WO2018070090A1 - 積層体およびその製造方法 - Google Patents
積層体およびその製造方法 Download PDFInfo
- Publication number
- WO2018070090A1 WO2018070090A1 PCT/JP2017/027394 JP2017027394W WO2018070090A1 WO 2018070090 A1 WO2018070090 A1 WO 2018070090A1 JP 2017027394 W JP2017027394 W JP 2017027394W WO 2018070090 A1 WO2018070090 A1 WO 2018070090A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- meth
- layer
- acrylate
- respect
- active energy
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Ceased
Links
Images
Classifications
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B32—LAYERED PRODUCTS
- B32B—LAYERED PRODUCTS, i.e. PRODUCTS BUILT-UP OF STRATA OF FLAT OR NON-FLAT, e.g. CELLULAR OR HONEYCOMB, FORM
- B32B27/00—Layered products comprising a layer of synthetic resin
- B32B27/06—Layered products comprising a layer of synthetic resin as the main or only constituent of a layer, which is next to another layer of the same or of a different material
- B32B27/08—Layered products comprising a layer of synthetic resin as the main or only constituent of a layer, which is next to another layer of the same or of a different material of synthetic resin
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B05—SPRAYING OR ATOMISING IN GENERAL; APPLYING FLUENT MATERIALS TO SURFACES, IN GENERAL
- B05D—PROCESSES FOR APPLYING FLUENT MATERIALS TO SURFACES, IN GENERAL
- B05D7/00—Processes, other than flocking, specially adapted for applying liquids or other fluent materials to particular surfaces or for applying particular liquids or other fluent materials
- B05D7/02—Processes, other than flocking, specially adapted for applying liquids or other fluent materials to particular surfaces or for applying particular liquids or other fluent materials to macromolecular substances, e.g. rubber
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B32—LAYERED PRODUCTS
- B32B—LAYERED PRODUCTS, i.e. PRODUCTS BUILT-UP OF STRATA OF FLAT OR NON-FLAT, e.g. CELLULAR OR HONEYCOMB, FORM
- B32B7/00—Layered products characterised by the relation between layers; Layered products characterised by the relative orientation of features between layers, or by the relative values of a measurable parameter between layers, i.e. products comprising layers having different physical, chemical or physicochemical properties; Layered products characterised by the interconnection of layers
- B32B7/02—Physical, chemical or physicochemical properties
- B32B7/023—Optical properties
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B05—SPRAYING OR ATOMISING IN GENERAL; APPLYING FLUENT MATERIALS TO SURFACES, IN GENERAL
- B05D—PROCESSES FOR APPLYING FLUENT MATERIALS TO SURFACES, IN GENERAL
- B05D7/00—Processes, other than flocking, specially adapted for applying liquids or other fluent materials to particular surfaces or for applying particular liquids or other fluent materials
- B05D7/24—Processes, other than flocking, specially adapted for applying liquids or other fluent materials to particular surfaces or for applying particular liquids or other fluent materials for applying particular liquids or other fluent materials
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B32—LAYERED PRODUCTS
- B32B—LAYERED PRODUCTS, i.e. PRODUCTS BUILT-UP OF STRATA OF FLAT OR NON-FLAT, e.g. CELLULAR OR HONEYCOMB, FORM
- B32B27/00—Layered products comprising a layer of synthetic resin
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B32—LAYERED PRODUCTS
- B32B—LAYERED PRODUCTS, i.e. PRODUCTS BUILT-UP OF STRATA OF FLAT OR NON-FLAT, e.g. CELLULAR OR HONEYCOMB, FORM
- B32B27/00—Layered products comprising a layer of synthetic resin
- B32B27/16—Layered products comprising a layer of synthetic resin specially treated, e.g. irradiated
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B32—LAYERED PRODUCTS
- B32B—LAYERED PRODUCTS, i.e. PRODUCTS BUILT-UP OF STRATA OF FLAT OR NON-FLAT, e.g. CELLULAR OR HONEYCOMB, FORM
- B32B27/00—Layered products comprising a layer of synthetic resin
- B32B27/28—Layered products comprising a layer of synthetic resin comprising synthetic resins not wholly covered by any one of the sub-groups B32B27/30 - B32B27/42
- B32B27/283—Layered products comprising a layer of synthetic resin comprising synthetic resins not wholly covered by any one of the sub-groups B32B27/30 - B32B27/42 comprising polysiloxanes
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B32—LAYERED PRODUCTS
- B32B—LAYERED PRODUCTS, i.e. PRODUCTS BUILT-UP OF STRATA OF FLAT OR NON-FLAT, e.g. CELLULAR OR HONEYCOMB, FORM
- B32B27/00—Layered products comprising a layer of synthetic resin
- B32B27/36—Layered products comprising a layer of synthetic resin comprising polyesters
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B32—LAYERED PRODUCTS
- B32B—LAYERED PRODUCTS, i.e. PRODUCTS BUILT-UP OF STRATA OF FLAT OR NON-FLAT, e.g. CELLULAR OR HONEYCOMB, FORM
- B32B27/00—Layered products comprising a layer of synthetic resin
- B32B27/36—Layered products comprising a layer of synthetic resin comprising polyesters
- B32B27/365—Layered products comprising a layer of synthetic resin comprising polyesters comprising polycarbonates
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08J—WORKING-UP; GENERAL PROCESSES OF COMPOUNDING; AFTER-TREATMENT NOT COVERED BY SUBCLASSES C08B, C08C, C08F, C08G or C08H
- C08J7/00—Chemical treatment or coating of shaped articles made of macromolecular substances
- C08J7/04—Coating
- C08J7/042—Coating with two or more layers, where at least one layer of a composition contains a polymer binder
- C08J7/0423—Coating with two or more layers, where at least one layer of a composition contains a polymer binder with at least one layer of inorganic material and at least one layer of a composition containing a polymer binder
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08J—WORKING-UP; GENERAL PROCESSES OF COMPOUNDING; AFTER-TREATMENT NOT COVERED BY SUBCLASSES C08B, C08C, C08F, C08G or C08H
- C08J7/00—Chemical treatment or coating of shaped articles made of macromolecular substances
- C08J7/08—Heat treatment
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08J—WORKING-UP; GENERAL PROCESSES OF COMPOUNDING; AFTER-TREATMENT NOT COVERED BY SUBCLASSES C08B, C08C, C08F, C08G or C08H
- C08J7/00—Chemical treatment or coating of shaped articles made of macromolecular substances
- C08J7/12—Chemical modification
- C08J7/16—Chemical modification with polymerisable compounds
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09D—COATING COMPOSITIONS, e.g. PAINTS, VARNISHES OR LACQUERS; FILLING PASTES; CHEMICAL PAINT OR INK REMOVERS; INKS; CORRECTING FLUIDS; WOODSTAINS; PASTES OR SOLIDS FOR COLOURING OR PRINTING; USE OF MATERIALS THEREFOR
- C09D183/00—Coating compositions based on macromolecular compounds obtained by reactions forming in the main chain of the macromolecule a linkage containing silicon, with or without sulfur, nitrogen, oxygen, or carbon only; Coating compositions based on derivatives of such polymers
- C09D183/02—Polysilicates
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B05—SPRAYING OR ATOMISING IN GENERAL; APPLYING FLUENT MATERIALS TO SURFACES, IN GENERAL
- B05D—PROCESSES FOR APPLYING FLUENT MATERIALS TO SURFACES, IN GENERAL
- B05D1/00—Processes for applying liquids or other fluent materials
- B05D1/30—Processes for applying liquids or other fluent materials performed by gravity only, i.e. flow coating
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B05—SPRAYING OR ATOMISING IN GENERAL; APPLYING FLUENT MATERIALS TO SURFACES, IN GENERAL
- B05D—PROCESSES FOR APPLYING FLUENT MATERIALS TO SURFACES, IN GENERAL
- B05D1/00—Processes for applying liquids or other fluent materials
- B05D1/62—Plasma-deposition of organic layers
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B05—SPRAYING OR ATOMISING IN GENERAL; APPLYING FLUENT MATERIALS TO SURFACES, IN GENERAL
- B05D—PROCESSES FOR APPLYING FLUENT MATERIALS TO SURFACES, IN GENERAL
- B05D2425/00—Indexing scheme corresponding to the position of each layer within a multilayer coating relative to the surface
- B05D2425/01—Indexing scheme corresponding to the position of each layer within a multilayer coating relative to the surface top layer/ last layer, i.e. first layer from the top surface
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B05—SPRAYING OR ATOMISING IN GENERAL; APPLYING FLUENT MATERIALS TO SURFACES, IN GENERAL
- B05D—PROCESSES FOR APPLYING FLUENT MATERIALS TO SURFACES, IN GENERAL
- B05D2451/00—Type of carrier, type of coating (Multilayers)
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B05—SPRAYING OR ATOMISING IN GENERAL; APPLYING FLUENT MATERIALS TO SURFACES, IN GENERAL
- B05D—PROCESSES FOR APPLYING FLUENT MATERIALS TO SURFACES, IN GENERAL
- B05D3/00—Pretreatment of surfaces to which liquids or other fluent materials are to be applied; After-treatment of applied coatings, e.g. intermediate treating of an applied coating preparatory to subsequent applications of liquids or other fluent materials
- B05D3/02—Pretreatment of surfaces to which liquids or other fluent materials are to be applied; After-treatment of applied coatings, e.g. intermediate treating of an applied coating preparatory to subsequent applications of liquids or other fluent materials by baking
- B05D3/0209—Multistage baking
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B05—SPRAYING OR ATOMISING IN GENERAL; APPLYING FLUENT MATERIALS TO SURFACES, IN GENERAL
- B05D—PROCESSES FOR APPLYING FLUENT MATERIALS TO SURFACES, IN GENERAL
- B05D3/00—Pretreatment of surfaces to which liquids or other fluent materials are to be applied; After-treatment of applied coatings, e.g. intermediate treating of an applied coating preparatory to subsequent applications of liquids or other fluent materials
- B05D3/02—Pretreatment of surfaces to which liquids or other fluent materials are to be applied; After-treatment of applied coatings, e.g. intermediate treating of an applied coating preparatory to subsequent applications of liquids or other fluent materials by baking
- B05D3/0254—After-treatment
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B05—SPRAYING OR ATOMISING IN GENERAL; APPLYING FLUENT MATERIALS TO SURFACES, IN GENERAL
- B05D—PROCESSES FOR APPLYING FLUENT MATERIALS TO SURFACES, IN GENERAL
- B05D3/00—Pretreatment of surfaces to which liquids or other fluent materials are to be applied; After-treatment of applied coatings, e.g. intermediate treating of an applied coating preparatory to subsequent applications of liquids or other fluent materials
- B05D3/06—Pretreatment of surfaces to which liquids or other fluent materials are to be applied; After-treatment of applied coatings, e.g. intermediate treating of an applied coating preparatory to subsequent applications of liquids or other fluent materials by exposure to radiation
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B05—SPRAYING OR ATOMISING IN GENERAL; APPLYING FLUENT MATERIALS TO SURFACES, IN GENERAL
- B05D—PROCESSES FOR APPLYING FLUENT MATERIALS TO SURFACES, IN GENERAL
- B05D5/00—Processes for applying liquids or other fluent materials to surfaces to obtain special surface effects, finishes or structures
- B05D5/06—Processes for applying liquids or other fluent materials to surfaces to obtain special surface effects, finishes or structures to obtain multicolour or other optical effects
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B32—LAYERED PRODUCTS
- B32B—LAYERED PRODUCTS, i.e. PRODUCTS BUILT-UP OF STRATA OF FLAT OR NON-FLAT, e.g. CELLULAR OR HONEYCOMB, FORM
- B32B2310/00—Treatment by energy or chemical effects
- B32B2310/08—Treatment by energy or chemical effects by wave energy or particle radiation
- B32B2310/0806—Treatment by energy or chemical effects by wave energy or particle radiation using electromagnetic radiation
- B32B2310/0825—Treatment by energy or chemical effects by wave energy or particle radiation using electromagnetic radiation using IR radiation
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B32—LAYERED PRODUCTS
- B32B—LAYERED PRODUCTS, i.e. PRODUCTS BUILT-UP OF STRATA OF FLAT OR NON-FLAT, e.g. CELLULAR OR HONEYCOMB, FORM
- B32B2310/00—Treatment by energy or chemical effects
- B32B2310/08—Treatment by energy or chemical effects by wave energy or particle radiation
- B32B2310/0806—Treatment by energy or chemical effects by wave energy or particle radiation using electromagnetic radiation
- B32B2310/0831—Treatment by energy or chemical effects by wave energy or particle radiation using electromagnetic radiation using UV radiation
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B32—LAYERED PRODUCTS
- B32B—LAYERED PRODUCTS, i.e. PRODUCTS BUILT-UP OF STRATA OF FLAT OR NON-FLAT, e.g. CELLULAR OR HONEYCOMB, FORM
- B32B2310/00—Treatment by energy or chemical effects
- B32B2310/08—Treatment by energy or chemical effects by wave energy or particle radiation
- B32B2310/0875—Treatment by energy or chemical effects by wave energy or particle radiation using particle radiation
- B32B2310/0887—Treatment by energy or chemical effects by wave energy or particle radiation using particle radiation using electron radiation, e.g. beta-rays
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08J—WORKING-UP; GENERAL PROCESSES OF COMPOUNDING; AFTER-TREATMENT NOT COVERED BY SUBCLASSES C08B, C08C, C08F, C08G or C08H
- C08J2369/00—Characterised by the use of polycarbonates; Derivatives of polycarbonates
Definitions
- the present invention relates to a laminate and a method for producing the same, and more specifically, relates to a laminate obtained by laminating an active energy ray-curable resin layer and an inorganic vapor deposition layer on an organic resin substrate, and a method for producing the same.
- a laminate in which an inorganic vapor deposition layer is provided on an organic resin substrate has excellent processability and lightness derived from an organic resin, and has excellent scratch resistance and chemical resistance derived from an inorganic vapor deposition layer. Therefore, it has attracted attention as a glass substitute material. Also, in such a laminate, for the purpose of supplementing the weather resistance of the organic resin base material and the purpose of improving the adhesion between the organic resin base material and the inorganic vapor deposition layer, generally, the organic resin base material and the inorganic resin base material are used. An intermediate layer is provided between the vapor deposition layer and the interlayer.
- thermosetting intermediate layer having a plurality of layers
- thermosetting material that does not require a primer even with a single layer polycarbonate resin base material.
- An intermediate layer (see Patent Document 2), a photo-curing intermediate layer that substantially requires a primer for a polycarbonate substrate (see Patent Document 3), a single-layer polycarbonate resin substrate that requires no primer
- Patent Document 4 a thermosetting intermediate layer having a plurality of layers
- thermosetting type is industrially disadvantageous because it requires a long time and heat energy for production, but it is said to be excellent in weather resistance.
- photo-curing type is industrially advantageous because it can be produced with low energy for a short time, but has insufficient weather resistance (see Non-Patent Document 1). Therefore, development of a laminate having an industrially advantageous photo-curing intermediate layer that can be produced in a short time and with low energy and having excellent weather resistance equivalent to or higher than that of a thermosetting type is required.
- count of coating is few is preferable, it is thought that the thing using the primer and excellent in adhesiveness and a weather resistance can be performed.
- Patent Document 6 discloses that the power spectrum is split when the reflected wave interference spectrum of the thin film is Fourier-transformed with respect to the wave number, but the split power spectrum ideally has almost the same height and spread ( [0006]) Further, if the intensity of the power spectrum is asymmetric, it is attributed to the difference in crystal structure, polarization, vibration of the measurement object, FFT processing, and their synergistic effect ([0007]).
- the present invention has been made in view of the above circumstances, and has a single intermediate layer made of an active energy ray-cured (photocured) film between an organic resin substrate and an inorganic vapor-deposited layer. It aims at providing the laminated body which shows the weather resistance and adhesiveness equivalent to or more than the laminated body which has this thermosetting film as an intermediate
- the present inventor has obtained a reflected wave spectrum with respect to the wave number in a coated article in which a cured product layer made of an active energy ray-curable resin composition is formed on an organic resin substrate.
- the Fourier-transformed power spectrum is split at a specific S / N ratio, the weather resistance and adhesion of a laminate in which an inorganic vapor-deposited layer is further laminated on the cured product layer is compared with that in which the power spectrum is not split.
- the present invention has been completed.
- the power spectrum obtained by Fourier transforming the reflected wave spectrum of the thin film is used for film thickness measurement, but can be used for characteristic analysis of the laminate. Until now, it has not been known at all that the performance of the laminate can be evaluated from a specific index of the spectrum.
- the present invention 1.
- a laminate having an organic resin base material and a single active energy ray-curable resin layer (i) and an inorganic vapor deposition layer (ii) sequentially laminated thereon, the layer (i) on the organic resin base material
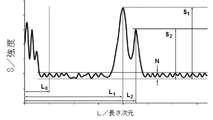
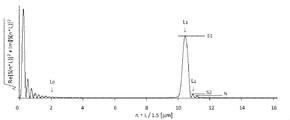
- the power spectrum obtained by Fourier-transforming the reflected wave spectrum by the reflectance spectroscopy in FIG. 5 with respect to the wave number and plotting the amplitude with respect to the length dimension is represented by L 1 and L 2 having a length dimension threshold L 0 or more.
- L 0 is an arbitrary value of 1 ⁇ 10 ⁇ 6 to 3 ⁇ 10 ⁇ 6 m
- L 0 or less in the power spectrum excluding the range of the domain of the first local maximum values S 1 in L 1 represents a 5 or more signal-to-noise ratio S 1 / N relative to the noise N, and first in the L 2 two Signal noise ratio S 2 / with a local maximum value S 2 of 2 or more with respect to noise N
- the active energy ray-curable resin layer (i) is an oligomer (A) of a silicate ester represented by the following general formula (1), and a bifunctional (meth) acrylate represented by the following general formula (2) ( Any one of 1 to 5 laminates comprising B),
- R is R 1 or R 2
- R 1 represents an alkyl group having 1 to 4 carbon atoms
- R 2 is a substituent represented by the following general formula (3)
- n is an integer of 1 to 10
- Z represents a divalent organic group containing a linear, branched or cyclic saturated hydrocarbon having 4 to 20 carbon atoms
- R 4 represents a hydrogen atom or a methyl group independently of each other.
- Y represents a linear alkylene group having 2 to 10 carbon atoms, and R 3 represents a hydrogen atom or a methyl group.
- L 1 and L 2 having a length dimension threshold L 0 or more.
- the first maximum value S 1 at L 1 indicates a signal noise ratio S 1 / N of 5 or more with respect to noise N
- the first maximum value at L 2 The second maximum value S 2 has a signal noise ratio S 2 / N of 2 or more with respect to the noise N.
- a method for producing a laminate characterized in that the range shown in the figure, 9.
- the step of laminating a single active energy ray-curable resin layer (i) on the organic resin base material is ( ⁇ ): a silicate ester represented by the following general formula (1) on the organic resin base material:
- R is R 1 or R 2
- R 1 represents an alkyl group having 1 to 4 carbon atoms
- R 2 is a substituent represented by the following general formula (3)
- n is an integer of 1 to 10
- Z represents a divalent organic group containing a linear, branched or cyclic saturated hydrocarbon having 4 to 20 carbon atoms
- R 4 represents a hydrogen atom or a methyl group independently of each other.
- Y represents a linear alkylene group having 2 to 10 carbon atoms
- R 3 represents a hydrogen atom or a methyl group.
- the reflected wave spectrum by the reflectance spectroscopy in the layer (i) on the organic resin substrate is Fourier-transformed with respect to the wave number, Provided is a method for manufacturing a laminated body according to any one of 8 to 14, including a step ( ⁇ ) of inspecting a power spectrum obtained by plotting an amplitude with respect to a vertical dimension.
- a laminate having an organic resin base material, a single-layer active energy ray-curable resin layer and an inorganic vapor deposition layer formed thereon, and excellent weather resistance and adhesion In addition, a manufacturing method thereof can be provided. In particular, it has excellent weather resistance and adhesion by using as an index the power spectrum obtained by Fourier transforming the reflected wave of the two-layered body in which the active energy ray-curable resin is formed on the organic resin base material with respect to the wave number. It is possible to design a laminate having The laminate of the present invention can be advantageously produced industrially because it does not require thermosetting, and peeling does not easily occur even under use conditions where peeling between the thermosetting acrylic resin layer and the thermosetting silicone resin layer becomes a problem.
- automobile headlamp covers protective films for LCDs used outdoors, building materials such as carports and sunroofs, motorcycle windshields and bullet trains, transportation equipment such as construction heavy machinery windows, automotive glazing, solar It can be suitably used for a protective film of a battery condensing mirror.
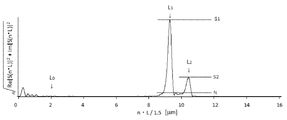
- FIG. It is a Fourier-transform result of Example 1.
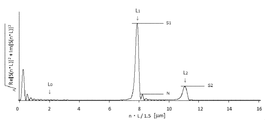
- FIG. It is a Fourier-transform result of Example 3.
- the laminate according to the present invention comprises an organic resin substrate, a single-layer active energy ray-curable resin layer (i) and an inorganic vapor deposition layer (ii) sequentially laminated thereon,
- the power spectrum obtained by Fourier-transforming the reflected wave spectrum by reflectance spectroscopy in the layer (i) on the material with respect to the wave number and plotting the amplitude with respect to the length dimension is equal to or greater than the threshold L 0 of the length dimension.
- L 1 and L 2 respectively have a first maximum value S 1 and a second maximum value S 2
- the threshold value L 0 is an arbitrary value between 1 ⁇ 10 ⁇ 6 and 3 ⁇ 10 ⁇ 6 m (for example, 2 ⁇ 10 ⁇ 6 m)
- the second maximum value S 2 at L 2 is A signal noise ratio S 2 / N of 2 or more is shown.
- the laminate of the present invention is a laminate obtained by sequentially laminating a single layer (i) made of an active energy ray-curable coating and a layer (ii) on at least one surface of an organic resin substrate. Even if the layers (i) and (ii) are laminated on both sides of the organic resin substrate, the layers (i) and (ii) are laminated on one surface of the organic resin substrate, and the other A configuration having layers may also be used.
- the organic resin constituting the substrate is not particularly limited, but specific examples thereof include polycarbonate resin, acrylic resin, epoxy resin, ABS resin, PET resin, PP resin, PE resin, One or more selected from POM resins and silicone resins are preferred, and polycarbonate resins are more preferred.
- the above parameters (L 0 , L 1 , L 2 , N, S 1 , S 2 ) used in the present invention are all Fourier transforms of the reflected wave spectrum of the layer (i) formed on the organic resin substrate. It is specified in the space.
- the reflected wave spectrum that is the premise of the Fourier transform will be described.
- the reflected wave spectrum is obtained by recording the reflectance of the film with a spectrophotometer. This reflectance measurement is preferably carried out in a range in which the layer (i) to be measured does not absorb light, and specifically, a reflection spectrum in the range of 300 to 10,000 nm is preferably measured. It is more preferable to measure a reflection spectrum in the range of ⁇ 5,000 nm.
- the obtained reflection spectrum is preferably converted to wave number on the horizontal axis, and the wavelength dependence of the reflection spectrum is eliminated by converting the horizontal axis to wave number, and the period depends only on the state of the film. You can get a function.
- the wavelength has the inverse dimension of the length, but after the Fourier transform, the dimension of the original function is reversed, and the Fourier transform of the wave number gives the dimension of the length, so the data interpretation after the Fourier transform is easy Can be.
- the reflected wave spectrum whose horizontal axis is converted into wave number units is then subjected to Fourier transform.
- Fourier transform numerical analysis methods such as discrete Fourier transform, fast Fourier transform, and Fourier numerical integration can be used.
- the data points may not be uniform by a method such as a photodiode array method, a diffraction grating, or in the process of wave number conversion.
- interpolation processing may be performed.
- the interpolation processing can be performed using linear interpolation, quadratic interpolation, Lagrangian interpolation, a spline function, or the like. When equal-width interpolation is performed, it may be preferable to apply to fast Fourier transform or discrete Fourier transform.
- the basic Fourier transform method is described below.
- the reflected wave spectrum f (k) expressed by the wave number axis and S (n ⁇ L) as a variable parameter in the present invention can be expressed by a Fourier transform as shown in Equation (1).
- Equation (1) the reflected wave spectrum f (k) is defined as a continuous function and an infinite interval
- Equation (1) the reflected wave spectrum f (k) is defined as a continuous function and an infinite interval
- Equation (1) can be used as it is, but f (k) is a discontinuous data set in actual measurement, The domain is not infinite. Therefore, generally, a discrete Fourier transform is used.
- the discrete Fourier transform in the present invention can be expressed by Equation (2).
- k j is a wave number that increases in the order of j
- ⁇ k j is a sampling interval
- n may be a function of L.
- the length dimension L in the present invention is defined as n ⁇ L calculated by dividing n when n is regarded as a constant. Can be done.
- n / L variable separation may be performed using constraint conditions, and n may be solved for L.
- this is different from the space of S (L) handled in the present invention, it is not mentioned in this specification.
- a program based on the above-described principle may be created, but a commercially available Fourier transform program, FFT analyzer, or the like can also be used.
- S (n ⁇ L) is a complex function, it cannot be plotted in real space as it is. Therefore, it is preferable to display the amplitude by coefficient conversion of the real part and the imaginary part. It is easy to understand that the vertical axis is the amplitude in practice, but it is possible to create a space that is energetically equivalent even if it is not necessarily the amplitude, according to Planschler's theorem. is not. When the vertical axis is other than the amplitude, it is preferable to re-formulate the parameter with an integrated ratio so as to be equivalent to the parameter in the case of the amplitude. When the fast Fourier transform is used, a full power spectrum that is symmetrical from the turning point can be obtained.
- the definition region of the power spectrum in the present invention is a one-sided power spectrum, and is a region excluding the threshold value L 0 described below.
- L 0 is a threshold value in the length dimension L.
- L is a threshold value in the length dimension L.
- the conversion function is extended, and if the original function is extended, the conversion function is compressed.
- the ⁇ function is a prominent example. This means that obtaining an accurate power spectrum in the region close to the origin in the length dimension L means that it is necessary to collect data having a longer period in the original function.
- the original function in the present invention is a reflected wave spectrum
- a long-period original function is not obtained indefinitely due to the light absorption characteristics of the layer (i) and the characteristics of the spectrometer.
- a signal derived from the sampling period of the original function also appears.
- a signal derived from the roughness of the outermost surface may be mixed.
- L 0 is 1 ⁇ 10 ⁇ 6 to 3 ⁇ 10 ⁇ 6 m, preferably 1.5 ⁇ 10 ⁇ 6 to 2.5 ⁇ 10 ⁇ 6 m, more preferably 2 ⁇ 10 ⁇ 6 m. It is. If L 0 is in the above range, the present invention is not greatly affected.
- S 1 is a main amplitude signal, which indicates the maximum intensity (first maximum value) in the defined region of the power spectrum excluding the region below L 0 .
- the value in the length dimension L when indicating S 1 is L 1 .
- S 1 has a signal-to-noise ratio (S 1 / N) of 5 or more, preferably 10 or more, more preferably 15 or more, and even more preferably 20 or more with respect to noise N described below. If the S 1 / N ratio is less than 5, the error of the measurement itself is large and the intended effect of the present invention is not achieved. There is no particular upper limit for the S 1 / N ratio.
- L 1 is a parameter appearing on the length dimension L, it has a specific length.
- L 1 can take a value of 3 ⁇ 10 ⁇ 6 to 3 ⁇ 10 ⁇ 5 m, preferably 5 ⁇ 10 ⁇ 6 to 2 ⁇ 10 ⁇ 5 m, more preferably 8 ⁇ 10 ⁇ 6 to 1.5. ⁇ 10 -5 m. If it is less than 5 ⁇ 10 ⁇ 6 m, thermal degradation may occur in the layer (ii) lamination, and if it exceeds 2 ⁇ 10 ⁇ 5 m, cracks may occur in the layer (ii) lamination.
- L 2 and S 2 relate to the associated amplitude signal.
- S 2 is an accompanying amplitude signal, and indicates an intensity (second maximum value) next to S 1 in the domain of the power spectrum excluding the region below L 0 .
- the value in the length dimension L when indicating S 2 is L 2 .
- S 2 has a signal / noise ratio (S 2 / N) of 2 or more, preferably 2.5 or more, more preferably 3 or more with respect to noise N described below. If the S 2 / N ratio is less than 2 times, the difference from noise is not clear, and an erroneous signal may be identified as S 2 .
- the upper limit of the S 2 / N ratio is not particularly defined, it is less than S 1 / N under the same N in terms of meaning.
- S 2 should be re-identified as the main amplitude signal and S 1 as the accompanying amplitude signal. Since L 2 is a parameter that appears on the length dimension L, it has a specific length. L 2 can take a range of 0.5L 1 to 2.0L 1 , but more preferably 1.0L 1 ⁇ L 2 ⁇ 1.5L 1 .
- the noise N may be white noise, colored noise, or the like.
- the noise N may be defined by a statistical or mathematical method.
- the noise N is white noise, it may be defined by a simple method described below. That is, the upper limit is the value of the signal having the third intensity after S 2 , and the lower limit is the value when the intensity is the smallest. A value obtained by subtracting the lower limit value from the upper limit value obtained here can be used as the noise N in the present invention. If it is colored noise, determine the baseline by regression analysis, visual inspection, etc., and then move the base line in parallel while maintaining the slope or curvature of the baseline. The noise N can be obtained by obtaining the lower limit value. Under the measurement conditions of the present invention, white noise generally appears rather than colored noise.
- S 2 is preferably 0.05 S 1 to 0.95 S 1 , more preferably 0.1 S 1 to 0.9 S 1 .
- the layer (i) constituting the laminate of the present invention is a single resin layer made of an active energy ray-curable paint.
- the active energy ray-curable coating material includes an oligomer (A) of a silicate ester represented by the following general formula (1) and a bifunctional (meth) acrylate (B) represented by the following general formula (2). Is preferred.
- R is R 1 or R 2
- R 1 represents an alkyl group having 1 to 4 carbon atoms
- R 2 represents a substituent represented by the following general formula (3)
- the molar ratio [R 1 ] / [R 2 ] of R 1 and R 2 in all R satisfies 0 to 10
- n is an integer of 1 to 10.
- Z represents a divalent organic group containing a linear, branched or cyclic saturated hydrocarbon having 4 to 20 carbon atoms
- R 4 independently of each other, represents a hydrogen atom or methyl Represents a group.
- Y represents a linear alkylene group having 2 to 10 carbon atoms
- R 3 represents a hydrogen atom or a methyl group.
- the silicate oligomer (A) represented by the general formula (1) can be produced, for example, by a reaction between a silicate and 1 mole equivalent or more of an ⁇ -functional (meth) acrylate alkylene alcohol.
- silicates include tetraalkoxysilanes such as tetramethoxysilane and tetraethoxysilane; methyl silicate 51, methyl silicate 53A, ethyl silicate 40, ethyl silicate 48 (above, manufactured by Colcoat Co., Ltd., product name), Examples thereof include silicate oligomers such as X-40-2308 (manufactured by Shin-Etsu Chemical Co., Ltd., product name).
- ⁇ -functional (meth) acrylic acid ester alkylene alcohols include hydroxymethyl acrylate, hydroxyethyl acrylate, hydroxypropyl acrylate, hydroxybutyl acrylate, hydroxyoctyl acrylate, and the like.
- the alkyl group having 1 to 4 carbon atoms may be linear, branched or cyclic, and specific examples thereof include methyl, ethyl, propyl, isopropyl, butyl, isobutyl, Examples thereof include a tert-butyl group.
- R 1 preferably contains one or more methyl groups, and when there are a plurality of R 1 , all of them are more preferably methyl groups.
- linear alkylene group having 2 to 10 carbon atoms examples include ethylene, trimethylene, tetramethylene, pentamethylene, hexamethylene, heptamethylene, octamethylene, nonamethylene, decamethylene group and the like. It is done. Among these, as Y, an ethylene group is preferable.
- R 3 is a hydrogen atom or a methyl group, preferably a hydrogen atom.
- the molar ratio [R 1 ] / [R 2 ] of R 1 and R 2 in the total R is 0 to 10, and this ratio is the synthesis of the oligomer (A) of the silicate ester
- it can be controlled by the number of equivalents of the reacting ⁇ -functional (meth) acrylate alkylene alcohols.
- the number of equivalents can be determined with reference to the molecular weight measurement by GPC of the silicate as the raw material.
- divalent organic group containing a linear, branched or cyclic saturated hydrocarbon having 4 to 20 carbon atoms include tetramethylene, pentamethylene, hexamethylene, heptamethylene, Linear alkylenes such as octamethylene, nonamethylene, decamethylene, undecamethylene, dodecamethylene, tridecamethylene group; branched such as neopentylene, 3-methyl-1,5-pentylene, 2-ethyl-1,6-hexylene group Chain alkylenes; and divalent organic groups containing cyclic saturated hydrocarbon groups such as 1,4-cyclohexylene, bis (methylidene) tricyclodecane, and decalylene groups.
- Z a hexamethylene, nonamethylene, or neopentylene group is preferable, and a hexamethylene group is more preferable.
- R 4 is a hydrogen atom or a methyl group.
- the active energy ray-curable coating material of the present invention may contain other components other than the components (A) and (B) described above as long as the layer (i) satisfies the parameters of the present invention.
- examples of such other components include ultraviolet absorbers (P), vinyl polymers (Q), acrylic esters (R) other than the above (B), and inorganic oxide fine particles such as titanium oxide and silicon oxide. (S), solvent (T), photoradical initiator (U) and the like.
- Examples of the ultraviolet absorber (P) include benzotriazoles, benzophenones, resorcinols, and triazines having a vinyl polymerizable group. More specifically, a (meth) acrylic monomer having an ultraviolet absorbing group in the molecule, that is, a benzotriazole compound represented by the following general formula (IX) and the following general formula (X) Benzophenone compounds and the like.
- X represents a hydrogen atom or a chlorine atom
- R 11 represents a hydrogen atom, a methyl group, or a tertiary alkyl group having 4 to 8 carbon atoms
- R 12 represents a linear or branched group. Represents a chain-like alkylene group having 2 to 10 carbon atoms
- R 13 represents a hydrogen atom or a methyl group
- q represents 0 or 1.
- R 13 has the same meaning as described above.
- R 14 represents a substituted or unsubstituted linear or branched alkylene group having 2 to 10 carbon atoms
- R 15 represents a hydrogen atom.
- R 16 represents a hydrogen atom, a hydroxyl group, or an alkoxy group having 1 to 6 carbon atoms.
- tertiary alkyl group having 4 to 8 carbon atoms examples include tert-butyl, tert-pentyl, tert-hexyl, tert-heptyl, tert-octyl, and ditert-octyl. Groups and the like.
- linear or branched alkylene group having 2 to 10 carbon atoms include ethylene, trimethylene, propylene, tetramethylene, 1,1-dimethyltetramethylene, butylene, octylene, decylene group and the like.
- examples of the linear or branched alkylene group having 2 to 10 carbon atoms include the same groups as those exemplified for R 12 above, and some of these hydrogen atoms. Is a group substituted with a halogen atom.
- Specific examples of the alkoxy group having 1 to 6 carbon atoms include methoxy, ethoxy, propoxy, and butoxy groups.
- benzotriazole compound represented by the general formula (IX) examples include 2- (2′-hydroxy-5 ′-(meth) acryloxyphenyl) -2H-benzotriazole, 2- (2′- Hydroxy-3'-tert-butyl-5 '-(meth) acryloxymethylphenyl) -2H-benzotriazole, 2- [2'-hydroxy-5'-(2- (meth) acryloxyethyl) phenyl]- 2H-benzotriazole, 2- [2′-hydroxy-3′-tert-butyl-5 ′-(2- (meth) acryloxyethyl) phenyl] -5-chloro-2H-benzotriazole, 2- [2 ′ And -hydroxy-3'-methyl-5 '-(8- (meth) acryloxyoctyl) phenyl] -2H-benzotriazole.
- benzophenone compound represented by the general formula (X) include 2-hydroxy-4- (2- (meth) acryloxyethoxy) benzophenone and 2-hydroxy-4- (4- (meth) acrylic. Roxybutoxy) benzophenone, 2,2′-dihydroxy-4- (2- (meth) acryloxyethoxy) benzophenone, 2,4-dihydroxy-4 ′-(2- (meth) acryloxyethoxy) benzophenone, 2,2 ', 4-trihydroxy-4'-(2- (meth) acryloxyethoxy) benzophenone, 2-hydroxy-4- (3- (meth) acryloxy-2-hydroxypropoxy) benzophenone, 2-hydroxy-4- ( And 3- (meth) acryloxy-1-hydroxypropoxy) benzophenone.
- the UV-absorbing vinyl monomer is preferably a benzotriazole compound represented by the formula (IX), among which 2- [2′-hydroxy-5 ′-(2- (meth) acryloxyethyl) phenyl ] -2H-benzotriazole is more preferred. Furthermore, the ultraviolet-absorbing vinyl monomers may be used singly or in combination of two or more.
- D represents a vinyl monomer having an alkoxysilyl group
- d is a mole fraction such that the monomer D is 1 to 50% by mass with respect to the total amount of the polymer
- E is Represents an ultraviolet-absorbing vinyl monomer
- e is a mole fraction such that the monomer E is 5 to 40% by mass with respect to the total amount of the polymer
- F is a copolymer with these vinyl monomers.
- the other monomer that can be polymerized represents f
- the monomer F is [100- (content of monomer D)-(content of monomer E)]% by mass with respect to the total amount of the polymer.
- the molar fraction is such that
- the vinyl monomer unit D is preferably formed by addition polymerization of a vinyl monomer having an alkoxysilyl group.
- vinyl monomers having an alkoxysilyl group include acryloyloxymethyltrimethoxysilane, acryloyloxymethyldimethoxymethylsilane, acryloyloxymethylmethoxydimethylsilane, methacryloyloxymethyltrimethoxysilane, and methacryloyloxymethyldimethoxy.
- Methylsilane methacryloyloxymethylmethoxydimethylsilane, 2-acryloyloxyethyltrimethoxysilane, 2-acryloyloxyethyldimethoxymethylsilane, 2-acryloyloxyethylmethoxydimethylsilane, 2-methacryloyloxyethyltrimethoxysilane, 2- Methacryloyloxyethyldimethoxymethylsilane, 2-methacryloyloxyethylmethoxydimethylsilane, -Acryloyloxypropyltrimethoxysilane, 3-acryloyloxypropyldimethoxymethylsilane, 3-acryloyloxypropylmethoxydimethylsilane, 3-methacryloyloxypropyltrimethoxysilane, 3-methacryloyloxypropyldimethoxymethylsilane, 3-methacryloyloxypropyldime
- Branched-chain alkenylalkoxysilanes aromatic unsaturated alkoxysilanes such as p-trimethoxysilylstyrene, 1,4-divinyl-2-trimethoxysilylbenzene, p-trimethoxysilyl- ⁇ -methylstyrene, etc.
- aromatic unsaturated alkoxysilanes such as p-trimethoxysilylstyrene, 1,4-divinyl-2-trimethoxysilylbenzene, p-trimethoxysilyl- ⁇ -methylstyrene, etc.
- 3-methacryloyloxypropyltrimethoxysilane for example, product name “KBM-503” manufactured by Shin-Etsu Chemical Co., Ltd.
- the vinyl monomer unit D has a molar fraction d such that it is 1 to 50% by mass, preferably 2 to 40% by mass, more preferably 5 to 35% by mass with respect to the total mass of the polymer (VIII).
- the monomer units E and F can be copolymerized. If the vinyl monomer unit D is less than 1% by mass relative to the total mass of the polymer, it may be difficult to form a network with the inorganic fine particle component, and if it exceeds 50% by mass, storage stability and weather resistance will be reduced. There is a case.
- the vinyl monomer unit E is preferably formed by addition polymerization of a vinyl monomer having an ultraviolet absorbing group.
- Any vinyl monomer having an ultraviolet absorbing group can be used as long as it has an ultraviolet absorbing group and a vinyl polymerizable group.
- the ultraviolet ray in the present invention refers to light having a wavelength of about 200 to 400 nm.
- Examples of ultraviolet absorbing groups include organic groups having benzotriazoles, benzophenones, resorcinols, triazines, etc.
- vinyl polymerizable groups include organic groups having vinyl, allyl, styryl, acryl, methacryl groups, etc. Groups.
- Examples of the vinyl monomer having an organic ultraviolet absorbing group include a (meth) acrylic monomer having an ultraviolet absorbing group in the molecule. Specific examples thereof include the general formula described above.
- the benzotriazole compound represented by (IX) and the benzophenone compound represented by the general formula (X) and each of the exemplified compounds included in these compounds can be mentioned. You may mix and use a seed
- benzotriazole compounds represented by the above formula (IX) are preferable, and in particular, 2- [2′-hydroxy-5 ′-(2- (meth) acryloxyethyl) phenyl] -2H-benzotriazole Is more preferable.
- the vinyl monomer unit E has a molar fraction e of 5 to 40% by mass, preferably 5 to 30% by mass, more preferably 8 to 25% by mass with respect to the total mass of the polymer (VIII).
- the monomer units D and F can be copolymerized.
- the weather resistance may be insufficient, and when it exceeds 40% by mass, the adhesion to the substrate may be reduced. .
- the other vinyl monomer unit F copolymerizable with the vinyl monomer units D and E is not particularly limited as long as it is a copolymerizable monomer, but a (meth) acrylic monomer having a cyclic hindered amine structure.
- Body (meth) acrylic acid ester, (meth) acrylonitrile, (meth) acrylamide, alkyl vinyl ether, alkyl vinyl ester, styrene, and derivatives thereof.
- (meth) acrylic monomer having a cyclic hindered amine structure examples include 2,2,6,6-tetramethyl-4-piperidinyl methacrylate, 1,2,2,6,6-pentamethyl-4 -Piperidinyl methacrylate and the like.
- (meth) acrylic acid esters and derivatives thereof include methyl (meth) acrylate, ethyl (meth) acrylate, n-propyl (meth) acrylate, isopropyl (meth) acrylate, (meth) acrylic acid.
- n-butyl isobutyl (meth) acrylate, sec-butyl (meth) acrylate, tert-butyl (meth) acrylate, n-pentyl (meth) acrylate, isopentyl (meth) acrylate, (meth) acrylic acid n-hexyl, isohexyl (meth) acrylate, n-heptyl (meth) acrylate, isoheptyl (meth) acrylate, 2-ethylhexyl (meth) acrylate, n-octyl (meth) acrylate, (meth) acrylic acid Isooctyl, n-nonyl (meth) acrylate, isononyl (meth) acrylate, (meth ) N-decyl acrylate, isodecyl (meth) acrylate, n-undecyl (meth) acrylate,
- Mono (meth) acrylic acid esters of monohydric alcohols ethylene glycol di (meth) acrylate, propylene glycol di (meth) acrylate, butylene glycol di (meth) acrylate, glycerin di (meth) acrylate, glycerin tri (meth) acrylate, Pentaerythritol di (meth) acrylate, pentaerythritol tetra (meth) acrylate, 1,4-cyclohexanediol di (meth) acrylate, polyethylene glycol di (meth) )
- Poly (meth) acrylic acid esters of polyhydric alcohols such as acrylate (number of ethylene glycol units is, for example, 2 to 20), polypropylene glycol di (meth) acrylate (number of propylene glycol units is, for example, 2 to 20); succinic acid mono [2- (meth) acryloyloxyethyl], di [2-
- (meth) acrylonitrile examples include ⁇ -chloroacrylonitrile, ⁇ -chloromethylacrylonitrile, ⁇ -trifluoromethylacrylonitrile, ⁇ -methoxyacrylonitrile, ⁇ -ethoxyacrylonitrile, vinylidene cyanide and the like.
- (meth) acrylamide derivatives include N-methyl (meth) acrylamide, N, N-dimethyl (meth) acrylamide, N-ethyl (meth) acrylamide, N, N-diethyl (meth) acrylamide, N- Methoxy (meth) acrylamide, N, N-dimethoxy (meth) acrylamide, N-ethoxy (meth) acrylamide, N, N-diethoxy (meth) acrylamide, diacetone (meth) acrylamide, N-methylol (meth) acrylamide, N- (2-hydroxyethyl) (meth) acrylamide, N, N-dimethylaminomethyl (meth) acrylamide, N- (2-dimethylamino) ethyl (meth) acrylamide, N, N′-methylenebis (meth) acrylamide, N, N'-ethylenebis (meth) acrylic Bromide, and the like.
- alkyl vinyl ether examples include methyl vinyl ether, ethyl vinyl ether, butyl vinyl ether, hexyl vinyl ether and the like.
- alkyl vinyl ester examples include vinyl formate, vinyl acetate, vinyl acrylate, vinyl butyrate, vinyl caproate, and vinyl stearate.
- styrene and its derivatives include styrene, ⁇ -methylstyrene, vinyltoluene and the like.
- (meth) acrylic acid esters are preferable, and in particular, methyl (meth) acrylate, ethyl (meth) acrylate, isopropyl (meth) acrylate, n-butyl (meth) acrylate, ( Isobutyl acrylate, n-hexyl (meth) acrylate, 2-ethylhexyl (meth) acrylate, isononyl (meth) acrylate, lauryl (meth) acrylate, cyclohexyl (meth) acrylate, (meth) acrylic acid 4-methylcyclohexyl, 4-tert-butylcyclohexyl (meth) acrylate, isobornyl (meth) acrylate, dicyclopentanyl (meth) acrylate, dicyclopentenyloxyethyl (meth) acrylate and the like are suitable.
- the vinyl-type monomer unit C can use the said monomer
- the vinyl monomer unit F is [100- (content of monomer D) ⁇ (content of monomer E)]% by mass, ie, 10 to 94% by mass with respect to the total mass of the polymer (VIII). %, Preferably 20 to 94% by mass, more preferably 35 to 90% by mass, with a molar fraction f so that it can be copolymerized with other monomer units D and E. If the vinyl monomer unit F is less than 10% by mass with respect to the total mass of the polymer, a coating appearance defect may occur. May decrease.
- Component (Q) is preferably obtained by copolymerizing vinyl-based monomer units D, E, and F.
- a radical polymerization initiator selected from peroxides such as dicumyl peroxide and benzoyl peroxide or azo compounds such as azobisisobutyronitrile is added to a solution containing these monomers, The reaction can be performed under heating (50 to 150 ° C., particularly 70 to 120 ° C. for 1 to 10 hours, particularly 3 to 8 hours).
- the polystyrene-reduced weight average molecular weight of the component (Q) by gel permeation chromatography (GPC) is not particularly limited, but is preferably 1,000 to 300,000, particularly 5,000 to 250,000. If the molecular weight is too large, the viscosity becomes too high and it may be difficult to synthesize, or it may be difficult to handle, and if it is too small, it may cause poor appearance such as whitening of the coating and weathering cracks, sufficient adhesion, Durability and weather resistance may not be obtained.
- Component (Q) may be manufactured using the components described above, or a commercially available product may be used.
- New Coat UVA-101, 102, 103, 104, Vanaresin UVA-5080, 55T, 55MHB, 7075, 73T above, manufactured by Shin-Nakamura Chemical Co., Ltd.
- the acrylic polymer of component (Q) may be a polycarbonate-based and / or polyester-based urethane-modified vinyl polymer.
- Polycarbonate-based and / or polyester-based urethane-modified vinyl polymers act as adhesion improvers. Specifically, they are layer-separated from other components in the cured film and have a concentration gradient in the thickness direction of the film. Thus, it is considered that the affinity to the organic resin substrate is increased and the adhesion is exhibited without reducing the scratch resistance.
- Such a polycarbonate-based and / or polyester-based urethane-modified vinyl polymer is obtained by grafting a polycarbonate-based or polyester-based polyurethane to a vinyl-based polymer.
- a vinyl polymer having a polycarbonate or polyester polyurethane obtained by the reaction of an aromatic polyester diol and an aromatic diisocyanate in the side chain is preferred, and a polycarbonate urethane obtained by the reaction of an aliphatic polycarbonate diol and an aromatic diisocyanate is preferred. More preferred are vinyl polymers in the chain.
- aliphatic polycarbonate diol examples include 1,4-tetramethylene type, 1,5-pentamethylene type, 1,6-hexamethylene type, 1,12-dodecane type, 1,4-cyclohexane type, A mixed type etc. are mentioned.
- aromatic isocyanate examples include 4,4'-diphenylmethane diisocyanate, 2,4-tolylene diisocyanate, 2,6-tolylene diisocyanate, m-xylene diisocyanate, naphthalene diisocyanate, and mixtures thereof.
- any monomer can be used as long as it contains a vinyl polymerizable group.
- Specific examples include methyl (meth) acrylate, ethyl (meth) acrylate, butyl (meth) acrylate, cyclohexyl (meth) acrylate, glycidyl (meth) acrylate, 2-hydroxyethyl (meth) acrylate, and (meth) acrylic acid. , Styrene, vinyl acetate and the like.
- a vinyl polymer is obtained by polymerizing these monomers by a known method.
- the urethane-modified vinyl polymer is preferably dissolved in an organic solvent in view of ease of synthesis, ease of handling, and the like.
- the organic solvent is not particularly limited as long as it dissolves a polycarbonate-based and / or polyester-based urethane-modified vinyl polymer component and is a relatively polar organic solvent.
- isopropyl alcohol Alcohols such as n-butanol, isobutanol, tert-butanol, diacetone alcohol; ketones such as methyl ethyl ketone, diethyl ketone, methyl isobutyl ketone, cyclohexanone, diacetone alcohol; dipropyl ether, dibutyl ether, anisole, dioxane, ethylene Ethers such as glycol monoethyl ether, ethylene glycol monobutyl ether, propylene glycol monomethyl ether, propylene glycol monomethyl ether acetate; ethyl acetate, vinegar Propyl, butyl acetate, esters such as cyclohexyl acetate and the like, These may be used alone, or may be used in combination of two or more.
- the molecular weight of the polycarbonate-based and / or polyester-based urethane-modified vinyl polymer is preferably 5,000 to 50,000, more preferably 7,000 to 40,000 in terms of weight average molecular weight by GPC analysis based on polystyrene. preferable. If the weight average molecular weight is less than 5,000, sufficient adhesion to the organic resin substrate may not be exhibited, and if it exceeds 50,000, the transparency when formed into a cured film may be impaired, There is a possibility that the solubility in the composition is lowered or separated.
- the hydroxyl value of the polycarbonate-based and / or polyester-based urethane-modified vinyl polymer is preferably 10 or more, more preferably in the range of 20 to 100, in terms of the solid content of this component.
- the solubility in the composition is lowered, and this component may be separated.
- polycarbonate-based and / or polyester-based urethane-modified vinyl polymer component commercially available products can be used. Examples of such commercially available products include Acryt 8UA-347 manufactured by Taisei Fine Chemical Co., Ltd. 357, 366 (polycarbonate), 140, 146, 301, 318 (polyester), and the like.
- the acrylic esters (R) are acrylic esters other than the substance (B), and specific examples thereof are not particularly limited, but are methyl methacrylate (abbreviation MMA), methyl acrylate (abbreviation MA). , Ethyl methacrylate, ethyl acrylate, hydroxyethyl acrylate (abbreviation HEA), hydroxyethyl methacrylate (abbreviation HEMA), hydroxypropyl acrylate, 4-hydroxyl acrylate, isobutyl acrylate, tert-butyl acrylate, acrylic N-octyl acid, isooctyl acrylate, isononyl acrylate, lauryl acrylate, stearyl acrylate, isostearyl acrylate, isonorbornyl acrylate, tetrahydrofurfuryl acrylate, acrylic acid (methoxyethyl), acrylic Methoxypolyethylene glycol, acrylic acid (2-methyl-2-e
- the amount of the substance (B) used is preferably 3 to 50% by mass, more preferably 5 to 40% by mass, and still more preferably 10 to 30% by mass with respect to the substance (R).
- (B) is less than 3% by mass, the power spectrum may be difficult to split, and when (B) is greater than 50% by mass, appearance abnormalities such as streaks and whitening may occur when the layer (i) is formed. is there.
- the inorganic oxide fine particles (S) include one or more selected from the group of inorganic oxide fine particles such as silicon oxide, zinc oxide, titanium oxide, cerium oxide, and aluminum oxide.
- the particle size of the inorganic oxide fine particles is preferably 5 to 200 nm, more preferably 10 to 150 nm, and even more preferably 15 to 100 nm. If it is less than 5 nm, it may easily aggregate, and if it is more than 200 nm, the transparency of the coating film may be lowered.
- the solvent (T) is not particularly limited, but pentane, hexane, heptane, octane, nonane, decane, undecane, dodecane, tridecane, tetradecane, pentadecane, hexadecane, heptadecane, octadecane, nonadecane, icosane, eicosane, Docosan, Triicosan, Tetricosan, Pentycosan, Hexaicosan, Heptycosan, Octaicosan, Nonaicosan, Triacontane, benzene, toluene, o-xylene, m-xylene, p-xylene, and mixtures containing these, petroleum ether, kerosene, ligroin, nujol Hydrocarbon compounds having 5 to 30 carbon atoms such as methanol, ethanol, 1-propanol
- the photo radical initiator (U) is not particularly limited, and examples thereof include 2,2-dimethoxy-1,2-diphenylethane-1-one, 1-hydroxycyclohexyl phenyl ketone, and 2-hydroxy-2-methyl.
- Alkylphenones such as -1-phenylpropan-one, 1- [4- (2-hydroxyethoxy) -phenyl] -2-hydroxy-2-methyl-1-propan-1-one, and phenylglyoxylic acid methyl ester; 2-methyl-1- (4-methylthiophenyl) -2-morpholinopropan-1-one, 2-benzyl-2-dimethylamino-1- (4-morpholinophenyl) -butanone-1,2- (dimethyl Amino) -2-[(4-methylphenyl) methyl] -1- [4- (4-morpholinyl) phenyl] -1-butanone and the like Roh alkylphenones; 2,4,6-trimethylbenzoyl over diphen
- the above-mentioned substances (A), substances (B), ultraviolet absorbers (P), vinyl polymers (Q), acrylic esters (R) other than (B), inorganic oxides The compounding amounts of the fine particles (S), the solvent (T), and the photo radical initiator (U) are 5 to 20% by mass for the substance (A), 3 to 10% by mass for the substance (B), and an ultraviolet absorber.
- (P) is 1 to 10% by mass
- vinyl copolymer (Q) is 1 to 10% by mass
- acrylic esters (R) is 5 to 30% by mass
- inorganic oxide fine particles (S) are 1 to 25% by mass.
- the solvent (T) is preferably 20 to 90% by mass
- the photo radical initiator (U) is preferably 0.1 to 10% by mass.
- the layer (ii) constituting the laminate of the present invention is an inorganic vapor-deposited layer as described above, and specific examples thereof include oxides of elements selected from the group consisting of silicon, boron, titanium, zirconium, aluminum, and the like.
- a layer formed from a compound such as nitride, carbide, or a mixture thereof is preferable, a layer containing at least silicon is more preferable, and a layer formed from a plasma polymer of an organosilicon compound is even more preferable.
- a single active energy ray-curable resin layer (i) and an inorganic vapor deposition layer (ii) are sequentially laminated on an organic resin substrate.
- the power spectrum obtained by Fourier-transforming the reflected wave spectrum by the reflectance spectroscopy in (i) with respect to the wave number and plotting the amplitude with respect to the length dimension is L 1 which is not less than the threshold L 0 of the length dimension.
- L 2 has a first maximum value S 1 and a second maximum value S 2 , respectively, and when the threshold value L 0 is an arbitrary value from 1 ⁇ 10 ⁇ 6 to 3 ⁇ 10 ⁇ 6 m, L
- the first maximum value S 1 at L 1 indicates a signal noise ratio S 1 / N of 5 or more with respect to the noise N
- the first maximum value at L 2 The second maximum value S 2 has a signal noise ratio S 2 / N of 2 or more with respect to the noise N. The range is shown.
- a step of laminating a single active energy ray-curable resin layer (i) on an organic resin substrate ( ⁇ ): An oligomer (A) of a silicate ester represented by the above general formula (1) and a bifunctional (meth) acrylate (B) represented by the above general formula (3) on an organic resin substrate.
- a step of applying the active energy ray-curable coating material containing only once ( ⁇ ): a step of heating at 60 to 100 ° C. for 3 to 15 minutes after application of the active energy ray-curable coating material and before curing; ( ⁇ ): a step of irradiating an active energy ray to cure the active energy ray-curable coating.
- the silicate ester oligomer (A) represented by the general formula (1) on the organic resin substrate and the bifunctional (meta) represented by the general formula (3) are used.
- the power spectrum is efficiently split by including both the silicate ester oligomer (A) and the bifunctional (meth) acrylate (B).
- the active energy ray-curable coating material used in this step the same materials as those described for the laminate can be used.
- Step ( ⁇ ) is a step of heating at 60 to 100 ° C., preferably 70 to 90 ° C. for 3 to 15 minutes, preferably 5 to 10 minutes after application of the active energy ray-curable coating material and before curing. is there.
- this step ( ⁇ ) the power spectrum obtained from the reflected wave spectrum of the layer (i) is efficiently split.
- the step ( ⁇ ) is a step of irradiating active energy rays and curing the active energy ray-curable coating material after the steps ( ⁇ ) and ( ⁇ ).
- the active energy ray is preferably at least one selected from the group of ultraviolet rays, electron beams, radiation, infrared rays, etc., and preferably contains at least ultraviolet rays.
- Cumulative energy amount in the active energy ray irradiation is preferably 300 ⁇ 3,000mJ ⁇ cm -2, more preferably 500 ⁇ 2,000mJ ⁇ cm -2, and still more preferably, 600 ⁇ 1,800mJ ⁇ cm -2.
- the inorganic vapor-deposited layer (ii) may be laminated after the steps ( ⁇ ) to ( ⁇ ), but prior to the lamination of the layer (ii), the step ( ⁇ ) And step ( ⁇ ) may further include a step ( ⁇ ) of inspecting the power spectrum obtained by the reflected wave spectrum measurement.
- the method described in the laminate can be used.
- the step ( ⁇ ) is a nondestructive inspection and can be easily performed as compared with other methods (for example, the method described in Patent Document 3).
- the inorganic vapor deposition layer (ii) can be laminated after the steps ( ⁇ ) to ( ⁇ ) and, if necessary, the step ( ⁇ ).
- the thickness of the inorganic vapor deposition layer is preferably 0.1 to 10 ⁇ m.
- the inorganic vapor deposition layer (ii) is not particularly limited as long as it is formed by a dry film forming method. For example, Si, Ti, Zn, Al, Ga, In, Ce, Bi, Sb, B And a layer mainly composed of at least one or more kinds of metals or metal oxides, nitrides, sulfides and the like having elements such as Zr, Sn, and Ta.
- a diamond-like carbon film layer having high hardness and excellent insulating properties can be used.
- the method for laminating the inorganic vapor deposition layer is not particularly limited as long as it is a dry film formation method, for example, physical vapor phase such as resistance heating vapor deposition, electron beam vapor deposition, molecular beam epitaxy, ion beam deposition, ion plating, sputtering, etc.
- Examples include a growth method and a dry film forming method such as a chemical vapor deposition method such as thermal CVD, plasma CVD, photo CVD, epitaxial CVD, atomic layer CVD, and catCVD.
- the inorganic vapor deposition layer (ii) in the present invention is more preferably one obtained by plasma polymerization of an organosilicon compound.
- an organosilicon compound As the plasma polymerization method, a known method (Journal of the American Chemical Society, 2006, Vol. 128, page 11018) or an organosilicon compound is supplied to a commercially available plasma apparatus (manufactured by Sakai Semiconductor Co., Ltd.). can do.
- the organosilicon compound that can be used in the present invention preferably has a molecular weight of 50 to 1,000.
- the inorganic vapor deposition layer (ii) preferably contains at least one organic silicon compound, but is combined with other known materials (Chemical Review, 2010, 110, 4417-4446) that can be used for CVD. Can be used.
- molecular weight is the number average molecular weight of polystyrene conversion calculated
- the viscosity is a value at 25 ° C. measured using a rotational viscometer.
- Monomer mixed solution prepared in advance here 20 g of ⁇ -methacryloxypropyltrimethoxysilane (manufactured by Shin-Etsu Chemical Co., Ltd., product name “KBM-503”) (corresponding to monomer unit D, the proportion of monomer unit D in the polymer being 20% by mass), 2- [2′-hydroxy-5 ′-(2-methacryloxyethyl) phenyl])-2H-benzotriazole (manufactured by Otsuka Chemical Co., Ltd., product name “RUVA-93”) 15 g (corresponding to monomer unit E, The proportion of the monomer unit E in the polymer is 15% by mass), 60 g of methyl methacrylate (MMA), 5 g of glycidyl methacrylate (GMA) (corresponding to the monomer unit F, the proportion of the monomer unit F in the polymer is 65% by mass), A mixture of 140 g of diacetone alcohol, And initiator solution: 2,2′-azobis (2-
- the obtained content is a diacetone alcohol solution containing 40% by mass of the polymer component (Q) -1.
- the viscosity of this polymer solution was 5 Pa ⁇ s, and the weight average molecular weight of the polymer component (Q) -1 by GPC analysis in terms of polystyrene was 6 ⁇ 10 4 .
- concentration was adjusted by adding pure water to obtain a translucent tin and manganese-containing peroxotitanic acid solution (solid content concentration 1% by mass).
- a 350 mL peroxotitanic acid solution synthesized as described above was charged into a 500 mL volume autoclave (product name: TEM-D500 manufactured by Pressure Glass Industry Co., Ltd.), and this was carried out for 240 minutes at 200 ° C. and 1.5 MPa. Hydrothermally treated.
- reaction mixture in the autoclave is discharged through a sampling tube to a container held in a water bath at 25 ° C., and rapidly cooled to stop the reaction, and the titanium oxide-tin oxide-manganese oxide composite oxidation A fine particle dispersion was obtained.
- the volume average 50% cumulative particle diameter of the fine particles by dynamic light scattering was 15 nm.
- 1,000 parts by mass of the composite oxide fine particle dispersion, 100 parts by mass of ethanol, and 2.0 parts by mass of ammonia were added at room temperature (25 ° C.) and magnetically stirred. .
- the separable flask was immersed in an ice bath and cooled until the content temperature reached 5 ° C. After adding 18 parts by mass of tetraethoxysilane (trade name “KBE-04”, manufactured by Shin-Etsu Chemical Co., Ltd.), a separable flask was installed in ⁇ ReactorEx (manufactured by Shikoku Keiki Kogyo Co., Ltd.). Then, magnetic stirring was performed while irradiating a microwave with a frequency of 2.45 GHz and an output of 1,000 W for 1 minute. Meanwhile, a thermometer was observed to confirm that the content temperature reached 85 ° C. The obtained mixture was filtered with a qualitative filter paper (Advantec 2B) to obtain a diluted colloidal solution.
- tetraethoxysilane trade name “KBE-04”, manufactured by Shin-Etsu Chemical Co., Ltd.
- This dilute colloidal solution was concentrated by ultrafiltration to a solid content concentration of 10% by mass to obtain an aqueous dispersion of core-shell type fine particles (S) -1 having the composite oxide fine particles as nuclei and silicon oxide as a shell.
- the core-shell type fine particles had a volume average 50% cumulative particle size of 20 nm by dynamic light scattering.
- RUVA-93 2- [3- (2H-benzotriazol-2-yl) -4-hydroxyphenyl] ethyl methacrylate
- TMPT-A Trimethylolpropane triacrylate
- DPHA Dipentaerythritol hexaacrylate
- PGM Propylene glycol monomethyl ether
- IPA isopropyl alcohol
- DAROCUR 1173 2-hydroxy-2-methyl-1-phenyl-1-propanone
- Irgacure TPO 2,4,6-trimethylbenzoyldiphenylphosphine oxide
- Irgacure 819 bis (2,4,6-trimethylbenzoyl) phenylphosphine Oxide
- IPA-ST Isopropyl alcohol-dispersed silica sol (manufactured by Nissan Chemical Industries, Ltd., SiO 2 concentration 30% by mass, body by dynamic light scattering method) (Product average 50% cumulative particle size 15nm)
- thermosetting acrylic resin composition (PR-1) 40 mass% diacetone alcohol solution (100 g) of vinyl polymer (Q) -1, propylene glycol monomethyl ether (100 g), silica sol (Manufactured by Nissan Chemical Industries, Ltd., product name “PMA-ST”, 20 g) was added and mixed well to obtain a thermosetting resin composition PR-1.
- thermosetting silicone resin composition (SC-1) A 500 mL flask was charged with 50 g of methyltrimethoxysilane (manufactured by Shin-Etsu Chemical Co., Ltd., product name “KBM-13”). 30 g of Snowtex O (manufactured by Nissan Chemical Industries, Ltd .: water-dispersed silica sol, average 15 to 20 nm, SiO 2 containing 20% by mass), water dispersion of inorganic oxide fine particles (S) -1 obtained in Synthesis Example 7 A liquid mixture of 45 g of liquid and 0.3 g of acetic acid was added. When the mixed solution was added, self-heating due to hydrolysis was observed, and the internal temperature rose to 50 ° C.
- Comparative Production Examples 3 to 7 Active energy ray-curable coatings were produced in the same manner as in Production Example 1 except that the respective components and blending amounts shown in Comparative Production Examples 3 to 7 in Table 2 were used.
- Comparative Production Examples 3 and 4 are paints that do not contain the component classified as (A).
- Comparative Production Examples 5 and 6 are paints that do not contain the component classified as (B).
- Comparative Production Example 7 is a paint that does not contain any of the components classified into (A) and (B). Even when these components were not contained, gelation or precipitation of specific components did not occur, and the same translucent uniform paint as in Production Examples 1 to 10 was obtained.
- a reflection accessory was attached to a spectrophotometer (manufactured by Hitachi High-Tech Science Co., Ltd., product name “U-3900H”) to obtain a reflected wave spectrum F ( ⁇ ) measured in wavelength units.
- the original function f (k) was obtained by wave number transforming F ( ⁇ ).
- ⁇ is the wavelength (nm)
- k is the wave number (m ⁇ 1 ). Since f (k) was a non-uniformly spaced data set, it was converted to a uniform width data set (f, k) by Lagrange linear interpolation.
- (F, k) was Fourier transformed to obtain a parameter function S (n ⁇ L).
- n ⁇ L is divided by the representative average refractive index (1.5) and plotted on the horizontal axis, and the square root of the sum of the square of the real part and the square of the imaginary part of S (n ⁇ L) is plotted on the vertical axis.
- Examples 2 to 8 A laminate was produced in the same manner as in Example 1 except that the conditions shown in Tables 3 and 4 were changed. In addition, the Fourier-transform result in the process ((delta)) of Example 3 and Example 7 is shown in FIG. 3 and FIG. 4, respectively.
- Example 9 to 16 For the formation of the inorganic vapor deposition layer (ii), for the step ( ⁇ ), methyltrimethoxysilane (“KBM-13” manufactured by Shin-Etsu Chemical Co., Ltd.) was supplied to the plasma device (Tsubaki Semiconductor Co., Ltd.). A laminated body was manufactured in the same manner as in Example 1 except that it was carried out.
- This inorganic vapor deposition method is abbreviated as ⁇ -2 in Tables 5-8. Tables 5 and 6 show the implementation details.
- the Fourier-transform result in the process ((delta)) of Example 13 is shown in FIG.
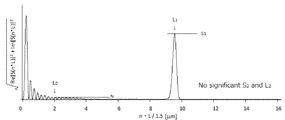
- Example 1 or Example 9 except that the paint of Comparative Production Examples 3 to 7 was used as the active energy ray-curable paint in the step ( ⁇ ) or the heating temperature and time in the step ( ⁇ ) were changed. Thus, a laminate was manufactured. Tables 9 and 10 show the implementation details. Moreover, the Fourier-transform result in the process ((delta)) of the comparative example 1 and the comparative example 4 is shown in FIG. 6 and FIG. 7, respectively.
- thermosetting acrylic resin composition PR-1 produced in Comparative Production Example 1 was applied to a polycarbonate substrate (5 mm thick) with a flow coat and allowed to stand at room temperature for 15 minutes. Thereafter, the thermosetting acrylic resin layer was cured at 120 ° C. for 1 hour (step ( ⁇ )). After the coating was cooled to room temperature, the thermosetting silicone resin composition SC-1 produced in Comparative Production Example 2 was further applied by flow coating and allowed to stand at room temperature for 15 minutes. Thereafter, the thermosetting silicone resin layer was cured at 120 ° C.
- thermosetting resin layer as a portion corresponding to the layer (i) of the present invention (step ( ⁇ )). Since Comparative Examples 7 and 8 were thermosetting, irradiation with active energy rays was unnecessary (step ( ⁇ )), and then reflected wave spectrum measurement and Fourier transform were performed in step ( ⁇ ) (FIG. 8).
- ⁇ -1 was performed to form the inorganic vapor deposition layer (ii).
- ⁇ -2 was performed to form the inorganic vapor deposition layer (ii). Table 10 shows the implementation details.
- Adhesion 500 MJ ⁇ cm -2
- a test piece that has undergone a weather resistance test 500 MJ ⁇ cm -2
- a razor blade a test piece that has undergone a weather resistance test (500 MJ ⁇ cm -2 ) is cut into 6 vertical and horizontal cuts at intervals of 2 mm using a razor blade to produce 25 grids.
- cellotape registered trademark, manufactured by Nichiban Co., Ltd.
- cellotape registered trademark, manufactured by Nichiban Co., Ltd.
- the number of squares remaining (X) was evaluated as X / 25.
- the case where X was 25 was evaluated as “ ⁇ ”, and the case where X was less than 25 was evaluated as “ ⁇ ”.
- Adhesion (1000 MJ ⁇ cm -2 ) Using a razor blade in accordance with JIS K5400, a test piece that has undergone a weather resistance test (1000 MJ ⁇ cm -2 ) is cut into two vertical and horizontal cuts at 2 mm intervals to produce 25 grids. Then, when cellotape (registered trademark, manufactured by Nichiban Co., Ltd.) is attached well and then peeled off suddenly in the 90 ° front direction, it peels off at any lamination interface between layer (i) and layer (ii). The number of squares remaining (X) was evaluated as X / 25. The case where X was 25 was evaluated as “ ⁇ ”, and the case where X was less than 25 was evaluated as “ ⁇ ”. As a special peeling mode, when the portion corresponding to the layer (i) is composed of a thermosetting acrylic resin layer and a thermosetting silicone resin layer (Comparative Examples 7 and 8), peeling between these layers is required. This was evaluated as “ ⁇ ”.
- the reflected wave spectrum obtained by reflectance spectroscopy in the active energy ray-curable resin layer (i) is Fourier-transformed with respect to the wave number, and the amplitude is plotted against the length dimension. It can be seen that when the obtained power spectrum is split at a specific S / N ratio (Examples 1 to 16), the obtained laminate is a laminate excellent in adhesion and weather resistance. In the examples, the power spectrum after Fourier transform was split with good reproducibility even when the reflected wave spectrum was repeatedly measured. On the other hand, it can be seen that the active energy ray-curable resin layer (i) is a laminate inferior in adhesion and weather resistance under the condition that no specific power spectrum splitting occurs (Comparative Examples 1 to 6).
- the portion corresponding to the layer (i) is formed of a multilayer of a thermosetting acrylic resin layer and a thermosetting silicone resin layer, but corresponds to the layer (i).
- the portion to be applied is a multi-layer, the number of times of coating is plural, and the noise N of the power spectrum tends to increase due to this.
- S 2 / N due to the multiple layers could be observed, S 1 / N was less than 10 and did not satisfy the scope of the present invention.
- a phenomenon of peeling occurs at the interface between the thermosetting acrylic resin and the thermosetting silicone resin layer, and although S 2 / N is observed, peeling / cohesive failure occurs in the layer (i). It can be seen that the unobserved Examples 1 to 16 are the result of a clear line.
- the active energy ray-curable component is spontaneously oriented in the layer (i), so that the refractive index can be physically interpreted as having a specific inflection point in the thickness direction of the film.
- the power spectrum is split because of the inflection point of the refractive index between the layers.
- peeling between the thermosetting acrylic resin layer and the thermosetting silicone resin layer may occur, and in the multilayer type, the manufacturing method becomes complicated.
- the present invention not only improves the curing mode of the coating material forming the layer (i) in the laminate to a more efficient active energy ray curing type, but also changes the refractive index in the thickness direction of the film.
- a method for predicting adhesion and weather resistance is provided. That is, by preparing the layer (i) so as to exhibit the specific power spectrum splitting of the present invention, it becomes possible to design a laminate having excellent adhesion and weather resistance.
- the refractive index inflection in a specific range contributes to the improvement of adhesion, and further, when the power spectrum of the single layer film is split
- the single-layer film had a birefringence or was considered to be an error in FFT conversion (see Patent Document 6), and a specific method for using the power spectrum was not known.
- the method using the power spectrum of the present invention has an advantage that it can be easily performed as compared with the conventional method (Patent Document 3) in which a destructive inspection of the film cross section is required in order to examine the orientation of the component.
Landscapes
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Engineering & Computer Science (AREA)
- Wood Science & Technology (AREA)
- Life Sciences & Earth Sciences (AREA)
- Polymers & Plastics (AREA)
- Health & Medical Sciences (AREA)
- Medicinal Chemistry (AREA)
- Materials Engineering (AREA)
- Physics & Mathematics (AREA)
- Plasma & Fusion (AREA)
- General Chemical & Material Sciences (AREA)
- Inorganic Chemistry (AREA)
- Thermal Sciences (AREA)
- Laminated Bodies (AREA)
- Application Of Or Painting With Fluid Materials (AREA)
- Paints Or Removers (AREA)
Priority Applications (4)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| KR1020197012899A KR102435248B1 (ko) | 2016-10-11 | 2017-07-28 | 적층체 및 그 제조 방법 |
| CN201780062289.1A CN109803826B (zh) | 2016-10-11 | 2017-07-28 | 层叠体及其制造方法 |
| EP17860022.7A EP3527366A4 (en) | 2016-10-11 | 2017-07-28 | LAMINATE BODY AND PROCESS FOR PRODUCING THE SAME |
| US16/340,977 US20190291394A1 (en) | 2016-10-11 | 2017-07-28 | Laminated body and production method therefor |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2016-199802 | 2016-10-11 | ||
| JP2016199802A JP6729270B2 (ja) | 2016-10-11 | 2016-10-11 | 積層体およびその製造方法 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2018070090A1 true WO2018070090A1 (ja) | 2018-04-19 |
Family
ID=61905496
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/JP2017/027394 Ceased WO2018070090A1 (ja) | 2016-10-11 | 2017-07-28 | 積層体およびその製造方法 |
Country Status (6)
| Country | Link |
|---|---|
| US (1) | US20190291394A1 (enExample) |
| EP (1) | EP3527366A4 (enExample) |
| JP (1) | JP6729270B2 (enExample) |
| KR (1) | KR102435248B1 (enExample) |
| CN (1) | CN109803826B (enExample) |
| WO (1) | WO2018070090A1 (enExample) |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP4480969A4 (en) * | 2022-02-14 | 2025-05-28 | Tokuyama Corporation | Photocurable composition, optical laminate, optical article, lens, and spectacles |
Families Citing this family (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP7085164B2 (ja) * | 2018-06-12 | 2022-06-16 | 東レ・ファインケミカル株式会社 | シロキサン樹脂組成物 |
Citations (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2013031794A (ja) * | 2011-08-01 | 2013-02-14 | Fujifilm Corp | 機能性フィルムの製造方法および機能性フィルム |
| JP2013035274A (ja) * | 2011-07-13 | 2013-02-21 | Kansai Paint Co Ltd | 積層体及び積層体の製造方法 |
Family Cites Families (9)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US9254506B2 (en) * | 2010-07-02 | 2016-02-09 | 3M Innovative Properties Company | Moisture resistant coating for barrier films |
| US8361607B2 (en) | 2011-04-14 | 2013-01-29 | Exatec Llc | Organic resin laminate |
| KR101702471B1 (ko) | 2011-08-26 | 2017-02-03 | 엑사테크 엘.엘.씨. | 유기 수지 라미네이트, 이의 제조 및 이용 방법, 및 이를 포함하는 제품 |
| JP5782353B2 (ja) | 2011-10-05 | 2015-09-24 | 株式会社東光高岳 | 膜厚測定装置および膜厚測定方法 |
| JP5770122B2 (ja) * | 2012-02-15 | 2015-08-26 | 富士フイルム株式会社 | 機能性フィルムの製造方法 |
| JP5934544B2 (ja) * | 2012-03-29 | 2016-06-15 | 富士フイルム株式会社 | ガスバリアフィルム |
| JP6256858B2 (ja) * | 2012-04-06 | 2018-01-10 | 三菱ケミカル株式会社 | ハードコート層を有する積層体及びその製造方法 |
| JP6239909B2 (ja) | 2013-09-17 | 2017-11-29 | 倉敷紡績株式会社 | 膜厚測定方法および装置 |
| US9758630B2 (en) * | 2014-03-27 | 2017-09-12 | Teijin Limited | Polymer substrate with hard coat layer and manufacturing method for such polymer substrate |
-
2016
- 2016-10-11 JP JP2016199802A patent/JP6729270B2/ja active Active
-
2017
- 2017-07-28 WO PCT/JP2017/027394 patent/WO2018070090A1/ja not_active Ceased
- 2017-07-28 KR KR1020197012899A patent/KR102435248B1/ko active Active
- 2017-07-28 EP EP17860022.7A patent/EP3527366A4/en not_active Withdrawn
- 2017-07-28 CN CN201780062289.1A patent/CN109803826B/zh active Active
- 2017-07-28 US US16/340,977 patent/US20190291394A1/en not_active Abandoned
Patent Citations (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2013035274A (ja) * | 2011-07-13 | 2013-02-21 | Kansai Paint Co Ltd | 積層体及び積層体の製造方法 |
| JP2013031794A (ja) * | 2011-08-01 | 2013-02-14 | Fujifilm Corp | 機能性フィルムの製造方法および機能性フィルム |
Non-Patent Citations (1)
| Title |
|---|
| See also references of EP3527366A4 * |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP4480969A4 (en) * | 2022-02-14 | 2025-05-28 | Tokuyama Corporation | Photocurable composition, optical laminate, optical article, lens, and spectacles |
Also Published As
| Publication number | Publication date |
|---|---|
| CN109803826A (zh) | 2019-05-24 |
| EP3527366A4 (en) | 2020-06-10 |
| CN109803826B (zh) | 2021-05-11 |
| EP3527366A1 (en) | 2019-08-21 |
| KR20190067832A (ko) | 2019-06-17 |
| KR102435248B1 (ko) | 2022-08-23 |
| JP2018062067A (ja) | 2018-04-19 |
| JP6729270B2 (ja) | 2020-07-22 |
| US20190291394A1 (en) | 2019-09-26 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| FI107616B (fi) | Menetelmä ja pinnoitusmassa UV- tai UV/IR-kovetetun pinnoitteen muodostamiseksi | |
| KR102244402B1 (ko) | 산화티타늄 함유 코팅 조성물 및 피복 물품 | |
| RU2514939C2 (ru) | Полисилоксановые покрытия с гибридными сополимерами | |
| JP6524261B2 (ja) | 積層体の製造方法 | |
| JPWO2010001949A1 (ja) | コーティング液、硬化膜及び樹脂積層体並びに該硬化膜及び樹脂積層体の製造方法 | |
| JP2016079395A (ja) | 活性エネルギー線硬化型シリコーンコーティング組成物及び被覆物品 | |
| CN104937055A (zh) | 涂层剂 | |
| JP2016132713A (ja) | 活性エネルギー線硬化型アクリルシリコーン樹脂組成物及び被覆物品 | |
| JP2024011542A (ja) | 積層体の製造方法 | |
| JP6911820B2 (ja) | コーティング剤及び表面被覆部材 | |
| JPWO2014196386A1 (ja) | シリコーンコーティング組成物及び被覆物品 | |
| JP6555185B2 (ja) | 被覆物品の製造方法、塗料及び積層体 | |
| WO2018070090A1 (ja) | 積層体およびその製造方法 | |
| JP6269853B2 (ja) | 有機樹脂積層体 | |
| JPWO2017104332A1 (ja) | ガスバリアーフィルム | |
| JP6304121B2 (ja) | 紫外線吸収性有機ケイ素化合物、塗料、及び積層体 | |
| JP5104788B2 (ja) | 積層体 | |
| JP2010188686A (ja) | 積層体 | |
| CN116261491B (zh) | 树脂玻璃用涂覆剂和树脂玻璃 | |
| JP3474964B2 (ja) | 表面を保護されたプラスチック成形体及びその製造方法 | |
| WO2022158031A1 (ja) | 樹脂ガラス用コーティング剤および樹脂ガラス | |
| JP2011184498A (ja) | 塗料組成物、及びそれを用いた反射防止部材の製造方法 | |
| JPH08300569A (ja) | 表面を保護されたプラスチック成形体及びその製造方法 | |
| JPH06271692A (ja) | 被覆成形物及びその製造方法 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 17860022 Country of ref document: EP Kind code of ref document: A1 |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| ENP | Entry into the national phase |
Ref document number: 20197012899 Country of ref document: KR Kind code of ref document: A |
|
| ENP | Entry into the national phase |
Ref document number: 2017860022 Country of ref document: EP Effective date: 20190513 |