WO2017002882A1 - クロマト分析装置およびクロマト分析方法 - Google Patents
クロマト分析装置およびクロマト分析方法 Download PDFInfo
- Publication number
- WO2017002882A1 WO2017002882A1 PCT/JP2016/069343 JP2016069343W WO2017002882A1 WO 2017002882 A1 WO2017002882 A1 WO 2017002882A1 JP 2016069343 W JP2016069343 W JP 2016069343W WO 2017002882 A1 WO2017002882 A1 WO 2017002882A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- chromatographic
- membrane
- substance
- sample
- analyzer
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Ceased
Links
Images
Classifications
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N33/00—Investigating or analysing materials by specific methods not covered by groups G01N1/00 - G01N31/00
- G01N33/48—Biological material, e.g. blood, urine; Haemocytometers
- G01N33/50—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing
- G01N33/53—Immunoassay; Biospecific binding assay; Materials therefor
- G01N33/543—Immunoassay; Biospecific binding assay; Materials therefor with an insoluble carrier for immobilising immunochemicals
- G01N33/54366—Apparatus specially adapted for solid-phase testing
- G01N33/54386—Analytical elements
- G01N33/54387—Immunochromatographic test strips
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N33/00—Investigating or analysing materials by specific methods not covered by groups G01N1/00 - G01N31/00
- G01N33/48—Biological material, e.g. blood, urine; Haemocytometers
- G01N33/50—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing
- G01N33/53—Immunoassay; Biospecific binding assay; Materials therefor
- G01N33/543—Immunoassay; Biospecific binding assay; Materials therefor with an insoluble carrier for immobilising immunochemicals
- G01N33/54366—Apparatus specially adapted for solid-phase testing
- G01N33/54386—Analytical elements
- G01N33/54387—Immunochromatographic test strips
- G01N33/54388—Immunochromatographic test strips based on lateral flow
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N33/00—Investigating or analysing materials by specific methods not covered by groups G01N1/00 - G01N31/00
- G01N33/48—Biological material, e.g. blood, urine; Haemocytometers
- G01N33/50—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing
- G01N33/53—Immunoassay; Biospecific binding assay; Materials therefor
- G01N33/543—Immunoassay; Biospecific binding assay; Materials therefor with an insoluble carrier for immobilising immunochemicals
- G01N33/54393—Improving reaction conditions or stability, e.g. by coating or irradiation of surface, by reduction of non-specific binding, by promotion of specific binding
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N2333/00—Assays involving biological materials from specific organisms or of a specific nature
- G01N2333/005—Assays involving biological materials from specific organisms or of a specific nature from viruses
- G01N2333/08—RNA viruses
- G01N2333/115—Paramyxoviridae, e.g. parainfluenza virus
- G01N2333/135—Respiratory syncytial virus
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N33/00—Investigating or analysing materials by specific methods not covered by groups G01N1/00 - G01N31/00
- G01N33/48—Biological material, e.g. blood, urine; Haemocytometers
- G01N33/50—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing
- G01N33/53—Immunoassay; Biospecific binding assay; Materials therefor
- G01N33/569—Immunoassay; Biospecific binding assay; Materials therefor for microorganisms, e.g. protozoa, bacteria, viruses
- G01N33/56983—Viruses
Definitions
- the present invention relates to a chromatographic analyzer and a chromatographic analysis method.
- immunochromatographic immunoassays that do not require pretreatment of specimens have been used as simple in-vitro diagnostic kits or portable diagnostic devices that detect antigens in sample solutions using the specific reactivity of antibodies.
- pathogen testing kits such as viruses and bacteria are familiar immunochromatographic analyzers widely used in general hospitals and clinics.
- the simplest structure of a conventional immunochromatography analyzer is that a sample addition unit, a labeling substance holding unit, a chromatographic medium unit carrying a detection unit, and an absorption unit that absorbs liquid that has passed through the detection unit are interconnected. Structure.
- Such an immunochromatography analyzer is currently required to have high sensitivity in order to detect a trace amount of antigen such as influenza virus.
- Patent Document 1 proposes a technique in which a metal ion masking agent coexists in a reaction solution.
- Patent Document 2 discloses a technique for forming a control line for preventing diffusion of a biomaterial-containing solution due to capillary action at a predetermined position, dropping the biomaterial-containing solution in the vicinity of the control line, and localizing the control line. Is disclosed.
- the conventional immunochromatography analyzer cannot increase the sensitivity to the required level while suppressing the non-specific reaction.
- an object of the present invention is to provide a chromatographic analysis apparatus and a chromatographic analysis method capable of increasing the detection sensitivity of a substance to be detected while suppressing non-specific reactions.
- the present invention is as follows. 1.
- a chromatographic analyzer including at least a chromatographic medium portion on which a detection unit for detecting a target substance contained in a specimen is supported,
- the chromatographic medium part has a configuration in which a membrane is provided on a support,
- the chromatographic analyzer wherein the membrane has an average film thickness of 110 ⁇ m to 130 ⁇ m, and the development flow rate of the membrane is 30 to 45 seconds / 40 mm.
- 2. The chromatographic analyzer according to 1, wherein a sample addition unit for adding the sample, a labeling substance holding unit for holding a labeling substance for recognizing a target substance contained in the sample, and the chromatographic medium part are sequentially provided. 3. 3.
- a chromatographic analysis method wherein the following steps (1) to (4) are sequentially performed using the chromatographic analyzer according to the above item 2.
- a step of adding the sample to the sample addition unit (2)
- a step of recognizing a substance to be detected contained in the sample by a labeling substance held in the labeling substance holding unit (3)
- a sample and a labeling substance Step of developing in a chromatographic medium part as a mobile phase (4) Step of detecting a substance to be detected in the developed mobile phase by a detection part 8.
- the average film thickness and the development flow rate of the membrane in the chromatographic medium part are set in a specific range, the adverse effect of the substance that causes the nonspecific reaction in the detection part can be prevented to the maximum. And the detection sensitivity can be increased at the same time.
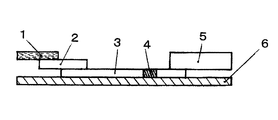
- FIG. 1 is a cross-sectional view for explaining an example of the structure of a chromatographic analyzer.
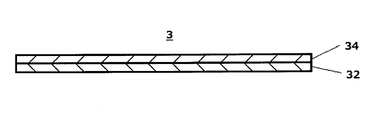
- FIG. 2 is a cross-sectional view for explaining an example of the structure of the support and membrane of the chromatographic medium part.
- FIG. 3 is a scanning electron microscope (SEM) photograph of a cross section of the chromatographic medium part of Example 6.
- the chromatographic analyzer and the chromatographic analysis method of the present invention are not particularly limited as long as they use specific binding based on the affinity of biomolecules.
- an immunochromatographic analyzer and method utilizing the binding of an antigen and an antibody a nucleic acid chromatographic analyzer and method utilizing a nucleic acid hybridization, etc., as well as a binding of a sugar and a lectin, a hormone and a receptor
- a chromatographic analysis apparatus and method utilizing the binding of an enzyme and an inhibitor.
- the immunochromatography analyzer of the present invention includes at least a chromatographic medium part on which a detection part for detecting a substance to be detected contained in a specimen is supported, and the membrane in the chromatographic medium part has an average described below. Although it will not restrict
- the immunochromatographic analyzer of the present invention comprises a sample adding part (also called a sample pad) (1), a labeling substance holding part (also called a conjugate pad) (2), and a chromatographic medium part (3).
- the detection unit (4), the absorption unit (5) and the backing sheet (6) are sequentially included.
- the sample addition part (1) is a part to which a sample as a specimen is added in the immunochromatography analyzer.
- the sample addition unit (1) may be any material as long as it is a material used in a normal immunochromatography analyzer. That is, in the sample addition part (1), although a sample is absorbed rapidly, it can be comprised with the porous sheet
- the porous sheet include cellulose filter paper, glass fiber, polyurethane, polyacetate, cellulose acetate, nylon, and cotton cloth.
- the labeling substance holding part (2) contains a labeling substance (marker substance), which will be described later, bound in advance to an antibody that binds to the substance to be detected. When the substance to be detected moves in the labeling substance holding part, it binds to the antibody and is labeled.
- the labeling substance holding part (2) is made of, for example, a glass fiber nonwoven fabric or a cellulose film.
- the chromatographic medium part (3) is a developed part of the chromatograph.
- the chromatographic medium part (3) is an inert film made of a microporous material that exhibits capillary action.
- the chromatographic medium part (3) has a structure in which a membrane (34) is provided on a support (32).
- the average film thickness of the membrane (34) is 110 ⁇ m to
- the development flow rate of the membrane (34) is 30 to 45 seconds / 40 mm.
- the membrane (34) When the average film thickness of the membrane (34) is less than 110 ⁇ m, the membrane (34) is detached from the support (32) or easily damaged, which is not practical. On the other hand, when the average film thickness of the membrane (34) exceeds 130 ⁇ m, non-specific reaction with respect to a highly viscous specimen tends to occur.
- the thickness of the membrane (34) can be determined, for example, from the scanning electron micrograph (for example, FIG. 3) of the cross section of the chromatographic medium part (3) comprising the support (32) and the membrane (34). ) And the membrane (34) can be identified, and the thickness of the membrane (34) can be measured.
- each film thickness can be measured with a film thickness measuring instrument such as a meter or a dial gauge, and the film thickness of the membrane (34) can be calculated from the difference in film thickness before and after dissolution.
- a plurality of different locations in the chromatographic medium part (3) are measured (preferably 9 to 10 or more locations), and the average value is calculated as the average film thickness of the membrane (34).
- the average film thickness of the membrane (34) is preferably 110 ⁇ m to 127 ⁇ m, more preferably 110 ⁇ m to 120 ⁇ m, and more preferably 114 ⁇ m to 117 ⁇ m in order to suppress non-specific reactions with higher sensitivity. Things are optimal. Further, if the thickness of the membrane (34) is in the range of 110 ⁇ m to 132 ⁇ m, the effect of the present invention can be sufficiently exerted, and a thickness of 110 ⁇ m to 122 ⁇ m is more preferably used.
- a more preferable development flow rate of the membrane (34) is 35 to 45 seconds / 40 mm.
- deployment flow velocity in this invention means the value obtained by measuring the time which developed 40 mm of water on the membrane in the perpendicular direction.
- the average film thickness of the membrane (34) becomes thin, the average pore diameter of the membrane (34) becomes small and the development flow rate becomes slow. Conversely, as the average film thickness of the membrane (34) increases, the development flow rate increases. In the present invention, the average film thickness of the membrane (34) is made thinner and the development flow rate is set faster than in the conventional chromatographic analyzer. As the average film thickness of the membrane (34) is reduced, the contact probability between the detected substance contained in the specimen and the antibody in the detection part (4) is increased, so that high sensitivity can be achieved and the development flow rate is increased. Thus, the adverse effect of the substance that causes the non-specific reaction in the detection unit (4) can be prevented to the maximum.
- nitrocellulose membrane a membrane made of cellulose acetate
- cellulose acetate membrane a membrane made of cellulose acetate
- Cellulose membranes, nylon membranes and porous plastic cloth polyethylene, polypropylene can also be used.
- nitrocellulose membrane it is only necessary to mainly contain nitrocellulose, and a membrane mainly composed of nitrocellulose such as a pure product or a nitrocellulose mixed product can be used.
- the nitrocellulose membrane exhibits a capillary phenomenon, but can further contain a substance that promotes the capillary phenomenon.
- a substance that lowers the surface tension of the film surface and brings about hydrophilicity is preferable.
- a substance having an amphipathic action such as sugars, amino acid derivatives, fatty acid esters, various synthetic surfactants or alcohols, which has no effect on the movement of the substance to be detected on the immunochromatograph and is a labeling substance
- Substances that do not affect the color development for example, gold particles are preferred.
- the chromatographic medium part (3) has the membrane (34) on the support (32).
- the configuration is provided.
- the support (32) examples include a support made of a water-impermeable plastic and the like, and examples thereof include a film-like support made of polyethylene terephthalate, polyethylene, and polyurethane.
- the support (32) preferably has a color that is not similar to the color caused by the labeling substance, and is usually colorless or white. Is preferred.
- the thickness of the support (32) is, for example, 50 ⁇ m to 130 ⁇ m, preferably 80 ⁇ m to 120 ⁇ m.
- the form and size of the chromatographic medium part (3) represented by the nitrocellulose membrane and cellulose acetate membrane as described above are not particularly limited, and are appropriate in terms of actual operation and observation of reaction results. If it is.
- the detection part (4) is formed on the chromatographic medium part (3), that is, an antibody that specifically binds to the substance to be detected is immobilized at an arbitrary position.
- antibodies include polyclonal antibodies and monoclonal antibodies.
- Monoclonal antibodies and polyclonal antibodies or fragments thereof are known and available, and can be prepared by known methods.
- the antibody can be immobilized at an arbitrary position as an immobilization reagent to form a detection part (4) as a reaction site.
- the immobilization reagent is immobilized on the chromatographic medium part (3) by directly immobilizing the immobilization reagent on the chromatographic medium part (3) by physical or chemical means, and the immobilization reagent is finely divided.
- a direct immobilization method physical adsorption may be used, or covalent bonding may be used. In the case of a nitrocellulose membrane, physical adsorption can be performed. In the covalent bond, cyanogen bromide, glutaraldehyde, carbodiimide or the like is generally used to activate the chromatographic medium part (3), but any method can be used.
- an immobilization reagent is bound to insoluble fine particles and then immobilization to the chromatographic medium part (3).
- the particle size of the insoluble fine particles can be selected so as to be captured by the chromatographic medium part (3) but cannot move, and is preferably fine particles having an average particle size of about 5 ⁇ m or less.
- organic polymers such as organic polymer latex particles obtained by emulsion polymerization methods such as polystyrene, styrene-butadiene copolymer, styrene-methacrylic acid copolymer, polyglycidyl methacrylate or acrolein-ethylene glycol dimethacrylate copolymer.
- Examples include fine particles of substances, fine particles such as gelatin, bentonite, agarose or cross-linked dextran, inorganic oxides such as silica, silica-alumina or alumina, or inorganic particles obtained by introducing a functional group to an inorganic oxide by silane coupling treatment, etc. .
- direct immobilization is preferable from the viewpoint of ease of sensitivity adjustment.
- Various methods can be used to immobilize the immobilization reagent on the chromatographic medium part (3).
- various techniques such as a microsyringe, a pen with a control pump, or ink jet printing can be used.
- the form of the reaction site is not particularly limited, but it may be fixed as a circular spot, a line extending perpendicularly to the development direction of the chromatographic medium, a numeral, a character, or a symbol such as +,-.
- the chromatographic medium part (3) may be subjected to a blocking treatment by a known method as necessary in order to further prevent the accuracy of the analysis from being lowered due to a nonspecific reaction.
- a blocking treatment by a known method as necessary in order to further prevent the accuracy of the analysis from being lowered due to a nonspecific reaction.
- proteins such as bovine serum albumin, skim milk, casein or gelatin are preferably used for the blocking treatment.
- one or a combination of two or more surfactants such as Tween 20, Triton X-100, or SDS may be washed.
- the absorption part (5) is installed at the end of the chromatographic medium part (3) as necessary to absorb the liquid such as the specimen and the developing solution that has passed through the detection part (4).
- the absorption part (5) contains, for example, a glass fiber, pulp, cellulose fiber or the like, or a non-woven fabric containing a hydrophilic drug having a polymer such as an acrylic acid polymer or an ethylene oxide group. Glass fiber is particularly preferable. When the absorption part (5) is made of glass fiber, the return of the sample liquid can be greatly reduced.
- the backing sheet (6) is a base material. By applying an adhesive on one side or sticking an adhesive tape, one side has adhesiveness, and the sample addition part (1), labeling substance holding part (2), chromatographic medium part ( 3) A part or all of the detection unit (4) and the absorption unit (5) are provided in close contact with each other.
- the backing sheet (6) is not particularly limited as long as the backing sheet (6) becomes impermeable and impermeable to the sample solution by the adhesive.
- the immunochromatographic analysis method of the present invention includes at least a step of detecting a substance to be detected contained in a sample by the detection unit (4) supported on the chromatographic medium unit (3) using the above-described immunochromatography analysis device. Although not particularly limited, it is preferable to sequentially perform the following steps (1) to (4).
- a step of adding the sample to the sample addition unit (2) A step of recognizing a substance to be detected contained in the sample by a labeling substance held in the labeling substance holding unit (3) A sample and a labeling substance Step of developing the chromatographic medium portion as a mobile phase (4) Step of detecting a substance to be detected in the developed mobile phase by the detection portion
- Step of adding specimen to sample addition section first, the concentration is such that the specimen moves smoothly in the immunochromatographic medium section (3) without reducing the measurement accuracy. It is preferable to prepare or dilute the sample with a sample diluent. Secondly, a predetermined amount (usually 0.1 to 2 ml) of the specimen-containing liquid is dropped onto the sample addition part (1). When the specimen-containing liquid is dropped, the specimen-containing liquid starts to move in the sample addition part (1).
- the sample diluent can also be used as a developing solution, but usually water is used as a solvent, and a buffer solution, a salt, and a nonionic surfactant, and further, for example, an antigen-antibody reaction is promoted. Alternatively, one or more of proteins, polymer compounds (PVP, etc.), ionic surfactants or polyanions, antibacterial agents, chelating agents, etc. for suppressing nonspecific reactions may be added.
- a sample and developing solution mixed in advance can be supplied and dropped onto the sample addition unit, or developed, or after the sample is first supplied and dropped onto the sample addition unit. Alternatively, the developing solution may be supplied and dropped on the sample addition unit to be developed.
- specimen containing the substance to be detected examples include biological samples, that is, nasal discharge, sputum, saliva, nasal wipe, pharyngeal wipe, stool, whole blood, serum, plasma, urine, spinal fluid, amniotic fluid, nipple discharge, In addition to tears, sweat, exudates from the skin, extracts from tissues, cells and stool, and the like, extracts from milk, eggs, wheat, beans, beef, pork, chicken, and foods containing them, and the like.
- the specimen is preferably at least one selected from nasal discharge, sputum, saliva, nasal wipe, pharyngeal wipe, and stool. That is, a so-called highly viscous specimen is preferable.
- a high-viscosity specimen contains a viscous substance such as mucin, and it is known that such a viscous substance tends to cause a nonspecific reaction.
- the average film thickness of the membrane (34) is set to 110 ⁇ m to 130 ⁇ m and the development flow rate of the membrane (34) is set to a range of 30 to 45 seconds / 40 mm as described above.
- the substance to be detected include carcinoembryonic antigen (CEA), HER2 protein, prostate specific antigen (PSA), CA19-9, ⁇ -fetoprotein (AFP), immunosuppressive acidic protein (IPA), CA15- 3, CA125, estrogen receptor, progesterone receptor, fecal occult blood, troponin I, troponin T, CK-MB, CRP, human chorionic gonadotropin (hCG), luteinizing hormone (LH), follicle stimulating hormone (FSH), syphilis antibody, Examples include, but are not limited to, influenza virus, RS virus, human hemoglobin, chlamydia antigen, group A ⁇ -streptococcal antigen, HBs antibody, HBs antigen, rotavirus, adenovirus, albumin or glycated albumin.
- fecal occult blood troponin I, troponin T, CK-MB, CRP, influenza virus, RS virus, human hemoglobin, chlamydia antigen, group A ⁇ streptococcal antigen, HBs antibody, HBs antigen, rotavirus, adenovirus, albumin or Glycated albumin is used as a substance to be detected.
- the labeling substance labels the antibody.
- Enzymes and the like are generally used for labeling the detection reagent in the immunochromatographic analysis method, but it is preferable to use an insoluble carrier as the labeling substance because it is suitable for visually determining the presence of the substance to be detected.
- a labeled detection reagent can be prepared by sensitizing the antibody to an insoluble carrier.
- the means for sensitizing the antibody to the insoluble carrier may be in accordance with a known method.
- the insoluble carrier as a labeling substance, metal particles such as gold, silver or platinum, metal oxide particles such as iron oxide, non-metallic particles such as sulfur and latex particles made of synthetic polymers, or other insoluble carriers are used. be able to.
- the insoluble carrier is a labeling substance suitable for visually determining the presence of the substance to be detected, and is preferably colored in order to facilitate the visual determination.
- the metal particles and the metal oxide particles themselves exhibit a specific natural color corresponding to the particle diameter, and the color can be used as a label.
- the average particle diameter of the gold particles is, for example, 10 nm to 250 nm, preferably 35 nm to 120 nm.
- the average particle diameter was measured by measuring the equivalent area diameter of 100 particles randomly using a projection photograph taken with a transmission electron microscope (TEM: manufactured by JEOL Ltd., JEM-2010). It can be calculated from the value.
- Step (3) is a step in which the substance to be detected is recognized after the substance to be detected is recognized by the labeling substance in the labeling substance holding part in step (2). In this step, the substance is passed through the chromatographic medium as a mobile phase.
- Step (4) is a step in which the substance to be detected in the specimen that has passed as a mobile phase on the chromatographic medium part is a specific antigen / antibody. This is a step in which the detection part is colored by specific reaction and binding so as to be sandwiched between the antibody and the labeling reagent that are held or immobilized on the detection part by the target binding reaction.
- the labeling reagent dissolved in the moisture of the sample does not cause a specific binding reaction even when it passes through the detection part on the chromatographic medium part, so that the detection part is not colored.
- the immunochromatography analyzer and the immunochromatography analysis method have been described as examples.
- the present invention is not limited to the above-described form, and the effect is achieved as long as the specific binding based on the affinity of the biomolecule is used. Can be enjoyed.
- sample addition part The nonwoven fabric (Millipore company_made: 300 mm x 30 mm) which consists of glass fiber was used as a sample addition part.
- labeling substance holding part 0.5 ml of colloidal gold suspension (manufactured by Tanaka Kikinzoku Kogyo Co., Ltd .: LC 40 nm) diluted with phosphate buffer (pH 7.4) to a concentration of 0.05 mg / ml 0.1 ml of the mouse-derived anti-RS virus monoclonal antibody was added and allowed to stand at room temperature for 10 minutes.
- a phosphate buffer pH 7.4 containing 1% by weight of bovine serum albumin (BSA) was added, and the mixture was allowed to stand at room temperature for 10 minutes. Then, after sufficiently stirring, centrifugation was performed at 8000 ⁇ g for 15 minutes to remove the supernatant, and 0.1 ml of a phosphate buffer solution (pH 7.4) containing 1% by mass of BSA was added.
- the labeling substance solution was prepared by the above procedure.
- the labeling substance holding part was produced by drying with a dryer.
- the film thickness is measured at three arbitrary positions from the observation, and the minimum film thickness, the maximum film thickness, and the average film thickness (average film thickness) of the chromatographic medium are measured from the total nine film thicknesses measured.
- 150 ⁇ L of a solution obtained by diluting a mouse-derived anti-RS virus monoclonal antibody with a phosphate buffer solution (pH 7.4) containing 5% by mass of isopropyl alcohol so as to have a concentration of 1.0 mg / ml was dried on the membrane. It is applied to the upper detection site (detection line) with a width of 1 mm, and has a wide affinity for gold nanoparticle labeling substances, etc.
- Sample diluent 1% by mass of nonionic surfactant (manufactured by NOF Corporation, trade name: MN811 and Nacalai Tesque, trade name: NP-40, 1: 1 mixture), 80 mM potassium chloride, 20 mM
- the average film thickness of the membrane is in the range of 110 ⁇ m to 130 ⁇ m, and the development flow rate of the membrane is in the range of 30 to 45 seconds / 40 mm. It was found that the non-specific reaction did not occur even in the analysis used. Sensitivity is also good. On the other hand, in Comparative Examples 1 and 2 in Table 2, the development flow rate is within the range of the present invention, but since the average film thickness of the membrane exceeds the upper limit specified in the present invention, nonspecific reaction is not caused. occured. In Comparative Example 3, the average film thickness of the membrane was within the range of the present invention, but the development flow rate exceeded the upper limit defined by the present invention, so the sensitivity was low compared to the Examples.
- Sample pad 2 Labeling substance holding part 3 Chromatographic medium part 32 Support body 34 Membrane 4 Detection part 5 Absorption part 6 Backing sheet
Landscapes
- Health & Medical Sciences (AREA)
- Immunology (AREA)
- Life Sciences & Earth Sciences (AREA)
- Engineering & Computer Science (AREA)
- Chemical & Material Sciences (AREA)
- Hematology (AREA)
- Urology & Nephrology (AREA)
- Molecular Biology (AREA)
- Biomedical Technology (AREA)
- General Health & Medical Sciences (AREA)
- Analytical Chemistry (AREA)
- Physics & Mathematics (AREA)
- Cell Biology (AREA)
- Food Science & Technology (AREA)
- Medicinal Chemistry (AREA)
- Biotechnology (AREA)
- Microbiology (AREA)
- Biochemistry (AREA)
- Pathology (AREA)
- General Physics & Mathematics (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Virology (AREA)
- Tropical Medicine & Parasitology (AREA)
- Investigating Or Analysing Biological Materials (AREA)
- Clinical Laboratory Science (AREA)
- Sampling And Sample Adjustment (AREA)
Priority Applications (4)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US15/739,833 US20180284115A1 (en) | 2015-06-30 | 2016-06-29 | Chromatographic analysis device and chromatographic analysis method |
| CN201680038667.8A CN107709994B (zh) | 2015-06-30 | 2016-06-29 | 层析分析装置及层析分析方法 |
| KR1020177037407A KR102102237B1 (ko) | 2015-06-30 | 2016-06-29 | 크로마토 분석 장치 및 크로마토 분석 방법 |
| EP16817990.1A EP3318875A1 (en) | 2015-06-30 | 2016-06-29 | Chromatographic analysis device and chromatographic analysis method |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2015-131804 | 2015-06-30 | ||
| JP2015131804A JP6270781B2 (ja) | 2015-06-30 | 2015-06-30 | クロマト分析装置およびクロマト分析方法 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2017002882A1 true WO2017002882A1 (ja) | 2017-01-05 |
Family
ID=57608710
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/JP2016/069343 Ceased WO2017002882A1 (ja) | 2015-06-30 | 2016-06-29 | クロマト分析装置およびクロマト分析方法 |
Country Status (6)
| Country | Link |
|---|---|
| US (1) | US20180284115A1 (enExample) |
| EP (1) | EP3318875A1 (enExample) |
| JP (1) | JP6270781B2 (enExample) |
| KR (1) | KR102102237B1 (enExample) |
| CN (1) | CN107709994B (enExample) |
| WO (1) | WO2017002882A1 (enExample) |
Families Citing this family (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP6943789B2 (ja) * | 2018-02-21 | 2021-10-06 | 田中貴金属工業株式会社 | モノクローナル抗体および非特異反応抑制剤 |
| JP6962834B2 (ja) * | 2018-02-21 | 2021-11-05 | 田中貴金属工業株式会社 | モノクローナル抗体および非特異反応抑制剤 |
| KR102766141B1 (ko) * | 2018-09-27 | 2025-02-12 | 세키스이 메디칼 가부시키가이샤 | 면역 크로마토그래피용 시험편 |
| WO2023034205A2 (en) * | 2021-08-30 | 2023-03-09 | Intellisafe Llc | Real-time virus and damaging agent detection |
Citations (11)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2004245831A (ja) * | 2003-01-21 | 2004-09-02 | Denka Seiken Co Ltd | メンブレンアッセイ法 |
| JP2005221473A (ja) * | 2004-02-09 | 2005-08-18 | Olympus Corp | 生体物質検出デバイス及びその製造方法及びその生体物質検出デバイスを用いる反応装置 |
| JP2008197038A (ja) * | 2007-02-15 | 2008-08-28 | Fujifilm Corp | イムノクロマトグラフィー法 |
| WO2009136476A1 (ja) * | 2008-05-07 | 2009-11-12 | パナソニック株式会社 | バイオセンサの製造方法およびバイオセンサ |
| WO2013088883A1 (ja) * | 2011-12-15 | 2013-06-20 | コニカミノルタ株式会社 | クロマトグラフィー分析装置及びクロマトグラフィー分析方法 |
| JP2013195403A (ja) * | 2012-03-22 | 2013-09-30 | Tanaka Kikinzoku Kogyo Kk | イムノクロマトグラフィー検出方法 |
| JP2013228308A (ja) * | 2012-04-26 | 2013-11-07 | Konica Minolta Inc | アナライトを検出または定量するためのラテラルフロー型クロマト法用テストストリップ |
| JP2014062820A (ja) * | 2012-09-21 | 2014-04-10 | Toyo Roshi Kaisha Ltd | イムノクロマトグラフ試験ストリップ用メンブレン、試験ストリップ及び検査方法 |
| JP5567932B2 (ja) * | 2010-08-03 | 2014-08-06 | 田中貴金属工業株式会社 | イムノクロマトグラフィー用試薬組成物およびそれを用いた測定方法 |
| WO2014122094A1 (en) * | 2013-02-08 | 2014-08-14 | Whatman Gmbh | Medical diagnostic test systems, and a matrix therefor |
| JP2014167439A (ja) * | 2013-02-28 | 2014-09-11 | Asahi Kasei Corp | マイコプラズマ・ニューモニアの検出方法 |
Family Cites Families (10)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP3580388B2 (ja) | 1996-01-25 | 2004-10-20 | 栄研化学株式会社 | 非特異反応の抑制方法 |
| JP4681198B2 (ja) * | 2000-03-17 | 2011-05-11 | 株式会社三和化学研究所 | 試験紙 |
| US8148057B2 (en) * | 2005-06-21 | 2012-04-03 | The United States Of America As Represented By The Secretary Of The Department Of Health And Human Services, Centers For Disease Control And Prevention | Methods, immunoassays and devices for detection of anti-lipoidal antibodies |
| JP5207290B2 (ja) | 2008-04-24 | 2013-06-12 | 国立大学法人北陸先端科学技術大学院大学 | クロマトストリップの作製方法、及びラテラルフロー型のクロマトストリップ |
| US9513300B2 (en) * | 2008-05-05 | 2016-12-06 | Cornell University | Determination of serum anti-mullerian hormone as a diagnostic test for spay in companion animals |
| US20100159599A1 (en) * | 2008-12-18 | 2010-06-24 | Xuedong Song | Lateral-flow porous membrane assay with flow rate control |
| EP2478369A4 (en) * | 2009-03-25 | 2012-11-14 | Lyzer Diagnostics Inc | APPARATUS AND METHODS FOR ANALYZING VARIABLES RELATED TO FLUIDS |
| EP2419216A1 (en) * | 2009-04-15 | 2012-02-22 | Koninklijke Philips Electronics N.V. | Microfluidic device comprising sensor |
| CL2011003002A1 (es) * | 2011-11-25 | 2012-05-25 | Univ Pontificia Catolica Chile | Anticuerpo monoclonal o un fragmento del mismo que se une a la proteina m2-1 del virus respiratorio sincicial (vrs) humano; secuencias nucleotidicas; composicion farmaceutica; metodo de diagnostico de infeccion producida por vrs; kit; y uso de dicho anticuerpo para preparar un medicamento. |
| JP6397224B2 (ja) * | 2014-06-04 | 2018-09-26 | 田中貴金属工業株式会社 | 免疫学的測定試薬におけるプロゾーン現象の解消法 |
-
2015
- 2015-06-30 JP JP2015131804A patent/JP6270781B2/ja active Active
-
2016
- 2016-06-29 US US15/739,833 patent/US20180284115A1/en not_active Abandoned
- 2016-06-29 WO PCT/JP2016/069343 patent/WO2017002882A1/ja not_active Ceased
- 2016-06-29 CN CN201680038667.8A patent/CN107709994B/zh active Active
- 2016-06-29 KR KR1020177037407A patent/KR102102237B1/ko active Active
- 2016-06-29 EP EP16817990.1A patent/EP3318875A1/en not_active Ceased
Patent Citations (11)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2004245831A (ja) * | 2003-01-21 | 2004-09-02 | Denka Seiken Co Ltd | メンブレンアッセイ法 |
| JP2005221473A (ja) * | 2004-02-09 | 2005-08-18 | Olympus Corp | 生体物質検出デバイス及びその製造方法及びその生体物質検出デバイスを用いる反応装置 |
| JP2008197038A (ja) * | 2007-02-15 | 2008-08-28 | Fujifilm Corp | イムノクロマトグラフィー法 |
| WO2009136476A1 (ja) * | 2008-05-07 | 2009-11-12 | パナソニック株式会社 | バイオセンサの製造方法およびバイオセンサ |
| JP5567932B2 (ja) * | 2010-08-03 | 2014-08-06 | 田中貴金属工業株式会社 | イムノクロマトグラフィー用試薬組成物およびそれを用いた測定方法 |
| WO2013088883A1 (ja) * | 2011-12-15 | 2013-06-20 | コニカミノルタ株式会社 | クロマトグラフィー分析装置及びクロマトグラフィー分析方法 |
| JP2013195403A (ja) * | 2012-03-22 | 2013-09-30 | Tanaka Kikinzoku Kogyo Kk | イムノクロマトグラフィー検出方法 |
| JP2013228308A (ja) * | 2012-04-26 | 2013-11-07 | Konica Minolta Inc | アナライトを検出または定量するためのラテラルフロー型クロマト法用テストストリップ |
| JP2014062820A (ja) * | 2012-09-21 | 2014-04-10 | Toyo Roshi Kaisha Ltd | イムノクロマトグラフ試験ストリップ用メンブレン、試験ストリップ及び検査方法 |
| WO2014122094A1 (en) * | 2013-02-08 | 2014-08-14 | Whatman Gmbh | Medical diagnostic test systems, and a matrix therefor |
| JP2014167439A (ja) * | 2013-02-28 | 2014-09-11 | Asahi Kasei Corp | マイコプラズマ・ニューモニアの検出方法 |
Non-Patent Citations (1)
| Title |
|---|
| See also references of EP3318875A4 * |
Also Published As
| Publication number | Publication date |
|---|---|
| CN107709994B (zh) | 2020-06-30 |
| EP3318875A4 (en) | 2018-05-09 |
| JP6270781B2 (ja) | 2018-01-31 |
| EP3318875A1 (en) | 2018-05-09 |
| KR20180014758A (ko) | 2018-02-09 |
| KR102102237B1 (ko) | 2020-04-20 |
| JP2017015533A (ja) | 2017-01-19 |
| US20180284115A1 (en) | 2018-10-04 |
| CN107709994A (zh) | 2018-02-16 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP5792859B1 (ja) | 免疫クロマト分析方法 | |
| JP6008670B2 (ja) | イムノクロマトグラフ試験ストリップ用メンブレン、試験ストリップ及び検査方法 | |
| JP6397224B2 (ja) | 免疫学的測定試薬におけるプロゾーン現象の解消法 | |
| KR101607715B1 (ko) | 면역 크로마토그래피법을 위한 전개액, 및 이를 이용한 측정 방법 | |
| JP6143818B2 (ja) | マイコプラズマ・ニューモニエ検出用免疫クロマト分析装置 | |
| KR102586991B1 (ko) | 면역크로마토그래피 장치 | |
| JP6270781B2 (ja) | クロマト分析装置およびクロマト分析方法 | |
| TWI639002B (zh) | Chromatographic medium | |
| JP6595818B2 (ja) | 免疫クロマト分析装置及び免疫クロマト分析方法 | |
| EP3686600A1 (en) | Substrate for chromatography medium, chromatography medium, and strip for immunochromatograph | |
| JP6533216B2 (ja) | 免疫クロマト分析装置及び免疫クロマト分析方法 | |
| JP2008197038A (ja) | イムノクロマトグラフィー法 | |
| JP6595215B2 (ja) | 免疫クロマト分析装置およびその製造方法並びに免疫クロマト分析方法 | |
| WO2016194986A1 (ja) | マイコプラズマ・ニューモニエ検出用免疫クロマト分析装置 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 16817990 Country of ref document: EP Kind code of ref document: A1 |
|
| DPE1 | Request for preliminary examination filed after expiration of 19th month from priority date (pct application filed from 20040101) | ||
| WWE | Wipo information: entry into national phase |
Ref document number: 15739833 Country of ref document: US |
|
| ENP | Entry into the national phase |
Ref document number: 20177037407 Country of ref document: KR Kind code of ref document: A |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2016817990 Country of ref document: EP |