EP3045397B1 - Dispositif d'emballage pour médicament - Google Patents
Dispositif d'emballage pour médicament Download PDFInfo
- Publication number
- EP3045397B1 EP3045397B1 EP15151360.3A EP15151360A EP3045397B1 EP 3045397 B1 EP3045397 B1 EP 3045397B1 EP 15151360 A EP15151360 A EP 15151360A EP 3045397 B1 EP3045397 B1 EP 3045397B1
- Authority
- EP
- European Patent Office
- Prior art keywords
- folding
- packaging material
- material web
- packaging
- guiding device
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Active
Links
Images
Classifications
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B65—CONVEYING; PACKING; STORING; HANDLING THIN OR FILAMENTARY MATERIAL
- B65B—MACHINES, APPARATUS OR DEVICES FOR, OR METHODS OF, PACKAGING ARTICLES OR MATERIALS; UNPACKING
- B65B9/00—Enclosing successive articles, or quantities of material, e.g. liquids or semiliquids, in flat, folded, or tubular webs of flexible sheet material; Subdividing filled flexible tubes to form packages
- B65B9/06—Enclosing successive articles, or quantities of material, in a longitudinally-folded web, or in a web folded into a tube about the articles or quantities of material placed upon it
- B65B9/08—Enclosing successive articles, or quantities of material, in a longitudinally-folded web, or in a web folded into a tube about the articles or quantities of material placed upon it in a web folded and sealed transversely to form pockets which are subsequently filled and then closed by sealing
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B65—CONVEYING; PACKING; STORING; HANDLING THIN OR FILAMENTARY MATERIAL
- B65B—MACHINES, APPARATUS OR DEVICES FOR, OR METHODS OF, PACKAGING ARTICLES OR MATERIALS; UNPACKING
- B65B55/00—Preserving, protecting or purifying packages or package contents in association with packaging
- B65B55/02—Sterilising, e.g. of complete packages
- B65B55/04—Sterilising wrappers or receptacles prior to, or during, packaging
- B65B55/10—Sterilising wrappers or receptacles prior to, or during, packaging by liquids or gases
- B65B55/103—Sterilising flat or tubular webs
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B65—CONVEYING; PACKING; STORING; HANDLING THIN OR FILAMENTARY MATERIAL
- B65B—MACHINES, APPARATUS OR DEVICES FOR, OR METHODS OF, PACKAGING ARTICLES OR MATERIALS; UNPACKING
- B65B61/00—Auxiliary devices, not otherwise provided for, for operating on sheets, blanks, webs, binding material, containers or packages
- B65B61/02—Auxiliary devices, not otherwise provided for, for operating on sheets, blanks, webs, binding material, containers or packages for perforating, scoring, slitting, or applying code or date marks on material prior to packaging
- B65B61/025—Auxiliary devices, not otherwise provided for, for operating on sheets, blanks, webs, binding material, containers or packages for perforating, scoring, slitting, or applying code or date marks on material prior to packaging for applying, e.g. printing, code or date marks on material prior to packaging
Definitions
- the present invention relates to a pharmaceutical packaging device, and more particularly to a packaging device for use in a blister machine.
- WO 2013/034504 A1 a blister machine which can be used in pharmacies and hospitals or, if dimensioned appropriately, also in blister centers, which assembles medicaments according to the individual patient according to the medically prescribed administration time.
- the packaging device of the blister machine packs the pharmaceutical compositions (which may contain only one drug or a plurality of individual drugs) in bags formed from an endless packaging material web, so-called blister bags, these bags leave the packaging device as "blister tube” for further use (as a blister tube strung together, not yet separated filled blister bag called).
- a blister bag regularly corresponds to a time of taking a patient, ie contains all medicines that a patient must take in the morning, for example.
- the blister machine according to the aforementioned WO document comprises a plurality of drug dispensing and dispensing devices which cooperate with a plurality of circulating guide means which supply the drugs also to circulating collection means through which the drug compositions are fed to a packaging device.
- the blister machine can provide a variety of drug compositions in a short time due to the special construction, which places special demands on the packaging device.
- the provided drug compositions of the device itself are supplied via appropriate transport devices and packed in blisters in the packaging device (this process is also referred to as blistering).
- the drug composition is transferred to the packaging device. This happens, for example, by temporarily opening the transport device, whereupon the pharmaceutical composition falls on a baffle plate provided for this purpose.
- the baffle plate simultaneously serves as a shaping / folding aid for a packaging material web guided past it (from which the packaging device forms the individual blister bags).
- the packaging material web is usually folded along the longitudinal axis such that the fold region is arranged "below". From the baffle the individual drugs of the drug composition slip into the fold area where a stop is regularly formed by the vertical seal of the preceding bag.
- the direction of movement of the packaging material web and the construction of the baffle plate require that the medicaments to be packaged regularly lie one behind the other in the prefolded packaging material web.
- the number of drugs so relatively long bags may result, resulting in a waste of material and increasing the time of packaging.
- the geometry of the impact plate causes drugs to be so unfavorably delivered to the prefolded portion of the packaging material web (eg, tablets in the shape of a flat circular cylinder on a face) that it will cause problems in closing the prefolded Section of the packaging material web and their further transport can come through the packaging device.
- the prefolded portion of the packaging material web eg, tablets in the shape of a flat circular cylinder on a face
- the object is achieved according to the invention by a packaging device for medicaments according to claim 1.
- the packaging device according to the invention comprises a packaging material supply for feeding a flexible, elongated packaging material web from a supply roll and a folding and guiding device arranged downstream of the packaging material supply (the designation "downstream"). refers to the direction of movement of the packaging material web, starting from the supply roll, by the packaging device).
- the folding and guiding device comprises a packaging material web receiving region and two packaging material web folding regions, which converge in a folding section, which is arranged opposite to the receiving region of the folding and guiding device.
- the folding and guiding device of the packaging device according to the invention folds the packaging material web in the longitudinal direction by guiding the edges of the packaging material web along the packaging material web folding regions and the folding section defines a fold region in the packaging material web, thereby producing a folded packaging material web having a partially U-shaped cross section.
- the folding and guiding device comprises an impact area for medicaments to be packaged, the medicaments being guided from this impact area into the area of the folded packaging material web (packaging area).
- the packaging device comprises a first joining device arranged downstream of the folding and guiding device, with which the folded packaging material web is joined vertically to the longitudinal direction, wherein usually two vertical joining regions are produced per pharmaceutical bag or blister bag to be produced. Since the blister pouches in the device are usually not separated after packaging or manufacturing, a joining region regularly serves as the "end" of a preceding blister pouch and "beginning" of a new blister pouch. Depending on the exact configuration of the first joining device, however, two separate vertical joint regions can also be produced per blister bag.
- the packaging device further comprises a second joining device arranged downstream of the folding and guiding device, with which the folded packaging material web is assembled parallel to the longitudinal direction and opposite or spaced from the folding region.
- first and second joining devices are arranged with respect to the direction of movement of the packaging material web within the packaging device.
- the folding and guiding device comprises a drug delivery section spaced from the folding section, through which the medicament to be packaged is vertically spaced from the fold section into the folded (and downstream of the feed section) vertical packaging material web (the packaging section).
- the medicaments are guided vertically spaced from the folding area into the packaging area, so that the medicaments initially, when stopped by the vertical joining area of the preceding medicament bag, fall down onto the folding area and subsequent medicaments possibly via already supplied medicaments to be ordered.
- the drugs to be delivered no longer interfere with each other, so that at the same speed (compared with known packaging devices), the same number of drugs can be packaged more quickly and into a "shorter" drug bag, since the drugs are arranged not only one behind the other, but also one on top of the other are.
- the supply of the individual medicaments of the medicament composition is accordingly improved in such a way that shorter medicament pouches can be produced, which i.a. leads to a saving of material with regard to the packaging material.
- a faster blistering is also possible, since the device can be operated faster due to the lower hinderance of the individual drugs during the delivery.
- the surface of the folding and guiding device providing the impact area is concave.
- a vertical joining area of a preceding drug bag forms a stop for the drugs, from which they fall toward the fold area, so that an arrangement over each other is more likely.
- the collision of the drugs in the impact area of the folding and guiding device regularly burst smallest drug particles and contaminate the surface of the folding and guiding device. Subsequent drugs may co-grind the contaminant particles so that over time, there may be increased contamination of the drugs in the drug sachets. It is therefore necessary to replace the folding and guiding device regularly, which is time-consuming and makes an unwanted interruption of the blistering necessary.
- the folding and guiding device comprises a releasable drug delivery member and a folding member, wherein the feed section is part of the releasable drug delivery member and the folding portion is part of the folding member.
- the pharmaceuticals are also arranged one above the other in the packaging area.
- An optimum arrangement of the medicaments in the folded region of the packaging material web is dependent on the distance of the feed section from the fold region of the packaging material web, more precisely from the distance between the top side of the feed section and the underside of the folding section.
- the detachable drug delivery member is pivotally mounted.
- the contamination of the surface of the folding and guiding device is problematic and requires that a cleaned surface is provided on a regular basis. This can be done, for example, by replacing the folding and guiding device, or at least one component of this device.
- the surface of the folding and guiding device which provides the impact area at least in sections provided with a non-stick coating, which impedes the adhesion of chipped drug particles. Possibly. peeling off drug particles are conducted with the drugs in the folded packaging material web and do not remain on the surface.
- the folding and guiding device is associated with a cleaning device, with which on the surface of the folding and guiding device adhering contaminants can be removed.
- a cleaning device with which on the surface of the folding and guiding device adhering contaminants can be removed.
- This can be done, for example, by the surface of the folding and guiding device is acted upon by a fluid which rinses the adhering contaminants in the packaging material web.
- the contaminated area of the packaging material web is assembled as usual into a bag and then disposed of.
- a cleaning fluid for example, a cleaning liquid can be used. Care must be taken in the design of the cleaning device and the guidance of the cleaning fluid that the contaminant particles are not flushed into other areas of the packaging device.
- the packaging material web is provided to the folding and guiding device via the packaging material supply, usually from a supply roll, which may be arranged in a special section of the packaging device. Particularly in the case of large blister centers, large amounts of drug are blistered in often repetitive order, so that the supply roll can already have finished printed or marked packaging material web areas. In such a case, a spontaneous change of the drug to be packaged is only possible if the supply roll is changed with the already marked packaging material web.
- the packaging device comprises a marking device with which the packaging material web corresponding to the packaging or already packaged medicinal products. Usually, corresponding markings are printed on the packaging material web, so that it is preferred that the marking device is arranged upstream of the folding and guiding device, so that a not yet folded packaging material web can be printed.
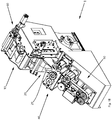
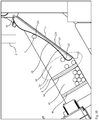
- Figures 1A and 1B show oblique views of an embodiment of the packaging device according to the invention.
- a packaging material web 2 guided through the device is shown, which leaves the device as a (only indicated) blister hose 2d.
- the blister hose is formed as it passes through the device; how this is done is explained with reference to the following figures.
- FIG. 1B the packaging material web and the blister hose is omitted, otherwise the figures are identical.
- the illustrated embodiment of the packaging device comprises a supply roll 11, on which a packaging material web 2 is stored, which is formed into bags as it passes through the packaging device and filled with drugs.
- the packaging material web 2 is supplied with a packaging material supply 10 a folding and guiding device 20, wherein in the embodiment shown between the supply roll and folding and guiding device 20 is still a marking device 60 is arranged, with which information is applied to the packaging material web can.
- the packaging material web 2 is folded in the longitudinal direction to form a U-shaped double web, the two "legs" of the double web are regularly ready or high. How exactly the actual folding process of the packaging material web and the filling of the folded packaging material web taking place in the same section of the packaging device will be carried out will be described in more detail with reference to the following figures.
- the folded and filled with pharmaceuticals packaging material web is vertical to the longitudinal direction or movement direction X of the packaging material web (see FIG. 1 ), wherein a joint area simultaneously represents the beginning of a new (not yet closed) blister bag and the end of the previous blister bag.
- the first joining device 40 is realized by a welding device, with which the folded double web is welded vertically to the longitudinal direction.
- Attached to the first joining device 40 is a cleaning device 29 with which a cleaning fluid can be applied to the surface of the folding and guiding device 20 in order to remove impurities from the surface.
- a second joining device 50 Downstream of the first joining device 40, a second joining device 50 is arranged, with which the folded double web, which is filled with medicaments and is already provided with vertical joining regions, is assembled parallel to the longitudinal direction and spaced from the fold of the packaging material web, this in the embodiment shown is again realized by a weld.
- the finished blister hose 2d is led out of the blister machine and fed to an inspection and (for example, patient-related) separation.
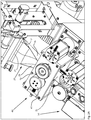
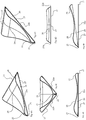
- FIG. 2A shows detailed views of an embodiment of the packaging device according to the invention in the region of the folding and guiding device 20.
- the packaging material web 2 is folded by means of the folding and guiding device 20 and the first joining device 40 to a double web 2c.
- the unfolded packaging material web 2 is guided under a triangular folding and guiding device 20 in a packaging material web receiving region 21.
- sections 2a, 2b of the packaging material web 2 protrude.
- the folding area is determined, ie the area in which the actual folding of the packaging material web takes place.
- the folding of the double web is maintained by the folding and guiding device 20 downstream first joining device 40.
- FIG. 2A also shows some details of the first joining device 40, namely two welding rollers 41, 42 oriented vertically to the longitudinal direction or movement direction of the packaging material web (or double web), with which welding regions or joining regions directed vertically to the longitudinal direction of the packaging material web are formed.
- each welding roller 41, 42 rotates, the rotational speed being adapted to the bag length and the moving speed of the packaging material web.
- each welding roller two opposite welding portions 41a, 41b, 42a, 42b (see Fig. 2B ) and only in these areas have the welding rollers during the rotation contact with the double track carried out between them.
- the welding sections are matched to one another, so that when the double web moves and the welding rollers rotate, they make contact with the double track every X cm and form a joining region 4.
- the path of "non-contact" between weld sections and dual track defines the length of the blister bag.
- FIG. 2B The representation according to Fig. 2B only serves to illustrate the rollers / contact areas.
- the joining region in the direction of movement of the packaging material web is at the level of the axes of the welding rolls, ie the joining region 4 was introduced "straight"; the rollers would have to be rotated by 90 ° C to reproduce this just happened welding. For clarity, however, this representation is selected.
- the folding and guiding device 20 can be designed in several parts and have a detachable drug delivery component and a folding component.
- the detachable drug delivery member 26 is omitted and it is only the newly formed, triangular-shaped folding member 27 can be seen.
- the packaging material web 2 after it has been guided under the folding and guiding device 20 at the packaging material receiving area 21, at the two other side regions of the folding and guiding device, the so-called packaging material web folding areas, of which only in the direction of movement "right" packaging material web folding area 22a can be seen, emerge.
- FIG. 2D the complete folding and guiding device 20 is shown, but the packaging material web 2 is omitted, and in this The illustration shows the "left" packaging material web folding region 22b.
- the first joining means is omitted to illustrate the area where the folded packaging material web portions 2a, 2b meet, this area 6 being illustrated only schematically.
- the packaging material web areas 2a, 2b converge in the area 6 in a pointed manner, which is not entirely true in practice, since the area is constantly filled with medicaments which deform the area.
- the packaging material web / double web is moved by the packaging device during blistering, and with the web also the joining region (in the direction of the arrow) moves on.
- the folded packaging material web and the folding and guiding device 20 form a kind of funnel, and in this funnel to be restrained drugs on the folding and guiding device passed (see also FIG. 3B ).
- both the first joining device and the packaging material web / double web are omitted, and it is the inventively designed folding and guiding device 20 can be seen.
- the folding and guiding device 20 is constructed in two parts, with a lower folding member 27 and an upper drug delivery member 26, wherein the drug delivery member is releasably secured to the folding member.
- the drug delivery member 26 provides a collision area 24 from which to package drugs to be delivered to the packaging area.
- the two packaging material web folding regions 22a, 22b which in the present case are provided by the folding component 27, converge in the folding section 23, which defines the fold region of the packaging material web.
- the drug delivery member 26 has the drug delivery section near the tip 25, which is formed by the folding portion 23 vertically (up) spaced. Furthermore, in Figure 2F already to guess that the surface of the drug delivery member 26 is concave.
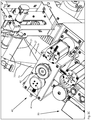
- FIGS. 3A and 3B show side views of the packaging device according to the invention in the region of the folding and guiding device 20.
- Bei FIG. 3A the first joining device is omitted to illustrate the course of the unfolding of the packaging material web 2 from the side.
- the folding and guiding device itself is arranged inclined in the packaging device, and the unfolded packaging material web 2c after folding is likewise guided further inclined through the device.
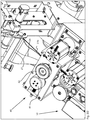
- FIG. 3B shows a further side view of the packaging device according to the invention, wherein in this representation, a part of the unfolded packaging material web, the area 2b from FIG. 3A is omitted, so that the folding and guiding device 20 can be seen.
- a plurality of medicaments 7 are shown in order, on the one hand, to illustrate the delivery of the medicaments 7 into the packaging region 6 and the arrangement of individual medicaments in a blister pack (to be finished) (delimited by joining regions 4a, 4b).
- the provided drug combination is fed via a feed 5 of the folding and guiding device 20, which they impinge on in the impact area 24.
- the concave configuration of the surface of the folding and guiding device 20, which in the embodiment shown consists of the drug delivery component 26 and the folding component 27, means that the angle in the impact region is steeper in comparison to a non-concave surface, so that the medicines are accelerated more.
- the medicament-feeding section 25 is formed, vertically spaced from the folding section 23.
- the embodiment of the folding and guiding device according to the invention requires that the medicaments 7 not in the area of the fold 3 are supplied to the packaging area 6, but, based on the direction of movement of the packaging material web, vertically spaced from the folding area 3.
- the folding and guiding device forms a kind of ski jump (this effect is enhanced by the concave configuration of the surface) , which has the consequence that the drugs 7 are not simply stored one behind the other in the folding area 3, but "jump" in the packaging area 6. In the packaging area they bounce, in the "snapshot” just shown, against the vertical joining region 4a and then fall in the direction of folding area 3. In this way, drugs can be arranged not only one behind the other (in the direction of movement of the packaging material web), but also one above the other, what leads to the same number of medicines being packaged in a smaller blister pack.
- a correspondingly manufactured blister bag is in FIG. 3B "left" of the joining region 4a shown. It can also be seen that a joining region 4a represents the "start” for the blister bag which has just been produced and the bag end for the preceding blister bag.
- the exact nature of the storage of the drugs in the packaging area depends on the medicines themselves and the length of the blister pack. In the state shown, the drugs collide at the joining area and then fall down. When moving the packaging material web, in which the joining region 4a moves on to the left, it may also happen that the drugs no longer bounce off the joining region, but simply arise in the direction of movement in the packaging area.
- FIGS. 4A-4C schematically show the difference in the production of blister packs with a known baffle plate and a folding and guiding device according to the invention, wherein the FIGS. 4A-4C the production with a known baffle plate 20 '.
- FIGS. 6A-6D show various views of an embodiment of a folding and invention Guide means 20, wherein the embodiment shown comprises a drug delivery member 26 and a folding member 27.
- the folding member 27 is formed as a flat triangular plate.
- the underside of the folding component can also be designed completely differently in other embodiments.
- FIG. 6C the concave configuration of the surface of the drug delivery member 26 is clearly visible. Furthermore, the packaging material web receiving region 21 and the packaging material web folding regions 22a, 22b, which are provided by the folding component 27 in the embodiment shown, can be seen.
- the drug delivery component can be designed to be pivotable relative to the folding component, with which it is possible to adapt the folding and guiding device to the medicaments to be packaged or to be restrained.
Landscapes
- Engineering & Computer Science (AREA)
- Mechanical Engineering (AREA)
- Containers And Plastic Fillers For Packaging (AREA)
- Basic Packing Technique (AREA)
- Medical Preparation Storing Or Oral Administration Devices (AREA)
Claims (7)
- Dispositif d'emballage (1) pour des médicaments, comportant
une alimentation (10) de la matière d'emballage, destinée à alimenter une bande (2) allongée, souple de matière d'emballage à partir d'une bobine de réserve (11),
un système de pliage et de guidage (20) placé en aval de l'alimentation (10) de la matière d'emballage, avec une zone de réception (21) de la bande de matière d'emballage et deux zones de pliage (22a, 22b) de la bande de matière d'emballage, qui convergent dans une partie de pliage (23), le système de pliage et de guidage (20) pliant la bande (2) de matière d'emballage dans la direction longitudinale, en ce que les bords de la bande de matière d'emballage sont guidés le long des zones de pliage (22a, 22b) de la bande de matière d'emballage et tronçon de pliage (23) immobilise une zone de pliage (3) dans la bande (2) de matière d'emballage et le système de pliage et de guidage (20) comporte une zone d'impact (24) pour des médicaments à emballer,
un premier système de jointage (40) placé en aval du système de pliage et de guidage (20), à l'aide duquel la bande (2) de matière d'emballage pliée est jointée à la verticale de la direction longitudinale,
un deuxième système de jointage (50) placé en aval du système de pliage et de guidage (20), à l'aide duquel la bande (2) de matière d'emballage pliée est jointée à la parallèle de la direction longitudinale,
le système de pliage et de guidage (20) comportant un tronçon d'alimentation (25) de médicaments écarté du tronçon de pliage (23), par l'intermédiaire duquel des médicaments écartés à la verticale sont dirigés de la zone de pliage (3) dans la bande (2) de matière d'emballage pliée. - Dispositif d'emballage (1) pour des médicaments selon la revendication 1, caractérisé en ce que la surface du système de pliage et de guidage (20) mettant à disposition la zone d'impact (24) est conçue de manière concave.
- Dispositif d'emballage (1) pour des médicaments selon la revendication 1 ou 2, caractérisé en ce que le système de pliage et de guidage (20) comporte un composant d'alimentation (26) de médicaments amovible et un composant de pliage (27), le tronçon d'alimentation (25) étant un élément du composant d'alimentation (26) de médicaments amovible et le tronçon de pliage (23) étant un élément du composant de pliage (27).
- Dispositif d'emballage (1) pour des médicaments selon la revendication 3, caractérisé en ce que le composant d'alimentation (26) de médicaments amovible est logé de manière pivotante de telle sorte que le tronçon d'alimentation (25) du composant d'alimentation (26) de médicaments soit réglable en hauteur par rapport à la zone de pliage (3).
- Dispositif d'emballage (1) pour des médicaments selon l'une quelconque des revendications 1 - 4, caractérisé en ce que la surface du système de pliage et de guidage (20) mettant à disposition la zone d'impact (24) est dotée au moins par tronçons d'un revêtement anti-adhérent (28).
- Dispositif d'emballage (1) pour des médicaments selon l'une quelconque des revendications 1 - 5, caractérisé en ce qu'au système de pliage et de guidage (20) est associé un système de nettoyage (29), à l'aide duquel des impuretés adhérant sur la surface du système de pliage et de guidage (20) peuvent être retirées.
- Dispositif d'emballage (1) pour des médicaments selon l'une quelconque des revendications 1 - 6, caractérisé en ce que le dispositif d'emballage (1) comprend un système de marquage (60) placé de préférence en amont du système de pliage et de guidage (20).
Priority Applications (12)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| EP15151360.3A EP3045397B1 (fr) | 2015-01-16 | 2015-01-16 | Dispositif d'emballage pour médicament |
| ES15151360.3T ES2618062T3 (es) | 2015-01-16 | 2015-01-16 | Dispositivo de empaquetado de medicamentos |
| DK15151360.3T DK3045397T3 (en) | 2015-01-16 | 2015-01-16 | Packaging device for pharmaceuticals |
| PT151513603T PT3045397T (pt) | 2015-01-16 | 2015-01-16 | Dispositivo de empacotamento para medicamentos |
| KR1020177016641A KR102423996B1 (ko) | 2015-01-16 | 2016-01-13 | 약제용 포장 장치 |
| CN201680005900.2A CN107428424B (zh) | 2015-01-16 | 2016-01-13 | 用于药物的包装装置 |
| CA2985677A CA2985677C (fr) | 2015-01-16 | 2016-01-13 | Dispositif d'emballage de medicaments |
| JP2017533793A JP6856531B2 (ja) | 2015-01-16 | 2016-01-13 | 薬剤用包装装置 |
| MX2017009240A MX364420B (es) | 2015-01-16 | 2016-01-13 | Dispositivo de empaquetado de medicamentos. |
| AU2016208113A AU2016208113B2 (en) | 2015-01-16 | 2016-01-13 | Packaging device for drugs |
| PCT/EP2016/050544 WO2016113291A1 (fr) | 2015-01-16 | 2016-01-13 | Dispositif d'emballage de médicaments |
| BR112017011839-4A BR112017011839B1 (pt) | 2015-01-16 | 2016-01-13 | Dispositivo de empacotamento para medicamentos |
Applications Claiming Priority (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| EP15151360.3A EP3045397B1 (fr) | 2015-01-16 | 2015-01-16 | Dispositif d'emballage pour médicament |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| EP3045397A1 EP3045397A1 (fr) | 2016-07-20 |
| EP3045397B1 true EP3045397B1 (fr) | 2017-01-11 |
Family
ID=52347207
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP15151360.3A Active EP3045397B1 (fr) | 2015-01-16 | 2015-01-16 | Dispositif d'emballage pour médicament |
Country Status (12)
| Country | Link |
|---|---|
| EP (1) | EP3045397B1 (fr) |
| JP (1) | JP6856531B2 (fr) |
| KR (1) | KR102423996B1 (fr) |
| CN (1) | CN107428424B (fr) |
| AU (1) | AU2016208113B2 (fr) |
| BR (1) | BR112017011839B1 (fr) |
| CA (1) | CA2985677C (fr) |
| DK (1) | DK3045397T3 (fr) |
| ES (1) | ES2618062T3 (fr) |
| MX (1) | MX364420B (fr) |
| PT (1) | PT3045397T (fr) |
| WO (1) | WO2016113291A1 (fr) |
Families Citing this family (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| KR102257372B1 (ko) * | 2019-03-13 | 2021-05-31 | (주)제이브이엠 | 약제 자동 포장기용 최종 호퍼 조립체 및 이를 가지는 약제 자동 포장기 |
| US11208223B2 (en) * | 2020-05-20 | 2021-12-28 | Becton Dickinson Rowa Germany Gmbh | Packaging apparatus for small piece goods and method for producing a blister tube comprising a plurality of blister bags |
| EP3912918B1 (fr) | 2020-05-20 | 2025-07-02 | Becton Dickinson Rowa Germany GmbH | Dispositif d'emballage pour petites marchandises et procédé de fabrication d'un tube blister comprenant plusieurs sacs blister |
| US12043464B2 (en) | 2020-11-09 | 2024-07-23 | Becton Dickinson Rowa Germany Gmbh | Method and device for producing a blister tube, and blister tube |
| EP3995401B1 (fr) | 2020-11-09 | 2025-10-08 | Becton Dickinson Rowa Germany GmbH | Procédure et dispositif pour fabriquer un tube blister, et tube blister |
Family Cites Families (19)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPH0640082Y2 (ja) * | 1990-02-08 | 1994-10-19 | 正二 湯山 | 錠剤包装装置 |
| JP2942769B2 (ja) * | 1995-03-02 | 1999-08-30 | 正二 湯山 | シール装置 |
| JP3524680B2 (ja) * | 1996-06-26 | 2004-05-10 | 株式会社湯山製作所 | 薬剤包装装置 |
| JP3607077B2 (ja) * | 1998-05-29 | 2005-01-05 | 理想科学工業株式会社 | 印刷装置の排紙台 |
| JP3608608B2 (ja) * | 2000-02-28 | 2005-01-12 | 日本精機株式会社 | 充填包装機におけるフィルム折返し案内装置 |
| JP2002046704A (ja) * | 2000-08-03 | 2002-02-12 | Seiko Engineering Kk | 粉末材の分包装置 |
| KR200251794Y1 (ko) * | 2001-07-24 | 2001-11-22 | (주)제이브이메디 | 약제분포기용 포장지의 폭접기조정장치 |
| KR100416227B1 (ko) * | 2001-10-23 | 2004-01-31 | (주)제이브이메디 | 약제분포기용 포장지의 세로접합 거리조절장치 |
| ES2222060B1 (es) * | 2001-12-21 | 2006-03-16 | Camilo Batalla Teixidor | Maquina de envasado automatico. |
| JP3936985B2 (ja) * | 2002-09-27 | 2007-06-27 | 三洋電機株式会社 | 薬剤供給装置 |
| JP4298343B2 (ja) * | 2003-03-24 | 2009-07-15 | 株式会社湯山製作所 | 薬剤包装装置 |
| JP4133966B2 (ja) * | 2004-08-11 | 2008-08-13 | 池田機械産業株式会社 | 粉粒体の充填包装装置 |
| JP2008087845A (ja) * | 2006-10-05 | 2008-04-17 | Sanko Kikai Kk | 自動包装機のエンボス充填シュート |
| JP2008298716A (ja) * | 2007-06-04 | 2008-12-11 | Ishida Co Ltd | 計量装置の洗浄方法、計量装置、計量包装装置および計量包装検査システム |
| JP4469007B2 (ja) * | 2008-10-02 | 2010-05-26 | 株式会社湯山製作所 | 薬剤包装装置 |
| JP2009083939A (ja) * | 2008-12-25 | 2009-04-23 | Takazono Sangyo Co Ltd | 薬剤包装装置 |
| JP5264573B2 (ja) * | 2009-03-11 | 2013-08-14 | 藤森工業株式会社 | 易開封加工装置、製袋充填方法および製袋充填装置 |
| NL2007384C2 (nl) | 2011-09-09 | 2013-03-12 | Ppm Engineering B V | Systeem en werkwijze voor het verpakken van gedoseerde hoeveelheden vaste medicijnen. |
| JP6108686B2 (ja) * | 2012-05-23 | 2017-04-05 | 株式会社タカゾノ | 薬剤分包装置 |
-
2015
- 2015-01-16 PT PT151513603T patent/PT3045397T/pt unknown
- 2015-01-16 DK DK15151360.3T patent/DK3045397T3/en active
- 2015-01-16 EP EP15151360.3A patent/EP3045397B1/fr active Active
- 2015-01-16 ES ES15151360.3T patent/ES2618062T3/es active Active
-
2016
- 2016-01-13 AU AU2016208113A patent/AU2016208113B2/en active Active
- 2016-01-13 JP JP2017533793A patent/JP6856531B2/ja active Active
- 2016-01-13 KR KR1020177016641A patent/KR102423996B1/ko active Active
- 2016-01-13 WO PCT/EP2016/050544 patent/WO2016113291A1/fr not_active Ceased
- 2016-01-13 CN CN201680005900.2A patent/CN107428424B/zh active Active
- 2016-01-13 BR BR112017011839-4A patent/BR112017011839B1/pt not_active IP Right Cessation
- 2016-01-13 MX MX2017009240A patent/MX364420B/es active IP Right Grant
- 2016-01-13 CA CA2985677A patent/CA2985677C/fr active Active
Non-Patent Citations (1)
| Title |
|---|
| None * |
Also Published As
| Publication number | Publication date |
|---|---|
| CA2985677A1 (fr) | 2016-07-21 |
| MX364420B (es) | 2019-04-25 |
| CN107428424A (zh) | 2017-12-01 |
| EP3045397A1 (fr) | 2016-07-20 |
| AU2016208113A1 (en) | 2017-06-29 |
| ES2618062T3 (es) | 2017-06-20 |
| BR112017011839A2 (pt) | 2018-02-27 |
| MX2017009240A (es) | 2018-03-28 |
| PT3045397T (pt) | 2017-03-15 |
| CA2985677C (fr) | 2022-08-16 |
| JP6856531B2 (ja) | 2021-04-07 |
| BR112017011839B1 (pt) | 2022-05-17 |
| AU2016208113B2 (en) | 2019-09-26 |
| JP2018502019A (ja) | 2018-01-25 |
| CN107428424B (zh) | 2019-06-25 |
| WO2016113291A1 (fr) | 2016-07-21 |
| DK3045397T3 (en) | 2017-03-20 |
| KR20170102866A (ko) | 2017-09-12 |
| KR102423996B1 (ko) | 2022-07-22 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| DE3213561C2 (fr) | ||
| EP3385174B1 (fr) | Dispositif d'emballage de portions de médicament | |
| DE69701057T2 (de) | Verfahren und Vorrichtung zum Öffnen und Füllen von Mehrkammerbeuteln und dabei erzeugte Beutel | |
| EP1973511B1 (fr) | Installation de production automatique d'emballages de produits medicaux et/ou pharmaceutiques et/ou de complements alimentaires | |
| EP3045397B1 (fr) | Dispositif d'emballage pour médicament | |
| DE69900236T2 (de) | Verfahren und Maschine zur Herstellung von Beuteln mit einem transversalen Reissverschluss | |
| DE3881607T2 (de) | Vorrichtung zum befestigen von füll- oder entleerelementen auf fortlaufenden folienbahnen. | |
| DE19929216B4 (de) | Mehrfachverpackungsmaschine | |
| DE102011075439A1 (de) | Verfahren und Vorrichtung zum Verpacken streifenförmiger Gegenstände, insbesondere Kaugummistreifen | |
| DE3807763C2 (de) | Vorrichtung zum Zuführen und Anbringen von Etiketten, Faden und Verbindungsabschnitten an Filterbeuteln für Aufgußprodukte | |
| DE2122089C3 (de) | Vorrichtung zum Verpacken von gleichartigen quaderförmigen Gegenständen | |
| EP1698554B1 (fr) | Tube d'alimentation pour comprimés | |
| EP2387495B1 (fr) | Dispositif de soudage et de séparation de matériaux d'emballage | |
| DE3807794C2 (de) | Dosiereinrichtung | |
| EP0164079B1 (fr) | Machine pour produire des emballages contenant des solvants | |
| DE69502995T2 (de) | Maschine zum Verpacken von Kleidungsstücken, wie Strümpfe oder dergleichen, in Schachteln | |
| EP3459865B1 (fr) | Procédé et dispositif d'emballage sous forme d'empilement des produits de petite taille | |
| WO2012076152A1 (fr) | Machine d'emballage présentant une évacuation de bordure latérale | |
| WO2023016693A1 (fr) | Appareil et procédé de fabrication d'emballages composites carton/plastique remplis | |
| EP0586926A2 (fr) | Dispositif pour couper et agrafer des produits imprimés à plusieurs couches dans des appareils de pliage | |
| EP3093826B1 (fr) | Dispositif d'emballage de portions de médicament | |
| WO2011113458A1 (fr) | Machine de traitement de sachets très performante | |
| DE102018008483B3 (de) | Verfahren zum Verpacken von portionierten, im Verarbeitungszustand flüssigen oder pastösen Produkten und Verpackungsmaschine zur Durchführung eines solchen Verfahrens | |
| EP3190057B1 (fr) | Machine d'emballage à feuilles tubulaires | |
| WO2004085137A1 (fr) | Procede et dispositif de positionnement et de soudage automatiques d'elements structuraux en plastique sur des segments en plastique plats |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PUAI | Public reference made under article 153(3) epc to a published international application that has entered the european phase |
Free format text: ORIGINAL CODE: 0009012 |
|
| AK | Designated contracting states |
Kind code of ref document: A1 Designated state(s): AL AT BE BG CH CY CZ DE DK EE ES FI FR GB GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO PL PT RO RS SE SI SK SM TR |
|
| AX | Request for extension of the european patent |
Extension state: BA ME |
|
| RAP1 | Party data changed (applicant data changed or rights of an application transferred) |
Owner name: BECTON DICKINSON ROWA GERMANY GMBH |
|
| 17P | Request for examination filed |
Effective date: 20160803 |
|
| RBV | Designated contracting states (corrected) |
Designated state(s): AL AT BE BG CH CY CZ DE DK EE ES FI FR GB GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO PL PT RO RS SE SI SK SM TR |
|
| GRAP | Despatch of communication of intention to grant a patent |
Free format text: ORIGINAL CODE: EPIDOSNIGR1 |
|
| INTG | Intention to grant announced |
Effective date: 20160919 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: GRANT OF PATENT IS INTENDED |
|
| GRAS | Grant fee paid |
Free format text: ORIGINAL CODE: EPIDOSNIGR3 |
|
| GRAA | (expected) grant |
Free format text: ORIGINAL CODE: 0009210 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: THE PATENT HAS BEEN GRANTED |
|
| AK | Designated contracting states |
Kind code of ref document: B1 Designated state(s): AL AT BE BG CH CY CZ DE DK EE ES FI FR GB GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO PL PT RO RS SE SI SK SM TR |
|
| REG | Reference to a national code |
Ref country code: GB Ref legal event code: FG4D Free format text: NOT ENGLISH |
|
| REG | Reference to a national code |
Ref country code: CH Ref legal event code: EP |
|
| REG | Reference to a national code |
Ref country code: AT Ref legal event code: REF Ref document number: 861016 Country of ref document: AT Kind code of ref document: T Effective date: 20170115 |
|
| REG | Reference to a national code |
Ref country code: FR Ref legal event code: PLFP Year of fee payment: 3 |
|
| REG | Reference to a national code |
Ref country code: IE Ref legal event code: FG4D Free format text: LANGUAGE OF EP DOCUMENT: GERMAN |
|
| REG | Reference to a national code |
Ref country code: DE Ref legal event code: R096 Ref document number: 502015000484 Country of ref document: DE |
|
| REG | Reference to a national code |
Ref country code: PT Ref legal event code: SC4A Ref document number: 3045397 Country of ref document: PT Date of ref document: 20170315 Kind code of ref document: T Free format text: AVAILABILITY OF NATIONAL TRANSLATION Effective date: 20170308 Ref country code: NL Ref legal event code: FP |
|
| REG | Reference to a national code |
Ref country code: DK Ref legal event code: T3 Effective date: 20170313 |
|
| REG | Reference to a national code |
Ref country code: LT Ref legal event code: MG4D |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: BE Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20170131 |
|
| REG | Reference to a national code |
Ref country code: ES Ref legal event code: FG2A Ref document number: 2618062 Country of ref document: ES Kind code of ref document: T3 Effective date: 20170620 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: NO Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20170411 Ref country code: FI Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20170111 Ref country code: IS Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20170511 Ref country code: LT Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20170111 Ref country code: GR Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20170412 Ref country code: HR Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20170111 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: RS Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20170111 Ref country code: SE Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20170111 Ref country code: BG Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20170411 Ref country code: PL Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20170111 Ref country code: LV Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20170111 |
|
| REG | Reference to a national code |
Ref country code: DE Ref legal event code: R097 Ref document number: 502015000484 Country of ref document: DE |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: CZ Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20170111 Ref country code: SK Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20170111 Ref country code: EE Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20170111 Ref country code: RO Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20170111 |
|
| REG | Reference to a national code |
Ref country code: IE Ref legal event code: MM4A |
|
| PLBE | No opposition filed within time limit |
Free format text: ORIGINAL CODE: 0009261 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: NO OPPOSITION FILED WITHIN TIME LIMIT |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: SM Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20170111 Ref country code: MC Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20170111 Ref country code: LU Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20170116 |
|
| 26N | No opposition filed |
Effective date: 20171012 |
|
| REG | Reference to a national code |
Ref country code: FR Ref legal event code: PLFP Year of fee payment: 4 |
|
| REG | Reference to a national code |
Ref country code: BE Ref legal event code: MM Effective date: 20170131 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: SI Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20170111 Ref country code: IE Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20170116 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: MT Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20170111 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: HU Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT; INVALID AB INITIO Effective date: 20150116 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: CY Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20170111 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: MK Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20170111 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: TR Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20170111 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: AL Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20170111 |
|
| P01 | Opt-out of the competence of the unified patent court (upc) registered |
Effective date: 20230530 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: PT Payment date: 20231219 Year of fee payment: 10 Ref country code: DK Payment date: 20231219 Year of fee payment: 10 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: ES Payment date: 20240202 Year of fee payment: 10 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: AT Payment date: 20231222 Year of fee payment: 10 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: CH Payment date: 20240202 Year of fee payment: 10 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: IT Payment date: 20240102 Year of fee payment: 10 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: NL Payment date: 20241219 Year of fee payment: 11 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: GB Payment date: 20241219 Year of fee payment: 11 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: FR Payment date: 20241220 Year of fee payment: 11 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: DE Payment date: 20241218 Year of fee payment: 11 |
|
| REG | Reference to a national code |
Ref country code: DK Ref legal event code: EBP Effective date: 20250131 |
|
| REG | Reference to a national code |
Ref country code: CH Ref legal event code: PL |
|
| REG | Reference to a national code |
Ref country code: AT Ref legal event code: MM01 Ref document number: 861016 Country of ref document: AT Kind code of ref document: T Effective date: 20250116 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: PT Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20250716 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: AT Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20250116 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: CH Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20250131 |