WO2015033926A1 - 細胞培養容器 - Google Patents
細胞培養容器 Download PDFInfo
- Publication number
- WO2015033926A1 WO2015033926A1 PCT/JP2014/073064 JP2014073064W WO2015033926A1 WO 2015033926 A1 WO2015033926 A1 WO 2015033926A1 JP 2014073064 W JP2014073064 W JP 2014073064W WO 2015033926 A1 WO2015033926 A1 WO 2015033926A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- microwell
- inclined surface
- cell culture
- culture container
- cells
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Ceased
Links
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12M—APPARATUS FOR ENZYMOLOGY OR MICROBIOLOGY; APPARATUS FOR CULTURING MICROORGANISMS FOR PRODUCING BIOMASS, FOR GROWING CELLS OR FOR OBTAINING FERMENTATION OR METABOLIC PRODUCTS, i.e. BIOREACTORS OR FERMENTERS
- C12M23/00—Constructional details, e.g. recesses, hinges
- C12M23/02—Form or structure of the vessel
- C12M23/12—Well or multiwell plates
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12M—APPARATUS FOR ENZYMOLOGY OR MICROBIOLOGY; APPARATUS FOR CULTURING MICROORGANISMS FOR PRODUCING BIOMASS, FOR GROWING CELLS OR FOR OBTAINING FERMENTATION OR METABOLIC PRODUCTS, i.e. BIOREACTORS OR FERMENTERS
- C12M21/00—Bioreactors or fermenters specially adapted for specific uses
- C12M21/06—Bioreactors or fermenters specially adapted for specific uses for in vitro fertilization
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12M—APPARATUS FOR ENZYMOLOGY OR MICROBIOLOGY; APPARATUS FOR CULTURING MICROORGANISMS FOR PRODUCING BIOMASS, FOR GROWING CELLS OR FOR OBTAINING FERMENTATION OR METABOLIC PRODUCTS, i.e. BIOREACTORS OR FERMENTERS
- C12M23/00—Constructional details, e.g. recesses, hinges
- C12M23/02—Form or structure of the vessel
Definitions
- the present invention relates to a cell culture vessel for culturing cells such as fertilized eggs that require individual management.
- the in vitro fertilized egg (zygote) is produced by fertilizing sperm and ovum in a culture system, and the fertilized egg goes through the cleavage, morula, and blastocyst stages, and then emerges from the zona pellucida It is possible to culture up to the blastocyst stage.
- Assistive reproductive technology (ART) to transfer a fertilized egg from the cleavage to the blastocyst stage to the uterus to give birth to a baby is not limited to the livestock region. Established in infertility medicine.
- the pregnancy success rate by in vitro fertilization is not necessarily high.
- the pregnancy success rate is still about 25 to 35%.
- One of the causes is that the probability of obtaining a high-quality fertilized egg suitable for transplantation into the uterus in culture is not high.
- a cultured fertilized egg is individually observed with a microscope to determine whether it is a high-quality fertilized egg suitable for transplantation into the uterus.
- a microdrop method In in vitro fertilization, a microdrop method is often used in which a culture solution is dropped in a container and fertilized eggs are placed in the container to culture in vitro.
- a petri dish having a single flat bottom and a diameter of 30 to 60 mm is used as a cell culture container, and a plurality of drops of culture solution are placed on the bottom of the petri dish at intervals. Methods of making and culturing cells therein have been used.
- the present inventors When carrying out a fertilized egg culture, the present inventors use a cell culture container having a microwell, so that the size of the well causes the bubbles to remain in the well when the culture solution is added, thereby removing the bubbles. It was found that there is a risk that the fertilized egg jumps out from the inside of the microwell if the work is complicated and the work is easy to remove bubbles.
- the present invention provides a method for culturing bubbles stably in a microwell in a cell culture by a microdrop method using a culture container having a microwell for containing cells and stably holding the cell in the microwell.
- An object of the present invention is to provide a cell culture container that can improve the culture workability and the observation workability.
- the present inventors have found that the above problem can be solved by using a cell culture vessel in which microwells having two types of inclined surfaces with different inclination angles are formed on the bottom surface.
- the present invention includes the following inventions.
- a cell culture container having a bottom surface and a side surface, One or more microwells for containing cells are arranged on the bottom, The first inclined surface formed so that the microwell becomes higher as it goes from the deepest part to the outer edge, and the second inclined surface that is connected to the first inclined surface and formed so as to become higher toward the outer edge and further toward the outer edge.
- An inclined surface, An angle ⁇ 1 formed by a line perpendicular to the bottom surface of the cell culture container and the first inclined surface is less than 90 °
- the angle ⁇ 2 formed by the line perpendicular to the bottom surface of the cell culture container and the second inclined surface is 3 ° or more and 45 ° or less
- ⁇ 1 is larger than ⁇ 2
- the depth of the microwell is 0.05 mm or more and 0.5 mm or less, Cell culture container.
- the first inclined surface of the microwell forms a conical surface or a side surface of the truncated cone
- the second inclined surface forms a side surface of the truncated cone
- a cell culture vessel having improved culture workability and observation workability is provided.
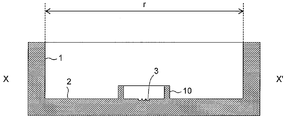
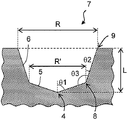
- FIGS. 1 A schematic view of one embodiment of the cell culture container of the present invention is shown in FIGS. 1 is a top view, FIG. 2 is a vertical sectional view, and FIG. 3 is an enlarged view of a microwell.
- the cell culture container of the present invention is A cell culture vessel having a side surface 1 and a bottom surface 2; A microwell 3 for accommodating cells is disposed on the bottom surface 2; The microwell 3 is formed so as to be higher as it goes from the deepest portion 4 to the outer edge, and to be higher on the outer edge side connected to the first inclined face and further toward the outer edge.
- Second inclined surface 6 The angle ⁇ 1 formed by the line perpendicular to the bottom surface of the cell culture container and the first inclined surface 5 is less than 90 °, The angle ⁇ 2 formed by the line perpendicular to the bottom surface of the cell culture container and the second inclined surface 6 is 3 ° or more and 45 ° or less, ⁇ 1 is larger than ⁇ 2, The depth L of the microwell is 0.05 mm or more and 0.5 mm or less.
- a microwell for containing cells has a first inclined surface and a second inclined surface having different inclination angles.
- the first inclined surface is formed so as to increase as it goes from the deepest portion to the outer edge, and the second inclined surface is formed so as to be connected to the outer edge of the first inclined surface.
- the second inclined surface is also formed so as to be steeper and higher than the first inclined surface as it proceeds from the connecting portion with the first inclined surface to the outer edge.
- the angle ⁇ 1 formed by the first inclined surface and the line perpendicular to the bottom surface of the cell culture vessel is less than 90 °, preferably 89 ° or less, more preferably 87 ° or less, and even more preferably 85 ° or less, Is 45 ° or more, more preferably 60 ° or more, and still more preferably 70 ° or more. Since the microwell of the cell culture container of the present invention has the first inclined surface inclined at such an angle ⁇ 1, the cells can be maintained at the center (deepest part) of the microwell, that is, some shaking. Since the cells do not move from the center position even if given, there is an advantage that it is not necessary to search for the cells under a microscope and the observation is easy.
- ⁇ 1 it is easy to move to a place (deepest part) where cells are to be placed using gravity as a driving source. Reflection and scattering on the surface are less likely to occur, and a clear observation image can be obtained. Furthermore, by setting the angle ⁇ 1 within the above range, it is possible to prevent bubbles from remaining in the microwell when the culture solution is added while maintaining the function of maintaining the cells at the center.
- the angle ⁇ 2 formed by the line perpendicular to the bottom surface of the cell culture container and the second inclined surface is 3 ° or more, preferably 5 ° or more, more preferably 7 ° or more, and 45 ° or less, preferably 40 °. Hereinafter, it is more preferably 30 ° or less. Since the microwell of the cell culture container of the present invention has the second inclined surface inclined at such an angle ⁇ 2, it is possible to prevent bubbles from remaining in the microwell when the culture solution is added. On the other hand, by setting the angle ⁇ 2 to 45 ° or less, the cells can be prevented from jumping out of the microwell, and the cells can be held in the microwell even if a slight vibration or roll is applied. Note that ⁇ 1 is larger than ⁇ 2.
- the size of the cell culture vessel is not particularly limited, but an opening having a circular shape and an opening width (for example, r in FIG. 2) of preferably 30 to 60 mm, particularly 35 mm is used.
- This is the same size as a petri dish used for conventional cell culture, and can be easily produced from a general-purpose petri dish, and is easily adaptable to an existing culture apparatus, etc., so that the size as described above is preferable. .
- Fig. 3 shows an enlarged view of the vertical cut surface of the microwell.
- the vertical cut surface of the microwell refers to a cut surface perpendicular to the bottom surface of the cell culture container and passing through the center of the microwell.
- the microwell is formed on the bottom surface of the cell culture container, has a first inclined surface 5 and a second inclined surface 6, and has an opening 7.
- the cell culture container is usually used in a state where its bottom surface is horizontal.
- the shape of the outer edge of the opening of the microwell is not particularly limited, but is preferably circular (including circular, substantially circular, elliptical, substantially elliptical, and semicircular), and particularly preferably circular.
- the first inclined surface 5 of the microwell is formed so as to be inclined so as to increase from the deepest part 4 of the microwell to the outer edge. It becomes higher as it goes to the outer edge.
- the first inclined surface of the microwell forms a gentle inclined surface, and preferably forms a conical surface or a side surface of a truncated cone.
- the deepest portion 4 of the microwell is configured such that the cone is arranged so as to correspond to the apex of the cone (FIG. 3).
- the deepest part of the microwell that is, the apex of the cone may be rounded.
- the truncated cone is arranged so that the smaller one of the upper and lower surfaces of the truncated cone corresponds to the deepest part of the microwell (FIG. 4). ).
- ⁇ 1 corresponds to an angle formed by the center line and the generatrix.
- the second inclined surface 6 of the microwell is formed so as to be connected to the outer edge end (opening portion of the first inclined surface) of the first inclined surface, and further proceeds from the connecting portion 8 with the first inclined surface to the outer edge. It is formed to be higher. As it goes to the outer edge, the height increases as shown in FIG. 3 in the vertical cut surface (cut surface perpendicular to the bottom surface of the cell culture container and passing through the center of the microwell) from the connecting portion 8 to the first inclined surface. It is said that it becomes high with a predetermined inclined structure toward the opening end 9 toward the outside.
- the second inclined surface of the microwell forms a gentle inclined surface, preferably the side surface of the truncated cone.
- the truncated cone is arranged such that the smaller of the upper surface and the lower surface of the truncated cone corresponds to the bottom side of the microwell (FIG. 3). And FIG. 4).
- ⁇ 2 corresponds to the angle formed by the center line and the bus line.
- the wall surface of the microwell may have a wall surface perpendicular to the bottom surface of the cell culture container on the opening side from the second inclined surface as described above.
- the angle ⁇ 3 formed between the first inclined surface and the second inclined surface inside the microwell is preferably 100 ° or more, more preferably 105 ° or more, preferably 150 ° or less, more preferably 145 ° or less.
- the connecting portion 8 of the first inclined surface and the second inclined surface and the opening end 9 of the microwell are rounded, that is, have a curvature.
- the curvature is preferably 0.01 mm or more, more preferably 0.02 mm or more, preferably 0.1 mm or less, more preferably 0.07 mm or less.
- the surface roughness of the inclined surface of the microwell is a large value, a clear contour cannot be obtained due to unevenness on the inclined surface when the image observed through transmission with a microscope is subjected to contour extraction processing. Since there is a fear, it is preferable that the value is as small as possible.
- the maximum height Ry extract only the reference length in the direction of the average line from the roughness curve, and the interval between the peak line and the valley line in the extracted part
- the surface roughness of the inclined surface can be reduced by increasing the processing accuracy of the mold, for example, by performing a polishing process when producing the mold for the culture vessel.
- the depth of the microwell means a depth (L in FIGS. 3 and 4) measured vertically from the opening to the deepest portion of the microwell, and is 0.05 mm or more, preferably 0.1 mm or more. 0.5 mm or less, preferably 0.4 mm or less. If the depth of the microwell is too shallow, the cells may move when the culture vessel is transported or when the cells divide, and the cells may come out of the microwell range. It is set so that it can be maintained. For example, in order to retain the cells in the microwell, the depth is preferably 1/3 or more of the maximum cell diameter, and more preferably 1/2 or more. On the other hand, if the depth is too deep, it becomes difficult to introduce the culture solution or cells into the microwell.
- the value is appropriately set so that the value is not too deep while the cells are held in the microwell.
- the upper limit of the depth can be 3 times or less the opening width of the opening of the microwell.
- the depth is preferably not more than 1 times the opening width of the microwell, and particularly preferably not more than 1/2.
- the opening of the microwell has an opening width that can accommodate cells.
- the opening width of the opening of the microwell refers to the length of the shortest diameter of the figure formed by the outer edge of the opening of the microwell. Therefore, when the outer edge of the opening of the microwell is circular, the opening width is equal to the diameter of the circle, and the diameter is larger than the maximum dimension of the cells to be cultured.
- the diameter of the circular opening is larger than the maximum cell size of the blastocyst stage It is desirable.
- the opening width is smaller than the pitch between the microwells.
- the opening width of the opening of the microwell is preferably 0.1 mm or more and 1 mm or less.
- it is 0.25 mm or more, More preferably, it is 0.26 mm or more, More preferably, it is 0.27 mm or more, Preferably it is less than 0.7 mm, More preferably, it is less than 0.45 mm.
- the opening width of the opening of the microwell can be defined as X + m (where X represents the maximum cell diameter).
- m is preferably 0.01 mm or more, and more preferably 0.02 mm or more.
- the opening width of the outer edge of the first inclined surface of the microwell is determined by ⁇ 1, ⁇ 2, and L although it may be automatically determined, it is preferably 0.15 mm or more, more preferably 0.2 mm or more, preferably less than 0.65 mm, more preferably less than 0.55 mm.
- At least one microwell is formed on the bottom surface of the cell culture container, preferably 4 or more, preferably 6 or more, more preferably 8 or more are formed in close proximity. It is sufficient that at least four microwells are formed close to each other, and microwells that are not close to each other may be formed separately. Further, a plurality of groups of microwells formed close to four or more may be arranged, and these groups may not be close to each other.
- the pitch between adjacent microwells is 1 mm or less. However, the pitch varies depending on the type of cells to be accommodated. By arranging the microwells closely at the pitch as described above, many cells can be cultured simultaneously while managing the cells individually, and more cells can enter a single field of view of the microscope. Images can be acquired.
- each group of adjacent microwells (cell storage units) is surrounded by an inner wall, and when a plurality of groups of microwells are present on the bottom surface of the cell culture container, each group is surrounded by an inner wall.
- each group is surrounded by an inner wall.
- the culture solution containing the fertilized egg is formed in a culture vessel, and the culture solution is prevented from drying by covering the droplet with oil.
- a group of four or more microwells formed close to each other is further surrounded by an inner wall, so that a culture solution can be accommodated therein to form a stable drop, thereby preventing dispersion of the culture solution.
- oil such as mineral oil.
- the material of the cell culture container of this embodiment is not particularly limited. Specifically, inorganic materials such as metal, glass, and silicon, plastics (for example, polystyrene resin, polyethylene resin, polypropylene resin, ABS resin, nylon, acrylic resin, fluororesin, polycarbonate resin, polyurethane resin, methylpentene resin, And organic materials represented by phenol resin, melamine resin, epoxy resin, and vinyl chloride resin).
- plastics for example, polystyrene resin, polyethylene resin, polypropylene resin, ABS resin, nylon, acrylic resin, fluororesin, polycarbonate resin, polyurethane resin, methylpentene resin, And organic materials represented by phenol resin, melamine resin, epoxy resin, and vinyl chloride resin.
- the cell culture container of this embodiment can be produced by a method known to those skilled in the art. For example, when a culture container made of a plastic material is manufactured, it can be manufactured by a conventional molding method such as injection molding.
- the cell culture container of this embodiment is preferably subjected to surface hydrophilization treatment such as plasma treatment from the viewpoint of preventing non-specific adhesion of cultured cells and preventing the drop of the culture solution from being biased by surface tension.
- surface hydrophilization treatment such as plasma treatment from the viewpoint of preventing non-specific adhesion of cultured cells and preventing the drop of the culture solution from being biased by surface tension.
- the number of bacteria (bioburden number) adhering to the container after production is preferably 100 cfu / container or less. Further, it is more preferable that sterilization treatment such as ⁇ -ray sterilization is performed.
- the cell culture container of this embodiment may be subjected to a surface treatment or a surface coat that promotes the development of fertilized eggs.
- a surface treatment or a surface coat that promotes the development of fertilized eggs.
- cells of other organs for example, endometrial cells or fallopian tube epithelial cells
- the cells to be cultured are not particularly limited, and examples include fertilized eggs, egg cells, ES cells (embryonic stem cells), and iPS cells (artificial pluripotent stem cells).
- An egg cell refers to an unfertilized egg cell, and includes an immature oocyte and a mature oocyte. After fertilization, the fertilized egg increases in number of cells from the 2 cell stage, the 4 cell stage, and the 8 cell stage by cleavage, and develops into a blastocyst through a morula.
- Fertilized eggs include early embryos such as 2-cell embryos, 4-cell embryos and 8-cell embryos, morulas, blastocysts (including early blastocysts, expanded blastocysts and escaped blastocysts).
- a blastocyst means an embryo composed of external cells with the potential to form the placenta and internal cell masses with the potential to form embryos.
- ES cells refer to undifferentiated pluripotent or totipotent cells obtained from the inner cell mass of a blastocyst.
- An iPS cell refers to a cell having a pluripotency similar to that of an ES cell by introducing several types of genes (transcription factors) into somatic cells (mainly fibroblasts). That is, in this embodiment, the cell includes an aggregate of a plurality of cells such as a fertilized egg or a blastocyst.
- the cell culture vessel of the present embodiment is preferably suitable for culturing mammalian and avian cells, particularly mammalian cells.
- Mammals refer to warm-blooded vertebrates, eg, primates such as humans and monkeys, rodents such as mice, rats and rabbits, pets such as dogs and cats, and livestock such as cattle, horses and pigs. Is mentioned.
- the cell culture container of this embodiment is particularly suitable for culturing human fertilized eggs.
- Cultivation is usually carried out by placing the cell culture vessel in an incubator that provides an environmental atmosphere containing gas necessary for the growth and maintenance of the cultured cells and a constant environmental temperature.
- Necessary gases include water vapor, free oxygen (O 2 ) and carbon dioxide (CO 2 ).
- O 2 free oxygen
- CO 2 carbon dioxide
- a stable pH is obtained by a stable CO 2 content and a stable temperature.
- a fertilized egg when a fertilized egg is cultured, it is usually determined whether it is a high-quality fertilized egg suitable for transplantation into the uterus after the culture.
- the determination may be performed automatically or manually with a microscope or the like.
- an image of the cells in the culture vessel obtained by a microscope is picked up by a detection device such as a CCD camera, the obtained image is subjected to contour extraction processing, and corresponds to the cells in the image
- the quality can be determined by extracting the portion and analyzing the extracted cell image with an image analysis apparatus.
- the image contour extraction processing for example, the processing described in JP-A-2006-337110 can be used.
- the cell When the microwell consists of a bottom surface parallel to the bottom surface of the cell culture container and a side surface perpendicular to the bottom surface, the cell may move in the microwell and come into contact with the side surface. There is a problem that it is difficult to extract an image of a cell by a contour extraction process in a photographed image.
- the wall surface of the microwell when the wall surface of the microwell has an inclined surface, it preferably includes a conical or frustoconical portion.
- the cells to be cultured automatically exist in the bottom part of the microwell, and even if the microwell has a side surface perpendicular to the bottom surface of the cell culture container on the opening side from the inclined surface, It does not remain in contact with this, and the contour extraction process of the captured cell image can be performed without any problem.
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Wood Science & Technology (AREA)
- Organic Chemistry (AREA)
- Engineering & Computer Science (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Chemical & Material Sciences (AREA)
- Zoology (AREA)
- Genetics & Genomics (AREA)
- Sustainable Development (AREA)
- Microbiology (AREA)
- Biotechnology (AREA)
- Biochemistry (AREA)
- General Engineering & Computer Science (AREA)
- General Health & Medical Sciences (AREA)
- Biomedical Technology (AREA)
- Clinical Laboratory Science (AREA)
- Molecular Biology (AREA)
- Apparatus Associated With Microorganisms And Enzymes (AREA)
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2013182221A JP5920296B2 (ja) | 2013-09-03 | 2013-09-03 | 細胞培養容器 |
| JP2013-182221 | 2013-09-03 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2015033926A1 true WO2015033926A1 (ja) | 2015-03-12 |
Family
ID=52628400
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/JP2014/073064 Ceased WO2015033926A1 (ja) | 2013-09-03 | 2014-09-02 | 細胞培養容器 |
Country Status (2)
| Country | Link |
|---|---|
| JP (1) | JP5920296B2 (enExample) |
| WO (1) | WO2015033926A1 (enExample) |
Cited By (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2020169985A (ja) * | 2019-03-29 | 2020-10-15 | エフ.ホフマン−ラ ロシュ アーゲーF. Hoffmann−La Roche Aktiengesellschaft | マイクロ流体デバイス |
| EP3640320A4 (en) * | 2017-06-12 | 2021-03-17 | Mitsubishi Paper Mills Limited | CRYOPRESERVATION TEMPLATE FOR THE CRYOPRESERVATION OF CELLS OR TISSUES |
| EP4524229A1 (en) | 2023-08-31 | 2025-03-19 | Lech Kiedrowski | Device for culturing and microcopic observations of cryopreserved cells |
Families Citing this family (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP6349847B2 (ja) * | 2013-03-26 | 2018-07-04 | 大日本印刷株式会社 | 細胞培養容器 |
| TW201623604A (zh) * | 2014-09-25 | 2016-07-01 | 住友電木股份有限公司 | 培養容器 |
| JP6123963B1 (ja) * | 2015-05-25 | 2017-05-10 | 大日本印刷株式会社 | 細胞処理容器 |
| KR102105301B1 (ko) * | 2019-04-02 | 2020-04-29 | 주식회사 엠케이바이오텍 | 각각의 수정란을 공동배양 할 수 있는 배양 접시 |
Citations (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPH08131153A (ja) * | 1994-09-16 | 1996-05-28 | Sumitomo Bakelite Co Ltd | 細胞培養容器とその製造方法、及び細胞培養方法 |
| JP2007185110A (ja) * | 2006-01-11 | 2007-07-26 | Ochanomizu Univ | 細胞培養容器 |
| JP2010531644A (ja) * | 2007-06-29 | 2010-09-30 | ウニセンス フェルティリテック アー/エス | 顕微鏡対象物の監視および/または培養用の機器、システムおよび方法 |
| JP4724854B2 (ja) * | 2009-02-09 | 2011-07-13 | 大日本印刷株式会社 | 細胞培養容器 |
| JP2012249547A (ja) * | 2011-05-31 | 2012-12-20 | Oji Holdings Corp | 細胞培養用基材及びその製造方法 |
-
2013
- 2013-09-03 JP JP2013182221A patent/JP5920296B2/ja active Active
-
2014
- 2014-09-02 WO PCT/JP2014/073064 patent/WO2015033926A1/ja not_active Ceased
Patent Citations (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPH08131153A (ja) * | 1994-09-16 | 1996-05-28 | Sumitomo Bakelite Co Ltd | 細胞培養容器とその製造方法、及び細胞培養方法 |
| JP2007185110A (ja) * | 2006-01-11 | 2007-07-26 | Ochanomizu Univ | 細胞培養容器 |
| JP2010531644A (ja) * | 2007-06-29 | 2010-09-30 | ウニセンス フェルティリテック アー/エス | 顕微鏡対象物の監視および/または培養用の機器、システムおよび方法 |
| JP4724854B2 (ja) * | 2009-02-09 | 2011-07-13 | 大日本印刷株式会社 | 細胞培養容器 |
| JP2012249547A (ja) * | 2011-05-31 | 2012-12-20 | Oji Holdings Corp | 細胞培養用基材及びその製造方法 |
Cited By (8)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP3640320A4 (en) * | 2017-06-12 | 2021-03-17 | Mitsubishi Paper Mills Limited | CRYOPRESERVATION TEMPLATE FOR THE CRYOPRESERVATION OF CELLS OR TISSUES |
| US11510408B2 (en) | 2017-06-12 | 2022-11-29 | Mitsubishi Paper Mills Limited | Cryopreservation jig for cryopreserving cells or tissues |
| JP2020169985A (ja) * | 2019-03-29 | 2020-10-15 | エフ.ホフマン−ラ ロシュ アーゲーF. Hoffmann−La Roche Aktiengesellschaft | マイクロ流体デバイス |
| JP2023027302A (ja) * | 2019-03-29 | 2023-03-01 | エフ. ホフマン-ラ ロシュ エージー. | マイクロ流体デバイス |
| JP7245191B2 (ja) | 2019-03-29 | 2023-03-23 | エフ. ホフマン-ラ ロシュ アーゲー | マイクロ流体デバイス |
| US12005439B2 (en) | 2019-03-29 | 2024-06-11 | Roche Molecular Systems, Inc. | Microfluidic device |
| JP7729802B2 (ja) | 2019-03-29 | 2025-08-26 | エフ. ホフマン-ラ ロシュ アーゲー | マイクロ流体デバイス |
| EP4524229A1 (en) | 2023-08-31 | 2025-03-19 | Lech Kiedrowski | Device for culturing and microcopic observations of cryopreserved cells |
Also Published As
| Publication number | Publication date |
|---|---|
| JP5920296B2 (ja) | 2016-05-18 |
| JP2015047136A (ja) | 2015-03-16 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP4724854B2 (ja) | 細胞培養容器 | |
| JP5920296B2 (ja) | 細胞培養容器 | |
| JP6344461B2 (ja) | 細胞培養容器 | |
| JP2015029431A (ja) | 細胞培養容器 | |
| JP5920375B2 (ja) | 細胞培養容器 | |
| JP5880786B2 (ja) | 細胞培養容器 | |
| JP6278590B2 (ja) | 細胞培養容器および細胞培養方法 | |
| JP2016082987A (ja) | 細胞培養容器 | |
| JP6657729B2 (ja) | 細胞取扱容器 | |
| JP6349847B2 (ja) | 細胞培養容器 | |
| JP6511817B2 (ja) | 細胞培養容器 | |
| JP6060625B2 (ja) | 細胞培養容器および細胞観察方法 | |
| JP6379529B2 (ja) | 細胞培養容器 | |
| JP6384501B2 (ja) | 細胞培養容器 | |
| JP6364817B2 (ja) | 細胞培養容器 | |
| JP6187065B2 (ja) | 細胞培養容器 | |
| JP6451728B2 (ja) | 細胞培養容器および細胞観察方法 | |
| JP6244747B2 (ja) | 特定の配列のマイクロウェルを有する細胞培養容器 | |
| JP6326905B2 (ja) | 細胞培養容器 | |
| JP6892215B2 (ja) | 細胞取扱容器 | |
| JP2017123801A (ja) | 細胞取扱容器 | |
| JP2016007178A (ja) | 細胞培養容器 | |
| JP2020072745A (ja) | 細胞取扱容器 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 14842773 Country of ref document: EP Kind code of ref document: A1 |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| 122 | Ep: pct application non-entry in european phase |
Ref document number: 14842773 Country of ref document: EP Kind code of ref document: A1 |