WO2012153763A1 - Co2のゼオライト膜分離回収システム - Google Patents
Co2のゼオライト膜分離回収システム Download PDFInfo
- Publication number
- WO2012153763A1 WO2012153763A1 PCT/JP2012/061874 JP2012061874W WO2012153763A1 WO 2012153763 A1 WO2012153763 A1 WO 2012153763A1 JP 2012061874 W JP2012061874 W JP 2012061874W WO 2012153763 A1 WO2012153763 A1 WO 2012153763A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- membrane
- membrane separation
- separation
- zeolite
- hydrogen
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Ceased
Links
Images
Classifications
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01D—SEPARATION
- B01D53/00—Separation of gases or vapours; Recovering vapours of volatile solvents from gases; Chemical or biological purification of waste gases, e.g. engine exhaust gases, smoke, fumes, flue gases, aerosols
- B01D53/22—Separation of gases or vapours; Recovering vapours of volatile solvents from gases; Chemical or biological purification of waste gases, e.g. engine exhaust gases, smoke, fumes, flue gases, aerosols by diffusion
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01D—SEPARATION
- B01D53/00—Separation of gases or vapours; Recovering vapours of volatile solvents from gases; Chemical or biological purification of waste gases, e.g. engine exhaust gases, smoke, fumes, flue gases, aerosols
- B01D53/22—Separation of gases or vapours; Recovering vapours of volatile solvents from gases; Chemical or biological purification of waste gases, e.g. engine exhaust gases, smoke, fumes, flue gases, aerosols by diffusion
- B01D53/228—Separation of gases or vapours; Recovering vapours of volatile solvents from gases; Chemical or biological purification of waste gases, e.g. engine exhaust gases, smoke, fumes, flue gases, aerosols by diffusion characterised by specific membranes
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01D—SEPARATION
- B01D53/00—Separation of gases or vapours; Recovering vapours of volatile solvents from gases; Chemical or biological purification of waste gases, e.g. engine exhaust gases, smoke, fumes, flue gases, aerosols
- B01D53/26—Drying gases or vapours
- B01D53/268—Drying gases or vapours by diffusion
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01D—SEPARATION
- B01D53/00—Separation of gases or vapours; Recovering vapours of volatile solvents from gases; Chemical or biological purification of waste gases, e.g. engine exhaust gases, smoke, fumes, flue gases, aerosols
- B01D53/34—Chemical or biological purification of waste gases
- B01D53/46—Removing components of defined structure
- B01D53/62—Carbon oxides
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01D—SEPARATION
- B01D67/00—Processes specially adapted for manufacturing semi-permeable membranes for separation processes or apparatus
- B01D67/0081—After-treatment of organic or inorganic membranes
- B01D67/0088—Physical treatment with compounds, e.g. swelling, coating or impregnation
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01D—SEPARATION
- B01D71/00—Semi-permeable membranes for separation processes or apparatus characterised by the material; Manufacturing processes specially adapted therefor
- B01D71/02—Inorganic material
- B01D71/028—Molecular sieves
- B01D71/0281—Zeolites
-
- C—CHEMISTRY; METALLURGY
- C01—INORGANIC CHEMISTRY
- C01B—NON-METALLIC ELEMENTS; COMPOUNDS THEREOF; METALLOIDS OR COMPOUNDS THEREOF NOT COVERED BY SUBCLASS C01C
- C01B3/00—Hydrogen; Gaseous mixtures containing hydrogen; Separation of hydrogen from mixtures containing it; Purification of hydrogen
- C01B3/50—Separation of hydrogen or hydrogen containing gases from gaseous mixtures, e.g. purification
- C01B3/501—Separation of hydrogen or hydrogen containing gases from gaseous mixtures, e.g. purification by diffusion
- C01B3/503—Separation of hydrogen or hydrogen containing gases from gaseous mixtures, e.g. purification by diffusion characterised by the membrane
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01D—SEPARATION
- B01D2253/00—Adsorbents used in seperation treatment of gases and vapours
- B01D2253/10—Inorganic adsorbents
- B01D2253/106—Silica or silicates
- B01D2253/108—Zeolites
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01D—SEPARATION
- B01D2256/00—Main component in the product gas stream after treatment
- B01D2256/16—Hydrogen
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01D—SEPARATION
- B01D2256/00—Main component in the product gas stream after treatment
- B01D2256/22—Carbon dioxide
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01D—SEPARATION
- B01D2257/00—Components to be removed
- B01D2257/50—Carbon oxides
- B01D2257/504—Carbon dioxide
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01D—SEPARATION
- B01D2323/00—Details relating to membrane preparation
- B01D2323/08—Specific temperatures applied
- B01D2323/081—Heating
-
- C—CHEMISTRY; METALLURGY
- C01—INORGANIC CHEMISTRY
- C01B—NON-METALLIC ELEMENTS; COMPOUNDS THEREOF; METALLOIDS OR COMPOUNDS THEREOF NOT COVERED BY SUBCLASS C01C
- C01B2203/00—Integrated processes for the production of hydrogen or synthesis gas
- C01B2203/04—Integrated processes for the production of hydrogen or synthesis gas containing a purification step for the hydrogen or the synthesis gas
- C01B2203/0405—Purification by membrane separation
-
- C—CHEMISTRY; METALLURGY
- C01—INORGANIC CHEMISTRY
- C01B—NON-METALLIC ELEMENTS; COMPOUNDS THEREOF; METALLOIDS OR COMPOUNDS THEREOF NOT COVERED BY SUBCLASS C01C
- C01B2203/00—Integrated processes for the production of hydrogen or synthesis gas
- C01B2203/04—Integrated processes for the production of hydrogen or synthesis gas containing a purification step for the hydrogen or the synthesis gas
- C01B2203/0465—Composition of the impurity
- C01B2203/0475—Composition of the impurity the impurity being carbon dioxide
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y02—TECHNOLOGIES OR APPLICATIONS FOR MITIGATION OR ADAPTATION AGAINST CLIMATE CHANGE
- Y02C—CAPTURE, STORAGE, SEQUESTRATION OR DISPOSAL OF GREENHOUSE GASES [GHG]
- Y02C20/00—Capture or disposal of greenhouse gases
- Y02C20/40—Capture or disposal of greenhouse gases of CO2
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y02—TECHNOLOGIES OR APPLICATIONS FOR MITIGATION OR ADAPTATION AGAINST CLIMATE CHANGE
- Y02P—CLIMATE CHANGE MITIGATION TECHNOLOGIES IN THE PRODUCTION OR PROCESSING OF GOODS
- Y02P20/00—Technologies relating to chemical industry
- Y02P20/151—Reduction of greenhouse gas [GHG] emissions, e.g. CO2
Definitions
- the present invention in the separation and recovery CO 2 in the hydrogen production process or the like, relates to a membrane separation system for recovering CO 2 at a high efficiency.
- hydrocarbon or the like is reformed into a gas mainly composed of hydrogen and carbon monoxide by steam reforming or partial oxidation, and then carbon monoxide according to the following chemical reaction formula.
- Hydrogen is produced by reacting water with steam.
- membrane separation can be operated continuously, and there is no need to regenerate the absorbent or adsorbent, so it is expected as an energy-saving process.
- Patent Documents 1 and 2 an organic polymer film that functions under wet conditions is used as the CO 2 facilitated transport film.
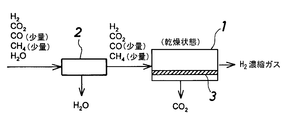
- FIG. 5 is a flow sheet showing a hydrogen production process for separating and recovering CO 2 using membrane separation of organic polymer membranes as described in Patent Documents 1 and 2.
- Hydrocarbons or alcohols as a raw material is reformed in the steam reforming reformer (10), H 2, CO 2, CO, CH 4 ( small amount) and H 2 O occurs, they are then water gas shift reactor Introduced in (11), the CO in the gas is shifted to CO 2 , and the CO in the gas is reduced to a small amount.
- the generated gas is sent to the separation module (12), and CO 2 is separated and recovered by the organic polymer membrane (13), whereby an H 2 concentrated gas is obtained.
- CO 2 / H 2 separation selectivity is 10 or more, and CO 2 can be recovered with high selectivity.
- the CO 2 permeability of these separation membranes is as small as 2 ⁇ 10 ⁇ 7 [mol / (m 2 ⁇ s ⁇ Pa)] at maximum, and considering application to a large-scale hydrogen production plant, CO 2 permeability It is desirable that the degree is 5 ⁇ 10 ⁇ 7 [mol / (m 2 ⁇ s ⁇ Pa)] or more and the CO 2 / H 2 separation selectivity is 10 or more.
- Non-patent document 1 reports the results of CO 2 / H 2 separation using a hydrophobic zeolite membrane. Under dry conditions, hydrogen with a small molecular diameter is preferentially permeated, and CO 2 is slightly preferred under wet conditions. However, CO 2 / H 2 separation selectivity is as small as about 2.9 to 6.2. JP 2008-36463 A Japanese Patent No. 4264194 Journal of Membrane Science, 2010, Vol. 360, p. 284-291.
- the present invention has been made in view of the above circumstances, and an object of the present invention is to provide a CO 2 membrane separation and recovery system excellent in CO 2 permeability and CO 2 separation selectivity in CO 2 recovery in a hydrogen production process or the like.
- membrane separation and recovery system of CO 2 of the present invention comprises a dehydration means in front of the CO 2 membrane separation means, and, CO 2 membrane separation unit shows the CO 2 selective permeability It comprises a hydrophilic zeolite membrane formed on a porous substrate, and the hydrophilic zeolite membrane has been dehydrated by heat treatment at 100 to 800 ° C., preferably 150 to 400 ° C. .
- the hydrophilic zeolite membrane is Li + , Na + , K + , Ag + , H + , NH 4 + , Ca 2+ , Sr 2+ , Ba 2+ , Cu 2+ , Zn serving as a selective adsorption site for CO 2.
- the hydrophilic zeolite is not particularly limited as long as it contains a lot of cation sites such as 2+, but as such a hydrophilic zeolite, from the viewpoint of CO 2 permeability, separation selectivity, and membrane durability.
- FAU or CHA type is mentioned.
- a noble metal membrane that selectively permeates hydrogen or a porous molecular sieve membrane having an effective pore diameter of 0.28 to 0.33 nm made of silica or zeolite is provided after the dehydration means.
- the “effective pore diameter” of the porous molecular sieve membrane is generally determined by hydrogen (0.28-0.29 nm), water (0.30 nm), CO 2 (0.33 nm), methane (0.38 nm). ) And other single component membrane permeation tests. For example, a membrane that allows hydrogen and water to permeate but does not allow CO 2 and methane to permeate is evaluated to have an effective pore size larger than hydrogen and 0.28 nm or more, smaller than CO 2 and less than 0.33 nm.
- Examples of the metal film that selectively permeates hydrogen include a Pd film.
- the present invention is a CO 2 membrane separation and recovery method using the membrane separation and recovery system of the above CO 2, comprising a dehydration step in front of the CO 2 membrane separation step, and, CO 2 membrane separation step
- the method is such that the feed gas dew point is kept dry at -80 to 0 ° C.
- CO 2 is preferably separated and recovered in a process for producing hydrogen from a hydrocarbon or alcohol.
- the above method is preferably performed by a noble metal membrane that is selectively provided for the hydrogen permeation, or a porous molecular sieve membrane having an effective pore diameter of 0.28 to 0.33 nm that is made of silica or zeolite, which is provided downstream of the dehydrating means. Including a step of performing hydrogen purification.
- CO 2 is separated and recovered from a mixed gas containing CO 2.
- the mixed gas is natural gas or biogas mainly composed of methane gas containing water vapor.
- CO 2 membrane separation means comprises a hydrophilic zeolite membrane was formed on a porous substrate showing a CO 2 selective permeability Since the hydrophilic zeolite membrane has been dehydrated by heat treatment at 100 to 800 ° C., preferably 150 to 400 ° C., in the recovery of CO 2 in a hydrogen production process or the like, the CO 2 permeability and CO 2 A CO 2 membrane separation and recovery system with excellent separation selectivity can be provided.
- the membrane separation and recovery system of CO 2 of the present invention is a flow sheet showing.
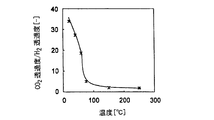
- Is a graph showing the results obtained for the separation and recovery of CO 2 / hydrogen represents the permeability of CO 2 and H 2 with respect to the temperature.
- Is a graph showing the results obtained for the separation and recovery of CO 2 / hydrogen represent CO 2 permeability / H 2 permeability against temperature.
- Is a graph showing the results obtained for the separation and recovery of CO 2 / hydrogen represents the permeated gas CO 2 concentration against temperature.
- It is a flow sheet showing a conventional membrane separation and recovery system for CO 2 .
- FIG. 1 is a flow sheet showing a CO 2 membrane separation and recovery system of the present invention.
- the CO 2 membrane separation system of the present invention includes a dehydration module (2) in front of the CO 2 membrane separation module (1).
- the CO 2 membrane module (1) includes a hydrophilic zeolite membrane (3) formed on a porous substrate exhibiting CO 2 selective permeability.
- the porous substrate include porous materials such as alumina, silica, cordierite, zirconia, titania, Vycor glass, and sintered metal, but are not limited to these, and various porous materials can be used.
- CO 2 membrane separation step in a CO 2 membrane module (1) the condition that the dew point is -80 ⁇ 0 ° C., made preferably so as to be kept at -20 ° C. or less.
- hydrophilic zeolite membrane (3) formed on a porous substrate is used as the CO 2 permeable separation membrane, not an organic polymer material.
- This hydrophilic zeolite membrane needs to be kept in a state in which the adsorbed water in the zeolite pores is removed by heating at 100 to 800 ° C., preferably 150 to 400 ° C.
- Hydrophilic Zeolite membranes zeolite species constituting the FAU type to indicate a CO 2 selective adsorptivity in CO 2 -H 2 mixed gas system, etc.
- CHA-type zeolite is preferred.
- the dehydration module (2) may be dehydrated by any method as long as water is removed until the dew point in the introduced gas is ⁇ 80 to 0 ° C., preferably ⁇ 20 ° C. or less.
- dehumidification can be performed with a membrane air dryer using, for example, a polymer hollow fiber membrane or a commercially available LTA type zeolite membrane (manufactured by Hitachi Zosen, NaA type zeolite membrane).
- LTA type zeolite membrane manufactured by Hitachi Zosen, NaA type zeolite membrane.
- water can be selectively permeated and removed, whereby the subsequent CO 2 membrane separation step can be made dry.
- moisture can be continuously removed by sweeping a part of the drying gas in the subsequent stage or by evacuation.
- a noble metal membrane that selectively permeates hydrogen or a porous molecular sieve membrane made of silica or zeolite and having an effective pore diameter of 0.28 nm to 0.33 nm is dehydrated. (Not shown). This makes it possible to purify hydrogen without being affected by membrane deterioration due to water vapor or the like.
- the hydrogen purification is performed before or after the CO 2 membrane separation is determined according to the required concentration of hydrogen to be recovered and CO 2 . For example, if you want to prioritize the transmission the CO 2 concentration increases to recover a CO 2 membrane separation step, to increase the CO 2 concentration of the gas supplied to the CO 2 membrane separation step, hydrogen purification step, CO 2 membrane separation step It is more advantageous to carry out in the preceding stage. On the other hand, when priority is given to increasing the concentration of permeated hydrogen to be recovered, it is advantageous to perform the hydrogen purification step after the CO 2 membrane separation step.
- the CO 2 separation and recovery process using the zeolite membrane of the present invention can also be applied to the separation and recovery of CO 2 from natural gas or biogas mainly composed of methane.
- Example 2 (CO 2 / hydrogen separation)
- the system of the present invention was used to separate and recover CO 2 from hydrogen.
- Example 1 using the hydrophilic zeolite membrane for CO 2 separation and recovery according to the present invention a commercially available tubular FAU type zeolite membrane (manufactured by Hitachi Zosen, NaY type zeolite membrane) was used.
- the membrane permeation separation ability was evaluated by cutting and dividing a tubular membrane element into 3 cm pieces, attaching them to a stainless steel membrane module, and performing heat drying at a temperature of 300 ° C. as a membrane dehydration treatment.
- the membrane permeability of CO 2 and hydrogen was calculated by supplying a mixed gas of CO 2 -hydrogen from the outside of the tubular zeolite membrane and measuring the flow rate and composition of the membrane permeating gas. Detailed conditions when performing CO 2 / hydrogen separation are shown below.
- Comparative Example 1 it was subjected to separation and recovery CO 2 using conventional organic polymer films as membranes for CO 2 separation and recovery.
- Comparative Example 2 is the same FAU type zeolite membrane of Example 1, those that did not perform the heat drying is used as film for CO 2 separation and recovery, carried out in the separation and recovery CO 2 under working atmosphere moistened It was.
- Comparative Example 3 is the same FAU type zeolite membrane of Example 1, those that did not perform the heat drying is used as film for CO 2 separation and recovery, carried out in the separation and recovery CO 2 under dry working atmosphere It was.
- the CO 2 permeability reached a maximum around 60 ° C., and showed a very high permeability of 10 ⁇ 6 [mol / m 2 ⁇ s ⁇ Pa] or more.
- the hydrogen permeability becomes smaller at lower temperature conditions, and the ratio of CO 2 and hydrogen permeability becomes higher at lower temperatures as shown in FIG. 3, and the CO 2 separation selectivity exceeds 10 at an operating condition of 60 ° C.
- CO 2 having a concentration of 90% or more could be separated and recovered.
- Table 1 below compares the performance of the CO 2 membrane separation and recovery system of the present invention and the conventional membrane separation system.
- the CO 2 permeability is at most from the order of 10 ⁇ 9 [mol / m 2 ⁇ s ⁇ Pa] on the condition that the CO 2 / H 2 separation selectivity exceeds 10. Although it is as small as 2 ⁇ 10 ⁇ 7 [mol / m 2 ⁇ s ⁇ Pa], CO 2 / H 2 separation selectivity is maintained at 10 or more by using the membrane separation system of the present invention. 2 permeability A very high CO 2 permeability of 10 ⁇ 6 [mol / m 2 ⁇ s ⁇ Pa] or more could be obtained.
- Feed gas total pressure absolute pressure 0.4 MPa, in the same manner as above as a feed gas total flow rate 300mL (STP) / min was the separated and recovered CO 2.
- the various supply gas compositions were 1: 1 with respect to CO 2 and the atmospheric pressure (absolute pressure 0.1 MPa) on the membrane permeation side.
- the CO 2 permeability is 10 ⁇ 8 mol / (m 2 under the operating condition of 60 ° C. or less in the wet condition and the undried FAU type zeolite membrane. ⁇ It was very small, less than s ⁇ Pa), and the separation ability was hardly expressed.
- the CO 2 permeability is 2 ⁇ 10 ⁇ 7 mol / (m 2) by setting the dew point of the supply gas to ⁇ 20 ° C. or lower under atmospheric pressure conditions and subjecting the membrane to a heat treatment of 150 ° C. or higher to keep it dehydrated. -S ⁇ Pa) and improved high separation performance with a permeability ratio of 10 to 100 times or more for each gas such as CH 4 and CO.
Landscapes
- Chemical & Material Sciences (AREA)
- Engineering & Computer Science (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Analytical Chemistry (AREA)
- General Chemical & Material Sciences (AREA)
- Oil, Petroleum & Natural Gas (AREA)
- Inorganic Chemistry (AREA)
- Organic Chemistry (AREA)
- Manufacturing & Machinery (AREA)
- Environmental & Geological Engineering (AREA)
- Health & Medical Sciences (AREA)
- Combustion & Propulsion (AREA)
- Biomedical Technology (AREA)
- Life Sciences & Earth Sciences (AREA)
- Geology (AREA)
- Separation Using Semi-Permeable Membranes (AREA)
- Hydrogen, Water And Hydrids (AREA)
- Carbon And Carbon Compounds (AREA)
- Drying Of Gases (AREA)
- Organic Low-Molecular-Weight Compounds And Preparation Thereof (AREA)
Priority Applications (4)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US14/116,347 US9333457B2 (en) | 2011-05-09 | 2012-05-09 | Zeolite membrane separation and recovery system for CO2 |
| CN201280022552.1A CN103635248B (zh) | 2011-05-09 | 2012-05-09 | Co2的沸石膜分离回收系统 |
| ES12781627T ES2734376T3 (es) | 2011-05-09 | 2012-05-09 | Sistema de separación/recuperación de CO2 mediante membrana de zeolita |
| EP12781627.0A EP2716347B1 (en) | 2011-05-09 | 2012-05-09 | Zeolite-membrane separation/recovery for co2 |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2011104382A JP5835937B2 (ja) | 2011-05-09 | 2011-05-09 | Co2のゼオライト膜分離回収システム |
| JP2011-104382 | 2011-05-09 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2012153763A1 true WO2012153763A1 (ja) | 2012-11-15 |
Family
ID=47139239
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/JP2012/061874 Ceased WO2012153763A1 (ja) | 2011-05-09 | 2012-05-09 | Co2のゼオライト膜分離回収システム |
Country Status (6)
| Country | Link |
|---|---|
| US (1) | US9333457B2 (enExample) |
| EP (1) | EP2716347B1 (enExample) |
| JP (1) | JP5835937B2 (enExample) |
| CN (1) | CN103635248B (enExample) |
| ES (1) | ES2734376T3 (enExample) |
| WO (1) | WO2012153763A1 (enExample) |
Families Citing this family (15)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2014091766A (ja) * | 2012-11-01 | 2014-05-19 | Ube Ind Ltd | バイオガス利用システムおよびバイオガス除湿方法 |
| US9051228B2 (en) * | 2013-05-31 | 2015-06-09 | Guild Associates | LNG pretreatment |
| CN107148312A (zh) | 2014-11-25 | 2017-09-08 | 日本碍子株式会社 | 分离膜结构体 |
| JP6626736B2 (ja) * | 2016-02-25 | 2019-12-25 | 日立造船株式会社 | ゼオライト膜複合体の再生方法 |
| WO2017169195A1 (ja) * | 2016-03-31 | 2017-10-05 | 日本ゼオン株式会社 | 膜分離方法および膜分離装置 |
| CN107159104B (zh) * | 2017-05-12 | 2020-07-17 | 太原理工大学 | 一种用于Chabazite分子筛模压成型的方法 |
| CN107376603B (zh) * | 2017-08-03 | 2020-04-28 | 中石化炼化工程(集团)股份有限公司 | 脱除制氢变换气变压吸附工艺尾气中co2的方法 |
| WO2020154358A1 (en) * | 2019-01-22 | 2020-07-30 | Rensselaer Polytechnic Institute | METHODS AND SYSTEMS FOR PRODUCING HIGH PURITY METHANOL FROM CARBON DIOXIDE HYDROGENATION USING NaA MEMBRANE REACTOR |
| WO2021186663A1 (ja) * | 2020-03-19 | 2021-09-23 | 株式会社日立製作所 | 一酸化炭素製造装置及び一酸化炭素製造システム |
| WO2022149318A1 (ja) * | 2021-01-07 | 2022-07-14 | 日本碍子株式会社 | 混合ガス分離方法および混合ガス分離装置 |
| WO2023277203A1 (ko) * | 2021-06-28 | 2023-01-05 | ㈜에어레인 | 막제습기에 의한 제습과정이 연계된 연소배가스의 이산화탄소 포집공정 |
| CN114011212A (zh) * | 2021-10-30 | 2022-02-08 | 雅邦绿色过程与新材料研究院南京有限公司 | 一种膜/mdea溶液耦合回收高浓度co2的节能工艺 |
| WO2025204263A1 (ja) * | 2024-03-27 | 2025-10-02 | 日本碍子株式会社 | ガス分離装置およびガス分離方法 |
| WO2025206099A1 (ja) * | 2024-03-28 | 2025-10-02 | 日本碍子株式会社 | ガス分離システム |
| WO2025206100A1 (ja) * | 2024-03-28 | 2025-10-02 | 日本碍子株式会社 | ガス分離システム |
Citations (8)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPH1036114A (ja) * | 1996-07-23 | 1998-02-10 | Fine Ceramics Center | ゼオライト膜、ゼオライト膜の製造方法及びゼオライト膜によるガス混合体の分離方法 |
| JP2006176399A (ja) * | 2004-11-29 | 2006-07-06 | National Institute Of Advanced Industrial & Technology | フィリップサイト型ゼオライト膜及びその製造方法 |
| JP2007313389A (ja) * | 2006-05-23 | 2007-12-06 | Asahi Kasei Corp | マーリノアイト型ゼオライト複合膜及びその製造方法 |
| JP2008036463A (ja) | 2006-08-01 | 2008-02-21 | Renaissance Energy Research:Kk | Co2促進輸送膜及びその製造方法 |
| JP2009011980A (ja) * | 2007-07-06 | 2009-01-22 | Research Institute Of Innovative Technology For The Earth | ガス分離用ゼオライト膜複合体の製造方法 |
| JP2009029675A (ja) * | 2007-07-27 | 2009-02-12 | Nippon Oil Corp | 水素製造および二酸化炭素回収方法ならびに装置 |
| JP4264194B2 (ja) | 1997-08-01 | 2009-05-13 | エクソンモービル リサーチ アンド エンジニアリング カンパニー | Co2選択膜プロセスおよび燃料を燃料電池用の水素に改質するためのシステム |
| JP2010254544A (ja) * | 2009-03-30 | 2010-11-11 | Tokyo Gas Co Ltd | 二酸化炭素分離回収装置を伴う水素分離型水素製造システム |
Family Cites Families (27)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5002590A (en) * | 1989-09-19 | 1991-03-26 | Bend Research, Inc. | Countercurrent dehydration by hollow fibers |
| US4952219A (en) * | 1989-09-29 | 1990-08-28 | Air Products And Chemicals, Inc. | Membrane drying of gas feeds to low temperature units |
| JPH07108368B2 (ja) * | 1990-11-02 | 1995-11-22 | 住友精化株式会社 | 混合ガス中の水分除去方法 |
| US5964923A (en) * | 1996-02-29 | 1999-10-12 | Membrane Technology And Research, Inc. | Natural gas treatment train |
| JP2001259417A (ja) * | 2000-03-21 | 2001-09-25 | Nissan Motor Co Ltd | 空調装置用吸着材,吸湿素子および除湿方法 |
| GB0028395D0 (en) | 2000-11-22 | 2001-01-03 | Ici Plc | Getters |
| CN1129547C (zh) * | 2001-10-24 | 2003-12-03 | 连云港中金医药包装有限公司 | 锂电池软包装膜及其复合方法 |
| US20040099138A1 (en) * | 2002-11-21 | 2004-05-27 | L'air Liquide, Societe Anonyme A Directoire Et Conseil De Surveillance Pour L'etude Et | Membrane separation process |
| US7316727B2 (en) * | 2004-03-19 | 2008-01-08 | The Regents Of The University Of Colorado | High-selectivity supported SAPO membranes |
| JP2006239663A (ja) * | 2005-03-07 | 2006-09-14 | Noritake Co Ltd | 水素ガス分離膜の製造方法 |
| JP5010109B2 (ja) * | 2005-04-28 | 2012-08-29 | 三菱重工業株式会社 | 水素製造装置および水素製造方法 |
| JP2007125543A (ja) * | 2005-10-07 | 2007-05-24 | Osaka Univ | 分離モジュール及び分離モジュールの製造方法 |
| US7938893B2 (en) * | 2006-04-18 | 2011-05-10 | Gas Technology Institute | Membrane reactor for H2S, CO2 and H2 separation |
| WO2007134094A2 (en) * | 2006-05-15 | 2007-11-22 | The Regents Of The University Of Colorado, A Body Corporate | High flux and selectivity sapo-34 membranes for co2/ch4 separations |
| EP2366447B1 (en) * | 2007-03-29 | 2014-12-17 | Nippon Oil Corporation | Method and apparatus for producing hydrogen and recovering carbon dioxide |
| US8911519B2 (en) * | 2007-07-27 | 2014-12-16 | Nippon Oil Corporation | Method and apparatus for hydrogen production and carbon dioxide recovery |
| CN101259384B (zh) * | 2008-05-05 | 2011-06-01 | 江西师范大学 | 富含毛沸石的t型分子筛膜及制备方法以及在气体分离中的应用 |
| DE102008057157A1 (de) * | 2008-11-13 | 2010-05-20 | Forschungszentrum Jülich GmbH | Verfahren zur CO2-Abtrennung aus dem Rauchgas eines Kraftwerkes |
| WO2011044366A1 (en) * | 2009-10-09 | 2011-04-14 | The Regents Of The University Of Colorado, A Body Corporate | Blocking defects in molecular sieve membranes with cyclodextrin |
| US8221524B2 (en) * | 2009-10-23 | 2012-07-17 | Guild Associates, Inc. | Oxygen removal from contaminated gases |
| JP5957828B2 (ja) * | 2010-08-26 | 2016-07-27 | 三菱化学株式会社 | ガス分離用ゼオライト膜複合体 |
| JP2012055501A (ja) * | 2010-09-09 | 2012-03-22 | Panasonic Corp | 空気清浄装置 |
| WO2012048078A1 (en) * | 2010-10-06 | 2012-04-12 | L'air Liquide Societe Anonyme Pour L'etude Et L'exploitation Des Procedes Georges Claude | Carbon dioxide removal process |
| JP2012106228A (ja) * | 2010-10-21 | 2012-06-07 | Hitachi Zosen Corp | 膜分離を利用した大気空気の除湿・除二酸化炭素連続処理方法および処理装置 |
| US8535638B2 (en) * | 2010-11-11 | 2013-09-17 | Air Liquide Large Industries U.S. | Process for recovering hydrogen and carbon dioxide |
| US20120118011A1 (en) * | 2010-11-11 | 2012-05-17 | Air Liquide Large Industries U.S. Lp | Process For The Production Of Hydrogen And Carbon Dioxide |
| JP2012236134A (ja) * | 2011-05-11 | 2012-12-06 | Hitachi Zosen Corp | 二酸化炭素分離システム |
-
2011
- 2011-05-09 JP JP2011104382A patent/JP5835937B2/ja active Active
-
2012
- 2012-05-09 WO PCT/JP2012/061874 patent/WO2012153763A1/ja not_active Ceased
- 2012-05-09 ES ES12781627T patent/ES2734376T3/es active Active
- 2012-05-09 CN CN201280022552.1A patent/CN103635248B/zh active Active
- 2012-05-09 US US14/116,347 patent/US9333457B2/en active Active
- 2012-05-09 EP EP12781627.0A patent/EP2716347B1/en active Active
Patent Citations (8)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPH1036114A (ja) * | 1996-07-23 | 1998-02-10 | Fine Ceramics Center | ゼオライト膜、ゼオライト膜の製造方法及びゼオライト膜によるガス混合体の分離方法 |
| JP4264194B2 (ja) | 1997-08-01 | 2009-05-13 | エクソンモービル リサーチ アンド エンジニアリング カンパニー | Co2選択膜プロセスおよび燃料を燃料電池用の水素に改質するためのシステム |
| JP2006176399A (ja) * | 2004-11-29 | 2006-07-06 | National Institute Of Advanced Industrial & Technology | フィリップサイト型ゼオライト膜及びその製造方法 |
| JP2007313389A (ja) * | 2006-05-23 | 2007-12-06 | Asahi Kasei Corp | マーリノアイト型ゼオライト複合膜及びその製造方法 |
| JP2008036463A (ja) | 2006-08-01 | 2008-02-21 | Renaissance Energy Research:Kk | Co2促進輸送膜及びその製造方法 |
| JP2009011980A (ja) * | 2007-07-06 | 2009-01-22 | Research Institute Of Innovative Technology For The Earth | ガス分離用ゼオライト膜複合体の製造方法 |
| JP2009029675A (ja) * | 2007-07-27 | 2009-02-12 | Nippon Oil Corp | 水素製造および二酸化炭素回収方法ならびに装置 |
| JP2010254544A (ja) * | 2009-03-30 | 2010-11-11 | Tokyo Gas Co Ltd | 二酸化炭素分離回収装置を伴う水素分離型水素製造システム |
Non-Patent Citations (1)
| Title |
|---|
| JOURNAL OF MEMBRANE SCIENCE, vol. 360, 2010, pages 284 - 291 |
Also Published As
| Publication number | Publication date |
|---|---|
| ES2734376T3 (es) | 2019-12-05 |
| EP2716347A1 (en) | 2014-04-09 |
| JP5835937B2 (ja) | 2015-12-24 |
| CN103635248B (zh) | 2017-09-12 |
| EP2716347B1 (en) | 2019-04-24 |
| JP2012232274A (ja) | 2012-11-29 |
| US20140174290A1 (en) | 2014-06-26 |
| EP2716347A4 (en) | 2014-11-19 |
| US9333457B2 (en) | 2016-05-10 |
| CN103635248A (zh) | 2014-03-12 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP5835937B2 (ja) | Co2のゼオライト膜分離回収システム | |
| KR102796453B1 (ko) | 메탄, 이산화탄소 및 황화수소를 함유하는 가스 혼합물로부터 메탄을 분리하기 위한 디바이스 및 프로세스 | |
| CN103596663B (zh) | 二氧化碳分离系统 | |
| US7575624B2 (en) | Molecular sieve and membrane system to purify natural gas | |
| RU2605593C2 (ru) | Способ извлечения гелия и устройство для его осуществления | |
| JP2012232274A5 (enExample) | ||
| JP2012236134A5 (enExample) | ||
| CA2911820A1 (en) | Methods and systems of enhanced carbon dioxide recovery | |
| KR19980070495A (ko) | 단화된 흡착막을 사용하여 다성분 기체 혼합물을 분리하는 방법 | |
| US20090270665A1 (en) | Device to separate olefins from paraffins and to purify olefins and use thereof | |
| JP2012236123A (ja) | ゼオライト膜による排ガス中の二酸化炭素分離回収システム | |
| JP5965945B2 (ja) | Co2のゼオライト膜分離回収システム | |
| WO2003020674A1 (en) | Co2 rejection from natural gas | |
| JP2012106228A (ja) | 膜分離を利用した大気空気の除湿・除二酸化炭素連続処理方法および処理装置 | |
| Ye et al. | Organic template residues confined in silicalite-1 zeolite membranes for tuning pore structures and boosting H2/CO2 separation | |
| JP2022184754A (ja) | 気体の分離方法 | |
| JP2014000535A (ja) | 二酸化炭素の分離方法及び二酸化炭素の分離膜 | |
| US20140357925A1 (en) | LNG Pretreatment | |
| JP2022169625A (ja) | 硫化水素分離用ゼオライト膜の再生方法 | |
| CN117246981B (zh) | 一种分离含氯化氢二氧化硫尾气的应用方法 | |
| JP2000262835A (ja) | 混合流体の分離装置及び分離法 | |
| DE102018109104A1 (de) | Gasaufbereitungsanlage zur Trennung von Wasserdampf aus einem Gasgemisch | |
| WO2025257285A1 (en) | Process and system for the capture of carbon dioxide directly from air | |
| Gu et al. | Enhancing High Temperature Hydrogen Separation On Mfi Zeolite Membranes By On-Stream Modification Of Zeolitic Pores |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 12781627 Country of ref document: EP Kind code of ref document: A1 |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2012781627 Country of ref document: EP |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 14116347 Country of ref document: US |