WO2015182120A1 - 銅含有シリコン材料及びその製造方法と負極活物質及び二次電池 - Google Patents
銅含有シリコン材料及びその製造方法と負極活物質及び二次電池 Download PDFInfo
- Publication number
- WO2015182120A1 WO2015182120A1 PCT/JP2015/002647 JP2015002647W WO2015182120A1 WO 2015182120 A1 WO2015182120 A1 WO 2015182120A1 JP 2015002647 W JP2015002647 W JP 2015002647W WO 2015182120 A1 WO2015182120 A1 WO 2015182120A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- copper
- silicon
- silicon material
- containing silicon
- calcium
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Ceased
Links
Images
Classifications
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M4/00—Electrodes
- H01M4/02—Electrodes composed of, or comprising, active material
- H01M4/36—Selection of substances as active materials, active masses, active liquids
- H01M4/38—Selection of substances as active materials, active masses, active liquids of elements or alloys
- H01M4/386—Silicon or alloys based on silicon
-
- C—CHEMISTRY; METALLURGY
- C01—INORGANIC CHEMISTRY
- C01B—NON-METALLIC ELEMENTS; COMPOUNDS THEREOF; METALLOIDS OR COMPOUNDS THEREOF NOT COVERED BY SUBCLASS C01C
- C01B33/00—Silicon; Compounds thereof
- C01B33/06—Metal silicides
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01G—CAPACITORS; CAPACITORS, RECTIFIERS, DETECTORS, SWITCHING DEVICES, LIGHT-SENSITIVE OR TEMPERATURE-SENSITIVE DEVICES OF THE ELECTROLYTIC TYPE
- H01G11/00—Hybrid capacitors, i.e. capacitors having different positive and negative electrodes; Electric double-layer [EDL] capacitors; Processes for the manufacture thereof or of parts thereof
- H01G11/22—Electrodes
- H01G11/30—Electrodes characterised by their material
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01G—CAPACITORS; CAPACITORS, RECTIFIERS, DETECTORS, SWITCHING DEVICES, LIGHT-SENSITIVE OR TEMPERATURE-SENSITIVE DEVICES OF THE ELECTROLYTIC TYPE
- H01G11/00—Hybrid capacitors, i.e. capacitors having different positive and negative electrodes; Electric double-layer [EDL] capacitors; Processes for the manufacture thereof or of parts thereof
- H01G11/22—Electrodes
- H01G11/30—Electrodes characterised by their material
- H01G11/50—Electrodes characterised by their material specially adapted for lithium-ion capacitors, e.g. for lithium-doping or for intercalation
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01G—CAPACITORS; CAPACITORS, RECTIFIERS, DETECTORS, SWITCHING DEVICES, LIGHT-SENSITIVE OR TEMPERATURE-SENSITIVE DEVICES OF THE ELECTROLYTIC TYPE
- H01G11/00—Hybrid capacitors, i.e. capacitors having different positive and negative electrodes; Electric double-layer [EDL] capacitors; Processes for the manufacture thereof or of parts thereof
- H01G11/84—Processes for the manufacture of hybrid or EDL capacitors, or components thereof
- H01G11/86—Processes for the manufacture of hybrid or EDL capacitors, or components thereof specially adapted for electrodes
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M10/00—Secondary cells; Manufacture thereof
- H01M10/05—Accumulators with non-aqueous electrolyte
- H01M10/052—Li-accumulators
- H01M10/0525—Rocking-chair batteries, i.e. batteries with lithium insertion or intercalation in both electrodes; Lithium-ion batteries
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M10/00—Secondary cells; Manufacture thereof
- H01M10/05—Accumulators with non-aqueous electrolyte
- H01M10/052—Li-accumulators
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y02—TECHNOLOGIES OR APPLICATIONS FOR MITIGATION OR ADAPTATION AGAINST CLIMATE CHANGE
- Y02E—REDUCTION OF GREENHOUSE GAS [GHG] EMISSIONS, RELATED TO ENERGY GENERATION, TRANSMISSION OR DISTRIBUTION
- Y02E60/00—Enabling technologies; Technologies with a potential or indirect contribution to GHG emissions mitigation
- Y02E60/10—Energy storage using batteries
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y02—TECHNOLOGIES OR APPLICATIONS FOR MITIGATION OR ADAPTATION AGAINST CLIMATE CHANGE
- Y02E—REDUCTION OF GREENHOUSE GAS [GHG] EMISSIONS, RELATED TO ENERGY GENERATION, TRANSMISSION OR DISTRIBUTION
- Y02E60/00—Enabling technologies; Technologies with a potential or indirect contribution to GHG emissions mitigation
- Y02E60/13—Energy storage using capacitors
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y02—TECHNOLOGIES OR APPLICATIONS FOR MITIGATION OR ADAPTATION AGAINST CLIMATE CHANGE
- Y02T—CLIMATE CHANGE MITIGATION TECHNOLOGIES RELATED TO TRANSPORTATION
- Y02T10/00—Road transport of goods or passengers
- Y02T10/60—Other road transportation technologies with climate change mitigation effect
- Y02T10/70—Energy storage systems for electromobility, e.g. batteries
Definitions
- the present invention relates to a copper-containing silicon material used for a negative electrode active material such as a lithium ion secondary battery, a manufacturing method thereof, and a secondary battery using the copper-containing silicon material as a negative electrode active material.
- a lithium ion secondary battery is a secondary battery having a high charge / discharge capacity and capable of high output.
- Lithium ion secondary batteries have active materials capable of inserting and extracting lithium (Li) in the positive electrode and the negative electrode, respectively. And it operates by moving lithium ions in the electrolyte provided between the two electrodes.
- lithium-containing metal composite oxides such as lithium cobalt composite oxide are mainly used as the active material for the positive electrode, and carbon materials having a multilayer structure are mainly used as the active material for the negative electrode.
- the performance of the lithium ion secondary battery depends on the materials of the positive electrode, the negative electrode, and the electrolyte constituting the secondary battery.
- active material that forms an active material is being actively conducted. For example, silicon or silicon oxide having a higher capacity than carbon has been studied as a negative electrode active material.
- silicon As the negative electrode active material, a battery having a higher capacity than that using a carbon material can be obtained.
- silicon has a large volume change due to insertion and extraction of Li during charge and discharge. Therefore, in a secondary battery using silicon as a negative electrode active material, silicon is pulverized during charge / discharge, causing a structural change, and dropping or peeling from the current collector, resulting in a short battery charge / discharge cycle life. There is a point. In view of this, a technique for suppressing volume change associated with insertion and extraction of Li during charge and discharge rather than silicon has been studied by using silicon oxide as a negative electrode active material.
- SiO x silicon oxide
- SiO x decomposes into Si and SiO 2 when heat-treated. This is called disproportionation reaction, and it is separated into two phases of Si phase and SiO 2 phase by solid internal reaction.
- the Si phase obtained by separation is very fine.
- the SiO 2 phase covering the Si phase has a function of suppressing the decomposition of the electrolytic solution. Therefore, the secondary battery using the negative electrode active material composed of SiO x decomposed into Si and SiO 2 has excellent cycle characteristics.
- Patent Document 1 describes a method of heating and sublimating metal silicon and SiO 2 to form silicon oxide gas and cooling it to produce SiO x .
- Patent Document 2 JP-A-2009-102219 discloses that a silicon raw material is decomposed to an elemental state in a high-temperature plasma and then rapidly cooled to liquid nitrogen temperature to obtain silicon nanoparticles. A manufacturing method for fixing in a SiO 2 —TiO 2 matrix by a sol-gel method or the like is described.
- the raw material is limited to a sublimable material. Furthermore, it is known that the SiO 2 phase covering the Si phase changes to lithium silicate when Li is occluded, thereby generating irreversible Li in the negative electrode, and it is necessary to add an excess active material to the positive electrode. Moreover, in the manufacturing method described in Patent Document 2, high energy is required for plasma discharge. Further, it is presumed that the silicon composites obtained by these production methods have low dispersibility of Si phase silicon particles and are likely to aggregate. When the silicon particles are aggregated to increase the particle size, a secondary battery using the same as a negative electrode active material has a low initial capacity and also deteriorates cycle characteristics.
- Non-patent Document 1 layered polysilane is synthesized by reacting hydrogen chloride (HCl) with calcium disilicide (CaSi 2 ). A method is described, and it is described that the layered polysilane thus obtained can be used for a light emitting device or the like.
- Non-Patent Document 2 Materials Research Bulletin, Vol.31, No.3, pp.307-316, 1996 (Non-Patent Document 2) is obtained by reacting hydrogen chloride (HCl) with calcium disilicide (CaSi 2 ). It is described that the layered polysilane was heat-treated at 900 ° C. to obtain a plate-like silicon crystal.
- HCl hydrogen chloride
- CaSi 2 calcium disilicide
- Patent Document 3 describes a lithium ion secondary battery using layered polysilane as a negative electrode active material.
- the secondary battery using the negative electrode active material composed of layered polysilane described in Patent Document 3 has insufficient rate characteristics and insufficient initial efficiency because the layered polysilane has low electron conductivity.
- the plate-like silicon crystal described in Non-Patent Document 2 has a high conductive resistance, it is difficult to use it as a negative electrode active material for a secondary battery as it is.
- the present invention has been made in view of such circumstances, a novel copper-containing silicon material having improved electron conductivity, a method for producing the same, a negative electrode active material using the copper-containing silicon material, and a negative electrode active material thereof.
- An object to be solved is to provide a secondary battery using a substance for a negative electrode.
- the feature of the method for producing a copper-containing silicon material of the present invention that solves the above problems is that a calcium source, a copper source, and a silicon source are prepared, and calcium (Ca), copper (Cu), and silicon (Si) are in an atomic ratio.
- the amorphous silicon phase contains copper and fine copper silicide is precipitated in the amorphous silicon phase, the electron conductivity is greatly improved.
- the XRD chart of the copper containing calcium silicide and copper containing silicon material which concern on Example 1 is shown.
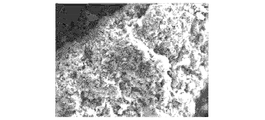
- 2 is an SEM image of a copper-containing silicon material according to Example 1.
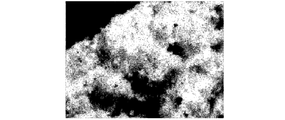
- 2 is a TEM-EDX image showing a distribution of silicon (Si) in a copper-containing silicon material according to Example 1.
- FIG. 2 is a TEM-EDX image showing the distribution of copper (Cu) in the copper-containing silicon material according to Example 1.
- FIG. 1 is a schematic diagram showing a structure of a copper-containing silicon material according to Example 1.
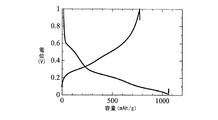
- FIG. The initial charge / discharge curve of the lithium ion secondary battery which concerns on Example 1 is shown.
- a calcium source, a copper source, and a silicon source are prepared, so that calcium (Ca), copper (Cu), and silicon (Si) have a predetermined ratio in atomic ratio. And molten to prepare a molten metal, and the molten metal is cooled to form a copper-containing calcium silicide.
- the calcium source calcium compounds such as calcium hydroxide, calcium oxide, calcium acetate, calcium carbonate, calcium chloride or metallic calcium can be used. From the viewpoint of reducing impurities, metallic calcium is preferred.
- copper compounds such as copper hydroxide, copper acetate, copper oxide, copper carbonate, copper cyanide, copper chloride, and organic copper compounds, or metallic copper can be used. From the viewpoint of reducing impurities, copper metal is preferable.
- silicon compounds such as organosilane, silicon monoxide, silicon dioxide, silicone, tetraethyl orthosilicate, or metal silicon can be used. From the viewpoint of reducing impurities, metal silicon is preferable.
- the above calcium source, copper source, and silicon source are mixed, melted and cast so that calcium (Ca), copper (Cu), and silicon (Si) are in a predetermined atomic ratio.
- the predetermined ratio is a ratio in which x and y satisfy 0.1 ⁇ x ⁇ 0.7, 1.33 ⁇ y ⁇ 2.1, and 1.8 ⁇ x + y ⁇ 2.2 when the composition formula is CaCu x Si y .
- X satisfies 0.1 ⁇ x ⁇ 0.7.
- the range of 0.2 ⁇ x ⁇ 0.6 is preferable, and the range of 0.2 ⁇ x ⁇ 0.3 is more preferable.
- y satisfies 1.33 ⁇ y ⁇ 2.1.
- the range of 1.5 ⁇ y ⁇ 2.1 is preferable, and the range of 1.65 ⁇ y ⁇ 1.85 is more preferable.
- the sum of x and y satisfies 1.8 ⁇ x + y ⁇ 2.2.
- the range of 1.85 ⁇ x + y ⁇ 2.15 is preferable, and the range of 1.9 ⁇ x + y ⁇ 2.0 is more preferable.
- the copper-containing calcium silicide may contain impurities such as raw materials in addition to Ca, Cu, and Si.
- the resulting copper-containing silicon material may have insufficient electronic conductivity.
- x exceeds 0.7 or y is less than 1.33 the initial capacity of a secondary battery using a copper-containing silicon material as a negative electrode active material may be reduced.
- the reaction in the second step may not easily proceed, or a large amount of impurity phase may be generated.
- the melting temperature can be 1100-1500 ° C., more preferably 1200-1400 ° C.
- the composition of Ca, Cu and Si is expressed by the formula CaCu x Si y (where x and y satisfy 0.1 ⁇ x ⁇ 0.7, 1.33 ⁇ y ⁇ 2.1, 1.8 ⁇ x + y ⁇ 2.2).
- a copper-containing calcium silicide is obtained.
- the copper-containing calcium silicide has a crystal structure of P6. It was found to belong to the / mmm space group. That is, the obtained copper-containing calcium silicide has a sheet-like graphite structure in which hexagonal silicon (Si) atoms and copper (Cu) atoms are sandwiched between calcium (Ca) atoms.
- a reaction between the copper-containing calcium silicide and an acid that extracts calcium (Ca) from the copper-containing calcium silicide is performed to form a silicon precursor.
- the copper-containing calcium silicide is desirably pulverized and classified in advance so that the reaction easily proceeds.
- the pulverization / classification means is not particularly limited, and conventionally used means may be employed.
- the particle size of the copper-containing calcium silicide powder used in the second step is not particularly limited, but is preferably 100 ⁇ m or less, more preferably 60 ⁇ m or less. Further, the lower limit of the particle size is preferably 1 ⁇ m or more because if it is too fine, the handling may be hindered.
- hydrochloric acid As an acid for extracting calcium (Ca) from copper-containing calcium silicide, hydrochloric acid (HCl) can be used as described in Non-Patent Document 2. However, when only hydrochloric acid (HCl) is used, the amount of oxygen and chlorine in the copper-containing silicon material as the final material may increase, which is not preferable as the negative electrode active material.
- an acid containing fluorine at least as an anion it is preferable to use an acid containing fluorine at least as an anion.
- an acid containing fluorine at least in the anion the amount of oxygen (O) contained in the obtained copper-containing silicon material can be reduced, and the amount of chlorine (Cl) can be reduced to zero by containing fluorine (F). Therefore, the initial capacity is improved when a copper-containing silicon material is used as a negative electrode active material of a lithium ion secondary battery.
- Acids containing at least fluorine in the anion include hydrofluoric acid, tetrafluoroboric acid, hexafluorophosphoric acid, hexafluoroarsenic acid, fluoroantimonic acid, hexafluorosilicic acid, hexafluorogermanic acid, hexafluorotin (IV)
- Examples include acids, trifluoroacetic acid, hexafluorotitanic acid, hexafluorozirconic acid, trifluoromethanesulfonic acid, fluorosulfonic acid, and the like.
- the acid for extracting calcium (Ca) from the copper-containing calcium silicide may contain other acids as long as it contains 0.01% by mass or more of at least one selected from the above acids.
- other acids include hydrochloric acid, hydrobromic acid, hydroiodic acid, sulfuric acid, methanesulfonic acid, nitric acid, phosphoric acid, formic acid, acetic acid and the like.
- the reaction between the acid and the copper-containing calcium silicide can be performed under the same conditions as described in Non-Patent Documents 1 and 2.
- the reaction is preferably performed at a low temperature of room temperature or lower, and it is desirable to carry out the reaction on an ice bath.
- the silicon precursor obtained using an acid containing fluorine at least as an anion has less oxygen and chlorine than the layered polysilane obtained by the methods described in Non-Patent Documents 1 and 2, and contains fluorine. It is out.
- hydrofluoric acid (HF) is used as an acid containing at least fluorine as an anion
- hydrochloric acid (HCl) is preferably mixed and used. Even when only hydrofluoric acid (HF) is used, a silicon precursor can be obtained. However, the obtained silicon precursor is not preferable because it has high activity and is oxidized by a small amount of air, increasing the amount of oxygen. When only hydrochloric acid (HCl) is used, the amount of oxygen in the silicon precursor may increase.
- the compounding ratio of the acid and the copper-containing calcium silicide it is desirable to make the acid excessive from the equivalent.
- the reaction atmosphere is preferably performed in an inert gas atmosphere. If the reaction time is too long, for example, Si and HF may further react to produce SiF 4, so that the reaction time is about 0.25 to 24 hours.
- the reaction time is about 0.25 to 24 hours.
- CaCl 2 is generated by the reaction in the second step, but can be easily removed by washing with water, and the silicon precursor is easily purified.
- tetrafluoroboric acid HHF 4
- hydrochloric acid HCl
- tetrafluoroboric acid HHF 4
- copper tetrafluoroboric acid
- the reaction conditions can be performed in the same manner as described above. According to this method, since the silicon precursor and the copper-containing silicon material obtained do not contain chlorine (Cl), the resistance can be further reduced when used as a negative electrode active material.
- the reaction proceeds by a mechanism that calcium (Ca) is extracted from the copper-containing calcium silicide belonging to the space group having a crystal structure of P6 / mmm. This is considered to be the same mechanism as the formation reaction of layered polysilane described in Non-Patent Documents 1 and 2.
- a silicon precursor having a layer structure having a sheet-like graphite structure including a hexagonal structure composed of silicon (Si) atoms and copper (Cu) atoms is formed.
- the silicon precursor is heat-treated in a non-oxidizing atmosphere to obtain the copper-containing silicon material of the present invention.
- the non-oxidizing atmosphere include a reduced-pressure atmosphere, a vacuum atmosphere, and an inert gas atmosphere.
- the heat treatment temperature is about 350 ° C. to 1100 ° C., preferably 400 ° C. or higher and lower than 1000 ° C., particularly preferably in the range of 500 ° C. or higher and 900 ° C. or lower.
- the heat treatment time varies depending on the heat treatment temperature, one hour is sufficient for heat treatment at 500 ° C. or higher.
- the copper-containing silicon material of the present invention obtained by the production method of the present invention may include an amorphous silicon phase and copper silicide such as Cu 3 Si and Cu 15 Si 4 deposited in the amorphous silicon phase. Since this copper-containing silicon material contains copper (Cu) in amorphous silicon and has high electron conductivity, it is useful as various semiconductor materials.
- the content of copper (Cu) is not particularly defined, but is preferably in the range of 1 to 50% by mass, more preferably in the range of 10 to 40% by mass, and particularly preferably in the range of 20 to 30% by mass. If the content of copper (Cu) is less than 1% by mass, the improvement of the electron conductivity is slight and is not practical. On the other hand, when the content of copper (Cu) exceeds 50% by mass, the initial capacity of a secondary battery using the copper-containing silicon material as a negative electrode active material is lowered.
- the silicon (Si) content is preferably in the range of 50 to 99% by mass, more preferably in the range of 60 to 95% by mass, and preferably in the range of 80 to 90% by mass. If the content of silicon (Si) is less than 50% by mass, the capacity of a secondary battery using it as a negative electrode active material is low and impractical. If it exceeds 99% by mass, the content of copper (Cu) is relative Therefore, the conductivity decreases.
- the copper-containing silicon material of the present invention has a structure in which fine copper silicide is precipitated in an amorphous phase containing copper and silicon.
- the copper-containing silicon material may contain calcium (Ca), fluorine (F), chlorine (Cl), oxygen (O), hydrogen (H), etc. derived from the raw material as impurities, and nano-sized silicon crystals. In some cases, it contains silicon or amorphous silicon.
- the copper-containing silicon material of the present invention contains lithium and electrochemically inactive copper silicide. Therefore, the secondary battery using the copper-containing silicon material of the present invention as the negative electrode active material is expected to improve cycle characteristics because the volume change of Si due to charge / discharge is suppressed.
- the copper-containing silicon material of the present invention can be used as a negative electrode active material in a secondary battery such as a lithium ion secondary battery.
- a secondary battery such as a lithium ion secondary battery.
- a copper-containing silicon material powder, a conductive auxiliary agent such as carbon powder, and a binder as necessary. Apply a suitable amount of an organic solvent and mix to make a slurry, and apply the binder on the current collector by roll coating, dip coating, doctor blade, spray coating, curtain coating, etc. It can be produced by drying or curing.
- Solvent-based binders include polyvinylidene fluoride (PolyVinylidene DiFluoride: PVdF), polytetrafluoroethylene (PTFE), styrene-butadiene rubber (SBR), polyimide (PI), polyamideimide (PAI), polyamide (PA), poly Examples include vinyl chloride (PVC), polymethacrylic acid (PMA), polyacrylonitrile (PAN), modified polyphenylene oxide (PPO), polyethylene oxide (PEO), polyethylene (PE), and polypropylene (PP).
- PVdF polyvinylidene fluoride
- PTFE polytetrafluoroethylene
- SBR styrene-butadiene rubber
- PI polyimide
- PAI polyamideimide
- PA polyamide
- PVC vinyl chloride
- PMA polymethacrylic acid
- PAN polyacrylonitrile
- PPO polyphenylene oxide
- PEO polyethylene oxide
- PE polyethylene
- a water-based binder means a binder used by mixing with an active material in a state where the binder is dispersed or dissolved in water, and typical examples include polyacrylic acid (PAA), styrene butadiene rubber (SBR), sodium alginate, Ammonium alginate can be used.
- PAA polyacrylic acid
- SBR styrene butadiene rubber
- Ammonium alginate can be used.
- a mixture of these binders with carboxymethyl cellulose (CMC) can be used as an aqueous binder, or CMC alone can be used as an aqueous binder in place of SBR and / or PAA.
- a water-soluble polymer cross-linked product may be used as the water-based binder, and a water-soluble cellulose ester cross-linked product such as a CMC cross-linked product, starch / acrylic acid graft polymer, or the like may be used.
- the potential of the negative electrode can be lowered, and the voltage of the secondary battery can be improved.
- PAI polyamideimide
- PAA polyacrylic acid
- a current collector is a chemically inert electronic high conductor that keeps current flowing through an electrode during discharging or charging.
- the current collector can adopt a shape such as a foil or a plate, but is not particularly limited as long as it has a shape according to the purpose.
- a copper foil or an aluminum foil can be suitably used as the current collector.
- the negative electrode active material known materials such as graphite, hard carbon, silicon, carbon fiber, tin (Sn), and silicon oxide can be mixed with the copper-containing silicon material of the present invention.
- a silicon oxide represented by SiO x (0.3 ⁇ x ⁇ 1.6) is particularly preferable.
- Each particle of the silicon oxide powder is composed of SiO x decomposed into fine Si and SiO 2 covering Si by a disproportionation reaction.
- x is less than the lower limit value, the Si ratio increases, so that the volume change during charge / discharge becomes too large and the cycle characteristics deteriorate.
- x exceeds the upper limit value the Si ratio decreases and the energy density decreases.
- a range of 0.5 ⁇ x ⁇ 1.5 is preferable, and a range of 0.7 ⁇ x ⁇ 1.2 is more desirable.
- a composite material of 1 to 50% by mass of a carbon material with respect to SiO x can also be used as the negative electrode active material.
- cycle characteristics are improved.
- Composite amount can not be obtained the effect of improving conductivity is less than 1% by weight of the carbon material, the negative electrode capacity ratio of SiO x exceeds 50 mass% is relatively decreased decreases.
- the composite amount of the carbon material is preferably in the range of 5 to 30% by mass, more preferably in the range of 5 to 20% by mass with respect to SiO x .
- a CVD method or the like can be used.
- the silicon oxide powder preferably has an average particle size in the range of 1 ⁇ m to 10 ⁇ m.
- the average particle size is larger than 10 ⁇ m, the durability of the secondary battery may be lowered.
- the average particle size is smaller than 1 ⁇ m, the particles are aggregated into coarse particles, and the durability of the secondary battery may be similarly reduced.
- Conductive aid is added to increase the conductivity of the electrode. Since the copper-containing silicon material of the present invention has high conductivity, a conductive aid is often unnecessary.
- Examples of conductive assistants include carbon black, natural graphite, granulated graphite, artificial graphite, flame retardant graphite, acetylene black (AB), ketjen black (KB) (registered trademark), and vapor grown carbon.
- Fiber (Vapor Grown Carbon Fiber: VGCF) or the like can be added alone or in combination of two or more.
- the amount of the conductive auxiliary agent used is not particularly limited, but can be, for example, about 20 to 100 parts by mass with respect to 100 parts by mass of the active material.
- the amount of the conductive auxiliary is less than 20 parts by mass, an efficient conductive path may not be formed. If the amount exceeds 100 parts by mass, the formability of the electrode deteriorates and the energy density decreases. Note that when the silicon oxide combined with the carbon material is used as the active material, the amount of the conductive auxiliary agent added can be reduced or eliminated.
- organic solvent there is no particular limitation on the organic solvent, and a mixture of a plurality of solvents may be used.
- N-methyl-2-pyrrolidone a mixed solvent of N-methyl-2-pyrrolidone and an ester solvent (ethyl acetate, n-butyl acetate, butyl cellosolve acetate, butyl carbitol acetate, etc.), or N-methyl-2- A mixed solvent of pyrrolidone and a glyme solvent (diglyme, triglyme, tetraglyme, etc.) is particularly preferred.
- the negative electrode can be predoped with lithium.
- an electrode formation method in which a half battery is assembled using metallic lithium as the counter electrode and electrochemically doped with lithium can be used.
- the amount of lithium doped is not particularly limited.
- the secondary battery of the present invention is a lithium ion secondary battery
- known positive electrodes, electrolytes, and separators that are not particularly limited can be used.
- the positive electrode may be anything that can be used in a lithium ion secondary battery.
- the positive electrode has a current collector and a positive electrode active material layer bound on the current collector.
- the positive electrode active material layer includes a positive electrode active material and a binder, and may further include a conductive additive.
- the positive electrode active material, the conductive additive, and the binder are not particularly limited as long as they can be used in the lithium ion secondary battery.
- positive electrode active materials include lithium metal, LiCoO 2 , Li x Ni a Co b Mn c O 2 , Li x Co b Mn c O 2 , Li x Ni a Mn c O 2 , Li x Ni a Co b O 2 and Examples include Li compounds or solid solutions selected from Li 2 MnO 3 (where 0.5 ⁇ x ⁇ 1.5, 0.1 ⁇ a ⁇ 1, 0.1 ⁇ b ⁇ 1, 0.1 ⁇ c ⁇ 1), Li 2 MnO 3 , sulfur, and the like.
- the current collector is not particularly limited as long as it is generally used for the positive electrode of a lithium ion secondary battery, such as aluminum, nickel, and stainless steel.
- the conductive auxiliary agent the same ones as described in the above negative electrode can be used.
- the electrolytic solution is obtained by dissolving a lithium metal salt as an electrolyte in an organic solvent.
- an aprotic organic solvent such as propylene carbonate (PC), ethylene carbonate (EC), dimethyl carbonate (DMC), diethyl carbonate (DEC), ethyl methyl carbonate (EMC) or the like should be used.
- PC propylene carbonate
- EC ethylene carbonate
- DMC dimethyl carbonate
- EMC diethyl carbonate
- EMC ethyl methyl carbonate
- a lithium metal salt soluble in an organic solvent such as LiPF 6 , LiBF 4 , LiAsF 6 , LiI, LiClO 4 , LiCF 3 SO 3 can be used.
- a lithium metal salt such as LiClO 4 , LiPF 6 , LiBF 4 , and LiCF 3 SO 3 in an organic solvent such as ethylene carbonate, dimethyl carbonate, propylene carbonate, and dimethyl carbonate is 0.5 to 1.7 mol / L.
- an organic solvent such as ethylene carbonate, dimethyl carbonate, propylene carbonate, and dimethyl carbonate.
- the separator is not particularly limited as long as it can be used for a non-aqueous secondary battery.
- the separator separates the positive electrode and the negative electrode and holds the electrolytic solution, and a thin microporous film such as polyethylene or polypropylene can be used.
- the shape of the secondary battery of the present invention is not particularly limited, and various shapes such as a cylindrical shape, a stacked shape, and a coin shape can be adopted. Regardless of the shape, a separator is sandwiched between the positive electrode and the negative electrode to form an electrode body, and the space between the positive electrode current collector and the negative electrode current collector to the positive electrode terminal and the negative electrode terminal is used for current collection. After connecting using a lead or the like, the electrode body is sealed in a battery case together with an electrolytic solution to form a battery.
- ⁇ Second step> A mixed solution of 20 ml of 55% by weight HF aqueous solution and 180 ml of 36% by weight HCl aqueous solution was brought to 0 ° C. in an ice bath, and 5 g of the above copper-containing calcium silicide was added thereto in an argon gas stream and stirred. did. After confirming the completion of foaming, the temperature was raised to room temperature, and the mixture was further stirred at room temperature for 1.5 hours. At this time, yellow powder floated. The obtained mixed solution was filtered, and the residue was washed with 200 ml of distilled water, then with 200 ml of acetone, and dried under vacuum for 12 hours to obtain 3.5 g of a silicon precursor.
- X-ray diffraction measurement using CuK ⁇ rays is performed on the obtained copper-containing silicon material, and the obtained XRD chart is shown in FIG. It can be seen that peaks attributed to Cu 3 Si and Cu 15 Si 4 exist and contain copper silicide.
- the total of the copper-containing calcium silicide is not 100, but it contains inevitable impurities such as those derived from raw materials.
- FIG. 2 shows the SEM image
- FIG. 3 shows the distribution of silicon (Si)
- FIG. 4 shows the distribution of copper (Cu).
- a part (a part indicated by an arrow) whose color is different from the surrounding is copper silicide, and the periphery is an amorphous phase.
- the amorphous phase contains not only Si but also Cu, and is considered to be a Si-Cu amorphous alloy.
- the copper-containing silicon material of this example is composed of a Si—Cu amorphous alloy phase 1 and a copper silicide 2 deposited almost uniformly in the Si—Cu amorphous alloy phase 1 as schematically shown in FIG. It is thought that.
- a solution obtained by dissolving 30% by mass of polyamideimide (PAI) resin in N-methyl-2-pyrrolidone (NMP) is used as the binder solution.
- PAI polyamideimide
- NMP N-methyl-2-pyrrolidone
- a lithium ion secondary battery (half cell) was produced using the negative electrode produced by the above procedure as an evaluation electrode.
- the counter electrode was a metal lithium foil (thickness 500 ⁇ m).
- the counter electrode was cut to ⁇ 14 mm and the evaluation electrode was cut to ⁇ 11 mm, and a separator (Hoechst Celanese glass filter and Celgard “Celgard2400”) was interposed between them to form an electrode body battery.
- This electrode body battery was accommodated in a battery case (CR2032 type coin battery member, manufactured by Hosen Co., Ltd.).
- a nonaqueous electrolyte solution in which LiPF 6 was dissolved at a concentration of 1M was injected into a mixed solvent in which ethylene carbonate and diethyl carbonate were mixed at a ratio of 1: 1 (volume ratio).
- a secondary battery was obtained.
- the silicon material of Comparative Example 1 does not contain copper (Cu) and has a high resistance that cannot be measured, whereas the copper-containing silicon material of Example 1 has a very low conductive resistance.
- the lithium secondary battery of Example 1 was subjected to a charge / discharge test under the conditions of temperature: 25 ° C., current: 0.1 C, voltage: 0.01-1.0 V.
- the charge / discharge curve is shown in FIG. 6, and the charge capacity, discharge capacity, and initial efficiency (100 ⁇ charge capacity / discharge capacity) are shown in Table 3.
- the silicon material of the comparative example 1 has too high resistance as shown in Table 2 and does not function as a battery, the battery formation and the battery characteristic test were not performed.
- the lithium ion secondary battery using the copper-containing silicon material of Example 1 as a negative electrode active material has a sufficient function as a secondary battery.
- the copper-containing silicon material of the present invention can be used as a negative electrode active material for power storage devices such as a secondary battery, an electric double layer capacitor, and a lithium ion capacitor.
- the secondary battery is useful as a non-aqueous secondary battery for motor driving of electric vehicles and hybrid vehicles, personal computers, portable communication devices, home appliances, office equipment, industrial equipment, etc. It can be optimally used for driving motors of electric vehicles and hybrid vehicles that require output.
- the copper-containing silicon material of the present invention has a high degree of freedom in heat treatment temperature and can be combined with other materials by controlling the size of the specific surface area. It can also be used as a material.
Landscapes
- Engineering & Computer Science (AREA)
- Chemical & Material Sciences (AREA)
- Power Engineering (AREA)
- Materials Engineering (AREA)
- Microelectronics & Electronic Packaging (AREA)
- Organic Chemistry (AREA)
- Manufacturing & Machinery (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Electrochemistry (AREA)
- General Chemical & Material Sciences (AREA)
- Inorganic Chemistry (AREA)
- Battery Electrode And Active Subsutance (AREA)
- Silicon Compounds (AREA)
- Electric Double-Layer Capacitors Or The Like (AREA)
Priority Applications (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US15/314,421 US20170200949A1 (en) | 2014-05-29 | 2015-05-26 | Copper-containing silicon material, method for producing same, negative electrode active material, and secondary battery |
| CN201580027663.5A CN106458610B (zh) | 2014-05-29 | 2015-05-26 | 含铜的硅材料及其制造方法和负极活性物质以及二次电池 |
| DE112015002533.2T DE112015002533B4 (de) | 2014-05-29 | 2015-05-26 | Kupfer-enthaltendes Siliciummaterial, Verfahren für dessen Herstellung, Negativelektrodenaktivmaterial und Sekundärbatterie |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2014-110821 | 2014-05-29 | ||
| JP2014110821A JP6318859B2 (ja) | 2014-05-29 | 2014-05-29 | 銅含有シリコン材料及びその製造方法と負極活物質及び二次電池 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2015182120A1 true WO2015182120A1 (ja) | 2015-12-03 |
Family
ID=54698467
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/JP2015/002647 Ceased WO2015182120A1 (ja) | 2014-05-29 | 2015-05-26 | 銅含有シリコン材料及びその製造方法と負極活物質及び二次電池 |
Country Status (5)
| Country | Link |
|---|---|
| US (1) | US20170200949A1 (enExample) |
| JP (1) | JP6318859B2 (enExample) |
| CN (1) | CN106458610B (enExample) |
| DE (1) | DE112015002533B4 (enExample) |
| WO (1) | WO2015182120A1 (enExample) |
Cited By (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2019053983A1 (ja) * | 2017-09-14 | 2019-03-21 | 株式会社豊田自動織機 | Al含有シリコン材料を含む負極活物質 |
| WO2019053984A1 (ja) * | 2017-09-14 | 2019-03-21 | 株式会社豊田自動織機 | Al含有シリコン材料を含む負極活物質 |
| JP2019052076A (ja) * | 2017-09-14 | 2019-04-04 | 株式会社豊田自動織機 | Al含有シリコン材料 |
| JP2019052077A (ja) * | 2017-09-14 | 2019-04-04 | 株式会社豊田自動織機 | Al含有シリコン材料 |
Families Citing this family (8)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN107074559B (zh) | 2014-09-19 | 2019-11-19 | 株式会社丰田自动织机 | 含MSix硅材料及其制造方法 |
| WO2016199358A1 (ja) * | 2015-06-12 | 2016-12-15 | 株式会社豊田自動織機 | シリコン材料およびその製造方法 |
| JP6642822B2 (ja) * | 2015-12-25 | 2020-02-12 | 株式会社豊田自動織機 | MSix(Mは第3〜9族元素から選択される少なくとも一元素。ただし、1/3≦x≦3)含有シリコン材料およびその製造方法 |
| JP6926873B2 (ja) | 2017-09-14 | 2021-08-25 | 株式会社豊田自動織機 | Al及びO含有シリコン材料 |
| CN109950542A (zh) * | 2019-04-03 | 2019-06-28 | 西安交通大学 | 一类含硅氧烷基团的接枝共聚物粘合剂及其应用以及基于其的二次电池 |
| JP7666259B2 (ja) * | 2021-09-16 | 2025-04-22 | 株式会社豊田中央研究所 | Cu5Ca系粉末及びその製造方法 |
| CN114613957B (zh) * | 2022-03-11 | 2023-08-11 | 山东大学 | 基于熔盐制备锂离子电池铜包覆硅负极材料的方法及应用 |
| CN115212882B (zh) * | 2022-06-30 | 2023-12-19 | 浙江工业大学 | 一种多孔硅化铜金属间化合物材料及其制备和应用 |
Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2011034839A (ja) * | 2009-08-03 | 2011-02-17 | Furukawa Electric Co Ltd:The | ナノサイズ粒子、ナノサイズ粒子を含むリチウムイオン二次電池用負極材料、リチウムイオン二次電池用負極、リチウムイオン二次電池、ナノサイズ粒子の製造方法 |
| JP2011090806A (ja) * | 2009-10-20 | 2011-05-06 | Toyota Central R&D Labs Inc | リチウム二次電池用電極及びそれを備えたリチウム二次電池 |
| JP2012072046A (ja) * | 2010-09-03 | 2012-04-12 | Toyota Central R&D Labs Inc | 遷移金属シリサイド−Si複合粉末及びその製造方法、並びに、遷移金属シリサイド−Si複合粉末製造用CaSiy系粉末及びその製造方法 |
| JP2013125743A (ja) * | 2011-12-13 | 2013-06-24 | Samsung Sdi Co Ltd | 負極活物質及びそれを含む二次電池 |
Family Cites Families (9)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| NO152551C (no) * | 1983-02-07 | 1985-10-16 | Elkem As | Fremgangsmaate til fremstilling av rent silisium. |
| EP1033767B9 (en) * | 1998-09-18 | 2010-09-01 | Canon Kabushiki Kaisha | Electrode material for negative pole of lithium secondary cell, electrode structure using said electrode material, lithium secondary cell using said electrode structure, and method for manufacturing said electrode structure and said lithium secondary cell |
| JP2005235397A (ja) * | 2000-01-25 | 2005-09-02 | Sanyo Electric Co Ltd | リチウム電池用電極並びにこれを用いたリチウム電池及びリチウム二次電池 |
| CN101179126B (zh) * | 2003-03-26 | 2011-09-28 | 佳能株式会社 | 电极材料、电极结构及具有该电极结构的二次电池 |
| JP4368139B2 (ja) * | 2003-05-08 | 2009-11-18 | パナソニック株式会社 | 非水電解質二次電池用負極材料 |
| KR101281277B1 (ko) * | 2005-03-23 | 2013-07-03 | 파이오닉스 가부시키가이샤 | 리튬이차전지용 음극 활물질 입자 및 음극의 제조 방법 |
| JP5128873B2 (ja) * | 2007-08-10 | 2013-01-23 | 株式会社豊田自動織機 | 二次電池用電極及びその製造方法 |
| CN102272983B (zh) * | 2008-12-30 | 2014-02-19 | 株式会社Lg化学 | 二次电池用阴极活性材料 |
| JP2013122905A (ja) * | 2011-11-10 | 2013-06-20 | Sanyo Special Steel Co Ltd | 鱗片状Si系合金負極材料 |
-
2014
- 2014-05-29 JP JP2014110821A patent/JP6318859B2/ja active Active
-
2015
- 2015-05-26 DE DE112015002533.2T patent/DE112015002533B4/de not_active Expired - Fee Related
- 2015-05-26 WO PCT/JP2015/002647 patent/WO2015182120A1/ja not_active Ceased
- 2015-05-26 CN CN201580027663.5A patent/CN106458610B/zh not_active Expired - Fee Related
- 2015-05-26 US US15/314,421 patent/US20170200949A1/en not_active Abandoned
Patent Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2011034839A (ja) * | 2009-08-03 | 2011-02-17 | Furukawa Electric Co Ltd:The | ナノサイズ粒子、ナノサイズ粒子を含むリチウムイオン二次電池用負極材料、リチウムイオン二次電池用負極、リチウムイオン二次電池、ナノサイズ粒子の製造方法 |
| JP2011090806A (ja) * | 2009-10-20 | 2011-05-06 | Toyota Central R&D Labs Inc | リチウム二次電池用電極及びそれを備えたリチウム二次電池 |
| JP2012072046A (ja) * | 2010-09-03 | 2012-04-12 | Toyota Central R&D Labs Inc | 遷移金属シリサイド−Si複合粉末及びその製造方法、並びに、遷移金属シリサイド−Si複合粉末製造用CaSiy系粉末及びその製造方法 |
| JP2013125743A (ja) * | 2011-12-13 | 2013-06-24 | Samsung Sdi Co Ltd | 負極活物質及びそれを含む二次電池 |
Non-Patent Citations (1)
| Title |
|---|
| W.J. HOR ET AL.: "Superconductivity in the copper-poor region of the Ca (Cu, Si)2-x system", PHYSICA C, vol. 434, 2006, pages 121 - 124, XP029168416 * |
Cited By (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2019053983A1 (ja) * | 2017-09-14 | 2019-03-21 | 株式会社豊田自動織機 | Al含有シリコン材料を含む負極活物質 |
| WO2019053984A1 (ja) * | 2017-09-14 | 2019-03-21 | 株式会社豊田自動織機 | Al含有シリコン材料を含む負極活物質 |
| JP2019052076A (ja) * | 2017-09-14 | 2019-04-04 | 株式会社豊田自動織機 | Al含有シリコン材料 |
| JP2019052077A (ja) * | 2017-09-14 | 2019-04-04 | 株式会社豊田自動織機 | Al含有シリコン材料 |
Also Published As
| Publication number | Publication date |
|---|---|
| DE112015002533B4 (de) | 2018-01-11 |
| JP6318859B2 (ja) | 2018-05-09 |
| JP2015224164A (ja) | 2015-12-14 |
| CN106458610A (zh) | 2017-02-22 |
| US20170200949A1 (en) | 2017-07-13 |
| DE112015002533T5 (de) | 2017-02-23 |
| CN106458610B (zh) | 2019-03-12 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP6318859B2 (ja) | 銅含有シリコン材料及びその製造方法と負極活物質及び二次電池 | |
| CN104937753B (zh) | 纳米硅材料的制造方法 | |
| KR101899701B1 (ko) | 나노 실리콘 재료와 그 제조 방법 및 이차 전지의 부극 | |
| JP5756781B2 (ja) | シリコン複合体及びその製造方法と負極活物質及び非水系二次電池 | |
| JP6176510B2 (ja) | シリコン材料及び二次電池の負極 | |
| JP5660403B2 (ja) | 負極活物質とその製造方法及び蓄電装置 | |
| WO2015068351A1 (ja) | 負極活物質及び蓄電装置 | |
| JP6011313B2 (ja) | 負極活物質とその製造方法及び蓄電装置 | |
| JP6065678B2 (ja) | 負極活物質とその製造方法及び蓄電装置 | |
| JP5534368B2 (ja) | 負極活物質及び蓄電装置 | |
| JP6176511B2 (ja) | シリコン材料及び二次電池の負極 | |
| JP6299154B2 (ja) | 負極活物質及び蓄電装置 | |
| JP5737447B2 (ja) | 銅含有層状ポリシランと負極活物質及び蓄電装置 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 15799924 Country of ref document: EP Kind code of ref document: A1 |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 15314421 Country of ref document: US |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 112015002533 Country of ref document: DE |
|
| 122 | Ep: pct application non-entry in european phase |
Ref document number: 15799924 Country of ref document: EP Kind code of ref document: A1 |