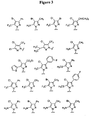
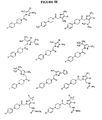
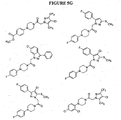
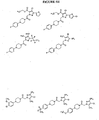
EP1531822B1 - 1-aryl-4-substituierte piperazin-derivate zur verwendung als ccr1-antagonisten zur behandlung von entzündungen und immunerkrankungen - Google Patents
1-aryl-4-substituierte piperazin-derivate zur verwendung als ccr1-antagonisten zur behandlung von entzündungen und immunerkrankungen Download PDFInfo
- Publication number
- EP1531822B1 EP1531822B1 EP03737057A EP03737057A EP1531822B1 EP 1531822 B1 EP1531822 B1 EP 1531822B1 EP 03737057 A EP03737057 A EP 03737057A EP 03737057 A EP03737057 A EP 03737057A EP 1531822 B1 EP1531822 B1 EP 1531822B1
- Authority
- EP
- European Patent Office
- Prior art keywords
- alkyl
- group
- chloro
- phenyl
- haloalkyl
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Expired - Lifetime
Links
- 230000004054 inflammatory process Effects 0.000 title abstract description 11
- 239000005557 antagonist Substances 0.000 title abstract description 10
- 206010061218 Inflammation Diseases 0.000 title abstract description 9
- 229940066771 systemic antihistamines piperazine derivative Drugs 0.000 title abstract description 4
- 208000026278 immune system disease Diseases 0.000 title description 7
- 150000004885 piperazines Chemical class 0.000 title description 5
- 150000001875 compounds Chemical class 0.000 claims abstract description 311
- -1 aryl piperazine derivatives Chemical class 0.000 claims abstract description 74
- 230000001404 mediated effect Effects 0.000 claims abstract description 62
- 102100031172 C-C chemokine receptor type 1 Human genes 0.000 claims abstract description 52
- 101710149814 C-C chemokine receptor type 1 Proteins 0.000 claims abstract description 52
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 claims abstract description 42
- 201000010099 disease Diseases 0.000 claims abstract description 39
- 238000000034 method Methods 0.000 claims abstract description 29
- 239000008194 pharmaceutical composition Substances 0.000 claims abstract description 17
- 125000004209 (C1-C8) alkyl group Chemical group 0.000 claims description 128
- 229910052739 hydrogen Inorganic materials 0.000 claims description 79
- 125000005913 (C3-C6) cycloalkyl group Chemical group 0.000 claims description 76
- 229910052736 halogen Inorganic materials 0.000 claims description 74
- 125000006648 (C1-C8) haloalkyl group Chemical group 0.000 claims description 70
- 239000001257 hydrogen Substances 0.000 claims description 69
- 125000004649 C2-C8 alkynyl group Chemical group 0.000 claims description 54
- 125000004648 C2-C8 alkenyl group Chemical group 0.000 claims description 53
- 150000002367 halogens Chemical class 0.000 claims description 53
- 125000001997 phenyl group Chemical group [H]C1=C([H])C([H])=C(*)C([H])=C1[H] 0.000 claims description 48
- 150000002431 hydrogen Chemical group 0.000 claims description 45
- 125000001424 substituent group Chemical group 0.000 claims description 45
- 125000004093 cyano group Chemical group *C#N 0.000 claims description 43
- 125000000449 nitro group Chemical group [O-][N+](*)=O 0.000 claims description 41
- 125000003118 aryl group Chemical group 0.000 claims description 40
- 125000004178 (C1-C4) alkyl group Chemical group 0.000 claims description 33
- 125000001544 thienyl group Chemical group 0.000 claims description 33
- 125000001072 heteroaryl group Chemical group 0.000 claims description 30
- 125000003226 pyrazolyl group Chemical group 0.000 claims description 27
- 150000003839 salts Chemical class 0.000 claims description 24
- 229910052757 nitrogen Inorganic materials 0.000 claims description 23
- UFHFLCQGNIYNRP-UHFFFAOYSA-N Hydrogen Chemical compound [H][H] UFHFLCQGNIYNRP-UHFFFAOYSA-N 0.000 claims description 22
- 125000002947 alkylene group Chemical group 0.000 claims description 20
- 125000005843 halogen group Chemical group 0.000 claims description 19
- 125000004765 (C1-C4) haloalkyl group Chemical group 0.000 claims description 18
- 125000004169 (C1-C6) alkyl group Chemical group 0.000 claims description 16
- 125000000217 alkyl group Chemical group 0.000 claims description 15
- 125000001246 bromo group Chemical group Br* 0.000 claims description 14
- 125000001309 chloro group Chemical group Cl* 0.000 claims description 14
- 239000003814 drug Substances 0.000 claims description 14
- 125000000171 (C1-C6) haloalkyl group Chemical group 0.000 claims description 11
- 125000004450 alkenylene group Chemical group 0.000 claims description 11
- 125000004419 alkynylene group Chemical group 0.000 claims description 11
- 125000004433 nitrogen atom Chemical group N* 0.000 claims description 11
- 125000001931 aliphatic group Chemical group 0.000 claims description 10
- WTKZEGDFNFYCGP-UHFFFAOYSA-N Pyrazole Chemical compound C=1C=NNC=1 WTKZEGDFNFYCGP-UHFFFAOYSA-N 0.000 claims description 9
- 125000003453 indazolyl group Chemical group N1N=C(C2=C1C=CC=C2)* 0.000 claims description 9
- 125000003373 pyrazinyl group Chemical group 0.000 claims description 9
- 125000004122 cyclic group Chemical group 0.000 claims description 8
- 125000002541 furyl group Chemical group 0.000 claims description 8
- 125000002883 imidazolyl group Chemical group 0.000 claims description 8
- 125000001786 isothiazolyl group Chemical group 0.000 claims description 8
- 125000000842 isoxazolyl group Chemical group 0.000 claims description 8
- 238000004519 manufacturing process Methods 0.000 claims description 8
- 125000001715 oxadiazolyl group Chemical group 0.000 claims description 8
- 125000002971 oxazolyl group Chemical group 0.000 claims description 8
- 239000000546 pharmaceutical excipient Substances 0.000 claims description 8
- 125000004076 pyridyl group Chemical group 0.000 claims description 8
- 125000000714 pyrimidinyl group Chemical group 0.000 claims description 8
- 125000003831 tetrazolyl group Chemical group 0.000 claims description 8
- 125000000335 thiazolyl group Chemical group 0.000 claims description 8
- 125000001425 triazolyl group Chemical group 0.000 claims description 8
- 229910003827 NRaRb Inorganic materials 0.000 claims description 7
- 201000006417 multiple sclerosis Diseases 0.000 claims description 7
- 206010039073 rheumatoid arthritis Diseases 0.000 claims description 7
- 229910052703 rhodium Inorganic materials 0.000 claims description 7
- 229910052794 bromium Inorganic materials 0.000 claims description 6
- 229910052717 sulfur Inorganic materials 0.000 claims description 5
- 201000004624 Dermatitis Diseases 0.000 claims description 4
- 206010052779 Transplant rejections Diseases 0.000 claims description 4
- 125000002777 acetyl group Chemical group [H]C([H])([H])C(*)=O 0.000 claims description 4
- 229910052801 chlorine Inorganic materials 0.000 claims description 4
- 229910052731 fluorine Inorganic materials 0.000 claims description 4
- 230000000699 topical effect Effects 0.000 claims description 4
- ZUDNYVKNAKHODP-UHFFFAOYSA-N 2-[4-bromo-5-methyl-3-(trifluoromethyl)pyrazol-1-yl]-1-[4-(5-chloro-2-methylphenyl)piperazin-1-yl]ethanone Chemical compound CC1=C(Br)C(C(F)(F)F)=NN1CC(=O)N1CCN(C=2C(=CC=C(Cl)C=2)C)CC1 ZUDNYVKNAKHODP-UHFFFAOYSA-N 0.000 claims description 3
- HTYBEXPZARGDNU-UHFFFAOYSA-N 2-[4-chloro-5-methyl-3-(trifluoromethyl)pyrazol-1-yl]-1-[4-(4-fluorophenyl)piperazin-1-yl]ethanone Chemical compound CC1=C(Cl)C(C(F)(F)F)=NN1CC(=O)N1CCN(C=2C=CC(F)=CC=2)CC1 HTYBEXPZARGDNU-UHFFFAOYSA-N 0.000 claims description 3
- 208000004262 Food Hypersensitivity Diseases 0.000 claims description 3
- 208000022559 Inflammatory bowel disease Diseases 0.000 claims description 3
- 125000003277 amino group Chemical group 0.000 claims description 3
- 239000002260 anti-inflammatory agent Substances 0.000 claims description 3
- 208000035475 disorder Diseases 0.000 claims description 3
- 235000020932 food allergy Nutrition 0.000 claims description 3
- 230000004957 immunoregulator effect Effects 0.000 claims description 3
- 229910052701 rubidium Inorganic materials 0.000 claims description 3
- 208000024780 Urticaria Diseases 0.000 claims description 2
- 206010047115 Vasculitis Diseases 0.000 claims description 2
- 239000000730 antalgic agent Substances 0.000 claims description 2
- 230000003110 anti-inflammatory effect Effects 0.000 claims description 2
- 208000010668 atopic eczema Diseases 0.000 claims description 2
- 125000004435 hydrogen atom Chemical group [H]* 0.000 claims description 2
- 125000004356 hydroxy functional group Chemical group O* 0.000 claims description 2
- LMIJMOOVLDNTLB-UHFFFAOYSA-N 2-(3,5-dimethyl-4-nitropyrazol-1-yl)-1-[4-(4-fluorophenyl)piperazin-1-yl]ethanone Chemical compound CC1=C([N+]([O-])=O)C(C)=NN1CC(=O)N1CCN(C=2C=CC(F)=CC=2)CC1 LMIJMOOVLDNTLB-UHFFFAOYSA-N 0.000 claims 2
- 206010016946 Food allergy Diseases 0.000 claims 1
- 201000002491 encephalomyelitis Diseases 0.000 claims 1
- 230000004968 inflammatory condition Effects 0.000 claims 1
- 238000003556 assay Methods 0.000 abstract description 13
- 102000004500 CCR1 Receptors Human genes 0.000 abstract description 4
- 108010017319 CCR1 Receptors Proteins 0.000 abstract description 4
- 241001465754 Metazoa Species 0.000 abstract description 4
- 238000012360 testing method Methods 0.000 abstract description 4
- 230000002860 competitive effect Effects 0.000 abstract description 3
- 230000003389 potentiating effect Effects 0.000 abstract description 3
- XEKOWRVHYACXOJ-UHFFFAOYSA-N Ethyl acetate Chemical compound CCOC(C)=O XEKOWRVHYACXOJ-UHFFFAOYSA-N 0.000 description 509
- HEDRZPFGACZZDS-MICDWDOJSA-N Trichloro(2H)methane Chemical compound [2H]C(Cl)(Cl)Cl HEDRZPFGACZZDS-MICDWDOJSA-N 0.000 description 269
- VLKZOEOYAKHREP-UHFFFAOYSA-N n-Hexane Chemical compound CCCCCC VLKZOEOYAKHREP-UHFFFAOYSA-N 0.000 description 236
- 238000003786 synthesis reaction Methods 0.000 description 204
- 230000015572 biosynthetic process Effects 0.000 description 203
- ZMXDDKWLCZADIW-UHFFFAOYSA-N N,N-Dimethylformamide Chemical compound CN(C)C=O ZMXDDKWLCZADIW-UHFFFAOYSA-N 0.000 description 201
- 235000019439 ethyl acetate Nutrition 0.000 description 170
- BWHMMNNQKKPAPP-UHFFFAOYSA-L potassium carbonate Chemical compound [K+].[K+].[O-]C([O-])=O BWHMMNNQKKPAPP-UHFFFAOYSA-L 0.000 description 168
- 238000005160 1H NMR spectroscopy Methods 0.000 description 138
- 239000007787 solid Substances 0.000 description 132
- 238000005859 coupling reaction Methods 0.000 description 93
- RTZKZFJDLAIYFH-UHFFFAOYSA-N Diethyl ether Chemical compound CCOCC RTZKZFJDLAIYFH-UHFFFAOYSA-N 0.000 description 92
- IAZDPXIOMUYVGZ-WFGJKAKNSA-N Dimethyl sulfoxide Chemical compound [2H]C([2H])([2H])S(=O)C([2H])([2H])[2H] IAZDPXIOMUYVGZ-WFGJKAKNSA-N 0.000 description 90
- 230000008878 coupling Effects 0.000 description 89
- 238000010168 coupling process Methods 0.000 description 89
- 229910000027 potassium carbonate Inorganic materials 0.000 description 84
- 238000004440 column chromatography Methods 0.000 description 83
- OKKJLVBELUTLKV-UHFFFAOYSA-N Methanol Chemical compound OC OKKJLVBELUTLKV-UHFFFAOYSA-N 0.000 description 81
- 239000000047 product Substances 0.000 description 79
- 239000011877 solvent mixture Substances 0.000 description 74
- 239000000203 mixture Substances 0.000 description 69
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 68
- YMWUJEATGCHHMB-UHFFFAOYSA-N Dichloromethane Chemical compound ClCCl YMWUJEATGCHHMB-UHFFFAOYSA-N 0.000 description 66
- 239000000243 solution Substances 0.000 description 64
- HEMHJVSKTPXQMS-UHFFFAOYSA-M Sodium hydroxide Chemical compound [OH-].[Na+] HEMHJVSKTPXQMS-UHFFFAOYSA-M 0.000 description 57
- 239000011541 reaction mixture Substances 0.000 description 56
- KOSFWARHNRKNOW-UHFFFAOYSA-N 2-chloro-1-[4-(4-fluorophenyl)piperazin-1-yl]ethanone Chemical compound C1=CC(F)=CC=C1N1CCN(C(=O)CCl)CC1 KOSFWARHNRKNOW-UHFFFAOYSA-N 0.000 description 52
- 238000001644 13C nuclear magnetic resonance spectroscopy Methods 0.000 description 50
- 239000007821 HATU Substances 0.000 description 44
- JRNVZBWKYDBUCA-UHFFFAOYSA-N N-chlorosuccinimide Chemical compound ClN1C(=O)CCC1=O JRNVZBWKYDBUCA-UHFFFAOYSA-N 0.000 description 44
- 238000006243 chemical reaction Methods 0.000 description 44
- HPALAKNZSZLMCH-UHFFFAOYSA-M sodium;chloride;hydrate Chemical class O.[Na+].[Cl-] HPALAKNZSZLMCH-UHFFFAOYSA-M 0.000 description 44
- IZBZQUREHISXFJ-UHFFFAOYSA-N 2-[4-chloro-5-methyl-3-(trifluoromethyl)pyrazol-1-yl]acetic acid Chemical compound CC1=C(Cl)C(C(F)(F)F)=NN1CC(O)=O IZBZQUREHISXFJ-UHFFFAOYSA-N 0.000 description 42
- WYURNTSHIVDZCO-UHFFFAOYSA-N Tetrahydrofuran Chemical compound C1CCOC1 WYURNTSHIVDZCO-UHFFFAOYSA-N 0.000 description 42
- 210000004027 cell Anatomy 0.000 description 41
- YXFVVABEGXRONW-UHFFFAOYSA-N Toluene Chemical compound CC1=CC=CC=C1 YXFVVABEGXRONW-UHFFFAOYSA-N 0.000 description 39
- 239000012071 phase Substances 0.000 description 39
- ZMANZCXQSJIPKH-UHFFFAOYSA-N Triethylamine Chemical compound CCN(CC)CC ZMANZCXQSJIPKH-UHFFFAOYSA-N 0.000 description 36
- 239000012267 brine Substances 0.000 description 36
- 239000003921 oil Substances 0.000 description 36
- 235000019198 oils Nutrition 0.000 description 36
- QTBSBXVTEAMEQO-UHFFFAOYSA-N Acetic acid Chemical compound CC(O)=O QTBSBXVTEAMEQO-UHFFFAOYSA-N 0.000 description 35
- WEVYAHXRMPXWCK-UHFFFAOYSA-N Acetonitrile Chemical compound CC#N WEVYAHXRMPXWCK-UHFFFAOYSA-N 0.000 description 27
- IJGRMHOSHXDMSA-UHFFFAOYSA-N Atomic nitrogen Chemical compound N#N IJGRMHOSHXDMSA-UHFFFAOYSA-N 0.000 description 26
- 102000019034 Chemokines Human genes 0.000 description 26
- 108010012236 Chemokines Proteins 0.000 description 26
- GLUUGHFHXGJENI-UHFFFAOYSA-N Piperazine Chemical compound C1CNCCN1 GLUUGHFHXGJENI-UHFFFAOYSA-N 0.000 description 22
- 239000003446 ligand Substances 0.000 description 22
- 108700012434 CCL3 Proteins 0.000 description 20
- 102000000013 Chemokine CCL3 Human genes 0.000 description 20
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 description 20
- 239000010410 layer Substances 0.000 description 20
- 239000012044 organic layer Substances 0.000 description 20
- HEDRZPFGACZZDS-UHFFFAOYSA-N CHCl3 Substances ClC(Cl)Cl HEDRZPFGACZZDS-UHFFFAOYSA-N 0.000 description 19
- VYPSYNLAJGMNEJ-UHFFFAOYSA-N Silicium dioxide Chemical compound O=[Si]=O VYPSYNLAJGMNEJ-UHFFFAOYSA-N 0.000 description 19
- 239000012230 colorless oil Substances 0.000 description 19
- 239000002904 solvent Substances 0.000 description 18
- 210000001616 monocyte Anatomy 0.000 description 17
- 229960000583 acetic acid Drugs 0.000 description 16
- 239000004480 active ingredient Substances 0.000 description 14
- 230000027455 binding Effects 0.000 description 14
- 238000001914 filtration Methods 0.000 description 13
- MFRIHAYPQRLWNB-UHFFFAOYSA-N sodium tert-butoxide Chemical compound [Na+].CC(C)(C)[O-] MFRIHAYPQRLWNB-UHFFFAOYSA-N 0.000 description 13
- UHOVQNZJYSORNB-UHFFFAOYSA-N Benzene Chemical compound C1=CC=CC=C1 UHOVQNZJYSORNB-UHFFFAOYSA-N 0.000 description 12
- UIIMBOGNXHQVGW-UHFFFAOYSA-M Sodium bicarbonate Chemical compound [Na+].OC([O-])=O UIIMBOGNXHQVGW-UHFFFAOYSA-M 0.000 description 12
- 239000012043 crude product Substances 0.000 description 12
- 239000003112 inhibitor Substances 0.000 description 12
- OAKJQQAXSVQMHS-UHFFFAOYSA-N Hydrazine Chemical compound NN OAKJQQAXSVQMHS-UHFFFAOYSA-N 0.000 description 11
- 238000001035 drying Methods 0.000 description 11
- JBSAVIITEALCKH-UHFFFAOYSA-N 2-chloro-1-[4-(4-chlorophenyl)piperazin-1-yl]ethanone Chemical compound C1CN(C(=O)CCl)CCN1C1=CC=C(Cl)C=C1 JBSAVIITEALCKH-UHFFFAOYSA-N 0.000 description 10
- KFZMGEQAYNKOFK-UHFFFAOYSA-N Isopropanol Chemical compound CC(C)O KFZMGEQAYNKOFK-UHFFFAOYSA-N 0.000 description 10
- CSNNHWWHGAXBCP-UHFFFAOYSA-L Magnesium sulfate Chemical compound [Mg+2].[O-][S+2]([O-])([O-])[O-] CSNNHWWHGAXBCP-UHFFFAOYSA-L 0.000 description 10
- 239000008346 aqueous phase Substances 0.000 description 10
- 230000000694 effects Effects 0.000 description 10
- 150000002148 esters Chemical class 0.000 description 10
- JFJRVIUVODVKJU-UHFFFAOYSA-N 2-chloro-1-[4-(4-chloro-3-methoxyphenyl)piperazin-1-yl]ethanone Chemical compound C1=C(Cl)C(OC)=CC(N2CCN(CC2)C(=O)CCl)=C1 JFJRVIUVODVKJU-UHFFFAOYSA-N 0.000 description 9
- PCLIMKBDDGJMGD-UHFFFAOYSA-N N-bromosuccinimide Chemical compound BrN1C(=O)CCC1=O PCLIMKBDDGJMGD-UHFFFAOYSA-N 0.000 description 9
- 239000002253 acid Substances 0.000 description 9
- 239000000706 filtrate Substances 0.000 description 9
- 230000006870 function Effects 0.000 description 9
- 230000002757 inflammatory effect Effects 0.000 description 9
- 239000000463 material Substances 0.000 description 9
- 238000002360 preparation method Methods 0.000 description 9
- 102000005962 receptors Human genes 0.000 description 9
- 108020003175 receptors Proteins 0.000 description 9
- 238000003756 stirring Methods 0.000 description 9
- 239000000725 suspension Substances 0.000 description 9
- 125000002023 trifluoromethyl group Chemical group FC(F)(F)* 0.000 description 9
- 230000005764 inhibitory process Effects 0.000 description 8
- 210000000265 leukocyte Anatomy 0.000 description 8
- 230000007935 neutral effect Effects 0.000 description 8
- 239000000741 silica gel Substances 0.000 description 8
- 229910002027 silica gel Inorganic materials 0.000 description 8
- LRHPLDYGYMQRHN-UHFFFAOYSA-N N-Butanol Chemical compound CCCCO LRHPLDYGYMQRHN-UHFFFAOYSA-N 0.000 description 7
- PMZURENOXWZQFD-UHFFFAOYSA-L Sodium Sulfate Chemical compound [Na+].[Na+].[O-]S([O-])(=O)=O PMZURENOXWZQFD-UHFFFAOYSA-L 0.000 description 7
- MUALRAIOVNYAIW-UHFFFAOYSA-N binap Chemical compound C1=CC=CC=C1P(C=1C(=C2C=CC=CC2=CC=1)C=1C2=CC=CC=C2C=CC=1P(C=1C=CC=CC=1)C=1C=CC=CC=1)C1=CC=CC=C1 MUALRAIOVNYAIW-UHFFFAOYSA-N 0.000 description 7
- 125000004432 carbon atom Chemical group C* 0.000 description 7
- 229940079593 drug Drugs 0.000 description 7
- 238000003818 flash chromatography Methods 0.000 description 7
- 239000012362 glacial acetic acid Substances 0.000 description 7
- 230000011664 signaling Effects 0.000 description 7
- RJIXEBOSMSSXJC-UHFFFAOYSA-N 4-chloro-3-phenyl-5-(trifluoromethyl)-1h-pyrazole Chemical compound ClC1=C(C(F)(F)F)NN=C1C1=CC=CC=C1 RJIXEBOSMSSXJC-UHFFFAOYSA-N 0.000 description 6
- QTBSBXVTEAMEQO-UHFFFAOYSA-M Acetate Chemical compound CC([O-])=O QTBSBXVTEAMEQO-UHFFFAOYSA-M 0.000 description 6
- 102000009410 Chemokine receptor Human genes 0.000 description 6
- VGCXGMAHQTYDJK-UHFFFAOYSA-N Chloroacetyl chloride Chemical compound ClCC(Cl)=O VGCXGMAHQTYDJK-UHFFFAOYSA-N 0.000 description 6
- 150000001412 amines Chemical class 0.000 description 6
- 239000011575 calcium Substances 0.000 description 6
- 230000035605 chemotaxis Effects 0.000 description 6
- 239000000284 extract Substances 0.000 description 6
- 239000000796 flavoring agent Substances 0.000 description 6
- 239000004615 ingredient Substances 0.000 description 6
- 230000002401 inhibitory effect Effects 0.000 description 6
- WXHIJDCHNDBCNY-UHFFFAOYSA-N palladium dihydride Chemical compound [PdH2] WXHIJDCHNDBCNY-UHFFFAOYSA-N 0.000 description 6
- 244000052769 pathogen Species 0.000 description 6
- 235000021251 pulses Nutrition 0.000 description 6
- 238000000746 purification Methods 0.000 description 6
- 238000010992 reflux Methods 0.000 description 6
- 238000007363 ring formation reaction Methods 0.000 description 6
- 239000003765 sweetening agent Substances 0.000 description 6
- 239000003826 tablet Substances 0.000 description 6
- VZGDMQKNWNREIO-UHFFFAOYSA-N tetrachloromethane Chemical compound ClC(Cl)(Cl)Cl VZGDMQKNWNREIO-UHFFFAOYSA-N 0.000 description 6
- YLQBMQCUIZJEEH-UHFFFAOYSA-N tetrahydrofuran Natural products C=1C=COC=1 YLQBMQCUIZJEEH-UHFFFAOYSA-N 0.000 description 6
- HCOKRIAXSMYGQC-UHFFFAOYSA-N 1-[4-(4-bromo-3-methoxyphenyl)piperazin-1-yl]-2-chloroethanone Chemical compound C1=C(Br)C(OC)=CC(N2CCN(CC2)C(=O)CCl)=C1 HCOKRIAXSMYGQC-UHFFFAOYSA-N 0.000 description 5
- KNTOBPVSOYISFT-UHFFFAOYSA-N 4-chloro-5-methyl-3-(trifluoromethyl)-1h-pyrazole Chemical compound CC=1NN=C(C(F)(F)F)C=1Cl KNTOBPVSOYISFT-UHFFFAOYSA-N 0.000 description 5
- 102100036850 C-C motif chemokine 23 Human genes 0.000 description 5
- OYPRJOBELJOOCE-UHFFFAOYSA-N Calcium Chemical compound [Ca] OYPRJOBELJOOCE-UHFFFAOYSA-N 0.000 description 5
- 108050000299 Chemokine receptor Proteins 0.000 description 5
- 101000713081 Homo sapiens C-C motif chemokine 23 Proteins 0.000 description 5
- CDBYLPFSWZWCQE-UHFFFAOYSA-L Sodium Carbonate Chemical compound [Na+].[Na+].[O-]C([O-])=O CDBYLPFSWZWCQE-UHFFFAOYSA-L 0.000 description 5
- 229910052791 calcium Inorganic materials 0.000 description 5
- 239000007859 condensation product Substances 0.000 description 5
- 235000014113 dietary fatty acids Nutrition 0.000 description 5
- 239000000194 fatty acid Substances 0.000 description 5
- 229930195729 fatty acid Natural products 0.000 description 5
- 150000004665 fatty acids Chemical class 0.000 description 5
- 235000003599 food sweetener Nutrition 0.000 description 5
- 238000009472 formulation Methods 0.000 description 5
- 210000002865 immune cell Anatomy 0.000 description 5
- 210000002540 macrophage Anatomy 0.000 description 5
- 229910052943 magnesium sulfate Inorganic materials 0.000 description 5
- 229910052751 metal Inorganic materials 0.000 description 5
- 239000002184 metal Substances 0.000 description 5
- 239000000041 non-steroidal anti-inflammatory agent Substances 0.000 description 5
- 229940021182 non-steroidal anti-inflammatory drug Drugs 0.000 description 5
- 231100000252 nontoxic Toxicity 0.000 description 5
- 230000003000 nontoxic effect Effects 0.000 description 5
- NLKNQRATVPKPDG-UHFFFAOYSA-M potassium iodide Chemical compound [K+].[I-] NLKNQRATVPKPDG-UHFFFAOYSA-M 0.000 description 5
- 230000002265 prevention Effects 0.000 description 5
- 150000003217 pyrazoles Chemical class 0.000 description 5
- 150000003254 radicals Chemical class 0.000 description 5
- 230000002285 radioactive effect Effects 0.000 description 5
- 239000011734 sodium Substances 0.000 description 5
- 229910000030 sodium bicarbonate Inorganic materials 0.000 description 5
- CWXPZXBSDSIRCS-UHFFFAOYSA-N tert-butyl piperazine-1-carboxylate Chemical compound CC(C)(C)OC(=O)N1CCNCC1 CWXPZXBSDSIRCS-UHFFFAOYSA-N 0.000 description 5
- RYHBNJHYFVUHQT-UHFFFAOYSA-N 1,4-Dioxane Chemical compound C1COCCO1 RYHBNJHYFVUHQT-UHFFFAOYSA-N 0.000 description 4
- DEZHHWXKURWGDQ-UHFFFAOYSA-N 1-[4-(4-chloro-3-methoxyphenyl)piperazin-1-yl]ethanone Chemical compound C1=C(Cl)C(OC)=CC(N2CCN(CC2)C(C)=O)=C1 DEZHHWXKURWGDQ-UHFFFAOYSA-N 0.000 description 4
- PDSOUBXNWWZCNB-UHFFFAOYSA-N 4-bromo-5-methyl-3-(trifluoromethyl)-1h-pyrazole Chemical compound CC=1NN=C(C(F)(F)F)C=1Br PDSOUBXNWWZCNB-UHFFFAOYSA-N 0.000 description 4
- QTNVXMOPTHGCII-UHFFFAOYSA-N 4-bromo-5-phenyl-1h-pyrazol-3-amine Chemical compound NC1=NNC(C=2C=CC=CC=2)=C1Br QTNVXMOPTHGCII-UHFFFAOYSA-N 0.000 description 4
- KZMGYPLQYOPHEL-UHFFFAOYSA-N Boron trifluoride etherate Chemical compound FB(F)F.CCOCC KZMGYPLQYOPHEL-UHFFFAOYSA-N 0.000 description 4
- 101800004538 Bradykinin Proteins 0.000 description 4
- VTYYLEPIZMXCLO-UHFFFAOYSA-L Calcium carbonate Chemical compound [Ca+2].[O-]C([O-])=O VTYYLEPIZMXCLO-UHFFFAOYSA-L 0.000 description 4
- 229910021589 Copper(I) bromide Inorganic materials 0.000 description 4
- PMATZTZNYRCHOR-CGLBZJNRSA-N Cyclosporin A Chemical compound CC[C@@H]1NC(=O)[C@H]([C@H](O)[C@H](C)C\C=C\C)N(C)C(=O)[C@H](C(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)N(C)C(=O)[C@H](C(C)C)NC(=O)[C@H](CC(C)C)N(C)C(=O)CN(C)C1=O PMATZTZNYRCHOR-CGLBZJNRSA-N 0.000 description 4
- 108010036949 Cyclosporine Proteins 0.000 description 4
- IAYPIBMASNFSPL-UHFFFAOYSA-N Ethylene oxide Chemical compound C1CO1 IAYPIBMASNFSPL-UHFFFAOYSA-N 0.000 description 4
- QXZGBUJJYSLZLT-UHFFFAOYSA-N H-Arg-Pro-Pro-Gly-Phe-Ser-Pro-Phe-Arg-OH Natural products NC(N)=NCCCC(N)C(=O)N1CCCC1C(=O)N1C(C(=O)NCC(=O)NC(CC=2C=CC=CC=2)C(=O)NC(CO)C(=O)N2C(CCC2)C(=O)NC(CC=2C=CC=CC=2)C(=O)NC(CCCN=C(N)N)C(O)=O)CCC1 QXZGBUJJYSLZLT-UHFFFAOYSA-N 0.000 description 4
- 239000012981 Hank's balanced salt solution Substances 0.000 description 4
- NTYJJOPFIAHURM-UHFFFAOYSA-N Histamine Chemical compound NCCC1=CN=CN1 NTYJJOPFIAHURM-UHFFFAOYSA-N 0.000 description 4
- 206010059176 Juvenile idiopathic arthritis Diseases 0.000 description 4
- 102100035792 Kininogen-1 Human genes 0.000 description 4
- WMFOQBRAJBCJND-UHFFFAOYSA-M Lithium hydroxide Chemical compound [Li+].[OH-] WMFOQBRAJBCJND-UHFFFAOYSA-M 0.000 description 4
- 229910019093 NaOCl Inorganic materials 0.000 description 4
- MWUXSHHQAYIFBG-UHFFFAOYSA-N Nitric oxide Chemical compound O=[N] MWUXSHHQAYIFBG-UHFFFAOYSA-N 0.000 description 4
- STSCVKRWJPWALQ-UHFFFAOYSA-N TRIFLUOROACETIC ACID ETHYL ESTER Chemical compound CCOC(=O)C(F)(F)F STSCVKRWJPWALQ-UHFFFAOYSA-N 0.000 description 4
- QJJXYPPXXYFBGM-LFZNUXCKSA-N Tacrolimus Chemical compound C1C[C@@H](O)[C@H](OC)C[C@@H]1\C=C(/C)[C@@H]1[C@H](C)[C@@H](O)CC(=O)[C@H](CC=C)/C=C(C)/C[C@H](C)C[C@H](OC)[C@H]([C@H](C[C@H]2C)OC)O[C@@]2(O)C(=O)C(=O)N2CCCC[C@H]2C(=O)O1 QJJXYPPXXYFBGM-LFZNUXCKSA-N 0.000 description 4
- 208000026935 allergic disease Diseases 0.000 description 4
- PNEYBMLMFCGWSK-UHFFFAOYSA-N aluminium oxide Inorganic materials [O-2].[O-2].[O-2].[Al+3].[Al+3] PNEYBMLMFCGWSK-UHFFFAOYSA-N 0.000 description 4
- 238000013459 approach Methods 0.000 description 4
- 239000007900 aqueous suspension Substances 0.000 description 4
- 125000004429 atom Chemical group 0.000 description 4
- LMEKQMALGUDUQG-UHFFFAOYSA-N azathioprine Chemical compound CN1C=NC([N+]([O-])=O)=C1SC1=NC=NC2=C1NC=N2 LMEKQMALGUDUQG-UHFFFAOYSA-N 0.000 description 4
- QXZGBUJJYSLZLT-FDISYFBBSA-N bradykinin Chemical compound NC(=N)NCCC[C@H](N)C(=O)N1CCC[C@H]1C(=O)N1[C@H](C(=O)NCC(=O)N[C@@H](CC=2C=CC=CC=2)C(=O)N[C@@H](CO)C(=O)N2[C@@H](CCC2)C(=O)N[C@@H](CC=2C=CC=CC=2)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O)CCC1 QXZGBUJJYSLZLT-FDISYFBBSA-N 0.000 description 4
- GDTBXPJZTBHREO-UHFFFAOYSA-N bromine Chemical compound BrBr GDTBXPJZTBHREO-UHFFFAOYSA-N 0.000 description 4
- RYYVLZVUVIJVGH-UHFFFAOYSA-N caffeine Chemical compound CN1C(=O)N(C)C(=O)C2=C1N=CN2C RYYVLZVUVIJVGH-UHFFFAOYSA-N 0.000 description 4
- OROGSEYTTFOCAN-DNJOTXNNSA-N codeine Chemical compound C([C@H]1[C@H](N(CC[C@@]112)C)C3)=C[C@H](O)[C@@H]1OC1=C2C3=CC=C1OC OROGSEYTTFOCAN-DNJOTXNNSA-N 0.000 description 4
- 239000003086 colorant Substances 0.000 description 4
- 229910000365 copper sulfate Inorganic materials 0.000 description 4
- ARUVKPQLZAKDPS-UHFFFAOYSA-L copper(II) sulfate Chemical compound [Cu+2].[O-][S+2]([O-])([O-])[O-] ARUVKPQLZAKDPS-UHFFFAOYSA-L 0.000 description 4
- NKNDPYCGAZPOFS-UHFFFAOYSA-M copper(i) bromide Chemical compound Br[Cu] NKNDPYCGAZPOFS-UHFFFAOYSA-M 0.000 description 4
- 238000002425 crystallisation Methods 0.000 description 4
- 230000008025 crystallization Effects 0.000 description 4
- 239000003085 diluting agent Substances 0.000 description 4
- 239000002270 dispersing agent Substances 0.000 description 4
- 238000006073 displacement reaction Methods 0.000 description 4
- 238000000132 electrospray ionisation Methods 0.000 description 4
- 239000000839 emulsion Substances 0.000 description 4
- NTCRDZSJYGZRIE-UHFFFAOYSA-N ethyl 5-thiophen-2-yl-1h-pyrazole-3-carboxylate Chemical compound N1N=C(C(=O)OCC)C=C1C1=CC=CS1 NTCRDZSJYGZRIE-UHFFFAOYSA-N 0.000 description 4
- 238000002474 experimental method Methods 0.000 description 4
- 235000013355 food flavoring agent Nutrition 0.000 description 4
- 238000007306 functionalization reaction Methods 0.000 description 4
- 210000003714 granulocyte Anatomy 0.000 description 4
- CGIGDMFJXJATDK-UHFFFAOYSA-N indomethacin Chemical compound CC1=C(CC(O)=O)C2=CC(OC)=CC=C2N1C(=O)C1=CC=C(Cl)C=C1 CGIGDMFJXJATDK-UHFFFAOYSA-N 0.000 description 4
- 208000015181 infectious disease Diseases 0.000 description 4
- 208000027866 inflammatory disease Diseases 0.000 description 4
- 125000005647 linker group Chemical group 0.000 description 4
- DLEDOFVPSDKWEF-UHFFFAOYSA-N lithium butane Chemical compound [Li+].CCC[CH2-] DLEDOFVPSDKWEF-UHFFFAOYSA-N 0.000 description 4
- 210000004698 lymphocyte Anatomy 0.000 description 4
- MZRVEZGGRBJDDB-UHFFFAOYSA-N n-Butyllithium Substances [Li]CCCC MZRVEZGGRBJDDB-UHFFFAOYSA-N 0.000 description 4
- 210000000056 organ Anatomy 0.000 description 4
- UNEIHNMKASENIG-UHFFFAOYSA-N para-chlorophenylpiperazine Chemical compound C1=CC(Cl)=CC=C1N1CCNCC1 UNEIHNMKASENIG-UHFFFAOYSA-N 0.000 description 4
- 230000036961 partial effect Effects 0.000 description 4
- 239000003208 petroleum Substances 0.000 description 4
- 125000004193 piperazinyl group Chemical group 0.000 description 4
- 229920001223 polyethylene glycol Polymers 0.000 description 4
- 239000003755 preservative agent Substances 0.000 description 4
- JHJLBTNAGRQEKS-UHFFFAOYSA-M sodium bromide Chemical compound [Na+].[Br-] JHJLBTNAGRQEKS-UHFFFAOYSA-M 0.000 description 4
- SUKJFIGYRHOWBL-UHFFFAOYSA-N sodium hypochlorite Chemical compound [Na+].Cl[O-] SUKJFIGYRHOWBL-UHFFFAOYSA-N 0.000 description 4
- LPXPTNMVRIOKMN-UHFFFAOYSA-M sodium nitrite Chemical compound [Na+].[O-]N=O LPXPTNMVRIOKMN-UHFFFAOYSA-M 0.000 description 4
- GEHJYWRUCIMESM-UHFFFAOYSA-L sodium sulfite Chemical compound [Na+].[Na+].[O-]S([O-])=O GEHJYWRUCIMESM-UHFFFAOYSA-L 0.000 description 4
- 239000000375 suspending agent Substances 0.000 description 4
- QAEDZJGFFMLHHQ-UHFFFAOYSA-N trifluoroacetic anhydride Chemical compound FC(F)(F)C(=O)OC(=O)C(F)(F)F QAEDZJGFFMLHHQ-UHFFFAOYSA-N 0.000 description 4
- 239000000080 wetting agent Substances 0.000 description 4
- CYPYTURSJDMMMP-WVCUSYJESA-N (1e,4e)-1,5-diphenylpenta-1,4-dien-3-one;palladium Chemical compound [Pd].[Pd].C=1C=CC=CC=1\C=C\C(=O)\C=C\C1=CC=CC=C1.C=1C=CC=CC=1\C=C\C(=O)\C=C\C1=CC=CC=C1.C=1C=CC=CC=1\C=C\C(=O)\C=C\C1=CC=CC=C1 CYPYTURSJDMMMP-WVCUSYJESA-N 0.000 description 3
- UECHCFXEJKAZEX-SECBINFHSA-N (3r)-1-(4-chlorophenyl)-3-methylpiperazine Chemical compound C1CN[C@H](C)CN1C1=CC=C(Cl)C=C1 UECHCFXEJKAZEX-SECBINFHSA-N 0.000 description 3
- FQUYSHZXSKYCSY-UHFFFAOYSA-N 1,4-diazepane Chemical group C1CNCCNC1 FQUYSHZXSKYCSY-UHFFFAOYSA-N 0.000 description 3
- YSJHCQGGIPTMQM-UHFFFAOYSA-N 1-(2,4-dichlorophenyl)piperazine Chemical compound ClC1=CC(Cl)=CC=C1N1CCNCC1 YSJHCQGGIPTMQM-UHFFFAOYSA-N 0.000 description 3
- VRVIKRZIQWEYFB-UHFFFAOYSA-N 1-(3,4-difluorophenyl)piperazine Chemical compound C1=C(F)C(F)=CC=C1N1CCNCC1 VRVIKRZIQWEYFB-UHFFFAOYSA-N 0.000 description 3
- YMAVACUOLRGMRE-UHFFFAOYSA-N 1-(4-chloro-3-methoxyphenyl)piperazine Chemical compound C1=C(Cl)C(OC)=CC(N2CCNCC2)=C1 YMAVACUOLRGMRE-UHFFFAOYSA-N 0.000 description 3
- LHOGNAPXWHWPQT-UHFFFAOYSA-N 3-(1,1,2,2,3,3,3-heptafluoropropyl)-5-methyl-1h-pyrazol-4-amine Chemical compound CC=1NN=C(C(F)(F)C(F)(F)C(F)(F)F)C=1N LHOGNAPXWHWPQT-UHFFFAOYSA-N 0.000 description 3
- UOFISFKBSXJEHZ-UHFFFAOYSA-N 3-(1,1,2,2,3,3,3-heptafluoropropyl)-5-methyl-4-nitro-1h-pyrazole Chemical compound CC=1NN=C(C(F)(F)C(F)(F)C(F)(F)F)C=1[N+]([O-])=O UOFISFKBSXJEHZ-UHFFFAOYSA-N 0.000 description 3
- YIEPWAOKLZRBEL-UHFFFAOYSA-N 3-tert-butyl-5-(trifluoromethyl)-1h-pyrazole Chemical compound CC(C)(C)C=1C=C(C(F)(F)F)NN=1 YIEPWAOKLZRBEL-UHFFFAOYSA-N 0.000 description 3
- NHUXQXHAFIITQR-UHFFFAOYSA-N 4-chloro-3-(3-fluorophenyl)-5-(trifluoromethyl)-1h-pyrazole Chemical compound FC1=CC=CC(C=2C(=C(NN=2)C(F)(F)F)Cl)=C1 NHUXQXHAFIITQR-UHFFFAOYSA-N 0.000 description 3
- SBKISCQYMYHQHK-UHFFFAOYSA-N 4-chloro-5-(4-fluorophenyl)-3-methylsulfanyl-1h-pyrazole Chemical compound ClC1=C(SC)NN=C1C1=CC=C(F)C=C1 SBKISCQYMYHQHK-UHFFFAOYSA-N 0.000 description 3
- FRXHUIQTYMOZLO-UHFFFAOYSA-N 4-chloro-5-methyl-1h-pyrazol-3-amine Chemical compound CC=1NN=C(N)C=1Cl FRXHUIQTYMOZLO-UHFFFAOYSA-N 0.000 description 3
- DIAOGBMBYWTBTJ-UHFFFAOYSA-N 4-chloro-5-propan-2-yl-3-(trifluoromethyl)-1h-pyrazole Chemical compound CC(C)C=1NN=C(C(F)(F)F)C=1Cl DIAOGBMBYWTBTJ-UHFFFAOYSA-N 0.000 description 3
- MJAMUSZUMAHFLH-UHFFFAOYSA-N 5-[2-cyclopropyl-1-(2-fluorophenyl)-2-oxoethyl]-4,6,7,7a-tetrahydrothieno[3,2-c]pyridin-2-one Chemical compound FC1=CC=CC=C1C(C(=O)C1CC1)N1CC2=CC(=O)SC2CC1 MJAMUSZUMAHFLH-UHFFFAOYSA-N 0.000 description 3
- WSFSSNUMVMOOMR-UHFFFAOYSA-N Formaldehyde Chemical compound O=C WSFSSNUMVMOOMR-UHFFFAOYSA-N 0.000 description 3
- PEDCQBHIVMGVHV-UHFFFAOYSA-N Glycerine Chemical compound OCC(O)CO PEDCQBHIVMGVHV-UHFFFAOYSA-N 0.000 description 3
- 241000282412 Homo Species 0.000 description 3
- FBOZXECLQNJBKD-ZDUSSCGKSA-N L-methotrexate Chemical compound C=1N=C2N=C(N)N=C(N)C2=NC=1CN(C)C1=CC=C(C(=O)N[C@@H](CCC(O)=O)C(O)=O)C=C1 FBOZXECLQNJBKD-ZDUSSCGKSA-N 0.000 description 3
- DNIAPMSPPWPWGF-UHFFFAOYSA-N Propylene glycol Chemical compound CC(O)CO DNIAPMSPPWPWGF-UHFFFAOYSA-N 0.000 description 3
- FAPWRFPIFSIZLT-UHFFFAOYSA-M Sodium chloride Chemical compound [Na+].[Cl-] FAPWRFPIFSIZLT-UHFFFAOYSA-M 0.000 description 3
- 210000001744 T-lymphocyte Anatomy 0.000 description 3
- 230000002378 acidificating effect Effects 0.000 description 3
- 150000007513 acids Chemical class 0.000 description 3
- 239000000739 antihistaminic agent Substances 0.000 description 3
- 238000006254 arylation reaction Methods 0.000 description 3
- 208000006673 asthma Diseases 0.000 description 3
- 239000000872 buffer Substances 0.000 description 3
- LWQQLNNNIPYSNX-UROSTWAQSA-N calcipotriol Chemical compound C1([C@H](O)/C=C/[C@@H](C)[C@@H]2[C@]3(CCCC(/[C@@H]3CC2)=C\C=C\2C([C@@H](O)C[C@H](O)C/2)=C)C)CC1 LWQQLNNNIPYSNX-UROSTWAQSA-N 0.000 description 3
- 238000005660 chlorination reaction Methods 0.000 description 3
- 239000000460 chlorine Substances 0.000 description 3
- 125000002668 chloroacetyl group Chemical group ClCC(=O)* 0.000 description 3
- 125000000753 cycloalkyl group Chemical group 0.000 description 3
- 210000004443 dendritic cell Anatomy 0.000 description 3
- 239000012954 diazonium Substances 0.000 description 3
- 150000001989 diazonium salts Chemical class 0.000 description 3
- LOKCTEFSRHRXRJ-UHFFFAOYSA-I dipotassium trisodium dihydrogen phosphate hydrogen phosphate dichloride Chemical compound P(=O)(O)(O)[O-].[K+].P(=O)(O)([O-])[O-].[Na+].[Na+].[Cl-].[K+].[Cl-].[Na+] LOKCTEFSRHRXRJ-UHFFFAOYSA-I 0.000 description 3
- NUWIFWDROFJEEC-UHFFFAOYSA-N ethyl 4-chloro-5-thiophen-2-yl-1h-pyrazole-3-carboxylate Chemical compound ClC1=C(C(=O)OCC)NN=C1C1=CC=CS1 NUWIFWDROFJEEC-UHFFFAOYSA-N 0.000 description 3
- SUALHSUMUQQLJP-UHFFFAOYSA-N ethyl 5-propyl-1h-pyrazole-3-carboxylate Chemical compound CCCC1=CC(C(=O)OCC)=NN1 SUALHSUMUQQLJP-UHFFFAOYSA-N 0.000 description 3
- PQJJJMRNHATNKG-UHFFFAOYSA-N ethyl bromoacetate Chemical compound CCOC(=O)CBr PQJJJMRNHATNKG-UHFFFAOYSA-N 0.000 description 3
- 238000001704 evaporation Methods 0.000 description 3
- 230000008020 evaporation Effects 0.000 description 3
- 238000002825 functional assay Methods 0.000 description 3
- 125000005842 heteroatom Chemical group 0.000 description 3
- 210000003630 histaminocyte Anatomy 0.000 description 3
- 150000002429 hydrazines Chemical class 0.000 description 3
- 150000002430 hydrocarbons Chemical group 0.000 description 3
- OROGSEYTTFOCAN-UHFFFAOYSA-N hydrocodone Natural products C1C(N(CCC234)C)C2C=CC(O)C3OC2=C4C1=CC=C2OC OROGSEYTTFOCAN-UHFFFAOYSA-N 0.000 description 3
- 230000008595 infiltration Effects 0.000 description 3
- 238000001764 infiltration Methods 0.000 description 3
- 230000028709 inflammatory response Effects 0.000 description 3
- 230000003834 intracellular effect Effects 0.000 description 3
- 229940057995 liquid paraffin Drugs 0.000 description 3
- 235000019341 magnesium sulphate Nutrition 0.000 description 3
- BFFGYMOQOGMTBM-UHFFFAOYSA-N methyl 4-piperazin-1-ylbenzoate Chemical compound C1=CC(C(=O)OC)=CC=C1N1CCNCC1 BFFGYMOQOGMTBM-UHFFFAOYSA-N 0.000 description 3
- 210000000822 natural killer cell Anatomy 0.000 description 3
- 239000012299 nitrogen atmosphere Substances 0.000 description 3
- 239000004006 olive oil Substances 0.000 description 3
- 235000008390 olive oil Nutrition 0.000 description 3
- 150000002894 organic compounds Chemical class 0.000 description 3
- 210000002997 osteoclast Anatomy 0.000 description 3
- 239000002953 phosphate buffered saline Substances 0.000 description 3
- 125000003386 piperidinyl group Chemical group 0.000 description 3
- 235000013855 polyvinylpyrrolidone Nutrition 0.000 description 3
- 238000000159 protein binding assay Methods 0.000 description 3
- 230000027425 release of sequestered calcium ion into cytosol Effects 0.000 description 3
- 239000000523 sample Substances 0.000 description 3
- 229920006395 saturated elastomer Polymers 0.000 description 3
- 239000000377 silicon dioxide Substances 0.000 description 3
- QFJCIRLUMZQUOT-HPLJOQBZSA-N sirolimus Chemical compound C1C[C@@H](O)[C@H](OC)C[C@@H]1C[C@@H](C)[C@H]1OC(=O)[C@@H]2CCCCN2C(=O)C(=O)[C@](O)(O2)[C@H](C)CC[C@H]2C[C@H](OC)/C(C)=C/C=C/C=C/[C@@H](C)C[C@@H](C)C(=O)[C@H](OC)[C@H](O)/C(C)=C/[C@@H](C)C(=O)C1 QFJCIRLUMZQUOT-HPLJOQBZSA-N 0.000 description 3
- 229910052938 sodium sulfate Inorganic materials 0.000 description 3
- 235000011152 sodium sulphate Nutrition 0.000 description 3
- 239000000126 substance Substances 0.000 description 3
- 229960001967 tacrolimus Drugs 0.000 description 3
- QJJXYPPXXYFBGM-SHYZHZOCSA-N tacrolimus Natural products CO[C@H]1C[C@H](CC[C@@H]1O)C=C(C)[C@H]2OC(=O)[C@H]3CCCCN3C(=O)C(=O)[C@@]4(O)O[C@@H]([C@H](C[C@H]4C)OC)[C@@H](C[C@H](C)CC(=C[C@@H](CC=C)C(=O)C[C@H](O)[C@H]2C)C)OC QJJXYPPXXYFBGM-SHYZHZOCSA-N 0.000 description 3
- 229940124597 therapeutic agent Drugs 0.000 description 3
- 230000001225 therapeutic effect Effects 0.000 description 3
- 210000001519 tissue Anatomy 0.000 description 3
- 238000005406 washing Methods 0.000 description 3
- PUPZLCDOIYMWBV-UHFFFAOYSA-N (+/-)-1,3-Butanediol Chemical compound CC(O)CCO PUPZLCDOIYMWBV-UHFFFAOYSA-N 0.000 description 2
- MKCLVAZPKSCYQH-UHFFFAOYSA-N (2-bromo-5-chlorophenyl)-phenylmethanone Chemical compound ClC1=CC=C(Br)C(C(=O)C=2C=CC=CC=2)=C1 MKCLVAZPKSCYQH-UHFFFAOYSA-N 0.000 description 2
- UECHCFXEJKAZEX-VIFPVBQESA-N (3s)-1-(4-chlorophenyl)-3-methylpiperazine Chemical compound C1CN[C@@H](C)CN1C1=CC=C(Cl)C=C1 UECHCFXEJKAZEX-VIFPVBQESA-N 0.000 description 2
- IAKHMKGGTNLKSZ-INIZCTEOSA-N (S)-colchicine Chemical compound C1([C@@H](NC(C)=O)CC2)=CC(=O)C(OC)=CC=C1C1=C2C=C(OC)C(OC)=C1OC IAKHMKGGTNLKSZ-INIZCTEOSA-N 0.000 description 2
- ZORQXIQZAOLNGE-UHFFFAOYSA-N 1,1-difluorocyclohexane Chemical compound FC1(F)CCCCC1 ZORQXIQZAOLNGE-UHFFFAOYSA-N 0.000 description 2
- CFKYXUKLXDAKBG-UHFFFAOYSA-N 1-(4-bromo-3-methoxyphenyl)piperazine Chemical compound C1=C(Br)C(OC)=CC(N2CCNCC2)=C1 CFKYXUKLXDAKBG-UHFFFAOYSA-N 0.000 description 2
- PJHPFAFEJNBIDC-UHFFFAOYSA-N 1-(4-bromophenyl)piperazine Chemical compound C1=CC(Br)=CC=C1N1CCNCC1 PJHPFAFEJNBIDC-UHFFFAOYSA-N 0.000 description 2
- BUCQPIGRGYVSMD-UHFFFAOYSA-N 1-(4-chloro-3-ethoxyphenyl)piperazine Chemical compound C1=C(Cl)C(OCC)=CC(N2CCNCC2)=C1 BUCQPIGRGYVSMD-UHFFFAOYSA-N 0.000 description 2
- RDIGKZWGNNEORL-UHFFFAOYSA-N 1-(4-chloro-3-propan-2-yloxyphenyl)piperazine Chemical compound C1=C(Cl)C(OC(C)C)=CC(N2CCNCC2)=C1 RDIGKZWGNNEORL-UHFFFAOYSA-N 0.000 description 2
- AVJKDKWRVSSJPK-UHFFFAOYSA-N 1-(4-fluorophenyl)piperazine Chemical compound C1=CC(F)=CC=C1N1CCNCC1 AVJKDKWRVSSJPK-UHFFFAOYSA-N 0.000 description 2
- KNQFDOLIXOOIGX-UHFFFAOYSA-N 1-(4-methylsulfonylphenyl)piperazine Chemical compound C1=CC(S(=O)(=O)C)=CC=C1N1CCNCC1 KNQFDOLIXOOIGX-UHFFFAOYSA-N 0.000 description 2
- MSYMTZAXOWPKOM-UHFFFAOYSA-N 1-[4-(4-chloro-3-methoxyphenyl)piperazin-1-yl]-2-[4-chloro-5-phenyl-3-(trifluoromethyl)pyrazol-1-yl]ethanone Chemical compound C1=C(Cl)C(OC)=CC(N2CCN(CC2)C(=O)CN2C(=C(Cl)C(=N2)C(F)(F)F)C=2C=CC=CC=2)=C1 MSYMTZAXOWPKOM-UHFFFAOYSA-N 0.000 description 2
- UWPIAGRNHBMGHQ-UHFFFAOYSA-N 1-[4-(trifluoromethoxy)phenyl]piperazine Chemical compound C1=CC(OC(F)(F)F)=CC=C1N1CCNCC1 UWPIAGRNHBMGHQ-UHFFFAOYSA-N 0.000 description 2
- FPNVMCMDWZNTEU-UHFFFAOYSA-N 1-bromo-4-chloro-2-fluorobenzene Chemical compound FC1=CC(Cl)=CC=C1Br FPNVMCMDWZNTEU-UHFFFAOYSA-N 0.000 description 2
- VBICKXHEKHSIBG-UHFFFAOYSA-N 1-monostearoylglycerol Chemical compound CCCCCCCCCCCCCCCCCC(=O)OCC(O)CO VBICKXHEKHSIBG-UHFFFAOYSA-N 0.000 description 2
- IIZPXYDJLKNOIY-JXPKJXOSSA-N 1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphocholine Chemical compound CCCCCCCCCCCCCCCC(=O)OC[C@H](COP([O-])(=O)OCC[N+](C)(C)C)OC(=O)CCC\C=C/C\C=C/C\C=C/C\C=C/CCCCC IIZPXYDJLKNOIY-JXPKJXOSSA-N 0.000 description 2
- CVGPCNYDFWPKDZ-UHFFFAOYSA-N 2,2,2-trifluoro-n-(5-methyl-1h-pyrazol-3-yl)acetamide Chemical compound CC1=CC(NC(=O)C(F)(F)F)=NN1 CVGPCNYDFWPKDZ-UHFFFAOYSA-N 0.000 description 2
- LLPKQRMDOFYSGZ-UHFFFAOYSA-N 2,5-dimethyl-1h-imidazole Chemical compound CC1=CN=C(C)N1 LLPKQRMDOFYSGZ-UHFFFAOYSA-N 0.000 description 2
- OZAIFHULBGXAKX-UHFFFAOYSA-N 2-(2-cyanopropan-2-yldiazenyl)-2-methylpropanenitrile Chemical compound N#CC(C)(C)N=NC(C)(C)C#N OZAIFHULBGXAKX-UHFFFAOYSA-N 0.000 description 2
- HZAXFHJVJLSVMW-UHFFFAOYSA-N 2-Aminoethan-1-ol Chemical compound NCCO HZAXFHJVJLSVMW-UHFFFAOYSA-N 0.000 description 2
- JKMHFZQWWAIEOD-UHFFFAOYSA-N 2-[4-(2-hydroxyethyl)piperazin-1-yl]ethanesulfonic acid Chemical compound OCC[NH+]1CCN(CCS([O-])(=O)=O)CC1 JKMHFZQWWAIEOD-UHFFFAOYSA-N 0.000 description 2
- CIOPDGJJOJXATR-UHFFFAOYSA-N 2-[4-chloro-5-methyl-3-(trifluoromethyl)pyrazol-1-yl]-1-[4-(4-chlorophenyl)piperazin-1-yl]-2-(3-methoxyphenyl)ethanone Chemical compound COC1=CC=CC(C(C(=O)N2CCN(CC2)C=2C=CC(Cl)=CC=2)N2C(=C(Cl)C(=N2)C(F)(F)F)C)=C1 CIOPDGJJOJXATR-UHFFFAOYSA-N 0.000 description 2
- UMDBLFLJZDATRX-UHFFFAOYSA-N 2-[4-chloro-5-methyl-3-(trifluoromethyl)pyrazol-1-yl]-1-piperazin-1-ylethanone Chemical compound CC1=C(Cl)C(C(F)(F)F)=NN1CC(=O)N1CCNCC1 UMDBLFLJZDATRX-UHFFFAOYSA-N 0.000 description 2
- QRJPTSGXZARBNX-UHFFFAOYSA-N 2-[5-(azidomethyl)-4-chloro-3-(trifluoromethyl)pyrazol-1-yl]-1-[4-(4-chlorophenyl)piperazin-1-yl]ethanone Chemical compound [N-]=[N+]=NCC1=C(Cl)C(C(F)(F)F)=NN1CC(=O)N1CCN(C=2C=CC(Cl)=CC=2)CC1 QRJPTSGXZARBNX-UHFFFAOYSA-N 0.000 description 2
- XWNIDICBAKIVFV-UHFFFAOYSA-N 2-[5-(azidomethyl)-4-chloro-3-(trifluoromethyl)pyrazol-1-yl]acetic acid Chemical compound OC(=O)CN1N=C(C(F)(F)F)C(Cl)=C1CN=[N+]=[N-] XWNIDICBAKIVFV-UHFFFAOYSA-N 0.000 description 2
- YMDZDFSUDFLGMX-UHFFFAOYSA-N 2-chloro-n-(2-chloroethyl)ethanamine;hydron;chloride Chemical compound [Cl-].ClCC[NH2+]CCCl YMDZDFSUDFLGMX-UHFFFAOYSA-N 0.000 description 2
- IZHVBANLECCAGF-UHFFFAOYSA-N 2-hydroxy-3-(octadecanoyloxy)propyl octadecanoate Chemical compound CCCCCCCCCCCCCCCCCC(=O)OCC(O)COC(=O)CCCCCCCCCCCCCCCCC IZHVBANLECCAGF-UHFFFAOYSA-N 0.000 description 2
- NGDDUAYSWPUSLX-UHFFFAOYSA-N 3,5-bis(trifluoromethyl)-1h-pyrazole Chemical compound FC(F)(F)C=1C=C(C(F)(F)F)NN=1 NGDDUAYSWPUSLX-UHFFFAOYSA-N 0.000 description 2
- PJSJVTZSFIRYIZ-UHFFFAOYSA-N 3,5-di(propan-2-yl)-1h-pyrazole Chemical compound CC(C)C=1C=C(C(C)C)NN=1 PJSJVTZSFIRYIZ-UHFFFAOYSA-N 0.000 description 2
- RLOPEUXBLPWZFO-UHFFFAOYSA-N 3-(3-fluorophenyl)-5-(trifluoromethyl)-1h-pyrazole Chemical compound FC1=CC=CC(C=2NN=C(C=2)C(F)(F)F)=C1 RLOPEUXBLPWZFO-UHFFFAOYSA-N 0.000 description 2
- ZYKLIOCPFODUAL-UHFFFAOYSA-N 3-[2-(5-methoxy-2-methylanilino)ethyl]-1,3-oxazolidin-2-one Chemical compound COC1=CC=C(C)C(NCCN2C(OCC2)=O)=C1 ZYKLIOCPFODUAL-UHFFFAOYSA-N 0.000 description 2
- JDMJMQIBTMLFGZ-UHFFFAOYSA-N 3-tert-butyl-4-chloro-5-(trifluoromethyl)-1h-pyrazole Chemical compound CC(C)(C)C=1NN=C(C(F)(F)F)C=1Cl JDMJMQIBTMLFGZ-UHFFFAOYSA-N 0.000 description 2
- UAMVKOTWSHJOSY-UHFFFAOYSA-N 4-bromo-1-chloro-2-methoxybenzene Chemical compound COC1=CC(Br)=CC=C1Cl UAMVKOTWSHJOSY-UHFFFAOYSA-N 0.000 description 2
- JJGQFOSYETWZLC-UHFFFAOYSA-N 4-bromo-1-chloro-2-propan-2-yloxybenzene Chemical compound CC(C)OC1=CC(Br)=CC=C1Cl JJGQFOSYETWZLC-UHFFFAOYSA-N 0.000 description 2
- BADSZRMNXWLUKO-UHFFFAOYSA-N 4-chloro-1h-pyrazole Chemical compound ClC=1C=NNC=1 BADSZRMNXWLUKO-UHFFFAOYSA-N 0.000 description 2
- FTCOPDZWVCMXEC-UHFFFAOYSA-N 4-chloro-3,5-di(propan-2-yl)-1h-pyrazole Chemical compound CC(C)C1=NNC(C(C)C)=C1Cl FTCOPDZWVCMXEC-UHFFFAOYSA-N 0.000 description 2
- YTLDTZLZQMKURR-UHFFFAOYSA-N 4-chloro-5-(trifluoromethyl)-1h-pyrazole Chemical compound FC(F)(F)C=1NN=CC=1Cl YTLDTZLZQMKURR-UHFFFAOYSA-N 0.000 description 2
- XPHOQLFXRQWBOK-UHFFFAOYSA-N 4-chloro-5-ethyl-3-(trifluoromethyl)-1h-pyrazole Chemical compound CCC=1NN=C(C(F)(F)F)C=1Cl XPHOQLFXRQWBOK-UHFFFAOYSA-N 0.000 description 2
- AXFZQBHZKSJNSJ-UHFFFAOYSA-N 4-chloro-5-propyl-3-(trifluoromethyl)-1h-pyrazole Chemical compound CCCC=1NN=C(C(F)(F)F)C=1Cl AXFZQBHZKSJNSJ-UHFFFAOYSA-N 0.000 description 2
- ZDFTZMJWTJLPRE-UHFFFAOYSA-N 5-(4-fluorophenyl)-3-methylsulfanyl-1h-pyrazole Chemical compound N1N=C(SC)C=C1C1=CC=C(F)C=C1 ZDFTZMJWTJLPRE-UHFFFAOYSA-N 0.000 description 2
- PYXNITNKYBLBMW-UHFFFAOYSA-N 5-(trifluoromethyl)-1h-pyrazole Chemical compound FC(F)(F)C1=CC=NN1 PYXNITNKYBLBMW-UHFFFAOYSA-N 0.000 description 2
- FYTLHYRDGXRYEY-UHFFFAOYSA-N 5-Methyl-3-pyrazolamine Chemical compound CC=1C=C(N)NN=1 FYTLHYRDGXRYEY-UHFFFAOYSA-N 0.000 description 2
- UEVFFMZHGNYDKM-UHFFFAOYSA-N 5-bromo-2-chlorophenol Chemical compound OC1=CC(Br)=CC=C1Cl UEVFFMZHGNYDKM-UHFFFAOYSA-N 0.000 description 2
- QCOCVGBRVUAGQF-UHFFFAOYSA-N 5-butyl-3-(trifluoromethyl)-1h-pyrazole Chemical compound CCCCC1=CC(C(F)(F)F)=NN1 QCOCVGBRVUAGQF-UHFFFAOYSA-N 0.000 description 2
- BTASDYJQIJBLJR-UHFFFAOYSA-N 5-butyl-4-chloro-3-(trifluoromethyl)-1h-pyrazole Chemical compound CCCCC=1NN=C(C(F)(F)F)C=1Cl BTASDYJQIJBLJR-UHFFFAOYSA-N 0.000 description 2
- KCNZLFVWIBQNTR-UHFFFAOYSA-N 5-ethyl-3-(trifluoromethyl)-1h-pyrazole Chemical compound CCC1=CC(C(F)(F)F)=NN1 KCNZLFVWIBQNTR-UHFFFAOYSA-N 0.000 description 2
- XKVUYEYANWFIJX-UHFFFAOYSA-N 5-methyl-1h-pyrazole Chemical compound CC1=CC=NN1 XKVUYEYANWFIJX-UHFFFAOYSA-N 0.000 description 2
- FPFMPASDBKDTNH-UHFFFAOYSA-N 5-propan-2-yl-3-(trifluoromethyl)-1h-pyrazole Chemical compound CC(C)C=1C=C(C(F)(F)F)NN=1 FPFMPASDBKDTNH-UHFFFAOYSA-N 0.000 description 2
- SGVOGAMSXGTMRX-UHFFFAOYSA-N 5-propyl-3-(trifluoromethyl)-1h-pyrazole Chemical compound CCCC1=CC(C(F)(F)F)=NN1 SGVOGAMSXGTMRX-UHFFFAOYSA-N 0.000 description 2
- TVNDPZYOQCCHTJ-UHFFFAOYSA-N 5-thiophen-2-yl-1h-pyrazole Chemical compound C1=CSC(C=2NN=CC=2)=C1 TVNDPZYOQCCHTJ-UHFFFAOYSA-N 0.000 description 2
- OZAIFHULBGXAKX-VAWYXSNFSA-N AIBN Substances N#CC(C)(C)\N=N\C(C)(C)C#N OZAIFHULBGXAKX-VAWYXSNFSA-N 0.000 description 2
- 244000215068 Acacia senegal Species 0.000 description 2
- 235000006491 Acacia senegal Nutrition 0.000 description 2
- IKHGUXGNUITLKF-UHFFFAOYSA-N Acetaldehyde Chemical compound CC=O IKHGUXGNUITLKF-UHFFFAOYSA-N 0.000 description 2
- RZVAJINKPMORJF-UHFFFAOYSA-N Acetaminophen Chemical compound CC(=O)NC1=CC=C(O)C=C1 RZVAJINKPMORJF-UHFFFAOYSA-N 0.000 description 2
- QGZKDVFQNNGYKY-UHFFFAOYSA-N Ammonia Chemical compound N QGZKDVFQNNGYKY-UHFFFAOYSA-N 0.000 description 2
- PAYRUJLWNCNPSJ-UHFFFAOYSA-N Aniline Chemical compound NC1=CC=CC=C1 PAYRUJLWNCNPSJ-UHFFFAOYSA-N 0.000 description 2
- 235000003911 Arachis Nutrition 0.000 description 2
- 244000105624 Arachis hypogaea Species 0.000 description 2
- CIWBSHSKHKDKBQ-JLAZNSOCSA-N Ascorbic acid Chemical compound OC[C@H](O)[C@H]1OC(=O)C(O)=C1O CIWBSHSKHKDKBQ-JLAZNSOCSA-N 0.000 description 2
- BSYNRYMUTXBXSQ-UHFFFAOYSA-N Aspirin Chemical compound CC(=O)OC1=CC=CC=C1C(O)=O BSYNRYMUTXBXSQ-UHFFFAOYSA-N 0.000 description 2
- 241000416162 Astragalus gummifer Species 0.000 description 2
- 208000023275 Autoimmune disease Diseases 0.000 description 2
- 208000035143 Bacterial infection Diseases 0.000 description 2
- WKBOTKDWSSQWDR-UHFFFAOYSA-N Bromine atom Chemical compound [Br] WKBOTKDWSSQWDR-UHFFFAOYSA-N 0.000 description 2
- BHPQYMZQTOCNFJ-UHFFFAOYSA-N Calcium cation Chemical compound [Ca+2] BHPQYMZQTOCNFJ-UHFFFAOYSA-N 0.000 description 2
- 208000024172 Cardiovascular disease Diseases 0.000 description 2
- 102000006573 Chemokine CXCL12 Human genes 0.000 description 2
- 108010008951 Chemokine CXCL12 Proteins 0.000 description 2
- 108010037462 Cyclooxygenase 2 Proteins 0.000 description 2
- CMSMOCZEIVJLDB-UHFFFAOYSA-N Cyclophosphamide Chemical compound ClCCN(CCCl)P1(=O)NCCCO1 CMSMOCZEIVJLDB-UHFFFAOYSA-N 0.000 description 2
- FBPFZTCFMRRESA-FSIIMWSLSA-N D-Glucitol Natural products OC[C@H](O)[C@H](O)[C@@H](O)[C@H](O)CO FBPFZTCFMRRESA-FSIIMWSLSA-N 0.000 description 2
- FBPFZTCFMRRESA-JGWLITMVSA-N D-glucitol Chemical compound OC[C@H](O)[C@@H](O)[C@H](O)[C@H](O)CO FBPFZTCFMRRESA-JGWLITMVSA-N 0.000 description 2
- 206010012438 Dermatitis atopic Diseases 0.000 description 2
- ROSDSFDQCJNGOL-UHFFFAOYSA-N Dimethylamine Chemical compound CNC ROSDSFDQCJNGOL-UHFFFAOYSA-N 0.000 description 2
- 102000004190 Enzymes Human genes 0.000 description 2
- 108090000790 Enzymes Proteins 0.000 description 2
- 108010008165 Etanercept Proteins 0.000 description 2
- QUSNBJAOOMFDIB-UHFFFAOYSA-N Ethylamine Chemical compound CCN QUSNBJAOOMFDIB-UHFFFAOYSA-N 0.000 description 2
- 108091006027 G proteins Proteins 0.000 description 2
- 102000030782 GTP binding Human genes 0.000 description 2
- 108091000058 GTP-Binding Proteins 0.000 description 2
- WQZGKKKJIJFFOK-GASJEMHNSA-N Glucose Natural products OC[C@H]1OC(O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-GASJEMHNSA-N 0.000 description 2
- 229920000084 Gum arabic Polymers 0.000 description 2
- 239000007995 HEPES buffer Substances 0.000 description 2
- HEFNNWSXXWATRW-UHFFFAOYSA-N Ibuprofen Chemical compound CC(C)CC1=CC=C(C(C)C(O)=O)C=C1 HEFNNWSXXWATRW-UHFFFAOYSA-N 0.000 description 2
- 108010050904 Interferons Proteins 0.000 description 2
- 102000014150 Interferons Human genes 0.000 description 2
- LPHGQDQBBGAPDZ-UHFFFAOYSA-N Isocaffeine Natural products CN1C(=O)N(C)C(=O)C2=C1N(C)C=N2 LPHGQDQBBGAPDZ-UHFFFAOYSA-N 0.000 description 2
- TWRXJAOTZQYOKJ-UHFFFAOYSA-L Magnesium chloride Chemical compound [Mg+2].[Cl-].[Cl-] TWRXJAOTZQYOKJ-UHFFFAOYSA-L 0.000 description 2
- 241000124008 Mammalia Species 0.000 description 2
- BAVYZALUXZFZLV-UHFFFAOYSA-N Methylamine Chemical compound NC BAVYZALUXZFZLV-UHFFFAOYSA-N 0.000 description 2
- YNAVUWVOSKDBBP-UHFFFAOYSA-N Morpholine Chemical compound C1COCCN1 YNAVUWVOSKDBBP-UHFFFAOYSA-N 0.000 description 2
- 238000005481 NMR spectroscopy Methods 0.000 description 2
- CMWTZPSULFXXJA-UHFFFAOYSA-N Naproxen Natural products C1=C(C(C)C(O)=O)C=CC2=CC(OC)=CC=C21 CMWTZPSULFXXJA-UHFFFAOYSA-N 0.000 description 2
- 206010028980 Neoplasm Diseases 0.000 description 2
- MITFXPHMIHQXPI-UHFFFAOYSA-N Oraflex Chemical compound N=1C2=CC(C(C(O)=O)C)=CC=C2OC=1C1=CC=C(Cl)C=C1 MITFXPHMIHQXPI-UHFFFAOYSA-N 0.000 description 2
- KDLHZDBZIXYQEI-UHFFFAOYSA-N Palladium Chemical compound [Pd] KDLHZDBZIXYQEI-UHFFFAOYSA-N 0.000 description 2
- ISWSIDIOOBJBQZ-UHFFFAOYSA-N Phenol Chemical compound OC1=CC=CC=C1 ISWSIDIOOBJBQZ-UHFFFAOYSA-N 0.000 description 2
- 229920002873 Polyethylenimine Polymers 0.000 description 2
- 102100038280 Prostaglandin G/H synthase 2 Human genes 0.000 description 2
- 201000004681 Psoriasis Diseases 0.000 description 2
- 201000001263 Psoriatic Arthritis Diseases 0.000 description 2
- 208000036824 Psoriatic arthropathy Diseases 0.000 description 2
- 206010039710 Scleroderma Diseases 0.000 description 2
- GIIZNNXWQWCKIB-UHFFFAOYSA-N Serevent Chemical compound C1=C(O)C(CO)=CC(C(O)CNCCCCCCOCCCCC=2C=CC=CC=2)=C1 GIIZNNXWQWCKIB-UHFFFAOYSA-N 0.000 description 2
- PXIPVTKHYLBLMZ-UHFFFAOYSA-N Sodium azide Chemical compound [Na+].[N-]=[N+]=[N-] PXIPVTKHYLBLMZ-UHFFFAOYSA-N 0.000 description 2
- UIIMBOGNXHQVGW-DEQYMQKBSA-M Sodium bicarbonate-14C Chemical compound [Na+].O[14C]([O-])=O UIIMBOGNXHQVGW-DEQYMQKBSA-M 0.000 description 2
- CZMRCDWAGMRECN-UGDNZRGBSA-N Sucrose Chemical compound O[C@H]1[C@H](O)[C@@H](CO)O[C@@]1(CO)O[C@@H]1[C@H](O)[C@@H](O)[C@H](O)[C@@H](CO)O1 CZMRCDWAGMRECN-UGDNZRGBSA-N 0.000 description 2
- 229930006000 Sucrose Natural products 0.000 description 2
- QAOWNCQODCNURD-UHFFFAOYSA-N Sulfuric acid Chemical compound OS(O)(=O)=O QAOWNCQODCNURD-UHFFFAOYSA-N 0.000 description 2
- GUGOEEXESWIERI-UHFFFAOYSA-N Terfenadine Chemical compound C1=CC(C(C)(C)C)=CC=C1C(O)CCCN1CCC(C(O)(C=2C=CC=CC=2)C=2C=CC=CC=2)CC1 GUGOEEXESWIERI-UHFFFAOYSA-N 0.000 description 2
- 229920001615 Tragacanth Polymers 0.000 description 2
- ZZHLYYDVIOPZBE-UHFFFAOYSA-N Trimeprazine Chemical compound C1=CC=C2N(CC(CN(C)C)C)C3=CC=CC=C3SC2=C1 ZZHLYYDVIOPZBE-UHFFFAOYSA-N 0.000 description 2
- 239000007983 Tris buffer Substances 0.000 description 2
- XSQUKJJJFZCRTK-UHFFFAOYSA-N Urea Chemical compound NC(N)=O XSQUKJJJFZCRTK-UHFFFAOYSA-N 0.000 description 2
- HUCJFAOMUPXHDK-UHFFFAOYSA-N Xylometazoline Chemical compound CC1=CC(C(C)(C)C)=CC(C)=C1CC1=NCCN1 HUCJFAOMUPXHDK-UHFFFAOYSA-N 0.000 description 2
- HCHKCACWOHOZIP-UHFFFAOYSA-N Zinc Chemical compound [Zn] HCHKCACWOHOZIP-UHFFFAOYSA-N 0.000 description 2
- 235000010489 acacia gum Nutrition 0.000 description 2
- 229960001138 acetylsalicylic acid Drugs 0.000 description 2
- 230000009471 action Effects 0.000 description 2
- 230000004913 activation Effects 0.000 description 2
- 125000003342 alkenyl group Chemical group 0.000 description 2
- 125000003545 alkoxy group Chemical group 0.000 description 2
- 125000000304 alkynyl group Chemical group 0.000 description 2
- 229910052782 aluminium Inorganic materials 0.000 description 2
- XAGFODPZIPBFFR-UHFFFAOYSA-N aluminium Chemical compound [Al] XAGFODPZIPBFFR-UHFFFAOYSA-N 0.000 description 2
- 229940024548 aluminum oxide Drugs 0.000 description 2
- 230000000202 analgesic effect Effects 0.000 description 2
- RDOXTESZEPMUJZ-UHFFFAOYSA-N anisole Chemical compound COC1=CC=CC=C1 RDOXTESZEPMUJZ-UHFFFAOYSA-N 0.000 description 2
- 239000003963 antioxidant agent Substances 0.000 description 2
- 235000006708 antioxidants Nutrition 0.000 description 2
- 206010003246 arthritis Diseases 0.000 description 2
- 125000003710 aryl alkyl group Chemical group 0.000 description 2
- 150000001499 aryl bromides Chemical class 0.000 description 2
- 239000012131 assay buffer Substances 0.000 description 2
- 201000008937 atopic dermatitis Diseases 0.000 description 2
- 229960002170 azathioprine Drugs 0.000 description 2
- 208000022362 bacterial infectious disease Diseases 0.000 description 2
- 239000011324 bead Substances 0.000 description 2
- WQZGKKKJIJFFOK-VFUOTHLCSA-N beta-D-glucose Chemical compound OC[C@H]1O[C@@H](O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-VFUOTHLCSA-N 0.000 description 2
- 230000037396 body weight Effects 0.000 description 2
- 210000000988 bone and bone Anatomy 0.000 description 2
- ILAHWRKJUDSMFH-UHFFFAOYSA-N boron tribromide Chemical compound BrB(Br)Br ILAHWRKJUDSMFH-UHFFFAOYSA-N 0.000 description 2
- 230000031709 bromination Effects 0.000 description 2
- 238000005893 bromination reaction Methods 0.000 description 2
- 229960001948 caffeine Drugs 0.000 description 2
- VJEONQKOZGKCAK-UHFFFAOYSA-N caffeine Natural products CN1C(=O)N(C)C(=O)C2=C1C=CN2C VJEONQKOZGKCAK-UHFFFAOYSA-N 0.000 description 2
- 229960002882 calcipotriol Drugs 0.000 description 2
- 229910000019 calcium carbonate Inorganic materials 0.000 description 2
- 235000010216 calcium carbonate Nutrition 0.000 description 2
- 229910001424 calcium ion Inorganic materials 0.000 description 2
- 239000001506 calcium phosphate Substances 0.000 description 2
- 229910000389 calcium phosphate Inorganic materials 0.000 description 2
- 235000011010 calcium phosphates Nutrition 0.000 description 2
- RZEKVGVHFLEQIL-UHFFFAOYSA-N celecoxib Chemical compound C1=CC(C)=CC=C1C1=CC(C(F)(F)F)=NN1C1=CC=C(S(N)(=O)=O)C=C1 RZEKVGVHFLEQIL-UHFFFAOYSA-N 0.000 description 2
- 239000001913 cellulose Substances 0.000 description 2
- 229920002678 cellulose Polymers 0.000 description 2
- 210000003169 central nervous system Anatomy 0.000 description 2
- 239000003153 chemical reaction reagent Substances 0.000 description 2
- 239000003795 chemical substances by application Substances 0.000 description 2
- 229960004126 codeine Drugs 0.000 description 2
- 238000009833 condensation Methods 0.000 description 2
- 230000005494 condensation Effects 0.000 description 2
- 238000010276 construction Methods 0.000 description 2
- 230000017858 demethylation Effects 0.000 description 2
- 238000010520 demethylation reaction Methods 0.000 description 2
- 238000013461 design Methods 0.000 description 2
- 238000011161 development Methods 0.000 description 2
- ZZVUWRFHKOJYTH-UHFFFAOYSA-N diphenhydramine Chemical compound C=1C=CC=CC=1C(OCCN(C)C)C1=CC=CC=C1 ZZVUWRFHKOJYTH-UHFFFAOYSA-N 0.000 description 2
- ZUOUZKKEUPVFJK-UHFFFAOYSA-N diphenyl Chemical group C1=CC=CC=C1C1=CC=CC=C1 ZUOUZKKEUPVFJK-UHFFFAOYSA-N 0.000 description 2
- 231100000673 dose–response relationship Toxicity 0.000 description 2
- 239000003937 drug carrier Substances 0.000 description 2
- 239000003974 emollient agent Substances 0.000 description 2
- 238000005516 engineering process Methods 0.000 description 2
- 238000006266 etherification reaction Methods 0.000 description 2
- QPFLIEFFEMYLBE-UHFFFAOYSA-N ethyl 2-[5-(bromomethyl)-4-chloro-3-(trifluoromethyl)pyrazol-1-yl]acetate Chemical compound CCOC(=O)CN1N=C(C(F)(F)F)C(Cl)=C1CBr QPFLIEFFEMYLBE-UHFFFAOYSA-N 0.000 description 2
- YEUFDJAHBFUZMI-UHFFFAOYSA-N ethyl 4-chloro-5-methyl-1h-pyrazole-3-carboxylate Chemical compound CCOC(=O)C1=NNC(C)=C1Cl YEUFDJAHBFUZMI-UHFFFAOYSA-N 0.000 description 2
- ILJRUIIBSAQSGA-UHFFFAOYSA-N ethyl 4-chloro-5-propyl-1h-pyrazole-3-carboxylate Chemical compound CCCC=1NN=C(C(=O)OCC)C=1Cl ILJRUIIBSAQSGA-UHFFFAOYSA-N 0.000 description 2
- OQEHTFFLOHTFSB-UHFFFAOYSA-N ethyl 4-piperazin-1-ylbenzoate Chemical compound C1=CC(C(=O)OCC)=CC=C1N1CCNCC1 OQEHTFFLOHTFSB-UHFFFAOYSA-N 0.000 description 2
- BOTXQJAHRCGJEG-UHFFFAOYSA-N ethyl 5-methyl-1h-pyrazole-3-carboxylate Chemical compound CCOC(=O)C=1C=C(C)NN=1 BOTXQJAHRCGJEG-UHFFFAOYSA-N 0.000 description 2
- 125000001495 ethyl group Chemical group [H]C([H])([H])C([H])([H])* 0.000 description 2
- HQMNCQVAMBCHCO-DJRRULDNSA-N etretinate Chemical compound CCOC(=O)\C=C(/C)\C=C\C=C(/C)\C=C\C1=C(C)C=C(OC)C(C)=C1C HQMNCQVAMBCHCO-DJRRULDNSA-N 0.000 description 2
- 229960002199 etretinate Drugs 0.000 description 2
- 230000028023 exocytosis Effects 0.000 description 2
- 230000004907 flux Effects 0.000 description 2
- 239000007903 gelatin capsule Substances 0.000 description 2
- 239000011521 glass Substances 0.000 description 2
- 239000008103 glucose Substances 0.000 description 2
- KWIUHFFTVRNATP-UHFFFAOYSA-N glycine betaine Chemical compound C[N+](C)(C)CC([O-])=O KWIUHFFTVRNATP-UHFFFAOYSA-N 0.000 description 2
- 239000008187 granular material Substances 0.000 description 2
- 229940093915 gynecological organic acid Drugs 0.000 description 2
- 230000036541 health Effects 0.000 description 2
- BXWNKGSJHAJOGX-UHFFFAOYSA-N hexadecan-1-ol Chemical compound CCCCCCCCCCCCCCCCO BXWNKGSJHAJOGX-UHFFFAOYSA-N 0.000 description 2
- 229960001340 histamine Drugs 0.000 description 2
- JYGXADMDTFJGBT-VWUMJDOOSA-N hydrocortisone Chemical compound O=C1CC[C@]2(C)[C@H]3[C@@H](O)C[C@](C)([C@@](CC4)(O)C(=O)CO)[C@@H]4[C@@H]3CCC2=C1 JYGXADMDTFJGBT-VWUMJDOOSA-N 0.000 description 2
- 230000009610 hypersensitivity Effects 0.000 description 2
- 229960001680 ibuprofen Drugs 0.000 description 2
- 210000000987 immune system Anatomy 0.000 description 2
- 230000001506 immunosuppresive effect Effects 0.000 description 2
- 229960003444 immunosuppressant agent Drugs 0.000 description 2
- 239000003018 immunosuppressive agent Substances 0.000 description 2
- 229960000905 indomethacin Drugs 0.000 description 2
- 239000012442 inert solvent Substances 0.000 description 2
- 229940079322 interferon Drugs 0.000 description 2
- 229910052740 iodine Inorganic materials 0.000 description 2
- 150000002500 ions Chemical class 0.000 description 2
- 235000010445 lecithin Nutrition 0.000 description 2
- 239000000787 lecithin Substances 0.000 description 2
- 229940067606 lecithin Drugs 0.000 description 2
- 239000007788 liquid Substances 0.000 description 2
- 239000000314 lubricant Substances 0.000 description 2
- HQKMJHAJHXVSDF-UHFFFAOYSA-L magnesium stearate Chemical compound [Mg+2].CCCCCCCCCCCCCCCCCC([O-])=O.CCCCCCCCCCCCCCCCCC([O-])=O HQKMJHAJHXVSDF-UHFFFAOYSA-L 0.000 description 2
- 238000004949 mass spectrometry Methods 0.000 description 2
- 239000002609 medium Substances 0.000 description 2
- GLVAUDGFNGKCSF-UHFFFAOYSA-N mercaptopurine Chemical compound S=C1NC=NC2=C1NC=N2 GLVAUDGFNGKCSF-UHFFFAOYSA-N 0.000 description 2
- BDAGIHXWWSANSR-UHFFFAOYSA-N methanoic acid Natural products OC=O BDAGIHXWWSANSR-UHFFFAOYSA-N 0.000 description 2
- 229960000485 methotrexate Drugs 0.000 description 2
- YWMCHHGKDMGQPQ-UHFFFAOYSA-N methyl 2-bromo-2-(3-methoxyphenyl)acetate Chemical compound COC(=O)C(Br)C1=CC=CC(OC)=C1 YWMCHHGKDMGQPQ-UHFFFAOYSA-N 0.000 description 2
- HPNSFSBZBAHARI-UHFFFAOYSA-N micophenolic acid Natural products OC1=C(CC=C(C)CCC(O)=O)C(OC)=C(C)C2=C1C(=O)OC2 HPNSFSBZBAHARI-UHFFFAOYSA-N 0.000 description 2
- 230000005012 migration Effects 0.000 description 2
- 238000013508 migration Methods 0.000 description 2
- 239000002480 mineral oil Substances 0.000 description 2
- 235000010446 mineral oil Nutrition 0.000 description 2
- 238000002156 mixing Methods 0.000 description 2
- 239000003068 molecular probe Substances 0.000 description 2
- 239000001788 mono and diglycerides of fatty acids Substances 0.000 description 2
- BQJCRHHNABKAKU-KBQPJGBKSA-N morphine Chemical compound O([C@H]1[C@H](C=C[C@H]23)O)C4=C5[C@@]12CCN(C)[C@@H]3CC5=CC=C4O BQJCRHHNABKAKU-KBQPJGBKSA-N 0.000 description 2
- 125000004123 n-propyl group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])* 0.000 description 2
- 229960002009 naproxen Drugs 0.000 description 2
- CMWTZPSULFXXJA-VIFPVBQESA-N naproxen Chemical compound C1=C([C@H](C)C(O)=O)C=CC2=CC(OC)=CC=C21 CMWTZPSULFXXJA-VIFPVBQESA-N 0.000 description 2
- 230000009871 nonspecific binding Effects 0.000 description 2
- 239000000346 nonvolatile oil Substances 0.000 description 2
- 239000002674 ointment Substances 0.000 description 2
- 150000007524 organic acids Chemical class 0.000 description 2
- 235000005985 organic acids Nutrition 0.000 description 2
- 239000012074 organic phase Substances 0.000 description 2
- 201000008482 osteoarthritis Diseases 0.000 description 2
- 229910052760 oxygen Inorganic materials 0.000 description 2
- WYWIFABBXFUGLM-UHFFFAOYSA-N oxymetazoline Chemical compound CC1=CC(C(C)(C)C)=C(O)C(C)=C1CC1=NCCN1 WYWIFABBXFUGLM-UHFFFAOYSA-N 0.000 description 2
- 230000001717 pathogenic effect Effects 0.000 description 2
- CPJSUEIXXCENMM-UHFFFAOYSA-N phenacetin Chemical compound CCOC1=CC=C(NC(C)=O)C=C1 CPJSUEIXXCENMM-UHFFFAOYSA-N 0.000 description 2
- WLJVXDMOQOGPHL-UHFFFAOYSA-N phenylacetic acid Chemical compound OC(=O)CC1=CC=CC=C1 WLJVXDMOQOGPHL-UHFFFAOYSA-N 0.000 description 2
- 239000008363 phosphate buffer Substances 0.000 description 2
- 229960002702 piroxicam Drugs 0.000 description 2
- QYSPLQLAKJAUJT-UHFFFAOYSA-N piroxicam Chemical compound OC=1C2=CC=CC=C2S(=O)(=O)N(C)C=1C(=O)NC1=CC=CC=N1 QYSPLQLAKJAUJT-UHFFFAOYSA-N 0.000 description 2
- 208000030428 polyarticular arthritis Diseases 0.000 description 2
- 229920000036 polyvinylpyrrolidone Polymers 0.000 description 2
- 239000001267 polyvinylpyrrolidone Substances 0.000 description 2
- 239000000843 powder Substances 0.000 description 2
- 239000002243 precursor Substances 0.000 description 2
- 108090000765 processed proteins & peptides Proteins 0.000 description 2
- ZAHRKKWIAAJSAO-UHFFFAOYSA-N rapamycin Natural products COCC(O)C(=C/C(C)C(=O)CC(OC(=O)C1CCCCN1C(=O)C(=O)C2(O)OC(CC(OC)C(=CC=CC=CC(C)CC(C)C(=O)C)C)CCC2C)C(C)CC3CCC(O)C(C3)OC)C ZAHRKKWIAAJSAO-UHFFFAOYSA-N 0.000 description 2
- 230000007115 recruitment Effects 0.000 description 2
- 230000002829 reductive effect Effects 0.000 description 2
- 230000004044 response Effects 0.000 description 2
- 125000006413 ring segment Chemical group 0.000 description 2
- RZJQGNCSTQAWON-UHFFFAOYSA-N rofecoxib Chemical compound C1=CC(S(=O)(=O)C)=CC=C1C1=C(C=2C=CC=CC=2)C(=O)OC1 RZJQGNCSTQAWON-UHFFFAOYSA-N 0.000 description 2
- 229960004017 salmeterol Drugs 0.000 description 2
- 238000012216 screening Methods 0.000 description 2
- 150000003335 secondary amines Chemical class 0.000 description 2
- 230000001624 sedative effect Effects 0.000 description 2
- 230000019491 signal transduction Effects 0.000 description 2
- 229960002930 sirolimus Drugs 0.000 description 2
- 235000017557 sodium bicarbonate Nutrition 0.000 description 2
- 229910000029 sodium carbonate Inorganic materials 0.000 description 2
- 235000010288 sodium nitrite Nutrition 0.000 description 2
- DAEPDZWVDSPTHF-UHFFFAOYSA-M sodium pyruvate Chemical compound [Na+].CC(=O)C([O-])=O DAEPDZWVDSPTHF-UHFFFAOYSA-M 0.000 description 2
- 235000010265 sodium sulphite Nutrition 0.000 description 2
- 238000000638 solvent extraction Methods 0.000 description 2
- 235000011069 sorbitan monooleate Nutrition 0.000 description 2
- 239000001593 sorbitan monooleate Substances 0.000 description 2
- 229940035049 sorbitan monooleate Drugs 0.000 description 2
- 239000000600 sorbitol Substances 0.000 description 2
- 239000007858 starting material Substances 0.000 description 2
- 230000003637 steroidlike Effects 0.000 description 2
- 239000005720 sucrose Substances 0.000 description 2
- 125000004434 sulfur atom Chemical group 0.000 description 2
- 239000004094 surface-active agent Substances 0.000 description 2
- 239000006188 syrup Substances 0.000 description 2
- 235000020357 syrup Nutrition 0.000 description 2
- YAPQBXQYLJRXSA-UHFFFAOYSA-N theobromine Chemical compound CN1C(=O)NC(=O)C2=C1N=CN2C YAPQBXQYLJRXSA-UHFFFAOYSA-N 0.000 description 2
- ZFXYFBGIUFBOJW-UHFFFAOYSA-N theophylline Chemical compound O=C1N(C)C(=O)N(C)C2=C1NC=N2 ZFXYFBGIUFBOJW-UHFFFAOYSA-N 0.000 description 2
- 238000002560 therapeutic procedure Methods 0.000 description 2
- 125000005309 thioalkoxy group Chemical group 0.000 description 2
- QORWJWZARLRLPR-UHFFFAOYSA-H tricalcium bis(phosphate) Chemical compound [Ca+2].[Ca+2].[Ca+2].[O-]P([O-])([O-])=O.[O-]P([O-])([O-])=O QORWJWZARLRLPR-UHFFFAOYSA-H 0.000 description 2
- GETQZCLCWQTVFV-UHFFFAOYSA-N trimethylamine Chemical compound CN(C)C GETQZCLCWQTVFV-UHFFFAOYSA-N 0.000 description 2
- RIOQSEWOXXDEQQ-UHFFFAOYSA-N triphenylphosphine Chemical compound C1=CC=CC=C1P(C=1C=CC=CC=1)C1=CC=CC=C1 RIOQSEWOXXDEQQ-UHFFFAOYSA-N 0.000 description 2
- LENZDBCJOHFCAS-UHFFFAOYSA-N tris Chemical compound OCC(N)(CO)CO LENZDBCJOHFCAS-UHFFFAOYSA-N 0.000 description 2
- 125000004417 unsaturated alkyl group Chemical group 0.000 description 2
- 235000015112 vegetable and seed oil Nutrition 0.000 description 2
- 239000008158 vegetable oil Substances 0.000 description 2
- 239000003981 vehicle Substances 0.000 description 2
- DGVVWUTYPXICAM-UHFFFAOYSA-N β‐Mercaptoethanol Chemical compound OCCS DGVVWUTYPXICAM-UHFFFAOYSA-N 0.000 description 2
- XWTYSIMOBUGWOL-UHFFFAOYSA-N (+-)-Terbutaline Chemical compound CC(C)(C)NCC(O)C1=CC(O)=CC(O)=C1 XWTYSIMOBUGWOL-UHFFFAOYSA-N 0.000 description 1
- RJMIEHBSYVWVIN-LLVKDONJSA-N (2r)-2-[4-(3-oxo-1h-isoindol-2-yl)phenyl]propanoic acid Chemical compound C1=CC([C@H](C(O)=O)C)=CC=C1N1C(=O)C2=CC=CC=C2C1 RJMIEHBSYVWVIN-LLVKDONJSA-N 0.000 description 1
- ZEYYDOLCHFETHQ-JOCHJYFZSA-N (2r)-2-cyclopentyl-2-[4-(quinolin-2-ylmethoxy)phenyl]acetic acid Chemical compound C1([C@@H](C(=O)O)C=2C=CC(OCC=3N=C4C=CC=CC4=CC=3)=CC=2)CCCC1 ZEYYDOLCHFETHQ-JOCHJYFZSA-N 0.000 description 1
- JOMNTHCQHJPVAZ-RXMQYKEDSA-N (2r)-2-methylpiperazine Chemical compound C[C@@H]1CNCCN1 JOMNTHCQHJPVAZ-RXMQYKEDSA-N 0.000 description 1
- LNAZSHAWQACDHT-XIYTZBAFSA-N (2r,3r,4s,5r,6s)-4,5-dimethoxy-2-(methoxymethyl)-3-[(2s,3r,4s,5r,6r)-3,4,5-trimethoxy-6-(methoxymethyl)oxan-2-yl]oxy-6-[(2r,3r,4s,5r,6r)-4,5,6-trimethoxy-2-(methoxymethyl)oxan-3-yl]oxyoxane Chemical compound CO[C@@H]1[C@@H](OC)[C@H](OC)[C@@H](COC)O[C@H]1O[C@H]1[C@H](OC)[C@@H](OC)[C@H](O[C@H]2[C@@H]([C@@H](OC)[C@H](OC)O[C@@H]2COC)OC)O[C@@H]1COC LNAZSHAWQACDHT-XIYTZBAFSA-N 0.000 description 1
- RDJGLLICXDHJDY-NSHDSACASA-N (2s)-2-(3-phenoxyphenyl)propanoic acid Chemical compound OC(=O)[C@@H](C)C1=CC=CC(OC=2C=CC=CC=2)=C1 RDJGLLICXDHJDY-NSHDSACASA-N 0.000 description 1
- GUHPRPJDBZHYCJ-SECBINFHSA-N (2s)-2-(5-benzoylthiophen-2-yl)propanoic acid Chemical compound S1C([C@H](C(O)=O)C)=CC=C1C(=O)C1=CC=CC=C1 GUHPRPJDBZHYCJ-SECBINFHSA-N 0.000 description 1
- MDKGKXOCJGEUJW-VIFPVBQESA-N (2s)-2-[4-(thiophene-2-carbonyl)phenyl]propanoic acid Chemical compound C1=CC([C@@H](C(O)=O)C)=CC=C1C(=O)C1=CC=CS1 MDKGKXOCJGEUJW-VIFPVBQESA-N 0.000 description 1
- PZIFPMYXXCAOCC-JWQCQUIFSA-N (2s,3r)-3-(2-carboxyethylsulfanyl)-2-hydroxy-3-[2-(8-phenyloctyl)phenyl]propanoic acid Chemical compound OC(=O)CCS[C@@H]([C@@H](O)C(O)=O)C1=CC=CC=C1CCCCCCCCC1=CC=CC=C1 PZIFPMYXXCAOCC-JWQCQUIFSA-N 0.000 description 1
- OQANPHBRHBJGNZ-FYJGNVAPSA-N (3e)-6-oxo-3-[[4-(pyridin-2-ylsulfamoyl)phenyl]hydrazinylidene]cyclohexa-1,4-diene-1-carboxylic acid Chemical compound C1=CC(=O)C(C(=O)O)=C\C1=N\NC1=CC=C(S(=O)(=O)NC=2N=CC=CC=2)C=C1 OQANPHBRHBJGNZ-FYJGNVAPSA-N 0.000 description 1
- BKAGJXUNCOKCTP-UHFFFAOYSA-N (5-chloro-2-piperazin-1-ylphenyl)-phenylmethanone Chemical compound C=1C=CC=CC=1C(=O)C1=CC(Cl)=CC=C1N1CCNCC1 BKAGJXUNCOKCTP-UHFFFAOYSA-N 0.000 description 1
- 125000000229 (C1-C4)alkoxy group Chemical group 0.000 description 1
- 239000001211 (E)-4-phenylbut-3-en-2-one Substances 0.000 description 1
- WRIDQFICGBMAFQ-UHFFFAOYSA-N (E)-8-Octadecenoic acid Natural products CCCCCCCCCC=CCCCCCCC(O)=O WRIDQFICGBMAFQ-UHFFFAOYSA-N 0.000 description 1
- SBRYTXXHTKCMFT-UHFFFAOYSA-N 1,1,1-trifluorohexane-2,4-dione Chemical compound CCC(=O)CC(=O)C(F)(F)F SBRYTXXHTKCMFT-UHFFFAOYSA-N 0.000 description 1
- LIKXJDINUMWKQA-UHFFFAOYSA-N 1-(2,3-dimethylphenyl)piperazine Chemical compound CC1=CC=CC(N2CCNCC2)=C1C LIKXJDINUMWKQA-UHFFFAOYSA-N 0.000 description 1
- CGQGZAHTFYOBCF-UHFFFAOYSA-N 1-(2,4-dichloro-5-methoxyphenyl)piperazine Chemical compound C1=C(Cl)C(OC)=CC(N2CCNCC2)=C1Cl CGQGZAHTFYOBCF-UHFFFAOYSA-N 0.000 description 1
- NUAYNFHITWMDMO-UHFFFAOYSA-N 1-(2,4-dichloro-5-methoxyphenyl)piperazine;hydrochloride Chemical compound Cl.C1=C(Cl)C(OC)=CC(N2CCNCC2)=C1Cl NUAYNFHITWMDMO-UHFFFAOYSA-N 0.000 description 1
- YRIFWVMRUFKWLM-UHFFFAOYSA-N 1-(2,5-dimethylphenyl)piperazine Chemical compound CC1=CC=C(C)C(N2CCNCC2)=C1 YRIFWVMRUFKWLM-UHFFFAOYSA-N 0.000 description 1
- GNNSHUNRDUGDAT-UHFFFAOYSA-N 1-(2-chloro-5-methoxyphenyl)piperazine Chemical compound COC1=CC=C(Cl)C(N2CCNCC2)=C1 GNNSHUNRDUGDAT-UHFFFAOYSA-N 0.000 description 1
- RACFVTCZAHYIGS-UHFFFAOYSA-N 1-(2-chloro-5-methoxyphenyl)piperazine;hydrochloride Chemical compound Cl.COC1=CC=C(Cl)C(N2CCNCC2)=C1 RACFVTCZAHYIGS-UHFFFAOYSA-N 0.000 description 1
- IVTZRJKKXSKXKO-UHFFFAOYSA-N 1-(2-fluorophenyl)piperazine Chemical compound FC1=CC=CC=C1N1CCNCC1 IVTZRJKKXSKXKO-UHFFFAOYSA-N 0.000 description 1
- VNZLQLYBRIOLFZ-UHFFFAOYSA-N 1-(2-methoxyphenyl)piperazine Chemical compound COC1=CC=CC=C1N1CCNCC1 VNZLQLYBRIOLFZ-UHFFFAOYSA-N 0.000 description 1
- WICKLEOONJPMEQ-UHFFFAOYSA-N 1-(2-methylphenyl)piperazine Chemical compound CC1=CC=CC=C1N1CCNCC1 WICKLEOONJPMEQ-UHFFFAOYSA-N 0.000 description 1
- PXFJLKKZSWWVRX-UHFFFAOYSA-N 1-(3,4-dichlorophenyl)piperazine Chemical compound C1=C(Cl)C(Cl)=CC=C1N1CCNCC1 PXFJLKKZSWWVRX-UHFFFAOYSA-N 0.000 description 1
- SFLNVAVCCYTHCQ-UHFFFAOYSA-N 1-(3,4-dimethylphenyl)piperazine Chemical compound C1=C(C)C(C)=CC=C1N1CCNCC1 SFLNVAVCCYTHCQ-UHFFFAOYSA-N 0.000 description 1
- KKIMDKMETPPURN-UHFFFAOYSA-N 1-(3-(trifluoromethyl)phenyl)piperazine Chemical compound FC(F)(F)C1=CC=CC(N2CCNCC2)=C1 KKIMDKMETPPURN-UHFFFAOYSA-N 0.000 description 1
- VHFVKMTVMIZMIK-UHFFFAOYSA-N 1-(3-chlorophenyl)piperazine Chemical compound ClC1=CC=CC(N2CCNCC2)=C1 VHFVKMTVMIZMIK-UHFFFAOYSA-N 0.000 description 1
- PZIBVWUXWNYTNL-UHFFFAOYSA-N 1-(3-methoxyphenyl)piperazine Chemical compound COC1=CC=CC(N2CCNCC2)=C1 PZIBVWUXWNYTNL-UHFFFAOYSA-N 0.000 description 1
- JIWHIRLNKIUYSM-UHFFFAOYSA-N 1-(3-methylphenyl)piperazine Chemical compound CC1=CC=CC(N2CCNCC2)=C1 JIWHIRLNKIUYSM-UHFFFAOYSA-N 0.000 description 1
- QJNWTYUDISMTMR-UHFFFAOYSA-N 1-(3-methylphenyl)piperazine;dihydrochloride Chemical compound Cl.Cl.CC1=CC=CC(N2CCNCC2)=C1 QJNWTYUDISMTMR-UHFFFAOYSA-N 0.000 description 1
- XCMHZUWCZNJMKA-UHFFFAOYSA-N 1-(3-methylsulfanylphenyl)piperazine Chemical compound CSC1=CC=CC(N2CCNCC2)=C1 XCMHZUWCZNJMKA-UHFFFAOYSA-N 0.000 description 1
- IBQMAPSJLHRQPE-UHFFFAOYSA-N 1-(4-(trifluoromethyl)phenyl)piperazine Chemical compound C1=CC(C(F)(F)F)=CC=C1N1CCNCC1 IBQMAPSJLHRQPE-UHFFFAOYSA-N 0.000 description 1
- WQACLMXRWZMQCO-UHFFFAOYSA-N 1-(4-bromo-3-methoxyphenyl)piperazine;hydrochloride Chemical compound Cl.C1=C(Br)C(OC)=CC(N2CCNCC2)=C1 WQACLMXRWZMQCO-UHFFFAOYSA-N 0.000 description 1
- KJFJJQOAKGKVFB-UHFFFAOYSA-N 1-(4-bromo-3-methylphenyl)piperazine Chemical compound C1=C(Br)C(C)=CC(N2CCNCC2)=C1 KJFJJQOAKGKVFB-UHFFFAOYSA-N 0.000 description 1
- PRBYRCRBYURQMR-UHFFFAOYSA-N 1-(4-bromo-3-methylphenyl)piperazine;hydrochloride Chemical compound Cl.C1=C(Br)C(C)=CC(N2CCNCC2)=C1 PRBYRCRBYURQMR-UHFFFAOYSA-N 0.000 description 1
- IPILNCXNYGCVDJ-UHFFFAOYSA-N 1-(4-chloro-2-fluorophenyl)piperazine Chemical compound FC1=CC(Cl)=CC=C1N1CCNCC1 IPILNCXNYGCVDJ-UHFFFAOYSA-N 0.000 description 1
- YSEWXFSLNGJJAO-UHFFFAOYSA-N 1-(4-chloro-2-methoxyphenyl)piperazine Chemical compound COC1=CC(Cl)=CC=C1N1CCNCC1 YSEWXFSLNGJJAO-UHFFFAOYSA-N 0.000 description 1
- DEESDIMYTYJVOS-UHFFFAOYSA-N 1-(4-chloro-3-methylphenyl)piperazine Chemical compound C1=C(Cl)C(C)=CC(N2CCNCC2)=C1 DEESDIMYTYJVOS-UHFFFAOYSA-N 0.000 description 1
- WXTTZXKXEGXAMA-UHFFFAOYSA-N 1-(4-chloro-3-propan-2-yloxyphenyl)piperazine;hydrochloride Chemical compound Cl.C1=C(Cl)C(OC(C)C)=CC(N2CCNCC2)=C1 WXTTZXKXEGXAMA-UHFFFAOYSA-N 0.000 description 1
- UECHCFXEJKAZEX-UHFFFAOYSA-N 1-(4-chlorophenyl)-3-methylpiperazine Chemical compound C1CNC(C)CN1C1=CC=C(Cl)C=C1 UECHCFXEJKAZEX-UHFFFAOYSA-N 0.000 description 1
- GGFWCDNEFMGGMQ-UHFFFAOYSA-N 1-(4-methoxypyridin-2-yl)piperazine Chemical compound COC1=CC=NC(N2CCNCC2)=C1 GGFWCDNEFMGGMQ-UHFFFAOYSA-N 0.000 description 1
- ONEYFZXGNFNRJH-UHFFFAOYSA-N 1-(4-methylphenyl)piperazine Chemical compound C1=CC(C)=CC=C1N1CCNCC1 ONEYFZXGNFNRJH-UHFFFAOYSA-N 0.000 description 1
- VWOJSRICSKDKAW-UHFFFAOYSA-N 1-(4-nitrophenyl)piperazine Chemical compound C1=CC([N+](=O)[O-])=CC=C1N1CCNCC1 VWOJSRICSKDKAW-UHFFFAOYSA-N 0.000 description 1
- KPXVKKBJROCIJB-UHFFFAOYSA-N 1-(4-piperazin-1-ylphenyl)ethanone Chemical compound C1=CC(C(=O)C)=CC=C1N1CCNCC1 KPXVKKBJROCIJB-UHFFFAOYSA-N 0.000 description 1
- LYPKLJIYXQVTPF-UHFFFAOYSA-N 1-(5-fluoro-2-methoxyphenyl)piperazine Chemical compound COC1=CC=C(F)C=C1N1CCNCC1 LYPKLJIYXQVTPF-UHFFFAOYSA-N 0.000 description 1
- WQWFTQWTQCGSDG-UHFFFAOYSA-N 1-(5-methoxy-2-methylphenyl)piperazine Chemical compound COC1=CC=C(C)C(N2CCNCC2)=C1 WQWFTQWTQCGSDG-UHFFFAOYSA-N 0.000 description 1
- ASOKPJOREAFHNY-UHFFFAOYSA-N 1-Hydroxybenzotriazole Chemical compound C1=CC=C2N(O)N=NC2=C1 ASOKPJOREAFHNY-UHFFFAOYSA-N 0.000 description 1
- ZVSACWYZRVCSEA-UHFFFAOYSA-N 1-[4-(4-acetylphenyl)piperazin-1-yl]-2-[4-chloro-5-methyl-3-(trifluoromethyl)pyrazol-1-yl]ethanone Chemical compound C1=CC(C(=O)C)=CC=C1N1CCN(C(=O)CN2C(=C(Cl)C(=N2)C(F)(F)F)C)CC1 ZVSACWYZRVCSEA-UHFFFAOYSA-N 0.000 description 1
- RWRSAPZIVXZAJU-UHFFFAOYSA-N 1-[4-(4-bromo-3-methoxyphenyl)piperazin-1-yl]-2-[4-chloro-3-phenyl-5-(trifluoromethyl)pyrazol-1-yl]ethanone Chemical compound C1=C(Br)C(OC)=CC(N2CCN(CC2)C(=O)CN2C(=C(Cl)C(=N2)C=2C=CC=CC=2)C(F)(F)F)=C1 RWRSAPZIVXZAJU-UHFFFAOYSA-N 0.000 description 1
- JLOCNTGKYFEQNF-UHFFFAOYSA-N 1-[4-(4-bromo-3-methoxyphenyl)piperazin-1-yl]-2-[4-chloro-5-methyl-3-(trifluoromethyl)pyrazol-1-yl]ethanone Chemical compound C1=C(Br)C(OC)=CC(N2CCN(CC2)C(=O)CN2C(=C(Cl)C(=N2)C(F)(F)F)C)=C1 JLOCNTGKYFEQNF-UHFFFAOYSA-N 0.000 description 1
- KWEFENWUBXZDMP-UHFFFAOYSA-N 1-[4-(4-bromo-3-methoxyphenyl)piperazin-1-yl]-2-[4-chloro-5-phenyl-3-(trifluoromethyl)pyrazol-1-yl]ethanone Chemical compound C1=C(Br)C(OC)=CC(N2CCN(CC2)C(=O)CN2C(=C(Cl)C(=N2)C(F)(F)F)C=2C=CC=CC=2)=C1 KWEFENWUBXZDMP-UHFFFAOYSA-N 0.000 description 1
- RRBMEMJIDRFDPZ-UHFFFAOYSA-N 1-[4-(4-bromo-3-methylphenyl)piperazin-1-yl]-2-[4-chloro-5-methyl-3-(trifluoromethyl)pyrazol-1-yl]ethanone Chemical compound CC1=C(Cl)C(C(F)(F)F)=NN1CC(=O)N1CCN(C=2C=C(C)C(Br)=CC=2)CC1 RRBMEMJIDRFDPZ-UHFFFAOYSA-N 0.000 description 1
- HBCMVDIOORTSMM-UHFFFAOYSA-N 1-[4-(4-bromophenyl)piperazin-1-yl]-2-[4-chloro-5-methyl-3-(trifluoromethyl)pyrazol-1-yl]ethanone Chemical compound CC1=C(Cl)C(C(F)(F)F)=NN1CC(=O)N1CCN(C=2C=CC(Br)=CC=2)CC1 HBCMVDIOORTSMM-UHFFFAOYSA-N 0.000 description 1
- PKYIAEVAYAMUHA-UHFFFAOYSA-N 1-[4-(4-chloro-2-methoxyphenyl)piperazin-1-yl]-2-[4-chloro-5-methyl-3-(trifluoromethyl)pyrazol-1-yl]ethanone Chemical compound COC1=CC(Cl)=CC=C1N1CCN(C(=O)CN2C(=C(Cl)C(=N2)C(F)(F)F)C)CC1 PKYIAEVAYAMUHA-UHFFFAOYSA-N 0.000 description 1
- DERJUQSPGPHSQT-UHFFFAOYSA-N 1-[4-(4-chloro-3-ethoxyphenyl)piperazin-1-yl]-2-[4-chloro-5-methyl-3-(trifluoromethyl)pyrazol-1-yl]ethanone Chemical compound C1=C(Cl)C(OCC)=CC(N2CCN(CC2)C(=O)CN2C(=C(Cl)C(=N2)C(F)(F)F)C)=C1 DERJUQSPGPHSQT-UHFFFAOYSA-N 0.000 description 1
- AMXPBXUAHHADJU-UHFFFAOYSA-N 1-[4-(4-chloro-3-methoxyphenyl)piperazin-1-yl]-2-[4-chloro-3-propan-2-yl-5-(trifluoromethyl)pyrazol-1-yl]ethanone Chemical compound C1=C(Cl)C(OC)=CC(N2CCN(CC2)C(=O)CN2C(=C(Cl)C(C(C)C)=N2)C(F)(F)F)=C1 AMXPBXUAHHADJU-UHFFFAOYSA-N 0.000 description 1
- OIFUSCWLFWSBRL-UHFFFAOYSA-N 1-[4-(4-chloro-3-methoxyphenyl)piperazin-1-yl]-2-[4-chloro-3-propyl-5-(trifluoromethyl)pyrazol-1-yl]ethanone Chemical compound FC(F)(F)C1=C(Cl)C(CCC)=NN1CC(=O)N1CCN(C=2C=C(OC)C(Cl)=CC=2)CC1 OIFUSCWLFWSBRL-UHFFFAOYSA-N 0.000 description 1
- PTHJVFQTMJMDKU-UHFFFAOYSA-N 1-[4-(4-chloro-3-methoxyphenyl)piperazin-1-yl]-2-[4-chloro-5-methyl-3-(trifluoromethyl)pyrazol-1-yl]ethanone Chemical compound C1=C(Cl)C(OC)=CC(N2CCN(CC2)C(=O)CN2C(=C(Cl)C(=N2)C(F)(F)F)C)=C1 PTHJVFQTMJMDKU-UHFFFAOYSA-N 0.000 description 1
- JMGHQLHLSYMMBM-UHFFFAOYSA-N 1-[4-(4-chloro-3-methoxyphenyl)piperazin-1-yl]-2-[4-chloro-5-propan-2-yl-3-(trifluoromethyl)pyrazol-1-yl]ethanone Chemical compound C1=C(Cl)C(OC)=CC(N2CCN(CC2)C(=O)CN2C(=C(Cl)C(=N2)C(F)(F)F)C(C)C)=C1 JMGHQLHLSYMMBM-UHFFFAOYSA-N 0.000 description 1
- JPTGBTQFDGNNAZ-UHFFFAOYSA-N 1-[4-(4-chloro-3-methylphenyl)piperazin-1-yl]-2-[4-chloro-5-methyl-3-(trifluoromethyl)pyrazol-1-yl]ethanone Chemical compound CC1=C(Cl)C(C(F)(F)F)=NN1CC(=O)N1CCN(C=2C=C(C)C(Cl)=CC=2)CC1 JPTGBTQFDGNNAZ-UHFFFAOYSA-N 0.000 description 1
- UNSXRMQCUFKGMT-UHFFFAOYSA-N 1-[4-(4-chlorophenyl)piperazin-1-yl]-2-(2,4-dimethylimidazol-1-yl)ethanone Chemical compound CC1=NC(C)=CN1CC(=O)N1CCN(C=2C=CC(Cl)=CC=2)CC1 UNSXRMQCUFKGMT-UHFFFAOYSA-N 0.000 description 1
- RIHYSNDQDZFBKY-UHFFFAOYSA-N 1-[4-(4-chlorophenyl)piperazin-1-yl]-2-[3-(1,1,2,2,3,3,3-heptafluoropropyl)-5-methyl-4-nitropyrazol-1-yl]ethanone Chemical compound CC1=C([N+]([O-])=O)C(C(F)(F)C(F)(F)C(F)(F)F)=NN1CC(=O)N1CCN(C=2C=CC(Cl)=CC=2)CC1 RIHYSNDQDZFBKY-UHFFFAOYSA-N 0.000 description 1
- MZUHBUYSTLUYDZ-UHFFFAOYSA-N 1-[4-(4-chlorophenyl)piperazin-1-yl]-2-[4-chloro-5-phenyl-3-(trifluoromethyl)pyrazol-1-yl]ethanone Chemical compound C=1C=CC=CC=1C1=C(Cl)C(C(F)(F)F)=NN1CC(=O)N(CC1)CCN1C1=CC=C(Cl)C=C1 MZUHBUYSTLUYDZ-UHFFFAOYSA-N 0.000 description 1
- IEQJROHYVJAVQJ-UHFFFAOYSA-N 1-[4-(4-fluorophenyl)piperazin-1-yl]-2-(3,4,5-tribromopyrazol-1-yl)ethanone Chemical compound C1=CC(F)=CC=C1N1CCN(C(=O)CN2C(=C(Br)C(Br)=N2)Br)CC1 IEQJROHYVJAVQJ-UHFFFAOYSA-N 0.000 description 1
- GKLZFXZRDAEWPC-UHFFFAOYSA-N 1-[4-(4-fluorophenyl)piperazin-1-yl]-2-(3-thiophen-2-ylpyrazol-1-yl)ethanone Chemical compound C1=CC(F)=CC=C1N1CCN(C(=O)CN2N=C(C=C2)C=2SC=CC=2)CC1 GKLZFXZRDAEWPC-UHFFFAOYSA-N 0.000 description 1
- HZHZRUCSCCZSGO-UHFFFAOYSA-N 1-[4-(4-fluorophenyl)piperazin-1-yl]-2-(4-iodo-3,5-dimethylpyrazol-1-yl)ethanone Chemical compound CC1=C(I)C(C)=NN1CC(=O)N1CCN(C=2C=CC(F)=CC=2)CC1 HZHZRUCSCCZSGO-UHFFFAOYSA-N 0.000 description 1
- BDJLGLVEQWFFSR-UHFFFAOYSA-N 1-[4-(4-fluorophenyl)piperazin-1-yl]-2-(5-nitroindazol-1-yl)ethanone Chemical compound N1=CC2=CC([N+](=O)[O-])=CC=C2N1CC(=O)N(CC1)CCN1C1=CC=C(F)C=C1 BDJLGLVEQWFFSR-UHFFFAOYSA-N 0.000 description 1
- ZOTBMIGHPNCNSB-UHFFFAOYSA-N 1-[4-(4-fluorophenyl)piperazin-1-yl]-2-(7-nitroindazol-1-yl)ethanone Chemical compound C1=2C([N+](=O)[O-])=CC=CC=2C=NN1CC(=O)N(CC1)CCN1C1=CC=C(F)C=C1 ZOTBMIGHPNCNSB-UHFFFAOYSA-N 0.000 description 1
- GMDHCUXCNVVKTA-UHFFFAOYSA-N 1-[4-(4-fluorophenyl)piperazin-1-yl]-2-[3-(1,1,2,2,3,3,3-heptafluoropropyl)-5-methyl-4-nitropyrazol-1-yl]ethanone Chemical compound CC1=C([N+]([O-])=O)C(C(F)(F)C(F)(F)C(F)(F)F)=NN1CC(=O)N1CCN(C=2C=CC(F)=CC=2)CC1 GMDHCUXCNVVKTA-UHFFFAOYSA-N 0.000 description 1
- JKYLHCCISUQFGT-UHFFFAOYSA-N 1-[4-(4-fluorophenyl)piperazin-1-yl]-2-pyrazol-1-ylethanone Chemical compound C1=CC(F)=CC=C1N1CCN(C(=O)CN2N=CC=C2)CC1 JKYLHCCISUQFGT-UHFFFAOYSA-N 0.000 description 1
- DDKKYGOXXAWEIT-UHFFFAOYSA-N 1-[[4-chloro-2-[2-[4-(4-chlorophenyl)piperazin-1-yl]-2-oxoethyl]-5-(trifluoromethyl)pyrazol-3-yl]methyl]-3-ethylurea Chemical compound CCNC(=O)NCC1=C(Cl)C(C(F)(F)F)=NN1CC(=O)N1CCN(C=2C=CC(Cl)=CC=2)CC1 DDKKYGOXXAWEIT-UHFFFAOYSA-N 0.000 description 1
- JGVAKMUCOOQHES-UHFFFAOYSA-N 1-[[4-chloro-2-[2-[4-(4-chlorophenyl)piperazin-1-yl]-2-oxoethyl]-5-(trifluoromethyl)pyrazol-3-yl]methyl]-3-methylurea Chemical compound CNC(=O)NCC1=C(Cl)C(C(F)(F)F)=NN1CC(=O)N1CCN(C=2C=CC(Cl)=CC=2)CC1 JGVAKMUCOOQHES-UHFFFAOYSA-N 0.000 description 1
- ISHYFWKKWKXXPL-UHFFFAOYSA-N 1-bromo-2,4-dichlorobenzene Chemical compound ClC1=CC=C(Br)C(Cl)=C1 ISHYFWKKWKXXPL-UHFFFAOYSA-N 0.000 description 1
- SEAOBYFQWJFORM-UHFFFAOYSA-N 1-bromo-4-(trifluoromethoxy)benzene Chemical compound FC(F)(F)OC1=CC=C(Br)C=C1 SEAOBYFQWJFORM-UHFFFAOYSA-N 0.000 description 1
- FJLFSYRGFJDJMQ-UHFFFAOYSA-N 1-bromo-4-methylsulfonylbenzene Chemical compound CS(=O)(=O)C1=CC=C(Br)C=C1 FJLFSYRGFJDJMQ-UHFFFAOYSA-N 0.000 description 1
- GWQSENYKCGJTRI-UHFFFAOYSA-N 1-chloro-4-iodobenzene Chemical compound ClC1=CC=C(I)C=C1 GWQSENYKCGJTRI-UHFFFAOYSA-N 0.000 description 1
- PTRUTZFCVFUTMW-UHFFFAOYSA-N 1-ethynyl-3-fluorobenzene Chemical compound FC1=CC=CC(C#C)=C1 PTRUTZFCVFUTMW-UHFFFAOYSA-N 0.000 description 1
- CGHIBGNXEGJPQZ-UHFFFAOYSA-N 1-hexyne Chemical compound CCCCC#C CGHIBGNXEGJPQZ-UHFFFAOYSA-N 0.000 description 1
- IXPNQXFRVYWDDI-UHFFFAOYSA-N 1-methyl-2,4-dioxo-1,3-diazinane-5-carboximidamide Chemical compound CN1CC(C(N)=N)C(=O)NC1=O IXPNQXFRVYWDDI-UHFFFAOYSA-N 0.000 description 1
- IBXNCJKFFQIKKY-UHFFFAOYSA-N 1-pentyne Chemical compound CCCC#C IBXNCJKFFQIKKY-UHFFFAOYSA-N 0.000 description 1
- CFJMRBQWBDQYMK-UHFFFAOYSA-N 1-phenyl-1-cyclopentanecarboxylic acid 2-[2-(diethylamino)ethoxy]ethyl ester Chemical compound C=1C=CC=CC=1C1(C(=O)OCCOCCN(CC)CC)CCCC1 CFJMRBQWBDQYMK-UHFFFAOYSA-N 0.000 description 1
- 125000004214 1-pyrrolidinyl group Chemical group [H]C1([H])N(*)C([H])([H])C([H])([H])C1([H])[H] 0.000 description 1
- 125000001462 1-pyrrolyl group Chemical group [*]N1C([H])=C([H])C([H])=C1[H] 0.000 description 1
- HYZJCKYKOHLVJF-UHFFFAOYSA-N 1H-benzimidazole Chemical compound C1=CC=C2NC=NC2=C1 HYZJCKYKOHLVJF-UHFFFAOYSA-N 0.000 description 1
- JVVRJMXHNUAPHW-UHFFFAOYSA-N 1h-pyrazol-5-amine Chemical class NC=1C=CNN=1 JVVRJMXHNUAPHW-UHFFFAOYSA-N 0.000 description 1
- 125000004206 2,2,2-trifluoroethyl group Chemical group [H]C([H])(*)C(F)(F)F 0.000 description 1
- KLIVRBFRQSOGQI-UHFFFAOYSA-N 2-(11-oxo-6h-benzo[c][1]benzothiepin-3-yl)acetic acid Chemical compound S1CC2=CC=CC=C2C(=O)C2=CC=C(CC(=O)O)C=C12 KLIVRBFRQSOGQI-UHFFFAOYSA-N 0.000 description 1
- NTVKSUPEUFXUGS-UHFFFAOYSA-N 2-(1h-pyrazol-5-yl)acetic acid Chemical class OC(=O)CC=1C=CNN=1 NTVKSUPEUFXUGS-UHFFFAOYSA-N 0.000 description 1
- MYQXHLQMZLTSDB-UHFFFAOYSA-N 2-(2-ethyl-2,3-dihydro-1-benzofuran-5-yl)acetic acid Chemical compound OC(=O)CC1=CC=C2OC(CC)CC2=C1 MYQXHLQMZLTSDB-UHFFFAOYSA-N 0.000 description 1
- KBVHXYDAYZDUML-UHFFFAOYSA-N 2-(2-oxo-1,3-oxazolidin-3-yl)ethyl 4-methylbenzenesulfonate Chemical compound C1=CC(C)=CC=C1S(=O)(=O)OCCN1C(=O)OCC1 KBVHXYDAYZDUML-UHFFFAOYSA-N 0.000 description 1
- DOHQVQXMPSXYGK-UHFFFAOYSA-N 2-(3-amino-4-bromo-5-methylpyrazol-1-yl)-1-[4-(4-fluorophenyl)piperazin-1-yl]ethanone Chemical compound CC1=C(Br)C(N)=NN1CC(=O)N1CCN(C=2C=CC(F)=CC=2)CC1 DOHQVQXMPSXYGK-UHFFFAOYSA-N 0.000 description 1
- HCHGCWMFYUPCBN-UHFFFAOYSA-N 2-(3-amino-4-bromo-5-phenylpyrazol-1-yl)-1-[4-(4-bromo-3-methoxyphenyl)piperazin-1-yl]ethanone Chemical compound C1=C(Br)C(OC)=CC(N2CCN(CC2)C(=O)CN2C(=C(Br)C(N)=N2)C=2C=CC=CC=2)=C1 HCHGCWMFYUPCBN-UHFFFAOYSA-N 0.000 description 1
- FSPKJDXLHHQPKS-UHFFFAOYSA-N 2-(3-amino-4-bromo-5-phenylpyrazol-1-yl)-1-[4-(4-chloro-3-methoxyphenyl)piperazin-1-yl]ethanone Chemical compound C1=C(Cl)C(OC)=CC(N2CCN(CC2)C(=O)CN2C(=C(Br)C(N)=N2)C=2C=CC=CC=2)=C1 FSPKJDXLHHQPKS-UHFFFAOYSA-N 0.000 description 1
- FWOPLEYCTNYZMD-UHFFFAOYSA-N 2-(3-amino-4-bromo-5-phenylpyrazol-1-yl)-1-[4-(4-chlorophenyl)piperazin-1-yl]ethanone Chemical compound C=1C=CC=CC=1C1=C(Br)C(N)=NN1CC(=O)N(CC1)CCN1C1=CC=C(Cl)C=C1 FWOPLEYCTNYZMD-UHFFFAOYSA-N 0.000 description 1
- XYEQVQHUDLONHA-UHFFFAOYSA-N 2-(3-amino-4-bromo-5-phenylpyrazol-1-yl)-1-[4-(4-fluorophenyl)piperazin-1-yl]ethanone Chemical compound C=1C=CC=CC=1C1=C(Br)C(N)=NN1CC(=O)N(CC1)CCN1C1=CC=C(F)C=C1 XYEQVQHUDLONHA-UHFFFAOYSA-N 0.000 description 1
- VGXBFQKKHHKYHN-UHFFFAOYSA-N 2-(3-amino-4-chloro-5-methylpyrazol-1-yl)-1-[4-(4-chloro-3-methoxyphenyl)piperazin-1-yl]ethanone Chemical compound C1=C(Cl)C(OC)=CC(N2CCN(CC2)C(=O)CN2C(=C(Cl)C(N)=N2)C)=C1 VGXBFQKKHHKYHN-UHFFFAOYSA-N 0.000 description 1
- DZKHLYCEVUUCRY-UHFFFAOYSA-N 2-(3-amino-4-chloro-5-methylpyrazol-1-yl)-1-[4-(4-chlorophenyl)piperazin-1-yl]ethanone Chemical compound CC1=C(Cl)C(N)=NN1CC(=O)N1CCN(C=2C=CC(Cl)=CC=2)CC1 DZKHLYCEVUUCRY-UHFFFAOYSA-N 0.000 description 1
- ADPJCUBOMJEHPR-UHFFFAOYSA-N 2-(3-amino-4-chloro-5-methylpyrazol-1-yl)-1-[4-(4-fluorophenyl)piperazin-1-yl]ethanone Chemical compound CC1=C(Cl)C(N)=NN1CC(=O)N1CCN(C=2C=CC(F)=CC=2)CC1 ADPJCUBOMJEHPR-UHFFFAOYSA-N 0.000 description 1
- AAZXGAWHAXIRJH-UHFFFAOYSA-N 2-(3-amino-4-tert-butylpyrazol-1-yl)-1-[4-(4-fluorophenyl)piperazin-1-yl]ethanone Chemical compound N1=C(N)C(C(C)(C)C)=CN1CC(=O)N1CCN(C=2C=CC(F)=CC=2)CC1 AAZXGAWHAXIRJH-UHFFFAOYSA-N 0.000 description 1
- GVRHDNGHLXYHEF-UHFFFAOYSA-N 2-(3-chloro-1$l^{3},2-iodazol-1-yl)-1-[4-(4-fluorophenyl)piperazin-1-yl]ethanone Chemical compound C1=CC(F)=CC=C1N1CCN(C(=O)CI2N=C(Cl)C=C2)CC1 GVRHDNGHLXYHEF-UHFFFAOYSA-N 0.000 description 1
- AFCMTYPBGJLTJB-UHFFFAOYSA-N 2-(4-bromo-3,5-dimethylpyrazol-1-yl)-1-[4-(4-fluorophenyl)piperazin-1-yl]ethanone Chemical compound CC1=C(Br)C(C)=NN1CC(=O)N1CCN(C=2C=CC(F)=CC=2)CC1 AFCMTYPBGJLTJB-UHFFFAOYSA-N 0.000 description 1
- WFDHAUDJYMBJTJ-UHFFFAOYSA-N 2-(benzimidazol-1-yl)-1-[4-(4-chlorophenyl)piperazin-1-yl]ethanone Chemical compound C1=CC(Cl)=CC=C1N1CCN(C(=O)CN2C3=CC=CC=C3N=C2)CC1 WFDHAUDJYMBJTJ-UHFFFAOYSA-N 0.000 description 1
- DCXHLPGLBYHNMU-UHFFFAOYSA-N 2-[1-(4-azidobenzoyl)-5-methoxy-2-methylindol-3-yl]acetic acid Chemical compound CC1=C(CC(O)=O)C2=CC(OC)=CC=C2N1C(=O)C1=CC=C(N=[N+]=[N-])C=C1 DCXHLPGLBYHNMU-UHFFFAOYSA-N 0.000 description 1
- APBSKHYXXKHJFK-UHFFFAOYSA-N 2-[2-(4-chlorophenyl)-1,3-thiazol-4-yl]acetic acid Chemical compound OC(=O)CC1=CSC(C=2C=CC(Cl)=CC=2)=N1 APBSKHYXXKHJFK-UHFFFAOYSA-N 0.000 description 1
- DITZWNURMIJXIF-UHFFFAOYSA-N 2-[3,5-bis(trifluoromethyl)pyrazol-1-yl]-1-[4-(4-fluorophenyl)piperazin-1-yl]ethanone Chemical compound C1=CC(F)=CC=C1N1CCN(C(=O)CN2C(=CC(=N2)C(F)(F)F)C(F)(F)F)CC1 DITZWNURMIJXIF-UHFFFAOYSA-N 0.000 description 1
- KMCVGYBEDGKOCE-UHFFFAOYSA-N 2-[3,5-di(propan-2-yl)pyrazol-1-yl]-1-[4-(4-fluorophenyl)piperazin-1-yl]ethanone Chemical compound N1=C(C(C)C)C=C(C(C)C)N1CC(=O)N1CCN(C=2C=CC(F)=CC=2)CC1 KMCVGYBEDGKOCE-UHFFFAOYSA-N 0.000 description 1
- ITTJHRNTLWBVRO-UHFFFAOYSA-N 2-[3-(4-fluorophenyl)-5-methylsulfanylpyrazol-1-yl]-1-[4-(4-fluorophenyl)piperazin-1-yl]ethanone Chemical compound CSC1=CC(C=2C=CC(F)=CC=2)=NN1CC(=O)N(CC1)CCN1C1=CC=C(F)C=C1 ITTJHRNTLWBVRO-UHFFFAOYSA-N 0.000 description 1
- CBOIJRXCKKKWEN-UHFFFAOYSA-N 2-[3-tert-butyl-4-chloro-5-(trifluoromethyl)pyrazol-1-yl]-1-[4-(4-fluorophenyl)piperazin-1-yl]ethanone Chemical compound FC(F)(F)C1=C(Cl)C(C(C)(C)C)=NN1CC(=O)N1CCN(C=2C=CC(F)=CC=2)CC1 CBOIJRXCKKKWEN-UHFFFAOYSA-N 0.000 description 1
- FBLONUHDENRCBU-UHFFFAOYSA-N 2-[3-tert-butyl-5-(trifluoromethyl)pyrazol-1-yl]-1-[4-(4-fluorophenyl)piperazin-1-yl]ethanone Chemical compound N1=C(C(C)(C)C)C=C(C(F)(F)F)N1CC(=O)N1CCN(C=2C=CC(F)=CC=2)CC1 FBLONUHDENRCBU-UHFFFAOYSA-N 0.000 description 1
- TYCOFFBAZNSQOJ-UHFFFAOYSA-N 2-[4-(3-fluorophenyl)phenyl]propanoic acid Chemical compound C1=CC(C(C(O)=O)C)=CC=C1C1=CC=CC(F)=C1 TYCOFFBAZNSQOJ-UHFFFAOYSA-N 0.000 description 1
- JIEKMACRVQTPRC-UHFFFAOYSA-N 2-[4-(4-chlorophenyl)-2-phenyl-5-thiazolyl]acetic acid Chemical compound OC(=O)CC=1SC(C=2C=CC=CC=2)=NC=1C1=CC=C(Cl)C=C1 JIEKMACRVQTPRC-UHFFFAOYSA-N 0.000 description 1
- YPFWPULCFVMIIJ-UHFFFAOYSA-N 2-[4-amino-3-(1,1,2,2,3,3,3-heptafluoropropyl)-5-methylpyrazol-1-yl]-1-[4-(4-fluorophenyl)piperazin-1-yl]ethanone Chemical compound CC1=C(N)C(C(F)(F)C(F)(F)C(F)(F)F)=NN1CC(=O)N1CCN(C=2C=CC(F)=CC=2)CC1 YPFWPULCFVMIIJ-UHFFFAOYSA-N 0.000 description 1
- ZMGUGNRUWFSIBM-UHFFFAOYSA-N 2-[4-bromo-3-phenyl-5-(trifluoromethyl)pyrazol-1-yl]-1-[4-(4-chloro-3-methoxyphenyl)piperazin-1-yl]ethanone Chemical compound C1=C(Cl)C(OC)=CC(N2CCN(CC2)C(=O)CN2C(=C(Br)C(=N2)C=2C=CC=CC=2)C(F)(F)F)=C1 ZMGUGNRUWFSIBM-UHFFFAOYSA-N 0.000 description 1
- VGCZVUUARDNYBH-UHFFFAOYSA-N 2-[4-bromo-5-methyl-3-(trifluoromethyl)pyrazol-1-yl]-1-[4-(4-chlorophenyl)piperazin-1-yl]ethanone Chemical compound CC1=C(Br)C(C(F)(F)F)=NN1CC(=O)N1CCN(C=2C=CC(Cl)=CC=2)CC1 VGCZVUUARDNYBH-UHFFFAOYSA-N 0.000 description 1
- KMUQMLXKTNGQED-UHFFFAOYSA-N 2-[4-bromo-5-methyl-3-(trifluoromethyl)pyrazol-1-yl]-1-[4-(4-fluorophenyl)piperazin-1-yl]ethanone Chemical compound CC1=C(Br)C(C(F)(F)F)=NN1CC(=O)N1CCN(C=2C=CC(F)=CC=2)CC1 KMUQMLXKTNGQED-UHFFFAOYSA-N 0.000 description 1
- PYJVMJRAFYMHER-UHFFFAOYSA-N 2-[4-chloro-3,5-di(propan-2-yl)pyrazol-1-yl]-1-[4-(4-fluorophenyl)piperazin-1-yl]ethanone Chemical compound CC(C)C1=C(Cl)C(C(C)C)=NN1CC(=O)N1CCN(C=2C=CC(F)=CC=2)CC1 PYJVMJRAFYMHER-UHFFFAOYSA-N 0.000 description 1
- PYAIEGJMALUWBL-UHFFFAOYSA-N 2-[4-chloro-3-(3-fluorophenyl)-5-(trifluoromethyl)pyrazol-1-yl]-1-[4-(4-chloro-3-methoxyphenyl)piperazin-1-yl]ethanone Chemical compound C1=C(Cl)C(OC)=CC(N2CCN(CC2)C(=O)CN2C(=C(Cl)C(=N2)C=2C=C(F)C=CC=2)C(F)(F)F)=C1 PYAIEGJMALUWBL-UHFFFAOYSA-N 0.000 description 1
- BBYGJBFHXDQMFI-UHFFFAOYSA-N 2-[4-chloro-3-(trifluoromethyl)pyrazol-1-yl]-1-[4-(4-fluorophenyl)piperazin-1-yl]ethanone Chemical compound C1=CC(F)=CC=C1N1CCN(C(=O)CN2N=C(C(Cl)=C2)C(F)(F)F)CC1 BBYGJBFHXDQMFI-UHFFFAOYSA-N 0.000 description 1
- HWXMVZWZQPXOBK-UHFFFAOYSA-N 2-[4-chloro-3-methyl-5-(trifluoromethyl)pyrazol-1-yl]-1-[4-(3-methylsulfanylphenyl)piperazin-1-yl]ethanone Chemical compound CSC1=CC=CC(N2CCN(CC2)C(=O)CN2C(=C(Cl)C(C)=N2)C(F)(F)F)=C1 HWXMVZWZQPXOBK-UHFFFAOYSA-N 0.000 description 1
- OWFVVQDLIKJEAO-UHFFFAOYSA-N 2-[4-chloro-3-phenyl-5-(trifluoromethyl)pyrazol-1-yl]-1-[1-(4-fluorophenyl)piperazin-2-yl]ethanone Chemical compound C1=CC(F)=CC=C1N1C(C(=O)CN2C(=C(Cl)C(=N2)C=2C=CC=CC=2)C(F)(F)F)CNCC1 OWFVVQDLIKJEAO-UHFFFAOYSA-N 0.000 description 1
- QHPNSSZJBXLMHB-UHFFFAOYSA-N 2-[4-chloro-5-(3-fluorophenyl)-3-(trifluoromethyl)pyrazol-1-yl]-1-[4-(4-chloro-3-methoxyphenyl)piperazin-1-yl]ethanone Chemical compound C1=C(Cl)C(OC)=CC(N2CCN(CC2)C(=O)CN2C(=C(Cl)C(=N2)C(F)(F)F)C=2C=C(F)C=CC=2)=C1 QHPNSSZJBXLMHB-UHFFFAOYSA-N 0.000 description 1
- RTEDZJQJFBHCBY-UHFFFAOYSA-N 2-[4-chloro-5-(4-fluorophenyl)-3-methylsulfanylpyrazol-1-yl]-1-[4-(4-fluorophenyl)piperazin-1-yl]ethanone Chemical compound C=1C=C(F)C=CC=1C1=C(Cl)C(SC)=NN1CC(=O)N(CC1)CCN1C1=CC=C(F)C=C1 RTEDZJQJFBHCBY-UHFFFAOYSA-N 0.000 description 1
- BGKOLGPDHIFFMC-UHFFFAOYSA-N 2-[4-chloro-5-ethyl-3-(trifluoromethyl)pyrazol-1-yl]-1-[4-(4-chloro-3-methoxyphenyl)piperazin-1-yl]ethanone Chemical compound CCC1=C(Cl)C(C(F)(F)F)=NN1CC(=O)N1CCN(C=2C=C(OC)C(Cl)=CC=2)CC1 BGKOLGPDHIFFMC-UHFFFAOYSA-N 0.000 description 1
- UDHKVYGWTIEFSC-LLVKDONJSA-N 2-[4-chloro-5-methyl-3-(trifluoromethyl)pyrazol-1-yl]-1-[(2r)-4-(4-chlorophenyl)-2-methylpiperazin-1-yl]ethanone Chemical compound C([C@H]1C)N(C=2C=CC(Cl)=CC=2)CCN1C(=O)CN1N=C(C(F)(F)F)C(Cl)=C1C UDHKVYGWTIEFSC-LLVKDONJSA-N 0.000 description 1
- UDHKVYGWTIEFSC-NSHDSACASA-N 2-[4-chloro-5-methyl-3-(trifluoromethyl)pyrazol-1-yl]-1-[(2s)-4-(4-chlorophenyl)-2-methylpiperazin-1-yl]ethanone Chemical compound C([C@@H]1C)N(C=2C=CC(Cl)=CC=2)CCN1C(=O)CN1N=C(C(F)(F)F)C(Cl)=C1C UDHKVYGWTIEFSC-NSHDSACASA-N 0.000 description 1
- OGOZNEJTEPWQMS-UHFFFAOYSA-N 2-[4-chloro-5-methyl-3-(trifluoromethyl)pyrazol-1-yl]-1-[2-(4-methylsulfonylphenyl)piperazin-1-yl]ethanone Chemical compound CC1=C(Cl)C(C(F)(F)F)=NN1CC(=O)N1C(C=2C=CC(=CC=2)S(C)(=O)=O)CNCC1 OGOZNEJTEPWQMS-UHFFFAOYSA-N 0.000 description 1
- SCGOCPFKFDQTOW-UHFFFAOYSA-N 2-[4-chloro-5-methyl-3-(trifluoromethyl)pyrazol-1-yl]-1-[3-methyl-4-(3-methylphenyl)piperazin-1-yl]ethanone Chemical compound CC1CN(C(=O)CN2C(=C(Cl)C(=N2)C(F)(F)F)C)CCN1C1=CC=CC(C)=C1 SCGOCPFKFDQTOW-UHFFFAOYSA-N 0.000 description 1
- JZDPMUOZNGLVNA-UHFFFAOYSA-N 2-[4-chloro-5-methyl-3-(trifluoromethyl)pyrazol-1-yl]-1-[4-(2,3-dimethylphenyl)piperazin-1-yl]ethanone Chemical compound CC1=C(Cl)C(C(F)(F)F)=NN1CC(=O)N1CCN(C=2C(=C(C)C=CC=2)C)CC1 JZDPMUOZNGLVNA-UHFFFAOYSA-N 0.000 description 1
- CXFISVNDRPEDHF-UHFFFAOYSA-N 2-[4-chloro-5-methyl-3-(trifluoromethyl)pyrazol-1-yl]-1-[4-(2,4-dichloro-5-methoxyphenyl)piperazin-1-yl]ethanone Chemical compound C1=C(Cl)C(OC)=CC(N2CCN(CC2)C(=O)CN2C(=C(Cl)C(=N2)C(F)(F)F)C)=C1Cl CXFISVNDRPEDHF-UHFFFAOYSA-N 0.000 description 1
- ZFNGCLARDBMAQV-UHFFFAOYSA-N 2-[4-chloro-5-methyl-3-(trifluoromethyl)pyrazol-1-yl]-1-[4-(2,4-dichlorophenyl)piperazin-1-yl]ethanone Chemical compound CC1=C(Cl)C(C(F)(F)F)=NN1CC(=O)N1CCN(C=2C(=CC(Cl)=CC=2)Cl)CC1 ZFNGCLARDBMAQV-UHFFFAOYSA-N 0.000 description 1
- UFRXFVKTDOXMOV-UHFFFAOYSA-N 2-[4-chloro-5-methyl-3-(trifluoromethyl)pyrazol-1-yl]-1-[4-(2,5-dimethylphenyl)piperazin-1-yl]ethanone Chemical compound CC1=C(Cl)C(C(F)(F)F)=NN1CC(=O)N1CCN(C=2C(=CC=C(C)C=2)C)CC1 UFRXFVKTDOXMOV-UHFFFAOYSA-N 0.000 description 1
- CHBOSKUCTRWENM-UHFFFAOYSA-N 2-[4-chloro-5-methyl-3-(trifluoromethyl)pyrazol-1-yl]-1-[4-(2-fluorophenyl)piperazin-1-yl]ethanone Chemical compound CC1=C(Cl)C(C(F)(F)F)=NN1CC(=O)N1CCN(C=2C(=CC=CC=2)F)CC1 CHBOSKUCTRWENM-UHFFFAOYSA-N 0.000 description 1
- NBXUBUNVRYUBLL-UHFFFAOYSA-N 2-[4-chloro-5-methyl-3-(trifluoromethyl)pyrazol-1-yl]-1-[4-(2-methoxyphenyl)piperazin-1-yl]ethanone Chemical compound COC1=CC=CC=C1N1CCN(C(=O)CN2C(=C(Cl)C(=N2)C(F)(F)F)C)CC1 NBXUBUNVRYUBLL-UHFFFAOYSA-N 0.000 description 1
- CCVNRSVXHKPGKN-UHFFFAOYSA-N 2-[4-chloro-5-methyl-3-(trifluoromethyl)pyrazol-1-yl]-1-[4-(2-methylphenyl)piperazin-1-yl]ethanone Chemical compound CC1=C(Cl)C(C(F)(F)F)=NN1CC(=O)N1CCN(C=2C(=CC=CC=2)C)CC1 CCVNRSVXHKPGKN-UHFFFAOYSA-N 0.000 description 1
- JTBVPOPERWZEFF-UHFFFAOYSA-N 2-[4-chloro-5-methyl-3-(trifluoromethyl)pyrazol-1-yl]-1-[4-(3,4-dichlorophenyl)piperazin-1-yl]ethanone Chemical compound CC1=C(Cl)C(C(F)(F)F)=NN1CC(=O)N1CCN(C=2C=C(Cl)C(Cl)=CC=2)CC1 JTBVPOPERWZEFF-UHFFFAOYSA-N 0.000 description 1
- LNHPFCCYGSZDMD-UHFFFAOYSA-N 2-[4-chloro-5-methyl-3-(trifluoromethyl)pyrazol-1-yl]-1-[4-(3,4-difluorophenyl)piperazin-1-yl]ethanone Chemical compound CC1=C(Cl)C(C(F)(F)F)=NN1CC(=O)N1CCN(C=2C=C(F)C(F)=CC=2)CC1 LNHPFCCYGSZDMD-UHFFFAOYSA-N 0.000 description 1
- WRYBEGXGLJXICP-UHFFFAOYSA-N 2-[4-chloro-5-methyl-3-(trifluoromethyl)pyrazol-1-yl]-1-[4-(3,4-dimethylphenyl)piperazin-1-yl]ethanone Chemical compound CC1=C(Cl)C(C(F)(F)F)=NN1CC(=O)N1CCN(C=2C=C(C)C(C)=CC=2)CC1 WRYBEGXGLJXICP-UHFFFAOYSA-N 0.000 description 1
- ZOUBLBCOBIKVFR-UHFFFAOYSA-N 2-[4-chloro-5-methyl-3-(trifluoromethyl)pyrazol-1-yl]-1-[4-(3-chlorophenyl)piperazin-1-yl]ethanone Chemical compound CC1=C(Cl)C(C(F)(F)F)=NN1CC(=O)N1CCN(C=2C=C(Cl)C=CC=2)CC1 ZOUBLBCOBIKVFR-UHFFFAOYSA-N 0.000 description 1
- UKTRVPJKUCEQTM-UHFFFAOYSA-N 2-[4-chloro-5-methyl-3-(trifluoromethyl)pyrazol-1-yl]-1-[4-(3-hydroxyphenyl)piperazin-1-yl]ethanone Chemical compound CC1=C(Cl)C(C(F)(F)F)=NN1CC(=O)N1CCN(C=2C=C(O)C=CC=2)CC1 UKTRVPJKUCEQTM-UHFFFAOYSA-N 0.000 description 1
- GJORGSVJQAGZKK-UHFFFAOYSA-N 2-[4-chloro-5-methyl-3-(trifluoromethyl)pyrazol-1-yl]-1-[4-(3-methoxyphenyl)piperazin-1-yl]ethanone Chemical compound COC1=CC=CC(N2CCN(CC2)C(=O)CN2C(=C(Cl)C(=N2)C(F)(F)F)C)=C1 GJORGSVJQAGZKK-UHFFFAOYSA-N 0.000 description 1
- WNQNOAKGEJFHNI-UHFFFAOYSA-N 2-[4-chloro-5-methyl-3-(trifluoromethyl)pyrazol-1-yl]-1-[4-(3-methylphenyl)piperazin-1-yl]ethanone Chemical compound CC1=C(Cl)C(C(F)(F)F)=NN1CC(=O)N1CCN(C=2C=C(C)C=CC=2)CC1 WNQNOAKGEJFHNI-UHFFFAOYSA-N 0.000 description 1
- FOCDXZMZPHBJOJ-UHFFFAOYSA-N 2-[4-chloro-5-methyl-3-(trifluoromethyl)pyrazol-1-yl]-1-[4-(4-chloro-3-propan-2-yloxyphenyl)piperazin-1-yl]ethanone Chemical compound C1=C(Cl)C(OC(C)C)=CC(N2CCN(CC2)C(=O)CN2C(=C(Cl)C(=N2)C(F)(F)F)C)=C1 FOCDXZMZPHBJOJ-UHFFFAOYSA-N 0.000 description 1
- UZEMDMOUTZGRHO-UHFFFAOYSA-N 2-[4-chloro-5-methyl-3-(trifluoromethyl)pyrazol-1-yl]-1-[4-(4-chlorophenyl)piperazin-1-yl]-2-phenylethanone Chemical compound CC1=C(Cl)C(C(F)(F)F)=NN1C(C=1C=CC=CC=1)C(=O)N1CCN(C=2C=CC(Cl)=CC=2)CC1 UZEMDMOUTZGRHO-UHFFFAOYSA-N 0.000 description 1
- PLVKJOZLUYCUSM-UHFFFAOYSA-N 2-[4-chloro-5-methyl-3-(trifluoromethyl)pyrazol-1-yl]-1-[4-(4-chlorophenyl)piperazin-1-yl]ethanone Chemical compound CC1=C(Cl)C(C(F)(F)F)=NN1CC(=O)N1CCN(C=2C=CC(Cl)=CC=2)CC1 PLVKJOZLUYCUSM-UHFFFAOYSA-N 0.000 description 1
- ZUASHDNIWCQJBE-UHFFFAOYSA-N 2-[4-chloro-5-methyl-3-(trifluoromethyl)pyrazol-1-yl]-1-[4-(4-fluorophenyl)piperazin-1-yl]propan-1-one Chemical compound N1=C(C(F)(F)F)C(Cl)=C(C)N1C(C)C(=O)N(CC1)CCN1C1=CC=C(F)C=C1 ZUASHDNIWCQJBE-UHFFFAOYSA-N 0.000 description 1
- CYBYHKCWHVLMLF-UHFFFAOYSA-N 2-[4-chloro-5-methyl-3-(trifluoromethyl)pyrazol-1-yl]-1-[4-(4-methoxyphenyl)piperazin-1-yl]ethanone Chemical compound C1=CC(OC)=CC=C1N1CCN(C(=O)CN2C(=C(Cl)C(=N2)C(F)(F)F)C)CC1 CYBYHKCWHVLMLF-UHFFFAOYSA-N 0.000 description 1
- QSBXVVOFEOVNCF-UHFFFAOYSA-N 2-[4-chloro-5-methyl-3-(trifluoromethyl)pyrazol-1-yl]-1-[4-(4-methoxypyridin-2-yl)piperazin-1-yl]ethanone Chemical compound COC1=CC=NC(N2CCN(CC2)C(=O)CN2C(=C(Cl)C(=N2)C(F)(F)F)C)=C1 QSBXVVOFEOVNCF-UHFFFAOYSA-N 0.000 description 1
- GPHYWGCAVGTJJE-UHFFFAOYSA-N 2-[4-chloro-5-methyl-3-(trifluoromethyl)pyrazol-1-yl]-1-[4-(4-methylphenyl)piperazin-1-yl]ethanone Chemical compound CC1=C(Cl)C(C(F)(F)F)=NN1CC(=O)N1CCN(C=2C=CC(C)=CC=2)CC1 GPHYWGCAVGTJJE-UHFFFAOYSA-N 0.000 description 1
- XYVPPGYWYCFKHX-UHFFFAOYSA-N 2-[4-chloro-5-methyl-3-(trifluoromethyl)pyrazol-1-yl]-1-[4-(4-nitrophenyl)piperazin-1-yl]ethanone Chemical compound CC1=C(Cl)C(C(F)(F)F)=NN1CC(=O)N1CCN(C=2C=CC(=CC=2)[N+]([O-])=O)CC1 XYVPPGYWYCFKHX-UHFFFAOYSA-N 0.000 description 1
- DHLYQYZVOHKNQP-UHFFFAOYSA-N 2-[4-chloro-5-methyl-3-(trifluoromethyl)pyrazol-1-yl]-1-[4-(5-fluoro-2-methoxyphenyl)piperazin-1-yl]ethanone Chemical compound COC1=CC=C(F)C=C1N1CCN(C(=O)CN2C(=C(Cl)C(=N2)C(F)(F)F)C)CC1 DHLYQYZVOHKNQP-UHFFFAOYSA-N 0.000 description 1
- JFOOIUAISBCDNT-UHFFFAOYSA-N 2-[4-chloro-5-methyl-3-(trifluoromethyl)pyrazol-1-yl]-1-[4-(5-methoxy-2-methylphenyl)piperazin-1-yl]ethanone Chemical compound COC1=CC=C(C)C(N2CCN(CC2)C(=O)CN2C(=C(Cl)C(=N2)C(F)(F)F)C)=C1 JFOOIUAISBCDNT-UHFFFAOYSA-N 0.000 description 1
- BAFCBMQTXHTUTP-UHFFFAOYSA-N 2-[4-chloro-5-methyl-3-(trifluoromethyl)pyrazol-1-yl]-1-[4-[3-(trifluoromethyl)phenyl]piperazin-1-yl]ethanone Chemical compound CC1=C(Cl)C(C(F)(F)F)=NN1CC(=O)N1CCN(C=2C=C(C=CC=2)C(F)(F)F)CC1 BAFCBMQTXHTUTP-UHFFFAOYSA-N 0.000 description 1
- QZRPAXNUDLMKJE-UHFFFAOYSA-N 2-[4-chloro-5-methyl-3-(trifluoromethyl)pyrazol-1-yl]-1-[4-[4-(trifluoromethoxy)phenyl]piperazin-1-yl]ethanone Chemical compound CC1=C(Cl)C(C(F)(F)F)=NN1CC(=O)N1CCN(C=2C=CC(OC(F)(F)F)=CC=2)CC1 QZRPAXNUDLMKJE-UHFFFAOYSA-N 0.000 description 1
- OQDNRWTXJVMQRZ-UHFFFAOYSA-N 2-[4-chloro-5-methyl-3-(trifluoromethyl)pyrazol-1-yl]-1-[4-[4-(trifluoromethyl)phenyl]piperazin-1-yl]ethanone Chemical compound CC1=C(Cl)C(C(F)(F)F)=NN1CC(=O)N1CCN(C=2C=CC(=CC=2)C(F)(F)F)CC1 OQDNRWTXJVMQRZ-UHFFFAOYSA-N 0.000 description 1
- CITWHPUZDXDVEK-UHFFFAOYSA-N 2-[4-chloro-5-methyl-3-(trifluoromethyl)pyrazol-1-yl]-2-(3-methoxyphenyl)acetic acid Chemical compound COC1=CC=CC(C(C(O)=O)N2C(=C(Cl)C(=N2)C(F)(F)F)C)=C1 CITWHPUZDXDVEK-UHFFFAOYSA-N 0.000 description 1
- MXIFLDVQWSXNGW-UHFFFAOYSA-N 2-[4-chloro-5-phenyl-3-(trifluoromethyl)pyrazol-1-yl]-1-[4-(4-fluorophenyl)piperazin-1-yl]ethanone Chemical compound C1=CC(F)=CC=C1N1CCN(C(=O)CN2C(=C(Cl)C(=N2)C(F)(F)F)C=2C=CC=CC=2)CC1 MXIFLDVQWSXNGW-UHFFFAOYSA-N 0.000 description 1
- LZDORDOFWNNTSN-UHFFFAOYSA-N 2-[5-amino-3-(furan-2-yl)pyrazol-1-yl]-1-[4-(4-fluorophenyl)piperazin-1-yl]ethanone Chemical compound NC1=CC(C=2OC=CC=2)=NN1CC(=O)N(CC1)CCN1C1=CC=C(F)C=C1 LZDORDOFWNNTSN-UHFFFAOYSA-N 0.000 description 1
- XXEXIPHOEOZYHR-UHFFFAOYSA-N 2-[5-butyl-3-(trifluoromethyl)pyrazol-1-yl]-1-[4-(4-chlorophenyl)piperazin-1-yl]ethanone Chemical compound CCCCC1=CC(C(F)(F)F)=NN1CC(=O)N1CCN(C=2C=CC(Cl)=CC=2)CC1 XXEXIPHOEOZYHR-UHFFFAOYSA-N 0.000 description 1
- RBPWIIWDHBTXDC-UHFFFAOYSA-N 2-[5-butyl-4-chloro-3-(trifluoromethyl)pyrazol-1-yl]-1-[4-(4-chlorophenyl)piperazin-1-yl]ethanone Chemical compound CCCCC1=C(Cl)C(C(F)(F)F)=NN1CC(=O)N1CCN(C=2C=CC(Cl)=CC=2)CC1 RBPWIIWDHBTXDC-UHFFFAOYSA-N 0.000 description 1
- OLFJYUSRRXBBMW-UHFFFAOYSA-N 2-[5-tert-butyl-3-(trifluoromethyl)pyrazol-1-yl]-1-[4-(4-fluorophenyl)piperazin-1-yl]ethanone Chemical compound CC(C)(C)C1=CC(C(F)(F)F)=NN1CC(=O)N1CCN(C=2C=CC(F)=CC=2)CC1 OLFJYUSRRXBBMW-UHFFFAOYSA-N 0.000 description 1
- WGDADRBTCPGSDG-UHFFFAOYSA-N 2-[[4,5-bis(4-chlorophenyl)-1,3-oxazol-2-yl]sulfanyl]propanoic acid Chemical compound O1C(SC(C)C(O)=O)=NC(C=2C=CC(Cl)=CC=2)=C1C1=CC=C(Cl)C=C1 WGDADRBTCPGSDG-UHFFFAOYSA-N 0.000 description 1
- XKSAJZSJKURQRX-UHFFFAOYSA-N 2-acetyloxy-5-(4-fluorophenyl)benzoic acid Chemical compound C1=C(C(O)=O)C(OC(=O)C)=CC=C1C1=CC=C(F)C=C1 XKSAJZSJKURQRX-UHFFFAOYSA-N 0.000 description 1
- 125000004174 2-benzimidazolyl group Chemical group [H]N1C(*)=NC2=C([H])C([H])=C([H])C([H])=C12 0.000 description 1
- OZGMODDEIHYPRY-UHFFFAOYSA-N 2-bromopropanoyl chloride Chemical compound CC(Br)C(Cl)=O OZGMODDEIHYPRY-UHFFFAOYSA-N 0.000 description 1
- CVHMCZLUHVAFON-SNVBAGLBSA-N 2-chloro-1-[(2r)-4-(4-chlorophenyl)-2-methylpiperazin-1-yl]ethanone Chemical compound C1CN(C(=O)CCl)[C@H](C)CN1C1=CC=C(Cl)C=C1 CVHMCZLUHVAFON-SNVBAGLBSA-N 0.000 description 1
- CVHMCZLUHVAFON-JTQLQIEISA-N 2-chloro-1-[(2s)-4-(4-chlorophenyl)-2-methylpiperazin-1-yl]ethanone Chemical compound C1CN(C(=O)CCl)[C@@H](C)CN1C1=CC=C(Cl)C=C1 CVHMCZLUHVAFON-JTQLQIEISA-N 0.000 description 1
- BFSVOASYOCHEOV-UHFFFAOYSA-N 2-diethylaminoethanol Chemical compound CCN(CC)CCO BFSVOASYOCHEOV-UHFFFAOYSA-N 0.000 description 1
- 229940013085 2-diethylaminoethanol Drugs 0.000 description 1
- 125000002941 2-furyl group Chemical group O1C([*])=C([H])C([H])=C1[H] 0.000 description 1
- QDMCWIHRLTVLIY-UHFFFAOYSA-N 2-methyl-1-(3-methylphenyl)piperazine Chemical compound CC1CNCCN1C1=CC=CC(C)=C1 QDMCWIHRLTVLIY-UHFFFAOYSA-N 0.000 description 1
- ILYSAKHOYBPSPC-UHFFFAOYSA-N 2-phenylbenzoic acid Chemical class OC(=O)C1=CC=CC=C1C1=CC=CC=C1 ILYSAKHOYBPSPC-UHFFFAOYSA-N 0.000 description 1
- 125000000094 2-phenylethyl group Chemical group [H]C1=C([H])C([H])=C(C([H])=C1[H])C([H])([H])C([H])([H])* 0.000 description 1
- 125000003903 2-propenyl group Chemical group [H]C([*])([H])C([H])=C([H])[H] 0.000 description 1
- 125000004105 2-pyridyl group Chemical group N1=C([*])C([H])=C([H])C([H])=C1[H] 0.000 description 1
- 125000000389 2-pyrrolyl group Chemical group [H]N1C([*])=C([H])C([H])=C1[H] 0.000 description 1
- 125000000175 2-thienyl group Chemical group S1C([*])=C([H])C([H])=C1[H] 0.000 description 1
- LQJBNNIYVWPHFW-UHFFFAOYSA-N 20:1omega9c fatty acid Natural products CCCCCCCCCCC=CCCCCCCCC(O)=O LQJBNNIYVWPHFW-UHFFFAOYSA-N 0.000 description 1
- TXQKCKQJBGFUBF-UHFFFAOYSA-N 3,4,5-tribromo-1h-pyrazole Chemical compound BrC1=NNC(Br)=C1Br TXQKCKQJBGFUBF-UHFFFAOYSA-N 0.000 description 1
- AXNUZKSSQHTNPZ-UHFFFAOYSA-N 3,4-difluoroaniline Chemical compound NC1=CC=C(F)C(F)=C1 AXNUZKSSQHTNPZ-UHFFFAOYSA-N 0.000 description 1
- FPQQSJJWHUJYPU-UHFFFAOYSA-N 3-(dimethylamino)propyliminomethylidene-ethylazanium;chloride Chemical compound Cl.CCN=C=NCCCN(C)C FPQQSJJWHUJYPU-UHFFFAOYSA-N 0.000 description 1
- KWZMHXZIEWCRFW-UHFFFAOYSA-N 3-[[4-chloro-2-[2-[4-(4-chlorophenyl)piperazin-1-yl]-2-oxoethyl]-5-(trifluoromethyl)pyrazol-3-yl]methyl]-1-methoxy-1-methylurea Chemical compound CON(C)C(=O)NCC1=C(Cl)C(C(F)(F)F)=NN1CC(=O)N1CCN(C=2C=CC(Cl)=CC=2)CC1 KWZMHXZIEWCRFW-UHFFFAOYSA-N 0.000 description 1
- WRDYUOZXHOGAPA-UHFFFAOYSA-N 3-amino-5-(cyanomethyl)-1-[2-[4-(4-fluorophenyl)piperazin-1-yl]-2-oxoethyl]pyrazole-4-carbonitrile Chemical compound N#CCC1=C(C#N)C(N)=NN1CC(=O)N1CCN(C=2C=CC(F)=CC=2)CC1 WRDYUOZXHOGAPA-UHFFFAOYSA-N 0.000 description 1
- GSXISOIYTHIBLC-UHFFFAOYSA-N 3-amino-5-(cyanomethyl)-1h-pyrazole-4-carbonitrile Chemical compound NC1=NNC(CC#N)=C1C#N GSXISOIYTHIBLC-UHFFFAOYSA-N 0.000 description 1
- 125000000474 3-butynyl group Chemical group [H]C#CC([H])([H])C([H])([H])* 0.000 description 1
- QPHAGNNWDZSKJH-UHFFFAOYSA-N 3-chloro-2h-indazole Chemical compound C1=CC=CC2=C(Cl)NN=C21 QPHAGNNWDZSKJH-UHFFFAOYSA-N 0.000 description 1
- 125000003682 3-furyl group Chemical group O1C([H])=C([*])C([H])=C1[H] 0.000 description 1
- USCSRAJGJYMJFZ-UHFFFAOYSA-N 3-methyl-1-butyne Chemical compound CC(C)C#C USCSRAJGJYMJFZ-UHFFFAOYSA-N 0.000 description 1
- AYGYICRITMSJOC-UHFFFAOYSA-N 3-piperazin-1-ylphenol Chemical compound OC1=CC=CC(N2CCNCC2)=C1 AYGYICRITMSJOC-UHFFFAOYSA-N 0.000 description 1
- 125000003349 3-pyridyl group Chemical group N1=C([H])C([*])=C([H])C([H])=C1[H] 0.000 description 1
- 125000001397 3-pyrrolyl group Chemical group [H]N1C([H])=C([*])C([H])=C1[H] 0.000 description 1
- 125000001541 3-thienyl group Chemical group S1C([H])=C([*])C([H])=C1[H] 0.000 description 1
- OSWFIVFLDKOXQC-UHFFFAOYSA-N 4-(3-methoxyphenyl)aniline Chemical compound COC1=CC=CC(C=2C=CC(N)=CC=2)=C1 OSWFIVFLDKOXQC-UHFFFAOYSA-N 0.000 description 1
- VVDDHNFZVXLQHN-UHFFFAOYSA-N 4-[4-[2-[4-chloro-5-methyl-3-(trifluoromethyl)pyrazol-1-yl]acetyl]piperazin-1-yl]-n,n-dimethylbenzenesulfonamide Chemical compound C1=CC(S(=O)(=O)N(C)C)=CC=C1N1CCN(C(=O)CN2C(=C(Cl)C(=N2)C(F)(F)F)C)CC1 VVDDHNFZVXLQHN-UHFFFAOYSA-N 0.000 description 1
- UWJGZCAHLZVAMT-UHFFFAOYSA-N 4-[4-[2-[4-chloro-5-methyl-3-(trifluoromethyl)pyrazol-1-yl]acetyl]piperazin-1-yl]benzonitrile Chemical compound CC1=C(Cl)C(C(F)(F)F)=NN1CC(=O)N1CCN(C=2C=CC(=CC=2)C#N)CC1 UWJGZCAHLZVAMT-UHFFFAOYSA-N 0.000 description 1
- OELYMZVJDKSMOJ-UHFFFAOYSA-N 4-bromo-1h-pyrazol-5-amine Chemical compound NC1=NNC=C1Br OELYMZVJDKSMOJ-UHFFFAOYSA-N 0.000 description 1
- WVGCPEDBFHEHEZ-UHFFFAOYSA-N 4-bromo-1h-pyrazole Chemical compound BrC=1C=NNC=1 WVGCPEDBFHEHEZ-UHFFFAOYSA-N 0.000 description 1
- RISOHYOEPYWKOB-UHFFFAOYSA-N 4-bromo-3,5-dimethyl-1h-pyrazole Chemical compound CC1=NNC(C)=C1Br RISOHYOEPYWKOB-UHFFFAOYSA-N 0.000 description 1
- OBKUMDOWKMRFPB-UHFFFAOYSA-N 4-bromo-3-phenyl-5-(trifluoromethyl)-1h-pyrazole Chemical compound BrC1=C(C(F)(F)F)NN=C1C1=CC=CC=C1 OBKUMDOWKMRFPB-UHFFFAOYSA-N 0.000 description 1
- WZMBDMWFVPKYCF-UHFFFAOYSA-N 4-bromo-5-methyl-1h-pyrazol-3-amine Chemical compound CC=1NN=C(N)C=1Br WZMBDMWFVPKYCF-UHFFFAOYSA-N 0.000 description 1
- WDFQBORIUYODSI-UHFFFAOYSA-N 4-bromoaniline Chemical compound NC1=CC=C(Br)C=C1 WDFQBORIUYODSI-UHFFFAOYSA-N 0.000 description 1
- TUXYZHVUPGXXQG-UHFFFAOYSA-N 4-bromobenzoic acid Chemical compound OC(=O)C1=CC=C(Br)C=C1 TUXYZHVUPGXXQG-UHFFFAOYSA-N 0.000 description 1
- CSFDTBRRIBJILD-UHFFFAOYSA-N 4-chloro-2-fluoroaniline Chemical compound NC1=CC=C(Cl)C=C1F CSFDTBRRIBJILD-UHFFFAOYSA-N 0.000 description 1
- CBZZNONQUSUHMH-UHFFFAOYSA-N 4-chloro-3,5-bis(trifluoromethyl)-1h-pyrazole Chemical compound FC(F)(F)C1=NNC(C(F)(F)F)=C1Cl CBZZNONQUSUHMH-UHFFFAOYSA-N 0.000 description 1
- JIDMZXNLLFOSIL-UHFFFAOYSA-N 4-chloro-5-(5-chlorothiophen-2-yl)-1h-pyrazole Chemical compound S1C(Cl)=CC=C1C1=NNC=C1Cl JIDMZXNLLFOSIL-UHFFFAOYSA-N 0.000 description 1
- LCDKUXJKMAFCTI-UHFFFAOYSA-N 4-chloro-5-methyl-1h-pyrazole Chemical compound CC=1NN=CC=1Cl LCDKUXJKMAFCTI-UHFFFAOYSA-N 0.000 description 1
- MIZPYLHXVUIZKM-UHFFFAOYSA-N 4-chloro-5-thiophen-2-yl-1h-pyrazole Chemical compound ClC1=CNN=C1C1=CC=CS1 MIZPYLHXVUIZKM-UHFFFAOYSA-N 0.000 description 1
- HVCNXQOWACZAFN-UHFFFAOYSA-N 4-ethylmorpholine Chemical compound CCN1CCOCC1 HVCNXQOWACZAFN-UHFFFAOYSA-N 0.000 description 1
- SYCHUQUJURZQMO-UHFFFAOYSA-N 4-hydroxy-2-methyl-1,1-dioxo-n-(1,3-thiazol-2-yl)-1$l^{6},2-benzothiazine-3-carboxamide Chemical compound OC=1C2=CC=CC=C2S(=O)(=O)N(C)C=1C(=O)NC1=NC=CS1 SYCHUQUJURZQMO-UHFFFAOYSA-N 0.000 description 1
- FJKROLUGYXJWQN-UHFFFAOYSA-M 4-hydroxybenzoate Chemical compound OC1=CC=C(C([O-])=O)C=C1 FJKROLUGYXJWQN-UHFFFAOYSA-M 0.000 description 1
- MZZXIXHKDJNBJQ-UHFFFAOYSA-N 4-iodo-3,5-dimethyl-1h-pyrazole Chemical compound CC1=NNC(C)=C1I MZZXIXHKDJNBJQ-UHFFFAOYSA-N 0.000 description 1
- LLNQWPTUJJYTTE-UHFFFAOYSA-N 4-iodopyrazole Chemical compound IC=1C=NNC=1 LLNQWPTUJJYTTE-UHFFFAOYSA-N 0.000 description 1
- DJJNYEXRPRQXPD-UHFFFAOYSA-N 4-piperazin-1-ylbenzonitrile Chemical compound C1=CC(C#N)=CC=C1N1CCNCC1 DJJNYEXRPRQXPD-UHFFFAOYSA-N 0.000 description 1
- 125000000339 4-pyridyl group Chemical group N1=C([H])C([H])=C([*])C([H])=C1[H] 0.000 description 1
- KDDQRKBRJSGMQE-UHFFFAOYSA-N 4-thiazolyl Chemical group [C]1=CSC=N1 KDDQRKBRJSGMQE-UHFFFAOYSA-N 0.000 description 1
- PJJGZPJJTHBVMX-UHFFFAOYSA-N 5,7-Dihydroxyisoflavone Chemical compound C=1C(O)=CC(O)=C(C2=O)C=1OC=C2C1=CC=CC=C1 PJJGZPJJTHBVMX-UHFFFAOYSA-N 0.000 description 1
- LSLYOANBFKQKPT-DIFFPNOSSA-N 5-[(1r)-1-hydroxy-2-[[(2r)-1-(4-hydroxyphenyl)propan-2-yl]amino]ethyl]benzene-1,3-diol Chemical compound C([C@@H](C)NC[C@H](O)C=1C=C(O)C=C(O)C=1)C1=CC=C(O)C=C1 LSLYOANBFKQKPT-DIFFPNOSSA-N 0.000 description 1
- FFNKBQRKZRMYCL-UHFFFAOYSA-N 5-amino-1h-pyrazole-4-carbonitrile Chemical compound NC1=NNC=C1C#N FFNKBQRKZRMYCL-UHFFFAOYSA-N 0.000 description 1
- XHZWFUVEKDDQPF-UHFFFAOYSA-N 5-bromo-1h-pyrazole Chemical class BrC1=CC=NN1 XHZWFUVEKDDQPF-UHFFFAOYSA-N 0.000 description 1
- RPJXLEZOFUNGNZ-UHFFFAOYSA-N 5-methoxy-2-methylaniline Chemical compound COC1=CC=C(C)C(N)=C1 RPJXLEZOFUNGNZ-UHFFFAOYSA-N 0.000 description 1
- DLCHCAYDSKIFIN-UHFFFAOYSA-N 5-methyl-3-(trifluoromethyl)-1h-pyrazole Chemical compound CC1=CC(C(F)(F)F)=NN1 DLCHCAYDSKIFIN-UHFFFAOYSA-N 0.000 description 1
- MZRUFMBFIKGOAL-UHFFFAOYSA-N 5-nitro-1h-pyrazole Chemical class [O-][N+](=O)C1=CC=NN1 MZRUFMBFIKGOAL-UHFFFAOYSA-N 0.000 description 1
- CWDWFSXUQODZGW-UHFFFAOYSA-N 5-thiazolyl Chemical group [C]1=CN=CS1 CWDWFSXUQODZGW-UHFFFAOYSA-N 0.000 description 1
- IXJCHVMUTFCRBH-SDUHDBOFSA-N 7-[(1r,2s,3e,5z)-10-(4-acetyl-3-hydroxy-2-propylphenoxy)-1-hydroxy-1-[3-(trifluoromethyl)phenyl]deca-3,5-dien-2-yl]sulfanyl-4-oxochromene-2-carboxylic acid Chemical compound CCCC1=C(O)C(C(C)=O)=CC=C1OCCCC\C=C/C=C/[C@@H]([C@H](O)C=1C=C(C=CC=1)C(F)(F)F)SC1=CC=C2C(=O)C=C(C(O)=O)OC2=C1 IXJCHVMUTFCRBH-SDUHDBOFSA-N 0.000 description 1
- PQCAUHUKTBHUSA-UHFFFAOYSA-N 7-nitro-1h-indazole Chemical compound [O-][N+](=O)C1=CC=CC2=C1NN=C2 PQCAUHUKTBHUSA-UHFFFAOYSA-N 0.000 description 1
- ZCYVEMRRCGMTRW-UHFFFAOYSA-N 7553-56-2 Chemical group [I] ZCYVEMRRCGMTRW-UHFFFAOYSA-N 0.000 description 1
- HBAQYPYDRFILMT-UHFFFAOYSA-N 8-[3-(1-cyclopropylpyrazol-4-yl)-1H-pyrazolo[4,3-d]pyrimidin-5-yl]-3-methyl-3,8-diazabicyclo[3.2.1]octan-2-one Chemical class C1(CC1)N1N=CC(=C1)C1=NNC2=C1N=C(N=C2)N1C2C(N(CC1CC2)C)=O HBAQYPYDRFILMT-UHFFFAOYSA-N 0.000 description 1
- QSBYPNXLFMSGKH-UHFFFAOYSA-N 9-Heptadecensaeure Natural products CCCCCCCC=CCCCCCCCC(O)=O QSBYPNXLFMSGKH-UHFFFAOYSA-N 0.000 description 1
- 229910016455 AlBN Inorganic materials 0.000 description 1
- 206010058284 Allergy to arthropod sting Diseases 0.000 description 1
- GUBGYTABKSRVRQ-XLOQQCSPSA-N Alpha-Lactose Chemical compound O[C@@H]1[C@@H](O)[C@@H](O)[C@@H](CO)O[C@H]1O[C@@H]1[C@@H](CO)O[C@H](O)[C@H](O)[C@H]1O GUBGYTABKSRVRQ-XLOQQCSPSA-N 0.000 description 1
- 239000005995 Aluminium silicate Substances 0.000 description 1
- 208000024827 Alzheimer disease Diseases 0.000 description 1
- NLXLAEXVIDQMFP-UHFFFAOYSA-N Ammonia chloride Chemical compound [NH4+].[Cl-] NLXLAEXVIDQMFP-UHFFFAOYSA-N 0.000 description 1
- QGZKDVFQNNGYKY-UHFFFAOYSA-O Ammonium Chemical compound [NH4+] QGZKDVFQNNGYKY-UHFFFAOYSA-O 0.000 description 1
- USFZMSVCRYTOJT-UHFFFAOYSA-N Ammonium acetate Chemical compound N.CC(O)=O USFZMSVCRYTOJT-UHFFFAOYSA-N 0.000 description 1
- 206010002198 Anaphylactic reaction Diseases 0.000 description 1
- 206010002556 Ankylosing Spondylitis Diseases 0.000 description 1
- 102000001381 Arachidonate 5-Lipoxygenase Human genes 0.000 description 1
- 108010093579 Arachidonate 5-lipoxygenase Proteins 0.000 description 1
- 239000004475 Arginine Substances 0.000 description 1
- 201000001320 Atherosclerosis Diseases 0.000 description 1
- 229930003347 Atropine Natural products 0.000 description 1
- XHVAWZZCDCWGBK-WYRLRVFGSA-M Aurothioglucose Chemical compound OC[C@H]1O[C@H](S[Au])[C@H](O)[C@@H](O)[C@@H]1O XHVAWZZCDCWGBK-WYRLRVFGSA-M 0.000 description 1
- 208000027496 Behcet disease Diseases 0.000 description 1
- 208000009137 Behcet syndrome Diseases 0.000 description 1
- ZBJJDYGJCNTNTH-UHFFFAOYSA-N Betahistine mesilate Chemical compound CS(O)(=O)=O.CS(O)(=O)=O.CNCCC1=CC=CC=N1 ZBJJDYGJCNTNTH-UHFFFAOYSA-N 0.000 description 1
- 206010065687 Bone loss Diseases 0.000 description 1
- 241000283690 Bos taurus Species 0.000 description 1
- 108091003079 Bovine Serum Albumin Proteins 0.000 description 1
- VOVIALXJUBGFJZ-KWVAZRHASA-N Budesonide Chemical compound C1CC2=CC(=O)C=C[C@]2(C)[C@@H]2[C@@H]1[C@@H]1C[C@H]3OC(CCC)O[C@@]3(C(=O)CO)[C@@]1(C)C[C@@H]2O VOVIALXJUBGFJZ-KWVAZRHASA-N 0.000 description 1
- COVZYZSDYWQREU-UHFFFAOYSA-N Busulfan Chemical compound CS(=O)(=O)OCCCCOS(C)(=O)=O COVZYZSDYWQREU-UHFFFAOYSA-N 0.000 description 1
- 102100031151 C-C chemokine receptor type 2 Human genes 0.000 description 1
- 101710149815 C-C chemokine receptor type 2 Proteins 0.000 description 1
- 102100024167 C-C chemokine receptor type 3 Human genes 0.000 description 1
- 101710149862 C-C chemokine receptor type 3 Proteins 0.000 description 1
- 102100037853 C-C chemokine receptor type 4 Human genes 0.000 description 1
- 101710149863 C-C chemokine receptor type 4 Proteins 0.000 description 1
- 102100035875 C-C chemokine receptor type 5 Human genes 0.000 description 1
- 101710149870 C-C chemokine receptor type 5 Proteins 0.000 description 1
- 102100036301 C-C chemokine receptor type 7 Human genes 0.000 description 1
- 102100028989 C-X-C chemokine receptor type 2 Human genes 0.000 description 1
- 102100028990 C-X-C chemokine receptor type 3 Human genes 0.000 description 1
- 102100025618 C-X-C chemokine receptor type 6 Human genes 0.000 description 1
- 101150041968 CDC13 gene Proteins 0.000 description 1
- UXVMQQNJUSDDNG-UHFFFAOYSA-L Calcium chloride Chemical compound [Cl-].[Cl-].[Ca+2] UXVMQQNJUSDDNG-UHFFFAOYSA-L 0.000 description 1
- 241000282472 Canis lupus familiaris Species 0.000 description 1
- 241000283707 Capra Species 0.000 description 1
- 239000004215 Carbon black (E152) Substances 0.000 description 1
- OKTJSMMVPCPJKN-NJFSPNSNSA-N Carbon-14 Chemical compound [14C] OKTJSMMVPCPJKN-NJFSPNSNSA-N 0.000 description 1
- ZKLPARSLTMPFCP-UHFFFAOYSA-N Cetirizine Chemical compound C1CN(CCOCC(=O)O)CCN1C(C=1C=CC(Cl)=CC=1)C1=CC=CC=C1 ZKLPARSLTMPFCP-UHFFFAOYSA-N 0.000 description 1
- 108010055165 Chemokine CCL4 Proteins 0.000 description 1
- 102000001326 Chemokine CCL4 Human genes 0.000 description 1
- KZBUYRJDOAKODT-UHFFFAOYSA-N Chlorine Chemical compound ClCl KZBUYRJDOAKODT-UHFFFAOYSA-N 0.000 description 1
- 208000017667 Chronic Disease Diseases 0.000 description 1
- 208000006545 Chronic Obstructive Pulmonary Disease Diseases 0.000 description 1
- OIRAEJWYWSAQNG-UHFFFAOYSA-N Clidanac Chemical compound ClC=1C=C2C(C(=O)O)CCC2=CC=1C1CCCCC1 OIRAEJWYWSAQNG-UHFFFAOYSA-N 0.000 description 1
- 208000015943 Coeliac disease Diseases 0.000 description 1
- 206010009900 Colitis ulcerative Diseases 0.000 description 1
- 208000035473 Communicable disease Diseases 0.000 description 1
- 206010010744 Conjunctivitis allergic Diseases 0.000 description 1
- RYGMFSIKBFXOCR-UHFFFAOYSA-N Copper Chemical compound [Cu] RYGMFSIKBFXOCR-UHFFFAOYSA-N 0.000 description 1
- 229920002261 Corn starch Polymers 0.000 description 1
- 208000011231 Crohn disease Diseases 0.000 description 1
- 229920000858 Cyclodextrin Polymers 0.000 description 1
- 229940093444 Cyclooxygenase 2 inhibitor Drugs 0.000 description 1
- 229940122204 Cyclooxygenase inhibitor Drugs 0.000 description 1
- 229930105110 Cyclosporin A Natural products 0.000 description 1
- FBPFZTCFMRRESA-KVTDHHQDSA-N D-Mannitol Chemical compound OC[C@@H](O)[C@@H](O)[C@H](O)[C@H](O)CO FBPFZTCFMRRESA-KVTDHHQDSA-N 0.000 description 1
- VVNCNSJFMMFHPL-VKHMYHEASA-N D-penicillamine Chemical compound CC(C)(S)[C@@H](N)C(O)=O VVNCNSJFMMFHPL-VKHMYHEASA-N 0.000 description 1
- 208000016192 Demyelinating disease Diseases 0.000 description 1
- 206010012442 Dermatitis contact Diseases 0.000 description 1
- 206010013700 Drug hypersensitivity Diseases 0.000 description 1
- 208000004232 Enteritis Diseases 0.000 description 1
- 241000283086 Equidae Species 0.000 description 1
- PIICEJLVQHRZGT-UHFFFAOYSA-N Ethylenediamine Chemical compound NCCN PIICEJLVQHRZGT-UHFFFAOYSA-N 0.000 description 1
- CWYNVVGOOAEACU-UHFFFAOYSA-N Fe2+ Chemical compound [Fe+2] CWYNVVGOOAEACU-UHFFFAOYSA-N 0.000 description 1
- 241000282326 Felis catus Species 0.000 description 1
- RBBWCVQDXDFISW-UHFFFAOYSA-N Feprazone Chemical compound O=C1C(CC=C(C)C)C(=O)N(C=2C=CC=CC=2)N1C1=CC=CC=C1 RBBWCVQDXDFISW-UHFFFAOYSA-N 0.000 description 1
- 229920001917 Ficoll Polymers 0.000 description 1
- PXGOKWXKJXAPGV-UHFFFAOYSA-N Fluorine Chemical compound FF PXGOKWXKJXAPGV-UHFFFAOYSA-N 0.000 description 1
- 102000003688 G-Protein-Coupled Receptors Human genes 0.000 description 1
- 108090000045 G-Protein-Coupled Receptors Proteins 0.000 description 1
- 108010010803 Gelatin Proteins 0.000 description 1
- 108010072051 Glatiramer Acetate Proteins 0.000 description 1
- 206010018364 Glomerulonephritis Diseases 0.000 description 1
- 244000068988 Glycine max Species 0.000 description 1
- 235000010469 Glycine max Nutrition 0.000 description 1
- 201000005569 Gout Diseases 0.000 description 1
- 208000031886 HIV Infections Diseases 0.000 description 1
- 208000037357 HIV infectious disease Diseases 0.000 description 1
- 229940122957 Histamine H2 receptor antagonist Drugs 0.000 description 1
- 101000716065 Homo sapiens C-C chemokine receptor type 7 Proteins 0.000 description 1
- 101000916050 Homo sapiens C-X-C chemokine receptor type 3 Proteins 0.000 description 1
- 101000856683 Homo sapiens C-X-C chemokine receptor type 6 Proteins 0.000 description 1
- VSNHCAURESNICA-UHFFFAOYSA-N Hydroxyurea Chemical compound NC(=O)NO VSNHCAURESNICA-UHFFFAOYSA-N 0.000 description 1
- RKUNBYITZUJHSG-UHFFFAOYSA-N Hyosciamin-hydrochlorid Natural products CN1C(C2)CCC1CC2OC(=O)C(CO)C1=CC=CC=C1 RKUNBYITZUJHSG-UHFFFAOYSA-N 0.000 description 1
- 206010020751 Hypersensitivity Diseases 0.000 description 1
- DGAQECJNVWCQMB-PUAWFVPOSA-M Ilexoside XXIX Chemical compound C[C@@H]1CC[C@@]2(CC[C@@]3(C(=CC[C@H]4[C@]3(CC[C@@H]5[C@@]4(CC[C@@H](C5(C)C)OS(=O)(=O)[O-])C)C)[C@@H]2[C@]1(C)O)C)C(=O)O[C@H]6[C@@H]([C@H]([C@@H]([C@H](O6)CO)O)O)O.[Na+] DGAQECJNVWCQMB-PUAWFVPOSA-M 0.000 description 1
- AMHAQOBUZCQMHN-UHFFFAOYSA-N Indo-1 dye Chemical compound CC1=CC=C(N(CC(O)=O)CC(O)=O)C(OCCOC=2C(=CC=C(C=2)C=2NC3=CC(=CC=C3C=2)C(O)=O)N(CC(O)=O)CC(O)=O)=C1 AMHAQOBUZCQMHN-UHFFFAOYSA-N 0.000 description 1
- 108010008212 Integrin alpha4beta1 Proteins 0.000 description 1
- 108010005716 Interferon beta-1a Proteins 0.000 description 1
- 108010005714 Interferon beta-1b Proteins 0.000 description 1
- 102000000589 Interleukin-1 Human genes 0.000 description 1
- 108010002352 Interleukin-1 Proteins 0.000 description 1
- 108010018951 Interleukin-8B Receptors Proteins 0.000 description 1
- 102000015696 Interleukins Human genes 0.000 description 1
- 108010063738 Interleukins Proteins 0.000 description 1
- HUYWAWARQUIQLE-UHFFFAOYSA-N Isoetharine Chemical compound CC(C)NC(CC)C(O)C1=CC=C(O)C(O)=C1 HUYWAWARQUIQLE-UHFFFAOYSA-N 0.000 description 1
- PWWVAXIEGOYWEE-UHFFFAOYSA-N Isophenergan Chemical compound C1=CC=C2N(CC(C)N(C)C)C3=CC=CC=C3SC2=C1 PWWVAXIEGOYWEE-UHFFFAOYSA-N 0.000 description 1
- SHGAZHPCJJPHSC-NUEINMDLSA-N Isotretinoin Chemical compound OC(=O)C=C(C)/C=C/C=C(C)C=CC1=C(C)CCCC1(C)C SHGAZHPCJJPHSC-NUEINMDLSA-N 0.000 description 1
- UETNIIAIRMUTSM-UHFFFAOYSA-N Jacareubin Natural products CC1(C)OC2=CC3Oc4c(O)c(O)ccc4C(=O)C3C(=C2C=C1)O UETNIIAIRMUTSM-UHFFFAOYSA-N 0.000 description 1
- ODKSFYDXXFIFQN-BYPYZUCNSA-P L-argininium(2+) Chemical compound NC(=[NH2+])NCCC[C@H]([NH3+])C(O)=O ODKSFYDXXFIFQN-BYPYZUCNSA-P 0.000 description 1
- ZDXPYRJPNDTMRX-VKHMYHEASA-N L-glutamine Chemical compound OC(=O)[C@@H](N)CCC(N)=O ZDXPYRJPNDTMRX-VKHMYHEASA-N 0.000 description 1
- 229930182816 L-glutamine Natural products 0.000 description 1
- HNDVDQJCIGZPNO-YFKPBYRVSA-N L-histidine Chemical compound OC(=O)[C@@H](N)CC1=CN=CN1 HNDVDQJCIGZPNO-YFKPBYRVSA-N 0.000 description 1
- KDXKERNSBIXSRK-YFKPBYRVSA-N L-lysine Chemical compound NCCCC[C@H](N)C(O)=O KDXKERNSBIXSRK-YFKPBYRVSA-N 0.000 description 1
- MKXZASYAUGDDCJ-SZMVWBNQSA-N LSM-2525 Chemical compound C1CCC[C@H]2[C@@]3([H])N(C)CC[C@]21C1=CC(OC)=CC=C1C3 MKXZASYAUGDDCJ-SZMVWBNQSA-N 0.000 description 1
- GUBGYTABKSRVRQ-QKKXKWKRSA-N Lactose Natural products OC[C@H]1O[C@@H](O[C@H]2[C@H](O)[C@@H](O)C(O)O[C@@H]2CO)[C@H](O)[C@@H](O)[C@H]1O GUBGYTABKSRVRQ-QKKXKWKRSA-N 0.000 description 1
- 239000004166 Lanolin Substances 0.000 description 1
- 240000007472 Leucaena leucocephala Species 0.000 description 1
- 235000010643 Leucaena leucocephala Nutrition 0.000 description 1
- 239000000867 Lipoxygenase Inhibitor Substances 0.000 description 1
- 229940122142 Lipoxygenase inhibitor Drugs 0.000 description 1
- WHXSMMKQMYFTQS-UHFFFAOYSA-N Lithium Chemical compound [Li] WHXSMMKQMYFTQS-UHFFFAOYSA-N 0.000 description 1
- 208000019693 Lung disease Diseases 0.000 description 1
- KDXKERNSBIXSRK-UHFFFAOYSA-N Lysine Natural products NCCCCC(N)C(O)=O KDXKERNSBIXSRK-UHFFFAOYSA-N 0.000 description 1
- 239000004472 Lysine Substances 0.000 description 1
- 102000009571 Macrophage Inflammatory Proteins Human genes 0.000 description 1
- 108010009474 Macrophage Inflammatory Proteins Proteins 0.000 description 1
- FYYHWMGAXLPEAU-UHFFFAOYSA-N Magnesium Chemical compound [Mg] FYYHWMGAXLPEAU-UHFFFAOYSA-N 0.000 description 1
- 229930195725 Mannitol Natural products 0.000 description 1
- SBDNJUWAMKYJOX-UHFFFAOYSA-N Meclofenamic Acid Chemical compound CC1=CC=C(Cl)C(NC=2C(=CC=CC=2)C(O)=O)=C1Cl SBDNJUWAMKYJOX-UHFFFAOYSA-N 0.000 description 1
- 201000009906 Meningitis Diseases 0.000 description 1
- FQISKWAFAHGMGT-SGJOWKDISA-M Methylprednisolone sodium succinate Chemical compound [Na+].C([C@@]12C)=CC(=O)C=C1[C@@H](C)C[C@@H]1[C@@H]2[C@@H](O)C[C@]2(C)[C@@](O)(C(=O)COC(=O)CCC([O-])=O)CC[C@H]21 FQISKWAFAHGMGT-SGJOWKDISA-M 0.000 description 1
- 102000029749 Microtubule Human genes 0.000 description 1
- 108091022875 Microtubule Proteins 0.000 description 1
- UCHDWCPVSPXUMX-TZIWLTJVSA-N Montelukast Chemical compound CC(C)(O)C1=CC=CC=C1CC[C@H](C=1C=C(\C=C\C=2N=C3C=C(Cl)C=CC3=CC=2)C=CC=1)SCC1(CC(O)=O)CC1 UCHDWCPVSPXUMX-TZIWLTJVSA-N 0.000 description 1
- 241000699670 Mus sp. Species 0.000 description 1
- RTGDFNSFWBGLEC-UHFFFAOYSA-N Mycophenolate mofetil Chemical compound COC1=C(C)C=2COC(=O)C=2C(O)=C1CC=C(C)CCC(=O)OCCN1CCOCC1 RTGDFNSFWBGLEC-UHFFFAOYSA-N 0.000 description 1
- 201000002481 Myositis Diseases 0.000 description 1
- IJHNSHDBIRRJRN-UHFFFAOYSA-N N,N-dimethyl-3-phenyl-3-(2-pyridinyl)-1-propanamine Chemical compound C=1C=CC=NC=1C(CCN(C)C)C1=CC=CC=C1 IJHNSHDBIRRJRN-UHFFFAOYSA-N 0.000 description 1
- UEEJHVSXFDXPFK-UHFFFAOYSA-N N-dimethylaminoethanol Chemical compound CN(C)CCO UEEJHVSXFDXPFK-UHFFFAOYSA-N 0.000 description 1
- HTLZVHNRZJPSMI-UHFFFAOYSA-N N-ethylpiperidine Chemical compound CCN1CCCCC1 HTLZVHNRZJPSMI-UHFFFAOYSA-N 0.000 description 1
- MBBZMMPHUWSWHV-BDVNFPICSA-N N-methylglucamine Chemical compound CNC[C@H](O)[C@@H](O)[C@H](O)[C@H](O)CO MBBZMMPHUWSWHV-BDVNFPICSA-N 0.000 description 1
- 229910017852 NH2NH2 Inorganic materials 0.000 description 1
- JAUOIFJMECXRGI-UHFFFAOYSA-N Neoclaritin Chemical compound C=1C(Cl)=CC=C2C=1CCC1=CC=CN=C1C2=C1CCNCC1 JAUOIFJMECXRGI-UHFFFAOYSA-N 0.000 description 1
- 206010029113 Neovascularisation Diseases 0.000 description 1
- JZFPYUNJRRFVQU-UHFFFAOYSA-N Niflumic acid Chemical compound OC(=O)C1=CC=CN=C1NC1=CC=CC(C(F)(F)F)=C1 JZFPYUNJRRFVQU-UHFFFAOYSA-N 0.000 description 1
- 239000005642 Oleic acid Substances 0.000 description 1
- ZQPPMHVWECSIRJ-UHFFFAOYSA-N Oleic acid Natural products CCCCCCCCC=CCCCCCCCC(O)=O ZQPPMHVWECSIRJ-UHFFFAOYSA-N 0.000 description 1
- 235000019502 Orange oil Nutrition 0.000 description 1
- 241000283973 Oryctolagus cuniculus Species 0.000 description 1
- 208000005141 Otitis Diseases 0.000 description 1
- 235000019483 Peanut oil Nutrition 0.000 description 1
- 241001494479 Pecora Species 0.000 description 1
- 239000004264 Petrolatum Substances 0.000 description 1
- 102000004861 Phosphoric Diester Hydrolases Human genes 0.000 description 1
- 108090001050 Phosphoric Diester Hydrolases Proteins 0.000 description 1
- NQRYJNQNLNOLGT-UHFFFAOYSA-N Piperidine Chemical compound C1CCNCC1 NQRYJNQNLNOLGT-UHFFFAOYSA-N 0.000 description 1
- VQDBNKDJNJQRDG-UHFFFAOYSA-N Pirbuterol Chemical compound CC(C)(C)NCC(O)C1=CC=C(O)C(CO)=N1 VQDBNKDJNJQRDG-UHFFFAOYSA-N 0.000 description 1
- 229920003171 Poly (ethylene oxide) Polymers 0.000 description 1
- 239000004698 Polyethylene Substances 0.000 description 1
- 208000007048 Polymyalgia Rheumatica Diseases 0.000 description 1
- ZLMJMSJWJFRBEC-UHFFFAOYSA-N Potassium Chemical compound [K] ZLMJMSJWJFRBEC-UHFFFAOYSA-N 0.000 description 1
- TVQZAMVBTVNYLA-UHFFFAOYSA-N Pranoprofen Chemical compound C1=CC=C2CC3=CC(C(C(O)=O)C)=CC=C3OC2=N1 TVQZAMVBTVNYLA-UHFFFAOYSA-N 0.000 description 1
- 241000288906 Primates Species 0.000 description 1
- 208000003251 Pruritus Diseases 0.000 description 1
- 239000012980 RPMI-1640 medium Substances 0.000 description 1
- 241000700159 Rattus Species 0.000 description 1
- 208000017442 Retinal disease Diseases 0.000 description 1
- 206010038923 Retinopathy Diseases 0.000 description 1
- 206010039085 Rhinitis allergic Diseases 0.000 description 1
- 206010040047 Sepsis Diseases 0.000 description 1
- XUIMIQQOPSSXEZ-UHFFFAOYSA-N Silicon Chemical compound [Si] XUIMIQQOPSSXEZ-UHFFFAOYSA-N 0.000 description 1
- VMHLLURERBWHNL-UHFFFAOYSA-M Sodium acetate Chemical compound [Na+].CC([O-])=O VMHLLURERBWHNL-UHFFFAOYSA-M 0.000 description 1
- 208000006045 Spondylarthropathies Diseases 0.000 description 1
- 229920002472 Starch Polymers 0.000 description 1
- 235000021355 Stearic acid Nutrition 0.000 description 1
- NINIDFKCEFEMDL-UHFFFAOYSA-N Sulfur Chemical compound [S] NINIDFKCEFEMDL-UHFFFAOYSA-N 0.000 description 1
- 230000006043 T cell recruitment Effects 0.000 description 1
- 239000012317 TBTU Substances 0.000 description 1
- 229910021626 Tin(II) chloride Inorganic materials 0.000 description 1
- UFLGIAIHIAPJJC-UHFFFAOYSA-N Tripelennamine Chemical compound C=1C=CC=NC=1N(CCN(C)C)CC1=CC=CC=C1 UFLGIAIHIAPJJC-UHFFFAOYSA-N 0.000 description 1
- YZCKVEUIGOORGS-NJFSPNSNSA-N Tritium Chemical compound [3H] YZCKVEUIGOORGS-NJFSPNSNSA-N 0.000 description 1
- 206010067584 Type 1 diabetes mellitus Diseases 0.000 description 1
- 201000006704 Ulcerative Colitis Diseases 0.000 description 1
- 206010046914 Vaginal infection Diseases 0.000 description 1
- 201000008100 Vaginitis Diseases 0.000 description 1
- 208000036142 Viral infection Diseases 0.000 description 1
- OGQICQVSFDPSEI-UHFFFAOYSA-N Zorac Chemical compound N1=CC(C(=O)OCC)=CC=C1C#CC1=CC=C(SCCC2(C)C)C2=C1 OGQICQVSFDPSEI-UHFFFAOYSA-N 0.000 description 1
- WERKSKAQRVDLDW-ANOHMWSOSA-N [(2s,3r,4r,5r)-2,3,4,5,6-pentahydroxyhexyl] (z)-octadec-9-enoate Chemical compound CCCCCCCC\C=C/CCCCCCCC(=O)OC[C@H](O)[C@@H](O)[C@H](O)[C@H](O)CO WERKSKAQRVDLDW-ANOHMWSOSA-N 0.000 description 1
- VAXMYJTZWJWBSI-UHFFFAOYSA-N [4-chloro-2-[2-[4-(4-chlorophenyl)piperazin-1-yl]-2-oxoethyl]-5-(trifluoromethyl)pyrazol-3-yl]methylurea Chemical compound NC(=O)NCC1=C(Cl)C(C(F)(F)F)=NN1CC(=O)N1CCN(C=2C=CC(Cl)=CC=2)CC1 VAXMYJTZWJWBSI-UHFFFAOYSA-N 0.000 description 1
- CLZISMQKJZCZDN-UHFFFAOYSA-N [benzotriazol-1-yloxy(dimethylamino)methylidene]-dimethylazanium Chemical compound C1=CC=C2N(OC(N(C)C)=[N+](C)C)N=NC2=C1 CLZISMQKJZCZDN-UHFFFAOYSA-N 0.000 description 1
- 238000010521 absorption reaction Methods 0.000 description 1
- 238000009825 accumulation Methods 0.000 description 1
- 229960004892 acemetacin Drugs 0.000 description 1
- FSQKKOOTNAMONP-UHFFFAOYSA-N acemetacin Chemical compound CC1=C(CC(=O)OCC(O)=O)C2=CC(OC)=CC=C2N1C(=O)C1=CC=C(Cl)C=C1 FSQKKOOTNAMONP-UHFFFAOYSA-N 0.000 description 1
- 150000001242 acetic acid derivatives Chemical class 0.000 description 1
- FHEAIOHRHQGZPC-KIWGSFCNSA-N acetic acid;(2s)-2-amino-3-(4-hydroxyphenyl)propanoic acid;(2s)-2-aminopentanedioic acid;(2s)-2-aminopropanoic acid;(2s)-2,6-diaminohexanoic acid Chemical compound CC(O)=O.C[C@H](N)C(O)=O.NCCCC[C@H](N)C(O)=O.OC(=O)[C@@H](N)CCC(O)=O.OC(=O)[C@@H](N)CC1=CC=C(O)C=C1 FHEAIOHRHQGZPC-KIWGSFCNSA-N 0.000 description 1
- DPXJVFZANSGRMM-UHFFFAOYSA-N acetic acid;2,3,4,5,6-pentahydroxyhexanal;sodium Chemical compound [Na].CC(O)=O.OCC(O)C(O)C(O)C(O)C=O DPXJVFZANSGRMM-UHFFFAOYSA-N 0.000 description 1
- CAWBRCOBJNWRLK-UHFFFAOYSA-N acetyloxymethyl 2-[4-[bis[2-(acetyloxymethoxy)-2-oxoethyl]amino]-3-[2-[2-[bis[2-(acetyloxymethoxy)-2-oxoethyl]amino]-5-methylphenoxy]ethoxy]phenyl]-1h-indole-6-carboxylate Chemical compound CC(=O)OCOC(=O)CN(CC(=O)OCOC(C)=O)C1=CC=C(C)C=C1OCCOC1=CC(C=2NC3=CC(=CC=C3C=2)C(=O)OCOC(C)=O)=CC=C1N(CC(=O)OCOC(C)=O)CC(=O)OCOC(C)=O CAWBRCOBJNWRLK-UHFFFAOYSA-N 0.000 description 1
- 239000011149 active material Substances 0.000 description 1
- 239000002671 adjuvant Substances 0.000 description 1
- NDAUXUAQIAJITI-UHFFFAOYSA-N albuterol Chemical compound CC(C)(C)NCC(O)C1=CC=C(O)C(CO)=C1 NDAUXUAQIAJITI-UHFFFAOYSA-N 0.000 description 1
- 229960005142 alclofenac Drugs 0.000 description 1
- ARHWPKZXBHOEEE-UHFFFAOYSA-N alclofenac Chemical compound OC(=O)CC1=CC=C(OCC=C)C(Cl)=C1 ARHWPKZXBHOEEE-UHFFFAOYSA-N 0.000 description 1
- 235000010443 alginic acid Nutrition 0.000 description 1
- 239000000783 alginic acid Substances 0.000 description 1
- 229920000615 alginic acid Polymers 0.000 description 1
- 229960001126 alginic acid Drugs 0.000 description 1
- 150000004781 alginic acids Chemical class 0.000 description 1
- 229960003790 alimemazine Drugs 0.000 description 1
- 150000001335 aliphatic alkanes Chemical class 0.000 description 1
- 125000003282 alkyl amino group Chemical group 0.000 description 1
- 150000001347 alkyl bromides Chemical class 0.000 description 1
- 125000004414 alkyl thio group Chemical group 0.000 description 1
- 201000009961 allergic asthma Diseases 0.000 description 1
- 208000002205 allergic conjunctivitis Diseases 0.000 description 1
- 208000002029 allergic contact dermatitis Diseases 0.000 description 1
- 201000010105 allergic rhinitis Diseases 0.000 description 1
- 201000010435 allergic urticaria Diseases 0.000 description 1
- FPHLBGOJWPEVME-UHFFFAOYSA-N alminoprofen Chemical compound OC(=O)C(C)C1=CC=C(NCC(C)=C)C=C1 FPHLBGOJWPEVME-UHFFFAOYSA-N 0.000 description 1
- 229960004663 alminoprofen Drugs 0.000 description 1
- HSFWRNGVRCDJHI-UHFFFAOYSA-N alpha-acetylene Natural products C#C HSFWRNGVRCDJHI-UHFFFAOYSA-N 0.000 description 1
- 235000012211 aluminium silicate Nutrition 0.000 description 1
- 238000005576 amination reaction Methods 0.000 description 1
- 150000001413 amino acids Chemical class 0.000 description 1
- 125000004202 aminomethyl group Chemical group [H]N([H])C([H])([H])* 0.000 description 1
- 238000005902 aminomethylation reaction Methods 0.000 description 1
- 229910021529 ammonia Inorganic materials 0.000 description 1
- 229940051880 analgesics and antipyretics pyrazolones Drugs 0.000 description 1
- 239000012491 analyte Substances 0.000 description 1
- 208000003455 anaphylaxis Diseases 0.000 description 1
- 230000033115 angiogenesis Effects 0.000 description 1
- 150000001448 anilines Chemical class 0.000 description 1
- 238000010171 animal model Methods 0.000 description 1
- 230000000954 anitussive effect Effects 0.000 description 1
- 229960002469 antazoline Drugs 0.000 description 1
- REYFJDPCWQRWAA-UHFFFAOYSA-N antazoline Chemical compound N=1CCNC=1CN(C=1C=CC=CC=1)CC1=CC=CC=C1 REYFJDPCWQRWAA-UHFFFAOYSA-N 0.000 description 1
- NUZWLKWWNNJHPT-UHFFFAOYSA-N anthralin Chemical compound C1C2=CC=CC(O)=C2C(=O)C2=C1C=CC=C2O NUZWLKWWNNJHPT-UHFFFAOYSA-N 0.000 description 1
- 230000001088 anti-asthma Effects 0.000 description 1
- 230000001387 anti-histamine Effects 0.000 description 1
- 229940121363 anti-inflammatory agent Drugs 0.000 description 1
- 230000000340 anti-metabolite Effects 0.000 description 1
- 239000000924 antiasthmatic agent Substances 0.000 description 1
- 238000009175 antibody therapy Methods 0.000 description 1
- 229940125715 antihistaminic agent Drugs 0.000 description 1
- 229940111133 antiinflammatory and antirheumatic drug oxicams Drugs 0.000 description 1
- 229940111131 antiinflammatory and antirheumatic product propionic acid derivative Drugs 0.000 description 1
- 229940100197 antimetabolite Drugs 0.000 description 1
- 239000002256 antimetabolite Substances 0.000 description 1
- 230000003078 antioxidant effect Effects 0.000 description 1
- 229940124584 antitussives Drugs 0.000 description 1
- 239000007864 aqueous solution Substances 0.000 description 1
- ODKSFYDXXFIFQN-UHFFFAOYSA-N arginine Natural products OC(=O)C(N)CCCNC(N)=N ODKSFYDXXFIFQN-UHFFFAOYSA-N 0.000 description 1
- 229960003121 arginine Drugs 0.000 description 1
- 125000005418 aryl aryl group Chemical group 0.000 description 1
- 150000001502 aryl halides Chemical class 0.000 description 1
- 125000005165 aryl thioxy group Chemical group 0.000 description 1
- 125000004104 aryloxy group Chemical group 0.000 description 1
- 235000010323 ascorbic acid Nutrition 0.000 description 1
- 229960005070 ascorbic acid Drugs 0.000 description 1
- 239000011668 ascorbic acid Substances 0.000 description 1
- GXDALQBWZGODGZ-UHFFFAOYSA-N astemizole Chemical compound C1=CC(OC)=CC=C1CCN1CCC(NC=2N(C3=CC=CC=C3N=2)CC=2C=CC(F)=CC=2)CC1 GXDALQBWZGODGZ-UHFFFAOYSA-N 0.000 description 1
- QVGXLLKOCUKJST-UHFFFAOYSA-N atomic oxygen Chemical compound [O] QVGXLLKOCUKJST-UHFFFAOYSA-N 0.000 description 1
- 208000024998 atopic conjunctivitis Diseases 0.000 description 1
- RKUNBYITZUJHSG-SPUOUPEWSA-N atropine Chemical compound O([C@H]1C[C@H]2CC[C@@H](C1)N2C)C(=O)C(CO)C1=CC=CC=C1 RKUNBYITZUJHSG-SPUOUPEWSA-N 0.000 description 1
- 229960000396 atropine Drugs 0.000 description 1
- AUJRCFUBUPVWSZ-XTZHGVARSA-M auranofin Chemical compound CCP(CC)(CC)=[Au]S[C@@H]1O[C@H](COC(C)=O)[C@@H](OC(C)=O)[C@H](OC(C)=O)[C@H]1OC(C)=O AUJRCFUBUPVWSZ-XTZHGVARSA-M 0.000 description 1
- 229960005207 auranofin Drugs 0.000 description 1
- 229960001799 aurothioglucose Drugs 0.000 description 1
- 230000006472 autoimmune response Effects 0.000 description 1
- 229940003504 avonex Drugs 0.000 description 1
- 229960001671 azapropazone Drugs 0.000 description 1
- WOIIIUDZSOLAIW-NSHDSACASA-N azapropazone Chemical compound C1=C(C)C=C2N3C(=O)[C@H](CC=C)C(=O)N3C(N(C)C)=NC2=C1 WOIIIUDZSOLAIW-NSHDSACASA-N 0.000 description 1
- 229960000383 azatadine Drugs 0.000 description 1
- SEBMTIQKRHYNIT-UHFFFAOYSA-N azatadine Chemical compound C1CN(C)CCC1=C1C2=NC=CC=C2CCC2=CC=CC=C21 SEBMTIQKRHYNIT-UHFFFAOYSA-N 0.000 description 1
- 125000002393 azetidinyl group Chemical group 0.000 description 1
- 210000003719 b-lymphocyte Anatomy 0.000 description 1
- 229960004669 basiliximab Drugs 0.000 description 1
- NBMKJKDGKREAPL-DVTGEIKXSA-N beclomethasone Chemical compound C1CC2=CC(=O)C=C[C@]2(C)[C@]2(Cl)[C@@H]1[C@@H]1C[C@H](C)[C@@](C(=O)CO)(O)[C@@]1(C)C[C@@H]2O NBMKJKDGKREAPL-DVTGEIKXSA-N 0.000 description 1
- 229940092705 beclomethasone Drugs 0.000 description 1
- 235000013871 bee wax Nutrition 0.000 description 1
- 239000012166 beeswax Substances 0.000 description 1
- 230000009286 beneficial effect Effects 0.000 description 1
- 229960005430 benoxaprofen Drugs 0.000 description 1
- JUHORIMYRDESRB-UHFFFAOYSA-N benzathine Chemical compound C=1C=CC=CC=1CNCCNCC1=CC=CC=C1 JUHORIMYRDESRB-UHFFFAOYSA-N 0.000 description 1
- WPYMKLBDIGXBTP-UHFFFAOYSA-N benzoic acid group Chemical group C(C1=CC=CC=C1)(=O)O WPYMKLBDIGXBTP-UHFFFAOYSA-N 0.000 description 1
- ZJRCIQAMTAINCB-UHFFFAOYSA-N benzoylacetonitrile Chemical compound N#CCC(=O)C1=CC=CC=C1 ZJRCIQAMTAINCB-UHFFFAOYSA-N 0.000 description 1
- 125000001797 benzyl group Chemical group [H]C1=C([H])C([H])=C(C([H])=C1[H])C([H])([H])* 0.000 description 1
- 229930008407 benzylideneacetone Natural products 0.000 description 1
- 229960003237 betaine Drugs 0.000 description 1
- 229960002537 betamethasone Drugs 0.000 description 1
- UREBDLICKHMUKA-DVTGEIKXSA-N betamethasone Chemical compound C1CC2=CC(=O)C=C[C@]2(C)[C@]2(F)[C@@H]1[C@@H]1C[C@H](C)[C@@](C(=O)CO)(O)[C@@]1(C)C[C@@H]2O UREBDLICKHMUKA-DVTGEIKXSA-N 0.000 description 1
- 229940021459 betaseron Drugs 0.000 description 1
- 125000002619 bicyclic group Chemical group 0.000 description 1
- GPRLTFBKWDERLU-UHFFFAOYSA-N bicyclo[2.2.2]octane Chemical compound C1CC2CCC1CC2 GPRLTFBKWDERLU-UHFFFAOYSA-N 0.000 description 1
- 239000011230 binding agent Substances 0.000 description 1
- 230000004071 biological effect Effects 0.000 description 1
- 230000033228 biological regulation Effects 0.000 description 1
- 239000004305 biphenyl Chemical group 0.000 description 1
- 235000010290 biphenyl Nutrition 0.000 description 1
- 229960004620 bitolterol Drugs 0.000 description 1
- FZGVEKPRDOIXJY-UHFFFAOYSA-N bitolterol Chemical compound C1=CC(C)=CC=C1C(=O)OC1=CC=C(C(O)CNC(C)(C)C)C=C1OC(=O)C1=CC=C(C)C=C1 FZGVEKPRDOIXJY-UHFFFAOYSA-N 0.000 description 1
- 230000000903 blocking effect Effects 0.000 description 1
- 210000001185 bone marrow Anatomy 0.000 description 1
- 229940098773 bovine serum albumin Drugs 0.000 description 1
- ZDIGNSYAACHWNL-UHFFFAOYSA-N brompheniramine Chemical compound C=1C=CC=NC=1C(CCN(C)C)C1=CC=C(Br)C=C1 ZDIGNSYAACHWNL-UHFFFAOYSA-N 0.000 description 1
- 229960000725 brompheniramine Drugs 0.000 description 1
- IJTPQQVCKPZIMV-UHFFFAOYSA-N bucloxic acid Chemical compound ClC1=CC(C(=O)CCC(=O)O)=CC=C1C1CCCCC1 IJTPQQVCKPZIMV-UHFFFAOYSA-N 0.000 description 1
- 229950005608 bucloxic acid Drugs 0.000 description 1
- 229960004436 budesonide Drugs 0.000 description 1
- 235000019437 butane-1,3-diol Nutrition 0.000 description 1
- 239000001110 calcium chloride Substances 0.000 description 1
- 229910001628 calcium chloride Inorganic materials 0.000 description 1
- 230000028956 calcium-mediated signaling Effects 0.000 description 1
- 201000011510 cancer Diseases 0.000 description 1
- OFAIGZWCDGNZGT-UHFFFAOYSA-N caramiphen Chemical compound C=1C=CC=CC=1C1(C(=O)OCCN(CC)CC)CCCC1 OFAIGZWCDGNZGT-UHFFFAOYSA-N 0.000 description 1
- 229960004160 caramiphen Drugs 0.000 description 1
- 239000004202 carbamide Substances 0.000 description 1
- PFKFTWBEEFSNDU-UHFFFAOYSA-N carbonyldiimidazole Chemical compound C1=CN=CN1C(=O)N1C=CN=C1 PFKFTWBEEFSNDU-UHFFFAOYSA-N 0.000 description 1
- 239000001768 carboxy methyl cellulose Substances 0.000 description 1
- 150000001732 carboxylic acid derivatives Chemical class 0.000 description 1
- 229960003184 carprofen Drugs 0.000 description 1
- IVUMCTKHWDRRMH-UHFFFAOYSA-N carprofen Chemical compound C1=CC(Cl)=C[C]2C3=CC=C(C(C(O)=O)C)C=C3N=C21 IVUMCTKHWDRRMH-UHFFFAOYSA-N 0.000 description 1
- 210000000845 cartilage Anatomy 0.000 description 1
- 230000015556 catabolic process Effects 0.000 description 1
- 229940047495 celebrex Drugs 0.000 description 1
- 229960000590 celecoxib Drugs 0.000 description 1
- 230000003915 cell function Effects 0.000 description 1
- 230000004663 cell proliferation Effects 0.000 description 1
- 229940107810 cellcept Drugs 0.000 description 1
- 230000001413 cellular effect Effects 0.000 description 1
- 229960001803 cetirizine Drugs 0.000 description 1
- 229960000541 cetyl alcohol Drugs 0.000 description 1
- PBAYDYUZOSNJGU-UHFFFAOYSA-N chelidonic acid Natural products OC(=O)C1=CC(=O)C=C(C(O)=O)O1 PBAYDYUZOSNJGU-UHFFFAOYSA-N 0.000 description 1
- 239000002975 chemoattractant Substances 0.000 description 1
- 239000007910 chewable tablet Substances 0.000 description 1
- 229940112822 chewing gum Drugs 0.000 description 1
- 235000015218 chewing gum Nutrition 0.000 description 1
- WORJEOGGNQDSOE-UHFFFAOYSA-N chloroform;methanol Chemical compound OC.ClC(Cl)Cl WORJEOGGNQDSOE-UHFFFAOYSA-N 0.000 description 1
- SOYKEARSMXGVTM-UHFFFAOYSA-N chlorphenamine Chemical compound C=1C=CC=NC=1C(CCN(C)C)C1=CC=C(Cl)C=C1 SOYKEARSMXGVTM-UHFFFAOYSA-N 0.000 description 1
- 229960003291 chlorphenamine Drugs 0.000 description 1
- OEYIOHPDSNJKLS-UHFFFAOYSA-N choline Chemical compound C[N+](C)(C)CCO OEYIOHPDSNJKLS-UHFFFAOYSA-N 0.000 description 1
- 229960001231 choline Drugs 0.000 description 1
- 238000004587 chromatography analysis Methods 0.000 description 1
- 229960001265 ciclosporin Drugs 0.000 description 1
- 229960002881 clemastine Drugs 0.000 description 1
- YNNUSGIPVFPVBX-NHCUHLMSSA-N clemastine Chemical compound CN1CCC[C@@H]1CCO[C@@](C)(C=1C=CC(Cl)=CC=1)C1=CC=CC=C1 YNNUSGIPVFPVBX-NHCUHLMSSA-N 0.000 description 1
- 229950010886 clidanac Drugs 0.000 description 1
- 229940110456 cocoa butter Drugs 0.000 description 1
- 235000019868 cocoa butter Nutrition 0.000 description 1
- 239000003240 coconut oil Substances 0.000 description 1
- 235000019864 coconut oil Nutrition 0.000 description 1
- 229960001338 colchicine Drugs 0.000 description 1
- 238000004590 computer program Methods 0.000 description 1
- 235000008504 concentrate Nutrition 0.000 description 1
- 239000012141 concentrate Substances 0.000 description 1
- 238000001816 cooling Methods 0.000 description 1
- 229910052802 copper Inorganic materials 0.000 description 1
- 239000010949 copper Substances 0.000 description 1
- 239000008120 corn starch Substances 0.000 description 1
- 239000003246 corticosteroid Substances 0.000 description 1
- 229960001334 corticosteroids Drugs 0.000 description 1
- 239000006071 cream Substances 0.000 description 1
- 229960000265 cromoglicic acid Drugs 0.000 description 1
- IMZMKUWMOSJXDT-UHFFFAOYSA-N cromoglycic acid Chemical compound O1C(C(O)=O)=CC(=O)C2=C1C=CC=C2OCC(O)COC1=CC=CC2=C1C(=O)C=C(C(O)=O)O2 IMZMKUWMOSJXDT-UHFFFAOYSA-N 0.000 description 1
- 238000006880 cross-coupling reaction Methods 0.000 description 1
- 239000013058 crude material Substances 0.000 description 1
- 239000013078 crystal Substances 0.000 description 1
- WZHCOOQXZCIUNC-UHFFFAOYSA-N cyclandelate Chemical compound C1C(C)(C)CC(C)CC1OC(=O)C(O)C1=CC=CC=C1 WZHCOOQXZCIUNC-UHFFFAOYSA-N 0.000 description 1
- 239000003255 cyclooxygenase 2 inhibitor Substances 0.000 description 1
- 229960004397 cyclophosphamide Drugs 0.000 description 1
- 229930182912 cyclosporin Natural products 0.000 description 1
- 229960001140 cyproheptadine Drugs 0.000 description 1
- JJCFRYNCJDLXIK-UHFFFAOYSA-N cyproheptadine Chemical compound C1CN(C)CCC1=C1C2=CC=CC=C2C=CC2=CC=CC=C21 JJCFRYNCJDLXIK-UHFFFAOYSA-N 0.000 description 1
- 210000001151 cytotoxic T lymphocyte Anatomy 0.000 description 1
- KWGRBVOPPLSCSI-UHFFFAOYSA-N d-ephedrine Natural products CNC(C)C(O)C1=CC=CC=C1 KWGRBVOPPLSCSI-UHFFFAOYSA-N 0.000 description 1
- 229960002806 daclizumab Drugs 0.000 description 1
- 239000000850 decongestant Substances 0.000 description 1
- 230000003247 decreasing effect Effects 0.000 description 1
- 230000007123 defense Effects 0.000 description 1
- 238000006731 degradation reaction Methods 0.000 description 1
- 230000002939 deleterious effect Effects 0.000 description 1
- 238000010511 deprotection reaction Methods 0.000 description 1
- 238000001212 derivatisation Methods 0.000 description 1
- 229960001271 desloratadine Drugs 0.000 description 1
- 230000001066 destructive effect Effects 0.000 description 1
- 229960003957 dexamethasone Drugs 0.000 description 1
- UREBDLICKHMUKA-CXSFZGCWSA-N dexamethasone Chemical compound C1CC2=CC(=O)C=C[C@]2(C)[C@]2(F)[C@@H]1[C@@H]1C[C@@H](C)[C@@](C(=O)CO)(O)[C@@]1(C)C[C@@H]2O UREBDLICKHMUKA-CXSFZGCWSA-N 0.000 description 1
- 229960001985 dextromethorphan Drugs 0.000 description 1
- 125000004663 dialkyl amino group Chemical group 0.000 description 1
- 229960001259 diclofenac Drugs 0.000 description 1
- DCOPUUMXTXDBNB-UHFFFAOYSA-N diclofenac Chemical compound OC(=O)CC1=CC=CC=C1NC1=C(Cl)C=CC=C1Cl DCOPUUMXTXDBNB-UHFFFAOYSA-N 0.000 description 1
- 235000005911 diet Nutrition 0.000 description 1
- 230000037213 diet Effects 0.000 description 1
- MDJHSSJHBRMILK-UHFFFAOYSA-N diethyl 1-[2-[4-(4-fluorophenyl)piperazin-1-yl]-2-oxoethyl]pyrazole-3,5-dicarboxylate Chemical compound N1=C(C(=O)OCC)C=C(C(=O)OCC)N1CC(=O)N1CCN(C=2C=CC(F)=CC=2)CC1 MDJHSSJHBRMILK-UHFFFAOYSA-N 0.000 description 1
- MBWXLICVQZUJOW-UHFFFAOYSA-N diethyl 1h-pyrazole-3,5-dicarboxylate Chemical compound CCOC(=O)C=1C=C(C(=O)OCC)NN=1 MBWXLICVQZUJOW-UHFFFAOYSA-N 0.000 description 1
- FAMRKDQNMBBFBR-BQYQJAHWSA-N diethyl azodicarboxylate Substances CCOC(=O)\N=N\C(=O)OCC FAMRKDQNMBBFBR-BQYQJAHWSA-N 0.000 description 1
- HPNMFZURTQLUMO-UHFFFAOYSA-N diethylamine Chemical compound CCNCC HPNMFZURTQLUMO-UHFFFAOYSA-N 0.000 description 1
- HUPFGZXOMWLGNK-UHFFFAOYSA-N diflunisal Chemical compound C1=C(O)C(C(=O)O)=CC(C=2C(=CC(F)=CC=2)F)=C1 HUPFGZXOMWLGNK-UHFFFAOYSA-N 0.000 description 1
- 229960000616 diflunisal Drugs 0.000 description 1
- XYYVYLMBEZUESM-UHFFFAOYSA-N dihydrocodeine Natural products C1C(N(CCC234)C)C2C=CC(=O)C3OC2=C4C1=CC=C2OC XYYVYLMBEZUESM-UHFFFAOYSA-N 0.000 description 1
- 238000007865 diluting Methods 0.000 description 1
- 230000003292 diminished effect Effects 0.000 description 1
- AMTWCFIAVKBGOD-UHFFFAOYSA-N dioxosilane;methoxy-dimethyl-trimethylsilyloxysilane Chemical compound O=[Si]=O.CO[Si](C)(C)O[Si](C)(C)C AMTWCFIAVKBGOD-UHFFFAOYSA-N 0.000 description 1
- 229960000520 diphenhydramine Drugs 0.000 description 1
- OWQUZNMMYNAXSL-UHFFFAOYSA-N diphenylpyraline Chemical compound C1CN(C)CCC1OC(C=1C=CC=CC=1)C1=CC=CC=C1 OWQUZNMMYNAXSL-UHFFFAOYSA-N 0.000 description 1
- 229960000879 diphenylpyraline Drugs 0.000 description 1
- 238000004821 distillation Methods 0.000 description 1
- 229960002311 dithranol Drugs 0.000 description 1
- 239000002934 diuretic Substances 0.000 description 1
- 230000001882 diuretic effect Effects 0.000 description 1
- DLNKOYKMWOXYQA-UHFFFAOYSA-N dl-pseudophenylpropanolamine Natural products CC(N)C(O)C1=CC=CC=C1 DLNKOYKMWOXYQA-UHFFFAOYSA-N 0.000 description 1
- 239000002552 dosage form Substances 0.000 description 1
- 229940075049 dovonex Drugs 0.000 description 1
- 239000000890 drug combination Substances 0.000 description 1
- 238000012377 drug delivery Methods 0.000 description 1
- 230000009977 dual effect Effects 0.000 description 1
- 239000000975 dye Substances 0.000 description 1
- 208000019258 ear infection Diseases 0.000 description 1
- 239000007911 effervescent powder Substances 0.000 description 1
- 239000007938 effervescent tablet Substances 0.000 description 1
- 238000004945 emulsification Methods 0.000 description 1
- 239000003995 emulsifying agent Substances 0.000 description 1
- 229940073621 enbrel Drugs 0.000 description 1
- 206010014599 encephalitis Diseases 0.000 description 1
- 210000003743 erythrocyte Anatomy 0.000 description 1
- 229960000403 etanercept Drugs 0.000 description 1
- OMTPOVVFJOLQAZ-UHFFFAOYSA-N ethyl 1-[2-[4-(4-fluorophenyl)piperazin-1-yl]-2-oxoethyl]-3-(trifluoromethyl)pyrazole-4-carboxylate Chemical compound N1=C(C(F)(F)F)C(C(=O)OCC)=CN1CC(=O)N1CCN(C=2C=CC(F)=CC=2)CC1 OMTPOVVFJOLQAZ-UHFFFAOYSA-N 0.000 description 1
- PBJRVPFJHVHHQQ-UHFFFAOYSA-N ethyl 1-[2-[4-(4-fluorophenyl)piperazin-1-yl]-2-oxoethyl]-5-propylpyrazole-3-carboxylate Chemical compound CCCC1=CC(C(=O)OCC)=NN1CC(=O)N1CCN(C=2C=CC(F)=CC=2)CC1 PBJRVPFJHVHHQQ-UHFFFAOYSA-N 0.000 description 1
- RHOCFVDCBOIWBS-UHFFFAOYSA-N ethyl 1-[2-[4-(4-fluorophenyl)piperazin-1-yl]-2-oxoethyl]pyrazole-4-carboxylate Chemical compound C1=C(C(=O)OCC)C=NN1CC(=O)N1CCN(C=2C=CC(F)=CC=2)CC1 RHOCFVDCBOIWBS-UHFFFAOYSA-N 0.000 description 1
- KACZQOKEFKFNDB-UHFFFAOYSA-N ethyl 1h-pyrazole-4-carboxylate Chemical compound CCOC(=O)C=1C=NNC=1 KACZQOKEFKFNDB-UHFFFAOYSA-N 0.000 description 1
- VOCDVDANMTYQMD-UHFFFAOYSA-N ethyl 2-[2-[4-(4-chlorophenyl)piperazin-1-yl]-2-oxoethyl]-5-thiophen-2-ylpyrazole-3-carboxylate Chemical compound CCOC(=O)C1=CC(C=2SC=CC=2)=NN1CC(=O)N(CC1)CCN1C1=CC=C(Cl)C=C1 VOCDVDANMTYQMD-UHFFFAOYSA-N 0.000 description 1
- VXFCWCWMUFXKLI-UHFFFAOYSA-N ethyl 2-[2-[4-(4-fluorophenyl)piperazin-1-yl]-2-oxoethyl]-5-methylpyrazole-3-carboxylate Chemical compound CCOC(=O)C1=CC(C)=NN1CC(=O)N1CCN(C=2C=CC(F)=CC=2)CC1 VXFCWCWMUFXKLI-UHFFFAOYSA-N 0.000 description 1
- IKDGVYVUTYLXAN-UHFFFAOYSA-N ethyl 2-[2-[4-(4-fluorophenyl)piperazin-1-yl]-2-oxoethyl]-5-propylpyrazole-3-carboxylate Chemical compound N1=C(CCC)C=C(C(=O)OCC)N1CC(=O)N1CCN(C=2C=CC(F)=CC=2)CC1 IKDGVYVUTYLXAN-UHFFFAOYSA-N 0.000 description 1
- LQJCYZYDTDOWKA-UHFFFAOYSA-N ethyl 2-[2-[4-(4-fluorophenyl)piperazin-1-yl]-2-oxoethyl]-5-thiophen-2-ylpyrazole-3-carboxylate Chemical compound CCOC(=O)C1=CC(C=2SC=CC=2)=NN1CC(=O)N(CC1)CCN1C1=CC=C(F)C=C1 LQJCYZYDTDOWKA-UHFFFAOYSA-N 0.000 description 1
- XVLVDRLHAACPOY-UHFFFAOYSA-N ethyl 2-[4-chloro-5-methyl-3-(trifluoromethyl)pyrazol-1-yl]-2-(3-methoxyphenyl)acetate Chemical compound N1=C(C(F)(F)F)C(Cl)=C(C)N1C(C(=O)OCC)C1=CC=CC(OC)=C1 XVLVDRLHAACPOY-UHFFFAOYSA-N 0.000 description 1
- VFJRSJKWRMVIAA-UHFFFAOYSA-N ethyl 2-[5-(bromomethyl)-4-chloro-3-methylpyrazol-1-yl]acetate Chemical compound CCOC(=O)CN1N=C(C)C(Cl)=C1CBr VFJRSJKWRMVIAA-UHFFFAOYSA-N 0.000 description 1
- INYWVNLRFQCNRR-UHFFFAOYSA-N ethyl 3-amino-1-[2-[4-(4-fluorophenyl)piperazin-1-yl]-2-oxoethyl]-5-methylpyrazole-4-carboxylate Chemical compound CC1=C(C(=O)OCC)C(N)=NN1CC(=O)N1CCN(C=2C=CC(F)=CC=2)CC1 INYWVNLRFQCNRR-UHFFFAOYSA-N 0.000 description 1
- WOCMIZZYXHVSPS-UHFFFAOYSA-N ethyl 3-amino-5-methyl-1h-pyrazole-4-carboxylate Chemical compound CCOC(=O)C1=C(C)NN=C1N WOCMIZZYXHVSPS-UHFFFAOYSA-N 0.000 description 1
- CBAYPEDSNOPXRY-UHFFFAOYSA-N ethyl 4-chloro-1-[2-[4-(4-fluorophenyl)piperazin-1-yl]-2-oxoethyl]-5-thiophen-2-ylpyrazole-3-carboxylate Chemical compound C=1C=CSC=1C1=C(Cl)C(C(=O)OCC)=NN1CC(=O)N(CC1)CCN1C1=CC=C(F)C=C1 CBAYPEDSNOPXRY-UHFFFAOYSA-N 0.000 description 1
- IXKIGCYXGRXWJO-UHFFFAOYSA-N ethyl 4-chloro-2-[2-[4-(4-fluorophenyl)piperazin-1-yl]-2-oxoethyl]-5-methylpyrazole-3-carboxylate Chemical compound CCOC(=O)C1=C(Cl)C(C)=NN1CC(=O)N1CCN(C=2C=CC(F)=CC=2)CC1 IXKIGCYXGRXWJO-UHFFFAOYSA-N 0.000 description 1
- HZYPILUIBJQBOU-UHFFFAOYSA-N ethyl 4-chloro-2-[2-[4-(4-fluorophenyl)piperazin-1-yl]-2-oxoethyl]-5-propylpyrazole-3-carboxylate Chemical compound CCOC(=O)C1=C(Cl)C(CCC)=NN1CC(=O)N1CCN(C=2C=CC(F)=CC=2)CC1 HZYPILUIBJQBOU-UHFFFAOYSA-N 0.000 description 1
- GDKWDJUNCZSRIC-UHFFFAOYSA-N ethyl 4-chloro-5-(5-chlorothiophen-2-yl)-1h-pyrazole-3-carboxylate Chemical compound ClC1=C(C(=O)OCC)NN=C1C1=CC=C(Cl)S1 GDKWDJUNCZSRIC-UHFFFAOYSA-N 0.000 description 1
- BZAWVCCAUDOUGZ-UHFFFAOYSA-N ethyl 4-chloro-5-(5-chlorothiophen-2-yl)-2-[2-[4-(4-fluorophenyl)piperazin-1-yl]-2-oxoethyl]pyrazole-3-carboxylate Chemical compound CCOC(=O)C1=C(Cl)C(C=2SC(Cl)=CC=2)=NN1CC(=O)N(CC1)CCN1C1=CC=C(F)C=C1 BZAWVCCAUDOUGZ-UHFFFAOYSA-N 0.000 description 1
- VYXIHSAEOXPAEY-UHFFFAOYSA-N ethyl 5-(trifluoromethyl)-1h-pyrazole-4-carboxylate Chemical compound CCOC(=O)C=1C=NNC=1C(F)(F)F VYXIHSAEOXPAEY-UHFFFAOYSA-N 0.000 description 1
- FAMRKDQNMBBFBR-UHFFFAOYSA-N ethyl n-ethoxycarbonyliminocarbamate Chemical compound CCOC(=O)N=NC(=O)OCC FAMRKDQNMBBFBR-UHFFFAOYSA-N 0.000 description 1
- 125000002534 ethynyl group Chemical group [H]C#C* 0.000 description 1
- 230000005284 excitation Effects 0.000 description 1
- 230000029142 excretion Effects 0.000 description 1
- 208000024711 extrinsic asthma Diseases 0.000 description 1
- ZWJINEZUASEZBH-UHFFFAOYSA-N fenamic acid Chemical class OC(=O)C1=CC=CC=C1NC1=CC=CC=C1 ZWJINEZUASEZBH-UHFFFAOYSA-N 0.000 description 1
- ZPAKPRAICRBAOD-UHFFFAOYSA-N fenbufen Chemical compound C1=CC(C(=O)CCC(=O)O)=CC=C1C1=CC=CC=C1 ZPAKPRAICRBAOD-UHFFFAOYSA-N 0.000 description 1
- 229960001395 fenbufen Drugs 0.000 description 1
- 229950006236 fenclofenac Drugs 0.000 description 1
- IDKAXRLETRCXKS-UHFFFAOYSA-N fenclofenac Chemical compound OC(=O)CC1=CC=CC=C1OC1=CC=C(Cl)C=C1Cl IDKAXRLETRCXKS-UHFFFAOYSA-N 0.000 description 1
- 229950011481 fenclozic acid Drugs 0.000 description 1
- 229960001419 fenoprofen Drugs 0.000 description 1
- 229960001022 fenoterol Drugs 0.000 description 1
- 229960002428 fentanyl Drugs 0.000 description 1
- IVLVTNPOHDFFCJ-UHFFFAOYSA-N fentanyl citrate Chemical compound OC(=O)CC(O)(C(O)=O)CC(O)=O.C=1C=CC=CC=1N(C(=O)CC)C(CC1)CCN1CCC1=CC=CC=C1 IVLVTNPOHDFFCJ-UHFFFAOYSA-N 0.000 description 1
- 229960002679 fentiazac Drugs 0.000 description 1
- 229960000489 feprazone Drugs 0.000 description 1
- 229960003592 fexofenadine Drugs 0.000 description 1
- RWTNPBWLLIMQHL-UHFFFAOYSA-N fexofenadine Chemical compound C1=CC(C(C)(C(O)=O)C)=CC=C1C(O)CCCN1CCC(C(O)(C=2C=CC=CC=2)C=2C=CC=CC=2)CC1 RWTNPBWLLIMQHL-UHFFFAOYSA-N 0.000 description 1
- 229960004369 flufenamic acid Drugs 0.000 description 1
- LPEPZBJOKDYZAD-UHFFFAOYSA-N flufenamic acid Chemical compound OC(=O)C1=CC=CC=C1NC1=CC=CC(C(F)(F)F)=C1 LPEPZBJOKDYZAD-UHFFFAOYSA-N 0.000 description 1
- 229950007979 flufenisal Drugs 0.000 description 1
- 239000012530 fluid Substances 0.000 description 1
- 239000007850 fluorescent dye Substances 0.000 description 1
- 239000011737 fluorine Substances 0.000 description 1
- 229950001284 fluprofen Drugs 0.000 description 1
- 229960002390 flurbiprofen Drugs 0.000 description 1
- SYTBZMRGLBWNTM-UHFFFAOYSA-N flurbiprofen Chemical compound FC1=CC(C(C(O)=O)C)=CC=C1C1=CC=CC=C1 SYTBZMRGLBWNTM-UHFFFAOYSA-N 0.000 description 1
- 229960002714 fluticasone Drugs 0.000 description 1
- MGNNYOODZCAHBA-GQKYHHCASA-N fluticasone Chemical compound C1([C@@H](F)C2)=CC(=O)C=C[C@]1(C)[C@]1(F)[C@@H]2[C@@H]2C[C@@H](C)[C@@](C(=O)SCF)(O)[C@@]2(C)C[C@@H]1O MGNNYOODZCAHBA-GQKYHHCASA-N 0.000 description 1
- RIKMMFOAQPJVMX-UHFFFAOYSA-N fomepizole Chemical compound CC=1C=NNC=1 RIKMMFOAQPJVMX-UHFFFAOYSA-N 0.000 description 1
- 235000019253 formic acid Nutrition 0.000 description 1
- 229950010931 furofenac Drugs 0.000 description 1
- 229940083124 ganglion-blocking antiadrenergic secondary and tertiary amines Drugs 0.000 description 1
- 239000007789 gas Substances 0.000 description 1
- 210000001035 gastrointestinal tract Anatomy 0.000 description 1
- 229920000159 gelatin Polymers 0.000 description 1
- 239000008273 gelatin Substances 0.000 description 1
- 235000019322 gelatine Nutrition 0.000 description 1
- 235000011852 gelatine desserts Nutrition 0.000 description 1
- 229960003776 glatiramer acetate Drugs 0.000 description 1
- 239000003862 glucocorticoid Substances 0.000 description 1
- 229940074045 glyceryl distearate Drugs 0.000 description 1
- 229940075507 glyceryl monostearate Drugs 0.000 description 1
- 150000002344 gold compounds Chemical class 0.000 description 1
- 150000004820 halides Chemical class 0.000 description 1
- 125000001188 haloalkyl group Chemical group 0.000 description 1
- 230000026030 halogenation Effects 0.000 description 1
- 238000005658 halogenation reaction Methods 0.000 description 1
- 239000007902 hard capsule Substances 0.000 description 1
- 210000002216 heart Anatomy 0.000 description 1
- 208000006454 hepatitis Diseases 0.000 description 1
- 231100000283 hepatitis Toxicity 0.000 description 1
- 125000004404 heteroalkyl group Chemical group 0.000 description 1
- FBPFZTCFMRRESA-UHFFFAOYSA-N hexane-1,2,3,4,5,6-hexol Chemical compound OCC(O)C(O)C(O)C(O)CO FBPFZTCFMRRESA-UHFFFAOYSA-N 0.000 description 1
- 238000004128 high performance liquid chromatography Methods 0.000 description 1
- 239000003485 histamine H2 receptor antagonist Substances 0.000 description 1
- HNDVDQJCIGZPNO-UHFFFAOYSA-N histidine Natural products OC(=O)C(N)CC1=CN=CN1 HNDVDQJCIGZPNO-UHFFFAOYSA-N 0.000 description 1
- 208000033519 human immunodeficiency virus infectious disease Diseases 0.000 description 1
- XGIHQYAWBCFNPY-AZOCGYLKSA-N hydrabamine Chemical compound C([C@@H]12)CC3=CC(C(C)C)=CC=C3[C@@]2(C)CCC[C@@]1(C)CNCCNC[C@@]1(C)[C@@H]2CCC3=CC(C(C)C)=CC=C3[C@@]2(C)CCC1 XGIHQYAWBCFNPY-AZOCGYLKSA-N 0.000 description 1
- 229930195733 hydrocarbon Natural products 0.000 description 1
- LLPOLZWFYMWNKH-CMKMFDCUSA-N hydrocodone Chemical compound C([C@H]1[C@H](N(CC[C@@]112)C)C3)CC(=O)[C@@H]1OC1=C2C3=CC=C1OC LLPOLZWFYMWNKH-CMKMFDCUSA-N 0.000 description 1
- 229960000240 hydrocodone Drugs 0.000 description 1
- 229960000890 hydrocortisone Drugs 0.000 description 1
- 229960001330 hydroxycarbamide Drugs 0.000 description 1
- XXSMGPRMXLTPCZ-UHFFFAOYSA-N hydroxychloroquine Chemical compound ClC1=CC=C2C(NC(C)CCCN(CCO)CC)=CC=NC2=C1 XXSMGPRMXLTPCZ-UHFFFAOYSA-N 0.000 description 1
- 229960004171 hydroxychloroquine Drugs 0.000 description 1
- 235000010979 hydroxypropyl methyl cellulose Nutrition 0.000 description 1
- 239000001866 hydroxypropyl methyl cellulose Substances 0.000 description 1
- 229920003088 hydroxypropyl methyl cellulose Polymers 0.000 description 1
- UFVKGYZPFZQRLF-UHFFFAOYSA-N hydroxypropyl methyl cellulose Chemical compound OC1C(O)C(OC)OC(CO)C1OC1C(O)C(O)C(OC2C(C(O)C(OC3C(C(O)C(O)C(CO)O3)O)C(CO)O2)O)C(CO)O1 UFVKGYZPFZQRLF-UHFFFAOYSA-N 0.000 description 1
- ZQDWXGKKHFNSQK-UHFFFAOYSA-N hydroxyzine Chemical compound C1CN(CCOCCO)CCN1C(C=1C=CC(Cl)=CC=1)C1=CC=CC=C1 ZQDWXGKKHFNSQK-UHFFFAOYSA-N 0.000 description 1
- 229960000930 hydroxyzine Drugs 0.000 description 1
- 229950009183 ibufenac Drugs 0.000 description 1
- CYWFCPPBTWOZSF-UHFFFAOYSA-N ibufenac Chemical compound CC(C)CC1=CC=C(CC(O)=O)C=C1 CYWFCPPBTWOZSF-UHFFFAOYSA-N 0.000 description 1
- 208000009326 ileitis Diseases 0.000 description 1
- 230000028993 immune response Effects 0.000 description 1
- 239000007943 implant Substances 0.000 description 1
- 229940073062 imuran Drugs 0.000 description 1
- 238000001727 in vivo Methods 0.000 description 1
- 229960004187 indoprofen Drugs 0.000 description 1
- 239000003701 inert diluent Substances 0.000 description 1
- 229960000598 infliximab Drugs 0.000 description 1
- 238000001802 infusion Methods 0.000 description 1
- 229940102223 injectable solution Drugs 0.000 description 1
- 229940102213 injectable suspension Drugs 0.000 description 1
- 238000002347 injection Methods 0.000 description 1
- 239000007924 injection Substances 0.000 description 1
- 150000007529 inorganic bases Chemical class 0.000 description 1
- 230000002452 interceptive effect Effects 0.000 description 1
- 238000007918 intramuscular administration Methods 0.000 description 1
- 238000007912 intraperitoneal administration Methods 0.000 description 1
- 238000001990 intravenous administration Methods 0.000 description 1
- ZCYVEMRRCGMTRW-YPZZEJLDSA-N iodine-125 Chemical compound [125I] ZCYVEMRRCGMTRW-YPZZEJLDSA-N 0.000 description 1
- 229940044173 iodine-125 Drugs 0.000 description 1
- 229960001361 ipratropium bromide Drugs 0.000 description 1
- KEWHKYJURDBRMN-ZEODDXGYSA-M ipratropium bromide hydrate Chemical compound O.[Br-].O([C@H]1C[C@H]2CC[C@@H](C1)[N@@+]2(C)C(C)C)C(=O)C(CO)C1=CC=CC=C1 KEWHKYJURDBRMN-ZEODDXGYSA-M 0.000 description 1
- 229950000831 iralukast Drugs 0.000 description 1
- 125000000959 isobutyl group Chemical group [H]C([H])([H])C([H])(C([H])([H])[H])C([H])([H])* 0.000 description 1
- 229960001268 isoetarine Drugs 0.000 description 1
- 238000002955 isolation Methods 0.000 description 1
- QXJSBBXBKPUZAA-UHFFFAOYSA-N isooleic acid Natural products CCCCCCCC=CCCCCCCCCC(O)=O QXJSBBXBKPUZAA-UHFFFAOYSA-N 0.000 description 1
- 125000001449 isopropyl group Chemical group [H]C([H])([H])C([H])(*)C([H])([H])[H] 0.000 description 1
- JJWLVOIRVHMVIS-UHFFFAOYSA-N isopropylamine Chemical compound CC(C)N JJWLVOIRVHMVIS-UHFFFAOYSA-N 0.000 description 1
- 230000000155 isotopic effect Effects 0.000 description 1
- 229960005280 isotretinoin Drugs 0.000 description 1
- 229950011455 isoxepac Drugs 0.000 description 1
- QFGMXJOBTNZHEL-UHFFFAOYSA-N isoxepac Chemical compound O1CC2=CC=CC=C2C(=O)C2=CC(CC(=O)O)=CC=C21 QFGMXJOBTNZHEL-UHFFFAOYSA-N 0.000 description 1
- 229950002252 isoxicam Drugs 0.000 description 1
- YYUAYBYLJSNDCX-UHFFFAOYSA-N isoxicam Chemical compound OC=1C2=CC=CC=C2S(=O)(=O)N(C)C=1C(=O)NC=1C=C(C)ON=1 YYUAYBYLJSNDCX-UHFFFAOYSA-N 0.000 description 1
- 235000015110 jellies Nutrition 0.000 description 1
- 210000001503 joint Anatomy 0.000 description 1
- 201000002215 juvenile rheumatoid arthritis Diseases 0.000 description 1
- NLYAJNPCOHFWQQ-UHFFFAOYSA-N kaolin Chemical compound O.O.O=[Al]O[Si](=O)O[Si](=O)O[Al]=O NLYAJNPCOHFWQQ-UHFFFAOYSA-N 0.000 description 1
- 239000003410 keratolytic agent Substances 0.000 description 1
- 150000002576 ketones Chemical class 0.000 description 1
- DKYWVDODHFEZIM-UHFFFAOYSA-N ketoprofen Chemical compound OC(=O)C(C)C1=CC=CC(C(=O)C=2C=CC=CC=2)=C1 DKYWVDODHFEZIM-UHFFFAOYSA-N 0.000 description 1
- 229960000991 ketoprofen Drugs 0.000 description 1
- 229960004752 ketorolac Drugs 0.000 description 1
- OZWKMVRBQXNZKK-UHFFFAOYSA-N ketorolac Chemical compound OC(=O)C1CCN2C1=CC=C2C(=O)C1=CC=CC=C1 OZWKMVRBQXNZKK-UHFFFAOYSA-N 0.000 description 1
- 210000003734 kidney Anatomy 0.000 description 1
- 239000008101 lactose Substances 0.000 description 1
- 229940039717 lanolin Drugs 0.000 description 1
- 235000019388 lanolin Nutrition 0.000 description 1
- 239000003199 leukotriene receptor blocking agent Substances 0.000 description 1
- 150000002617 leukotrienes Chemical class 0.000 description 1
- 230000000670 limiting effect Effects 0.000 description 1
- 229910052744 lithium Inorganic materials 0.000 description 1
- 210000004185 liver Anatomy 0.000 description 1
- 238000011068 loading method Methods 0.000 description 1
- 229960003088 loratadine Drugs 0.000 description 1
- JCCNYMKQOSZNPW-UHFFFAOYSA-N loratadine Chemical compound C1CN(C(=O)OCC)CCC1=C1C2=NC=CC=C2CCC2=CC(Cl)=CC=C21 JCCNYMKQOSZNPW-UHFFFAOYSA-N 0.000 description 1
- 239000007937 lozenge Substances 0.000 description 1
- 210000004072 lung Anatomy 0.000 description 1
- 210000003563 lymphoid tissue Anatomy 0.000 description 1
- 208000002780 macular degeneration Diseases 0.000 description 1
- 239000011777 magnesium Substances 0.000 description 1
- 229910052749 magnesium Inorganic materials 0.000 description 1
- 229910001629 magnesium chloride Inorganic materials 0.000 description 1
- VTHJTEIRLNZDEV-UHFFFAOYSA-L magnesium dihydroxide Chemical compound [OH-].[OH-].[Mg+2] VTHJTEIRLNZDEV-UHFFFAOYSA-L 0.000 description 1
- 239000000347 magnesium hydroxide Substances 0.000 description 1
- 229910001862 magnesium hydroxide Inorganic materials 0.000 description 1
- 229960000816 magnesium hydroxide Drugs 0.000 description 1
- 235000019359 magnesium stearate Nutrition 0.000 description 1
- 239000000594 mannitol Substances 0.000 description 1
- 235000010355 mannitol Nutrition 0.000 description 1
- 229960003803 meclofenamic acid Drugs 0.000 description 1
- 229960003464 mefenamic acid Drugs 0.000 description 1
- 229960000582 mepyramine Drugs 0.000 description 1
- YECBIJXISLIIDS-UHFFFAOYSA-N mepyramine Chemical compound C1=CC(OC)=CC=C1CN(CCN(C)C)C1=CC=CC=N1 YECBIJXISLIIDS-UHFFFAOYSA-N 0.000 description 1
- 229960001428 mercaptopurine Drugs 0.000 description 1
- QSHDDOUJBYECFT-UHFFFAOYSA-N mercury Chemical compound [Hg] QSHDDOUJBYECFT-UHFFFAOYSA-N 0.000 description 1
- 229910052753 mercury Inorganic materials 0.000 description 1
- KBOPZPXVLCULAV-UHFFFAOYSA-N mesalamine Chemical compound NC1=CC=C(O)C(C(O)=O)=C1 KBOPZPXVLCULAV-UHFFFAOYSA-N 0.000 description 1
- 229960004963 mesalazine Drugs 0.000 description 1
- 230000002503 metabolic effect Effects 0.000 description 1
- LMOINURANNBYCM-UHFFFAOYSA-N metaproterenol Chemical compound CC(C)NCC(O)C1=CC(O)=CC(O)=C1 LMOINURANNBYCM-UHFFFAOYSA-N 0.000 description 1
- HTMIBDQKFHUPSX-UHFFFAOYSA-N methdilazine Chemical compound C1N(C)CCC1CN1C2=CC=CC=C2SC2=CC=CC=C21 HTMIBDQKFHUPSX-UHFFFAOYSA-N 0.000 description 1
- 229960004056 methdilazine Drugs 0.000 description 1
- UZKWTJUDCOPSNM-UHFFFAOYSA-N methoxybenzene Substances CCCCOC=C UZKWTJUDCOPSNM-UHFFFAOYSA-N 0.000 description 1
- BSVIOYCZTJRBDB-UHFFFAOYSA-N methyl 2-(3-methoxyphenyl)acetate Chemical compound COC(=O)CC1=CC=CC(OC)=C1 BSVIOYCZTJRBDB-UHFFFAOYSA-N 0.000 description 1
- CIOSZUHLXJCWSF-UHFFFAOYSA-N methyl 2-[4-chloro-5-methyl-3-(trifluoromethyl)pyrazol-1-yl]-2-(3-methoxyphenyl)acetate Chemical compound N1=C(C(F)(F)F)C(Cl)=C(C)N1C(C(=O)OC)C1=CC=CC(OC)=C1 CIOSZUHLXJCWSF-UHFFFAOYSA-N 0.000 description 1
- LIEDGYIZNKIFFX-UHFFFAOYSA-N methyl 4-[4-[2-[4-chloro-5-methyl-3-(trifluoromethyl)pyrazol-1-yl]acetyl]piperazin-1-yl]benzoate Chemical compound C1=CC(C(=O)OC)=CC=C1N1CCN(C(=O)CN2C(=C(Cl)C(=N2)C(F)(F)F)C)CC1 LIEDGYIZNKIFFX-UHFFFAOYSA-N 0.000 description 1
- 229920000609 methyl cellulose Polymers 0.000 description 1
- 125000002496 methyl group Chemical group [H]C([H])([H])* 0.000 description 1
- 235000010981 methylcellulose Nutrition 0.000 description 1
- 239000001923 methylcellulose Substances 0.000 description 1
- 229960004584 methylprednisolone Drugs 0.000 description 1
- 210000004688 microtubule Anatomy 0.000 description 1
- 150000007522 mineralic acids Chemical class 0.000 description 1
- OJGQFYYLKNCIJD-UHFFFAOYSA-N miroprofen Chemical compound C1=CC(C(C(O)=O)C)=CC=C1C1=CN(C=CC=C2)C2=N1 OJGQFYYLKNCIJD-UHFFFAOYSA-N 0.000 description 1
- 229950006616 miroprofen Drugs 0.000 description 1
- 230000004048 modification Effects 0.000 description 1
- 238000012986 modification Methods 0.000 description 1
- 229960005285 mofebutazone Drugs 0.000 description 1
- REOJLIXKJWXUGB-UHFFFAOYSA-N mofebutazone Chemical compound O=C1C(CCCC)C(=O)NN1C1=CC=CC=C1 REOJLIXKJWXUGB-UHFFFAOYSA-N 0.000 description 1
- 125000006682 monohaloalkyl group Chemical group 0.000 description 1
- 229960005127 montelukast Drugs 0.000 description 1
- 229960005181 morphine Drugs 0.000 description 1
- 125000004573 morpholin-4-yl group Chemical group N1(CCOCC1)* 0.000 description 1
- 239000002324 mouth wash Substances 0.000 description 1
- 229940014456 mycophenolate Drugs 0.000 description 1
- RTGDFNSFWBGLEC-SYZQJQIISA-N mycophenolate mofetil Chemical compound COC1=C(C)C=2COC(=O)C=2C(O)=C1C\C=C(/C)CCC(=O)OCCN1CCOCC1 RTGDFNSFWBGLEC-SYZQJQIISA-N 0.000 description 1
- 229960004866 mycophenolate mofetil Drugs 0.000 description 1
- HPNSFSBZBAHARI-RUDMXATFSA-N mycophenolic acid Chemical compound OC1=C(C\C=C(/C)CCC(O)=O)C(OC)=C(C)C2=C1C(=O)OC2 HPNSFSBZBAHARI-RUDMXATFSA-N 0.000 description 1
- 239000003703 n methyl dextro aspartic acid receptor blocking agent Substances 0.000 description 1
- GGCBAJNALZWVKD-UHFFFAOYSA-N n,n-dimethyl-4-piperazin-1-ylbenzenesulfonamide Chemical compound C1=CC(S(=O)(=O)N(C)C)=CC=C1N1CCNCC1 GGCBAJNALZWVKD-UHFFFAOYSA-N 0.000 description 1
- KRKPYFLIYNGWTE-UHFFFAOYSA-N n,o-dimethylhydroxylamine Chemical compound CNOC KRKPYFLIYNGWTE-UHFFFAOYSA-N 0.000 description 1
- YFLRDYPWQHIZGD-UHFFFAOYSA-N n-(4-chloro-5-methyl-1h-pyrazol-3-yl)-2,2,2-trifluoroacetamide Chemical compound CC=1NN=C(NC(=O)C(F)(F)F)C=1Cl YFLRDYPWQHIZGD-UHFFFAOYSA-N 0.000 description 1
- 125000004108 n-butyl group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])C([H])([H])* 0.000 description 1
- 125000003136 n-heptyl group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])* 0.000 description 1
- 125000001280 n-hexyl group Chemical group C(CCCCC)* 0.000 description 1
- MYWUZJCMWCOHBA-UHFFFAOYSA-N n-methyl-1-phenylpropan-2-amine Chemical compound CNC(C)CC1=CC=CC=C1 MYWUZJCMWCOHBA-UHFFFAOYSA-N 0.000 description 1
- 125000000740 n-pentyl group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])* 0.000 description 1
- 125000001624 naphthyl group Chemical group 0.000 description 1
- 230000001613 neoplastic effect Effects 0.000 description 1
- 229940063121 neoral Drugs 0.000 description 1
- 201000008383 nephritis Diseases 0.000 description 1
- 230000004770 neurodegeneration Effects 0.000 description 1
- 208000015122 neurodegenerative disease Diseases 0.000 description 1
- 229960000916 niflumic acid Drugs 0.000 description 1
- 231100000344 non-irritating Toxicity 0.000 description 1
- UMRZSTCPUPJPOJ-KNVOCYPGSA-N norbornane Chemical compound C1C[C@H]2CC[C@@H]1C2 UMRZSTCPUPJPOJ-KNVOCYPGSA-N 0.000 description 1
- 239000003865 nucleic acid synthesis inhibitor Substances 0.000 description 1
- 150000007523 nucleic acids Chemical class 0.000 description 1
- 102000039446 nucleic acids Human genes 0.000 description 1
- 108020004707 nucleic acids Proteins 0.000 description 1
- QIQXTHQIDYTFRH-UHFFFAOYSA-N octadecanoic acid Chemical compound CCCCCCCCCCCCCCCCCC(O)=O QIQXTHQIDYTFRH-UHFFFAOYSA-N 0.000 description 1
- OQCDKBAXFALNLD-UHFFFAOYSA-N octadecanoic acid Natural products CCCCCCCC(C)CCCCCCCCC(O)=O OQCDKBAXFALNLD-UHFFFAOYSA-N 0.000 description 1
- ZQPPMHVWECSIRJ-KTKRTIGZSA-N oleic acid Chemical compound CCCCCCCC\C=C/CCCCCCCC(O)=O ZQPPMHVWECSIRJ-KTKRTIGZSA-N 0.000 description 1
- 239000003402 opiate agonist Substances 0.000 description 1
- 230000003287 optical effect Effects 0.000 description 1
- 229940041672 oral gel Drugs 0.000 description 1
- 239000010502 orange oil Substances 0.000 description 1
- 229960002657 orciprenaline Drugs 0.000 description 1
- 150000007530 organic bases Chemical class 0.000 description 1
- 230000003204 osmotic effect Effects 0.000 description 1
- 229960002739 oxaprozin Drugs 0.000 description 1
- OFPXSFXSNFPTHF-UHFFFAOYSA-N oxaprozin Chemical compound O1C(CCC(=O)O)=NC(C=2C=CC=CC=2)=C1C1=CC=CC=C1 OFPXSFXSNFPTHF-UHFFFAOYSA-N 0.000 description 1
- AICOOMRHRUFYCM-ZRRPKQBOSA-N oxazine, 1 Chemical compound C([C@@H]1[C@H](C(C[C@]2(C)[C@@H]([C@H](C)N(C)C)[C@H](O)C[C@]21C)=O)CC1=CC2)C[C@H]1[C@@]1(C)[C@H]2N=C(C(C)C)OC1 AICOOMRHRUFYCM-ZRRPKQBOSA-N 0.000 description 1
- TWNQGVIAIRXVLR-UHFFFAOYSA-N oxo(oxoalumanyloxy)alumane Chemical compound O=[Al]O[Al]=O TWNQGVIAIRXVLR-UHFFFAOYSA-N 0.000 description 1
- 239000001301 oxygen Substances 0.000 description 1
- 125000004430 oxygen atom Chemical group O* 0.000 description 1
- 229960001528 oxymetazoline Drugs 0.000 description 1
- 229960000649 oxyphenbutazone Drugs 0.000 description 1
- HFHZKZSRXITVMK-UHFFFAOYSA-N oxyphenbutazone Chemical compound O=C1C(CCCC)C(=O)N(C=2C=CC=CC=2)N1C1=CC=C(O)C=C1 HFHZKZSRXITVMK-UHFFFAOYSA-N 0.000 description 1
- 229910052763 palladium Inorganic materials 0.000 description 1
- 210000000496 pancreas Anatomy 0.000 description 1
- MRDGZSKYFPGAKP-UHFFFAOYSA-N para-methoxyphenylpiperazine Chemical compound C1=CC(OC)=CC=C1N1CCNCC1 MRDGZSKYFPGAKP-UHFFFAOYSA-N 0.000 description 1
- 229960005489 paracetamol Drugs 0.000 description 1
- 239000012188 paraffin wax Substances 0.000 description 1
- 230000007170 pathology Effects 0.000 description 1
- 239000000312 peanut oil Substances 0.000 description 1
- 229960001639 penicillamine Drugs 0.000 description 1
- 229960003436 pentoxyverine Drugs 0.000 description 1
- 210000003819 peripheral blood mononuclear cell Anatomy 0.000 description 1
- 229940066842 petrolatum Drugs 0.000 description 1
- 235000019271 petrolatum Nutrition 0.000 description 1
- 239000008024 pharmaceutical diluent Substances 0.000 description 1
- 229960003893 phenacetin Drugs 0.000 description 1
- 229960001190 pheniramine Drugs 0.000 description 1
- 229960003424 phenylacetic acid Drugs 0.000 description 1
- 239000003279 phenylacetic acid Substances 0.000 description 1
- 229960002895 phenylbutazone Drugs 0.000 description 1
- VYMDGNCVAMGZFE-UHFFFAOYSA-N phenylbutazonum Chemical compound O=C1C(CCCC)C(=O)N(C=2C=CC=CC=2)N1C1=CC=CC=C1 VYMDGNCVAMGZFE-UHFFFAOYSA-N 0.000 description 1
- 229960001802 phenylephrine Drugs 0.000 description 1
- SONNWYBIRXJNDC-VIFPVBQESA-N phenylephrine Chemical compound CNC[C@H](O)C1=CC=CC(O)=C1 SONNWYBIRXJNDC-VIFPVBQESA-N 0.000 description 1
- YZTJYBJCZXZGCT-UHFFFAOYSA-N phenylpiperazine Chemical class C1CNCCN1C1=CC=CC=C1 YZTJYBJCZXZGCT-UHFFFAOYSA-N 0.000 description 1
- DLNKOYKMWOXYQA-APPZFPTMSA-N phenylpropanolamine Chemical compound C[C@@H](N)[C@H](O)C1=CC=CC=C1 DLNKOYKMWOXYQA-APPZFPTMSA-N 0.000 description 1
- 229960000395 phenylpropanolamine Drugs 0.000 description 1
- OJMIONKXNSYLSR-UHFFFAOYSA-N phosphorous acid Chemical class OP(O)O OJMIONKXNSYLSR-UHFFFAOYSA-N 0.000 description 1
- 230000000704 physical effect Effects 0.000 description 1
- 229960005414 pirbuterol Drugs 0.000 description 1
- PIDSZXPFGCURGN-UHFFFAOYSA-N pirprofen Chemical compound ClC1=CC(C(C(O)=O)C)=CC=C1N1CC=CC1 PIDSZXPFGCURGN-UHFFFAOYSA-N 0.000 description 1
- 229960000851 pirprofen Drugs 0.000 description 1
- 229950011515 pobilukast Drugs 0.000 description 1
- 239000002798 polar solvent Substances 0.000 description 1
- 229920000768 polyamine Polymers 0.000 description 1
- 239000004417 polycarbonate Substances 0.000 description 1
- 229920000515 polycarbonate Polymers 0.000 description 1
- 125000003367 polycyclic group Chemical group 0.000 description 1
- 229920000573 polyethylene Polymers 0.000 description 1
- 125000006684 polyhaloalkyl group Polymers 0.000 description 1
- 235000010482 polyoxyethylene sorbitan monooleate Nutrition 0.000 description 1
- 239000000244 polyoxyethylene sorbitan monooleate Substances 0.000 description 1
- 229920000053 polysorbate 80 Polymers 0.000 description 1
- 239000011148 porous material Substances 0.000 description 1
- 239000011591 potassium Substances 0.000 description 1
- 229910052700 potassium Inorganic materials 0.000 description 1
- LPNYRYFBWFDTMA-UHFFFAOYSA-N potassium tert-butoxide Chemical compound [K+].CC(C)(C)[O-] LPNYRYFBWFDTMA-UHFFFAOYSA-N 0.000 description 1
- UAJUXJSXCLUTNU-UHFFFAOYSA-N pranlukast Chemical compound C=1C=C(OCCCCC=2C=CC=CC=2)C=CC=1C(=O)NC(C=1)=CC=C(C(C=2)=O)C=1OC=2C=1N=NNN=1 UAJUXJSXCLUTNU-UHFFFAOYSA-N 0.000 description 1
- 229960004583 pranlukast Drugs 0.000 description 1
- 229960003101 pranoprofen Drugs 0.000 description 1
- 238000001556 precipitation Methods 0.000 description 1
- 229960005205 prednisolone Drugs 0.000 description 1
- OIGNJSKKLXVSLS-VWUMJDOOSA-N prednisolone Chemical compound O=C1C=C[C@]2(C)[C@H]3[C@@H](O)C[C@](C)([C@@](CC4)(O)C(=O)CO)[C@@H]4[C@@H]3CCC2=C1 OIGNJSKKLXVSLS-VWUMJDOOSA-N 0.000 description 1
- 229960004618 prednisone Drugs 0.000 description 1
- XOFYZVNMUHMLCC-ZPOLXVRWSA-N prednisone Chemical compound O=C1C=C[C@]2(C)[C@H]3C(=O)C[C@](C)([C@@](CC4)(O)C(=O)CO)[C@@H]4[C@@H]3CCC2=C1 XOFYZVNMUHMLCC-ZPOLXVRWSA-N 0.000 description 1
- 230000002335 preservative effect Effects 0.000 description 1
- 150000003141 primary amines Chemical class 0.000 description 1
- MFDFERRIHVXMIY-UHFFFAOYSA-N procaine Chemical compound CCN(CC)CCOC(=O)C1=CC=C(N)C=C1 MFDFERRIHVXMIY-UHFFFAOYSA-N 0.000 description 1
- 229960004919 procaine Drugs 0.000 description 1
- 230000008569 process Effects 0.000 description 1
- 102000004196 processed proteins & peptides Human genes 0.000 description 1
- 229940002612 prodrug Drugs 0.000 description 1
- 239000000651 prodrug Substances 0.000 description 1
- 229940072288 prograf Drugs 0.000 description 1
- 230000000135 prohibitive effect Effects 0.000 description 1
- 229960003910 promethazine Drugs 0.000 description 1
- 230000001737 promoting effect Effects 0.000 description 1
- 150000005599 propionic acid derivatives Chemical class 0.000 description 1
- JCRIVQIOJSSCQD-UHFFFAOYSA-N propylhexedrine Chemical compound CNC(C)CC1CCCCC1 JCRIVQIOJSSCQD-UHFFFAOYSA-N 0.000 description 1
- 229960000786 propylhexedrine Drugs 0.000 description 1
- 239000002599 prostaglandin synthase inhibitor Substances 0.000 description 1
- 102000004169 proteins and genes Human genes 0.000 description 1
- 108090000623 proteins and genes Proteins 0.000 description 1
- KWGRBVOPPLSCSI-WCBMZHEXSA-N pseudoephedrine Chemical compound CN[C@@H](C)[C@@H](O)C1=CC=CC=C1 KWGRBVOPPLSCSI-WCBMZHEXSA-N 0.000 description 1
- 229960003908 pseudoephedrine Drugs 0.000 description 1
- 150000003212 purines Chemical class 0.000 description 1
- 125000000561 purinyl group Chemical group N1=C(N=C2N=CNC2=C1)* 0.000 description 1
- JEXVQSWXXUJEMA-UHFFFAOYSA-N pyrazol-3-one Chemical class O=C1C=CN=N1 JEXVQSWXXUJEMA-UHFFFAOYSA-N 0.000 description 1
- 125000005344 pyridylmethyl group Chemical group [H]C1=C([H])C([H])=C([H])C(=N1)C([H])([H])* 0.000 description 1
- 125000000719 pyrrolidinyl group Chemical group 0.000 description 1
- VMXUWOKSQNHOCA-LCYFTJDESA-N ranitidine Chemical compound [O-][N+](=O)/C=C(/NC)NCCSCC1=CC=C(CN(C)C)O1 VMXUWOKSQNHOCA-LCYFTJDESA-N 0.000 description 1
- 229960000620 ranitidine Drugs 0.000 description 1
- 229940099538 rapamune Drugs 0.000 description 1
- 230000008707 rearrangement Effects 0.000 description 1
- 238000006462 rearrangement reaction Methods 0.000 description 1
- 238000001953 recrystallisation Methods 0.000 description 1
- 210000000664 rectum Anatomy 0.000 description 1
- 230000009467 reduction Effects 0.000 description 1
- 230000001105 regulatory effect Effects 0.000 description 1
- 229940116176 remicade Drugs 0.000 description 1
- 239000011347 resin Substances 0.000 description 1
- 229920005989 resin Polymers 0.000 description 1
- 230000000241 respiratory effect Effects 0.000 description 1
- 238000006049 ring expansion reaction Methods 0.000 description 1
- 238000007142 ring opening reaction Methods 0.000 description 1
- 229960000371 rofecoxib Drugs 0.000 description 1
- 238000002390 rotary evaporation Methods 0.000 description 1
- CVHZOJJKTDOEJC-UHFFFAOYSA-N saccharin Chemical compound C1=CC=C2C(=O)NS(=O)(=O)C2=C1 CVHZOJJKTDOEJC-UHFFFAOYSA-N 0.000 description 1
- 229940081974 saccharin Drugs 0.000 description 1
- 235000019204 saccharin Nutrition 0.000 description 1
- 239000000901 saccharin and its Na,K and Ca salt Substances 0.000 description 1
- 229960002052 salbutamol Drugs 0.000 description 1
- 150000003873 salicylate salts Chemical class 0.000 description 1
- 229940063122 sandimmune Drugs 0.000 description 1
- 201000000306 sarcoidosis Diseases 0.000 description 1
- HFHDHCJBZVLPGP-UHFFFAOYSA-N schardinger α-dextrin Chemical compound O1C(C(C2O)O)C(CO)OC2OC(C(C2O)O)C(CO)OC2OC(C(C2O)O)C(CO)OC2OC(C(O)C2O)C(CO)OC2OC(C(C2O)O)C(CO)OC2OC2C(O)C(O)C1OC2CO HFHDHCJBZVLPGP-UHFFFAOYSA-N 0.000 description 1
- 125000002914 sec-butyl group Chemical group [H]C([H])([H])C([H])([H])C([H])(*)C([H])([H])[H] 0.000 description 1
- 238000000926 separation method Methods 0.000 description 1
- 239000008159 sesame oil Substances 0.000 description 1
- 235000011803 sesame oil Nutrition 0.000 description 1
- 238000007493 shaping process Methods 0.000 description 1
- 229910052710 silicon Inorganic materials 0.000 description 1
- 239000010703 silicon Substances 0.000 description 1
- 235000012239 silicon dioxide Nutrition 0.000 description 1
- 229940083037 simethicone Drugs 0.000 description 1
- 229940115586 simulect Drugs 0.000 description 1
- 201000009890 sinusitis Diseases 0.000 description 1
- 210000003491 skin Anatomy 0.000 description 1
- 208000017520 skin disease Diseases 0.000 description 1
- 239000002002 slurry Substances 0.000 description 1
- 229940083542 sodium Drugs 0.000 description 1
- 229910052708 sodium Inorganic materials 0.000 description 1
- 235000015424 sodium Nutrition 0.000 description 1
- 239000001632 sodium acetate Substances 0.000 description 1
- 235000017281 sodium acetate Nutrition 0.000 description 1
- 235000010413 sodium alginate Nutrition 0.000 description 1
- 239000000661 sodium alginate Substances 0.000 description 1
- 229940005550 sodium alginate Drugs 0.000 description 1
- 229910001467 sodium calcium phosphate Inorganic materials 0.000 description 1
- 235000019812 sodium carboxymethyl cellulose Nutrition 0.000 description 1
- 229920001027 sodium carboxymethylcellulose Polymers 0.000 description 1
- 239000011780 sodium chloride Substances 0.000 description 1
- BEOOHQFXGBMRKU-UHFFFAOYSA-N sodium cyanoborohydride Chemical compound [Na+].[B-]C#N BEOOHQFXGBMRKU-UHFFFAOYSA-N 0.000 description 1
- 239000001488 sodium phosphate Substances 0.000 description 1
- 229940054269 sodium pyruvate Drugs 0.000 description 1
- 239000007901 soft capsule Substances 0.000 description 1
- 239000012265 solid product Substances 0.000 description 1
- 241000894007 species Species 0.000 description 1
- 201000005671 spondyloarthropathy Diseases 0.000 description 1
- 239000007921 spray Substances 0.000 description 1
- 238000010561 standard procedure Methods 0.000 description 1
- 235000011150 stannous chloride Nutrition 0.000 description 1
- 239000008107 starch Substances 0.000 description 1
- 235000019698 starch Nutrition 0.000 description 1
- 239000008117 stearic acid Substances 0.000 description 1
- 230000000638 stimulation Effects 0.000 description 1
- 238000010254 subcutaneous injection Methods 0.000 description 1
- 239000007929 subcutaneous injection Substances 0.000 description 1
- 239000000758 substrate Substances 0.000 description 1
- 229950005175 sudoxicam Drugs 0.000 description 1
- GGCSSNBKKAUURC-UHFFFAOYSA-N sufentanil Chemical group C1CN(CCC=2SC=CC=2)CCC1(COC)N(C(=O)CC)C1=CC=CC=C1 GGCSSNBKKAUURC-UHFFFAOYSA-N 0.000 description 1
- 229960001940 sulfasalazine Drugs 0.000 description 1
- NCEXYHBECQHGNR-UHFFFAOYSA-N sulfasalazine Natural products C1=C(O)C(C(=O)O)=CC(N=NC=2C=CC(=CC=2)S(=O)(=O)NC=2N=CC=CC=2)=C1 NCEXYHBECQHGNR-UHFFFAOYSA-N 0.000 description 1
- 239000011593 sulfur Substances 0.000 description 1
- 229960000894 sulindac Drugs 0.000 description 1
- MLKXDPUZXIRXEP-MFOYZWKCSA-N sulindac Chemical compound CC1=C(CC(O)=O)C2=CC(F)=CC=C2\C1=C/C1=CC=C(S(C)=O)C=C1 MLKXDPUZXIRXEP-MFOYZWKCSA-N 0.000 description 1
- 239000000829 suppository Substances 0.000 description 1
- 230000001629 suppression Effects 0.000 description 1
- 229960004492 suprofen Drugs 0.000 description 1
- 230000002459 sustained effect Effects 0.000 description 1
- 210000002437 synoviocyte Anatomy 0.000 description 1
- 201000000596 systemic lupus erythematosus Diseases 0.000 description 1
- 239000000454 talc Substances 0.000 description 1
- 235000012222 talc Nutrition 0.000 description 1
- 229910052623 talc Inorganic materials 0.000 description 1
- 229960000565 tazarotene Drugs 0.000 description 1
- 229960003676 tenidap Drugs 0.000 description 1
- LXIKEPCNDFVJKC-QXMHVHEDSA-N tenidap Chemical compound C12=CC(Cl)=CC=C2N(C(=O)N)C(=O)\C1=C(/O)C1=CC=CS1 LXIKEPCNDFVJKC-QXMHVHEDSA-N 0.000 description 1
- 229960000195 terbutaline Drugs 0.000 description 1
- 229960000351 terfenadine Drugs 0.000 description 1
- BCALDKXBXSNSBC-UHFFFAOYSA-N tert-butyl 4-(4-chloro-3-methoxyphenyl)piperazine-1-carboxylate Chemical compound C1=C(Cl)C(OC)=CC(N2CCN(CC2)C(=O)OC(C)(C)C)=C1 BCALDKXBXSNSBC-UHFFFAOYSA-N 0.000 description 1
- 125000000999 tert-butyl group Chemical group [H]C([H])([H])C(*)(C([H])([H])[H])C([H])([H])[H] 0.000 description 1
- DPKBAXPHAYBPRL-UHFFFAOYSA-M tetrabutylazanium;iodide Chemical compound [I-].CCCC[N+](CCCC)(CCCC)CCCC DPKBAXPHAYBPRL-UHFFFAOYSA-M 0.000 description 1
- 229960004559 theobromine Drugs 0.000 description 1
- 229960000278 theophylline Drugs 0.000 description 1
- 239000002562 thickening agent Substances 0.000 description 1
- 229960001312 tiaprofenic acid Drugs 0.000 description 1
- AXZWODMDQAVCJE-UHFFFAOYSA-L tin(II) chloride (anhydrous) Chemical compound [Cl-].[Cl-].[Sn+2] AXZWODMDQAVCJE-UHFFFAOYSA-L 0.000 description 1
- 229950002345 tiopinac Drugs 0.000 description 1
- 229950006150 tioxaprofen Drugs 0.000 description 1
- 229960002905 tolfenamic acid Drugs 0.000 description 1
- YEZNLOUZAIOMLT-UHFFFAOYSA-N tolfenamic acid Chemical compound CC1=C(Cl)C=CC=C1NC1=CC=CC=C1C(O)=O YEZNLOUZAIOMLT-UHFFFAOYSA-N 0.000 description 1
- 229960001017 tolmetin Drugs 0.000 description 1
- UPSPUYADGBWSHF-UHFFFAOYSA-N tolmetin Chemical compound C1=CC(C)=CC=C1C(=O)C1=CC=C(CC(O)=O)N1C UPSPUYADGBWSHF-UHFFFAOYSA-N 0.000 description 1
- BWHOZHOGCMHOBV-BQYQJAHWSA-N trans-benzylideneacetone Chemical compound CC(=O)\C=C\C1=CC=CC=C1 BWHOZHOGCMHOBV-BQYQJAHWSA-N 0.000 description 1
- LLPOLZWFYMWNKH-UHFFFAOYSA-N trans-dihydrocodeinone Natural products C1C(N(CCC234)C)C2CCC(=O)C3OC2=C4C1=CC=C2OC LLPOLZWFYMWNKH-UHFFFAOYSA-N 0.000 description 1
- 230000037317 transdermal delivery Effects 0.000 description 1
- 229960005294 triamcinolone Drugs 0.000 description 1
- GFNANZIMVAIWHM-OBYCQNJPSA-N triamcinolone Chemical compound O=C1C=C[C@]2(C)[C@@]3(F)[C@@H](O)C[C@](C)([C@@]([C@H](O)C4)(O)C(=O)CO)[C@@H]4[C@@H]3CCC2=C1 GFNANZIMVAIWHM-OBYCQNJPSA-N 0.000 description 1
- 238000005583 trifluoroacetylation reaction Methods 0.000 description 1
- 229960003223 tripelennamine Drugs 0.000 description 1
- 229960001128 triprolidine Drugs 0.000 description 1
- CBEQULMOCCWAQT-WOJGMQOQSA-N triprolidine Chemical compound C1=CC(C)=CC=C1C(\C=1N=CC=CC=1)=C/CN1CCCC1 CBEQULMOCCWAQT-WOJGMQOQSA-N 0.000 description 1
- YFTHZRPMJXBUME-UHFFFAOYSA-N tripropylamine Chemical compound CCCN(CCC)CCC YFTHZRPMJXBUME-UHFFFAOYSA-N 0.000 description 1
- RYFMWSXOAZQYPI-UHFFFAOYSA-K trisodium phosphate Chemical compound [Na+].[Na+].[Na+].[O-]P([O-])([O-])=O RYFMWSXOAZQYPI-UHFFFAOYSA-K 0.000 description 1
- 229910052722 tritium Inorganic materials 0.000 description 1
- 229960000281 trometamol Drugs 0.000 description 1
- 208000001072 type 2 diabetes mellitus Diseases 0.000 description 1
- 125000000391 vinyl group Chemical group [H]C([*])=C([H])[H] 0.000 description 1
- 229920002554 vinyl polymer Polymers 0.000 description 1
- 229940087652 vioxx Drugs 0.000 description 1
- 230000009385 viral infection Effects 0.000 description 1
- 150000003704 vitamin D3 derivatives Chemical class 0.000 description 1
- 230000003442 weekly effect Effects 0.000 description 1
- 238000010626 work up procedure Methods 0.000 description 1
- 229910009112 xH2O Inorganic materials 0.000 description 1
- 229960000833 xylometazoline Drugs 0.000 description 1
- 229950007802 zidometacin Drugs 0.000 description 1
- MWLSOWXNZPKENC-SSDOTTSWSA-N zileuton Chemical compound C1=CC=C2SC([C@H](N(O)C(N)=O)C)=CC2=C1 MWLSOWXNZPKENC-SSDOTTSWSA-N 0.000 description 1
- 229960005332 zileuton Drugs 0.000 description 1
- 150000003751 zinc Chemical class 0.000 description 1
- 229910052725 zinc Inorganic materials 0.000 description 1
- 239000011701 zinc Substances 0.000 description 1
- 229960003414 zomepirac Drugs 0.000 description 1
- ZXVNMYWKKDOREA-UHFFFAOYSA-N zomepirac Chemical compound C1=C(CC(O)=O)N(C)C(C(=O)C=2C=CC(Cl)=CC=2)=C1C ZXVNMYWKKDOREA-UHFFFAOYSA-N 0.000 description 1
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D401/00—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom
- C07D401/02—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom containing two hetero rings
- C07D401/06—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom containing two hetero rings linked by a carbon chain containing only aliphatic carbon atoms
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D473/00—Heterocyclic compounds containing purine ring systems
- C07D473/26—Heterocyclic compounds containing purine ring systems with an oxygen, sulphur, or nitrogen atom directly attached in position 2 or 6, but not in both
- C07D473/32—Nitrogen atom
- C07D473/34—Nitrogen atom attached in position 6, e.g. adenine
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/495—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with two or more nitrogen atoms as the only ring heteroatoms, e.g. piperazine or tetrazines
- A61K31/496—Non-condensed piperazines containing further heterocyclic rings, e.g. rifampin, thiothixene or sparfloxacin
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P1/00—Drugs for disorders of the alimentary tract or the digestive system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P17/00—Drugs for dermatological disorders
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
- A61P25/04—Centrally acting analgesics, e.g. opioids
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P29/00—Non-central analgesic, antipyretic or antiinflammatory agents, e.g. antirheumatic agents; Non-steroidal antiinflammatory drugs [NSAID]
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P37/00—Drugs for immunological or allergic disorders
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P37/00—Drugs for immunological or allergic disorders
- A61P37/08—Antiallergic agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P43/00—Drugs for specific purposes, not provided for in groups A61P1/00-A61P41/00
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P9/00—Drugs for disorders of the cardiovascular system
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D231/00—Heterocyclic compounds containing 1,2-diazole or hydrogenated 1,2-diazole rings
- C07D231/02—Heterocyclic compounds containing 1,2-diazole or hydrogenated 1,2-diazole rings not condensed with other rings
- C07D231/10—Heterocyclic compounds containing 1,2-diazole or hydrogenated 1,2-diazole rings not condensed with other rings having two or three double bonds between ring members or between ring members and non-ring members
- C07D231/12—Heterocyclic compounds containing 1,2-diazole or hydrogenated 1,2-diazole rings not condensed with other rings having two or three double bonds between ring members or between ring members and non-ring members with only hydrogen atoms, hydrocarbon or substituted hydrocarbon radicals, directly attached to ring carbon atoms
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D231/00—Heterocyclic compounds containing 1,2-diazole or hydrogenated 1,2-diazole rings
- C07D231/02—Heterocyclic compounds containing 1,2-diazole or hydrogenated 1,2-diazole rings not condensed with other rings
- C07D231/10—Heterocyclic compounds containing 1,2-diazole or hydrogenated 1,2-diazole rings not condensed with other rings having two or three double bonds between ring members or between ring members and non-ring members
- C07D231/14—Heterocyclic compounds containing 1,2-diazole or hydrogenated 1,2-diazole rings not condensed with other rings having two or three double bonds between ring members or between ring members and non-ring members with hetero atoms or with carbon atoms having three bonds to hetero atoms with at the most one bond to halogen, e.g. ester or nitrile radicals, directly attached to ring carbon atoms
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D231/00—Heterocyclic compounds containing 1,2-diazole or hydrogenated 1,2-diazole rings
- C07D231/02—Heterocyclic compounds containing 1,2-diazole or hydrogenated 1,2-diazole rings not condensed with other rings
- C07D231/10—Heterocyclic compounds containing 1,2-diazole or hydrogenated 1,2-diazole rings not condensed with other rings having two or three double bonds between ring members or between ring members and non-ring members
- C07D231/14—Heterocyclic compounds containing 1,2-diazole or hydrogenated 1,2-diazole rings not condensed with other rings having two or three double bonds between ring members or between ring members and non-ring members with hetero atoms or with carbon atoms having three bonds to hetero atoms with at the most one bond to halogen, e.g. ester or nitrile radicals, directly attached to ring carbon atoms
- C07D231/16—Halogen atoms or nitro radicals
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D231/00—Heterocyclic compounds containing 1,2-diazole or hydrogenated 1,2-diazole rings
- C07D231/02—Heterocyclic compounds containing 1,2-diazole or hydrogenated 1,2-diazole rings not condensed with other rings
- C07D231/10—Heterocyclic compounds containing 1,2-diazole or hydrogenated 1,2-diazole rings not condensed with other rings having two or three double bonds between ring members or between ring members and non-ring members
- C07D231/14—Heterocyclic compounds containing 1,2-diazole or hydrogenated 1,2-diazole rings not condensed with other rings having two or three double bonds between ring members or between ring members and non-ring members with hetero atoms or with carbon atoms having three bonds to hetero atoms with at the most one bond to halogen, e.g. ester or nitrile radicals, directly attached to ring carbon atoms
- C07D231/18—One oxygen or sulfur atom
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D231/00—Heterocyclic compounds containing 1,2-diazole or hydrogenated 1,2-diazole rings
- C07D231/02—Heterocyclic compounds containing 1,2-diazole or hydrogenated 1,2-diazole rings not condensed with other rings
- C07D231/10—Heterocyclic compounds containing 1,2-diazole or hydrogenated 1,2-diazole rings not condensed with other rings having two or three double bonds between ring members or between ring members and non-ring members
- C07D231/14—Heterocyclic compounds containing 1,2-diazole or hydrogenated 1,2-diazole rings not condensed with other rings having two or three double bonds between ring members or between ring members and non-ring members with hetero atoms or with carbon atoms having three bonds to hetero atoms with at the most one bond to halogen, e.g. ester or nitrile radicals, directly attached to ring carbon atoms
- C07D231/38—Nitrogen atoms
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D231/00—Heterocyclic compounds containing 1,2-diazole or hydrogenated 1,2-diazole rings
- C07D231/54—Heterocyclic compounds containing 1,2-diazole or hydrogenated 1,2-diazole rings condensed with carbocyclic rings or ring systems
- C07D231/56—Benzopyrazoles; Hydrogenated benzopyrazoles
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D233/00—Heterocyclic compounds containing 1,3-diazole or hydrogenated 1,3-diazole rings, not condensed with other rings
- C07D233/54—Heterocyclic compounds containing 1,3-diazole or hydrogenated 1,3-diazole rings, not condensed with other rings having two double bonds between ring members or between ring members and non-ring members
- C07D233/56—Heterocyclic compounds containing 1,3-diazole or hydrogenated 1,3-diazole rings, not condensed with other rings having two double bonds between ring members or between ring members and non-ring members with only hydrogen atoms or radicals containing only hydrogen and carbon atoms, attached to ring carbon atoms
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D235/00—Heterocyclic compounds containing 1,3-diazole or hydrogenated 1,3-diazole rings, condensed with other rings
- C07D235/02—Heterocyclic compounds containing 1,3-diazole or hydrogenated 1,3-diazole rings, condensed with other rings condensed with carbocyclic rings or ring systems
- C07D235/04—Benzimidazoles; Hydrogenated benzimidazoles
- C07D235/06—Benzimidazoles; Hydrogenated benzimidazoles with only hydrogen atoms, hydrocarbon or substituted hydrocarbon radicals, directly attached in position 2
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D249/00—Heterocyclic compounds containing five-membered rings having three nitrogen atoms as the only ring hetero atoms
- C07D249/02—Heterocyclic compounds containing five-membered rings having three nitrogen atoms as the only ring hetero atoms not condensed with other rings
- C07D249/08—1,2,4-Triazoles; Hydrogenated 1,2,4-triazoles
- C07D249/10—1,2,4-Triazoles; Hydrogenated 1,2,4-triazoles with hetero atoms or with carbon atoms having three bonds to hetero atoms with at the most one bond to halogen, e.g. ester or nitrile radicals, directly attached to ring carbon atoms
- C07D249/12—Oxygen or sulfur atoms
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D257/00—Heterocyclic compounds containing rings having four nitrogen atoms as the only ring hetero atoms
- C07D257/02—Heterocyclic compounds containing rings having four nitrogen atoms as the only ring hetero atoms not condensed with other rings
- C07D257/04—Five-membered rings
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D401/00—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom
- C07D401/02—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom containing two hetero rings
- C07D401/04—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom containing two hetero rings directly linked by a ring-member-to-ring-member bond
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D401/00—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom
- C07D401/02—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom containing two hetero rings
- C07D401/12—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom containing two hetero rings linked by a chain containing hetero atoms as chain links
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D401/00—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom
- C07D401/14—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom containing three or more hetero rings
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D403/00—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, not provided for by group C07D401/00
- C07D403/02—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, not provided for by group C07D401/00 containing two hetero rings
- C07D403/04—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, not provided for by group C07D401/00 containing two hetero rings directly linked by a ring-member-to-ring-member bond
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D403/00—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, not provided for by group C07D401/00
- C07D403/02—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, not provided for by group C07D401/00 containing two hetero rings
- C07D403/12—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, not provided for by group C07D401/00 containing two hetero rings linked by a chain containing hetero atoms as chain links
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D405/00—Heterocyclic compounds containing both one or more hetero rings having oxygen atoms as the only ring hetero atoms, and one or more rings having nitrogen as the only ring hetero atom
- C07D405/02—Heterocyclic compounds containing both one or more hetero rings having oxygen atoms as the only ring hetero atoms, and one or more rings having nitrogen as the only ring hetero atom containing two hetero rings
- C07D405/04—Heterocyclic compounds containing both one or more hetero rings having oxygen atoms as the only ring hetero atoms, and one or more rings having nitrogen as the only ring hetero atom containing two hetero rings directly linked by a ring-member-to-ring-member bond
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D409/00—Heterocyclic compounds containing two or more hetero rings, at least one ring having sulfur atoms as the only ring hetero atoms
- C07D409/02—Heterocyclic compounds containing two or more hetero rings, at least one ring having sulfur atoms as the only ring hetero atoms containing two hetero rings
- C07D409/04—Heterocyclic compounds containing two or more hetero rings, at least one ring having sulfur atoms as the only ring hetero atoms containing two hetero rings directly linked by a ring-member-to-ring-member bond
Definitions
- the present invention provides compounds, pharmaceutical compositions containing one or more of these compounds or their pharmaceutically acceptable salts, which are effective in inhibiting the binding of various chemokine, such as MIP-1 ⁇ , leukotactin, MPIF-1 and RANTES, to the CCR1 receptor.
- various chemokine such as MIP-1 ⁇ , leukotactin, MPIF-1 and RANTES
- the compounds and compositions have utility in treating inflammatory and immune disorder conditions and diseases.
- the immune system which comprises leukocytes (white blood cells (WBCs): T and B lymphocytes, monocytes, macrophages granulocytes, NK cell, mast cells, dendritic cell, and immune derived cells (for example; osteoclasts)), lymphoid tissues and lymphoid vessels, is the body's defense system.
- WBCs white blood cells
- monocytes monocytes
- macrophages macrophages granulocytes
- NK cell mast cells
- dendritic cell dendritic cell
- immune derived cells for example; osteoclasts
- Chemokines act as molecular beacons for the recruitment and activation of immune cells, such as lymphocytes, monocytes and granulocytes, identifying sites where pathogens exist.
- chemokine signaling can develop and has been attributed to triggering or sustaining inflammatory disorders, such as rheumatoid arthritis, multiple sclerosis and others.
- rheumatoid arthritis unregulated chemokine accumulation in bone joints attracts and activates infiltrating macrophages and T-cells.
- the activities of these cells induce synovial cell proliferation that leads, at least in part, to inflammation and eventual bone and cartilage loss (see, DeVries, M.E., et al., Semin Immunol 11(2):95-104 (1999 )).
- chemokine-mediated monocyte/macrophage and T cell recruitment to the central nervous system A hallmark of some demyelinating diseases such as multiple sclerosis is the chemokine-mediated monocyte/macrophage and T cell recruitment to the central nervous system (see, Kennedy, et al., J. Clin. Immunol. 19(5):273-279 (1999 )). Chemokine recruitment of destructive WBCs to transplants has been implicated in their subsequent rejection. See, DeVries, M.E., et al., ibid Because chemokines play pivotal roles in inflammation and lymphocyte development, the ability to specifically manipulate their activity has enormous impact on ameliorating and halting diseases that currently have no satisfactory treatment. In addition, transplant rejection may be minimized without the generalized and complicating effects of costly immunosuppressive pharmaceuticals:
- Chemokines a group of greater than 40 small peptides (7-10 kD), ligate receptors expressed primarily on WBCs or immune derived cells, and signal through G-protein-coupled signaling cascades to mediate their chemoattractant and chemostimulant functions.
- Receptors may bind more than one ligand; for example, the receptor CCR1 ligates RANTES (regulated on activation normal T cell expressed), MIP-1 ⁇ (macrophage inflammatory p rotein), MPIF-1/CK ⁇ , and Leukotactin chemokines (among others with lesser affinities).
- 24 chemokines receptors are known.
- Chemokine activity can be controlled through the modulation of their corresponding receptor, treating related inflammatory and immunological diseases and enabling organ and tissue transplants.
- the receptor CCR1 and its chemokine ligands represent significant therapeutic targets (see Saeki, et al., Current Pharmaceutical Design 9:1201-1208 (2003 )) since they have been implicated in rheumatoid arthritis, transplant rejection (see, DeVries, M.E., et al., ibid.), and multiple sclerosis (see, Fischer, et al., J Neuroimmunol. 110(1-2):195-208 (2000 ); Izikson, et al J. Exp. Med 192(7):1075-1080 (2000 ); and Rottman, et al., Eur.
- the present invention provides the use of a compound having the formula: or a pharmaceutically acceptable salt thereof in the manufacture of in the manufacture of a medicament for use in a method of treating CCR1 mediated diseases or conditions comprising administering to a subject in need thereof a therapeutically effective amount of said compound.
- the subscript n represents an integer of from 1 to 2, preferably 1 .
- the subscript m represents an integer of from 0 to 10, limited by the number of available substituents positions on the piperazine or homopiperazine ring to which it is attached.
- piperazine derivatives (n is 1) can have from 0 to 8 R 1 groups, preferably 0 to 4 R 1 groups, and more preferably, 0, 1 or 2 R 1 groups.
- Each R 1 is a substituent independently selected from C 1-8 alkyl, C 1-8 haloalkyl, C 3-6 cycloalkyl, C 2-8 alkenyl and C 2-8 alkynyl, -COR a , -CO 2 R 3 , -CONR a R b , -NR a COR b , -SO 2 R a , -X 1 CO 2 R a , -X 1 CONR a R b , -X 1 NR a COR b , -X 1 SO 2 R a , -X 1 SO 2 NR a R b , X 1 NR a R b , -X 1 OR a , wherein X 1 is a member selected from the group consisting Of C 1-4 alkylene, C 2-4 alkenylene and C 2-4 alkynylene and each R a and R b is independently selected from the group consisting of hydrogen, C 1-8 alkyl, C
- Ar 1 represents phenyl substituted with from one to three R 2 substituents independently selected from halogen, -OR c , -OC(O)R c , -NR c R d , -SR c , -R e , -CN, -NO 2 , -CO 2 R c , -CONR c R d , -C(O)R c , -OC(O)NR c R d , -NR d C(O)R c , -NR d C(O) 2 R c , -NR c -C(O)NR c R d , -S(O)R e , -S(O) 2 R e , -NR c S(O) 2 R e , -S(O) 2 NR c R d and -N 3 wherein each R c and R d is independently selected from hydrogen, C 1-8 alkyl, C 1
- HAr represents an optionally substituted heteroaryl group selected from the group consisting of pyrazolyl and benzopyrazolyl and is bound to the remainder of the molecule via a nitrogen atom of the pyrazolyl ring or via a nitrogen atom of the pyrazole portion of the fused ring respectively, each of which is substituted with from one to five R 3 substituents independently selected from the group consisting of halogen, phenyl, thienyl, furanyl, pyridyl, pyrimidinyl, pyrazinyl, pyridizinyl, pyrazolyl, imidazolyl, thiazolyl, oxazolyl, isoxazolyl, isothiazolyl, triazolyl, tetrazolyl, oxadiazolyl, -OR f , - OC(O)R f , -NR f R g , -SR f , -R h
- L 1 represents a -CH 2 - and is optionally substituted with phenyl, -R k , -X 4 OR i , -X 4 OC(O)R i , -X 4 NR i R j , -X 4 SR i , -X 4 CN or -X 4 NO 2
- X 4 is selected from the group consisting of C 1-4 alkylene, C 2-4 alkenylene and C 2-4 alkynylene and each R i and R j is independently selected from hydrogen, C 1-8 alkyl, C 1-8 haloalkyl, C 3 - 6 cycloalkyl, C 2-8 alkenyl, C 2-8 alkynyl, aryl, heteroaryl, aryl-C 1-4 alkyl, and aryloxy-C 1-4 alkyl, and each R k is independently selected from the group consisting of C 1-8 alkyl, C 1-8 haloalkyl, C 3-6
- the present invention further provides compounds and pharmaceutical compositions containing one or more of these compounds.
- alkyl by itself or as part of another substituent, means, unless otherwise stated, a straight or branched chain hydrocarbon radical, having the number of carbon atoms designated (i.e. C 1-8 means one to eight carbons).
- alkyl groups include methyl, ethyl, n-propyl, isopropyl, n-butyl, t-butyl, isobutyl, sec-butyl, n-pentyl, n-hexyl, n-heptyl, n-octyl, and the like.
- alkenyl refers to an unsaturated alkyl group having one or more double bonds.
- alkynyl refers to an unsaturated alkyl group having one or more triple bonds.
- unsaturated alkyle groups include vinyl, 2-propenyl, crotyl, 2-isopentenyl, 2-(butadienyl), 2,4-pentadienyl, 3-(1,4-pentadienyl), ethynyl, 1- and 3-propynyl, 3-butynyl, and the higher homologs and isomers.
- cycloalkyl refers to hydrocarbon rings having the indicated number of ring atoms (e.g., C 3-6 cycloalkyl) and being fully saturated or having no more than one double bond between ring vertices.
- Cycloalkyl is also meant to refer to bicyclic and polycyclic hydrocarbon rings such as, for example, bicyclo[2.2.1]heptane, bicyclo[2.2.2]octane, etc.
- alkylene by itself or as part of another substituent means a divalent radical derived from an alkane, as exemplified by -CH 2 CH 2 CH 2 CH 2 -.
- an alkyl (or alkylene) group will have from 1 to 24 carbon atoms, with those groups having 10 or fewer carbon atoms being preferred in the present invention.
- a “lower alkyl” or “lower alkylene” is a shorter chain alkyl or alkylene group, generally having four or fewer carbon atoms.
- alkoxy alkylamino and “alkylthio” (or thioalkoxy) are used in their conventional sense, and refer to those alkyl groups attached to the remainder of the molecule via an oxygen atom, an amino group, or a sulfur atom, respectively. Additionally, for dialkylamino groups, the alkyl portions can be the same or different and can also be combined to form a 3-7 membered ring with the nitrogen atom to which each is attached. Accordingly, a group represented as -NR a R b is meant to include piperidinyl, pyrrolidinyl, morpholiny), azetidinyl and the like.
- halo or halogen
- substituents mean, unless otherwise stated, a fluorine, chlorine, bromine, or iodine atom.
- terms such as “haloalkyl,” are meant to include monohaloalkyl and polyhaloalkyl.
- C 1-4 haloalkyl is mean to include trifluoromethyl, 2,2,2-trifluoroethyl, 4-chlorobutyl, 3-bromopropyl, and the like.
- aryl means, unless otherwise stated, a polyunsaturared, typically aromatic, hydrocarbon group which can be a single ring or multiple rings (up to three rings) which are fused together or linked covalently.
- heteroaryl refers to aryl groups (or rings) that contain from one to five heteroatoms selected from N, O, and S, wherein the nitrogen and sulfur atoms are optionally oxidized, and the nitrogen atom(s) are optionally quaternized.
- a heteroaryl group can be attached to the remainder of the molecule through a heteroatom.
- Non-limiting examples of aryl groups include phenyl, naphthyl and biphenyl, while non-limiting examples of heteroaryl groups include 1-pyrrolyl, 2-pyrrolyl, 3-pyrrolyl, 1-pyrazoly], 3-pyrazolyl, 2-imidazolyl, 4-imidazolyl, pyrazinyl, 2-oxazolyl, 4-oxazolyl, 5-oxazolyl, 3-isoxazolyl, 4-isoxazolyl, 5-isoxazolyl, 2-thiazolyl, 4-thiazolyl, 5-thiazolyl, 2-furyl, 3-furyl, 2-thienyl, 3-thienyl, 2-pyridyl, 3-pyridyl, 4-pyridyl, 2-pyrimidyl, 4-pyrimidyl, 5-benzothiazolyl, purinyl, 2-benzimidazolyl, benzopyrazolyl, 5-indolyl, 1-isoquinolyl, 5-isoquinoly
- aryl when used in combination with other terms (e.g ., aryloxy, arylthioxy, arylalkyl) includes both aryl and heteroaryl rings as defined above.
- arylalkyl is meant to include those radicals in which an aryl group is attached to an alkyl group (e.g ., benzyl, phenethyl, pyridylmethyl and the like).
- alkyl in some embodiments, will include both substituted and unsubstituted forms of the indicated radical. Preferred substituents for each type of radical are provided below.
- aryl and heteroaryl will refer to substituted or unsubstituted versions as provided below, while the term “alkyl” and related aliphatic radicals is meant to refer to unsubstituted version, unless indicated to be substituted.
- -NR'C(NH 2 ) NH
- -NH-C(NH 2 ) NR', -S(O)R', -S(O) 2 R', -S(O) 2 NR'R", -NR'S(O) 2 R", -CN and -NO 2 in a number ranging from zero to (2m'+1), where m' is the total number of carbon atoms in such radical.
- R', R" and R"' each independently refer to hydrogen, unsubstituted C 1-8 alkyl, unsubstituted heteroalkyl, unsubstituted aryl, aryl substituted with 1-3 halogens, unsubstituted C 1-8 alkyl, C 1-8 alkoxy or C 1-8 thioalkoxy groups, or unsubstituted aryl-C 1-4 alkyl groups.
- R' and R" are attached to the same nitrogen atom, they can be combined with the nitrogen atom to form a 3-, 4-, 5-,6-, or 7-membered ring.
- -NR'R is meant to include 1-pyrrolidinyl and 4-morpholinyl.
- R', R" and R"' are independently selected from hydrogen, C 1-8 alkyl, C 3-6 cycloalkyl, C 2-8 alkenyl, C 2-8 alkynyl, unsubstituted aryl and heteroaryl, (unsubstituted aryl)-C 1-4 alkyl, and unsubstituted aryloxy-C 1-4 alkyl.
- R', R" and R"' are independently selected from hydrogen, C 1-8 alkyl, C 3-6 cycloalkyl, C 2-8 alkenyl, C 2-8 alkynyl, unsubstituted aryl and heteroaryl, (unsubstituted aryl)-C 1-4 alkyl, and unsubstituted aryloxy-C 1-4 alkyl.
- Other suitable substituents include each of the above aryl substituents attached to a ring atom by an alkylene tether of from 1-4 carbon atoms.
- Two of the substituents on adjacent atoms of the aryl or heteroaryl ring may optionally be replaced with a substituent of the formula -T-C(O)-(CH 2 ) q -U-, wherein T and U are independently -NH-, -O-, -CH 2 - or a single bond, and q is an integer of from 0 to 2.
- two of the substituents on adjacent atoms of the aryl or heteroaryl ring may optionally be replaced with a substituent of the formula -A-(CH 2 ) r -B-, wherein A and B are independently -CH 2 -, -O-, -NH-, -S-, -S(O)-, -S(O) 2 -, -S(O) 2 NR'- or a single bond, and r is an integer of from 1 to 3.
- One of the single bonds of the new ring so formed may optionally be replaced with a double bond.
- two of the substituents on adjacent atoms of the aryl or heteroaryl ring may optionally be replaced with a substituent of the formula -(CH 2 ) s -X-(CH 2 ) t -, where s and t are independently integers of from 0 to 3, and X is -O-, -NR'-, -S-, - S(O)-, -S(O) 2 -, or -S(O) 2 NR'-.
- the substituent R' in -NR'- and -S(O) 2 NR'- is selected from hydrogen or unsubstituted C 1-6 alkyl.
- heteroatom is meant to include oxygen (O), nitrogen (N), sulfur (S) and silicon (Si).
- salts are meant to include salts of the active compounds which are prepared with relatively nontoxic acids or bases, depending on the particular substituents found on the compounds described herein.
- base addition salts can be obtained by contacting the neutral form of such compounds with a sufficient amount of the desired base, either neat or in a suitable inert solvent.
- salts derived from phannaceutically-acceptable inorganic bases include aluminum, ammonium, calcium, copper, ferric, ferrous, lithium, magnesium, manganic, manganous, potassium, sodium, zinc and the like.
- Salts derived from pharmaceutically-acceptable organic bases include salts of primary, secondary and tertiary amines, including substituted amines, cyclic amines, naturally- occuring amines and the like, such as arginine, betaine, caffeine, choline, N,N'-dibenzylethylenediamine, diethylamine, 2-diethylaminoethanol, 2-dimethylaminoethanol, ethanolamine, ethylenediamine, N-ethylmorpholine, N-ethylpiperidine, glucamine, glucasamine, histidine, hydrabamine, isopropylamine, lysine, methylglucamine, morpholine, piperazine, piperadine, polyamine resin, procaine, purines, theobromine, triethylamine, trimethylamine, tripropylamine, tromethamine and the like.
- acid addition salts can be obtained by contacting the neutral form of such compounds with a sufficient amount of the desired acid, either neat or in a suitable inert solvent.
- pharmaceutically acceptable acid addition salts include those derived from inorganic acids like hydrochloric, hydrobromic, nitric, carbonic, monohydrogencarbonic, phosphoric, monohydrogenphosphoric, dihydrogenphosphoric, sulfuric, monohydrogensulfuric, hydriodic, or phosphorous acids and the like, as well as the salts derived from relatively nontoxic organic acids like acetic, propionic, isobutyric, malonic, benzoic, succinic, suberic, fumaric, mandelic, phthalic, benzenesulfonic, p-tolylsulfonic, citric, tartaric, methanesulfonic, and the like.
- salts of amino acids such as arginate and the like, and salts of organic acids like glucuronic or galactunoric acids and the like (see, for example, Berge, S.M., et al, "Pharmaceutical Salts", Journal of Pharmaceutical Science, 1977, 66, 1-19 ).
- Certain specific compounds of the present invention contain both basic and acidic functionalities that allow the compounds to be converted into either base or acid addition salts.
- the neutral forms of the compounds may be regenerated by contacting the salt with a base or acid and isolating the parent compound in the conventional manner.
- the parent form of the compound differs from the various salt forms in certain physical properties, such as solubility in polar solvents, but otherwise the salts are equivalent to the parent form of the compound for the purposes of the present invention.
- Certain compounds of the present invention can exist in unsolvated forms as well as solvated forms, including hydrated forms.
- the solvated forms are equivalent to unsolvated forms and are intended to be encompassed within the scope of the present invention
- Certain compounds of the present invention may exist in multiple crystalline or amorphous forms. In general, all physical forms are equivalent for the uses contemplated by the present invention and are intended to be within the scope of the present invention.
- Certain compounds of the present invention possess asymmetric carbon atoms (optical centers) or double bonds; the racemates, diastereomers, geometric isomers, regioisomers and individual isomers (e.g., separate enantiomers) are all intended to be encompassed within the scope of the present invention.
- the compounds of the present invention may also contain unnatural proportions of atomic isotopes at one or more of the atoms that constitute such compounds.
- the compounds may be radiolabeled with radioactive isotopes, such as for example tritium ( 3 H), iodine-125 ( 125 I) or carbon-14 ( 14 C). All isotopic variations of the compounds of the present invention, whether radioactive or not, arc intended to be encompassed within the scope of the present invention.
- the present invention derives from the discovery that compounds of formula I (as well as the subgeneric formulae II, III and IV) act as potent antagonists of the CCR1 receptor. This antagonist activity has been further confirmed in animal testing for inflammation, one of the hallmark disease states for CCR1. Accordingly, the compounds provided herein are useful in pharmaceutical compositions, methods for the treatment of CCR1-mediated diseases, and as controls in assays for the identification of competitive CCR1 antagonists.
- the compounds used in the present invention have the formula: or a pharmaceutically acceptable salt thereof.
- n represents an integer of from 1 to 2, preferably 1.
- m represents an integer of from 0 to 10, limited by the number of available substituents positions on the piperazine or homopiperazine ring to which it is attached.
- piperazine derivatives (n is 1) can have from 0 to 8 R 1 groups, preferably 0 to 4 R 1 groups, and more preferably, 0, 1 or 2 R 1 groups.
- Ar 1 represents phenyl substituted with from one to three R 2 substituents independently selected from halogen, -OR c , -OC(O)R c , -NR c R d , -SR c , -R c -CN, -NO 2 , -CO 2 R c , -CONR c R d , -C(O)R c , -OC(O)NR c R d , -NR d C(O)R c , -NR d C(O) 2 R c , -NR c -C(O)NR c R d , -S(O)R e , -S(O) 2 R c , -NR c S(O) 2 R e , -S(O) 2 NR c R d and -N 3 wherein each R c and R d is independently selected from hydrogen, C 1-8 alkyl, C 1-8 alky
- HAr is a heteroaryl group selected from the group consisting of pyrozolyl and benzopyrazolyl and is bound to the remainder of the molecule via a nitrogen atom of the pyrazolyl ring or via a nitrogen atom of the pyrazole portion of the fused ring system respectively, each of which is substituted with from one to five R 3 substituents independently selected from the group consisting of halogen, phenyl, thienyl, furanyl, pyridyl, pyrimidinyl, pyrazinyl, pyridizinyl, pyrazolyl, imidazolyl, thiazolyl, oxazolyl, isoxazolyl, isothiazolyl, triazolyl, tetrazolyl, oxadiazolyl, -OR f , -OC(O)R f , -NR f R g , -SR f , -R h , -
- L 1 represents a linking group which is -CH 2 - optionally substituted with -R k , -X 4 OR i , -X 4 OC(O)R i , -X 4 NR i R j , -X 4 SR i , -X 4 CN or -X 4 NO 2 , wherein X 4 is selected from the group consisting of C 1-4 alkylene, C 2-4 alkenylene and C 2-4 alkynylene.
- each R i and R j is independently selected from hydrogen, C 1-8 alkyl, C 1-8 haloalkyl, C 3-6 cycloalkyl, C 2-8 alkenyl, C 2-8 alkynyl, aryl, heteroaryl, aryl-C 1-4 alkyl, and aryloxy-C 1-4 alkyl
- each R k is independently selected from the group consisting of C 1-8 alkyl, C 1-8 haloalkyl, C 3-6 cycloalkyl, C 2-8 alkenyl, C 2-8 alkynyl, aryl, heteroaryl, aryl-C 1-4 alkyl, and aryloxy-C 1-4 alkyl.
- the linking group is unsubstituted, while in other preferred embodiments, substituents are present that can increase partitioning into selected solvents or into selected tissues.
- each R 1 is a substituent independently selected from C 1-8 alkyl, C 1-8 haloalkyl, C 3-6 cycloalkyl, C 2-8 alkenyl and C 2-8 alkynyl, -COR a , -CO 2 R a , -CONR 3 R b , -NR a COR b , -SO 2 R a , -X 1 COR a .
- X 1 is a member selected from the group consisting of C 1-4 alkylene, C 2-4 alkenylene and C 2-4 alkynylene and each R a and R b is independently selected from the group consisting of hydrogen, C 1-8 alkyl, C 1-8 haloalkyl and C 3-6 cycloalkyl, and wherein the aliphatic portions of each of said R 1 substituents is optionally substituted with from one to three members selected from the group consisting of OH, O(C 1-8 alkyl), SH, S(C 1-8 alkyl), CN, NO 2 , NH 2 , NH(C 1-8 alkyl) and N(C
- the compounds are represented by formula I in which Ar 1 is phenyl substituted with from 1 to 3 R 2 groups wherein Ar 1 is selected from those represented by: wherein Hal is F, Cl or Br and each R is independently C 1-6 alkyl or C 3-6 cycloalkyl.
- HAr is selected from pyrazolyl optionally substituted from one to three R 3 groups independently selected from halogen, phenyl, thienyl, -OR f , -OC(O)R f , -NR f R g , -SR f , -R h , -CN, -NO 2 , -CO 2 R f , -CONR f R g , -C(O)R f , -OC(O)NR f R g , -NR g C(O)R f , -NR g C(O) 2 R h , -NR f -C(O)NR f R g , -S(O)R h , -S(O) 2 Rh, -S(O) 2 NR f R g , -NR f S(O) 2 R h , -NR f S(O) 2 NR f R g
- n 1, m is 0, 1 or 2
- Ar 1 is phenyl substituted with from one to three R 2 groups
- HAr is pyrazolyl which is substituted with three R 3 groups
- L 1 is -CH 2 -.
- Ar 1 is selected from those substituted phenyl moieties provided in Figure 1 .
- the compounds are represented by formula I in which Ar 1 is selected from phenyl, substituted with from one to three R 2 substituents wherein each R 2 is a member independently selected from the group consisting of halogen.
- the compounds are represented by formula I in which HAr is selected from pyrazolyl and, benzopyrazolyl, each of which is optionally substituted with from one to five R 3 groups independently selected from the group consisting of halogen, phenyl, thienyl, -OR f , -COR f , -CO 2 R f , -CONR f R g , -NO 2 , -R h , -CN, -SR f , -S(O)R h , -S(O) 2 R h and -NR f R g , wherein R f and R g are each independently selected from the group consisting of H, C 1-8 alkyl, C 3-6 cycloalkyl and C 1-8 haloalkyl, and each R h is independently selected from the group consisting of C 1-8 alkyl, C 3-6 cycloalkyl and C 1-8 haloalkyl, and each R h is
- the compounds are represented by formula II: or a pharmaceutically acceptable salt thereof, wherein each of R 1a , R 1b , R 1c , R 1d , R 1e , R 1f , R 1g and R 1h represents a member independently selected from the group consisting of H, C 1-8 alkyl, C 1-8 haloalkyl, C 3-6 cycloalkyl, C 2-8 alkenyl and C 2-8 alkynyl.
- the remaining groups have the meanings provided above with reference to formula I in their most complete interpretation.
- L 1 is -CH 2 - optionally substituted with one or more substituents independently selected from the group consisting of C 1-4 alkyl, C 1-4 haloalkyl and phenyl.
- HAr is selected from pyrazolyl and benzopyrazolyl, each of which is optionally substituted with from one to five R 3 groups independently selected from halogen, phenyl, thienyl, -OR f , -OC(O)R f , -NR f R g , -SR f , -R h , -CN, -NO 2 , -CO 2 R f , -CONR f R g , -C(O)R f , -OC(O)NR f R g , -NR g C(O)R f , -NR g C(O) 2 R h , -NR f -C(O)NR f R
- HAr is pyrazolyl or benzopyrazolyl, each of which is optionally substituted with from one to three R 3 groups independently selected from halogen, phenyl, thienyl, -OR f , -CO 2 R f , -COR f , -CONR f R g , -NO 2 , -R h , -CN, SR f , -S(O)R h , -S(O) 2 R h and -NR f R g , wherein each R f and R g is independently selected from H, C 1-8 alkyl and C 1-8 haloalkyl, each R h is independently selected from C 1-8 alkyl and C 1-8 haloalkyl. Most preferably, HAr is selected from the substituted pyrazolyl moieties provided in Figures 2 and 3 .
- the compound is represent by formula II above, wherein Ar 1 is phenyl substituted with from one to three R 2 substituents independently selected from the group consisting of halogen, -OR c , -OC(O)R c , -NR c R d , -SR c , -R c , -CN, -NO 2 , -CO 2 R c , -CONR c R d , -C(O)R c , -OC(O)NR c R d , -NR d C(O)R c , -NR d C(O) 2 R c , -NR c -C(O)NR c R d , -S(O)R c , -S(O) 2 R e , -S(O) 2 NR c R d and -N 3 wherein each R c and R d is independently selected from hydrogen,
- each R 1 is selected from C 1-4 alkyl and C 1-4 haloalkyl
- R 2a , R 2b , R 2c , R 2d and R 2e are each members independently selected from hydrogen, halogen -OR c , -OC(O)R c , -NR c R d , -SR c , -R e , -CN, NO 2 , -CO 2 R c , -CONR c R d , -C(O)R c , -OC(O)NR c R d , -NR d C(O)R c , -NR d C(O) 2 R e , -NR c -C(O)NR c R d , -S(O)R e , -S(
- the subscript m is 0 or 1 and at least one of R 2a or R 2e is hydrogen. More preferably, at least one of R 3a , R 3b and R 3c is selected from halogen and C 1-4 haloalkyl. Still more preferably, R 2d is hydrogen and at least two of R 3a , R 3b and R 3c are selected from halogen and C 1-4 haloalkyl with the remaining member being other than hydrogen.
- m is 0 or 1 and at least one of R 2a or R 2e is hydrogen, R 2d is hydrogen, R 2c is selected from F, Cl, Br, CN, NO 2 , CO 2 CH 3 , C(O)CH 3 and S(O) 2 CH 3 , and at least two of R 3a , R 3b and R 3c are selected from halogen and C 1-4 haloalkyl with the remaining member being other than hydrogen.
- the subscript m is 0 or 1; and R 2a and R 2e are both hydrogen. More preferably, at least one of R 3a , R 3b and R 3c is selected from halogen and C 1-4 haloalkyl.
- R 3a , R 3b and R 3c is selected from halogen and C 1-4 haloalkyl, and the remaining members of R 3a , R 3b and R 3c are other than hydrogen.
- the subscript m is 0 or 1; and R 2b and R 2c are both hydrogen.
- at least one of R 3a , R 3b and R 3c is selected from halogen and C 1-4 haloalkyl.
- at least one of R 3a , R 3b and R 3c is selected from halogen and C 1-4 haloalkyl, and the remaining members of R 3a , R 3b and R 3c are other than hydrogen.
- R 3b is preferably halogen, nitro or cyano, more preferably halogen and most preferably chloro or bromo;
- R 3c is preferably C 1-6 alkyl, C 1-6 haloalkyl or C 3-6 cycloalkyl;
- R 2c is halogen and R 2b is -OR c or R e wherein R c is selected from hydrogen, C 1-8 alkyl, C 1-8 haloalkyl, C 3-6 cycloalkyl, C 2-8 alkenyl and C 2-8 alkynyl, and R e is selected from the group consisting of C 1-8 alkyl, C 1-8 haloalkyl, C 3-6 cycloalkyl, C 2-8 alkenyl and C 2-8 alkynyl, and each of R c and R e is optionally further substituted with from one to three members selected from the group consisting of OH, O(C 1-8 alkyl), SH, S(C
- R 3b is preferably halogen, nitro or cyano, more preferably halogen and most preferably chloro or bromo;
- R 3a is preferably C 1-6 alkyl, C 1-6 haloalkyl or C 3-6 cycloalkyl;
- R 2c is preferably halogen and R 2b is preferably -OR c or R c wherein R c is selected from hydrogen, C 1-8 alkyl, C 1-8 haloalkyl, C 3-6 cycloalkyl, C 2-8 alkenyl and C 2-8 alkynyl, and R c is selected from the group consisting of C 1-8 alkyl, C 1-8 haloalkyl, C 3-6 cycloalkyl, C 2-8 alkenyl and C 2-8 alkynyl, and each of R c and R e is optionally further substituted with from one to three members selected from the group consisting of OH, O(C 1-8 alkyl), SH, S(
- R 3a is selected from NH 2 , CF 3 , SCH 3 , Ph and thienyl;
- R 3b is chloro or bromo;
- R 3c is preferably C 1-6 alkyl, C 1-6 haloalkyl or C 3-6 cycloalkyl;
- R 2c is hydrogen, halogen, cyano or nitro; and
- R 2b is selected from hydrogen, halogen.
- R c and R d is independently selected from hydrogen, C 1-8 alkyl, C 1-8 haloalkyl, C 3-6 cycloalkyl, C 2-8 alkenyl and C 2-8 alkynyl
- each R e is independently selected from the group consisting of C 1-8 alkyl, C 1-8 haloalkyl, C 3-6 cycloalkyl, C 2-8 alkenyl and C 2-8 alkynyl
- each of R c , R d and R e is optionally further substituted with from one to three members selected from the group consisting of OH, O(C 1-8 al
- R 2c is halogen, cyano or nitro
- R 2b is R e or -OR c
- R 3a is selected from the group consisting of NH 2 , CF 3 , SCH 3 , Ph and thienyl
- R 3b is chloro or bromo
- R 3c is selected from the group consisting of C 1-6 alkyl and C 3-6 cycloalkyl.
- R 3c is selected from NH 2 , CF 3 , SCH 3 , Ph and thienyl;
- R 3b is chloro or bromo;
- R 3a is preferably C 1-6 alkyl, C 1-6 haloalkyl or C 3-6 cycloalkyl;
- R 2c is hydrogen, halogen, cyano or nitro, preferably halogen; and
- R 2b is selected from hydrogen, halogen, -OR c , -OC(O)R c , -NR c R d , -SR c , -R e , -CO 2 R c , -CONR c R d , -C(O)R c , -S(O)R e and -S(O) 2 R e , wherein R c and R d is independently selected from hydrogen, C 1-8 alkyl, C 1-8 haloalkyl, C
- R 2c is halogen, cyano or nitro
- R 2b is R e or -OR c
- R 3a is selected from the group consisting of C 1- 6 alkyl and C 3-6 cycloalkyl
- R 3c is selected from the group consisting of NH 2 , CF 3 , SCH 3 , Ph and thienyl
- R 3b is chloro or bromo.
- R 3a is selected from NH 2 , CF 3 , SCH 3 , Ph and thienyl;
- R 3b is chloro or bromo;
- R 3c is preferably C 1-6 alkyl, C 1-6 haloalkyl or C 3-6 cycloalkyl;
- R 2a is preferably other than hydrogen, and is selected from halogen, -OR c , -OC(O)R c , -NR c R d , -SR c , -R e , -CO 2 R c , -CONR c R d , -C(O)R c , -S(O)R e and -S(O) 2 R e ;
- R2 c is hydrogen, halogen, cyano or nitro, preferably halogen; and
- R 2d is selected from hydrogen, halogen, -OR c , -OC(O)R c , -NR
- each of R 2a and R 2d is other than hydrogen.
- R 2a is other than hydrogen
- R 2c is halogen, cyano or nitro
- R 2d is R e or -OR c
- R 3a is selected from the group consisting of C 1-6 alkyl and C 3-6 cycloalkyl
- R 3b is chloro or bromo
- R 3c is selected from the group consisting of NH 2 , CF 3 , SHC 3 , Ph and thienyl.
- R 3c is selected from NH 2 , CF 3 , SCH 3 , Ph and thienyl;
- R 3b is chloro or bromo;
- R 3a is preferably C 1-6 alkyl, C 1-6 haloalkyl or C 3-6 cycloalkyl;
- R 2a is hydrogen, halogen, -OR c , -OC(O)R c , -NR c R d , -SR c , -R e , -CO 2 R c , -CONR c R d , -C(O)R c , -S(O)R e and -S(O) 2 R e ;
- R 2c is hydrogen, halogen, cyano or nitro; and
- R 2d is selected from hydrogen, halogen, -OR c , -OC(O)R c , -NR c R d , -SR
- each of R 2a and R 2d is other than hydrogen.
- R 20 is other than hydrogen;
- R 2c is halogen, cyano or nitro;
- R 2d is R e or -OR c ;
- R 3a is selected from the group consisting of NH 2 , CF 3 , SCH 3 , Ph and thienyl;
- R 3b is chloro or bromo;
- R 3c is selected from the group consisting of C 1-6 alkyl and C 3-6 cycloalkyl.
- compositions for modulating CCR1 activity in humans and animals will typically contain a pharmaceutical carrier or diluent.
- composition as used herein is intended to encompass a product comprising the specified ingredients in the specified amounts, as well as any product which results, directly or indirectly, from combination of the specified ingredients in the specified amounts.
- pharmaceutically acceptable it is meant the carrier, diluent or excipient must be compatible with the other ingredients of the formulation and not deleterious to the recipient thereof.
- compositions for the administration of the compounds of this invention may conveniently be presented in unit dosage form and may be prepared by any of the methods well known in the art of pharmacy and drug delivery. All methods include the step of bringing the active ingredient into association with the carrier which constitutes one or more accessory ingredients.
- the pharmaceutical compositions are prepared by uniformly and intimately bringing the active ingredient into association with a liquid carrier or a finely divided solid carrier or both, and then, if necessary, shaping the product into the desired formulation.
- the active object compound is included in an amount sufficient to produce the desired effect upon the process or condition of diseases.
- compositions containing the active ingredient may be in a form suitable for oral use, for example, as tablets, troches, lozenges, aqueous or oily suspensions, dispersible powders or granules, emulsions and self emulsifications as described in U.S. Patent Application 20020012680 , hard or soft capsules, syrups, elixirs, solutions, buccal patch, oral gel, chewing gum, chewable tablets, effervescent powder and effervescent tablets.
- compositions intended for oral use may be prepared according to any method known to the art for the manufacture of pharmaceutical compositions and such compositions may contain one or more agents selected from the group consisting of sweetening agents, flavoring agents, coloring agents, antioxidants and preserving agents in order to provide pharmaceutically elegant and palatable preparations.
- Tablets contain the active ingredient in admixture with non-toxic pharmaceutically acceptable excipients which are suitable for the manufacture of tablets.
- excipients may be for example, inert diluents, such as cellulose, silicon dioxide, aluminum oxide, calcium carbonate, sodium carbonate, glucose, mannitol, sorbitol, lactose, calcium phosphate or sodium phosphate; granulating and disintegrating agents, for example, corn starch, or alginic acid; binding agents, for example PVP, cellulose, PEG, starch, gelatin or acacia, and lubricating agents, for example magnesium stearate, stearic acid or talc.
- the tablets may be uncoated or they may be coated, enterically or otherwise, by known techniques to delay disintegration and absorption in the gastrointestinal tract and thereby provide a sustained action over a longer period.
- a time delay material such as glyceryl monostearate or glyceryl distearate may be employed, They may also be coated by the techniques described in the U.S. Pat. Nos. 4,256,108 ; 4,1666,452 ; and 4,265,874 to form osmotic therapeutic tablets for control release.
- Formulations for oral use may also be presented as hard gelatin capsules wherein the active ingredient is mixed with an inert solid diluent, for example, calcium carbonate, calcium phosphate or kaolin, or as soft gelatin capsules wherein the active ingredient is mixed with water or an oil medium, for example peanut oil, liquid paraffin, or olive oil.
- emulsions can be prepared with a non-water miscible ingredient such as oils and stabilized with surfactants such as mono-diglycerides, PEG esters and the like.
- Aqueous suspensions contain the active materials in admixture with excipients suitable for the manufacture of aqueous suspensions.
- excipients are suspending agents, for example sodium carboxymethylcellulose, methylcellulose, hydroxypropylmethylcellulose, sodium alginate, polyvinyl-pyrrolidone, gum tragacanth and gum acacia; dispersing or wetting agents may be a naturally-occurring phosphatide, for example lecithin, or condensation products of an alkylene oxide with fatty acids, for example polyoxyethylene stearate, or condensation products of ethylene oxide with long chain aliphatic alcohols, for example heptadecaethyleneoxycetanol, or condensation products of ethylene oxide with partial esters derived from fatty acids and a hexitol such as polyoxyethylene sorbitol monooleate, or condensation products of ethylene oxide with partial esters derived from fatty acids and hexitol anhydrides, for example polyethylene sorbitan monoole
- the aqueous suspensions may also contain one or more preservatives, for example ethyl, or n-propyl, p-hydroxybenzoate, one or more coloring agents, one or more flavoring agents, and one or more sweetening agents, such as sucrose or saccharin.
- preservatives for example ethyl, or n-propyl, p-hydroxybenzoate
- coloring agents for example ethyl, or n-propyl, p-hydroxybenzoate
- coloring agents for example ethyl, or n-propyl, p-hydroxybenzoate
- flavoring agents for example ethyl, or n-propyl, p-hydroxybenzoate
- sweetening agents such as sucrose or saccharin.
- Oily suspensions may be formulated by suspending the active ingredient in a vegetable oil, for example arachis oil, olive oil, sesame oil or coconut oil, or in a mineral oil such as liquid paraffin.
- the oily suspensions may contain a thickening agent, for example beeswax, hard paraffin or cetyl alcohol. Sweetening agents such as those set forth above, and flavoring agents may be added to provide a palatable oral preparation. These compositions may be preserved by the addition of an anti-oxidant such as ascorbic acid.
- Dispersible powders and granules suitable for preparation of an aqueous suspension by the addition of water provide the active ingredient in admixture with a dispersing or wetting agent, suspending agent and one or more preservatives.
- a dispersing or wetting agent e.g., glycerol, glycerol, glycerol, glycerol, glycerol, glycerol, glycerin, glycerin, glycerin, glycerin, glycerin, sorbitol, sorbitol, sorbitol, sorbitol, sorbitol, sorbitol, sorbitol, sorbitol, sorbitol, sorbitol, glycerol, glycerol, glycerol, glycerol, glycerol, glycerol, glycerol, glycerol, glycerol
- the pharmaceutical compositions of the invention may also be in the form of oil-in-water emulsions
- the oily phase may be a vegetable oil, for example olive oil or arachis oil, or a mineral oil, for example liquid paraffin or mixtures of these.
- Suitable emulsifying agents may be naturally-occurring gums, for example gum acacia or gum tragacanth, naturally-occurring phosphatides, for example soy bean, lecithin, and esters or partial esters derived form fatty acids and hexitol anhydrides, for example sorbitan monooleate, and condensation products of the said partial esters with ethylene oxide, for example polyoxyethylene sorbitan monooleate.
- the emulsions may also contain sweetening and flavoring agents.
- Syrups and elixirs may be formulated with sweetening agents, for example glycerol, propylene glycol, sorbitol or sucrose. Such formulations may also contain a demulcent, a preservative and flavoring and coloring agents.
- Oral solutions can be prepared in combination with, for example, cyclodextrin, PEG and surfactants.
- the pharmaceutical compositions may be in the form of a sterile injectable aqueous or oleagenous suspension.
- This suspension may be formulated according to the known art using those suitable dispersing or wetting agents and suspending agents which have been mentioned above.
- the sterile injectable preparation may also be a sterile injectable solution or suspension in a non-toxic parenterally-acceptable diluent or solvent, for example as a solution in 1,3-butane diol.
- the acceptable vehicles and solvents that may be employed are water, Ringer's solution and isotonic sodium chloride solution.
- sterile, fixed oils are conventionally employed as a solvent or suspending medium.
- any bland fixed oil may be employed including synthetic mono- or diglycerides.
- fatty acids such as oleic acid find use in the preparation of injectables.
- the compounds may also be administered in the form of suppositories for rectal administration of the drug.
- These compositions can be prepared by mixing the drug with a suitable non-irritating excipient which is solid at ordinary temperatures but liquid at the rectal temperature and will therefore melt in the rectum to release the drug.
- a suitable non-irritating excipient which is solid at ordinary temperatures but liquid at the rectal temperature and will therefore melt in the rectum to release the drug.
- Such material include cocoa butter and polyethylene glycols.
- the compound can be administered via ocular delivery by means of solutions or ointments.
- transdermal delivery of the subject compounds can be accomplished by means of iontophoretic patches and the like.
- creams, ointments, jellies, solutions or suspensions, etc., containing the compounds of the present invention are employed.
- topical application is also meant to include the use of mouth washes and gargles.
- the present invention concerns treating CCR1-mediated conditions or diseases by administering to a subject having such a disease or condition, a therapeutically effective amount of a compound of formula I above.
- the "subject” is defined herein to include animals such as mammals, including, but not limited to, primates (e.g., humans), cows, sheep, goats, horses, dogs, cats, rabbits, rats, mice and the like.
- CCR1 provides a target for interfering with or promoting specific aspects of immune cell functions, or more generally, with functions associated with CCR1 expression on a wide range of cell types in a mammal, such as a human.
- Compounds that inhibit CCR1 are particularly useful for modulating monocyte, macrophage, lymphocyte, granulocyte, NK cell, mast cells, dendritic cell, and certain immune derived cell (for example, osteoclasts) function for therapeutic purposes.
- the present invention is directed to compounds which are useful in the prevention and/or treatment of a wide variety of inflammatory and immunoregulatory disorders and diseases (see Saeki, et al., Current Pharmaceutical Design 9:1201-1208 (2003 )).
- an instant compound that inhibits one or more functions of CCR1 may be administered to inhibit (i.e., reduce or prevent) inflammation or cellular infiltration associated with an immune disorder.
- one or more inflammatory processes such as leukocyte emigration or infiltration, chemotaxis, exocytosis (e.g., of enzymes, histamine) or inflammatory mediator release, can be inhibited.
- monocyte infiltration to an inflammatory site e.g., an affected joint in arthritis, or into the CNS in MS
- an inflammatory site e.g., an affected joint in arthritis, or into the CNS in MS
- an instant compound that promotes one or more functions of CCR1 is administered to stimulate (induce or enhance) an inflammatory response, such as leukocyte emigration, chemotaxis, exocytosis (e.g., of enzymes, histamine) or inflammatory mediator release, resulting in the beneficial stimulation of inflammatory processes.
- an inflammatory response such as leukocyte emigration, chemotaxis, exocytosis (e.g., of enzymes, histamine) or inflammatory mediator release, resulting in the beneficial stimulation of inflammatory processes.
- monocytes can be recruited to combat bacterial infections.
- the disease or condition is one in which the actions of immune cells such monocyte, macrophage, lymphocyte, granulocyte, NK cell, mast cell, dendritic cell, or certain immune derived cell (for example, osteoclasts) are to be inhibited or promoted, in order to modulate the inflammatory or autoimmune response.
- immune cells such monocyte, macrophage, lymphocyte, granulocyte, NK cell, mast cell, dendritic cell, or certain immune derived cell (for example, osteoclasts) are to be inhibited or promoted, in order to modulate the inflammatory or autoimmune response.
- diseases or conditions including chronic diseases, of humans or other species can treated with modulators of CCR1 function.
- diseases or conditions include: (1) allergic diseases such as systemic anaphylaxis or hypersensitivity responses, drug allergies, insect sting allergies and food allergies, (2) inflammatory bowel diseases, such as Crohn's disease, ulcerative colitis, ileitis and enteritis, (3) vaginitis, (4) psoriasis and inflammatory dermatoses such as dermatitis, eczema, atopic dermatitis, allergic contact dermatitis, urticaria and pruritus, (5) vasculitis, (6) spondyloarthropathies, (7) scleroderma, (8) asthma and respiratory allergic diseases such as allergic asthma, allergic rhinitis, hypersensitivity lung diseases and the like, (9) autoimmune diseases, such as fibromyalagia, scleroderma, ankylosing spondylitis, juvenile RA, Still's disease, poly
- diseases or conditions can be treated with modulators of CCR1 function.
- diseases to be treated with modulators of CCR1 function include cancers, cardiovascular diseases, diseases in which angiogenesis or neovascularization play a role (neoplastic diseases, retinopathy and macular degeneration), infectious diseases (viral infections, e.g. , HIV infection, and bacterial infections) and immunosuppressive diseases such as organ transplant conditions and skin transplant conditions.
- organ transplant conditions is meant to include bone marrow transplant conditions and solid organ (e.g. , kidney, liver, lung, heart, pancreas or combination thereof) transplant conditions.
- the compounds used in the present invention are accordingly useful in the prevention and treatment of a wide variety of inflammatory and immunoregulatory disorders and diseases.
- the compounds may be administered by oral, parenteral, (e.g. , intramuscular, intraperitoneal, intravenous, ICV, intracisternal injection or infusion, subcutaneous injection, or implant), by inhalation spray, nasal, vaginal, rectal, sublingual, or topical routes of administration and may be formulated, alone or together, in suitable dosage unit formulations containing conventional non-toxic pharmaceutically acceptable carriers, adjuvants and vehicles appropriate for each route of administration.
- parenteral e.g. , intramuscular, intraperitoneal, intravenous, ICV, intracisternal injection or infusion, subcutaneous injection, or implant
- an appropriate dosage level will generally be 0.001 to 100 mg per kg patient body weight per day which can be administered in single or multiple doses.
- the dosage level will be 0.01 to 25 mg/kg per day; more preferably 0.05 to 10 mg/kg per day.
- a suitable dosage level may be 0.01 to 25 mg/kg per day, 0.05 to 10 mg/kg per day, or 0.1 to 5 mg/kg per day. Within this range the dosage may be 0.005 to 0.05, 0.05 to 0.5 or 0.5 to 5.0 mg/kg per day.
- the compositions are preferably provided in the form of tablets containing 1.0 to 1000 milligrams of the active ingredient, particularly 1.0, 5.0, 10.0, 15.0.
- the compounds may be administered on a regimen of I to 4 times per day, preferably once or twice per day.
- the specific dose level and frequency of dosage for any particular patient may be varied and will depend upon a variety of factors including the activity of the specific compound employed, the metabolic stability and length of action of that compound, the age, body weight, hereditary characteristics, general health, sex and diet of the subject, as well as the mode and time of administration, rate of excretion, drug combination, and the severity of the particular condition for the subject undergoing therapy.
- the compounds and compositions used in the present invention can be combined with other compounds and compositions having related utilities to prevent and treat the condition or disease of interest, such as inflammatory or autoimmune disorders, conditions and diseases, including inflammatory bowel disease, rheumatoid arthritis, osteoarthritis, psoriatic arthritis, polyarticular arthritis, multiple sclerosis, allergic diseases, psoriasis, atopic dermatitis and asthma, and those pathologies noted above.
- inflammatory or autoimmune disorders including inflammatory bowel disease, rheumatoid arthritis, osteoarthritis, psoriatic arthritis, polyarticular arthritis, multiple sclerosis, allergic diseases, psoriasis, atopic dermatitis and asthma, and those pathologies noted above.
- the present compounds and compositions may be used in conjunction with an anti-inflammatory or analgesic agent such as an opiate agonist, a lipoxygenase inhibitor, such as an inhibitor of 5-lipoxygenase, a cyclooxygenase inhibitor, such as a cyclooxygenase-2 inhibitor, an interleukin inhibitor, such as an interleukin-1 inhibitor, an NMDA antagonist, an inhibitor of nitric oxide or an inhibitor of the synthesis of nitric oxide, a non steroidal anti-inflammatory agent, or a cytokine-suppressing anti-inflammatory agent, for example with a compound such as acetaminophen, aspirin, codeine, fentanyl, ibuprofen, indomethacin, ketorolac, morphine, naproxen, phenacetin, piroxicam, a steroidal analgesic,
- an anti-inflammatory or analgesic agent such as an opiate agonist, a
- the instant compounds and compositions may be administered with an analgesic listed above; a potentiator such as caffeine, an H2 antagonist (e.g. , ranitidine), simethicone, aluminum or magnesium hydroxide; a decongestant such as phenylephrine, phenylpropanolamine, pseudoephedrine, oxymetazoline, ephinephrine, naphazolinc, xylometazoline, propylhexedrine, or levo desoxy ephedrine; an antitussive such as codeine, hydrocodone, caramiphen, carbetapentane, or dextromethorphan; a diuretic; and a sedating or non sedating antihistamine.
- a potentiator such as caffeine, an H2 antagonist (e.g. , ranitidine), simethicone, aluminum or magnesium hydroxide
- a decongestant such as phenylephrine, pheny
- compounds and compositions used in the present invention may be used in combination with other drugs that are used in the treatment, prevention, suppression or amelioration of the diseases or conditions for which compounds and compositions of the present invention are useful.
- Such other drugs may be administered, by a route and in an amount commonly used therefor, contemporaneously or sequentially with a compound or composition of the present invention.
- a pharmaceutical composition containing such other drugs in addition to the compound or composition used in the present invention is preferred.
- the pharmaceutical compositions include those that also contain one or more other active ingredients or therapeutic agents, in addition to a compound or composition used in the present invention
- other therapeutic agents include, but are not limited to: (a) VLA-4 antagonists, (b) corticosteroids, such as beclomethasone, methylprednisolone, betamethasone, prednisone, prenisolone, dexamethasone, fluticasone, hydrocortisone, budesonide, triamcinolone, salmeterol, salmeterol, salbutamol, formeterol; (c) immunosuppressants such as cyclosporine (cyclosporine A, Sandimmune®, Neoral®), tacrolimus (FK-506, Prograf®), rapamycin (sirolimus, Rapamune®) and other FK-506 type immunosuppressants, and mycophenol
- H1-histamine antagonists such as bromopheniramine, chlorpheniramine, dexchloipheniramine, triprolidine, clemastine, diphenhydramine, diphenylpyraline, tripelennamine, hydroxyzine, methdilazine, promethazine, trimeprazine, azatadine, cyproheptadine, antazoline, pheniramine pyrilamine, astemizole, terfenadine, loratadine, cetirizine, fexofenadine, descarboethoxyloratadine, and the like; (e) non steroidal anti asthmatics (e.g.
- terbutaline metaproterenol
- fenoterol isoetharine, albutetol, bitolterol and pirbuterol
- theophylline cromolyn sodium, atropine, ipratropium bromide
- leukotriene antagonists e.g. , zafmlukast, montelukast, pranlukast, iralukast, pobilukast and SKB-106,203
- leukotriene biosynthesis inhibitors zileuton, BAY-1005
- non steroidal anti-inflammatory agents such as propionic acid derivatives (e.g.
- alminoprofen alminoprofen, benoxaprofen, bucloxic acid, carprofen, fenbufen, fenoprofen, fluprofen, flurbiprofen, ibuprofen, indoprofen, ketoprofen, miroprofen, naproxen, oxaprozin, pirprofen, pranoprofen, suprofen, tiaprofenic acid and tioxaprofen), acetic acid derivatives (e.g.
- indomethacin acemetacin, alclofenac, clidanac, diclofenac, fenclofenac, fenclozic acid, fentiazac, furofenac, ibufenac, isoxepac, oxpinac, sulindac, tiopinac, tolmetin, zidometacin and zomepirac
- fenamic acid derivatives e.g. , flufenamic acid, meclofenamic acid, mefenamic acid, niflumic acid and tolfenamic acid
- biphenylcarboxylic acid derivatives e.g.
- oxicams e.g. , isoxicam, piroxicam, sudoxicam and tenoxican
- salicylates e.g. , acetyl salicylic acid and sulfasalazine
- pyrazolones e.g.
- cyclooxygenase-2 (COX-2) inhibitors such as celecoxib (Celebrex®) and rofecoxib (Vioxx®);
- COX-2 (COX-2) inhibitors such as celecoxib (Celebrex®) and rofecoxib (Vioxx®);
- PDE IV inhibitors of phosphodiesterase type IV (PDE IV);
- gold compounds such as auranofin and aurothioglucose,
- etanercept Enbrel®
- antibody therapies such as orthoclone (OKT3), daclizumab (Zenapax®), basiliximab (Simulect®) and infliximab (Remicade®), (1) other antagonists of the chemokine receptors, especially CCR5, CXCR2, CXCR3, CCR2, CCR3, CCR4, CCR7, GX 3 CR1 and CXCR6;
- vitamin D 3 derivatives e.g. , calcipotriene or calcipotriol (Dovonex®), (p) PUVA, (q) anthralin (Drithrocreme®); (r) etretinate (Tegison®) and isotretinoin and (s) multiple sclerosis therapeutic agents such as interferon ⁇ -1 ⁇ (Betaseron®), interferon ( ⁇ -1 ⁇ (Avonex®), azathioprine (Imurek®, Imuran®), glatiramer acetate (Capoxone®), a glucocorticoid (e.g.
- DMARDS such as methotrexate (u) other compounds such as 5-aminosalicylic acid and prodrugs thereof; hydroxychloroquine; D-penicillamine; antimetabolites such as azathioprine, 6-mercaptopurine and methotrexate; DNA synthesis inhibitors such as hydroxyurea and microtubule disrupters such as colchicine.
- the weight ratio of the compound used in the present invention to the second active ingredient may be varied and will depend upon the effective dose of each ingredient. Generally, an effective dose of each will be used.
- the weight ratio of the compound used in the present invention to the NSAID will generally range from 1000:1 to 1:1000, preferably 200:1 to 1:200.
- Combinations of a compound used in the present invention and other active ingredients will generally also be within the aforementioned range, but in each case, an effective dose of each active ingredient should be used.
- Reagents and solvents used below can be obtained from commercial sources such as Aldrich Chemical Co. (Milwaukee, Wisconsin, USA). 1 H-NMR were recorded on a Varian Mercury 400 MHz NMR spectrometer. Significant peaks are tabulated in the order: multiplicity (s, singlet; d, doublet; t, triplet; q, quartet; m, multiplet) and number of protons. Mass spectrometry results are reported as the ratio of mass over charge, followed by the relative abundance of each ion (in parenthesis). In tables, a single m/e value is reported for the M+H (or, as noted, M-H) ion containing the most common atomic isotopes.
- Electrospray ionization (ESI) mass spectrometry analysis was conducted on a Hewlett-Packard MSD electrospray mass spectrometer using the HP1100 HPLC for sample delivery. Normally the analyte was dissolved in methanol at 0.1 mg/mL and 1 microlitre was infused with the delivery solvent into the mass spectrometer, which scanned from 100 to 1500 daltons. All compounds could be analyzed in the positive ESI mode, using acetonitrile / water with 1% formic acid as the delivery solvent. The compounds provided below could also be analyzed in the negative ESI mode, using 2mM NH 4 OAc in acetonitrile / water as delivery system.
- ESI Electrospray ionization
- Two regioisomers can sometimes exist for certain compounds used in the invention.
- compounds such as those of formula III can be prepared wherein the pyrazole moiety is linked to the remainder of the molecule via either of the nitrogen atoms in the pyrazole ring.
- both regioisomeric types have demonstrated biological properties and are meant to be within the scope of all the appended claims, whether explicitly drawn or not.
- the piperazine ring can be formally attached to the terminal aryl unit in a number of ways: by aromatic nuclephilic displacement reactions, metal catalyzed coupling reactions (arylation reactions of secondary amines), ring expansion, rearrangement and cyclization reactions and the like. Also, different protection / deprotection strategies can be utilized. Hence, either all or only part of the final molecular architecture can be present during the key aryl coupling step. Examples for a variety of such aryl coupling strategies are listed below.
- PROTOCOL A Metal catalysed arylation reactions of secondary amines
- reaction mixture was refluxed at 110°C for 20 hrs, filtered through a celite bed, washed with toluene, concentrated, taken in ethyl acetate and extracted with 1.5 (N) HCl solution three times.
- the combined aqueous layers were washed with diethyl ether.
- the aqueous layer was neutralized with 10% aqueous sodium hydroxide solution and then extracted with ethyl acetate three times.
- the combined ethyl acetate layers were washed with water and saturated brine solution, dried over anhydrous sodium sulfate and concentrated. Purification by flash chromatography (eluted with CHCl3-MeOH) afforded the title compound as product.
- Piperazine (0.588g, 6.84mmol), Pd(II)acetate (0.027g , 0.123mmol), sodium t-butoxide (0.837g, 10.06mmol) and BINAP (0.154g, 0.286mmol) were stirred at room temperature in 10 mL dry toluene for 15 min.
- 4-trifluoromethoxy bromo benzene (1.5 g, 6.22mmol) in 10 mL dry toluene was added into the reaction mixture. Then the reaction mixture was refluxed at 110°C for 20 hrs.
- reaction mixture was filtered through a celite bed, washed with toluene, concentrated, ethyl acetate added and then extracted with 1.5 (N) aqueous HCl solution three times.
- the combined aqueous layers were washed with diethyl ether.
- the aqueous layer was neutralized with 10% aqueous sodium hydroxide solution and then extracted with ethyl acetate three times.
- the combined ethyl acetate layers were washed with water and saturated brine solution, dried over anhydrous sodium sulfate and concentrated to afford the product.
- the toluene was concentrated and the reaction mixture was taken in ethyl acetate and extracted with 1.5 (N) HCl solution three times. The combined aqueous layers were washed with diethyl ether. The aqueous layer was neutralized with 10% aqueous sodium hydroxide solution and then extracted with ethyl acetate three times. The combined ethyl acetate layers were washed with water and saturated brine solution, dried over anhydrous sodium sulfate, concentrated and chromatographed (9/1-CHCl3/MeOH) to afford the product.
- 1-Bromo-3-isopropoxy-4-chlorobenzene (preparation described elsewhere) was combined with 1.11g (6 mmol) of 1-Bocpiperazine, 672mg (7.0 mmol) of sodium tert-butoxide, 93mg (0.15 mmol) of rac-2,2'-Bis(diphenylphosphine)-1,1'-binaphthyl, and 45mg (0.05 mmol) Tris(dibenzylidencacetone)dipalladium (0) in a flask under an N2 atmosphere, and the mixture was heated at 85°C for 3.5 hours.
- the resulting residue was partitioned between a 1/1 mixture of ether and ethyl acetate and water, and the phases were separated.
- the final organic phase was dried over Na2SO4, filtered, and concentrated in vacuo to an oil.
- the oil was dissolved in ethyl acetate, 10mL each of 2M HCl in ether and methanol were added, and the product was isolated by filtration after crystallization.
- a single neck round bottom flask was charged with 1-chloro-4-iodo benzene (1.0 g, 0.0041 mol) and R(-)-2-methylpiperazine (0.5 g, 0.005 mol), potassium t-butoxide (0.705 g, 0.0062 mol), tris(benzylideneacetone)dipalladium(0) (0.095 g, 0.0002 mol) and 1,3 bis(2,6-diisopropylphenyl)imidazole-2-ylidene) (0.073 g, 0.0001 mol).
- the flask was evacuated and filled with nitrogen. Dry dioxane (20 mL) was added and stirred at 70 °C overnight.
- reaction mixture was diluted with dichloromethane and filtered. Crude compound was purified by column chromatography. The compound was dissolved in ether and purged with HCl gas to yield 1-(4-Chloro-phenyl)-3-methyl-piperazine.
- reaction mixture was filtered through a celite bed and washed with toluene, then concentrated and the reaction mixture was taken into ethyl acetate and extracted with 1.5 (N) HCl solution three times.
- the combined aqueous layer was washed with diethyl ether.
- the aqueous layer was neutralized with 10% aqueous sodium hydroxide solution and then extracted with ethyl acetate three times.
- the combined ethyl acetate layers were washed with water and saturated brine solution, dried over anhydrous sodium sulfate, and concentrated to afford the product as a white solid.
- PROTOCOL B Piperidine ring formation via cyclization reactions
- 3,4-Difluoro-aniline (1g, 7.7mmol) was dissolved in dry n-butanol (10mL) and dry sodium carbonate (3.2g, 30mmol) was added to it and the reaction mixture stirred for 1. hour under nitrogen.
- Bis(2-chloroethyl) amine hydrochloride (1.38g, 7.7mmol) in nBuOH (10mL) were then added to the mixture via a syringe. The reaction was then heated at 120°C for 48h. The nBuOH was evaporated in vacuo and the residue was extracted with ethyl acetate. Drying of the organic layer with Na 2 SO 4 followed by concentration afforded the crude product. Purification using flash column chromatography (chloroform/methanol) afforded 1-(3,4-Difluoro-phenyl)-piperazine as an off white solid.
- PROTOCOL C Piperidine ring formation via a ring opening / ring cyclization strategy
- the solution was then concentrated in vacuo to give a residue that was partitioned between ether and water.
- the phases were separated, and the aqueous phase as basified with 1M NaOH.
- the aqueous phase was then extracted twice with ethyl acetate.
- the combined ethyl acetate phases were washed once with brine, dried over Na2S04, filtered, and acidified with 2M HCl in ether.
- the product was isolated via filtration.
- a direct halogen displacement strategy can be complimentary to the metal mediated approaches, discussed above, for the construction of the ring systems provided herein.
- 4-bromoethyl benzoate (10:0 g, 0.0437 mol) was taken into 250 mL of dry DMF, piperazine (37g, 0.437 mol) was added, followed by 30g (0.2185 mol) of dry potassium carbonate, 1.0 g of TBAI and 1.5 g of potassium iodide.
- the reaction mixture was heated at 135 °C for over night.
- the reaction mixture was quenched with water and extracted with ethyl acetate.
- the extracts were washed with water, then brine and then concentrated to yield 4-Piperazin-1-yl-benzoic acid ethyl ester as an off-white solid.
- PROTOCOL D Synthesis and addition of elaborated piperazines to aryl and heteroaryl halides via aryl-halogen displacement methodologies
- Functionalization of the aryl ring within the arylpiperazine ring system can, in general, take place either before or after introduction of the piperazine ring, as illustrated in the examples below.
- PROTOCOLE Selected examples of halogenation of aromatic systems after attachment of the piperazine ring system
- PROTOCOL F Selected examples of demethylation / etherification of aromatic precursors for attachment of the piperazine ring system to access key arylpiperazine moieties
- PROTOCOL F Additional examples of analogous ring systems constructed using similar demethylation / etherification strategies.
- PROTOCOL G General procedure for the synthesis of elaborated aryl bromides from anilines
- the reaction mixture was cooled to room temperature and solid formed was washed with water to afford white solid cuprous bromide.
- the diazonium salt was portion wise added into the freshly prepared cuprous bromide in 40 mL HBr at -10°C bath temperature and the reaction mixture was then warmed to room temperature.
- the reaction mixture was heated at 55°C for 20 min, cooled and then extracted with ethyl acetate three times.
- the combined organic layer was washed with water and saturated brine solution, dried over sodium sulfate and concentrated.
- the crude material was purified by column chromatography (5:95 ethyl acetate: pet ether) to afford solid product.
- Diazonium salt was portion wise added into the freshly prepared cuprous bromide in 40 mL HBr at -10°C bath temperature and the reaction mixture warmed to room temperature. Then the reaction mixture was heated at 55°C for 20 min, cooled to room temperature and extracted with ethyl acetate three times. The combined organic layer was washed with water and saturated brine solution, dried over sodium sulfate and concentrated. The product was purified by crystallization from DCM/Pet ether.
- PROTOCOL G Additional examples of analogous ring systems constructed using similar Sandmeyer type strategies
- PROTOCOL H Pyrazole synthesis via addition of hydrazines to ⁇ -acetylenic ketones
- PROTOCOL I General procedure for the synthesis of pyrazoles via condensation of hydrazines with ⁇ -diketones:
- PROTOCOL J Pyrazole synthesis via condensation or hydrazines with ⁇ -Cyanoketones
- PROTOCOL K chlorination of pyrazoles with NAOCl in glacial Acetic acid
- PROTOCOL L chlorination or bromination of pyrazoles with N-chlorosuccinimide (NCS) or N-bromosuccinimide (NBS):
- PROTOCOL M General procedure for reduction of Nitropyrazoles
- PROTOCOL N Functionalization of alkyl substituted heteroaryl ring systems: aminomethylation
- This intermediate ester (5g, 0.019mol) was taken in CCl 4 (100mL) and AIBN (0.053g, 0.33mmol) was added to it under nitrogen. The mixture was irradiated with a regular light bulb. The mixture was brought to reflux and then NBS (3.42g, 0.019mol), in four portions in 15min intervals, was added to the mixture. After complete addition the mixture was left refluxing under the influence of light for 3h. The reaction mixture was then filtered and the filtrate was washed with water and brine.
- PROTOCOL O Synthesis of (5-Azidomethyl-4-chloro-3-trifluoromethyl-pyrazol-1-yl)-acetic acid:
- PROTOCOL P (vide infra): Synthesis of 2-(5-Azidomethyl-4-chloro-3-trifluromethyl-pyrazol-1-yl)-1-[4-(4-chlorophenyl)-piperazin-1-yl]-ethanone:
- PROTOCOL Q Synthesis of 2-(5-Aminometyl-4-chloro-3-trifluromethyl-pyrazol-1-yl)-1-[4-(4-chlorophenyl)-piperazin-1-yl]-ethanone
- the acidic aqueous phase was basified with 1M NaOH, and was extracted once with ethyl acetate. The final ethyl acetate phase was washed once with brine, dried over Na2S04, filtered, and concentrated to an oil.
- PROTOCOL R Urea derivatization of aminomethyl functionality on pyrazole ring system.
- the ester (14.8g, 0.0565 mol) was dissolved in THF (100mL) and a solution of LiOH (6.9g) in water (50mL) was added to it. The mixture was stirred for 10h at room temperature Excess THF was evaporated under reduced pressure and the aqueous layer was washed with ethyl acetate to remove any unhydrolysed material. The aqueous layer was then acidified with 1.5N HCl and extracted with ethyl acetate. The ethyl acetate layer was dried and concentrated to obtain the crude acid. On re-crystallization from ether/pet, priduct was obtained as while: crystals.
- PROTOCOL P Compounds prepared by HATU mediated coupling:
- the ethyl acetate phase was then dried over Na2SO4, filtered, and concentrated to a residue in vacuo.
- the residue was dissolved in a minimum volume of 5M HCl in isopropanol, and was precipitated by diluting the solution with ethyl acetate.
- PROTOCOL S preparation of chloroacetyl arylpiperazines
- Protocol S was followed using 1-(4-chloro-phenyl) piperazine, Et 3 N, chloroacetyl chloride and methylene chloride.
- Protocol S was followed using 1-(4-Chloro-phenyl)-3-(S)-methyl-piperazine, Et 3 N, chloroacetyl chloride and methylene chloride. Column chromatography afforded the title compound.
- PROTOCOL T K 2 CO 3 mediated coupling reaction of chloroacetyl arylpiperazines with pyrazoles
- Protocol T was followed using 4-Chloro-5-phenyl-3-trifluoromethyl-1H-pyrazole, K 2 CO 3 , 2-Chloro-1-[4-(4-fluoro-phenyl)-piperazin-1-yl]-ethanone and DMF.
- Protocol T was followed using 4-Chloro-5-phenyl-3-trifluoromethyl-1H-pyrazole, K 2 CO 3 , 2-Chloro-1-[4-(4-chloro-phenyl)-piperazin-1-yl]-ethanone and DMF.
- 13 C NMR 400 MHz, CDCl 3 ): 162, 146.4, 142.2, 118.5, 116.2, 52, 50.4, 46.2, 42.2, 15.2.
- Protocol T was followed using 3-Heptafluoropropyl-5-methyl-4-nitro-1H-pyrazole, K 2 CO 3 , 2-Chloro-1-[4-(4-chloro-phenyl)-piperazin-1-yl)-ethanone and DMF.
- Protocol T was followed using 4-Chloro-5-phenyl-3-trifluoromethyl-1H-pyrazole, K 2 CO 3 , 2-Chloro-1-[4-(4-chloro-3-methoxy-phenyl)-piperazin-1-yl]-ethanone and DMF.
- Protocol T was followed using 4-Chloro-5-phenyl-3-trifluoromethyl-1H-pyrazole, K 2 CO 3 , 2-Chloro-1-[4-(4-bromo-3-methoxy-phenyl)-piperazin-1-yl]-ethanone and DMF.
- Protocol T was followed using 4-Bromo-5-methyl-3-trifluoromethyl-1H-pyrazole, K 2 CO 3 , 2-Chloro-1-[4-(4-chloro-3-methoxy-phenyl)-piperazin-1-yl)-ethanone and DMF.
- Protocol T was followed using 4-Bromo-5-phenyl-1H-pyrazol-3-ylamine, K 2 CO 3 , 2-Chloro-1-[4-(4-chloro-3-methoxy-phenyl)-piperazin-1-yl]-ethanone and DMF.
- Protocol T was followed using 4-Chloro-5-methyl-1H-pyrazol-3-ylamine, K 2 CO 3 , 2-Chloro-1-[4-(4-chloro-3-methoxy-phenyl)-piperazin-1-yl]-ethanone and DMF.
- Protocol T was followed using 4-Chloro-3-trifluoromethyl-1H-pyrazole, K 2 CO 3 , 2-Chloro-1-[4-(4-fluoro-phenyl)-piperazin-1-yl]-ethanone and DMF.
- Protocol T was followed using 3-(4-Fluoro-phenyl)-5-methylsulfanyl-1H-pyrazole, K 2 CO 3 , 2-Chloro-1-[4-(4-fluoro-phenyl)-piperazin-1-yl]-ethanone and DMF.
- Protocol T was followed using 4-Chloro-3-(4-Fluoro-phenyl)-5 -methylsulfanyl-1H-pyrazole, K 2 CO 3 , 2-Chloro-1-[4-(4-fluoro-phenyl)-piperazin-1-yl]-ethanone and DMF.
- Protocol T was followed using 4-Chloro-3-(4-Fluoro-phenyl)-5-methylsulfanyl-1H-pyrazole, K 2 CO 3 , 2-Chloro-1-[4-(4-fluoro-phenyl)-piperazin-1-yl]-ethanone and DMF.
- Protocol T was followed using 4-Chloro-3-Thiophen-2-yl-2H-pyrazole-5-carboxylic acid ethyl ester, K 2 CO 3 , 2-Chloro-1-[4-(4-fluoro-phenyl)-piperazin-1-yl]-ethanone and DMF.
- 1 H NMR 400 MHz, CDCl 3 ); 6.92-7.02 (m, 4H), 5.14 (s, 2H), 3.64-3.82 (m, 4H), 3.6 (s, 2H), 3.1-3.22 (m, 4H), 2.16 (s, 3H).
- 1 H NMR 400 MHz, CDCl 3 ): 7.18-7.24 (m. 2H), 6.78-6.84 (m, 2H).
- Protocol T was followed using 4-Chloro-5-n-butyl-3-trifluoromethyl-1H-pyrazole, K 2 CO 3 , 2-Chloro-1-[4-(4-fluoro-phenyl)-piperazin-1-yl]-ethanone and DMF.
- 1 H NMR 400 MHz, CDCl 3 ); 7.58-7.62 (d, 1H), 7.52 (s, 1H), 6.95-7.1 (m, 2H), 6.84-6.92 (dd, 2H), 5.00 (s, 2H).
- Protocol T was followed using 3-Trifluoromethyl-1H-pyrazole-4-carboxylic acid ethyl ester, K 2 CO 3 , 2-Chloro-1-[4-(4-fluoro-phenyl)-piperazin-1-yl]-ethanone and DMF.
- 1 H NMR 400 MHz, CDCl 3 ); 6.95.7.1 (m, 2H), 6.84-6.92 (dd, 2H), 5.00 (s, 2H), 3.62-3.82 (m, 4H), 3.02-3.12 (m, 4H), 2.22-2.32 (d, 6H).
- Protocol T was followed using 5-Propyl-2H-pyrazole-3-carboxylic acid ethyl ester, K 2 CO 3 , 2-Chloro-1-[4-(4-fluoro-phenyl)-piperazin-1-yl]-ethanone and DMF.
- Protocol T was followed using 5-Propyl-2H-pyrazole-3-carboxylic acid ethyl ester, K 2 CO 3 , 2-Chloro-1-[4-(4-fluoro-phenyl)-piperazin-1-yl]-ethanone and DMF. Column chromatography using a solvent mixture (hexane/ ethyl acetate 1/1) afforded the title compound as white solid.
- Protocol T was followed using 3,5-Bis-trifluoromethyl-1H-pyrazole, K 2 CO 3 , 2-Chloro-1-[4-(4-fluoro-phenyl)-piperazin-1-yl]-ethanone and DMF.
- Protocol T was followed using 1H-Pyrazole-3,5-dicarboxylic acid diethyl ester, K 2 CO 3 , 2-Chloro-1-[4-(4-fluoro-phenyl)-piperazin-1-yl]-ethanone and DMF.
- Protocol T was followed using 4-Chloro-5-Propyl-2H-pyrazole-3-carboxylic acid ethyl ester, K 2 CO 3 , 2-Chloro-1-[4-(4-fluoro-phenyl)-piperazin-1-yl]-ethanone and DMF.
- Protocol T was followed using 5-tert-Butyl-3-trifluoromethyl-1H-pyrazole, K 2 CO 3 , 2-Chloro-1-[4-(4-fluoro-phenyl)-piperazin-1-yl]-ethanone and DMF.
- 1 H NMR 400 MHz, CDCl 3 ): 6.95-7.1 (m, 2H), 6.84-6.92 (dd, 2H), 4.90 (s, 2H), 3.62-3.82 (m, 4H), 3.02-3.12 (m, 4H), 2.24-2.34 (d, 6H).
- Protocol T was followed using 4-Chloro-3-(5-chloro-thiophen-2-yl)-1H-pyrazole, K 2 CO 3 , 2-Chloro-1-[4-(4-fluoro-phenyl)-piperazin-1-yl]-ethanone and DMF.
- Protocol T was followed using 4-Chloro-5-methyl-2H-pyrazole-3-carboxylic acid ethyl ester, K 2 CO 3 , 2-Chloro-1-[4-(4-fluoro-phenyl)-piperazin-1-yl]-ethanone and DMF.
- Protocol T was followed using 4-Chloro-5-(5-chloro-thiophen-2-yl)-2H-pyrazole-3-carboxylic acid ethyl ester, K 2 CO 3 , 2-Chloro-1-[4-(4-fluoro-phenyl)-piperazin-1-yl]-ethanone and DMF.
- 1 H NMR 400 MH 2 , CDCl 3 ): 7.18-7.22 (d, 1H), 6.78-6.84 (d, 2H), 4.8 (s, 2H). 4.4 (s, 2H), 3.72-3.82 (m, 4H), 3.08-3.18 (m, 4H), 2.14 (s, 3H).
- Protocol T was followed using 4-Chloro-5-phenyl-3-trifluoromethyl-1H-pyrazole, K 2 CO 3 , 2-Chloro-1-[4-(4-bromo-3-methoxy-phenyl)-piperazin-1-yl]-ethanone and DMF.
- Protocol T was followed using 4-Bromo-5-methyl-1H-pyrazol-3-ylamine, K 2 CO 3 , 2-Chloro-1-[4-(4-fluoro-phenyl)-piperazin-1-yl]-ethanone and DMF. Column chromatography ethyl acetate afforded the title compound as a colorless oil.
- Protocol T was followed using 5-methyl-2H-pyrazole-3-carboxylic acid ethyl ester, K 2 CO 3 , 2-Chloro-1-[4-(4-fluoro-phenyl)-piperazin-1-yl]-ethanone and DMF.
- Protocol T was followed using 3,5-Diisopropyl-4-chloro-1H-pyrazole, K 2 CO 3 , 2-Chloro-1-[4-(4-fluoro-phenyl)-piperazin-1-yl]-ethanone and DMF.
- MS (ES) M+H) expected 406.9. found 407.1.
- 1 H NMR 400 MHz, CDCl 3 ): 7.34-7.38 (m, 1H), 7.24-7.26 (m, 1H), 7.12 (s, 1H).
- Protocol T was followed using 4-Amino-3-heptafluoropropyl-5-methyl-1H-pyrazole, K 2 CO 3 , 2-Chloro-1-[4-(4-chloro-phenyl)-piperazin-1-yl]-ethanone and DMF.
- Protocol T was followed using 4-Chloro-5-ethyl-3-trifluoromethyl-1-H-pyrazol, K 2 CO 3 , 1-[4-(4-Chloro-3-methoxyphenyl)-piperazine-1-yl]-ethanone and DMF.
- Protocol T was followed using 4-Chloro-5-isopropyl-3-trifluoromethyl-1-H-pyrazol, K 2 CO 3 , 1-[4-(4-Chloro-3-methoxyphenyl)-piperazine-1-yl]-ethanone and DMF.
- Protocol T was followed using 4-Chloro-3-isopropyl-5-trifluoromethyl-1-H-pyrazol, K 2 CO 3 , 1-[4-(4-Chloro-3-methoxyphenyl)-piperazine-1-yl]-ethanone and DMF.
- Protocol T was followed using 4-Chloro-3-n-propyl-5-trifluoromethyl-1-H-pyrazol, K 2 CO 3 , 1-[4-(4-Chloro-3-methoxyphenyl)-piperazine-1-yl]-ethanone and DMF.
- Protocol T was followed using 4-Bromo-3-phenyl-5-trifluoromethyl-1H-pyrazole, K 2 CO 3 , 2-Chloro-1-[4-(4-chloro-3-methoxy-phenyl)-piperazin-1-yl]-ethanone and DMF.
- Protocol T was followed using 4-Chloro-5-phenyl-3-trifluoromethyl-1H-pyrazole, K 2 CO 3 , 2-Chloro-1-[4-(4-chloro-3-methoxy-phenyl)-piperazin-1-yl]-ethanone and DMF.
- 1 H NMR 400 MHz, CDCl 3 ): 7.78-7.84 (m, 2H), 7.36-7.52 (m, 4H).
- Protocol T was followed using 4-Chloro-3-[3-Fluorophenyl]-5-trifluoromethyl-1H-pyrazole, K 2 CO 3 , 2-Chloro-1-[4-(4-chloro-3-methoxy-phenyl)-piperazin-1-yl]-ethanone and DMF.
- Protocol T was followed using 4-Chloro-5-[3-Fluorophenyl]-3-trifluoromethyl-1H-pyrazole, K 2 CO 3 , 2-Chloro-1-[4-(4-chloro-3-methoxy-phenyl)-piperazin-1-yl]-ethanone and DMF.
- 1 H NMR 400 MHz, CDCl 3 ): 7.64-7.68 (d, 1H), 7.56-7.62 (d, 1H), 7.36-7.42 (m, 1H), 7.22-7.24 (m. 2H), 7.08-7.12 (m, 1H), 6.42-6.52 (m, 2H), 5.2 (s, 2H), 3.9 (s, 3H), 3.62-3.82 (m, 4H), 3.12-3.22 (m, 4H).
- PROTOCOL U for the K 2 CO 3 mediated coupling reaction of chloroacetyl substituted arylpiperazines with novel heteraryl ring systems
- Benzimidazole (0.785g, 0.7mmol) was taken in dry DMF (15ml) and dry potassium carbonate (340mg) and KI (20mg) was added to it and the reaction mixture stirred at room temperature for 1h under nitrogen.
- 2-Chloro-1-[4-(4-chloro-phenyl)-piperazin-1-yl]-ethanone (200mg, 1.1mmol) in DMF (5ml) was then added to the mixture through a syringe. The reaction was then heated at 140°C for 14h, cooled and then quenched with water and extracted with ethyl acetate.
- 2,4-dimethylimidazole (0.633g, 0.7mmol) was taken up in dry DMF (15ml) and dry potassium carbonate (340mg) and KI (20mg) was added and the reaction mixture was stirred at room temperature for 1h under nitrogen.
- 2-Chloro-1-[4-(4-chloro-phenyl)-piperazin-1-yl]-ethanone (200mg, 1.1mmol) in DMF (5ml) was then added to the mixture through a syringe. The reaction was then heated at 140°C for 14h, cooled and quenched with water and extracted with ethyl acetate.
- This compound (90mg, 0.275mmol) was taken into dry CH 2 Cl 2 (10ml) and cooled to 0°C. To this cold mixture was first added 4-chlorophcnyl-piperazine (0.059g, 0.31 mmol) followed by the addition of T3P (0.35g, 0.55mmol, 50% solution in EtOAc). The reaction was left overnight at room temperature. The mixture was diluted with CH 2 Cl 2 , and then washed sequentially with saturated NaHCO3 solution, brine, dried over Na2S04, and concentrated to afford the crude product.
- This example illustrates the activity associated with representative compounds of the invention.
- THP-1 cells were obtained from ATCC and cultured as a suspension in RPMI-1640 medium supplemented with 2 mM L-glutamine, 1.5 g/L sodium bicarbonate, 4.5 g/L glucose, 10 mM HEPES, 1 mM sodium pyruvate, 0.05% 2-mercaptoethanol and 10% FBS. Cells were grown under 5% CO 2 /95% air, 100% humidity at 37°C and subcultured twice weekly at 1:5 and harvested at 1 x 10 6 cells/ml. THP-1 cells express CCR1 and can be used in CCR1 binding and functional assays.
- Monocytes were isolated from human buffy coats using the Miltenyi bead isolation system (Miltenyi, Auburn, CA). Briefly, following a Ficoll gradient separation to isolate peripheral blood mononuclear cells, cells were washed with PBS and the red blood cells lysed using standard procedures. Remaining cells were labeled with anti-CD14 antibodies coupled to magnetic beads (Miltenyi Biotech, Auburn, CA). Labeled cells were passed through AutoMACS (Miltenyi, Auburn, CA) and positive fraction collected. Monocytes express CCR1 and can be used in CCR1 binding and functional assays.
- Miltenyi bead isolation system Miltenyi, Auburn, CA.
- CCR1 expressing cells were centrifuged and resuspended in assay buffer (20 mM HEPES pH 7.1, 1.40 mM NaCl, 1 mM CaCl 2 , 5 mM MgCl 2 , and with 0.2% bovine serum albumin) to a concentration of 2.2 x 10 5 cells/ml for THP-1 cells and 1.1 x 10 6 for monocytes.
- Binding assays were set up as follows. First, 0.09 ml of cells (1 x 10 5 THP-1 cells/well or 5 x 10 5 monocytes) was added to the assay plates containing the compounds, giving a final concentration of ⁇ 2-10 ⁇ M each compound for screening (or part of a dose response for compound IC 50 determinations).
- Control wells containing either diluent only (for total counts) or excess MIP-1 ⁇ or MIP-1 ⁇ (1 ⁇ g/ml, for non-specific binding) were used to calculate the percent of total inhibition for compound.
- the computer program Prism from GraphPad, Inc. (San Diego, Ca) was used to calculate IC 50 values. IC 50 values are those concentrations required to reduce the binding of labeled MIP-1 ⁇ to the receptor by 50%.
- THP-1 or monocytes were incubated with 3 ⁇ M of INDO-1AM dye (Molecular Probes; Eugene, OR) in cell media for 45 minutes at room temperature and washed with phosphate buffered saline (PBS). After INDO-1AM loading, the cells were resuspended in flux buffer (Hank's balanced salt solution (HBSS) and 1% FBS). Calcium mobilization was measured using a Photon Technology International spectrophotometer (Photon Technology International; New Jersey) with excitation at 350 nm and dual simultaneous recording of fluorescence emission at 400 nm and 490 nm. Relative intracellular calcium levels were expressed as the 400 nm/490 nm emission ratio.
- INDO-1AM dye Molecular Probes; Eugene, OR
- chemokine ligands may be used over a range from 1 to 100 nM. The emission ratio was plotted over time (typically 2-3 minutes).
- Candidate ligand blocking compounds up to 10 ⁇ M were added at 10 seconds, followed by chemokines at 60 seconds (i.e., MIP-1 ⁇ ; R&D Systems; Minneapolis, MN) and control chemokine (i.e., SDF-1 ⁇ ; R&D Systems; Minneapolis, MN) at 150 seconds.
- Chemotaxis assays were performed using 5 ⁇ m pore polycarbonate, polyvinylpyrrolidone-coated filters in 96-well chemotaxis chambers (Neuroprobe; Gaithersburg, MD) using chemotaxis buffer (Hank's balanced salt solution (HBSS) and 1% FBS).
- CCR1 chemokine ligands i.e, MIP-1 ⁇ , Leukotactin; R&D Systems; Minneapolis, MN
- Other chemokines i.e., SDF-1 ⁇ , R&D Systems; Minneapolis, MN are used as specificity controls.
- the lower chamber was loaded with 29 ⁇ l of chemokine (i.e., 0.1 nM MIP-1 ⁇ ) and varying amounts of compound; the top chamber contained 100,000 THP-1 or monocyte cells in 20 ⁇ l.
- the chambers were incubated 1-2 hours at 37°C, and the number of cells in the lower chamber quantified either by direct cell counts in five high powered fields per well or by the CyQuant assay (Molecular Probes), a fluorescent dye method that measures nucleic acid content and microscopic observation.
- radioactive ligand i.e, MIP-1 ⁇ or leukotactin binding to cells expressing CCR1 on the cell surface (for example, THP-1 cells or isolated human monocytes).
- MIP-1 ⁇ or leukotactin binding to cells expressing CCR1 on the cell surface
- CCX-105 was identified from the compound screening effort.
- inhibitory activity was titered over a 1 x 10 -10 to 1 x 10 -4 M range of compound concentrations. In the assay, the amount of compound was varied; while cell number and ligand concentration were held constant. Compound CCX-105 was titered and found to be a potent inhibitor of CCR I specific chemokine binding (see Table, for compound 1.001).
- CCR1 is a seven transmembrane, G-protein linked receptor.
- a hallmark of signaling cascades induced by the ligation of some such receptors is the pulse-like release of calcium ions from intracellular stores.
- Calcium mobilization assays were performed to determine if the candidate CCR1 inhibitory compounds were able to also block aspects of CCR1 signaling.
- Candidate compounds able to inhibit ligand binding and signaling with an enhanced specificity over other chemokine and non-chemokine receptors were desired.
- CCR1 chemokine ligands i.e., MIP-1 ⁇ , MPIF-1, Leukotactin, etc.
- CCR1 chemokine ligands i.e., MIP-1 ⁇ , MPIF-1, Leukotactin, etc.
- THP-1 cells or monocytes were loaded with INDO-1/AM and assayed for calcium release in response to CCR1 chemokine ligand (i.e., MIP-1 ⁇ ) addition.
- non-CCR1 ligands, specifically bradykinin was added, which also signals via a seven transmembrane receptor. Without compound, a pulse of fluorescent signal will be seen upon MIP-1 ⁇ addition.
- CCX-105 was able to significantly and specifically inhibit signaling from CCR1.
- Table 2 Inhibition of calcium signaling Compound MIP-1 ⁇ 1 Bradykinin 1
- chemokines One of the primary functions of chemokines is their ability to mediate the migration of chemokine receptor-expressing cells, such as white blood cells.
- chemokine receptor-expressing cells such as white blood cells.
- a chemotaxis assay was employed. THP-1 myelomonocytic leukemia cells, which resemble monocytes, as wells as freshly isolated monocytes, were used as targets for chemoattraction by CCR1 chemokine ligands (i.e., MIP-1 ⁇ , CCL15/leukotactin).
- IC 50 values are those compound concentrations required to inhibit the number of cells responding to a CCR1 agonist by 50%.
- LPS bacterial membrane component lipopolysaccharide
- CCX-105 was able to significantly and specifically inhibit the inflammatory response in this in vivo assay. Table 3. CCX-105 efficacy in a rabbit model of destructive joint inflammation synovium inflammation score Vehicle 3 CCX-105 (dose 1) 2 CCX-105 (dose 2) 1
- Rat collagen arthritis is an experimental model of polyarthritis that has been widely used for preclinical testing of numerous anti-arthritic agents (see Trentharn, et al., J. Exp. Med. 146(3):857-868 (1977 ), Bendele, et al., Toxicologic Pathol. 27:134-142 (1999 ), Bendele, et al., Arthritis Rheum. 42:498-506 (1999 ))
- the hallmarks of this model are reliable onset and progression of robust, easily measurable polyarticular inflammation, marked cartilage destruction in association with pannus formation and mild to moderate bone resorption and periosteal bone proliferation.
- Female Lewis rats (approximately 0.2 kilograms) were anesthetized with isoflurane and injected with Freund's Incomplete Adjuvant containing 2 mg/ml bovine type II collagen at the base of the tail and two sites on the back on days 0 sand 6 of this 17 day study.
- Compound 1.028 was dosed daily in a sub-cutaneous manner from day 0 till day 17 at a dose of 25 mg/kg and a volume of 1 ml/kg in the following vehicle (20% N,N-dimethylacetamide, 75% corn oil, 5% Tween-80). Caliper measurements of the ankle joint diameter were taken, and reducing joint swelling was taken as a measure of efficacy.
Landscapes
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Health & Medical Sciences (AREA)
- Veterinary Medicine (AREA)
- Public Health (AREA)
- General Health & Medical Sciences (AREA)
- Animal Behavior & Ethology (AREA)
- Life Sciences & Earth Sciences (AREA)
- Medicinal Chemistry (AREA)
- Pharmacology & Pharmacy (AREA)
- General Chemical & Material Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Engineering & Computer Science (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Pain & Pain Management (AREA)
- Neurology (AREA)
- Neurosurgery (AREA)
- Biomedical Technology (AREA)
- Immunology (AREA)
- Rheumatology (AREA)
- Heart & Thoracic Surgery (AREA)
- Pulmonology (AREA)
- Cardiology (AREA)
- Dermatology (AREA)
- Epidemiology (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
- Plural Heterocyclic Compounds (AREA)
- Medicines That Contain Protein Lipid Enzymes And Other Medicines (AREA)
Claims (38)
- Verwendung einer Verbindung mit der Formel
der Index n eine ganze Zahl von 1 bis 2 ist;
der Index m eine ganze Zahl von 0 bis 10 ist;
jedes R1 ein Substituent ist, unabhängig ausgewählt ist aus der Gruppe, bestehend aus C1-8-Alkyl, C1-8-Haloalkyl, C3-6-Cycloalkyl, C2-8-Alkenyl und
C2-8-Alkinyl, -CORa, -CO2Ra, -CONRaRb, -NRaCORb, -SO2Ra, -X1CORa, -X1CO2Ra X1CONRaRb, -X1NRaCORb, -X1SO2Ra, -X1SO2NRaRb -X1NRaRb, -X1ORa, worin X1 ein Element ist, ausgewählt aus der Gruppe, bestehend aus C1-4-Alkylen, C2-4-Alkenylen und C2-4-Alkinylen und jedes Ra und Rb unabhängig ausgewählt ist aus der Gruppe, bestehend aus Wasserstoff, C1-8-Alkyl, C1-8-Haloalkyl und C3-6-Cyloalkyl, und worin die aliphatischen Teile von jedem der R1-Substituenten optional substituiert sind mit von einem bis drei Elementen, ausgewählt aus der Gruppe, bestehend aus OH, O(C1-8-Alkyl), SH, S(C1-8-Alkyl), CN, NO2, NH2, NH(C1-8-Alkyl) und N(C1-8-Alkyl)2; Ar1 Phenyl ist, substituiert mit von einem bis drei R2-Substituenten, unabhängig ausgewählt aus der Gruppe, bestehend aus Halogen, -ORc, -OC(O)Rc, -NRcRd, -SRc, -Re, -CN, -NO2, -CO2Rc, -CONRcRd, C(O)Rc, -OC(O)NRcRd, -NRdC(O)Rc, -NRdC(O)2Re, -NRc-C(O)NRcRd, -S(O)Re, -S(O)2Re, -NRcS(O)2Re, -S(O)2NRcRd und -N3 worin jedes Rc und Rd unabhängig ausgewählt ist aus Wasserstoff, C1-8-Alkyl, C1-8-Haloalkyl, C3-6-Cycloalkyl, C2-8-Alkenyl und C2-8-Alkinyl und jedes Re unabhängig ausgewählt ist aus der Gruppe, bestehend aus C1-8-Alkyl, C1-8-Haloalkyl, C3-6-Cycloalkyl, C2-8-Alkenyl und C2-8-Alkinyl und worin der aliphatische Teil von Rc, Rd und Re optional weiter substituiert ist mit von einem bis drei Elementen, ausgewählt aus der Gruppe, bestehend aus OH, O(C1-8-Alkyl), SH, S(C1-8-Alkyl), CN, NO2, NH2, NH(C1-8-Alkyl) und N(C1-8-Alkyl)2; HAr eine Heteroarylgruppe ist, ausgewählt aus der Gruppe, bestehend aus Pyrazolyl und Benzopyrazolyl, und gebunden ist an den Rest des Moleküls über ein Stickstoffatom des Pyrazolylrings bzw. über ein Stickstoffatom des Pyrazolteils des fusionierten Ringsystems, wobei jedes davon optional substituiert ist mit von einem bis fünf R3-Substituenten, unabhängig ausgewählt aus der Gruppe, bestehend aus Halogen, Phenyl, Thienyl, Furanyl, Pyridyl, Pyrimidinyl, Pyrazinyl, Pyridizinyl, Pyrazolyl, Imidazolyl, Thiazolyl, Oxazolyl, Isoxazolyl, Isothiazolyl, Triazolyl, Tetrazolyl, Oxadiazolyl, -ORf, -OC(O)Rf, -NRfRg, -SRf, -Rh, -CN, -NO2, -CO2Rf, -CONRfRg, -C(O)Rf, -OC(O)NRfRg, -NRgC(O)Rf, -NRgC(O)2Rh, -NRf-C(O)NRfRg, -NH-C(NH2)=NH, -NRhC(NH2)=NH, -NH-C(NH2)=NRh, -NH-C/NHRh)=NH, -S(O)Rh, -S(O)2Rh, -NRfS(O)2Rh, -S(O)2NRfRg, -NRfS(O)2Rh, -NRfS(O)2NRfRg, -N3, -X3ORf, - XIOC(O)Rf, -X3NRfRg, -X3SRf, -X3CN, -X3NO2, -X3CO2Rf, -X3CONRfRg, - X3C(O)Rf, -X3OC(O)NRfRg, -X3NRgC(O)Rf, -X3NRgC(O)2Rh, -X3NRf-C(O)NRfR9, -X3NH-C(NH2)=NH, -X3NRhC(NH2)=NH, -X3NH-C(NH2)=NR , -X3NH-C(NHRh)=NH, -X3S(O)Rh, -X3S(O)2Rh, -X3NRfS(O)2Rh, -X3S(O)2NRfRg und -X3N3 worin X3 ausgewählt ist aus der Gruppe, bestehend aus C1-4-Alkylen, C2-4-Alkenylen und C2-4-Alkinylen und jedes Rf und Rg unabhängig ausgewählt ist aus Wasserstoff, C1-8-Alkyl, C1-8-Haloalkyl, C3-6-Cycloalkyl, C2-8-Alkenyl, C2-8-Alkinyl, Aryl, Heteroaryl, Aryl-C1-4-Alkyl und Aryloxy-C1-4-Alkyl und jedes Rh unabhängig ausgewählt ist aus der Gruppe, bestehend aus C1-8-Alkyl, C1-8-Haloalkoyl, C3-6-Cycloalkyl, C2-8-Alkenyl, C2-8-Alkinyl, Aryl, Heteroaryl, Aryl-C1-4-Alkyl und Aryloxy-C1-4-Alkyl, worin die aliphatischen Teile von Rf, Rg und Rh optional weiter substituiert sind mit von einem bis drei Mitgliedern, ausgewählt aus der Gruppe, bestehend aus OH, O(C1-8-Alkyl), SH, S(C1-8-Alkyl), CN, NO2, NH2, NH(C1-8-Alkyl) und N(C1-8-Alkyl)2; und worin jede Phenyl-, Thienyl-, Furanyl-, Pyridyl-, Pyrimidinyl-, Pyrazinyl-, Pyridizinyl-, Pyrazolyl-, Imidazolyl-, Thiazolyl-, Oxazolyl-, Isoxazolyl-, Isothiazolyl-, Triazolyl-, Tetrazolyl- oder Oxadiazolyl-Gruppe, die vorliegt, optional substituiert ist mit von einem bis drei Substituenten, ausgewählt aus der Gruppe, bestehend aus Halogen, -ORf, -NRfRg, --Rh, -CN, -NO2, -CO2Rf, -CONRfRg, -C(O)Rf, -X3ORf, -X3NRfRg, -X3NRfS(O)2Rh und -X3S(O)2NRfRg, L1 -CH2- ist und optional substituiert ist mit Phenyl, -Rk, -X4ORi, -X4OC(O)Ri, -X4NRiRj, -X4RSi, -X4CN oder -X4NO2, worin X4 ausgewählt ist aus der Gruppe, bestehend aus C-1-4-Alkylen, C2-4-Alkenylen und C2-4-Alkinylen und jedes Ri und Rj unabhängig ausgewählt ist aus Wasserstoff, C-1-8-Alkyl, C-1-8-Haloalkyl, C3-6-Cycloalkyl, C2-8-Alkenyl, C2-8-Alkinyl, Aryl, Heteroaryl, Aryl-C-1-4-Alkyl und Aryloxy-C-1-4-Alkyl und jedes Rk unabhängig ausgewählt ist aus der Gruppe, bestehend aus C1-8-Alkyl, C1-8-Haloalkyl, C3-6-Cycloalkyl, C2-8-Alkenyl, C2-8-Alkinyl, Aryl, Heteroaryl, Aryl-C-1-4-Alkyl und Aryloxy-C-1-4-Alkyl, bei der Herstellung eines Medikaments zur Verwendung in einem Verfahren zur Behandlung von CCR1-vermittelten Krankheiten oder Zuständen, umfassend das Verabreichen an ein Subjekt, das eine therapeutisch wirkungsvolle Menge der Verbindung erfordert. - Verwendung nach Anspruch 1, worin HAr Pyrazolyl ist, das optional substituiert ist mit von ein bis drei R3-Gruppen, unabhängig ausgewählt aus der Gruppe, bestehend aus Halogen, Phenyl, Thienyl, -ORf, -OC(O)Rf, -NRfRg, -SRf, -Rh, -CN, -NO2, -CO2Rf, -CONRfRg, -C(O)Rf, -OC(O)NRf,Rg, -NRgC(O)Rf, -NRgC(O)2Rh, -NRf-C(O)NRfRg, -S(O)Rh, -S(O)2Rh, -S(O)2NRfRg, -NRfS(O)2Rh, -NRfS(O)2NRfRg, -N3, -X3ORf, -X3OC(O)Rf, -X3NRfRg, -X3SRf, -X3CN, X3NO2, -X3CO2Rf, -X3CONRfRg, -X3C(O)Rf, -X3OC(O)NRfRg, -X3NRgC(O)Rf-, X3NRgC(O)2Rh, -X3NRf-C(O)NRfRg, -X3S(O)Rh, -X3S(O)2Rh, -X3NRfS(O)2Rh, -X3S(O)2NRfR9 und -X3N3, worin Rf und Rg jeweils unabhängig ausgewählt sind aus der Gruppe, bestehend aus H, C1-8-Alkyl und C1-8-Haloalkyl und jedes Rh unabhängig ausgewählt ist aus der Gruppe, bestehend aus C1-8-Alkyl und C1-8-Haloalkyl.
- Verwendung nach Anspruch 2, worin n 1 ist, m 0-2 ist, HAr Pyrazolyl ist, das substituiert ist mit drei R3-Gruppen und L1 -CH2 ist.
- Verwendung nach Anspruch 1, worin die Verbindung die Formel:
- Verwendung nach Anspruch 5, worin die ein bis drei R2-Substituenten unabhängig ausgewählt sind aus der Gruppe, bestehend aus Halogen, -ORc, -OC(O)Rc, -NRcRd, -SRc, -Re, -CN, -NO2, -CO2Rc, -CONRcR d, -C(O)Rc, -OC(O)NRcRd, -NRdC(O)Rc, -NRdC(O)2Re, -NRc-C(O)NRcRd, -S(O)Re, -S(O)2Re, -NRcS(O)2NRcRd und -N3, worin jedes Rc und Rd unabhängig ausgewählt ist aus Wasserstoff, C1-8-Alkyl, C1-8-Haloalkyl, C3-6-Cycloalkyl, C2-8-Alkenyl und C2-8-Alkinyl und jedes Re unabhängig ausgewählt ist aus der Gruppe, bestehend aus C1-8-Alkyl, C1-8-Haloalkyl, C3-6-Cycloalkyl, C2-8-Alkenyl und C2-8-Alkinyl.
- Verwendung nach Anspruch 6, worin L1 -CH2- ist, optional substituiert mit einem oder mehreren Substituenten, unabhängig ausgewählt aus der Gruppe, bestehend aus C1-4-Alkyl, C-1-4-Haloalkyl und Phenyl.
- Verwendung nach Anspruch 7, worin die eine bis fünf R3-Gruppen unabhängig ausgewählt sind aus der Gruppe, bestehend aus Halogen, Phenyl, Thienyl, -ORf, -OC(O)Rf, -NRfR9, -SRf, -Rh, -CN, -NO2, -CO2Rf, -CONRfRg, -C(O)Rf, -OC(O)NRfR9, -NRgC(O)Rf, -NRgC(O)2Rh, -NRf-C(O)NRfRg, -S(O)Rh, -S(O)2Rh, -S(O)2Rh, -S(O)2NRfRg, -NRfS(O)2Rh, -NRfS(O)2NRfRg, -X3ORf, -X3OC(O)Rf, -X3NRfRg, -X3SRf, -X3CN, -X3NO2, -X3CO2Rf, -X3CONRfRg, -X3C(O)Rf, -X3OC(O)NRfRg, -X3NRgC(O)Rf, -X3NRgC(O)Rh, -X3NRf-C(O)NRfRg, -X3S(O)Rh, -X3S(O)2Rh, -X3NRfS(O)2Rh und -X3S(O)2NRfRg, worin X3 ausgewählt ist aus der Gruppe, bestehend aus C1-4-Alkylen, C2-4-Alkenylen und C2-4-Alkinylen, und jedes Rf und Rg unabhängig ausgewählt ist aus Wasserstoff, C1-8-Alkyl, C1-8-Haloalkyl, C3-6-Cycloalkyl, C2-8-Alkenyl und C2-8-Alkinyl und jedes Rh unabhängig ausgewählt ist aus der Gruppe, bestehend aus C1-8-Alkyl, C1-8-Haloalkyl, C3-6-Cycloalkyl, C2-8-Alkenyl, C2-8-Alkinyl und worin jede vorliegende Phenyl- oder Thienylgruppe, optional substituiert ist mit von einem bis drei Substituenten, ausgewählt aus der Gruppe, bestehend aus Halogen, -ORf, -NRfRg, -Rh, -CN, -NO2, -CO2Rf, -CONRfRg, -C(O)Rf, -X3ORf, -X3NRfRg, -X3NRfS(O)2Rh und -X3S(O)2NRfRg.
- Verwendung nach Anspruch 8, worin HAr optional substituiert ist mit von einer bis drei R3-Gruppen, unabhängig ausgewählt aus der Gruppe, bestehend aus Halogen, Phenyl, Thienyl, -ORf, -CO2Rf, -CORf, -CONRfRg, -NO2, -Rh, -CN, -SRf, -S(O)Rh, -S(O)2Rh und -NRfRg, worin jedes Rf und Rg unabhängig ausgewählt ist aus der Gruppe, bestehend aus H, C1-8-Alkyl und C1-8-Haloalkyl und jedes Rh unabhängig ausgewählt ist aus der Gruppe, bestehend aus C1-8-Alkyl und C1-8-Haloalkyl.
- Verwendung nach Anspruch 9, worin L1 -CH2- ist.
- Verwendung nach Anspruch 5, worin Ar1 Phenyl ist, optional substituiert mit einem bis drei R2-Substituenten, unabhängig ausgewählt aus der Gruppe, bestehend aus Halogen, -ORc, OC(O)Rc, -NRcRd, -SRc, -Re, - CN,
-NO2, -CO2Rc, -CONRcRd, -C(O)Rc, -OC(O)NRcRd, -NRdC(O)Rc,
-NRdC(O)2Re, -NRc-C(O)NRcRd, -S(O)Re, -S(O)2Re, -S(O)2NRcRd und -N3, worin jedes Rc und Rd unabhängig ausgewählt ist aus Wasserstoff, C1-8-Alkyl, C1-8-Haloalkyl, C3-6-Cycloalkyl, C2-8-Alkenyl und C2-8-Alkinyl und jedes Re unabhängig ausgewählt ist aus der Gruppe, bestehend aus C1-8-Alkyl, C1-8-Haloalkyl, C3-6-Cycloalkyl, C2-8-Alkenyl und C2-8-Alkinyl, worin die Alkylteile der Substituenten optional substituiert sind mit einer oder zwei Hydroxy- oder Aminogruppen; L1 -CH2- ist; HAr Pyrazolyl oder Benzopyrazolyl ist, jedes davon optional substituiert ist mit von einer bis drei R3-Gruppen, unabhängig ausgewählt aus der Gruppe, bestehend aus Halogen, Phenyl, Thienyl, ORf, CO2Rf, CONRfRg, NO2, Rh, CN, SRf, S(O)Rh, S(O)2Rh und NRfRg, worin jedes Rf und Rg unabhängig ausgewählt ist aus der Gruppe, bestehend aus H, C1-8-Alkyl und C1-8-Haloalkyl, und jedes Rh unabhängig ausgewählt ist aus der Gruppe, bestehend aus C1-8-Alkyl und C1-8-Haloalkyl; und jedes R1a, R1b, R1c, R1d, R1e, R1f R1g und R1h Elemente sind, unabhängig ausgewählt aus der Gruppe, bestehend aus H, C-1-4-Alkyl und C-1-4-Haloalkyl, worin mindestens sechs aus R1a bis R1h H sind. - Verwendung nach Anspruch 1, worin die Verbindung die Formel
jedes R1 ein Element ist, ausgewählt aus der Gruppe, bestehend aus C1-4-Alkyl und C1-4-Haloalkyl; R2a, R2b, R2c, R2d und R2e Elemente sind, unabhängig ausgewählt aus der Gruppe, bestehend aus Wasserstoff, Halogen, -ORc, -OC(O)Rc, -NRcRd, -SRc, -Re, -CN, NO2, -CO2Rc, -CONRcRd, -C(O)RC, -OC(O)NRcRd, -NRdC(O)Rc, -NRdC(O)2Re, -NRc-C(O)NRcRd, -S(O)Re, -S(O)2Re, -NRcS(O)2Re, -S(O)2NRcRd und -N3, worin jedes Rc und Rd unabhängig ausgewählt ist aus Wasserstoff, C1-8-Alkyl, C1-8-Halolalkyl, C3-6-Cycloalkyl, C2-8-Alkenyl und C2-8-Alkinyl und jedes Re unabhängig ausgewählt ist aus der Gruppe, bestehend aus C1-8-Alkyl, C1-8-Haloalkyl, C3-6-Cycloalkyl, C2-8-Alkenyl und C2-8-Alkinyl und jedes Rc, Rd und Re optional weiter substituiert ist mit von einem bis drei Elementen, ausgewählt aus der Gruppe, bestehend aus OH, O(C1-8-Alkyl), SH, S(C1-8-Alkyl), CN, NO2, NH2, NH(C1-8-Alkyl) und N(C1-8-Alkyl)2; R3a, R3b und R3c jeweils Mitglieder sind, unabhängig ausgewählt aus der Gruppe, bestehend aus Wasserstoff, Halogen, Phenyl, Thienyl, -ORf, -OC(O)RF, -NRfR9, -SRf, -Rh, -CN, -NO2, -CO2Rf, -CONRfRg, -C(O)Rf, - OC(O)NRfRg, -NRgC(O)Rf, -NRgC(O)2Rh, -NRfC(O)NRfRg, -S(O)Rh, -S(O)2Rh, -NRfS(O)2Rh, -S(O)2NRfRg, -NRfS(O)2Rh, -NRfS(O)2NRfRg, -X3ORf, -X3OC(O)Rf, -X3NRfRg, -X3SRf, -X3CN, -X3NO2, -X3CO2Rf, -X3CONRfRg, -X3C(O)Rf, -X3C(O)Rf, -X3OC(O)NRfRg, -X3NR9C(O)Rf, -X3NRgC(O)2Rh, -X3NRf-C(O)NRfRg, -X3S(O)Rh, -X3S(O)2Rh, -X3NRfS(O)2Rh und X3S(O)2NRfRg, worin X3 C1-4-Alkylen ist, jedes Rf und Rg unabhängig ausgewählt ist aus Wasserstoff, C1-8-Alkyl, C1-8-Haloalkyl, C3-6-Cycloalkyl, C2-8-Alkenyl und C2-8-Alkinyl, und jedes Rh unabhängig ausgewählt ist aus der Gruppe, bestehend aus C1-8-Alkyl, C1-8-Haloalkyl, C3-6-Cycloalkyl, C2-8-Alkenyl und C2-8-Alkinyl und worin jede vorliegende Phenyl- oder Thienylgruppe optional substituiert ist mit von einem bis drei Substituenten, ausgewählt aus der Gruppe, bestehend aus Halogen, -ORf, -NRfRg, -Rh, -CN, -NO2, -CO2Rf, -CONRfRg, -C(O)Rf, -X3NRfRg, -X3NRfS(O)2Rh und -X3S(O)2NRfRg. - Verwendung nach Anspruch 14, worin m 0 oder 1 ist und mindestens eines von R2a und R2e Wasserstoff ist.
- Verwendung nach Anspruch 15, worin mindestens eines von R3a, R3b und R3c ausgewählt ist aus der Gruppe, bestehend aus Halogen und C-1-4-Haloalkyl.
- Verwendung nach Anspruch 16, worin R2d Wasserstoff ist und mindestens zwei von R3a, R3b und R3c ausgewählt sind aus der Gruppe, bestehend aus Halogen und C-1-4-Haloalkyl.
- Verwendung nach Anspruch 17, worin R2c ausgewählt ist aus der Gruppe, bestehend aus F, Cl, Br, CN, NO2, CO2CH3, C(O)CH3 und S(O)2CH3 und jedes von R3a, R3b und Rc von Wasserstoff verschieden ist.
- Verwendung nach Anspruch 14, worin m 0 oder 1 ist und R2a und R2e jeweils Wasserstoff sind.
- Verwendung nach Anspruch 19, worin mindestens eines von R3a, R3b und R3c ausgewählt ist aus der Gruppe, bestehend aus Halogen und C1-4-Haloalkyl.
- Verwendung nach Anspruch 20, worin jedes von R3a, R3b und R3c von Wasserstoff verschieden ist.
- Verwendung nach Anspruch 14, worin m 0 oder 1 ist und R2b und R2e jeweils Wasserstoff sind.
- Verwendung nach Anspruch 23, worin R3c und R3a jeweils unabhängig ausgewählt sind aus der Gruppe, bestehend aus C1-6-Alkyl, C1-6-Haloalkyl und C3-6-Cycloalkyl.
- Verwendung nach Anspruch 14, worin die Verbindung die Formel
- Verwendung nach Anspruch 14, worin die Verbindung die Formel
- Verwendung nach Anspruch 14, worin die Verbindung die Formel
- Verwendung nach Anspruch 14, worin die Verbindung die Formel
- Verwendung nach Anspruch 22, worin mindestens eines von R3a, R3b und R3c ausgewählt ist aus der Gruppe, bestehend aus Halogen und C1-4-Halolalkyl.
- Verwendung nach Anspruch 29, worin jedes von R3a, R3b und R3c verschieden von Wasserstoff ist.
- Verwendung nach einem der Ansprüche 1 bis 30, worin CCR1-vermittelte Krankheit oder Zustand ein Entzündungszustand ist.
- Verwendung nach einem der Ansprüche 1 bis 30, worin die CCR1-vermittelte Krankheit oder der CC1-vermittelte Zustand eine Immunregulationsstörung ist.
- Verwendung nach einem der Ansprüche 1 bis 30, worin die CCR1-vermittelte Krankheit oder der CCR1-vermittelte Zustand ausgewählt ist aus der Gruppe, bestehend aus rheumatischer Arthritis, Multiple Sklerose, Transplantatabstoßung, Dermatitis, Ekzem, Urtikaria, Vasculitis, entzündliche Darmerkrankung, Lebensmittelallergie und Enzephalomyelitis.
- Verwendung nach einem der Ansprüche 1 bis 30, worin die Verabreichung oral, parenteral, rektal, transdermal, sublingual, nasal oder topisch ist.
- Verwendung nach einem der Ansprüche 1 bis 30, worin die Verbindung in Kombination mit einem entzündungshemmenden oder analgetischen Mittel verwendet wird.
- Verbindung nach einem der Ansprüche 1 bis 30 zur Verwendung in einem Verfahren zur Behandlung von CCR1-vermittelten Krankheiten oder Zuständen, wie in einem der Ansprüche 1 und 31 bis 35 definiert.
- Pharmazeutische Zusammensetzung, umfassend einen pharmazeutsch verträglichen Exzipienten und eine Verbindung nach einem der Ansprüche 1 bis 30 mit der Maßgabe, dass die Verbindung verschieden ist von CAS Reg. Nr. 492422-98-7, 1-[[4-Brom-5-methyl-3-(trifluormethyl)-1 H-pyrazol-1-yl]acetyl]-4-(5-chlor-2-methylphenyl)piperazin; CAS Reg. Nr. 351986-92-0, 1-[[4-Chlor-5-methyl-3-(trilfuormethyl)-1 H-pyrazol-1-yl]acetyl]-4-(4-fluorphenyl)-piperazin oder CAS Reg. Nr. 356039-23-1, 1-[(3,5-Dimethyl-4-nitro-1H-pyrazol-1-yl)acetyl]-4-(4-fluorphenyl)-piperazin.
- Verbindung nach einem der Ansprüche 1 bis 30, mit der Maßgabe, dass die Verbindung verschieden ist von CAS Reg. Nr. 492422-98-7, 1-[[4-Brom-5-methyl-3-(trifluormethyl)-1 H-pyrazol-1-yl]acetyl]-4-(5-chlor-2-methylphenyl)piperazin; CAS Reg. Nr. 351986-92-0, 1-[[4-Chlor-5-methyl-3-(trifluormethyl)-1 H-pyrazol-1-yl]acetyl]-4-(4-fluorphenyl)-piperazin oder CAS Reg. Nr. 356039-23-1, 1-[(3,5-Dimethyl-4-nitro-1H-pyrazol-1-yl)acetyl]-4-(4-fluorphenyl)-piperazin.
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US45371102P | 2002-06-12 | 2002-06-12 | |
| US453711P | 2002-06-12 | ||
| PCT/US2003/018660 WO2003105853A1 (en) | 2002-06-12 | 2003-06-11 | 1-aryl-4-substituted piperazines derivatives for use as ccr1 antagonists for the treatment of inflammation and immune disorders |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| EP1531822A1 EP1531822A1 (de) | 2005-05-25 |
| EP1531822B1 true EP1531822B1 (de) | 2009-08-05 |
Family
ID=29736828
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP03737057A Expired - Lifetime EP1531822B1 (de) | 2002-06-12 | 2003-06-11 | 1-aryl-4-substituierte piperazin-derivate zur verwendung als ccr1-antagonisten zur behandlung von entzündungen und immunerkrankungen |
Country Status (14)
| Country | Link |
|---|---|
| US (2) | US7157464B2 (de) |
| EP (1) | EP1531822B1 (de) |
| JP (1) | JP4723242B2 (de) |
| KR (1) | KR101255356B1 (de) |
| CN (1) | CN1867336B (de) |
| AT (1) | ATE438401T1 (de) |
| AU (1) | AU2003236500B9 (de) |
| CA (1) | CA2488202C (de) |
| DE (1) | DE60328690D1 (de) |
| DK (1) | DK1531822T3 (de) |
| ES (1) | ES2329356T3 (de) |
| HK (1) | HK1099206A1 (de) |
| MX (1) | MXPA04012393A (de) |
| WO (1) | WO2003105853A1 (de) |
Families Citing this family (63)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP4723242B2 (ja) * | 2002-06-12 | 2011-07-13 | ケモセントリックス インコーポレーティッド | 炎症および免疫障害治療用ccr1アンタゴニストとして使用するための1−アリール−4−置換ピペラジン誘導体 |
| US7589199B2 (en) | 2002-06-12 | 2009-09-15 | Chemocentryx, Inc. | Substituted piperazines |
| US20050256130A1 (en) * | 2002-06-12 | 2005-11-17 | Chemocentryx, Inc. | Substituted piperazines |
| US7842693B2 (en) * | 2002-06-12 | 2010-11-30 | Chemocentryx, Inc. | Substituted piperazines |
| US8013156B2 (en) * | 2003-03-19 | 2011-09-06 | Exelixis, Inc. | Tie-2 modulators and methods of use |
| US7754711B2 (en) * | 2003-07-30 | 2010-07-13 | Xenon Pharmaceuticals Inc. | Pyridazine derivatives and their use as therapeutic agents |
| AU2011265309B2 (en) * | 2003-11-21 | 2014-06-05 | Array Biopharma, Inc. | AKT protein kinase inhibitors |
| CN1882347A (zh) | 2003-11-21 | 2006-12-20 | 阿雷生物药品公司 | Akt蛋白激酶抑制剂 |
| GB0401269D0 (en) * | 2004-01-21 | 2004-02-25 | Astrazeneca Ab | Compounds |
| US7288563B2 (en) | 2004-02-19 | 2007-10-30 | Bristol-Myers Squibb Company | Substituted bicycloalkylamine derivatives as modulators of chemokine receptor activity |
| US7479496B2 (en) | 2004-02-19 | 2009-01-20 | Bristol-Myers Squibb Company | Substituted spiro azabicyclics as modulators of chemokine receptor activity |
| US7381738B2 (en) | 2004-02-19 | 2008-06-03 | Bristol-Myers Squibb Company | Substituted bicycloalkylamine derivatives as modulators of chemokine receptor activity |
| US7230022B2 (en) | 2004-02-19 | 2007-06-12 | Bristol-Myers Squibb Company | Substituted fused bicyclic amines as modulators of chemokine receptor activity |
| US7435831B2 (en) | 2004-03-03 | 2008-10-14 | Chemocentryx, Inc. | Bicyclic and bridged nitrogen heterocycles |
| CA2558211C (en) | 2004-03-03 | 2013-09-03 | Chemocentryx, Inc. | Bicyclic and bridged nitrogen heterocycles |
| RU2006140687A (ru) * | 2004-06-18 | 2008-07-27 | Ньюросерч А/С (DK) | Новые алкил-замещенные производные пиперазина и их применение в качестве ингибиторов обратного захвата моноаминовых нейротрансмиттеров |
| PE20060484A1 (es) * | 2004-07-14 | 2006-07-06 | Ucb Farchim Sa | Preparaciones farmaceuticas liquidas que comprenden un compuesto de benzhidril piperizina |
| KR101220615B1 (ko) | 2004-07-26 | 2013-01-11 | 메르크 세로노 에스.에이. | N-히드록시아미드 유도체 및 그의 용도 |
| US20080167321A1 (en) † | 2004-09-20 | 2008-07-10 | Xenon Pharmaceuticals Inc. | Pyridine Derivatives For Inhibiting Human Stearoyl-Coa-Desaturase |
| US7482353B2 (en) | 2005-06-15 | 2009-01-27 | Applied Biosystems Inc. | Compositions and methods pertaining to PNA synthons and oligomers comprising a universal base |
| KR101418024B1 (ko) * | 2005-06-22 | 2014-07-16 | 케모센트릭스, 인크. | 아자인다졸 화합물 및 사용방법 |
| US7777035B2 (en) | 2005-06-22 | 2010-08-17 | Chemocentryx, Inc. | Azaindazole compounds and methods of use |
| US7790726B2 (en) * | 2005-08-16 | 2010-09-07 | Chemocentryx, Inc. | Monocyclic and bicyclic compounds and methods of use |
| WO2007044885A2 (en) | 2005-10-11 | 2007-04-19 | Chemocentryx, Inc. | Piperidine derivatives and methods of use |
| EP1847543A1 (de) | 2006-04-19 | 2007-10-24 | Boehringer Ingelheim Pharma GmbH & Co. KG | Dihydrothienopyrimidine zur Behandlung von entzündlichen Erkrankungen |
| WO2007146230A2 (en) * | 2006-06-14 | 2007-12-21 | Merck & Co., Inc. | Non-nucleoside reverse transcriptase inhibitors |
| EP2044043B2 (de) | 2006-06-16 | 2021-03-03 | H. Lundbeck A/S | 1-[2-(2,4-dimethylphenylsulfanyl)phenyl]piperazin hydrobromid als verbindung mit kombinierter serotoninwiederaufnahme-, 5-ht3- und 5-ht1a-aktivität zur behandlung kognitiver störungen |
| US20090252779A1 (en) * | 2006-06-22 | 2009-10-08 | Chemocentryx, Inc. | Azaindazole compounds and methods of use |
| EA015529B1 (ru) | 2007-05-22 | 2011-08-30 | Кемосентрикс, Инк. | 3-(имидазолил)пиразоло[3,4-b]пиридины |
| WO2009015166A1 (en) | 2007-07-24 | 2009-01-29 | Bristol-Myers Squibb Company | Piperidine derivatives as modulators of chemokine receptor activity |
| EP2285783B1 (de) | 2008-04-29 | 2014-05-21 | Boehringer Ingelheim International GmbH | Indazolverbindungen als ccr1-rezeptorantagonisten |
| BRPI0910837B1 (pt) * | 2008-04-30 | 2017-03-07 | Bayer Cropscience Ag | ésteres e tioésteres tiazol-4-carboxílicos, seus usos, e método e composição para controlar fungos fitopatogênicos prejudiciais |
| EP2297112B1 (de) * | 2008-05-06 | 2013-04-03 | Boehringer Ingelheim International GmbH | Pyrazolverbindungen als ccr1-antagonisten |
| TWI433838B (zh) | 2008-06-25 | 2014-04-11 | 必治妥美雅史谷比公司 | 作為趨化因子受體活性調節劑之六氫吡啶衍生物 |
| US8343975B2 (en) | 2008-09-11 | 2013-01-01 | Penglie Zhang | 4-amino-3-(imidazolyl)-pyrazolo[3,4-D]pyrimidines |
| CA2737472A1 (en) * | 2008-09-26 | 2010-04-01 | Boehringer Ingelheim International Gmbh | Azaindazole compounds as ccr1 receptor antagonists |
| EP2352501B1 (de) * | 2008-11-03 | 2014-01-01 | ChemoCentryx, Inc. | Verbindung zur verwendung in der behandlung von osteoporose |
| US8362249B2 (en) * | 2009-04-27 | 2013-01-29 | Boehringer Ingelheim International Gmbh | CXCR3 receptor antagonists |
| EP2424840B1 (de) * | 2009-04-27 | 2014-08-06 | Boehringer Ingelheim International GmbH | Cxcr3-rezeptor antagonisten |
| WO2011049917A1 (en) | 2009-10-21 | 2011-04-28 | Boehringer Ingelheim International Gmbh | Indazole and pyrazolopyridine compounds as ccr1 receptor antagonists |
| WO2011056440A1 (en) | 2009-10-27 | 2011-05-12 | Boehringer Ingelheim International Gmbh | Heterocyclic compounds as ccr1 receptor antagonists |
| JP2013517281A (ja) * | 2010-01-13 | 2013-05-16 | テンペロ、ファーマシューティカルズ、インコーポレイテッド | 化合物及び方法 |
| JP5793182B2 (ja) | 2010-04-30 | 2015-10-14 | ベーリンガー インゲルハイム インターナショナル ゲゼルシャフト ミット ベシュレンクテル ハフツング | Ccr1受容体アンタゴニストとしてのアザインダゾールアミド化合物 |
| EP2655371B1 (de) | 2010-12-23 | 2015-02-25 | Boehringer Ingelheim International GmbH | Pyrazolopiperidinverbindungen als ccr1-rezeptorantagonisten |
| CA2852160A1 (en) | 2011-10-28 | 2013-05-02 | Galderma Research & Development | New leukocyte infiltrate markers for rosacea and uses thereof |
| US8822464B2 (en) * | 2011-11-28 | 2014-09-02 | Boehringer Ingelheim International Gmbh | N-aryl-piperazine derivatives and their use as positive allosteric modulators of mGluR5 receptors |
| US8741892B2 (en) | 2011-12-05 | 2014-06-03 | Boehringer Ingelheim International Gmbh | Compounds |
| US8642774B2 (en) | 2011-12-08 | 2014-02-04 | Boehringer Ingelheim International Gmbh | Compounds |
| US8796467B2 (en) | 2011-12-13 | 2014-08-05 | Boehringer Ingelheim International Gmbh | Compounds |
| US8846948B2 (en) | 2011-12-13 | 2014-09-30 | Boehringer Ingelheim International Gmbh | Compounds |
| US8937176B2 (en) | 2011-12-14 | 2015-01-20 | Boehringer Ingelheim International Gmbh | Compounds |
| US8716277B2 (en) | 2011-12-14 | 2014-05-06 | Boehringer Ingelheim International Gmbh | Substituted imidazole compounds useful as positive allosteric modulators of mGlu5 receptor activity |
| US8883789B2 (en) | 2011-12-14 | 2014-11-11 | Boehringer Ingelheim International Gmbh | Piperazine derivatives and their use as positive allosteric modulators of mGluR5 receptors |
| US8889677B2 (en) | 2012-01-17 | 2014-11-18 | Boehringer Ingellheim International GmbH | Substituted triazoles useful as mGlu5 receptor modulators |
| ES2671323T3 (es) | 2013-07-22 | 2018-06-06 | Idorsia Pharmaceuticals Ltd | Derivados 1¿(piperazin¿1¿il)¿2¿([1,2,4]triazol¿1¿il)¿etanona |
| AR099789A1 (es) | 2014-03-24 | 2016-08-17 | Actelion Pharmaceuticals Ltd | Derivados de 8-(piperazin-1-il)-1,2,3,4-tetrahidro-isoquinolina |
| PL3245203T3 (pl) | 2015-01-15 | 2019-05-31 | Idorsia Pharmaceuticals Ltd | Pochodne hydroksyalkilopiperazyny jako modulatory receptora cxcr3 |
| AR103399A1 (es) | 2015-01-15 | 2017-05-10 | Actelion Pharmaceuticals Ltd | Derivados de (r)-2-metil-piperazina como moduladores del receptor cxcr3 |
| EP3474667A4 (de) * | 2016-06-23 | 2019-11-27 | St. Jude Children's Research Hospital, Inc. | Kleinmolekülige modulatoren von pantothenatkinasen |
| BR112020012875A2 (pt) | 2017-12-27 | 2021-01-05 | St. Jude Children¿S Research Hospital, Inc. | Moduladores de pequenas moléculas das pantotenato quinases |
| WO2019133632A1 (en) | 2017-12-27 | 2019-07-04 | St. Jude Children's Research Hospital | Methods of treating disorders associated with castor |
| US11872217B2 (en) | 2019-07-10 | 2024-01-16 | Chemocentryx, Inc. | Indanes as PD-L1 inhibitors |
| WO2021039961A1 (ja) * | 2019-08-30 | 2021-03-04 | 大日本住友製薬株式会社 | 縮環ピラゾール誘導体 |
Family Cites Families (91)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| BE663344A (de) * | 1964-05-04 | |||
| US3362956A (en) * | 1965-08-19 | 1968-01-09 | Sterling Drug Inc | 1-[(heterocyclyl)-lower-alkyl]-4-substituted-piperazines |
| US3491098A (en) * | 1967-05-29 | 1970-01-20 | Sterling Drug Inc | 1-((imidazolyl)-lower-alkyl)-4-substituted-piperazines |
| US3778433A (en) * | 1969-04-18 | 1973-12-11 | Sumitomo Chemical Co | Process for producing benzodiazepine derivatives |
| DE2247187A1 (de) * | 1972-09-26 | 1974-03-28 | Bayer Ag | Imidazolylessigsaeureamide, verfahren zu ihrer herstellung sowie ihre verwendung als arzneimittel |
| JPS5944313B2 (ja) * | 1974-11-26 | 1984-10-29 | 中外製薬株式会社 | インダゾ−ル誘導体の製法 |
| US3994890A (en) * | 1974-01-31 | 1976-11-30 | Chugai Seiyaku Kabushiki Kaisha | 1-Aminoalkyl, 3-phenyl indazoles |
| US4174393A (en) * | 1975-07-09 | 1979-11-13 | Duphar International Research B.V. | 1,3,4-Substituted pyrazoline derivatives |
| US4166452A (en) * | 1976-05-03 | 1979-09-04 | Generales Constantine D J Jr | Apparatus for testing human responses to stimuli |
| US4256108A (en) * | 1977-04-07 | 1981-03-17 | Alza Corporation | Microporous-semipermeable laminated osmotic system |
| US4243619A (en) * | 1978-03-31 | 1981-01-06 | Union Carbide Corporation | Process for making film from low density ethylene hydrocarbon copolymer |
| US4310429A (en) * | 1978-06-19 | 1982-01-12 | The B. F. Goodrich Company | Stabilized polymers, novel stabilizers, and synthesis thereof |
| DE2842539A1 (de) * | 1978-09-29 | 1980-04-10 | Bayer Ag | 1-isocyanato-anthrachinone |
| US4265874A (en) | 1980-04-25 | 1981-05-05 | Alza Corporation | Method of delivering drug with aid of effervescent activity generated in environment of use |
| DE3132915A1 (de) * | 1981-08-20 | 1983-03-03 | Kali-Chemie Pharma Gmbh, 3000 Hannover | 1,5-diphenylpyrazolin-3-on-verbindungen sowie verfahren und zwischenprodukte zu ihrer herstellung und diese verbindungen enthaltende arzneimittel |
| JPS5852256A (ja) * | 1981-09-24 | 1983-03-28 | Nippon Nohyaku Co Ltd | 置換又は非置換脂肪酸アミド誘導体及びその塩類 |
| DE3306771A1 (de) * | 1983-02-25 | 1984-08-30 | Bayer Ag, 5090 Leverkusen | Chinoloncarbonsaeuren, verfahren zu ihrer herstellung sowie diese enthaltende antibakterielle mittel |
| US4547505A (en) | 1983-03-25 | 1985-10-15 | Degussa Aktiengesellschaft | N-Phenyl-N-'-cycloalkylalkanoylpiperazine useful as analgetics and process for its production |
| US4562189A (en) * | 1984-10-09 | 1985-12-31 | American Cyanamid Company | Pyrazolylpiperazines |
| DE3442860A1 (de) * | 1984-11-24 | 1986-05-28 | Kali-Chemie Pharma Gmbh, 3000 Hannover | 5-alkyl-1-phenyl-2-piperazinoalkylpyrazolin-3- on-verbindungen sowie verfahren und zwischenprodukte zu ihrer herstellung und diese verbindungen enthaltende arzneimittel |
| ZA871335B (en) * | 1986-02-27 | 1987-09-30 | Duphar Int Res | New aryl-substituted(n-piperidinyl)methyl-and(n-piperazinyl)-methylazoles having antipsychotic properties |
| IL85700A0 (en) * | 1987-03-24 | 1988-08-31 | Takeda Chemical Industries Ltd | 1,4-disubstituted piperazine compounds,their production and use |
| US4997836A (en) * | 1988-11-11 | 1991-03-05 | Takeda Chemical Industries, Ltd. | Trisubstituted piperazine compounds, their production and use |
| EP0385043A1 (de) * | 1989-02-28 | 1990-09-05 | Fabrica Espanola De Productos Quimicos Y Farmaceuticos, S.A. (Faes) | 4-Substituierte Piperazinderivate |
| US5215989A (en) * | 1989-12-08 | 1993-06-01 | Merck & Co., Inc. | Nitrogen-containing heterocyclic compounds as class III antiarrhythmic agents |
| FR2672052B1 (fr) * | 1991-01-28 | 1995-05-24 | Esteve Labor Dr | Derives d'aryl (ou heteroaryl)-piperazinyl-alkyl-azoles, leur preparation et leur application en tant que medicaments. |
| GB9021535D0 (en) * | 1990-10-03 | 1990-11-14 | Wyeth John & Brother Ltd | Piperazine derivatives |
| GB9021453D0 (en) * | 1990-10-03 | 1990-11-14 | Wyeth John & Brother Ltd | Piperazine derivatives |
| FR2673628B1 (fr) * | 1991-03-07 | 1993-07-09 | Esteve Labor Dr | Procede de preparation de derives d'aryl (ou heteroaryl)-piperazinyl-butyl-azoles. |
| GB9305623D0 (en) * | 1993-03-18 | 1993-05-05 | Merck Sharp & Dohme | Therapeutic agents |
| US5681954A (en) * | 1993-05-14 | 1997-10-28 | Daiichi Pharmaceutical Co., Ltd. | Piperazine derivatives |
| US5464788A (en) * | 1994-03-24 | 1995-11-07 | Merck & Co., Inc. | Tocolytic oxytocin receptor antagonists |
| US5486534A (en) * | 1994-07-21 | 1996-01-23 | G. D. Searle & Co. | 3,4-substituted pyrazoles for the treatment of inflammation |
| US5607936A (en) * | 1994-09-30 | 1997-03-04 | Merck & Co., Inc. | Substituted aryl piperazines as neurokinin antagonists |
| US5719156A (en) * | 1995-05-02 | 1998-02-17 | Schering Corporation | Piperazino derivatives as neurokinin antagonists |
| JP4108747B2 (ja) * | 1995-07-13 | 2008-06-25 | アボット ゲゼルシャフト ミット ベシュレンクテル ハフツング ウント コンパニー コマンディトゲゼルシャフト | 治療薬としてのピペラジン誘導体 |
| GB9518552D0 (en) | 1995-09-11 | 1995-11-08 | Fujisawa Pharmaceutical Co | New heterocyclic compounds |
| US5760028A (en) * | 1995-12-22 | 1998-06-02 | The Dupont Merck Pharmaceutical Company | Integrin receptor antagonists |
| US5646151A (en) * | 1996-03-08 | 1997-07-08 | Adolor Corporation | Kappa agonist compounds and pharmaceutical formulations thereof |
| WO1997044329A1 (en) | 1996-05-20 | 1997-11-27 | Teijin Limited | Diarylalkyl cyclic diamine derivatives as chemokine receptor antagonists |
| US5760225A (en) * | 1996-11-15 | 1998-06-02 | Neurogen Corporation | Certain pyrazole derivatives as corticotropin-releasing factor receptor CRF1 specific ligands |
| DE69720051T2 (de) * | 1996-12-03 | 2003-09-04 | Banyu Pharmaceutical Co., Ltd. | Harnstoffderivate |
| WO1998025617A1 (en) | 1996-12-13 | 1998-06-18 | Merck & Co., Inc. | Substituted aryl piperazines as modulators of chemokine receptor activity |
| JP4095676B2 (ja) | 1997-03-03 | 2008-06-04 | エーザイ株式会社 | 注意障害を治療するためのコリンエステラーゼ阻害剤の使用 |
| US6979686B1 (en) | 2001-12-07 | 2005-12-27 | Pharmacia Corporation | Substituted pyrazoles as p38 kinase inhibitors |
| US6207665B1 (en) * | 1997-06-12 | 2001-03-27 | Schering Aktiengesellschaft | Piperazine derivatives and their use as anti-inflammatory agents |
| US5968938A (en) * | 1997-06-18 | 1999-10-19 | Merck & Co., Inc. | Piperazine oxytocin receptor antagonists |
| ES2125206B1 (es) * | 1997-07-21 | 1999-11-16 | Esteve Labor Dr | Derivados de acil-piperazinil-pirimidinas, su preparacion y su aplicacion como medicamentos. |
| GB9716657D0 (en) | 1997-08-07 | 1997-10-15 | Zeneca Ltd | Chemical compounds |
| CA2298813A1 (en) | 1997-08-28 | 1999-03-04 | Merck & Co., Inc. | Pyrrolidine and piperidine modulators of chemokine receptor activity |
| US6384035B1 (en) * | 1997-10-09 | 2002-05-07 | Ortho-Mcneil Pharmaceutical, Inc. | Heterocycles useful in the treatment of benign prostatic hyperplasia |
| PL192083B1 (pl) | 1997-11-18 | 2006-08-31 | Dupont Pharmaceuticals Res Lab | Pochodne amin cyklicznych i ich zastosowanie |
| ZA9811576B (en) | 1997-12-19 | 2000-06-19 | Takeda Chemical Industries Ltd | Anilide derivative, production and use thereof. |
| CA2319077A1 (en) | 1998-01-21 | 1999-07-29 | Yoshisuke Nakasato | Chemokine receptor antagonists and methods of use therefor |
| EP1047675A1 (de) | 1998-01-21 | 2000-11-02 | Millennium Pharmaceuticals, Inc. | Chemokinrezeptor-antagonisten und verfahren zur verwendund |
| DE69920888T2 (de) * | 1998-03-27 | 2006-02-02 | Bristol-Myers Squibb Pharma Co. | Disubstituierte pyrazoline und triazoline als faktor xa inhibitoren |
| US6492375B2 (en) * | 1998-06-30 | 2002-12-10 | Neuromed Technologies, Inc. | Partially saturated calcium channel blockers |
| US6288083B1 (en) * | 1998-09-04 | 2001-09-11 | Millennium Pharmaceuticals, Inc. | Chemokine receptor antagonists and methods of use therefor |
| CA2350903A1 (en) | 1998-11-20 | 2000-06-02 | F. Hoffmann-La Roche Ag | Pyrrolidine derivatives-ccr-3 receptor antagonists |
| GB9902459D0 (en) | 1999-02-05 | 1999-03-24 | Zeneca Ltd | Chemical compounds |
| GB9902461D0 (en) | 1999-02-05 | 1999-03-24 | Zeneca Ltd | Chemical compounds |
| GB9902452D0 (en) | 1999-02-05 | 1999-03-24 | Zeneca Ltd | Chemical compounds |
| GB9902453D0 (en) | 1999-02-05 | 1999-03-24 | Zeneca Ltd | Chemical compounds |
| JP3533134B2 (ja) | 1999-02-15 | 2004-05-31 | 三井化学株式会社 | フッ素化剤及びその製法と使用 |
| GB9905010D0 (en) * | 1999-03-04 | 1999-04-28 | Merck Sharp & Dohme | Therapeutic agents |
| WO2000053600A1 (fr) | 1999-03-11 | 2000-09-14 | Banyu Pharmaceutical Co., Ltd. | Derives piperidiniques |
| AU4796700A (en) | 1999-05-13 | 2000-12-05 | Dupont Pharmaceuticals Research Laboratories, Inc. | Ureido-substituted cyclic amine derivatives and their use as drug |
| US6492516B1 (en) | 1999-05-14 | 2002-12-10 | Merck & Co., Inc. | Compounds having cytokine inhibitory activity |
| ATE277903T1 (de) | 1999-05-14 | 2004-10-15 | Bristol Myers Squibb Pharma Re | Zyklische aminderivate und ihre verwendung |
| ES2293980T3 (es) * | 2000-01-13 | 2008-04-01 | Amgen Inc. | Agentes antibacterianos. |
| AU2001250404A1 (en) * | 2000-03-27 | 2001-10-08 | Basf Aktiengesellschaft | Use of dopamine-d3 receptor ligands for the treatment of diseases of the central nervous system |
| KR20020084273A (ko) * | 2000-03-31 | 2002-11-04 | 화이자 프로덕츠 인코포레이티드 | 신규한 피페라진 유도체 |
| US20020045613A1 (en) * | 2000-04-27 | 2002-04-18 | Heinz Pauls | 1-aroyl-piperidinyl benzamidines |
| US6673923B2 (en) * | 2000-05-03 | 2004-01-06 | Tularik Inc. | Pyrazole antimicrobial agents |
| AU2001269821A1 (en) * | 2000-06-15 | 2001-12-24 | Barbara Chen | Cycloalkyl alkanoic acids as integrin receptor antagonists |
| GB0017256D0 (en) * | 2000-07-13 | 2000-08-30 | Merck Sharp & Dohme | Therapeutic agents |
| KR20030024799A (ko) | 2000-07-20 | 2003-03-26 | 뉴로젠 코포레이션 | 캡사이신 수용체 리간드 |
| EP1309591B1 (de) * | 2000-08-14 | 2007-01-24 | Ortho-McNeil Pharmaceutical, Inc. | Substituierte pyrazole |
| EA200300392A1 (ru) * | 2000-10-19 | 2003-10-30 | Пфайзер Продактс Инк. | Производные пиперазина с мостиковой связью |
| US6451399B1 (en) | 2001-02-05 | 2002-09-17 | Daniel G. Boyce | Display mat |
| CA2440803A1 (en) | 2001-03-07 | 2002-09-12 | Pfizer Products Inc. | Modulators of chemokine receptor activity |
| CN1157388C (zh) * | 2001-05-29 | 2004-07-14 | 北京大学 | 哌嗪基二硫代甲酸酯类化合物,它们的制备方法和在抗肿瘤药物中的应用 |
| ES2180456B1 (es) * | 2001-07-20 | 2004-05-01 | Laboratorios S.A.L.V.A.T., S.A. | Isoxazoles sustituidos y su utilizacion como antibioticos. |
| WO2003024450A1 (en) | 2001-09-20 | 2003-03-27 | Eisai Co., Ltd. | Methods for treating prion diseases |
| US7067517B2 (en) | 2001-12-14 | 2006-06-27 | Nero Nordisk A/S | Use of compounds for decreasing activity of hormone-sensitive lipase |
| NO315724B1 (no) * | 2001-12-20 | 2003-10-13 | Sten Hellvik | Datainnsamlingssystem for et fartöy |
| JP4723242B2 (ja) | 2002-06-12 | 2011-07-13 | ケモセントリックス インコーポレーティッド | 炎症および免疫障害治療用ccr1アンタゴニストとして使用するための1−アリール−4−置換ピペラジン誘導体 |
| US20050256130A1 (en) | 2002-06-12 | 2005-11-17 | Chemocentryx, Inc. | Substituted piperazines |
| US7842693B2 (en) * | 2002-06-12 | 2010-11-30 | Chemocentryx, Inc. | Substituted piperazines |
| PA8575901A1 (es) | 2002-07-18 | 2004-07-20 | Pfizer Prod Inc | Derivados de piperidina novedosos |
| MXPA05001334A (es) * | 2002-08-02 | 2005-09-08 | Argenta Discovery Ltd | Acidos tienil-hidroxamicos sustituidos como inhibidores de la desacetilasa de histona. |
-
2003
- 2003-06-11 JP JP2004512756A patent/JP4723242B2/ja not_active Expired - Lifetime
- 2003-06-11 KR KR1020047020177A patent/KR101255356B1/ko active IP Right Grant
- 2003-06-11 AU AU2003236500A patent/AU2003236500B9/en not_active Ceased
- 2003-06-11 MX MXPA04012393A patent/MXPA04012393A/es active IP Right Grant
- 2003-06-11 CN CN038193027A patent/CN1867336B/zh not_active Expired - Fee Related
- 2003-06-11 EP EP03737057A patent/EP1531822B1/de not_active Expired - Lifetime
- 2003-06-11 CA CA2488202A patent/CA2488202C/en not_active Expired - Fee Related
- 2003-06-11 DE DE60328690T patent/DE60328690D1/de not_active Expired - Lifetime
- 2003-06-11 AT AT03737057T patent/ATE438401T1/de active
- 2003-06-11 ES ES03737057T patent/ES2329356T3/es not_active Expired - Lifetime
- 2003-06-11 US US10/460,752 patent/US7157464B2/en not_active Expired - Lifetime
- 2003-06-11 DK DK03737057T patent/DK1531822T3/da active
- 2003-06-11 WO PCT/US2003/018660 patent/WO2003105853A1/en active Search and Examination
-
2006
- 2006-07-21 US US11/491,540 patent/US7449576B1/en not_active Expired - Lifetime
-
2007
- 2007-05-22 HK HK07105429A patent/HK1099206A1/xx not_active IP Right Cessation
Also Published As
| Publication number | Publication date |
|---|---|
| US20040082571A1 (en) | 2004-04-29 |
| CA2488202A1 (en) | 2003-12-24 |
| CN1867336A (zh) | 2006-11-22 |
| MXPA04012393A (es) | 2005-06-17 |
| WO2003105853A1 (en) | 2003-12-24 |
| DK1531822T3 (da) | 2009-12-07 |
| EP1531822A1 (de) | 2005-05-25 |
| AU2003236500A1 (en) | 2003-12-31 |
| KR101255356B1 (ko) | 2013-04-17 |
| ATE438401T1 (de) | 2009-08-15 |
| AU2003236500B9 (en) | 2009-07-02 |
| CN1867336B (zh) | 2010-05-12 |
| JP2006508036A (ja) | 2006-03-09 |
| DE60328690D1 (de) | 2009-09-17 |
| ES2329356T3 (es) | 2009-11-25 |
| CA2488202C (en) | 2011-03-08 |
| HK1099206A1 (en) | 2007-08-10 |
| US7157464B2 (en) | 2007-01-02 |
| US7449576B1 (en) | 2008-11-11 |
| AU2003236500B2 (en) | 2009-01-08 |
| KR20050042754A (ko) | 2005-05-10 |
| JP4723242B2 (ja) | 2011-07-13 |
| US20080261987A1 (en) | 2008-10-23 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| EP1531822B1 (de) | 1-aryl-4-substituierte piperazin-derivate zur verwendung als ccr1-antagonisten zur behandlung von entzündungen und immunerkrankungen | |
| US7842693B2 (en) | Substituted piperazines | |
| AU2004296879B2 (en) | Substituted piperazines | |
| US8324216B2 (en) | Substituted piperazines | |
| EP1720545B1 (de) | Bizyklische und überbrückte stickstoff-heterozyklen | |
| EP1906965B1 (de) | Azaindazol-verbindungen und anwendungsverfahren | |
| AU621461B2 (en) | 4,5,6-Substituted-N-(substituted-phenyl)-2-pyrimidinamines | |
| EP2323663B1 (de) | 4-amino-3-(imidazolyl)-pyrazolo-[3,4-d]-pyrimidine | |
| DK2935227T3 (en) | DIAZOLAMIDES AS CCR1 RECEPTOR ANTAGONISTS | |
| CN100542534C (zh) | 取代的哌嗪 | |
| MXPA06006518A (es) | Piperazinas sustituidas. | |
| CN116396278A (zh) | 作为多巴胺d3受体选择性调节剂的咔唑类衍生物 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PUAI | Public reference made under article 153(3) epc to a published international application that has entered the european phase |
Free format text: ORIGINAL CODE: 0009012 |
|
| 17P | Request for examination filed |
Effective date: 20041222 |
|
| AK | Designated contracting states |
Kind code of ref document: A1 Designated state(s): AT BE BG CH CY CZ DE DK EE ES FI FR GB GR HU IE IT LI LU MC NL PT RO SE SI SK TR |
|
| AX | Request for extension of the european patent |
Extension state: AL LT LV MK |
|
| RAP1 | Party data changed (applicant data changed or rights of an application transferred) |
Owner name: CHEMOCENTRYX, INC. |
|
| 17Q | First examination report despatched |
Effective date: 20060426 |
|
| RIN1 | Information on inventor provided before grant (corrected) |
Inventor name: PENNELL, ANDREW, M., K. Inventor name: MCMASTER, BRIAN, E. Inventor name: SEN, SUBRABRATA Inventor name: AGGEN, JAMES, B. Inventor name: DAIRAGHI, DANIEL, JOSEPH Inventor name: WRIGHT, J., J., KIM |
|
| RIN1 | Information on inventor provided before grant (corrected) |
Inventor name: SEN, SUBRABRATA Inventor name: MCMASTER, BRIAN, E. Inventor name: DAIRAGHI, DANIEL, JOSEPH Inventor name: WRIGHT, J., J., KIM Inventor name: MARTICHONOK, VALERI V. Inventor name: PENNELL, ANDREW, M., K. Inventor name: AGGEN, JAMES, B. |
|
| RIN1 | Information on inventor provided before grant (corrected) |
Inventor name: MARTICHONOK, VALERI V. Inventor name: MCMASTER, BRIAN, E. Inventor name: SEN, SUBHABRATA Inventor name: WRIGHT, J., J., KIM Inventor name: DAIRAGHI, DANIEL, JOSEPH Inventor name: AGGEN, JAMES, B. Inventor name: PENNELL, ANDREW, M., K. |
|
| GRAP | Despatch of communication of intention to grant a patent |
Free format text: ORIGINAL CODE: EPIDOSNIGR1 |
|
| GRAS | Grant fee paid |
Free format text: ORIGINAL CODE: EPIDOSNIGR3 |
|
| GRAA | (expected) grant |
Free format text: ORIGINAL CODE: 0009210 |
|
| AK | Designated contracting states |
Kind code of ref document: B1 Designated state(s): AT BE BG CH CY CZ DE DK EE ES FI FR GB GR HU IE IT LI LU MC NL PT RO SE SI SK TR |
|
| AX | Request for extension of the european patent |
Extension state: AL LT LV MK |
|
| REG | Reference to a national code |
Ref country code: GB Ref legal event code: FG4D |
|
| REG | Reference to a national code |
Ref country code: CH Ref legal event code: NV Representative=s name: KIRKER & CIE S.A. Ref country code: CH Ref legal event code: EP |
|
| REG | Reference to a national code |
Ref country code: IE Ref legal event code: FG4D |
|
| REF | Corresponds to: |
Ref document number: 60328690 Country of ref document: DE Date of ref document: 20090917 Kind code of ref document: P |
|
| REG | Reference to a national code |
Ref country code: SE Ref legal event code: TRGR |
|
| REG | Reference to a national code |
Ref country code: ES Ref legal event code: FG2A Ref document number: 2329356 Country of ref document: ES Kind code of ref document: T3 |
|
| REG | Reference to a national code |
Ref country code: DK Ref legal event code: T3 |
|
| LTIE | Lt: invalidation of european patent or patent extension |
Effective date: 20090805 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: FI Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20090805 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: SI Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20090805 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: BG Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20091105 Ref country code: PT Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20091205 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: CZ Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20090805 Ref country code: EE Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20090805 Ref country code: RO Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20090805 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: SK Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20090805 |
|
| PLBE | No opposition filed within time limit |
Free format text: ORIGINAL CODE: 0009261 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: NO OPPOSITION FILED WITHIN TIME LIMIT |
|
| 26N | No opposition filed |
Effective date: 20100507 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: GR Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20091106 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: MC Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20100630 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: CY Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20090805 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: LU Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20100611 Ref country code: HU Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20100206 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: TR Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20090805 |
|
| REG | Reference to a national code |
Ref country code: FR Ref legal event code: PLFP Year of fee payment: 14 |
|
| REG | Reference to a national code |
Ref country code: FR Ref legal event code: PLFP Year of fee payment: 15 |
|
| REG | Reference to a national code |
Ref country code: FR Ref legal event code: PLFP Year of fee payment: 16 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: DK Payment date: 20180612 Year of fee payment: 16 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: BE Payment date: 20180417 Year of fee payment: 16 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: IE Payment date: 20190610 Year of fee payment: 17 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: SE Payment date: 20190611 Year of fee payment: 17 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: CH Payment date: 20190614 Year of fee payment: 17 |
|
| REG | Reference to a national code |
Ref country code: DK Ref legal event code: EBP Effective date: 20190630 |
|
| REG | Reference to a national code |
Ref country code: AT Ref legal event code: MM01 Ref document number: 438401 Country of ref document: AT Kind code of ref document: T Effective date: 20190611 |
|
| REG | Reference to a national code |
Ref country code: BE Ref legal event code: MM Effective date: 20190630 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: AT Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20190611 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: BE Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20190630 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: DK Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20190630 |
|
| REG | Reference to a national code |
Ref country code: CH Ref legal event code: PL |
|
| REG | Reference to a national code |
Ref country code: NL Ref legal event code: MM Effective date: 20200701 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: NL Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20200701 Ref country code: CH Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20200630 Ref country code: LI Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20200630 Ref country code: IE Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20200611 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: SE Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20200612 |
|
| REG | Reference to a national code |
Ref country code: SE Ref legal event code: EUG |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: IT Payment date: 20220510 Year of fee payment: 20 Ref country code: GB Payment date: 20220425 Year of fee payment: 20 Ref country code: FR Payment date: 20220408 Year of fee payment: 20 Ref country code: DE Payment date: 20220420 Year of fee payment: 20 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: ES Payment date: 20220706 Year of fee payment: 20 |
|
| REG | Reference to a national code |
Ref country code: DE Ref legal event code: R071 Ref document number: 60328690 Country of ref document: DE |
|
| REG | Reference to a national code |
Ref country code: ES Ref legal event code: FD2A Effective date: 20230626 |
|
| REG | Reference to a national code |
Ref country code: GB Ref legal event code: PE20 Expiry date: 20230610 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: ES Free format text: LAPSE BECAUSE OF EXPIRATION OF PROTECTION Effective date: 20230612 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: GB Free format text: LAPSE BECAUSE OF EXPIRATION OF PROTECTION Effective date: 20230610 |