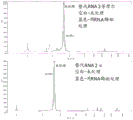
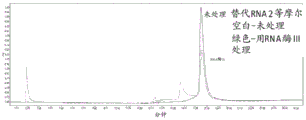
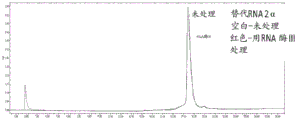
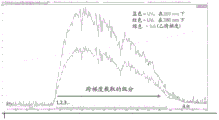
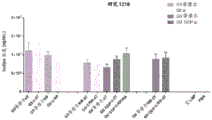
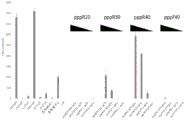
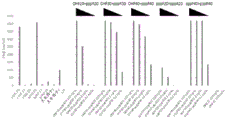
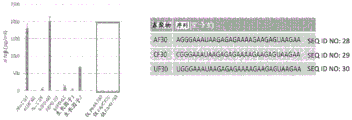
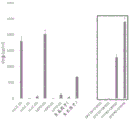
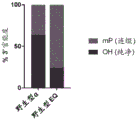
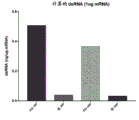
|
US6100024A
(en)
|
1991-02-08 |
2000-08-08 |
Promega Corporation |
Methods and compositions for nucleic acid detection by target extension and probe amplification
|
|
US5298422A
(en)
|
1991-11-06 |
1994-03-29 |
Baylor College Of Medicine |
Myogenic vector systems
|
|
US5256555A
(en)
*
|
1991-12-20 |
1993-10-26 |
Ambion, Inc. |
Compositions and methods for increasing the yields of in vitro RNA transcription and other polynucleotide synthetic reactions
|
|
US5605798A
(en)
|
1993-01-07 |
1997-02-25 |
Sequenom, Inc. |
DNA diagnostic based on mass spectrometry
|
|
WO1995008626A1
(en)
|
1993-09-20 |
1995-03-30 |
The Regents Of The University Of Colorado |
Strategy for the production of rna from immobilized templates
|
|
US6096503A
(en)
|
1993-11-12 |
2000-08-01 |
The Scripps Research Institute |
Method for simultaneous identification of differentially expresses mRNAs and measurement of relative concentrations
|
|
FR2733762B1
(fr)
|
1995-05-02 |
1997-08-01 |
Genset Sa |
Methode de couplage specifique de la coiffe de l'extremite 5' d'un fragment d'arnm et preparation d'arnm et d'adnc complet
|
|
US6251665B1
(en)
|
1997-02-07 |
2001-06-26 |
Cem Cezayirli |
Directed maturation of stem cells and production of programmable antigen presenting dentritic cells therefrom
|
|
AU7863200A
(en)
|
1999-10-06 |
2001-05-10 |
Quark Biotech, Inc. |
Method for enrichment of natural antisense messenger rna
|
|
WO2002044196A1
(en)
*
|
2000-11-28 |
2002-06-06 |
University Of Massachusetts |
Methods and reagents for introducing a sulfhydryl group into the 5'-terminus of rna
|
|
AU2002230997A1
(en)
|
2000-12-15 |
2002-06-24 |
Genetics Institute, Llc |
Methods and compositions for diagnosing and treating rheumatoid arthritis
|
|
US8426194B2
(en)
|
2003-01-21 |
2013-04-23 |
Ptc Therapeutics, Inc. |
Methods and agents for screening for compounds capable of modulating VEGF expression
|
|
GB0316089D0
(en)
|
2003-07-09 |
2003-08-13 |
Xo Bioscience Ltd |
Differentiation method
|
|
EP2311994A1
(en)
*
|
2003-08-01 |
2011-04-20 |
Life Technologies Corporation |
Compositions and methods for preparing short RNA molecules and other nucleic acids
|
|
US20140142290A1
(en)
|
2003-08-01 |
2014-05-22 |
Life Technologies Corporation |
Compositions and methods for preparing short rna molecules and other nucleicacids
|
|
US20050287539A1
(en)
*
|
2004-06-29 |
2005-12-29 |
Emmanuel Labourier |
Methods and compositions for preparing capped RNA
|
|
US20080220471A1
(en)
|
2005-07-27 |
2008-09-11 |
Genentech, Inc. |
Vectors and Methods Using Same
|
|
US9012219B2
(en)
*
|
2005-08-23 |
2015-04-21 |
The Trustees Of The University Of Pennsylvania |
RNA preparations comprising purified modified RNA for reprogramming cells
|
|
PL2578685T3
(pl)
|
2005-08-23 |
2020-01-31 |
The Trustees Of The University Of Pennsylvania |
Rna zawierający zmodyfikowane nukleozydy i sposoby jego zastosowania
|
|
EP2010659B1
(en)
|
2006-04-14 |
2014-06-18 |
CellScript, Inc. |
Kits and methods for generating 5' capped RNA
|
|
CN101611145B
(zh)
|
2006-12-21 |
2013-08-14 |
诺维信股份有限公司 |
用于在细菌细胞中表达基因的修饰型信使rna稳定化序列
|
|
DE102006061015A1
(de)
|
2006-12-22 |
2008-06-26 |
Curevac Gmbh |
Verfahren zur Reinigung von RNA im präparativen Maßstab mittels HPLC
|
|
WO2009046738A1
(en)
|
2007-10-09 |
2009-04-16 |
Curevac Gmbh |
Composition for treating lung cancer, particularly of non-small lung cancers (nsclc)
|
|
EP2451475A2
(en)
|
2009-07-06 |
2012-05-16 |
Novartis AG |
Self replicating rna molecules and uses thereof
|
|
KR102505097B1
(ko)
|
2009-12-07 |
2023-03-02 |
더 트러스티스 오브 더 유니버시티 오브 펜실베니아 |
세포 리프로그래밍을 위한 정제된 변형 rna를 포함하는 rna 제제
|
|
JP2013531634A
(ja)
|
2010-05-24 |
2013-08-08 |
メルク・シャープ・エンド・ドーム・コーポレイション |
オリゴヌクレオチド送達のための新規なアミノアルコールカチオン性脂質
|
|
EP2600901B1
(en)
|
2010-08-06 |
2019-03-27 |
ModernaTX, Inc. |
A pharmaceutical formulation comprising engineered nucleic acids and medical use thereof
|
|
WO2012038450A1
(en)
|
2010-09-21 |
2012-03-29 |
Riboxx Gmbh |
Microwave-driven rna polymerization by rna polymerases of caliciviruses
|
|
MX2013003681A
(es)
|
2010-10-01 |
2013-11-20 |
Moderna Therapeutics Inc |
Ácidos nucleicos manipulados y métodos de uso de los mismos.
|
|
CA3012765A1
(en)
|
2010-11-19 |
2012-05-24 |
The Regents Of The University Of Michigan |
Ncrna and uses thereof
|
|
WO2012135805A2
(en)
|
2011-03-31 |
2012-10-04 |
modeRNA Therapeutics |
Delivery and formulation of engineered nucleic acids
|
|
EP2710136A4
(en)
|
2011-05-17 |
2015-01-21 |
Moderna Therapeutics Inc |
MANIPULATED NUCLEIC ACIDS AND USE METHOD FOR NON-HUMAN SPINE
|
|
US9464124B2
(en)
|
2011-09-12 |
2016-10-11 |
Moderna Therapeutics, Inc. |
Engineered nucleic acids and methods of use thereof
|
|
EP3384938A1
(en)
|
2011-09-12 |
2018-10-10 |
Moderna Therapeutics, Inc. |
Engineered nucleic acids and methods of use thereof
|
|
US20140378538A1
(en)
|
2011-12-14 |
2014-12-25 |
Moderma Therapeutics, Inc. |
Methods of responding to a biothreat
|
|
SMT202200355T1
(it)
|
2011-12-16 |
2022-11-18 |
Modernatx Inc |
Composizioni di mrna modificato
|
|
CA2859691A1
(en)
|
2011-12-21 |
2013-06-27 |
Moderna Therapeutics, Inc. |
Methods of increasing the viability or longevity of an organ or organ explant
|
|
PL3144389T3
(pl)
*
|
2011-12-30 |
2018-10-31 |
Cellscript, Llc |
WYTWARZANIE I STOSOWANIE ZSYNTETYZOWANEGO IN VITRO ssRNA DO WPROWADZANIA DO SSACZYCH KOMÓREK W CELU INDUKCJI EFEKTU BIOLOGICZNEGO LUB BIOCHEMICZNEGO
|
|
EP2834260A4
(en)
|
2012-04-02 |
2016-08-10 |
Moderna Therapeutics Inc |
MODIFIED POLYNUCLEOTIDES FOR THE PREPARATION OF MEMBRANE PROTEINS
|
|
US10501512B2
(en)
|
2012-04-02 |
2019-12-10 |
Modernatx, Inc. |
Modified polynucleotides
|
|
US10501513B2
(en)
|
2012-04-02 |
2019-12-10 |
Modernatx, Inc. |
Modified polynucleotides for the production of oncology-related proteins and peptides
|
|
US9283287B2
(en)
|
2012-04-02 |
2016-03-15 |
Moderna Therapeutics, Inc. |
Modified polynucleotides for the production of nuclear proteins
|
|
EP2885419A4
(en)
|
2012-08-14 |
2016-05-25 |
Moderna Therapeutics Inc |
ENZYMES AND POLYMERASES FOR RNA SYNTHESIS
|
|
EP2922554B1
(en)
|
2012-11-26 |
2022-02-23 |
ModernaTX, Inc. |
Terminally modified rna
|
|
EP2931319B1
(en)
|
2012-12-13 |
2019-08-21 |
ModernaTX, Inc. |
Modified nucleic acid molecules and uses thereof
|
|
WO2014093574A1
(en)
|
2012-12-13 |
2014-06-19 |
Moderna Therapeutics, Inc. |
Modified polynucleotides for altering cell phenotype
|
|
WO2014159813A1
(en)
|
2013-03-13 |
2014-10-02 |
Moderna Therapeutics, Inc. |
Long-lived polynucleotide molecules
|
|
EP2971010B1
(en)
|
2013-03-14 |
2020-06-10 |
ModernaTX, Inc. |
Formulation and delivery of modified nucleoside, nucleotide, and nucleic acid compositions
|
|
MX2015012865A
(es)
|
2013-03-14 |
2016-07-21 |
Shire Human Genetic Therapies |
Metodos para purificacion de arn mensajero.
|
|
JP2016514970A
(ja)
|
2013-03-14 |
2016-05-26 |
シャイアー ヒューマン ジェネティック セラピーズ インコーポレイテッド |
メッセンジャーrnaのキャップ効率の定量的評価
|
|
US20160032316A1
(en)
|
2013-03-14 |
2016-02-04 |
The Trustees Of The University Of Pennsylvania |
Purification and Purity Assessment of RNA Molecules Synthesized with Modified Nucleosides
|
|
CN105283548A
(zh)
|
2013-03-15 |
2016-01-27 |
葛兰素史密斯克莱生物公司 |
Rna纯化方法
|
|
US10590161B2
(en)
|
2013-03-15 |
2020-03-17 |
Modernatx, Inc. |
Ion exchange purification of mRNA
|
|
US20160017313A1
(en)
|
2013-03-15 |
2016-01-21 |
Moderna Therapeutics, Inc. |
Analysis of mrna heterogeneity and stability
|
|
EP2971165A4
(en)
|
2013-03-15 |
2016-11-23 |
Moderna Therapeutics Inc |
DISSOLUTION OF DNA FRAGMENTS IN MRNA MANUFACTURING METHODS
|
|
US8980864B2
(en)
|
2013-03-15 |
2015-03-17 |
Moderna Therapeutics, Inc. |
Compositions and methods of altering cholesterol levels
|
|
US10138507B2
(en)
|
2013-03-15 |
2018-11-27 |
Modernatx, Inc. |
Manufacturing methods for production of RNA transcripts
|
|
WO2014144039A1
(en)
|
2013-03-15 |
2014-09-18 |
Moderna Therapeutics, Inc. |
Characterization of mrna molecules
|
|
EP4279610A3
(en)
|
2013-03-15 |
2024-01-03 |
ModernaTX, Inc. |
Ribonucleic acid purification
|
|
PL3019619T3
(pl)
|
2013-07-11 |
2022-01-10 |
Modernatx, Inc. |
Kompozycje zawierające syntetyczne polinkleotydy kodujące białka powiązane z crispr i syntetyczne sgrna oraz sposoby ich stosowania
|
|
EP3041938A1
(en)
|
2013-09-03 |
2016-07-13 |
Moderna Therapeutics, Inc. |
Circular polynucleotides
|
|
CA2923029A1
(en)
|
2013-09-03 |
2015-03-12 |
Moderna Therapeutics, Inc. |
Chimeric polynucleotides
|
|
EP3052106A4
(en)
|
2013-09-30 |
2017-07-19 |
ModernaTX, Inc. |
Polynucleotides encoding immune modulating polypeptides
|
|
EP3052511A4
(en)
|
2013-10-02 |
2017-05-31 |
Moderna Therapeutics, Inc. |
Polynucleotide molecules and uses thereof
|
|
JP2016538829A
(ja)
|
2013-10-03 |
2016-12-15 |
モデルナ セラピューティクス インコーポレイテッドModerna Therapeutics,Inc. |
低密度リポタンパク質受容体をコードするポリヌクレオチド
|
|
AU2014337156A1
(en)
|
2013-10-18 |
2016-05-12 |
Modernatx, Inc. |
Compositions and methods for tolerizing cellular systems
|
|
US20170173128A1
(en)
|
2013-12-06 |
2017-06-22 |
Moderna TX, Inc. |
Targeted adaptive vaccines
|
|
EP3119873A4
(en)
|
2014-03-20 |
2018-01-03 |
The Board of Regents of The University of Texas System |
T7 rna polymerase variants with expanded substrate range and enhanced transcriptional yield
|
|
RS66380B1
(sr)
|
2014-04-23 |
2025-02-28 |
Modernatx Inc |
Vakcine nukleinske kiseline
|
|
US10286086B2
(en)
|
2014-06-19 |
2019-05-14 |
Modernatx, Inc. |
Alternative nucleic acid molecules and uses thereof
|
|
US20170204152A1
(en)
|
2014-07-16 |
2017-07-20 |
Moderna Therapeutics, Inc. |
Chimeric polynucleotides
|
|
EP3169783A4
(en)
|
2014-07-17 |
2018-10-03 |
Modernatx, Inc. |
Terminal modifications of polynucleotides
|
|
EP3247363A4
(en)
|
2015-01-21 |
2018-10-03 |
Moderna Therapeutics, Inc. |
Lipid nanoparticle compositions
|
|
WO2016164762A1
(en)
|
2015-04-08 |
2016-10-13 |
Moderna Therapeutics, Inc. |
Polynucleotides encoding low density lipoprotein receptor egf-a and intracellular domain mutants and methods of using the same
|
|
EP3289077B1
(en)
|
2015-04-30 |
2020-04-15 |
CureVac AG |
Method for in vitro transcription using an immobilized restriction enzyme
|
|
WO2016201377A1
(en)
|
2015-06-10 |
2016-12-15 |
Moderna Therapeutics, Inc. |
Targeted adaptive vaccines
|
|
WO2017011773A2
(en)
|
2015-07-15 |
2017-01-19 |
Modernatx, Inc. |
Codon-optimized nucleic acids encoding antibodies
|
|
WO2017015457A1
(en)
|
2015-07-21 |
2017-01-26 |
Modernatx, Inc. |
Ebola vaccine
|
|
EP4218805A1
(en)
|
2015-07-21 |
2023-08-02 |
ModernaTX, Inc. |
Infectious disease vaccines
|
|
WO2017020026A1
(en)
|
2015-07-30 |
2017-02-02 |
Modernatx, Inc. |
Concatemeric peptide epitopes rnas
|
|
WO2017019935A1
(en)
|
2015-07-30 |
2017-02-02 |
Modernatx, Inc. |
Multimeric mrna
|
|
US20180237849A1
(en)
|
2015-08-17 |
2018-08-23 |
Modernatx, Inc. |
Rna mapping/fingerprinting
|
|
WO2017031232A1
(en)
|
2015-08-17 |
2017-02-23 |
Modernatx, Inc. |
Methods for preparing particles and related compositions
|
|
ES2910425T3
(es)
|
2015-09-17 |
2022-05-12 |
Modernatx Inc |
Compuestos y composiciones para la administración intracelular de agentes terapéuticos
|
|
ES2810701T5
(es)
|
2015-10-05 |
2024-07-11 |
Modernatx Inc |
Procedimientos para la administración terapéutica de medicamentos de ácido ribonucleico mensajero
|
|
WO2017066789A1
(en)
|
2015-10-16 |
2017-04-20 |
Modernatx, Inc. |
Mrna cap analogs with modified sugar
|
|
JP7687767B2
(ja)
|
2015-10-22 |
2025-06-03 |
モデルナティエックス インコーポレイテッド |
呼吸器合胞体ウイルスワクチン
|
|
EP3364982A4
(en)
|
2015-10-22 |
2019-04-17 |
ModernaTX, Inc. |
VACCINES AGAINST SEXUALLY TRANSMITTED DISEASES
|
|
WO2017070624A1
(en)
|
2015-10-22 |
2017-04-27 |
Modernatx, Inc. |
Tropical disease vaccines
|
|
JP6921833B2
(ja)
|
2015-10-22 |
2021-08-18 |
モデルナティーエックス, インコーポレイテッド |
ヒトサイトメガロウイルスワクチン
|
|
EP4349405A3
(en)
|
2015-10-22 |
2024-06-19 |
ModernaTX, Inc. |
Respiratory virus vaccines
|
|
CN118846026A
(zh)
|
2015-10-22 |
2024-10-29 |
摩登纳特斯有限公司 |
广谱流感病毒疫苗
|
|
AU2016342049B2
(en)
|
2015-10-22 |
2023-05-18 |
Modernatx, Inc. |
Herpes simplex virus vaccine
|
|
TN2018000152A1
(en)
|
2015-10-22 |
2019-10-04 |
Modernatx Inc |
Nucleic acid vaccines for varicella zoster virus (vzv)
|
|
US20180318409A1
(en)
|
2015-10-22 |
2018-11-08 |
Modernatx, Inc. |
Cancer vaccines
|
|
WO2017099823A1
(en)
|
2015-12-10 |
2017-06-15 |
Modernatx, Inc. |
Compositions and methods for delivery of therapeutic agents
|
|
CA3007108A1
(en)
|
2015-12-17 |
2017-06-22 |
Modernatx, Inc. |
Polynucleotides encoding methylmalonyl-coa mutase
|
|
EP4036079B1
(en)
|
2015-12-22 |
2025-11-19 |
ModernaTX, Inc. |
Compounds and compositions for intracellular delivery of agents
|
|
US10465190B1
(en)
|
2015-12-23 |
2019-11-05 |
Modernatx, Inc. |
In vitro transcription methods and constructs
|
|
EP3402881B1
(en)
|
2016-01-13 |
2021-04-28 |
New England Biolabs, Inc. |
Thermostable variants of t7 rna polymerase
|
|
US20210206818A1
(en)
|
2016-01-22 |
2021-07-08 |
Modernatx, Inc. |
Messenger ribonucleic acids for the production of intracellular binding polypeptides and methods of use thereof
|
|
WO2017201332A1
(en)
|
2016-05-18 |
2017-11-23 |
Modernatx, Inc. |
Polynucleotides encoding acyl-coa dehydrogenase, very long-chain for the treatment of very long-chain acyl-coa dehydrogenase deficiency
|
|
WO2017201333A1
(en)
|
2016-05-18 |
2017-11-23 |
Modernatx, Inc. |
Polynucleotides encoding lipoprotein lipase for the treatment of hyperlipidemia
|
|
AU2017268394A1
(en)
|
2016-05-18 |
2019-01-03 |
Modernatx, Inc. |
Polynucleotides encoding relaxin
|
|
CA3024507A1
(en)
|
2016-05-18 |
2017-11-23 |
Modernatx, Inc. |
Polynucleotides encoding .alpha.-galactosidase a for the treatment of fabry disease
|
|
JP2019519516A
(ja)
|
2016-05-18 |
2019-07-11 |
モデルナティーエックス, インコーポレイテッド |
がんの治療のためのmRNA併用療法
|
|
JP2019519601A
(ja)
|
2016-05-18 |
2019-07-11 |
モダーナティエックス・インコーポレイテッドModernaTX, Inc. |
急性間欠性ポルフィリン症の治療のためのポルホビリノゲン脱アミノ酵素をコードするポリヌクレオチド
|
|
MA45041A
(fr)
|
2016-05-18 |
2019-03-27 |
Modernatx Inc |
Polynucléotides codant pour la galactose-1-phosphate uridylyltransférase destinés au traitement de la galactosémie de type 1
|
|
US11801227B2
(en)
|
2016-05-18 |
2023-10-31 |
Modernatx, Inc. |
Polynucleotides encoding cystic fibrosis transmembrane conductance regulator for the treatment of cystic fibrosis
|
|
US20190382774A1
(en)
|
2016-05-18 |
2019-12-19 |
Modernatx, Inc. |
Polyribonucleotides containing reduced uracil content and uses thereof
|
|
WO2017201342A1
(en)
|
2016-05-18 |
2017-11-23 |
Modernatx, Inc. |
Polynucleotides encoding jagged1 for the treatment of alagille syndrome
|
|
ES2941411T3
(es)
|
2016-05-18 |
2023-05-22 |
Modernatx Inc |
Polinucleótidos que codifican interleucina-12 (IL12) y usos de los mismos
|
|
SG11201810162PA
(en)
|
2016-05-18 |
2018-12-28 |
Modernatx Inc |
Polynucleotides encoding citrin for the treatment of citrullinemia type 2
|
|
EP3468537A1
(en)
|
2016-06-14 |
2019-04-17 |
Modernatx, Inc. |
Stabilized formulations of lipid nanoparticles
|
|
CN106244608B
(zh)
|
2016-08-08 |
2019-10-25 |
中国科学技术大学 |
诱导的t7 rna聚合酶
|
|
CA3036831A1
(en)
|
2016-09-14 |
2018-03-22 |
Modernatx, Inc. |
High purity rna compositions and methods for preparation thereof
|
|
EP3528821A4
(en)
|
2016-10-21 |
2020-07-01 |
ModernaTX, Inc. |
HUMAN CYTOMEGALOVIRUS VACCINE
|
|
AU2017347837A1
(en)
|
2016-10-26 |
2019-06-06 |
Modernatx, Inc. |
Messenger ribonucleic acids for enhancing immune responses and methods of use thereof
|
|
EP3532613A4
(en)
|
2016-10-26 |
2020-05-06 |
ModernaTX, Inc. |
METHOD AND COMPOSITIONS FOR RNA MAPPING
|
|
EP3538067A1
(en)
|
2016-11-08 |
2019-09-18 |
Modernatx, Inc. |
Stabilized formulations of lipid nanoparticles
|
|
EP3538146A4
(en)
|
2016-11-11 |
2020-07-15 |
ModernaTX, Inc. |
INFLUENZA VACCINE
|
|
US11103578B2
(en)
|
2016-12-08 |
2021-08-31 |
Modernatx, Inc. |
Respiratory virus nucleic acid vaccines
|
|
US11384352B2
(en)
|
2016-12-13 |
2022-07-12 |
Modernatx, Inc. |
RNA affinity purification
|
|
US20180243225A1
(en)
|
2017-01-25 |
2018-08-30 |
Modernatx, Inc. |
Ebola/marburg vaccines
|
|
SG11201906895WA
(en)
|
2017-02-01 |
2019-08-27 |
Modernatx Inc |
Rna cancer vaccines
|
|
AU2018214556A1
(en)
|
2017-02-01 |
2019-08-15 |
Modernatx, Inc. |
Immunomodulatory therapeutic mRNA compositions encoding activating oncogene mutation peptides
|
|
WO2018144778A1
(en)
|
2017-02-01 |
2018-08-09 |
Modernatx, Inc. |
Polynucleotide secondary structure
|
|
EP3582790A4
(en)
|
2017-02-16 |
2020-11-25 |
ModernaTX, Inc. |
VERY POWERFUL IMMUNOGENIC COMPOSITIONS
|
|
WO2018157009A1
(en)
|
2017-02-24 |
2018-08-30 |
Modernatx, Inc. |
Nucleic acid-based therapy of muscular dystrophies
|
|
US11752206B2
(en)
|
2017-03-15 |
2023-09-12 |
Modernatx, Inc. |
Herpes simplex virus vaccine
|
|
WO2018170270A1
(en)
|
2017-03-15 |
2018-09-20 |
Modernatx, Inc. |
Varicella zoster virus (vzv) vaccine
|
|
US11464848B2
(en)
|
2017-03-15 |
2022-10-11 |
Modernatx, Inc. |
Respiratory syncytial virus vaccine
|
|
WO2018170336A1
(en)
|
2017-03-15 |
2018-09-20 |
Modernatx, Inc. |
Lipid nanoparticle formulation
|
|
RS63953B1
(sr)
|
2017-03-15 |
2023-02-28 |
Modernatx Inc |
Jedinjenje i kompozicije za intracelularnu isporuku terapeutskih sredstava
|
|
US11576961B2
(en)
|
2017-03-15 |
2023-02-14 |
Modernatx, Inc. |
Broad spectrum influenza virus vaccine
|
|
MA47790A
(fr)
|
2017-03-17 |
2021-05-05 |
Modernatx Inc |
Vaccins à base d'arn contre des maladies zoonotiques
|
|
US20200038499A1
(en)
|
2017-03-22 |
2020-02-06 |
Modernatx, Inc. |
Rna bacterial vaccines
|
|
EP3607074A4
(en)
|
2017-04-05 |
2021-07-07 |
Modernatx, Inc. |
REDUCTION OR ELIMINATION OF IMMUNE RESPONSES TO NON-INTRAVENOUS THERAPEUTIC PROTEINS, FOR EXAMPLE SUBCUTANEOUSLY
|
|
MA49463A
(fr)
|
2017-04-26 |
2021-05-05 |
Modernatx Inc |
Vaccin contre le virus de l'herpès simplex
|
|
EP3638215A4
(en)
|
2017-06-15 |
2021-03-24 |
Modernatx, Inc. |
RNA FORMULATIONS
|
|
WO2018232355A1
(en)
|
2017-06-15 |
2018-12-20 |
Modernatx, Inc. |
Rna antibodies
|
|
JP7588391B2
(ja)
|
2017-06-30 |
2024-11-22 |
コデクシス, インコーポレイテッド |
T7 rnaポリメラーゼバリアント
|
|
EP3655040A1
(en)
|
2017-07-21 |
2020-05-27 |
Modernatx, Inc. |
Modified mrna encoding a propionyl-coa carboxylase and uses thereof
|
|
EP3668971B8
(en)
|
2017-08-18 |
2024-05-29 |
ModernaTX, Inc. |
Rna polymerase variants
|
|
EP3668977A4
(en)
|
2017-08-18 |
2021-04-21 |
Modernatx, Inc. |
HPLC ANALYTICAL PROCESSES
|
|
EP3668979A4
(en)
|
2017-08-18 |
2021-06-02 |
Modernatx, Inc. |
METHOD OF HPLC ANALYSIS
|
|
US20200254086A1
(en)
|
2017-08-18 |
2020-08-13 |
Moderna TX, Inc. |
Efficacious mrna vaccines
|
|
CA3073211A1
(en)
|
2017-08-31 |
2019-03-07 |
Modernatx, Inc. |
Methods of making lipid nanoparticles
|
|
WO2019055807A1
(en)
|
2017-09-14 |
2019-03-21 |
Modernatx, Inc. |
RNA VACCINES AGAINST ZIKA VIRUS
|
|
EA202090919A1
(ru)
|
2017-10-31 |
2020-09-09 |
Модернатикс, Инк. |
Липидные наночастицы для доставки модифицированной рнк, кодирующей полипептид vegf-a
|
|
US20190192646A1
(en)
|
2017-11-03 |
2019-06-27 |
Modernatx, Inc. |
Salmonella vaccines
|
|
CA3083102A1
(en)
|
2017-11-21 |
2019-05-31 |
Modernatx, Inc. |
Epstein-barr virus vaccines
|
|
US11911453B2
(en)
|
2018-01-29 |
2024-02-27 |
Modernatx, Inc. |
RSV RNA vaccines
|
|
SG11202012770RA
(en)
|
2018-06-27 |
2021-01-28 |
Modernatx Inc |
Personalized cancer vaccine epitope selection
|
|
CA3111836A1
(en)
|
2018-09-13 |
2020-03-19 |
Modernatx, Inc. |
Modified mrna for the treatment of progressive familial intrahepatic cholestasis disorders
|
|
EP3852732A1
(en)
|
2018-09-19 |
2021-07-28 |
ModernaTX, Inc. |
Peg lipids and uses thereof
|
|
CN112996854B
(zh)
|
2018-09-19 |
2024-08-30 |
摩登纳特斯有限公司 |
高纯度peg脂质和其用途
|
|
EP3853202A1
(en)
|
2018-09-19 |
2021-07-28 |
ModernaTX, Inc. |
Compounds and compositions for intracellular delivery of therapeutic agents
|
|
WO2020061457A1
(en)
|
2018-09-20 |
2020-03-26 |
Modernatx, Inc. |
Preparation of lipid nanoparticles and methods of administration thereof
|
|
JP2022506839A
(ja)
|
2018-11-07 |
2022-01-17 |
モデルナティエックス インコーポレイテッド |
Rnaがんワクチン
|
|
CN113939282A
(zh)
|
2019-01-31 |
2022-01-14 |
摩登纳特斯有限公司 |
制备脂质纳米颗粒的方法
|
|
US11351242B1
(en)
|
2019-02-12 |
2022-06-07 |
Modernatx, Inc. |
HMPV/hPIV3 mRNA vaccine composition
|
|
US11851694B1
(en)
|
2019-02-20 |
2023-12-26 |
Modernatx, Inc. |
High fidelity in vitro transcription
|
|
MA55037A
(fr)
|
2019-02-20 |
2021-12-29 |
Modernatx Inc |
Variants d'arn polymérase pour le coiffage co-transcriptionnel
|
|
KR20210149727A
(ko)
|
2019-03-11 |
2021-12-09 |
모더나티엑스, 인크. |
유가식(fed-batch) 시험관내 전사 공정
|
|
WO2020190750A1
(en)
|
2019-03-15 |
2020-09-24 |
Modernatx, Inc. |
Hiv rna vaccines
|
|
WO2020243561A1
(en)
|
2019-05-31 |
2020-12-03 |
Modernatx, Inc. |
Expanded t cell assay
|
|
BR112022002548A2
(pt)
|
2019-08-14 |
2022-06-14 |
Modernatx Inc |
Processos para purificar produtos a jusante de transcrição in vitro
|
|
EP4028030A4
(en)
|
2019-09-11 |
2023-09-27 |
ModernaTX, Inc. |
VACCINE AGAINST HUMAN CYTOMEGALOVIRUS
|
|
AR120080A1
(es)
|
2019-09-19 |
2022-02-02 |
Modernatx Inc |
Compuestos lipídicos de cola ramificada y composiciones para la administración intracelular de agentes terapéuticos
|
|
US20220349006A1
(en)
|
2019-09-19 |
2022-11-03 |
Moderna TX, Inc. |
Cap guides and methods of use thereof for rna mapping
|
|
US20210217484A1
(en)
|
2020-01-10 |
2021-07-15 |
Modernatx, Inc. |
Variational autoencoder for biological sequence generation
|
|
US20210228707A1
(en)
|
2020-01-28 |
2021-07-29 |
Modernatx, Inc. |
Coronavirus rna vaccines
|
|
CN115103682A
(zh)
|
2020-01-30 |
2022-09-23 |
摩登纳特斯有限公司 |
呼吸道病毒免疫组合物
|
|
MX2022009410A
(es)
|
2020-01-31 |
2022-10-18 |
Modernatx Inc |
Metodos de preparacion de nanoparticulas lipidicas.
|
|
EP4100052A2
(en)
|
2020-02-07 |
2022-12-14 |
ModernaTX, Inc. |
Sars-cov-2 mrna domain vaccines
|
|
WO2021211343A1
(en)
|
2020-04-13 |
2021-10-21 |
Modernatx, Inc. |
Zika virus mrna vaccines
|
|
WO2021222304A1
(en)
|
2020-04-27 |
2021-11-04 |
Modernatx, Inc. |
Sars-cov-2 rna vaccines
|
|
WO2021159130A2
(en)
|
2020-05-15 |
2021-08-12 |
Modernatx, Inc. |
Coronavirus rna vaccines and methods of use
|
|
EP4149484A4
(en)
|
2020-05-15 |
2024-10-02 |
ModernaTX, Inc. |
RNA FORMULATIONS FOR HIGH VOLUME DELIVERY
|
|
US20230190761A1
(en)
|
2020-05-21 |
2023-06-22 |
Modernatx, Inc. |
Methylene blue stabilized mrna compositions
|
|
US20230287437A1
(en)
|
2020-06-05 |
2023-09-14 |
Modernatx, Inc. |
Bacterial strains for dna production
|
|
MX2023001461A
(es)
|
2020-08-06 |
2023-04-26 |
Modernatx Inc |
Composiciones para la administracion de moleculas de carga activa al epitelio de las vias respiratorias.
|
|
US11406703B2
(en)
|
2020-08-25 |
2022-08-09 |
Modernatx, Inc. |
Human cytomegalovirus vaccine
|
|
US20230355743A1
(en)
|
2020-09-25 |
2023-11-09 |
Modernatx, Inc. |
Multi-proline-substituted coronavirus spike protein vaccines
|
|
US12329811B2
(en)
|
2021-01-11 |
2025-06-17 |
Modernatx, Inc. |
Seasonal RNA influenza virus vaccines
|
|
AU2022208057A1
(en)
|
2021-01-15 |
2023-08-03 |
Modernatx, Inc. |
Variant strain-based coronavirus vaccines
|
|
EP4277653A1
(en)
|
2021-01-15 |
2023-11-22 |
ModernaTX, Inc. |
Variant strain-based coronavirus vaccines
|
|
US20240100145A1
(en)
|
2021-03-05 |
2024-03-28 |
Moderna TX, Inc. |
Vlp enteroviral vaccines
|
|
US20240299531A1
(en)
|
2021-03-15 |
2024-09-12 |
Moderna TX, Inc |
Therapeutic use of sars-cov-2 mrna domain vaccines
|
|
EP4313072A1
(en)
|
2021-03-26 |
2024-02-07 |
ModernaTX, Inc. |
Pertussis vaccine
|
|
WO2022212442A1
(en)
|
2021-03-31 |
2022-10-06 |
Modernatx, Inc. |
Synthesis of trinucleotide and tetranucleotide caps for mrna production
|
|
EP4314046A4
(en)
|
2021-04-01 |
2025-03-19 |
ModernaTX, Inc. |
Mucosal expression of antibody structures and isotypes by mRNA
|
|
JP2024512780A
(ja)
|
2021-04-01 |
2024-03-19 |
モデルナティエックス インコーポレイテッド |
多価rna組成物中のrna種の識別及び比率決定のための方法
|
|
CA3216490A1
(en)
|
2021-04-13 |
2022-10-20 |
Modernatx, Inc. |
Epstein-barr virus mrna vaccines
|
|
WO2022221336A1
(en)
|
2021-04-13 |
2022-10-20 |
Modernatx, Inc. |
Respiratory syncytial virus mrna vaccines
|
|
EP4322993A1
(en)
|
2021-04-13 |
2024-02-21 |
ModernaTX, Inc. |
Respiratory virus combination vaccines
|
|
EP4322994A1
(en)
|
2021-04-14 |
2024-02-21 |
ModernaTX, Inc. |
Influenza-coronavirus combination vaccines
|
|
WO2022226277A1
(en)
|
2021-04-23 |
2022-10-27 |
Modernatx, Inc. |
Stabilized formulations
|
|
EP4326241A1
(en)
|
2021-04-23 |
2024-02-28 |
ModernaTX, Inc. |
Isoquinoline-stabilized lipid nanoparticle formulations
|
|
WO2022232585A1
(en)
|
2021-04-29 |
2022-11-03 |
Modernatx, Inc. |
Lyophilization methods for preparing lipid formulated therapeutics
|
|
US20220363937A1
(en)
|
2021-05-14 |
2022-11-17 |
Armstrong World Industries, Inc. |
Stabilization of antimicrobial coatings
|
|
WO2022245888A1
(en)
|
2021-05-19 |
2022-11-24 |
Modernatx, Inc. |
Seasonal flu rna vaccines and methods of use
|
|
EP4355761A1
(en)
|
2021-06-14 |
2024-04-24 |
ModernaTX, Inc. |
Mrna vaccines encoding flexible coronavirus spike proteins
|
|
WO2022266012A1
(en)
|
2021-06-14 |
2022-12-22 |
Modernatx, Inc. |
Coronavirus glycosylation variant vaccines
|
|
WO2022266389A1
(en)
|
2021-06-17 |
2022-12-22 |
Modernatx, Inc. |
Alternative rna purification strategies
|
|
WO2023283642A2
(en)
|
2021-07-09 |
2023-01-12 |
Modernatx, Inc. |
Pan-human coronavirus concatemeric vaccines
|
|
WO2023283645A1
(en)
|
2021-07-09 |
2023-01-12 |
Modernatx, Inc. |
Pan-human coronavirus domain vaccines
|
|
EP4366768A4
(en)
|
2021-07-09 |
2025-05-21 |
ModernaTX, Inc. |
Pan-human coronavirus vaccines
|
|
US20250092440A1
(en)
|
2021-08-02 |
2025-03-20 |
Modernatx, Inc. |
Extraction-less reverse phase (rp) chromatography of mrna encapsulated in lipid nanoparticles for mrna purity assessment
|
|
WO2023019181A1
(en)
|
2021-08-11 |
2023-02-16 |
Modernatx, Inc. |
Sars-cov-2 lipid nanoparticle vaccine formulations
|
|
WO2023018773A1
(en)
|
2021-08-11 |
2023-02-16 |
Modernatx, Inc. |
Lipid nanoparticle formulations and methods of synthesis thereof
|
|
WO2023018923A1
(en)
|
2021-08-13 |
2023-02-16 |
Modernatx, Inc. |
Multicolumn chromatography mrna purification
|
|
WO2023056401A1
(en)
|
2021-10-01 |
2023-04-06 |
Modernatx, Inc. |
Rna formulations for high volume distribution, and methods of using the same for treating a disease or condition caused by or associated with human cytomegalovirus
|
|
US20250011798A1
(en)
|
2021-10-18 |
2025-01-09 |
Modernatx, Inc. |
Markerless dna production
|
|
US20250051736A1
(en)
|
2021-10-18 |
2025-02-13 |
Modernatx, Inc. |
Custom bacterial strain for recombinant protein production
|
|
US20250231165A1
(en)
|
2021-10-20 |
2025-07-17 |
Modernatx, Inc. |
Drug product surrogate solutions
|
|
US20250362278A1
(en)
|
2021-11-01 |
2025-11-27 |
Modernatx, Inc. |
Mass spectrometry of mrna
|
|
EP4426855A1
(en)
|
2021-11-05 |
2024-09-11 |
ModernaTX, Inc. |
Methods of purifying dna for gene synthesis
|
|
WO2023092069A1
(en)
|
2021-11-18 |
2023-05-25 |
Modernatx, Inc. |
Sars-cov-2 mrna domain vaccines and methods of use
|
|
JP2024545152A
(ja)
|
2021-12-08 |
2024-12-05 |
モデルナティエックス インコーポレイテッド |
単純ヘルペスウイルスmRNAワクチン
|
|
EP4448777A1
(en)
|
2021-12-15 |
2024-10-23 |
ModernaTX, Inc. |
Determination of encapsulation efficiency of lipid nanoparticles
|
|
US20250084397A1
(en)
|
2022-01-04 |
2025-03-13 |
Modernatx, Inc. |
Methods of purifying dna for gene synthesis
|
|
EP4463545A1
(en)
|
2022-01-14 |
2024-11-20 |
ModernaTX, Inc. |
In vitro transcription dna purification and recycling
|
|
US20250136970A1
(en)
|
2022-02-03 |
2025-05-01 |
Modernatx, Inc. |
Continuous precipitation for mrna purification
|
|
EP4475882A1
(en)
|
2022-02-09 |
2024-12-18 |
ModernaTX, Inc. |
Mucosal administration methods and formulations
|
|
WO2023196914A1
(en)
|
2022-04-08 |
2023-10-12 |
Modernatx, Inc. |
Influenza nucleic acid compositions and uses thereof
|
|
WO2023201204A1
(en)
|
2022-04-11 |
2023-10-19 |
Modernatx, Inc. |
Detection of mrna purity in a mixture
|
|
CN119317710A
(zh)
|
2022-04-14 |
2025-01-14 |
摩登纳特斯有限公司 |
Rna聚合酶变体
|
|
WO2023201296A1
(en)
|
2022-04-15 |
2023-10-19 |
Modernatx, Inc. |
Ribosomal engagement potency assay
|
|
WO2023212696A1
(en)
|
2022-04-29 |
2023-11-02 |
Modernatx, Inc. |
Lyophilized human cytomegalovirus vaccines
|
|
WO2023225524A1
(en)
|
2022-05-17 |
2023-11-23 |
Modernatx, Inc. |
Preparation of highly concentrated mrna
|
|
EP4544074A1
(en)
|
2022-06-24 |
2025-04-30 |
ModernaTX, Inc. |
Methods of producing rna
|
|
WO2024010993A1
(en)
|
2022-07-06 |
2024-01-11 |
Modernatx, Inc. |
Primer design for cell-free dna production
|
|
EP4554617A1
(en)
|
2022-07-13 |
2025-05-21 |
ModernaTX, Inc. |
Norovirus mrna vaccines
|
|
EP4562141A1
(en)
|
2022-07-28 |
2025-06-04 |
ModernaTX, Inc. |
Methods of rna purification
|
|
WO2024030369A1
(en)
|
2022-08-01 |
2024-02-08 |
Modernatx, Inc. |
Extraction-less reverse phase (rp) chromatography for mrna purity assessment
|
|
WO2024050483A1
(en)
|
2022-08-31 |
2024-03-07 |
Modernatx, Inc. |
Variant strain-based coronavirus vaccines and uses thereof
|
|
EP4612301A1
(en)
|
2022-11-03 |
2025-09-10 |
ModernaTX, Inc. |
Chemical stability of mrna
|
|
EP4630397A1
(en)
|
2022-12-08 |
2025-10-15 |
ModernaTX, Inc. |
Ionizable lipids with malonate tails
|
|
EP4648793A1
(en)
|
2023-01-11 |
2025-11-19 |
ModernaTX, Inc. |
Personalized cancer vaccines
|
|
WO2024163465A1
(en)
|
2023-01-30 |
2024-08-08 |
Modernatx, Inc. |
Epstein-barr virus mrna vaccines
|