KR20180127407A - 암 요법용 조작된 trail - Google Patents
암 요법용 조작된 trail Download PDFInfo
- Publication number
- KR20180127407A KR20180127407A KR1020187029745A KR20187029745A KR20180127407A KR 20180127407 A KR20180127407 A KR 20180127407A KR 1020187029745 A KR1020187029745 A KR 1020187029745A KR 20187029745 A KR20187029745 A KR 20187029745A KR 20180127407 A KR20180127407 A KR 20180127407A
- Authority
- KR
- South Korea
- Prior art keywords
- ser
- gly
- leu
- lys
- glu
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Withdrawn
Links
- 238000011275 oncology therapy Methods 0.000 title description 4
- 230000035772 mutation Effects 0.000 claims abstract description 72
- 102000046283 TNF-Related Apoptosis-Inducing Ligand Human genes 0.000 claims description 324
- 108090000765 processed proteins & peptides Proteins 0.000 claims description 303
- 102000004196 processed proteins & peptides Human genes 0.000 claims description 291
- 229920001184 polypeptide Polymers 0.000 claims description 289
- 239000000178 monomer Substances 0.000 claims description 153
- 230000004927 fusion Effects 0.000 claims description 98
- 108090000623 proteins and genes Proteins 0.000 claims description 89
- 125000000539 amino acid group Chemical group 0.000 claims description 82
- 102000004169 proteins and genes Human genes 0.000 claims description 82
- 235000018102 proteins Nutrition 0.000 claims description 75
- 235000001014 amino acid Nutrition 0.000 claims description 63
- 150000001413 amino acids Chemical class 0.000 claims description 61
- 229940024606 amino acid Drugs 0.000 claims description 54
- 238000006467 substitution reaction Methods 0.000 claims description 48
- 206010028980 Neoplasm Diseases 0.000 claims description 47
- 238000000034 method Methods 0.000 claims description 37
- 241000282414 Homo sapiens Species 0.000 claims description 36
- 210000002966 serum Anatomy 0.000 claims description 35
- 101000830565 Homo sapiens Tumor necrosis factor ligand superfamily member 10 Proteins 0.000 claims description 28
- 102000044949 human TNFSF10 Human genes 0.000 claims description 26
- 239000013638 trimer Substances 0.000 claims description 23
- 230000000087 stabilizing effect Effects 0.000 claims description 21
- 201000011510 cancer Diseases 0.000 claims description 18
- 238000002844 melting Methods 0.000 claims description 17
- 230000008018 melting Effects 0.000 claims description 17
- 108091006905 Human Serum Albumin Proteins 0.000 claims description 14
- 102000008100 Human Serum Albumin Human genes 0.000 claims description 14
- AGPKZVBTJJNPAG-WHFBIAKZSA-N L-isoleucine Chemical compound CC[C@H](C)[C@H](N)C(O)=O AGPKZVBTJJNPAG-WHFBIAKZSA-N 0.000 claims description 10
- 238000011534 incubation Methods 0.000 claims description 10
- 229960000310 isoleucine Drugs 0.000 claims description 10
- AGPKZVBTJJNPAG-UHFFFAOYSA-N isoleucine Natural products CCC(C)C(N)C(O)=O AGPKZVBTJJNPAG-UHFFFAOYSA-N 0.000 claims description 10
- 238000006471 dimerization reaction Methods 0.000 claims description 7
- KZSNJWFQEVHDMF-UHFFFAOYSA-N Valine Natural products CC(C)C(N)C(O)=O KZSNJWFQEVHDMF-UHFFFAOYSA-N 0.000 claims description 6
- 125000003630 glycyl group Chemical group [H]N([H])C([H])([H])C(*)=O 0.000 claims description 6
- 239000004474 valine Substances 0.000 claims description 6
- DHMQDGOQFOQNFH-UHFFFAOYSA-N Glycine Natural products NCC(O)=O DHMQDGOQFOQNFH-UHFFFAOYSA-N 0.000 claims description 5
- 239000004471 Glycine Substances 0.000 claims description 5
- QNAYBMKLOCPYGJ-REOHCLBHSA-N L-alanine Chemical compound C[C@H](N)C(O)=O QNAYBMKLOCPYGJ-REOHCLBHSA-N 0.000 claims description 5
- 235000004279 alanine Nutrition 0.000 claims description 5
- ROHFNLRQFUQHCH-YFKPBYRVSA-N L-leucine Chemical compound CC(C)C[C@H](N)C(O)=O ROHFNLRQFUQHCH-YFKPBYRVSA-N 0.000 claims description 4
- KZSNJWFQEVHDMF-BYPYZUCNSA-N L-valine Chemical compound CC(C)[C@H](N)C(O)=O KZSNJWFQEVHDMF-BYPYZUCNSA-N 0.000 claims description 4
- ROHFNLRQFUQHCH-UHFFFAOYSA-N Leucine Natural products CC(C)CC(N)C(O)=O ROHFNLRQFUQHCH-UHFFFAOYSA-N 0.000 claims description 4
- 230000000977 initiatory effect Effects 0.000 claims description 2
- 108700012411 TNFSF10 Proteins 0.000 claims 15
- 125000002987 valine group Chemical group [H]N([H])C([H])(C(*)=O)C([H])(C([H])([H])[H])C([H])([H])[H] 0.000 claims 2
- 101000873843 Homo sapiens Sorting and assembly machinery component 50 homolog Proteins 0.000 claims 1
- 102100035853 Sorting and assembly machinery component 50 homolog Human genes 0.000 claims 1
- 239000002246 antineoplastic agent Substances 0.000 abstract description 9
- 229940124597 therapeutic agent Drugs 0.000 abstract description 4
- 210000004027 cell Anatomy 0.000 description 160
- 125000003275 alpha amino acid group Chemical group 0.000 description 70
- 108010044374 isoleucyl-tyrosine Proteins 0.000 description 50
- 108010050475 glycyl-leucyl-tyrosine Proteins 0.000 description 44
- 108010025306 histidylleucine Proteins 0.000 description 42
- 230000000694 effects Effects 0.000 description 39
- 241000880493 Leptailurus serval Species 0.000 description 36
- 108010062166 Lys-Asn-Asp Proteins 0.000 description 36
- 108010028188 glycyl-histidyl-serine Proteins 0.000 description 35
- 108010072637 phenylalanyl-arginyl-phenylalanine Proteins 0.000 description 35
- 108010069117 seryl-lysyl-aspartic acid Proteins 0.000 description 34
- WQVFQXXBNHHPLX-ZKWXMUAHSA-N Ala-Ala-His Chemical compound C[C@H](N)C(=O)N[C@@H](C)C(=O)N[C@@H](Cc1cnc[nH]1)C(O)=O WQVFQXXBNHHPLX-ZKWXMUAHSA-N 0.000 description 33
- 108010001064 glycyl-glycyl-glycyl-glycine Proteins 0.000 description 33
- 108010084525 phenylalanyl-phenylalanyl-glycine Proteins 0.000 description 33
- 108010034529 leucyl-lysine Proteins 0.000 description 31
- 102100031940 Epithelial cell adhesion molecule Human genes 0.000 description 29
- 101000920667 Homo sapiens Epithelial cell adhesion molecule Proteins 0.000 description 29
- 108020004414 DNA Proteins 0.000 description 28
- YBKKLDBBPFIXBQ-MBLNEYKQSA-N Ile-Thr-Gly Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H]([C@@H](C)O)C(=O)NCC(=O)O)N YBKKLDBBPFIXBQ-MBLNEYKQSA-N 0.000 description 28
- XVHAUVJXBFGUPC-RPTUDFQQSA-N Thr-Tyr-Phe Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](CC1=CC=CC=C1)C(O)=O XVHAUVJXBFGUPC-RPTUDFQQSA-N 0.000 description 27
- 238000003556 assay Methods 0.000 description 27
- 230000014509 gene expression Effects 0.000 description 26
- 108010001271 arginyl-glutamyl-arginine Proteins 0.000 description 25
- 238000011282 treatment Methods 0.000 description 25
- 230000006870 function Effects 0.000 description 24
- OGMDXNFGPOPZTK-GUBZILKMSA-N Asn-Glu-His Chemical compound C1=C(NC=N1)C[C@@H](C(=O)O)NC(=O)[C@H](CCC(=O)O)NC(=O)[C@H](CC(=O)N)N OGMDXNFGPOPZTK-GUBZILKMSA-N 0.000 description 23
- VAZZOGXDUQSVQF-NUMRIWBASA-N Glu-Asn-Thr Chemical compound C[C@H]([C@@H](C(=O)O)NC(=O)[C@H](CC(=O)N)NC(=O)[C@H](CCC(=O)O)N)O VAZZOGXDUQSVQF-NUMRIWBASA-N 0.000 description 23
- KYNNSEJZFVCDIV-ZPFDUUQYSA-N Lys-Ile-Asn Chemical compound [H]N[C@@H](CCCCN)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CC(N)=O)C(O)=O KYNNSEJZFVCDIV-ZPFDUUQYSA-N 0.000 description 23
- INHMISZWLJZQGH-ULQDDVLXSA-N Phe-Leu-Val Chemical compound CC(C)[C@@H](C(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](N)CC1=CC=CC=C1 INHMISZWLJZQGH-ULQDDVLXSA-N 0.000 description 23
- KRXFXDCNKLANCP-CXTHYWKRSA-N Tyr-Tyr-Ile Chemical compound C([C@@H](C(=O)N[C@@H]([C@@H](C)CC)C(O)=O)NC(=O)[C@@H](N)CC=1C=CC(O)=CC=1)C1=CC=C(O)C=C1 KRXFXDCNKLANCP-CXTHYWKRSA-N 0.000 description 23
- 108010012581 phenylalanylglutamate Proteins 0.000 description 23
- MYCSPQIARXTUTP-SRVKXCTJSA-N Asn-Leu-His Chemical compound CC(C)C[C@@H](C(=O)N[C@@H](CC1=CN=CN1)C(=O)O)NC(=O)[C@H](CC(=O)N)N MYCSPQIARXTUTP-SRVKXCTJSA-N 0.000 description 22
- IVGJYOOGJLFKQE-AVGNSLFASA-N Glu-Leu-Lys Chemical compound CC(C)C[C@@H](C(=O)N[C@@H](CCCCN)C(=O)O)NC(=O)[C@H](CCC(=O)O)N IVGJYOOGJLFKQE-AVGNSLFASA-N 0.000 description 22
- HBJZFCIVFIBNSV-DCAQKATOSA-N Leu-Arg-Asn Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](CC(N)=O)C(O)=O HBJZFCIVFIBNSV-DCAQKATOSA-N 0.000 description 22
- KOSWSHVQIVTVQF-ZPFDUUQYSA-N Leu-Ile-Asp Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CC(O)=O)C(O)=O KOSWSHVQIVTVQF-ZPFDUUQYSA-N 0.000 description 22
- BYPMOIFBQPEWOH-CIUDSAMLSA-N Lys-Asn-Asp Chemical compound C(CCN)C[C@@H](C(=O)N[C@@H](CC(=O)N)C(=O)N[C@@H](CC(=O)O)C(=O)O)N BYPMOIFBQPEWOH-CIUDSAMLSA-N 0.000 description 22
- ZXEUFAVXODIPHC-GUBZILKMSA-N Lys-Glu-Asn Chemical compound NCCCC[C@H](N)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(N)=O)C(O)=O ZXEUFAVXODIPHC-GUBZILKMSA-N 0.000 description 22
- CUXJENOFJXOSOZ-BIIVOSGPSA-N Ser-Ser-Pro Chemical compound C1C[C@@H](N(C1)C(=O)[C@H](CO)NC(=O)[C@H](CO)N)C(=O)O CUXJENOFJXOSOZ-BIIVOSGPSA-N 0.000 description 22
- SDFUZKIAHWRUCS-QEJZJMRPSA-N Ser-Trp-Glu Chemical compound C1=CC=C2C(=C1)C(=CN2)C[C@@H](C(=O)N[C@@H](CCC(=O)O)C(=O)O)NC(=O)[C@H](CO)N SDFUZKIAHWRUCS-QEJZJMRPSA-N 0.000 description 22
- IEWKKXZRJLTIOV-AVGNSLFASA-N Tyr-Ser-Gln Chemical compound [H]N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](CO)C(=O)N[C@@H](CCC(N)=O)C(O)=O IEWKKXZRJLTIOV-AVGNSLFASA-N 0.000 description 22
- LOKCTEFSRHRXRJ-UHFFFAOYSA-I dipotassium trisodium dihydrogen phosphate hydrogen phosphate dichloride Chemical compound P(=O)(O)(O)[O-].[K+].P(=O)(O)([O-])[O-].[Na+].[Na+].[Cl-].[K+].[Cl-].[Na+] LOKCTEFSRHRXRJ-UHFFFAOYSA-I 0.000 description 22
- 230000001976 improved effect Effects 0.000 description 22
- 230000001965 increasing effect Effects 0.000 description 22
- 239000002953 phosphate buffered saline Substances 0.000 description 22
- 108010020532 tyrosyl-proline Proteins 0.000 description 22
- HKZAAJSTFUZYTO-LURJTMIESA-N (2s)-2-[[2-[[2-[[2-[(2-aminoacetyl)amino]acetyl]amino]acetyl]amino]acetyl]amino]-3-hydroxypropanoic acid Chemical compound NCC(=O)NCC(=O)NCC(=O)NCC(=O)N[C@@H](CO)C(O)=O HKZAAJSTFUZYTO-LURJTMIESA-N 0.000 description 21
- 108010002311 N-glycylglutamic acid Proteins 0.000 description 21
- YFNOUBWUIIJQHF-LPEHRKFASA-N Pro-Asp-Pro Chemical compound C1C[C@H](NC1)C(=O)N[C@@H](CC(=O)O)C(=O)N2CCC[C@@H]2C(=O)O YFNOUBWUIIJQHF-LPEHRKFASA-N 0.000 description 21
- HUPLKEHTTQBXSC-YJRXYDGGSA-N Thr-Ser-Tyr Chemical compound C[C@@H](O)[C@H](N)C(=O)N[C@@H](CO)C(=O)N[C@H](C(O)=O)CC1=CC=C(O)C=C1 HUPLKEHTTQBXSC-YJRXYDGGSA-N 0.000 description 21
- KNYHAWKHFQRYOX-PYJNHQTQSA-N Val-Ile-His Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CC1=CN=CN1)C(=O)O)NC(=O)[C@H](C(C)C)N KNYHAWKHFQRYOX-PYJNHQTQSA-N 0.000 description 21
- 108010062796 arginyllysine Proteins 0.000 description 21
- XKUKSGPZAADMRA-UHFFFAOYSA-N glycyl-glycyl-glycine Natural products NCC(=O)NCC(=O)NCC(O)=O XKUKSGPZAADMRA-UHFFFAOYSA-N 0.000 description 21
- 239000002609 medium Substances 0.000 description 21
- 102000005962 receptors Human genes 0.000 description 21
- XTZDZAXYPDISRR-MNXVOIDGSA-N Glu-Ile-Lys Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CCCCN)C(=O)O)NC(=O)[C@H](CCC(=O)O)N XTZDZAXYPDISRR-MNXVOIDGSA-N 0.000 description 20
- BABSVXFGKFLIGW-UWVGGRQHSA-N Leu-Gly-Arg Chemical compound CC(C)C[C@H](N)C(=O)NCC(=O)N[C@H](C(O)=O)CCCNC(N)=N BABSVXFGKFLIGW-UWVGGRQHSA-N 0.000 description 20
- IBSGMIPRBMPMHE-IHRRRGAJSA-N Leu-Met-Lys Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](CCCCN)C(O)=O IBSGMIPRBMPMHE-IHRRRGAJSA-N 0.000 description 20
- PBLLTSKBTAHDNA-KBPBESRZSA-N Lys-Gly-Phe Chemical compound [H]N[C@@H](CCCCN)C(=O)NCC(=O)N[C@@H](CC1=CC=CC=C1)C(O)=O PBLLTSKBTAHDNA-KBPBESRZSA-N 0.000 description 20
- JJHVFCUWLSKADD-ONGXEEELSA-N Phe-Gly-Ala Chemical compound [H]N[C@@H](CC1=CC=CC=C1)C(=O)NCC(=O)N[C@@H](C)C(O)=O JJHVFCUWLSKADD-ONGXEEELSA-N 0.000 description 20
- SIEBDTCABMZCLF-XGEHTFHBSA-N Ser-Val-Thr Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H]([C@@H](C)O)C(O)=O SIEBDTCABMZCLF-XGEHTFHBSA-N 0.000 description 20
- TWLMXDWFVNEFFK-FJXKBIBVSA-N Thr-Arg-Gly Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)NCC(O)=O TWLMXDWFVNEFFK-FJXKBIBVSA-N 0.000 description 20
- RYSNTWVRSLCAJZ-RYUDHWBXSA-N Tyr-Gln-Gly Chemical compound OC(=O)CNC(=O)[C@H](CCC(N)=O)NC(=O)[C@@H](N)CC1=CC=C(O)C=C1 RYSNTWVRSLCAJZ-RYUDHWBXSA-N 0.000 description 20
- 230000003833 cell viability Effects 0.000 description 20
- 238000003570 cell viability assay Methods 0.000 description 20
- 108010078144 glutaminyl-glycine Proteins 0.000 description 20
- 108010009298 lysylglutamic acid Proteins 0.000 description 20
- 108020003175 receptors Proteins 0.000 description 20
- JBDLMLZNDRLDIX-HJGDQZAQSA-N Asn-Thr-Leu Chemical compound [H]N[C@@H](CC(N)=O)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(C)C)C(O)=O JBDLMLZNDRLDIX-HJGDQZAQSA-N 0.000 description 19
- CKSBRMUOQDNPKZ-SRVKXCTJSA-N Lys-Gln-Met Chemical compound [H]N[C@@H](CCCCN)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCSC)C(O)=O CKSBRMUOQDNPKZ-SRVKXCTJSA-N 0.000 description 19
- YCCUXNNKXDGMAM-KKUMJFAQSA-N Phe-Leu-Ser Chemical compound [H]N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CO)C(O)=O YCCUXNNKXDGMAM-KKUMJFAQSA-N 0.000 description 19
- FIXILCYTSAUERA-FXQIFTODSA-N Ser-Ala-Arg Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](C)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O FIXILCYTSAUERA-FXQIFTODSA-N 0.000 description 19
- 230000006907 apoptotic process Effects 0.000 description 19
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 description 19
- 239000012634 fragment Substances 0.000 description 19
- UGXYFDQFLVCDFC-CIUDSAMLSA-N Asn-Ser-Lys Chemical compound NCCCC[C@@H](C(O)=O)NC(=O)[C@H](CO)NC(=O)[C@@H](N)CC(N)=O UGXYFDQFLVCDFC-CIUDSAMLSA-N 0.000 description 18
- YKLNMGJYMNPBCP-ACZMJKKPSA-N Glu-Asn-Asp Chemical compound C(CC(=O)O)[C@@H](C(=O)N[C@@H](CC(=O)N)C(=O)N[C@@H](CC(=O)O)C(=O)O)N YKLNMGJYMNPBCP-ACZMJKKPSA-N 0.000 description 18
- HAGKYCXGTRUUFI-RYUDHWBXSA-N Glu-Tyr-Gly Chemical compound C1=CC(=CC=C1C[C@@H](C(=O)NCC(=O)O)NC(=O)[C@H](CCC(=O)O)N)O HAGKYCXGTRUUFI-RYUDHWBXSA-N 0.000 description 18
- YYPFZVIXAVDHIK-IUCAKERBSA-N Gly-Glu-Leu Chemical compound CC(C)C[C@@H](C(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)CN YYPFZVIXAVDHIK-IUCAKERBSA-N 0.000 description 18
- HPCFRQWLTRDGHT-AJNGGQMLSA-N Ile-Leu-Leu Chemical compound CC[C@H](C)[C@H](N)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(C)C)C(O)=O HPCFRQWLTRDGHT-AJNGGQMLSA-N 0.000 description 18
- KQNDIKOYWZTZIX-FXQIFTODSA-N Ser-Ser-Arg Chemical compound OC[C@H](N)C(=O)N[C@@H](CO)C(=O)N[C@H](C(O)=O)CCCNC(N)=N KQNDIKOYWZTZIX-FXQIFTODSA-N 0.000 description 18
- CNNVVEPJTFOGHI-ACRUOGEOSA-N Tyr-Lys-Tyr Chemical compound [H]N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(O)=O CNNVVEPJTFOGHI-ACRUOGEOSA-N 0.000 description 18
- HTONZBWRYUKUKC-RCWTZXSCSA-N Val-Thr-Val Chemical compound CC(C)[C@H](N)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](C(C)C)C(O)=O HTONZBWRYUKUKC-RCWTZXSCSA-N 0.000 description 18
- 108010005233 alanylglutamic acid Proteins 0.000 description 18
- KOSRFJWDECSPRO-UHFFFAOYSA-N alpha-L-glutamyl-L-glutamic acid Natural products OC(=O)CCC(N)C(=O)NC(CCC(O)=O)C(O)=O KOSRFJWDECSPRO-UHFFFAOYSA-N 0.000 description 18
- 108010050848 glycylleucine Proteins 0.000 description 18
- 239000000203 mixture Substances 0.000 description 18
- 108010073969 valyllysine Proteins 0.000 description 18
- AMIQZQAAYGYKOP-FXQIFTODSA-N Arg-Ser-Asn Chemical compound [H]N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC(N)=O)C(O)=O AMIQZQAAYGYKOP-FXQIFTODSA-N 0.000 description 17
- FCKPEGOCSVZPNC-WHOFXGATSA-N Gly-Ile-Phe Chemical compound NCC(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@H](C(O)=O)CC1=CC=CC=C1 FCKPEGOCSVZPNC-WHOFXGATSA-N 0.000 description 17
- HJARVELKOSZUEW-YUMQZZPRSA-N Gly-Pro-Gln Chemical compound [H]NCC(=O)N1CCC[C@H]1C(=O)N[C@@H](CCC(N)=O)C(O)=O HJARVELKOSZUEW-YUMQZZPRSA-N 0.000 description 17
- HKCCVDWHHTVVPN-CIUDSAMLSA-N Lys-Asp-Ala Chemical compound [H]N[C@@H](CCCCN)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](C)C(O)=O HKCCVDWHHTVVPN-CIUDSAMLSA-N 0.000 description 17
- DRINJBAHUGXNFC-DCAQKATOSA-N Met-Asp-His Chemical compound [H]N[C@@H](CCSC)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CC1=CNC=N1)C(O)=O DRINJBAHUGXNFC-DCAQKATOSA-N 0.000 description 17
- 241000699666 Mus <mouse, genus> Species 0.000 description 17
- SGAOHNPSEPVAFP-ZDLURKLDSA-N Thr-Ser-Gly Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CO)C(=O)NCC(O)=O SGAOHNPSEPVAFP-ZDLURKLDSA-N 0.000 description 17
- 108010055341 glutamyl-glutamic acid Proteins 0.000 description 17
- 108010061238 threonyl-glycine Proteins 0.000 description 17
- QAODJPUKWNNNRP-DCAQKATOSA-N Arg-Glu-Arg Chemical compound NC(N)=NCCC[C@H](N)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCCN=C(N)N)C(O)=O QAODJPUKWNNNRP-DCAQKATOSA-N 0.000 description 16
- KYQJHBWHRASMKG-ZLUOBGJFSA-N Asn-Ser-Cys Chemical compound NC(=O)C[C@H](N)C(=O)N[C@@H](CO)C(=O)N[C@@H](CS)C(O)=O KYQJHBWHRASMKG-ZLUOBGJFSA-N 0.000 description 16
- XEDQMTWEYFBOIK-ACZMJKKPSA-N Asp-Ala-Glu Chemical compound [H]N[C@@H](CC(O)=O)C(=O)N[C@@H](C)C(=O)N[C@@H](CCC(O)=O)C(O)=O XEDQMTWEYFBOIK-ACZMJKKPSA-N 0.000 description 16
- CRNKLABLTICXDV-GUBZILKMSA-N Asp-His-Glu Chemical compound C1=C(NC=N1)C[C@@H](C(=O)N[C@@H](CCC(=O)O)C(=O)O)NC(=O)[C@H](CC(=O)O)N CRNKLABLTICXDV-GUBZILKMSA-N 0.000 description 16
- 102000004091 Caspase-8 Human genes 0.000 description 16
- 108090000538 Caspase-8 Proteins 0.000 description 16
- XKPACHRGOWQHFH-IRIUXVKKSA-N Gln-Thr-Tyr Chemical compound [H]N[C@@H](CCC(N)=O)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(O)=O XKPACHRGOWQHFH-IRIUXVKKSA-N 0.000 description 16
- CGUYGMFQZCYJSG-DCAQKATOSA-N Met-Lys-Ser Chemical compound [H]N[C@@H](CCSC)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CO)C(O)=O CGUYGMFQZCYJSG-DCAQKATOSA-N 0.000 description 16
- UDCHKDYNMRJYMI-QEJZJMRPSA-N Trp-Glu-Ser Chemical compound [H]N[C@@H](CC1=CNC2=C1C=CC=C2)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CO)C(O)=O UDCHKDYNMRJYMI-QEJZJMRPSA-N 0.000 description 16
- WSMVEHPVOYXPAQ-XIRDDKMYSA-N Trp-Ser-Lys Chemical compound C1=CC=C2C(=C1)C(=CN2)C[C@@H](C(=O)N[C@@H](CO)C(=O)N[C@@H](CCCCN)C(=O)O)N WSMVEHPVOYXPAQ-XIRDDKMYSA-N 0.000 description 16
- YMUQBRQQCPQEQN-CXTHYWKRSA-N Tyr-Ile-Tyr Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CC1=CC=C(C=C1)O)C(=O)O)NC(=O)[C@H](CC2=CC=C(C=C2)O)N YMUQBRQQCPQEQN-CXTHYWKRSA-N 0.000 description 16
- AAOPYWQQBXHINJ-DZKIICNBSA-N Val-Gln-Tyr Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CCC(=O)N)C(=O)N[C@@H](CC1=CC=C(C=C1)O)C(=O)O)N AAOPYWQQBXHINJ-DZKIICNBSA-N 0.000 description 16
- PEEYDECOOVQKRZ-DLOVCJGASA-N Ala-Ser-Phe Chemical compound [H]N[C@@H](C)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC1=CC=CC=C1)C(O)=O PEEYDECOOVQKRZ-DLOVCJGASA-N 0.000 description 15
- OFIYLHVAAJYRBC-HJWJTTGWSA-N Arg-Ile-Phe Chemical compound CC[C@H](C)[C@H](NC(=O)[C@@H](N)CCCNC(N)=N)C(=O)N[C@@H](Cc1ccccc1)C(O)=O OFIYLHVAAJYRBC-HJWJTTGWSA-N 0.000 description 15
- VEAIMHJZTIDCIH-KKUMJFAQSA-N Arg-Phe-Gln Chemical compound [H]N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H](CCC(N)=O)C(O)=O VEAIMHJZTIDCIH-KKUMJFAQSA-N 0.000 description 15
- OLGCWMNDJTWQAG-GUBZILKMSA-N Asn-Glu-Lys Chemical compound NCCCC[C@@H](C(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@@H](N)CC(N)=O OLGCWMNDJTWQAG-GUBZILKMSA-N 0.000 description 15
- SPJRFUJMDJGDRO-UBHSHLNASA-N Cys-Trp-Ser Chemical compound C1=CC=C2C(C[C@H](NC(=O)[C@H](CS)N)C(=O)N[C@@H](CO)C(O)=O)=CNC2=C1 SPJRFUJMDJGDRO-UBHSHLNASA-N 0.000 description 15
- GJBUAAAIZSRCDC-GVXVVHGQSA-N Glu-Leu-Val Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C(C)C)C(O)=O GJBUAAAIZSRCDC-GVXVVHGQSA-N 0.000 description 15
- OQXDUSZKISQQSS-GUBZILKMSA-N Glu-Lys-Ala Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](C)C(O)=O OQXDUSZKISQQSS-GUBZILKMSA-N 0.000 description 15
- OCJRHJZKGGSPRW-IUCAKERBSA-N Glu-Lys-Gly Chemical compound NCCCC[C@@H](C(=O)NCC(O)=O)NC(=O)[C@@H](N)CCC(O)=O OCJRHJZKGGSPRW-IUCAKERBSA-N 0.000 description 15
- GWCRIHNSVMOBEQ-BQBZGAKWSA-N Gly-Arg-Ser Chemical compound [H]NCC(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CO)C(O)=O GWCRIHNSVMOBEQ-BQBZGAKWSA-N 0.000 description 15
- YKLOMBNBQUTJDT-HVTMNAMFSA-N Ile-His-Glu Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CC1=CN=CN1)C(=O)N[C@@H](CCC(=O)O)C(=O)O)N YKLOMBNBQUTJDT-HVTMNAMFSA-N 0.000 description 15
- XQLGNKLSPYCRMZ-HJWJTTGWSA-N Ile-Phe-Val Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H](C(C)C)C(=O)O)N XQLGNKLSPYCRMZ-HJWJTTGWSA-N 0.000 description 15
- VUBIPAHVHMZHCM-KKUMJFAQSA-N Leu-Tyr-Ser Chemical compound CC(C)C[C@H](N)C(=O)N[C@H](C(=O)N[C@@H](CO)C(O)=O)CC1=CC=C(O)C=C1 VUBIPAHVHMZHCM-KKUMJFAQSA-N 0.000 description 15
- QYOXSYXPHUHOJR-GUBZILKMSA-N Lys-Asn-Glu Chemical compound [H]N[C@@H](CCCCN)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CCC(O)=O)C(O)=O QYOXSYXPHUHOJR-GUBZILKMSA-N 0.000 description 15
- 241000699670 Mus sp. Species 0.000 description 15
- UNLYPPYNDXHGDG-IHRRRGAJSA-N Phe-Gln-Glu Chemical compound OC(=O)CC[C@@H](C(O)=O)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@@H](N)CC1=CC=CC=C1 UNLYPPYNDXHGDG-IHRRRGAJSA-N 0.000 description 15
- HDBOEVPDIDDEPC-CIUDSAMLSA-N Ser-Lys-Asn Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(N)=O)C(O)=O HDBOEVPDIDDEPC-CIUDSAMLSA-N 0.000 description 15
- KCPFDGNYAMKZQP-KBPBESRZSA-N Tyr-Gly-Leu Chemical compound [H]N[C@@H](CC1=CC=C(O)C=C1)C(=O)NCC(=O)N[C@@H](CC(C)C)C(O)=O KCPFDGNYAMKZQP-KBPBESRZSA-N 0.000 description 15
- IGXLNVIYDYONFB-UFYCRDLUSA-N Tyr-Phe-Arg Chemical compound C([C@H](N)C(=O)N[C@@H](CC=1C=CC=CC=1)C(=O)N[C@@H](CCCN=C(N)N)C(O)=O)C1=CC=C(O)C=C1 IGXLNVIYDYONFB-UFYCRDLUSA-N 0.000 description 15
- QPOUERMDWKKZEG-HJPIBITLSA-N Tyr-Ser-Ile Chemical compound CC[C@H](C)[C@@H](C(O)=O)NC(=O)[C@H](CO)NC(=O)[C@@H](N)CC1=CC=C(O)C=C1 QPOUERMDWKKZEG-HJPIBITLSA-N 0.000 description 15
- 201000010099 disease Diseases 0.000 description 15
- 108010049041 glutamylalanine Proteins 0.000 description 15
- 108010080629 tryptophan-leucine Proteins 0.000 description 15
- MXOODARRORARSU-ACZMJKKPSA-N Glu-Ala-Ser Chemical compound C[C@@H](C(=O)N[C@@H](CO)C(=O)O)NC(=O)[C@H](CCC(=O)O)N MXOODARRORARSU-ACZMJKKPSA-N 0.000 description 14
- LPCKHUXOGVNZRS-YUMQZZPRSA-N Gly-His-Ser Chemical compound [H]NCC(=O)N[C@@H](CC1=CNC=N1)C(=O)N[C@@H](CO)C(O)=O LPCKHUXOGVNZRS-YUMQZZPRSA-N 0.000 description 14
- JBCLFWXMTIKCCB-UHFFFAOYSA-N H-Gly-Phe-OH Natural products NCC(=O)NC(C(O)=O)CC1=CC=CC=C1 JBCLFWXMTIKCCB-UHFFFAOYSA-N 0.000 description 14
- 239000003814 drug Substances 0.000 description 14
- 230000004048 modification Effects 0.000 description 14
- 238000012986 modification Methods 0.000 description 14
- MVRGBQGZSDJBSM-GMOBBJLQSA-N Asp-Pro-Ile Chemical compound CC[C@H](C)[C@@H](C(=O)O)NC(=O)[C@@H]1CCCN1C(=O)[C@H](CC(=O)O)N MVRGBQGZSDJBSM-GMOBBJLQSA-N 0.000 description 13
- AKDOUBMVLRCHBD-SIUGBPQLSA-N Gln-Tyr-Ile Chemical compound [H]N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H]([C@@H](C)CC)C(O)=O AKDOUBMVLRCHBD-SIUGBPQLSA-N 0.000 description 13
- AIQWYVFNBNNOLU-RHYQMDGZSA-N Leu-Thr-Val Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](C(C)C)C(O)=O AIQWYVFNBNNOLU-RHYQMDGZSA-N 0.000 description 13
- YQFZRHYZLARWDY-IHRRRGAJSA-N Leu-Val-Lys Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H](C(C)C)C(=O)N[C@H](C(O)=O)CCCCN YQFZRHYZLARWDY-IHRRRGAJSA-N 0.000 description 13
- 230000004913 activation Effects 0.000 description 13
- 108010011559 alanylphenylalanine Proteins 0.000 description 13
- 230000010056 antibody-dependent cellular cytotoxicity Effects 0.000 description 13
- 108010064235 lysylglycine Proteins 0.000 description 13
- 108010017391 lysylvaline Proteins 0.000 description 13
- NONSEUUPKITYQT-BQBZGAKWSA-N Arg-Asn-Gly Chemical compound C(C[C@@H](C(=O)N[C@@H](CC(=O)N)C(=O)NCC(=O)O)N)CN=C(N)N NONSEUUPKITYQT-BQBZGAKWSA-N 0.000 description 12
- XMPXVJIDADUOQB-RCOVLWMOSA-N Gly-Gly-Ile Chemical compound CC[C@H](C)[C@@H](C([O-])=O)NC(=O)CNC(=O)C[NH3+] XMPXVJIDADUOQB-RCOVLWMOSA-N 0.000 description 12
- CCHSQWLCOOZREA-GMOBBJLQSA-N Ile-Asp-Met Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CC(=O)O)C(=O)N[C@@H](CCSC)C(=O)O)N CCHSQWLCOOZREA-GMOBBJLQSA-N 0.000 description 12
- CSFVADKICPDRRF-KKUMJFAQSA-N Leu-His-Leu Chemical compound CC(C)C[C@H]([NH3+])C(=O)N[C@H](C(=O)N[C@@H](CC(C)C)C([O-])=O)CC1=CN=CN1 CSFVADKICPDRRF-KKUMJFAQSA-N 0.000 description 12
- KCXUCYYZNZFGLL-SRVKXCTJSA-N Lys-Ala-Leu Chemical compound [H]N[C@@H](CCCCN)C(=O)N[C@@H](C)C(=O)N[C@@H](CC(C)C)C(O)=O KCXUCYYZNZFGLL-SRVKXCTJSA-N 0.000 description 12
- KJJROSNFBRWPHS-JYJNAYRXSA-N Phe-Glu-Leu Chemical compound [H]N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(C)C)C(O)=O KJJROSNFBRWPHS-JYJNAYRXSA-N 0.000 description 12
- IXCHOHLPHNGFTJ-YUMQZZPRSA-N Ser-Gly-His Chemical compound C1=C(NC=N1)C[C@@H](C(=O)O)NC(=O)CNC(=O)[C@H](CO)N IXCHOHLPHNGFTJ-YUMQZZPRSA-N 0.000 description 12
- JLKWJWPDXPKKHI-FXQIFTODSA-N Ser-Pro-Asn Chemical compound C1C[C@H](N(C1)C(=O)[C@H](CO)N)C(=O)N[C@@H](CC(=O)N)C(=O)O JLKWJWPDXPKKHI-FXQIFTODSA-N 0.000 description 12
- VGQVAVQWKJLIRM-FXQIFTODSA-N Ser-Ser-Val Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CO)C(=O)N[C@@H](C(C)C)C(O)=O VGQVAVQWKJLIRM-FXQIFTODSA-N 0.000 description 12
- YOOAQCZYZHGUAZ-KATARQTJSA-N Thr-Leu-Ser Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CO)C(O)=O YOOAQCZYZHGUAZ-KATARQTJSA-N 0.000 description 12
- 239000000427 antigen Substances 0.000 description 12
- 102000036639 antigens Human genes 0.000 description 12
- 108091007433 antigens Proteins 0.000 description 12
- 238000004091 panning Methods 0.000 description 12
- 108010026333 seryl-proline Proteins 0.000 description 12
- DVWVZSJAYIJZFI-FXQIFTODSA-N Ala-Arg-Asn Chemical compound [H]N[C@@H](C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(N)=O)C(O)=O DVWVZSJAYIJZFI-FXQIFTODSA-N 0.000 description 11
- WVCJSDCHTUTONA-FXQIFTODSA-N Asn-Asp-Arg Chemical compound [H]N[C@@H](CC(N)=O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O WVCJSDCHTUTONA-FXQIFTODSA-N 0.000 description 11
- RSMZEHCMIOKNMW-GSSVUCPTSA-N Asp-Thr-Thr Chemical compound [H]N[C@@H](CC(O)=O)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H]([C@@H](C)O)C(O)=O RSMZEHCMIOKNMW-GSSVUCPTSA-N 0.000 description 11
- SOEGEPHNZOISMT-BYPYZUCNSA-N Gly-Ser-Gly Chemical compound NCC(=O)N[C@@H](CO)C(=O)NCC(O)=O SOEGEPHNZOISMT-BYPYZUCNSA-N 0.000 description 11
- ZUWSVOYKBCHLRR-MGHWNKPDSA-N Ile-Tyr-Lys Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CC1=CC=C(C=C1)O)C(=O)N[C@@H](CCCCN)C(=O)O)N ZUWSVOYKBCHLRR-MGHWNKPDSA-N 0.000 description 11
- UBZGNBKMIJHOHL-BZSNNMDCSA-N Leu-Leu-Phe Chemical compound CC(C)C[C@H]([NH3+])C(=O)N[C@@H](CC(C)C)C(=O)N[C@H](C([O-])=O)CC1=CC=CC=C1 UBZGNBKMIJHOHL-BZSNNMDCSA-N 0.000 description 11
- FOBUGKUBUJOWAD-IHPCNDPISA-N Leu-Leu-Trp Chemical compound C1=CC=C2C(C[C@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](N)CC(C)C)C(O)=O)=CNC2=C1 FOBUGKUBUJOWAD-IHPCNDPISA-N 0.000 description 11
- KZZCOWMDDXDKSS-CIUDSAMLSA-N Leu-Ser-Asn Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC(N)=O)C(O)=O KZZCOWMDDXDKSS-CIUDSAMLSA-N 0.000 description 11
- SXWQMBGNFXAGAT-FJXKBIBVSA-N Met-Gly-Thr Chemical compound CSCC[C@H](N)C(=O)NCC(=O)N[C@@H]([C@@H](C)O)C(O)=O SXWQMBGNFXAGAT-FJXKBIBVSA-N 0.000 description 11
- CNFMPVYIVQUJOO-NHCYSSNCSA-N Met-Val-Gln Chemical compound CSCC[C@H](N)C(=O)N[C@@H](C(C)C)C(=O)N[C@H](C(O)=O)CCC(N)=O CNFMPVYIVQUJOO-NHCYSSNCSA-N 0.000 description 11
- AJLVKXCNXIJHDV-CIUDSAMLSA-N Pro-Ala-Gln Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H](C)C(=O)N[C@@H](CCC(N)=O)C(O)=O AJLVKXCNXIJHDV-CIUDSAMLSA-N 0.000 description 11
- 210000001744 T-lymphocyte Anatomy 0.000 description 11
- BDGBHYCAZJPLHX-HJGDQZAQSA-N Thr-Lys-Asn Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(N)=O)C(O)=O BDGBHYCAZJPLHX-HJGDQZAQSA-N 0.000 description 11
- 238000000338 in vitro Methods 0.000 description 11
- QWTLUPDHBKBULE-UHFFFAOYSA-N 2-[[2-[[2-[[2-[[2-[[2-[[2-[[2-[[2-[(2-aminoacetyl)amino]acetyl]amino]acetyl]amino]acetyl]amino]acetyl]amino]acetyl]amino]acetyl]amino]acetyl]amino]acetyl]amino]acetic acid Chemical compound NCC(=O)NCC(=O)NCC(=O)NCC(=O)NCC(=O)NCC(=O)NCC(=O)NCC(=O)NCC(=O)NCC(O)=O QWTLUPDHBKBULE-UHFFFAOYSA-N 0.000 description 10
- MNZHHDPWDWQJCQ-YUMQZZPRSA-N Ala-Leu-Gly Chemical compound C[C@H](N)C(=O)N[C@@H](CC(C)C)C(=O)NCC(O)=O MNZHHDPWDWQJCQ-YUMQZZPRSA-N 0.000 description 10
- HMQDRBKQMLRCCG-GMOBBJLQSA-N Asp-Arg-Ile Chemical compound [H]N[C@@H](CC(O)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H]([C@@H](C)CC)C(O)=O HMQDRBKQMLRCCG-GMOBBJLQSA-N 0.000 description 10
- OSCLNNWLKKIQJM-WDSKDSINSA-N Gln-Ser-Gly Chemical compound [H]N[C@@H](CCC(N)=O)C(=O)N[C@@H](CO)C(=O)NCC(O)=O OSCLNNWLKKIQJM-WDSKDSINSA-N 0.000 description 10
- AUTNXSQEVVHSJK-YVNDNENWSA-N Glu-Glu-Ile Chemical compound CC[C@H](C)[C@@H](C(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@@H](N)CCC(O)=O AUTNXSQEVVHSJK-YVNDNENWSA-N 0.000 description 10
- WCORRBXVISTKQL-WHFBIAKZSA-N Gly-Ser-Ser Chemical compound NCC(=O)N[C@@H](CO)C(=O)N[C@@H](CO)C(O)=O WCORRBXVISTKQL-WHFBIAKZSA-N 0.000 description 10
- HIAHVKLTHNOENC-HGNGGELXSA-N His-Glu-Ala Chemical compound [H]N[C@@H](CC1=CNC=N1)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](C)C(O)=O HIAHVKLTHNOENC-HGNGGELXSA-N 0.000 description 10
- IAYPZSHNZQHQNO-KKUMJFAQSA-N His-Ser-Phe Chemical compound C1=CC=C(C=C1)C[C@@H](C(=O)O)NC(=O)[C@H](CO)NC(=O)[C@H](CC2=CN=CN2)N IAYPZSHNZQHQNO-KKUMJFAQSA-N 0.000 description 10
- GNXGAVNTVNOCLL-SIUGBPQLSA-N Ile-Tyr-Gln Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CC1=CC=C(C=C1)O)C(=O)N[C@@H](CCC(=O)N)C(=O)O)N GNXGAVNTVNOCLL-SIUGBPQLSA-N 0.000 description 10
- GPICTNQYKHHHTH-GUBZILKMSA-N Leu-Gln-Ser Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CO)C(O)=O GPICTNQYKHHHTH-GUBZILKMSA-N 0.000 description 10
- PPNCMJARTHYNEC-MEYUZBJRSA-N Lys-Tyr-Thr Chemical compound NCCCC[C@H](N)C(=O)N[C@H](C(=O)N[C@@H]([C@H](O)C)C(O)=O)CC1=CC=C(O)C=C1 PPNCMJARTHYNEC-MEYUZBJRSA-N 0.000 description 10
- 101100531027 Oryza sativa subsp. japonica RPS3A gene Proteins 0.000 description 10
- IWZRODDWOSIXPZ-IRXDYDNUSA-N Phe-Phe-Gly Chemical compound C([C@H](N)C(=O)N[C@@H](CC=1C=CC=CC=1)C(=O)NCC(O)=O)C1=CC=CC=C1 IWZRODDWOSIXPZ-IRXDYDNUSA-N 0.000 description 10
- KIQUCMUULDXTAZ-HJOGWXRNSA-N Phe-Tyr-Tyr Chemical compound N[C@@H](Cc1ccccc1)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1ccc(O)cc1)C(O)=O KIQUCMUULDXTAZ-HJOGWXRNSA-N 0.000 description 10
- KJMOINFQVCCSDX-XKBZYTNZSA-N Ser-Gln-Thr Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H]([C@@H](C)O)C(O)=O KJMOINFQVCCSDX-XKBZYTNZSA-N 0.000 description 10
- UPLYXVPQLJVWMM-KKUMJFAQSA-N Ser-Phe-Leu Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H](CC(C)C)C(O)=O UPLYXVPQLJVWMM-KKUMJFAQSA-N 0.000 description 10
- PCJLFYBAQZQOFE-KATARQTJSA-N Ser-Thr-Lys Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](CCCCN)C(=O)O)NC(=O)[C@H](CO)N)O PCJLFYBAQZQOFE-KATARQTJSA-N 0.000 description 10
- NZYNRRGJJVSSTJ-GUBZILKMSA-N Val-Ser-Val Chemical compound CC(C)[C@H](N)C(=O)N[C@@H](CO)C(=O)N[C@@H](C(C)C)C(O)=O NZYNRRGJJVSSTJ-GUBZILKMSA-N 0.000 description 10
- 230000030833 cell death Effects 0.000 description 10
- 108010025198 decaglycine Proteins 0.000 description 10
- 229940079593 drug Drugs 0.000 description 10
- 108010063718 gamma-glutamylaspartic acid Proteins 0.000 description 10
- 239000000499 gel Substances 0.000 description 10
- VPZXBVLAVMBEQI-UHFFFAOYSA-N glycyl-DL-alpha-alanine Natural products OC(=O)C(C)NC(=O)CN VPZXBVLAVMBEQI-UHFFFAOYSA-N 0.000 description 10
- 239000003446 ligand Substances 0.000 description 10
- MXHCPCSDRGLRER-UHFFFAOYSA-N pentaglycine Chemical compound NCC(=O)NCC(=O)NCC(=O)NCC(=O)NCC(O)=O MXHCPCSDRGLRER-UHFFFAOYSA-N 0.000 description 10
- 230000002829 reductive effect Effects 0.000 description 10
- UCSJYZPVAKXKNQ-HZYVHMACSA-N streptomycin Chemical compound CN[C@H]1[C@H](O)[C@@H](O)[C@H](CO)O[C@H]1O[C@@H]1[C@](C=O)(O)[C@H](C)O[C@H]1O[C@@H]1[C@@H](NC(N)=N)[C@H](O)[C@@H](NC(N)=N)[C@H](O)[C@H]1O UCSJYZPVAKXKNQ-HZYVHMACSA-N 0.000 description 10
- WXERCAHAIKMTKX-ZLUOBGJFSA-N Ala-Asp-Asp Chemical compound [H]N[C@@H](C)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CC(O)=O)C(O)=O WXERCAHAIKMTKX-ZLUOBGJFSA-N 0.000 description 9
- IIABBYGHLYWVOS-FXQIFTODSA-N Arg-Asn-Ser Chemical compound [H]N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CO)C(O)=O IIABBYGHLYWVOS-FXQIFTODSA-N 0.000 description 9
- CZUHPNLXLWMYMG-UBHSHLNASA-N Arg-Phe-Ala Chemical compound NC(N)=NCCC[C@H](N)C(=O)N[C@H](C(=O)N[C@@H](C)C(O)=O)CC1=CC=CC=C1 CZUHPNLXLWMYMG-UBHSHLNASA-N 0.000 description 9
- DAPLJWATMAXPPZ-CIUDSAMLSA-N Asn-Asn-Leu Chemical compound CC(C)C[C@@H](C(O)=O)NC(=O)[C@H](CC(N)=O)NC(=O)[C@@H](N)CC(N)=O DAPLJWATMAXPPZ-CIUDSAMLSA-N 0.000 description 9
- YQPSDMUGFKJZHR-QRTARXTBSA-N Asn-Trp-Val Chemical compound CC(C)[C@@H](C(=O)O)NC(=O)[C@H](CC1=CNC2=CC=CC=C21)NC(=O)[C@H](CC(=O)N)N YQPSDMUGFKJZHR-QRTARXTBSA-N 0.000 description 9
- QNIACYURSSCLRP-GUBZILKMSA-N Asp-Lys-Gln Chemical compound [H]N[C@@H](CC(O)=O)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCC(N)=O)C(O)=O QNIACYURSSCLRP-GUBZILKMSA-N 0.000 description 9
- NVFSJIXJZCDICF-SRVKXCTJSA-N Asp-Lys-Lys Chemical compound C(CCN)C[C@@H](C(=O)N[C@@H](CCCCN)C(=O)O)NC(=O)[C@H](CC(=O)O)N NVFSJIXJZCDICF-SRVKXCTJSA-N 0.000 description 9
- MNQMTYSEKZHIDF-GCJQMDKQSA-N Asp-Thr-Ala Chemical compound [H]N[C@@H](CC(O)=O)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](C)C(O)=O MNQMTYSEKZHIDF-GCJQMDKQSA-N 0.000 description 9
- SQIARYGNVQWOSB-BZSNNMDCSA-N Asp-Tyr-Phe Chemical compound [H]N[C@@H](CC(O)=O)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](CC1=CC=CC=C1)C(O)=O SQIARYGNVQWOSB-BZSNNMDCSA-N 0.000 description 9
- AMRLSQGGERHDHJ-FXQIFTODSA-N Cys-Ala-Arg Chemical compound [H]N[C@@H](CS)C(=O)N[C@@H](C)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O AMRLSQGGERHDHJ-FXQIFTODSA-N 0.000 description 9
- OZHXXYOHPLLLMI-CIUDSAMLSA-N Cys-Lys-Ala Chemical compound [H]N[C@@H](CS)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](C)C(O)=O OZHXXYOHPLLLMI-CIUDSAMLSA-N 0.000 description 9
- LGIKBBLQVSWUGK-DCAQKATOSA-N Gln-Leu-Gln Chemical compound [H]N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(N)=O)C(O)=O LGIKBBLQVSWUGK-DCAQKATOSA-N 0.000 description 9
- RWCBJYUPAUTWJD-NHCYSSNCSA-N Gln-Met-Val Chemical compound [H]N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](C(C)C)C(O)=O RWCBJYUPAUTWJD-NHCYSSNCSA-N 0.000 description 9
- JWNZHMSRZXXGTM-XKBZYTNZSA-N Glu-Ser-Thr Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H]([C@@H](C)O)C(O)=O JWNZHMSRZXXGTM-XKBZYTNZSA-N 0.000 description 9
- MXJYXYDREQWUMS-XKBZYTNZSA-N Glu-Thr-Ser Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CO)C(O)=O MXJYXYDREQWUMS-XKBZYTNZSA-N 0.000 description 9
- DLISPGXMKZTWQG-IFFSRLJSSA-N Glu-Thr-Val Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](C(C)C)C(O)=O DLISPGXMKZTWQG-IFFSRLJSSA-N 0.000 description 9
- UGVQELHRNUDMAA-BYPYZUCNSA-N Gly-Ala-Gly Chemical compound [NH3+]CC(=O)N[C@@H](C)C(=O)NCC([O-])=O UGVQELHRNUDMAA-BYPYZUCNSA-N 0.000 description 9
- VSVZIEVNUYDAFR-YUMQZZPRSA-N Gly-Ala-Leu Chemical compound CC(C)C[C@@H](C(O)=O)NC(=O)[C@H](C)NC(=O)CN VSVZIEVNUYDAFR-YUMQZZPRSA-N 0.000 description 9
- MZZSCEANQDPJER-ONGXEEELSA-N Gly-Ala-Phe Chemical compound NCC(=O)N[C@@H](C)C(=O)N[C@H](C(O)=O)CC1=CC=CC=C1 MZZSCEANQDPJER-ONGXEEELSA-N 0.000 description 9
- KFMBRBPXHVMDFN-UWVGGRQHSA-N Gly-Arg-Lys Chemical compound NCCCC[C@@H](C(O)=O)NC(=O)[C@@H](NC(=O)CN)CCCNC(N)=N KFMBRBPXHVMDFN-UWVGGRQHSA-N 0.000 description 9
- QPDUVFSVVAOUHE-XVKPBYJWSA-N Gly-Gln-Val Chemical compound CC(C)[C@H](NC(=O)[C@H](CCC(N)=O)NC(=O)CN)C(O)=O QPDUVFSVVAOUHE-XVKPBYJWSA-N 0.000 description 9
- YXTFLTJYLIAZQG-FJXKBIBVSA-N Gly-Thr-Arg Chemical compound NCC(=O)N[C@@H]([C@H](O)C)C(=O)N[C@H](C(O)=O)CCCN=C(N)N YXTFLTJYLIAZQG-FJXKBIBVSA-N 0.000 description 9
- TVTZEOHWHUVYCG-KYNKHSRBSA-N Gly-Thr-Thr Chemical compound [H]NCC(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H]([C@@H](C)O)C(O)=O TVTZEOHWHUVYCG-KYNKHSRBSA-N 0.000 description 9
- PEDCQBHIVMGVHV-UHFFFAOYSA-N Glycerine Chemical compound OCC(O)CO PEDCQBHIVMGVHV-UHFFFAOYSA-N 0.000 description 9
- NCSIQAFSIPHVAN-IUKAMOBKSA-N Ile-Asn-Thr Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CC(=O)N)C(=O)N[C@@H]([C@@H](C)O)C(=O)O)N NCSIQAFSIPHVAN-IUKAMOBKSA-N 0.000 description 9
- FADXGVVLSPPEQY-GHCJXIJMSA-N Ile-Cys-Asn Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CS)C(=O)N[C@@H](CC(=O)N)C(=O)O)N FADXGVVLSPPEQY-GHCJXIJMSA-N 0.000 description 9
- KAFOIVJDVSZUMD-UHFFFAOYSA-N Leu-Gln-Gln Natural products CC(C)CC(N)C(=O)NC(CCC(N)=O)C(=O)NC(CCC(N)=O)C(O)=O KAFOIVJDVSZUMD-UHFFFAOYSA-N 0.000 description 9
- RSFGIMMPWAXNML-MNXVOIDGSA-N Leu-Gln-Ile Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H]([C@@H](C)CC)C(O)=O RSFGIMMPWAXNML-MNXVOIDGSA-N 0.000 description 9
- QJUWBDPGGYVRHY-YUMQZZPRSA-N Leu-Gly-Cys Chemical compound CC(C)C[C@@H](C(=O)NCC(=O)N[C@@H](CS)C(=O)O)N QJUWBDPGGYVRHY-YUMQZZPRSA-N 0.000 description 9
- HYMLKESRWLZDBR-WEDXCCLWSA-N Leu-Gly-Thr Chemical compound CC(C)C[C@H](N)C(=O)NCC(=O)N[C@@H]([C@@H](C)O)C(O)=O HYMLKESRWLZDBR-WEDXCCLWSA-N 0.000 description 9
- FIICHHJDINDXKG-IHPCNDPISA-N Leu-Lys-Trp Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC1=CNC2=C1C=CC=C2)C(O)=O FIICHHJDINDXKG-IHPCNDPISA-N 0.000 description 9
- FBNPMTNBFFAMMH-UHFFFAOYSA-N Leu-Val-Arg Natural products CC(C)CC(N)C(=O)NC(C(C)C)C(=O)NC(C(O)=O)CCCN=C(N)N FBNPMTNBFFAMMH-UHFFFAOYSA-N 0.000 description 9
- DFXQCCBKGUNYGG-GUBZILKMSA-N Lys-Gln-Ala Chemical compound OC(=O)[C@H](C)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@@H](N)CCCCN DFXQCCBKGUNYGG-GUBZILKMSA-N 0.000 description 9
- GPJGFSFYBJGYRX-YUMQZZPRSA-N Lys-Gly-Asp Chemical compound NCCCC[C@H](N)C(=O)NCC(=O)N[C@H](C(O)=O)CC(O)=O GPJGFSFYBJGYRX-YUMQZZPRSA-N 0.000 description 9
- PRSBSVAVOQOAMI-BJDJZHNGSA-N Lys-Ile-Ser Chemical compound OC[C@@H](C(O)=O)NC(=O)[C@H]([C@@H](C)CC)NC(=O)[C@@H](N)CCCCN PRSBSVAVOQOAMI-BJDJZHNGSA-N 0.000 description 9
- PDIDTSZKKFEDMB-UWVGGRQHSA-N Lys-Pro-Gly Chemical compound [H]N[C@@H](CCCCN)C(=O)N1CCC[C@H]1C(=O)NCC(O)=O PDIDTSZKKFEDMB-UWVGGRQHSA-N 0.000 description 9
- CTJUSALVKAWFFU-CIUDSAMLSA-N Lys-Ser-Cys Chemical compound C(CCN)C[C@@H](C(=O)N[C@@H](CO)C(=O)N[C@@H](CS)C(=O)O)N CTJUSALVKAWFFU-CIUDSAMLSA-N 0.000 description 9
- MHQXIBRPDKXDGZ-ZFWWWQNUSA-N Met-Gly-Trp Chemical compound C1=CC=C2C(C[C@H](NC(=O)CNC(=O)[C@@H](N)CCSC)C(O)=O)=CNC2=C1 MHQXIBRPDKXDGZ-ZFWWWQNUSA-N 0.000 description 9
- YBAFDPFAUTYYRW-UHFFFAOYSA-N N-L-alpha-glutamyl-L-leucine Natural products CC(C)CC(C(O)=O)NC(=O)C(N)CCC(O)=O YBAFDPFAUTYYRW-UHFFFAOYSA-N 0.000 description 9
- SITLTJHOQZFJGG-UHFFFAOYSA-N N-L-alpha-glutamyl-L-valine Natural products CC(C)C(C(O)=O)NC(=O)C(N)CCC(O)=O SITLTJHOQZFJGG-UHFFFAOYSA-N 0.000 description 9
- DFEVBOYEUQJGER-JURCDPSOSA-N Phe-Ala-Ile Chemical compound N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H](C)C(=O)N[C@@H]([C@@H](C)CC)C(=O)O DFEVBOYEUQJGER-JURCDPSOSA-N 0.000 description 9
- AUJWXNGCAQWLEI-KBPBESRZSA-N Phe-Lys-Gly Chemical compound [H]N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H](CCCCN)C(=O)NCC(O)=O AUJWXNGCAQWLEI-KBPBESRZSA-N 0.000 description 9
- JLLJTMHNXQTMCK-UBHSHLNASA-N Phe-Pro-Ala Chemical compound OC(=O)[C@H](C)NC(=O)[C@@H]1CCCN1C(=O)[C@@H](N)CC1=CC=CC=C1 JLLJTMHNXQTMCK-UBHSHLNASA-N 0.000 description 9
- WWPAHTZOWURIMR-ULQDDVLXSA-N Phe-Pro-Leu Chemical compound CC(C)C[C@@H](C(O)=O)NC(=O)[C@@H]1CCCN1C(=O)[C@@H](N)CC1=CC=CC=C1 WWPAHTZOWURIMR-ULQDDVLXSA-N 0.000 description 9
- UNBFGVQVQGXXCK-KKUMJFAQSA-N Phe-Ser-Leu Chemical compound [H]N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC(C)C)C(O)=O UNBFGVQVQGXXCK-KKUMJFAQSA-N 0.000 description 9
- JHSRGEODDALISP-XVSYOHENSA-N Phe-Thr-Asn Chemical compound [H]N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(N)=O)C(O)=O JHSRGEODDALISP-XVSYOHENSA-N 0.000 description 9
- IEIFEYBAYFSRBQ-IHRRRGAJSA-N Phe-Val-Ser Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CO)C(=O)O)NC(=O)[C@H](CC1=CC=CC=C1)N IEIFEYBAYFSRBQ-IHRRRGAJSA-N 0.000 description 9
- NXEYSLRNNPWCRN-SRVKXCTJSA-N Pro-Glu-Leu Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(C)C)C(O)=O NXEYSLRNNPWCRN-SRVKXCTJSA-N 0.000 description 9
- LGSANCBHSMDFDY-GARJFASQSA-N Pro-Glu-Pro Chemical compound C1C[C@H](NC1)C(=O)N[C@@H](CCC(=O)O)C(=O)N2CCC[C@@H]2C(=O)O LGSANCBHSMDFDY-GARJFASQSA-N 0.000 description 9
- DMKWYMWNEKIPFC-IUCAKERBSA-N Pro-Gly-Arg Chemical compound [H]N1CCC[C@H]1C(=O)NCC(=O)N[C@@H](CCCNC(N)=N)C(O)=O DMKWYMWNEKIPFC-IUCAKERBSA-N 0.000 description 9
- OWQXAJQZLWHPBH-FXQIFTODSA-N Pro-Ser-Asn Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H](CO)C(=O)N[C@@H](CC(N)=O)C(O)=O OWQXAJQZLWHPBH-FXQIFTODSA-N 0.000 description 9
- PZZJMBYSYAKYPK-UWJYBYFXSA-N Ser-Ala-Tyr Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](C)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(O)=O PZZJMBYSYAKYPK-UWJYBYFXSA-N 0.000 description 9
- GZFAWAQTEYDKII-YUMQZZPRSA-N Ser-Gly-Leu Chemical compound CC(C)C[C@@H](C(O)=O)NC(=O)CNC(=O)[C@@H](N)CO GZFAWAQTEYDKII-YUMQZZPRSA-N 0.000 description 9
- OQPNSDWGAMFJNU-QWRGUYRKSA-N Ser-Gly-Tyr Chemical compound OC[C@H](N)C(=O)NCC(=O)N[C@H](C(O)=O)CC1=CC=C(O)C=C1 OQPNSDWGAMFJNU-QWRGUYRKSA-N 0.000 description 9
- MQQBBLVOUUJKLH-HJPIBITLSA-N Ser-Ile-Tyr Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(O)=O MQQBBLVOUUJKLH-HJPIBITLSA-N 0.000 description 9
- RRVFEDGUXSYWOW-BZSNNMDCSA-N Ser-Phe-Phe Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H](CC1=CC=CC=C1)C(O)=O RRVFEDGUXSYWOW-BZSNNMDCSA-N 0.000 description 9
- HHJFMHQYEAAOBM-ZLUOBGJFSA-N Ser-Ser-Ala Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CO)C(=O)N[C@@H](C)C(O)=O HHJFMHQYEAAOBM-ZLUOBGJFSA-N 0.000 description 9
- XQJCEKXQUJQNNK-ZLUOBGJFSA-N Ser-Ser-Ser Chemical compound OC[C@H](N)C(=O)N[C@@H](CO)C(=O)N[C@@H](CO)C(O)=O XQJCEKXQUJQNNK-ZLUOBGJFSA-N 0.000 description 9
- KIEIJCFVGZCUAS-MELADBBJSA-N Ser-Tyr-Pro Chemical compound C1C[C@@H](N(C1)C(=O)[C@H](CC2=CC=C(C=C2)O)NC(=O)[C@H](CO)N)C(=O)O KIEIJCFVGZCUAS-MELADBBJSA-N 0.000 description 9
- FAPWRFPIFSIZLT-UHFFFAOYSA-M Sodium chloride Chemical compound [Na+].[Cl-] FAPWRFPIFSIZLT-UHFFFAOYSA-M 0.000 description 9
- FQPDRTDDEZXCEC-SVSWQMSJSA-N Thr-Ile-Ser Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CO)C(O)=O FQPDRTDDEZXCEC-SVSWQMSJSA-N 0.000 description 9
- DXPURPNJDFCKKO-RHYQMDGZSA-N Thr-Lys-Val Chemical compound CC(C)[C@H](NC(=O)[C@H](CCCCN)NC(=O)[C@@H](N)[C@@H](C)O)C(O)=O DXPURPNJDFCKKO-RHYQMDGZSA-N 0.000 description 9
- UKINEYBQXPMOJO-UBHSHLNASA-N Trp-Asn-Ser Chemical compound C1=CC=C2C(=C1)C(=CN2)C[C@@H](C(=O)N[C@@H](CC(=O)N)C(=O)N[C@@H](CO)C(=O)O)N UKINEYBQXPMOJO-UBHSHLNASA-N 0.000 description 9
- OLWFDNLLBWQWCP-STQMWFEESA-N Tyr-Gly-Met Chemical compound [H]N[C@@H](CC1=CC=C(O)C=C1)C(=O)NCC(=O)N[C@@H](CCSC)C(O)=O OLWFDNLLBWQWCP-STQMWFEESA-N 0.000 description 9
- MQGGXGKQSVEQHR-KKUMJFAQSA-N Tyr-Ser-Leu Chemical compound CC(C)C[C@@H](C(O)=O)NC(=O)[C@H](CO)NC(=O)[C@@H](N)CC1=CC=C(O)C=C1 MQGGXGKQSVEQHR-KKUMJFAQSA-N 0.000 description 9
- ZZDYJFVIKVSUFA-WLTAIBSBSA-N Tyr-Thr-Gly Chemical compound [H]N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H]([C@@H](C)O)C(=O)NCC(O)=O ZZDYJFVIKVSUFA-WLTAIBSBSA-N 0.000 description 9
- NUQZCPSZHGIYTA-HKUYNNGSSA-N Tyr-Trp-Gly Chemical compound C1=CC=C2C(=C1)C(=CN2)C[C@@H](C(=O)NCC(=O)O)NC(=O)[C@H](CC3=CC=C(C=C3)O)N NUQZCPSZHGIYTA-HKUYNNGSSA-N 0.000 description 9
- GNWUWQAVVJQREM-NHCYSSNCSA-N Val-Asn-His Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CC(=O)N)C(=O)N[C@@H](CC1=CN=CN1)C(=O)O)N GNWUWQAVVJQREM-NHCYSSNCSA-N 0.000 description 9
- WDIGUPHXPBMODF-UMNHJUIQSA-N Val-Glu-Pro Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CCC(=O)O)C(=O)N1CCC[C@@H]1C(=O)O)N WDIGUPHXPBMODF-UMNHJUIQSA-N 0.000 description 9
- ZIGZPYJXIWLQFC-QTKMDUPCSA-N Val-His-Thr Chemical compound C[C@H]([C@@H](C(=O)O)NC(=O)[C@H](CC1=CN=CN1)NC(=O)[C@H](C(C)C)N)O ZIGZPYJXIWLQFC-QTKMDUPCSA-N 0.000 description 9
- PQSNETRGCRUOGP-KKHAAJSZSA-N Val-Thr-Asn Chemical compound CC(C)[C@H](N)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@H](C(O)=O)CC(N)=O PQSNETRGCRUOGP-KKHAAJSZSA-N 0.000 description 9
- 108010069020 alanyl-prolyl-glycine Proteins 0.000 description 9
- 108010086434 alanyl-seryl-glycine Proteins 0.000 description 9
- 108010069205 aspartyl-phenylalanine Proteins 0.000 description 9
- 108010068265 aspartyltyrosine Proteins 0.000 description 9
- 238000004113 cell culture Methods 0.000 description 9
- 239000003795 chemical substances by application Substances 0.000 description 9
- 230000000295 complement effect Effects 0.000 description 9
- 108010060199 cysteinylproline Proteins 0.000 description 9
- 108010000434 glycyl-alanyl-leucine Proteins 0.000 description 9
- 108010067216 glycyl-glycyl-glycine Proteins 0.000 description 9
- 108010079413 glycyl-prolyl-glutamic acid Proteins 0.000 description 9
- 108010037850 glycylvaline Proteins 0.000 description 9
- RAXXELZNTBOGNW-UHFFFAOYSA-N imidazole Natural products C1=CNC=N1 RAXXELZNTBOGNW-UHFFFAOYSA-N 0.000 description 9
- 238000004519 manufacturing process Methods 0.000 description 9
- 230000001404 mediated effect Effects 0.000 description 9
- 125000003729 nucleotide group Chemical group 0.000 description 9
- 108010024654 phenylalanyl-prolyl-alanine Proteins 0.000 description 9
- 108010053725 prolylvaline Proteins 0.000 description 9
- 210000001519 tissue Anatomy 0.000 description 9
- YBJHBAHKTGYVGT-ZKWXMUAHSA-N (+)-Biotin Chemical compound N1C(=O)N[C@@H]2[C@H](CCCCC(=O)O)SC[C@@H]21 YBJHBAHKTGYVGT-ZKWXMUAHSA-N 0.000 description 8
- XWFWAXPOLRTDFZ-FXQIFTODSA-N Ala-Pro-Ser Chemical compound C[C@H](N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CO)C(O)=O XWFWAXPOLRTDFZ-FXQIFTODSA-N 0.000 description 8
- 102000009027 Albumins Human genes 0.000 description 8
- 108010088751 Albumins Proteins 0.000 description 8
- 239000012591 Dulbecco’s Phosphate Buffered Saline Substances 0.000 description 8
- DVLZZEPUNFEUBW-AVGNSLFASA-N Glu-His-Leu Chemical compound CC(C)C[C@@H](C(=O)O)NC(=O)[C@H](CC1=CN=CN1)NC(=O)[C@H](CCC(=O)O)N DVLZZEPUNFEUBW-AVGNSLFASA-N 0.000 description 8
- UJMNFCAHLYKWOZ-DCAQKATOSA-N Glu-Lys-Gln Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCC(N)=O)C(O)=O UJMNFCAHLYKWOZ-DCAQKATOSA-N 0.000 description 8
- FFJQHWKSGAWSTJ-BFHQHQDPSA-N Gly-Thr-Ala Chemical compound [H]NCC(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](C)C(O)=O FFJQHWKSGAWSTJ-BFHQHQDPSA-N 0.000 description 8
- HDODQNPMSHDXJT-GHCJXIJMSA-N Ile-Asn-Ser Chemical compound CC[C@H](C)[C@H](N)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CO)C(O)=O HDODQNPMSHDXJT-GHCJXIJMSA-N 0.000 description 8
- ZGKVPOSSTGHJAF-HJPIBITLSA-N Ile-Tyr-Ser Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CC1=CC=C(C=C1)O)C(=O)N[C@@H](CO)C(=O)O)N ZGKVPOSSTGHJAF-HJPIBITLSA-N 0.000 description 8
- AAKRWBIIGKPOKQ-ONGXEEELSA-N Leu-Val-Gly Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H](C(C)C)C(=O)NCC(O)=O AAKRWBIIGKPOKQ-ONGXEEELSA-N 0.000 description 8
- HQTKVSCNCDLXSX-BQBZGAKWSA-N Ser-Arg-Gly Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CCCNC(N)=N)C(=O)NCC(O)=O HQTKVSCNCDLXSX-BQBZGAKWSA-N 0.000 description 8
- YMTLKLXDFCSCNX-BYPYZUCNSA-N Ser-Gly-Gly Chemical compound OC[C@H](N)C(=O)NCC(=O)NCC(O)=O YMTLKLXDFCSCNX-BYPYZUCNSA-N 0.000 description 8
- PPNPDKGQRFSCAC-CIUDSAMLSA-N Ser-Lys-Asp Chemical compound NCCCC[C@H](NC(=O)[C@@H](N)CO)C(=O)N[C@@H](CC(O)=O)C(O)=O PPNPDKGQRFSCAC-CIUDSAMLSA-N 0.000 description 8
- FPCGZYMRFFIYIH-CIUDSAMLSA-N Ser-Lys-Ser Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CO)C(O)=O FPCGZYMRFFIYIH-CIUDSAMLSA-N 0.000 description 8
- QGXCWPNQVCYJEL-NUMRIWBASA-N Thr-Asn-Glu Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CCC(O)=O)C(O)=O QGXCWPNQVCYJEL-NUMRIWBASA-N 0.000 description 8
- QGVBFDIREUUSHX-IFFSRLJSSA-N Thr-Val-Gln Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCC(N)=O)C(O)=O QGVBFDIREUUSHX-IFFSRLJSSA-N 0.000 description 8
- WQOHKVRQDLNDIL-YJRXYDGGSA-N Tyr-Thr-Ser Chemical compound [H]N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CO)C(O)=O WQOHKVRQDLNDIL-YJRXYDGGSA-N 0.000 description 8
- 238000006243 chemical reaction Methods 0.000 description 8
- 239000000463 material Substances 0.000 description 8
- 239000002773 nucleotide Substances 0.000 description 8
- 239000011347 resin Substances 0.000 description 8
- 229920005989 resin Polymers 0.000 description 8
- OZFAFGSSMRRTDW-UHFFFAOYSA-N (2,4-dichlorophenyl) benzenesulfonate Chemical compound ClC1=CC(Cl)=CC=C1OS(=O)(=O)C1=CC=CC=C1 OZFAFGSSMRRTDW-UHFFFAOYSA-N 0.000 description 7
- QMOQBVOBWVNSNO-UHFFFAOYSA-N 2-[[2-[[2-[(2-azaniumylacetyl)amino]acetyl]amino]acetyl]amino]acetate Chemical compound NCC(=O)NCC(=O)NCC(=O)NCC(O)=O QMOQBVOBWVNSNO-UHFFFAOYSA-N 0.000 description 7
- ZBLQIYPCUWZSRZ-QEJZJMRPSA-N Ala-Phe-Leu Chemical compound CC(C)C[C@@H](C(O)=O)NC(=O)[C@@H](NC(=O)[C@H](C)N)CC1=CC=CC=C1 ZBLQIYPCUWZSRZ-QEJZJMRPSA-N 0.000 description 7
- BNYNOWJESJJIOI-XUXIUFHCSA-N Arg-Lys-Ile Chemical compound CC[C@H](C)[C@@H](C(=O)O)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CCCN=C(N)N)N BNYNOWJESJJIOI-XUXIUFHCSA-N 0.000 description 7
- QISZHYWZHJRDAO-CIUDSAMLSA-N Asn-Asp-Lys Chemical compound C(CCN)C[C@@H](C(=O)O)NC(=O)[C@H](CC(=O)O)NC(=O)[C@H](CC(=O)N)N QISZHYWZHJRDAO-CIUDSAMLSA-N 0.000 description 7
- 108020004705 Codon Proteins 0.000 description 7
- 108010066687 Epithelial Cell Adhesion Molecule Proteins 0.000 description 7
- 102000018651 Epithelial Cell Adhesion Molecule Human genes 0.000 description 7
- HVHRPWQEQHIQJF-AVGNSLFASA-N Leu-Lys-Glu Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCC(O)=O)C(O)=O HVHRPWQEQHIQJF-AVGNSLFASA-N 0.000 description 7
- BRTVHXHCUSXYRI-CIUDSAMLSA-N Leu-Ser-Ser Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H](CO)C(=O)N[C@@H](CO)C(O)=O BRTVHXHCUSXYRI-CIUDSAMLSA-N 0.000 description 7
- CGBYDGAJHSOGFQ-LPEHRKFASA-N Pro-Ala-Pro Chemical compound C[C@@H](C(=O)N1CCC[C@@H]1C(=O)O)NC(=O)[C@@H]2CCCN2 CGBYDGAJHSOGFQ-LPEHRKFASA-N 0.000 description 7
- IHCXPSYCHXFXKT-DCAQKATOSA-N Pro-Arg-Glu Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCC(O)=O)C(O)=O IHCXPSYCHXFXKT-DCAQKATOSA-N 0.000 description 7
- 240000004808 Saccharomyces cerevisiae Species 0.000 description 7
- QVOGDCQNGLBNCR-FXQIFTODSA-N Ser-Arg-Ser Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CO)C(O)=O QVOGDCQNGLBNCR-FXQIFTODSA-N 0.000 description 7
- FIDMVVBUOCMMJG-CIUDSAMLSA-N Ser-Asn-Leu Chemical compound CC(C)C[C@@H](C(O)=O)NC(=O)[C@H](CC(N)=O)NC(=O)[C@@H](N)CO FIDMVVBUOCMMJG-CIUDSAMLSA-N 0.000 description 7
- GRRAECZXRONTEE-UBHSHLNASA-N Ser-Cys-Trp Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CS)C(=O)N[C@@H](CC1=CNC2=C1C=CC=C2)C(O)=O GRRAECZXRONTEE-UBHSHLNASA-N 0.000 description 7
- UOLGINIHBRIECN-FXQIFTODSA-N Ser-Glu-Glu Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(O)=O UOLGINIHBRIECN-FXQIFTODSA-N 0.000 description 7
- 238000004458 analytical method Methods 0.000 description 7
- 108010052670 arginyl-glutamyl-glutamic acid Proteins 0.000 description 7
- 102000055104 bcl-X Human genes 0.000 description 7
- 108700000711 bcl-X Proteins 0.000 description 7
- 150000001875 compounds Chemical class 0.000 description 7
- 238000004132 cross linking Methods 0.000 description 7
- 230000003247 decreasing effect Effects 0.000 description 7
- 108010075431 glycyl-alanyl-phenylalanine Proteins 0.000 description 7
- 238000005259 measurement Methods 0.000 description 7
- 238000000746 purification Methods 0.000 description 7
- 230000002441 reversible effect Effects 0.000 description 7
- 239000013598 vector Substances 0.000 description 7
- SEKBHZJLARBNPB-GHCJXIJMSA-N Asn-Ile-Ser Chemical compound [H]N[C@@H](CC(N)=O)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CO)C(O)=O SEKBHZJLARBNPB-GHCJXIJMSA-N 0.000 description 6
- XHTUGJCAEYOZOR-UBHSHLNASA-N Asn-Ser-Trp Chemical compound [H]N[C@@H](CC(N)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC1=CNC2=C1C=CC=C2)C(O)=O XHTUGJCAEYOZOR-UBHSHLNASA-N 0.000 description 6
- 108010049207 Death Domain Receptors Proteins 0.000 description 6
- 102000009058 Death Domain Receptors Human genes 0.000 description 6
- NLKVNZUFDPWPNL-YUMQZZPRSA-N Glu-Arg-Gly Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)NCC(O)=O NLKVNZUFDPWPNL-YUMQZZPRSA-N 0.000 description 6
- AFWYPMDMDYCKMD-KBPBESRZSA-N Gly-Leu-Tyr Chemical compound NCC(=O)N[C@@H](CC(C)C)C(=O)N[C@H](C(O)=O)CC1=CC=C(O)C=C1 AFWYPMDMDYCKMD-KBPBESRZSA-N 0.000 description 6
- BNMRSWQOHIQTFL-JSGCOSHPSA-N Gly-Val-Phe Chemical compound NCC(=O)N[C@@H](C(C)C)C(=O)N[C@H](C(O)=O)CC1=CC=CC=C1 BNMRSWQOHIQTFL-JSGCOSHPSA-N 0.000 description 6
- SKYULSWNBYAQMG-IHRRRGAJSA-N His-Leu-Arg Chemical compound [H]N[C@@H](CC1=CNC=N1)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O SKYULSWNBYAQMG-IHRRRGAJSA-N 0.000 description 6
- BXOLYFJYQQRQDJ-MXAVVETBSA-N His-Leu-Ile Chemical compound CC[C@H](C)[C@@H](C(=O)O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC1=CN=CN1)N BXOLYFJYQQRQDJ-MXAVVETBSA-N 0.000 description 6
- JHNJNTMTZHEDLJ-NAKRPEOUSA-N Ile-Ser-Arg Chemical compound CC[C@H](C)[C@H](N)C(=O)N[C@@H](CO)C(=O)N[C@@H](CCCN=C(N)N)C(O)=O JHNJNTMTZHEDLJ-NAKRPEOUSA-N 0.000 description 6
- KZNQNBZMBZJQJO-UHFFFAOYSA-N N-glycyl-L-proline Natural products NCC(=O)N1CCCC1C(O)=O KZNQNBZMBZJQJO-UHFFFAOYSA-N 0.000 description 6
- GNUCSNWOCQFMMC-UFYCRDLUSA-N Phe-Arg-Phe Chemical compound C([C@H](N)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](CC=1C=CC=CC=1)C(O)=O)C1=CC=CC=C1 GNUCSNWOCQFMMC-UFYCRDLUSA-N 0.000 description 6
- 101710179684 Poly [ADP-ribose] polymerase Proteins 0.000 description 6
- 102100023712 Poly [ADP-ribose] polymerase 1 Human genes 0.000 description 6
- 229920000776 Poly(Adenosine diphosphate-ribose) polymerase Polymers 0.000 description 6
- DNIAPMSPPWPWGF-UHFFFAOYSA-N Propylene glycol Chemical compound CC(O)CO DNIAPMSPPWPWGF-UHFFFAOYSA-N 0.000 description 6
- XJPXTYLVMUZGNW-IHRRRGAJSA-N Tyr-Pro-Asp Chemical compound [H]N[C@@H](CC1=CC=C(O)C=C1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(O)=O)C(O)=O XJPXTYLVMUZGNW-IHRRRGAJSA-N 0.000 description 6
- 239000000556 agonist Substances 0.000 description 6
- 238000013459 approach Methods 0.000 description 6
- 108010093581 aspartyl-proline Proteins 0.000 description 6
- 230000015572 biosynthetic process Effects 0.000 description 6
- 239000000872 buffer Substances 0.000 description 6
- -1 carboxyl methyl Chemical group 0.000 description 6
- UQLDLKMNUJERMK-UHFFFAOYSA-L di(octadecanoyloxy)lead Chemical compound [Pb+2].CCCCCCCCCCCCCCCCCC([O-])=O.CCCCCCCCCCCCCCCCCC([O-])=O UQLDLKMNUJERMK-UHFFFAOYSA-L 0.000 description 6
- 108010077515 glycylproline Proteins 0.000 description 6
- 230000028993 immune response Effects 0.000 description 6
- 238000001727 in vivo Methods 0.000 description 6
- 230000006882 induction of apoptosis Effects 0.000 description 6
- 238000004020 luminiscence type Methods 0.000 description 6
- 239000000047 product Substances 0.000 description 6
- 239000011780 sodium chloride Substances 0.000 description 6
- 239000000243 solution Substances 0.000 description 6
- 238000001890 transfection Methods 0.000 description 6
- 238000012546 transfer Methods 0.000 description 6
- 108010003137 tyrosyltyrosine Proteins 0.000 description 6
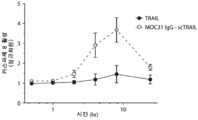
- 238000002689 xenotransplantation Methods 0.000 description 6
- XJFPXLWGZWAWRQ-UHFFFAOYSA-N 2-[[2-[[2-[[2-[[2-[(2-azaniumylacetyl)amino]acetyl]amino]acetyl]amino]acetyl]amino]acetyl]amino]acetate Chemical compound NCC(=O)NCC(=O)NCC(=O)NCC(=O)NCC(=O)NCC(O)=O XJFPXLWGZWAWRQ-UHFFFAOYSA-N 0.000 description 5
- ROLXPVQSRCPVGK-XDTLVQLUSA-N Ala-Glu-Tyr Chemical compound N[C@@H](C)C(=O)N[C@@H](CCC(=O)O)C(=O)N[C@@H](CC1=CC=C(C=C1)O)C(=O)O ROLXPVQSRCPVGK-XDTLVQLUSA-N 0.000 description 5
- ZATRYQNPUHGXCU-DTWKUNHWSA-N Arg-Gly-Pro Chemical compound C1C[C@@H](N(C1)C(=O)CNC(=O)[C@H](CCCN=C(N)N)N)C(=O)O ZATRYQNPUHGXCU-DTWKUNHWSA-N 0.000 description 5
- OPEPUCYIGFEGSW-WDSKDSINSA-N Asn-Gly-Glu Chemical compound [H]N[C@@H](CC(N)=O)C(=O)NCC(=O)N[C@@H](CCC(O)=O)C(O)=O OPEPUCYIGFEGSW-WDSKDSINSA-N 0.000 description 5
- DONWIPDSZZJHHK-HJGDQZAQSA-N Asp-Lys-Thr Chemical compound C[C@H]([C@@H](C(=O)O)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CC(=O)O)N)O DONWIPDSZZJHHK-HJGDQZAQSA-N 0.000 description 5
- WWOYXVBGHAHQBG-FXQIFTODSA-N Asp-Met-Asp Chemical compound [H]N[C@@H](CC(O)=O)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](CC(O)=O)C(O)=O WWOYXVBGHAHQBG-FXQIFTODSA-N 0.000 description 5
- SNLOOPZHAQDMJG-CIUDSAMLSA-N Gln-Glu-Glu Chemical compound NC(=O)CC[C@H](N)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(O)=O SNLOOPZHAQDMJG-CIUDSAMLSA-N 0.000 description 5
- VNCNWQPIQYAMAK-ACZMJKKPSA-N Glu-Ser-Ser Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](CO)C(O)=O VNCNWQPIQYAMAK-ACZMJKKPSA-N 0.000 description 5
- QAMMIGULQSIRCD-IRXDYDNUSA-N Gly-Phe-Tyr Chemical compound C([C@H](NC(=O)C[NH3+])C(=O)N[C@@H](CC=1C=CC(O)=CC=1)C([O-])=O)C1=CC=CC=C1 QAMMIGULQSIRCD-IRXDYDNUSA-N 0.000 description 5
- HAOUOFNNJJLVNS-BQBZGAKWSA-N Gly-Pro-Ser Chemical compound NCC(=O)N1CCC[C@H]1C(=O)N[C@@H](CO)C(O)=O HAOUOFNNJJLVNS-BQBZGAKWSA-N 0.000 description 5
- ZRSJXIKQXUGKRB-TUBUOCAGSA-N His-Ile-Thr Chemical compound [H]N[C@@H](CC1=CNC=N1)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H]([C@@H](C)O)C(O)=O ZRSJXIKQXUGKRB-TUBUOCAGSA-N 0.000 description 5
- HZWWOGWOBQBETJ-CUJWVEQBSA-N His-Thr-Cys Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](CS)C(=O)O)NC(=O)[C@H](CC1=CN=CN1)N)O HZWWOGWOBQBETJ-CUJWVEQBSA-N 0.000 description 5
- 102100026120 IgG receptor FcRn large subunit p51 Human genes 0.000 description 5
- 101710177940 IgG receptor FcRn large subunit p51 Proteins 0.000 description 5
- ADDYYRVQQZFIMW-MNXVOIDGSA-N Ile-Lys-Glu Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCC(=O)O)C(=O)O)N ADDYYRVQQZFIMW-MNXVOIDGSA-N 0.000 description 5
- 108060003951 Immunoglobulin Proteins 0.000 description 5
- ZDXPYRJPNDTMRX-VKHMYHEASA-N L-glutamine Chemical compound OC(=O)[C@@H](N)CCC(N)=O ZDXPYRJPNDTMRX-VKHMYHEASA-N 0.000 description 5
- 229930182555 Penicillin Natural products 0.000 description 5
- JGSARLDLIJGVTE-MBNYWOFBSA-N Penicillin G Chemical compound N([C@H]1[C@H]2SC([C@@H](N2C1=O)C(O)=O)(C)C)C(=O)CC1=CC=CC=C1 JGSARLDLIJGVTE-MBNYWOFBSA-N 0.000 description 5
- SJRQWEDYTKYHHL-SLFFLAALSA-N Phe-Tyr-Pro Chemical compound C1C[C@@H](N(C1)C(=O)[C@H](CC2=CC=C(C=C2)O)NC(=O)[C@H](CC3=CC=CC=C3)N)C(=O)O SJRQWEDYTKYHHL-SLFFLAALSA-N 0.000 description 5
- FUVBEZJCRMHWEM-FXQIFTODSA-N Pro-Asn-Ser Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CO)C(O)=O FUVBEZJCRMHWEM-FXQIFTODSA-N 0.000 description 5
- LSIWVWRUTKPXDS-DCAQKATOSA-N Pro-Gln-Arg Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O LSIWVWRUTKPXDS-DCAQKATOSA-N 0.000 description 5
- VZKBJNBZMZHKRC-XUXIUFHCSA-N Pro-Ile-Leu Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CC(C)C)C(O)=O VZKBJNBZMZHKRC-XUXIUFHCSA-N 0.000 description 5
- SUENWIFTSTWUKD-AVGNSLFASA-N Pro-Leu-Val Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C(C)C)C(O)=O SUENWIFTSTWUKD-AVGNSLFASA-N 0.000 description 5
- IAOHCSQDQDWRQU-GUBZILKMSA-N Ser-Val-Arg Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O IAOHCSQDQDWRQU-GUBZILKMSA-N 0.000 description 5
- XKWABWFMQXMUMT-HJGDQZAQSA-N Thr-Pro-Glu Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCC(O)=O)C(O)=O XKWABWFMQXMUMT-HJGDQZAQSA-N 0.000 description 5
- 241000723873 Tobacco mosaic virus Species 0.000 description 5
- NHOVZGFNTGMYMI-KKUMJFAQSA-N Tyr-Ser-Lys Chemical compound NCCCC[C@@H](C(O)=O)NC(=O)[C@H](CO)NC(=O)[C@@H](N)CC1=CC=C(O)C=C1 NHOVZGFNTGMYMI-KKUMJFAQSA-N 0.000 description 5
- HJSLDXZAZGFPDK-ULQDDVLXSA-N Val-Phe-Leu Chemical compound CC(C)C[C@@H](C(=O)O)NC(=O)[C@H](CC1=CC=CC=C1)NC(=O)[C@H](C(C)C)N HJSLDXZAZGFPDK-ULQDDVLXSA-N 0.000 description 5
- KRAHMIJVUPUOTQ-DCAQKATOSA-N Val-Ser-His Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CO)C(=O)N[C@@H](CC1=CN=CN1)C(=O)O)N KRAHMIJVUPUOTQ-DCAQKATOSA-N 0.000 description 5
- ZLNYBMWGPOKSLW-LSJOCFKGSA-N Val-Val-Asp Chemical compound CC(C)[C@H](N)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC(O)=O)C(O)=O ZLNYBMWGPOKSLW-LSJOCFKGSA-N 0.000 description 5
- 239000002253 acid Substances 0.000 description 5
- 230000037396 body weight Effects 0.000 description 5
- 238000012054 celltiter-glo Methods 0.000 description 5
- 230000001413 cellular effect Effects 0.000 description 5
- 108020001507 fusion proteins Proteins 0.000 description 5
- 102000037865 fusion proteins Human genes 0.000 description 5
- 102000018358 immunoglobulin Human genes 0.000 description 5
- 230000001939 inductive effect Effects 0.000 description 5
- 230000003993 interaction Effects 0.000 description 5
- 239000012528 membrane Substances 0.000 description 5
- 150000007523 nucleic acids Chemical class 0.000 description 5
- 229940049954 penicillin Drugs 0.000 description 5
- 239000013612 plasmid Substances 0.000 description 5
- 238000002360 preparation method Methods 0.000 description 5
- 238000001742 protein purification Methods 0.000 description 5
- 230000019491 signal transduction Effects 0.000 description 5
- 238000001542 size-exclusion chromatography Methods 0.000 description 5
- 238000002415 sodium dodecyl sulfate polyacrylamide gel electrophoresis Methods 0.000 description 5
- 238000013112 stability test Methods 0.000 description 5
- 229960005322 streptomycin Drugs 0.000 description 5
- 208000024891 symptom Diseases 0.000 description 5
- 238000012360 testing method Methods 0.000 description 5
- YJHKTAMKPGFJCT-NRPADANISA-N Ala-Val-Glu Chemical compound [H]N[C@@H](C)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCC(O)=O)C(O)=O YJHKTAMKPGFJCT-NRPADANISA-N 0.000 description 4
- 108091093088 Amplicon Proteins 0.000 description 4
- PNQWAUXQDBIJDY-GUBZILKMSA-N Arg-Glu-Glu Chemical compound [H]N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(O)=O PNQWAUXQDBIJDY-GUBZILKMSA-N 0.000 description 4
- ZUVMUOOHJYNJPP-XIRDDKMYSA-N Arg-Trp-Gln Chemical compound [H]N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC1=CNC2=C1C=CC=C2)C(=O)N[C@@H](CCC(N)=O)C(O)=O ZUVMUOOHJYNJPP-XIRDDKMYSA-N 0.000 description 4
- WONGRTVAMHFGBE-WDSKDSINSA-N Asn-Gly-Gln Chemical compound C(CC(=O)N)[C@@H](C(=O)O)NC(=O)CNC(=O)[C@H](CC(=O)N)N WONGRTVAMHFGBE-WDSKDSINSA-N 0.000 description 4
- PUUPMDXIHCOPJU-HJGDQZAQSA-N Asn-Thr-Lys Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](CCCCN)C(=O)O)NC(=O)[C@H](CC(=O)N)N)O PUUPMDXIHCOPJU-HJGDQZAQSA-N 0.000 description 4
- SNDBKTFJWVEVPO-WHFBIAKZSA-N Asp-Gly-Ser Chemical compound [H]N[C@@H](CC(O)=O)C(=O)NCC(=O)N[C@@H](CO)C(O)=O SNDBKTFJWVEVPO-WHFBIAKZSA-N 0.000 description 4
- DPNWSMBUYCLEDG-CIUDSAMLSA-N Asp-Lys-Ser Chemical compound [H]N[C@@H](CC(O)=O)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CO)C(O)=O DPNWSMBUYCLEDG-CIUDSAMLSA-N 0.000 description 4
- JSNWZMFSLIWAHS-HJGDQZAQSA-N Asp-Thr-Leu Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](CC(C)C)C(=O)O)NC(=O)[C@H](CC(=O)O)N)O JSNWZMFSLIWAHS-HJGDQZAQSA-N 0.000 description 4
- IJGRMHOSHXDMSA-UHFFFAOYSA-N Atomic nitrogen Chemical compound N#N IJGRMHOSHXDMSA-UHFFFAOYSA-N 0.000 description 4
- 241000283707 Capra Species 0.000 description 4
- BWGNESOTFCXPMA-UHFFFAOYSA-N Dihydrogen disulfide Chemical compound SS BWGNESOTFCXPMA-UHFFFAOYSA-N 0.000 description 4
- 238000002965 ELISA Methods 0.000 description 4
- 108010087819 Fc receptors Proteins 0.000 description 4
- 102000009109 Fc receptors Human genes 0.000 description 4
- LJEPDHWNQXPXMM-NHCYSSNCSA-N Gln-Arg-Val Chemical compound [H]N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](C(C)C)C(O)=O LJEPDHWNQXPXMM-NHCYSSNCSA-N 0.000 description 4
- DHNWZLGBTPUTQQ-QEJZJMRPSA-N Gln-Asp-Trp Chemical compound C1=CC=C2C(=C1)C(=CN2)C[C@@H](C(=O)O)NC(=O)[C@H](CC(=O)O)NC(=O)[C@H](CCC(=O)N)N DHNWZLGBTPUTQQ-QEJZJMRPSA-N 0.000 description 4
- KDXKFBSNIJYNNR-YVNDNENWSA-N Gln-Glu-Ile Chemical compound [H]N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H]([C@@H](C)CC)C(O)=O KDXKFBSNIJYNNR-YVNDNENWSA-N 0.000 description 4
- CKOFNWCLWRYUHK-XHNCKOQMSA-N Glu-Asp-Pro Chemical compound C1C[C@@H](N(C1)C(=O)[C@H](CC(=O)O)NC(=O)[C@H](CCC(=O)O)N)C(=O)O CKOFNWCLWRYUHK-XHNCKOQMSA-N 0.000 description 4
- MWMJCGBSIORNCD-AVGNSLFASA-N Glu-Leu-Leu Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(C)C)C(O)=O MWMJCGBSIORNCD-AVGNSLFASA-N 0.000 description 4
- QDMVXRNLOPTPIE-WDCWCFNPSA-N Glu-Lys-Thr Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H]([C@@H](C)O)C(O)=O QDMVXRNLOPTPIE-WDCWCFNPSA-N 0.000 description 4
- ZYRXTRTUCAVNBQ-GVXVVHGQSA-N Glu-Val-Lys Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CCCCN)C(=O)O)NC(=O)[C@H](CCC(=O)O)N ZYRXTRTUCAVNBQ-GVXVVHGQSA-N 0.000 description 4
- YWAQATDNEKZFFK-BYPYZUCNSA-N Gly-Gly-Ser Chemical compound NCC(=O)NCC(=O)N[C@@H](CO)C(O)=O YWAQATDNEKZFFK-BYPYZUCNSA-N 0.000 description 4
- LCRDMSSAKLTKBU-ZDLURKLDSA-N Gly-Ser-Thr Chemical compound C[C@@H](O)[C@@H](C(O)=O)NC(=O)[C@H](CO)NC(=O)CN LCRDMSSAKLTKBU-ZDLURKLDSA-N 0.000 description 4
- ZLCLYFGMKFCDCN-XPUUQOCRSA-N Gly-Ser-Val Chemical compound CC(C)[C@H](NC(=O)[C@H](CO)NC(=O)CN)C(O)=O ZLCLYFGMKFCDCN-XPUUQOCRSA-N 0.000 description 4
- MAABHGXCIBEYQR-XVYDVKMFSA-N His-Asn-Ala Chemical compound C[C@@H](C(=O)O)NC(=O)[C@H](CC(=O)N)NC(=O)[C@H](CC1=CN=CN1)N MAABHGXCIBEYQR-XVYDVKMFSA-N 0.000 description 4
- JCOSMKPAOYDKRO-AVGNSLFASA-N His-Glu-Lys Chemical compound C1=C(NC=N1)C[C@@H](C(=O)N[C@@H](CCC(=O)O)C(=O)N[C@@H](CCCCN)C(=O)O)N JCOSMKPAOYDKRO-AVGNSLFASA-N 0.000 description 4
- VGSPNSSCMOHRRR-BJDJZHNGSA-N Ile-Ser-Lys Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CO)C(=O)N[C@@H](CCCCN)C(=O)O)N VGSPNSSCMOHRRR-BJDJZHNGSA-N 0.000 description 4
- 229930182816 L-glutamine Natural products 0.000 description 4
- RCFDOSNHHZGBOY-UHFFFAOYSA-N L-isoleucyl-L-alanine Natural products CCC(C)C(N)C(=O)NC(C)C(O)=O RCFDOSNHHZGBOY-UHFFFAOYSA-N 0.000 description 4
- TYYLDKGBCJGJGW-UHFFFAOYSA-N L-tryptophan-L-tyrosine Natural products C=1NC2=CC=CC=C2C=1CC(N)C(=O)NC(C(O)=O)CC1=CC=C(O)C=C1 TYYLDKGBCJGJGW-UHFFFAOYSA-N 0.000 description 4
- OIARJGNVARWKFP-YUMQZZPRSA-N Leu-Asn-Gly Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](CC(N)=O)C(=O)NCC(O)=O OIARJGNVARWKFP-YUMQZZPRSA-N 0.000 description 4
- PVMPDMIKUVNOBD-CIUDSAMLSA-N Leu-Asp-Ser Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CO)C(O)=O PVMPDMIKUVNOBD-CIUDSAMLSA-N 0.000 description 4
- BTNXKBVLWJBTNR-SRVKXCTJSA-N Leu-His-Asn Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](CC1=CNC=N1)C(=O)N[C@@H](CC(N)=O)C(O)=O BTNXKBVLWJBTNR-SRVKXCTJSA-N 0.000 description 4
- LINKCQUOMUDLKN-KATARQTJSA-N Leu-Thr-Cys Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](CS)C(=O)O)NC(=O)[C@H](CC(C)C)N)O LINKCQUOMUDLKN-KATARQTJSA-N 0.000 description 4
- MWVUEPNEPWMFBD-SRVKXCTJSA-N Lys-Cys-Lys Chemical compound NCCCC[C@H](N)C(=O)N[C@@H](CS)C(=O)N[C@H](C(O)=O)CCCCN MWVUEPNEPWMFBD-SRVKXCTJSA-N 0.000 description 4
- ODUQLUADRKMHOZ-JYJNAYRXSA-N Lys-Glu-Tyr Chemical compound C1=CC(=CC=C1C[C@@H](C(=O)O)NC(=O)[C@H](CCC(=O)O)NC(=O)[C@H](CCCCN)N)O ODUQLUADRKMHOZ-JYJNAYRXSA-N 0.000 description 4
- LUTDBHBIHHREDC-IHRRRGAJSA-N Lys-Pro-Lys Chemical compound NCCCC[C@H](N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCCN)C(O)=O LUTDBHBIHHREDC-IHRRRGAJSA-N 0.000 description 4
- HKXSZKJMDBHOTG-CIUDSAMLSA-N Lys-Ser-Ala Chemical compound OC(=O)[C@H](C)NC(=O)[C@H](CO)NC(=O)[C@@H](N)CCCCN HKXSZKJMDBHOTG-CIUDSAMLSA-N 0.000 description 4
- YKBSXQFZWFXFIB-VOAKCMCISA-N Lys-Thr-Lys Chemical compound NCCCC[C@H](N)C(=O)N[C@@H]([C@H](O)C)C(=O)N[C@@H](CCCCN)C(O)=O YKBSXQFZWFXFIB-VOAKCMCISA-N 0.000 description 4
- 241000699660 Mus musculus Species 0.000 description 4
- 108091028043 Nucleic acid sequence Proteins 0.000 description 4
- JDMKQHSHKJHAHR-UHFFFAOYSA-N Phe-Phe-Leu-Tyr Natural products C=1C=C(O)C=CC=1CC(C(O)=O)NC(=O)C(CC(C)C)NC(=O)C(NC(=O)C(N)CC=1C=CC=CC=1)CC1=CC=CC=C1 JDMKQHSHKJHAHR-UHFFFAOYSA-N 0.000 description 4
- ZJPGOXWRFNKIQL-JYJNAYRXSA-N Phe-Pro-Pro Chemical compound C([C@H](N)C(=O)N1[C@@H](CCC1)C(=O)N1[C@@H](CCC1)C(O)=O)C1=CC=CC=C1 ZJPGOXWRFNKIQL-JYJNAYRXSA-N 0.000 description 4
- RVGRUAULSDPKGF-UHFFFAOYSA-N Poloxamer Chemical compound C1CO1.CC1CO1 RVGRUAULSDPKGF-UHFFFAOYSA-N 0.000 description 4
- UAYHMOIGIQZLFR-NHCYSSNCSA-N Pro-Gln-Val Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](C(C)C)C(O)=O UAYHMOIGIQZLFR-NHCYSSNCSA-N 0.000 description 4
- HAAQQNHQZBOWFO-LURJTMIESA-N Pro-Gly-Gly Chemical compound OC(=O)CNC(=O)CNC(=O)[C@@H]1CCCN1 HAAQQNHQZBOWFO-LURJTMIESA-N 0.000 description 4
- HWLKHNDRXWTFTN-GUBZILKMSA-N Pro-Pro-Cys Chemical compound C1C[C@H](NC1)C(=O)N2CCC[C@H]2C(=O)N[C@@H](CS)C(=O)O HWLKHNDRXWTFTN-GUBZILKMSA-N 0.000 description 4
- FDMKYQQYJKYCLV-GUBZILKMSA-N Pro-Pro-Ser Chemical compound OC[C@@H](C(O)=O)NC(=O)[C@@H]1CCCN1C(=O)[C@H]1NCCC1 FDMKYQQYJKYCLV-GUBZILKMSA-N 0.000 description 4
- 102000002067 Protein Subunits Human genes 0.000 description 4
- 108010001267 Protein Subunits Proteins 0.000 description 4
- UGJRQLURDVGULT-LKXGYXEUSA-N Ser-Asn-Thr Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H]([C@@H](C)O)C(O)=O UGJRQLURDVGULT-LKXGYXEUSA-N 0.000 description 4
- QPFJSHSJFIYDJZ-GHCJXIJMSA-N Ser-Asp-Ile Chemical compound CC[C@H](C)[C@@H](C(O)=O)NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H](N)CO QPFJSHSJFIYDJZ-GHCJXIJMSA-N 0.000 description 4
- PWKMJDQXKCENMF-MEYUZBJRSA-N Tyr-Thr-Leu Chemical compound [H]N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(C)C)C(O)=O PWKMJDQXKCENMF-MEYUZBJRSA-N 0.000 description 4
- XTDDIVQWDXMRJL-IHRRRGAJSA-N Val-Leu-His Chemical compound CC(C)C[C@@H](C(=O)N[C@@H](CC1=CN=CN1)C(=O)O)NC(=O)[C@H](C(C)C)N XTDDIVQWDXMRJL-IHRRRGAJSA-N 0.000 description 4
- SYSWVVCYSXBVJG-RHYQMDGZSA-N Val-Leu-Thr Chemical compound C[C@H]([C@@H](C(=O)O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](C(C)C)N)O SYSWVVCYSXBVJG-RHYQMDGZSA-N 0.000 description 4
- BZDGLJPROOOUOZ-XGEHTFHBSA-N Val-Thr-Cys Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](CS)C(=O)O)NC(=O)[C@H](C(C)C)N)O BZDGLJPROOOUOZ-XGEHTFHBSA-N 0.000 description 4
- LLJLBRRXKZTTRD-GUBZILKMSA-N Val-Val-Ser Chemical compound CC(C)[C@@H](C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CO)C(=O)O)N LLJLBRRXKZTTRD-GUBZILKMSA-N 0.000 description 4
- HCHKCACWOHOZIP-UHFFFAOYSA-N Zinc Chemical compound [Zn] HCHKCACWOHOZIP-UHFFFAOYSA-N 0.000 description 4
- 239000005557 antagonist Substances 0.000 description 4
- 210000003719 b-lymphocyte Anatomy 0.000 description 4
- 229960002685 biotin Drugs 0.000 description 4
- 235000020958 biotin Nutrition 0.000 description 4
- 239000011616 biotin Substances 0.000 description 4
- 238000006664 bond formation reaction Methods 0.000 description 4
- 238000010367 cloning Methods 0.000 description 4
- 230000004540 complement-dependent cytotoxicity Effects 0.000 description 4
- 238000011161 development Methods 0.000 description 4
- 230000018109 developmental process Effects 0.000 description 4
- 239000003085 diluting agent Substances 0.000 description 4
- 239000000539 dimer Substances 0.000 description 4
- 208000035475 disorder Diseases 0.000 description 4
- 239000012636 effector Substances 0.000 description 4
- 238000005516 engineering process Methods 0.000 description 4
- 238000002795 fluorescence method Methods 0.000 description 4
- 108010013768 glutamyl-aspartyl-proline Proteins 0.000 description 4
- 230000005764 inhibitory process Effects 0.000 description 4
- 238000002955 isolation Methods 0.000 description 4
- 108010073472 leucyl-prolyl-proline Proteins 0.000 description 4
- 239000002502 liposome Substances 0.000 description 4
- 239000007788 liquid Substances 0.000 description 4
- 108010003700 lysyl aspartic acid Proteins 0.000 description 4
- 102000039446 nucleic acids Human genes 0.000 description 4
- 108020004707 nucleic acids Proteins 0.000 description 4
- 238000011580 nude mouse model Methods 0.000 description 4
- 108010064486 phenylalanyl-leucyl-valine Proteins 0.000 description 4
- 229920001993 poloxamer 188 Polymers 0.000 description 4
- 230000000069 prophylactic effect Effects 0.000 description 4
- 230000004044 response Effects 0.000 description 4
- 108010048397 seryl-lysyl-leucine Proteins 0.000 description 4
- 230000011664 signaling Effects 0.000 description 4
- 239000006228 supernatant Substances 0.000 description 4
- 230000001225 therapeutic effect Effects 0.000 description 4
- 238000002560 therapeutic procedure Methods 0.000 description 4
- 108010071097 threonyl-lysyl-proline Proteins 0.000 description 4
- 108010044292 tryptophyltyrosine Proteins 0.000 description 4
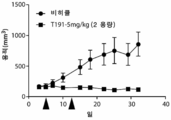
- 230000004614 tumor growth Effects 0.000 description 4
- 108010051110 tyrosyl-lysine Proteins 0.000 description 4
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 4
- SUHLZMHFRALVSY-YUMQZZPRSA-N Ala-Lys-Gly Chemical compound NCCCC[C@H](NC(=O)[C@@H](N)C)C(=O)NCC(O)=O SUHLZMHFRALVSY-YUMQZZPRSA-N 0.000 description 3
- HQIZDMIGUJOSNI-IUCAKERBSA-N Arg-Gly-Arg Chemical compound N[C@@H](CCCNC(N)=N)C(=O)NCC(=O)N[C@@H](CCCNC(N)=N)C(O)=O HQIZDMIGUJOSNI-IUCAKERBSA-N 0.000 description 3
- QKSAZKCRVQYYGS-UWVGGRQHSA-N Arg-Gly-His Chemical compound N[C@@H](CCCN=C(N)N)C(=O)NCC(=O)N[C@@H](Cc1cnc[nH]1)C(O)=O QKSAZKCRVQYYGS-UWVGGRQHSA-N 0.000 description 3
- DNLQVHBBMPZUGJ-BQBZGAKWSA-N Arg-Ser-Gly Chemical compound [H]N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CO)C(=O)NCC(O)=O DNLQVHBBMPZUGJ-BQBZGAKWSA-N 0.000 description 3
- SRUUBQBAVNQZGJ-LAEOZQHASA-N Asn-Gln-Val Chemical compound CC(C)[C@@H](C(=O)O)NC(=O)[C@H](CCC(=O)N)NC(=O)[C@H](CC(=O)N)N SRUUBQBAVNQZGJ-LAEOZQHASA-N 0.000 description 3
- HNXWVVHIGTZTBO-LKXGYXEUSA-N Asn-Ser-Thr Chemical compound C[C@@H](O)[C@@H](C(O)=O)NC(=O)[C@H](CO)NC(=O)[C@@H](N)CC(N)=O HNXWVVHIGTZTBO-LKXGYXEUSA-N 0.000 description 3
- QNNBHTFDFFFHGC-KKUMJFAQSA-N Asn-Tyr-Lys Chemical compound C1=CC(=CC=C1C[C@@H](C(=O)N[C@@H](CCCCN)C(=O)O)NC(=O)[C@H](CC(=O)N)N)O QNNBHTFDFFFHGC-KKUMJFAQSA-N 0.000 description 3
- NDNZRWUDUMTITL-FXQIFTODSA-N Cys-Ser-Val Chemical compound [H]N[C@@H](CS)C(=O)N[C@@H](CO)C(=O)N[C@@H](C(C)C)C(O)=O NDNZRWUDUMTITL-FXQIFTODSA-N 0.000 description 3
- 241000588724 Escherichia coli Species 0.000 description 3
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 description 3
- WMOMPXKOKASNBK-PEFMBERDSA-N Gln-Asn-Ile Chemical compound [H]N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H]([C@@H](C)CC)C(O)=O WMOMPXKOKASNBK-PEFMBERDSA-N 0.000 description 3
- XKBASPWPBXNVLQ-WDSKDSINSA-N Gln-Gly-Asn Chemical compound [H]N[C@@H](CCC(N)=O)C(=O)NCC(=O)N[C@@H](CC(N)=O)C(O)=O XKBASPWPBXNVLQ-WDSKDSINSA-N 0.000 description 3
- ZEEPYMXTJWIMSN-GUBZILKMSA-N Gln-Lys-Ser Chemical compound NCCCC[C@@H](C(=O)N[C@@H](CO)C(O)=O)NC(=O)[C@@H](N)CCC(N)=O ZEEPYMXTJWIMSN-GUBZILKMSA-N 0.000 description 3
- HUFCEIHAFNVSNR-IHRRRGAJSA-N Glu-Gln-Tyr Chemical compound OC(=O)CC[C@H](N)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@H](C(O)=O)CC1=CC=C(O)C=C1 HUFCEIHAFNVSNR-IHRRRGAJSA-N 0.000 description 3
- OLPPXYMMIARYAL-QMMMGPOBSA-N Gly-Gly-Val Chemical compound CC(C)[C@@H](C(O)=O)NC(=O)CNC(=O)CN OLPPXYMMIARYAL-QMMMGPOBSA-N 0.000 description 3
- SYOJVRNQCXYEOV-XVKPBYJWSA-N Gly-Val-Glu Chemical compound [H]NCC(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCC(O)=O)C(O)=O SYOJVRNQCXYEOV-XVKPBYJWSA-N 0.000 description 3
- 102100031181 Glyceraldehyde-3-phosphate dehydrogenase Human genes 0.000 description 3
- CSTDQOOBZBAJKE-BWAGICSOSA-N His-Tyr-Thr Chemical compound C[C@H]([C@@H](C(=O)O)NC(=O)[C@H](CC1=CC=C(C=C1)O)NC(=O)[C@H](CC2=CN=CN2)N)O CSTDQOOBZBAJKE-BWAGICSOSA-N 0.000 description 3
- 101000610602 Homo sapiens Tumor necrosis factor receptor superfamily member 10C Proteins 0.000 description 3
- 101000610609 Homo sapiens Tumor necrosis factor receptor superfamily member 10D Proteins 0.000 description 3
- XOWMDXHFSBCAKQ-SRVKXCTJSA-N Leu-Ser-Leu Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H](CO)C(=O)N[C@H](C(O)=O)CC(C)C XOWMDXHFSBCAKQ-SRVKXCTJSA-N 0.000 description 3
- FBNPMTNBFFAMMH-AVGNSLFASA-N Leu-Val-Arg Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H](C(C)C)C(=O)N[C@H](C(O)=O)CCCN=C(N)N FBNPMTNBFFAMMH-AVGNSLFASA-N 0.000 description 3
- IHRFZLQEQVHXFA-RHYQMDGZSA-N Met-Thr-Lys Chemical compound CSCC[C@H](N)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@H](C(O)=O)CCCCN IHRFZLQEQVHXFA-RHYQMDGZSA-N 0.000 description 3
- JOXIIFVCSATTDH-IHPCNDPISA-N Phe-Asn-Trp Chemical compound C1=CC=C(C=C1)C[C@@H](C(=O)N[C@@H](CC(=O)N)C(=O)N[C@@H](CC2=CNC3=CC=CC=C32)C(=O)O)N JOXIIFVCSATTDH-IHPCNDPISA-N 0.000 description 3
- 229920001213 Polysorbate 20 Polymers 0.000 description 3
- KIPIKSXPPLABPN-CIUDSAMLSA-N Pro-Glu-Asn Chemical compound NC(=O)C[C@@H](C(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@@H]1CCCN1 KIPIKSXPPLABPN-CIUDSAMLSA-N 0.000 description 3
- 239000012980 RPMI-1640 medium Substances 0.000 description 3
- FKYWFUYPVKLJLP-DCAQKATOSA-N Ser-Pro-Leu Chemical compound CC(C)C[C@@H](C(O)=O)NC(=O)[C@@H]1CCCN1C(=O)[C@@H](N)CO FKYWFUYPVKLJLP-DCAQKATOSA-N 0.000 description 3
- FZXOPYUEQGDGMS-ACZMJKKPSA-N Ser-Ser-Gln Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CO)C(=O)N[C@@H](CCC(N)=O)C(O)=O FZXOPYUEQGDGMS-ACZMJKKPSA-N 0.000 description 3
- PYTKULIABVRXSC-BWBBJGPYSA-N Ser-Ser-Thr Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CO)C(=O)N[C@@H]([C@@H](C)O)C(O)=O PYTKULIABVRXSC-BWBBJGPYSA-N 0.000 description 3
- 108010088160 Staphylococcal Protein A Proteins 0.000 description 3
- KBBRNEDOYWMIJP-KYNKHSRBSA-N Thr-Gly-Thr Chemical compound C[C@H]([C@@H](C(=O)NCC(=O)N[C@@H]([C@@H](C)O)C(=O)O)N)O KBBRNEDOYWMIJP-KYNKHSRBSA-N 0.000 description 3
- ZESGVALRVJIVLZ-VFCFLDTKSA-N Thr-Thr-Pro Chemical compound C[C@H]([C@@H](C(=O)N[C@@H]([C@@H](C)O)C(=O)N1CCC[C@@H]1C(=O)O)N)O ZESGVALRVJIVLZ-VFCFLDTKSA-N 0.000 description 3
- 102100040115 Tumor necrosis factor receptor superfamily member 10C Human genes 0.000 description 3
- 102100040110 Tumor necrosis factor receptor superfamily member 10D Human genes 0.000 description 3
- SQUMHUZLJDUROQ-YDHLFZDLSA-N Tyr-Val-Asp Chemical compound [H]N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC(O)=O)C(O)=O SQUMHUZLJDUROQ-YDHLFZDLSA-N 0.000 description 3
- COYSIHFOCOMGCF-WPRPVWTQSA-N Val-Arg-Gly Chemical compound CC(C)[C@H](N)C(=O)N[C@H](C(=O)NCC(O)=O)CCCN=C(N)N COYSIHFOCOMGCF-WPRPVWTQSA-N 0.000 description 3
- COYSIHFOCOMGCF-UHFFFAOYSA-N Val-Arg-Gly Natural products CC(C)C(N)C(=O)NC(C(=O)NCC(O)=O)CCCN=C(N)N COYSIHFOCOMGCF-UHFFFAOYSA-N 0.000 description 3
- PIFJAFRUVWZRKR-QMMMGPOBSA-N Val-Gly-Gly Chemical compound CC(C)[C@H]([NH3+])C(=O)NCC(=O)NCC([O-])=O PIFJAFRUVWZRKR-QMMMGPOBSA-N 0.000 description 3
- KISFXYYRKKNLOP-IHRRRGAJSA-N Val-Phe-Ser Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H](CO)C(=O)O)N KISFXYYRKKNLOP-IHRRRGAJSA-N 0.000 description 3
- KSFXWENSJABBFI-ZKWXMUAHSA-N Val-Ser-Asn Chemical compound [H]N[C@@H](C(C)C)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC(N)=O)C(O)=O KSFXWENSJABBFI-ZKWXMUAHSA-N 0.000 description 3
- 150000007513 acids Chemical class 0.000 description 3
- 238000001042 affinity chromatography Methods 0.000 description 3
- 230000003321 amplification Effects 0.000 description 3
- 229940034982 antineoplastic agent Drugs 0.000 description 3
- 230000001640 apoptogenic effect Effects 0.000 description 3
- 108010091092 arginyl-glycyl-proline Proteins 0.000 description 3
- 108010047857 aspartylglycine Proteins 0.000 description 3
- 230000009286 beneficial effect Effects 0.000 description 3
- 230000008901 benefit Effects 0.000 description 3
- 210000004369 blood Anatomy 0.000 description 3
- 239000008280 blood Substances 0.000 description 3
- 210000004899 c-terminal region Anatomy 0.000 description 3
- 230000005754 cellular signaling Effects 0.000 description 3
- 210000001072 colon Anatomy 0.000 description 3
- 125000004122 cyclic group Chemical group 0.000 description 3
- 125000000151 cysteine group Chemical group N[C@@H](CS)C(=O)* 0.000 description 3
- 230000003111 delayed effect Effects 0.000 description 3
- 210000004443 dendritic cell Anatomy 0.000 description 3
- 239000006185 dispersion Substances 0.000 description 3
- 239000003937 drug carrier Substances 0.000 description 3
- 238000001502 gel electrophoresis Methods 0.000 description 3
- 108020004445 glyceraldehyde-3-phosphate dehydrogenase Proteins 0.000 description 3
- 230000012010 growth Effects 0.000 description 3
- 239000000710 homodimer Substances 0.000 description 3
- 230000006872 improvement Effects 0.000 description 3
- 230000006698 induction Effects 0.000 description 3
- 239000003112 inhibitor Substances 0.000 description 3
- 230000002401 inhibitory effect Effects 0.000 description 3
- 238000003780 insertion Methods 0.000 description 3
- 230000037431 insertion Effects 0.000 description 3
- 108010057821 leucylproline Proteins 0.000 description 3
- 230000000670 limiting effect Effects 0.000 description 3
- 238000012417 linear regression Methods 0.000 description 3
- 230000003211 malignant effect Effects 0.000 description 3
- 230000001394 metastastic effect Effects 0.000 description 3
- 206010061289 metastatic neoplasm Diseases 0.000 description 3
- 238000003199 nucleic acid amplification method Methods 0.000 description 3
- 239000008188 pellet Substances 0.000 description 3
- 239000000256 polyoxyethylene sorbitan monolaurate Substances 0.000 description 3
- 235000010486 polyoxyethylene sorbitan monolaurate Nutrition 0.000 description 3
- 230000008569 process Effects 0.000 description 3
- 108010020755 prolyl-glycyl-glycine Proteins 0.000 description 3
- 108010077112 prolyl-proline Proteins 0.000 description 3
- 108010031719 prolyl-serine Proteins 0.000 description 3
- 230000009467 reduction Effects 0.000 description 3
- 241000894007 species Species 0.000 description 3
- 238000003860 storage Methods 0.000 description 3
- 238000004114 suspension culture Methods 0.000 description 3
- 239000012137 tryptone Substances 0.000 description 3
- 108010052774 valyl-lysyl-glycyl-phenylalanyl-tyrosine Proteins 0.000 description 3
- 229910052725 zinc Inorganic materials 0.000 description 3
- 239000011701 zinc Substances 0.000 description 3
- DGVVWUTYPXICAM-UHFFFAOYSA-N β‐Mercaptoethanol Chemical compound OCCS DGVVWUTYPXICAM-UHFFFAOYSA-N 0.000 description 3
- NFGXHKASABOEEW-UHFFFAOYSA-N 1-methylethyl 11-methoxy-3,7,11-trimethyl-2,4-dodecadienoate Chemical compound COC(C)(C)CCCC(C)CC=CC(C)=CC(=O)OC(C)C NFGXHKASABOEEW-UHFFFAOYSA-N 0.000 description 2
- IVLXQGJVBGMLRR-UHFFFAOYSA-N 2-aminoacetic acid;hydron;chloride Chemical compound Cl.NCC(O)=O IVLXQGJVBGMLRR-UHFFFAOYSA-N 0.000 description 2
- JEPNLGMEZMCFEX-QSFUFRPTSA-N Ala-His-Ile Chemical compound CC[C@H](C)[C@@H](C(=O)O)NC(=O)[C@H](CC1=CN=CN1)NC(=O)[C@H](C)N JEPNLGMEZMCFEX-QSFUFRPTSA-N 0.000 description 2
- 102000014914 Carrier Proteins Human genes 0.000 description 2
- 206010009944 Colon cancer Diseases 0.000 description 2
- 102000004127 Cytokines Human genes 0.000 description 2
- 108090000695 Cytokines Proteins 0.000 description 2
- 239000006144 Dulbecco’s modified Eagle's medium Substances 0.000 description 2
- 102000004190 Enzymes Human genes 0.000 description 2
- 108090000790 Enzymes Proteins 0.000 description 2
- 108091006020 Fc-tagged proteins Proteins 0.000 description 2
- UQJNXZSSGQIPIQ-FBCQKBJTSA-N Gly-Gly-Thr Chemical compound C[C@@H](O)[C@@H](C(O)=O)NC(=O)CNC(=O)CN UQJNXZSSGQIPIQ-FBCQKBJTSA-N 0.000 description 2
- SAVXZJYTTQQQDD-QEWYBTABSA-N Ile-Phe-Glu Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H](CCC(=O)O)C(=O)O)N SAVXZJYTTQQQDD-QEWYBTABSA-N 0.000 description 2
- 102000001706 Immunoglobulin Fab Fragments Human genes 0.000 description 2
- 108010054477 Immunoglobulin Fab Fragments Proteins 0.000 description 2
- 102100020870 La-related protein 6 Human genes 0.000 description 2
- 108050008265 La-related protein 6 Proteins 0.000 description 2
- 102000003960 Ligases Human genes 0.000 description 2
- 108090000364 Ligases Proteins 0.000 description 2
- 102100029193 Low affinity immunoglobulin gamma Fc region receptor III-A Human genes 0.000 description 2
- 108010035042 Osteoprotegerin Proteins 0.000 description 2
- 102000008108 Osteoprotegerin Human genes 0.000 description 2
- 229920002873 Polyethylenimine Polymers 0.000 description 2
- SHAQGFGGJSLLHE-BQBZGAKWSA-N Pro-Gln Chemical compound NC(=O)CC[C@@H](C([O-])=O)NC(=O)[C@@H]1CCC[NH2+]1 SHAQGFGGJSLLHE-BQBZGAKWSA-N 0.000 description 2
- RNMRYWZYFHHOEV-CIUDSAMLSA-N Ser-Gln-Arg Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O RNMRYWZYFHHOEV-CIUDSAMLSA-N 0.000 description 2
- VFWQQZMRKFOGLE-ZLUOBGJFSA-N Ser-Ser-Cys Chemical compound C([C@@H](C(=O)N[C@@H](CO)C(=O)N[C@@H](CS)C(=O)O)N)O VFWQQZMRKFOGLE-ZLUOBGJFSA-N 0.000 description 2
- SNXUIBACCONSOH-BWBBJGPYSA-N Ser-Thr-Ser Chemical compound OC[C@H](N)C(=O)N[C@@H]([C@H](O)C)C(=O)N[C@@H](CO)C(O)=O SNXUIBACCONSOH-BWBBJGPYSA-N 0.000 description 2
- 108010090804 Streptavidin Proteins 0.000 description 2
- XPNSAQMEAVSQRD-FBCQKBJTSA-N Thr-Gly-Gly Chemical compound C[C@@H](O)[C@H](N)C(=O)NCC(=O)NCC(O)=O XPNSAQMEAVSQRD-FBCQKBJTSA-N 0.000 description 2
- XHWCDRUPDNSDAZ-XKBZYTNZSA-N Thr-Ser-Glu Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](CO)C(=O)N[C@@H](CCC(=O)O)C(=O)O)N)O XHWCDRUPDNSDAZ-XKBZYTNZSA-N 0.000 description 2
- 239000007983 Tris buffer Substances 0.000 description 2
- 101710097160 Tumor necrosis factor ligand superfamily member 10 Proteins 0.000 description 2
- 102100024598 Tumor necrosis factor ligand superfamily member 10 Human genes 0.000 description 2
- KKHRWGYHBZORMQ-NHCYSSNCSA-N Val-Arg-Glu Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](CCC(=O)O)C(=O)O)N KKHRWGYHBZORMQ-NHCYSSNCSA-N 0.000 description 2
- 241000251539 Vertebrata <Metazoa> Species 0.000 description 2
- KYIKRXIYLAGAKQ-UHFFFAOYSA-N abcn Chemical compound C1CCCCC1(C#N)N=NC1(C#N)CCCCC1 KYIKRXIYLAGAKQ-UHFFFAOYSA-N 0.000 description 2
- 230000002159 abnormal effect Effects 0.000 description 2
- 238000010521 absorption reaction Methods 0.000 description 2
- 230000009471 action Effects 0.000 description 2
- 230000003213 activating effect Effects 0.000 description 2
- 239000012190 activator Substances 0.000 description 2
- 230000006978 adaptation Effects 0.000 description 2
- 238000007792 addition Methods 0.000 description 2
- 108091008324 binding proteins Proteins 0.000 description 2
- 230000004071 biological effect Effects 0.000 description 2
- OWMVSZAMULFTJU-UHFFFAOYSA-N bis-tris Chemical compound OCCN(CCO)C(CO)(CO)CO OWMVSZAMULFTJU-UHFFFAOYSA-N 0.000 description 2
- 230000000903 blocking effect Effects 0.000 description 2
- AIYUHDOJVYHVIT-UHFFFAOYSA-M caesium chloride Chemical compound [Cl-].[Cs+] AIYUHDOJVYHVIT-UHFFFAOYSA-M 0.000 description 2
- 239000013592 cell lysate Substances 0.000 description 2
- 239000003153 chemical reaction reagent Substances 0.000 description 2
- 238000004587 chromatography analysis Methods 0.000 description 2
- 238000003776 cleavage reaction Methods 0.000 description 2
- 230000037011 constitutive activity Effects 0.000 description 2
- 235000018417 cysteine Nutrition 0.000 description 2
- XUJNEKJLAYXESH-UHFFFAOYSA-N cysteine Natural products SCC(N)C(O)=O XUJNEKJLAYXESH-UHFFFAOYSA-N 0.000 description 2
- 230000006378 damage Effects 0.000 description 2
- 238000012217 deletion Methods 0.000 description 2
- 230000037430 deletion Effects 0.000 description 2
- 230000001419 dependent effect Effects 0.000 description 2
- 238000001514 detection method Methods 0.000 description 2
- 238000004090 dissolution Methods 0.000 description 2
- 239000012893 effector ligand Substances 0.000 description 2
- 229940088598 enzyme Drugs 0.000 description 2
- 238000000684 flow cytometry Methods 0.000 description 2
- 238000012921 fluorescence analysis Methods 0.000 description 2
- 238000002866 fluorescence resonance energy transfer Methods 0.000 description 2
- 238000001943 fluorescence-activated cell sorting Methods 0.000 description 2
- 108010089804 glycyl-threonine Proteins 0.000 description 2
- 230000012447 hatching Effects 0.000 description 2
- 210000000987 immune system Anatomy 0.000 description 2
- 238000001095 inductively coupled plasma mass spectrometry Methods 0.000 description 2
- 108010078274 isoleucylvaline Proteins 0.000 description 2
- 239000007951 isotonicity adjuster Substances 0.000 description 2
- 150000002632 lipids Chemical class 0.000 description 2
- 239000012516 mab select resin Substances 0.000 description 2
- 238000007898 magnetic cell sorting Methods 0.000 description 2
- 108010082117 matrigel Proteins 0.000 description 2
- 230000005012 migration Effects 0.000 description 2
- 238000013508 migration Methods 0.000 description 2
- 229910052757 nitrogen Inorganic materials 0.000 description 2
- 238000006384 oligomerization reaction Methods 0.000 description 2
- 229960003552 other antineoplastic agent in atc Drugs 0.000 description 2
- 244000052769 pathogen Species 0.000 description 2
- 239000008194 pharmaceutical composition Substances 0.000 description 2
- 229920001223 polyethylene glycol Polymers 0.000 description 2
- 108091033319 polynucleotide Proteins 0.000 description 2
- 102000040430 polynucleotide Human genes 0.000 description 2
- 239000002157 polynucleotide Substances 0.000 description 2
- 238000012545 processing Methods 0.000 description 2
- 230000035755 proliferation Effects 0.000 description 2
- 230000002035 prolonged effect Effects 0.000 description 2
- 108020001580 protein domains Proteins 0.000 description 2
- 238000002818 protein evolution Methods 0.000 description 2
- 238000003127 radioimmunoassay Methods 0.000 description 2
- 238000002708 random mutagenesis Methods 0.000 description 2
- 230000000306 recurrent effect Effects 0.000 description 2
- 230000001105 regulatory effect Effects 0.000 description 2
- 238000005070 sampling Methods 0.000 description 2
- 230000007017 scission Effects 0.000 description 2
- 239000011734 sodium Substances 0.000 description 2
- PUZPDOWCWNUUKD-UHFFFAOYSA-M sodium fluoride Chemical compound [F-].[Na+] PUZPDOWCWNUUKD-UHFFFAOYSA-M 0.000 description 2
- 239000002904 solvent Substances 0.000 description 2
- 238000010561 standard procedure Methods 0.000 description 2
- 230000004936 stimulating effect Effects 0.000 description 2
- 230000004083 survival effect Effects 0.000 description 2
- 239000000725 suspension Substances 0.000 description 2
- 238000010189 synthetic method Methods 0.000 description 2
- 108091005703 transmembrane proteins Proteins 0.000 description 2
- 102000035160 transmembrane proteins Human genes 0.000 description 2
- LENZDBCJOHFCAS-UHFFFAOYSA-N tris Chemical compound OCC(N)(CO)CO LENZDBCJOHFCAS-UHFFFAOYSA-N 0.000 description 2
- 238000010798 ubiquitination Methods 0.000 description 2
- 230000034512 ubiquitination Effects 0.000 description 2
- PCDUALPXEOKZPE-DXCABUDRSA-N (2s)-2-[[(2s)-2-[[(2s)-2-[[(2s)-2-[[(2s)-2-[[(2s)-2-[[(2s)-2-amino-3-hydroxypropanoyl]amino]-3-hydroxypropanoyl]amino]-3-hydroxypropanoyl]amino]-3-hydroxypropanoyl]amino]-3-hydroxypropanoyl]amino]-3-hydroxypropanoyl]amino]-3-hydroxypropanoic acid Chemical compound OC[C@H](N)C(=O)N[C@@H](CO)C(=O)N[C@@H](CO)C(=O)N[C@@H](CO)C(=O)N[C@@H](CO)C(=O)N[C@@H](CO)C(=O)N[C@@H](CO)C(O)=O PCDUALPXEOKZPE-DXCABUDRSA-N 0.000 description 1
- 108091032973 (ribonucleotides)n+m Proteins 0.000 description 1
- 125000000022 2-aminoethyl group Chemical group [H]C([*])([H])C([H])([H])N([H])[H] 0.000 description 1
- FWMNVWWHGCHHJJ-SKKKGAJSSA-N 4-amino-1-[(2r)-6-amino-2-[[(2r)-2-[[(2r)-2-[[(2r)-2-amino-3-phenylpropanoyl]amino]-3-phenylpropanoyl]amino]-4-methylpentanoyl]amino]hexanoyl]piperidine-4-carboxylic acid Chemical compound C([C@H](C(=O)N[C@H](CC(C)C)C(=O)N[C@H](CCCCN)C(=O)N1CCC(N)(CC1)C(O)=O)NC(=O)[C@H](N)CC=1C=CC=CC=1)C1=CC=CC=C1 FWMNVWWHGCHHJJ-SKKKGAJSSA-N 0.000 description 1
- ODHCTXKNWHHXJC-VKHMYHEASA-N 5-oxo-L-proline Chemical compound OC(=O)[C@@H]1CCC(=O)N1 ODHCTXKNWHHXJC-VKHMYHEASA-N 0.000 description 1
- 230000005730 ADP ribosylation Effects 0.000 description 1
- CXRCVCURMBFFOL-FXQIFTODSA-N Ala-Ala-Pro Chemical compound C[C@H](N)C(=O)N[C@@H](C)C(=O)N1CCC[C@H]1C(O)=O CXRCVCURMBFFOL-FXQIFTODSA-N 0.000 description 1
- KUDREHRZRIVKHS-UWJYBYFXSA-N Ala-Asp-Tyr Chemical compound [H]N[C@@H](C)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(O)=O KUDREHRZRIVKHS-UWJYBYFXSA-N 0.000 description 1
- WCBVQNZTOKJWJS-ACZMJKKPSA-N Ala-Cys-Glu Chemical compound [H]N[C@@H](C)C(=O)N[C@@H](CS)C(=O)N[C@@H](CCC(O)=O)C(O)=O WCBVQNZTOKJWJS-ACZMJKKPSA-N 0.000 description 1
- IYCZBJXFSZSHPN-DLOVCJGASA-N Ala-Cys-Phe Chemical compound [H]N[C@@H](C)C(=O)N[C@@H](CS)C(=O)N[C@@H](CC1=CC=CC=C1)C(O)=O IYCZBJXFSZSHPN-DLOVCJGASA-N 0.000 description 1
- OYJCVIGKMXUVKB-GARJFASQSA-N Ala-Leu-Pro Chemical compound C[C@@H](C(=O)N[C@@H](CC(C)C)C(=O)N1CCC[C@@H]1C(=O)O)N OYJCVIGKMXUVKB-GARJFASQSA-N 0.000 description 1
- MDNAVFBZPROEHO-UHFFFAOYSA-N Ala-Lys-Val Natural products CC(C)C(C(O)=O)NC(=O)C(NC(=O)C(C)N)CCCCN MDNAVFBZPROEHO-UHFFFAOYSA-N 0.000 description 1
- IORKCNUBHNIMKY-CIUDSAMLSA-N Ala-Pro-Glu Chemical compound C[C@H](N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCC(O)=O)C(O)=O IORKCNUBHNIMKY-CIUDSAMLSA-N 0.000 description 1
- WQLDNOCHHRISMS-NAKRPEOUSA-N Ala-Pro-Ile Chemical compound [H]N[C@@H](C)C(=O)N1CCC[C@H]1C(=O)N[C@@H]([C@@H](C)CC)C(O)=O WQLDNOCHHRISMS-NAKRPEOUSA-N 0.000 description 1
- DYXOFPBJBAHWFY-JBDRJPRFSA-N Ala-Ser-Ile Chemical compound CC[C@H](C)[C@@H](C(O)=O)NC(=O)[C@H](CO)NC(=O)[C@H](C)N DYXOFPBJBAHWFY-JBDRJPRFSA-N 0.000 description 1
- QGZKDVFQNNGYKY-UHFFFAOYSA-O Ammonium Chemical compound [NH4+] QGZKDVFQNNGYKY-UHFFFAOYSA-O 0.000 description 1
- HPKSHFSEXICTLI-CIUDSAMLSA-N Arg-Glu-Ala Chemical compound [H]N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](C)C(O)=O HPKSHFSEXICTLI-CIUDSAMLSA-N 0.000 description 1
- FRBAHXABMQXSJQ-FXQIFTODSA-N Arg-Ser-Ser Chemical compound [H]N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CO)C(=O)N[C@@H](CO)C(O)=O FRBAHXABMQXSJQ-FXQIFTODSA-N 0.000 description 1
- XYOVHPDDWCEUDY-CIUDSAMLSA-N Asn-Ala-Leu Chemical compound [H]N[C@@H](CC(N)=O)C(=O)N[C@@H](C)C(=O)N[C@@H](CC(C)C)C(O)=O XYOVHPDDWCEUDY-CIUDSAMLSA-N 0.000 description 1
- HZYFHQOWCFUSOV-IMJSIDKUSA-N Asn-Asp Chemical compound NC(=O)C[C@H](N)C(=O)N[C@@H](CC(O)=O)C(O)=O HZYFHQOWCFUSOV-IMJSIDKUSA-N 0.000 description 1
- BHQQRVARKXWXPP-ACZMJKKPSA-N Asn-Asp-Glu Chemical compound C(CC(=O)O)[C@@H](C(=O)O)NC(=O)[C@H](CC(=O)O)NC(=O)[C@H](CC(=O)N)N BHQQRVARKXWXPP-ACZMJKKPSA-N 0.000 description 1
- HDHZCEDPLTVHFZ-GUBZILKMSA-N Asn-Leu-Glu Chemical compound [H]N[C@@H](CC(N)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(O)=O)C(O)=O HDHZCEDPLTVHFZ-GUBZILKMSA-N 0.000 description 1
- JWQWPRCDYWNVNM-ACZMJKKPSA-N Asn-Ser-Gln Chemical compound C(CC(=O)N)[C@@H](C(=O)O)NC(=O)[C@H](CO)NC(=O)[C@H](CC(=O)N)N JWQWPRCDYWNVNM-ACZMJKKPSA-N 0.000 description 1
- HPNDKUOLNRVRAY-BIIVOSGPSA-N Asn-Ser-Pro Chemical compound C1C[C@@H](N(C1)C(=O)[C@H](CO)NC(=O)[C@H](CC(=O)N)N)C(=O)O HPNDKUOLNRVRAY-BIIVOSGPSA-N 0.000 description 1
- LGCVSPFCFXWUEY-IHPCNDPISA-N Asn-Trp-Tyr Chemical compound C1=CC=C2C(=C1)C(=CN2)C[C@@H](C(=O)N[C@@H](CC3=CC=C(C=C3)O)C(=O)O)NC(=O)[C@H](CC(=O)N)N LGCVSPFCFXWUEY-IHPCNDPISA-N 0.000 description 1
- QXHVOUSPVAWEMX-ZLUOBGJFSA-N Asp-Asp-Ser Chemical compound OC(=O)C[C@H](N)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CO)C(O)=O QXHVOUSPVAWEMX-ZLUOBGJFSA-N 0.000 description 1
- DCXYFEDJOCDNAF-UHFFFAOYSA-N Asparagine Natural products OC(=O)C(N)CC(N)=O DCXYFEDJOCDNAF-UHFFFAOYSA-N 0.000 description 1
- 241000894006 Bacteria Species 0.000 description 1
- 108091003079 Bovine Serum Albumin Proteins 0.000 description 1
- 206010006187 Breast cancer Diseases 0.000 description 1
- 208000026310 Breast neoplasm Diseases 0.000 description 1
- 102100024217 CAMPATH-1 antigen Human genes 0.000 description 1
- 108010065524 CD52 Antigen Proteins 0.000 description 1
- 101100283604 Caenorhabditis elegans pigk-1 gene Proteins 0.000 description 1
- 201000009030 Carcinoma Diseases 0.000 description 1
- 102000011727 Caspases Human genes 0.000 description 1
- 108010076667 Caspases Proteins 0.000 description 1
- 241000282693 Cercopithecidae Species 0.000 description 1
- 102000000989 Complement System Proteins Human genes 0.000 description 1
- 108010069112 Complement System Proteins Proteins 0.000 description 1
- 239000004971 Cross linker Substances 0.000 description 1
- XMTDCXXLDZKAGI-ACZMJKKPSA-N Cys-Ala-Gln Chemical compound C[C@@H](C(=O)N[C@@H](CCC(=O)N)C(=O)O)NC(=O)[C@H](CS)N XMTDCXXLDZKAGI-ACZMJKKPSA-N 0.000 description 1
- XKDHARKYRGHLKO-QEJZJMRPSA-N Cys-Trp-Gln Chemical compound C1=CC=C2C(=C1)C(=CN2)C[C@@H](C(=O)N[C@@H](CCC(=O)N)C(=O)O)NC(=O)[C@H](CS)N XKDHARKYRGHLKO-QEJZJMRPSA-N 0.000 description 1
- FBPFZTCFMRRESA-FSIIMWSLSA-N D-Glucitol Natural products OC[C@H](O)[C@H](O)[C@@H](O)[C@H](O)CO FBPFZTCFMRRESA-FSIIMWSLSA-N 0.000 description 1
- FBPFZTCFMRRESA-KVTDHHQDSA-N D-Mannitol Chemical compound OC[C@@H](O)[C@@H](O)[C@H](O)[C@H](O)CO FBPFZTCFMRRESA-KVTDHHQDSA-N 0.000 description 1
- FBPFZTCFMRRESA-JGWLITMVSA-N D-glucitol Chemical compound OC[C@H](O)[C@@H](O)[C@H](O)[C@H](O)CO FBPFZTCFMRRESA-JGWLITMVSA-N 0.000 description 1
- 238000001712 DNA sequencing Methods 0.000 description 1
- 238000012286 ELISA Assay Methods 0.000 description 1
- 239000006145 Eagle's minimal essential medium Substances 0.000 description 1
- 241000283074 Equus asinus Species 0.000 description 1
- 101000658547 Escherichia coli (strain K12) Type I restriction enzyme EcoKI endonuclease subunit Proteins 0.000 description 1
- 101000658543 Escherichia coli Type I restriction enzyme EcoAI endonuclease subunit Proteins 0.000 description 1
- 101000658546 Escherichia coli Type I restriction enzyme EcoEI endonuclease subunit Proteins 0.000 description 1
- 101000658530 Escherichia coli Type I restriction enzyme EcoR124II endonuclease subunit Proteins 0.000 description 1
- 101000658540 Escherichia coli Type I restriction enzyme EcoprrI endonuclease subunit Proteins 0.000 description 1
- 108010010803 Gelatin Proteins 0.000 description 1
- CITDWMLWXNUQKD-FXQIFTODSA-N Gln-Gln-Asn Chemical compound C(CC(=O)N)[C@@H](C(=O)N[C@@H](CCC(=O)N)C(=O)N[C@@H](CC(=O)N)C(=O)O)N CITDWMLWXNUQKD-FXQIFTODSA-N 0.000 description 1
- MAGNEQBFSBREJL-DCAQKATOSA-N Gln-Glu-Lys Chemical compound C(CCN)C[C@@H](C(=O)O)NC(=O)[C@H](CCC(=O)O)NC(=O)[C@H](CCC(=O)N)N MAGNEQBFSBREJL-DCAQKATOSA-N 0.000 description 1
- JEFZIKRIDLHOIF-BYPYZUCNSA-N Gln-Gly Chemical compound NC(=O)CC[C@H](N)C(=O)NCC(O)=O JEFZIKRIDLHOIF-BYPYZUCNSA-N 0.000 description 1
- KUBFPYIMAGXGBT-ACZMJKKPSA-N Gln-Ser-Ala Chemical compound [H]N[C@@H](CCC(N)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](C)C(O)=O KUBFPYIMAGXGBT-ACZMJKKPSA-N 0.000 description 1
- XIYWAJQIWLXXAF-XKBZYTNZSA-N Gln-Thr-Cys Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](CS)C(=O)O)NC(=O)[C@H](CCC(=O)N)N)O XIYWAJQIWLXXAF-XKBZYTNZSA-N 0.000 description 1
- WPJDPEOQUIXXOY-AVGNSLFASA-N Gln-Tyr-Asn Chemical compound C1=CC(=CC=C1C[C@@H](C(=O)N[C@@H](CC(=O)N)C(=O)O)NC(=O)[C@H](CCC(=O)N)N)O WPJDPEOQUIXXOY-AVGNSLFASA-N 0.000 description 1
- FITIQFSXXBKFFM-NRPADANISA-N Gln-Val-Ser Chemical compound [H]N[C@@H](CCC(N)=O)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CO)C(O)=O FITIQFSXXBKFFM-NRPADANISA-N 0.000 description 1
- GLWXKFRTOHKGIT-ACZMJKKPSA-N Glu-Asn-Asn Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC(N)=O)C(O)=O GLWXKFRTOHKGIT-ACZMJKKPSA-N 0.000 description 1
- WATXSTJXNBOHKD-LAEOZQHASA-N Glu-Asp-Val Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](C(C)C)C(O)=O WATXSTJXNBOHKD-LAEOZQHASA-N 0.000 description 1
- PVBBEKPHARMPHX-DCAQKATOSA-N Glu-Gln-Leu Chemical compound CC(C)C[C@@H](C(O)=O)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@@H](N)CCC(O)=O PVBBEKPHARMPHX-DCAQKATOSA-N 0.000 description 1
- KASDBWKLWJKTLJ-GUBZILKMSA-N Glu-Glu-Met Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCSC)C(O)=O KASDBWKLWJKTLJ-GUBZILKMSA-N 0.000 description 1
- PHONAZGUEGIOEM-GLLZPBPUSA-N Glu-Glu-Thr Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H]([C@@H](C)O)C(O)=O PHONAZGUEGIOEM-GLLZPBPUSA-N 0.000 description 1
- YGLCLCMAYUYZSG-AVGNSLFASA-N Glu-Lys-His Chemical compound OC(=O)CC[C@H](N)C(=O)N[C@@H](CCCCN)C(=O)N[C@H](C(O)=O)CC1=CN=CN1 YGLCLCMAYUYZSG-AVGNSLFASA-N 0.000 description 1
- GUOWMVFLAJNPDY-CIUDSAMLSA-N Glu-Ser-Met Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](CCSC)C(O)=O GUOWMVFLAJNPDY-CIUDSAMLSA-N 0.000 description 1
- DMYACXMQUABZIQ-NRPADANISA-N Glu-Ser-Val Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](C(C)C)C(O)=O DMYACXMQUABZIQ-NRPADANISA-N 0.000 description 1
- WQZGKKKJIJFFOK-GASJEMHNSA-N Glucose Natural products OC[C@H]1OC(O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-GASJEMHNSA-N 0.000 description 1
- LJPIRKICOISLKN-WHFBIAKZSA-N Gly-Ala-Ser Chemical compound NCC(=O)N[C@@H](C)C(=O)N[C@@H](CO)C(O)=O LJPIRKICOISLKN-WHFBIAKZSA-N 0.000 description 1
- GRIRDMVMJJDZKV-RCOVLWMOSA-N Gly-Asn-Val Chemical compound [H]NCC(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](C(C)C)C(O)=O GRIRDMVMJJDZKV-RCOVLWMOSA-N 0.000 description 1
- XBWMTPAIUQIWKA-BYULHYEWSA-N Gly-Asp-Ile Chemical compound CC[C@H](C)[C@@H](C(O)=O)NC(=O)[C@H](CC(O)=O)NC(=O)CN XBWMTPAIUQIWKA-BYULHYEWSA-N 0.000 description 1
- BIRKKBCSAIHDDF-WDSKDSINSA-N Gly-Glu-Cys Chemical compound NCC(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CS)C(O)=O BIRKKBCSAIHDDF-WDSKDSINSA-N 0.000 description 1
- BEQGFMIBZFNROK-JGVFFNPUSA-N Gly-Glu-Pro Chemical compound C1C[C@@H](N(C1)C(=O)[C@H](CCC(=O)O)NC(=O)CN)C(=O)O BEQGFMIBZFNROK-JGVFFNPUSA-N 0.000 description 1
- BUEFQXUHTUZXHR-LURJTMIESA-N Gly-Gly-Pro zwitterion Chemical compound NCC(=O)NCC(=O)N1CCC[C@H]1C(O)=O BUEFQXUHTUZXHR-LURJTMIESA-N 0.000 description 1
- SWQALSGKVLYKDT-UHFFFAOYSA-N Gly-Ile-Ala Natural products NCC(=O)NC(C(C)CC)C(=O)NC(C)C(O)=O SWQALSGKVLYKDT-UHFFFAOYSA-N 0.000 description 1
- NNCSJUBVFBDDLC-YUMQZZPRSA-N Gly-Leu-Ser Chemical compound NCC(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CO)C(O)=O NNCSJUBVFBDDLC-YUMQZZPRSA-N 0.000 description 1
- CSMYMGFCEJWALV-WDSKDSINSA-N Gly-Ser-Gln Chemical compound NCC(=O)N[C@@H](CO)C(=O)N[C@H](C(O)=O)CCC(N)=O CSMYMGFCEJWALV-WDSKDSINSA-N 0.000 description 1
- FFALDIDGPLUDKV-ZDLURKLDSA-N Gly-Thr-Ser Chemical compound [H]NCC(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CO)C(O)=O FFALDIDGPLUDKV-ZDLURKLDSA-N 0.000 description 1
- IZVICCORZOSGPT-JSGCOSHPSA-N Gly-Val-Tyr Chemical compound [H]NCC(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(O)=O IZVICCORZOSGPT-JSGCOSHPSA-N 0.000 description 1
- 101710088172 HTH-type transcriptional regulator RipA Proteins 0.000 description 1
- 206010066476 Haematological malignancy Diseases 0.000 description 1
- 101000658545 Haemophilus influenzae (strain ATCC 51907 / DSM 11121 / KW20 / Rd) Type I restriction enyme HindI endonuclease subunit Proteins 0.000 description 1
- 208000002250 Hematologic Neoplasms Diseases 0.000 description 1
- 108091006054 His-tagged proteins Proteins 0.000 description 1
- 241000282412 Homo Species 0.000 description 1
- 101001012157 Homo sapiens Receptor tyrosine-protein kinase erbB-2 Proteins 0.000 description 1
- 108010073807 IgG Receptors Proteins 0.000 description 1
- FHPZJWJWTWZKNA-LLLHUVSDSA-N Ile-Phe-Pro Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CC1=CC=CC=C1)C(=O)N2CCC[C@@H]2C(=O)O)N FHPZJWJWTWZKNA-LLLHUVSDSA-N 0.000 description 1
- SVZFKLBRCYCIIY-CYDGBPFRSA-N Ile-Pro-Arg Chemical compound CC[C@H](C)[C@H](N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(O)=O SVZFKLBRCYCIIY-CYDGBPFRSA-N 0.000 description 1
- JNLSTRPWUXOORL-MMWGEVLESA-N Ile-Ser-Pro Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CO)C(=O)N1CCC[C@@H]1C(=O)O)N JNLSTRPWUXOORL-MMWGEVLESA-N 0.000 description 1
- RQJUKVXWAKJDBW-SVSWQMSJSA-N Ile-Ser-Thr Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CO)C(=O)N[C@@H]([C@@H](C)O)C(=O)O)N RQJUKVXWAKJDBW-SVSWQMSJSA-N 0.000 description 1
- DGTOKVBDZXJHNZ-WZLNRYEVSA-N Ile-Thr-Tyr Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC1=CC=C(C=C1)O)C(=O)O)N DGTOKVBDZXJHNZ-WZLNRYEVSA-N 0.000 description 1
- YJRSIJZUIUANHO-NAKRPEOUSA-N Ile-Val-Ala Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](C)C(=O)O)N YJRSIJZUIUANHO-NAKRPEOUSA-N 0.000 description 1
- 102000018071 Immunoglobulin Fc Fragments Human genes 0.000 description 1
- 108010091135 Immunoglobulin Fc Fragments Proteins 0.000 description 1
- 108010021625 Immunoglobulin Fragments Proteins 0.000 description 1
- 102000008394 Immunoglobulin Fragments Human genes 0.000 description 1
- 102000006496 Immunoglobulin Heavy Chains Human genes 0.000 description 1
- 108010019476 Immunoglobulin Heavy Chains Proteins 0.000 description 1
- 206010061218 Inflammation Diseases 0.000 description 1
- 108010065920 Insulin Lispro Proteins 0.000 description 1
- ONIBWKKTOPOVIA-BYPYZUCNSA-N L-Proline Chemical compound OC(=O)[C@@H]1CCCN1 ONIBWKKTOPOVIA-BYPYZUCNSA-N 0.000 description 1
- DCXYFEDJOCDNAF-REOHCLBHSA-N L-asparagine Chemical compound OC(=O)[C@@H](N)CC(N)=O DCXYFEDJOCDNAF-REOHCLBHSA-N 0.000 description 1
- GRZSCTXVCDUIPO-SRVKXCTJSA-N Leu-Arg-Gln Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCC(N)=O)C(O)=O GRZSCTXVCDUIPO-SRVKXCTJSA-N 0.000 description 1
- STAVRDQLZOTNKJ-RHYQMDGZSA-N Leu-Arg-Thr Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H]([C@@H](C)O)C(O)=O STAVRDQLZOTNKJ-RHYQMDGZSA-N 0.000 description 1
- DBVWMYGBVFCRBE-CIUDSAMLSA-N Leu-Asn-Asn Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC(N)=O)C(O)=O DBVWMYGBVFCRBE-CIUDSAMLSA-N 0.000 description 1
- WCTCIIAGNMFYAO-DCAQKATOSA-N Leu-Cys-Val Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](CS)C(=O)N[C@@H](C(C)C)C(O)=O WCTCIIAGNMFYAO-DCAQKATOSA-N 0.000 description 1
- HPBCTWSUJOGJSH-MNXVOIDGSA-N Leu-Glu-Ile Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H]([C@@H](C)CC)C(O)=O HPBCTWSUJOGJSH-MNXVOIDGSA-N 0.000 description 1
- YOKVEHGYYQEQOP-QWRGUYRKSA-N Leu-Leu-Gly Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H](CC(C)C)C(=O)NCC(O)=O YOKVEHGYYQEQOP-QWRGUYRKSA-N 0.000 description 1
- KYIIALJHAOIAHF-KKUMJFAQSA-N Leu-Leu-His Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H](CC(C)C)C(=O)N[C@H](C(O)=O)CC1=CN=CN1 KYIIALJHAOIAHF-KKUMJFAQSA-N 0.000 description 1
- FAELBUXXFQLUAX-AJNGGQMLSA-N Leu-Leu-Ile Chemical compound CC[C@H](C)[C@@H](C(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](N)CC(C)C FAELBUXXFQLUAX-AJNGGQMLSA-N 0.000 description 1
- ZGUMORRUBUCXEH-AVGNSLFASA-N Leu-Lys-Gln Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCC(N)=O)C(O)=O ZGUMORRUBUCXEH-AVGNSLFASA-N 0.000 description 1
- AMSSKPUHBUQBOQ-SRVKXCTJSA-N Leu-Ser-Lys Chemical compound CC(C)C[C@@H](C(=O)N[C@@H](CO)C(=O)N[C@@H](CCCCN)C(=O)O)N AMSSKPUHBUQBOQ-SRVKXCTJSA-N 0.000 description 1
- SBANPBVRHYIMRR-UHFFFAOYSA-N Leu-Ser-Pro Natural products CC(C)CC(N)C(=O)NC(CO)C(=O)N1CCCC1C(O)=O SBANPBVRHYIMRR-UHFFFAOYSA-N 0.000 description 1
- SEOXPEFQEOYURL-PMVMPFDFSA-N Leu-Tyr-Trp Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](CC1=CNC2=C1C=CC=C2)C(O)=O SEOXPEFQEOYURL-PMVMPFDFSA-N 0.000 description 1
- 101710099301 Low affinity immunoglobulin gamma Fc region receptor III-A Proteins 0.000 description 1
- 206010025323 Lymphomas Diseases 0.000 description 1
- SWWCDAGDQHTKIE-RHYQMDGZSA-N Lys-Arg-Thr Chemical compound [H]N[C@@H](CCCCN)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H]([C@@H](C)O)C(O)=O SWWCDAGDQHTKIE-RHYQMDGZSA-N 0.000 description 1
- MRWXLRGAFDOILG-DCAQKATOSA-N Lys-Gln-Gln Chemical compound [H]N[C@@H](CCCCN)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCC(N)=O)C(O)=O MRWXLRGAFDOILG-DCAQKATOSA-N 0.000 description 1
- XNKDCYABMBBEKN-IUCAKERBSA-N Lys-Gly-Gln Chemical compound NCCCC[C@H](N)C(=O)NCC(=O)N[C@H](C(O)=O)CCC(N)=O XNKDCYABMBBEKN-IUCAKERBSA-N 0.000 description 1
- WWEWGPOLIJXGNX-XUXIUFHCSA-N Lys-Met-Ile Chemical compound CC[C@H](C)[C@@H](C(=O)O)NC(=O)[C@H](CCSC)NC(=O)[C@H](CCCCN)N WWEWGPOLIJXGNX-XUXIUFHCSA-N 0.000 description 1
- SBQDRNOLGSYHQA-YUMQZZPRSA-N Lys-Ser-Gly Chemical compound [H]N[C@@H](CCCCN)C(=O)N[C@@H](CO)C(=O)NCC(O)=O SBQDRNOLGSYHQA-YUMQZZPRSA-N 0.000 description 1
- IOQWIOPSKJOEKI-SRVKXCTJSA-N Lys-Ser-Leu Chemical compound [H]N[C@@H](CCCCN)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC(C)C)C(O)=O IOQWIOPSKJOEKI-SRVKXCTJSA-N 0.000 description 1
- DIBZLYZXTSVGLN-CIUDSAMLSA-N Lys-Ser-Ser Chemical compound [H]N[C@@H](CCCCN)C(=O)N[C@@H](CO)C(=O)N[C@@H](CO)C(O)=O DIBZLYZXTSVGLN-CIUDSAMLSA-N 0.000 description 1
- OEYKVQKYCHATHO-SZMVWBNQSA-N Lys-Trp-Gln Chemical compound C1=CC=C2C(=C1)C(=CN2)C[C@@H](C(=O)N[C@@H](CCC(=O)N)C(=O)O)NC(=O)[C@H](CCCCN)N OEYKVQKYCHATHO-SZMVWBNQSA-N 0.000 description 1
- XABXVVSWUVCZST-GVXVVHGQSA-N Lys-Val-Gln Chemical compound NC(=O)CC[C@@H](C(O)=O)NC(=O)[C@H](C(C)C)NC(=O)[C@@H](N)CCCCN XABXVVSWUVCZST-GVXVVHGQSA-N 0.000 description 1
- HMZPYMSEAALNAE-ULQDDVLXSA-N Lys-Val-Tyr Chemical compound [H]N[C@@H](CCCCN)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(O)=O HMZPYMSEAALNAE-ULQDDVLXSA-N 0.000 description 1
- 241000282567 Macaca fascicularis Species 0.000 description 1
- 229930195725 Mannitol Natural products 0.000 description 1
- VTKPSXWRUGCOAC-GUBZILKMSA-N Met-Ala-Met Chemical compound CSCC[C@H](N)C(=O)N[C@@H](C)C(=O)N[C@H](C(O)=O)CCSC VTKPSXWRUGCOAC-GUBZILKMSA-N 0.000 description 1
- HLYIDXAXQIJYIG-CIUDSAMLSA-N Met-Gln-Asp Chemical compound CSCC[C@H](N)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC(O)=O)C(O)=O HLYIDXAXQIJYIG-CIUDSAMLSA-N 0.000 description 1
- OOSPRDCGTLQLBP-NHCYSSNCSA-N Met-Glu-Val Chemical compound [H]N[C@@H](CCSC)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](C(C)C)C(O)=O OOSPRDCGTLQLBP-NHCYSSNCSA-N 0.000 description 1
- 206010027476 Metastases Diseases 0.000 description 1
- 101000658548 Methanocaldococcus jannaschii (strain ATCC 43067 / DSM 2661 / JAL-1 / JCM 10045 / NBRC 100440) Putative type I restriction enzyme MjaIXP endonuclease subunit Proteins 0.000 description 1
- 101000658542 Methanocaldococcus jannaschii (strain ATCC 43067 / DSM 2661 / JAL-1 / JCM 10045 / NBRC 100440) Putative type I restriction enzyme MjaVIIIP endonuclease subunit Proteins 0.000 description 1
- 101000658529 Methanocaldococcus jannaschii (strain ATCC 43067 / DSM 2661 / JAL-1 / JCM 10045 / NBRC 100440) Putative type I restriction enzyme MjaVIIP endonuclease subunit Proteins 0.000 description 1
- AUEJLPRZGVVDNU-UHFFFAOYSA-N N-L-tyrosyl-L-leucine Natural products CC(C)CC(C(O)=O)NC(=O)C(N)CC1=CC=C(O)C=C1 AUEJLPRZGVVDNU-UHFFFAOYSA-N 0.000 description 1
- 239000000020 Nitrocellulose Substances 0.000 description 1
- 238000012408 PCR amplification Methods 0.000 description 1
- 108090000526 Papain Proteins 0.000 description 1
- 102000035195 Peptidases Human genes 0.000 description 1
- 108091005804 Peptidases Proteins 0.000 description 1
- HXSUFWQYLPKEHF-IHRRRGAJSA-N Phe-Asn-Arg Chemical compound C1=CC=C(C=C1)C[C@@H](C(=O)N[C@@H](CC(=O)N)C(=O)N[C@@H](CCCN=C(N)N)C(=O)O)N HXSUFWQYLPKEHF-IHRRRGAJSA-N 0.000 description 1
- YMIZSYUAZJSOFL-SRVKXCTJSA-N Phe-Ser-Asn Chemical compound [H]N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC(N)=O)C(O)=O YMIZSYUAZJSOFL-SRVKXCTJSA-N 0.000 description 1
- IIEOLPMQYRBZCN-SRVKXCTJSA-N Phe-Ser-Cys Chemical compound N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H](CO)C(=O)N[C@@H](CS)C(=O)O IIEOLPMQYRBZCN-SRVKXCTJSA-N 0.000 description 1
- 229940122907 Phosphatase inhibitor Drugs 0.000 description 1
- 239000002202 Polyethylene glycol Substances 0.000 description 1
- JARJPEMLQAWNBR-GUBZILKMSA-N Pro-Asp-Arg Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O JARJPEMLQAWNBR-GUBZILKMSA-N 0.000 description 1
- TUYWCHPXKQTISF-LPEHRKFASA-N Pro-Cys-Pro Chemical compound C1C[C@H](NC1)C(=O)N[C@@H](CS)C(=O)N2CCC[C@@H]2C(=O)O TUYWCHPXKQTISF-LPEHRKFASA-N 0.000 description 1
- WGAQWMRJUFQXMF-ZPFDUUQYSA-N Pro-Gln-Ile Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H]([C@@H](C)CC)C(O)=O WGAQWMRJUFQXMF-ZPFDUUQYSA-N 0.000 description 1
- JMVQDLDPDBXAAX-YUMQZZPRSA-N Pro-Gly-Gln Chemical compound NC(=O)CC[C@@H](C(O)=O)NC(=O)CNC(=O)[C@@H]1CCCN1 JMVQDLDPDBXAAX-YUMQZZPRSA-N 0.000 description 1
- ZLXKLMHAMDENIO-DCAQKATOSA-N Pro-Lys-Asp Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(O)=O)C(O)=O ZLXKLMHAMDENIO-DCAQKATOSA-N 0.000 description 1
- PCWLNNZTBJTZRN-AVGNSLFASA-N Pro-Pro-Lys Chemical compound NCCCC[C@@H](C(O)=O)NC(=O)[C@@H]1CCCN1C(=O)[C@H]1NCCC1 PCWLNNZTBJTZRN-AVGNSLFASA-N 0.000 description 1
- GMJDSFYVTAMIBF-FXQIFTODSA-N Pro-Ser-Asp Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H](CO)C(=O)N[C@@H](CC(O)=O)C(O)=O GMJDSFYVTAMIBF-FXQIFTODSA-N 0.000 description 1
- ONIBWKKTOPOVIA-UHFFFAOYSA-N Proline Natural products OC(=O)C1CCCN1 ONIBWKKTOPOVIA-UHFFFAOYSA-N 0.000 description 1
- 239000004365 Protease Substances 0.000 description 1
- 229940124158 Protease/peptidase inhibitor Drugs 0.000 description 1
- 102000001708 Protein Isoforms Human genes 0.000 description 1
- 108010029485 Protein Isoforms Proteins 0.000 description 1
- 108010076504 Protein Sorting Signals Proteins 0.000 description 1
- 108091030071 RNAI Proteins 0.000 description 1
- 102100030086 Receptor tyrosine-protein kinase erbB-2 Human genes 0.000 description 1
- 206010038111 Recurrent cancer Diseases 0.000 description 1
- AUNGANRZJHBGPY-SCRDCRAPSA-N Riboflavin Chemical compound OC[C@@H](O)[C@@H](O)[C@@H](O)CN1C=2C=C(C)C(C)=CC=2N=C2C1=NC(=O)NC2=O AUNGANRZJHBGPY-SCRDCRAPSA-N 0.000 description 1
- 241000235070 Saccharomyces Species 0.000 description 1
- 206010039491 Sarcoma Diseases 0.000 description 1
- 229920002684 Sepharose Polymers 0.000 description 1
- 238000012300 Sequence Analysis Methods 0.000 description 1
- VAUMZJHYZQXZBQ-WHFBIAKZSA-N Ser-Asn-Gly Chemical compound OC[C@H](N)C(=O)N[C@@H](CC(N)=O)C(=O)NCC(O)=O VAUMZJHYZQXZBQ-WHFBIAKZSA-N 0.000 description 1
- VGNYHOBZJKWRGI-CIUDSAMLSA-N Ser-Asn-Lys Chemical compound NCCCC[C@@H](C(O)=O)NC(=O)[C@H](CC(N)=O)NC(=O)[C@@H](N)CO VGNYHOBZJKWRGI-CIUDSAMLSA-N 0.000 description 1
- BLPYXIXXCFVIIF-FXQIFTODSA-N Ser-Cys-Arg Chemical compound C(C[C@@H](C(=O)O)NC(=O)[C@H](CS)NC(=O)[C@H](CO)N)CN=C(N)N BLPYXIXXCFVIIF-FXQIFTODSA-N 0.000 description 1
- AEGUWTFAQQWVLC-BQBZGAKWSA-N Ser-Gly-Arg Chemical compound [H]N[C@@H](CO)C(=O)NCC(=O)N[C@@H](CCCNC(N)=N)C(O)=O AEGUWTFAQQWVLC-BQBZGAKWSA-N 0.000 description 1
- IOVHBRCQOGWAQH-ZKWXMUAHSA-N Ser-Gly-Ile Chemical compound [H]N[C@@H](CO)C(=O)NCC(=O)N[C@@H]([C@@H](C)CC)C(O)=O IOVHBRCQOGWAQH-ZKWXMUAHSA-N 0.000 description 1
- XXXAXOWMBOKTRN-XPUUQOCRSA-N Ser-Gly-Val Chemical compound [H]N[C@@H](CO)C(=O)NCC(=O)N[C@@H](C(C)C)C(O)=O XXXAXOWMBOKTRN-XPUUQOCRSA-N 0.000 description 1
- XNCUYZKGQOCOQH-YUMQZZPRSA-N Ser-Leu-Gly Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CC(C)C)C(=O)NCC(O)=O XNCUYZKGQOCOQH-YUMQZZPRSA-N 0.000 description 1
- YUJLIIRMIAGMCQ-CIUDSAMLSA-N Ser-Leu-Ser Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CO)C(O)=O YUJLIIRMIAGMCQ-CIUDSAMLSA-N 0.000 description 1
- WNDUPCKKKGSKIQ-CIUDSAMLSA-N Ser-Pro-Gln Chemical compound OC[C@H](N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCC(N)=O)C(O)=O WNDUPCKKKGSKIQ-CIUDSAMLSA-N 0.000 description 1
- CKDXFSPMIDSMGV-GUBZILKMSA-N Ser-Pro-Val Chemical compound [H]N[C@@H](CO)C(=O)N1CCC[C@H]1C(=O)N[C@@H](C(C)C)C(O)=O CKDXFSPMIDSMGV-GUBZILKMSA-N 0.000 description 1
- SRSPTFBENMJHMR-WHFBIAKZSA-N Ser-Ser-Gly Chemical compound OC[C@H](N)C(=O)N[C@@H](CO)C(=O)NCC(O)=O SRSPTFBENMJHMR-WHFBIAKZSA-N 0.000 description 1
- OZPDGESCTGGNAD-CIUDSAMLSA-N Ser-Ser-Lys Chemical compound NCCCC[C@@H](C(O)=O)NC(=O)[C@H](CO)NC(=O)[C@@H](N)CO OZPDGESCTGGNAD-CIUDSAMLSA-N 0.000 description 1
- ZKOKTQPHFMRSJP-YJRXYDGGSA-N Ser-Thr-Tyr Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(O)=O ZKOKTQPHFMRSJP-YJRXYDGGSA-N 0.000 description 1
- RCOUFINCYASMDN-GUBZILKMSA-N Ser-Val-Met Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCSC)C(O)=O RCOUFINCYASMDN-GUBZILKMSA-N 0.000 description 1
- HNDMFDBQXYZSRM-IHRRRGAJSA-N Ser-Val-Phe Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC1=CC=CC=C1)C(O)=O HNDMFDBQXYZSRM-IHRRRGAJSA-N 0.000 description 1
- 201000004283 Shwachman-Diamond syndrome Diseases 0.000 description 1
- 101001042773 Staphylococcus aureus (strain COL) Type I restriction enzyme SauCOLORF180P endonuclease subunit Proteins 0.000 description 1
- 101000838760 Staphylococcus aureus (strain MRSA252) Type I restriction enzyme SauMRSORF196P endonuclease subunit Proteins 0.000 description 1
- 101000838761 Staphylococcus aureus (strain MSSA476) Type I restriction enzyme SauMSSORF170P endonuclease subunit Proteins 0.000 description 1
- 101000838758 Staphylococcus aureus (strain MW2) Type I restriction enzyme SauMW2ORF169P endonuclease subunit Proteins 0.000 description 1
- 101001042566 Staphylococcus aureus (strain Mu50 / ATCC 700699) Type I restriction enzyme SauMu50ORF195P endonuclease subunit Proteins 0.000 description 1
- 101000838763 Staphylococcus aureus (strain N315) Type I restriction enzyme SauN315I endonuclease subunit Proteins 0.000 description 1
- 101000838759 Staphylococcus epidermidis (strain ATCC 35984 / RP62A) Type I restriction enzyme SepRPIP endonuclease subunit Proteins 0.000 description 1
- 101000838756 Staphylococcus saprophyticus subsp. saprophyticus (strain ATCC 15305 / DSM 20229 / NCIMB 8711 / NCTC 7292 / S-41) Type I restriction enzyme SsaAORF53P endonuclease subunit Proteins 0.000 description 1
- 102000007000 Tenascin Human genes 0.000 description 1
- 108010008125 Tenascin Proteins 0.000 description 1
- 108020005038 Terminator Codon Proteins 0.000 description 1
- DWYAUVCQDTZIJI-VZFHVOOUSA-N Thr-Ala-Ser Chemical compound C[C@@H](O)[C@H](N)C(=O)N[C@@H](C)C(=O)N[C@@H](CO)C(O)=O DWYAUVCQDTZIJI-VZFHVOOUSA-N 0.000 description 1
- KRPKYGOFYUNIGM-XVSYOHENSA-N Thr-Asp-Phe Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](CC(=O)O)C(=O)N[C@@H](CC1=CC=CC=C1)C(=O)O)N)O KRPKYGOFYUNIGM-XVSYOHENSA-N 0.000 description 1
- DSLHSTIUAPKERR-XGEHTFHBSA-N Thr-Cys-Val Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CS)C(=O)N[C@@H](C(C)C)C(O)=O DSLHSTIUAPKERR-XGEHTFHBSA-N 0.000 description 1
- GKWNLDNXMMLRMC-GLLZPBPUSA-N Thr-Glu-Gln Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](CCC(=O)O)C(=O)N[C@@H](CCC(=O)N)C(=O)O)N)O GKWNLDNXMMLRMC-GLLZPBPUSA-N 0.000 description 1
- AMXMBCAXAZUCFA-RHYQMDGZSA-N Thr-Leu-Arg Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O AMXMBCAXAZUCFA-RHYQMDGZSA-N 0.000 description 1
- FIFDDJFLNVAVMS-RHYQMDGZSA-N Thr-Leu-Met Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCSC)C(O)=O FIFDDJFLNVAVMS-RHYQMDGZSA-N 0.000 description 1
- VRUFCJZQDACGLH-UVOCVTCTSA-N Thr-Leu-Thr Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H]([C@@H](C)O)C(O)=O VRUFCJZQDACGLH-UVOCVTCTSA-N 0.000 description 1
- XSEPSRUDSPHMPX-KATARQTJSA-N Thr-Lys-Ser Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CO)C(O)=O XSEPSRUDSPHMPX-KATARQTJSA-N 0.000 description 1
- BIBYEFRASCNLAA-CDMKHQONSA-N Thr-Phe-Gly Chemical compound C[C@@H](O)[C@H](N)C(=O)N[C@H](C(=O)NCC(O)=O)CC1=CC=CC=C1 BIBYEFRASCNLAA-CDMKHQONSA-N 0.000 description 1
- MROIJTGJGIDEEJ-RCWTZXSCSA-N Thr-Pro-Pro Chemical compound C[C@@H](O)[C@H](N)C(=O)N1CCC[C@H]1C(=O)N1[C@H](C(O)=O)CCC1 MROIJTGJGIDEEJ-RCWTZXSCSA-N 0.000 description 1
- BKVICMPZWRNWOC-RHYQMDGZSA-N Thr-Val-Leu Chemical compound CC(C)C[C@@H](C(O)=O)NC(=O)[C@H](C(C)C)NC(=O)[C@@H](N)[C@@H](C)O BKVICMPZWRNWOC-RHYQMDGZSA-N 0.000 description 1
- 101710120037 Toxin CcdB Proteins 0.000 description 1
- WACMTVIJWRNVSO-CWRNSKLLSA-N Trp-Asp-Pro Chemical compound C1C[C@@H](N(C1)C(=O)[C@H](CC(=O)O)NC(=O)[C@H](CC2=CNC3=CC=CC=C32)N)C(=O)O WACMTVIJWRNVSO-CWRNSKLLSA-N 0.000 description 1
- NLWCSMOXNKBRLC-WDSOQIARSA-N Trp-Lys-Val Chemical compound [H]N[C@@H](CC1=CNC2=C1C=CC=C2)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](C(C)C)C(O)=O NLWCSMOXNKBRLC-WDSOQIARSA-N 0.000 description 1
- 102000004142 Trypsin Human genes 0.000 description 1
- 108090000631 Trypsin Proteins 0.000 description 1
- DXUVJJRTVACXSO-KKUMJFAQSA-N Tyr-Gln-Met Chemical compound CSCC[C@@H](C(=O)O)NC(=O)[C@H](CCC(=O)N)NC(=O)[C@H](CC1=CC=C(C=C1)O)N DXUVJJRTVACXSO-KKUMJFAQSA-N 0.000 description 1
- NKUGCYDFQKFVOJ-JYJNAYRXSA-N Tyr-Leu-Gln Chemical compound NC(=O)CC[C@@H](C(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](N)CC1=CC=C(O)C=C1 NKUGCYDFQKFVOJ-JYJNAYRXSA-N 0.000 description 1
- SINRIKQYQJRGDQ-MEYUZBJRSA-N Tyr-Lys-Thr Chemical compound C[C@@H](O)[C@@H](C(O)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@@H](N)CC1=CC=C(O)C=C1 SINRIKQYQJRGDQ-MEYUZBJRSA-N 0.000 description 1
- RIVVDNTUSRVTQT-IRIUXVKKSA-N Tyr-Thr-Gln Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](CCC(=O)N)C(=O)O)NC(=O)[C@H](CC1=CC=C(C=C1)O)N)O RIVVDNTUSRVTQT-IRIUXVKKSA-N 0.000 description 1
- QHDXUYOYTPWCSK-RCOVLWMOSA-N Val-Asp-Gly Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CC(=O)O)C(=O)NCC(=O)O)N QHDXUYOYTPWCSK-RCOVLWMOSA-N 0.000 description 1
- QHFQQRKNGCXTHL-AUTRQRHGSA-N Val-Gln-Glu Chemical compound CC(C)[C@H](N)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCC(O)=O)C(O)=O QHFQQRKNGCXTHL-AUTRQRHGSA-N 0.000 description 1
- XGJLNBNZNMVJRS-NRPADANISA-N Val-Glu-Ala Chemical compound CC(C)[C@H](N)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](C)C(O)=O XGJLNBNZNMVJRS-NRPADANISA-N 0.000 description 1
- UEHRGZCNLSWGHK-DLOVCJGASA-N Val-Glu-Val Chemical compound CC(C)[C@H](N)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](C(C)C)C(O)=O UEHRGZCNLSWGHK-DLOVCJGASA-N 0.000 description 1
- JTWIMNMUYLQNPI-WPRPVWTQSA-N Val-Gly-Arg Chemical compound CC(C)[C@H](N)C(=O)NCC(=O)N[C@H](C(O)=O)CCCNC(N)=N JTWIMNMUYLQNPI-WPRPVWTQSA-N 0.000 description 1
- APEBUJBRGCMMHP-HJWJTTGWSA-N Val-Ile-Phe Chemical compound CC(C)[C@H](N)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@H](C(O)=O)CC1=CC=CC=C1 APEBUJBRGCMMHP-HJWJTTGWSA-N 0.000 description 1
- DAVNYIUELQBTAP-XUXIUFHCSA-N Val-Leu-Ile Chemical compound CC[C@H](C)[C@@H](C(=O)O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](C(C)C)N DAVNYIUELQBTAP-XUXIUFHCSA-N 0.000 description 1
- QPPZEDOTPZOSEC-RCWTZXSCSA-N Val-Met-Thr Chemical compound C[C@H]([C@@H](C(=O)O)NC(=O)[C@H](CCSC)NC(=O)[C@H](C(C)C)N)O QPPZEDOTPZOSEC-RCWTZXSCSA-N 0.000 description 1
- TVGWMCTYUFBXAP-QTKMDUPCSA-N Val-Thr-His Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](CC1=CN=CN1)C(=O)O)NC(=O)[C@H](C(C)C)N)O TVGWMCTYUFBXAP-QTKMDUPCSA-N 0.000 description 1
- LCHZBEUVGAVMKS-RHYQMDGZSA-N Val-Thr-Leu Chemical compound CC(C)C[C@H](NC(=O)[C@@H](NC(=O)[C@@H](N)C(C)C)[C@@H](C)O)C(O)=O LCHZBEUVGAVMKS-RHYQMDGZSA-N 0.000 description 1
- YLBNZCJFSVJDRJ-KJEVXHAQSA-N Val-Thr-Tyr Chemical compound CC(C)[C@H](N)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](Cc1ccc(O)cc1)C(O)=O YLBNZCJFSVJDRJ-KJEVXHAQSA-N 0.000 description 1
- PGBMPFKFKXYROZ-UFYCRDLUSA-N Val-Tyr-Phe Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CC1=CC=C(C=C1)O)C(=O)N[C@@H](CC2=CC=CC=C2)C(=O)O)N PGBMPFKFKXYROZ-UFYCRDLUSA-N 0.000 description 1
- ZHWZDZFWBXWPDW-GUBZILKMSA-N Val-Val-Cys Chemical compound CC(C)[C@H](N)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CS)C(O)=O ZHWZDZFWBXWPDW-GUBZILKMSA-N 0.000 description 1
- IOUPEELXVYPCPG-UHFFFAOYSA-N Valylglycine Chemical compound CC(C)C(N)C(=O)NCC(O)=O IOUPEELXVYPCPG-UHFFFAOYSA-N 0.000 description 1
- 238000002835 absorbance Methods 0.000 description 1
- 239000003070 absorption delaying agent Substances 0.000 description 1
- 108010081404 acein-2 Proteins 0.000 description 1
- 230000021736 acetylation Effects 0.000 description 1
- 238000006640 acetylation reaction Methods 0.000 description 1
- 239000013543 active substance Substances 0.000 description 1
- 230000010933 acylation Effects 0.000 description 1
- 238000005917 acylation reaction Methods 0.000 description 1
- 238000005377 adsorption chromatography Methods 0.000 description 1
- 239000011543 agarose gel Substances 0.000 description 1
- 238000000246 agarose gel electrophoresis Methods 0.000 description 1
- 230000002776 aggregation Effects 0.000 description 1
- 238000004220 aggregation Methods 0.000 description 1
- 108010087924 alanylproline Proteins 0.000 description 1
- 229960000548 alemtuzumab Drugs 0.000 description 1
- 229940060587 alpha e Drugs 0.000 description 1
- 108010050025 alpha-glutamyltryptophan Proteins 0.000 description 1
- 230000004075 alteration Effects 0.000 description 1
- 230000009435 amidation Effects 0.000 description 1
- 238000007112 amidation reaction Methods 0.000 description 1
- BFNBIHQBYMNNAN-UHFFFAOYSA-N ammonium sulfate Chemical compound N.N.OS(O)(=O)=O BFNBIHQBYMNNAN-UHFFFAOYSA-N 0.000 description 1
- 229910052921 ammonium sulfate Inorganic materials 0.000 description 1
- 235000011130 ammonium sulphate Nutrition 0.000 description 1
- 229960000723 ampicillin Drugs 0.000 description 1
- AVKUERGKIZMTKX-NJBDSQKTSA-N ampicillin Chemical compound C1([C@@H](N)C(=O)N[C@H]2[C@H]3SC([C@@H](N3C2=O)C(O)=O)(C)C)=CC=CC=C1 AVKUERGKIZMTKX-NJBDSQKTSA-N 0.000 description 1
- 238000012436 analytical size exclusion chromatography Methods 0.000 description 1
- 238000005349 anion exchange Methods 0.000 description 1
- 239000003242 anti bacterial agent Substances 0.000 description 1
- 230000000844 anti-bacterial effect Effects 0.000 description 1
- 230000000259 anti-tumor effect Effects 0.000 description 1
- 230000005888 antibody-dependent cellular phagocytosis Effects 0.000 description 1
- 229940121375 antifungal agent Drugs 0.000 description 1
- 239000003429 antifungal agent Substances 0.000 description 1
- 230000005775 apoptotic pathway Effects 0.000 description 1
- 239000012062 aqueous buffer Substances 0.000 description 1
- 239000007864 aqueous solution Substances 0.000 description 1
- 125000000637 arginyl group Chemical group N[C@@H](CCCNC(N)=N)C(=O)* 0.000 description 1
- 108010009111 arginyl-glycyl-glutamic acid Proteins 0.000 description 1
- 230000010516 arginylation Effects 0.000 description 1
- 125000003118 aryl group Chemical group 0.000 description 1
- 229960001230 asparagine Drugs 0.000 description 1
- 235000009582 asparagine Nutrition 0.000 description 1
- 108010077245 asparaginyl-proline Proteins 0.000 description 1
- 108010038633 aspartylglutamate Proteins 0.000 description 1
- 108010092854 aspartyllysine Proteins 0.000 description 1
- 230000001363 autoimmune Effects 0.000 description 1
- 239000011324 bead Substances 0.000 description 1
- WQZGKKKJIJFFOK-VFUOTHLCSA-N beta-D-glucose Chemical compound OC[C@H]1O[C@@H](O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-VFUOTHLCSA-N 0.000 description 1
- 238000013357 binding ELISA Methods 0.000 description 1
- 239000003124 biologic agent Substances 0.000 description 1
- 230000008512 biological response Effects 0.000 description 1
- 238000010241 blood sampling Methods 0.000 description 1
- 229940098773 bovine serum albumin Drugs 0.000 description 1
- 239000007975 buffered saline Substances 0.000 description 1
- 208000035269 cancer or benign tumor Diseases 0.000 description 1
- 238000005251 capillar electrophoresis Methods 0.000 description 1
- 229960003669 carbenicillin Drugs 0.000 description 1
- FPPNZSSZRUTDAP-UWFZAAFLSA-N carbenicillin Chemical compound N([C@H]1[C@H]2SC([C@@H](N2C1=O)C(O)=O)(C)C)C(=O)C(C(O)=O)C1=CC=CC=C1 FPPNZSSZRUTDAP-UWFZAAFLSA-N 0.000 description 1
- 150000001720 carbohydrates Chemical class 0.000 description 1
- 229910052799 carbon Inorganic materials 0.000 description 1
- 125000003178 carboxy group Chemical group [H]OC(*)=O 0.000 description 1
- 230000000747 cardiac effect Effects 0.000 description 1
- 239000000969 carrier Substances 0.000 description 1
- 238000005341 cation exchange Methods 0.000 description 1
- 229960005395 cetuximab Drugs 0.000 description 1
- 230000008859 change Effects 0.000 description 1
- 239000013522 chelant Substances 0.000 description 1
- 238000007385 chemical modification Methods 0.000 description 1
- 210000004978 chinese hamster ovary cell Anatomy 0.000 description 1
- 238000011260 co-administration Methods 0.000 description 1
- 238000000576 coating method Methods 0.000 description 1
- 238000004440 column chromatography Methods 0.000 description 1
- 238000010961 commercial manufacture process Methods 0.000 description 1
- 230000006957 competitive inhibition Effects 0.000 description 1
- 238000012790 confirmation Methods 0.000 description 1
- 238000010276 construction Methods 0.000 description 1
- 239000000356 contaminant Substances 0.000 description 1
- 230000008878 coupling Effects 0.000 description 1
- 238000010168 coupling process Methods 0.000 description 1
- 238000005859 coupling reaction Methods 0.000 description 1
- 238000005520 cutting process Methods 0.000 description 1
- 230000001461 cytolytic effect Effects 0.000 description 1
- 230000001472 cytotoxic effect Effects 0.000 description 1
- 230000003013 cytotoxicity Effects 0.000 description 1
- 231100000135 cytotoxicity Toxicity 0.000 description 1
- 230000034994 death Effects 0.000 description 1
- 230000003412 degenerative effect Effects 0.000 description 1
- 230000001934 delay Effects 0.000 description 1
- 230000017858 demethylation Effects 0.000 description 1
- 238000010520 demethylation reaction Methods 0.000 description 1
- 238000013461 design Methods 0.000 description 1
- 230000001627 detrimental effect Effects 0.000 description 1
- 239000008121 dextrose Substances 0.000 description 1
- 238000010586 diagram Methods 0.000 description 1
- 229910003460 diamond Inorganic materials 0.000 description 1
- 239000010432 diamond Substances 0.000 description 1
- 230000029087 digestion Effects 0.000 description 1
- 238000010790 dilution Methods 0.000 description 1
- 239000012895 dilution Substances 0.000 description 1
- 239000013024 dilution buffer Substances 0.000 description 1
- 239000002612 dispersion medium Substances 0.000 description 1
- 238000009826 distribution Methods 0.000 description 1
- 239000002552 dosage form Substances 0.000 description 1
- 230000009977 dual effect Effects 0.000 description 1
- 239000000975 dye Substances 0.000 description 1
- 210000003162 effector t lymphocyte Anatomy 0.000 description 1
- 238000001962 electrophoresis Methods 0.000 description 1
- 238000004520 electroporation Methods 0.000 description 1
- 239000000839 emulsion Substances 0.000 description 1
- 230000002708 enhancing effect Effects 0.000 description 1
- 230000002255 enzymatic effect Effects 0.000 description 1
- 210000003979 eosinophil Anatomy 0.000 description 1
- 125000001495 ethyl group Chemical group [H]C([H])([H])C([H])([H])* 0.000 description 1
- 210000003527 eukaryotic cell Anatomy 0.000 description 1
- 238000011156 evaluation Methods 0.000 description 1
- 230000007717 exclusion Effects 0.000 description 1
- 230000001747 exhibiting effect Effects 0.000 description 1
- 239000013604 expression vector Substances 0.000 description 1
- 239000011536 extraction buffer Substances 0.000 description 1
- 230000022244 formylation Effects 0.000 description 1
- 238000006170 formylation reaction Methods 0.000 description 1
- 230000005714 functional activity Effects 0.000 description 1
- 229930182830 galactose Natural products 0.000 description 1
- 230000006251 gamma-carboxylation Effects 0.000 description 1
- 239000008273 gelatin Substances 0.000 description 1
- 229920000159 gelatin Polymers 0.000 description 1
- 235000019322 gelatine Nutrition 0.000 description 1
- 235000011852 gelatine desserts Nutrition 0.000 description 1
- 238000002523 gelfiltration Methods 0.000 description 1
- 230000009368 gene silencing by RNA Effects 0.000 description 1
- 238000007429 general method Methods 0.000 description 1
- 102000054766 genetic haplotypes Human genes 0.000 description 1
- 239000011521 glass Substances 0.000 description 1
- 230000036252 glycation Effects 0.000 description 1
- 108010020688 glycylhistidine Proteins 0.000 description 1
- 108010015792 glycyllysine Proteins 0.000 description 1
- 239000001963 growth medium Substances 0.000 description 1
- 238000004128 high performance liquid chromatography Methods 0.000 description 1
- 210000003630 histaminocyte Anatomy 0.000 description 1
- 108010018006 histidylserine Proteins 0.000 description 1
- 210000005260 human cell Anatomy 0.000 description 1
- 230000002209 hydrophobic effect Effects 0.000 description 1
- 230000033444 hydroxylation Effects 0.000 description 1
- 238000005805 hydroxylation reaction Methods 0.000 description 1
- 238000003384 imaging method Methods 0.000 description 1
- 210000002865 immune cell Anatomy 0.000 description 1
- 230000001900 immune effect Effects 0.000 description 1
- 230000000899 immune system response Effects 0.000 description 1
- 238000003119 immunoblot Methods 0.000 description 1
- 230000005847 immunogenicity Effects 0.000 description 1
- 229940072221 immunoglobulins Drugs 0.000 description 1
- 230000004957 immunoregulator effect Effects 0.000 description 1
- 238000009169 immunotherapy Methods 0.000 description 1
- 230000001771 impaired effect Effects 0.000 description 1
- 231100000405 induce cancer Toxicity 0.000 description 1
- 230000004054 inflammatory process Effects 0.000 description 1
- 238000001802 infusion Methods 0.000 description 1
- 239000004615 ingredient Substances 0.000 description 1
- 108091008042 inhibitory receptors Proteins 0.000 description 1
- 239000007972 injectable composition Substances 0.000 description 1
- 238000002347 injection Methods 0.000 description 1
- 239000007924 injection Substances 0.000 description 1
- 238000011081 inoculation Methods 0.000 description 1
- 238000007918 intramuscular administration Methods 0.000 description 1
- 238000001990 intravenous administration Methods 0.000 description 1
- 230000026045 iodination Effects 0.000 description 1
- 238000006192 iodination reaction Methods 0.000 description 1
- 238000004255 ion exchange chromatography Methods 0.000 description 1
- 230000000155 isotopic effect Effects 0.000 description 1
- 108010053037 kyotorphin Proteins 0.000 description 1
- 230000003902 lesion Effects 0.000 description 1
- 150000002614 leucines Chemical class 0.000 description 1
- 108010044311 leucyl-glycyl-glycine Proteins 0.000 description 1
- 208000032839 leukemia Diseases 0.000 description 1
- 239000012139 lysis buffer Substances 0.000 description 1
- 229920002521 macromolecule Polymers 0.000 description 1
- 210000002540 macrophage Anatomy 0.000 description 1
- 230000014759 maintenance of location Effects 0.000 description 1
- 230000036210 malignancy Effects 0.000 description 1
- 239000000594 mannitol Substances 0.000 description 1
- 235000010355 mannitol Nutrition 0.000 description 1
- 239000011159 matrix material Substances 0.000 description 1
- 230000007246 mechanism Effects 0.000 description 1
- 229910052751 metal Inorganic materials 0.000 description 1
- 239000002184 metal Substances 0.000 description 1
- 229910021645 metal ion Inorganic materials 0.000 description 1
- 230000009401 metastasis Effects 0.000 description 1
- 108010085203 methionylmethionine Proteins 0.000 description 1
- 230000011987 methylation Effects 0.000 description 1
- 238000007069 methylation reaction Methods 0.000 description 1
- 239000004530 micro-emulsion Substances 0.000 description 1
- 230000000813 microbial effect Effects 0.000 description 1
- 230000006667 mitochondrial pathway Effects 0.000 description 1
- 210000001616 monocyte Anatomy 0.000 description 1
- 238000002703 mutagenesis Methods 0.000 description 1
- 231100000350 mutagenesis Toxicity 0.000 description 1
- 230000007498 myristoylation Effects 0.000 description 1
- 210000004898 n-terminal fragment Anatomy 0.000 description 1
- 210000000440 neutrophil Anatomy 0.000 description 1
- 229920001220 nitrocellulos Polymers 0.000 description 1
- 230000036963 noncompetitive effect Effects 0.000 description 1
- 238000001543 one-way ANOVA Methods 0.000 description 1
- 210000000056 organ Anatomy 0.000 description 1
- 230000001009 osteoporotic effect Effects 0.000 description 1
- 230000003647 oxidation Effects 0.000 description 1
- 238000007254 oxidation reaction Methods 0.000 description 1
- 239000006179 pH buffering agent Substances 0.000 description 1
- 239000005022 packaging material Substances 0.000 description 1
- 235000019834 papain Nutrition 0.000 description 1
- 229940055729 papain Drugs 0.000 description 1
- 230000001575 pathological effect Effects 0.000 description 1
- 239000000137 peptide hydrolase inhibitor Substances 0.000 description 1
- 102000013415 peroxidase activity proteins Human genes 0.000 description 1
- 108040007629 peroxidase activity proteins Proteins 0.000 description 1
- 239000000546 pharmaceutical excipient Substances 0.000 description 1
- 108010070409 phenylalanyl-glycyl-glycine Proteins 0.000 description 1
- 108010073025 phenylalanylphenylalanine Proteins 0.000 description 1
- HXITXNWTGFUOAU-UHFFFAOYSA-N phenylboronic acid Chemical compound OB(O)C1=CC=CC=C1 HXITXNWTGFUOAU-UHFFFAOYSA-N 0.000 description 1
- 230000026731 phosphorylation Effects 0.000 description 1
- 238000006366 phosphorylation reaction Methods 0.000 description 1
- 239000004033 plastic Substances 0.000 description 1
- 229920003023 plastic Polymers 0.000 description 1
- 229920000744 poly(arginines) Polymers 0.000 description 1
- 229920005862 polyol Polymers 0.000 description 1
- 150000003077 polyols Chemical class 0.000 description 1
- 230000004481 post-translational protein modification Effects 0.000 description 1
- 230000001323 posttranslational effect Effects 0.000 description 1
- 239000000843 powder Substances 0.000 description 1
- 230000013823 prenylation Effects 0.000 description 1
- 239000003755 preservative agent Substances 0.000 description 1
- 125000002924 primary amino group Chemical group [H]N([H])* 0.000 description 1
- 230000000861 pro-apoptotic effect Effects 0.000 description 1
- 210000001236 prokaryotic cell Anatomy 0.000 description 1
- 210000002307 prostate Anatomy 0.000 description 1
- 235000019833 protease Nutrition 0.000 description 1
- 238000000159 protein binding assay Methods 0.000 description 1
- 230000012846 protein folding Effects 0.000 description 1
- 230000002797 proteolythic effect Effects 0.000 description 1
- 229940043131 pyroglutamate Drugs 0.000 description 1
- 230000006340 racemization Effects 0.000 description 1
- 238000010814 radioimmunoprecipitation assay Methods 0.000 description 1
- 239000002994 raw material Substances 0.000 description 1
- 239000000018 receptor agonist Substances 0.000 description 1
- 229940044601 receptor agonist Drugs 0.000 description 1
- 238000010188 recombinant method Methods 0.000 description 1
- 210000003289 regulatory T cell Anatomy 0.000 description 1
- 108091008146 restriction endonucleases Proteins 0.000 description 1
- 238000012552 review Methods 0.000 description 1
- 238000007363 ring formation reaction Methods 0.000 description 1
- 229960004641 rituximab Drugs 0.000 description 1
- 150000003839 salts Chemical class 0.000 description 1
- 239000006152 selective media Substances 0.000 description 1
- 238000012163 sequencing technique Methods 0.000 description 1
- 238000013207 serial dilution Methods 0.000 description 1
- 125000003607 serino group Chemical group [H]N([H])[C@]([H])(C(=O)[*])C(O[H])([H])[H] 0.000 description 1
- 150000003384 small molecules Chemical class 0.000 description 1
- FQENQNTWSFEDLI-UHFFFAOYSA-J sodium diphosphate Chemical compound [Na+].[Na+].[Na+].[Na+].[O-]P([O-])(=O)OP([O-])([O-])=O FQENQNTWSFEDLI-UHFFFAOYSA-J 0.000 description 1
- 235000013024 sodium fluoride Nutrition 0.000 description 1
- 239000011775 sodium fluoride Substances 0.000 description 1
- SUKJFIGYRHOWBL-UHFFFAOYSA-N sodium hypochlorite Chemical compound [Na+].Cl[O-] SUKJFIGYRHOWBL-UHFFFAOYSA-N 0.000 description 1
- 229940048086 sodium pyrophosphate Drugs 0.000 description 1
- 239000002594 sorbent Substances 0.000 description 1
- 239000000600 sorbitol Substances 0.000 description 1
- 230000006641 stabilisation Effects 0.000 description 1
- 238000011105 stabilization Methods 0.000 description 1
- 239000003381 stabilizer Substances 0.000 description 1
- 239000007858 starting material Substances 0.000 description 1
- 238000007920 subcutaneous administration Methods 0.000 description 1
- 239000000126 substance Substances 0.000 description 1
- 239000000758 substrate Substances 0.000 description 1
- 235000000346 sugar Nutrition 0.000 description 1
- 150000005846 sugar alcohols Polymers 0.000 description 1
- 150000008163 sugars Chemical class 0.000 description 1
- 230000002195 synergetic effect Effects 0.000 description 1
- 230000008685 targeting Effects 0.000 description 1
- 230000002123 temporal effect Effects 0.000 description 1
- 235000019818 tetrasodium diphosphate Nutrition 0.000 description 1
- 239000001577 tetrasodium phosphonato phosphate Substances 0.000 description 1
- 231100000331 toxic Toxicity 0.000 description 1
- 230000002588 toxic effect Effects 0.000 description 1
- 230000001988 toxicity Effects 0.000 description 1
- 231100000419 toxicity Toxicity 0.000 description 1
- 238000011830 transgenic mouse model Methods 0.000 description 1
- 229960000575 trastuzumab Drugs 0.000 description 1
- 238000011269 treatment regimen Methods 0.000 description 1
- IHIXIJGXTJIKRB-UHFFFAOYSA-N trisodium vanadate Chemical compound [Na+].[Na+].[Na+].[O-][V]([O-])([O-])=O IHIXIJGXTJIKRB-UHFFFAOYSA-N 0.000 description 1
- 239000012588 trypsin Substances 0.000 description 1
- 210000004881 tumor cell Anatomy 0.000 description 1
- 108010079202 tyrosyl-alanyl-cysteine Proteins 0.000 description 1
- 108010071635 tyrosyl-prolyl-arginine Proteins 0.000 description 1
- 108010078580 tyrosylleucine Proteins 0.000 description 1
- 230000004222 uncontrolled growth Effects 0.000 description 1
- 210000003462 vein Anatomy 0.000 description 1
- 230000035899 viability Effects 0.000 description 1
- 230000000007 visual effect Effects 0.000 description 1
- 239000011534 wash buffer Substances 0.000 description 1
- 238000001262 western blot Methods 0.000 description 1
- 238000009736 wetting Methods 0.000 description 1
- 108010027345 wheylin-1 peptide Proteins 0.000 description 1
- 210000005253 yeast cell Anatomy 0.000 description 1
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/18—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans
- C07K16/28—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants
- C07K16/2878—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants against the NGF-receptor/TNF-receptor superfamily, e.g. CD27, CD30, CD40, CD95
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K14/00—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- C07K14/435—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans
- C07K14/705—Receptors; Cell surface antigens; Cell surface determinants
- C07K14/70575—NGF/TNF-superfamily, e.g. CD70, CD95L, CD153, CD154
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K38/00—Medicinal preparations containing peptides
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K38/00—Medicinal preparations containing peptides
- A61K38/16—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- A61K38/17—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N15/00—Mutation or genetic engineering; DNA or RNA concerning genetic engineering, vectors, e.g. plasmids, or their isolation, preparation or purification; Use of hosts therefor
- C12N15/09—Recombinant DNA-technology
- C12N15/11—DNA or RNA fragments; Modified forms thereof; Non-coding nucleic acids having a biological activity
- C12N15/62—DNA sequences coding for fusion proteins
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2319/00—Fusion polypeptide
- C07K2319/30—Non-immunoglobulin-derived peptide or protein having an immunoglobulin constant or Fc region, or a fragment thereof, attached thereto
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Genetics & Genomics (AREA)
- General Health & Medical Sciences (AREA)
- Engineering & Computer Science (AREA)
- Zoology (AREA)
- Medicinal Chemistry (AREA)
- Molecular Biology (AREA)
- Immunology (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Biophysics (AREA)
- Biochemistry (AREA)
- Biomedical Technology (AREA)
- Gastroenterology & Hepatology (AREA)
- Animal Behavior & Ethology (AREA)
- Pharmacology & Pharmacy (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
- General Engineering & Computer Science (AREA)
- Biotechnology (AREA)
- Wood Science & Technology (AREA)
- Cell Biology (AREA)
- Toxicology (AREA)
- Epidemiology (AREA)
- General Chemical & Material Sciences (AREA)
- Microbiology (AREA)
- Plant Pathology (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Physics & Mathematics (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Peptides Or Proteins (AREA)
- Medicines That Contain Protein Lipid Enzymes And Other Medicines (AREA)
- Micro-Organisms Or Cultivation Processes Thereof (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
- Preparation Of Compounds By Using Micro-Organisms (AREA)
Applications Claiming Priority (7)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US201662309352P | 2016-03-16 | 2016-03-16 | |
| US62/309,352 | 2016-03-16 | ||
| US201662323501P | 2016-04-15 | 2016-04-15 | |
| US62/323,501 | 2016-04-15 | ||
| US201762445556P | 2017-01-12 | 2017-01-12 | |
| US62/445,556 | 2017-01-12 | ||
| PCT/US2017/022789 WO2017161173A1 (en) | 2016-03-16 | 2017-03-16 | Engineered trail for cancer therapy |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| KR20180127407A true KR20180127407A (ko) | 2018-11-28 |
Family
ID=58413224
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020187029745A Withdrawn KR20180127407A (ko) | 2016-03-16 | 2017-03-16 | 암 요법용 조작된 trail |
Country Status (10)
| Country | Link |
|---|---|
| US (1) | US20190077870A1 (cg-RX-API-DMAC7.html) |
| EP (1) | EP3430034A1 (cg-RX-API-DMAC7.html) |
| JP (1) | JP2019518713A (cg-RX-API-DMAC7.html) |
| KR (1) | KR20180127407A (cg-RX-API-DMAC7.html) |
| CN (1) | CN108884142A (cg-RX-API-DMAC7.html) |
| AU (1) | AU2017234679A1 (cg-RX-API-DMAC7.html) |
| CA (1) | CA3017622A1 (cg-RX-API-DMAC7.html) |
| IL (1) | IL261267A (cg-RX-API-DMAC7.html) |
| MX (1) | MX2018011219A (cg-RX-API-DMAC7.html) |
| WO (1) | WO2017161173A1 (cg-RX-API-DMAC7.html) |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2022080865A3 (ko) * | 2020-10-13 | 2022-08-11 | 신동준 | 반려견의 항암용 재조합 단백질 및 이를 포함하는 반려견을 위한 항암용 조성물 |
Families Citing this family (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN108026181B (zh) * | 2015-10-22 | 2021-07-02 | 成都华创生物技术有限公司 | 一种TRAIL双靶点突变蛋白MuR6S4TR、其制备方法及其应用 |
| CN109641033A (zh) * | 2016-06-13 | 2019-04-16 | 梅里麦克制药股份有限公司 | 用基于trail的治疗剂或死亡受体激动剂选择和治疗患者的方法 |
| CN109125709B (zh) * | 2018-08-23 | 2021-10-22 | 成都华创生物技术有限公司 | Trail突变体在制备治疗痤疮药物中的应用及一种制剂 |
Family Cites Families (39)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| DE3883899T3 (de) | 1987-03-18 | 1999-04-22 | Sb2, Inc., Danville, Calif. | Geänderte antikörper. |
| US5677425A (en) | 1987-09-04 | 1997-10-14 | Celltech Therapeutics Limited | Recombinant antibody |
| JPH0822239B2 (ja) * | 1988-07-07 | 1996-03-06 | 株式会社蛋白工学研究所 | 変異ヒト腫傷壊死因子 |
| CA2118508A1 (en) | 1992-04-24 | 1993-11-11 | Elizabeth S. Ward | Recombinant production of immunoglobulin-like domains in prokaryotic cells |
| CA2163345A1 (en) | 1993-06-16 | 1994-12-22 | Susan Adrienne Morgan | Antibodies |
| US5869046A (en) | 1995-04-14 | 1999-02-09 | Genentech, Inc. | Altered polypeptides with increased half-life |
| US6121022A (en) | 1995-04-14 | 2000-09-19 | Genentech, Inc. | Altered polypeptides with increased half-life |
| US6096871A (en) | 1995-04-14 | 2000-08-01 | Genentech, Inc. | Polypeptides altered to contain an epitope from the Fc region of an IgG molecule for increased half-life |
| JP4046354B2 (ja) | 1996-03-18 | 2008-02-13 | ボード オブ リージェンツ,ザ ユニバーシティ オブ テキサス システム | 増大した半減期を有する免疫グロブリン様ドメイン |
| US6277375B1 (en) | 1997-03-03 | 2001-08-21 | Board Of Regents, The University Of Texas System | Immunoglobulin-like domains with increased half-lives |
| US6194551B1 (en) | 1998-04-02 | 2001-02-27 | Genentech, Inc. | Polypeptide variants |
| US6737056B1 (en) | 1999-01-15 | 2004-05-18 | Genentech, Inc. | Polypeptide variants with altered effector function |
| PL220113B1 (pl) | 1999-01-15 | 2015-08-31 | Genentech Inc | Wariant macierzystego polipeptydu zawierającego region Fc, polipeptyd zawierający wariant regionu Fc o zmienionym powinowactwie wiązania receptora Fc gamma (FcγR), polipeptyd zawierający wariant regionu Fc o zmienionym powinowactwie wiązania noworodkowego receptora Fc (FcRn), kompozycja, wyizolowany kwas nukleinowy, wektor, komórka gospodarza, sposób otrzymywania wariantu polipeptydu, zastosowanie wariantu polipeptydu i sposób otrzymywania wariantu regionu Fc |
| CA2377585A1 (en) * | 1999-06-28 | 2001-01-04 | Genentech, Inc. | Methods for making apo-2 ligand using divalent metal ions |
| US7091321B2 (en) | 2000-02-11 | 2006-08-15 | Emd Lexigen Research Center Corp. | Enhancing the circulating half-life of antibody-based fusion proteins |
| US6725230B2 (en) | 2000-07-18 | 2004-04-20 | Aegis Analytical Corporation | System, method and computer program for assembling process data of multi-database origins using a hierarchical display |
| US20040002587A1 (en) | 2002-02-20 | 2004-01-01 | Watkins Jeffry D. | Fc region variants |
| US20040132101A1 (en) | 2002-09-27 | 2004-07-08 | Xencor | Optimized Fc variants and methods for their generation |
| US7317091B2 (en) | 2002-03-01 | 2008-01-08 | Xencor, Inc. | Optimized Fc variants |
| EP1534335B9 (en) | 2002-08-14 | 2016-01-13 | Macrogenics, Inc. | Fcgammariib-specific antibodies and methods of use thereof |
| EP2298805A3 (en) | 2002-09-27 | 2011-04-13 | Xencor, Inc. | Optimized Fc variants and methods for their generation |
| DK1562972T3 (da) | 2002-10-15 | 2010-12-06 | Facet Biotech Corp | Modifikation af FcRn-bindingsaffiniteter eller serumhalveringstider for antistoffer ved mutagenese |
| CA2512729C (en) | 2003-01-09 | 2014-09-16 | Macrogenics, Inc. | Identification and engineering of antibodies with variant fc regions and methods of using same |
| US8101720B2 (en) | 2004-10-21 | 2012-01-24 | Xencor, Inc. | Immunoglobulin insertions, deletions and substitutions |
| GB0324368D0 (en) | 2003-10-17 | 2003-11-19 | Univ Cambridge Tech | Polypeptides including modified constant regions |
| GB0328261D0 (en) * | 2003-12-05 | 2004-01-07 | Univ Groningen | Improved cytokine design |
| CA2552788C (en) | 2004-01-12 | 2013-09-24 | Applied Molecular Evolution, Inc. | Fc region variants |
| EP1737890A2 (en) | 2004-03-24 | 2007-01-03 | Xencor, Inc. | Immunoglobulin variants outside the fc region |
| CN101001873B (zh) | 2004-08-04 | 2013-03-13 | 曼璀克生物科技有限责任公司 | Fc区变体 |
| GB0724532D0 (en) * | 2007-12-17 | 2008-01-30 | Nat Univ Ireland | Trail variants for treating cancer |
| DK2604693T3 (en) * | 2008-07-21 | 2016-05-30 | Apogenix Gmbh | Single-chain TNFSF molecules |
| JP5677972B2 (ja) | 2008-11-18 | 2015-02-25 | メリマック ファーマシューティカルズ インコーポレーティッド | ヒト血清アルブミンリンカーおよびそのコンジュゲート |
| ES2548030T3 (es) * | 2009-06-01 | 2015-10-13 | Medimmune, Llc | Moléculas con semividas prolongadas y usos de las mismas |
| EP2564188A4 (en) | 2010-04-26 | 2013-09-25 | Merrimack Pharmaceuticals Inc | TESTS FOR ANTI-MEDICATION ANTIBODIES IN THE PRESENCE OF ENDOGENOUS PROTEIN COMPOUNDS OF THE MEDICINAL PRODUCT |
| EP2468764A1 (en) * | 2010-12-24 | 2012-06-27 | Rijksuniversiteit te Groningen | TNF family ligand variants |
| US9822179B2 (en) * | 2011-04-01 | 2017-11-21 | Universitat Stuttgart | Recombinant TNF ligand family member polypeptides with antibody binding domain and uses therefor |
| US20130150566A1 (en) * | 2011-07-06 | 2013-06-13 | Targetpharma Laboratories (Changzhou) Co., Ltd | Tumor-targeted tnf-related apoptosis-inducing ligand's variant and the application thereof |
| NO2776305T3 (cg-RX-API-DMAC7.html) * | 2014-04-23 | 2018-01-27 | ||
| US10428149B2 (en) * | 2015-03-18 | 2019-10-01 | Universitat Stuttgart | Single-chain tumor necrosis factor (TNF) ligand family molecules, fusion proteins and derivatives thereof |
-
2017
- 2017-03-16 AU AU2017234679A patent/AU2017234679A1/en not_active Abandoned
- 2017-03-16 CA CA3017622A patent/CA3017622A1/en not_active Abandoned
- 2017-03-16 MX MX2018011219A patent/MX2018011219A/es unknown
- 2017-03-16 US US16/084,447 patent/US20190077870A1/en not_active Abandoned
- 2017-03-16 EP EP17713861.7A patent/EP3430034A1/en not_active Withdrawn
- 2017-03-16 WO PCT/US2017/022789 patent/WO2017161173A1/en not_active Ceased
- 2017-03-16 CN CN201780016988.2A patent/CN108884142A/zh active Pending
- 2017-03-16 JP JP2018548878A patent/JP2019518713A/ja active Pending
- 2017-03-16 KR KR1020187029745A patent/KR20180127407A/ko not_active Withdrawn
-
2018
- 2018-08-21 IL IL261267A patent/IL261267A/en unknown
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2022080865A3 (ko) * | 2020-10-13 | 2022-08-11 | 신동준 | 반려견의 항암용 재조합 단백질 및 이를 포함하는 반려견을 위한 항암용 조성물 |
Also Published As
| Publication number | Publication date |
|---|---|
| EP3430034A1 (en) | 2019-01-23 |
| MX2018011219A (es) | 2019-01-10 |
| CN108884142A (zh) | 2018-11-23 |
| CA3017622A1 (en) | 2017-09-21 |
| IL261267A (en) | 2018-10-31 |
| AU2017234679A1 (en) | 2018-08-30 |
| US20190077870A1 (en) | 2019-03-14 |
| WO2017161173A1 (en) | 2017-09-21 |
| JP2019518713A (ja) | 2019-07-04 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP7587656B2 (ja) | Pd-l1及びcd137に結合する抗体分子 | |
| KR102427192B1 (ko) | 항-인간 4-1bb 항체 및 그의 용도 | |
| US11377477B2 (en) | PD-1 targeted IL-15/IL-15RALPHA fc fusion proteins and uses in combination therapies thereof | |
| TWI853809B (zh) | 包含4-1bbl之靶向her2的抗原結合分子 | |
| TW202227494A (zh) | 多特異性免疫靶向分子及其用途 | |
| KR20220016945A (ko) | 다중특이적 단백질 | |
| KR20180031728A (ko) | 다가 및 다중특이적 gitr 결합 융합 단백질 | |
| JP2020512814A (ja) | 免疫抱合体 | |
| CA2956126A1 (en) | Sirp-alpha immunoglobulin fusion proteins | |
| KR20140054268A (ko) | 탠덤 fc 이중특이적 항체 | |
| CN107250161A (zh) | 包含dr5‑结合结构域的多价分子 | |
| JP2022531306A (ja) | CLEC12a結合性ポリペプチド及びその使用 | |
| JP2022531765A (ja) | Cd33結合性ポリペプチド及びその使用 | |
| KR20180127407A (ko) | 암 요법용 조작된 trail | |
| CN111465618A (zh) | 双特异性cd16-结合分子及其在疾病治疗中的用途 | |
| JP2023524995A (ja) | イヌpd-1結合性ポリペプチド及びその使用 | |
| KR20190117467A (ko) | IFN-γ-유도성 조절 T 세포 전환가능 항암 (IRTCA) 항체 및 그의 용도 | |
| KR20230024408A (ko) | 항-cldn-18.2 항체 및 그 용도 | |
| RU2826084C2 (ru) | МОЛЕКУЛЫ АНТИТЕЛА, КОТОРЫЕ СВЯЗЫВАЮТ PD-L1 и CD137 | |
| RU2777573C2 (ru) | Антитела против 4-1bb человека и их применение |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PA0105 | International application |
Patent event date: 20181015 Patent event code: PA01051R01D Comment text: International Patent Application |
|
| PG1501 | Laying open of application | ||
| PA0201 | Request for examination |
Patent event code: PA02012R01D Patent event date: 20200313 Comment text: Request for Examination of Application |
|
| PC1202 | Submission of document of withdrawal before decision of registration |
Comment text: [Withdrawal of Procedure relating to Patent, etc.] Withdrawal (Abandonment) Patent event code: PC12021R01D Patent event date: 20210305 |