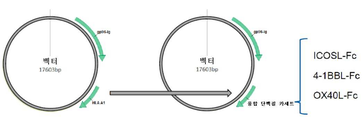
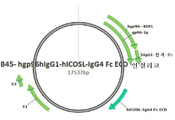
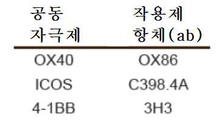
KR20170109582A - 벡터 공동 발현 백신 및 공동 자극 분자 - Google Patents
벡터 공동 발현 백신 및 공동 자극 분자 Download PDFInfo
- Publication number
- KR20170109582A KR20170109582A KR1020177022218A KR20177022218A KR20170109582A KR 20170109582 A KR20170109582 A KR 20170109582A KR 1020177022218 A KR1020177022218 A KR 1020177022218A KR 20177022218 A KR20177022218 A KR 20177022218A KR 20170109582 A KR20170109582 A KR 20170109582A
- Authority
- KR
- South Korea
- Prior art keywords
- cell
- leu
- ser
- val
- cells
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Ceased
Links
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K39/39—Medicinal preparations containing antigens or antibodies characterised by the immunostimulating additives, e.g. chemical adjuvants
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K39/0005—Vertebrate antigens
- A61K39/0011—Cancer antigens
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K39/0005—Vertebrate antigens
- A61K39/0011—Cancer antigens
- A61K39/001102—Receptors, cell surface antigens or cell surface determinants
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K39/395—Antibodies; Immunoglobulins; Immune serum, e.g. antilymphocytic serum
- A61K39/39533—Antibodies; Immunoglobulins; Immune serum, e.g. antilymphocytic serum against materials from animals
- A61K39/3955—Antibodies; Immunoglobulins; Immune serum, e.g. antilymphocytic serum against materials from animals against proteinaceous materials, e.g. enzymes, hormones, lymphokines
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K39/395—Antibodies; Immunoglobulins; Immune serum, e.g. antilymphocytic serum
- A61K39/42—Antibodies; Immunoglobulins; Immune serum, e.g. antilymphocytic serum viral
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K48/00—Medicinal preparations containing genetic material which is inserted into cells of the living body to treat genetic diseases; Gene therapy
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P31/00—Antiinfectives, i.e. antibiotics, antiseptics, chemotherapeutics
- A61P31/12—Antivirals
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P31/00—Antiinfectives, i.e. antibiotics, antiseptics, chemotherapeutics
- A61P31/12—Antivirals
- A61P31/14—Antivirals for RNA viruses
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P31/00—Antiinfectives, i.e. antibiotics, antiseptics, chemotherapeutics
- A61P31/12—Antivirals
- A61P31/14—Antivirals for RNA viruses
- A61P31/18—Antivirals for RNA viruses for HIV
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P31/00—Antiinfectives, i.e. antibiotics, antiseptics, chemotherapeutics
- A61P31/12—Antivirals
- A61P31/20—Antivirals for DNA viruses
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P33/00—Antiparasitic agents
- A61P33/02—Antiprotozoals, e.g. for leishmaniasis, trichomoniasis, toxoplasmosis
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P43/00—Drugs for specific purposes, not provided for in groups A61P1/00-A61P41/00
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K14/00—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- C07K14/435—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans
- C07K14/46—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans from vertebrates
- C07K14/47—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans from vertebrates from mammals
- C07K14/4701—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans from vertebrates from mammals not used
- C07K14/4702—Regulators; Modulating activity
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K14/00—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- C07K14/435—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans
- C07K14/705—Receptors; Cell surface antigens; Cell surface determinants
- C07K14/70503—Immunoglobulin superfamily
- C07K14/70532—B7 molecules, e.g. CD80, CD86
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K14/00—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- C07K14/435—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans
- C07K14/705—Receptors; Cell surface antigens; Cell surface determinants
- C07K14/70575—NGF/TNF-superfamily, e.g. CD70, CD95L, CD153, CD154
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/18—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans
- C07K16/28—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants
- C07K16/2878—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants against the NGF-receptor/TNF-receptor superfamily, e.g. CD27, CD30, CD40, CD95
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N15/00—Mutation or genetic engineering; DNA or RNA concerning genetic engineering, vectors, e.g. plasmids, or their isolation, preparation or purification; Use of hosts therefor
- C12N15/09—Recombinant DNA-technology
- C12N15/63—Introduction of foreign genetic material using vectors; Vectors; Use of hosts therefor; Regulation of expression
- C12N15/79—Vectors or expression systems specially adapted for eukaryotic hosts
- C12N15/85—Vectors or expression systems specially adapted for eukaryotic hosts for animal cells
- C12N15/86—Viral vectors
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K2039/505—Medicinal preparations containing antigens or antibodies comprising antibodies
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K2039/51—Medicinal preparations containing antigens or antibodies comprising whole cells, viruses or DNA/RNA
- A61K2039/515—Animal cells
- A61K2039/5152—Tumor cells
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K2039/51—Medicinal preparations containing antigens or antibodies comprising whole cells, viruses or DNA/RNA
- A61K2039/515—Animal cells
- A61K2039/5156—Animal cells expressing foreign proteins
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K2039/51—Medicinal preparations containing antigens or antibodies comprising whole cells, viruses or DNA/RNA
- A61K2039/525—Virus
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K2039/51—Medicinal preparations containing antigens or antibodies comprising whole cells, viruses or DNA/RNA
- A61K2039/525—Virus
- A61K2039/5258—Virus-like particles
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K2039/51—Medicinal preparations containing antigens or antibodies comprising whole cells, viruses or DNA/RNA
- A61K2039/53—DNA (RNA) vaccination
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K2039/555—Medicinal preparations containing antigens or antibodies characterised by a specific combination antigen/adjuvant
- A61K2039/55511—Organic adjuvants
- A61K2039/55516—Proteins; Peptides
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K2039/57—Medicinal preparations containing antigens or antibodies characterised by the type of response, e.g. Th1, Th2
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/70—Immunoglobulins specific features characterized by effect upon binding to a cell or to an antigen
- C07K2317/75—Agonist effect on antigen
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2319/00—Fusion polypeptide
- C07K2319/30—Non-immunoglobulin-derived peptide or protein having an immunoglobulin constant or Fc region, or a fragment thereof, attached thereto
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2319/00—Fusion polypeptide
- C07K2319/40—Fusion polypeptide containing a tag for immunodetection, or an epitope for immunisation
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2319/00—Fusion polypeptide
- C07K2319/70—Fusion polypeptide containing domain for protein-protein interaction
- C07K2319/74—Fusion polypeptide containing domain for protein-protein interaction containing a fusion for binding to a cell surface receptor
- C07K2319/75—Fusion polypeptide containing domain for protein-protein interaction containing a fusion for binding to a cell surface receptor containing a fusion for activation of a cell surface receptor, e.g. thrombopoeitin, NPY and other peptide hormones
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y02—TECHNOLOGIES OR APPLICATIONS FOR MITIGATION OR ADAPTATION AGAINST CLIMATE CHANGE
- Y02A—TECHNOLOGIES FOR ADAPTATION TO CLIMATE CHANGE
- Y02A50/00—TECHNOLOGIES FOR ADAPTATION TO CLIMATE CHANGE in human health protection, e.g. against extreme weather
- Y02A50/30—Against vector-borne diseases, e.g. mosquito-borne, fly-borne, tick-borne or waterborne diseases whose impact is exacerbated by climate change
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Medicinal Chemistry (AREA)
- General Health & Medical Sciences (AREA)
- Organic Chemistry (AREA)
- Immunology (AREA)
- Veterinary Medicine (AREA)
- Public Health (AREA)
- Animal Behavior & Ethology (AREA)
- Pharmacology & Pharmacy (AREA)
- Genetics & Genomics (AREA)
- Engineering & Computer Science (AREA)
- Molecular Biology (AREA)
- Microbiology (AREA)
- Epidemiology (AREA)
- Virology (AREA)
- Mycology (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Zoology (AREA)
- Biochemistry (AREA)
- Biophysics (AREA)
- Oncology (AREA)
- Chemical Kinetics & Catalysis (AREA)
- General Chemical & Material Sciences (AREA)
- Biotechnology (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Cell Biology (AREA)
- General Engineering & Computer Science (AREA)
- Wood Science & Technology (AREA)
- Biomedical Technology (AREA)
- Gastroenterology & Hepatology (AREA)
- Toxicology (AREA)
- Communicable Diseases (AREA)
- Plant Pathology (AREA)
- Physics & Mathematics (AREA)
- Tropical Medicine & Parasitology (AREA)
- Endocrinology (AREA)
- AIDS & HIV (AREA)
Applications Claiming Priority (5)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US201562113153P | 2015-02-06 | 2015-02-06 | |
| US62/113,153 | 2015-02-06 | ||
| US201562174942P | 2015-06-12 | 2015-06-12 | |
| US62/174,942 | 2015-06-12 | ||
| PCT/US2016/016682 WO2016127015A1 (en) | 2015-02-06 | 2016-02-05 | Vector co-expressing vaccine and costimulatory molecules |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| KR20170109582A true KR20170109582A (ko) | 2017-09-29 |
Family
ID=56564722
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020177022218A Ceased KR20170109582A (ko) | 2015-02-06 | 2016-02-05 | 벡터 공동 발현 백신 및 공동 자극 분자 |
Country Status (15)
| Country | Link |
|---|---|
| US (4) | US10046047B2 (enExample) |
| EP (1) | EP3253876B1 (enExample) |
| JP (1) | JP6744318B2 (enExample) |
| KR (1) | KR20170109582A (enExample) |
| CN (1) | CN107208099B (enExample) |
| AU (1) | AU2016215175B2 (enExample) |
| BR (1) | BR112017016681A2 (enExample) |
| CA (1) | CA2975191C (enExample) |
| DK (1) | DK3253876T3 (enExample) |
| ES (1) | ES2845727T3 (enExample) |
| IL (1) | IL253423B (enExample) |
| MX (1) | MX384422B (enExample) |
| RU (1) | RU2714157C2 (enExample) |
| SG (1) | SG11201705844SA (enExample) |
| WO (1) | WO2016127015A1 (enExample) |
Families Citing this family (22)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| SG11201705844SA (en) * | 2015-02-06 | 2017-08-30 | Heat Biologics Inc | Vector co-expressing vaccine and costimulatory molecules |
| CN107708725A (zh) * | 2015-04-02 | 2018-02-16 | 坎库雷有限公司 | 用于引发免疫应答的剂和组合物 |
| CN116063566A (zh) | 2015-10-01 | 2023-05-05 | 热生物制品有限公司 | 作为异源嵌合蛋白邻接i型和ii型胞外结构域的组合物和方法 |
| EP3515486A4 (en) * | 2016-09-26 | 2020-05-20 | Advantagene, Inc. | METHOD FOR TREATING TIM-3 INCREASE |
| US11666649B2 (en) | 2016-10-11 | 2023-06-06 | University Of Miami | Vectors and vaccine cells for immunity against Zika virus |
| EP3577133A1 (en) | 2017-02-06 | 2019-12-11 | Orionis Biosciences NV | Targeted chimeric proteins and uses thereof |
| CN110381974A (zh) * | 2017-02-27 | 2019-10-25 | 沙塔克实验室有限公司 | 基于csf1r的嵌合蛋白 |
| CN110234345A (zh) | 2017-02-27 | 2019-09-13 | 沙塔克实验室有限公司 | 制备和使用基于细胞外结构域的嵌合蛋白的方法 |
| US11192933B2 (en) * | 2017-02-27 | 2021-12-07 | Shattuck Labs, Inc. | VSIG8-based chimeric proteins |
| CA3058938A1 (en) | 2017-04-04 | 2018-10-11 | Heat Biologics, Inc. | Intratumoral vaccination |
| WO2019104327A1 (en) * | 2017-11-27 | 2019-05-31 | Heat Biologics, Inc. | Gp96-based cancer therapy |
| EP3720450A4 (en) * | 2017-12-04 | 2021-07-28 | Heat Biologics, Inc. | CELL-BASED VACCINE PRODUCTION |
| US12084497B2 (en) | 2018-08-08 | 2024-09-10 | Orionis Biosciences, Inc. | SIRP1α targeted chimeric proteins and uses thereof |
| CN112888706A (zh) * | 2018-08-29 | 2021-06-01 | 沙塔克实验室有限公司 | 包含基于tim-3的嵌合蛋白的组合疗法 |
| WO2020047319A1 (en) * | 2018-08-29 | 2020-03-05 | Shattuck Labs, Inc. | Combination therapies comprising sirp alpha-based chimeric proteins |
| US10780121B2 (en) | 2018-08-29 | 2020-09-22 | Shattuck Labs, Inc. | FLT3L-based chimeric proteins |
| EP3860647A4 (en) * | 2018-10-01 | 2022-10-19 | Heat Biologics, Inc. | CELL-BASED COMBINATION THERAPIES |
| WO2020106621A1 (en) * | 2018-11-19 | 2020-05-28 | Board Of Regents, The University Of Texas System | A modular, polycistronic vector for car and tcr transduction |
| EP4021494A1 (en) * | 2019-08-30 | 2022-07-06 | Pelican Therapeutics, Inc. | Methods of treating cancer using tnfrsf25 antibodies |
| US20210283242A1 (en) * | 2020-03-02 | 2021-09-16 | Heat Biologics, Inc. | Immune-mediated coronavirus treatments |
| US20240409608A1 (en) * | 2021-10-26 | 2024-12-12 | Navicure Biopharmaceuticals Limited | Combination therapies against cancer and infectious diseases |
| WO2025227519A1 (zh) * | 2024-04-29 | 2025-11-06 | 北京臻知医学科技有限责任公司 | 一种肿瘤疫苗及其应用 |
Family Cites Families (136)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4690915A (en) | 1985-08-08 | 1987-09-01 | The United States Of America As Represented By The Department Of Health And Human Services | Adoptive immunotherapy as a treatment modality in humans |
| US5217891A (en) | 1987-07-28 | 1993-06-08 | Chiron Corporation | DNA constructs containing a kluyveromyces α factor leader sequence for directing secretion of heterologous polypeptides |
| CA1338778C (en) | 1988-06-15 | 1996-12-10 | Richard A. Young | Stress proteins and uses therefor |
| WO1990002564A1 (en) | 1988-09-12 | 1990-03-22 | Codon | Vaccine diagnostic employing proteins homologous to heat shock proteins of trypanosoma cruzi |
| US5232833A (en) | 1988-09-14 | 1993-08-03 | Stressgen Biotechnologies Corporation | Accumulation of heat shock proteins for evaluating biological damage due to chronic exposure of an organism to sublethal levels of pollutants |
| EP0484440A4 (en) | 1989-07-28 | 1993-01-07 | Us Health | Efficient directional genetic cloning system |
| GB9007194D0 (en) | 1990-03-30 | 1990-05-30 | Wellcome Found | Live vaccines |
| US5348945A (en) | 1990-04-06 | 1994-09-20 | Wake Forest University | Method of treatment with hsp70 |
| US5188964A (en) | 1990-04-12 | 1993-02-23 | Board Of Regents, The University Of Texas System | Method and kit for the prognostication of breast cancer patient via heat shock/stress protein determination |
| GB9016315D0 (en) | 1990-07-25 | 1990-09-12 | Burnie James P | Medicaments |
| GB9024320D0 (en) | 1990-11-08 | 1990-12-19 | Univ London | Treatment of uveitis |
| JPH06501479A (ja) | 1990-11-08 | 1994-02-17 | スタンフオード・ルツク・リミテツド | 抗原担体 |
| GB2251186A (en) | 1990-12-04 | 1992-07-01 | Randall Neal Gatz | Polypeptide for use in treatment of autoimmune disease |
| US5177942A (en) | 1991-08-15 | 1993-01-12 | The Toro Company | Mower deck with improved belt drive arrangement |
| GB9200949D0 (en) | 1992-01-17 | 1992-03-11 | Medical Res Council | Diagnostic peptides |
| FR2688227A1 (fr) | 1992-03-04 | 1993-09-10 | Inst Nat Sante Rech Med | Proteines formant des complexes avec des chaperones et leurs ligands, leurs fragments, leur obtention et leurs applications biologiques. |
| IT1262896B (it) | 1992-03-06 | 1996-07-22 | Composti coniugati formati da proteine heat shock (hsp) e oligo-poli- saccaridi, loro uso per la produzione di vaccini. | |
| HUT70972A (en) | 1992-03-09 | 1995-11-28 | Ist Naz Stud Cura Dei Tumori | Protein compound capable of inhibiting tumoral growth |
| WO1993021529A1 (en) | 1992-04-14 | 1993-10-28 | Duke University | Method of detecting tumors containing complexes of p53 and hsp70 |
| JPH07509694A (ja) | 1992-05-14 | 1995-10-26 | ベイラー・カレッジ・オブ・メディシン | 突然変異したステロイドホルモン受容体,その使用方法および遺伝子治療のための分子スイッチ |
| IL102687A (en) | 1992-07-30 | 1997-06-10 | Yeda Res & Dev | Conjugates of poorly immunogenic antigens and synthetic pepide carriers and vaccines comprising them |
| WO1994003599A1 (fr) | 1992-08-04 | 1994-02-17 | Sagami Chemical Research Center | ADNc HUMAIN ET PROTEINE POUR LAQUELLE CODE CET ADNc |
| GB2270076A (en) | 1992-08-18 | 1994-03-02 | Univ Manchester | Human HSP 90 Epitopes |
| GB9223816D0 (en) | 1992-11-13 | 1993-01-06 | Medical Res Council | Heat shock proteins and the treatment of tumours |
| NZ263550A (en) | 1993-03-19 | 1996-12-20 | Boehringer Ingelheim Int | Preparing cancer vaccines containing autologous tumour cells characterised in that tumour cells or firbroblasts are cultivated and transfected ex vivo with a composition of dna and a dna binding molecule conjugate |
| US5496934A (en) | 1993-04-14 | 1996-03-05 | Yissum Research Development Company Of The Hebrew University Of Jerusalem | Nucleic acids encoding a cellulose binding domain |
| US6004941A (en) | 1993-06-14 | 1999-12-21 | Basf Aktiengesellschaft | Methods for regulating gene expression |
| WO1995004824A1 (en) | 1993-08-05 | 1995-02-16 | Medvet Science Pty. Ltd. | Generation of dna libraries and retroviral vectors for same |
| US5705159A (en) | 1993-08-31 | 1998-01-06 | John Wayne Cancer Institute | Immunoreactive peptide sequence from a 43 KD human cancer antigen |
| US5997873A (en) | 1994-01-13 | 1999-12-07 | Mount Sinai School Of Medicine Of The City University Of New York | Method of preparation of heat shock protein 70-peptide complexes |
| US5750119A (en) | 1994-01-13 | 1998-05-12 | Mount Sinai School Of Medicine Of The City University Of New York | Immunotherapeutic stress protein-peptide complexes against cancer |
| US5961979A (en) | 1994-03-16 | 1999-10-05 | Mount Sinai School Of Medicine Of The City University Of New York | Stress protein-peptide complexes as prophylactic and therapeutic vaccines against intracellular pathogens |
| WO1996001611A1 (en) | 1994-07-08 | 1996-01-25 | Baxter International Inc. | Implanted device containing tumor cells for the treatment of cancer |
| US5772995A (en) | 1994-07-18 | 1998-06-30 | Sidney Kimmel Cancer Center | Compositions and methods for enhanced tumor cell immunity in vivo |
| JP3907698B2 (ja) | 1994-10-03 | 2007-04-18 | アメリカ合衆国 | 抗原を発現する組み換えウイルスと免疫刺激分子を発現する組み換えウイルスとを含む組成物 |
| US5703057A (en) | 1995-04-07 | 1997-12-30 | Board Of Regents The University Of Texas System | Expression library immunization |
| IL123219A0 (en) | 1995-08-18 | 1998-09-24 | Sloan Kettering Inst Cancer | Method for treatment of cancer and infectious diseases and compositions useful in same |
| US6761892B1 (en) | 1995-08-18 | 2004-07-13 | Sloan-Kettering Institute For Cancer Research | Heat shock protein-based vaccines and immunotherapies |
| US6719974B1 (en) | 1995-08-18 | 2004-04-13 | Sloan-Kettering Institute For Cancer Research | Heat shock protein-based vaccines and immunotherapies |
| US6331299B1 (en) | 1995-08-18 | 2001-12-18 | Sloan-Kettering Institute For Cancer Research | Method for treatment of cancer and infectious disease and compositions useful in same |
| EP0851765A4 (en) | 1995-09-13 | 2000-01-19 | Univ Fordham | THERAPEUTIC AND PROPHYLACTIC PROCESSES THAT USE HEAT SHOCK PROTEINS |
| US5985270A (en) | 1995-09-13 | 1999-11-16 | Fordham University | Adoptive immunotherapy using macrophages sensitized with heat shock protein-epitope complexes |
| US5935576A (en) | 1995-09-13 | 1999-08-10 | Fordham University | Compositions and methods for the treatment and prevention of neoplastic diseases using heat shock proteins complexed with exogenous antigens |
| US5837251A (en) | 1995-09-13 | 1998-11-17 | Fordham University | Compositions and methods using complexes of heat shock proteins and antigenic molecules for the treatment and prevention of neoplastic diseases |
| DE19602985A1 (de) | 1996-01-27 | 1997-07-31 | Max Delbrueck Centrum | Tumorzellimpfstoff für die Immuntheraphie von malignen Tumoren |
| WO1997026910A2 (de) | 1996-01-27 | 1997-07-31 | Max-Delbrück-Centrum für Molekulare Medizin | Tumorimpfstoff für die immuntherapie von malignen tumoren |
| CA2249354A1 (en) | 1996-03-28 | 1997-10-02 | Genitrix, L.L.C. | Opsonin-enhanced cells, and methods of modulating an immune response to an antigen |
| US5747332A (en) | 1996-09-20 | 1998-05-05 | University Of New Mexico | Methods for purifying and synthesizing heat shock protein complexes |
| US6130087A (en) | 1996-10-07 | 2000-10-10 | Fordham University | Methods for generating cytotoxic T cells in vitro |
| DK0941315T3 (da) | 1996-11-26 | 2006-05-15 | Stressgen Biotechnologies Corp | Fusionsproteiner indeholdende stressproteiner til induktion af immunreaktioner |
| US6017540A (en) | 1997-02-07 | 2000-01-25 | Fordham University | Prevention and treatment of primary and metastatic neoplastic diseases and infectious diseases with heat shock/stress protein-peptide complexes |
| US5830464A (en) | 1997-02-07 | 1998-11-03 | Fordham University | Compositions and methods for the treatment and growth inhibition of cancer using heat shock/stress protein-peptide complexes in combination with adoptive immunotherapy |
| US6015709A (en) | 1997-08-26 | 2000-01-18 | Ariad Pharmaceuticals, Inc. | Transcriptional activators, and compositions and uses related thereto |
| US6007821A (en) | 1997-10-16 | 1999-12-28 | Fordham University | Method and compositions for the treatment of autoimmune disease using heat shock proteins |
| US5948646A (en) | 1997-12-11 | 1999-09-07 | Fordham University | Methods for preparation of vaccines against cancer comprising heat shock protein-peptide complexes |
| DE69942810D1 (de) | 1998-02-20 | 2010-11-11 | Univ Miami | Modifizierter Hitzeschockprotein-Antigenpeptid-Komplex |
| US5989910A (en) | 1998-08-03 | 1999-11-23 | University Of Lausanne | Potent genetic switch allowing regulated gene expression in eukaryotic cells |
| US6797480B1 (en) | 1998-10-05 | 2004-09-28 | University Of Connecticut Health Center | Purification of heat shock/stress protein cell surface receptors and their use as immunotherapeutic agents |
| US6451316B1 (en) | 1998-10-05 | 2002-09-17 | University Of Conneticut Health Center | Methods for generating antigen-reactive T cells in vitro |
| US6475490B1 (en) | 1998-10-19 | 2002-11-05 | Fordham University | Compositions and methods for promoting tissue repair using heat shock proteins |
| US6734172B2 (en) * | 1998-11-18 | 2004-05-11 | Pacific Northwest Research Institute | Surface receptor antigen vaccines |
| CA2362126C (en) * | 1999-02-03 | 2017-01-17 | Amgen Inc. | B7rp-1 polypeptides |
| CN101070540B (zh) * | 2000-04-28 | 2015-04-01 | 约翰霍普金斯大学 | 新的树突状细胞共刺激分子 |
| US7132109B1 (en) | 2000-10-20 | 2006-11-07 | University Of Connecticut Health Center | Using heat shock proteins to increase immune response |
| CA2452517A1 (en) | 2001-07-11 | 2003-01-23 | University Of Miami | Recombinant vsv for the treatment of tumor cells |
| JP4384489B2 (ja) * | 2001-08-20 | 2009-12-16 | ユニバーシティー オブ コネティカット ヘルス センター | 癌及び感染性疾患の治療に有用な熱ショックタンパク質又はα−2−マクログロブリンを含む組成物の調製方法 |
| ATE435655T1 (de) * | 2001-10-09 | 2009-07-15 | Mayo Foundation | Verwendung von agonistischen 4-1bb antikörpern zur erhöhung der immunantwort |
| EP1456376A4 (en) | 2001-11-16 | 2006-09-06 | Us Gov Health & Human Serv | NEW CHIMERAL REV, TAT AND NEF ANTIGENES |
| WO2003057171A2 (en) * | 2002-01-03 | 2003-07-17 | The Trustees Of The University Of Pennsylvania | Activation and expansion of t-cells using an engineered multivalent signaling platform |
| US20030170756A1 (en) | 2002-02-01 | 2003-09-11 | Thomas Jefferson University | Treatment of tumor cells for use in immunotherapy of cancer |
| CN1440812A (zh) * | 2002-02-27 | 2003-09-10 | 上海中信国健药业有限公司 | 一种使用cd137结合激动剂增强免疫应答的方法 |
| WO2003072595A2 (en) * | 2002-02-28 | 2003-09-04 | Antigenics Inc. | Methods and products based on oligomerization of stress proteins |
| IL164376A0 (en) * | 2002-04-03 | 2005-12-18 | Applied Research Systems | Ox4or binding agents, their preparation and pharmaceutical compositions containing them |
| AU2003231098A1 (en) * | 2002-04-25 | 2003-11-10 | University Of Connecticut Health Center | Using heat shock proteins to improve the therapeutic benefit of a non-vaccine treatment modality |
| US6984389B2 (en) * | 2002-04-25 | 2006-01-10 | University Of Connecticut Health Center | Using heat shock proteins to improve the therapeutic benefit of a non-vaccine treatment modality |
| CA2489004C (en) * | 2002-06-13 | 2013-01-08 | Crucell Holland B.V. | Agonistic binding molecules to the human ox40 receptor |
| WO2004032865A2 (en) | 2002-10-09 | 2004-04-22 | Central Iowa Health System | Antitumor vaccination using allogeneic tumor cells expressing alpha (1,3)-galactosyltransferase |
| US20040197312A1 (en) * | 2003-04-02 | 2004-10-07 | Marina Moskalenko | Cytokine-expressing cellular vaccine combinations |
| AU2004274430A1 (en) * | 2003-09-12 | 2005-03-31 | Antigenics, Inc. | Vaccine for treatment and prevention of herpes simplex virus infection |
| US20070141666A1 (en) | 2003-09-26 | 2007-06-21 | Applied Research Systems Ars Holding N.V. | Leader sequences for use in production of proteins |
| EP1667701A4 (en) | 2003-09-26 | 2007-02-14 | Univ Miami | TUMOR VACCINE |
| CA2548347A1 (en) * | 2003-12-11 | 2005-06-30 | Sidney Kimmel Cancer Center | Methods for generating immunity to antigen |
| FR2867982B1 (fr) | 2004-03-26 | 2007-07-20 | Jean Marie Andrieu | Procede pour amplifier l'activite de vaccins therapeutiques |
| WO2005124346A1 (en) * | 2004-05-17 | 2005-12-29 | The Board Of Trustees Of The University Of Illinois | 4-1bb receptors are expressed on regulatory t-cells |
| EP2243788A1 (en) | 2005-02-24 | 2010-10-27 | Medical Research Council | HIVCON: An HIV immunogen and uses thereof |
| EP2650020B1 (en) * | 2005-05-06 | 2016-08-24 | Providence Health & Services - Oregon | Trimeric OX40-immunoglobulin fusion protein and methods of use |
| CA2621083C (en) * | 2005-08-30 | 2017-04-11 | University Of Miami | Immunomodulating tumor necrosis factor receptor 25 (tnfr25) agonists, antagonists and immunotoxins |
| CA2652599C (en) * | 2006-05-03 | 2019-09-24 | Ross Kedl | Cd40 agonist antibody/type1 interferon synergistic adjuvant combination, conjugates containing and use thereof as a therapeutic to enhance cellular immunity |
| WO2008108808A2 (en) * | 2006-08-24 | 2008-09-12 | Trustees Of Boston University | Complexes derived from heterohybrid cells and uses thereof |
| PT2856876T (pt) * | 2007-03-30 | 2018-03-28 | Memorial Sloan Kettering Cancer Center | Expressão constitutiva de ligantes co-estimulatórios em linfócitos t adotivamente transferidos |
| ITPD20070252A1 (it) * | 2007-07-24 | 2009-01-25 | Primiero Paolo | Complessi di grp94 ed immunoglobuline g umane |
| US9408909B2 (en) * | 2007-09-14 | 2016-08-09 | Vrije Universiteit Brussel | Enhancing the T-cell stimulatory capacity of human antigen presenting cells in vitro and in vivo and its use in vaccination |
| GB0719509D0 (en) * | 2007-10-05 | 2007-11-14 | Isis Innovation | Molecular adjuvant |
| US8475785B2 (en) | 2008-03-03 | 2013-07-02 | The University Of Miami | Allogeneic cancer cell-based immunotherapy |
| AU2009223784A1 (en) * | 2008-03-08 | 2009-09-17 | Immungene, Inc. | Engineered fusion molecules immunotherapy in cancer and inflammatory diseases |
| US8968720B2 (en) | 2008-03-20 | 2015-03-03 | University Of Miami | Heat shock protein GP96 vaccination and methods of using same |
| US9931386B2 (en) * | 2008-06-16 | 2018-04-03 | Atsuo Ochi | Recombinant multiple domain fusion protein mitogens and use thereof for inducing enhancement or repression of antigen-specific immunity |
| CN102223894A (zh) | 2008-11-21 | 2011-10-19 | 迈阿密大学 | 用于产生粘膜和系统免疫的hiv/siv疫苗 |
| WO2010077634A1 (en) | 2008-12-09 | 2010-07-08 | Genentech, Inc. | Anti-pd-l1 antibodies and their use to enhance t-cell function |
| CA2757524A1 (en) * | 2009-04-03 | 2010-10-07 | Agenus Inc. | Methods for preparing and using multichaperone-antigen complexes |
| JP5883384B2 (ja) * | 2009-08-13 | 2016-03-15 | ザ ジョンズ ホプキンス ユニバーシティー | 免疫機能を調節する方法 |
| US9459246B2 (en) | 2009-09-08 | 2016-10-04 | Nodality, Inc. | Induced intercellular communication |
| CA2775761C (en) * | 2009-09-30 | 2018-08-28 | Memorial Sloan-Kettering Cancer Center | Combination immunotherapy for the treatment of cancer |
| WO2011069529A1 (en) | 2009-12-09 | 2011-06-16 | Curevac Gmbh | Mannose-containing solution for lyophilization, transfection and/or injection of nucleic acids |
| JP2013527753A (ja) * | 2010-03-23 | 2013-07-04 | イントレキソン コーポレーション | 治療タンパク質を条件的に発現するベクター、該ベクターを含む宿主細胞およびそれらの使用 |
| US8828944B2 (en) | 2010-04-22 | 2014-09-09 | Institut Gustave Roussy | Compounds and uses thereof to induce an immunogenic cancer cell death in a subject |
| US9527912B2 (en) * | 2010-05-17 | 2016-12-27 | The Board Of Trustees Of The Leland Stanford Junior University | Prevention of immunological rejection of transplanted stem cells by leukocyte costimulatory molecule blockade |
| CA2837059A1 (en) | 2010-05-21 | 2011-11-24 | University Of Miami | Cancer treatment |
| US9234042B2 (en) * | 2010-10-18 | 2016-01-12 | Delphi Genetics Sa | Method for producing antibody using “naked” expression vector expressing type II transmembrane fusion protein |
| CN102462841A (zh) * | 2010-11-09 | 2012-05-23 | 中国科学院微生物研究所 | 一种促进gp96蛋白的免疫活性的方法及其应用 |
| JP2014508762A (ja) * | 2011-02-23 | 2014-04-10 | ユニバーシティー オブ マイアミ | SIV/HIVからの防御を目的とする、組合せ細胞ベースのgp96−Ig−SIV/HIV、組換えgp120タンパク質のワクチン接種 |
| WO2012166617A2 (en) | 2011-05-27 | 2012-12-06 | Cytocure Llc | Methods, compositions, and kits for the treatment of cancer |
| SG11201404677TA (en) * | 2011-12-12 | 2014-11-27 | Cell Medica Ltd | Process of expanding t cells |
| US9567606B2 (en) * | 2012-02-14 | 2017-02-14 | Merial Inc. | Recombinant poxviral vectors expressing both rabies and OX40 proteins, and vaccines made therefrom |
| DK2925350T3 (da) * | 2012-12-03 | 2019-05-13 | Bristol Myers Squibb Co | Øgning af virksomheden mod cancer af immunomodulatoriske fc- fusionsproteiner |
| ES2689080T3 (es) * | 2013-01-02 | 2018-11-08 | Glenmark Pharmaceuticals S.A. | Anticuerpos que se unen a TL1A y sus usos |
| US20140193410A1 (en) * | 2013-01-09 | 2014-07-10 | University Of Miami | Compositions and Methods for the Regulation of T Regulatory Cells Using TL1A-Ig Fusion Protein |
| EP2951209A4 (en) * | 2013-01-31 | 2016-06-22 | Univ Jefferson | CD40 OX40 AGONIST FUSION PROTEIN AND USES THEREOF |
| CA2906356A1 (en) | 2013-03-15 | 2014-09-18 | Novelogics Biotechnology, Inc. | Antibodies to mica and micb proteins |
| WO2014140884A2 (en) | 2013-03-15 | 2014-09-18 | Novelogics Biotechnology, Inc. | Methods and devices for removal of immunosuppressive ligands |
| US10196435B2 (en) * | 2013-11-18 | 2019-02-05 | University Of Southern California | OX40L fusion protein for the immunotherapy of tumors of veterinary animals |
| CA2931322A1 (en) * | 2013-11-22 | 2015-05-28 | Dnatrix, Inc. | Adenovirus expressing immune cell stimulatory receptor agonist(s) |
| EP3082853A2 (en) * | 2013-12-20 | 2016-10-26 | The Broad Institute, Inc. | Combination therapy with neoantigen vaccine |
| WO2015131176A1 (en) * | 2014-02-28 | 2015-09-03 | Podack Eckhard R | Compositions, methods, and kits for treatment of cancer |
| KR20170002410A (ko) * | 2014-04-03 | 2017-01-06 | 오거스타 유니버시티 리서치 인스티튜트, 인크. | 종양 유도 면역 반응의 효능을 향상시키는 방법 |
| BR112016027845A2 (pt) * | 2014-05-29 | 2017-10-31 | Medimmune Llc | proteínas de fusão de ox40l e usos das mesmas |
| MA41414A (fr) * | 2015-01-28 | 2017-12-05 | Centre Nat Rech Scient | Protéines de liaison agonistes d' icos |
| MA41460A (fr) * | 2015-02-03 | 2017-12-12 | Oncomed Pharm Inc | Agents de liaison à la tnfrsf et leurs utilisations |
| SG11201705844SA (en) * | 2015-02-06 | 2017-08-30 | Heat Biologics Inc | Vector co-expressing vaccine and costimulatory molecules |
| TWI716405B (zh) * | 2015-05-07 | 2021-01-21 | 美商艾吉納斯公司 | 抗ox40抗體及其使用方法 |
| CN109219620B (zh) * | 2016-06-09 | 2023-01-31 | 派立卡恩治疗公司 | 抗-tnfrsf25抗体 |
| CA3058938A1 (en) * | 2017-04-04 | 2018-10-11 | Heat Biologics, Inc. | Intratumoral vaccination |
| WO2019104327A1 (en) * | 2017-11-27 | 2019-05-31 | Heat Biologics, Inc. | Gp96-based cancer therapy |
| EP3860647A4 (en) * | 2018-10-01 | 2022-10-19 | Heat Biologics, Inc. | CELL-BASED COMBINATION THERAPIES |
| EP4019536A4 (en) * | 2019-08-19 | 2023-09-06 | Nantong Yichen Biopharma. Co. Ltd. | IMMUNOCYTOTIN, ITS PRODUCTION AND ITS USE |
| US20210283242A1 (en) * | 2020-03-02 | 2021-09-16 | Heat Biologics, Inc. | Immune-mediated coronavirus treatments |
-
2016
- 2016-02-05 SG SG11201705844SA patent/SG11201705844SA/en unknown
- 2016-02-05 EP EP16747310.7A patent/EP3253876B1/en active Active
- 2016-02-05 RU RU2017127025A patent/RU2714157C2/ru active
- 2016-02-05 CN CN201680009177.5A patent/CN107208099B/zh not_active Expired - Fee Related
- 2016-02-05 CA CA2975191A patent/CA2975191C/en active Active
- 2016-02-05 ES ES16747310T patent/ES2845727T3/es active Active
- 2016-02-05 KR KR1020177022218A patent/KR20170109582A/ko not_active Ceased
- 2016-02-05 BR BR112017016681A patent/BR112017016681A2/pt not_active Application Discontinuation
- 2016-02-05 DK DK16747310.7T patent/DK3253876T3/da active
- 2016-02-05 AU AU2016215175A patent/AU2016215175B2/en not_active Ceased
- 2016-02-05 JP JP2017539659A patent/JP6744318B2/ja not_active Expired - Fee Related
- 2016-02-05 US US15/016,375 patent/US10046047B2/en active Active
- 2016-02-05 WO PCT/US2016/016682 patent/WO2016127015A1/en not_active Ceased
- 2016-02-05 MX MX2017010159A patent/MX384422B/es unknown
-
2017
- 2017-07-11 IL IL253423A patent/IL253423B/en unknown
-
2018
- 2018-06-25 US US16/016,770 patent/US10780161B2/en not_active Expired - Fee Related
- 2018-06-25 US US16/016,771 patent/US10758611B2/en not_active Expired - Fee Related
-
2020
- 2020-07-24 US US16/937,766 patent/US20210000945A1/en not_active Abandoned
Also Published As
| Publication number | Publication date |
|---|---|
| BR112017016681A2 (pt) | 2018-04-10 |
| CA2975191C (en) | 2023-05-09 |
| EP3253876A4 (en) | 2018-09-19 |
| AU2016215175A1 (en) | 2017-09-21 |
| AU2016215175B2 (en) | 2021-09-16 |
| US10046047B2 (en) | 2018-08-14 |
| WO2016127015A1 (en) | 2016-08-11 |
| JP6744318B2 (ja) | 2020-08-26 |
| RU2714157C2 (ru) | 2020-02-12 |
| CN107208099A (zh) | 2017-09-26 |
| CA2975191A1 (en) | 2016-08-11 |
| US20180360955A1 (en) | 2018-12-20 |
| CN107208099B (zh) | 2022-05-13 |
| RU2017127025A (ru) | 2019-03-07 |
| IL253423B (en) | 2021-08-31 |
| SG11201705844SA (en) | 2017-08-30 |
| EP3253876B1 (en) | 2020-11-04 |
| RU2017127025A3 (enExample) | 2019-07-17 |
| EP3253876A1 (en) | 2017-12-13 |
| MX2017010159A (es) | 2017-12-18 |
| IL253423A0 (en) | 2017-09-28 |
| ES2845727T3 (es) | 2021-07-27 |
| US10758611B2 (en) | 2020-09-01 |
| DK3253876T3 (da) | 2021-01-25 |
| US20210000945A1 (en) | 2021-01-07 |
| MX384422B (es) | 2025-03-14 |
| US20190000967A1 (en) | 2019-01-03 |
| US20160250322A1 (en) | 2016-09-01 |
| US10780161B2 (en) | 2020-09-22 |
| JP2018509888A (ja) | 2018-04-12 |
| HK1247960A1 (zh) | 2018-10-05 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| CN107208099B (zh) | 共表达疫苗和共刺激分子的载体 | |
| US20200308238A1 (en) | Heterodimeric proteins for modulating gamma delta t cells | |
| US20230303659A1 (en) | Intratumoral vaccination | |
| US20210046177A1 (en) | Compositions and methods for combination cancer vaccine and immunologic adjuvant therapy | |
| JP2022516964A (ja) | ガンマデルタt細胞を調節するためのヘテロ二量体タンパク質 | |
| US20210346486A1 (en) | Combination cell-based therapies | |
| KR20200092964A (ko) | Gp96-기반 암 치료법 | |
| US20220298252A1 (en) | Methods of treating cancer using tnfrsf25 antibodies | |
| HK1247960B (en) | Vector co-expressing vaccine and costimulatory molecules |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PA0105 | International application |
Patent event date: 20170808 Patent event code: PA01051R01D Comment text: International Patent Application |
|
| PG1501 | Laying open of application | ||
| A201 | Request for examination | ||
| PA0201 | Request for examination |
Patent event code: PA02012R01D Patent event date: 20210203 Comment text: Request for Examination of Application |
|
| E902 | Notification of reason for refusal | ||
| PE0902 | Notice of grounds for rejection |
Comment text: Notification of reason for refusal Patent event date: 20230424 Patent event code: PE09021S01D |
|
| E601 | Decision to refuse application | ||
| PE0601 | Decision on rejection of patent |
Patent event date: 20230705 Comment text: Decision to Refuse Application Patent event code: PE06012S01D Patent event date: 20230424 Comment text: Notification of reason for refusal Patent event code: PE06011S01I |