EP1126478A1 - Dispositif a coefficient de temperature positif et son procede de fabrication - Google Patents
Dispositif a coefficient de temperature positif et son procede de fabrication Download PDFInfo
- Publication number
- EP1126478A1 EP1126478A1 EP00946362A EP00946362A EP1126478A1 EP 1126478 A1 EP1126478 A1 EP 1126478A1 EP 00946362 A EP00946362 A EP 00946362A EP 00946362 A EP00946362 A EP 00946362A EP 1126478 A1 EP1126478 A1 EP 1126478A1
- Authority
- EP
- European Patent Office
- Prior art keywords
- composition material
- powder
- conductive
- formed composition
- electrodes
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Withdrawn
Links
- 238000004519 manufacturing process Methods 0.000 title claims description 22
- 239000002131 composite material Substances 0.000 claims abstract description 102
- 239000000843 powder Substances 0.000 claims abstract description 101
- 229920000642 polymer Polymers 0.000 claims abstract description 30
- 239000000945 filler Substances 0.000 claims abstract description 27
- 239000004020 conductor Substances 0.000 claims abstract description 23
- 229910003178 Mo2C Inorganic materials 0.000 claims abstract description 20
- 238000007747 plating Methods 0.000 claims abstract description 16
- 238000000034 method Methods 0.000 claims description 28
- 230000004888 barrier function Effects 0.000 claims description 17
- 239000000463 material Substances 0.000 claims description 15
- OKTJSMMVPCPJKN-UHFFFAOYSA-N Carbon Chemical compound [C] OKTJSMMVPCPJKN-UHFFFAOYSA-N 0.000 claims description 7
- 239000011248 coating agent Substances 0.000 claims description 6
- 238000000576 coating method Methods 0.000 claims description 6
- 238000004898 kneading Methods 0.000 claims description 6
- 238000002156 mixing Methods 0.000 claims description 6
- 239000000956 alloy Substances 0.000 claims description 3
- 229910045601 alloy Inorganic materials 0.000 claims description 3
- 229920001169 thermoplastic Polymers 0.000 claims description 3
- 230000005611 electricity Effects 0.000 abstract description 3
- 230000000052 comparative effect Effects 0.000 description 41
- 239000002184 metal Substances 0.000 description 23
- 229910052751 metal Inorganic materials 0.000 description 23
- PXHVJJICTQNCMI-UHFFFAOYSA-N Nickel Chemical compound [Ni] PXHVJJICTQNCMI-UHFFFAOYSA-N 0.000 description 17
- 239000002861 polymer material Substances 0.000 description 8
- 230000007613 environmental effect Effects 0.000 description 5
- 229920001903 high density polyethylene Polymers 0.000 description 5
- 239000004700 high-density polyethylene Substances 0.000 description 5
- 238000005259 measurement Methods 0.000 description 5
- 230000001681 protective effect Effects 0.000 description 5
- 239000004816 latex Substances 0.000 description 4
- 229920000126 latex Polymers 0.000 description 4
- 229920005989 resin Polymers 0.000 description 4
- 239000011347 resin Substances 0.000 description 4
- 238000007789 sealing Methods 0.000 description 4
- 239000011231 conductive filler Substances 0.000 description 3
- 230000007423 decrease Effects 0.000 description 3
- -1 polyethylene Polymers 0.000 description 3
- 239000000126 substance Substances 0.000 description 3
- 239000004743 Polypropylene Substances 0.000 description 2
- 229920001328 Polyvinylidene chloride Polymers 0.000 description 2
- 238000003556 assay Methods 0.000 description 2
- 230000000694 effects Effects 0.000 description 2
- 238000007772 electroless plating Methods 0.000 description 2
- 230000020169 heat generation Effects 0.000 description 2
- 229910052759 nickel Inorganic materials 0.000 description 2
- 239000002245 particle Substances 0.000 description 2
- 229920001155 polypropylene Polymers 0.000 description 2
- 239000005033 polyvinylidene chloride Substances 0.000 description 2
- 238000003825 pressing Methods 0.000 description 2
- 239000004698 Polyethylene Substances 0.000 description 1
- 230000002159 abnormal effect Effects 0.000 description 1
- 150000001721 carbon Chemical class 0.000 description 1
- 239000006229 carbon black Substances 0.000 description 1
- 239000011365 complex material Substances 0.000 description 1
- 230000003247 decreasing effect Effects 0.000 description 1
- 230000006866 deterioration Effects 0.000 description 1
- 239000003792 electrolyte Substances 0.000 description 1
- 239000003822 epoxy resin Substances 0.000 description 1
- 239000011888 foil Substances 0.000 description 1
- 229910002804 graphite Inorganic materials 0.000 description 1
- 239000010439 graphite Substances 0.000 description 1
- WABPQHHGFIMREM-UHFFFAOYSA-N lead(0) Chemical compound [Pb] WABPQHHGFIMREM-UHFFFAOYSA-N 0.000 description 1
- 229920001684 low density polyethylene Polymers 0.000 description 1
- 239000004702 low-density polyethylene Substances 0.000 description 1
- 239000000203 mixture Substances 0.000 description 1
- 239000005416 organic matter Substances 0.000 description 1
- 230000035699 permeability Effects 0.000 description 1
- 229920002037 poly(vinyl butyral) polymer Polymers 0.000 description 1
- 229920000647 polyepoxide Polymers 0.000 description 1
- 229920000573 polyethylene Polymers 0.000 description 1
- 229920006395 saturated elastomer Polymers 0.000 description 1
- 230000035939 shock Effects 0.000 description 1
- 238000005476 soldering Methods 0.000 description 1
- 239000002904 solvent Substances 0.000 description 1
- 239000010935 stainless steel Substances 0.000 description 1
- 229910001220 stainless steel Inorganic materials 0.000 description 1
Images
Classifications
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01C—RESISTORS
- H01C7/00—Non-adjustable resistors formed as one or more layers or coatings; Non-adjustable resistors made from powdered conducting material or powdered semi-conducting material with or without insulating material
- H01C7/02—Non-adjustable resistors formed as one or more layers or coatings; Non-adjustable resistors made from powdered conducting material or powdered semi-conducting material with or without insulating material having positive temperature coefficient
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01C—RESISTORS
- H01C17/00—Apparatus or processes specially adapted for manufacturing resistors
- H01C17/06—Apparatus or processes specially adapted for manufacturing resistors adapted for coating resistive material on a base
- H01C17/065—Apparatus or processes specially adapted for manufacturing resistors adapted for coating resistive material on a base by thick film techniques, e.g. serigraphy
- H01C17/06506—Precursor compositions therefor, e.g. pastes, inks, glass frits
- H01C17/06513—Precursor compositions therefor, e.g. pastes, inks, glass frits characterised by the resistive component
- H01C17/0652—Precursor compositions therefor, e.g. pastes, inks, glass frits characterised by the resistive component containing carbon or carbides
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01C—RESISTORS
- H01C1/00—Details
- H01C1/14—Terminals or tapping points or electrodes specially adapted for resistors; Arrangements of terminals or tapping points or electrodes on resistors
- H01C1/142—Terminals or tapping points or electrodes specially adapted for resistors; Arrangements of terminals or tapping points or electrodes on resistors the terminals or tapping points being coated on the resistive element
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01C—RESISTORS
- H01C7/00—Non-adjustable resistors formed as one or more layers or coatings; Non-adjustable resistors made from powdered conducting material or powdered semi-conducting material with or without insulating material
- H01C7/02—Non-adjustable resistors formed as one or more layers or coatings; Non-adjustable resistors made from powdered conducting material or powdered semi-conducting material with or without insulating material having positive temperature coefficient
- H01C7/027—Non-adjustable resistors formed as one or more layers or coatings; Non-adjustable resistors made from powdered conducting material or powdered semi-conducting material with or without insulating material having positive temperature coefficient consisting of conducting or semi-conducting material dispersed in a non-conductive organic material
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01C—RESISTORS
- H01C7/00—Non-adjustable resistors formed as one or more layers or coatings; Non-adjustable resistors made from powdered conducting material or powdered semi-conducting material with or without insulating material
- H01C7/04—Non-adjustable resistors formed as one or more layers or coatings; Non-adjustable resistors made from powdered conducting material or powdered semi-conducting material with or without insulating material having negative temperature coefficient
- H01C7/049—Non-adjustable resistors formed as one or more layers or coatings; Non-adjustable resistors made from powdered conducting material or powdered semi-conducting material with or without insulating material having negative temperature coefficient mainly consisting of organic or organo-metal substances
-
- H—ELECTRICITY
- H05—ELECTRIC TECHNIQUES NOT OTHERWISE PROVIDED FOR
- H05B—ELECTRIC HEATING; ELECTRIC LIGHT SOURCES NOT OTHERWISE PROVIDED FOR; CIRCUIT ARRANGEMENTS FOR ELECTRIC LIGHT SOURCES, IN GENERAL
- H05B3/00—Ohmic-resistance heating
- H05B3/10—Heating elements characterised by the composition or nature of the materials or by the arrangement of the conductor
- H05B3/12—Heating elements characterised by the composition or nature of the materials or by the arrangement of the conductor characterised by the composition or nature of the conductive material
- H05B3/14—Heating elements characterised by the composition or nature of the materials or by the arrangement of the conductor characterised by the composition or nature of the conductive material the material being non-metallic
- H05B3/146—Conductive polymers, e.g. polyethylene, thermoplastics
Definitions
- the present invention relates to a PTC element composed of a conductive composition material (hereinafter referred to PTC composition material) showing positive temperature properties, so-called PTC (Positive Temperature Coefficient) properties that mean its resistance value suddenly rises when a fixed temperature (hereinafter referred to as a switching temperature) region is reached.
- PTC composition material a conductive composition material showing positive temperature properties
- PTC Positive Temperature Coefficient
- PTC elements are used as an protective element of electrical circuits to prevent over-current that flows when an abnormal state is happen in these apparatus.
- a PTC element is composed of a PTC composition material obtained by blending and kneading conductive powder with a crystalline polymer and of electrodes formed on said PTC composition material, and shows sudden increase in its resistance value when the switching temperature is reached. That is, a PTC composition material produces heat in accordance with joule heat (I 2 R heat) generated by resistance value R peculiar to the material and electric current value I flowed in the element through the above-mentioned electrodes. Like this, if relatively high current flows in the PTC composition material, heat generation occurs to make its resistance value increase.
- PTC elements are used as a sheet heat generation material using the generation of above-mentioned joule heat, an over-current protective element using increase in resistance value, and the like.
- the contact resistance value between the PTC composition material and the electrode becomes high and good ohmic contact cannot be obtained.
- the PTC composition material relating to this first former example because resistance value becomes high at room temperature, it is difficult to use the PTC composition material as an over-current protective element and the like.
- the adhesion between the PTC composition material and the electrodes is insufficient, resistance value increases greatly in cases where the PTC element is repeatedly operated (turning on electricity).
- the contact resistance value between the PTC composition material and the electrode becomes lower than that in the above-mentioned first former example and the adhesion between the two also becomes better, but it is not yet reached to obtain good ohmic contact.
- the adhesion between the PTC composition material and the plated coat is not sufficient and the contact resistance value between the two becomes high. And great increase in resistance value due to repeated operation is also inevitable.
- a PTC composition material is a complex material of an organic matter and an inorganic substance
- the composition material is greatly affected by environmental humidity during being stocked and used and the change of its resistance becomes to be great as the passage of time when switching operation is repeatedly carried out.
- it is needed to fill up the conductive filler in large amount.
- the resulting composition material is easily affected by environmental humidity during being stocked and used.
- the element itself becomes to be apt to peel off from the electrodes as the passage of time when switching operation is repeatedly carried out, the composition material could not obtain sufficient reliability for the long time usage (repeated usage).
- conductive powder filler composed of at least one out of TiC, WC, W 2 C, ZrC, VC, NbC, TaC, and Mo 2 C is blended and kneaded with a crystalline polymer component by 35 to 60 volume percent to form a formed composition material, a conductive material is pressure sealed and buried so that the material is partly exposed from the surface of said formed composition material, and then electrodes are formed by plating on said surface of said formed composition material.
- a PTC element can be obtained, and the PTC element is characterized in that it has a formed composition material in which conductive powder filler is blended and kneaded with a crystalline polymer component by 35 to 60 volume percent, a conductive material pressure sealed and buried so that the material is partly exposed from the surface of said formed composition material, and electrodes formed by plating on said surface of said formed composition material, and in that at least one out of TiC, WC, W 2 C, ZrC, VC, NbC, TaC, and Mo 2 C is used as said conductive powder filler.
- Said conductive material is preferable to include Ni powder, Al powder, Cu powder, Fe powder, Ag powder, or graphite powder.
- a formed composition material by blending and kneading said conductive powder filler with a crystalline polymer component by 45 to 60 volume percent.
- a production method of a PTC element that is characterized in that the method has a process in which a formed composition material is obtained by means of blending and kneading at least one out of TiC, WC, W 2 C, ZrC, VC, NbC, TaC, and Mo 2 C with a crystalline polymer component by 35 to 60 volume percent as a conductive powder filler, a process in which after coated said formed composition material with a conductive paste containing a conductive powder, said conductive powder is treated by pressure sealing to bury said conductive powder so that part of said conductive powder is exposed from the surface of said formed composition material, and a process in which said surface of said formed composition material is plated to form electrodes.
- Ni powder, Al powder, Cu powder, Fe powder, Ag powder, or graphite powder is used as said conductive powder.
- said conductive powder filler is blended and kneaded with a crystalline polymer component by 45 to 60 volume percent to form a formed composition material.
- conductive powder filler composed of at least one out of TiC, WC, W 2 C, ZrC, VC, NbC, TaC, and Mo 2 C is blended and kneaded with a crystalline polymer component by 35 to 60 volume percent to form a formed composition material, a conductive material is pressure sealed and buried so that the material is partly exposed from the surface of said formed composition material, then electrodes are formed by plating on said surface of said formed composition material, moreover, a steam barrier layer is formed in the part other than said plated electrode of the PCT element.
- a PTC element that is characterized in that the PTC element has a formed composition material in which a conductive powder filler using at least one out of TiC, WC, W 2 C, ZrC, VC, NbC, TaC, and Mo 2 C is blended and kneaded with a crystalline polymer component by 35 to 60 volume percent, a conductive material which is pressure sealed and buried so that it is partly exposed from the surface of said formed composition material, electrodes formed by plating on said surface of said formed composition material, and a steam barrier layer formed in the part other than said plated electrode.
- said crystalline polymer component is a polymer alloy in which at least one kind of thermoplastic polymer is mixed.
- a production method of a PTC element that is characterized in that the method has a process in which a formed composition material is obtained by means of blending and kneading at least one out of TiC, WC, W 2 C, ZrC, VC, NbC, TaC, and Mo 2 C with a crystalline polymer component by 35 to 60 volume percent as a conductive powder filler, a process in which after coated said formed composition material with a conductive paste containing a conductive powder, said conductive powder is treated by pressure sealing to bury said conductive powder so that part of said conductive powder is exposed from the surface of said formed composition material, a process in which said surface of said formed composition material is plated to form electrodes, and a process in which a steam barrier treatment is carried out in the part other than said plated electrodes.
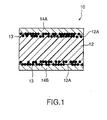
- PTC element 10 related to the first embodiment of the present invention has formed composition material 12 in which conductive powder filler (not shown in the figure) was blended and kneaded with a crystalline polymer component by 35 to 60 volume percent, conductive material 13 pressure sealed and buried so that it is partly exposed from surface 12A of formed composition material 12, and electrodes 14A and 14B formed by means of plate treatment on surface 12A of formed composition material 12.
- conductive powder filler one or two kinds or more out of TiC, WC, W 2 C, ZrC, VC, NbC, TaC, and Mo 2 C can be used.
- conductive material 13 is preferable to include Ni powder, Al powder, Cu powder, Fe powder, Ag powder, or graphite powder.
- crystalline high-density polyethylene having softening point of about 130°C as a polymer component and conductive powder filler of 1 to 5 ⁇ m in particle size were blended and kneaded on a roller mill heated about 140 to 200°C so that the content of the conductive powder was 35 to 60 volume percent, and kneaded polymer material was obtained.
- one or two kinds or more out of TiC, WC, W 2 C, ZrC, VC, NbC, TaC, and Mo 2 C can be used as conductive, powder [See Fig. 2(a)].
- test piece of 1 cm 2 in area was punched out from the Ni plated sheet obtained as mentioned above and used as a sample for assay (hereinafter, this sample for assay is referred to as "the example” ). Furthermore, as conductive powder buried in the sheet by pressure sealing, it is acceptable to use Al powder, Cu powder, Fe powder, Ag powder, or graphite powder, as well as Ni powder.
- comparative sample 1 was prepared as in the following (hereinafter, this comparative sample is referred to as "comparative example 1")
- comparative sample 2 was prepared as in the following (hereinafter, this comparative sample is referred to as "comparative example 2" ). Also in this comparative example 2, the same treatment as in the above-mentioned example was carried out until kneaded polymer material was sheeted. After that, metal plates, their one side to be contacted with the kneaded material sheet (one side of each metal plate) were roughened with electrolyte, were agglutinated on both sides of the kneaded material sheet by heat pressing at temperatures of about 140 to 200°C to form electrodes, And, a test piece of 1 cm 2 in area was punched out from this sheet and a PTC element was obtained (comparative example 2).
- comparative sample 3 was prepared as in the following (hereinafter, this comparative sample is referred to as "comparative example 3" ). Also in this comparative example 3, the same treatment as in the above-mentioned example was carried out until kneaded polymer material was sheeted. After that, the kneaded material sheet was defatted and then Ni electroless plating was carried out on the sheet to form electrodes. And, a test piece of 1 cm 2 in area was punched out from this sheet and a PTC element was obtained (comparative example 3).
- comparative sample 4 was prepared as in the following (hereinafter, this comparative sample is referred to as "comparative example 4" ).
- Crystalline high-density polyethylene having softening point of about 130°C as a polymer component and conductive powder of 1 to 5 ⁇ m in particle size were blended and kneaded on a roller mill heated about 140 to 200°C so that the content of the conductive powder was 34 volume percent, and kneaded polymer material was obtained.
- TiC, WC, W 2 C, ZrC, VC, NbC, TaC, and Mo 2 C was used as conductive powder.
- the same treatment as in the above-mentioned example was carried out, then a test piece of 1 cm 2 in area was punched out from this sheet and a PTC element was obtained (comparative example 4).
- Characteristic properties were tested on the example and comparative example 1 to 3 obtained as mentioned above.
- target properties of the PTC element were decided as follows: bonding strength of the electrode is 500 gf/cm 2 or more, which means sufficient reliability as an electrode, resistance at room temperature is 2 ⁇ ⁇ cm or less, and the ratio of resistance value after resistance value was suddenly increased to temperatures (after switching) to resistance value at room temperature (after switching R/room temperature R) is 10 4 or more, which means that the PTC element sufficiently operates as over-current protective element and can be fully used as a sheet heater. Further, the target of resistance value at room temperature in cases of repeated switching of the PTC was decided to be 2 ⁇ ⁇ cm or less even after 50 times switching.
- the bonding strength of electrodes in the example was higher than those in comparative example 1 where a metal plate not roughened was used as electrodes and comparative example 3 where only plating was performed to form electrodes, and was about the same value as that in comparative example 2 where a roughened metal plate was used as electrodes.
- the bonding strength of electrodes of the example was confirmed to be more than 500 gf/cm 2 , the value is possible to sufficiently holding reliability as an electrode.
- resistivity at room temperature was confirmed to be as low as target values of 2 ⁇ ⁇ cm or less.
- resistivity at room temperature is as low as target value of 2 ⁇ ⁇ cm or less and the curve of temperature-resistivity greatly increases at temperatures roughly corresponding to the softening point (about 130°C) of crystalline high-density polyethylene used in the example.
- the ratio of resistance value after resistance value was suddenly increased to temperatures (after switching) to resistance value at room temperature (after switching R/room temperature R) is higher than 10 8 and greatly exceeds the target value of 10 4 which means that the PTC element sufficiently operates as over-current protective element and can be fully used as a sheet heater.
- initial resistivity at room temperature was as low as target value of 2 ⁇ cm or less and continued to keep target values of 2 ⁇ ⁇ cm or less after repeated current flow. And after several times of repeated current flow, increase in resistivity at room temperature was found to be saturated.
- the filling amount of conductive powder is less than 35 volume percent, as mentioned above, stability to repeated current flow decreases and resistivity at room temperature may exceed the target value of 2 ⁇ ⁇ cm according as the frequency of operations.
- the filling amount of conductive powder is more than 60 volume percent, it will be actually difficult to manufacture elements because of decrease in their manufacturing efficiency.
- a conductive material was pressure sealed and buried on the surface of a formed composition material, in which conductive powder filler was blended and kneaded with a crystalline polymer component by 35 to 60 volume percent, so that the conductive material is partly exposed, and electrodes were formed by plating treatment on the surface of the formed composition material where the conductive material was partly exposed, and further, as conductive powder filler, at least one out of TiC, WC, W 2 C, ZrC, VC, NbC, TaC, and Mo 2 C was used. Consequently, the adhesion between the PTC composition material and electrodes becomes good and the contact resistance between the two can be lowered. And it is possible to obtain a PTC element excellent in stability to repeated current flow.
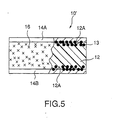
- Fig. 5 is a partial sectional view showing the PTC element related to the second embodiment of the present invention.
- the PTC element 10 in the embodiment has formed composition material 12, conductive material 13 which is pressure sealed and buried so that the material is partly exposed from the surface 12A of formed composition material 12, and electrodes 14A and 14B formed by plating treatment on the surface 12A of formed composition material 12. And on the part where formed composition material 12 other than (plated) electrodes 14A and 14B is exposed, steam barrier layer 16 is coated.
- composition material 12 is quite the same as that in the above-mentioned embodiment 1 and was formed as follows: conductive powder filler using at least one out of TiC, WC, W 2 C, ZrC, VC, NbC, TaC, and Mo 2 C was blended and kneaded with a crystalline polymer component by 35 to 60 volume percent and then the mixture was formed.
- the crystalline polymer component in formed composition material 12 is composed of a polymer alloy in which one kind or two or more kinds of thermoplastic polymers, for example, modified polyethylene or modified polypropylene, are mixed.
- Nickel (Ni) foil is used in each of electrode 14A and 14B.
- Steam barrier layer 16 is formed by conducting steam barrier treatment, for example, as mention later, such as coating with PVDC latex and others.
- Fig. 6 indicates drawings to explain the production method of the PTC element of the example, (a) shows the formed composition material, (b) shows a state in which a conductive material is pressure sealed and buried so that the material is partly exposed from the surface of the formed composition material, (c) shows a state in which electrodes are formed by plating treatment on the formed composition material, (d) shows steam barrier treatment, and (e) shows the PTC element treated with steam barrier treatment, respectively.
- steam barrier layer 16 was formed by coating PVCD latex 16a on the part except for plated electrodes (nickel foil) 14A and 14B in formed composition material 12, that is, on part 12 C where the surface of formed composition material 12 is exposed, as shown in Fig. 6 (d), and PTC (resistant) element 10 of the example shown Fig. 6 (e) was consequently made.
- high-density polyethylene resin was used as a main component of the formed composition material, but the main component is not limited to the resin.
- main components of formed composition materials polypropylene type, low-density polyethylene type and other type resins can be used, as well as high-density polyethylene resin.
- steam barrier was formed by coating with PVCD latex, but in addition to this, methods of steam barrier treatment using substances with low steam permeability, for example, polyvinylidene chloride and the like are considered.
- a conductive material is pressure sealed and buried so that part of the conductive material is exposed, and the surface of the formed composition material with partly exposed conductive material is plated to form electrodes.
- at least one out of TiC, WC, W 2 C, ZrC, VC, NbC, TaC, and Mo 2 C is used as conductive powder filler. Consequently, the adhesion between the PTC composition material and the electrodes becomes good and contact resistance value between the two can be reduced. And a PTC element with excellent stability to repeated turning on electricity and its production. method can be obtained.
- the PTC element and its production method of the above-mentioned first embodiment it is further possible to effectively prevent the PTC element itself from peeling from electrodes due to the effect of environmental humidity. And a high reliable PTC element with good stability and reproducibility to repeated use and its production method can be provided.
Landscapes
- Engineering & Computer Science (AREA)
- Microelectronics & Electronic Packaging (AREA)
- Physics & Mathematics (AREA)
- Electromagnetism (AREA)
- Ceramic Engineering (AREA)
- Chemical & Material Sciences (AREA)
- Dispersion Chemistry (AREA)
- Manufacturing & Machinery (AREA)
- Thermistors And Varistors (AREA)
- Resistance Heating (AREA)
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP20261799 | 1999-07-16 | ||
| JP11202617A JP2001035640A (ja) | 1999-07-16 | 1999-07-16 | Ptc素子及びその製造方法 |
| PCT/JP2000/004777 WO2001006521A1 (fr) | 1999-07-16 | 2000-07-14 | Dispositif a coefficient de temperature positif et son procede de fabrication |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| EP1126478A1 true EP1126478A1 (fr) | 2001-08-22 |
| EP1126478A4 EP1126478A4 (fr) | 2002-01-09 |
Family
ID=16460357
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP00946362A Withdrawn EP1126478A4 (fr) | 1999-07-16 | 2000-07-14 | Dispositif a coefficient de temperature positif et son procede de fabrication |
Country Status (8)
| Country | Link |
|---|---|
| EP (1) | EP1126478A4 (fr) |
| JP (1) | JP2001035640A (fr) |
| KR (1) | KR20010079845A (fr) |
| CN (1) | CN1318201A (fr) |
| CA (1) | CA2344532A1 (fr) |
| NO (1) | NO20011325L (fr) |
| TW (1) | TW472499B (fr) |
| WO (1) | WO2001006521A1 (fr) |
Families Citing this family (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| KR100420470B1 (ko) * | 2001-10-31 | 2004-03-02 | 엘지전선 주식회사 | 정온도 특성 소자 제조를 위한 솔더링 공법 |
| WO2005031886A1 (fr) * | 2003-09-25 | 2005-04-07 | Matsushita Electric Industrial Co., Ltd. | Element piezoelectrique, tete jet d'encre pourvue dudit element, et leurs procedes de fabrication |
| JPWO2014077366A1 (ja) * | 2012-11-19 | 2017-01-05 | 株式会社Uacj | 集電体、電極構造体、蓄電部品および集電体用組成物 |
| DE102017121041A1 (de) * | 2017-05-24 | 2018-11-29 | Webasto SE | Heizgerät und Verfahren zur Herstellung desselben |
| CN112153765B (zh) * | 2020-11-25 | 2021-03-09 | 广东康烯科技有限公司 | 多孔碳化钼MXene/还原氧化石墨烯基发热膜 |
Citations (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPH0969409A (ja) * | 1995-08-31 | 1997-03-11 | Mitsubishi Electric Corp | Ptc素子 |
| JPH10125504A (ja) * | 1996-10-17 | 1998-05-15 | Tdk Corp | 有機質正特性サーミスタとその製造方法 |
| JPH10140004A (ja) * | 1996-11-05 | 1998-05-26 | Daicel Huels Ltd | 高分子感温体用樹脂組成物および高分子感温体 |
| JPH10208902A (ja) * | 1997-01-21 | 1998-08-07 | Tdk Corp | 有機ptcサーミスタの製造方法 |
| JPH11144906A (ja) * | 1997-11-13 | 1999-05-28 | Tokin Corp | Ptc組成物 |
Family Cites Families (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPH06318504A (ja) * | 1993-05-10 | 1994-11-15 | Daito Tsushinki Kk | Ptc素子 |
| JP2810351B2 (ja) * | 1995-09-27 | 1998-10-15 | ティーディーケイ株式会社 | 有機質正特性サーミスタ |
| JP3214546B2 (ja) * | 1996-11-08 | 2001-10-02 | ティーディーケイ株式会社 | 有機質正特性サーミスタの製造方法および有機質正特性サーミスタ |
| JPH10241907A (ja) * | 1997-02-28 | 1998-09-11 | Mitsubishi Electric Corp | 回路保護装置 |
| JPH1116707A (ja) * | 1997-06-27 | 1999-01-22 | Tdk Corp | 有機質正特性サーミスタ |
| JPH1187106A (ja) * | 1997-09-08 | 1999-03-30 | Unitika Ltd | Ptc素子の製造方法 |
-
1999
- 1999-07-16 JP JP11202617A patent/JP2001035640A/ja not_active Withdrawn
-
2000
- 2000-07-14 EP EP00946362A patent/EP1126478A4/fr not_active Withdrawn
- 2000-07-14 CN CN00801438A patent/CN1318201A/zh active Pending
- 2000-07-14 WO PCT/JP2000/004777 patent/WO2001006521A1/fr not_active Application Discontinuation
- 2000-07-14 CA CA002344532A patent/CA2344532A1/fr not_active Abandoned
- 2000-07-14 KR KR1020017003431A patent/KR20010079845A/ko not_active Application Discontinuation
- 2000-07-15 TW TW089114201A patent/TW472499B/zh not_active IP Right Cessation
-
2001
- 2001-03-15 NO NO20011325A patent/NO20011325L/no unknown
Patent Citations (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPH0969409A (ja) * | 1995-08-31 | 1997-03-11 | Mitsubishi Electric Corp | Ptc素子 |
| JPH10125504A (ja) * | 1996-10-17 | 1998-05-15 | Tdk Corp | 有機質正特性サーミスタとその製造方法 |
| JPH10140004A (ja) * | 1996-11-05 | 1998-05-26 | Daicel Huels Ltd | 高分子感温体用樹脂組成物および高分子感温体 |
| JPH10208902A (ja) * | 1997-01-21 | 1998-08-07 | Tdk Corp | 有機ptcサーミスタの製造方法 |
| JPH11144906A (ja) * | 1997-11-13 | 1999-05-28 | Tokin Corp | Ptc組成物 |
Non-Patent Citations (6)
| Title |
|---|
| PATENT ABSTRACTS OF JAPAN vol. 1997, no. 07, 31 July 1997 (1997-07-31) & JP 09 069409 A (MITSUBISHI ELECTRIC CORP), 11 March 1997 (1997-03-11) * |
| PATENT ABSTRACTS OF JAPAN vol. 1998, no. 10, 31 August 1998 (1998-08-31) & JP 10 125504 A (TDK CORP), 15 May 1998 (1998-05-15) * |
| PATENT ABSTRACTS OF JAPAN vol. 1998, no. 10, 31 August 1998 (1998-08-31) & JP 10 140004 A (DAICEL HUELS LTD), 26 May 1998 (1998-05-26) * |
| PATENT ABSTRACTS OF JAPAN vol. 1998, no. 13, 30 November 1998 (1998-11-30) & JP 10 208902 A (TDK CORP), 7 August 1998 (1998-08-07) * |
| PATENT ABSTRACTS OF JAPAN vol. 1999, no. 10, 31 August 1999 (1999-08-31) & JP 11 144906 A (TOKIN CORP), 28 May 1999 (1999-05-28) * |
| See also references of WO0106521A1 * |
Also Published As
| Publication number | Publication date |
|---|---|
| NO20011325L (no) | 2001-05-16 |
| CN1318201A (zh) | 2001-10-17 |
| EP1126478A4 (fr) | 2002-01-09 |
| CA2344532A1 (fr) | 2001-01-25 |
| JP2001035640A (ja) | 2001-02-09 |
| TW472499B (en) | 2002-01-11 |
| NO20011325D0 (no) | 2001-03-15 |
| KR20010079845A (ko) | 2001-08-22 |
| WO2001006521A1 (fr) | 2001-01-25 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| EP0852801B2 (fr) | Compositions polymeres ameliorees a coefficient de temperature positif | |
| JP6598231B2 (ja) | 高分子系導電性複合材料及びptc素子 | |
| US5928547A (en) | High power current limiting polymer devices for circuit breaker applications | |
| US6358438B1 (en) | Electrically conductive polymer composition | |
| CN106679844A (zh) | 高分子ptc温度传感器 | |
| EP1126478A1 (fr) | Dispositif a coefficient de temperature positif et son procede de fabrication | |
| CN109448943A (zh) | 一种高分子ptc过电流保护元件 | |
| CN207199390U (zh) | 一种表面贴装ptc过电流保护元件 | |
| US7304562B2 (en) | Organic PTC thermistor and production | |
| CN212782901U (zh) | 高可靠性过流保护元件 | |
| CN206410798U (zh) | 高分子ptc温度传感器 | |
| CN108447634B (zh) | 表面贴装型热敏电阻组件 | |
| CN106189219A (zh) | 一种聚合物基导电复合材料及电路保护元件 | |
| CN105427976A (zh) | 具有电阻正温度效应的表面贴装型过电流保护元件及其制造方法 | |
| CN1278340C (zh) | 一种高分子ptc热敏电阻器及其制造方法 | |
| CN106898446A (zh) | 过电流保护元件 | |
| TW463443B (en) | A PTC circuit protection device | |
| CN104733144B (zh) | 表面贴装型电路保护元件及制造方法 | |
| CN118198983A (zh) | 集成过电流保护装置 | |
| JP2002124402A (ja) | Ptc素子及びその製造方法 | |
| US20030020591A1 (en) | Electrical device having ptc conductive polymer | |
| CN1988063A (zh) | 一种快速动作高分子ptc热敏电阻器及其制造方法 | |
| JP2001110603A (ja) | Ptc素子及びその製造方法 | |
| JP3609573B2 (ja) | 有機ptc組成物 | |
| WO2001078453A1 (fr) | Dispositif electrique presentant un polymere conducteur ctp |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PUAI | Public reference made under article 153(3) epc to a published international application that has entered the european phase |
Free format text: ORIGINAL CODE: 0009012 |
|
| 17P | Request for examination filed |
Effective date: 20010316 |
|
| AK | Designated contracting states |
Kind code of ref document: A1 Designated state(s): DE FR GB SE |
|
| AX | Request for extension of the european patent |
Free format text: AL;LT;LV;MK;RO;SI |
|
| A4 | Supplementary search report drawn up and despatched |
Effective date: 20011127 |
|
| AK | Designated contracting states |
Kind code of ref document: A4 Designated state(s): DE FR GB SE |
|
| 17Q | First examination report despatched |
Effective date: 20020923 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: THE APPLICATION IS DEEMED TO BE WITHDRAWN |
|
| 18D | Application deemed to be withdrawn |
Effective date: 20030404 |