WO2019167441A1 - 毒素分離器具 - Google Patents
毒素分離器具 Download PDFInfo
- Publication number
- WO2019167441A1 WO2019167441A1 PCT/JP2019/000595 JP2019000595W WO2019167441A1 WO 2019167441 A1 WO2019167441 A1 WO 2019167441A1 JP 2019000595 W JP2019000595 W JP 2019000595W WO 2019167441 A1 WO2019167441 A1 WO 2019167441A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- toxin
- activated carbon
- blood
- biological fluid
- protein
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Ceased
Links
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M1/00—Suction or pumping devices for medical purposes; Devices for carrying-off, for treatment of, or for carrying-over, body-liquids; Drainage systems
- A61M1/36—Other treatment of blood in a by-pass of the natural circulatory system, e.g. temperature adaptation, irradiation ; Extra-corporeal blood circuits
- A61M1/3679—Other treatment of blood in a by-pass of the natural circulatory system, e.g. temperature adaptation, irradiation ; Extra-corporeal blood circuits by absorption
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M1/00—Suction or pumping devices for medical purposes; Devices for carrying-off, for treatment of, or for carrying-over, body-liquids; Drainage systems
- A61M1/34—Filtering material out of the blood by passing it through a membrane, i.e. hemofiltration or diafiltration
- A61M1/3413—Diafiltration
- A61M1/3417—Diafiltration using distinct filters for dialysis and ultra-filtration
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M1/00—Suction or pumping devices for medical purposes; Devices for carrying-off, for treatment of, or for carrying-over, body-liquids; Drainage systems
- A61M1/34—Filtering material out of the blood by passing it through a membrane, i.e. hemofiltration or diafiltration
- A61M1/3472—Filtering material out of the blood by passing it through a membrane, i.e. hemofiltration or diafiltration with treatment of the filtrate
- A61M1/3486—Biological, chemical treatment, e.g. chemical precipitation; treatment by absorbents
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01D—SEPARATION
- B01D15/00—Separating processes involving the treatment of liquids with solid sorbents; Apparatus therefor
- B01D15/08—Selective adsorption, e.g. chromatography
- B01D15/26—Selective adsorption, e.g. chromatography characterised by the separation mechanism
- B01D15/38—Selective adsorption, e.g. chromatography characterised by the separation mechanism involving specific interaction not covered by one or more of groups B01D15/265 and B01D15/30 - B01D15/36, e.g. affinity, ligand exchange or chiral chromatography
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J20/00—Solid sorbent compositions or filter aid compositions; Sorbents for chromatography; Processes for preparing, regenerating or reactivating thereof
- B01J20/02—Solid sorbent compositions or filter aid compositions; Sorbents for chromatography; Processes for preparing, regenerating or reactivating thereof comprising inorganic material
- B01J20/20—Solid sorbent compositions or filter aid compositions; Sorbents for chromatography; Processes for preparing, regenerating or reactivating thereof comprising inorganic material comprising free carbon; comprising carbon obtained by carbonising processes
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J20/00—Solid sorbent compositions or filter aid compositions; Sorbents for chromatography; Processes for preparing, regenerating or reactivating thereof
- B01J20/28—Solid sorbent compositions or filter aid compositions; Sorbents for chromatography; Processes for preparing, regenerating or reactivating thereof characterised by their form or physical properties
- B01J20/28014—Solid sorbent compositions or filter aid compositions; Sorbents for chromatography; Processes for preparing, regenerating or reactivating thereof characterised by their form or physical properties characterised by their form
- B01J20/28016—Particle form
- B01J20/28019—Spherical, ellipsoidal or cylindrical
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J20/00—Solid sorbent compositions or filter aid compositions; Sorbents for chromatography; Processes for preparing, regenerating or reactivating thereof
- B01J20/28—Solid sorbent compositions or filter aid compositions; Sorbents for chromatography; Processes for preparing, regenerating or reactivating thereof characterised by their form or physical properties
- B01J20/28054—Solid sorbent compositions or filter aid compositions; Sorbents for chromatography; Processes for preparing, regenerating or reactivating thereof characterised by their form or physical properties characterised by their surface properties or porosity
- B01J20/28057—Surface area, e.g. B.E.T specific surface area
- B01J20/28059—Surface area, e.g. B.E.T specific surface area being less than 100 m2/g
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J20/00—Solid sorbent compositions or filter aid compositions; Sorbents for chromatography; Processes for preparing, regenerating or reactivating thereof
- B01J20/28—Solid sorbent compositions or filter aid compositions; Sorbents for chromatography; Processes for preparing, regenerating or reactivating thereof characterised by their form or physical properties
- B01J20/28054—Solid sorbent compositions or filter aid compositions; Sorbents for chromatography; Processes for preparing, regenerating or reactivating thereof characterised by their form or physical properties characterised by their surface properties or porosity
- B01J20/28069—Pore volume, e.g. total pore volume, mesopore volume, micropore volume
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J20/00—Solid sorbent compositions or filter aid compositions; Sorbents for chromatography; Processes for preparing, regenerating or reactivating thereof
- B01J20/28—Solid sorbent compositions or filter aid compositions; Sorbents for chromatography; Processes for preparing, regenerating or reactivating thereof characterised by their form or physical properties
- B01J20/28054—Solid sorbent compositions or filter aid compositions; Sorbents for chromatography; Processes for preparing, regenerating or reactivating thereof characterised by their form or physical properties characterised by their surface properties or porosity
- B01J20/28069—Pore volume, e.g. total pore volume, mesopore volume, micropore volume
- B01J20/28071—Pore volume, e.g. total pore volume, mesopore volume, micropore volume being less than 0.5 ml/g
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J20/00—Solid sorbent compositions or filter aid compositions; Sorbents for chromatography; Processes for preparing, regenerating or reactivating thereof
- B01J20/28—Solid sorbent compositions or filter aid compositions; Sorbents for chromatography; Processes for preparing, regenerating or reactivating thereof characterised by their form or physical properties
- B01J20/28054—Solid sorbent compositions or filter aid compositions; Sorbents for chromatography; Processes for preparing, regenerating or reactivating thereof characterised by their form or physical properties characterised by their surface properties or porosity
- B01J20/28078—Pore diameter
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J20/00—Solid sorbent compositions or filter aid compositions; Sorbents for chromatography; Processes for preparing, regenerating or reactivating thereof
- B01J20/28—Solid sorbent compositions or filter aid compositions; Sorbents for chromatography; Processes for preparing, regenerating or reactivating thereof characterised by their form or physical properties
- B01J20/28054—Solid sorbent compositions or filter aid compositions; Sorbents for chromatography; Processes for preparing, regenerating or reactivating thereof characterised by their form or physical properties characterised by their surface properties or porosity
- B01J20/28078—Pore diameter
- B01J20/2808—Pore diameter being less than 2 nm, i.e. micropores or nanopores
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J20/00—Solid sorbent compositions or filter aid compositions; Sorbents for chromatography; Processes for preparing, regenerating or reactivating thereof
- B01J20/28—Solid sorbent compositions or filter aid compositions; Sorbents for chromatography; Processes for preparing, regenerating or reactivating thereof characterised by their form or physical properties
- B01J20/28054—Solid sorbent compositions or filter aid compositions; Sorbents for chromatography; Processes for preparing, regenerating or reactivating thereof characterised by their form or physical properties characterised by their surface properties or porosity
- B01J20/28078—Pore diameter
- B01J20/28083—Pore diameter being in the range 2-50 nm, i.e. mesopores
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J20/00—Solid sorbent compositions or filter aid compositions; Sorbents for chromatography; Processes for preparing, regenerating or reactivating thereof
- B01J20/28—Solid sorbent compositions or filter aid compositions; Sorbents for chromatography; Processes for preparing, regenerating or reactivating thereof characterised by their form or physical properties
- B01J20/28054—Solid sorbent compositions or filter aid compositions; Sorbents for chromatography; Processes for preparing, regenerating or reactivating thereof characterised by their form or physical properties characterised by their surface properties or porosity
- B01J20/28078—Pore diameter
- B01J20/28085—Pore diameter being more than 50 nm, i.e. macropores
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M1/00—Suction or pumping devices for medical purposes; Devices for carrying-off, for treatment of, or for carrying-over, body-liquids; Drainage systems
- A61M1/14—Dialysis systems; Artificial kidneys; Blood oxygenators ; Reciprocating systems for treatment of body fluids, e.g. single needle systems for hemofiltration or pheresis
- A61M1/16—Dialysis systems; Artificial kidneys; Blood oxygenators ; Reciprocating systems for treatment of body fluids, e.g. single needle systems for hemofiltration or pheresis with membranes
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M2202/00—Special media to be introduced, removed or treated
- A61M2202/04—Liquids
- A61M2202/0496—Urine
- A61M2202/0498—Urea
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01D—SEPARATION
- B01D15/00—Separating processes involving the treatment of liquids with solid sorbents; Apparatus therefor
Definitions
- the present invention relates to a toxin separation instrument for separating toxin from a biological fluid.
- a blood circulation path is formed outside the patient's body, the patient's blood is taken out of the body, and the purification process outside the body is performed, and then again. Treatment methods have been used to return blood to the patient.
- a porous separation membrane such as a polysulfone dialysis membrane has been conventionally used.
- a specific example is a hollow fiber dialyzer in which a housing is filled with a polysulfone hollow fiber membrane (porous separation membrane).
- a polysulfone hollow fiber membrane porous separation membrane
- the conventional porous separation membrane has a controlled pore size, and removes low molecular weight toxins such as urea and creatine by filtration and diffusion from the blood side to the dialysate side, while blood cells, platelets, Useful proteins such as albumin and complement are prevented from passing. Therefore, it is difficult to remove a solute having a relatively large molecular weight from blood.
- a method using activated carbon is known to remove toxins having a relatively large molecular weight.
- the diameter is 0.1 to 1 mm
- H / C is 0.14 or less
- the pore volume of pores having a pore diameter of 5 to 1000 nm is 0.25 to 0.00.
- This spherical activated carbon for direct perfusion has an adsorption capacity for toxic substances having a molecular weight of about 100 to 1000, which is at least the same as that of conventional spherical activated carbon, but has an adsorption capacity for toxic substances having a molecular weight of about 1000 to 10,000, compared to conventional spherical activated carbon. It is described that it improves.
- indoxyl sulfate which is a toxin
- albumin molecular weight of about 66000
- toxins bound to these proteins can hardly be removed by dialysis using a conventional porous separation membrane.
- Patent Document 1 does not mention a toxin bound to a protein as an adsorption target. Moreover, in the spherical activated carbon described in Patent Document 1, not only the toxin but also the protein may be removed.
- An oral adsorbent containing spherical activated carbon as described in Patent Document 2 cannot be reduced by directly adsorbing toxins such as indoxyl sulfate already present in blood.
- the use in dialysis patients is practically off-label, and the current situation is limited to use in patients with chronic renal failure in conservative phase for the purpose of delaying introduction into hemodialysis.
- an object of one embodiment of the present invention is to isolate a toxin that can selectively separate the toxin from the toxin and the protein with respect to the toxin that is bound to the protein and present in the biological fluid. It is to provide equipment.
- a toxin separation device is a toxin separation device that separates a toxin from a biological fluid, and the toxin binds to a protein in the biological fluid.
- activated carbon having a pore diameter of 1.4 to 35 nm measured by a nitrogen adsorption method and having a pore volume of 0.06 cm 3 / g or more.
- One embodiment of the present invention also provides a blood purification system comprising the toxin separating instrument and a dialyzer.
- One embodiment of the present invention is also a method for separating a toxin from a biological fluid, wherein the toxin is bound to a protein and is present in the biological fluid, and the biological fluid is attached to the toxin separation instrument.
- a method for separating toxins comprising a step of passing through.
- One embodiment of the present invention is also activated carbon for separating toxin from biological fluid, wherein the toxin is bound to protein and present in the biological fluid, and is measured by a nitrogen adsorption method.
- an activated carbon for toxin separation wherein the pore volume of pores having a pore diameter of 1.4 to 35 nm is 0.06 cm 3 / g or more.
- the toxin can be selectively separated from the toxin and the protein with respect to the toxin bound to the protein and present in the biological fluid. Therefore, harmful toxicity can be efficiently removed from the biological fluid while the useful protein remains in the biological fluid.
- a toxin separation device is a toxin separation device for separating a toxin from a biological fluid, wherein the toxin is bound to a protein and present in the biological fluid, and a nitrogen adsorption method.
- Activated carbon having a pore diameter of 1.4 to 35 nm measured by the above and having a pore volume of 0.06 cm 3 / g or more.
- the biological fluid refers to a liquid obtained from a biological body.
- the biological fluid include body fluids such as blood, plasma, serum, urine and ascites, and cell culture fluid.
- the biological fluid may be untreated or may be subjected to any treatment.
- the biological fluid is blood obtained from a patient in need of hemodialysis.
- the origin organism is not particularly limited, and includes mammals, birds, reptiles, etc., preferably pets such as dogs and cats; domestic animals such as cows, horses and pigs; and mammals including humans.
- the toxin separated by the toxin separation device is a toxin (protein-bound toxin) that is bound to a protein and is present in a biological fluid.
- toxins include uremic toxins.
- Protein binding uremic toxins include indoxyl sulfate, 3-carboxy-4-methyl-5-propyl-2-furanpropionic acid (CMPF), hippuric acid, homocysteine, indole-3-acetic acid, N-carboxyl Examples include uremic toxins of low molecular weight (less than 500 molecular weight) such as methyl lysine, p-cresyl sulfate, pentosidine, phenyl sulfate, quinolinic acid, spermidine, and glyoxal. In one example, the molecular weight of the toxin can be greater than or equal to 58 and less than 500.
- the toxin does not need to be 100% bound to the protein in the biological fluid, and at least a part (for example, 10% or more, 20% or more, 30% or more, 40% or more, 50% or more, 60% or more, 70% or more, 80% or more, 90% or more, 95% or more, or 99% or more) may be bonded.
- Proteins to which the toxin binds include albumin and ⁇ 1-acid glycoprotein.
- the protein is a protein useful for a living body.
- the molecular weight of the protein can be, for example, 10,000 or more, 30000 or more, or 60000 or more.
- the toxin separation device separates the toxin from a biological fluid for one or more types of protein-binding toxins.
- a toxin separation device has a pore volume (mesopore volume) of a pore (referred to herein as “mesopore”) having a pore diameter measured by a nitrogen adsorption method of 1.4 to 35 nm. ) Is provided with activated carbon having 0.06 cm 3 / g or more.
- Mesopore volume is preferably 0.08 cm 3 / g or more, more preferably 0.10 cm 3 / g or more, further preferably 0.12 cm 3 / g or more. It is not an upper limit particularly limited mesopore volume, from the viewpoint of strength, preferably at 1.0 cm 3 / g or less, and more preferably less 0.80 cm 3 / g.
- the specific method of measuring the mesopore volume by the nitrogen adsorption method is as in the examples described later.
- micropore volume a pore volume of a pore having a pore diameter of 0.5 to 1.4 nm
- micropore The pore volume (macropore volume) of pores having a pore diameter of 50 to 10,000 nm (referred to herein as “macropores”) has no correlation with the adsorption rate of the toxin to be separated, and only the mesopores It has been found that there is a correlation with the adsorption rate of the toxin to be separated.
- the micropore volume refers to that measured by the carbon dioxide adsorption method
- macropore volume refers to that measured by the mercury intrusion method.
- the toxin separation device separates toxins from biological fluids by the action of activated carbon. Specifically, activated carbon selectively adsorbs the toxin out of the toxin and the protein. Therefore, the protein is left in the biological fluid.
- the adsorption rate of the protein is lower than the adsorption rate of the toxin. In one example, the adsorption rate of the protein is less than 1%, preferably 0%.
- the activated carbon preferably has a pore (macropore) surface area of 0.10 m 2 / g or more with a pore diameter of 50 to 10,000 nm measured by mercury porosimetry.
- a pore (macropore) surface area of 0.10 m 2 / g or more with a pore diameter of 50 to 10,000 nm measured by mercury porosimetry.
- the toxin adsorption rate (particularly, the initial adsorption rate) is increased.
- Macropore surface area is more preferably 0.25 m 2 / g or more, more preferably 0.40 m 2 / g or more.
- the specific method of measuring the macropore surface area by the mercury intrusion method is as in the examples described later.
- the bulk density of the activated carbon is not particularly limited, but is preferably 0.30 g / cm 3 or more and more preferably 0.40 g / cm 3 or more from the viewpoint of crushing strength. In terms of adsorption properties, it is preferably 0.70 g / cm 3 or less, more preferably 0.60 g / cm 3 or less.
- the bulk density is a value obtained by dividing the dry weight W (g) of activated carbon when the container is filled with activated carbon by the volume V (cm 3 ) of the activated carbon filled.
- the crushing strength of the activated carbon is not particularly limited, but is preferably 100 g / grain or more, and more preferably 200 g / grain or more from the viewpoint of reducing the generation of fine powder.
- the crushing strength of activated carbon can be measured according to the examples described later.
- the crushing strength of the activated carbon is not particularly limited, but is preferably 2.0 kg / mm 3 or more and more preferably 3.5 mg / mm 3 or more from the viewpoint of reducing the generation of fine powder.
- the crushing strength of activated carbon can be measured according to the below-mentioned Example.
- the shape of the activated carbon is not particularly limited, and examples thereof include a spherical shape, a fibrous shape, a powder shape, and a granulated product.
- the activated carbon is preferably spherical.
- a spherical shape since the contact area between the activated carbons is small, it is easy for the biological fluid to pass between the activated carbons. Further, since the structure has no corners, it is difficult to chip and fine powder is not easily generated, so that clogging can be reduced. Therefore, it is easy for the biological fluid to pass through and the safety is improved.
- the average particle size is not particularly limited, but is preferably 100 ⁇ m or more, more preferably 200 ⁇ m or more, and further preferably 250 ⁇ m or more from the viewpoint of pressure loss. Further, from the viewpoint of the adsorption rate, it is preferably 1500 ⁇ m or less, more preferably 800 ⁇ m or less, and even more preferably 600 ⁇ m or less.
- the average particle diameter means a particle diameter (D v50 ) at a particle size accumulation ratio of 50% in a volume-based particle size accumulation diagram.
- activated carbon produced as a lump rather than granulated with fine powder activated carbon with a binder or the like is preferable to use.
- Activated carbon can use any carbon-containing material as a carbon source.
- the carbon-containing material include synthetic resin and pitch.
- the synthetic resin any of a heat-meltable resin (thermosetting resin) and a heat-infusible resin (thermoplastic resin) can be used.
- the pitch include petroleum pitch and coal pitch.
- Activated carbon used as a plant raw material may be used, and examples include fruit shells such as wood, charcoal, rice husk, coconut husk and palm husk. From the viewpoint of a small amount of impurities, a synthetic resin or pitch is preferable.
- Activated carbon can be manufactured according to the below-mentioned Example, for example.
- the toxin separation device includes the activated carbon.
- the toxin separation device is a column.
- the amount of activated carbon in the column is not particularly limited, and may be appropriately selected depending on the type of biological fluid, the amount of biological fluid, the shape of activated carbon, and the like.
- the toxin separation device is a blood purification column, and the amount of activated carbon is preferably, for example, 50 to 500 g / column, more preferably 75 to 400 g / column, and 100 to 300 g / column. More preferably, it is a column.
- the size of the column housing is not particularly limited, and may be appropriately selected according to the purpose.
- the toxin separation device is a filter.
- the size of the filter is not particularly limited, and may be appropriately selected depending on the type of biological fluid, the amount of biological fluid, the shape of activated carbon, and the like.
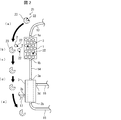
- FIG. 1 is a schematic diagram showing an example of a blood purification system 100 according to an embodiment of the present invention.
- the blood purification system 100 includes at least a toxin separation instrument 1 and a dialyzer 3.
- the toxin separation instrument 1 is a column (blood purification column) having a large number of activated carbons 2 therein.
- the toxin separation device 1 has a blood inlet 1a for allowing blood to flow in and a blood outlet 1b for discharging purified blood.
- the dialyzer 3 is an existing dialyzer (for example, a hollow fiber type dialyzer).
- the dialyzer 3 has a pore size of the porous separation membrane adjusted, and removes low molecular weight toxins such as urea and creatine by filtration and diffusion from the blood side to the dialysate side, while blood cells and platelets in the blood are removed. Proteins such as albumin and complement are prevented from passing through.
- the dialyzer 3 has a blood inlet 3a for allowing blood to flow in and a blood outlet 3b for allowing purified blood to flow out.
- the dialyzer 3 has a dialysate inlet 3c for allowing dialysate to flow in and a dialysate outlet 3d for allowing dialysate to flow out.
- the dialysate inflow port 3 c is connected to a dialysate supply unit (for example, including a dialysate supply device and a dialysis monitoring device) 16 via a tube 17.
- a tube 18 is connected to the dialysate outlet 3d.
- the dialysate flows from the dialysate supply unit 16 in the order of the tube 17, dialyzer 3, and tube 18, and the dialysate recovery unit (not shown, for example, in the dialysis monitoring device) downstream of the tube 18 It may be).
- the toxin separation device 1 is disposed on the upstream side of the dialyzer 3, and the blood outlet 1b of the toxin separation device 1 and the blood inlet 3a of the dialyzer 3 are connected by a tube 14. Therefore, the blood that has passed through the toxin separation device 1 is configured to flow into the dialyzer 3.
- a tube 13 is connected to the blood inlet 1a of the toxin separation instrument 1. Further, a pump 12 for feeding blood is connected to the tube 13. Further, a tube 11 is connected to the pump 12. The tube 11 is inserted into a blood vessel of a dialysis patient when the blood purification system 100 is used.
- a tube 15 is connected to the blood outlet 3 b of the dialyzer 3.
- the tube 15 is inserted into a blood vessel of a dialysis patient when the blood purification system 100 is used.
- the blood purification system 100 is configured by further disposing the toxin separating device 1 in front of the dialyzer 3 (upstream side) in a conventional typical hemodialysis configuration.
- the other member may be inserted in arbitrary positions.
- the blood purification system 100 is configured to include at least the toxin separation instrument 1 and the dialyzer 3, but other members (for example, the above-described members) may be optionally included in the blood purification system 100.
- the toxin separation device 1 may be disposed on the downstream side of the dialyzer 3.
- purification means that at least a part of at least one (one or more) protein-binding toxins in a biological fluid is separated from the biological fluid.
- 40% or more, preferably 70% or more, more preferably 90% or more of at least one (one or more) protein-binding toxins in the biological fluid are derived from the biological fluid. To be separated.
- a toxin separation method is a method for separating a toxin from a biological fluid, wherein the toxin is bound to a protein and is present in the biological fluid. A step of passing the biological fluid.
- FIG. 2 is a schematic diagram for explaining the separation of protein-binding toxins when the blood purification system 100 is used.
- the blood that has flowed into the toxin separation device 1 contacts the activated carbon 2 in the toxin separation device 1.
- the specific mechanism is not particularly limited, but the activated carbon 2 adsorbs the toxin 22 in the toxin-protein complex 21.
- activated carbon 2 does not adsorb protein 23.
- the removal amount of the toxin 22 existing in the blood by binding to the protein 23 is dramatically increased.
- a plurality of types of toxins 22 may be separated by the toxin separation instrument 1.
- toxins that do not bind to proteins may also be separated by the toxin separation instrument 1.
- the toxin separation method further includes a step of passing the biological fluid through a dialyzer before or after the step of passing the biological fluid through the toxin separation instrument.
- the “toxin separation method” can also be referred to as the “biological fluid purification method”.
- toxin separation device is used in vitro, and therefore the toxin separation method is performed in vitro.
- the activated carbon for toxin separation is activated carbon for separating a toxin from a biological fluid, wherein the toxin is bound to a protein and present in the biological fluid, and is adsorbed with nitrogen.
- the pore volume of pores having a pore diameter measured by the method of 1.4 to 35 nm is 0.06 cm 3 / g or more.
- the present invention also provides a dialysis method using the above-described toxin separation device or the above-described blood purification system.
- the present invention also provides a method for treating or preventing uremia using the above-described toxin separation device or the above-described blood purification system.
- the method treats or prevents uremia caused by protein-bound uremic toxins.
- the present invention also provides activated carbon having a pore diameter of 1.4 to 35 nm measured by a nitrogen adsorption method and a volume of 0.06 cm 3 / g or more.
- the present invention also provides a column packing material for packing a column for separating toxin from a biological fluid, wherein the toxin is present in the biological fluid by binding to a protein and measured by a nitrogen adsorption method.
- a column filler made of activated carbon having a pore diameter of 1.4 to 35 nm and a pore volume of 0.06 cm 3 / g or more.
- a particle size cumulative diagram was prepared according to JIS K 1474.
- the horizontal line is drawn on the horizontal axis from the intersection of the vertical line at the 50% point on the horizontal axis and the particle size cumulative line to obtain the mesh size (mm) of the sieve indicated by the intersection.
- the average particle size was used.
- the bulk density After filling the graduated cylinder with activated carbon, tapping was repeated until the bulk did not change. The bulk density was calculated by dividing the dry weight W (g) of the activated carbon at this time by the volume V (cm 3 ) of the activated carbon.
- crushing strength was calculated by dividing the crushing strength by the volume calculated from the average particle diameter.
- the specific surface area Based on the method defined in JIS Z 8830, the specific surface area (SSA) was measured. That is, the specific surface area can be calculated by the following equation by measuring the gas adsorption amount of activated carbon using a specific surface area measuring instrument (for example, “ASAP2010” manufactured by MICROMERITICS) by a gas adsorption method. Specifically, activated carbon as a sample was filled in a sample tube, dried under reduced pressure at 300 ° C., and the weight of the sample after drying was measured.
- a specific surface area measuring instrument for example, “ASAP2010” manufactured by MICROMERITICS
- the sample tube was cooled to ⁇ 196 ° C., nitrogen was introduced into the sample tube, nitrogen was adsorbed on the spherical activated carbon sample, and the relationship between nitrogen partial pressure and adsorption amount (adsorption isotherm) was measured.
- MA is the cross-sectional area of the nitrogen molecule, and 0.162 nm 2 was substituted.
- [Micropore volume] Measurement was carried out using “Autosorb (registered trademark) -iQ” manufactured by Cantachrome Instruments. First, a sample was filled in a sample tube and dried under reduced pressure at 200 ° C. for 12 hours to perform a pretreatment for removing impurities such as moisture in pores in the sample. Next, the sample temperature was lowered to ⁇ 78 ° C. using dry ice ethanol while reducing the pressure. Here, it introduce
- NLDFT delocalized density functional method
- [Mesopore volume] The pore volume was measured using “Autosorb (registered trademark) -iQ” manufactured by Cantachrome Instruments. First, a sample was filled in a sample tube and dried under reduced pressure at 200 ° C. for 12 hours to perform a pretreatment for removing impurities such as moisture in pores in the sample. Next, the sample temperature was lowered to -196 ° C. using liquid nitrogen while reducing the pressure. Here, it introduced in steps, changing the pressure of nitrogen, and the amount of nitrogen adsorption of the sample at each pressure was measured. From the relationship between the obtained pressure and the amount of nitrogen adsorbed, the volume (cm 3 / g) of pores having a pore diameter of 1.5 to 35 nm was measured using the Barrett Joyner Hallenda method.
- a mercury porosimeter for example, “AUTOPOREIV 9500” manufactured by MICROMERITICS.
- the volume of mercury injected into the sample from a pressure (0.01 MPa) corresponding to a pore diameter of 89 ⁇ m to a maximum pressure (414 MPa: equivalent to a pore diameter of 3 nm) was measured.
- the surface tension of mercury is 484 dyne / cm
- the contact angle between mercury and carbon is 130 degrees
- the pressure P is MPa
- the relationship between the pressure P and the pore diameter D was determined.
- the pore volume having a pore diameter in the range of 50 nm to 300 nm in the present invention corresponds to the volume V of mercury injected from a mercury intrusion pressure of 4.2 MPa to 25.4 MPa.
- a container for example, “Mobicol Polypropylene Column” manufactured by Mobitech Co., Ltd.
- the membrane filter after the filtration treatment was dried at 110 ° C. for 1 hour, then allowed to cool in a desiccator for 30 minutes, and weighed to 0.1 mg with a precision chemical balance.
- Indoxyl sulfate adsorption rate (%) (C 0 -C) / C 0 ⁇ 100
- C 0 Indoxyl sulfate initial concentration before treatment
- C Indoxyl sulfate residual concentration after treatment
- Indoxyl sulfate initial concentration before treatment C 0 is obtained by adding 0.1 mL of physiological saline to a round bottom tube made of polypropylene (PP). After mixing, 0.9 mL of the test solution was added and immediately centrifuged at 3000 g for 10 minutes using a centrifuge, and the supernatant was obtained as a pre-treatment plasma sample. The indoxyl sulfate concentration in the bovine blood at this time was 3.5 to 3.8 mg / dL.
- the initial albumin concentration and the residual concentration were measured using a biochemical automatic analyzer (for example, “Unicel DxC600 Clinical System” manufactured by Beckman Coulter, Inc.), and the albumin adsorption rate was calculated using the above-described adsorption rate calculation formula. Calculated.
- the initial adsorption rate of indoxyl sulfate was calculated by dividing the amount of adsorbed indoxyl sulfate after 5 minutes by time (5 minutes).
- Adsorption test 2 immersion method
- adsorption experiments of indole-3-acetic acid, phenylsulfuric acid, p-cresylsulfuric acid, and hippuric acid were performed by the following method.
- a plasma sample after treatment was obtained in the same manner as in adsorption test 1 except that a 5-component mixed solution obtained by adding the above-mentioned 4 components was added instead of adding an aqueous solution of indoxyl sulfate.
- the adsorption rate was calculated in the same manner as indoxyl sulfate.
- a mini-module was manufactured by filling 200 mg of the activated carbon sample into a container (for example, “Mobicol Polypropylene Column” manufactured by Mobitech Co., Ltd.) having an inlet for inflow and an outlet for outflow.
- An aqueous solution of indoxyl sulfate (potassium salt, manufactured by Sigma-Aldrich) prepared to 5 mg / mL was added to bovine blood anticoagulated by addition of heparin (10 U / mL) to prepare a test solution.
- test solution was passed through the minimodule using a peristaltic pump, the test solution flowing out from the outlet was collected 30 minutes later, centrifuged at 3000 g for 10 minutes using a centrifuge, and the supernatant was obtained as a plasma sample after processing.
- the indoxyl sulfate adsorption rate was calculated in the same manner as in adsorption test 1.
- Example 1 A 70 kg petroleum pitch with a softening point of 205 ° C. and an H / C atomic ratio of 0.65 and 30 kg of naphthalene are charged into a 300 liter pressure vessel equipped with a stirring blade and an outlet nozzle, and heated, melted and mixed at 190 ° C. After cooling to 80 to 90 ° C., the inside of the pressure vessel was pressurized with nitrogen gas, and the contents were extruded from the outlet nozzle to obtain a string-like molded body having a diameter of about 500 ⁇ m.
- this string-like molded body was pulverized so that the ratio (L / D) of the diameter (D) to the length (L) was about 1.5, and the obtained crushed material was heated to 93 ° C.
- the solution was poured into an aqueous solution in which 53% by mass of polyvinyl alcohol (saponification degree 88%) was dissolved, stirred and dispersed, and cooled to obtain a spherical pitch molded body slurry. After most of the water was removed by filtration, naphthalene in the pitch formed body was extracted and removed with n-hexane having a mass about 6 times that of the spherical pitch formed body.
- the porous spherical pitch obtained in this manner was heated to 260 ° C. while passing through heated air using a fluidized bed, oxidized at a temperature of 260 ° C. for 2 hours, and insoluble to heat. Spherical oxidized pitch was obtained.
- a porous spherical oxidation pitch is put in a vertical tubular furnace having a diameter of 50 mm, heated to 200 ° C./h up to 850 ° C. under nitrogen gas flow, held at 850 ° C. for 1 hour, and pre-baked.
- a carbon precursor was obtained.
- the obtained carbon precursor was subjected to activation treatment at 850 ° C. in a nitrogen gas containing 50 vol% of water vapor until the bulk density became 0.46 g / cm 3 to obtain spherical activated carbon 1.
- Example 2 A porous spherical pitch similar to that in Example 1 was heated to 240 ° C. while passing through heated air using a fluidized bed, and maintained at 240 ° C. for 2 hours to oxidize and porous infusible to heat. Spherical oxidized pitch was obtained. Next, 40 g of porous spherical oxidation pitch is put in a tubular furnace, heated to 1350 ° C. at 250 ° C./h under a nitrogen gas flow, and kept at 1350 ° C. for 4 hours to carry out preliminary firing to obtain a carbon precursor. It was. Next, the obtained carbon precursor was subjected to activation treatment at 900 ° C. in a nitrogen gas containing 50 vol% of water vapor until the bulk density became 0.47 g / cm 3 to obtain spherical activated carbon 2.
- Example 3 Spherical activated carbon 3 was obtained in the same manner as in Example 1 except that the activation treatment was performed until the bulk density reached 0.60 g / cm 3 .
- Example 4 A porous spherical pitch similar to that in Example 1 was heated to 210 ° C. while passing through heated air using a fluidized bed, oxidized at a temperature of 210 ° C. for 2 hours, and insoluble to heat. Spherical oxidized pitch was obtained. 100 g was put into a vertical tubular furnace having a diameter of 50 mm, heated to 200 ° C./h up to 850 ° C. under a nitrogen gas flow, and kept at 850 ° C. for 1 hour to perform preliminary firing to obtain a carbon precursor. Next, the obtained carbon precursor was subjected to activation treatment at 850 ° C. in nitrogen gas containing 50 vol% of water vapor until the bulk density became 0.60 g / cm 3 to obtain spherical activated carbon 4.
- Example 5 40 g of porous spherical oxidation pitch similar to that in Example 4 was put in a tubular furnace, heated to 1200 ° C. at 250 ° C./h under nitrogen gas flow, held at 1200 ° C. for 4 hours, pre-fired, and carbon A precursor was obtained. Next, the obtained carbon precursor was subjected to activation treatment at 900 ° C. in a nitrogen gas containing 50 vol% of water vapor until the bulk density became 0.45 g / cm 3 to obtain spherical activated carbon 5.
- Example 6 Spherical white birch (manufactured by Osaka Gas Chemical Company) was used as the activated carbon.
- this activated carbon is not manufactured as a lump, but is obtained by granulating finely powdered activated carbon with a binder.
- Example 7 Petroleum pitch and SiO 2 were homogeneously mixed with each other at a weight ratio of 25:75, stirred while heating at 300 ° C., and then cooled to room temperature to prepare a composite pitch material in which SiO 2 was dispersed.
- the obtained composite pitch was roughly pulverized with a hammer mill and then pulverized with a jet mill (Hosokawa Micron Corporation / 100-AFG) to obtain a finely pulverized composite pitch.
- the finely pulverized composite pitch was heated to an air atmosphere of 260 ° C. and held at 260 ° C. for 1 hour to perform an infusibilization treatment.
- the obtained infusible finely pulverized composite pitch was held at 650 ° C. for 1 hour, and then treated with a hydrofluoric acid aqueous solution to remove SiO 2 to obtain a porous carbon material. After putting 30 g of this porous carbon material in a crucible and holding it at 850 ° C. for 1 hour under a nitrogen gas flow in a vertical tubular furnace, the bulk density becomes 0.16 g / cm 3 in nitrogen gas containing 50 vol% of water vapor. Until then, activation treatment was performed at 850 ° C., and powdered activated carbon 1 was obtained.
- Example 8 Powdered activated carbon 2 was obtained in the same manner as in Example 7 except that the activation treatment was performed until the bulk density became 0.20 g / cm 3 .
- Example 9 30 g of a phenol resin (Asahi Organic Material: BEAPS) was put in a crucible, and pre-baked by holding at 850 ° C. for 1 hour under a nitrogen gas flow in a vertical tubular furnace to obtain a carbon precursor. Next, the obtained carbon precursor was subjected to activation treatment at 850 ° C. in nitrogen gas containing 50 vol% of water vapor until the bulk density became 0.51 g / cm 3 to obtain spherical activated carbon 8.
- a phenol resin Asahi Organic Material: BEAPS
- Example 10 In place of the spherical activated carbon, activated carbon fiber (Soene Co.) was used.
- Example 11 220 g of deionized water and 58 g of methylcellulose were placed in a 1 L separable flask, and 105 g of styrene, 57% divinylbenzene (57% divinylbenzene and 43% ethylvinylbenzene) 184 g, 2,2'-azobis 2.68 g of 2,4-dimethylvaleronitrile) and 63 g of 1-butanol as a porogen were appropriately added. Next, the inside of the system was replaced with nitrogen gas, and this two-phase system was stirred at 150 rpm, heated to 55 ° C., and held for 20 hours.
- the obtained resin was filtered and dried on a rotary evaporator, and then 1-butanol was removed from the resin by distillation in a vacuum dryer, and then dried at 90 ° C. for 12 hours, and spherical porous particles having an average particle size of 180 ⁇ m.
- a synthetic resin was obtained. 100 g of the obtained spherical porous synthetic resin was charged into a reaction tube with a mesh dish and subjected to infusibilization treatment in a vertical tubular furnace.
- the infusibilization condition is that a spherical porous oxide resin is obtained by flowing dry air from the lower part of the reaction tube to the upper part at 3 L / min, raising the temperature to 260 ° C.
- the bulk density is 0.50 g / cm 3 at 820 ° C. in a nitrogen gas atmosphere containing 64.5 vol% of water vapor using a fluidized bed.
- the activation treatment was performed until the spherical activated carbon 9 was obtained.
- Powdered activated carbon 3 was obtained in the same manner as in Example 7 except that the activation treatment was not performed.
- Example 8 having a large macropore surface area had a higher adsorption rate (initial adsorption rate).
- indole-3-acetic acid, phenylsulfuric acid, p-cresylsulfuric acid, and hippuric acid also showed the same adsorption as indoxylsulfuric acid.
- the same results as in the case of indoxyl sulfate were obtained.
- the albumin adsorption rate was 0% in experiments using indole-3-acetic acid, phenylsulfuric acid, p-cresylsulfuric acid, and hippuric acid.
- Table 4 shows the results of the liquid passing method. Here, the result after 30 minutes of Example 1 is shown as a representative.
- the present invention can be used for hemodialysis and the like.
Landscapes
- Chemical & Material Sciences (AREA)
- Health & Medical Sciences (AREA)
- Analytical Chemistry (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Organic Chemistry (AREA)
- Engineering & Computer Science (AREA)
- Heart & Thoracic Surgery (AREA)
- Vascular Medicine (AREA)
- Nanotechnology (AREA)
- Life Sciences & Earth Sciences (AREA)
- Biomedical Technology (AREA)
- Public Health (AREA)
- Anesthesiology (AREA)
- Hematology (AREA)
- Veterinary Medicine (AREA)
- Animal Behavior & Ethology (AREA)
- General Health & Medical Sciences (AREA)
- Inorganic Chemistry (AREA)
- Cardiology (AREA)
- Biodiversity & Conservation Biology (AREA)
- Cell Biology (AREA)
- Molecular Biology (AREA)
- Solid-Sorbent Or Filter-Aiding Compositions (AREA)
- External Artificial Organs (AREA)
- Treatment Of Liquids With Adsorbents In General (AREA)
- Carbon And Carbon Compounds (AREA)
- Medicines Containing Material From Animals Or Micro-Organisms (AREA)
- Medicines That Contain Protein Lipid Enzymes And Other Medicines (AREA)
- Medicines Containing Antibodies Or Antigens For Use As Internal Diagnostic Agents (AREA)
Priority Applications (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US16/969,590 US10967115B2 (en) | 2018-03-01 | 2019-01-10 | Toxin separator |
| ES19761567T ES2917609T3 (es) | 2018-03-01 | 2019-01-10 | Separador de toxinas |
| EP19761567.7A EP3760247B1 (en) | 2018-03-01 | 2019-01-10 | Toxin separator |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2018036930A JP7008540B2 (ja) | 2018-03-01 | 2018-03-01 | 毒素分離器具 |
| JP2018-036930 | 2018-03-01 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2019167441A1 true WO2019167441A1 (ja) | 2019-09-06 |
Family
ID=67804985
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/JP2019/000595 Ceased WO2019167441A1 (ja) | 2018-03-01 | 2019-01-10 | 毒素分離器具 |
Country Status (6)
| Country | Link |
|---|---|
| US (1) | US10967115B2 (enExample) |
| EP (1) | EP3760247B1 (enExample) |
| JP (1) | JP7008540B2 (enExample) |
| ES (1) | ES2917609T3 (enExample) |
| TW (1) | TWI712414B (enExample) |
| WO (1) | WO2019167441A1 (enExample) |
Cited By (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2021147252A (ja) * | 2020-03-17 | 2021-09-27 | 株式会社クラレ | 活性炭およびそれを用いたカビ臭を抑制する方法 |
| JPWO2021229885A1 (enExample) * | 2020-05-11 | 2021-11-18 | ||
| WO2023085229A1 (ja) * | 2021-11-15 | 2023-05-19 | パナソニックIpマネジメント株式会社 | 脱硫剤 |
Families Citing this family (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| ES2971056T3 (es) * | 2018-07-25 | 2024-06-03 | Univ Twente | Composiciones para usar en la eliminación simultánea de endotoxinas y solutos urémicos durante el tratamiento de pacientes |
| WO2025027992A1 (ja) * | 2023-08-03 | 2025-02-06 | フタムラ化学株式会社 | 活性炭吸着剤 |
| JP7610765B1 (ja) * | 2023-08-03 | 2025-01-08 | フタムラ化学株式会社 | 活性炭吸着剤 |
| CN119432563B (zh) * | 2025-01-10 | 2025-06-03 | 泰州市食品检验院 | 一种蜡样芽孢杆菌呕吐毒素分离装置及培养方法 |
Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2004256324A (ja) | 2003-02-24 | 2004-09-16 | Kureha Chem Ind Co Ltd | 血液の直接灌流用球状活性炭及びその製造方法 |
| JP2008303193A (ja) * | 2007-06-11 | 2008-12-18 | Teikoku Medix Kk | 医療用吸着剤 |
| WO2016031908A1 (ja) | 2014-08-27 | 2016-03-03 | 株式会社クレハ | 経口投与用吸着剤並びに腎疾患治療剤及び肝疾患治療剤 |
| JP2016117650A (ja) * | 2013-02-22 | 2016-06-30 | 株式会社クレハ | 経口投与用吸着剤並びに腎疾患治療剤及び肝疾患治療剤 |
Family Cites Families (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| GB0921528D0 (en) * | 2009-12-09 | 2010-01-27 | Mast Carbon Internat Ltd | Carbon and its use in blood cleansing applications |
| TW201440819A (zh) * | 2013-02-22 | 2014-11-01 | Kureha Corp | 經口投予用吸附劑及腎疾病治療劑及肝疾病治療劑 |
-
2018
- 2018-03-01 JP JP2018036930A patent/JP7008540B2/ja active Active
-
2019
- 2019-01-10 US US16/969,590 patent/US10967115B2/en active Active
- 2019-01-10 ES ES19761567T patent/ES2917609T3/es active Active
- 2019-01-10 EP EP19761567.7A patent/EP3760247B1/en active Active
- 2019-01-10 WO PCT/JP2019/000595 patent/WO2019167441A1/ja not_active Ceased
- 2019-01-22 TW TW108102343A patent/TWI712414B/zh active
Patent Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2004256324A (ja) | 2003-02-24 | 2004-09-16 | Kureha Chem Ind Co Ltd | 血液の直接灌流用球状活性炭及びその製造方法 |
| JP2008303193A (ja) * | 2007-06-11 | 2008-12-18 | Teikoku Medix Kk | 医療用吸着剤 |
| JP2016117650A (ja) * | 2013-02-22 | 2016-06-30 | 株式会社クレハ | 経口投与用吸着剤並びに腎疾患治療剤及び肝疾患治療剤 |
| WO2016031908A1 (ja) | 2014-08-27 | 2016-03-03 | 株式会社クレハ | 経口投与用吸着剤並びに腎疾患治療剤及び肝疾患治療剤 |
Cited By (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2021147252A (ja) * | 2020-03-17 | 2021-09-27 | 株式会社クラレ | 活性炭およびそれを用いたカビ臭を抑制する方法 |
| JP7477999B2 (ja) | 2020-03-17 | 2024-05-02 | 株式会社クラレ | 活性炭およびそれを用いたカビ臭を抑制する方法 |
| JPWO2021229885A1 (enExample) * | 2020-05-11 | 2021-11-18 | ||
| WO2021229885A1 (ja) * | 2020-05-11 | 2021-11-18 | 株式会社クレハ | タンパク質の精製方法、カラム、キットおよび活性炭粒子 |
| WO2023085229A1 (ja) * | 2021-11-15 | 2023-05-19 | パナソニックIpマネジメント株式会社 | 脱硫剤 |
Also Published As
| Publication number | Publication date |
|---|---|
| TW201936202A (zh) | 2019-09-16 |
| JP7008540B2 (ja) | 2022-01-25 |
| EP3760247B1 (en) | 2022-05-18 |
| US10967115B2 (en) | 2021-04-06 |
| EP3760247A4 (en) | 2021-05-05 |
| US20210001034A1 (en) | 2021-01-07 |
| JP2019150261A (ja) | 2019-09-12 |
| ES2917609T3 (es) | 2022-07-11 |
| TWI712414B (zh) | 2020-12-11 |
| EP3760247A1 (en) | 2021-01-06 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| WO2019167441A1 (ja) | 毒素分離器具 | |
| JP4908216B2 (ja) | 水不溶性微粒子を除去した吸着材を充填した直接血液灌流用吸着器、および水不溶性微粒子を除去した直接血液灌流用吸着材の取得方法 | |
| AU2013349976B2 (en) | Filter device combining beads and fibers | |
| AU2013349975B2 (en) | Integrated device for liver support system | |
| EP1993631B1 (de) | Regenerierbares filter zur extrakorporalen behandlung partikelhaltiger flüssigkeiten und deren anwendung | |
| US20100276359A1 (en) | Adsorption column for purifying body fluid | |
| JPWO2017082423A1 (ja) | 血液処理用リン吸着剤、血液処理システム及び血液処理方法 | |
| JP4578405B2 (ja) | 全血処理が可能な低密度リポ蛋白およびフィブリノーゲンの吸着材、及び吸着器 | |
| CN113613776B (zh) | 血液净化器 | |
| WO1997027889A1 (en) | Adsorbent for disease-related factors in body fluids, method of elimination by adsorption, body fluid purifier, and apparatus for purifying body fluids | |
| JP6621414B2 (ja) | 濾過装置 | |
| EP4007618A1 (en) | Devices, systems and methods for the broad-spectrum reduction of pro-inflammatory cytokines in blood | |
| WO2020218291A1 (ja) | 可溶性腫瘍壊死因子受容体の吸着材料 | |
| JP5017996B2 (ja) | 白血球およびサイトカインの吸着器 | |
| JP7125892B2 (ja) | アミロイドβ除去器具、生体由来液浄化システム、アミロイドβ除去方法およびアミロイドβ除去用吸着材 | |
| JP2008206753A (ja) | 炎症性疾患処置カラム | |
| WO2005099789A1 (en) | Treatment of sepsis | |
| JP4859520B2 (ja) | 血圧降下性ペプチドの吸着材、吸着方法および吸着装置 | |
| CN113631256B (zh) | 血液净化器 | |
| JP4837221B2 (ja) | 強心配糖体の吸着材、吸着除去方法および吸着器 | |
| CN108175888A (zh) | 血液毒素吸附器 | |
| JP2014128451A (ja) | 血液浄化システム |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 19761567 Country of ref document: EP Kind code of ref document: A1 |
|
| DPE1 | Request for preliminary examination filed after expiration of 19th month from priority date (pct application filed from 20040101) | ||
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2019761567 Country of ref document: EP |
|
| ENP | Entry into the national phase |
Ref document number: 2019761567 Country of ref document: EP Effective date: 20201001 |