WO2011040200A1 - 血管モデル - Google Patents
血管モデル Download PDFInfo
- Publication number
- WO2011040200A1 WO2011040200A1 PCT/JP2010/065473 JP2010065473W WO2011040200A1 WO 2011040200 A1 WO2011040200 A1 WO 2011040200A1 JP 2010065473 W JP2010065473 W JP 2010065473W WO 2011040200 A1 WO2011040200 A1 WO 2011040200A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- blood vessel
- vessel model
- polyvinyl alcohol
- model
- silica particles
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Ceased
Links
Images
Classifications
-
- G—PHYSICS
- G09—EDUCATION; CRYPTOGRAPHY; DISPLAY; ADVERTISING; SEALS
- G09B—EDUCATIONAL OR DEMONSTRATION APPLIANCES; APPLIANCES FOR TEACHING, OR COMMUNICATING WITH, THE BLIND, DEAF OR MUTE; MODELS; PLANETARIA; GLOBES; MAPS; DIAGRAMS
- G09B23/00—Models for scientific, medical, or mathematical purposes, e.g. full-sized devices for demonstration purposes
- G09B23/28—Models for scientific, medical, or mathematical purposes, e.g. full-sized devices for demonstration purposes for medicine
- G09B23/30—Anatomical models
- G09B23/303—Anatomical models specially adapted to simulate circulation of bodily fluids
-
- G—PHYSICS
- G09—EDUCATION; CRYPTOGRAPHY; DISPLAY; ADVERTISING; SEALS
- G09B—EDUCATIONAL OR DEMONSTRATION APPLIANCES; APPLIANCES FOR TEACHING, OR COMMUNICATING WITH, THE BLIND, DEAF OR MUTE; MODELS; PLANETARIA; GLOBES; MAPS; DIAGRAMS
- G09B23/00—Models for scientific, medical, or mathematical purposes, e.g. full-sized devices for demonstration purposes
- G09B23/28—Models for scientific, medical, or mathematical purposes, e.g. full-sized devices for demonstration purposes for medicine
- G09B23/285—Models for scientific, medical, or mathematical purposes, e.g. full-sized devices for demonstration purposes for medicine for injections, endoscopy, bronchoscopy, sigmoidscopy, insertion of contraceptive devices or enemas
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/02—Prostheses implantable into the body
- A61F2/04—Hollow or tubular parts of organs, e.g. bladders, tracheae, bronchi or bile ducts
- A61F2/06—Blood vessels
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/0012—Galenical forms characterised by the site of application
- A61K9/0019—Injectable compositions; Intramuscular, intravenous, arterial, subcutaneous administration; Compositions to be administered through the skin in an invasive manner
- A61K9/0024—Solid, semi-solid or solidifying implants, which are implanted or injected in body tissue
Definitions
- the present invention relates to a blood vessel model. More specifically, it is a blood vessel model imitating a human blood vessel, and can be suitably used as, for example, a blood vessel model for practice of inserting a stent graft into an aneurysm, or a blood vessel model for practice of resection / suture of blood vessels. It relates to a blood vessel model.
- any of the above materials such as silicone rubber has a very strong water repellency, so it does not have hydrophilicity like human blood vessels and does not have flexibility like human blood vessels. Therefore, it cannot be said that a blood vessel model made of the material is suitable for a vascular surgeon or the like to practice a technique.
- An object of the present invention is to have a moderate hydrophilicity and flexibility (elasticity), the surface of which is not sticky, a blood vessel model for practicing insertion of a stent graft into an aneurysm, and a blood vessel model for practicing resection and suturing of a blood vessel
- An object of the present invention is to provide a blood vessel model that can be suitably used as a device.
- the present invention (1) A blood vessel model imitating a human blood vessel, comprising an aqueous gel composed of polyvinyl alcohol having an average polymerization degree of 300 to 3500 and a saponification degree of 90 mol% or more, and silica particles, (2) The blood vessel model according to (1), wherein colloidal silica is used as the silica particles, (3) The blood vessel model according to (1) or (2), wherein the amount of silica particles is 0.01 to 50 parts by weight per 100 parts by weight of polyvinyl alcohol, (4) The blood vessel model according to any one of (1) to (3), wherein the mixed solution containing polyvinyl alcohol, silica particles, and water is frozen at a temperature of ⁇ 10 ° C. or less and then thawed.
- the cross-linked gel is a cross-linked gel formed by using dimethyl sulfoxide.
- a method for producing a blood vessel model imitating a human blood vessel comprising an average polymerization degree of 300 to 3500, a mixed solution containing polyvinyl alcohol, silica particles and water having a saponification degree of 90 mol% or more In a blood vessel model molding die, frozen at a temperature of ⁇ 10 ° C.
- the present invention relates to the method for producing a blood vessel model according to (13), wherein the volume ratio) is 50/50 to 95/5.
- the blood vessel model of the present invention has moderate hydrophilicity and flexibility (elasticity), and its surface is not sticky. Therefore, a blood vessel model for practicing insertion of a stent graft into an aneurysm, practicing resection and suturing of a blood vessel It can be suitably used as a blood vessel model.
- the blood vessel model of the present invention is a blood vessel model imitating a human blood vessel, and contains an aqueous gel and silica particles made of polyvinyl alcohol having an average polymerization degree of 300 to 3500 and a saponification degree of 90 mol% or more. It is characterized by that.
- a mixed solution containing polyvinyl alcohol, water, and silica particles having an average polymerization degree of 300 to 3500 and a saponification degree of 90 mol% or more is frozen at a temperature of ⁇ 10 ° C. or less. Thereafter, it can be easily manufactured by thawing.
- the average degree of polymerization determined by the viscosity method of polyvinyl alcohol is preferably 300 or more, more preferably 500 or more, still more preferably 1000 or more, from the viewpoint of increasing mechanical strength such as tensile strength of the blood vessel model of the present invention. From the viewpoint of imparting appropriate flexibility (elasticity), it is preferably 3500 or less, more preferably 3000 or less, and even more preferably 2500 or less.
- the saponification degree of polyvinyl alcohol is preferably 90 mol% or more, more preferably 95 mol% or more, from the viewpoint of increasing mechanical strength such as tensile strength and flexibility (elasticity) of the blood vessel model of the present invention. More preferably, it is 98 mol% or more.
- the upper limit of the degree of saponification of polyvinyl alcohol and the higher it is, the more preferable it is, and the completely saponified polyvinyl alcohol is more preferable.
- Polyvinyl alcohol can usually be used as an aqueous solution.
- the concentration of polyvinyl alcohol in the aqueous polyvinyl alcohol solution is preferably 1% by weight or more, more preferably 3% by weight or more, and further preferably 5% by weight or more, from the viewpoint of increasing the mechanical strength of the blood vessel model of the present invention. From the viewpoint of sufficiently dissolving alcohol in water and improving moldability, it is preferably 40% by weight or less, more preferably 30% by weight or less, and still more preferably 20% by weight or less.
- the blood vessel model of the present invention has one major feature in that it contains silica particles. Since the blood vessel model of the present invention contains silica particles in this way, the surface is not sticky and has appropriate hydrophilicity and flexibility (elasticity). Accordingly, the blood vessel model of the present invention can be suitably used as a blood vessel model for practicing insertion of a stent graft into an aneurysm or practicing resection / suture of a blood vessel.
- the mixed solution containing silica particles and polyvinyl alcohol can be used without repeating the operation of cold thawing of the polyvinyl alcohol solution a plurality of times as in the prior art.
- the blood vessel model having an appropriate hydrophilicity and flexibility (elasticity) and having excellent mechanical strength such as tensile strength can be efficiently obtained.
- the particle diameter of the silica particles is preferably about 3 to 100 nm from the viewpoint of improving the dispersion stability in polyvinyl alcohol and the smoothness of the blood vessel model of the present invention.
- the amount of silica particles is preferably 0.01 parts by weight or more, more preferably 0.05 parts by weight from the viewpoint of increasing the mechanical strength and flexibility (elasticity) of the blood vessel model of the present invention per 100 parts by weight of polyvinyl alcohol. Part or more, more preferably 0.1 part by weight or more, and preferably 50 parts by weight or less, more preferably 30 parts by weight or less, and still more preferably 20 parts by weight, from the viewpoint of preventing the blood vessel model of the present invention from becoming hard. Or less. Silica particles can usually be mixed with polyvinyl alcohol or an aqueous solution thereof.
- colloidal silica as the silica particles.
- colloidal silica When colloidal silica is used as the silica particle, it has a non-sticky surface, has moderate hydrophilicity and flexibility (elasticity), and is a blood vessel model for practicing insertion of a stent graft into an aneurysm or practicing resection and suturing of a blood vessel As a result, a blood vessel model that can be suitably used is obtained.
- the content of the silica particles in the colloidal silica is preferably about 3 to 40% by weight from the viewpoint of the dispersion stability of the silica particles in the colloidal silica.
- Colloidal silica can be easily obtained commercially, for example, as a product name: Snowtex (registered trademark) manufactured by Nissan Chemical Industries, Ltd.
- the blood vessel model of the present invention has moderate hydrophilicity and flexibility (elasticity), its surface is not sticky, and has a high tensile strength, so that the aqueous gel is preferably a crosslinked gel.
- the crosslinked gel is a crosslinked gel crosslinked with dimethyl sulfoxide from the viewpoint of producing a blood vessel model having moderate hydrophilicity and flexibility (elasticity), having a non-sticky surface and high tensile strength. Is preferred.
- the blood vessel model containing the crosslinked gel and silica particles of the present invention contains, for example, polyvinyl alcohol having an average polymerization degree of 300 to 3500 and a saponification degree of 90 mol% or more, silica particles, dimethyl sulfoxide and water.
- the mixed solution can be easily produced by cooling to a temperature of ⁇ 10 ° C. or lower and then thawing.
- Polyvinyl alcohol can be added to a mixed solvent of dimethyl sulfoxide and water, water used for the mixed solvent, or a mixture in which silica particles are added to the mixed solvent.
- the heating temperature at that time is not particularly limited, but it is usually preferably about 60 to 95 ° C.
- the content of polyvinyl alcohol in the mixed solution containing polyvinyl alcohol, silica particles, dimethyl sulfoxide and water depends on the mechanical strength such as the tensile strength of the blood vessel model of the present invention. From the viewpoint of increasing the strength, it is preferably 1% by weight or more, more preferably 3% by weight or more, and further preferably 5% by weight or more. From the viewpoint of improving the solubility of polyvinyl alcohol and preventing stickiness, it is preferably 40%. % By weight or less, more preferably 30% by weight or less, still more preferably 20% by weight or less.
- the ratio of dimethyl sulfoxide to water is preferably 50/50 or more, more preferably 60/40 or more, from the viewpoint of increasing mechanical strength such as tensile strength of the blood vessel model of the present invention. More preferably, the ratio is 70/30 or more, and from the viewpoint of suppressing the stickiness of the surface of the blood vessel model of the present invention, and improving flexibility (elasticity) and hydrophilicity, preferably 95/5 or less, more preferably 90 / 10 or less, more preferably 85/15 or less.
- the amount of silica particles increases the mechanical strength such as the tensile strength of the blood vessel model of the present invention per 100 parts by weight of water, prevents stickiness, From the viewpoint of imparting hydrophilicity, it is preferably 0.01 parts by weight or more, more preferably 0.05 parts by weight or more, and still more preferably 0.1 parts by weight or more, and the flexibility (elasticity) of the blood vessel model of the present invention. ) Is preferably 50 parts by weight or less, more preferably 30 parts by weight or less, and still more preferably 20 parts by weight or less.
- the amount of water includes the amount of water contained in the colloidal silica.
- the mixed solution is frozen by cooling the mixed solution containing polyvinyl alcohol, silica particles, dimethyl sulfoxide and water at a desired temperature for a desired time.
- the mixed solution freezes, the mixed solution is gelated by crosslinking, so that a molded body including the crosslinked gel and silica particles is formed.
- Polyvinyl alcohol is preferably added to polyvinyl alcohol from the viewpoint of preventing the surface layer from drying.
- the polysaccharide is preferably added to the mixed solution from the viewpoint of dispersion stability.
- polysaccharides include chitin, deacetylated chitin, chitosan, chitosan acetate, chitosan maleate, chitosan glycolate, chitosan sorbate, chitosan formate, chitosan salicylate, chitosan propionate, chitosan lactate, chitosan itako Nate, chitosan niacate, chitosan gallate, chitosan glutamate, carboxymethyl chitosan, alkyl cellulose, nitrocellulose, hydroxypropyl cellulose, starch, collagen, alginate, hyaluronic acid, heparin, etc. It is not limited to only. Of these, chitosan and its derivatives are preferable, and chitosan is more preferable from the viewpoint of preventing the blood vessel model of the present invention from being dried.
- chitosan examples include deacetylated chitin derived from crustaceans such as shrimp, crab and squid. Chitosan is readily available commercially. Chitosan can usually be used in the form of a powder. The molecular weight of chitosan is not particularly limited, but is usually preferably 10,000 to 200,000, more preferably 10,000 to 40,000.
- the amount of polysaccharide cannot be determined unconditionally because it varies depending on the type of the polysaccharide.
- the viewpoint of preventing drying of the blood vessel model of the present invention per 100 parts by weight of polyvinyl alcohol preferably 0.3 weight. Part or more, more preferably 0.5 part by weight or more, further preferably 1 part by weight or more. From the viewpoint of allowing the blood vessel model of the present invention to have appropriate elasticity, preferably 300 parts by weight or less, more preferably Is 250 parts by weight or less, more preferably 200 parts by weight or less.
- the polysaccharide is usually preferably used as an aqueous solution from the viewpoint of enhancing dispersion stability.
- the aqueous polysaccharide solution can be obtained, for example, by dissolving the polysaccharide in an aqueous solution of an acid such as acetic acid, hydrochloric acid, or lactic acid so that the concentration is about 0.5 to 10% by weight. If necessary, the polysaccharide aqueous solution may be adjusted to neutral to basic with a basic substance such as sodium hydroxide or potassium hydroxide.
- additives such as colorants such as pigments and dyes, fragrances, antioxidants, antifungal agents, and antibacterial agents may be added in appropriate amounts within a range that does not impair the object of the present invention. .
- colorants such as pigments and dyes, fragrances, antioxidants, antifungal agents, and antibacterial agents
- additives are usually preferably added to the mixed solution from the viewpoint of improving dispersion stability.
- the blood vessel model of the present invention is obtained by filling a mixed solution containing polyvinyl alcohol, water, silica particles and, if necessary, dimethyl sulfoxide into a blood vessel model mold, freezing at a temperature of ⁇ 10 ° C. or lower, and then thawing. Can be manufactured.
- the blood vessel model of the present invention is, for example, filled with a mixed solution into a tube having an inner diameter corresponding to the diameter of a human blood vessel, frozen at a temperature of ⁇ 10 ° C. or lower, and then thawed.
- a linear body such as a wire, a wire, or a metal wire having a diameter corresponding to the inner diameter of the blood vessel is inserted into the central portion of the molded body formed in step B to form a blood passage, and the tube is formed from the formed body. And it can manufacture by removing a linear body.
- the formed molded body is taken out from the inside of the tube by suction, extrusion, etc. May be formed.
- the tube examples include a rubber tube made of silicone rubber, a tube made of elastomer, a resin tube made of resin such as polypropylene, acrylic resin, polycarbonate, etc., but the present invention is limited to such examples only. It is not a thing.
- the mixed solution is filled into a straight tube having an inner diameter corresponding to the outer shape of the blood vessel, and a core material having a diameter corresponding to the inner diameter of the blood vessel is inserted into the central portion of the straight tube.
- the blood vessel model can be manufactured by freezing at a temperature of ⁇ 10 ° C. or lower, thawing, and removing the straight pipe and the core material from the formed body.
- the straight pipe examples include a resin pipe made of a synthetic resin such as polypropylene, hard polyethylene, hard vinyl chloride, acrylic resin, polyester, and polycarbonate, and a glass pipe.
- a resin pipe made of a synthetic resin such as polypropylene, hard polyethylene, hard vinyl chloride, acrylic resin, polyester, and polycarbonate, and a glass pipe.
- the inner diameter of the straight pipe is preferably determined according to the diameter of the blood vessel of the living body.
- Examples of the core material inserted into the straight pipe include a core material made of a synthetic resin such as polypropylene, hard polyethylene, hard vinyl chloride, acrylic resin, polyester, and polycarbonate, a glass core material, and a metal core material.
- a core material made of a synthetic resin such as polypropylene, hard polyethylene, hard vinyl chloride, acrylic resin, polyester, and polycarbonate
- acrylic resin acrylic resin
- polyester polycarbonate
- an opening at one end of the straight pipe with a sealing plug having a through hole for inserting the core material in the center part It is preferable to seal and insert a core material into the through hole.
- the opening at one end of the straight pipe may be sealed with the sealing plug.
- the sealing plug include a rubber plug made of rubber such as silicone rubber and natural rubber, a cork plug, and the like, but the present invention is not limited to such examples.
- the straight pipe in which the opening at one end of the straight pipe is sealed with a sealing plug and the core material is inserted after the mixed solution is injected into the gap between the straight pipe and the core material, in the same manner as described above, it is preferable to seal the opening at the other end of the straight pipe with a sealing plug having a through hole for inserting a core material at the center.
- the straight tube in which the core material is inserted in the center portion, filled with the mixed solution and sealed at both ends with sealing plugs has a temperature of ⁇ 10 ° C. or lower in order to gel the mixed solution. To be frozen.
- the freezing temperature of the mixed solution is preferably ⁇ 10 ° C. or lower, more preferably ⁇ 15 ° C. or lower, and further preferably ⁇ 20 ° C. or lower from the viewpoint of increasing the mechanical strength of the blood vessel model of the present invention. From the viewpoint of increasing the production efficiency of the blood vessel model, it is preferably ⁇ 35 ° C. or higher, more preferably ⁇ 30 ° C. or higher.
- the time for cooling the mixed solution at the above temperature is preferably about 1 to 10 hours, more preferably about 3 to 8 hours, from the viewpoint of increasing the mechanical strength of the blood vessel model of the present invention and increasing the production efficiency thereof. .
- the mixed solution When the mixed solution is cooled at a desired temperature for a desired time, the mixed solution is frozen. At that time, the mixed solution is gelled, so that a molded body containing an aqueous gel and silica particles is formed.
- the molded body thus formed is thawed.
- the molded body may be naturally thawed by being left at room temperature, or may be thawed by heating. Among them, natural thawing is preferable from the viewpoint of increasing energy efficiency.
- the temperature at which the molded body is thawed is not particularly limited, and is usually room temperature to about 40 ° C, preferably about 10 to 40 ° C.
- the blood vessel model of the present invention can be obtained by thawing the molded body in this way.
- the obtained blood vessel model can be used as it is without being dried.
- the blood vessel model may be dried to approximate the blood vessel of a living body. The degree of drying varies depending on the type of blood vessels in the living body and cannot be determined unconditionally, so it is preferable to appropriately adjust according to the type of blood vessels.
- the aqueous gel tissue constituting the blood vessel model can be made uniform.
- the blood vessel model when the blood vessel model is dried by heating the blood vessel model, the blood vessel model can be dried in a drying chamber.
- the temperature of the blood vessel model at the time of drying the blood vessel model is preferably 35 ° C. or higher, more preferably 40 ° C. or higher, from the viewpoint of homogenizing the aqueous gel tissue, and the gel elasticity and flexibility (elasticity) of the blood vessel model. ) Is preferably 80 ° C or lower, more preferably 75 ° C or lower.
- the time for adjusting the temperature of the blood vessel model to the above temperature varies depending on the temperature, it cannot be determined unconditionally. However, from the viewpoint of homogenizing the aqueous gel tissue of the blood vessel model, it is usually 0.5 to 3 hours. It is preferable that it is a grade.
- the blood vessel model After adjusting the temperature of the blood vessel model, the blood vessel model may be cooled to room temperature by cooling.
- the blood vessel model of the present invention is usually manufactured to have the same diameter and inner diameter as a human blood vessel. Therefore, it is usually preferable to adjust the blood vessel model of the present invention so that the outer diameter is about 2 to 5 mm and the inner diameter is about 1 to 3 mm.
- the blood vessel model of the present invention can be used as it is as a blood vessel model, but if necessary, it may be cut to a predetermined length. Furthermore, a blood vessel model having a predetermined diameter and inner diameter can also be produced by making the diameter and inner diameter of the blood vessel model of the present invention larger than the blood vessel of a human body and stretching the blood vessel model.
- the inside of the blood vessel model of the present invention may be left hollow, but if necessary, it can be filled with a liquid similar to blood.
- the blood vessel model can be used as a blood vessel model in which a liquid similar to blood is filled.
- an aneurysm-like blood vessel model that looks like an aneurysm having a diameter of several centimeters, for example, about 3 to 6 cm may be formed between the blood vessel model and the blood vessel model.
- An aneurysm-like blood vessel model has, for example, a predetermined diameter, and the mixed solution is applied to the surface of a balloon-like spherical body that is inflated by introducing air into the inside, and the method for producing a blood vessel model of the present invention is applied. Therefore, it can be manufactured by cold thawing.
- the spherical body in the aneurysm-like blood vessel model can be removed by contraction.
- a hole can be provided in the blood vessel model. This hole can be used as a blood passage by connecting to the internal hole of a straight tubular blood vessel model.
- the aneurysm-like blood vessel model obtained in this way is, for example, a practice blood vessel model in which a stent graft is inserted into an aortic aneurysm, a practice blood vessel model in which an operation is performed by excising the aortic aneurysm and embedding an artificial blood vessel instead. Can be suitably used. An example thereof will be described with reference to the drawings.
- FIG. 2 is a schematic explanatory diagram when performing a procedure practice by inserting a stent graft using a blood vessel model in which an aortic aneurysm is formed according to the present invention.
- FIG. 2 (a) shows a blood vessel model having an aortic aneurysm 2 generated in the aorta 1. If the aortic aneurysm 2 existing in the human body is left as it is, the aortic aneurysm 2 may rupture and may die. Therefore, as shown in FIG. 2 (b), the catheter 4 with the stained graft 3 inserted therein is inserted into the place where the aortic aneurysm 2 exists, and the catheter is inserted at the place where the aortic aneurysm 2 exists. The stent graft 3 is taken out from 4 and the stent graft 3 is expanded in the aorta 1 so as to cover the aortic aneurysm 2.
- aortic aneurysm 2 Since the aortic aneurysm 2 is covered with the stent graft 3 in this way, blood does not flow into the aortic aneurysm 2, so that the aortic aneurysm 2 contracts as shown in FIG. Can be prevented from bursting.
- the blood vessel model of the present invention can be used as a blood vessel model for practice in which a blood vessel portion having an aortic aneurysm is excised and an artificial blood vessel is implanted instead.
- blood vessels before and after the aortic reservoir 2 of the blood vessel model are clamped with forceps, and the blood vessel model having the aortic aneurysm 2 between both forceps is excised, and then a healthy blood vessel shape is formed at the excised portion.
- the blood vessel model is applied, and the both ends of this blood vessel model and the blood vessel model from which the aortic aneurysm 2 has been removed are sutured to complete the treatment practice.
- the blood vessel model of the present invention as a blood vessel model for practicing the procedure of excising a blood vessel portion having an aortic aneurysm and embedding an artificial blood vessel instead, practice for excising the blood vessel having an aneurysm in the aorta In addition, it is possible to practice for suturing the both ends of the resected blood vessel and a healthy blood vessel.
- the cold thawing of the mixed solution is performed once without repeating the cold thawing operation of the mixed solution several times.
- a blood vessel model that has hydrophilic properties such as blood vessels in the human body, its surface is not sticky, and has flexibility (elasticity) and incision feeling like human blood vessels, and has good mechanical strength. Can be obtained efficiently.
- the cold thawing operation may be repeated a plurality of times as necessary.
- the blood vessel model of the present invention has hydrophilicity like a blood vessel of a human body, its surface is not sticky, and has flexibility (elasticity) and a feeling of incision like a blood vessel of a human body.
- the blood vessel model of the present invention containing a crosslinked gel and silica particles has a non-sticky surface, moderate hydrophilicity and flexibility (elasticity), and suitable tensile strength.
- the blood vessel model of the present invention can be suitably used as, for example, a practice blood vessel model for inserting a stent graft into a blood vessel having an aneurysm, and a practice blood vessel model for blood vessel resection / suture operation.
- Example 1 Polyvinyl alcohol having an average degree of polymerization of 1700 and a degree of saponification of about 98 to 99 mol% (manufactured by Kuraray Co., Ltd., trade name: Kuraray Poval PVA-117) is dissolved in water, and the concentration of polyvinyl alcohol is 10 A weight% aqueous polyvinyl alcohol solution was prepared. The resulting polyvinyl alcohol aqueous solution was stirred for 15 minutes while being heated to 80 ° C., and then allowed to cool to room temperature. 500 mL of this aqueous polyvinyl alcohol solution was placed in a 1 L (liter) beaker.
- colloidal silica manufactured by Nissan Chemical Industries, Ltd., trade name: Snowtex XP, silica particle size: about 5 nm, silica content: 5% by weight
- the mixed solution was obtained by stirring the contents in the beaker so as to have a uniform composition.
- a rubber plug made of silicone rubber is inserted into an opening at one end of a straight pipe made of acrylic resin having a diameter of 5 mm, an inner diameter of 4 mm, and a length of 200 mm, and a diameter of 2 mm is inserted into the core material insertion hole provided at the center of the rubber plug.
- a core material made of acrylic resin having a length of 250 mm was inserted.
- the colored mixed solution liquid temperature: 20 ° C.
- the core material was inserted into the core material insertion hole of a silicone rubber rubber plug having a core material insertion hole in the center, and the rubber plug was inserted into the opening of the straight pipe.
- this straight pipe was placed in a freezer (freezer room temperature: ⁇ 20 ° C.), cooled for 5 hours, removed from the freezer, and allowed to stand at room temperature until it reached room temperature.
- this straight pipe was put in a dryer, heated to 60 ° C., held at the same temperature for 10 minutes, then taken out of the dryer and allowed to cool.
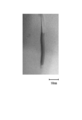
- FIG. 1 is a drawing substitute photograph of the blood vessel model. As shown in FIG. 1, it can be seen that a liquid (black portion in the figure) that approximates blood exists in the obtained blood vessel model, and has a form that approximates a human blood vessel.
- Example 2 polyvinyl alcohol having an average degree of polymerization of 1000 and a degree of saponification of about 98 to 99 mol% [manufactured by Kuraray Co., Ltd., trade name: Kuraray Poval PVA-110] was used as the polyvinyl alcohol.
- a blood vessel model was prepared in the same manner as in Example 1 except that.
- Example 3 polyvinyl alcohol having an average degree of polymerization of 2000 and a degree of saponification of about 98 to 99 mol% (manufactured by Kuraray Co., Ltd., trade name: Kuraray Poval PVA-120) was used as the polyvinyl alcohol.
- a blood vessel model was prepared in the same manner as in Example 1 except that.
- Example 4 A blood vessel model was prepared in the same manner as in Example 1 except that the amount of colloidal silica in Example 1 was changed to 1 mL.
- Example 5 A blood vessel model was produced in the same manner as in Example 1 except that the amount of colloidal silica in Example 1 was changed to 80 mL.
- Example 1 a blood vessel model was produced in the same manner as in Example 1 except that colloidal silica was not used.
- Comparative Example 2 80 g of polyvinyl alcohol powder (average polymerization degree: 1700, saponification degree: 99.0 mol%) and 20 g of polyvinyl alcohol powder (average polymerization degree: 1800, saponification degree: 86 to 90 mol%) are mixed, An alcohol mixture was obtained. The obtained polyvinyl alcohol mixture was dissolved in a mixed solvent of dimethyl sulfoxide and water [dimethyl sulfoxide / water (weight ratio): 80/20] while heating at 120 ° C. to obtain a polyvinyl alcohol solution having a water content of 80% by weight. Prepared.
- Example 1 instead of the mixed solution, the polyvinyl alcohol obtained above was inserted in the gap between the straight pipe and the core material in the same manner as in Example 1 except that the polyvinyl alcohol solution obtained above was used.
- the solution liquid temperature: 45 ° C
- the core into the core material insertion hole of the silicone rubber rubber plug that has the core material insertion hole in the center. The material was passed through and a rubber plug was inserted into the opening of the straight pipe.
- this straight pipe was placed in a freezer (freezer room temperature: ⁇ 20 ° C.), cooled for 6 hours, taken out of the freezer, and allowed to stand at room temperature until it reached room temperature.
- the rubber plugs at both ends of the straight pipe were removed, and the core material was pulled out.
- dimethyl sulfoxide was replaced with ethanol to remove it, and after immersing in 25 ° C. water, the straight tube was taken out of the water and the obtained blood vessel The model was removed from the straight pipe.
- polyvinyl alcohol having an average degree of polymerization of 1700 and a saponification degree of 99.0 mol% and polyvinyl alcohol having an average degree of polymerization of 1800 and a saponification degree of 86 to 90 mol% Mixing at a weight ratio of 20 and dissolving in a mixed solvent of water and dimethyl sulfoxide, and cooling the resulting polyvinyl alcohol to room temperature does not proceed sufficiently to gel, it is understood that a blood vessel model cannot be obtained. .
- Comparative Example 3 In Comparative Example 1, after pouring the polyvinyl alcohol solution into a straight tube made of acrylic resin having a capacity of 200 mL, the temperature for cooling the resin container was changed from room temperature to ⁇ 20 ° C., frozen at this temperature for 24 hours, A gel was prepared in the same manner as in Comparative Example 1 except that the solution was returned to room temperature and thawed. As a result, unlike the comparative example 1, a gel was obtained, but it was confirmed that the obtained gel had low elasticity and its surface was sticky.
- Comparative Example 4 A blood vessel model was prepared by cutting a commercially available silicone rubber tube having a diameter of 2 mm into a length of 20 cm.
- Example 6 In the same manner as in Example 1, two blood vessel models having a diameter of 4 mm, an inner diameter of 2 mm, and a length of 200 mm were produced. In order to seal the intersection after making these two blood vessel models intersect, and providing a hole with a diameter of about 2 mm in each blood vessel model at the intersection, the inside of the two blood vessel models communicates. The mixed solution obtained in Example 1 was applied.
- an opening having a diameter of about 2 mm was provided on the side surface of one of the blood vessel models.
- an aortic aneurysm-like blood vessel model was prepared using the mixed solution obtained in Example 1 as an aneurysm. More specifically, in this aneurysm-like blood vessel model, the mixed solution obtained in Example 1 is applied to the surface of a rubber balloon inflated to a diameter of about 8 mm, and cold thawing is performed in the same manner as in Example 1. An aneurysm-like blood vessel model is prepared by this, and then a needle is inserted into the blood vessel model, the balloon inside is ruptured, the needle is extracted, and the balloon is taken out from the formed opening having a diameter of about 2 mm. A blood vessel model was prepared.
- the opening of the aneurysm-like blood vessel model obtained above and the two blood vessel models obtained above are crossed and joined to the side opening of the integrated blood vessel model to communicate the inside.
- cold thawing is performed in the same manner as in Example 1 to manufacture a blood vessel model having an aneurysm-like blood vessel model. did.
- the obtained blood vessel model was filled with a red acrylic poster color (trade name: Delta Serum Coat, manufactured by Delta) approximating the color of human blood.
- the blood vessel model at that time is shown in FIG.
- FIG. 3 is a drawing substitute photograph of the blood vessel model obtained above. As shown in FIG. 3, a liquid (black portion in the figure) similar to blood exists inside the obtained blood vessel model, and in the blood vessel model that runs in the left-right direction toward the drawing, two blood vessels It can be seen that there is an aneurysm that approximates an aneurysm on the left side of the intersection of the model.
- this blood vessel model was sufficiently used for practicing surgery for implanting an artificial blood vessel in an aneurysm, practicing insertion of a stent graft in an aneurysm, etc. We were able to get a good evaluation that we could expect.
- the blood vessel model of the present invention can be suitably used as, for example, a blood vessel model for inserting a stent graft into an aneurysm and a blood vessel model for practicing resection / suture of blood vessels.
- Example 7 A mixed solvent was prepared by adding 80 mL of dimethyl sulfoxide and 20 mL of water to a 500 mL beaker and mixing well. 20 mL of colloidal silica (manufactured by Nissan Chemical Industries, Ltd., trade name: Snowtex XP, silica particle size: about 5 nm, silica content: 5% by weight) is added to 100 mL of the obtained mixed solvent in the beaker. Then, the contents in the beaker were stirred so as to have a uniform composition.
- colloidal silica manufactured by Nissan Chemical Industries, Ltd., trade name: Snowtex XP, silica particle size: about 5 nm, silica content: 5% by weight
- polyvinyl alcohol having an average polymerization degree of 1700 and a saponification degree of about 98 to 99 mol% manufactured by Kuraray Co., Ltd., trade name: Kuraray Poval PVA-117
- the mixture solution was obtained by stirring for 15 minutes while heating to 80 ° C.
- a rubber plug made of silicone rubber is inserted into an opening at one end of a straight pipe made of acrylic resin having a diameter of 5 mm, an inner diameter of 4 mm, and a length of 200 mm, and a diameter of 2 mm is inserted into the core material insertion hole provided at the center of the rubber plug.
- a core material made of acrylic resin having a length of 250 mm was inserted.
- the colored mixed solution liquid temperature: 45 ° C.
- the core material was inserted into the core material insertion hole of a silicone rubber rubber plug having a core material insertion hole in the center, and the rubber plug was inserted into the opening of the straight pipe.
- this straight pipe was placed in a freezer (freezer room temperature: ⁇ 20 ° C.), cooled for 6 hours, taken out of the freezer, and allowed to stand at room temperature until it reached room temperature.
- the obtained blood vessel model is taken out from the straight tube, and the core material is pulled out from the blood vessel model. Then, the blood vessel model is immersed in water in a container containing 5 L of water at 25 ° C. It was left for 24 hours while replenishing at a flow rate of min, and then removed from the container.
- FIG. 4 is a drawing substitute photograph of the blood vessel model obtained above. As shown in FIG. 4, it can be seen that a liquid (black portion in the figure) that approximates blood exists in the obtained blood vessel model, and has a form that approximates a human blood vessel.
- Example 8 polyvinyl alcohol having an average degree of polymerization of 1000 and a degree of saponification of about 98 to 99 mol% [manufactured by Kuraray Co., Ltd., trade name: Kuraray Poval PVA-110] was used as the polyvinyl alcohol.
- a blood vessel model was prepared in the same manner as in Example 7 except that.
- Example 9 polyvinyl alcohol having an average degree of polymerization of 2000 and a degree of saponification of about 98 to 99 mol% (manufactured by Kuraray Co., Ltd., trade name: Kuraray Poval PVA-120) was used as the polyvinyl alcohol.
- a blood vessel model was prepared in the same manner as in Example 7 except that.
- Example 10 In Example 7, a blood vessel model was prepared in the same manner as in Example 7 except that the amount of colloidal silica was changed to 5 mL.
- Example 11 In Example 7, a blood vessel model was prepared in the same manner as in Example 7 except that the amount of colloidal silica was changed to 50 mL.
- Example 12 a blood vessel model was prepared in the same manner as in Example 7 except that 75 mL of dimethyl sulfoxide and 25 mL of water were used instead of 80 mL of dimethyl sulfoxide and 20 mL of water.
- Example 13 a blood vessel model was prepared in the same manner as in Example 7 except that 85 mL of dimethyl sulfoxide and 15 mL of water were used instead of 80 mL of dimethyl sulfoxide and 20 mL of water.
- Example 7 a blood vessel model was prepared in the same manner as in Example 7 except that colloidal silica was not used.
- Comparative Example 6 80 g of polyvinyl alcohol powder (average polymerization degree: 1700, saponification degree: 99.0 mol%) and 20 g of polyvinyl alcohol powder (average polymerization degree: 1800, saponification degree: 86 to 90 mol%) are mixed, An alcohol mixed solution was obtained. The obtained polyvinyl alcohol mixed solution was dissolved in a mixed solvent of dimethyl sulfoxide and water [dimethyl sulfoxide / water (weight ratio): 80/20] while heating at 120 ° C., and a polyvinyl alcohol solution having a water content of 80% by weight. Was prepared.
- Example 7 the polyvinyl alcohol obtained above was inserted into the gap between the straight pipe and the core in the same manner as in Example 7 except that the polyvinyl alcohol solution obtained above was used instead of the mixed solution.
- the solution liquid temperature: 45 ° C
- the material was passed through and a rubber plug was inserted into the opening of the straight pipe.
- this straight pipe was placed in a freezer (freezer room temperature: ⁇ 20 ° C.), cooled for 6 hours, taken out of the freezer, and allowed to stand at room temperature until it reached room temperature.
- the rubber plugs at both ends of the straight pipe were removed, and the core material was pulled out.
- dimethyl sulfoxide was replaced with ethanol to remove it, and after immersing in 25 ° C. water, the straight tube was taken out of the water and the obtained blood vessel The model was removed from the straight pipe.
- polyvinyl alcohol having an average degree of polymerization of 1700 and a saponification degree of 99.0 mol% and polyvinyl alcohol having an average degree of polymerization of 1800 and a saponification degree of 86 to 90 mol% Mixing at a weight ratio of 20 and dissolving in a mixed solvent of water and dimethyl sulfoxide, and cooling the resulting polyvinyl alcohol to room temperature does not proceed sufficiently to gel, it is understood that a blood vessel model cannot be obtained. .
- Comparative Example 7 In Comparative Example 5, after pouring the polyvinyl alcohol solution into a straight tube made of acrylic resin having a capacity of 200 mL, the temperature for cooling the resin container was changed from room temperature to ⁇ 20 ° C., frozen at this temperature for 24 hours, A blood vessel model was produced in the same manner as in Comparative Example 5 except that the sample was returned to room temperature and thawed. As a result, unlike the comparative example 5, a gel-like blood vessel model was obtained. However, it was confirmed that the obtained blood vessel model had small flexibility (elasticity) and the surface thereof was sticky.
- each of the blood vessel models obtained in each example is excellent in transparency, has moderate hydrophilicity and flexibility, has a non-sticky surface, and has a high tensile strength. I understand.
- Example 14 In the same manner as in Example 7, two blood vessel models having a diameter of 4 mm, an inner diameter of 2 mm, and a length of 200 mm were produced. In order to seal the intersection after making these two blood vessel models intersect, and providing a hole with a diameter of about 2 mm in each blood vessel model at the intersection, the inside of the two blood vessel models communicates. The mixed solution obtained in Example 7 was applied.
- an opening having a diameter of about 2 mm was provided on the side surface of one of the blood vessel models.
- an aneurysm-like blood vessel model was prepared using the mixed solution obtained in Example 7 as an aneurysm. More specifically, in this aneurysm-like blood vessel model, the mixed aqueous solution obtained in Example 7 is applied to the surface of a rubber balloon inflated to a diameter of about 8 mm, and cold thawing is performed in the same manner as in Example 7. An aneurysm-like blood vessel model is prepared by this, and then a needle is inserted into the blood vessel model, the balloon inside is ruptured, the needle is extracted, and the balloon is taken out from the formed opening having a diameter of about 2 mm. A blood vessel model was prepared.
- the opening of the aneurysm-like blood vessel model obtained above and the two blood vessel models obtained above are crossed and joined to the side opening of the integrated blood vessel model to communicate the inside.
- cold thawing is performed in the same manner as in Example 7 to manufacture a blood vessel model having an aneurysm-like blood vessel model. did.
- the obtained blood vessel model was filled with a red acrylic poster color (trade name: Delta Serum Coat, manufactured by Delta) approximating the color of human blood.
- the blood vessel model at that time is shown in FIG.
- FIG. 5 is a drawing substitute photograph of the blood vessel model obtained above. As shown in FIG. 5, in the obtained blood vessel model, there is a liquid (black portion in the drawing) that approximates blood, and in the blood vessel model that runs vertically toward the drawing, two blood vessels It can be seen that there is an aneurysm that resembles an aneurysm at the top of the intersection of the model.
- the blood vessel model of the present invention can be suitably used, for example, as a blood vessel model for practicing insertion of a stent graft into an aneurysm, a blood vessel model for practicing blood vessel resection / suture operation, and the like. .
Landscapes
- Health & Medical Sciences (AREA)
- Engineering & Computer Science (AREA)
- General Physics & Mathematics (AREA)
- Physics & Mathematics (AREA)
- General Health & Medical Sciences (AREA)
- Medicinal Chemistry (AREA)
- Chemical & Material Sciences (AREA)
- Educational Technology (AREA)
- Business, Economics & Management (AREA)
- Algebra (AREA)
- Computational Mathematics (AREA)
- Medical Informatics (AREA)
- Mathematical Analysis (AREA)
- Mathematical Optimization (AREA)
- Mathematical Physics (AREA)
- Pure & Applied Mathematics (AREA)
- Theoretical Computer Science (AREA)
- Educational Administration (AREA)
- Pulmonology (AREA)
- Life Sciences & Earth Sciences (AREA)
- Biomedical Technology (AREA)
- Veterinary Medicine (AREA)
- Public Health (AREA)
- Animal Behavior & Ethology (AREA)
- Radiology & Medical Imaging (AREA)
- Dermatology (AREA)
- Epidemiology (AREA)
- Pharmacology & Pharmacy (AREA)
- Neurosurgery (AREA)
- Gastroenterology & Hepatology (AREA)
- Cardiology (AREA)
- Oral & Maxillofacial Surgery (AREA)
- Transplantation (AREA)
- Heart & Thoracic Surgery (AREA)
- Vascular Medicine (AREA)
- Instructional Devices (AREA)
- Prostheses (AREA)
- Materials For Medical Uses (AREA)
Priority Applications (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US13/392,177 US8758797B2 (en) | 2009-09-30 | 2010-09-09 | PVA and silica particle blood vessel model |
| EP10820320.9A EP2485207B1 (en) | 2009-09-30 | 2010-09-09 | Blood vessel model |
| US14/272,624 US9202389B2 (en) | 2009-09-30 | 2014-05-08 | Blood vessel model comprising polyvinyl alcohol and silica particles |
Applications Claiming Priority (4)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2009228305A JP4790055B2 (ja) | 2009-05-29 | 2009-09-30 | 動脈瘤に対するステントグラフトの挿入練習用または血管の切除・縫合手術練習用の血管モデル |
| JP2009-228296 | 2009-09-30 | ||
| JP2009228296A JP4841663B2 (ja) | 2009-09-30 | 2009-09-30 | 動脈瘤に対するステントグラフトの挿入練習用または血管の切除・縫合手術練習用の血管モデル |
| JP2009-228305 | 2009-09-30 |
Related Child Applications (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US13/392,177 A-371-Of-International US8758797B2 (en) | 2009-09-30 | 2010-09-09 | PVA and silica particle blood vessel model |
| US14/272,624 Division US9202389B2 (en) | 2009-09-30 | 2014-05-08 | Blood vessel model comprising polyvinyl alcohol and silica particles |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2011040200A1 true WO2011040200A1 (ja) | 2011-04-07 |
Family
ID=43826028
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/JP2010/065473 Ceased WO2011040200A1 (ja) | 2009-09-30 | 2010-09-09 | 血管モデル |
Country Status (4)
| Country | Link |
|---|---|
| US (2) | US8758797B2 (enExample) |
| EP (1) | EP2485207B1 (enExample) |
| JP (1) | JP4841663B2 (enExample) |
| WO (1) | WO2011040200A1 (enExample) |
Cited By (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN103284765A (zh) * | 2012-02-28 | 2013-09-11 | 常州奥斯特医疗器械有限公司 | 一种动脉瘤血管模型及其制作方法 |
| US20150170547A1 (en) * | 2011-10-21 | 2015-06-18 | Nitta Casings Inc. | Collagen-polysaccharide materials mimicking blood vessels, tissues and bones for medical, pharmaceutical and orthopedic applications, and processes for producing the same |
| CN105448170A (zh) * | 2014-08-19 | 2016-03-30 | 中山大学附属第三医院 | 一种内含树状管道结构的仿体模型 |
| CN110491231A (zh) * | 2019-08-22 | 2019-11-22 | 潍坊医学院 | 一种血管内支架植入的生物力学实验模拟装置 |
| CN110640950A (zh) * | 2019-09-24 | 2020-01-03 | 中国人民解放军东部战区总医院 | 一种个体化肠瘘支架制作方法及其适用模具 |
| JP2021067794A (ja) * | 2019-10-23 | 2021-04-30 | 国立大学法人横浜国立大学 | 生体モデル |
Families Citing this family (13)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP6120504B2 (ja) * | 2012-08-06 | 2017-04-26 | 有限会社湘南化成 | 管状モデルの製造方法、血管モデル、血管モデルシミュレータ及び成形型 |
| WO2016109879A1 (en) | 2015-01-06 | 2016-07-14 | The Hospital For Sick Children | Simulator for practicing trans-oral surgery and method of use thereof |
| US10510268B2 (en) | 2016-04-05 | 2019-12-17 | Synaptive Medical (Barbados) Inc. | Multi-metric surgery simulator and methods |
| CN106141593B (zh) * | 2016-08-11 | 2019-06-25 | 成都嘉宝祥生物科技有限公司 | 一种分叉血管模型制作方法 |
| CN107049486B (zh) * | 2017-03-29 | 2019-04-26 | 广州博敏科技有限公司 | 动脉瘤血管模型及其制备方法和应用 |
| US10692402B2 (en) * | 2017-05-05 | 2020-06-23 | Synaptive Medical (Barbados) Inc. | Simulated fibrous tissue for surgical training |
| WO2019004374A1 (ja) | 2017-06-28 | 2019-01-03 | デンカ株式会社 | 止血を含む手技を練習するために用いられる潰瘍模型 |
| CN109674558B (zh) * | 2019-03-08 | 2021-03-16 | 北京工业大学 | 一种基于等离子电晕法制备高度光滑和透明主动脉根部的方法 |
| EP4083969A4 (en) | 2019-12-23 | 2023-06-07 | Denka Company Limited | MUCOUS TISSUE MODEL |
| TWI787902B (zh) * | 2020-12-08 | 2022-12-21 | 日商泰爾茂股份有限公司 | 手法模擬器及模擬方法 |
| CN113838353A (zh) * | 2021-08-29 | 2021-12-24 | 北京工业大学 | 一种高透明的弹性脑动脉瘤模型的制备方法 |
| CN115536965A (zh) * | 2022-09-27 | 2022-12-30 | 中新巨成医学科技有限公司 | 一种生物仿真材料及其制备方法和用途 |
| CN117304539B (zh) * | 2023-11-30 | 2024-02-09 | 上海汇禾医疗器械有限公司 | 一种血管模型、其制备方法和栓塞剂体外模拟测试装置 |
Citations (9)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPH0226567A (ja) * | 1988-07-15 | 1990-01-29 | Nippon Oil Co Ltd | ハイパーサーミア用ファントム |
| JPH0527776Y2 (enExample) | 1988-04-01 | 1993-07-15 | ||
| JPH064768Y2 (ja) | 1989-07-10 | 1994-02-09 | ホシザキ電機株式会社 | 貯氷式陳列台 |
| JPH11167342A (ja) | 1997-12-05 | 1999-06-22 | Koken Co Ltd | 動物実験手技訓練用動物血管モデル |
| JP2005195696A (ja) | 2003-12-26 | 2005-07-21 | Colin Medical Technology Corp | 血管模型セット |
| JP2006126686A (ja) | 2004-11-01 | 2006-05-18 | Crosswell:Kk | 血管モデル |
| JP2007316343A (ja) | 2006-05-25 | 2007-12-06 | Univ Waseda | 手技訓練用血管モデル |
| JP2007316434A (ja) * | 2006-05-26 | 2007-12-06 | Tohoku Techno Arch Co Ltd | 生体模型のための粘膜材 |
| JP2008261990A (ja) | 2007-04-11 | 2008-10-30 | Kobe Univ | 手術訓練用脳モデル |
Family Cites Families (19)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US3592936A (en) | 1969-04-25 | 1971-07-13 | Merck & Co Inc | Method of treatment using pharmaceutical composition containing dimethyl sulfoxide |
| US3738957A (en) * | 1971-03-18 | 1973-06-12 | Du Pont | Coacervates of polyvinyl alcohol and colloidal silica |
| ATE49725T1 (de) | 1983-09-28 | 1990-02-15 | Idemitsu Petrochemical Co | Verfahren zur herstellung von filmen oder folien aus thermoplastischem kunststoff. |
| JPS62230609A (ja) * | 1986-04-01 | 1987-10-09 | Asahi Glass Co Ltd | シリカ系粒子 |
| CA2058416C (en) | 1990-04-05 | 1998-11-24 | Shigeki Takada | Process for suspension polymerization of vinyl compound |
| JPH0462010A (ja) | 1990-06-23 | 1992-02-27 | Bando Chem Ind Ltd | 袋状中空体の成形用マンドレルおよび加硫装置 |
| US5266224A (en) | 1991-05-24 | 1993-11-30 | Zirconium Technology Corporation | Borate cross-linking solutions |
| JP2541332Y2 (ja) | 1991-09-12 | 1997-07-16 | 株式会社高研 | 装着型注射採血手技練習模型 |
| JPH064768U (ja) | 1992-05-13 | 1994-01-21 | 株式会社高研 | 注射採血輸液手技練習模型 |
| JPH062010A (ja) | 1992-06-18 | 1994-01-11 | Kobe Steel Ltd | 粉末成形体の焼結方法 |
| JP2683992B2 (ja) | 1993-01-29 | 1997-12-03 | 博 田中 | 成形用型およびその型を用いた成形方法 |
| US5977021A (en) | 1996-12-24 | 1999-11-02 | Oji Paper Co., Ltd | Heat-sensitive recording adhesive sheet |
| JP2000080126A (ja) * | 1998-09-02 | 2000-03-21 | Fujikura Ltd | ホウ素架橋ポリビニルアルコール成形物およびその製造方法 |
| US7857626B2 (en) | 2000-10-23 | 2010-12-28 | Toly Christopher C | Medical physiological simulator including a conductive elastomer layer |
| EP1848332A4 (en) | 2005-02-03 | 2011-11-02 | Christopher Sakezles | MODELS AND METHODS USING THESE MODELS FOR TESTING MEDICAL DEVICES |
| US7521434B2 (en) | 2005-06-27 | 2009-04-21 | Luromed Llc | Cross-linked gels of hyaluronic acid with hydrophobic polymers and processes for making them |
| KR20090058516A (ko) | 2006-08-08 | 2009-06-09 | 클라리언트 파이넌스 (비브이아이)리미티드 | 광학 증백제 수용액 |
| US8361503B2 (en) | 2007-03-02 | 2013-01-29 | University of Pittsburgh—of the Commonwealth System of Higher Education | Extracellular matrix-derived gels and related methods |
| JP4448153B2 (ja) | 2007-03-27 | 2010-04-07 | 村中医療器株式会社 | 脳神経外科手術のトレーニング装置、この装置に用いられる脳モデル及びモデル主体 |
-
2009
- 2009-09-30 JP JP2009228296A patent/JP4841663B2/ja active Active
-
2010
- 2010-09-09 US US13/392,177 patent/US8758797B2/en active Active
- 2010-09-09 WO PCT/JP2010/065473 patent/WO2011040200A1/ja not_active Ceased
- 2010-09-09 EP EP10820320.9A patent/EP2485207B1/en active Active
-
2014
- 2014-05-08 US US14/272,624 patent/US9202389B2/en active Active
Patent Citations (9)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPH0527776Y2 (enExample) | 1988-04-01 | 1993-07-15 | ||
| JPH0226567A (ja) * | 1988-07-15 | 1990-01-29 | Nippon Oil Co Ltd | ハイパーサーミア用ファントム |
| JPH064768Y2 (ja) | 1989-07-10 | 1994-02-09 | ホシザキ電機株式会社 | 貯氷式陳列台 |
| JPH11167342A (ja) | 1997-12-05 | 1999-06-22 | Koken Co Ltd | 動物実験手技訓練用動物血管モデル |
| JP2005195696A (ja) | 2003-12-26 | 2005-07-21 | Colin Medical Technology Corp | 血管模型セット |
| JP2006126686A (ja) | 2004-11-01 | 2006-05-18 | Crosswell:Kk | 血管モデル |
| JP2007316343A (ja) | 2006-05-25 | 2007-12-06 | Univ Waseda | 手技訓練用血管モデル |
| JP2007316434A (ja) * | 2006-05-26 | 2007-12-06 | Tohoku Techno Arch Co Ltd | 生体模型のための粘膜材 |
| JP2008261990A (ja) | 2007-04-11 | 2008-10-30 | Kobe Univ | 手術訓練用脳モデル |
Non-Patent Citations (1)
| Title |
|---|
| See also references of EP2485207A4 |
Cited By (9)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20150170547A1 (en) * | 2011-10-21 | 2015-06-18 | Nitta Casings Inc. | Collagen-polysaccharide materials mimicking blood vessels, tissues and bones for medical, pharmaceutical and orthopedic applications, and processes for producing the same |
| CN103284765A (zh) * | 2012-02-28 | 2013-09-11 | 常州奥斯特医疗器械有限公司 | 一种动脉瘤血管模型及其制作方法 |
| CN103284765B (zh) * | 2012-02-28 | 2016-02-24 | 常州奥斯特医疗器械有限公司 | 一种动脉瘤血管模型及其制作方法 |
| CN105448170A (zh) * | 2014-08-19 | 2016-03-30 | 中山大学附属第三医院 | 一种内含树状管道结构的仿体模型 |
| CN105448170B (zh) * | 2014-08-19 | 2019-10-08 | 中山大学附属第三医院 | 一种内含树状管道结构的仿体模型 |
| CN110491231A (zh) * | 2019-08-22 | 2019-11-22 | 潍坊医学院 | 一种血管内支架植入的生物力学实验模拟装置 |
| CN110640950A (zh) * | 2019-09-24 | 2020-01-03 | 中国人民解放军东部战区总医院 | 一种个体化肠瘘支架制作方法及其适用模具 |
| JP2021067794A (ja) * | 2019-10-23 | 2021-04-30 | 国立大学法人横浜国立大学 | 生体モデル |
| JP7416375B2 (ja) | 2019-10-23 | 2024-01-17 | 国立大学法人横浜国立大学 | 生体モデル |
Also Published As
| Publication number | Publication date |
|---|---|
| JP2011075907A (ja) | 2011-04-14 |
| US9202389B2 (en) | 2015-12-01 |
| EP2485207B1 (en) | 2015-08-26 |
| US8758797B2 (en) | 2014-06-24 |
| EP2485207A4 (en) | 2013-05-01 |
| EP2485207A1 (en) | 2012-08-08 |
| US20120156666A1 (en) | 2012-06-21 |
| US20140246798A1 (en) | 2014-09-04 |
| JP4841663B2 (ja) | 2011-12-21 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP4841663B2 (ja) | 動脈瘤に対するステントグラフトの挿入練習用または血管の切除・縫合手術練習用の血管モデル | |
| JP4790055B2 (ja) | 動脈瘤に対するステントグラフトの挿入練習用または血管の切除・縫合手術練習用の血管モデル | |
| US20210125524A1 (en) | Organ model | |
| JP4993519B2 (ja) | 手術練習用または手術用切除具の切れ味の確認用の臓器モデル | |
| JP4993518B2 (ja) | 手術練習用または手術用切除具の切れ味の確認用の臓器モデル | |
| JP6055069B1 (ja) | 臓器、組織又は器官モデル | |
| EP3253315B1 (en) | Synthetic tissue structures for electrosurgical training and simulation | |
| JP4675414B2 (ja) | 臓器モデル | |
| JP2011022522A (ja) | 皮膚モデル | |
| JP5745155B1 (ja) | 臓器組織質感モデル | |
| JP7365020B2 (ja) | ゲルを有する物体の製造法 | |
| JP2015194708A (ja) | 生体臓器模型用水性ゲル組成物および生体臓器模型 | |
| WO2016047329A1 (ja) | 生体臓器模型用水性ゲル組成物および生体臓器模型 | |
| JP2010262119A (ja) | 骨モデル | |
| EP3300057B1 (en) | Use of a simulated animal organ | |
| JP4993516B2 (ja) | 手技練習用シート | |
| JP4993517B2 (ja) | 刃物の切れ味検査用シート |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 10820320 Country of ref document: EP Kind code of ref document: A1 |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 13392177 Country of ref document: US |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2010820320 Country of ref document: EP |