JP2006524986A - Fc融合体 - Google Patents
Fc融合体 Download PDFInfo
- Publication number
- JP2006524986A JP2006524986A JP2004563348A JP2004563348A JP2006524986A JP 2006524986 A JP2006524986 A JP 2006524986A JP 2004563348 A JP2004563348 A JP 2004563348A JP 2004563348 A JP2004563348 A JP 2004563348A JP 2006524986 A JP2006524986 A JP 2006524986A
- Authority
- JP
- Japan
- Prior art keywords
- dab
- antibody
- effector group
- effector
- variable domain
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Pending
Links
- 230000004927 fusion Effects 0.000 title claims description 36
- 239000012636 effector Substances 0.000 claims abstract description 267
- 238000000034 method Methods 0.000 claims abstract description 118
- 230000027455 binding Effects 0.000 claims abstract description 76
- 238000001727 in vivo Methods 0.000 claims abstract description 18
- 108090000623 proteins and genes Proteins 0.000 claims description 105
- 210000004027 cell Anatomy 0.000 claims description 80
- 239000013598 vector Substances 0.000 claims description 78
- 102000004169 proteins and genes Human genes 0.000 claims description 66
- 108090000765 processed proteins & peptides Proteins 0.000 claims description 61
- 229920001184 polypeptide Polymers 0.000 claims description 57
- 102000004196 processed proteins & peptides Human genes 0.000 claims description 57
- 230000014509 gene expression Effects 0.000 claims description 47
- 150000007523 nucleic acids Chemical class 0.000 claims description 41
- 239000000203 mixture Substances 0.000 claims description 37
- 102000039446 nucleic acids Human genes 0.000 claims description 34
- 108020004707 nucleic acids Proteins 0.000 claims description 34
- 108060003951 Immunoglobulin Proteins 0.000 claims description 33
- 102000018358 immunoglobulin Human genes 0.000 claims description 33
- 238000011282 treatment Methods 0.000 claims description 27
- 108060008682 Tumor Necrosis Factor Proteins 0.000 claims description 22
- 102000000852 Tumor Necrosis Factor-alpha Human genes 0.000 claims description 22
- 201000010099 disease Diseases 0.000 claims description 16
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 claims description 16
- 230000002265 prevention Effects 0.000 claims description 15
- 150000001413 amino acids Chemical class 0.000 claims description 14
- 101000611183 Homo sapiens Tumor necrosis factor Proteins 0.000 claims description 12
- 241001465754 Metazoa Species 0.000 claims description 11
- 239000003814 drug Substances 0.000 claims description 11
- 238000002360 preparation method Methods 0.000 claims description 11
- 210000004602 germ cell Anatomy 0.000 claims description 10
- 102000057041 human TNF Human genes 0.000 claims description 10
- 208000027866 inflammatory disease Diseases 0.000 claims description 10
- NFGXHKASABOEEW-UHFFFAOYSA-N 1-methylethyl 11-methoxy-3,7,11-trimethyl-2,4-dodecadienoate Chemical compound COC(C)(C)CCCC(C)CC=CC(C)=CC(=O)OC(C)C NFGXHKASABOEEW-UHFFFAOYSA-N 0.000 claims description 7
- 230000003053 immunization Effects 0.000 claims description 7
- 238000002649 immunization Methods 0.000 claims description 7
- 206010006895 Cachexia Diseases 0.000 claims description 6
- 208000022559 Inflammatory bowel disease Diseases 0.000 claims description 6
- 239000000539 dimer Substances 0.000 claims description 6
- 230000001404 mediated effect Effects 0.000 claims description 5
- 206010039073 rheumatoid arthritis Diseases 0.000 claims description 5
- 208000011231 Crohn disease Diseases 0.000 claims description 4
- 108010076504 Protein Sorting Signals Proteins 0.000 claims description 4
- 108010026228 mRNA guanylyltransferase Proteins 0.000 claims description 4
- 201000006417 multiple sclerosis Diseases 0.000 claims description 4
- 206010011091 Coronary artery thrombosis Diseases 0.000 claims description 3
- 201000004681 Psoriasis Diseases 0.000 claims description 3
- 206010040070 Septic Shock Diseases 0.000 claims description 3
- 230000001684 chronic effect Effects 0.000 claims description 3
- 208000002528 coronary thrombosis Diseases 0.000 claims description 3
- 201000008383 nephritis Diseases 0.000 claims description 3
- 230000036303 septic shock Effects 0.000 claims description 3
- 230000002194 synthesizing effect Effects 0.000 claims description 3
- 239000003085 diluting agent Substances 0.000 claims description 2
- 239000003937 drug carrier Substances 0.000 claims description 2
- 239000000546 pharmaceutical excipient Substances 0.000 claims description 2
- 101000686985 Mouse mammary tumor virus (strain C3H) Protein PR73 Proteins 0.000 claims 1
- 210000000805 cytoplasm Anatomy 0.000 claims 1
- 230000029142 excretion Effects 0.000 claims 1
- 210000004072 lung Anatomy 0.000 claims 1
- 230000000414 obstructive effect Effects 0.000 claims 1
- 231100000617 superantigen Toxicity 0.000 claims 1
- 239000013638 trimer Substances 0.000 claims 1
- 239000000427 antigen Substances 0.000 description 68
- 108091007433 antigens Proteins 0.000 description 68
- 102000036639 antigens Human genes 0.000 description 68
- 235000018102 proteins Nutrition 0.000 description 65
- 108020001507 fusion proteins Proteins 0.000 description 34
- 102000037865 fusion proteins Human genes 0.000 description 34
- 238000010367 cloning Methods 0.000 description 25
- 206010003246 arthritis Diseases 0.000 description 21
- 239000013604 expression vector Substances 0.000 description 21
- 238000004458 analytical method Methods 0.000 description 18
- 230000010076 replication Effects 0.000 description 18
- 108020004414 DNA Proteins 0.000 description 17
- 238000000338 in vitro Methods 0.000 description 16
- FAPWRFPIFSIZLT-UHFFFAOYSA-M Sodium chloride Chemical compound [Na+].[Cl-] FAPWRFPIFSIZLT-UHFFFAOYSA-M 0.000 description 15
- 241000588724 Escherichia coli Species 0.000 description 14
- 240000004808 Saccharomyces cerevisiae Species 0.000 description 14
- 235000014680 Saccharomyces cerevisiae Nutrition 0.000 description 14
- 108060008683 Tumor Necrosis Factor Receptor Proteins 0.000 description 13
- 230000000694 effects Effects 0.000 description 13
- 239000012634 fragment Substances 0.000 description 13
- 239000013612 plasmid Substances 0.000 description 13
- 102000003298 tumor necrosis factor receptor Human genes 0.000 description 13
- 238000002965 ELISA Methods 0.000 description 12
- 241000699670 Mus sp. Species 0.000 description 12
- 239000003446 ligand Substances 0.000 description 12
- 210000004962 mammalian cell Anatomy 0.000 description 12
- 239000002773 nucleotide Substances 0.000 description 12
- 125000003729 nucleotide group Chemical group 0.000 description 12
- 108010087819 Fc receptors Proteins 0.000 description 11
- 102000009109 Fc receptors Human genes 0.000 description 11
- 108091006020 Fc-tagged proteins Proteins 0.000 description 11
- 108010021625 Immunoglobulin Fragments Proteins 0.000 description 11
- 102000008394 Immunoglobulin Fragments Human genes 0.000 description 11
- 241000235058 Komagataella pastoris Species 0.000 description 11
- 230000006870 function Effects 0.000 description 11
- 241001515965 unidentified phage Species 0.000 description 11
- 108091026890 Coding region Proteins 0.000 description 10
- 239000000047 product Substances 0.000 description 10
- 210000002966 serum Anatomy 0.000 description 10
- OKKJLVBELUTLKV-UHFFFAOYSA-N Methanol Chemical compound OC OKKJLVBELUTLKV-UHFFFAOYSA-N 0.000 description 9
- IXKSXJFAGXLQOQ-XISFHERQSA-N WHWLQLKPGQPMY Chemical compound C([C@@H](C(=O)N[C@@H](CC=1C2=CC=CC=C2NC=1)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC(C)C)C(=O)N1CCC[C@H]1C(=O)NCC(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC(O)=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCSC)C(=O)N[C@@H](CC=1C=CC(O)=CC=1)C(O)=O)NC(=O)[C@@H](N)CC=1C2=CC=CC=C2NC=1)C1=CNC=N1 IXKSXJFAGXLQOQ-XISFHERQSA-N 0.000 description 9
- 235000001014 amino acid Nutrition 0.000 description 9
- 229940024606 amino acid Drugs 0.000 description 9
- 230000001580 bacterial effect Effects 0.000 description 9
- 230000035772 mutation Effects 0.000 description 9
- 239000011780 sodium chloride Substances 0.000 description 9
- 239000001963 growth medium Substances 0.000 description 8
- 239000003550 marker Substances 0.000 description 8
- 239000002953 phosphate buffered saline Substances 0.000 description 8
- 239000013615 primer Substances 0.000 description 8
- 238000000746 purification Methods 0.000 description 8
- 230000001225 therapeutic effect Effects 0.000 description 8
- 241000894006 Bacteria Species 0.000 description 7
- WQZGKKKJIJFFOK-GASJEMHNSA-N Glucose Natural products OC[C@H]1OC(O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-GASJEMHNSA-N 0.000 description 7
- PEDCQBHIVMGVHV-UHFFFAOYSA-N Glycerine Chemical compound OCC(O)CO PEDCQBHIVMGVHV-UHFFFAOYSA-N 0.000 description 7
- 238000003556 assay Methods 0.000 description 7
- 238000010276 construction Methods 0.000 description 7
- 230000013595 glycosylation Effects 0.000 description 7
- 238000006206 glycosylation reaction Methods 0.000 description 7
- 239000007924 injection Substances 0.000 description 7
- 238000002347 injection Methods 0.000 description 7
- 241000894007 species Species 0.000 description 7
- 101710117290 Aldo-keto reductase family 1 member C4 Proteins 0.000 description 6
- 102000002260 Alkaline Phosphatase Human genes 0.000 description 6
- 108020004774 Alkaline Phosphatase Proteins 0.000 description 6
- 108090000204 Dipeptidase 1 Proteins 0.000 description 6
- 101000917826 Homo sapiens Low affinity immunoglobulin gamma Fc region receptor II-a Proteins 0.000 description 6
- 101000917824 Homo sapiens Low affinity immunoglobulin gamma Fc region receptor II-b Proteins 0.000 description 6
- 241000124008 Mammalia Species 0.000 description 6
- 101150117115 V gene Proteins 0.000 description 6
- LOKCTEFSRHRXRJ-UHFFFAOYSA-I dipotassium trisodium dihydrogen phosphate hydrogen phosphate dichloride Chemical compound P(=O)(O)(O)[O-].[K+].P(=O)(O)([O-])[O-].[Na+].[Na+].[Cl-].[K+].[Cl-].[Na+] LOKCTEFSRHRXRJ-UHFFFAOYSA-I 0.000 description 6
- 238000005516 engineering process Methods 0.000 description 6
- 239000000499 gel Substances 0.000 description 6
- 230000001900 immune effect Effects 0.000 description 6
- 230000001965 increasing effect Effects 0.000 description 6
- 238000004519 manufacturing process Methods 0.000 description 6
- 238000002703 mutagenesis Methods 0.000 description 6
- 231100000350 mutagenesis Toxicity 0.000 description 6
- 238000002823 phage display Methods 0.000 description 6
- 102000005962 receptors Human genes 0.000 description 6
- 108020003175 receptors Proteins 0.000 description 6
- 238000002415 sodium dodecyl sulfate polyacrylamide gel electrophoresis Methods 0.000 description 6
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Chemical compound O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 6
- -1 GF-β1 Proteins 0.000 description 5
- DHMQDGOQFOQNFH-UHFFFAOYSA-N Glycine Chemical compound NCC(O)=O DHMQDGOQFOQNFH-UHFFFAOYSA-N 0.000 description 5
- 108010001336 Horseradish Peroxidase Proteins 0.000 description 5
- 241000699666 Mus <mouse, genus> Species 0.000 description 5
- 241000235648 Pichia Species 0.000 description 5
- 238000002835 absorbance Methods 0.000 description 5
- 230000003321 amplification Effects 0.000 description 5
- 230000015572 biosynthetic process Effects 0.000 description 5
- 239000000872 buffer Substances 0.000 description 5
- 230000000295 complement effect Effects 0.000 description 5
- 239000000470 constituent Substances 0.000 description 5
- 238000011161 development Methods 0.000 description 5
- 238000001943 fluorescence-activated cell sorting Methods 0.000 description 5
- 230000006698 induction Effects 0.000 description 5
- 238000003199 nucleic acid amplification method Methods 0.000 description 5
- 239000013641 positive control Substances 0.000 description 5
- 230000000069 prophylactic effect Effects 0.000 description 5
- 239000000126 substance Substances 0.000 description 5
- 238000003786 synthesis reaction Methods 0.000 description 5
- YBJHBAHKTGYVGT-ZKWXMUAHSA-N (+)-Biotin Chemical compound N1C(=O)N[C@@H]2[C@H](CCCCC(=O)O)SC[C@@H]21 YBJHBAHKTGYVGT-ZKWXMUAHSA-N 0.000 description 4
- 108091032973 (ribonucleotides)n+m Proteins 0.000 description 4
- IJGRMHOSHXDMSA-UHFFFAOYSA-N Atomic nitrogen Chemical compound N#N IJGRMHOSHXDMSA-UHFFFAOYSA-N 0.000 description 4
- 102100026189 Beta-galactosidase Human genes 0.000 description 4
- 102100036845 C-C motif chemokine 22 Human genes 0.000 description 4
- FBPFZTCFMRRESA-FSIIMWSLSA-N D-Glucitol Natural products OC[C@H](O)[C@H](O)[C@@H](O)[C@H](O)CO FBPFZTCFMRRESA-FSIIMWSLSA-N 0.000 description 4
- 101710121366 Disintegrin and metalloproteinase domain-containing protein 11 Proteins 0.000 description 4
- 102000004190 Enzymes Human genes 0.000 description 4
- 108090000790 Enzymes Proteins 0.000 description 4
- 238000012413 Fluorescence activated cell sorting analysis Methods 0.000 description 4
- 241000282412 Homo Species 0.000 description 4
- VEXZGXHMUGYJMC-UHFFFAOYSA-N Hydrochloric acid Chemical compound Cl VEXZGXHMUGYJMC-UHFFFAOYSA-N 0.000 description 4
- 108091028043 Nucleic acid sequence Proteins 0.000 description 4
- 108091034117 Oligonucleotide Proteins 0.000 description 4
- 108091005804 Peptidases Proteins 0.000 description 4
- 239000001888 Peptone Substances 0.000 description 4
- 108010080698 Peptones Proteins 0.000 description 4
- 239000004365 Protease Substances 0.000 description 4
- 229960000723 ampicillin Drugs 0.000 description 4
- AVKUERGKIZMTKX-NJBDSQKTSA-N ampicillin Chemical compound C1([C@@H](N)C(=O)N[C@H]2[C@H]3SC([C@@H](N3C2=O)C(O)=O)(C)C)=CC=CC=C1 AVKUERGKIZMTKX-NJBDSQKTSA-N 0.000 description 4
- 238000000137 annealing Methods 0.000 description 4
- WQZGKKKJIJFFOK-VFUOTHLCSA-N beta-D-glucose Chemical compound OC[C@H]1O[C@@H](O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-VFUOTHLCSA-N 0.000 description 4
- 108010005774 beta-Galactosidase Proteins 0.000 description 4
- 229940079593 drug Drugs 0.000 description 4
- 239000000839 emulsion Substances 0.000 description 4
- 229940088598 enzyme Drugs 0.000 description 4
- GNBHRKFJIUUOQI-UHFFFAOYSA-N fluorescein Chemical compound O1C(=O)C2=CC=CC=C2C21C1=CC=C(O)C=C1OC1=CC(O)=CC=C21 GNBHRKFJIUUOQI-UHFFFAOYSA-N 0.000 description 4
- 230000002068 genetic effect Effects 0.000 description 4
- 239000008103 glucose Substances 0.000 description 4
- 208000025750 heavy chain disease Diseases 0.000 description 4
- 210000004408 hybridoma Anatomy 0.000 description 4
- 230000001939 inductive effect Effects 0.000 description 4
- 239000003112 inhibitor Substances 0.000 description 4
- 230000003993 interaction Effects 0.000 description 4
- 230000004048 modification Effects 0.000 description 4
- 238000012986 modification Methods 0.000 description 4
- 239000013642 negative control Substances 0.000 description 4
- 235000019319 peptone Nutrition 0.000 description 4
- 238000012216 screening Methods 0.000 description 4
- 239000000600 sorbitol Substances 0.000 description 4
- 230000003442 weekly effect Effects 0.000 description 4
- 101100348617 Candida albicans (strain SC5314 / ATCC MYA-2876) NIK1 gene Proteins 0.000 description 3
- 102000004127 Cytokines Human genes 0.000 description 3
- 108090000695 Cytokines Proteins 0.000 description 3
- 230000004543 DNA replication Effects 0.000 description 3
- 241000701959 Escherichia virus Lambda Species 0.000 description 3
- 108090001047 Fibroblast growth factor 10 Proteins 0.000 description 3
- 241000724791 Filamentous phage Species 0.000 description 3
- 102100026122 High affinity immunoglobulin gamma Fc receptor I Human genes 0.000 description 3
- 101000913074 Homo sapiens High affinity immunoglobulin gamma Fc receptor I Proteins 0.000 description 3
- 108010054477 Immunoglobulin Fab Fragments Proteins 0.000 description 3
- 102000001706 Immunoglobulin Fab Fragments Human genes 0.000 description 3
- FBOZXECLQNJBKD-ZDUSSCGKSA-N L-methotrexate Chemical compound C=1N=C2N=C(N)N=C(N)C2=NC=1CN(C)C1=CC=C(C(=O)N[C@@H](CCC(O)=O)C(O)=O)C=C1 FBOZXECLQNJBKD-ZDUSSCGKSA-N 0.000 description 3
- 102100029204 Low affinity immunoglobulin gamma Fc region receptor II-a Human genes 0.000 description 3
- 206010028980 Neoplasm Diseases 0.000 description 3
- 241000700159 Rattus Species 0.000 description 3
- 102100037486 Reverse transcriptase/ribonuclease H Human genes 0.000 description 3
- 101100007329 Saccharomyces cerevisiae (strain ATCC 204508 / S288c) COS1 gene Proteins 0.000 description 3
- 238000012300 Sequence Analysis Methods 0.000 description 3
- 108091008874 T cell receptors Proteins 0.000 description 3
- 102000016266 T-Cell Antigen Receptors Human genes 0.000 description 3
- 206010067584 Type 1 diabetes mellitus Diseases 0.000 description 3
- JLCPHMBAVCMARE-UHFFFAOYSA-N [3-[[3-[[3-[[3-[[3-[[3-[[3-[[3-[[3-[[3-[[3-[[5-(2-amino-6-oxo-1H-purin-9-yl)-3-[[3-[[3-[[3-[[3-[[3-[[5-(2-amino-6-oxo-1H-purin-9-yl)-3-[[5-(2-amino-6-oxo-1H-purin-9-yl)-3-hydroxyoxolan-2-yl]methoxy-hydroxyphosphoryl]oxyoxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxyoxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methyl [5-(6-aminopurin-9-yl)-2-(hydroxymethyl)oxolan-3-yl] hydrogen phosphate Polymers Cc1cn(C2CC(OP(O)(=O)OCC3OC(CC3OP(O)(=O)OCC3OC(CC3O)n3cnc4c3nc(N)[nH]c4=O)n3cnc4c3nc(N)[nH]c4=O)C(COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3CO)n3cnc4c(N)ncnc34)n3ccc(N)nc3=O)n3cnc4c(N)ncnc34)n3ccc(N)nc3=O)n3ccc(N)nc3=O)n3ccc(N)nc3=O)n3cnc4c(N)ncnc34)n3cnc4c(N)ncnc34)n3cc(C)c(=O)[nH]c3=O)n3cc(C)c(=O)[nH]c3=O)n3ccc(N)nc3=O)n3cc(C)c(=O)[nH]c3=O)n3cnc4c3nc(N)[nH]c4=O)n3cnc4c(N)ncnc34)n3cnc4c(N)ncnc34)n3cnc4c(N)ncnc34)n3cnc4c(N)ncnc34)O2)c(=O)[nH]c1=O JLCPHMBAVCMARE-UHFFFAOYSA-N 0.000 description 3
- 230000003213 activating effect Effects 0.000 description 3
- 230000009831 antigen interaction Effects 0.000 description 3
- 210000003719 b-lymphocyte Anatomy 0.000 description 3
- 230000008901 benefit Effects 0.000 description 3
- 102000006635 beta-lactamase Human genes 0.000 description 3
- 230000004071 biological effect Effects 0.000 description 3
- 210000004369 blood Anatomy 0.000 description 3
- 239000008280 blood Substances 0.000 description 3
- 230000037396 body weight Effects 0.000 description 3
- 210000004899 c-terminal region Anatomy 0.000 description 3
- 201000011510 cancer Diseases 0.000 description 3
- 229940041514 candida albicans extract Drugs 0.000 description 3
- 210000004978 chinese hamster ovary cell Anatomy 0.000 description 3
- 238000004587 chromatography analysis Methods 0.000 description 3
- 238000003776 cleavage reaction Methods 0.000 description 3
- 230000009260 cross reactivity Effects 0.000 description 3
- 238000001514 detection method Methods 0.000 description 3
- 239000008121 dextrose Substances 0.000 description 3
- 238000004520 electroporation Methods 0.000 description 3
- 230000012010 growth Effects 0.000 description 3
- 210000000987 immune system Anatomy 0.000 description 3
- 229940072221 immunoglobulins Drugs 0.000 description 3
- 230000033001 locomotion Effects 0.000 description 3
- 239000002609 medium Substances 0.000 description 3
- 229960000485 methotrexate Drugs 0.000 description 3
- 239000003094 microcapsule Substances 0.000 description 3
- 230000000051 modifying effect Effects 0.000 description 3
- 238000010369 molecular cloning Methods 0.000 description 3
- 239000000178 monomer Substances 0.000 description 3
- 206010028417 myasthenia gravis Diseases 0.000 description 3
- 235000015097 nutrients Nutrition 0.000 description 3
- 210000001322 periplasm Anatomy 0.000 description 3
- 238000001525 receptor binding assay Methods 0.000 description 3
- 230000007017 scission Effects 0.000 description 3
- 238000012163 sequencing technique Methods 0.000 description 3
- 238000010561 standard procedure Methods 0.000 description 3
- 239000000758 substrate Substances 0.000 description 3
- 239000006228 supernatant Substances 0.000 description 3
- 230000001629 suppression Effects 0.000 description 3
- 239000000725 suspension Substances 0.000 description 3
- 201000000596 systemic lupus erythematosus Diseases 0.000 description 3
- 238000012360 testing method Methods 0.000 description 3
- 210000001519 tissue Anatomy 0.000 description 3
- 230000009466 transformation Effects 0.000 description 3
- 230000003612 virological effect Effects 0.000 description 3
- 238000001262 western blot Methods 0.000 description 3
- 239000012138 yeast extract Substances 0.000 description 3
- SBKVPJHMSUXZTA-MEJXFZFPSA-N (2S)-2-[[(2S)-2-[[(2S)-1-[(2S)-5-amino-2-[[2-[[(2S)-1-[(2S)-6-amino-2-[[(2S)-2-[[(2S)-5-amino-2-[[(2S)-2-[[(2S)-2-[[(2S)-2-[[(2S)-2-amino-3-(1H-indol-3-yl)propanoyl]amino]-3-(1H-imidazol-4-yl)propanoyl]amino]-3-(1H-indol-3-yl)propanoyl]amino]-4-methylpentanoyl]amino]-5-oxopentanoyl]amino]-4-methylpentanoyl]amino]hexanoyl]pyrrolidine-2-carbonyl]amino]acetyl]amino]-5-oxopentanoyl]pyrrolidine-2-carbonyl]amino]-4-methylsulfanylbutanoyl]amino]-3-(4-hydroxyphenyl)propanoic acid Chemical compound C([C@@H](C(=O)N[C@@H](CC=1C2=CC=CC=C2NC=1)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCCN)C(=O)N1CCC[C@H]1C(=O)NCC(=O)N[C@@H](CCC(N)=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCSC)C(=O)N[C@@H](CC=1C=CC(O)=CC=1)C(O)=O)NC(=O)[C@@H](N)CC=1C2=CC=CC=C2NC=1)C1=CNC=N1 SBKVPJHMSUXZTA-MEJXFZFPSA-N 0.000 description 2
- QKNYBSVHEMOAJP-UHFFFAOYSA-N 2-amino-2-(hydroxymethyl)propane-1,3-diol;hydron;chloride Chemical compound Cl.OCC(N)(CO)CO QKNYBSVHEMOAJP-UHFFFAOYSA-N 0.000 description 2
- FTOAOBMCPZCFFF-UHFFFAOYSA-N 5,5-diethylbarbituric acid Chemical compound CCC1(CC)C(=O)NC(=O)NC1=O FTOAOBMCPZCFFF-UHFFFAOYSA-N 0.000 description 2
- 101100136076 Aspergillus oryzae (strain ATCC 42149 / RIB 40) pel1 gene Proteins 0.000 description 2
- 102100023995 Beta-nerve growth factor Human genes 0.000 description 2
- 206010051728 Bone erosion Diseases 0.000 description 2
- 102100021943 C-C motif chemokine 2 Human genes 0.000 description 2
- 101001007681 Candida albicans (strain WO-1) Kexin Proteins 0.000 description 2
- 101710167800 Capsid assembly scaffolding protein Proteins 0.000 description 2
- 101710132601 Capsid protein Proteins 0.000 description 2
- OKTJSMMVPCPJKN-UHFFFAOYSA-N Carbon Chemical compound [C] OKTJSMMVPCPJKN-UHFFFAOYSA-N 0.000 description 2
- 208000006545 Chronic Obstructive Pulmonary Disease Diseases 0.000 description 2
- 101710094648 Coat protein Proteins 0.000 description 2
- 108020004705 Codon Proteins 0.000 description 2
- 102000053602 DNA Human genes 0.000 description 2
- 108010014303 DNA-directed DNA polymerase Proteins 0.000 description 2
- 102000016928 DNA-directed DNA polymerase Human genes 0.000 description 2
- AOJJSUZBOXZQNB-TZSSRYMLSA-N Doxorubicin Chemical compound O([C@H]1C[C@@](O)(CC=2C(O)=C3C(=O)C=4C=CC=C(C=4C(=O)C3=C(O)C=21)OC)C(=O)CO)[C@H]1C[C@H](N)[C@H](O)[C@H](C)O1 AOJJSUZBOXZQNB-TZSSRYMLSA-N 0.000 description 2
- 108010074860 Factor Xa Proteins 0.000 description 2
- 102000004864 Fibroblast growth factor 10 Human genes 0.000 description 2
- 108700028146 Genetic Enhancer Elements Proteins 0.000 description 2
- 239000004471 Glycine Substances 0.000 description 2
- 102100021181 Golgi phosphoprotein 3 Human genes 0.000 description 2
- 102100034221 Growth-regulated alpha protein Human genes 0.000 description 2
- 108010067060 Immunoglobulin Variable Region Proteins 0.000 description 2
- 102000017727 Immunoglobulin Variable Region Human genes 0.000 description 2
- QIVBCDIJIAJPQS-VIFPVBQESA-N L-tryptophane Chemical compound C1=CC=C2C(C[C@H](N)C(O)=O)=CNC2=C1 QIVBCDIJIAJPQS-VIFPVBQESA-N 0.000 description 2
- GUBGYTABKSRVRQ-QKKXKWKRSA-N Lactose Natural products OC[C@H]1O[C@@H](O[C@H]2[C@H](O)[C@@H](O)C(O)O[C@@H]2CO)[C@H](O)[C@@H](O)[C@H]1O GUBGYTABKSRVRQ-QKKXKWKRSA-N 0.000 description 2
- 101710125418 Major capsid protein Proteins 0.000 description 2
- 108010038049 Mating Factor Proteins 0.000 description 2
- 241000699660 Mus musculus Species 0.000 description 2
- 229930193140 Neomycin Natural products 0.000 description 2
- 108091005461 Nucleic proteins Proteins 0.000 description 2
- 101710141454 Nucleoprotein Proteins 0.000 description 2
- 238000012408 PCR amplification Methods 0.000 description 2
- 102000003992 Peroxidases Human genes 0.000 description 2
- 108010002747 Pfu DNA polymerase Proteins 0.000 description 2
- 101710130420 Probable capsid assembly scaffolding protein Proteins 0.000 description 2
- 101710083689 Probable capsid protein Proteins 0.000 description 2
- 108020004511 Recombinant DNA Proteins 0.000 description 2
- 101100221606 Saccharomyces cerevisiae (strain ATCC 204508 / S288c) COS7 gene Proteins 0.000 description 2
- 101710204410 Scaffold protein Proteins 0.000 description 2
- 108010003723 Single-Domain Antibodies Proteins 0.000 description 2
- 108020004682 Single-Stranded DNA Proteins 0.000 description 2
- 239000004098 Tetracycline Substances 0.000 description 2
- QIVBCDIJIAJPQS-UHFFFAOYSA-N Tryptophan Natural products C1=CC=C2C(CC(N)C(O)=O)=CNC2=C1 QIVBCDIJIAJPQS-UHFFFAOYSA-N 0.000 description 2
- 108091008605 VEGF receptors Proteins 0.000 description 2
- 102000009484 Vascular Endothelial Growth Factor Receptors Human genes 0.000 description 2
- 241000700605 Viruses Species 0.000 description 2
- 239000003242 anti bacterial agent Substances 0.000 description 2
- 229940088710 antibiotic agent Drugs 0.000 description 2
- 238000013459 approach Methods 0.000 description 2
- 210000001188 articular cartilage Anatomy 0.000 description 2
- 210000000544 articulatio talocruralis Anatomy 0.000 description 2
- 210000004507 artificial chromosome Anatomy 0.000 description 2
- 230000003115 biocidal effect Effects 0.000 description 2
- 229960002685 biotin Drugs 0.000 description 2
- 235000020958 biotin Nutrition 0.000 description 2
- 239000011616 biotin Substances 0.000 description 2
- 229910052799 carbon Inorganic materials 0.000 description 2
- 238000005119 centrifugation Methods 0.000 description 2
- 239000003153 chemical reaction reagent Substances 0.000 description 2
- 239000013611 chromosomal DNA Substances 0.000 description 2
- 239000013599 cloning vector Substances 0.000 description 2
- 230000024203 complement activation Effects 0.000 description 2
- 238000007796 conventional method Methods 0.000 description 2
- 239000013078 crystal Substances 0.000 description 2
- 102000003675 cytokine receptors Human genes 0.000 description 2
- 108010057085 cytokine receptors Proteins 0.000 description 2
- 230000006378 damage Effects 0.000 description 2
- 230000007812 deficiency Effects 0.000 description 2
- 238000004925 denaturation Methods 0.000 description 2
- 230000036425 denaturation Effects 0.000 description 2
- 230000029087 digestion Effects 0.000 description 2
- 238000010790 dilution Methods 0.000 description 2
- 239000012895 dilution Substances 0.000 description 2
- 125000002228 disulfide group Chemical group 0.000 description 2
- 239000012530 fluid Substances 0.000 description 2
- 238000004108 freeze drying Methods 0.000 description 2
- 238000002523 gelfiltration Methods 0.000 description 2
- 230000036541 health Effects 0.000 description 2
- 239000000710 homodimer Substances 0.000 description 2
- 238000003365 immunocytochemistry Methods 0.000 description 2
- 239000002596 immunotoxin Substances 0.000 description 2
- 229940051026 immunotoxin Drugs 0.000 description 2
- 230000002637 immunotoxin Effects 0.000 description 2
- 231100000608 immunotoxin Toxicity 0.000 description 2
- 238000007901 in situ hybridization Methods 0.000 description 2
- 239000000893 inhibin Substances 0.000 description 2
- ZPNFWUPYTFPOJU-LPYSRVMUSA-N iniprol Chemical compound C([C@H]1C(=O)NCC(=O)NCC(=O)N[C@H]2CSSC[C@H]3C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@H](C(N[C@H](C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC=4C=CC(O)=CC=4)C(=O)N[C@@H](CC=4C=CC=CC=4)C(=O)N[C@@H](CC=4C=CC(O)=CC=4)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](C)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](C)C(=O)NCC(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CSSC[C@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](C)NC(=O)[C@H](CO)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CC=4C=CC=CC=4)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCCN)NC(=O)[C@H](C)NC(=O)[C@H](CCCNC(N)=N)NC2=O)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CSSC[C@H](NC(=O)[C@H](CC=2C=CC=CC=2)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H]2N(CCC2)C(=O)[C@@H](N)CCCNC(N)=N)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(O)=O)C(=O)N2[C@@H](CCC2)C(=O)N2[C@@H](CCC2)C(=O)N[C@@H](CC=2C=CC(O)=CC=2)C(=O)N[C@@H]([C@@H](C)O)C(=O)NCC(=O)N2[C@@H](CCC2)C(=O)N3)C(=O)NCC(=O)NCC(=O)N[C@@H](C)C(O)=O)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@H](C(=O)N[C@@H](CC=2C=CC=CC=2)C(=O)N[C@H](C(=O)N1)C(C)C)[C@@H](C)O)[C@@H](C)CC)=O)[C@@H](C)CC)C1=CC=C(O)C=C1 ZPNFWUPYTFPOJU-LPYSRVMUSA-N 0.000 description 2
- NOESYZHRGYRDHS-UHFFFAOYSA-N insulin Chemical compound N1C(=O)C(NC(=O)C(CCC(N)=O)NC(=O)C(CCC(O)=O)NC(=O)C(C(C)C)NC(=O)C(NC(=O)CN)C(C)CC)CSSCC(C(NC(CO)C(=O)NC(CC(C)C)C(=O)NC(CC=2C=CC(O)=CC=2)C(=O)NC(CCC(N)=O)C(=O)NC(CC(C)C)C(=O)NC(CCC(O)=O)C(=O)NC(CC(N)=O)C(=O)NC(CC=2C=CC(O)=CC=2)C(=O)NC(CSSCC(NC(=O)C(C(C)C)NC(=O)C(CC(C)C)NC(=O)C(CC=2C=CC(O)=CC=2)NC(=O)C(CC(C)C)NC(=O)C(C)NC(=O)C(CCC(O)=O)NC(=O)C(C(C)C)NC(=O)C(CC(C)C)NC(=O)C(CC=2NC=NC=2)NC(=O)C(CO)NC(=O)CNC2=O)C(=O)NCC(=O)NC(CCC(O)=O)C(=O)NC(CCCNC(N)=N)C(=O)NCC(=O)NC(CC=3C=CC=CC=3)C(=O)NC(CC=3C=CC=CC=3)C(=O)NC(CC=3C=CC(O)=CC=3)C(=O)NC(C(C)O)C(=O)N3C(CCC3)C(=O)NC(CCCCN)C(=O)NC(C)C(O)=O)C(=O)NC(CC(N)=O)C(O)=O)=O)NC(=O)C(C(C)CC)NC(=O)C(CO)NC(=O)C(C(C)O)NC(=O)C1CSSCC2NC(=O)C(CC(C)C)NC(=O)C(NC(=O)C(CCC(N)=O)NC(=O)C(CC(N)=O)NC(=O)C(NC(=O)C(N)CC=1C=CC=CC=1)C(C)C)CC1=CN=CN1 NOESYZHRGYRDHS-UHFFFAOYSA-N 0.000 description 2
- 238000001990 intravenous administration Methods 0.000 description 2
- 125000001449 isopropyl group Chemical group [H]C([H])([H])C([H])(*)C([H])([H])[H] 0.000 description 2
- 230000002147 killing effect Effects 0.000 description 2
- 239000008101 lactose Substances 0.000 description 2
- 210000002414 leg Anatomy 0.000 description 2
- 210000001616 monocyte Anatomy 0.000 description 2
- 229960004927 neomycin Drugs 0.000 description 2
- 229910052757 nitrogen Inorganic materials 0.000 description 2
- 239000002245 particle Substances 0.000 description 2
- 101150040383 pel2 gene Proteins 0.000 description 2
- 101150050446 pelB gene Proteins 0.000 description 2
- 108040007629 peroxidase activity proteins Proteins 0.000 description 2
- 108700010839 phage proteins Proteins 0.000 description 2
- 239000008194 pharmaceutical composition Substances 0.000 description 2
- 230000000704 physical effect Effects 0.000 description 2
- 210000002729 polyribosome Anatomy 0.000 description 2
- 230000001681 protective effect Effects 0.000 description 2
- 238000005215 recombination Methods 0.000 description 2
- 230000006798 recombination Effects 0.000 description 2
- 230000003362 replicative effect Effects 0.000 description 2
- 238000011160 research Methods 0.000 description 2
- 238000012552 review Methods 0.000 description 2
- 230000028327 secretion Effects 0.000 description 2
- 239000013589 supplement Substances 0.000 description 2
- 230000004083 survival effect Effects 0.000 description 2
- 230000008685 targeting Effects 0.000 description 2
- 229960002180 tetracycline Drugs 0.000 description 2
- 229930101283 tetracycline Natural products 0.000 description 2
- 235000019364 tetracycline Nutrition 0.000 description 2
- 150000003522 tetracyclines Chemical class 0.000 description 2
- 238000002560 therapeutic procedure Methods 0.000 description 2
- 239000003053 toxin Substances 0.000 description 2
- 231100000765 toxin Toxicity 0.000 description 2
- 108700012359 toxins Proteins 0.000 description 2
- 230000005030 transcription termination Effects 0.000 description 2
- 230000009261 transgenic effect Effects 0.000 description 2
- 238000011830 transgenic mouse model Methods 0.000 description 2
- 208000035408 type 1 diabetes mellitus 1 Diseases 0.000 description 2
- 241000701161 unidentified adenovirus Species 0.000 description 2
- 238000011144 upstream manufacturing Methods 0.000 description 2
- 239000003981 vehicle Substances 0.000 description 2
- 210000003462 vein Anatomy 0.000 description 2
- MZOFCQQQCNRIBI-VMXHOPILSA-N (3s)-4-[[(2s)-1-[[(2s)-1-[[(1s)-1-carboxy-2-hydroxyethyl]amino]-4-methyl-1-oxopentan-2-yl]amino]-5-(diaminomethylideneamino)-1-oxopentan-2-yl]amino]-3-[[2-[[(2s)-2,6-diaminohexanoyl]amino]acetyl]amino]-4-oxobutanoic acid Chemical compound OC[C@@H](C(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](CC(O)=O)NC(=O)CNC(=O)[C@@H](N)CCCCN MZOFCQQQCNRIBI-VMXHOPILSA-N 0.000 description 1
- KISWVXRQTGLFGD-UHFFFAOYSA-N 2-[[2-[[6-amino-2-[[2-[[2-[[5-amino-2-[[2-[[1-[2-[[6-amino-2-[(2,5-diamino-5-oxopentanoyl)amino]hexanoyl]amino]-5-(diaminomethylideneamino)pentanoyl]pyrrolidine-2-carbonyl]amino]-3-hydroxypropanoyl]amino]-5-oxopentanoyl]amino]-5-(diaminomethylideneamino)p Chemical compound C1CCN(C(=O)C(CCCN=C(N)N)NC(=O)C(CCCCN)NC(=O)C(N)CCC(N)=O)C1C(=O)NC(CO)C(=O)NC(CCC(N)=O)C(=O)NC(CCCN=C(N)N)C(=O)NC(CO)C(=O)NC(CCCCN)C(=O)NC(C(=O)NC(CC(C)C)C(O)=O)CC1=CC=C(O)C=C1 KISWVXRQTGLFGD-UHFFFAOYSA-N 0.000 description 1
- IJJWOSAXNHWBPR-HUBLWGQQSA-N 5-[(3as,4s,6ar)-2-oxo-1,3,3a,4,6,6a-hexahydrothieno[3,4-d]imidazol-4-yl]-n-(6-hydrazinyl-6-oxohexyl)pentanamide Chemical compound N1C(=O)N[C@@H]2[C@H](CCCCC(=O)NCCCCCC(=O)NN)SC[C@@H]21 IJJWOSAXNHWBPR-HUBLWGQQSA-N 0.000 description 1
- FHVDTGUDJYJELY-UHFFFAOYSA-N 6-{[2-carboxy-4,5-dihydroxy-6-(phosphanyloxy)oxan-3-yl]oxy}-4,5-dihydroxy-3-phosphanyloxane-2-carboxylic acid Chemical compound O1C(C(O)=O)C(P)C(O)C(O)C1OC1C(C(O)=O)OC(OP)C(O)C1O FHVDTGUDJYJELY-UHFFFAOYSA-N 0.000 description 1
- 102100038222 60 kDa heat shock protein, mitochondrial Human genes 0.000 description 1
- 229920001817 Agar Polymers 0.000 description 1
- 108010032595 Antibody Binding Sites Proteins 0.000 description 1
- 108010025628 Apolipoproteins E Proteins 0.000 description 1
- 102000013918 Apolipoproteins E Human genes 0.000 description 1
- 101000716807 Arabidopsis thaliana Protein SCO1 homolog 1, mitochondrial Proteins 0.000 description 1
- 208000023275 Autoimmune disease Diseases 0.000 description 1
- 208000035143 Bacterial infection Diseases 0.000 description 1
- 108010081589 Becaplermin Proteins 0.000 description 1
- 101710129634 Beta-nerve growth factor Proteins 0.000 description 1
- 102000004506 Blood Proteins Human genes 0.000 description 1
- 108010017384 Blood Proteins Proteins 0.000 description 1
- 101001069913 Bos taurus Growth-regulated protein homolog beta Proteins 0.000 description 1
- 101001069912 Bos taurus Growth-regulated protein homolog gamma Proteins 0.000 description 1
- 102000004219 Brain-derived neurotrophic factor Human genes 0.000 description 1
- 108090000715 Brain-derived neurotrophic factor Proteins 0.000 description 1
- 102100023702 C-C motif chemokine 13 Human genes 0.000 description 1
- 101710112613 C-C motif chemokine 13 Proteins 0.000 description 1
- 102100023698 C-C motif chemokine 17 Human genes 0.000 description 1
- 102100023701 C-C motif chemokine 18 Human genes 0.000 description 1
- 101710155857 C-C motif chemokine 2 Proteins 0.000 description 1
- 102100036846 C-C motif chemokine 21 Human genes 0.000 description 1
- 102100036850 C-C motif chemokine 23 Human genes 0.000 description 1
- 102100036849 C-C motif chemokine 24 Human genes 0.000 description 1
- 102100032367 C-C motif chemokine 5 Human genes 0.000 description 1
- 102100032366 C-C motif chemokine 7 Human genes 0.000 description 1
- 101710155834 C-C motif chemokine 7 Proteins 0.000 description 1
- 102100034871 C-C motif chemokine 8 Human genes 0.000 description 1
- 101710155833 C-C motif chemokine 8 Proteins 0.000 description 1
- 102100025248 C-X-C motif chemokine 10 Human genes 0.000 description 1
- 102100039398 C-X-C motif chemokine 2 Human genes 0.000 description 1
- 102100036189 C-X-C motif chemokine 3 Human genes 0.000 description 1
- 102100036150 C-X-C motif chemokine 5 Human genes 0.000 description 1
- 102100036153 C-X-C motif chemokine 6 Human genes 0.000 description 1
- 101710085504 C-X-C motif chemokine 6 Proteins 0.000 description 1
- 102100036170 C-X-C motif chemokine 9 Human genes 0.000 description 1
- 101710085500 C-X-C motif chemokine 9 Proteins 0.000 description 1
- 102100032528 C-type lectin domain family 11 member A Human genes 0.000 description 1
- 108700012434 CCL3 Proteins 0.000 description 1
- 101150093802 CXCL1 gene Proteins 0.000 description 1
- 101100123850 Caenorhabditis elegans her-1 gene Proteins 0.000 description 1
- 101100314454 Caenorhabditis elegans tra-1 gene Proteins 0.000 description 1
- 241000222120 Candida <Saccharomycetales> Species 0.000 description 1
- 241000283707 Capra Species 0.000 description 1
- 229920002134 Carboxymethyl cellulose Polymers 0.000 description 1
- 102100028892 Cardiotrophin-1 Human genes 0.000 description 1
- 102000014914 Carrier Proteins Human genes 0.000 description 1
- 102000000844 Cell Surface Receptors Human genes 0.000 description 1
- 108010001857 Cell Surface Receptors Proteins 0.000 description 1
- 101710104316 Cell surface-binding protein Proteins 0.000 description 1
- 108010058432 Chaperonin 60 Proteins 0.000 description 1
- 108010082155 Chemokine CCL18 Proteins 0.000 description 1
- 108010082161 Chemokine CCL19 Proteins 0.000 description 1
- 102000003805 Chemokine CCL19 Human genes 0.000 description 1
- 108010083647 Chemokine CCL24 Proteins 0.000 description 1
- 102000000013 Chemokine CCL3 Human genes 0.000 description 1
- 108010055165 Chemokine CCL4 Proteins 0.000 description 1
- 102000001326 Chemokine CCL4 Human genes 0.000 description 1
- 108010055166 Chemokine CCL5 Proteins 0.000 description 1
- 108010078239 Chemokine CX3CL1 Proteins 0.000 description 1
- 102000000503 Collagen Type II Human genes 0.000 description 1
- 108010041390 Collagen Type II Proteins 0.000 description 1
- 102000016917 Complement C1 Human genes 0.000 description 1
- 108010028774 Complement C1 Proteins 0.000 description 1
- 206010010356 Congenital anomaly Diseases 0.000 description 1
- PMATZTZNYRCHOR-CGLBZJNRSA-N Cyclosporin A Chemical compound CC[C@@H]1NC(=O)[C@H]([C@H](O)[C@H](C)C\C=C\C)N(C)C(=O)[C@H](C(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)N(C)C(=O)[C@H](C(C)C)NC(=O)[C@H](CC(C)C)N(C)C(=O)CN(C)C1=O PMATZTZNYRCHOR-CGLBZJNRSA-N 0.000 description 1
- 108010036949 Cyclosporine Proteins 0.000 description 1
- 239000003155 DNA primer Substances 0.000 description 1
- 102000052510 DNA-Binding Proteins Human genes 0.000 description 1
- 108700020911 DNA-Binding Proteins Proteins 0.000 description 1
- 208000016192 Demyelinating disease Diseases 0.000 description 1
- 102000001301 EGF receptor Human genes 0.000 description 1
- 108060006698 EGF receptor Proteins 0.000 description 1
- 241000196324 Embryophyta Species 0.000 description 1
- 102100023688 Eotaxin Human genes 0.000 description 1
- 101710139422 Eotaxin Proteins 0.000 description 1
- 101150021185 FGF gene Proteins 0.000 description 1
- 102100028412 Fibroblast growth factor 10 Human genes 0.000 description 1
- 102100028071 Fibroblast growth factor 7 Human genes 0.000 description 1
- 108090000385 Fibroblast growth factor 7 Proteins 0.000 description 1
- 102000013818 Fractalkine Human genes 0.000 description 1
- 101710115997 Gamma-tubulin complex component 2 Proteins 0.000 description 1
- 108010010803 Gelatin Proteins 0.000 description 1
- 102000034615 Glial cell line-derived neurotrophic factor Human genes 0.000 description 1
- 108091010837 Glial cell line-derived neurotrophic factor Proteins 0.000 description 1
- 102000003886 Glycoproteins Human genes 0.000 description 1
- 108090000288 Glycoproteins Proteins 0.000 description 1
- 108010017080 Granulocyte Colony-Stimulating Factor Proteins 0.000 description 1
- 102000004269 Granulocyte Colony-Stimulating Factor Human genes 0.000 description 1
- 108010017213 Granulocyte-Macrophage Colony-Stimulating Factor Proteins 0.000 description 1
- 102100039620 Granulocyte-macrophage colony-stimulating factor Human genes 0.000 description 1
- HVLSXIKZNLPZJJ-TXZCQADKSA-N HA peptide Chemical group C([C@@H](C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](C(C)C)C(=O)N1[C@@H](CCC1)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CC=1C=CC(O)=CC=1)C(=O)N[C@@H](C)C(O)=O)NC(=O)[C@H]1N(CCC1)C(=O)[C@@H](N)CC=1C=CC(O)=CC=1)C1=CC=C(O)C=C1 HVLSXIKZNLPZJJ-TXZCQADKSA-N 0.000 description 1
- 108010004889 Heat-Shock Proteins Proteins 0.000 description 1
- 102000002812 Heat-Shock Proteins Human genes 0.000 description 1
- 101000690301 Homo sapiens Aldo-keto reductase family 1 member C4 Proteins 0.000 description 1
- 101000978362 Homo sapiens C-C motif chemokine 17 Proteins 0.000 description 1
- 101000897480 Homo sapiens C-C motif chemokine 2 Proteins 0.000 description 1
- 101000713085 Homo sapiens C-C motif chemokine 21 Proteins 0.000 description 1
- 101000713081 Homo sapiens C-C motif chemokine 23 Proteins 0.000 description 1
- 101000947193 Homo sapiens C-X-C motif chemokine 3 Proteins 0.000 description 1
- 101000947186 Homo sapiens C-X-C motif chemokine 5 Proteins 0.000 description 1
- 101000942297 Homo sapiens C-type lectin domain family 11 member A Proteins 0.000 description 1
- 101000777461 Homo sapiens Disintegrin and metalloproteinase domain-containing protein 17 Proteins 0.000 description 1
- 101001069921 Homo sapiens Growth-regulated alpha protein Proteins 0.000 description 1
- 101001076418 Homo sapiens Interleukin-1 receptor type 1 Proteins 0.000 description 1
- 101000960954 Homo sapiens Interleukin-18 Proteins 0.000 description 1
- 101000973997 Homo sapiens Nucleosome assembly protein 1-like 4 Proteins 0.000 description 1
- 101000947178 Homo sapiens Platelet basic protein Proteins 0.000 description 1
- 101001116548 Homo sapiens Protein CBFA2T1 Proteins 0.000 description 1
- 101001076715 Homo sapiens RNA-binding protein 39 Proteins 0.000 description 1
- 101000851030 Homo sapiens Vascular endothelial growth factor receptor 3 Proteins 0.000 description 1
- 206010020751 Hypersensitivity Diseases 0.000 description 1
- 102000004877 Insulin Human genes 0.000 description 1
- 108090001061 Insulin Proteins 0.000 description 1
- 102000048143 Insulin-Like Growth Factor II Human genes 0.000 description 1
- 108090001117 Insulin-Like Growth Factor II Proteins 0.000 description 1
- 102100037850 Interferon gamma Human genes 0.000 description 1
- 108010074328 Interferon-gamma Proteins 0.000 description 1
- 102000000589 Interleukin-1 Human genes 0.000 description 1
- 108010002352 Interleukin-1 Proteins 0.000 description 1
- 102100026016 Interleukin-1 receptor type 1 Human genes 0.000 description 1
- 102000003810 Interleukin-18 Human genes 0.000 description 1
- 108090000171 Interleukin-18 Proteins 0.000 description 1
- 102100039898 Interleukin-18 Human genes 0.000 description 1
- 108010002350 Interleukin-2 Proteins 0.000 description 1
- 108010002386 Interleukin-3 Proteins 0.000 description 1
- 108090000978 Interleukin-4 Proteins 0.000 description 1
- 108010002616 Interleukin-5 Proteins 0.000 description 1
- 108090001005 Interleukin-6 Proteins 0.000 description 1
- 108010002586 Interleukin-7 Proteins 0.000 description 1
- 108090001007 Interleukin-8 Proteins 0.000 description 1
- 108010002335 Interleukin-9 Proteins 0.000 description 1
- 102000000585 Interleukin-9 Human genes 0.000 description 1
- 108010092277 Leptin Proteins 0.000 description 1
- 102000016267 Leptin Human genes 0.000 description 1
- 102100032352 Leukemia inhibitory factor Human genes 0.000 description 1
- 108090000581 Leukemia inhibitory factor Proteins 0.000 description 1
- 208000019693 Lung disease Diseases 0.000 description 1
- 102100035304 Lymphotactin Human genes 0.000 description 1
- 108090000542 Lymphotoxin-alpha Proteins 0.000 description 1
- 108010046938 Macrophage Colony-Stimulating Factor Proteins 0.000 description 1
- 102000007651 Macrophage Colony-Stimulating Factor Human genes 0.000 description 1
- 102100039364 Metalloproteinase inhibitor 1 Human genes 0.000 description 1
- 108010006519 Molecular Chaperones Proteins 0.000 description 1
- 108010014251 Muramidase Proteins 0.000 description 1
- 102000016943 Muramidase Human genes 0.000 description 1
- 102000047918 Myelin Basic Human genes 0.000 description 1
- 101710107068 Myelin basic protein Proteins 0.000 description 1
- 108010062010 N-Acetylmuramoyl-L-alanine Amidase Proteins 0.000 description 1
- 108091007491 NSP3 Papain-like protease domains Proteins 0.000 description 1
- 108010025020 Nerve Growth Factor Proteins 0.000 description 1
- 102000003683 Neurotrophin-4 Human genes 0.000 description 1
- 108090000099 Neurotrophin-4 Proteins 0.000 description 1
- 102100021584 Neurturin Human genes 0.000 description 1
- 108010015406 Neurturin Proteins 0.000 description 1
- 206010061876 Obstruction Diseases 0.000 description 1
- 241000320412 Ogataea angusta Species 0.000 description 1
- 241001452677 Ogataea methanolica Species 0.000 description 1
- 108090000630 Oncostatin M Proteins 0.000 description 1
- 102000004140 Oncostatin M Human genes 0.000 description 1
- 241000283973 Oryctolagus cuniculus Species 0.000 description 1
- 108091008606 PDGF receptors Proteins 0.000 description 1
- 241001111421 Pannus Species 0.000 description 1
- 102000035195 Peptidases Human genes 0.000 description 1
- 241000157426 Pernis Species 0.000 description 1
- 102100036154 Platelet basic protein Human genes 0.000 description 1
- 108090000778 Platelet factor 4 Proteins 0.000 description 1
- 102000011653 Platelet-Derived Growth Factor Receptors Human genes 0.000 description 1
- 206010036030 Polyarthritis Diseases 0.000 description 1
- 229920001213 Polysorbate 20 Polymers 0.000 description 1
- 101710193132 Pre-hexon-linking protein VIII Proteins 0.000 description 1
- 102100033237 Pro-epidermal growth factor Human genes 0.000 description 1
- 101710098940 Pro-epidermal growth factor Proteins 0.000 description 1
- 102100036352 Protein disulfide-isomerase Human genes 0.000 description 1
- 102100023361 SAP domain-containing ribonucleoprotein Human genes 0.000 description 1
- 229920002684 Sepharose Polymers 0.000 description 1
- 239000012505 Superdex™ Substances 0.000 description 1
- 108010006785 Taq Polymerase Proteins 0.000 description 1
- 102000009843 Thyroglobulin Human genes 0.000 description 1
- 108010034949 Thyroglobulin Proteins 0.000 description 1
- 102100027188 Thyroid peroxidase Human genes 0.000 description 1
- 101710113649 Thyroid peroxidase Proteins 0.000 description 1
- 108091023040 Transcription factor Proteins 0.000 description 1
- 102000040945 Transcription factor Human genes 0.000 description 1
- 108090001012 Transforming Growth Factor beta Proteins 0.000 description 1
- 102000004887 Transforming Growth Factor beta Human genes 0.000 description 1
- 102000011117 Transforming Growth Factor beta2 Human genes 0.000 description 1
- 102400001320 Transforming growth factor alpha Human genes 0.000 description 1
- 101800004564 Transforming growth factor alpha Proteins 0.000 description 1
- 101800000304 Transforming growth factor beta-2 Proteins 0.000 description 1
- 102000056172 Transforming growth factor beta-3 Human genes 0.000 description 1
- 108090000097 Transforming growth factor beta-3 Proteins 0.000 description 1
- 239000007983 Tris buffer Substances 0.000 description 1
- 102000005789 Vascular Endothelial Growth Factors Human genes 0.000 description 1
- 108010019530 Vascular Endothelial Growth Factors Proteins 0.000 description 1
- 102100033179 Vascular endothelial growth factor receptor 3 Human genes 0.000 description 1
- 208000036142 Viral infection Diseases 0.000 description 1
- 108010084455 Zeocin Proteins 0.000 description 1
- 230000002378 acidificating effect Effects 0.000 description 1
- 230000009471 action Effects 0.000 description 1
- 230000004913 activation Effects 0.000 description 1
- 239000000654 additive Substances 0.000 description 1
- 239000002671 adjuvant Substances 0.000 description 1
- 229940009456 adriamycin Drugs 0.000 description 1
- 238000001042 affinity chromatography Methods 0.000 description 1
- 230000009824 affinity maturation Effects 0.000 description 1
- 239000008272 agar Substances 0.000 description 1
- 239000000556 agonist Substances 0.000 description 1
- 230000001476 alcoholic effect Effects 0.000 description 1
- 229940072056 alginate Drugs 0.000 description 1
- 235000010443 alginic acid Nutrition 0.000 description 1
- 229920000615 alginic acid Polymers 0.000 description 1
- 208000026935 allergic disease Diseases 0.000 description 1
- 230000000172 allergic effect Effects 0.000 description 1
- 125000000539 amino acid group Chemical group 0.000 description 1
- 238000010171 animal model Methods 0.000 description 1
- 235000021120 animal protein Nutrition 0.000 description 1
- 238000005349 anion exchange Methods 0.000 description 1
- 210000003423 ankle Anatomy 0.000 description 1
- 239000005557 antagonist Substances 0.000 description 1
- 230000000845 anti-microbial effect Effects 0.000 description 1
- 239000003963 antioxidant agent Substances 0.000 description 1
- 108010026054 apolipoprotein SAA Proteins 0.000 description 1
- 239000007864 aqueous solution Substances 0.000 description 1
- 230000002917 arthritic effect Effects 0.000 description 1
- 125000000613 asparagine group Chemical group N[C@@H](CC(N)=O)C(=O)* 0.000 description 1
- QVGXLLKOCUKJST-UHFFFAOYSA-N atomic oxygen Chemical compound [O] QVGXLLKOCUKJST-UHFFFAOYSA-N 0.000 description 1
- 208000010668 atopic eczema Diseases 0.000 description 1
- 208000022362 bacterial infectious disease Diseases 0.000 description 1
- 229960002319 barbital Drugs 0.000 description 1
- 239000011230 binding agent Substances 0.000 description 1
- 238000002306 biochemical method Methods 0.000 description 1
- 230000000903 blocking effect Effects 0.000 description 1
- BPKIGYQJPYCAOW-FFJTTWKXSA-I calcium;potassium;disodium;(2s)-2-hydroxypropanoate;dichloride;dihydroxide;hydrate Chemical compound O.[OH-].[OH-].[Na+].[Na+].[Cl-].[Cl-].[K+].[Ca+2].C[C@H](O)C([O-])=O BPKIGYQJPYCAOW-FFJTTWKXSA-I 0.000 description 1
- 238000004364 calculation method Methods 0.000 description 1
- 210000000234 capsid Anatomy 0.000 description 1
- 239000001768 carboxy methyl cellulose Substances 0.000 description 1
- 235000010948 carboxy methyl cellulose Nutrition 0.000 description 1
- 239000008112 carboxymethyl-cellulose Substances 0.000 description 1
- 108010041776 cardiotrophin 1 Proteins 0.000 description 1
- 239000000969 carrier Substances 0.000 description 1
- 230000015556 catabolic process Effects 0.000 description 1
- 230000021164 cell adhesion Effects 0.000 description 1
- 238000004113 cell culture Methods 0.000 description 1
- 210000004671 cell-free system Anatomy 0.000 description 1
- 230000001413 cellular effect Effects 0.000 description 1
- 230000008614 cellular interaction Effects 0.000 description 1
- 238000012512 characterization method Methods 0.000 description 1
- 239000002738 chelating agent Substances 0.000 description 1
- 238000001311 chemical methods and process Methods 0.000 description 1
- 239000003795 chemical substances by application Substances 0.000 description 1
- 229960001265 ciclosporin Drugs 0.000 description 1
- 238000011260 co-administration Methods 0.000 description 1
- 230000004154 complement system Effects 0.000 description 1
- 238000010924 continuous production Methods 0.000 description 1
- 239000012228 culture supernatant Substances 0.000 description 1
- 229930182912 cyclosporin Natural products 0.000 description 1
- 125000000151 cysteine group Chemical group N[C@@H](CS)C(=O)* 0.000 description 1
- 230000009089 cytolysis Effects 0.000 description 1
- 231100000433 cytotoxic Toxicity 0.000 description 1
- 230000001472 cytotoxic effect Effects 0.000 description 1
- 230000007423 decrease Effects 0.000 description 1
- 230000002950 deficient Effects 0.000 description 1
- 230000006735 deficit Effects 0.000 description 1
- 238000006731 degradation reaction Methods 0.000 description 1
- 239000008367 deionised water Substances 0.000 description 1
- 229910021641 deionized water Inorganic materials 0.000 description 1
- 230000001419 dependent effect Effects 0.000 description 1
- 238000009795 derivation Methods 0.000 description 1
- 238000013461 design Methods 0.000 description 1
- 230000001627 detrimental effect Effects 0.000 description 1
- 238000003745 diagnosis Methods 0.000 description 1
- 239000013024 dilution buffer Substances 0.000 description 1
- 230000008034 disappearance Effects 0.000 description 1
- 238000009826 distribution Methods 0.000 description 1
- 101150107911 eae gene Proteins 0.000 description 1
- 239000003792 electrolyte Substances 0.000 description 1
- 239000002158 endotoxin Substances 0.000 description 1
- 230000002255 enzymatic effect Effects 0.000 description 1
- 208000033068 episodic angioedema with eosinophilia Diseases 0.000 description 1
- 230000003628 erosive effect Effects 0.000 description 1
- 125000001495 ethyl group Chemical group [H]C([H])([H])C([H])([H])* 0.000 description 1
- 238000002474 experimental method Methods 0.000 description 1
- 239000000284 extract Substances 0.000 description 1
- 108700014844 flt3 ligand Proteins 0.000 description 1
- 238000010230 functional analysis Methods 0.000 description 1
- 230000002538 fungal effect Effects 0.000 description 1
- 238000001502 gel electrophoresis Methods 0.000 description 1
- 239000008273 gelatin Substances 0.000 description 1
- 229920000159 gelatin Polymers 0.000 description 1
- 235000019322 gelatine Nutrition 0.000 description 1
- 235000011852 gelatine desserts Nutrition 0.000 description 1
- 238000010353 genetic engineering Methods 0.000 description 1
- 102000054766 genetic haplotypes Human genes 0.000 description 1
- PCHJSUWPFVWCPO-UHFFFAOYSA-N gold Chemical compound [Au] PCHJSUWPFVWCPO-UHFFFAOYSA-N 0.000 description 1
- 239000010931 gold Substances 0.000 description 1
- 229910052737 gold Inorganic materials 0.000 description 1
- 239000003102 growth factor Substances 0.000 description 1
- 108010021083 hen egg lysozyme Proteins 0.000 description 1
- 238000007489 histopathology method Methods 0.000 description 1
- 102000054751 human RUNX1T1 Human genes 0.000 description 1
- 238000009396 hybridization Methods 0.000 description 1
- 206010020718 hyperplasia Diseases 0.000 description 1
- 230000009610 hypersensitivity Effects 0.000 description 1
- 238000003125 immunofluorescent labeling Methods 0.000 description 1
- 238000012151 immunohistochemical method Methods 0.000 description 1
- 239000002955 immunomodulating agent Substances 0.000 description 1
- 238000009169 immunotherapy Methods 0.000 description 1
- 230000006872 improvement Effects 0.000 description 1
- 238000000099 in vitro assay Methods 0.000 description 1
- 238000011065 in-situ storage Methods 0.000 description 1
- 230000000415 inactivating effect Effects 0.000 description 1
- 238000010348 incorporation Methods 0.000 description 1
- 239000011261 inert gas Substances 0.000 description 1
- 208000015181 infectious disease Diseases 0.000 description 1
- 230000002458 infectious effect Effects 0.000 description 1
- 230000008595 infiltration Effects 0.000 description 1
- 238000001764 infiltration Methods 0.000 description 1
- 230000004968 inflammatory condition Effects 0.000 description 1
- 230000002401 inhibitory effect Effects 0.000 description 1
- 230000005764 inhibitory process Effects 0.000 description 1
- 229940125396 insulin Drugs 0.000 description 1
- 230000004068 intracellular signaling Effects 0.000 description 1
- 238000007918 intramuscular administration Methods 0.000 description 1
- 238000007912 intraperitoneal administration Methods 0.000 description 1
- 239000007928 intraperitoneal injection Substances 0.000 description 1
- 238000011835 investigation Methods 0.000 description 1
- 238000004255 ion exchange chromatography Methods 0.000 description 1
- 238000002955 isolation Methods 0.000 description 1
- 210000000629 knee joint Anatomy 0.000 description 1
- NRYBAZVQPHGZNS-ZSOCWYAHSA-N leptin Chemical compound O=C([C@H](CO)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CC=1C2=CC=CC=C2NC=1)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CO)NC(=O)CNC(=O)[C@H](CCC(N)=O)NC(=O)[C@@H](N)CC(C)C)CCSC)N1CCC[C@H]1C(=O)NCC(=O)N[C@@H](CS)C(O)=O NRYBAZVQPHGZNS-ZSOCWYAHSA-N 0.000 description 1
- 229940039781 leptin Drugs 0.000 description 1
- 230000000670 limiting effect Effects 0.000 description 1
- 239000007788 liquid Substances 0.000 description 1
- 238000011068 loading method Methods 0.000 description 1
- 108010019677 lymphotactin Proteins 0.000 description 1
- 229960000274 lysozyme Drugs 0.000 description 1
- 239000004325 lysozyme Substances 0.000 description 1
- 235000010335 lysozyme Nutrition 0.000 description 1
- 239000000463 material Substances 0.000 description 1
- 239000011159 matrix material Substances 0.000 description 1
- AEUKDPKXTPNBNY-XEYRWQBLSA-N mcp 2 Chemical compound C([C@@H](C(=O)N[C@@H](CS)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CCCNC(N)=N)C(=O)NCC(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CC=1NC=NC=1)C(=O)N1[C@@H](CCC1)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CS)C(=O)N[C@@H](CS)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O)NC(=O)CNC(=O)[C@H](C)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H]1N(CCC1)C(=O)[C@H](CC(C)C)NC(=O)[C@H](CS)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](C)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CS)NC(=O)[C@H](C)NC(=O)[C@H](CS)NC(=O)[C@@H](NC(=O)[C@@H](N)C(C)C)C(C)C)C1=CC=CC=C1 AEUKDPKXTPNBNY-XEYRWQBLSA-N 0.000 description 1
- 239000012528 membrane Substances 0.000 description 1
- 239000002480 mineral oil Substances 0.000 description 1
- 235000010446 mineral oil Nutrition 0.000 description 1
- 238000001823 molecular biology technique Methods 0.000 description 1
- 229940053128 nerve growth factor Drugs 0.000 description 1
- 239000003921 oil Substances 0.000 description 1
- 238000005457 optimization Methods 0.000 description 1
- 229940043515 other immunoglobulins in atc Drugs 0.000 description 1
- 239000001301 oxygen Substances 0.000 description 1
- 229910052760 oxygen Inorganic materials 0.000 description 1
- 238000012856 packing Methods 0.000 description 1
- 230000007170 pathology Effects 0.000 description 1
- 239000008188 pellet Substances 0.000 description 1
- CWCMIVBLVUHDHK-ZSNHEYEWSA-N phleomycin D1 Chemical compound N([C@H](C(=O)N[C@H](C)[C@@H](O)[C@H](C)C(=O)N[C@@H]([C@H](O)C)C(=O)NCCC=1SC[C@@H](N=1)C=1SC=C(N=1)C(=O)NCCCCNC(N)=N)[C@@H](O[C@H]1[C@H]([C@@H](O)[C@H](O)[C@H](CO)O1)O[C@@H]1[C@H]([C@@H](OC(N)=O)[C@H](O)[C@@H](CO)O1)O)C=1N=CNC=1)C(=O)C1=NC([C@H](CC(N)=O)NC[C@H](N)C(N)=O)=NC(N)=C1C CWCMIVBLVUHDHK-ZSNHEYEWSA-N 0.000 description 1
- 108010017843 platelet-derived growth factor A Proteins 0.000 description 1
- 108010000685 platelet-derived growth factor AB Proteins 0.000 description 1
- 208000030428 polyarticular arthritis Diseases 0.000 description 1
- 238000003752 polymerase chain reaction Methods 0.000 description 1
- 239000000256 polyoxyethylene sorbitan monolaurate Substances 0.000 description 1
- 235000010486 polyoxyethylene sorbitan monolaurate Nutrition 0.000 description 1
- 239000001267 polyvinylpyrrolidone Substances 0.000 description 1
- 235000013855 polyvinylpyrrolidone Nutrition 0.000 description 1
- 229920000036 polyvinylpyrrolidone Polymers 0.000 description 1
- 239000002243 precursor Substances 0.000 description 1
- 239000003755 preservative agent Substances 0.000 description 1
- 230000008569 process Effects 0.000 description 1
- 229940002612 prodrug Drugs 0.000 description 1
- 239000000651 prodrug Substances 0.000 description 1
- 230000000750 progressive effect Effects 0.000 description 1
- 238000011321 prophylaxis Methods 0.000 description 1
- 238000002331 protein detection Methods 0.000 description 1
- 108020003519 protein disulfide isomerase Proteins 0.000 description 1
- 230000002797 proteolythic effect Effects 0.000 description 1
- 230000006337 proteolytic cleavage Effects 0.000 description 1
- 230000002685 pulmonary effect Effects 0.000 description 1
- 238000002708 random mutagenesis Methods 0.000 description 1
- 239000011541 reaction mixture Substances 0.000 description 1
- 230000008707 rearrangement Effects 0.000 description 1
- 239000000018 receptor agonist Substances 0.000 description 1
- 229940044601 receptor agonist Drugs 0.000 description 1
- 239000011347 resin Substances 0.000 description 1
- 229920005989 resin Polymers 0.000 description 1
- 230000003248 secreting effect Effects 0.000 description 1
- 238000010187 selection method Methods 0.000 description 1
- 239000006152 selective media Substances 0.000 description 1
- 239000011734 sodium Substances 0.000 description 1
- RGHFKWPGWBFQLN-UHFFFAOYSA-M sodium;5,5-diethylpyrimidin-3-ide-2,4,6-trione Chemical compound [Na+].CCC1(CC)C([O-])=NC(=O)NC1=O RGHFKWPGWBFQLN-UHFFFAOYSA-M 0.000 description 1
- 239000002195 soluble material Substances 0.000 description 1
- 239000000243 solution Substances 0.000 description 1
- 125000006850 spacer group Chemical group 0.000 description 1
- 238000010186 staining Methods 0.000 description 1
- 210000000130 stem cell Anatomy 0.000 description 1
- 230000000638 stimulation Effects 0.000 description 1
- 238000003860 storage Methods 0.000 description 1
- 230000008961 swelling Effects 0.000 description 1
- 208000024891 symptom Diseases 0.000 description 1
- 229960000814 tetanus toxoid Drugs 0.000 description 1
- ZRKFYGHZFMAOKI-QMGMOQQFSA-N tgfbeta Chemical compound C([C@H](NC(=O)[C@H](C(C)C)NC(=O)CNC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H]([C@@H](C)O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H]([C@@H](C)O)NC(=O)[C@H](CC(C)C)NC(=O)CNC(=O)[C@H](C)NC(=O)[C@H](CO)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@@H](NC(=O)[C@H](C)NC(=O)[C@H](C)NC(=O)[C@@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](N)CCSC)C(C)C)[C@@H](C)CC)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC=1C=CC=CC=1)C(=O)N[C@@H](C)C(=O)N1[C@@H](CCC1)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](C)C(=O)N[C@@H](CC=1C=CC=CC=1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](C)C(=O)N[C@@H](CC(C)C)C(=O)N1[C@@H](CCC1)C(=O)N1[C@@H](CCC1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CO)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(C)C)C(O)=O)C1=CC=C(O)C=C1 ZRKFYGHZFMAOKI-QMGMOQQFSA-N 0.000 description 1
- 239000002562 thickening agent Substances 0.000 description 1
- 229960002175 thyroglobulin Drugs 0.000 description 1
- 206010043778 thyroiditis Diseases 0.000 description 1
- 239000003104 tissue culture media Substances 0.000 description 1
- 230000009772 tissue formation Effects 0.000 description 1
- 239000012096 transfection reagent Substances 0.000 description 1
- 238000012301 transgenic model Methods 0.000 description 1
- 238000003146 transient transfection Methods 0.000 description 1
- 238000013519 translation Methods 0.000 description 1
- LENZDBCJOHFCAS-UHFFFAOYSA-N tris Chemical compound OCC(N)(CO)CO LENZDBCJOHFCAS-UHFFFAOYSA-N 0.000 description 1
- 210000004881 tumor cell Anatomy 0.000 description 1
- 230000009385 viral infection Effects 0.000 description 1
- 238000005406 washing Methods 0.000 description 1
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P1/00—Drugs for disorders of the alimentary tract or the digestive system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P1/00—Drugs for disorders of the alimentary tract or the digestive system
- A61P1/04—Drugs for disorders of the alimentary tract or the digestive system for ulcers, gastritis or reflux esophagitis, e.g. antacids, inhibitors of acid secretion, mucosal protectants
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P11/00—Drugs for disorders of the respiratory system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P13/00—Drugs for disorders of the urinary system
- A61P13/12—Drugs for disorders of the urinary system of the kidneys
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P17/00—Drugs for dermatological disorders
- A61P17/06—Antipsoriatics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P19/00—Drugs for skeletal disorders
- A61P19/02—Drugs for skeletal disorders for joint disorders, e.g. arthritis, arthrosis
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
- A61P25/28—Drugs for disorders of the nervous system for treating neurodegenerative disorders of the central nervous system, e.g. nootropic agents, cognition enhancers, drugs for treating Alzheimer's disease or other forms of dementia
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P29/00—Non-central analgesic, antipyretic or antiinflammatory agents, e.g. antirheumatic agents; Non-steroidal antiinflammatory drugs [NSAID]
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P31/00—Antiinfectives, i.e. antibiotics, antiseptics, chemotherapeutics
- A61P31/04—Antibacterial agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P7/00—Drugs for disorders of the blood or the extracellular fluid
- A61P7/02—Antithrombotic agents; Anticoagulants; Platelet aggregation inhibitors
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P9/00—Drugs for disorders of the cardiovascular system
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/18—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans
- C07K16/24—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against cytokines, lymphokines or interferons
- C07K16/241—Tumor Necrosis Factors
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/40—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against enzymes
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K2039/505—Medicinal preparations containing antigens or antibodies comprising antibodies
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/50—Immunoglobulins specific features characterized by immunoglobulin fragments
- C07K2317/52—Constant or Fc region; Isotype
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/50—Immunoglobulins specific features characterized by immunoglobulin fragments
- C07K2317/56—Immunoglobulins specific features characterized by immunoglobulin fragments variable (Fv) region, i.e. VH and/or VL
- C07K2317/569—Single domain, e.g. dAb, sdAb, VHH, VNAR or nanobody®
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/90—Immunoglobulins specific features characterized by (pharmaco)kinetic aspects or by stability of the immunoglobulin
- C07K2317/94—Stability, e.g. half-life, pH, temperature or enzyme-resistance
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2319/00—Fusion polypeptide
Landscapes
- Health & Medical Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- General Health & Medical Sciences (AREA)
- Medicinal Chemistry (AREA)
- Life Sciences & Earth Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- General Chemical & Material Sciences (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Pharmacology & Pharmacy (AREA)
- Animal Behavior & Ethology (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Engineering & Computer Science (AREA)
- Immunology (AREA)
- Biochemistry (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Molecular Biology (AREA)
- Genetics & Genomics (AREA)
- Biophysics (AREA)
- Biomedical Technology (AREA)
- Neurology (AREA)
- Neurosurgery (AREA)
- Rheumatology (AREA)
- Orthopedic Medicine & Surgery (AREA)
- Hospice & Palliative Care (AREA)
- Psychiatry (AREA)
- Pulmonology (AREA)
- Dermatology (AREA)
- Oncology (AREA)
- Cardiology (AREA)
- Physical Education & Sports Medicine (AREA)
- Communicable Diseases (AREA)
- Pain & Pain Management (AREA)
- Hematology (AREA)
- Diabetes (AREA)
- Heart & Thoracic Surgery (AREA)
- Urology & Nephrology (AREA)
- Peptides Or Proteins (AREA)
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
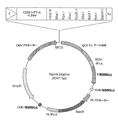
| GBGB0230203.2A GB0230203D0 (en) | 2002-12-27 | 2002-12-27 | Fc fusion |
| PCT/GB2003/005597 WO2004058820A2 (en) | 2002-12-27 | 2003-12-24 | Single-domain-effector group and its uses |
Related Child Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2010165687A Division JP2011004748A (ja) | 2002-12-27 | 2010-07-23 | Fc融合体 |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| JP2006524986A true JP2006524986A (ja) | 2006-11-09 |
| JP2006524986A5 JP2006524986A5 (enExample) | 2007-02-08 |
Family
ID=9950455
Family Applications (3)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2004563348A Pending JP2006524986A (ja) | 2002-12-27 | 2003-12-24 | Fc融合体 |
| JP2010165687A Pending JP2011004748A (ja) | 2002-12-27 | 2010-07-23 | Fc融合体 |
| JP2013142295A Pending JP2014011996A (ja) | 2002-12-27 | 2013-07-08 | Fc融合体 |
Family Applications After (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2010165687A Pending JP2011004748A (ja) | 2002-12-27 | 2010-07-23 | Fc融合体 |
| JP2013142295A Pending JP2014011996A (ja) | 2002-12-27 | 2013-07-08 | Fc融合体 |
Country Status (10)
| Country | Link |
|---|---|
| US (1) | US20060083747A1 (enExample) |
| EP (3) | EP1878751A2 (enExample) |
| JP (3) | JP2006524986A (enExample) |
| AT (1) | ATE472557T1 (enExample) |
| AU (1) | AU2003295139B2 (enExample) |
| CA (1) | CA2511959C (enExample) |
| DE (1) | DE60333229D1 (enExample) |
| ES (1) | ES2346431T3 (enExample) |
| GB (1) | GB0230203D0 (enExample) |
| WO (1) | WO2004058820A2 (enExample) |
Cited By (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2012514997A (ja) * | 2009-01-14 | 2012-07-05 | エフィミュヌ | 組換え一価抗体 |
| JP2012527875A (ja) * | 2009-05-28 | 2012-11-12 | グラクソ グループ リミテッド | 抗原結合タンパク質 |
| JP2013519389A (ja) * | 2010-02-18 | 2013-05-30 | エフィミュヌ | 抗cd28ヒト化抗体 |
| JP2016054746A (ja) * | 2010-08-02 | 2016-04-21 | リジェネロン・ファーマシューティカルズ・インコーポレイテッドRegeneron Pharmaceuticals, Inc. | Vlドメインを含む結合タンパク質を作製するマウス |
| JP2018523673A (ja) * | 2015-08-14 | 2018-08-23 | アラーガン、インコーポレイテッドAllergan,Incorporated | Pdgfに対する重鎖のみ抗体 |
Families Citing this family (181)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US6765087B1 (en) * | 1992-08-21 | 2004-07-20 | Vrije Universiteit Brussel | Immunoglobulins devoid of light chains |
| US20060002935A1 (en) * | 2002-06-28 | 2006-01-05 | Domantis Limited | Tumor Necrosis Factor Receptor 1 antagonists and methods of use therefor |
| US20130216538A1 (en) * | 2002-12-27 | 2013-08-22 | Domantis Limited | Compositions and Methods for Treating Inflammatory Disorders |
| DE10303974A1 (de) | 2003-01-31 | 2004-08-05 | Abbott Gmbh & Co. Kg | Amyloid-β(1-42)-Oligomere, Verfahren zu deren Herstellung und deren Verwendung |
| EP2275448A3 (en) * | 2003-12-19 | 2013-02-06 | Genentech, Inc. | Monovalent antibody fragments useful as therapeutics |
| LT2311874T (lt) | 2004-07-22 | 2017-11-27 | Erasmus University Medical Center Rotterdam | Rišančiosios molekulės |
| PT1791423E (pt) * | 2004-08-02 | 2013-08-02 | Sami Labs Ltd | Composições e métodos para o tratamento de condições dermatológicas hiperproliferativas |
| US7476724B2 (en) | 2004-08-05 | 2009-01-13 | Genentech, Inc. | Humanized anti-cmet antibodies |
| CN101243191B (zh) | 2004-11-29 | 2014-04-16 | 塞昆纳姆股份有限公司 | 用于检测甲基化dna的手段和方法 |
| AU2005308916B2 (en) | 2004-11-29 | 2011-07-21 | Sequenom, Inc. | Kits and methods for detecting methylated DNA |
| FR2879605B1 (fr) | 2004-12-16 | 2008-10-17 | Centre Nat Rech Scient Cnrse | Production de formats d'anticorps et applications immunologiques de ces formats |
| CN103254309B (zh) | 2005-05-18 | 2017-09-26 | 埃博灵克斯股份有限公司 | 针对肿瘤坏死因子α的改进的纳米体TM |
| EP1917977B1 (en) * | 2005-08-23 | 2011-12-28 | Akira Matsumori | Therapeutic drug for heart disease and virus disease |
| TW200732350A (en) * | 2005-10-21 | 2007-09-01 | Amgen Inc | Methods for generating monovalent IgG |
| DK1976877T4 (en) | 2005-11-30 | 2017-01-16 | Abbvie Inc | Monoclonal antibodies to amyloid beta protein and uses thereof |
| CA2628703C (en) | 2005-11-30 | 2019-10-29 | Abbott Laboratories | Anti-a.beta. globulomer antibodies, antigen-binding moieties thereof, corresponding hybridomas, nucleic acids, vectors, host cells, methods of producing said antibodies, compositions comprising said antibodies, uses of said antibodies and methods of using said antibodies |
| WO2007066109A1 (en) * | 2005-12-06 | 2007-06-14 | Domantis Limited | Bispecific ligands with binding specificity to cell surface targets and methods of use therefor |
| WO2007070979A1 (en) | 2005-12-20 | 2007-06-28 | Arana Therapeutics Limited | Chimeric antibodies with part new world primate binding regions |
| WO2007087673A1 (en) | 2006-02-01 | 2007-08-09 | Arana Therapeutics Limited | Domain antibody construct |
| WO2007146163A2 (en) * | 2006-06-09 | 2007-12-21 | Welson Pharmaceuticals, Inc. | Fc-fusion proteins with reduced fc-mediated effector activities |
| US8455626B2 (en) | 2006-11-30 | 2013-06-04 | Abbott Laboratories | Aβ conformer selective anti-aβ globulomer monoclonal antibodies |
| EP2115004A2 (en) | 2006-12-19 | 2009-11-11 | Ablynx N.V. | Amino acid sequences directed against gpcrs and polypeptides comprising the same for the treatment of gpcr-related diseases and disorders |
| US9156914B2 (en) | 2006-12-19 | 2015-10-13 | Ablynx N.V. | Amino acid sequences directed against a metalloproteinase from the ADAM family and polypeptides comprising the same for the treatment of ADAM-related diseases and disorders |
| WO2008104386A2 (en) | 2007-02-27 | 2008-09-04 | Abbott Gmbh & Co. Kg | Method for the treatment of amyloidoses |
| GB0724331D0 (en) * | 2007-12-13 | 2008-01-23 | Domantis Ltd | Compositions for pulmonary delivery |
| CA2683791A1 (en) * | 2007-06-06 | 2008-12-11 | Domantis Limited | Polypeptides, antibody variable domains & antagonists |
| CA2696263C (en) * | 2007-08-15 | 2017-06-13 | Bing Liu | Monospecific and multispecific antibodies and method of use |
| CA2700714C (en) | 2007-09-26 | 2018-09-11 | Ucb Pharma S.A. | Dual specificity antibody fusions |
| CA2706200A1 (en) | 2007-11-27 | 2009-06-04 | Ablynx N.V. | Immunoglobulin constructs comprising multiple single variable domains and an fc portion |
| US8557965B2 (en) | 2008-04-07 | 2013-10-15 | Ablynx N.V. | Single variable domains against notch pathway members |
| KR20110020860A (ko) * | 2008-05-23 | 2011-03-03 | 알리바 바이오파마수티컬스, 아이엔씨. | 유전자 삽입동물에서 단일 vl 도메인 항체를 생산하는 방법 |
| CN102164965B (zh) | 2008-09-26 | 2016-03-30 | Ucb医药有限公司 | 生物产品 |
| BRPI1008014A2 (pt) | 2009-02-19 | 2016-10-04 | Glaxo Group Ltd | domínio variável simples, ligante multiespecífico, antagonistas de tnfr1, e de receptor de tnf-alfa, uso do agonista de tnfr, antagonista anti-tnfr1, polipeptídeo ou ligante multiespecífico, método para tratar e/ou prevenir uma condição ou doença, ácido nucleico, vetor, e, hospedeiro |
| KR20130119990A (ko) | 2009-04-10 | 2013-11-01 | 아블린쓰 엔.브이. | Il-6r에 대한 개선된 아미노산 서열 및 il-6r 관련 질환 및 질병의 치료를 위한 그를 포함하는 폴리펩티드 |
| CA2764398A1 (en) | 2009-06-05 | 2010-12-09 | Erik Depla | Improved amino acid sequences directed against human respiratory syncytial virus (hrsv) and polypeptides comprising the same for the prevention and/or treatment of respiratory tract infections |
| EP2453920A2 (en) | 2009-07-16 | 2012-05-23 | Glaxo Group Limited | Antagonists, uses & methods for partially inhibiting tnfr1 |
| GB201005063D0 (en) | 2010-03-25 | 2010-05-12 | Ucb Pharma Sa | Biological products |
| ES2552177T3 (es) | 2009-10-27 | 2015-11-26 | Glaxo Group Limited | Polipéptidos anti-TNFR1 estables, dominios variables de anticuerpos y antagonistas |
| EP2507262A1 (en) | 2009-11-30 | 2012-10-10 | Ablynx N.V. | Improved amino acid sequences directed against human respiratory syncytial virus (hrsv) and polypeptides comprising the same for the prevention and/or treatment of respiratory tract infections |
| SMT201900251T1 (it) | 2009-12-10 | 2019-07-11 | Regeneron Pharma | Topi che producono anticorpi della catena pesante |
| EP2513145B1 (en) | 2009-12-14 | 2018-01-24 | Ablynx N.V. | Single variable domain antibodies against ox40l, constructs and therapeutic use |
| WO2011083141A2 (en) | 2010-01-08 | 2011-07-14 | Ablynx Nv | Method for generation of immunoglobulin sequences by using lipoprotein particles |
| ES2603559T5 (es) | 2010-02-08 | 2021-02-22 | Regeneron Pharma | Cadena ligera común de ratón |
| US9796788B2 (en) | 2010-02-08 | 2017-10-24 | Regeneron Pharmaceuticals, Inc. | Mice expressing a limited immunoglobulin light chain repertoire |
| US20130045492A1 (en) | 2010-02-08 | 2013-02-21 | Regeneron Pharmaceuticals, Inc. | Methods For Making Fully Human Bispecific Antibodies Using A Common Light Chain |
| US9120855B2 (en) | 2010-02-10 | 2015-09-01 | Novartis Ag | Biologic compounds directed against death receptor 5 |
| WO2011118739A1 (ja) | 2010-03-26 | 2011-09-29 | 協和発酵キリン株式会社 | 新規修飾部位導入抗体および抗体フラグメント |
| CN104744591B (zh) | 2010-04-15 | 2022-09-27 | Abbvie德国有限责任两合公司 | β淀粉样蛋白结合蛋白 |
| RU2012149227A (ru) | 2010-05-20 | 2014-06-27 | Аблинкс Нв | Биологические материалы, относящиеся к her3 |
| US8911728B2 (en) | 2010-05-21 | 2014-12-16 | The United States Of America, As Represented By The Secretary Of The Department Of Health And Human Services | High-affinity fully functional soluble single-domain human CD4, antibodies, and related fusion proteins |
| WO2012024187A1 (en) | 2010-08-14 | 2012-02-23 | Abbott Laboratories | Amyloid-beta binding proteins |
| ES2723775T3 (es) | 2010-11-08 | 2019-09-02 | Ablynx Nv | Polipéptidos que se unen a CXCR2 |
| CA2827170A1 (en) | 2011-02-11 | 2012-08-16 | David M. Hilbert | Monovalent and multivalent multispecific complexes and uses thereof |
| EP2699597B1 (en) | 2011-04-21 | 2016-06-01 | Garvan Institute of Medical Research | Modified variable domain molecules and methods for producing and using them b |
| UA117218C2 (uk) | 2011-05-05 | 2018-07-10 | Мерк Патент Гмбх | Поліпептид, спрямований проти il-17a, il-17f та/або il17-a/f |
| AR086823A1 (es) | 2011-06-30 | 2014-01-22 | Genentech Inc | Formulaciones de anticuerpo anti-c-met, metodos |
| FI3865581T3 (fi) | 2011-08-05 | 2024-11-02 | Regeneron Pharma | Humanisoituja universaaleja kevytketjun hiiriä |
| US9706759B2 (en) | 2011-12-20 | 2017-07-18 | Regeneron Pharmaceuticals, Inc. | Humanized light chain mice |
| JP2015504674A (ja) * | 2012-01-11 | 2015-02-16 | アリゾナ ボード オブ リージェンツ ア ボディ コーポレート オブ ザ ステイト オブ アリゾナ アクティング フォー アンド オン ビハーフ オブ アリゾナ ステイト ユニバーシティーArizona Board Of Regents, A Body Corporate Of The State Of Arizona Acting For And On Behalf Of Arizona State University | 神経疾患タンパク質の二重特異性抗体フラグメントおよび使用方法 |
| US9328174B2 (en) | 2012-05-09 | 2016-05-03 | Novartis Ag | Chemokine receptor binding polypeptides |
| EP2905290B1 (en) | 2012-10-05 | 2019-12-04 | Kyowa Kirin Co., Ltd. | Heterodimeric protein composition |
| US10993420B2 (en) | 2013-03-15 | 2021-05-04 | Erasmus University Medical Center | Production of heavy chain only antibodies in transgenic mammals |
| JOP20200094A1 (ar) | 2014-01-24 | 2017-06-16 | Dana Farber Cancer Inst Inc | جزيئات جسم مضاد لـ pd-1 واستخداماتها |
| JOP20200096A1 (ar) | 2014-01-31 | 2017-06-16 | Children’S Medical Center Corp | جزيئات جسم مضاد لـ tim-3 واستخداماتها |
| CA2936962C (en) | 2014-03-14 | 2024-03-05 | Novartis Ag | Antibody molecules to lag-3 and uses thereof |
| ES2939760T3 (es) | 2014-03-15 | 2023-04-26 | Novartis Ag | Tratamiento de cáncer utilizando un receptor quimérico para antígenos |
| SG11201607015VA (en) | 2014-03-21 | 2016-09-29 | Regeneron Pharma | V<sb>L</sb> ANTIGEN BINDING PROTEINS EXHIBITING DISTINCT BINDING CHARACTERISTICS |
| CA3124228C (en) * | 2014-03-21 | 2024-05-14 | Regeneron Pharmaceuticals, Inc. | Non-human animals that make single domain binding proteins |
| CN107002119A (zh) | 2014-03-24 | 2017-08-01 | 豪夫迈·罗氏有限公司 | 使用c‑met拮抗剂的癌症治疗及前者与hgf表达的关联 |
| KR20250099289A (ko) | 2014-05-16 | 2025-07-01 | 아블린쓰 엔.브이. | 개선된 면역글로불린 가변 도메인 |
| SG10201810124PA (en) | 2014-05-16 | 2018-12-28 | Ablynx Nv | Improved immunoglobulin variable domains |
| NL2013661B1 (en) | 2014-10-21 | 2016-10-05 | Ablynx Nv | KV1.3 Binding immunoglobulins. |
| US11542488B2 (en) | 2014-07-21 | 2023-01-03 | Novartis Ag | Sortase synthesized chimeric antigen receptors |
| JP2017528433A (ja) | 2014-07-21 | 2017-09-28 | ノバルティス アーゲー | 低い免疫増強用量のmTOR阻害剤とCARの組み合わせ |
| JP7054622B2 (ja) | 2014-07-21 | 2022-04-14 | ノバルティス アーゲー | ヒト化抗bcmaキメラ抗原受容体を使用した癌の処置 |
| EP3722316A1 (en) | 2014-07-21 | 2020-10-14 | Novartis AG | Treatment of cancer using a cd33 chimeric antigen receptor |
| ES2781175T3 (es) | 2014-07-31 | 2020-08-31 | Novartis Ag | Subconjunto optimizado de células T que contienen un receptor de antígeno quimérico |
| US10851149B2 (en) | 2014-08-14 | 2020-12-01 | The Trustees Of The University Of Pennsylvania | Treatment of cancer using GFR α-4 chimeric antigen receptor |
| ES2791248T3 (es) | 2014-08-19 | 2020-11-03 | Novartis Ag | Receptor antigénico quimérico (CAR) anti-CD123 para su uso en el tratamiento del cáncer |
| US10577417B2 (en) | 2014-09-17 | 2020-03-03 | Novartis Ag | Targeting cytotoxic cells with chimeric receptors for adoptive immunotherapy |
| CA2964367C (en) | 2014-10-14 | 2024-01-30 | Novartis Ag | Antibody molecules to pd-l1 and uses thereof |
| WO2016061632A1 (en) | 2014-10-23 | 2016-04-28 | La Trobe University | Fn14-binding proteins and uses thereof |
| US20180334490A1 (en) | 2014-12-03 | 2018-11-22 | Qilong H. Wu | Methods for b cell preconditioning in car therapy |
| WO2016149678A1 (en) | 2015-03-19 | 2016-09-22 | Regeneron Pharmaceuticals, Inc. | Non-human animals that select for light chain variable regions that bind antigen |
| MX387517B (es) | 2015-04-06 | 2025-03-18 | Subdomain Llc | Polipeptidos que contienen dominios de union de novo y usos de los mismos. |
| JP6961490B2 (ja) | 2015-04-08 | 2021-11-05 | ノバルティス アーゲー | Cd20療法、cd22療法、およびcd19キメラ抗原受容体(car)発現細胞との併用療法 |
| US12128069B2 (en) | 2015-04-23 | 2024-10-29 | The Trustees Of The University Of Pennsylvania | Treatment of cancer using chimeric antigen receptor and protein kinase a blocker |
| WO2017019896A1 (en) | 2015-07-29 | 2017-02-02 | Novartis Ag | Combination therapies comprising antibody molecules to pd-1 |
| US20180207273A1 (en) | 2015-07-29 | 2018-07-26 | Novartis Ag | Combination therapies comprising antibody molecules to tim-3 |
| EP3792279A3 (en) * | 2015-07-29 | 2021-07-07 | Allergan, Inc. | Heavy chain only antibodies to ang-2 |
| DK3317301T3 (da) | 2015-07-29 | 2021-06-28 | Immutep Sas | Kombinationsterapier omfattende antistofmolekyler mod lag-3 |
| WO2017106656A1 (en) | 2015-12-17 | 2017-06-22 | Novartis Ag | Antibody molecules to pd-1 and uses thereof |
| KR20180094977A (ko) | 2015-12-17 | 2018-08-24 | 노파르티스 아게 | c-Met 억제제와 PD-1에 대한 항체 분자의 조합물 및 그의 용도 |
| EP3393504B1 (en) | 2015-12-22 | 2025-09-24 | Novartis AG | Mesothelin chimeric antigen receptor (car) and antibody against pd-l1 inhibitor for combined use in anticancer therapy |
| US20210198368A1 (en) | 2016-01-21 | 2021-07-01 | Novartis Ag | Multispecific molecules targeting cll-1 |
| WO2017149515A1 (en) | 2016-03-04 | 2017-09-08 | Novartis Ag | Cells expressing multiple chimeric antigen receptor (car) molecules and uses therefore |
| EP3432924A1 (en) | 2016-03-23 | 2019-01-30 | Novartis AG | Cell secreted minibodies and uses thereof |
| EP3443096B1 (en) | 2016-04-15 | 2023-03-01 | Novartis AG | Compositions and methods for selective expression of chimeric antigen receptors |
| KR102460040B1 (ko) | 2016-04-27 | 2022-11-01 | 애브비 인코포레이티드 | 항-il-13 항체를 이용한 il-13 활성이 유해한 질환의 치료 방법 |
| US20210177896A1 (en) | 2016-06-02 | 2021-06-17 | Novartis Ag | Therapeutic regimens for chimeric antigen receptor (car)- expressing cells |
| CN110461315B (zh) | 2016-07-15 | 2025-05-02 | 诺华股份有限公司 | 使用与激酶抑制剂组合的嵌合抗原受体治疗和预防细胞因子释放综合征 |
| IL316970A (en) | 2016-07-28 | 2025-01-01 | Novartis Ag | Combination therapies of chimeric antigen receptors and PD-1 inhibitors |
| KR20190036551A (ko) | 2016-08-01 | 2019-04-04 | 노파르티스 아게 | Pro-m2 대식세포 분자의 억제제를 병용하는, 키메라 항원 수용체를 이용한 암의 치료 |
| WO2018067992A1 (en) | 2016-10-07 | 2018-04-12 | Novartis Ag | Chimeric antigen receptors for the treatment of cancer |
| IL266506B2 (en) | 2016-11-16 | 2025-05-01 | Ablynx Nv | T cell recruiting polypeptides capable of binding cd123 and tcr alpha/beta |
| US11535662B2 (en) | 2017-01-26 | 2022-12-27 | Novartis Ag | CD28 compositions and methods for chimeric antigen receptor therapy |
| EP3589647A1 (en) | 2017-02-28 | 2020-01-08 | Novartis AG | Shp inhibitor compositions and uses for chimeric antigen receptor therapy |
| US20200055948A1 (en) | 2017-04-28 | 2020-02-20 | Novartis Ag | Cells expressing a bcma-targeting chimeric antigen receptor, and combination therapy with a gamma secretase inhibitor |
| EP3615068A1 (en) | 2017-04-28 | 2020-03-04 | Novartis AG | Bcma-targeting agent, and combination therapy with a gamma secretase inhibitor |
| MY204117A (en) | 2017-06-22 | 2024-08-08 | Novartis Ag | Antibody molecules to cd73 and uses thereof |
| MX2019015738A (es) | 2017-06-27 | 2020-02-20 | Novartis Ag | Regimen de dosificacion para anticuerpos anti-tim-3 y usos de los mismos. |
| SG11201913137VA (en) | 2017-07-11 | 2020-01-30 | Compass Therapeutics Llc | Agonist antibodies that bind human cd137 and uses thereof |
| AU2018302283B2 (en) | 2017-07-20 | 2025-07-10 | Novartis Ag | Dosage regimens of anti-LAG-3 antibodies and uses thereof |
| IL273202B2 (en) | 2017-09-11 | 2024-08-01 | Univ Monash | Binding proteins to the human thrombin receptor, par4 |
| WO2019089753A2 (en) | 2017-10-31 | 2019-05-09 | Compass Therapeutics Llc | Cd137 antibodies and pd-1 antagonists and uses thereof |
| WO2019089798A1 (en) | 2017-10-31 | 2019-05-09 | Novartis Ag | Anti-car compositions and methods |
| EA202091198A1 (ru) | 2017-11-14 | 2020-09-09 | Эрселлкс, Инк. | Полипептиды, содержащие домен d, и их применение |
| CA3081602A1 (en) | 2017-11-16 | 2019-05-23 | Novartis Ag | Combination therapies |
| EP3713961A2 (en) | 2017-11-20 | 2020-09-30 | Compass Therapeutics LLC | Cd137 antibodies and tumor antigen-targeting antibodies and uses thereof |
| WO2019139987A1 (en) | 2018-01-09 | 2019-07-18 | Elstar Therapeutics, Inc. | Calreticulin binding constructs and engineered t cells for the treatment of diseases |
| EP3746116A1 (en) | 2018-01-31 | 2020-12-09 | Novartis AG | Combination therapy using a chimeric antigen receptor |
| US12152073B2 (en) | 2018-03-14 | 2024-11-26 | Marengo Therapeutics, Inc. | Multifunctional molecules that bind to calreticulin and uses thereof |
| US20210147547A1 (en) | 2018-04-13 | 2021-05-20 | Novartis Ag | Dosage Regimens For Anti-Pd-L1 Antibodies And Uses Thereof |
| WO2019210153A1 (en) | 2018-04-27 | 2019-10-31 | Novartis Ag | Car t cell therapies with enhanced efficacy |
| WO2019226658A1 (en) | 2018-05-21 | 2019-11-28 | Compass Therapeutics Llc | Multispecific antigen-binding compositions and methods of use |
| JP2021525243A (ja) | 2018-05-21 | 2021-09-24 | コンパス セラピューティクス リミテッド ライアビリティ カンパニー | Nk細胞による標的細胞の殺傷を増進するための組成物および方法 |
| US20210213063A1 (en) | 2018-05-25 | 2021-07-15 | Novartis Ag | Combination therapy with chimeric antigen receptor (car) therapies |
| US20210214459A1 (en) | 2018-05-31 | 2021-07-15 | Novartis Ag | Antibody molecules to cd73 and uses thereof |
| TWI890660B (zh) | 2018-06-13 | 2025-07-21 | 瑞士商諾華公司 | Bcma 嵌合抗原受體及其用途 |
| MX2020013798A (es) | 2018-06-19 | 2021-08-11 | Atarga Llc | Moléculas de anticuerpo de componente de complemento 5 y sus usos. |
| CN112955465A (zh) | 2018-07-03 | 2021-06-11 | 马伦戈治疗公司 | 抗tcr抗体分子及其用途 |
| AR116109A1 (es) | 2018-07-10 | 2021-03-31 | Novartis Ag | Derivados de 3-(5-amino-1-oxoisoindolin-2-il)piperidina-2,6-diona y usos de los mismos |
| WO2020021465A1 (en) | 2018-07-25 | 2020-01-30 | Advanced Accelerator Applications (Italy) S.R.L. | Method of treatment of neuroendocrine tumors |
| CN119735694A (zh) | 2018-11-13 | 2025-04-01 | 指南针制药有限责任公司 | 对抗检查点分子的多特异性结合构建体及其用途 |
| JP2022514280A (ja) | 2018-12-20 | 2022-02-10 | ノバルティス アーゲー | Mdm2阻害剤のための延長低用量レジメン |
| KR20210106437A (ko) | 2018-12-20 | 2021-08-30 | 노파르티스 아게 | 3-(1-옥소이소인돌린-2-일)피페리딘-2,6-디온 유도체를 포함하는 투약 요법 및 약학적 조합물 |
| AU2020222345B2 (en) | 2019-02-15 | 2022-11-17 | Novartis Ag | 3-(1-oxo-5-(piperidin-4-yl)isoindolin-2-yl)piperidine-2,6-dione derivatives and uses thereof |
| US12479817B2 (en) | 2019-02-15 | 2025-11-25 | Novartis Ag | Substituted 3-(1-oxoisoindolin-2-yl)piperidine-2,6-dione derivatives and uses thereof |
| US10871640B2 (en) | 2019-02-15 | 2020-12-22 | Perkinelmer Cellular Technologies Germany Gmbh | Methods and systems for automated imaging of three-dimensional objects |
| EP3927828A4 (en) | 2019-02-21 | 2023-02-01 | Enosi Life Sciences Corp. | ANTIBODIES AND ENONOMERS |
| CN119661722A (zh) | 2019-02-21 | 2025-03-21 | 马伦戈治疗公司 | 结合t细胞相关癌细胞的多功能分子及其用途 |
| CN119039441A (zh) | 2019-02-21 | 2024-11-29 | 马伦戈治疗公司 | 与nkp30结合的抗体分子及其用途 |
| US20220088075A1 (en) | 2019-02-22 | 2022-03-24 | The Trustees Of The University Of Pennsylvania | Combination therapies of egfrviii chimeric antigen receptors and pd-1 inhibitors |
| JP2022527790A (ja) | 2019-03-29 | 2022-06-06 | アターガ,エルエルシー | 抗fgf23抗体分子 |
| CN114786680A (zh) | 2019-10-21 | 2022-07-22 | 诺华股份有限公司 | Tim-3抑制剂及其用途 |
| KR20220103947A (ko) | 2019-10-21 | 2022-07-25 | 노파르티스 아게 | 베네토클락스 및 tim-3 억제제를 사용한 조합 요법 |
| AR120566A1 (es) | 2019-11-26 | 2022-02-23 | Novartis Ag | Receptores de antígeno quiméricos y sus usos |
| JP2023506958A (ja) | 2019-12-20 | 2023-02-20 | ノバルティス アーゲー | 骨髄線維症および骨髄異形成症候群を処置するための、デシタビンまたは抗pd-1抗体スパルタリズマブを伴うかまたは伴わない抗tim-3抗体mbg453および抗tgf-ベータ抗体nis793の組合せ |
| GB2609554B (en) | 2020-01-03 | 2025-08-20 | Marengo Therapeutics Inc | Anti-TCR antibody molecules and uses thereof |
| CN115298322A (zh) | 2020-01-17 | 2022-11-04 | 贝克顿迪金森公司 | 用于单细胞分泌组学的方法和组合物 |
| IL293752A (en) | 2020-01-17 | 2022-08-01 | Novartis Ag | Combination comprising a tim-3 inhibitor and a hypomethylating agent for use in treating myelodysplastic syndrome or chronic myelomonocytic leukemia |
| KR20220147109A (ko) | 2020-02-27 | 2022-11-02 | 노파르티스 아게 | 키메라 항원 수용체 발현 세포의 제조 방법 |
| WO2021260528A1 (en) | 2020-06-23 | 2021-12-30 | Novartis Ag | Dosing regimen comprising 3-(1-oxoisoindolin-2-yl)piperidine-2,6-dione derivatives |
| CR20230009A (es) | 2020-07-16 | 2023-01-25 | Novartis Ag | Anticuerpos anti-betacelulina, fragmentos de los mismos, y moléculas de unión multiespecíficas |
| WO2022026592A2 (en) | 2020-07-28 | 2022-02-03 | Celltas Bio, Inc. | Antibody molecules to coronavirus and uses thereof |
| WO2022029573A1 (en) | 2020-08-03 | 2022-02-10 | Novartis Ag | Heteroaryl substituted 3-(1-oxoisoindolin-2-yl)piperidine-2,6-dione derivatives and uses thereof |
| CN116847887A (zh) | 2020-08-27 | 2023-10-03 | 伊诺西治疗公司 | 治疗自身免疫性疾病和癌症的方法和组合物 |
| EP4204020A1 (en) | 2020-08-31 | 2023-07-05 | Advanced Accelerator Applications International S.A. | Method of treating psma-expressing cancers |
| WO2022043557A1 (en) | 2020-08-31 | 2022-03-03 | Advanced Accelerator Applications International Sa | Method of treating psma-expressing cancers |
| EP4240765A2 (en) | 2020-11-06 | 2023-09-13 | Novartis AG | Antibody fc variants |
| IL302700A (en) | 2020-11-13 | 2023-07-01 | Novartis Ag | Combination therapies with chimeric antigen receptor (car)-expressing cells |
| US20240141060A1 (en) | 2021-01-29 | 2024-05-02 | Novartis Ag | Dosage regimes for anti-cd73 and anti-entpd2 antibodies and uses thereof |
| IL305301A (en) | 2021-02-19 | 2023-10-01 | Us Health | SINGLE DOMAIN ANTIBODIES THAT NEUTRALIZE SARS-CoV-2 |
| TW202304979A (zh) | 2021-04-07 | 2023-02-01 | 瑞士商諾華公司 | 抗TGFβ抗體及其他治療劑用於治療增殖性疾病之用途 |
| AR125874A1 (es) | 2021-05-18 | 2023-08-23 | Novartis Ag | Terapias de combinación |
| WO2023044483A2 (en) | 2021-09-20 | 2023-03-23 | Voyager Therapeutics, Inc. | Compositions and methods for the treatment of her2 positive cancer |
| US20250034559A1 (en) | 2021-11-17 | 2025-01-30 | Voyager Therapeutics, Inc. | Compositions and methods for the treatment of tau-related disorders |
| US20230383010A1 (en) | 2022-02-07 | 2023-11-30 | Visterra, Inc. | Anti-idiotype antibody molecules and uses thereof |
| US20250295809A1 (en) | 2022-05-13 | 2025-09-25 | Voyager Therapeutics, Inc. | Compositions and methods for the treatment of her2 positive cancer |
| JP2025528068A (ja) | 2022-08-03 | 2025-08-26 | ボイジャー セラピューティクス インコーポレイテッド | 血液脳関門を通過させるための組成物及び方法 |
| EP4605077A1 (en) | 2022-10-18 | 2025-08-27 | Confo Therapeutics N.V. | Amino acid sequences directed against the melanocortin 4 receptor and polypeptides comprising the same for the treatment of mc4r-related diseases and disorders |
| WO2024168061A2 (en) | 2023-02-07 | 2024-08-15 | Ayan Therapeutics Inc. | Antibody molecules binding to sars-cov-2 |
| WO2025049818A1 (en) | 2023-08-29 | 2025-03-06 | Enosi Therapeutics Corporation | Tnfr1 antagonists lacking agonist activity and uses thereof |
| WO2025099632A1 (en) | 2023-11-08 | 2025-05-15 | Sanofi | Cd25 based lysosomal degrader and uses thereof |
| WO2025122634A1 (en) | 2023-12-05 | 2025-06-12 | Voyager Therapeutics, Inc. | Compositions and methods for the treatment of tau-related disorders |
| US12357539B1 (en) | 2024-05-16 | 2025-07-15 | Genzyme Corporation | Vial adapter and injection kit for withdrawing a liquid medicament from an injection vial |
| US12357538B1 (en) | 2024-11-25 | 2025-07-15 | Genzyme Corporation | Vial adapter and injection kit for withdrawing a liquid medicament from an injection vial |
| US12377023B1 (en) | 2024-12-02 | 2025-08-05 | Genzyme Corporation | Fluid transfer device |
| US12420017B1 (en) | 2025-02-26 | 2025-09-23 | Genzyme Corporation | Damping device for a medicament delivery device |
| US12434008B1 (en) | 2025-02-26 | 2025-10-07 | Genzyme Corporation | Lock ring for a medicament delivery device |
| US12465697B1 (en) | 2025-02-26 | 2025-11-11 | Genzyme Corporation | Medicament delivery device |
Citations (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2002044215A2 (en) * | 2000-12-01 | 2002-06-06 | Cockbain, Julian | Hybrid antibodies |
| WO2002061071A2 (en) * | 2000-12-18 | 2002-08-08 | Dyax Corp. | Focused libraries of genetic packages |
Family Cites Families (13)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US3907502A (en) * | 1973-12-11 | 1975-09-23 | Miless L Brink | Method for identifying Bence Jones proteins |
| WO1988009344A1 (en) * | 1987-05-21 | 1988-12-01 | Creative Biomolecules, Inc. | Targeted multifunctional proteins |
| WO1990005144A1 (en) * | 1988-11-11 | 1990-05-17 | Medical Research Council | Single domain ligands, receptors comprising said ligands, methods for their production, and use of said ligands and receptors |
| US5116964A (en) * | 1989-02-23 | 1992-05-26 | Genentech, Inc. | Hybrid immunoglobulins |
| WO1993008210A1 (en) * | 1991-10-18 | 1993-04-29 | Beth Israel Hospital Association | Vascular permeability factor targeted compounds |
| ATE427968T1 (de) * | 1992-08-21 | 2009-04-15 | Univ Bruxelles | Immunoglobuline ohne leichtkette |
| US5869046A (en) * | 1995-04-14 | 1999-02-09 | Genentech, Inc. | Altered polypeptides with increased half-life |
| WO1998020140A1 (en) * | 1996-11-06 | 1998-05-14 | The Regents Of The University Of California | Isolated tumor necrosis factor receptor releasing enzyme, compositions comprising the enzyme and methods of the use thereof |
| US6277375B1 (en) * | 1997-03-03 | 2001-08-21 | Board Of Regents, The University Of Texas System | Immunoglobulin-like domains with increased half-lives |
| WO1999002671A1 (en) | 1997-07-07 | 1999-01-21 | Medical Research Council | In vitro sorting method |
| WO2002051870A2 (en) * | 2000-12-22 | 2002-07-04 | GRAD, Carole Legal Representative of KAPLAN, Howard | Phage display libraries of human vh fragments |
| GB0110029D0 (en) * | 2001-04-24 | 2001-06-13 | Grosveld Frank | Transgenic animal |
| US7438910B2 (en) * | 2002-09-06 | 2008-10-21 | Amgen Inc. | Therapeutic human anti-IL1-R1 monoclonal antibody |
-
2002
- 2002-12-27 GB GBGB0230203.2A patent/GB0230203D0/en not_active Ceased
-
2003
- 2003-12-24 EP EP07001933A patent/EP1878751A2/en not_active Withdrawn
- 2003-12-24 AU AU2003295139A patent/AU2003295139B2/en not_active Ceased
- 2003-12-24 AT AT03786140T patent/ATE472557T1/de not_active IP Right Cessation
- 2003-12-24 EP EP07001932.8A patent/EP1878750A3/en not_active Withdrawn
- 2003-12-24 JP JP2004563348A patent/JP2006524986A/ja active Pending
- 2003-12-24 CA CA2511959A patent/CA2511959C/en not_active Expired - Lifetime
- 2003-12-24 DE DE60333229T patent/DE60333229D1/de not_active Expired - Lifetime
- 2003-12-24 ES ES03786140T patent/ES2346431T3/es not_active Expired - Lifetime
- 2003-12-24 WO PCT/GB2003/005597 patent/WO2004058820A2/en not_active Ceased
- 2003-12-24 EP EP03786140A patent/EP1581559B1/en not_active Revoked
-
2005
- 2005-06-24 US US11/166,496 patent/US20060083747A1/en not_active Abandoned
-
2010
- 2010-07-23 JP JP2010165687A patent/JP2011004748A/ja active Pending
-
2013
- 2013-07-08 JP JP2013142295A patent/JP2014011996A/ja active Pending
Patent Citations (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2002044215A2 (en) * | 2000-12-01 | 2002-06-06 | Cockbain, Julian | Hybrid antibodies |
| WO2002061071A2 (en) * | 2000-12-18 | 2002-08-08 | Dyax Corp. | Focused libraries of genetic packages |
Cited By (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2012514997A (ja) * | 2009-01-14 | 2012-07-05 | エフィミュヌ | 組換え一価抗体 |
| JP2012527875A (ja) * | 2009-05-28 | 2012-11-12 | グラクソ グループ リミテッド | 抗原結合タンパク質 |
| JP2013519389A (ja) * | 2010-02-18 | 2013-05-30 | エフィミュヌ | 抗cd28ヒト化抗体 |
| JP2016054746A (ja) * | 2010-08-02 | 2016-04-21 | リジェネロン・ファーマシューティカルズ・インコーポレイテッドRegeneron Pharmaceuticals, Inc. | Vlドメインを含む結合タンパク質を作製するマウス |
| JP2018046872A (ja) * | 2010-08-02 | 2018-03-29 | リジェネロン・ファーマシューティカルズ・インコーポレイテッドRegeneron Pharmaceuticals, Inc. | Vlドメインを含む結合タンパク質を作製するマウス |
| JP2018523673A (ja) * | 2015-08-14 | 2018-08-23 | アラーガン、インコーポレイテッドAllergan,Incorporated | Pdgfに対する重鎖のみ抗体 |
| US11028163B2 (en) | 2015-08-14 | 2021-06-08 | Allergan, Inc. | Heavy chain only antibodies to PDGF |
Also Published As
| Publication number | Publication date |
|---|---|
| EP1878750A3 (en) | 2013-07-31 |
| DE60333229D1 (de) | 2010-08-12 |
| US20060083747A1 (en) | 2006-04-20 |
| ATE472557T1 (de) | 2010-07-15 |
| EP1581559B1 (en) | 2010-06-30 |
| AU2003295139A1 (en) | 2004-07-22 |
| WO2004058820A2 (en) | 2004-07-15 |
| WO2004058820A3 (en) | 2005-09-29 |
| GB0230203D0 (en) | 2003-02-05 |
| JP2011004748A (ja) | 2011-01-13 |
| ES2346431T3 (es) | 2010-10-15 |
| JP2014011996A (ja) | 2014-01-23 |
| CA2511959A1 (en) | 2004-07-15 |
| AU2003295139B2 (en) | 2012-02-02 |
| CA2511959C (en) | 2014-12-16 |
| EP1878750A2 (en) | 2008-01-16 |
| EP1581559A2 (en) | 2005-10-05 |
| EP1878751A2 (en) | 2008-01-16 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| EP1581559B1 (en) | VL DAb FC FUSION | |
| ES2263984T3 (es) | Ligandos doble-especificos con una vida media serica aumentada. | |
| AU2005250216B2 (en) | Bispecific fusion antibodies with enhanced serum half-life | |
| EP1558645B1 (en) | Stabilized single domain antibodies in pharmaceutical composition adapted for inhalation | |
| AU2004220325B2 (en) | Polypeptides | |
| US20120076787A1 (en) | Combination of a tnf-alpha antagonist and a vegf antagonist for use in the treatment or prevention of diseases of the eye | |
| US20110305692A1 (en) | Antigen-binding contructs | |
| US20060257406A1 (en) | Ligand | |
| US20060002935A1 (en) | Tumor Necrosis Factor Receptor 1 antagonists and methods of use therefor | |
| JP4602090B2 (ja) | 再標的化 | |
| JP2013018785A (ja) | 炎症性疾患を治療するための組成物及び方法 | |
| JP2012527875A (ja) | 抗原結合タンパク質 | |
| JP2012527878A (ja) | 抗原結合タンパク質 | |
| HK1110340A (en) | Single domain - fc fusion constructs | |
| HK1110339A (en) | Single domain - fc fusion constructs | |
| HK1070081B (en) | Dual specific ligands with increased serum half-life | |
| HK1138017A (en) | Ligand | |
| HK1156053A (en) | Ligand |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20061212 |
|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20061212 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20090820 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20091110 |
|
| A602 | Written permission of extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A602 Effective date: 20091117 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20100222 |
|
| A02 | Decision of refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A02 Effective date: 20100323 |