KR20200030594A - B형 간염 감염을 치료하기 위한 PAPD5 및 PAPD7 mRNA의 감소용 핵산 분자 - Google Patents
B형 간염 감염을 치료하기 위한 PAPD5 및 PAPD7 mRNA의 감소용 핵산 분자 Download PDFInfo
- Publication number
- KR20200030594A KR20200030594A KR1020207005096A KR20207005096A KR20200030594A KR 20200030594 A KR20200030594 A KR 20200030594A KR 1020207005096 A KR1020207005096 A KR 1020207005096A KR 20207005096 A KR20207005096 A KR 20207005096A KR 20200030594 A KR20200030594 A KR 20200030594A
- Authority
- KR
- South Korea
- Prior art keywords
- oligonucleotide
- nucleosides
- nucleic acid
- oligonucleotides
- papd5
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Ceased
Links
- 102000039446 nucleic acids Human genes 0.000 title claims abstract description 217
- 108020004707 nucleic acids Proteins 0.000 title claims abstract description 217
- 150000007523 nucleic acids Chemical class 0.000 title claims abstract description 214
- 101000735431 Homo sapiens Terminal nucleotidyltransferase 4A Proteins 0.000 title claims abstract description 183
- 102100034939 Terminal nucleotidyltransferase 4A Human genes 0.000 title claims abstract description 173
- 101000735429 Homo sapiens Terminal nucleotidyltransferase 4B Proteins 0.000 title claims abstract description 160
- 102100034938 Terminal nucleotidyltransferase 4B Human genes 0.000 title claims abstract description 150
- 230000001603 reducing effect Effects 0.000 title claims description 9
- 108020004999 messenger RNA Proteins 0.000 title description 93
- 208000002672 hepatitis B Diseases 0.000 title description 9
- 230000014509 gene expression Effects 0.000 claims abstract description 72
- 208000015181 infectious disease Diseases 0.000 claims abstract description 63
- 230000000295 complement effect Effects 0.000 claims abstract description 49
- 239000008194 pharmaceutical composition Substances 0.000 claims abstract description 35
- 238000011282 treatment Methods 0.000 claims abstract description 32
- 230000001684 chronic effect Effects 0.000 claims abstract description 16
- 230000002265 prevention Effects 0.000 claims abstract description 14
- 108091034117 Oligonucleotide Proteins 0.000 claims description 624
- 239000002777 nucleoside Substances 0.000 claims description 329
- 125000003835 nucleoside group Chemical group 0.000 claims description 280
- 150000001875 compounds Chemical class 0.000 claims description 270
- 239000000074 antisense oligonucleotide Substances 0.000 claims description 210
- 238000012230 antisense oligonucleotides Methods 0.000 claims description 210
- 241000700721 Hepatitis B virus Species 0.000 claims description 193
- 125000003729 nucleotide group Chemical group 0.000 claims description 190
- 239000002773 nucleotide Substances 0.000 claims description 187
- 125000005647 linker group Chemical group 0.000 claims description 151
- 150000003833 nucleoside derivatives Chemical class 0.000 claims description 109
- 238000000034 method Methods 0.000 claims description 94
- 235000000346 sugar Nutrition 0.000 claims description 61
- RYYWUUFWQRZTIU-UHFFFAOYSA-K thiophosphate Chemical compound [O-]P([O-])([O-])=S RYYWUUFWQRZTIU-UHFFFAOYSA-K 0.000 claims description 60
- 150000003839 salts Chemical class 0.000 claims description 59
- -1 LNA nucleoside Chemical class 0.000 claims description 52
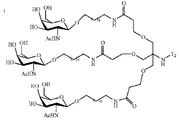
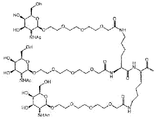
- OVRNDRQMDRJTHS-KEWYIRBNSA-N N-acetyl-D-galactosamine Chemical group CC(=O)N[C@H]1C(O)O[C@H](CO)[C@H](O)[C@@H]1O OVRNDRQMDRJTHS-KEWYIRBNSA-N 0.000 claims description 52
- 238000000338 in vitro Methods 0.000 claims description 40
- 102100034343 Integrase Human genes 0.000 claims description 35
- 101710203526 Integrase Proteins 0.000 claims description 35
- 150000004713 phosphodiesters Chemical class 0.000 claims description 27
- FWMNVWWHGCHHJJ-SKKKGAJSSA-N 4-amino-1-[(2r)-6-amino-2-[[(2r)-2-[[(2r)-2-[[(2r)-2-amino-3-phenylpropanoyl]amino]-3-phenylpropanoyl]amino]-4-methylpentanoyl]amino]hexanoyl]piperidine-4-carboxylic acid Chemical compound C([C@H](C(=O)N[C@H](CC(C)C)C(=O)N[C@H](CCCCN)C(=O)N1CCC(N)(CC1)C(O)=O)NC(=O)[C@H](N)CC=1C=CC=CC=1)C1=CC=CC=C1 FWMNVWWHGCHHJJ-SKKKGAJSSA-N 0.000 claims description 21
- 239000003814 drug Substances 0.000 claims description 13
- 230000002401 inhibitory effect Effects 0.000 claims description 13
- 238000001727 in vivo Methods 0.000 claims description 12
- 230000000692 anti-sense effect Effects 0.000 claims description 11
- 239000003085 diluting agent Substances 0.000 claims description 10
- 239000002671 adjuvant Substances 0.000 claims description 6
- 230000005764 inhibitory process Effects 0.000 abstract description 27
- 210000004027 cell Anatomy 0.000 description 194
- JLCPHMBAVCMARE-UHFFFAOYSA-N [3-[[3-[[3-[[3-[[3-[[3-[[3-[[3-[[3-[[3-[[3-[[5-(2-amino-6-oxo-1H-purin-9-yl)-3-[[3-[[3-[[3-[[3-[[3-[[5-(2-amino-6-oxo-1H-purin-9-yl)-3-[[5-(2-amino-6-oxo-1H-purin-9-yl)-3-hydroxyoxolan-2-yl]methoxy-hydroxyphosphoryl]oxyoxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxyoxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methyl [5-(6-aminopurin-9-yl)-2-(hydroxymethyl)oxolan-3-yl] hydrogen phosphate Polymers Cc1cn(C2CC(OP(O)(=O)OCC3OC(CC3OP(O)(=O)OCC3OC(CC3O)n3cnc4c3nc(N)[nH]c4=O)n3cnc4c3nc(N)[nH]c4=O)C(COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3CO)n3cnc4c(N)ncnc34)n3ccc(N)nc3=O)n3cnc4c(N)ncnc34)n3ccc(N)nc3=O)n3ccc(N)nc3=O)n3ccc(N)nc3=O)n3cnc4c(N)ncnc34)n3cnc4c(N)ncnc34)n3cc(C)c(=O)[nH]c3=O)n3cc(C)c(=O)[nH]c3=O)n3ccc(N)nc3=O)n3cc(C)c(=O)[nH]c3=O)n3cnc4c3nc(N)[nH]c4=O)n3cnc4c(N)ncnc34)n3cnc4c(N)ncnc34)n3cnc4c(N)ncnc34)n3cnc4c(N)ncnc34)O2)c(=O)[nH]c1=O JLCPHMBAVCMARE-UHFFFAOYSA-N 0.000 description 185
- 108020004414 DNA Proteins 0.000 description 142
- 101710142246 External core antigen Proteins 0.000 description 63
- 230000008685 targeting Effects 0.000 description 63
- 108091032973 (ribonucleotides)n+m Proteins 0.000 description 57
- 238000013461 design Methods 0.000 description 50
- 108020000948 Antisense Oligonucleotides Proteins 0.000 description 47
- MBLBDJOUHNCFQT-UHFFFAOYSA-N N-acetyl-D-galactosamine Natural products CC(=O)NC(C=O)C(O)C(O)C(O)CO MBLBDJOUHNCFQT-UHFFFAOYSA-N 0.000 description 47
- 230000000694 effects Effects 0.000 description 40
- 229910052739 hydrogen Inorganic materials 0.000 description 39
- 239000001257 hydrogen Substances 0.000 description 39
- 239000000203 mixture Substances 0.000 description 34
- 102000005427 Asialoglycoprotein Receptor Human genes 0.000 description 31
- 108010006523 asialoglycoprotein receptor Proteins 0.000 description 31
- 101710163270 Nuclease Proteins 0.000 description 29
- 230000001976 improved effect Effects 0.000 description 28
- 241000282414 Homo sapiens Species 0.000 description 26
- 241001465754 Metazoa Species 0.000 description 25
- LOKCTEFSRHRXRJ-UHFFFAOYSA-I dipotassium trisodium dihydrogen phosphate hydrogen phosphate dichloride Chemical compound P(=O)(O)(O)[O-].[K+].P(=O)(O)([O-])[O-].[Na+].[Na+].[Cl-].[K+].[Cl-].[Na+] LOKCTEFSRHRXRJ-UHFFFAOYSA-I 0.000 description 25
- 239000000463 material Substances 0.000 description 25
- 239000002953 phosphate buffered saline Substances 0.000 description 25
- 210000003494 hepatocyte Anatomy 0.000 description 24
- 108090000623 proteins and genes Proteins 0.000 description 24
- 230000009467 reduction Effects 0.000 description 24
- 210000004185 liver Anatomy 0.000 description 23
- 239000002609 medium Substances 0.000 description 23
- 239000000126 substance Substances 0.000 description 23
- 230000004048 modification Effects 0.000 description 22
- 238000012986 modification Methods 0.000 description 22
- 238000004519 manufacturing process Methods 0.000 description 20
- UFHFLCQGNIYNRP-UHFFFAOYSA-N Hydrogen Chemical compound [H][H] UFHFLCQGNIYNRP-UHFFFAOYSA-N 0.000 description 19
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 description 19
- IAZDPXIOMUYVGZ-UHFFFAOYSA-N Dimethylsulphoxide Chemical compound CS(C)=O IAZDPXIOMUYVGZ-UHFFFAOYSA-N 0.000 description 18
- 238000003197 gene knockdown Methods 0.000 description 18
- 239000000427 antigen Substances 0.000 description 17
- 201000010099 disease Diseases 0.000 description 17
- 125000002496 methyl group Chemical group [H]C([H])([H])* 0.000 description 17
- 108091007433 antigens Proteins 0.000 description 16
- 102000036639 antigens Human genes 0.000 description 16
- 230000027455 binding Effects 0.000 description 16
- 230000028327 secretion Effects 0.000 description 16
- 241000699666 Mus <mouse, genus> Species 0.000 description 15
- 108091028043 Nucleic acid sequence Proteins 0.000 description 15
- 239000003795 chemical substances by application Substances 0.000 description 15
- 239000011734 sodium Substances 0.000 description 15
- LRSASMSXMSNRBT-UHFFFAOYSA-N 5-methylcytosine Chemical compound CC1=CNC(=O)N=C1N LRSASMSXMSNRBT-UHFFFAOYSA-N 0.000 description 14
- 238000003556 assay Methods 0.000 description 14
- 150000002431 hydrogen Chemical class 0.000 description 14
- 150000001768 cations Chemical class 0.000 description 13
- WEVYAHXRMPXWCK-UHFFFAOYSA-N Acetonitrile Chemical compound CC#N WEVYAHXRMPXWCK-UHFFFAOYSA-N 0.000 description 12
- 241000699670 Mus sp. Species 0.000 description 12
- 230000004069 differentiation Effects 0.000 description 12
- 230000035755 proliferation Effects 0.000 description 12
- 238000011529 RT qPCR Methods 0.000 description 11
- 108091027967 Small hairpin RNA Proteins 0.000 description 11
- 241000700605 Viruses Species 0.000 description 11
- 239000001963 growth medium Substances 0.000 description 11
- 238000003753 real-time PCR Methods 0.000 description 11
- 230000002829 reductive effect Effects 0.000 description 11
- 238000011160 research Methods 0.000 description 11
- 238000012216 screening Methods 0.000 description 11
- 125000006850 spacer group Chemical group 0.000 description 11
- 210000001519 tissue Anatomy 0.000 description 11
- 238000012228 RNA interference-mediated gene silencing Methods 0.000 description 10
- OPTASPLRGRRNAP-UHFFFAOYSA-N cytosine Chemical group NC=1C=CNC(=O)N=1 OPTASPLRGRRNAP-UHFFFAOYSA-N 0.000 description 10
- 238000011161 development Methods 0.000 description 10
- 230000018109 developmental process Effects 0.000 description 10
- 230000009368 gene silencing by RNA Effects 0.000 description 10
- 239000004055 small Interfering RNA Substances 0.000 description 10
- 239000006228 supernatant Substances 0.000 description 10
- 102100026031 Beta-glucuronidase Human genes 0.000 description 9
- 241000711549 Hepacivirus C Species 0.000 description 9
- 101000933465 Homo sapiens Beta-glucuronidase Proteins 0.000 description 9
- 108010050904 Interferons Proteins 0.000 description 9
- 102000014150 Interferons Human genes 0.000 description 9
- OVRNDRQMDRJTHS-CBQIKETKSA-N N-Acetyl-D-Galactosamine Chemical group CC(=O)N[C@H]1[C@@H](O)O[C@H](CO)[C@H](O)[C@@H]1O OVRNDRQMDRJTHS-CBQIKETKSA-N 0.000 description 9
- FAPWRFPIFSIZLT-UHFFFAOYSA-M Sodium chloride Chemical compound [Na+].[Cl-] FAPWRFPIFSIZLT-UHFFFAOYSA-M 0.000 description 9
- 125000000217 alkyl group Chemical group 0.000 description 9
- 230000007423 decrease Effects 0.000 description 9
- 102000044901 human TENT4B Human genes 0.000 description 9
- 238000009396 hybridization Methods 0.000 description 9
- 230000003834 intracellular effect Effects 0.000 description 9
- 239000002245 particle Substances 0.000 description 9
- 238000003786 synthesis reaction Methods 0.000 description 9
- PYMYPHUHKUWMLA-LMVFSUKVSA-N Ribose Natural products OC[C@@H](O)[C@@H](O)[C@@H](O)C=O PYMYPHUHKUWMLA-LMVFSUKVSA-N 0.000 description 8
- HMFHBZSHGGEWLO-UHFFFAOYSA-N alpha-D-Furanose-Ribose Natural products OCC1OC(O)C(O)C1O HMFHBZSHGGEWLO-UHFFFAOYSA-N 0.000 description 8
- 125000004103 aminoalkyl group Chemical group 0.000 description 8
- 102000051440 human TENT4A Human genes 0.000 description 8
- 229940079322 interferon Drugs 0.000 description 8
- 238000010172 mouse model Methods 0.000 description 8
- 239000011780 sodium chloride Substances 0.000 description 8
- 230000003612 virological effect Effects 0.000 description 8
- HMFHBZSHGGEWLO-SOOFDHNKSA-N D-ribofuranose Chemical compound OC[C@H]1OC(O)[C@H](O)[C@@H]1O HMFHBZSHGGEWLO-SOOFDHNKSA-N 0.000 description 7
- 241000124008 Mammalia Species 0.000 description 7
- 230000015572 biosynthetic process Effects 0.000 description 7
- 230000015556 catabolic process Effects 0.000 description 7
- 238000006731 degradation reaction Methods 0.000 description 7
- 238000002474 experimental method Methods 0.000 description 7
- 239000003112 inhibitor Substances 0.000 description 7
- 238000005259 measurement Methods 0.000 description 7
- 235000018102 proteins Nutrition 0.000 description 7
- 102000004169 proteins and genes Human genes 0.000 description 7
- 210000002966 serum Anatomy 0.000 description 7
- 208000024891 symptom Diseases 0.000 description 7
- 230000001225 therapeutic effect Effects 0.000 description 7
- 241000282567 Macaca fascicularis Species 0.000 description 6
- 102000000574 RNA-Induced Silencing Complex Human genes 0.000 description 6
- 108010016790 RNA-Induced Silencing Complex Proteins 0.000 description 6
- WYURNTSHIVDZCO-UHFFFAOYSA-N Tetrahydrofuran Chemical compound C1CCOC1 WYURNTSHIVDZCO-UHFFFAOYSA-N 0.000 description 6
- ISAKRJDGNUQOIC-UHFFFAOYSA-N Uracil Chemical compound O=C1C=CNC(=O)N1 ISAKRJDGNUQOIC-UHFFFAOYSA-N 0.000 description 6
- 230000033228 biological regulation Effects 0.000 description 6
- 238000003776 cleavage reaction Methods 0.000 description 6
- 230000021615 conjugation Effects 0.000 description 6
- 230000008878 coupling Effects 0.000 description 6
- 238000010168 coupling process Methods 0.000 description 6
- 238000005859 coupling reaction Methods 0.000 description 6
- 229940104302 cytosine Drugs 0.000 description 6
- 230000003828 downregulation Effects 0.000 description 6
- UYTPUPDQBNUYGX-UHFFFAOYSA-N guanine Chemical compound O=C1NC(N)=NC2=C1N=CN2 UYTPUPDQBNUYGX-UHFFFAOYSA-N 0.000 description 6
- 239000012139 lysis buffer Substances 0.000 description 6
- 230000001483 mobilizing effect Effects 0.000 description 6
- 239000000178 monomer Substances 0.000 description 6
- 238000002515 oligonucleotide synthesis Methods 0.000 description 6
- 125000002924 primary amino group Chemical group [H]N([H])* 0.000 description 6
- 238000000746 purification Methods 0.000 description 6
- 238000004007 reversed phase HPLC Methods 0.000 description 6
- 125000000548 ribosyl group Chemical group C1([C@H](O)[C@H](O)[C@H](O1)CO)* 0.000 description 6
- 230000007017 scission Effects 0.000 description 6
- 229910052717 sulfur Inorganic materials 0.000 description 6
- 238000012360 testing method Methods 0.000 description 6
- KDCGOANMDULRCW-UHFFFAOYSA-N 7H-purine Chemical compound N1=CNC2=NC=NC2=C1 KDCGOANMDULRCW-UHFFFAOYSA-N 0.000 description 5
- YMWUJEATGCHHMB-UHFFFAOYSA-N Dichloromethane Chemical compound ClCCl YMWUJEATGCHHMB-UHFFFAOYSA-N 0.000 description 5
- 239000004472 Lysine Substances 0.000 description 5
- 101100462829 Mus musculus Tent4b gene Proteins 0.000 description 5
- ZMXDDKWLCZADIW-UHFFFAOYSA-N N,N-Dimethylformamide Chemical compound CN(C)C=O ZMXDDKWLCZADIW-UHFFFAOYSA-N 0.000 description 5
- 229910052799 carbon Inorganic materials 0.000 description 5
- 238000006243 chemical reaction Methods 0.000 description 5
- 239000003153 chemical reaction reagent Substances 0.000 description 5
- 239000002299 complementary DNA Substances 0.000 description 5
- 238000003745 diagnosis Methods 0.000 description 5
- 238000009826 distribution Methods 0.000 description 5
- 230000002708 enhancing effect Effects 0.000 description 5
- 125000000524 functional group Chemical group 0.000 description 5
- 125000004435 hydrogen atom Chemical group [H]* 0.000 description 5
- 239000012535 impurity Substances 0.000 description 5
- 230000001965 increasing effect Effects 0.000 description 5
- 230000001404 mediated effect Effects 0.000 description 5
- 238000002844 melting Methods 0.000 description 5
- 230000008018 melting Effects 0.000 description 5
- 229920001223 polyethylene glycol Polymers 0.000 description 5
- 238000003762 quantitative reverse transcription PCR Methods 0.000 description 5
- 230000004044 response Effects 0.000 description 5
- 125000001424 substituent group Chemical group 0.000 description 5
- RHCSKNNOAZULRK-APZFVMQVSA-N 2,2-dideuterio-2-(3,4,5-trimethoxyphenyl)ethanamine Chemical compound NCC([2H])([2H])C1=CC(OC)=C(OC)C(OC)=C1 RHCSKNNOAZULRK-APZFVMQVSA-N 0.000 description 4
- GFFGJBXGBJISGV-UHFFFAOYSA-N Adenine Chemical compound NC1=NC=NC2=C1N=CN2 GFFGJBXGBJISGV-UHFFFAOYSA-N 0.000 description 4
- 229930024421 Adenine Natural products 0.000 description 4
- 108091003079 Bovine Serum Albumin Proteins 0.000 description 4
- 102000012422 Collagen Type I Human genes 0.000 description 4
- 108010022452 Collagen Type I Proteins 0.000 description 4
- 102000004190 Enzymes Human genes 0.000 description 4
- 108090000790 Enzymes Proteins 0.000 description 4
- 108700039887 Essential Genes Proteins 0.000 description 4
- 241000282412 Homo Species 0.000 description 4
- JUJWROOIHBZHMG-UHFFFAOYSA-N Pyridine Chemical compound C1=CC=NC=C1 JUJWROOIHBZHMG-UHFFFAOYSA-N 0.000 description 4
- 108020004459 Small interfering RNA Proteins 0.000 description 4
- 210000001744 T-lymphocyte Anatomy 0.000 description 4
- 108010067390 Viral Proteins Proteins 0.000 description 4
- 230000001154 acute effect Effects 0.000 description 4
- 230000000840 anti-viral effect Effects 0.000 description 4
- 125000003118 aryl group Chemical group 0.000 description 4
- UCMIRNVEIXFBKS-UHFFFAOYSA-N beta-alanine Chemical compound NCCC(O)=O UCMIRNVEIXFBKS-UHFFFAOYSA-N 0.000 description 4
- 238000004364 calculation method Methods 0.000 description 4
- 150000001720 carbohydrates Chemical class 0.000 description 4
- 230000002759 chromosomal effect Effects 0.000 description 4
- 208000016350 chronic hepatitis B virus infection Diseases 0.000 description 4
- 238000002648 combination therapy Methods 0.000 description 4
- 239000002552 dosage form Substances 0.000 description 4
- 229960000980 entecavir Drugs 0.000 description 4
- YXPVEXCTPGULBZ-WQYNNSOESA-N entecavir hydrate Chemical compound O.C1=NC=2C(=O)NC(N)=NC=2N1[C@H]1C[C@H](O)[C@@H](CO)C1=C YXPVEXCTPGULBZ-WQYNNSOESA-N 0.000 description 4
- 229940088598 enzyme Drugs 0.000 description 4
- 239000012091 fetal bovine serum Substances 0.000 description 4
- 229910052757 nitrogen Inorganic materials 0.000 description 4
- 210000004940 nucleus Anatomy 0.000 description 4
- 210000000056 organ Anatomy 0.000 description 4
- 229910052760 oxygen Inorganic materials 0.000 description 4
- 150000008300 phosphoramidites Chemical class 0.000 description 4
- 230000003389 potentiating effect Effects 0.000 description 4
- 102000004196 processed proteins & peptides Human genes 0.000 description 4
- 108090000765 processed proteins & peptides Proteins 0.000 description 4
- RXWNCPJZOCPEPQ-NVWDDTSBSA-N puromycin Chemical compound C1=CC(OC)=CC=C1C[C@H](N)C(=O)N[C@H]1[C@@H](O)[C@H](N2C3=NC=NC(=C3N=C2)N(C)C)O[C@@H]1CO RXWNCPJZOCPEPQ-NVWDDTSBSA-N 0.000 description 4
- 238000011084 recovery Methods 0.000 description 4
- 230000007115 recruitment Effects 0.000 description 4
- 230000010076 replication Effects 0.000 description 4
- 239000000523 sample Substances 0.000 description 4
- 239000000243 solution Substances 0.000 description 4
- 239000013589 supplement Substances 0.000 description 4
- IQFYYKKMVGJFEH-CSMHCCOUSA-N telbivudine Chemical compound O=C1NC(=O)C(C)=CN1[C@H]1O[C@@H](CO)[C@H](O)C1 IQFYYKKMVGJFEH-CSMHCCOUSA-N 0.000 description 4
- VCMJCVGFSROFHV-WZGZYPNHSA-N tenofovir disoproxil fumarate Chemical compound OC(=O)\C=C\C(O)=O.N1=CN=C2N(C[C@@H](C)OCP(=O)(OCOC(=O)OC(C)C)OCOC(=O)OC(C)C)C=NC2=C1N VCMJCVGFSROFHV-WZGZYPNHSA-N 0.000 description 4
- 229940124597 therapeutic agent Drugs 0.000 description 4
- 229940035893 uracil Drugs 0.000 description 4
- 102000040650 (ribonucleotides)n+m Human genes 0.000 description 3
- 101710132601 Capsid protein Proteins 0.000 description 3
- OKTJSMMVPCPJKN-UHFFFAOYSA-N Carbon Chemical compound [C] OKTJSMMVPCPJKN-UHFFFAOYSA-N 0.000 description 3
- 108020004635 Complementary DNA Proteins 0.000 description 3
- 102000004533 Endonucleases Human genes 0.000 description 3
- 108010042407 Endonucleases Proteins 0.000 description 3
- LYCAIKOWRPUZTN-UHFFFAOYSA-N Ethylene glycol Chemical compound OCCO LYCAIKOWRPUZTN-UHFFFAOYSA-N 0.000 description 3
- VZCYOOQTPOCHFL-OWOJBTEDSA-N Fumaric acid Chemical compound OC(=O)\C=C\C(O)=O VZCYOOQTPOCHFL-OWOJBTEDSA-N 0.000 description 3
- 238000012404 In vitro experiment Methods 0.000 description 3
- 102100040018 Interferon alpha-2 Human genes 0.000 description 3
- 108010079944 Interferon-alpha2b Proteins 0.000 description 3
- 108700026244 Open Reading Frames Proteins 0.000 description 3
- MUBZPKHOEPUJKR-UHFFFAOYSA-N Oxalic acid Chemical compound OC(=O)C(O)=O MUBZPKHOEPUJKR-UHFFFAOYSA-N 0.000 description 3
- 229910019142 PO4 Inorganic materials 0.000 description 3
- CZPWVGJYEJSRLH-UHFFFAOYSA-N Pyrimidine Chemical compound C1=CN=CN=C1 CZPWVGJYEJSRLH-UHFFFAOYSA-N 0.000 description 3
- NINIDFKCEFEMDL-UHFFFAOYSA-N Sulfur Chemical compound [S] NINIDFKCEFEMDL-UHFFFAOYSA-N 0.000 description 3
- RYYWUUFWQRZTIU-UHFFFAOYSA-N Thiophosphoric acid Chemical class OP(O)(S)=O RYYWUUFWQRZTIU-UHFFFAOYSA-N 0.000 description 3
- 102100024333 Toll-like receptor 2 Human genes 0.000 description 3
- 108020000999 Viral RNA Proteins 0.000 description 3
- 239000002253 acid Substances 0.000 description 3
- 230000004913 activation Effects 0.000 description 3
- 229960000643 adenine Drugs 0.000 description 3
- 238000000137 annealing Methods 0.000 description 3
- 238000009175 antibody therapy Methods 0.000 description 3
- 239000003443 antiviral agent Substances 0.000 description 3
- 239000012298 atmosphere Substances 0.000 description 3
- 230000009286 beneficial effect Effects 0.000 description 3
- 230000008901 benefit Effects 0.000 description 3
- MSWZFWKMSRAUBD-UHFFFAOYSA-N beta-D-galactosamine Natural products NC1C(O)OC(CO)C(O)C1O MSWZFWKMSRAUBD-UHFFFAOYSA-N 0.000 description 3
- IQFYYKKMVGJFEH-UHFFFAOYSA-N beta-L-thymidine Natural products O=C1NC(=O)C(C)=CN1C1OC(CO)C(O)C1 IQFYYKKMVGJFEH-UHFFFAOYSA-N 0.000 description 3
- 239000000872 buffer Substances 0.000 description 3
- 235000014633 carbohydrates Nutrition 0.000 description 3
- 125000003178 carboxy group Chemical group [H]OC(*)=O 0.000 description 3
- 239000000969 carrier Substances 0.000 description 3
- 238000004113 cell culture Methods 0.000 description 3
- 230000001413 cellular effect Effects 0.000 description 3
- KRKNYBCHXYNGOX-UHFFFAOYSA-N citric acid Chemical compound OC(=O)CC(O)(C(O)=O)CC(O)=O KRKNYBCHXYNGOX-UHFFFAOYSA-N 0.000 description 3
- 230000000052 comparative effect Effects 0.000 description 3
- 239000013068 control sample Substances 0.000 description 3
- 210000000805 cytoplasm Anatomy 0.000 description 3
- 238000012217 deletion Methods 0.000 description 3
- 230000037430 deletion Effects 0.000 description 3
- 238000004925 denaturation Methods 0.000 description 3
- 230000036425 denaturation Effects 0.000 description 3
- 230000001419 dependent effect Effects 0.000 description 3
- 230000002222 downregulating effect Effects 0.000 description 3
- 238000011156 evaluation Methods 0.000 description 3
- 238000009472 formulation Methods 0.000 description 3
- 229930182830 galactose Natural products 0.000 description 3
- 150000002256 galaktoses Chemical class 0.000 description 3
- 208000006454 hepatitis Diseases 0.000 description 3
- 125000001072 heteroaryl group Chemical group 0.000 description 3
- 230000006872 improvement Effects 0.000 description 3
- 239000007924 injection Substances 0.000 description 3
- 238000002347 injection Methods 0.000 description 3
- 230000003993 interaction Effects 0.000 description 3
- 238000001990 intravenous administration Methods 0.000 description 3
- 210000003734 kidney Anatomy 0.000 description 3
- JTEGQNOMFQHVDC-NKWVEPMBSA-N lamivudine Chemical compound O=C1N=C(N)C=CN1[C@H]1O[C@@H](CO)SC1 JTEGQNOMFQHVDC-NKWVEPMBSA-N 0.000 description 3
- 239000003446 ligand Substances 0.000 description 3
- 201000007270 liver cancer Diseases 0.000 description 3
- 210000004962 mammalian cell Anatomy 0.000 description 3
- 125000000325 methylidene group Chemical group [H]C([H])=* 0.000 description 3
- 238000002156 mixing Methods 0.000 description 3
- 230000010412 perfusion Effects 0.000 description 3
- 239000010452 phosphate Substances 0.000 description 3
- 150000003254 radicals Chemical class 0.000 description 3
- 238000013207 serial dilution Methods 0.000 description 3
- 150000003384 small molecules Chemical class 0.000 description 3
- 239000007787 solid Substances 0.000 description 3
- 230000000707 stereoselective effect Effects 0.000 description 3
- 238000007920 subcutaneous administration Methods 0.000 description 3
- 125000000446 sulfanediyl group Chemical group *S* 0.000 description 3
- 239000011593 sulfur Substances 0.000 description 3
- 229940021747 therapeutic vaccine Drugs 0.000 description 3
- 239000003053 toxin Substances 0.000 description 3
- 231100000765 toxin Toxicity 0.000 description 3
- 108700012359 toxins Proteins 0.000 description 3
- 238000013518 transcription Methods 0.000 description 3
- 230000035897 transcription Effects 0.000 description 3
- 230000014616 translation Effects 0.000 description 3
- 229960005486 vaccine Drugs 0.000 description 3
- PMJWDPGOWBRILU-UHFFFAOYSA-N (2,5-dioxopyrrolidin-1-yl) 4-[4-(2,5-dioxopyrrol-1-yl)phenyl]butanoate Chemical compound O=C1CCC(=O)N1OC(=O)CCCC(C=C1)=CC=C1N1C(=O)C=CC1=O PMJWDPGOWBRILU-UHFFFAOYSA-N 0.000 description 2
- WCMOHMXWOOBVMZ-UHFFFAOYSA-N (2,5-dioxopyrrolidin-1-yl) 6-[3-(2,5-dioxopyrrol-1-yl)propanoylamino]hexanoate Chemical compound O=C1CCC(=O)N1OC(=O)CCCCCNC(=O)CCN1C(=O)C=CC1=O WCMOHMXWOOBVMZ-UHFFFAOYSA-N 0.000 description 2
- 125000004454 (C1-C6) alkoxycarbonyl group Chemical group 0.000 description 2
- 125000004916 (C1-C6) alkylcarbonyl group Chemical group 0.000 description 2
- 125000006619 (C1-C6) dialkylamino group Chemical group 0.000 description 2
- XGDRLCRGKUCBQL-UHFFFAOYSA-N 1h-imidazole-4,5-dicarbonitrile Chemical compound N#CC=1N=CNC=1C#N XGDRLCRGKUCBQL-UHFFFAOYSA-N 0.000 description 2
- RUVRGYVESPRHSZ-UHFFFAOYSA-N 2-[2-(2-azaniumylethoxy)ethoxy]acetate Chemical compound NCCOCCOCC(O)=O RUVRGYVESPRHSZ-UHFFFAOYSA-N 0.000 description 2
- MSWZFWKMSRAUBD-GASJEMHNSA-N 2-amino-2-deoxy-D-galactopyranose Chemical compound N[C@H]1C(O)O[C@H](CO)[C@H](O)[C@@H]1O MSWZFWKMSRAUBD-GASJEMHNSA-N 0.000 description 2
- QUBNFZFTFXTLKH-UHFFFAOYSA-N 2-aminododecanoic acid Chemical compound CCCCCCCCCCC(N)C(O)=O QUBNFZFTFXTLKH-UHFFFAOYSA-N 0.000 description 2
- DRNGLYHKYPNTEA-UHFFFAOYSA-N 4-azaniumylcyclohexane-1-carboxylate Chemical compound NC1CCC(C(O)=O)CC1 DRNGLYHKYPNTEA-UHFFFAOYSA-N 0.000 description 2
- LRFVTYWOQMYALW-UHFFFAOYSA-N 9H-xanthine Chemical compound O=C1NC(=O)NC2=C1NC=N2 LRFVTYWOQMYALW-UHFFFAOYSA-N 0.000 description 2
- HJCMDXDYPOUFDY-WHFBIAKZSA-N Ala-Gln Chemical compound C[C@H](N)C(=O)N[C@H](C(O)=O)CCC(N)=O HJCMDXDYPOUFDY-WHFBIAKZSA-N 0.000 description 2
- 108091029792 Alkylated DNA Proteins 0.000 description 2
- 108700028369 Alleles Proteins 0.000 description 2
- 108091023037 Aptamer Proteins 0.000 description 2
- 101150075175 Asgr1 gene Proteins 0.000 description 2
- 125000005947 C1-C6 alkylsulfonyloxy group Chemical group 0.000 description 2
- 125000000882 C2-C6 alkenyl group Chemical group 0.000 description 2
- 125000003601 C2-C6 alkynyl group Chemical group 0.000 description 2
- 102000000844 Cell Surface Receptors Human genes 0.000 description 2
- 108010001857 Cell Surface Receptors Proteins 0.000 description 2
- 208000017667 Chronic Disease Diseases 0.000 description 2
- 206010008909 Chronic Hepatitis Diseases 0.000 description 2
- 102000008186 Collagen Human genes 0.000 description 2
- 108010035532 Collagen Proteins 0.000 description 2
- 102000029816 Collagenase Human genes 0.000 description 2
- 108060005980 Collagenase Proteins 0.000 description 2
- 108020004394 Complementary RNA Proteins 0.000 description 2
- 102000053602 DNA Human genes 0.000 description 2
- 238000012286 ELISA Assay Methods 0.000 description 2
- 238000008157 ELISA kit Methods 0.000 description 2
- 239000006145 Eagle's minimal essential medium Substances 0.000 description 2
- 108060002716 Exonuclease Proteins 0.000 description 2
- 241000285366 HBV genotype D Species 0.000 description 2
- 239000012981 Hank's balanced salt solution Substances 0.000 description 2
- 101100215371 Homo sapiens ACTB gene Proteins 0.000 description 2
- 101000785944 Homo sapiens Asialoglycoprotein receptor 1 Proteins 0.000 description 2
- 101000693243 Homo sapiens Paternally-expressed gene 3 protein Proteins 0.000 description 2
- VEXZGXHMUGYJMC-UHFFFAOYSA-N Hydrochloric acid Chemical compound Cl VEXZGXHMUGYJMC-UHFFFAOYSA-N 0.000 description 2
- ZGUNAGUHMKGQNY-ZETCQYMHSA-N L-alpha-phenylglycine zwitterion Chemical compound OC(=O)[C@@H](N)C1=CC=CC=C1 ZGUNAGUHMKGQNY-ZETCQYMHSA-N 0.000 description 2
- 240000003183 Manihot esculenta Species 0.000 description 2
- 235000016735 Manihot esculenta subsp esculenta Nutrition 0.000 description 2
- AFVFQIVMOAPDHO-UHFFFAOYSA-N Methanesulfonic acid Chemical compound CS(O)(=O)=O AFVFQIVMOAPDHO-UHFFFAOYSA-N 0.000 description 2
- 241000699660 Mus musculus Species 0.000 description 2
- 101100462831 Mus musculus Tent4a gene Proteins 0.000 description 2
- FVMMQJUBNMOPPR-WLDMJGECSA-N N-[(3R,4R,5R,6R)-2,4,5-trihydroxy-6-(hydroxymethyl)oxan-3-yl]formamide Chemical compound OC[C@H]1OC(O)[C@H](NC=O)[C@@H](O)[C@H]1O FVMMQJUBNMOPPR-WLDMJGECSA-N 0.000 description 2
- RTEOJYOKWPEKKN-HXQZNRNWSA-N N-[(3R,4R,5R,6R)-2,4,5-trihydroxy-6-(hydroxymethyl)oxan-3-yl]propanamide Chemical compound CCC(=O)N[C@H]1C(O)O[C@H](CO)[C@H](O)[C@@H]1O RTEOJYOKWPEKKN-HXQZNRNWSA-N 0.000 description 2
- 102100025757 Paternally-expressed gene 3 protein Human genes 0.000 description 2
- 108091093037 Peptide nucleic acid Proteins 0.000 description 2
- 241000233805 Phoenix Species 0.000 description 2
- NBIIXXVUZAFLBC-UHFFFAOYSA-N Phosphoric acid Chemical compound OP(O)(O)=O NBIIXXVUZAFLBC-UHFFFAOYSA-N 0.000 description 2
- 239000004698 Polyethylene Substances 0.000 description 2
- 229920002594 Polyethylene Glycol 8000 Polymers 0.000 description 2
- 238000002123 RNA extraction Methods 0.000 description 2
- 238000003559 RNA-seq method Methods 0.000 description 2
- IWUCXVSUMQZMFG-AFCXAGJDSA-N Ribavirin Chemical compound N1=C(C(=O)N)N=CN1[C@H]1[C@H](O)[C@H](O)[C@@H](CO)O1 IWUCXVSUMQZMFG-AFCXAGJDSA-N 0.000 description 2
- QAOWNCQODCNURD-UHFFFAOYSA-N Sulfuric acid Chemical compound OS(O)(=O)=O QAOWNCQODCNURD-UHFFFAOYSA-N 0.000 description 2
- IQFYYKKMVGJFEH-XLPZGREQSA-N Thymidine Chemical compound O=C1NC(=O)C(C)=CN1[C@@H]1O[C@H](CO)[C@@H](O)C1 IQFYYKKMVGJFEH-XLPZGREQSA-N 0.000 description 2
- 108010060888 Toll-like receptor 2 Proteins 0.000 description 2
- DRTQHJPVMGBUCF-XVFCMESISA-N Uridine Chemical compound O[C@@H]1[C@H](O)[C@@H](CO)O[C@H]1N1C(=O)NC(=O)C=C1 DRTQHJPVMGBUCF-XVFCMESISA-N 0.000 description 2
- 108020005202 Viral DNA Proteins 0.000 description 2
- 238000010521 absorption reaction Methods 0.000 description 2
- 230000009471 action Effects 0.000 description 2
- 229960001997 adefovir Drugs 0.000 description 2
- WOZSCQDILHKSGG-UHFFFAOYSA-N adefovir depivoxil Chemical compound N1=CN=C2N(CCOCP(=O)(OCOC(=O)C(C)(C)C)OCOC(=O)C(C)(C)C)C=NC2=C1N WOZSCQDILHKSGG-UHFFFAOYSA-N 0.000 description 2
- WQZGKKKJIJFFOK-PHYPRBDBSA-N alpha-D-galactose Chemical group OC[C@H]1O[C@H](O)[C@H](O)[C@@H](O)[C@H]1O WQZGKKKJIJFFOK-PHYPRBDBSA-N 0.000 description 2
- 150000001413 amino acids Chemical class 0.000 description 2
- 235000011114 ammonium hydroxide Nutrition 0.000 description 2
- 125000005129 aryl carbonyl group Chemical group 0.000 description 2
- 125000005161 aryl oxy carbonyl group Chemical group 0.000 description 2
- 125000004104 aryloxy group Chemical group 0.000 description 2
- 125000004429 atom Chemical group 0.000 description 2
- 229940000635 beta-alanine Drugs 0.000 description 2
- 230000003115 biocidal effect Effects 0.000 description 2
- 230000004071 biological effect Effects 0.000 description 2
- 230000000903 blocking effect Effects 0.000 description 2
- 210000004369 blood Anatomy 0.000 description 2
- 239000008280 blood Substances 0.000 description 2
- 230000004700 cellular uptake Effects 0.000 description 2
- 125000003636 chemical group Chemical group 0.000 description 2
- HVYWMOMLDIMFJA-DPAQBDIFSA-N cholesterol Chemical compound C1C=C2C[C@@H](O)CC[C@]2(C)[C@@H]2[C@@H]1[C@@H]1CC[C@H]([C@H](C)CCCC(C)C)[C@@]1(C)CC2 HVYWMOMLDIMFJA-DPAQBDIFSA-N 0.000 description 2
- 208000019425 cirrhosis of liver Diseases 0.000 description 2
- 230000001149 cognitive effect Effects 0.000 description 2
- 229920001436 collagen Polymers 0.000 description 2
- 229960002424 collagenase Drugs 0.000 description 2
- 239000003184 complementary RNA Substances 0.000 description 2
- 230000034994 death Effects 0.000 description 2
- 231100000517 death Toxicity 0.000 description 2
- 230000000593 degrading effect Effects 0.000 description 2
- 210000004443 dendritic cell Anatomy 0.000 description 2
- 208000035475 disorder Diseases 0.000 description 2
- 238000012377 drug delivery Methods 0.000 description 2
- 238000009509 drug development Methods 0.000 description 2
- 229940088679 drug related substance Drugs 0.000 description 2
- 238000001962 electrophoresis Methods 0.000 description 2
- 238000005516 engineering process Methods 0.000 description 2
- 102000013165 exonuclease Human genes 0.000 description 2
- 125000002485 formyl group Chemical group [H]C(*)=O 0.000 description 2
- ZDXPYRJPNDTMRX-UHFFFAOYSA-N glutamine Natural products OC(=O)C(N)CCC(N)=O ZDXPYRJPNDTMRX-UHFFFAOYSA-N 0.000 description 2
- 229910052736 halogen Inorganic materials 0.000 description 2
- 150000002367 halogens Chemical class 0.000 description 2
- 125000005223 heteroarylcarbonyl group Chemical group 0.000 description 2
- 125000005553 heteroaryloxy group Chemical group 0.000 description 2
- 125000005226 heteroaryloxycarbonyl group Chemical group 0.000 description 2
- 238000004128 high performance liquid chromatography Methods 0.000 description 2
- 229940088597 hormone Drugs 0.000 description 2
- 239000005556 hormone Substances 0.000 description 2
- 230000005745 host immune response Effects 0.000 description 2
- 125000002887 hydroxy group Chemical group [H]O* 0.000 description 2
- FDGQSTZJBFJUBT-UHFFFAOYSA-N hypoxanthine Chemical compound O=C1NC=NC2=C1NC=N2 FDGQSTZJBFJUBT-UHFFFAOYSA-N 0.000 description 2
- 210000002865 immune cell Anatomy 0.000 description 2
- 238000012606 in vitro cell culture Methods 0.000 description 2
- 238000011534 incubation Methods 0.000 description 2
- 230000000977 initiatory effect Effects 0.000 description 2
- 238000003780 insertion Methods 0.000 description 2
- 230000037431 insertion Effects 0.000 description 2
- JVTAAEKCZFNVCJ-UHFFFAOYSA-N lactic acid Chemical compound CC(O)C(O)=O JVTAAEKCZFNVCJ-UHFFFAOYSA-N 0.000 description 2
- 229960001627 lamivudine Drugs 0.000 description 2
- 210000005229 liver cell Anatomy 0.000 description 2
- 208000014018 liver neoplasm Diseases 0.000 description 2
- 238000004020 luminiscence type Methods 0.000 description 2
- 239000006166 lysate Substances 0.000 description 2
- 239000003550 marker Substances 0.000 description 2
- 150000007522 mineralic acids Chemical class 0.000 description 2
- 238000010899 nucleation Methods 0.000 description 2
- 238000007899 nucleic acid hybridization Methods 0.000 description 2
- 230000009437 off-target effect Effects 0.000 description 2
- PBLZLIFKVPJDCO-UHFFFAOYSA-N omega-Aminododecanoic acid Natural products NCCCCCCCCCCCC(O)=O PBLZLIFKVPJDCO-UHFFFAOYSA-N 0.000 description 2
- 150000007524 organic acids Chemical class 0.000 description 2
- 239000008188 pellet Substances 0.000 description 2
- WEXRUCMBJFQVBZ-UHFFFAOYSA-N pentobarbital Chemical compound CCCC(C)C1(CC)C(=O)NC(=O)NC1=O WEXRUCMBJFQVBZ-UHFFFAOYSA-N 0.000 description 2
- 230000003285 pharmacodynamic effect Effects 0.000 description 2
- 230000000144 pharmacologic effect Effects 0.000 description 2
- NBIIXXVUZAFLBC-UHFFFAOYSA-K phosphate Chemical compound [O-]P([O-])([O-])=O NBIIXXVUZAFLBC-UHFFFAOYSA-K 0.000 description 2
- 125000002467 phosphate group Chemical group [H]OP(=O)(O[H])O[*] 0.000 description 2
- 230000001766 physiological effect Effects 0.000 description 2
- 229920000642 polymer Polymers 0.000 description 2
- 230000003449 preventive effect Effects 0.000 description 2
- 150000003212 purines Chemical class 0.000 description 2
- 229950010131 puromycin Drugs 0.000 description 2
- UMJSCPRVCHMLSP-UHFFFAOYSA-N pyridine Natural products COC1=CC=CN=C1 UMJSCPRVCHMLSP-UHFFFAOYSA-N 0.000 description 2
- 238000011002 quantification Methods 0.000 description 2
- 102000005962 receptors Human genes 0.000 description 2
- 108020003175 receptors Proteins 0.000 description 2
- 238000010839 reverse transcription Methods 0.000 description 2
- 229960000329 ribavirin Drugs 0.000 description 2
- HZCAHMRRMINHDJ-DBRKOABJSA-N ribavirin Natural products O[C@@H]1[C@H](O)[C@@H](CO)O[C@H]1N1N=CN=C1 HZCAHMRRMINHDJ-DBRKOABJSA-N 0.000 description 2
- 108010052833 ribonuclease HI Proteins 0.000 description 2
- YGSDEFSMJLZEOE-UHFFFAOYSA-N salicylic acid Chemical compound OC(=O)C1=CC=CC=C1O YGSDEFSMJLZEOE-UHFFFAOYSA-N 0.000 description 2
- 238000012163 sequencing technique Methods 0.000 description 2
- 239000002356 single layer Substances 0.000 description 2
- 108010062513 snake venom phosphodiesterase I Proteins 0.000 description 2
- 239000007790 solid phase Substances 0.000 description 2
- 238000010532 solid phase synthesis reaction Methods 0.000 description 2
- 239000002904 solvent Substances 0.000 description 2
- 241000894007 species Species 0.000 description 2
- 230000002269 spontaneous effect Effects 0.000 description 2
- 238000006467 substitution reaction Methods 0.000 description 2
- 239000000758 substrate Substances 0.000 description 2
- JJAHTWIKCUJRDK-UHFFFAOYSA-N succinimidyl 4-(N-maleimidomethyl)cyclohexane-1-carboxylate Chemical compound C1CC(CN2C(C=CC2=O)=O)CCC1C(=O)ON1C(=O)CCC1=O JJAHTWIKCUJRDK-UHFFFAOYSA-N 0.000 description 2
- 150000008163 sugars Chemical class 0.000 description 2
- CYFLXLSBHQBMFT-UHFFFAOYSA-N sulfamoxole Chemical group O1C(C)=C(C)N=C1NS(=O)(=O)C1=CC=C(N)C=C1 CYFLXLSBHQBMFT-UHFFFAOYSA-N 0.000 description 2
- 229960005311 telbivudine Drugs 0.000 description 2
- 229960004556 tenofovir Drugs 0.000 description 2
- WGTYBPLFGIVFAS-UHFFFAOYSA-M tetramethylammonium hydroxide Chemical compound [OH-].C[N+](C)(C)C WGTYBPLFGIVFAS-UHFFFAOYSA-M 0.000 description 2
- RWQNBRDOKXIBIV-UHFFFAOYSA-N thymine Chemical compound CC1=CNC(=O)NC1=O RWQNBRDOKXIBIV-UHFFFAOYSA-N 0.000 description 2
- 229940113082 thymine Drugs 0.000 description 2
- JOXIMZWYDAKGHI-UHFFFAOYSA-N toluene-4-sulfonic acid Chemical compound CC1=CC=C(S(O)(=O)=O)C=C1 JOXIMZWYDAKGHI-UHFFFAOYSA-N 0.000 description 2
- VZCYOOQTPOCHFL-UHFFFAOYSA-N trans-butenedioic acid Natural products OC(=O)C=CC(O)=O VZCYOOQTPOCHFL-UHFFFAOYSA-N 0.000 description 2
- 238000013519 translation Methods 0.000 description 2
- 210000002845 virion Anatomy 0.000 description 2
- 239000011782 vitamin Substances 0.000 description 2
- 235000013343 vitamin Nutrition 0.000 description 2
- 229940088594 vitamin Drugs 0.000 description 2
- 229930003231 vitamin Natural products 0.000 description 2
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 2
- TYKASZBHFXBROF-UHFFFAOYSA-N (2,5-dioxopyrrolidin-1-yl) 2-(2,5-dioxopyrrol-1-yl)acetate Chemical compound O=C1CCC(=O)N1OC(=O)CN1C(=O)C=CC1=O TYKASZBHFXBROF-UHFFFAOYSA-N 0.000 description 1
- VLARLSIGSPVYHX-UHFFFAOYSA-N (2,5-dioxopyrrolidin-1-yl) 6-(2,5-dioxopyrrol-1-yl)hexanoate Chemical compound O=C1CCC(=O)N1OC(=O)CCCCCN1C(=O)C=CC1=O VLARLSIGSPVYHX-UHFFFAOYSA-N 0.000 description 1
- YIMATHOGWXZHFX-WCTZXXKLSA-N (2r,3r,4r,5r)-5-(hydroxymethyl)-3-(2-methoxyethoxy)oxolane-2,4-diol Chemical compound COCCO[C@H]1[C@H](O)O[C@H](CO)[C@H]1O YIMATHOGWXZHFX-WCTZXXKLSA-N 0.000 description 1
- GUAHPAJOXVYFON-ZETCQYMHSA-N (8S)-8-amino-7-oxononanoic acid zwitterion Chemical compound C[C@H](N)C(=O)CCCCCC(O)=O GUAHPAJOXVYFON-ZETCQYMHSA-N 0.000 description 1
- 125000006700 (C1-C6) alkylthio group Chemical group 0.000 description 1
- BJEPYKJPYRNKOW-REOHCLBHSA-N (S)-malic acid Chemical compound OC(=O)[C@@H](O)CC(O)=O BJEPYKJPYRNKOW-REOHCLBHSA-N 0.000 description 1
- JKMHFZQWWAIEOD-UHFFFAOYSA-N 2-[4-(2-hydroxyethyl)piperazin-1-yl]ethanesulfonic acid Chemical compound OCC[NH+]1CCN(CCS([O-])(=O)=O)CC1 JKMHFZQWWAIEOD-UHFFFAOYSA-N 0.000 description 1
- XQCZBXHVTFVIFE-UHFFFAOYSA-N 2-amino-4-hydroxypyrimidine Chemical compound NC1=NC=CC(O)=N1 XQCZBXHVTFVIFE-UHFFFAOYSA-N 0.000 description 1
- MWBWWFOAEOYUST-UHFFFAOYSA-N 2-aminopurine Chemical compound NC1=NC=C2N=CNC2=N1 MWBWWFOAEOYUST-UHFFFAOYSA-N 0.000 description 1
- HBJGQJWNMZDFKL-UHFFFAOYSA-N 2-chloro-7h-purin-6-amine Chemical compound NC1=NC(Cl)=NC2=C1NC=N2 HBJGQJWNMZDFKL-UHFFFAOYSA-N 0.000 description 1
- KMEMIMRPZGDOMG-UHFFFAOYSA-N 2-cyanoethoxyphosphonamidous acid Chemical compound NP(O)OCCC#N KMEMIMRPZGDOMG-UHFFFAOYSA-N 0.000 description 1
- KXTUJUVCAGXOBN-WQXQQRIOSA-N 2-methyl-N-[(3R,4R,5R,6R)-2,4,5-trihydroxy-6-(hydroxymethyl)oxan-3-yl]propanamide Chemical compound CC(C)C(=O)N[C@H]1C(O)O[C@H](CO)[C@H](O)[C@@H]1O KXTUJUVCAGXOBN-WQXQQRIOSA-N 0.000 description 1
- 108020005065 3' Flanking Region Proteins 0.000 description 1
- BMYNFMYTOJXKLE-UHFFFAOYSA-N 3-azaniumyl-2-hydroxypropanoate Chemical compound NCC(O)C(O)=O BMYNFMYTOJXKLE-UHFFFAOYSA-N 0.000 description 1
- 125000002103 4,4'-dimethoxytriphenylmethyl group Chemical group [H]C1=C([H])C([H])=C(C([H])=C1[H])C(*)(C1=C([H])C([H])=C(OC([H])([H])[H])C([H])=C1[H])C1=C([H])C([H])=C(OC([H])([H])[H])C([H])=C1[H] 0.000 description 1
- FLCPHISGULLHCH-UHFFFAOYSA-N 4-amino-2-(6-aminohexoxy)butanoic acid Chemical compound NCCCCCCOC(C(=O)O)CCN FLCPHISGULLHCH-UHFFFAOYSA-N 0.000 description 1
- 108020005029 5' Flanking Region Proteins 0.000 description 1
- LQLQRFGHAALLLE-UHFFFAOYSA-N 5-bromouracil Chemical compound BrC1=CNC(=O)NC1=O LQLQRFGHAALLLE-UHFFFAOYSA-N 0.000 description 1
- UJBCLAXPPIDQEE-UHFFFAOYSA-N 5-prop-1-ynyl-1h-pyrimidine-2,4-dione Chemical compound CC#CC1=CNC(=O)NC1=O UJBCLAXPPIDQEE-UHFFFAOYSA-N 0.000 description 1
- QNNARSZPGNJZIX-UHFFFAOYSA-N 6-amino-5-prop-1-ynyl-1h-pyrimidin-2-one Chemical compound CC#CC1=CNC(=O)N=C1N QNNARSZPGNJZIX-UHFFFAOYSA-N 0.000 description 1
- SLXKOJJOQWFEFD-UHFFFAOYSA-N 6-aminohexanoic acid Chemical compound NCCCCCC(O)=O SLXKOJJOQWFEFD-UHFFFAOYSA-N 0.000 description 1
- ZCYVEMRRCGMTRW-UHFFFAOYSA-N 7553-56-2 Chemical compound [I] ZCYVEMRRCGMTRW-UHFFFAOYSA-N 0.000 description 1
- VKKXEIQIGGPMHT-UHFFFAOYSA-N 7h-purine-2,8-diamine Chemical compound NC1=NC=C2NC(N)=NC2=N1 VKKXEIQIGGPMHT-UHFFFAOYSA-N 0.000 description 1
- MSSXOMSJDRHRMC-UHFFFAOYSA-N 9H-purine-2,6-diamine Chemical compound NC1=NC(N)=C2NC=NC2=N1 MSSXOMSJDRHRMC-UHFFFAOYSA-N 0.000 description 1
- 208000004998 Abdominal Pain Diseases 0.000 description 1
- QTBSBXVTEAMEQO-UHFFFAOYSA-M Acetate Chemical compound CC([O-])=O QTBSBXVTEAMEQO-UHFFFAOYSA-M 0.000 description 1
- 206010059193 Acute hepatitis B Diseases 0.000 description 1
- QGZKDVFQNNGYKY-UHFFFAOYSA-O Ammonium Chemical compound [NH4+] QGZKDVFQNNGYKY-UHFFFAOYSA-O 0.000 description 1
- USFZMSVCRYTOJT-UHFFFAOYSA-N Ammonium acetate Chemical compound N.CC(O)=O USFZMSVCRYTOJT-UHFFFAOYSA-N 0.000 description 1
- 239000005695 Ammonium acetate Substances 0.000 description 1
- VHUUQVKOLVNVRT-UHFFFAOYSA-N Ammonium hydroxide Chemical compound [NH4+].[OH-] VHUUQVKOLVNVRT-UHFFFAOYSA-N 0.000 description 1
- 102000008682 Argonaute Proteins Human genes 0.000 description 1
- 108010088141 Argonaute Proteins Proteins 0.000 description 1
- 231100000699 Bacterial toxin Toxicity 0.000 description 1
- DWRXFEITVBNRMK-UHFFFAOYSA-N Beta-D-1-Arabinofuranosylthymine Natural products O=C1NC(=O)C(C)=CN1C1C(O)C(O)C(CO)O1 DWRXFEITVBNRMK-UHFFFAOYSA-N 0.000 description 1
- 108010017384 Blood Proteins Proteins 0.000 description 1
- 102000004506 Blood Proteins Human genes 0.000 description 1
- 241000283690 Bos taurus Species 0.000 description 1
- 238000011746 C57BL/6J (JAX™ mouse strain) Methods 0.000 description 1
- 108091079001 CRISPR RNA Proteins 0.000 description 1
- 241000282836 Camelus dromedarius Species 0.000 description 1
- 201000009030 Carcinoma Diseases 0.000 description 1
- 102000053642 Catalytic RNA Human genes 0.000 description 1
- 108090000994 Catalytic RNA Proteins 0.000 description 1
- 241000700199 Cavia porcellus Species 0.000 description 1
- 241000282693 Cercopithecidae Species 0.000 description 1
- 241001432959 Chernes Species 0.000 description 1
- 208000000419 Chronic Hepatitis B Diseases 0.000 description 1
- 108020004638 Circular DNA Proteins 0.000 description 1
- 108020004705 Codon Proteins 0.000 description 1
- 208000035473 Communicable disease Diseases 0.000 description 1
- 241000699800 Cricetinae Species 0.000 description 1
- 102100025620 Cytochrome b-245 light chain Human genes 0.000 description 1
- 102000004127 Cytokines Human genes 0.000 description 1
- 108090000695 Cytokines Proteins 0.000 description 1
- 238000007400 DNA extraction Methods 0.000 description 1
- 238000013382 DNA quantification Methods 0.000 description 1
- 230000006820 DNA synthesis Effects 0.000 description 1
- 101150018489 DNA-C gene Proteins 0.000 description 1
- 241000702421 Dependoparvovirus Species 0.000 description 1
- KCXVZYZYPLLWCC-UHFFFAOYSA-N EDTA Chemical compound OC(=O)CN(CC(O)=O)CCN(CC(O)=O)CC(O)=O KCXVZYZYPLLWCC-UHFFFAOYSA-N 0.000 description 1
- 238000002965 ELISA Methods 0.000 description 1
- 108010093099 Endoribonucleases Proteins 0.000 description 1
- 102000002494 Endoribonucleases Human genes 0.000 description 1
- 101710091045 Envelope protein Proteins 0.000 description 1
- 241000283073 Equus caballus Species 0.000 description 1
- 239000005977 Ethylene Substances 0.000 description 1
- 241000282326 Felis catus Species 0.000 description 1
- 206010016654 Fibrosis Diseases 0.000 description 1
- 241000287828 Gallus gallus Species 0.000 description 1
- 229930182566 Gentamicin Natural products 0.000 description 1
- CEAZRRDELHUEMR-URQXQFDESA-N Gentamicin Chemical compound O1[C@H](C(C)NC)CC[C@@H](N)[C@H]1O[C@H]1[C@H](O)[C@@H](O[C@@H]2[C@@H]([C@@H](NC)[C@@](C)(O)CO2)O)[C@H](N)C[C@@H]1N CEAZRRDELHUEMR-URQXQFDESA-N 0.000 description 1
- 101150001754 Gusb gene Proteins 0.000 description 1
- 239000007995 HEPES buffer Substances 0.000 description 1
- 229940121759 Helicase inhibitor Drugs 0.000 description 1
- 206010019851 Hepatotoxicity Diseases 0.000 description 1
- 101000785948 Homo sapiens Asialoglycoprotein receptor 2 Proteins 0.000 description 1
- 101000856723 Homo sapiens Cytochrome b-245 light chain Proteins 0.000 description 1
- 101000831567 Homo sapiens Toll-like receptor 2 Proteins 0.000 description 1
- 102000004157 Hydrolases Human genes 0.000 description 1
- 108090000604 Hydrolases Proteins 0.000 description 1
- UGQMRVRMYYASKQ-UHFFFAOYSA-N Hypoxanthine nucleoside Natural products OC1C(O)C(CO)OC1N1C(NC=NC2=O)=C2N=C1 UGQMRVRMYYASKQ-UHFFFAOYSA-N 0.000 description 1
- DGAQECJNVWCQMB-PUAWFVPOSA-M Ilexoside XXIX Chemical compound C[C@@H]1CC[C@@]2(CC[C@@]3(C(=CC[C@H]4[C@]3(CC[C@@H]5[C@@]4(CC[C@@H](C5(C)C)OS(=O)(=O)[O-])C)C)[C@@H]2[C@]1(C)O)C)C(=O)O[C@H]6[C@@H]([C@H]([C@@H]([C@H](O6)CO)O)O)O.[Na+] DGAQECJNVWCQMB-PUAWFVPOSA-M 0.000 description 1
- 229930010555 Inosine Natural products 0.000 description 1
- UGQMRVRMYYASKQ-KQYNXXCUSA-N Inosine Chemical compound O[C@@H]1[C@H](O)[C@@H](CO)O[C@H]1N1C2=NC=NC(O)=C2N=C1 UGQMRVRMYYASKQ-KQYNXXCUSA-N 0.000 description 1
- 108010047761 Interferon-alpha Proteins 0.000 description 1
- 102000006992 Interferon-alpha Human genes 0.000 description 1
- PIWKPBJCKXDKJR-UHFFFAOYSA-N Isoflurane Chemical compound FC(F)OC(Cl)C(F)(F)F PIWKPBJCKXDKJR-UHFFFAOYSA-N 0.000 description 1
- 241000725296 JDV virus Species 0.000 description 1
- 206010023126 Jaundice Diseases 0.000 description 1
- QNAYBMKLOCPYGJ-REOHCLBHSA-N L-alanine Chemical compound C[C@H](N)C(O)=O QNAYBMKLOCPYGJ-REOHCLBHSA-N 0.000 description 1
- 241000713666 Lentivirus Species 0.000 description 1
- KDXKERNSBIXSRK-UHFFFAOYSA-N Lysine Natural products NCCCCC(N)C(O)=O KDXKERNSBIXSRK-UHFFFAOYSA-N 0.000 description 1
- 240000004658 Medicago sativa Species 0.000 description 1
- 235000010624 Medicago sativa Nutrition 0.000 description 1
- 108700011259 MicroRNAs Proteins 0.000 description 1
- 108010008699 Mucin-4 Proteins 0.000 description 1
- 102100022693 Mucin-4 Human genes 0.000 description 1
- 241000282341 Mustela putorius furo Species 0.000 description 1
- 244000061176 Nicotiana tabacum Species 0.000 description 1
- 235000002637 Nicotiana tabacum Nutrition 0.000 description 1
- GRYLNZFGIOXLOG-UHFFFAOYSA-N Nitric acid Chemical compound O[N+]([O-])=O GRYLNZFGIOXLOG-UHFFFAOYSA-N 0.000 description 1
- 108090001074 Nucleocapsid Proteins Proteins 0.000 description 1
- 102000003832 Nucleotidyltransferases Human genes 0.000 description 1
- 108090000119 Nucleotidyltransferases Proteins 0.000 description 1
- 241000842783 Orna Species 0.000 description 1
- 241000283973 Oryctolagus cuniculus Species 0.000 description 1
- 241001494479 Pecora Species 0.000 description 1
- 108091005804 Peptidases Proteins 0.000 description 1
- 241000009328 Perro Species 0.000 description 1
- 208000037581 Persistent Infection Diseases 0.000 description 1
- 239000002202 Polyethylene glycol Substances 0.000 description 1
- 102000015623 Polynucleotide Adenylyltransferase Human genes 0.000 description 1
- 108010024055 Polynucleotide adenylyltransferase Proteins 0.000 description 1
- ZLMJMSJWJFRBEC-UHFFFAOYSA-N Potassium Chemical compound [K] ZLMJMSJWJFRBEC-UHFFFAOYSA-N 0.000 description 1
- 241000288906 Primates Species 0.000 description 1
- 239000004365 Protease Substances 0.000 description 1
- 101710188315 Protein X Proteins 0.000 description 1
- 108010067787 Proteoglycans Proteins 0.000 description 1
- 102000016611 Proteoglycans Human genes 0.000 description 1
- 102000005583 Pyrin Human genes 0.000 description 1
- 108010059278 Pyrin Proteins 0.000 description 1
- 241000700159 Rattus Species 0.000 description 1
- 102100037486 Reverse transcriptase/ribonuclease H Human genes 0.000 description 1
- 102000006382 Ribonucleases Human genes 0.000 description 1
- 108010083644 Ribonucleases Proteins 0.000 description 1
- 238000011579 SCID mouse model Methods 0.000 description 1
- 108091006611 SLC10A1 Proteins 0.000 description 1
- 108020004682 Single-Stranded DNA Proteins 0.000 description 1
- 102100021988 Sodium/bile acid cotransporter Human genes 0.000 description 1
- ANLMVXSIPASBFL-UHFFFAOYSA-N Streptamin D Natural products NC1C(O)C(N)C(O)C(O)C1O ANLMVXSIPASBFL-UHFFFAOYSA-N 0.000 description 1
- KDYFGRWQOYBRFD-UHFFFAOYSA-N Succinic acid Natural products OC(=O)CCC(O)=O KDYFGRWQOYBRFD-UHFFFAOYSA-N 0.000 description 1
- 102000002689 Toll-like receptor Human genes 0.000 description 1
- 108020000411 Toll-like receptor Proteins 0.000 description 1
- 108700009124 Transcription Initiation Site Proteins 0.000 description 1
- 108700019146 Transgenes Proteins 0.000 description 1
- 241000251539 Vertebrata <Metazoa> Species 0.000 description 1
- 206010058874 Viraemia Diseases 0.000 description 1
- 208000036142 Viral infection Diseases 0.000 description 1
- 206010047700 Vomiting Diseases 0.000 description 1
- 101710086987 X protein Proteins 0.000 description 1
- 238000002835 absorbance Methods 0.000 description 1
- 238000004847 absorption spectroscopy Methods 0.000 description 1
- 150000007513 acids Chemical class 0.000 description 1
- 239000012190 activator Substances 0.000 description 1
- 208000037628 acute hepatitis B virus infection Diseases 0.000 description 1
- 235000004279 alanine Nutrition 0.000 description 1
- 125000004414 alkyl thio group Chemical group 0.000 description 1
- BJEPYKJPYRNKOW-UHFFFAOYSA-N alpha-hydroxysuccinic acid Natural products OC(=O)C(O)CC(O)=O BJEPYKJPYRNKOW-UHFFFAOYSA-N 0.000 description 1
- 229910000147 aluminium phosphate Inorganic materials 0.000 description 1
- 235000001014 amino acid Nutrition 0.000 description 1
- 125000003277 amino group Chemical group 0.000 description 1
- 229960002684 aminocaproic acid Drugs 0.000 description 1
- QGZKDVFQNNGYKY-UHFFFAOYSA-N ammonia Natural products N QGZKDVFQNNGYKY-UHFFFAOYSA-N 0.000 description 1
- 229940043376 ammonium acetate Drugs 0.000 description 1
- 235000019257 ammonium acetate Nutrition 0.000 description 1
- 230000000202 analgesic effect Effects 0.000 description 1
- 238000010171 animal model Methods 0.000 description 1
- 230000000507 anthelmentic effect Effects 0.000 description 1
- 230000001142 anti-diarrhea Effects 0.000 description 1
- 230000003474 anti-emetic effect Effects 0.000 description 1
- 230000003110 anti-inflammatory effect Effects 0.000 description 1
- 239000002111 antiemetic agent Substances 0.000 description 1
- 239000003429 antifungal agent Substances 0.000 description 1
- 229940121375 antifungal agent Drugs 0.000 description 1
- 239000002246 antineoplastic agent Substances 0.000 description 1
- 238000013459 approach Methods 0.000 description 1
- PYMYPHUHKUWMLA-WDCZJNDASA-N arabinose Chemical compound OC[C@@H](O)[C@@H](O)[C@H](O)C=O PYMYPHUHKUWMLA-WDCZJNDASA-N 0.000 description 1
- PYMYPHUHKUWMLA-UHFFFAOYSA-N arabinose Natural products OCC(O)C(O)C(O)C=O PYMYPHUHKUWMLA-UHFFFAOYSA-N 0.000 description 1
- QVGXLLKOCUKJST-UHFFFAOYSA-N atomic oxygen Chemical compound [O] QVGXLLKOCUKJST-UHFFFAOYSA-N 0.000 description 1
- 239000000688 bacterial toxin Substances 0.000 description 1
- 239000011324 bead Substances 0.000 description 1
- 125000003236 benzoyl group Chemical group [H]C1=C([H])C([H])=C(C([H])=C1[H])C(*)=O 0.000 description 1
- 235000021028 berry Nutrition 0.000 description 1
- SRBFZHDQGSBBOR-UHFFFAOYSA-N beta-D-Pyranose-Lyxose Natural products OC1COC(O)C(O)C1O SRBFZHDQGSBBOR-UHFFFAOYSA-N 0.000 description 1
- DRTQHJPVMGBUCF-PSQAKQOGSA-N beta-L-uridine Natural products O[C@H]1[C@@H](O)[C@H](CO)O[C@@H]1N1C(=O)NC(=O)C=C1 DRTQHJPVMGBUCF-PSQAKQOGSA-N 0.000 description 1
- 125000002619 bicyclic group Chemical group 0.000 description 1
- 210000000941 bile Anatomy 0.000 description 1
- 230000005540 biological transmission Effects 0.000 description 1
- 239000000090 biomarker Substances 0.000 description 1
- 230000037396 body weight Effects 0.000 description 1
- KDYFGRWQOYBRFD-NUQCWPJISA-N butanedioic acid Chemical compound O[14C](=O)CC[14C](O)=O KDYFGRWQOYBRFD-NUQCWPJISA-N 0.000 description 1
- 238000004422 calculation algorithm Methods 0.000 description 1
- 230000000711 cancerogenic effect Effects 0.000 description 1
- 210000000234 capsid Anatomy 0.000 description 1
- 229940124765 capsid inhibitor Drugs 0.000 description 1
- 231100000357 carcinogen Toxicity 0.000 description 1
- 239000003183 carcinogenic agent Substances 0.000 description 1
- 230000003197 catalytic effect Effects 0.000 description 1
- 239000006285 cell suspension Substances 0.000 description 1
- 230000036755 cellular response Effects 0.000 description 1
- 238000005119 centrifugation Methods 0.000 description 1
- 239000000919 ceramic Substances 0.000 description 1
- 230000002490 cerebral effect Effects 0.000 description 1
- 230000008859 change Effects 0.000 description 1
- 238000007385 chemical modification Methods 0.000 description 1
- QSHQKIURKJITMZ-BRPMRXRMSA-N cholane Chemical compound C1CC2CCCC[C@]2(C)[C@@H]2[C@@H]1[C@@H]1CC[C@H]([C@H](C)CCC)[C@@]1(C)CC2 QSHQKIURKJITMZ-BRPMRXRMSA-N 0.000 description 1
- 235000012000 cholesterol Nutrition 0.000 description 1
- 231100000749 chronicity Toxicity 0.000 description 1
- 230000007882 cirrhosis Effects 0.000 description 1
- 235000015165 citric acid Nutrition 0.000 description 1
- 230000001268 conjugating effect Effects 0.000 description 1
- 238000010276 construction Methods 0.000 description 1
- 239000012228 culture supernatant Substances 0.000 description 1
- 238000011461 current therapy Methods 0.000 description 1
- 125000004122 cyclic group Chemical group 0.000 description 1
- 125000001559 cyclopropyl group Chemical group [H]C1([H])C([H])([H])C1([H])* 0.000 description 1
- 230000009089 cytolysis Effects 0.000 description 1
- 229940127089 cytotoxic agent Drugs 0.000 description 1
- 231100000135 cytotoxicity Toxicity 0.000 description 1
- 230000003013 cytotoxicity Effects 0.000 description 1
- 230000005860 defense response to virus Effects 0.000 description 1
- 238000010511 deprotection reaction Methods 0.000 description 1
- 238000010586 diagram Methods 0.000 description 1
- 238000007865 diluting Methods 0.000 description 1
- 238000010790 dilution Methods 0.000 description 1
- 239000012895 dilution Substances 0.000 description 1
- 229940042399 direct acting antivirals protease inhibitors Drugs 0.000 description 1
- 238000010494 dissociation reaction Methods 0.000 description 1
- 230000005593 dissociations Effects 0.000 description 1
- 229940079593 drug Drugs 0.000 description 1
- 230000004064 dysfunction Effects 0.000 description 1
- 238000002330 electrospray ionisation mass spectrometry Methods 0.000 description 1
- 230000002255 enzymatic effect Effects 0.000 description 1
- 125000004185 ester group Chemical group 0.000 description 1
- DEFVIWRASFVYLL-UHFFFAOYSA-N ethylene glycol bis(2-aminoethyl)tetraacetic acid Chemical compound OC(=O)CN(CC(O)=O)CCOCCOCCN(CC(O)=O)CC(O)=O DEFVIWRASFVYLL-UHFFFAOYSA-N 0.000 description 1
- 230000029142 excretion Effects 0.000 description 1
- 230000001747 exhibiting effect Effects 0.000 description 1
- 238000013401 experimental design Methods 0.000 description 1
- 206010016256 fatigue Diseases 0.000 description 1
- 230000001605 fetal effect Effects 0.000 description 1
- 238000000684 flow cytometry Methods 0.000 description 1
- 239000012634 fragment Substances 0.000 description 1
- 239000001530 fumaric acid Substances 0.000 description 1
- 235000011087 fumaric acid Nutrition 0.000 description 1
- 238000010230 functional analysis Methods 0.000 description 1
- 230000004927 fusion Effects 0.000 description 1
- 239000007789 gas Substances 0.000 description 1
- 210000001035 gastrointestinal tract Anatomy 0.000 description 1
- 230000030279 gene silencing Effects 0.000 description 1
- 238000012226 gene silencing method Methods 0.000 description 1
- 230000002068 genetic effect Effects 0.000 description 1
- 229960002518 gentamicin Drugs 0.000 description 1
- PCHJSUWPFVWCPO-UHFFFAOYSA-N gold Chemical compound [Au] PCHJSUWPFVWCPO-UHFFFAOYSA-N 0.000 description 1
- 239000010931 gold Substances 0.000 description 1
- 229910052737 gold Inorganic materials 0.000 description 1
- 230000005802 health problem Effects 0.000 description 1
- 231100000283 hepatitis Toxicity 0.000 description 1
- 231100000304 hepatotoxicity Toxicity 0.000 description 1
- 230000007686 hepatotoxicity Effects 0.000 description 1
- 150000002402 hexoses Chemical group 0.000 description 1
- 210000005260 human cell Anatomy 0.000 description 1
- XMBWDFGMSWQBCA-UHFFFAOYSA-N hydrogen iodide Chemical compound I XMBWDFGMSWQBCA-UHFFFAOYSA-N 0.000 description 1
- 229940071870 hydroiodic acid Drugs 0.000 description 1
- 238000010191 image analysis Methods 0.000 description 1
- 125000001841 imino group Chemical group [H]N=* 0.000 description 1
- 230000002519 immonomodulatory effect Effects 0.000 description 1
- 230000001900 immune effect Effects 0.000 description 1
- 230000028993 immune response Effects 0.000 description 1
- 208000026278 immune system disease Diseases 0.000 description 1
- 238000010166 immunofluorescence Methods 0.000 description 1
- 230000005847 immunogenicity Effects 0.000 description 1
- 230000003308 immunostimulating effect Effects 0.000 description 1
- 230000001506 immunosuppresive effect Effects 0.000 description 1
- 238000007901 in situ hybridization Methods 0.000 description 1
- 238000010348 incorporation Methods 0.000 description 1
- 230000006698 induction Effects 0.000 description 1
- 230000001939 inductive effect Effects 0.000 description 1
- 238000001802 infusion Methods 0.000 description 1
- 230000015788 innate immune response Effects 0.000 description 1
- 150000007529 inorganic bases Chemical class 0.000 description 1
- 229960003786 inosine Drugs 0.000 description 1
- 229940047124 interferons Drugs 0.000 description 1
- 230000000968 intestinal effect Effects 0.000 description 1
- 238000001361 intraarterial administration Methods 0.000 description 1
- 230000004068 intracellular signaling Effects 0.000 description 1
- 238000007918 intramuscular administration Methods 0.000 description 1
- 238000010255 intramuscular injection Methods 0.000 description 1
- 239000007927 intramuscular injection Substances 0.000 description 1
- 238000007912 intraperitoneal administration Methods 0.000 description 1
- 239000007928 intraperitoneal injection Substances 0.000 description 1
- 238000007913 intrathecal administration Methods 0.000 description 1
- 238000007914 intraventricular administration Methods 0.000 description 1
- 229910052740 iodine Inorganic materials 0.000 description 1
- 239000011630 iodine Substances 0.000 description 1
- 238000004255 ion exchange chromatography Methods 0.000 description 1
- 229960002725 isoflurane Drugs 0.000 description 1
- 125000001449 isopropyl group Chemical group [H]C([H])([H])C([H])(*)C([H])([H])[H] 0.000 description 1
- 239000004310 lactic acid Substances 0.000 description 1
- 235000014655 lactic acid Nutrition 0.000 description 1
- 230000000670 limiting effect Effects 0.000 description 1
- 150000002632 lipids Chemical group 0.000 description 1
- 238000012153 long-term therapy Methods 0.000 description 1
- 239000001630 malic acid Substances 0.000 description 1
- 235000011090 malic acid Nutrition 0.000 description 1
- 230000008774 maternal effect Effects 0.000 description 1
- 239000011159 matrix material Substances 0.000 description 1
- 230000007246 mechanism Effects 0.000 description 1
- 230000004060 metabolic process Effects 0.000 description 1
- 229940098779 methanesulfonic acid Drugs 0.000 description 1
- 230000011987 methylation Effects 0.000 description 1
- 238000007069 methylation reaction Methods 0.000 description 1
- YACKEPLHDIMKIO-UHFFFAOYSA-N methylphosphonic acid Chemical class CP(O)(O)=O YACKEPLHDIMKIO-UHFFFAOYSA-N 0.000 description 1
- 239000002679 microRNA Substances 0.000 description 1
- 210000001616 monocyte Anatomy 0.000 description 1
- 125000004573 morpholin-4-yl group Chemical group N1(CCOCC1)* 0.000 description 1
- LNOPIUAQISRISI-UHFFFAOYSA-N n'-hydroxy-2-propan-2-ylsulfonylethanimidamide Chemical compound CC(C)S(=O)(=O)CC(N)=NO LNOPIUAQISRISI-UHFFFAOYSA-N 0.000 description 1
- 230000024717 negative regulation of secretion Effects 0.000 description 1
- 230000007935 neutral effect Effects 0.000 description 1
- 229910017604 nitric acid Inorganic materials 0.000 description 1
- 125000000449 nitro group Chemical group [O-][N+](*)=O 0.000 description 1
- 239000000041 non-steroidal anti-inflammatory agent Substances 0.000 description 1
- 229940021182 non-steroidal anti-inflammatory drug Drugs 0.000 description 1
- 231100000252 nontoxic Toxicity 0.000 description 1
- 230000003000 nontoxic effect Effects 0.000 description 1
- 238000002414 normal-phase solid-phase extraction Methods 0.000 description 1
- 238000010606 normalization Methods 0.000 description 1
- 238000005457 optimization Methods 0.000 description 1
- 235000005985 organic acids Nutrition 0.000 description 1
- 150000007530 organic bases Chemical class 0.000 description 1
- 230000002018 overexpression Effects 0.000 description 1
- 235000006408 oxalic acid Nutrition 0.000 description 1
- 230000003647 oxidation Effects 0.000 description 1
- 238000007254 oxidation reaction Methods 0.000 description 1
- 125000004043 oxo group Chemical group O=* 0.000 description 1
- 239000001301 oxygen Substances 0.000 description 1
- 125000004430 oxygen atom Chemical group O* 0.000 description 1
- FJKROLUGYXJWQN-UHFFFAOYSA-N papa-hydroxy-benzoic acid Natural products OC(=O)C1=CC=C(O)C=C1 FJKROLUGYXJWQN-UHFFFAOYSA-N 0.000 description 1
- 244000052769 pathogen Species 0.000 description 1
- 230000037361 pathway Effects 0.000 description 1
- 229960001412 pentobarbital Drugs 0.000 description 1
- 239000000137 peptide hydrolase inhibitor Substances 0.000 description 1
- 230000035699 permeability Effects 0.000 description 1
- 239000000546 pharmaceutical excipient Substances 0.000 description 1
- 239000012071 phase Substances 0.000 description 1
- 230000004962 physiological condition Effects 0.000 description 1
- 210000002826 placenta Anatomy 0.000 description 1
- 239000013612 plasmid Substances 0.000 description 1
- 239000004417 polycarbonate Substances 0.000 description 1
- 229920000515 polycarbonate Polymers 0.000 description 1
- 102000054765 polymorphisms of proteins Human genes 0.000 description 1
- 108091033319 polynucleotide Proteins 0.000 description 1
- 102000040430 polynucleotide Human genes 0.000 description 1
- 239000002157 polynucleotide Substances 0.000 description 1
- 229920001184 polypeptide Polymers 0.000 description 1
- 231100000683 possible toxicity Toxicity 0.000 description 1
- 239000011591 potassium Substances 0.000 description 1
- 229910052700 potassium Inorganic materials 0.000 description 1
- 159000000001 potassium salts Chemical class 0.000 description 1
- 238000001556 precipitation Methods 0.000 description 1
- 238000002360 preparation method Methods 0.000 description 1
- 230000008569 process Effects 0.000 description 1
- 239000000651 prodrug Substances 0.000 description 1
- 229940002612 prodrug Drugs 0.000 description 1
- 239000000047 product Substances 0.000 description 1
- 230000000750 progressive effect Effects 0.000 description 1
- 230000002062 proliferating effect Effects 0.000 description 1
- 230000000069 prophylactic effect Effects 0.000 description 1
- 238000011321 prophylaxis Methods 0.000 description 1
- 150000003230 pyrimidines Chemical class 0.000 description 1
- 238000012797 qualification Methods 0.000 description 1
- 125000001453 quaternary ammonium group Chemical group 0.000 description 1
- 230000008929 regeneration Effects 0.000 description 1
- 238000011069 regeneration method Methods 0.000 description 1
- 230000001105 regulatory effect Effects 0.000 description 1
- 230000000717 retained effect Effects 0.000 description 1
- 238000010242 retro-orbital bleeding Methods 0.000 description 1
- 238000004366 reverse phase liquid chromatography Methods 0.000 description 1
- 230000002441 reversible effect Effects 0.000 description 1
- 238000012552 review Methods 0.000 description 1
- 108091092562 ribozyme Proteins 0.000 description 1
- 229960004889 salicylic acid Drugs 0.000 description 1
- 230000035945 sensitivity Effects 0.000 description 1
- 230000011664 signaling Effects 0.000 description 1
- 229910052708 sodium Inorganic materials 0.000 description 1
- 159000000000 sodium salts Chemical class 0.000 description 1
- JAJWGJBVLPIOOH-IZYKLYLVSA-M sodium taurocholate Chemical compound [Na+].C([C@H]1C[C@H]2O)[C@H](O)CC[C@]1(C)[C@@H]1[C@@H]2[C@@H]2CC[C@H]([C@@H](CCC(=O)NCCS([O-])(=O)=O)C)[C@@]2(C)[C@@H](O)C1 JAJWGJBVLPIOOH-IZYKLYLVSA-M 0.000 description 1
- 230000009870 specific binding Effects 0.000 description 1
- 230000006641 stabilisation Effects 0.000 description 1
- 238000011105 stabilization Methods 0.000 description 1
- 230000000087 stabilizing effect Effects 0.000 description 1
- 230000010473 stable expression Effects 0.000 description 1
- 230000003637 steroidlike Effects 0.000 description 1
- ANLMVXSIPASBFL-FAEUDGQSSA-N streptamine Chemical compound N[C@H]1[C@H](O)[C@@H](N)[C@H](O)[C@@H](O)[C@@H]1O ANLMVXSIPASBFL-FAEUDGQSSA-N 0.000 description 1
- 239000007929 subcutaneous injection Substances 0.000 description 1
- 238000005486 sulfidation Methods 0.000 description 1
- 230000002459 sustained effect Effects 0.000 description 1
- 238000010189 synthetic method Methods 0.000 description 1
- 108091035539 telomere Proteins 0.000 description 1
- 102000055501 telomere Human genes 0.000 description 1
- 210000003411 telomere Anatomy 0.000 description 1
- 229960004693 tenofovir disoproxil fumarate Drugs 0.000 description 1
- YLQBMQCUIZJEEH-UHFFFAOYSA-N tetrahydrofuran Natural products C=1C=COC=1 YLQBMQCUIZJEEH-UHFFFAOYSA-N 0.000 description 1
- 238000002560 therapeutic procedure Methods 0.000 description 1
- 125000003396 thiol group Chemical group [H]S* 0.000 description 1
- 238000006177 thiolation reaction Methods 0.000 description 1
- ZEMGGZBWXRYJHK-UHFFFAOYSA-N thiouracil Chemical compound O=C1C=CNC(=S)N1 ZEMGGZBWXRYJHK-UHFFFAOYSA-N 0.000 description 1
- 125000000464 thioxo group Chemical group S=* 0.000 description 1
- 229940104230 thymidine Drugs 0.000 description 1
- 238000004448 titration Methods 0.000 description 1
- 229940044616 toll-like receptor 7 agonist Drugs 0.000 description 1
- 231100000331 toxic Toxicity 0.000 description 1
- 230000002588 toxic effect Effects 0.000 description 1
- 231100000419 toxicity Toxicity 0.000 description 1
- 230000001988 toxicity Effects 0.000 description 1
- 230000009466 transformation Effects 0.000 description 1
- 230000001131 transforming effect Effects 0.000 description 1
- 230000032258 transport Effects 0.000 description 1
- 238000004704 ultra performance liquid chromatography Methods 0.000 description 1
- 230000003827 upregulation Effects 0.000 description 1
- DRTQHJPVMGBUCF-UHFFFAOYSA-N uracil arabinoside Natural products OC1C(O)C(CO)OC1N1C(=O)NC(=O)C=C1 DRTQHJPVMGBUCF-UHFFFAOYSA-N 0.000 description 1
- 229940045145 uridine Drugs 0.000 description 1
- 210000002700 urine Anatomy 0.000 description 1
- 210000003462 vein Anatomy 0.000 description 1
- 230000035899 viability Effects 0.000 description 1
- 125000000391 vinyl group Chemical group [H]C([*])=C([H])[H] 0.000 description 1
- 229920002554 vinyl polymer Polymers 0.000 description 1
- 230000009385 viral infection Effects 0.000 description 1
- 230000008673 vomiting Effects 0.000 description 1
- 239000011534 wash buffer Substances 0.000 description 1
- 229920001285 xanthan gum Polymers 0.000 description 1
- 229940075420 xanthine Drugs 0.000 description 1
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/70—Carbohydrates; Sugars; Derivatives thereof
- A61K31/7088—Compounds having three or more nucleosides or nucleotides
- A61K31/7115—Nucleic acids or oligonucleotides having modified bases, i.e. other than adenine, guanine, cytosine, uracil or thymine
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N15/00—Mutation or genetic engineering; DNA or RNA concerning genetic engineering, vectors, e.g. plasmids, or their isolation, preparation or purification; Use of hosts therefor
- C12N15/09—Recombinant DNA-technology
- C12N15/11—DNA or RNA fragments; Modified forms thereof; Non-coding nucleic acids having a biological activity
- C12N15/113—Non-coding nucleic acids modulating the expression of genes, e.g. antisense oligonucleotides; Antisense DNA or RNA; Triplex- forming oligonucleotides; Catalytic nucleic acids, e.g. ribozymes; Nucleic acids used in co-suppression or gene silencing
- C12N15/1131—Non-coding nucleic acids modulating the expression of genes, e.g. antisense oligonucleotides; Antisense DNA or RNA; Triplex- forming oligonucleotides; Catalytic nucleic acids, e.g. ribozymes; Nucleic acids used in co-suppression or gene silencing against viruses
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/70—Carbohydrates; Sugars; Derivatives thereof
- A61K31/7088—Compounds having three or more nucleosides or nucleotides
- A61K31/7105—Natural ribonucleic acids, i.e. containing only riboses attached to adenine, guanine, cytosine or uracil and having 3'-5' phosphodiester links
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/70—Carbohydrates; Sugars; Derivatives thereof
- A61K31/7088—Compounds having three or more nucleosides or nucleotides
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/70—Carbohydrates; Sugars; Derivatives thereof
- A61K31/7088—Compounds having three or more nucleosides or nucleotides
- A61K31/712—Nucleic acids or oligonucleotides having modified sugars, i.e. other than ribose or 2'-deoxyribose
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/70—Carbohydrates; Sugars; Derivatives thereof
- A61K31/7088—Compounds having three or more nucleosides or nucleotides
- A61K31/7125—Nucleic acids or oligonucleotides having modified internucleoside linkage, i.e. other than 3'-5' phosphodiesters
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/70—Carbohydrates; Sugars; Derivatives thereof
- A61K31/7088—Compounds having three or more nucleosides or nucleotides
- A61K31/713—Double-stranded nucleic acids or oligonucleotides
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/50—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates
- A61K47/51—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent
- A61K47/54—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent the modifying agent being an organic compound
- A61K47/549—Sugars, nucleosides, nucleotides or nucleic acids
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/50—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates
- A61K47/51—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent
- A61K47/62—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent the modifying agent being a protein, peptide or polyamino acid
- A61K47/64—Drug-peptide, drug-protein or drug-polyamino acid conjugates, i.e. the modifying agent being a peptide, protein or polyamino acid which is covalently bonded or complexed to a therapeutically active agent
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P1/00—Drugs for disorders of the alimentary tract or the digestive system
- A61P1/16—Drugs for disorders of the alimentary tract or the digestive system for liver or gallbladder disorders, e.g. hepatoprotective agents, cholagogues, litholytics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P31/00—Antiinfectives, i.e. antibiotics, antiseptics, chemotherapeutics
- A61P31/12—Antivirals
- A61P31/20—Antivirals for DNA viruses
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07H—SUGARS; DERIVATIVES THEREOF; NUCLEOSIDES; NUCLEOTIDES; NUCLEIC ACIDS
- C07H21/00—Compounds containing two or more mononucleotide units having separate phosphate or polyphosphate groups linked by saccharide radicals of nucleoside groups, e.g. nucleic acids
- C07H21/02—Compounds containing two or more mononucleotide units having separate phosphate or polyphosphate groups linked by saccharide radicals of nucleoside groups, e.g. nucleic acids with ribosyl as saccharide radical
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N15/00—Mutation or genetic engineering; DNA or RNA concerning genetic engineering, vectors, e.g. plasmids, or their isolation, preparation or purification; Use of hosts therefor
- C12N15/09—Recombinant DNA-technology
- C12N15/11—DNA or RNA fragments; Modified forms thereof; Non-coding nucleic acids having a biological activity
- C12N15/113—Non-coding nucleic acids modulating the expression of genes, e.g. antisense oligonucleotides; Antisense DNA or RNA; Triplex- forming oligonucleotides; Catalytic nucleic acids, e.g. ribozymes; Nucleic acids used in co-suppression or gene silencing
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N15/00—Mutation or genetic engineering; DNA or RNA concerning genetic engineering, vectors, e.g. plasmids, or their isolation, preparation or purification; Use of hosts therefor
- C12N15/09—Recombinant DNA-technology
- C12N15/11—DNA or RNA fragments; Modified forms thereof; Non-coding nucleic acids having a biological activity
- C12N15/113—Non-coding nucleic acids modulating the expression of genes, e.g. antisense oligonucleotides; Antisense DNA or RNA; Triplex- forming oligonucleotides; Catalytic nucleic acids, e.g. ribozymes; Nucleic acids used in co-suppression or gene silencing
- C12N15/1137—Non-coding nucleic acids modulating the expression of genes, e.g. antisense oligonucleotides; Antisense DNA or RNA; Triplex- forming oligonucleotides; Catalytic nucleic acids, e.g. ribozymes; Nucleic acids used in co-suppression or gene silencing against enzymes
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2310/00—Structure or type of the nucleic acid
- C12N2310/10—Type of nucleic acid
- C12N2310/11—Antisense
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2310/00—Structure or type of the nucleic acid
- C12N2310/10—Type of nucleic acid
- C12N2310/14—Type of nucleic acid interfering nucleic acids [NA]
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2310/00—Structure or type of the nucleic acid
- C12N2310/30—Chemical structure
- C12N2310/31—Chemical structure of the backbone
- C12N2310/315—Phosphorothioates
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2310/00—Structure or type of the nucleic acid
- C12N2310/30—Chemical structure
- C12N2310/32—Chemical structure of the sugar
- C12N2310/323—Chemical structure of the sugar modified ring structure
- C12N2310/3231—Chemical structure of the sugar modified ring structure having an additional ring, e.g. LNA, ENA
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2310/00—Structure or type of the nucleic acid
- C12N2310/30—Chemical structure
- C12N2310/34—Spatial arrangement of the modifications
- C12N2310/341—Gapmers, i.e. of the type ===---===
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2310/00—Structure or type of the nucleic acid
- C12N2310/30—Chemical structure
- C12N2310/34—Spatial arrangement of the modifications
- C12N2310/343—Spatial arrangement of the modifications having patterns, e.g. ==--==--==--
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2310/00—Structure or type of the nucleic acid
- C12N2310/30—Chemical structure
- C12N2310/34—Spatial arrangement of the modifications
- C12N2310/346—Spatial arrangement of the modifications having a combination of backbone and sugar modifications
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2310/00—Structure or type of the nucleic acid
- C12N2310/30—Chemical structure
- C12N2310/35—Nature of the modification
- C12N2310/351—Conjugate
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12Y—ENZYMES
- C12Y207/00—Transferases transferring phosphorus-containing groups (2.7)
- C12Y207/07—Nucleotidyltransferases (2.7.7)
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12Y—ENZYMES
- C12Y207/00—Transferases transferring phosphorus-containing groups (2.7)
- C12Y207/07—Nucleotidyltransferases (2.7.7)
- C12Y207/07007—DNA-directed DNA polymerase (2.7.7.7), i.e. DNA replicase
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y02—TECHNOLOGIES OR APPLICATIONS FOR MITIGATION OR ADAPTATION AGAINST CLIMATE CHANGE
- Y02A—TECHNOLOGIES FOR ADAPTATION TO CLIMATE CHANGE
- Y02A50/00—TECHNOLOGIES FOR ADAPTATION TO CLIMATE CHANGE in human health protection, e.g. against extreme weather
- Y02A50/30—Against vector-borne diseases, e.g. mosquito-borne, fly-borne, tick-borne or waterborne diseases whose impact is exacerbated by climate change
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Engineering & Computer Science (AREA)
- Molecular Biology (AREA)
- Genetics & Genomics (AREA)
- General Health & Medical Sciences (AREA)
- Organic Chemistry (AREA)
- Biochemistry (AREA)
- Biotechnology (AREA)
- Biomedical Technology (AREA)
- Medicinal Chemistry (AREA)
- Veterinary Medicine (AREA)
- Public Health (AREA)
- Animal Behavior & Ethology (AREA)
- Pharmacology & Pharmacy (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Zoology (AREA)
- General Engineering & Computer Science (AREA)
- Wood Science & Technology (AREA)
- Virology (AREA)
- Epidemiology (AREA)
- Physics & Mathematics (AREA)
- Plant Pathology (AREA)
- Biophysics (AREA)
- Microbiology (AREA)
- General Chemical & Material Sciences (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Communicable Diseases (AREA)
- Oncology (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Gastroenterology & Hepatology (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
- Medicines That Contain Protein Lipid Enzymes And Other Medicines (AREA)
- Medicinal Preparation (AREA)
- Saccharide Compounds (AREA)
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| KR1020207021922A KR102431353B1 (ko) | 2017-10-16 | 2018-10-16 | B형 간염 감염을 치료하기 위한 PAPD5 및 PAPD7 mRNA의 감소용 핵산 분자 |
Applications Claiming Priority (5)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| EP17196554 | 2017-10-16 | ||
| EP17196554.4 | 2017-10-16 | ||
| EP17208056 | 2017-12-18 | ||
| EP17208056.6 | 2017-12-18 | ||
| PCT/EP2018/078136 WO2019076842A1 (en) | 2017-10-16 | 2018-10-16 | NUCLEIC ACID MOLECULE FOR THE REDUCTION OF PAPD5 AND PAPD7 MRNA FOR THE TREATMENT OF HEPATITIS B INFECTION |
Related Child Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020207021922A Division KR102431353B1 (ko) | 2017-10-16 | 2018-10-16 | B형 간염 감염을 치료하기 위한 PAPD5 및 PAPD7 mRNA의 감소용 핵산 분자 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| KR20200030594A true KR20200030594A (ko) | 2020-03-20 |
Family
ID=63799031
Family Applications (5)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020207005096A Ceased KR20200030594A (ko) | 2017-10-16 | 2018-10-16 | B형 간염 감염을 치료하기 위한 PAPD5 및 PAPD7 mRNA의 감소용 핵산 분자 |
| KR1020227027132A Active KR102585898B1 (ko) | 2017-10-16 | 2018-10-16 | B형 간염 감염을 치료하기 위한 PAPD5 및 PAPD7 mRNA의 감소용 핵산 분자 |
| KR1020237033451A Active KR102769100B1 (ko) | 2017-10-16 | 2018-10-16 | B형 간염 감염을 치료하기 위한 PAPD5 및 PAPD7 mRNA의 감소용 핵산 분자 |
| KR1020207021922A Active KR102431353B1 (ko) | 2017-10-16 | 2018-10-16 | B형 간염 감염을 치료하기 위한 PAPD5 및 PAPD7 mRNA의 감소용 핵산 분자 |
| KR1020257004579A Pending KR20250025042A (ko) | 2017-10-16 | 2018-10-16 | B형 간염 감염을 치료하기 위한 PAPD5 및 PAPD7 mRNA의 감소용 핵산 분자 |
Family Applications After (4)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020227027132A Active KR102585898B1 (ko) | 2017-10-16 | 2018-10-16 | B형 간염 감염을 치료하기 위한 PAPD5 및 PAPD7 mRNA의 감소용 핵산 분자 |
| KR1020237033451A Active KR102769100B1 (ko) | 2017-10-16 | 2018-10-16 | B형 간염 감염을 치료하기 위한 PAPD5 및 PAPD7 mRNA의 감소용 핵산 분자 |
| KR1020207021922A Active KR102431353B1 (ko) | 2017-10-16 | 2018-10-16 | B형 간염 감염을 치료하기 위한 PAPD5 및 PAPD7 mRNA의 감소용 핵산 분자 |
| KR1020257004579A Pending KR20250025042A (ko) | 2017-10-16 | 2018-10-16 | B형 간염 감염을 치료하기 위한 PAPD5 및 PAPD7 mRNA의 감소용 핵산 분자 |
Country Status (20)
| Country | Link |
|---|---|
| US (4) | US10953034B2 (enExample) |
| EP (1) | EP3645723A1 (enExample) |
| JP (3) | JP2021500013A (enExample) |
| KR (5) | KR20200030594A (enExample) |
| CN (2) | CN118048356A (enExample) |
| AU (3) | AU2018350693B2 (enExample) |
| BR (2) | BR122020018622A2 (enExample) |
| CA (1) | CA3072314A1 (enExample) |
| CL (1) | CL2020000945A1 (enExample) |
| CR (2) | CR20200205A (enExample) |
| IL (2) | IL272566B2 (enExample) |
| MA (1) | MA52151A (enExample) |
| MX (1) | MX2020003464A (enExample) |
| PE (1) | PE20200868A1 (enExample) |
| PH (1) | PH12020550436A1 (enExample) |
| RU (1) | RU2766360C2 (enExample) |
| SG (1) | SG11202003461TA (enExample) |
| TW (3) | TW202424191A (enExample) |
| UA (1) | UA126931C2 (enExample) |
| WO (1) | WO2019076842A1 (enExample) |
Families Citing this family (10)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| MA45496A (fr) * | 2016-06-17 | 2019-04-24 | Hoffmann La Roche | Molécules d'acide nucléique pour la réduction de l'arnm de padd5 ou pad7 pour le traitement d'une infection par l'hépatite b |
| RU2766360C2 (ru) | 2017-10-16 | 2022-03-15 | Ф. Хоффманн-Ля Рош Аг | МОЛЕКУЛЫ НУКЛЕИНОВЫХ КИСЛОТ ДЛЯ УМЕНЬШЕНИЯ УРОВНЯ мРНК PAPD5 ИЛИ PAPD7 ДЛЯ ЛЕЧЕНИЯ ИНФЕКЦИОННОГО ГЕПАТИТА В |
| WO2019157531A1 (en) * | 2018-02-12 | 2019-08-15 | Ionis Pharmaceuticals, Inc. | Modified compounds and uses thereof |
| CA3103054A1 (en) | 2018-07-03 | 2020-01-09 | F. Hoffmann-La Roche Ag | Oligonucleotides for modulating tau expression |
| JP7616987B2 (ja) | 2018-07-13 | 2025-01-17 | エフ. ホフマン-ラ ロシュ アーゲー | Rtel1発現の調節用のオリゴヌクレオチド |
| US20220220483A1 (en) * | 2019-05-30 | 2022-07-14 | Seoul National University R&Db Foundation | Method for inhibiting infection and activation of virus |
| BR112021023488A2 (pt) * | 2019-05-31 | 2022-01-18 | Aligos Therapeutics Inc | Oligonucleotídeos de gapmer modificados e métodos de uso |
| CA3163646A1 (en) * | 2019-12-24 | 2021-07-01 | F. Hoffman-La Roche Ag | Pharmaceutical combination of antiviral agents targeting hbv and/or an immune modulator for treatment of hbv |
| AU2021383758A1 (en) * | 2020-11-20 | 2023-06-15 | Aligos Therapeutics, Inc. | Conjugates of s-antigen transport inhibiting oligonucleotide polymers having enhanced liver targeting |
| WO2025199466A1 (en) | 2024-03-22 | 2025-09-25 | Purdue Research Foundation | Liver-specific asialoglycoprotein receptor targeting ligands, conjugates comprising same, and related compositions and methods of use |
Family Cites Families (100)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4711955A (en) | 1981-04-17 | 1987-12-08 | Yale University | Modified nucleotides and methods of preparing and using same |
| CA1223831A (en) | 1982-06-23 | 1987-07-07 | Dean Engelhardt | Modified nucleotides, methods of preparing and utilizing and compositions containing the same |
| DE3538677A1 (de) | 1985-10-31 | 1987-05-07 | Schloemann Siemag Ag | Wendehaspel |
| WO1993007883A1 (en) | 1991-10-24 | 1993-04-29 | Isis Pharmaceuticals, Inc. | Derivatized oligonucleotides having improved uptake and other properties |
| US5792608A (en) | 1991-12-12 | 1998-08-11 | Gilead Sciences, Inc. | Nuclease stable and binding competent oligomers and methods for their use |
| NL9201440A (nl) | 1992-08-11 | 1994-03-01 | Univ Leiden | Triantennaire clusterglycosiden, hun bereiding en toepassing. |
| ATE207119T1 (de) * | 1992-11-06 | 2001-11-15 | Sandoz Ltd | Die herstellung von menschlichen, monoklonalen antikörpern, die gegen das oberflächenantigen von hepatitis b aktiv sind |
| US20040157780A1 (en) | 1993-11-29 | 2004-08-12 | Epimmune Inc. | CTL inducing peptides from c-erb2 (HER-2/neu) |
| EP0754238A4 (en) | 1994-04-05 | 1998-01-28 | Pharmagenics Inc | DETERMINATION AND IDENTIFICATION OF ACTIVE SUBSTANCES IN SUBSTANCE LIBRARIES |
| US5525711A (en) | 1994-05-18 | 1996-06-11 | The United States Of America As Represented By The Secretary Of The Department Of Health And Human Services | Pteridine nucleotide analogs as fluorescent DNA probes |
| JP3756313B2 (ja) | 1997-03-07 | 2006-03-15 | 武 今西 | 新規ビシクロヌクレオシド及びオリゴヌクレオチド類縁体 |
| EP2253639A1 (en) | 1997-09-12 | 2010-11-24 | Exiqon A/S | Bi- and tri-cyclic nucleoside, nucleotide and oligonucleoide analogues |
| EP1152009B2 (en) | 1999-02-12 | 2017-09-06 | Daiichi Sankyo Company, Limited | Novel nucleosides and oligonucleotide analogues |
| CN102180924A (zh) | 1999-05-04 | 2011-09-14 | 桑塔里斯制药公司 | L-核糖-lna类似物 |
| US6617442B1 (en) | 1999-09-30 | 2003-09-09 | Isis Pharmaceuticals, Inc. | Human Rnase H1 and oligonucleotide compositions thereof |
| WO2003022987A2 (en) | 2001-07-26 | 2003-03-20 | Eos Biotechnology, Inc. | Methods of diagnosis of hepatitis c infection, compositions and methods of screening for modulators of hepatitis c infection |
| US8090542B2 (en) | 2002-11-14 | 2012-01-03 | Dharmacon Inc. | Functional and hyperfunctional siRNA |
| DK1569661T3 (da) | 2002-11-18 | 2010-01-11 | Santaris Pharma As | Antisense design |
| US20060257851A1 (en) | 2002-11-26 | 2006-11-16 | Itzhak Bentwich | Bioinformatically detectable group of novel viral regulatory genes and uses thereof |
| SG173218A1 (en) | 2003-06-12 | 2011-08-29 | Alnylam Pharmaceuticals Inc | Conserved hbv and hcv sequences useful for gene silencing |
| US7374927B2 (en) | 2004-05-03 | 2008-05-20 | Affymetrix, Inc. | Methods of analysis of degraded nucleic acid samples |
| CN101052717A (zh) | 2004-05-11 | 2007-10-10 | α基因株式会社 | 诱发rna干扰的多核苷酸以及使用该多核苷酸的基因表达抑制方法 |
| US20060263798A1 (en) | 2005-02-11 | 2006-11-23 | International Business Machines Corporation | System and method for identification of MicroRNA precursor sequences and corresponding mature MicroRNA sequences from genomic sequences |
| WO2007031091A2 (en) | 2005-09-15 | 2007-03-22 | Santaris Pharma A/S | Rna antagonist compounds for the modulation of p21 ras expression |
| CN102908630B (zh) | 2006-01-27 | 2014-11-19 | Isis制药公司 | 6-修饰的双环核酸类似物 |
| AU2007225238A1 (en) | 2006-03-10 | 2007-09-20 | Wyeth | Microarray for monitoring gene expression in multiple strains of Streptococcus pneumoniae |
| US20090292006A1 (en) | 2006-05-05 | 2009-11-26 | Sanjay Bhanot | Compounds and methods for modulating expression of dgat2 |
| US7666854B2 (en) | 2006-05-11 | 2010-02-23 | Isis Pharmaceuticals, Inc. | Bis-modified bicyclic nucleic acid analogs |
| JP5441688B2 (ja) | 2006-05-11 | 2014-03-12 | アイシス ファーマシューティカルズ, インコーポレーテッド | 5’修飾二環式核酸類似体 |
| BRPI0716944A2 (pt) | 2006-09-19 | 2013-09-17 | Novartis Ag | biomarcadores de modulaÇço alvo, eficÁcia, diagnàstico, e/ou prognàstico para inibidores de raf |
| WO2008049085A1 (en) | 2006-10-18 | 2008-04-24 | Isis Pharmaceuticals, Inc. | Antisense compounds |
| DK2149605T3 (da) | 2007-03-22 | 2013-09-30 | Santaris Pharma As | Korte RNA antagonist forbindelser til modulering af det ønskede mRNA |
| DK2170917T3 (da) | 2007-05-30 | 2012-10-08 | Isis Pharmaceuticals Inc | N-Substituerede bicycliske nukleinsyreanaloge med aminomethylenbro |
| ES2386492T3 (es) | 2007-06-08 | 2012-08-21 | Isis Pharmaceuticals, Inc. | Análogos de ácidos nucleicos bicíclicos carbocíclicos |
| ES2376507T5 (es) | 2007-07-05 | 2015-08-31 | Isis Pharmaceuticals, Inc. | Análogos de ácidos nucleicos bicíclicos 6-disustituidos |
| WO2009043759A2 (en) | 2007-10-04 | 2009-04-09 | Santaris Pharma A/S | Short rna antagonist compounds for the modulation of hif1alpha |
| WO2009067647A1 (en) | 2007-11-21 | 2009-05-28 | Isis Pharmaceuticals, Inc. | Carbocyclic alpha-l-bicyclic nucleic acid analogs |
| CA3023889A1 (en) | 2007-12-27 | 2009-07-09 | Abbott Laboratories | Anti-t. cruzi antibodies and methods of use |
| WO2009090182A1 (en) | 2008-01-14 | 2009-07-23 | Santaris Pharma A/S | C4'-substituted - dna nucleotide gapmer oligonucleotides |
| DK2285819T3 (da) | 2008-04-04 | 2013-12-02 | Isis Pharmaceuticals Inc | Oligomere forbindelser omfattende neutralt bundne, terminale bicykliske nukleosider |
| EP2310021A4 (en) | 2008-07-10 | 2012-06-27 | Merck Sharp & Dohme | METHOD OF USE OF COMPOSITIONS WITH MIR-192 AND / OR MIR-215 FOR THE TREATMENT OF CANCER |
| EP2356129B1 (en) | 2008-09-24 | 2013-04-03 | Isis Pharmaceuticals, Inc. | Substituted alpha-l-bicyclic nucleosides |
| EP2177615A1 (en) | 2008-10-10 | 2010-04-21 | Fraunhofer-Gesellschaft zur Förderung der angewandten Forschung e.V. | Method for a genome wide identification of expression regulatory sequences and use of genes and molecules derived thereof for the diagnosis and therapy of metabolic and/or tumorous diseases |
| US20100249214A1 (en) * | 2009-02-11 | 2010-09-30 | Dicerna Pharmaceuticals | Multiplex dicer substrate rna interference molecules having joining sequences |
| KR20110110776A (ko) | 2008-12-18 | 2011-10-07 | 다이서나 파마수이티컬, 인크. | 유전자 발현의 특이적 억제를 위한 연장된 다이서 기질 제제 및 방법 |
| US9012421B2 (en) | 2009-08-06 | 2015-04-21 | Isis Pharmaceuticals, Inc. | Bicyclic cyclohexose nucleic acid analogs |
| JP5892938B2 (ja) | 2009-10-16 | 2016-03-30 | グラクソ グループ リミテッドGlaxo Group Limited | Hbvアンチセンス阻害剤 |
| JP2013126374A (ja) | 2010-03-04 | 2013-06-27 | Kyushu Univ | イソフラボン類の作用に関連する遺伝子 |
| US8846637B2 (en) | 2010-06-08 | 2014-09-30 | Isis Pharmaceuticals, Inc. | Substituted 2′-amino and 2′-thio-bicyclic nucleosides and oligomeric compounds prepared therefrom |
| SI2606134T1 (sl) | 2010-08-17 | 2019-08-30 | Sirna Therapeutics, Inc. | RNA-INTERFERENČNO POSREDOVANO ZAVIRANJE IZRAŽANJA GENA VIRUSA HEPATITISA B (HBV) Z UPORABO KRATKE INTERFERENČNE NUKLEINSKE KISLINE (siNA) |
| BR112013010525A2 (pt) | 2010-10-28 | 2016-08-02 | Benitec Biopharma Ltd | tratamento de hbv |
| EP3467109A1 (en) | 2011-02-08 | 2019-04-10 | Ionis Pharmaceuticals, Inc. | Oligomeric compounds comprising bicyclic nucleotides and uses thereof |
| KR102055331B1 (ko) | 2011-04-21 | 2019-12-12 | 아이오니스 파마수티컬즈, 인코포레이티드 | B형 간염 바이러스(hbv) 발현 조절 |
| EA202191537A1 (ru) | 2011-06-30 | 2022-01-31 | Эрроухэд Фармасьютикалс, Инк. | Композиции и способы ингибирования экспрессии генов вируса гепатита в |
| EP2742056B2 (en) | 2011-08-11 | 2020-06-10 | Ionis Pharmaceuticals, Inc. | Selective antisense compounds and uses thereof |
| EP3640332A1 (en) | 2011-08-29 | 2020-04-22 | Ionis Pharmaceuticals, Inc. | Oligomer-conjugate complexes and their use |
| CN104136451A (zh) | 2011-09-07 | 2014-11-05 | 玛瑞纳生物技术有限公司 | 具有构象限制的单体的核酸化合物的合成和用途 |
| WO2013113326A1 (en) | 2012-01-31 | 2013-08-08 | Curevac Gmbh | Pharmaceutical composition comprising a polymeric carrier cargo complex and at least one protein or peptide antigen |
| EP2850092B1 (en) | 2012-04-09 | 2017-03-01 | Ionis Pharmaceuticals, Inc. | Tricyclic nucleic acid analogs |
| CN104379765A (zh) * | 2012-04-10 | 2015-02-25 | 非营利性组织佛兰芒综合大学生物技术研究所 | 用于检测癌症中的微卫星不稳定性和测定与dna碱基切除修复途径抑制的合成致死性的新标记 |
| WO2013159109A1 (en) | 2012-04-20 | 2013-10-24 | Isis Pharmaceuticals, Inc. | Modulation of hepatitis b virus (hbv) expression |
| WO2013166264A2 (en) | 2012-05-02 | 2013-11-07 | University Of Georgia Research Foundation, Inc. | Methods for altering virus replication |
| ES2940887T3 (es) | 2012-07-13 | 2023-05-12 | Wave Life Sciences Ltd | Método de preparación de oligonucleótidos quirales |
| WO2014036429A1 (en) | 2012-08-31 | 2014-03-06 | Aptamir Therapeutics, Inc. | Mirna modulators of chronic visceral inflammation |
| WO2014076196A1 (en) | 2012-11-15 | 2014-05-22 | Santaris Pharma A/S | Anti apob antisense conjugate compounds |
| JP6580993B2 (ja) * | 2013-01-30 | 2019-09-25 | エフ.ホフマン−ラ ロシュ アーゲーF. Hoffmann−La Roche Aktiengesellschaft | Lnaオリゴヌクレオチド糖質コンジュゲート |
| HUE050394T2 (hu) | 2013-05-01 | 2020-11-30 | Ionis Pharmaceuticals Inc | Apolipoprotein(a) expressziójának módosítására szolgáló eljárások és készítmények |
| WO2014204723A1 (en) * | 2013-06-17 | 2014-12-24 | The Broad Institute Inc. | Oncogenic models based on delivery and use of the crispr-cas systems, vectors and compositions |
| HRP20200163T1 (hr) | 2013-06-27 | 2020-08-07 | Roche Innovation Center Copenhagen A/S | Antisens oligomeri i konjugati koji ciljno djeluju na pcsk9 |
| CA2922689A1 (en) | 2013-08-28 | 2015-03-05 | Caris Life Sciences Switzerland Holdings Gmbh | Oligonucleotide probes and uses thereof |
| EP2845607A1 (en) * | 2013-09-09 | 2015-03-11 | University of Vienna | Antisense oligonucleotides with improved pharmacokinetic properties |
| AU2015212903A1 (en) | 2014-01-30 | 2016-05-19 | F. Hoffmann-La Roche Ag | Novel dihydroquinolizinones for the treatment and prophylaxis of hepatitis B virus infection |
| US10358643B2 (en) | 2014-01-30 | 2019-07-23 | Hoffmann-La Roche, Inc. | Poly oligomer compound with biocleavable conjugates |
| CA2948080A1 (en) | 2014-05-13 | 2015-11-19 | F. Hoffmann-La Roche Ag | Novel dihydroquinolizinones for the treatment and prophylaxis of hepatitis b virus infection |
| GB201408623D0 (en) * | 2014-05-15 | 2014-07-02 | Santaris Pharma As | Oligomers and oligomer conjugates |
| TW201610169A (zh) | 2014-07-01 | 2016-03-16 | 泰瓦藥品工業有限公司 | 利用哺乳動物及人類細胞進行之格拉雷姆(glatiramer)乙酸鹽相關藥物產品之生物特徵化 |
| FR3026868B1 (fr) * | 2014-10-02 | 2019-08-16 | Dav | Dispositif et procede de commande pour vehicule automobile |
| BR112017006679A2 (pt) | 2014-10-02 | 2017-12-26 | Protiva Biotherapeutics Inc | moléculas, composição, partícula, composição farmacêutica, métodos para silenciar a expressão de um gene, usos de uma partícula, métodos para melhorar um ou mais sintomas, métodos para tratar uma infecção, usos de uma composição, método para inibir a replicação do vírus da hepatite d |
| US20170327524A1 (en) | 2014-10-10 | 2017-11-16 | Hoffmann-La Roche, Inc. | Galnac phosphoramidites, nucleic acid conjugates thereof and their use |
| US9637485B2 (en) | 2014-11-03 | 2017-05-02 | Hoffmann-La Roche Inc. | 6,7-dihydrobenzo[a]quinolizin-2-one derivatives for the treatment and prophylaxis of hepatitis B virus infection |
| EP3221329A1 (en) | 2014-11-19 | 2017-09-27 | Roche Innovation Center Copenhagen A/S | Lna gapmer oligonucleotides comprising chiral phosphorothioate linkages |
| EP3221452A4 (en) * | 2014-11-21 | 2018-11-21 | Caris Science, Inc. | Oligonucleotide probes and uses thereof |
| WO2016096938A1 (en) | 2014-12-16 | 2016-06-23 | Roche Innovation Center Copenhagen A/S | Chiral toxicity screening method |
| JP6506845B2 (ja) | 2014-12-30 | 2019-04-24 | エフ.ホフマン−ラ ロシュ アーゲーF. Hoffmann−La Roche Aktiengesellschaft | B型肝炎ウイルス感染症の治療及び予防のための新規テトラヒドロピリドピリミジン類及びテトラヒドロピリドピリジン類 |
| WO2016127002A1 (en) * | 2015-02-04 | 2016-08-11 | Bristol-Myers Squibb Company | Lna oligonucleotides with alternating flanks |
| WO2016128335A1 (en) | 2015-02-11 | 2016-08-18 | F. Hoffmann-La Roche Ag | Novel 2-oxo-6,7-dihydrobenzo[a]quinolizine-3-carboxylic acid derivatives for the treatment and prophylaxis of hepatitis b virus infection |
| JP6462155B2 (ja) | 2015-05-04 | 2019-01-30 | エフ.ホフマン−ラ ロシュ アーゲーF. Hoffmann−La Roche Aktiengesellschaft | B型肝炎ウイルス感染症の治療用のHBsAg(HBV表面抗原)及びHBV DNA産生の阻害剤としてのテトラヒドロピリドピリミジン類及びテトラヒドロピリドピリジン類 |
| CN108136206B (zh) | 2015-07-17 | 2021-10-15 | 阿克丘勒斯治疗公司 | 抗乙型肝炎病毒的组合物和药剂及其用途 |
| JP7336191B2 (ja) | 2015-08-07 | 2023-08-31 | アローヘッド ファーマシューティカルズ インコーポレイテッド | B型肝炎ウイルス感染に対するRNAi療法 |
| WO2017066712A2 (en) | 2015-10-16 | 2017-04-20 | The Children's Medical Center Corporation | Modulators of telomere disease |
| WO2017066796A2 (en) * | 2015-10-16 | 2017-04-20 | The Children's Medical Center Corporation | Modulators of telomere disease |
| MX2018010830A (es) * | 2016-03-14 | 2019-02-07 | Hoffmann La Roche | Oligonucleotidos para reducir la expresion de pd-l1. |
| JP6985288B2 (ja) | 2016-04-14 | 2021-12-22 | エフ.ホフマン−ラ ロシュ アーゲーF. Hoffmann−La Roche Aktiengesellschaft | トリチル−モノ−GalNAc化合物とその利用 |
| MA45496A (fr) | 2016-06-17 | 2019-04-24 | Hoffmann La Roche | Molécules d'acide nucléique pour la réduction de l'arnm de padd5 ou pad7 pour le traitement d'une infection par l'hépatite b |
| CN109311880B (zh) | 2016-06-29 | 2021-09-03 | 豪夫迈·罗氏有限公司 | 用于治疗和预防乙型肝炎病毒感染的新的四氢吡啶并嘧啶类化合物 |
| WO2018059718A1 (en) | 2016-09-30 | 2018-04-05 | Rijk Zwaan Zaadteelt En Zaadhandel B.V. | Peronospora resistance in spinacia oleracea |
| CL2020001638A1 (es) | 2017-10-16 | 2020-10-16 | Hoffmann La Roche | Molécula de ácidos nucleicos para la reducción del arnm de papd5 y papd7 en el tratamiento de la infección de la hepatitis b. (divisional solicitud 202000945) |
| RU2766360C2 (ru) | 2017-10-16 | 2022-03-15 | Ф. Хоффманн-Ля Рош Аг | МОЛЕКУЛЫ НУКЛЕИНОВЫХ КИСЛОТ ДЛЯ УМЕНЬШЕНИЯ УРОВНЯ мРНК PAPD5 ИЛИ PAPD7 ДЛЯ ЛЕЧЕНИЯ ИНФЕКЦИОННОГО ГЕПАТИТА В |
| CN111699007A (zh) * | 2018-01-29 | 2020-09-22 | 豪夫迈·罗氏有限公司 | 用于制备GalNAc寡核苷酸缀合物的方法 |
| CL2019000945A1 (es) | 2019-04-09 | 2019-07-12 | Energy Harvest As | Dispositivo de válvula rotatoria y sus métodos. |
-
2018
- 2018-10-16 RU RU2020115761A patent/RU2766360C2/ru active
- 2018-10-16 CN CN202311504811.0A patent/CN118048356A/zh active Pending
- 2018-10-16 IL IL272566A patent/IL272566B2/en unknown
- 2018-10-16 KR KR1020207005096A patent/KR20200030594A/ko not_active Ceased
- 2018-10-16 CR CR20200205A patent/CR20200205A/es unknown
- 2018-10-16 CR CR20210262A patent/CR20210262A/es unknown
- 2018-10-16 TW TW112131957A patent/TW202424191A/zh unknown
- 2018-10-16 WO PCT/EP2018/078136 patent/WO2019076842A1/en not_active Ceased
- 2018-10-16 UA UAA202002832A patent/UA126931C2/uk unknown
- 2018-10-16 CN CN201880080967.1A patent/CN111511914B/zh active Active
- 2018-10-16 KR KR1020227027132A patent/KR102585898B1/ko active Active
- 2018-10-16 US US16/162,279 patent/US10953034B2/en active Active
- 2018-10-16 IL IL310631A patent/IL310631A/en unknown
- 2018-10-16 CA CA3072314A patent/CA3072314A1/en active Pending
- 2018-10-16 KR KR1020237033451A patent/KR102769100B1/ko active Active
- 2018-10-16 KR KR1020207021922A patent/KR102431353B1/ko active Active
- 2018-10-16 TW TW107136344A patent/TWI762732B/zh active
- 2018-10-16 MX MX2020003464A patent/MX2020003464A/es unknown
- 2018-10-16 PE PE2020000398A patent/PE20200868A1/es unknown
- 2018-10-16 AU AU2018350693A patent/AU2018350693B2/en active Active
- 2018-10-16 KR KR1020257004579A patent/KR20250025042A/ko active Pending
- 2018-10-16 BR BR122020018622-4A patent/BR122020018622A2/pt unknown
- 2018-10-16 JP JP2020518675A patent/JP2021500013A/ja not_active Ceased
- 2018-10-16 MA MA052151A patent/MA52151A/fr unknown
- 2018-10-16 SG SG11202003461TA patent/SG11202003461TA/en unknown
- 2018-10-16 TW TW109139355A patent/TWI816066B/zh active
- 2018-10-16 EP EP18783511.1A patent/EP3645723A1/en active Pending
- 2018-10-16 BR BR112020007417-9A patent/BR112020007417A2/pt unknown
-
2019
- 2019-10-23 US US16/661,959 patent/US11484546B2/en active Active
-
2020
- 2020-04-08 CL CL2020000945A patent/CL2020000945A1/es unknown
- 2020-04-16 PH PH12020550436A patent/PH12020550436A1/en unknown
-
2021
- 2021-02-12 JP JP2021020438A patent/JP7348922B2/ja active Active
- 2021-05-21 AU AU2021203300A patent/AU2021203300B2/en active Active
-
2022
- 2022-07-19 US US17/813,576 patent/US12303525B2/en active Active
-
2023
- 2023-08-08 JP JP2023129333A patent/JP2023138687A/ja active Pending
-
2024
- 2024-03-22 AU AU2024201873A patent/AU2024201873A1/en active Pending
-
2025
- 2025-04-11 US US19/177,040 patent/US20250319114A1/en active Pending
Also Published As
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| KR102391812B1 (ko) | B형 간염 감염을 치료하기 위해 PAPD5 또는 PAPD7 mRNA를 감소시키기 위한 핵산 분자 | |
| AU2021203300B2 (en) | Nucleic acid molecule for reduction of PAPD5 and PAPD7 mRNA for treating hepatitis B infection | |
| AU2017276286B2 (en) | Modulation of huntingtin expression | |
| KR20220062517A (ko) | 결합 변형된 올리고머 화합물 및 이의 용도 | |
| KR102149483B1 (ko) | 예측변수 인자들을 이용하여 동정된 환자 부분 모집단에서 암 치료를 위한 마시티닙의 용도 | |
| KR20220012230A (ko) | 스플라이싱 및 번역을 조절하기 위한 방법 및 조성물 | |
| KR102585973B1 (ko) | 타우 발현을 조절하기 위한 올리고뉴클레오티드 | |
| TW202221014A (zh) | 用於減少app表現之化合物及方法 | |
| TW202241462A (zh) | 調節杭丁頓蛋白(huntingtin)之化合物及方法 | |
| US20040115641A1 (en) | Modulation of ROCK 1 expression | |
| KR20230005933A (ko) | Atxn1을 조정하는 화합물 및 방법 | |
| CN111344408A (zh) | 用于调控fndc3b表达的寡核苷酸 | |
| CN111556894A (zh) | 用于调控gsk3b表达的寡核苷酸 | |
| HK40033739A (en) | Oligonucleotides for modulating fndc3b expression |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A201 | Request for examination | ||
| PA0105 | International application |
Patent event date: 20200221 Patent event code: PA01051R01D Comment text: International Patent Application |
|
| PA0201 | Request for examination |
Patent event code: PA02012R01D Patent event date: 20200221 Comment text: Request for Examination of Application |
|
| PG1501 | Laying open of application | ||
| A107 | Divisional application of patent | ||
| PA0104 | Divisional application for international application |
Comment text: Divisional Application for International Patent Patent event code: PA01041R01D Patent event date: 20200728 |
|
| E902 | Notification of reason for refusal | ||
| PE0902 | Notice of grounds for rejection |
Comment text: Notification of reason for refusal Patent event date: 20201218 Patent event code: PE09021S01D |
|
| E601 | Decision to refuse application | ||
| PE0601 | Decision on rejection of patent |
Patent event date: 20210225 Comment text: Decision to Refuse Application Patent event code: PE06012S01D Patent event date: 20201218 Comment text: Notification of reason for refusal Patent event code: PE06011S01I |