EP0761748B1 - Polysiloxan-enthaltende Kautschukzusammensetzung - Google Patents
Polysiloxan-enthaltende Kautschukzusammensetzung Download PDFInfo
- Publication number
- EP0761748B1 EP0761748B1 EP96113916A EP96113916A EP0761748B1 EP 0761748 B1 EP0761748 B1 EP 0761748B1 EP 96113916 A EP96113916 A EP 96113916A EP 96113916 A EP96113916 A EP 96113916A EP 0761748 B1 EP0761748 B1 EP 0761748B1
- Authority
- EP
- European Patent Office
- Prior art keywords
- polysiloxane
- weight
- group
- silica
- rubber composition
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Expired - Lifetime
Links
- -1 Polysiloxane Polymers 0.000 title claims description 181
- 229920001296 polysiloxane Polymers 0.000 title claims description 167
- 239000000203 mixture Substances 0.000 title claims description 145
- 229920001971 elastomer Polymers 0.000 title claims description 123
- 239000005060 rubber Substances 0.000 title claims description 123
- VYPSYNLAJGMNEJ-UHFFFAOYSA-N Silicium dioxide Chemical compound O=[Si]=O VYPSYNLAJGMNEJ-UHFFFAOYSA-N 0.000 claims description 211
- 239000000377 silicon dioxide Substances 0.000 claims description 99
- 239000006087 Silane Coupling Agent Substances 0.000 claims description 78
- 229910044991 metal oxide Inorganic materials 0.000 claims description 40
- 150000004706 metal oxides Chemical class 0.000 claims description 40
- 238000004073 vulcanization Methods 0.000 claims description 37
- 239000006229 carbon black Substances 0.000 claims description 31
- 239000000945 filler Substances 0.000 claims description 23
- 238000002156 mixing Methods 0.000 claims description 23
- 125000005372 silanol group Chemical group 0.000 claims description 21
- 239000003054 catalyst Substances 0.000 claims description 18
- 239000003795 chemical substances by application Substances 0.000 claims description 18
- 239000000843 powder Substances 0.000 claims description 17
- VTYYLEPIZMXCLO-UHFFFAOYSA-L Calcium carbonate Chemical compound [Ca+2].[O-]C([O-])=O VTYYLEPIZMXCLO-UHFFFAOYSA-L 0.000 claims description 14
- 235000021355 Stearic acid Nutrition 0.000 claims description 12
- QIQXTHQIDYTFRH-UHFFFAOYSA-N octadecanoic acid Chemical compound CCCCCCCCCCCCCCCCCC(O)=O QIQXTHQIDYTFRH-UHFFFAOYSA-N 0.000 claims description 12
- OQCDKBAXFALNLD-UHFFFAOYSA-N octadecanoic acid Natural products CCCCCCCC(C)CCCCCCCCC(O)=O OQCDKBAXFALNLD-UHFFFAOYSA-N 0.000 claims description 12
- 238000010058 rubber compounding Methods 0.000 claims description 12
- 239000008117 stearic acid Substances 0.000 claims description 12
- 239000010936 titanium Substances 0.000 claims description 12
- 229910052719 titanium Inorganic materials 0.000 claims description 12
- RTAQQCXQSZGOHL-UHFFFAOYSA-N Titanium Chemical compound [Ti] RTAQQCXQSZGOHL-UHFFFAOYSA-N 0.000 claims description 11
- 229920003244 diene elastomer Polymers 0.000 claims description 9
- 125000002496 methyl group Chemical group [H]C([H])([H])* 0.000 claims description 9
- 125000000962 organic group Chemical group 0.000 claims description 9
- 229910052739 hydrogen Inorganic materials 0.000 claims description 8
- 239000001257 hydrogen Substances 0.000 claims description 8
- UFHFLCQGNIYNRP-UHFFFAOYSA-N Hydrogen Chemical compound [H][H] UFHFLCQGNIYNRP-UHFFFAOYSA-N 0.000 claims description 7
- 229910000019 calcium carbonate Inorganic materials 0.000 claims description 7
- 238000004381 surface treatment Methods 0.000 claims description 7
- 125000001495 ethyl group Chemical group [H]C([H])([H])C([H])([H])* 0.000 claims description 6
- 125000001997 phenyl group Chemical group [H]C1=C([H])C([H])=C(*)C([H])=C1[H] 0.000 claims description 6
- 125000000217 alkyl group Chemical group 0.000 claims description 5
- 229910052681 coesite Inorganic materials 0.000 claims description 4
- 229910052906 cristobalite Inorganic materials 0.000 claims description 4
- 229910052682 stishovite Inorganic materials 0.000 claims description 4
- 229910052905 tridymite Inorganic materials 0.000 claims description 4
- 229910000077 silane Inorganic materials 0.000 claims description 3
- BLRPTPMANUNPDV-UHFFFAOYSA-N Silane Chemical compound [SiH4] BLRPTPMANUNPDV-UHFFFAOYSA-N 0.000 claims description 2
- 239000007822 coupling agent Substances 0.000 claims description 2
- 229910020175 SiOH Inorganic materials 0.000 claims 4
- XUIMIQQOPSSXEZ-UHFFFAOYSA-N Silicon Chemical group [Si] XUIMIQQOPSSXEZ-UHFFFAOYSA-N 0.000 claims 1
- 229910052710 silicon Inorganic materials 0.000 claims 1
- 239000010703 silicon Substances 0.000 claims 1
- KFZMGEQAYNKOFK-UHFFFAOYSA-N Isopropanol Chemical compound CC(C)O KFZMGEQAYNKOFK-UHFFFAOYSA-N 0.000 description 36
- 230000000704 physical effect Effects 0.000 description 34
- 238000006243 chemical reaction Methods 0.000 description 29
- 238000005299 abrasion Methods 0.000 description 27
- 239000000126 substance Substances 0.000 description 26
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 description 25
- OKTJSMMVPCPJKN-UHFFFAOYSA-N Carbon Chemical compound [C] OKTJSMMVPCPJKN-UHFFFAOYSA-N 0.000 description 20
- NINIDFKCEFEMDL-UHFFFAOYSA-N Sulfur Chemical compound [S] NINIDFKCEFEMDL-UHFFFAOYSA-N 0.000 description 20
- 229910052717 sulfur Inorganic materials 0.000 description 20
- 239000011593 sulfur Substances 0.000 description 20
- MTHSVFCYNBDYFN-UHFFFAOYSA-N diethylene glycol Chemical compound OCCOCCO MTHSVFCYNBDYFN-UHFFFAOYSA-N 0.000 description 18
- 229910052799 carbon Inorganic materials 0.000 description 17
- 238000011156 evaluation Methods 0.000 description 17
- XLOMVQKBTHCTTD-UHFFFAOYSA-N Zinc monoxide Chemical compound [Zn]=O XLOMVQKBTHCTTD-UHFFFAOYSA-N 0.000 description 16
- 229920003048 styrene butadiene rubber Polymers 0.000 description 16
- 239000002174 Styrene-butadiene Substances 0.000 description 15
- 230000000052 comparative effect Effects 0.000 description 15
- 239000002253 acid Substances 0.000 description 14
- 238000009472 formulation Methods 0.000 description 13
- 244000043261 Hevea brasiliensis Species 0.000 description 12
- 229920003052 natural elastomer Polymers 0.000 description 12
- 229920001194 natural rubber Polymers 0.000 description 12
- 230000009467 reduction Effects 0.000 description 12
- 230000000694 effects Effects 0.000 description 10
- DEQZTKGFXNUBJL-UHFFFAOYSA-N n-(1,3-benzothiazol-2-ylsulfanyl)cyclohexanamine Chemical compound C1CCCCC1NSC1=NC2=CC=CC=C2S1 DEQZTKGFXNUBJL-UHFFFAOYSA-N 0.000 description 10
- 239000011787 zinc oxide Substances 0.000 description 10
- 235000014692 zinc oxide Nutrition 0.000 description 10
- OKKJLVBELUTLKV-UHFFFAOYSA-N Methanol Chemical compound OC OKKJLVBELUTLKV-UHFFFAOYSA-N 0.000 description 9
- 239000004594 Masterbatch (MB) Substances 0.000 description 8
- 125000005370 alkoxysilyl group Chemical group 0.000 description 8
- 239000004615 ingredient Substances 0.000 description 8
- 238000000034 method Methods 0.000 description 8
- 229920000459 Nitrile rubber Polymers 0.000 description 7
- 239000003963 antioxidant agent Substances 0.000 description 7
- 230000003078 antioxidant effect Effects 0.000 description 7
- 239000004927 clay Substances 0.000 description 7
- 238000004898 kneading Methods 0.000 description 7
- 239000003921 oil Substances 0.000 description 7
- 238000012360 testing method Methods 0.000 description 7
- CSCPPACGZOOCGX-UHFFFAOYSA-N Acetone Chemical compound CC(C)=O CSCPPACGZOOCGX-UHFFFAOYSA-N 0.000 description 6
- 125000000524 functional group Chemical group 0.000 description 6
- 238000004519 manufacturing process Methods 0.000 description 6
- 239000005062 Polybutadiene Substances 0.000 description 5
- 239000000654 additive Substances 0.000 description 5
- 150000001875 compounds Chemical class 0.000 description 5
- QUPDWYMUPZLYJZ-UHFFFAOYSA-N ethyl Chemical compound C[CH2] QUPDWYMUPZLYJZ-UHFFFAOYSA-N 0.000 description 5
- 230000003014 reinforcing effect Effects 0.000 description 5
- 239000000454 talc Substances 0.000 description 5
- 229910052623 talc Inorganic materials 0.000 description 5
- MYRTYDVEIRVNKP-UHFFFAOYSA-N 1,2-Divinylbenzene Chemical group C=CC1=CC=CC=C1C=C MYRTYDVEIRVNKP-UHFFFAOYSA-N 0.000 description 4
- PPBRXRYQALVLMV-UHFFFAOYSA-N Styrene Chemical compound C=CC1=CC=CC=C1 PPBRXRYQALVLMV-UHFFFAOYSA-N 0.000 description 4
- KPUWHANPEXNPJT-UHFFFAOYSA-N disiloxane Chemical class [SiH3]O[SiH3] KPUWHANPEXNPJT-UHFFFAOYSA-N 0.000 description 4
- 239000006185 dispersion Substances 0.000 description 4
- 238000001125 extrusion Methods 0.000 description 4
- XMGQYMWWDOXHJM-UHFFFAOYSA-N limonene Chemical compound CC(=C)C1CCC(C)=CC1 XMGQYMWWDOXHJM-UHFFFAOYSA-N 0.000 description 4
- 230000001050 lubricating effect Effects 0.000 description 4
- 239000002075 main ingredient Substances 0.000 description 4
- 229920000642 polymer Polymers 0.000 description 4
- 238000006116 polymerization reaction Methods 0.000 description 4
- 238000002360 preparation method Methods 0.000 description 4
- 238000010998 test method Methods 0.000 description 4
- RIOQSEWOXXDEQQ-UHFFFAOYSA-N triphenylphosphine Chemical compound C1=CC=CC=C1P(C=1C=CC=CC=1)C1=CC=CC=C1 RIOQSEWOXXDEQQ-UHFFFAOYSA-N 0.000 description 4
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 4
- OWRCNXZUPFZXOS-UHFFFAOYSA-N 1,3-diphenylguanidine Chemical compound C=1C=CC=CC=1NC(=N)NC1=CC=CC=C1 OWRCNXZUPFZXOS-UHFFFAOYSA-N 0.000 description 3
- ZZMVLMVFYMGSMY-UHFFFAOYSA-N 4-n-(4-methylpentan-2-yl)-1-n-phenylbenzene-1,4-diamine Chemical compound C1=CC(NC(C)CC(C)C)=CC=C1NC1=CC=CC=C1 ZZMVLMVFYMGSMY-UHFFFAOYSA-N 0.000 description 3
- 0 CCC(CC)(N(*)O)OC Chemical compound CCC(CC)(N(*)O)OC 0.000 description 3
- 125000003277 amino group Chemical group 0.000 description 3
- 125000003178 carboxy group Chemical group [H]OC(*)=O 0.000 description 3
- 229910052570 clay Inorganic materials 0.000 description 3
- 238000013329 compounding Methods 0.000 description 3
- 238000004132 cross linking Methods 0.000 description 3
- 230000003247 decreasing effect Effects 0.000 description 3
- 238000001035 drying Methods 0.000 description 3
- 125000003700 epoxy group Chemical group 0.000 description 3
- 230000020169 heat generation Effects 0.000 description 3
- SCPYDCQAZCOKTP-UHFFFAOYSA-N silanol Chemical compound [SiH3]O SCPYDCQAZCOKTP-UHFFFAOYSA-N 0.000 description 3
- 229920002545 silicone oil Polymers 0.000 description 3
- 125000003396 thiol group Chemical group [H]S* 0.000 description 3
- VTHOKNTVYKTUPI-UHFFFAOYSA-N triethoxy-[3-(3-triethoxysilylpropyltetrasulfanyl)propyl]silane Chemical compound CCO[Si](OCC)(OCC)CCCSSSSCCC[Si](OCC)(OCC)OCC VTHOKNTVYKTUPI-UHFFFAOYSA-N 0.000 description 3
- 239000004636 vulcanized rubber Substances 0.000 description 3
- WYTZZXDRDKSJID-UHFFFAOYSA-N (3-aminopropyl)triethoxysilane Chemical compound CCO[Si](OCC)(OCC)CCCN WYTZZXDRDKSJID-UHFFFAOYSA-N 0.000 description 2
- KBPLFHHGFOOTCA-UHFFFAOYSA-N 1-Octanol Chemical compound CCCCCCCCO KBPLFHHGFOOTCA-UHFFFAOYSA-N 0.000 description 2
- BBMCTIGTTCKYKF-UHFFFAOYSA-N 1-heptanol Chemical compound CCCCCCCO BBMCTIGTTCKYKF-UHFFFAOYSA-N 0.000 description 2
- 239000005995 Aluminium silicate Substances 0.000 description 2
- IJGRMHOSHXDMSA-UHFFFAOYSA-N Atomic nitrogen Chemical compound N#N IJGRMHOSHXDMSA-UHFFFAOYSA-N 0.000 description 2
- OYPRJOBELJOOCE-UHFFFAOYSA-N Calcium Chemical compound [Ca] OYPRJOBELJOOCE-UHFFFAOYSA-N 0.000 description 2
- UQSXHKLRYXJYBZ-UHFFFAOYSA-N Iron oxide Chemical compound [Fe]=O UQSXHKLRYXJYBZ-UHFFFAOYSA-N 0.000 description 2
- LRHPLDYGYMQRHN-UHFFFAOYSA-N N-Butanol Chemical compound CCCCO LRHPLDYGYMQRHN-UHFFFAOYSA-N 0.000 description 2
- AMQJEAYHLZJPGS-UHFFFAOYSA-N N-Pentanol Chemical compound CCCCCO AMQJEAYHLZJPGS-UHFFFAOYSA-N 0.000 description 2
- ISWSIDIOOBJBQZ-UHFFFAOYSA-N Phenol Chemical compound OC1=CC=CC=C1 ISWSIDIOOBJBQZ-UHFFFAOYSA-N 0.000 description 2
- GWEVSGVZZGPLCZ-UHFFFAOYSA-N Titan oxide Chemical compound O=[Ti]=O GWEVSGVZZGPLCZ-UHFFFAOYSA-N 0.000 description 2
- YKTSYUJCYHOUJP-UHFFFAOYSA-N [O--].[Al+3].[Al+3].[O-][Si]([O-])([O-])[O-] Chemical compound [O--].[Al+3].[Al+3].[O-][Si]([O-])([O-])[O-] YKTSYUJCYHOUJP-UHFFFAOYSA-N 0.000 description 2
- 235000012211 aluminium silicate Nutrition 0.000 description 2
- 239000010692 aromatic oil Substances 0.000 description 2
- TZCXTZWJZNENPQ-UHFFFAOYSA-L barium sulfate Chemical compound [Ba+2].[O-]S([O-])(=O)=O TZCXTZWJZNENPQ-UHFFFAOYSA-L 0.000 description 2
- 230000015572 biosynthetic process Effects 0.000 description 2
- 229920005549 butyl rubber Polymers 0.000 description 2
- 229910052791 calcium Inorganic materials 0.000 description 2
- 239000011575 calcium Substances 0.000 description 2
- 239000000378 calcium silicate Substances 0.000 description 2
- 229910052918 calcium silicate Inorganic materials 0.000 description 2
- OSGAYBCDTDRGGQ-UHFFFAOYSA-L calcium sulfate Chemical compound [Ca+2].[O-]S([O-])(=O)=O OSGAYBCDTDRGGQ-UHFFFAOYSA-L 0.000 description 2
- OYACROKNLOSFPA-UHFFFAOYSA-N calcium;dioxido(oxo)silane Chemical compound [Ca+2].[O-][Si]([O-])=O OYACROKNLOSFPA-UHFFFAOYSA-N 0.000 description 2
- 150000001732 carboxylic acid derivatives Chemical class 0.000 description 2
- 239000002131 composite material Substances 0.000 description 2
- 229920001577 copolymer Polymers 0.000 description 2
- NKSJNEHGWDZZQF-UHFFFAOYSA-N ethenyl(trimethoxy)silane Chemical compound CO[Si](OC)(OC)C=C NKSJNEHGWDZZQF-UHFFFAOYSA-N 0.000 description 2
- 238000007429 general method Methods 0.000 description 2
- 238000010438 heat treatment Methods 0.000 description 2
- NLYAJNPCOHFWQQ-UHFFFAOYSA-N kaolin Chemical compound O.O.O=[Al]O[Si](=O)O[Si](=O)O[Al]=O NLYAJNPCOHFWQQ-UHFFFAOYSA-N 0.000 description 2
- 238000009940 knitting Methods 0.000 description 2
- 229940087305 limonene Drugs 0.000 description 2
- 235000001510 limonene Nutrition 0.000 description 2
- 125000000396 limonene group Chemical group 0.000 description 2
- 229910052751 metal Inorganic materials 0.000 description 2
- 239000002184 metal Substances 0.000 description 2
- GLDOVTGHNKAZLK-UHFFFAOYSA-N octadecan-1-ol Chemical compound CCCCCCCCCCCCCCCCCCO GLDOVTGHNKAZLK-UHFFFAOYSA-N 0.000 description 2
- 239000011368 organic material Substances 0.000 description 2
- 230000009257 reactivity Effects 0.000 description 2
- 239000012744 reinforcing agent Substances 0.000 description 2
- 239000007787 solid Substances 0.000 description 2
- 239000007858 starting material Substances 0.000 description 2
- 125000003011 styrenyl group Chemical group [H]\C(*)=C(/[H])C1=C([H])C([H])=C([H])C([H])=C1[H] 0.000 description 2
- 239000012756 surface treatment agent Substances 0.000 description 2
- 238000003786 synthesis reaction Methods 0.000 description 2
- LFQCEHFDDXELDD-UHFFFAOYSA-N tetramethyl orthosilicate Chemical compound CO[Si](OC)(OC)OC LFQCEHFDDXELDD-UHFFFAOYSA-N 0.000 description 2
- 150000003609 titanium compounds Chemical class 0.000 description 2
- OGIDPMRJRNCKJF-UHFFFAOYSA-N titanium oxide Inorganic materials [Ti]=O OGIDPMRJRNCKJF-UHFFFAOYSA-N 0.000 description 2
- UEXTVLKDFZEPMH-PAEMJXPASA-N (4r)-4-[(3r,5s,6r,7r,8s,9s,10s,11s,13r,14s,17r)-6-ethyl-3,7,11-trihydroxy-10,13-dimethyl-2,3,4,5,6,7,8,9,11,12,14,15,16,17-tetradecahydro-1h-cyclopenta[a]phenanthren-17-yl]pentanoic acid Chemical compound C([C@@]12C)C[C@@H](O)C[C@H]1[C@@H](CC)[C@@H](O)[C@@H]1[C@@H]2[C@@H](O)C[C@]2(C)[C@@H]([C@H](C)CCC(O)=O)CC[C@H]21 UEXTVLKDFZEPMH-PAEMJXPASA-N 0.000 description 1
- ODIGIKRIUKFKHP-UHFFFAOYSA-N (n-propan-2-yloxycarbonylanilino) acetate Chemical compound CC(C)OC(=O)N(OC(C)=O)C1=CC=CC=C1 ODIGIKRIUKFKHP-UHFFFAOYSA-N 0.000 description 1
- SDRZFSPCVYEJTP-UHFFFAOYSA-N 1-ethenylcyclohexene Chemical compound C=CC1=CCCCC1 SDRZFSPCVYEJTP-UHFFFAOYSA-N 0.000 description 1
- SBASXUCJHJRPEV-UHFFFAOYSA-N 2-(2-methoxyethoxy)ethanol Chemical compound COCCOCCO SBASXUCJHJRPEV-UHFFFAOYSA-N 0.000 description 1
- XNWFRZJHXBZDAG-UHFFFAOYSA-N 2-METHOXYETHANOL Chemical compound COCCO XNWFRZJHXBZDAG-UHFFFAOYSA-N 0.000 description 1
- 229920002943 EPDM rubber Polymers 0.000 description 1
- RRHGJUQNOFWUDK-UHFFFAOYSA-N Isoprene Chemical group CC(=C)C=C RRHGJUQNOFWUDK-UHFFFAOYSA-N 0.000 description 1
- 229910002666 PdCl2 Inorganic materials 0.000 description 1
- 229910008051 Si-OH Inorganic materials 0.000 description 1
- 229910006358 Si—OH Inorganic materials 0.000 description 1
- 239000004902 Softening Agent Substances 0.000 description 1
- 229910021536 Zeolite Inorganic materials 0.000 description 1
- 239000000853 adhesive Substances 0.000 description 1
- 230000001070 adhesive effect Effects 0.000 description 1
- 150000001298 alcohols Chemical class 0.000 description 1
- XYLMUPLGERFSHI-UHFFFAOYSA-N alpha-Methylstyrene Chemical compound CC(=C)C1=CC=CC=C1 XYLMUPLGERFSHI-UHFFFAOYSA-N 0.000 description 1
- PNEYBMLMFCGWSK-UHFFFAOYSA-N aluminium oxide Inorganic materials [O-2].[O-2].[O-2].[Al+3].[Al+3] PNEYBMLMFCGWSK-UHFFFAOYSA-N 0.000 description 1
- DIZPMCHEQGEION-UHFFFAOYSA-H aluminium sulfate (anhydrous) Chemical compound [Al+3].[Al+3].[O-]S([O-])(=O)=O.[O-]S([O-])(=O)=O.[O-]S([O-])(=O)=O DIZPMCHEQGEION-UHFFFAOYSA-H 0.000 description 1
- 230000003712 anti-aging effect Effects 0.000 description 1
- 125000003118 aryl group Chemical group 0.000 description 1
- 239000000440 bentonite Substances 0.000 description 1
- 229910000278 bentonite Inorganic materials 0.000 description 1
- KAKZBPTYRLMSJV-UHFFFAOYSA-N butadiene group Chemical group C=CC=C KAKZBPTYRLMSJV-UHFFFAOYSA-N 0.000 description 1
- 229910000420 cerium oxide Inorganic materials 0.000 description 1
- 239000013522 chelant Substances 0.000 description 1
- 239000008199 coating composition Substances 0.000 description 1
- 230000002860 competitive effect Effects 0.000 description 1
- 239000002537 cosmetic Substances 0.000 description 1
- 230000008878 coupling Effects 0.000 description 1
- 238000010168 coupling process Methods 0.000 description 1
- 238000005859 coupling reaction Methods 0.000 description 1
- 239000003431 cross linking reagent Substances 0.000 description 1
- 230000007423 decrease Effects 0.000 description 1
- 230000003111 delayed effect Effects 0.000 description 1
- 235000014113 dietary fatty acids Nutrition 0.000 description 1
- IJKVHSBPTUYDLN-UHFFFAOYSA-N dihydroxy(oxo)silane Chemical compound O[Si](O)=O IJKVHSBPTUYDLN-UHFFFAOYSA-N 0.000 description 1
- FSBVERYRVPGNGG-UHFFFAOYSA-N dimagnesium dioxido-bis[[oxido(oxo)silyl]oxy]silane hydrate Chemical compound O.[Mg+2].[Mg+2].[O-][Si](=O)O[Si]([O-])([O-])O[Si]([O-])=O FSBVERYRVPGNGG-UHFFFAOYSA-N 0.000 description 1
- HNPSIPDUKPIQMN-UHFFFAOYSA-N dioxosilane;oxo(oxoalumanyloxy)alumane Chemical compound O=[Si]=O.O=[Al]O[Al]=O HNPSIPDUKPIQMN-UHFFFAOYSA-N 0.000 description 1
- 238000005516 engineering process Methods 0.000 description 1
- 239000000194 fatty acid Substances 0.000 description 1
- 229930195729 fatty acid Natural products 0.000 description 1
- 150000004665 fatty acids Chemical class 0.000 description 1
- 239000010433 feldspar Substances 0.000 description 1
- 239000006260 foam Substances 0.000 description 1
- 125000004435 hydrogen atom Chemical group [H]* 0.000 description 1
- 230000002209 hydrophobic effect Effects 0.000 description 1
- 238000005470 impregnation Methods 0.000 description 1
- 239000011256 inorganic filler Substances 0.000 description 1
- 229910003475 inorganic filler Inorganic materials 0.000 description 1
- 125000001449 isopropyl group Chemical group [H]C([H])([H])C([H])(*)C([H])([H])[H] 0.000 description 1
- 229910052622 kaolinite Inorganic materials 0.000 description 1
- 239000000391 magnesium silicate Substances 0.000 description 1
- 229910052919 magnesium silicate Inorganic materials 0.000 description 1
- 235000019792 magnesium silicate Nutrition 0.000 description 1
- 239000010445 mica Substances 0.000 description 1
- 229910052618 mica group Inorganic materials 0.000 description 1
- 229910052757 nitrogen Inorganic materials 0.000 description 1
- 230000003287 optical effect Effects 0.000 description 1
- 150000002894 organic compounds Chemical class 0.000 description 1
- TWNQGVIAIRXVLR-UHFFFAOYSA-N oxo(oxoalumanyloxy)alumane Chemical compound O=[Al]O[Al]=O TWNQGVIAIRXVLR-UHFFFAOYSA-N 0.000 description 1
- BMMGVYCKOGBVEV-UHFFFAOYSA-N oxo(oxoceriooxy)cerium Chemical compound [Ce]=O.O=[Ce]=O BMMGVYCKOGBVEV-UHFFFAOYSA-N 0.000 description 1
- 125000004430 oxygen atom Chemical group O* 0.000 description 1
- RVTZCBVAJQQJTK-UHFFFAOYSA-N oxygen(2-);zirconium(4+) Chemical compound [O-2].[O-2].[Zr+4] RVTZCBVAJQQJTK-UHFFFAOYSA-N 0.000 description 1
- PIBWKRNGBLPSSY-UHFFFAOYSA-L palladium(II) chloride Chemical compound Cl[Pd]Cl PIBWKRNGBLPSSY-UHFFFAOYSA-L 0.000 description 1
- 150000002978 peroxides Chemical class 0.000 description 1
- WVDDGKGOMKODPV-ZQBYOMGUSA-N phenyl(114C)methanol Chemical compound O[14CH2]C1=CC=CC=C1 WVDDGKGOMKODPV-ZQBYOMGUSA-N 0.000 description 1
- 125000000286 phenylethyl group Chemical group [H]C1=C([H])C([H])=C(C([H])=C1[H])C([H])([H])C([H])([H])* 0.000 description 1
- 239000004014 plasticizer Substances 0.000 description 1
- 229920002857 polybutadiene Polymers 0.000 description 1
- 229920001195 polyisoprene Polymers 0.000 description 1
- 230000002265 prevention Effects 0.000 description 1
- 239000010734 process oil Substances 0.000 description 1
- FZYCEURIEDTWNS-UHFFFAOYSA-N prop-1-en-2-ylbenzene Chemical compound CC(=C)C1=CC=CC=C1.CC(=C)C1=CC=CC=C1 FZYCEURIEDTWNS-UHFFFAOYSA-N 0.000 description 1
- BDERNNFJNOPAEC-UHFFFAOYSA-N propan-1-ol Chemical compound CCCO BDERNNFJNOPAEC-UHFFFAOYSA-N 0.000 description 1
- 230000002787 reinforcement Effects 0.000 description 1
- 239000011369 resultant mixture Substances 0.000 description 1
- 238000005096 rolling process Methods 0.000 description 1
- 150000003839 salts Chemical class 0.000 description 1
- 150000003377 silicon compounds Chemical class 0.000 description 1
- 229910052814 silicon oxide Inorganic materials 0.000 description 1
- 239000002904 solvent Substances 0.000 description 1
- 238000001179 sorption measurement Methods 0.000 description 1
- 239000000057 synthetic resin Substances 0.000 description 1
- 229920003002 synthetic resin Polymers 0.000 description 1
- 150000003606 tin compounds Chemical class 0.000 description 1
- 125000000026 trimethylsilyl group Chemical group [H]C([H])([H])[Si]([*])(C([H])([H])[H])C([H])([H])[H] 0.000 description 1
- 239000010457 zeolite Substances 0.000 description 1
- 229910001928 zirconium oxide Inorganic materials 0.000 description 1
Classifications
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08K—Use of inorganic or non-macromolecular organic substances as compounding ingredients
- C08K9/00—Use of pretreated ingredients
- C08K9/04—Ingredients treated with organic substances
- C08K9/06—Ingredients treated with organic substances with silicon-containing compounds
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08K—Use of inorganic or non-macromolecular organic substances as compounding ingredients
- C08K9/00—Use of pretreated ingredients
- C08K9/08—Ingredients agglomerated by treatment with a binding agent
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08L—COMPOSITIONS OF MACROMOLECULAR COMPOUNDS
- C08L21/00—Compositions of unspecified rubbers
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08L—COMPOSITIONS OF MACROMOLECULAR COMPOUNDS
- C08L7/00—Compositions of natural rubber
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08L—COMPOSITIONS OF MACROMOLECULAR COMPOUNDS
- C08L9/00—Compositions of homopolymers or copolymers of conjugated diene hydrocarbons
- C08L9/06—Copolymers with styrene
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y10—TECHNICAL SUBJECTS COVERED BY FORMER USPC
- Y10T—TECHNICAL SUBJECTS COVERED BY FORMER US CLASSIFICATION
- Y10T428/00—Stock material or miscellaneous articles
- Y10T428/29—Coated or structually defined flake, particle, cell, strand, strand portion, rod, filament, macroscopic fiber or mass thereof
- Y10T428/2982—Particulate matter [e.g., sphere, flake, etc.]
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y10—TECHNICAL SUBJECTS COVERED BY FORMER USPC
- Y10T—TECHNICAL SUBJECTS COVERED BY FORMER US CLASSIFICATION
- Y10T428/00—Stock material or miscellaneous articles
- Y10T428/29—Coated or structually defined flake, particle, cell, strand, strand portion, rod, filament, macroscopic fiber or mass thereof
- Y10T428/2982—Particulate matter [e.g., sphere, flake, etc.]
- Y10T428/2991—Coated
- Y10T428/2998—Coated including synthetic resin or polymer
Definitions
- the present invention relates to a polysiloxane-containing rubber composition having improved properties and a rubber compounding agent therefor.
- Rubber compositions comprising various rubbers and a filler such as silica are known in the art and are used, for example, as rubber compositions for tire treads having a low heat generation characteristic and superior in abrasion resistance etc.
- tire treads in which silica is formulated have a low rolling resistance and a good grip on wet roads, but there were the problems that the viscosity of the unvulcanized compound increases, the vulcanization is slowed, the kneading performance in the mixing falls, etc. and the productivity deteriorates.
- Various proposals have been made in the past to solve these problems, but unfortunately these are not practically satisfactory.
- JP-A-8-48910 discloses a method for treating a metal oxide with methyl hydrogen polysiloxane, in which the metal oxides are necessary for treating at a high temperature.
- JP-A-8-53630 discloses the treatment with an alkoxy silane, but this is not still sufficient.
- rubber compositions comprising various rubbers and metal oxides such as silica, clay, talc are known and are used as, for example, rubber compositions for tire tread having excellent low heat build-up and abrasion resistance.
- metal oxides when metal oxides are compounded, there are disadvantages, when compared in the case of carbon black, the modulus is decreased and the abrasion resistance is low.
- JP-A-6-248116 has been proposed.
- silica is surface-treated under heating with, for example, a hydrophobic agent such as silicone oil comprising an organic silicon compound at, for example, 250°C for 1 hour.
- this method has a disadvantage of a short scorching due to heating at a high temperature.
- the objects of the present invention are to eliminate the above-mentioned disadvantageous of the prior art and to provide a filler-containing vulcanizing rubber composition which improves the processability of the unvulcanized rubber composition, without substantially impairing the properties of the filler-containing vulcanized rubber composition such as the low heat generation characteristic and the abrasion resistance.
- Another object of the present invention is to provide a rubber compounding agent improving the vulcanized physical properties of a silica-containing vulcanizing rubber composition, in particular the modulus, abrasion resistance, and tan ⁇ balance etc. and a silica-containing vulcanizing rubber composition using the same.
- a further object of the present invention is to provide a surface-treated metal oxide capable of increasing the reinforceability when compounding into rubber and of improving the modulus and the abrasion resistance of the rubber composition and a rubber composition containing the same.
- a still further object of the present invention is to provide a silica-containing vulcanizing rubber composition for a tire tread which improves the processability of the unvulcanized rubber composition, without substantially impairing the properties of the silica-containing vulcanizing rubber composition, for example, the low heat generation characteristic and the abrasion resistance.
- a rubber composition comprising a filler, said rubber composition containing a polysiloxane having a repeating unit of the formula (III) as defined in claim 1 and having a number average molecular weight of up to 100,000.
- a rubber composition comprising 100 parts by weight of a starting rubber, 5 to 100 parts by weight of silica, and an amount of the above-mentioned rubber compounding agent to give a content of the polysiloxane contained therein of 0.2 to 30% by weight in the total composition.
- a polysiloxane surface-treated metal oxide comprising 100 parts by weight of a metal oxide surface treated with 0.1 - 50 parts by weight of a polysiloxane having a repeating unit of the formula (III) as defined in claim 8 and having a number average molecular weight of up to 100,000.
- a rubber composition comprising 100 parts by weight of a starting rubber and 5 to 100 parts by weight of the above-mentioned polysiloxane surface-treated metal oxide.
- a rubber composition for a pneumatic tire tread comprising 100 parts by weight of a diene rubber, 2 to 80 parts by weight of carbon black, 5 to 80 parts by weight of silica, a silane coupling agent, and a polysiloxane having a repeating unit of the formula (III) as defined in claim 13 and having an average molecular weight of up to 100,000.
- filler used herein means any conventional inorganic fillers (e.g., calcium carbonate, clay, talc, diatomaceous silica, mica, alumina, aluminum sulfate, barium sulfate, calcium sulfate, etc.) and so-called reinforcing agents, (e.g., carbon black, silica, etc.).
- sica used herein means wet silica and dry silica and silica having a nitrogen specific surface area of 50 to 400 m 2 /g. The use of the wet silica is preferable.
- vulcanization used herein includes cross-linking by sulfur, peroxide, etc. in addition to the usual vulcanization by sulfur.
- the present invention provides a rubber composition comprising a filler, said rubber composition containing a polysiloxane as defined above.
- a rubber composition further containing in the rubber composition a silane coupling agent in an amount of 40% by weight or less of the silica content.
- silane coupling agents can be jointly used in a silica-containing rubber composition to reinforce the rubber, but silanol groups are also present inside the silica particles and there was the problem that these would react with the silane coupling agent to cause a loss of the silane coupling agent and reduce the reinforcing effect, making inclusion of a large amount of a silane coupling agent necessary.
- diethylene glycol or other polarized substance is added to this, it is possible to prevent to a certain extent the phenomenon of adsorption of the vulcanization accelerator or other polarized compounding agents, but complete prevention is not possible and it was not possible to prevent the substances chemically bonding with a silane coupling agent or other silica particles from bonding inside.
- the alkoxysilyl groups react with the silanol groups and covers the surface of the silica particles, and therefore, the problems in the prior art are solved and it is possible to effectively suppress the rise in viscosity caused by the cohesiveness and polarity of the silanol groups and the wasted consumption of the vulcanization accelerator or other polarized additives or silane coupling agent etc.
- the polysiloxane blended in the rubber composition according to the present invention must have an alkoxysilyl group reacting with a silanol group and be a polymer (or oligomer) of a size covering the surface of the silica particles and exhibit a lubricating effect, for example, an average degree of polymerization of 3 to 10,000, preferably 10 to 1,000 (or a number average molecular weight of 200 to 300,000, preferably 500 to 50,000).
- the polysiloxane is a known substance.
- it may be manufactured as follows:
- the polysiloxane containing an alkoxysilyl group is synthesized by causing a reaction between an Si-H group containing polysiloxane and alcohol in the presence of a catalyst.
- methanol, ethanol, propanol, butanol, pentanol, heptanol, octanol, octadecanol, phenol, benzyl alcohol, and also ethylene glycol monomethyl ether, diethylene glycol monomethyl ether, and other alcohols having oxygen atoms may be illustrated.
- chloroplatinic acid platinum-ether complexes, platinum-olefin complexes, PdCl 2 (PPh 3 ) 2 , RhCl 2 (PPh 3 ) 2 , or basic catalysts may be used.
- the corresponding ⁇ Si-H group containing polysiloxane and alcohol are reacted in the presence of the catalyst for synthesis.
- introduction is easy by causing a reaction of ⁇ Si-H and an organic compound having a double bond.
- a compound having a double bond there are styrene, ⁇ -methylstyrene, ⁇ -methylstyrene dimer, limonene, vinylcyclohexene, etc.
- the polysiloxane used in the present invention may be synthesized by causing a reaction between a silanol terminal polysiloxane and an alkoxysilane in the presence of a bivalent tin compound or other catalyst.
- a silanol terminal polysiloxane examples include: wherein n is 1 to 2000
- alkoxysilane examples are the following alkoxysilanes. Further, the silane coupling agents shown in Table I are exemplified.
- the polysiloxane usable in the present invention may further be synthesized by a reaction between polysiloxane having a reactive functional group at its side chain or terminal and a silane coupling agent of Table I.
- Examples of the polysiloxane having a reactive functional group are an epoxy group, amine group, mercapto group, carboxyl group, etc.
- polysiloxane used in the present invention is not particularly limited in its terminal groups and side chains and is determined by the type of the starting material used during manufacture.
- the amount of the polysiloxane used in the present invention is preferably 100% by weight or less, preferably 1 to 100% by weight, particularly preferably 2 to 40% by weight, of the weight of the filler in the rubber composition.
- the content of the polysiloxane is too small, the desired effect cannot be obtained, while conversely if too great, substances not bonding with the filler (e.g., silica) will leak out from the vulcanized product in some cases, which is not desired.
- the amount of the filler to be kneaded preferably 5 to 150 parts by weight, more preferably 20 to 120 parts by weight, particularly preferably 50 to 90 parts by weight of the filler based upon 100 parts by weight of the rubber component can be used.
- the rubber contained as the main ingredient in the vulcanizable rubber composition according to the present invention may be any rubber generally contained in various rubber compositions in the past, for example, natural rubber (NR), polyisoprene rubber (IR), various styrene-butadiene copolymer rubbers (SBR), various polybutadiene rubbers (BR), acrylonitrile-butadiene copolymer rubbers (NBR), butyl rubber (IIR), and other diene rubbers or ethylene-propylene copolymer rubbers (EPR, EPDM) etc. alone or as any blends.
- natural rubber NR
- IR polyisoprene rubber
- SBR various styrene-butadiene copolymer rubbers
- BR polybutadiene rubbers
- BR acrylonitrile-butadiene copolymer rubbers
- IIR butyl rubber
- EPR ethylene-propylene copolymer rubbers
- the vulcanizable rubber composition according to a preferred mode of the present invention further contains a silane coupling agent.
- the silane coupling agent used in the present invention may be made any silane coupling agent used together with silica fillers in the past.
- the typical examples are shown in Table I. Of these, bis-[3-(triethoxysilica)-propyl]tetrasulfide and the silane coupling agents shown in Table I' below is most preferred from the viewpoint of the processability.
- a silane coupling agent When a silane coupling agent is mixed into the vulcanizing rubber composition according to the present invention, it is possible to reduce the amount of the silane coupling agent used compared with the past and it is possible to further improve the abrasion resistance.
- the preferable amount of the silane coupling agent used in the present invention is 40% by weight or less, preferably 0.5 to 40% by weight, particularly preferably 1 to 20% by weight, based upon the amount of the silica in the composition.
- the amount of the silane coupling agent is too small, the desired effects cannot be obtained, while conversely when too great, scorching will easily occur in the mixing or extrusion step, which is not desirable.
- a rubber composition comprising 100 parts by weight of the starting rubber, 5 to 100 parts by weight of silica, and an amount of the rubber compounding agent composed of (A) and (B) or (A) and (C) to give a content of the polysiloxane contained therein of 0.2 to 30% by weight in the total composition.
- the alkoxysilyl groups react with the silanol groups and cover the surface of the silica particles, and therefore, the problems in the prior art are solved and it is possible to effectively suppress the rise in viscosity caused by the cohesiveness and polarity of the silanol groups and the wasted consumption of the vulcanization accelerator or other polarized additives or silane coupling agent etc.
- the silane coupling agent for increasing the reinforcing nature of the silica and the polysiloxane for improving the processability of the silica both react with the silanol groups on the surface of the silica in competitive reactions.
- the physical properties of the rubber differed depending on the method of mixture (or order). That is, if the silica and polysiloxane react first, the reinforcing nature falls, which is not desirable. Therefore, in the present invention, the coupling agent and polysiloxane are mixed in advance into the rubber, so reaction of just the polysiloxane first is prevented or the polysiloxane is pre-impregnated into carbon black or another nonreactive filler (i.e., inert powder) or silica or another powder so as to delay the reaction with the silica and thereby prevent a decline in the vulcanized physical properties of the rubber.
- the coupling agent and polysiloxane are mixed in advance into the rubber, so reaction of just the polysiloxane first is prevented or the polysiloxane is pre-impregnated into carbon black or another nonreactive filler (i.e., inert powder) or silica or another powder so as to delay the reaction with the silica and
- the polysiloxane (A) blended in the rubber composition according to the present invention must have an alkoxysilyl group reacting with a silanol group and be a polymer (or oligomer) of a size covering the surface of the silica particles and exhibit a lubricating effect, for example, an average degree of polymerization of 3 to 10,000, preferably 10 to 1,000.
- the polysiloxane is a known substance, as mentioned above.
- the polysiloxane (A) used in the present invention may further be synthesized by a reaction between polysiloxane having a reactive functional group at its side chain or terminal and a silane coupling agent of Table I.
- a reaction between polysiloxane having a reactive functional group at its side chain or terminal and a silane coupling agent of Table I mention may be made of an epoxy group, amine group, mercapto group, carboxyl group, etc., also as mentioned above.
- polysiloxane (A) used in the present invention is not particularly limited in its terminal groups and side chains and is determined by the type of the feedstock used during manufacture.
- the polysiloxane (A) used in the present invention is mixed in to give 0.2 to 30% by weight, preferably 1.0 to 10% by weight, based upon the rubber composition. If the content of the polysiloxane (A) is too small, the desired effect cannot be obtained, while conversely if too great, substances not bonding with the silica will leak out from the vulcanized product in some cases, which is not desired.
- the rubber contained as the main ingredient in the vulcanizing rubber composition according to the present invention may be any rubber generally contained in various rubber compositions in the past, as mentioned above.
- the silane coupling agent (B) used in the vulcanizing rubber composition of the present invention may be made any silane coupling agent used together with silica fillers in the past.
- any agent shown in Table I may be mentioned.
- bis-[3-(triethoxysilica)-propyl]tetrasulfide and the silane coupling agents shown in Table I' below is most preferred from the viewpoint of the processability.
- a silane coupling agent (B) is mixed into the vulcanizing rubber composition according to the present invention, it is possible to reduce the amount of the silane coupling agent (B) used compared with the past and it is possible to further improve the abrasion resistance.
- the preferable amount of the silane coupling agent (B) used in the present invention is, in terms of a ratio (ratio by weight) of the polysiloxane (A) in the composition and the silane coupling agent (B) of 95/5 to 5/95, particularly preferably 60/40 to 80/20. If the amount of the silane coupling agent (B) is too small, the desired effects cannot be obtained, while conversely if too great, scorching will easily occur in the mixing or extrusion step, which is not desirable.
- the rubber composition of the second embodiment according to the second aspect of the present invention is composed of at least one powder (C) generally mixed with rubber compositions in the past mixed with the polysiloxane (A).
- the powder (C) are carbon black, calcium carbonate, stearic acid, and other inert powders, silica, etc.
- inert powder means a powder with a small reactivity with the polysiloxane (A) of the present invention.
- the amount of this inert powder (C) is, in terms of the ratio of weight of (A)/(C), 70/30 to 5/95, more preferably 60/40 to 30/70.
- the method of impregnation of the polysiloxane (A) to the powder (C) is not particularly limited, but use may be made of a mixer, kneader, mill, etc. usually used according to need.
- the silica-containing rubber composition according to the present invention is composed of 5 to 100 parts by weight, preferably 5 to 80 parts by weight, of rubber use silica based upon 100 parts by weight of the starting rubber and an amount of the rubber compounding agent (that is, the premix of the polysiloxane (A) and the silane coupling agent (B) or powder (C)) so as to give 0.2 to 30% by weight, preferably 1 to 10% by weight, of polysiloxane (A) in the total composition.
- the rubber compounding agent that is, the premix of the polysiloxane (A) and the silane coupling agent (B) or powder (C)
- the present invention provides a polysiloxane surface-treated metal oxide comprising 100 parts by weight of a metal oxide surface treated with 0.1 - 50 parts by weight of a polysiloxane as defined above.
- a polysiloxane surface-treated metal oxide wherein the metal oxide is SiO 2 or SiO 2 -containing metal oxide or wherein 0.05 to 50% by weight of a titanium catalyst, based upon the amount of the polysiloxane used, is further used during the surface treatment.
- a rubber composition comprising 100 parts by weight of a starting rubber and 5 to 100 parts by weight of the above-mentioned polysiloxane surface-treated metal oxide, preferably silica and optionally a silane coupling agent in an amount of 0.5 - 40% by weight of the amount of the silica is further contained in the composition.
- the polysiloxane used according to the present invention must have an alkoxysilyl group reacting with a silanol group and be a polymer (or oligomer) of a size covering the surface of the silica particles and exhibit a lubricating effect, for example, an average degree of polymerization of 3 to 10,000, preferably 10 to 1,000.
- the polysiloxane is a known substance and can be synthesized by the reaction between an Si-H group containing polysiloxane and alcohol in the presence of a catalyst, as mentioned above.
- the polysiloxane usable in the present invention may further be synthesized by a reaction between polysiloxane having a reactive functional group at its side chain or terminal and a silane coupling agent of Table I shown above.
- Examples of the polysiloxane having a reactive functional group are an epoxy group, amine group, mercapto group, carboxyl group, etc.
- the polysiloxane used in the present invention is not particularly limited in its terminal groups and side chains and is determined by the type of the starting material used during manufacture.
- the surface of the metal oxide is surface treated with the polysiloxane.
- the surface treating method is not specifically limited.
- the metal oxide can be impregnated or coated with the polysiloxane at room temperature in an appropriate solvent (e.g., acetone, methanol, ethanol), followed by heat drying at room temperature to 120°C.
- the amount of the polysiloxane to be used for the surface treatment according to the present invention is generally 0.1 to 50 parts by weight, preferably 1 to 20 parts by weight. If the amount of the polysiloxane is too small, the desired results cannot be obtained, while conversely it too large, the polysiloxane which does not react with the metal oxide is unpreferably exdudated from the vulcanized product.
- the desired reinforcing effects can be obtained.
- the amount is less than 5 parts by weight, the desired effects of the present invention are not sufficient, while more than 100 parts by weight, the processability unpreferably becomes poor.
- metal oxides according to the present invention are metal oxides comprising a single metal such as silicon oxide, titanium oxide, aluminum oxide, iron oxide, zirconium oxide, cerium oxide and complex or composite oxides such as calcium silicate, aluminum silicate, magnesium silicate, zeolite, feldspar, kaolinite, clay, talc.
- silicate fillers such as silicic anhydride, silicic hydrate, calcium silicate, aluminum silicate, kaolin, talc are preferable.
- the titanium catalysts usable in the preferable embodiment of the present invention are titanium compounds such as alkoxy titanium, titanium chelate, titanium acylate, complex or composite titanate.
- titanium compounds TA-10 and TC-100 available from Matsumoto Koushou K.K. Japan having the following structure can be effectively used.
- the above titanium catalyst is used in an amount of 0.05 to 50% by weight, more preferably 0.1 to 10% by weight, of the amount of the polysiloxane. If the amount is too small, the desired effect of the surface treatment cannot be efficiently obtained, while conversely too large, the processability tends to be decreased when the rubber composition is prepared.
- the rubber contained as the main ingredient in the vulcanizable rubber composition according to the present invention may be any conventional rubber as mentioned previously.
- the vulcanizable rubber composition according to a preferred mode of the present invention may further contains a silane coupling agent.
- the silane coupling agent usable in the present invention may be any silane coupling agent used together with silica fillers in the past.
- the typical examples, are shown in Table I. Further, the following special sulfur-containing silane coupling agents shown in Table I' below can also be used. Of these, bis-[3-(triethoxysilica)-propyljtetrasulfide is most preferred from the viewpoint of the processability.
- the preferable amount of the silane coupling agent usable in the present invention is 1 to 20% by weight, preferably 2 to 10% by weight, based upon the amount of the silica in the composition.
- the amount of the silane coupling agent is too small, the desired effects cannot be obtained, while conversely when too large, scorching will easily occur in the mixing or extrusion step, which is not desirable.
- a rubber composition for a tire tread including 100 parts by weight of a diene rubber, 2 to 80 parts by weight, preferably 5 to 60 parts by weight, of carbon black, 5 to 80 parts by weight, preferably 10 to 50 parts by weight, of silica, a silane coupling agent, and the polysiloxane mentioned above.
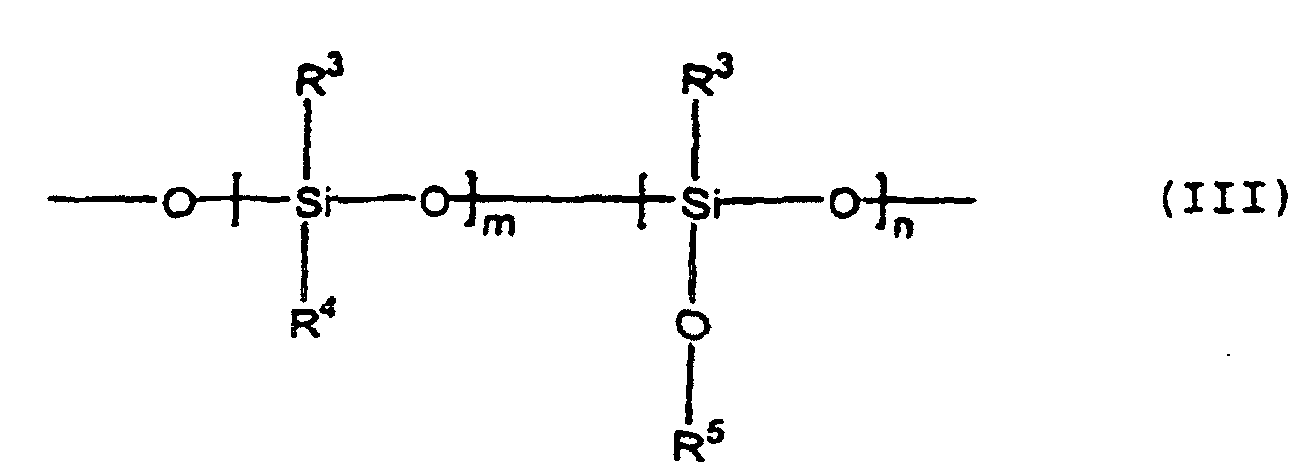
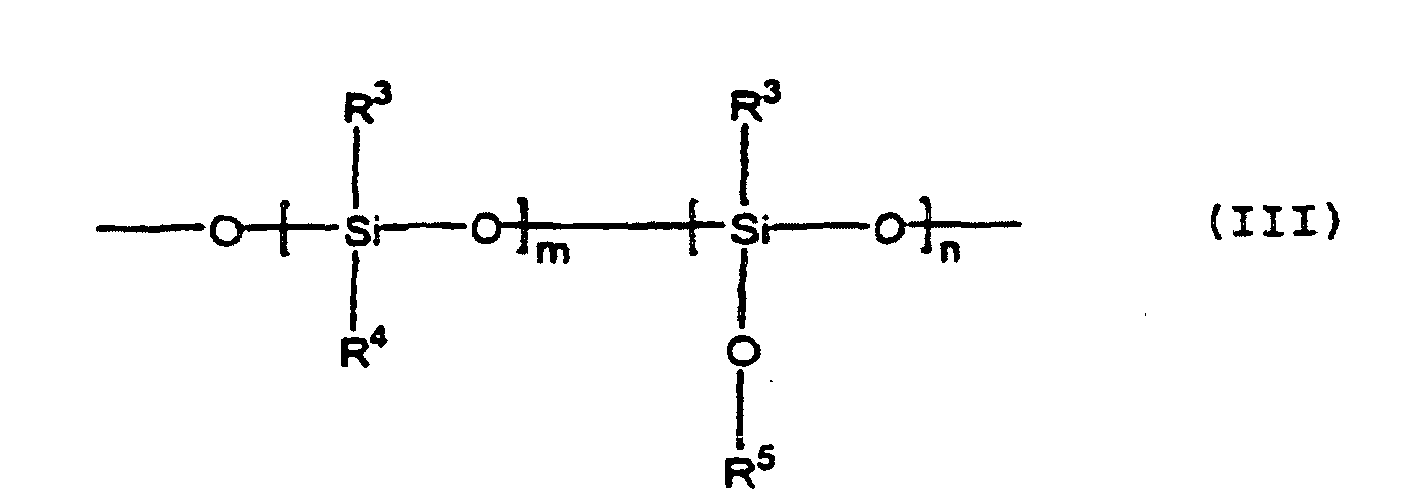
- the polysiloxane has repeating units of the formula (III): wherein R 3 represents independently a methyl group, ethyl group, or phenyl group, R 4 indicates independently hydrogen or an organic group (e.g., methyl, phenylethyl, 2-(4-methyl-3-cyclohexenyl)-propyl, 2,4-diphenyl-4-methylpentyl, R 5 indicates independently an alkyl group m is 0 or an integer of 1 or more, and n is an integer of 5 to 1000.
- a rubber composition for a tire tread as mentioned above, wherein the amounts of the polysiloxane and silane coupling agent are: 0.5 ⁇ (W PS /W SC ) ⁇ 7 Content of silica x 1 wt% ⁇ W PS + W SC ⁇ Content of silica x 40 wt% wherein, W PS : content of polysiloxane (parts by weight), and W SC : content of silane coupling agent (parts by weight).
- a rubber composition for a tire tread as mentioned above, wherein the compound except for the vulcanization system is obtained by mixing at a temperature of at least 120°C in a simultaneous step.
- the alkoxysiloxane reacts with the silanol groups and covers the surface of the silica particles, and therefore, the problems in the prior art are solved and it is possible to effectively suppress the rise in viscosity caused by the cohesiveness and polarity of the silanol groups and the wasted consumption of the vulcanization accelerator or other polarized additives or silane coupling agent etc.
- the polysiloxane of the above formula (III) contained in the rubber composition according to the present invention must have an alkoxysilyl group or acyloxysilyl group reacting with a silanol group and be a polymer (or oligomer) of a size covering the surface of the silica particles and exhibit a lubricating effect, for example, an average degree of polymerization of 3 to 10,000, preferably 10 to 1,000. Accordingly, in the repeating unit of the above formula (III), it is essential that a ⁇ Si-O-R 3 group be present. Accordingly, n is 5 to 1000 and m may be zero, but a hydrogen group or other organic group is also possible.
- the polysiloxane is a known substance. For example, it may be manufactured as follows:
- the compound having the siloxane structure of formula (III) is synthesized by causing a reaction between the corresponding polyalkylhydrogensiloxane and alcohol or carboxylic acid in the presence of a catalyst.
- Examples of the polyalkylhydrogensiloxane are those mentioned above.
- the polysiloxane used in the present invention is not particularly limited in its terminal groups and is determined by the type of the feedstock used during manufacture. For example, it may have a trimethylsilyl group, methyldiphenylsilyl group, triphenylsilyl group, and also organic groups.
- R 3 represents a methyl group, ethyl group, or phenyl group
- R 4 represents hydrogen or an organic group, for example, CH 3 , C 2 H 5 , styrene residue, divinylbenzene residue, limonene residue, butadiene residue, isoprene residue, etc.
- the diene rubber contained as the main ingredient in the vulcanizing rubber composition according to the present invention may be any diene rubber generally contained in various rubber compositions as mentioned above.
- the silane coupling agent used in the present invention may be made any silane coupling agent used together with silica fillers in the past. Typical examples are shown in Table I above. Of these, bis-[3-(triethoxysilica)-propyl]tetrasulfide is most preferred from the viewpoint of the processability.
- the amounts of the polysiloxane and silane coupling agent used in the present invention are a total weight of the polysiloxane and silane coupling agent of 0.5 to 40% by weight, preferably 1 to 20% by weight based upon the amount of the silica, and a weight ratio (of polysiloxane/silane coupling agent) of 0.5 to 7, preferably a range of 1 to 4. If the amount of the polysiloxane is too small, the desired effects cannot be obtained, while conversely if too great, the substances not bonding with the silica will leak out from the vulcanized product in some cases, which is not desirable.
- the rubber composition according to the present invention may contain, in addition to the above-mentioned essential ingredients, a vulcanization or cross-linking agent, a vulcanization or cross-linking accelerator, various types of oils, an antiaging agent, reinforcing agent, plasticizer, softening agent, or other various additives generally mixed into general rubbers.
- the compounds are kneaded and vulcanized by general methods to make the composition which may then be used for vulcanization or cross-linking.
- the amounts of these additives added may be made the amounts generally added in the past so long as they do not run counter to the object of the present invention.
- the polysiloxanes 1 to 6 obtained above are deduced Polysiloxane no.
- silanol terminated polymethylhydrogensiloxane molecular weight of 35000
- tetramethoxysilane 100 g was mixed with silanol terminated polymethylhydrogensiloxane (molecular weight of 35000) and 20 g of tetramethoxysilane.
- stannous dioctylate as a catalyst. The reaction was caused at 90°C for 4 hours, followed by distilling off the residual tetramethoxysilane at the same temperature under reduced pressure.
- the polysiloxane having the following structure was synthesized.
- the components other than the vulcanization accelerator and the sulfur were mixed in a 1.8 liter internal mixer for 3 to 5 minutes.
- the vulcanization accelerator and sulfur were kneaded by an 8-inch open roll to the master batch discharged when reaching 165 ⁇ 5°C to obtain the rubber composition.
- the unvulcanized physical properties of the obtained rubber composition were measured.
- composition was then pressed and vulcanized in a 15 x 15 x 0.2 cm mold at 160°C for 20 minutes to prepare the desired test piece and the vulcanized physical properties were evaluated.
- Example 1 (Standard Example) and Examples 2 to 3 (Examples of Invention)
- the ingredients for mixture in the first step were mixed in a 1.8 liter internal mixer for 3 to 5 minutes.
- the ingredients of the second step were mixed into the master batch discharged when reaching 165 ⁇ 5°C.
- To this were kneaded the vulcanization accelerator and sulfur by an 8-inch open roll to obtain the rubber composition.
- the obtained rubber composition was pressed and vulcanized in a 15 x 15 x 0.2 cm mold at 160°C for 20 minutes to prepare the desired test piece (rubber sheet) and the vulcanized physical properties were evaluated.
- a polysiloxane (III) and a silane coupling agent or inert filler by adding mixtures of a polysiloxane (III) and a silane coupling agent or inert filler, mixing becomes possible without overreaction with the silanol groups on the surface of the silica in one-step mixing and the physical properties (tensile strength, abrasion resistance, tan ⁇ ) are improved as shown in Examples 24, 26, Examples 29, 30, and Examples 34, 36 compared with Examples 22, 23 and 24, Examples 27, 28 and 30, and Examples 22 to 33 and 35.
- the superiority of the tan ⁇ balance in the present invention is shown by the size of the gradient between 0°C and 60°C.
- Example 39 (Reference Example): Preparation of polymethylethoxysiloxane (Surface-treatment agent)
- Polysiloxane surface-treated metal oxide was obtained by bring into contact the metal oxide shown in Table VIII with 3% acetone solution of the polymethylethoxy siloxane obtained above, followed by drying at 80°C. Thus, the polysiloxane surface-treated metal oxide was obtained.
- the hydrophobicity of the polysiloxane surface-treated metal oxide was determined as follows.
- the components other than the sulfur and the vulcanization accelerator were kneaded in a 1.8 later closed type mixer for 3 to 5 minutes and when the temperature reached at 165 ⁇ 5°C, the masterbatch was dischanged. The masterbatch was then kneaded with the valcanization accelerator and surfur by an 8-inch open rolls to obtain the rubber composition.
- the unvulcanized physical properties of the obtained rubber composition were measured. Next, the composition was pressed and vulcanized in a 15 x 15 x 0.2 cm mold at 160°C for 20 minutes to prepare the desired test piece and the vulcanized physical properties were evaluated.
- Scorch time Time for viscosity to rise 5 points at 125°C measured based on JIS K 6300.
- Example 47 (Standard), Examples 48 - 51 (Present Invention) and Example 52 (Comparative)
- the surface-treated silica-1, 2, 3 and 4 are those obtained by surface treating the surface of silica (Nipsil AQ manufactured by Japan Silica) 5, 10, 20 and 30 parts by weight of polymethylethoxy siloxane prepared in Reference Example 39, based upon 100 parts by weight of silica, respectively.
- the surface treatment was carried out by contacting the silica with 3% acetane solution of polymethylethoxy siloxane at room temperature followed by drying at 120°C.
- Example 53 (Standard), Example 54 (Present Invention) and Example 55 (Comparative Example)
- All the surface-treated silica obtained improved the processability of the unvulcanized rubber composition and the tensile stress and antiabrasion of the vulcanized product, when evaluated in the same manner as in Examples 47 - 50.
- the present invention by mixing into the rubber composition a metal oxide surface-treated with a polysiloxane (III) it is possible to remarkably improve the processability (i.e., scorching time) of the unvulcanized rubber composition and 300% modulus and antiabrasion as shown by Examples 48 to 51. Furthermore, as shown in Example 55, when carbon black and surface-treated silica were formulated, the processability (i.e., scorching time) of the unvulcanized rubber composition can be improved. As shown in Examples 56 - 60, the surface treatment efficiency can be improved when a titanium catalyst is used.
- the components other than the vulcanization accelerator and the sulfur were mixed in a 1.8 liter internal mixer for 3 to 5 minutes.
- the vulcanization accelerator and sulfur were kneaded by an 8-inch open roll to the master batch discharged when reaching 165 ⁇ 5°C to obtain the rubber composition.
- the first step consists of mixing in a 1.8 liter internal mixer for 3 to 4 minutes.
- the master batch discharged when reaching 150 ⁇ 5°C was mixed together with the remaining components in the second step by a 1.8 liter internal mixer for 3 to 5 minutes.
- the vulcanization accelerator and sulfur were mixed with the master batch of the second step discharged when reaching 165 ⁇ 5°C by an 8 inch open roll to obtain the rubber composition.
- the unvulcanized physical properties of the obtained rubber composition were measured.
- composition was pressed and vulcanized in a 15 x 15 x 0.2 cm mold at 160°C for 20 minutes to prepare the desired test piece and the vulcanized physical properties were evaluated.
- the processability of the unvulcanized rubber composition is improved and further the physical properties of the vulcanized rubber are equivalent or become better as shown by Examples 63 to 66, Examples 70 to 73, and Examples 76 to 80; when the silane coupling agent and polysiloxane of formula (III) are added, even if the silica and carbon are mixed in a simultaneous step, the state of dispersion of the carbon/silica is improved and equivalent or better physical properties are exhibited compared with other mixtures as shown by Examples 63 to 64, Examples 70 to 77, and Examples 76 to 78; and the abrasion resistance is particularly improved, as clear compared with Comparative Examples 65 to 66, Comparative Example 73, and Comparative Examples 79 to 80.
Landscapes
- Chemical & Material Sciences (AREA)
- Health & Medical Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Medicinal Chemistry (AREA)
- Polymers & Plastics (AREA)
- Organic Chemistry (AREA)
- Compositions Of Macromolecular Compounds (AREA)
Claims (15)
- Kautschukzusammensetzung, die einen Füllstoff umfaßt, wobei die Kautschukzusammensetzung ein Polysiloxan mit einem Zahlenmittelwert des Molekulargewichts von bis zu 100 000 und mit einer Struktureinheit der Formel (III) enthält. worin R3 unabhängig eine Methyl-Gruppe, Ethyl-Gruppe oder Phenyl-Gruppe darstellt, R4 unabhängig Wasserstoff oder eine organische Gruppe darstellt, R5 unabhängig eine Alkyl-Gruppe darstellt, m 0 oder eine ganze Zahl von 1 oder mehr ist und n eine ganze Zahl von 5 bis 1000 ist, mit der Maßgabe, daß die terminale Gruppe nicht eine Silanol-Gruppe SiOH ist.
- Kautschukzusammensetzung gemäß Anspruch 1, worin der Füllstoff ein siliciumhaltiger Füllstoff ist.
- Kautschukzusammensetzung gemäß Anspruch 1, worin das Polysiloxan in einer Menge von 100 Gew.-% oder weniger des Gehalts des Füllstoffs enthalten ist.
- Kautschukzusammensetzung gemäß Anspruch 1, die ferner einen Silan-Kuppler in einer Menge von 40 Gew.-% oder weniger des Gehalts des Füllstoffs umfaßt.
- Kautschuk-Kompoundierungsmittel, umfassend (A) ein Polysiloxan mit einem Zahlenmittelwert des Molekulargewichts von bis 100 000 und mit einer Struktureinheit der Formel (III): worin R3 unabhängig eine Methyl-Gruppe, Ethyl-Gruppe oder Phenyl-Gruppe darstellt, R4 unabhängig Wasserstoff oder eine organische Gruppe darstellt, R5 unabhängig eine Alkyl-Gruppe darstellt, m 0 oder eine ganze Zahl von 1 oder mehr ist und n eine ganze Zahl von 5 bis 1000 ist, mit der Maßgabe, daß die terminale Gruppe nicht eine Silanol-Gruppe SiOH ist, und (B) einen Silan-Kuppler in einem Verhältnis von (A)/(B) = 95/5 bis 5/95.
- Kautschuk-Kompoundierungsmittel gemäß Anspruch 5, worin das Polysiloxan (A) in wenigstens einem Pulver (C), das aus der Gruppe ausgewählt ist, die aus Ruß, Calciumcarbonat, Stearinsäure und Kieselerde besteht, in einem Verhältnis von (A)/(C) = 70/30 bis 5/95 imprägniert ist.
- Kautschukzusammensetzung, die 100 Gew.-Teile eines Ausgangskautschuks, 5 bis 100 Gew.-Teile Kieselerde und eine Menge des Kautschuk-Kompoundierungsmittels gemäß Anspruch 5 zum Erhalt eines Gehalts des darin enthaltenen Polysiloxans von 0,2 bis 30 Gew.-% in der Gesamtzusammensetzung umfaßt.
- Mit Polysiloxan oberflächenbehandeltes Metalloxid, das 100 Gew.-Teile einer Metalloxidoberfläche umfaßt, die mit 0,1 bis 50 Gew.-Teilen eines Polysiloxans mit einem Zahlenmittelwert des Molekulargewichts von bis zu 100 000 und mit einer Struktureinheit in der Formel (III) behandelt ist: worin R3 unabhängig eine Methyl-Gruppe, Ethyl-Gruppe oder Phenyl-Gruppe darstellt, R4 unabhängig Wasserstoff oder eine organische Gruppe darstellt, R5 unabhängig eine Alkyl-Gruppe darstellt, m 0 oder eine ganze Zahl von 1 oder mehr ist und n eine ganze Zahl von 5 bis 1000 ist, mit der Maßgabe, daß die terminale Gruppe nicht eine Silanol-Gruppe SiOH ist.
- Mit Polysiloxan oberflächenbehandeltes Metalloxid gemäß Anspruch 8, worin das Metalloxid SiO2 oder SiO2haltiges Metalloxid ist.
- Mit Polysiloxan oberflächenbehandeltes Metalloxid gemäß Anspruch 8, worin während der Oberflächenbehandlung ferner 0,05 bis 50 Gew.-% eines Titankatalysators auf Basis der Menge des verwendeten Polysiloxans verwendet werden.
- Kautschukzusammensetzung, die 100 Gew.-Teile eines Ausgangskautschuks und 5 bis 100 Gew.-Teile des mit Polysiloxan oberflächenbehandelten Metalloxids gemäß Anspruch 8 umfaßt.
- Kautschukzusammensetzung gemäß Anspruch 11, worin das Metalloxid Kieselerde ist und ferner ein Silan-Kuppler in einer Menge von 0,5 bis 40 Gew.-% der Menge der Kieselerde in der Zusammensetzung enthalten ist.
- Kautschukzusammensetzung für eine Luftreifen-Lauffläche, die 100 Gew.-Teile eines Dien-Kautschuks, 2 bis 80 Gew.-Teile Ruß, 5 bis 80 Gew.-Teile Kieselerde, eines Silan-Kupplers und eines Polysiloxans mit einem Zahlenmittelwert des Molekulargewichts von bis zu 100 000 und mit einer Struktureinheit der Formel (III) umfaßt: worin R3 unabhängig eine Methyl-Gruppe, Ethyl-Gruppe oder Phenyl-Gruppe darstellt, R4 unabhängig Wasserstoff oder eine organische Gruppe darstellt, R5 unabhängig eine Alkyl-Gruppe darstellt, m 0 oder eine ganze Zahl von 1 oder mehr ist und n eine ganze Zahl von 5 bis 1000 ist, mit der Maßgabe, daß die terminale Gruppe nicht eine Silanol-Gruppe SiOH ist.
- Kautschukzusammensetzung gemäß Anspruch 13, worin die Mengen des Polysiloxans und des Silan-Kupplers wie folgt sind:worin WPS der Polysiloxan-Gehalt (Gew.-Teile) und WSC der Gehalt an Silan-Kuppler (Gew.-Teile) ist.0.5 ≤ (WPS/WSC) ≤ 7Kieselerdegehalt x 1 Gew.-% ≤ WPS + WSC ≤Kieselerdegehalt x 40 Gew.-%
- Kautschukzusammensetzung gemäß Anspruch 13, worin die Zusammensetzung, ausgenommen das Vulkanisationssystem, durch Vermischen bei einer Temperatur von wenigstens 120°C in einem simultanen Schritt erhalten wird.
Applications Claiming Priority (21)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP223079/95 | 1995-08-31 | ||
| JP22307995 | 1995-08-31 | ||
| JP22307995 | 1995-08-31 | ||
| JP27285995 | 1995-10-20 | ||
| JP7272859A JP2853980B2 (ja) | 1995-10-20 | 1995-10-20 | タイヤトレッド用ゴム組成物 |
| JP272859/95 | 1995-10-20 | ||
| JP34154095 | 1995-12-27 | ||
| JP34154095 | 1995-12-27 | ||
| JP341540/95 | 1995-12-27 | ||
| JP7643/96 | 1996-01-19 | ||
| JP7663/96 | 1996-01-19 | ||
| JP8007663A JP2853986B2 (ja) | 1996-01-19 | 1996-01-19 | ゴム用配合剤及びそれを用いたゴム組成物 |
| JP764396 | 1996-01-19 | ||
| JP764396 | 1996-01-19 | ||
| JP766396 | 1996-01-19 | ||
| JP11676396 | 1996-05-10 | ||
| JP116763/96 | 1996-05-10 | ||
| JP11676396 | 1996-05-10 | ||
| JP20059696 | 1996-07-30 | ||
| JP200596/96 | 1996-07-30 | ||
| JP20059696A JP3604237B2 (ja) | 1996-01-19 | 1996-07-30 | ポリシロキサン表面処理金属酸化物及びそれを用いたゴム組成物 |
Publications (3)
| Publication Number | Publication Date |
|---|---|
| EP0761748A2 EP0761748A2 (de) | 1997-03-12 |
| EP0761748A3 EP0761748A3 (de) | 1998-11-11 |
| EP0761748B1 true EP0761748B1 (de) | 2004-05-19 |
Family
ID=27563417
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP96113916A Expired - Lifetime EP0761748B1 (de) | 1995-08-31 | 1996-08-30 | Polysiloxan-enthaltende Kautschukzusammensetzung |
Country Status (3)
| Country | Link |
|---|---|
| US (5) | US6177505B1 (de) |
| EP (1) | EP0761748B1 (de) |
| DE (1) | DE69632512T2 (de) |
Cited By (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US9758639B2 (en) | 2014-04-30 | 2017-09-12 | Bridgestone Corporation | Rubber composition with imidazole-based silica shielding agent |
| US9951208B2 (en) | 2014-11-06 | 2018-04-24 | Bridgestone Corporation | Silica shielding agents and related methods |
Families Citing this family (42)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US6177505B1 (en) * | 1995-08-31 | 2001-01-23 | The Yokohama Rubber Co., Ltd. | Polysiloxane-containing rubber composition |
| FR2743565B1 (fr) * | 1996-01-11 | 1998-02-20 | Rhone Poulenc Chimie | Utilisation d'une association de composes silicones comme agent de couplage dans les compositions d'elastomeres chargees de silice |
| CN1168768C (zh) | 1996-06-26 | 2004-09-29 | 株式会社普利司通 | 橡胶组合物 |
| EP1213323A3 (de) * | 1996-09-11 | 2003-07-16 | The Yokohama Rubber Co., Ltd. | Verwendung einer Polysiloxan enthaltenden Kautschukzusammensetzung für Reifen |
| KR19990071884A (ko) * | 1996-10-04 | 1999-09-27 | 하기와라 세이지 | 실리카함유디엔고무조성물 |
| AU764335B2 (en) * | 1999-05-14 | 2003-08-14 | Sri Sports Limited | Painted golf ball |
| US6784255B1 (en) * | 1999-07-23 | 2004-08-31 | Mitsui Chemicals Inc. | Rubber composition for extrusion molding and for molding with mold and use thereof |
| US6342560B1 (en) | 1999-08-19 | 2002-01-29 | Ppg Industries Ohio, Inc. | Chemically modified fillers and polymeric compositions containing same |
| US7704552B2 (en) | 1999-08-19 | 2010-04-27 | Ppg Industries Ohio, Inc. | Process for producing chemically treated amorphous precipitated silica |
| US7687107B2 (en) * | 1999-08-19 | 2010-03-30 | Ppg Industries Ohio, Inc. | Process for producing chemically modified amorphous precipitated silica |
| US6649684B1 (en) | 1999-08-19 | 2003-11-18 | Ppg Industries Ohio, Inc. | Chemically treated fillers and polymeric compositions containing same |
| US6476110B1 (en) * | 2000-02-18 | 2002-11-05 | Continental Ag | Rubber composition containing solid magnetizable particles of greater stiffness than the rubbery compounds |
| US6313220B1 (en) * | 2000-03-03 | 2001-11-06 | Thierry Florent Edme Materne | Preparation of reinforced elastomer, elastomer composite, and tire having component thereof |
| WO2002004558A1 (en) * | 2000-07-11 | 2002-01-17 | Mitsui Chemicals, Inc. | Rubber composition and use thereof |
| KR100734496B1 (ko) * | 2000-07-25 | 2007-07-03 | 미쓰이 가가쿠 가부시키가이샤 | 경화성 조성물 및 그 용도 |
| US7176269B2 (en) * | 2000-07-25 | 2007-02-13 | Mitsui Chemicals, Inc. | Curable composition and its use |
| DE10044989A1 (de) | 2000-09-11 | 2002-03-21 | Bayer Ag | Flüssige schwefelhaltige Oligosiloxane und ihre Verwendung in Kautschukmischungen |
| US20040014869A1 (en) * | 2001-05-09 | 2004-01-22 | Wong Wai Keung | Method for preparing silica filled elastomeric compositions |
| DE10130500A1 (de) * | 2001-06-25 | 2003-01-09 | Degussa | Kautschukmischungen |
| US20030119960A1 (en) * | 2001-09-04 | 2003-06-26 | Hong Sung W. | Rubber compositions and method for decreasing the tangent delta value |
| DE10208590B4 (de) * | 2002-02-27 | 2007-02-01 | Degussa Ag | Kautschukmischungen |
| DE10238369A1 (de) * | 2002-08-22 | 2004-03-04 | Degussa Ag | Mittel als Haftvermittler für gefüllte und peroxidisch zu vernetzende Gummicompounds |
| US6743509B2 (en) † | 2002-10-01 | 2004-06-01 | Dow Corning Corporation | Method of treating precipitated calcium carbonate fillers |
| TWI254730B (en) * | 2004-03-26 | 2006-05-11 | Tsrc Corp | Conjugated diene-vinyl aromatic hydrocarbon copolymer containing siloxane compound for promoting abrasive resistance |
| CN101180344B (zh) * | 2005-03-24 | 2012-01-11 | 株式会社普利司通 | 具有低挥发性有机化合物(voc)释放的配混二氧化硅补强橡胶 |
| US7968630B2 (en) * | 2005-09-08 | 2011-06-28 | The Goodyear Tire & Rubber Company | Pneumatic tire containing zinc porphyrin compound |
| DE102006005100A1 (de) | 2006-02-04 | 2007-08-09 | Goldschmidt Gmbh | Verfahren zur Herstellung organomodifizierter Siloxane |
| US8501895B2 (en) * | 2007-05-23 | 2013-08-06 | Bridgestone Corporation | Method for making alkoxy-modified silsesquioxanes and amino alkoxy-modified silsesquioxanes |
| US7915368B2 (en) * | 2007-05-23 | 2011-03-29 | Bridgestone Corporation | Method for making alkoxy-modified silsesquioxanes |
| US8962746B2 (en) * | 2007-12-27 | 2015-02-24 | Bridgestone Corporation | Methods of making blocked-mercapto alkoxy-modified silsesquioxane compounds |
| US8097674B2 (en) | 2007-12-31 | 2012-01-17 | Bridgestone Corporation | Amino alkoxy-modified silsesquioxanes in silica-filled rubber with low volatile organic chemical evolution |
| US8794282B2 (en) | 2007-12-31 | 2014-08-05 | Bridgestone Corporation | Amino alkoxy-modified silsesquioxane adhesives for improved metal adhesion and metal adhesion retention to cured rubber |
| GB2474474A (en) * | 2009-10-14 | 2011-04-20 | Dow Corning | Silicone rubber compositions |
| US8642691B2 (en) | 2009-12-28 | 2014-02-04 | Bridgestone Corporation | Amino alkoxy-modified silsesquioxane adhesives for improved metal adhesion and metal adhesion retention to cured rubber |
| CN104411760B (zh) * | 2012-06-27 | 2016-08-24 | 横滨橡胶株式会社 | 轮胎胎面用橡胶组合物以及充气轮胎 |
| TWI486384B (zh) * | 2012-12-28 | 2015-06-01 | Chi Mei Corp | 聚矽氧烷化合物、經改質的共軛二烯系-乙烯基芳香烴系共聚物及其製造方法 |
| EP3086928B1 (de) | 2013-12-27 | 2019-09-11 | Bridgestone Corporation | Aus zusammensetzungen mit mercaptofunktionellen siloxanen hergestellte vulkanisate und reifenkomponenten |
| GB2525420A (en) * | 2014-04-24 | 2015-10-28 | Henkel Ag & Co Kgaa | New modified silicone oil for low temperature cure die casting lubricants |
| CN107406689A (zh) | 2014-12-31 | 2017-11-28 | 株式会社普利司通 | 用于将钢合金粘附到橡胶的氨基烷氧基改性倍半硅氧烷粘合剂 |
| US20240158615A1 (en) | 2022-11-02 | 2024-05-16 | The Goodyear Tire & Rubber Company | Precipitated silica pretreated with a coupling agent and polyethylene glycol for a rubber composition |
| US20240384066A1 (en) | 2023-05-15 | 2024-11-21 | The Goodyear Tire & Rubber Company | Voc-free coupling agents for rubber compositions |
| US20250019526A1 (en) | 2023-07-10 | 2025-01-16 | The Goodyear Tire & Rubber Company | Winter tread composition including aluminum hydroxide |
Family Cites Families (36)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US3255140A (en) * | 1961-10-17 | 1966-06-07 | Bayer Ag | Siloxane heat sensitizing agents for latex mixtures |
| DE2357184A1 (de) * | 1973-11-16 | 1975-05-22 | Merck Patent Gmbh | Verfahren zur herstellung von organisch modifizierten siliciumdioxiden |
| DE2837117A1 (de) * | 1977-08-30 | 1979-03-15 | Shinetsu Chemical Co | Gummizusammenstellung |
| US4176296A (en) * | 1978-06-22 | 1979-11-27 | General Electric Company | Core mounting for solenoidal electric field lamps |
| JPS5531817A (en) | 1978-08-25 | 1980-03-06 | Shin Etsu Chem Co Ltd | Rubber composition |
| JPS5667348A (en) * | 1979-11-08 | 1981-06-06 | Mitsuboshi Belting Ltd | Rubber composition |
| JPS5676440A (en) | 1979-11-28 | 1981-06-24 | Dainichi Nippon Cables Ltd | Nonsilicone rubber composition |
| US4369289A (en) * | 1980-09-30 | 1983-01-18 | Union Carbide Corporation | Masterbatch composition comprising a matrix having a polysiloxane dispersed therein and a method for the preparation thereof |
| JPS57172925A (en) | 1981-04-16 | 1982-10-25 | Shin Etsu Chem Co Ltd | High-filling of organic polymer with inorganic filler |
| JPS5855181B2 (ja) | 1981-05-29 | 1983-12-08 | 信越化学工業株式会社 | ゴム組成物 |
| JPS58198547A (ja) * | 1982-05-16 | 1983-11-18 | Bando Chem Ind Ltd | ゴム組成物 |
| US4474908A (en) * | 1982-05-27 | 1984-10-02 | Ppg Industries, Inc. | Rubber compositions |
| JPS5945906A (ja) | 1982-09-09 | 1984-03-15 | Nippon Yunikaa Kk | 無機固体粒子組成物 |
| US4526922A (en) * | 1983-04-15 | 1985-07-02 | Union Carbide Corporation | Organofunctional silane-siloxane oligomer coupling compositions, curable and cured elastomeric compositions containing same and novel electric cable containing said cured elastomeric compositions |
| JPS624873A (ja) | 1985-06-28 | 1987-01-10 | Toshiba Corp | 成膜装置 |
| JPS6248743A (ja) | 1985-08-28 | 1987-03-03 | Shin Etsu Chem Co Ltd | ゴム組成物 |
| JPS63199253A (ja) * | 1987-02-13 | 1988-08-17 | Shin Etsu Chem Co Ltd | ゴム組成物 |
| FR2613708B1 (fr) | 1987-04-13 | 1990-10-12 | Rhone Poulenc Chimie | Silice de precipitation hydrophobe, son procede de preparation et son application au renforcement des elastomeres silicones |
| JP2670855B2 (ja) * | 1989-06-26 | 1997-10-29 | 日本合成ゴム株式会社 | シリコーン複合ゴム組成物およびその用途 |
| US5094829A (en) | 1990-06-21 | 1992-03-10 | Ppg Industries, Inc. | Reinforced precipitated silica |
| FR2674515B1 (fr) | 1991-03-29 | 1993-09-03 | Talc Luzenac | Substances talqueuses presentant des proprietes specifiques de surface et procedes de fabrication. |
| JP2626302B2 (ja) | 1991-04-09 | 1997-07-02 | 信越化学工業株式会社 | 硬化性オルガノポリシロキサン組成物及び硬化物 |
| DE4233077A1 (de) * | 1992-10-01 | 1994-04-07 | Wacker Chemie Gmbh | Dichtungsmassen auf der Grundlage von Polymerisaten ethylenisch ungesättigter Monomeren |
| US5430087A (en) * | 1993-09-02 | 1995-07-04 | Hydril Company | Carbon black pair with different particle size and improved rubber stock |
| JP3403747B2 (ja) | 1993-02-23 | 2003-05-06 | 株式会社ブリヂストン | タイヤ用ゴム組成物 |
| JP2725573B2 (ja) | 1993-11-12 | 1998-03-11 | 松下電工株式会社 | 疎水性エアロゲルの製法 |
| FR2721615A1 (fr) | 1994-06-24 | 1995-12-29 | Rhone Poulenc Chimie | Procédé de préparation de particules d'oxyde métallique organophiles. |
| JPH0848910A (ja) | 1994-08-03 | 1996-02-20 | Asahi Glass Co Ltd | 表面処理金属酸化物およびその製造方法 |
| JP3582860B2 (ja) | 1994-08-08 | 2004-10-27 | Thk株式会社 | 曲線案内装置 |
| JP3465391B2 (ja) | 1994-12-05 | 2003-11-10 | 信越化学工業株式会社 | 反応性オルガノポリシロキサン |
| KR100394129B1 (ko) | 1995-03-17 | 2003-10-22 | 제온 코포레이션 | 고무조성물 |
| JP2853980B2 (ja) | 1995-10-20 | 1999-02-03 | 横浜ゴム株式会社 | タイヤトレッド用ゴム組成物 |
| US6177505B1 (en) * | 1995-08-31 | 2001-01-23 | The Yokohama Rubber Co., Ltd. | Polysiloxane-containing rubber composition |
| US6191247B1 (en) * | 1996-04-10 | 2001-02-20 | The Yokohama Rubber Co., Ltd. | Polysiloxane composition having superior storage stability and rubber composition containing same |
| CN1168768C (zh) * | 1996-06-26 | 2004-09-29 | 株式会社普利司通 | 橡胶组合物 |
| JPH10237229A (ja) * | 1997-02-28 | 1998-09-08 | Yokohama Rubber Co Ltd:The | ゴム組成物 |
-
1996
- 1996-08-30 US US08/708,060 patent/US6177505B1/en not_active Expired - Fee Related
- 1996-08-30 EP EP96113916A patent/EP0761748B1/de not_active Expired - Lifetime
- 1996-08-30 DE DE69632512T patent/DE69632512T2/de not_active Expired - Fee Related
-
1998
- 1998-11-10 US US09/188,695 patent/US6121347A/en not_active Expired - Fee Related
- 1998-11-10 US US09/188,694 patent/US6033597A/en not_active Expired - Fee Related
-
2000
- 2000-03-16 US US09/526,733 patent/US6337361B1/en not_active Expired - Fee Related
- 2000-11-16 US US09/713,298 patent/US6525110B1/en not_active Expired - Fee Related
Cited By (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US9758639B2 (en) | 2014-04-30 | 2017-09-12 | Bridgestone Corporation | Rubber composition with imidazole-based silica shielding agent |
| US9951208B2 (en) | 2014-11-06 | 2018-04-24 | Bridgestone Corporation | Silica shielding agents and related methods |
Also Published As
| Publication number | Publication date |
|---|---|
| US6121347A (en) | 2000-09-19 |
| US6177505B1 (en) | 2001-01-23 |
| US6337361B1 (en) | 2002-01-08 |
| US6033597A (en) | 2000-03-07 |
| EP0761748A3 (de) | 1998-11-11 |
| EP0761748A2 (de) | 1997-03-12 |
| DE69632512T2 (de) | 2005-05-25 |
| DE69632512D1 (de) | 2004-06-24 |
| US6525110B1 (en) | 2003-02-25 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| EP0761748B1 (de) | Polysiloxan-enthaltende Kautschukzusammensetzung | |
| US6191247B1 (en) | Polysiloxane composition having superior storage stability and rubber composition containing same | |
| US6140450A (en) | Modified polysiloxanes, rubber compositions and tire tread rubber compositions containing the same, and reactive plasticizer | |
| US7307121B2 (en) | Silica containing rubber composition | |
| EP0861872B1 (de) | Verwendung einer Polysiloxan enthaltenden Kautschukzusammensetzung für Reifen | |
| JP2006089754A (ja) | タイヤケーシング | |
| BR102020004308A2 (pt) | Borracha reforçada contendo óleo de triglicerídeo sililado | |
| JP4036519B2 (ja) | 加工性を改良したタイヤトレッド用ゴム組成物 | |
| HUP0101533A2 (hu) | Hidrofobizált oxidos vagy szilikátor töltőanyagot tartalmazó emulziós kaucsukkeverék és eljárás az előállítására | |
| JP3369883B2 (ja) | ゴム組成物および空気入りタイヤ | |
| WO1997048267A2 (en) | Rubber composition and pneumatic tires produced therefrom | |
| JP2853980B2 (ja) | タイヤトレッド用ゴム組成物 | |
| JPH1135735A (ja) | ゴム組成物 | |
| JP3105174B2 (ja) | ゴム組成物 | |
| JPH11106512A (ja) | 変性ポリシロキサン、それを用いたゴム組成物およびタイヤトレッド用ゴム組成物 | |
| JPH1095204A (ja) | 空気入りタイヤ | |
| WO1998014515A1 (en) | Silica-containing diene rubber composition | |
| JP3608892B2 (ja) | シリカ配合ゴム組成物 | |
| JP2008163125A (ja) | ゴム組成物およびそれを用いた空気入りタイヤ | |
| JP3604237B2 (ja) | ポリシロキサン表面処理金属酸化物及びそれを用いたゴム組成物 | |
| JP2000313809A (ja) | ポリシロキサン組成物およびそれを用いたゴム組成物 | |
| JP4336920B2 (ja) | ゴム組成物 | |
| JP2853986B2 (ja) | ゴム用配合剤及びそれを用いたゴム組成物 | |
| JPH1053707A (ja) | ゴム用配合剤及びそれを用いたゴム組成物 | |
| JPH11189680A (ja) | ゴム組成物 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PUAI | Public reference made under article 153(3) epc to a published international application that has entered the european phase |
Free format text: ORIGINAL CODE: 0009012 |
|
| AK | Designated contracting states |
Kind code of ref document: A2 Designated state(s): DE FR IT |
|
| 17P | Request for examination filed |
Effective date: 19970724 |
|
| PUAL | Search report despatched |
Free format text: ORIGINAL CODE: 0009013 |
|
| AK | Designated contracting states |
Kind code of ref document: A3 Designated state(s): DE FR IT |
|
| 17Q | First examination report despatched |
Effective date: 19990611 |
|
| APAB | Appeal dossier modified |
Free format text: ORIGINAL CODE: EPIDOS NOAPE |
|
| APAB | Appeal dossier modified |
Free format text: ORIGINAL CODE: EPIDOS NOAPE |
|
| APBJ | Interlocutory revision of appeal recorded |
Free format text: ORIGINAL CODE: EPIDOS IRAPE |
|
| GRAH | Despatch of communication of intention to grant a patent |
Free format text: ORIGINAL CODE: EPIDOS IGRA |
|
| GRAS | Grant fee paid |
Free format text: ORIGINAL CODE: EPIDOSNIGR3 |
|
| GRAA | (expected) grant |
Free format text: ORIGINAL CODE: 0009210 |
|
| AK | Designated contracting states |
Kind code of ref document: B1 Designated state(s): DE FR IT |
|
| REF | Corresponds to: |
Ref document number: 69632512 Country of ref document: DE Date of ref document: 20040624 Kind code of ref document: P |
|
| ET | Fr: translation filed | ||
| PLBE | No opposition filed within time limit |
Free format text: ORIGINAL CODE: 0009261 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: NO OPPOSITION FILED WITHIN TIME LIMIT |
|
| 26N | No opposition filed |
Effective date: 20050222 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: DE Payment date: 20060824 Year of fee payment: 11 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: IT Payment date: 20060831 Year of fee payment: 11 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: DE Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20080301 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: FR Payment date: 20080818 Year of fee payment: 13 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: IT Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20070830 |
|
| REG | Reference to a national code |
Ref country code: FR Ref legal event code: ST Effective date: 20100430 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: FR Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20090831 |