JP2010514432A - 植物における異種タンパク質の改善された発現のための、5’(5’−utr)における最適化されたリーダー機能を有する人工dna配列 - Google Patents
植物における異種タンパク質の改善された発現のための、5’(5’−utr)における最適化されたリーダー機能を有する人工dna配列 Download PDFInfo
- Publication number
- JP2010514432A JP2010514432A JP2009543473A JP2009543473A JP2010514432A JP 2010514432 A JP2010514432 A JP 2010514432A JP 2009543473 A JP2009543473 A JP 2009543473A JP 2009543473 A JP2009543473 A JP 2009543473A JP 2010514432 A JP2010514432 A JP 2010514432A
- Authority
- JP
- Japan
- Prior art keywords
- sequence
- sequence according
- synthesis
- plant
- promoter
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Pending
Links
- 230000014509 gene expression Effects 0.000 title claims abstract description 32
- 108020003589 5' Untranslated Regions Proteins 0.000 title claims abstract description 19
- 108090000623 proteins and genes Proteins 0.000 title claims description 47
- 102000004169 proteins and genes Human genes 0.000 title claims description 25
- 108091028043 Nucleic acid sequence Proteins 0.000 title claims description 5
- 239000002773 nucleotide Substances 0.000 claims abstract description 14
- 125000003729 nucleotide group Chemical group 0.000 claims abstract description 14
- 241000196324 Embryophyta Species 0.000 claims description 80
- 238000003786 synthesis reaction Methods 0.000 claims description 38
- 230000015572 biosynthetic process Effects 0.000 claims description 30
- 210000004027 cell Anatomy 0.000 claims description 24
- 239000013604 expression vector Substances 0.000 claims description 22
- 230000000694 effects Effects 0.000 claims description 21
- 238000000034 method Methods 0.000 claims description 15
- 102000007056 Recombinant Fusion Proteins Human genes 0.000 claims description 13
- 108010008281 Recombinant Fusion Proteins Proteins 0.000 claims description 13
- 108020004999 messenger RNA Proteins 0.000 claims description 12
- 238000004519 manufacturing process Methods 0.000 claims description 11
- 239000013612 plasmid Substances 0.000 claims description 7
- 241000894007 species Species 0.000 claims description 7
- 229960005486 vaccine Drugs 0.000 claims description 7
- 108700009124 Transcription Initiation Site Proteins 0.000 claims description 6
- 230000001580 bacterial effect Effects 0.000 claims description 6
- 230000000295 complement effect Effects 0.000 claims description 6
- 230000035772 mutation Effects 0.000 claims description 6
- 241001465754 Metazoa Species 0.000 claims description 5
- 108091034117 Oligonucleotide Proteins 0.000 claims description 5
- 230000003321 amplification Effects 0.000 claims description 5
- 238000012217 deletion Methods 0.000 claims description 5
- 230000037430 deletion Effects 0.000 claims description 5
- 230000001939 inductive effect Effects 0.000 claims description 5
- 238000003780 insertion Methods 0.000 claims description 5
- 230000037431 insertion Effects 0.000 claims description 5
- 238000003199 nucleic acid amplification method Methods 0.000 claims description 5
- 241000219193 Brassicaceae Species 0.000 claims description 4
- 230000001419 dependent effect Effects 0.000 claims description 4
- 239000002994 raw material Substances 0.000 claims description 4
- 241000589155 Agrobacterium tumefaciens Species 0.000 claims description 3
- 241000588724 Escherichia coli Species 0.000 claims description 3
- 229920000704 biodegradable plastic Polymers 0.000 claims description 3
- 238000004520 electroporation Methods 0.000 claims description 3
- 241001233957 eudicotyledons Species 0.000 claims description 3
- 239000004009 herbicide Substances 0.000 claims description 3
- 239000003262 industrial enzyme Substances 0.000 claims description 3
- 239000000203 mixture Substances 0.000 claims description 3
- 239000002245 particle Substances 0.000 claims description 3
- 230000008569 process Effects 0.000 claims description 3
- 210000001519 tissue Anatomy 0.000 claims description 3
- 241000589156 Agrobacterium rhizogenes Species 0.000 claims description 2
- 241000701489 Cauliflower mosaic virus Species 0.000 claims description 2
- 241000209504 Poaceae Species 0.000 claims description 2
- 241000208292 Solanaceae Species 0.000 claims description 2
- 241000700605 Viruses Species 0.000 claims description 2
- 244000052616 bacterial pathogen Species 0.000 claims description 2
- 210000003763 chloroplast Anatomy 0.000 claims description 2
- 235000014113 dietary fatty acids Nutrition 0.000 claims description 2
- 229930195729 fatty acid Natural products 0.000 claims description 2
- 239000000194 fatty acid Substances 0.000 claims description 2
- 150000004665 fatty acids Chemical group 0.000 claims description 2
- 239000000446 fuel Substances 0.000 claims description 2
- 244000053095 fungal pathogen Species 0.000 claims description 2
- 208000019420 lymphoid neoplasm Diseases 0.000 claims description 2
- 210000003470 mitochondria Anatomy 0.000 claims description 2
- 235000016709 nutrition Nutrition 0.000 claims description 2
- 230000006798 recombination Effects 0.000 claims description 2
- 238000005215 recombination Methods 0.000 claims description 2
- 229930000044 secondary metabolite Natural products 0.000 claims description 2
- 230000010474 transient expression Effects 0.000 claims description 2
- 230000007704 transition Effects 0.000 claims description 2
- 244000052613 viral pathogen Species 0.000 claims description 2
- 239000013603 viral vector Substances 0.000 claims description 2
- 230000003612 virological effect Effects 0.000 claims description 2
- 238000001243 protein synthesis Methods 0.000 claims 1
- 230000002194 synthesizing effect Effects 0.000 claims 1
- 230000005758 transcription activity Effects 0.000 claims 1
- 230000014616 translation Effects 0.000 claims 1
- 102000053187 Glucuronidase Human genes 0.000 description 24
- 108010060309 Glucuronidase Proteins 0.000 description 24
- 238000013518 transcription Methods 0.000 description 18
- 230000035897 transcription Effects 0.000 description 18
- 238000013519 translation Methods 0.000 description 15
- 230000009261 transgenic effect Effects 0.000 description 13
- 239000013598 vector Substances 0.000 description 12
- 244000061176 Nicotiana tabacum Species 0.000 description 11
- 235000002637 Nicotiana tabacum Nutrition 0.000 description 11
- 108020004707 nucleic acids Proteins 0.000 description 10
- 102000039446 nucleic acids Human genes 0.000 description 10
- 150000007523 nucleic acids Chemical class 0.000 description 10
- 230000002441 reversible effect Effects 0.000 description 9
- 239000000243 solution Substances 0.000 description 9
- 102000004190 Enzymes Human genes 0.000 description 8
- 108090000790 Enzymes Proteins 0.000 description 8
- 108700019146 Transgenes Proteins 0.000 description 8
- 230000009466 transformation Effects 0.000 description 8
- 238000006243 chemical reaction Methods 0.000 description 7
- 239000012634 fragment Substances 0.000 description 7
- 108020004463 18S ribosomal RNA Proteins 0.000 description 6
- 239000002609 medium Substances 0.000 description 6
- 238000003753 real-time PCR Methods 0.000 description 6
- 241000589158 Agrobacterium Species 0.000 description 5
- 108091026890 Coding region Proteins 0.000 description 5
- 241000723873 Tobacco mosaic virus Species 0.000 description 5
- 238000004458 analytical method Methods 0.000 description 5
- 108091032973 (ribonucleotides)n+m Proteins 0.000 description 4
- BHELIUBJHYAEDK-OAIUPTLZSA-N Aspoxicillin Chemical compound C1([C@H](C(=O)N[C@@H]2C(N3[C@H](C(C)(C)S[C@@H]32)C(O)=O)=O)NC(=O)[C@H](N)CC(=O)NC)=CC=C(O)C=C1 BHELIUBJHYAEDK-OAIUPTLZSA-N 0.000 description 4
- 238000003556 assay Methods 0.000 description 4
- 238000002474 experimental method Methods 0.000 description 4
- 238000007421 fluorometric assay Methods 0.000 description 4
- 239000000499 gel Substances 0.000 description 4
- 230000006872 improvement Effects 0.000 description 4
- 229930027917 kanamycin Natural products 0.000 description 4
- SBUJHOSQTJFQJX-NOAMYHISSA-N kanamycin Chemical compound O[C@@H]1[C@@H](O)[C@H](O)[C@@H](CN)O[C@@H]1O[C@H]1[C@H](O)[C@@H](O[C@@H]2[C@@H]([C@@H](N)[C@H](O)[C@@H](CO)O2)O)[C@H](N)C[C@@H]1N SBUJHOSQTJFQJX-NOAMYHISSA-N 0.000 description 4
- 229960000318 kanamycin Drugs 0.000 description 4
- 229930182823 kanamycin A Natural products 0.000 description 4
- 238000005259 measurement Methods 0.000 description 4
- 239000000047 product Substances 0.000 description 4
- 230000014621 translational initiation Effects 0.000 description 4
- 108020004414 DNA Proteins 0.000 description 3
- 241000282412 Homo Species 0.000 description 3
- 108091081024 Start codon Proteins 0.000 description 3
- 229930006000 Sucrose Natural products 0.000 description 3
- CZMRCDWAGMRECN-UGDNZRGBSA-N Sucrose Chemical compound O[C@H]1[C@H](O)[C@@H](CO)O[C@@]1(CO)O[C@@H]1[C@H](O)[C@@H](O)[C@H](O)[C@@H](CO)O1 CZMRCDWAGMRECN-UGDNZRGBSA-N 0.000 description 3
- 238000000540 analysis of variance Methods 0.000 description 3
- 238000010367 cloning Methods 0.000 description 3
- 238000000338 in vitro Methods 0.000 description 3
- 239000000543 intermediate Substances 0.000 description 3
- 230000001105 regulatory effect Effects 0.000 description 3
- 108091008146 restriction endonucleases Proteins 0.000 description 3
- 238000013207 serial dilution Methods 0.000 description 3
- 239000000758 substrate Substances 0.000 description 3
- 239000005720 sucrose Substances 0.000 description 3
- PRPINYUDVPFIRX-UHFFFAOYSA-N 1-naphthaleneacetic acid Chemical compound C1=CC=C2C(CC(=O)O)=CC=CC2=C1 PRPINYUDVPFIRX-UHFFFAOYSA-N 0.000 description 2
- 239000005972 6-Benzyladenine Substances 0.000 description 2
- 229920001817 Agar Polymers 0.000 description 2
- 108020004705 Codon Proteins 0.000 description 2
- 102000012410 DNA Ligases Human genes 0.000 description 2
- 108010061982 DNA Ligases Proteins 0.000 description 2
- 230000006820 DNA synthesis Effects 0.000 description 2
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 description 2
- 241000209510 Liliopsida Species 0.000 description 2
- NWBJYWHLCVSVIJ-UHFFFAOYSA-N N-benzyladenine Chemical compound N=1C=NC=2NC=NC=2C=1NCC1=CC=CC=C1 NWBJYWHLCVSVIJ-UHFFFAOYSA-N 0.000 description 2
- 238000002123 RNA extraction Methods 0.000 description 2
- 238000011529 RT qPCR Methods 0.000 description 2
- 238000010818 SYBR green PCR Master Mix Methods 0.000 description 2
- 238000012300 Sequence Analysis Methods 0.000 description 2
- 239000008049 TAE buffer Substances 0.000 description 2
- HGEVZDLYZYVYHD-UHFFFAOYSA-N acetic acid;2-amino-2-(hydroxymethyl)propane-1,3-diol;2-[2-[bis(carboxymethyl)amino]ethyl-(carboxymethyl)amino]acetic acid Chemical compound CC(O)=O.OCC(N)(CO)CO.OC(=O)CN(CC(O)=O)CCN(CC(O)=O)CC(O)=O HGEVZDLYZYVYHD-UHFFFAOYSA-N 0.000 description 2
- 239000008272 agar Substances 0.000 description 2
- 238000000246 agarose gel electrophoresis Methods 0.000 description 2
- 238000011088 calibration curve Methods 0.000 description 2
- GPRBEKHLDVQUJE-VINNURBNSA-N cefotaxime Chemical compound N([C@@H]1C(N2C(=C(COC(C)=O)CS[C@@H]21)C(O)=O)=O)C(=O)/C(=N/OC)C1=CSC(N)=N1 GPRBEKHLDVQUJE-VINNURBNSA-N 0.000 description 2
- 229960004261 cefotaxime Drugs 0.000 description 2
- 239000003623 enhancer Substances 0.000 description 2
- 230000008995 epigenetic change Effects 0.000 description 2
- 238000001727 in vivo Methods 0.000 description 2
- JTEDVYBZBROSJT-UHFFFAOYSA-N indole-3-butyric acid Chemical compound C1=CC=C2C(CCCC(=O)O)=CNC2=C1 JTEDVYBZBROSJT-UHFFFAOYSA-N 0.000 description 2
- 230000008092 positive effect Effects 0.000 description 2
- 230000003252 repetitive effect Effects 0.000 description 2
- 230000010076 replication Effects 0.000 description 2
- 238000006467 substitution reaction Methods 0.000 description 2
- 238000012360 testing method Methods 0.000 description 2
- 238000012546 transfer Methods 0.000 description 2
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 2
- 108020005065 3' Flanking Region Proteins 0.000 description 1
- FWMNVWWHGCHHJJ-SKKKGAJSSA-N 4-amino-1-[(2r)-6-amino-2-[[(2r)-2-[[(2r)-2-[[(2r)-2-amino-3-phenylpropanoyl]amino]-3-phenylpropanoyl]amino]-4-methylpentanoyl]amino]hexanoyl]piperidine-4-carboxylic acid Chemical compound C([C@H](C(=O)N[C@H](CC(C)C)C(=O)N[C@H](CCCCN)C(=O)N1CCC(N)(CC1)C(O)=O)NC(=O)[C@H](N)CC=1C=CC=CC=1)C1=CC=CC=C1 FWMNVWWHGCHHJJ-SKKKGAJSSA-N 0.000 description 1
- HSHNITRMYYLLCV-UHFFFAOYSA-N 4-methylumbelliferone Chemical compound C1=C(O)C=CC2=C1OC(=O)C=C2C HSHNITRMYYLLCV-UHFFFAOYSA-N 0.000 description 1
- ARQXEQLMMNGFDU-JHZZJYKESA-N 4-methylumbelliferone beta-D-glucuronide Chemical compound C1=CC=2C(C)=CC(=O)OC=2C=C1O[C@@H]1O[C@H](C(O)=O)[C@@H](O)[C@H](O)[C@H]1O ARQXEQLMMNGFDU-JHZZJYKESA-N 0.000 description 1
- 108020005029 5' Flanking Region Proteins 0.000 description 1
- 101000717362 Acetabularia peniculus Ribulose bisphosphate carboxylase small subunit, chloroplastic 7 Proteins 0.000 description 1
- 102000002260 Alkaline Phosphatase Human genes 0.000 description 1
- 108020004774 Alkaline Phosphatase Proteins 0.000 description 1
- 238000000846 Bartlett's test Methods 0.000 description 1
- 102100026031 Beta-glucuronidase Human genes 0.000 description 1
- 238000009010 Bradford assay Methods 0.000 description 1
- 102000004127 Cytokines Human genes 0.000 description 1
- 108090000695 Cytokines Proteins 0.000 description 1
- 108010014303 DNA-directed DNA polymerase Proteins 0.000 description 1
- 102000016928 DNA-directed DNA polymerase Human genes 0.000 description 1
- 101100437498 Escherichia coli (strain K12) uidA gene Proteins 0.000 description 1
- 102100024165 G1/S-specific cyclin-D1 Human genes 0.000 description 1
- 101000980756 Homo sapiens G1/S-specific cyclin-D1 Proteins 0.000 description 1
- 206010020649 Hyperkeratosis Diseases 0.000 description 1
- 102100034343 Integrase Human genes 0.000 description 1
- 241000208125 Nicotiana Species 0.000 description 1
- 240000007594 Oryza sativa Species 0.000 description 1
- 235000007164 Oryza sativa Nutrition 0.000 description 1
- 238000002944 PCR assay Methods 0.000 description 1
- 108010002747 Pfu DNA polymerase Proteins 0.000 description 1
- 108010092799 RNA-directed DNA polymerase Proteins 0.000 description 1
- 239000005708 Sodium hypochlorite Substances 0.000 description 1
- 244000061456 Solanum tuberosum Species 0.000 description 1
- 235000002595 Solanum tuberosum Nutrition 0.000 description 1
- 206010042566 Superinfection Diseases 0.000 description 1
- 108010006785 Taq Polymerase Proteins 0.000 description 1
- JLCPHMBAVCMARE-UHFFFAOYSA-N [3-[[3-[[3-[[3-[[3-[[3-[[3-[[3-[[3-[[3-[[3-[[5-(2-amino-6-oxo-1H-purin-9-yl)-3-[[3-[[3-[[3-[[3-[[3-[[5-(2-amino-6-oxo-1H-purin-9-yl)-3-[[5-(2-amino-6-oxo-1H-purin-9-yl)-3-hydroxyoxolan-2-yl]methoxy-hydroxyphosphoryl]oxyoxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxyoxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methyl [5-(6-aminopurin-9-yl)-2-(hydroxymethyl)oxolan-3-yl] hydrogen phosphate Polymers Cc1cn(C2CC(OP(O)(=O)OCC3OC(CC3OP(O)(=O)OCC3OC(CC3O)n3cnc4c3nc(N)[nH]c4=O)n3cnc4c3nc(N)[nH]c4=O)C(COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3CO)n3cnc4c(N)ncnc34)n3ccc(N)nc3=O)n3cnc4c(N)ncnc34)n3ccc(N)nc3=O)n3ccc(N)nc3=O)n3ccc(N)nc3=O)n3cnc4c(N)ncnc34)n3cnc4c(N)ncnc34)n3cc(C)c(=O)[nH]c3=O)n3cc(C)c(=O)[nH]c3=O)n3ccc(N)nc3=O)n3cc(C)c(=O)[nH]c3=O)n3cnc4c3nc(N)[nH]c4=O)n3cnc4c(N)ncnc34)n3cnc4c(N)ncnc34)n3cnc4c(N)ncnc34)n3cnc4c(N)ncnc34)O2)c(=O)[nH]c1=O JLCPHMBAVCMARE-UHFFFAOYSA-N 0.000 description 1
- 230000036579 abiotic stress Effects 0.000 description 1
- 239000011543 agarose gel Substances 0.000 description 1
- 101150036080 at gene Proteins 0.000 description 1
- 230000010165 autogamy Effects 0.000 description 1
- 102000006995 beta-Glucosidase Human genes 0.000 description 1
- 108010047754 beta-Glucosidase Proteins 0.000 description 1
- 239000002551 biofuel Substances 0.000 description 1
- 239000000872 buffer Substances 0.000 description 1
- 238000010804 cDNA synthesis Methods 0.000 description 1
- 238000004364 calculation method Methods 0.000 description 1
- 230000015556 catabolic process Effects 0.000 description 1
- 238000005119 centrifugation Methods 0.000 description 1
- 239000013599 cloning vector Substances 0.000 description 1
- 239000002299 complementary DNA Substances 0.000 description 1
- 150000001875 compounds Chemical class 0.000 description 1
- 238000012790 confirmation Methods 0.000 description 1
- 239000000470 constituent Substances 0.000 description 1
- 238000010276 construction Methods 0.000 description 1
- 239000007799 cork Substances 0.000 description 1
- 238000006731 degradation reaction Methods 0.000 description 1
- 238000001514 detection method Methods 0.000 description 1
- 238000011161 development Methods 0.000 description 1
- 230000018109 developmental process Effects 0.000 description 1
- 230000029087 digestion Effects 0.000 description 1
- 239000012153 distilled water Substances 0.000 description 1
- 239000003814 drug Substances 0.000 description 1
- 230000002708 enhancing effect Effects 0.000 description 1
- 238000012869 ethanol precipitation Methods 0.000 description 1
- 238000011156 evaluation Methods 0.000 description 1
- 239000000835 fiber Substances 0.000 description 1
- 238000001506 fluorescence spectroscopy Methods 0.000 description 1
- 235000013305 food Nutrition 0.000 description 1
- 239000003102 growth factor Substances 0.000 description 1
- 230000001744 histochemical effect Effects 0.000 description 1
- 238000005286 illumination Methods 0.000 description 1
- 238000011065 in-situ storage Methods 0.000 description 1
- 208000015181 infectious disease Diseases 0.000 description 1
- 239000003550 marker Substances 0.000 description 1
- 230000004066 metabolic change Effects 0.000 description 1
- 238000000520 microinjection Methods 0.000 description 1
- 238000002703 mutagenesis Methods 0.000 description 1
- 231100000350 mutagenesis Toxicity 0.000 description 1
- 102000042567 non-coding RNA Human genes 0.000 description 1
- 238000010606 normalization Methods 0.000 description 1
- 239000003415 peat Substances 0.000 description 1
- 229920000642 polymer Polymers 0.000 description 1
- 230000001915 proofreading effect Effects 0.000 description 1
- 238000011002 quantification Methods 0.000 description 1
- 238000010791 quenching Methods 0.000 description 1
- 230000000171 quenching effect Effects 0.000 description 1
- 239000011535 reaction buffer Substances 0.000 description 1
- 238000011897 real-time detection Methods 0.000 description 1
- 230000008929 regeneration Effects 0.000 description 1
- 238000011069 regeneration method Methods 0.000 description 1
- 238000007634 remodeling Methods 0.000 description 1
- 210000003705 ribosome Anatomy 0.000 description 1
- 235000009566 rice Nutrition 0.000 description 1
- 239000007320 rich medium Substances 0.000 description 1
- 230000035945 sensitivity Effects 0.000 description 1
- SUKJFIGYRHOWBL-UHFFFAOYSA-N sodium hypochlorite Chemical compound [Na+].Cl[O-] SUKJFIGYRHOWBL-UHFFFAOYSA-N 0.000 description 1
- 239000007787 solid Substances 0.000 description 1
- 239000006228 supernatant Substances 0.000 description 1
- 229920001059 synthetic polymer Polymers 0.000 description 1
- 230000002103 transcriptional effect Effects 0.000 description 1
- 238000001890 transfection Methods 0.000 description 1
- 238000011144 upstream manufacturing Methods 0.000 description 1
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N15/00—Mutation or genetic engineering; DNA or RNA concerning genetic engineering, vectors, e.g. plasmids, or their isolation, preparation or purification; Use of hosts therefor
- C12N15/09—Recombinant DNA-technology
- C12N15/63—Introduction of foreign genetic material using vectors; Vectors; Use of hosts therefor; Regulation of expression
- C12N15/79—Vectors or expression systems specially adapted for eukaryotic hosts
- C12N15/82—Vectors or expression systems specially adapted for eukaryotic hosts for plant cells, e.g. plant artificial chromosomes (PACs)
- C12N15/8216—Methods for controlling, regulating or enhancing expression of transgenes in plant cells
-
- A—HUMAN NECESSITIES
- A01—AGRICULTURE; FORESTRY; ANIMAL HUSBANDRY; HUNTING; TRAPPING; FISHING
- A01H—NEW PLANTS OR NON-TRANSGENIC PROCESSES FOR OBTAINING THEM; PLANT REPRODUCTION BY TISSUE CULTURE TECHNIQUES
- A01H1/00—Processes for modifying genotypes ; Plants characterised by associated natural traits
-
- A—HUMAN NECESSITIES
- A01—AGRICULTURE; FORESTRY; ANIMAL HUSBANDRY; HUNTING; TRAPPING; FISHING
- A01H—NEW PLANTS OR NON-TRANSGENIC PROCESSES FOR OBTAINING THEM; PLANT REPRODUCTION BY TISSUE CULTURE TECHNIQUES
- A01H6/00—Angiosperms, i.e. flowering plants, characterised by their botanic taxonomy
- A01H6/46—Gramineae or Poaceae, e.g. ryegrass, rice, wheat or maize
- A01H6/4636—Oryza sp. [rice]
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N15/00—Mutation or genetic engineering; DNA or RNA concerning genetic engineering, vectors, e.g. plasmids, or their isolation, preparation or purification; Use of hosts therefor
- C12N15/09—Recombinant DNA-technology
- C12N15/63—Introduction of foreign genetic material using vectors; Vectors; Use of hosts therefor; Regulation of expression
- C12N15/66—General methods for inserting a gene into a vector to form a recombinant vector using cleavage and ligation; Use of non-functional linkers or adaptors, e.g. linkers containing the sequence for a restriction endonuclease
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Genetics & Genomics (AREA)
- Engineering & Computer Science (AREA)
- Organic Chemistry (AREA)
- Chemical & Material Sciences (AREA)
- Zoology (AREA)
- Bioinformatics & Cheminformatics (AREA)
- General Engineering & Computer Science (AREA)
- Biotechnology (AREA)
- Biomedical Technology (AREA)
- Wood Science & Technology (AREA)
- General Health & Medical Sciences (AREA)
- Molecular Biology (AREA)
- Biochemistry (AREA)
- Plant Pathology (AREA)
- Physics & Mathematics (AREA)
- Biophysics (AREA)
- Microbiology (AREA)
- Cell Biology (AREA)
- Medicinal Chemistry (AREA)
- Developmental Biology & Embryology (AREA)
- Animal Behavior & Ethology (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
- General Chemical & Material Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Environmental Sciences (AREA)
- Pharmacology & Pharmacy (AREA)
- Botany (AREA)
- Physiology (AREA)
- Natural Medicines & Medicinal Plants (AREA)
- Breeding Of Plants And Reproduction By Means Of Culturing (AREA)
- Micro-Organisms Or Cultivation Processes Thereof (AREA)
- Preparation Of Compounds By Using Micro-Organisms (AREA)
- Medicines Containing Antibodies Or Antigens For Use As Internal Diagnostic Agents (AREA)
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| IT000280A ITUD20060280A1 (it) | 2006-12-29 | 2006-12-29 | Sequenza artificiale di dna evente funzione di leader in 5' (5'-utr) ottimizzata per la sovraespressione di proteine eterologhe in pianta |
| PCT/EP2007/064590 WO2008080954A1 (en) | 2006-12-29 | 2007-12-27 | Artificial dna sequence with optimized leader function in 5' (5'-utr) for the improved expression of heterologous proteins in plants |
Related Child Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2014105535A Division JP5913434B2 (ja) | 2006-12-29 | 2014-05-21 | 植物における異種タンパク質の改善された発現のための、5’(5’−utr)における最適化されたリーダー機能を有する人工dna配列 |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| JP2010514432A true JP2010514432A (ja) | 2010-05-06 |
| JP2010514432A5 JP2010514432A5 (enExample) | 2011-02-17 |
Family
ID=39205172
Family Applications (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2009543473A Pending JP2010514432A (ja) | 2006-12-29 | 2007-12-27 | 植物における異種タンパク質の改善された発現のための、5’(5’−utr)における最適化されたリーダー機能を有する人工dna配列 |
| JP2014105535A Active JP5913434B2 (ja) | 2006-12-29 | 2014-05-21 | 植物における異種タンパク質の改善された発現のための、5’(5’−utr)における最適化されたリーダー機能を有する人工dna配列 |
Family Applications After (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2014105535A Active JP5913434B2 (ja) | 2006-12-29 | 2014-05-21 | 植物における異種タンパク質の改善された発現のための、5’(5’−utr)における最適化されたリーダー機能を有する人工dna配列 |
Country Status (18)
| Country | Link |
|---|---|
| US (1) | US9085775B2 (enExample) |
| EP (1) | EP2118291B1 (enExample) |
| JP (2) | JP2010514432A (enExample) |
| KR (1) | KR101596229B1 (enExample) |
| CN (1) | CN101617048B (enExample) |
| AU (1) | AU2007341217A1 (enExample) |
| BR (1) | BRPI0720641A8 (enExample) |
| CA (1) | CA2674170C (enExample) |
| CY (1) | CY1116649T1 (enExample) |
| DK (1) | DK2118291T3 (enExample) |
| ES (1) | ES2546184T3 (enExample) |
| HU (1) | HUE027484T2 (enExample) |
| IT (1) | ITUD20060280A1 (enExample) |
| NZ (1) | NZ578518A (enExample) |
| PL (1) | PL2118291T3 (enExample) |
| PT (1) | PT2118291E (enExample) |
| RU (1) | RU2009129091A (enExample) |
| WO (1) | WO2008080954A1 (enExample) |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2016502864A (ja) * | 2013-01-16 | 2016-02-01 | ロディナ ホールディング ソシエタ アノニマ | 植物における組換えタンパク質の過剰発現のための5’(5’−utr)に最適化されたリーダー機能を持つ人工dna配列と植物における組換えタンパク質の産生方法 |
Families Citing this family (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| ITUD20080055A1 (it) * | 2008-03-13 | 2009-09-14 | Transactiva S R L | Procedimento per la produzione di una proteina umana in pianta, in particolare un enzima lisosomiale umano ricombinante in endosperma di cereali |
| WO2013183863A1 (ko) * | 2012-06-04 | 2013-12-12 | 대한민국(관리부서:국립수산과학원) | 3'영역의 염기 변이를 통한 전체 단백질의 발현 조절 방법 |
| EP3013981B1 (en) * | 2013-06-25 | 2021-10-20 | The University of Utah Research Foundation | Methods of performing polymerase chain reaction and related uses thereof |
| IT201700042052A1 (it) | 2017-04-14 | 2018-10-14 | Transactiva S R L | Vettore di espressione e metodo per la produzione di una proteina umana in pianta, in particolare un anticorpo ricombinante in endosperma di cereale |
| CN121022839A (zh) * | 2025-02-06 | 2025-11-28 | 北京臻知医学科技有限责任公司 | 一种5’UTR序列及其在提高mRNA翻译效率中的应用 |
Family Cites Families (13)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| GB8613481D0 (en) * | 1986-06-04 | 1986-07-09 | Diatech Ltd | Translation of mrna |
| GB9206016D0 (en) | 1992-03-19 | 1992-04-29 | Sandoz Ltd | Improvements in or relating to organic compounds |
| US5362865A (en) * | 1993-09-02 | 1994-11-08 | Monsanto Company | Enhanced expression in plants using non-translated leader sequences |
| GB9515941D0 (en) * | 1995-08-03 | 1995-10-04 | Zeneca Ltd | DNA constructs |
| JP3761628B2 (ja) * | 1995-07-14 | 2006-03-29 | 住友化学株式会社 | 植物プロモーターおよびその利用 |
| GB9516241D0 (en) | 1995-08-08 | 1995-10-11 | Zeneca Ltd | Dna constructs |
| ZA9811228B (en) | 1997-12-12 | 1999-06-14 | Mogen Int | New constitutive plant promoters |
| US6376747B1 (en) | 1999-08-27 | 2002-04-23 | Her Majesty The Queen In Right Of Canada As Represented By The Minister Of Agriculture And Agri-Food Canada | Plant-derived map kinase kinase |
| FR2815969B1 (fr) * | 2000-10-30 | 2004-12-10 | Aventis Cropscience Sa | Plantes tolerantes aux herbicides par contournement de voie metabolique |
| KR100474115B1 (ko) | 2001-06-01 | 2005-03-08 | 대한민국 | 유전자변형식물에 대한 pcr 검정용 프라이머 |
| US7847064B2 (en) * | 2004-07-09 | 2010-12-07 | Donald Danforth Plant Science Center | Methods and compositions for regulating gene expression in plant cells |
| US7517689B2 (en) * | 2004-07-09 | 2009-04-14 | Donald Danforth Plant Science Center | Methods and compositions for regulating gene expression in plant cells |
| US8065093B2 (en) * | 2004-10-06 | 2011-11-22 | Agency For Science, Technology, And Research | Methods, systems, and compositions for classification, prognosis, and diagnosis of cancers |
-
2006
- 2006-12-29 IT IT000280A patent/ITUD20060280A1/it unknown
-
2007
- 2007-12-27 ES ES07858186.5T patent/ES2546184T3/es active Active
- 2007-12-27 BR BRPI0720641A patent/BRPI0720641A8/pt not_active IP Right Cessation
- 2007-12-27 JP JP2009543473A patent/JP2010514432A/ja active Pending
- 2007-12-27 CA CA2674170A patent/CA2674170C/en not_active Expired - Fee Related
- 2007-12-27 US US12/521,355 patent/US9085775B2/en not_active Expired - Fee Related
- 2007-12-27 CN CN2007800518216A patent/CN101617048B/zh not_active Expired - Fee Related
- 2007-12-27 KR KR1020097015452A patent/KR101596229B1/ko not_active Expired - Fee Related
- 2007-12-27 AU AU2007341217A patent/AU2007341217A1/en not_active Abandoned
- 2007-12-27 DK DK07858186.5T patent/DK2118291T3/en active
- 2007-12-27 HU HUE07858186A patent/HUE027484T2/en unknown
- 2007-12-27 PT PT78581865T patent/PT2118291E/pt unknown
- 2007-12-27 PL PL07858186T patent/PL2118291T3/pl unknown
- 2007-12-27 EP EP07858186.5A patent/EP2118291B1/en active Active
- 2007-12-27 RU RU2009129091/10A patent/RU2009129091A/ru not_active Application Discontinuation
- 2007-12-27 WO PCT/EP2007/064590 patent/WO2008080954A1/en not_active Ceased
- 2007-12-27 NZ NZ578518A patent/NZ578518A/xx not_active IP Right Cessation
-
2014
- 2014-05-21 JP JP2014105535A patent/JP5913434B2/ja active Active
-
2015
- 2015-08-26 CY CY20151100750T patent/CY1116649T1/el unknown
Non-Patent Citations (3)
| Title |
|---|
| JPN5010000010; DOWSON DAY M J: PLANT MOLECULAR BIOLOGY V23 N1, 1993, P97-109, SPRINGER * |
| JPN6012058590; Journal of Virology Vol. 70(12), 1996, pp. 8411-8421 * |
| JPN6012058593; Proceedings of the National Academy of Sciences of the United States of America Vol. 86, 1989, pp. 129-132 * |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2016502864A (ja) * | 2013-01-16 | 2016-02-01 | ロディナ ホールディング ソシエタ アノニマ | 植物における組換えタンパク質の過剰発現のための5’(5’−utr)に最適化されたリーダー機能を持つ人工dna配列と植物における組換えタンパク質の産生方法 |
Also Published As
| Publication number | Publication date |
|---|---|
| PL2118291T3 (pl) | 2015-11-30 |
| CN101617048A (zh) | 2009-12-30 |
| BRPI0720641A8 (pt) | 2017-12-19 |
| CN101617048B (zh) | 2012-11-07 |
| DK2118291T3 (en) | 2015-08-31 |
| EP2118291A1 (en) | 2009-11-18 |
| US20110041222A1 (en) | 2011-02-17 |
| NZ578518A (en) | 2012-08-31 |
| KR101596229B1 (ko) | 2016-03-07 |
| ES2546184T3 (es) | 2015-09-21 |
| CY1116649T1 (el) | 2017-03-15 |
| KR20090102825A (ko) | 2009-09-30 |
| AU2007341217A1 (en) | 2008-07-10 |
| BRPI0720641A2 (pt) | 2014-03-18 |
| CA2674170A1 (en) | 2008-07-10 |
| EP2118291B1 (en) | 2015-06-03 |
| US9085775B2 (en) | 2015-07-21 |
| HUE027484T2 (en) | 2016-09-28 |
| PT2118291E (pt) | 2015-09-24 |
| JP5913434B2 (ja) | 2016-04-27 |
| ITUD20060280A1 (it) | 2008-06-30 |
| WO2008080954A1 (en) | 2008-07-10 |
| RU2009129091A (ru) | 2011-02-10 |
| CA2674170C (en) | 2019-03-12 |
| JP2014221040A (ja) | 2014-11-27 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| RU2692924C2 (ru) | Регуляторные молекулы нуклеиновых кислот для усиления семя-специфичной и/или семя-предпочтительной генной экспрессии в растениях | |
| JP5913434B2 (ja) | 植物における異種タンパク質の改善された発現のための、5’(5’−utr)における最適化されたリーダー機能を有する人工dna配列 | |
| AU2017234672B2 (en) | Zea mays regulatory elements and uses thereof | |
| AU2017235944B2 (en) | Zea mays regulatory elements and uses thereof | |
| AU2014329590B2 (en) | Zea mays metallothionein-like regulatory elements and uses thereof | |
| CN105567687B (zh) | 一种花生种子特异启动子ahssp1及其应用 | |
| AU2014329590A1 (en) | Zea mays metallothionein-like regulatory elements and uses thereof | |
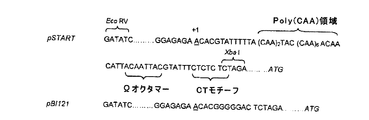
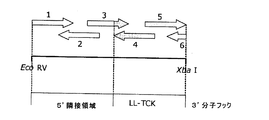
| US20150284738A1 (en) | Artificial dna sequence with optimized leader function in 5' (5'-utr) for the improved expression of heterologous proteins in plants | |
| CN101182530B (zh) | 一种诱导增强性组成型启动子及其应用 | |
| KR20150113013A (ko) | 식물에서 재조합 단백질의 과다발현을 위한 5''(5''-utr)에서 최적화된 리더 기능을 가진 인공 dna 서열 및 식물에서 재조합 단백질의 생산 방법 | |
| JP5360702B2 (ja) | 植物の篩管を通して遺伝子の転写物を輸送する方法 | |
| CA2883151C (en) | Stress-inducible promoter and uses thereof | |
| CN110157710A (zh) | 花烟草NaD1基因启动子及其用途 | |
| JP4439844B2 (ja) | 植物の鉄欠乏応答性及び/又は根特異的発現を付与するシスエレメント |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20100422 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20101224 |
|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20101224 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20121113 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20130212 |
|
| A602 | Written permission of extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A602 Effective date: 20130219 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20130312 |
|
| A602 | Written permission of extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A602 Effective date: 20130319 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20130411 |
|
| A602 | Written permission of extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A602 Effective date: 20130418 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20130513 |
|
| A02 | Decision of refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A02 Effective date: 20140128 |