WO2017170023A1 - 被覆銀粒子とその製造方法、導電性組成物、および導電体 - Google Patents
被覆銀粒子とその製造方法、導電性組成物、および導電体 Download PDFInfo
- Publication number
- WO2017170023A1 WO2017170023A1 PCT/JP2017/011388 JP2017011388W WO2017170023A1 WO 2017170023 A1 WO2017170023 A1 WO 2017170023A1 JP 2017011388 W JP2017011388 W JP 2017011388W WO 2017170023 A1 WO2017170023 A1 WO 2017170023A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- silver
- particles
- coated silver
- carboxylic acid
- aliphatic carboxylic
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Ceased
Links
Images
Classifications
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B22—CASTING; POWDER METALLURGY
- B22F—WORKING METALLIC POWDER; MANUFACTURE OF ARTICLES FROM METALLIC POWDER; MAKING METALLIC POWDER; APPARATUS OR DEVICES SPECIALLY ADAPTED FOR METALLIC POWDER
- B22F1/00—Metallic powder; Treatment of metallic powder, e.g. to facilitate working or to improve properties
- B22F1/05—Metallic powder characterised by the size or surface area of the particles
- B22F1/054—Nanosized particles
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B22—CASTING; POWDER METALLURGY
- B22F—WORKING METALLIC POWDER; MANUFACTURE OF ARTICLES FROM METALLIC POWDER; MAKING METALLIC POWDER; APPARATUS OR DEVICES SPECIALLY ADAPTED FOR METALLIC POWDER
- B22F1/00—Metallic powder; Treatment of metallic powder, e.g. to facilitate working or to improve properties
- B22F1/10—Metallic powder containing lubricating or binding agents; Metallic powder containing organic material
- B22F1/102—Metallic powder coated with organic material
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B22—CASTING; POWDER METALLURGY
- B22F—WORKING METALLIC POWDER; MANUFACTURE OF ARTICLES FROM METALLIC POWDER; MAKING METALLIC POWDER; APPARATUS OR DEVICES SPECIALLY ADAPTED FOR METALLIC POWDER
- B22F9/00—Making metallic powder or suspensions thereof
- B22F9/16—Making metallic powder or suspensions thereof using chemical processes
- B22F9/18—Making metallic powder or suspensions thereof using chemical processes with reduction of metal compounds
- B22F9/24—Making metallic powder or suspensions thereof using chemical processes with reduction of metal compounds starting from liquid metal compounds, e.g. solutions
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B22—CASTING; POWDER METALLURGY
- B22F—WORKING METALLIC POWDER; MANUFACTURE OF ARTICLES FROM METALLIC POWDER; MAKING METALLIC POWDER; APPARATUS OR DEVICES SPECIALLY ADAPTED FOR METALLIC POWDER
- B22F9/00—Making metallic powder or suspensions thereof
- B22F9/16—Making metallic powder or suspensions thereof using chemical processes
- B22F9/30—Making metallic powder or suspensions thereof using chemical processes with decomposition of metal compounds, e.g. by pyrolysis
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B22—CASTING; POWDER METALLURGY
- B22F—WORKING METALLIC POWDER; MANUFACTURE OF ARTICLES FROM METALLIC POWDER; MAKING METALLIC POWDER; APPARATUS OR DEVICES SPECIALLY ADAPTED FOR METALLIC POWDER
- B22F9/00—Making metallic powder or suspensions thereof
- B22F9/16—Making metallic powder or suspensions thereof using chemical processes
- B22F9/30—Making metallic powder or suspensions thereof using chemical processes with decomposition of metal compounds, e.g. by pyrolysis
- B22F9/305—Making metallic powder or suspensions thereof using chemical processes with decomposition of metal compounds, e.g. by pyrolysis of metal carbonyls
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01B—CABLES; CONDUCTORS; INSULATORS; SELECTION OF MATERIALS FOR THEIR CONDUCTIVE, INSULATING OR DIELECTRIC PROPERTIES
- H01B1/00—Conductors or conductive bodies characterised by the conductive materials; Selection of materials as conductors
- H01B1/02—Conductors or conductive bodies characterised by the conductive materials; Selection of materials as conductors mainly consisting of metals or alloys
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01B—CABLES; CONDUCTORS; INSULATORS; SELECTION OF MATERIALS FOR THEIR CONDUCTIVE, INSULATING OR DIELECTRIC PROPERTIES
- H01B1/00—Conductors or conductive bodies characterised by the conductive materials; Selection of materials as conductors
- H01B1/20—Conductive material dispersed in non-conductive organic material
- H01B1/22—Conductive material dispersed in non-conductive organic material the conductive material comprising metals or alloys
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01B—CABLES; CONDUCTORS; INSULATORS; SELECTION OF MATERIALS FOR THEIR CONDUCTIVE, INSULATING OR DIELECTRIC PROPERTIES
- H01B13/00—Apparatus or processes specially adapted for manufacturing conductors or cables
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01B—CABLES; CONDUCTORS; INSULATORS; SELECTION OF MATERIALS FOR THEIR CONDUCTIVE, INSULATING OR DIELECTRIC PROPERTIES
- H01B5/00—Non-insulated conductors or conductive bodies characterised by their form
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B22—CASTING; POWDER METALLURGY
- B22F—WORKING METALLIC POWDER; MANUFACTURE OF ARTICLES FROM METALLIC POWDER; MAKING METALLIC POWDER; APPARATUS OR DEVICES SPECIALLY ADAPTED FOR METALLIC POWDER
- B22F2301/00—Metallic composition of the powder or its coating
- B22F2301/25—Noble metals, i.e. Ag Au, Ir, Os, Pd, Pt, Rh, Ru
- B22F2301/255—Silver or gold
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B22—CASTING; POWDER METALLURGY
- B22F—WORKING METALLIC POWDER; MANUFACTURE OF ARTICLES FROM METALLIC POWDER; MAKING METALLIC POWDER; APPARATUS OR DEVICES SPECIALLY ADAPTED FOR METALLIC POWDER
- B22F2304/00—Physical aspects of the powder
- B22F2304/05—Submicron size particles
- B22F2304/054—Particle size between 1 and 100 nm
Definitions
- the present invention relates to coated silver particles and a method for producing the same, a conductive composition containing the coated silver particles, and a conductor manufactured using the conductive composition.
- a paste-like conductive composition containing metal powder, a sintering agent, and a medium is directly pattern-printed instead of the photolithography method with many steps.
- Printing methods are attracting attention. Examples of the printing method include an inkjet printing method, a screen printing method, a flexographic printing method, and a dispensing printing method.
- metal particles having a particle diameter smaller than that of the metal powder are preferably used.
- the metal particles for the sintering agent include gold particles, silver particles, and copper particles.
- Patent Document 1 Compared to gold and silver which are noble metals, copper is relatively easily oxidized and tends to form an oxide film on the surface.
- Patent Document 2 the present inventors disclose coated copper particles including copper core particles and a coating layer mainly composed of a long-chain aliphatic amine, and a method for producing the same (claims 1 and 2). 4 etc.).
- Patent Document 2 a coated copper particle whose surface is coated with an aliphatic carboxylic acid and a method for producing the same (Claim 1).
- the coated copper particles described in Patent Documents 1 and 2 are excellent in oxidation resistance, particle size stability, and particle dispersibility in a medium because the surface is coated with an organic substance. Further, since the organic matter is simply adsorbed (physical adsorption or ion adsorption) on the copper core particles, it can be easily detached from the copper core particles during sintering. Therefore, the coated copper particles described in Patent Documents 1 and 2 are excellent in sinterability.
- JP 2014-001443 A Japanese Patent Laying-Open No. 2015-227476
- Patent Documents 1 and 2 are inventions related to copper particles and do not disclose application to silver particles. Silver particles are excellent in oxidation resistance, but corrosive to sulfurized gas and the like.
- the present invention has been made in view of the above problems, and aims to provide coated silver particles excellent in corrosion resistance, particle size stability, particle dispersibility in a medium, and sinterability. is there.
- the coated silver particles of the present invention contain silver core particles and a plurality of aliphatic carboxylic acid molecules arranged at a density of 2.5 to 5.2 molecules per nm 2 on the surface of the silver core particles.
- the aliphatic carboxylic acid molecule preferably has an aliphatic group having 5 to 26 carbon atoms.
- the DSEM is 0.02 to 5.0 ⁇ m.
- the particle diameter variation rate defined by the general formula SD / D SEM is preferably 0.01 to 0.5.
- the method for producing coated silver particles of the present invention includes a step (A) of thermally decomposing an aliphatic carboxylic acid silver complex in a medium.
- Step (A) A step (A1) of preparing a reaction liquid containing a silver carboxylate, an aliphatic carboxylic acid, and a medium; It is preferable to include a step (A2) of producing a metallic silver by thermally decomposing a complex compound produced in the reaction solution.
- the reaction solution preferably further contains a complexing agent.
- the complexing agent is preferably an amino alcohol.
- the thermal decomposition temperature of the silver carboxylate is preferably 100 ° C. or higher.
- the conductive composition of the present invention comprises the above-described coated silver particles of the present invention and a medium.
- the conductor of the present invention is a heat-treated product of the above-described conductive composition of the present invention.
- Examples of the conductor of the present invention include wiring and a conductor layer.
- particle size In the present specification, unless otherwise specified, the “particle diameter” means the primary particle diameter.
- the “average primary particle diameter of particles (silver core particles or coated silver particles)” is any 20 particles (silver cores) determined by observation with a scanning electron microscope (SEM). It is an arithmetic average value (D SEM ) of primary particle diameters of particles or coated silver particles. It should be noted that the average primary particle diameter of the silver nucleus particles and the average primary particle diameter of the coated silver particles containing the silver nucleus particles can be regarded as substantially the same.
- “Particle diameter variation rate” is a value of standard deviation (SD) / average primary particle diameter (D SEM ) of the primary particle diameter of any 20 particles (silver core particles or coated silver particles) obtained by SEM observation. It is.
- the “organic component amount of the coated silver particles” is measured by thermogravimetric / differential heat (TG-DTA) analysis. The measurement conditions are as follows. Temperature increase rate: 10 ° C./min, Measurement temperature range: 25-500 ° C Measurement atmosphere: nitrogen (100 ml / min). In the TG-DTA analysis, the loss on heating is determined as the amount of organic component.
- TG-DTA thermogravimetric / differential heat
- Crossing density of aliphatic carboxylic acid molecules In the present specification, unless otherwise specified, the “coating density of aliphatic carboxylic acid molecules” on the surface of silver core particles is calculated by the following method.
- Samples for LC measurement are prepared as follows. In a sample bottle, 1 g of coated silver particles and 9 mL of acetonitrile are placed. To this, 1 mL of 0.36 mass% hydrochloric acid aqueous solution is added. The contents are irradiated with ultrasonic waves for 30 minutes and mixed by stirring. Next, the obtained slurry liquid is allowed to stand for solid-liquid separation, and then the supernatant liquid is collected. The supernatant is filtered through a 0.2 ⁇ m diameter filter to obtain a sample for LC measurement.
- thermogravimetry / differential heat TG-DTA
- the aliphatic carboxylic acid molecular weight contained in the coated silver particles is calculated by combining the LC analysis result and the TG-DTA analysis result.
- the number of aliphatic carboxylic acid molecules contained in 1 g of the coated silver core particles is represented by the following formula (a).
- [Number of aliphatic carboxylic acid molecules] Macid / (Mw / NA) (a)
- Macid is the aliphatic carboxylic acid molecular weight (g) contained in 1 g of the coated silver particles
- Mw is the molecular weight (g / mol) of the aliphatic carboxylic acid molecule
- NA is the Avogadro constant.
- the silver nucleus particle amount MAg (g) is obtained by approximating the shape of the silver nucleus particle to be spherical and subtracting the amount of organic component from the mass of the coated silver particle. From the silver core particle amount MAg (g), the number of silver core particles in 1 g of the coated silver particles is represented by the following formula (b).
- [Number of silver core particles in 1 g of coated silver particles] MAg / [(4 ⁇ r3 / 3) ⁇ d ⁇ 10 ⁇ 21 ]
- MAg is the amount (g) of silver core particles contained in 1 g of the coated silver particles
- r is the radius (nm) of the primary particle diameter of silver core particles calculated by SEM image observation
- the surface area of the silver core particles contained in 1 g of the coated silver particles is represented by the following formula (c) from the formula (b).
- [Surface area (nm 2 ) of silver core particles contained in 1 g of coated silver particles] [number of silver core particles] ⁇ 4 ⁇ r 2 (c)
- the coating density (molecules / nm 2 ) of silver core particles with aliphatic carboxylic acid molecules is calculated by the following formula (d) using the formulas (a) and (c).
- [Coating density (molecules / nm 2 )] [number of aliphatic carboxylic acid molecules] / [silver core particle surface area] (d)
- coated silver particles having excellent corrosion resistance, particle size stability, particle dispersibility in a medium, and sinterability can be provided.
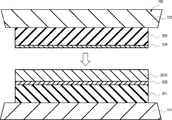
- FIG. 6 is a process diagram illustrating a method for manufacturing a laminate in Example 3.
- FIG. 6 is a process diagram illustrating a method for manufacturing a laminate in Example 3.
- 2 is a TG curve of the coated silver particles (AgP1) obtained in Example 1-1.
- 2 is a SEM photograph of coated silver particles (AgP1) obtained in Example 1-1.
- 3 is a SEM photograph of coated silver particles (AgP2) obtained in Example 1-2.
- coated silver particles contain silver core particles and a plurality of aliphatic carboxylic acid molecules arranged at a density of 2.5 to 5.2 molecules per nm 2 on the surface of the silver core particles.
- coated silver particles of the present invention can be used as metal particles alone or in combination with other metal particles in applications where silver particles are used.
- the coated silver particles of the present invention can be used in combination with, for example, a metal powder having a particle size larger than that of the silver core particles.
- the coated silver particles of the present invention can be used as a metal powder sintering agent.
- the mass ratio of the coated silver particles of the present invention to the metal powder Is not particularly limited, and is preferably 20:80 to 80:20, more preferably 30:70 to 70:30, and particularly preferably 40:60 to 60:40.
- the conductive composition of the present invention comprises the above-described coated silver particles of the present invention and a medium.
- the conductive composition of the present invention contains a metal powder having a particle size larger than that of the coated silver particles.
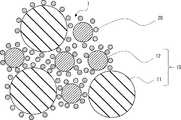
- FIG. 1 the schematic diagram of the electroconductive composition of one Embodiment which concerns on this invention is shown.
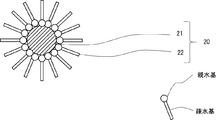
- FIG. 2 the schematic diagram of the covering silver particle of one Embodiment concerning this invention is shown.
- “hydrophilic groups” are schematically shown as circles and hydrophobic groups as bars.
- the conductive composition 1 of the present embodiment includes metal powder 10, coated silver particles 20, and a medium (not shown).
- the shape, particle diameter, distribution, and the like of each particle of the metal powder 10 and each particle of the coated silver particle 20 are schematic.
- the metal powder 10 a known metal powder for a conductive composition can be used.
- the metal powder 10 include copper powder and silver powder.
- the metal powder 10 it is preferable to use a plurality of types of metal powders having different average primary particle diameters. By using multiple types of metal powders with different average primary particle diameters, metal powders with relatively small average primary particle diameters enter the gaps between metal powders with relatively large average primary particle diameters, improving the packing density of metal powders Can be made.
- the metal powder 10 includes a first metal powder 11 having a relatively large average primary particle diameter and a second metal powder 12 having a relatively small average primary particle diameter is illustrated.
- the average primary particle diameter of the first metal powder 11 having a relatively large average primary particle diameter is not particularly limited, and is preferably 1 to 100 ⁇ m, more preferably 1 to 50 ⁇ m.
- the average primary particle diameter of the second metal powder 12 having a relatively small average primary particle diameter is not particularly limited, and is preferably 0.2 to 10 ⁇ m, more preferably 0.2 to 5 ⁇ m.
- the conductive composition 1 of the present embodiment includes coated silver particles 20 that act as a sintering agent.
- the coated silver particles 20 include silver core particles 21 having a particle diameter smaller than that of the metal powder 10 and a plurality of aliphatic carboxylic acid molecules 22 that cover the surfaces of the silver core particles 21.
- the plurality of aliphatic carboxylic acid molecules 22 are adsorbed to the surface of the silver core particle 21.
- the mode of adsorption is not particularly limited, and examples include physical adsorption and ion adsorption.
- the plurality of aliphatic carboxylic acid molecules 22 are physically adsorbed on the surface of the silver nucleus particle 21 with the carboxy group, which is a hydrophilic group, on the silver nucleus particle 21 side, and an LB film (Langmuir-Blodgett film).
- a monomolecular film can be formed.
- the coating mode is adsorption such as physical adsorption.
- the coated silver particle 20 in which the silver nucleus particle 21 is coated with a plurality of aliphatic carboxylic acid molecules 22 has an aliphatic group (hydrophobic group) of the aliphatic carboxylic acid molecule 22 on the outermost surface.
- silver particles are excellent in oxidation resistance but corrosive to sulfurized gas and the like.
- the coated silver particles 20 whose surface is coated with the aliphatic carboxylic acid molecules 22 are excellent in oxidation resistance and excellent in corrosion resistance against sulfur gas and the like.
- the hydrophobic groups of the coated silver particles 20 interact with each other, and aggregation of the coated silver particles 20 is suppressed. Therefore, the coated silver particles 20 having the above structure are excellent in particle size stability after production and particle dispersibility in a medium.
- the coated silver particles 20 are also excellent in sinterability.
- the conductive composition 1 of the present embodiment including the coated silver particles 20 having the above structure is excellent in particle dispersibility and sinterability of the coated silver particles 20 acting as the metal powder 10 and the sintering agent.
- the coated silver particles 20 excellent in oxidation resistance, corrosion resistance, particle size stability, particle dispersibility in a medium, and sinterability.
- the electrically conductive composition 1 excellent in particle dispersibility and sinterability can be provided.
- the average primary particle diameter of the silver core particles is not particularly limited and may be within a range suitable as a sintering agent.
- the average primary particle diameter of the silver core particles is preferably 0.02 ⁇ m (20 nm) to 5.0 ⁇ m, more preferably 0.02 ⁇ m (20 nm) to 1.0 ⁇ m, and still more preferably 0.02 ⁇ m (20 nm) to 0.5 ⁇ m. Particularly preferred is 0.02 ⁇ m (20 nm) to 0.2 ⁇ m. If the average primary particle diameter is less than 0.02 ⁇ m (20 nm), it is difficult to produce particles, and if it exceeds 5.0 ⁇ m, the filling effect may be insufficient.
- the purity of the silver core particles is not particularly limited, and a higher one is preferable because a highly conductive conductor can be obtained.
- the purity of the silver core particles is preferably 95% by mass or more, more preferably 97% by mass or more.
- the kind of the aliphatic carboxylic acid molecule that covers the surface of the silver core particle is not particularly limited.
- the number of carboxy groups contained in the aliphatic carboxylic acid molecule is not particularly limited, and is preferably 1 to 2, more preferably 1.
- the aliphatic carboxylic acid molecule may be a saturated aliphatic carboxylic acid molecule or an unsaturated aliphatic carboxylic acid molecule.
- the number of unsaturated bonds contained in the unsaturated aliphatic group is preferably 1 to 3, more preferably 1 to 2.
- the aliphatic group contained in the aliphatic carboxylic acid molecule may be linear or branched, and is preferably linear.
- the number of carbon atoms in the aliphatic group of the aliphatic carboxylic acid molecule is , Preferably 5 or more.
- the aliphatic carboxylic acid having 5 or more carbon atoms in the aliphatic group is also referred to as “long-chain carboxylic acid”.
- the particle diameter variation rate of the coated silver particles tends to be small.
- the length of the carbon chain is highly correlated with the magnitude of the van der Waals force that influences the association force.
- a carboxylic acid having a long carbon chain has a strong associative force, and can contribute to water-in-oil Emulsion-like phase stabilization that is a microreaction field in the production method described later. Thus, it is considered that coated silver particles having a uniform particle diameter can be produced efficiently.
- the carbon number of the aliphatic group is preferably 5 to 26, more preferably 5 to 20, still more preferably 5 to 17, particularly preferably 7 to 17, and most preferably 9 to 17.
- the boiling point of the aliphatic carboxylic acid molecule is preferably higher than the thermal decomposition temperature of the silver aliphatic carboxylate complex in the production method described later. Specifically, the boiling point of the aliphatic carboxylic acid molecule is preferably 100 ° C. or higher, more preferably 120 ° C. or higher. The boiling point of the aliphatic carboxylic acid molecule is preferably 400 ° C. or lower because the thermal decomposability during sintering of the aliphatic carboxylic acid molecule becomes good.
- Unsaturated aliphatic carboxylic acid molecules such as oleic acid and linoleic acid; and, Examples include saturated aliphatic carboxylic acid molecules such as stearic acid, heptadecanoic acid, lauric acid, and octanoic acid.
- saturated aliphatic carboxylic acid molecules such as stearic acid, heptadecanoic acid, lauric acid, and octanoic acid.
- One or more aliphatic carboxylic acid molecules can be used.
- the coating density of the plurality of aliphatic carboxylic acid molecules on the surface of the silver core particles is 2.5 to 5.2 molecules / nm 2 , preferably 3.0 to 5.2 molecules / nm 2 , more preferably 3.5 to 5.2 molecules / nm 2 .
- the medium a known medium used for a general conductive composition can be used.
- the medium include hydrocarbon solvents, higher alcohol solvents, cellosolve, and cellosolve acetate solvents.
- One type or two or more types of media can be used.
- the solid content concentration of the conductive composition is not particularly limited and is selected according to the printing method, and is, for example, 10 to 99% by mass, preferably 40 to 95% by mass.
- the electrically conductive composition of this invention can contain 1 type, or 2 or more types of arbitrary components as needed.
- Dispersant> known polymer dispersants such as polyester dispersants and polyacrylic acid dispersants can be used as the dispersant.
- Thickener> If necessary, a known polymer thickener such as a polymethacrylic acid thickener can be used as the thickener.
- Coupling agent> A coupling agent such as a silane coupling agent and a titanate coupling agent can be used as necessary.
- the method for producing coated silver particles of the present invention includes a step (A) of thermally decomposing an aliphatic carboxylate silver complex in a medium.
- a step (A) of thermally decomposing an aliphatic carboxylate silver complex silver nucleus particles and aliphatic carboxylic acid are generated, and a plurality of aliphatic carboxylic acid molecules are adsorbed on the surface of the generated single silver nucleus particle.
- Physical adsorption or ion adsorption Physical adsorption or ion adsorption
- Step (A) A step (A1) of preparing a reaction liquid containing a silver carboxylate (silver carboxylate), an aliphatic carboxylic acid, and a medium; A step (A2) of producing a metallic silver by thermally decomposing a complex compound (aliphatic carboxylate silver complex) produced in the reaction solution.
- the reaction solution can further contain a complexing agent as required.
- the thermal decomposition temperature of the aliphatic carboxylate silver complex affects the particle diameter of the coated silver particles to be produced. If the thermal decomposition temperature of the aliphatic carboxylate complex is too low, the thermal decomposition reaction is accelerated by the heat of reaction during the complexing reaction, which may make it difficult to control the particle size. Since coated silver particles having a particle size in a range suitable as a sintering agent can be stably obtained, the thermal decomposition temperature of the raw silver carboxylate is preferably 100 ° C. or higher. For example, the thermal decomposition temperature of silver formate is about 110 ° C., and the thermal decomposition temperature of silver oxalate is about 210 ° C.
- the raw material silver carboxylate is not particularly limited, and silver formate, silver oxalate, silver carbonate, and the like from the viewpoints of reducibility of silver ions, thermal decomposition temperature, availability of raw materials, ease of production of raw materials, and the like. Silver citrate and the like are preferable. Of these, silver oxalate is preferred because of its high thermal decomposition temperature.
- Silver oxalate is composed of 2 moles of monovalent silver ions and 1 mole of oxalate ions.
- a commercially available product may be used for silver oxalate, and it may be produced by a known method. Since oxalic acid has reducibility, when silver oxalate is thermally decomposed, monovalent silver ions are reduced and reduced silver particles are generated.
- the content of silver oxalate in the reaction solution is not particularly limited, and is preferably 0.5 to 2.5 mol / L, more preferably 1.0 to 2.5 mol / L, particularly preferably from the viewpoint of production efficiency and the like. Is 1.5 to 2.0 mol / L.
- the starting aliphatic carboxylic acid is not particularly limited, and is selected according to the structure of the aliphatic carboxylic acid molecule in the desired coated silver particles.
- the carbon number of the starting aliphatic carboxylic acid matches the carbon number of the aliphatic group of the aliphatic carboxylic acid molecule in the desired coated silver particle. Since the coated silver particles having the same particle diameter can be efficiently produced, and the effect of improving the corrosion resistance and particle dispersibility of the coated silver particles is effectively expressed, the number of carbon atoms of the starting aliphatic carboxylic acid is preferably 5 or more.
- the carbon number of the starting aliphatic carboxylic acid is preferably 5 to 26, more preferably 5 to 20, still more preferably 5 to 17, particularly preferably 7 to 17, and most preferably 9 to 17.
- the boiling point of the starting aliphatic carboxylic acid is preferably higher than the heating temperature of the reaction solution.
- the boiling point of the aliphatic carboxylic acid molecule is preferably 100 ° C. or higher, more preferably 120 ° C. or higher. Since the thermal decomposability at the time of sintering of the aliphatic carboxylic acid molecules in the coated silver particles becomes good, the boiling point of the raw aliphatic carboxylic acid molecules is preferably 400 ° C. or lower.
- Unsaturated aliphatic carboxylic acids such as oleic acid and linoleic acid; and, Examples include saturated aliphatic carboxylic acids such as stearic acid, heptadecanoic acid, lauric acid, and octanoic acid.
- the raw material aliphatic carboxylic acid may be used alone or in combination of two or more.
- the content of the aliphatic carboxylic acid in the reaction solution is not particularly limited, and is preferably 2.5 to 25 mol%, more preferably 5.0 to 15 mol%.
- the content of the aliphatic carboxylic acid in the reaction solution is 2.5 mol% or more, a sufficient reaction rate tends to be obtained and the productivity tends to be improved, and the particle diameter variation rate of the coated silver particles tends to be small. is there.
- the content of the aliphatic carboxylic acid in the reaction solution is 25 mol% or less, an increase in the viscosity of the reaction system is suppressed, and good stirring properties are obtained.
- the complexing agent is not particularly limited, and amino alcohol and the like are preferable.
- the presence of a complexing agent such as amino alcohol in the reaction solution effectively produces a complex compound from silver carboxylate.
- the complex compound is easily solubilized in the medium.
- An amino alcohol is an alcohol compound having at least one amino group.
- the number of amino groups is not particularly limited, and is preferably one. That is, as the amino alcohol, monoamino monoalcohol is preferable. Of these, monoamino monoalcohols having no amino group substitution and monodentate monoamino monoalcohols are preferred.
- the boiling point of amino alcohol is not particularly limited and is preferably higher than the heating temperature of the reaction solution. Specifically, the boiling point of amino alcohol is preferably 120 ° C. or higher, more preferably 130 ° C. or higher. The boiling point of amino alcohol is preferably 400 ° C. or lower, more preferably 300 ° C. or lower.
- the SP value of the amino alcohol is preferably 11.0 or more, more preferably 12.0 or more, and particularly preferably 13.0 or more.
- the SP value of amino alcohol is preferably 18.0 or less, more preferably 17.0 or less.
- SP value is a solubility parameter defined by Hildebrand, and is the square root of intermolecular bond energy E1 per 1 mL of sample at 25 ° C.
- the SP value is specifically calculated as follows.
- Intermolecular bond energy E1 is a value obtained by subtracting gas energy from latent heat of vaporization Hb.
- H25 Hb ⁇ [1 + 0.175 ⁇ (Tb ⁇ 25) / 100]
- E1 E ⁇ D / Mw (In the above formula, D is the density of the sample, and Mw is the molecular weight of the sample.)
- amino alcohol As amino alcohol, 2-aminoethanol (boiling point: 170 ° C., SP value: 14.54), 3-amino-1-propanol (boiling point: 187 ° C., SP value: 13.45), 5-amino-1-pentanol (boiling point: 245 ° C., SP value: 12.78), DL-1-amino-2-propanol (boiling point: 160 ° C., SP value: 12.74), and, And N-methyldiethanolamine (boiling point: 247 ° C., SP value: 13.26). These can be used alone or in combination of two or more.
- the content of amino alcohol in the reaction solution is not particularly limited, and is preferably 1.5 to 4.0 times mol, more preferably 1.5 to 3.0 times mol with respect to silver ions in the reaction solution. is there.
- the content of amino alcohol is 1.5 times mol or more with respect to silver ions, the solubility of silver carboxylate is improved, and the reaction time can be shortened.
- the content of amino alcohol is 4.0 times mol or less with respect to silver ions, it is possible to suppress unnecessary adhesion of amino alcohol to the produced coated silver particles.
- ⁇ Medium> One type or two or more types of media can be used.
- the medium one or more kinds can be selected from organic media generally used for chemical reactions.
- the medium a medium that does not inhibit the reduction reaction of silver ions by carboxylic acid and satisfies the ⁇ SP value of 4.2 or more, which is the difference between the SP value of amino alcohol and the SP value of the medium, is preferable.
- the ⁇ SP value is 4.2 or more, the width of the particle size distribution of the produced coated silver particles is narrowed, and coated silver particles having a uniform particle diameter tend to be obtained.
- the ⁇ SP value is preferably 4.5 or more, more preferably 5.0 or more, and particularly preferably 7.0 or more.
- the ⁇ SP value is preferably 11.0 or less, more preferably 10.0 or less.
- the SP value of the medium is preferably smaller than that of amino alcohol.
- the SP value of the media is defined by an average SP value considering the SP value and the mole fraction of each medium included in the media.
- the medium preferably includes at least a medium that is incompatible with amino alcohol (hereinafter referred to as “main medium”).
- main medium a medium that is incompatible with amino alcohol
- auxiliary medium a medium that is compatible with amino alcohol
- the boiling point of the main medium is preferably higher than the heating temperature of the reaction solution. Specifically, the boiling point of the main medium is preferably 120 ° C. or higher, more preferably 130 ° C. or higher. The boiling point of the main medium is preferably 400 ° C. or lower, more preferably 300 ° C. or lower.
- the main medium is preferably one that can form an azeotropic mixture with water. If an azeotropic mixture with water can be formed, water generated in the reaction system can be easily removed in the heating step of the reaction solution.
- ethylcyclohexane (boiling point: 132 ° C., SP value: 8.18), C9 alkylcyclohexane mixture [for example, “Swaclean 150” manufactured by Gordo (boiling point: 149 ° C., SP value: 7.9) , And n-octane (boiling point: 125 ° C., SP value: 7.54).
- One or more main media can be used.
- the preferred boiling point of the auxiliary medium is the same as that of the main medium.
- the SP value of the auxiliary medium is preferably larger than that of the main medium, and more preferably high enough to be compatible with amino alcohol.
- auxiliary medium examples include ethylene glycol (EO) glycol ether, propylene glycol (PO) glycol ether, and dialkyl glycol ether.
- EO glycol ether examples include methyl diglycol, isopropyl glycol, and butyl glycol.
- PO glycol ether examples include methylpropylene diglycol, methylpropylene triglycol, propylpropylene glycol, and butylpropylene glycol.
- dialkyl glycol ether examples include dimethyl diglycol.
- the amount of medium in the reaction solution is adjusted so that the silver ion concentration is preferably 0.5 to 2.5 mol / L, more preferably 1.0 to 2.0 mol / L.
- Productivity improves that the silver ion concentration in a reaction liquid is 1.0 mol / L or more.
- the silver ion concentration in the reaction solution is 2.5 mol / L or less, an increase in the viscosity of the reaction solution is suppressed, and good stirring properties are obtained.
- the reaction solution can contain one or more optional components other than those described above as required.
- ⁇ Complex compound> In the reaction solution containing silver carboxylate, aliphatic carboxylic acid (preferably long chain carboxylic acid), and medium, one or more complex compounds derived from silver carboxylate (aliphatic carboxylate silver complex) Is generated.
- the structure of the complex compound is not particularly limited, and the structure of the complex compound in the reaction solution may change as the reaction proceeds.
- the complex compound can contain a silver ion and an aliphatic carboxylic acid or its ion as a ligand.
- the complex compound can contain silver ions, aliphatic carboxylic acids or ions thereof, and amino alcohol as a ligand.
- the thermal decomposition temperature of a complex compound it is considered that a carboxylate ion derived from silver carboxylate is ionically bonded to the silver ion.
- Various types of ligands and the number of ligands in the complex compound can be considered.
- the complex compound produced in the reaction solution can produce silver core particles by thermal decomposition treatment.
- the temperature of the thermal decomposition treatment is appropriately selected according to the structure of the complex compound.
- silver carboxylate tends to lower the thermal decomposition temperature by forming a complex compound with amino alcohol.
- the thermal decomposition temperature of silver oxalate is about 210 to 250 ° C.
- the thermal decomposition temperature of silver oxalate can be lowered to about 70 to 120 ° C.
- the heating temperature (thermal decomposition treatment temperature) of the reaction solution using amino alcohol as the complexing agent is preferably 60 to 130 ° C, more preferably 80 to 130 ° C.
- Silver nucleus particles are generated by thermal decomposition of the complex compound, and the surface of the silver nucleus particles is adsorbed on the surface of the generated silver nucleus particles (physical adsorption or ion adsorption, etc.) so that the surface of the silver nucleus particles has a plurality of fats. Coated silver particles coated with a group carboxylic acid molecule can be obtained.
- the time for the pyrolysis treatment can be appropriately selected according to the temperature for the pyrolysis treatment, and is preferably, for example, 30 to 180 minutes.
- the atmosphere for the thermal decomposition treatment is not particularly limited, and may be an air atmosphere or an inert atmosphere such as a nitrogen atmosphere.
- the particle size distribution of the coated silver particles is adjusted by adjusting the type and amount of the aliphatic carboxylic acid, the concentration of the silver carboxylate complex, and the ratio of the mixed medium (main medium / auxiliary medium). Thus, it can be adjusted to a narrow range.
- the size of the coated silver particles can be made uniform by appropriately maintaining the heating rate that governs the number of metal nuclei generated, that is, the stirring rate related to the amount of heat input to the reaction system and the size of the micro reaction field.
- coated silver particles having a narrow particle size distribution are obtained. This can be considered as follows, for example.
- the ⁇ SP value which is the difference in SP value between the amino alcohol as a complexing agent for solubilizing silver carboxylate in the reaction medium and the medium, is preferably 4.2 or more.
- the complex compound produced in the reaction solution can be dissolved in the reaction solution.
- the complex compound is thermally decomposed to liberate amino alcohol as a complexing agent, the liberated amino alcohol is incompatible with the medium. It cannot melt and begins to form two phases.
- the liberated amino alcohol has a high affinity with silver carboxylate and complex compounds, and can act as a new complexing agent or medium for silver carboxylate.
- the liberated amino alcohol forms a highly polar inner core (droplet), and the outside of the medium is surrounded by a less polar medium, so that a two-phase structure similar to Water in oil Emulsion is formed. It is presumed that this functions as a micro reaction field.
- the metal nucleus and its growing particles, silver carboxylate amino alcohol complex, water, and carboxylic acid are isolated from the medium in the amino alcohol layer, and the reaction is considered to proceed.
- the production method of the coated silver particles may further include post-processes such as a washing process, a separating process, and a drying process of the coated silver particles after the thermal decomposition treatment process, if necessary.
- post-processes such as a washing process, a separating process, and a drying process of the coated silver particles after the thermal decomposition treatment process, if necessary.
- a known method can be applied to these post processes.
- the cleaning step can be performed using an organic medium, for example.
- the organic medium used in the washing step is not particularly limited, and examples thereof include alcohol media such as methanol, ketone media such as acetone, and the like. These can be used alone or in combination of two or more.
- the conductor of the present invention is a heat-treated product of the above-described conductive composition of the present invention. It does not restrict
- the conductor layer include an electrode layer and a bonding layer.
- the bonding layer include a bonding layer that bonds a base material and a semiconductor element such as an IC (Integrated Circuit) chip.
- the thickness of the conductor of the present invention is not particularly limited, and is preferably about 1 to 100 ⁇ m, for example.
- the conductor of the present invention is manufactured by a manufacturing method including a step of coating the above-described conductive composition of the present invention on a substrate and a step of sintering the coated conductive composition. Can do.
- the substrate includes at least a substrate body, and may include one or more elements such as a layer and a member formed on the substrate body as necessary.
- the substrate body is, for example, Resins such as polyimide; Glass; Ceramics such as silica and alumina; Metals such as stainless steel, copper and titanium; Includes semiconductors such as silicon.
- the base body may be made of a composite material. In applications such as semiconductor parts and electronic devices, lead frames and substrates are preferably used as the base body.
- the thickness of the substrate is preferably about 0.01 to 5 mm, for example.
- the coating method is not particularly limited, and a known printing method such as an inkjet printing method, a screen printing method, a flexographic printing method, or a dispense printing method can be employed.
- the conductive composition of the present invention can be patterned by the above printing method.
- the sintering temperature of the conductive composition is not particularly limited, and is, for example, 100 to 600 ° C., preferably 150 to 350 ° C.
- the sintering time is selected according to the sintering temperature, and is, for example, 1 to 120 minutes, preferably 1 to 60 minutes.
- pressure sintering may be performed as necessary.
- the applied pressure is not particularly limited, and is preferably 0.1 to 100 MPa, more preferably 0.1 to 50 MPa.
- the sintering atmosphere is not particularly limited, and may be an air atmosphere or an inert atmosphere having a low oxygen concentration.
- the inert atmosphere having a low oxygen concentration include an inert gas atmosphere such as nitrogen and argon, and a reduced pressure atmosphere.
- the reaction solution was heated to 40 ° C. using an oil bath while stirring and mixing the reaction solution, and the heating and stirring at this reaction temperature was continued. White crystals gradually precipitated immediately after the start of the reaction.
- the reaction was terminated after 3 hours from the end of dropping, and the reaction solution was naturally cooled to room temperature.
- the resulting precipitate was filtered and washed with 1000 mL of ion exchange water.
- the obtained filtrate was a white solid.
- the filtrate was dried under reduced pressure (vacuum drying) under conditions of a temperature of 40 ° C. or lower / pressure of 3 kPa or lower to obtain 167 g of white silver oxalate.
- the identification of the crystal structure by PXRD analysis was implemented, and the loss
- Example 1-1 “Production of coated silver particles (AgP1)”
- a 300 mL glass three-necked flask equipped with a stirrer, a thermometer, and a reflux condenser was placed in an oil bath.
- 30 g of silver oxalate obtained in Production Example 1 4 g of lauric acid (manufactured by Tokyo Chemical Industry Co., Ltd.) 10 g of tripropylene glycol monomethyl ether (manufactured by Tokyo Chemical Industry Co., Ltd., boiling point: 242 ° C., SP value: 9.20) as a medium (auxiliary medium) 54 g of petroleum-based hydrocarbon (C9 alkylcyclohexane mixture) as a medium (main medium) (“Swclean 150” manufactured by Gordo, boiling point: 149 ° C., SP value: 7.9) Stir and mix.
- the reaction solution was heated to 40 ° C. using an oil bath while stirring and mixing the reaction solution. While continuing heating and stirring at this reaction temperature, 53 g of 3-amino-1-propanol (manufactured by Tokyo Chemical Industry Co., Ltd.) as a complexing agent was slowly added dropwise to the reaction solution. After completion of the dropwise addition, the mixture was heated with stirring at a rate of temperature increase of about 1 ° C./min until the liquid temperature reached about 85 ° C., and the heating and stirring at this temperature was continued. Three hours after the end of dropping, heating of the oil bath was stopped to complete the reaction, and the reaction solution was naturally cooled to room temperature.
- Example 1-2 “Production of coated silver particles (AgP2)” 18 g of purple coated silver particles (AgP2) were obtained in the same manner as in Example 1-1 except that the reaction temperature (heating temperature after addition of 3-amino-1-propanol) was 100 ° C.
- TG-DTA analysis Thermogravimetric / differential thermal analysis (TG-DTA analysis) TG-DTA analysis was performed to measure organic coverage.
- the organic coating amount was in the range of 1.0 to 1.3% by mass.
- the organic coating amount of the coated silver particles (AgP1) of Example 1-1 was 1.2% by mass.
- the organic coating amount of the coated silver particles (AgP2) of Example 1-2 was 1.3% by mass.
- the TG curve of the coated silver particles (AgP1) of Example 1-1 is shown in FIG.
- SEM observation SEM observation was carried out to evaluate the particle shape, average primary particle size D SEM , and particle size variation rate.
- SEM photographs of the coated silver particles (AgP1) and (AgP2) are shown in FIGS. 5A and 5B.
- Particle shape is spherical, average primary particle diameter D SEM ranged 0.02 ⁇ 5.0 .mu.m.
- the average primary particle diameter of the coated silver particles (AgP1) of Example 1-1 was 81.5 nm.
- the average primary particle diameter of the coated silver particles (AgP2) of Example 1-2 was 58.1 nm.
- the variation rate of the particle size was in the range of 0.01 to 0.5.
- coated silver particles having a uniform particle size were obtained.
- Example 2 "Production of conductive composition” A conductive composition (conductive paste composition) was produced using the coated silver particles (AgP1) obtained in Example 1-1.
- the first metal powder having a relatively large average particle diameter silver powder having an average particle diameter of 3.6 ⁇ m (“SPN30J” manufactured by Mitsui Kinzoku Co., Ltd.) was prepared.
- silver powder having an average particle diameter of 1.3 ⁇ m (“SPN05S” manufactured by Mitsui Kinzoku Co., Ltd.) was prepared.
- a polyacrylic acid type dispersant (“Mariarim” manufactured by NOF Corporation) was prepared as a dispersant.
- a polymethacrylic acid thickener (“KC1100” manufactured by NOF Corporation) was prepared.
- As a medium 2,2,4-trimethyl-1,3-pentanediol monoisobutyrate (“Kyowanol M” manufactured by NH Neochem) was prepared.
- the coated silver particles (AgP1) obtained in Example 1-1, the first metal powder, the second metal powder, the dispersant, the thickener, and the medium are as follows. It mix
- Example 3 "Formation of bonding layer"
- a copper lead frame (silver plated lead frame) having a surface plated with silver was prepared.
- the conductive composition obtained in Example 2 was applied in a thickness of 50 ⁇ m in a 9 mm square pattern on the chip mounting portion (9 mm square square shape in plan view) of the lead frame.
- an IC (Integrated Circuit) chip having a silicon wafer as a substrate and silver plating on the surface as a barrier layer was prepared.
- Chip bonding was performed using a chip bonding apparatus including a hot stage and a bonding head that is disposed opposite to the hot stage and sucks and holds an IC chip.
- the above-described silver-plated lead frame coated with a silver paste composition is placed on the hot stage in a state where the hot stage and the bonding head are sufficiently separated from each other.
- the above silver-plated IC chip was held by suction.
- the bonding head was lowered and the coating film of the silver paste composition was pressure-sintered to form a silver bonding layer (bonding conductor layer).
- the conditions for pressure sintering were as follows. Sintering temperature: 300 ° C Applied pressure: 30 MPa Heating and pressing time: 10 minutes.
- Sintering temperature 300 ° C
- Applied pressure 30 MPa
- Heating and pressing time 10 minutes.
- a laminate composed of IC chip / barrier layer (silver plating layer) / silver bonding layer / silver plating layer / lead frame was obtained.
- 3A and 3B are schematic cross-sectional views. Each code
- the silver joining layer of the obtained laminated body was a dense and highly uniform conductor layer.
- Example 4 "Formation of conductor layer"
- the conductive composition obtained in Example 2 was applied in a 9 mm square pattern in a 10 ⁇ m thickness on a 40 ⁇ m thick polyimide film having a 12 ⁇ m copper foil laminated on the back side. Subsequently, the said coating film was heated at 350 degreeC for 1 hour, and the conductor layer was obtained. When the volume resistivity value of the obtained conductor layer was measured, it was 5 ⁇ ⁇ cm, and it had the same high conductivity as the Ag bulk body.
- conductive composition 10 metal powder 11: first metal powder 12: second metal powder 20: coated silver particle 21: silver core particle 22: aliphatic carboxylic acid molecule 100: chip bonding apparatus 101: hot stage 102: Bonding head 201: Lead frame 202: Silver plating layer 203X: Coating film 203: Silver bonding layer (conductor layer) 204: Barrier layer (silver plating layer) 205: IC chip 200: Laminate
Landscapes
- Chemical & Material Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- General Chemical & Material Sciences (AREA)
- Engineering & Computer Science (AREA)
- Physics & Mathematics (AREA)
- Spectroscopy & Molecular Physics (AREA)
- Dispersion Chemistry (AREA)
- Inorganic Chemistry (AREA)
- Nanotechnology (AREA)
- Manufacturing & Machinery (AREA)
- Powder Metallurgy (AREA)
- Conductive Materials (AREA)
- Manufacture Of Metal Powder And Suspensions Thereof (AREA)
- Non-Insulated Conductors (AREA)
Priority Applications (4)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN201780020261.1A CN108883466A (zh) | 2016-03-28 | 2017-03-22 | 被覆银颗粒及其制造方法、导电性组合物和导体 |
| US16/084,265 US20190076921A1 (en) | 2016-03-28 | 2017-03-22 | Coated silver particle and manufacturing method therefor, conductive composition, and conductor |
| EP17774564.3A EP3437760B1 (en) | 2016-03-28 | 2017-03-22 | Production method for coated silver particle |
| KR1020187025466A KR20180126468A (ko) | 2016-03-28 | 2017-03-22 | 피복 은 입자와 그 제조 방법, 도전성 조성물, 및 도전체 |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2016-064296 | 2016-03-28 | ||
| JP2016064296A JP6979150B2 (ja) | 2016-03-28 | 2016-03-28 | 被覆銀粒子とその製造方法、導電性組成物、および導電体 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2017170023A1 true WO2017170023A1 (ja) | 2017-10-05 |
Family
ID=59965494
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/JP2017/011388 Ceased WO2017170023A1 (ja) | 2016-03-28 | 2017-03-22 | 被覆銀粒子とその製造方法、導電性組成物、および導電体 |
Country Status (6)
| Country | Link |
|---|---|
| US (1) | US20190076921A1 (enExample) |
| EP (1) | EP3437760B1 (enExample) |
| JP (1) | JP6979150B2 (enExample) |
| KR (1) | KR20180126468A (enExample) |
| CN (1) | CN108883466A (enExample) |
| WO (1) | WO2017170023A1 (enExample) |
Cited By (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2019169377A1 (en) * | 2018-03-02 | 2019-09-06 | E. I. Du Pont De Nemours And Company | Method of manufacturing an electronic device and conductive paste for the same |
| WO2023132051A1 (ja) * | 2022-01-07 | 2023-07-13 | 住友ベークライト株式会社 | ペースト状樹脂組成物、高熱伝導性材料、および半導体装置 |
Families Citing this family (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP6920029B2 (ja) | 2016-04-04 | 2021-08-18 | 日亜化学工業株式会社 | 金属粉焼結ペースト及びその製造方法、導電性材料の製造方法 |
| JP6879512B2 (ja) * | 2018-09-20 | 2021-06-02 | 協立化学産業株式会社 | 封止用組成物 |
| EP3636718B1 (en) * | 2018-10-12 | 2023-02-01 | Karlsruher Institut für Technologie | Highly conductive, printable ink for highly stretchable soft electronics |
| JP7029182B2 (ja) * | 2019-05-22 | 2022-03-03 | 協立化学産業株式会社 | 接合体の製造方法 |
| JP7333055B2 (ja) * | 2019-07-19 | 2023-08-24 | 協立化学産業株式会社 | 接合用組成物、接合体及びその製造方法 |
| JP7333056B2 (ja) * | 2019-07-19 | 2023-08-24 | 協立化学産業株式会社 | 接合用組成物、接合体及びその製造方法 |
| CN112053796B (zh) * | 2020-08-20 | 2022-03-29 | 广东风华高新科技股份有限公司 | 一种抗硫化银电极浆料及其制备方法 |
Citations (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPS569301A (en) * | 1979-06-29 | 1981-01-30 | Du Pont | Flake silver powder |
| JP2003320255A (ja) * | 2002-04-26 | 2003-11-11 | Mitsubishi Chemicals Corp | オレフィンオキシド製造用触媒および該触媒を用いるオレフィンオキシドの製造方法 |
| JP2011224558A (ja) * | 2004-11-26 | 2011-11-10 | Seoul National Univ Industry Foundation | 単分散ナノ粒子の新しい大量製造方法 |
| JP2012088242A (ja) | 2010-10-21 | 2012-05-10 | Sumitomo Metal Mining Co Ltd | 金属微粒子表面の脂肪酸の定量方法 |
| JP2014001443A (ja) | 2012-06-21 | 2014-01-09 | Kyoritsu Kagaku Sangyo Kk | 酸化物被覆銅微粒子及びその製造方法 |
| JP2015227476A (ja) | 2014-05-30 | 2015-12-17 | 協立化学産業株式会社 | 被覆銅粒子及びその製造方法 |
Family Cites Families (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4331714A (en) * | 1979-06-29 | 1982-05-25 | E. I. Dupont De Nemours And Company | Process of making flake silver powders with chemisorbed monolayer of dispersant |
| US7749300B2 (en) * | 2008-06-05 | 2010-07-06 | Xerox Corporation | Photochemical synthesis of bimetallic core-shell nanoparticles |
| US9682447B2 (en) * | 2010-08-20 | 2017-06-20 | Henkel IP & Holding GmbH | Organic acid- or latent organic acid-functionalized polymer-coated metal powders for solder pastes |
| JP6037494B2 (ja) * | 2012-01-11 | 2016-12-07 | 国立大学法人山形大学 | 銀ナノ粒子の製造方法及び銀ナノ粒子、並びに銀塗料組成物 |
| US20150231698A1 (en) * | 2012-08-02 | 2015-08-20 | National University Corporation Yamagata University | Process for producing coated silver fine particles and coated silver fine particles produced by said production process |
| JP6428339B2 (ja) * | 2015-02-13 | 2018-11-28 | 三菱マテリアル株式会社 | 銀粉及びペースト状組成物並びに銀粉の製造方法 |
-
2016
- 2016-03-28 JP JP2016064296A patent/JP6979150B2/ja active Active
-
2017
- 2017-03-22 CN CN201780020261.1A patent/CN108883466A/zh active Pending
- 2017-03-22 WO PCT/JP2017/011388 patent/WO2017170023A1/ja not_active Ceased
- 2017-03-22 US US16/084,265 patent/US20190076921A1/en not_active Abandoned
- 2017-03-22 EP EP17774564.3A patent/EP3437760B1/en active Active
- 2017-03-22 KR KR1020187025466A patent/KR20180126468A/ko not_active Ceased
Patent Citations (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPS569301A (en) * | 1979-06-29 | 1981-01-30 | Du Pont | Flake silver powder |
| JP2003320255A (ja) * | 2002-04-26 | 2003-11-11 | Mitsubishi Chemicals Corp | オレフィンオキシド製造用触媒および該触媒を用いるオレフィンオキシドの製造方法 |
| JP2011224558A (ja) * | 2004-11-26 | 2011-11-10 | Seoul National Univ Industry Foundation | 単分散ナノ粒子の新しい大量製造方法 |
| JP2012088242A (ja) | 2010-10-21 | 2012-05-10 | Sumitomo Metal Mining Co Ltd | 金属微粒子表面の脂肪酸の定量方法 |
| JP2014001443A (ja) | 2012-06-21 | 2014-01-09 | Kyoritsu Kagaku Sangyo Kk | 酸化物被覆銅微粒子及びその製造方法 |
| JP2015227476A (ja) | 2014-05-30 | 2015-12-17 | 協立化学産業株式会社 | 被覆銅粒子及びその製造方法 |
Non-Patent Citations (2)
| Title |
|---|
| "Determining cross-sectional area of stearic acid molecule, -- Experimental values and Calculated values", CHEMISTRY AND EDUCATION, vol. 40, no. 2, 1992 |
| See also references of EP3437760A4 |
Cited By (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2019169377A1 (en) * | 2018-03-02 | 2019-09-06 | E. I. Du Pont De Nemours And Company | Method of manufacturing an electronic device and conductive paste for the same |
| EP3759181A1 (en) * | 2018-03-02 | 2021-01-06 | DuPont Electronics, Inc. | Method of manufacturing an electronic device and conductive paste for the same |
| WO2023132051A1 (ja) * | 2022-01-07 | 2023-07-13 | 住友ベークライト株式会社 | ペースト状樹脂組成物、高熱伝導性材料、および半導体装置 |
Also Published As
| Publication number | Publication date |
|---|---|
| CN108883466A (zh) | 2018-11-23 |
| JP6979150B2 (ja) | 2021-12-08 |
| EP3437760A1 (en) | 2019-02-06 |
| JP2017179403A (ja) | 2017-10-05 |
| EP3437760B1 (en) | 2021-05-05 |
| EP3437760A4 (en) | 2019-08-14 |
| US20190076921A1 (en) | 2019-03-14 |
| KR20180126468A (ko) | 2018-11-27 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP6979150B2 (ja) | 被覆銀粒子とその製造方法、導電性組成物、および導電体 | |
| EP2671655B1 (en) | Method for manufacturing coated metal fine particles | |
| JP6716809B2 (ja) | 銅ペースト組成物の製造方法 | |
| JP6491753B2 (ja) | 低温焼結性に優れる金属ペースト及び該金属ペーストの製造方法 | |
| EP3150306B1 (en) | Coated copper particles and method for manufacturing same | |
| JP4496216B2 (ja) | 導電性金属ペースト | |
| EP2990142B1 (en) | Metal nanoparticle dispersion, process for producing metal nanoparticle dispersion, and bonding method | |
| TWI648111B (zh) | Coated copper particles, a method for producing the same, and a conductive composition and circuit formation | |
| JP6037893B2 (ja) | 金属微粒子組成物、接合材、電子部品、接合層の形成方法、導体層の形成方法及びインク組成物 | |
| TWI527069B (zh) | And a method for producing metal powder paste | |
| JP6938057B2 (ja) | 被覆銀粒子とその製造方法、導電性組成物、および導電体 | |
| JP6892120B2 (ja) | 被覆金属粒子、導電性組成物、導電体、接合用積層体、回路形成物及び焼結体の製造方法 | |
| JPWO2018190246A1 (ja) | 銅粒子混合物及びその製造方法、銅粒子混合物分散液、銅粒子混合物含有インク、銅粒子混合物の保存方法及び銅粒子混合物の焼結方法 | |
| JP2016176146A (ja) | 被覆銅粒子 | |
| JP7139590B2 (ja) | 導体形成用組成物、並びに接合体及びその製造方法 | |
| JP6414085B2 (ja) | 金属ナノ微粒子の製造方法 | |
| WO2016080544A1 (ja) | 金属表面の処理方法並びに当該方法により処理された銀被着銅及び複合金属体 | |
| JP2020090725A (ja) | 複合粒子、銅ペースト組成物、および導電体 | |
| JP2021147684A (ja) | 銅および酸化銅含有微粒子及びその製造方法 | |
| TWI757412B (zh) | 銀奈米粒子的製造方法 | |
| JP6823856B1 (ja) | 接合体の製造方法 | |
| JP6944734B2 (ja) | 接合体および電子装置 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| ENP | Entry into the national phase |
Ref document number: 20187025466 Country of ref document: KR Kind code of ref document: A |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2017774564 Country of ref document: EP |
|
| ENP | Entry into the national phase |
Ref document number: 2017774564 Country of ref document: EP Effective date: 20181029 |
|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 17774564 Country of ref document: EP Kind code of ref document: A1 |