WO2013147223A1 - ポリカーボネート樹脂の製造方法 - Google Patents
ポリカーボネート樹脂の製造方法 Download PDFInfo
- Publication number
- WO2013147223A1 WO2013147223A1 PCT/JP2013/059671 JP2013059671W WO2013147223A1 WO 2013147223 A1 WO2013147223 A1 WO 2013147223A1 JP 2013059671 W JP2013059671 W JP 2013059671W WO 2013147223 A1 WO2013147223 A1 WO 2013147223A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- polycarbonate resin
- producing
- stirring shaft
- scraper
- resin according
- Prior art date
Links
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08G—MACROMOLECULAR COMPOUNDS OBTAINED OTHERWISE THAN BY REACTIONS ONLY INVOLVING UNSATURATED CARBON-TO-CARBON BONDS
- C08G64/00—Macromolecular compounds obtained by reactions forming a carbonic ester link in the main chain of the macromolecule
- C08G64/20—General preparatory processes
- C08G64/30—General preparatory processes using carbonates
- C08G64/307—General preparatory processes using carbonates and phenols
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08G—MACROMOLECULAR COMPOUNDS OBTAINED OTHERWISE THAN BY REACTIONS ONLY INVOLVING UNSATURATED CARBON-TO-CARBON BONDS
- C08G64/00—Macromolecular compounds obtained by reactions forming a carbonic ester link in the main chain of the macromolecule
- C08G64/20—General preparatory processes
- C08G64/205—General preparatory processes characterised by the apparatus used
Definitions
- the present invention relates to a method for producing a polycarbonate resin, and more particularly, to a method for producing a polycarbonate resin that reduces the amount of deposits on a stirring shaft of a polycondensation reaction apparatus and suppresses the mixing of foreign substances into a product.
- a melting method in which a dihydroxy compound and a carbonic acid diester are polycondensed is known.
- Patent Document 1 a method of producing a polycarbonate resin by continuously performing a polycondensation reaction using a plurality of reaction apparatuses is known.
- the fixed substance of the polycarbonate resin is poorly compatible with a polycarbonate resin that is usually produced, and is often yellowed. For this reason, if it drops and removes from the stirring shaft or the like, it is mixed as a foreign substance in the polycarbonate resin, which causes a deterioration in color tone and an increase in foreign substances. Moreover, when this fallen object is large, the polymer filter etc. provided in the reaction apparatus may be damaged.
- an object of the present invention is to suppress the generation of foreign matters and maintain the quality of the obtained polycarbonate resin in the production of polycarbonate resin.
- the present invention has the following gist.
- Method. The method for producing a polycarbonate resin according to (1) above, wherein the polycarbonate resin is obtained by subjecting a carbonic acid diester and a dihydroxy compound to a polycondensation reaction in the presence of a transesterification catalyst.
- the transesterification catalyst is a long-period periodic table group 1 element (excluding hydrogen) compound, long-period periodic table group 2 element compound, basic boron compound, basic phosphorus compound, basic
- the scraper since the scraper is disposed near the stirring shaft in the reactor, the polycarbonate resin adhering to the stirring shaft can be scraped off. As a result, it is possible to prevent foreign substances that are high molecular weight substances or highly branched substances from being mixed in, and to efficiently produce a polymer with few foreign substances, and to stably produce a polycarbonate resin with stable quality. And the damage of the polymer filter provided in the reaction apparatus can also be prevented.
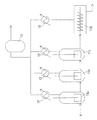
- FIG. 1 It is an example of the polycondensation reaction process figure which shows the example of a polycondensation reaction apparatus.
- A is a cross-sectional view of a polycondensation reaction apparatus provided with a scraper
- (b) is a bb cross-sectional view of the cross-sectional view of (a).
- (A), (b), (c), and (d) are figures which show the mode of the deposit
- the present invention is a method for producing a polycarbonate resin in which a polycarbonate resin is continuously produced using a plurality of polycondensation reaction apparatuses.
- the polycarbonate resin that is the production object in the present invention is preferably a polymer compound produced by polycondensation reaction (ester exchange reaction) of a carbonic acid diester and a dihydroxy compound.
- Carbonated diester examples include substituted diphenyl carbonates such as diphenyl carbonate (DPC) and ditolyl carbonate, and dialkyl carbonates such as dimethyl carbonate, diethyl carbonate, and di-t-butyl carbonate. These carbonic acid diesters can be used alone or in admixture of two or more.
- DPC diphenyl carbonate
- dialkyl carbonates such as dimethyl carbonate, diethyl carbonate, and di-t-butyl carbonate.
- the carbonic acid diester may preferably be substituted with dicarboxylic acid or dicarboxylic acid ester in an amount of 50 mol% or less, more preferably 30 mol% or less.
- Representative dicarboxylic acids or dicarboxylic acid esters include terephthalic acid, isophthalic acid, diphenyl terephthalate, and diphenyl isophthalate. When substituted with such a dicarboxylic acid or dicarboxylic acid ester, a polyester carbonate is obtained.
- carbonic acid diesters including the above substituted dicarboxylic acid or dicarboxylic acid ester; the same shall apply hereinafter
- the carbonic acid diester is used in an amount of 1.01 to 1.30 times (molar ratio), preferably 1.02 to 1.20 times (molar ratio) with respect to the dihydroxy compound.
- the molar ratio is too small, the amount of terminal hydroxyl groups of the obtained polycarbonate resin increases, and the thermal stability of the polycarbonate resin tends to deteriorate.
- the dihydroxy compound is a compound having two hydroxyl groups in the molecule.
- the molecule has one or more aromatic rings, and each of the two hydroxyl groups is bonded to the aromatic ring.
- Aromatic dihydroxy compounds are preferably used.
- aromatic dihydroxy compounds include, for example, bis (4-hydroxydiphenyl) methane, 2,2-bis (4-hydroxyphenyl) propane, and 2,2-bis (4-hydroxy-3-methyl).
- Phenyl) propane 2,2-bis (4-hydroxy-3-tert-butylphenyl) propane, 2,2-bis (4-hydroxy-3,5-dimethylphenyl) propane, 2,2-bis (4- Bisphenols such as hydroxy-3,5-dibromophenyl) propane, 4,4-bis (4-hydroxyphenyl) heptane, 1,1-bis (4-hydroxyphenyl) cyclohexane; 4,4′-dihydroxybiphenyl, 3 , 3 ′, 5,5′-tetramethyl-4,4′-dihydroxybiphenyl and the like; bis (4-hydroxyphenol) Nyl) sulfone, bis (4-hydroxyphenyl) sulfide, bis (4-hydroxyphenyl) ether, bis (4-hydroxyphenyl) ketone and
- transesterification catalyst In the transesterification reaction, a transesterification catalyst is used.
- the transesterification catalyst include a catalyst usually used for producing a polycarbonate by a transesterification method, and are not particularly limited.
- group 1 element excluding hydrogen
- long-period group 2 At least one basic compound selected from the group consisting of elemental compounds (hereinafter sometimes referred to as “Group 2 elements”), basic boron compounds, basic phosphorus compounds, basic ammonium compounds, and amine compounds.
- Group 2 elements At least one basic compound selected from the group consisting of elemental compounds (hereinafter sometimes referred to as “Group 2 elements”), basic boron compounds, basic phosphorus compounds, basic ammonium compounds, and amine compounds.
- Compounds Compounds.
- these transesterification catalysts practically, at least one compound selected from the group consisting of compounds of group 1 elements (excluding hydrogen) and compounds of group 2 elements is preferable.
- These transesterification catalysts may be used alone or in combination of
- the amount of the transesterification catalyst used is usually preferably 1 ⁇ 10 ⁇ 9 to 1 ⁇ 10 ⁇ 1 mol, more preferably 1 ⁇ 10 ⁇ 7 to 1 ⁇ 10 ⁇ 3 mol, still more preferably 1 mol of the dihydroxy compound. Used at 1 ⁇ 10 ⁇ 7 to 1 ⁇ 10 ⁇ 5 mol.
- Examples of the compounds of Group 1 elements include inorganic compounds such as hydroxides, carbonates, hydrogen carbonate compounds of Group 1 elements (excluding hydrogen); Group 1 elements (excluding hydrogen) And organic compounds such as salts with alcohols, phenols and organic carboxylic acids.
- a group 1 element except hydrogen
- lithium, sodium, potassium, rubidium, and cesium are mentioned, for example.
- cesium compounds and potassium compounds are preferable, and cesium carbonate, cesium hydrogen carbonate, cesium hydroxide, potassium acetate, and potassium carbonate are particularly preferable.
- Group 2 element compounds examples include hydroxides such as beryllium, magnesium, calcium, strontium and barium, inorganic compounds such as carbonates; alcohols such as beryllium, magnesium, calcium, strontium and barium. And salts with phenols and organic carboxylic acids. Of these Group 2 elements (excluding hydrogen), magnesium compounds are preferred.
- Examples of the basic boron compound include sodium salts, potassium salts, lithium salts, calcium salts, magnesium salts, barium salts, and strontium salts of boron compounds.
- the boron compound for example, tetramethylboron, tetraethylboron, tetrapropylboron, tetrabutylboron, trimethylethylboron, trimethylbenzylboron, trimethylphenylboron, triethylmethylboron, triethylbenzylboron, triethylphenylboron, tributyl Examples include benzylboron, tributylphenylboron, tetraphenylboron, benzyltriphenylboron, methyltriphenylboron, and butyltriphenylboron.
- Examples of the basic phosphorus compound include triethylphosphine, tri-n-propylphosphine, triisopropylphosphine, tri-n-butylphosphine, triphenylphosphine, tris (pt-butylphenyl) phosphine, and tributyl.
- Examples thereof include trivalent phosphorus compounds such as phosphine, and quaternary phosphonium salts derived from these compounds. Of these, triphenylphosphine, tris (pt-butylphenyl) phosphine, and the like are preferable.
- Examples of the basic ammonium compound include tetramethylammonium hydroxide, tetraethylammonium hydroxide, tetrapropylammonium hydroxide, tetrabutylammonium hydroxide, trimethylethylammonium hydroxide, trimethylbenzylammonium hydroxide, trimethylphenylammonium hydroxide.
- triethylmethylammonium hydroxide triethylbenzylammonium hydroxide, triethylphenylammonium hydroxide, tributylbenzylammonium hydroxide, tributylphenylammonium hydroxide, tetraphenylammonium hydroxide, benzyltriphenylammonium hydroxide, Tilt Li phenyl ammonium hydroxide, butyltriphenyl ammonium hydroxide, and the like. Of these, tetramethylammonium hydroxide and the like are preferable.
- Examples of the amine compound include 4-aminopyridine, 2-aminopyridine, N, N-dimethyl-4-aminopyridine, 4-diethylaminopyridine, 2-hydroxypyridine, 2-methoxypyridine, 4- Examples include methoxypyridine, 2-dimethylaminoimidazole, 2-methoxyimidazole, imidazole, 2-mercaptoimidazole, 2-methylimidazole, and aminoquinoline.
- the polycarbonate resin is prepared by preparing a mixture of the above-mentioned dihydroxy compound and carbonic acid diester using a raw material preparation device (raw material preparation step), and subjecting the raw material mixture to a polycondensation reaction using a reaction device in the presence of the transesterification catalyst. (Polycondensation step).
- a raw material preparation device raw material preparation step
- a polycondensation reaction using a reaction device in the presence of the transesterification catalyst.
- Polycondensation step As the reaction system, a batch system, a continuous system, a combination thereof, or the like can be used. In the present invention, the raw material preparation process and the polycondensation process are performed in a continuous system.
- Polycarbonate resin after the polycondensation step, stop the reaction and devolatilize and remove unreacted raw materials and reaction by-products in the polymerization reaction solution, add a heat stabilizer, release agent, colorant, etc., necessary Depending on the process, it is manufactured through a process of forming pellets having a predetermined particle diameter.
- the raw material mixture is sent in a molten state to a plurality of reactors as shown in FIG. 1 and subjected to a polycondensation reaction.
- This polycondensation reaction is usually carried out continuously in a reactor of 2 or more, preferably 3 to 7 groups.
- Specific reaction conditions include temperature: 150 ° C. to 320 ° C., pressure: normal pressure to 0.01 Torr (1.3 Pa), average residence time: 5 to 300 minutes, preferably temperature: 180 to 310 ° C. Pressure: 20 to 0.05 Torr (2.7 kPa to 6.7 Pa), Average residence time: 60 to 150 minutes
- reaction conditions are gradually increased to higher temperatures and higher vacuums. Set to.
- a plurality of reactors including a vertical reactor are provided to increase the average molecular weight of the polycarbonate resin.
- 3 to 6 groups, preferably 4 to 5 groups are installed.
- FIG. 1 As a specific example, in FIG. 1, three vertical reactors 11a, 11b, and 11c and one horizontal reactor 11d are used.
- the melt of the raw material mixture A is supplied to the first vertical reactor 11a, and the polycondensation reaction is started in the presence of the transesterification catalyst.
- the reaction mixture is sequentially sent to the vertical reactor 11b, vertical reactor 11c, and horizontal reactor 11d to advance the polycondensation reaction.
- phenol is produced as a by-product, which is liquefied by a heat exchanger and sent to the phenol tank 13.
- the phenol in the phenol tank 13 is appropriately treated and reused as a raw material for a dihydroxy compound or a carbonic acid diester compound.
- a horizontal reactor 11d is used as the latter stage reactor of the group of reactors. This increases the viscosity as the polycondensation reaction proceeds, so that the latter stage has a high viscosity. This is to make the stirring in the easier.
- the polycarbonate resin obtained in this polycondensation step is then devolatilized and cooled.
- Examples of the vertical and horizontal reactors include, for example, a stirred tank reactor, a thin film reactor, a centrifugal thin film evaporation reactor, a surface renewal biaxial kneading reactor, a biaxial horizontal stirred reactor, and a wet wall type A reactor, a perforated plate type reactor that polymerizes while freely dropping, a perforated plate type reactor with wire that polymerizes while dropping along a wire, and the like are used.
- a stirring tank type reactor and a biaxial horizontal type stirring reactor are preferable.
- Examples of the type of agitator blades in the vertical reactor include turbine blades, paddle blades, fiddler blades, anchor blades, full zone blades (manufactured by Shinko Pantech Co., Ltd.), Sunmeler blades (manufactured by Mitsubishi Heavy Industries, Ltd.), Max Blend blades (Sumitomo) Heavy machinery industry), helical ribbon blades, twisted lattice blades (manufactured by Hitachi, Ltd.), and the like.
- a stirring blade a Max blend blade (made by Sumitomo Heavy Industries, Ltd.) and a helical ribbon blade are preferable.
- the horizontal reactor refers to a reactor in which the rotating shaft of the stirring blade is horizontal (horizontal direction).
- a stirring blade of a horizontal reactor for example, a uniaxial stirring blade such as a disk type or a paddle type; HVR, SCR, N-SCR (manufactured by Mitsubishi Heavy Industries, Ltd.), Vivolac (manufactured by Sumitomo Heavy Industries, Ltd.), or And a biaxial stirring blade such as a spectacle blade or a lattice blade (manufactured by Hitachi, Ltd.).
- the transesterification catalyst used for the polycondensation reaction of a dihydroxy compound and a carbonic acid diester is usually used in advance as an aqueous solution.
- concentration of the aqueous solution is not particularly limited, and is adjusted to an arbitrary concentration according to the solubility of the transesterification catalyst in water. Moreover, it can replace with water and other solvents, such as acetone, alcohol, toluene, and phenol, can also be selected.
- the property of water used for dissolving the transesterification catalyst is not particularly limited as long as the kind and concentration of impurities contained are constant, but usually distilled water, deionized water, and the like are preferably used.
- a polycarbonate resin is continuously produced using a plurality of reaction apparatuses.
- At least one has a stirring shaft and a scraper.
- the polycarbonate resin has a viscosity average molecular weight of preferably 14,000 or more, more preferably 18,000 or more. If the viscosity average molecular weight is less than 10,000, adhesion to the stirring shaft 15 is unlikely to occur, so there is no need to provide a scraper.
- the upper limit of the viscosity average molecular weight is preferably 30,000, and more preferably 28,000.
- the viscosity average molecular weight of the target polycarbonate resin is 30,000.
- a substance in the middle of polymerization in the reactor (hereinafter sometimes referred to as “polymerization intermediate substance”) is likely to adhere to the stirring shaft. , Will come off immediately.
- the scraper is provided in such a place, it is effective to peel off a substance that is difficult to peel off during polymerization.
- FIG. 2A shows the case where the scraper 14 is attached to the horizontal reactor 11d.
- a horizontal reactor 11d shown in FIG. 2 (a) is a device in which a stirring blade 16 is attached to a horizontally arranged stirring shaft 15, and the polymerization melt inserted from the inlet 17 is stirred by the stirring blade 16.
- the polycondensation reaction proceeds while being discharged from the discharge port 18 to the outside.
- the stirring shaft 16 is often not provided with the stirring blade 16. In this case, a substance in the middle of polymerization adheres to the stirring shaft 15 in the vicinity of the discharge port 18 and is difficult to peel off.
- the reactor equipped with the scraper needs to be provided at least one of the reactors within the range of the viscosity average molecular weight at the outlet of the reactor.
- a scraper for all of them.
- this reactor it is preferable to use a horizontal reactor arranged last, from the viewpoint of viscosity average molecular weight.
- the melt viscosity at the reactor outlet of the reactor equipped with the scraper is 500 poise or more, preferably 1000 poise or more, more preferably 5000 poise or more. If the melt viscosity is less than 500 poise, it is difficult for the agitation shaft 15 to adhere to the melt, so that it is not necessary to provide a scraper.
- the upper limit of melt viscosity is 50,000 poise, preferably 30,000 poise, more preferably 25,000 poise, and most preferably 15,000 poise. If the melt viscosity exceeds 50,000 poise, there is a possibility that deposits may also be generated on the scraper, which is not preferable.
- the temperature of the polycarbonate resin at the reactor outlet of the reactor equipped with the scraper is 260 ° C. or higher, preferably 265 ° C. or higher, more preferably 270 ° C. or higher. If the temperature of the polycarbonate resin is lower than 260 ° C., the melt viscosity of the polycarbonate resin becomes too high, and there is a possibility that deposits may also be generated on the scraper.
- the upper limit of the temperature of the polycarbonate resin is 320 ° C, preferably 310 ° C, and most preferably 300 ° C. If the temperature of the polycarbonate resin exceeds 320 ° C., the polycarbonate resin may be yellowed, which is not preferable.
- the scraper 14 is thinned to form a scraping portion, and is disposed in the vicinity of the stirring shaft 15.
- the tip portion As shown in FIG. 2 (b), it is possible to more easily remove the deposits on the stirring shaft 15.
- the distance between the scraping portion 14a of the scraper 14 and the stirring shaft 15 is preferably 30 mm or less, and more preferably 20 mm or less. When it exceeds 30 mm, there is a possibility that the attached substance cannot be sufficiently peeled off.
- the distance between the scraping part and the stirring shaft 15 is preferably 1 mm or more, and more preferably 5 mm or more. If it is less than 1 mm, the scraping part and the stirring shaft 15 may come into contact with each other.
- the scraping portion 14a of the scraper 14 is below the shaft center of the stirring shaft. By doing in this way, the scraped deposits can be dropped downward without coming into contact with the stirring shaft again.
- the length of the scraping portion 14a of the scraper 14 is preferably 30% or less, more preferably 20% or less with respect to the total length of the stirring shaft 15. If it is longer than 30%, the resin adheres to the central portion of the scraper, and there is a possibility that a fixed matter is generated on the scraper.
- the lower limit of the length of the scraping portion 14a is preferably 3% and more preferably 5% with respect to the total length of the stirring shaft. If it is shorter than 3%, the scraping efficiency of the deposit may be reduced. Note that the length of the scraping portion 14a of the scraper 14 is premised on a length that does not contact the stirring blade 16.
- the installation position of the scraper is on the outlet side from the center. This is because the viscosity average molecular weight increases toward the downstream side, and deposits are easily generated.
- the peripheral speed of the stirring shaft 15 is preferably 5 cm / s or more, more preferably 7 cm / s or more, and still more preferably 8 cm / s or more. When it is slower than 5 cm / s, the strength of the stirring shaft is lowered, and the stirring shaft may be broken when operated for a long time.
- the upper limit of the peripheral speed is preferably 15 cm / s, and more preferably 10 cm / s. When the speed is higher than 15 cm / s, the polymerizing substance adhering to the stirring shaft 15 may be lifted above the rotating shaft before falling below the stirring shaft 15, and the adhesion may proceed without being able to peel off.
- the diameter of the stirring shaft 15 is preferably 200 mm or more, more preferably 300 mm or more, and still more preferably 400 mm or more. If it is smaller than 200 mm, the strength of the stirring shaft is lowered, and the stirring shaft may be broken when it is operated for a long time.
- the upper limit of the diameter of the stirring shaft 15 is preferably 600 mm, and more preferably 500 mm. If it is larger than 600 mm, even if the peripheral speed of the stirring shaft 15 is in the above range, the linear speed on the surface of the stirring shaft 15 becomes too high, and the substance in the middle of polymerization adhering to the stirring shaft 15 cannot be peeled off and proceeds instead of adhering. There is a case.
- the material of the scraper 14 is preferably made of stainless steel, more preferably SUS304, SUS304L, SUS310S, SUS316, SUS316L, and most preferably SUS316L, SUS310S.
- the surface of the scraper 14 is usually surface-treated, and preferable surface treatment is buffing, electrolytic polishing, acid treatment, heat treatment, various platings, coating treatment, and more preferable surface treatment is buffing. Electrolytic polishing is performed later.
- the surface roughness (Rmax) of the scraper 14 is preferably 100 ⁇ m or less, more preferably 10 ⁇ m or less, and most preferably 1 ⁇ m or less. If this surface roughness (Rmax) is too large, the peeled deposits remain on the scraper, and foreign matter may increase.
- the surface roughness (Rmax) is preferably low, but the construction is difficult, so 0.01 ⁇ m or more is preferable, and 0.1 ⁇ m or more is more preferable.
- the surface roughness of the scraper 14 is sufficient if it is 0.1 ⁇ m or more.
- melt viscosity The polycarbonate resin dried at 120 ° C. for 5 hours was heated to 280 ° C. using a capillary rheometer (manufactured by Toyo Seiki Co., Ltd.) having a die diameter of 1 mm ⁇ ⁇ 30 mm, and the melt viscosity was adjusted at a shear rate of 122 (sec ⁇ 1 ). It was measured.
- an aqueous cesium carbonate solution was used as a catalyst at a ratio of 0.5 ⁇ mol (1.0 ⁇ mol per 1 mol of bisphenol A as a metal amount) per 1 mol of bisphenol A Continuous supply.
- the reaction liquid discharged from the bottom of the reactor is continuously continuously supplied to the second and third vertical stirring reactors (capacity 10 m 3 ) and the fourth horizontal reactor (capacity 15 m 3 ), and the fourth reaction. It was extracted from the polymer outlet at the bottom of the vessel.
- the fourth reactor a biaxial horizontal reactor was used.
- the peripheral speed of the stirring shaft of this fourth polymerization tank was 8.8 cm / s, and the diameter of the stirring shaft was 560 mm.
- the reaction conditions in the second to fourth reactors were the second reactor (260 ° C., 4.00 ⁇ 10 3 Pa, 75 rpm), the third reactor (270 ° C., 200 Pa, 75 rpm), and the fourth reaction, respectively.
- a vessel (280 ° C., 67 Pa, 4 rpm) was set to high temperature and high vacuum as the reaction progressed.
- the liquid level was controlled so that the average residence time of the second and third reactors was 60 minutes and the average residence time of the fourth reactor was 90 minutes. Distillation was also performed.
- the viscosity average molecular weight (Mv) of the reaction solution at the outlet of the fourth reactor was 21,000, and the melt viscosity at 280 ° C. was about 10,000 Poise.
- Example 1 As shown in FIG. 2, the horizontal reactor 11d was operated in the same manner as in Comparative Example 1 except that a scraper was installed. Moreover, as shown in FIG.2 (b), the shape of a scraper is thin like a blade, and this front-end
- the number of fish eyes of the polycarbonate resin pellets was 25. Further, 7 days after the start of operation, a slight amount of deposit was generated between the scraper and the stirring shaft, but no deposit was dropped. The number of fish eyes of the polycarbonate resin pellet at this time was 31. Further, even after 2 months of operation, no deposits were dropped, and the number of fish eyes in the polycarbonate resin pellets at this time was 28.
- Example 2 As shown in FIG. 2, a simulation was performed in the same manner as in Comparative Example 3 except that a scraper was installed in the horizontal reactor 11d. Moreover, as shown in FIG.2 (b), the shape of the scraper was thin like a blade, and this tip portion was used as a scraping portion. The distance between the tip of the scraper and the stirring shaft was set to 10 mm. The length of the tip of the scraper was 6% of the total length of the stirring shaft. The result of this polymer flow simulation is shown in FIG. Moreover, the average thickness of the deposit in the polymer flow simulation was 10.3 mm in Example 2.
- the method for producing a polycarbonate resin according to the present invention can prevent the introduction of foreign matter that is a high molecular weight product or a highly branched product, and can efficiently and stably produce a polycarbonate resin having a small amount of foreign matter and a stable quality. It is possible, and the industrial applicability is high, such as preventing damage to the polymer filter provided in the reaction apparatus. It should be noted that the entire contents of the specification, claims, drawings and abstract of Japanese Patent Application No. 2012-078641 filed on March 30, 2012 are cited herein as disclosure of the specification of the present invention. Incorporated.
Landscapes
- Chemical & Material Sciences (AREA)
- Health & Medical Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Medicinal Chemistry (AREA)
- Polymers & Plastics (AREA)
- Organic Chemistry (AREA)
- Polyesters Or Polycarbonates (AREA)
Priority Applications (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN201380016901.3A CN104204032A (zh) | 2012-03-30 | 2013-03-29 | 聚碳酸酯树脂的制造方法 |
| KR1020147027078A KR20140145136A (ko) | 2012-03-30 | 2013-03-29 | 폴리카보네이트 수지의 제조 방법 |
| IN8297DEN2014 IN2014DN08297A (ko) | 2012-03-30 | 2013-03-29 |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2012-078641 | 2012-03-30 | ||
| JP2012078641 | 2012-03-30 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2013147223A1 true WO2013147223A1 (ja) | 2013-10-03 |
Family
ID=49260463
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/JP2013/059671 WO2013147223A1 (ja) | 2012-03-30 | 2013-03-29 | ポリカーボネート樹脂の製造方法 |
Country Status (5)
| Country | Link |
|---|---|
| JP (1) | JP2013227511A (ko) |
| KR (1) | KR20140145136A (ko) |
| CN (1) | CN104204032A (ko) |
| IN (1) | IN2014DN08297A (ko) |
| WO (1) | WO2013147223A1 (ko) |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2022202130A1 (ja) * | 2021-03-26 | 2022-09-29 | 旭化成株式会社 | ポリカーボネートの製造装置の組み立て方法、及びポリカーボネートの製造装置 |
Families Citing this family (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2018150453A (ja) * | 2017-03-13 | 2018-09-27 | 三菱ケミカル株式会社 | ポリブチレンサクシネートの製造方法 |
Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPS4636525Y1 (ko) * | 1968-09-17 | 1971-12-15 | ||
| JPH02126934A (ja) * | 1987-12-18 | 1990-05-15 | Davy Mckee Ag | 高粘性物質処理装置 |
| WO2002040569A1 (fr) * | 2000-11-14 | 2002-05-23 | Teijin Limited | Polycarbonate aromatique, son procede de production et composition contenant celui-ci |
| JP2008050591A (ja) * | 2006-07-25 | 2008-03-06 | Mitsubishi Chemicals Corp | 芳香族ポリカーボネートの製造方法 |
Family Cites Families (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPH0636525A (ja) * | 1992-07-17 | 1994-02-10 | Sanyo Electric Co Ltd | ディスク再生装置の選曲回路 |
| JPH0827265A (ja) * | 1994-07-20 | 1996-01-30 | Mitsubishi Chem Corp | 芳香族ポリカーボネートの製造方法 |
| JPH0912703A (ja) * | 1995-06-26 | 1997-01-14 | Teijin Ltd | 芳香族ポリカーボネートの製造方法 |
| JPH11310631A (ja) * | 1998-04-28 | 1999-11-09 | Daicel Chem Ind Ltd | ポリカーボネートの製造法 |
| CN2487736Y (zh) * | 2001-05-10 | 2002-04-24 | 天津大学 | 生产聚碳酸酯的卧式单转筒搅拌脱气反应器 |
| BRPI0702912A2 (pt) * | 2006-07-25 | 2011-03-29 | Mitsubishi Chem Corp | processo para a produção de policarbonato aromático |
-
2013
- 2013-03-07 JP JP2013045717A patent/JP2013227511A/ja active Pending
- 2013-03-29 KR KR1020147027078A patent/KR20140145136A/ko not_active Application Discontinuation
- 2013-03-29 WO PCT/JP2013/059671 patent/WO2013147223A1/ja active Application Filing
- 2013-03-29 IN IN8297DEN2014 patent/IN2014DN08297A/en unknown
- 2013-03-29 CN CN201380016901.3A patent/CN104204032A/zh active Pending
Patent Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPS4636525Y1 (ko) * | 1968-09-17 | 1971-12-15 | ||
| JPH02126934A (ja) * | 1987-12-18 | 1990-05-15 | Davy Mckee Ag | 高粘性物質処理装置 |
| WO2002040569A1 (fr) * | 2000-11-14 | 2002-05-23 | Teijin Limited | Polycarbonate aromatique, son procede de production et composition contenant celui-ci |
| JP2008050591A (ja) * | 2006-07-25 | 2008-03-06 | Mitsubishi Chemicals Corp | 芳香族ポリカーボネートの製造方法 |
Cited By (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2022202130A1 (ja) * | 2021-03-26 | 2022-09-29 | 旭化成株式会社 | ポリカーボネートの製造装置の組み立て方法、及びポリカーボネートの製造装置 |
| JP2022150566A (ja) * | 2021-03-26 | 2022-10-07 | 旭化成株式会社 | ポリカーボネートの製造装置の組み立て方法、及びポリカーボネートの製造装置 |
| KR20220144412A (ko) * | 2021-03-26 | 2022-10-26 | 아사히 가세이 가부시키가이샤 | 폴리카르보네이트의 제조 장치의 조립 방법 및 폴리카르보네이트의 제조 장치 |
| KR102503433B1 (ko) | 2021-03-26 | 2023-02-24 | 아사히 가세이 가부시키가이샤 | 폴리카르보네이트의 제조 장치의 조립 방법 및 폴리카르보네이트의 제조 장치 |
| JP7258069B2 (ja) | 2021-03-26 | 2023-04-14 | 旭化成株式会社 | ポリカーボネートの製造装置の組み立て方法、及びポリカーボネートの製造装置 |
Also Published As
| Publication number | Publication date |
|---|---|
| KR20140145136A (ko) | 2014-12-22 |
| IN2014DN08297A (ko) | 2015-05-15 |
| CN104204032A (zh) | 2014-12-10 |
| JP2013227511A (ja) | 2013-11-07 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| RU2413739C2 (ru) | Непрерывный способ получения и установка для получения ароматического поликарбоната | |
| RU2380382C2 (ru) | Способ получения ароматического поликарбоната | |
| KR101211979B1 (ko) | 폴리카보네이트 수지의 제조 방법 | |
| WO2013147223A1 (ja) | ポリカーボネート樹脂の製造方法 | |
| JP6167570B2 (ja) | 芳香族ポリカーボネートの製造方法及び製造設備 | |
| JP5347225B2 (ja) | ポリカーボネート樹脂の製造方法 | |
| JP5303847B2 (ja) | 炭酸ジエステルの精製方法及びポリカーボネート樹脂の製造方法 | |
| JP4836299B2 (ja) | 芳香族ポリカーボネート樹脂の製造方法 | |
| JP2011006553A (ja) | ポリカーボネートの製造方法 | |
| JP4957311B2 (ja) | 芳香族ポリカーボネートの連続製造方法 | |
| JP2008214552A (ja) | 芳香族ポリカーボネート樹脂ペレットの製造方法、その製造方法により得られた芳香族ポリカーボネート樹脂ペレット及び成形品 | |
| JP5754465B2 (ja) | 芳香族ポリカーボネートの連続製造方法 | |
| JP5589560B2 (ja) | 芳香族ポリカーボネート樹脂の製造方法 | |
| JP5464166B2 (ja) | 炭酸ジエステルの精製方法及びポリカーボネート樹脂の製造方法 | |
| JP3366781B2 (ja) | ポリカーボネートの製造方法 | |
| JP2009279754A (ja) | 芳香族ポリカーボネート樹脂組成物の製造方法 | |
| JP2013177636A (ja) | ポリカーボネート樹脂の製造方法 | |
| JP5168844B2 (ja) | 芳香族ポリカーボネートの製造方法 | |
| JP5003045B2 (ja) | 芳香族ポリカーボネートの製造方法 | |
| JP5942548B2 (ja) | ポリカーボネート樹脂ペレット | |
| JP5605287B2 (ja) | 連続反応装置およびポリカーボネートの連続製造方法 | |
| JP5245311B2 (ja) | 芳香族ポリカーボネートの製造方法 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 13768333 Country of ref document: EP Kind code of ref document: A1 |
|
| ENP | Entry into the national phase |
Ref document number: 20147027078 Country of ref document: KR Kind code of ref document: A |
|
| 122 | Ep: pct application non-entry in european phase |
Ref document number: 13768333 Country of ref document: EP Kind code of ref document: A1 |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |