WO2018088389A1 - 生体組織活着面、インプラント、生体組織活着面の形成方法、インプラントの製造方法 - Google Patents
生体組織活着面、インプラント、生体組織活着面の形成方法、インプラントの製造方法 Download PDFInfo
- Publication number
- WO2018088389A1 WO2018088389A1 PCT/JP2017/040076 JP2017040076W WO2018088389A1 WO 2018088389 A1 WO2018088389 A1 WO 2018088389A1 JP 2017040076 W JP2017040076 W JP 2017040076W WO 2018088389 A1 WO2018088389 A1 WO 2018088389A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- living tissue
- living
- implant
- tissue
- surface according
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Ceased
Links
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61C—DENTISTRY; APPARATUS OR METHODS FOR ORAL OR DENTAL HYGIENE
- A61C13/00—Dental prostheses; Making same
- A61C13/0003—Making bridge-work, inlays, implants or the like
- A61C13/0006—Production methods
- A61C13/0018—Production methods using laser
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61C—DENTISTRY; APPARATUS OR METHODS FOR ORAL OR DENTAL HYGIENE
- A61C8/00—Means to be fixed to the jaw-bone for consolidating natural teeth or for fixing dental prostheses thereon; Dental implants; Implanting tools
- A61C8/0012—Means to be fixed to the jaw-bone for consolidating natural teeth or for fixing dental prostheses thereon; Dental implants; Implanting tools characterised by the material or composition, e.g. ceramics, surface layer, metal alloy
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61C—DENTISTRY; APPARATUS OR METHODS FOR ORAL OR DENTAL HYGIENE
- A61C8/00—Means to be fixed to the jaw-bone for consolidating natural teeth or for fixing dental prostheses thereon; Dental implants; Implanting tools
- A61C8/0018—Means to be fixed to the jaw-bone for consolidating natural teeth or for fixing dental prostheses thereon; Dental implants; Implanting tools characterised by the shape
- A61C8/0037—Details of the shape
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61C—DENTISTRY; APPARATUS OR METHODS FOR ORAL OR DENTAL HYGIENE
- A61C13/00—Dental prostheses; Making same
- A61C13/01—Palates or other bases or supports for the artificial teeth; Making same
- A61C13/02—Palates or other bases or supports for the artificial teeth; Making same made by galvanoplastic methods or by plating; Surface treatment; Enamelling; Perfuming; Making antiseptic
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61C—DENTISTRY; APPARATUS OR METHODS FOR ORAL OR DENTAL HYGIENE
- A61C8/00—Means to be fixed to the jaw-bone for consolidating natural teeth or for fixing dental prostheses thereon; Dental implants; Implanting tools
- A61C8/0003—Not used, see subgroups
- A61C8/0004—Consolidating natural teeth
- A61C8/0006—Periodontal tissue or bone regeneration
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61C—DENTISTRY; APPARATUS OR METHODS FOR ORAL OR DENTAL HYGIENE
- A61C8/00—Means to be fixed to the jaw-bone for consolidating natural teeth or for fixing dental prostheses thereon; Dental implants; Implanting tools
- A61C8/0018—Means to be fixed to the jaw-bone for consolidating natural teeth or for fixing dental prostheses thereon; Dental implants; Implanting tools characterised by the shape
- A61C8/0022—Self-screwing
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/02—Prostheses implantable into the body
- A61F2/30—Joints
- A61F2/30767—Special external or bone-contacting surface, e.g. coating for improving bone ingrowth
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/02—Prostheses implantable into the body
- A61F2/30—Joints
- A61F2/3094—Designing or manufacturing processes
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/02—Prostheses implantable into the body
- A61F2/30—Joints
- A61F2/3094—Designing or manufacturing processes
- A61F2/30942—Designing or manufacturing processes for designing or making customized prostheses, e.g. using templates, CT or NMR scans, finite-element analysis or CAD-CAM techniques
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/02—Prostheses implantable into the body
- A61F2/30—Joints
- A61F2/32—Joints for the hip
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/02—Prostheses implantable into the body
- A61F2/30—Joints
- A61F2/32—Joints for the hip
- A61F2/36—Femoral heads ; Femoral endoprostheses
- A61F2/3662—Femoral shafts
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61C—DENTISTRY; APPARATUS OR METHODS FOR ORAL OR DENTAL HYGIENE
- A61C8/00—Means to be fixed to the jaw-bone for consolidating natural teeth or for fixing dental prostheses thereon; Dental implants; Implanting tools
- A61C8/0018—Means to be fixed to the jaw-bone for consolidating natural teeth or for fixing dental prostheses thereon; Dental implants; Implanting tools characterised by the shape
- A61C8/0037—Details of the shape
- A61C2008/0046—Textured surface, e.g. roughness, microstructure
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/02—Prostheses implantable into the body
- A61F2/30—Joints
- A61F2/3094—Designing or manufacturing processes
- A61F2/30965—Reinforcing the prosthesis by embedding particles or fibres during moulding or dipping
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/02—Prostheses implantable into the body
- A61F2/30—Joints
- A61F2/30767—Special external or bone-contacting surface, e.g. coating for improving bone ingrowth
- A61F2/30771—Special external or bone-contacting surface, e.g. coating for improving bone ingrowth applied in original prostheses, e.g. holes or grooves
- A61F2002/3084—Nanostructures
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/02—Prostheses implantable into the body
- A61F2/30—Joints
- A61F2/3094—Designing or manufacturing processes
- A61F2002/3097—Designing or manufacturing processes using laser
Definitions
- the present invention relates to a living tissue survival surface, an implant, a method for forming a living tissue survival surface, and a method for manufacturing an implant.
- Implants that are implanted in the body are drawing attention.
- Implants include dental implants, artificial joints, and artificial bones.
- the dental implant is formed of a biocompatible material such as a biocompatible metal material or a biocompatible ceramic material.
- the biocompatible metal material is titanium, a titanium alloy, a cobalt chromium alloy, or the like.
- the biocompatible ceramic material is alumina or zirconia.
- Artificial hip joints, artificial bones, and the like are formed of biocompatible resin materials in addition to biocompatible metal materials and biocompatible ceramic materials.
- the outer surface of the implant is closely bonded to the bone (hard tissue). By roughening the outer surface of the implant (such as making it porous), internal bone growth is promoted and high osseointegration is obtained.
- resin-made implants for example, a resin surface is coated with metal or ceramics, and this coating is roughened.
- FIG. 19 is a photograph of the outer surface of a conventional titanium fixture taken with an SEM (magnification rate 2000 times) (4 items). These outer surfaces are roughened (made porous) by edging or blasting with hydrochloric acid or the like.
- ⁇ Implant adheres not only to bone but also to mucosal tissue (soft tissue) around the bone, so adhesion (affinity) with soft tissue is also important.
- soft tissue mucosal tissue
- adhesion affinity
- the dental implant has low adhesion to the gingiva, the gingiva is inflamed, and the gingiva (gum) is retracted or the alveolar bone is reduced (bone resorption). For this reason, it is necessary to increase the adhesion between the dental implant and the gingiva (adhesion to the soft tissue) and prevent (block) the entry of bacteria.
- Implants are required to improve the adhesion to living tissue (bonding to hard tissue, adhesion to soft tissue) to accelerate the healing of living tissue.
- An object of the present invention is to provide a living tissue survival surface, an implant, a method for forming a living tissue survival surface, and a method for manufacturing an implant, which can improve the ability to adhere to living tissue.
- the first embodiment of the living tissue survival surface of the present invention is a living tissue survival surface that is activated on a biological tissue, and is characterized by having a large number of fingertip-shaped microvillous bodies made of a biocompatible material.
- the second embodiment of the living tissue survival surface of the present invention is characterized in that, in the first embodiment, the microvillus has a tip diameter of nanometer size.
- the third embodiment of the living tissue activating surface of the present invention is characterized in that, in the second embodiment, the tip diameter is 50 nm or more and less than 500 nm.
- the fourth embodiment of the living tissue occluding surface of the present invention is characterized in that, in any of the first to third embodiments, the three-dimensional surface roughness Sa is a nanometer size.
- the fifth embodiment of the living tissue survival surface of the present invention is characterized in that, in any of the first to fourth embodiments, the developed area ratio Sdr of the interface is 0.1 or more and 2.0 or less.
- the sixth embodiment of the living tissue survival surface of the present invention is characterized in that in any one of the first to fifth embodiments, a plurality of first grooves having a width of 1 ⁇ m or more and 50 ⁇ m or less are provided.
- the seventh embodiment of the living tissue activating surface of the present invention is characterized in that, in the sixth embodiment, the first groove has a depth of 1 ⁇ m or more and 20 ⁇ m or less.
- the eighth embodiment of the living tissue activating surface of the present invention is characterized in that, in the sixth or seventh embodiment, the first grooves are arranged in parallel or in a lattice shape.
- the ninth embodiment of the living tissue survival surface of the present invention is characterized in that in any one of the first to eighth embodiments, there are a plurality of second grooves having a width of 10 ⁇ m or more and 500 ⁇ m or less.
- the tenth embodiment of the living tissue activating surface of the present invention is characterized in that, in the ninth embodiment, the second groove has a depth of 5 ⁇ m or more and 500 ⁇ m or less.
- the eleventh embodiment of the living tissue occluding surface of the present invention is characterized in that, in the ninth or tenth embodiment, the second grooves are arranged in parallel or in a lattice shape.
- the twelfth embodiment of the living tissue occluding surface of the present invention is any one of the first to eleventh embodiments, wherein the biocompatible material is a biocompatible ceramic material.
- the thirteenth embodiment of the living tissue occupying surface of the present invention is characterized in that, in the twelfth embodiment, the biocompatible ceramic material contains zirconia.
- the biocompatible material is a biocompatible metal material.
- the biocompatible metal material includes titanium, a titanium alloy, or a cobalt chromium alloy.
- the sixteenth embodiment of the living tissue occupying surface of the present invention is any one of the first to eleventh embodiments, wherein the biocompatible material is a biocompatible resin material.
- the seventeenth embodiment of the living tissue activating surface of the present invention is characterized in that, in the sixteenth embodiment, the biocompatible resin material includes a polyether ether ketone resin.
- the first embodiment of the implant of the present invention is an implant that is attached to a living tissue, and has one of the first to seventeenth embodiments of the living tissue attaching surface of the present invention on a surface that is attached to the living tissue. It is characterized by that.
- a second embodiment of the implant of the present invention is the screw-type fixture of a dental implant according to the first embodiment, wherein the living tissue attachment surface is any one or more of a screw surface, a collar surface, and a tip surface. It is provided in.
- a third embodiment of the implant according to the present invention is a cylindrical fixture of a dental implant according to the first embodiment, and the living tissue attachment surface is provided on any one or more of a distal end surface and an outer peripheral surface. It is characterized by that.
- a fourth embodiment of the implant of the present invention is an abutment for a dental implant in the first embodiment, wherein the living tissue attachment surface is provided on a gingival margin surface.
- a fifth embodiment of the implant according to the present invention is the stem of the artificial hip joint in the first embodiment, wherein the living tissue attachment surface is provided on the surface of the site to be embedded in the femur.
- the first embodiment of the method for forming a living tissue activating surface of the present invention is a method for forming a living tissue activating surface for activating a living tissue, wherein laser non-thermal processing for irradiating laser light in the air is a biocompatible material. A large number of fingertip-shaped microvillous bodies are formed on the surface of the substrate.
- the second embodiment of the method for forming a living tissue survival surface of the present invention is characterized in that, in the first embodiment, the laser beam is a laser beam of an ultrashort pulse laser.
- the third embodiment of the method for forming a living tissue living surface of the present invention is characterized in that, in the second aspect, the laser beam is a laser beam of a picosecond laser or a femtosecond laser.
- the fourth embodiment of the method for forming a living tissue survival surface of the present invention is characterized in that, in any one of the first to third embodiments, the microvillus has a tip diameter of nanometer size.
- laser non-thermal processing is performed on a ceramic sintered body made of a biocompatible ceramic material. It is characterized by.
- the sixth embodiment of the method for forming a living tissue survival surface of the present invention is characterized in that, in the fifth embodiment, the biocompatible ceramic material contains zirconia.
- a laser non-heat is applied to an acid-etched metal workpiece made of a biocompatible metal material. It is characterized by processing.
- the eighth embodiment of the method for forming a living tissue survival surface of the present invention is characterized in that, in the seventh embodiment, the biocompatible metal material includes titanium, a titanium alloy, or a cobalt chromium alloy.
- laser non-thermal processing is performed on a resin molded body made of a biocompatible resin material.
- the tenth embodiment of the method for forming a living tissue survival surface of the present invention is characterized in that, in the ninth embodiment, the biocompatible resin material includes a polyether ether ketone resin.
- the laser beam is scanned, the width is 1 ⁇ m or more, 50 ⁇ m or less, and the depth is 1 ⁇ m. As described above, a plurality of first grooves of 20 ⁇ m or less are formed.
- the laser light is scanned in a parallel direction or a crossing direction, and the first grooves are parallel or latticed. It is formed in the shape.
- the laser beam is scanned, the width is 10 ⁇ m or more, 500 ⁇ m or less, and the depth is A plurality of second grooves of 5 ⁇ m or more and 500 ⁇ m or less are formed.
- the laser beams are scanned in a parallel direction or a crossing direction, and the second grooves are parallel or latticed. It is formed in the shape.
- a first embodiment of the method for producing an implant of the present invention is a method for producing an implant that is to be engrafted in a living tissue, in the step of forming a surface to be engrafted in the living tissue, Any one of the first to fourteenth embodiments is included.
- the second embodiment of the implant manufacturing method of the present invention is characterized in that, in the first embodiment, it is a fixture for a dental implant.
- a third embodiment of the method for producing an implant of the present invention is characterized in that, in the first embodiment, the abutment is a dental implant.
- a fourth embodiment of the method for manufacturing an implant of the present invention is characterized in that, in the first embodiment, the stem is an artificial hip joint.
- the living tissue survival surface, the implant, the method for forming a living tissue survival surface, and the method for producing an implant of the present invention can improve the ability to adhere to the living tissue.
- FIG. 1 It is a figure which shows the dental implant 1 which concerns on 1st embodiment of this invention, Comprising: It is (a) screw type dental implant 1A, (b) cylinder type dental implant 1B. It is the photograph which image
- FIG. 1 It is a figure which shows the artificial hip joint 101 which concerns on 3rd embodiment of this invention. It is the photograph which image
- FIG. 1 is a diagram showing a dental implant 1 according to a first embodiment of the present invention, in which (a) a screw-type dental implant 1A and (b) a cylinder-type dental implant 1B.
- the dental implant 1 is a zirconia implant.
- the dental implant 1 includes a screw-type dental implant 1A and a cylinder-type dental implant 1B.
- the dental implant 1 includes a fixture 10 that is fixed to an alveolar bone (biological tissue, hard tissue) H, and an abutment 20 that is fitted to the fixture 10.
- a crown 6 called an artificial tooth crown is attached to the abutment 20.
- the root end side of the crown 6 is covered with a gum (biological tissue, soft tissue) S.
- the longitudinal direction (direction along the central axis) of the dental implant 1 is referred to as vertical.
- the crown 6 side is referred to as the tip side.
- the tip is also called the first end.
- the fixture 10 side is referred to as the root end side.
- the root end is also called the second end.
- the direction perpendicular to the vertical direction is called horizontal.
- the direction around the central axis of the dental implant 1 is referred to as the circumferential direction.
- the fixture (implant) 10 is a shaft-like member having a center hole (not shown), and is formed of ceramics (biocompatible material, biocompatible ceramic material) containing zirconia.
- the fixture 10 includes a screw-type fixture 10 ⁇ / b> A in which a male screw 15 is formed on the outer surface 11 and a cylinder-type fixture 10 ⁇ / b> B without the male screw 15.
- the screw-type fixture 10A and the cylinder-type fixture 10B differ only in the presence or absence of the male screw 15.
- the screw-type fixture 10 ⁇ / b> A is fixed to the alveolar bone H by screwing into a screw hole formed in the alveolar bone H.
- the cylinder-type fixture 10 ⁇ / b> B is fixed to the alveolar bone H by fitting into a circular hole formed in the alveolar bone H.
- a center hole is opened at the center of the tip surface 13 of the fixture 10.
- the central hole is formed along the vertical direction.
- the shape (length, thickness, etc.) of the fixture 10 is arbitrary. It may be the case where there is no center hole.
- the fixture 10 is formed with a living tissue survival surface 30 (biological tissue survival surfaces 31 to 35).
- the living tissue survival surface 30 is provided on the outer surface 11 of the fixture 10.
- the outer surface 11 includes a tip surface 13, a collar surface 12, and a screw surface (male screw 15).
- the outer surface 11 includes a tip surface 13 and an outer peripheral surface 14.
- the living tissue survival surface 30 is a surface excellent in the binding property to the alveolar bone H and the adhesion property to the gum S.
- the living tissue survival surface 30 has a large number of microvilli 41 described later.
- the microvillous bodies 41 are dense.
- the living tissue survival surface 30 is a fine villi dense surface.
- the living tissue living surface 30 may have one or both of a small groove 43 and a large groove 45 described later.
- the fixture 10 adheres not only to the alveolar bone H but also to the mucosal tissue (soft tissue) around the alveolar bone H.
- the distal end surface 13 is in close contact with the gum S.
- the fixture 10 also has an important adhesion property (affinity) with the soft tissue. If the fixture 10 has low adhesion to the gum S, the gum S is inflamed and the gum S retreats or the alveolar bone H decreases (bone resorption). Therefore, it is necessary to increase adhesion between the fixture 10 and the gum S (adhesion to soft tissue) and prevent (block) bacteria from entering.
- the living tissue survival surface 30 improves the bone bonding and gum adhesion of the fixture 10 by the microvillus 41, the small groove 43, and the large groove 45.
- the living tissue survival surface 30 improves the adhesion of the fixture 10 to the living tissue (bondability to hard tissue, adhesion to soft tissue) and accelerates the healing of the living tissue.
- Bio tissue survival surface 31) 2 and 3 are photographs obtained by photographing the living tissue survival surface 30 (biological tissue survival surface 31) according to the first embodiment of the present invention with an SEM, wherein (a) is a magnification of 30 times, and (b). Is an enlargement ratio of 200 times, (c) is an enlargement ratio of 500 times, (d) is an enlargement ratio of 2000 times, (e) is an enlargement ratio of 5000 times, and (f) is an enlargement ratio of 10,000 times.
- the living tissue living surface 31 is an example of the living tissue living surface 30, and is formed on the outer surface 11 of the fixture 10A.
- the living tissue survival surface 31 has a large number of microvillus bodies 41 whose tips are fingertip-shaped. A large number of microvillus bodies 41 are densely arranged.
- the fingertip shape means a shape having a round tip (hemispherical shape) like a fingertip.
- the microvillous body 41 has a hemispherical protrusion at the tip and is not sharp.
- the microvillous body 41 is formed so that the outer diameter (diameter) of the tip is nanometer size.
- the nanometer size is sometimes referred to as nanometer order, nanometer scale, or nanometer class. In English, it is called “order of magnitude”. In Japanese, it is called “grade”, “class”, “scale” or “digit”.
- the tip diameter of the microvillous body 41 is 1 nm or more and less than 1000 nm.
- the tip diameter of the microvillus 41 is, for example, not less than 50 nm and less than 500 nm. For example, it may be 100 nm or more and less than 300 nm.
- the living tissue survival surface 31 has a three-dimensional surface roughness Sa (arithmetic average height: ISO25178) of nanometer size (1 nm or more and less than 1000 nm).
- the three-dimensional roughness Sa of the living tissue survival surface 31 is, for example, 500 nm or more and 800 nm.
- the living tissue survival surface 31 has an interface development area ratio Sdr (ISO25178) of 0.1 or more and 2.0 or less.
- the living tissue activating surface 31 has an interface development area ratio Sdr of, for example, 0.5 or more and 1.0 or less.
- the living tissue survival surface 31 has a plurality of large grooves 45.
- the plurality of large grooves 45 are arranged to intersect each other.
- a plurality of large grooves 45 are arranged in a lattice pattern. This is to ensure that osteoblasts having an oval shape of about 20 to 30 ⁇ m are firmly established inside the large groove 45.
- the large groove (second groove) 57 has a width of 10 ⁇ m or more and 500 ⁇ m or less.
- the large groove 45 has a width of, for example, 20 ⁇ m or more and 100 ⁇ m or less. It may be 30 ⁇ m or more and 50 ⁇ m or less. This is to prevent preosteoblasts from spreading too much.
- the large groove 45 has a depth of 5 ⁇ m or more and 500 ⁇ m or less. For example, it may be 10 ⁇ m or more and 100 ⁇ m or less. This is to prevent the preosteoblasts from getting over the large groove 45.
- the large grooves 45 arranged in parallel in the vertical direction intersect with the large grooves 45 arranged in parallel in the horizontal direction (circumferential direction).
- the intersecting large grooves 45 may have an intersecting angle of 60 ° or more.
- Bio tissue survival surface 32) 4 and 5 are photographs obtained by photographing the living tissue survival surface 30 (biological tissue survival surface 32) according to the first embodiment of the present invention with an SEM, wherein (a) is an enlargement ratio of 200 times, and (b). Is an enlargement ratio of 500 times, (c) is an enlargement ratio of 2000 times, (d) is an enlargement ratio of 5000 times, (e) is an enlargement ratio of 10,000 times, and (f) is an enlargement ratio of 20000 times.
- the living tissue living surface 32 is an example of the living tissue living surface 30, and is formed on the outer surface 11 of the fixture 10B.
- the living tissue living surface 32 is formed in the same manner as the living tissue living surface 31.
- FIG. 6A and 6B are reference photographs obtained by photographing the surface of the small intestine with an SEM.
- FIG. 6A shows an enlargement ratio of about 100 times
- FIG. 6B shows an enlargement ratio of about 5000 times
- FIG. 6C shows an enlargement ratio of about 10,000 times.
- Villi are fine protrusions protruding from the surface of an organ, and are present in the small intestine and placenta.
- FIGS. 6B and 6C a large number of microvilluss are further concentrated on the surface of the villus.
- the microvillus itself may be called soft hair or soft process.
- the villi and microvilli are protrusions having a fingertip shape at the tip.
- the tip diameter of the microvilli is less than 1 ⁇ m.
- the surface area is remarkably increased by villi and microvilli, and absorption and binding are performed efficiently and effectively.
- the living tissue survival surfaces 31 and 32 have a structure similar to the inner surface of the small intestine or the like.
- the large groove 45 resembles the villi of a living tissue
- the microvilli 41 resembles the villi of a living tissue.
- the living tissue active surface 31 has high connectivity and adhesion with living tissue (hard tissue such as bone, soft tissue such as mucosal tissue).
- the biological tissue activating surface 31 is considered to have a shape close to an ideal as a surface that adheres to and adheres to the biological tissue.
- FIG. 7 is a photograph of the living tissue survival surface 30 (biological tissue survival surface 33) according to the first embodiment of the present invention taken with an SEM, wherein (a) is an enlargement factor of 200 times and (b) is an enlargement factor. 2000 times, (c) is an enlargement rate of 5000 times, and (d) is an enlargement rate of 10,000 times.
- the biological tissue survival surface 33 is an example of the biological tissue survival surface 30 and is formed on the outer surface 11 of the fixture 10B. Similar to the living tissue living surfaces 31 and 32, the living tissue living surface 33 has a large number of microvilli 41. The three-dimensional roughness Sa of the living tissue living surface 33 and the developed area ratio Sdr of the interface are the same as those of the living tissue living surfaces 31 and 32.
- the living tissue survival surface 33 has a plurality of large grooves 45 arranged in parallel. The shape and the like of the large groove 45 are as described above.
- FIG. 8 is a photograph of the living tissue survival surface 30 (biological tissue survival surface 34) according to the first embodiment of the present invention taken with an SEM, wherein (a) is an enlargement factor of 200 times and (b) is an enlargement factor. 500 times (c) is an enlargement factor of 10,000 times.
- the living tissue living surface 34 is an example of the living tissue living surface 30, and is formed on the outer surface 11 of the fixture 10B.
- the living tissue activating surface 34 has a large number of microvillous bodies 41, like the living tissue activating surfaces 31 to 33.
- the three-dimensional roughness Sa of the living tissue living surface 34 and the developed area ratio Sdr of the interface are the same as those of the living tissue living surfaces 31 to 33.
- the living tissue survival surface 34 includes a plurality of small grooves 43 and large grooves 45.
- the plurality of small grooves 43 are arranged in parallel.
- the plurality of large grooves 45 are also arranged in parallel.
- the small grooves 43 and the large grooves 45 intersect in a lattice pattern.
- the small groove 43 and the large groove 45 may have an intersection angle of 60 ° or more.
- the shape and the like of the large groove 45 are as described above.
- the small grooves (first grooves) 43 have a width of 1 ⁇ m or more and 50 ⁇ m or less, and are arranged in parallel.
- the small groove 43 has a width of, for example, 1 ⁇ m or more and 20 ⁇ m. For example, it may be 5 ⁇ m or more and 10 ⁇ m or less.
- the small groove 43 has a depth of 1 ⁇ m or more and 20 ⁇ m or less. For example, it may be 2 ⁇ m or more and 5 ⁇ m or less. This is to give mechanical stimulation
- FIG. 9 is a photograph of the living tissue survival surface 30 (biological tissue survival surface 35) according to the first embodiment of the present invention taken with an SEM, wherein (a) is an enlargement factor of 500 times and (b) is an enlargement factor. 10,000 times.
- the biological tissue survival surface 35 is an example of the biological tissue survival surface 30, and is formed on the outer surface 11 of the fixture 10A.
- the biological tissue survival surface 35 has a large number of microvilli 41 as in the biological tissue survival surfaces 31 to 34.
- the three-dimensional roughness Sa of the living tissue living surface 35 and the developed area ratio Sdr of the interface are the same as those of the living tissue living surfaces 31 to 34.
- the living tissue survival surface 35 has a plurality of small grooves 43 arranged in parallel and a plurality of large grooves 45 arranged in parallel.
- the small groove 43 and the large groove 45 are arranged in parallel.
- a plurality of small grooves 43 are arranged (overlapped) inside the large groove 45.
- the small groove 43 and the large groove 45 are parallel.
- the small groove 43 and the large groove 45 may have an intersection angle of 30 ° or less.
- the shape and the like of the small groove 43 and the large groove 45 are as described above.
- FIG. 19 is a reference photograph in which the outer surface of a conventional titanium fixture is taken with an SEM (magnification rate 2000 times), where (a) is a product of Company A, (b) is a product of Company B, and (c) is Company C product, (d) is Company D product.
- the outer surface is roughened (porous). These outer surfaces are roughened by edging or blasting with hydrochloric acid or the like. These outer surfaces have a large number of pores, and further have a large number of protrusions with sharp tips around the pores.
- the outer surface of the conventional fixture has a three-dimensional roughness Sa of 2 ⁇ m or more. However, any of the outer surfaces of the conventional fixture does not have a hemispherical (finger-tip-shaped) protrusion (microvillus) and cannot be said to be a microvilli dense surface.
- the abutment (implant) 20 is a shaft-like member and is formed of ceramics containing zirconia.
- the abutment 20 has a main body portion 23 and a tapered shaft portion 25.
- the taper shaft portion 25 is fitted into the center hole of the fixture 10, and the main body portion 23 is disposed so as to be exposed from the front end side of the fixture 10.
- the main body portion 23 is formed in a truncated cone shape or the like, and the crown 6 is attached using an adhesive, cement, or the like.
- the root end side region not covered by the crown 6) than the crown 6 is referred to as a gingival margin 24 (gingival margin).
- the gingival margin 24 is exposed between the fixture 10 and the crown 6.
- the abutment 20 is formed with a living tissue survival surface 30 (biological tissue survival surfaces 31 to 35).
- the biological tissue survival surface 30 is provided on the outer surface 21 of the abutment 20.
- the living tissue survival surface 30 is provided on the gingival margin surface (gingival margin 24).
- the gingival margin 24 is in close contact with the gum S in the same manner as the distal end surface 13 of the fixture 10. For this reason, the gum S is easily adhered by providing the living tissue survival surface 30 (biological tissue survival surfaces 31 to 35) in the gingival margin 24. Thereby, the fusion of the abutment 20 and the gum S is strengthened as compared with the conventional case.
- the fixture 10 (10A, 10B) has a living tissue active surface 30 (biological tissue active surfaces 31 to 35) on the outer surface 11. Thereby, the fixation (adhesion) to the outer surface 11 of an anterior osteoblast or an osteoblast can be accelerated
- the biological tissue survival surface 30 (biological tissue survival surfaces 31 to 35) has a large number of small grooves 43 and large grooves 45. This can promote the proliferation of preosteoblasts. Since various modes (number, shape, arrangement) of the small grooves 43 and the large grooves 45 can be set, a mechanical stimulus (mechanical stress) can be effectively applied to the preosteoblasts. Therefore, differentiation into osteoblasts is promoted, and the bone bonding period is shortened.
- the living tissue survival surface 30 includes a plurality of ups and downs having different scale sizes.
- the microvillous body 41 forms nanometer-sized irregularities.
- the small groove 43 forms a single-micron size unevenness.
- the large groove 45 forms irregularities having a larger scale size than these. Therefore, the living tissue survival surface 30 can efficiently and effectively give mechanical stimulation to the preosteoblasts. Therefore, the bond between the fixture 10 and the alveolar bone H becomes stronger than before, and the bone bond period is also shortened.
- the fixture 10 ⁇ / b> B can bind well to the alveolar bone H because the living tissue survival surface 30 exhibits high bone-binding properties.
- the abutment 20 has a living tissue attachment surface 30 (biological tissue attachment surfaces 31 to 35) on the outer surface 21 (gingival margin 24). Since the living tissue survival surface 30 has a large number of microvillous bodies 41, adhesion to the gums (adhesion to soft tissues) is enhanced and bacteria can be prevented from entering (sealing).
- the dental implant 1 (10A, 10B) is provided with the living tissue attachment surface 30 on the outer surface 11 of the fixture 10 and the outer surface 21 of the abutment 20, respectively, the bonding to the human body becomes stronger.
- the dental implant 1A and the dental implant 1B exhibit the same function and effect.
- the living tissue living surface 30 is provided in a region of the outer surfaces 11 and 21 that is in close contact with (being attached to) the living tissue. As long as the region is in close contact with the living tissue, there may be one place or a plurality of places.
- the area of the living tissue survival surface 30 is arbitrary.
- the living tissue living surface 30 may be provided on almost the entire outer surfaces 11 and 21.
- the living tissue survival surface 30 may be provided over the entire surface of the outer surface 11 that is in close contact with the alveolar bone H (collar surface 12, outer peripheral surface 14, male screw 15).
- the living tissue survival surface 30 may be provided over the entire surface of the outer surface 11 that is in close contact with the gum S (tip surface 13).
- the living tissue survival surface 30 may be provided only on the outer surface 11 or only on the outer surface 21.
- the living tissue activation surface 30 may have different surface properties (surface roughness). This is because the alveolar bone H is coupled to the collar surface 12 and the male screw 15 and the gum S is adhered to the distal end surface 13.
- the living tissue living surface 30 of the gingival margin 24 may be formed to have the same surface properties (surface roughness) as the living tissue living surface 30 of the distal end surface 13. In either case, the gums S are adhered.
- the small groove 43 and the large groove 45 are formed in a semicircular arc shape in cross section.
- the cross-sectional shape may be, for example, a triangle (isosceles triangle) or a rectangle.
- the small groove 43 and the large groove 45 may have a uniform width or a different width in the extending direction.
- the depth may be uniform over the extending direction or different depths.
- the plurality of small grooves 43 and the plurality of large grooves 45 may have uniform widths or different widths.
- the depth may be uniform or different.
- the number of the small grooves 43 and the large grooves 45 is arbitrary.
- the small groove 43 and the large groove 45 are not limited to a straight line and may be curved. It is preferable that the adjacent small grooves 43 and the large grooves 45 are arranged with no gap as much as possible.
- the extending direction of the small groove 43 and the large groove 45 is an arbitrary angle with respect to the vertical direction of the fixture 10.
- the biological tissue survival surface 30 may be mixed on the outer surfaces 11 and 21. Any one or more of the living tissue survival surfaces 31 to 35 may be provided.
- the number, shape, and arrangement of the small grooves 43 and the large grooves 45 can be arbitrarily set on the living tissue survival surface 30.
- the small groove 43 and the large groove 45 may have a form other than that in the living tissue living surface 31 to 35.
- a plurality of large grooves 45 may be arranged in a lattice shape, and a plurality of small grooves 43 may be arranged in a lattice shape (the large grooves 45 and the small grooves 43 intersect and overlap).
- a plurality of large grooves 45 may be arranged in parallel, and a plurality of small grooves 43 may be arranged in a lattice shape (the large grooves 45 and the small grooves 43 intersect and overlap).
- the living tissue survival surface 30 may have only a large number of microvilli 41 and may not have the small grooves 43 and the large grooves 45 (see the biological tissue survival surface 131 of the third embodiment).
- the dental implant 1 (1A, 1B) is formed from a biocompatible ceramic material.
- the fixture 10 (10A, 10B) and the abutment 20 are formed from a ceramic material containing zirconium oxide.
- the manufacturing process of the fixture 10 (10A, 10B) includes a molding process, a sintering process, and a surface processing process.
- the surface processing step is a process for forming a living tissue active surface and includes a laser non-thermal processing step. Since the manufacturing process of the abutment 20 is the same as the manufacturing process of the fixture 10, the description thereof is omitted.
- the outer surface 11 of the zirconia sintered body is irradiated with laser light to form the living tissue active surface 30 (the biological tissue active surfaces 31 to 35) on the outer surface 11.
- a laser beam of an ultrashort pulse laser is used.
- a laser beam of a picosecond laser or a femtosecond laser can be used.
- the ultrashort pulse laser is a laser with a very short pulse having a pulse width (time width) of several picoseconds to several femtoseconds.
- the several picosecond laser is a laser having a pulse width of 1 trillionth of a second.
- the femtosecond laser is a laser having a pulse width of 1/1000 trillion seconds.
- Non-thermal processing is processing in which laser light is irradiated under atmospheric pressure (in air containing moisture) to instantaneously melt, evaporate, and scatter. Since the melted portion is instantly evaporated and scattered and removed, there is very little thermal influence (thermal damage) around the processed part.
- a pulse laser having a large laser beam output peak power or energy density
- a living tissue living surface 30 having a large number of microvilli 41 is formed.
- the number or shape (size) of the microvilli 41 can be changed.
- the small grooves 43 and the large grooves 45 are dug into the outer surface 11 by scanning while irradiating laser light.
- a plurality of small grooves 43 and large grooves 45 are formed by scanning the laser light a plurality of times.
- the processing width (light diameter) by the laser beam can be changed.
- the width and depth of the small groove 43 and the large groove 45 can be changed by adjusting the output (processing width) of the laser beam.
- the widths and depths of the small grooves 43 and the large grooves 45 can be changed according to the number of times of irradiation, the scanning speed, the laser light output, and the like for the same location.
- the large groove 45 is formed first, and then the small groove 43 is formed.
- the small grooves 43, the large grooves 45, or the small grooves 43 and the large grooves 45 are arranged in a lattice shape, scanning is performed in two directions that intersect (directly) the laser beams.
- the crossing angle of scanning at this time is the crossing angle between the small grooves 43, the large grooves 45, or the small grooves 43 and the large grooves 45.
- the outer surface 11 When the outer surface 11 is shaved with laser light to form the large grooves 45 and the small grooves 43, a large number of microvillus bodies 41 are simultaneously formed on the inner surfaces of the large grooves 45 and the small grooves 43.
- the microvillous body 41, the small groove 43, and the large groove 45 are formed at the same time, and become the living tissue active surface 30 (the biological tissue active surfaces 31 to 35).
- laser non-thermal processing of the collar surface 12, the tip surface 13, and the male screw 15 may be performed with different laser beam outputs.
- the surface properties (surface roughness) of the living tissue tissue attachment surface 30 in the collar surface 12, the tip surface 13, and the male screw 15 are varied. This is because the alveolar bone H is coupled to the collar surface 12 and the male screw 15 and the gum S is adhered to the distal end surface 13.
- the fixture 10 After forming the living tissue survival surface 30 (biological tissue survival surfaces 31 to 35), washing and sterilization are performed. In this way, the fixture 10 is manufactured.
- the present invention is not limited to the case where the surface processing step (formation of the living tissue active surface) is performed after the fixture 10 is subjected to the main sintering process.
- a surface processing step may be performed on the zirconia sintered body, followed by a main sintering process.
- the zirconia sintered body contracts, and the microvillus 41, the small groove 43, and the large groove 45 are also reduced. Therefore, the living body tissue attachment surface 30 is formed to be large in consideration of the shrinkage of the zirconia sintered body.
- the fixture 10 biological tissue activating surface 30 of the same shape as the case where it forms after this sintering process is obtained.
- the small groove 43 and the large groove 45 are not limited to being formed by laser non-thermal processing.
- the small groove 43 and the large groove 45 may be molded on the outer surface 11.
- the outer surface 11 Prior to laser non-thermal processing, the outer surface 11 may be laser-heat processed to form the small grooves 43 and the large grooves 45.
- the living tissue active surface 30 (the biological tissue active surfaces 31 to 35) can be formed on the fixture 10 made of zirconium or the like.
- FIG. 10 is a diagram showing a dental implant 3 according to a second embodiment of the present invention, in which (a) a screw-type dental implant 3A and (b) a cylinder-type dental implant 3B.
- the members having the same shape as those in the first embodiment are denoted by the same reference numerals, and the description thereof is omitted.
- the dental implant 3 is a metal (titanium alloy) implant.
- the dental implant 3 includes a screw type dental implant 3A and a cylinder type dental implant 3B.
- the dental implant 3 includes a fixture 50 that is fixed to the alveolar bone H, and an abutment 60 that is fitted to the fixture 50.
- the fixture (implant) 50 is a shaft-like member having a central hole (not shown), and is formed of a titanium alloy (biocompatible material, biocompatible metal material).
- the fixture 50 differs from the fixture 10 only in material.
- the fixture 50 includes a screw type fixture 50 ⁇ / b> A in which a male screw 15 is formed on the outer surface 51 and a cylinder type fixture 50 ⁇ / b> B without the male screw 15.
- the screw-type fixture 50A and the cylinder-type fixture 50B differ only in the presence or absence of the male screw 15.
- the fixture 50 is formed with a living tissue survival surface 70 (a biological tissue survival surface 71).
- the living tissue survival surface 70 is provided on the outer surface 51 of the fixture 50.
- the outer surface 51 includes a tip surface 13, a collar surface 12, and a screw surface (male screw 15).
- the outer surface 51 includes the tip surface 13 and the outer peripheral surface 14.
- the living tissue survival surface 70 is a surface that is excellent in the binding property to the alveolar bone H and the adhesion property to the gum S.
- the living tissue survival surface 70 has a large number of microvilli 81 described later.
- the microvillus 81 is dense. Similar to the biological tissue survival surface 30, the biological tissue survival surface 70 is a fine villi dense surface.
- the living tissue survival surface 70 may have one or both of a small groove 83 and a large groove 85 described later.
- the living tissue living surface 70 is different from the living tissue living surface 30 only in material.
- the microvillus 81 corresponds to the microvilli 41
- the small groove 83 corresponds to the small groove 43
- the large groove 85 corresponds to the large groove 45.
- FIG. 11 and FIG. 12 are photographs taken by SEM of the living tissue survival surface 70 (biological tissue survival surface 71) according to the second embodiment of the present invention, where (a) is an enlargement ratio of 200 times, and (b). Is an enlargement ratio of 500 times, (c) is an enlargement ratio of 2000 times, (d) is an enlargement ratio of 5000 times, and (e) is an enlargement ratio of 10,000 times.
- the biological tissue survival surface 71 is an example of the biological tissue survival surface 70 and has a large number of microvilli 81.
- the shape and the like of the microvilli 81 are the same as those of the microvilli 41.
- the three-dimensional surface roughness Sa of the living tissue living surface 71 and the developed area ratio Sdr of the interface are the same as those of the living tissue living surface 30.
- the living tissue survival surface 71 has a plurality of large grooves 85 arranged in an intersecting manner. A plurality of large grooves 85 are arranged in a lattice pattern. The number and shape of the large grooves 85 are the same as those of the large grooves 45.
- the biological tissue survival surface 71 has the same form as the biological tissue survival surface 31.
- the living tissue living surface 70 a surface having the same form as the living tissue living surfaces 32 to 35 may be formed.
- the number and shape of the small grooves 83 are the same as those of the small grooves 43.
- the living tissue living surface 70 may include only a large number of microvillus bodies 81 and may not include the small grooves 83 and the large grooves 85 (see the living tissue living surface 131 of the third embodiment).
- the abutment (implant) 80 is made of a titanium alloy.
- the abutment 60 is formed with a living tissue survival surface 70 (a biological tissue survival surface 71).
- the living tissue survival surface 70 is provided on the outer surface 61 of the abutment 60.
- the living tissue survival surface 70 is provided on the gingival margin surface (gingival margin 24).
- the abutment 60 is different from the abutment 20 only in material.
- the fixture 50 (50A, 50B) and the abutment 60 have the same functions and effects as the fixture 10 (10A, 10B) and the abutment 20.
- the biological tissue survival surface 70 has the same effects as the biological tissue survival surface 30.
- the living tissue survival surface 70 improves the adhesion to the living tissue (bondability to hard tissue, adhesion to soft tissue) and accelerates the healing of the living tissue. Therefore, the dental implant 3 (3A, 3B) exhibits the same function and effect as the dental implant 1 (1A, 1B).
- the dental implant 3 (3A, 3B) is formed from a biocompatible metal material.
- Fixture 50 (50A, 50B) and abutment 60 are formed from a titanium alloy material.
- the manufacturing process of the fixture 50 includes a machining process and a surface processing process.
- the surface processing step is a process for forming a living tissue active surface, and includes an acid etching step and a laser non-thermal processing step. Since the manufacturing process of the abutment 60 is the same as the manufacturing process of the fixture 50, description thereof is omitted.
- the titanium alloy material is cut with a composite lathe or the like, or plastically processed to form a titanium workpiece (metal workpiece). Blasting is performed on the outer surface 51 of the titanium workpiece. This is to increase the efficiency of acid etching in the subsequent process.
- the blasting process for the titanium workpiece is optional. After machining, the titanium workpiece is washed with water or alcohol.
- the surface processing step first, the outer surface 51 of the titanium processed body is subjected to acid etching.
- the titanium processed body is dipped in hydrochloric acid for edging.
- the concentration of hydrochloric acid is, for example, 1 to 20%
- the liquid temperature is, for example, 30 ° C. to 80 ° C.
- the immersion time is, for example, 10 minutes to 60 minutes.
- the acid used for edging may be other than hydrochloric acid. Sulfuric acid, hydrofluoric acid, nitric acid, and the like, and mixed acids thereof can be used.
- the titanium processed body is ultrasonically cleaned with pure water.
- FIGS. 13 and 14 are photographs obtained by photographing the outer surface 51 of the acid-etched titanium processed body with an SEM.
- FIG. 13A is an enlargement factor of 200 times
- FIG. 13B is an enlargement factor of 500 times
- FIG. The enlargement rate is 2000 times
- (d) is the enlargement rate 5000 times
- (e) is the enlargement rate 10000 times.
- FIGS. 13 and 14 are photographs obtained by photographing the outer surface 51 of the acid-etched titanium processed body with an SEM.
- FIG. 13A is an enlargement factor of 200 times
- FIG. 13B is an enlargement factor of 500 times
- the enlargement ratio 2000 is the enlargement ratio 5000 times
- (e) is the enlargement ratio 10,000 times.
- the outer surface 51 of the acid-etched titanium processed body (acid-etched metal body) has a large number of pores, and further has a number of protrusions with sharp tips around the pores.
- the outer surface 51 is a rough surface (porous), and the conventional fixture is the same as the outer surface (see FIG. 19).
- the microvilli 41 is not yet present on the outer surface 51.
- the outer surface 51 of the titanium processed body is irradiated with laser light to form a living tissue activating surface 70 (biological tissue activating surface 71) on the outer surface 51.
- This laser non-thermal processing step is the same as the laser non-thermal processing step of the living tissue living surface 30.
- the outer surface 51 of the acid-etched titanium workpiece is irradiated with a laser beam such as a femtosecond laser, the outer surface 51 is non-thermally processed (laser non-thermal processing).
- laser non-thermal processing When the outer surface 51 is non-thermally processed with laser light, a large number of microvillus bodies 81 are formed.
- the small groove 83 and the large groove 85 are dug into the outer surface 51 by scanning while irradiating laser light.
- the microvillus body 81, the small groove 83, and the large groove 85 are simultaneously formed to become the living tissue active surface 70 (the biological tissue active surface 71).
- the fixture 50 is manufactured.
- the small groove 83 and the large groove 85 are not limited to being formed by laser non-thermal processing.
- a small groove 83 and a large groove 85 may be formed on the outer surface 51.
- the outer surface 51 Prior to laser non-thermal processing, the outer surface 51 may be laser-heated to form the small groove 83 and the large groove 85.
- the living tissue survival surface 70 (biological tissue survival surface 71) can be formed on the titanium alloy fixture 50 and the like.
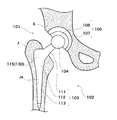
- FIG. 15 is a view showing an artificial hip joint 101 according to the third embodiment of the present invention.
- the artificial hip joint 101 is replaced with a hip joint in order to restore its function when the hip joint is damaged.
- the hip prosthesis 101 is composed of a femoral component 102 embedded in the femur J and an acetabular component 106 embedded in the acetabulum K.
- the femoral component 102 includes a stem 103 and a head 104.
- the stem 103 is embedded in a femur (biological tissue, hard tissue) J and supports the head 104.
- the head 104 is a spherical member that serves as the head of the femur J, and is formed of a biocompatible ceramic material such as zirconia.
- the acetabular component 106 includes a cup 107 and a liner 108.
- the cup 107 is a hook-shaped member embedded in the acetabulum (biological tissue, hard tissue) K, and is formed of a biocompatible ceramic material such as zirconia or a biocompatible metal material such as titanium alloy.
- the living tissue living surface 30, 70 may be formed on the outer surface of the cup 107.
- the liner 108 is a bowl-shaped member fixed to the inside of the cup 107, and is formed of, for example, an ultra high molecular polyethylene resin.
- the liner 108 slidably supports the head 104 and serves as an articulating surface.
- the direction in which the femoral component 102 extends is referred to as the longitudinal direction.
- the head 104 side is referred to as the tip (first end), and the stem 103 side is referred to as the end (second end).
- the width direction of the stem 103 is referred to as a lateral direction.
- the thickness direction of the stem 103 is referred to as the front-rear direction.
- the stem (implant) 103 is inserted into a stricture Ja formed in the femur J to be bone-bonded.
- the stem 103 supports the head 104 and transmits a load to the femur J.
- the stem 103 is formed of a biocompatible resin material (biocompatible material).
- the stem 103 is made of a polyether ether ketone resin (PEKK).
- the stem 103 has a body part 111, a leg part 112, and a neck part 113, which are integrally formed of polyetheretherketone resin.
- the body part 111 is a block-like part extending in the longitudinal direction, and is inserted into the stricture Ja to be bone-bonded.
- the body portion 111 has a vertical length of about 50 mm.
- the width of the body part 111 gradually decreases from the front end side toward the end side.
- the tip side is about 33 mm and the end side is about 15 mm.
- the thickness of the body part 111 is substantially constant from the distal end side toward the distal end side.
- the tip side is about 13 mm and the end side is about 11 mm.
- the leg portion 112 is a substantially quadrangular prism-shaped portion extending in the vertical direction, and is disposed on the terminal side of the body portion 111.
- the leg portion 112 guides the insertion of the body portion 111 into the constriction hole Ja, and maintains the posture after the stem 103 is embedded.
- the leg portion 112 has a vertical length of about 90 mm.
- the leg part 112 becomes gradually thinner from the tip toward the end.
- the neck portion 113 is a substantially cylindrical portion extending in the vertical direction, and is disposed on the distal end side of the body portion 111.
- the neck portion 113 projects from the stricture Ja to introduce a load from the acetabulum side.
- the neck 113 has a length of about 22 mm.
- the neck portion 113 gradually becomes thicker from the tip toward the end.
- a head coupling portion is formed at the tip of the neck portion 113.
- the stem 103 is formed with a living tissue survival surface 130 (a biological tissue survival surface 131).
- the living tissue survival surface 130 is provided on the outer surface 115 of the portion (body portion 111, leg portion 112) to be embedded in the stricture Ja among the outer surface of the stem 103.
- the biological tissue survival surface 130 is also provided at least on the outer surface 115 of the body portion 111.
- the biological tissue survival surface 130 is a surface excellent in the binding property to the natural bone (femur J) similarly to the biological tissue survival surfaces 30 and 70.
- the living tissue survival surface 130 has a large number of microvilli 141 described later.
- the microvillus 141 is dense.
- the biological tissue survival surface 130 is a microvillus dense surface, like the biological tissue survival surfaces 30 and 70.
- the living tissue survival surface 130 includes the microvillus 141, and may further include one or both of a small groove and a large groove.
- the living tissue living surface 130 is different from the living tissue living surfaces 30, 70 only in material.
- the microvilli 141 corresponds to the microvilli 41, 81
- the small grooves correspond to the small grooves 43, 83
- the large grooves correspond to the large grooves 45, 85.
- FIGS. 16 and 17 are photographs taken by SEM of the living tissue survival surface 130 (biological tissue survival surface 131) according to the third embodiment of the present invention, where (a) is an enlargement ratio of 200 times, and (b). Is an enlargement ratio of 500 times, (c) is an enlargement ratio of 2000 times, (d) is an enlargement ratio of 5000 times, and (e) is an enlargement ratio of 10,000 times.
- the biological tissue survival surface 131 is an example of the biological tissue survival surface 130 and has a large number of microvilli 141.
- the shape and the like of the microvilli 141 are the same as those of the microvilli 41 and 81.
- the three-dimensional surface roughness Sa of the living tissue living surface 131 and the developed area ratio Sdr of the interface are the same as those of the living tissue living surfaces 30 and 70.
- the living tissue living surface 130 a surface having the same form as the living tissue living surfaces 31 to 35, 71 and the like may be formed.
- the biological tissue survival surface 130 may have a small groove or a large groove.
- the small grooves and the large grooves correspond to the small grooves 43 and 83 and the large grooves 45 and 85, respectively.
- the number and shape of the small grooves and large grooves on the living tissue active surface 130 are the same as those of the small grooves 43 and 83 and the large grooves 45 and 85.
- the stem 103 has the same effect as the fixtures 10 and 50.
- the biological tissue survival surface 130 has the same effects as the biological tissue survival surfaces 30 and 70.
- the living tissue adhesion surface 130 improves the adhesion to the living tissue (bonding property to the hard tissue) and accelerates the healing of the living tissue.
- the living tissue survival surface 130 can exhibit the ability to adhere to the living tissue only on the resin surface. For this reason, it is not necessary to coat the resin surface with metal or ceramics, or to mix metal or ceramics into the resin material. Since the artificial hip joint 101 is provided with the living tissue survival surface 130 on the outer surface 115 of the stem 103, the connection to the human body becomes stronger. Therefore, the artificial hip joint 101 exhibits the same function and effect as the dental implants 1 and 3.
- the artificial hip joint 101 is formed from a biocompatible resin material.
- the stem 103 is formed from a polyetheretherketone resin.
- the manufacturing process of the stem 103 includes a molding process and a surface processing process.
- the surface processing step is a process for forming a living tissue active surface and includes a laser non-thermal processing step. Since the manufacturing process of the head 104, the cup 107, and the liner 108 is the same as the conventional process, the description thereof is omitted.
- the outer surface 115 of the polyether ether ketone molded body is irradiated with laser light to form a living tissue active surface 130 (biological tissue active surface 131) on the outer surface 115.
- This laser non-thermal processing step is the same as the laser non-thermal processing step of the living tissue living surface 30,70.
- the outer surface 115 of the polyether ether ketone molded body is irradiated with a laser beam such as a femtosecond laser, the outer surface 115 is subjected to non-thermal processing (laser non-thermal processing).
- laser non-thermal processing When the outer surface 115 is non-thermally processed with a laser beam, a living tissue survival surface 131 having a large number of microvilli 141 is formed.
- a small groove or a large groove may be dug into the outer surface 115 by scanning while irradiating with laser light.
- the microvillus 141, the small groove, and the large groove are simultaneously formed to become the living tissue living surface 130.
- the stem 103 is manufactured.
- the small groove 83 and the large groove 85 are not limited to being formed by laser non-thermal processing.
- a small groove 83 and a large groove 85 may be formed on the outer surface 51.
- the outer surface 51 Prior to laser non-thermal processing, the outer surface 51 may be laser-heated to form the small groove 83 and the large groove 85.
- the living tissue survival surface 130 is provided in a region (outer surface 115) of the outer surface of the stem 103 that is in close contact with (is attached to) the femur J. As long as the surface is in close contact with the femur J, there may be one place or a plurality of places.
- the area of the living tissue survival surface 130 is arbitrary.
- the living tissue living surface 130 may be provided on almost the entire outer surface 115.
- the living tissue survival surface 130 may be provided only on the outer surface of the body portion 111 (the surface excluding the outer surface of the leg portion 112) of the outer surface 115.
- the living tissue survival surface 130 (biological tissue survival surface 131) can be formed on the stem 103 made of polyetheretherketone resin.
- the stem 103 is not limited to the polyether ether ketone resin, but may be formed of a biocompatible ceramic material such as zirconia or a biocompatible metal material such as a titanium alloy.
- the living tissue living surface 30, 70 may be formed on the outer surface 115 of the stem 103.
- FIG. 18A and 18B are photographs showing the results of animal experiments, where FIG. 18A is an X-ray photograph of the left mandibular alveolar bone H, FIG. 18B is a photograph of the right mandibular gum S, and FIG. 18C is the left mandibular alveolar bone.
- An X-ray photograph of H, (d) is a photograph of the gum S of the right lower jaw.
- One fixture 10A and one fixture 10B were embedded in the alveolar bone H of the left and right lower jaws of the dog. Four weeks after the implantation, the state of the fixture 10 was photographed with an X-ray or the like.
- Fixation 10 (10A, 10B) and gum S had high adhesion (adhesion to soft tissue), and could prevent (block) bacterial invasion.
- the fixtures (10A, 10B) were bone-bonded so that they could not be extracted from the alveolar bone H by human power.
- the fixtures (10A, 10B) and the alveolar bone H had high bone bonding (bondability to hard tissue), and the bone bonding period could be shortened.
- the fixture 10 (10A, 10B) has improved adhesiveness (bonding property to hard tissue and adhesion property to soft tissue), and has been able to enhance (accelerate) the healing of living tissue.
- the present invention is not limited to the above-described embodiment, and includes various modifications made to the above-described embodiment without departing from the spirit of the present invention. That is, the specific shapes, configurations, and the like given in the embodiment are merely examples, and can be changed as appropriate.
- the implant of the present invention may be an artificial bone, a bone grafting material, or the like. Artificial bones and bone prosthetic materials are used to supplement bone-deleted parts caused by fractures or tumor resections or cartilage removed by lumbar spine surgery.
- the implant of the present invention may be an artificial joint member, an osteosynthesis material used for fixing a fracture site, or a fixing device such as a spine (a spinal implant or a lumbar implant).
- the implant is not limited to being implanted in the living body (inside the body), and may be fixed to the body surface. Implants may be applied not only to humans but also to pets and livestock.
- the living tissue survival surface may have irregularities such as other grooves and wrinkles in addition to or instead of the small grooves and large grooves.
- zirconia zirconium oxide
- a combination of zirconia and carbon, resin, glass, or the like may be used.
- Zirconia (zirconium oxide) may be contained by 50% or more in the volume ratio of the implant.
- Zirconia (zirconium oxide) is contained 90% or more in the volume ratio of the implant.
- alumina aluminum oxide
- yttrium oxide hafnium oxide
- silicone oxide magnesium oxide
- cerium oxide cerium oxide
- the biocompatible metal material may be copper, titanium, titanium alloy, cobalt chromium alloy, or the like.
- the biocompatible resin material may be silicon, nylon, POM, composite material, or the like.
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Oral & Maxillofacial Surgery (AREA)
- Veterinary Medicine (AREA)
- Public Health (AREA)
- General Health & Medical Sciences (AREA)
- Animal Behavior & Ethology (AREA)
- Orthopedic Medicine & Surgery (AREA)
- Engineering & Computer Science (AREA)
- Epidemiology (AREA)
- Dentistry (AREA)
- Biomedical Technology (AREA)
- Cardiology (AREA)
- Heart & Thoracic Surgery (AREA)
- Transplantation (AREA)
- Vascular Medicine (AREA)
- Manufacturing & Machinery (AREA)
- Physics & Mathematics (AREA)
- Ceramic Engineering (AREA)
- Developmental Biology & Embryology (AREA)
- Optics & Photonics (AREA)
- Geometry (AREA)
- Dental Prosthetics (AREA)
- Prostheses (AREA)
- Surgical Instruments (AREA)
Priority Applications (11)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2018519064A JP6455634B2 (ja) | 2016-11-10 | 2017-11-07 | 生体組織活着面の形成方法、インプラントの製造方法 |
| US16/339,571 US11051915B2 (en) | 2016-11-10 | 2017-11-07 | Biological tissue rootage face, implant, method for forming biological tissue rootage face, and method for producing implant |
| KR1020197010160A KR102085312B1 (ko) | 2016-11-10 | 2017-11-07 | 생체 조직 활착면, 임플란트, 생체 조직 활착면의 형성 방법, 임플란트의 제조 방법 |
| ES17868814T ES2903424T3 (es) | 2016-11-10 | 2017-11-07 | Superficie de adherencia de tejido biológico, implante, método de formación de superficie de adhesión de tejido biológico y método de fabricación del implante |
| CN201780069718.8A CN109963523A (zh) | 2016-11-10 | 2017-11-07 | 生物体组织附着面、植入物、生物体组织附着面的形成方法、植入物的制造方法 |
| AU2017356025A AU2017356025B2 (en) | 2016-11-10 | 2017-11-07 | Biological tissue rootage face, implant, method for forming biological tissue rootage face, and method for producing implant |
| EP17868814.9A EP3539502B1 (en) | 2016-11-10 | 2017-11-07 | Biological tissue adhesion surface, implant, method of forming biological tissue adhesion surface, and method of manufacturing implant |
| IL266454A IL266454B (en) | 2016-11-10 | 2019-05-05 | Implant and method of creating the implant |
| AU2020200112A AU2020200112B9 (en) | 2016-11-10 | 2020-01-07 | Biological tissue rootage face, implant, method for forming biological tissue rootage face, and method for producing implant |
| US16/912,083 US11596505B2 (en) | 2016-11-10 | 2020-06-25 | Biological tissue rootage face, implant, method for forming biological tissue rootage face, and method for producing implant |
| IL276562A IL276562B (en) | 2016-11-10 | 2020-08-06 | A method for creating root surfaces for biological tissue |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2016219467 | 2016-11-10 | ||
| JP2016-219467 | 2016-11-10 |
Related Child Applications (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US16/339,571 A-371-Of-International US11051915B2 (en) | 2016-11-10 | 2017-11-07 | Biological tissue rootage face, implant, method for forming biological tissue rootage face, and method for producing implant |
| US16/912,083 Division US11596505B2 (en) | 2016-11-10 | 2020-06-25 | Biological tissue rootage face, implant, method for forming biological tissue rootage face, and method for producing implant |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2018088389A1 true WO2018088389A1 (ja) | 2018-05-17 |
Family
ID=62109574
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/JP2017/040076 Ceased WO2018088389A1 (ja) | 2016-11-10 | 2017-11-07 | 生体組織活着面、インプラント、生体組織活着面の形成方法、インプラントの製造方法 |
Country Status (9)
| Country | Link |
|---|---|
| US (2) | US11051915B2 (enExample) |
| EP (1) | EP3539502B1 (enExample) |
| JP (4) | JP6455634B2 (enExample) |
| KR (1) | KR102085312B1 (enExample) |
| CN (1) | CN109963523A (enExample) |
| AU (2) | AU2017356025B2 (enExample) |
| ES (1) | ES2903424T3 (enExample) |
| IL (2) | IL266454B (enExample) |
| WO (1) | WO2018088389A1 (enExample) |
Cited By (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2019217187A (ja) * | 2018-06-22 | 2019-12-26 | 慶達科技股▲ふん▼有限公司 | インプラント材 |
| US20230181292A1 (en) * | 2020-08-20 | 2023-06-15 | B2Lab Co.,Ltd. | Surface-treated implant structure |
| JP2023550531A (ja) * | 2020-12-18 | 2023-12-01 | スパイン・ウェーヴ・インコーポレイテッド | 拡張可能tlifデバイスおよび関連する挿入移植器具 |
Families Citing this family (12)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20220054713A1 (en) * | 2018-10-23 | 2022-02-24 | Osaka University | Implant material and method of manufacturing the implant material |
| CN110590401A (zh) * | 2019-09-18 | 2019-12-20 | 北京大学口腔医学院 | 一种烧结氧化锆材料的表面处理方法及其应用 |
| JP7355332B2 (ja) * | 2019-12-26 | 2023-10-03 | 国立大学法人大阪大学 | インプラント |
| JP2021126479A (ja) * | 2020-02-10 | 2021-09-02 | 明俊 町田 | Cad/camシステムによる陶材の一つであるジルコニア製品の製作方法と装飾方法 |
| JP7512045B2 (ja) | 2020-02-14 | 2024-07-08 | Dgshape株式会社 | 義歯用アバットメントの保持アタッチメント、切削加工機、および義歯用アバットメントの製作方法 |
| KR102195610B1 (ko) * | 2020-09-17 | 2020-12-28 | 장천석 | 치과용 임플란트의 표면마찰 계수를 증가시키는 레이저 표면 처리 방법 |
| KR102195615B1 (ko) * | 2020-09-17 | 2020-12-28 | 장천석 | 치과용 임플란트의 표면마찰 계수를 증가시키는 그릿 및 레이저 융합 표면 처리 방법 |
| US12029653B1 (en) | 2023-06-14 | 2024-07-09 | Spine Wave, Inc. | Medical implant having a textured tissue contact surface |
| CN117550922A (zh) * | 2023-11-10 | 2024-02-13 | 天津医科大学口腔医院 | 一种通过飞秒激光增加氧化锆表面粘接性的方法 |
| KR20250104219A (ko) * | 2023-12-29 | 2025-07-08 | 오스템임플란트 주식회사 | 치과용 임플란트 표면처리방법 및 그 방법에 의해 표면 처리된 치과용 임플란트 |
| KR20250148762A (ko) | 2024-04-03 | 2025-10-15 | 단국대학교 천안캠퍼스 산학협력단 | 레이저 처리된 나노 웨이브 구조의 표면을 갖는 생체 적합성 재료를 포함하는 골 재생 촉진용 임플란트 및 이의 제조방법 |
| CN120036965B (zh) * | 2025-02-07 | 2025-12-05 | 北京瑞瓷医疗科技有限公司 | 一种一体化高强度种植体及制造方法 |
Citations (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20010039454A1 (en) * | 1993-11-02 | 2001-11-08 | John Ricci | Orthopedic implants having ordered microgeometric surface patterns |
| JP2007525280A (ja) * | 2004-07-29 | 2007-09-06 | バイオロック インターナショナル, インコーポレイテッド | 骨統合の促進のための外科用インプラント |
| JP2010227551A (ja) * | 2009-03-03 | 2010-10-14 | Puente:Kk | 骨芽細胞系細胞の増殖と分化を促進する医療用ナノ表面加工チタンおよびその製造方法 |
| JP2011509098A (ja) * | 2007-07-09 | 2011-03-24 | アストラ・テック・アクチエボラーグ | ナノ表面 |
| JP2014124500A (ja) * | 2012-12-27 | 2014-07-07 | Nanto Precision Co Ltd | インプラント体、アバットメント体、インプラント、インプラントの製造方法 |
Family Cites Families (25)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5310343A (en) | 1992-10-14 | 1994-05-10 | Jiro Hasegawa | Endo-osseous implant |
| US6419491B1 (en) | 1993-11-02 | 2002-07-16 | Bio-Lok International, Inc. | Dental implant system with repeating microgeometric surface patterns |
| US5639237A (en) * | 1995-06-08 | 1997-06-17 | Fontenot; Mark G | Dental prosthesis having indentations |
| JP3648968B2 (ja) * | 1998-02-09 | 2005-05-18 | 松下電工株式会社 | 生体用ジルコニア系複合セラミック焼結体 |
| DE59800332D1 (de) * | 1998-12-11 | 2000-12-14 | Dinkelacker Wolfgang | Zahnimplantat und Verfahren zu seiner Herstellung |
| US7018418B2 (en) * | 2001-01-25 | 2006-03-28 | Tecomet, Inc. | Textured surface having undercut micro recesses in a surface |
| EP1601301A1 (de) | 2003-05-03 | 2005-12-07 | Wolfgang Dinkelacker | Einschraubbares knochenimplantat |
| US7666522B2 (en) * | 2003-12-03 | 2010-02-23 | IMDS, Inc. | Laser based metal deposition (LBMD) of implant structures |
| US20090035723A1 (en) * | 2007-08-03 | 2009-02-05 | Claus Daniel | Material with a repetitive pattern of micro-features for application in a living organism and method of fabrication |
| JP5207480B2 (ja) | 2008-05-30 | 2013-06-12 | 株式会社ナントー精密 | インプラント体及びその製造方法並びに歯科用インプラント |
| US8758012B2 (en) | 2008-07-14 | 2014-06-24 | Nobel Biocare Services Ag | Compact dental implant |
| JP5356859B2 (ja) | 2009-02-26 | 2013-12-04 | 菊水化学工業株式会社 | インプラントフィクスチャー |
| EP2233108B1 (en) | 2009-03-23 | 2015-04-29 | Dentsply IH AB | Bone fixture |
| EP2263991A1 (en) * | 2009-06-19 | 2010-12-22 | Nobel Biocare Services AG | Dental application coating |
| EP2662051A1 (en) | 2012-05-11 | 2013-11-13 | Dentsply IH AB | Medical device having a surface comprising nanoparticles |
| KR101311990B1 (ko) * | 2013-07-18 | 2013-09-27 | 오스템임플란트 주식회사 | 임플란트 표면의 생체활성과 골결합력 및 골융합을 증진시키는 물질이 코팅된 임플란트와 그 제조방법 및 임플란트의 보관 방법 |
| CN106029931A (zh) * | 2013-10-09 | 2016-10-12 | 怡康医疗股份有限公司 | 用于医疗装置的改善的金属合金 |
| CN103519911B (zh) * | 2013-10-22 | 2016-04-13 | 福建医科大学附属口腔医院 | 牙种植体 |
| CN103892930B (zh) * | 2014-04-18 | 2017-04-05 | 南宁越洋科技有限公司 | 氧化锆陶瓷熔附生物活性玻璃陶瓷人工骨粉的牙种植体 |
| JP6397228B2 (ja) | 2014-06-12 | 2018-09-26 | 富士フィルター工業株式会社 | 歯科用アバットメント及び製造方法 |
| JP6590343B2 (ja) * | 2014-09-11 | 2019-10-16 | 国立研究開発法人産業技術総合研究所 | ジルコニア系セラミックスの表面構造形成方法及びジルコニア系セラミックス |
| EP3034033A1 (en) * | 2014-12-16 | 2016-06-22 | Nobel Biocare Services AG | Dental implant |
| JP2019136472A (ja) * | 2017-07-07 | 2019-08-22 | ダイセルポリマー株式会社 | インプラントとその製造方法 |
| JP6596639B2 (ja) * | 2017-08-28 | 2019-10-30 | 株式会社ナントー | ステム、大腿骨コンポーネント、人工股関節 |
| EP4000555B1 (en) * | 2020-11-13 | 2024-07-10 | Common Sense Engineering and Consult | An anatomical dental implant arranged to be implanted in a naturally occurring cavity of the jawbone |
-
2017
- 2017-11-07 EP EP17868814.9A patent/EP3539502B1/en active Active
- 2017-11-07 CN CN201780069718.8A patent/CN109963523A/zh active Pending
- 2017-11-07 WO PCT/JP2017/040076 patent/WO2018088389A1/ja not_active Ceased
- 2017-11-07 ES ES17868814T patent/ES2903424T3/es active Active
- 2017-11-07 AU AU2017356025A patent/AU2017356025B2/en active Active
- 2017-11-07 US US16/339,571 patent/US11051915B2/en active Active
- 2017-11-07 JP JP2018519064A patent/JP6455634B2/ja active Active
- 2017-11-07 KR KR1020197010160A patent/KR102085312B1/ko active Active
-
2018
- 2018-04-16 JP JP2018078125A patent/JP6531301B2/ja active Active
- 2018-10-15 JP JP2018194164A patent/JP6574963B2/ja active Active
-
2019
- 2019-05-05 IL IL266454A patent/IL266454B/en active IP Right Grant
- 2019-06-28 JP JP2019122229A patent/JP6647503B2/ja active Active
-
2020
- 2020-01-07 AU AU2020200112A patent/AU2020200112B9/en active Active
- 2020-06-25 US US16/912,083 patent/US11596505B2/en active Active
- 2020-08-06 IL IL276562A patent/IL276562B/en unknown
Patent Citations (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20010039454A1 (en) * | 1993-11-02 | 2001-11-08 | John Ricci | Orthopedic implants having ordered microgeometric surface patterns |
| JP2007525280A (ja) * | 2004-07-29 | 2007-09-06 | バイオロック インターナショナル, インコーポレイテッド | 骨統合の促進のための外科用インプラント |
| JP2011509098A (ja) * | 2007-07-09 | 2011-03-24 | アストラ・テック・アクチエボラーグ | ナノ表面 |
| JP2010227551A (ja) * | 2009-03-03 | 2010-10-14 | Puente:Kk | 骨芽細胞系細胞の増殖と分化を促進する医療用ナノ表面加工チタンおよびその製造方法 |
| JP2014124500A (ja) * | 2012-12-27 | 2014-07-07 | Nanto Precision Co Ltd | インプラント体、アバットメント体、インプラント、インプラントの製造方法 |
Non-Patent Citations (3)
| Title |
|---|
| See also references of EP3539502A4 * |
| SHINONAGA, TOGO ET AL: "Creation of New Functional Biomaterials by Periodic Nanostructures Formation with Femtosecond Laser", JOURNAL OF THE JAPAN SOCIETY OF PRECISION ENGINEERING, vol. 81, no. 8, 5 August 2015 (2015-08-05), pages 726 - 730, XP055502941 * |
| SHINONAGA, TOGO: "Cell Spreading on titanium dioxide film formed and modified with aerosol beam and femtosecond laser", APPLIED SURFACE SCIENCE, vol. 288, 1 January 2014 (2014-01-01), pages 649 - 653, XP028780756 * |
Cited By (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2019217187A (ja) * | 2018-06-22 | 2019-12-26 | 慶達科技股▲ふん▼有限公司 | インプラント材 |
| US20230181292A1 (en) * | 2020-08-20 | 2023-06-15 | B2Lab Co.,Ltd. | Surface-treated implant structure |
| JP2023550531A (ja) * | 2020-12-18 | 2023-12-01 | スパイン・ウェーヴ・インコーポレイテッド | 拡張可能tlifデバイスおよび関連する挿入移植器具 |
Also Published As
| Publication number | Publication date |
|---|---|
| EP3539502A1 (en) | 2019-09-18 |
| AU2017356025B2 (en) | 2020-05-14 |
| JP2019034177A (ja) | 2019-03-07 |
| US20200038150A1 (en) | 2020-02-06 |
| IL276562B (en) | 2022-04-01 |
| ES2903424T3 (es) | 2022-04-01 |
| US11051915B2 (en) | 2021-07-06 |
| IL276562A (en) | 2020-09-30 |
| JP6455634B2 (ja) | 2019-01-23 |
| EP3539502A4 (en) | 2020-06-17 |
| JPWO2018088389A1 (ja) | 2018-11-15 |
| AU2020200112B2 (en) | 2020-07-23 |
| JP6574963B2 (ja) | 2019-09-18 |
| EP3539502B1 (en) | 2021-10-13 |
| US20200345465A1 (en) | 2020-11-05 |
| US11596505B2 (en) | 2023-03-07 |
| JP6647503B2 (ja) | 2020-02-14 |
| CN109963523A (zh) | 2019-07-02 |
| JP2019188197A (ja) | 2019-10-31 |
| AU2017356025A1 (en) | 2019-05-16 |
| KR102085312B1 (ko) | 2020-03-06 |
| JP2018138180A (ja) | 2018-09-06 |
| KR20190052689A (ko) | 2019-05-16 |
| AU2020200112A1 (en) | 2020-01-30 |
| AU2020200112B9 (en) | 2021-06-17 |
| IL266454B (en) | 2021-05-31 |
| IL266454A (en) | 2019-06-30 |
| JP6531301B2 (ja) | 2019-06-19 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP6574963B2 (ja) | 生体組織活着面、インプラント、生体組織活着面の形成方法、インプラントの製造方法 | |
| JP6284169B2 (ja) | 多孔質な3次元構造を有するインプラント用材料 | |
| JP2018138180A5 (enExample) | ||
| JP2014124500A (ja) | インプラント体、アバットメント体、インプラント、インプラントの製造方法 | |
| JP6589214B1 (ja) | ヘッド、大腿骨コンポーネント、人工股関節 | |
| JP2015051240A (ja) | インプラント体、インプラント、インプラント体の製造方法 | |
| KR20230028093A (ko) | 표면 처리된 임플란트 구조체 | |
| JP6488459B2 (ja) | フィクスチャー、インプラント | |
| JP2009045246A (ja) | 骨芽細胞系細胞の増殖と分化を促進する医療用ナノ表面加工チタンおよびその製造方法 | |
| Mohammed et al. | Enhancing Osteoblast Response and Biointegration in vivo: Grown Zirconium Oxide Nanotube Bundles on Zirconium Surgical Screw | |
| Mahrous et al. | The effect of atmospheric plasma-sprayed peek implants on osseointegration | |
| KR20120014307A (ko) | 하이드록시 아파타이트가 코팅된 다공성 임플란트 픽스쳐 및 그의 제조방법 | |
| Mohammadi et al. | Investigation of Dental Implant’s Titanium Surface Hydrophilicity Effect on Biocompatibility and Osseointegration | |
| CN107280788B (zh) | 一种牙种植体 | |
| KR101198694B1 (ko) | 표면에 하이드록시 아파타이트가 석출된 다공성 임플란트 픽스쳐 및 그의 제조방법 | |
| Faiz et al. | Comprehensive Review of Dental Implant Surface Characterization: Techniques and Clinical Implications | |
| Figliuzzi | Carlo Mangano, Francesco Mangano, Jamil Awad Shibli, Giuseppe Luongo, Michele De Franco, Francesco Briguglio |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| WWE | Wipo information: entry into national phase |
Ref document number: 2018519064 Country of ref document: JP |
|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 17868814 Country of ref document: EP Kind code of ref document: A1 |
|
| ENP | Entry into the national phase |
Ref document number: 20197010160 Country of ref document: KR Kind code of ref document: A |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| ENP | Entry into the national phase |
Ref document number: 2017356025 Country of ref document: AU Date of ref document: 20171107 Kind code of ref document: A |
|
| ENP | Entry into the national phase |
Ref document number: 2017868814 Country of ref document: EP Effective date: 20190611 |