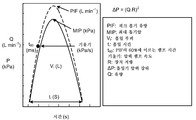
KR20140103939A - 흡입 프로파일-비의존적 약물 전달을 위한 에어로졸화 장치 - Google Patents
흡입 프로파일-비의존적 약물 전달을 위한 에어로졸화 장치 Download PDFInfo
- Publication number
- KR20140103939A KR20140103939A KR1020147015785A KR20147015785A KR20140103939A KR 20140103939 A KR20140103939 A KR 20140103939A KR 1020147015785 A KR1020147015785 A KR 1020147015785A KR 20147015785 A KR20147015785 A KR 20147015785A KR 20140103939 A KR20140103939 A KR 20140103939A
- Authority
- KR
- South Korea
- Prior art keywords
- powder
- flow
- outlet
- opening
- pharmaceutical formulation
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Withdrawn
Links
- 0 *C1CCCC1 Chemical compound *C1CCCC1 0.000 description 1
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M15/00—Inhalators
- A61M15/0028—Inhalators using prepacked dosages, one for each application, e.g. capsules to be perforated or broken-up
- A61M15/003—Inhalators using prepacked dosages, one for each application, e.g. capsules to be perforated or broken-up using capsules, e.g. to be perforated or broken-up
- A61M15/0033—Details of the piercing or cutting means
- A61M15/0035—Piercing means
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/66—Phosphorus compounds
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M11/00—Sprayers or atomisers specially adapted for therapeutic purposes
- A61M11/001—Particle size control
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M15/00—Inhalators
- A61M15/0001—Details of inhalators; Constructional features thereof
- A61M15/0021—Mouthpieces therefor
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M15/00—Inhalators
- A61M15/0028—Inhalators using prepacked dosages, one for each application, e.g. capsules to be perforated or broken-up
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M15/00—Inhalators
- A61M15/0028—Inhalators using prepacked dosages, one for each application, e.g. capsules to be perforated or broken-up
- A61M15/003—Inhalators using prepacked dosages, one for each application, e.g. capsules to be perforated or broken-up using capsules, e.g. to be perforated or broken-up
- A61M15/0033—Details of the piercing or cutting means
- A61M15/0041—Details of the piercing or cutting means with movable piercing or cutting means
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M15/00—Inhalators
- A61M15/0028—Inhalators using prepacked dosages, one for each application, e.g. capsules to be perforated or broken-up
- A61M15/0045—Inhalators using prepacked dosages, one for each application, e.g. capsules to be perforated or broken-up using multiple prepacked dosages on a same carrier, e.g. blisters
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M15/00—Inhalators
- A61M15/0091—Inhalators mechanically breath-triggered
- A61M15/0093—Inhalators mechanically breath-triggered without arming or cocking, e.g. acting directly on the delivery valve
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M15/00—Inhalators
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M2202/00—Special media to be introduced, removed or treated
- A61M2202/06—Solids
- A61M2202/064—Powder
Landscapes
- Health & Medical Sciences (AREA)
- Engineering & Computer Science (AREA)
- Life Sciences & Earth Sciences (AREA)
- Veterinary Medicine (AREA)
- Public Health (AREA)
- General Health & Medical Sciences (AREA)
- Animal Behavior & Ethology (AREA)
- Biomedical Technology (AREA)
- Hematology (AREA)
- Heart & Thoracic Surgery (AREA)
- Anesthesiology (AREA)
- Pulmonology (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Medicinal Chemistry (AREA)
- Chemical & Material Sciences (AREA)
- Pharmacology & Pharmacy (AREA)
- Epidemiology (AREA)
- Medicinal Preparation (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
- Medical Preparation Storing Or Oral Administration Devices (AREA)
- Physical Or Chemical Processes And Apparatus (AREA)
Applications Claiming Priority (5)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US201161576768P | 2011-12-16 | 2011-12-16 | |
| US201161576735P | 2011-12-16 | 2011-12-16 | |
| US61/576,735 | 2011-12-16 | ||
| US61/576,768 | 2011-12-16 | ||
| PCT/US2012/069938 WO2013090841A2 (en) | 2011-12-16 | 2012-12-14 | Aerosolization apparatus for inhalation profile-independent drug delivery |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| KR20140103939A true KR20140103939A (ko) | 2014-08-27 |
Family
ID=47501481
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020147015785A Withdrawn KR20140103939A (ko) | 2011-12-16 | 2012-12-14 | 흡입 프로파일-비의존적 약물 전달을 위한 에어로졸화 장치 |
Country Status (13)
| Country | Link |
|---|---|
| US (1) | US9744318B2 (enExample) |
| EP (1) | EP2790761B1 (enExample) |
| JP (1) | JP2015500712A (enExample) |
| KR (1) | KR20140103939A (enExample) |
| CN (1) | CN103998087B (enExample) |
| AU (1) | AU2012351947B2 (enExample) |
| BR (1) | BR112014014399A2 (enExample) |
| CA (1) | CA2858247A1 (enExample) |
| ES (1) | ES2924032T3 (enExample) |
| IL (1) | IL232762A0 (enExample) |
| MX (1) | MX2014007277A (enExample) |
| RU (1) | RU2615076C2 (enExample) |
| WO (1) | WO2013090841A2 (enExample) |
Families Citing this family (26)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US8822663B2 (en) | 2010-08-06 | 2014-09-02 | Moderna Therapeutics, Inc. | Engineered nucleic acids and methods of use thereof |
| EP2622064B1 (en) | 2010-10-01 | 2019-05-29 | Modernatx, Inc. | Modified nucleosides, nucleotides, and nucleic acids, and uses thereof |
| US8710200B2 (en) | 2011-03-31 | 2014-04-29 | Moderna Therapeutics, Inc. | Engineered nucleic acids encoding a modified erythropoietin and their expression |
| US9464124B2 (en) | 2011-09-12 | 2016-10-11 | Moderna Therapeutics, Inc. | Engineered nucleic acids and methods of use thereof |
| EP3492109B1 (en) | 2011-10-03 | 2020-03-04 | ModernaTX, Inc. | Modified nucleosides, nucleotides, and nucleic acids, and uses thereof |
| HRP20220717T1 (hr) | 2011-12-16 | 2022-07-22 | Modernatx, Inc. | Modificirani pripravci mrna |
| US9283287B2 (en) | 2012-04-02 | 2016-03-15 | Moderna Therapeutics, Inc. | Modified polynucleotides for the production of nuclear proteins |
| WO2013151665A2 (en) | 2012-04-02 | 2013-10-10 | modeRNA Therapeutics | Modified polynucleotides for the production of proteins associated with human disease |
| US9572897B2 (en) | 2012-04-02 | 2017-02-21 | Modernatx, Inc. | Modified polynucleotides for the production of cytoplasmic and cytoskeletal proteins |
| US10501512B2 (en) | 2012-04-02 | 2019-12-10 | Modernatx, Inc. | Modified polynucleotides |
| US9597380B2 (en) | 2012-11-26 | 2017-03-21 | Modernatx, Inc. | Terminally modified RNA |
| US8980864B2 (en) | 2013-03-15 | 2015-03-17 | Moderna Therapeutics, Inc. | Compositions and methods of altering cholesterol levels |
| AR095222A1 (es) * | 2013-04-03 | 2015-09-30 | Sanofi Sa | Elemento de medición para un dispositivo de inhalación y montaje para un dispositivo de inhalación que comprende un elemento de medición |
| EP3024523B1 (en) | 2013-07-22 | 2018-08-29 | Inhalation Sciences Sweden AB | Apparatus and method for generating an aerosol |
| SG11201602503TA (en) | 2013-10-03 | 2016-04-28 | Moderna Therapeutics Inc | Polynucleotides encoding low density lipoprotein receptor |
| JP2017047130A (ja) * | 2015-09-04 | 2017-03-09 | 原田車両設計株式会社 | 薬剤吸入装置 |
| KR20180050320A (ko) * | 2015-09-09 | 2018-05-14 | 노파르티스 아게 | 분무-건조된 제제의 폐로의 표적화된 전달 |
| CN205916436U (zh) * | 2016-07-13 | 2017-02-01 | 深圳市合川医疗科技有限公司 | 一种泡罩穿刺元件 |
| EP3612259B1 (en) * | 2017-04-17 | 2022-11-09 | Respira Therapeutics, Inc. | Unit dose dry powder inhaler |
| IL273553B2 (en) * | 2017-10-02 | 2024-09-01 | Novartis Ag | A method for preparing a pharmacy product |
| CN108635643B (zh) * | 2018-06-04 | 2025-01-10 | 润生药业有限公司 | 一种干粉吸入装置 |
| US11864587B2 (en) * | 2018-06-15 | 2024-01-09 | Valley Product Concepts, LLC | Capsules for use in personal vaporizers |
| WO2020135920A1 (en) * | 2018-12-28 | 2020-07-02 | Université Libre de Bruxelles | Kit for inhaled chemotherapy, and treatment of lung cancer with said kit |
| EP3947385A1 (en) * | 2019-04-04 | 2022-02-09 | Novartis AG | Siremadlin succinate |
| CN110286064B (zh) * | 2019-08-05 | 2021-07-27 | 贵州大学 | 一种矿物颗粒润湿性测量装置及其测量方法 |
| CN115089825A (zh) * | 2022-07-06 | 2022-09-23 | 苏州易合医药有限公司 | 肺部给药装置 |
Family Cites Families (30)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5785049A (en) | 1994-09-21 | 1998-07-28 | Inhale Therapeutic Systems | Method and apparatus for dispersion of dry powder medicaments |
| US6582728B1 (en) | 1992-07-08 | 2003-06-24 | Inhale Therapeutic Systems, Inc. | Spray drying of macromolecules to produce inhaleable dry powders |
| CH684746A5 (de) | 1993-02-25 | 1994-12-15 | Alusuisse Lonza Services Ag | Laminat. |
| KR100419037B1 (ko) | 1994-03-07 | 2004-06-12 | 넥타르 테라퓨틱스 | 폐를통한인슐린의전달방법및그조성물 |
| US6051256A (en) | 1994-03-07 | 2000-04-18 | Inhale Therapeutic Systems | Dispersible macromolecule compositions and methods for their preparation and use |
| EP0846009B1 (en) * | 1994-09-21 | 2008-03-05 | Nektar Therapeutics | Apparatus and method for dispersing dry powder medicaments |
| US5693609A (en) | 1994-11-17 | 1997-12-02 | Eli Lilly And Company | Acylated insulin analogs |
| JP3708553B2 (ja) | 1995-04-14 | 2005-10-19 | ネクター セラピューティクス | 向上した分散性を有する粉末型薬理組成物 |
| US6258341B1 (en) | 1995-04-14 | 2001-07-10 | Inhale Therapeutic Systems, Inc. | Stable glassy state powder formulations |
| US6503480B1 (en) | 1997-05-23 | 2003-01-07 | Massachusetts Institute Of Technology | Aerodynamically light particles for pulmonary drug delivery |
| US5985309A (en) | 1996-05-24 | 1999-11-16 | Massachusetts Institute Of Technology | Preparation of particles for inhalation |
| US5874064A (en) | 1996-05-24 | 1999-02-23 | Massachusetts Institute Of Technology | Aerodynamically light particles for pulmonary drug delivery |
| US6565885B1 (en) | 1997-09-29 | 2003-05-20 | Inhale Therapeutic Systems, Inc. | Methods of spray drying pharmaceutical compositions |
| US20060165606A1 (en) | 1997-09-29 | 2006-07-27 | Nektar Therapeutics | Pulmonary delivery particles comprising water insoluble or crystalline active agents |
| US6309623B1 (en) | 1997-09-29 | 2001-10-30 | Inhale Therapeutic Systems, Inc. | Stabilized preparations for use in metered dose inhalers |
| US6270869B1 (en) | 1998-12-02 | 2001-08-07 | Alusuisse Technology & Management Ltd. | Cold formable laminate films |
| US6606992B1 (en) | 1999-06-30 | 2003-08-19 | Nektar Therapeutics | Systems and methods for aerosolizing pharmaceutical formulations |
| DK1666028T3 (da) | 1999-10-29 | 2010-06-21 | Novartis Ag | Tørre pulversammensætninger med forbedret dispersitet |
| US7871598B1 (en) | 2000-05-10 | 2011-01-18 | Novartis Ag | Stable metal ion-lipid powdered pharmaceutical compositions for drug delivery and methods of use |
| CA2382133C (en) | 2000-05-10 | 2010-11-23 | Alliance Pharmaceutical Corporation | Phospholipid-based powders for drug delivery |
| US6668827B2 (en) * | 2000-05-16 | 2003-12-30 | Nektar Therapeutics | Systems devices and methods for opening receptacles having a powder to be fluidized |
| SE0101825D0 (sv) * | 2001-05-22 | 2001-05-22 | Astrazeneca Ab | An Inhalation device |
| GB0315509D0 (en) * | 2003-07-02 | 2003-08-06 | Meridica Ltd | Dispensing device |
| GB2405798A (en) * | 2003-09-15 | 2005-03-16 | Vectura Ltd | Dry powder inhaler with primary and secondary piercing elements and a medicament pack for use with an inhalation device. |
| GB0427856D0 (en) * | 2004-12-20 | 2005-01-19 | Glaxo Group Ltd | Maniflod for use in medicament dispenser |
| BRPI0718315A2 (pt) | 2006-10-25 | 2013-11-12 | Novartis Ag | Aparelho e dispersão de pó, método de fabricação e utilização do aparelho, e componentes que possam ser usados no aparelho e em outros dispositivos. |
| EP2082764A1 (en) * | 2008-01-24 | 2009-07-29 | Boehringer Ingelheim International GmbH | Inhaler |
| CA2724009C (en) * | 2008-05-15 | 2016-10-11 | Novartis Ag | Pulmonary delivery of a fluoroquinolone |
| CA3086027C (en) * | 2008-06-13 | 2022-05-17 | Mannkind Corporation | A dry powder inhaler and system for drug delivery |
| WO2010039200A2 (en) | 2008-09-30 | 2010-04-08 | Oriel Therapeutics, Inc. | Dry powder inhalers with endless strips and cooperating piercers and related methods |
-
2012
- 2012-12-14 EP EP12809952.0A patent/EP2790761B1/en active Active
- 2012-12-14 WO PCT/US2012/069938 patent/WO2013090841A2/en not_active Ceased
- 2012-12-14 ES ES12809952T patent/ES2924032T3/es active Active
- 2012-12-14 JP JP2014547529A patent/JP2015500712A/ja not_active Ceased
- 2012-12-14 KR KR1020147015785A patent/KR20140103939A/ko not_active Withdrawn
- 2012-12-14 CA CA2858247A patent/CA2858247A1/en not_active Abandoned
- 2012-12-14 BR BR112014014399A patent/BR112014014399A2/pt not_active IP Right Cessation
- 2012-12-14 RU RU2014128828A patent/RU2615076C2/ru not_active IP Right Cessation
- 2012-12-14 US US14/364,478 patent/US9744318B2/en not_active Expired - Fee Related
- 2012-12-14 CN CN201280062267.2A patent/CN103998087B/zh not_active Expired - Fee Related
- 2012-12-14 AU AU2012351947A patent/AU2012351947B2/en not_active Expired - Fee Related
- 2012-12-14 MX MX2014007277A patent/MX2014007277A/es unknown
-
2014
- 2014-05-22 IL IL232762A patent/IL232762A0/en unknown
Also Published As
| Publication number | Publication date |
|---|---|
| AU2012351947A1 (en) | 2014-06-26 |
| US9744318B2 (en) | 2017-08-29 |
| ES2924032T3 (es) | 2022-10-04 |
| CN103998087B (zh) | 2017-04-05 |
| WO2013090841A2 (en) | 2013-06-20 |
| EP2790761B1 (en) | 2022-05-11 |
| CA2858247A1 (en) | 2013-06-20 |
| EP2790761A2 (en) | 2014-10-22 |
| JP2015500712A (ja) | 2015-01-08 |
| RU2014128828A (ru) | 2016-02-10 |
| BR112014014399A2 (pt) | 2017-06-13 |
| MX2014007277A (es) | 2014-07-28 |
| CN103998087A (zh) | 2014-08-20 |
| US20140318539A1 (en) | 2014-10-30 |
| WO2013090841A3 (en) | 2013-08-08 |
| RU2615076C2 (ru) | 2017-04-03 |
| AU2012351947B2 (en) | 2017-08-17 |
| IL232762A0 (en) | 2014-07-31 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| RU2615076C2 (ru) | Устройство аэрозолизации для независимой от профиля вдоха доставки лекарственного средства | |
| KR100652532B1 (ko) | 유동 저항이 조절된 에어로졸화 활성 약물의 송달 | |
| JP4542090B2 (ja) | カプセル穿刺整合ガイドを有するエアロゾル化装置 | |
| JP6348501B2 (ja) | 超低密度肺粉剤 | |
| JP6312262B2 (ja) | 乾燥粉末薬物送達システム | |
| JP2006527046A (ja) | 粉末エアロゾル用送給装置 | |
| KR20050114720A (ko) | 공기 입구 차폐 부재를 갖춘 에어로졸화 장치 | |
| JP2007530671A (ja) | 事前計量されるdpiのための乾燥粉末調合剤 | |
| CN115919780A (zh) | 含有硬脂酸镁的干粉组合物 | |
| KR20200105857A (ko) | 폐 투여에 의한 우울증의 치료에 사용하기 위한 건조 분말 케타민 조성물 | |
| KR20210117265A (ko) | 건조 분말 흡입기 장치 | |
| MXPA06014502A (es) | Reduccion de retencion de polvo en superficies. | |
| KR20200115560A (ko) | 흡입형 치료제의 고용량 전달 | |
| JP4266399B2 (ja) | 粉末状吸入用医薬品組成物 | |
| CN113038941A (zh) | 用于通过肺部施用治疗抑郁症的方法中的氯胺酮组合物 | |
| D’Lima | Characterization of a Novel Dry Powder Inhaler | |
| HK1116391A (en) | Minimizing powder retention on surfaces |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PA0105 | International application |
Patent event date: 20140611 Patent event code: PA01051R01D Comment text: International Patent Application |
|
| PG1501 | Laying open of application | ||
| PC1203 | Withdrawal of no request for examination | ||
| WITN | Application deemed withdrawn, e.g. because no request for examination was filed or no examination fee was paid |