFFFAOYSA-N H-Arg-Pro-Pro-Gly-Phe-Ser-Pro-Phe-Arg-OH Natural products NC(N)=NCCCC(N)C(=O)N1CCCC1C(=O)N1C(C(=O)NCC(=O)NC(CC=2C=CC=CC=2)C(=O)NC(CO)C(=O)N2C(CCC2)C(=O)NC(CC=2C=CC=CC=2)C(=O)NC(CCCN=C(N)N)C(O)=O)CCC1 QXZGBUJJYSLZLT-UHFFFAOYSA-N 0.000 description 3
- KFZMGEQAYNKOFK-UHFFFAOYSA-N Isopropanol Chemical compound CC(C)O KFZMGEQAYNKOFK-UHFFFAOYSA-N 0.000 description 3
- 102100035792 Kininogen-1 Human genes 0.000 description 3
- NPPQSCRMBWNHMW-UHFFFAOYSA-N Meprobamate Chemical compound NC(=O)OCC(C)(CCC)COC(N)=O NPPQSCRMBWNHMW-UHFFFAOYSA-N 0.000 description 3
- OKKJLVBELUTLKV-UHFFFAOYSA-N Methanol Chemical compound OC OKKJLVBELUTLKV-UHFFFAOYSA-N 0.000 description 3
- 108700020962 Peroxidase Proteins 0.000 description 3
- 102000003992 Peroxidases Human genes 0.000 description 3
- OFOBLEOULBTSOW-UHFFFAOYSA-N Propanedioic acid Natural products OC(=O)CC(O)=O OFOBLEOULBTSOW-UHFFFAOYSA-N 0.000 description 3
- 238000000692 Student's t-test Methods 0.000 description 3
- 241000251539 Vertebrata <Metazoa> Species 0.000 description 3
- 238000009825 accumulation Methods 0.000 description 3
- 229960000583 acetic acid Drugs 0.000 description 3
- 230000002378 acidificating effect Effects 0.000 description 3
- 239000002671 adjuvant Substances 0.000 description 3
- QXZGBUJJYSLZLT-FDISYFBBSA-N bradykinin Chemical compound NC(=N)NCCC[C@H](N)C(=O)N1CCC[C@H]1C(=O)N1[C@H](C(=O)NCC(=O)N[C@@H](CC=2C=CC=CC=2)C(=O)N[C@@H](CO)C(=O)N2[C@@H](CCC2)C(=O)N[C@@H](CC=2C=CC=CC=2)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O)CCC1 QXZGBUJJYSLZLT-FDISYFBBSA-N 0.000 description 3
- 239000000872 buffer Substances 0.000 description 3
- 239000002775 capsule Substances 0.000 description 3
- 235000013339 cereals Nutrition 0.000 description 3
- KRKNYBCHXYNGOX-UHFFFAOYSA-N citric acid Chemical compound OC(=O)CC(O)(C(O)=O)CC(O)=O KRKNYBCHXYNGOX-UHFFFAOYSA-N 0.000 description 3
- 238000007796 conventional method Methods 0.000 description 3
- 208000035475 disorder Diseases 0.000 description 3
- 239000002552 dosage form Substances 0.000 description 3
- 239000008157 edible vegetable oil Substances 0.000 description 3
- 239000007903 gelatin capsule Substances 0.000 description 3
- 230000002068 genetic effect Effects 0.000 description 3
- 229940093915 gynecological organic acid Drugs 0.000 description 3
- 229910052736 halogen Inorganic materials 0.000 description 3
- 150000002367 halogens Chemical class 0.000 description 3
- 238000004128 high performance liquid chromatography Methods 0.000 description 3
- 125000001867 hydroperoxy group Chemical group [*]OO[H] 0.000 description 3
- 208000015181 infectious disease Diseases 0.000 description 3
- 239000004615 ingredient Substances 0.000 description 3
- 150000007529 inorganic bases Chemical class 0.000 description 3
- 150000002500 ions Chemical class 0.000 description 3
- 238000002514 liquid chromatography mass spectrum Methods 0.000 description 3
- 239000002609 medium Substances 0.000 description 3
- 229910052751 metal Inorganic materials 0.000 description 3
- 239000002184 metal Substances 0.000 description 3
- 230000004048 modification Effects 0.000 description 3
- 238000012986 modification Methods 0.000 description 3
- 210000004498 neuroglial cell Anatomy 0.000 description 3
- 235000020665 omega-6 fatty acid Nutrition 0.000 description 3
- 229940033080 omega-6 fatty acid Drugs 0.000 description 3
- 235000005985 organic acids Nutrition 0.000 description 3
- 239000008194 pharmaceutical composition Substances 0.000 description 3
- 239000000843 powder Substances 0.000 description 3
- CRDZYJSQHCXHEG-SFVBTVKNSA-N protectin D1 Chemical compound CC\C=C/C[C@H](O)\C=C/C=C/C=C/[C@H](O)C\C=C/C\C=C/CCC(O)=O CRDZYJSQHCXHEG-SFVBTVKNSA-N 0.000 description 3
- 230000001105 regulatory effect Effects 0.000 description 3
- 230000001953 sensory effect Effects 0.000 description 3
- 239000007790 solid phase Substances 0.000 description 3
- 235000014347 soups Nutrition 0.000 description 3
- 239000006228 supernatant Substances 0.000 description 3
- 239000013589 supplement Substances 0.000 description 3
- 150000005671 trienes Chemical class 0.000 description 3
- UFTFJSFQGQCHQW-UHFFFAOYSA-N triformin Chemical compound O=COCC(OC=O)COC=O UFTFJSFQGQCHQW-UHFFFAOYSA-N 0.000 description 3
- 230000002792 vascular Effects 0.000 description 3
- 239000003981 vehicle Substances 0.000 description 3
- TWSWSIQAPQLDBP-CGRWFSSPSA-N (7e,10e,13e,16e)-docosa-7,10,13,16-tetraenoic acid Chemical compound CCCCC\C=C\C\C=C\C\C=C\C\C=C\CCCCCC(O)=O TWSWSIQAPQLDBP-CGRWFSSPSA-N 0.000 description 2
- 241000003610 Aplanochytrium Species 0.000 description 2
- 235000004977 Brassica sinapistrum Nutrition 0.000 description 2
- 108700012434 CCL3 Proteins 0.000 description 2
- 201000004624 Dermatitis Diseases 0.000 description 2
- FEWJPZIEWOKRBE-JCYAYHJZSA-N Dextrotartaric acid Chemical compound OC(=O)[C@H](O)[C@@H](O)C(O)=O FEWJPZIEWOKRBE-JCYAYHJZSA-N 0.000 description 2
- 238000008157 ELISA kit Methods 0.000 description 2
- 241000239366 Euphausiacea Species 0.000 description 2
- 230000005526 G1 to G0 transition Effects 0.000 description 2
- IAJILQKETJEXLJ-UHFFFAOYSA-N Galacturonsaeure Natural products O=CC(O)C(O)C(O)C(O)C(O)=O IAJILQKETJEXLJ-UHFFFAOYSA-N 0.000 description 2
- DHMQDGOQFOQNFH-UHFFFAOYSA-N Glycine Chemical compound NCC(O)=O DHMQDGOQFOQNFH-UHFFFAOYSA-N 0.000 description 2
- 239000004471 Glycine Substances 0.000 description 2
- AEMRFAOFKBGASW-UHFFFAOYSA-N Glycolic acid Chemical compound OCC(O)=O AEMRFAOFKBGASW-UHFFFAOYSA-N 0.000 description 2
- 241000282412 Homo Species 0.000 description 2
- 101000620009 Homo sapiens Polyunsaturated fatty acid 5-lipoxygenase Proteins 0.000 description 2
- VEXZGXHMUGYJMC-UHFFFAOYSA-N Hydrochloric acid Chemical compound Cl VEXZGXHMUGYJMC-UHFFFAOYSA-N 0.000 description 2
- 108010093096 Immobilized Enzymes Proteins 0.000 description 2
- 102100037850 Interferon gamma Human genes 0.000 description 2
- 102000008070 Interferon-gamma Human genes 0.000 description 2
- 102000000589 Interleukin-1 Human genes 0.000 description 2
- 108010002352 Interleukin-1 Proteins 0.000 description 2
- 108010065805 Interleukin-12 Proteins 0.000 description 2
- 102000004125 Interleukin-1alpha Human genes 0.000 description 2
- 108010082786 Interleukin-1alpha Proteins 0.000 description 2
- 108090001005 Interleukin-6 Proteins 0.000 description 2
- XEEYBQQBJWHFJM-UHFFFAOYSA-N Iron Chemical compound [Fe] XEEYBQQBJWHFJM-UHFFFAOYSA-N 0.000 description 2
- 241000003482 Japonochytrium Species 0.000 description 2
- 241001491672 Labyrinthulaceae Species 0.000 description 2
- 239000004472 Lysine Substances 0.000 description 2
- 102000008109 Mixed Function Oxygenases Human genes 0.000 description 2
- 108010074633 Mixed Function Oxygenases Proteins 0.000 description 2
- 241000699670 Mus sp. Species 0.000 description 2
- 208000002193 Pain Diseases 0.000 description 2
- NBIIXXVUZAFLBC-UHFFFAOYSA-N Phosphoric acid Chemical compound OP(O)(O)=O NBIIXXVUZAFLBC-UHFFFAOYSA-N 0.000 description 2
- 206010037660 Pyrexia Diseases 0.000 description 2
- LCTONWCANYUPML-UHFFFAOYSA-N Pyruvic acid Chemical compound CC(=O)C(O)=O LCTONWCANYUPML-UHFFFAOYSA-N 0.000 description 2
- LOUPRKONTZGTKE-WZBLMQSHSA-N Quinine Chemical compound C([C@H]([C@H](C1)C=C)C2)C[N@@]1[C@@H]2[C@H](O)C1=CC=NC2=CC=C(OC)C=C21 LOUPRKONTZGTKE-WZBLMQSHSA-N 0.000 description 2
- 241000700159 Rattus Species 0.000 description 2
- 241000598397 Schizochytrium sp. Species 0.000 description 2
- 206010040047 Sepsis Diseases 0.000 description 2
- VYPSYNLAJGMNEJ-UHFFFAOYSA-N Silicium dioxide Chemical compound O=[Si]=O VYPSYNLAJGMNEJ-UHFFFAOYSA-N 0.000 description 2
- QAOWNCQODCNURD-UHFFFAOYSA-N Sulfuric acid Chemical compound OS(O)(=O)=O QAOWNCQODCNURD-UHFFFAOYSA-N 0.000 description 2
- 241000144181 Thraustochytrium aureum Species 0.000 description 2
- 102100040247 Tumor necrosis factor Human genes 0.000 description 2
- 206010067584 Type 1 diabetes mellitus Diseases 0.000 description 2
- 241001491678 Ulkenia Species 0.000 description 2
- 241000700605 Viruses Species 0.000 description 2
- 201000006793 Walker-Warburg syndrome Diseases 0.000 description 2
- 238000010521 absorption reaction Methods 0.000 description 2
- 125000002252 acyl group Chemical group 0.000 description 2
- 230000006978 adaptation Effects 0.000 description 2
- TWSWSIQAPQLDBP-UHFFFAOYSA-N adrenic acid Natural products CCCCCC=CCC=CCC=CCC=CCCCCCC(O)=O TWSWSIQAPQLDBP-UHFFFAOYSA-N 0.000 description 2
- 150000001299 aldehydes Chemical class 0.000 description 2
- 229910052784 alkaline earth metal Inorganic materials 0.000 description 2
- 150000001342 alkaline earth metals Chemical class 0.000 description 2
- 125000000217 alkyl group Chemical group 0.000 description 2
- 125000003277 amino group Chemical group 0.000 description 2
- 206010002026 amyotrophic lateral sclerosis Diseases 0.000 description 2
- 238000004458 analytical method Methods 0.000 description 2
- 229940121363 anti-inflammatory agent Drugs 0.000 description 2
- 229940053200 antiepileptics fatty acid derivative Drugs 0.000 description 2
- 208000006673 asthma Diseases 0.000 description 2
- 239000002585 base Substances 0.000 description 2
- WPYMKLBDIGXBTP-UHFFFAOYSA-N benzoic acid Chemical compound OC(=O)C1=CC=CC=C1 WPYMKLBDIGXBTP-UHFFFAOYSA-N 0.000 description 2
- 230000008033 biological extinction Effects 0.000 description 2
- 229910021538 borax Inorganic materials 0.000 description 2
- 210000004556 brain Anatomy 0.000 description 2
- 239000011575 calcium Substances 0.000 description 2
- 239000001569 carbon dioxide Substances 0.000 description 2
- 229910002092 carbon dioxide Inorganic materials 0.000 description 2
- 239000000969 carrier Substances 0.000 description 2
- 230000015556 catabolic process Effects 0.000 description 2
- 238000005119 centrifugation Methods 0.000 description 2
- 230000001684 chronic effect Effects 0.000 description 2
- 235000009508 confectionery Nutrition 0.000 description 2
- 238000006731 degradation reaction Methods 0.000 description 2
- 238000013461 design Methods 0.000 description 2
- 235000005911 diet Nutrition 0.000 description 2
- 230000037213 diet Effects 0.000 description 2
- 238000001035 drying Methods 0.000 description 2
- 235000013601 eggs Nutrition 0.000 description 2
- 238000010828 elution Methods 0.000 description 2
- 238000005516 engineering process Methods 0.000 description 2
- 210000003414 extremity Anatomy 0.000 description 2
- 238000000855 fermentation Methods 0.000 description 2
- 230000004151 fermentation Effects 0.000 description 2
- 239000012467 final product Substances 0.000 description 2
- 235000004426 flaxseed Nutrition 0.000 description 2
- 238000005194 fractionation Methods 0.000 description 2
- 235000011389 fruit/vegetable juice Nutrition 0.000 description 2
- 239000007789 gas Substances 0.000 description 2
- 239000008187 granular material Substances 0.000 description 2
- 230000012010 growth Effects 0.000 description 2
- 238000003306 harvesting Methods 0.000 description 2
- 229960001340 histamine Drugs 0.000 description 2
- 239000001257 hydrogen Substances 0.000 description 2
- 229910052739 hydrogen Inorganic materials 0.000 description 2
- 125000004435 hydrogen atom Chemical group [H]* 0.000 description 2
- 201000008319 inclusion body myositis Diseases 0.000 description 2
- 230000002401 inhibitory effect Effects 0.000 description 2
- 230000005764 inhibitory process Effects 0.000 description 2
- 229910052500 inorganic mineral Inorganic materials 0.000 description 2
- 229960003130 interferon gamma Drugs 0.000 description 2
- 150000002576 ketones Chemical class 0.000 description 2
- 208000014987 limb edema Diseases 0.000 description 2
- 210000004185 liver Anatomy 0.000 description 2
- 150000004668 long chain fatty acids Chemical class 0.000 description 2
- 235000010598 long-chain omega-6 fatty acid Nutrition 0.000 description 2
- 208000002780 macular degeneration Diseases 0.000 description 2
- VZCYOOQTPOCHFL-UPHRSURJSA-N maleic acid Chemical compound OC(=O)\C=C/C(O)=O VZCYOOQTPOCHFL-UPHRSURJSA-N 0.000 description 2
- 235000013372 meat Nutrition 0.000 description 2
- 230000002503 metabolic effect Effects 0.000 description 2
- 230000004060 metabolic process Effects 0.000 description 2
- 150000002739 metals Chemical class 0.000 description 2
- QPJVMBTYPHYUOC-UHFFFAOYSA-N methyl benzoate Chemical compound COC(=O)C1=CC=CC=C1 QPJVMBTYPHYUOC-UHFFFAOYSA-N 0.000 description 2
- 239000011707 mineral Substances 0.000 description 2
- 150000007522 mineralic acids Chemical class 0.000 description 2
- 238000002156 mixing Methods 0.000 description 2
- 201000006417 multiple sclerosis Diseases 0.000 description 2
- 125000000449 nitro group Chemical group [O-][N+](*)=O 0.000 description 2
- 229910052757 nitrogen Inorganic materials 0.000 description 2
- 150000007530 organic bases Chemical class 0.000 description 2
- 239000007800 oxidant agent Substances 0.000 description 2
- 150000002924 oxiranes Chemical class 0.000 description 2
- 229940094443 oxytocics prostaglandins Drugs 0.000 description 2
- 239000002245 particle Substances 0.000 description 2
- 150000002978 peroxides Chemical group 0.000 description 2
- 239000003614 peroxisome proliferator Substances 0.000 description 2
- 239000000546 pharmaceutical excipient Substances 0.000 description 2
- 230000002265 prevention Effects 0.000 description 2
- 150000003138 primary alcohols Chemical class 0.000 description 2
- 125000006239 protecting group Chemical group 0.000 description 2
- 239000002994 raw material Substances 0.000 description 2
- 239000011541 reaction mixture Substances 0.000 description 2
- 238000011160 research Methods 0.000 description 2
- 208000023504 respiratory system disease Diseases 0.000 description 2
- YGSDEFSMJLZEOE-UHFFFAOYSA-N salicylic acid Chemical compound OC(=O)C1=CC=CC=C1O YGSDEFSMJLZEOE-UHFFFAOYSA-N 0.000 description 2
- 229930000044 secondary metabolite Natural products 0.000 description 2
- 239000000741 silica gel Substances 0.000 description 2
- 229910002027 silica gel Inorganic materials 0.000 description 2
- 235000010339 sodium tetraborate Nutrition 0.000 description 2
- 239000012453 solvate Substances 0.000 description 2
- 150000003431 steroids Chemical class 0.000 description 2
- 238000003756 stirring Methods 0.000 description 2
- 238000010189 synthetic method Methods 0.000 description 2
- 238000012360 testing method Methods 0.000 description 2
- 238000004809 thin layer chromatography Methods 0.000 description 2
- 150000003595 thromboxanes Chemical class 0.000 description 2
- JOXIMZWYDAKGHI-UHFFFAOYSA-N toluene-4-sulfonic acid Chemical compound CC1=CC=C(S(O)(=O)=O)C=C1 JOXIMZWYDAKGHI-UHFFFAOYSA-N 0.000 description 2
- BSVBQGMMJUBVOD-UHFFFAOYSA-N trisodium borate Chemical compound [Na+].[Na+].[Na+].[O-]B([O-])[O-] BSVBQGMMJUBVOD-UHFFFAOYSA-N 0.000 description 2
- DCXXMTOCNZCJGO-UHFFFAOYSA-N tristearoylglycerol Chemical compound CCCCCCCCCCCCCCCCCC(=O)OCC(OC(=O)CCCCCCCCCCCCCCCCC)COC(=O)CCCCCCCCCCCCCCCCC DCXXMTOCNZCJGO-UHFFFAOYSA-N 0.000 description 2
- 239000013598 vector Substances 0.000 description 2
- 239000011782 vitamin Substances 0.000 description 2
- 229930003231 vitamin Natural products 0.000 description 2
- 229940088594 vitamin Drugs 0.000 description 2
- QBYIENPQHBMVBV-HFEGYEGKSA-N (2R)-2-hydroxy-2-phenylacetic acid Chemical compound O[C@@H](C(O)=O)c1ccccc1.O[C@@H](C(O)=O)c1ccccc1 QBYIENPQHBMVBV-HFEGYEGKSA-N 0.000 description 1
- MJYQFWSXKFLTAY-OVEQLNGDSA-N (2r,3r)-2,3-bis[(4-hydroxy-3-methoxyphenyl)methyl]butane-1,4-diol;(2r,3r,4s,5s,6r)-6-(hydroxymethyl)oxane-2,3,4,5-tetrol Chemical compound OC[C@H]1O[C@@H](O)[C@H](O)[C@@H](O)[C@@H]1O.C1=C(O)C(OC)=CC(C[C@@H](CO)[C@H](CO)CC=2C=C(OC)C(O)=CC=2)=C1 MJYQFWSXKFLTAY-OVEQLNGDSA-N 0.000 description 1
- LDDMACCNBZAMSG-BDVNFPICSA-N (2r,3r,4s,5r)-3,4,5,6-tetrahydroxy-2-(methylamino)hexanal Chemical compound CN[C@@H](C=O)[C@@H](O)[C@H](O)[C@H](O)CO LDDMACCNBZAMSG-BDVNFPICSA-N 0.000 description 1
- YOVXRIACERVBAG-AJQRHIRFSA-N (3e,5e)-6-hydroxy-2-oxo-6-phenylhexa-3,5-dienoic acid Chemical compound OC(=O)C(=O)\C=C\C=C(\O)C1=CC=CC=C1 YOVXRIACERVBAG-AJQRHIRFSA-N 0.000 description 1
- OYHQOLUKZRVURQ-NTGFUMLPSA-N (9Z,12Z)-9,10,12,13-tetratritiooctadeca-9,12-dienoic acid Chemical compound C(CCCCCCC\C(=C(/C\C(=C(/CCCCC)\[3H])\[3H])\[3H])\[3H])(=O)O OYHQOLUKZRVURQ-NTGFUMLPSA-N 0.000 description 1
- WBYWAXJHAXSJNI-VOTSOKGWSA-M .beta-Phenylacrylic acid Natural products [O-]C(=O)\C=C\C1=CC=CC=C1 WBYWAXJHAXSJNI-VOTSOKGWSA-M 0.000 description 1
- TZCPCKNHXULUIY-RGULYWFUSA-N 1,2-distearoyl-sn-glycero-3-phosphoserine Chemical compound CCCCCCCCCCCCCCCCCC(=O)OC[C@H](COP(O)(=O)OC[C@H](N)C(O)=O)OC(=O)CCCCCCCCCCCCCCCCC TZCPCKNHXULUIY-RGULYWFUSA-N 0.000 description 1
- GMRQFYUYWCNGIN-ZVUFCXRFSA-N 1,25-dihydroxy vitamin D3 Chemical compound C1([C@@H]2CC[C@@H]([C@]2(CCC1)C)[C@@H](CCCC(C)(C)O)C)=CC=C1C[C@@H](O)C[C@H](O)C1=C GMRQFYUYWCNGIN-ZVUFCXRFSA-N 0.000 description 1
- LTERDCBCHFKFRI-BGKMTWLOSA-N 11-HDoHE Chemical compound CC\C=C/C\C=C/C\C=C/CC(O)\C=C\C=C/C\C=C/CCC(O)=O LTERDCBCHFKFRI-BGKMTWLOSA-N 0.000 description 1
- VQLZUPRMDSZAFO-UHFFFAOYSA-N 13,14-dihydroxydocosa-2,4,6,8,10-pentaenoic acid Chemical compound CCCCCCCCC(O)C(O)CC=CC=CC=CC=CC=CC(O)=O VQLZUPRMDSZAFO-UHFFFAOYSA-N 0.000 description 1
- WLKCSMCLEKGITB-MXTKCVSJSA-N 15(R)-HEPE Chemical compound CC\C=C/C[C@@H](O)\C=C\C=C/C\C=C/C\C=C/CCCC(O)=O WLKCSMCLEKGITB-MXTKCVSJSA-N 0.000 description 1
- SWTYBBUBEPPYCX-NGHKTGSUSA-N 17(R)-HDoHE Chemical compound CC\C=C/C[C@@H](O)\C=C\C=C/C\C=C/C\C=C/C\C=C/CCC(O)=O SWTYBBUBEPPYCX-NGHKTGSUSA-N 0.000 description 1
- LRWYBGFSVUBWMO-UAAZXLHOSA-N 18(R)-HEPE Chemical compound CC[C@@H](O)\C=C\C=C/C\C=C/C\C=C/C\C=C/CCCC(O)=O LRWYBGFSVUBWMO-UAAZXLHOSA-N 0.000 description 1
- HCSBTDBGTNZOAB-UHFFFAOYSA-N 2,3-dinitrobenzoic acid Chemical compound OC(=O)C1=CC=CC([N+]([O-])=O)=C1[N+]([O-])=O HCSBTDBGTNZOAB-UHFFFAOYSA-N 0.000 description 1
- HZAXFHJVJLSVMW-UHFFFAOYSA-N 2-Aminoethan-1-ol Chemical class NCCO HZAXFHJVJLSVMW-UHFFFAOYSA-N 0.000 description 1
- QKNYBSVHEMOAJP-UHFFFAOYSA-N 2-amino-2-(hydroxymethyl)propane-1,3-diol;hydron;chloride Chemical compound Cl.OCC(N)(CO)CO QKNYBSVHEMOAJP-UHFFFAOYSA-N 0.000 description 1
- GRWKNBPOGBTZMN-UHFFFAOYSA-N 2-benzyl-3-phenylpropane-1,2-diamine Chemical class C=1C=CC=CC=1CC(N)(CN)CC1=CC=CC=C1 GRWKNBPOGBTZMN-UHFFFAOYSA-N 0.000 description 1
- JIXCQMYEHYPJOI-UHFFFAOYSA-N 2-pentadeca-10,12,14-trienyl-3-pentyloxirane Chemical compound CCCCCC1OC1CCCCCCCCCC=CC=CC=C JIXCQMYEHYPJOI-UHFFFAOYSA-N 0.000 description 1
- ZSLUVFAKFWKJRC-IGMARMGPSA-N 232Th Chemical compound [232Th] ZSLUVFAKFWKJRC-IGMARMGPSA-N 0.000 description 1
- BMYNFMYTOJXKLE-UHFFFAOYSA-N 3-azaniumyl-2-hydroxypropanoate Chemical compound NCC(O)C(O)=O BMYNFMYTOJXKLE-UHFFFAOYSA-N 0.000 description 1
- SJZRECIVHVDYJC-UHFFFAOYSA-N 4-hydroxybutyric acid Chemical compound OCCCC(O)=O SJZRECIVHVDYJC-UHFFFAOYSA-N 0.000 description 1
- OBKXEAXTFZPCHS-UHFFFAOYSA-N 4-phenylbutyric acid Chemical compound OC(=O)CCCC1=CC=CC=C1 OBKXEAXTFZPCHS-UHFFFAOYSA-N 0.000 description 1
- OZXAIGIRPOOJTI-VLGMZSPHSA-N 7-hdohe Chemical compound CC\C=C/C\C=C/C\C=C/C\C=C/C=C\C(O)C\C=C/CCC(O)=O OZXAIGIRPOOJTI-VLGMZSPHSA-N 0.000 description 1
- QTBSBXVTEAMEQO-UHFFFAOYSA-M Acetate Chemical compound CC([O-])=O QTBSBXVTEAMEQO-UHFFFAOYSA-M 0.000 description 1
- 208000002874 Acne Vulgaris Diseases 0.000 description 1
- NIXOWILDQLNWCW-UHFFFAOYSA-M Acrylate Chemical compound [O-]C(=O)C=C NIXOWILDQLNWCW-UHFFFAOYSA-M 0.000 description 1
- 102100022089 Acyl-[acyl-carrier-protein] hydrolase Human genes 0.000 description 1
- 208000026872 Addison Disease Diseases 0.000 description 1
- QGZKDVFQNNGYKY-UHFFFAOYSA-O Ammonium Chemical class [NH4+] QGZKDVFQNNGYKY-UHFFFAOYSA-O 0.000 description 1
- USFZMSVCRYTOJT-UHFFFAOYSA-N Ammonium acetate Chemical compound N.CC(O)=O USFZMSVCRYTOJT-UHFFFAOYSA-N 0.000 description 1
- 239000005695 Ammonium acetate Substances 0.000 description 1
- 208000019901 Anxiety disease Diseases 0.000 description 1
- 239000004475 Arginine Substances 0.000 description 1
- 208000006820 Arthralgia Diseases 0.000 description 1
- 208000012657 Atopic disease Diseases 0.000 description 1
- 208000006096 Attention Deficit Disorder with Hyperactivity Diseases 0.000 description 1
- 208000036864 Attention deficit/hyperactivity disease Diseases 0.000 description 1
- 241000003595 Aurantiochytrium limacinum Species 0.000 description 1
- 206010003805 Autism Diseases 0.000 description 1
- 208000020706 Autistic disease Diseases 0.000 description 1
- 208000023275 Autoimmune disease Diseases 0.000 description 1
- 241000206761 Bacillariophyta Species 0.000 description 1
- 239000005711 Benzoic acid Substances 0.000 description 1
- 208000020925 Bipolar disease Diseases 0.000 description 1
- LSNNMFCWUKXFEE-UHFFFAOYSA-M Bisulfite Chemical compound OS([O-])=O LSNNMFCWUKXFEE-UHFFFAOYSA-M 0.000 description 1
- 108091003079 Bovine Serum Albumin Proteins 0.000 description 1
- 235000014698 Brassica juncea var multisecta Nutrition 0.000 description 1
- 240000002791 Brassica napus Species 0.000 description 1
- 235000006008 Brassica napus var napus Nutrition 0.000 description 1
- 235000006618 Brassica rapa subsp oleifera Nutrition 0.000 description 1
- 244000188595 Brassica sinapistrum Species 0.000 description 1
- CPELXLSAUQHCOX-UHFFFAOYSA-M Bromide Chemical compound [Br-] CPELXLSAUQHCOX-UHFFFAOYSA-M 0.000 description 1
- 102100021943 C-C motif chemokine 2 Human genes 0.000 description 1
- 101710155857 C-C motif chemokine 2 Proteins 0.000 description 1
- 101710155856 C-C motif chemokine 3 Proteins 0.000 description 1
- IXAQOQZEOGMIQS-JEWNPAEBSA-M CCCCC[C@@H](O)\C=C\C=C/C=C/C=C/[C@@H](O)[C@@H](O)CCCC([O-])=O Chemical compound CCCCC[C@@H](O)\C=C\C=C/C=C/C=C/[C@@H](O)[C@@H](O)CCCC([O-])=O IXAQOQZEOGMIQS-JEWNPAEBSA-M 0.000 description 1
- OYPRJOBELJOOCE-UHFFFAOYSA-N Calcium Chemical compound [Ca] OYPRJOBELJOOCE-UHFFFAOYSA-N 0.000 description 1
- OKTJSMMVPCPJKN-OUBTZVSYSA-N Carbon-13 Chemical compound [13C] OKTJSMMVPCPJKN-OUBTZVSYSA-N 0.000 description 1
- 208000024172 Cardiovascular disease Diseases 0.000 description 1
- 244000020518 Carthamus tinctorius Species 0.000 description 1
- 235000003255 Carthamus tinctorius Nutrition 0.000 description 1
- 206010053684 Cerebrohepatorenal syndrome Diseases 0.000 description 1
- 241000283153 Cetacea Species 0.000 description 1
- 208000010693 Charcot-Marie-Tooth Disease Diseases 0.000 description 1
- VEXZGXHMUGYJMC-UHFFFAOYSA-M Chloride anion Chemical compound [Cl-] VEXZGXHMUGYJMC-UHFFFAOYSA-M 0.000 description 1
- 241000195628 Chlorophyta Species 0.000 description 1
- 235000001258 Cinchona calisaya Nutrition 0.000 description 1
- WBYWAXJHAXSJNI-SREVYHEPSA-N Cinnamic acid Chemical compound OC(=O)\C=C/C1=CC=CC=C1 WBYWAXJHAXSJNI-SREVYHEPSA-N 0.000 description 1
- KRKNYBCHXYNGOX-UHFFFAOYSA-K Citrate Chemical compound [O-]C(=O)CC(O)(CC([O-])=O)C([O-])=O KRKNYBCHXYNGOX-UHFFFAOYSA-K 0.000 description 1
- 208000015943 Coeliac disease Diseases 0.000 description 1
- 241001633026 Coenocystis Species 0.000 description 1
- 206010010904 Convulsion Diseases 0.000 description 1
- RYGMFSIKBFXOCR-UHFFFAOYSA-N Copper Chemical compound [Cu] RYGMFSIKBFXOCR-UHFFFAOYSA-N 0.000 description 1
- 201000006306 Cor pulmonale Diseases 0.000 description 1
- 241000392488 Corallochytrium Species 0.000 description 1
- 208000011231 Crohn disease Diseases 0.000 description 1
- 241000238424 Crustacea Species 0.000 description 1
- 241000199913 Crypthecodinium Species 0.000 description 1
- 241000199912 Crypthecodinium cohnii Species 0.000 description 1
- 201000003883 Cystic fibrosis Diseases 0.000 description 1
- 102100031461 Cytochrome P450 2J2 Human genes 0.000 description 1
- 102100024899 Cytochrome P450 4F8 Human genes 0.000 description 1
- 102100022028 Cytochrome P450 4V2 Human genes 0.000 description 1
- 102100022027 Cytochrome P450 4X1 Human genes 0.000 description 1
- 108020004414 DNA Proteins 0.000 description 1
- 241000238557 Decapoda Species 0.000 description 1
- 208000019505 Deglutition disease Diseases 0.000 description 1
- 206010012289 Dementia Diseases 0.000 description 1
- BWLUMTFWVZZZND-UHFFFAOYSA-N Dibenzylamine Chemical class C=1C=CC=CC=1CNCC1=CC=CC=C1 BWLUMTFWVZZZND-UHFFFAOYSA-N 0.000 description 1
- 201000010374 Down Syndrome Diseases 0.000 description 1
- 239000012591 Dulbecco’s Phosphate Buffered Saline Substances 0.000 description 1
- KCXVZYZYPLLWCC-UHFFFAOYSA-N EDTA Chemical compound OC(=O)CN(CC(O)=O)CCN(CC(O)=O)CC(O)=O KCXVZYZYPLLWCC-UHFFFAOYSA-N 0.000 description 1
- 238000002965 ELISA Methods 0.000 description 1
- 108010039731 Fatty Acid Synthases Proteins 0.000 description 1
- BDAGIHXWWSANSR-UHFFFAOYSA-M Formate Chemical compound [O-]C=O BDAGIHXWWSANSR-UHFFFAOYSA-M 0.000 description 1
- 238000005863 Friedel-Crafts acylation reaction Methods 0.000 description 1
- 108010010803 Gelatin Proteins 0.000 description 1
- WHUUTDBJXJRKMK-UHFFFAOYSA-N Glutamic acid Natural products OC(=O)C(N)CCC(O)=O WHUUTDBJXJRKMK-UHFFFAOYSA-N 0.000 description 1
- ZWZWYGMENQVNFU-UHFFFAOYSA-N Glycerophosphorylserin Natural products OC(=O)C(N)COP(O)(=O)OCC(O)CO ZWZWYGMENQVNFU-UHFFFAOYSA-N 0.000 description 1
- AEMRFAOFKBGASW-UHFFFAOYSA-M Glycolate Chemical compound OCC([O-])=O AEMRFAOFKBGASW-UHFFFAOYSA-M 0.000 description 1
- 108010017213 Granulocyte-Macrophage Colony-Stimulating Factor Proteins 0.000 description 1
- 102100039620 Granulocyte-macrophage colony-stimulating factor Human genes 0.000 description 1
- 208000030836 Hashimoto thyroiditis Diseases 0.000 description 1
- 206010019233 Headaches Diseases 0.000 description 1
- 244000020551 Helianthus annuus Species 0.000 description 1
- 235000003222 Helianthus annuus Nutrition 0.000 description 1
- 101000941723 Homo sapiens Cytochrome P450 2J2 Proteins 0.000 description 1
- 101000909112 Homo sapiens Cytochrome P450 4F8 Proteins 0.000 description 1
- 101000896951 Homo sapiens Cytochrome P450 4V2 Proteins 0.000 description 1
- 208000023105 Huntington disease Diseases 0.000 description 1
- OAKJQQAXSVQMHS-UHFFFAOYSA-N Hydrazine Chemical compound NN OAKJQQAXSVQMHS-UHFFFAOYSA-N 0.000 description 1
- MHAJPDPJQMAIIY-UHFFFAOYSA-N Hydrogen peroxide Chemical compound OO MHAJPDPJQMAIIY-UHFFFAOYSA-N 0.000 description 1
- 206010058490 Hyperoxia Diseases 0.000 description 1
- 206010020751 Hypersensitivity Diseases 0.000 description 1
- DGAQECJNVWCQMB-PUAWFVPOSA-M Ilexoside XXIX Chemical compound C[C@@H]1CC[C@@]2(CC[C@@]3(C(=CC[C@H]4[C@]3(CC[C@@H]5[C@@]4(CC[C@@H](C5(C)C)OS(=O)(=O)[O-])C)C)[C@@H]2[C@]1(C)O)C)C(=O)O[C@H]6[C@@H]([C@H]([C@@H]([C@H](O6)CO)O)O)O.[Na+] DGAQECJNVWCQMB-PUAWFVPOSA-M 0.000 description 1
- 208000022559 Inflammatory bowel disease Diseases 0.000 description 1
- 102000003816 Interleukin-13 Human genes 0.000 description 1
- 108090000176 Interleukin-13 Proteins 0.000 description 1
- 102000004388 Interleukin-4 Human genes 0.000 description 1
- 108090000978 Interleukin-4 Proteins 0.000 description 1
- 108090001007 Interleukin-8 Proteins 0.000 description 1
- 241001441073 Japonochytrium marinum Species 0.000 description 1
- 206010023230 Joint stiffness Diseases 0.000 description 1
- AHLPHDHHMVZTML-BYPYZUCNSA-N L-Ornithine Chemical compound NCCC[C@H](N)C(O)=O AHLPHDHHMVZTML-BYPYZUCNSA-N 0.000 description 1
- ZGUNAGUHMKGQNY-ZETCQYMHSA-N L-alpha-phenylglycine zwitterion Chemical compound OC(=O)[C@@H](N)C1=CC=CC=C1 ZGUNAGUHMKGQNY-ZETCQYMHSA-N 0.000 description 1
- ODKSFYDXXFIFQN-BYPYZUCNSA-P L-argininium(2+) Chemical compound NC(=[NH2+])NCCC[C@H]([NH3+])C(O)=O ODKSFYDXXFIFQN-BYPYZUCNSA-P 0.000 description 1
- CKLJMWTZIZZHCS-REOHCLBHSA-N L-aspartic acid Chemical compound OC(=O)[C@@H](N)CC(O)=O CKLJMWTZIZZHCS-REOHCLBHSA-N 0.000 description 1
- WHUUTDBJXJRKMK-VKHMYHEASA-N L-glutamic acid Chemical compound OC(=O)[C@@H](N)CCC(O)=O WHUUTDBJXJRKMK-VKHMYHEASA-N 0.000 description 1
- HNDVDQJCIGZPNO-YFKPBYRVSA-N L-histidine Chemical compound OC(=O)[C@@H](N)CC1=CN=CN1 HNDVDQJCIGZPNO-YFKPBYRVSA-N 0.000 description 1
- KDXKERNSBIXSRK-YFKPBYRVSA-N L-lysine Chemical compound NCCCC[C@H](N)C(O)=O KDXKERNSBIXSRK-YFKPBYRVSA-N 0.000 description 1
- 241001467310 Labyrinthuloides minuta Species 0.000 description 1
- JVTAAEKCZFNVCJ-UHFFFAOYSA-M Lactate Chemical compound CC(O)C([O-])=O JVTAAEKCZFNVCJ-UHFFFAOYSA-M 0.000 description 1
- 240000006240 Linum usitatissimum Species 0.000 description 1
- WHXSMMKQMYFTQS-UHFFFAOYSA-N Lithium Chemical compound [Li] WHXSMMKQMYFTQS-UHFFFAOYSA-N 0.000 description 1
- 102000004317 Lyases Human genes 0.000 description 1
- 108090000856 Lyases Proteins 0.000 description 1
- KDXKERNSBIXSRK-UHFFFAOYSA-N Lysine Natural products NCCCCC(N)C(O)=O KDXKERNSBIXSRK-UHFFFAOYSA-N 0.000 description 1
- FYYHWMGAXLPEAU-UHFFFAOYSA-N Magnesium Chemical compound [Mg] FYYHWMGAXLPEAU-UHFFFAOYSA-N 0.000 description 1
- OFOBLEOULBTSOW-UHFFFAOYSA-L Malonate Chemical compound [O-]C(=O)CC([O-])=O OFOBLEOULBTSOW-UHFFFAOYSA-L 0.000 description 1
- 241000124008 Mammalia Species 0.000 description 1
- 208000001145 Metabolic Syndrome Diseases 0.000 description 1
- AFVFQIVMOAPDHO-UHFFFAOYSA-N Methanesulfonic acid Chemical compound CS(O)(=O)=O AFVFQIVMOAPDHO-UHFFFAOYSA-N 0.000 description 1
- 239000004909 Moisturizer Substances 0.000 description 1
- 102000014962 Monocyte Chemoattractant Proteins Human genes 0.000 description 1
- 108010064136 Monocyte Chemoattractant Proteins Proteins 0.000 description 1
- 241000235575 Mortierella Species 0.000 description 1
- 206010052904 Musculoskeletal stiffness Diseases 0.000 description 1
- 208000000112 Myalgia Diseases 0.000 description 1
- 206010051606 Necrotising colitis Diseases 0.000 description 1
- 206010028980 Neoplasm Diseases 0.000 description 1
- 101100137378 Neurospora crassa (strain ATCC 24698 / 74-OR23-1A / CBS 708.71 / DSM 1257 / FGSC 987) cyp41 gene Proteins 0.000 description 1
- 244000061176 Nicotiana tabacum Species 0.000 description 1
- 235000002637 Nicotiana tabacum Nutrition 0.000 description 1
- GRYLNZFGIOXLOG-UHFFFAOYSA-N Nitric acid Chemical compound O[N+]([O-])=O GRYLNZFGIOXLOG-UHFFFAOYSA-N 0.000 description 1
- 239000006057 Non-nutritive feed additive Substances 0.000 description 1
- RSPISYXLHRIGJD-UHFFFAOYSA-N OOOO Chemical class OOOO RSPISYXLHRIGJD-UHFFFAOYSA-N 0.000 description 1
- AHLPHDHHMVZTML-UHFFFAOYSA-N Orn-delta-NH2 Natural products NCCCC(N)C(O)=O AHLPHDHHMVZTML-UHFFFAOYSA-N 0.000 description 1
- UTJLXEIPEHZYQJ-UHFFFAOYSA-N Ornithine Natural products OC(=O)C(C)CCCN UTJLXEIPEHZYQJ-UHFFFAOYSA-N 0.000 description 1
- 229910019142 PO4 Inorganic materials 0.000 description 1
- 241001138688 Parietichytrium sarkarianum Species 0.000 description 1
- 208000018737 Parkinson disease Diseases 0.000 description 1
- 241000199919 Phaeophyceae Species 0.000 description 1
- NBIIXXVUZAFLBC-UHFFFAOYSA-L Phosphate ion(2-) Chemical compound OP([O-])([O-])=O NBIIXXVUZAFLBC-UHFFFAOYSA-L 0.000 description 1
- OAICVXFJPJFONN-UHFFFAOYSA-N Phosphorus Chemical compound [P] OAICVXFJPJFONN-UHFFFAOYSA-N 0.000 description 1
- 108010064851 Plant Proteins Proteins 0.000 description 1
- 241000958364 Pohlia Species 0.000 description 1
- 229920001213 Polysorbate 20 Polymers 0.000 description 1
- ZLMJMSJWJFRBEC-UHFFFAOYSA-N Potassium Chemical compound [K] ZLMJMSJWJFRBEC-UHFFFAOYSA-N 0.000 description 1
- 241000288906 Primates Species 0.000 description 1
- XBDQKXXYIPTUBI-UHFFFAOYSA-M Propionate Chemical compound CCC([O-])=O XBDQKXXYIPTUBI-UHFFFAOYSA-M 0.000 description 1
- 201000004681 Psoriasis Diseases 0.000 description 1
- 208000028017 Psychotic disease Diseases 0.000 description 1
- 208000006311 Pyoderma Diseases 0.000 description 1
- IWYDHOAUDWTVEP-UHFFFAOYSA-N R-2-phenyl-2-hydroxyacetic acid Natural products OC(=O)C(O)C1=CC=CC=C1 IWYDHOAUDWTVEP-UHFFFAOYSA-N 0.000 description 1
- 239000012979 RPMI medium Substances 0.000 description 1
- 239000012980 RPMI-1640 medium Substances 0.000 description 1
- 208000017442 Retinal disease Diseases 0.000 description 1
- 208000007014 Retinitis pigmentosa Diseases 0.000 description 1
- 206010038923 Retinopathy Diseases 0.000 description 1
- 241000206572 Rhodophyta Species 0.000 description 1
- 241001303601 Rosacea Species 0.000 description 1
- 240000004808 Saccharomyces cerevisiae Species 0.000 description 1
- 241000233673 Schizochytrium aggregatum Species 0.000 description 1
- 241000003597 Schizochytrium minutum Species 0.000 description 1
- 241000555745 Sciuridae Species 0.000 description 1
- 206010039966 Senile dementia Diseases 0.000 description 1
- 241000863430 Shewanella Species 0.000 description 1
- 241001138690 Sicyoidochytrium minutum Species 0.000 description 1
- 244000061456 Solanum tuberosum Species 0.000 description 1
- 235000002595 Solanum tuberosum Nutrition 0.000 description 1
- 229930182558 Sterol Natural products 0.000 description 1
- 241001466451 Stramenopiles Species 0.000 description 1
- KDYFGRWQOYBRFD-UHFFFAOYSA-N Succinic acid Natural products OC(=O)CCC(O)=O KDYFGRWQOYBRFD-UHFFFAOYSA-N 0.000 description 1
- QAOWNCQODCNURD-UHFFFAOYSA-L Sulfate Chemical compound [O-]S([O-])(=O)=O QAOWNCQODCNURD-UHFFFAOYSA-L 0.000 description 1
- LSNNMFCWUKXFEE-UHFFFAOYSA-N Sulfurous acid Chemical compound OS(O)=O LSNNMFCWUKXFEE-UHFFFAOYSA-N 0.000 description 1
- FEWJPZIEWOKRBE-UHFFFAOYSA-N Tartaric acid Natural products [H+].[H+].[O-]C(=O)C(O)C(O)C([O-])=O FEWJPZIEWOKRBE-UHFFFAOYSA-N 0.000 description 1
- 229910052776 Thorium Inorganic materials 0.000 description 1
- 241000003599 Thraustochytrium aggregatum Species 0.000 description 1
- 241001501879 Thraustochytrium kinnei Species 0.000 description 1
- 241000323188 Thraustochytrium motivum Species 0.000 description 1
- 241000817756 Thraustochytrium roseum Species 0.000 description 1
- 241000003603 Thraustochytrium striatum Species 0.000 description 1
- 108010018242 Transcription Factor AP-1 Proteins 0.000 description 1
- 102100023118 Transcription factor JunD Human genes 0.000 description 1
- 229920004890 Triton X-100 Polymers 0.000 description 1
- 241001501884 Ulkenia profunda Species 0.000 description 1
- 241000003605 Ulkenia visurgensis Species 0.000 description 1
- 208000024780 Urticaria Diseases 0.000 description 1
- 241000607598 Vibrio Species 0.000 description 1
- 239000004164 Wax ester Substances 0.000 description 1
- ZZXDRXVIRVJQBT-UHFFFAOYSA-M Xylenesulfonate Chemical compound CC1=CC=CC(S([O-])(=O)=O)=C1C ZZXDRXVIRVJQBT-UHFFFAOYSA-M 0.000 description 1
- 240000008042 Zea mays Species 0.000 description 1
- 235000005824 Zea mays ssp. parviglumis Nutrition 0.000 description 1
- 235000002017 Zea mays subsp mays Nutrition 0.000 description 1
- 201000004525 Zellweger Syndrome Diseases 0.000 description 1
- 208000036813 Zellweger spectrum disease Diseases 0.000 description 1
- HCHKCACWOHOZIP-UHFFFAOYSA-N Zinc Chemical compound [Zn] HCHKCACWOHOZIP-UHFFFAOYSA-N 0.000 description 1
- 201000000690 abdominal obesity-metabolic syndrome Diseases 0.000 description 1
- 238000002835 absorbance Methods 0.000 description 1
- IPBVNPXQWQGGJP-UHFFFAOYSA-N acetic acid phenyl ester Natural products CC(=O)OC1=CC=CC=C1 IPBVNPXQWQGGJP-UHFFFAOYSA-N 0.000 description 1
- 238000010306 acid treatment Methods 0.000 description 1
- 206010000496 acne Diseases 0.000 description 1
- 230000004913 activation Effects 0.000 description 1
- 239000012190 activator Substances 0.000 description 1
- 239000008186 active pharmaceutical agent Substances 0.000 description 1
- 230000001154 acute effect Effects 0.000 description 1
- 230000006022 acute inflammation Effects 0.000 description 1
- 208000038016 acute inflammation Diseases 0.000 description 1
- 239000000654 additive Substances 0.000 description 1
- 210000000577 adipose tissue Anatomy 0.000 description 1
- 238000012382 advanced drug delivery Methods 0.000 description 1
- 239000000443 aerosol Substances 0.000 description 1
- 206010064930 age-related macular degeneration Diseases 0.000 description 1
- IAJILQKETJEXLJ-RSJOWCBRSA-N aldehydo-D-galacturonic acid Chemical compound O=C[C@H](O)[C@@H](O)[C@@H](O)[C@H](O)C(O)=O IAJILQKETJEXLJ-RSJOWCBRSA-N 0.000 description 1
- IAJILQKETJEXLJ-QTBDOELSSA-N aldehydo-D-glucuronic acid Chemical compound O=C[C@H](O)[C@@H](O)[C@H](O)[C@H](O)C(O)=O IAJILQKETJEXLJ-QTBDOELSSA-N 0.000 description 1
- 229910052783 alkali metal Inorganic materials 0.000 description 1
- 150000001340 alkali metals Chemical class 0.000 description 1
- 150000001336 alkenes Chemical group 0.000 description 1
- HQPCSDADVLFHHO-LTKCOYKYSA-N all-cis-8,11,14,17-icosatetraenoic acid Chemical compound CC\C=C/C\C=C/C\C=C/C\C=C/CCCCCCC(O)=O HQPCSDADVLFHHO-LTKCOYKYSA-N 0.000 description 1
- AHANXAKGNAKFSK-PDBXOOCHSA-N all-cis-icosa-11,14,17-trienoic acid Chemical compound CC\C=C/C\C=C/C\C=C/CCCCCCCCCC(O)=O AHANXAKGNAKFSK-PDBXOOCHSA-N 0.000 description 1
- 230000009285 allergic inflammation Effects 0.000 description 1
- 229940061720 alpha hydroxy acid Drugs 0.000 description 1
- 150000001280 alpha hydroxy acids Chemical class 0.000 description 1
- DTOSIQBPPRVQHS-PDBXOOCHSA-N alpha-linolenic acid Chemical compound CC\C=C/C\C=C/C\C=C/CCCCCCCC(O)=O DTOSIQBPPRVQHS-PDBXOOCHSA-N 0.000 description 1
- 235000020661 alpha-linolenic acid Nutrition 0.000 description 1
- 229910000147 aluminium phosphate Inorganic materials 0.000 description 1
- 230000009435 amidation Effects 0.000 description 1
- 238000007112 amidation reaction Methods 0.000 description 1
- 229940043376 ammonium acetate Drugs 0.000 description 1
- 235000019257 ammonium acetate Nutrition 0.000 description 1
- 230000003698 anagen phase Effects 0.000 description 1
- 229940035676 analgesics Drugs 0.000 description 1
- 210000001557 animal structure Anatomy 0.000 description 1
- 208000008303 aniridia Diseases 0.000 description 1
- 239000000730 antalgic agent Substances 0.000 description 1
- 230000036506 anxiety Effects 0.000 description 1
- 230000004596 appetite loss Effects 0.000 description 1
- 238000009360 aquaculture Methods 0.000 description 1
- 244000144974 aquaculture Species 0.000 description 1
- ODKSFYDXXFIFQN-UHFFFAOYSA-N arginine Natural products OC(=O)C(N)CCCNC(N)=N ODKSFYDXXFIFQN-UHFFFAOYSA-N 0.000 description 1
- 159000000032 aromatic acids Chemical class 0.000 description 1
- 125000003118 aryl group Chemical group 0.000 description 1
- 235000003704 aspartic acid Nutrition 0.000 description 1
- 238000003556 assay Methods 0.000 description 1
- QVGXLLKOCUKJST-UHFFFAOYSA-N atomic oxygen Chemical compound [O] QVGXLLKOCUKJST-UHFFFAOYSA-N 0.000 description 1
- 208000010668 atopic eczema Diseases 0.000 description 1
- 230000005784 autoimmunity Effects 0.000 description 1
- 235000015173 baked goods and baking mixes Nutrition 0.000 description 1
- 229910052788 barium Inorganic materials 0.000 description 1
- DSAJWYNOEDNPEQ-UHFFFAOYSA-N barium atom Chemical compound [Ba] DSAJWYNOEDNPEQ-UHFFFAOYSA-N 0.000 description 1
- 239000002199 base oil Substances 0.000 description 1
- OGBUMNBNEWYMNJ-UHFFFAOYSA-N batilol Chemical class CCCCCCCCCCCCCCCCCCOCC(O)CO OGBUMNBNEWYMNJ-UHFFFAOYSA-N 0.000 description 1
- 239000011324 bead Substances 0.000 description 1
- 230000003542 behavioural effect Effects 0.000 description 1
- 235000010233 benzoic acid Nutrition 0.000 description 1
- 150000001558 benzoic acid derivatives Chemical class 0.000 description 1
- WGQKYBSKWIADBV-UHFFFAOYSA-O benzylaminium Chemical class [NH3+]CC1=CC=CC=C1 WGQKYBSKWIADBV-UHFFFAOYSA-O 0.000 description 1
- OQFSQFPPLPISGP-UHFFFAOYSA-N beta-carboxyaspartic acid Natural products OC(=O)C(N)C(C(O)=O)C(O)=O OQFSQFPPLPISGP-UHFFFAOYSA-N 0.000 description 1
- 230000004071 biological effect Effects 0.000 description 1
- 229960000074 biopharmaceutical Drugs 0.000 description 1
- 230000036983 biotransformation Effects 0.000 description 1
- ZBCBWPMODOFKDW-UHFFFAOYSA-O bis(2-hydroxyethyl)azanium Chemical class OCC[NH2+]CCO ZBCBWPMODOFKDW-UHFFFAOYSA-O 0.000 description 1
- 210000004369 blood Anatomy 0.000 description 1
- 239000008280 blood Substances 0.000 description 1
- 235000015496 breakfast cereal Nutrition 0.000 description 1
- 244000309464 bull Species 0.000 description 1
- KDYFGRWQOYBRFD-NUQCWPJISA-N butanedioic acid Chemical compound O[14C](=O)CC[14C](O)=O KDYFGRWQOYBRFD-NUQCWPJISA-N 0.000 description 1
- 229910052791 calcium Inorganic materials 0.000 description 1
- 201000011510 cancer Diseases 0.000 description 1
- 239000007894 caplet Substances 0.000 description 1
- 150000004657 carbamic acid derivatives Chemical class 0.000 description 1
- 235000014633 carbohydrates Nutrition 0.000 description 1
- 150000001720 carbohydrates Chemical class 0.000 description 1
- OKTJSMMVPCPJKN-YPZZEJLDSA-N carbon-10 atom Chemical compound [10C] OKTJSMMVPCPJKN-YPZZEJLDSA-N 0.000 description 1
- OKTJSMMVPCPJKN-BJUDXGSMSA-N carbon-11 Chemical compound [11C] OKTJSMMVPCPJKN-BJUDXGSMSA-N 0.000 description 1
- 150000001732 carboxylic acid derivatives Chemical class 0.000 description 1
- 150000001735 carboxylic acids Chemical class 0.000 description 1
- 239000000679 carrageenan Substances 0.000 description 1
- 235000010418 carrageenan Nutrition 0.000 description 1
- 229940113118 carrageenan Drugs 0.000 description 1
- 229920001525 carrageenan Polymers 0.000 description 1
- 238000006555 catalytic reaction Methods 0.000 description 1
- 239000003518 caustics Substances 0.000 description 1
- 238000004113 cell culture Methods 0.000 description 1
- 239000013592 cell lysate Substances 0.000 description 1
- 230000004663 cell proliferation Effects 0.000 description 1
- 239000006285 cell suspension Substances 0.000 description 1
- 230000010307 cell transformation Effects 0.000 description 1
- 230000001413 cellular effect Effects 0.000 description 1
- 230000005754 cellular signaling Effects 0.000 description 1
- 206010008129 cerebral palsy Diseases 0.000 description 1
- 230000008859 change Effects 0.000 description 1
- 235000013351 cheese Nutrition 0.000 description 1
- 239000003153 chemical reaction reagent Substances 0.000 description 1
- 239000002975 chemoattractant Substances 0.000 description 1
- 239000007910 chewable tablet Substances 0.000 description 1
- 230000001055 chewing effect Effects 0.000 description 1
- 229940112822 chewing gum Drugs 0.000 description 1
- 235000015218 chewing gum Nutrition 0.000 description 1
- KVSASDOGYIBWTA-UHFFFAOYSA-N chloro benzoate Chemical compound ClOC(=O)C1=CC=CC=C1 KVSASDOGYIBWTA-UHFFFAOYSA-N 0.000 description 1
- 238000013375 chromatographic separation Methods 0.000 description 1
- 208000025302 chronic primary adrenal insufficiency Diseases 0.000 description 1
- LOUPRKONTZGTKE-UHFFFAOYSA-N cinchonine Natural products C1C(C(C2)C=C)CCN2C1C(O)C1=CC=NC2=CC=C(OC)C=C21 LOUPRKONTZGTKE-UHFFFAOYSA-N 0.000 description 1
- 229930016911 cinnamic acid Natural products 0.000 description 1
- 235000013985 cinnamic acid Nutrition 0.000 description 1
- DERZBLKQOCDDDZ-JLHYYAGUSA-N cinnarizine Chemical compound C1CN(C(C=2C=CC=CC=2)C=2C=CC=CC=2)CCN1C\C=C\C1=CC=CC=C1 DERZBLKQOCDDDZ-JLHYYAGUSA-N 0.000 description 1
- 235000015165 citric acid Nutrition 0.000 description 1
- 238000003776 cleavage reaction Methods 0.000 description 1
- 239000011248 coating agent Substances 0.000 description 1
- 206010009887 colitis Diseases 0.000 description 1
- 230000000295 complement effect Effects 0.000 description 1
- 230000036461 convulsion Effects 0.000 description 1
- 229910052802 copper Inorganic materials 0.000 description 1
- 239000010949 copper Substances 0.000 description 1
- 235000005822 corn Nutrition 0.000 description 1
- 239000010779 crude oil Substances 0.000 description 1
- 238000012136 culture method Methods 0.000 description 1
- 125000004093 cyano group Chemical group *C#N 0.000 description 1
- 108010026647 cytochrome P-450 4X1 Proteins 0.000 description 1
- 230000006240 deamidation Effects 0.000 description 1
- GHVNFZFCNZKVNT-UHFFFAOYSA-N decanoic acid Chemical compound CCCCCCCCCC(O)=O GHVNFZFCNZKVNT-UHFFFAOYSA-N 0.000 description 1
- 238000007257 deesterification reaction Methods 0.000 description 1
- 230000007547 defect Effects 0.000 description 1
- 230000006735 deficit Effects 0.000 description 1
- 238000001514 detection method Methods 0.000 description 1
- 230000018109 developmental process Effects 0.000 description 1
- UREBDLICKHMUKA-CXSFZGCWSA-N dexamethasone Chemical compound C1CC2=CC(=O)C=C[C@]2(C)[C@]2(F)[C@@H]1[C@@H]1C[C@@H](C)[C@@](C(=O)CO)(O)[C@@]1(C)C[C@@H]2O UREBDLICKHMUKA-CXSFZGCWSA-N 0.000 description 1
- 229960003957 dexamethasone Drugs 0.000 description 1
- 206010012601 diabetes mellitus Diseases 0.000 description 1
- 238000003745 diagnosis Methods 0.000 description 1
- 125000004663 dialkyl amino group Chemical group 0.000 description 1
- 125000000664 diazo group Chemical group [N-]=[N+]=[*] 0.000 description 1
- 238000006193 diazotization reaction Methods 0.000 description 1
- 230000000378 dietary effect Effects 0.000 description 1
- 229940079919 digestives enzyme preparation Drugs 0.000 description 1
- NBIIXXVUZAFLBC-UHFFFAOYSA-M dihydrogenphosphate Chemical compound OP(O)([O-])=O NBIIXXVUZAFLBC-UHFFFAOYSA-M 0.000 description 1
- 239000003085 diluting agent Substances 0.000 description 1
- XEYBRNLFEZDVAW-ARSRFYASSA-N dinoprostone Chemical compound CCCCC[C@H](O)\C=C\[C@H]1[C@H](O)CC(=O)[C@@H]1C\C=C/CCCC(O)=O XEYBRNLFEZDVAW-ARSRFYASSA-N 0.000 description 1
- 229960002986 dinoprostone Drugs 0.000 description 1
- XPPKVPWEQAFLFU-UHFFFAOYSA-J diphosphate(4-) Chemical compound [O-]P([O-])(=O)OP([O-])([O-])=O XPPKVPWEQAFLFU-UHFFFAOYSA-J 0.000 description 1
- 235000011180 diphosphates Nutrition 0.000 description 1
- 238000006073 displacement reaction Methods 0.000 description 1
- 238000011143 downstream manufacturing Methods 0.000 description 1
- 238000009510 drug design Methods 0.000 description 1
- 238000009509 drug development Methods 0.000 description 1
- 230000000142 dyskinetic effect Effects 0.000 description 1
- 206010013932 dyslexia Diseases 0.000 description 1
- 239000007938 effervescent tablet Substances 0.000 description 1
- PRHHYVQTPBEDFE-UHFFFAOYSA-N eicosatrienoic acid Natural products CCCCCC=CCC=CCCCCC=CCCCC(O)=O PRHHYVQTPBEDFE-UHFFFAOYSA-N 0.000 description 1
- 230000007613 environmental effect Effects 0.000 description 1
- 206010015037 epilepsy Diseases 0.000 description 1
- 210000003743 erythrocyte Anatomy 0.000 description 1
- 230000032050 esterification Effects 0.000 description 1
- 238000005886 esterification reaction Methods 0.000 description 1
- CCIVGXIOQKPBKL-UHFFFAOYSA-M ethanesulfonate Chemical compound CCS([O-])(=O)=O CCIVGXIOQKPBKL-UHFFFAOYSA-M 0.000 description 1
- 150000002170 ethers Chemical class 0.000 description 1
- 238000002474 experimental method Methods 0.000 description 1
- 230000004136 fatty acid synthesis Effects 0.000 description 1
- 239000012894 fetal calf serum Substances 0.000 description 1
- 239000000835 fiber Substances 0.000 description 1
- 229940125753 fibrate Drugs 0.000 description 1
- 239000012997 ficoll-paque Substances 0.000 description 1
- 239000010419 fine particle Substances 0.000 description 1
- 229940013317 fish oils Drugs 0.000 description 1
- 239000012530 fluid Substances 0.000 description 1
- 239000013022 formulation composition Substances 0.000 description 1
- 235000020509 fortified beverage Nutrition 0.000 description 1
- 235000014106 fortified food Nutrition 0.000 description 1
- 239000012458 free base Substances 0.000 description 1
- 239000012737 fresh medium Substances 0.000 description 1
- 235000015203 fruit juice Nutrition 0.000 description 1
- 239000001530 fumaric acid Substances 0.000 description 1
- 229960002598 fumaric acid Drugs 0.000 description 1
- 235000013376 functional food Nutrition 0.000 description 1
- 210000001035 gastrointestinal tract Anatomy 0.000 description 1
- 239000000499 gel Substances 0.000 description 1
- 239000008273 gelatin Substances 0.000 description 1
- 229920000159 gelatin Polymers 0.000 description 1
- 235000019322 gelatine Nutrition 0.000 description 1
- 235000011852 gelatine desserts Nutrition 0.000 description 1
- 238000012239 gene modification Methods 0.000 description 1
- 230000009395 genetic defect Effects 0.000 description 1
- 230000005017 genetic modification Effects 0.000 description 1
- 235000013617 genetically modified food Nutrition 0.000 description 1
- 235000003869 genetically modified organism Nutrition 0.000 description 1
- 239000012362 glacial acetic acid Substances 0.000 description 1
- 239000003862 glucocorticoid Substances 0.000 description 1
- 229940097043 glucuronic acid Drugs 0.000 description 1
- 239000004220 glutamic acid Substances 0.000 description 1
- 235000013922 glutamic acid Nutrition 0.000 description 1
- 229960004275 glycolic acid Drugs 0.000 description 1
- 235000011868 grain product Nutrition 0.000 description 1
- 231100000869 headache Toxicity 0.000 description 1
- MNWFXJYAOYHMED-UHFFFAOYSA-N heptanoic acid Chemical compound CCCCCCC(O)=O MNWFXJYAOYHMED-UHFFFAOYSA-N 0.000 description 1
- 125000001072 heteroaryl group Chemical group 0.000 description 1
- 125000000623 heterocyclic group Chemical group 0.000 description 1
- FUZZWVXGSFPDMH-UHFFFAOYSA-M hexanoate Chemical compound CCCCCC([O-])=O FUZZWVXGSFPDMH-UHFFFAOYSA-M 0.000 description 1
- HNDVDQJCIGZPNO-UHFFFAOYSA-N histidine Natural products OC(=O)C(N)CC1=CN=CN1 HNDVDQJCIGZPNO-UHFFFAOYSA-N 0.000 description 1
- 229940088597 hormone Drugs 0.000 description 1
- 239000005556 hormone Substances 0.000 description 1
- 235000020256 human milk Nutrition 0.000 description 1
- 229930195733 hydrocarbon Natural products 0.000 description 1
- 150000002430 hydrocarbons Chemical class 0.000 description 1
- XMBWDFGMSWQBCA-UHFFFAOYSA-N hydrogen iodide Chemical compound I XMBWDFGMSWQBCA-UHFFFAOYSA-N 0.000 description 1
- QAOWNCQODCNURD-UHFFFAOYSA-M hydrogensulfate Chemical compound OS([O-])(=O)=O QAOWNCQODCNURD-UHFFFAOYSA-M 0.000 description 1
- 230000007062 hydrolysis Effects 0.000 description 1
- 238000006460 hydrolysis reaction Methods 0.000 description 1
- 230000003301 hydrolyzing effect Effects 0.000 description 1
- 150000002432 hydroperoxides Chemical class 0.000 description 1
- 230000000222 hyperoxic effect Effects 0.000 description 1
- 210000002865 immune cell Anatomy 0.000 description 1
- 230000036737 immune function Effects 0.000 description 1
- 239000000411 inducer Substances 0.000 description 1
- 230000001939 inductive effect Effects 0.000 description 1
- 239000012678 infectious agent Substances 0.000 description 1
- 230000008595 infiltration Effects 0.000 description 1
- 238000001764 infiltration Methods 0.000 description 1
- 230000004968 inflammatory condition Effects 0.000 description 1
- 230000028709 inflammatory response Effects 0.000 description 1
- 238000001802 infusion Methods 0.000 description 1
- 230000003993 interaction Effects 0.000 description 1
- 230000019189 interleukin-1 beta production Effects 0.000 description 1
- 229940028885 interleukin-4 Drugs 0.000 description 1
- 210000000936 intestine Anatomy 0.000 description 1
- 238000005040 ion trap Methods 0.000 description 1
- 229910052742 iron Inorganic materials 0.000 description 1
- 230000007794 irritation Effects 0.000 description 1
- 208000028867 ischemia Diseases 0.000 description 1
- KQNPFQTWMSNSAP-UHFFFAOYSA-N isobutyric acid Chemical compound CC(C)C(O)=O KQNPFQTWMSNSAP-UHFFFAOYSA-N 0.000 description 1
- 239000010977 jade Substances 0.000 description 1
- 150000002611 lead compounds Chemical class 0.000 description 1
- 210000000265 leukocyte Anatomy 0.000 description 1
- VNYSSYRCGWBHLG-AMOLWHMGSA-N leukotriene B4 Chemical compound CCCCC\C=C/C[C@@H](O)\C=C\C=C\C=C/[C@@H](O)CCCC(O)=O VNYSSYRCGWBHLG-AMOLWHMGSA-N 0.000 description 1
- 229960004488 linolenic acid Drugs 0.000 description 1
- KQQKGWQCNNTQJW-UHFFFAOYSA-N linolenic acid Natural products CC=CCCC=CCC=CCCCCCCCC(O)=O KQQKGWQCNNTQJW-UHFFFAOYSA-N 0.000 description 1
- 238000004811 liquid chromatography Methods 0.000 description 1
- 238000004895 liquid chromatography mass spectrometry Methods 0.000 description 1
- 229910052744 lithium Inorganic materials 0.000 description 1
- 244000144972 livestock Species 0.000 description 1
- 230000033001 locomotion Effects 0.000 description 1
- 230000007774 longterm Effects 0.000 description 1
- 235000021266 loss of appetite Nutrition 0.000 description 1
- 208000019017 loss of appetite Diseases 0.000 description 1
- 210000003141 lower extremity Anatomy 0.000 description 1
- WORCCYVLMMTGFR-UHFFFAOYSA-M loxoprofen sodium Chemical compound [Na+].C1=CC(C(C([O-])=O)C)=CC=C1CC1C(=O)CCC1 WORCCYVLMMTGFR-UHFFFAOYSA-M 0.000 description 1
- 239000007937 lozenge Substances 0.000 description 1
- 229910052749 magnesium Inorganic materials 0.000 description 1
- 239000011777 magnesium Substances 0.000 description 1
- 238000012423 maintenance Methods 0.000 description 1
- 230000014759 maintenance of location Effects 0.000 description 1
- 239000011976 maleic acid Substances 0.000 description 1
- 229940098895 maleic acid Drugs 0.000 description 1
- 238000007726 management method Methods 0.000 description 1
- IWYDHOAUDWTVEP-UHFFFAOYSA-M mandelate Chemical compound [O-]C(=O)C(O)C1=CC=CC=C1 IWYDHOAUDWTVEP-UHFFFAOYSA-M 0.000 description 1
- 229960002510 mandelic acid Drugs 0.000 description 1
- 238000004949 mass spectrometry Methods 0.000 description 1
- 235000013622 meat product Nutrition 0.000 description 1
- 230000037353 metabolic pathway Effects 0.000 description 1
- 125000005341 metaphosphate group Chemical group 0.000 description 1
- IZYBEMGNIUSSAX-UHFFFAOYSA-N methyl benzenecarboperoxoate Chemical compound COOC(=O)C1=CC=CC=C1 IZYBEMGNIUSSAX-UHFFFAOYSA-N 0.000 description 1
- 229940095102 methyl benzoate Drugs 0.000 description 1
- WBYWAXJHAXSJNI-UHFFFAOYSA-N methyl p-hydroxycinnamate Natural products OC(=O)C=CC1=CC=CC=C1 WBYWAXJHAXSJNI-UHFFFAOYSA-N 0.000 description 1
- 235000020786 mineral supplement Nutrition 0.000 description 1
- 230000001333 moisturizer Effects 0.000 description 1
- 238000010369 molecular cloning Methods 0.000 description 1
- 210000003205 muscle Anatomy 0.000 description 1
- 208000013465 muscle pain Diseases 0.000 description 1
- 208000011042 muscle-eye-brain disease Diseases 0.000 description 1
- 201000006938 muscular dystrophy Diseases 0.000 description 1
- 230000035772 mutation Effects 0.000 description 1
- 206010028417 myasthenia gravis Diseases 0.000 description 1
- HSZCJVZRHXPCIA-UHFFFAOYSA-N n-benzyl-n-ethylaniline Chemical class C=1C=CC=CC=1N(CC)CC1=CC=CC=C1 HSZCJVZRHXPCIA-UHFFFAOYSA-N 0.000 description 1
- PSZYNBSKGUBXEH-UHFFFAOYSA-N naphthalene-1-sulfonic acid Chemical compound C1=CC=C2C(S(=O)(=O)O)=CC=CC2=C1 PSZYNBSKGUBXEH-UHFFFAOYSA-N 0.000 description 1
- KVBGVZZKJNLNJU-UHFFFAOYSA-N naphthalene-2-sulfonic acid Chemical compound C1=CC=CC2=CC(S(=O)(=O)O)=CC=C21 KVBGVZZKJNLNJU-UHFFFAOYSA-N 0.000 description 1
- 208000004995 necrotizing enterocolitis Diseases 0.000 description 1
- 201000010193 neural tube defect Diseases 0.000 description 1
- 208000015122 neurodegenerative disease Diseases 0.000 description 1
- 230000007823 neuropathy Effects 0.000 description 1
- 201000001119 neuropathy Diseases 0.000 description 1
- 238000006396 nitration reaction Methods 0.000 description 1
- 229910017604 nitric acid Inorganic materials 0.000 description 1
- 231100000252 nontoxic Toxicity 0.000 description 1
- 230000003000 nontoxic effect Effects 0.000 description 1
- 238000002414 normal-phase solid-phase extraction Methods 0.000 description 1
- 150000007523 nucleic acids Chemical class 0.000 description 1
- 108020004707 nucleic acids Proteins 0.000 description 1
- 102000039446 nucleic acids Human genes 0.000 description 1
- WWZKQHOCKIZLMA-UHFFFAOYSA-M octanoate Chemical compound CCCCCCCC([O-])=O WWZKQHOCKIZLMA-UHFFFAOYSA-M 0.000 description 1
- 229960003104 ornithine Drugs 0.000 description 1
- 229940116315 oxalic acid Drugs 0.000 description 1
- 235000006408 oxalic acid Nutrition 0.000 description 1
- 238000010525 oxidative degradation reaction Methods 0.000 description 1
- 230000001590 oxidative effect Effects 0.000 description 1
- 230000004783 oxidative metabolism Effects 0.000 description 1
- 239000001301 oxygen Substances 0.000 description 1
- 229910052760 oxygen Inorganic materials 0.000 description 1
- FJKROLUGYXJWQN-UHFFFAOYSA-N papa-hydroxy-benzoic acid Natural products OC(=O)C1=CC=C(O)C=C1 FJKROLUGYXJWQN-UHFFFAOYSA-N 0.000 description 1
- 244000045947 parasite Species 0.000 description 1
- 125000001151 peptidyl group Chemical group 0.000 description 1
- 201000006195 perinatal necrotizing enterocolitis Diseases 0.000 description 1
- 210000003819 peripheral blood mononuclear cell Anatomy 0.000 description 1
- 208000033808 peripheral neuropathy Diseases 0.000 description 1
- 125000000864 peroxy group Chemical group O(O*)* 0.000 description 1
- 239000003208 petroleum Substances 0.000 description 1
- DYUMLJSJISTVPV-UHFFFAOYSA-N phenyl propanoate Chemical compound CCC(=O)OC1=CC=CC=C1 DYUMLJSJISTVPV-UHFFFAOYSA-N 0.000 description 1
- 229940049953 phenylacetate Drugs 0.000 description 1
- WLJVXDMOQOGPHL-UHFFFAOYSA-N phenylacetic acid Chemical compound OC(=O)CC1=CC=CC=C1 WLJVXDMOQOGPHL-UHFFFAOYSA-N 0.000 description 1
- 229950009215 phenylbutanoic acid Drugs 0.000 description 1
- NBIIXXVUZAFLBC-UHFFFAOYSA-K phosphate Chemical compound [O-]P([O-])([O-])=O NBIIXXVUZAFLBC-UHFFFAOYSA-K 0.000 description 1
- 239000010452 phosphate Substances 0.000 description 1
- 229910052698 phosphorus Inorganic materials 0.000 description 1
- 239000011574 phosphorus Substances 0.000 description 1
- XNGIFLGASWRNHJ-UHFFFAOYSA-L phthalate(2-) Chemical compound [O-]C(=O)C1=CC=CC=C1C([O-])=O XNGIFLGASWRNHJ-UHFFFAOYSA-L 0.000 description 1
- 235000021118 plant-derived protein Nutrition 0.000 description 1
- 210000002706 plastid Anatomy 0.000 description 1
- 239000002798 polar solvent Substances 0.000 description 1
- 239000000256 polyoxyethylene sorbitan monolaurate Substances 0.000 description 1
- 235000010486 polyoxyethylene sorbitan monolaurate Nutrition 0.000 description 1
- 239000013641 positive control Substances 0.000 description 1
- 229910052700 potassium Inorganic materials 0.000 description 1
- 239000011591 potassium Substances 0.000 description 1
- 244000144977 poultry Species 0.000 description 1
- 235000013594 poultry meat Nutrition 0.000 description 1
- 235000013613 poultry product Nutrition 0.000 description 1
- 230000035935 pregnancy Effects 0.000 description 1
- 230000002028 premature Effects 0.000 description 1
- 238000003825 pressing Methods 0.000 description 1
- 230000003449 preventive effect Effects 0.000 description 1
- 125000002924 primary amino group Chemical group [H]N([H])* 0.000 description 1
- MFDFERRIHVXMIY-UHFFFAOYSA-N procaine Chemical compound CCN(CC)CCOC(=O)C1=CC=C(N)C=C1 MFDFERRIHVXMIY-UHFFFAOYSA-N 0.000 description 1
- 229960004919 procaine Drugs 0.000 description 1
- 235000020991 processed meat Nutrition 0.000 description 1
- KCXFHTAICRTXLI-UHFFFAOYSA-N propane-1-sulfonic acid Chemical compound CCCS(O)(=O)=O KCXFHTAICRTXLI-UHFFFAOYSA-N 0.000 description 1
- UORVCLMRJXCDCP-UHFFFAOYSA-M propynoate Chemical compound [O-]C(=O)C#C UORVCLMRJXCDCP-UHFFFAOYSA-M 0.000 description 1
- 150000003815 prostacyclins Chemical class 0.000 description 1
- XEYBRNLFEZDVAW-UHFFFAOYSA-N prostaglandin E2 Natural products CCCCCC(O)C=CC1C(O)CC(=O)C1CC=CCCCC(O)=O XEYBRNLFEZDVAW-UHFFFAOYSA-N 0.000 description 1
- 229940127293 prostanoid Drugs 0.000 description 1
- 150000003814 prostanoids Chemical class 0.000 description 1
- 235000011962 puddings Nutrition 0.000 description 1
- RYVMUASDIZQXAA-UHFFFAOYSA-N pyranoside Natural products O1C2(OCC(C)C(OC3C(C(O)C(O)C(CO)O3)O)C2)C(C)C(C2(CCC3C4(C)CC5O)C)C1CC2C3CC=C4CC5OC(C(C1O)O)OC(CO)C1OC(C1OC2C(C(OC3C(C(O)C(O)C(CO)O3)O)C(O)C(CO)O2)O)OC(CO)C(O)C1OC1OCC(O)C(O)C1O RYVMUASDIZQXAA-UHFFFAOYSA-N 0.000 description 1
- 229940107700 pyruvic acid Drugs 0.000 description 1
- 229960000948 quinine Drugs 0.000 description 1
- 238000011084 recovery Methods 0.000 description 1
- 238000001953 recrystallisation Methods 0.000 description 1
- 238000006722 reduction reaction Methods 0.000 description 1
- 238000007670 refining Methods 0.000 description 1
- 230000008929 regeneration Effects 0.000 description 1
- 238000011069 regeneration method Methods 0.000 description 1
- OIWTWACQMDFHJG-CCFUIAGSSA-N resolvin D1 Chemical compound CC\C=C/C[C@H](O)\C=C\C=C/C=C/C=C/[C@@H](O)[C@@H](O)C\C=C/CCC(O)=O OIWTWACQMDFHJG-CCFUIAGSSA-N 0.000 description 1
- IKFAUGXNBOBQDM-XFMPMKITSA-N resolvin D2 Chemical compound CC\C=C/C[C@H](O)[C@H](O)\C=C\C=C\C=C/C=C/[C@@H](O)C\C=C/CCC(O)=O IKFAUGXNBOBQDM-XFMPMKITSA-N 0.000 description 1
- QBTJOLCUKWLTIC-UZAFJXHNSA-N resolvin D3 Chemical compound CC\C=C/C[C@H](O)\C=C\C=C/C[C@@H](O)\C=C\C=C\C=C/[C@@H](O)CCC(O)=O QBTJOLCUKWLTIC-UZAFJXHNSA-N 0.000 description 1
- YKPLJNOOLKUEBS-RIYRYSNMSA-N resolvin D4 Chemical compound CC\C=C/C[C@H](O)\C=C\C=C/C\C=C/C=C/C=C/[C@@H](O)[C@@H](O)CCC(O)=O YKPLJNOOLKUEBS-RIYRYSNMSA-N 0.000 description 1
- JKPUWSZSJINVLB-OSKNXYPTSA-N resolvin D6 Chemical compound CC\C=C/C[C@H](O)\C=C\C=C/C\C=C/C\C=C/C=C/[C@@H](O)CCC(O)=O JKPUWSZSJINVLB-OSKNXYPTSA-N 0.000 description 1
- AOPOCGPBAIARAV-OTBJXLELSA-N resolvin E1 Chemical compound CC[C@@H](O)\C=C\C=C/C[C@@H](O)\C=C\C=C\C=C/[C@@H](O)CCCC(O)=O AOPOCGPBAIARAV-OTBJXLELSA-N 0.000 description 1
- KPRHYAOSTOHNQA-NNQKPOSRSA-N resolvin E2 Chemical compound CC[C@@H](O)\C=C\C=C/C\C=C/C\C=C/C=C/[C@@H](O)CCCC(O)=O KPRHYAOSTOHNQA-NNQKPOSRSA-N 0.000 description 1
- JBRPFYYLEQERPG-XTIXYJHRSA-N resolvin d5 Chemical compound CC\C=C/C[C@H](O)\C=C\C=C/C\C=C/C=C/[C@@H](O)\C=C/CCCC(O)=O JBRPFYYLEQERPG-XTIXYJHRSA-N 0.000 description 1
- 230000000717 retained effect Effects 0.000 description 1
- 238000012552 review Methods 0.000 description 1
- 206010039073 rheumatoid arthritis Diseases 0.000 description 1
- JQXXHWHPUNPDRT-WLSIYKJHSA-N rifampicin Chemical compound O([C@](C1=O)(C)O/C=C/[C@@H]([C@H]([C@@H](OC(C)=O)[C@H](C)[C@H](O)[C@H](C)[C@@H](O)[C@@H](C)\C=C\C=C(C)/C(=O)NC=2C(O)=C3C([O-])=C4C)C)OC)C4=C1C3=C(O)C=2\C=N\N1CC[NH+](C)CC1 JQXXHWHPUNPDRT-WLSIYKJHSA-N 0.000 description 1
- 229960001225 rifampicin Drugs 0.000 description 1
- 201000004700 rosacea Diseases 0.000 description 1
- YGSDEFSMJLZEOE-UHFFFAOYSA-M salicylate Chemical compound OC1=CC=CC=C1C([O-])=O YGSDEFSMJLZEOE-UHFFFAOYSA-M 0.000 description 1
- 229960004889 salicylic acid Drugs 0.000 description 1
- 201000000980 schizophrenia Diseases 0.000 description 1
- 230000007017 scission Effects 0.000 description 1
- 238000012216 screening Methods 0.000 description 1
- 229940116351 sebacate Drugs 0.000 description 1
- CXMXRPHRNRROMY-UHFFFAOYSA-L sebacate(2-) Chemical compound [O-]C(=O)CCCCCCCCC([O-])=O CXMXRPHRNRROMY-UHFFFAOYSA-L 0.000 description 1
- 150000003333 secondary alcohols Chemical class 0.000 description 1
- 210000002966 serum Anatomy 0.000 description 1
- 230000035939 shock Effects 0.000 description 1
- 208000019116 sleep disease Diseases 0.000 description 1
- 239000000344 soap Substances 0.000 description 1
- 229910052708 sodium Inorganic materials 0.000 description 1
- 239000011734 sodium Substances 0.000 description 1
- 239000007787 solid Substances 0.000 description 1
- 238000010532 solid phase synthesis reaction Methods 0.000 description 1
- 239000002904 solvent Substances 0.000 description 1
- 230000003595 spectral effect Effects 0.000 description 1
- 150000003408 sphingolipids Chemical class 0.000 description 1
- 238000013222 sprague-dawley male rat Methods 0.000 description 1
- 230000006641 stabilisation Effects 0.000 description 1
- 238000011105 stabilization Methods 0.000 description 1
- 230000003637 steroidlike Effects 0.000 description 1
- 235000003702 sterols Nutrition 0.000 description 1
- TYFQFVWCELRYAO-UHFFFAOYSA-L suberate(2-) Chemical compound [O-]C(=O)CCCCCCC([O-])=O TYFQFVWCELRYAO-UHFFFAOYSA-L 0.000 description 1
- KDYFGRWQOYBRFD-UHFFFAOYSA-L succinate(2-) Chemical compound [O-]C(=O)CCC([O-])=O KDYFGRWQOYBRFD-UHFFFAOYSA-L 0.000 description 1
- 235000000346 sugar Nutrition 0.000 description 1
- BDHFUVZGWQCTTF-UHFFFAOYSA-M sulfonate Chemical compound [O-]S(=O)=O BDHFUVZGWQCTTF-UHFFFAOYSA-M 0.000 description 1
- 150000003457 sulfones Chemical class 0.000 description 1
- 150000003460 sulfonic acids Chemical class 0.000 description 1
- 150000003462 sulfoxides Chemical class 0.000 description 1
- 230000000475 sunscreen effect Effects 0.000 description 1
- 239000000516 sunscreening agent Substances 0.000 description 1
- 230000001502 supplementing effect Effects 0.000 description 1
- 208000035581 susceptibility to neural tube defects Diseases 0.000 description 1
- 239000000725 suspension Substances 0.000 description 1
- 238000004114 suspension culture Methods 0.000 description 1
- 230000009885 systemic effect Effects 0.000 description 1
- 229940037128 systemic glucocorticoids Drugs 0.000 description 1
- 201000000596 systemic lupus erythematosus Diseases 0.000 description 1
- 235000002906 tartaric acid Nutrition 0.000 description 1
- 239000011975 tartaric acid Substances 0.000 description 1
- 229940095064 tartrate Drugs 0.000 description 1
- 238000002560 therapeutic procedure Methods 0.000 description 1
- 150000007970 thio esters Chemical class 0.000 description 1
- 150000003568 thioethers Chemical class 0.000 description 1
- 125000003396 thiol group Chemical group [H]S* 0.000 description 1
- 150000003573 thiols Chemical class 0.000 description 1
- 230000006032 tissue transformation Effects 0.000 description 1
- 239000003860 topical agent Substances 0.000 description 1
- 230000000699 topical effect Effects 0.000 description 1
- 231100000331 toxic Toxicity 0.000 description 1
- 230000002588 toxic effect Effects 0.000 description 1
- 229910052723 transition metal Inorganic materials 0.000 description 1
- 150000003624 transition metals Chemical class 0.000 description 1
- 208000001072 type 2 diabetes mellitus Diseases 0.000 description 1
- 208000034373 type A muscular dystrophy-dystroglycanopathy Diseases 0.000 description 1
- 235000013343 vitamin Nutrition 0.000 description 1
- 235000019195 vitamin supplement Nutrition 0.000 description 1
- 235000019386 wax ester Nutrition 0.000 description 1
- 230000029663 wound healing Effects 0.000 description 1
- 229940071104 xylenesulfonate Drugs 0.000 description 1
- 229910052725 zinc Inorganic materials 0.000 description 1
- 239000011701 zinc Substances 0.000 description 1
- UHVMMEOXYDMDKI-JKYCWFKZSA-L zinc;1-(5-cyanopyridin-2-yl)-3-[(1s,2s)-2-(6-fluoro-2-hydroxy-3-propanoylphenyl)cyclopropyl]urea;diacetate Chemical compound [Zn+2].CC([O-])=O.CC([O-])=O.CCC(=O)C1=CC=C(F)C([C@H]2[C@H](C2)NC(=O)NC=2N=CC(=CC=2)C#N)=C1O UHVMMEOXYDMDKI-JKYCWFKZSA-L 0.000 description 1
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12P—FERMENTATION OR ENZYME-USING PROCESSES TO SYNTHESISE A DESIRED CHEMICAL COMPOUND OR COMPOSITION OR TO SEPARATE OPTICAL ISOMERS FROM A RACEMIC MIXTURE
- C12P7/00—Preparation of oxygen-containing organic compounds
- C12P7/64—Fats; Fatty oils; Ester-type waxes; Higher fatty acids, i.e. having at least seven carbon atoms in an unbroken chain bound to a carboxyl group; Oxidised oils or fats
- C12P7/6436—Fatty acid esters
- C12P7/6445—Glycerides
- C12P7/6472—Glycerides containing polyunsaturated fatty acid [PUFA] residues, i.e. having two or more double bonds in their backbone
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/185—Acids; Anhydrides, halides or salts thereof, e.g. sulfur acids, imidic, hydrazonic or hydroximic acids
- A61K31/19—Carboxylic acids, e.g. valproic acid
- A61K31/20—Carboxylic acids, e.g. valproic acid having a carboxyl group bound to a chain of seven or more carbon atoms, e.g. stearic, palmitic, arachidic acids
- A61K31/202—Carboxylic acids, e.g. valproic acid having a carboxyl group bound to a chain of seven or more carbon atoms, e.g. stearic, palmitic, arachidic acids having three or more double bonds, e.g. linolenic
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P17/00—Drugs for dermatological disorders
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
- A61P25/28—Drugs for disorders of the nervous system for treating neurodegenerative disorders of the central nervous system, e.g. nootropic agents, cognition enhancers, drugs for treating Alzheimer's disease or other forms of dementia
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P29/00—Non-central analgesic, antipyretic or antiinflammatory agents, e.g. antirheumatic agents; Non-steroidal antiinflammatory drugs [NSAID]
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P43/00—Drugs for specific purposes, not provided for in groups A61P1/00-A61P41/00
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P7/00—Drugs for disorders of the blood or the extracellular fluid
- A61P7/10—Antioedematous agents; Diuretics
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C59/00—Compounds having carboxyl groups bound to acyclic carbon atoms and containing any of the groups OH, O—metal, —CHO, keto, ether, groups, groups, or groups
- C07C59/40—Unsaturated compounds
- C07C59/42—Unsaturated compounds containing hydroxy or O-metal groups
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D303/00—Compounds containing three-membered rings having one oxygen atom as the only ring hetero atom
- C07D303/02—Compounds containing oxirane rings
- C07D303/38—Compounds containing oxirane rings with hydrocarbon radicals, substituted by carbon atoms having three bonds to hetero atoms with at the most one bond to halogen, e.g. ester or nitrile radicals
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12P—FERMENTATION OR ENZYME-USING PROCESSES TO SYNTHESISE A DESIRED CHEMICAL COMPOUND OR COMPOSITION OR TO SEPARATE OPTICAL ISOMERS FROM A RACEMIC MIXTURE
- C12P7/00—Preparation of oxygen-containing organic compounds
- C12P7/64—Fats; Fatty oils; Ester-type waxes; Higher fatty acids, i.e. having at least seven carbon atoms in an unbroken chain bound to a carboxyl group; Oxidised oils or fats
- C12P7/6409—Fatty acids
- C12P7/6427—Polyunsaturated fatty acids [PUFA], i.e. having two or more double bonds in their backbone
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12P—FERMENTATION OR ENZYME-USING PROCESSES TO SYNTHESISE A DESIRED CHEMICAL COMPOUND OR COMPOSITION OR TO SEPARATE OPTICAL ISOMERS FROM A RACEMIC MIXTURE
- C12P7/00—Preparation of oxygen-containing organic compounds
- C12P7/64—Fats; Fatty oils; Ester-type waxes; Higher fatty acids, i.e. having at least seven carbon atoms in an unbroken chain bound to a carboxyl group; Oxidised oils or fats
- C12P7/6409—Fatty acids
- C12P7/6427—Polyunsaturated fatty acids [PUFA], i.e. having two or more double bonds in their backbone
- C12P7/6432—Eicosapentaenoic acids [EPA]
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12P—FERMENTATION OR ENZYME-USING PROCESSES TO SYNTHESISE A DESIRED CHEMICAL COMPOUND OR COMPOSITION OR TO SEPARATE OPTICAL ISOMERS FROM A RACEMIC MIXTURE
- C12P7/00—Preparation of oxygen-containing organic compounds
- C12P7/64—Fats; Fatty oils; Ester-type waxes; Higher fatty acids, i.e. having at least seven carbon atoms in an unbroken chain bound to a carboxyl group; Oxidised oils or fats
- C12P7/6409—Fatty acids
- C12P7/6427—Polyunsaturated fatty acids [PUFA], i.e. having two or more double bonds in their backbone
- C12P7/6434—Docosahexenoic acids [DHA]
Landscapes
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Engineering & Computer Science (AREA)
- Zoology (AREA)
- Wood Science & Technology (AREA)
- General Health & Medical Sciences (AREA)
- General Chemical & Material Sciences (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Oil, Petroleum & Natural Gas (AREA)
- Genetics & Genomics (AREA)
- General Engineering & Computer Science (AREA)
- Biochemistry (AREA)
- Microbiology (AREA)
- Biotechnology (AREA)
- Public Health (AREA)
- Medicinal Chemistry (AREA)
- Pharmacology & Pharmacy (AREA)
- Animal Behavior & Ethology (AREA)
- Veterinary Medicine (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Biomedical Technology (AREA)
- Neurosurgery (AREA)
- Neurology (AREA)
- Dermatology (AREA)
- Hospice & Palliative Care (AREA)
- Rheumatology (AREA)
- Diabetes (AREA)
- Pain & Pain Management (AREA)
- Hematology (AREA)
- Psychiatry (AREA)
- Epidemiology (AREA)
- Acyclic And Carbocyclic Compounds In Medicinal Compositions (AREA)
- Organic Low-Molecular-Weight Compounds And Preparation Thereof (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
- Cosmetics (AREA)
- Epoxy Compounds (AREA)
- Medicines That Contain Protein Lipid Enzymes And Other Medicines (AREA)
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US62984204P | 2004-11-19 | 2004-11-19 | |
| US72903805P | 2005-10-21 | 2005-10-21 | |
| PCT/US2005/042462 WO2006055965A2 (en) | 2004-11-19 | 2005-11-21 | Oxylipins from long chain polyunsaturated fatty acids and methods of making and using the same |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| JP2008520739A true JP2008520739A (ja) | 2008-06-19 |
| JP2008520739A5 JP2008520739A5 (enExample) | 2009-01-15 |
Family
ID=36407879
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2007543444A Pending JP2008520739A (ja) | 2004-11-19 | 2005-11-21 | 長鎖多価不飽和脂肪酸由来のオキシリピンならびにその作製および使用方法 |
Country Status (13)
| Country | Link |
|---|---|
| US (2) | US7884131B2 (enExample) |
| EP (1) | EP1824809A4 (enExample) |
| JP (1) | JP2008520739A (enExample) |
| KR (1) | KR20070090928A (enExample) |
| CN (1) | CN101102988B (enExample) |
| AU (1) | AU2005306320B2 (enExample) |
| BR (1) | BRPI0516803A (enExample) |
| CA (1) | CA2588166A1 (enExample) |
| EA (1) | EA010802B1 (enExample) |
| IL (1) | IL183246A0 (enExample) |
| MX (1) | MX2007006049A (enExample) |
| NZ (1) | NZ555394A (enExample) |
| WO (1) | WO2006055965A2 (enExample) |
Cited By (10)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2012026106A1 (ja) * | 2010-08-25 | 2012-03-01 | 国立大学法人 東京大学 | 光学活性な新規抗炎症性化合物及びその製造方法 |
| JP2013500966A (ja) * | 2009-07-31 | 2013-01-10 | ユニバーシティー オブ ピッツバーグ − オブ ザ コモンウェルス システム オブ ハイヤー エデュケーション | 抗炎症剤としての脂肪酸 |
| JP2015522535A (ja) * | 2012-05-10 | 2015-08-06 | ソルテックス エヌエー エルエルシー | 天然の特異的炎症収束性メディエータおよびその前駆物質を含有する、抗炎症活性を有する油 |
| JP2016505016A (ja) * | 2013-01-11 | 2016-02-18 | マサチューセッツ アイ アンド イヤー インファーマリー | 炎症及び血管形成を減少させるcyp450脂質メタボライト |
| JP2016520612A (ja) * | 2013-05-30 | 2016-07-14 | ザ ブリガム アンド ウィメンズ ホスピタル インコーポレイテッドThe Brigham and Women’s Hospital, Inc. | 新規n−3イムノリソルベント:構造及び作用 |
| JP2018506584A (ja) * | 2015-02-09 | 2018-03-08 | ボード オブ スーパーバイザーズ オブ ルイジアナ ステート ユニバーシティ アンド アグリカルチュアル アンド メカニカル カレッジ | 炎症性、変性及び神経変性疾患の治療のための化合物、組成物及び方法 |
| US10537541B2 (en) | 2015-10-02 | 2020-01-21 | Complexa Inc. | Treatment of focal segmental glomerular sclerosis (FSGS) using therapeutically effective oral doses of 10-nitro-9(E)-octadec-9-enoic acid |
| US11608342B2 (en) | 2015-07-07 | 2023-03-21 | H. Lundbeck A/S | PDE9 inhibitors with imidazo triazinone backbone and imidazo pyrazinone backbone for treatment of peripheral diseases |
| US12006319B2 (en) | 2018-05-25 | 2024-06-11 | Cardurion Pharmaceuticals, Inc. | Monohydrate and crystalline forms of 6-[(3S,4S)-4-methyl-1-(pyrimidin-2-ylmethyl)pyrrolidin-3-yl]-3-tetrahydropyran-4-yl-7H-imidazo[1,5-a]pyrazin-8-one |
| US12213975B2 (en) | 2018-08-31 | 2025-02-04 | Cardurion Pharmaceuticals, Inc. | PDE9 inhibitors for treating sickle cell disease |
Families Citing this family (60)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US7893106B2 (en) * | 2004-11-19 | 2011-02-22 | Martek Biosciences, Corporation | Oxylipins from stearidonic acid and γ-linolenic acid and methods of making and using the same |
| US20090318394A1 (en) * | 2004-11-19 | 2009-12-24 | Julie Nauroth | Long Chain Polyunsaturated Fatty Acids and Methods of Making and Using the Same |
| MX336435B (es) * | 2005-07-08 | 2016-01-19 | Dsm Ip Assets Bv | Acidos grasos poliinsaturados para el tratamiento de demencia y condiciones relacionadas con pre-demencia. |
| WO2007041440A2 (en) * | 2005-10-03 | 2007-04-12 | The Brigham And Women's Hospital, Inc. | Anti-inflammatory actions of neuroprotectin d1/protectin d1 and its natural stereoisomers |
| WO2007090162A2 (en) * | 2006-01-31 | 2007-08-09 | Martek Biosciences Corporation | OXYLIPINS FROM STEARIDONIC ACID AND γ-LINOLENIC ACID AND METHODS OF MAKING AND USING THE SAME |
| GB0604647D0 (en) | 2006-03-08 | 2006-04-19 | Shchepinov Mikhail | Stabilized food supplements and their derivatives |
| CA2650607A1 (en) * | 2006-04-28 | 2007-11-08 | Resolvyx Pharmaceuticals, Inc. | Compositions and methods for the treatment of cardiovascular disease |
| WO2008011085A1 (en) * | 2006-07-19 | 2008-01-24 | Resolvyx Pharmaceuticals, Inc. | Compositions and methods for the treatment of mucositis |
| US20080161274A1 (en) * | 2006-10-26 | 2008-07-03 | Resolvyx Pharmaceuticals, Inc. | Compounds and methods for inhibition of bone loss |
| WO2008070129A2 (en) * | 2006-12-05 | 2008-06-12 | Resolvyx Pharmaceuticals, Inc. | Compositions and methods for the treatment of inflammatory disease |
| US20110190389A1 (en) * | 2007-02-20 | 2011-08-04 | Linda Arterburn | Oxylipins from long chain polyunsaturated fatty acids and methods of making and using the same |
| PL2124973T3 (pl) * | 2007-03-23 | 2015-10-30 | Novartis Ag | Kompozycja zawierająca kwasy tłuszczowe omega-3 oraz zamaskowane lub powleczone sole miedzi |
| WO2008118457A2 (en) * | 2007-03-26 | 2008-10-02 | University Of Medicine And Dentistry Of Nj | Synthesis of resolvins and intermediates, compounds prepared thereby, and uses thereof |
| US8324277B2 (en) | 2007-08-01 | 2012-12-04 | University of Pittsburgh—of the Commonwealth System of Higher Education | Nitrated-fatty acids modulation of type II diabetes |
| CA2699483A1 (en) * | 2007-09-14 | 2009-03-26 | Resolvyx Pharmaceuticals, Inc. | Compositions and methods for modulating immune function |
| KR20150115959A (ko) * | 2007-10-12 | 2015-10-14 | 레솔빅스 파마슈티칼즈, 인코퍼레이티드 | 안과 질환의 치료를 위한 옥실리핀 화합물 |
| US20110027841A1 (en) * | 2007-12-21 | 2011-02-03 | Martek Biosciences Corporation | Method for preparation of oxylipins |
| CN102083787A (zh) | 2008-05-01 | 2011-06-01 | 康普雷克萨公司 | 乙烯基取代的脂肪酸 |
| US20140024713A1 (en) | 2008-06-19 | 2014-01-23 | University Of Utah Research Foundation | Use of nitrated lipids for treatment of side effects of toxic medical therapies |
| WO2009155439A2 (en) | 2008-06-19 | 2009-12-23 | University Of Utah Research Foundation | Use of nitrated lipids for treatment of side effects of toxic medical therapies |
| US8927747B2 (en) * | 2008-09-16 | 2015-01-06 | The Brigham And Women's Hospital, Inc. | 14-hydroxy-docosahexaenoic acid compounds |
| CA2742227C (en) * | 2008-10-31 | 2017-01-24 | Lipid Pharmaceuticals Ehf. | The use of fatty acid compositions as laxatives |
| WO2010095706A1 (ja) * | 2009-02-20 | 2010-08-26 | 国立大学法人東京大学 | 新規抗炎症性化合物 |
| ES2345241B1 (es) * | 2009-03-16 | 2011-09-08 | Lipopharma Therapeutics | Uso de 2-hidroxiderivados de acidos grasos poliinsaturados como medicamentos. |
| WO2010118761A1 (en) * | 2009-04-17 | 2010-10-21 | Eolas Science Limited | Compositions rich in omega-3 fatty acids with a low content in phytanic acid |
| LT2443246T (lt) | 2009-06-15 | 2018-03-26 | Amarin Pharmaceuticals Ireland Limited | Kompozicijos ir būdai, skirti trigliceridų sumažinimui, nepakeliant ldl-c lygio subjekte, kartu taikant statino terapiją |
| USRE46608E1 (en) | 2009-09-01 | 2017-11-14 | Catabasis Pharmaceuticals, Inc. | Fatty acid niacin conjugates and their uses |
| SG178948A1 (en) | 2009-09-01 | 2012-04-27 | Catabasis Pharmaceuticals Inc | Fatty acid niacin conjugates and their uses |
| US8686167B2 (en) | 2009-10-02 | 2014-04-01 | Complexa, Inc. | Heteroatom containing substituted fatty acids |
| AU2010313249B2 (en) | 2009-10-30 | 2016-05-19 | Biojiva Llc | Alleviating oxidative stress disorders with PUFA derivatives |
| WO2012023254A1 (ja) * | 2010-08-19 | 2012-02-23 | 国立大学法人 東京大学 | オメガ3系脂肪酸由来の新規抗炎症性代謝物 |
| AU2011336856A1 (en) | 2010-11-29 | 2013-07-04 | Amarin Pharmaceuticals Ireland Limited | Low eructation composition and methods for treating and/or preventing cardiovascular disease in a subject with fish allergy/hypersensitivity |
| US11712429B2 (en) | 2010-11-29 | 2023-08-01 | Amarin Pharmaceuticals Ireland Limited | Low eructation composition and methods for treating and/or preventing cardiovascular disease in a subject with fish allergy/hypersensitivity |
| CA2834342C (en) | 2011-04-26 | 2021-08-31 | Retrotope, Inc. | Impaired energy processing disorders and mitochondrial deficiency |
| KR102110175B1 (ko) | 2011-04-26 | 2020-05-13 | 레트로토프 인코포레이티드 | Pufa 산화와 관련된 장애 |
| US10154983B2 (en) | 2011-04-26 | 2018-12-18 | Retrotope, Inc. | Neurodegenerative disorders and muscle diseases implicating PUFAs |
| DK2701697T3 (da) | 2011-04-26 | 2020-06-29 | Retrotope Inc | Oxidative nethindesygdomme |
| US9416118B2 (en) | 2011-06-10 | 2016-08-16 | The Brigham And Women's Hospital, Inc. | Docosahexaenoyl ethanolamides |
| WO2013028501A1 (en) | 2011-08-19 | 2013-02-28 | The University Of Utah Research Foundation | Combination therapy with nitrated lipids and inhibitors of the renin-angiotensin-aldosterone system |
| US10322118B2 (en) * | 2012-04-10 | 2019-06-18 | Trustees Of Dartmouth College | Compounds and methods for inhibiting Cif virulence factor |
| EP2861227A4 (en) | 2012-06-17 | 2016-01-27 | Matinas Biopharma Inc | OMEGA-3 PENTAIC ACID COMPOSITIONS AND METHODS OF USE |
| PL2887923T3 (pl) | 2012-08-24 | 2023-08-14 | Sun Pharmaceutical Industries Limited | Preparat okulistyczny polioksylipidu lub polioksylowego kwasu tłuszczowego i leczenie chorób oczu |
| WO2014130894A1 (en) * | 2013-02-21 | 2014-08-28 | University Of Southern California | Compositions for the treatment of inflammatory diseases |
| EP2968246A4 (en) * | 2013-03-13 | 2016-08-03 | Matinas Biopharma Inc | OMEGA 3 PENTANOIC ACID COMPOSITIONS AND METHODS OF USE |
| EP3325438A4 (en) * | 2015-07-20 | 2019-04-03 | The Brigham and Women's Hospital, Inc. | STRUCTURAL EXPLANATION OF NOVEL 13-SERIES RESOLVINES THAT INCREASE WITH ATORVASTATIN AND REMOVAL INFECTIONS |
| WO2017041094A1 (en) | 2015-09-03 | 2017-03-09 | Solutex Na Llc | Compositions comprising omega-3 fatty acids, 17-hdha and 18- hepe and methods of using same |
| JP2018532809A (ja) | 2015-11-10 | 2018-11-08 | サン ファーマ グローバル エフジーイー | 局所製剤およびその使用 |
| EP3950649A1 (en) | 2015-11-23 | 2022-02-09 | Retrotope, Inc. | Site-specific isotopic labeling of 1, 4-diene systems |
| WO2019104311A1 (en) * | 2017-11-27 | 2019-05-31 | Brown University | Compositions and methods for suppressing neurological disease |
| WO2019104315A1 (en) * | 2017-11-27 | 2019-05-31 | Brown University | Compositions and methods using lipids for treating neurological disease |
| WO2019203540A2 (ko) | 2018-04-16 | 2019-10-24 | 한국생명공학연구원 | 다중불포화지방산의 멀티-하이드록시 유도체 생산 방법 |
| MA51766A (fr) | 2018-09-24 | 2020-12-16 | Amarin Pharmaceuticals Ie Ltd | Procédés de réduction du risque d'événements cardiovasculaires chez un sujet |
| KR102673270B1 (ko) * | 2018-10-15 | 2024-06-07 | (주)아모레퍼시픽 | 피부장벽 강화용 조성물 |
| KR102704402B1 (ko) | 2018-10-24 | 2024-09-06 | (주)아모레퍼시픽 | 피부장벽 강화용 조성물 |
| CN114980973A (zh) | 2019-11-12 | 2022-08-30 | 阿马里纳药物爱尔兰有限公司 | 降低心房纤颤和/或心房扑动受试者心血管事件风险的方法 |
| KR20220143931A (ko) | 2020-02-21 | 2022-10-25 | 레트로토프 인코포레이티드 | 고도불포화 지방산 및 이의 유도체의 동위원소 변형을 위한 공정 |
| US12109194B2 (en) | 2021-02-05 | 2024-10-08 | Biojiva Llc | Synergistic combination therapy for treating ALS |
| AU2022263358A1 (en) | 2021-04-21 | 2023-11-30 | Amarin Pharmaceuticals Ireland Limited | Methods of reducing the risk of heart failure |
| KR20220159751A (ko) | 2021-05-26 | 2022-12-05 | (주)아모레퍼시픽 | 미백용 조성물 및 이를 이용한 피부 미백방법 |
| WO2024229108A1 (en) * | 2023-05-01 | 2024-11-07 | The Regents Of The University Of California | In-situ production of anti-inflammatory lipids using milk fat globules |
Citations (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPS63238075A (ja) * | 1987-03-24 | 1988-10-04 | Mitsumaru Kagaku Kk | 高度不飽和脂肪酸からのラクトン類の製造法 |
| JPH05286956A (ja) * | 1992-04-06 | 1993-11-02 | Nippon Suisan Kaisha Ltd | ドコサペンタエン酸モノエポキシ体、及びその製造法 |
| JPH11209259A (ja) * | 1998-01-20 | 1999-08-03 | Noevir Co Ltd | 皮膚化粧料 |
| WO2002092540A1 (en) * | 2001-05-14 | 2002-11-21 | Martek Biosciences Corporation | Production and use of a polar lipid-rich fraction containing omega-3 and/or omega-6 highly unsatruated fatty acids from microbes, genetically modified plant seeds and marine organisms |
| JP2003525880A (ja) * | 2000-02-16 | 2003-09-02 | ザ・ブリガーム・アンド・ウーメンズ・ホスピタル・インコーポレーテッド | アスピリン誘発脂質メディエータ |
Family Cites Families (22)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4866046A (en) * | 1988-05-31 | 1989-09-12 | Top Laboratories, Inc. | Low-dosage sublingual aspirin |
| US5102670A (en) | 1990-09-14 | 1992-04-07 | Abraham Nader G | Method for treating eye disorders by reducing 12(r)-hydroxyeicosatetraenoic acid and 12(r)-dihydroxyeicosatrienoic acid levels |
| US6887901B1 (en) | 1993-06-15 | 2005-05-03 | Brigham & Women's Hospital, Inc. | Lipoxin compounds and their use in treating cell proliferative disorders |
| GB9403857D0 (en) * | 1994-03-01 | 1994-04-20 | Scotia Holdings Plc | Fatty acid derivatives |
| CZ281096B6 (cs) * | 1994-06-30 | 1996-06-12 | Jindřich Mudr. Drsc. Mourek | Tuková složka mléčné výživy a dětská výživa |
| US5955496A (en) | 1996-08-13 | 1999-09-21 | The Regents Of The University Of California | Dihydroxy-oxy-eicosadienoates |
| DK0956013T3 (da) | 1996-10-11 | 2003-08-04 | Scarista Ltd | Farmaceutisk præparat indeholdende eicosapentaensyre og/eller stearidonsyre |
| US6174695B1 (en) * | 1997-08-12 | 2001-01-16 | The Regents Of The University Of California | Epoxide hydrolase inhibitor methods |
| DE19757414A1 (de) | 1997-12-23 | 1999-07-01 | Nutricia Nv | Fettmischung |
| ES2286999T5 (es) | 1999-03-04 | 2016-11-17 | Suntory Holdings Limited | Utilización de un material que contiene ácido docosapentaenoico |
| EP1197560A4 (en) | 1999-07-07 | 2005-08-03 | Kyowa Hakko Kogyo Kk | PROCESS FOR PREPARING HYDROXILATED FATTY ACID AND DELTA LACTONE |
| BR0015404A (pt) | 1999-11-09 | 2002-06-25 | Alcon Inc | Compostos relacionados com ácido 15-hidroxieicosatetraenóico e métodos de uso |
| DE10046541A1 (de) | 2000-09-19 | 2002-03-28 | Knoll Ag | Mechanisch stabile darreichungsformen, enthaltend Ubichinone |
| WO2002029018A2 (en) | 2000-10-06 | 2002-04-11 | Michigan State University | Divinyl ether synthase gene and protein, and uses thereof |
| WO2003053423A2 (en) * | 2001-12-18 | 2003-07-03 | The Brigham And Women's Hospital | Inhibition or prevention of infection by bacteria with epa, dha or analogs |
| US7527935B2 (en) | 2002-03-19 | 2009-05-05 | Mitsubishi Tanabe Pharma Corporation | G-protein coupled receptor having eicosanoid as ligand and gene thereof |
| SI2022775T1 (sl) | 2002-04-01 | 2015-03-31 | University Of Southern California | Trihidroksi polinenasiäśeni eikosanoidi |
| US7582785B2 (en) | 2002-04-01 | 2009-09-01 | University Of Southern California | Trihydroxy polyunsaturated eicosanoid derivatives |
| FR2843124B1 (fr) | 2002-08-02 | 2004-10-15 | Goemar Lab Sa | Procede de preparation d'acides gras polyinsatures libres et de leurs metabolites d'oxydation |
| DK2216318T3 (en) * | 2002-08-12 | 2018-12-10 | Brigham & Womens Hospital | Resolvins: Bio templates for therapeutic interventions |
| US20040166130A1 (en) | 2002-12-23 | 2004-08-26 | L'oreal | Lanolin-free cosmetic composition comprising a hydroxylated fatty acid aromatic ester |
| US7893106B2 (en) | 2004-11-19 | 2011-02-22 | Martek Biosciences, Corporation | Oxylipins from stearidonic acid and γ-linolenic acid and methods of making and using the same |
-
2005
- 2005-11-21 KR KR1020077013738A patent/KR20070090928A/ko not_active Ceased
- 2005-11-21 BR BRPI0516803-1A patent/BRPI0516803A/pt not_active IP Right Cessation
- 2005-11-21 US US11/284,790 patent/US7884131B2/en not_active Expired - Fee Related
- 2005-11-21 CA CA002588166A patent/CA2588166A1/en not_active Abandoned
- 2005-11-21 CN CN2005800468877A patent/CN101102988B/zh not_active Expired - Fee Related
- 2005-11-21 JP JP2007543444A patent/JP2008520739A/ja active Pending
- 2005-11-21 MX MX2007006049A patent/MX2007006049A/es active IP Right Grant
- 2005-11-21 NZ NZ555394A patent/NZ555394A/en not_active IP Right Cessation
- 2005-11-21 EP EP05846821A patent/EP1824809A4/en not_active Withdrawn
- 2005-11-21 AU AU2005306320A patent/AU2005306320B2/en not_active Ceased
- 2005-11-21 WO PCT/US2005/042462 patent/WO2006055965A2/en not_active Ceased
- 2005-11-21 EA EA200701100A patent/EA010802B1/ru not_active IP Right Cessation
-
2007
- 2007-05-15 IL IL183246A patent/IL183246A0/en unknown
-
2010
- 2010-12-29 US US12/981,261 patent/US20110178047A1/en not_active Abandoned
Patent Citations (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPS63238075A (ja) * | 1987-03-24 | 1988-10-04 | Mitsumaru Kagaku Kk | 高度不飽和脂肪酸からのラクトン類の製造法 |
| JPH05286956A (ja) * | 1992-04-06 | 1993-11-02 | Nippon Suisan Kaisha Ltd | ドコサペンタエン酸モノエポキシ体、及びその製造法 |
| JPH11209259A (ja) * | 1998-01-20 | 1999-08-03 | Noevir Co Ltd | 皮膚化粧料 |
| JP2003525880A (ja) * | 2000-02-16 | 2003-09-02 | ザ・ブリガーム・アンド・ウーメンズ・ホスピタル・インコーポレーテッド | アスピリン誘発脂質メディエータ |
| WO2002092540A1 (en) * | 2001-05-14 | 2002-11-21 | Martek Biosciences Corporation | Production and use of a polar lipid-rich fraction containing omega-3 and/or omega-6 highly unsatruated fatty acids from microbes, genetically modified plant seeds and marine organisms |
| JP2004536059A (ja) * | 2001-05-14 | 2004-12-02 | マーテック バイオサイエンシズ ボールダー コーポレーション | 微生物、遺伝的に改変された植物種子および海洋生物由来のω−3および/またはω−6高度不飽和脂肪酸を含有する極性脂質の豊富な画分の生成および使用 |
Non-Patent Citations (7)
| Title |
|---|
| JPN6011060206; MILKS,M.M. and SPRECHER,H.: 'Metabolism of 4,7,10,13,16-docosapentaenoic acid by human platelet cyclooxygenase and lipoxygenase' Biochimica et Biophysica Acta, Lipids and Lipid Metabolism Vol.835, No.1, 1985, p.29-35 * |
| JPN6011060209; SPRECHER,H. and CAREAGA,M.M.: 'Metabolism of (n-6) and (n-3) polyunsaturated fatty acids by human platelets' Prostaglandins, Leukotrienes and Medicine Vol.23, No.2-3, 1986, p.129-134 * |
| JPN6011060211; OLIW,E.H. and SPRECHER,H.: 'Metabolism of polyunsaturated fatty acids by an (n-6)-lipoxygenase associated with human ejaculates' Biochimica et Biophysica Acta, Lipids and Lipid Metabolism Vol.1002, No.3, 1989, p.283-291 * |
| JPN6011060212; GUERRIERO,A. et al.: 'Hydroxyeicosatetraenoic, hydroxyeicosapentaenoic, hydroxydocosapentaenoic, and hydroxydocosahexaenoi' Journal of Natural Products Vol.53, No.1, 1990, p.57-61 * |
| JPN6011060213; KARANIAN,J.W. et al.: 'Inhibitory effects of n-6 and n-3 hydroxy fatty acids on thromboxane (U46619)-induced smooth muscle' Journal of Pharmacology and Experimental Therapeutics Vol.270, No.3, 1994, p.1105-1109 * |
| JPN6011060215; HAVIV,F. et al.: 'Structural requirements for the inhibition of 5-lipoxygenase by 15-hydroxyeicosa-5,8,11,13-tetraenoi' Journal of Medicinal Chemistry Vol.30, No.2, 1987, p.254-263 * |
| JPN6011060216; MANCINI,I. et al.: 'Novel 10-hydroxydocosapolyenoic acids from deep-water scleractinian corals' Helvetica Chimica Acta Vol.82, No.5, 1999, p.677-684 * |
Cited By (17)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2017114897A (ja) * | 2009-07-31 | 2017-06-29 | ユニバーシティー オブ ピッツバーグ − オブ ザ コモンウェルス システム オブ ハイヤー エデュケーション | 抗炎症剤としての脂肪酸 |
| JP2013500966A (ja) * | 2009-07-31 | 2013-01-10 | ユニバーシティー オブ ピッツバーグ − オブ ザ コモンウェルス システム オブ ハイヤー エデュケーション | 抗炎症剤としての脂肪酸 |
| JPWO2012026106A1 (ja) * | 2010-08-25 | 2013-10-28 | 国立大学法人 東京大学 | 光学活性な新規抗炎症性化合物及びその製造方法 |
| WO2012026106A1 (ja) * | 2010-08-25 | 2012-03-01 | 国立大学法人 東京大学 | 光学活性な新規抗炎症性化合物及びその製造方法 |
| JP2015522535A (ja) * | 2012-05-10 | 2015-08-06 | ソルテックス エヌエー エルエルシー | 天然の特異的炎症収束性メディエータおよびその前駆物質を含有する、抗炎症活性を有する油 |
| JP2018193378A (ja) * | 2012-05-10 | 2018-12-06 | ソルテックス エヌエー エルエルシー | 天然の特異的炎症収束性メディエータおよびその前駆物質を含有する、抗炎症活性を有する油 |
| JP2016505016A (ja) * | 2013-01-11 | 2016-02-18 | マサチューセッツ アイ アンド イヤー インファーマリー | 炎症及び血管形成を減少させるcyp450脂質メタボライト |
| JP2016520612A (ja) * | 2013-05-30 | 2016-07-14 | ザ ブリガム アンド ウィメンズ ホスピタル インコーポレイテッドThe Brigham and Women’s Hospital, Inc. | 新規n−3イムノリソルベント:構造及び作用 |
| JP2019163265A (ja) * | 2013-05-30 | 2019-09-26 | ザ ブリガム アンド ウィメンズ ホスピタル インコーポレイテッドThe Brigham and Women’s Hospital, Inc. | 新規n−3イムノリソルベント:構造及び作用 |
| US11667598B2 (en) | 2013-05-30 | 2023-06-06 | The Brigham & Women's Hospital Inc. | N-3 immunoresolvents: structures and actions |
| JP2018506584A (ja) * | 2015-02-09 | 2018-03-08 | ボード オブ スーパーバイザーズ オブ ルイジアナ ステート ユニバーシティ アンド アグリカルチュアル アンド メカニカル カレッジ | 炎症性、変性及び神経変性疾患の治療のための化合物、組成物及び方法 |
| JP7025212B2 (ja) | 2015-02-09 | 2022-02-24 | ボード オブ スーパーバイザーズ オブ ルイジアナ ステート ユニバーシティ アンド アグリカルチュアル アンド メカニカル カレッジ | 炎症性、変性及び神経変性疾患の治療のための化合物、組成物及び方法 |
| US11608342B2 (en) | 2015-07-07 | 2023-03-21 | H. Lundbeck A/S | PDE9 inhibitors with imidazo triazinone backbone and imidazo pyrazinone backbone for treatment of peripheral diseases |
| US10537541B2 (en) | 2015-10-02 | 2020-01-21 | Complexa Inc. | Treatment of focal segmental glomerular sclerosis (FSGS) using therapeutically effective oral doses of 10-nitro-9(E)-octadec-9-enoic acid |
| US12006319B2 (en) | 2018-05-25 | 2024-06-11 | Cardurion Pharmaceuticals, Inc. | Monohydrate and crystalline forms of 6-[(3S,4S)-4-methyl-1-(pyrimidin-2-ylmethyl)pyrrolidin-3-yl]-3-tetrahydropyran-4-yl-7H-imidazo[1,5-a]pyrazin-8-one |
| US12466832B2 (en) | 2018-05-25 | 2025-11-11 | Cardurion Pharmaceuticals, Inc. | Monohydrate and crystalline forms of 6-[(3S,4S)-4-methyl-1-(pyrimidin-2-ylmethyl)pyrrolidin-3-yl]-3-tetrahydropyran-4-yl-7H-imidazo[1,5-a]pyrazin-8-one |
| US12213975B2 (en) | 2018-08-31 | 2025-02-04 | Cardurion Pharmaceuticals, Inc. | PDE9 inhibitors for treating sickle cell disease |
Also Published As
| Publication number | Publication date |
|---|---|
| BRPI0516803A (pt) | 2008-09-23 |
| EA010802B1 (ru) | 2008-12-30 |
| AU2005306320B2 (en) | 2011-09-08 |
| KR20070090928A (ko) | 2007-09-06 |
| AU2005306320A1 (en) | 2006-05-26 |
| US7884131B2 (en) | 2011-02-08 |
| CN101102988A (zh) | 2008-01-09 |
| EP1824809A2 (en) | 2007-08-29 |
| EA200701100A1 (ru) | 2007-10-26 |
| IL183246A0 (en) | 2007-08-19 |
| US20110178047A1 (en) | 2011-07-21 |
| WO2006055965A3 (en) | 2006-11-30 |
| EP1824809A4 (en) | 2010-03-03 |
| MX2007006049A (es) | 2007-07-24 |
| CN101102988B (zh) | 2011-12-14 |
| CA2588166A1 (en) | 2006-05-26 |
| US20060241088A1 (en) | 2006-10-26 |
| NZ555394A (en) | 2010-07-30 |
| WO2006055965A2 (en) | 2006-05-26 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP2008520739A (ja) | 長鎖多価不飽和脂肪酸由来のオキシリピンならびにその作製および使用方法 | |
| JP2010519311A (ja) | 長鎖多価不飽和脂肪酸由来のオキシリピンならびにその作製およびその使用方法 | |
| US7893106B2 (en) | Oxylipins from stearidonic acid and γ-linolenic acid and methods of making and using the same | |
| US20090318394A1 (en) | Long Chain Polyunsaturated Fatty Acids and Methods of Making and Using the Same | |
| DE60124256T3 (de) | Aspirin-ausgelöste lipidmediatoren | |
| US20090320148A1 (en) | Oxylipins from stearidonic acid and gamma-linolenic acid and methods of making and using the same | |
| JP2010519311A5 (enExample) | ||
| AU2014358123B2 (en) | Dihomo-gamma-linolenic acid-containing microbial oil and dihomo-gamma-linolenic acid-containing microbial biomass | |
| JP7242769B2 (ja) | 遊離多価不飽和脂肪酸含有組成物及びその製造方法 | |
| AU2011253846A1 (en) | Oxylipins from long chain polyunsaturated fatty acids and methods of making and using the same | |
| US20110027841A1 (en) | Method for preparation of oxylipins | |
| HK1105948B (en) | Aspirin triggered lipid mediators |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20081120 |
|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20081120 |
|
| A977 | Report on retrieval |
Free format text: JAPANESE INTERMEDIATE CODE: A971007 Effective date: 20111109 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20111116 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20120214 |
|
| A602 | Written permission of extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A602 Effective date: 20120221 |
|
| A02 | Decision of refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A02 Effective date: 20121017 |