WO2009087918A1 - 銀微粉、銀インクおよび銀塗料ならびにそれらの製造法 - Google Patents
銀微粉、銀インクおよび銀塗料ならびにそれらの製造法 Download PDFInfo
- Publication number
- WO2009087918A1 WO2009087918A1 PCT/JP2008/073623 JP2008073623W WO2009087918A1 WO 2009087918 A1 WO2009087918 A1 WO 2009087918A1 JP 2008073623 W JP2008073623 W JP 2008073623W WO 2009087918 A1 WO2009087918 A1 WO 2009087918A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- silver
- particles
- hexylamine
- organic medium
- fine powder
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Ceased
Links
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09C—TREATMENT OF INORGANIC MATERIALS, OTHER THAN FIBROUS FILLERS, TO ENHANCE THEIR PIGMENTING OR FILLING PROPERTIES ; PREPARATION OF CARBON BLACK ; PREPARATION OF INORGANIC MATERIALS WHICH ARE NO SINGLE CHEMICAL COMPOUNDS AND WHICH ARE MAINLY USED AS PIGMENTS OR FILLERS
- C09C1/00—Treatment of specific inorganic materials other than fibrous fillers; Preparation of carbon black
- C09C1/62—Metallic pigments or fillers
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B22—CASTING; POWDER METALLURGY
- B22F—WORKING METALLIC POWDER; MANUFACTURE OF ARTICLES FROM METALLIC POWDER; MAKING METALLIC POWDER; APPARATUS OR DEVICES SPECIALLY ADAPTED FOR METALLIC POWDER
- B22F1/00—Metallic powder; Treatment of metallic powder, e.g. to facilitate working or to improve properties
- B22F1/05—Metallic powder characterised by the size or surface area of the particles
- B22F1/054—Nanosized particles
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B22—CASTING; POWDER METALLURGY
- B22F—WORKING METALLIC POWDER; MANUFACTURE OF ARTICLES FROM METALLIC POWDER; MAKING METALLIC POWDER; APPARATUS OR DEVICES SPECIALLY ADAPTED FOR METALLIC POWDER
- B22F1/00—Metallic powder; Treatment of metallic powder, e.g. to facilitate working or to improve properties
- B22F1/05—Metallic powder characterised by the size or surface area of the particles
- B22F1/054—Nanosized particles
- B22F1/0545—Dispersions or suspensions of nanosized particles
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B22—CASTING; POWDER METALLURGY
- B22F—WORKING METALLIC POWDER; MANUFACTURE OF ARTICLES FROM METALLIC POWDER; MAKING METALLIC POWDER; APPARATUS OR DEVICES SPECIALLY ADAPTED FOR METALLIC POWDER
- B22F9/00—Making metallic powder or suspensions thereof
- B22F9/16—Making metallic powder or suspensions thereof using chemical processes
- B22F9/18—Making metallic powder or suspensions thereof using chemical processes with reduction of metal compounds
- B22F9/24—Making metallic powder or suspensions thereof using chemical processes with reduction of metal compounds starting from liquid metal compounds, e.g. solutions
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B82—NANOTECHNOLOGY
- B82Y—SPECIFIC USES OR APPLICATIONS OF NANOSTRUCTURES; MEASUREMENT OR ANALYSIS OF NANOSTRUCTURES; MANUFACTURE OR TREATMENT OF NANOSTRUCTURES
- B82Y30/00—Nanotechnology for materials or surface science, e.g. nanocomposites
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09D—COATING COMPOSITIONS, e.g. PAINTS, VARNISHES OR LACQUERS; FILLING PASTES; CHEMICAL PAINT OR INK REMOVERS; INKS; CORRECTING FLUIDS; WOODSTAINS; PASTES OR SOLIDS FOR COLOURING OR PRINTING; USE OF MATERIALS THEREFOR
- C09D11/00—Inks
- C09D11/52—Electrically conductive inks
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09D—COATING COMPOSITIONS, e.g. PAINTS, VARNISHES OR LACQUERS; FILLING PASTES; CHEMICAL PAINT OR INK REMOVERS; INKS; CORRECTING FLUIDS; WOODSTAINS; PASTES OR SOLIDS FOR COLOURING OR PRINTING; USE OF MATERIALS THEREFOR
- C09D5/00—Coating compositions, e.g. paints, varnishes or lacquers, characterised by their physical nature or the effects produced; Filling pastes
- C09D5/24—Electrically-conducting paints
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01B—CABLES; CONDUCTORS; INSULATORS; SELECTION OF MATERIALS FOR THEIR CONDUCTIVE, INSULATING OR DIELECTRIC PROPERTIES
- H01B1/00—Conductors or conductive bodies characterised by the conductive materials; Selection of materials as conductors
- H01B1/20—Conductive material dispersed in non-conductive organic material
- H01B1/22—Conductive material dispersed in non-conductive organic material the conductive material comprising metals or alloys
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B22—CASTING; POWDER METALLURGY
- B22F—WORKING METALLIC POWDER; MANUFACTURE OF ARTICLES FROM METALLIC POWDER; MAKING METALLIC POWDER; APPARATUS OR DEVICES SPECIALLY ADAPTED FOR METALLIC POWDER
- B22F2998/00—Supplementary information concerning processes or compositions relating to powder metallurgy
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B22—CASTING; POWDER METALLURGY
- B22F—WORKING METALLIC POWDER; MANUFACTURE OF ARTICLES FROM METALLIC POWDER; MAKING METALLIC POWDER; APPARATUS OR DEVICES SPECIALLY ADAPTED FOR METALLIC POWDER
- B22F2999/00—Aspects linked to processes or compositions used in powder metallurgy
-
- C—CHEMISTRY; METALLURGY
- C01—INORGANIC CHEMISTRY
- C01P—INDEXING SCHEME RELATING TO STRUCTURAL AND PHYSICAL ASPECTS OF SOLID INORGANIC COMPOUNDS
- C01P2002/00—Crystal-structural characteristics
- C01P2002/80—Crystal-structural characteristics defined by measured data other than those specified in group C01P2002/70
- C01P2002/88—Crystal-structural characteristics defined by measured data other than those specified in group C01P2002/70 by thermal analysis data, e.g. TGA, DTA, DSC
-
- C—CHEMISTRY; METALLURGY
- C01—INORGANIC CHEMISTRY
- C01P—INDEXING SCHEME RELATING TO STRUCTURAL AND PHYSICAL ASPECTS OF SOLID INORGANIC COMPOUNDS
- C01P2004/00—Particle morphology
- C01P2004/01—Particle morphology depicted by an image
- C01P2004/04—Particle morphology depicted by an image obtained by TEM, STEM, STM or AFM
-
- C—CHEMISTRY; METALLURGY
- C01—INORGANIC CHEMISTRY
- C01P—INDEXING SCHEME RELATING TO STRUCTURAL AND PHYSICAL ASPECTS OF SOLID INORGANIC COMPOUNDS
- C01P2004/00—Particle morphology
- C01P2004/60—Particles characterised by their size
- C01P2004/64—Nanometer sized, i.e. from 1-100 nanometer
-
- C—CHEMISTRY; METALLURGY
- C01—INORGANIC CHEMISTRY
- C01P—INDEXING SCHEME RELATING TO STRUCTURAL AND PHYSICAL ASPECTS OF SOLID INORGANIC COMPOUNDS
- C01P2006/00—Physical properties of inorganic compounds
- C01P2006/40—Electric properties
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y10—TECHNICAL SUBJECTS COVERED BY FORMER USPC
- Y10T—TECHNICAL SUBJECTS COVERED BY FORMER US CLASSIFICATION
- Y10T428/00—Stock material or miscellaneous articles
- Y10T428/29—Coated or structually defined flake, particle, cell, strand, strand portion, rod, filament, macroscopic fiber or mass thereof
- Y10T428/2982—Particulate matter [e.g., sphere, flake, etc.]
- Y10T428/2991—Coated
Definitions
- the present invention relates to a silver fine powder composed of silver nanoparticles coated with an organic substance, a silver ink using the silver fine powder, a silver paint, and a method for producing them.
- nanoparticle means a particle having a particle diameter of about 40 nm or less
- fine powder means a powder composed of nanoparticles.
- Metal fine powder is highly active, and since sintering proceeds even at low temperatures, it has long been noted as a patterning material for materials with low heat resistance. In recent years, in particular, due to advances in nanotechnology, it has become possible to manufacture single nanoclass particles relatively easily.
- Patent Document 1 discloses a method for synthesizing a large amount of silver nanoparticles using an amine compound using silver oxide as a starting material.
- Patent Document 2 discloses a method of synthesizing silver nanoparticles by mixing and melting an amine and a silver compound raw material.
- Non-Patent Document 1 describes making a paste using silver nanoparticles.
- Patent Document 4 discloses a technique for producing silver nanoparticles having extremely good dispersibility in a liquid.
- Patent Document 3 discloses a polar solvent in which an organic protective material B having a functional group such as a mercapto group having a good affinity for metal particles is dissolved in a nonpolar solvent in which metal nanoparticles protected by the organic protective material A are present. Is added, and a method of exchanging the protective material for metal nanoparticles from A to B by stirring and mixing is disclosed.
- JP 2006-219693 A International Publication No. 04/012884 Pamphlet JP 2006-89786 A Japanese Patent Laid-Open No. 2007-39718 Nakami Masami et al., “Application of Silver Nanoparticles to Conductive Pastes”, Chemical Industry, Chemical Industry, October 2005, p.749-754
- the surface of the metal fine powder is generally covered with an organic protective material.
- This protective material has a role of separating the particles from each other during the silver particle synthesis reaction. Therefore, it is advantageous to select one having a molecular weight that is somewhat large. If the molecular weight is small, the distance between particles becomes narrow, and in a wet synthesis reaction, sintering may progress during the reaction. If it becomes so, particle
- organic protective materials having a large molecular weight are generally difficult to volatilize and remove even when heated.
- a highly conductive sintered body (wiring) is constructed unless exposed to a high temperature of 250 ° C. or higher. It is difficult. For this reason, the types of applicable substrates are limited to some materials having a high heat-resistant temperature, such as polyimide, glass, and aramid.
- the organic protective material has a relatively large molecular weight
- the organic protective material has an effect of facilitating the sintering of metallic silver particles in a thin film drawn with ink or paint containing the silver particles. This is thought to be because the organic protective material itself tends to oxidize and decompose during firing due to having an unsaturated bond in the molecule of the organic protective material, and detachment from the metallic silver particles occurs relatively easily.
- the conductive film can be formed even by low-temperature baking at about 180 ° C.
- a metal fine powder that can be sintered at a low temperature of about 100 to 180 ° C., preferably about 100 to 150 ° C. can be produced by a simple method, its application will inevitably expand.
- transparent polycarbonate it becomes possible to draw fine wiring directly on the surface of a medium such as a CD or DVD or a lens, and various functions can be imparted.
- An inexpensive antenna in which fine wiring is drawn on a PET (polyethylene terephthalate) substrate, an IC tag using paper as a material, and the like are also feasible.
- Patent Document 3 discloses a technique for replacing a protective material covering the surface of metal particles with another protective material.
- this technique employs a method of obtaining metal particles covered with a protective material by dropping a reducing agent into a solvent in which the metal supply substance and the protective material are dissolved at the stage of synthesizing the metal nanoparticles. Is.
- the reaction in which the reducing agent is dropped into the solvent in this way it is necessary to use a reducing agent having strong reducing properties because the reducing agent itself is diluted with the solvent, and even if the liquid is stirred, it is completely uniform. It is not easy to deposit metal nanoparticles with a good reducing power.
- the reducing agent component is likely to be mixed into the particles.
- Patent Document 3 shows an example in which an organic compound having a molecular weight as small as about 100, such as naphthenic acid or octylamine, is used as a protective material formed at the particle synthesis stage. No specific technique for synthesizing protected metal nanoparticles is shown. The metal nanoparticles having a small molecular weight of the protective material tend to aggregate and settle in the liquid medium. Actually, in the invention of Patent Document 3, a process of precipitating and collecting metal nanoparticle aggregates at the synthesis stage is essential.
- Patent Document 3 is further applied to industrial implementation in that it is difficult to control a uniform reduction reaction and the particles are likely to aggregate and settle (dispersibility is not so good). Improvement is desired.
- the present invention is intended to provide a silver fine powder coated with a protective material capable of significantly reducing the sintering temperature as compared with the prior art, a silver ink using the same, and a silver paint by a simple method.
- hexylamine (C 6 H 13 —NH 2 ) adsorbed on the surface has an average particle diameter D TEM : 3 to 20 nm or an X-ray crystal particle diameter D X : 1 to 20 nm.
- Silver fine powder composed of silver particles is provided.
- distributed this silver fine powder in the liquid organic medium S is provided.
- aromatic hydrocarbons are suitable, and examples thereof include decalin (C 10 H 18 ).
- the silver coating material formed by mixing this silver fine powder with an organic medium is provided.
- This silver paint has the property of forming a conductive film having a specific resistance of 25 ⁇ ⁇ cm or less when a coating film coated with the silver paint is baked at 120 ° C. in the atmosphere.
- a conductive film having a specific resistance of 25 ⁇ ⁇ cm or less can be obtained even when baked at 100 ° C.
- average particle diameter D TEM 3 to 20 nm or X-ray crystal grain diameter D coated with primary amine A having a molecular weight of 200 to 400 having an unsaturated bond X :
- a production method including a step of generating precipitated particles (precipitation step) and a step of recovering the precipitated particles as a solid content by solid-liquid separation operation (solid-liquid separation step).
- the recovered solid content is composed of low-temperature sinterable silver fine powder.
- the “sedimented particles” are particles that settle when the stirring of the liquid is stopped. Since the liquid is stirred during the sedimentation step, many of the precipitated particles are floating in the liquid.
- the silver ink of the present invention is a step of washing the solid content (silver fine powder) collected as described above (washing step), and a step of dispersing the solid content after washing in the liquid organic medium S (inking step). It can manufacture by the method which has this. Furthermore, the low-temperature sinterable silver paint of the present invention can be applied by mixing the solid content (silver fine powder) collected as described above (cleaning step), and mixing the solid content after cleaning with an organic medium. It can be manufactured by a method having a process of forming properties (painting process).
- the condition that the specific resistance of the fired film is evaluated to be 20 ⁇ ⁇ cm or less when the sample to be measured is fired at 200 ° C. in the atmosphere is applied to 120 ° C. firing or 100 ° C. firing, and the 120 ° C. fired film or 100 ° C.
- the conductivity of the fired film is evaluated. That is, the conditions for preparing, applying, firing and measuring the paint are the same as those when the specific resistance is 20 ⁇ ⁇ cm or less after firing at 200 ° C.
- the specific resistance of the baked film at 100 ° C. or the baked film at 100 ° C. is measured. If it is a technique (a publicly known general technique) that can confirm that sintering has occurred by firing at 200 ° C., the presence or absence of sintering can be determined even when applied to 120 ° C. firing or 100 ° C. firing.
- the silver fine powder or silver paint which cannot find the conditions under which a fired film having a specific resistance of 20 ⁇ ⁇ cm or less is originally formed by firing at 200 ° C. in the atmosphere is out of the scope of the present invention.
- hexylamine is adsorbed on the surface means that the surface of metallic silver is coated with a protective material formed by adsorbing hexylamine molecules on the surface, and metallic silver of individual particles. It means a state where the particles can exist as independent particles without being combined.
- the silver fine powder composed of such silver nanoparticles has the property of becoming a conductive film having a specific resistance of 25 ⁇ ⁇ cm or less when fired at 120 ° C. in the atmosphere as described above, other impurities may be used.
- Organic substances for example, an amine A component such as oleylamine may be contained.
- a silver fine powder that can be sintered at a firing temperature as low as 120 ° C., and a silver ink and a silver paint using the same are realized.
- the firing temperature is lowered to about 100 ° C., it is difficult for sintering failure to occur, so the degree of freedom in controlling the firing temperature is expanded as compared with the conventional case.
- the silver fine powder, silver ink and silver paint of the present invention can be produced relatively easily, and it is considered that industrial implementation is sufficiently possible.
- grains used for the silver coating material in the example of n 6 of Example 1.
- the silver fine powder having excellent low-temperature sinterability according to the present invention is characterized in that silver particles as its constituent elements are coated with an organic protective material that adsorbs hexylamine.
- an organic compound having a function as a surfactant has an R—X structure having a hydrophobic group R and a hydrophilic group X.
- the hydrophobic group R is typically an alkyl group in which hydrogen is bonded to the carbon skeleton.
- hydrophilic groups X can also be used as an organic protective material for protecting the active outermost surface of the metal silver particles.
- the hydrophilic group X is bonded to the surface of the metallic silver, and the hydrophobic group R is oriented toward the outside of the particles covered with the organic protective material.
- metal nanoparticles are extremely active, normally, the surface of the particles cannot be stably present unless they are covered with a protective material.
- a protective material in order to impart conductivity to the thin film drawn with the silver nanoparticle paint, it is necessary that the silver particles of the silver particles sinter at as low a temperature as possible.
- the particle surface protective material In addition to being extremely fine such as D TEM of 20 nm or less, the particle surface protective material must be easily detached from the particle surface during volatilization and removed by volatilization.
- silver nanoparticles are synthesized by a “wet process”, which is advantageous for mass production compared to synthesis from the gas phase, if silver particles coated directly with a low molecular weight amine are produced during synthesis, they are dispersed by aggregation or the like. It is difficult to obtain silver fine powder having good properties, and it tends to hinder the operation of preparing a paint through a process such as washing after the synthesis reaction. Therefore, in the present invention, silver nanoparticles having a good dispersibility coated with amine A having a molecular weight of 200 to 400 are obtained in advance, and thereafter, amine A is replaced with amine B having a low molecular weight, whereby organic protection of amine B is achieved. Silver nanoparticles coated with the material are obtained.
- hexylamine (C 6 H 13 —NH 2 , molecular weight 101.2) is applied in the present invention.
- silver nanoparticles adsorbed with octylamine (C 8 H 17 —NH 2 ), which is a primary amine having 8 carbon atoms, can be sufficiently sintered at a firing temperature of 120 ° C.
- the resulting property is exhibited, when the temperature reaches about 100 ° C., the resistance of the conductive film tends to increase rapidly. For this reason, for example, when a low firing temperature condition of 120 ° C. is employed, it is difficult to stably obtain a desired conductive film unless temperature control is strict.
- a primary amine having an unsaturated bond and having a molecular weight of 200 to 400 is adopted as amine A.
- Such a primary amine is suitable as a protective material to be present when silver particles are synthesized by a wet process. Since amine has a weak coordinating force on the surface of silver particles, desorption from the surface of silver particles is relatively angry, and replacement with hexylamine is easy. However, if the molecular weight is excessively large, smooth desorption may be hindered. In addition, due to the presence of unsaturated bonds, even if the molecular weight is somewhat large, such as 200 to 400, it exhibits a liquid state at around room temperature, so there is no need for heating in the subsequent precipitation step or solid-liquid separation step. There is an easy advantage.
- the heating during the process causes the particles to sinter, which becomes an obstacle to the production of high-quality paints and inks.
- oleylamine is very suitable due to the ease of silver particle synthesis.
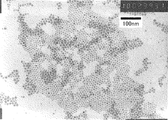
- the particle size of the silver particles coated with the organic protective material can be represented by the average particle diameter D TEM or X-ray crystal particle diameter D X is measured from the image of the TEM (transmission electron microscope).
- D TEM silver particles having a DTEM of 3 to 20 nm or silver particles having an X-ray crystal grain size D X of 1 to 20 nm are preferable targets.
- a silver fine powder having such a particle size range is advantageous for producing inks and paints having good characteristics.
- D TEM is 6 ⁇ 20 nm
- the silver particles having a particle size of about 4 ⁇ 20 nm D X is the easily synthesized by methods described below.
- D TEM is 3 ⁇ 7 nm
- very fine silver particles D X of about 1 ⁇ 5 nm, for example oleylamine can be synthesized by such method of reducing the direct silver compound as the solvent.
- impurities are likely to be mixed into the crystal grain boundaries of the synthesized metallic silver, and if the amount of impurities increases, pores are generated when firing fine wiring, and good conductivity cannot be secured, or migration resistance Inconvenience that is inferior to that.
- silver particles having a single crystallinity represented by D TEM / D X of 2.5 or less are desirable, and 2.0 or less is even more preferable.
- Silver particles using hexylamine as a protective material are more likely to settle in a liquid medium than those coated with an organic protective material having a high molecular weight.
- an appropriate liquid organic medium S it is preferable to use an appropriate liquid organic medium S.
- “silver ink” exhibiting dispersibility was obtained.
- aromatic hydrocarbons are suitable.
- good dispersibility can be obtained in cyclohexane, toluene, cumene, diethylbenzene, tetralin, decalin, and the like.
- decalin C 10 H 18
- This silver fine powder excellent in low-temperature sinterability can be obtained as follows.
- Synthesis of silver particles It is important that the silver nanoparticle raw material used in the present invention has a stable particle property such as a particle size distribution and has a property of being difficult to aggregate and settle in a liquid medium.
- the synthesis method disclosed in Patent Document 4 will be briefly described. That is, in this synthesis method, silver particles are precipitated by reducing the silver compound in alcohol or polyol using alcohol or polyol as a reducing agent.
- alcohol or polyol is a solvent and a reducing agent.
- the reduction reaction can proceed by raising the temperature of the solvent solution, preferably by bringing it to a reflux state.
- amine A examples include oleylamine.
- the amount of the primary amine A that coexists in the solvent during the reduction reaction can be 0.1 to 20 equivalents relative to silver, more preferably 1.0 to 15 equivalents, and 2.0 to 10 equivalents. Is more preferable.
- 1 mol of amine corresponds to 1 equivalent per 1 mol of silver. If the amount of primary amine used is too small, the amount of protective material on the surface of the silver particles is insufficient, and monodispersion in the liquid cannot be realized. If the amount is too large, there is a possibility that the reaction of replacing amine A with hexylamine in the subsequent step cannot be performed efficiently.
- the reducing agent As the reducing agent, alcohol or polyol as a solvent is used. In the reaction, it is efficient to perform a reflux operation. Therefore, the boiling point of the alcohol or polyol is preferably low, specifically 80 to 300 ° C., preferably 80 to 200 ° C., more preferably 80 to 150 ° C.
- Various materials disclosed in Patent Document 4 and the like can be used, among which isobutanol and n-butanol are preferable.
- a reduction auxiliary agent may be added.
- a reduction adjuvant should just select 1 or more types from what is indicated by patent documents 4, it is especially preferred to use diethanolamine and triethanolamine among these.
- the silver compound which is a supply source of silver various compounds can be applied as long as they can be dissolved in the above solvent, and examples thereof include silver chloride, silver nitrate, silver oxide, and silver carbonate. preferable.
- the concentration of Ag ions in the solution during the reduction reaction can be 0.05 mol / L or more, preferably 0.05 to 5.0 mol / L.
- the amine A / Ag molar ratio can be in the range of 0.05 to 5.0.
- the molar ratio of reducing aid / Ag can be in the range of 0.1-20.
- the temperature of the reduction reaction is preferably in the range of 50 to 200 ° C.
- a temperature of 50 to 150 ° C. is more preferable, and a range of 60 to 140 ° C. is more preferable.
- Silver particles covered with amine A (synthesized by the above reduction) have a ratio of amine A to the total of silver particles and amine A (hereinafter simply referred to as “amine A ratio”) of 0.05 to 25 mass. It is desirable to adjust to%. If the amine A ratio is too low, particles are likely to aggregate. On the contrary, if the amine A ratio is high, there is a possibility that the reaction of replacing amine A with amine B in the subsequent step cannot be performed efficiently.
- the silver particles covered with amine A are synthesized, for example, by a reduction reaction in a wet process as described above, and then subjected to solid-liquid separation and washing. Then, it mixes with a liquid organic medium and makes a dispersion liquid.
- a liquid organic medium a substance in which silver particles covered with amine A are well dispersed is selected.
- a hydrocarbon system can be suitably used.
- aliphatic hydrocarbons such as hexane, heptane, octane, nonane, decane, undecane, dodecane, tridecane, and tetradecane can be used. Petroleum solvents such as kerosene may be used.
- One or more of these substances may be used to form a liquid organic medium.
- a silver particle dispersion in which silver particles coated with amine A are monodispersed.
- “monodispersed” means that individual silver particles exist in a liquid medium in a state where they can move independently without aggregating with each other.
- a liquid containing silver particles is subjected to a solid-liquid separation operation by centrifugation, a liquid (supernatant) in which the particles remain dispersed can be adopted as the silver particle dispersion here. it can.
- the replacement reaction proceeds at about 5 ° C. or higher, but if the reaction is conducted at a low liquid temperature, a part of amine A tends to remain adsorbed on the surface of the metallic silver. That is, a protective material in which a large amount of impurity amine A is present in hexylamine is likely to be formed. In this case, the dispersibility in the aromatic organic compound is lowered, which is disadvantageous in producing an inexpensive liquid ink using the aromatic organic compound as a dispersion medium. Therefore, the replacement with hexylamine is preferably performed at 20 ° C. or higher, and more preferably performed at 50 ° C. or higher. However, if the temperature is raised too much, inadvertent sintering may occur, so the temperature is preferably 80 ° C. or lower, and more preferably 70 ° C. or lower.
- the effect of the “floating ring” by the high molecular weight amine A gradually decreases, and amine A still remains.
- the particles will settle even when the When the precipitated particles are deposited on the bottom of the reaction vessel, the particles cannot be “enclosed by hexylamine”, and the replacement reaction is less likely to proceed. Therefore, in the present invention, the liquid is stirred during the replacement reaction. However, it is not necessary to stir too strongly. It is sufficient if the particles to which amine A is still attached can be exposed to the “enclosed state by hexylamine”. Therefore, it is desirable to give a stirring force that does not allow sedimentation particles to accumulate on the bottom of the reaction vessel.
- hexylamine to be mixed is sufficient to realize an “enclosed state by hexylamine”. It is desirable to add a considerably large molar ratio to the amount of amine A present as a protective material before mixing. Specifically, in the equivalent ratio (hexylamine / Ag) to Ag existing as silver particles before mixing, it is desirable to mix 1 equivalent or more of hexylamine, although it depends on the liquid volume. In the experiments so far, good results have been obtained with a hexylamine / Ag equivalent ratio of about 2 to 20 equivalents. In addition, 1 mol of hexylamine corresponds to 1 equivalent with respect to 1 mol of Ag.
- amine A is oleylamine, for example, isopropanol can be suitably added.
- Solid-liquid separation As described above, since the particles after the replacement reaction are settled, the particles after the replacement reaction (precipitation step) can be recovered as a solid content by solid-liquid separation of the liquid after the completion of the reaction. Centrifugation is desirable as the solid-liquid separation.
- the obtained solid content is mainly composed of silver nanoparticles coated with an organic protective film composed of hexylamine. Thus, the silver fine powder of the present invention is obtained.
- the solid content is desirably washed using a solvent such as alcohol.
- the solid content obtained after the solid-liquid separation after one or more washing operations is used for the paint.
- the silver content of the present invention is obtained by mixing the solid content after washing (silver fine powder in which the protective material is replaced with hexylamine) and an appropriate liquid organic medium S and dispersing the silver fine powder in the liquid organic medium S. Ink is obtained.
- hexylamine has a low molecular weight, its ability as a “floating ring” is inherently small, but by using an appropriate liquid organic medium S, a good dispersion state can be realized.
- aromatic hydrocarbons are relatively effective.
- decalin can be exemplified as a suitable target.
- the silver paint of the present invention is obtained by mixing the solid content after washing (silver fine powder with the protective material replaced with hexylamine) and an appropriate organic medium so as to be coated. It is important that the organic medium to be mixed here is one that is easy to volatilize and remove at a temperature of about 120 ° C.
- ⁇ Comparative Example 1 As a reference, a silver paint was prepared using silver fine powder synthesized by the alcohol reduction method disclosed in Patent Document 4 and the like, and the specific resistance of the fired film fired at firing temperatures of 200 ° C. and 120 ° C. was examined.
- the silver fine powder is such that the surface of each particle is covered with an organic protective material made of amine A (here, oleylamine). Specifically, the experiment was conducted as follows.
- Tetradecane was prepared as a liquid organic medium. The solid component after washing was mixed and dispersed therein, and solid-liquid separation was performed for 30 minutes with a centrifuge, and the separated liquid was recovered. In this liquid, silver particles covered with amine A (oleylamine) are monodispersed.
- This silver particle dispersion was observed with a transmission electron microscope (TEM) to obtain an average particle diameter DTEM . That is, among the particles observed at a magnification of 600,000 by TEM (JEM-2010 manufactured by JEOL Ltd.), the particle diameters of 300 independent silver particles that did not overlap were measured, and the average particle diameter was calculated. . As a result, D TEM was 8.5 nm. In this example, as will be described later, since this silver particle dispersion is used for the silver paint, Table 1 shows the D TEM values.
- the coating amount of amine A (oleylamine) on the silver particles in this silver particle dispersion was 8.0% by mass as a result of measurement by the method disclosed in Japanese Patent Application No. 2007-233501.
- TG-DTA measurement of protective material TG-DTA measurement at a heating rate of 10 ° C./min was performed on the solid content after washing (in a wet state) obtained according to the above “synthesis of silver particles”.
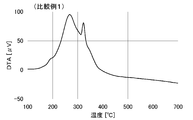
- the DTA curve is shown in FIG. In FIG. 1, a large peak between 200 and 300 ° C. and a peak between 300 and 330 ° C. are considered to be attributed to oleylamine, which is amine A.
- ⁇ is the X-ray wavelength of the Cu—K ⁇ ray
- ⁇ is the half width of the diffraction peak
- ⁇ is the Bragg angle of the diffraction line.
- a silver paint using silver particles coated with a protective material made of amine A was prepared.
- the viscosity of the silver paint dispersion was measured with a rotary viscometer (RE550L manufactured by Toki Sangyo Co., Ltd.), the viscosity was 5.8 mPa ⁇ s.
- the silver concentration in this silver particle dispersion was 60% by mass as measured using a TG-DTA apparatus. Since this silver particle dispersion was judged to have properties that can be applied as an ink, it was decided to use this as it is as a silver paint.
- a coating film was formed by coating the silver paint on a glass substrate by spin coating.
- the substrate on which the coating film has been formed is first pre-baked on a hot plate at 60 ° C. for 30 minutes in the air, and then held at 200 ° C. for 1 hour in the air on the hot plate to obtain a “200 ° C. baking film”. It was. Similarly, after pre-baking at 60 ° C., it was held on a 120 ° C. hot plate for 1 hour to obtain a “120 ° C. baking film”.
- the specific resistance of the 200 ° C. fired film is greatly reduced. It can be said that ending occurs.
- the 120 ° C. fired film was not recognized as having conductivity. Therefore, it can be said that the sintering of silver particles sufficient to impart conductivity is not occurring under the condition of 120 ° C. ⁇ 1 hour.
- Comparative Example 2 Silver nanoparticles were synthesized according to “Synthesis of silver particles” described in Comparative Example 1 to obtain a silver particle dispersion in which silver particles coated with amine A (oleylamine) were monodispersed in tetradecane. In Comparative Example 2, octylamine was adopted as the amine B to be substituted.
- amine A oleylamine
- octylamine-substituted particles 107.8 g of octylamine (C 8 H 17 —NH 2 , a special grade manufactured by Wako Pure Chemical Industries, Ltd.) as a reagent was prepared. This is an amount that is 10.0 equivalents to Ag. Further, 100.3 g of isopropyl alcohol (special grade manufactured by Wako Pure Chemical Industries, Ltd.) was prepared for the purpose of promoting the reaction, and octylamine and isopropyl alcohol were mixed in a glass container.
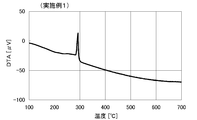
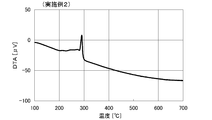
- TG-DTA measurement of protective material For the solid content after washing, TG-DTA measurement was performed in the same manner as in Comparative Example 1. The DTA curve is shown in FIG. From the comparison between before substitution (FIG. 1) and after substitution (FIG. 2), after substitution, the peak seen in FIG. 1 disappeared and a new peak was observed. From this, it is considered that the protective material was changed from amine A (oleylamine) to octylamine.
- the coating film was formed by apply
- hexylamine-substituted particles As the amine B, 84.4 g of hexylamine (C 6 H 13 —NH 2 , a special grade manufactured by Wako Pure Chemical Industries, Ltd.) as a reagent was prepared. This is an amount that is 10.0 equivalents to Ag. Further, 100.3 g of isopropyl alcohol (special grade made by Wako Pure Chemical Industries, Ltd.) was prepared for the purpose of promoting the reaction, and hexylamine and isopropyl alcohol were mixed.
- Solid-liquid separation and washing 405.3 g of methanol (corresponding to twice the mass of the reaction solution) was added to the above reaction solution. This was added to promote sedimentation. The liquid was subjected to solid-liquid separation by centrifugation for 5 minutes. The obtained solid content was recovered, and 80.1 g of methanol was further added to the solid content, and ultrasonic dispersion was performed for 30 minutes. Thereafter, the solid content was recovered by solid-liquid separation by centrifugation for 5 minutes.
- a coating film was formed in the same manner as in Comparative Example 2.
- Example 2 Silver nanoparticles were synthesized according to “Synthesis of silver particles” described in Comparative Example 1 to obtain a silver particle dispersion in which silver particles coated with amine A (oleylamine) were monodispersed in tetradecane.
- amine A oleylamine
- hexylamine was employed in the same manner as in Example 1.
- the time dependence of the low-temperature sintering property was confirmed using a silver particle dispersion in which silver particles covered with hexylamine B are dispersed.
- hexylamine-substituted particles As amine B, 42.2 g of hexylamine (C 6 H 13 —NH 2 , a special grade manufactured by Wako Pure Chemical Industries, Ltd.) as a reagent was prepared. This is an amount that gives 5.0 equivalents to Ag. Further, 50.1 g of isopropyl alcohol (special grade manufactured by Wako Pure Chemical Industries, Ltd.) was prepared for the purpose of promoting the reaction, and hexylamine and isopropyl alcohol were mixed.
- Decalin was prepared as a liquid organic medium. The solid component after washing was mixed and dispersed therein, and solid-liquid separation was performed for 30 minutes with a centrifuge, and the separated liquid was recovered. In this liquid, silver particles covered with amine A are monodispersed.
- TG-DTA measurement of protective material For the solid content after washing, TG-DTA measurement was performed in the same manner as in Comparative Example 1. The DTA curve is shown in FIG. From the comparison between before replacement (FIG. 1) and after replacement (FIG. 4), the peak seen in FIG. 1 disappeared after replacement, and a new peak was observed. From this, it is considered that the protective material was changed from amine A (oleylamine) to hexylamine. Further, the heat loss at this time was 3.3%.
- a coating film was formed by the spin coat method in the same manner as in Comparative Example 1.
- a fired film was further formed by the following method.
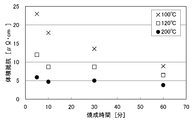
- the substrate on which the coating film was formed was baked on the hot plate at 200 ° C., 120 ° C., and 100 ° C. in the air.
- pre-baking is not performed, and by changing the baking time (holding time at the above temperature) to 5, 10, 30, 60 minutes, “200 ° C. fired film” and “120 ° C. baking at each holding time” Films "and" 100 ° C fired film "were obtained.
Landscapes
- Chemical & Material Sciences (AREA)
- Engineering & Computer Science (AREA)
- Nanotechnology (AREA)
- Materials Engineering (AREA)
- Organic Chemistry (AREA)
- Physics & Mathematics (AREA)
- Life Sciences & Earth Sciences (AREA)
- Wood Science & Technology (AREA)
- Dispersion Chemistry (AREA)
- Inorganic Chemistry (AREA)
- Spectroscopy & Molecular Physics (AREA)
- General Chemical & Material Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Composite Materials (AREA)
- Condensed Matter Physics & Semiconductors (AREA)
- General Physics & Mathematics (AREA)
- Crystallography & Structural Chemistry (AREA)
- Powder Metallurgy (AREA)
- Manufacture Of Metal Powder And Suspensions Thereof (AREA)
- Conductive Materials (AREA)
- Pigments, Carbon Blacks, Or Wood Stains (AREA)
- Paints Or Removers (AREA)
- Inks, Pencil-Leads, Or Crayons (AREA)
- Non-Insulated Conductors (AREA)
Priority Applications (4)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US12/811,583 US8916068B2 (en) | 2008-01-06 | 2008-12-25 | Silver micropowder, silver ink, silver coating, and methods for production of these materials |
| KR1020107013493A KR101526233B1 (ko) | 2008-01-06 | 2008-12-25 | 은 미분, 은 잉크 및 은 도료 및 그 제조법 |
| CN200880124131.3A CN101909786B (zh) | 2008-01-06 | 2008-12-25 | 银微粉、银油墨及银涂料以及它们的制造方法 |
| EP20080869325 EP2233230B1 (en) | 2008-01-06 | 2008-12-25 | Silver micropowder, silver ink, silver coating, and methods for production of these materials |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2008000220A JP5371247B2 (ja) | 2008-01-06 | 2008-01-06 | 銀塗料およびその製造法 |
| JP2008-000220 | 2008-01-06 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2009087918A1 true WO2009087918A1 (ja) | 2009-07-16 |
Family
ID=40853042
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/JP2008/073623 Ceased WO2009087918A1 (ja) | 2008-01-06 | 2008-12-25 | 銀微粉、銀インクおよび銀塗料ならびにそれらの製造法 |
Country Status (7)
| Country | Link |
|---|---|
| US (1) | US8916068B2 (enExample) |
| EP (1) | EP2233230B1 (enExample) |
| JP (1) | JP5371247B2 (enExample) |
| KR (1) | KR101526233B1 (enExample) |
| CN (1) | CN101909786B (enExample) |
| TW (1) | TWI389751B (enExample) |
| WO (1) | WO2009087918A1 (enExample) |
Cited By (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20110305821A1 (en) * | 2010-06-09 | 2011-12-15 | Xerox Corporation | Silver nanoparticle composition comprising solvents with specific hansen solubility parameters |
| WO2012043440A1 (ja) * | 2010-09-29 | 2012-04-05 | トッパン・フォームズ株式会社 | 銀インク組成物 |
| CN102816462A (zh) * | 2012-08-13 | 2012-12-12 | 中国科学院宁波材料技术与工程研究所 | 一种表面包覆着有机保护剂的纳米银颗粒的制备方法 |
| JP2013079430A (ja) * | 2011-10-05 | 2013-05-02 | Nippon Synthetic Chem Ind Co Ltd:The | 金属複合超微粒子の製造方法 |
| JP2013079431A (ja) * | 2011-10-05 | 2013-05-02 | Nippon Synthetic Chem Ind Co Ltd:The | 金属複合超微粒子の製造方法 |
| US10941304B2 (en) | 2016-04-04 | 2021-03-09 | Nichia Corporation | Metal powder sintering paste and method of producing the same, and method of producing conductive material |
Families Citing this family (34)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP5275342B2 (ja) | 2007-05-11 | 2013-08-28 | エスディーシー マテリアルズ インコーポレイテッド | 粒子生産システム及び粒子生産方法 |
| US8575059B1 (en) | 2007-10-15 | 2013-11-05 | SDCmaterials, Inc. | Method and system for forming plug and play metal compound catalysts |
| JP5246096B2 (ja) * | 2009-08-10 | 2013-07-24 | 日立電線株式会社 | 複合金属微粒子材料、金属膜及び金属膜の製造方法、並びにプリント配線板及び電線ケーブル |
| US9126191B2 (en) | 2009-12-15 | 2015-09-08 | SDCmaterials, Inc. | Advanced catalysts for automotive applications |
| US8652992B2 (en) | 2009-12-15 | 2014-02-18 | SDCmaterials, Inc. | Pinning and affixing nano-active material |
| DE112010004973T5 (de) * | 2009-12-22 | 2013-02-28 | Dic Corp. | Leitpaste für Siebdruckverfahren |
| US8673049B2 (en) | 2010-08-27 | 2014-03-18 | Dowa Electronics Materials Co., Ltd. | Low-temperature sintered silver nanoparticle composition and electronic articles formed using the same |
| JP5623861B2 (ja) | 2010-10-14 | 2014-11-12 | 株式会社東芝 | 金属ナノ粒子分散組成物 |
| US8669202B2 (en) | 2011-02-23 | 2014-03-11 | SDCmaterials, Inc. | Wet chemical and plasma methods of forming stable PtPd catalysts |
| US20120288697A1 (en) * | 2011-05-13 | 2012-11-15 | Xerox Corporation | Coating methods using silver nanoparticles |
| EP2812139B1 (en) * | 2012-02-10 | 2017-12-27 | Lockheed Martin Corporation | Nanoparticle paste formulations and methods for production and use thereof |
| JP5850784B2 (ja) * | 2012-03-29 | 2016-02-03 | Dowaエレクトロニクス株式会社 | ブースターアンテナおよびその製造方法 |
| JP5991067B2 (ja) * | 2012-08-02 | 2016-09-14 | 東ソー株式会社 | 耐溶剤性透明導電膜用塗工液及びこれよりなる耐溶剤性透明導電膜 |
| WO2014030310A1 (ja) * | 2012-08-23 | 2014-02-27 | バンドー化学株式会社 | 導電性ペースト |
| CN108907178B (zh) * | 2012-10-29 | 2020-12-15 | 阿尔法组装解决方案公司 | 烧结粉末 |
| US9156025B2 (en) | 2012-11-21 | 2015-10-13 | SDCmaterials, Inc. | Three-way catalytic converter using nanoparticles |
| US9511352B2 (en) | 2012-11-21 | 2016-12-06 | SDCmaterials, Inc. | Three-way catalytic converter using nanoparticles |
| CN103194117B (zh) * | 2013-04-08 | 2014-12-03 | 电子科技大学 | 一种免烧结超细银纳米油墨的制备方法及其应用 |
| CN103194118A (zh) * | 2013-04-23 | 2013-07-10 | 电子科技大学 | 一种免烧结超细银纳米油墨的制备方法及其应用 |
| JP6099472B2 (ja) * | 2013-04-26 | 2017-03-22 | Dowaエレクトロニクス株式会社 | 金属ナノ粒子分散体、金属ナノ粒子分散体の製造方法および接合方法 |
| CN105592921A (zh) | 2013-07-25 | 2016-05-18 | Sdc材料公司 | 用于催化转化器的洗涂层和经涂覆基底及其制造和使用方法 |
| WO2015061477A1 (en) | 2013-10-22 | 2015-04-30 | SDCmaterials, Inc. | Catalyst design for heavy-duty diesel combustion engines |
| EP3068517A4 (en) | 2013-10-22 | 2017-07-05 | SDCMaterials, Inc. | Compositions of lean nox trap |
| TWI634165B (zh) | 2014-02-13 | 2018-09-01 | 日商大阪曹達股份有限公司 | 金屬奈米微粒子的製造方法 |
| US9687811B2 (en) | 2014-03-21 | 2017-06-27 | SDCmaterials, Inc. | Compositions for passive NOx adsorption (PNA) systems and methods of making and using same |
| CN104087023B (zh) * | 2014-07-02 | 2015-11-25 | 广东新劲刚新材料科技股份有限公司 | 一种表面包银核壳复合粒子的表面处理方法及其在制备导电与电磁屏蔽复合材料中的应用 |
| JP6282616B2 (ja) * | 2014-07-30 | 2018-02-21 | Dowaエレクトロニクス株式会社 | 銀粉およびその製造方法 |
| WO2016033526A1 (en) * | 2014-08-29 | 2016-03-03 | SDCmaterials, Inc. | Composition comprising nanoparticles with desired sintering and melting point temperatures and methods of making thereof |
| JP6657573B2 (ja) * | 2015-03-06 | 2020-03-04 | コニカミノルタ株式会社 | 導電性インク |
| US11236248B2 (en) | 2016-03-04 | 2022-02-01 | Hp Indigo B.V. | Metallic pigment particles |
| CN109562448B (zh) * | 2016-08-10 | 2021-01-22 | 阪东化学株式会社 | 金属银微粒子的制造方法 |
| WO2018180286A1 (ja) * | 2017-03-31 | 2018-10-04 | 富士フイルム株式会社 | 金被覆銀平板状粒子、金被覆銀平板状粒子分散液及びその製造方法、塗布膜、並びに、反射防止光学部材 |
| JP7710990B2 (ja) | 2019-03-15 | 2025-07-22 | ベーアーエスエフ・エスエー | ブチルメトキシジベンゾイルメタン、トリアジン誘導体及び光安定剤を含む日焼け止め組成物 |
| JP7490629B2 (ja) * | 2020-12-22 | 2024-05-27 | 花王株式会社 | 金属微粒子含有インク |
Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2006169613A (ja) * | 2004-12-20 | 2006-06-29 | Ulvac Japan Ltd | 金属薄膜の形成方法及び金属薄膜 |
| JP2006219693A (ja) * | 2005-02-08 | 2006-08-24 | Harima Chem Inc | 金属銀微粒子の製造方法 |
| JP2007046072A (ja) * | 2005-08-05 | 2007-02-22 | Dowa Holdings Co Ltd | 銀粒子粉末の製造法 |
| JP2007138250A (ja) * | 2005-11-18 | 2007-06-07 | Mitsubishi Materials Corp | 銀粒子の製造方法及び得られた該銀粒子を含有する銀粒子含有組成物並びにその用途 |
Family Cites Families (10)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP3646784B2 (ja) * | 2000-03-31 | 2005-05-11 | セイコーエプソン株式会社 | 薄膜パタ−ンの製造方法および微細構造体 |
| TWI242478B (en) | 2002-08-01 | 2005-11-01 | Masami Nakamoto | Metal nanoparticle and process for producing the same |
| JP2006089786A (ja) | 2004-09-22 | 2006-04-06 | Mitsuboshi Belting Ltd | 極性溶媒に分散した金属ナノ粒子の製造方法 |
| JP4660780B2 (ja) * | 2005-03-01 | 2011-03-30 | Dowaエレクトロニクス株式会社 | 銀粒子粉末の製造方法 |
| JP5151476B2 (ja) * | 2005-04-12 | 2013-02-27 | 旭硝子株式会社 | インク組成物及び金属質材料 |
| KR100690360B1 (ko) * | 2005-05-23 | 2007-03-09 | 삼성전기주식회사 | 도전성 잉크, 그 제조방법 및 도전성 기판 |
| JP4674375B2 (ja) * | 2005-08-01 | 2011-04-20 | Dowaエレクトロニクス株式会社 | 銀粒子粉末の製造法 |
| JP5139659B2 (ja) * | 2006-09-27 | 2013-02-06 | Dowaエレクトロニクス株式会社 | 銀粒子複合粉末およびその製造法 |
| US7737497B2 (en) * | 2007-11-29 | 2010-06-15 | Xerox Corporation | Silver nanoparticle compositions |
| JP5226293B2 (ja) * | 2007-12-25 | 2013-07-03 | Dowaエレクトロニクス株式会社 | 銀導電膜の製造方法 |
-
2008
- 2008-01-06 JP JP2008000220A patent/JP5371247B2/ja active Active
- 2008-12-25 US US12/811,583 patent/US8916068B2/en not_active Expired - Fee Related
- 2008-12-25 EP EP20080869325 patent/EP2233230B1/en not_active Not-in-force
- 2008-12-25 WO PCT/JP2008/073623 patent/WO2009087918A1/ja not_active Ceased
- 2008-12-25 CN CN200880124131.3A patent/CN101909786B/zh not_active Expired - Fee Related
- 2008-12-25 KR KR1020107013493A patent/KR101526233B1/ko active Active
-
2009
- 2009-01-06 TW TW98100148A patent/TWI389751B/zh active
Patent Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2006169613A (ja) * | 2004-12-20 | 2006-06-29 | Ulvac Japan Ltd | 金属薄膜の形成方法及び金属薄膜 |
| JP2006219693A (ja) * | 2005-02-08 | 2006-08-24 | Harima Chem Inc | 金属銀微粒子の製造方法 |
| JP2007046072A (ja) * | 2005-08-05 | 2007-02-22 | Dowa Holdings Co Ltd | 銀粒子粉末の製造法 |
| JP2007138250A (ja) * | 2005-11-18 | 2007-06-07 | Mitsubishi Materials Corp | 銀粒子の製造方法及び得られた該銀粒子を含有する銀粒子含有組成物並びにその用途 |
Non-Patent Citations (1)
| Title |
|---|
| See also references of EP2233230A4 * |
Cited By (9)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20110305821A1 (en) * | 2010-06-09 | 2011-12-15 | Xerox Corporation | Silver nanoparticle composition comprising solvents with specific hansen solubility parameters |
| US8765025B2 (en) * | 2010-06-09 | 2014-07-01 | Xerox Corporation | Silver nanoparticle composition comprising solvents with specific hansen solubility parameters |
| WO2012043440A1 (ja) * | 2010-09-29 | 2012-04-05 | トッパン・フォームズ株式会社 | 銀インク組成物 |
| JP2013079430A (ja) * | 2011-10-05 | 2013-05-02 | Nippon Synthetic Chem Ind Co Ltd:The | 金属複合超微粒子の製造方法 |
| JP2013079431A (ja) * | 2011-10-05 | 2013-05-02 | Nippon Synthetic Chem Ind Co Ltd:The | 金属複合超微粒子の製造方法 |
| CN102816462A (zh) * | 2012-08-13 | 2012-12-12 | 中国科学院宁波材料技术与工程研究所 | 一种表面包覆着有机保护剂的纳米银颗粒的制备方法 |
| US10941304B2 (en) | 2016-04-04 | 2021-03-09 | Nichia Corporation | Metal powder sintering paste and method of producing the same, and method of producing conductive material |
| US11634596B2 (en) | 2016-04-04 | 2023-04-25 | Nichia Corporation | Metal powder sintering paste and method of producing the same, and method of producing conductive material |
| US12125607B2 (en) | 2016-04-04 | 2024-10-22 | Nichia Corporation | Metal powder sintering paste and method of producing the same, and method of producing conductive material |
Also Published As
| Publication number | Publication date |
|---|---|
| US8916068B2 (en) | 2014-12-23 |
| EP2233230B1 (en) | 2014-04-16 |
| KR101526233B1 (ko) | 2015-06-05 |
| CN101909786A (zh) | 2010-12-08 |
| KR20100112555A (ko) | 2010-10-19 |
| JP5371247B2 (ja) | 2013-12-18 |
| JP2009161808A (ja) | 2009-07-23 |
| TWI389751B (zh) | 2013-03-21 |
| US20100283013A1 (en) | 2010-11-11 |
| EP2233230A1 (en) | 2010-09-29 |
| TW200936276A (en) | 2009-09-01 |
| EP2233230A4 (en) | 2012-02-01 |
| CN101909786B (zh) | 2014-01-08 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP5371247B2 (ja) | 銀塗料およびその製造法 | |
| JP4294705B2 (ja) | 有機物質で被覆された銀微粉の製法および銀微粉 | |
| CN101583449B (zh) | 银微粉及其制造方法以及油墨 | |
| JP5213420B2 (ja) | 液中分散性および耐食性に優れた銅粉並びにその製造法 | |
| JP5274000B2 (ja) | 低温焼結性銀微粉および銀塗料ならびにそれらの製造法 | |
| JP4674375B2 (ja) | 銀粒子粉末の製造法 | |
| JP5162383B2 (ja) | 銀被覆銅微粉の製造方法 | |
| JP4897624B2 (ja) | 低温焼結性銀微粉および銀塗料ならびにそれらの製造法 | |
| JP5139848B2 (ja) | 没食子酸の誘導体に被覆された銀ナノ粒子 | |
| KR20100110791A (ko) | 극성 매체와의 친화성이 우수한 은 미분 및 은 잉크 | |
| JP2010275580A (ja) | 低温焼結性金属ナノ粒子の製造方法および金属ナノ粒子およびそれを用いた分散液の製造方法 | |
| JP2009102716A (ja) | 銀ナノ粒子の製造方法 | |
| JP5232016B2 (ja) | 配線形成用材料 | |
| JP5176060B2 (ja) | 銀粒子分散液の製造法 | |
| JP2009215502A (ja) | 脂環式・芳香族炭化水素を溶媒とする銀インク | |
| JP4674376B2 (ja) | 銀粒子粉末の製造法 | |
| JP2009215503A (ja) | 非極性炭化水素を溶媒とする分散性に優れた銀インク | |
| JP2009091634A (ja) | 銀微粉の製造方法 | |
| JP5139846B2 (ja) | ケトンとの親和性に優れた銀微粉および銀インク | |
| TWI683322B (zh) | 導電性糊劑之製造方法 | |
| WO2017073364A1 (ja) | 印刷用導電性ペーストおよびその調製方法、ならびに銀ナノ粒子分散液の調製方法 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| WWE | Wipo information: entry into national phase |
Ref document number: 200880124131.3 Country of ref document: CN |
|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 08869325 Country of ref document: EP Kind code of ref document: A1 |
|
| ENP | Entry into the national phase |
Ref document number: 20107013493 Country of ref document: KR Kind code of ref document: A |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2008869325 Country of ref document: EP |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 12811583 Country of ref document: US |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |