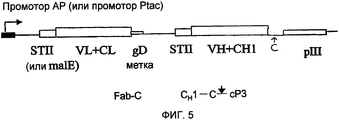
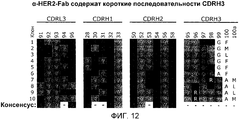
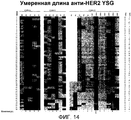
RU2470941C2 - Связывающие полипептиды и их применения - Google Patents
Связывающие полипептиды и их применения Download PDFInfo
- Publication number
- RU2470941C2 RU2470941C2 RU2008126948/10A RU2008126948A RU2470941C2 RU 2470941 C2 RU2470941 C2 RU 2470941C2 RU 2008126948/10 A RU2008126948/10 A RU 2008126948/10A RU 2008126948 A RU2008126948 A RU 2008126948A RU 2470941 C2 RU2470941 C2 RU 2470941C2
- Authority
- RU
- Russia
- Prior art keywords
- amino acid
- acid sequence
- seq
- polypeptide
- target antigen
- Prior art date
Links
- 108090000765 processed proteins & peptides Proteins 0.000 title claims abstract description 509
- 102000004196 processed proteins & peptides Human genes 0.000 title claims abstract description 502
- 229920001184 polypeptide Polymers 0.000 title claims abstract description 499
- 230000027455 binding Effects 0.000 title claims abstract description 141
- 125000003275 alpha amino acid group Chemical group 0.000 claims abstract description 340
- 239000000427 antigen Substances 0.000 claims abstract description 305
- 108091007433 antigens Proteins 0.000 claims abstract description 305
- 102000036639 antigens Human genes 0.000 claims abstract description 305
- 238000000034 method Methods 0.000 claims abstract description 153
- 101000610604 Homo sapiens Tumor necrosis factor receptor superfamily member 10B Proteins 0.000 claims abstract description 70
- 239000000203 mixture Substances 0.000 claims abstract description 46
- 102000053594 human TNFRSF10B Human genes 0.000 claims abstract description 32
- 206010028980 Neoplasm Diseases 0.000 claims abstract description 26
- 239000013598 vector Substances 0.000 claims abstract description 26
- 239000003814 drug Substances 0.000 claims abstract description 23
- 201000011510 cancer Diseases 0.000 claims abstract description 22
- 238000004519 manufacturing process Methods 0.000 claims abstract description 14
- 239000013604 expression vector Substances 0.000 claims abstract description 7
- 229910052727 yttrium Inorganic materials 0.000 claims description 643
- 229910052717 sulfur Inorganic materials 0.000 claims description 639
- 235000001014 amino acid Nutrition 0.000 claims description 245
- 150000001413 amino acids Chemical class 0.000 claims description 226
- NFGXHKASABOEEW-UHFFFAOYSA-N 1-methylethyl 11-methoxy-3,7,11-trimethyl-2,4-dodecadienoate Chemical compound COC(C)(C)CCCC(C)CC=CC(C)=CC(=O)OC(C)C NFGXHKASABOEEW-UHFFFAOYSA-N 0.000 claims description 151
- 108090000623 proteins and genes Proteins 0.000 claims description 66
- 210000004027 cell Anatomy 0.000 claims description 53
- 229910052721 tungsten Inorganic materials 0.000 claims description 49
- 102000004169 proteins and genes Human genes 0.000 claims description 45
- 229910052731 fluorine Inorganic materials 0.000 claims description 44
- 235000018102 proteins Nutrition 0.000 claims description 41
- 238000006471 dimerization reaction Methods 0.000 claims description 38
- 241000124008 Mammalia Species 0.000 claims description 33
- 102000040430 polynucleotide Human genes 0.000 claims description 33
- 108091033319 polynucleotide Proteins 0.000 claims description 33
- 239000002157 polynucleotide Substances 0.000 claims description 33
- 102000006496 Immunoglobulin Heavy Chains Human genes 0.000 claims description 31
- 108010019476 Immunoglobulin Heavy Chains Proteins 0.000 claims description 31
- 239000003795 chemical substances by application Substances 0.000 claims description 31
- 239000012634 fragment Substances 0.000 claims description 29
- 229910052739 hydrogen Inorganic materials 0.000 claims description 27
- 229910052698 phosphorus Inorganic materials 0.000 claims description 26
- 229910052757 nitrogen Inorganic materials 0.000 claims description 25
- -1 poly-his Proteins 0.000 claims description 24
- 238000011282 treatment Methods 0.000 claims description 22
- 230000004927 fusion Effects 0.000 claims description 21
- 229910052720 vanadium Inorganic materials 0.000 claims description 20
- 239000002246 antineoplastic agent Substances 0.000 claims description 19
- 108010003533 Viral Envelope Proteins Proteins 0.000 claims description 18
- 241000700605 Viruses Species 0.000 claims description 18
- 230000004071 biological effect Effects 0.000 claims description 18
- 229940127089 cytotoxic agent Drugs 0.000 claims description 17
- 235000018417 cysteine Nutrition 0.000 claims description 14
- 229940124597 therapeutic agent Drugs 0.000 claims description 14
- XUJNEKJLAYXESH-UHFFFAOYSA-N cysteine Natural products SCC(N)C(O)=O XUJNEKJLAYXESH-UHFFFAOYSA-N 0.000 claims description 13
- 239000011159 matrix material Substances 0.000 claims description 13
- 229910052799 carbon Inorganic materials 0.000 claims description 11
- 101710091045 Envelope protein Proteins 0.000 claims description 10
- 101710188315 Protein X Proteins 0.000 claims description 10
- 208000027866 inflammatory disease Diseases 0.000 claims description 10
- 125000000151 cysteine group Chemical group N[C@@H](CS)C(=O)* 0.000 claims description 9
- 208000026278 immune system disease Diseases 0.000 claims description 9
- 230000002757 inflammatory effect Effects 0.000 claims description 8
- 239000002254 cytotoxic agent Substances 0.000 claims description 7
- 231100000599 cytotoxic agent Toxicity 0.000 claims description 7
- 206010039073 rheumatoid arthritis Diseases 0.000 claims description 7
- 102000013463 Immunoglobulin Light Chains Human genes 0.000 claims description 6
- 108010065825 Immunoglobulin Light Chains Proteins 0.000 claims description 6
- ROHFNLRQFUQHCH-YFKPBYRVSA-N L-leucine Chemical compound CC(C)C[C@H](N)C(O)=O ROHFNLRQFUQHCH-YFKPBYRVSA-N 0.000 claims description 6
- ROHFNLRQFUQHCH-UHFFFAOYSA-N Leucine Natural products CC(C)CC(N)C(O)=O ROHFNLRQFUQHCH-UHFFFAOYSA-N 0.000 claims description 6
- 239000004037 angiogenesis inhibitor Substances 0.000 claims description 6
- 208000014018 liver neoplasm Diseases 0.000 claims description 5
- 208000002154 non-small cell lung carcinoma Diseases 0.000 claims description 5
- 238000010187 selection method Methods 0.000 claims description 5
- 208000029729 tumor suppressor gene on chromosome 11 Diseases 0.000 claims description 5
- 102100038895 Myc proto-oncogene protein Human genes 0.000 claims description 4
- 101710135898 Myc proto-oncogene protein Proteins 0.000 claims description 4
- 208000015914 Non-Hodgkin lymphomas Diseases 0.000 claims description 4
- 206010033128 Ovarian cancer Diseases 0.000 claims description 4
- 206010061535 Ovarian neoplasm Diseases 0.000 claims description 4
- 101710193132 Pre-hexon-linking protein VIII Proteins 0.000 claims description 4
- 101710150448 Transcriptional regulator Myc Proteins 0.000 claims description 4
- 238000012258 culturing Methods 0.000 claims description 4
- 230000003247 decreasing effect Effects 0.000 claims description 4
- 201000007270 liver cancer Diseases 0.000 claims description 4
- 206010006187 Breast cancer Diseases 0.000 claims description 3
- 208000026310 Breast neoplasm Diseases 0.000 claims description 3
- 208000008839 Kidney Neoplasms Diseases 0.000 claims description 3
- 206010038389 Renal cancer Diseases 0.000 claims description 3
- 229940034982 antineoplastic agent Drugs 0.000 claims description 3
- 210000004899 c-terminal region Anatomy 0.000 claims description 3
- 208000029742 colonic neoplasm Diseases 0.000 claims description 3
- 102000034287 fluorescent proteins Human genes 0.000 claims description 3
- 108091006047 fluorescent proteins Proteins 0.000 claims description 3
- 201000010536 head and neck cancer Diseases 0.000 claims description 3
- 208000014829 head and neck neoplasm Diseases 0.000 claims description 3
- 201000010982 kidney cancer Diseases 0.000 claims description 3
- 206010027406 Mesothelioma Diseases 0.000 claims description 2
- 208000034578 Multiple myelomas Diseases 0.000 claims description 2
- 206010060862 Prostate cancer Diseases 0.000 claims description 2
- 208000000236 Prostatic Neoplasms Diseases 0.000 claims description 2
- 208000015634 Rectal Neoplasms Diseases 0.000 claims description 2
- 210000001072 colon Anatomy 0.000 claims description 2
- 201000001441 melanoma Diseases 0.000 claims description 2
- 102100021696 Syncytin-1 Human genes 0.000 claims 2
- 101100112922 Candida albicans CDR3 gene Proteins 0.000 claims 1
- 206010035226 Plasma cell myeloma Diseases 0.000 claims 1
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 abstract description 51
- 150000007523 nucleic acids Chemical class 0.000 abstract description 29
- 102000039446 nucleic acids Human genes 0.000 abstract description 21
- 108020004707 nucleic acids Proteins 0.000 abstract description 21
- 230000014509 gene expression Effects 0.000 abstract description 17
- 239000000126 substance Substances 0.000 abstract description 15
- 230000000694 effects Effects 0.000 abstract description 14
- 210000000987 immune system Anatomy 0.000 abstract description 7
- 238000004113 cell culture Methods 0.000 abstract description 6
- 108010067390 Viral Proteins Proteins 0.000 abstract 1
- 210000004779 membrane envelope Anatomy 0.000 abstract 1
- 229940024606 amino acid Drugs 0.000 description 177
- 102100035360 Cerebellar degeneration-related antigen 1 Human genes 0.000 description 95
- 101710117290 Aldo-keto reductase family 1 member C4 Proteins 0.000 description 91
- 239000011230 binding agent Substances 0.000 description 76
- 108020004705 Codon Proteins 0.000 description 48
- 101001012157 Homo sapiens Receptor tyrosine-protein kinase erbB-2 Proteins 0.000 description 48
- 102100030086 Receptor tyrosine-protein kinase erbB-2 Human genes 0.000 description 38
- 102100040112 Tumor necrosis factor receptor superfamily member 10B Human genes 0.000 description 33
- 108020004414 DNA Proteins 0.000 description 32
- 208000035475 disorder Diseases 0.000 description 28
- 239000002245 particle Substances 0.000 description 23
- 201000010099 disease Diseases 0.000 description 22
- 108091034117 Oligonucleotide Proteins 0.000 description 21
- 125000000539 amino acid group Chemical group 0.000 description 20
- 239000000523 sample Substances 0.000 description 20
- JLCPHMBAVCMARE-UHFFFAOYSA-N [3-[[3-[[3-[[3-[[3-[[3-[[3-[[3-[[3-[[3-[[3-[[5-(2-amino-6-oxo-1H-purin-9-yl)-3-[[3-[[3-[[3-[[3-[[3-[[5-(2-amino-6-oxo-1H-purin-9-yl)-3-[[5-(2-amino-6-oxo-1H-purin-9-yl)-3-hydroxyoxolan-2-yl]methoxy-hydroxyphosphoryl]oxyoxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxyoxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methyl [5-(6-aminopurin-9-yl)-2-(hydroxymethyl)oxolan-3-yl] hydrogen phosphate Polymers Cc1cn(C2CC(OP(O)(=O)OCC3OC(CC3OP(O)(=O)OCC3OC(CC3O)n3cnc4c3nc(N)[nH]c4=O)n3cnc4c3nc(N)[nH]c4=O)C(COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3CO)n3cnc4c(N)ncnc34)n3ccc(N)nc3=O)n3cnc4c(N)ncnc34)n3ccc(N)nc3=O)n3ccc(N)nc3=O)n3ccc(N)nc3=O)n3cnc4c(N)ncnc34)n3cnc4c(N)ncnc34)n3cc(C)c(=O)[nH]c3=O)n3cc(C)c(=O)[nH]c3=O)n3ccc(N)nc3=O)n3cc(C)c(=O)[nH]c3=O)n3cnc4c3nc(N)[nH]c4=O)n3cnc4c(N)ncnc34)n3cnc4c(N)ncnc34)n3cnc4c(N)ncnc34)n3cnc4c(N)ncnc34)O2)c(=O)[nH]c1=O JLCPHMBAVCMARE-UHFFFAOYSA-N 0.000 description 19
- 102000002259 TNF-Related Apoptosis-Inducing Ligand Receptors Human genes 0.000 description 17
- 108010000449 TNF-Related Apoptosis-Inducing Ligand Receptors Proteins 0.000 description 17
- 238000006467 substitution reaction Methods 0.000 description 17
- 238000002823 phage display Methods 0.000 description 15
- 229940002612 prodrug Drugs 0.000 description 15
- 239000000651 prodrug Substances 0.000 description 15
- AOJJSUZBOXZQNB-TZSSRYMLSA-N Doxorubicin Chemical compound O([C@H]1C[C@@](O)(CC=2C(O)=C3C(=O)C=4C=CC=C(C=4C(=O)C3=C(O)C=21)OC)C(=O)CO)[C@H]1C[C@H](N)[C@H](O)[C@H](C)O1 AOJJSUZBOXZQNB-TZSSRYMLSA-N 0.000 description 14
- 239000002904 solvent Substances 0.000 description 14
- 238000012216 screening Methods 0.000 description 13
- 108091035707 Consensus sequence Proteins 0.000 description 12
- 108091028043 Nucleic acid sequence Proteins 0.000 description 12
- 108700012411 TNFSF10 Proteins 0.000 description 12
- 102100024598 Tumor necrosis factor ligand superfamily member 10 Human genes 0.000 description 12
- 241000894007 species Species 0.000 description 12
- FBOZXECLQNJBKD-ZDUSSCGKSA-N L-methotrexate Chemical compound C=1N=C2N=C(N)N=C(N)C2=NC=1CN(C)C1=CC=C(C(=O)N[C@@H](CCC(O)=O)C(O)=O)C=C1 FBOZXECLQNJBKD-ZDUSSCGKSA-N 0.000 description 11
- 239000000556 agonist Substances 0.000 description 11
- 239000005557 antagonist Substances 0.000 description 11
- 230000006907 apoptotic process Effects 0.000 description 11
- 230000006870 function Effects 0.000 description 11
- 102000051957 human ERBB2 Human genes 0.000 description 11
- 229960000485 methotrexate Drugs 0.000 description 11
- 108060003951 Immunoglobulin Proteins 0.000 description 10
- XUJNEKJLAYXESH-REOHCLBHSA-N L-Cysteine Chemical compound SC[C@H](N)C(O)=O XUJNEKJLAYXESH-REOHCLBHSA-N 0.000 description 10
- 241001465754 Metazoa Species 0.000 description 10
- 238000003556 assay Methods 0.000 description 10
- 102000018358 immunoglobulin Human genes 0.000 description 10
- 230000009870 specific binding Effects 0.000 description 10
- 230000001419 dependent effect Effects 0.000 description 9
- 238000001727 in vivo Methods 0.000 description 9
- 239000003446 ligand Substances 0.000 description 9
- 102100038132 Endogenous retrovirus group K member 6 Pro protein Human genes 0.000 description 8
- 230000012010 growth Effects 0.000 description 8
- 238000000338 in vitro Methods 0.000 description 8
- 238000002955 isolation Methods 0.000 description 8
- 102000005962 receptors Human genes 0.000 description 8
- 108020003175 receptors Proteins 0.000 description 8
- 102000008394 Immunoglobulin Fragments Human genes 0.000 description 7
- 108010021625 Immunoglobulin Fragments Proteins 0.000 description 7
- NKANXQFJJICGDU-QPLCGJKRSA-N Tamoxifen Chemical compound C=1C=CC=CC=1C(/CC)=C(C=1C=CC(OCCN(C)C)=CC=1)/C1=CC=CC=C1 NKANXQFJJICGDU-QPLCGJKRSA-N 0.000 description 7
- 108020001507 fusion proteins Proteins 0.000 description 7
- 102000037865 fusion proteins Human genes 0.000 description 7
- 238000011065 in-situ storage Methods 0.000 description 7
- 230000002401 inhibitory effect Effects 0.000 description 7
- 239000000243 solution Substances 0.000 description 7
- 230000001225 therapeutic effect Effects 0.000 description 7
- 101710132601 Capsid protein Proteins 0.000 description 6
- 101710094648 Coat protein Proteins 0.000 description 6
- 102000001301 EGF receptor Human genes 0.000 description 6
- 238000002965 ELISA Methods 0.000 description 6
- 108010008165 Etanercept Proteins 0.000 description 6
- 241000724791 Filamentous phage Species 0.000 description 6
- 101710125418 Major capsid protein Proteins 0.000 description 6
- 101710141454 Nucleoprotein Proteins 0.000 description 6
- 101710083689 Probable capsid protein Proteins 0.000 description 6
- 108020005038 Terminator Codon Proteins 0.000 description 6
- 230000015572 biosynthetic process Effects 0.000 description 6
- 238000012217 deletion Methods 0.000 description 6
- 230000037430 deletion Effects 0.000 description 6
- 229960004679 doxorubicin Drugs 0.000 description 6
- 230000003211 malignant effect Effects 0.000 description 6
- 230000001404 mediated effect Effects 0.000 description 6
- 230000001105 regulatory effect Effects 0.000 description 6
- 230000010076 replication Effects 0.000 description 6
- 229960005486 vaccine Drugs 0.000 description 6
- 108010047041 Complementarity Determining Regions Proteins 0.000 description 5
- 108060006698 EGF receptor Proteins 0.000 description 5
- 102100021181 Golgi phosphoprotein 3 Human genes 0.000 description 5
- 241000282412 Homo Species 0.000 description 5
- 241000699666 Mus <mouse, genus> Species 0.000 description 5
- ZDZOTLJHXYCWBA-VCVYQWHSSA-N N-debenzoyl-N-(tert-butoxycarbonyl)-10-deacetyltaxol Chemical compound O([C@H]1[C@H]2[C@@](C([C@H](O)C3=C(C)[C@@H](OC(=O)[C@H](O)[C@@H](NC(=O)OC(C)(C)C)C=4C=CC=CC=4)C[C@]1(O)C3(C)C)=O)(C)[C@@H](O)C[C@H]1OC[C@]12OC(=O)C)C(=O)C1=CC=CC=C1 ZDZOTLJHXYCWBA-VCVYQWHSSA-N 0.000 description 5
- 201000004681 Psoriasis Diseases 0.000 description 5
- 230000004913 activation Effects 0.000 description 5
- 230000001580 bacterial effect Effects 0.000 description 5
- 230000008901 benefit Effects 0.000 description 5
- 238000004590 computer program Methods 0.000 description 5
- 239000003623 enhancer Substances 0.000 description 5
- 239000012535 impurity Substances 0.000 description 5
- 230000001965 increasing effect Effects 0.000 description 5
- 239000002773 nucleotide Substances 0.000 description 5
- 125000003729 nucleotide group Chemical group 0.000 description 5
- 230000008569 process Effects 0.000 description 5
- 230000028327 secretion Effects 0.000 description 5
- RCINICONZNJXQF-MZXODVADSA-N taxol Chemical compound O([C@@H]1[C@@]2(C[C@@H](C(C)=C(C2(C)C)[C@H](C([C@]2(C)[C@@H](O)C[C@H]3OC[C@]3([C@H]21)OC(C)=O)=O)OC(=O)C)OC(=O)[C@H](O)[C@@H](NC(=O)C=1C=CC=CC=1)C=1C=CC=CC=1)O)C(=O)C1=CC=CC=C1 RCINICONZNJXQF-MZXODVADSA-N 0.000 description 5
- 241001515965 unidentified phage Species 0.000 description 5
- 229960004528 vincristine Drugs 0.000 description 5
- OGWKCGZFUXNPDA-XQKSVPLYSA-N vincristine Chemical compound C([N@]1C[C@@H](C[C@]2(C(=O)OC)C=3C(=CC4=C([C@]56[C@H]([C@@]([C@H](OC(C)=O)[C@]7(CC)C=CCN([C@H]67)CC5)(O)C(=O)OC)N4C=O)C=3)OC)C[C@@](C1)(O)CC)CC1=C2NC2=CC=CC=C12 OGWKCGZFUXNPDA-XQKSVPLYSA-N 0.000 description 5
- OGWKCGZFUXNPDA-UHFFFAOYSA-N vincristine Natural products C1C(CC)(O)CC(CC2(C(=O)OC)C=3C(=CC4=C(C56C(C(C(OC(C)=O)C7(CC)C=CCN(C67)CC5)(O)C(=O)OC)N4C=O)C=3)OC)CN1CCC1=C2NC2=CC=CC=C12 OGWKCGZFUXNPDA-UHFFFAOYSA-N 0.000 description 5
- 230000003612 virological effect Effects 0.000 description 5
- 108091026890 Coding region Proteins 0.000 description 4
- CMSMOCZEIVJLDB-UHFFFAOYSA-N Cyclophosphamide Chemical compound ClCCN(CCCl)P1(=O)NCCCO1 CMSMOCZEIVJLDB-UHFFFAOYSA-N 0.000 description 4
- GHASVSINZRGABV-UHFFFAOYSA-N Fluorouracil Chemical compound FC1=CNC(=O)NC1=O GHASVSINZRGABV-UHFFFAOYSA-N 0.000 description 4
- DHMQDGOQFOQNFH-UHFFFAOYSA-N Glycine Chemical compound NCC(O)=O DHMQDGOQFOQNFH-UHFFFAOYSA-N 0.000 description 4
- 208000022559 Inflammatory bowel disease Diseases 0.000 description 4
- 238000004458 analytical method Methods 0.000 description 4
- 239000003242 anti bacterial agent Substances 0.000 description 4
- 229940088710 antibiotic agent Drugs 0.000 description 4
- 230000000903 blocking effect Effects 0.000 description 4
- 230000004663 cell proliferation Effects 0.000 description 4
- 238000003776 cleavage reaction Methods 0.000 description 4
- 230000006378 damage Effects 0.000 description 4
- 238000010494 dissociation reaction Methods 0.000 description 4
- 230000005593 dissociations Effects 0.000 description 4
- 229940079593 drug Drugs 0.000 description 4
- 229960000403 etanercept Drugs 0.000 description 4
- 229960002949 fluorouracil Drugs 0.000 description 4
- 229940088597 hormone Drugs 0.000 description 4
- 210000004408 hybridoma Anatomy 0.000 description 4
- 238000003780 insertion Methods 0.000 description 4
- 230000037431 insertion Effects 0.000 description 4
- 230000004048 modification Effects 0.000 description 4
- 238000012986 modification Methods 0.000 description 4
- 238000000159 protein binding assay Methods 0.000 description 4
- 230000007017 scission Effects 0.000 description 4
- WYWHKKSPHMUBEB-UHFFFAOYSA-N tioguanine Chemical compound N1C(N)=NC(=S)C2=C1N=CN2 WYWHKKSPHMUBEB-UHFFFAOYSA-N 0.000 description 4
- 210000001519 tissue Anatomy 0.000 description 4
- JXLYSJRDGCGARV-CFWMRBGOSA-N vinblastine Chemical compound C([C@H](C[C@]1(C(=O)OC)C=2C(=CC3=C([C@]45[C@H]([C@@]([C@H](OC(C)=O)[C@]6(CC)C=CCN([C@H]56)CC4)(O)C(=O)OC)N3C)C=2)OC)C[C@@](C2)(O)CC)N2CCC2=C1NC1=CC=CC=C21 JXLYSJRDGCGARV-CFWMRBGOSA-N 0.000 description 4
- VVIAGPKUTFNRDU-UHFFFAOYSA-N 6S-folinic acid Natural products C1NC=2NC(N)=NC(=O)C=2N(C=O)C1CNC1=CC=C(C(=O)NC(CCC(O)=O)C(O)=O)C=C1 VVIAGPKUTFNRDU-UHFFFAOYSA-N 0.000 description 3
- STQGQHZAVUOBTE-UHFFFAOYSA-N 7-Cyan-hept-2t-en-4,6-diinsaeure Natural products C1=2C(O)=C3C(=O)C=4C(OC)=CC=CC=4C(=O)C3=C(O)C=2CC(O)(C(C)=O)CC1OC1CC(N)C(O)C(C)O1 STQGQHZAVUOBTE-UHFFFAOYSA-N 0.000 description 3
- 241000894006 Bacteria Species 0.000 description 3
- 108091003079 Bovine Serum Albumin Proteins 0.000 description 3
- 201000009030 Carcinoma Diseases 0.000 description 3
- 206010009944 Colon cancer Diseases 0.000 description 3
- 208000011231 Crohn disease Diseases 0.000 description 3
- PMATZTZNYRCHOR-CGLBZJNRSA-N Cyclosporin A Chemical compound CC[C@@H]1NC(=O)[C@H]([C@H](O)[C@H](C)C\C=C\C)N(C)C(=O)[C@H](C(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)N(C)C(=O)[C@H](C(C)C)NC(=O)[C@H](CC(C)C)N(C)C(=O)CN(C)C1=O PMATZTZNYRCHOR-CGLBZJNRSA-N 0.000 description 3
- 108010036949 Cyclosporine Proteins 0.000 description 3
- UHDGCWIWMRVCDJ-CCXZUQQUSA-N Cytarabine Chemical compound O=C1N=C(N)C=CN1[C@H]1[C@@H](O)[C@H](O)[C@@H](CO)O1 UHDGCWIWMRVCDJ-CCXZUQQUSA-N 0.000 description 3
- 238000012286 ELISA Assay Methods 0.000 description 3
- 102000004190 Enzymes Human genes 0.000 description 3
- 108090000790 Enzymes Proteins 0.000 description 3
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 description 3
- 101000611183 Homo sapiens Tumor necrosis factor Proteins 0.000 description 3
- 108010054477 Immunoglobulin Fab Fragments Proteins 0.000 description 3
- 102000001706 Immunoglobulin Fab Fragments Human genes 0.000 description 3
- NWIBSHFKIJFRCO-WUDYKRTCSA-N Mytomycin Chemical compound C1N2C(C(C(C)=C(N)C3=O)=O)=C3[C@@H](COC(N)=O)[C@@]2(OC)[C@@H]2[C@H]1N2 NWIBSHFKIJFRCO-WUDYKRTCSA-N 0.000 description 3
- 206010030113 Oedema Diseases 0.000 description 3
- 241000283973 Oryctolagus cuniculus Species 0.000 description 3
- 229930012538 Paclitaxel Natural products 0.000 description 3
- 208000034038 Pathologic Neovascularization Diseases 0.000 description 3
- IIDJRNMFWXDHID-UHFFFAOYSA-N Risedronic acid Chemical compound OP(=O)(O)C(P(O)(O)=O)(O)CC1=CC=CN=C1 IIDJRNMFWXDHID-UHFFFAOYSA-N 0.000 description 3
- 230000018199 S phase Effects 0.000 description 3
- FOCVUCIESVLUNU-UHFFFAOYSA-N Thiotepa Chemical compound C1CN1P(N1CC1)(=S)N1CC1 FOCVUCIESVLUNU-UHFFFAOYSA-N 0.000 description 3
- JXLYSJRDGCGARV-WWYNWVTFSA-N Vinblastine Natural products O=C(O[C@H]1[C@](O)(C(=O)OC)[C@@H]2N(C)c3c(cc(c(OC)c3)[C@]3(C(=O)OC)c4[nH]c5c(c4CCN4C[C@](O)(CC)C[C@H](C3)C4)cccc5)[C@@]32[C@H]2[C@@]1(CC)C=CCN2CC3)C JXLYSJRDGCGARV-WWYNWVTFSA-N 0.000 description 3
- 230000002159 abnormal effect Effects 0.000 description 3
- 239000002253 acid Substances 0.000 description 3
- 150000007513 acids Chemical class 0.000 description 3
- 230000001270 agonistic effect Effects 0.000 description 3
- 230000003042 antagnostic effect Effects 0.000 description 3
- 102000025171 antigen binding proteins Human genes 0.000 description 3
- 108091000831 antigen binding proteins Proteins 0.000 description 3
- 230000003115 biocidal effect Effects 0.000 description 3
- 229940098773 bovine serum albumin Drugs 0.000 description 3
- 239000000872 buffer Substances 0.000 description 3
- 238000004422 calculation algorithm Methods 0.000 description 3
- HXCHCVDVKSCDHU-LULTVBGHSA-N calicheamicin Chemical compound C1[C@H](OC)[C@@H](NCC)CO[C@H]1O[C@H]1[C@H](O[C@@H]2C\3=C(NC(=O)OC)C(=O)C[C@](C/3=C/CSSSC)(O)C#C\C=C/C#C2)O[C@H](C)[C@@H](NO[C@@H]2O[C@H](C)[C@@H](SC(=O)C=3C(=C(OC)C(O[C@H]4[C@@H]([C@H](OC)[C@@H](O)[C@H](C)O4)O)=C(I)C=3C)OC)[C@@H](O)C2)[C@@H]1O HXCHCVDVKSCDHU-LULTVBGHSA-N 0.000 description 3
- 229930195731 calicheamicin Natural products 0.000 description 3
- 208000035269 cancer or benign tumor Diseases 0.000 description 3
- 230000030833 cell death Effects 0.000 description 3
- 230000008859 change Effects 0.000 description 3
- 229960004630 chlorambucil Drugs 0.000 description 3
- JCKYGMPEJWAADB-UHFFFAOYSA-N chlorambucil Chemical compound OC(=O)CCCC1=CC=C(N(CCCl)CCCl)C=C1 JCKYGMPEJWAADB-UHFFFAOYSA-N 0.000 description 3
- 229960001265 ciclosporin Drugs 0.000 description 3
- 230000000295 complement effect Effects 0.000 description 3
- 229960004397 cyclophosphamide Drugs 0.000 description 3
- 229930182912 cyclosporin Natural products 0.000 description 3
- 231100000433 cytotoxic Toxicity 0.000 description 3
- 230000001472 cytotoxic effect Effects 0.000 description 3
- STQGQHZAVUOBTE-VGBVRHCVSA-N daunorubicin Chemical compound O([C@H]1C[C@@](O)(CC=2C(O)=C3C(=O)C=4C=CC=C(C=4C(=O)C3=C(O)C=21)OC)C(C)=O)[C@H]1C[C@H](N)[C@H](O)[C@H](C)O1 STQGQHZAVUOBTE-VGBVRHCVSA-N 0.000 description 3
- CYQFCXCEBYINGO-IAGOWNOFSA-N delta1-THC Chemical compound C1=C(C)CC[C@H]2C(C)(C)OC3=CC(CCCCC)=CC(O)=C3[C@@H]21 CYQFCXCEBYINGO-IAGOWNOFSA-N 0.000 description 3
- 239000000539 dimer Substances 0.000 description 3
- LOKCTEFSRHRXRJ-UHFFFAOYSA-I dipotassium trisodium dihydrogen phosphate hydrogen phosphate dichloride Chemical compound P(=O)(O)(O)[O-].[K+].P(=O)(O)([O-])[O-].[Na+].[Na+].[Cl-].[K+].[Cl-].[Na+] LOKCTEFSRHRXRJ-UHFFFAOYSA-I 0.000 description 3
- 229960003668 docetaxel Drugs 0.000 description 3
- 238000005516 engineering process Methods 0.000 description 3
- 230000002708 enhancing effect Effects 0.000 description 3
- 229940088598 enzyme Drugs 0.000 description 3
- VJJPUSNTGOMMGY-MRVIYFEKSA-N etoposide Chemical compound COC1=C(O)C(OC)=CC([C@@H]2C3=CC=4OCOC=4C=C3[C@@H](O[C@H]3[C@@H]([C@@H](O)[C@@H]4O[C@H](C)OC[C@H]4O3)O)[C@@H]3[C@@H]2C(OC3)=O)=C1 VJJPUSNTGOMMGY-MRVIYFEKSA-N 0.000 description 3
- 210000003527 eukaryotic cell Anatomy 0.000 description 3
- 235000008191 folinic acid Nutrition 0.000 description 3
- 239000011672 folinic acid Substances 0.000 description 3
- VVIAGPKUTFNRDU-ABLWVSNPSA-N folinic acid Chemical compound C1NC=2NC(N)=NC(=O)C=2N(C=O)C1CNC1=CC=C(C(=O)N[C@@H](CCC(O)=O)C(O)=O)C=C1 VVIAGPKUTFNRDU-ABLWVSNPSA-N 0.000 description 3
- 230000005714 functional activity Effects 0.000 description 3
- 239000001963 growth medium Substances 0.000 description 3
- 230000036541 health Effects 0.000 description 3
- 206010073071 hepatocellular carcinoma Diseases 0.000 description 3
- 239000005556 hormone Substances 0.000 description 3
- 230000001900 immune effect Effects 0.000 description 3
- 229940072221 immunoglobulins Drugs 0.000 description 3
- 238000011534 incubation Methods 0.000 description 3
- 229960000598 infliximab Drugs 0.000 description 3
- 230000003993 interaction Effects 0.000 description 3
- UWKQSNNFCGGAFS-XIFFEERXSA-N irinotecan Chemical compound C1=C2C(CC)=C3CN(C(C4=C([C@@](C(=O)OC4)(O)CC)C=4)=O)C=4C3=NC2=CC=C1OC(=O)N(CC1)CCC1N1CCCCC1 UWKQSNNFCGGAFS-XIFFEERXSA-N 0.000 description 3
- VHOGYURTWQBHIL-UHFFFAOYSA-N leflunomide Chemical compound O1N=CC(C(=O)NC=2C=CC(=CC=2)C(F)(F)F)=C1C VHOGYURTWQBHIL-UHFFFAOYSA-N 0.000 description 3
- 229960001691 leucovorin Drugs 0.000 description 3
- RGLRXNKKBLIBQS-XNHQSDQCSA-N leuprolide acetate Chemical compound CC(O)=O.CCNC(=O)[C@@H]1CCCN1C(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](CC(C)C)NC(=O)[C@@H](NC(=O)[C@H](CO)NC(=O)[C@H](CC=1C2=CC=CC=C2NC=1)NC(=O)[C@H](CC=1N=CNC=1)NC(=O)[C@H]1NC(=O)CC1)CC1=CC=C(O)C=C1 RGLRXNKKBLIBQS-XNHQSDQCSA-N 0.000 description 3
- GLVAUDGFNGKCSF-UHFFFAOYSA-N mercaptopurine Chemical compound S=C1NC=NC2=C1NC=N2 GLVAUDGFNGKCSF-UHFFFAOYSA-N 0.000 description 3
- ZAHQPTJLOCWVPG-UHFFFAOYSA-N mitoxantrone dihydrochloride Chemical compound Cl.Cl.O=C1C2=C(O)C=CC(O)=C2C(=O)C2=C1C(NCCNCCO)=CC=C2NCCNCCO ZAHQPTJLOCWVPG-UHFFFAOYSA-N 0.000 description 3
- 231100000219 mutagenic Toxicity 0.000 description 3
- 230000003505 mutagenic effect Effects 0.000 description 3
- 230000035772 mutation Effects 0.000 description 3
- 230000003472 neutralizing effect Effects 0.000 description 3
- 229940021182 non-steroidal anti-inflammatory drug Drugs 0.000 description 3
- 229960001592 paclitaxel Drugs 0.000 description 3
- 230000001575 pathological effect Effects 0.000 description 3
- 239000002953 phosphate buffered saline Substances 0.000 description 3
- 239000013612 plasmid Substances 0.000 description 3
- 230000000069 prophylactic effect Effects 0.000 description 3
- 238000000746 purification Methods 0.000 description 3
- 230000002285 radioactive effect Effects 0.000 description 3
- 230000002829 reductive effect Effects 0.000 description 3
- 238000013207 serial dilution Methods 0.000 description 3
- 230000019491 signal transduction Effects 0.000 description 3
- 229960001603 tamoxifen Drugs 0.000 description 3
- 210000001685 thyroid gland Anatomy 0.000 description 3
- 239000003053 toxin Substances 0.000 description 3
- 231100000765 toxin Toxicity 0.000 description 3
- 108700012359 toxins Proteins 0.000 description 3
- 229960003048 vinblastine Drugs 0.000 description 3
- XRASPMIURGNCCH-UHFFFAOYSA-N zoledronic acid Chemical compound OP(=O)(O)C(P(O)(O)=O)(O)CN1C=CN=C1 XRASPMIURGNCCH-UHFFFAOYSA-N 0.000 description 3
- FDKXTQMXEQVLRF-ZHACJKMWSA-N (E)-dacarbazine Chemical compound CN(C)\N=N\c1[nH]cnc1C(N)=O FDKXTQMXEQVLRF-ZHACJKMWSA-N 0.000 description 2
- HZAXFHJVJLSVMW-UHFFFAOYSA-N 2-Aminoethan-1-ol Chemical compound NCCO HZAXFHJVJLSVMW-UHFFFAOYSA-N 0.000 description 2
- GOJUJUVQIVIZAV-UHFFFAOYSA-N 2-amino-4,6-dichloropyrimidine-5-carbaldehyde Chemical group NC1=NC(Cl)=C(C=O)C(Cl)=N1 GOJUJUVQIVIZAV-UHFFFAOYSA-N 0.000 description 2
- LSBDFXRDZJMBSC-UHFFFAOYSA-N 2-phenylacetamide Chemical class NC(=O)CC1=CC=CC=C1 LSBDFXRDZJMBSC-UHFFFAOYSA-N 0.000 description 2
- FPQQSJJWHUJYPU-UHFFFAOYSA-N 3-(dimethylamino)propyliminomethylidene-ethylazanium;chloride Chemical compound Cl.CCN=C=NCCCN(C)C FPQQSJJWHUJYPU-UHFFFAOYSA-N 0.000 description 2
- AOJJSUZBOXZQNB-VTZDEGQISA-N 4'-epidoxorubicin Chemical compound O([C@H]1C[C@@](O)(CC=2C(O)=C3C(=O)C=4C=CC=C(C=4C(=O)C3=C(O)C=21)OC)C(=O)CO)[C@H]1C[C@H](N)[C@@H](O)[C@H](C)O1 AOJJSUZBOXZQNB-VTZDEGQISA-N 0.000 description 2
- OGSPWJRAVKPPFI-UHFFFAOYSA-N Alendronic Acid Chemical compound NCCCC(O)(P(O)(O)=O)P(O)(O)=O OGSPWJRAVKPPFI-UHFFFAOYSA-N 0.000 description 2
- 108020004774 Alkaline Phosphatase Proteins 0.000 description 2
- 102000002260 Alkaline Phosphatase Human genes 0.000 description 2
- 101100493732 Arabidopsis thaliana BBX22 gene Proteins 0.000 description 2
- 101100421335 Arabidopsis thaliana SFH3 gene Proteins 0.000 description 2
- 101100150076 Arabidopsis thaliana SPH3 gene Proteins 0.000 description 2
- BFYIZQONLCFLEV-DAELLWKTSA-N Aromasine Chemical compound O=C1C=C[C@]2(C)[C@H]3CC[C@](C)(C(CC4)=O)[C@@H]4[C@@H]3CC(=C)C2=C1 BFYIZQONLCFLEV-DAELLWKTSA-N 0.000 description 2
- IJGRMHOSHXDMSA-UHFFFAOYSA-N Atomic nitrogen Chemical compound N#N IJGRMHOSHXDMSA-UHFFFAOYSA-N 0.000 description 2
- 208000023275 Autoimmune disease Diseases 0.000 description 2
- 108010006654 Bleomycin Proteins 0.000 description 2
- KLWPJMFMVPTNCC-UHFFFAOYSA-N Camptothecin Natural products CCC1(O)C(=O)OCC2=C1C=C3C4Nc5ccccc5C=C4CN3C2=O KLWPJMFMVPTNCC-UHFFFAOYSA-N 0.000 description 2
- GAGWJHPBXLXJQN-UORFTKCHSA-N Capecitabine Chemical compound C1=C(F)C(NC(=O)OCCCCC)=NC(=O)N1[C@H]1[C@H](O)[C@H](O)[C@@H](C)O1 GAGWJHPBXLXJQN-UORFTKCHSA-N 0.000 description 2
- 108010076667 Caspases Proteins 0.000 description 2
- 102000011727 Caspases Human genes 0.000 description 2
- 208000001333 Colorectal Neoplasms Diseases 0.000 description 2
- 241000196324 Embryophyta Species 0.000 description 2
- 108010042407 Endonucleases Proteins 0.000 description 2
- 102000004533 Endonucleases Human genes 0.000 description 2
- HTIJFSOGRVMCQR-UHFFFAOYSA-N Epirubicin Natural products COc1cccc2C(=O)c3c(O)c4CC(O)(CC(OC5CC(N)C(=O)C(C)O5)c4c(O)c3C(=O)c12)C(=O)CO HTIJFSOGRVMCQR-UHFFFAOYSA-N 0.000 description 2
- 239000004471 Glycine Substances 0.000 description 2
- 101000610605 Homo sapiens Tumor necrosis factor receptor superfamily member 10A Proteins 0.000 description 2
- 108010009817 Immunoglobulin Constant Regions Proteins 0.000 description 2
- 102000009786 Immunoglobulin Constant Regions Human genes 0.000 description 2
- OUYCCCASQSFEME-QMMMGPOBSA-N L-tyrosine Chemical compound OC(=O)[C@@H](N)CC1=CC=C(O)C=C1 OUYCCCASQSFEME-QMMMGPOBSA-N 0.000 description 2
- 239000005551 L01XE03 - Erlotinib Substances 0.000 description 2
- 108010000817 Leuprolide Proteins 0.000 description 2
- 102000003960 Ligases Human genes 0.000 description 2
- 108090000364 Ligases Proteins 0.000 description 2
- 230000027311 M phase Effects 0.000 description 2
- 102000029749 Microtubule Human genes 0.000 description 2
- 108091022875 Microtubule Proteins 0.000 description 2
- 229930192392 Mitomycin Natural products 0.000 description 2
- 241001529936 Murinae Species 0.000 description 2
- 206010061902 Pancreatic neoplasm Diseases 0.000 description 2
- 229920000776 Poly(Adenosine diphosphate-ribose) polymerase Polymers 0.000 description 2
- 229920001213 Polysorbate 20 Polymers 0.000 description 2
- 241000700159 Rattus Species 0.000 description 2
- 241000283984 Rodentia Species 0.000 description 2
- 101100519256 Saccharomyces cerevisiae (strain ATCC 204508 / S288c) PDR16 gene Proteins 0.000 description 2
- 101100094106 Saccharomyces cerevisiae (strain ATCC 204508 / S288c) RSC8 gene Proteins 0.000 description 2
- VYPSYNLAJGMNEJ-UHFFFAOYSA-N Silicium dioxide Chemical compound O=[Si]=O VYPSYNLAJGMNEJ-UHFFFAOYSA-N 0.000 description 2
- CDBYLPFSWZWCQE-UHFFFAOYSA-L Sodium Carbonate Chemical compound [Na+].[Na+].[O-]C([O-])=O CDBYLPFSWZWCQE-UHFFFAOYSA-L 0.000 description 2
- 101100054666 Streptomyces halstedii sch3 gene Proteins 0.000 description 2
- CYQFCXCEBYINGO-UHFFFAOYSA-N THC Natural products C1=C(C)CCC2C(C)(C)OC3=CC(CCCCC)=CC(O)=C3C21 CYQFCXCEBYINGO-UHFFFAOYSA-N 0.000 description 2
- 229940123237 Taxane Drugs 0.000 description 2
- 208000031981 Thrombocytopenic Idiopathic Purpura Diseases 0.000 description 2
- 102100040247 Tumor necrosis factor Human genes 0.000 description 2
- 102100040113 Tumor necrosis factor receptor superfamily member 10A Human genes 0.000 description 2
- 230000003213 activating effect Effects 0.000 description 2
- 229960002964 adalimumab Drugs 0.000 description 2
- 230000009824 affinity maturation Effects 0.000 description 2
- 229960003437 aminoglutethimide Drugs 0.000 description 2
- ROBVIMPUHSLWNV-UHFFFAOYSA-N aminoglutethimide Chemical compound C=1C=C(N)C=CC=1C1(CC)CCC(=O)NC1=O ROBVIMPUHSLWNV-UHFFFAOYSA-N 0.000 description 2
- YBBLVLTVTVSKRW-UHFFFAOYSA-N anastrozole Chemical compound N#CC(C)(C)C1=CC(C(C)(C#N)C)=CC(CN2N=CN=C2)=C1 YBBLVLTVTVSKRW-UHFFFAOYSA-N 0.000 description 2
- 229940041181 antineoplastic drug Drugs 0.000 description 2
- 230000001640 apoptogenic effect Effects 0.000 description 2
- 210000003719 b-lymphocyte Anatomy 0.000 description 2
- 108010005774 beta-Galactosidase Proteins 0.000 description 2
- QZPQTZZNNJUOLS-UHFFFAOYSA-N beta-lapachone Chemical compound C12=CC=CC=C2C(=O)C(=O)C2=C1OC(C)(C)CC2 QZPQTZZNNJUOLS-UHFFFAOYSA-N 0.000 description 2
- 229960001561 bleomycin Drugs 0.000 description 2
- OYVAGSVQBOHSSS-UAPAGMARSA-O bleomycin A2 Chemical compound N([C@H](C(=O)N[C@H](C)[C@@H](O)[C@H](C)C(=O)N[C@@H]([C@H](O)C)C(=O)NCCC=1SC=C(N=1)C=1SC=C(N=1)C(=O)NCCC[S+](C)C)[C@@H](O[C@H]1[C@H]([C@@H](O)[C@H](O)[C@H](CO)O1)O[C@@H]1[C@H]([C@@H](OC(N)=O)[C@H](O)[C@@H](CO)O1)O)C=1N=CNC=1)C(=O)C1=NC([C@H](CC(N)=O)NC[C@H](N)C(N)=O)=NC(N)=C1C OYVAGSVQBOHSSS-UAPAGMARSA-O 0.000 description 2
- 210000001772 blood platelet Anatomy 0.000 description 2
- 229940127093 camptothecin Drugs 0.000 description 2
- 230000010261 cell growth Effects 0.000 description 2
- 238000006243 chemical reaction Methods 0.000 description 2
- DQLATGHUWYMOKM-UHFFFAOYSA-L cisplatin Chemical compound N[Pt](N)(Cl)Cl DQLATGHUWYMOKM-UHFFFAOYSA-L 0.000 description 2
- 229960004316 cisplatin Drugs 0.000 description 2
- ACSIXWWBWUQEHA-UHFFFAOYSA-N clodronic acid Chemical compound OP(O)(=O)C(Cl)(Cl)P(O)(O)=O ACSIXWWBWUQEHA-UHFFFAOYSA-N 0.000 description 2
- 238000010367 cloning Methods 0.000 description 2
- 238000002648 combination therapy Methods 0.000 description 2
- 238000012875 competitive assay Methods 0.000 description 2
- 230000002860 competitive effect Effects 0.000 description 2
- 150000001875 compounds Chemical class 0.000 description 2
- 238000010276 construction Methods 0.000 description 2
- 238000007796 conventional method Methods 0.000 description 2
- 229940111134 coxibs Drugs 0.000 description 2
- PSNOPSMXOBPNNV-VVCTWANISA-N cryptophycin 1 Chemical compound C1=C(Cl)C(OC)=CC=C1C[C@@H]1C(=O)NC[C@@H](C)C(=O)O[C@@H](CC(C)C)C(=O)O[C@H]([C@H](C)[C@@H]2[C@H](O2)C=2C=CC=CC=2)C/C=C/C(=O)N1 PSNOPSMXOBPNNV-VVCTWANISA-N 0.000 description 2
- PSNOPSMXOBPNNV-UHFFFAOYSA-N cryptophycin-327 Natural products C1=C(Cl)C(OC)=CC=C1CC1C(=O)NCC(C)C(=O)OC(CC(C)C)C(=O)OC(C(C)C2C(O2)C=2C=CC=CC=2)CC=CC(=O)N1 PSNOPSMXOBPNNV-UHFFFAOYSA-N 0.000 description 2
- 239000003255 cyclooxygenase 2 inhibitor Substances 0.000 description 2
- OPTASPLRGRRNAP-UHFFFAOYSA-N cytosine Chemical compound NC=1C=CNC(=O)N=1 OPTASPLRGRRNAP-UHFFFAOYSA-N 0.000 description 2
- 229960003901 dacarbazine Drugs 0.000 description 2
- 229960000975 daunorubicin Drugs 0.000 description 2
- VSJKWCGYPAHWDS-UHFFFAOYSA-N dl-camptothecin Natural products C1=CC=C2C=C(CN3C4=CC5=C(C3=O)COC(=O)C5(O)CC)C4=NC2=C1 VSJKWCGYPAHWDS-UHFFFAOYSA-N 0.000 description 2
- 238000012377 drug delivery Methods 0.000 description 2
- 239000012636 effector Substances 0.000 description 2
- VLCYCQAOQCDTCN-UHFFFAOYSA-N eflornithine Chemical compound NCCCC(N)(C(F)F)C(O)=O VLCYCQAOQCDTCN-UHFFFAOYSA-N 0.000 description 2
- 229940073621 enbrel Drugs 0.000 description 2
- 229960001904 epirubicin Drugs 0.000 description 2
- AAKJLRGGTJKAMG-UHFFFAOYSA-N erlotinib Chemical compound C=12C=C(OCCOC)C(OCCOC)=CC2=NC=NC=1NC1=CC=CC(C#C)=C1 AAKJLRGGTJKAMG-UHFFFAOYSA-N 0.000 description 2
- 229960005420 etoposide Drugs 0.000 description 2
- OVBPIULPVIDEAO-LBPRGKRZSA-N folic acid Chemical compound C=1N=C2NC(N)=NC(=O)C2=NC=1CNC1=CC=C(C(=O)N[C@@H](CCC(O)=O)C(O)=O)C=C1 OVBPIULPVIDEAO-LBPRGKRZSA-N 0.000 description 2
- 238000013467 fragmentation Methods 0.000 description 2
- 238000006062 fragmentation reaction Methods 0.000 description 2
- CHPZKNULDCNCBW-UHFFFAOYSA-N gallium nitrate Chemical compound [Ga+3].[O-][N+]([O-])=O.[O-][N+]([O-])=O.[O-][N+]([O-])=O CHPZKNULDCNCBW-UHFFFAOYSA-N 0.000 description 2
- PCHJSUWPFVWCPO-UHFFFAOYSA-N gold Chemical compound [Au] PCHJSUWPFVWCPO-UHFFFAOYSA-N 0.000 description 2
- 229910052737 gold Inorganic materials 0.000 description 2
- 239000010931 gold Substances 0.000 description 2
- 230000012447 hatching Effects 0.000 description 2
- 208000005252 hepatitis A Diseases 0.000 description 2
- 201000008110 hereditary spherocytosis type 3 Diseases 0.000 description 2
- 229960001101 ifosfamide Drugs 0.000 description 2
- HOMGKSMUEGBAAB-UHFFFAOYSA-N ifosfamide Chemical compound ClCCNP1(=O)OCCCN1CCCl HOMGKSMUEGBAAB-UHFFFAOYSA-N 0.000 description 2
- YLMAHDNUQAMNNX-UHFFFAOYSA-N imatinib methanesulfonate Chemical compound CS(O)(=O)=O.C1CN(C)CCN1CC1=CC=C(C(=O)NC=2C=C(NC=3N=C(C=CN=3)C=3C=NC=CC=3)C(C)=CC=2)C=C1 YLMAHDNUQAMNNX-UHFFFAOYSA-N 0.000 description 2
- 230000001976 improved effect Effects 0.000 description 2
- 230000006698 induction Effects 0.000 description 2
- 208000015181 infectious disease Diseases 0.000 description 2
- 230000002458 infectious effect Effects 0.000 description 2
- 230000005764 inhibitory process Effects 0.000 description 2
- 238000002347 injection Methods 0.000 description 2
- 239000007924 injection Substances 0.000 description 2
- NOESYZHRGYRDHS-UHFFFAOYSA-N insulin Chemical compound N1C(=O)C(NC(=O)C(CCC(N)=O)NC(=O)C(CCC(O)=O)NC(=O)C(C(C)C)NC(=O)C(NC(=O)CN)C(C)CC)CSSCC(C(NC(CO)C(=O)NC(CC(C)C)C(=O)NC(CC=2C=CC(O)=CC=2)C(=O)NC(CCC(N)=O)C(=O)NC(CC(C)C)C(=O)NC(CCC(O)=O)C(=O)NC(CC(N)=O)C(=O)NC(CC=2C=CC(O)=CC=2)C(=O)NC(CSSCC(NC(=O)C(C(C)C)NC(=O)C(CC(C)C)NC(=O)C(CC=2C=CC(O)=CC=2)NC(=O)C(CC(C)C)NC(=O)C(C)NC(=O)C(CCC(O)=O)NC(=O)C(C(C)C)NC(=O)C(CC(C)C)NC(=O)C(CC=2NC=NC=2)NC(=O)C(CO)NC(=O)CNC2=O)C(=O)NCC(=O)NC(CCC(O)=O)C(=O)NC(CCCNC(N)=N)C(=O)NCC(=O)NC(CC=3C=CC=CC=3)C(=O)NC(CC=3C=CC=CC=3)C(=O)NC(CC=3C=CC(O)=CC=3)C(=O)NC(C(C)O)C(=O)N3C(CCC3)C(=O)NC(CCCCN)C(=O)NC(C)C(O)=O)C(=O)NC(CC(N)=O)C(O)=O)=O)NC(=O)C(C(C)CC)NC(=O)C(CO)NC(=O)C(C(C)O)NC(=O)C1CSSCC2NC(=O)C(CC(C)C)NC(=O)C(NC(=O)C(CCC(N)=O)NC(=O)C(CC(N)=O)NC(=O)C(NC(=O)C(N)CC=1C=CC=CC=1)C(C)C)CC1=CN=CN1 NOESYZHRGYRDHS-UHFFFAOYSA-N 0.000 description 2
- 229960000681 leflunomide Drugs 0.000 description 2
- 229960004338 leuprorelin Drugs 0.000 description 2
- 210000004072 lung Anatomy 0.000 description 2
- 230000036210 malignancy Effects 0.000 description 2
- 208000015486 malignant pancreatic neoplasm Diseases 0.000 description 2
- 239000003550 marker Substances 0.000 description 2
- HAWPXGHAZFHHAD-UHFFFAOYSA-N mechlorethamine Chemical compound ClCCN(C)CCCl HAWPXGHAZFHHAD-UHFFFAOYSA-N 0.000 description 2
- 229960004961 mechlorethamine Drugs 0.000 description 2
- SGDBTWWWUNNDEQ-LBPRGKRZSA-N melphalan Chemical compound OC(=O)[C@@H](N)CC1=CC=C(N(CCCl)CCCl)C=C1 SGDBTWWWUNNDEQ-LBPRGKRZSA-N 0.000 description 2
- 229960001924 melphalan Drugs 0.000 description 2
- 229960001428 mercaptopurine Drugs 0.000 description 2
- 210000004688 microtubule Anatomy 0.000 description 2
- 229960004857 mitomycin Drugs 0.000 description 2
- 229960001156 mitoxantrone Drugs 0.000 description 2
- KKZJGLLVHKMTCM-UHFFFAOYSA-N mitoxantrone Chemical compound O=C1C2=C(O)C=CC(O)=C2C(=O)C2=C1C(NCCNCCO)=CC=C2NCCNCCO KKZJGLLVHKMTCM-UHFFFAOYSA-N 0.000 description 2
- 201000006417 multiple sclerosis Diseases 0.000 description 2
- QZGIWPZCWHMVQL-UIYAJPBUSA-N neocarzinostatin chromophore Chemical compound O1[C@H](C)[C@H](O)[C@H](O)[C@@H](NC)[C@H]1O[C@@H]1C/2=C/C#C[C@H]3O[C@@]3([C@@H]3OC(=O)OC3)C#CC\2=C[C@H]1OC(=O)C1=C(O)C=CC2=C(C)C=C(OC)C=C12 QZGIWPZCWHMVQL-UIYAJPBUSA-N 0.000 description 2
- 229960001756 oxaliplatin Drugs 0.000 description 2
- DWAFYCQODLXJNR-BNTLRKBRSA-L oxaliplatin Chemical compound O1C(=O)C(=O)O[Pt]11N[C@@H]2CCCC[C@H]2N1 DWAFYCQODLXJNR-BNTLRKBRSA-L 0.000 description 2
- WRUUGTRCQOWXEG-UHFFFAOYSA-N pamidronate Chemical compound NCCC(O)(P(O)(O)=O)P(O)(O)=O WRUUGTRCQOWXEG-UHFFFAOYSA-N 0.000 description 2
- 201000002528 pancreatic cancer Diseases 0.000 description 2
- 208000008443 pancreatic carcinoma Diseases 0.000 description 2
- 230000026731 phosphorylation Effects 0.000 description 2
- 238000006366 phosphorylation reaction Methods 0.000 description 2
- BASFCYQUMIYNBI-UHFFFAOYSA-N platinum Chemical compound [Pt] BASFCYQUMIYNBI-UHFFFAOYSA-N 0.000 description 2
- 230000008488 polyadenylation Effects 0.000 description 2
- 239000000256 polyoxyethylene sorbitan monolaurate Substances 0.000 description 2
- 235000010486 polyoxyethylene sorbitan monolaurate Nutrition 0.000 description 2
- 238000001556 precipitation Methods 0.000 description 2
- XOFYZVNMUHMLCC-ZPOLXVRWSA-N prednisone Chemical compound O=C1C=C[C@]2(C)[C@H]3C(=O)C[C@](C)([C@@](CC4)(O)C(=O)CO)[C@@H]4[C@@H]3CCC2=C1 XOFYZVNMUHMLCC-ZPOLXVRWSA-N 0.000 description 2
- 229960004618 prednisone Drugs 0.000 description 2
- 238000002360 preparation method Methods 0.000 description 2
- 238000012545 processing Methods 0.000 description 2
- 239000000047 product Substances 0.000 description 2
- 230000002062 proliferating effect Effects 0.000 description 2
- 230000035755 proliferation Effects 0.000 description 2
- RXWNCPJZOCPEPQ-NVWDDTSBSA-N puromycin Chemical compound C1=CC(OC)=CC=C1C[C@H](N)C(=O)N[C@H]1[C@@H](O)[C@H](N2C3=NC=NC(=C3N=C2)N(C)C)O[C@@H]1CO RXWNCPJZOCPEPQ-NVWDDTSBSA-N 0.000 description 2
- GZUITABIAKMVPG-UHFFFAOYSA-N raloxifene Chemical compound C1=CC(O)=CC=C1C1=C(C(=O)C=2C=CC(OCCN3CCCCC3)=CC=2)C2=CC=C(O)C=C2S1 GZUITABIAKMVPG-UHFFFAOYSA-N 0.000 description 2
- 238000010188 recombinant method Methods 0.000 description 2
- 229940116176 remicade Drugs 0.000 description 2
- 108091008146 restriction endonucleases Proteins 0.000 description 2
- 150000003839 salts Chemical class 0.000 description 2
- 201000000306 sarcoidosis Diseases 0.000 description 2
- 239000000333 selective estrogen receptor modulator Substances 0.000 description 2
- 229940095743 selective estrogen receptor modulator Drugs 0.000 description 2
- 238000010532 solid phase synthesis reaction Methods 0.000 description 2
- 230000004936 stimulating effect Effects 0.000 description 2
- 230000000638 stimulation Effects 0.000 description 2
- PVYJZLYGTZKPJE-UHFFFAOYSA-N streptonigrin Chemical compound C=1C=C2C(=O)C(OC)=C(N)C(=O)C2=NC=1C(C=1N)=NC(C(O)=O)=C(C)C=1C1=CC=C(OC)C(OC)=C1O PVYJZLYGTZKPJE-UHFFFAOYSA-N 0.000 description 2
- 208000024891 symptom Diseases 0.000 description 2
- 238000003786 synthesis reaction Methods 0.000 description 2
- 238000010189 synthetic method Methods 0.000 description 2
- 201000000596 systemic lupus erythematosus Diseases 0.000 description 2
- 229940063683 taxotere Drugs 0.000 description 2
- 229960001196 thiotepa Drugs 0.000 description 2
- 206010043778 thyroiditis Diseases 0.000 description 2
- 229960003087 tioguanine Drugs 0.000 description 2
- 230000000699 topical effect Effects 0.000 description 2
- UCFGDBYHRUNTLO-QHCPKHFHSA-N topotecan Chemical class C1=C(O)C(CN(C)C)=C2C=C(CN3C4=CC5=C(C3=O)COC(=O)[C@]5(O)CC)C4=NC2=C1 UCFGDBYHRUNTLO-QHCPKHFHSA-N 0.000 description 2
- 231100000331 toxic Toxicity 0.000 description 2
- 230000002588 toxic effect Effects 0.000 description 2
- 210000004881 tumor cell Anatomy 0.000 description 2
- 229940121358 tyrosine kinase inhibitor Drugs 0.000 description 2
- 239000005483 tyrosine kinase inhibitor Substances 0.000 description 2
- 150000004917 tyrosine kinase inhibitor derivatives Chemical class 0.000 description 2
- 229960004355 vindesine Drugs 0.000 description 2
- UGGWPQSBPIFKDZ-KOTLKJBCSA-N vindesine Chemical compound C([C@@H](C[C@]1(C(=O)OC)C=2C(=CC3=C([C@]45[C@H]([C@@]([C@H](O)[C@]6(CC)C=CCN([C@H]56)CC4)(O)C(N)=O)N3C)C=2)OC)C[C@@](C2)(O)CC)N2CCC2=C1N=C1[C]2C=CC=C1 UGGWPQSBPIFKDZ-KOTLKJBCSA-N 0.000 description 2
- 229950009268 zinostatin Drugs 0.000 description 2
- 229960004276 zoledronic acid Drugs 0.000 description 2
- NNJPGOLRFBJNIW-HNNXBMFYSA-N (-)-demecolcine Chemical compound C1=C(OC)C(=O)C=C2[C@@H](NC)CCC3=CC(OC)=C(OC)C(OC)=C3C2=C1 NNJPGOLRFBJNIW-HNNXBMFYSA-N 0.000 description 1
- YXTKHLHCVFUPPT-YYFJYKOTSA-N (2s)-2-[[4-[(2-amino-5-formyl-4-oxo-1,6,7,8-tetrahydropteridin-6-yl)methylamino]benzoyl]amino]pentanedioic acid;(1r,2r)-1,2-dimethanidylcyclohexane;5-fluoro-1h-pyrimidine-2,4-dione;oxalic acid;platinum(2+) Chemical compound [Pt+2].OC(=O)C(O)=O.[CH2-][C@@H]1CCCC[C@H]1[CH2-].FC1=CNC(=O)NC1=O.C1NC=2NC(N)=NC(=O)C=2N(C=O)C1CNC1=CC=C(C(=O)N[C@@H](CCC(O)=O)C(O)=O)C=C1 YXTKHLHCVFUPPT-YYFJYKOTSA-N 0.000 description 1
- FLWWDYNPWOSLEO-HQVZTVAUSA-N (2s)-2-[[4-[1-(2-amino-4-oxo-1h-pteridin-6-yl)ethyl-methylamino]benzoyl]amino]pentanedioic acid Chemical compound C=1N=C2NC(N)=NC(=O)C2=NC=1C(C)N(C)C1=CC=C(C(=O)N[C@@H](CCC(O)=O)C(O)=O)C=C1 FLWWDYNPWOSLEO-HQVZTVAUSA-N 0.000 description 1
- CGMTUJFWROPELF-YPAAEMCBSA-N (3E,5S)-5-[(2S)-butan-2-yl]-3-(1-hydroxyethylidene)pyrrolidine-2,4-dione Chemical compound CC[C@H](C)[C@@H]1NC(=O)\C(=C(/C)O)C1=O CGMTUJFWROPELF-YPAAEMCBSA-N 0.000 description 1
- OQANPHBRHBJGNZ-FYJGNVAPSA-N (3e)-6-oxo-3-[[4-(pyridin-2-ylsulfamoyl)phenyl]hydrazinylidene]cyclohexa-1,4-diene-1-carboxylic acid Chemical compound C1=CC(=O)C(C(=O)O)=C\C1=N\NC1=CC=C(S(=O)(=O)NC=2N=CC=CC=2)C=C1 OQANPHBRHBJGNZ-FYJGNVAPSA-N 0.000 description 1
- TVIRNGFXQVMMGB-OFWIHYRESA-N (3s,6r,10r,13e,16s)-16-[(2r,3r,4s)-4-chloro-3-hydroxy-4-phenylbutan-2-yl]-10-[(3-chloro-4-methoxyphenyl)methyl]-6-methyl-3-(2-methylpropyl)-1,4-dioxa-8,11-diazacyclohexadec-13-ene-2,5,9,12-tetrone Chemical compound C1=C(Cl)C(OC)=CC=C1C[C@@H]1C(=O)NC[C@@H](C)C(=O)O[C@@H](CC(C)C)C(=O)O[C@H]([C@H](C)[C@@H](O)[C@@H](Cl)C=2C=CC=CC=2)C/C=C/C(=O)N1 TVIRNGFXQVMMGB-OFWIHYRESA-N 0.000 description 1
- FFTVPQUHLQBXQZ-KVUCHLLUSA-N (4s,4as,5ar,12ar)-4,7-bis(dimethylamino)-1,10,11,12a-tetrahydroxy-3,12-dioxo-4a,5,5a,6-tetrahydro-4h-tetracene-2-carboxamide Chemical compound C1C2=C(N(C)C)C=CC(O)=C2C(O)=C2[C@@H]1C[C@H]1[C@H](N(C)C)C(=O)C(C(N)=O)=C(O)[C@@]1(O)C2=O FFTVPQUHLQBXQZ-KVUCHLLUSA-N 0.000 description 1
- XRBSKUSTLXISAB-XVVDYKMHSA-N (5r,6r,7r,8r)-8-hydroxy-7-(hydroxymethyl)-5-(3,4,5-trimethoxyphenyl)-5,6,7,8-tetrahydrobenzo[f][1,3]benzodioxole-6-carboxylic acid Chemical compound COC1=C(OC)C(OC)=CC([C@@H]2C3=CC=4OCOC=4C=C3[C@H](O)[C@@H](CO)[C@@H]2C(O)=O)=C1 XRBSKUSTLXISAB-XVVDYKMHSA-N 0.000 description 1
- AESVUZLWRXEGEX-DKCAWCKPSA-N (7S,9R)-7-[(2S,4R,5R,6R)-4-amino-5-hydroxy-6-methyloxan-2-yl]oxy-6,9,11-trihydroxy-9-(2-hydroxyacetyl)-4-methoxy-8,10-dihydro-7H-tetracene-5,12-dione iron(3+) Chemical compound [Fe+3].COc1cccc2C(=O)c3c(O)c4C[C@@](O)(C[C@H](O[C@@H]5C[C@@H](N)[C@@H](O)[C@@H](C)O5)c4c(O)c3C(=O)c12)C(=O)CO AESVUZLWRXEGEX-DKCAWCKPSA-N 0.000 description 1
- RCFNNLSZHVHCEK-IMHLAKCZSA-N (7s,9s)-7-(4-amino-6-methyloxan-2-yl)oxy-6,9,11-trihydroxy-9-(2-hydroxyacetyl)-4-methoxy-8,10-dihydro-7h-tetracene-5,12-dione;hydrochloride Chemical compound [Cl-].O([C@H]1C[C@@](O)(CC=2C(O)=C3C(=O)C=4C=CC=C(C=4C(=O)C3=C(O)C=21)OC)C(=O)CO)C1CC([NH3+])CC(C)O1 RCFNNLSZHVHCEK-IMHLAKCZSA-N 0.000 description 1
- NOPNWHSMQOXAEI-PUCKCBAPSA-N (7s,9s)-7-[(2r,4s,5s,6s)-4-(2,3-dihydropyrrol-1-yl)-5-hydroxy-6-methyloxan-2-yl]oxy-6,9,11-trihydroxy-9-(2-hydroxyacetyl)-4-methoxy-8,10-dihydro-7h-tetracene-5,12-dione Chemical group N1([C@H]2C[C@@H](O[C@@H](C)[C@H]2O)O[C@H]2C[C@@](O)(CC=3C(O)=C4C(=O)C=5C=CC=C(C=5C(=O)C4=C(O)C=32)OC)C(=O)CO)CCC=C1 NOPNWHSMQOXAEI-PUCKCBAPSA-N 0.000 description 1
- FPVKHBSQESCIEP-UHFFFAOYSA-N (8S)-3-(2-deoxy-beta-D-erythro-pentofuranosyl)-3,6,7,8-tetrahydroimidazo[4,5-d][1,3]diazepin-8-ol Natural products C1C(O)C(CO)OC1N1C(NC=NCC2O)=C2N=C1 FPVKHBSQESCIEP-UHFFFAOYSA-N 0.000 description 1
- LKJPYSCBVHEWIU-KRWDZBQOSA-N (R)-bicalutamide Chemical compound C([C@@](O)(C)C(=O)NC=1C=C(C(C#N)=CC=1)C(F)(F)F)S(=O)(=O)C1=CC=C(F)C=C1 LKJPYSCBVHEWIU-KRWDZBQOSA-N 0.000 description 1
- AGNGYMCLFWQVGX-AGFFZDDWSA-N (e)-1-[(2s)-2-amino-2-carboxyethoxy]-2-diazonioethenolate Chemical compound OC(=O)[C@@H](N)CO\C([O-])=C\[N+]#N AGNGYMCLFWQVGX-AGFFZDDWSA-N 0.000 description 1
- WNXJIVFYUVYPPR-UHFFFAOYSA-N 1,3-dioxolane Chemical compound C1COCO1 WNXJIVFYUVYPPR-UHFFFAOYSA-N 0.000 description 1
- AVDPRCFLZAVJQF-UHFFFAOYSA-N 2,3,4-trihydroxy-8-(2-hydroxyacetyl)-1-methoxytetracene-5,12-dione Chemical compound OCC(=O)C1=CC=C2C=C(C(=O)C=3C(OC)=C(O)C(O)=C(O)C=3C3=O)C3=CC2=C1 AVDPRCFLZAVJQF-UHFFFAOYSA-N 0.000 description 1
- BTOTXLJHDSNXMW-POYBYMJQSA-N 2,3-dideoxyuridine Chemical compound O1[C@H](CO)CC[C@@H]1N1C(=O)NC(=O)C=C1 BTOTXLJHDSNXMW-POYBYMJQSA-N 0.000 description 1
- BOMZMNZEXMAQQW-UHFFFAOYSA-N 2,5,11-trimethyl-6h-pyrido[4,3-b]carbazol-2-ium-9-ol;acetate Chemical compound CC([O-])=O.C[N+]1=CC=C2C(C)=C(NC=3C4=CC(O)=CC=3)C4=C(C)C2=C1 BOMZMNZEXMAQQW-UHFFFAOYSA-N 0.000 description 1
- BGFTWECWAICPDG-UHFFFAOYSA-N 2-[bis(4-chlorophenyl)methyl]-4-n-[3-[bis(4-chlorophenyl)methyl]-4-(dimethylamino)phenyl]-1-n,1-n-dimethylbenzene-1,4-diamine Chemical compound C1=C(C(C=2C=CC(Cl)=CC=2)C=2C=CC(Cl)=CC=2)C(N(C)C)=CC=C1NC(C=1)=CC=C(N(C)C)C=1C(C=1C=CC(Cl)=CC=1)C1=CC=C(Cl)C=C1 BGFTWECWAICPDG-UHFFFAOYSA-N 0.000 description 1
- QCXJFISCRQIYID-IAEPZHFASA-N 2-amino-1-n-[(3s,6s,7r,10s,16s)-3-[(2s)-butan-2-yl]-7,11,14-trimethyl-2,5,9,12,15-pentaoxo-10-propan-2-yl-8-oxa-1,4,11,14-tetrazabicyclo[14.3.0]nonadecan-6-yl]-4,6-dimethyl-3-oxo-9-n-[(3s,6s,7r,10s,16s)-7,11,14-trimethyl-2,5,9,12,15-pentaoxo-3,10-di(propa Chemical compound C[C@H]1OC(=O)[C@H](C(C)C)N(C)C(=O)CN(C)C(=O)[C@@H]2CCCN2C(=O)[C@H](C(C)C)NC(=O)[C@H]1NC(=O)C1=C(N=C2C(C(=O)N[C@@H]3C(=O)N[C@H](C(N4CCC[C@H]4C(=O)N(C)CC(=O)N(C)[C@@H](C(C)C)C(=O)O[C@@H]3C)=O)[C@@H](C)CC)=C(N)C(=O)C(C)=C2O2)C2=C(C)C=C1 QCXJFISCRQIYID-IAEPZHFASA-N 0.000 description 1
- FDAYLTPAFBGXAB-UHFFFAOYSA-N 2-chloro-n,n-bis(2-chloroethyl)ethanamine Chemical compound ClCCN(CCCl)CCCl FDAYLTPAFBGXAB-UHFFFAOYSA-N 0.000 description 1
- LGEXGKUJMFHVSY-UHFFFAOYSA-N 2-n,4-n,6-n-trimethyl-1,3,5-triazine-2,4,6-triamine Chemical compound CNC1=NC(NC)=NC(NC)=N1 LGEXGKUJMFHVSY-UHFFFAOYSA-N 0.000 description 1
- CTRPRMNBTVRDFH-UHFFFAOYSA-N 2-n-methyl-1,3,5-triazine-2,4,6-triamine Chemical class CNC1=NC(N)=NC(N)=N1 CTRPRMNBTVRDFH-UHFFFAOYSA-N 0.000 description 1
- AOPRXJXHLWYPQR-UHFFFAOYSA-N 2-phenoxyacetamide Chemical class NC(=O)COC1=CC=CC=C1 AOPRXJXHLWYPQR-UHFFFAOYSA-N 0.000 description 1
- NDMPLJNOPCLANR-UHFFFAOYSA-N 3,4-dihydroxy-15-(4-hydroxy-18-methoxycarbonyl-5,18-seco-ibogamin-18-yl)-16-methoxy-1-methyl-6,7-didehydro-aspidospermidine-3-carboxylic acid methyl ester Natural products C1C(CC)(O)CC(CC2(C(=O)OC)C=3C(=CC4=C(C56C(C(C(O)C7(CC)C=CCN(C67)CC5)(O)C(=O)OC)N4C)C=3)OC)CN1CCC1=C2NC2=CC=CC=C12 NDMPLJNOPCLANR-UHFFFAOYSA-N 0.000 description 1
- XMTQQYYKAHVGBJ-UHFFFAOYSA-N 3-(3,4-DICHLOROPHENYL)-1,1-DIMETHYLUREA Chemical compound CN(C)C(=O)NC1=CC=C(Cl)C(Cl)=C1 XMTQQYYKAHVGBJ-UHFFFAOYSA-N 0.000 description 1
- PWMYMKOUNYTVQN-UHFFFAOYSA-N 3-(8,8-diethyl-2-aza-8-germaspiro[4.5]decan-2-yl)-n,n-dimethylpropan-1-amine Chemical compound C1C[Ge](CC)(CC)CCC11CN(CCCN(C)C)CC1 PWMYMKOUNYTVQN-UHFFFAOYSA-N 0.000 description 1
- QGJZLNKBHJESQX-UHFFFAOYSA-N 3-Epi-Betulin-Saeure Natural products C1CC(O)C(C)(C)C2CCC3(C)C4(C)CCC5(C(O)=O)CCC(C(=C)C)C5C4CCC3C21C QGJZLNKBHJESQX-UHFFFAOYSA-N 0.000 description 1
- CLOUCVRNYSHRCF-UHFFFAOYSA-N 3beta-Hydroxy-20(29)-Lupen-3,27-oic acid Natural products C1CC(O)C(C)(C)C2CCC3(C)C4(C(O)=O)CCC5(C)CCC(C(=C)C)C5C4CCC3C21C CLOUCVRNYSHRCF-UHFFFAOYSA-N 0.000 description 1
- CLPFFLWZZBQMAO-UHFFFAOYSA-N 4-(5,6,7,8-tetrahydroimidazo[1,5-a]pyridin-5-yl)benzonitrile Chemical compound C1=CC(C#N)=CC=C1C1N2C=NC=C2CCC1 CLPFFLWZZBQMAO-UHFFFAOYSA-N 0.000 description 1
- QFVHZQCOUORWEI-UHFFFAOYSA-N 4-[(4-anilino-5-sulfonaphthalen-1-yl)diazenyl]-5-hydroxynaphthalene-2,7-disulfonic acid Chemical compound C=12C(O)=CC(S(O)(=O)=O)=CC2=CC(S(O)(=O)=O)=CC=1N=NC(C1=CC=CC(=C11)S(O)(=O)=O)=CC=C1NC1=CC=CC=C1 QFVHZQCOUORWEI-UHFFFAOYSA-N 0.000 description 1
- DODQJNMQWMSYGS-QPLCGJKRSA-N 4-[(z)-1-[4-[2-(dimethylamino)ethoxy]phenyl]-1-phenylbut-1-en-2-yl]phenol Chemical compound C=1C=C(O)C=CC=1C(/CC)=C(C=1C=CC(OCCN(C)C)=CC=1)/C1=CC=CC=C1 DODQJNMQWMSYGS-QPLCGJKRSA-N 0.000 description 1
- FWMNVWWHGCHHJJ-SKKKGAJSSA-N 4-amino-1-[(2r)-6-amino-2-[[(2r)-2-[[(2r)-2-[[(2r)-2-amino-3-phenylpropanoyl]amino]-3-phenylpropanoyl]amino]-4-methylpentanoyl]amino]hexanoyl]piperidine-4-carboxylic acid Chemical compound C([C@H](C(=O)N[C@H](CC(C)C)C(=O)N[C@H](CCCCN)C(=O)N1CCC(N)(CC1)C(O)=O)NC(=O)[C@H](N)CC=1C=CC=CC=1)C1=CC=CC=C1 FWMNVWWHGCHHJJ-SKKKGAJSSA-N 0.000 description 1
- TVZGACDUOSZQKY-LBPRGKRZSA-N 4-aminofolic acid Chemical compound C1=NC2=NC(N)=NC(N)=C2N=C1CNC1=CC=C(C(=O)N[C@@H](CCC(O)=O)C(O)=O)C=C1 TVZGACDUOSZQKY-LBPRGKRZSA-N 0.000 description 1
- IDPUKCWIGUEADI-UHFFFAOYSA-N 5-[bis(2-chloroethyl)amino]uracil Chemical compound ClCCN(CCCl)C1=CNC(=O)NC1=O IDPUKCWIGUEADI-UHFFFAOYSA-N 0.000 description 1
- NMUSYJAQQFHJEW-KVTDHHQDSA-N 5-azacytidine Chemical compound O=C1N=C(N)N=CN1[C@H]1[C@H](O)[C@H](O)[C@@H](CO)O1 NMUSYJAQQFHJEW-KVTDHHQDSA-N 0.000 description 1
- FHIDNBAQOFJWCA-UAKXSSHOSA-N 5-fluorouridine Chemical compound O[C@@H]1[C@H](O)[C@@H](CO)O[C@H]1N1C(=O)NC(=O)C(F)=C1 FHIDNBAQOFJWCA-UAKXSSHOSA-N 0.000 description 1
- WYXSYVWAUAUWLD-SHUUEZRQSA-N 6-azauridine Chemical compound O[C@@H]1[C@H](O)[C@@H](CO)O[C@H]1N1C(=O)NC(=O)C=N1 WYXSYVWAUAUWLD-SHUUEZRQSA-N 0.000 description 1
- ZGXJTSGNIOSYLO-UHFFFAOYSA-N 88755TAZ87 Chemical compound NCC(=O)CCC(O)=O ZGXJTSGNIOSYLO-UHFFFAOYSA-N 0.000 description 1
- FUXVKZWTXQUGMW-FQEVSTJZSA-N 9-Aminocamptothecin Chemical compound C1=CC(N)=C2C=C(CN3C4=CC5=C(C3=O)COC(=O)[C@]5(O)CC)C4=NC2=C1 FUXVKZWTXQUGMW-FQEVSTJZSA-N 0.000 description 1
- HDZZVAMISRMYHH-UHFFFAOYSA-N 9beta-Ribofuranosyl-7-deazaadenin Natural products C1=CC=2C(N)=NC=NC=2N1C1OC(CO)C(O)C1O HDZZVAMISRMYHH-UHFFFAOYSA-N 0.000 description 1
- ZOZKYEHVNDEUCO-DKXJUACHSA-N Aceglatone Chemical compound O1C(=O)[C@H](OC(C)=O)[C@H]2OC(=O)[C@@H](OC(=O)C)[C@H]21 ZOZKYEHVNDEUCO-DKXJUACHSA-N 0.000 description 1
- 241000208140 Acer Species 0.000 description 1
- REAQAWSENITKJL-DDWPSWQVSA-N Ala-Met-Asp-Tyr Chemical compound [H]N[C@@H](C)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(O)=O REAQAWSENITKJL-DDWPSWQVSA-N 0.000 description 1
- 108010012934 Albumin-Bound Paclitaxel Proteins 0.000 description 1
- 102000009027 Albumins Human genes 0.000 description 1
- 108010088751 Albumins Proteins 0.000 description 1
- CEIZFXOZIQNICU-UHFFFAOYSA-N Alternaria alternata Crofton-weed toxin Natural products CCC(C)C1NC(=O)C(C(C)=O)=C1O CEIZFXOZIQNICU-UHFFFAOYSA-N 0.000 description 1
- 206010002556 Ankylosing Spondylitis Diseases 0.000 description 1
- 108020000948 Antisense Oligonucleotides Proteins 0.000 description 1
- 101100107610 Arabidopsis thaliana ABCF4 gene Proteins 0.000 description 1
- 101100067974 Arabidopsis thaliana POP2 gene Proteins 0.000 description 1
- 239000004475 Arginine Substances 0.000 description 1
- 208000002109 Argyria Diseases 0.000 description 1
- 108010078554 Aromatase Proteins 0.000 description 1
- 206010003210 Arteriosclerosis Diseases 0.000 description 1
- 206010003445 Ascites Diseases 0.000 description 1
- 206010003827 Autoimmune hepatitis Diseases 0.000 description 1
- 206010050245 Autoimmune thrombocytopenia Diseases 0.000 description 1
- 108010008014 B-Cell Maturation Antigen Proteins 0.000 description 1
- 102000006942 B-Cell Maturation Antigen Human genes 0.000 description 1
- 102100022005 B-lymphocyte antigen CD20 Human genes 0.000 description 1
- VGGGPCQERPFHOB-MCIONIFRSA-N Bestatin Chemical compound CC(C)C[C@H](C(O)=O)NC(=O)[C@@H](O)[C@H](N)CC1=CC=CC=C1 VGGGPCQERPFHOB-MCIONIFRSA-N 0.000 description 1
- 102100026189 Beta-galactosidase Human genes 0.000 description 1
- DIZWSDNSTNAYHK-XGWVBXMLSA-N Betulinic acid Natural products CC(=C)[C@@H]1C[C@H]([C@H]2CC[C@]3(C)[C@H](CC[C@@H]4[C@@]5(C)CC[C@H](O)C(C)(C)[C@@H]5CC[C@@]34C)[C@@H]12)C(=O)O DIZWSDNSTNAYHK-XGWVBXMLSA-N 0.000 description 1
- 208000008439 Biliary Liver Cirrhosis Diseases 0.000 description 1
- 208000033222 Biliary cirrhosis primary Diseases 0.000 description 1
- 229940122361 Bisphosphonate Drugs 0.000 description 1
- 206010005003 Bladder cancer Diseases 0.000 description 1
- 241000283690 Bos taurus Species 0.000 description 1
- 208000003174 Brain Neoplasms Diseases 0.000 description 1
- 206010048962 Brain oedema Diseases 0.000 description 1
- MBABCNBNDNGODA-LTGLSHGVSA-N Bullatacin Natural products O=C1C(C[C@H](O)CCCCCCCCCC[C@@H](O)[C@@H]2O[C@@H]([C@@H]3O[C@H]([C@@H](O)CCCCCCCCCC)CC3)CC2)=C[C@H](C)O1 MBABCNBNDNGODA-LTGLSHGVSA-N 0.000 description 1
- 108010037003 Buserelin Proteins 0.000 description 1
- PYMDEDHDQYLBRT-DRIHCAFSSA-N Buserelin acetate Chemical compound CC(O)=O.CCNC(=O)[C@@H]1CCCN1C(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](COC(C)(C)C)NC(=O)[C@@H](NC(=O)[C@H](CO)NC(=O)[C@H](CC=1C2=CC=CC=C2NC=1)NC(=O)[C@H](CC=1NC=NC=1)NC(=O)[C@H]1NC(=O)CC1)CC1=CC=C(O)C=C1 PYMDEDHDQYLBRT-DRIHCAFSSA-N 0.000 description 1
- COVZYZSDYWQREU-UHFFFAOYSA-N Busulfan Chemical compound CS(=O)(=O)OCCCCOS(C)(=O)=O COVZYZSDYWQREU-UHFFFAOYSA-N 0.000 description 1
- 102100035351 Cadherin-related family member 2 Human genes 0.000 description 1
- 102100035355 Cadherin-related family member 3 Human genes 0.000 description 1
- 241000282472 Canis lupus familiaris Species 0.000 description 1
- GAGWJHPBXLXJQN-UHFFFAOYSA-N Capecitabine Natural products C1=C(F)C(NC(=O)OCCCCC)=NC(=O)N1C1C(O)C(O)C(C)O1 GAGWJHPBXLXJQN-UHFFFAOYSA-N 0.000 description 1
- 208000010667 Carcinoma of liver and intrahepatic biliary tract Diseases 0.000 description 1
- DLGOEMSEDOSKAD-UHFFFAOYSA-N Carmustine Chemical compound ClCCNC(=O)N(N=O)CCCl DLGOEMSEDOSKAD-UHFFFAOYSA-N 0.000 description 1
- 206010008342 Cervix carcinoma Diseases 0.000 description 1
- JWBOIMRXGHLCPP-UHFFFAOYSA-N Chloditan Chemical compound C=1C=CC=C(Cl)C=1C(C(Cl)Cl)C1=CC=C(Cl)C=C1 JWBOIMRXGHLCPP-UHFFFAOYSA-N 0.000 description 1
- MKQWTWSXVILIKJ-LXGUWJNJSA-N Chlorozotocin Chemical compound OC[C@@H](O)[C@@H](O)[C@H](O)[C@H](C=O)NC(=O)N(N=O)CCCl MKQWTWSXVILIKJ-LXGUWJNJSA-N 0.000 description 1
- 208000030939 Chronic inflammatory demyelinating polyneuropathy Diseases 0.000 description 1
- 108091033380 Coding strand Proteins 0.000 description 1
- 206010009900 Colitis ulcerative Diseases 0.000 description 1
- 208000035473 Communicable disease Diseases 0.000 description 1
- 229930188224 Cryptophycin Natural products 0.000 description 1
- 102000004127 Cytokines Human genes 0.000 description 1
- 108090000695 Cytokines Proteins 0.000 description 1
- 150000008574 D-amino acids Chemical class 0.000 description 1
- VVNCNSJFMMFHPL-VKHMYHEASA-N D-penicillamine Chemical compound CC(C)(S)[C@@H](N)C(O)=O VVNCNSJFMMFHPL-VKHMYHEASA-N 0.000 description 1
- 102000012410 DNA Ligases Human genes 0.000 description 1
- 108010061982 DNA Ligases Proteins 0.000 description 1
- 102000004594 DNA Polymerase I Human genes 0.000 description 1
- 108010017826 DNA Polymerase I Proteins 0.000 description 1
- 239000012624 DNA alkylating agent Substances 0.000 description 1
- 229940124087 DNA topoisomerase II inhibitor Drugs 0.000 description 1
- 102000016928 DNA-directed DNA polymerase Human genes 0.000 description 1
- 108010014303 DNA-directed DNA polymerase Proteins 0.000 description 1
- 108010092160 Dactinomycin Proteins 0.000 description 1
- WEAHRLBPCANXCN-UHFFFAOYSA-N Daunomycin Natural products CCC1(O)CC(OC2CC(N)C(O)C(C)O2)c3cc4C(=O)c5c(OC)cccc5C(=O)c4c(O)c3C1 WEAHRLBPCANXCN-UHFFFAOYSA-N 0.000 description 1
- XXGMIHXASFDFSM-UHFFFAOYSA-N Delta9-tetrahydrocannabinol Natural products CCCCCc1cc2OC(C)(C)C3CCC(=CC3c2c(O)c1O)C XXGMIHXASFDFSM-UHFFFAOYSA-N 0.000 description 1
- 206010012438 Dermatitis atopic Diseases 0.000 description 1
- 206010012442 Dermatitis contact Diseases 0.000 description 1
- 206010012646 Diabetic blindness Diseases 0.000 description 1
- 206010012689 Diabetic retinopathy Diseases 0.000 description 1
- AUGQEEXBDZWUJY-ZLJUKNTDSA-N Diacetoxyscirpenol Chemical compound C([C@]12[C@]3(C)[C@H](OC(C)=O)[C@@H](O)[C@H]1O[C@@H]1C=C(C)CC[C@@]13COC(=O)C)O2 AUGQEEXBDZWUJY-ZLJUKNTDSA-N 0.000 description 1
- 206010061818 Disease progression Diseases 0.000 description 1
- ZQZFYGIXNQKOAV-OCEACIFDSA-N Droloxifene Chemical compound C=1C=CC=CC=1C(/CC)=C(C=1C=C(O)C=CC=1)\C1=CC=C(OCCN(C)C)C=C1 ZQZFYGIXNQKOAV-OCEACIFDSA-N 0.000 description 1
- CYQFCXCEBYINGO-DLBZAZTESA-N Dronabinol Natural products C1=C(C)CC[C@H]2C(C)(C)OC3=CC(CCCCC)=CC(O)=C3[C@H]21 CYQFCXCEBYINGO-DLBZAZTESA-N 0.000 description 1
- 102000043859 Dynamin Human genes 0.000 description 1
- 108700021058 Dynamin Proteins 0.000 description 1
- 206010014733 Endometrial cancer Diseases 0.000 description 1
- 206010014759 Endometrial neoplasm Diseases 0.000 description 1
- 201000009273 Endometriosis Diseases 0.000 description 1
- 241000701867 Enterobacteria phage T7 Species 0.000 description 1
- OBMLHUPNRURLOK-XGRAFVIBSA-N Epitiostanol Chemical compound C1[C@@H]2S[C@@H]2C[C@]2(C)[C@H]3CC[C@](C)([C@H](CC4)O)[C@@H]4[C@@H]3CC[C@H]21 OBMLHUPNRURLOK-XGRAFVIBSA-N 0.000 description 1
- 241000283086 Equidae Species 0.000 description 1
- 206010015150 Erythema Diseases 0.000 description 1
- 206010015218 Erythema multiforme Diseases 0.000 description 1
- 241000588724 Escherichia coli Species 0.000 description 1
- 241000701959 Escherichia virus Lambda Species 0.000 description 1
- 241001524679 Escherichia virus M13 Species 0.000 description 1
- JOYRKODLDBILNP-UHFFFAOYSA-N Ethyl urethane Chemical compound CCOC(N)=O JOYRKODLDBILNP-UHFFFAOYSA-N 0.000 description 1
- DBVJJBKOTRCVKF-UHFFFAOYSA-N Etidronic acid Chemical compound OP(=O)(O)C(O)(C)P(O)(O)=O DBVJJBKOTRCVKF-UHFFFAOYSA-N 0.000 description 1
- 208000004332 Evans syndrome Diseases 0.000 description 1
- 241000282326 Felis catus Species 0.000 description 1
- MPJKWIXIYCLVCU-UHFFFAOYSA-N Folinic acid Natural products NC1=NC2=C(N(C=O)C(CNc3ccc(cc3)C(=O)NC(CCC(=O)O)CC(=O)O)CN2)C(=O)N1 MPJKWIXIYCLVCU-UHFFFAOYSA-N 0.000 description 1
- 208000004262 Food Hypersensitivity Diseases 0.000 description 1
- 206010016946 Food allergy Diseases 0.000 description 1
- 230000010190 G1 phase Effects 0.000 description 1
- 108700012941 GNRH1 Proteins 0.000 description 1
- 206010017993 Gastrointestinal neoplasms Diseases 0.000 description 1
- 108700028146 Genetic Enhancer Elements Proteins 0.000 description 1
- 108700039691 Genetic Promoter Regions Proteins 0.000 description 1
- 108010068370 Glutens Proteins 0.000 description 1
- 108090000288 Glycoproteins Proteins 0.000 description 1
- 102000003886 Glycoproteins Human genes 0.000 description 1
- 239000000579 Gonadotropin-Releasing Hormone Substances 0.000 description 1
- 108010069236 Goserelin Proteins 0.000 description 1
- 208000035895 Guillain-Barré syndrome Diseases 0.000 description 1
- HVLSXIKZNLPZJJ-TXZCQADKSA-N HA peptide Chemical compound C([C@@H](C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](C(C)C)C(=O)N1[C@@H](CCC1)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CC=1C=CC(O)=CC=1)C(=O)N[C@@H](C)C(O)=O)NC(=O)[C@H]1N(CCC1)C(=O)[C@@H](N)CC=1C=CC(O)=CC=1)C1=CC=C(O)C=C1 HVLSXIKZNLPZJJ-TXZCQADKSA-N 0.000 description 1
- 208000001204 Hashimoto Disease Diseases 0.000 description 1
- 208000030836 Hashimoto thyroiditis Diseases 0.000 description 1
- 208000035186 Hemolytic Autoimmune Anemia Diseases 0.000 description 1
- 206010073069 Hepatic cancer Diseases 0.000 description 1
- 208000005176 Hepatitis C Diseases 0.000 description 1
- 208000005331 Hepatitis D Diseases 0.000 description 1
- 208000037319 Hepatitis infectious Diseases 0.000 description 1
- 101000897405 Homo sapiens B-lymphocyte antigen CD20 Proteins 0.000 description 1
- 101000737811 Homo sapiens Cadherin-related family member 2 Proteins 0.000 description 1
- 101000737802 Homo sapiens Cadherin-related family member 3 Proteins 0.000 description 1
- 101100118549 Homo sapiens EGFR gene Proteins 0.000 description 1
- 101000610602 Homo sapiens Tumor necrosis factor receptor superfamily member 10C Proteins 0.000 description 1
- 101000610609 Homo sapiens Tumor necrosis factor receptor superfamily member 10D Proteins 0.000 description 1
- 108010000521 Human Growth Hormone Proteins 0.000 description 1
- 102000002265 Human Growth Hormone Human genes 0.000 description 1
- 239000000854 Human Growth Hormone Substances 0.000 description 1
- VSNHCAURESNICA-UHFFFAOYSA-N Hydroxyurea Chemical compound NC(=O)NO VSNHCAURESNICA-UHFFFAOYSA-N 0.000 description 1
- 101150098499 III gene Proteins 0.000 description 1
- MPBVHIBUJCELCL-UHFFFAOYSA-N Ibandronate Chemical compound CCCCCN(C)CCC(O)(P(O)(O)=O)P(O)(O)=O MPBVHIBUJCELCL-UHFFFAOYSA-N 0.000 description 1
- XDXDZDZNSLXDNA-TZNDIEGXSA-N Idarubicin Chemical compound C1[C@H](N)[C@H](O)[C@H](C)O[C@H]1O[C@@H]1C2=C(O)C(C(=O)C3=CC=CC=C3C3=O)=C3C(O)=C2C[C@@](O)(C(C)=O)C1 XDXDZDZNSLXDNA-TZNDIEGXSA-N 0.000 description 1
- XDXDZDZNSLXDNA-UHFFFAOYSA-N Idarubicin Natural products C1C(N)C(O)C(C)OC1OC1C2=C(O)C(C(=O)C3=CC=CC=C3C3=O)=C3C(O)=C2CC(O)(C(C)=O)C1 XDXDZDZNSLXDNA-UHFFFAOYSA-N 0.000 description 1
- 206010021245 Idiopathic thrombocytopenic purpura Diseases 0.000 description 1
- 208000029462 Immunodeficiency disease Diseases 0.000 description 1
- 108010067060 Immunoglobulin Variable Region Proteins 0.000 description 1
- 102000017727 Immunoglobulin Variable Region Human genes 0.000 description 1
- 102000004877 Insulin Human genes 0.000 description 1
- 108090001061 Insulin Proteins 0.000 description 1
- 102000014150 Interferons Human genes 0.000 description 1
- 108010050904 Interferons Proteins 0.000 description 1
- 108091029795 Intergenic region Proteins 0.000 description 1
- UETNIIAIRMUTSM-UHFFFAOYSA-N Jacareubin Natural products CC1(C)OC2=CC3Oc4c(O)c(O)ccc4C(=O)C3C(=C2C=C1)O UETNIIAIRMUTSM-UHFFFAOYSA-N 0.000 description 1
- 208000003456 Juvenile Arthritis Diseases 0.000 description 1
- QNAYBMKLOCPYGJ-REOHCLBHSA-N L-alanine Chemical compound C[C@H](N)C(O)=O QNAYBMKLOCPYGJ-REOHCLBHSA-N 0.000 description 1
- ODKSFYDXXFIFQN-BYPYZUCNSA-P L-argininium(2+) Chemical compound NC(=[NH2+])NCCC[C@H]([NH3+])C(O)=O ODKSFYDXXFIFQN-BYPYZUCNSA-P 0.000 description 1
- FFEARJCKVFRZRR-BYPYZUCNSA-N L-methionine Chemical compound CSCC[C@H](N)C(O)=O FFEARJCKVFRZRR-BYPYZUCNSA-N 0.000 description 1
- LRQKBLKVPFOOQJ-YFKPBYRVSA-N L-norleucine Chemical compound CCCC[C@H]([NH3+])C([O-])=O LRQKBLKVPFOOQJ-YFKPBYRVSA-N 0.000 description 1
- COLNVLDHVKWLRT-QMMMGPOBSA-N L-phenylalanine Chemical compound OC(=O)[C@@H](N)CC1=CC=CC=C1 COLNVLDHVKWLRT-QMMMGPOBSA-N 0.000 description 1
- QIVBCDIJIAJPQS-VIFPVBQESA-N L-tryptophane Chemical compound C1=CC=C2C(C[C@H](N)C(O)=O)=CNC2=C1 QIVBCDIJIAJPQS-VIFPVBQESA-N 0.000 description 1
- 125000002707 L-tryptophyl group Chemical group [H]C1=C([H])C([H])=C2C(C([C@](N([H])[H])(C(=O)[*])[H])([H])[H])=C([H])N([H])C2=C1[H] 0.000 description 1
- 239000005517 L01XE01 - Imatinib Substances 0.000 description 1
- 229920001491 Lentinan Polymers 0.000 description 1
- MEPSBMMZQBMKHM-UHFFFAOYSA-N Lomatiol Natural products CC(=C/CC1=C(O)C(=O)c2ccccc2C1=O)CO MEPSBMMZQBMKHM-UHFFFAOYSA-N 0.000 description 1
- GQYIWUVLTXOXAJ-UHFFFAOYSA-N Lomustine Chemical compound ClCCN(N=O)C(=O)NC1CCCCC1 GQYIWUVLTXOXAJ-UHFFFAOYSA-N 0.000 description 1
- 208000019693 Lung disease Diseases 0.000 description 1
- 206010058467 Lung neoplasm malignant Diseases 0.000 description 1
- 206010025323 Lymphomas Diseases 0.000 description 1
- 231100000002 MTT assay Toxicity 0.000 description 1
- 238000000134 MTT assay Methods 0.000 description 1
- 101710155913 Major envelope protein Proteins 0.000 description 1
- 229930126263 Maytansine Natural products 0.000 description 1
- 206010027476 Metastases Diseases 0.000 description 1
- 206010049567 Miller Fisher syndrome Diseases 0.000 description 1
- VFKZTMPDYBFSTM-KVTDHHQDSA-N Mitobronitol Chemical compound BrC[C@@H](O)[C@@H](O)[C@H](O)[C@H](O)CBr VFKZTMPDYBFSTM-KVTDHHQDSA-N 0.000 description 1
- 241000699670 Mus sp. Species 0.000 description 1
- 201000002481 Myositis Diseases 0.000 description 1
- NQTADLQHYWFPDB-UHFFFAOYSA-N N-Hydroxysuccinimide Chemical compound ON1C(=O)CCC1=O NQTADLQHYWFPDB-UHFFFAOYSA-N 0.000 description 1
- OVBPIULPVIDEAO-UHFFFAOYSA-N N-Pteroyl-L-glutaminsaeure Natural products C=1N=C2NC(N)=NC(=O)C2=NC=1CNC1=CC=C(C(=O)NC(CCC(O)=O)C(O)=O)C=C1 OVBPIULPVIDEAO-UHFFFAOYSA-N 0.000 description 1
- 108091007491 NSP3 Papain-like protease domains Proteins 0.000 description 1
- 206010029113 Neovascularisation Diseases 0.000 description 1
- 208000012902 Nervous system disease Diseases 0.000 description 1
- SYNHCENRCUAUNM-UHFFFAOYSA-N Nitrogen mustard N-oxide hydrochloride Chemical compound Cl.ClCC[N+]([O-])(C)CCCl SYNHCENRCUAUNM-UHFFFAOYSA-N 0.000 description 1
- KGTDRFCXGRULNK-UHFFFAOYSA-N Nogalamycin Natural products COC1C(OC)(C)C(OC)C(C)OC1OC1C2=C(O)C(C(=O)C3=C(O)C=C4C5(C)OC(C(C(C5O)N(C)C)O)OC4=C3C3=O)=C3C=C2C(C(=O)OC)C(C)(O)C1 KGTDRFCXGRULNK-UHFFFAOYSA-N 0.000 description 1
- 229930187135 Olivomycin Natural products 0.000 description 1
- 102000043276 Oncogene Human genes 0.000 description 1
- 108700020796 Oncogene Proteins 0.000 description 1
- 229910019142 PO4 Inorganic materials 0.000 description 1
- 241000609499 Palicourea Species 0.000 description 1
- 108010057150 Peplomycin Proteins 0.000 description 1
- 108010067902 Peptide Library Proteins 0.000 description 1
- 108090000608 Phosphoric Monoester Hydrolases Proteins 0.000 description 1
- 102000004160 Phosphoric Monoester Hydrolases Human genes 0.000 description 1
- 108010051742 Platelet-Derived Growth Factor beta Receptor Proteins 0.000 description 1
- 102100026547 Platelet-derived growth factor receptor beta Human genes 0.000 description 1
- 208000021738 Plummer disease Diseases 0.000 description 1
- 102000012338 Poly(ADP-ribose) Polymerases Human genes 0.000 description 1
- 108010061844 Poly(ADP-ribose) Polymerases Proteins 0.000 description 1
- 206010036049 Polycystic ovaries Diseases 0.000 description 1
- HFVNWDWLWUCIHC-GUPDPFMOSA-N Prednimustine Chemical compound O=C([C@@]1(O)CC[C@H]2[C@H]3[C@@H]([C@]4(C=CC(=O)C=C4CC3)C)[C@@H](O)C[C@@]21C)COC(=O)CCCC1=CC=C(N(CCCl)CCCl)C=C1 HFVNWDWLWUCIHC-GUPDPFMOSA-N 0.000 description 1
- 208000012654 Primary biliary cholangitis Diseases 0.000 description 1
- 241000288906 Primates Species 0.000 description 1
- 108010076504 Protein Sorting Signals Proteins 0.000 description 1
- 102100024924 Protein kinase C alpha type Human genes 0.000 description 1
- 101710109947 Protein kinase C alpha type Proteins 0.000 description 1
- 201000001263 Psoriatic Arthritis Diseases 0.000 description 1
- 208000036824 Psoriatic arthropathy Diseases 0.000 description 1
- AHHFEZNOXOZZQA-ZEBDFXRSSA-N Ranimustine Chemical compound CO[C@H]1O[C@H](CNC(=O)N(CCCl)N=O)[C@@H](O)[C@H](O)[C@H]1O AHHFEZNOXOZZQA-ZEBDFXRSSA-N 0.000 description 1
- 101710100968 Receptor tyrosine-protein kinase erbB-2 Proteins 0.000 description 1
- 108020004511 Recombinant DNA Proteins 0.000 description 1
- 208000006265 Renal cell carcinoma Diseases 0.000 description 1
- PLXBWHJQWKZRKG-UHFFFAOYSA-N Resazurin Chemical compound C1=CC(=O)C=C2OC3=CC(O)=CC=C3[N+]([O-])=C21 PLXBWHJQWKZRKG-UHFFFAOYSA-N 0.000 description 1
- 208000017442 Retinal disease Diseases 0.000 description 1
- 206010038923 Retinopathy Diseases 0.000 description 1
- 206010039085 Rhinitis allergic Diseases 0.000 description 1
- OWPCHSCAPHNHAV-UHFFFAOYSA-N Rhizoxin Natural products C1C(O)C2(C)OC2C=CC(C)C(OC(=O)C2)CC2CC2OC2C(=O)OC1C(C)C(OC)C(C)=CC=CC(C)=CC1=COC(C)=N1 OWPCHSCAPHNHAV-UHFFFAOYSA-N 0.000 description 1
- NSFWWJIQIKBZMJ-YKNYLIOZSA-N Roridin A Chemical compound C([C@]12[C@]3(C)[C@H]4C[C@H]1O[C@@H]1C=C(C)CC[C@@]13COC(=O)[C@@H](O)[C@H](C)CCO[C@H](\C=C\C=C/C(=O)O4)[C@H](O)C)O2 NSFWWJIQIKBZMJ-YKNYLIOZSA-N 0.000 description 1
- CIEYTVIYYGTCCI-UHFFFAOYSA-N SJ000286565 Natural products C1=CC=C2C(=O)C(CC=C(C)C)=C(O)C(=O)C2=C1 CIEYTVIYYGTCCI-UHFFFAOYSA-N 0.000 description 1
- 101100378956 Saccharomyces cerevisiae (strain ATCC 204508 / S288c) AMD2 gene Proteins 0.000 description 1
- 101100068078 Saccharomyces cerevisiae (strain ATCC 204508 / S288c) GCN4 gene Proteins 0.000 description 1
- 101100123851 Saccharomyces cerevisiae (strain ATCC 204508 / S288c) HER1 gene Proteins 0.000 description 1
- 206010039491 Sarcoma Diseases 0.000 description 1
- 206010039710 Scleroderma Diseases 0.000 description 1
- 206010040047 Sepsis Diseases 0.000 description 1
- 241000700584 Simplexvirus Species 0.000 description 1
- 208000021386 Sjogren Syndrome Diseases 0.000 description 1
- 206010041067 Small cell lung cancer Diseases 0.000 description 1
- VMHLLURERBWHNL-UHFFFAOYSA-M Sodium acetate Chemical compound [Na+].CC([O-])=O VMHLLURERBWHNL-UHFFFAOYSA-M 0.000 description 1
- 208000006045 Spondylarthropathies Diseases 0.000 description 1
- 108010088160 Staphylococcal Protein A Proteins 0.000 description 1
- QAOWNCQODCNURD-UHFFFAOYSA-L Sulfate Chemical compound [O-]S([O-])(=O)=O QAOWNCQODCNURD-UHFFFAOYSA-L 0.000 description 1
- 201000009594 Systemic Scleroderma Diseases 0.000 description 1
- 208000004732 Systemic Vasculitis Diseases 0.000 description 1
- 206010042953 Systemic sclerosis Diseases 0.000 description 1
- 230000024932 T cell mediated immunity Effects 0.000 description 1
- BXFOFFBJRFZBQZ-QYWOHJEZSA-N T-2 toxin Chemical compound C([C@@]12[C@]3(C)[C@H](OC(C)=O)[C@@H](O)[C@H]1O[C@H]1[C@]3(COC(C)=O)C[C@@H](C(=C1)C)OC(=O)CC(C)C)O2 BXFOFFBJRFZBQZ-QYWOHJEZSA-N 0.000 description 1
- 210000001744 T-lymphocyte Anatomy 0.000 description 1
- 241001116500 Taxus Species 0.000 description 1
- 241001116498 Taxus baccata Species 0.000 description 1
- CGMTUJFWROPELF-UHFFFAOYSA-N Tenuazonic acid Natural products CCC(C)C1NC(=O)C(=C(C)/O)C1=O CGMTUJFWROPELF-UHFFFAOYSA-N 0.000 description 1
- 241000473945 Theria <moth genus> Species 0.000 description 1
- 208000024770 Thyroid neoplasm Diseases 0.000 description 1
- DKJJVAGXPKPDRL-UHFFFAOYSA-N Tiludronic acid Chemical compound OP(O)(=O)C(P(O)(O)=O)SC1=CC=C(Cl)C=C1 DKJJVAGXPKPDRL-UHFFFAOYSA-N 0.000 description 1
- 101710183280 Topoisomerase Proteins 0.000 description 1
- 239000000317 Topoisomerase II Inhibitor Substances 0.000 description 1
- 101100218648 Toxoplasma gondii (strain ATCC 50611 / Me49) BFD1 gene Proteins 0.000 description 1
- 108700029229 Transcriptional Regulatory Elements Proteins 0.000 description 1
- UMILHIMHKXVDGH-UHFFFAOYSA-N Triethylene glycol diglycidyl ether Chemical compound C1OC1COCCOCCOCCOCC1CO1 UMILHIMHKXVDGH-UHFFFAOYSA-N 0.000 description 1
- FYAMXEPQQLNQDM-UHFFFAOYSA-N Tris(1-aziridinyl)phosphine oxide Chemical compound C1CN1P(N1CC1)(=O)N1CC1 FYAMXEPQQLNQDM-UHFFFAOYSA-N 0.000 description 1
- QIVBCDIJIAJPQS-UHFFFAOYSA-N Tryptophan Natural products C1=CC=C2C(CC(N)C(O)=O)=CNC2=C1 QIVBCDIJIAJPQS-UHFFFAOYSA-N 0.000 description 1
- 102000004243 Tubulin Human genes 0.000 description 1
- 108090000704 Tubulin Proteins 0.000 description 1
- 108060008683 Tumor Necrosis Factor Receptor Proteins 0.000 description 1
- 102100040115 Tumor necrosis factor receptor superfamily member 10C Human genes 0.000 description 1
- 102100040110 Tumor necrosis factor receptor superfamily member 10D Human genes 0.000 description 1
- 206010067584 Type 1 diabetes mellitus Diseases 0.000 description 1
- 201000006704 Ulcerative Colitis Diseases 0.000 description 1
- 208000007097 Urinary Bladder Neoplasms Diseases 0.000 description 1
- 208000025609 Urogenital disease Diseases 0.000 description 1
- 208000024780 Urticaria Diseases 0.000 description 1
- 208000006105 Uterine Cervical Neoplasms Diseases 0.000 description 1
- 206010046798 Uterine leiomyoma Diseases 0.000 description 1
- 108091008605 VEGF receptors Proteins 0.000 description 1
- 102000009484 Vascular Endothelial Growth Factor Receptors Human genes 0.000 description 1
- 206010047115 Vasculitis Diseases 0.000 description 1
- 241000863480 Vinca Species 0.000 description 1
- 229940122803 Vinca alkaloid Drugs 0.000 description 1
- 206010047741 Vulval cancer Diseases 0.000 description 1
- 208000004354 Vulvar Neoplasms Diseases 0.000 description 1
- 208000027207 Whipple disease Diseases 0.000 description 1
- VWQVUPCCIRVNHF-OUBTZVSYSA-N Yttrium-90 Chemical compound [90Y] VWQVUPCCIRVNHF-OUBTZVSYSA-N 0.000 description 1
- OGQICQVSFDPSEI-UHFFFAOYSA-N Zorac Chemical compound N1=CC(C(=O)OCC)=CC=C1C#CC1=CC=C(SCCC2(C)C)C2=C1 OGQICQVSFDPSEI-UHFFFAOYSA-N 0.000 description 1
- IFJUINDAXYAPTO-UUBSBJJBSA-N [(8r,9s,13s,14s,17s)-17-[2-[4-[4-[bis(2-chloroethyl)amino]phenyl]butanoyloxy]acetyl]oxy-13-methyl-6,7,8,9,11,12,14,15,16,17-decahydrocyclopenta[a]phenanthren-3-yl] benzoate Chemical compound C([C@@H]1[C@@H](C2=CC=3)CC[C@]4([C@H]1CC[C@@H]4OC(=O)COC(=O)CCCC=1C=CC(=CC=1)N(CCCl)CCCl)C)CC2=CC=3OC(=O)C1=CC=CC=C1 IFJUINDAXYAPTO-UUBSBJJBSA-N 0.000 description 1
- IHGLINDYFMDHJG-UHFFFAOYSA-N [2-(4-methoxyphenyl)-3,4-dihydronaphthalen-1-yl]-[4-(2-pyrrolidin-1-ylethoxy)phenyl]methanone Chemical compound C1=CC(OC)=CC=C1C(CCC1=CC=CC=C11)=C1C(=O)C(C=C1)=CC=C1OCCN1CCCC1 IHGLINDYFMDHJG-UHFFFAOYSA-N 0.000 description 1
- 108010023617 abarelix Proteins 0.000 description 1
- AIWRTTMUVOZGPW-HSPKUQOVSA-N abarelix Chemical compound C([C@@H](C(=O)N[C@H](CC(N)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCCNC(C)C)C(=O)N1[C@@H](CCC1)C(=O)N[C@H](C)C(N)=O)N(C)C(=O)[C@H](CO)NC(=O)[C@@H](CC=1C=NC=CC=1)NC(=O)[C@@H](CC=1C=CC(Cl)=CC=1)NC(=O)[C@@H](CC=1C=C2C=CC=CC2=CC=1)NC(C)=O)C1=CC=C(O)C=C1 AIWRTTMUVOZGPW-HSPKUQOVSA-N 0.000 description 1
- 229960002184 abarelix Drugs 0.000 description 1
- 229940028652 abraxane Drugs 0.000 description 1
- 239000002250 absorbent Substances 0.000 description 1
- 230000002745 absorbent Effects 0.000 description 1
- 208000033017 acquired idiopathic inflammatory myopathy Diseases 0.000 description 1
- 229930183665 actinomycin Natural products 0.000 description 1
- 239000013543 active substance Substances 0.000 description 1
- 229940037127 actonel Drugs 0.000 description 1
- 230000001154 acute effect Effects 0.000 description 1
- 238000007792 addition Methods 0.000 description 1
- 230000001919 adrenal effect Effects 0.000 description 1
- 210000004100 adrenal gland Anatomy 0.000 description 1
- 229940009456 adriamycin Drugs 0.000 description 1
- 238000001261 affinity purification Methods 0.000 description 1
- 206010064930 age-related macular degeneration Diseases 0.000 description 1
- 238000003349 alamar blue assay Methods 0.000 description 1
- 235000004279 alanine Nutrition 0.000 description 1
- 229940062527 alendronate Drugs 0.000 description 1
- 229930013930 alkaloid Natural products 0.000 description 1
- 229940045714 alkyl sulfonate alkylating agent Drugs 0.000 description 1
- 150000008052 alkyl sulfonates Chemical class 0.000 description 1
- 229940100198 alkylating agent Drugs 0.000 description 1
- 239000002168 alkylating agent Substances 0.000 description 1
- SHGAZHPCJJPHSC-YCNIQYBTSA-N all-trans-retinoic acid Chemical compound OC(=O)\C=C(/C)\C=C\C=C(/C)\C=C\C1=C(C)CCCC1(C)C SHGAZHPCJJPHSC-YCNIQYBTSA-N 0.000 description 1
- 208000026935 allergic disease Diseases 0.000 description 1
- 201000010105 allergic rhinitis Diseases 0.000 description 1
- 229960000473 altretamine Drugs 0.000 description 1
- 229960002749 aminolevulinic acid Drugs 0.000 description 1
- 229960003896 aminopterin Drugs 0.000 description 1
- 230000003321 amplification Effects 0.000 description 1
- 229960001220 amsacrine Drugs 0.000 description 1
- XCPGHVQEEXUHNC-UHFFFAOYSA-N amsacrine Chemical compound COC1=CC(NS(C)(=O)=O)=CC=C1NC1=C(C=CC=C2)C2=NC2=CC=CC=C12 XCPGHVQEEXUHNC-UHFFFAOYSA-N 0.000 description 1
- 229960002932 anastrozole Drugs 0.000 description 1
- BBDAGFIXKZCXAH-CCXZUQQUSA-N ancitabine Chemical compound N=C1C=CN2[C@@H]3O[C@H](CO)[C@@H](O)[C@@H]3OC2=N1 BBDAGFIXKZCXAH-CCXZUQQUSA-N 0.000 description 1
- 229950000242 ancitabine Drugs 0.000 description 1
- 239000003098 androgen Substances 0.000 description 1
- 229940030486 androgens Drugs 0.000 description 1
- 239000003817 anthracycline antibiotic agent Substances 0.000 description 1
- NUZWLKWWNNJHPT-UHFFFAOYSA-N anthralin Chemical compound C1C2=CC=CC(O)=C2C(=O)C2=C1C=CC=C2O NUZWLKWWNNJHPT-UHFFFAOYSA-N 0.000 description 1
- 230000002280 anti-androgenic effect Effects 0.000 description 1
- 229940046836 anti-estrogen Drugs 0.000 description 1
- 230000001833 anti-estrogenic effect Effects 0.000 description 1
- 230000003388 anti-hormonal effect Effects 0.000 description 1
- 230000000340 anti-metabolite Effects 0.000 description 1
- 230000000118 anti-neoplastic effect Effects 0.000 description 1
- 230000000259 anti-tumor effect Effects 0.000 description 1
- 239000000051 antiandrogen Substances 0.000 description 1
- 229940030495 antiandrogen sex hormone and modulator of the genital system Drugs 0.000 description 1
- 230000000890 antigenic effect Effects 0.000 description 1
- 229940100197 antimetabolite Drugs 0.000 description 1
- 239000002256 antimetabolite Substances 0.000 description 1
- 229940045687 antimetabolites folic acid analogs Drugs 0.000 description 1
- 229940045719 antineoplastic alkylating agent nitrosoureas Drugs 0.000 description 1
- 229940045713 antineoplastic alkylating drug ethylene imines Drugs 0.000 description 1
- 239000003435 antirheumatic agent Substances 0.000 description 1
- 239000000074 antisense oligonucleotide Substances 0.000 description 1
- 238000012230 antisense oligonucleotides Methods 0.000 description 1
- 238000003782 apoptosis assay Methods 0.000 description 1
- 238000013459 approach Methods 0.000 description 1
- 150000008209 arabinosides Chemical class 0.000 description 1
- 229940059756 arava Drugs 0.000 description 1
- ODKSFYDXXFIFQN-UHFFFAOYSA-N arginine Natural products OC(=O)C(N)CCCNC(N)=N ODKSFYDXXFIFQN-UHFFFAOYSA-N 0.000 description 1
- 229940078010 arimidex Drugs 0.000 description 1
- 229940087620 aromasin Drugs 0.000 description 1
- 239000003886 aromatase inhibitor Substances 0.000 description 1
- 229940046844 aromatase inhibitors Drugs 0.000 description 1
- 208000011775 arteriosclerosis disease Diseases 0.000 description 1
- 206010003246 arthritis Diseases 0.000 description 1
- 208000006673 asthma Diseases 0.000 description 1
- 230000003140 astrocytic effect Effects 0.000 description 1
- 201000008937 atopic dermatitis Diseases 0.000 description 1
- 230000001363 autoimmune Effects 0.000 description 1
- 201000000448 autoimmune hemolytic anemia Diseases 0.000 description 1
- 201000003710 autoimmune thrombocytopenic purpura Diseases 0.000 description 1
- 229960002756 azacitidine Drugs 0.000 description 1
- VSRXQHXAPYXROS-UHFFFAOYSA-N azanide;cyclobutane-1,1-dicarboxylic acid;platinum(2+) Chemical compound [NH2-].[NH2-].[Pt+2].OC(=O)C1(C(O)=O)CCC1 VSRXQHXAPYXROS-UHFFFAOYSA-N 0.000 description 1
- 229960002170 azathioprine Drugs 0.000 description 1
- LMEKQMALGUDUQG-UHFFFAOYSA-N azathioprine Chemical compound CN1C=NC([N+]([O-])=O)=C1SC1=NC=NC2=C1NC=N2 LMEKQMALGUDUQG-UHFFFAOYSA-N 0.000 description 1
- 150000001541 aziridines Chemical class 0.000 description 1
- 230000009286 beneficial effect Effects 0.000 description 1
- 102000005936 beta-Galactosidase Human genes 0.000 description 1
- QGJZLNKBHJESQX-FZFNOLFKSA-N betulinic acid Chemical compound C1C[C@H](O)C(C)(C)[C@@H]2CC[C@@]3(C)[C@]4(C)CC[C@@]5(C(O)=O)CC[C@@H](C(=C)C)[C@@H]5[C@H]4CC[C@@H]3[C@]21C QGJZLNKBHJESQX-FZFNOLFKSA-N 0.000 description 1
- 229960000997 bicalutamide Drugs 0.000 description 1
- 230000000975 bioactive effect Effects 0.000 description 1
- 229920001222 biopolymer Polymers 0.000 description 1
- 150000004663 bisphosphonates Chemical class 0.000 description 1
- 201000000053 blastoma Diseases 0.000 description 1
- 208000006752 brain edema Diseases 0.000 description 1
- 238000009395 breeding Methods 0.000 description 1
- 230000001488 breeding effect Effects 0.000 description 1
- 229960005520 bryostatin Drugs 0.000 description 1
- MJQUEDHRCUIRLF-TVIXENOKSA-N bryostatin 1 Chemical compound C([C@@H]1CC(/[C@@H]([C@@](C(C)(C)/C=C/2)(O)O1)OC(=O)/C=C/C=C/CCC)=C\C(=O)OC)[C@H]([C@@H](C)O)OC(=O)C[C@H](O)C[C@@H](O1)C[C@H](OC(C)=O)C(C)(C)[C@]1(O)C[C@@H]1C\C(=C\C(=O)OC)C[C@H]\2O1 MJQUEDHRCUIRLF-TVIXENOKSA-N 0.000 description 1
- MUIWQCKLQMOUAT-AKUNNTHJSA-N bryostatin 20 Natural products COC(=O)C=C1C[C@@]2(C)C[C@]3(O)O[C@](C)(C[C@@H](O)CC(=O)O[C@](C)(C[C@@]4(C)O[C@](O)(CC5=CC(=O)O[C@]45C)C(C)(C)C=C[C@@](C)(C1)O2)[C@@H](C)O)C[C@H](OC(=O)C(C)(C)C)C3(C)C MUIWQCKLQMOUAT-AKUNNTHJSA-N 0.000 description 1
- MBABCNBNDNGODA-LUVUIASKSA-N bullatacin Chemical compound O1[C@@H]([C@@H](O)CCCCCCCCCC)CC[C@@H]1[C@@H]1O[C@@H]([C@H](O)CCCCCCCCCC[C@@H](O)CC=2C(O[C@@H](C)C=2)=O)CC1 MBABCNBNDNGODA-LUVUIASKSA-N 0.000 description 1
- 208000019748 bullous skin disease Diseases 0.000 description 1
- 229960005064 buserelin acetate Drugs 0.000 description 1
- 229960002092 busulfan Drugs 0.000 description 1
- 108700002839 cactinomycin Proteins 0.000 description 1
- 229950009908 cactinomycin Drugs 0.000 description 1
- LWQQLNNNIPYSNX-UROSTWAQSA-N calcipotriol Chemical compound C1([C@H](O)/C=C/[C@@H](C)[C@@H]2[C@]3(CCCC(/[C@@H]3CC2)=C\C=C\2C([C@@H](O)C[C@H](O)C/2)=C)C)CC1 LWQQLNNNIPYSNX-UROSTWAQSA-N 0.000 description 1
- 229960002882 calcipotriol Drugs 0.000 description 1
- 238000004364 calculation method Methods 0.000 description 1
- 244000309466 calf Species 0.000 description 1
- IVFYLRMMHVYGJH-PVPPCFLZSA-N calusterone Chemical compound C1C[C@]2(C)[C@](O)(C)CC[C@H]2[C@@H]2[C@@H](C)CC3=CC(=O)CC[C@]3(C)[C@H]21 IVFYLRMMHVYGJH-PVPPCFLZSA-N 0.000 description 1
- 229950009823 calusterone Drugs 0.000 description 1
- 229940088954 camptosar Drugs 0.000 description 1
- VSJKWCGYPAHWDS-FQEVSTJZSA-N camptothecin Chemical compound C1=CC=C2C=C(CN3C4=CC5=C(C3=O)COC(=O)[C@]5(O)CC)C4=NC2=C1 VSJKWCGYPAHWDS-FQEVSTJZSA-N 0.000 description 1
- 229960004117 capecitabine Drugs 0.000 description 1
- 229960004562 carboplatin Drugs 0.000 description 1
- 125000003178 carboxy group Chemical group [H]OC(*)=O 0.000 description 1
- XREUEWVEMYWFFA-CSKJXFQVSA-N carminomycin Chemical compound C1[C@H](N)[C@H](O)[C@H](C)O[C@H]1O[C@@H]1C2=C(O)C(C(=O)C3=C(O)C=CC=C3C3=O)=C3C(O)=C2C[C@@](O)(C(C)=O)C1 XREUEWVEMYWFFA-CSKJXFQVSA-N 0.000 description 1
- 229930188550 carminomycin Natural products 0.000 description 1
- XREUEWVEMYWFFA-UHFFFAOYSA-N carminomycin I Natural products C1C(N)C(O)C(C)OC1OC1C2=C(O)C(C(=O)C3=C(O)C=CC=C3C3=O)=C3C(O)=C2CC(O)(C(C)=O)C1 XREUEWVEMYWFFA-UHFFFAOYSA-N 0.000 description 1
- 229960005243 carmustine Drugs 0.000 description 1
- 229950001725 carubicin Drugs 0.000 description 1
- BBZDXMBRAFTCAA-AREMUKBSSA-N carzelesin Chemical compound C1=2NC=C(C)C=2C([C@H](CCl)CN2C(=O)C=3NC4=CC=C(C=C4C=3)NC(=O)C3=CC4=CC=C(C=C4O3)N(CC)CC)=C2C=C1OC(=O)NC1=CC=CC=C1 BBZDXMBRAFTCAA-AREMUKBSSA-N 0.000 description 1
- 229950007509 carzelesin Drugs 0.000 description 1
- 238000006555 catalytic reaction Methods 0.000 description 1
- 229960000590 celecoxib Drugs 0.000 description 1
- RZEKVGVHFLEQIL-UHFFFAOYSA-N celecoxib Chemical compound C1=CC(C)=CC=C1C1=CC(C(F)(F)F)=NN1C1=CC=C(S(N)(=O)=O)C=C1 RZEKVGVHFLEQIL-UHFFFAOYSA-N 0.000 description 1
- 230000006369 cell cycle progression Effects 0.000 description 1
- 210000000170 cell membrane Anatomy 0.000 description 1
- 230000003833 cell viability Effects 0.000 description 1
- 238000003570 cell viability assay Methods 0.000 description 1
- 210000003169 central nervous system Anatomy 0.000 description 1
- 201000010881 cervical cancer Diseases 0.000 description 1
- 239000003153 chemical reaction reagent Substances 0.000 description 1
- 238000002512 chemotherapy Methods 0.000 description 1
- YTRQFSDWAXHJCC-UHFFFAOYSA-N chloroform;phenol Chemical compound ClC(Cl)Cl.OC1=CC=CC=C1 YTRQFSDWAXHJCC-UHFFFAOYSA-N 0.000 description 1
- 229960001480 chlorozotocin Drugs 0.000 description 1
- 239000013611 chromosomal DNA Substances 0.000 description 1
- 210000000349 chromosome Anatomy 0.000 description 1
- 230000001684 chronic effect Effects 0.000 description 1
- 201000005795 chronic inflammatory demyelinating polyneuritis Diseases 0.000 description 1
- 238000005352 clarification Methods 0.000 description 1
- 229960002842 clobetasol Drugs 0.000 description 1
- CBGUOGMQLZIXBE-XGQKBEPLSA-N clobetasol propionate Chemical compound C1CC2=CC(=O)C=C[C@]2(C)[C@]2(F)[C@@H]1[C@@H]1C[C@H](C)[C@@](C(=O)CCl)(OC(=O)CC)[C@@]1(C)C[C@@H]2O CBGUOGMQLZIXBE-XGQKBEPLSA-N 0.000 description 1
- 229960002286 clodronic acid Drugs 0.000 description 1
- HJKBJIYDJLVSAO-UHFFFAOYSA-L clodronic acid disodium salt Chemical compound [Na+].[Na+].OP([O-])(=O)C(Cl)(Cl)P(O)([O-])=O HJKBJIYDJLVSAO-UHFFFAOYSA-L 0.000 description 1
- 239000002299 complementary DNA Substances 0.000 description 1
- 238000010668 complexation reaction Methods 0.000 description 1
- 238000009833 condensation Methods 0.000 description 1
- 230000005494 condensation Effects 0.000 description 1
- 208000010247 contact dermatitis Diseases 0.000 description 1
- 239000003246 corticosteroid Substances 0.000 description 1
- 229960001334 corticosteroids Drugs 0.000 description 1
- COFJBSXICYYSKG-OAUVCNBTSA-N cph2u7dndy Chemical compound OS(O)(=O)=O.C([C@H](C[C@]1(C(=O)OC)C=2C(=CC3=C([C@]45[C@H]([C@@]([C@H](O)[C@]6(CC)C=CCN([C@H]56)CC4)(O)C(N)=O)N3C)C=2)OC)C[C@@](C2)(O)CC)N2CCC2=C1NC1=CC=CC=C21 COFJBSXICYYSKG-OAUVCNBTSA-N 0.000 description 1
- 108010006226 cryptophycin Proteins 0.000 description 1
- 108010089438 cryptophycin 1 Proteins 0.000 description 1
- 108010090203 cryptophycin 8 Proteins 0.000 description 1
- 229960000684 cytarabine Drugs 0.000 description 1
- 210000000805 cytoplasm Anatomy 0.000 description 1
- 229940104302 cytosine Drugs 0.000 description 1
- ATWWYGQDYGSWQA-UHFFFAOYSA-N demecolceine Natural products C1=C(O)C(=O)C=C2C(NC)CCC3=CC(OC)=C(OC)C(OC)=C3C2=C1 ATWWYGQDYGSWQA-UHFFFAOYSA-N 0.000 description 1
- 229960005052 demecolcine Drugs 0.000 description 1
- 230000003210 demyelinating effect Effects 0.000 description 1
- 239000005547 deoxyribonucleotide Substances 0.000 description 1
- 201000001981 dermatomyositis Diseases 0.000 description 1
- 238000001514 detection method Methods 0.000 description 1
- 238000011161 development Methods 0.000 description 1
- 230000018109 developmental process Effects 0.000 description 1
- 206010012601 diabetes mellitus Diseases 0.000 description 1
- 208000010643 digestive system disease Diseases 0.000 description 1
- PZXJOHSZQAEJFE-UHFFFAOYSA-N dihydrobetulinic acid Natural products C1CC(O)C(C)(C)C2CCC3(C)C4(C)CCC5(C(O)=O)CCC(C(C)C)C5C4CCC3C21C PZXJOHSZQAEJFE-UHFFFAOYSA-N 0.000 description 1
- 239000003085 diluting agent Substances 0.000 description 1
- 238000010790 dilution Methods 0.000 description 1
- 239000012895 dilution Substances 0.000 description 1
- 239000002988 disease modifying antirheumatic drug Substances 0.000 description 1
- 230000005750 disease progression Effects 0.000 description 1
- 238000009826 distribution Methods 0.000 description 1
- 229960002311 dithranol Drugs 0.000 description 1
- 239000003534 dna topoisomerase inhibitor Substances 0.000 description 1
- AMRJKAQTDDKMCE-UHFFFAOYSA-N dolastatin Chemical compound CC(C)C(N(C)C)C(=O)NC(C(C)C)C(=O)N(C)C(C(C)C)C(OC)CC(=O)N1CCCC1C(OC)C(C)C(=O)NC(C=1SC=CN=1)CC1=CC=CC=C1 AMRJKAQTDDKMCE-UHFFFAOYSA-N 0.000 description 1
- 229930188854 dolastatin Natural products 0.000 description 1
- 229950004203 droloxifene Drugs 0.000 description 1
- NOTIQUSPUUHHEH-UXOVVSIBSA-N dromostanolone propionate Chemical compound C([C@@H]1CC2)C(=O)[C@H](C)C[C@]1(C)[C@@H]1[C@@H]2[C@@H]2CC[C@H](OC(=O)CC)[C@@]2(C)CC1 NOTIQUSPUUHHEH-UXOVVSIBSA-N 0.000 description 1
- 229960004242 dronabinol Drugs 0.000 description 1
- 229950004683 drostanolone propionate Drugs 0.000 description 1
- 239000003937 drug carrier Substances 0.000 description 1
- 229960005501 duocarmycin Drugs 0.000 description 1
- VQNATVDKACXKTF-XELLLNAOSA-N duocarmycin Chemical compound COC1=C(OC)C(OC)=C2NC(C(=O)N3C4=CC(=O)C5=C([C@@]64C[C@@H]6C3)C=C(N5)C(=O)OC)=CC2=C1 VQNATVDKACXKTF-XELLLNAOSA-N 0.000 description 1
- 229930184221 duocarmycin Natural products 0.000 description 1
- 230000008482 dysregulation Effects 0.000 description 1
- FSIRXIHZBIXHKT-MHTVFEQDSA-N edatrexate Chemical compound C=1N=C2N=C(N)N=C(N)C2=NC=1CC(CC)C1=CC=C(C(=O)N[C@@H](CCC(O)=O)C(O)=O)C=C1 FSIRXIHZBIXHKT-MHTVFEQDSA-N 0.000 description 1
- 229950006700 edatrexate Drugs 0.000 description 1
- 229960002759 eflornithine Drugs 0.000 description 1
- 229940121647 egfr inhibitor Drugs 0.000 description 1
- XOPYFXBZMVTEJF-PDACKIITSA-N eleutherobin Chemical compound C(/[C@H]1[C@H](C(=CC[C@@H]1C(C)C)C)C[C@@H]([C@@]1(C)O[C@@]2(C=C1)OC)OC(=O)\C=C\C=1N=CN(C)C=1)=C2\CO[C@@H]1OC[C@@H](O)[C@@H](O)[C@@H]1OC(C)=O XOPYFXBZMVTEJF-PDACKIITSA-N 0.000 description 1
- XOPYFXBZMVTEJF-UHFFFAOYSA-N eleutherobin Natural products C1=CC2(OC)OC1(C)C(OC(=O)C=CC=1N=CN(C)C=1)CC(C(=CCC1C(C)C)C)C1C=C2COC1OCC(O)C(O)C1OC(C)=O XOPYFXBZMVTEJF-UHFFFAOYSA-N 0.000 description 1
- 229950000549 elliptinium acetate Drugs 0.000 description 1
- 229940120655 eloxatin Drugs 0.000 description 1
- 201000008184 embryoma Diseases 0.000 description 1
- 201000003914 endometrial carcinoma Diseases 0.000 description 1
- 230000002357 endometrial effect Effects 0.000 description 1
- JOZGNYDSEBIJDH-UHFFFAOYSA-N eniluracil Chemical compound O=C1NC=C(C#C)C(=O)N1 JOZGNYDSEBIJDH-UHFFFAOYSA-N 0.000 description 1
- 229950010213 eniluracil Drugs 0.000 description 1
- 208000037902 enteropathy Diseases 0.000 description 1
- 230000002255 enzymatic effect Effects 0.000 description 1
- 201000009580 eosinophilic pneumonia Diseases 0.000 description 1
- YJGVMLPVUAXIQN-UHFFFAOYSA-N epipodophyllotoxin Natural products COC1=C(OC)C(OC)=CC(C2C3=CC=4OCOC=4C=C3C(O)C3C2C(OC3)=O)=C1 YJGVMLPVUAXIQN-UHFFFAOYSA-N 0.000 description 1
- 210000000981 epithelium Anatomy 0.000 description 1
- 229950002973 epitiostanol Drugs 0.000 description 1
- 229930013356 epothilone Natural products 0.000 description 1
- 150000003883 epothilone derivatives Chemical class 0.000 description 1
- 229960001433 erlotinib Drugs 0.000 description 1
- ITSGNOIFAJAQHJ-BMFNZSJVSA-N esorubicin Chemical compound O([C@H]1C[C@@](O)(CC=2C(O)=C3C(=O)C=4C=CC=C(C=4C(=O)C3=C(O)C=21)OC)C(=O)CO)[C@H]1C[C@H](N)C[C@H](C)O1 ITSGNOIFAJAQHJ-BMFNZSJVSA-N 0.000 description 1
- 229950002017 esorubicin Drugs 0.000 description 1
- 229960001842 estramustine Drugs 0.000 description 1
- FRPJXPJMRWBBIH-RBRWEJTLSA-N estramustine Chemical compound ClCCN(CCCl)C(=O)OC1=CC=C2[C@H]3CC[C@](C)([C@H](CC4)O)[C@@H]4[C@@H]3CCC2=C1 FRPJXPJMRWBBIH-RBRWEJTLSA-N 0.000 description 1
- 229940011871 estrogen Drugs 0.000 description 1
- 239000000262 estrogen Substances 0.000 description 1
- 239000000328 estrogen antagonist Substances 0.000 description 1
- 102000015694 estrogen receptors Human genes 0.000 description 1
- 108010038795 estrogen receptors Proteins 0.000 description 1
- QSRLNKCNOLVZIR-KRWDZBQOSA-N ethyl (2s)-2-[[2-[4-[bis(2-chloroethyl)amino]phenyl]acetyl]amino]-4-methylsulfanylbutanoate Chemical compound CCOC(=O)[C@H](CCSC)NC(=O)CC1=CC=C(N(CCCl)CCCl)C=C1 QSRLNKCNOLVZIR-KRWDZBQOSA-N 0.000 description 1
- 229960004585 etidronic acid Drugs 0.000 description 1
- 229960005237 etoglucid Drugs 0.000 description 1
- 229940085363 evista Drugs 0.000 description 1
- 229960000255 exemestane Drugs 0.000 description 1
- 230000001747 exhibiting effect Effects 0.000 description 1
- 238000000605 extraction Methods 0.000 description 1
- 229950011548 fadrozole Drugs 0.000 description 1
- 230000003176 fibrotic effect Effects 0.000 description 1
- XRECTZIEBJDKEO-UHFFFAOYSA-N flucytosine Chemical compound NC1=NC(=O)NC=C1F XRECTZIEBJDKEO-UHFFFAOYSA-N 0.000 description 1
- 229960004413 flucytosine Drugs 0.000 description 1
- 229960000390 fludarabine Drugs 0.000 description 1
- GIUYCYHIANZCFB-FJFJXFQQSA-N fludarabine phosphate Chemical compound C1=NC=2C(N)=NC(F)=NC=2N1[C@@H]1O[C@H](COP(O)(O)=O)[C@@H](O)[C@@H]1O GIUYCYHIANZCFB-FJFJXFQQSA-N 0.000 description 1
- 238000001943 fluorescence-activated cell sorting Methods 0.000 description 1
- 229960002074 flutamide Drugs 0.000 description 1
- MKXKFYHWDHIYRV-UHFFFAOYSA-N flutamide Chemical compound CC(C)C(=O)NC1=CC=C([N+]([O-])=O)C(C(F)(F)F)=C1 MKXKFYHWDHIYRV-UHFFFAOYSA-N 0.000 description 1
- 235000019152 folic acid Nutrition 0.000 description 1
- 239000011724 folic acid Substances 0.000 description 1
- 229960000304 folic acid Drugs 0.000 description 1
- 150000002224 folic acids Chemical class 0.000 description 1
- 229960004421 formestane Drugs 0.000 description 1
- OSVMTWJCGUFAOD-KZQROQTASA-N formestane Chemical compound O=C1CC[C@]2(C)[C@H]3CC[C@](C)(C(CC4)=O)[C@@H]4[C@@H]3CCC2=C1O OSVMTWJCGUFAOD-KZQROQTASA-N 0.000 description 1
- 229940001490 fosamax Drugs 0.000 description 1
- 229960004783 fotemustine Drugs 0.000 description 1
- YAKWPXVTIGTRJH-UHFFFAOYSA-N fotemustine Chemical compound CCOP(=O)(OCC)C(C)NC(=O)N(CCCl)N=O YAKWPXVTIGTRJH-UHFFFAOYSA-N 0.000 description 1
- 229940044658 gallium nitrate Drugs 0.000 description 1
- 239000000499 gel Substances 0.000 description 1
- 229960005277 gemcitabine Drugs 0.000 description 1
- SDUQYLNIPVEERB-QPPQHZFASA-N gemcitabine Chemical compound O=C1N=C(N)C=CN1[C@H]1C(F)(F)[C@H](O)[C@@H](CO)O1 SDUQYLNIPVEERB-QPPQHZFASA-N 0.000 description 1
- 229940020967 gemzar Drugs 0.000 description 1
- 238000001415 gene therapy Methods 0.000 description 1
- 210000004602 germ cell Anatomy 0.000 description 1
- 210000004907 gland Anatomy 0.000 description 1
- 229940080856 gleevec Drugs 0.000 description 1
- 230000002518 glial effect Effects 0.000 description 1
- 208000005017 glioblastoma Diseases 0.000 description 1
- 235000021312 gluten Nutrition 0.000 description 1
- 150000004676 glycans Chemical class 0.000 description 1
- 229930182470 glycoside Natural products 0.000 description 1
- 229940076085 gold Drugs 0.000 description 1
- 229960003690 goserelin acetate Drugs 0.000 description 1
- 201000007192 granulomatous hepatitis Diseases 0.000 description 1
- 239000003102 growth factor Substances 0.000 description 1
- 230000009931 harmful effect Effects 0.000 description 1
- 201000001505 hemoglobinuria Diseases 0.000 description 1
- 208000006454 hepatitis Diseases 0.000 description 1
- 231100000283 hepatitis Toxicity 0.000 description 1
- 208000002672 hepatitis B Diseases 0.000 description 1
- 201000010284 hepatitis E Diseases 0.000 description 1
- 230000001553 hepatotropic effect Effects 0.000 description 1
- 229940022353 herceptin Drugs 0.000 description 1
- UUVWYPNAQBNQJQ-UHFFFAOYSA-N hexamethylmelamine Chemical compound CN(C)C1=NC(N(C)C)=NC(N(C)C)=N1 UUVWYPNAQBNQJQ-UHFFFAOYSA-N 0.000 description 1
- 102000057041 human TNF Human genes 0.000 description 1
- 229940048921 humira Drugs 0.000 description 1
- 230000028996 humoral immune response Effects 0.000 description 1
- 238000009396 hybridization Methods 0.000 description 1
- 229940088013 hycamtin Drugs 0.000 description 1
- 230000002209 hydrophobic effect Effects 0.000 description 1
- 229960001330 hydroxycarbamide Drugs 0.000 description 1
- XXSMGPRMXLTPCZ-UHFFFAOYSA-N hydroxychloroquine Chemical compound ClC1=CC=C2C(NC(C)CCCN(CCO)CC)=CC=NC2=C1 XXSMGPRMXLTPCZ-UHFFFAOYSA-N 0.000 description 1
- 229960004171 hydroxychloroquine Drugs 0.000 description 1
- KNOSIOWNDGUGFJ-UHFFFAOYSA-N hydroxysesamone Natural products C1=CC(O)=C2C(=O)C(CC=C(C)C)=C(O)C(=O)C2=C1O KNOSIOWNDGUGFJ-UHFFFAOYSA-N 0.000 description 1
- 230000002267 hypothalamic effect Effects 0.000 description 1
- 229940015872 ibandronate Drugs 0.000 description 1
- 229960000908 idarubicin Drugs 0.000 description 1
- 229960003685 imatinib mesylate Drugs 0.000 description 1
- 230000028993 immune response Effects 0.000 description 1
- 230000003053 immunization Effects 0.000 description 1
- 238000002649 immunization Methods 0.000 description 1
- DBIGHPPNXATHOF-UHFFFAOYSA-N improsulfan Chemical compound CS(=O)(=O)OCCCNCCCOS(C)(=O)=O DBIGHPPNXATHOF-UHFFFAOYSA-N 0.000 description 1
- 229950008097 improsulfan Drugs 0.000 description 1
- 230000006872 improvement Effects 0.000 description 1
- 230000002779 inactivation Effects 0.000 description 1
- 230000006882 induction of apoptosis Effects 0.000 description 1
- 239000003112 inhibitor Substances 0.000 description 1
- 229940125396 insulin Drugs 0.000 description 1
- 239000000138 intercalating agent Substances 0.000 description 1
- 229940047124 interferons Drugs 0.000 description 1
- 239000000543 intermediate Substances 0.000 description 1
- 208000028774 intestinal disease Diseases 0.000 description 1
- 210000000936 intestine Anatomy 0.000 description 1
- 238000007918 intramuscular administration Methods 0.000 description 1
- 229960004768 irinotecan Drugs 0.000 description 1
- YWXYYJSYQOXTPL-SLPGGIOYSA-N isosorbide mononitrate Chemical compound [O-][N+](=O)O[C@@H]1CO[C@@H]2[C@@H](O)CO[C@@H]21 YWXYYJSYQOXTPL-SLPGGIOYSA-N 0.000 description 1
- 230000000366 juvenile effect Effects 0.000 description 1
- CWPGNVFCJOPXFB-UHFFFAOYSA-N lapachol Chemical compound C1=CC=C2C(=O)C(=O)C(CC=C(C)C)=C(O)C2=C1 CWPGNVFCJOPXFB-UHFFFAOYSA-N 0.000 description 1
- SIUGQQMOYSVTAT-UHFFFAOYSA-N lapachol Natural products CC(=CCC1C(O)C(=O)c2ccccc2C1=O)C SIUGQQMOYSVTAT-UHFFFAOYSA-N 0.000 description 1
- 229960001320 lapatinib ditosylate Drugs 0.000 description 1
- 201000010260 leiomyoma Diseases 0.000 description 1
- 229940115286 lentinan Drugs 0.000 description 1
- 229960003881 letrozole Drugs 0.000 description 1
- HPJKCIUCZWXJDR-UHFFFAOYSA-N letrozole Chemical compound C1=CC(C#N)=CC=C1C(N1N=CN=C1)C1=CC=C(C#N)C=C1 HPJKCIUCZWXJDR-UHFFFAOYSA-N 0.000 description 1
- 208000032839 leukemia Diseases 0.000 description 1
- 230000000670 limiting effect Effects 0.000 description 1
- 201000002250 liver carcinoma Diseases 0.000 description 1
- 229960002247 lomustine Drugs 0.000 description 1
- YROQEQPFUCPDCP-UHFFFAOYSA-N losoxantrone Chemical compound OCCNCCN1N=C2C3=CC=CC(O)=C3C(=O)C3=C2C1=CC=C3NCCNCCO YROQEQPFUCPDCP-UHFFFAOYSA-N 0.000 description 1
- 229950008745 losoxantrone Drugs 0.000 description 1
- 201000005249 lung adenocarcinoma Diseases 0.000 description 1
- 201000005202 lung cancer Diseases 0.000 description 1
- 208000020816 lung neoplasm Diseases 0.000 description 1
- 201000005243 lung squamous cell carcinoma Diseases 0.000 description 1
- 108010078259 luprolide acetate gel depot Proteins 0.000 description 1
- 229940087857 lupron Drugs 0.000 description 1
- RVFGKBWWUQOIOU-NDEPHWFRSA-N lurtotecan Chemical compound O=C([C@]1(O)CC)OCC(C(N2CC3=4)=O)=C1C=C2C3=NC1=CC=2OCCOC=2C=C1C=4CN1CCN(C)CC1 RVFGKBWWUQOIOU-NDEPHWFRSA-N 0.000 description 1
- 229950002654 lurtotecan Drugs 0.000 description 1
- 230000000527 lymphocytic effect Effects 0.000 description 1
- 210000003563 lymphoid tissue Anatomy 0.000 description 1
- 210000002540 macrophage Anatomy 0.000 description 1
- 208000002780 macular degeneration Diseases 0.000 description 1
- 230000014759 maintenance of location Effects 0.000 description 1
- 210000004962 mammalian cell Anatomy 0.000 description 1
- 238000007726 management method Methods 0.000 description 1
- MQXVYODZCMMZEM-ZYUZMQFOSA-N mannomustine Chemical compound ClCCNC[C@@H](O)[C@@H](O)[C@H](O)[C@H](O)CNCCCl MQXVYODZCMMZEM-ZYUZMQFOSA-N 0.000 description 1
- 229950008612 mannomustine Drugs 0.000 description 1
- 229940099262 marinol Drugs 0.000 description 1
- WKPWGQKGSOKKOO-RSFHAFMBSA-N maytansine Chemical compound CO[C@@H]([C@@]1(O)C[C@](OC(=O)N1)([C@H]([C@@H]1O[C@@]1(C)[C@@H](OC(=O)[C@H](C)N(C)C(C)=O)CC(=O)N1C)C)[H])\C=C\C=C(C)\CC2=CC(OC)=C(Cl)C1=C2 WKPWGQKGSOKKOO-RSFHAFMBSA-N 0.000 description 1
- 238000005259 measurement Methods 0.000 description 1
- 239000002609 medium Substances 0.000 description 1
- 229960004296 megestrol acetate Drugs 0.000 description 1
- RQZAXGRLVPAYTJ-GQFGMJRRSA-N megestrol acetate Chemical compound C1=C(C)C2=CC(=O)CC[C@]2(C)[C@@H]2[C@@H]1[C@@H]1CC[C@@](C(C)=O)(OC(=O)C)[C@@]1(C)CC2 RQZAXGRLVPAYTJ-GQFGMJRRSA-N 0.000 description 1
- 239000012528 membrane Substances 0.000 description 1
- 229930182817 methionine Natural products 0.000 description 1
- HPNSFSBZBAHARI-UHFFFAOYSA-N micophenolic acid Natural products OC1=C(CC=C(C)CCC(O)=O)C(OC)=C(C)C2=C1C(=O)OC2 HPNSFSBZBAHARI-UHFFFAOYSA-N 0.000 description 1
- 210000000110 microvilli Anatomy 0.000 description 1
- 229960004023 minocycline Drugs 0.000 description 1
- 229960005485 mitobronitol Drugs 0.000 description 1
- 230000004898 mitochondrial function Effects 0.000 description 1
- 229960003539 mitoguazone Drugs 0.000 description 1
- MXWHMTNPTTVWDM-NXOFHUPFSA-N mitoguazone Chemical compound NC(N)=N\N=C(/C)\C=N\N=C(N)N MXWHMTNPTTVWDM-NXOFHUPFSA-N 0.000 description 1
- VFKZTMPDYBFSTM-GUCUJZIJSA-N mitolactol Chemical compound BrC[C@H](O)[C@@H](O)[C@@H](O)[C@H](O)CBr VFKZTMPDYBFSTM-GUCUJZIJSA-N 0.000 description 1
- 229950010913 mitolactol Drugs 0.000 description 1
- 229960000350 mitotane Drugs 0.000 description 1
- 238000012544 monitoring process Methods 0.000 description 1
- 238000010172 mouse model Methods 0.000 description 1
- 238000002703 mutagenesis Methods 0.000 description 1
- 231100000350 mutagenesis Toxicity 0.000 description 1
- 229960000951 mycophenolic acid Drugs 0.000 description 1
- HPNSFSBZBAHARI-RUDMXATFSA-N mycophenolic acid Chemical compound OC1=C(C\C=C(/C)CCC(O)=O)C(OC)=C(C)C2=C1C(=O)OC2 HPNSFSBZBAHARI-RUDMXATFSA-N 0.000 description 1
- AZBFJBJXUQUQLF-UHFFFAOYSA-N n-(1,5-dimethylpyrrolidin-3-yl)pyrrolidine-1-carboxamide Chemical compound C1N(C)C(C)CC1NC(=O)N1CCCC1 AZBFJBJXUQUQLF-UHFFFAOYSA-N 0.000 description 1
- BLCLNMBMMGCOAS-UHFFFAOYSA-N n-[1-[[1-[[1-[[1-[[1-[[1-[[1-[2-[(carbamoylamino)carbamoyl]pyrrolidin-1-yl]-5-(diaminomethylideneamino)-1-oxopentan-2-yl]amino]-4-methyl-1-oxopentan-2-yl]amino]-3-[(2-methylpropan-2-yl)oxy]-1-oxopropan-2-yl]amino]-3-(4-hydroxyphenyl)-1-oxopropan-2-yl]amin Chemical compound C1CCC(C(=O)NNC(N)=O)N1C(=O)C(CCCN=C(N)N)NC(=O)C(CC(C)C)NC(=O)C(COC(C)(C)C)NC(=O)C(NC(=O)C(CO)NC(=O)C(CC=1C2=CC=CC=C2NC=1)NC(=O)C(CC=1NC=NC=1)NC(=O)C1NC(=O)CC1)CC1=CC=C(O)C=C1 BLCLNMBMMGCOAS-UHFFFAOYSA-N 0.000 description 1
- 239000002105 nanoparticle Substances 0.000 description 1
- 229930014626 natural product Natural products 0.000 description 1
- 229940086322 navelbine Drugs 0.000 description 1
- 230000027498 negative regulation of mitosis Effects 0.000 description 1
- 230000009826 neoplastic cell growth Effects 0.000 description 1
- MQYXUWHLBZFQQO-UHFFFAOYSA-N nepehinol Natural products C1CC(O)C(C)(C)C2CCC3(C)C4(C)CCC5(C)CCC(C(=C)C)C5C4CCC3C21C MQYXUWHLBZFQQO-UHFFFAOYSA-N 0.000 description 1
- 230000001537 neural effect Effects 0.000 description 1
- 229960002653 nilutamide Drugs 0.000 description 1
- XWXYUMMDTVBTOU-UHFFFAOYSA-N nilutamide Chemical compound O=C1C(C)(C)NC(=O)N1C1=CC=C([N+]([O-])=O)C(C(F)(F)F)=C1 XWXYUMMDTVBTOU-UHFFFAOYSA-N 0.000 description 1
- 229960001420 nimustine Drugs 0.000 description 1
- VFEDRRNHLBGPNN-UHFFFAOYSA-N nimustine Chemical compound CC1=NC=C(CNC(=O)N(CCCl)N=O)C(N)=N1 VFEDRRNHLBGPNN-UHFFFAOYSA-N 0.000 description 1
- KGTDRFCXGRULNK-JYOBTZKQSA-N nogalamycin Chemical compound CO[C@@H]1[C@@](OC)(C)[C@@H](OC)[C@H](C)O[C@H]1O[C@@H]1C2=C(O)C(C(=O)C3=C(O)C=C4[C@@]5(C)O[C@H]([C@H]([C@@H]([C@H]5O)N(C)C)O)OC4=C3C3=O)=C3C=C2[C@@H](C(=O)OC)[C@@](C)(O)C1 KGTDRFCXGRULNK-JYOBTZKQSA-N 0.000 description 1
- 229950009266 nogalamycin Drugs 0.000 description 1
- 229940085033 nolvadex Drugs 0.000 description 1
- 238000003199 nucleic acid amplification method Methods 0.000 description 1
- 230000001293 nucleolytic effect Effects 0.000 description 1
- 239000002777 nucleoside Substances 0.000 description 1
- CZDBNBLGZNWKMC-MWQNXGTOSA-N olivomycin Chemical class O([C@@H]1C[C@@H](O[C@H](C)[C@@H]1O)OC=1C=C2C=C3C[C@H]([C@@H](C(=O)C3=C(O)C2=C(O)C=1)O[C@H]1O[C@@H](C)[C@H](O)[C@@H](OC2O[C@@H](C)[C@H](O)[C@@H](O)C2)C1)[C@H](OC)C(=O)[C@@H](O)[C@@H](C)O)[C@H]1C[C@H](O)[C@H](OC)[C@H](C)O1 CZDBNBLGZNWKMC-MWQNXGTOSA-N 0.000 description 1
- 230000002611 ovarian Effects 0.000 description 1
- 238000004806 packaging method and process Methods 0.000 description 1
- 229940046231 pamidronate Drugs 0.000 description 1
- 230000001314 paroxysmal effect Effects 0.000 description 1
- 230000007170 pathology Effects 0.000 description 1
- 229960001639 penicillamine Drugs 0.000 description 1
- 229960002340 pentostatin Drugs 0.000 description 1
- FPVKHBSQESCIEP-JQCXWYLXSA-N pentostatin Chemical compound C1[C@H](O)[C@@H](CO)O[C@H]1N1C(N=CNC[C@H]2O)=C2N=C1 FPVKHBSQESCIEP-JQCXWYLXSA-N 0.000 description 1
- QIMGFXOHTOXMQP-GFAGFCTOSA-N peplomycin Chemical compound N([C@H](C(=O)N[C@H](C)[C@@H](O)[C@H](C)C(=O)N[C@@H]([C@H](O)C)C(=O)NCCC=1SC=C(N=1)C=1SC=C(N=1)C(=O)NCCCN[C@@H](C)C=1C=CC=CC=1)[C@@H](O[C@H]1[C@H]([C@@H](O)[C@H](O)[C@H](CO)O1)O[C@@H]1[C@H]([C@@H](OC(N)=O)[C@H](O)[C@@H](CO)O1)O)C=1NC=NC=1)C(=O)C1=NC([C@H](CC(N)=O)NC[C@H](N)C(N)=O)=NC(N)=C1C QIMGFXOHTOXMQP-GFAGFCTOSA-N 0.000 description 1
- 229950003180 peplomycin Drugs 0.000 description 1
- 210000001428 peripheral nervous system Anatomy 0.000 description 1
- 210000001322 periplasm Anatomy 0.000 description 1
- 201000002628 peritoneum cancer Diseases 0.000 description 1
- 108700010839 phage proteins Proteins 0.000 description 1
- NBIIXXVUZAFLBC-UHFFFAOYSA-K phosphate Chemical compound [O-]P([O-])([O-])=O NBIIXXVUZAFLBC-UHFFFAOYSA-K 0.000 description 1
- 239000010452 phosphate Substances 0.000 description 1
- AQSJGOWTSHOLKH-UHFFFAOYSA-N phosphite(3-) Chemical class [O-]P([O-])[O-] AQSJGOWTSHOLKH-UHFFFAOYSA-N 0.000 description 1
- 150000008300 phosphoramidites Chemical class 0.000 description 1
- 230000000704 physical effect Effects 0.000 description 1
- 230000004962 physiological condition Effects 0.000 description 1
- 229960000952 pipobroman Drugs 0.000 description 1
- NJBFOOCLYDNZJN-UHFFFAOYSA-N pipobroman Chemical compound BrCCC(=O)N1CCN(C(=O)CCBr)CC1 NJBFOOCLYDNZJN-UHFFFAOYSA-N 0.000 description 1
- NUKCGLDCWQXYOQ-UHFFFAOYSA-N piposulfan Chemical compound CS(=O)(=O)OCCC(=O)N1CCN(C(=O)CCOS(C)(=O)=O)CC1 NUKCGLDCWQXYOQ-UHFFFAOYSA-N 0.000 description 1
- 229950001100 piposulfan Drugs 0.000 description 1
- 239000013600 plasmid vector Substances 0.000 description 1
- 229910052697 platinum Inorganic materials 0.000 description 1
- 150000003057 platinum Chemical class 0.000 description 1
- 229960001237 podophyllotoxin Drugs 0.000 description 1
- YJGVMLPVUAXIQN-XVVDYKMHSA-N podophyllotoxin Chemical compound COC1=C(OC)C(OC)=CC([C@@H]2C3=CC=4OCOC=4C=C3[C@H](O)[C@@H]3[C@@H]2C(OC3)=O)=C1 YJGVMLPVUAXIQN-XVVDYKMHSA-N 0.000 description 1
- YVCVYCSAAZQOJI-UHFFFAOYSA-N podophyllotoxin Natural products COC1=C(O)C(OC)=CC(C2C3=CC=4OCOC=4C=C3C(O)C3C2C(OC3)=O)=C1 YVCVYCSAAZQOJI-UHFFFAOYSA-N 0.000 description 1
- 229920002401 polyacrylamide Polymers 0.000 description 1
- 201000010065 polycystic ovary syndrome Diseases 0.000 description 1
- 238000003752 polymerase chain reaction Methods 0.000 description 1
- 208000005987 polymyositis Diseases 0.000 description 1
- 229920001282 polysaccharide Polymers 0.000 description 1
- 239000005017 polysaccharide Substances 0.000 description 1
- 239000002243 precursor Substances 0.000 description 1
- 229960004694 prednimustine Drugs 0.000 description 1
- CPTBDICYNRMXFX-UHFFFAOYSA-N procarbazine Chemical compound CNNCC1=CC=C(C(=O)NC(C)C)C=C1 CPTBDICYNRMXFX-UHFFFAOYSA-N 0.000 description 1
- 229960000624 procarbazine Drugs 0.000 description 1
- 230000003623 progesteronic effect Effects 0.000 description 1
- 238000004393 prognosis Methods 0.000 description 1
- 210000001236 prokaryotic cell Anatomy 0.000 description 1
- 238000011321 prophylaxis Methods 0.000 description 1
- WOLQREOUPKZMEX-UHFFFAOYSA-N pteroyltriglutamic acid Chemical compound C=1N=C2NC(N)=NC(=O)C2=NC=1CNC1=CC=C(C(=O)NC(CCC(=O)NC(CCC(=O)NC(CCC(O)=O)C(O)=O)C(O)=O)C(O)=O)C=C1 WOLQREOUPKZMEX-UHFFFAOYSA-N 0.000 description 1
- 230000005180 public health Effects 0.000 description 1
- 201000009732 pulmonary eosinophilia Diseases 0.000 description 1
- 150000003212 purines Chemical class 0.000 description 1
- 229950010131 puromycin Drugs 0.000 description 1
- 150000003230 pyrimidines Chemical class 0.000 description 1
- 238000001959 radiotherapy Methods 0.000 description 1
- 229960004622 raloxifene Drugs 0.000 description 1
- 238000002708 random mutagenesis Methods 0.000 description 1
- 229960002185 ranimustine Drugs 0.000 description 1
- BMKDZUISNHGIBY-UHFFFAOYSA-N razoxane Chemical compound C1C(=O)NC(=O)CN1C(C)CN1CC(=O)NC(=O)C1 BMKDZUISNHGIBY-UHFFFAOYSA-N 0.000 description 1
- 229960000460 razoxane Drugs 0.000 description 1
- 230000006798 recombination Effects 0.000 description 1
- 238000005215 recombination Methods 0.000 description 1
- 230000022983 regulation of cell cycle Effects 0.000 description 1
- 230000003362 replicative effect Effects 0.000 description 1
- 238000011160 research Methods 0.000 description 1
- 230000004044 response Effects 0.000 description 1
- 229930002330 retinoic acid Natural products 0.000 description 1
- 238000012552 review Methods 0.000 description 1
- OWPCHSCAPHNHAV-LMONGJCWSA-N rhizoxin Chemical compound C/C([C@H](OC)[C@@H](C)[C@@H]1C[C@H](O)[C@]2(C)O[C@@H]2/C=C/[C@@H](C)[C@]2([H])OC(=O)C[C@@](C2)(C[C@@H]2O[C@H]2C(=O)O1)[H])=C\C=C\C(\C)=C\C1=COC(C)=N1 OWPCHSCAPHNHAV-LMONGJCWSA-N 0.000 description 1
- 229940089617 risedronate Drugs 0.000 description 1
- 238000005096 rolling process Methods 0.000 description 1
- MBABCNBNDNGODA-WPZDJQSSSA-N rolliniastatin 1 Natural products O1[C@@H]([C@@H](O)CCCCCCCCCC)CC[C@H]1[C@H]1O[C@@H]([C@H](O)CCCCCCCCCC[C@@H](O)CC=2C(O[C@@H](C)C=2)=O)CC1 MBABCNBNDNGODA-WPZDJQSSSA-N 0.000 description 1
- IMUQLZLGWJSVMV-UOBFQKKOSA-N roridin A Natural products CC(O)C1OCCC(C)C(O)C(=O)OCC2CC(=CC3OC4CC(OC(=O)C=C/C=C/1)C(C)(C23)C45CO5)C IMUQLZLGWJSVMV-UOBFQKKOSA-N 0.000 description 1
- VHXNKPBCCMUMSW-FQEVSTJZSA-N rubitecan Chemical compound C1=CC([N+]([O-])=O)=C2C=C(CN3C4=CC5=C(C3=O)COC(=O)[C@]5(O)CC)C4=NC2=C1 VHXNKPBCCMUMSW-FQEVSTJZSA-N 0.000 description 1
- RCINICONZNJXQF-VAZQATRQSA-N s1150_selleck Chemical compound O([C@@H]1[C@@]2(C[C@@H](C(C)=C(C2(C)C)[C@H](C([C@]2(C)[C@@H](O)C[C@H]3OC[C@]3(C21)OC(C)=O)=O)OC(=O)C)OC(=O)[C@H](O)[C@@H](NC(=O)C=1C=CC=CC=1)C=1C=CC=CC=1)O)C(=O)C1=CC=CC=C1 RCINICONZNJXQF-VAZQATRQSA-N 0.000 description 1
- 230000011218 segmentation Effects 0.000 description 1
- 210000002966 serum Anatomy 0.000 description 1
- 230000011664 signaling Effects 0.000 description 1
- 230000007781 signaling event Effects 0.000 description 1
- 239000000377 silicon dioxide Substances 0.000 description 1
- 229940112726 skelid Drugs 0.000 description 1
- 208000017520 skin disease Diseases 0.000 description 1
- 208000000587 small cell lung carcinoma Diseases 0.000 description 1
- 239000001632 sodium acetate Substances 0.000 description 1
- 235000017281 sodium acetate Nutrition 0.000 description 1
- 229910000029 sodium carbonate Inorganic materials 0.000 description 1
- 238000002415 sodium dodecyl sulfate polyacrylamide gel electrophoresis Methods 0.000 description 1
- 239000007787 solid Substances 0.000 description 1
- 229950006315 spirogermanium Drugs 0.000 description 1
- 201000005671 spondyloarthropathy Diseases 0.000 description 1
- ICXJVZHDZFXYQC-UHFFFAOYSA-N spongistatin 1 Natural products OC1C(O2)(O)CC(O)C(C)C2CCCC=CC(O2)CC(O)CC2(O2)CC(OC)CC2CC(=O)C(C)C(OC(C)=O)C(C)C(=C)CC(O2)CC(C)(O)CC2(O2)CC(OC(C)=O)CC2CC(=O)OC2C(O)C(CC(=C)CC(O)C=CC(Cl)=C)OC1C2C ICXJVZHDZFXYQC-UHFFFAOYSA-N 0.000 description 1
- 206010041823 squamous cell carcinoma Diseases 0.000 description 1
- 230000000087 stabilizing effect Effects 0.000 description 1
- 150000003431 steroids Chemical class 0.000 description 1
- 229960001052 streptozocin Drugs 0.000 description 1
- ZSJLQEPLLKMAKR-GKHCUFPYSA-N streptozocin Chemical compound O=NN(C)C(=O)N[C@H]1[C@@H](O)O[C@H](CO)[C@@H](O)[C@@H]1O ZSJLQEPLLKMAKR-GKHCUFPYSA-N 0.000 description 1
- 210000002536 stromal cell Anatomy 0.000 description 1
- 239000000758 substrate Substances 0.000 description 1
- NCEXYHBECQHGNR-UHFFFAOYSA-N sulfasalazine Natural products C1=C(O)C(C(=O)O)=CC(N=NC=2C=CC(=CC=2)S(=O)(=O)NC=2N=CC=CC=2)=C1 NCEXYHBECQHGNR-UHFFFAOYSA-N 0.000 description 1
- 229960001940 sulfasalazine Drugs 0.000 description 1
- 238000002198 surface plasmon resonance spectroscopy Methods 0.000 description 1
- 208000011580 syndromic disease Diseases 0.000 description 1
- 230000009885 systemic effect Effects 0.000 description 1
- 229940120982 tarceva Drugs 0.000 description 1
- RCINICONZNJXQF-XAZOAEDWSA-N taxol® Chemical compound O([C@@H]1[C@@]2(CC(C(C)=C(C2(C)C)[C@H](C([C@]2(C)[C@@H](O)C[C@H]3OC[C@]3(C21)OC(C)=O)=O)OC(=O)C)OC(=O)[C@H](O)[C@@H](NC(=O)C=1C=CC=CC=1)C=1C=CC=CC=1)O)C(=O)C1=CC=CC=C1 RCINICONZNJXQF-XAZOAEDWSA-N 0.000 description 1
- 229960000565 tazarotene Drugs 0.000 description 1
- NRUKOCRGYNPUPR-QBPJDGROSA-N teniposide Chemical compound COC1=C(O)C(OC)=CC([C@@H]2C3=CC=4OCOC=4C=C3[C@@H](O[C@H]3[C@@H]([C@@H](O)[C@@H]4O[C@@H](OC[C@H]4O3)C=3SC=CC=3)O)[C@@H]3[C@@H]2C(OC3)=O)=C1 NRUKOCRGYNPUPR-QBPJDGROSA-N 0.000 description 1
- 229960001278 teniposide Drugs 0.000 description 1
- 229960005353 testolactone Drugs 0.000 description 1
- BPEWUONYVDABNZ-DZBHQSCQSA-N testolactone Chemical compound O=C1C=C[C@]2(C)[C@H]3CC[C@](C)(OC(=O)CC4)[C@@H]4[C@@H]3CCC2=C1 BPEWUONYVDABNZ-DZBHQSCQSA-N 0.000 description 1
- 238000002560 therapeutic procedure Methods 0.000 description 1
- RYYWUUFWQRZTIU-UHFFFAOYSA-K thiophosphate Chemical compound [O-]P([O-])([O-])=S RYYWUUFWQRZTIU-UHFFFAOYSA-K 0.000 description 1
- 206010043554 thrombocytopenia Diseases 0.000 description 1
- 201000002510 thyroid cancer Diseases 0.000 description 1
- 230000006016 thyroid dysfunction Effects 0.000 description 1
- YFTWHEBLORWGNI-UHFFFAOYSA-N tiamiprine Chemical compound CN1C=NC([N+]([O-])=O)=C1SC1=NC(N)=NC2=C1NC=N2 YFTWHEBLORWGNI-UHFFFAOYSA-N 0.000 description 1
- 229950011457 tiamiprine Drugs 0.000 description 1
- 238000004448 titration Methods 0.000 description 1
- 229940044693 topoisomerase inhibitor Drugs 0.000 description 1
- 231100000041 toxicology testing Toxicity 0.000 description 1
- 238000013518 transcription Methods 0.000 description 1
- 230000035897 transcription Effects 0.000 description 1
- 230000005030 transcription termination Effects 0.000 description 1
- 230000002103 transcriptional effect Effects 0.000 description 1
- 238000012546 transfer Methods 0.000 description 1
- 230000009466 transformation Effects 0.000 description 1
- 238000013519 translation Methods 0.000 description 1
- 229950001353 tretamine Drugs 0.000 description 1
- IUCJMVBFZDHPDX-UHFFFAOYSA-N tretamine Chemical compound C1CN1C1=NC(N2CC2)=NC(N2CC2)=N1 IUCJMVBFZDHPDX-UHFFFAOYSA-N 0.000 description 1
- 229960001727 tretinoin Drugs 0.000 description 1
- PXSOHRWMIRDKMP-UHFFFAOYSA-N triaziquone Chemical compound O=C1C(N2CC2)=C(N2CC2)C(=O)C=C1N1CC1 PXSOHRWMIRDKMP-UHFFFAOYSA-N 0.000 description 1
- 229960004560 triaziquone Drugs 0.000 description 1
- 229930013292 trichothecene Natural products 0.000 description 1
- 150000003327 trichothecene derivatives Chemical class 0.000 description 1
- 229960001670 trilostane Drugs 0.000 description 1
- KVJXBPDAXMEYOA-CXANFOAXSA-N trilostane Chemical compound OC1=C(C#N)C[C@]2(C)[C@H]3CC[C@](C)([C@H](CC4)O)[C@@H]4[C@@H]3CC[C@@]32O[C@@H]31 KVJXBPDAXMEYOA-CXANFOAXSA-N 0.000 description 1
- 229950000212 trioxifene Drugs 0.000 description 1
- 239000001226 triphosphate Substances 0.000 description 1
- 235000011178 triphosphate Nutrition 0.000 description 1
- 229960000875 trofosfamide Drugs 0.000 description 1
- UMKFEPPTGMDVMI-UHFFFAOYSA-N trofosfamide Chemical compound ClCCN(CCCl)P1(=O)OCCCN1CCCl UMKFEPPTGMDVMI-UHFFFAOYSA-N 0.000 description 1
- 229950010147 troxacitabine Drugs 0.000 description 1
- RXRGZNYSEHTMHC-BQBZGAKWSA-N troxacitabine Chemical compound O=C1N=C(N)C=CN1[C@H]1O[C@@H](CO)OC1 RXRGZNYSEHTMHC-BQBZGAKWSA-N 0.000 description 1
- HDZZVAMISRMYHH-LITAXDCLSA-N tubercidin Chemical compound C1=CC=2C(N)=NC=NC=2N1[C@@H]1O[C@@H](CO)[C@H](O)[C@H]1O HDZZVAMISRMYHH-LITAXDCLSA-N 0.000 description 1
- 102000003298 tumor necrosis factor receptor Human genes 0.000 description 1
- OUYCCCASQSFEME-UHFFFAOYSA-N tyrosine Natural products OC(=O)C(N)CC1=CC=C(O)C=C1 OUYCCCASQSFEME-UHFFFAOYSA-N 0.000 description 1
- 235000002374 tyrosine Nutrition 0.000 description 1
- 108010002164 tyrosine receptor Proteins 0.000 description 1
- 229950009811 ubenimex Drugs 0.000 description 1
- 229960001055 uracil mustard Drugs 0.000 description 1
- 201000005112 urinary bladder cancer Diseases 0.000 description 1
- 206010046766 uterine cancer Diseases 0.000 description 1
- 208000012991 uterine carcinoma Diseases 0.000 description 1
- 230000002792 vascular Effects 0.000 description 1
- GBABOYUKABKIAF-IELIFDKJSA-N vinorelbine Chemical compound C1N(CC=2C3=CC=CC=C3NC=22)CC(CC)=C[C@H]1C[C@]2(C(=O)OC)C1=CC([C@]23[C@H]([C@@]([C@H](OC(C)=O)[C@]4(CC)C=CCN([C@H]34)CC2)(O)C(=O)OC)N2C)=C2C=C1OC GBABOYUKABKIAF-IELIFDKJSA-N 0.000 description 1
- 229960002066 vinorelbine Drugs 0.000 description 1
- CILBMBUYJCWATM-PYGJLNRPSA-N vinorelbine ditartrate Chemical compound OC(=O)[C@H](O)[C@@H](O)C(O)=O.OC(=O)[C@H](O)[C@@H](O)C(O)=O.C1N(CC=2C3=CC=CC=C3NC=22)CC(CC)=C[C@H]1C[C@]2(C(=O)OC)C1=CC([C@]23[C@H]([C@@]([C@H](OC(C)=O)[C@]4(CC)C=CCN([C@H]34)CC2)(O)C(=O)OC)N2C)=C2C=C1OC CILBMBUYJCWATM-PYGJLNRPSA-N 0.000 description 1
- 210000002845 virion Anatomy 0.000 description 1
- XLMPPFTZALNBFS-INIZCTEOSA-N vorozole Chemical compound C1([C@@H](C2=CC=C3N=NN(C3=C2)C)N2N=CN=C2)=CC=C(Cl)C=C1 XLMPPFTZALNBFS-INIZCTEOSA-N 0.000 description 1
- 201000005102 vulva cancer Diseases 0.000 description 1
- 229940053867 xeloda Drugs 0.000 description 1
- 229940002005 zometa Drugs 0.000 description 1
- 229960000641 zorubicin Drugs 0.000 description 1
- FBTUMDXHSRTGRV-ALTNURHMSA-N zorubicin Chemical compound O([C@H]1C[C@@](O)(CC=2C(O)=C3C(=O)C=4C=CC=C(C=4C(=O)C3=C(O)C=21)OC)C(\C)=N\NC(=O)C=1C=CC=CC=1)[C@H]1C[C@H](N)[C@H](O)[C@H](C)O1 FBTUMDXHSRTGRV-ALTNURHMSA-N 0.000 description 1
- 150000003952 β-lactams Chemical class 0.000 description 1
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/18—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans
- C07K16/32—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against translation products of oncogenes
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P1/00—Drugs for disorders of the alimentary tract or the digestive system
- A61P1/04—Drugs for disorders of the alimentary tract or the digestive system for ulcers, gastritis or reflux esophagitis, e.g. antacids, inhibitors of acid secretion, mucosal protectants
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P1/00—Drugs for disorders of the alimentary tract or the digestive system
- A61P1/16—Drugs for disorders of the alimentary tract or the digestive system for liver or gallbladder disorders, e.g. hepatoprotective agents, cholagogues, litholytics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P11/00—Drugs for disorders of the respiratory system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P13/00—Drugs for disorders of the urinary system
- A61P13/08—Drugs for disorders of the urinary system of the prostate
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P13/00—Drugs for disorders of the urinary system
- A61P13/12—Drugs for disorders of the urinary system of the kidneys
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P15/00—Drugs for genital or sexual disorders; Contraceptives
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P17/00—Drugs for dermatological disorders
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P19/00—Drugs for skeletal disorders
- A61P19/02—Drugs for skeletal disorders for joint disorders, e.g. arthritis, arthrosis
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P29/00—Non-central analgesic, antipyretic or antiinflammatory agents, e.g. antirheumatic agents; Non-steroidal antiinflammatory drugs [NSAID]
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
- A61P35/02—Antineoplastic agents specific for leukemia
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P37/00—Drugs for immunological or allergic disorders
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P37/00—Drugs for immunological or allergic disorders
- A61P37/02—Immunomodulators
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P43/00—Drugs for specific purposes, not provided for in groups A61P1/00-A61P41/00
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P7/00—Drugs for disorders of the blood or the extracellular fluid
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/005—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies constructed by phage libraries
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/18—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans
- C07K16/28—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants
- C07K16/2878—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants against the NGF-receptor/TNF-receptor superfamily, e.g. CD27, CD30, CD40, CD95
-
- C—CHEMISTRY; METALLURGY
- C40—COMBINATORIAL TECHNOLOGY
- C40B—COMBINATORIAL CHEMISTRY; LIBRARIES, e.g. CHEMICAL LIBRARIES
- C40B30/00—Methods of screening libraries
- C40B30/04—Methods of screening libraries by measuring the ability to specifically bind a target molecule, e.g. antibody-antigen binding, receptor-ligand binding
-
- C—CHEMISTRY; METALLURGY
- C40—COMBINATORIAL TECHNOLOGY
- C40B—COMBINATORIAL CHEMISTRY; LIBRARIES, e.g. CHEMICAL LIBRARIES
- C40B40/00—Libraries per se, e.g. arrays, mixtures
- C40B40/04—Libraries containing only organic compounds
- C40B40/06—Libraries containing nucleotides or polynucleotides, or derivatives thereof
- C40B40/08—Libraries containing RNA or DNA which encodes proteins, e.g. gene libraries
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N33/00—Investigating or analysing materials by specific methods not covered by groups G01N1/00 - G01N31/00
- G01N33/48—Biological material, e.g. blood, urine; Haemocytometers
- G01N33/50—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing
- G01N33/53—Immunoassay; Biospecific binding assay; Materials therefor
- G01N33/574—Immunoassay; Biospecific binding assay; Materials therefor for cancer
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N33/00—Investigating or analysing materials by specific methods not covered by groups G01N1/00 - G01N31/00
- G01N33/48—Biological material, e.g. blood, urine; Haemocytometers
- G01N33/50—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing
- G01N33/68—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing involving proteins, peptides or amino acids
- G01N33/6854—Immunoglobulins
- G01N33/6857—Antibody fragments
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/20—Immunoglobulins specific features characterized by taxonomic origin
- C07K2317/24—Immunoglobulins specific features characterized by taxonomic origin containing regions, domains or residues from different species, e.g. chimeric, humanized or veneered
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/50—Immunoglobulins specific features characterized by immunoglobulin fragments
- C07K2317/55—Fab or Fab'
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/50—Immunoglobulins specific features characterized by immunoglobulin fragments
- C07K2317/56—Immunoglobulins specific features characterized by immunoglobulin fragments variable (Fv) region, i.e. VH and/or VL
- C07K2317/565—Complementarity determining region [CDR]
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/90—Immunoglobulins specific features characterized by (pharmaco)kinetic aspects or by stability of the immunoglobulin
- C07K2317/92—Affinity (KD), association rate (Ka), dissociation rate (Kd) or EC50 value
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N2333/00—Assays involving biological materials from specific organisms or of a specific nature
- G01N2333/435—Assays involving biological materials from specific organisms or of a specific nature from animals; from humans
- G01N2333/705—Assays involving receptors, cell surface antigens or cell surface determinants
- G01N2333/70503—Immunoglobulin superfamily, e.g. VCAMs, PECAM, LFA-3
- G01N2333/70539—MHC-molecules, e.g. HLA-molecules
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N2333/00—Assays involving biological materials from specific organisms or of a specific nature
- G01N2333/435—Assays involving biological materials from specific organisms or of a specific nature from animals; from humans
- G01N2333/705—Assays involving receptors, cell surface antigens or cell surface determinants
- G01N2333/71—Assays involving receptors, cell surface antigens or cell surface determinants for growth factors; for growth regulators
Landscapes
- Health & Medical Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Organic Chemistry (AREA)
- Immunology (AREA)
- Medicinal Chemistry (AREA)
- General Health & Medical Sciences (AREA)
- Engineering & Computer Science (AREA)
- Molecular Biology (AREA)
- Chemical Kinetics & Catalysis (AREA)
- General Chemical & Material Sciences (AREA)
- Animal Behavior & Ethology (AREA)
- Pharmacology & Pharmacy (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Biochemistry (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Hematology (AREA)
- Urology & Nephrology (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Biomedical Technology (AREA)
- Genetics & Genomics (AREA)
- Biophysics (AREA)
- Oncology (AREA)
- Cell Biology (AREA)
- Biotechnology (AREA)
- Pathology (AREA)
- General Physics & Mathematics (AREA)
- Analytical Chemistry (AREA)
- Physics & Mathematics (AREA)
- Food Science & Technology (AREA)
- Microbiology (AREA)
- Virology (AREA)
- Hospice & Palliative Care (AREA)
- Rheumatology (AREA)
- Orthopedic Medicine & Surgery (AREA)
- Diabetes (AREA)
- Gastroenterology & Hepatology (AREA)
- Endocrinology (AREA)
Applications Claiming Priority (5)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US74218505P | 2005-12-02 | 2005-12-02 | |
| US60/742,185 | 2005-12-02 | ||
| US80555306P | 2006-06-22 | 2006-06-22 | |
| US60/805,553 | 2006-06-22 | ||
| PCT/US2006/046046 WO2007094842A2 (en) | 2005-12-02 | 2006-12-01 | Binding polypeptides and uses thereof |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| RU2008126948A RU2008126948A (ru) | 2010-01-10 |
| RU2470941C2 true RU2470941C2 (ru) | 2012-12-27 |
Family
ID=38371942
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| RU2008126948/10A RU2470941C2 (ru) | 2005-12-02 | 2006-12-01 | Связывающие полипептиды и их применения |
Country Status (10)
| Country | Link |
|---|---|
| US (1) | US8957187B2 (enExample) |
| EP (1) | EP1957540B1 (enExample) |
| JP (1) | JP5808070B2 (enExample) |
| KR (1) | KR101434682B1 (enExample) |
| AU (1) | AU2006338198B2 (enExample) |
| CA (1) | CA2631327C (enExample) |
| ES (1) | ES2388932T3 (enExample) |
| RU (1) | RU2470941C2 (enExample) |
| WO (1) | WO2007094842A2 (enExample) |
| ZA (1) | ZA200805045B (enExample) |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| RU2644681C2 (ru) * | 2011-04-28 | 2018-02-13 | Зе Боард Оф Трастиз Оф Зе Леланд Стэнфорд Джуниор Юниверсити | Идентификация ассоциированных с образцом полинуклеотидов |
Families Citing this family (33)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| ES2577292T3 (es) | 2005-11-07 | 2016-07-14 | Genentech, Inc. | Polipéptidos de unión con secuencias hipervariables de VH/VL diversificadas y consenso |
| US20070237764A1 (en) * | 2005-12-02 | 2007-10-11 | Genentech, Inc. | Binding polypeptides with restricted diversity sequences |
| US8877688B2 (en) | 2007-09-14 | 2014-11-04 | Adimab, Llc | Rationally designed, synthetic antibody libraries and uses therefor |
| CN101855242B (zh) * | 2007-09-14 | 2014-07-30 | 阿迪马布有限责任公司 | 合理设计的合成抗体文库及其用途 |
| EP2889370A1 (en) | 2007-12-11 | 2015-07-01 | The Scripps Research Institute | In vivo unnatural amino acid expression in the methylotrophic yeast Pichia pastoris |
| US9102711B2 (en) | 2007-12-31 | 2015-08-11 | Xoma Technology Ltd. | Methods and materials for targeted mutagenesis |
| JP5487570B2 (ja) * | 2008-07-09 | 2014-05-07 | 富士フイルム株式会社 | 抗体と蛋白質との融合蛋白質の製造方法 |
| AU2009274129B2 (en) * | 2008-07-21 | 2016-02-25 | Immunomedics Inc. | Structural variants of antibodies for improved therapeutic characteristics |
| UA112050C2 (uk) * | 2008-08-04 | 2016-07-25 | БАЄР ХЕЛСКЕР ЛЛСі | Терапевтична композиція, що містить моноклональне антитіло проти інгібітора шляху тканинного фактора (tfpi) |
| WO2010019565A2 (en) * | 2008-08-12 | 2010-02-18 | Medlmmune, Llc | Anti-ephrin b2 antibodies and their use in treatment of disease |
| CN102753193A (zh) | 2008-10-31 | 2012-10-24 | 比奥根艾迪克Ma公司 | Light靶向分子及其用途 |
| WO2010068278A2 (en) * | 2008-12-10 | 2010-06-17 | The Scripps Research Institute | Production of carrier-peptide conjugates using chemically reactive unnatural amino acids |
| US20090197339A1 (en) * | 2008-12-10 | 2009-08-06 | The Scripps Research Institute | In vivo unnatural amino acid expression in the methylotrophic yeast pichia pastoris |
| JP5756802B2 (ja) | 2009-08-13 | 2015-07-29 | クリスタル バイオサイエンス インク.Crystal Bioscience Inc. | 最小cdrを有する抗体を産生するトランスジェニック動物 |
| CA2775350A1 (en) * | 2009-09-24 | 2011-03-31 | Seattle Genetics, Inc. | Dr5 ligand drug conjugates |
| WO2011084496A1 (en) * | 2009-12-16 | 2011-07-14 | Abbott Biotherapeutics Corp. | Anti-her2 antibodies and their uses |
| AU2011223710B2 (en) | 2010-03-01 | 2016-04-14 | Bayer Healthcare Llc | Optimized monoclonal antibodies against tissue factor pathway inhibitor (TFPI) |
| FR2959994B1 (fr) * | 2010-05-12 | 2012-08-24 | Lfb Biotechnologies | Nouveaux anticorps humanises 12g4 mutes et leurs fragments diriges contre le recepteur humain de l'hormone anti-mullerienne de type ii |
| NZ701444A (en) | 2010-08-27 | 2016-06-24 | Gilead Biologics Inc | Antibodies to matrix metalloproteinase 9 |
| US20140121123A1 (en) * | 2010-10-29 | 2014-05-01 | Kevin Caili Wang | Methods for diversifying antibodies, antibodies derived therefrom and uses thereof |
| CA2826142A1 (en) * | 2011-02-03 | 2012-08-09 | Xoma Technology Ltd. | Methods and materials for enhancing functional protein expression in bacteria |
| EP2699586B1 (en) * | 2011-04-21 | 2019-03-06 | Ology Bioservices, Inc. | Methods for isolating and quantifying antigen from vaccines |
| MD20140107A2 (ro) * | 2012-02-29 | 2015-03-31 | Gilead Biologics, Inc. | Anticorpi împotriva metaloproteinazei 9 din matrice |
| WO2013130905A1 (en) * | 2012-02-29 | 2013-09-06 | Gilead Biologics, Inc. | Antibodies to matrix metalloproteinase 9 |
| KR101505157B1 (ko) * | 2012-05-08 | 2015-03-24 | 주식회사 종근당 | 항―ErbB2 항체 변이체 |
| WO2016007625A1 (en) * | 2014-07-08 | 2016-01-14 | Tdw Group | Antibodies to argininosuccinate synthase and related methods |
| US11254738B2 (en) * | 2016-09-07 | 2022-02-22 | The Governing Council Of The University Of Toronto Banting Institute | Synthetic antibodies against VEGF and their uses |
| CN106946990B (zh) * | 2017-02-23 | 2021-02-23 | 南昌大佳科技有限公司 | 一种针对c-Myc标签的纳米抗体 |
| KR102344616B1 (ko) | 2017-05-30 | 2021-12-31 | 더 보드 오브 리젠츠 오브 더 유니버시티 오브 오클라호마 | 항-더블코르틴-유사 키나제 1 항체 및 사용 방법 |
| EP3720481A4 (en) | 2017-12-04 | 2021-10-13 | Coare Holdings, Inc. | ANTI-DCLK1 ANTIBODIES AND CHIMERA ANTIGEN RECEPTORS AND COMPOSITIONS AND METHODS OF USING THEREOF |
| CN109627319B (zh) * | 2019-01-11 | 2022-06-21 | 北京协同创新研究院 | 一种抗her-2的重链抗体及其应用 |
| AU2020368789B2 (en) | 2019-10-16 | 2024-01-18 | Naturesense Co., Ltd. | Peptide for improving memory and preventing or alleviating cognitive impairment, composition containing same and preparation method therefor |
| KR102107808B1 (ko) * | 2019-11-12 | 2020-05-07 | 주식회사 네이처센스 농업회사법인 | 기억력 및 인지기능 개선용 펩타이드 |
Citations (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2004050895A2 (en) * | 2002-11-27 | 2004-06-17 | Irm Llc | Methods and compositions for inducing apoptosis in cancer cells |
| WO2005012359A2 (en) * | 2003-08-01 | 2005-02-10 | Genentech, Inc. | Anti-vegf antibodies |
| WO2005012531A2 (en) * | 2003-08-01 | 2005-02-10 | Genentech, Inc. | Antibody cdr polypeptide sequences with restricted diversity |
Family Cites Families (107)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US3773919A (en) * | 1969-10-23 | 1973-11-20 | Du Pont | Polylactide-drug mixtures |
| US3896111A (en) * | 1973-02-20 | 1975-07-22 | Research Corp | Ansa macrolides |
| US4151042A (en) * | 1977-03-31 | 1979-04-24 | Takeda Chemical Industries, Ltd. | Method for producing maytansinol and its derivatives |
| US4137230A (en) * | 1977-11-14 | 1979-01-30 | Takeda Chemical Industries, Ltd. | Method for the production of maytansinoids |
| USRE30985E (en) * | 1978-01-01 | 1982-06-29 | Serum-free cell culture media | |
| US4265814A (en) * | 1978-03-24 | 1981-05-05 | Takeda Chemical Industries | Matansinol 3-n-hexadecanoate |
| US4307016A (en) * | 1978-03-24 | 1981-12-22 | Takeda Chemical Industries, Ltd. | Demethyl maytansinoids |
| JPS5562090A (en) * | 1978-10-27 | 1980-05-10 | Takeda Chem Ind Ltd | Novel maytansinoid compound and its preparation |
| JPS5566585A (en) * | 1978-11-14 | 1980-05-20 | Takeda Chem Ind Ltd | Novel maytansinoid compound and its preparation |
| US4256746A (en) * | 1978-11-14 | 1981-03-17 | Takeda Chemical Industries | Dechloromaytansinoids, their pharmaceutical compositions and method of use |
| JPS55164687A (en) * | 1979-06-11 | 1980-12-22 | Takeda Chem Ind Ltd | Novel maytansinoid compound and its preparation |
| JPS55102583A (en) * | 1979-01-31 | 1980-08-05 | Takeda Chem Ind Ltd | 20-acyloxy-20-demethylmaytansinoid compound |
| JPS55162791A (en) * | 1979-06-05 | 1980-12-18 | Takeda Chem Ind Ltd | Antibiotic c-15003pnd and its preparation |
| JPS55164685A (en) * | 1979-06-08 | 1980-12-22 | Takeda Chem Ind Ltd | Novel maytansinoid compound and its preparation |
| JPS55164686A (en) * | 1979-06-11 | 1980-12-22 | Takeda Chem Ind Ltd | Novel maytansinoid compound and its preparation |
| US4309428A (en) * | 1979-07-30 | 1982-01-05 | Takeda Chemical Industries, Ltd. | Maytansinoids |
| JPS5645483A (en) * | 1979-09-19 | 1981-04-25 | Takeda Chem Ind Ltd | C-15003phm and its preparation |
| JPS5645485A (en) * | 1979-09-21 | 1981-04-25 | Takeda Chem Ind Ltd | Production of c-15003pnd |
| EP0028683A1 (en) * | 1979-09-21 | 1981-05-20 | Takeda Chemical Industries, Ltd. | Antibiotic C-15003 PHO and production thereof |
| WO1982001188A1 (en) * | 1980-10-08 | 1982-04-15 | Takeda Chemical Industries Ltd | 4,5-deoxymaytansinoide compounds and process for preparing same |
| US4450254A (en) * | 1980-11-03 | 1984-05-22 | Standard Oil Company | Impact improvement of high nitrile resins |
| US4419446A (en) * | 1980-12-31 | 1983-12-06 | The United States Of America As Represented By The Department Of Health And Human Services | Recombinant DNA process utilizing a papilloma virus DNA as a vector |
| US4313946A (en) * | 1981-01-27 | 1982-02-02 | The United States Of America As Represented By The Secretary Of Agriculture | Chemotherapeutically active maytansinoids from Trewia nudiflora |
| US4315929A (en) * | 1981-01-27 | 1982-02-16 | The United States Of America As Represented By The Secretary Of Agriculture | Method of controlling the European corn borer with trewiasine |
| JPS57192389A (en) * | 1981-05-20 | 1982-11-26 | Takeda Chem Ind Ltd | Novel maytansinoid |
| US4670393A (en) * | 1982-03-22 | 1987-06-02 | Genentech, Inc. | DNA vectors encoding a novel human growth hormone-variant protein |
| US4601978A (en) * | 1982-11-24 | 1986-07-22 | The Regents Of The University Of California | Mammalian metallothionein promoter system |
| US4560655A (en) * | 1982-12-16 | 1985-12-24 | Immunex Corporation | Serum-free cell culture medium and process for making same |
| US4657866A (en) * | 1982-12-21 | 1987-04-14 | Sudhir Kumar | Serum-free, synthetic, completely chemically defined tissue culture media |
| US4816567A (en) * | 1983-04-08 | 1989-03-28 | Genentech, Inc. | Recombinant immunoglobin preparations |
| US4755465A (en) * | 1983-04-25 | 1988-07-05 | Genentech, Inc. | Secretion of correctly processed human growth hormone in E. coli and Pseudomonas |
| IL71991A (en) | 1983-06-06 | 1994-05-30 | Genentech Inc | Preparation of human FGI and FGE in their processed form through recombinant AND tranology in prokaryotes |
| US4767704A (en) * | 1983-10-07 | 1988-08-30 | Columbia University In The City Of New York | Protein-free culture medium |
| US4965199A (en) * | 1984-04-20 | 1990-10-23 | Genentech, Inc. | Preparation of functional human factor VIII in mammalian cells using methotrexate based selection |
| US4970198A (en) * | 1985-10-17 | 1990-11-13 | American Cyanamid Company | Antitumor antibiotics (LL-E33288 complex) |
| GB8516415D0 (en) | 1985-06-28 | 1985-07-31 | Celltech Ltd | Culture of animal cells |
| SE8505922D0 (sv) | 1985-12-13 | 1985-12-13 | Kabigen Ab | Construction of an igg binding protein to facilitate downstream processing using protein engineering |
| US4927762A (en) * | 1986-04-01 | 1990-05-22 | Cell Enterprises, Inc. | Cell culture medium with antioxidant |
| EP0266032A1 (en) | 1986-08-29 | 1988-05-04 | Beecham Group Plc | Modified fibrinolytic enzyme |
| IL85035A0 (en) * | 1987-01-08 | 1988-06-30 | Int Genetic Eng | Polynucleotide molecule,a chimeric antibody with specificity for human b cell surface antigen,a process for the preparation and methods utilizing the same |
| US5079233A (en) * | 1987-01-30 | 1992-01-07 | American Cyanamid Company | N-acyl derivatives of the LL-E33288 antitumor antibiotics, composition and methods for using the same |
| EP0307434B2 (en) * | 1987-03-18 | 1998-07-29 | Scotgen Biopharmaceuticals, Inc. | Altered antibodies |
| EP0288451B1 (en) | 1987-04-23 | 1994-08-10 | Monsanto Company | Secretion of insulin-like growth factor 1 in E. coli |
| US4975278A (en) * | 1988-02-26 | 1990-12-04 | Bristol-Myers Company | Antibody-enzyme conjugates in combination with prodrugs for the delivery of cytotoxic agents to tumor cells |
| US5770701A (en) * | 1987-10-30 | 1998-06-23 | American Cyanamid Company | Process for preparing targeted forms of methyltrithio antitumor agents |
| US5606040A (en) * | 1987-10-30 | 1997-02-25 | American Cyanamid Company | Antitumor and antibacterial substituted disulfide derivatives prepared from compounds possessing a methyl-trithio group |
| US5053394A (en) * | 1988-09-21 | 1991-10-01 | American Cyanamid Company | Targeted forms of methyltrithio antitumor agents |
| JP3040121B2 (ja) * | 1988-01-12 | 2000-05-08 | ジェネンテク,インコーポレイテッド | 増殖因子レセプターの機能を阻害することにより腫瘍細胞を処置する方法 |
| EP0435911B1 (en) | 1988-09-23 | 1996-03-13 | Cetus Oncology Corporation | Cell culture medium for enhanced cell growth, culture longevity and product expression |
| DE68929151T2 (de) | 1988-10-28 | 2000-07-20 | Genentech, Inc. | Verfahren zum nachweis von aktiven domänen und aminosäureresten in polypeptiden und hormonvarianten |
| WO1990005144A1 (en) | 1988-11-11 | 1990-05-17 | Medical Research Council | Single domain ligands, receptors comprising said ligands, methods for their production, and use of said ligands and receptors |
| US5530101A (en) * | 1988-12-28 | 1996-06-25 | Protein Design Labs, Inc. | Humanized immunoglobulins |
| DE3920358A1 (de) | 1989-06-22 | 1991-01-17 | Behringwerke Ag | Bispezifische und oligospezifische, mono- und oligovalente antikoerperkonstrukte, ihre herstellung und verwendung |
| CA2026147C (en) | 1989-10-25 | 2006-02-07 | Ravi J. Chari | Cytotoxic agents comprising maytansinoids and their therapeutic use |
| US5208020A (en) * | 1989-10-25 | 1993-05-04 | Immunogen Inc. | Cytotoxic agents comprising maytansinoids and their therapeutic use |
| US5427908A (en) * | 1990-05-01 | 1995-06-27 | Affymax Technologies N.V. | Recombinant library screening methods |
| US5723286A (en) * | 1990-06-20 | 1998-03-03 | Affymax Technologies N.V. | Peptide library and screening systems |
| GB9015198D0 (en) * | 1990-07-10 | 1990-08-29 | Brien Caroline J O | Binding substance |
| US6172197B1 (en) * | 1991-07-10 | 2001-01-09 | Medical Research Council | Methods for producing members of specific binding pairs |
| ES2139598T3 (es) | 1990-07-10 | 2000-02-16 | Medical Res Council | Procedimientos para la produccion de miembros de parejas de union especifica. |
| US5122469A (en) * | 1990-10-03 | 1992-06-16 | Genentech, Inc. | Method for culturing Chinese hamster ovary cells to improve production of recombinant proteins |
| US5508192A (en) * | 1990-11-09 | 1996-04-16 | Board Of Regents, The University Of Texas System | Bacterial host strains for producing proteolytically sensitive polypeptides |
| US5264365A (en) * | 1990-11-09 | 1993-11-23 | Board Of Regents, The University Of Texas System | Protease-deficient bacterial strains for production of proteolytically sensitive polypeptides |
| WO1992009690A2 (en) | 1990-12-03 | 1992-06-11 | Genentech, Inc. | Enrichment method for variant proteins with altered binding properties |
| EP1471142B1 (en) * | 1991-04-10 | 2008-11-19 | The Scripps Research Institute | Heterodimeric receptor libraries using phagemids |
| WO1994004679A1 (en) * | 1991-06-14 | 1994-03-03 | Genentech, Inc. | Method for making humanized antibodies |
| CA2103059C (en) * | 1991-06-14 | 2005-03-22 | Paul J. Carter | Method for making humanized antibodies |
| JP3951062B2 (ja) * | 1991-09-19 | 2007-08-01 | ジェネンテック・インコーポレーテッド | 少なくとも遊離のチオールとして存在するシステインを有する抗体フラグメントの大腸菌での発現、2官能性F(ab’)2抗体の産生のための使用 |
| EP0605522B1 (en) | 1991-09-23 | 1999-06-23 | Medical Research Council | Methods for the production of humanized antibodies |
| US5362852A (en) * | 1991-09-27 | 1994-11-08 | Pfizer Inc. | Modified peptide derivatives conjugated at 2-hydroxyethylamine moieties |
| US5270170A (en) * | 1991-10-16 | 1993-12-14 | Affymax Technologies N.V. | Peptide library and screening method |
| EP1136556B1 (en) | 1991-11-25 | 2005-06-08 | Enzon, Inc. | Method of producing multivalent antigen-binding proteins |
| WO1993011236A1 (en) | 1991-12-02 | 1993-06-10 | Medical Research Council | Production of anti-self antibodies from antibody segment repertoires and displayed on phage |
| US5733743A (en) * | 1992-03-24 | 1998-03-31 | Cambridge Antibody Technology Limited | Methods for producing members of specific binding pairs |
| JP3507073B2 (ja) | 1992-03-24 | 2004-03-15 | ケンブリッジ アンティボディー テクノロジー リミティド | 特異的結合対の成員の製造方法 |
| ZA932522B (en) | 1992-04-10 | 1993-12-20 | Res Dev Foundation | Immunotoxins directed against c-erbB-2(HER/neu) related surface antigens |
| US6083713A (en) * | 1992-08-31 | 2000-07-04 | Bristol-Myers Squibb Company | Cloning and expression of βAPP-C100 receptor (C100-R) |
| PT752248E (pt) | 1992-11-13 | 2001-01-31 | Idec Pharma Corp | Aplicacao terapeutica de anticorpos quimericos e marcados radioactivamente contra antigenios de diferenciacao restrita de linfocitos b humanos para o tratamento do linfoma de celulas b |
| EP0672142B1 (en) * | 1992-12-04 | 2001-02-28 | Medical Research Council | Multivalent and multispecific binding proteins, their manufacture and use |
| WO1994029351A2 (en) | 1993-06-16 | 1994-12-22 | Celltech Limited | Antibodies |
| US5534615A (en) * | 1994-04-25 | 1996-07-09 | Genentech, Inc. | Cardiac hypertrophy factor and uses therefor |
| US5773001A (en) * | 1994-06-03 | 1998-06-30 | American Cyanamid Company | Conjugates of methyltrithio antitumor agents and intermediates for their synthesis |
| US5639635A (en) * | 1994-11-03 | 1997-06-17 | Genentech, Inc. | Process for bacterial production of polypeptides |
| US5770567A (en) * | 1994-11-14 | 1998-06-23 | Genentech, Inc. | Sensory and motor neuron derived factor (SMDF) |
| US5840523A (en) * | 1995-03-01 | 1998-11-24 | Genetech, Inc. | Methods and compositions for secretion of heterologous polypeptides |
| US5731168A (en) * | 1995-03-01 | 1998-03-24 | Genentech, Inc. | Method for making heteromultimeric polypeptides |
| US5739277A (en) * | 1995-04-14 | 1998-04-14 | Genentech Inc. | Altered polypeptides with increased half-life |
| US5714586A (en) * | 1995-06-07 | 1998-02-03 | American Cyanamid Company | Methods for the preparation of monomeric calicheamicin derivative/carrier conjugates |
| US5712374A (en) * | 1995-06-07 | 1998-01-27 | American Cyanamid Company | Method for the preparation of substantiallly monomeric calicheamicin derivative/carrier conjugates |
| US6027888A (en) * | 1996-04-05 | 2000-02-22 | Board Of Regents, The University Of Texas System | Methods for producing soluble, biologically-active disulfide-bond containing eukaryotic proteins in bacterial cells |
| CA2269204C (en) | 1996-10-18 | 2012-01-24 | Genentech, Inc. | Anti-erbb2 antibodies |
| US6072047A (en) | 1997-02-13 | 2000-06-06 | Immunex Corporation | Receptor that binds trail |
| ATE516354T1 (de) | 1997-05-15 | 2011-07-15 | Genentech Inc | Apo-2-rezeptor |
| US6083715A (en) | 1997-06-09 | 2000-07-04 | Board Of Regents, The University Of Texas System | Methods for producing heterologous disulfide bond-containing polypeptides in bacterial cells |
| DK1068241T3 (da) | 1998-04-02 | 2008-02-04 | Genentech Inc | Antistofvarianter og fragmenter deraf |
| ES2694002T3 (es) | 1999-01-15 | 2018-12-17 | Genentech, Inc. | Polipéptido que comprende una región Fc de IgG1 humana variante |
| US6436401B1 (en) * | 1999-09-14 | 2002-08-20 | Milkhaus Laboratory, Inc. | Methods for alleviating symptoms associated with diabetes and diabetic neuropathy comprising administration of low levels of antibodies |
| EP1226161A2 (en) | 1999-09-15 | 2002-07-31 | Genentech, Inc. | Apo-2 receptor antibodies |
| DE60041564D1 (de) | 1999-12-24 | 2009-03-26 | Genentech Inc | Verfahren und Zusammensetzungen zur Verlängerung der Entsorgungshalbwertszeit von biowirksamen Verbindungen |
| ES2649037T3 (es) | 2000-12-12 | 2018-01-09 | Medimmune, Llc | Moléculas con semividas prolongadas, composiciones y usos de las mismas |
| EP1360288B1 (en) * | 2000-12-18 | 2011-02-16 | Dyax Corp. | Focused libraries of genetic packages |
| ES2401428T3 (es) * | 2002-04-10 | 2013-04-19 | Genentech, Inc. | Variantes de anticuerpos anti-HER2 |
| CA2488441C (en) * | 2002-06-03 | 2015-01-27 | Genentech, Inc. | Synthetic antibody phage libraries |
| EP1391213A1 (en) | 2002-08-21 | 2004-02-25 | Boehringer Ingelheim International GmbH | Compositions and methods for treating cancer using maytansinoid CD44 antibody immunoconjugates and chemotherapeutic agents |
| WO2004065416A2 (en) | 2003-01-16 | 2004-08-05 | Genentech, Inc. | Synthetic antibody phage libraries |
| KR20070010046A (ko) | 2004-04-06 | 2007-01-19 | 제넨테크, 인크. | Dr5 항체 및 그의 용도 |
| ES2577292T3 (es) | 2005-11-07 | 2016-07-14 | Genentech, Inc. | Polipéptidos de unión con secuencias hipervariables de VH/VL diversificadas y consenso |
-
2006
- 2006-12-01 RU RU2008126948/10A patent/RU2470941C2/ru active
- 2006-12-01 ES ES06849911T patent/ES2388932T3/es active Active
- 2006-12-01 WO PCT/US2006/046046 patent/WO2007094842A2/en not_active Ceased
- 2006-12-01 AU AU2006338198A patent/AU2006338198B2/en not_active Expired - Fee Related
- 2006-12-01 KR KR1020087016105A patent/KR101434682B1/ko active Active
- 2006-12-01 EP EP06849911A patent/EP1957540B1/en active Active
- 2006-12-01 JP JP2008543499A patent/JP5808070B2/ja active Active
- 2006-12-01 US US11/566,120 patent/US8957187B2/en active Active
- 2006-12-01 CA CA2631327A patent/CA2631327C/en active Active
-
2008
- 2008-06-10 ZA ZA2008/05045A patent/ZA200805045B/en unknown
Patent Citations (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2004050895A2 (en) * | 2002-11-27 | 2004-06-17 | Irm Llc | Methods and compositions for inducing apoptosis in cancer cells |
| WO2005012359A2 (en) * | 2003-08-01 | 2005-02-10 | Genentech, Inc. | Anti-vegf antibodies |
| WO2005012531A2 (en) * | 2003-08-01 | 2005-02-10 | Genentech, Inc. | Antibody cdr polypeptide sequences with restricted diversity |
Non-Patent Citations (2)
| Title |
|---|
| FELLOUSE et al., "Molecular recognition by a binary code", Journal of molecular biology, vol.348, no.5, 20.05.2005, с.1153-1162. * |
| РОЙТ и др. Иммунология. - М.: Мир, 2000, с.110-116. * |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| RU2644681C2 (ru) * | 2011-04-28 | 2018-02-13 | Зе Боард Оф Трастиз Оф Зе Леланд Стэнфорд Джуниор Юниверсити | Идентификация ассоциированных с образцом полинуклеотидов |
Also Published As
| Publication number | Publication date |
|---|---|
| ES2388932T3 (es) | 2012-10-19 |
| AU2006338198B2 (en) | 2012-04-26 |
| ZA200805045B (en) | 2009-12-30 |
| WO2007094842A3 (en) | 2008-02-28 |
| RU2008126948A (ru) | 2010-01-10 |
| EP1957540B1 (en) | 2012-06-13 |
| KR20080077246A (ko) | 2008-08-21 |
| WO2007094842A2 (en) | 2007-08-23 |
| KR101434682B1 (ko) | 2014-08-27 |
| EP1957540A2 (en) | 2008-08-20 |
| US20070202552A1 (en) | 2007-08-30 |
| AU2006338198A1 (en) | 2007-08-23 |
| CA2631327A1 (en) | 2007-08-23 |
| HK1121470A1 (en) | 2009-04-24 |
| JP5808070B2 (ja) | 2015-11-10 |
| US8957187B2 (en) | 2015-02-17 |
| JP2009518011A (ja) | 2009-05-07 |
| CA2631327C (en) | 2015-10-13 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| RU2470941C2 (ru) | Связывающие полипептиды и их применения | |
| US11753464B2 (en) | Methods and compositions for targeting polyubiquitin | |
| US8679490B2 (en) | Binding polypeptides with diversified and consensus VH/VL hypervariable sequences | |
| US20050106667A1 (en) | Binding polypeptides with restricted diversity sequences | |
| US20090023602A1 (en) | Binding polypeptides with restricted diversity sequences | |
| US9487578B2 (en) | Methods and compositions for targeting polyubiquitin | |
| US20070237764A1 (en) | Binding polypeptides with restricted diversity sequences | |
| JP2009518011A5 (enExample) | ||
| RU2451031C2 (ru) | Антитела к эфрину в2 и способы их применения | |
| CN101370832B (zh) | 结合多肽及其用途 | |
| AU2012204022B2 (en) | Binding polypeptides and uses thereof | |
| BRPI0620505A2 (pt) | polipeptìdeos, anticorpo, polipeptìdeo de fusão, composição, polinucleotìdeo, vetor, célula hospedeira, método de produção de um polipeptìdeo, biblioteca de polipeptìdeos, métodos para gerar uma composição, método para a seleção de um domìnio variável ligante ao antìgeno, método para selecionar polipeptìdeos, método para isolar um ou mais polipeptìdeos, método para o tratamento de cáncer e método para tratar |