KR20140096373A - 폴리에틸렌글리콜이 수식된 인테그린 차단제 hm-3 및 그 응용 - Google Patents
폴리에틸렌글리콜이 수식된 인테그린 차단제 hm-3 및 그 응용 Download PDFInfo
- Publication number
- KR20140096373A KR20140096373A KR1020147016557A KR20147016557A KR20140096373A KR 20140096373 A KR20140096373 A KR 20140096373A KR 1020147016557 A KR1020147016557 A KR 1020147016557A KR 20147016557 A KR20147016557 A KR 20147016557A KR 20140096373 A KR20140096373 A KR 20140096373A
- Authority
- KR
- South Korea
- Prior art keywords
- tumor
- once
- mpeg
- group
- human
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Ceased
Links
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/50—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates
- A61K47/51—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent
- A61K47/56—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent the modifying agent being an organic macromolecular compound, e.g. an oligomeric, polymeric or dendrimeric molecule
- A61K47/59—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent the modifying agent being an organic macromolecular compound, e.g. an oligomeric, polymeric or dendrimeric molecule obtained otherwise than by reactions only involving carbon-to-carbon unsaturated bonds, e.g. polyureas or polyurethanes
- A61K47/60—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent the modifying agent being an organic macromolecular compound, e.g. an oligomeric, polymeric or dendrimeric molecule obtained otherwise than by reactions only involving carbon-to-carbon unsaturated bonds, e.g. polyureas or polyurethanes the organic macromolecular compound being a polyoxyalkylene oligomer, polymer or dendrimer, e.g. PEG, PPG, PEO or polyglycerol
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K17/00—Carrier-bound or immobilised peptides; Preparation thereof
- C07K17/02—Peptides being immobilised on, or in, an organic carrier
- C07K17/04—Peptides being immobilised on, or in, an organic carrier entrapped within the carrier, e.g. gel, hollow fibre
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K38/00—Medicinal preparations containing peptides
- A61K38/04—Peptides having up to 20 amino acids in a fully defined sequence; Derivatives thereof
- A61K38/10—Peptides having 12 to 20 amino acids
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/50—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K14/00—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- C07K14/435—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans
- C07K14/78—Connective tissue peptides, e.g. collagen, elastin, laminin, fibronectin, vitronectin or cold insoluble globulin [CIG]
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K17/00—Carrier-bound or immobilised peptides; Preparation thereof
- C07K17/02—Peptides being immobilised on, or in, an organic carrier
- C07K17/08—Peptides being immobilised on, or in, an organic carrier the carrier being a synthetic polymer
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K7/00—Peptides having 5 to 20 amino acids in a fully defined sequence; Derivatives thereof
- C07K7/04—Linear peptides containing only normal peptide links
- C07K7/08—Linear peptides containing only normal peptide links having 12 to 20 amino acids
Landscapes
- Health & Medical Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- General Health & Medical Sciences (AREA)
- Medicinal Chemistry (AREA)
- Organic Chemistry (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Pharmacology & Pharmacy (AREA)
- Animal Behavior & Ethology (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Epidemiology (AREA)
- Engineering & Computer Science (AREA)
- Molecular Biology (AREA)
- Biochemistry (AREA)
- Biophysics (AREA)
- Genetics & Genomics (AREA)
- Gastroenterology & Hepatology (AREA)
- Immunology (AREA)
- Toxicology (AREA)
- Zoology (AREA)
- General Chemical & Material Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Medicines That Contain Protein Lipid Enzymes And Other Medicines (AREA)
- Peptides Or Proteins (AREA)
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN2011103705299A CN102417540A (zh) | 2011-11-21 | 2011-11-21 | 一种聚乙二醇修饰的整合素阻断剂hm-3及其应用 |
| CN201110370529.9 | 2011-11-21 | ||
| PCT/CN2012/084788 WO2013075600A1 (zh) | 2011-11-21 | 2012-11-17 | 一种聚乙二醇修饰的整合素阻断剂hm-3及其应用 |
Related Child Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020197020874A Division KR102106485B1 (ko) | 2011-11-21 | 2012-11-17 | 폴리에틸렌글리콜이 수식된 인테그린 차단제 hm-3 및 그 응용 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| KR20140096373A true KR20140096373A (ko) | 2014-08-05 |
Family
ID=45942198
Family Applications (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020197020874A Active KR102106485B1 (ko) | 2011-11-21 | 2012-11-17 | 폴리에틸렌글리콜이 수식된 인테그린 차단제 hm-3 및 그 응용 |
| KR1020147016557A Ceased KR20140096373A (ko) | 2011-11-21 | 2012-11-17 | 폴리에틸렌글리콜이 수식된 인테그린 차단제 hm-3 및 그 응용 |
Family Applications Before (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020197020874A Active KR102106485B1 (ko) | 2011-11-21 | 2012-11-17 | 폴리에틸렌글리콜이 수식된 인테그린 차단제 hm-3 및 그 응용 |
Country Status (7)
| Country | Link |
|---|---|
| US (2) | US20140329759A1 (enExample) |
| EP (1) | EP2784093B1 (enExample) |
| KR (2) | KR102106485B1 (enExample) |
| CN (1) | CN102417540A (enExample) |
| AU (1) | AU2012343020B2 (enExample) |
| IN (1) | IN2014CN04482A (enExample) |
| WO (1) | WO2013075600A1 (enExample) |
Families Citing this family (19)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN102417540A (zh) * | 2011-11-21 | 2012-04-18 | 中国药科大学 | 一种聚乙二醇修饰的整合素阻断剂hm-3及其应用 |
| CN103656615B (zh) * | 2013-12-11 | 2017-01-04 | 南京安吉生物科技有限公司 | 一种hm-3和铂类、紫杉醇类或他滨类药物在制备实体瘤药物中的用途 |
| CN103623394A (zh) * | 2013-12-11 | 2014-03-12 | 南京安吉生物科技有限公司 | 一种peg-hm-3和铂类、紫杉醇类或他滨类药物在制备实体瘤药物中的用途 |
| CN103739671B (zh) * | 2013-12-31 | 2015-08-26 | 郭向华 | 一种聚乙二醇修饰的抑制核因子-κB多肽及其应用 |
| CN103739673B (zh) * | 2013-12-31 | 2015-08-19 | 浙江元太生物科技有限公司 | 一种聚乙二醇修饰的抑制白介素-6多肽及其应用 |
| CN103739669B (zh) * | 2013-12-31 | 2015-08-26 | 浙江元太生物科技有限公司 | 一种抑制白介素-6多肽及其应用 |
| CN103736078B (zh) * | 2014-01-09 | 2017-12-12 | 南京安吉生物科技有限公司 | mPEG‑HM‑3多肽冻干粉针制剂及其制备方法和用途 |
| CN103720667B (zh) * | 2014-01-09 | 2016-04-13 | 中国药科大学 | Ap-25多肽冻干粉针制剂及其制备方法和用途 |
| CN103819542A (zh) * | 2014-02-28 | 2014-05-28 | 中国药科大学 | 一种聚乙二醇修饰及蛋白质融合表达的整合素阻断剂ap-25及其应用 |
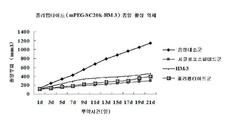
| CN104127378A (zh) * | 2014-08-11 | 2014-11-05 | 中国药科大学 | mPEG-SC20K-HM-3多肽注射液及其制备方法和用途 |
| CN105622721A (zh) * | 2015-12-16 | 2016-06-01 | 李斯文 | 一种聚乙二醇修饰的血管生成抑制剂hs-1及其应用 |
| CN105646667A (zh) * | 2016-04-06 | 2016-06-08 | 南京安吉生物科技有限公司 | 聚乙二醇修饰的血管生成抑制剂hm-1及其应用 |
| CN108623693B (zh) * | 2017-03-20 | 2022-03-25 | 徐寒梅 | 一种融合蛋白及其制备方法和其在制备治疗眼科疾病、抗炎、抗肿瘤药物中的应用 |
| CN109503700A (zh) * | 2017-09-14 | 2019-03-22 | 南京安吉生物科技有限公司 | 马来酰亚胺基团修饰的血管生成抑制剂hm-1及其应用 |
| CN107857800B (zh) * | 2017-11-09 | 2020-05-05 | 北京赛升药业股份有限公司 | 一种长效整合素抑制剂及其应用 |
| CN109879969B (zh) | 2017-12-06 | 2024-04-09 | 天士力生物医药股份有限公司 | 一种hm-3融合蛋白及其应用 |
| CN111855876B (zh) * | 2020-07-10 | 2023-05-02 | 北京赛升药业股份有限公司 | 一种安替安吉肽有关物质的检测方法 |
| CN113488512A (zh) * | 2021-06-23 | 2021-10-08 | 深圳市华星光电半导体显示技术有限公司 | 显示面板及其制备方法 |
| CN114230676B (zh) * | 2021-12-22 | 2024-06-11 | 天士力生物医药股份有限公司 | 重组hm-3融合蛋白及其应用 |
Family Cites Families (10)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5595756A (en) * | 1993-12-22 | 1997-01-21 | Inex Pharmaceuticals Corporation | Liposomal compositions for enhanced retention of bioactive agents |
| US6521593B1 (en) * | 1999-02-01 | 2003-02-18 | Childrens Hospital Los Angeles | Methods for inhibiting brain tumor growth |
| MXPA05005469A (es) * | 2002-11-25 | 2005-09-08 | Attenuon Llc | Peptidos que inhibien angiogenesis, migracion celular, invasion celular y proliferacion celular, composiciones y usos de los mismos. |
| CN1314705C (zh) * | 2005-06-03 | 2007-05-09 | 中国药科大学 | 高效抑制血管生成多肽及其制备方法和应用 |
| KR100863060B1 (ko) * | 2006-06-02 | 2008-10-10 | 베이징 선바이오 바이오테크, 코오퍼레이션, 리미티드 | 암 억제 활동을 하는 재조합 단백질, 그 암호화 유전자 및 재조합 단백질을 활성성분으로 포함하는 암치료용 약학적 조성물 |
| CN102145161A (zh) * | 2011-04-07 | 2011-08-10 | 中国药科大学 | 整合素阻断剂在制备治疗肿瘤药物中的应用 |
| CN102178656B (zh) * | 2011-05-12 | 2012-08-22 | 内蒙古奇特生物高科技(集团)有限公司 | Hm-3多肽冻干粉制剂及其制备方法 |
| CN102205110B (zh) * | 2011-05-18 | 2014-03-26 | 中国药科大学 | 整合素阻断剂在制备治疗新生血管性眼病药物中的应用 |
| CN102417540A (zh) * | 2011-11-21 | 2012-04-18 | 中国药科大学 | 一种聚乙二醇修饰的整合素阻断剂hm-3及其应用 |
| CN104127378A (zh) * | 2014-08-11 | 2014-11-05 | 中国药科大学 | mPEG-SC20K-HM-3多肽注射液及其制备方法和用途 |
-
2011
- 2011-11-21 CN CN2011103705299A patent/CN102417540A/zh active Pending
-
2012
- 2012-11-17 KR KR1020197020874A patent/KR102106485B1/ko active Active
- 2012-11-17 US US14/359,462 patent/US20140329759A1/en not_active Abandoned
- 2012-11-17 WO PCT/CN2012/084788 patent/WO2013075600A1/zh not_active Ceased
- 2012-11-17 IN IN4482CHN2014 patent/IN2014CN04482A/en unknown
- 2012-11-17 EP EP12851929.5A patent/EP2784093B1/en active Active
- 2012-11-17 KR KR1020147016557A patent/KR20140096373A/ko not_active Ceased
- 2012-11-17 AU AU2012343020A patent/AU2012343020B2/en active Active
-
2016
- 2016-10-24 US US15/332,539 patent/US20170100489A1/en not_active Abandoned
Also Published As
| Publication number | Publication date |
|---|---|
| US20170100489A1 (en) | 2017-04-13 |
| KR102106485B1 (ko) | 2020-05-04 |
| EP2784093A1 (en) | 2014-10-01 |
| AU2012343020A1 (en) | 2014-06-12 |
| WO2013075600A1 (zh) | 2013-05-30 |
| KR20190090872A (ko) | 2019-08-02 |
| AU2012343020B2 (en) | 2016-05-19 |
| EP2784093B1 (en) | 2019-10-09 |
| IN2014CN04482A (enExample) | 2015-09-04 |
| US20140329759A1 (en) | 2014-11-06 |
| EP2784093A4 (en) | 2015-08-05 |
| CN102417540A (zh) | 2012-04-18 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| KR102106485B1 (ko) | 폴리에틸렌글리콜이 수식된 인테그린 차단제 hm-3 및 그 응용 | |
| BRPI0512286B1 (pt) | Proteínas quiméricas inibidoras da angiogênese e o uso | |
| WO2011011315A1 (en) | POLYPEPTIDES SELECTIVE FOR AV β3 INTEGRIN CONJUGATED WITH A VARIANT OF HUMAN SERUM ALBUMIN (HSA) AND PHARMACEUTICAL USES THEREOF | |
| US10870684B2 (en) | Disintegrin variants and pharmaceutical uses thereof | |
| KR102003422B1 (ko) | 인테그린 차단제 폴리펩타이드 및 그의 응용 | |
| JP2007527206A (ja) | がん治療のためのペプタボディ | |
| Meng et al. | Improved immunocompatibility of active targeting liposomes by attenuating nucleophilic attack of cyclic RGD peptides on complement 3 | |
| CN106466485B (zh) | 一种具有细胞内吞介导功能的靶向配体-药物偶联体 | |
| CN105315350A (zh) | 抗肿瘤血管生成多肽mPEG-Mal-Cys-AS16 | |
| Chen et al. | Unleashing the potential of natural biological peptide Macropin: Hydrocarbon stapling for effective breast cancer treatment | |
| US20230212233A1 (en) | Novel mutant of recombinant ganoderma lucidum immunomodulatory protein and use thereof | |
| CN102372770A (zh) | 一种抑血管生成素、纯化方法及含有它们的药物组合物 | |
| WO2019158720A1 (en) | C/ebp alpha sarna compositions and methods of use | |
| KR101323669B1 (ko) | 암세포 특이적 세포괴사 유도 및 암 소멸 효과를 나타내는 세포사 유도 융합 펩타이드 | |
| US20240424071A1 (en) | Novel mutant of recombinant ganoderma lucidum immunomodulatory protein and use thereof | |
| Pei et al. | Anti-tumor activity and pharmacokinetics of AP25-Fc fusion protein | |
| US20240082346A1 (en) | Composition Comprising an Antiviral Agent and a CXCR4 Selective Antagonist and Methods For Using the Same | |
| CN103172745A (zh) | 包含免疫球蛋白Fc片段的长效人内皮抑素 | |
| CN107857800B (zh) | 一种长效整合素抑制剂及其应用 | |
| BR112017003334B1 (pt) | Variante de desintegrina, seu uso, composição farmacêutica e seu uso | |
| CN107163134A (zh) | 一种胸腺法新聚乙二醇聚合物、包含其的药物组合物及应用 | |
| HK1233930A1 (en) | Cyclic prosaposin peptides and uses thereof | |
| HK1233930B (en) | Cyclic prosaposin peptides and uses thereof |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PA0105 | International application |
Patent event date: 20140617 Patent event code: PA01051R01D Comment text: International Patent Application |
|
| PG1501 | Laying open of application | ||
| A201 | Request for examination | ||
| PA0201 | Request for examination |
Patent event code: PA02012R01D Patent event date: 20170427 Comment text: Request for Examination of Application |
|
| E902 | Notification of reason for refusal | ||
| PE0902 | Notice of grounds for rejection |
Comment text: Notification of reason for refusal Patent event date: 20180905 Patent event code: PE09021S01D |
|
| AMND | Amendment | ||
| E601 | Decision to refuse application | ||
| PE0601 | Decision on rejection of patent |
Patent event date: 20190318 Comment text: Decision to Refuse Application Patent event code: PE06012S01D Patent event date: 20180905 Comment text: Notification of reason for refusal Patent event code: PE06011S01I |
|
| AMND | Amendment | ||
| PX0901 | Re-examination |
Patent event code: PX09011S01I Patent event date: 20190318 Comment text: Decision to Refuse Application Patent event code: PX09012R01I Patent event date: 20181105 Comment text: Amendment to Specification, etc. |
|
| PX0601 | Decision of rejection after re-examination |
Comment text: Decision to Refuse Application Patent event code: PX06014S01D Patent event date: 20190615 Comment text: Amendment to Specification, etc. Patent event code: PX06012R01I Patent event date: 20190520 Comment text: Decision to Refuse Application Patent event code: PX06011S01I Patent event date: 20190318 Comment text: Amendment to Specification, etc. Patent event code: PX06012R01I Patent event date: 20181105 Comment text: Notification of reason for refusal Patent event code: PX06013S01I Patent event date: 20180905 |
|
| PA0104 | Divisional application for international application |
Comment text: Divisional Application for International Patent Patent event code: PA01041R01D Patent event date: 20190717 |