JP4875489B2 - プロトン伝導性膜およびその使用 - Google Patents
プロトン伝導性膜およびその使用 Download PDFInfo
- Publication number
- JP4875489B2 JP4875489B2 JP2006521484A JP2006521484A JP4875489B2 JP 4875489 B2 JP4875489 B2 JP 4875489B2 JP 2006521484 A JP2006521484 A JP 2006521484A JP 2006521484 A JP2006521484 A JP 2006521484A JP 4875489 B2 JP4875489 B2 JP 4875489B2
- Authority
- JP
- Japan
- Prior art keywords
- acid
- polymer
- affinity
- membrane
- aromatic
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Expired - Fee Related
Links
- 239000012528 membrane Substances 0.000 title claims description 40
- 229920000642 polymer Polymers 0.000 claims description 59
- 229910019142 PO4 Inorganic materials 0.000 claims description 52
- NBIIXXVUZAFLBC-UHFFFAOYSA-K phosphate Chemical compound [O-]P([O-])([O-])=O NBIIXXVUZAFLBC-UHFFFAOYSA-K 0.000 claims description 52
- 239000010452 phosphate Substances 0.000 claims description 52
- NBIIXXVUZAFLBC-UHFFFAOYSA-N Phosphoric acid Chemical compound OP(O)(O)=O NBIIXXVUZAFLBC-UHFFFAOYSA-N 0.000 claims description 47
- 125000003118 aryl group Chemical group 0.000 claims description 33
- -1 aromatic carboxylic acids Chemical class 0.000 claims description 27
- 239000002253 acid Substances 0.000 claims description 22
- 229910000147 aluminium phosphate Inorganic materials 0.000 claims description 22
- 239000000178 monomer Substances 0.000 claims description 22
- 150000001875 compounds Chemical class 0.000 claims description 19
- 239000000203 mixture Substances 0.000 claims description 18
- 239000000446 fuel Substances 0.000 claims description 17
- 229920000137 polyphosphoric acid Polymers 0.000 claims description 17
- 238000002156 mixing Methods 0.000 claims description 15
- 150000002148 esters Chemical class 0.000 claims description 9
- 238000000034 method Methods 0.000 claims description 9
- 239000006185 dispersion Substances 0.000 claims description 7
- SCKXCAADGDQQCS-UHFFFAOYSA-N Performic acid Chemical compound OOC=O SCKXCAADGDQQCS-UHFFFAOYSA-N 0.000 claims description 6
- QQVIHTHCMHWDBS-UHFFFAOYSA-N isophthalic acid Chemical compound OC(=O)C1=CC=CC(C(O)=O)=C1 QQVIHTHCMHWDBS-UHFFFAOYSA-N 0.000 claims description 6
- KKEYFWRCBNTPAC-UHFFFAOYSA-N Terephthalic acid Chemical compound OC(=O)C1=CC=C(C(O)=O)C=C1 KKEYFWRCBNTPAC-UHFFFAOYSA-N 0.000 claims description 4
- 229920001940 conductive polymer Polymers 0.000 claims description 4
- 238000010438 heat treatment Methods 0.000 claims description 4
- XNGIFLGASWRNHJ-UHFFFAOYSA-N phthalic acid Chemical compound OC(=O)C1=CC=CC=C1C(O)=O XNGIFLGASWRNHJ-UHFFFAOYSA-N 0.000 claims description 4
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 claims description 4
- HSTOKWSFWGCZMH-UHFFFAOYSA-N 3,3'-diaminobenzidine Chemical group C1=C(N)C(N)=CC=C1C1=CC=C(N)C(N)=C1 HSTOKWSFWGCZMH-UHFFFAOYSA-N 0.000 claims description 3
- 125000003785 benzimidazolyl group Chemical group N1=C(NC2=C1C=CC=C2)* 0.000 claims description 3
- 239000002322 conducting polymer Substances 0.000 claims description 3
- 230000008569 process Effects 0.000 claims description 3
- 238000001632 acidimetric titration Methods 0.000 claims description 2
- ANUAIBBBDSEVKN-UHFFFAOYSA-N benzene-1,2,4,5-tetramine Chemical compound NC1=CC(N)=C(N)C=C1N ANUAIBBBDSEVKN-UHFFFAOYSA-N 0.000 claims description 2
- HYZJCKYKOHLVJF-UHFFFAOYSA-N 1H-benzimidazole Chemical compound C1=CC=C2NC=NC2=C1 HYZJCKYKOHLVJF-UHFFFAOYSA-N 0.000 claims 1
- WPYMKLBDIGXBTP-UHFFFAOYSA-N benzoic acid Chemical compound OC(=O)C1=CC=CC=C1 WPYMKLBDIGXBTP-UHFFFAOYSA-N 0.000 claims 1
- 230000003301 hydrolyzing effect Effects 0.000 claims 1
- 235000011007 phosphoric acid Nutrition 0.000 description 19
- 125000001072 heteroaryl group Chemical group 0.000 description 15
- 125000002950 monocyclic group Chemical group 0.000 description 12
- 125000003367 polycyclic group Chemical group 0.000 description 12
- 229920005597 polymer membrane Polymers 0.000 description 12
- 238000006116 polymerization reaction Methods 0.000 description 10
- 239000007789 gas Substances 0.000 description 8
- 239000000654 additive Substances 0.000 description 7
- 229910052760 oxygen Inorganic materials 0.000 description 7
- UHOVQNZJYSORNB-UHFFFAOYSA-N Benzene Chemical compound C1=CC=CC=C1 UHOVQNZJYSORNB-UHFFFAOYSA-N 0.000 description 6
- 239000004693 Polybenzimidazole Substances 0.000 description 6
- HEMHJVSKTPXQMS-UHFFFAOYSA-M Sodium hydroxide Chemical compound [OH-].[Na+] HEMHJVSKTPXQMS-UHFFFAOYSA-M 0.000 description 6
- 125000000217 alkyl group Chemical group 0.000 description 6
- 150000001735 carboxylic acids Chemical class 0.000 description 6
- 230000007062 hydrolysis Effects 0.000 description 6
- 238000006460 hydrolysis reaction Methods 0.000 description 6
- 239000011261 inert gas Substances 0.000 description 6
- 239000001301 oxygen Chemical group 0.000 description 6
- 229920002480 polybenzimidazole Polymers 0.000 description 6
- 229920006254 polymer film Polymers 0.000 description 6
- FXHOOIRPVKKKFG-UHFFFAOYSA-N N,N-Dimethylacetamide Chemical compound CN(C)C(C)=O FXHOOIRPVKKKFG-UHFFFAOYSA-N 0.000 description 5
- QVGXLLKOCUKJST-UHFFFAOYSA-N atomic oxygen Chemical compound [O] QVGXLLKOCUKJST-UHFFFAOYSA-N 0.000 description 5
- 230000015572 biosynthetic process Effects 0.000 description 5
- 238000006243 chemical reaction Methods 0.000 description 5
- USIUVYZYUHIAEV-UHFFFAOYSA-N diphenyl ether Chemical compound C=1C=CC=CC=1OC1=CC=CC=C1 USIUVYZYUHIAEV-UHFFFAOYSA-N 0.000 description 5
- 150000000000 tetracarboxylic acids Chemical class 0.000 description 5
- 125000003837 (C1-C20) alkyl group Chemical group 0.000 description 4
- KLSJWNVTNUYHDU-UHFFFAOYSA-N Amitrole Chemical group NC1=NC=NN1 KLSJWNVTNUYHDU-UHFFFAOYSA-N 0.000 description 4
- SMWDFEZZVXVKRB-UHFFFAOYSA-N Quinoline Chemical compound N1=CC=CC2=CC=CC=C21 SMWDFEZZVXVKRB-UHFFFAOYSA-N 0.000 description 4
- QMKYBPDZANOJGF-UHFFFAOYSA-N benzene-1,3,5-tricarboxylic acid Chemical compound OC(=O)C1=CC(C(O)=O)=CC(C(O)=O)=C1 QMKYBPDZANOJGF-UHFFFAOYSA-N 0.000 description 4
- WJJMNDUMQPNECX-UHFFFAOYSA-N dipicolinic acid Chemical compound OC(=O)C1=CC=CC(C(O)=O)=N1 WJJMNDUMQPNECX-UHFFFAOYSA-N 0.000 description 4
- 229910052757 nitrogen Inorganic materials 0.000 description 4
- BASFCYQUMIYNBI-UHFFFAOYSA-N platinum Chemical compound [Pt] BASFCYQUMIYNBI-UHFFFAOYSA-N 0.000 description 4
- 230000000379 polymerizing effect Effects 0.000 description 4
- XSCHRSMBECNVNS-UHFFFAOYSA-N quinoxaline Chemical compound N1=CC=NC2=CC=CC=C21 XSCHRSMBECNVNS-UHFFFAOYSA-N 0.000 description 4
- 150000003628 tricarboxylic acids Chemical class 0.000 description 4
- ARCGXLSVLAOJQL-UHFFFAOYSA-N trimellitic acid Chemical compound OC(=O)C1=CC=C(C(O)=O)C(C(O)=O)=C1 ARCGXLSVLAOJQL-UHFFFAOYSA-N 0.000 description 4
- JUJWROOIHBZHMG-UHFFFAOYSA-N Pyridine Chemical compound C1=CC=NC=C1 JUJWROOIHBZHMG-UHFFFAOYSA-N 0.000 description 3
- 150000008065 acid anhydrides Chemical class 0.000 description 3
- 230000009471 action Effects 0.000 description 3
- 239000003054 catalyst Substances 0.000 description 3
- 239000000945 filler Substances 0.000 description 3
- 229910052739 hydrogen Inorganic materials 0.000 description 3
- 125000004435 hydrogen atom Chemical group [H]* 0.000 description 3
- 238000005342 ion exchange Methods 0.000 description 3
- 238000004519 manufacturing process Methods 0.000 description 3
- IJGRMHOSHXDMSA-UHFFFAOYSA-N nitrogen Substances N#N IJGRMHOSHXDMSA-UHFFFAOYSA-N 0.000 description 3
- ZUOUZKKEUPVFJK-UHFFFAOYSA-N phenylbenzene Natural products C1=CC=CC=C1C1=CC=CC=C1 ZUOUZKKEUPVFJK-UHFFFAOYSA-N 0.000 description 3
- 239000005518 polymer electrolyte Substances 0.000 description 3
- 230000009257 reactivity Effects 0.000 description 3
- 229910003209 (NH4)3H(SeO4)2 Inorganic materials 0.000 description 2
- PIPQOFRJDBZPFR-UHFFFAOYSA-N 1h-benzimidazole-5,6-dicarboxylic acid Chemical compound C1=C(C(O)=O)C(C(=O)O)=CC2=C1NC=N2 PIPQOFRJDBZPFR-UHFFFAOYSA-N 0.000 description 2
- YDMVPJZBYSWOOP-UHFFFAOYSA-N 1h-pyrazole-3,5-dicarboxylic acid Chemical compound OC(=O)C=1C=C(C(O)=O)NN=1 YDMVPJZBYSWOOP-UHFFFAOYSA-N 0.000 description 2
- OYFRNYNHAZOYNF-UHFFFAOYSA-N 2,5-dihydroxyterephthalic acid Chemical compound OC(=O)C1=CC(O)=C(C(O)=O)C=C1O OYFRNYNHAZOYNF-UHFFFAOYSA-N 0.000 description 2
- NGNBDVOYPDDBFK-UHFFFAOYSA-N 2-[2,4-di(pentan-2-yl)phenoxy]acetyl chloride Chemical compound CCCC(C)C1=CC=C(OCC(Cl)=O)C(C(C)CCC)=C1 NGNBDVOYPDDBFK-UHFFFAOYSA-N 0.000 description 2
- CDOWNLMZVKJRSC-UHFFFAOYSA-N 2-hydroxyterephthalic acid Chemical compound OC(=O)C1=CC=C(C(O)=O)C(O)=C1 CDOWNLMZVKJRSC-UHFFFAOYSA-N 0.000 description 2
- BCEQKAQCUWUNML-UHFFFAOYSA-N 4-hydroxybenzene-1,3-dicarboxylic acid Chemical compound OC(=O)C1=CC=C(O)C(C(O)=O)=C1 BCEQKAQCUWUNML-UHFFFAOYSA-N 0.000 description 2
- XFFZVIRSYFJKEX-UHFFFAOYSA-N 4-phenylpyridine-2,5-dicarboxylic acid Chemical compound C1=NC(C(=O)O)=CC(C=2C=CC=CC=2)=C1C(O)=O XFFZVIRSYFJKEX-UHFFFAOYSA-N 0.000 description 2
- QNVNLUSHGRBCLO-UHFFFAOYSA-N 5-hydroxybenzene-1,3-dicarboxylic acid Chemical compound OC(=O)C1=CC(O)=CC(C(O)=O)=C1 QNVNLUSHGRBCLO-UHFFFAOYSA-N 0.000 description 2
- UJOBWOGCFQCDNV-UHFFFAOYSA-N 9H-carbazole Chemical compound C1=CC=C2C3=CC=CC=C3NC2=C1 UJOBWOGCFQCDNV-UHFFFAOYSA-N 0.000 description 2
- CURLTUGMZLYLDI-UHFFFAOYSA-N Carbon dioxide Chemical compound O=C=O CURLTUGMZLYLDI-UHFFFAOYSA-N 0.000 description 2
- SIKJAQJRHWYJAI-UHFFFAOYSA-N Indole Chemical compound C1=CC=C2NC=CC2=C1 SIKJAQJRHWYJAI-UHFFFAOYSA-N 0.000 description 2
- XUMBMVFBXHLACL-UHFFFAOYSA-N Melanin Chemical compound O=C1C(=O)C(C2=CNC3=C(C(C(=O)C4=C32)=O)C)=C2C4=CNC2=C1C XUMBMVFBXHLACL-UHFFFAOYSA-N 0.000 description 2
- 229920000557 Nafion® Polymers 0.000 description 2
- UFWIBTONFRDIAS-UHFFFAOYSA-N Naphthalene Chemical compound C1=CC=CC2=CC=CC=C21 UFWIBTONFRDIAS-UHFFFAOYSA-N 0.000 description 2
- PCNDJXKNXGMECE-UHFFFAOYSA-N Phenazine Natural products C1=CC=CC2=NC3=CC=CC=C3N=C21 PCNDJXKNXGMECE-UHFFFAOYSA-N 0.000 description 2
- KYQCOXFCLRTKLS-UHFFFAOYSA-N Pyrazine Chemical compound C1=CN=CC=N1 KYQCOXFCLRTKLS-UHFFFAOYSA-N 0.000 description 2
- CZPWVGJYEJSRLH-UHFFFAOYSA-N Pyrimidine Chemical compound C1=CN=CN=C1 CZPWVGJYEJSRLH-UHFFFAOYSA-N 0.000 description 2
- KAESVJOAVNADME-UHFFFAOYSA-N Pyrrole Chemical compound C=1C=CNC=1 KAESVJOAVNADME-UHFFFAOYSA-N 0.000 description 2
- NINIDFKCEFEMDL-UHFFFAOYSA-N Sulfur Chemical group [S] NINIDFKCEFEMDL-UHFFFAOYSA-N 0.000 description 2
- QAOWNCQODCNURD-UHFFFAOYSA-N Sulfuric acid Chemical compound OS(O)(=O)=O QAOWNCQODCNURD-UHFFFAOYSA-N 0.000 description 2
- 150000007513 acids Chemical class 0.000 description 2
- DZBUGLKDJFMEHC-UHFFFAOYSA-N acridine Chemical compound C1=CC=CC2=CC3=CC=CC=C3N=C21 DZBUGLKDJFMEHC-UHFFFAOYSA-N 0.000 description 2
- 125000003277 amino group Chemical group 0.000 description 2
- 150000008064 anhydrides Chemical class 0.000 description 2
- MWPLVEDNUUSJAV-UHFFFAOYSA-N anthracene Chemical compound C1=CC=CC2=CC3=CC=CC=C3C=C21 MWPLVEDNUUSJAV-UHFFFAOYSA-N 0.000 description 2
- 239000003963 antioxidant agent Substances 0.000 description 2
- WZJYKHNJTSNBHV-UHFFFAOYSA-N benzo[h]quinoline Chemical compound C1=CN=C2C3=CC=CC=C3C=CC2=C1 WZJYKHNJTSNBHV-UHFFFAOYSA-N 0.000 description 2
- 239000004305 biphenyl Substances 0.000 description 2
- 235000010290 biphenyl Nutrition 0.000 description 2
- 239000003990 capacitor Substances 0.000 description 2
- 125000004432 carbon atom Chemical group C* 0.000 description 2
- 239000000969 carrier Substances 0.000 description 2
- 150000001805 chlorine compounds Chemical class 0.000 description 2
- 239000011248 coating agent Substances 0.000 description 2
- 238000000576 coating method Methods 0.000 description 2
- 238000004132 cross linking Methods 0.000 description 2
- MPFLRYZEEAQMLQ-UHFFFAOYSA-N dinicotinic acid Chemical compound OC(=O)C1=CN=CC(C(O)=O)=C1 MPFLRYZEEAQMLQ-UHFFFAOYSA-N 0.000 description 2
- KZTYYGOKRVBIMI-UHFFFAOYSA-N diphenyl sulfone Chemical compound C=1C=CC=CC=1S(=O)(=O)C1=CC=CC=C1 KZTYYGOKRVBIMI-UHFFFAOYSA-N 0.000 description 2
- 125000001495 ethyl group Chemical group [H]C([H])([H])C([H])([H])* 0.000 description 2
- 239000001257 hydrogen Substances 0.000 description 2
- AMWRITDGCCNYAT-UHFFFAOYSA-L hydroxy(oxo)manganese;manganese Chemical compound [Mn].O[Mn]=O.O[Mn]=O AMWRITDGCCNYAT-UHFFFAOYSA-L 0.000 description 2
- 150000002443 hydroxylamines Chemical class 0.000 description 2
- LVPMIMZXDYBCDF-UHFFFAOYSA-N isocinchomeronic acid Chemical compound OC(=O)C1=CC=C(C(O)=O)N=C1 LVPMIMZXDYBCDF-UHFFFAOYSA-N 0.000 description 2
- 238000011068 loading method Methods 0.000 description 2
- MJIVRKPEXXHNJT-UHFFFAOYSA-N lutidinic acid Chemical compound OC(=O)C1=CC=NC(C(O)=O)=C1 MJIVRKPEXXHNJT-UHFFFAOYSA-N 0.000 description 2
- 239000000463 material Substances 0.000 description 2
- 238000005259 measurement Methods 0.000 description 2
- 125000002496 methyl group Chemical group [H]C([H])([H])* 0.000 description 2
- YNPNZTXNASCQKK-UHFFFAOYSA-N phenanthrene Chemical compound C1=CC=C2C3=CC=CC=C3C=CC2=C1 YNPNZTXNASCQKK-UHFFFAOYSA-N 0.000 description 2
- 229910052615 phyllosilicate Inorganic materials 0.000 description 2
- 229910052697 platinum Inorganic materials 0.000 description 2
- 239000000843 powder Substances 0.000 description 2
- 238000000746 purification Methods 0.000 description 2
- GMIOYJQLNFNGPR-UHFFFAOYSA-N pyrazine-2,5-dicarboxylic acid Chemical compound OC(=O)C1=CN=C(C(O)=O)C=N1 GMIOYJQLNFNGPR-UHFFFAOYSA-N 0.000 description 2
- CHGYKYXGIWNSCD-UHFFFAOYSA-N pyridine-2,4,6-tricarboxylic acid Chemical compound OC(=O)C1=CC(C(O)=O)=NC(C(O)=O)=C1 CHGYKYXGIWNSCD-UHFFFAOYSA-N 0.000 description 2
- HLRLQGYRJSKVNX-UHFFFAOYSA-N pyrimidine-2,4-dicarboxylic acid Chemical compound OC(=O)C1=CC=NC(C(O)=O)=N1 HLRLQGYRJSKVNX-UHFFFAOYSA-N 0.000 description 2
- CYIDZMCFTVVTJO-UHFFFAOYSA-N pyromellitic acid Chemical compound OC(=O)C1=CC(C(O)=O)=C(C(O)=O)C=C1C(O)=O CYIDZMCFTVVTJO-UHFFFAOYSA-N 0.000 description 2
- 230000009467 reduction Effects 0.000 description 2
- 150000003839 salts Chemical class 0.000 description 2
- 238000005728 strengthening Methods 0.000 description 2
- 229910052717 sulfur Inorganic materials 0.000 description 2
- 239000011593 sulfur Chemical group 0.000 description 2
- 229910052645 tectosilicate Inorganic materials 0.000 description 2
- 238000010998 test method Methods 0.000 description 2
- 238000010626 work up procedure Methods 0.000 description 2
- 239000010457 zeolite Substances 0.000 description 2
- WBYWAXJHAXSJNI-VOTSOKGWSA-M .beta-Phenylacrylic acid Natural products [O-]C(=O)\C=C\C1=CC=CC=C1 WBYWAXJHAXSJNI-VOTSOKGWSA-M 0.000 description 1
- UZCCMCYUIFRHDR-UHFFFAOYSA-N 1,1,2,2,3,3,4,4,5,5,6,6,6-tridecafluorohexane-1-sulfonate triethylazanium Chemical compound CC[NH+](CC)CC.[O-]S(=O)(=O)C(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)F UZCCMCYUIFRHDR-UHFFFAOYSA-N 0.000 description 1
- OWQPOVKKUWUEKE-UHFFFAOYSA-N 1,2,3-benzotriazine Chemical compound N1=NN=CC2=CC=CC=C21 OWQPOVKKUWUEKE-UHFFFAOYSA-N 0.000 description 1
- SLLFVLKNXABYGI-UHFFFAOYSA-N 1,2,3-benzoxadiazole Chemical compound C1=CC=C2ON=NC2=C1 SLLFVLKNXABYGI-UHFFFAOYSA-N 0.000 description 1
- JYEUMXHLPRZUAT-UHFFFAOYSA-N 1,2,3-triazine Chemical compound C1=CN=NN=C1 JYEUMXHLPRZUAT-UHFFFAOYSA-N 0.000 description 1
- FOMVFKTYQSZBMJ-UHFFFAOYSA-N 1,5-dihydroxycyclohexa-3,5-diene-1,2-dicarboxylic acid Chemical compound OC(=O)C1C=CC(O)=CC1(O)C(O)=O FOMVFKTYQSZBMJ-UHFFFAOYSA-N 0.000 description 1
- VMLKTERJLVWEJJ-UHFFFAOYSA-N 1,5-naphthyridine Chemical compound C1=CC=NC2=CC=CN=C21 VMLKTERJLVWEJJ-UHFFFAOYSA-N 0.000 description 1
- UKGMFBZPIQCNPM-UHFFFAOYSA-N 1,6-dihydroxycyclohexa-3,5-diene-1,2-dicarboxylic acid Chemical compound OC(=O)C1C=CC=C(O)C1(O)C(O)=O UKGMFBZPIQCNPM-UHFFFAOYSA-N 0.000 description 1
- TZMSYXZUNZXBOL-UHFFFAOYSA-N 10H-phenoxazine Chemical compound C1=CC=C2NC3=CC=CC=C3OC2=C1 TZMSYXZUNZXBOL-UHFFFAOYSA-N 0.000 description 1
- WFNRNCNCXRGUKN-UHFFFAOYSA-N 2,3,5,6-tetrafluoroterephthalic acid Chemical compound OC(=O)C1=C(F)C(F)=C(C(O)=O)C(F)=C1F WFNRNCNCXRGUKN-UHFFFAOYSA-N 0.000 description 1
- KKTUQAYCCLMNOA-UHFFFAOYSA-N 2,3-diaminobenzoic acid Chemical compound NC1=CC=CC(C(O)=O)=C1N KKTUQAYCCLMNOA-UHFFFAOYSA-N 0.000 description 1
- VEPOHXYIFQMVHW-XOZOLZJESA-N 2,3-dihydroxybutanedioic acid (2S,3S)-3,4-dimethyl-2-phenylmorpholine Chemical compound OC(C(O)C(O)=O)C(O)=O.C[C@H]1[C@@H](OCCN1C)c1ccccc1 VEPOHXYIFQMVHW-XOZOLZJESA-N 0.000 description 1
- PGRIMKUYGUHAKH-UHFFFAOYSA-N 2,4,5,6-tetrafluorobenzene-1,3-dicarboxylic acid Chemical compound OC(=O)C1=C(F)C(F)=C(F)C(C(O)=O)=C1F PGRIMKUYGUHAKH-UHFFFAOYSA-N 0.000 description 1
- YWJNJZBDYHRABW-UHFFFAOYSA-N 2,4-dihydroxybenzene-1,3-dicarboxylic acid Chemical compound OC(=O)C1=CC=C(O)C(C(O)=O)=C1O YWJNJZBDYHRABW-UHFFFAOYSA-N 0.000 description 1
- YUWKPDBHJFNMAD-UHFFFAOYSA-N 2-fluoroterephthalic acid Chemical compound OC(=O)C1=CC=C(C(O)=O)C(F)=C1 YUWKPDBHJFNMAD-UHFFFAOYSA-N 0.000 description 1
- GRFXXWWBAWFSRF-UHFFFAOYSA-N 2-nitrosobutane Chemical compound CCC(C)N=O GRFXXWWBAWFSRF-UHFFFAOYSA-N 0.000 description 1
- MILSYCKGLDDVLM-UHFFFAOYSA-N 2-phenylpropan-2-ylbenzene Chemical compound C=1C=CC=CC=1C(C)(C)C1=CC=CC=C1 MILSYCKGLDDVLM-UHFFFAOYSA-N 0.000 description 1
- YJLVXRPNNDKMMO-UHFFFAOYSA-N 3,4,5,6-tetrafluorophthalic acid Chemical compound OC(=O)C1=C(F)C(F)=C(F)C(F)=C1C(O)=O YJLVXRPNNDKMMO-UHFFFAOYSA-N 0.000 description 1
- QXGJCWSBOZXWOV-UHFFFAOYSA-N 3,4-dihydroxyphthalic acid Chemical compound OC(=O)C1=CC=C(O)C(O)=C1C(O)=O QXGJCWSBOZXWOV-UHFFFAOYSA-N 0.000 description 1
- GWHLJVMSZRKEAQ-UHFFFAOYSA-N 3-(2,3-dicarboxyphenyl)phthalic acid Chemical compound OC(=O)C1=CC=CC(C=2C(=C(C(O)=O)C=CC=2)C(O)=O)=C1C(O)=O GWHLJVMSZRKEAQ-UHFFFAOYSA-N 0.000 description 1
- BBCQSMSCEJBIRD-UHFFFAOYSA-N 3-fluorophthalic acid Chemical compound OC(=O)C1=CC=CC(F)=C1C(O)=O BBCQSMSCEJBIRD-UHFFFAOYSA-N 0.000 description 1
- WAJQSFFBBJKSBB-UHFFFAOYSA-N 4,5-dihydroxynaphthalene-2,7-dicarboxylic acid Chemical compound OC1=CC(C(O)=O)=CC2=CC(C(=O)O)=CC(O)=C21 WAJQSFFBBJKSBB-UHFFFAOYSA-N 0.000 description 1
- MZGVIIXFGJCRDR-UHFFFAOYSA-N 4,6-dihydroxybenzene-1,3-dicarboxylic acid Chemical compound OC(=O)C1=CC(C(O)=O)=C(O)C=C1O MZGVIIXFGJCRDR-UHFFFAOYSA-N 0.000 description 1
- KTWGCHVHXBORDH-UHFFFAOYSA-N 4,6-diphenylbenzene-1,3-dicarboxylic acid Chemical compound OC(=O)C1=CC(C(O)=O)=C(C=2C=CC=CC=2)C=C1C1=CC=CC=C1 KTWGCHVHXBORDH-UHFFFAOYSA-N 0.000 description 1
- RQBIGPMJQUKYAH-UHFFFAOYSA-N 4-(3,4-diaminophenoxy)benzene-1,2-diamine Chemical compound C1=C(N)C(N)=CC=C1OC1=CC=C(N)C(N)=C1 RQBIGPMJQUKYAH-UHFFFAOYSA-N 0.000 description 1
- JKETWUADWJKEKN-UHFFFAOYSA-N 4-(3,4-diaminophenyl)sulfonylbenzene-1,2-diamine Chemical compound C1=C(N)C(N)=CC=C1S(=O)(=O)C1=CC=C(N)C(N)=C1 JKETWUADWJKEKN-UHFFFAOYSA-N 0.000 description 1
- UITKHKNFVCYWNG-UHFFFAOYSA-N 4-(3,4-dicarboxybenzoyl)phthalic acid Chemical compound C1=C(C(O)=O)C(C(=O)O)=CC=C1C(=O)C1=CC=C(C(O)=O)C(C(O)=O)=C1 UITKHKNFVCYWNG-UHFFFAOYSA-N 0.000 description 1
- LFBALUPVVFCEPA-UHFFFAOYSA-N 4-(3,4-dicarboxyphenyl)phthalic acid Chemical compound C1=C(C(O)=O)C(C(=O)O)=CC=C1C1=CC=C(C(O)=O)C(C(O)=O)=C1 LFBALUPVVFCEPA-UHFFFAOYSA-N 0.000 description 1
- LFEWXDOYPCWFHR-UHFFFAOYSA-N 4-(4-carboxybenzoyl)benzoic acid Chemical compound C1=CC(C(=O)O)=CC=C1C(=O)C1=CC=C(C(O)=O)C=C1 LFEWXDOYPCWFHR-UHFFFAOYSA-N 0.000 description 1
- NEQFBGHQPUXOFH-UHFFFAOYSA-N 4-(4-carboxyphenyl)benzoic acid Chemical compound C1=CC(C(=O)O)=CC=C1C1=CC=C(C(O)=O)C=C1 NEQFBGHQPUXOFH-UHFFFAOYSA-N 0.000 description 1
- VNLYHYHJIXGBFX-UHFFFAOYSA-N 4-(trifluoromethyl)phthalic acid Chemical compound OC(=O)C1=CC=C(C(F)(F)F)C=C1C(O)=O VNLYHYHJIXGBFX-UHFFFAOYSA-N 0.000 description 1
- ILPWTQGYOZFLBN-UHFFFAOYSA-N 4-[(3,4-diaminophenyl)methyl]benzene-1,2-diamine Chemical compound C1=C(N)C(N)=CC=C1CC1=CC=C(N)C(N)=C1 ILPWTQGYOZFLBN-UHFFFAOYSA-N 0.000 description 1
- UPVPYSXPEKPCSM-UHFFFAOYSA-N 4-[2-(4-carboxyphenyl)ethenyl]benzoic acid 4-[2-(4-carboxyphenyl)-1,1,1,3,3,3-hexafluoropropan-2-yl]benzoic acid Chemical compound C1(=CC=C(C=C1)C(=O)O)C=CC1=CC=C(C=C1)C(=O)O.C(=O)(O)C1=CC=C(C=C1)C(C(F)(F)F)(C(F)(F)F)C1=CC=C(C=C1)C(=O)O UPVPYSXPEKPCSM-UHFFFAOYSA-N 0.000 description 1
- QURGMSIQFRADOZ-UHFFFAOYSA-N 5-(3,5-dicarboxyphenyl)benzene-1,3-dicarboxylic acid Chemical compound OC(=O)C1=CC(C(O)=O)=CC(C=2C=C(C=C(C=2)C(O)=O)C(O)=O)=C1 QURGMSIQFRADOZ-UHFFFAOYSA-N 0.000 description 1
- MMHLSHSAOIJBHI-UHFFFAOYSA-N 5-(3-carboxyphenyl)benzene-1,3-dicarboxylic acid Chemical compound OC(=O)C1=CC=CC(C=2C=C(C=C(C=2)C(O)=O)C(O)=O)=C1 MMHLSHSAOIJBHI-UHFFFAOYSA-N 0.000 description 1
- LQEZHWGJSWHXPJ-UHFFFAOYSA-N 5-(4-carboxyphenyl)benzene-1,3-dicarboxylic acid Chemical compound C1=CC(C(=O)O)=CC=C1C1=CC(C(O)=O)=CC(C(O)=O)=C1 LQEZHWGJSWHXPJ-UHFFFAOYSA-N 0.000 description 1
- KBZFDRWPMZESDI-UHFFFAOYSA-N 5-aminobenzene-1,3-dicarboxylic acid Chemical compound NC1=CC(C(O)=O)=CC(C(O)=O)=C1 KBZFDRWPMZESDI-UHFFFAOYSA-N 0.000 description 1
- AUIOTTUHAZONIC-UHFFFAOYSA-N 5-fluorobenzene-1,3-dicarboxylic acid Chemical compound OC(=O)C1=CC(F)=CC(C(O)=O)=C1 AUIOTTUHAZONIC-UHFFFAOYSA-N 0.000 description 1
- KDCGOANMDULRCW-UHFFFAOYSA-N 7H-purine Chemical compound N1=CNC2=NC=NC2=C1 KDCGOANMDULRCW-UHFFFAOYSA-N 0.000 description 1
- 229910018072 Al 2 O 3 Inorganic materials 0.000 description 1
- QGZKDVFQNNGYKY-UHFFFAOYSA-O Ammonium Chemical compound [NH4+] QGZKDVFQNNGYKY-UHFFFAOYSA-O 0.000 description 1
- ROFVEXUMMXZLPA-UHFFFAOYSA-N Bipyridyl Chemical compound N1=CC=CC=C1C1=CC=CC=N1 ROFVEXUMMXZLPA-UHFFFAOYSA-N 0.000 description 1
- UGFAIRIUMAVXCW-UHFFFAOYSA-N Carbon monoxide Chemical compound [O+]#[C-] UGFAIRIUMAVXCW-UHFFFAOYSA-N 0.000 description 1
- WBYWAXJHAXSJNI-SREVYHEPSA-N Cinnamic acid Chemical compound OC(=O)\C=C/C1=CC=CC=C1 WBYWAXJHAXSJNI-SREVYHEPSA-N 0.000 description 1
- MYMOFIZGZYHOMD-UHFFFAOYSA-N Dioxygen Chemical group O=O MYMOFIZGZYHOMD-UHFFFAOYSA-N 0.000 description 1
- 239000002000 Electrolyte additive Substances 0.000 description 1
- PXGOKWXKJXAPGV-UHFFFAOYSA-N Fluorine Chemical compound FF PXGOKWXKJXAPGV-UHFFFAOYSA-N 0.000 description 1
- OFOBLEOULBTSOW-UHFFFAOYSA-N Malonic acid Chemical compound OC(=O)CC(O)=O OFOBLEOULBTSOW-UHFFFAOYSA-N 0.000 description 1
- BPQQTUXANYXVAA-UHFFFAOYSA-N Orthosilicate Chemical compound [O-][Si]([O-])([O-])[O-] BPQQTUXANYXVAA-UHFFFAOYSA-N 0.000 description 1
- ISWSIDIOOBJBQZ-UHFFFAOYSA-N Phenol Chemical compound OC1=CC=CC=C1 ISWSIDIOOBJBQZ-UHFFFAOYSA-N 0.000 description 1
- 229920000388 Polyphosphate Polymers 0.000 description 1
- WTKZEGDFNFYCGP-UHFFFAOYSA-N Pyrazole Chemical compound C=1C=NNC=1 WTKZEGDFNFYCGP-UHFFFAOYSA-N 0.000 description 1
- 229910018287 SbF 5 Inorganic materials 0.000 description 1
- 229910006404 SnO 2 Inorganic materials 0.000 description 1
- QAOWNCQODCNURD-UHFFFAOYSA-L Sulfate Chemical compound [O-]S([O-])(=O)=O QAOWNCQODCNURD-UHFFFAOYSA-L 0.000 description 1
- DPOPAJRDYZGTIR-UHFFFAOYSA-N Tetrazine Chemical compound C1=CN=NN=N1 DPOPAJRDYZGTIR-UHFFFAOYSA-N 0.000 description 1
- 229910021536 Zeolite Inorganic materials 0.000 description 1
- DGEZNRSVGBDHLK-UHFFFAOYSA-N [1,10]phenanthroline Chemical compound C1=CN=C2C3=NC=CC=C3C=CC2=C1 DGEZNRSVGBDHLK-UHFFFAOYSA-N 0.000 description 1
- 230000000996 additive effect Effects 0.000 description 1
- 239000003570 air Substances 0.000 description 1
- 125000003545 alkoxy group Chemical group 0.000 description 1
- 150000003973 alkyl amines Chemical class 0.000 description 1
- 239000012080 ambient air Substances 0.000 description 1
- 150000001412 amines Chemical class 0.000 description 1
- IVHKZGYFKJRXBD-UHFFFAOYSA-N amino carbamate Chemical class NOC(N)=O IVHKZGYFKJRXBD-UHFFFAOYSA-N 0.000 description 1
- 229910052908 analcime Inorganic materials 0.000 description 1
- 150000004982 aromatic amines Chemical class 0.000 description 1
- SQTGBVURPMTXBT-UHFFFAOYSA-N azanium;1,1,2,2,3,3,4,4,4-nonafluorobutane-1-sulfonate Chemical compound [NH4+].[O-]S(=O)(=O)C(F)(F)C(F)(F)C(F)(F)C(F)(F)F SQTGBVURPMTXBT-UHFFFAOYSA-N 0.000 description 1
- BMWDUGHMODRTLU-UHFFFAOYSA-N azanium;trifluoromethanesulfonate Chemical compound [NH4+].[O-]S(=O)(=O)C(F)(F)F BMWDUGHMODRTLU-UHFFFAOYSA-N 0.000 description 1
- SCJFJWNHJWAJSX-UHFFFAOYSA-N benzaldehyde N-(2,2-dimethylpropylidene)hydroxylamine Chemical compound CC(C)(C)C=NO.O=CC1=CC=CC=C1 SCJFJWNHJWAJSX-UHFFFAOYSA-N 0.000 description 1
- RWCCWEUUXYIKHB-UHFFFAOYSA-N benzophenone Chemical compound C=1C=CC=CC=1C(=O)C1=CC=CC=C1 RWCCWEUUXYIKHB-UHFFFAOYSA-N 0.000 description 1
- 239000012965 benzophenone Substances 0.000 description 1
- QRUDEWIWKLJBPS-UHFFFAOYSA-N benzotriazole Chemical compound C1=CC=C2N[N][N]C2=C1 QRUDEWIWKLJBPS-UHFFFAOYSA-N 0.000 description 1
- 239000012964 benzotriazole Substances 0.000 description 1
- 125000006267 biphenyl group Chemical group 0.000 description 1
- 125000002529 biphenylenyl group Chemical group C1(=CC=CC=2C3=CC=CC=C3C12)* 0.000 description 1
- NLNRQJQXCQVDQJ-UHFFFAOYSA-N bis(3,4-diaminophenyl)methanone Chemical compound C1=C(N)C(N)=CC=C1C(=O)C1=CC=C(N)C(N)=C1 NLNRQJQXCQVDQJ-UHFFFAOYSA-N 0.000 description 1
- 229910052799 carbon Inorganic materials 0.000 description 1
- 229910002092 carbon dioxide Inorganic materials 0.000 description 1
- 239000001569 carbon dioxide Substances 0.000 description 1
- 229910002091 carbon monoxide Inorganic materials 0.000 description 1
- 238000005266 casting Methods 0.000 description 1
- VGNIIAPVRLMILS-UHFFFAOYSA-M cesium;1,1,2,2,3,3,4,4,4-nonafluorobutane-1-sulfonate Chemical compound [Cs+].[O-]S(=O)(=O)C(F)(F)C(F)(F)C(F)(F)C(F)(F)F VGNIIAPVRLMILS-UHFFFAOYSA-M 0.000 description 1
- 229930016911 cinnamic acid Natural products 0.000 description 1
- 235000013985 cinnamic acid Nutrition 0.000 description 1
- 229910000428 cobalt oxide Inorganic materials 0.000 description 1
- IVMYJDGYRUAWML-UHFFFAOYSA-N cobalt(ii) oxide Chemical compound [Co]=O IVMYJDGYRUAWML-UHFFFAOYSA-N 0.000 description 1
- 239000011231 conductive filler Substances 0.000 description 1
- 239000004020 conductor Substances 0.000 description 1
- 238000010276 construction Methods 0.000 description 1
- 238000011109 contamination Methods 0.000 description 1
- 229920001577 copolymer Polymers 0.000 description 1
- UFULAYFCSOUIOV-UHFFFAOYSA-N cysteamine Chemical compound NCCS UFULAYFCSOUIOV-UHFFFAOYSA-N 0.000 description 1
- 230000003247 decreasing effect Effects 0.000 description 1
- 230000001419 dependent effect Effects 0.000 description 1
- 238000009792 diffusion process Methods 0.000 description 1
- NEQVFHFOWYYPBS-UHFFFAOYSA-M dimethyl(3-triphenylphosphaniumylpropyl)azanium;dibromide Chemical compound Br.[Br-].C=1C=CC=CC=1[P+](C=1C=CC=CC=1)(CCCN(C)C)C1=CC=CC=C1 NEQVFHFOWYYPBS-UHFFFAOYSA-M 0.000 description 1
- HNPSIPDUKPIQMN-UHFFFAOYSA-N dioxosilane;oxo(oxoalumanyloxy)alumane Chemical compound O=[Si]=O.O=[Al]O[Al]=O HNPSIPDUKPIQMN-UHFFFAOYSA-N 0.000 description 1
- GWZCCUDJHOGOSO-UHFFFAOYSA-N diphenic acid Chemical compound OC(=O)C1=CC=CC=C1C1=CC=CC=C1C(O)=O GWZCCUDJHOGOSO-UHFFFAOYSA-N 0.000 description 1
- CZZYITDELCSZES-UHFFFAOYSA-N diphenylmethane Chemical compound C=1C=CC=CC=1CC1=CC=CC=C1 CZZYITDELCSZES-UHFFFAOYSA-N 0.000 description 1
- 238000001035 drying Methods 0.000 description 1
- 230000000694 effects Effects 0.000 description 1
- 238000005868 electrolysis reaction Methods 0.000 description 1
- 239000003792 electrolyte Substances 0.000 description 1
- 230000002349 favourable effect Effects 0.000 description 1
- 239000000835 fiber Substances 0.000 description 1
- 229910052731 fluorine Inorganic materials 0.000 description 1
- 239000011737 fluorine Substances 0.000 description 1
- 230000008014 freezing Effects 0.000 description 1
- 238000007710 freezing Methods 0.000 description 1
- 239000002737 fuel gas Substances 0.000 description 1
- 239000003502 gasoline Substances 0.000 description 1
- 238000012812 general test Methods 0.000 description 1
- 239000011521 glass Substances 0.000 description 1
- 239000003365 glass fiber Substances 0.000 description 1
- 125000005843 halogen group Chemical group 0.000 description 1
- 230000020169 heat generation Effects 0.000 description 1
- 229930195733 hydrocarbon Natural products 0.000 description 1
- 150000002430 hydrocarbons Chemical class 0.000 description 1
- 125000002887 hydroxy group Chemical group [H]O* 0.000 description 1
- QWPPOHNGKGFGJK-UHFFFAOYSA-N hypochlorous acid Chemical compound ClO QWPPOHNGKGFGJK-UHFFFAOYSA-N 0.000 description 1
- 238000001566 impedance spectroscopy Methods 0.000 description 1
- 239000012535 impurity Substances 0.000 description 1
- HOBCFUWDNJPFHB-UHFFFAOYSA-N indolizine Chemical compound C1=CC=CN2C=CC=C21 HOBCFUWDNJPFHB-UHFFFAOYSA-N 0.000 description 1
- 125000001449 isopropyl group Chemical group [H]C([H])([H])C([H])(*)C([H])([H])[H] 0.000 description 1
- 238000003475 lamination Methods 0.000 description 1
- 229910000464 lead oxide Inorganic materials 0.000 description 1
- FEDFHMISXKDOJI-UHFFFAOYSA-M lithium;1,1,2,2,3,3,4,4,4-nonafluorobutane-1-sulfonate Chemical compound [Li+].[O-]S(=O)(=O)C(F)(F)C(F)(F)C(F)(F)C(F)(F)F FEDFHMISXKDOJI-UHFFFAOYSA-M 0.000 description 1
- VAXNCPZUCRECEW-UHFFFAOYSA-M lithium;1,1,2,2,3,3,4,4,5,5,6,6,6-tridecafluorohexane-1-sulfonate Chemical compound [Li+].[O-]S(=O)(=O)C(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)F VAXNCPZUCRECEW-UHFFFAOYSA-M 0.000 description 1
- MCVFFRWZNYZUIJ-UHFFFAOYSA-M lithium;trifluoromethanesulfonate Chemical compound [Li+].[O-]S(=O)(=O)C(F)(F)F MCVFFRWZNYZUIJ-UHFFFAOYSA-M 0.000 description 1
- 230000007774 longterm Effects 0.000 description 1
- 229960003151 mercaptamine Drugs 0.000 description 1
- 150000001247 metal acetylides Chemical class 0.000 description 1
- WBYWAXJHAXSJNI-UHFFFAOYSA-N methyl p-hydroxycinnamate Natural products OC(=O)C=CC1=CC=CC=C1 WBYWAXJHAXSJNI-UHFFFAOYSA-N 0.000 description 1
- 229910052901 montmorillonite Inorganic materials 0.000 description 1
- 229910052680 mordenite Inorganic materials 0.000 description 1
- 125000004123 n-propyl group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])* 0.000 description 1
- OBKARQMATMRWQZ-UHFFFAOYSA-N naphthalene-1,2,5,6-tetracarboxylic acid Chemical compound OC(=O)C1=C(C(O)=O)C=CC2=C(C(O)=O)C(C(=O)O)=CC=C21 OBKARQMATMRWQZ-UHFFFAOYSA-N 0.000 description 1
- OLAPPGSPBNVTRF-UHFFFAOYSA-N naphthalene-1,4,5,8-tetracarboxylic acid Chemical compound C1=CC(C(O)=O)=C2C(C(=O)O)=CC=C(C(O)=O)C2=C1C(O)=O OLAPPGSPBNVTRF-UHFFFAOYSA-N 0.000 description 1
- ABMFBCRYHDZLRD-UHFFFAOYSA-N naphthalene-1,4-dicarboxylic acid Chemical compound C1=CC=C2C(C(=O)O)=CC=C(C(O)=O)C2=C1 ABMFBCRYHDZLRD-UHFFFAOYSA-N 0.000 description 1
- DFFZOPXDTCDZDP-UHFFFAOYSA-N naphthalene-1,5-dicarboxylic acid Chemical compound C1=CC=C2C(C(=O)O)=CC=CC2=C1C(O)=O DFFZOPXDTCDZDP-UHFFFAOYSA-N 0.000 description 1
- RXOHFPCZGPKIRD-UHFFFAOYSA-N naphthalene-2,6-dicarboxylic acid Chemical compound C1=C(C(O)=O)C=CC2=CC(C(=O)O)=CC=C21 RXOHFPCZGPKIRD-UHFFFAOYSA-N 0.000 description 1
- WPUMVKJOWWJPRK-UHFFFAOYSA-N naphthalene-2,7-dicarboxylic acid Chemical compound C1=CC(C(O)=O)=CC2=CC(C(=O)O)=CC=C21 WPUMVKJOWWJPRK-UHFFFAOYSA-N 0.000 description 1
- 125000001624 naphthyl group Chemical group 0.000 description 1
- 229910052674 natrolite Inorganic materials 0.000 description 1
- 229910000480 nickel oxide Inorganic materials 0.000 description 1
- QJGQUHMNIGDVPM-UHFFFAOYSA-N nitrogen group Chemical group [N] QJGQUHMNIGDVPM-UHFFFAOYSA-N 0.000 description 1
- NLRKCXQQSUWLCH-UHFFFAOYSA-N nitrosobenzene Chemical class O=NC1=CC=CC=C1 NLRKCXQQSUWLCH-UHFFFAOYSA-N 0.000 description 1
- 229910000510 noble metal Inorganic materials 0.000 description 1
- YEXPOXQUZXUXJW-UHFFFAOYSA-N oxolead Chemical compound [Pb]=O YEXPOXQUZXUXJW-UHFFFAOYSA-N 0.000 description 1
- GNRSAWUEBMWBQH-UHFFFAOYSA-N oxonickel Chemical compound [Ni]=O GNRSAWUEBMWBQH-UHFFFAOYSA-N 0.000 description 1
- 229960004624 perflexane Drugs 0.000 description 1
- ZJIJAJXFLBMLCK-UHFFFAOYSA-N perfluorohexane Chemical compound FC(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)F ZJIJAJXFLBMLCK-UHFFFAOYSA-N 0.000 description 1
- QZHDEAJFRJCDMF-UHFFFAOYSA-N perfluorohexanesulfonic acid Chemical compound OS(=O)(=O)C(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)F QZHDEAJFRJCDMF-UHFFFAOYSA-N 0.000 description 1
- 125000001997 phenyl group Chemical group [H]C1=C([H])C([H])=C(*)C([H])=C1[H] 0.000 description 1
- 125000000843 phenylene group Chemical group C1(=C(C=CC=C1)*)* 0.000 description 1
- AQSJGOWTSHOLKH-UHFFFAOYSA-N phosphite(3-) Chemical class [O-]P([O-])[O-] AQSJGOWTSHOLKH-UHFFFAOYSA-N 0.000 description 1
- 125000004437 phosphorous atom Chemical group 0.000 description 1
- 229910052698 phosphorus Chemical group 0.000 description 1
- 239000004033 plastic Substances 0.000 description 1
- 229920003023 plastic Polymers 0.000 description 1
- 239000003880 polar aprotic solvent Substances 0.000 description 1
- 229920002577 polybenzoxazole Polymers 0.000 description 1
- 238000012667 polymer degradation Methods 0.000 description 1
- 239000001205 polyphosphate Substances 0.000 description 1
- 235000011176 polyphosphates Nutrition 0.000 description 1
- LVTHXRLARFLXNR-UHFFFAOYSA-M potassium;1,1,2,2,3,3,4,4,4-nonafluorobutane-1-sulfonate Chemical compound [K+].[O-]S(=O)(=O)C(F)(F)C(F)(F)C(F)(F)C(F)(F)F LVTHXRLARFLXNR-UHFFFAOYSA-M 0.000 description 1
- RSCGQEBKFSGWJT-UHFFFAOYSA-M potassium;1,1,2,2,3,3,4,4,5,5,6,6,6-tridecafluorohexane-1-sulfonate Chemical compound [K+].[O-]S(=O)(=O)C(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)F RSCGQEBKFSGWJT-UHFFFAOYSA-M 0.000 description 1
- GLGXXYFYZWQGEL-UHFFFAOYSA-M potassium;trifluoromethanesulfonate Chemical compound [K+].[O-]S(=O)(=O)C(F)(F)F GLGXXYFYZWQGEL-UHFFFAOYSA-M 0.000 description 1
- PBMFSQRYOILNGV-UHFFFAOYSA-N pyridazine Chemical compound C1=CC=NN=C1 PBMFSQRYOILNGV-UHFFFAOYSA-N 0.000 description 1
- UMJSCPRVCHMLSP-UHFFFAOYSA-N pyridine Natural products COC1=CC=CN=C1 UMJSCPRVCHMLSP-UHFFFAOYSA-N 0.000 description 1
- IAYUQKZZQKUOFL-UHFFFAOYSA-N pyridine-2,3,5,6-tetramine Chemical compound NC1=CC(N)=C(N)N=C1N IAYUQKZZQKUOFL-UHFFFAOYSA-N 0.000 description 1
- JWVCLYRUEFBMGU-UHFFFAOYSA-N quinazoline Chemical compound N1=CN=CC2=CC=CC=C21 JWVCLYRUEFBMGU-UHFFFAOYSA-N 0.000 description 1
- 230000005855 radiation Effects 0.000 description 1
- 230000000284 resting effect Effects 0.000 description 1
- 229910052665 sodalite Inorganic materials 0.000 description 1
- QBJDFZSOZNDVDE-UHFFFAOYSA-M sodium;1,1,2,2,3,3,4,4,4-nonafluorobutane-1-sulfonate Chemical compound [Na+].[O-]S(=O)(=O)C(F)(F)C(F)(F)C(F)(F)C(F)(F)F QBJDFZSOZNDVDE-UHFFFAOYSA-M 0.000 description 1
- WXNIEINRHBIHRE-UHFFFAOYSA-M sodium;1,1,2,2,3,3,4,4,5,5,6,6,6-tridecafluorohexane-1-sulfonate Chemical compound [Na+].[O-]S(=O)(=O)C(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)F WXNIEINRHBIHRE-UHFFFAOYSA-M 0.000 description 1
- XGPOMXSYOKFBHS-UHFFFAOYSA-M sodium;trifluoromethanesulfonate Chemical compound [Na+].[O-]S(=O)(=O)C(F)(F)F XGPOMXSYOKFBHS-UHFFFAOYSA-M 0.000 description 1
- 239000007790 solid phase Substances 0.000 description 1
- 238000001179 sorption measurement Methods 0.000 description 1
- 238000001228 spectrum Methods 0.000 description 1
- 238000005507 spraying Methods 0.000 description 1
- 230000006641 stabilisation Effects 0.000 description 1
- 238000011105 stabilization Methods 0.000 description 1
- 239000000126 substance Substances 0.000 description 1
- 125000001424 substituent group Chemical group 0.000 description 1
- 238000006467 substitution reaction Methods 0.000 description 1
- 150000003457 sulfones Chemical class 0.000 description 1
- 150000003460 sulfonic acids Chemical class 0.000 description 1
- 230000003319 supportive effect Effects 0.000 description 1
- 239000000725 suspension Substances 0.000 description 1
- 238000010345 tape casting Methods 0.000 description 1
- 125000000999 tert-butyl group Chemical group [H]C([H])([H])C(*)(C([H])([H])[H])C([H])([H])[H] 0.000 description 1
- 238000012360 testing method Methods 0.000 description 1
- 238000004448 titration Methods 0.000 description 1
- ITMCEJHCFYSIIV-UHFFFAOYSA-N triflic acid Chemical compound OS(=O)(=O)C(F)(F)F ITMCEJHCFYSIIV-UHFFFAOYSA-N 0.000 description 1
Classifications
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M8/00—Fuel cells; Manufacture thereof
- H01M8/10—Fuel cells with solid electrolytes
- H01M8/1016—Fuel cells with solid electrolytes characterised by the electrolyte material
- H01M8/1018—Polymeric electrolyte materials
- H01M8/1069—Polymeric electrolyte materials characterised by the manufacturing processes
- H01M8/1072—Polymeric electrolyte materials characterised by the manufacturing processes by chemical reactions, e.g. in situ polymerisation or in situ crosslinking
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M8/00—Fuel cells; Manufacture thereof
- H01M8/02—Details
- H01M8/0289—Means for holding the electrolyte
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M8/00—Fuel cells; Manufacture thereof
- H01M8/10—Fuel cells with solid electrolytes
- H01M8/1004—Fuel cells with solid electrolytes characterised by membrane-electrode assemblies [MEA]
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M8/00—Fuel cells; Manufacture thereof
- H01M8/10—Fuel cells with solid electrolytes
- H01M8/1016—Fuel cells with solid electrolytes characterised by the electrolyte material
- H01M8/1018—Polymeric electrolyte materials
- H01M8/102—Polymeric electrolyte materials characterised by the chemical structure of the main chain of the ion-conducting polymer
- H01M8/103—Polymeric electrolyte materials characterised by the chemical structure of the main chain of the ion-conducting polymer having nitrogen, e.g. sulfonated polybenzimidazoles [S-PBI], polybenzimidazoles with phosphoric acid, sulfonated polyamides [S-PA] or sulfonated polyphosphazenes [S-PPh]
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M8/00—Fuel cells; Manufacture thereof
- H01M8/10—Fuel cells with solid electrolytes
- H01M8/1016—Fuel cells with solid electrolytes characterised by the electrolyte material
- H01M8/1018—Polymeric electrolyte materials
- H01M8/1041—Polymer electrolyte composites, mixtures or blends
- H01M8/1046—Mixtures of at least one polymer and at least one additive
- H01M8/1048—Ion-conducting additives, e.g. ion-conducting particles, heteropolyacids, metal phosphate or polybenzimidazole with phosphoric acid
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y02—TECHNOLOGIES OR APPLICATIONS FOR MITIGATION OR ADAPTATION AGAINST CLIMATE CHANGE
- Y02E—REDUCTION OF GREENHOUSE GAS [GHG] EMISSIONS, RELATED TO ENERGY GENERATION, TRANSMISSION OR DISTRIBUTION
- Y02E60/00—Enabling technologies; Technologies with a potential or indirect contribution to GHG emissions mitigation
- Y02E60/30—Hydrogen technology
- Y02E60/50—Fuel cells
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y02—TECHNOLOGIES OR APPLICATIONS FOR MITIGATION OR ADAPTATION AGAINST CLIMATE CHANGE
- Y02P—CLIMATE CHANGE MITIGATION TECHNOLOGIES IN THE PRODUCTION OR PROCESSING OF GOODS
- Y02P70/00—Climate change mitigation technologies in the production process for final industrial or consumer products
- Y02P70/50—Manufacturing or production processes characterised by the final manufactured product
Landscapes
- Chemical & Material Sciences (AREA)
- Engineering & Computer Science (AREA)
- Manufacturing & Machinery (AREA)
- General Chemical & Material Sciences (AREA)
- Sustainable Energy (AREA)
- Life Sciences & Earth Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Electrochemistry (AREA)
- Sustainable Development (AREA)
- Composite Materials (AREA)
- Crystallography & Structural Chemistry (AREA)
- Macromolecular Compounds Obtained By Forming Nitrogen-Containing Linkages In General (AREA)
- Conductive Materials (AREA)
- Manufacture Of Macromolecular Shaped Articles (AREA)
- Fuel Cell (AREA)
- Other Resins Obtained By Reactions Not Involving Carbon-To-Carbon Unsaturated Bonds (AREA)
- Inert Electrodes (AREA)
Description
A)溶液および/または分散液を形成するために、ポリリン酸中において、高リン酸親和性を有する1以上の芳香族および/またはヘテロ芳香族ジアミノカルボン酸を混合するか、あるいは、高リン酸親和性または低リン酸親和性を有する1以上の芳香族テトラアミノ化合物と、1以上の芳香族カルボン酸またはそれらのエステル(これらは、1カルボン酸モノマー当たり少なくとも2つの酸基を含み、高リン酸親和性または低リン酸親和性を有する)とを混合する工程、
B)好ましくは不活性ガス下で、工程A)からの混合物を加熱し、そして1.5dl/gまで、好ましくは0.3〜1.0dl/g、特には0.5〜0.8dl/gの固有粘度が得られるまで重合して、そのリン酸親和性が、工程D)において形成されるポリマーのリン酸親和性よりも高いポリマーを形成する工程、
C)ポリリン酸中において、高リン酸親和性または低リン酸親和性を有する1以上の芳香族テトラアミノ化合物と、1以上の芳香族カルボン酸またはそれらのエステル(これらは、1カルボン酸モノマー当たり少なくとも2つの酸基を含み、高リン酸親和性または低リン酸親和性を有する)とを混合して、溶液および/または分散液を形成する工程、
D)好ましくは不活性ガス下で、工程C)からの混合物を加熱し、そして1.5dl/gまで、好ましくは0.3〜1.0dl/g、特には0.5〜0.8dl/gの固有粘度が得られるまで重合して、そのリン酸親和性が、工程B)において形成されるポリマーのリン酸親和性よりも低いポリマーを形成する工程、
E)工程B)からのポリマーと工程D)からのポリマーとを混合する工程(工程Bからのポリマーのリン酸親和性は、工程Dからのポリマーのリン酸親和性よりも高い)、
F)工程E)に従う混合物を使用して、担体上または電極上に層を適用する工程、
G)1.5dl/g超、好ましくは1.8dl/g超、特には1.9dl/g超の固有粘度が得られるまで、好ましくは不活性ガス下で、工程F)に従って得られ得るシート様構造/層(sheetlike structure/layer)を加熱して、ポリアゾールブロックポリマーを形成する工程、
H)工程G)において形成される膜を処理する工程(それが自己支持性になるまで)、
を包含する方法によって得られ得る、ポリアゾールに基づくプロトン伝導性ポリマー膜を提供する。
Arは、同一または異なり、そして各々、四価の芳香族またはヘテロ芳香族基(これは、単または多環式であり得る)であり、
Ar1は、同一または異なり、そして各々、二価の芳香族またはヘテロ芳香族基(これは、単または多環式であり得る)であり、
Ar2は、同一または異なり、そして各々、二価または三価の芳香族またはヘテロ芳香族基(これは、単または多環式であり得る)であり、
Ar3は、同一または異なり、そして各々、三価の芳香族またはヘテロ芳香族基(これは、単または多環式であり得る)であり、
Ar4は、同一または異なり、そして各々、三価の芳香族またはヘテロ芳香族基(これは、単または多環式であり得る)であり、
Ar5は、同一または異なり、そして各々、四価の芳香族またはヘテロ芳香族基(これは、単または多環式であり得る)であり、
Ar6は、同一または異なり、そして各々、二価の芳香族またはヘテロ芳香族基(これは、単または多環式であり得る)であり、
Ar7は、同一または異なり、そして各々、二価の芳香族またはヘテロ芳香族基(これは、単または多環式であり得る)であり、
Ar8は、同一または異なり、そして各々、三価の芳香族またはヘテロ芳香族基(これは、単または多環式であり得る)であり、
Ar9は、同一または異なり、そして各々、二価または三価または四価の芳香族またはヘテロ芳香族基(これは、単または多環式であり得る)であり、
Ar10は、同一または異なり、そして各々、二価または三価の芳香族またはヘテロ芳香族基(これは、単または多環式であり得る)であり、
Ar11は、同一または異なり、そして各々、二価の芳香族またはヘテロ芳香族基(これは、単または多環式であり得る)であり、
Xは、同一または異なり、そして各々、酸素、硫黄またはアミノ基{これは、さらなる基として、水素原子、1〜20の炭素原子を有する基(好ましくは、分枝または非分枝のアルキルまたはアルコキシ基、あるいはアリール基)を保有する}であり、
Rは、同一または異なり、そして水素、アルキル基または芳香族基であり、但し、式(XX)におけるRは水素ではなく、そして
n、mは、各々、10以上、好ましくは100以上の整数である]
の繰り返しアゾール単位を含む。
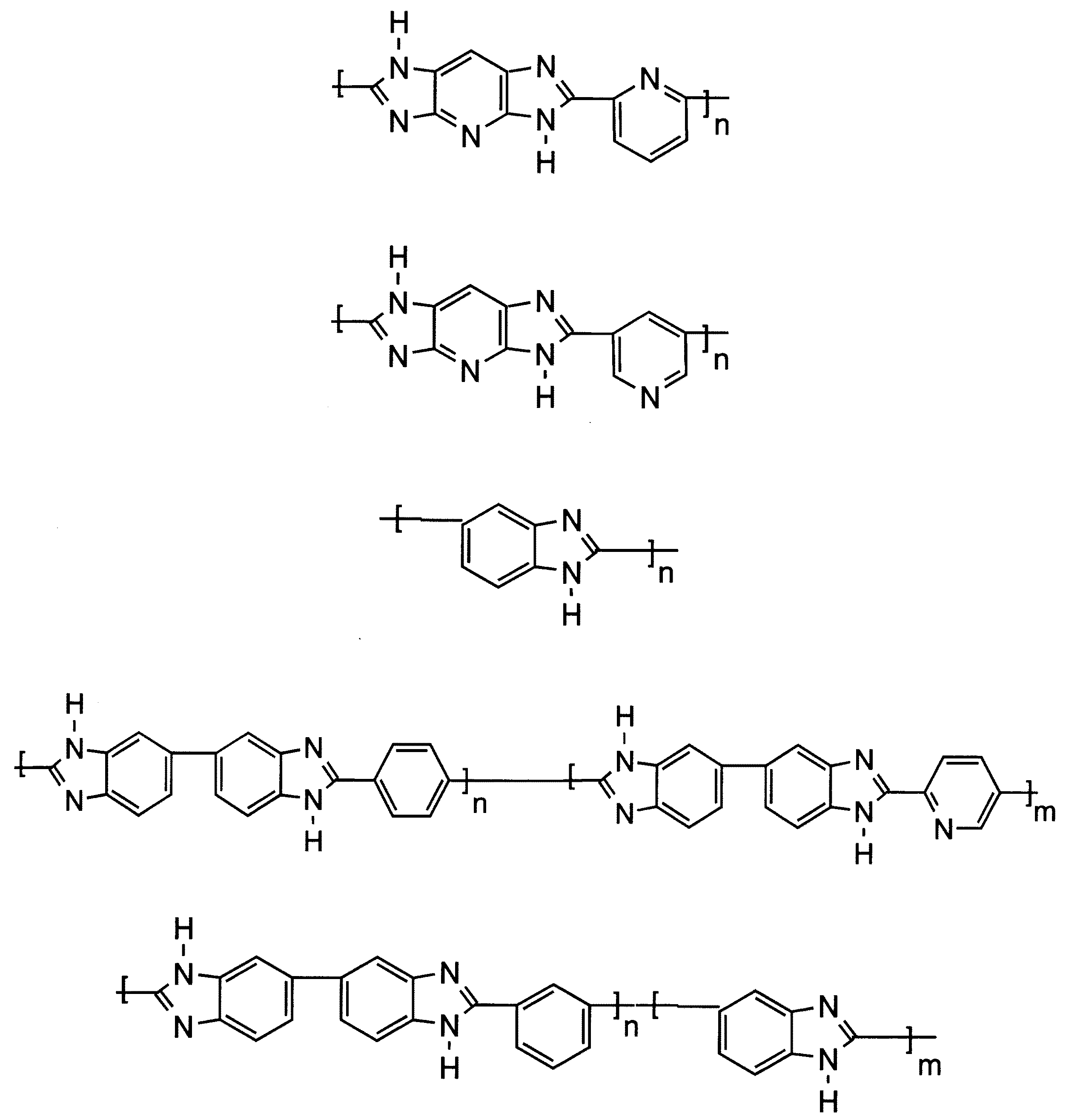
本発明の文脈において、好ましいのは、繰り返しベンゾイミダゾール単位を含むブロックポリマーである。繰り返しベンゾイミダゾール単位を含む非常に好適なポリマーのいくつかの例は、以下の式によって示される:
スルフェート: 例えば、CsHSO4、Fe(SO4)2、(NH4)3H(SO4)2、LiHSO4、NaHSO4、KHSO4、RbSO4、LiN2H5SO4、NH4HSO4;
ホスフェート: 例えば、Zr3(PO4)4、Zr(HPO4)2、HZr2(PO4)3、UO2PO4.3H2O、H8UO2PO4、Ce(HPO4)2、Ti(HPO4)2、KH2PO4、NaH2PO4、LiH2PO4、NH4H2PO4、CsH2PO4、CaHPO4、MgHPO4、HSbP2O8、HSb3P2O14、H5Sb5P2O20;
ポリ酸: 例えば、H3PW12O40.nH2O(n=21−29)、H3SiW12O40.nH2O(n=21−29)、HxWO3、HSbWO6、H3PMo12O40、H2Sb4O11、HTaWO6、HNbO3、HTiNbO5、HTiTaO5、HSbTeO6、H5Ti4O9、HSbO3、H2MoO4;
セレン化物(selenites)およびヒ化物: 例えば、(NH4)3H(SeO4)2、UO2AsO4、(NH4)3H(SeO4)2、KH2AsO4、Cs3H(SeO4)2、Rb3H(SeO4)2;
酸化物: 例えば、Al2O3、Sb2O5、ThO2、SnO2、ZrO2、MoO3;
シリケート: 例えば、ゼオライト、ゼオライト(NH4 +)、シートシリケート(sheet silicates)、フレームワークシリケート(framework silicates)、H−ソーダ沸石、H−モルデン沸石、NH4−方沸石(NH4-analcines)、NH4−方ソーダ石、NH4−ガレート(NH4-gallates)、H−モンモリロナイト;
酸: 例えば、HClO4、SbF5;
充填剤: 例えば、炭化物、特にSiC、Si3N4、繊維、特にガラス繊維、ガラス粉末および/またはポリマー粉末(好ましくは、ポリアゾールに基づく)。
トリフルオロメタンスルホン酸、トリフルオロメタンスルホン酸カリウム、トリフルオロメタンスルホン酸ナトリウム、トリフルオロメタンスルホン酸リチウム、トリフルオロメタンスルホン酸アンモニウム、ペルフルオロヘキサンスルホン酸カリウム、ペルフルオロヘキサンスルホン酸ナトリウム、ペルフルオロヘキサンスルホン酸リチウム、ペルフルオロヘキサンスルホン酸アンモニウム、ペルフルオロヘキサンスルホン酸、ノナフルオロブタンスルホン酸カリウム、ノナフルオロブタンスルホン酸ナトリウム、ノナフルオロブタンスルホン酸リチウム、ノナフルオロブタンスルホン酸アンモニウム、ノナフルオロブタンスルホン酸セシウム、トリエチルアンモニウムペルフルオロヘキサンスルホネート、ペルフルオロスルホンイミドおよびナフィオン(Nafion)。
ビス(トリフルオロメチル)ニトロキサイド、2,2−ジフェニル−1−ピクリニルヒドラジル、フェノール、アルキルフェノール、立体的に込み合った(sterically hindered)アルキルフェノール(例えば、Irganox)、芳香族アミン、立体的に込み合ったアミン(例えば、Chimassorb);立体的に込み合ったヒドロキシルアミン、立体的に込み合ったアルキルアミン、立体的に込み合ったヒドロキシルアミン、立体的に込み合ったヒドロキシルアミンエーテル、ホスファイト(例えば、Irgafos)、ニトロソベンゼン、メチル−2−ニトロソプロパン、ベンゾフェノン、ベンズアルデヒド−tert−ブチルニトロン、システアミン、メラニン、酸化鉛、酸化マンガン、酸化ニッケル、酸化コバルト。
A)溶液および/または分散液を形成するために、ポリリン酸中において、高リン酸親和性を有する1以上の芳香族および/またはヘテロ芳香族ジアミノカルボン酸を混合するか、あるいは、高リン酸親和性または低リン酸親和性を有する1以上の芳香族テトラアミノ化合物と、1以上の芳香族カルボン酸またはそれらのエステル(これらは、1カルボン酸モノマー当たり少なくとも2つの酸基を含み、高リン酸親和性または低リン酸親和性を有する)とを混合する工程、
B)好ましくは不活性ガス下で、工程A)からの混合物を加熱し、そして1.5dl/gまで、好ましくは0.3〜1.0dl/g、特には0.5〜0.8dl/gの固有粘度が得られるまで重合して、そのリン酸親和性が、工程D)において形成されるポリマーのリン酸親和性よりも高いポリマーを形成する工程、
C)ポリリン酸中において、高リン酸親和性または低リン酸親和性を有する1以上の芳香族テトラアミノ化合物と、1以上の芳香族カルボン酸またはそれらのエステル(これらは、1カルボン酸モノマー当たり少なくとも2つの酸基を含み、高リン酸親和性または低リン酸親和性を有する)とを混合して、溶液および/または分散液を形成する工程、
D)好ましくは不活性ガス下で、工程C)からの混合物を加熱し、そして1.5dl/gまで、好ましくは0.3〜1.0dl/g、特には0.5〜0.8dl/gの固有粘度が得られるまで重合して、そのリン酸親和性が、工程B)において形成されるポリマーのリン酸親和性よりも低いポリマーを形成する工程、
E)工程B)からのポリマーと工程D)からのポリマーとを混合する工程(工程Bからのポリマーのリン酸親和性は、工程Dからのポリマーのリン酸親和性よりも高い)、
F)工程E)に従う混合物を使用して、電極上に層を適用する工程、
G)1.5dl/g超、好ましくは1.8dl/g超、特には2.0dl/g超の固有粘度が得られるまで、好ましくは不活性ガス下で、工程F)に従って得られ得るシート様構造/層を加熱して、ポリアゾールブロックポリマーを形成する工程、
H)工程G)において形成される膜を処理する工程、
を包含する方法によって得られ得る、ポリアゾールに基づくプロトン伝導性ポリマーコーティングを有する、電極を提供する。
IECについての試験方法
膜の伝導性は、いわゆるイオン交換容量(ion exchange capacity)(IEC)によって表される酸基の含有量に大きく依存する。イオン交換容量を測定するために、3cmの直径を有するサンプルを打ち抜き、そして100mlの水で満たしたビーカー中に入れる。放出された酸を0.1MNaOHで滴定する。引き続いて、該サンプルを取り出し、過剰量の水を取り除き、そして該サンプルを160℃で4時間乾燥する。次いで、乾燥重量、m0を、0.1mgの精度で重量測定によって測定する。次いで、イオン交換容量を、以下の式によって、第一滴定終点までの0.1MNaOHの消費量、V1(ml)および乾燥重量、m0(mg)から計算する:
IEC=V1 *300/m0
比伝導率についての試験方法
比伝導率を、定電位モードにおける4極配置でのインピーダンス分光法(impedance spectroscopy in a 4-pole arrangement in potentiostatic mode)によって、そして白金電極(ワイヤ、直径0.25mm)を使用して、測定する。集電電極間の距離は2cmである。得られるスペクトルを、オーム抵抗およびキャパシタの並列配置からなるシンプルなモデルで評価する。リン酸ドープされた膜のサンプル断面を、サンプル搭載の直前に測定する。温度依存性を測定するために、テストセルを、オーブン中において所望の温度までもっていき、そして該サンプルのすぐ隣に配置されたPt−100サーモエレメントによって制御する。温度が達成されると、該サンプルを、測定開始前の10分間、この温度で維持する。
Claims (10)
- 以下の工程:
A)溶液および/または分散液を形成するために、ポリリン酸中において、
(i)高リン酸親和性を有する1以上の芳香族テトラアミノ化合物と、
(ii)1以上の芳香族カルボン酸またはそれらのエステル(これらは、1カルボン酸モノマー当たり少なくとも2つの酸基を含み、低リン酸親和性を有する)とを
混合する工程、
B)工程A)からの混合物を加熱し、そして1.5dl/gまでの固有粘度が得られるまで重合して、そのリン酸親和性が、工程D)において形成されるポリマーのリン酸親和性よりも高いポリマーを形成する工程、
C)ポリリン酸中において、低リン酸親和性を有する1以上の芳香族テトラアミノ化合物と、1以上の芳香族カルボン酸またはそれらのエステル(これらは、1カルボン酸モノマー当たり少なくとも2つの酸基を含み、低リン酸親和性を有する)とを混合して、溶液および/または分散液を形成する工程、
D)工程C)からの混合物を加熱し、そして1.5dl/gまでの固有粘度が得られるまで重合して、そのリン酸親和性が、工程B)において形成されるポリマーのリン酸親和性よりも低いポリマーを形成する工程、
E)工程B)からのポリマーと工程D)からのポリマーとを混合する工程(工程Bからのポリマーのリン酸親和性は、工程Dからのポリマーのリン酸親和性よりも高い)、
F)工程E)に従う混合物を使用して、担体上または電極上に層を適用する工程、
G)1.5dl/g超の固有粘度が得られるまで、工程F)に従って得られ得るシート様構造/層を加熱して、ポリアゾールブロックポリマーを形成する工程、
H)工程G)において形成される膜のポリリン酸を、該膜が自己支持性となりかつ損傷無しに前記担体から除去され得るまでの時間および温度で、湿気の存在下において、部分加水分解し、処理する工程、
を包含し、
工程A)において使用される全てのモノマーに基づく低リン酸親和性を有するモノマーの総含有量が、40重量%までであり、
(a)前記使用される高リン酸親和性を有する芳香族テトラアミノ化合物が、3,3`,4,4`−テトラアミノジフェニルスルホンであり、
(b)前記使用される低リン酸親和性を有する芳香族テトラアミノ化合物が、3,3`,4,4`−テトラアミノビフェニル及び1,2,4,5−テトラアミノベンゼンから選ばれる化合物であり、
(c)前記使用される低リン酸親和性を有する芳香族カルボン酸が、イソフタル酸、テレフタル酸及びフタル酸から選ばれる化合物である、
ことを包含する方法によって得られ得る、
ポリアゾールに基づくプロトン伝導性ポリマー膜。 - (酸滴定によって)P2O5として計算すると、少なくとも83%の含有量を有するポリリン酸が、工程A)および工程C)において得られることを特徴とする、請求項1に記載の膜。
- ポリベンゾイミダゾールの繰り返しセグメントを含有するブロックポリマーが、工程G)において形成されることを特徴とする、請求項1又は2に記載の膜。
- 前記膜が、湿気または水および/またはスチームの存在下において、0℃超〜150℃の温度で、工程H)において処理されることを特徴とする、請求項1〜4のいずれかに記載の膜。
- 工程H)における膜の処理が、10秒〜300時間であることを特徴とする、請求項1〜5のいずれかに記載の膜。
- 20〜4000μmの厚みを有する層が、工程F)において得られることを特徴とする、請求項1〜6のいずれかに記載の膜。
- 工程H)によって形成される膜が、15〜3000μmの厚みを有することを特徴とする、請求項1〜7のいずれかに記載の膜。
- 請求項1〜8の1以上に記載の少なくとも1つの膜および少なくとも1つの電極を備える、膜−電極ユニット。
- 請求項9に記載の1以上の膜−電極ユニットを備える、燃料電池。
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| EP03017027 | 2003-07-27 | ||
| EP03017027.8 | 2003-07-27 | ||
| PCT/EP2004/008229 WO2005011039A2 (de) | 2003-07-27 | 2004-07-23 | Protonenleitende membran und deren verwendung |
Related Child Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2011206354A Division JP5468051B2 (ja) | 2003-07-27 | 2011-09-21 | プロトン伝導性膜およびその使用 |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| JP2007500416A JP2007500416A (ja) | 2007-01-11 |
| JP4875489B2 true JP4875489B2 (ja) | 2012-02-15 |
Family
ID=34089580
Family Applications (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2006521484A Expired - Fee Related JP4875489B2 (ja) | 2003-07-27 | 2004-07-23 | プロトン伝導性膜およびその使用 |
| JP2011206354A Expired - Fee Related JP5468051B2 (ja) | 2003-07-27 | 2011-09-21 | プロトン伝導性膜およびその使用 |
Family Applications After (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2011206354A Expired - Fee Related JP5468051B2 (ja) | 2003-07-27 | 2011-09-21 | プロトン伝導性膜およびその使用 |
Country Status (4)
| Country | Link |
|---|---|
| US (2) | US7820314B2 (ja) |
| EP (1) | EP1652259A2 (ja) |
| JP (2) | JP4875489B2 (ja) |
| WO (1) | WO2005011039A2 (ja) |
Families Citing this family (37)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| DE10117686A1 (de) * | 2001-04-09 | 2002-10-24 | Celanese Ventures Gmbh | Protonenleitende Membran und deren Verwendung |
| DE10209419A1 (de) * | 2002-03-05 | 2003-09-25 | Celanese Ventures Gmbh | Verfahren zur Herstellung einer Polymerelektrolytmembran und deren Anwendung in Brennstoffzellen |
| DE10213540A1 (de) * | 2002-03-06 | 2004-02-19 | Celanese Ventures Gmbh | Lösung aus Vinylphosphonsäure, Verfahren zur Herstellung einer Polymerelektrolytmembran aus Polyvinylphosphaonsäure und deren Anwendung in Brennstoffzellen |
| US7846982B2 (en) * | 2002-03-06 | 2010-12-07 | Pemeas Gmbh | Proton conducting electrolyte membrane having reduced methanol permeability and the use thereof in fuel cells |
| US20050118478A1 (en) * | 2002-03-06 | 2005-06-02 | Joachim Kiefer | Mixture comprising sulphonic acid containing vinyl, polymer electrolyte membrane comprising polyvinylsulphonic acid and the use thereof in fuel cells |
| DE10228657A1 (de) * | 2002-06-27 | 2004-01-15 | Celanese Ventures Gmbh | Protonenleitende Membran und deren Verwendung |
| DE10235358A1 (de) * | 2002-08-02 | 2004-02-12 | Celanese Ventures Gmbh | Protonenleitende Polymermembran umfassend Phosphonsäuregruppen enthaltende Polymere und deren Anwendung in Brennstoffzellen |
| DE10242708A1 (de) * | 2002-09-13 | 2004-05-19 | Celanese Ventures Gmbh | Protonenleitende Membranen und deren Verwendung |
| DE10246372A1 (de) * | 2002-10-04 | 2004-04-15 | Celanese Ventures Gmbh | Mit einer Katalysatorschicht beschichtete protonenleitende Polymermembran enthaltend Polyazole und deren Anwendung in Brennstoffzellen |
| DE10246461A1 (de) * | 2002-10-04 | 2004-04-15 | Celanese Ventures Gmbh | Protonenleitende Polymermembran enthaltend Polyazolblends und deren Anwendung in Brennstoffzellen |
| JP4875489B2 (ja) | 2003-07-27 | 2012-02-15 | ベーアーエスエフ フューエル セル ゲーエムベーハー | プロトン伝導性膜およびその使用 |
| DE10361832A1 (de) * | 2003-12-30 | 2005-07-28 | Celanese Ventures Gmbh | Protonenleitende Membran und deren Verwendung |
| DE102005020604A1 (de) * | 2005-05-03 | 2006-11-16 | Pemeas Gmbh | Brennstoffzellen mit geringerem Gewicht und Volumen |
| WO2007003363A1 (en) * | 2005-07-01 | 2007-01-11 | Basf Fuel Cell Gmbh | Gas diffusion electrodes, membrane-electrode assemblies and method for the production thereof |
| US8945736B2 (en) | 2005-09-10 | 2015-02-03 | Basf Fuel Cell Gmbh | Method for conditioning membrane-electrode-units for fuel cells |
| DE102005051887A1 (de) * | 2005-10-29 | 2007-05-03 | Pemeas Gmbh | Membran für Brennstoffzellen, enthaltend Polymere, die Phosphonsäure-und/oder Sulfonsäuregruppen umfassen, Membran-Elektroden-Einheit und deren Anwendung in Brennstoffzellen |
| DE102005052378A1 (de) * | 2005-10-31 | 2007-05-03 | Pemeas Gmbh | Verbesserte Membran-Elektrodeneinheiten und Brennstoffzellen mit hoher Lebensdauer |
| KR100819676B1 (ko) * | 2005-11-14 | 2008-04-03 | 주식회사 엘지화학 | 브랜치된 멀티블록 폴리벤즈이미다졸-벤즈아마이드공중합체 및 제조방법, 이를 이용한 전해질막 및 전해질페이스트/겔 |
| DE102007007879A1 (de) * | 2007-02-14 | 2008-08-21 | Gkss-Forschungszentrum Geesthacht Gmbh | Beschichtung eines Bauteils |
| WO2010063489A1 (de) * | 2008-12-06 | 2010-06-10 | Basf Se | Verfahren zur herstellung einer protonenleitenden membran |
| US9096725B2 (en) * | 2009-04-24 | 2015-08-04 | Samsung Electronics Co., Ltd. | Cross-linked polyazole, method of preparing the polyazole, electrode for fuel cell including the cross-linked polyazole, electrolyte membrane for fuel cell including the cross-linked polyazole, method of manufacturing the electrolyte membrane, and fuel cell including the cross-linked polyazole |
| KR20120047856A (ko) * | 2009-06-20 | 2012-05-14 | 바스프 에스이 | 고분자량 폴리아졸의 제조방법 |
| DE112010003228A5 (de) | 2009-07-16 | 2013-06-06 | Basf Se | Verfahren zum Betrieb einer Brennstoffzelle und zugehörige Brennstoffzelle |
| DE112010002924A5 (de) | 2009-07-16 | 2012-11-29 | Basf Se | Verfahren zum Betrieb einer Brennstoffzelle und zugehörige Brennstoffzelle |
| US20120225360A1 (en) | 2009-07-16 | 2012-09-06 | Basf Se | Method for operating a fuel cell |
| DK2804889T3 (da) * | 2012-01-17 | 2019-06-17 | Basf Se | Protonledende membran, fremgangsmåde til fremstilling heraf og anvendelse af denne i elektrokemiske celler |
| EP2804888B1 (en) * | 2012-01-17 | 2017-03-15 | Basf Se | Proton-conducting membrane, method for their production and their use in electrochemical cells |
| US9812725B2 (en) | 2012-01-17 | 2017-11-07 | Basf Se | Proton-conducting membrane and use thereof |
| US20130183603A1 (en) | 2012-01-17 | 2013-07-18 | Basf Se | Proton-conducting membrane, method for their production and their use in electrochemical cells |
| US9130208B2 (en) * | 2012-05-08 | 2015-09-08 | Basf Se | Membrane electrode assemblies and fuel cells with long lifetime |
| EP2847253A4 (en) * | 2012-05-08 | 2016-01-13 | Basf Se | ENHANCED MEMBRANE-ELECTRODE ASSEMBLIES AND LONG-TERM FUEL CELLS |
| CN103304817B (zh) * | 2013-05-14 | 2015-12-02 | 徐州斯尔克纤维科技股份有限公司 | 一种改性聚苯并二噁唑树脂聚合物的制备方法 |
| SG10202006578WA (en) * | 2016-04-20 | 2020-08-28 | Jsr Corp | Polymer, composition, molded article, cured product, and laminate |
| WO2020056268A2 (en) | 2018-09-14 | 2020-03-19 | University Of South Carolina | Low permeability polybenzimidazole (pbi) membranes for redox flow batteries |
| KR102877929B1 (ko) | 2018-09-14 | 2025-10-30 | 유니버시티 오브 싸우스 캐롤라이나 | 산화환원 유동 배터리용 폴리벤즈이미다졸 (pbi) 멤브레인 |
| JP7533963B2 (ja) * | 2018-09-14 | 2024-08-14 | ユニバーシティー オブ サウス カロライナ | 有機溶媒なしでpbiフィルムを製造するための新規な方法 |
| US11777124B2 (en) | 2020-03-06 | 2023-10-03 | University Of South Carolina | Proton-conducting PBI membrane processing with enhanced performance and durability |
Citations (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2002022412A1 (en) * | 2000-09-14 | 2002-03-21 | Nissan Motor Co., Ltd. | Brake pedal apparatus for vehicle |
| WO2002036249A1 (de) * | 2000-10-21 | 2002-05-10 | Celanese Ventures Gmbh | Neue membranen für den einsatz in brennstoffzellen mit einer verbesserten mechanik |
| WO2002071518A1 (de) * | 2001-03-01 | 2002-09-12 | Celanese Ventures Gmbh | Polymermembran, verfahren zu deren herstellung sowie deren verwendung |
| WO2002081547A1 (de) * | 2001-04-09 | 2002-10-17 | Celanese Ventures Gmbh | Protonenleitende membran und deren verwendung |
| WO2002088219A1 (de) * | 2001-04-09 | 2002-11-07 | Celanese Ventures Gmbh | Protonenleitende membran und deren verwendung |
Family Cites Families (80)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| NL257772A (ja) * | 1959-11-18 | |||
| NL268724A (ja) * | 1960-08-31 | |||
| GB1000525A (en) | 1962-07-20 | 1965-08-04 | Teijin Ltd | Process for preparation of polybenzimidazoles |
| JPS501707B1 (ja) * | 1969-12-20 | 1975-01-21 | ||
| US3783137A (en) * | 1971-06-09 | 1974-01-01 | Horizons Inc | Process for the preparation of heterocyclic polymers fromaromatic tetra-mines and derivatives of polycarboxylic acids |
| US3808305A (en) * | 1971-07-27 | 1974-04-30 | H Gregor | Crosslinked,interpolymer fixed-charge membranes |
| US4187333A (en) * | 1973-05-23 | 1980-02-05 | California Institute Of Technology | Ion-exchange hollow fibers |
| DE2450670A1 (de) * | 1974-10-25 | 1976-04-29 | Benckiser Gmbh Joh A | Verfahren zur abtrennung von citrat oder citronensaeure aus fermentationsloesungen |
| US4012303A (en) * | 1974-12-23 | 1977-03-15 | Hooker Chemicals & Plastics Corporation | Trifluorostyrene sulfonic acid membranes |
| JPS5397988A (en) | 1977-02-08 | 1978-08-26 | Toyo Soda Mfg Co Ltd | Production of cation exchange membrane |
| US4191618A (en) * | 1977-12-23 | 1980-03-04 | General Electric Company | Production of halogens in an electrolysis cell with catalytic electrodes bonded to an ion transporting membrane and an oxygen depolarized cathode |
| US4212714A (en) * | 1979-05-14 | 1980-07-15 | General Electric Company | Electrolysis of alkali metal halides in a three compartment cell with self-pressurized buffer compartment |
| US4333805A (en) * | 1980-05-02 | 1982-06-08 | General Electric Company | Halogen evolution with improved anode catalyst |
| FR2485395B1 (fr) * | 1980-06-24 | 1986-04-11 | Commissariat Energie Atomique | Membrane echangeuse de cations, son procede de fabrication et son application en tant qu'electrolyte solide |
| US4634530A (en) * | 1980-09-29 | 1987-01-06 | Celanese Corporation | Chemical modification of preformed polybenzimidazole semipermeable membrane |
| US4622276A (en) * | 1983-12-16 | 1986-11-11 | Stauffer Chemical Company | Fuel cell electrolyte |
| US4775864A (en) * | 1986-08-07 | 1988-10-04 | Standard Microsystems Corporation | Local area network with multiple node bus topology |
| US4775215A (en) | 1986-10-31 | 1988-10-04 | Hoechst Celanese Corporation | Nonlinear optical devices |
| US5098985A (en) * | 1988-10-11 | 1992-03-24 | The Dow Chemical Company | Copolymers containing polybenzoxazole, polybenzothiazole and polybenzimidazole moieties |
| US5218076A (en) * | 1989-08-31 | 1993-06-08 | The Dow Chemical Company | Branch polybenzazole polymer and method of preparation |
| US5091500A (en) | 1990-09-21 | 1992-02-25 | The Dow Chemical Company | Polybenzazole polymer containing perfluorocyclobutane rings |
| US5211984A (en) * | 1991-02-19 | 1993-05-18 | The Regents Of The University Of California | Membrane catalyst layer for fuel cells |
| KR960700297A (ko) * | 1993-01-15 | 1996-01-19 | 로날드 에이. 블리이커 | 이온 교환막 및 그 제조방법 |
| US5312895A (en) * | 1993-03-12 | 1994-05-17 | The United States Of America As Represented By The Secretary Of The Air Force | Benzobisazole copolymer system soluble in aprotic solvents |
| CN1041208C (zh) | 1993-04-28 | 1998-12-16 | 阿克佐诺贝尔公司 | 吡啶并双咪唑基刚性棒形聚合物 |
| CH691209A5 (de) * | 1993-09-06 | 2001-05-15 | Scherrer Inst Paul | Herstellungsverfahren für einen Polmerelektrolyten und elektrochemische Zelle mit diesem Polymerelektrolyten. |
| US5633337A (en) * | 1995-01-26 | 1997-05-27 | The United States Of America As Represented By The Secretary Of The Air Force | Aromatic benzobisazole polymers and copolymers incorporating diphenylamino moieties |
| US5492996A (en) * | 1995-02-21 | 1996-02-20 | The United States Of America As Represented By The Secretary Of The Air Force | Alcohol soluble benzazole polymers |
| US5599639A (en) * | 1995-08-31 | 1997-02-04 | Hoechst Celanese Corporation | Acid-modified polybenzimidazole fuel cell elements |
| DE19548421B4 (de) * | 1995-12-22 | 2004-06-03 | Celanese Ventures Gmbh | Verfahren zur kontinuierlichen Herstellung von Membranelektrodeneinheiten |
| DE19632285A1 (de) * | 1996-08-09 | 1998-02-19 | Hoechst Ag | Protonenleiter mit einer Temperaturbeständigkeit in einem weiten Bereich und guten Protonenleitfähigkeiten |
| DE19650478A1 (de) | 1996-12-05 | 1998-06-10 | Daimler Benz Ag | Lackiertes metallisches Substrat mit einer korrosionsschützenden Haftschicht auf Basis von Polysäuren und Verfahren zum Aufbringen der Haftschicht |
| DE19653484A1 (de) | 1996-12-20 | 1998-06-25 | Fraunhofer Ges Forschung | Verfahren zur Herstellung von Membran-Elektroden-Einheiten und eine so hergestellte Membran-Elektroden-Einheit |
| DE19727554A1 (de) * | 1997-06-28 | 1999-01-07 | Huels Chemische Werke Ag | Verfahren zur Hydrophilierung der Oberfläche polymerer Substrate mit einem Makroinitiator als Primer |
| EP0893165A3 (de) | 1997-06-28 | 2000-09-20 | Degussa-Hüls Aktiengesellschaft | Bioaktive Beschichtung von Oberflächen unter Verwendung von Makroinitiatoren |
| US6248469B1 (en) * | 1997-08-29 | 2001-06-19 | Foster-Miller, Inc. | Composite solid polymer electrolyte membranes |
| CA2300934C (en) | 1997-08-29 | 2008-08-26 | Foster-Miller, Inc. | Composite solid polymer electrolyte membranes |
| US6030718A (en) * | 1997-11-20 | 2000-02-29 | Avista Corporation | Proton exchange membrane fuel cell power system |
| US6110616A (en) * | 1998-01-30 | 2000-08-29 | Dais-Analytic Corporation | Ion-conducting membrane for fuel cell |
| US6124060A (en) * | 1998-05-20 | 2000-09-26 | Honda Giken Kogyo Kabushiki Kaisha | Solid polymer electrolytes |
| US6087032A (en) * | 1998-08-13 | 2000-07-11 | Asahi Glass Company Ltd. | Solid polymer electrolyte type fuel cell |
| FI107932B (fi) | 1999-02-16 | 2001-10-31 | Mikael Paronen | Polymeerikalvo ja menetelmä sen valmistamiseksi |
| US7344791B1 (en) | 1999-03-08 | 2008-03-18 | Toudai Tlo, Ltd. | Electrolytic membrane for fuel cell and its manufacturing method, and fuel cell and its manufacturing method |
| US6517962B1 (en) * | 1999-08-23 | 2003-02-11 | Ballard Power Systems Inc. | Fuel cell anode structures for voltage reversal tolerance |
| EP1110992B1 (en) | 1999-11-29 | 2006-08-02 | Kabushiki Kaisha Toyota Chuo Kenkyusho | Solid polymer electrolyte having high-durability |
| JP3656244B2 (ja) * | 1999-11-29 | 2005-06-08 | 株式会社豊田中央研究所 | 高耐久性固体高分子電解質及びその高耐久性固体高分子電解質を用いた電極−電解質接合体並びにその電極−電解質接合体を用いた電気化学デバイス |
| DE60018533T2 (de) * | 1999-12-06 | 2006-04-13 | Toyo Boseki K.K. | Polybenzazol und Fasern daraus |
| EP1245056A1 (en) | 1999-12-16 | 2002-10-02 | Proton Energy Systems, Inc. | Low gravity electrochemical cell |
| GB0006428D0 (en) * | 2000-03-17 | 2000-05-03 | Johnson Matthey Plc | Electrochemical cell |
| GB0006429D0 (en) * | 2000-03-17 | 2000-05-03 | Johnson Matthey Plc | Electrochemical cell |
| DE10024576A1 (de) | 2000-05-19 | 2001-11-22 | Univ Stuttgart | Kovalent und ionisch vernetzte Polymere und Polymermembranen |
| CN1439032A (zh) | 2000-06-02 | 2003-08-27 | Sri国际公司 | 聚合物组合物 |
| JP3690589B2 (ja) * | 2000-11-13 | 2005-08-31 | 東洋紡績株式会社 | スルホン酸含有ポリイミダゾール化合物およびその成型物 |
| WO2002038650A1 (en) | 2000-11-13 | 2002-05-16 | Toyo Boseki Kabusiki Kaisha | Polybenzazole compound having sulfo group and/or phosphono group, resin composition containing the same, molded resin, solid polymer electrolyte film, solid electrolyte film/electrode catalyst layer composite, and process for producing the composite |
| JP2002146014A (ja) | 2000-11-15 | 2002-05-22 | Toyobo Co Ltd | イオン伝導性ホスホン酸含有ポリアゾール |
| JP2002146016A (ja) | 2000-11-15 | 2002-05-22 | Toyobo Co Ltd | イオン伝導性ホスホン酸含有ポリアゾール |
| DE10129458A1 (de) | 2001-06-19 | 2003-01-02 | Celanese Ventures Gmbh | Verbesserte Polymerfolien auf Basis von Polyazolen |
| JP2003022709A (ja) | 2001-07-09 | 2003-01-24 | Toyobo Co Ltd | ブレンドポリマー電解質、該電解質を主成分とする電解質膜、及び該電解質を用いた膜/電極接合体 |
| DE10133738A1 (de) | 2001-07-11 | 2003-02-06 | Joerg Mueller | Verfahren zur Herstellung einer plasmapolymerisierten Polymer-Elektrolytmembran |
| DE10144815A1 (de) * | 2001-09-12 | 2003-03-27 | Celanese Ventures Gmbh | Protonenleitende Membran und deren Verwendung |
| DE10148131B4 (de) * | 2001-09-28 | 2010-07-01 | Gkss-Forschungszentrum Geesthacht Gmbh | Verfahren zur Herstellung eines Polymers, Polymer und protonenleitfähige Membran für elektrochemische Anwendungen |
| JP4093408B2 (ja) * | 2001-10-20 | 2008-06-04 | ビーエーエスエフ・フュエル・セル・ゲーエムベーハー | 改良された機械的性質をもつ、燃料電池に使用するための新規な膜 |
| DE10209419A1 (de) * | 2002-03-05 | 2003-09-25 | Celanese Ventures Gmbh | Verfahren zur Herstellung einer Polymerelektrolytmembran und deren Anwendung in Brennstoffzellen |
| DE10213540A1 (de) * | 2002-03-06 | 2004-02-19 | Celanese Ventures Gmbh | Lösung aus Vinylphosphonsäure, Verfahren zur Herstellung einer Polymerelektrolytmembran aus Polyvinylphosphaonsäure und deren Anwendung in Brennstoffzellen |
| US7846982B2 (en) * | 2002-03-06 | 2010-12-07 | Pemeas Gmbh | Proton conducting electrolyte membrane having reduced methanol permeability and the use thereof in fuel cells |
| US20050118478A1 (en) * | 2002-03-06 | 2005-06-02 | Joachim Kiefer | Mixture comprising sulphonic acid containing vinyl, polymer electrolyte membrane comprising polyvinylsulphonic acid and the use thereof in fuel cells |
| CA2483015A1 (en) * | 2002-04-25 | 2003-11-06 | Pemeas Gmbh | Multilayer electrolyte membrane |
| DE10220817A1 (de) * | 2002-05-10 | 2003-11-27 | Celanese Ventures Gmbh | Verfahren zur Herstellung einer gepfropften Polymerelektrolytmembran und deren Anwendung in Brennstoffzellen |
| DE10220818A1 (de) * | 2002-05-10 | 2003-11-20 | Celanese Ventures Gmbh | Verfahren zur Herstellung einer gepfropften Polymerelektrolytmembran und deren Anwendung in Brennstoffzellen |
| DE10228657A1 (de) | 2002-06-27 | 2004-01-15 | Celanese Ventures Gmbh | Protonenleitende Membran und deren Verwendung |
| WO2004015803A1 (de) * | 2002-08-02 | 2004-02-19 | Pemeas Gmbh | Protonenleitende polymembran umfassend sulfonsäuregruppen enthaltende polymere und deren anwendung in brennstoffzellen |
| WO2004024796A1 (de) * | 2002-08-29 | 2004-03-25 | Pemeas Gmbh | Verfahren zur herstellung von protonenleitenden polymermembranen, verbesserte polymermembranen und deren anwendung in brennstoffzellen |
| DE10239701A1 (de) | 2002-08-29 | 2004-03-11 | Celanese Ventures Gmbh | Polymerfolie auf Basis von Polyazolen und deren Verwendung |
| DE10242708A1 (de) | 2002-09-13 | 2004-05-19 | Celanese Ventures Gmbh | Protonenleitende Membranen und deren Verwendung |
| US6860905B2 (en) | 2002-10-01 | 2005-03-01 | Peach State Labs, Inc. | Anionic phthalic acid ester compounds and stain resistant compositions |
| DE10246373A1 (de) * | 2002-10-04 | 2004-04-15 | Celanese Ventures Gmbh | Protonenleitende Polymermembran umfassend Sulfonsäuregruppen enthaltende Polyazole und deren Anwendung in Brennstoffzellen |
| DE10246459A1 (de) * | 2002-10-04 | 2004-04-15 | Celanese Ventures Gmbh | Protonenleitende Polymermembran umfassend Phosphonsäuregruppen enthaltende Polyazole und deren Anwendung in Brennstoffzellen |
| US7834131B2 (en) * | 2003-07-11 | 2010-11-16 | Basf Fuel Cell Gmbh | Asymmetric polymer film, method for the production and utilization thereof |
| JP4875489B2 (ja) * | 2003-07-27 | 2012-02-15 | ベーアーエスエフ フューエル セル ゲーエムベーハー | プロトン伝導性膜およびその使用 |
| US20080038624A1 (en) * | 2003-09-04 | 2008-02-14 | Jorg Belack | Proton-conducting polymer membrane coated with a catalyst layer, said polymer membrane comprising phosphonic acid polymers, membrane/electrode unit and use thereof in fuel cells |
-
2004
- 2004-07-23 JP JP2006521484A patent/JP4875489B2/ja not_active Expired - Fee Related
- 2004-07-23 EP EP04763421A patent/EP1652259A2/de not_active Withdrawn
- 2004-07-23 WO PCT/EP2004/008229 patent/WO2005011039A2/de not_active Ceased
- 2004-07-23 US US10/566,135 patent/US7820314B2/en not_active Expired - Fee Related
-
2010
- 2010-10-19 US US12/907,368 patent/US8323810B2/en not_active Expired - Fee Related
-
2011
- 2011-09-21 JP JP2011206354A patent/JP5468051B2/ja not_active Expired - Fee Related
Patent Citations (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2002022412A1 (en) * | 2000-09-14 | 2002-03-21 | Nissan Motor Co., Ltd. | Brake pedal apparatus for vehicle |
| WO2002036249A1 (de) * | 2000-10-21 | 2002-05-10 | Celanese Ventures Gmbh | Neue membranen für den einsatz in brennstoffzellen mit einer verbesserten mechanik |
| WO2002071518A1 (de) * | 2001-03-01 | 2002-09-12 | Celanese Ventures Gmbh | Polymermembran, verfahren zu deren herstellung sowie deren verwendung |
| WO2002081547A1 (de) * | 2001-04-09 | 2002-10-17 | Celanese Ventures Gmbh | Protonenleitende membran und deren verwendung |
| WO2002088219A1 (de) * | 2001-04-09 | 2002-11-07 | Celanese Ventures Gmbh | Protonenleitende membran und deren verwendung |
Also Published As
| Publication number | Publication date |
|---|---|
| US8323810B2 (en) | 2012-12-04 |
| US20110033777A1 (en) | 2011-02-10 |
| WO2005011039A3 (de) | 2005-03-03 |
| JP2007500416A (ja) | 2007-01-11 |
| US7820314B2 (en) | 2010-10-26 |
| WO2005011039A2 (de) | 2005-02-03 |
| JP2012046754A (ja) | 2012-03-08 |
| EP1652259A2 (de) | 2006-05-03 |
| US20060210881A1 (en) | 2006-09-21 |
| JP5468051B2 (ja) | 2014-04-09 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP4875489B2 (ja) | プロトン伝導性膜およびその使用 | |
| US7384552B2 (en) | Proton-conducting membrane and the use thereof | |
| US7235320B2 (en) | Proton-conducting membrane and use thereof | |
| JP5226751B2 (ja) | プロトン伝導性膜およびその使用 | |
| JP4537199B2 (ja) | プロトン伝導性膜およびその使用 | |
| JP2006502266A (ja) | ポリアゾールブレンドを含むプロトン伝導性高分子膜および燃料電池におけるその使用 | |
| JP2011080075A (ja) | スルホン酸基含有ポリアゾールを含むプロトン伝導性高分子膜及び燃料電池におけるその使用。 | |
| JP2006501991A (ja) | ホスホン酸基含有ポリアゾールを含むプロトン伝導性高分子膜及び燃料電池におけるその使用。 | |
| EP2804889A1 (en) | Proton-conducting membrane, method for their production and their use in electrochemical cells | |
| JP5279771B2 (ja) | プロトン伝導性ポリマー膜 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20070601 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20101130 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20110225 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A821 Effective date: 20110225 |
|
| A02 | Decision of refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A02 Effective date: 20110524 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20110921 |
|
| A911 | Transfer to examiner for re-examination before appeal (zenchi) |
Free format text: JAPANESE INTERMEDIATE CODE: A911 Effective date: 20110928 |
|
| TRDD | Decision of grant or rejection written | ||
| A01 | Written decision to grant a patent or to grant a registration (utility model) |
Free format text: JAPANESE INTERMEDIATE CODE: A01 Effective date: 20111101 |
|
| A01 | Written decision to grant a patent or to grant a registration (utility model) |
Free format text: JAPANESE INTERMEDIATE CODE: A01 |
|
| A61 | First payment of annual fees (during grant procedure) |
Free format text: JAPANESE INTERMEDIATE CODE: A61 Effective date: 20111125 |
|
| FPAY | Renewal fee payment (event date is renewal date of database) |
Free format text: PAYMENT UNTIL: 20141202 Year of fee payment: 3 |
|
| R150 | Certificate of patent or registration of utility model |
Free format text: JAPANESE INTERMEDIATE CODE: R150 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| LAPS | Cancellation because of no payment of annual fees |