JP6899397B2 - 磁性材料の製造方法 - Google Patents
磁性材料の製造方法 Download PDFInfo
- Publication number
- JP6899397B2 JP6899397B2 JP2018548544A JP2018548544A JP6899397B2 JP 6899397 B2 JP6899397 B2 JP 6899397B2 JP 2018548544 A JP2018548544 A JP 2018548544A JP 2018548544 A JP2018548544 A JP 2018548544A JP 6899397 B2 JP6899397 B2 JP 6899397B2
- Authority
- JP
- Japan
- Prior art keywords
- magnetic material
- raw material
- alkali metal
- product
- magnetic
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Expired - Fee Related
Links
- 239000000696 magnetic material Substances 0.000 title claims description 49
- 238000004519 manufacturing process Methods 0.000 title claims description 47
- 239000002994 raw material Substances 0.000 claims description 63
- 229910052783 alkali metal Inorganic materials 0.000 claims description 37
- 150000001340 alkali metals Chemical class 0.000 claims description 37
- 150000001875 compounds Chemical class 0.000 claims description 35
- 229910052742 iron Inorganic materials 0.000 claims description 20
- 238000001816 cooling Methods 0.000 claims description 18
- 229910052710 silicon Inorganic materials 0.000 claims description 16
- 229910052748 manganese Inorganic materials 0.000 claims description 14
- 229910052698 phosphorus Inorganic materials 0.000 claims description 12
- 229910052796 boron Inorganic materials 0.000 claims description 10
- 239000000155 melt Substances 0.000 claims description 9
- 229910052804 chromium Inorganic materials 0.000 claims description 6
- 229910052759 nickel Inorganic materials 0.000 claims description 6
- 229910052799 carbon Inorganic materials 0.000 claims description 5
- 230000000694 effects Effects 0.000 claims description 5
- 229910052684 Cerium Inorganic materials 0.000 claims description 3
- 229910052779 Neodymium Inorganic materials 0.000 claims description 3
- 229910052777 Praseodymium Inorganic materials 0.000 claims description 3
- 229910052782 aluminium Inorganic materials 0.000 claims description 3
- 229910052787 antimony Inorganic materials 0.000 claims description 3
- 229910052785 arsenic Inorganic materials 0.000 claims description 3
- 229910052733 gallium Inorganic materials 0.000 claims description 3
- 229910052739 hydrogen Inorganic materials 0.000 claims description 3
- 229910052757 nitrogen Inorganic materials 0.000 claims description 3
- 229910052718 tin Inorganic materials 0.000 claims description 3
- 238000010587 phase diagram Methods 0.000 claims description 2
- XEEYBQQBJWHFJM-UHFFFAOYSA-N iron Substances [Fe] XEEYBQQBJWHFJM-UHFFFAOYSA-N 0.000 description 59
- 238000010438 heat treatment Methods 0.000 description 42
- 239000011572 manganese Substances 0.000 description 27
- 229910052751 metal Inorganic materials 0.000 description 12
- 239000002184 metal Substances 0.000 description 12
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 description 8
- 239000007789 gas Substances 0.000 description 8
- 238000002844 melting Methods 0.000 description 8
- 230000008018 melting Effects 0.000 description 8
- 238000006243 chemical reaction Methods 0.000 description 7
- 238000000034 method Methods 0.000 description 7
- OAICVXFJPJFONN-UHFFFAOYSA-N Phosphorus Chemical compound [P] OAICVXFJPJFONN-UHFFFAOYSA-N 0.000 description 6
- 239000000463 material Substances 0.000 description 6
- 229910052746 lanthanum Inorganic materials 0.000 description 5
- 239000000843 powder Substances 0.000 description 5
- 239000000126 substance Substances 0.000 description 5
- 238000002441 X-ray diffraction Methods 0.000 description 4
- PNEYBMLMFCGWSK-UHFFFAOYSA-N aluminium oxide Inorganic materials [O-2].[O-2].[O-2].[Al+3].[Al+3] PNEYBMLMFCGWSK-UHFFFAOYSA-N 0.000 description 4
- 239000011651 chromium Substances 0.000 description 4
- 239000013078 crystal Substances 0.000 description 4
- PXHVJJICTQNCMI-UHFFFAOYSA-N nickel Substances [Ni] PXHVJJICTQNCMI-UHFFFAOYSA-N 0.000 description 4
- 238000005191 phase separation Methods 0.000 description 4
- 239000011574 phosphorus Substances 0.000 description 4
- QTBSBXVTEAMEQO-UHFFFAOYSA-N Acetic acid Chemical compound CC(O)=O QTBSBXVTEAMEQO-UHFFFAOYSA-N 0.000 description 3
- OKKJLVBELUTLKV-UHFFFAOYSA-N Methanol Chemical compound OC OKKJLVBELUTLKV-UHFFFAOYSA-N 0.000 description 3
- KRKNYBCHXYNGOX-UHFFFAOYSA-N citric acid Chemical compound OC(=O)CC(O)(C(O)=O)CC(O)=O KRKNYBCHXYNGOX-UHFFFAOYSA-N 0.000 description 3
- 239000000470 constituent Substances 0.000 description 3
- 229910052744 lithium Inorganic materials 0.000 description 3
- 239000000203 mixture Substances 0.000 description 3
- BASFCYQUMIYNBI-UHFFFAOYSA-N platinum Chemical compound [Pt] BASFCYQUMIYNBI-UHFFFAOYSA-N 0.000 description 3
- 229910052700 potassium Inorganic materials 0.000 description 3
- 239000003870 refractory metal Substances 0.000 description 3
- 229910021332 silicide Inorganic materials 0.000 description 3
- FVBUAEGBCNSCDD-UHFFFAOYSA-N silicide(4-) Chemical compound [Si-4] FVBUAEGBCNSCDD-UHFFFAOYSA-N 0.000 description 3
- 229910052708 sodium Inorganic materials 0.000 description 3
- 239000002904 solvent Substances 0.000 description 3
- 230000002194 synthesizing effect Effects 0.000 description 3
- XKRFYHLGVUSROY-UHFFFAOYSA-N Argon Chemical compound [Ar] XKRFYHLGVUSROY-UHFFFAOYSA-N 0.000 description 2
- OKTJSMMVPCPJKN-UHFFFAOYSA-N Carbon Chemical compound [C] OKTJSMMVPCPJKN-UHFFFAOYSA-N 0.000 description 2
- PEDCQBHIVMGVHV-UHFFFAOYSA-N Glycerine Chemical compound OCC(O)CO PEDCQBHIVMGVHV-UHFFFAOYSA-N 0.000 description 2
- ZOKXTWBITQBERF-UHFFFAOYSA-N Molybdenum Chemical compound [Mo] ZOKXTWBITQBERF-UHFFFAOYSA-N 0.000 description 2
- 229910018487 Ni—Cr Inorganic materials 0.000 description 2
- 238000004458 analytical method Methods 0.000 description 2
- 238000000137 annealing Methods 0.000 description 2
- 229910052792 caesium Inorganic materials 0.000 description 2
- 230000008859 change Effects 0.000 description 2
- VNNRSPGTAMTISX-UHFFFAOYSA-N chromium nickel Chemical compound [Cr].[Ni] VNNRSPGTAMTISX-UHFFFAOYSA-N 0.000 description 2
- 229910052730 francium Inorganic materials 0.000 description 2
- 229910052732 germanium Inorganic materials 0.000 description 2
- 239000011261 inert gas Substances 0.000 description 2
- -1 iron-chromium-aluminum Chemical compound 0.000 description 2
- FZLIPJUXYLNCLC-UHFFFAOYSA-N lanthanum atom Chemical compound [La] FZLIPJUXYLNCLC-UHFFFAOYSA-N 0.000 description 2
- 150000002739 metals Chemical class 0.000 description 2
- NFFIWVVINABMKP-UHFFFAOYSA-N methylidynetantalum Chemical compound [Ta]#C NFFIWVVINABMKP-UHFFFAOYSA-N 0.000 description 2
- 238000012986 modification Methods 0.000 description 2
- 230000004048 modification Effects 0.000 description 2
- 229910052750 molybdenum Inorganic materials 0.000 description 2
- 239000011733 molybdenum Substances 0.000 description 2
- 150000004767 nitrides Chemical class 0.000 description 2
- 150000007524 organic acids Chemical class 0.000 description 2
- 230000009257 reactivity Effects 0.000 description 2
- 229910052701 rubidium Inorganic materials 0.000 description 2
- 229910003468 tantalcarbide Inorganic materials 0.000 description 2
- 229910052715 tantalum Inorganic materials 0.000 description 2
- GUVRBAGPIYLISA-UHFFFAOYSA-N tantalum atom Chemical compound [Ta] GUVRBAGPIYLISA-UHFFFAOYSA-N 0.000 description 2
- WFKWXMTUELFFGS-UHFFFAOYSA-N tungsten Chemical compound [W] WFKWXMTUELFFGS-UHFFFAOYSA-N 0.000 description 2
- 229910052721 tungsten Inorganic materials 0.000 description 2
- 239000010937 tungsten Substances 0.000 description 2
- 229910018072 Al 2 O 3 Inorganic materials 0.000 description 1
- PIGFYZPCRLYGLF-UHFFFAOYSA-N Aluminum nitride Chemical compound [Al]#N PIGFYZPCRLYGLF-UHFFFAOYSA-N 0.000 description 1
- ZOXJGFHDIHLPTG-UHFFFAOYSA-N Boron Chemical compound [B] ZOXJGFHDIHLPTG-UHFFFAOYSA-N 0.000 description 1
- PZNSFCLAULLKQX-UHFFFAOYSA-N Boron nitride Chemical compound N#B PZNSFCLAULLKQX-UHFFFAOYSA-N 0.000 description 1
- 229910000640 Fe alloy Inorganic materials 0.000 description 1
- 229910002060 Fe-Cr-Al alloy Inorganic materials 0.000 description 1
- 229910052688 Gadolinium Inorganic materials 0.000 description 1
- UFHFLCQGNIYNRP-UHFFFAOYSA-N Hydrogen Chemical compound [H][H] UFHFLCQGNIYNRP-UHFFFAOYSA-N 0.000 description 1
- 229910000858 La alloy Inorganic materials 0.000 description 1
- 229910018251 LaSi 2 Inorganic materials 0.000 description 1
- PWHULOQIROXLJO-UHFFFAOYSA-N Manganese Chemical compound [Mn] PWHULOQIROXLJO-UHFFFAOYSA-N 0.000 description 1
- 229910000914 Mn alloy Inorganic materials 0.000 description 1
- 229910000676 Si alloy Inorganic materials 0.000 description 1
- NRTOMJZYCJJWKI-UHFFFAOYSA-N Titanium nitride Chemical compound [Ti]#N NRTOMJZYCJJWKI-UHFFFAOYSA-N 0.000 description 1
- 150000001298 alcohols Chemical class 0.000 description 1
- 239000003513 alkali Substances 0.000 description 1
- 229910045601 alloy Inorganic materials 0.000 description 1
- 239000000956 alloy Substances 0.000 description 1
- 229910052786 argon Inorganic materials 0.000 description 1
- 230000015572 biosynthetic process Effects 0.000 description 1
- JRACIMOSEUMYIP-UHFFFAOYSA-N bis($l^{2}-silanylidene)iron Chemical compound [Si]=[Fe]=[Si] JRACIMOSEUMYIP-UHFFFAOYSA-N 0.000 description 1
- FHTCLMVMBMJAEE-UHFFFAOYSA-N bis($l^{2}-silanylidene)manganese Chemical compound [Si]=[Mn]=[Si] FHTCLMVMBMJAEE-UHFFFAOYSA-N 0.000 description 1
- YXTPWUNVHCYOSP-UHFFFAOYSA-N bis($l^{2}-silanylidene)molybdenum Chemical compound [Si]=[Mo]=[Si] YXTPWUNVHCYOSP-UHFFFAOYSA-N 0.000 description 1
- 239000000919 ceramic Substances 0.000 description 1
- 238000007796 conventional method Methods 0.000 description 1
- 238000010586 diagram Methods 0.000 description 1
- 230000004907 flux Effects 0.000 description 1
- 238000007716 flux method Methods 0.000 description 1
- UIWYJDYFSGRHKR-UHFFFAOYSA-N gadolinium atom Chemical compound [Gd] UIWYJDYFSGRHKR-UHFFFAOYSA-N 0.000 description 1
- 235000011187 glycerol Nutrition 0.000 description 1
- 229910002804 graphite Inorganic materials 0.000 description 1
- 239000010439 graphite Substances 0.000 description 1
- 229910052734 helium Inorganic materials 0.000 description 1
- 239000001307 helium Substances 0.000 description 1
- SWQJXJOGLNCZEY-UHFFFAOYSA-N helium atom Chemical compound [He] SWQJXJOGLNCZEY-UHFFFAOYSA-N 0.000 description 1
- 238000000265 homogenisation Methods 0.000 description 1
- 239000001257 hydrogen Substances 0.000 description 1
- VAKIVKMUBMZANL-UHFFFAOYSA-N iron phosphide Chemical compound P.[Fe].[Fe].[Fe] VAKIVKMUBMZANL-UHFFFAOYSA-N 0.000 description 1
- 150000001247 metal acetylides Chemical class 0.000 description 1
- 150000002736 metal compounds Chemical class 0.000 description 1
- 239000007769 metal material Substances 0.000 description 1
- 239000011812 mixed powder Substances 0.000 description 1
- 229910052754 neon Inorganic materials 0.000 description 1
- GKAOGPIIYCISHV-UHFFFAOYSA-N neon atom Chemical compound [Ne] GKAOGPIIYCISHV-UHFFFAOYSA-N 0.000 description 1
- 229910052755 nonmetal Inorganic materials 0.000 description 1
- 235000005985 organic acids Nutrition 0.000 description 1
- 239000003960 organic solvent Substances 0.000 description 1
- 239000002245 particle Substances 0.000 description 1
- 150000002989 phenols Chemical class 0.000 description 1
- 150000003018 phosphorus compounds Chemical class 0.000 description 1
- 229910052697 platinum Inorganic materials 0.000 description 1
- 229910052761 rare earth metal Inorganic materials 0.000 description 1
- 150000002910 rare earth metals Chemical class 0.000 description 1
- 229910052594 sapphire Inorganic materials 0.000 description 1
- 239000010980 sapphire Substances 0.000 description 1
- 238000004904 shortening Methods 0.000 description 1
- HBMJWWWQQXIZIP-UHFFFAOYSA-N silicon carbide Chemical compound [Si+]#[C-] HBMJWWWQQXIZIP-UHFFFAOYSA-N 0.000 description 1
- 238000007711 solidification Methods 0.000 description 1
- 230000008023 solidification Effects 0.000 description 1
- 238000003786 synthesis reaction Methods 0.000 description 1
- 238000005979 thermal decomposition reaction Methods 0.000 description 1
- UONOETXJSWQNOL-UHFFFAOYSA-N tungsten carbide Chemical compound [W+]#[C-] UONOETXJSWQNOL-UHFFFAOYSA-N 0.000 description 1
- ZVWKZXLXHLZXLS-UHFFFAOYSA-N zirconium nitride Chemical compound [Zr]#N ZVWKZXLXHLZXLS-UHFFFAOYSA-N 0.000 description 1
Images
Classifications
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01F—MAGNETS; INDUCTANCES; TRANSFORMERS; SELECTION OF MATERIALS FOR THEIR MAGNETIC PROPERTIES
- H01F1/00—Magnets or magnetic bodies characterised by the magnetic materials therefor; Selection of materials for their magnetic properties
- H01F1/01—Magnets or magnetic bodies characterised by the magnetic materials therefor; Selection of materials for their magnetic properties of inorganic materials
- H01F1/012—Magnets or magnetic bodies characterised by the magnetic materials therefor; Selection of materials for their magnetic properties of inorganic materials adapted for magnetic entropy change by magnetocaloric effect, e.g. used as magnetic refrigerating material
- H01F1/017—Compounds
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B22—CASTING; POWDER METALLURGY
- B22F—WORKING METALLIC POWDER; MANUFACTURE OF ARTICLES FROM METALLIC POWDER; MAKING METALLIC POWDER; APPARATUS OR DEVICES SPECIALLY ADAPTED FOR METALLIC POWDER
- B22F1/00—Metallic powder; Treatment of metallic powder, e.g. to facilitate working or to improve properties
- B22F1/14—Treatment of metallic powder
- B22F1/142—Thermal or thermo-mechanical treatment
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B22—CASTING; POWDER METALLURGY
- B22F—WORKING METALLIC POWDER; MANUFACTURE OF ARTICLES FROM METALLIC POWDER; MAKING METALLIC POWDER; APPARATUS OR DEVICES SPECIALLY ADAPTED FOR METALLIC POWDER
- B22F1/00—Metallic powder; Treatment of metallic powder, e.g. to facilitate working or to improve properties
- B22F1/14—Treatment of metallic powder
- B22F1/145—Chemical treatment, e.g. passivation or decarburisation
-
- C—CHEMISTRY; METALLURGY
- C22—METALLURGY; FERROUS OR NON-FERROUS ALLOYS; TREATMENT OF ALLOYS OR NON-FERROUS METALS
- C22C—ALLOYS
- C22C1/00—Making non-ferrous alloys
-
- C—CHEMISTRY; METALLURGY
- C22—METALLURGY; FERROUS OR NON-FERROUS ALLOYS; TREATMENT OF ALLOYS OR NON-FERROUS METALS
- C22C—ALLOYS
- C22C19/00—Alloys based on nickel or cobalt
- C22C19/03—Alloys based on nickel or cobalt based on nickel
-
- C—CHEMISTRY; METALLURGY
- C22—METALLURGY; FERROUS OR NON-FERROUS ALLOYS; TREATMENT OF ALLOYS OR NON-FERROUS METALS
- C22C—ALLOYS
- C22C19/00—Alloys based on nickel or cobalt
- C22C19/07—Alloys based on nickel or cobalt based on cobalt
-
- C—CHEMISTRY; METALLURGY
- C22—METALLURGY; FERROUS OR NON-FERROUS ALLOYS; TREATMENT OF ALLOYS OR NON-FERROUS METALS
- C22C—ALLOYS
- C22C22/00—Alloys based on manganese
-
- C—CHEMISTRY; METALLURGY
- C22—METALLURGY; FERROUS OR NON-FERROUS ALLOYS; TREATMENT OF ALLOYS OR NON-FERROUS METALS
- C22C—ALLOYS
- C22C27/00—Alloys based on rhenium or a refractory metal not mentioned in groups C22C14/00 or C22C16/00
- C22C27/06—Alloys based on chromium
-
- C—CHEMISTRY; METALLURGY
- C22—METALLURGY; FERROUS OR NON-FERROUS ALLOYS; TREATMENT OF ALLOYS OR NON-FERROUS METALS
- C22C—ALLOYS
- C22C38/00—Ferrous alloys, e.g. steel alloys
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01F—MAGNETS; INDUCTANCES; TRANSFORMERS; SELECTION OF MATERIALS FOR THEIR MAGNETIC PROPERTIES
- H01F1/00—Magnets or magnetic bodies characterised by the magnetic materials therefor; Selection of materials for their magnetic properties
- H01F1/01—Magnets or magnetic bodies characterised by the magnetic materials therefor; Selection of materials for their magnetic properties of inorganic materials
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B22—CASTING; POWDER METALLURGY
- B22F—WORKING METALLIC POWDER; MANUFACTURE OF ARTICLES FROM METALLIC POWDER; MAKING METALLIC POWDER; APPARATUS OR DEVICES SPECIALLY ADAPTED FOR METALLIC POWDER
- B22F2301/00—Metallic composition of the powder or its coating
- B22F2301/35—Iron
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B22—CASTING; POWDER METALLURGY
- B22F—WORKING METALLIC POWDER; MANUFACTURE OF ARTICLES FROM METALLIC POWDER; MAKING METALLIC POWDER; APPARATUS OR DEVICES SPECIALLY ADAPTED FOR METALLIC POWDER
- B22F2301/00—Metallic composition of the powder or its coating
- B22F2301/45—Rare earth metals, i.e. Sc, Y, Lanthanides (57-71)
Landscapes
- Chemical & Material Sciences (AREA)
- Engineering & Computer Science (AREA)
- Materials Engineering (AREA)
- Organic Chemistry (AREA)
- Metallurgy (AREA)
- Mechanical Engineering (AREA)
- Power Engineering (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Thermal Sciences (AREA)
- Physics & Mathematics (AREA)
- General Chemical & Material Sciences (AREA)
- Hard Magnetic Materials (AREA)
- Silicon Compounds (AREA)
Description
La1−aAa(FebSi1−bB1−b−c)13Cd (1)
式中、AはCe,Pr,Ndから選択される少なくとも1つの元素であり、BはAl,Mn,Co,Ni,Crから選択される少なくとも1つの元素であり、CはB,Hから選択される少なくとも1つの元素であり、a,b,c,dは、0≦a≦1、0.8≦b≦0.92、0.08≦c≦0.2、0≦d≦1である。
(AxB1−x)2+y(CzD1−z) (2)
式中、AはMn又はCoであり、BはFe,Cr又はNiであり、CはP,B,Se,Ge,Ga,Si,Sn,N,As又はSbであり、DはGe又はSiであり、x,y,zは、0<x<1、−0.1≦y≦0.1、0<z<1である。
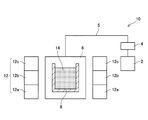
図1を参照し、磁性材料(化合物)の製造装置について説明する。製造装置10は、加熱室6と、加熱室6内に配置される容器8と、ヒータ12を備えている。Arガスタンク2が、配管5を介して加熱室6に接続されている。Arガスは、配管5を通じて加熱室6に供給される。配管5には、圧力制御装置4が設けられている。圧力制御装置4は、加熱室6内の圧力を調節する。容器8内には、磁性部材を構成原料とアルカリ金属の混合原料14が配置される。ヒータ12は、各々発熱量(温度)を独立して制御可能な発熱体12a,12b,12cを備えている。発熱体12a,12b,12cを独立して制御することにより、融液14内において、位置毎に温度差が生じることを抑制する。
(Mn,Fe)2(P,Si)系の磁性部材の合成例を説明する。(Mn,Fe)2(P,Si)系の磁性部材も、図1の製造装置10で、図2のフローに従って合成した。
Claims (6)
- 磁気熱量効果を有する化合物の磁性材料の製造方法であり、
前記磁性材料は、少なくともFe元素を含み、包晶型の状態図を有し、
前記磁性材料を構成する原料を、アルカリ金属を含む融液中で包晶温度以下で反応させて生成物を生成する工程と、
前記生成物を冷却した後、前記アルカリ金属を除去する工程と、
を備える磁性材料の製造方法。 - 前記アルカリ金属は、少なくともNaを含む請求項1に記載の製造方法。
- 前記磁性材料は、下記式(1)で示される化合物である請求項1又は2に記載の製造方法。
La1−aAa(FebSi1−bB1−b−c)13Cd (1)
(式中、AはCe,Pr,Ndから選択される少なくとも1つの元素であり、BはAl,Mn,Co,Ni,Crから選択される少なくとも1つの元素であり、CはB,Hから選択される少なくとも1つの元素であり、a,b,c,dは、0≦a≦1、0.8≦b≦0.92、0.08≦c≦0.2、0≦d≦1である。) - 前記磁性材料は、La(FebSi1−b)13で示される化合物である請求項3に記載の製造方法。
(式中、bは、0.8≦b≦0.92である。) - 前記磁性材料は、下記式(2)で示される、4元系の化合物である請求項1又は2に記載の製造方法。
(AxB1−x)2+y(CzD1−z) (2)
(式中、AはMn又はCoであり、BはFe,Cr又はNiであり、CはP,B,Se,Ge,Ga,Si,Sn,N,As又はSbであり、DはGe又はSiであり、x,y,zは、0<x<1、−0.1≦y≦0.1、0<z<1である。) - 前記磁性材料は、(MnxFe1−x)2(PzSi1−z)で示される化合物である請求項5に記載の製造方法。
(式中、x,zは、0<x<1、0<z<1である。)
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2016215578 | 2016-11-02 | ||
| JP2016215578 | 2016-11-02 | ||
| PCT/JP2017/007950 WO2018083819A1 (ja) | 2016-11-02 | 2017-02-28 | 磁性材料の製造方法 |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| JPWO2018083819A1 JPWO2018083819A1 (ja) | 2019-09-19 |
| JP6899397B2 true JP6899397B2 (ja) | 2021-07-07 |
Family
ID=62075540
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2018548544A Expired - Fee Related JP6899397B2 (ja) | 2016-11-02 | 2017-02-28 | 磁性材料の製造方法 |
Country Status (4)
| Country | Link |
|---|---|
| US (1) | US20190252098A1 (ja) |
| EP (1) | EP3537456A4 (ja) |
| JP (1) | JP6899397B2 (ja) |
| WO (1) | WO2018083819A1 (ja) |
Families Citing this family (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP7134906B2 (ja) * | 2019-03-18 | 2022-09-12 | 大電株式会社 | 磁気冷凍材料 |
| CN110605386B (zh) * | 2019-07-24 | 2021-09-03 | 南京理工大学 | Mo掺杂的Mn-Fe-P-Si基磁制冷材料及其制备方法 |
| JP7187431B2 (ja) * | 2019-12-18 | 2022-12-12 | 大電株式会社 | 磁気冷凍材料 |
Family Cites Families (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2007031831A (ja) * | 2005-06-23 | 2007-02-08 | Sumitomo Metal Mining Co Ltd | 磁気冷凍用希土類−鉄−水素系合金粉末とその製造方法、および得られる押出構造体とその製造方法、並びにそれを用いた磁気冷凍システム |
| JP4735857B2 (ja) * | 2007-03-30 | 2011-07-27 | 国立大学法人 東京大学 | β−YbAlB4、β−YbAlB4を有してなる磁気冷凍作業物質及びその製造方法、並びにそれを用いた磁気冷凍方法及び磁気冷凍装置 |
| JP2009068077A (ja) | 2007-09-13 | 2009-04-02 | Tohoku Univ | 合金材料、磁性材料、磁性材料の製造方法およびその製造方法により製造した磁性材料 |
| AU2009242216C1 (en) | 2008-04-28 | 2014-09-04 | Technology Foundation Stw | Method for producing metal-based materials for magnetic cooling or heat pumps |
| FR2988206B1 (fr) * | 2012-03-16 | 2014-05-02 | Erasteel | Procede de fabrication d'un element magnetocalorique, et element magnetocalorique ainsi obtenu |
-
2017
- 2017-02-28 EP EP17867640.9A patent/EP3537456A4/en not_active Withdrawn
- 2017-02-28 JP JP2018548544A patent/JP6899397B2/ja not_active Expired - Fee Related
- 2017-02-28 WO PCT/JP2017/007950 patent/WO2018083819A1/ja not_active Ceased
-
2019
- 2019-04-29 US US16/396,955 patent/US20190252098A1/en not_active Abandoned
Also Published As
| Publication number | Publication date |
|---|---|
| US20190252098A1 (en) | 2019-08-15 |
| JPWO2018083819A1 (ja) | 2019-09-19 |
| WO2018083819A1 (ja) | 2018-05-11 |
| EP3537456A4 (en) | 2020-06-10 |
| EP3537456A1 (en) | 2019-09-11 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP4450074B2 (ja) | 炭化珪素単結晶の成長方法 | |
| Chen et al. | Structural refinement and thermal expansion of hexaborides | |
| JP4277926B1 (ja) | 炭化珪素単結晶の成長法 | |
| US20080173543A1 (en) | Low oxygen content, crack-free heusler and heusler-like alloys & deposition sources & methods of making same | |
| JP6899397B2 (ja) | 磁性材料の製造方法 | |
| JP2020059633A (ja) | 酸化ガリウム結晶の製造装置及び酸化ガリウム結晶の製造方法並びにこれらに用いる酸化ガリウム結晶育成用のるつぼ | |
| JP5049590B2 (ja) | 炭化珪素(SiC)単結晶の製造方法 | |
| CN100477309C (zh) | 填充方钴矿基合金、其形成方法及利用其的热电转换器件 | |
| JP2009167045A (ja) | 炭化珪素単結晶の成長方法 | |
| JP5919961B2 (ja) | セラミック複合体の製造方法 | |
| US9938605B1 (en) | Methods for making zirconium based alloys and bulk metallic glasses | |
| JP7072146B2 (ja) | 鉄ガリウム合金の単結晶育成方法 | |
| TWI620714B (zh) | Method for producing metal hydride | |
| JP5688589B2 (ja) | 融液組成制御一方向凝固結晶成長装置および結晶成長方法 | |
| JP2010143782A (ja) | 融液組成制御一方向凝固結晶成長装置および結晶成長方法 | |
| JP4595909B2 (ja) | 窒化アルミニウム単結晶の製造方法 | |
| JP7613156B2 (ja) | 鉄アルミニウム合金の製造方法 | |
| JP2000054009A (ja) | 合金粉末の製造方法及びそれを用いた熱電素子の製造方法 | |
| JP2015518812A (ja) | 共晶複合材料形成のための、金属酸化物のケイ素熱還元 | |
| Levytskyy et al. | Crystal structure and hydrogenation properties of the hexagonal Dy2M17 and Dy2M17Cx (M= Fe, Co, Ni; x< 0.5) compounds | |
| CN119020848B (zh) | 一种制备Cr(23-x)FexC6单晶的方法及制备得到的晶体材料 | |
| KR20200059684A (ko) | 일차원 산화물계 전자화물 및 이의 제조방법 | |
| JP2997762B2 (ja) | 六ホウ化カルシウム結晶の育成法 | |
| JP7394374B2 (ja) | 熱電変換材料 | |
| JP2008288267A (ja) | typeIクラスレート化合物の製造方法、熱電変換材料、及び熱電変換素子 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20191023 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20201104 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20201228 |
|
| TRDD | Decision of grant or rejection written | ||
| A01 | Written decision to grant a patent or to grant a registration (utility model) |
Free format text: JAPANESE INTERMEDIATE CODE: A01 Effective date: 20210608 |
|
| A61 | First payment of annual fees (during grant procedure) |
Free format text: JAPANESE INTERMEDIATE CODE: A61 Effective date: 20210614 |
|
| R150 | Certificate of patent or registration of utility model |
Ref document number: 6899397 Country of ref document: JP Free format text: JAPANESE INTERMEDIATE CODE: R150 |
|
| LAPS | Cancellation because of no payment of annual fees |