EP3303522B1 - Procede de conversion de charges comprenant une etape d'hydrocraquage, une etape de precipitation et une etape de separation des sediments pour la production de fiouls - Google Patents
Procede de conversion de charges comprenant une etape d'hydrocraquage, une etape de precipitation et une etape de separation des sediments pour la production de fiouls Download PDFInfo
- Publication number
- EP3303522B1 EP3303522B1 EP16719813.4A EP16719813A EP3303522B1 EP 3303522 B1 EP3303522 B1 EP 3303522B1 EP 16719813 A EP16719813 A EP 16719813A EP 3303522 B1 EP3303522 B1 EP 3303522B1
- Authority
- EP
- European Patent Office
- Prior art keywords
- fraction
- hydrocracking
- process according
- weight
- separation
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Active
Links
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10G—CRACKING HYDROCARBON OILS; PRODUCTION OF LIQUID HYDROCARBON MIXTURES, e.g. BY DESTRUCTIVE HYDROGENATION, OLIGOMERISATION, POLYMERISATION; RECOVERY OF HYDROCARBON OILS FROM OIL-SHALE, OIL-SAND, OR GASES; REFINING MIXTURES MAINLY CONSISTING OF HYDROCARBONS; REFORMING OF NAPHTHA; MINERAL WAXES
- C10G67/00—Treatment of hydrocarbon oils by at least one hydrotreatment process and at least one process for refining in the absence of hydrogen only
- C10G67/02—Treatment of hydrocarbon oils by at least one hydrotreatment process and at least one process for refining in the absence of hydrogen only plural serial stages only
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10G—CRACKING HYDROCARBON OILS; PRODUCTION OF LIQUID HYDROCARBON MIXTURES, e.g. BY DESTRUCTIVE HYDROGENATION, OLIGOMERISATION, POLYMERISATION; RECOVERY OF HYDROCARBON OILS FROM OIL-SHALE, OIL-SAND, OR GASES; REFINING MIXTURES MAINLY CONSISTING OF HYDROCARBONS; REFORMING OF NAPHTHA; MINERAL WAXES
- C10G31/00—Refining of hydrocarbon oils, in the absence of hydrogen, by methods not otherwise provided for
- C10G31/06—Refining of hydrocarbon oils, in the absence of hydrogen, by methods not otherwise provided for by heating, cooling, or pressure treatment
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10G—CRACKING HYDROCARBON OILS; PRODUCTION OF LIQUID HYDROCARBON MIXTURES, e.g. BY DESTRUCTIVE HYDROGENATION, OLIGOMERISATION, POLYMERISATION; RECOVERY OF HYDROCARBON OILS FROM OIL-SHALE, OIL-SAND, OR GASES; REFINING MIXTURES MAINLY CONSISTING OF HYDROCARBONS; REFORMING OF NAPHTHA; MINERAL WAXES
- C10G31/00—Refining of hydrocarbon oils, in the absence of hydrogen, by methods not otherwise provided for
- C10G31/09—Refining of hydrocarbon oils, in the absence of hydrogen, by methods not otherwise provided for by filtration
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10G—CRACKING HYDROCARBON OILS; PRODUCTION OF LIQUID HYDROCARBON MIXTURES, e.g. BY DESTRUCTIVE HYDROGENATION, OLIGOMERISATION, POLYMERISATION; RECOVERY OF HYDROCARBON OILS FROM OIL-SHALE, OIL-SAND, OR GASES; REFINING MIXTURES MAINLY CONSISTING OF HYDROCARBONS; REFORMING OF NAPHTHA; MINERAL WAXES
- C10G47/00—Cracking of hydrocarbon oils, in the presence of hydrogen or hydrogen- generating compounds, to obtain lower boiling fractions
- C10G47/24—Cracking of hydrocarbon oils, in the presence of hydrogen or hydrogen- generating compounds, to obtain lower boiling fractions with moving solid particles
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10G—CRACKING HYDROCARBON OILS; PRODUCTION OF LIQUID HYDROCARBON MIXTURES, e.g. BY DESTRUCTIVE HYDROGENATION, OLIGOMERISATION, POLYMERISATION; RECOVERY OF HYDROCARBON OILS FROM OIL-SHALE, OIL-SAND, OR GASES; REFINING MIXTURES MAINLY CONSISTING OF HYDROCARBONS; REFORMING OF NAPHTHA; MINERAL WAXES
- C10G67/00—Treatment of hydrocarbon oils by at least one hydrotreatment process and at least one process for refining in the absence of hydrogen only
- C10G67/02—Treatment of hydrocarbon oils by at least one hydrotreatment process and at least one process for refining in the absence of hydrogen only plural serial stages only
- C10G67/12—Treatment of hydrocarbon oils by at least one hydrotreatment process and at least one process for refining in the absence of hydrogen only plural serial stages only including oxidation as the refining step in the absence of hydrogen
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10G—CRACKING HYDROCARBON OILS; PRODUCTION OF LIQUID HYDROCARBON MIXTURES, e.g. BY DESTRUCTIVE HYDROGENATION, OLIGOMERISATION, POLYMERISATION; RECOVERY OF HYDROCARBON OILS FROM OIL-SHALE, OIL-SAND, OR GASES; REFINING MIXTURES MAINLY CONSISTING OF HYDROCARBONS; REFORMING OF NAPHTHA; MINERAL WAXES
- C10G2300/00—Aspects relating to hydrocarbon processing covered by groups C10G1/00 - C10G99/00
- C10G2300/10—Feedstock materials
- C10G2300/1037—Hydrocarbon fractions
- C10G2300/1048—Middle distillates
- C10G2300/1059—Gasoil having a boiling range of about 330 - 427 °C
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10G—CRACKING HYDROCARBON OILS; PRODUCTION OF LIQUID HYDROCARBON MIXTURES, e.g. BY DESTRUCTIVE HYDROGENATION, OLIGOMERISATION, POLYMERISATION; RECOVERY OF HYDROCARBON OILS FROM OIL-SHALE, OIL-SAND, OR GASES; REFINING MIXTURES MAINLY CONSISTING OF HYDROCARBONS; REFORMING OF NAPHTHA; MINERAL WAXES
- C10G2300/00—Aspects relating to hydrocarbon processing covered by groups C10G1/00 - C10G99/00
- C10G2300/10—Feedstock materials
- C10G2300/1077—Vacuum residues
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10G—CRACKING HYDROCARBON OILS; PRODUCTION OF LIQUID HYDROCARBON MIXTURES, e.g. BY DESTRUCTIVE HYDROGENATION, OLIGOMERISATION, POLYMERISATION; RECOVERY OF HYDROCARBON OILS FROM OIL-SHALE, OIL-SAND, OR GASES; REFINING MIXTURES MAINLY CONSISTING OF HYDROCARBONS; REFORMING OF NAPHTHA; MINERAL WAXES
- C10G2300/00—Aspects relating to hydrocarbon processing covered by groups C10G1/00 - C10G99/00
- C10G2300/20—Characteristics of the feedstock or the products
- C10G2300/201—Impurities
- C10G2300/202—Heteroatoms content, i.e. S, N, O, P
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10G—CRACKING HYDROCARBON OILS; PRODUCTION OF LIQUID HYDROCARBON MIXTURES, e.g. BY DESTRUCTIVE HYDROGENATION, OLIGOMERISATION, POLYMERISATION; RECOVERY OF HYDROCARBON OILS FROM OIL-SHALE, OIL-SAND, OR GASES; REFINING MIXTURES MAINLY CONSISTING OF HYDROCARBONS; REFORMING OF NAPHTHA; MINERAL WAXES
- C10G2300/00—Aspects relating to hydrocarbon processing covered by groups C10G1/00 - C10G99/00
- C10G2300/20—Characteristics of the feedstock or the products
- C10G2300/201—Impurities
- C10G2300/205—Metal content
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10G—CRACKING HYDROCARBON OILS; PRODUCTION OF LIQUID HYDROCARBON MIXTURES, e.g. BY DESTRUCTIVE HYDROGENATION, OLIGOMERISATION, POLYMERISATION; RECOVERY OF HYDROCARBON OILS FROM OIL-SHALE, OIL-SAND, OR GASES; REFINING MIXTURES MAINLY CONSISTING OF HYDROCARBONS; REFORMING OF NAPHTHA; MINERAL WAXES
- C10G2300/00—Aspects relating to hydrocarbon processing covered by groups C10G1/00 - C10G99/00
- C10G2300/20—Characteristics of the feedstock or the products
- C10G2300/201—Impurities
- C10G2300/205—Metal content
- C10G2300/206—Asphaltenes
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10G—CRACKING HYDROCARBON OILS; PRODUCTION OF LIQUID HYDROCARBON MIXTURES, e.g. BY DESTRUCTIVE HYDROGENATION, OLIGOMERISATION, POLYMERISATION; RECOVERY OF HYDROCARBON OILS FROM OIL-SHALE, OIL-SAND, OR GASES; REFINING MIXTURES MAINLY CONSISTING OF HYDROCARBONS; REFORMING OF NAPHTHA; MINERAL WAXES
- C10G2300/00—Aspects relating to hydrocarbon processing covered by groups C10G1/00 - C10G99/00
- C10G2300/20—Characteristics of the feedstock or the products
- C10G2300/30—Physical properties of feedstocks or products
- C10G2300/301—Boiling range
Definitions
- the present invention relates to the refining and the conversion of heavy hydrocarbon fractions containing, inter alia, sulfur-containing impurities. It relates more particularly to a process for converting heavy petroleum feeds of the atmospheric residue type and / or vacuum residue for the production of heavy fractions that can be used as fuel bases, in particular bunker oil bases, with a low sediment content.
- the process according to the invention also makes it possible to produce atmospheric distillates (naphtha, kerosene and diesel), vacuum distillates and light gases (C1 to C4).
- sediment content after aging is a measurement carried out according to the method described in the ISO 10307-2 standard (also known to those skilled in the art under the name of IP390). In the rest of the text will therefore read "sediment content after aging", the sediment content measured according to the ISO 10307-2 method.
- the reference to IP390 will also indicate that the measurement of the sediment content after aging is performed according to the ISO 10307-2 method.
- the sediment content according to ISO 10307-1 (also known as IP375) is different from the sediment content after aging according to ISO 10307-2 (also known as IP390).
- the sediment content after aging according to ISO 10307-2 is a much more stringent specification and corresponds to the specification for bunker fuels.
- terrestrial fuel oils in particular fuel oils that can be used for the production of heat and / or electricity, may also be subject to stability specifications, in particular maximum sediment contents, the thresholds of which vary according to the places of production. Because there is no international harmonization as in the case of maritime transport. There is, however, an interest in reducing the sediment content of terrestrial fuel oils.
- Residue hydrocracking processes convert low value residues to higher value added distillates.
- the resulting heavy fraction corresponding to the unconverted residual cut is generally unstable. It contains sediments that are mainly precipitated asphaltenes. This unstable residual cut can not therefore be efficiently be efficiently converted as fuel oil, especially as bunker oil without a specific treatment since the hydrocracking is operated under severe conditions leading to a high conversion rate.
- the patent US6447671 discloses a process for converting heavy petroleum fractions comprising a first bubbling bed hydrocracking step, a step of removing the catalyst particles contained in the hydrocracking effluent, and then a fixed bed hydrotreating step.
- the demand US2014 / 0034549 discloses a residue conversion process using a bubbling bed hydrocracking step and a step with an upflow reactor associated with a so-called "stripper" reactor.
- the sediment content of the final effluent is reduced relative to the effluent of the boiling bed stage.
- the sediment content after aging is not less than 0.1% by weight, as required for marketing as a residual type marine fuel.
- the patent FR2981659 discloses a petroleum heavy fraction conversion process comprising a first bubbling bed hydrocracking step and a fixed bed hydrotreating step comprising permutable reactors.
- the hydrocracking process partially converts heavy feeds to produce atmospheric distillates and / or vacuum distillates.
- ebullated bed technology is known to be suitable for heavy loads loaded with impurities, the bubbling bed inherently produces catalyst fines and sediments that must be removed to meet product quality such as heating oil. hold. The fines come mainly from the attrition of the catalyst in the bubbling bed.
- the sediments may be precipitated asphaltenes.
- the hydrocracking conditions and in particular the temperature cause them to undergo reactions (dealkylation, polycondensation, etc.) leading to their precipitation.
- reactions dealkylation, polycondensation, etc.
- conversion rates for compounds boiling above 540 ° C: 540 + ° C, for example greater than 30, 40 or 50% depending of the nature of the charge.
- An advantage of the method according to the invention is to avoid in particular the risk of clogging of boat engines.
- Another advantage of the process of the invention is to avoid the risk of fouling, in the case of any processing steps carried out downstream of the hydrocracking step of avoiding clogging of the bed (s) ( s) catalytic (s) implemented.
- the heavy fractions obtained by the present process can be mixed with fluxing bases so as to achieve the target viscosity of the desired fuel grade as well as the specification in sediment content after aging.
- Another point of interest of the process is the partial conversion of the feedstock making it possible to produce, particularly by hydrocracking, atmospheric distillates or vacuum distillates (naphtha, kerosene, diesel, vacuum distillate), which can be used as bases in plants.
- fuel pools directly or after passing through another refining process such as hydrotreating, reforming, isomerization, hydrocracking or catalytic cracking.
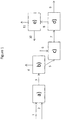
- the figure 1 illustrates a schematic view of the process according to the invention showing a hydrocracking zone, a separation zone, a precipitation zone, a physical separation zone of the sediments and a recovery zone of the fraction of interest.
- the feedstocks treated in the process according to the invention are advantageously chosen from atmospheric residues, vacuum residues from direct distillation, crude oils, crude head oils, deasphalted oils, deasphalting resins, asphalts or pitches. deasphalting, residues resulting from conversion processes, aromatic extracts from lubricant base production lines, oil sands or their derivatives, oil shales or their derivatives, whether alone or as a mixture.
- fillers can advantageously be used as they are or else diluted by a hydrocarbon fraction or a mixture of hydrocarbon fractions which may be chosen from products resulting from a fluid catalytic cracking process (FCC according to the initials of the English name of "Fluid Catalytic Cracking"), a light cutting oil (LCO), a heavy cutting oil (HCO), a decanted oil (OD according to the initials of the English name “Decanted Oil”), a residue of FCC , or which may come from the distillation, gas oil fractions including those obtained by atmospheric or vacuum distillation, such as vacuum gas oil.
- the heavy charges can also advantageously comprise cuts resulting from the process of liquefying coal or biomass, aromatic extracts, or any other hydrocarbon cuts or non-petroleum fillers such as pyrolysis oil from lignocellulosic biomasses.
- the fillers according to the invention generally have a sulfur content of at least 0.1% by weight, an initial boiling point of at least 340 ° C. and a final boiling point of at least 440 ° C. preferably a final boiling temperature of at least 540 ° C.
- the feedstock may contain at least 1% C7 asphaltenes and at least 5 ppm metals, preferably at least 2% C7 asphaltenes and at least 25 ppm metals.
- the fillers according to the invention are preferably atmospheric residues or residues under vacuum, or mixtures of these residues.
- Step a) Hydrocracking in a bubbling bed
- the filler according to the invention is subjected to a hydrocracking step which is carried out in at least one reactor containing a catalyst supported in a bubbling bed and preferably operating with an upward flow of liquid and gas.
- the objective of the hydrocracking step is to convert the heavy fraction into lighter cuts while partially refining the charge.
- Bubbling bed technologies use extruded bed catalysts supported in the form of extrudates, the diameter of which is generally of the order of 1 mm or less than 1 mm.
- the catalysts remain inside the reactors and are not evacuated with the products.
- the temperature levels are high in order to obtain high conversions while minimizing the amounts of catalysts used.
- the catalytic activity can be kept constant by replacing the catalyst in line. It is therefore not necessary to stop the unit to change the spent catalyst, nor to increase the reaction temperatures along the cycle to compensate for the deactivation.
- operating at constant operating conditions provides consistent yields and product qualities along the cycle. Also, because the catalyst is kept agitated by a large recycling of liquid, the pressure drop on the reactor remains low and constant.
- the conditions of hydrocracking step a) in the presence of hydrogen are usually conventional bubbling bed hydrocracking conditions of a liquid hydrocarbon fraction. It is advantageously carried out under a hydrogen partial pressure of 5 to 35 MPa, often 8 to 25 MPa and usually 12 to 20 MPa at a temperature of 330 to 500 ° C and often 350 to 450 ° C.
- the hourly space velocity (VVH) and the hydrogen partial pressure are important factors that are chosen according to the characteristics of the product to be treated and the desired conversion.
- the VVH defined as the volumetric flow rate of the feed divided by the total volume of the reactor, is generally in a range from 0.05 h -1 to 5 h -1 , preferably from 0.1 h -1 to 2 h -1 and more preferably from 0.2 h -1 to 1 h -1 .
- the amount of hydrogen mixed with the feedstock is usually 50 to 5000 Nm 3 / m 3 (normal cubic meters (Nm 3 ) per cubic meter (m 3 ) of liquid charge) and most often from 100 to 1000 Nm 3 / m 3 and preferably from 200 to 500 Nm 3 / m 3 .
- a conventional granular hydrocracking catalyst comprising, on an amorphous support, at least one metal or metal compound having a hydrodehydrogenating function.
- This catalyst may be a catalyst comprising Group VIII metals, for example nickel and / or cobalt, most often in combination with at least one Group VIB metal, for example molybdenum and / or tungsten.
- a catalyst comprising from 0.5 to 10% by weight of nickel and preferably from 1 to 5% by weight of nickel (expressed as nickel oxide NiO) and from 1 to 30% by weight of molybdenum of preferably from 5 to 20% by weight of molybdenum (expressed as MoO 3 molybdenum oxide) on an amorphous mineral support.
- This support will for example be chosen from the group formed by alumina, silica, silica-aluminas, magnesia, clays and mixtures of at least two of these minerals.
- This support may also contain other compounds and for example oxides chosen from the group formed by boron oxide, zirconia, titanium oxide and phosphoric anhydride. Most often an alumina support is used and very often a support of alumina doped with phosphorus and possibly boron.
- phosphorus pentoxide P 2 O 5 When phosphorus pentoxide P 2 O 5 is present, its concentration is usually less than 20% by weight and most often less than 10% by weight.
- the concentration of boron trioxide B 2 O 3 is usually from 0 to 10% by weight.
- the alumina used is usually a gamma or eta alumina. This catalyst is most often in the form of extrudates.
- the total content of metal oxides of groups VI and VIII is often from 5 to 40% by weight and in general from 7 to 30% by weight and the weight ratio expressed as metal oxide between metal (or metals) of Group VI on metal (or metals) of group VIII is usually 20 to 1 and most often 10 to 2.
- the spent catalyst is partly replaced by fresh catalyst, generally by withdrawal at the bottom of the reactor and introduction to the top of the fresh or new catalyst reactor at a regular time interval, that is to say for example by puff or almost keep on going.
- the catalyst can also be introduced from below and withdrawn from the top of the reactor. For example, fresh catalyst can be introduced every day.
- the replacement rate of spent catalyst with fresh catalyst may be, for example, from about 0.05 kilograms to about 10 kilograms per cubic meter of charge. This withdrawal and this replacement are performed using devices allowing the continuous operation of this hydrocracking step.
- the unit usually includes a recirculation pump allowing the maintaining the catalyst in a bubbling bed by continuously recycling at least a portion of the liquid withdrawn at the top of the reactor and reinjected at the bottom of the reactor. It is also possible to send the spent catalyst withdrawn from the reactor into a regeneration zone in which the carbon and the sulfur contained therein are eliminated before it is reinjected in the hydrocracking step a).
- hydrocracking step a) is carried out under the conditions of the H-OIL® process as described, for example, in US6270654 .
- the hydrocracking can be carried out in a single reactor or in several (generally two) reactors arranged in series.
- the use of at least two ebullated bed reactors in series makes it possible to obtain products of better quality and with a better yield, thus limiting the energy and hydrogen needs in possible post-treatments.
- the hydrocracking into two reactors makes it possible to have improved operability in terms of the flexibility of the operating conditions and of the catalytic system.
- the temperature of the second reactor is preferably at least 5 ° C higher than that of the first bubbling bed reactor.
- the pressure of the second reactor is 0.1 to 1 MPa lower than for the first reactor to allow the flow of at least a portion of the effluent from the first step without pumping is necessary.
- the different operating conditions in terms of temperature in the two hydrocracking reactors are selected to be able to control the hydrogenation and the conversion of the feedstock into the desired products in each reactor.
- the effluent obtained at the end of the first hydrocracking reactor is subjected to a separation of the light fraction and at least a portion, preferably all, of the residual effluent is treated in the second hydrocracking reactor .
- This separation can be performed in an inter-floor separator as described in the patent US 6270654 and in particular makes it possible to avoid excessive hydrocracking of the light fraction in the second hydrocracking reactor.
- the hydrocracking step may also be carried out in at least one reactor operating in a hybrid bed mode, that is to say operating in a bubbling bed with a supported catalyst associated with a dispersed catalyst consisting of very fine catalyst particles. forming a suspension with the charge to be treated.
- a hybrid bed has two populations of catalyst, a population of bubbling bed catalyst to which is added a population of "dispersed” type catalyst.
- the term “dispersed” refers to an implementation of the reactor in which the catalyst is in the form of very fine particles, that is to say generally a size of between 1 nanometer (ie 10 -9 m) and 150 microns, preferably between 0.1 and 100 microns, and even more preferably between 10 and 80 microns.
- the hydrocracking stage may comprise a first bubbling bed reactor followed by a second hybrid bed type reactor (that is to say bubbling bed type with "dispersed" type catalyst injection).
- the hydrocracking step may comprise a first hybrid bed type reactor followed by a second hybrid type reactor.
- the hydrocracking step may comprise a single hybrid bed type reactor.
- the "disperse" catalyst used in the hybrid bed reactor may be a sulfide catalyst preferably containing at least one member selected from the group consisting of Mo, Fe, Ni, W, Co, V, Ru. These catalysts are generally monometallic or bimetallic (by combining, for example, a non-noble group VIIIB element (Co, Ni, Fe) and a group VIB element (Mo, W) .
- the catalysts used may be heterogeneous solid powders (such as natural ores, iron sulphate, etc.), dispersed catalysts derived from water-soluble precursors such as phosphomolybdic acid, ammonium molybdate, or a mixture of Mo or Ni oxide. With aqueous ammonia, the catalysts used are preferably derived from soluble precursors in an organic phase (oil-soluble catalysts).
- the precursors are generally organometallic compounds such as the naphthenates of Mo, Co, Fe, or Ni, or the Mo octoates, or the multi-carbonyl compounds of these metals, for example 2-ethyl hexanoates of Mo or Ni , Mo or Ni acetylacetonates, C7-C12 fatty acid salts of Mo or W, etc. They can be used in the presence a surfactant to improve the dispersion of the metals, when the catalyst is bimetallic.
- the catalysts are in the form of dispersed particles, colloidal or otherwise depending on the nature of the catalyst. Such precursors and catalysts that can be used in the process according to the invention are widely described in the literature.
- the catalysts are prepared before being injected into the feed.
- the preparation process is adapted according to the state in which the precursor is and of its nature. In all cases, the precursor is sulfided ( ex-situ or in-situ ) to form the catalyst dispersed in the feedstock.
- the precursor is advantageously mixed with a carbonaceous feedstock (which may be a part of the feedstock to be treated, an external feedstock, a recycled fraction, etc.), the mixture is then sulphurized. by addition of a sulfur compound (preferred hydrogen sulphide or optionally an organic sulphide such as DMDS in the presence of hydrogen) and heated.
- a sulfur compound preferred hydrogen sulphide or optionally an organic sulphide such as DMDS in the presence of hydrogen
- the preparations of these catalysts are described in the literature.
- the "dispersed" catalyst particles as defined above generally have a size of between 1 nanometer and 150 microns, preferably between 0.1 and 100 microns, and even more preferably between 10 and 80 microns.
- the content of catalytic compounds (expressed as weight percentage of metal elements of group VIII and / or of group VIB) is between 0 and 10% by weight, preferably between 0 and 1% by weight.
- Additives may be added during the preparation of the catalyst or to the "dispersed" catalyst before it is injected into the reactor. These additives are described in the literature.
- the preferred solid additives are inorganic oxides such as alumina, silica, mixed Al / Si oxides, supported spent catalysts (for example, on alumina and / or silica) containing at least one group VIII element (such as Ni, Co) and / or at least one group VIB element (such as Mo, W).
- group VIII element such as Ni, Co
- group VIB element such as Mo, W
- the catalysts described in the application US2008 / 177124 Carbonaceous solids with a low hydrogen content (for example 4% hydrogen), such as coke or ground activated carbon, optionally pretreated, can also be used. Mixtures of such additives can also be used.
- the particle size of the additive is generally between 10 and 750 microns, preferably between 100 and 600 microns.
- the content of any solid additive present at the inlet of the reaction zone of the "dispersed" hydrocracking process is between 0 and 10% by weight, preferably between 1 and 3% by weight, and the content of catalytic compounds (expressed as weight percentage of Group VIII and / or Group VIB metal elements) is between 0 and 10% by weight, preferably between 0 and 1% by weight.
- the hybrid bed reactor (s) used in the hydrocracking zone therefore consist of two populations of catalysts, a first population using supported catalysts in the form of extrudates whose diameter is advantageously between 0.8 and 1.2 mm. , generally equal to 0.9 mm or 1.1 mm and a second population of "dispersed" type catalyst discussed above.
- the fluidization of the catalyst particles in the bubbling bed is enabled by the use of a boiling pump which allows a recycle of liquid, generally inside the reactor.
- the flow rate of liquid recycled by the boiling pump is adjusted so that the supported catalyst particles are fluidized but not transported, so that these particles remain in the bubbling bed reactor (with the exception of catalyst fines that can be formed by attrition and entrained with the liquid since these fines are small).
- the "dispersed" type catalyst is also entrained with the liquid since the "dispersed” type catalyst consists of particles of very small size.
- Step b) Separation of the hydrocracking effluent
- the effluent obtained at the end of the hydrocracking step a) undergoes at least one separation step, optionally supplemented by further additional separation steps, making it possible to separate at least one light hydrocarbon fraction containing bases. fuels and a heavy fraction containing boiling compounds at least 350 ° C.
- the separation step may advantageously be carried out by any method known to those skilled in the art such as, for example, the combination of one or more high and / or low pressure separators, and / or distillation stages and / or or high and / or low pressure stripping.
- the separation step b) makes it possible to obtain a gaseous phase, at least a light fraction of hydrocarbons of the naphtha, kerosene and / or diesel type, a vacuum distillate fraction and a vacuum residue fraction and / or a fraction of atmospheric residue.
- the heavy fraction sent in the precipitation step c) corresponds at least in part to an atmospheric residue fraction.
- the separation may be carried out in a fractionation section which may first comprise a high temperature high pressure separator (HPHT), and optionally a low temperature high pressure separator (HPBT), and / or atmospheric distillation and / or distillation under empty.
- HPHT high temperature high pressure separator
- HPBT low temperature high pressure separator
- the effluent obtained at the end of step a) is separated (generally in an HPHT separator) into a light fraction and a heavy fraction containing predominantly boiling compounds at least 350 ° C.
- the cutting point of the separation is advantageously between 200 and 400 ° C.
- the effluent from the hydrocracking may also undergo a succession of flashes comprising at least one high temperature high pressure balloon (HPHT) and a low pressure balloon high temperature (BPHT) for separating a heavy fraction which is sent in a steam stripping step for removing from said heavy fraction at least a light fraction rich in hydrogen sulfide.
- HPHT high temperature high pressure balloon
- BPHT low pressure balloon high temperature
- the heavy fraction recovered at the bottom of the stripping column contains compounds boiling at least 350 ° C. but also atmospheric distillates.
- said heavy fraction separated from the light fraction rich in hydrogen sulphide is then sent to the precipitation step c) and then to the sediment separation step d).
- At least a portion of the so-called heavy fraction from step b) is fractionated by atmospheric distillation into at least one atmospheric distillate fraction containing at least one light fraction of naphtha, kerosene and / or diesel type hydrocarbons. and an atmospheric residue fraction.
- At least a part of the fraction atmospheric residue, corresponding at least in part to the heavy fraction resulting from step b), can be sent in the precipitation step c) and then in step d) of physical separation of the sediments .
- the atmospheric residue may also be at least partially fractionated by vacuum distillation into a vacuum distillate fraction containing vacuum gas oil and a vacuum residue fraction.
- Said fraction vacuum residue corresponding at least in part to the heavy fraction from step b), is advantageously sent at least partly in the precipitation step c) and then in step d) of physical separation of the sediments d).
- At least a portion of the vacuum distillate and / or the vacuum residue may also be recycled to the hydrocracking step a).
- the light fraction (s) obtained may (may) undergo further separation steps, possibly in the presence of the light fraction obtained from the internal separator. stage between the two hydrocracking reactors.
- it (s) is (are) subject (s) to atmospheric distillation to obtain a gaseous fraction, at least a light fraction of naphtha, kerosene and / or diesel type hydrocarbons and a vacuum distillate fraction.
- Part of the atmospheric distillate and / or the vacuum distillate from the separation step b) may constitute a part of a fuel oil as a fluxing agent. These cuts can also be marine fuels with low viscosity (MGO or MGO, Marine Diesel Oil or Marine Gas Oil according to English terminology). Another part of the vacuum distillate can still be upgraded by hydrocracking and / or catalytic cracking in a fluidized bed.
- the gaseous fractions resulting from the separation step preferably undergo a purification treatment to recover the hydrogen and recycle it to the hydrocracking reactors (step a)). Part of the purified hydrogen can be used during the precipitation step.
- the recovery of different fuel base cuts (LPG, naphtha, kerosene, diesel and / or vacuum gas oil) obtained from the present invention is well known to those skilled in the art.
- the products obtained can be integrated in fuel tanks (also called “pools" fuels according to the English terminology) or undergo additional refining steps.
- the fraction (s) naphtha, kerosene, gas oil and vacuum gas oil may be subjected to one or more treatments (hydrotreatment, hydrocracking, alkylation, isomerization, catalytic reforming, catalytic cracking or thermal or other) to bring them to the specifications. required (sulfur content, smoke point, octane, cetane, etc ...) separately or in mixture.
- the vacuum distillate leaving the bubbling bed after separation can be hydrotreated.
- This hydrotreated vacuum distillate may be used as a fluxing agent for the fuel oil pool having a sulfur content of less than or equal to 0.5% by weight or may be used directly as oil with a sulfur content of less than or equal to 0.1% by weight.
- Part of the atmospheric residue, vacuum distillate and / or vacuum residue may undergo further refining steps, such as hydrotreatment, hydrocracking, or fluidized catalytic cracking.
- the heavy fraction obtained at the end of the separation step b) contains organic sediments which result from the hydrocracking conditions and the catalyst residues.
- Part of the sediments consist of asphaltenes precipitated under hydrocracking conditions and are analyzed as existing sediments (IP375).
- the process according to the invention comprises a precipitation step making it possible to improve the sediment separation efficiency and thus to obtain stable fuel oils or bases, that is to say a sediment content after aging less than or equal to 0.1% by weight.
- the precipitation step according to the invention makes it possible to form all the existing and potential sediments (by converting the potential sediments into existing sediments) so as to separate them more effectively and thus respect the sediment content after aging (measured according to the ISO 10307-2 method) of 0.1% maximum weight.
- the precipitation step according to the invention comprises bringing the heavy fraction resulting from the separation step b) into contact with a distillate cut of which at least 20% by weight has a boiling temperature greater than or equal to 100 ° C. C, preferably greater than or equal to 120 ° C, more preferably greater than or equal to 150 ° C.
- the distillate cut is characterized in that it comprises at least 25% by weight having a boiling point greater than or equal to 100 ° C. preferably greater than or equal to 120 ° C, more preferably greater than or equal to 150 ° C.
- At least 5% by weight or even 10% by weight of the distillate cut according to the invention has a boiling point of at least 252 ° C.
- At least 5 wt.% Or even 10 wt.% Of the distillate cut according to the invention has a boiling point of at least 255 ° C.
- the precipitation step c) according to the invention is advantageously carried out for a residence time of less than 500 minutes, preferably less than 300 minutes, more preferably less than 60 minutes, at a temperature between 25 and 350 °. C, preferably between 50 and 350 ° C, preferably between 65 and 300 ° C and more preferably between 80 and 250 ° C.
- the pressure of the precipitation step is advantageously less than 20 MPa, preferably less than 10 MPa, more preferably less than 3 MPa and even more preferably less than 1.5 MPa.
- the distillate cut according to the invention advantageously comprises hydrocarbons having more than 12 carbon atoms, preferably hydrocarbons having more than 13 carbon atoms, more preferably hydrocarbons having between 13 and 40 carbon atoms.
- Said distillate fraction can partly or entirely from the separation step b) of the invention or another refining process or another chemical process.
- Said distillate cut may be used in a mixture with a naphtha-type cut and / or a vacuum-type gas oil cut and / or vacuum residue.
- Said distillate cut may be used in a mixture with the light fraction obtained after step b), the atmospheric distillate fraction resulting from step b) and / or the vacuum distillate fraction from step b ) of seperation.
- the distillate cut according to the invention is mixed with another cut, a light fraction and / or a heavy fraction as indicated above, the proportions are chosen so that the resulting mixture respects the characteristics of the the distillate cut according to the invention.
- distillate cut according to the invention has the advantage of avoiding the majority use of high value added cuts such as petrochemical cuts, naphtha ...
- the mass ratio between the distillate cut according to the invention and the heavy fraction obtained at the end of the separation step b) is between 0.01 and 100, preferably between 0.05 and 10, more preferably between 0, 1 and 5, and even more preferably between 0.1 and 2.
- the distillate cut according to the invention is at least drawn from the process, it is possible to accumulate this cut during a start-up period so as to reach the desired ratio.
- the precipitation step can be carried out using an exchanger or a heating furnace followed by one or more capacity (s) in series or in parallel such (s) as a horizontal or vertical balloon, optionally with a settling function to remove some of the heavier solids, and / or a piston reactor.
- a stirred and heated tank may also be used, and may be provided with a bottom draw to remove some of the heavier solids.
- the precipitation step can be carried out online, without buffer capacity, possibly using a static mixer.
- step c) of precipitation of the heavy fraction resulting from step b) is carried out in the presence of an inert gas and / or an oxidizing gas and / or an oxidizing liquid and / or hydrogen, preferably from the separation steps of the process of the invention, in particular from the separation step b).
- the precipitation step c) can be carried out in the presence of an inert gas such as dinitrogen, or in the presence of an oxidizing gas such as dioxygen, ozone or nitrogen oxides, or in the presence of a mixture containing an inert gas and an oxidizing gas such as air or air depleted by nitrogen, or in the presence of an oxidizing liquid to accelerate the precipitation process.
- oxidizing liquid means an oxygenated compound, for example a peroxide such as hydrogen peroxide, or an inorganic oxidizing solution such as a solution of potassium permanganate or a mineral acid such as sulfuric acid. According to this variant, the oxidizing liquid is then mixed with the heavy fraction from the separation step b) and the distillate cut according to the invention during the implementation of step c).
- Step d) Separation of sediments
- the method according to the invention further comprises a step d) of physical separation of sediments and catalyst residues.
- the heavy fraction obtained at the end of the precipitation step c) contains precipitated asphaltene-type organic sediments which result from hydrocracking and precipitation conditions. This heavy fraction may also contain catalyst fines resulting from the attrition of extruded type catalysts in the implementation of hydrocracking reactor. This heavy fraction may optionally contain "dispersed" catalyst residues in the case of the implementation of a hybrid reactor.
- At least a portion of the heavy fraction from step c) of precipitation is subjected to a physical separation of sediments and catalyst residues, by means of at least one physical separation means selected from a filter, a separation membrane, a bed of organic or inorganic type filtering solids, electrostatic precipitation, a centrifugation system, decantation, auger withdrawal.
- a combination, in series and / or in parallel, of several separation means of the same type or different type can be used during this step d) separation of sediments and catalyst residues.
- One of these solid-liquid separation techniques may require the periodic use of a light rinsing fraction, resulting from the process or not, allowing for example the cleaning of a filter and the evacuation of sediments.
- the heavy fraction (with a sediment content after aging of less than or equal to 0.1% by weight) is obtained, comprising a portion of the distillate cut according to US Pat. invention introduced in step c).
- Step e) Recovery of the heavy fraction at the end of step d) of separation
- the mixture resulting from stage d) is advantageously introduced into a stage e) of recovery of the heavy fraction having a sediment content after aging less than or equal to 0.1% by weight, said step consisting in separating the heavy fraction from step d) of the distillate cut introduced in step c).
- Step e) is a separation step similar to separation step b).
- Step e) can be implemented by means of separator balloon and / or distillation distillation equipment so as to separate on the one hand at least part of the distillate cup introduced during step c) of precipitation and on the other hand the heavy fraction having a sediment content after aging less than or equal to 0.1% by weight.
- a part of the distillate cut separated from step e) is recycled in step c) of precipitation.
- Said recovered heavy fraction may advantageously be used as a base of fuel oil or as fuel oil, especially as a base of bunker oil or as bunker oil, having a sediment content after aging less than 0.1% by weight.
- said heavy fraction is mixed with one or more fluxing bases selected from the group consisting of catalytic cracking light cutting oils, catalytic cracking heavy cutting oils, catalytic cracking residue, kerosene, a gas oil, a vacuum distillate and / or a decanted oil.
- one or more fluxing bases selected from the group consisting of catalytic cracking light cutting oils, catalytic cracking heavy cutting oils, catalytic cracking residue, kerosene, a gas oil, a vacuum distillate and / or a decanted oil.
- part of the distillate cut according to the invention can be left in the sediment-reduced heavy fraction so that the viscosity of the mixture is directly that of a desired fuel grade, for example 180 or 380 cSt at 50 ° C.
- the sulfur content of the heavy fraction resulting from stage d) or e), and containing predominantly compounds boiling at least 350 ° C., is a function of the operating conditions of the hydrocracking stage but also of the content of the hydrocracking stage. sulfur of the original charge.
- a step f) of hydrotreatment in a fixed bed is made necessary in the case where the refiner wishes to reduce the sulfur content, in particular for a bunker oil base or a bunker oil intended to be burned on a ship without smoke treatment.
- Stage f) of hydrotreatment in a fixed bed is carried out on at least a part of the heavy fraction resulting from stage d) or e).
- the heavy fraction from step f) can advantageously be used as a base of fuel oil or as fuel oil, especially as a base of bunker oil or as bunker oil, having a sediment content after aging less than 0.1% by weight.
- said heavy fraction is mixed with one or more fluxing bases selected from the group consisting of catalytic cracking light cutting oils, catalytic cracking heavy cutting oils, catalytic cracking residue, kerosene, a gas oil, a vacuum distillate and / or a decanted oil.
- the heavy fraction resulting from step d) or e) is sent to the hydrotreatment step f) comprising one or more hydrotreatment zones in fixed beds.
- the sending in a fixed bed of a heavy fraction devoid of sediment is an advantage of the present invention since the fixed bed will be less subject to clogging and increased pressure drop.
- Hydroprocessing is understood to mean, in particular, hydrodesulphurization (HDS) reactions, hydrodenitrogenation (HDN) reactions and hydrodemetallation (HDM) reactions, but also hydrogenation, hydrodeoxygenation, hydrodearomatization, hydrodenetration, hydroisomerization, hydrodealkylation, hydrocracking, hydro-deasphalting and Conradson carbon reduction.
- HDS hydrodesulphurization
- HDN hydrodenitrogenation
- HDM hydrodemetallation
- Such a method of hydrotreating heavy cuts is widely known and can be related to the process known as HYVAHL-F TM described in US Pat. US5417846 .
- hydrodemetallation reactions are mainly carried out but also part of the hydrodesulfurization reactions.
- hydrodesulphurization reactions are mainly carried out but also part of the hydrodemetallation reactions.
- a co-charge may be introduced with the heavy fraction in the hydrotreatment step f).
- This co-charge can be chosen from atmospheric residues, vacuum residues from direct distillation, deasphalted oils, aromatic extracts from lubricant base production lines, hydrocarbon fractions or a mixture of hydrocarbon fractions that can be chosen. from products resulting from a fluid-bed catalytic cracking process: a light cutting oil (LCO), a heavy cutting oil (HCO), a decanted oil, or possibly derived from distillation, the gas oil fractions, in particular those obtained by distillation atmospheric or vacuum, such as vacuum gas oil.
- LCO light cutting oil
- HCO heavy cutting oil
- decanted oil or possibly derived from distillation
- the gas oil fractions in particular those obtained by distillation atmospheric or vacuum, such as vacuum gas oil.
- the hydrotreatment step can advantageously be carried out at a temperature of between 300 and 500 ° C., preferably 350 ° C. to 420 ° C. and under a hydrogen partial pressure advantageously between 2 MPa and 25 MPa, preferably between 10 and 20 MPa, an overall hourly space velocity (VVH) which is defined as the volumetric flow rate of the feed divided by the total volume of the catalyst, being in a range from 0.1 hr-1 to 5 hr -1 and preferably from 0.1 hr -1 to 2 hr -1, a quantity of hydrogen mixed with the load usually of 100 to 5000 Nm3 / m3 (normal cubic meters (Nm3) per cubic meter (m3) of filler liquid), most often from 200 to 2000 Nm3 / m3 and preferably from 300 to 1500 Nm3 / m3.
- VVH hourly space velocity
- the hydrotreating step is carried out industrially in one or more liquid downflow reactors.
- the hydrotreatment temperature is generally adjusted according to the desired level of hydrotreatment.
- the hydrotreatment catalysts used are preferably known catalysts and are generally granular catalysts comprising, on a support, at least one metal or metal compound having a hydrodehydrogenating function. These catalysts are advantageously catalysts comprising at least one Group VIII metal, generally selected from the group consisting of nickel and / or cobalt, and / or at least one Group VIB metal, preferably molybdenum and / or tungsten. .
- a catalyst comprising from 0.5 to 10% by weight of nickel and preferably from 1 to 5% by weight of nickel (expressed as nickel oxide NiO) and from 1 to 30% by weight of molybdenum, preferably from 5 to 20% by weight of molybdenum (expressed as molybdenum oxide MoO 3 ) on a mineral support.
- This support will, for example, be selected from the group formed by alumina, silica, silica-aluminas, magnesia, clays and mixtures of at least two of these minerals.
- this support contains other doping compounds, in particular oxides chosen from the group formed by boron oxide, zirconia, ceria, titanium oxide, phosphoric anhydride and a mixture of these oxides.
- an alumina support is used and very often a support of alumina doped with phosphorus and possibly boron.
- concentration of phosphorus pentoxide P 2 O 5 is usually between 0 or 0.1% and 10% by weight.
- concentration of trioxide boron B 2 O 5 is usually from 0 or 0.1% to 10% by weight.
- the alumina used is usually a ⁇ or ⁇ alumina. This catalyst is most often in the form of extrudates.
- the total content of metal oxides of groups VIB and VIII is often from 5 to 40% by weight and in general from 7 to 30% by weight and the weight ratio expressed as metal oxide between metal (or metals) of Group VIB on metal (or metals) of group VIII is usually 20 to 1 and most often 10 to 2.
- hydrotreatment step including a hydrodemetallation step (HDM), then a hydrodesulfurization step (HDS), it is most often used specific catalysts adapted to each step.
- HDM hydrodemetallation step
- HDS hydrodesulfurization step
- Catalysts that can be used in the hydrodemetallation (HDM) stage are for example indicated in the patents EP113297 , EP113284 , US5221656 , US5827421 , US7119045 , US5622616 and US5089463 .
- Hydrodemetallation (HDM) catalysts are preferably used in the reactive reactors.
- Catalysts that can be used in the hydrodesulfurization (HDS) step are, for example, indicated in the patents EP113297 , EP113284 , US6589908 , US4818743 or US6332976 . It is also possible to use a mixed catalyst that is active in hydrodemetallization and in hydrodesulfurization for both the hydrodemetallation (HDM) section and the hydrodesulfurization (HDS) section as described in the patent. FR2940143 .
- the catalysts used in the process according to the present invention are preferably subjected to an in-situ or ex-situ sulphurization treatment .
- Step g) Optional step of separation of the hydrotreatment effluent
- the process according to the invention may comprise a step g) of separating the effluents from the hydrotreating step f).
- the optional separation step g) may advantageously be carried out by any method known to those skilled in the art such as, for example, the combination of one or more high and / or low pressure separators, and / or distillation and / or high and / or low pressure stripping.
- This optional separation step g) is similar to the separation step b) and will not be further described.
- the effluent obtained in step f) may be at least partly, and often entirely, sent to a separation step g), comprising atmospheric distillation and / or vacuum distillation.
- the effluent of the hydrotreatment step is fractionated by atmospheric distillation into a gaseous fraction, at least one atmospheric distillate fraction containing the fuels bases (naphtha, kerosene and / or diesel) and an atmospheric residue fraction. At least a portion of the atmospheric residue can then be fractionated by vacuum distillation into a vacuum distillate fraction containing vacuum gas oil and a vacuum residue fraction.
- the vacuum residue fraction and / or the vacuum distillate fraction and / or the atmospheric residue fraction may be at least partly the bases of low-sulfur fuel oils having a sulfur content of less than or equal to 0.5% by weight and a sediment content after aging less than or equal to 0.1%.
- the vacuum distillate fraction can constitute a fuel oil base having a sulfur content of less than or equal to 0.1% by weight.
- Part of the vacuum residue and / or the atmospheric residue can also be recycled to the hydrocracking step a).
- the heavy fractions resulting from steps d) and / or e) and / or f) and / or g) can be mixed with one or more fluxing bases chosen from the group consisting of light cutting oils.
- kerosene, gas oil and / or vacuum distillate produced in the process of the invention will be used.
- use will be kerosene, gas oil and / or vacuum distillate obtained (s) in the separation steps b) or g) of the process.
- the figure 1 schematically describes an example of implementation of the invention without limiting the scope.
- the hydrocarbon feedstock (1) and hydrogen (2) are contacted in a bubbling bed hydrocracking zone a).
- the effluent (3) from the hydrocracking zone a) is sent to a separation zone b) to obtain at least a light hydrocarbon fraction (4) and a heavy liquid fraction (5) containing compounds boiling at at least 350 ° C.
- This heavy fraction (5) is brought into contact with a distillate cut (6) during a precipitation step in zone c).
- the effluent (7) consists of a heavy fraction and sediment is treated in a physical separation step in zone d) to remove a sediment-containing fraction (9) and recover a sediment-reduced liquid hydrocarbon fraction (8).
- the liquid hydrocarbon fraction (8) is then treated in a recovery step in zone e) firstly of the liquid hydrocarbon fraction (11) having a sediment content after aging less than or equal to 0.1% by weight, and on the other hand a fraction (10) containing at least a portion of the distillate cut introduced during step c).
- a vacuum residue feedstock (RSV Ural) containing 84% by weight of compounds boiling at a temperature above 520 ° C, having a density of 9.5 ° API and a sulfur content of 2.6% by weight is treated.
- the feedstock was subjected to a hydrocracking step comprising two successive bubbling bed reactors.
- the operating conditions of the hydrocracking step are given in Table 1.
- Table 1 Operating conditions of the hydrocracking section ⁇ / u> 2 bubbling beds catalysts NiMo on alumina Temperature R1 (° C) 430 Temperature R2 (° C) 430 H2 partial pressure (MPa) 15 VVH "reactors" (h-1, Sm3 / h fresh load / m3 of reactors) 0.35 VVH "bubbling bed catalysts" (h-1, Sm3 / h fresh load / m3 bubbling bed catalysts) 0.65 H2 / HC entry hydrocracking section excluding H2 consumption (Nm3 / m3 fresh load) 600
- NiMo catalyst on Alumina used is sold by the company Axens under the reference HOC-458.
- the effluent of the hydrocracking step is then subjected to a separation step for separating a gaseous fraction and a heavy liquid fraction by means of separators.
- the heavy liquid fraction is then distilled in an atmospheric distillation column so as to recover distillates and an atmospheric residue.
- the atmospheric residue corresponding to the 350 ° C + fraction of the effluent in the proportion 44% by weight of DSV and 56% by weight of RSV of the hydrocracking step of the invention is characterized by a sediment content ( IP375) of 0.4% w / w and sediment content after aging (IP390) of 0.9% w / w.
- the mixture is then subjected to a step of separating the sediments and catalyst residues by means of a Pall® brand porous metal filter.
- This step of physical separation of the sediments is followed by a distillation step of the mixture making it possible to recover, on the one hand, the atmospheric residue with a reduced sediment content, and on the other hand the distillate cut.
- the operating conditions of the hydrocracking step coupled with the different treatment variants (separation of sediments with precipitation step (B) according to the invention or without precipitation step (A)) of the atmospheric residue (RA) have an impact on the stability of the effluents obtained. This is illustrated by the post-aging sediment concentrations measured in RA atmospheric residues (350 ° C + cut) before and after the sediment precipitation and separation step.
- the atmospheric residue obtained according to the invention is an excellent fuel oil base, especially a bunker oil base having a sediment content after aging (IP390) less than 0.1% by weight.
- the RA atmospheric residue treated according to the mixture of Table 4 has a sediment content after aging of less than 0.1%, a sulfur content of 0.93% w / w and a viscosity of 380 cSt at 50 ° C.
- This atmospheric residue thus constitutes a quality bunker oil, which can be sold according to the RMG or IFO 380 grade, with low sediment content.
- it may be burned in the ECA zone or outside the ECA zones by 2020-25 provided that the vessel is equipped with flue-gas scrubbers to cut down the sulfur oxides.
Landscapes
- Chemical & Material Sciences (AREA)
- Oil, Petroleum & Natural Gas (AREA)
- Engineering & Computer Science (AREA)
- Chemical Kinetics & Catalysis (AREA)
- General Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Production Of Liquid Hydrocarbon Mixture For Refining Petroleum (AREA)
- Catalysts (AREA)
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| FR1554962A FR3036703B1 (fr) | 2015-06-01 | 2015-06-01 | Procede de conversion de charges comprenant une etape d'hydrocraquage, une etape de precipitation et une etape de separation des sediments pour la production de fiouls |
| PCT/EP2016/058747 WO2016192892A1 (fr) | 2015-06-01 | 2016-04-20 | Procede de conversion de charges comprenant une etape d'hydrocraquage, une etape de precipitation et une etape de separation des sediments pour la production de fiouls |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| EP3303522A1 EP3303522A1 (fr) | 2018-04-11 |
| EP3303522B1 true EP3303522B1 (fr) | 2019-03-06 |
Family
ID=54199789
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP16719813.4A Active EP3303522B1 (fr) | 2015-06-01 | 2016-04-20 | Procede de conversion de charges comprenant une etape d'hydrocraquage, une etape de precipitation et une etape de separation des sediments pour la production de fiouls |
Country Status (11)
| Country | Link |
|---|---|
| US (1) | US11702603B2 (enExample) |
| EP (1) | EP3303522B1 (enExample) |
| JP (1) | JP6670855B2 (enExample) |
| KR (1) | KR102529350B1 (enExample) |
| CN (1) | CN107849466A (enExample) |
| ES (1) | ES2728566T3 (enExample) |
| FR (1) | FR3036703B1 (enExample) |
| PT (1) | PT3303522T (enExample) |
| SA (1) | SA517390453B1 (enExample) |
| TW (1) | TWI700361B (enExample) |
| WO (1) | WO2016192892A1 (enExample) |
Families Citing this family (28)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| DE202016007978U1 (de) | 2016-12-27 | 2018-03-28 | Rudolf King | GPS-basierte Methode zur effektiven, mobilen Nachbarschaftshilfe im Notfall mittels statischen und/oder dynamischen und zeitlich unbegrenzten oder definierten sozialen Netzwerken (SENI) und mobilen Endgeräten |
| US10604709B2 (en) | 2017-02-12 | 2020-03-31 | Magēmā Technology LLC | Multi-stage device and process for production of a low sulfur heavy marine fuel oil from distressed heavy fuel oil materials |
| US11788017B2 (en) | 2017-02-12 | 2023-10-17 | Magëmã Technology LLC | Multi-stage process and device for reducing environmental contaminants in heavy marine fuel oil |
| US12025435B2 (en) | 2017-02-12 | 2024-07-02 | Magēmã Technology LLC | Multi-stage device and process for production of a low sulfur heavy marine fuel oil |
| US12071592B2 (en) | 2017-02-12 | 2024-08-27 | Magēmā Technology LLC | Multi-stage process and device utilizing structured catalyst beds and reactive distillation for the production of a low sulfur heavy marine fuel oil |
| US10655074B2 (en) | 2017-02-12 | 2020-05-19 | Mag{hacek over (e)}m{hacek over (a)} Technology LLC | Multi-stage process and device for reducing environmental contaminates in heavy marine fuel oil |
| US12281266B2 (en) | 2017-02-12 | 2025-04-22 | Magẽmã Technology LLC | Heavy marine fuel oil composition |
| US10696906B2 (en) | 2017-09-29 | 2020-06-30 | Marathon Petroleum Company Lp | Tower bottoms coke catching device |
| CN109988629B (zh) * | 2017-12-29 | 2021-08-31 | 中国石油化工股份有限公司 | 一种蜡油加氢裂化方法和系统 |
| FR3084372B1 (fr) * | 2018-07-24 | 2020-08-07 | Ifp Energies Now | Procede de traitement d'une charge hydrocarbonee lourde comprenant un hydrotraitement en lit fixe, deux desasphaltages et un hydrocraquage en lit bouillonnant de l'asphalte |
| FR3084371B1 (fr) * | 2018-07-24 | 2020-08-07 | Ifp Energies Now | Procede de traitement d'une charge hydrocarbonee lourde comprenant un hydrotraitement en lit fixe, un desasphaltage et un hydrocraquage en lit bouillonnant de l'asphalte |
| US12000720B2 (en) | 2018-09-10 | 2024-06-04 | Marathon Petroleum Company Lp | Product inventory monitoring |
| US12031676B2 (en) | 2019-03-25 | 2024-07-09 | Marathon Petroleum Company Lp | Insulation securement system and associated methods |
| US11975316B2 (en) | 2019-05-09 | 2024-05-07 | Marathon Petroleum Company Lp | Methods and reforming systems for re-dispersing platinum on reforming catalyst |
| CA3081520C (en) | 2019-05-30 | 2023-10-31 | Marathon Petroleum Company Lp | Methods and systems for minimizing nox and co emissions in natural draft heaters |
| KR20210072217A (ko) * | 2019-12-06 | 2021-06-17 | 현대오일뱅크 주식회사 | 안정화된 연료유의 제조방법 및 그로부터 얻는 안정화된 연료유 |
| US11124714B2 (en) | 2020-02-19 | 2021-09-21 | Marathon Petroleum Company Lp | Low sulfur fuel oil blends for stability enhancement and associated methods |
| US11898109B2 (en) | 2021-02-25 | 2024-02-13 | Marathon Petroleum Company Lp | Assemblies and methods for enhancing control of hydrotreating and fluid catalytic cracking (FCC) processes using spectroscopic analyzers |
| US12473500B2 (en) | 2021-02-25 | 2025-11-18 | Marathon Petroleum Company Lp | Assemblies and methods for enhancing control of fluid catalytic cracking (FCC) processes using spectroscopic analyzers |
| US20250012744A1 (en) | 2021-02-25 | 2025-01-09 | Marathon Petroleum Company Lp | Methods and assemblies for enhancing control of refining processes using spectroscopic analyzers |
| US12461022B2 (en) | 2021-02-25 | 2025-11-04 | Marathon Petroleum Company Lp | Methods and assemblies for determining and using standardized spectral responses for calibration of spectroscopic analyzers |
| US11702600B2 (en) | 2021-02-25 | 2023-07-18 | Marathon Petroleum Company Lp | Assemblies and methods for enhancing fluid catalytic cracking (FCC) processes during the FCC process using spectroscopic analyzers |
| US11905468B2 (en) | 2021-02-25 | 2024-02-20 | Marathon Petroleum Company Lp | Assemblies and methods for enhancing control of fluid catalytic cracking (FCC) processes using spectroscopic analyzers |
| US11692141B2 (en) | 2021-10-10 | 2023-07-04 | Marathon Petroleum Company Lp | Methods and systems for enhancing processing of hydrocarbons in a fluid catalytic cracking unit using a renewable additive |
| US11802257B2 (en) | 2022-01-31 | 2023-10-31 | Marathon Petroleum Company Lp | Systems and methods for reducing rendered fats pour point |
| US12311305B2 (en) | 2022-12-08 | 2025-05-27 | Marathon Petroleum Company Lp | Removable flue gas strainer and associated methods |
| US12306076B2 (en) | 2023-05-12 | 2025-05-20 | Marathon Petroleum Company Lp | Systems, apparatuses, and methods for sample cylinder inspection, pressurization, and sample disposal |
| US12415962B2 (en) | 2023-11-10 | 2025-09-16 | Marathon Petroleum Company Lp | Systems and methods for producing aviation fuel |
Family Cites Families (17)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US2988501A (en) * | 1958-08-18 | 1961-06-13 | Union Oil Co | Hydrorefining of crude oils |
| US4883581A (en) * | 1986-10-03 | 1989-11-28 | Exxon Chemical Patents Inc. | Pretreatment for reducing oxidative reactivity of baseoils |
| US5108580A (en) * | 1989-03-08 | 1992-04-28 | Texaco Inc. | Two catalyst stage hydrocarbon cracking process |
| FR2753984B1 (fr) * | 1996-10-02 | 1999-05-28 | Inst Francais Du Petrole | Procede de conversion d'une fraction lourde d'hydrocarbures impliquant une hydrodemetallisation en lit bouillonnant de catalyseur |
| FR2866897B1 (fr) * | 2004-03-01 | 2007-08-31 | Inst Francais Du Petrole | Utilisation de gaz pour le preraffinage de petrole conventionnel et optionnellement sequestration de co2 |
| US7811444B2 (en) * | 2006-06-08 | 2010-10-12 | Marathon Oil Canada Corporation | Oxidation of asphaltenes |
| FR2933709B1 (fr) * | 2008-07-10 | 2011-07-22 | Inst Francais Du Petrole | Procede de conversion comprenant une hydroconversion d'une charge, un fractionnement, puis un desasphatage de la fraction residu sous vide |
| EA025489B1 (ru) * | 2009-11-17 | 2016-12-30 | Эйч А Ди Корпорейшн | Способ удаления асфальтенов из тяжелой нефти путем воздействия с высокой скоростью сдвига |
| PL2348091T3 (pl) * | 2010-01-12 | 2013-04-30 | Ifp Energies Now | Sposób bezpośredniego hydroupłynniania biomasy obejmujący dwa etapy hydrokonwersji na złożu wrzącym |
| US8790508B2 (en) * | 2010-09-29 | 2014-07-29 | Saudi Arabian Oil Company | Integrated deasphalting and oxidative removal of heteroatom hydrocarbon compounds from liquid hydrocarbon feedstocks |
| JP6073882B2 (ja) * | 2011-07-29 | 2017-02-01 | サウジ アラビアン オイル カンパニー | 重質炭化水素の安定化方法 |
| US10400184B2 (en) * | 2011-08-31 | 2019-09-03 | Exxonmobil Research And Engineering Company | Hydroprocessing of heavy hydrocarbon feeds using small pore catalysts |
| FR2981659B1 (fr) * | 2011-10-20 | 2013-11-01 | Ifp Energies Now | Procede de conversion de charges petrolieres comprenant une etape d'hydroconversion en lit bouillonnant et une etape d'hydrotraitement en lit fixe pour la production de fiouls a basse teneur en soufre |
| FR2983866B1 (fr) * | 2011-12-07 | 2015-01-16 | Ifp Energies Now | Procede d'hydroconversion de charges petrolieres en lits fixes pour la production de fiouls a basse teneur en soufre |
| FR3000097B1 (fr) * | 2012-12-20 | 2014-12-26 | Ifp Energies Now | Procede integre de traitement de charges petrolieres pour la production de fiouls a basse teneur en soufre |
| FR3000098B1 (fr) * | 2012-12-20 | 2014-12-26 | IFP Energies Nouvelles | Procede avec separation de traitement de charges petrolieres pour la production de fiouls a basse teneur en soufre |
| JP6654622B2 (ja) * | 2014-07-25 | 2020-02-26 | サウジ アラビアン オイル カンパニーSaudi Arabian Oil Company | アスファルト、石油生コークス、並びに液体及びガスコークス化ユニット生成物の統合製造プロセス |
-
2015
- 2015-06-01 FR FR1554962A patent/FR3036703B1/fr not_active Expired - Fee Related
-
2016
- 2016-04-20 JP JP2017562068A patent/JP6670855B2/ja active Active
- 2016-04-20 PT PT16719813T patent/PT3303522T/pt unknown
- 2016-04-20 CN CN201680032073.6A patent/CN107849466A/zh active Pending
- 2016-04-20 WO PCT/EP2016/058747 patent/WO2016192892A1/fr not_active Ceased
- 2016-04-20 KR KR1020177037761A patent/KR102529350B1/ko active Active
- 2016-04-20 US US15/578,467 patent/US11702603B2/en active Active
- 2016-04-20 EP EP16719813.4A patent/EP3303522B1/fr active Active
- 2016-04-20 ES ES16719813T patent/ES2728566T3/es active Active
- 2016-06-01 TW TW105117261A patent/TWI700361B/zh active
-
2017
- 2017-11-30 SA SA517390453A patent/SA517390453B1/ar unknown
Non-Patent Citations (1)
| Title |
|---|
| None * |
Also Published As
| Publication number | Publication date |
|---|---|
| FR3036703B1 (fr) | 2017-05-26 |
| TWI700361B (zh) | 2020-08-01 |
| PT3303522T (pt) | 2019-06-12 |
| KR102529350B1 (ko) | 2023-05-04 |
| ES2728566T3 (es) | 2019-10-25 |
| TW201715033A (zh) | 2017-05-01 |
| SA517390453B1 (ar) | 2021-06-28 |
| JP6670855B2 (ja) | 2020-03-25 |
| US20180134974A1 (en) | 2018-05-17 |
| FR3036703A1 (fr) | 2016-12-02 |
| KR20180014776A (ko) | 2018-02-09 |
| WO2016192892A1 (fr) | 2016-12-08 |
| CN107849466A (zh) | 2018-03-27 |
| JP2018520228A (ja) | 2018-07-26 |
| EP3303522A1 (fr) | 2018-04-11 |
| US11702603B2 (en) | 2023-07-18 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| EP3303522B1 (fr) | Procede de conversion de charges comprenant une etape d'hydrocraquage, une etape de precipitation et une etape de separation des sediments pour la production de fiouls | |
| EP3018187B1 (fr) | Procede de conversion de charges petrolieres comprenant une etape d'hydrocraquage en lit bouillonnant, une etape de maturation et une etape de separation des sediments pour la production de fiouls a basse teneur en sediments | |
| EP3303523B1 (fr) | Procede de conversion de charges comprenant une etape d'hydrotraitement, une etape d'hydrocraquage, une etape de precipitation et une etape de separation des sediments pour la production de fiouls | |
| EP3026097B1 (fr) | Procede de production de combustibles de type fuel lourd a partir d'une charge hydrocarbonee lourde utilisant une separation entre l'etape d'hydrotraitement et l'etape d'hydrocraquage | |
| EP3018188B1 (fr) | Procede de conversion de charges petrolieres comprenant une etape d'hydrotraitement en lit fixe, une etape d'hydrocraquage en lit bouillonnant, une etape de maturation et une etape de separation des sediments pour la production de fiouls a basse teneur en sediments | |
| CA3021600C (fr) | Procede de conversion comprenant des lits de garde permutables d'hydrodemetallation, une etape d'hydrotraitement en lit fixe et une etape d'hydrocraquage en reacteurs permutables | |
| EP2788458B1 (fr) | Procede d'hydroconversion de charges petrolieres en lits fixes pour la production de fiouls a basse teneur en soufre | |
| EP3018189B1 (fr) | Procede de conversion de charges petrolieres comprenant une etape de viscoreduction, une etape de maturation et une etape de separation des sediments pour la production de fiouls a basse teneur en sediments | |
| FR3027909A1 (fr) | Procede integre de production de combustibles de type fuel lourd a partir d'une charge hydrocarbonee lourde sans separation intermediaire entre l'etape d'hydrotraitement et l'etape d'hydrocraquage | |
| WO2014096704A1 (fr) | Procédé avec separation de traitement de charges petrolieres pour la production de fiouls a basse teneur en soufre | |
| WO2015091033A1 (fr) | Nouveau procede integre de traitement de charges petrolieres pour la production de fiouls a basse teneur en soufre et en sediments | |
| WO2013057389A1 (fr) | Procédé de conversion de charges petrolieres comprenant une etape d'hydroconversion en lit bouillonnant et une etape d'hydrotraitement en lit fixe pour la production de fiouls a basse teneur en soufre | |
| WO2012085407A1 (fr) | Procède de conversion de charge hydrocarbonate comprenant une huile de schiste par hydre conversion en lit bouillonnant, fractionnement par distillation atmosphérique, et hydrocraquage | |
| WO2012085406A1 (fr) | Procede de conversion de charge hydrocarbonee comprenant une huile de schiste par hydroconversion en lit bouillonnant, fractionnement par distillation atmospherique et extraction liquide/liquide de la fraction lourde. | |
| FR3084371A1 (fr) | Procede de traitement d'une charge hydrocarbonee lourde comprenant un hydrotraitement en lit fixe, un desasphaltage et un hydrocraquage en lit bouillonnant de l'asphalte | |
| WO2012085408A1 (fr) | Procede de conversion de charge hydrocarbonee comprenant une huile de schiste par decontamination, hydroconversion en lit bouillonnant, et fractionnement par distillation atmospherique | |
| FR3084372A1 (fr) | Procede de traitement d'une charge hydrocarbonee lourde comprenant un hydrotraitement en lit fixe, deux desasphaltages et un hydrocraquage en lit bouillonnant de l'asphalte | |
| WO2016192893A1 (fr) | Procédé de conversion de charges comprenant une étape de viscoréduction, une étape de précipitation et une étape de séparation des sédiments pour la production de fiouls |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: THE INTERNATIONAL PUBLICATION HAS BEEN MADE |
|
| PUAI | Public reference made under article 153(3) epc to a published international application that has entered the european phase |
Free format text: ORIGINAL CODE: 0009012 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: REQUEST FOR EXAMINATION WAS MADE |
|
| 17P | Request for examination filed |
Effective date: 20180102 |
|
| AK | Designated contracting states |
Kind code of ref document: A1 Designated state(s): AL AT BE BG CH CY CZ DE DK EE ES FI FR GB GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO PL PT RO RS SE SI SK SM TR |
|
| AX | Request for extension of the european patent |
Extension state: BA ME |
|
| DAV | Request for validation of the european patent (deleted) | ||
| DAX | Request for extension of the european patent (deleted) | ||
| GRAP | Despatch of communication of intention to grant a patent |
Free format text: ORIGINAL CODE: EPIDOSNIGR1 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: GRANT OF PATENT IS INTENDED |
|
| RIC1 | Information provided on ipc code assigned before grant |
Ipc: C10G 31/06 20060101ALI20180913BHEP Ipc: C10G 67/12 20060101ALI20180913BHEP Ipc: C10G 47/24 20060101AFI20180913BHEP Ipc: C10G 31/09 20060101ALI20180913BHEP Ipc: C10G 67/02 20060101ALI20180913BHEP |
|
| INTG | Intention to grant announced |
Effective date: 20180928 |
|
| GRAS | Grant fee paid |
Free format text: ORIGINAL CODE: EPIDOSNIGR3 |
|
| GRAA | (expected) grant |
Free format text: ORIGINAL CODE: 0009210 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: THE PATENT HAS BEEN GRANTED |
|
| AK | Designated contracting states |
Kind code of ref document: B1 Designated state(s): AL AT BE BG CH CY CZ DE DK EE ES FI FR GB GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO PL PT RO RS SE SI SK SM TR |
|
| REG | Reference to a national code |
Ref country code: GB Ref legal event code: FG4D Free format text: NOT ENGLISH |
|
| REG | Reference to a national code |
Ref country code: CH Ref legal event code: EP Ref country code: AT Ref legal event code: REF Ref document number: 1104530 Country of ref document: AT Kind code of ref document: T Effective date: 20190315 |
|
| REG | Reference to a national code |
Ref country code: DE Ref legal event code: R096 Ref document number: 602016010745 Country of ref document: DE |
|
| REG | Reference to a national code |
Ref country code: IE Ref legal event code: FG4D Free format text: LANGUAGE OF EP DOCUMENT: FRENCH |
|
| REG | Reference to a national code |
Ref country code: PT Ref legal event code: SC4A Ref document number: 3303522 Country of ref document: PT Date of ref document: 20190612 Kind code of ref document: T Free format text: AVAILABILITY OF NATIONAL TRANSLATION Effective date: 20190603 |
|
| REG | Reference to a national code |
Ref country code: NL Ref legal event code: FP |
|
| REG | Reference to a national code |
Ref country code: LT Ref legal event code: MG4D |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: NO Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20190606 Ref country code: LT Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20190306 Ref country code: SE Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20190306 Ref country code: FI Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20190306 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: HR Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20190306 Ref country code: RS Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20190306 Ref country code: LV Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20190306 Ref country code: BG Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20190606 Ref country code: GR Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20190607 |
|
| REG | Reference to a national code |
Ref country code: AT Ref legal event code: MK05 Ref document number: 1104530 Country of ref document: AT Kind code of ref document: T Effective date: 20190306 |
|
| REG | Reference to a national code |
Ref country code: ES Ref legal event code: FG2A Ref document number: 2728566 Country of ref document: ES Kind code of ref document: T3 Effective date: 20191025 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: EE Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20190306 Ref country code: AL Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20190306 Ref country code: SK Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20190306 Ref country code: IT Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20190306 Ref country code: RO Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20190306 Ref country code: CZ Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20190306 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: SM Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20190306 Ref country code: PL Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20190306 |
|
| REG | Reference to a national code |
Ref country code: CH Ref legal event code: PL |
|
| REG | Reference to a national code |
Ref country code: DE Ref legal event code: R097 Ref document number: 602016010745 Country of ref document: DE |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: AT Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20190306 Ref country code: LU Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20190420 Ref country code: IS Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20190706 |
|
| PLBE | No opposition filed within time limit |
Free format text: ORIGINAL CODE: 0009261 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: NO OPPOSITION FILED WITHIN TIME LIMIT |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: LI Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20190430 Ref country code: DK Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20190306 Ref country code: CH Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20190430 Ref country code: MC Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20190306 |
|
| 26N | No opposition filed |
Effective date: 20191209 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: SI Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20190306 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: TR Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20190306 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: IE Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20190420 |
|
| GBPC | Gb: european patent ceased through non-payment of renewal fee |
Effective date: 20200420 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: GB Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20200420 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: CY Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20190306 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: MT Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20190306 Ref country code: HU Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT; INVALID AB INITIO Effective date: 20160420 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: MK Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20190306 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: NL Payment date: 20250424 Year of fee payment: 10 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: DE Payment date: 20250428 Year of fee payment: 10 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: ES Payment date: 20250513 Year of fee payment: 10 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: BE Payment date: 20250424 Year of fee payment: 10 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: PT Payment date: 20250407 Year of fee payment: 10 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: FR Payment date: 20250424 Year of fee payment: 10 |