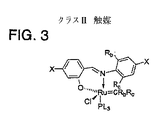
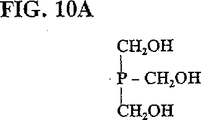
JP4943612B2 - フェロモン又はその成分のメタセシス合成方法 - Google Patents
フェロモン又はその成分のメタセシス合成方法 Download PDFInfo
- Publication number
- JP4943612B2 JP4943612B2 JP2001538323A JP2001538323A JP4943612B2 JP 4943612 B2 JP4943612 B2 JP 4943612B2 JP 2001538323 A JP2001538323 A JP 2001538323A JP 2001538323 A JP2001538323 A JP 2001538323A JP 4943612 B2 JP4943612 B2 JP 4943612B2
- Authority
- JP
- Japan
- Prior art keywords
- acetate
- metathesis
- product
- catalyst
- omega
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Expired - Lifetime
Links
- 0 C*(C)C1(CO2)C2(CO2)P2OC1 Chemical compound C*(C)C1(CO2)C2(CO2)P2OC1 0.000 description 4
- XQPFRDLDHNFCCR-MLCCFXAWSA-N CCCCCCCCCC[C@@H](C(CCC1)OC1=O)O Chemical compound CCCCCCCCCC[C@@H](C(CCC1)OC1=O)O XQPFRDLDHNFCCR-MLCCFXAWSA-N 0.000 description 1
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07F—ACYCLIC, CARBOCYCLIC OR HETEROCYCLIC COMPOUNDS CONTAINING ELEMENTS OTHER THAN CARBON, HYDROGEN, HALOGEN, OXYGEN, NITROGEN, SULFUR, SELENIUM OR TELLURIUM
- C07F9/00—Compounds containing elements of Groups 5 or 15 of the Periodic Table
- C07F9/02—Phosphorus compounds
- C07F9/28—Phosphorus compounds with one or more P—C bonds
- C07F9/50—Organo-phosphines
- C07F9/505—Preparation; Separation; Purification; Stabilisation
- C07F9/5086—Preparation; Separation; Purification; Stabilisation from phosphonium salts as starting materials
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C29/00—Preparation of compounds having hydroxy or O-metal groups bound to a carbon atom not belonging to a six-membered aromatic ring
- C07C29/09—Preparation of compounds having hydroxy or O-metal groups bound to a carbon atom not belonging to a six-membered aromatic ring by hydrolysis
- C07C29/095—Preparation of compounds having hydroxy or O-metal groups bound to a carbon atom not belonging to a six-membered aromatic ring by hydrolysis of esters of organic acids
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C29/00—Preparation of compounds having hydroxy or O-metal groups bound to a carbon atom not belonging to a six-membered aromatic ring
- C07C29/44—Preparation of compounds having hydroxy or O-metal groups bound to a carbon atom not belonging to a six-membered aromatic ring increasing the number of carbon atoms by addition reactions, i.e. reactions involving at least one carbon-to-carbon double or triple bond
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C45/00—Preparation of compounds having >C = O groups bound only to carbon or hydrogen atoms; Preparation of chelates of such compounds
- C07C45/51—Preparation of compounds having >C = O groups bound only to carbon or hydrogen atoms; Preparation of chelates of such compounds by pyrolysis, rearrangement or decomposition
- C07C45/511—Preparation of compounds having >C = O groups bound only to carbon or hydrogen atoms; Preparation of chelates of such compounds by pyrolysis, rearrangement or decomposition involving transformation of singly bound oxygen functional groups to >C = O groups
- C07C45/515—Preparation of compounds having >C = O groups bound only to carbon or hydrogen atoms; Preparation of chelates of such compounds by pyrolysis, rearrangement or decomposition involving transformation of singly bound oxygen functional groups to >C = O groups the singly bound functional group being an acetalised, ketalised hemi-acetalised, or hemi-ketalised hydroxyl group
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C6/00—Preparation of hydrocarbons from hydrocarbons containing a different number of carbon atoms by redistribution reactions
- C07C6/02—Metathesis reactions at an unsaturated carbon-to-carbon bond
- C07C6/04—Metathesis reactions at an unsaturated carbon-to-carbon bond at a carbon-to-carbon double bond
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C67/00—Preparation of carboxylic acid esters
- C07C67/12—Preparation of carboxylic acid esters from asymmetrical anhydrides
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C67/00—Preparation of carboxylic acid esters
- C07C67/28—Preparation of carboxylic acid esters by modifying the hydroxylic moiety of the ester, such modification not being an introduction of an ester group
- C07C67/293—Preparation of carboxylic acid esters by modifying the hydroxylic moiety of the ester, such modification not being an introduction of an ester group by isomerisation; by change of size of the carbon skeleton
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C67/00—Preparation of carboxylic acid esters
- C07C67/28—Preparation of carboxylic acid esters by modifying the hydroxylic moiety of the ester, such modification not being an introduction of an ester group
- C07C67/297—Preparation of carboxylic acid esters by modifying the hydroxylic moiety of the ester, such modification not being an introduction of an ester group by splitting-off hydrogen or functional groups; by hydrogenolysis of functional groups
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C67/00—Preparation of carboxylic acid esters
- C07C67/30—Preparation of carboxylic acid esters by modifying the acid moiety of the ester, such modification not being an introduction of an ester group
- C07C67/333—Preparation of carboxylic acid esters by modifying the acid moiety of the ester, such modification not being an introduction of an ester group by isomerisation; by change of size of the carbon skeleton
- C07C67/343—Preparation of carboxylic acid esters by modifying the acid moiety of the ester, such modification not being an introduction of an ester group by isomerisation; by change of size of the carbon skeleton by increase in the number of carbon atoms
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C67/00—Preparation of carboxylic acid esters
- C07C67/475—Preparation of carboxylic acid esters by splitting of carbon-to-carbon bonds and redistribution, e.g. disproportionation or migration of groups between different molecules
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07F—ACYCLIC, CARBOCYCLIC OR HETEROCYCLIC COMPOUNDS CONTAINING ELEMENTS OTHER THAN CARBON, HYDROGEN, HALOGEN, OXYGEN, NITROGEN, SULFUR, SELENIUM OR TELLURIUM
- C07F5/00—Compounds containing elements of Groups 3 or 13 of the Periodic Table
- C07F5/02—Boron compounds
- C07F5/025—Boronic and borinic acid compounds
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07F—ACYCLIC, CARBOCYCLIC OR HETEROCYCLIC COMPOUNDS CONTAINING ELEMENTS OTHER THAN CARBON, HYDROGEN, HALOGEN, OXYGEN, NITROGEN, SULFUR, SELENIUM OR TELLURIUM
- C07F5/00—Compounds containing elements of Groups 3 or 13 of the Periodic Table
- C07F5/02—Boron compounds
- C07F5/04—Esters of boric acids
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07B—GENERAL METHODS OF ORGANIC CHEMISTRY; APPARATUS THEREFOR
- C07B2200/00—Indexing scheme relating to specific properties of organic compounds
- C07B2200/09—Geometrical isomers
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y02—TECHNOLOGIES OR APPLICATIONS FOR MITIGATION OR ADAPTATION AGAINST CLIMATE CHANGE
- Y02P—CLIMATE CHANGE MITIGATION TECHNOLOGIES IN THE PRODUCTION OR PROCESSING OF GOODS
- Y02P20/00—Technologies relating to chemical industry
- Y02P20/50—Improvements relating to the production of bulk chemicals
- Y02P20/582—Recycling of unreacted starting or intermediate materials
Landscapes
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Chemical Kinetics & Catalysis (AREA)
- General Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Biochemistry (AREA)
- Health & Medical Sciences (AREA)
- Molecular Biology (AREA)
- Organic Low-Molecular-Weight Compounds And Preparation Thereof (AREA)
- Agricultural Chemicals And Associated Chemicals (AREA)
- Low-Molecular Organic Synthesis Reactions Using Catalysts (AREA)
- Catalysts (AREA)
- Pyrane Compounds (AREA)
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US16654399P | 1999-11-18 | 1999-11-18 | |
| US60/166,543 | 1999-11-18 | ||
| PCT/US2000/031549 WO2001036368A2 (en) | 1999-11-18 | 2000-11-17 | Metathesis syntheses of pheromones or their components |
Related Child Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2011084025A Division JP5687114B2 (ja) | 1999-11-18 | 2011-04-05 | フェロモン又はその成分のメタセシス合成方法 |
Publications (3)
| Publication Number | Publication Date |
|---|---|
| JP2003531818A JP2003531818A (ja) | 2003-10-28 |
| JP2003531818A5 JP2003531818A5 (enExample) | 2008-01-17 |
| JP4943612B2 true JP4943612B2 (ja) | 2012-05-30 |
Family
ID=22603759
Family Applications (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2001538323A Expired - Lifetime JP4943612B2 (ja) | 1999-11-18 | 2000-11-17 | フェロモン又はその成分のメタセシス合成方法 |
| JP2011084025A Expired - Lifetime JP5687114B2 (ja) | 1999-11-18 | 2011-04-05 | フェロモン又はその成分のメタセシス合成方法 |
Family Applications After (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2011084025A Expired - Lifetime JP5687114B2 (ja) | 1999-11-18 | 2011-04-05 | フェロモン又はその成分のメタセシス合成方法 |
Country Status (11)
| Country | Link |
|---|---|
| EP (1) | EP1230207B1 (enExample) |
| JP (2) | JP4943612B2 (enExample) |
| CN (1) | CN100528829C (enExample) |
| AT (1) | ATE298318T1 (enExample) |
| AU (1) | AU1921201A (enExample) |
| BR (1) | BR0015712B1 (enExample) |
| CA (1) | CA2392049C (enExample) |
| DE (1) | DE60020987T2 (enExample) |
| IL (2) | IL149720A0 (enExample) |
| WO (1) | WO2001036368A2 (enExample) |
| ZA (1) | ZA200204712B (enExample) |
Families Citing this family (57)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2002020395A (ja) * | 2000-07-04 | 2002-01-23 | Sekisui Chem Co Ltd | 新規な高メタセシス活性の有機金属錯体化合物、これを含有してなるメタセシス反応触媒、この触媒を用いた重合方法、並びにこの重合方法により得られた樹脂組成物 |
| WO2002079126A1 (en) | 2001-03-30 | 2002-10-10 | California Institute Of Technology | Cross-metathesis reaction of functionalized and substituted olefins using group 8 transition metal carbene complexes as metathesis catalysts |
| BRPI0406756A (pt) | 2003-01-13 | 2005-12-20 | Cargill Inc | Método para fabricação de agentes quìmicos industriais |
| BRPI0606718A2 (pt) | 2005-01-10 | 2009-07-14 | Cargill Inc | vela e cera para vela contendo produtos de metátese e semelhantes a metátese |
| KR101269568B1 (ko) * | 2005-07-04 | 2013-06-04 | 자난 사이텍 컴퍼니 리미티드 | 루테늄 착물 리간드, 루테늄 착물, 고정 루테늄 착물 촉매및 그의 제조방법과 용도 |
| WO2007081987A2 (en) * | 2006-01-10 | 2007-07-19 | Elevance Renewable Sciences, Inc. | Method of making hydrogenated metathesis products |
| US8888908B2 (en) | 2006-03-07 | 2014-11-18 | Elevance Renewable Sciences, Inc. | Colorant compositions comprising metathesized unsaturated polyol esters |
| CN102525829B (zh) | 2006-03-07 | 2014-08-06 | 埃莱文斯可更新科学公司 | 含有复分解不饱和多元醇酯的组合物 |
| EP2046908B1 (en) | 2006-07-12 | 2017-01-11 | Elevance Renewable Sciences, Inc. | Hot melt adhesive compositions comprising metathesized unsaturated polyol ester wax |
| WO2008010961A2 (en) | 2006-07-13 | 2008-01-24 | Elevance Renewable Sciences, Inc. | Synthesis of terminal alkenes from internal alkenes and ethylene via olefin metathesis |
| CN101129138B (zh) * | 2006-08-25 | 2010-06-23 | 北京中捷四方生物科技有限公司 | 一种思茅松毛虫性引诱剂和诱芯及其制备方法 |
| CN106083579A (zh) | 2006-10-13 | 2016-11-09 | 埃莱文斯可更新科学公司 | 通过烯烃复分解由内烯烃合成末端烯烃的方法 |
| WO2008048520A2 (en) | 2006-10-13 | 2008-04-24 | Elevance Renewable Sciences, Inc. | Methods of making organic compounds by metathesis and hydrocyanation |
| DK2121546T3 (en) | 2006-10-13 | 2018-03-12 | Elevance Renewable Sciences | Process for preparing omega-dicarboxylic acid olefin derivative by metathesis |
| ATE519725T1 (de) | 2006-10-13 | 2011-08-15 | Elevance Renewable Sciences | Metatheseverfahren mit hydrierung, und damit in zusammenhang stehende zusammensetzungen |
| BRPI0814994A2 (pt) * | 2007-08-09 | 2015-02-03 | Elevance Renewable Sciences | Métodos químicos para tratamento de uma matéria-prima de metátese |
| FR2921363B1 (fr) * | 2007-09-20 | 2009-11-06 | Arkema France | Procedes de synthese de diacides gras par metathese de diacides insatures obtenus par fermentation d'acides gras naturels |
| JP4594371B2 (ja) * | 2007-11-30 | 2010-12-08 | 信越化学工業株式会社 | (e3,z5)−3,5−アルカジエニルアセタートの製造方法 |
| CN101502262B (zh) * | 2009-03-20 | 2012-06-20 | 中国农业科学院草原研究所 | 草地螟性诱剂的合成及其应用 |
| EP2473038A4 (en) * | 2009-09-04 | 2013-10-23 | United Paragon Associates Inc | COMPOSITIONS FOR TREATING SUFFICIENT OR ILLNESSES PROVIDED BY NEUROKININ-2-RECEPTOR ACTIVITY |
| US8735640B2 (en) | 2009-10-12 | 2014-05-27 | Elevance Renewable Sciences, Inc. | Methods of refining and producing fuel and specialty chemicals from natural oil feedstocks |
| US9175231B2 (en) | 2009-10-12 | 2015-11-03 | Elevance Renewable Sciences, Inc. | Methods of refining natural oils and methods of producing fuel compositions |
| US9000246B2 (en) | 2009-10-12 | 2015-04-07 | Elevance Renewable Sciences, Inc. | Methods of refining and producing dibasic esters and acids from natural oil feedstocks |
| US9382502B2 (en) | 2009-10-12 | 2016-07-05 | Elevance Renewable Sciences, Inc. | Methods of refining and producing isomerized fatty acid esters and fatty acids from natural oil feedstocks |
| US9365487B2 (en) | 2009-10-12 | 2016-06-14 | Elevance Renewable Sciences, Inc. | Methods of refining and producing dibasic esters and acids from natural oil feedstocks |
| US9051519B2 (en) | 2009-10-12 | 2015-06-09 | Elevance Renewable Sciences, Inc. | Diene-selective hydrogenation of metathesis derived olefins and unsaturated esters |
| US9169447B2 (en) | 2009-10-12 | 2015-10-27 | Elevance Renewable Sciences, Inc. | Methods of refining natural oils, and methods of producing fuel compositions |
| US9222056B2 (en) | 2009-10-12 | 2015-12-29 | Elevance Renewable Sciences, Inc. | Methods of refining natural oils, and methods of producing fuel compositions |
| US8957268B2 (en) | 2009-10-12 | 2015-02-17 | Elevance Renewable Sciences, Inc. | Methods of refining natural oil feedstocks |
| ES2608556T3 (es) * | 2011-01-14 | 2017-04-12 | Basf Se | Composición atrayente que contiene etil 3-metilbutanoato y (E,E)-8-,10-dodecadien-1-ol |
| US9150468B2 (en) * | 2011-09-28 | 2015-10-06 | Nalco Company | Method of producing olefins via metathesis |
| US9133416B2 (en) | 2011-12-22 | 2015-09-15 | Elevance Renewable Sciences, Inc. | Methods for suppressing isomerization of olefin metathesis products |
| MX348233B (es) | 2011-12-22 | 2017-05-30 | Elevance Renewable Sciences | Metodos para suprimir la isomerizacion de productos de metatesis de olefina, metodos de refinación de aceites naturales, y metodos de producción de composiciones de combustible. |
| US9139493B2 (en) | 2011-12-22 | 2015-09-22 | Elevance Renewable Sciences, Inc. | Methods for suppressing isomerization of olefin metathesis products |
| US9169174B2 (en) | 2011-12-22 | 2015-10-27 | Elevance Renewable Sciences, Inc. | Methods for suppressing isomerization of olefin metathesis products |
| EP2804936B1 (en) | 2012-01-10 | 2016-03-23 | Elevance Renewable Sciences, Inc. | Renewable fatty acid waxes and methods of making |
| EP2859068A1 (en) * | 2012-06-12 | 2015-04-15 | Elevance Renewable Sciences, Inc. | Methods for suppressing dehydrogenation |
| EP2864447B3 (en) | 2012-06-20 | 2019-07-17 | Elevance Renewable Sciences, Inc. | Natural oil metathesis compositions |
| US9388098B2 (en) | 2012-10-09 | 2016-07-12 | Elevance Renewable Sciences, Inc. | Methods of making high-weight esters, acids, and derivatives thereof |
| ES2662827T3 (es) * | 2013-04-12 | 2018-04-09 | Shin-Etsu Chemical Co., Ltd | Omega-halo-2-alquinal, procedimiento de producción del mismo y procedimiento de producción de acetato de Z-alquen-in-ilo conjugado usando el mismo |
| GB201404468D0 (en) | 2014-03-13 | 2014-04-30 | Givaudan Sa | Process |
| JP6572966B2 (ja) * | 2015-03-03 | 2019-09-11 | Agc株式会社 | 含フッ素オレフィン化合物の製造方法 |
| AU2016357756B2 (en) * | 2015-11-18 | 2021-09-30 | Provivi, Inc. | Production of fatty olefin derivatives via olefin metathesis |
| WO2017100585A1 (en) * | 2015-12-10 | 2017-06-15 | Materia, Inc. | Olefin metathesis catalysts |
| CN105794775B (zh) * | 2016-03-30 | 2018-05-08 | 中国检验检疫科学研究院 | 一种长小蠹科昆虫引诱剂及其制备方法与应用 |
| WO2018069146A1 (fr) | 2016-10-11 | 2018-04-19 | Demeta | Procede de synthese de pheromones |
| CN110603240B (zh) * | 2017-02-17 | 2022-11-15 | 普罗维维公司 | 通过烯烃复分解合成信息素和相关物质 |
| JP7023166B2 (ja) * | 2018-04-18 | 2022-02-21 | 信越化学工業株式会社 | (9e,11z)-9,11-ヘキサデカジエナールの製造方法 |
| WO2020123534A1 (en) | 2018-12-10 | 2020-06-18 | Provivi, Inc. | Synthesis of conjugated diene pheromones and related compounds |
| FR3098515B1 (fr) * | 2019-07-12 | 2022-11-04 | Melchior Material & Life Science France | Nouvelles formulations stables de composés 1-Z-bromo alcènes-1 et leur utilisation dans la fabrication de phéromones |
| WO2021247583A1 (en) * | 2020-06-01 | 2021-12-09 | Provivi, Inc. | Synthesis of pheromone derivatives via z-selective olefin metathesis |
| JP7291105B2 (ja) * | 2020-06-24 | 2023-06-14 | 信越化学工業株式会社 | (9z,11e)-9,11-ヘキサデカジエナールの製造方法 |
| CN112782309B (zh) * | 2020-12-29 | 2022-10-28 | 常州大学 | 一种稳定长链不饱和烯醛的方法 |
| CN114507122B (zh) * | 2022-01-28 | 2024-07-05 | 宜都市华阳化工有限责任公司 | 3-(4-甲基苯亚甲基)-樟脑合成副产物的回收利用方法 |
| KR102473703B1 (ko) | 2022-07-12 | 2022-12-01 | 전종열 | 사피엔산과 그 트랜스 이성질체 및 사피엔산 에스터를 제조하는 방법 |
| CN115452991B (zh) * | 2022-09-15 | 2024-07-12 | 江苏科技大学 | 十四碳烯酸和十六碳烯醛在检测检疫性害虫中的应用 |
| CN117682940A (zh) * | 2023-11-13 | 2024-03-12 | 中捷四方生物科技股份有限公司 | 无患子在制备昆虫信息素中的应用 |
Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5916983A (en) * | 1997-05-27 | 1999-06-29 | Bend Research, Inc. | Biologically active compounds by catalytic olefin metathesis |
| JPH11262667A (ja) * | 1992-04-03 | 1999-09-28 | California Inst Of Technol | オレフィンメタセシス反応用高活性ルテニウムまたはオスミウム金属カルベン錯体の製造法 |
| WO1999051344A1 (de) * | 1998-04-06 | 1999-10-14 | Aventis Research & Technologies Gmbh & Co. Kg | Alkylidenkomplexe des rutheniums mit n-heterozyklischen carbenliganden; verwendung als hochaktive, selektive katalysatoren für die olefin-metathese |
| JP2002535297A (ja) * | 1999-01-26 | 2002-10-22 | カリフォルニア インスティチュート オブ テクノロジー | 末端オレフィンのクロスメタセシスのための方法 |
Family Cites Families (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP3942122B2 (ja) * | 1997-12-26 | 2007-07-11 | 高砂香料工業株式会社 | ルテニウムメタセシス触媒およびそれを用いたメタセ シス反応によるオレフィン反応生成物を製造する方法 |
| DE19907519A1 (de) * | 1999-02-22 | 2000-08-31 | Basf Ag | Verfahren zur Herstellung von substituierten Olefinen |
| JP4410422B2 (ja) * | 1999-05-24 | 2010-02-03 | カリフォルニア インスティチュート オブ テクノロジー | イミダゾリジンに基づく金属カルベンメタセシス触媒 |
-
2000
- 2000-11-17 CN CNB008181101A patent/CN100528829C/zh not_active Expired - Lifetime
- 2000-11-17 BR BRPI0015712-0A patent/BR0015712B1/pt not_active IP Right Cessation
- 2000-11-17 DE DE60020987T patent/DE60020987T2/de not_active Expired - Lifetime
- 2000-11-17 EP EP00982144A patent/EP1230207B1/en not_active Expired - Lifetime
- 2000-11-17 CA CA2392049A patent/CA2392049C/en not_active Expired - Lifetime
- 2000-11-17 JP JP2001538323A patent/JP4943612B2/ja not_active Expired - Lifetime
- 2000-11-17 AU AU19212/01A patent/AU1921201A/en not_active Abandoned
- 2000-11-17 AT AT00982144T patent/ATE298318T1/de not_active IP Right Cessation
- 2000-11-17 WO PCT/US2000/031549 patent/WO2001036368A2/en not_active Ceased
- 2000-11-17 IL IL14972000A patent/IL149720A0/xx active IP Right Grant
-
2002
- 2002-05-19 IL IL149720A patent/IL149720A/en unknown
- 2002-06-12 ZA ZA200204712A patent/ZA200204712B/en unknown
-
2011
- 2011-04-05 JP JP2011084025A patent/JP5687114B2/ja not_active Expired - Lifetime
Patent Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPH11262667A (ja) * | 1992-04-03 | 1999-09-28 | California Inst Of Technol | オレフィンメタセシス反応用高活性ルテニウムまたはオスミウム金属カルベン錯体の製造法 |
| US5916983A (en) * | 1997-05-27 | 1999-06-29 | Bend Research, Inc. | Biologically active compounds by catalytic olefin metathesis |
| WO1999051344A1 (de) * | 1998-04-06 | 1999-10-14 | Aventis Research & Technologies Gmbh & Co. Kg | Alkylidenkomplexe des rutheniums mit n-heterozyklischen carbenliganden; verwendung als hochaktive, selektive katalysatoren für die olefin-metathese |
| JP2002535297A (ja) * | 1999-01-26 | 2002-10-22 | カリフォルニア インスティチュート オブ テクノロジー | 末端オレフィンのクロスメタセシスのための方法 |
Also Published As
| Publication number | Publication date |
|---|---|
| BR0015712B1 (pt) | 2011-01-25 |
| CN1423629A (zh) | 2003-06-11 |
| ZA200204712B (en) | 2004-04-08 |
| BR0015712A (pt) | 2004-04-27 |
| JP2003531818A (ja) | 2003-10-28 |
| CN100528829C (zh) | 2009-08-19 |
| ATE298318T1 (de) | 2005-07-15 |
| JP2011178789A (ja) | 2011-09-15 |
| EP1230207A2 (en) | 2002-08-14 |
| JP5687114B2 (ja) | 2015-03-18 |
| CA2392049C (en) | 2012-01-31 |
| DE60020987D1 (de) | 2005-07-28 |
| IL149720A (en) | 2008-03-20 |
| EP1230207B1 (en) | 2005-06-22 |
| IL149720A0 (en) | 2002-11-10 |
| DE60020987T2 (de) | 2006-05-11 |
| WO2001036368A3 (en) | 2002-01-10 |
| AU1921201A (en) | 2001-05-30 |
| WO2001036368A2 (en) | 2001-05-25 |
| CA2392049A1 (en) | 2001-05-25 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP4943612B2 (ja) | フェロモン又はその成分のメタセシス合成方法 | |
| US6696597B2 (en) | Metathesis syntheses of pheromones or their components | |
| US6215019B1 (en) | Synthesis of 5-decenyl acetate and other pheromone components | |
| JPH0212942B2 (enExample) | ||
| Hiyama et al. | Asymmetric coupling of arylmagnesium bromides with allylic esters | |
| KR950004892B1 (ko) | 살충제용 에테르의 제조방법 | |
| NO141752B (no) | Substituerte eddiksyreestere for anvendelse som pesticider | |
| EP1192117B1 (en) | (e8,z10)-tetradeca-8,10-dienal, the method of its preparation and its use as sexual attractant for leafminer moths | |
| JPS6229422B2 (enExample) | ||
| KR920002126B1 (ko) | 살충제 화합물의 제조방법 | |
| CN101815439A (zh) | 具有高杀虫活性的聚烯基环丙羧酸酯 | |
| US4188374A (en) | Attractant for the potato moth and the preparation of the former | |
| US12344573B2 (en) | Synthesis of straight-chain lepidopteran pheromones through one- or two-carbon homologation of fatty alkenes | |
| US4484007A (en) | Process for preparation of arylterpenoid insect maturation inhibitors | |
| CA2187277C (en) | Novel derivative of ester of carboxylic acid, the process of manufacturing the same, and insecticide and insect proofing agent containing the same | |
| US4567265A (en) | Cyclopropanoid cyanoesters and method of making same | |
| Chen et al. | Preparative scale syntheses of isomerically pure (10E, 12E, 14Z)-and (10E, 12E, 14E)-hexadeca-10, 12, 14-trienals, sex pheromone components of Manduca sexta | |
| GB2142632A (en) | Process for the preparation of the pheromone 3,11-dimethyl-2-nonacosanone | |
| JPH0372053B2 (enExample) | ||
| EA046714B1 (ru) | Способ получения z11-гексадецен-1-илацетата - компонента феромона золотистой двухпятнистой совки chrysodeixis chalcites, и других насекомых | |
| Fiandanese et al. | A general approach to the synthesis of mono-olefinic insect sex pheromones of Z-or E-configuration. | |
| CN1026649C (zh) | 改进的杀虫剂组合物及其生产方法及用途 | |
| JPS5833845B2 (ja) | 1 1 1− トリハロ −4− メチル −3− ベンテンノ セイゾウホウホウ | |
| PL215367B1 (pl) | Pochodne (5Z,7E)-dodeka-5,7-dienalu, sposób ich wytwarzania, środek zawierający te związki oraz sposób jego wytwarzania i zastosowanie | |
| GB2025962A (en) | Cyclopropane Derivatives |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20071108 |
|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20071108 |
|
| RD03 | Notification of appointment of power of attorney |
Free format text: JAPANESE INTERMEDIATE CODE: A7423 Effective date: 20080409 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A821 Effective date: 20080409 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20101005 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20101227 |
|
| A602 | Written permission of extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A602 Effective date: 20110107 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20110304 |
|
| A602 | Written permission of extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A602 Effective date: 20110311 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20110405 |
|
| TRDD | Decision of grant or rejection written | ||
| A01 | Written decision to grant a patent or to grant a registration (utility model) |
Free format text: JAPANESE INTERMEDIATE CODE: A01 Effective date: 20120131 |
|
| A01 | Written decision to grant a patent or to grant a registration (utility model) |
Free format text: JAPANESE INTERMEDIATE CODE: A01 |
|
| A61 | First payment of annual fees (during grant procedure) |
Free format text: JAPANESE INTERMEDIATE CODE: A61 Effective date: 20120301 |
|
| R150 | Certificate of patent or registration of utility model |
Ref document number: 4943612 Country of ref document: JP Free format text: JAPANESE INTERMEDIATE CODE: R150 Free format text: JAPANESE INTERMEDIATE CODE: R150 |
|
| FPAY | Renewal fee payment (event date is renewal date of database) |
Free format text: PAYMENT UNTIL: 20150309 Year of fee payment: 3 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| EXPY | Cancellation because of completion of term |