JP2011503012A - 予後に関するバイオマーカーおよび治療の分子標的としての細胞外ヒストン - Google Patents
予後に関するバイオマーカーおよび治療の分子標的としての細胞外ヒストン Download PDFInfo
- Publication number
- JP2011503012A JP2011503012A JP2010532342A JP2010532342A JP2011503012A JP 2011503012 A JP2011503012 A JP 2011503012A JP 2010532342 A JP2010532342 A JP 2010532342A JP 2010532342 A JP2010532342 A JP 2010532342A JP 2011503012 A JP2011503012 A JP 2011503012A
- Authority
- JP
- Japan
- Prior art keywords
- histone
- inhibitor
- subject
- peptide
- cytotoxicity
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Pending
Links
- 108010033040 Histones Proteins 0.000 title claims abstract description 335
- 102000006947 Histones Human genes 0.000 title claims abstract description 191
- 238000002560 therapeutic procedure Methods 0.000 title claims description 10
- 239000000092 prognostic biomarker Substances 0.000 title description 2
- 239000003112 inhibitor Substances 0.000 claims abstract description 62
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 claims abstract description 32
- 201000010099 disease Diseases 0.000 claims abstract description 28
- 238000004393 prognosis Methods 0.000 claims abstract description 4
- 108090000765 processed proteins & peptides Proteins 0.000 claims description 139
- 238000000034 method Methods 0.000 claims description 132
- 230000003013 cytotoxicity Effects 0.000 claims description 61
- 231100000135 cytotoxicity Toxicity 0.000 claims description 61
- 102000004196 processed proteins & peptides Human genes 0.000 claims description 50
- 229960000856 protein c Drugs 0.000 claims description 40
- 230000002583 anti-histone Effects 0.000 claims description 39
- 101800004937 Protein C Proteins 0.000 claims description 35
- 101800001700 Saposin-D Proteins 0.000 claims description 35
- 208000014674 injury Diseases 0.000 claims description 35
- 239000012634 fragment Substances 0.000 claims description 34
- 238000001356 surgical procedure Methods 0.000 claims description 23
- 241000282414 Homo sapiens Species 0.000 claims description 22
- 201000000596 systemic lupus erythematosus Diseases 0.000 claims description 20
- 230000008733 trauma Effects 0.000 claims description 18
- 230000001472 cytotoxic effect Effects 0.000 claims description 17
- 206010001052 Acute respiratory distress syndrome Diseases 0.000 claims description 16
- 208000013616 Respiratory Distress Syndrome Diseases 0.000 claims description 15
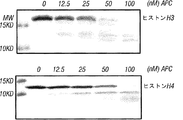
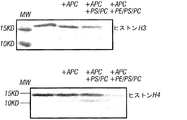
- 238000001262 western blot Methods 0.000 claims description 15
- 208000024172 Cardiovascular disease Diseases 0.000 claims description 13
- 201000000028 adult respiratory distress syndrome Diseases 0.000 claims description 13
- 238000002965 ELISA Methods 0.000 claims description 12
- 241000283973 Oryctolagus cuniculus Species 0.000 claims description 12
- 231100000433 cytotoxic Toxicity 0.000 claims description 12
- 210000002966 serum Anatomy 0.000 claims description 12
- 230000001988 toxicity Effects 0.000 claims description 12
- 231100000419 toxicity Toxicity 0.000 claims description 12
- 210000002889 endothelial cell Anatomy 0.000 claims description 11
- 241000283707 Capra Species 0.000 claims description 9
- 239000008194 pharmaceutical composition Substances 0.000 claims description 9
- 102000004127 Cytokines Human genes 0.000 claims description 8
- 108090000695 Cytokines Proteins 0.000 claims description 8
- 230000000740 bleeding effect Effects 0.000 claims description 8
- 230000000770 proinflammatory effect Effects 0.000 claims description 8
- 230000001225 therapeutic effect Effects 0.000 claims description 8
- 241000282693 Cercopithecidae Species 0.000 claims description 7
- 108090001005 Interleukin-6 Proteins 0.000 claims description 7
- 241001494479 Pecora Species 0.000 claims description 7
- 206010063837 Reperfusion injury Diseases 0.000 claims description 7
- 241000283073 Equus caballus Species 0.000 claims description 6
- 241000282326 Felis catus Species 0.000 claims description 6
- 230000003511 endothelial effect Effects 0.000 claims description 6
- 230000002401 inhibitory effect Effects 0.000 claims description 6
- 230000009885 systemic effect Effects 0.000 claims description 6
- 201000001320 Atherosclerosis Diseases 0.000 claims description 5
- 108060005986 Granzyme Proteins 0.000 claims description 5
- 102000001398 Granzyme Human genes 0.000 claims description 5
- 229940121363 anti-inflammatory agent Drugs 0.000 claims description 5
- 239000002260 anti-inflammatory agent Substances 0.000 claims description 5
- 208000023275 Autoimmune disease Diseases 0.000 claims description 4
- 108010023321 Factor VII Proteins 0.000 claims description 4
- 206010030113 Oedema Diseases 0.000 claims description 4
- 206010033645 Pancreatitis Diseases 0.000 claims description 4
- 206010033647 Pancreatitis acute Diseases 0.000 claims description 4
- 201000003229 acute pancreatitis Diseases 0.000 claims description 4
- 230000001684 chronic effect Effects 0.000 claims description 4
- 230000035699 permeability Effects 0.000 claims description 4
- 238000001959 radiotherapy Methods 0.000 claims description 4
- 241000193738 Bacillus anthracis Species 0.000 claims description 3
- HTTJABKRGRZYRN-UHFFFAOYSA-N Heparin Chemical compound OC1C(NC(=O)C)C(O)OC(COS(O)(=O)=O)C1OC1C(OS(O)(=O)=O)C(O)C(OC2C(C(OS(O)(=O)=O)C(OC3C(C(O)C(O)C(O3)C(O)=O)OS(O)(=O)=O)C(CO)O2)NS(O)(=O)=O)C(C(O)=O)O1 HTTJABKRGRZYRN-UHFFFAOYSA-N 0.000 claims description 3
- 239000004365 Protease Substances 0.000 claims description 3
- 230000005796 circulatory shock Effects 0.000 claims description 3
- 229920000669 heparin Polymers 0.000 claims description 3
- 229960002897 heparin Drugs 0.000 claims description 3
- 230000000451 tissue damage Effects 0.000 claims description 3
- 231100000827 tissue damage Toxicity 0.000 claims description 3
- 230000005747 tumor angiogenesis Effects 0.000 claims description 3
- 230000002792 vascular Effects 0.000 claims description 3
- 206010061818 Disease progression Diseases 0.000 claims description 2
- 108010088842 Fibrinolysin Proteins 0.000 claims description 2
- 206010058872 Fungal sepsis Diseases 0.000 claims description 2
- 108090001007 Interleukin-8 Proteins 0.000 claims description 2
- 108091005804 Peptidases Proteins 0.000 claims description 2
- 230000003213 activating effect Effects 0.000 claims description 2
- 201000005008 bacterial sepsis Diseases 0.000 claims description 2
- 230000016396 cytokine production Effects 0.000 claims description 2
- 230000005750 disease progression Effects 0.000 claims description 2
- 208000012947 ischemia reperfusion injury Diseases 0.000 claims description 2
- 229940012957 plasmin Drugs 0.000 claims description 2
- 102400000827 Saposin-D Human genes 0.000 claims 2
- 102100037486 Reverse transcriptase/ribonuclease H Human genes 0.000 claims 1
- 206010025135 lupus erythematosus Diseases 0.000 claims 1
- 206010040047 Sepsis Diseases 0.000 abstract description 57
- 230000034994 death Effects 0.000 abstract description 12
- 231100000517 death Toxicity 0.000 abstract description 12
- 206010061218 Inflammation Diseases 0.000 abstract description 10
- 230000004054 inflammatory process Effects 0.000 abstract description 10
- 230000004044 response Effects 0.000 abstract description 8
- 239000000090 biomarker Substances 0.000 abstract description 4
- 206010053159 Organ failure Diseases 0.000 abstract description 3
- 230000002757 inflammatory effect Effects 0.000 abstract description 3
- 206010048554 Endothelial dysfunction Diseases 0.000 abstract 1
- 230000008694 endothelial dysfunction Effects 0.000 abstract 1
- 210000004027 cell Anatomy 0.000 description 80
- 108090000623 proteins and genes Proteins 0.000 description 62
- 102000004169 proteins and genes Human genes 0.000 description 57
- 235000018102 proteins Nutrition 0.000 description 50
- 239000000203 mixture Substances 0.000 description 49
- 239000002158 endotoxin Substances 0.000 description 47
- 229920006008 lipopolysaccharide Polymers 0.000 description 47
- 241000699666 Mus <mouse, genus> Species 0.000 description 39
- 102000017975 Protein C Human genes 0.000 description 38
- 241001465754 Metazoa Species 0.000 description 35
- 235000001014 amino acid Nutrition 0.000 description 35
- 238000011282 treatment Methods 0.000 description 35
- 241000699670 Mus sp. Species 0.000 description 31
- 230000027455 binding Effects 0.000 description 29
- 238000009739 binding Methods 0.000 description 29
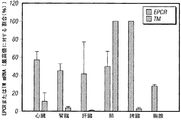
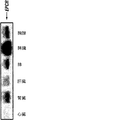
- 108010009900 Endothelial Protein C Receptor Proteins 0.000 description 28
- 102000009839 Endothelial Protein C Receptor Human genes 0.000 description 28
- 230000006378 damage Effects 0.000 description 27
- 102000008070 Interferon-gamma Human genes 0.000 description 25
- 108010074328 Interferon-gamma Proteins 0.000 description 25
- 229960003130 interferon gamma Drugs 0.000 description 25
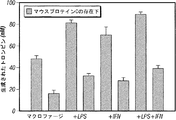
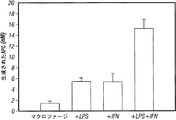
- 210000002540 macrophage Anatomy 0.000 description 25
- 229940024606 amino acid Drugs 0.000 description 24
- 150000001413 amino acids Chemical class 0.000 description 24
- 239000000523 sample Substances 0.000 description 22
- 208000027418 Wounds and injury Diseases 0.000 description 21
- 230000000694 effects Effects 0.000 description 20
- 210000003038 endothelium Anatomy 0.000 description 20
- 210000001519 tissue Anatomy 0.000 description 20
- 239000002609 medium Substances 0.000 description 19
- 238000000746 purification Methods 0.000 description 19
- 239000000427 antigen Substances 0.000 description 18
- 108091007433 antigens Proteins 0.000 description 18
- 102000036639 antigens Human genes 0.000 description 18
- 239000003636 conditioned culture medium Substances 0.000 description 18
- 238000001727 in vivo Methods 0.000 description 18
- 239000000243 solution Substances 0.000 description 18
- 229960004072 thrombin Drugs 0.000 description 18
- 108020004414 DNA Proteins 0.000 description 17
- 241000588724 Escherichia coli Species 0.000 description 17
- 108090000190 Thrombin Proteins 0.000 description 17
- 108010079274 Thrombomodulin Proteins 0.000 description 17
- 102000012607 Thrombomodulin Human genes 0.000 description 17
- 230000004913 activation Effects 0.000 description 17
- 239000003795 chemical substances by application Substances 0.000 description 17
- 239000000463 material Substances 0.000 description 17
- 239000003446 ligand Substances 0.000 description 16
- 210000000056 organ Anatomy 0.000 description 16
- 241000283690 Bos taurus Species 0.000 description 15
- 125000003275 alpha amino acid group Chemical group 0.000 description 15
- 229920001184 polypeptide Polymers 0.000 description 15
- 108091003079 Bovine Serum Albumin Proteins 0.000 description 14
- 230000000844 anti-bacterial effect Effects 0.000 description 14
- 238000001514 detection method Methods 0.000 description 14
- 238000002415 sodium dodecyl sulfate polyacrylamide gel electrophoresis Methods 0.000 description 14
- 238000006467 substitution reaction Methods 0.000 description 14
- 206010035226 Plasma cell myeloma Diseases 0.000 description 13
- 210000004369 blood Anatomy 0.000 description 13
- 239000008280 blood Substances 0.000 description 13
- 238000003776 cleavage reaction Methods 0.000 description 13
- 230000004927 fusion Effects 0.000 description 13
- 208000015181 infectious disease Diseases 0.000 description 13
- 201000000050 myeloid neoplasm Diseases 0.000 description 13
- 230000007017 scission Effects 0.000 description 13
- 210000001541 thymus gland Anatomy 0.000 description 13
- 244000309466 calf Species 0.000 description 12
- LOKCTEFSRHRXRJ-UHFFFAOYSA-I dipotassium trisodium dihydrogen phosphate hydrogen phosphate dichloride Chemical compound P(=O)(O)(O)[O-].[K+].P(=O)(O)([O-])[O-].[Na+].[Na+].[Cl-].[K+].[Cl-].[Na+] LOKCTEFSRHRXRJ-UHFFFAOYSA-I 0.000 description 12
- 238000002347 injection Methods 0.000 description 12
- 239000007924 injection Substances 0.000 description 12
- 238000004519 manufacturing process Methods 0.000 description 12
- 108020004999 messenger RNA Proteins 0.000 description 12
- 239000002953 phosphate buffered saline Substances 0.000 description 12
- 210000002381 plasma Anatomy 0.000 description 12
- 238000005406 washing Methods 0.000 description 12
- 102000004190 Enzymes Human genes 0.000 description 11
- 108090000790 Enzymes Proteins 0.000 description 11
- 108090001090 Lectins Proteins 0.000 description 11
- 102000004856 Lectins Human genes 0.000 description 11
- 239000012124 Opti-MEM Substances 0.000 description 11
- 239000003814 drug Substances 0.000 description 11
- 229940088598 enzyme Drugs 0.000 description 11
- 238000000338 in vitro Methods 0.000 description 11
- 239000002523 lectin Substances 0.000 description 11
- 231100000636 lethal dose Toxicity 0.000 description 11
- XJMOSONTPMZWPB-UHFFFAOYSA-M propidium iodide Chemical compound [I-].[I-].C12=CC(N)=CC=C2C2=CC=C(N)C=C2[N+](CCC[N+](C)(CC)CC)=C1C1=CC=CC=C1 XJMOSONTPMZWPB-UHFFFAOYSA-M 0.000 description 11
- 101000924577 Homo sapiens Adenomatous polyposis coli protein Proteins 0.000 description 10
- 102000055691 human APC Human genes 0.000 description 10
- FDGQSTZJBFJUBT-UHFFFAOYSA-N hypoxanthine Chemical compound O=C1NC=NC2=C1NC=N2 FDGQSTZJBFJUBT-UHFFFAOYSA-N 0.000 description 10
- 230000001965 increasing effect Effects 0.000 description 10
- 238000001802 infusion Methods 0.000 description 10
- 230000007246 mechanism Effects 0.000 description 10
- 230000008569 process Effects 0.000 description 10
- 210000000952 spleen Anatomy 0.000 description 10
- 238000010186 staining Methods 0.000 description 10
- 241000700159 Rattus Species 0.000 description 9
- 238000001042 affinity chromatography Methods 0.000 description 9
- 238000003745 diagnosis Methods 0.000 description 9
- 238000000684 flow cytometry Methods 0.000 description 9
- 230000004048 modification Effects 0.000 description 9
- 238000012986 modification Methods 0.000 description 9
- 239000000126 substance Substances 0.000 description 9
- 208000024891 symptom Diseases 0.000 description 9
- 238000012360 testing method Methods 0.000 description 9
- JZNWSCPGTDBMEW-UHFFFAOYSA-N Glycerophosphorylethanolamin Natural products NCCOP(O)(=O)OCC(O)CO JZNWSCPGTDBMEW-UHFFFAOYSA-N 0.000 description 8
- ROHFNLRQFUQHCH-YFKPBYRVSA-N L-leucine Chemical compound CC(C)C[C@H](N)C(O)=O ROHFNLRQFUQHCH-YFKPBYRVSA-N 0.000 description 8
- ROHFNLRQFUQHCH-UHFFFAOYSA-N Leucine Natural products CC(C)CC(N)C(O)=O ROHFNLRQFUQHCH-UHFFFAOYSA-N 0.000 description 8
- KDXKERNSBIXSRK-UHFFFAOYSA-N Lysine Natural products NCCCCC(N)C(O)=O KDXKERNSBIXSRK-UHFFFAOYSA-N 0.000 description 8
- 239000004472 Lysine Substances 0.000 description 8
- 241001529936 Murinae Species 0.000 description 8
- 108010047956 Nucleosomes Proteins 0.000 description 8
- FAPWRFPIFSIZLT-UHFFFAOYSA-M Sodium chloride Chemical compound [Na+].[Cl-] FAPWRFPIFSIZLT-UHFFFAOYSA-M 0.000 description 8
- 206010051379 Systemic Inflammatory Response Syndrome Diseases 0.000 description 8
- QTBSBXVTEAMEQO-UHFFFAOYSA-N acetic acid Substances CC(O)=O QTBSBXVTEAMEQO-UHFFFAOYSA-N 0.000 description 8
- 238000003556 assay Methods 0.000 description 8
- 150000001875 compounds Chemical class 0.000 description 8
- 239000012091 fetal bovine serum Substances 0.000 description 8
- 210000004408 hybridoma Anatomy 0.000 description 8
- 238000003018 immunoassay Methods 0.000 description 8
- 230000003993 interaction Effects 0.000 description 8
- 210000001623 nucleosome Anatomy 0.000 description 8
- 239000000816 peptidomimetic Substances 0.000 description 8
- 210000003024 peritoneal macrophage Anatomy 0.000 description 8
- 239000012071 phase Substances 0.000 description 8
- 150000008104 phosphatidylethanolamines Chemical class 0.000 description 8
- 208000032843 Hemorrhage Diseases 0.000 description 7
- AGPKZVBTJJNPAG-WHFBIAKZSA-N L-isoleucine Chemical compound CC[C@H](C)[C@H](N)C(O)=O AGPKZVBTJJNPAG-WHFBIAKZSA-N 0.000 description 7
- 241000282520 Papio Species 0.000 description 7
- MTCFGRXMJLQNBG-UHFFFAOYSA-N Serine Natural products OCC(N)C(O)=O MTCFGRXMJLQNBG-UHFFFAOYSA-N 0.000 description 7
- 239000002671 adjuvant Substances 0.000 description 7
- 208000034158 bleeding Diseases 0.000 description 7
- 239000012530 fluid Substances 0.000 description 7
- 230000006870 function Effects 0.000 description 7
- 210000002216 heart Anatomy 0.000 description 7
- 230000003053 immunization Effects 0.000 description 7
- 230000002163 immunogen Effects 0.000 description 7
- 229960000310 isoleucine Drugs 0.000 description 7
- AGPKZVBTJJNPAG-UHFFFAOYSA-N isoleucine Natural products CCC(C)C(N)C(O)=O AGPKZVBTJJNPAG-UHFFFAOYSA-N 0.000 description 7
- 231100000225 lethality Toxicity 0.000 description 7
- 210000004072 lung Anatomy 0.000 description 7
- 238000002360 preparation method Methods 0.000 description 7
- 150000003839 salts Chemical class 0.000 description 7
- TZCPCKNHXULUIY-RGULYWFUSA-N 1,2-distearoyl-sn-glycero-3-phosphoserine Chemical compound CCCCCCCCCCCCCCCCCC(=O)OC[C@H](COP(O)(=O)OC[C@H](N)C(O)=O)OC(=O)CCCCCCCCCCCCCCCCC TZCPCKNHXULUIY-RGULYWFUSA-N 0.000 description 6
- 239000004475 Arginine Substances 0.000 description 6
- DCXYFEDJOCDNAF-UHFFFAOYSA-N Asparagine Natural products OC(=O)C(N)CC(N)=O DCXYFEDJOCDNAF-UHFFFAOYSA-N 0.000 description 6
- PEDCQBHIVMGVHV-UHFFFAOYSA-N Glycerine Chemical compound OCC(O)CO PEDCQBHIVMGVHV-UHFFFAOYSA-N 0.000 description 6
- ZWZWYGMENQVNFU-UHFFFAOYSA-N Glycerophosphorylserin Natural products OC(=O)C(N)COP(O)(=O)OCC(O)CO ZWZWYGMENQVNFU-UHFFFAOYSA-N 0.000 description 6
- DCXYFEDJOCDNAF-REOHCLBHSA-N L-asparagine Chemical compound OC(=O)[C@@H](N)CC(N)=O DCXYFEDJOCDNAF-REOHCLBHSA-N 0.000 description 6
- KZSNJWFQEVHDMF-BYPYZUCNSA-N L-valine Chemical compound CC(C)[C@H](N)C(O)=O KZSNJWFQEVHDMF-BYPYZUCNSA-N 0.000 description 6
- 241001504519 Papio ursinus Species 0.000 description 6
- IQFYYKKMVGJFEH-XLPZGREQSA-N Thymidine Chemical compound O=C1NC(=O)C(C)=CN1[C@@H]1O[C@H](CO)[C@@H](O)C1 IQFYYKKMVGJFEH-XLPZGREQSA-N 0.000 description 6
- KZSNJWFQEVHDMF-UHFFFAOYSA-N Valine Natural products CC(C)C(N)C(O)=O KZSNJWFQEVHDMF-UHFFFAOYSA-N 0.000 description 6
- ODKSFYDXXFIFQN-UHFFFAOYSA-N arginine Natural products OC(=O)C(N)CCCNC(N)=N ODKSFYDXXFIFQN-UHFFFAOYSA-N 0.000 description 6
- 229960001230 asparagine Drugs 0.000 description 6
- 235000009582 asparagine Nutrition 0.000 description 6
- 230000008901 benefit Effects 0.000 description 6
- 230000015572 biosynthetic process Effects 0.000 description 6
- 229940098773 bovine serum albumin Drugs 0.000 description 6
- 239000000872 buffer Substances 0.000 description 6
- 238000004587 chromatography analysis Methods 0.000 description 6
- 229940079593 drug Drugs 0.000 description 6
- ZDXPYRJPNDTMRX-UHFFFAOYSA-N glutamine Natural products OC(=O)C(N)CCC(N)=O ZDXPYRJPNDTMRX-UHFFFAOYSA-N 0.000 description 6
- 230000001404 mediated effect Effects 0.000 description 6
- 230000037361 pathway Effects 0.000 description 6
- WTJKGGKOPKCXLL-RRHRGVEJSA-N phosphatidylcholine Chemical compound CCCCCCCCCCCCCCCC(=O)OC[C@H](COP([O-])(=O)OCC[N+](C)(C)C)OC(=O)CCCCCCCC=CCCCCCCCC WTJKGGKOPKCXLL-RRHRGVEJSA-N 0.000 description 6
- 229920001223 polyethylene glycol Polymers 0.000 description 6
- 238000000926 separation method Methods 0.000 description 6
- 210000003491 skin Anatomy 0.000 description 6
- 239000004474 valine Substances 0.000 description 6
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 6
- TVZGACDUOSZQKY-LBPRGKRZSA-N 4-aminofolic acid Chemical compound C1=NC2=NC(N)=NC(N)=C2N=C1CNC1=CC=C(C(=O)N[C@@H](CCC(O)=O)C(O)=O)C=C1 TVZGACDUOSZQKY-LBPRGKRZSA-N 0.000 description 5
- 108010014172 Factor V Proteins 0.000 description 5
- 108010014173 Factor X Proteins 0.000 description 5
- DHMQDGOQFOQNFH-UHFFFAOYSA-N Glycine Chemical compound NCC(O)=O DHMQDGOQFOQNFH-UHFFFAOYSA-N 0.000 description 5
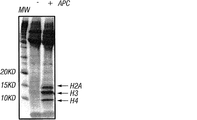
- 102000017286 Histone H2A Human genes 0.000 description 5
- 108050005231 Histone H2A Proteins 0.000 description 5
- UGQMRVRMYYASKQ-UHFFFAOYSA-N Hypoxanthine nucleoside Natural products OC1C(O)C(CO)OC1N1C(NC=NC2=O)=C2N=C1 UGQMRVRMYYASKQ-UHFFFAOYSA-N 0.000 description 5
- CKLJMWTZIZZHCS-REOHCLBHSA-N L-aspartic acid Chemical compound OC(=O)[C@@H](N)CC(O)=O CKLJMWTZIZZHCS-REOHCLBHSA-N 0.000 description 5
- WHUUTDBJXJRKMK-VKHMYHEASA-N L-glutamic acid Chemical compound OC(=O)[C@@H](N)CCC(O)=O WHUUTDBJXJRKMK-VKHMYHEASA-N 0.000 description 5
- 108090000143 Mouse Proteins Proteins 0.000 description 5
- 206010028980 Neoplasm Diseases 0.000 description 5
- 239000002202 Polyethylene glycol Substances 0.000 description 5
- 206010040070 Septic Shock Diseases 0.000 description 5
- AYFVYJQAPQTCCC-UHFFFAOYSA-N Threonine Natural products CC(O)C(N)C(O)=O AYFVYJQAPQTCCC-UHFFFAOYSA-N 0.000 description 5
- 239000004473 Threonine Substances 0.000 description 5
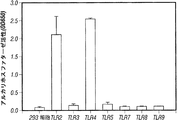
- 102000002689 Toll-like receptor Human genes 0.000 description 5
- 108020000411 Toll-like receptor Proteins 0.000 description 5
- 230000001154 acute effect Effects 0.000 description 5
- -1 amide glycosides Chemical class 0.000 description 5
- 229960003896 aminopterin Drugs 0.000 description 5
- 229940009098 aspartate Drugs 0.000 description 5
- 210000003719 b-lymphocyte Anatomy 0.000 description 5
- 230000033228 biological regulation Effects 0.000 description 5
- 210000000988 bone and bone Anatomy 0.000 description 5
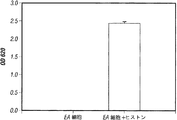
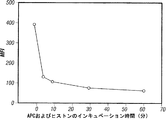
- 238000002784 cytotoxicity assay Methods 0.000 description 5
- 231100000263 cytotoxicity test Toxicity 0.000 description 5
- 229930195712 glutamate Natural products 0.000 description 5
- 238000004128 high performance liquid chromatography Methods 0.000 description 5
- HNDVDQJCIGZPNO-UHFFFAOYSA-N histidine Natural products OC(=O)C(N)CC1=CN=CN1 HNDVDQJCIGZPNO-UHFFFAOYSA-N 0.000 description 5
- 238000011534 incubation Methods 0.000 description 5
- 230000017306 interleukin-6 production Effects 0.000 description 5
- 230000021995 interleukin-8 production Effects 0.000 description 5
- 210000000265 leukocyte Anatomy 0.000 description 5
- 238000007726 management method Methods 0.000 description 5
- 239000000843 powder Substances 0.000 description 5
- 230000002829 reductive effect Effects 0.000 description 5
- 239000006152 selective media Substances 0.000 description 5
- 230000036303 septic shock Effects 0.000 description 5
- 230000011664 signaling Effects 0.000 description 5
- 239000011780 sodium chloride Substances 0.000 description 5
- 238000007920 subcutaneous administration Methods 0.000 description 5
- YBJHBAHKTGYVGT-ZKWXMUAHSA-N (+)-Biotin Chemical compound N1C(=O)N[C@@H]2[C@H](CCCCC(=O)O)SC[C@@H]21 YBJHBAHKTGYVGT-ZKWXMUAHSA-N 0.000 description 4
- KWPACVJPAFGBEQ-IKGGRYGDSA-N (2s)-1-[(2r)-2-amino-3-phenylpropanoyl]-n-[(3s)-1-chloro-6-(diaminomethylideneamino)-2-oxohexan-3-yl]pyrrolidine-2-carboxamide Chemical compound C([C@@H](N)C(=O)N1[C@@H](CCC1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)CCl)C1=CC=CC=C1 KWPACVJPAFGBEQ-IKGGRYGDSA-N 0.000 description 4
- 101100339431 Arabidopsis thaliana HMGB2 gene Proteins 0.000 description 4
- 239000006144 Dulbecco’s modified Eagle's medium Substances 0.000 description 4
- 108700010013 HMGB1 Proteins 0.000 description 4
- 101150021904 HMGB1 gene Proteins 0.000 description 4
- 102100037907 High mobility group protein B1 Human genes 0.000 description 4
- 101000669447 Homo sapiens Toll-like receptor 4 Proteins 0.000 description 4
- FFEARJCKVFRZRR-BYPYZUCNSA-N L-methionine Chemical compound CSCC[C@H](N)C(O)=O FFEARJCKVFRZRR-BYPYZUCNSA-N 0.000 description 4
- COLNVLDHVKWLRT-QMMMGPOBSA-N L-phenylalanine Chemical compound OC(=O)[C@@H](N)CC1=CC=CC=C1 COLNVLDHVKWLRT-QMMMGPOBSA-N 0.000 description 4
- 102100039360 Toll-like receptor 4 Human genes 0.000 description 4
- 206010052428 Wound Diseases 0.000 description 4
- 239000002253 acid Substances 0.000 description 4
- 239000004480 active ingredient Substances 0.000 description 4
- 238000013459 approach Methods 0.000 description 4
- 230000001363 autoimmune Effects 0.000 description 4
- 210000000170 cell membrane Anatomy 0.000 description 4
- 239000003593 chromogenic compound Substances 0.000 description 4
- 238000000576 coating method Methods 0.000 description 4
- DDRJAANPRJIHGJ-UHFFFAOYSA-N creatinine Chemical compound CN1CC(=O)NC1=N DDRJAANPRJIHGJ-UHFFFAOYSA-N 0.000 description 4
- 230000001120 cytoprotective effect Effects 0.000 description 4
- 238000009472 formulation Methods 0.000 description 4
- 238000001990 intravenous administration Methods 0.000 description 4
- 208000028867 ischemia Diseases 0.000 description 4
- 150000002632 lipids Chemical class 0.000 description 4
- 239000002502 liposome Substances 0.000 description 4
- 239000007788 liquid Substances 0.000 description 4
- 239000011159 matrix material Substances 0.000 description 4
- 229930182817 methionine Natural products 0.000 description 4
- 230000003278 mimic effect Effects 0.000 description 4
- 230000004001 molecular interaction Effects 0.000 description 4
- 230000004768 organ dysfunction Effects 0.000 description 4
- 239000002245 particle Substances 0.000 description 4
- COLNVLDHVKWLRT-UHFFFAOYSA-N phenylalanine Natural products OC(=O)C(N)CC1=CC=CC=C1 COLNVLDHVKWLRT-UHFFFAOYSA-N 0.000 description 4
- 239000000047 product Substances 0.000 description 4
- 238000003753 real-time PCR Methods 0.000 description 4
- 241000894007 species Species 0.000 description 4
- 230000000638 stimulation Effects 0.000 description 4
- 230000004083 survival effect Effects 0.000 description 4
- 238000003786 synthesis reaction Methods 0.000 description 4
- 231100000331 toxic Toxicity 0.000 description 4
- 230000002588 toxic effect Effects 0.000 description 4
- 230000003827 upregulation Effects 0.000 description 4
- AGNGYMCLFWQVGX-AGFFZDDWSA-N (e)-1-[(2s)-2-amino-2-carboxyethoxy]-2-diazonioethenolate Chemical compound OC(=O)[C@@H](N)CO\C([O-])=C\[N+]#N AGNGYMCLFWQVGX-AGFFZDDWSA-N 0.000 description 3
- 108091032973 (ribonucleotides)n+m Proteins 0.000 description 3
- 102000002260 Alkaline Phosphatase Human genes 0.000 description 3
- 108020004774 Alkaline Phosphatase Proteins 0.000 description 3
- DWRXFEITVBNRMK-UHFFFAOYSA-N Beta-D-1-Arabinofuranosylthymine Natural products O=C1NC(=O)C(C)=CN1C1C(O)C(O)C(CO)O1 DWRXFEITVBNRMK-UHFFFAOYSA-N 0.000 description 3
- 208000009043 Chemical Burns Diseases 0.000 description 3
- 238000011537 Coomassie blue staining Methods 0.000 description 3
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 description 3
- 108010074105 Factor Va Proteins 0.000 description 3
- 101710103773 Histone H2B Proteins 0.000 description 3
- 102100021639 Histone H2B type 1-K Human genes 0.000 description 3
- 241000282412 Homo Species 0.000 description 3
- 208000001953 Hypotension Diseases 0.000 description 3
- QNAYBMKLOCPYGJ-REOHCLBHSA-N L-alanine Chemical compound C[C@H](N)C(O)=O QNAYBMKLOCPYGJ-REOHCLBHSA-N 0.000 description 3
- ZDXPYRJPNDTMRX-VKHMYHEASA-N L-glutamine Chemical compound OC(=O)[C@@H](N)CCC(N)=O ZDXPYRJPNDTMRX-VKHMYHEASA-N 0.000 description 3
- FBOZXECLQNJBKD-ZDUSSCGKSA-N L-methotrexate Chemical compound C=1N=C2N=C(N)N=C(N)C2=NC=1CN(C)C1=CC=C(C(=O)N[C@@H](CCC(O)=O)C(O)=O)C=C1 FBOZXECLQNJBKD-ZDUSSCGKSA-N 0.000 description 3
- OUYCCCASQSFEME-QMMMGPOBSA-N L-tyrosine Chemical compound OC(=O)[C@@H](N)CC1=CC=C(O)C=C1 OUYCCCASQSFEME-QMMMGPOBSA-N 0.000 description 3
- MUBZPKHOEPUJKR-UHFFFAOYSA-N Oxalic acid Chemical compound OC(=O)C(O)=O MUBZPKHOEPUJKR-UHFFFAOYSA-N 0.000 description 3
- 241000009328 Perro Species 0.000 description 3
- DNIAPMSPPWPWGF-UHFFFAOYSA-N Propylene glycol Chemical compound CC(O)CO DNIAPMSPPWPWGF-UHFFFAOYSA-N 0.000 description 3
- 108010094028 Prothrombin Proteins 0.000 description 3
- 102100027378 Prothrombin Human genes 0.000 description 3
- 239000012980 RPMI-1640 medium Substances 0.000 description 3
- HEMHJVSKTPXQMS-UHFFFAOYSA-M Sodium hydroxide Chemical compound [OH-].[Na+] HEMHJVSKTPXQMS-UHFFFAOYSA-M 0.000 description 3
- 241000282898 Sus scrofa Species 0.000 description 3
- 206010053476 Traumatic haemorrhage Diseases 0.000 description 3
- 108060008682 Tumor Necrosis Factor Proteins 0.000 description 3
- 108010093894 Xanthine oxidase Proteins 0.000 description 3
- 230000009471 action Effects 0.000 description 3
- 235000004279 alanine Nutrition 0.000 description 3
- 238000002399 angioplasty Methods 0.000 description 3
- 239000003242 anti bacterial agent Substances 0.000 description 3
- 210000000628 antibody-producing cell Anatomy 0.000 description 3
- 239000007864 aqueous solution Substances 0.000 description 3
- QVGXLLKOCUKJST-UHFFFAOYSA-N atomic oxygen Chemical compound [O] QVGXLLKOCUKJST-UHFFFAOYSA-N 0.000 description 3
- 229950011321 azaserine Drugs 0.000 description 3
- 239000002585 base Substances 0.000 description 3
- IQFYYKKMVGJFEH-UHFFFAOYSA-N beta-L-thymidine Natural products O=C1NC(=O)C(C)=CN1C1OC(CO)C(O)C1 IQFYYKKMVGJFEH-UHFFFAOYSA-N 0.000 description 3
- 230000003115 biocidal effect Effects 0.000 description 3
- 230000004071 biological effect Effects 0.000 description 3
- 239000012472 biological sample Substances 0.000 description 3
- 230000036765 blood level Effects 0.000 description 3
- 201000011510 cancer Diseases 0.000 description 3
- 230000005779 cell damage Effects 0.000 description 3
- 208000037887 cell injury Diseases 0.000 description 3
- 230000008859 change Effects 0.000 description 3
- 230000004087 circulation Effects 0.000 description 3
- 239000011248 coating agent Substances 0.000 description 3
- 235000018417 cysteine Nutrition 0.000 description 3
- XUJNEKJLAYXESH-UHFFFAOYSA-N cysteine Natural products SCC(N)C(O)=O XUJNEKJLAYXESH-UHFFFAOYSA-N 0.000 description 3
- 210000004207 dermis Anatomy 0.000 description 3
- 208000035475 disorder Diseases 0.000 description 3
- 239000006185 dispersion Substances 0.000 description 3
- 239000002612 dispersion medium Substances 0.000 description 3
- 239000002552 dosage form Substances 0.000 description 3
- 230000004064 dysfunction Effects 0.000 description 3
- 230000008497 endothelial barrier function Effects 0.000 description 3
- 238000005516 engineering process Methods 0.000 description 3
- 239000000835 fiber Substances 0.000 description 3
- 238000005227 gel permeation chromatography Methods 0.000 description 3
- 239000001257 hydrogen Substances 0.000 description 3
- 229910052739 hydrogen Inorganic materials 0.000 description 3
- 230000028993 immune response Effects 0.000 description 3
- 238000002649 immunization Methods 0.000 description 3
- 230000005847 immunogenicity Effects 0.000 description 3
- 230000001976 improved effect Effects 0.000 description 3
- 208000027866 inflammatory disease Diseases 0.000 description 3
- 239000004615 ingredient Substances 0.000 description 3
- 230000005764 inhibitory process Effects 0.000 description 3
- 238000007918 intramuscular administration Methods 0.000 description 3
- 238000007912 intraperitoneal administration Methods 0.000 description 3
- 238000004255 ion exchange chromatography Methods 0.000 description 3
- 108010045069 keyhole-limpet hemocyanin Proteins 0.000 description 3
- 230000007774 longterm Effects 0.000 description 3
- 210000001165 lymph node Anatomy 0.000 description 3
- 239000003550 marker Substances 0.000 description 3
- 238000004949 mass spectrometry Methods 0.000 description 3
- 238000005399 mechanical ventilation Methods 0.000 description 3
- 239000012528 membrane Substances 0.000 description 3
- 210000004379 membrane Anatomy 0.000 description 3
- 229960000485 methotrexate Drugs 0.000 description 3
- 230000011987 methylation Effects 0.000 description 3
- 238000007069 methylation reaction Methods 0.000 description 3
- 244000005700 microbiome Species 0.000 description 3
- 238000002156 mixing Methods 0.000 description 3
- 210000003205 muscle Anatomy 0.000 description 3
- 230000014508 negative regulation of coagulation Effects 0.000 description 3
- 239000002773 nucleotide Substances 0.000 description 3
- 125000003729 nucleotide group Chemical group 0.000 description 3
- 239000001301 oxygen Substances 0.000 description 3
- 229910052760 oxygen Inorganic materials 0.000 description 3
- 230000008506 pathogenesis Effects 0.000 description 3
- 230000001575 pathological effect Effects 0.000 description 3
- 238000002264 polyacrylamide gel electrophoresis Methods 0.000 description 3
- 229920000136 polysorbate Polymers 0.000 description 3
- XOJVVFBFDXDTEG-UHFFFAOYSA-N pristane Chemical compound CC(C)CCCC(C)CCCC(C)CCCC(C)C XOJVVFBFDXDTEG-UHFFFAOYSA-N 0.000 description 3
- 230000001681 protective effect Effects 0.000 description 3
- 238000001742 protein purification Methods 0.000 description 3
- 229940039716 prothrombin Drugs 0.000 description 3
- 210000001147 pulmonary artery Anatomy 0.000 description 3
- 238000003127 radioimmunoassay Methods 0.000 description 3
- 238000011084 recovery Methods 0.000 description 3
- 238000011160 research Methods 0.000 description 3
- 230000002441 reversible effect Effects 0.000 description 3
- 230000035939 shock Effects 0.000 description 3
- 230000019491 signal transduction Effects 0.000 description 3
- 239000002904 solvent Substances 0.000 description 3
- 239000000758 substrate Substances 0.000 description 3
- 239000006228 supernatant Substances 0.000 description 3
- 229940104230 thymidine Drugs 0.000 description 3
- 208000037816 tissue injury Diseases 0.000 description 3
- 102000003390 tumor necrosis factor Human genes 0.000 description 3
- OUYCCCASQSFEME-UHFFFAOYSA-N tyrosine Natural products OC(=O)C(N)CC1=CC=C(O)C=C1 OUYCCCASQSFEME-UHFFFAOYSA-N 0.000 description 3
- 239000013598 vector Substances 0.000 description 3
- 238000009423 ventilation Methods 0.000 description 3
- 230000005730 ADP ribosylation Effects 0.000 description 2
- 208000009304 Acute Kidney Injury Diseases 0.000 description 2
- 241000894006 Bacteria Species 0.000 description 2
- 108010039209 Blood Coagulation Factors Proteins 0.000 description 2
- 102000015081 Blood Coagulation Factors Human genes 0.000 description 2
- 101000651448 Bos taurus Prothrombin Proteins 0.000 description 2
- 101800000407 Brain natriuretic peptide 32 Proteins 0.000 description 2
- 206010006802 Burns second degree Diseases 0.000 description 2
- 102000014914 Carrier Proteins Human genes 0.000 description 2
- 108010078791 Carrier Proteins Proteins 0.000 description 2
- 108010077544 Chromatin Proteins 0.000 description 2
- KCXVZYZYPLLWCC-UHFFFAOYSA-N EDTA Chemical compound OC(=O)CN(CC(O)=O)CCN(CC(O)=O)CC(O)=O KCXVZYZYPLLWCC-UHFFFAOYSA-N 0.000 description 2
- 238000008157 ELISA kit Methods 0.000 description 2
- 206010015150 Erythema Diseases 0.000 description 2
- KRHYYFGTRYWZRS-UHFFFAOYSA-N Fluorane Chemical compound F KRHYYFGTRYWZRS-UHFFFAOYSA-N 0.000 description 2
- 239000004471 Glycine Substances 0.000 description 2
- 102000003886 Glycoproteins Human genes 0.000 description 2
- 108090000288 Glycoproteins Proteins 0.000 description 2
- 108010054964 H-hexahydrotyrosyl-alanyl-arginine-4-nitroanilide Proteins 0.000 description 2
- 239000012981 Hank's balanced salt solution Substances 0.000 description 2
- 102000007625 Hirudins Human genes 0.000 description 2
- 108010007267 Hirudins Proteins 0.000 description 2
- 102100039869 Histone H2B type F-S Human genes 0.000 description 2
- 101001035372 Homo sapiens Histone H2B type F-S Proteins 0.000 description 2
- 101500025568 Homo sapiens Saposin-D Proteins 0.000 description 2
- VEXZGXHMUGYJMC-UHFFFAOYSA-N Hydrochloric acid Chemical compound Cl VEXZGXHMUGYJMC-UHFFFAOYSA-N 0.000 description 2
- 206010020772 Hypertension Diseases 0.000 description 2
- 102000018251 Hypoxanthine Phosphoribosyltransferase Human genes 0.000 description 2
- 108010091358 Hypoxanthine Phosphoribosyltransferase Proteins 0.000 description 2
- 206010021143 Hypoxia Diseases 0.000 description 2
- 108010021625 Immunoglobulin Fragments Proteins 0.000 description 2
- 102000008394 Immunoglobulin Fragments Human genes 0.000 description 2
- 108010063738 Interleukins Proteins 0.000 description 2
- 102000015696 Interleukins Human genes 0.000 description 2
- HNDVDQJCIGZPNO-YFKPBYRVSA-N L-histidine Chemical compound OC(=O)[C@@H](N)CC1=CN=CN1 HNDVDQJCIGZPNO-YFKPBYRVSA-N 0.000 description 2
- QIVBCDIJIAJPQS-VIFPVBQESA-N L-tryptophane Chemical compound C1=CC=C2C(C[C@H](N)C(O)=O)=CNC2=C1 QIVBCDIJIAJPQS-VIFPVBQESA-N 0.000 description 2
- 101000763311 Mus musculus Thrombomodulin Proteins 0.000 description 2
- 102000003945 NF-kappa B Human genes 0.000 description 2
- 108010057466 NF-kappa B Proteins 0.000 description 2
- MWUXSHHQAYIFBG-UHFFFAOYSA-N Nitric oxide Chemical compound O=[N] MWUXSHHQAYIFBG-UHFFFAOYSA-N 0.000 description 2
- 229910019142 PO4 Inorganic materials 0.000 description 2
- 102000003992 Peroxidases Human genes 0.000 description 2
- ISWSIDIOOBJBQZ-UHFFFAOYSA-N Phenol Chemical compound OC1=CC=CC=C1 ISWSIDIOOBJBQZ-UHFFFAOYSA-N 0.000 description 2
- NBIIXXVUZAFLBC-UHFFFAOYSA-N Phosphoric acid Chemical compound OP(O)(O)=O NBIIXXVUZAFLBC-UHFFFAOYSA-N 0.000 description 2
- ONIBWKKTOPOVIA-UHFFFAOYSA-N Proline Natural products OC(=O)C1CCCN1 ONIBWKKTOPOVIA-UHFFFAOYSA-N 0.000 description 2
- 201000005660 Protein C Deficiency Diseases 0.000 description 2
- 206010037423 Pulmonary oedema Diseases 0.000 description 2
- 208000001647 Renal Insufficiency Diseases 0.000 description 2
- 208000033626 Renal failure acute Diseases 0.000 description 2
- 229920002684 Sepharose Polymers 0.000 description 2
- 108010071390 Serum Albumin Proteins 0.000 description 2
- 102000007562 Serum Albumin Human genes 0.000 description 2
- PXIPVTKHYLBLMZ-UHFFFAOYSA-N Sodium azide Chemical compound [Na+].[N-]=[N+]=[N-] PXIPVTKHYLBLMZ-UHFFFAOYSA-N 0.000 description 2
- QAOWNCQODCNURD-UHFFFAOYSA-N Sulfuric acid Chemical compound OS(O)(=O)=O QAOWNCQODCNURD-UHFFFAOYSA-N 0.000 description 2
- OUUQCZGPVNCOIJ-UHFFFAOYSA-M Superoxide Chemical compound [O-][O] OUUQCZGPVNCOIJ-UHFFFAOYSA-M 0.000 description 2
- 108010000499 Thromboplastin Proteins 0.000 description 2
- 102000002262 Thromboplastin Human genes 0.000 description 2
- 101710120037 Toxin CcdB Proteins 0.000 description 2
- 239000013504 Triton X-100 Substances 0.000 description 2
- 229920004890 Triton X-100 Polymers 0.000 description 2
- QIVBCDIJIAJPQS-UHFFFAOYSA-N Tryptophan Natural products C1=CC=C2C(CC(N)C(O)=O)=CNC2=C1 QIVBCDIJIAJPQS-UHFFFAOYSA-N 0.000 description 2
- 206010053614 Type III immune complex mediated reaction Diseases 0.000 description 2
- XSQUKJJJFZCRTK-UHFFFAOYSA-N Urea Chemical compound NC(N)=O XSQUKJJJFZCRTK-UHFFFAOYSA-N 0.000 description 2
- 102100033220 Xanthine oxidase Human genes 0.000 description 2
- 238000010521 absorption reaction Methods 0.000 description 2
- 230000021736 acetylation Effects 0.000 description 2
- 238000006640 acetylation reaction Methods 0.000 description 2
- 201000011040 acute kidney failure Diseases 0.000 description 2
- 206010069351 acute lung injury Diseases 0.000 description 2
- 208000012998 acute renal failure Diseases 0.000 description 2
- 230000001464 adherent effect Effects 0.000 description 2
- 150000001408 amides Chemical class 0.000 description 2
- 230000003024 amidolytic effect Effects 0.000 description 2
- 125000003277 amino group Chemical group 0.000 description 2
- BFNBIHQBYMNNAN-UHFFFAOYSA-N ammonium sulfate Chemical compound N.N.OS(O)(=O)=O BFNBIHQBYMNNAN-UHFFFAOYSA-N 0.000 description 2
- 229910052921 ammonium sulfate Inorganic materials 0.000 description 2
- 235000011130 ammonium sulphate Nutrition 0.000 description 2
- 230000003321 amplification Effects 0.000 description 2
- 238000004458 analytical method Methods 0.000 description 2
- 238000010171 animal model Methods 0.000 description 2
- 230000003110 anti-inflammatory effect Effects 0.000 description 2
- 239000003146 anticoagulant agent Substances 0.000 description 2
- 229940127219 anticoagulant drug Drugs 0.000 description 2
- 229940121375 antifungal agent Drugs 0.000 description 2
- 239000003429 antifungal agent Substances 0.000 description 2
- 239000012736 aqueous medium Substances 0.000 description 2
- 206010003246 arthritis Diseases 0.000 description 2
- 230000001580 bacterial effect Effects 0.000 description 2
- 230000031018 biological processes and functions Effects 0.000 description 2
- 229960002685 biotin Drugs 0.000 description 2
- 235000020958 biotin Nutrition 0.000 description 2
- 239000011616 biotin Substances 0.000 description 2
- 239000010836 blood and blood product Substances 0.000 description 2
- 239000003114 blood coagulation factor Substances 0.000 description 2
- 238000004820 blood count Methods 0.000 description 2
- 230000036770 blood supply Effects 0.000 description 2
- 210000004204 blood vessel Anatomy 0.000 description 2
- 210000001124 body fluid Anatomy 0.000 description 2
- 239000010839 body fluid Substances 0.000 description 2
- 230000036760 body temperature Effects 0.000 description 2
- 150000001720 carbohydrates Chemical class 0.000 description 2
- 235000014633 carbohydrates Nutrition 0.000 description 2
- 210000000748 cardiovascular system Anatomy 0.000 description 2
- 239000000969 carrier Substances 0.000 description 2
- 238000004113 cell culture Methods 0.000 description 2
- 230000001413 cellular effect Effects 0.000 description 2
- 238000005119 centrifugation Methods 0.000 description 2
- 238000006243 chemical reaction Methods 0.000 description 2
- 239000003153 chemical reaction reagent Substances 0.000 description 2
- 238000002512 chemotherapy Methods 0.000 description 2
- OSASVXMJTNOKOY-UHFFFAOYSA-N chlorobutanol Chemical compound CC(C)(O)C(Cl)(Cl)Cl OSASVXMJTNOKOY-UHFFFAOYSA-N 0.000 description 2
- 210000003483 chromatin Anatomy 0.000 description 2
- 210000000349 chromosome Anatomy 0.000 description 2
- 230000006329 citrullination Effects 0.000 description 2
- 230000015271 coagulation Effects 0.000 description 2
- 238000005345 coagulation Methods 0.000 description 2
- 238000007906 compression Methods 0.000 description 2
- 230000006835 compression Effects 0.000 description 2
- 230000008878 coupling Effects 0.000 description 2
- 238000010168 coupling process Methods 0.000 description 2
- 238000005859 coupling reaction Methods 0.000 description 2
- 229940109239 creatinine Drugs 0.000 description 2
- 238000012258 culturing Methods 0.000 description 2
- 230000002939 deleterious effect Effects 0.000 description 2
- 238000002405 diagnostic procedure Methods 0.000 description 2
- 230000029087 digestion Effects 0.000 description 2
- 238000010790 dilution Methods 0.000 description 2
- 239000012895 dilution Substances 0.000 description 2
- 239000000539 dimer Substances 0.000 description 2
- 239000002270 dispersing agent Substances 0.000 description 2
- 230000002222 downregulating effect Effects 0.000 description 2
- 230000003828 downregulation Effects 0.000 description 2
- 108010008250 drotrecogin alfa activated Proteins 0.000 description 2
- 239000003937 drug carrier Substances 0.000 description 2
- 230000002500 effect on skin Effects 0.000 description 2
- 238000001962 electrophoresis Methods 0.000 description 2
- 238000010828 elution Methods 0.000 description 2
- 210000002615 epidermis Anatomy 0.000 description 2
- 238000002474 experimental method Methods 0.000 description 2
- 239000000284 extract Substances 0.000 description 2
- 239000011536 extraction buffer Substances 0.000 description 2
- 238000001914 filtration Methods 0.000 description 2
- 238000005194 fractionation Methods 0.000 description 2
- 239000007789 gas Substances 0.000 description 2
- 239000000499 gel Substances 0.000 description 2
- 238000001502 gel electrophoresis Methods 0.000 description 2
- 150000004676 glycans Chemical class 0.000 description 2
- 230000012010 growth Effects 0.000 description 2
- 230000036541 health Effects 0.000 description 2
- 208000019622 heart disease Diseases 0.000 description 2
- 208000013746 hereditary thrombophilia due to congenital protein C deficiency Diseases 0.000 description 2
- 229940006607 hirudin Drugs 0.000 description 2
- WQPDUTSPKFMPDP-OUMQNGNKSA-N hirudin Chemical compound C([C@@H](C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N1[C@@H](CCC1)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC=1C=CC(OS(O)(=O)=O)=CC=1)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(N)=O)C(O)=O)NC(=O)[C@H](CC(O)=O)NC(=O)CNC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CC=1NC=NC=1)NC(=O)[C@H](CO)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H]1N(CCC1)C(=O)[C@H](CCCCN)NC(=O)[C@H]1N(CCC1)C(=O)[C@@H](NC(=O)CNC(=O)[C@H](CCC(O)=O)NC(=O)CNC(=O)[C@@H](NC(=O)[C@@H](NC(=O)[C@H]1NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CCC(O)=O)NC(=O)CNC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CO)NC(=O)CNC(=O)[C@H](CC(C)C)NC(=O)[C@H]([C@@H](C)CC)NC(=O)[C@@H]2CSSC[C@@H](C(=O)N[C@@H](CCC(O)=O)C(=O)NCC(=O)N[C@@H](CO)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@H](C(=O)N[C@H](C(NCC(=O)N[C@@H](CCC(N)=O)C(=O)NCC(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CCCCN)C(=O)N2)=O)CSSC1)C(C)C)NC(=O)[C@H](CC(C)C)NC(=O)[C@H]1NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CCC(N)=O)NC(=O)CNC(=O)[C@H](CO)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H]([C@@H](C)O)NC(=O)[C@@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H](NC(=O)[C@H](CC=2C=CC(O)=CC=2)NC(=O)[C@@H](NC(=O)[C@@H](N)C(C)C)C(C)C)[C@@H](C)O)CSSC1)C(C)C)[C@@H](C)O)[C@@H](C)O)C1=CC=CC=C1 WQPDUTSPKFMPDP-OUMQNGNKSA-N 0.000 description 2
- 229940100689 human protein c Drugs 0.000 description 2
- JYGXADMDTFJGBT-VWUMJDOOSA-N hydrocortisone Chemical compound O=C1CC[C@]2(C)[C@H]3[C@@H](O)C[C@](C)([C@@](CC4)(O)C(=O)CO)[C@@H]4[C@@H]3CCC2=C1 JYGXADMDTFJGBT-VWUMJDOOSA-N 0.000 description 2
- 208000000122 hyperventilation Diseases 0.000 description 2
- 230000000870 hyperventilation Effects 0.000 description 2
- 230000007954 hypoxia Effects 0.000 description 2
- 230000003100 immobilizing effect Effects 0.000 description 2
- 230000016178 immune complex formation Effects 0.000 description 2
- 238000001114 immunoprecipitation Methods 0.000 description 2
- 230000001771 impaired effect Effects 0.000 description 2
- 230000028709 inflammatory response Effects 0.000 description 2
- 229940047122 interleukins Drugs 0.000 description 2
- 230000000302 ischemic effect Effects 0.000 description 2
- 239000007951 isotonicity adjuster Substances 0.000 description 2
- 210000003734 kidney Anatomy 0.000 description 2
- 201000006370 kidney failure Diseases 0.000 description 2
- 230000003907 kidney function Effects 0.000 description 2
- 230000001665 lethal effect Effects 0.000 description 2
- 210000004698 lymphocyte Anatomy 0.000 description 2
- 238000012423 maintenance Methods 0.000 description 2
- 238000005259 measurement Methods 0.000 description 2
- 230000011278 mitosis Effects 0.000 description 2
- 208000029744 multiple organ dysfunction syndrome Diseases 0.000 description 2
- 230000007935 neutral effect Effects 0.000 description 2
- 210000000440 neutrophil Anatomy 0.000 description 2
- 230000009871 nonspecific binding Effects 0.000 description 2
- 238000003199 nucleic acid amplification method Methods 0.000 description 2
- 239000003921 oil Substances 0.000 description 2
- 235000019198 oils Nutrition 0.000 description 2
- 210000004789 organ system Anatomy 0.000 description 2
- 238000007500 overflow downdraw method Methods 0.000 description 2
- 230000036961 partial effect Effects 0.000 description 2
- 244000052769 pathogen Species 0.000 description 2
- 108040007629 peroxidase activity proteins Proteins 0.000 description 2
- NBIIXXVUZAFLBC-UHFFFAOYSA-K phosphate Chemical compound [O-]P([O-])([O-])=O NBIIXXVUZAFLBC-UHFFFAOYSA-K 0.000 description 2
- 239000010452 phosphate Substances 0.000 description 2
- 230000026731 phosphorylation Effects 0.000 description 2
- 238000006366 phosphorylation reaction Methods 0.000 description 2
- 229920000642 polymer Polymers 0.000 description 2
- 229920001282 polysaccharide Polymers 0.000 description 2
- 239000005017 polysaccharide Substances 0.000 description 2
- 239000011148 porous material Substances 0.000 description 2
- 230000004481 post-translational protein modification Effects 0.000 description 2
- 238000011533 pre-incubation Methods 0.000 description 2
- 238000001556 precipitation Methods 0.000 description 2
- 230000002265 prevention Effects 0.000 description 2
- 230000002633 protecting effect Effects 0.000 description 2
- 239000002510 pyrogen Substances 0.000 description 2
- 238000011002 quantification Methods 0.000 description 2
- 230000005855 radiation Effects 0.000 description 2
- 238000006722 reduction reaction Methods 0.000 description 2
- 230000001105 regulatory effect Effects 0.000 description 2
- 230000008085 renal dysfunction Effects 0.000 description 2
- 230000010410 reperfusion Effects 0.000 description 2
- 239000011347 resin Substances 0.000 description 2
- 229920005989 resin Polymers 0.000 description 2
- 206010039073 rheumatoid arthritis Diseases 0.000 description 2
- 238000012163 sequencing technique Methods 0.000 description 2
- SQGYOTSLMSWVJD-UHFFFAOYSA-N silver(1+) nitrate Chemical compound [Ag+].[O-]N(=O)=O SQGYOTSLMSWVJD-UHFFFAOYSA-N 0.000 description 2
- 210000001082 somatic cell Anatomy 0.000 description 2
- 238000001179 sorption measurement Methods 0.000 description 2
- 238000010561 standard procedure Methods 0.000 description 2
- 238000003860 storage Methods 0.000 description 2
- 235000000346 sugar Nutrition 0.000 description 2
- 230000010741 sumoylation Effects 0.000 description 2
- 239000004094 surface-active agent Substances 0.000 description 2
- 208000008203 tachypnea Diseases 0.000 description 2
- 206010043089 tachypnoea Diseases 0.000 description 2
- 230000008685 targeting Effects 0.000 description 2
- GETQZCLCWQTVFV-UHFFFAOYSA-N trimethylamine Chemical compound CN(C)C GETQZCLCWQTVFV-UHFFFAOYSA-N 0.000 description 2
- 230000034512 ubiquitination Effects 0.000 description 2
- 238000010798 ubiquitination Methods 0.000 description 2
- 210000002700 urine Anatomy 0.000 description 2
- 210000003462 vein Anatomy 0.000 description 2
- QBYIENPQHBMVBV-HFEGYEGKSA-N (2R)-2-hydroxy-2-phenylacetic acid Chemical compound O[C@@H](C(O)=O)c1ccccc1.O[C@@H](C(O)=O)c1ccccc1 QBYIENPQHBMVBV-HFEGYEGKSA-N 0.000 description 1
- MTCFGRXMJLQNBG-REOHCLBHSA-N (2S)-2-Amino-3-hydroxypropansäure Chemical compound OC[C@H](N)C(O)=O MTCFGRXMJLQNBG-REOHCLBHSA-N 0.000 description 1
- OEDPHAKKZGDBEV-GFPBKZJXSA-N (2s)-6-amino-2-[[(2s)-6-amino-2-[[(2s)-6-amino-2-[[(2s)-6-amino-2-[[(2s)-2-[[(2r)-3-[2,3-di(hexadecanoyloxy)propylsulfanyl]-2-(hexadecanoylamino)propanoyl]amino]-3-hydroxypropanoyl]amino]hexanoyl]amino]hexanoyl]amino]hexanoyl]amino]hexanoic acid Chemical compound NCCCC[C@@H](C(O)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CO)NC(=O)[C@@H](NC(=O)CCCCCCCCCCCCCCC)CSCC(COC(=O)CCCCCCCCCCCCCCC)OC(=O)CCCCCCCCCCCCCCC OEDPHAKKZGDBEV-GFPBKZJXSA-N 0.000 description 1
- LOGFVTREOLYCPF-KXNHARMFSA-N (2s,3r)-2-[[(2r)-1-[(2s)-2,6-diaminohexanoyl]pyrrolidine-2-carbonyl]amino]-3-hydroxybutanoic acid Chemical compound C[C@@H](O)[C@@H](C(O)=O)NC(=O)[C@H]1CCCN1C(=O)[C@@H](N)CCCCN LOGFVTREOLYCPF-KXNHARMFSA-N 0.000 description 1
- IIZPXYDJLKNOIY-JXPKJXOSSA-N 1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphocholine Chemical compound CCCCCCCCCCCCCCCC(=O)OC[C@H](COP([O-])(=O)OCC[N+](C)(C)C)OC(=O)CCC\C=C/C\C=C/C\C=C/C\C=C/CCCCC IIZPXYDJLKNOIY-JXPKJXOSSA-N 0.000 description 1
- OWEGMIWEEQEYGQ-UHFFFAOYSA-N 100676-05-9 Natural products OC1C(O)C(O)C(CO)OC1OCC1C(O)C(O)C(O)C(OC2C(OC(O)C(O)C2O)CO)O1 OWEGMIWEEQEYGQ-UHFFFAOYSA-N 0.000 description 1
- NHBKXEKEPDILRR-UHFFFAOYSA-N 2,3-bis(butanoylsulfanyl)propyl butanoate Chemical compound CCCC(=O)OCC(SC(=O)CCC)CSC(=O)CCC NHBKXEKEPDILRR-UHFFFAOYSA-N 0.000 description 1
- JKMHFZQWWAIEOD-UHFFFAOYSA-N 2-[4-(2-hydroxyethyl)piperazin-1-yl]ethanesulfonic acid Chemical compound OCC[NH+]1CCN(CCS([O-])(=O)=O)CC1 JKMHFZQWWAIEOD-UHFFFAOYSA-N 0.000 description 1
- QKNYBSVHEMOAJP-UHFFFAOYSA-N 2-amino-2-(hydroxymethyl)propane-1,3-diol;hydron;chloride Chemical compound Cl.OCC(N)(CO)CO QKNYBSVHEMOAJP-UHFFFAOYSA-N 0.000 description 1
- MSWZFWKMSRAUBD-GASJEMHNSA-N 2-amino-2-deoxy-D-galactopyranose Chemical compound N[C@H]1C(O)O[C@H](CO)[C@H](O)[C@@H]1O MSWZFWKMSRAUBD-GASJEMHNSA-N 0.000 description 1
- LPXQRXLUHJKZIE-UHFFFAOYSA-N 8-azaguanine Chemical compound NC1=NC(O)=C2NN=NC2=N1 LPXQRXLUHJKZIE-UHFFFAOYSA-N 0.000 description 1
- 229960005508 8-azaguanine Drugs 0.000 description 1
- 101710197241 Accessory gland-specific peptide 70A Proteins 0.000 description 1
- 102000007469 Actins Human genes 0.000 description 1
- 108010085238 Actins Proteins 0.000 description 1
- 101710112282 Adenomatous polyposis coli protein Proteins 0.000 description 1
- 102100034540 Adenomatous polyposis coli protein Human genes 0.000 description 1
- 206010001367 Adrenal insufficiency Diseases 0.000 description 1
- 229920000936 Agarose Polymers 0.000 description 1
- 108010088751 Albumins Proteins 0.000 description 1
- 102000009027 Albumins Human genes 0.000 description 1
- QGZKDVFQNNGYKY-UHFFFAOYSA-O Ammonium Chemical compound [NH4+] QGZKDVFQNNGYKY-UHFFFAOYSA-O 0.000 description 1
- 206010002091 Anaesthesia Diseases 0.000 description 1
- 206010002329 Aneurysm Diseases 0.000 description 1
- 206010002383 Angina Pectoris Diseases 0.000 description 1
- 206010002556 Ankylosing Spondylitis Diseases 0.000 description 1
- 102000004411 Antithrombin III Human genes 0.000 description 1
- 108090000935 Antithrombin III Proteins 0.000 description 1
- 244000105624 Arachis hypogaea Species 0.000 description 1
- 200000000007 Arterial disease Diseases 0.000 description 1
- 206010003267 Arthritis reactive Diseases 0.000 description 1
- 206010003274 Arthritis viral Diseases 0.000 description 1
- 206010003445 Ascites Diseases 0.000 description 1
- BSYNRYMUTXBXSQ-UHFFFAOYSA-N Aspirin Chemical compound CC(=O)OC1=CC=CC=C1C(O)=O BSYNRYMUTXBXSQ-UHFFFAOYSA-N 0.000 description 1
- 206010003757 Atypical pneumonia Diseases 0.000 description 1
- BTBUEUYNUDRHOZ-UHFFFAOYSA-N Borate Chemical compound [O-]B([O-])[O-] BTBUEUYNUDRHOZ-UHFFFAOYSA-N 0.000 description 1
- 206010006803 Burns third degree Diseases 0.000 description 1
- 108010074051 C-Reactive Protein Proteins 0.000 description 1
- 102100032752 C-reactive protein Human genes 0.000 description 1
- OYPRJOBELJOOCE-UHFFFAOYSA-N Calcium Chemical compound [Ca] OYPRJOBELJOOCE-UHFFFAOYSA-N 0.000 description 1
- 244000045232 Canavalia ensiformis Species 0.000 description 1
- 235000010520 Canavalia ensiformis Nutrition 0.000 description 1
- 239000004215 Carbon black (E152) Substances 0.000 description 1
- 206010007559 Cardiac failure congestive Diseases 0.000 description 1
- 208000031229 Cardiomyopathies Diseases 0.000 description 1
- 108010076119 Caseins Proteins 0.000 description 1
- 241000700199 Cavia porcellus Species 0.000 description 1
- 206010009192 Circulatory collapse Diseases 0.000 description 1
- 108091026890 Coding region Proteins 0.000 description 1
- 206010009900 Colitis ulcerative Diseases 0.000 description 1
- 208000035473 Communicable disease Diseases 0.000 description 1
- 108010062580 Concanavalin A Proteins 0.000 description 1
- 208000002330 Congenital Heart Defects Diseases 0.000 description 1
- 206010056370 Congestive cardiomyopathy Diseases 0.000 description 1
- 239000000055 Corticotropin-Releasing Hormone Substances 0.000 description 1
- 241000699800 Cricetinae Species 0.000 description 1
- 208000011231 Crohn disease Diseases 0.000 description 1
- 150000008574 D-amino acids Chemical class 0.000 description 1
- LEVWYRKDKASIDU-QWWZWVQMSA-N D-cystine Chemical compound OC(=O)[C@H](N)CSSC[C@@H](N)C(O)=O LEVWYRKDKASIDU-QWWZWVQMSA-N 0.000 description 1
- SHZGCJCMOBCMKK-UHFFFAOYSA-N D-mannomethylose Natural products CC1OC(O)C(O)C(O)C1O SHZGCJCMOBCMKK-UHFFFAOYSA-N 0.000 description 1
- 102000053602 DNA Human genes 0.000 description 1
- 230000033616 DNA repair Effects 0.000 description 1
- 102000052510 DNA-Binding Proteins Human genes 0.000 description 1
- 101710096438 DNA-binding protein Proteins 0.000 description 1
- FEWJPZIEWOKRBE-JCYAYHJZSA-N Dextrotartaric acid Chemical compound OC(=O)[C@H](O)[C@@H](O)C(O)=O FEWJPZIEWOKRBE-JCYAYHJZSA-N 0.000 description 1
- 208000008960 Diabetic foot Diseases 0.000 description 1
- 238000009007 Diagnostic Kit Methods 0.000 description 1
- 206010052337 Diastolic dysfunction Diseases 0.000 description 1
- 201000010046 Dilated cardiomyopathy Diseases 0.000 description 1
- MYMOFIZGZYHOMD-UHFFFAOYSA-N Dioxygen Chemical compound O=O MYMOFIZGZYHOMD-UHFFFAOYSA-N 0.000 description 1
- 208000000059 Dyspnea Diseases 0.000 description 1
- 206010013975 Dyspnoeas Diseases 0.000 description 1
- 206010014357 Electric shock Diseases 0.000 description 1
- 206010069808 Electrical burn Diseases 0.000 description 1
- 206010014522 Embolism venous Diseases 0.000 description 1
- 241001596967 Escherichia coli M15 Species 0.000 description 1
- 102000001690 Factor VIII Human genes 0.000 description 1
- 108010061932 Factor VIIIa Proteins 0.000 description 1
- 206010016207 Familial Mediterranean fever Diseases 0.000 description 1
- 108010049003 Fibrinogen Proteins 0.000 description 1
- 102000008946 Fibrinogen Human genes 0.000 description 1
- 241000233866 Fungi Species 0.000 description 1
- 108010010803 Gelatin Proteins 0.000 description 1
- CEAZRRDELHUEMR-URQXQFDESA-N Gentamicin Chemical compound O1[C@H](C(C)NC)CC[C@@H](N)[C@H]1O[C@H]1[C@H](O)[C@@H](O[C@@H]2[C@@H]([C@@H](NC)[C@@](C)(O)CO2)O)[C@H](N)C[C@@H]1N CEAZRRDELHUEMR-URQXQFDESA-N 0.000 description 1
- 229930182566 Gentamicin Natural products 0.000 description 1
- WQZGKKKJIJFFOK-GASJEMHNSA-N Glucose Natural products OC[C@H]1OC(O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-GASJEMHNSA-N 0.000 description 1
- 108010015776 Glucose oxidase Proteins 0.000 description 1
- 239000004366 Glucose oxidase Substances 0.000 description 1
- SXRSQZLOMIGNAQ-UHFFFAOYSA-N Glutaraldehyde Chemical compound O=CCCCC=O SXRSQZLOMIGNAQ-UHFFFAOYSA-N 0.000 description 1
- 244000068988 Glycine max Species 0.000 description 1
- 235000010469 Glycine max Nutrition 0.000 description 1
- 229920002683 Glycosaminoglycan Polymers 0.000 description 1
- 239000007995 HEPES buffer Substances 0.000 description 1
- 229940121710 HMGCoA reductase inhibitor Drugs 0.000 description 1
- 206010019280 Heart failures Diseases 0.000 description 1
- 108010058611 Helix lectin Proteins 0.000 description 1
- 208000032456 Hemorrhagic Shock Diseases 0.000 description 1
- 101000917858 Homo sapiens Low affinity immunoglobulin gamma Fc region receptor III-A Proteins 0.000 description 1
- 101000917839 Homo sapiens Low affinity immunoglobulin gamma Fc region receptor III-B Proteins 0.000 description 1
- 101001098529 Homo sapiens Proteinase-activated receptor 1 Proteins 0.000 description 1
- 101000713169 Homo sapiens Solute carrier family 52, riboflavin transporter, member 2 Proteins 0.000 description 1
- 229920002153 Hydroxypropyl cellulose Polymers 0.000 description 1
- 206010058558 Hypoperfusion Diseases 0.000 description 1
- DGAQECJNVWCQMB-PUAWFVPOSA-M Ilexoside XXIX Chemical compound C[C@@H]1CC[C@@]2(CC[C@@]3(C(=CC[C@H]4[C@]3(CC[C@@H]5[C@@]4(CC[C@@H](C5(C)C)OS(=O)(=O)[O-])C)C)[C@@H]2[C@]1(C)O)C)C(=O)O[C@H]6[C@@H]([C@H]([C@@H]([C@H](O6)CO)O)O)O.[Na+] DGAQECJNVWCQMB-PUAWFVPOSA-M 0.000 description 1
- 108060003951 Immunoglobulin Proteins 0.000 description 1
- 208000004575 Infectious Arthritis Diseases 0.000 description 1
- 102000003777 Interleukin-1 beta Human genes 0.000 description 1
- 108090000193 Interleukin-1 beta Proteins 0.000 description 1
- 206010061245 Internal injury Diseases 0.000 description 1
- SHZGCJCMOBCMKK-PQMKYFCFSA-N L-Fucose Natural products C[C@H]1O[C@H](O)[C@@H](O)[C@@H](O)[C@@H]1O SHZGCJCMOBCMKK-PQMKYFCFSA-N 0.000 description 1
- SHZGCJCMOBCMKK-DHVFOXMCSA-N L-fucopyranose Chemical compound C[C@@H]1OC(O)[C@@H](O)[C@H](O)[C@@H]1O SHZGCJCMOBCMKK-DHVFOXMCSA-N 0.000 description 1
- FFFHZYDWPBMWHY-VKHMYHEASA-N L-homocysteine Chemical compound OC(=O)[C@@H](N)CCS FFFHZYDWPBMWHY-VKHMYHEASA-N 0.000 description 1
- PNNNRSAQSRJVSB-UHFFFAOYSA-N L-rhamnose Natural products CC(O)C(O)C(O)C(O)C=O PNNNRSAQSRJVSB-UHFFFAOYSA-N 0.000 description 1
- AYFVYJQAPQTCCC-GBXIJSLDSA-N L-threonine Chemical compound C[C@@H](O)[C@H](N)C(O)=O AYFVYJQAPQTCCC-GBXIJSLDSA-N 0.000 description 1
- JVTAAEKCZFNVCJ-UHFFFAOYSA-M Lactate Chemical compound CC(O)C([O-])=O JVTAAEKCZFNVCJ-UHFFFAOYSA-M 0.000 description 1
- GUBGYTABKSRVRQ-QKKXKWKRSA-N Lactose Natural products OC[C@H]1O[C@@H](O[C@H]2[C@H](O)[C@@H](O)C(O)O[C@@H]2CO)[C@H](O)[C@@H](O)[C@H]1O GUBGYTABKSRVRQ-QKKXKWKRSA-N 0.000 description 1
- 235000014647 Lens culinaris subsp culinaris Nutrition 0.000 description 1
- 244000043158 Lens esculenta Species 0.000 description 1
- 108091036060 Linker DNA Proteins 0.000 description 1
- 108090001030 Lipoproteins Proteins 0.000 description 1
- 102000004895 Lipoproteins Human genes 0.000 description 1
- 102100029185 Low affinity immunoglobulin gamma Fc region receptor III-B Human genes 0.000 description 1
- 208000004852 Lung Injury Diseases 0.000 description 1
- 208000019693 Lung disease Diseases 0.000 description 1
- 208000032376 Lung infection Diseases 0.000 description 1
- 239000006142 Luria-Bertani Agar Substances 0.000 description 1
- GUBGYTABKSRVRQ-PICCSMPSSA-N Maltose Natural products O[C@@H]1[C@@H](O)[C@H](O)[C@@H](CO)O[C@@H]1O[C@@H]1[C@@H](CO)OC(O)[C@H](O)[C@H]1O GUBGYTABKSRVRQ-PICCSMPSSA-N 0.000 description 1
- 208000003430 Mitral Valve Prolapse Diseases 0.000 description 1
- 208000034486 Multi-organ failure Diseases 0.000 description 1
- 208000010718 Multiple Organ Failure Diseases 0.000 description 1
- 241000711408 Murine respirovirus Species 0.000 description 1
- 101000924587 Mus musculus Adenomatous polyposis coli protein Proteins 0.000 description 1
- 241000187479 Mycobacterium tuberculosis Species 0.000 description 1
- 208000009525 Myocarditis Diseases 0.000 description 1
- YDGMGEXADBMOMJ-LURJTMIESA-N N(g)-dimethylarginine Chemical compound CN(C)C(\N)=N\CCC[C@H](N)C(O)=O YDGMGEXADBMOMJ-LURJTMIESA-N 0.000 description 1
- OVRNDRQMDRJTHS-CBQIKETKSA-N N-Acetyl-D-Galactosamine Chemical compound CC(=O)N[C@H]1[C@@H](O)O[C@H](CO)[C@H](O)[C@@H]1O OVRNDRQMDRJTHS-CBQIKETKSA-N 0.000 description 1
- MBLBDJOUHNCFQT-UHFFFAOYSA-N N-acetyl-D-galactosamine Natural products CC(=O)NC(C=O)C(O)C(O)C(O)CO MBLBDJOUHNCFQT-UHFFFAOYSA-N 0.000 description 1
- 240000002853 Nelumbo nucifera Species 0.000 description 1
- 235000006508 Nelumbo nucifera Nutrition 0.000 description 1
- 235000006510 Nelumbo pentapetala Nutrition 0.000 description 1
- GRYLNZFGIOXLOG-UHFFFAOYSA-N Nitric acid Chemical compound O[N+]([O-])=O GRYLNZFGIOXLOG-UHFFFAOYSA-N 0.000 description 1
- 102000007999 Nuclear Proteins Human genes 0.000 description 1
- 108010089610 Nuclear Proteins Proteins 0.000 description 1
- 108091028043 Nucleic acid sequence Proteins 0.000 description 1
- 108010058846 Ovalbumin Proteins 0.000 description 1
- 239000002033 PVDF binder Substances 0.000 description 1
- 208000002193 Pain Diseases 0.000 description 1
- 108090000526 Papain Proteins 0.000 description 1
- 206010034246 Pelvic fractures Diseases 0.000 description 1
- 102000057297 Pepsin A Human genes 0.000 description 1
- 108090000284 Pepsin A Proteins 0.000 description 1
- 102000035195 Peptidases Human genes 0.000 description 1
- 108010022233 Plasminogen Activator Inhibitor 1 Proteins 0.000 description 1
- 102100039418 Plasminogen activator inhibitor 1 Human genes 0.000 description 1
- 241000276498 Pollachius virens Species 0.000 description 1
- 239000004793 Polystyrene Substances 0.000 description 1
- ZLMJMSJWJFRBEC-UHFFFAOYSA-N Potassium Chemical compound [K] ZLMJMSJWJFRBEC-UHFFFAOYSA-N 0.000 description 1
- 208000004210 Pressure Ulcer Diseases 0.000 description 1
- 206010036790 Productive cough Diseases 0.000 description 1
- 229940124158 Protease/peptidase inhibitor Drugs 0.000 description 1
- 101710121440 Proteinase-activated receptor 1 Proteins 0.000 description 1
- 102100037136 Proteinase-activated receptor 1 Human genes 0.000 description 1
- 201000004681 Psoriasis Diseases 0.000 description 1
- 201000001263 Psoriatic Arthritis Diseases 0.000 description 1
- 208000036824 Psoriatic arthropathy Diseases 0.000 description 1
- 206010037370 Pulmonary contusion Diseases 0.000 description 1
- 206010037660 Pyrexia Diseases 0.000 description 1
- IWYDHOAUDWTVEP-UHFFFAOYSA-N R-2-phenyl-2-hydroxyacetic acid Natural products OC(=O)C(O)C1=CC=CC=C1 IWYDHOAUDWTVEP-UHFFFAOYSA-N 0.000 description 1
- 108020004511 Recombinant DNA Proteins 0.000 description 1
- 108700008625 Reporter Genes Proteins 0.000 description 1
- 208000021063 Respiratory fume inhalation disease Diseases 0.000 description 1
- 240000000528 Ricinus communis Species 0.000 description 1
- 235000004443 Ricinus communis Nutrition 0.000 description 1
- 241000283984 Rodentia Species 0.000 description 1
- 241000293869 Salmonella enterica subsp. enterica serovar Typhimurium Species 0.000 description 1
- 206010039509 Scab Diseases 0.000 description 1
- 102100028904 Serine/threonine-protein kinase MARK2 Human genes 0.000 description 1
- 206010049771 Shock haemorrhagic Diseases 0.000 description 1
- 208000021386 Sjogren Syndrome Diseases 0.000 description 1
- 229930006000 Sucrose Natural products 0.000 description 1
- CZMRCDWAGMRECN-UGDNZRGBSA-N Sucrose Chemical compound O[C@H]1[C@H](O)[C@@H](CO)O[C@@]1(CO)O[C@@H]1[C@H](O)[C@@H](O)[C@H](O)[C@@H](CO)O1 CZMRCDWAGMRECN-UGDNZRGBSA-N 0.000 description 1
- 208000001871 Tachycardia Diseases 0.000 description 1
- FEWJPZIEWOKRBE-UHFFFAOYSA-N Tartaric acid Natural products [H+].[H+].[O-]C(=O)C(O)C(O)C([O-])=O FEWJPZIEWOKRBE-UHFFFAOYSA-N 0.000 description 1
- 208000030886 Traumatic Brain injury Diseases 0.000 description 1
- 206010069363 Traumatic lung injury Diseases 0.000 description 1
- 241000209140 Triticum Species 0.000 description 1
- 235000021307 Triticum Nutrition 0.000 description 1
- 108090000631 Trypsin Proteins 0.000 description 1
- 102000004142 Trypsin Human genes 0.000 description 1
- 208000037386 Typhoid Diseases 0.000 description 1
- 108090000848 Ubiquitin Proteins 0.000 description 1
- 102000044159 Ubiquitin Human genes 0.000 description 1
- 201000006704 Ulcerative Colitis Diseases 0.000 description 1
- 108010046334 Urease Proteins 0.000 description 1
- LEHOTFFKMJEONL-UHFFFAOYSA-N Uric Acid Chemical compound N1C(=O)NC(=O)C2=C1NC(=O)N2 LEHOTFFKMJEONL-UHFFFAOYSA-N 0.000 description 1
- TVWHNULVHGKJHS-UHFFFAOYSA-N Uric acid Natural products N1C(=O)NC(=O)C2NC(=O)NC21 TVWHNULVHGKJHS-UHFFFAOYSA-N 0.000 description 1
- 208000024248 Vascular System injury Diseases 0.000 description 1
- 208000012339 Vascular injury Diseases 0.000 description 1
- 206010047642 Vitiligo Diseases 0.000 description 1
- 108010046516 Wheat Germ Agglutinins Proteins 0.000 description 1
- 102000005773 Xanthine dehydrogenase Human genes 0.000 description 1
- 108010091383 Xanthine dehydrogenase Proteins 0.000 description 1
- 230000002159 abnormal effect Effects 0.000 description 1
- 239000003070 absorption delaying agent Substances 0.000 description 1
- 235000011054 acetic acid Nutrition 0.000 description 1
- 125000000218 acetic acid group Chemical group C(C)(=O)* 0.000 description 1
- 229960001138 acetylsalicylic acid Drugs 0.000 description 1
- 150000007513 acids Chemical class 0.000 description 1
- 239000013543 active substance Substances 0.000 description 1
- 208000038016 acute inflammation Diseases 0.000 description 1
- 230000006022 acute inflammation Effects 0.000 description 1
- 208000017515 adrenocortical insufficiency Diseases 0.000 description 1
- 208000011341 adult acute respiratory distress syndrome Diseases 0.000 description 1
- 239000000556 agonist Substances 0.000 description 1
- 230000000172 allergic effect Effects 0.000 description 1
- WNROFYMDJYEPJX-UHFFFAOYSA-K aluminium hydroxide Chemical compound [OH-].[OH-].[OH-].[Al+3] WNROFYMDJYEPJX-UHFFFAOYSA-K 0.000 description 1
- 229910000147 aluminium phosphate Inorganic materials 0.000 description 1
- 125000000539 amino acid group Chemical group 0.000 description 1
- 229960000723 ampicillin Drugs 0.000 description 1
- AVKUERGKIZMTKX-NJBDSQKTSA-N ampicillin Chemical compound C1([C@@H](N)C(=O)N[C@H]2[C@H]3SC([C@@H](N3C2=O)C(O)=O)(C)C)=CC=CC=C1 AVKUERGKIZMTKX-NJBDSQKTSA-N 0.000 description 1
- 238000002266 amputation Methods 0.000 description 1
- 206010002026 amyotrophic lateral sclerosis Diseases 0.000 description 1
- 230000037005 anaesthesia Effects 0.000 description 1
- 230000002421 anti-septic effect Effects 0.000 description 1
- 229940088710 antibiotic agent Drugs 0.000 description 1
- 230000009830 antibody antigen interaction Effects 0.000 description 1
- 230000009833 antibody interaction Effects 0.000 description 1
- 239000003529 anticholesteremic agent Substances 0.000 description 1
- 229940127226 anticholesterol agent Drugs 0.000 description 1
- 230000010100 anticoagulation Effects 0.000 description 1
- 230000009831 antigen interaction Effects 0.000 description 1
- 230000000890 antigenic effect Effects 0.000 description 1
- 229960005348 antithrombin iii Drugs 0.000 description 1
- 230000001640 apoptogenic effect Effects 0.000 description 1
- 230000006907 apoptotic process Effects 0.000 description 1
- 230000006793 arrhythmia Effects 0.000 description 1
- 206010003119 arrhythmia Diseases 0.000 description 1
- 210000001367 artery Anatomy 0.000 description 1
- 208000010668 atopic eczema Diseases 0.000 description 1
- 208000014171 autosomal dominant thrombophilia due to protein C deficiency Diseases 0.000 description 1
- 244000052616 bacterial pathogen Species 0.000 description 1
- 239000011324 bead Substances 0.000 description 1
- 230000009286 beneficial effect Effects 0.000 description 1
- HFACYLZERDEVSX-UHFFFAOYSA-N benzidine Chemical compound C1=CC(N)=CC=C1C1=CC=C(N)C=C1 HFACYLZERDEVSX-UHFFFAOYSA-N 0.000 description 1
- 239000003782 beta lactam antibiotic agent Substances 0.000 description 1
- MSWZFWKMSRAUBD-UHFFFAOYSA-N beta-D-galactosamine Natural products NC1C(O)OC(CO)C(O)C1O MSWZFWKMSRAUBD-UHFFFAOYSA-N 0.000 description 1
- WQZGKKKJIJFFOK-VFUOTHLCSA-N beta-D-glucose Chemical compound OC[C@H]1O[C@@H](O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-VFUOTHLCSA-N 0.000 description 1
- 125000002619 bicyclic group Chemical group 0.000 description 1
- 230000002146 bilateral effect Effects 0.000 description 1
- 230000008827 biological function Effects 0.000 description 1
- 229960000074 biopharmaceutical Drugs 0.000 description 1
- 238000001574 biopsy Methods 0.000 description 1
- 230000000903 blocking effect Effects 0.000 description 1
- 210000000601 blood cell Anatomy 0.000 description 1
- 230000017531 blood circulation Effects 0.000 description 1
- 230000023555 blood coagulation Effects 0.000 description 1
- 230000036772 blood pressure Effects 0.000 description 1
- 229940125691 blood product Drugs 0.000 description 1
- 230000037396 body weight Effects 0.000 description 1
- 239000011575 calcium Substances 0.000 description 1
- 229910052791 calcium Inorganic materials 0.000 description 1
- 229940087373 calcium oxide Drugs 0.000 description 1
- BPKIGYQJPYCAOW-FFJTTWKXSA-I calcium;potassium;disodium;(2s)-2-hydroxypropanoate;dichloride;dihydroxide;hydrate Chemical compound O.[OH-].[OH-].[Na+].[Na+].[Cl-].[Cl-].[K+].[Ca+2].C[C@H](O)C([O-])=O BPKIGYQJPYCAOW-FFJTTWKXSA-I 0.000 description 1
- 239000002775 capsule Substances 0.000 description 1
- 239000004202 carbamide Substances 0.000 description 1
- 150000001718 carbodiimides Chemical class 0.000 description 1
- 238000003763 carbonization Methods 0.000 description 1
- 125000003178 carboxy group Chemical group [H]OC(*)=O 0.000 description 1
- 238000012754 cardiac puncture Methods 0.000 description 1
- 230000001269 cardiogenic effect Effects 0.000 description 1
- 239000005018 casein Substances 0.000 description 1
- BECPQYXYKAMYBN-UHFFFAOYSA-N casein, tech. Chemical compound NCCCCC(C(O)=O)N=C(O)C(CC(O)=O)N=C(O)C(CCC(O)=N)N=C(O)C(CC(C)C)N=C(O)C(CCC(O)=O)N=C(O)C(CC(O)=O)N=C(O)C(CCC(O)=O)N=C(O)C(C(C)O)N=C(O)C(CCC(O)=N)N=C(O)C(CCC(O)=N)N=C(O)C(CCC(O)=N)N=C(O)C(CCC(O)=O)N=C(O)C(CCC(O)=O)N=C(O)C(COP(O)(O)=O)N=C(O)C(CCC(O)=N)N=C(O)C(N)CC1=CC=CC=C1 BECPQYXYKAMYBN-UHFFFAOYSA-N 0.000 description 1
- 235000021240 caseins Nutrition 0.000 description 1
- 230000015556 catabolic process Effects 0.000 description 1
- 238000005341 cation exchange Methods 0.000 description 1
- 239000003518 caustics Substances 0.000 description 1
- 230000007910 cell fusion Effects 0.000 description 1
- 210000003855 cell nucleus Anatomy 0.000 description 1
- 230000002490 cerebral effect Effects 0.000 description 1
- 208000026106 cerebrovascular disease Diseases 0.000 description 1
- 239000003638 chemical reducing agent Substances 0.000 description 1
- 231100001157 chemotherapeutic toxicity Toxicity 0.000 description 1
- 229960004926 chlorobutanol Drugs 0.000 description 1
- 238000011210 chromatographic step Methods 0.000 description 1
- 230000005794 circulatory dysfunction Effects 0.000 description 1
- 238000004440 column chromatography Methods 0.000 description 1
- 230000002301 combined effect Effects 0.000 description 1
- 239000002299 complementary DNA Substances 0.000 description 1
- 238000002591 computed tomography Methods 0.000 description 1
- 238000009833 condensation Methods 0.000 description 1
- 230000005494 condensation Effects 0.000 description 1
- 208000028831 congenital heart disease Diseases 0.000 description 1
- 230000001268 conjugating effect Effects 0.000 description 1
- 239000000470 constituent Substances 0.000 description 1
- 238000007796 conventional method Methods 0.000 description 1
- 239000007771 core particle Substances 0.000 description 1
- 210000004087 cornea Anatomy 0.000 description 1
- 208000029078 coronary artery disease Diseases 0.000 description 1
- IDLFZVILOHSSID-OVLDLUHVSA-N corticotropin Chemical compound C([C@@H](C(=O)N[C@@H](CO)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC=1NC=NC=1)C(=O)N[C@@H](CC=1C=CC=CC=1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC=1C2=CC=CC=C2NC=1)C(=O)NCC(=O)N[C@@H](CCCCN)C(=O)N1[C@@H](CCC1)C(=O)N[C@@H](C(C)C)C(=O)NCC(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1[C@@H](CCC1)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC=1C=CC(O)=CC=1)C(=O)N1[C@@H](CCC1)C(=O)N[C@@H](CC(N)=O)C(=O)NCC(=O)N[C@@H](C)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](C)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](C)C(=O)N[C@@H](CC=1C=CC=CC=1)C(=O)N1[C@@H](CCC1)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC=1C=CC=CC=1)C(O)=O)NC(=O)[C@@H](N)CO)C1=CC=C(O)C=C1 IDLFZVILOHSSID-OVLDLUHVSA-N 0.000 description 1
- 229960000258 corticotropin Drugs 0.000 description 1
- 238000002316 cosmetic surgery Methods 0.000 description 1
- 238000005520 cutting process Methods 0.000 description 1
- ATDGTVJJHBUTRL-UHFFFAOYSA-N cyanogen bromide Chemical compound BrC#N ATDGTVJJHBUTRL-UHFFFAOYSA-N 0.000 description 1
- 125000004122 cyclic group Chemical group 0.000 description 1
- 229960003067 cystine Drugs 0.000 description 1
- 230000007423 decrease Effects 0.000 description 1
- 230000007812 deficiency Effects 0.000 description 1
- 238000006731 degradation reaction Methods 0.000 description 1
- 230000000593 degrading effect Effects 0.000 description 1
- 230000001934 delay Effects 0.000 description 1
- 230000003111 delayed effect Effects 0.000 description 1
- 230000001419 dependent effect Effects 0.000 description 1
- 238000003795 desorption Methods 0.000 description 1
- 238000011161 development Methods 0.000 description 1
- 238000006193 diazotization reaction Methods 0.000 description 1
- CGMRCMMOCQYHAD-UHFFFAOYSA-J dicalcium hydroxide phosphate Chemical compound [OH-].[Ca++].[Ca++].[O-]P([O-])([O-])=O CGMRCMMOCQYHAD-UHFFFAOYSA-J 0.000 description 1
- UGMCXQCYOVCMTB-UHFFFAOYSA-K dihydroxy(stearato)aluminium Chemical compound CCCCCCCCCCCCCCCCCC(=O)O[Al](O)O UGMCXQCYOVCMTB-UHFFFAOYSA-K 0.000 description 1
- 238000007865 diluting Methods 0.000 description 1
- 239000003085 diluting agent Substances 0.000 description 1
- 229910001882 dioxygen Inorganic materials 0.000 description 1
- 231100000673 dose–response relationship Toxicity 0.000 description 1
- 238000003110 dot immunobinding assay Methods 0.000 description 1
- 238000001647 drug administration Methods 0.000 description 1
- 238000002283 elective surgery Methods 0.000 description 1
- 230000005611 electricity Effects 0.000 description 1
- 238000002297 emergency surgery Methods 0.000 description 1
- 206010014665 endocarditis Diseases 0.000 description 1
- 239000006274 endogenous ligand Substances 0.000 description 1
- 238000001839 endoscopy Methods 0.000 description 1
- 230000002255 enzymatic effect Effects 0.000 description 1
- 231100000321 erythema Toxicity 0.000 description 1
- BEFDCLMNVWHSGT-UHFFFAOYSA-N ethenylcyclopentane Chemical compound C=CC1CCCC1 BEFDCLMNVWHSGT-UHFFFAOYSA-N 0.000 description 1
- XJRPTMORGOIMMI-UHFFFAOYSA-N ethyl 2-amino-4-(trifluoromethyl)-1,3-thiazole-5-carboxylate Chemical compound CCOC(=O)C=1SC(N)=NC=1C(F)(F)F XJRPTMORGOIMMI-UHFFFAOYSA-N 0.000 description 1
- 238000002270 exclusion chromatography Methods 0.000 description 1
- 239000013604 expression vector Substances 0.000 description 1
- 238000001125 extrusion Methods 0.000 description 1
- 210000000416 exudates and transudate Anatomy 0.000 description 1
- 229960000301 factor viii Drugs 0.000 description 1
- 229960004887 ferric hydroxide Drugs 0.000 description 1
- 230000001605 fetal effect Effects 0.000 description 1
- 229940012952 fibrinogen Drugs 0.000 description 1
- 239000010419 fine particle Substances 0.000 description 1
- 238000002637 fluid replacement therapy Methods 0.000 description 1
- 239000012458 free base Substances 0.000 description 1
- 238000004108 freeze drying Methods 0.000 description 1
- 230000005714 functional activity Effects 0.000 description 1
- 230000002538 fungal effect Effects 0.000 description 1
- 108020001507 fusion proteins Proteins 0.000 description 1
- 102000037865 fusion proteins Human genes 0.000 description 1
- 108010074605 gamma-Globulins Proteins 0.000 description 1
- 238000004868 gas analysis Methods 0.000 description 1
- 238000011902 gastrointestinal surgery Methods 0.000 description 1
- 210000001035 gastrointestinal tract Anatomy 0.000 description 1
- 238000001641 gel filtration chromatography Methods 0.000 description 1
- 239000008273 gelatin Substances 0.000 description 1
- 229920000159 gelatin Polymers 0.000 description 1
- 235000019322 gelatine Nutrition 0.000 description 1
- 235000011852 gelatine desserts Nutrition 0.000 description 1
- 238000002523 gelfiltration Methods 0.000 description 1
- 102000034356 gene-regulatory proteins Human genes 0.000 description 1
- 108091006104 gene-regulatory proteins Proteins 0.000 description 1
- 230000002068 genetic effect Effects 0.000 description 1
- 229960002518 gentamicin Drugs 0.000 description 1
- 230000001434 glomerular Effects 0.000 description 1
- 239000008103 glucose Substances 0.000 description 1
- 229940116332 glucose oxidase Drugs 0.000 description 1
- 235000019420 glucose oxidase Nutrition 0.000 description 1
- 229930182470 glycoside Natural products 0.000 description 1
- 230000013595 glycosylation Effects 0.000 description 1
- 238000006206 glycosylation reaction Methods 0.000 description 1
- 239000001963 growth medium Substances 0.000 description 1
- 238000001631 haemodialysis Methods 0.000 description 1
- 210000005003 heart tissue Anatomy 0.000 description 1
- 238000003505 heat denaturation Methods 0.000 description 1
- 230000000322 hemodialysis Effects 0.000 description 1
- 230000003284 homeostatic effect Effects 0.000 description 1
- 210000005260 human cell Anatomy 0.000 description 1
- 229930195733 hydrocarbon Natural products 0.000 description 1
- 150000002430 hydrocarbons Chemical class 0.000 description 1
- 229960000890 hydrocortisone Drugs 0.000 description 1
- 150000002431 hydrogen Chemical class 0.000 description 1
- 239000001863 hydroxypropyl cellulose Substances 0.000 description 1
- 235000010977 hydroxypropyl cellulose Nutrition 0.000 description 1
- 206010020871 hypertrophic cardiomyopathy Diseases 0.000 description 1
- 230000036543 hypotension Effects 0.000 description 1
- 208000021822 hypotensive Diseases 0.000 description 1
- 230000001077 hypotensive effect Effects 0.000 description 1
- 210000002865 immune cell Anatomy 0.000 description 1
- 210000000987 immune system Anatomy 0.000 description 1
- 102000018358 immunoglobulin Human genes 0.000 description 1
- 230000016784 immunoglobulin production Effects 0.000 description 1
- 239000012133 immunoprecipitate Substances 0.000 description 1
- 239000012535 impurity Substances 0.000 description 1
- 238000011065 in-situ storage Methods 0.000 description 1
- 230000002779 inactivation Effects 0.000 description 1
- 238000010348 incorporation Methods 0.000 description 1
- 230000006698 induction Effects 0.000 description 1
- 230000001939 inductive effect Effects 0.000 description 1
- 230000036512 infertility Effects 0.000 description 1
- 230000004968 inflammatory condition Effects 0.000 description 1
- 230000006749 inflammatory damage Effects 0.000 description 1
- 206010022000 influenza Diseases 0.000 description 1
- 239000003978 infusion fluid Substances 0.000 description 1
- 230000000977 initiatory effect Effects 0.000 description 1
- 239000007972 injectable composition Substances 0.000 description 1
- 150000007529 inorganic bases Chemical class 0.000 description 1
- 238000003780 insertion Methods 0.000 description 1
- 230000037431 insertion Effects 0.000 description 1
- 230000002452 interceptive effect Effects 0.000 description 1
- 238000001361 intraarterial administration Methods 0.000 description 1
- 230000003834 intracellular effect Effects 0.000 description 1
- 238000010253 intravenous injection Methods 0.000 description 1
- 238000011835 investigation Methods 0.000 description 1
- IEECXTSVVFWGSE-UHFFFAOYSA-M iron(3+);oxygen(2-);hydroxide Chemical compound [OH-].[O-2].[Fe+3] IEECXTSVVFWGSE-UHFFFAOYSA-M 0.000 description 1
- 230000001788 irregular Effects 0.000 description 1
- 208000002551 irritable bowel syndrome Diseases 0.000 description 1
- 230000004171 ischemic cascade Effects 0.000 description 1
- 238000001155 isoelectric focusing Methods 0.000 description 1
- 238000002955 isolation Methods 0.000 description 1
- JJWLVOIRVHMVIS-UHFFFAOYSA-N isopropylamine Chemical compound CC(C)N JJWLVOIRVHMVIS-UHFFFAOYSA-N 0.000 description 1
- 230000000366 juvenile effect Effects 0.000 description 1
- 208000017169 kidney disease Diseases 0.000 description 1
- 230000002147 killing effect Effects 0.000 description 1
- 239000008101 lactose Substances 0.000 description 1
- 238000000608 laser ablation Methods 0.000 description 1
- 238000002430 laser surgery Methods 0.000 description 1
- 229940067606 lecithin Drugs 0.000 description 1
- 239000000787 lecithin Substances 0.000 description 1
- 235000010445 lecithin Nutrition 0.000 description 1
- 108010034897 lentil lectin Proteins 0.000 description 1
- 231100000518 lethal Toxicity 0.000 description 1
- 206010024378 leukocytosis Diseases 0.000 description 1
- 230000000670 limiting effect Effects 0.000 description 1
- 238000004811 liquid chromatography Methods 0.000 description 1
- 210000004185 liver Anatomy 0.000 description 1
- 239000012160 loading buffer Substances 0.000 description 1
- 230000033001 locomotion Effects 0.000 description 1
- 230000005980 lung dysfunction Effects 0.000 description 1
- 231100000515 lung injury Toxicity 0.000 description 1
- 201000004792 malaria Diseases 0.000 description 1
- 210000004962 mammalian cell Anatomy 0.000 description 1
- 229960002510 mandelic acid Drugs 0.000 description 1
- 238000001840 matrix-assisted laser desorption--ionisation time-of-flight mass spectrometry Methods 0.000 description 1
- 230000021121 meiosis Effects 0.000 description 1
- 230000034217 membrane fusion Effects 0.000 description 1
- 230000004060 metabolic process Effects 0.000 description 1
- 230000031864 metaphase Effects 0.000 description 1
- 125000002496 methyl group Chemical group [H]C([H])([H])* 0.000 description 1
- 230000000813 microbial effect Effects 0.000 description 1
- 238000009629 microbiological culture Methods 0.000 description 1
- 238000002406 microsurgery Methods 0.000 description 1
- 235000013336 milk Nutrition 0.000 description 1
- 239000008267 milk Substances 0.000 description 1
- 210000004080 milk Anatomy 0.000 description 1
- 150000007522 mineralic acids Chemical class 0.000 description 1
- 238000002324 minimally invasive surgery Methods 0.000 description 1
- 238000010369 molecular cloning Methods 0.000 description 1
- 230000009456 molecular mechanism Effects 0.000 description 1
- 239000002808 molecular sieve Substances 0.000 description 1
- 210000001616 monocyte Anatomy 0.000 description 1
- 229940126619 mouse monoclonal antibody Drugs 0.000 description 1
- 201000006417 multiple sclerosis Diseases 0.000 description 1
- 230000035772 mutation Effects 0.000 description 1
- 208000010125 myocardial infarction Diseases 0.000 description 1
- 230000002988 nephrogenic effect Effects 0.000 description 1
- 210000005036 nerve Anatomy 0.000 description 1
- 210000001640 nerve ending Anatomy 0.000 description 1
- 229910017604 nitric acid Inorganic materials 0.000 description 1
- 231100001160 nonlethal Toxicity 0.000 description 1
- 238000010606 normalization Methods 0.000 description 1
- 230000004145 nucleotide salvage Effects 0.000 description 1
- 210000004940 nucleus Anatomy 0.000 description 1
- 235000015097 nutrients Nutrition 0.000 description 1
- 230000035764 nutrition Effects 0.000 description 1
- 235000016709 nutrition Nutrition 0.000 description 1
- 238000011369 optimal treatment Methods 0.000 description 1
- 150000007524 organic acids Chemical class 0.000 description 1
- 235000005985 organic acids Nutrition 0.000 description 1
- 150000007530 organic bases Chemical class 0.000 description 1
- 230000000399 orthopedic effect Effects 0.000 description 1
- 229940092253 ovalbumin Drugs 0.000 description 1
- 235000006408 oxalic acid Nutrition 0.000 description 1
- 239000007800 oxidant agent Substances 0.000 description 1
- 230000003647 oxidation Effects 0.000 description 1
- 238000007254 oxidation reaction Methods 0.000 description 1
- 230000004792 oxidative damage Effects 0.000 description 1
- 230000036542 oxidative stress Effects 0.000 description 1
- 230000036407 pain Effects 0.000 description 1
- 210000000496 pancreas Anatomy 0.000 description 1
- 238000004091 panning Methods 0.000 description 1
- 229940055729 papain Drugs 0.000 description 1
- 235000019834 papain Nutrition 0.000 description 1
- 238000007911 parenteral administration Methods 0.000 description 1
- 235000016236 parenteral nutrition Nutrition 0.000 description 1
- 238000004810 partition chromatography Methods 0.000 description 1
- 230000001717 pathogenic effect Effects 0.000 description 1
- 230000007170 pathology Effects 0.000 description 1
- 235000020232 peanut Nutrition 0.000 description 1
- 230000000149 penetrating effect Effects 0.000 description 1
- 229940111202 pepsin Drugs 0.000 description 1
- 239000000137 peptide hydrolase inhibitor Substances 0.000 description 1
- 230000007030 peptide scission Effects 0.000 description 1
- 238000010647 peptide synthesis reaction Methods 0.000 description 1
- 230000010412 perfusion Effects 0.000 description 1
- 210000003200 peritoneal cavity Anatomy 0.000 description 1
- 230000002085 persistent effect Effects 0.000 description 1
- 239000000825 pharmaceutical preparation Substances 0.000 description 1
- 239000002831 pharmacologic agent Substances 0.000 description 1
- 230000000144 pharmacologic effect Effects 0.000 description 1
- 229960003742 phenol Drugs 0.000 description 1
- 125000002467 phosphate group Chemical group [H]OP(=O)(O[H])O[*] 0.000 description 1
- 150000003904 phospholipids Chemical class 0.000 description 1
- 210000003720 plasmablast Anatomy 0.000 description 1
- 229920005862 polyol Polymers 0.000 description 1
- 150000003077 polyols Chemical class 0.000 description 1
- 229920002223 polystyrene Polymers 0.000 description 1
- 229920002981 polyvinylidene fluoride Polymers 0.000 description 1
- 230000034190 positive regulation of NF-kappaB transcription factor activity Effects 0.000 description 1
- 230000002980 postoperative effect Effects 0.000 description 1
- 230000001124 posttranscriptional effect Effects 0.000 description 1
- 239000011591 potassium Substances 0.000 description 1
- 229910052700 potassium Inorganic materials 0.000 description 1
- 229940124606 potential therapeutic agent Drugs 0.000 description 1
- 230000003389 potentiating effect Effects 0.000 description 1
- 239000003755 preservative agent Substances 0.000 description 1
- 230000002335 preservative effect Effects 0.000 description 1
- 238000002203 pretreatment Methods 0.000 description 1
- 230000003449 preventive effect Effects 0.000 description 1
- 230000009862 primary prevention Effects 0.000 description 1
- 238000011809 primate model Methods 0.000 description 1
- 230000003244 pro-oxidative effect Effects 0.000 description 1
- MFDFERRIHVXMIY-UHFFFAOYSA-N procaine Chemical compound CCN(CC)CCOC(=O)C1=CC=C(N)C=C1 MFDFERRIHVXMIY-UHFFFAOYSA-N 0.000 description 1
- 229960004919 procaine Drugs 0.000 description 1
- 230000002035 prolonged effect Effects 0.000 description 1
- 230000000644 propagated effect Effects 0.000 description 1
- 238000012342 propidium iodide staining Methods 0.000 description 1
- 235000019419 proteases Nutrition 0.000 description 1
- 238000000159 protein binding assay Methods 0.000 description 1
- 235000004252 protein component Nutrition 0.000 description 1
- 238000001243 protein synthesis Methods 0.000 description 1
- 230000017854 proteolysis Effects 0.000 description 1
- 230000006337 proteolytic cleavage Effects 0.000 description 1
- 208000005333 pulmonary edema Diseases 0.000 description 1
- 230000006825 purine synthesis Effects 0.000 description 1
- 150000003212 purines Chemical class 0.000 description 1
- 150000003230 pyrimidines Chemical class 0.000 description 1
- 239000002516 radical scavenger Substances 0.000 description 1
- 230000002285 radioactive effect Effects 0.000 description 1
- 238000002673 radiosurgery Methods 0.000 description 1
- 239000012429 reaction media Substances 0.000 description 1
- 208000002574 reactive arthritis Diseases 0.000 description 1
- 239000003642 reactive oxygen metabolite Substances 0.000 description 1
- 230000009257 reactivity Effects 0.000 description 1
- 238000003259 recombinant expression Methods 0.000 description 1
- 238000010188 recombinant method Methods 0.000 description 1
- 238000002278 reconstructive surgery Methods 0.000 description 1
- 230000009467 reduction Effects 0.000 description 1
- 230000008439 repair process Effects 0.000 description 1
- 238000002271 resection Methods 0.000 description 1
- 230000036387 respiratory rate Effects 0.000 description 1
- 238000004366 reverse phase liquid chromatography Methods 0.000 description 1
- 238000002432 robotic surgery Methods 0.000 description 1
- 210000003296 saliva Anatomy 0.000 description 1
- 238000003118 sandwich ELISA Methods 0.000 description 1
- 238000007423 screening assay Methods 0.000 description 1
- 230000035945 sensitivity Effects 0.000 description 1
- 208000013223 septicemia Diseases 0.000 description 1
- 208000037974 severe injury Diseases 0.000 description 1
- 230000009528 severe injury Effects 0.000 description 1
- 231100000004 severe toxicity Toxicity 0.000 description 1
- 206010040560 shock Diseases 0.000 description 1
- 208000013220 shortness of breath Diseases 0.000 description 1
- 229910001961 silver nitrate Inorganic materials 0.000 description 1
- 239000011734 sodium Substances 0.000 description 1
- 229910052708 sodium Inorganic materials 0.000 description 1
- URGAHOPLAPQHLN-UHFFFAOYSA-N sodium aluminosilicate Chemical compound [Na+].[Al+3].[O-][Si]([O-])=O.[O-][Si]([O-])=O URGAHOPLAPQHLN-UHFFFAOYSA-N 0.000 description 1
- 239000007787 solid Substances 0.000 description 1
- 239000007790 solid phase Substances 0.000 description 1
- 230000000392 somatic effect Effects 0.000 description 1
- 239000004334 sorbic acid Substances 0.000 description 1
- 229940075582 sorbic acid Drugs 0.000 description 1
- 235000010199 sorbic acid Nutrition 0.000 description 1
- 210000004989 spleen cell Anatomy 0.000 description 1
- 210000004988 splenocyte Anatomy 0.000 description 1
- 201000005671 spondyloarthropathy Diseases 0.000 description 1
- 210000003802 sputum Anatomy 0.000 description 1
- 208000024794 sputum Diseases 0.000 description 1
- 230000006641 stabilisation Effects 0.000 description 1
- 238000011105 stabilization Methods 0.000 description 1
- 238000011272 standard treatment Methods 0.000 description 1
- 230000001954 sterilising effect Effects 0.000 description 1
- 125000001424 substituent group Chemical group 0.000 description 1
- 239000005720 sucrose Substances 0.000 description 1
- 150000008163 sugars Chemical class 0.000 description 1
- 238000009120 supportive therapy Methods 0.000 description 1
- 238000007460 surgical drainage Methods 0.000 description 1
- 230000002459 sustained effect Effects 0.000 description 1
- 239000012730 sustained-release form Substances 0.000 description 1
- 230000008718 systemic inflammatory response Effects 0.000 description 1
- 230000006794 tachycardia Effects 0.000 description 1
- 239000011975 tartaric acid Substances 0.000 description 1
- 235000002906 tartaric acid Nutrition 0.000 description 1
- 229940124597 therapeutic agent Drugs 0.000 description 1
- RTKIYNMVFMVABJ-UHFFFAOYSA-L thimerosal Chemical compound [Na+].CC[Hg]SC1=CC=CC=C1C([O-])=O RTKIYNMVFMVABJ-UHFFFAOYSA-L 0.000 description 1
- 229940033663 thimerosal Drugs 0.000 description 1
- 239000007143 thioglycolate medium Substances 0.000 description 1
- 230000036964 tight binding Effects 0.000 description 1
- 239000003104 tissue culture media Substances 0.000 description 1
- 238000004448 titration Methods 0.000 description 1
- 238000002627 tracheal intubation Methods 0.000 description 1
- 230000014616 translation Effects 0.000 description 1
- 238000002054 transplantation Methods 0.000 description 1
- 239000012588 trypsin Substances 0.000 description 1
- 239000001974 tryptic soy broth Substances 0.000 description 1
- 108010050327 trypticase-soy broth Proteins 0.000 description 1
- 210000004881 tumor cell Anatomy 0.000 description 1
- 201000008297 typhoid fever Diseases 0.000 description 1
- 229940116269 uric acid Drugs 0.000 description 1
- 238000001291 vacuum drying Methods 0.000 description 1
- 235000015112 vegetable and seed oil Nutrition 0.000 description 1
- 239000008158 vegetable oil Substances 0.000 description 1
- 208000004043 venous thromboembolism Diseases 0.000 description 1
- 238000001429 visible spectrum Methods 0.000 description 1
- 239000011800 void material Substances 0.000 description 1
- 239000002132 β-lactam antibiotic Substances 0.000 description 1
- 229940124586 β-lactam antibiotics Drugs 0.000 description 1
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K38/00—Medicinal preparations containing peptides
- A61K38/04—Peptides having up to 20 amino acids in a fully defined sequence; Derivatives thereof
- A61K38/10—Peptides having 12 to 20 amino acids
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/70—Carbohydrates; Sugars; Derivatives thereof
- A61K31/715—Polysaccharides, i.e. having more than five saccharide radicals attached to each other by glycosidic linkages; Derivatives thereof, e.g. ethers, esters
- A61K31/726—Glycosaminoglycans, i.e. mucopolysaccharides
- A61K31/727—Heparin; Heparan
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K38/00—Medicinal preparations containing peptides
- A61K38/16—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- A61K38/17—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans
- A61K38/1703—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans from vertebrates
- A61K38/1709—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans from vertebrates from mammals
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K38/00—Medicinal preparations containing peptides
- A61K38/16—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- A61K38/17—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans
- A61K38/36—Blood coagulation or fibrinolysis factors
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K38/00—Medicinal preparations containing peptides
- A61K38/16—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- A61K38/43—Enzymes; Proenzymes; Derivatives thereof
- A61K38/46—Hydrolases (3)
- A61K38/48—Hydrolases (3) acting on peptide bonds (3.4)
- A61K38/482—Serine endopeptidases (3.4.21)
- A61K38/4833—Thrombin (3.4.21.5)
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K38/00—Medicinal preparations containing peptides
- A61K38/16—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- A61K38/43—Enzymes; Proenzymes; Derivatives thereof
- A61K38/46—Hydrolases (3)
- A61K38/48—Hydrolases (3) acting on peptide bonds (3.4)
- A61K38/482—Serine endopeptidases (3.4.21)
- A61K38/4846—Factor VII (3.4.21.21); Factor IX (3.4.21.22); Factor Xa (3.4.21.6); Factor XI (3.4.21.27); Factor XII (3.4.21.38)
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K38/00—Medicinal preparations containing peptides
- A61K38/16—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- A61K38/43—Enzymes; Proenzymes; Derivatives thereof
- A61K38/46—Hydrolases (3)
- A61K38/48—Hydrolases (3) acting on peptide bonds (3.4)
- A61K38/482—Serine endopeptidases (3.4.21)
- A61K38/4866—Protein C (3.4.21.69)
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K39/395—Antibodies; Immunoglobulins; Immune serum, e.g. antilymphocytic serum
- A61K39/39533—Antibodies; Immunoglobulins; Immune serum, e.g. antilymphocytic serum against materials from animals
- A61K39/3955—Antibodies; Immunoglobulins; Immune serum, e.g. antilymphocytic serum against materials from animals against proteinaceous materials, e.g. enzymes, hormones, lymphokines
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K45/00—Medicinal preparations containing active ingredients not provided for in groups A61K31/00 - A61K41/00
- A61K45/06—Mixtures of active ingredients without chemical characterisation, e.g. antiphlogistics and cardiaca
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P1/00—Drugs for disorders of the alimentary tract or the digestive system
- A61P1/18—Drugs for disorders of the alimentary tract or the digestive system for pancreatic disorders, e.g. pancreatic enzymes
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P11/00—Drugs for disorders of the respiratory system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P17/00—Drugs for dermatological disorders
- A61P17/02—Drugs for dermatological disorders for treating wounds, ulcers, burns, scars, keloids, or the like
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P31/00—Antiinfectives, i.e. antibiotics, antiseptics, chemotherapeutics
- A61P31/04—Antibacterial agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P31/00—Antiinfectives, i.e. antibiotics, antiseptics, chemotherapeutics
- A61P31/10—Antimycotics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P37/00—Drugs for immunological or allergic disorders
- A61P37/02—Immunomodulators
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P43/00—Drugs for specific purposes, not provided for in groups A61P1/00-A61P41/00
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P9/00—Drugs for disorders of the cardiovascular system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P9/00—Drugs for disorders of the cardiovascular system
- A61P9/10—Drugs for disorders of the cardiovascular system for treating ischaemic or atherosclerotic diseases, e.g. antianginal drugs, coronary vasodilators, drugs for myocardial infarction, retinopathy, cerebrovascula insufficiency, renal arteriosclerosis
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/18—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/40—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against enzymes
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K7/00—Peptides having 5 to 20 amino acids in a fully defined sequence; Derivatives thereof
- C07K7/04—Linear peptides containing only normal peptide links
- C07K7/08—Linear peptides containing only normal peptide links having 12 to 20 amino acids
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12Y—ENZYMES
- C12Y304/00—Hydrolases acting on peptide bonds, i.e. peptidases (3.4)
- C12Y304/21—Serine endopeptidases (3.4.21)
- C12Y304/21005—Thrombin (3.4.21.5)
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12Y—ENZYMES
- C12Y304/00—Hydrolases acting on peptide bonds, i.e. peptidases (3.4)
- C12Y304/21—Serine endopeptidases (3.4.21)
- C12Y304/21006—Coagulation factor Xa (3.4.21.6)
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N33/00—Investigating or analysing materials by specific methods not covered by groups G01N1/00 - G01N31/00
- G01N33/48—Biological material, e.g. blood, urine; Haemocytometers
- G01N33/50—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing
- G01N33/68—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing involving proteins, peptides or amino acids
- G01N33/6875—Nucleoproteins
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K2039/505—Medicinal preparations containing antigens or antibodies comprising antibodies
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/70—Immunoglobulins specific features characterized by effect upon binding to a cell or to an antigen
- C07K2317/76—Antagonist effect on antigen, e.g. neutralization or inhibition of binding
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12Y—ENZYMES
- C12Y304/00—Hydrolases acting on peptide bonds, i.e. peptidases (3.4)
- C12Y304/21—Serine endopeptidases (3.4.21)
- C12Y304/21069—Protein C activated (3.4.21.69)
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Chemical & Material Sciences (AREA)
- General Health & Medical Sciences (AREA)
- Medicinal Chemistry (AREA)
- Engineering & Computer Science (AREA)
- Organic Chemistry (AREA)
- Animal Behavior & Ethology (AREA)
- Veterinary Medicine (AREA)
- Public Health (AREA)
- Pharmacology & Pharmacy (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Immunology (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Epidemiology (AREA)
- Gastroenterology & Hepatology (AREA)
- Molecular Biology (AREA)
- Biochemistry (AREA)
- Genetics & Genomics (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Zoology (AREA)
- General Chemical & Material Sciences (AREA)
- Hematology (AREA)
- Biophysics (AREA)
- Urology & Nephrology (AREA)
- Biomedical Technology (AREA)
- General Engineering & Computer Science (AREA)
- Wood Science & Technology (AREA)
- Microbiology (AREA)
- Dermatology (AREA)
- General Physics & Mathematics (AREA)
- Pathology (AREA)
- Analytical Chemistry (AREA)
- Physics & Mathematics (AREA)
- Marine Sciences & Fisheries (AREA)
- Food Science & Technology (AREA)
- Biotechnology (AREA)
- Cell Biology (AREA)
- Communicable Diseases (AREA)
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US98588607P | 2007-11-06 | 2007-11-06 | |
| PCT/US2008/082632 WO2009061918A1 (en) | 2007-11-06 | 2008-11-06 | Extracellular histones as biomarkers for prognosis and molecular targets for therapy |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| JP2011503012A true JP2011503012A (ja) | 2011-01-27 |
| JP2011503012A5 JP2011503012A5 (enExample) | 2012-11-08 |
Family
ID=40270490
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2010532342A Pending JP2011503012A (ja) | 2007-11-06 | 2008-11-06 | 予後に関するバイオマーカーおよび治療の分子標的としての細胞外ヒストン |
Country Status (6)
| Country | Link |
|---|---|
| US (5) | US8716218B2 (enExample) |
| EP (1) | EP2209485B1 (enExample) |
| JP (1) | JP2011503012A (enExample) |
| AU (1) | AU2008323947B2 (enExample) |
| CA (1) | CA2704974C (enExample) |
| WO (1) | WO2009061918A1 (enExample) |
Cited By (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2013125687A1 (ja) * | 2012-02-22 | 2013-08-29 | 学校法人 城西大学 | 炎症疾患治療剤 |
| JPWO2013125687A1 (ja) * | 2013-02-22 | 2015-07-30 | 学校法人 城西大学 | 炎症疾患治療剤 |
| US9701739B2 (en) | 2010-08-20 | 2017-07-11 | Josai University Corporation | Monoclonal antibody having immunosuppressive activity or antigen binding fragment thereof |
| WO2018164027A1 (ja) * | 2017-03-06 | 2018-09-13 | 学校法人順天堂 | ヒストンによる細胞傷害を抑制するための薬剤 |
| JP2019536062A (ja) * | 2016-11-04 | 2019-12-12 | セントロ デ インベスティガシオン ビオメディカ エン レッド | 敗血症又は敗血症性ショック(ss)患者に由来する血漿中の循環ヒストンh3及びh2bを検出する質量分析ベースの方法 |
| JP2020037578A (ja) * | 2014-07-02 | 2020-03-12 | インフレクティス・バイオサイエンス | プロテオパチーの処置のためのベンジリデングアニジン誘導体の新規な治療的使用 |
Families Citing this family (24)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2009061918A1 (en) | 2007-11-06 | 2009-05-14 | Oklahoma Medical Research Foundation | Extracellular histones as biomarkers for prognosis and molecular targets for therapy |
| ES2524669T3 (es) * | 2007-11-16 | 2014-12-10 | Biocartis Nv | Biomarcadores y procedimientos de diagnóstico, predicción y/o pronóstico de septicemia y usos de los mismos |
| JPWO2012067152A1 (ja) * | 2010-11-16 | 2014-05-12 | 三菱化学株式会社 | EndothelialProteinCReceptor蛋白質による脳梗塞の検査方法 |
| WO2012071611A1 (en) * | 2010-12-01 | 2012-06-07 | The Australian National University | Histone inhibition |
| TWI442934B (zh) * | 2011-03-11 | 2014-07-01 | Univ Taipei Medical | 組蛋白h4抗體的用途 |
| CA2898472C (en) | 2013-02-15 | 2023-03-07 | Immunomedics, Inc. | Chimeric and humanized anti-histone antibodies |
| FR3007411B1 (fr) * | 2013-06-21 | 2015-07-03 | Agronomique Inst Nat Rech | Anticorps monocatenaire a chaine lourde de camelide dirige contre la chromatine et utilisations |
| EP3160504B1 (en) | 2014-06-24 | 2020-09-16 | Immunomedics, Inc. | Anti-histone therapy for vascular necrosis in severe glomerulonephritis |
| US10351621B2 (en) | 2014-06-24 | 2019-07-16 | Immunomedics, Inc. | Anti-histone therapy in acute kidney injury |
| US10633410B2 (en) | 2014-12-22 | 2020-04-28 | University Of Iowa Research Foundation | Nucleic acid aptamers to treat histone-induced disease states |
| US11391742B2 (en) | 2015-02-10 | 2022-07-19 | B.R.A.H.M.S. Gmbh | Free histone proteins as biomarkers |
| CN106611094B (zh) * | 2015-10-15 | 2021-08-06 | 北京寻因生物科技有限公司 | 基于肠道微生物菌群预测及干预化疗药物毒副反应的系统 |
| JP6738652B2 (ja) * | 2016-05-26 | 2020-08-12 | シスメックス株式会社 | 試料分析方法、試料分析装置および試薬 |
| CN109564226A (zh) * | 2016-08-09 | 2019-04-02 | B.R.A.H.M.S 有限公司 | 作为指示器官功能障碍的标志物的组蛋白和/或proADM |
| CA3033094A1 (en) | 2016-08-09 | 2018-02-15 | B.R.A.H.M.S Gmbh | Histones and/or proadm as markers indicating an adverse event |
| CN106397575A (zh) * | 2016-10-20 | 2017-02-15 | 上海懿贝瑞生物医药科技有限公司 | 一种具有胞外组蛋白毒性抑制作用的多肽制备方法及用途 |
| EP3577465B1 (en) | 2017-02-02 | 2023-03-29 | B.R.A.H.M.S GmbH | Proadm as marker indicating an adverse event |
| AU2018332326B2 (en) | 2017-09-18 | 2025-04-03 | Santersus Ag | Method and device for purification of blood from circulating cell free DNA |
| GB201804833D0 (en) * | 2018-03-26 | 2018-05-09 | Univ Liverpool | Pro-coagulant histones |
| CN110101838B (zh) * | 2019-04-29 | 2022-07-01 | 陕西师范大学 | 一种提高组蛋白抗大肠杆菌性能的方法 |
| CN111202839A (zh) * | 2020-02-21 | 2020-05-29 | 佛山科学技术学院 | 一种组蛋白h4在抑制禽流感病毒中的应用 |
| US20230149453A1 (en) * | 2020-04-03 | 2023-05-18 | Medsenic | Use of an arsenic compound for treating a short or long cytokine storm in various autoimmune/inflammatory diseases in humans or animals |
| CN112679579B (zh) * | 2020-12-29 | 2022-02-01 | 潍坊医学院 | 一种分离自华蟾素注射液的镇痛肽ci5及其应用 |
| WO2023126672A1 (en) | 2021-12-27 | 2023-07-06 | Santersus Ag | Method and device for removal of circulating cell free dna |
Citations (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2007246473A (ja) * | 2006-03-17 | 2007-09-27 | Chemo Sero Therapeut Res Inst | 臓器障害抑制剤 |
Family Cites Families (8)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2000064466A1 (en) | 1999-04-28 | 2000-11-02 | Northwestern University | Localization of major peptide autoepitopes for nucleosome specific t cells of systemic lupus erythematosus |
| US7238656B2 (en) | 2001-08-29 | 2007-07-03 | Yissum Research Development Company Of The Hebrew University Of Jerusalem | Protective factors against inflammation, burns and noxious stimuli |
| US7528227B2 (en) | 2004-03-23 | 2009-05-05 | Yissum Research Development Company Of The Hebrew University Of Jerusalem | Histone H2A peptide derivatives and uses thereof |
| TW200407335A (en) * | 2002-07-22 | 2004-05-16 | Chugai Pharmaceutical Co Ltd | Non-neutralizing antibody to inhibit the inactivation of activated protein C |
| JP4834826B2 (ja) | 2003-07-22 | 2011-12-14 | 独立行政法人産業技術総合研究所 | ヒストンh3及びh4リンカー領域を認識するモノクローナル抗体及びその産生ハイブリドーマ |
| MXPA06014075A (es) * | 2004-06-03 | 2007-03-15 | Novimmune Sa | Anticuerpos anti-cd3 y metodos de uso de los mismos. |
| EP2011490A1 (en) | 2007-07-05 | 2009-01-07 | Sanofi-Aventis | Beta adrenergic receptor ligand derivatives for modulating apoptosis |
| WO2009061918A1 (en) * | 2007-11-06 | 2009-05-14 | Oklahoma Medical Research Foundation | Extracellular histones as biomarkers for prognosis and molecular targets for therapy |
-
2008
- 2008-11-06 WO PCT/US2008/082632 patent/WO2009061918A1/en not_active Ceased
- 2008-11-06 CA CA2704974A patent/CA2704974C/en active Active
- 2008-11-06 JP JP2010532342A patent/JP2011503012A/ja active Pending
- 2008-11-06 US US12/266,336 patent/US8716218B2/en active Active
- 2008-11-06 EP EP08847874.8A patent/EP2209485B1/en active Active
- 2008-11-06 AU AU2008323947A patent/AU2008323947B2/en active Active
-
2014
- 2014-05-06 US US14/270,470 patent/US10238711B2/en active Active
-
2015
- 2015-06-09 US US14/734,238 patent/US10350261B2/en active Active
-
2017
- 2017-12-12 US US15/838,639 patent/US20180092961A1/en not_active Abandoned
-
2019
- 2019-01-31 US US16/263,124 patent/US11458187B2/en active Active
Patent Citations (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2007246473A (ja) * | 2006-03-17 | 2007-09-27 | Chemo Sero Therapeut Res Inst | 臓器障害抑制剤 |
Non-Patent Citations (2)
| Title |
|---|
| JPN5010015263; ABAKUSHIN,D.N. et al: 'HISTONES EVOKE THYMOCYTE DEATH IN VITRO : HISTONE-BINDING IMMUNOGLOBULINS DECREASE THEIR CYTOTOXICIT' BIOCHEMISTRY Vol.64, No.6, 199906, p.693-698 * |
| JPN6013033354; CURRIE,J.R. et al: Biochimica et Biophysica Acta Vol.1355, 1997, p.248-258 * |
Cited By (10)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US9701739B2 (en) | 2010-08-20 | 2017-07-11 | Josai University Corporation | Monoclonal antibody having immunosuppressive activity or antigen binding fragment thereof |
| WO2013125687A1 (ja) * | 2012-02-22 | 2013-08-29 | 学校法人 城西大学 | 炎症疾患治療剤 |
| KR20140138699A (ko) * | 2012-02-22 | 2014-12-04 | 조사이 유니버시티 코포레이션 | 염증 질환 치료제 |
| KR102159412B1 (ko) * | 2012-02-22 | 2020-09-23 | 조사이 유니버시티 코포레이션 | 염증 질환 치료제 |
| JPWO2013125687A1 (ja) * | 2013-02-22 | 2015-07-30 | 学校法人 城西大学 | 炎症疾患治療剤 |
| JP2020037578A (ja) * | 2014-07-02 | 2020-03-12 | インフレクティス・バイオサイエンス | プロテオパチーの処置のためのベンジリデングアニジン誘導体の新規な治療的使用 |
| JP2019536062A (ja) * | 2016-11-04 | 2019-12-12 | セントロ デ インベスティガシオン ビオメディカ エン レッド | 敗血症又は敗血症性ショック(ss)患者に由来する血漿中の循環ヒストンh3及びh2bを検出する質量分析ベースの方法 |
| JP7173495B2 (ja) | 2016-11-04 | 2022-11-16 | セントロ デ インベスティガシオン ビオメディカ エン レッド | 敗血症又は敗血症性ショック(ss)患者に由来する血漿中の循環ヒストンh3及びh2bを検出する質量分析ベースの方法 |
| US11740245B2 (en) | 2016-11-04 | 2023-08-29 | Centro De Investigación Biomédica En Red (Ciber-Isciii) | Mass spectrometry-based methods for the detection of circulating histones H3 and H2B in plasma from sepsis or septic shock (SS) patients |
| WO2018164027A1 (ja) * | 2017-03-06 | 2018-09-13 | 学校法人順天堂 | ヒストンによる細胞傷害を抑制するための薬剤 |
Also Published As
| Publication number | Publication date |
|---|---|
| US11458187B2 (en) | 2022-10-04 |
| US20150344556A1 (en) | 2015-12-03 |
| WO2009061918A1 (en) | 2009-05-14 |
| EP2209485B1 (en) | 2016-03-30 |
| US10350261B2 (en) | 2019-07-16 |
| CA2704974A1 (en) | 2009-05-14 |
| AU2008323947A1 (en) | 2009-05-14 |
| US8716218B2 (en) | 2014-05-06 |
| AU2008323947B2 (en) | 2014-02-20 |
| US20090117099A1 (en) | 2009-05-07 |
| EP2209485A1 (en) | 2010-07-28 |
| US10238711B2 (en) | 2019-03-26 |
| US20180092961A1 (en) | 2018-04-05 |
| US20190216884A1 (en) | 2019-07-18 |
| US20140308289A1 (en) | 2014-10-16 |
| CA2704974C (en) | 2019-06-04 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US11458187B2 (en) | Extracellular histones as biomarkers for prognosis and molecular targets for therapy | |
| JP2012136536A (ja) | アテローム血栓症およびプラーク破壊を予防するためのアネキシンv | |
| EP3260133B1 (en) | Antibodies against mac-1 | |
| JP6198277B2 (ja) | 血液凝固促進に使用するための化合物 | |
| US10646556B2 (en) | Methods for treatment of and prophylaxis against inflammatory disorders | |
| KR20200116098A (ko) | 항응고제 단백질 및 호중구의 활성화와 관련된 질환을 치료하기 위한 이의 용도 | |
| JP6989377B2 (ja) | Kv1.3カリウムチャネルアンタゴニスト | |
| US8173595B2 (en) | Methods and compositions for the inhibition of thrombus formation | |
| WO2008038777A1 (en) | Therapeutic agent for disseminated intravascular coagulation syndrome | |
| US20170042981A1 (en) | Half-life extended factor fviia protein for prevention and treatment of bleeding and dosing regimens therefor | |
| JP2012224549A (ja) | 抗凝固薬及びその用途 | |
| US20190092814A1 (en) | Peptide | |
| Bertin | Leukocytes in venous thrombosis | |
| Lim | Effects of thrombin and its related peptide fragments on neutrophils | |
| Konatschnig et al. | Targeting complement resistance on human tumours: Cloning, expression and functional characterisation of a novel chimeric anti-CD59 miniantibody | |
| JP2009502849A (ja) | 血栓症及び癌の治療、及び/又は予防のためのacrp30の使用 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20111104 |
|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20111104 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20120910 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20130708 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20130930 |
|
| A602 | Written permission of extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A602 Effective date: 20131007 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20131220 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20140122 |
|
| A02 | Decision of refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A02 Effective date: 20140630 |