WO2016024600A1 - Composition de résine colorée pour filtre coloré, filtre coloré et dispositif d'affichage - Google Patents
Composition de résine colorée pour filtre coloré, filtre coloré et dispositif d'affichage Download PDFInfo
- Publication number
- WO2016024600A1 WO2016024600A1 PCT/JP2015/072807 JP2015072807W WO2016024600A1 WO 2016024600 A1 WO2016024600 A1 WO 2016024600A1 JP 2015072807 W JP2015072807 W JP 2015072807W WO 2016024600 A1 WO2016024600 A1 WO 2016024600A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- group
- resin composition
- mass
- colored resin
- color filter
- Prior art date
Links
Images
Classifications
-
- G—PHYSICS
- G03—PHOTOGRAPHY; CINEMATOGRAPHY; ANALOGOUS TECHNIQUES USING WAVES OTHER THAN OPTICAL WAVES; ELECTROGRAPHY; HOLOGRAPHY
- G03F—PHOTOMECHANICAL PRODUCTION OF TEXTURED OR PATTERNED SURFACES, e.g. FOR PRINTING, FOR PROCESSING OF SEMICONDUCTOR DEVICES; MATERIALS THEREFOR; ORIGINALS THEREFOR; APPARATUS SPECIALLY ADAPTED THEREFOR
- G03F7/00—Photomechanical, e.g. photolithographic, production of textured or patterned surfaces, e.g. printing surfaces; Materials therefor, e.g. comprising photoresists; Apparatus specially adapted therefor
- G03F7/004—Photosensitive materials
- G03F7/09—Photosensitive materials characterised by structural details, e.g. supports, auxiliary layers
- G03F7/105—Photosensitive materials characterised by structural details, e.g. supports, auxiliary layers having substances, e.g. indicators, for forming visible images
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09B—ORGANIC DYES OR CLOSELY-RELATED COMPOUNDS FOR PRODUCING DYES, e.g. PIGMENTS; MORDANTS; LAKES
- C09B63/00—Lakes
-
- G—PHYSICS
- G02—OPTICS
- G02B—OPTICAL ELEMENTS, SYSTEMS OR APPARATUS
- G02B5/00—Optical elements other than lenses
- G02B5/20—Filters
-
- G—PHYSICS
- G02—OPTICS
- G02F—OPTICAL DEVICES OR ARRANGEMENTS FOR THE CONTROL OF LIGHT BY MODIFICATION OF THE OPTICAL PROPERTIES OF THE MEDIA OF THE ELEMENTS INVOLVED THEREIN; NON-LINEAR OPTICS; FREQUENCY-CHANGING OF LIGHT; OPTICAL LOGIC ELEMENTS; OPTICAL ANALOGUE/DIGITAL CONVERTERS
- G02F1/00—Devices or arrangements for the control of the intensity, colour, phase, polarisation or direction of light arriving from an independent light source, e.g. switching, gating or modulating; Non-linear optics
- G02F1/01—Devices or arrangements for the control of the intensity, colour, phase, polarisation or direction of light arriving from an independent light source, e.g. switching, gating or modulating; Non-linear optics for the control of the intensity, phase, polarisation or colour
- G02F1/13—Devices or arrangements for the control of the intensity, colour, phase, polarisation or direction of light arriving from an independent light source, e.g. switching, gating or modulating; Non-linear optics for the control of the intensity, phase, polarisation or colour based on liquid crystals, e.g. single liquid crystal display cells
- G02F1/133—Constructional arrangements; Operation of liquid crystal cells; Circuit arrangements
- G02F1/1333—Constructional arrangements; Manufacturing methods
- G02F1/1335—Structural association of cells with optical devices, e.g. polarisers or reflectors
-
- G—PHYSICS
- G02—OPTICS
- G02F—OPTICAL DEVICES OR ARRANGEMENTS FOR THE CONTROL OF LIGHT BY MODIFICATION OF THE OPTICAL PROPERTIES OF THE MEDIA OF THE ELEMENTS INVOLVED THEREIN; NON-LINEAR OPTICS; FREQUENCY-CHANGING OF LIGHT; OPTICAL LOGIC ELEMENTS; OPTICAL ANALOGUE/DIGITAL CONVERTERS
- G02F1/00—Devices or arrangements for the control of the intensity, colour, phase, polarisation or direction of light arriving from an independent light source, e.g. switching, gating or modulating; Non-linear optics
- G02F1/01—Devices or arrangements for the control of the intensity, colour, phase, polarisation or direction of light arriving from an independent light source, e.g. switching, gating or modulating; Non-linear optics for the control of the intensity, phase, polarisation or colour
- G02F1/13—Devices or arrangements for the control of the intensity, colour, phase, polarisation or direction of light arriving from an independent light source, e.g. switching, gating or modulating; Non-linear optics for the control of the intensity, phase, polarisation or colour based on liquid crystals, e.g. single liquid crystal display cells
- G02F1/133—Constructional arrangements; Operation of liquid crystal cells; Circuit arrangements
- G02F1/1333—Constructional arrangements; Manufacturing methods
- G02F1/1335—Structural association of cells with optical devices, e.g. polarisers or reflectors
- G02F1/133509—Filters, e.g. light shielding masks
- G02F1/133514—Colour filters
-
- G—PHYSICS
- G03—PHOTOGRAPHY; CINEMATOGRAPHY; ANALOGOUS TECHNIQUES USING WAVES OTHER THAN OPTICAL WAVES; ELECTROGRAPHY; HOLOGRAPHY
- G03F—PHOTOMECHANICAL PRODUCTION OF TEXTURED OR PATTERNED SURFACES, e.g. FOR PRINTING, FOR PROCESSING OF SEMICONDUCTOR DEVICES; MATERIALS THEREFOR; ORIGINALS THEREFOR; APPARATUS SPECIALLY ADAPTED THEREFOR
- G03F7/00—Photomechanical, e.g. photolithographic, production of textured or patterned surfaces, e.g. printing surfaces; Materials therefor, e.g. comprising photoresists; Apparatus specially adapted therefor
- G03F7/0005—Production of optical devices or components in so far as characterised by the lithographic processes or materials used therefor
- G03F7/0007—Filters, e.g. additive colour filters; Components for display devices
-
- G—PHYSICS
- G03—PHOTOGRAPHY; CINEMATOGRAPHY; ANALOGOUS TECHNIQUES USING WAVES OTHER THAN OPTICAL WAVES; ELECTROGRAPHY; HOLOGRAPHY
- G03F—PHOTOMECHANICAL PRODUCTION OF TEXTURED OR PATTERNED SURFACES, e.g. FOR PRINTING, FOR PROCESSING OF SEMICONDUCTOR DEVICES; MATERIALS THEREFOR; ORIGINALS THEREFOR; APPARATUS SPECIALLY ADAPTED THEREFOR
- G03F7/00—Photomechanical, e.g. photolithographic, production of textured or patterned surfaces, e.g. printing surfaces; Materials therefor, e.g. comprising photoresists; Apparatus specially adapted therefor
- G03F7/004—Photosensitive materials
-
- G—PHYSICS
- G03—PHOTOGRAPHY; CINEMATOGRAPHY; ANALOGOUS TECHNIQUES USING WAVES OTHER THAN OPTICAL WAVES; ELECTROGRAPHY; HOLOGRAPHY
- G03F—PHOTOMECHANICAL PRODUCTION OF TEXTURED OR PATTERNED SURFACES, e.g. FOR PRINTING, FOR PROCESSING OF SEMICONDUCTOR DEVICES; MATERIALS THEREFOR; ORIGINALS THEREFOR; APPARATUS SPECIALLY ADAPTED THEREFOR
- G03F7/00—Photomechanical, e.g. photolithographic, production of textured or patterned surfaces, e.g. printing surfaces; Materials therefor, e.g. comprising photoresists; Apparatus specially adapted therefor
- G03F7/004—Photosensitive materials
- G03F7/027—Non-macromolecular photopolymerisable compounds having carbon-to-carbon double bonds, e.g. ethylenic compounds
-
- G—PHYSICS
- G03—PHOTOGRAPHY; CINEMATOGRAPHY; ANALOGOUS TECHNIQUES USING WAVES OTHER THAN OPTICAL WAVES; ELECTROGRAPHY; HOLOGRAPHY
- G03F—PHOTOMECHANICAL PRODUCTION OF TEXTURED OR PATTERNED SURFACES, e.g. FOR PRINTING, FOR PROCESSING OF SEMICONDUCTOR DEVICES; MATERIALS THEREFOR; ORIGINALS THEREFOR; APPARATUS SPECIALLY ADAPTED THEREFOR
- G03F7/00—Photomechanical, e.g. photolithographic, production of textured or patterned surfaces, e.g. printing surfaces; Materials therefor, e.g. comprising photoresists; Apparatus specially adapted therefor
- G03F7/004—Photosensitive materials
- G03F7/027—Non-macromolecular photopolymerisable compounds having carbon-to-carbon double bonds, e.g. ethylenic compounds
- G03F7/028—Non-macromolecular photopolymerisable compounds having carbon-to-carbon double bonds, e.g. ethylenic compounds with photosensitivity-increasing substances, e.g. photoinitiators
- G03F7/031—Organic compounds not covered by group G03F7/029
-
- G—PHYSICS
- G03—PHOTOGRAPHY; CINEMATOGRAPHY; ANALOGOUS TECHNIQUES USING WAVES OTHER THAN OPTICAL WAVES; ELECTROGRAPHY; HOLOGRAPHY
- G03F—PHOTOMECHANICAL PRODUCTION OF TEXTURED OR PATTERNED SURFACES, e.g. FOR PRINTING, FOR PROCESSING OF SEMICONDUCTOR DEVICES; MATERIALS THEREFOR; ORIGINALS THEREFOR; APPARATUS SPECIALLY ADAPTED THEREFOR
- G03F7/00—Photomechanical, e.g. photolithographic, production of textured or patterned surfaces, e.g. printing surfaces; Materials therefor, e.g. comprising photoresists; Apparatus specially adapted therefor
- G03F7/004—Photosensitive materials
- G03F7/038—Macromolecular compounds which are rendered insoluble or differentially wettable
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09B—ORGANIC DYES OR CLOSELY-RELATED COMPOUNDS FOR PRODUCING DYES, e.g. PIGMENTS; MORDANTS; LAKES
- C09B11/00—Diaryl- or thriarylmethane dyes
- C09B11/28—Pyronines ; Xanthon, thioxanthon, selenoxanthan, telluroxanthon dyes
Definitions
- the present invention relates to a colored resin composition for a color filter, a color filter, and a display device.
- Color filters are used in these liquid crystal display devices and organic light emitting display devices.
- a backlight is used as a light source, the amount of light is controlled by electrically driving the liquid crystal, and color expression is performed by the light passing through a color filter. Therefore, a color filter must be present in the color representation of a liquid crystal television and plays a major role in determining the performance of the display.
- color adjustment of pixels may be performed using a color filter, or a color image may be formed in the same manner as a liquid crystal display device using a color filter for a white light emitting organic light emitting element.
- An image display device including a color filter affects the design and performance of a mobile terminal in order to be directly linked to the usable time and charging frequency of the mobile terminal.
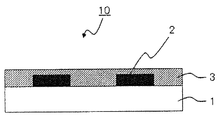
- the color filter is generally formed on a transparent substrate, a transparent layer formed on the transparent substrate, and composed of a colored layer of three primary colors of red, green, and blue, and on the transparent substrate so as to partition each colored pattern. And a light shielding portion formed.
- a pigment dispersion method using a pigment having excellent heat resistance and light resistance as a coloring material has been widely used.
- photosensitive resin compositions for color filters using dyes with high transmittance are being studied, and in order to further improve the heat resistance and light resistance of dyes.
- the use of a rake color material in which a dye is insolubilized has been studied.
- Patent Document 1 discloses a blue pigment for a color filter comprising an anion containing one or more elements selected from tungsten, silicon, and phosphorus as basic elements and oxygen as essential elements. Therefore, it is said that the use of the pigment can provide a liquid crystal display device capable of providing a liquid crystal display with little color change during high-temperature firing and excellent luminance over a long period of time.
- Patent Documents 2 and 3 a heteropolyoxo containing a specific ratio of phosphorus or silicon, molybdenum, and tungsten as a counter anion of a triarylmethane dye cation as a rake colorant having excellent heat resistance and light resistance Lake color materials using metalate anions are described.
- Patent Document 4 includes a specific color filter colored resin composition containing a specific colorant containing a divalent or higher cation in which a plurality of dye skeletons are cross-linked by a crosslinkable group and a divalent or higher anion, A color filter using a color material is disclosed. According to Patent Document 4, it is possible to form a colored layer having high contrast and excellent solvent resistance and electrical reliability by using a colored resin composition for a color filter containing the color material.
- a colored resin composition for forming a colored layer of a color filter usually contains a curable binder component for forming a layer, and in order to improve adhesion to the substrate, a silane coupling agent is used. Widely used (for example, Patent Documents 4 to 6).
- the colored resin composition for a color filter according to the present invention contains a lake color material, a dispersant, an alkali-soluble resin, a polyfunctional monomer, an initiator, and a solvent.
- the alkali-soluble resin is a resin having a hydrocarbon ring and an ethylenic double bond, and an acid value of 80 mgKOH / g or more and 300 mgKOH / g or less,
- the content ratio of the silane coupling agent is 1% by mass or less based on the total solid content in the colored resin composition.
- the lake color material is preferably a lake color material containing a polyacid anion from the viewpoint of excellent heat resistance and light resistance.
- the lake color material includes a lake color material having a triarylmethane dye from the viewpoint of improving the luminance of the color filter.
- the rake color material includes a color material represented by the following general formula (I), whereby a color filter having excellent heat resistance and light resistance and high brightness is obtained. It is preferable from the point which can form.
- A is an a-valent organic group in which the carbon atom directly bonded to N has no ⁇ bond, and the organic group is saturated aliphatic carbonized at least at the terminal directly bonded to N.
- R i to R v each independently represents a hydrogen atom, an optionally substituted alkyl group or an optionally substituted aryl group, and R ii and R iii , R iv and R v may combine to form a ring structure
- Ar 1 represents a divalent aromatic group which may have a substituent
- a plurality of R i to R v and Ar 1 may be the same or different.
- a and c represent an integer of 2 or more
- b and d represent an integer of 1 or more.
- e is 0 or 1, and when e is 0, there is no bond.
- a plurality of e may be the same or different.
- the hydrocarbon ring of the alkali-soluble resin has a cyclopentyl group, a cyclohexyl group, a bornyl group, an isobornyl group, a dicyclopentanyl group, a dicyclopentenyl group, an adamantyl group, And at least one selected from the group consisting of substituents represented by the following chemical formula (A) is excellent in heat resistance and solvent resistance by improving the curability of the colored layer, and suppresses water stain. From the viewpoint of excellent substrate adhesion of the colored layer.
- the alkali-soluble resin is a compound represented by the following general formula (B) to improve the solvent resistance by improving the curability of the colored layer, This is preferable because water stain is improved and the remaining film ratio of the colored layer is increased.
- X represents a group represented by the following general formula (D)
- Y each independently represents a residue of a polyvalent carboxylic acid or its acid anhydride
- R i represents a group represented by the following general formula (C)
- j is an integer of 0 to 4
- k is an integer of 0 to 3
- n is an integer of 1 or more.
- R ii represents a hydrogen atom or a methyl group, and R iii independently represents a hydrogen atom or a methyl group.
- R iv is independently a hydrogen atom, an alkyl group having 1 to 5 carbon atoms, a phenyl group, or a halogen atom, and R v is —O— or —OCH 2 CH. 2 O- is shown.
- the initiator is preferably an oxime ester photopolymerization initiator from the viewpoint of suppressing water stain and being excellent in solvent resistance and sensitivity.
- the colored resin composition for a color filter of the present invention preferably further contains at least one selected from a dioxazine-based color material and a xanthene-based color material from the viewpoint that it can be adjusted to a desired color tone.
- the color filter according to the present invention is a color filter comprising at least a transparent substrate and a colored layer provided on the transparent substrate, and at least one of the colored layers is a colored resin composition for a color filter according to the present invention. It has a colored layer formed by curing a product.
- the display device according to the present invention includes the color filter according to the present invention.
- the present invention has been made in view of the above circumstances, and is a colored resin composition for a color filter that can form a colored layer having excellent sensitivity over time, substrate adhesion, heat resistance, and solvent resistance, A color filter formed using the colored resin composition for the color filter and a display device using the color filter can be provided.
- FIG. 1 is a schematic sectional view showing an example of the color filter of the present invention.
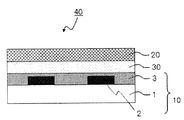
- FIG. 2 is a schematic sectional view showing an example of the display device of the present invention.
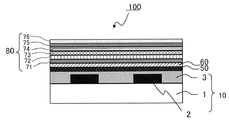
- FIG. 3 is a schematic cross-sectional view showing another example of the display device of the present invention.
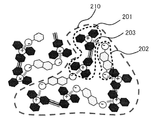
- FIG. 4 is a schematic diagram showing a molecular association state of the coloring material represented by the general formula (I).
- light includes electromagnetic waves having wavelengths in the visible and invisible regions, and further includes radiation, and the radiation includes, for example, microwaves and electron beams. Specifically, it means an electromagnetic wave having a wavelength of 5 ⁇ m or less and an electron beam.
- (meth) acryl represents each of acryl and methacryl
- (meth) acrylate represents each of acrylate and methacrylate.
- the organic group means a group having one or more carbon atoms.
- solid content means all components other than the solvent which comprises a colored resin composition, and even if it is a liquid monomer, it shall be contained in the said solid content.
- the colored resin composition for a color filter according to the present invention contains a rake colorant, a dispersant, an alkali-soluble resin, a polyfunctional monomer, an initiator, and a solvent.
- the alkali-soluble resin is a resin having a hydrocarbon ring and an ethylenic double bond, and an acid value of 80 mgKOH / g or more and 300 mgKOH / g or less,
- the content ratio of the silane coupling agent is 1% by mass or less based on the total solid content in the colored resin composition.
- the colored resin composition for a color filter of the present invention is selected from the rake colorant and the specific alkali-soluble resin, and the content ratio of the silane coupling agent is 1 with respect to the total solid content in the colored resin composition.
- the present inventors prepared a colored resin composition containing a lake colorant and a silane coupling agent and repeatedly used it while being stored. Even when the storage period was short, the colored resin composition was used. It was found that the adhesion of the colored layer formed in this way to the substrate may decrease. As a result of studying this, the resin composition using a combination of the silane coupling agent and the rake colorant was more time-consuming than the resin composition containing no rake colorant. It became clear that the change was remarkable.
- the line width of the patterned colored layer is It has also been clarified that the photosensitive resin composition becomes thinner as the storage period becomes longer.
- the change in the line width was due to a change in the sensitivity of the photosensitive resin composition, and the change in the sensitivity was estimated to be caused by the content ratio of the silane coupling agent.
- the change in sensitivity over time causes a pattern with a different line width to be formed depending on the storage period of the photosensitive resin composition when a patterned colored layer is produced under the same conditions. It becomes a problem that it becomes difficult.
- the reason why the silane coupling agent changes over time due to the above combination is not yet elucidated. It is estimated that hydrolysis of the silyl group is promoted.
- the ionic component generated from the lake color material has a low molecular weight as compared with alkali-soluble resins and the like, and thus is estimated to be close to the silane coupling agent as compared with alkali-soluble resins and the like. It is presumed that the change with time is greatly influenced by the ionic component generated from the rake color material.
- the present inventors have selected and used a hydrocarbon ring and an ethylenic double bond as an alkali-soluble resin, and an acid value of 80 mgKOH / g or more and 300 mgKOH / g or less. Even when not using the said silane coupling agent, the knowledge that it was excellent in adhesiveness with a board
- the alkali-soluble resins or the alkali-soluble resin and the polyfunctional monomer, etc. in the curing step of the resin composition at the time of manufacturing the color filter Can form crosslinks.
- the shrinkage at the time of curing the colored layer is suppressed and the adhesiveness with the substrate is excellent.
- such a crosslinking reaction improves the resistance to an alkaline developer during development.
- the present inventors have found that the use of an alkali-soluble resin having a hydrocarbon ring suppresses the solvent resistance of the obtained colored layer, particularly the swelling of the colored layer.
- the presence of bulky hydrocarbon rings in the colored layer suppresses the movement of molecules in the colored layer and increases the strength of the coating film. It is estimated that From these facts, by controlling the content ratio of the silane coupling agent to 1% by mass or less based on the total solid content in the colored composition, it is possible to suppress changes in adhesiveness and sensitivity over time. In addition, even when the content of the silane coupling agent is 1% by mass or less based on the total solid content in the colored composition, a colored resin composition having excellent adhesion to the substrate can be obtained. it can.
- an alkali-soluble resin having a relatively large number of acidic groups which is a combination of a lake color material and an alkali-soluble resin having an acid value of 80 mgKOH / g or more, is formed on the surface of the fine particles of the lake color material. It is presumed that it is likely to interact with the basic group of the existing dye or lake agent, and as a result, the alkali-soluble resin is likely to be adsorbed on the surface of the lake color material. Since the alkali-soluble resin has a relatively high acid value, once adsorbed, it is less likely to dissociate even during high-temperature heating, can further suppress the decomposition of the coloring material, suppress the decrease in luminance, and significantly improve heat resistance. Estimated.
- the colored resin composition for color filters of the present invention improves the temporal stability of the sensitivity of the colored resin composition even when a rake color material is used, and the colored resin composition is used after long-term storage. In addition, a colored layer having excellent substrate adhesion and excellent colorability can be obtained.
- the colored resin composition for a color filter of the present invention contains at least a lake colorant, a dispersant, an alkali-soluble resin, a polyfunctional monomer, an initiator, and a solvent, and the above-mentioned specific content ratio
- a silane coupling agent may be contained, and other components may be further contained as necessary as long as the effects of the present invention are not impaired.
- each component of the colored resin composition for a color filter of the present invention will be described in detail in order.
- the lake color material of the present invention refers to a color material in which a solvent-soluble color material is insolubilized by salt formation with counter ions.
- the rake color material can be usually obtained by mixing a color material described later and a rake agent described later in a solvent.
- a dye having a high transmittance is preferably used from the viewpoint of increasing the brightness of the color filter.
- the dye may be appropriately selected according to the desired color tone, and may be any basic skeleton (color development site) such as an azo dye, anthraquinone dye, triarylmethane dye, xanthene dye, cyanine dye, or indigo dye. ) May be used.
- the dye may be a dye classified into any of an acidic dye having an anionic substituent and a basic dye having a cationic substituent.
- an acidic dye having an anionic substituent and a basic dye having a cationic substituent.
- triarylmethane dyes, xanthene dyes, cyanine dyes are preferable, and triarylmethane dyes are more preferable.
- Examples of the acid dye include C.I. I. Acid Violet 29, 31, 33, 34, 36, 36: 1, 39, 41, 42, 43, 47, 51, 63, 76, 103, 118, 126, C.I. I. Acid Blue 2, 8, 14, 25, 27, 35, 37, 40, 41, 41: 1, 41: 2, 43, 45, 46, 47, 49, 50, 51, 51, 53, 54, 55, 56, 57, 58, 62, 62: 1, 63, 64, 65, 68, 69, 70, 78, 79, 80, 81, 96, 111, 124, 127, 127: 1, 129, 137, 138, 143,145,150,175,176,183,198,203,204,205,208,215,220,221,225,226,227,230,231,232,233,235,239,245,247, 253, 257, 258, 260, 261, 264, 266, 270, 271, 272, 273, 274, 277,
- Acid violet 9, 30, 102 sulforhodamine G, sulforhodamine B, sulforhodamine 101, xanthene acid dyes such as sulforhodamine 640 and the like.
- xanthene acid dyes C.I. I. Acid Red 50, C.I. I. Acid Red 52, C.I. I. Acid Red 289, C.I. I. Acid Violet 9, C.I. I. Acid Violet 30, C.I. I. A rhodamine acid dye such as Acid Blue 19 is preferred.
- Examples of commercially available basic dyes include C.I. I. Basic violet 1, 3, 14, C.I. I. Basic Blue 1, 5, 7, 8, 11, 26, C.I. I.
- Triarylmethane basic dyes such as Basic Green 1, 4; I. Basic Yellow 13, C.I. I. Cyanine basic dyes such as Basic Red 14; C.I. I. Azo basic dyes such as Basic Red 29; I. And xanthene-based basic dyes such as Basic Violet 11.
- Triarylmethane basic dyes are C.I. I. Basic blue 1, 5, 7, 8, 11, 26 are preferred.
- the dye which has the cation of the coloring material represented by general formula (I ') mentioned later is also mentioned as a suitable thing. These dyes can be used alone or in combination of two or more.
- the counter ion differs depending on the type of the dye, the counter ion of the acid dye is a cation, and the counter ion of the basic dye is an anion. Therefore, the rake agent is appropriately selected and used depending on the dye. That is, when the acid dye is insolubilized, a compound that generates a counter cation of the dye is used as a rake agent. When the basic dye is insolubilized, a counter anion of the dye is generated as a rake agent. A compound is used.
- Examples of the counter cation of the acid dye include an ammonium cation, a metal cation, and an inorganic polymer.
- a rake agent that generates ammonium ions for example, primary amine compounds, secondary amine compounds, tertiary amine compounds, and the like are preferable. Among them, secondary amines are preferred because of their excellent heat resistance and light resistance. It is preferable to use an amine compound or a tertiary amine compound.
- what is necessary is just to select suitably from the metal salt which has a desired metal ion as a lake agent which generate
- the counter cation of the acid dye can be used alone or in combination of two or more.
- the rake color material containing an acid dye is preferably a rake color material containing a xanthene-based dye from the viewpoint that high luminance can be achieved.
- the xanthene acid dye in the lake color material it is preferable to have a compound represented by the following general formula (VI), that is, a rhodamine acid dye.
- R 10 to R 13 each independently represents a hydrogen atom, an alkyl group, an aryl group, or a heteroaryl group, and R 10 and R 12 , R 11 and R 13 are bonded to each other.
- R 14 represents an acidic group
- X represents a halogen atom
- m represents an integer of 0 to 5.
- the general formula (VI) has one or more acidic groups.
- N is an integer of 0 or more.
- the alkyl group in R 10 to R 13 is not particularly limited. Examples thereof include a linear or branched alkyl group having 1 to 20 carbon atoms which may have a substituent. Among them, a linear or branched alkyl group having 1 to 8 carbon atoms is preferable. More preferably, it is a linear or branched alkyl group having 1 to 5 carbon atoms.
- the substituent that the alkyl group may have is not particularly limited, and examples thereof include an aryl group, a halogen atom, a hydroxyl group, and the like, and the substituted alkyl group includes a benzyl group, and further, a substituent. May have a halogen atom or an acidic group.
- the aryl group in R 10 to R 13 is not particularly limited.
- an aryl group which may have a substituent having 6 to 20 carbon atoms is exemplified, and among them, a group having a phenyl group, a naphthyl group or the like is preferable.
- the heteroaryl group in R 10 to R 13 include heteroaryl groups which may have a substituent having 5 to 20 carbon atoms, and those containing a nitrogen atom, an oxygen atom or a sulfur atom as the heteroatom are preferable .
- Examples of the substituent that the aryl group or heteroaryl group may have include an alkyl group having 1 to 5 carbon atoms, a halogen atom, an acidic group, a hydroxyl group, an alkoxy group, a carbamoyl group, and a carboxylic acid ester group.
- R 10 to R 13 may be the same or different.
- the acidic group or a salt thereof include a carboxy group (—COOH), a carboxylate group (—COO ⁇ ), a carboxylate group (—COOM, where M represents a metal atom), a sulfonate group (—SOO). 3 -), a sulfo group (-SO 3 H), sulfonate (-SO 3 M, wherein M represents a metal atom), and among them, a sulfonato group (-SO 3 -.), a sulfo group It is preferable to have at least one of (—SO 3 H) or a sulfonate group (—SO 3 M).
- the metal atom M include a sodium atom and a potassium atom.
- Acid Red 50, Acid Red 52, Acid Red 289, Acid Violet 9, Acid Violet 30, Acid Blue 19 and the like are preferable from the viewpoint of increasing brightness.
- the metal lake color material of the xanthene acid dye includes a metal atom-containing material as a lake agent.
- a rake agent containing a metal atom By using a rake agent containing a metal atom, the heat resistance of the coloring material is increased.
- a rake agent containing a metal atom that becomes a divalent or higher valent metal cation is preferable.
- the counter anion of the basic dye may be an organic anion or an inorganic anion.
- organic anion include organic compounds having an anionic group as a substituent.
- the lake color material is an acid dye and a basic dye present as an ion pair.
- rake agents that generate these organic anions include alkali metal salts and alkaline earth metal salts of the above organic anions.
- the inorganic anion for example, an anion of oxo acid (phosphate ion, sulfate ion, chromate ion, tungstate ion (WO 4 2 ⁇ ), molybdate ion (MoO 4 2 ⁇ ), etc.) Mention may be made of inorganic anions such as polyacid anions condensed with oxo acids and mixtures thereof.
- the polyacid may be an isopolyacid anion (M m O n ) c- or a heteropoly acid anion (X l M m O n ) c- .
- M represents a poly atom
- X represents a hetero atom
- m represents a composition ratio of poly atoms
- n represents a composition ratio of oxygen atoms.
- the poly atom M include Mo, W, V, Ti, and Nb.
- the hetero atom X include Si, P, As, S, Fe, and Co.
- a polyacid anion containing at least one of molybdenum (Mo) and tungsten (W) is preferable, and a c-valent polyacid anion containing at least tungsten is more preferable.
- rake agents that generate inorganic anions include alkali salts and alkali metal salts of the above inorganic anions.
- the counter anion of the basic dye in the lake color material can be used alone or in combination of two or more.
- the rake color material is preferably a rake color material composed of a basic dye and an inorganic anion, more preferably a basic dye and a polyacid anion, from the viewpoint of heat resistance and light resistance. .
- the silane coupling agent is easily changed over time, but in the present invention, the content of the silane coupling agent is the total solid content in the colored resin composition.
- the influence of the change with time is small, while the heat resistance and light resistance are high.
- the lake color material includes a lake color material having a triarylmethane dye from the viewpoint that the brightness of the color filter can be improved.
- the rake color material is excellent in heat resistance and light resistance and achieves high brightness of the color filter, among others, the color material represented by the following general formula (I) and the following general formula ( It is preferable that the color material is one or more selected from the color materials represented by II), and that the color material represented by the following general formula (I) forms a molecular association state and is more excellent It is preferable at the point which shows heat resistance.
- A is an a-valent organic group in which the carbon atom directly bonded to N has no ⁇ bond, and the organic group is saturated aliphatic carbonized at least at the terminal directly bonded to N.
- R i to R v each independently represents a hydrogen atom, an optionally substituted alkyl group or an optionally substituted aryl group, and R ii and R iii , R iv and R v may combine to form a ring structure
- Ar 1 represents a divalent aromatic group which may have a substituent
- a plurality of R i to R v and Ar 1 may be the same or different.
- a and c represent an integer of 2 or more
- b and d represent an integer of 1 or more.
- e is 0 or 1, and when e is 0, there is no bond.
- a plurality of e may be the same or different.
- R I , R II , R III , R IV , R V and R VI each independently represent a hydrogen atom, an alkyl group having 1 to 3 carbon atoms, or a phenyl group
- the color material represented by the general formula (I) includes a divalent or higher valent anion 202 and a divalent or higher cation 201. It is presumed that the cation and the cation are not simply ion-bonded by one molecule to one molecule but form a molecular aggregate 210 in which a plurality of molecules are associated through the ion bond 203. Therefore, the apparent molecular weight of the color material represented by the general formula (I) is remarkably increased as compared with the molecular weight of the conventional lake color material. It is presumed that the formation of such molecular aggregates increases the cohesive force in the solid state, lowers the thermal motion, suppresses dissociation of ion pairs and decomposition of the cation part, and improves heat resistance.
- a in the general formula (I) is an a-valent organic group in which the carbon atom directly bonded to N (nitrogen atom) does not have a ⁇ bond, and the organic group is saturated at least at the terminal directly bonded to N.
- A preferably does not have a siloxane bond, and more preferably does not have Si (silicon atom).
- an aliphatic hydrocarbon group having a saturated aliphatic hydrocarbon group at the terminal directly bonded to N is linear, branched or cyclic unless the terminal carbon atom directly bonded to N has a ⁇ bond.
- the carbon atom other than the terminal may have an unsaturated bond, may have a substituent, and the carbon chain contains O, S, and N. Also good.
- a carbonyl group, a carboxyl group, an oxycarbonyl group, an amide group or the like may be contained, and a hydrogen atom may be further substituted with a halogen atom or the like.
- the aromatic group having an aliphatic hydrocarbon group in A is a monocyclic or polycyclic aromatic group having an aliphatic hydrocarbon group having a saturated aliphatic hydrocarbon group at the terminal directly bonded to N. And may have a substituent, and may be a heterocyclic ring containing O, S, and N. Especially, it is preferable that A contains a cyclic
- the cyclic aliphatic hydrocarbon groups a bridged alicyclic hydrocarbon group is preferable from the viewpoint of skeleton fastness.
- the bridged alicyclic hydrocarbon group means a polycyclic aliphatic hydrocarbon group having a bridged structure in the aliphatic ring and having a polycyclic structure, for example, norbornane, bicyclo [2,2,2]. Examples include octane and adamantane.
- norbornane is preferable.
- the group containing a benzene ring and a naphthalene ring is mentioned, for example, Among these, the group containing a benzene ring is preferable.
- A is a divalent organic group, a linear, branched or cyclic alkylene group having 1 to 20 carbon atoms, or an aromatic group substituted with two alkylene groups having 1 to 20 carbon atoms such as a xylylene group Etc.
- the valence a in the general formula (I) is the number of chromogenic cation sites constituting the cation, and a is an integer of 2 or more. In the color material of the present invention, since the valence a of the cation is 2 or more, it has excellent heat resistance.
- the upper limit of a is not particularly limited, but a is preferably 4 or less, and more preferably 3 or less, from the viewpoint of ease of production.
- the alkyl group for R i to R v is not particularly limited. Examples thereof include straight-chain or branched alkyl groups having 1 to 20 carbon atoms. Among them, straight-chain or branched alkyl groups having 1 to 8 carbon atoms are preferable, and straight chain having 1 to 5 carbon atoms. A chain or branched alkyl group is more preferable from the viewpoint of luminance and heat resistance. Of these, the alkyl group in R i to R v is particularly preferably an ethyl group or a methyl group.
- the substituent that the alkyl group may have is not particularly limited, and examples thereof include an aryl group, a halogen atom, and a hydroxyl group, and examples of the substituted alkyl group include a benzyl group.
- the aryl group in R i to R v is not particularly limited. For example, a phenyl group, a naphthyl group, etc. are mentioned. Examples of the substituent that the aryl group may have include an alkyl group and a halogen atom.
- R i to R v are each independently a hydrogen atom, an alkyl group having 1 to 5 carbon atoms, a phenyl group, or R ii and R iii , or R iv and R v.
- R i to R v are each independently a hydrogen atom, an alkyl group having 1 to 5 carbon atoms, a phenyl group, or R ii and R iii , or R iv and R v.
- R i to R v can each independently have the above-described structure, and among these, R i is preferably a hydrogen atom from the viewpoint of color purity, and R ii to R ii from the viewpoint of ease of production and raw material procurement. More preferably, R v are all the same.
- the divalent aromatic group in Ar 1 is not particularly limited.
- the aromatic group for Ar 1 the same aromatic groups as those described for the aromatic group for A can be used.
- Ar 1 is preferably an aromatic group having 6 to 20 carbon atoms, more preferably an aromatic group composed of a condensed polycyclic carbocycle having 10 to 14 carbon atoms. Among these, a phenylene group or a naphthylene group is more preferable because the structure is simple and the raw material is inexpensive.
- a plurality of R i to R v and Ar 1 in one molecule may be the same or different.
- the combination of R i to R v and Ar 1 can be adjusted to a desired color.
- the anion portion (B c ⁇ ) represents a c-valent polyacid anion containing at least tungsten and optionally containing molybdenum.
- the polyacid anion in the colorant represented by the general formula (I) can be used alone or in combination of two or more of the above-mentioned anions.
- the overall ratio of tungsten to molybdenum is preferably 90:10 to 100: 0 from the viewpoint of heat resistance and light resistance.
- b represents the number of cations
- d represents the number of anions in the molecular aggregate
- b and d represent an integer of 1 or more.
- a plurality of cations in the molecular aggregate may be one kind alone, or two or more kinds may be combined.
- the anion present in the molecular aggregate may be a single anion or a combination of two or more, and an organic anion and an inorganic anion may be used in combination. .
- E in the general formula (I) is an integer of 0 or 1.
- a plurality of e may be the same or different. That is, for example, it may be a cation moiety having only a triarylmethane skeleton or a plurality of xanthene skeletons, or may be a cation moiety containing both a triarylmethane skeleton and a xanthene skeleton in one molecule. From the viewpoint of color purity, an anion portion having only the same skeleton is preferable.
- the color material represented by the general formula (I) can be adjusted to a desired color by using a cation portion including both a triarylmethane skeleton and a xanthene skeleton.
- e in the color material represented by the general formula (I) is 0, that is, the use of the color material represented by the following general formula (I ′) is easy to adjust to a desired color. More preferred.
- the color material represented by the general formula (I) has a xanthene skeleton, it may be included in a xanthene dye described later, but as long as the color material represented by the general formula (I) is applicable, the present invention. In this case, it is assumed that it corresponds to the color material represented by the general formula (I).
- the method for producing the color material represented by the general formula (I) is not particularly limited. For example, it can be obtained by the production method described in International Publication No. 2012/144520 pamphlet.
- the color material represented by the general formula (II) is a color material obtained by rake formation of a triarylmethane dye, and therefore is suitable for increasing the brightness as in the case of conventional dyes. Furthermore, since the specific heteropolyoxometalate anion is used, it is excellent in heat resistance and light resistance as compared with conventional color materials.
- Examples of the alkyl group having 1 to 3 carbon atoms of R I to R VI in the general formula (II) include a methyl group, an ethyl group, an n-propyl group, and an iso-propyl group.
- the structure of the cation moiety having a triarylmethane skeleton may be appropriately selected depending on the desired chromaticity and the like. Among these, from the viewpoint of easily achieving high brightness and high contrast, it is preferable to have the same structure as the cation portion of a conventionally known triarylmethane dye.
- Basic Blue 7 in which R I to R V are ethyl groups and R VI is a hydrogen atom, R I to R IV are methyl groups, R V is a phenyl group
- Basic Blue 26 in which R VI is a hydrogen atom, R I to R IV are methyl groups
- R V is an ethyl group
- Basic Blue 11 in which R VI is a hydrogen atom, R I to R V are methyl groups
- R VI is phenyl
- Examples thereof include a cation portion such as basic blue 8 and the like, and it is preferable to have a cation portion structure similar to that of basic blue 7 from the viewpoint of easily achieving high luminance and high contrast.
- X ⁇ in the colorant represented by the above general formula (I) is only one of (SiMoW 11 O 40 ) 4- / 4 or P 2 Mo y W 18-y O 62 ) 6- / 6 Or (SiMoW 11 O 40 ) 4- / 4 and P 2 Mo y W 18-y O 62 ) 6- / 6 may be used in combination.
- the heteropolyoxometalate anion represented by (SiMoW 11 O 40 ) 4- / 4 is used as a corresponding heteropolyacid or a corresponding heteropolyoxometalate alkali metal salt, for example, Journal of American Chemical Society, 104 (1982). It can be obtained according to the method described in p3194. More specifically, a heteropolyacid can be obtained by mixing and stirring a nitric acid aqueous solution and an alkali metal molybdate aqueous solution, adding K 8 ( ⁇ -type SiW 11 O 39 ) thereto, and stirring for 2 to 6 hours. Furthermore, a heteropolyoxometalate alkali metal salt can be obtained by reacting the obtained heteropolyacid with an alkali metal chloride.
- the heteropolyoxometalate anion represented by (P 2 Mo y W 18-y O 62 ) 6- / 6 is used as a corresponding heteropolyacid or a corresponding heteropolyoxometalate alkali metal salt, for example, Inorganic Chemistry , vol 47, p3679.
- a heteropolyacid can be obtained by dissolving an alkali metal tungstate and an alkali metal molybdate in water, adding phosphoric acid thereto, and heating to reflux for 5 to 10 hours with heating and stirring. it can.
- a heteropolyoxometalate alkali metal salt can be obtained by reacting the obtained heteropolyacid with an alkali metal salt compound.
- the number y of molybdenum in the heteropolyoxometalate anion can be adjusted in the range of 1 to 3 by appropriately adjusting the amounts of the alkali metal tungstate and alkali metal molybdate.
- an alkali metal molybdate salt is dissolved in water, hydrochloric acid is added thereto, and then an ⁇ 2-type deficient Dawson-type phosphotungstic acid alkali metal salt such as K 10 ( ⁇ 2-type P 2 W 17 O 61 ) is added.
- K 10 ⁇ 2-type P 2 W 17 O 61
- P 2 Mo 1 W 17 O 62 having no y distribution can be obtained by stirring at 10 to 30 ° C. for 30 minutes to 2 hours.
- heteropolyoxometalate alkali metal salt examples include K 4 (SiMoW 11 O 40 ), K 6 (P 2 MoW 17 O 62 ), K 6 (P 2 Mo 2 W 16 O 62 ), and K 6 (P 2 ). Mo 3 W 15 O 62 ) and the like.
- the colorant represented by the above general formula (II) is obtained by salt-substituting a heteropolyacid or heteropolyoxometalate alkali metal salt corresponding to the obtained heteropolyoxometalate anion with a dye having a desired structure. Can be obtained. From the viewpoint of high reaction yield of salt substitution, it is preferable to use a heteropolyoxometalate alkali metal salt rather than a heteropolyacid.
- the color material represented by the general formula (II) may be a hydrate having crystal water or an anhydride. Moreover, you may use the color material represented by the said general formula (II) individually by 1 type or in combination of 2 or more types.
- the rake colorant can be used alone or in combination of two or more.
- the colored resin composition for a color filter of the present invention may further contain another color material for the purpose of controlling the color tone within a range not impairing the effects of the present invention.
- Other pigments include known pigments and dyes, and one or more can be used.
- dioxazine-based color materials and xanthene-based color materials as other color materials from the viewpoint of obtaining a desired color tone.
- preferable dioxazine pigments include Pigment Violet 23 and the like.
- preferable xanthene color materials include Acid Red 51, 52, 87, 92, 94, 289, 388, C.I. I. Acid Violet 9, 30, 102, sulforhodamine G, sulforhodamine B, sulforhodamine 101, sulforhodamine 640, etc., as well as JP2010-32999, JP2010-211198, and Japanese Patent No. 4492760 And the xanthene dyes described.
- Acid Red 289 or xanthene color materials represented by the following general formula (III) is preferably used from the viewpoint of heat resistance and light resistance.
- R 21 and R 22 are each independently an alkyl group or an aryl group, and R 23 and R 24 are each independently an aryl group or a heteroaryl group.
- the alkyl group in R 21 and R 22 is not particularly limited. Examples thereof include a linear or branched alkyl group having 1 to 20 carbon atoms which may have a substituent. Among them, a linear or branched alkyl group having 1 to 8 carbon atoms is preferable. More preferably, it is a linear or branched alkyl group having 1 to 5 carbon atoms.
- the substituent that the alkyl group may have is not particularly limited, and examples thereof include an aryl group, a halogen atom, a hydroxyl group, and the like, and the substituted alkyl group includes a benzyl group, and further, a substituent. May have a halogen atom.
- the aryl group in R 21 to R 24 is not particularly limited.
- an aryl group which may have a substituent having 6 to 20 carbon atoms is exemplified, and among them, a group having a phenyl group, a naphthyl group or the like is preferable.
- the heteroaryl group in R 23 and R 24 include heteroaryl groups which may have a substituent having 5 to 20 carbon atoms, and those containing a nitrogen atom, an oxygen atom or a sulfur atom as a heteroatom are preferable. .
- the substituent that the aryl group or heteroaryl group may have include an alkyl group having 1 to 5 carbon atoms, a halogen atom, a hydroxyl group, an alkoxy group, a carbamoyl group, and a carboxylic acid ester group.
- R 21 to R 24 do not have an acidic group from the viewpoint of light resistance.
- the substitution position of the sulfonate group (—SO 3 ⁇ ) of the benzene ring that substitutes the xanthene skeleton is not particularly limited, but is preferably in the ortho or para position with respect to the xanthene skeleton. From the viewpoint of light resistance, the sulfonate group is preferably substituted in the ortho position relative to the xanthene skeleton.
- the compound represented by the general formula (III) has one sulfonate group and does not have a metal ion or a halogen atom.
- R 21 to R 24 are not hydrogen atoms, and R 23 and R 24 are either aryl groups or heteroaryl groups.
- the method for producing the compound represented by the general formula (III) is not particularly limited, and can be obtained by referring to, for example, JP2010-211198A.
- the blending ratio of the color material may be appropriately adjusted so as to obtain a desired color tone.
- the rake color material is 30 to 100 parts by mass with respect to 100 parts by mass of the total amount of the color material including the rake color material and other color materials.
- the amount is 60 to 99 parts by mass, and more preferably 70 to 98 parts by mass.
- the lake color material is used by being dispersed in a solvent with a dispersant.
- the dispersant can be appropriately selected from conventionally known dispersants.
- the dispersant that can be used include cationic, anionic, nonionic, amphoteric, silicone, and fluorine surfactants.
- the surfactants a polymer dispersant is preferable because it can be uniformly and finely dispersed.
- polymer dispersant examples include (co) polymers of unsaturated carboxylic acid esters such as polyacrylic acid esters; (partial) amine salts of (co) polymers of unsaturated carboxylic acid such as polyacrylic acid; (Partial) ammonium salts and (partial) alkylamine salts; (co) polymers of hydroxyl group-containing unsaturated carboxylic acid esters such as hydroxyl group-containing polyacrylates and their modified products; polyurethanes; unsaturated polyamides; polysiloxanes Long chain polyaminoamide phosphates; polyethylenimine derivatives (amides and their bases obtained by reaction of poly (lower alkylene imines) with free carboxyl group-containing polyesters); polyallylamine derivatives (polyallylamine and free carboxyls) Polyester, polyamide, or ester and amide co-condensation Object reaction product obtained by reacting one or more compound selected from among the three compounds of (polyester amide)), and the
- a polymer dispersant containing a nitrogen atom in the main chain or side chain and having an amine value is preferable from the viewpoint that the rake colorant can be suitably dispersed and the dispersion stability is good.
- it is a polymer dispersant made of a polymer containing a repeating unit having a tertiary amine, because the dispersibility is good, no foreign matter is deposited at the time of coating film formation, and the resolvability in a solvent is excellent.
- a polymer containing a repeating unit having a tertiary amine as a dispersant, the dispersibility and dispersion stability of the lake color material are improved.
- the repeating unit having a tertiary amine is a site having an affinity for the lake colorant.
- the polymer dispersant made of a polymer containing a repeating unit having a tertiary amine usually contains a repeating unit that becomes a site having an affinity for a solvent.
- a polymer containing a repeating unit having a tertiary amine a block copolymer having a block part composed of a repeating unit having a tertiary amine and a block part having a solvent affinity is particularly preferable. It is preferable at the point which can form the coating film which is excellent in it and becomes high-intensity.
- the repeating unit having a tertiary amine only needs to have a tertiary amine, and the tertiary amine may be contained in the side chain of the block polymer or may constitute the main chain.
- a repeating unit having a tertiary amine in the side chain is preferable, and among them, the structure represented by the following general formula (IV) is preferable because the main chain skeleton is hardly thermally decomposed and has high heat resistance. Is more preferable.
- R 1 is a hydrogen atom or a methyl group
- Q is a divalent linking group
- R 2 is an alkylene group having 1 to 8 carbon atoms, — [CH (R 5 ) —CH
- R 3 And R 4 each independently represents an optionally substituted chain or cyclic hydrocarbon group, or R 3 and R 4 are bonded to each other to form a cyclic structure
- R 5 and R 6 are Each independently represents a hydrogen atom or a methyl group.
- x represents an integer of 1 to 18, y represents an integer of 1 to 5, and z represents an integer of 1 to 18.
- Examples of the divalent linking group Q in the general formula (IV) include, for example, an alkylene group having 1 to 10 carbon atoms, an arylene group, a —CONH— group, a —COO— group, an ether group having a carbon number of 1 to 10 (— R′—OR ′′ —: R ′ and R ′′ are each independently an alkylene group) and combinations thereof.
- Q is a —COO— group because of the heat resistance of the polymer obtained, solubility in propylene glycol monomethyl ether acetate (PGMEA) suitably used as a solvent, and a relatively inexpensive material. preferable.
- the divalent organic group R 2 in the general formula (IV) is an alkylene group having 1 to 8 carbon atoms, — [CH (R 5 ) —CH (R 6 ) —O] x —CH (R 5 ) —CH (R 6 ) — or — [(CH 2 ) y —O] z — (CH 2 ) y —.
- the alkylene group having 1 to 8 carbon atoms may be linear or branched. For example, methylene group, ethylene group, trimethylene group, propylene group, various butylene groups, various pentylene groups, various hexylenes. Groups, various octylene groups and the like.
- R 5 and R 6 are each independently a hydrogen atom or a methyl group.
- R 2 is preferably an alkylene group having 1 to 8 carbon atoms from the viewpoint of dispersibility. Among them, R 2 is more preferably a methylene group, an ethylene group, a propylene group, or a butylene group. Groups are more preferred.
- Examples of the cyclic structure formed by combining R 3 and R 4 in the general formula (IV) include a 5- to 7-membered nitrogen-containing heterocyclic monocycle or a condensed ring formed by condensing two of these. It is done.
- the nitrogen-containing heterocycle preferably has no aromaticity, more preferably a saturated ring.
- repeating unit represented by the general formula (IV) examples include (meth) acryloyloxypropyldimethylamine, (meth) acryloyloxyethyldimethylamine, (meth) acryloyloxypropyldiethylamine, (meth) acryloyloxyethyldiethylamine, and the like. Examples include, but are not limited to, derived repeating units.
- the block part having solvent affinity in the copolymer from the viewpoint of improving solvent affinity and improving dispersibility, the block unit does not have the structural unit represented by the general formula (IV), and the general formula It has a solvent affinity block part having a structural unit copolymerizable with (IV).
- the arrangement of each block of the block copolymer is not particularly limited, and for example, an AB block copolymer, an ABA block copolymer, a BAB block copolymer, and the like can be used.
- the constitutional unit copolymerizable with the general formula (IV) is a constitutional unit represented by the following general formula (V) from the viewpoint of improving heat resistance while improving dispersibility and dispersion stability of the rake colorant. Preferably it is a unit.
- R 7 is a hydrogen atom or a methyl group
- A is a direct bond or a divalent linking group
- R 8 is an alkyl group having 1 to 18 carbon atoms, or an alkenyl group having 2 to 18 carbon atoms.
- R 9 and R 10 are each independently a hydrogen atom or a methyl group
- R 11 is a hydrogen atom, an alkyl group having 1 to 18 carbon atoms, an alkenyl group having 2 to 18 carbon atoms, an aralkyl group
- m represents an integer of 3 to 200
- n represents an integer of 10 to
- the divalent linking group A of the general formula (V) can be the same as Q in the general formula (IV), and propylene glycol which is suitably used as the heat resistance and solvent of the obtained polymer.
- A is preferably a —COO— group.
- the alkyl group having 1 to 18 carbon atoms may be linear, branched or cyclic, for example, methyl group, ethyl group, n-propyl group, isopropyl group, n-butyl.
- the alkenyl group having 2 to 18 carbon atoms may be linear, branched or cyclic.
- alkenyl groups include vinyl groups, allyl groups, propenyl groups, various butenyl groups, various hexenyl groups, various octenyl groups, various decenyl groups, various dodecenyl groups, various tetradecenyl groups, various hexadecenyl groups, various octadecenyl groups, A cyclopentenyl group, a cyclohexenyl group, a cyclooctenyl group, etc. can be mentioned.
- R 8 is preferably a methyl group, various butyl groups, various hexyl groups, benzyl groups, cyclohexyl groups, or hydroxyethyl groups from the viewpoint of dispersibility and substrate adhesion.
- Examples of the aryl group which may have a substituent include a phenyl group, a biphenyl group, a naphthyl group, a tolyl group, and a xylyl group.
- the aryl group preferably has 6 to 24 carbon atoms, more preferably 6 to 12 carbon atoms.
- Examples of the aralkyl group which may have a substituent include a benzyl group, a phenethyl group, a naphthylmethyl group, and a biphenylmethyl group.
- the aralkyl group preferably has 7 to 20 carbon atoms, more preferably 7 to 14 carbon atoms.
- Examples of the substituent of the aromatic ring such as an aryl group and an aralkyl group include an alkenyl group, a nitro group, and a halogen atom in addition to a linear or branched alkyl group having 1 to 4 carbon atoms.
- R 11 may have a hydrogen atom or a substituent, an alkyl group having 1 to 18 carbon atoms, an alkenyl group having 2 to 18 carbon atoms, an aralkyl group, an aryl group, —CHO, —CH 2 CHO, or A monovalent group represented by —CH 2 COOR 12 , wherein R 12 is a hydrogen atom or a linear, branched or cyclic alkyl group having 1 to 5 carbon atoms;
- examples of the substituent that may be included include linear, branched or cyclic alkyl groups having 1 to 4 carbon atoms, and halogen atoms such as F, Cl, and Br. And so on.
- the alkyl group having 1 to 18 carbon atoms and the alkenyl group, aralkyl group and aryl group having 2 to 18 carbon atoms in R 11 are as described for R 8 above.
- R 8 x, y and z are the same as R 2 in the general formula (IV).
- the glass transition temperature (Tg) of the solvent-compatible block part of the block copolymer may be appropriately selected.
- the glass transition temperature (Tg) of the solvent-compatible block part is preferably 80 ° C. or higher, and more preferably 100 ° C. or higher.
- the glass transition temperature (Tg) of the solvent-affinity block part in the present invention can be calculated by the following formula.
- Tgi is the glass transition temperature (absolute temperature) of the homopolymer of the i-th monomer.
- Tgi the value of the homopolymer glass transition temperature of each monomer
- the number of structural units constituting the solvent-affinity block part may be appropriately adjusted within a range where the amine value of the dispersant is 70 mgKOH / g or more and 160 mgKOH / g.
- the number of structural units constituting the solvent-affinity block part is 10 to 200 from the viewpoint that the solvent-affinity part and the colorant affinity part act effectively and improve the dispersibility of the colorant. It is preferably 10 to 100, more preferably 10 to 70.
- the solvent-affinity block part may be selected so as to function as a solvent-affinity site, and the repeating unit constituting the solvent-affinity block part may be composed of one kind, or two or more kinds.
- the repeating unit may be included.
- the ratio between the number m of the structural unit represented by the general formula (IV) and the number n of other structural units constituting the solvent-affinity block unit m / n is preferably in the range of 0.01 to 1, and more preferably in the range of 0.05 to 0.7 from the viewpoint of dispersibility and dispersion stability of the color material.
- a dispersant having an amine value of 70 mgKOH / g or more and 160 mgKOH / g or less is selected and used.
- the amine value is within the above range, the viscosity is excellent in stability over time and heat resistance, and is also excellent in alkali developability and solvent resolubility.
- the amine value of the dispersant is preferably 80 mgKOH / g or more, more preferably 90 mgKOH / g or more, from the viewpoint of dispersibility and dispersion stability.
- the amine value of the dispersant is preferably 120 mgKOH / g or less, and more preferably 105 mgKOH / g or less.
- the amine value refers to the number of mg of potassium hydroxide equivalent to perchloric acid required to neutralize the amine component contained in 1 g of a sample, and can be measured by the method defined in JIS-K7237. When measured by this method, since the organic acid compound usually dissociates in the amino group that forms a salt with the organic acid compound in the dispersant, the amine value of the block copolymer itself used as the dispersant is Can be measured.
- the acid value of the dispersant used in the present invention is preferably 1 mg KOH / g or more as a lower limit from the viewpoint that a development residue suppressing effect is exhibited.
- the acid value of the dispersant is more preferably 2 mgKOH / g or more from the viewpoint of more excellent development residue suppression effect.
- the acid value of the dispersant used in the present invention is preferably 18 mgKOH / g or less as the upper limit from the viewpoint of preventing the deterioration of the development adhesion and the solvent resolubility.
- the acid value of the dispersant is more preferably 16 mgKOH / g or less, and even more preferably 14 mgKOH / g or less, from the viewpoint of improving the development adhesion and the solvent resolubility.
- the acid value of the block copolymer before salt formation is preferably 1 mgKOH / g or more, and more preferably 2 mgKOH / g or more. This is because the effect of suppressing the development residue is improved.
- the upper limit of the acid value of the block copolymer before salt formation is preferably 18 mgKOH / g or less, more preferably 16 mgKOH / g or less, and even more preferably 14 mgKOH / g or less.
- the development adhesiveness and the solvent resolubility are improved.
- the colorant concentration is increased and the dispersant content is increased, the amount of the binder is relatively decreased. Therefore, the colored resin layer is easily peeled off from the base substrate during development.
- the dispersant contains a B block containing a structural unit derived from a carboxy group-containing monomer and has the specific acid value and glass transition temperature, the development adhesion is improved. If the acid value is too high, the developability is excellent, but it is presumed that the polarity is too high and peeling easily occurs during development.
- the glass transition temperature of a dispersing agent is 30 degreeC or more from the point which image development adhesiveness improves. That is, whether the dispersant is a block copolymer before salt formation or a salt block copolymer, the glass transition temperature is preferably 30 ° C. or higher.
- the glass transition temperature of the dispersant is low, it is particularly close to the developer temperature (usually about 23 ° C.), and the development adhesion may be lowered. This is presumably because when the glass transition temperature is close to the developer temperature, the movement of the dispersant increases during development, resulting in poor development adhesion.
- the glass transition temperature is 30 ° C.
- the glass transition temperature of the dispersant is preferably 32 ° C. or higher, more preferably 35 ° C. or higher, from the viewpoint of development adhesion. On the other hand, the temperature is preferably 200 ° C. or lower from the viewpoint of operability during use, such as easy precision weighing.
- the glass transition temperature of the dispersant in the present invention can be determined by measuring by differential scanning calorimetry (DSC) according to JIS K7121.
- the dispersant has an acid value of 1 mgKOH / g or more and 18 mgKOH / g or less and a glass transition temperature of 30 ° C. or more. Furthermore, it is preferable from the viewpoint of excellent solvent resolubility and further high development adhesiveness while suppressing generation of development residues.
- the colorant concentration is increased and the dispersant content is increased, the amount of the binder is relatively decreased. Therefore, the colored resin layer is easily peeled off from the base substrate during development.
- the dispersant contains a B block containing a structural unit derived from a carboxy group-containing monomer and has the specific acid value and glass transition temperature, the development adhesion is improved.
- the acid value is too high, it is presumed that although the developability is excellent, the polarity is too high and peeling easily occurs during development.
- the carboxy group-containing monomer a monomer that can be copolymerized with a monomer having a structural unit represented by the general formula (II) and contains an unsaturated double bond and a carboxy group can be used.
- monomers include (meth) acrylic acid, vinyl benzoic acid, maleic acid, maleic acid monoalkyl ester, fumaric acid, itaconic acid, crotonic acid, cinnamic acid, acrylic acid dimer, and the like.
- an addition reaction product of a monomer having a hydroxyl group such as 2-hydroxyethyl (meth) acrylate and a cyclic anhydride such as maleic anhydride, phthalic anhydride, or cyclohexanedicarboxylic anhydride, ⁇ -carboxy-polycaprolactone Mono (meth) acrylates can also be used.
- a cyclic anhydride such as maleic anhydride, phthalic anhydride, or cyclohexanedicarboxylic anhydride, ⁇ -carboxy-polycaprolactone Mono (meth) acrylates
- acid anhydride group containing monomers such as maleic anhydride, itaconic anhydride, and citraconic anhydride, as a precursor of a carboxy group.
- (meth) acrylic acid is particularly preferable from the viewpoints of copolymerizability, cost, solubility, glass transition temperature, and the like.
- the content ratio of the structural unit derived from the carboxy group-containing monomer may be appropriately set so that the acid value of the block copolymer is within the range of the specific acid value. Although not limited, it is preferably 0.05 to 4.5% by mass and more preferably 0.07 to 3.7% by mass with respect to the total mass of all the structural units of the block copolymer. Since the content ratio of the structural unit derived from the carboxy group-containing monomer is not less than the lower limit value, the effect of suppressing the development residue is expressed, and since it is not more than the upper limit value, the development adhesiveness is deteriorated and the solvent resolubility is reduced. Deterioration can be prevented. In addition, the structural unit derived from a carboxy group containing monomer should just become said specific acid value, may consist of 1 type, and may contain 2 or more types of structural units.
- the monomer whose glass transition temperature (Tgi) of the homopolymer of the monomer is 10 ° C. or higher from the viewpoint that the glass transition temperature of the dispersant used in the present invention is a specific value or higher and development adhesion is improved.
- Tgi glass transition temperature of the homopolymer of the monomer
- the ratio m / n of the unit number m of the structural unit of the A block and the unit number n of the structural unit of the B block is in the range of 0.05 to 1.5.
- the range of 0.1 to 1.0 is more preferable from the viewpoint of the dispersibility and dispersion stability of the color material.
- the weight average molecular weight Mw of the block copolymer is not particularly limited, but is preferably 1000 to 20000, and preferably 2000 to 15000 from the viewpoint of good colorant dispersibility and dispersion stability. More preferably, it is more preferably 3000 to 12000.
- the weight average molecular weight is determined as a standard polystyrene conversion value by (Mw) and gel permeation chromatography (GPC).
- Mw polystyrene conversion value by
- GPC gel permeation chromatography
- the method for producing the block copolymer is not particularly limited. Although a block copolymer can be produced by a known method, it is preferable to produce it by a living polymerization method. This is because chain transfer and deactivation are unlikely to occur, a copolymer having a uniform molecular weight can be produced, and dispersibility and the like can be improved.
- the living polymerization method include a living anionic polymerization method such as a living radical polymerization method and a group transfer polymerization method, and a living cation polymerization method.
- a copolymer can be produced by sequentially polymerizing monomers by these methods.
- a block copolymer can be produced by first producing the A block and polymerizing the structural units constituting the B block into the A block.
- the order of polymerization of the A block and the B block can be reversed.
- the A block and the B block can be manufactured separately, and then the A block and the B block can be coupled.
- a block copolymer having a block part composed of a repeating unit having a tertiary amine and a block part having a solvent affinity for example, a block copolymer described in Japanese Patent No. 4911253 is used. It can be mentioned as a suitable thing.
- the repeating unit having the tertiary amine is included with respect to 100 parts by mass of the lake color material.
- the content of the polymer is preferably 15 to 300 parts by mass, and more preferably 20 to 250 parts by mass. Within the above range, the dispersibility and dispersion stability are excellent.
- the third grade from the viewpoint of dispersibility and dispersion stability of the rake color material, in particular, the color material represented by the general formula (I) and the color material represented by the general formula (II), the third grade.
- a dispersant a salt in which at least a part of an amino group in a polymer containing a repeating unit having an amine and an organic acid compound form a salt (hereinafter, such a polymer is Sometimes referred to as a salt-type polymer).
- a salt-type polymer By using the salt polymer, the dispersibility and dispersion stability of the color material represented by the general formula (I) and the color material represented by the general formula (II) are improved.
- the rake colorant particularly the general formula (I)
- the polymer containing a repeating unit having a tertiary amine is a block copolymer
- the organic acid compound is an acidic organic phosphorus compound. From the viewpoint of excellent dispersibility and dispersion stability of the coloring material and the coloring material represented by the general formula (II).
- the organic acid compound is not particularly limited as long as it is a compound having one or more carbon atoms and an acidic group.
- the acidic group possessed by the organic acid compound include a carboxy group, a sulfo group, and a phosphoric acid group.
- the acidic group is a sulfo group or a phosphoric acid group. Is preferred.
- the number of acidic groups contained in one molecule of the organic acid compound is not particularly limited.
- the number of acidic groups in one molecule may be 1 to 3. Preferably, it is 1 to 2 and more preferably.
- the valence of the acidic group is not particularly limited, but is preferably a monovalent to trivalent acid from the viewpoint of dispersion stability, heat resistance, and developability, and more preferably a monovalent to divalent acid. preferable.
- the organic acid compound preferably has a molecular weight of 5000 or less, more preferably 100 or more and 1000 or less, and more preferably 150 or more and 500 or less from the viewpoint of easily forming a salt with the amino group of the dispersant. Is more preferable.
- the organic acid compound is at least one selected from the group consisting of the following general formula (VI) and the following general formula (VII): dispersibility, dispersion stability, heat resistance and alkali developability From the point which is excellent in it.
- R a and R a ′ are each independently a hydrogen atom, a hydroxyl group, an alkyl group having 1 to 18 carbon atoms, an alkenyl group having 2 to 18 carbon atoms, an aralkyl group, or an aryl group. 1 represented by the group, — [CH (R c ) —CH (R d ) —O] s —R e , — [(CH 2 ) t —O] u —R e , or —O—R a ′′.
- R a and R a ′ each contain a carbon atom.
- R a ′′ is an alkyl group having 1 to 18 carbon atoms, an alkenyl group having 2 to 18 carbon atoms, an aralkyl group, or an aryl group. , — [CH (R c ) —CH (R d ) —O] s —R e , — [(CH 2 ) t —O] u —R e .
- R b represents an alkyl group having 1 to 18 carbon atoms, an alkenyl group having 2 to 18 carbon atoms, an aralkyl group, an aryl group, — [CH (R c ) —CH (R d ) —O] s —R e , — A monovalent group represented by [(CH 2 ) t —O] u —R e or —O—R b ′ .
- R b ′ is an alkyl group having 1 to 18 carbon atoms, an alkenyl group having 2 to 18 carbon atoms, an aralkyl group, an aryl group, — [CH (R c ) —CH (R d ) —O] s —R e , Or, it is a monovalent group represented by — [(CH 2 ) t —O] u —R e .
- R c and R d are each independently a hydrogen atom or a methyl group
- R e is a hydrogen atom, an alkyl group having 1 to 18 carbon atoms, an alkenyl group having 2 to 18 carbon atoms, an aralkyl group, an aryl group, —CHO, —CH 2 CHO, —CO—CH ⁇ CH 2 , —CO—C (CH 3 ) ⁇ CH 2 or a monovalent group represented by —CH 2 COOR f
- R f is a hydrogen atom or carbon
- each of the alkyl group, alkenyl group, aralkyl group, and aryl group may have a substituent.
- s represents an integer of 1 to 18, t represents an integer of 1 to 5, and u represents an integer of 1 to 18.
- R a and R a ′ have an aromatic ring
- an appropriate substituent for example, a linear or branched alkyl group having 1 to 4 carbon atoms, is provided on the aromatic ring. You may have.
- the alkyl group having 1 to 18 carbon atoms, the aralkyl group, and the aryl group may be the same as R 8 in the dispersant.
- R a and / or R a ′ is —O—R a ′′ , it is an acidic phosphate ester.
- R a ′′ has an aromatic ring, it may have an appropriate substituent on the aromatic ring, for example, a linear or branched alkyl group having 1 to 4 carbon atoms.
- R e In the monovalent group represented by R e, Examples of the substituent which may have, for example, C 1-4 straight, branched or cyclic alkyl group, F, Cl, halogen atom such as Br And so on.
- R e the alkyl group having 1 to 18 carbon atoms is as shown in the above R 8
- the alkenyl group having 2 to 18 carbon atoms is as shown in the above R a and R a ′.
- s is an integer of 1 to 18
- t is an integer of 1 to 5
- u is an integer of 1 to 18.
- s is preferably an integer of 1 to 4, and more Preferably, it is an integer of 1 to 2, and t is preferably an integer of 1 to 4, more preferably 2 or 3. u is preferably an integer of 1 to 4, more preferably an integer of 1 to 2. is there.
- R b when R b has an aromatic ring, it may have an appropriate substituent on the aromatic ring, for example, a linear or branched alkyl group having 1 to 4 carbon atoms. Good.
- R b When R b is —O—R b ′, it is an acidic sulfate ester.
- R b ′ is an alkyl group having 1 to 18 carbon atoms, an alkenyl group having 2 to 18 carbon atoms, an aralkyl group, an aryl group, — [CH (R c ) —CH (R d ) —O] s —R e Or a monovalent group represented by — [(CH 2 ) t —O] u —R e .
- the alkyl group having 1 to 18 carbon atoms, the aralkyl group and the aryl group are as described above for R 8
- the alkenyl group having 2 to 18 carbon atoms are as described above for R a and R a ′. It is.
- R b ′ When R b ′ has an aromatic ring, it may have an appropriate substituent on the aromatic ring, for example, a linear or branched alkyl group having 1 to 4 carbon atoms.
- R c , R d and R e are the same as described above.
- s is an integer of 1 to 18
- t is an integer of 1 to 5
- u is an integer of 1 to 18.
- Preferred s, t, and u are the same as R a , R a ′, and R a ′′ above.
- R a and R a ′ in the general formula (VI) each independently have a hydrogen atom, a hydroxyl group, a methyl group, an ethyl group, or a substituent.
- Rb in general formula (VII) is a methyl group, an ethyl group, the aryl group which may have a substituent, an aralkyl group, a vinyl group.
- R b ′ is a methyl group, an ethyl group, an aryl group or an aralkyl group which may have a substituent, a vinyl group, an allyl group, — [CH (R c ) —CH (R d ) —O] s —R e , or — [(CH 2 ) t —O] u —R e , and R c and R d are each independently a hydrogen atom or a methyl group, Improves the dispersibility of the coloring material, and the resulting colored layer has high contrast and excellent heat resistance.
- R b is an aryl group which may have a substituent
- An aromatic ring is preferable from the viewpoints of improving the dispersibility of the coloring material, high contrast of the obtained colored layer, and excellent heat resistance.
- the other of R a and R a ′ is preferably a hydrogen atom or a hydroxyl group.
- the organic acid compounds represented by the general formula (VI) and the general formula (VII) include phosphorus (P), sulfur (S), and carbon.
- a compound in which atoms are directly bonded is preferable, and R a and R a ′ each independently represent a hydrogen atom, a hydroxyl group, an alkyl group having 1 to 18 carbon atoms, an alkenyl group having 2 to 18 carbon atoms, an aralkyl group, an aryl
- R b is an alkyl group having 1 to 18 carbon atoms, an alkenyl group having 2 to 18 carbon atoms, an aralkyl group, an aryl group, — [CH (R c ) —CH (R d ) —O] s —R e , — [(CH 2 ) t —O] u —R e is preferably a monovalent group.
- the organic acid compound is preferably benzenesulfonic acid, p-toluenesulfonic acid, monobutylphosphoric acid, dibutylphosphoric acid, methylphosphoric acid, dibenzylphosphoric acid, diphenylphosphoric acid, phenylphosphonic acid, etc. from the viewpoint of dispersibility and heat resistance.
- p-toluenesulfonic acid and phenylphosphonic acid are more preferable.
- the organic acid compound may be a hydrate such as p-toluenesulfonic acid monohydrate.
- an organic acid compound can be used individually by 1 type or in combination of 2 or more types.
- a group consisting of the general formulas (VI) and (VII) in a solvent in which a polymer having the structural unit represented by the general formula (IV) is dissolved or dispersed is used. And a method of adding one or more compounds selected from the above, stirring, and heating if necessary.
- a method of adding one or more compounds selected from the above, stirring, and heating is used.
- the fact that one or more compounds selected from the group form a salt and the proportion thereof can be confirmed by a known method such as NMR.
- the content of the dispersant is from 3 to 45 parts by weight, more preferably from 5 to 35 parts by weight, based on 100 parts by weight of the total solid content in the colorant dispersion. It is preferable to mix. In particular, when a coating film or a colored layer having a high color material concentration is formed, the content of the dispersant is 3 to 25 parts by mass, more preferably 100 parts by mass of the total solid content in the color material dispersion. It is preferable to blend at a ratio of 5 to 20 parts by mass.
- the solid content is everything except the above-mentioned solvent, and includes monomers dissolved in the solvent.
- the total content of the dispersant and the organic acid compound is not particularly limited, but from the viewpoint of dispersibility and dispersion stability, the total content of the dispersant and the organic acid compound with respect to 100 parts by mass of the colorant.
- the content is preferably 10 to 120 parts by mass, more preferably 20 to 100 parts by mass, and even more preferably 30 to 80 parts by mass.
- the content of the salt type polymer with respect to 100 parts by mass of the color material Is preferably 10 to 120 parts by mass, and more preferably 20 to 80 parts by mass. Within the above range, the dispersibility and dispersion stability are excellent.
- Alkali-soluble resin In the present invention, a resin having a hydrocarbon ring and an ethylenic double bond and having an acid value of 80 mgKOH / g or more and 300 mgKOH / g or less is selected and used as the alkali-soluble resin.
- the alkali-soluble resin is a resin that acts as a binder resin and is soluble in a developer used for forming a pattern, preferably an alkali developer.
- the alkali-soluble resin is a resin having an acid value of 80 mgKOH / g or more, the number of acidic groups contained in the resin is relatively large, and it is estimated that the acidic groups contribute to adhesion to the substrate. Is done.
- the said alkali-soluble resins or the said alkali-soluble resin, a polyfunctional monomer, etc. can form a crosslinking bond.
- the film strength of the cured film of the colored layer is improved, the thermal shrinkage of the cured film is suppressed, and the adhesion to the substrate is excellent.
- the colored layer formed by the colored resin composition for a color filter of the present invention has excellent substrate adhesion even when no silane coupling agent is used.
- the colored resin composition for a color filter of the present invention does not deteriorate the adhesion to the substrate even after long-term storage, and the change in sensitivity is suppressed, so that a patterned colored layer as designed can be obtained.
- the alkali-soluble resin having an acid value of 80 mgKOH / g or more is a resin having a relatively large amount of acidic groups, a dye existing near the surface of the molecular aggregate of the colorant represented by the general formula (I) or It is presumed that the alkali-soluble resin is likely to be adsorbed on the surface of the molecular aggregate as a result of easily interacting with the basic group of the rake agent.
- the alkali-soluble resin Since the alkali-soluble resin has a relatively high acid value, once adsorbed, it is less likely to dissociate even during high-temperature heating, can further suppress the decomposition of the coloring material, suppress the decrease in luminance, and significantly improve heat resistance. Estimated. Furthermore, the present inventors have found that the use of an alkali-soluble resin having a hydrocarbon ring suppresses the solvent resistance of the obtained colored layer, particularly the swelling of the colored layer. Although the action is unclear, the bulky hydrocarbon ring in the colored layer suppresses the movement of molecules in the colored layer, resulting in an increase in the strength of the coating and suppression of swelling by the solvent. It is estimated that.
- the alkali-soluble resin in the present invention has at least a hydrocarbon ring and an ethylenic double bond, and usually further has a carboxyl group.
- Specific examples include an acrylic copolymer having a carboxyl group and an ethylenic double bond, and an epoxy (meth) acrylate resin having a carboxyl group and an ethylenic double bond.
- an acrylic copolymer having a structural unit having a carboxyl group in the side chain and a structural unit having a double bond in the side chain is particularly preferable.
- An acrylic copolymer having a constitutional unit having a carboxyl group and a constitutional unit having an ethylenic double bond includes, for example, a carboxyl group-containing ethylenically unsaturated monomer, and other monomers that can be copolymerized as necessary.
- a compound having both an epoxy group and an ethylenic double bond in the molecule for example, glycidyl (meth) acrylate, is added to the carboxyl group of the (co) polymer obtained by (co) polymerization, and two side chains are added. It is obtained by introducing a double bond.
- carboxyl group-containing ethylenically unsaturated monomer examples include (meth) acrylic acid, vinyl benzoic acid, maleic acid, maleic acid monoalkyl ester, fumaric acid, itaconic acid, crotonic acid, cinnamic acid, and acrylic acid dimer. It is done. Also, an addition reaction product of a monomer having a hydroxyl group such as 2-hydroxyethyl (meth) acrylate and a cyclic anhydride such as maleic anhydride, phthalic anhydride, or cyclohexanedicarboxylic anhydride, ⁇ -carboxy-polycaprolactone Mono (meth) acrylates can also be used.
- anhydride containing monomers such as maleic anhydride, itaconic anhydride, and citraconic anhydride, as a precursor of a carboxyl group.
- (meth) acrylic acid is particularly preferable from the viewpoints of copolymerizability, cost, solubility, glass transition temperature, and the like.
- the method of introducing a structural unit having an ethylenically unsaturated bond in the side chain is not limited to the above method, and a known method can be used as appropriate.
- a structural unit having a hydroxyl group may be introduced into the copolymer, a compound having an isocyanate group and an ethylenic double bond in the molecule may be added, and a double bond may be introduced into the side chain.
- the alkali-soluble resin further has a hydrocarbon ring from the viewpoint of excellent adhesion of the colored layer.
- a hydrocarbon ring include a cyclic aliphatic hydrocarbon ring which may have a substituent, an aromatic ring which may have a substituent, and combinations thereof. May have a substituent such as a carbonyl group, a carboxyl group, an oxycarbonyl group or an amide group.
- hydrocarbon ring examples include aliphatic hydrocarbons such as cyclopropane, cyclobutane, cyclopentane, cyclohexane, norbornane, tricyclo [5.2.1.0 (2,6)] decane (dicyclopentane), and adamantane.
- Rings aromatic rings such as benzene, naphthalene, anthracene, phenanthrene, fluorene; chain polycycles such as biphenyl, terphenyl, diphenylmethane, triphenylmethane, stilbene, and cardo structures represented by the following chemical formula (A) It is done.
- the hydrocarbon ring When an aliphatic ring is included as the hydrocarbon ring, it is preferable from the viewpoint of improving the heat resistance and adhesion of the colored layer and improving the luminance of the obtained colored layer. Moreover, when including the cardo structure shown by the said Chemical formula (A), the sclerosis
- the use of a resin having a structural unit having a carboxyl group and the structural unit having the hydrocarbon ring makes it easy to adjust the amount of each structural unit, and has the hydrocarbon ring. This is preferable from the viewpoint that the amount of the structural unit is increased to easily improve the function of the structural unit.
- An acrylic copolymer having a carboxyl-containing structural unit and the hydrocarbon ring is prepared by using an ethylenically unsaturated monomer having a hydrocarbon ring as the aforementioned “other copolymerizable monomer”. Can do.
- Examples of the ethylenically unsaturated monomer having a hydrocarbon ring include cyclohexyl (meth) acrylate, dicyclopentanyl (meth) acrylate, isobornyl (meth) acrylate, benzyl (meth) acrylate, phenoxyethyl (meth) acrylate, and the like. Can be mentioned.
- Acrylic copolymers having a structural unit having a carboxyl group and a structural unit having an ethylenic double bond include, for example, methyl (meth) acrylate, ethyl (meth) acrylate, and other structural units having an ester group. These structural units may be contained.
- the structural unit having an ester group not only functions as a component that suppresses alkali solubility of the colored resin composition for a color filter, but also functions as a component that improves the solubility in a solvent and further the solvent resolubility.
- the alkali-soluble resin in the present invention is preferably an acrylic copolymer having a structural unit having a carboxyl group and a structural unit having a hydrocarbon ring, and has a structural unit having a carboxyl group and a hydrocarbon ring.
- An acrylic copolymer having a structural unit and a structural unit having an ethylenic double bond is more preferable.
- an epoxy (meth) acrylate resin having a carboxy group containing a cardo structure (hereinafter referred to as a cardo resin) can be preferably used.
- the fluorene skeleton contains a ⁇ -conjugated system, so it is highly sensitive to radicals. It is believed that there is.
- an oxime ester photopolymerization initiator which will be described later
- a cardo resin the required performance such as solvent resistance, water stain and sensitivity can be further improved.
- cardo resin examples include a polymerizable compound represented by the following general formula (B) described in JP-A-2007-119720 and an epoxy having a fluorene skeleton described in JP-A-2006-308698 ( Preferable examples include a reaction product (polycondensate) of meth) acrylate and polybasic acid.
- X represents a group represented by the following general formula (D)
- Y each independently represents a residue of a polyvalent carboxylic acid or its acid anhydride
- R i represents a group represented by the following general formula (C)
- j is an integer of 0 to 4
- k is an integer of 0 to 3
- n is an integer of 1 or more.
- R ii represents a hydrogen atom or a methyl group, and R iii independently represents a hydrogen atom or a methyl group.
- R iv is independently a hydrogen atom, an alkyl group having 1 to 5 carbon atoms, a phenyl group, or a halogen atom, and R v is —O— or —OCH 2 CH. 2 O- is shown.
- the cardo resin used in the present invention is, for example, epoxidizing a fluorene bisphenol compound to obtain an epoxy compound of a fluorene bisphenol compound, which is reacted with (meth) acrylic acid to form an epoxy (meth) acrylate, and this epoxy (meth) acrylate. Can be obtained by reacting with a polyvalent carboxylic acid or an acid anhydride thereof.
- the fluorene bisphenol compound, in the general formula (D), a R v is -O-, what this -O- becomes -OH may preferably be mentioned.
- fluorene bisphenol compound examples include 9,9-bis (4-hydroxyphenyl) fluorene, 9,9-bis (4-hydroxy-3-methylphenyl) fluorene, and 9,9-bis (4-hydroxy-3-methoxyphenyl).
- Fluorene 9,9-bis (4-hydroxy-3,5-dimethylphenyl) fluorene, 9,9-bis (4-hydroxy-3-fluorophenyl) fluorene, 9,9-bis (4-hydroxy-3) -Chlorophenyl) fluorene, 9,9-bis (4-hydroxy-3-bromophenyl) fluorene, 9,9-bis (4-hydroxy-3,5-dichlorophenyl) fluorene, 9,9-bis (4-hydroxy- Bisphenol compounds such as 3,5-dibromophenyl) fluorene, and mixtures thereof.
- Examples of the polyvalent carboxylic acid used in the reaction of the epoxy (meth) acrylate resin having a fluorene skeleton and the acid anhydride thereof include maleic acid, succinic acid, itaconic acid, phthalic acid, tetrahydrophthalic acid, and hexahydrophthalic acid.
- Dicarboxylic acids such as methyltetrahydrophthalic acid, methylendomethylenetetrahydrophthalic acid, chlorendic acid, methyltetrahydrophthalic acid, glutaric acid or their anhydrides; biphenyltetracarboxylic acid, benzophenonetetracarboxylic acid, biphenylethertetracarboxylic acid, Biphenylsulfone tetracarboxylic acid, 4- (1,2-dicarboxyethyl) -1,2,3,4-tetrahydronaphthalene-1,2-dicarboxylic acid, butanetetracarboxylic acid, tetracarboxylic acid such as pyromellitic acid, or That Dianhydride; trimellitic acid or tricarboxylic acids or their anhydrides of the acid anhydrides and the like.
- the cardo resin used in the present invention is preferably an epoxy (meth) acrylate having a fluorene skeleton which is an addition product of a fluorene epoxy (meth) acrylic acid derivative and a dicarboxylic acid anhydride and / or tetracarboxylic dianhydride. An acid adduct is mentioned.
- Examples of commercial names of cardo resins that can be used in the present invention include INR-16M (manufactured by Nagase Chemtech Co., Ltd.), trade name V259ME, (manufactured by Nippon Steel & Sumikin Chemical Co., Ltd.), and the like.
- the epoxy (meth) acrylate resin having a carboxy group may be used alone or in combination of two or more.
- the alkali-soluble resin used in the colored resin composition for color filters may be used alone or in combination of two or more, and the content is not particularly limited, but for color filters.
- the alkali-soluble resin is preferably in the range of 5 to 60% by mass, more preferably 10 to 40% by mass, based on the total solid content of the colored resin composition. If the content of the alkali-soluble resin is less than the above lower limit, sufficient alkali developability may not be obtained, and if the content of the alkali-soluble resin is more than the above upper limit, the film may be rough during development. Pattern chipping may occur.
- the solid content is everything except the above-mentioned solvent, and includes a liquid polyfunctional monomer.
- the alkali-soluble resin can be made into an alkali-soluble resin having desired performance by appropriately adjusting the charged amount of each structural unit.
- the charged amount of the carboxyl group-containing ethylenically unsaturated monomer is preferably 5% or more, more preferably 10% or more, based on the total amount of the monomer from the viewpoint of obtaining a good pattern.
- the amount of the ruboxyl group-containing ethylenically unsaturated monomer is preferably 50% by mass or less, and 40% by mass or less based on the total amount of monomers. More preferably.
- the ratio of the carboxyl group-containing ethylenically unsaturated monomer is less than 5% by mass, the solubility of the resulting coating film in an alkaline developer is lowered, and pattern formation becomes difficult.
- the proportion of the carboxyl group-containing ethylenically unsaturated monomer exceeds 50% by mass, the formed pattern tends to drop off from the substrate or the pattern surface becomes rough during development with an alkaline developer.
- the charged amount of the carboxyl group-containing ethylenically unsaturated monomer is the total amount of monomers.
- the content is preferably 5 to 50% by mass, more preferably 10 to 40% by mass.
- the charged amount of the hydrocarbon ring group-containing ethylenically unsaturated monomer is preferably 30 to 80% by mass, and preferably 40 to 75% by mass with respect to the total amount of monomers. More preferred.
- an acrylic copolymer having a carboxyl group-containing structural unit, a hydrocarbon ring-containing structural unit, and an ethylenic double bond-containing structural unit which is more preferably used as an alkali-soluble resin
- a carboxyl group-containing When a compound having both an epoxy group and an ethylenic double bond in the molecule is added to the ethylenically unsaturated monomer to introduce an ethylenic double bond, the charged amount of the carboxyl group-containing ethylenically unsaturated monomer is The amount is preferably 5 to 50% by mass, more preferably 10 to 40% by mass, based on the total amount of monomers.
- the charging amount of the hydrocarbon ring group-containing ethylenically unsaturated monomer is preferably 30 to 80% by mass, more preferably 40 to 75% by mass with respect to the total amount of the monomer. .
- the compound having both an epoxy group and an ethylenic double bond is preferably 10 to 95% by mass with respect to the charged amount of the carboxyl group-containing ethylenically unsaturated monomer. More preferably, it is -90 mass%.
- the acrylic copolymer has a structural unit having a carboxyl group and a hydrocarbon ring, the structural unit is included in each of the structural unit having a carboxyl group and the structural unit having a hydrocarbon ring.
- alkali-soluble resin those having an acid value of 80 mgKOH / g or more and 300 mgKOH / g or less are selected and used from the viewpoint of developability (solubility) in an aqueous alkali solution used for the developer and the adhesion to the substrate. . Especially, it is preferable that they are 90 mgKOH / g or more and 280 mgKOH / g or less, and it is more preferable that they are 100 mgKOH / g or more and 250 mgKOH / g or less.
- An alkali-soluble resin having an acid value of 100 mgKOH / g or more is preferable in combination with a rake color material from the viewpoint of improving heat resistance and improving luminance of the colored layer.
- an alkali-soluble resin having an acid value of 90 mgKOH / g or more from the viewpoint of improving heat resistance.
- the alkali-soluble resin having an acid value of not less than the above lower limit value easily interacts with a basic group of an anion present near the surface of the molecular aggregate of the colorant represented by the general formula (I). It is presumed that the alkali-soluble resin is easily adsorbed on the surface of the molecular aggregate.
- the alkali-soluble resin has a relatively high acid value, once adsorbed, it is less likely to dissociate even during high-temperature heating, can further suppress the decomposition of the coloring material, suppress the decrease in luminance, and significantly improve heat resistance. Estimated.
- the acid value can be measured according to JIS K 0070.
- the ethylenically unsaturated bond equivalent is preferably in the range of 100 to 2000, particularly preferably in the range of 140 to 1500.
- the ethylenically unsaturated bond equivalent is 2000 or less, the development resistance and adhesion are excellent.
- the ratio of other structural units, such as the structural unit which has the said carboxyl group, and the structural unit which has a hydrocarbon ring can be increased relatively if it is 100 or more, it is excellent in developability and heat resistance.
- the ethylenically unsaturated bond equivalent is a weight average molecular weight per mole of the ethylenically unsaturated bond in the alkali-soluble resin, and is represented by the following formula (1).
- W represents the mass (g) of alkali-soluble resin, and M is alkali-soluble.
- the ethylenically unsaturated bond equivalent is determined, for example, by measuring the number of ethylenic double bonds contained in 1 g of the alkali-soluble resin in accordance with the test method for the iodine value described in JIS K 0070: 1992. It may be calculated.
- the colored resin composition of the present invention may further contain other resins as long as the effects of the present invention are not impaired.
- resins include, for example, polycarbonate resin, polyvinyl alcohol resin, polyvinyl pyrrolidone resin, hydroxyethyl cellulose resin, carboxymethyl cellulose resin, polyvinyl chloride resin, melamine resin, phenol resin, alkyd resin, epoxy resin, polyurethane resin, Polyester resin, maleic acid resin, polyamide resin, polyimide resin and the like are mentioned.
- polyamide resin or polyimide resin is preferable from the viewpoint of heat resistance, and further, polyamide resin having a cyclic structure or polyimide resin having a cyclic structure. More preferably.
- These resins may have alkali solubility or may not have alkali solubility. These resins can be used alone or in combination of two or more.
- the weight average molecular weight of the alkali-soluble resin is not particularly limited. Among these, the range is preferably 1,000 to 500,000, and more preferably 3,000 to 200,000. If it is less than 1,000, the binder function after curing is remarkably lowered, and if it exceeds 500,000, pattern formation may be difficult during development with an alkaline developer.
- the alkali-soluble resin used in the colored resin composition for a color filter of the present invention may be used singly or in combination of two or more, and is alkali-soluble with respect to the total solid content of the colored resin composition.
- the resin content is not particularly limited, but is preferably 5 to 60% by mass, and more preferably 10 to 40% by mass. If the content of the alkali-soluble resin is too small, sufficient alkali developability may not be obtained, and if the content of the alkali-soluble resin is too large, the ratio of the coloring material is relatively low, which is sufficient. The coloring density may not be obtained.
- the solid content is everything except the above-mentioned solvent, and includes a liquid polyfunctional monomer.
- the polyfunctional monomer used in the colored resin composition for a color filter of the present invention is not particularly limited as long as it can be polymerized by an initiator described later, and usually has two or more ethylenically unsaturated double bonds.
- a polyfunctional (meth) acrylate having two or more acryloyl groups or methacryloyl groups is preferable, and a trifunctional or higher polyfunctional (meth) acrylate is more preferable.
- trifunctional or higher polyfunctional (meth) acrylates include, for example, trimethylolpropane tri (meth) acrylate, trimethylolethane tri (meth) acrylate, glycerol tri (meth) acrylate, and pentaerythritol.
- the polyfunctional monomer in terms of improving photocurability (high sensitivity), preferably has three (trifunctional) or more polymerizable double bonds, for example, trivalent or higher.
- Preferred examples include poly (meth) acrylates of polyhydric alcohols.
- a polyfunctional monomer has a carboxyl group from the point which improves alkali developability.
- Examples of the polyfunctional monomer having a carboxyl group include carboxylic acid-modified products of the poly (meth) acrylates of the polyhydric alcohol.
- poly (meth) acrylates of polyhydric alcohols include trimethylolpropane tri (meth) acrylate, pentaerythritol tri (meth) acrylate, pentaerythritol tetra (meth) acrylate, dipentaerythritol tetra (meth) acrylate, Examples thereof include dipentaerythritol penta (meth) acrylate and dipentaerythritol hexa (meth) acrylate.
- Examples of the carboxylic acid-modified products of poly (meth) acrylates of polyhydric alcohols include succinic acid-modified products of pentaerythritol tri (meth) acrylate and succinic acid-modified products of dipentaerythritol penta (meth) acrylate. .
- These polyfunctional monomers may be used individually by 1 type, and may be used in combination of 2 or more type.
- a polyfunctional monomer having a carboxyl group and a polyfunctional monomer having no carboxyl group may be used in combination.
- a succinic acid modified product of pentaerythritol tri (meth) acrylate having a carboxyl group and a succinic acid modified product of dipentaerythritol penta (meth) acrylate are preferred.
- Such a polyfunctional monomer may be a commercially available product as appropriate.
- a commercially available product containing a succinic acid modified product of dipentaerythritol penta (meth) acrylate trade names M-520D and TO-2371 (Toagosei Co., Ltd.). Etc.).
- the content of the polyfunctional monomer used in the colored resin composition for color filters of the present invention is not particularly limited, but the content of the polyfunctional monomer with respect to the total solid content of the colored resin composition is 5 to 60% by mass. 10 to 40% by mass is more preferable. If the content of the polyfunctional monomer is less than the above range, the photocuring may not sufficiently proceed and the exposed part may be eluted, and if the content of the polyfunctional monomer is more than the above range, the alkali developability may be deteriorated. There is.
- an initiator used in the colored resin composition for color filters of this invention there is no restriction
- Initiators include aromatic ketones, benzoin ethers, halomethyl oxadiazole compounds, ⁇ -amino ketones, biimidazoles, N, N-dimethylaminobenzophenone, halomethyl-S-triazine compounds, thioxanthone, etc. Can do.
- Specific examples of the initiator include aromatic ketones such as benzophenone, 4,4′-bisdiethylaminobenzophenone and 4-methoxy-4′-dimethylaminobenzophenone, benzoin ethers such as benzoin methyl ether, and benzoin such as ethylbenzoin.
- Biimidazoles such as 2- (o-chlorophenyl) -4,5-phenylimidazole dimer, halo such as 2-trichloromethyl-5- (p-methoxystyryl) -1,3,4-oxadiazole Methyloxadiazole compounds, halomethyl-S-triazine compounds such as 2- (4-butoxy-naphth-1-yl) -4,6-bis-trichloromethyl-S-triazine, 2,2-dimethoxy-1, 2-diphenylethane-1-one, 2-methyl-1- [4- (methylthio) phenyl] -2 Morpholinopropanone, 1,2-benzyl-2-dimethylamino-1- (4-morpholinophenyl) -butanone-1,1-hydroxy-cyclohexyl-phenyl ketone, benzyl, benzoylbenzoic acid, methyl benzoylbenzoate, 4 -Benzoyl
- 2-methyl-1- [4- (methylthio) phenyl] -2-morpholinopropan-1-one, 2-benzyl-2- (dimethylamino) -1- (4-morpholinophenyl) -1- Butanone, 4,4′-bis (diethylamino) benzophenone, and diethylthioxanthone are preferably used.
- the sensitivity is obtained by combining an ⁇ -aminoacetophenone initiator such as 2-methyl-1- [4- (methylthio) phenyl] -2-morpholinopropan-1-one with a thioxan initiator such as diethylthioxanthone.
- the content of the ⁇ -aminoacetophenone initiator and the thioxan initiator is preferably 5 to 15% by mass with respect to the total solid content of the colored resin composition.
- the amount of the initiator is 15% by mass or less, sublimates during the production process are reduced, which is preferable.
- the amount of the initiator is 5% by mass or more, development resistance such as water stain is improved.
- the initiator preferably contains an oxime ester photoinitiator from the viewpoint of a high water stain generation suppressing effect.
- the water stain refers to this phenomenon in which a trace of water stain is generated after rinsing with pure water after alkali development. Such water stain disappears after post-baking, so there is no problem as a product, but it is detected as unevenness in the appearance inspection of the patterning surface after development, and there is a problem that it is impossible to distinguish between normal products and abnormal products. Arise. Therefore, if the inspection sensitivity of the inspection apparatus is lowered in the appearance inspection, the yield of the final color filter product is lowered as a result, which becomes a problem.
- oxime ester-based photoinitiator those having an aromatic ring are preferable from the viewpoint of reducing contamination of the colored resin composition for color filters and degradation of the apparatus due to decomposition products, and having condensed rings including aromatic rings. More preferred are those having a condensed ring containing a benzene ring and a heterocycle.
- oxime ester photoinitiators examples include 1,2-octadion-1- [4- (phenylthio)-, 2- (o-benzoyloxime)], ethanone, 1- [9-ethyl-6- (2-methyl) Benzoyl) -9H-carbazol-3-yl]-, 1- (o-acetyloxime), JP 2000-80068 A, JP 2001-233842 A, Special Table 2010-527339, Special Table 2010-527338, It can be appropriately selected from oxime ester photoinitiators described in JP2013-041153A.
- the oxime ester photoinitiator used in the present invention is preferably an oxime ester photoinitiator that generates an aryl radical, particularly a phenyl radical, and more preferably an oxime ester type that generates an alkyl radical, particularly a methyl radical. It is preferable to use a photoinitiator from the viewpoint of excellent solvent resistance, development resistance, and water stain generation suppression effect. It is presumed that the radical transfer of the alkyl radical is easier to activate than the aryl radical.
- an oxime ester photoinitiator and a photoinitiator having a tertiary amine structure from the viewpoint of suppressing water stain and improving sensitivity. Since the photoinitiator having a tertiary amine structure has a tertiary amine structure that is an oxygen quencher in the molecule, radicals generated from the initiator are hardly deactivated by oxygen, and sensitivity can be improved. is there.
- Examples of commercially available photoinitiators having the tertiary amine structure include 2-methyl-1- (4-methylthiophenyl) -2-morpholinopropan-1-one (for example, Irgacure 907, manufactured by BASF), 2-benzyl-2- (dimethylamino) -1- (4-morpholinophenyl) -1-butanone (eg Irgacure 369, manufactured by BASF), 4,4′-bis (diethylamino) benzophenone (eg Hycure ABP, Kawaguchi Pharmaceutical).
- 2-methyl-1- (4-methylthiophenyl) -2-morpholinopropan-1-one for example, Irgacure 907, manufactured by BASF
- 2-benzyl-2- (dimethylamino) -1- (4-morpholinophenyl) -1-butanone eg Irgacure 369, manufactured by BASF
- an oxime ester photoinitiator as the initiator, and it is preferable to include an alkyl radical oxime ester compound.
- an alkyl radical oxime ester compound and the ⁇ -aminoalkylphenone-based initiator are combined, it is possible to obtain a coating film having an excellent effect of inhibiting water stain and to easily adjust the sensitivity.
- an alkyl radical oxime ester compound and an aryl radical oxime ester compound are used in combination, a coating film particularly excellent in solvent resistance and water stain suppression can be obtained with a small amount of initiator. Adjustment is also easy.
- the amount of the alkyl radical oxime ester compound is preferably 2 to 7% by mass based on the total solid content of the colored resin composition. If the amount of the initiator is less than 7 masses, it is preferable because the pattern does not become too thick with respect to the mask opening. When the amount of the initiator is 2% by mass or more, the solvent resistance is good.
- the content of the initiator used in the colored resin composition of the present invention is not particularly limited, but is preferably 1 to 40% by mass and more preferably 2 to 30% by mass with respect to the total solid content of the colored resin composition. Preferable is 3 to 20% by mass. If the content is less than the above range, the polymerization reaction cannot be sufficiently caused, so that the hardness of the colored layer may not be sufficient. In some cases, the content of the coloring material or the like in the solid content is relatively small, and a sufficient coloring density cannot be obtained.
- the solvent is not particularly limited, and may be appropriately selected from solvents that can disperse the lake color material. From the viewpoint of dispersibility of the rake color material, it is preferable to select a solvent having a solubility of the rake color material at 23 ° C. of 0.1 (g / 10 ml solvent) or less.
- a coloring resin composition excellent in heat resistance and light resistance can be used by dispersing the coloring material as fine particles by using such a solvent that is substantially insoluble or hardly soluble in the coloring material. Can be obtained.
- the solvent used in the present invention has excellent dispersibility and heat resistance, and a high-brightness coating film can be obtained, so that the solubility of the lake color material at 23 ° C. is 0.05 (g / 10 ml solvent) or less. It is preferable that in this invention, the solvent whose solubility of the lake color material in 23 degreeC is 0.1 (g / 10ml solvent) or less can be determined easily with the following evaluation methods. First, it can be determined by the following method whether it is a solvent which does not melt
- a 20 mL sample tube bottle is charged with 0.1 g of a colorant whose solubility is to be judged, solvent S is charged with a 10 ml hole pipette, and after further capping, it is treated with ultrasound for 3 minutes.
- the obtained liquid is stored in a 23 ° C. water bath for 60 minutes.
- 5 ml of this supernatant is filtered through a PTFE 5 ⁇ m membrane filter, and further filtered through a 0.25 ⁇ m membrane filter to remove insolubles.
- the absorption spectrum of the obtained filtrate is measured using a 1 cm cell with an ultraviolet-visible spectrophotometer (for example, UV-2500PC manufactured by Shimadzu Corporation). Absorbance (abs) at the maximum absorption wavelength of each colorant is obtained.
- the solubility is further determined by the following evaluation method.
- a good colorant solvent for example, alcohol such as methanol
- a solubility of the color material in the solvent S is calculated from the absorbance and dilution ratio of the color material solution of the solvent S and the color material solution of the good solvent.
- a solvent having a solubility of the coloring material of 0.1 (g / 10 ml solvent) or less is judged that the coloring material is a poorly soluble solvent that can be used in the present invention.
- the solvent having a solubility of the coloring material of 0.1 (g / 10 ml solvent) or less is 95% by mass or more in the total solvent of the coloring material dispersion from the viewpoint of improving the dispersion stability of the coloring material dispersion. It is preferable to further contain 98% by mass or more, and most preferably 100% by mass.
- ester solvents include ethyl acetate, butyl acetate, methyl methoxypropionate, ethyl ethoxypropionate, ethyl lactate, methoxyethyl acetate, propylene glycol monomethyl ether acetate, 3-methoxy-3-methyl-1-butyl acetate, 3-methoxybutyl acetate, methoxybutyl acetate, ethoxyethyl acetate, ethyl cellosolve acetate, dipropylene glycol methyl ether acetate, propylene glycol diacetate, 1,3-butylene glycol diacetate, cyclohexanol acetate, 1,6-hexanediol di Examples include acetate, diethylene glycol monoethyl ether acetate,
- the solvent used in the present invention includes propylene glycol monomethyl ether acetate (PGMEA), 3-methoxy-3-methyl-1-butyl acetate, ethyl ethoxypropionate, ethyl lactate, and 3-methoxybutyl acetate. It is preferable from the point of the solubility and application
- PGMEA propylene glycol monomethyl ether acetate
- the solvent used in the present invention preferably contains propylene glycol monomethyl ether acetate in an amount of 50% by mass or more, more preferably 70% by mass or more, and further preferably 90% by mass or more. These solvents may be used alone or in combination of two or more.
- the colored resin composition of the present invention may contain various additives as necessary within a range that does not impair the object of the present invention.
- the additive include an antioxidant, a polymerization terminator, a chain transfer agent, a leveling agent, a plasticizer, a surfactant, an antifoaming agent, a silane coupling agent, an ultraviolet absorber, and an adhesion promoter.
- silane coupling agent In this invention, you may use a silane coupling agent in 1 mass% or less with respect to the total solid of a colored resin composition.
- the silane coupling agent represents a compound having one or more groups selected from a silanol group and an alkoxysilyl group.
- adhesion to the substrate is improved by using a silane coupling agent.
- the silane coupling agent include known silane coupling agents, and silane coupling agents used in the technical field may be appropriately selected and used.
- silane coupling agent examples include vinyl silanes such as vinyltris ( ⁇ -methoxyethoxy) silane, vinylethoxysilane, vinyltrimethoxysilane, ⁇ -methacryloxypropyltrimethoxysilane, 3-methacryloxypropyltrimethoxysilane, etc.
- 3-methacryloxypropyltrimethoxysilane 3-methacryloxypropylmethyldimethoxysilane, 3-methacryloxypropylmethyldiethoxysilane, 3- Methacryloki Propyl triethoxysilane is preferred.
- the colored resin composition of the present invention preferably contains an antioxidant from the viewpoint of heat resistance and light resistance.
- the antioxidant may be appropriately selected from conventionally known antioxidants. Specific examples of antioxidants include, for example, hindered phenol antioxidants, amine antioxidants, phosphorus antioxidants, sulfur antioxidants, hydrazine antioxidants, and the like. From the viewpoint of light resistance, it is preferable to use a hindered phenol-based antioxidant.
- hindered phenol-based antioxidant for example, pentaerythritol tetrakis [3- (3,5-di-tert-butyl-4-hydroxyphenyl) propionate] (trade name: trade name: IRGANOX 1010, manufactured by BASF), 1,3,5-tris (3,5-di-tert-butyl-4-hydroxybenzyl) isocyanurate (trade name: Irganox 3114, manufactured by BASF), 2,4,6-tris (4-hydroxy-3 , 5-di-tert-butylbenzyl) mesitylene (trade name: Irganox 1330, manufactured by BASF), 2,2′-methylenebis (6-tert-butyl-4-methylphenol) (trade name: Sumilyzer MDP-S, Manufactured by Sumitomo Chemical Co., Ltd., 6,6'-thiobis (2-tert-butyl-4-methylphenol) (Trade name: Irganox 1081, manufactured
- pentaerythritol tetrakis [3- (3,5-di-tert-butyl-4-hydroxyphenyl) propionate] (trade name: trade name: IRGANOX1010, manufactured by BASF) is preferable from the viewpoint of heat resistance and light resistance. .
- the amount of the antioxidant is not particularly limited as long as the effect of the present invention is not impaired.
- the blending amount of the antioxidant is preferably 0.1 to 5.0 parts by mass with respect to 100 parts by mass of the total solid content in the colored resin composition, and preferably 0.5 to 4. More preferably, it is 0 parts by mass. If it is more than the said lower limit, it is excellent in heat resistance and light resistance. On the other hand, if it is below the said upper limit, the colored resin composition of this invention can be made into a highly sensitive photosensitive resin composition.
- surfactant examples include polyoxyethylene lauryl ether, polyoxyethylene stearyl ether, polyoxyethylene oleyl ether, polyoxyethylene octyl phenyl ether, polyoxyethylene nonyl phenyl ether, polyethylene glycol dilaurate, polyethylene glycol distearate. Sorbitan fatty acid esters, fatty acid-modified polyesters, tertiary amine-modified polyurethanes, and the like.
- a fluorosurfactant can also be used.
- plasticizer examples include dibutyl phthalate, dioctyl phthalate, and tricresyl.
- antifoaming agent and leveling agent examples include silicon-based, fluorine-based, and acrylic compounds.
- Chain transfer agent for example, a monofunctional thiol compound and a polyfunctional thiol compound are preferable, and among them, a polyfunctional thiol compound is preferable.
- the monofunctional thiol compound examples include 2-mercaptobenzothiazole, 2-mercaptobenzimidazole, 2-mercaptobenzoxazole, 2-mercaptomethylbenzimidazole, 2-mercaptomethylbenzoxazole, 2-mercaptomethylbenzothiazole and the like.
- the monofunctional thiol compound is preferably 2-mercaptomethylbenzothiazole from the viewpoint of chain transfer of radicals generated by the photopolymerization initiator and improving curability.
- the polyfunctional thiol compound is not particularly limited, and various compounds can be used.
- Examples of polyfunctional thiol compounds include 1,2-ethanedithiol, 1,3-propanedithiol, 1,4-butanedithiol, 1,6-hexanedithiol, 1,8-octanedithiol, 1,2-cyclohexanedithiol , Decanedithiol, ethylene glycol bisthioglycolate, ethylene glycol bis (3-mercaptopropionate), ethylene glycol bisthioglycolate, 1,4-butanediol dithioglycolate, 1,4-butanediol bis (3- Mercaptopropionate), trimethylolpropane tristhioglycolate, trimethylolpropane tris (3-mercaptopropionate), pentaerythritol tetrakisthioglycolate, pentaerythritol t
- polyfunctional thiol compound examples include trimercaptopropionic acid tris (2-hydroxyethyl) isocyanurate, 1,4-dimethylmercaptobenzene, 2,4,6-trimercapto-s-triazine, 2- (N, N -Dibutylamino) -4,6-dimercapto-s-triazine and the like.
- Polyfunctional thiol compounds include 2,5-hexanedithiol, 2,9-decanedithiol, 1,4-bis (1-mercaptoethyl) benzene, diphthalic acid (1-mercaptoethyl ester), diphthalic acid ( 2-mercaptopropyl ester), phthalic acid di (3-mercaptobutyl ester), phthalic acid di (3-mercaptoisobutyl ester) and the like having a substituent at the carbon atom at the ⁇ -position and / or ⁇ -position
- Polyfunctional thiol compounds ethylene glycol bis (3-mercaptobutyrate), propylene glycol bis (3-mercaptobutyrate), diethylene glycol bis (3-mercaptobutyrate), butanediol bis (3-mercaptobutyrate), octanediol Bis (3-mercaptobutyrate), trimethylo Propanetris (3-mercaptobutyrate), pentaerythr
- the polyfunctional thiol compound is pentaerythritol tetrakis (3-mercaptobutyrate).
- the product include pentaerythritol tetrakis (3-mercaptobutyrate) (PTMP; manufactured by Showa Denko KK, “Karenz MT (trademark) PE1”).
- the total content of the lake color material and other color materials is preferably 3 to 65% by mass, more preferably 4 to 55% by mass, based on the total solid content of the colored resin composition. If it is at least the lower limit, the colored layer has a sufficient color density when the colored resin composition is applied to a predetermined film thickness (usually 1.0 to 5.0 ⁇ m). Moreover, if it is below the said upper limit, while being excellent in the dispersibility and dispersion stability, the colored layer which has sufficient hardness and adhesiveness with a board
- the total content of the alkali-soluble resin, the polyfunctional monomer, and the initiator is 10 to 92% by mass, preferably 15 to 87% by mass with respect to the total solid content of the colored resin composition. preferable.
- substrate can be obtained.
- it is below the said upper limit it is excellent in developability and generation
- a solvent suitably in the range which can form a colored layer accurately.
- it is preferably in the range of 55 to 95% by mass, more preferably in the range of 65 to 88% by mass, based on the total amount of the colored resin composition containing the solvent.
- the content of the solvent is within the above range, the coating property can be excellent.
- the method for producing a colored resin composition for a color filter of the present invention comprises a lake colorant, a dispersant, an alkali-soluble resin, a polyfunctional monomer, an initiator, a solvent, and various additive components used as desired. Any method can be used as long as the rake colorant can be uniformly dispersed in the solvent by the dispersant, and the rake colorant can be prepared by mixing using a known mixing means.
- a dispersion of a rake colorant is prepared, and an alkali-soluble resin, a polyfunctional monomer, an initiator, and various additions used as desired are added to the dispersion.
- Method of mixing the components (2) A rake colorant, a dispersant, an alkali-soluble resin, a polyfunctional monomer, an initiator, and various additive components used as desired are simultaneously added and mixed in a solvent.
- a colorant, a dispersant, and an alkali-soluble resin are added to a solvent to prepare a dispersion, and the alkali-soluble resin, polyfunctional monomer, and initiator are further added to the dispersion. And if desired And the like; by adding various additive components to be needed, mixing methods. Among these methods, the method (4) is preferable because it can effectively prevent aggregation of the color material and can be uniformly dispersed.
- the method for preparing the dispersion of the rake color material can be appropriately selected from conventionally known dispersion methods. For example, (1) A dispersant is mixed and stirred in advance in a solvent to prepare a dispersant solution, and then an organic acid compound is mixed to form a salt between the amino group of the dispersant and the organic acid compound.
- the method (1) is preferable from the viewpoint of dispersion stability of the coloring material.
- the dispersing machine for performing the dispersion treatment examples include a roll mill such as a two-roll or a three-roll, a ball mill such as a ball mill or a vibration ball mill, a bead mill such as a paint conditioner, a continuous disk type bead mill, or a continuous annular type bead mill.
- the bead diameter to be used is preferably 0.03 to 2.00 mm, more preferably 0.10 to 1.0 mm.
- preliminary dispersion is performed with 2 mm zirconia beads having a relatively large bead diameter, and the main dispersion is further performed with 0.1 mm zirconia beads having a relatively small bead diameter. Further, after dispersion, it is preferably filtered through a membrane filter of 0.5 to 0.1 ⁇ m.
- Color filter is a color filter comprising at least a transparent substrate and a colored layer provided on the transparent substrate, wherein at least one of the colored layers is the colored resin composition according to the present invention. It has a colored layer formed by curing a product.
- FIG. 1 is a schematic sectional view showing an example of the color filter of the present invention.
- the color filter 10 of the present invention has a transparent substrate 1, a light shielding part 2, and a colored layer 3.
- At least one of the colored layers used in the color filter of the present invention is a colored layer formed by curing the colored resin composition for a color filter according to the present invention.
- the colored layer is usually formed in an opening of a light shielding part on a transparent substrate, which will be described later, and is usually composed of three or more colored patterns.
- the arrangement of the colored layers is not particularly limited, and for example, a general arrangement such as a stripe type, a mosaic type, a triangle type, or a four-pixel arrangement type can be used.
- variety, area, etc. of a colored layer can be set arbitrarily.
- the thickness of the colored layer is appropriately controlled by adjusting the coating method, the solid content concentration, the viscosity, and the like of the colored resin composition, but is usually preferably in the range of 1 to 5 ⁇ m.
- the colored layer can be formed by the following method.
- a coating means such as a spray coating method, a dip coating method, a bar coating method, a coal coating method, a spin coating method, or a die coating method.
- a wet coating After drying the wet coating film using a hot plate or oven, it is exposed to light through a mask having a predetermined pattern, and an alkali-soluble resin and a polyfunctional monomer are photopolymerized.
- a photosensitive coating film is used.
- Examples of the light source used for exposure include ultraviolet rays such as a low-pressure mercury lamp, a high-pressure mercury lamp, and a metal halide lamp, and an electron beam.
- the exposure amount is appropriately adjusted depending on the light source used, the thickness of the coating film, and the like.
- the heating conditions are appropriately selected depending on the blending ratio of each component in the colored resin composition to be used, the thickness of the coating film, and the like.
- a coating film is formed with a desired pattern by melt
- a solution in which an alkali is dissolved in water or a water-soluble solvent is usually used.
- An appropriate amount of a surfactant or the like may be added to the alkaline solution.
- a general method can be adopted as the developing method.
- the developer is usually washed and the cured coating film of the colored resin composition is dried to form a colored layer.
- the heating conditions are not particularly limited and are appropriately selected depending on the application of the coating film.
- the chromaticity of the colored layer of the color filter may be appropriately adjusted according to the light source and the like, and is not particularly limited.
- x is 0 or less in the chromaticity (x, y) of the C light source. It is preferable that 12 to 0.27 and y be in the range of 0.04 to 0.18.
- the light shielding part in the color filter of the present invention is formed in a pattern on a transparent substrate described later, and can be the same as that used as a light shielding part in a general color filter.
- the pattern shape of the light shielding portion is not particularly limited, and examples thereof include a stripe shape and a matrix shape.
- Examples of the light-shielding portion include those obtained by dispersing or dissolving a black pigment in a binder resin, and metal thin films such as chromium and chromium oxide.
- the metal thin film may be a CrO x film (x is an arbitrary number) and a laminate of two Cr films, and a CrO x film (x is an arbitrary number) with a reduced reflectance.
- the light shielding part is a material in which a black color material is dispersed or dissolved in a binder resin
- the light shielding part can be formed by any method that can pattern the light shielding part, and is not particularly limited.
- a photolithography method, a printing method, an ink jet method and the like using the colored resin composition for the light shielding part can be exemplified.
- the patterned light-shielding portion can be formed, for example, by the same method as that for forming the colored layer.
- the thickness of the light shielding portion is set to about 0.2 to 0.4 ⁇ m in the case of a metal thin film, and about 0.5 to 2 ⁇ m in the case where a black color material is dispersed or dissolved in a binder resin. Is set.
- the transparent substrate in the color filter of the present invention is not particularly limited as long as it is a base material transparent to visible light, and a transparent substrate used for a general color filter can be used.
- transparent flexible rigid materials such as quartz glass, alkali-free glass, and synthetic quartz plates, or transparent flexible flexible materials such as transparent resin films, optical resin plates, and flexible glasses. Materials.
- the thickness of the transparent substrate is not particularly limited, but for example, a thickness of about 100 ⁇ m to 1 mm can be used according to the use of the color filter of the present invention.
- the color filter of the present invention is one in which, for example, an overcoat layer, a transparent electrode layer, an alignment film, an alignment protrusion, a columnar spacer, and the like are formed in addition to the transparent substrate, the light shielding portion, and the colored layer. May be.
- Display Device A display device according to the present invention includes the color filter according to the present invention.
- the configuration of the display device is not particularly limited, and can be appropriately selected from conventionally known display devices, such as a liquid crystal display device and an organic light emitting display device.
- the liquid crystal display device includes the above-described color filter according to the present invention, a counter substrate, and a liquid crystal layer formed between the color filter and the counter substrate.
- a liquid crystal display device of the present invention will be described with reference to the drawings.
- FIG. 2 is a schematic diagram illustrating an example of a liquid crystal display device.
- the liquid crystal display device 40 includes a color filter 10, a counter substrate 20 having a TFT array substrate and the like, and a liquid crystal layer 30 formed between the color filter 10 and the counter substrate 20.
- the liquid crystal display device of the present invention is not limited to the configuration shown in FIG. 2, but can be a configuration generally known as a liquid crystal display device using a color filter.
- the driving method of the liquid crystal display device of the present invention is not particularly limited, and a driving method generally used for a liquid crystal display device can be employed. Examples of such a drive method include a TN method, an IPS method, an OCB method, and an MVA method. In the present invention, any of these methods can be preferably used. Further, the counter substrate can be appropriately selected and used according to the driving method of the liquid crystal display device of the present invention. Furthermore, as the liquid crystal constituting the liquid crystal layer, various liquid crystals having different dielectric anisotropy and mixtures thereof can be used according to the driving method of the liquid crystal display device of the present invention.
- a method for forming a liquid crystal layer a method generally used as a method for producing a liquid crystal cell can be used, and examples thereof include a vacuum injection method and a liquid crystal dropping method.
- FIG. 3 is a schematic cross-sectional view illustrating an example of an organic light emitting display device.
- the organic light emitting display device 100 of the present invention includes a color filter 10 and an organic light emitter 80.
- An organic protective layer 50 and an inorganic oxide film 60 may be provided between the color filter 10 and the organic light emitter 80.
- the transparent anode 71, the hole injection layer 72, the hole transport layer 73, the light emitting layer 74, the electron injection layer 75, and the cathode 76 are sequentially formed on the upper surface of the color filter. Examples thereof include a method and a method in which an organic light emitter 80 formed on another substrate is bonded onto the inorganic oxide film 60.
- the transparent anode 71, the hole injection layer 72, the hole transport layer 73, the light emitting layer 74, the electron injection layer 75, the cathode 76, and other configurations in the organic light emitting body 80 known structures can be appropriately used.
- the organic light emitting display device 100 manufactured as described above can be applied to, for example, a passive drive type organic EL display or an active drive type organic EL display.
- the organic light emitting display device of the present invention is not limited to the configuration shown in FIG. 3, and may be a known configuration as an organic light emitting display device that generally uses a color filter.
- the weight average molecular weight Mw of the block copolymer is determined as a standard polystyrene conversion value by GPC (gel permeation chromatography).
- HLC-8120GPC manufactured by Tosoh Corporation was used, the elution solvent was N-methylpyrrolidone to which 0.01 mol / liter of lithium bromide was added, and polystyrene standards for calibration curves were Mw377400, 210500, 96000, 50400. , 20650, 10850, 5460, 2930, 1300, 580 (Easy PS-2 series manufactured by Polymer Laboratories) and Mw1090000 (manufactured by Tosoh Corporation), and TSK-GEL ALPHA-M ⁇ 2 (Tosoh Corporation) (Made by Co., Ltd.).
- the obtained block copolymer A was dissolved in PGMEA to prepare a 60 wt% block copolymer A solution.
- the weight average molecular weight was measured by a Shodex GPC System-21H (Shorex GPC System-21H) using polystyrene as a standard substance and THF as an eluent.
- the acid value was measured based on JIS K 0070.
- Photosensitive binders B to G were obtained in the same manner as in Production Example 10 except that the alkali soluble resins B to G solutions were used in place of the alkali soluble resin A solution in Production Example 10, respectively.
- Photosensitive binders H to J were obtained in the same manner as in Production Example 10, except that the alkali-soluble resins H to J were used in place of the alkali-soluble resin A solution in Production Example 10, respectively.
- Example 1 Preparation of colored resin composition 1
- 30.9 parts by mass of the colorant dispersion A obtained in Production Example 2 26.9 parts by mass of the photosensitive binder A obtained in Production Example 10, 0.04 parts by mass of a surfactant MegaFac R08MH (manufactured by DIC), 42.2 parts by mass of PGMEA was mixed to obtain a colored resin composition 1 of Example 1.
- Examples 2 to 4, 6 to 9 Preparation of colored resin compositions 2 to 4 and 6 to 9
- the photosensitive binder A was changed to photosensitive binders B to D, F to G, A-1, and A-2 in Example 1
- Examples 2 to 4, and 6 to 9 were used. Colored resin compositions 2 to 4 and 6 to 9 were obtained.
- Comparative Example 5a Preparation of Comparative Colored Resin Composition 5a
- a comparative colored resin composition 5a of Comparative Example 5a was obtained in the same manner as in Example 1 except that the photosensitive binder A was changed to the photosensitive binder E in Example 1.
- Example 10 Preparation of colored resin composition 10
- 30.8 parts by mass of the colorant dispersion A obtained in Production Example 2 26.9 parts by mass of the photosensitive binder A obtained in Production Example 10, 0.04 parts by mass of a surfactant MegaFac R08MH (manufactured by DIC), KBM503 (manufactured by Shin-Etsu Chemical; silane coupling agent (3-methacryloxypropyltrimethoxysilane)) 0.09 parts by mass (0.5% based on the total solid content) 42.1 parts by mass of PGMEA were mixed, and Example 10 colored resin compositions 10 were obtained.
- a surfactant MegaFac R08MH manufactured by DIC
- KBM503 manufactured by Shin-Etsu Chemical; silane coupling agent (3-methacryloxypropyltrimethoxysilane)
- Example 11 Preparation of colored resin composition 11
- 30.8 parts by mass of the colorant dispersion A obtained in Production Example 2 26.9 parts by mass of the photosensitive binder A obtained in Production Example 10, 0.04 parts by mass of a surfactant MegaFac R08MH (manufactured by DIC), KBM503 (manufactured by Shin-Etsu Chemical Co., Ltd.) 0.18 parts by mass (1.0% with respect to the total solid content) and PGMEA 42.1 parts by mass were mixed to obtain a colored resin composition 11 of Example 11.
- a surfactant MegaFac R08MH manufactured by DIC
- KBM503 manufactured by Shin-Etsu Chemical Co., Ltd.
- PGMEA 42.1 parts by mass were mixed to obtain a colored resin composition 11 of Example 11.
- Comparative Example 1 Preparation of comparative colored resin composition 1
- 30.8 parts by mass of the colorant dispersion A obtained in Production Example 2 26.8 parts by mass of the photosensitive binder A obtained in Production Example 10, 0.04 parts by mass of a surfactant MegaFac R08MH (manufactured by DIC), KBM503 (manufactured by Shin-Etsu Chemical Co., Ltd.) 0.36 parts by mass (2.0% with respect to the total solid content) and 42.0 parts by mass of PGMEA were mixed to obtain a comparative colored resin composition 1 of Comparative Example 1.
- a surfactant MegaFac R08MH manufactured by DIC
- KBM503 manufactured by Shin-Etsu Chemical Co., Ltd.
- Comparative Example 2 Preparation of comparative colored resin composition 2
- a comparative colored resin composition 2 of Comparative Example 2 was obtained in the same manner as in Example 1 except that the photosensitive binder A was changed to the photosensitive binder H in Example 1.
- Comparative Example 3 Preparation of comparative colored resin composition 3
- a comparative colored resin composition 3 of Comparative Example 3 was obtained in the same manner as in Example 11 except that the photosensitive binder A was changed to the photosensitive binder H in Example 11.
- Comparative Example 4 Preparation of comparative colored resin composition 4
- a comparative colored resin composition 4 of Comparative Example 4 was obtained in the same manner as in Comparative Example 1 except that the photosensitive binder A was changed to the photosensitive binder H in Comparative Example 1.
- Comparative Examples 5 to 6 Preparation of comparative colored resin compositions 5 to 6) Comparative colored resin compositions 5 to 6 of Comparative Examples 5 to 6 were obtained in the same manner as in Example 1 except that the photosensitive binder A was changed to photosensitive binders I to J in Example 1.
- Comparative Example 7 Preparation of comparative colored resin composition 7
- Comparative Example 7 a comparative colored resin composition 7 of Comparative Example 7 was obtained in the same manner as Comparative Example 1, except that the colorant dispersion A was changed to the pigment dispersion B of Comparative Production Example 1.
- Comparative Example 8 Preparation of comparative colored resin composition 8
- Comparative Example 4 a comparative colored resin composition 8 of Comparative Example 8 was obtained in the same manner as Comparative Example 4 except that the colorant dispersion A was changed to the pigment dispersion B of Comparative Production Example 1.
- the resulting colored film was cut into a grid-like cut with a cutter knife, and then a mending tape was applied thereon and quickly peeled off in the vertical direction.
- B was the one completely peeled off by a grid-like film, and A was the others. If the evaluation result is A, the adhesion to the substrate is excellent and the practical range is evaluated. The results are shown in Table 2.
- T2 / T1 ⁇ 100 (%) was calculated, and the case of 95% or more was A, and the case of less than 95% was B.
- the results are shown in Table 2. If the evaluation result is A, the development resistance is excellent, and it is evaluated as a practical range.
- the thickness of the colored resin composition of the example after post-baking using a spin coater is 2 on a glass substrate (NH Techno Glass Co., Ltd., “NA35”) having a thickness of 0.7 mm and a size of 100 mm ⁇ 100 mm. It was applied so as to be about 5 ⁇ m. Then, it heat-dried for 3 minutes on an 80 degreeC hotplate. A cured film (blue colored film) was obtained by irradiating ultraviolet rays of 40 mJ / cm 2 using an ultrahigh pressure mercury lamp without using a photomask and post-baking in a clean oven at 220 ° C. for 30 minutes. The thickness of the obtained cured film is measured and set to T1.
- T2 / T1 ⁇ 100 (%) is calculated.
- AA is less than 103%
- A is 103% or more and less than 105%
- B is 105% or more and less than 110%
- C and 115% or more was D. If the above evaluation criteria are A, B or C, they can be used practically, but if the evaluation result is B and further A, the effect is more excellent. The results are shown in 2.
- a rake colorant and an alkali-soluble resin having an ethylenic double bond and an acid value of 80 mgKOH / g or more and 300 mgKOH / g or less, and the content ratio of the silane coupling agent is the total content in the colored resin composition
- the colored layers formed using the colored resin compositions of Examples 1 to 4 and 6 to 11 that are 1% by mass or less based on the solid content are excellent in substrate adhesion and development resistance, even after storage for 1 month. It was revealed that there was no change in substrate adhesion and line width, and the stability over time was excellent. It was also revealed that the colored layers of Examples 1 to 4 and 6 to 11 all had high luminance Y and excellent heat resistance.
- Example 8 even when an antioxidant is not used, the substrate adhesion and development resistance are excellent, and there is no change in substrate adhesion and line width even after storage for 1 month, and the stability over time is improved. Although a colored layer having excellent and high luminance can be obtained, it was revealed from the comparison with Example 1 that a colored layer having higher luminance can be obtained by using an antioxidant. Moreover, it became clear from the comparison of Examples 1, 10, 11 and Comparative Example 1 that the colored resin composition is excellent in stability over time if the content ratio of the silane coupling agent is 1% or less. .
- the colored resin composition of Comparative Example 5a using the alkali-soluble resin E having no hydrocarbon ring was good in substrate adhesion and developability, but easily swelled with NMP and easily caused water stain.
- Example 12 to 16 Preparation of colored resin compositions 12 to 16
- the colored resin compositions 12 to 16 of Examples 12 to 16 were obtained in the same manner as in Examples 1 to 4 and 11, except that the color material dispersion A was changed to the color material dispersion C, respectively. It was.
- Comparative Examples 9 to 13 Preparation of comparative colored resin compositions 9 to 13
- Comparative colored resin compositions 9 to 13 of Comparative Examples 9 to 13 were the same as those of Comparative Examples 1 to 2, 4 to 6 except that the color material dispersion A was changed to the color material dispersion C, respectively. Got.
- Example 17 to 21 Preparation of colored resin compositions 17 to 21
- Colored resin compositions 17 to 21 of Examples 17 to 21 were obtained in the same manner as the Examples except that Color Material Dispersion A was changed to Color Material Dispersion D in Examples 1 to 4 and 11, respectively. It was.
- Comparative Examples 14-18 Preparation of Comparative Colored Resin Compositions 14-18
- Comparative colored resin compositions 14 to 18 of Comparative Examples 14 to 18 were the same as the comparative examples except that the color material dispersion A was changed to the color material dispersion D in Comparative Examples 1 to 2, 4 to 6, respectively. Got.
- the color material D represented by the following structural formula was synthesized by the following procedure. In a 500 ml four-necked flask, 40.2 parts by weight of a sulfofluorane compound of the following chemical formula (4), 312 parts by weight of methanol, 6.8 parts by weight of N-methyl-2,6-xylidine and N-methyl-o- 6.0 parts by weight of toluidine was charged and refluxed for 30 hours. The reaction solution was filtered at 60 ° C. to remove insoluble components, and then the solvent was removed under reduced pressure until the reaction solution was about 70 ml, and the mixture was poured into 200 parts by weight of 6% hydrochloric acid.
- a sulfofluorane compound of the following chemical formula (4) 312 parts by weight of methanol, 6.8 parts by weight of N-methyl-2,6-xylidine and N-methyl-o- 6.0 parts by weight of toluidine was charged and refluxed for 30 hours.
- the reaction solution was
- a dispersing agent BYK-LPN6919 (trade name, manufactured by Big Chemie Co., Ltd., a block polymer containing a block part composed of a repeating unit having a tertiary amine, nonvolatile content 60% by weight, amine value 120 mgKOH / g, weight average molecular weight 8000) was added and stirred. Thereafter, a reflux condenser was connected, the temperature was raised to 80 ° C. with a water bath, and the reaction was performed for 4 hours after reaching 80 ° C.
- a dispersing agent BYK-LPN6919 trade name, manufactured by Big Chemie Co., Ltd., a block polymer containing a block part composed of a repeating unit having a tertiary amine, nonvolatile content 60% by weight, amine value 120 mgKOH / g, weight average molecular weight 8000
- a dispersing agent BYK-LPN6919 (trade name, manufactured by BYK Chemie Co., Ltd., block polymer including a block part composed of a repeating unit having a tertiary amine, nonvolatile content 60% by mass, amine value 120 mgKOH / g, weight average molecular weight 8000) was added and stirred. Thereafter, a reflux condenser was connected, the temperature was raised to 80 ° C. with a water bath, and the reaction was performed for 4 hours after reaching 80 ° C.
- a dispersing agent BYK-LPN6919 trade name, manufactured by BYK Chemie Co., Ltd., block polymer including a block part composed of a repeating unit having a tertiary amine, nonvolatile content 60% by mass, amine value 120 mgKOH / g, weight average molecular weight 8000
- Example 22 Preparation of colored resin composition 22
- 26.9 parts by mass of the colorant dispersion A obtained in Production Example 2 2.9 parts by mass of the colorant dispersion E obtained in Production Example 21, and 20.7 parts of the photosensitive binder A obtained in Production Example 11.
- a colored resin composition 22 of Example 22 was obtained by mixing parts by mass, 0.01 parts by mass of surfactant Megafac R08MH (manufactured by DIC), and 49.6 parts by mass of PGMEA.
- Example 23 Preparation of colored resin composition 23
- 26.9 parts by mass of the colorant dispersion A obtained in Production Example 2 2.9 parts by mass of the colorant dispersion E obtained in Production Example 21, and 20.7 parts of the photosensitive binder A obtained in Production Example 11.
- surfactant MegaFac R08MH manufactured by DIC
- KBM503 manufactured by Shin-Etsu Chemical
- PGMEA 49.6 parts by weight
- Comparative Example 19 Preparation of comparative colored resin composition 19
- 26.9 parts by mass of the colorant dispersion A obtained in Production Example 2 2.9 parts by mass of the colorant dispersion E obtained in Production Example 21, and 20.7 parts of the photosensitive binder A obtained in Production Example 11.
- surfactant Megafac R08MH manufactured by DIC
- KBM503 manufactured by Shin-Etsu Chemical
- PGMEA a comparative colored resin composition 19 of Comparative Example 19 was obtained.
- Comparative Example 20 Preparation of comparative colored resin composition 20
- a comparative colored resin composition 20 of Comparative Example 20 was obtained in the same manner as in Example 22 except that the photosensitive binder A was changed to the photosensitive binder H in Example 22.
- Examples 24 to 25 Preparation of colored resin compositions 24 to 25
- the amount of the color material dispersion was changed to 27.2 parts by mass of the color material dispersion A, 4.1 parts by mass of the color material dispersion F, and 48.2 parts by mass of PGMEA.
- colored resin compositions 24 to 25 of Examples 24 to 25 were obtained.
- Comparative Examples 21 to 22 Preparation of comparative colored resin compositions 21 to 22
- the amount of the coloring material dispersion was 27.2 parts by weight of the coloring material dispersion A, 4.1 parts by weight of the coloring material dispersion F, and 48.2 parts by weight of PGMEA.
- Comparative Examples 19 to 20 Comparative colored resin compositions 21 to 22 of Comparative Examples 21 to 22 were obtained.
- Examples 26 to 27 Preparation of colored resin compositions 26 to 27
- the amount of the color material dispersion was changed to 26.3 parts by mass of color material dispersion A, 2.3 parts by mass of color material dispersion G, and 50.3 parts by mass of PGMEA.
- colored resin compositions 26 to 27 of Examples 26 to 27 were obtained.
- Comparative Examples 23 to 24 Preparation of comparative colored resin compositions 23 to 24
- the blending amount of the color material dispersion was 26.3 parts by mass of the color material dispersion A, 2.3 parts by mass of the color material dispersion G, and 50.3 parts by mass of PGMEA.
- Comparative Examples 19 to 20 comparative colored resin compositions 23 to 24 of Comparative Examples 23 to 24 were obtained.
- the post-baking temperature was set to 230 ° C., and in the same manner as in each of the above evaluation methods, evaluation of substrate adhesion, evaluation of substrate adhesion change, evaluation of line width change, evaluation of development resistance Then, solvent resistance evaluation, water stain evaluation and optical property evaluation were performed. The results are shown in Table 4.
- EEMA 1-ethoxyethyl methacrylate
- TMSMA 2- (trimethylsilyloxy) ethyl methacrylate
- EHMA 2-ethylhexyl methacrylate
- BMA n-butyl methacrylate
- BzMA benzyl methacrylate
- MMA methyl methacrylate
- DMMA dimethylaminoethyl methacrylate
- MAA methacrylic acid
- the structural unit was derived from 2-hydroxyethyl methacrylate (HEMA).
- HEMA 2-hydroxyethyl methacrylate
- the obtained block copolymer PGMEA solution was reprecipitated in hexane, purified by filtration and vacuum drying, and a block copolymer X (acid value 8 mg KOH / g, Tg 38 ° C.) was obtained.
- the block copolymer X thus obtained was confirmed by GPC (gel permeation chromatography).
- the weight average molecular weight Mw was 7730.
- the amine value was 95 mgKOH / g.
- Example 28 to 81 Preparation of colored resin composition
- the content ratio of the colorant dispersion, the photosensitive binder component, and the silane coupling agent (KBM503) was changed to the combinations shown in Tables 5 to 7 below, and pentaerythritol tetrakis (3-mercaptobutyrate) ( PTMP; “Karenz MT (trademark) PE1” manufactured by Showa Denko Co., Ltd.))
- PTMP pentaerythritol tetrakis (3-mercaptobutyrate)
- PTMP pentaerythritol tetrakis (3-mercaptobutyrate)
- PTMP pentaerythritol tetrakis (3-mercaptobutyrate)
- PTMP pentaerythritol tetrakis (3-mercaptobutyrate)
- PTMP pentaerythritol tetrakis (3-mercap
- Example 1 the content ratio of the colorant dispersion, the photosensitive binder component, and the silane coupling agent (KBM503) was changed to the combinations shown in Tables 5 to 6 below, and pentaerythritol tetrakis (3-mercaptobutyrate) ( PTMP; “Karenz MT (trademark) PE1” manufactured by Showa Denko KK))) was added in the same manner as in Example 1 except that 0.47 parts by weight were obtained, and colored resin compositions 25 to 32 of Comparative Examples 25 to 32 were obtained. It was.
- PTMP pentaerythritol tetrakis (3-mercaptobutyrate)
- Example 82 Preparation of colored resin composition 82
- 26.9 parts by weight of the colorant dispersion I obtained in Production Example 49, 2.9 parts by weight of the colorant dispersion L obtained in Production Example 53, and photosensitive binder A-6 20 obtained in Production Example 28 0.7 parts by mass, 0.01 parts by mass of a surfactant Megafac R08MH (manufactured by DIC), pentaerythritol tetrakis (3-mercaptobutyrate) (PTMP; Showa Denko KK, “Karenz MT (trademark) PE1”)) .47 parts by weight and 49.6 parts by mass of PGMEA were mixed to obtain a colored resin composition 82 of Example 82.
- a surfactant Megafac R08MH manufactured by DIC
- pentaerythritol tetrakis (3-mercaptobutyrate) PTMP; Showa Denko KK, “Karenz MT (trademark) PE1”
- Example 83 Preparation of colored resin composition 83
- a colored resin composition 83 of Example 83 was obtained in the same manner as in Example 82 except that the photosensitive binder A-6 was changed to the photosensitive binder M-2 obtained in Production Example 35 in Example 82. .
- Examples 84 to 85 Preparation of colored resin compositions 84 to 85
- the blending amount of the color material dispersion was 27.2 parts by mass of color material dispersion I, 4.1 parts by mass of color material dispersion M obtained in Production Example 54, and 48 PGMEA was further added.
- the colored resin compositions 84 to 85 of Examples 84 to 85 were obtained in the same manner as Examples 82 to 83, respectively, except that the amount was changed to 2 parts by mass.
- Example 86 to 87 Preparation of colored resin compositions 86 to 87
- the mixing amount of the color material dispersion was 26.3 parts by mass of color material dispersion I, 2.3 parts by mass of color material dispersion N, and 50.3 parts by mass of PGMEA.
- colored resin compositions 86 to 87 of Examples 86 to 87 were obtained.
- Each evaluation method of the colored resin composition is the same as each evaluation method except that the post-baking temperature is 230 ° C. (for Examples 78 to 81 using the coloring material B or coloring material C, the post-baking temperature is 200 ° C.).
- the post-baking temperature is 200 ° C.
Landscapes
- Physics & Mathematics (AREA)
- General Physics & Mathematics (AREA)
- Chemical & Material Sciences (AREA)
- Nonlinear Science (AREA)
- Optics & Photonics (AREA)
- Organic Chemistry (AREA)
- Spectroscopy & Molecular Physics (AREA)
- Mathematical Physics (AREA)
- Crystallography & Structural Chemistry (AREA)
- Engineering & Computer Science (AREA)
- Architecture (AREA)
- Structural Engineering (AREA)
- Optical Filters (AREA)
- Materials For Photolithography (AREA)
- Liquid Crystal (AREA)
- Addition Polymer Or Copolymer, Post-Treatments, Or Chemical Modifications (AREA)
- Macromonomer-Based Addition Polymer (AREA)
- Polymerisation Methods In General (AREA)
- Graft Or Block Polymers (AREA)
Abstract
La présente invention concerne une composition de résine colorée pour un filtre coloré qui peut former une couche colorée présentant une excellente stabilité de sensibilité au cours du temps et une excellente adhésion au substrat, de résistance à la chaleur et de résistance au solvant, et dans lequel les taches d'humidité pendant le développement sont supprimées. Cette composition de résine colorée pour un filtre coloré contient un colorant laqué, un dispersant, une résine soluble dans un alcali, un monomère polyfonctionnel, un initiateur et un solvant. La résine soluble dans un alcali a un cycle hydrocarboné et une double liaison éthylénique, et a un indice d'acide de 80 à 300 mg de KOH/g. Le rapport de teneur d'un agent de couplage de silane à la teneur totale en solides dans la composition de résine colorée est de 1 % en masse ou moins.
Priority Applications (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN201580043097.7A CN106574999B (zh) | 2014-08-12 | 2015-08-11 | 彩色滤光片用着色树脂组合物、彩色滤光片及显示设备 |
| KR1020177003608A KR20170042583A (ko) | 2014-08-12 | 2015-08-11 | 컬러 필터용 착색 수지 조성물, 컬러 필터 및 표시 장치 |
Applications Claiming Priority (4)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2014164264 | 2014-08-12 | ||
| JP2014-164264 | 2014-08-12 | ||
| JP2014-248393 | 2014-12-08 | ||
| JP2014248393 | 2014-12-08 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2016024600A1 true WO2016024600A1 (fr) | 2016-02-18 |
Family
ID=55304227
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/JP2015/072807 WO2016024600A1 (fr) | 2014-08-12 | 2015-08-11 | Composition de résine colorée pour filtre coloré, filtre coloré et dispositif d'affichage |
Country Status (5)
| Country | Link |
|---|---|
| JP (1) | JP6664905B2 (fr) |
| KR (1) | KR20170042583A (fr) |
| CN (1) | CN106574999B (fr) |
| TW (1) | TWI589995B (fr) |
| WO (1) | WO2016024600A1 (fr) |
Cited By (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN107783373A (zh) * | 2016-08-24 | 2018-03-09 | 东友精细化工有限公司 | 着色固化性树脂组合物、滤色器和显示装置 |
| JP2018194747A (ja) * | 2017-05-19 | 2018-12-06 | 東友ファインケム株式会社Dongwoo Fine−Chem Co., Ltd. | 着色硬化性樹脂組成物、カラーフィルタ及び表示装置 |
| JPWO2017170473A1 (ja) * | 2016-03-29 | 2019-02-07 | 株式会社Adeka | 光重合開始剤組成物及び感光性組成物 |
Families Citing this family (9)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP6841283B2 (ja) * | 2016-08-05 | 2021-03-10 | 大日本印刷株式会社 | 着色組成物、カラーフィルタ及びその製造方法、液晶表示装置、並びに、発光表示装置 |
| WO2018151044A1 (fr) * | 2017-02-17 | 2018-08-23 | 東レ株式会社 | Composition colorante, et substrat de filtre coloré et dispositif d'affichage l'utilisant |
| KR102403787B1 (ko) * | 2017-03-31 | 2022-05-30 | 동우 화인켐 주식회사 | 청색 감광성 수지 조성물, 이를 이용하여 제조된 컬러필터 및 화상 표시 장치 |
| JP7051321B2 (ja) * | 2017-07-21 | 2022-04-11 | 東京応化工業株式会社 | 感光性組成物、パターン形成方法、硬化物、及び表示装置 |
| KR102361604B1 (ko) * | 2017-08-07 | 2022-02-10 | 동우 화인켐 주식회사 | 착색 감광성 수지 조성물, 이를 포함하는 컬러필터 및 이를 포함하는 표시장치 |
| JP7153651B2 (ja) * | 2017-08-10 | 2022-10-14 | 株式会社Dnpファインケミカル | 感光性着色樹脂組成物及びその硬化物、カラーフィルタ、並びに表示装置 |
| TWI739970B (zh) * | 2017-12-12 | 2021-09-21 | 奇美實業股份有限公司 | 負型感光性樹脂組成物、間隙體、保護膜,及液晶顯示元件 |
| JP7440016B2 (ja) * | 2019-07-10 | 2024-02-28 | サカタインクス株式会社 | カラーフィルター用染料分散組成物及びカラーフィルター用染料分散レジスト組成物 |
| JP7569213B2 (ja) * | 2019-12-26 | 2024-10-17 | 住友化学株式会社 | 感光性組成物 |
Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2011133851A (ja) * | 2009-12-22 | 2011-07-07 | Lg Chem Ltd | 基板接着力が向上した高遮光性ブラックマトリックス組成物 |
| JP2012194523A (ja) * | 2011-02-28 | 2012-10-11 | Toyo Ink Sc Holdings Co Ltd | カラーフィルタ用着色組成物、およびカラーフィルタ |
| JP2013057053A (ja) * | 2011-04-21 | 2013-03-28 | Dainippon Printing Co Ltd | 色材分散液、カラーフィルター用着色樹脂組成物、カラーフィルター、液晶表示装置及び有機発光表示装置 |
| JP2013114184A (ja) * | 2011-11-30 | 2013-06-10 | Dnp Fine Chemicals Co Ltd | 着色樹脂組成物、カラーフィルタ、及び液晶表示装置 |
Family Cites Families (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP3148429B2 (ja) * | 1992-02-04 | 2001-03-19 | 新日本製鐵株式会社 | 光重合性不飽和化合物及びアルカリ現像型感光性樹脂組成物 |
| JP2002088136A (ja) * | 2000-09-12 | 2002-03-27 | Nagase Kasei Kogyo Kk | 光重合性不飽和樹脂および該樹脂を含有する感光性樹脂組成物 |
| TWI524143B (zh) * | 2011-04-18 | 2016-03-01 | Sumitomo Chemical Co | Coloring hardening resin composition |
| JP5900372B2 (ja) * | 2013-02-08 | 2016-04-06 | 富士ゼロックス株式会社 | 静電荷像現像用トナー、静電荷像現像剤、トナーカートリッジ、プロセスカートリッジ、画像形成装置、及び、画像形成方法 |
-
2015
- 2015-08-11 KR KR1020177003608A patent/KR20170042583A/ko unknown
- 2015-08-11 CN CN201580043097.7A patent/CN106574999B/zh active Active
- 2015-08-11 JP JP2015159250A patent/JP6664905B2/ja active Active
- 2015-08-11 WO PCT/JP2015/072807 patent/WO2016024600A1/fr active Application Filing
- 2015-08-12 TW TW104126242A patent/TWI589995B/zh active
Patent Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2011133851A (ja) * | 2009-12-22 | 2011-07-07 | Lg Chem Ltd | 基板接着力が向上した高遮光性ブラックマトリックス組成物 |
| JP2012194523A (ja) * | 2011-02-28 | 2012-10-11 | Toyo Ink Sc Holdings Co Ltd | カラーフィルタ用着色組成物、およびカラーフィルタ |
| JP2013057053A (ja) * | 2011-04-21 | 2013-03-28 | Dainippon Printing Co Ltd | 色材分散液、カラーフィルター用着色樹脂組成物、カラーフィルター、液晶表示装置及び有機発光表示装置 |
| JP2013114184A (ja) * | 2011-11-30 | 2013-06-10 | Dnp Fine Chemicals Co Ltd | 着色樹脂組成物、カラーフィルタ、及び液晶表示装置 |
Cited By (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPWO2017170473A1 (ja) * | 2016-03-29 | 2019-02-07 | 株式会社Adeka | 光重合開始剤組成物及び感光性組成物 |
| CN107783373A (zh) * | 2016-08-24 | 2018-03-09 | 东友精细化工有限公司 | 着色固化性树脂组合物、滤色器和显示装置 |
| JP2018194747A (ja) * | 2017-05-19 | 2018-12-06 | 東友ファインケム株式会社Dongwoo Fine−Chem Co., Ltd. | 着色硬化性樹脂組成物、カラーフィルタ及び表示装置 |
| JP7017867B2 (ja) | 2017-05-19 | 2022-02-09 | 東友ファインケム株式会社 | 着色硬化性樹脂組成物、カラーフィルタ及び表示装置 |
Also Published As
| Publication number | Publication date |
|---|---|
| JP6664905B2 (ja) | 2020-03-13 |
| TWI589995B (zh) | 2017-07-01 |
| KR20170042583A (ko) | 2017-04-19 |
| TW201612638A (en) | 2016-04-01 |
| CN106574999B (zh) | 2019-03-22 |
| JP2016110065A (ja) | 2016-06-20 |
| CN106574999A (zh) | 2017-04-19 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP5897754B1 (ja) | 色材分散液、カラーフィルタ用着色樹脂組成物、カラーフィルタ及び表示装置 | |
| WO2016024600A1 (fr) | Composition de résine colorée pour filtre coloré, filtre coloré et dispositif d'affichage | |
| KR101390535B1 (ko) | 염료 분산액, 컬러 필터용 감광성 수지 조성물, 컬러 필터, 액정 표시 장치 및 유기 발광 표시 장치 | |
| US8853717B2 (en) | Dye dispersion liquid, photosensitive resin composition for color filters, color filter, liquid crystal display device and organic light emitting display device | |
| JP6494875B2 (ja) | カラーフィルタ用色材分散液、カラーフィルタ用着色樹脂組成物、カラーフィルタ、及び表示装置 | |
| JP2020013138A (ja) | カラーフィルタ用色材分散液、カラーフィルタ用感光性着色樹脂組成物、カラーフィルタ、及び表示装置 | |
| TWI748029B (zh) | 彩色濾光片用色材分散液、彩色濾光片用著色樹脂組成物、彩色濾光片及顯示裝置 | |
| JP6779772B2 (ja) | カラーフィルタ用着色樹脂組成物、色材分散液、カラーフィルタ、及び表示装置 | |
| JPWO2018062105A1 (ja) | カラーフィルタ用感光性着色樹脂組成物、カラーフィルタ、及び表示装置 | |
| WO2018159458A1 (fr) | Liquide dispersé dans un colorant destiné à des filtres colorés, dispersant, composition de résine colorante photosensible destinée à des filtres colorés, filtre coloré et dispositif d'affichage | |
| JP2013213982A (ja) | カラーフィルター用感光性樹脂組成物、及びその製造方法、カラーフィルター、液晶表示装置、並びに、有機発光表示装置 | |
| JP5110222B2 (ja) | 顔料分散液、カラーフィルタ用ネガ型レジスト組成物、カラーフィルタ、並びに、液晶表示装置及び有機発光表示装置 | |
| TW202104172A (zh) | 彩色濾光片用感光性著色樹脂組合物、硬化物、彩色濾光片及顯示裝置 | |
| JP2017146426A (ja) | カラーフィルタ用着色樹脂組成物、カラーフィルタ、及び表示装置 | |
| WO2019031292A1 (fr) | Composition de résine colorante photosensible, produit durci de celle-ci, filtre coloré et dispositif d'affichage | |
| JP6817805B2 (ja) | カラーフィルタ用着色樹脂組成物、色材分散液、カラーフィルタ、及び表示装置 | |
| JP2020003614A (ja) | 感光性着色樹脂組成物及びその硬化物、カラーフィルタ、並びに表示装置 | |
| JP6950070B2 (ja) | カラーフィルタ用着色樹脂組成物、色材分散液、カラーフィルタ、及び表示装置 | |
| JP2016122073A (ja) | 色材分散液、カラーフィルタ用感光性着色樹脂組成物、カラーフィルタ、液晶表示装置、及び有機発光表示装置 | |
| JP6450057B1 (ja) | 感光性着色樹脂組成物及びその硬化物、カラーフィルタ、並びに表示装置 | |
| JP5724274B2 (ja) | カラーフィルタ用赤色顔料分散液、カラーフィルタ用赤色感光性樹脂組成物、カラーフィルタ、及び液晶表示装置 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 15832005 Country of ref document: EP Kind code of ref document: A1 |
|
| ENP | Entry into the national phase |
Ref document number: 20177003608 Country of ref document: KR Kind code of ref document: A |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| 122 | Ep: pct application non-entry in european phase |
Ref document number: 15832005 Country of ref document: EP Kind code of ref document: A1 |