WO2019078220A1 - 定量噴射型エアゾール製品および定量噴射型エアゾール製品の噴射方法 - Google Patents
定量噴射型エアゾール製品および定量噴射型エアゾール製品の噴射方法 Download PDFInfo
- Publication number
- WO2019078220A1 WO2019078220A1 PCT/JP2018/038549 JP2018038549W WO2019078220A1 WO 2019078220 A1 WO2019078220 A1 WO 2019078220A1 JP 2018038549 W JP2018038549 W JP 2018038549W WO 2019078220 A1 WO2019078220 A1 WO 2019078220A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- aerosol
- injection
- aerosol product
- propellant
- valve
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Ceased
Links
Images
Classifications
-
- A—HUMAN NECESSITIES
- A01—AGRICULTURE; FORESTRY; ANIMAL HUSBANDRY; HUNTING; TRAPPING; FISHING
- A01N—PRESERVATION OF BODIES OF HUMANS OR ANIMALS OR PLANTS OR PARTS THEREOF; BIOCIDES, e.g. AS DISINFECTANTS, AS PESTICIDES OR AS HERBICIDES; PEST REPELLANTS OR ATTRACTANTS; PLANT GROWTH REGULATORS
- A01N25/00—Biocides, pest repellants or attractants, or plant growth regulators, characterised by their forms, or by their non-active ingredients or by their methods of application, e.g. seed treatment or sequential application; Substances for reducing the noxious effect of the active ingredients to organisms other than pests
- A01N25/02—Biocides, pest repellants or attractants, or plant growth regulators, characterised by their forms, or by their non-active ingredients or by their methods of application, e.g. seed treatment or sequential application; Substances for reducing the noxious effect of the active ingredients to organisms other than pests containing liquids as carriers, diluents or solvents
- A01N25/04—Dispersions, emulsions, suspoemulsions, suspension concentrates or gels
- A01N25/06—Aerosols
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/10—Dispersions; Emulsions
- A61K9/12—Aerosols; Foams
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L9/00—Disinfection, sterilisation or deodorisation of air
- A61L9/01—Deodorant compositions
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L9/00—Disinfection, sterilisation or deodorisation of air
- A61L9/015—Disinfection, sterilisation or deodorisation of air using gaseous or vaporous substances, e.g. ozone
- A61L9/04—Disinfection, sterilisation or deodorisation of air using gaseous or vaporous substances, e.g. ozone using substances evaporated in the air without heating
- A61L9/12—Apparatus, e.g. holders, therefor
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B65—CONVEYING; PACKING; STORING; HANDLING THIN OR FILAMENTARY MATERIAL
- B65D—CONTAINERS FOR STORAGE OR TRANSPORT OF ARTICLES OR MATERIALS, e.g. BAGS, BARRELS, BOTTLES, BOXES, CANS, CARTONS, CRATES, DRUMS, JARS, TANKS, HOPPERS, FORWARDING CONTAINERS; ACCESSORIES, CLOSURES, OR FITTINGS THEREFOR; PACKAGING ELEMENTS; PACKAGES
- B65D83/00—Containers or packages with special means for dispensing contents
- B65D83/14—Containers for dispensing liquid or semi-liquid contents by internal gaseous pressure, i.e. aerosol containers comprising propellant
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B65—CONVEYING; PACKING; STORING; HANDLING THIN OR FILAMENTARY MATERIAL
- B65D—CONTAINERS FOR STORAGE OR TRANSPORT OF ARTICLES OR MATERIALS, e.g. BAGS, BARRELS, BOTTLES, BOXES, CANS, CARTONS, CRATES, DRUMS, JARS, TANKS, HOPPERS, FORWARDING CONTAINERS; ACCESSORIES, CLOSURES, OR FITTINGS THEREFOR; PACKAGING ELEMENTS; PACKAGES
- B65D83/00—Containers or packages with special means for dispensing contents
- B65D83/14—Containers for dispensing liquid or semi-liquid contents by internal gaseous pressure, i.e. aerosol containers comprising propellant
- B65D83/44—Valves specially adapted for the discharge of contents; Regulating devices
- B65D83/52—Metering valves; Metering devices
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B05—SPRAYING OR ATOMISING IN GENERAL; APPLYING FLUENT MATERIALS TO SURFACES, IN GENERAL
- B05B—SPRAYING APPARATUS; ATOMISING APPARATUS; NOZZLES
- B05B9/00—Spraying apparatus for discharge of liquids or other fluent material, without essentially mixing with gas or vapour
Definitions
- the present invention relates to a metered dose aerosol product and a method for jetting a metered dose aerosol product. More particularly, the present invention relates to a metered-dose aerosol product and a method for jetting a metered-dose aerosol product, wherein the jetted aerosol composition is easily visible and the volatile compounds can be widely diffused.
- the present invention has been made in view of such conventional problems, and it is possible to make the jetted aerosol composition easy to be seen and to spread the aerosol composition widely, and the metered jet aerosol product and the measurement can be made. It is an object of the present invention to provide a method of injecting a jet aerosol product.
- the inventor of the present invention diligently studied the conditions for solving the above problems and obtained the following findings. That is, the undiluted solution and the propellant are contained so as to be a specific blending ratio, and by adjusting the amount of injection once to a specific amount, the injected aerosol is enhanced while enhancing the visibility of the injected aerosol composition. The inventors have found that the composition can be widely diffused, and complete the present invention.
- the quantitative injection-type aerosol product of the present invention for solving the above problems comprises a stock solution and a propellant, and the blending ratio (volume ratio) of the stock solution and the propellant is 1/99 to 10/90, It is a metered-dose aerosol product with an injection volume of 1.0 to 3.0 mL per injection.
- the injection method of the fixed quantity injection type aerosol product of the present invention which solves the above-mentioned subject, the blending ratio (volume ratio) of the undiluted solution, the propellant, and the undiluted solution and the propellant
- the aerosol composition according to the present invention is a method for injecting a metered-dose aerosol product, wherein the aerosol composition is injected so that the injection amount per injection is 1.0 to 3.0 mL.
- the present invention it is possible to provide a metered-dose aerosol product and a method for jetting a metered-dose aerosol product, in which the jetted aerosol composition is easily visible and the aerosol composition can be widely diffused. .
- FIG. 1 is a schematic view for explaining the method of measuring the drug diffusion rate in the examples.
- FIG. 2 is a schematic view for explaining the method of measuring the drug diffusion rate in the example.
- FIG. 3 is a photograph explaining the injection state of the aerosol composition of Example 4 evaluated as (circle) in visibility evaluation.
- FIG. 4 is a photograph explaining the injection state of the aerosol composition of Comparative Example 1 evaluated as x in visibility evaluation.
- an aerosol container filled with an aerosol composition containing a stock solution and a propellant, and an aerosol meter valve attached to the aerosol container.
- a stem mechanism attached to the aerosol metering valve, and a stem mechanism and an injection button for operating the aerosol metering valve.
- the blend ratio (volume ratio) of the stock solution and the propellant is 1/99 to 10/90.
- the injection amount per injection is 1.0 to 3.0 mL.
- the aerosol product of the present embodiment is characterized in that the mixing ratio of the undiluted solution to the propellant and the injection amount of the aerosol composition per one time are adjusted to be in the above-described specific range.
- the stock solution may incorporate the desired components to be diffused. Such components are not particularly limited.
- the stock solution suitably contains a volatile compound having a vapor pressure of 1.0 ⁇ 10 ⁇ 5 mmHg (25 ° C.) or more.
- the volatile compound having a vapor pressure of 1.0 ⁇ 10 ⁇ 5 mmHg (25 ° C.) or more is not particularly limited.
- the vapor pressure of the volatile compound is preferably 1.0 ⁇ 10 ⁇ 5 mmHg (25 ° C.) or more, more preferably 1.5 ⁇ 10 ⁇ 5 mmHg (25 ° C.) or more, 2.0 More preferably, it is not less than 10 -5 mmHg (25 ° C).
- the vapor pressure of the volatile compound is preferably 5.0 ⁇ 10 ⁇ 3 mmHg (25 ° C.) or less, more preferably 3.0 ⁇ 10 ⁇ 3 mmHg (25 ° C.) or less.
- the type of volatile compound is not particularly limited.
- the volatile compound is appropriately selected so that the aerosol product of the present embodiment can impart the intended medicinal effect.
- the volatile compounds include insecticidal compounds such as pyrethroid compounds, carbamate compounds, and oxadiazole compounds.
- the volatile compounds include perfume compounds such as menthol, citral, geraniol and the like.
- the volatile compounds include antifungal compounds such as isothiocyanate antifungal agents and phenolic antifungal agents. These volatile compounds may be used in combination. That is, for example, when the aerosol product is used for pest control and fragrance, the respective volatile compounds may be used in combination.
- Pyrethroid compounds include metfluthrin (vapor pressure: 1.5 ⁇ 10 ⁇ 5 mmHg (25 ° C.), profluthrin (vapor pressure: 7.7 ⁇ 10 ⁇ 5 mmHg (25 ° C.)), transfluthrin (vapor pressure: 2) 7 ⁇ 10 ⁇ 5 mmHg (25 ° C.), terralessrin (vapor pressure: 6.6 ⁇ 10 ⁇ 4 mmHg (25 ° C.), etc.
- the insecticidal effect is high and the effect of the present invention can be obtained.
- the pyrethroid-based compound preferably contains transfluthrin.
- the carbamate compounds include propoxur (vapor pressure: 2.1 ⁇ 10 ⁇ 5 mmHg (25 ° C.)), carbaryl (vapor pressure: 4.1 ⁇ 10 ⁇ 5 mmHg (23.5 ° C.)) and the like.
- carbamate compounds are preferably propoxur because they have high vapor pressure and high insecticidal activity.
- the vapor pressure of carbaryl is 4.1 ⁇ 10 ⁇ 5 mmHg or more at 25 ° C.
- the oxadiazole compound is, for example, methoxadiazone (vapor pressure: 1.1 ⁇ 10 ⁇ 3 mmHg (25 ° C.)) and the like.
- insecticidal compounds are dichlorvos (vapor pressure: 1.6 ⁇ 10 ⁇ 2 mmHg (25 ° C.)) as an organophosphate agent system, and hydroprene (immature pressure: 1.9) which is a juvenile hormone-like substance. It may be ⁇ 10 ⁇ 4 mmHg (25 ° C.)), amidoflumeth (vapor pressure: 1.1 ⁇ 10 ⁇ 3 mmHg (20 ° C.), etc. The vapor pressure of amidoflumetho is at least 1.1 ⁇ 10 ⁇ 3 mmHg at 25 ° C.
- insecticidal compounds for example, cockroaches such as the black-nosed cockroach, the cockroach, the cockroach, the cockroach, the cockroach, the cockroach, the spider, the centipede, the ants, the geese, the pokeweed, the louses, the louse beetles, the termites, the worms, the mites Insect pests such as fleas, bedbugs and lice, fly pests such as mosquitoes, flies, moths, bees, bugs, cutworms, beetles, bark beetles, moths, moths and the like can be suitably controlled.
- cockroaches such as the black-nosed cockroach, the cockroach, the cockroach, the cockroach, the cockroach, the cockroach, the spider, the centipede, the ants, the geese, the pokeweed, the louses, the louse beetles, the termites, the
- the perfume compound is menthol (vapor pressure: 6.3 ⁇ 10 ⁇ 2 mmHg (25 ° C.)), citral (vapor pressure: 9.1 ⁇ 10 ⁇ 2 mmHg (mPa), for reasons such as high palatability and low threshold. 25 ° C.)), geraniol (vapor pressure: 3.0 ⁇ 10 ⁇ 2 mmHg (25 ° C.)), and the like.
- the isothiocyanate antibacterial agents are methyl isothiocyanate (vapor pressure: 16 mmHg (30 ° C.)), allyl isothiocyanate (vapor pressure: 3.7 mmHg (20 ° C.)) and the like.
- the isothiocyanate antibacterial agent is preferably allyl isothiocyanate.
- the vapor pressure of methyl isothiocyanate is 1.0 ⁇ 10 ⁇ 5 mmHg or more at 25 ° C.
- the vapor pressure of allyl isothiocyanate is 3.7 mmHg or more at 25 ° C.
- Phenolic antibacterial agents include thymol (vapor pressure: 2.2 ⁇ 10 ⁇ 3 mmHg (25 ° C.)), isopropylmethylphenol (vapor pressure: 2.0 ⁇ 10 ⁇ 3 mmHg (20 ° C.)) and the like.
- the phenol-based antibacterial agent is preferably isopropylmethylphenol.
- the vapor pressure of isopropylmethylphenol is at least 2.0 ⁇ 10 ⁇ 3 mmHg at 25 ° C.
- the content of the volatile compound is not particularly limited.
- the content of the volatile compound may be appropriately adjusted depending on the type of the volatile compound.
- the content of the insecticidal compound is preferably 0.1 w / v% or more, more preferably 10 w / v% or more in the stock solution.
- the content of the insecticidal compound may be 100 w / v% in the stock solution.
- the aerosol product may not sufficiently exert the desired insecticidal effect or may not be spread widely.
- the content of the perfume compound is preferably 0.05 w / v% or more, more preferably 10 w / v% or more in the stock solution.
- the content of the flavor compound may be 100 w / v% in the stock solution. If the content of the perfume compound is less than 1 w / v%, the aerosol product may not be able to provide sufficient aroma or be less likely to be spread widely.
- the content of the antibacterial compound is preferably 1 w / v% or more, and more preferably 10 w / v% or more in the stock solution.
- the content of the antibacterial compound may be 100 w / v% in the stock solution.
- the aerosol product may not sufficiently exhibit the desired antibacterial effect or may not be easily diffused widely.
- the stock solution may optionally contain a solvent.
- a solvent may be contained to appropriately dissolve the volatile compound to facilitate mixing with the propellant in the aerosol container or to aid diffusion of the volatile compound into the room.
- the solvent is not particularly limited.
- the solvent is ethanol, isopropyl alcohol (IPA), propylene glycol monomethyl ether, isopropyl myristate (IPM) or the like.
- the solvent is preferably ethanol or IPA from the viewpoint of being easy to dissolve the volatile compound, being inexpensive, and easy to handle.
- the content of the solvent in the stock solution is preferably more than 0% by mass, and more preferably 10% by mass or more.
- the content of the solvent in the stock solution is preferably 99% by mass or less, and more preferably 90% by mass or less.
- the content of the solvent exceeds 99% by mass, the amount of the volatilizable compound in the aerosol product decreases, and the effect of the combination of the volatilizable compound may not be sufficiently obtained.
- the stock solution may contain optional components in addition to the above-mentioned volatilizable compound and solvent suitably blended.
- optional components are non-volatile or non-volatile compounds; nonionic, anionic or cationic surfactants; antioxidants such as butyl hydroxytoluene; stabilizers such as citric acid, ascorbic acid; And inorganic powders such as talc and silicic acid, deodorants, pigments and the like.
- the propellant is the content that is pressure-filled into the aerosol container with the stock solution.
- the propellant is injected from the injection port of the injection button described later together with the undiluted solution by operating an aerosol metering valve described later.
- the propellant imparts a propulsive force at the time of spraying the stock solution, and atomizes the aerosol composition and diffuses it into space.
- the propellant is a liquefied gas.
- liquefied gas examples include liquefied petroleum gas, aliphatic hydrocarbons having 3 to 5 carbon atoms such as propane, normal butane, isobutane, normal pentane, and isopentane, and trans-1,3,3,3-tetrafluoroprop-1-ene. Examples thereof include hydrofluoroolefins such as ene and trans-2,3,3,3-tetrafluoroprop-1-ene, dimethyl ether, and mixtures thereof.
- a liquefied gas may be used in combination with a compressed gas. Examples of the compressed gas include nitrogen gas, carbon dioxide gas, nitrous oxide gas, compressed air and the like.
- the propellant is preferably liquefied petroleum gas.
- the mixing ratio (volume ratio) of the undiluted solution to the propellant at 20 ° C. may be 1/99 to 10/90, preferably 1/99 to 9/91, and 1 More preferably, it is 5 / 98.5 to 8/92.
- the blending ratio (volume ratio) of the propellant exceeds 99% by volume, the aerosol composition may have a small amount of the active ingredient and may hardly exhibit its effect.
- the blending ratio (volume ratio) of the propellant is less than 90% by volume, the aerosol composition may have a reduced diffusivity after the injection.
- the internal pressure of the aerosol metering valve at 25 ° C. is preferably 0.2 MPa or more, more preferably 0.25 MPa or more, in the state of being filled with the stock solution and the propellant.
- the internal pressure of the aerosol metering valve at 25 ° C. is preferably 0.8 MPa or less, more preferably 0.7 MPa or less.
- the aerosol composition may drip from the injection port after injection.
- the internal pressure of the aerosol metering valve exceeds 0.8 MPa, the aerosol composition may leak from the aerosol container.
- the internal pressure of the aerosol metering valve can be determined, for example, by PGM-E compact pressure sensor (manufactured by Kyowaden Kogyo) attached to a WGA-710C instrumentation conditioner (manufactured by Kyowaden Kogyo) at 25 ° C. It can be measured by connecting to a valve.
- PGM-E compact pressure sensor manufactured by Kyowaden Kogyo
- WGA-710C instrumentation conditioner manufactured by Kyowaden Kogyo
- an aerosol product comprising the above-described stock solution and propellant is pressure-filled into the aerosol container.
- the aerosol container is not particularly limited.
- the aerosol container is a substantially cylindrical pressure-resistant container having an opening at the top. The opening is a filling port for filling the aerosol composition, and after being filled with the stock solution, it is closed by an aerosol metering valve described later.
- the material of the aerosol container is not particularly limited.
- the material of the aerosol container is a metal such as aluminum or tin, a synthetic resin such as polyethylene terephthalate, a pressure resistant glass, or the like.
- the aerosol metering valve is a mechanism for removing the aerosol composition filled in the aerosol container, is attached to the opening of the aerosol container, and closes the opening. Further, the aerosol metering valve of the present embodiment is formed with a metering chamber for temporarily storing the aerosol composition taken out of the aerosol container. The volume of the metering chamber corresponds to the volume of the aerosol composition sprayed by one injection. The volume of the metering chamber of the present embodiment is 1.0 to 3.0 mL.
- the stem mechanism is a part attached to the aerosol metering valve, and an internal passage is formed for sending the stock solution and the propellant taken in the aerosol metering valve to the injection button described later.
- the internal passage is suitably opened and closed by the stem rubber of the stem mechanism.
- the injection button is a member for injecting the undiluted solution taken in from the aerosol container via the stem mechanism by the aerosol metering valve together with the propellant.
- the injection button is formed with an injection hole for injecting the aerosol composition.
- the number, size and shape of the injection ports are not particularly limited. As an example, the number of injection holes may be one, or two or more. Further, the size of the injection port (injection port diameter) is preferably 0.2 mm or more, more preferably 0.3 mm or more, and still more preferably 0.5 mm or more. The diameter of the injection port is preferably 4.5 mm or less, more preferably 3.0 mm or less, and still more preferably 0.9 mm or less.
- the shape (cross-sectional shape) of the injection port may be circular, oval, square, or various irregular shapes. With respect to the total area of nozzle hole, it is preferably 0.03 mm 2 or more, more preferably 0.1 mm 2 or more, more preferably 0.25 mm 2 or more. The total area of the injection port is preferably 16 mm 2 or less, more preferably 5 mm 2 or less, and still more preferably 3.5 mm 2 or less.
- the stem mechanism and the aerosol metering valve are activated, and the inside of the aerosol container communicates with the outside.
- the aerosol composition in the aerosol container is taken out of the aerosol container in a fixed amount according to the pressure difference between the inside and the outside of the aerosol container, and is jetted from the injection port of the injection member.
- the aerosol product of the present embodiment is adjusted so that the injection amount per injection is 1.0 to 3.0 mL.
- the injection amount per injection may be 1.0 mL or more.
- the injection amount per injection may be 3.0 mL or less, preferably 2.5 mL or less, and more preferably 2.2 mL or less.
- the injection amount per injection is less than 1.0 mL, the injected aerosol composition is less visible.
- the injection amount per time exceeds 3.0 mL, the injected aerosol composition has a large particle size, and does not diffuse widely and is likely to fall.
- the aerosol product of this embodiment is injected by specifying the mixture ratio of the above-mentioned undiluted solution and propellant and adjusting the injection amount per time to be 1.0 to 3.0 mL. It is possible to achieve wide spread of the aerosol composition in the stock solution while improving the visibility of the aerosol composition. Therefore, the method of adjusting the injection amount per time described above is not particularly limited. The injection amount per injection can be adjusted, for example, by appropriately adjusting the volume of the metering chamber in the aerosol metering valve, the time when the inside of the aerosol container and the outside are in communication when the injection button is operated, and the like.
- the aerosol composition sprayed by the aerosol product of the present embodiment preferably has an injection load of 5 gf or more, more preferably 9 gf or more at a position 20 cm away from the injection port.
- the aerosol composition preferably has an injection load of 40 gf or less at a position 20 cm away from the injection port, and more preferably 30 gf or less.
- the injection load of the injected aerosol composition is a ⁇ 60 mm circular flat plate attached to a digital force gauge (model: DS2-2N, manufactured by Imada Co., Ltd.) from a distance of 20 cm from the injection port at room temperature of 25 ° C.
- the maximum value when the aerosol composition is injected horizontally toward the center can be used as the injection load, and can be calculated by calculating the average.
- the method for producing the aerosol product of the present embodiment is not particularly limited.
- an aerosol product is manufactured by filling the aerosol container with the undiluted solution, closing the opening of the aerosol container by means of an aerosol metering valve provided with a metering chamber, and pressure filling the propellant via a stem mechanism.
- the injection button may be a trigger button, a push-down button, or the like.
- the sprayed aerosol composition is easily visible, and the volatile compound is widely diffused.
- the injection method (hereinafter, also referred to as injection method) of the fixed injection type aerosol product according to one embodiment of the present invention is such that the mixing ratio (volume ratio) of the stock solution and the propellant is 1/99 to In this method, an aerosol composition containing 10/90 is injected so that the injection amount per injection becomes 1.0 to 3.0 mL.
- the aerosol composition and the aerosol product may be similar to those described in the above embodiment.
- the number of injections may be one. According to the injection method of the present embodiment, since the injection amount per injection is 1.0 to 3.0 mL, the user visually recognizes the injected aerosol composition even if the number of injections is one. It's easy to do. In addition, even if the number of injections is one, the injected aerosol composition is easy to spread and can be widely effective. According to the injection method of the present embodiment, the desired effect can be obtained even if the number of injections is one, but if necessary, the number of injections may be two or more.
- the injected aerosol composition is easily visible, and the volatile compound is diffused in a wide range.
- Aerosol 1 Button diameter ⁇ 0.6 mm button, valve 0.2 cc valve aerosol 2: Button diameter ⁇ 0.6 mm button, valve 1.0 cc valve aerosol 3: Button diameter ⁇ 1.0 mm button, valve 1.0 cc valve aerosol 4 : Button injection diameter ⁇ 2.0 mm button, valve 1.0 cc valve aerosol 5: Button injection diameter ⁇ 2.0 mm button, valve 2.2 cc valve aerosol 6: button injection diameter ⁇ 0.5 mm ⁇ 8 buttons, valve 1.0 cc valve aerosol 7: Button injection diameter ⁇ 1.0 mm ⁇ 3 buttons, valve 1.0 cc valve
- Example 1 100 parts by mass of transfluthrin as a volatile compound (stock solution, vapor pressure: 2.7 ⁇ 10 -5 mmHg (25 ° C)) is filled in an aerosol container with a volume of 200 mL and a valve is attached, and then the bulk and injection Pressurize the propellant (Liquefied petroleum gas saturated vapor pressure: 0.49 MPa (25 ° C)) so that the compounding ratio (volume ratio) with the agent is 1.6 / 98.4, attach the injection button and attach the aerosol product Was made (Valve, button followed aerosol 4).
- propellant Liiquefied petroleum gas saturated vapor pressure: 0.49 MPa (25 ° C)
- the internal pressure of the aerosol product (the internal pressure of the valve; the same applies hereinafter) (25 ° C.) was 0.45 MPa, and the injection load at a horizontal position 20 cm away from the injection port was 19.5 gf.
- 16 mg of drug substance is injected at a time.
- Example 2 Bulk material transfluthrin (vapor pressure: 2.7 ⁇ 10 -5 mmHg (25 ° C)) with 72 parts by weight of isopropyl alcohol (IPA) so that the concentration in the stock solution is 25% w / v.
- Propellant Liiquefied petroleum gas saturated vapor pressure: 0.49MPa
- the internal pressure (25 ° C.) of the aerosol product was 0.42 MPa, and the injection load at a horizontal position 20 cm away from the injection port was 25.4 gf.
- 16 mg of drug substance is injected at a time.
- Example 3 An aerosol product was produced in the same manner as in Example 2 except that the valve and the button were in accordance with Aerosol 5.
- the internal pressure (25 ° C.) of the aerosol product was 0.43 MPa, and the injection load at a horizontal position 20 cm away from the injection port was 24.8 gf.
- 35 mg of drug substance is injected at a time.
- Example 4 An aerosol product was produced in the same manner as in Example 2 except that the valve and the button were in accordance with Aerosol 2.
- the internal pressure (25 ° C.) of the aerosol product was 0.42 MPa, and the injection load at a horizontal position 20 cm away from the injection port was 9.4 gf.
- 16 mg of drug substance is injected at a time.
- Example 5 An aerosol product was produced in the same manner as in Example 2, except that the valve and the button were in accordance with Aerosol 3.
- the internal pressure (25 ° C.) of the aerosol product was 0.42 MPa, and the injection load at a horizontal position 20 cm away from the injection port was 11.6 gf.
- 16 mg of drug substance is injected at a time.
- Example 6 As the drug substance, transfluthrin (vapor pressure: 2.7 ⁇ 10 -5 mmHg (25 ° C)) and 31.6 parts by mass of isopropyl alcohol (so that the concentration in the stock solution is 62.5% by weight / volume) After filling an aerosol container with a volume of 200 mL with IPA and attaching a valve, the propellant (Liquefied petroleum gas saturated vapor pressure) so that the blending ratio (volume ratio) between the undiluted solution and the propellant becomes 2.6 / 97.4 : 0.49 MPa (25 ° C.)) pressurized and fitted with a spray button to make an aerosol product (valve, button followed aerosol 2).
- the propellant Liiquefied petroleum gas saturated vapor pressure
- the internal pressure (25 ° C.) of the aerosol product was 0.45 MPa, and the injection load at a horizontal position 20 cm away from the injection port was 9.8 gf.
- 16 mg of drug substance is injected at a time.
- Example 7 An aerosol product was produced in the same manner as in Example 2 except that the valve and the button were in accordance with Aerosol 6.
- the internal pressure (25 ° C.) of the aerosol product was 0.42 MPa, and the injection load at a horizontal position 20 cm away from the injection port was 21.2 gf.
- 16 mg of drug substance is injected at a time.
- Example 8 100 parts by weight of phthalthrin (vapor pressure: 4.5 ⁇ 10 ⁇ 6 mmHg (25 ° C.)) as an ingredient, and 46 parts by weight of isopropyl alcohol (IPA) so that the concentration in the stock solution is 50% by weight / volume Together with a volume of 200 mL in an aerosol container, and after installing a valve, the propellant (liquefied petroleum gas saturated vapor pressure: 0) so that the blending ratio (volume ratio) between the undiluted solution and the propellant becomes 6.4 / 93.6. Pressurized at .49 MPa (25 ° C.) and fitted with spray buttons to produce an aerosol product (valve, button followed aerosol 2).
- IPA isopropyl alcohol
- the internal pressure (25 ° C.) of the aerosol product was 0.48 MPa, and the injection load at a horizontal position 20 cm away from the injection port was 10.3 gf.
- 32 mg of drug substance is injected at a time.
- Example 9 100 parts by weight of linalool (flavouring compound, vapor pressure: 0.16 mmHg (25 ° C.)) as the drug substance, and the volume together with 69 parts by weight of isopropyl alcohol (IPA) so that the concentration in the stock solution is 25% by weight / volume
- Propellant Liiquefied petroleum gas saturated vapor pressure: 0.49MPa
- the internal pressure (25 ° C.) of the aerosol product was 0.50 MPa, and the injection load at a horizontal position 20 cm away from the injection port was 21.4 gf.
- 16 mg of drug substance is injected at a time.
- Example 10 As raw material, transfluthrin (vapor pressure: 2.7 ⁇ 10 -5 mmHg (25 ° C)) and 72.0 parts by mass of isopropyl myristate (IPM) so that the concentration in the stock solution becomes 25% by weight / volume )
- the propellant liquefied petroleum gas saturated vapor pressure: so that the blending ratio (volume ratio) between the undiluted solution and the propellant becomes 6.4 / 93.6
- the pressure was 0.49 MPa (25 ° C.)
- the spray button was attached to make an aerosol product (valve, button followed aerosol 6).
- the internal pressure (25 ° C.) of the aerosol product was 0.42 MPa, and the injection load at a horizontal position 20 cm away from the injection port was 19.5 gf.
- 16 mg of drug substance is injected at a time.
- Example 11 An aerosol product was produced in the same manner as in Example 2, except that the valve and the button were in accordance with Aerosol 7.
- the internal pressure (25 ° C.) of the aerosol product was 0.42 MPa, and the injection load at a horizontal position 20 cm away from the injection port was 19.6 gf.
- 16 mg of drug substance is injected at a time.
- the volatilizable compound diffusion rate was measured for the obtained aerosol products of Examples 1 to 11 and Comparative Examples 1 to 4 according to the following evaluation method. The results are shown in Table 1 or Table 2. In the column of “injection diameter” in Table 1 and Table 2, the number of the injection ports is also shown in the embodiment using the button in which the number of the injection ports is plural.
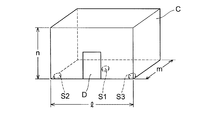
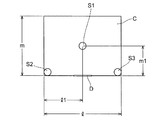
- FIGS. 1 and 2 are schematic views of the 8-compartment chamber C for explaining the measurement method of the volatile compound diffusion rate, FIG. 1 is a perspective view, and FIG. 2 is a plan view.
- the dimensions of the 8 tatami chamber C are: width l is 360 cm, depth m is 360 cm, and height n is 270 cm.
- the metal petri dish is placed at one place (metal petri dish S1, 9 9 cm stainless steel petri dish 10 units) at the center of the 8 tatami chamber C (180 cm in lateral position l1 and 180 cm in depth position m1) and the remaining 2 places (metal A petri dish S2 and a metal petri dish S3 were placed at both ends of the entrance door D side of the 8-mat chamber C, respectively.
- FIG. 3 is a photograph explaining the injection state of the aerosol composition of Example 4 evaluated as (circle) in visibility evaluation.
- FIG. 4 is a photograph explaining the injection state of the aerosol composition of Comparative Example 1 evaluated as x in visibility evaluation. (Evaluation criteria) ⁇ : The aerosol composition was visually visible. X: The aerosol composition was difficult to visually recognize.
- the aerosol product of the present invention is capable of easily visualizing the jetted aerosol composition and spreading the volatile compound in a wide range by a single jet.
- the aerosol products of Comparative Examples 1 and 2 were used, it was difficult to visually recognize the sprayed aerosol composition at the time of spraying.
Landscapes
- Health & Medical Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Dispersion Chemistry (AREA)
- General Health & Medical Sciences (AREA)
- Epidemiology (AREA)
- Animal Behavior & Ethology (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
- Engineering & Computer Science (AREA)
- Mechanical Engineering (AREA)
- Pharmacology & Pharmacy (AREA)
- Wood Science & Technology (AREA)
- Agronomy & Crop Science (AREA)
- Pest Control & Pesticides (AREA)
- Plant Pathology (AREA)
- Toxicology (AREA)
- Dentistry (AREA)
- Medicinal Chemistry (AREA)
- Zoology (AREA)
- Environmental Sciences (AREA)
- Agricultural Chemicals And Associated Chemicals (AREA)
- Fats And Perfumes (AREA)
- Containers And Packaging Bodies Having A Special Means To Remove Contents (AREA)
- Disinfection, Sterilisation Or Deodorisation Of Air (AREA)
Priority Applications (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2019549302A JP7166269B2 (ja) | 2017-10-20 | 2018-10-16 | 定量噴射型エアゾール製品および定量噴射型エアゾール製品の噴射方法 |
| JP2022170693A JP2023017810A (ja) | 2017-10-20 | 2022-10-25 | 定量噴射型エアゾール製品および定量噴射型エアゾール製品の噴射方法 |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2017-203736 | 2017-10-20 | ||
| JP2017203736 | 2017-10-20 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2019078220A1 true WO2019078220A1 (ja) | 2019-04-25 |
Family
ID=66174151
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/JP2018/038549 Ceased WO2019078220A1 (ja) | 2017-10-20 | 2018-10-16 | 定量噴射型エアゾール製品および定量噴射型エアゾール製品の噴射方法 |
Country Status (2)
| Country | Link |
|---|---|
| JP (2) | JP7166269B2 (enExample) |
| WO (1) | WO2019078220A1 (enExample) |
Cited By (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2023039210A (ja) * | 2021-09-08 | 2023-03-20 | 大日本除蟲菊株式会社 | 定量噴射エアゾール組成物及び定量噴射エアゾール製品 |
| JP2023108909A (ja) * | 2022-01-26 | 2023-08-07 | ライセンスインターナショナル株式会社 | エアゾール殺虫剤 |
| US12239128B2 (en) | 2021-03-03 | 2025-03-04 | S. C. Johnson & Son, Inc. | Methods and systems for spraying a pest control composition |
Citations (8)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2006121988A (ja) * | 2004-10-29 | 2006-05-18 | Fumakilla Ltd | 害虫防除方法 |
| JP2006325489A (ja) * | 2005-05-26 | 2006-12-07 | Earth Chem Corp Ltd | 害虫防除方法 |
| JP2010155809A (ja) * | 2008-12-29 | 2010-07-15 | Earth Chem Corp Ltd | 孵化阻害材 |
| JP2010280633A (ja) * | 2009-06-05 | 2010-12-16 | Dainippon Jochugiku Co Ltd | 害虫防除方法 |
| JP2011162244A (ja) * | 2010-02-12 | 2011-08-25 | Dainippon Jochugiku Co Ltd | 定量噴射型のエアゾール製品 |
| JP2014031342A (ja) * | 2012-08-03 | 2014-02-20 | Fumakilla Ltd | 殺虫エアゾール用組成物 |
| JP2016150760A (ja) * | 2015-02-16 | 2016-08-22 | 株式会社ダイゾー | エアゾール製品 |
| WO2017073478A1 (ja) * | 2015-10-27 | 2017-05-04 | アース製薬株式会社 | エアゾール型芳香剤 |
Family Cites Families (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP3289836B2 (ja) * | 1991-03-20 | 2002-06-10 | 石原産業株式会社 | 水性懸濁状除草組成物 |
| JP3199444B2 (ja) * | 1992-04-10 | 2001-08-20 | アース製薬株式会社 | 害虫防除方法 |
| JP2002154925A (ja) * | 2000-11-15 | 2002-05-28 | Sekisui Plastics Co Ltd | エアゾール用の球状凝集粒子及びこれを含むエアゾール剤 |
| JP2009131243A (ja) * | 2007-10-29 | 2009-06-18 | Earth Chem Corp Ltd | 害虫防除装置 |
| JP6034658B2 (ja) * | 2012-10-29 | 2016-11-30 | 大日本除蟲菊株式会社 | 衣類害虫防除方法 |
| JP6633280B2 (ja) * | 2014-03-04 | 2020-01-22 | 大日本除蟲菊株式会社 | 蚊類防除用エアゾール、及び蚊類防除方法 |
| JP6506892B2 (ja) * | 2015-02-24 | 2019-04-24 | 大日本除蟲菊株式会社 | 多足類害虫防除用エアゾール |
-
2018
- 2018-10-16 WO PCT/JP2018/038549 patent/WO2019078220A1/ja not_active Ceased
- 2018-10-16 JP JP2019549302A patent/JP7166269B2/ja active Active
-
2022
- 2022-10-25 JP JP2022170693A patent/JP2023017810A/ja active Pending
Patent Citations (8)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2006121988A (ja) * | 2004-10-29 | 2006-05-18 | Fumakilla Ltd | 害虫防除方法 |
| JP2006325489A (ja) * | 2005-05-26 | 2006-12-07 | Earth Chem Corp Ltd | 害虫防除方法 |
| JP2010155809A (ja) * | 2008-12-29 | 2010-07-15 | Earth Chem Corp Ltd | 孵化阻害材 |
| JP2010280633A (ja) * | 2009-06-05 | 2010-12-16 | Dainippon Jochugiku Co Ltd | 害虫防除方法 |
| JP2011162244A (ja) * | 2010-02-12 | 2011-08-25 | Dainippon Jochugiku Co Ltd | 定量噴射型のエアゾール製品 |
| JP2014031342A (ja) * | 2012-08-03 | 2014-02-20 | Fumakilla Ltd | 殺虫エアゾール用組成物 |
| JP2016150760A (ja) * | 2015-02-16 | 2016-08-22 | 株式会社ダイゾー | エアゾール製品 |
| WO2017073478A1 (ja) * | 2015-10-27 | 2017-05-04 | アース製薬株式会社 | エアゾール型芳香剤 |
Cited By (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US12239128B2 (en) | 2021-03-03 | 2025-03-04 | S. C. Johnson & Son, Inc. | Methods and systems for spraying a pest control composition |
| JP2023039210A (ja) * | 2021-09-08 | 2023-03-20 | 大日本除蟲菊株式会社 | 定量噴射エアゾール組成物及び定量噴射エアゾール製品 |
| JP7716936B2 (ja) | 2021-09-08 | 2025-08-01 | 大日本除蟲菊株式会社 | 定量噴射エアゾール組成物及び定量噴射エアゾール製品 |
| JP2023108909A (ja) * | 2022-01-26 | 2023-08-07 | ライセンスインターナショナル株式会社 | エアゾール殺虫剤 |
Also Published As
| Publication number | Publication date |
|---|---|
| JPWO2019078220A1 (ja) | 2020-11-05 |
| JP2023017810A (ja) | 2023-02-07 |
| JP7166269B2 (ja) | 2022-11-07 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP7529854B2 (ja) | 害虫防除用定量噴射型エアゾール製品および害虫防除方法 | |
| JP2023017810A (ja) | 定量噴射型エアゾール製品および定量噴射型エアゾール製品の噴射方法 | |
| JP7681631B2 (ja) | 害虫防除用定量噴射型エアゾール製品および害虫防除方法 | |
| AU2019399077B2 (en) | Method for Controlling Insect Pests and Acarines, and Aerosol for Controlling Insect Pests and Acarines | |
| JP7665062B2 (ja) | 匍匐害虫の忌避方法 | |
| JP7704651B2 (ja) | 特定有害物防除用定量噴射エアゾール製品を用いた特定有害物防除方法 | |
| WO2019117164A1 (ja) | 定量噴射型エアゾール、定量噴射型エアゾールの噴射方法及び薬剤の効力向上方法 | |
| JP2016155774A (ja) | 多足類害虫防除用エアゾール | |
| JP2018095565A (ja) | エアゾール製品および害虫防除方法 | |
| JP5070037B2 (ja) | アリ防除用エアゾール剤およびこれを用いたアリの防除方法 | |
| JP2014152132A (ja) | 鱗翅目飛翔害虫防除用エアゾール剤 | |
| JP5934583B2 (ja) | 害虫を殺虫するための組成物 | |
| JPWO2020111071A1 (ja) | 匍匐害虫の防除方法および匍匐害虫防除用エアゾール装置 | |
| CN114599226B (zh) | 空间处理用定量喷射气溶胶 | |
| TW202439970A (zh) | 害蟲防治製品 | |
| JP7152613B2 (ja) | ゴキブリ類駆除用エアゾール、及びゴキブリ類駆除方法 | |
| JP2023039210A (ja) | 定量噴射エアゾール組成物及び定量噴射エアゾール製品 | |
| JP7097692B2 (ja) | 害虫防除エアゾール装置 | |
| JP2024180336A (ja) | ダニ類防除方法及びダニ類防除用噴射製剤 | |
| JP2022111683A (ja) | アリ駆除用エアゾール製品及びアリ駆除方法 | |
| JP2022109097A (ja) | アリ防除用空間処理エアゾール製品及びアリ防除方法 | |
| WO2023037840A1 (ja) | 有害微生物防除用定量噴射エアゾール製品、及びこれを用いた有害微生物防除方法 | |
| HK40073208A (en) | Metered spray aerosol product for controlling specific harmful matter, and method for controlling specific harmful matter using same | |
| HK40068417A (en) | Metered spray aerosol for treating space | |
| HK40072744A (en) | Method and aerosol for controlling crawling insect pest |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 18867352 Country of ref document: EP Kind code of ref document: A1 |
|
| ENP | Entry into the national phase |
Ref document number: 2019549302 Country of ref document: JP Kind code of ref document: A |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| 122 | Ep: pct application non-entry in european phase |
Ref document number: 18867352 Country of ref document: EP Kind code of ref document: A1 |