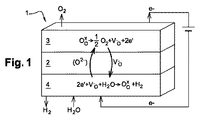
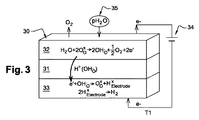
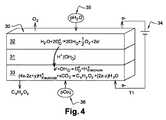
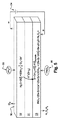
JP2014532119A - 水蒸気の電気分解によって水素と酸素を発生させる方法 - Google Patents
水蒸気の電気分解によって水素と酸素を発生させる方法 Download PDFInfo
- Publication number
- JP2014532119A JP2014532119A JP2014535087A JP2014535087A JP2014532119A JP 2014532119 A JP2014532119 A JP 2014532119A JP 2014535087 A JP2014535087 A JP 2014535087A JP 2014535087 A JP2014535087 A JP 2014535087A JP 2014532119 A JP2014532119 A JP 2014532119A
- Authority
- JP
- Japan
- Prior art keywords
- cathode
- anode
- current
- compound
- proton conducting
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Pending
Links
- 238000000034 method Methods 0.000 title claims abstract description 55
- 239000001257 hydrogen Substances 0.000 title claims abstract description 38
- 229910052739 hydrogen Inorganic materials 0.000 title claims abstract description 38
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Chemical compound O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 title claims abstract description 36
- UFHFLCQGNIYNRP-UHFFFAOYSA-N Hydrogen Chemical compound [H][H] UFHFLCQGNIYNRP-UHFFFAOYSA-N 0.000 title claims abstract description 28
- 238000005868 electrolysis reaction Methods 0.000 title claims abstract description 28
- QVGXLLKOCUKJST-UHFFFAOYSA-N atomic oxygen Chemical compound [O] QVGXLLKOCUKJST-UHFFFAOYSA-N 0.000 title claims abstract description 23
- 239000001301 oxygen Substances 0.000 title claims abstract description 23
- 229910052760 oxygen Inorganic materials 0.000 title claims abstract description 23
- 239000000919 ceramic Substances 0.000 claims abstract description 39
- 125000004435 hydrogen atom Chemical group [H]* 0.000 claims abstract description 19
- 238000007254 oxidation reaction Methods 0.000 claims abstract description 19
- 230000003647 oxidation Effects 0.000 claims abstract description 17
- 239000007784 solid electrolyte Substances 0.000 claims abstract description 12
- 239000003463 adsorbent Substances 0.000 claims abstract description 8
- 150000001875 compounds Chemical class 0.000 claims description 33
- 229910002091 carbon monoxide Inorganic materials 0.000 claims description 30
- UGFAIRIUMAVXCW-UHFFFAOYSA-N Carbon monoxide Chemical compound [O+]#[C-] UGFAIRIUMAVXCW-UHFFFAOYSA-N 0.000 claims description 29
- CURLTUGMZLYLDI-UHFFFAOYSA-N Carbon dioxide Chemical compound O=C=O CURLTUGMZLYLDI-UHFFFAOYSA-N 0.000 claims description 16
- IJGRMHOSHXDMSA-UHFFFAOYSA-N Atomic nitrogen Chemical compound N#N IJGRMHOSHXDMSA-UHFFFAOYSA-N 0.000 claims description 14
- 230000015572 biosynthetic process Effects 0.000 claims description 12
- 230000009467 reduction Effects 0.000 claims description 11
- 239000011195 cermet Substances 0.000 claims description 10
- 229910002092 carbon dioxide Inorganic materials 0.000 claims description 8
- 239000001569 carbon dioxide Substances 0.000 claims description 8
- 229910052747 lanthanoid Inorganic materials 0.000 claims description 8
- 150000002602 lanthanoids Chemical class 0.000 claims description 8
- 230000036961 partial effect Effects 0.000 claims description 8
- 229910052757 nitrogen Inorganic materials 0.000 claims description 7
- -1 nitrogen-containing compound Chemical class 0.000 claims description 6
- VYZAMTAEIAYCRO-UHFFFAOYSA-N Chromium Chemical compound [Cr] VYZAMTAEIAYCRO-UHFFFAOYSA-N 0.000 claims description 5
- 229910052804 chromium Inorganic materials 0.000 claims description 5
- 239000011651 chromium Substances 0.000 claims description 5
- 239000004020 conductor Substances 0.000 claims description 5
- 238000004517 catalytic hydrocracking Methods 0.000 claims description 4
- 230000001939 inductive effect Effects 0.000 claims description 4
- 239000000463 material Substances 0.000 claims description 4
- 239000000203 mixture Substances 0.000 claims description 4
- 150000001491 aromatic compounds Chemical class 0.000 claims description 3
- 238000002844 melting Methods 0.000 claims description 3
- 230000008018 melting Effects 0.000 claims description 3
- 229910052799 carbon Inorganic materials 0.000 claims description 2
- 230000001590 oxidative effect Effects 0.000 claims description 2
- 239000003792 electrolyte Substances 0.000 abstract description 23
- 239000002156 adsorbate Substances 0.000 abstract description 4
- QGZKDVFQNNGYKY-UHFFFAOYSA-N Ammonia Chemical compound N QGZKDVFQNNGYKY-UHFFFAOYSA-N 0.000 description 22
- 239000007789 gas Substances 0.000 description 21
- 239000012528 membrane Substances 0.000 description 15
- 238000004519 manufacturing process Methods 0.000 description 13
- 229910021529 ammonia Inorganic materials 0.000 description 11
- 238000006243 chemical reaction Methods 0.000 description 11
- 150000002431 hydrogen Chemical class 0.000 description 10
- 238000005984 hydrogenation reaction Methods 0.000 description 9
- 238000006722 reduction reaction Methods 0.000 description 9
- 230000032798 delamination Effects 0.000 description 8
- 238000005755 formation reaction Methods 0.000 description 8
- 150000002500 ions Chemical class 0.000 description 8
- 238000005245 sintering Methods 0.000 description 8
- 239000000243 solution Substances 0.000 description 8
- 229910045601 alloy Inorganic materials 0.000 description 6
- 239000000956 alloy Substances 0.000 description 6
- 229910052758 niobium Inorganic materials 0.000 description 6
- 239000010955 niobium Substances 0.000 description 6
- GUCVJGMIXFAOAE-UHFFFAOYSA-N niobium atom Chemical compound [Nb] GUCVJGMIXFAOAE-UHFFFAOYSA-N 0.000 description 6
- 230000002829 reductive effect Effects 0.000 description 6
- 229910052715 tantalum Inorganic materials 0.000 description 5
- GUVRBAGPIYLISA-UHFFFAOYSA-N tantalum atom Chemical compound [Ta] GUVRBAGPIYLISA-UHFFFAOYSA-N 0.000 description 5
- 229930195733 hydrocarbon Natural products 0.000 description 4
- 150000002430 hydrocarbons Chemical class 0.000 description 4
- 238000003786 synthesis reaction Methods 0.000 description 4
- 229910000599 Cr alloy Inorganic materials 0.000 description 3
- OAICVXFJPJFONN-UHFFFAOYSA-N Phosphorus Chemical compound [P] OAICVXFJPJFONN-UHFFFAOYSA-N 0.000 description 3
- 150000001450 anions Chemical class 0.000 description 3
- 229910052787 antimony Inorganic materials 0.000 description 3
- WATWJIUSRGPENY-UHFFFAOYSA-N antimony atom Chemical compound [Sb] WATWJIUSRGPENY-UHFFFAOYSA-N 0.000 description 3
- 229910052785 arsenic Inorganic materials 0.000 description 3
- RQNWIZPPADIBDY-UHFFFAOYSA-N arsenic atom Chemical compound [As] RQNWIZPPADIBDY-UHFFFAOYSA-N 0.000 description 3
- 229910052797 bismuth Inorganic materials 0.000 description 3
- JCXGWMGPZLAOME-UHFFFAOYSA-N bismuth atom Chemical compound [Bi] JCXGWMGPZLAOME-UHFFFAOYSA-N 0.000 description 3
- 239000003054 catalyst Substances 0.000 description 3
- 238000009903 catalytic hydrogenation reaction Methods 0.000 description 3
- 238000010531 catalytic reduction reaction Methods 0.000 description 3
- 238000002347 injection Methods 0.000 description 3
- 239000007924 injection Substances 0.000 description 3
- 229910052751 metal Inorganic materials 0.000 description 3
- 239000002184 metal Substances 0.000 description 3
- MWUXSHHQAYIFBG-UHFFFAOYSA-N nitrogen oxide Inorganic materials O=[N] MWUXSHHQAYIFBG-UHFFFAOYSA-N 0.000 description 3
- 229910052698 phosphorus Inorganic materials 0.000 description 3
- 239000011574 phosphorus Substances 0.000 description 3
- 239000011148 porous material Substances 0.000 description 3
- 230000008569 process Effects 0.000 description 3
- 239000000047 product Substances 0.000 description 3
- 239000012078 proton-conducting electrolyte Substances 0.000 description 3
- 241000894007 species Species 0.000 description 3
- 238000000629 steam reforming Methods 0.000 description 3
- 229910052720 vanadium Inorganic materials 0.000 description 3
- GPPXJZIENCGNKB-UHFFFAOYSA-N vanadium Chemical compound [V]#[V] GPPXJZIENCGNKB-UHFFFAOYSA-N 0.000 description 3
- XKRFYHLGVUSROY-UHFFFAOYSA-N Argon Chemical compound [Ar] XKRFYHLGVUSROY-UHFFFAOYSA-N 0.000 description 2
- 229910052691 Erbium Inorganic materials 0.000 description 2
- XEEYBQQBJWHFJM-UHFFFAOYSA-N Iron Chemical compound [Fe] XEEYBQQBJWHFJM-UHFFFAOYSA-N 0.000 description 2
- PXHVJJICTQNCMI-UHFFFAOYSA-N Nickel Chemical compound [Ni] PXHVJJICTQNCMI-UHFFFAOYSA-N 0.000 description 2
- QAOWNCQODCNURD-UHFFFAOYSA-N Sulfuric acid Chemical compound OS(O)(=O)=O QAOWNCQODCNURD-UHFFFAOYSA-N 0.000 description 2
- RAHZWNYVWXNFOC-UHFFFAOYSA-N Sulphur dioxide Chemical compound O=S=O RAHZWNYVWXNFOC-UHFFFAOYSA-N 0.000 description 2
- 150000001335 aliphatic alkanes Chemical class 0.000 description 2
- 210000000621 bronchi Anatomy 0.000 description 2
- 150000001722 carbon compounds Chemical class 0.000 description 2
- 238000012512 characterization method Methods 0.000 description 2
- 239000002800 charge carrier Substances 0.000 description 2
- 239000000788 chromium alloy Substances 0.000 description 2
- 238000002485 combustion reaction Methods 0.000 description 2
- 150000001924 cycloalkanes Chemical class 0.000 description 2
- 230000000694 effects Effects 0.000 description 2
- 238000003487 electrochemical reaction Methods 0.000 description 2
- UYAHIZSMUZPPFV-UHFFFAOYSA-N erbium Chemical compound [Er] UYAHIZSMUZPPFV-UHFFFAOYSA-N 0.000 description 2
- 239000002803 fossil fuel Substances 0.000 description 2
- 238000009776 industrial production Methods 0.000 description 2
- 239000003209 petroleum derivative Substances 0.000 description 2
- 238000011160 research Methods 0.000 description 2
- 229920006395 saturated elastomer Polymers 0.000 description 2
- 230000009919 sequestration Effects 0.000 description 2
- 229910052723 transition metal Inorganic materials 0.000 description 2
- 150000003624 transition metals Chemical class 0.000 description 2
- 238000013519 translation Methods 0.000 description 2
- OKTJSMMVPCPJKN-UHFFFAOYSA-N Carbon Chemical compound [C] OKTJSMMVPCPJKN-UHFFFAOYSA-N 0.000 description 1
- YZCKVEUIGOORGS-UHFFFAOYSA-N Hydrogen atom Chemical compound [H] YZCKVEUIGOORGS-UHFFFAOYSA-N 0.000 description 1
- ZOKXTWBITQBERF-UHFFFAOYSA-N Molybdenum Chemical compound [Mo] ZOKXTWBITQBERF-UHFFFAOYSA-N 0.000 description 1
- RTAQQCXQSZGOHL-UHFFFAOYSA-N Titanium Chemical compound [Ti] RTAQQCXQSZGOHL-UHFFFAOYSA-N 0.000 description 1
- 238000010521 absorption reaction Methods 0.000 description 1
- 230000002411 adverse Effects 0.000 description 1
- 229910052786 argon Inorganic materials 0.000 description 1
- 208000006673 asthma Diseases 0.000 description 1
- 230000033228 biological regulation Effects 0.000 description 1
- 210000003123 bronchiole Anatomy 0.000 description 1
- 239000006227 byproduct Substances 0.000 description 1
- 239000000969 carrier Substances 0.000 description 1
- 238000006555 catalytic reaction Methods 0.000 description 1
- 229910002110 ceramic alloy Inorganic materials 0.000 description 1
- 229910010293 ceramic material Inorganic materials 0.000 description 1
- 229910017052 cobalt Inorganic materials 0.000 description 1
- 239000010941 cobalt Substances 0.000 description 1
- GUTLYIVDDKVIGB-UHFFFAOYSA-N cobalt atom Chemical compound [Co] GUTLYIVDDKVIGB-UHFFFAOYSA-N 0.000 description 1
- 238000007796 conventional method Methods 0.000 description 1
- 230000009849 deactivation Effects 0.000 description 1
- 230000007423 decrease Effects 0.000 description 1
- 230000001419 dependent effect Effects 0.000 description 1
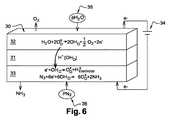
- 238000010586 diagram Methods 0.000 description 1
- 239000002283 diesel fuel Substances 0.000 description 1
- 239000011532 electronic conductor Substances 0.000 description 1
- 238000010304 firing Methods 0.000 description 1
- 239000005431 greenhouse gas Substances 0.000 description 1
- 230000035874 hyperreactivity Effects 0.000 description 1
- 238000010348 incorporation Methods 0.000 description 1
- 230000003993 interaction Effects 0.000 description 1
- 239000010416 ion conductor Substances 0.000 description 1
- 229910052742 iron Inorganic materials 0.000 description 1
- 239000003350 kerosene Substances 0.000 description 1
- 239000010410 layer Substances 0.000 description 1
- 230000007774 longterm Effects 0.000 description 1
- 229910001092 metal group alloy Inorganic materials 0.000 description 1
- 229910052750 molybdenum Inorganic materials 0.000 description 1
- 239000011733 molybdenum Substances 0.000 description 1
- 229910052759 nickel Inorganic materials 0.000 description 1
- 125000004430 oxygen atom Chemical group O* 0.000 description 1
- 244000052769 pathogen Species 0.000 description 1
- 238000012545 processing Methods 0.000 description 1
- 239000011241 protective layer Substances 0.000 description 1
- LVTJOONKWUXEFR-FZRMHRINSA-N protoneodioscin Natural products O(C[C@@H](CC[C@]1(O)[C@H](C)[C@@H]2[C@]3(C)[C@H]([C@H]4[C@@H]([C@]5(C)C(=CC4)C[C@@H](O[C@@H]4[C@H](O[C@H]6[C@@H](O)[C@@H](O)[C@@H](O)[C@H](C)O6)[C@@H](O)[C@H](O[C@H]6[C@@H](O)[C@@H](O)[C@@H](O)[C@H](C)O6)[C@H](CO)O4)CC5)CC3)C[C@@H]2O1)C)[C@H]1[C@H](O)[C@H](O)[C@H](O)[C@@H](CO)O1 LVTJOONKWUXEFR-FZRMHRINSA-N 0.000 description 1
- 239000000376 reactant Substances 0.000 description 1
- 230000000241 respiratory effect Effects 0.000 description 1
- 230000000717 retained effect Effects 0.000 description 1
- 238000000926 separation method Methods 0.000 description 1
- 239000007787 solid Substances 0.000 description 1
- 238000001179 sorption measurement Methods 0.000 description 1
- 238000003860 storage Methods 0.000 description 1
- 239000000126 substance Substances 0.000 description 1
- 238000001308 synthesis method Methods 0.000 description 1
- 229910052719 titanium Inorganic materials 0.000 description 1
- 239000010936 titanium Substances 0.000 description 1
- WFKWXMTUELFFGS-UHFFFAOYSA-N tungsten Chemical compound [W] WFKWXMTUELFFGS-UHFFFAOYSA-N 0.000 description 1
- 229910052721 tungsten Inorganic materials 0.000 description 1
- 239000010937 tungsten Substances 0.000 description 1
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C25—ELECTROLYTIC OR ELECTROPHORETIC PROCESSES; APPARATUS THEREFOR
- C25B—ELECTROLYTIC OR ELECTROPHORETIC PROCESSES FOR THE PRODUCTION OF COMPOUNDS OR NON-METALS; APPARATUS THEREFOR
- C25B1/00—Electrolytic production of inorganic compounds or non-metals
- C25B1/01—Products
- C25B1/02—Hydrogen or oxygen
- C25B1/04—Hydrogen or oxygen by electrolysis of water
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01D—SEPARATION
- B01D53/00—Separation of gases or vapours; Recovering vapours of volatile solvents from gases; Chemical or biological purification of waste gases, e.g. engine exhaust gases, smoke, fumes, flue gases, aerosols
- B01D53/32—Separation of gases or vapours; Recovering vapours of volatile solvents from gases; Chemical or biological purification of waste gases, e.g. engine exhaust gases, smoke, fumes, flue gases, aerosols by electrical effects other than those provided for in group B01D61/00
- B01D53/326—Separation of gases or vapours; Recovering vapours of volatile solvents from gases; Chemical or biological purification of waste gases, e.g. engine exhaust gases, smoke, fumes, flue gases, aerosols by electrical effects other than those provided for in group B01D61/00 in electrochemical cells
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10G—CRACKING HYDROCARBON OILS; PRODUCTION OF LIQUID HYDROCARBON MIXTURES, e.g. BY DESTRUCTIVE HYDROGENATION, OLIGOMERISATION, POLYMERISATION; RECOVERY OF HYDROCARBON OILS FROM OIL-SHALE, OIL-SAND, OR GASES; REFINING MIXTURES MAINLY CONSISTING OF HYDROCARBONS; REFORMING OF NAPHTHA; MINERAL WAXES
- C10G45/00—Refining of hydrocarbon oils using hydrogen or hydrogen-generating compounds
- C10G45/44—Hydrogenation of the aromatic hydrocarbons
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10G—CRACKING HYDROCARBON OILS; PRODUCTION OF LIQUID HYDROCARBON MIXTURES, e.g. BY DESTRUCTIVE HYDROGENATION, OLIGOMERISATION, POLYMERISATION; RECOVERY OF HYDROCARBON OILS FROM OIL-SHALE, OIL-SAND, OR GASES; REFINING MIXTURES MAINLY CONSISTING OF HYDROCARBONS; REFORMING OF NAPHTHA; MINERAL WAXES
- C10G47/00—Cracking of hydrocarbon oils, in the presence of hydrogen or hydrogen- generating compounds, to obtain lower boiling fractions
- C10G47/22—Non-catalytic cracking in the presence of hydrogen
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10G—CRACKING HYDROCARBON OILS; PRODUCTION OF LIQUID HYDROCARBON MIXTURES, e.g. BY DESTRUCTIVE HYDROGENATION, OLIGOMERISATION, POLYMERISATION; RECOVERY OF HYDROCARBON OILS FROM OIL-SHALE, OIL-SAND, OR GASES; REFINING MIXTURES MAINLY CONSISTING OF HYDROCARBONS; REFORMING OF NAPHTHA; MINERAL WAXES
- C10G49/00—Treatment of hydrocarbon oils, in the presence of hydrogen or hydrogen-generating compounds, not provided for in a single one of groups C10G45/02, C10G45/32, C10G45/44, C10G45/58 or C10G47/00
- C10G49/007—Treatment of hydrocarbon oils, in the presence of hydrogen or hydrogen-generating compounds, not provided for in a single one of groups C10G45/02, C10G45/32, C10G45/44, C10G45/58 or C10G47/00 in the presence of hydrogen from a special source or of a special composition or having been purified by a special treatment
-
- C—CHEMISTRY; METALLURGY
- C25—ELECTROLYTIC OR ELECTROPHORETIC PROCESSES; APPARATUS THEREFOR
- C25B—ELECTROLYTIC OR ELECTROPHORETIC PROCESSES FOR THE PRODUCTION OF COMPOUNDS OR NON-METALS; APPARATUS THEREFOR
- C25B11/00—Electrodes; Manufacture thereof not otherwise provided for
- C25B11/02—Electrodes; Manufacture thereof not otherwise provided for characterised by shape or form
- C25B11/03—Electrodes; Manufacture thereof not otherwise provided for characterised by shape or form perforated or foraminous
- C25B11/031—Porous electrodes
-
- C—CHEMISTRY; METALLURGY
- C25—ELECTROLYTIC OR ELECTROPHORETIC PROCESSES; APPARATUS THEREFOR
- C25B—ELECTROLYTIC OR ELECTROPHORETIC PROCESSES FOR THE PRODUCTION OF COMPOUNDS OR NON-METALS; APPARATUS THEREFOR
- C25B11/00—Electrodes; Manufacture thereof not otherwise provided for
- C25B11/04—Electrodes; Manufacture thereof not otherwise provided for characterised by the material
-
- C—CHEMISTRY; METALLURGY
- C25—ELECTROLYTIC OR ELECTROPHORETIC PROCESSES; APPARATUS THEREFOR
- C25B—ELECTROLYTIC OR ELECTROPHORETIC PROCESSES FOR THE PRODUCTION OF COMPOUNDS OR NON-METALS; APPARATUS THEREFOR
- C25B15/00—Operating or servicing cells
- C25B15/08—Supplying or removing reactants or electrolytes; Regeneration of electrolytes
-
- C—CHEMISTRY; METALLURGY
- C25—ELECTROLYTIC OR ELECTROPHORETIC PROCESSES; APPARATUS THEREFOR
- C25B—ELECTROLYTIC OR ELECTROPHORETIC PROCESSES FOR THE PRODUCTION OF COMPOUNDS OR NON-METALS; APPARATUS THEREFOR
- C25B3/00—Electrolytic production of organic compounds
- C25B3/20—Processes
- C25B3/25—Reduction
-
- C—CHEMISTRY; METALLURGY
- C25—ELECTROLYTIC OR ELECTROPHORETIC PROCESSES; APPARATUS THEREFOR
- C25B—ELECTROLYTIC OR ELECTROPHORETIC PROCESSES FOR THE PRODUCTION OF COMPOUNDS OR NON-METALS; APPARATUS THEREFOR
- C25B9/00—Cells or assemblies of cells; Constructional parts of cells; Assemblies of constructional parts, e.g. electrode-diaphragm assemblies; Process-related cell features
- C25B9/17—Cells comprising dimensionally-stable non-movable electrodes; Assemblies of constructional parts thereof
- C25B9/19—Cells comprising dimensionally-stable non-movable electrodes; Assemblies of constructional parts thereof with diaphragms
- C25B9/23—Cells comprising dimensionally-stable non-movable electrodes; Assemblies of constructional parts thereof with diaphragms comprising ion-exchange membranes in or on which electrode material is embedded
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01D—SEPARATION
- B01D2257/00—Components to be removed
- B01D2257/30—Sulfur compounds
- B01D2257/302—Sulfur oxides
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01D—SEPARATION
- B01D2257/00—Components to be removed
- B01D2257/40—Nitrogen compounds
- B01D2257/404—Nitrogen oxides other than dinitrogen oxide
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01D—SEPARATION
- B01D2257/00—Components to be removed
- B01D2257/50—Carbon oxides
- B01D2257/502—Carbon monoxide
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01D—SEPARATION
- B01D2257/00—Components to be removed
- B01D2257/50—Carbon oxides
- B01D2257/504—Carbon dioxide
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y02—TECHNOLOGIES OR APPLICATIONS FOR MITIGATION OR ADAPTATION AGAINST CLIMATE CHANGE
- Y02E—REDUCTION OF GREENHOUSE GAS [GHG] EMISSIONS, RELATED TO ENERGY GENERATION, TRANSMISSION OR DISTRIBUTION
- Y02E60/00—Enabling technologies; Technologies with a potential or indirect contribution to GHG emissions mitigation
- Y02E60/30—Hydrogen technology
- Y02E60/36—Hydrogen production from non-carbon containing sources, e.g. by water electrolysis
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y02—TECHNOLOGIES OR APPLICATIONS FOR MITIGATION OR ADAPTATION AGAINST CLIMATE CHANGE
- Y02P—CLIMATE CHANGE MITIGATION TECHNOLOGIES IN THE PRODUCTION OR PROCESSING OF GOODS
- Y02P20/00—Technologies relating to chemical industry
- Y02P20/151—Reduction of greenhouse gas [GHG] emissions, e.g. CO2
Landscapes
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Engineering & Computer Science (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Electrochemistry (AREA)
- Oil, Petroleum & Natural Gas (AREA)
- Metallurgy (AREA)
- Materials Engineering (AREA)
- General Chemical & Material Sciences (AREA)
- Inorganic Chemistry (AREA)
- Analytical Chemistry (AREA)
- Electrolytic Production Of Non-Metals, Compounds, Apparatuses Therefor (AREA)
- Electrodes For Compound Or Non-Metal Manufacture (AREA)
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| FR1159221A FR2981368B1 (fr) | 2011-10-12 | 2011-10-12 | Procede de generation d'hydrogene et d'oxygene par electrolyse de vapeur d'eau |
| FR1159221 | 2011-10-12 | ||
| PCT/EP2012/070214 WO2013053858A1 (fr) | 2011-10-12 | 2012-10-11 | Procédé de génération d'hydrogène et d'oxygène par électrolyse de vapeur d'eau |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| JP2014532119A true JP2014532119A (ja) | 2014-12-04 |
Family
ID=47040711
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2014535087A Pending JP2014532119A (ja) | 2011-10-12 | 2012-10-11 | 水蒸気の電気分解によって水素と酸素を発生させる方法 |
Country Status (9)
| Country | Link |
|---|---|
| US (1) | US20140284220A1 (enExample) |
| EP (1) | EP2766512A1 (enExample) |
| JP (1) | JP2014532119A (enExample) |
| CN (1) | CN103987878A (enExample) |
| BR (1) | BR112014008732A2 (enExample) |
| FR (1) | FR2981368B1 (enExample) |
| IN (1) | IN2014DN03034A (enExample) |
| RU (1) | RU2014118792A (enExample) |
| WO (1) | WO2013053858A1 (enExample) |
Cited By (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2017078204A (ja) * | 2015-10-20 | 2017-04-27 | 東京瓦斯株式会社 | 高温水蒸気電解セル及び高温水蒸気電解システム |
| JP2017081815A (ja) * | 2015-10-29 | 2017-05-18 | 学校法人玉川学園 | 水素生成装置及び水素生成方法 |
| JP2018519414A (ja) * | 2015-04-16 | 2018-07-19 | サウジ アラビアン オイル カンパニーSaudi Arabian Oil Company | 二酸化炭素および硫化水素を共処理する方法 |
| JP2022513860A (ja) * | 2018-12-18 | 2022-02-09 | オプス-12 インコーポレイテッド | 電解槽及び使用方法 |
| US12043912B2 (en) | 2018-11-28 | 2024-07-23 | Twelve Benefit Corporation | Electrolyzer and method of use |
| US12305304B2 (en) | 2022-10-13 | 2025-05-20 | Twelve Benefit Corporation | Interface for carbon oxide electrolyzer bipolar membrane |
Families Citing this family (17)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| AU2014295915A1 (en) | 2013-07-31 | 2016-02-11 | Aquahydrex Pty Ltd | Modular electrochemical cells |
| US10480083B2 (en) | 2014-07-28 | 2019-11-19 | Nippon Shokubai Co., Ltd. | Steam electrolysis cell |
| WO2016017251A1 (ja) * | 2014-07-28 | 2016-02-04 | 株式会社日本触媒 | 水蒸気電解用セル |
| JP6747778B2 (ja) * | 2014-07-28 | 2020-08-26 | 株式会社日本触媒 | 水蒸気電解用セル |
| JP6363425B2 (ja) * | 2014-08-08 | 2018-07-25 | 株式会社東芝 | 水素製造システム及び水素製造方法 |
| GB2539233B (en) * | 2015-06-10 | 2019-12-18 | Siemens Plc | Electrochemical cell |
| GB2544485B (en) * | 2015-11-16 | 2018-09-19 | Siemens Ag | Electrochemical cell comprising a steam inlet and a solid oxide layer |
| EP3390694A4 (en) * | 2015-12-14 | 2019-10-23 | AquaHydrex Pty Ltd | METHOD AND SYSTEM FOR THE EFFICIENT OPERATION OF ELECTROCHEMICAL CELLS |
| EP3421443B1 (en) * | 2016-02-25 | 2020-11-04 | Kyocera Corporation | Light-absorbing member, hydrogen production member, and hydrogen production device |
| CN106185984B (zh) * | 2016-07-23 | 2021-06-29 | 陈志强 | 基于水蒸汽电解法联合生产氨与硝酸的系统 |
| US11421330B2 (en) * | 2017-03-16 | 2022-08-23 | Battelle Energy Alliance, Llc | Methods for carbon dioxide hydrogenation |
| DE102017218012A1 (de) | 2017-10-10 | 2019-04-11 | Technische Universität Bergakademie Freiberg | Elektrolyse- und/oder Brennstoffzelle umfassend ein Elektrodenmaterial enthaltend einen metallokeramischen Verbundwerkstoff und Verfahren zur Herstellung dieser |
| CA3127358A1 (en) | 2019-02-01 | 2020-08-06 | Aquahydrex, Inc. | Electrochemical system with confined electrolyte |
| CN110804468B (zh) * | 2019-11-27 | 2023-03-31 | 浙江天禄环境科技有限公司 | 一种合成气的干法脱硫工艺 |
| CN111282410B (zh) * | 2020-02-19 | 2021-07-06 | 华中师范大学 | 电化学法降解气态污染物的装置及其方法 |
| GB2616256A (en) * | 2022-02-24 | 2023-09-06 | Ceres Ip Co Ltd | Treatment plant electrolyser system |
| FR3157815A1 (fr) * | 2023-12-27 | 2025-07-04 | Genvia | Installation et procédé de traitement de gaz sulfurés et de récupération de soufre par couplage d’unité d’électrolyse haute température |
Family Cites Families (12)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4402804A (en) * | 1982-05-17 | 1983-09-06 | Ppg Industries, Inc. | Electrolytic synthesis of aryl alcohols, aryl aldehydes, and aryl acids |
| US4547273A (en) * | 1984-06-07 | 1985-10-15 | Energy Conversion Devices, Inc. | Mobile atom insertion reaction, mobile atom transmissive membrane for carrying out the reaction, and reactor incorporating the mobile atom transmissive membrane |
| JP5011127B2 (ja) * | 2005-01-21 | 2012-08-29 | エクソンモービル リサーチ アンド エンジニアリング カンパニー | 水素源からの水素含有ストリーム中の水素の管理 |
| CN101163536B (zh) * | 2005-01-21 | 2011-12-07 | 埃克森美孚研究工程公司 | 采用精炼工艺单元如加氢处理、加氢裂化的快速循环压力摆动吸附的改进的集成 |
| DE102006035893A1 (de) * | 2006-07-31 | 2008-02-07 | Wolf, Bodo M., Dr. | Verfahren zur Wiederaufarbeitung von Verbrennungsprodukten fossiler Brennstoffe |
| US20080038621A1 (en) * | 2006-08-10 | 2008-02-14 | Ngk Insulators, Ltd. | Electrochemical devices |
| FR2916653B1 (fr) | 2007-06-01 | 2011-05-06 | Areva Np | Procede d'optimisation de la conductivite ionique d'une membrane conductrice ionique. |
| FR2919618B1 (fr) * | 2007-08-02 | 2009-11-13 | Commissariat Energie Atomique | Electrolyseur haute temperature et haute pression a fonctionnement allothermique et forte capacite de production |
| FR2931168B1 (fr) * | 2008-05-15 | 2010-07-30 | Areva | Procede de production de composes du type cxhyoz par reduction de dioxyde de carbone (co2) et/ou de monoxyde de carbone (co) |
| FR2939450B1 (fr) * | 2008-12-05 | 2013-11-01 | Alex Hr Roustaei | Systeme de production, conversion et restitution de h2 en cycle gaz-liquide-gaz avec absorption du co2 a chaque changement d'etat, utilisant une double electrolyse alcaline a base des nanoparticules |
| US20100314235A1 (en) * | 2009-06-16 | 2010-12-16 | Exxonmobil Research And Engineering Company | High temperature hydropyrolysis of carbonaceous materials |
| KR20110094969A (ko) * | 2010-02-18 | 2011-08-24 | 삼성전자주식회사 | 나노다이아몬드를 포함하는 전기화학적 수처리용 전극 및 상기 전극을 포함하는 전기화학적 수처리 장치 |
-
2011
- 2011-10-12 FR FR1159221A patent/FR2981368B1/fr not_active Expired - Fee Related
-
2012
- 2012-10-11 CN CN201280057387.3A patent/CN103987878A/zh active Pending
- 2012-10-11 JP JP2014535087A patent/JP2014532119A/ja active Pending
- 2012-10-11 IN IN3034DEN2014 patent/IN2014DN03034A/en unknown
- 2012-10-11 WO PCT/EP2012/070214 patent/WO2013053858A1/fr not_active Ceased
- 2012-10-11 EP EP12773302.0A patent/EP2766512A1/fr not_active Withdrawn
- 2012-10-11 BR BR112014008732A patent/BR112014008732A2/pt not_active Application Discontinuation
- 2012-10-11 RU RU2014118792/04A patent/RU2014118792A/ru not_active Application Discontinuation
- 2012-10-11 US US14/350,844 patent/US20140284220A1/en not_active Abandoned
Cited By (8)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2018519414A (ja) * | 2015-04-16 | 2018-07-19 | サウジ アラビアン オイル カンパニーSaudi Arabian Oil Company | 二酸化炭素および硫化水素を共処理する方法 |
| JP2017078204A (ja) * | 2015-10-20 | 2017-04-27 | 東京瓦斯株式会社 | 高温水蒸気電解セル及び高温水蒸気電解システム |
| JP2017081815A (ja) * | 2015-10-29 | 2017-05-18 | 学校法人玉川学園 | 水素生成装置及び水素生成方法 |
| US12043912B2 (en) | 2018-11-28 | 2024-07-23 | Twelve Benefit Corporation | Electrolyzer and method of use |
| JP2022513860A (ja) * | 2018-12-18 | 2022-02-09 | オプス-12 インコーポレイテッド | 電解槽及び使用方法 |
| US11888191B2 (en) | 2018-12-18 | 2024-01-30 | Twelve Benefit Corporation | Electrolyzer and method of use |
| JP7686558B2 (ja) | 2018-12-18 | 2025-06-02 | トゥエルブ ベネフィット コーポレーション | 電解槽及び使用方法 |
| US12305304B2 (en) | 2022-10-13 | 2025-05-20 | Twelve Benefit Corporation | Interface for carbon oxide electrolyzer bipolar membrane |
Also Published As
| Publication number | Publication date |
|---|---|
| US20140284220A1 (en) | 2014-09-25 |
| BR112014008732A2 (pt) | 2017-04-25 |
| IN2014DN03034A (enExample) | 2015-05-08 |
| CN103987878A (zh) | 2014-08-13 |
| RU2014118792A (ru) | 2015-11-20 |
| EP2766512A1 (fr) | 2014-08-20 |
| FR2981368B1 (fr) | 2013-11-15 |
| WO2013053858A1 (fr) | 2013-04-18 |
| FR2981368A1 (fr) | 2013-04-19 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP2014532119A (ja) | 水蒸気の電気分解によって水素と酸素を発生させる方法 | |
| Guo et al. | Electrochemical nitrogen fixation and utilization: theories, advanced catalyst materials and system design | |
| CN104272513B (zh) | 用于制氢的氧化还原液流电池 | |
| US20140291162A1 (en) | METHOD AND SYSTEM FOR TREATING CARBON GASES BY ELECTROCHEMICAL HYDROGENATION IN ORDER TO OBTAIN A CxHyOz COMPOUND | |
| JP2011521104A (ja) | 二酸化炭素(co2)および/または一酸化炭素(co)の還元によってcxhyozタイプの化合物を製造するための方法 | |
| JP4907745B2 (ja) | 二酸化炭素を還元する方法 | |
| KR101724690B1 (ko) | 양극산화를 통한 철-니켈 기반 물분해 촉매전극 제조방법 및 이에 따라 제조된 물 분해 촉매전극 | |
| US11050076B1 (en) | Flow cell systems, flow cell batteries, and hydrogen production processes | |
| JP5916045B2 (ja) | 触媒部材の製造方法、触媒部材及びそれを用いた有機合成方法 | |
| Schreiber | Industrial CO2 electroreduction to ethylene: main technical challenges | |
| JP2014167146A (ja) | 二酸化炭素ガスの電気分解方法。 | |
| Navaee et al. | Review on CO2 Management: From CO2 Sources, Capture, and Conversion to Future Perspectives of Gas-Phase Electrochemical Conversion and Utilization | |
| KR101695622B1 (ko) | 알코올 기반의 전해질을 이용한 전기화학적 암모니아 합성방법 | |
| CN116322983B (zh) | 碱水电解用阳极和其制造方法 | |
| Pandey et al. | Value addition of MXenes as photo-/electrocatalysts in water splitting for sustainable hydrogen production | |
| Zhang et al. | Bridge‐Oxygen Bond: An Active Group for Energy Electrocatalysis | |
| Katsounaros et al. | Electrocatalysis for the hydrogen economy | |
| Dipu et al. | Carbon dioxide reduction in a tubular solid oxide electrolysis cell for a carbon recycling energy system | |
| Liu et al. | Enhance Water Electrolysis for Green Hydrogen Production with Material Engineering: A Review | |
| JP2012122126A (ja) | 表面改質銅部材 | |
| Hong et al. | Electrochemical activation of Pt electrode for efficient and stable anion exchange membrane ammonia electrolyzer | |
| CN116507413B (zh) | 碱水电解用阳极和其制造方法 | |
| Ding et al. | Joule Heating for Metal Oxide Electrocatalyst Fabrication: A Fast‐Track Strategy for Water Electrolysis | |
| Jain et al. | Electrocatalytic applications of heteroatom-doped carbon nanostructures: thinking beyond PEM fuel cells | |
| JP2005048247A (ja) | 固体電解質型水素処理装置 |