JP4914345B2 - 吸入器 - Google Patents
吸入器 Download PDFInfo
- Publication number
- JP4914345B2 JP4914345B2 JP2007512106A JP2007512106A JP4914345B2 JP 4914345 B2 JP4914345 B2 JP 4914345B2 JP 2007512106 A JP2007512106 A JP 2007512106A JP 2007512106 A JP2007512106 A JP 2007512106A JP 4914345 B2 JP4914345 B2 JP 4914345B2
- Authority
- JP
- Japan
- Prior art keywords
- inhaler
- mouthpiece
- recess
- capsule
- cap
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Expired - Lifetime
Links
- 239000002775 capsule Substances 0.000 claims description 48
- 239000003814 drug Substances 0.000 claims description 19
- 239000000843 powder Substances 0.000 claims description 12
- 229940079593 drug Drugs 0.000 claims description 8
- 150000003839 salts Chemical class 0.000 claims description 6
- 208000006545 Chronic Obstructive Pulmonary Disease Diseases 0.000 claims description 5
- 208000006673 asthma Diseases 0.000 claims description 5
- 230000002685 pulmonary effect Effects 0.000 claims description 5
- NDAUXUAQIAJITI-UHFFFAOYSA-N albuterol Chemical group CC(C)(C)NCC(O)C1=CC=C(O)C(CO)=C1 NDAUXUAQIAJITI-UHFFFAOYSA-N 0.000 claims description 4
- 229940124630 bronchodilator Drugs 0.000 claims description 4
- 229960002052 salbutamol Drugs 0.000 claims description 4
- 239000002260 anti-inflammatory agent Substances 0.000 claims description 3
- 229940124599 anti-inflammatory drug Drugs 0.000 claims description 3
- SMNDYUVBFMFKNZ-UHFFFAOYSA-N 2-furoic acid Chemical compound OC(=O)C1=CC=CO1 SMNDYUVBFMFKNZ-UHFFFAOYSA-N 0.000 claims description 2
- VOVIALXJUBGFJZ-KWVAZRHASA-N Budesonide Chemical compound C1CC2=CC(=O)C=C[C@]2(C)[C@@H]2[C@@H]1[C@@H]1C[C@H]3OC(CCC)O[C@@]3(C(=O)CO)[C@@]1(C)C[C@@H]2O VOVIALXJUBGFJZ-KWVAZRHASA-N 0.000 claims description 2
- LUKZNWIVRBCLON-GXOBDPJESA-N Ciclesonide Chemical compound C1([C@H]2O[C@@]3([C@H](O2)C[C@@H]2[C@@]3(C[C@H](O)[C@@H]3[C@@]4(C)C=CC(=O)C=C4CC[C@H]32)C)C(=O)COC(=O)C(C)C)CCCCC1 LUKZNWIVRBCLON-GXOBDPJESA-N 0.000 claims description 2
- VPNYRYCIDCJBOM-UHFFFAOYSA-M Glycopyrronium bromide Chemical compound [Br-].C1[N+](C)(C)CCC1OC(=O)C(O)(C=1C=CC=CC=1)C1CCCC1 VPNYRYCIDCJBOM-UHFFFAOYSA-M 0.000 claims description 2
- GIIZNNXWQWCKIB-UHFFFAOYSA-N Serevent Chemical compound C1=C(O)C(CO)=CC(C(O)CNCCCCCCOCCCCC=2C=CC=CC=2)=C1 GIIZNNXWQWCKIB-UHFFFAOYSA-N 0.000 claims description 2
- 229960004436 budesonide Drugs 0.000 claims description 2
- 229960003728 ciclesonide Drugs 0.000 claims description 2
- 229960000289 fluticasone propionate Drugs 0.000 claims description 2
- WMWTYOKRWGGJOA-CENSZEJFSA-N fluticasone propionate Chemical compound C1([C@@H](F)C2)=CC(=O)C=C[C@]1(C)[C@]1(F)[C@@H]2[C@@H]2C[C@@H](C)[C@@](C(=O)SCF)(OC(=O)CC)[C@@]2(C)C[C@@H]1O WMWTYOKRWGGJOA-CENSZEJFSA-N 0.000 claims description 2
- 229960002848 formoterol Drugs 0.000 claims description 2
- BPZSYCZIITTYBL-UHFFFAOYSA-N formoterol Chemical compound C1=CC(OC)=CC=C1CC(C)NCC(O)C1=CC=C(O)C(NC=O)=C1 BPZSYCZIITTYBL-UHFFFAOYSA-N 0.000 claims description 2
- 229940015042 glycopyrrolate Drugs 0.000 claims description 2
- 229960001361 ipratropium bromide Drugs 0.000 claims description 2
- KEWHKYJURDBRMN-ZEODDXGYSA-M ipratropium bromide hydrate Chemical compound O.[Br-].O([C@H]1C[C@H]2CC[C@@H](C1)[N@@+]2(C)C(C)C)C(=O)C(CO)C1=CC=CC=C1 KEWHKYJURDBRMN-ZEODDXGYSA-M 0.000 claims description 2
- 229960001609 oxitropium bromide Drugs 0.000 claims description 2
- LCELQERNWLBPSY-KHSTUMNDSA-M oxitropium bromide Chemical compound [Br-].C1([C@@H](CO)C(=O)O[C@H]2C[C@@H]3[N+]([C@H](C2)[C@@H]2[C@H]3O2)(C)CC)=CC=CC=C1 LCELQERNWLBPSY-KHSTUMNDSA-M 0.000 claims description 2
- 238000003825 pressing Methods 0.000 claims description 2
- 238000004080 punching Methods 0.000 claims description 2
- 229960004017 salmeterol Drugs 0.000 claims description 2
- LERNTVKEWCAPOY-DZZGSBJMSA-N tiotropium Chemical compound O([C@H]1C[C@@H]2[N+]([C@H](C1)[C@@H]1[C@H]2O1)(C)C)C(=O)C(O)(C=1SC=CC=1)C1=CC=CS1 LERNTVKEWCAPOY-DZZGSBJMSA-N 0.000 claims description 2
- 229940110309 tiotropium Drugs 0.000 claims description 2
- 239000002253 acid Substances 0.000 claims 1
- 229940092705 beclomethasone Drugs 0.000 claims 1
- NBMKJKDGKREAPL-DVTGEIKXSA-N beclomethasone Chemical group C1CC2=CC(=O)C=C[C@]2(C)[C@]2(Cl)[C@@H]1[C@@H]1C[C@H](C)[C@@](C(=O)CO)(O)[C@@]1(C)C[C@@H]2O NBMKJKDGKREAPL-DVTGEIKXSA-N 0.000 claims 1
- 239000003570 air Substances 0.000 description 13
- 210000003811 finger Anatomy 0.000 description 6
- 239000000463 material Substances 0.000 description 4
- 150000001875 compounds Chemical class 0.000 description 3
- 238000005553 drilling Methods 0.000 description 3
- 150000003431 steroids Chemical class 0.000 description 3
- 210000003813 thumb Anatomy 0.000 description 3
- 239000000168 bronchodilator agent Substances 0.000 description 2
- 238000001647 drug administration Methods 0.000 description 2
- 229920012128 methyl methacrylate acrylonitrile butadiene styrene Polymers 0.000 description 2
- KUVIULQEHSCUHY-XYWKZLDCSA-N Beclometasone Chemical compound C1CC2=CC(=O)C=C[C@]2(C)[C@]2(Cl)[C@@H]1[C@@H]1C[C@H](C)[C@@](C(=O)COC(=O)CC)(OC(=O)CC)[C@@]1(C)C[C@@H]2O KUVIULQEHSCUHY-XYWKZLDCSA-N 0.000 description 1
- 206010047571 Visual impairment Diseases 0.000 description 1
- 239000004676 acrylonitrile butadiene styrene Substances 0.000 description 1
- 230000003213 activating effect Effects 0.000 description 1
- 239000000048 adrenergic agonist Substances 0.000 description 1
- 229940126157 adrenergic receptor agonist Drugs 0.000 description 1
- 239000012080 ambient air Substances 0.000 description 1
- 229940121363 anti-inflammatory agent Drugs 0.000 description 1
- 229950000210 beclometasone dipropionate Drugs 0.000 description 1
- 102000016966 beta-2 Adrenergic Receptors Human genes 0.000 description 1
- 108010014499 beta-2 Adrenergic Receptors Proteins 0.000 description 1
- BZDKYAZTCWRUDZ-UHFFFAOYSA-N buta-1,3-diene;methyl 2-methylprop-2-enoate;prop-2-enenitrile;styrene Chemical compound C=CC=C.C=CC#N.COC(=O)C(C)=C.C=CC1=CC=CC=C1 BZDKYAZTCWRUDZ-UHFFFAOYSA-N 0.000 description 1
- 239000003795 chemical substances by application Substances 0.000 description 1
- 238000004140 cleaning Methods 0.000 description 1
- 230000000295 complement effect Effects 0.000 description 1
- -1 for example Substances 0.000 description 1
- 239000003862 glucocorticoid Substances 0.000 description 1
- 238000003780 insertion Methods 0.000 description 1
- 230000037431 insertion Effects 0.000 description 1
- 238000004519 manufacturing process Methods 0.000 description 1
- 239000003149 muscarinic antagonist Substances 0.000 description 1
- 239000004033 plastic Substances 0.000 description 1
- 229920003023 plastic Polymers 0.000 description 1
- 208000023504 respiratory system disease Diseases 0.000 description 1
- 239000012453 solvate Substances 0.000 description 1
- 238000003860 storage Methods 0.000 description 1
- 208000029257 vision disease Diseases 0.000 description 1
- 230000000007 visual effect Effects 0.000 description 1
- 230000004393 visual impairment Effects 0.000 description 1
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M15/00—Inhalators
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M15/00—Inhalators
- A61M15/0028—Inhalators using prepacked dosages, one for each application, e.g. capsules to be perforated or broken-up
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M15/00—Inhalators
- A61M15/0001—Details of inhalators; Constructional features thereof
- A61M15/0021—Mouthpieces therefor
- A61M15/0025—Mouthpieces therefor with caps
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M15/00—Inhalators
- A61M15/0028—Inhalators using prepacked dosages, one for each application, e.g. capsules to be perforated or broken-up
- A61M15/003—Inhalators using prepacked dosages, one for each application, e.g. capsules to be perforated or broken-up using capsules, e.g. to be perforated or broken-up
- A61M15/0033—Details of the piercing or cutting means
Landscapes
- Health & Medical Sciences (AREA)
- Engineering & Computer Science (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Pulmonology (AREA)
- Anesthesiology (AREA)
- Biomedical Technology (AREA)
- Heart & Thoracic Surgery (AREA)
- Hematology (AREA)
- Life Sciences & Earth Sciences (AREA)
- Animal Behavior & Ethology (AREA)
- General Health & Medical Sciences (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
- Medicinal Preparation (AREA)
- Medical Preparation Storing Or Oral Administration Devices (AREA)
- Acyclic And Carbocyclic Compounds In Medicinal Compositions (AREA)
Description
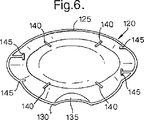
5 本体
10 前部
15 後部
20 第1の側部
25 第2の側部
30 マウスピース
32 吸気口
35 プッシュボタン
40 プッシュボタン
45 カプセルチャンバ
50 凹部
55 フランジ
60 管
65 穴あきプレートまたはグリッド
70 吸入通路
75 ヒンジ部材
80 溝
85 軸方向穴
90 空気通路
95 針またはピン
105 ばね
110 肩部
115 把持部材
120 キャップ
125 前部
130 後部
132 凹部
135 溝付き領域
140 マウスピースガイド
145 リブ
Claims (9)
- 吸入される粉末薬を収容するカプセルを保持するための凹部を有する本体と、
前記凹部に対して接線方向に配置された少なくとも1つの空気通路と、
前記本体の前記凹部と連通するように同軸状に配置された吸入通路を含むマウスピースと、
空気が前記空気通路を通って前記凹部に引き込まれて凹部内で渦巻くときに前記粉末薬が放出されるように、前記凹部に装填されたとき前記カプセルに穴を開けるための前記本体上のカプセル穴開け手段とを備える、粉末薬用の吸入器において、
前記カプセル穴開け手段は、少なくとも1つの穴開け部材をそれぞれ含んで対向して互いに離れる方向にばね付勢された一対のプッシュボタンを備え、
前記マウスピースは、ユーザが当該マウスピースに押圧力を加えることにより、前記吸入器の縦軸に垂直な軸を中心として開放装填位置と閉鎖放出位置との間で回転可能なように前記本体の後縁部に回転可能に取り付けられており、
前記本体は、前記縦軸に垂直な軸に沿って前記マウスピースのヒンジ部材から突出するヒンジ軸を収容する一対の軸方向穴を有し、
前記一対のプッシュボタンは、前記ヒンジ軸に平行な方向に移動するように前記本体に設けられている、
ことを特徴とする吸入器。 - 前記穴開け部材が針または尖ったピンであることを特徴とする請求項1に記載の吸入器。
- 前記凹部は、前記カプセルが前記吸入器の縦軸を中心に前記凹部内で回転できるように形成されていることを特徴とする請求項1に記載の吸入器。
- 前記本体は、前記本体の前記縁部に前記マウスピースを固定する2つ以上の相互連結本体部分を有することを特徴とする請求項1に記載の吸入器。
- 取り外し可能なキャップをさらに含み、前記マウスピースと係合するマウスピースガイドが前記キャップの内面上に設けられていることを特徴とする請求項1に記載の吸入器。
- 前記吸入器の前記本体に係合するリブが、前記キャップの開口部に隣接する前記キャップの内面に設けられていることを特徴とする請求項5に記載の吸入器。
- 粉末薬を収容したカプセルを含むことを特徴とする請求項1から6の何れか一に記載の吸入器。
- 前記粉末薬が、肺吸入によるぜんそくまたは慢性閉塞性肺疾患の治療に適していること
を特徴とする請求項7に記載の吸入器。 - 前記粉末薬は、気管支拡張薬、抗炎症薬、およびそれらの組合せの少なくとも1つであり、前記気管支拡張薬は、アルブテロール(サルブタモール)、サルメテロール、フォルモテロール、およびそれらの薬学的に許容可能な塩、臭化イプラトロピウム、臭化オキシトロピウム、チオトロピウム、グリコピロレート、およびそれらの薬学的に許容可能な塩、で成るグループから選択されるものであり、前記抗炎症薬は、ブデソニド、ジプロピオン酸ベクロメタゾン、プロピオン酸フルチカゾン、シクレソナイド、およびフランカルボン酸モタメゾンで成るグループから選択されるものである、ことを特徴とする請求項1から8の何れか一に記載の吸入器。
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| GB0410712.4 | 2004-05-13 | ||
| GBGB0410712.4A GB0410712D0 (en) | 2004-05-13 | 2004-05-13 | Organic compounds |
| PCT/EP2005/005182 WO2005113042A1 (en) | 2004-05-13 | 2005-05-12 | Inhaler device |
Related Child Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2011241398A Division JP2012071146A (ja) | 2004-05-13 | 2011-11-02 | 吸入器 |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| JP2007536962A JP2007536962A (ja) | 2007-12-20 |
| JP4914345B2 true JP4914345B2 (ja) | 2012-04-11 |
Family
ID=32526997
Family Applications (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2007512106A Expired - Lifetime JP4914345B2 (ja) | 2004-05-13 | 2005-05-12 | 吸入器 |
| JP2011241398A Pending JP2012071146A (ja) | 2004-05-13 | 2011-11-02 | 吸入器 |
Family Applications After (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2011241398A Pending JP2012071146A (ja) | 2004-05-13 | 2011-11-02 | 吸入器 |
Country Status (31)
| Country | Link |
|---|---|
| US (1) | US8479730B2 (ja) |
| EP (1) | EP1747036B1 (ja) |
| JP (2) | JP4914345B2 (ja) |
| KR (1) | KR101170187B1 (ja) |
| CN (1) | CN1953779B (ja) |
| AR (1) | AR051353A1 (ja) |
| AT (1) | ATE502666T1 (ja) |
| AU (1) | AU2005245095B2 (ja) |
| BR (1) | BRPI0511073B8 (ja) |
| CA (1) | CA2563573C (ja) |
| CY (1) | CY1111555T1 (ja) |
| DE (1) | DE602005027064D1 (ja) |
| DK (1) | DK1747036T3 (ja) |
| EC (1) | ECSP066987A (ja) |
| ES (1) | ES2362429T3 (ja) |
| GB (1) | GB0410712D0 (ja) |
| IL (1) | IL178878A (ja) |
| MA (1) | MA28612B1 (ja) |
| MX (1) | MXPA06013081A (ja) |
| MY (1) | MY144563A (ja) |
| NO (1) | NO334854B1 (ja) |
| NZ (1) | NZ550625A (ja) |
| PE (1) | PE20060098A1 (ja) |
| PL (1) | PL1747036T3 (ja) |
| PT (1) | PT1747036E (ja) |
| RU (1) | RU2363501C2 (ja) |
| SI (1) | SI1747036T1 (ja) |
| TN (1) | TNSN06367A1 (ja) |
| TW (1) | TWI353857B (ja) |
| WO (1) | WO2005113042A1 (ja) |
| ZA (1) | ZA200608728B (ja) |
Families Citing this family (120)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US9006175B2 (en) | 1999-06-29 | 2015-04-14 | Mannkind Corporation | Potentiation of glucose elimination |
| SI1494732T1 (sl) | 2002-03-20 | 2008-08-31 | Mannking Corp | Inhalacijski aparat |
| KR101273120B1 (ko) | 2004-08-20 | 2013-06-13 | 맨카인드 코포레이션 | 다이케토피페라진 합성의 촉매 작용 |
| BR122019022692B1 (pt) | 2004-08-23 | 2023-01-10 | Mannkind Corporation | Composição terapêutica em pó seco contendo dicetopiperazina, pelo menos um tipo de cátion e um agente biologicamente ativo |
| KR101486829B1 (ko) | 2005-09-14 | 2015-01-29 | 맨카인드 코포레이션 | 결정질 미립자 표면에 대한 활성제의 친화력의 증가를 기반으로 하는 약물 제제의 방법 |
| EP1986679B1 (en) | 2006-02-22 | 2017-10-25 | MannKind Corporation | A method for improving the pharmaceutic properties of microparticles comprising diketopiperazine and an active agent |
| CL2007001477S1 (es) * | 2006-11-27 | 2008-03-14 | Cipla Ltd | Camara de capsula. |
| DE102007036411A1 (de) * | 2007-07-20 | 2009-02-12 | Boehringer Ingelheim Pharma Gmbh & Co. Kg | Pulverinhalator |
| EP2020249A1 (de) | 2007-08-01 | 2009-02-04 | Boehringer Ingelheim Pharma GmbH & Co. KG | Inhalator |
| JP5456681B2 (ja) | 2007-10-17 | 2014-04-02 | ノバルティス アーゲー | ALK阻害剤として有用なイミダゾ[1,2−a]ピリジン誘導体 |
| JP5570996B2 (ja) | 2007-12-14 | 2014-08-13 | エアロデザインズ インコーポレイテッド | エアロゾル化可能な食料品の送達 |
| US20090165783A1 (en) * | 2007-12-31 | 2009-07-02 | Mcdonald Molly | Nebulizer Apparatus and Method |
| WO2009087224A1 (en) | 2008-01-11 | 2009-07-16 | Novartis Ag | Pyrimidines as kinase inhibitors |
| EP2082769B1 (en) | 2008-01-24 | 2015-06-03 | Vectura Delivery Devices Limited | Inhaler |
| TR200803522A1 (tr) | 2008-05-16 | 2009-12-21 | Bi̇lgi̇ç Mahmut | Kolay kullanımlı inhalasyon cihazı. |
| TR200803524A2 (tr) * | 2008-05-16 | 2009-12-21 | Bi̇lgi̇ç Mahmut | İnhalasyon cihazı. |
| TWI677355B (zh) | 2008-06-13 | 2019-11-21 | 美商曼凱公司 | 用於藥物傳輸之乾粉吸入器及系統 |
| US8485180B2 (en) | 2008-06-13 | 2013-07-16 | Mannkind Corporation | Dry powder drug delivery system |
| ES2421385T3 (es) | 2008-06-20 | 2013-09-02 | Mannkind Corp | Aparato interactivo y procedimiento para establecer el perfil, en tiempo real, de esfuerzos de inhalación |
| TWI614024B (zh) | 2008-08-11 | 2018-02-11 | 曼凱公司 | 超快起作用胰島素之用途 |
| GB0818476D0 (en) * | 2008-10-09 | 2008-11-12 | Vectura Delivery Device Ltd | Inhaler |
| US8314106B2 (en) | 2008-12-29 | 2012-11-20 | Mannkind Corporation | Substituted diketopiperazine analogs for use as drug delivery agents |
| EP2676695A3 (en) | 2009-03-11 | 2017-03-01 | MannKind Corporation | Apparatus, system and method for measuring resistance of an inhaler |
| US20120022127A1 (en) | 2009-04-09 | 2012-01-26 | Thomas Allmendinger | Process for preparing pyrrolidinium salts |
| MY186975A (en) | 2009-06-12 | 2021-08-26 | Mannkind Corp | Diketopiperazine microparticles with defined specific surface areas |
| US9016147B2 (en) | 2009-11-03 | 2015-04-28 | Mannkind Corporation | Apparatus and method for simulating inhalation efforts |
| WO2011110970A1 (en) | 2010-03-12 | 2011-09-15 | Ranbaxy Laboratories Limited | Single dose dry powder inhaler device |
| MX359281B (es) | 2010-06-21 | 2018-09-21 | Mannkind Corp | Sistema y metodos para suministrar un farmaco en polvo seco. |
| US8372845B2 (en) | 2010-09-17 | 2013-02-12 | Novartis Ag | Pyrazine derivatives as enac blockers |
| GB201020638D0 (en) * | 2010-12-06 | 2011-01-19 | Liconsa Laboratorios Sa | Inhalator |
| CN102553040B (zh) * | 2010-12-17 | 2014-07-02 | 陈庆堂 | 滤出药粉吸入器 |
| CN102553038B (zh) * | 2010-12-17 | 2014-07-02 | 陈庆堂 | 药粉吸嘴放置盒 |
| JOP20120023B1 (ar) | 2011-02-04 | 2022-03-14 | Novartis Ag | صياغات مساحيق جافة من جسيمات تحتوي على واحد أو اثنين من المواد الفعالة لعلاج امراض ممرات الهواء الانسدادية او الالتهابية |
| CA2829708C (en) | 2011-03-15 | 2019-09-03 | Novartis Ag | Inhaler |
| US8925726B2 (en) | 2011-04-01 | 2015-01-06 | Mannkind Corporation | Blister package for pharmaceutical cartridges |
| WO2012174472A1 (en) | 2011-06-17 | 2012-12-20 | Mannkind Corporation | High capacity diketopiperazine microparticles |
| WO2013016784A1 (en) | 2011-08-04 | 2013-02-07 | Victor Esteve | Dry powder inhaler |
| UY34305A (es) | 2011-09-01 | 2013-04-30 | Novartis Ag | Derivados de heterociclos bicíclicos para el tratamiento de la hipertensión arterial pulmonar |
| CA2852536A1 (en) | 2011-10-24 | 2013-05-02 | Mannkind Corporation | Methods and compositions for treating pain |
| CN104010592B (zh) | 2011-12-23 | 2016-08-03 | 皇家飞利浦有限公司 | 用于递送牙齿增白剂的设备、系统和机构 |
| US9174985B2 (en) | 2012-01-13 | 2015-11-03 | Novartis Ag | Salts of 7-(2,3-di-p-tolyl-7,8-dihydropyrido[2,3-b]pyrazin-5(6H)-yl)heptanoic acid as IP receptor agonists |
| US9326547B2 (en) | 2012-01-31 | 2016-05-03 | Altria Client Services Llc | Electronic vaping article |
| US8809340B2 (en) | 2012-03-19 | 2014-08-19 | Novartis Ag | Crystalline form |
| EP2666465A1 (en) | 2012-05-25 | 2013-11-27 | Almirall, S.A. | Novel dosage and formulation |
| EP2668941A1 (en) | 2012-05-31 | 2013-12-04 | Almirall, S.A. | Novel dosage form and formulation of abediterol |
| CA2878457C (en) | 2012-07-12 | 2021-01-19 | Mannkind Corporation | Dry powder drug delivery systems and methods |
| EP2911690A1 (en) | 2012-10-26 | 2015-09-02 | MannKind Corporation | Inhalable influenza vaccine compositions and methods |
| BR112015014372A8 (pt) | 2012-12-19 | 2019-10-29 | Novartis Ag | inibidores de autotaxina, seus usos, e composição e combinação farmacêuticas". |
| WO2014106727A1 (en) | 2013-01-03 | 2014-07-10 | Vectura Limited | Inhaler and formulation |
| US9073921B2 (en) | 2013-03-01 | 2015-07-07 | Novartis Ag | Salt forms of bicyclic heterocyclic derivatives |
| JP6232079B2 (ja) | 2013-03-14 | 2017-11-15 | ノバルティス アーゲー | スプレーブレンディングによるスプレー乾燥製剤の脱アモルファス化 |
| US9452139B2 (en) | 2013-03-14 | 2016-09-27 | Novartis Ag | Respirable agglomerates of porous carrier particles and micronized drug |
| EP2970149B1 (en) | 2013-03-15 | 2019-08-21 | MannKind Corporation | Microcrystalline diketopiperazine compositions and methods |
| US20160158470A1 (en) | 2013-07-16 | 2016-06-09 | Victor Esteve | Powder inhaler |
| EP3021834A1 (en) | 2013-07-18 | 2016-05-25 | MannKind Corporation | Heat-stable dry powder pharmaceutical compositions and methods |
| KR20160033726A (ko) | 2013-07-18 | 2016-03-28 | 노파르티스 아게 | 헤테로방향족 고리-벤질-아미드-사이클 코어를 포함하는 오토탁신 억제제 |
| EP3022201A1 (en) | 2013-07-18 | 2016-05-25 | Novartis AG | Autotaxin inhibitors |
| CN105517607A (zh) | 2013-08-05 | 2016-04-20 | 曼金德公司 | 吹入设备和方法 |
| WO2015053361A1 (ja) * | 2013-10-11 | 2015-04-16 | 株式会社リーチハイアー | 経口粉末の吸入補助器具 |
| WO2015091287A1 (en) | 2013-12-19 | 2015-06-25 | Almirall S.A. | Dosage formulation comprising salmeterol and fluticasone propionate |
| CA2940599A1 (en) | 2014-03-27 | 2015-10-01 | Novartis Ag | Spray-dried solid-in-oil-in-water dispersions for inhalation of active pharmaceutical ingredients |
| US10307464B2 (en) | 2014-03-28 | 2019-06-04 | Mannkind Corporation | Use of ultrarapid acting insulin |
| JP6404944B2 (ja) | 2014-04-24 | 2018-10-17 | ノバルティス アーゲー | ホスファチジルイノシトール3−キナーゼ阻害薬としてのピラジン誘導体 |
| EP3134397A1 (en) | 2014-04-24 | 2017-03-01 | Novartis AG | Amino pyrazine derivatives as phosphatidylinositol 3-kinase inhibitors |
| EA201692140A1 (ru) | 2014-04-24 | 2017-04-28 | Новартис Аг | Производные аминопиридина в качестве ингибиторов фосфатидилинозитол 3-киназы |
| EP3134398A1 (en) | 2014-04-24 | 2017-03-01 | Novartis Ag | Autotaxin inhibitors |
| PT107627B (pt) * | 2014-04-29 | 2017-03-08 | Hovione Farmaciência S A | Inalador de cápsulas com dobradiça |
| US20170088546A1 (en) | 2014-05-14 | 2017-03-30 | Novartis Ag | Carboxamide inhibitors |
| WO2015173656A1 (en) | 2014-05-14 | 2015-11-19 | Novartis Ag | Carboxamide derivatives |
| US10561806B2 (en) * | 2014-10-02 | 2020-02-18 | Mannkind Corporation | Mouthpiece cover for an inhaler |
| WO2016053525A1 (en) * | 2014-10-03 | 2016-04-07 | Illinois Tool Works Inc. | Reusable holder for a radioactive source capsule |
| JP2018510668A (ja) | 2015-01-12 | 2018-04-19 | ケダリオン セラピューティックス,インコーポレイテッド | 微小液滴の繰り出し装置及び方法 |
| CA2973817A1 (en) | 2015-01-20 | 2016-07-28 | Novartis Ag | Application unlock using a connected physical device and transfer of data therebetween |
| WO2016116629A1 (en) | 2015-01-23 | 2016-07-28 | Sandoz Ag | An apparatus and method for producing a flow profile |
| EP3111978B1 (en) * | 2015-07-03 | 2021-09-01 | Novartis AG | Inhaler adapted to read information stored in a data storage means of a container |
| US9877520B2 (en) * | 2015-07-29 | 2018-01-30 | Nitesh Rastogi | Hinged vaping system |
| WO2017042696A1 (en) | 2015-09-09 | 2017-03-16 | Novartis Ag | Targeted delivery of spray-dried formulations to the lungs |
| RU2731644C2 (ru) | 2015-09-09 | 2020-09-07 | Новартис Аг | Молекулы, связывающиеся с тимусным стромальным лимфопоэтином (tslp), и способы применения таких молекул |
| EA038332B1 (ru) | 2015-09-09 | 2021-08-10 | Новартис Аг | Молекулы, связывающиеся с тимусным стромальным лимфопоэтином (tslp), и способы применения таких молекул |
| USD867575S1 (en) * | 2015-11-19 | 2019-11-19 | Emphasys Importadora Exportadora e Distribuidora | Inhaler |
| HUE051868T2 (hu) | 2016-01-28 | 2021-03-29 | Novartis Ag | Eljárási áramlási tulajdonság mérésére inhalátorban, inhalátor és rendszer |
| GB201605105D0 (en) | 2016-03-24 | 2016-05-11 | Nicoventures Holdings Ltd | Vapour provision apparatus |
| GB201605100D0 (en) | 2016-03-24 | 2016-05-11 | Nicoventures Holdings Ltd | Vapour provision system |
| AU2017369977A1 (en) * | 2016-11-30 | 2019-04-11 | Philip Morris Products S.A. | Inhaler with swirl end plug |
| CN110234382B (zh) * | 2016-12-20 | 2022-04-15 | 埃姆弗西斯进出口及分销有限公司 | 干粉末吸入器 |
| EP3570986B1 (en) | 2017-01-20 | 2025-03-19 | Bausch + Lomb Ireland Limited | Piezoelectric fluid dispenser |
| CN110730674B (zh) * | 2017-04-18 | 2022-02-01 | 励志私人有限公司 | 干粉吸入器和用于干粉吸入器的间隔装置 |
| AU2018308199B2 (en) | 2017-07-25 | 2023-08-31 | Adherium (Nz) Limited | Adherence monitoring method and device |
| CN107551368A (zh) * | 2017-10-12 | 2018-01-09 | 上海华瑞气雾剂有限公司 | 一种防划伤的干粉吸入器 |
| JP7209715B2 (ja) * | 2017-11-23 | 2023-01-20 | チア タイ ティエンチン ファーマシューティカル グループ カンパニー リミテッド | 新型乾燥粉末吸入装置 |
| JP7436363B2 (ja) | 2017-12-08 | 2024-02-21 | ノバルティス アーゲー | 流体送達アライメントシステム |
| WO2019122361A1 (en) | 2017-12-22 | 2019-06-27 | Dr. August Wolff Gmbh & Co. Kg Arzneimittel | Asimadoline for use in treating pulmonary diseases, vascular diseases, and sepsis |
| CN111465423B (zh) | 2017-12-28 | 2022-08-16 | 菲利普莫里斯生产公司 | 具有涡旋通道的吸入器 |
| US20190314196A1 (en) | 2018-04-12 | 2019-10-17 | Kedalion Therapeutics, Inc. | Topical Ocular Delivery Methods and Devices for Use in the Same |
| US12350194B1 (en) | 2018-04-12 | 2025-07-08 | Bausch + Lomb Ireland Limited | Topical ocular delivery of fluids with controlled mass dosing and wireless communication |
| DE102018108958A1 (de) | 2018-04-16 | 2019-10-17 | Emphasys Importadora Exportadora E Distribuidora Ltda. | Trockenpulverinhalator |
| AU2019297326B2 (en) | 2018-07-03 | 2025-01-09 | Bausch + Lomb Ireland Limited | Topical ocular delivery devices and methods for using the same |
| CN109078245A (zh) * | 2018-10-11 | 2018-12-25 | 上海博极给药医药科技有限公司 | 一种双胶囊型干粉吸入装置 |
| US12097145B2 (en) | 2019-03-06 | 2024-09-24 | Bausch + Lomb Ireland Limited | Vented multi-dose ocular fluid delivery system |
| US11679028B2 (en) | 2019-03-06 | 2023-06-20 | Novartis Ag | Multi-dose ocular fluid delivery system |
| US20220296521A1 (en) | 2019-06-10 | 2022-09-22 | Respira Therapeutics, Inc. | Carrier-based formulations and related methods |
| US11793951B2 (en) * | 2019-06-24 | 2023-10-24 | De Motu Cordis Pty Ltd | Automatic dispenser for respiratory delivery device and method |
| US11717621B2 (en) * | 2019-06-24 | 2023-08-08 | De Motu Cordis Pty Ltd | Automatic dispenser for respiratory delivery device |
| IT201900017417A1 (it) * | 2019-09-27 | 2021-03-27 | Amiko S R L | Inalatore a polvere secca per somministrazione nasale o polmonare |
| US12496218B1 (en) | 2019-11-12 | 2025-12-16 | Bausch + Lomb Ireland Limited | Fractionated topical ocular drug delivery methods and devices for use in the same |
| GB2589635B (en) * | 2019-12-06 | 2021-12-01 | Merxin Ltd | Elongate form medicament carrier and medicament dispenser |
| CA3159735A1 (en) | 2019-12-09 | 2021-06-17 | Avi Eliahu | Dry powder suction inhaler device, medication formulations used therewith and methods of manufacture |
| JP2023511763A (ja) | 2020-01-31 | 2023-03-22 | アドビタ ライフサイエンス ゲゼルシャフト ミット ベシュレンクテル ハフツング | 炎症性肺疾患の吸入治療用のヒト抗炎症性ペプチド |
| EP4120974A4 (en) | 2020-04-17 | 2024-05-22 | Bausch + Lomb Ireland Limited | Hydrodynamically actuated preservative free dispensing system |
| US12290472B2 (en) | 2020-04-17 | 2025-05-06 | Bausch + Lomb Ireland Limited | Hydrodynamically actuated preservative free dispensing system |
| US12090087B2 (en) | 2020-04-17 | 2024-09-17 | Bausch + Lomb Ireland Limited | Hydrodynamically actuated preservative free dispensing system having a collapsible liquid reservoir |
| US11938057B2 (en) | 2020-04-17 | 2024-03-26 | Bausch + Lomb Ireland Limited | Hydrodynamically actuated preservative free dispensing system |
| CN113018612B (zh) * | 2021-04-20 | 2024-07-19 | 苏州新劢德医疗器械科技有限公司 | 吸入装置 |
| CN119255833A (zh) | 2022-05-24 | 2025-01-03 | 基因泰克公司 | 多单位剂量干粉吸入器及使用方法 |
| US12171934B2 (en) * | 2023-05-23 | 2024-12-24 | De Motu Cordis Pty Ltd | Respiratory delivery device |
| US20250352747A1 (en) | 2024-05-16 | 2025-11-20 | Astrazeneca Ab | Chimney shuttling mechanism for an inhaler |
| US20250352745A1 (en) | 2024-05-16 | 2025-11-20 | Astrazeneca Ab | Inhaler |
| US20250352743A1 (en) | 2024-05-16 | 2025-11-20 | Astrazeneca Ab | Spin chamber for an inhaler |
| WO2025242605A1 (en) | 2024-05-20 | 2025-11-27 | Medimmune Limited | Inhaler and capsule for delivering thymic stromal lymphopoietin (tslp)-binding antibodies |
| KR20250167233A (ko) * | 2024-05-22 | 2025-12-01 | 주식회사 케이티앤지 | 흡입기 및 이를 포함하는 흡입기 시스템 |
Citations (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US3991761A (en) * | 1974-03-18 | 1976-11-16 | Salvatore Cocozza | Inhaler for powdered medicaments |
| JPH10234827A (ja) * | 1997-02-20 | 1998-09-08 | Ishikawa Seisakusho:Kk | 吸引器 |
Family Cites Families (24)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US3931671A (en) * | 1974-09-30 | 1976-01-13 | Amp Incorporated | Terminal locator and retainer device |
| EP0005585B1 (en) * | 1978-05-03 | 1981-08-12 | FISONS plc | Inhalation device |
| GB8804069D0 (en) | 1988-02-22 | 1988-03-23 | Britains Petite Ltd | Dispensers for powdered medication |
| DE3927170A1 (de) | 1989-08-17 | 1991-02-21 | Boehringer Ingelheim Kg | Inhalator |
| NZ238489A (en) * | 1990-06-14 | 1995-09-26 | Rhone Poulenc Rorer Ltd | Inhaler with capsule in swirling chamber: capsule pierced in chamber |
| US5797391A (en) * | 1991-03-28 | 1998-08-25 | Rhone-Poulenc Rorer Limited | Inhaler |
| US5492112A (en) * | 1991-05-20 | 1996-02-20 | Dura Pharmaceuticals, Inc. | Dry powder inhaler |
| RU2002467C1 (ru) * | 1991-06-25 | 1993-11-15 | Чучалин Александр Григорьевич; Бабарсков Евгений Викторович; Опенев В чеслав Иванович; Зезин Сергей Борисович; Коркина Людмила Георгиевна; Казначеев Владимир Александрович; Лох- мачев Александр Викторович | Ингал тор дл введени лекарственных средств в виде порошка |
| JPH0611588U (ja) * | 1992-07-15 | 1994-02-15 | 富士通化成株式会社 | 表裏判別構造を有する耳かき |
| AU119600S (en) * | 1993-01-21 | 1994-03-07 | Boehringer Ingelheim Kg | Inhaler device |
| IL108780A (en) | 1993-02-27 | 1999-06-20 | Fisons Plc | Inhalation device |
| DE4318455A1 (de) * | 1993-06-03 | 1994-12-08 | Boehringer Ingelheim Kg | Kapselhalterung |
| SE9302550D0 (sv) * | 1993-07-30 | 1993-07-30 | Ernst Hoerlin | Powder inhaler |
| US6209538B1 (en) * | 1995-08-02 | 2001-04-03 | Robert A. Casper | Dry powder medicament inhalator having an inhalation-activated flow diverting means for triggering delivery of medicament |
| ES2172763T3 (es) * | 1996-02-21 | 2002-10-01 | Schering Corp | Inhalador de medicamento en polvo. |
| GB9913083D0 (en) | 1999-06-04 | 1999-08-04 | Novartis Ag | Organic compounds |
| JP2001266903A (ja) * | 2000-03-21 | 2001-09-28 | Matsushita Electric Ind Co Ltd | 偏平型電池 |
| GB0015876D0 (en) | 2000-06-28 | 2000-08-23 | Novartis Ag | Organic compounds |
| ITMI20010357U1 (it) | 2001-06-28 | 2002-12-30 | Plastiape Spa | Dispositivo inalatore |
| GR1004350B (el) | 2002-03-29 | 2003-09-26 | Συσκευη εισπνοων ξηρης σκονης | |
| AR040962A1 (es) | 2002-08-09 | 2005-04-27 | Novartis Ag | Compuestos derivados de tiazol 1,3-2-ona, composicion farmaceutica y proceso de preparacion del compuesto |
| AR044134A1 (es) | 2003-05-02 | 2005-08-24 | Novartis Ag | Derivados de quinuclidina, metodo de preparacion y composiciones farmaceuticas. |
| PE20050231A1 (es) | 2003-06-24 | 2005-05-20 | Novartis Ag | Derivados de piperidinium y pirrolidinium como antagonistas del receptor muscarinico m3 |
| DE10352277A1 (de) * | 2003-11-08 | 2005-06-02 | Boehringer Ingelheim Pharma Gmbh & Co. Kg | Pulverinhalator |
-
2004
- 2004-05-13 GB GBGB0410712.4A patent/GB0410712D0/en not_active Ceased
-
2005
- 2005-05-11 AR ARP050101913A patent/AR051353A1/es active IP Right Grant
- 2005-05-11 PE PE2005000523A patent/PE20060098A1/es active IP Right Grant
- 2005-05-12 DK DK05745323.5T patent/DK1747036T3/da active
- 2005-05-12 WO PCT/EP2005/005182 patent/WO2005113042A1/en not_active Ceased
- 2005-05-12 PL PL05745323T patent/PL1747036T3/pl unknown
- 2005-05-12 US US11/568,466 patent/US8479730B2/en active Active
- 2005-05-12 NZ NZ550625A patent/NZ550625A/en not_active IP Right Cessation
- 2005-05-12 KR KR1020067023534A patent/KR101170187B1/ko not_active Expired - Lifetime
- 2005-05-12 JP JP2007512106A patent/JP4914345B2/ja not_active Expired - Lifetime
- 2005-05-12 CA CA2563573A patent/CA2563573C/en not_active Expired - Lifetime
- 2005-05-12 RU RU2006143840/14A patent/RU2363501C2/ru active
- 2005-05-12 MX MXPA06013081A patent/MXPA06013081A/es active IP Right Grant
- 2005-05-12 SI SI200531319T patent/SI1747036T1/sl unknown
- 2005-05-12 TW TW094115456A patent/TWI353857B/zh not_active IP Right Cessation
- 2005-05-12 CN CN2005800153538A patent/CN1953779B/zh not_active Expired - Lifetime
- 2005-05-12 PT PT05745323T patent/PT1747036E/pt unknown
- 2005-05-12 ES ES05745323T patent/ES2362429T3/es not_active Expired - Lifetime
- 2005-05-12 BR BRPI0511073A patent/BRPI0511073B8/pt active IP Right Grant
- 2005-05-12 DE DE602005027064T patent/DE602005027064D1/de not_active Expired - Lifetime
- 2005-05-12 AT AT05745323T patent/ATE502666T1/de active
- 2005-05-12 EP EP05745323A patent/EP1747036B1/en not_active Expired - Lifetime
- 2005-05-12 AU AU2005245095A patent/AU2005245095B2/en not_active Expired
- 2005-05-12 MY MYPI20052149A patent/MY144563A/en unknown
-
2006
- 2006-10-19 ZA ZA200608728A patent/ZA200608728B/xx unknown
- 2006-10-26 IL IL178878A patent/IL178878A/en active IP Right Grant
- 2006-11-10 TN TNP2006000367A patent/TNSN06367A1/en unknown
- 2006-11-10 EC EC2006006987A patent/ECSP066987A/es unknown
- 2006-11-29 MA MA29497A patent/MA28612B1/fr unknown
- 2006-12-12 NO NO20065718A patent/NO334854B1/no unknown
-
2011
- 2011-06-08 CY CY20111100552T patent/CY1111555T1/el unknown
- 2011-11-02 JP JP2011241398A patent/JP2012071146A/ja active Pending
Patent Citations (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US3991761A (en) * | 1974-03-18 | 1976-11-16 | Salvatore Cocozza | Inhaler for powdered medicaments |
| JPH10234827A (ja) * | 1997-02-20 | 1998-09-08 | Ishikawa Seisakusho:Kk | 吸引器 |
Also Published As
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP4914345B2 (ja) | 吸入器 | |
| RU2456026C2 (ru) | Усовершенствованный ингалятор для сухого порошкообразного препарата | |
| US6732732B2 (en) | Inhalation device and method | |
| JP6683613B2 (ja) | 乾燥粉末吸入器 | |
| JPS6155978B2 (ja) | ||
| JPS636024B2 (ja) | ||
| EP1485154B1 (en) | Powder inhalation device | |
| HK1102919B (en) | Inhaler device | |
| HK1061658B (en) | Inhalation device |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20080430 |
|
| RD04 | Notification of resignation of power of attorney |
Free format text: JAPANESE INTERMEDIATE CODE: A7424 Effective date: 20090212 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20100803 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20101104 |
|
| A602 | Written permission of extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A602 Effective date: 20101111 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20101203 |
|
| A602 | Written permission of extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A602 Effective date: 20101210 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20101228 |
|
| A602 | Written permission of extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A602 Effective date: 20110111 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20110202 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20110802 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20110927 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20111101 |
|
| A602 | Written permission of extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A602 Effective date: 20111109 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20111115 |
|
| A602 | Written permission of extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A602 Effective date: 20111122 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20111216 |
|
| TRDD | Decision of grant or rejection written | ||
| A01 | Written decision to grant a patent or to grant a registration (utility model) |
Free format text: JAPANESE INTERMEDIATE CODE: A01 Effective date: 20120117 |
|
| A01 | Written decision to grant a patent or to grant a registration (utility model) |
Free format text: JAPANESE INTERMEDIATE CODE: A01 |
|
| A61 | First payment of annual fees (during grant procedure) |
Free format text: JAPANESE INTERMEDIATE CODE: A61 Effective date: 20120120 |
|
| R150 | Certificate of patent or registration of utility model |
Ref document number: 4914345 Country of ref document: JP Free format text: JAPANESE INTERMEDIATE CODE: R150 Free format text: JAPANESE INTERMEDIATE CODE: R150 |
|
| FPAY | Renewal fee payment (event date is renewal date of database) |
Free format text: PAYMENT UNTIL: 20150127 Year of fee payment: 3 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| RD02 | Notification of acceptance of power of attorney |
Free format text: JAPANESE INTERMEDIATE CODE: R3D02 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R153 | Grant of patent term extension |
Free format text: JAPANESE INTERMEDIATE CODE: R153 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R153 | Grant of patent term extension |
Free format text: JAPANESE INTERMEDIATE CODE: R153 |
|
| R153 | Grant of patent term extension |
Free format text: JAPANESE INTERMEDIATE CODE: R153 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |