WO2016143217A1 - 薄葉紙 - Google Patents
薄葉紙 Download PDFInfo
- Publication number
- WO2016143217A1 WO2016143217A1 PCT/JP2015/085157 JP2015085157W WO2016143217A1 WO 2016143217 A1 WO2016143217 A1 WO 2016143217A1 JP 2015085157 W JP2015085157 W JP 2015085157W WO 2016143217 A1 WO2016143217 A1 WO 2016143217A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- thin paper
- less
- paper according
- porous
- acid
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Ceased
Links
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F13/00—Bandages or dressings; Absorbent pads
- A61F13/15—Absorbent pads, e.g. sanitary towels, swabs or tampons for external or internal application to the body; Supporting or fastening means therefor; Tampon applicators
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F13/00—Bandages or dressings; Absorbent pads
- A61F13/15—Absorbent pads, e.g. sanitary towels, swabs or tampons for external or internal application to the body; Supporting or fastening means therefor; Tampon applicators
- A61F13/45—Absorbent pads, e.g. sanitary towels, swabs or tampons for external or internal application to the body; Supporting or fastening means therefor; Tampon applicators characterised by the shape
- A61F13/49—Absorbent pads, e.g. sanitary towels, swabs or tampons for external or internal application to the body; Supporting or fastening means therefor; Tampon applicators characterised by the shape specially adapted to be worn around the waist, e.g. diapers, nappies
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F13/00—Bandages or dressings; Absorbent pads
- A61F13/15—Absorbent pads, e.g. sanitary towels, swabs or tampons for external or internal application to the body; Supporting or fastening means therefor; Tampon applicators
- A61F13/53—Absorbent pads, e.g. sanitary towels, swabs or tampons for external or internal application to the body; Supporting or fastening means therefor; Tampon applicators characterised by the absorbing medium
-
- D—TEXTILES; PAPER
- D21—PAPER-MAKING; PRODUCTION OF CELLULOSE
- D21H—PULP COMPOSITIONS; PREPARATION THEREOF NOT COVERED BY SUBCLASSES D21C OR D21D; IMPREGNATING OR COATING OF PAPER; TREATMENT OF FINISHED PAPER NOT COVERED BY CLASS B31 OR SUBCLASS D21G; PAPER NOT OTHERWISE PROVIDED FOR
- D21H17/00—Non-fibrous material added to the pulp, characterised by its constitution; Paper-impregnating material characterised by its constitution
- D21H17/63—Inorganic compounds
- D21H17/67—Water-insoluble compounds, e.g. fillers, pigments
-
- D—TEXTILES; PAPER
- D21—PAPER-MAKING; PRODUCTION OF CELLULOSE
- D21H—PULP COMPOSITIONS; PREPARATION THEREOF NOT COVERED BY SUBCLASSES D21C OR D21D; IMPREGNATING OR COATING OF PAPER; TREATMENT OF FINISHED PAPER NOT COVERED BY CLASS B31 OR SUBCLASS D21G; PAPER NOT OTHERWISE PROVIDED FOR
- D21H19/00—Coated paper; Coating material
- D21H19/10—Coatings without pigments
-
- D—TEXTILES; PAPER
- D21—PAPER-MAKING; PRODUCTION OF CELLULOSE
- D21H—PULP COMPOSITIONS; PREPARATION THEREOF NOT COVERED BY SUBCLASSES D21C OR D21D; IMPREGNATING OR COATING OF PAPER; TREATMENT OF FINISHED PAPER NOT COVERED BY CLASS B31 OR SUBCLASS D21G; PAPER NOT OTHERWISE PROVIDED FOR
- D21H27/00—Special paper not otherwise provided for, e.g. made by multi-step processes
Definitions
- the present invention relates to a thin paper used for absorbent articles such as disposable diapers and urine pads.
- bacteria act on urine when they remain on the skin or face sheet. Specifically, when bacteria and urine come into contact, the bacteria-derived enzyme decomposes odorous precursors such as urea, protein, and glucuronic acid conjugates contained in urine, fatty acids such as acetic acid, ammonia, Various odor components from acidic to neutral to alkaline such as amines such as trimethylamine, phenols, mercaptans and the like are generated in the liquid. This odor component volatilizes and feels as urine odor. Such a rotting odor has a large amount of urine, such as a disposable diaper for adults, and when wearing for a relatively long time (for example, wearing for 6 hours), it grows at an accelerated rate with the growth of bacteria.
- odorous precursors such as urea, protein, and glucuronic acid conjugates contained in urine
- fatty acids such as acetic acid, ammonia
- Various odor components from acidic to neutral to alkaline such as amines such as tri
- This strong rotting odor may cause strong discomfort due to the rotting odor drifting not only around the diaper changing area, but also in the living room, living room, and around the trash disposal bin (stored for a certain period of time until garbage collection). . It is possible to improve the working environment in diaper wearers, surrounding families, caregivers' hygiene, nursing care facilities, hospitals, etc., and deodorizing performance to reduce unpleasant rotten odors may be imparted to absorbent articles It is strongly desired. On the other hand, it must be assumed to be a severe wearing environment such as the generation of a long-time rot odor caused by normal bacteria or enteric bacteria. However, conventional deodorization techniques still have much room for improvement in these respects.
- the present invention provides a thin paper in which 0.05 g / m 2 or more of a cationic antibacterial agent, porous particles, and a pH buffering deodorant are contained in a cellulose fiber sheet substrate.
- FIG. 1 It is a schematic diagram which shows an example of the preparation apparatus of the aqueous coating liquid used in the manufacturing process of the thin paper of this invention. It is a schematic diagram which shows an example of the spray apparatus of the aqueous coating liquid used in the manufacturing process of the thin paper of this invention. It is sectional drawing which shows typically an example of the absorbent article using the thin paper of this invention. The graph which showed the result of the smell sensory evaluation with respect to the sample of an Example in time series. The graph which showed the result of the odor sensory evaluation with respect to the sample of a comparative example in time series.
- each cross-sectional view is schematically shown.
- the constituent element is a sheet or the like having a small thickness, it is indicated by a line, and the constituent elements are shown with an interval in order to easily distinguish the constituent elements. Therefore, in practice, the components are in contact or bonded unless otherwise specified.
- the present invention suppresses the odor to a level where it does not feel uncomfortable even if the absorbent article is worn for a long time under the harsh conditions in which normal skin bacteria or intestinal bacteria grow. It is related to thin paper.
- the thin paper of the present invention can suppress the odor to a level where it does not feel uncomfortable even if the absorbent article is worn for a long time under severe conditions with many bacteria.
- the thin paper of the present invention comprises a cellulose fiber sheet base material containing 0.05 g / m 2 or more of a cationic antibacterial agent, porous particles, and a pH buffering deodorant.
- a cationic antibacterial agent e.g., a cationic antibacterial agent
- porous particles e.g., a cationic antibacterial agent
- a pH buffering deodorant e.g., a cationic antibacterial agent, porous particles, and a pH buffering deodorant.
- the above three kinds of agents are mixedly arranged on one sheet.
- the combination of this cationic antibacterial agent, porous particles, and pH buffering deodorant shows a wide deodorant spectrum when excrement liquid such as urine touches thin paper, and various kinds of malodorous components derived from excreted urine. Production suppression and deodorization become possible.
- the rot odor is strongly suppressed by the action of continuous and effective antibacterial and deodorizing. And it can maintain a weak odor equivalent to or less than the initial excretion.
- the cationic antibacterial agent and the pH buffering deodorant are contained in the cellulose fibers constituting the thin paper, and are dried and solid.
- the cationic antibacterial agent and the pH buffering deodorant in the fiber are gradually dissolved in contact with the excretory liquid, so that moderate antibacterial and neutralizing deodorant action effects can be obtained.
- This is a long-term odor control at a high level, which is different from the effect that the mixed solution of the cationic antibacterial agent and the buffering agent exists in a liquid state and is brought into contact with excrement.
- the deodorization mechanism of the present invention includes a time axis deodorization mechanism along each stage until malodor occurs, and a pH axis deodorization mechanism corresponding to a wide range of rot odors having various pH at each generation stage. Broadly divided.
- the time axis deodorization mechanism has the following three stages.
- the cationic antibacterial agent eliminates the source of odorous components (biological deodorant action). That is, in the liquid phase of excretory liquid, the growth of microorganisms and microorganism-derived enzymes that are the cause of production of odorous components is suppressed.
- the pH buffering deodorant neutralizes the odor component produced in the liquid phase without being suppressed in the first stage, and stays in the liquid phase as a neutralized salt (chemical deodorization action) ).
- the porous particles are adsorbed or adsorbed with odorous components that cannot be suppressed in the previous stage and cannot be neutralized, increase over time, cannot be completely dissolved in the liquid phase, and volatilize in the gas phase. Inclusion (physical deodorizing effect).
- the deodorization mechanism on the pH axis is prevention of the generation of a wide variety of acidic and alkaline odors (complex odors) at each generation stage.
- the generation of various odors from acidic to alkaline such as lower fatty acids, phenols, mercaptans and amines is prevented.
- the generation of various odorous components is suppressed by suppressing the growth of microorganisms and microorganism-derived enzymes.
- changes in the pH of the excretory fluid are captured and buffered to neutralize a wide range of acidic and alkaline odorous components and suppress the generation of odorous gas.
- the porous particles adsorb and confine a wide variety of odorous gases.
- the deodorization mechanism by the combination of the above three types of agents can be comprehensively contained in the time axis and pH axis matrix against the mechanism of malodor generation.
- each stage until the bad odor is generated does not always proceed sequentially in time series, and each stage may be mixed at a certain point in time. That is, the above-mentioned three kinds of agents mixed in thin paper act simultaneously and contain odors comprehensively.
- the cationic antibacterial agent acts on microorganisms that are the source of odor (antibacterial) and controls the production of odorous components, the amount of odorous components in the liquid and the odorous components that evaporate out of the liquid is extremely low. It is done.
- Cationic antimicrobial agent used in the present invention to suppress even the occurrence of a strong rancidity as control of malodor production, it is contained in the 0.05 g / m 2 basis weight of not less than, 0.1 g / m 2 or more Preferably, 0.2 g / m 2 or more is more preferable.
- the upper limit is not particularly limited from the viewpoint of controlling malodor production, and can be set as appropriate depending on the application of the article used. For example, in the use of absorbent articles, it may be in contact with the user's skin directly or through excreted urine for a long time. There is a possibility that the influence and the permeability of excreted urine passing through the sheet may be inhibited. Therefore, the upper limit is preferably 1.0 g / m 2 or less, more preferably 0.5 g / m 2 or less, 0.3 g / m 2 or less is more preferable.
- the content basis weight of the cationic antibacterial agent can be measured by the following method. When analyzing from a commercial product etc., each member is peeled off using a dryer, a cold spray, etc., and the target thin paper is obtained. Thereafter, the basis weight of the cationic antibacterial agent in the thin paper can be measured with a liquid chromatograph / mass spectrometer (6140 LC / MS manufactured by Agilent Technologies, ionization method: ESI). Alternatively, a calibration curve can be created, and the content of the cationic antibacterial agent can be measured based on the calibration curve.
- a liquid chromatograph / mass spectrometer (6140 LC / MS manufactured by Agilent Technologies, ionization method: ESI).
- a calibration curve can be created, and the content of the cationic antibacterial agent can be measured based on the calibration curve.
- cationic antibacterial agent those having the above-mentioned antibacterial action can be used without particular limitation. For example, there are those described in paragraphs [0015] to [0018] of the specification of JP-A-8-99841. These cationic antibacterial agents may be used alone or in combination of two or more.
- Cationic antibacterial agents are organic compound-based antibacterial agents, and are more leached into excretory fluids such as urine and have a wider antibacterial action than metal ion systems such as silver, zinc and copper.
- quaternary ammonium salts such as didecyldimethylammonium salt, alkylpyridinium salt, benzethonium salt, benzalkonium salt, monoalkyltrimethylammonium salt, dialkyldimethylammonium salt, and the like, and at least one selected from the group consisting of these are included.
- it contains seeds.
- These cationic antibacterial agents may be used individually by 1 type, and may be used in combination of 2 or more type.
- a benzalkonium salt represented by the following formula (1) is preferable from the viewpoint of antibacterial properties and safety (low irritation to skin).
- R 1 and R 2 represent the same or different methyl group, ethyl group, or a linear or branched alkyl group or alkenyl group having 8 to 20 carbon atoms.
- X ⁇ represents a monovalent anion.
- One of the agents represented by formula (1) can be used alone or in combination of two or more.
- R 1 and R 2 for example, a combination in which R 1 is a methyl group and R 2 is a linear or branched alkyl group having 8 to 20 carbon atoms
- examples include a combination in which 1 and R 2 are the same group and the group is a linear or branched alkyl group having 8 to 20 carbon atoms.
- the alkyl group preferably has 10 to 18 carbon atoms.
- X - a monovalent anion represented by is preferably, for example, halide ions or anions active group.
- the “anionic active group” refers to an ion having an anionic surface active ability. Examples of the anionic active group include an anionic active group having 6 or more carbon atoms, particularly 10 or more carbon atoms, and a linear or branched alkyl group or alkenyl group having 20 or less carbon atoms, particularly 18 or less carbon atoms. Is preferably used.
- alkyl phosphate ester salt for example, alkyl phosphate ester salt, alkyl carboxylate salt, alkyl sulfonate salt, and alkyl sulfate ester salt are preferably used from the viewpoint of antibacterial activity.
- alkyl phosphoric acid represented by the following formula (2) it is preferable to use an alkyl phosphoric acid represented by the following formula (2) from the viewpoint that the antibacterial ability is further enhanced.
- one of R 3 and R 4 represents a linear or branched alkyl group or alkenyl group having 6 to 20 carbon atoms, and the other represents a hydrogen atom, a methyl group, or an ethyl group.
- a preferable combination of R 3 and R 4 includes a combination in which R 3 is a hydrogen atom and R 4 is a linear or branched alkyl group having 8 to 20 carbon atoms. More preferably, the alkyl group has 10 to 18 carbon atoms. Having a long-chain alkyl cationic active group (quaternary amine) improves the texture of the paper (bulkness and flexibility), and is preferable as a thin paper.
- Benzalkonium cetyl phosphate is an organic cationic antibacterial agent that has a good balance between antibacterial properties and low skin irritation (low water solubility), and is relatively safe. It is a preferred agent for use in an article sheet.
- the pH buffering deodorant used in the present invention is an agent that neutralizes acidic and alkaline odorous components generated in a liquid phase such as excretory liquid and reduces pH change. That is, it is a neutralizing deodorant by an equilibrium action.
- Such an agent is not particularly limited, and examples thereof include weak acids and their conjugate bases, or mixtures thereof, weak bases and co-liquid acids thereof, and mixtures thereof. Specifically, citric acid etc. are mentioned as a weak acid.
- the salt include alkali metal salts such as Na, K, and Ca.
- the weak base include polyhydroxyamine.
- the pH buffering deodorant does not necessarily need to neutralize all acid and alkali odors at the same time.
- citric acid as a weak acid it is possible to neutralize and deodorize alkali odors such as amine and ammonia which are one of odor components. Furthermore, neutralization produces a salt of citric acid, and a secondary buffering action occurs. Moreover, even if it is only a sodium citrate simple substance, and it is a mixture of a citric acid and its salt, a buffer effect will arise. The same applies to Tris as a weak base and its salt.
- the basis weight of the pH buffering deodorant is to effectively neutralize and deodorize acidic and alkaline odor components that are continuously generated in the excrement of the absorbent article for a long period of time, and not volatilize in the gas phase.
- 0.05 g / m 2 or more preferably, 0.1 g / m 2 or more preferably, 0.15 g / m 2 or more is more preferable.
- the upper limit is not particularly limited, but is preferably 2.0 g / m 2 or less, more preferably 1.0 g / m 2 or less, and still more preferably 0.5 g / m 2 or less from the viewpoint of production cost.
- the content basis weight of the pH buffering deodorant can be measured using a known ion chromatography analysis method or the like.
- a known ion chromatography analysis method or the like for example, for the analysis of anions such as organic acids and phosphoric acids, alkanolamines and the like, qualitative analysis can be performed using Dionex ICS-2100 (manufactured by Thermo Fisher Scientific Co., Ltd., conductivity detector, gradient elution method).
- the content basis weight can be measured.
- the pH buffering deodorant is also referred to as at least one selected from the group consisting of organic acids and salts thereof having at least one acid dissociation index pKa (25 ° C.) of 5.0 or more (hereinafter also referred to as “agent A”). ) Is preferably included.
- the organic acid salt is a metal salt such as Na, K, or Ca. By containing this A agent, it acts on the neutralization deodorization of acidic odor components, such as fatty acids, and alkaline odor components, such as amines.
- the acid dissociation index is, for example, (a) the method described in The Journal of Physical Chemistry vol. 68, number 6, page 1560 (1964), (b) an automatic potentiometric titrator (COM-980Win (trade name) manufactured by Hiranuma Sangyo Co., Ltd.) Etc.), and (c) the acid dissociation index described in Chemical Handbook of the Chemical Society of Japan (Revised 3rd edition, published June 25, 1984, published by Maruzen Co., Ltd.) (D) A database such as pKaBASE (trade name) manufactured by CompuDrug can be used.
- the basis weight contained in the thin paper of the agent A varies depending on the target excrement, malodor concentration, and form of use, but from the viewpoint of deodorizing performance, 0.01 g / m 2 or more is preferable, and 0.03 g / m. 2 or more is more preferable, and 0.1 g / m 2 or more is still more preferable.
- the upper limit, excessive basis weight for impairing the strength reduction and texture of the thin paper preferably 3 g / m 2 or less, more preferably 1 g / m 2 or less, 0.5 g / m 2 or less is more preferable.
- the organic acid having at least one acid dissociation index pKa (25 ° C.) of 5.0 or more is, for example, maleic acid (second-stage pKa value: 5.83) as an organic dibasic acid.
- the numerical value in () below is 2 Stage pKa value), malonic acid (5.28), 2-methylmalonic acid (5.76), 2-ethylmalonic acid (5.81), 2-isopropylmalonic acid (5.88), 2,2-dimethylmalonic acid (5.73), 2-ethyl-2-methylmalonic acid (6.55), 2,2-diethylmalonic acid (7.42), 2,2-diisopropylmalonic acid (8 .85), m-hydroxybenzoic acid (9.96), p-hydroxybenzoic acid (9.46), 1,2-cyclohexanedicarboxylic acid (trans isomer: 6.06, cis isomer: 6.74), 1 , 2-cyclopentanedicarboxylic acid (trans isomer: 5.9,
- citric acid third-stage pKa value: 5.69 and the like can be mentioned.
- phosphoric acid, citric acid, succinic acid having an acid dissociation index (pKa) of at least 5.2 in at least one dissociation stage from the viewpoint of storage stability such as easy acquisition and increase in internal pressure due to gas generation. It is preferable to include at least one selected from the group consisting of acid, maleic acid, malonic acid and the like, and citric acid is particularly preferable.
- the pH buffering deodorant preferably contains at least one selected from the group consisting of a polyhydroxyamine compound and a salt thereof (hereinafter also referred to as “B agent”).
- the polyhydroxyamine compound of agent B is a compound represented by the following formula (3).
- R 5 represents a hydrogen atom, an alkyl group having 1 to 5 carbon atoms, or a hydroxyalkyl group having 1 to 5 carbon atoms.
- the alkyl group having 1 to 5 carbon atoms may be either linear or branched, and examples thereof include a methyl group, an ethyl group, an n-propyl group, an isopropyl group, various butyl groups, and various pentyl groups.
- Examples of the hydroxyalkyl group having 1 to 5 carbon atoms include a hydroxymethyl group, a 2-hydroxyethyl group, a 2-hydroxypropyl group, a 3-hydroxypropyl group, a 2-hydroxybutyl group, a 3-hydroxybutyl group, 4- Examples thereof include a hydroxybutyl group.
- R 5 is preferably a hydrogen atom, a methyl group, an ethyl group, a hydroxymethyl group or a 2-hydroxyethyl group from the viewpoints of deodorizing performance and availability, and in particular, a hydrogen atom, a hydroxymethyl group, a 2-hydroxyethyl group.
- An ethyl group is preferred.
- R 6 represents a hydrogen atom, an alkyl group having 1 to 6 carbon atoms, or a hydroxyalkyl group having 1 to 5 carbon atoms.
- the alkyl group having 1 to 6 carbon atoms may be linear, branched or cyclic. Specific examples of the alkyl group include methyl, ethyl, n-propyl, isopropyl, various butyl, various pentyl, various hexyl, cyclopentyl, cyclohexyl and the like.
- Examples of the hydroxyalkyl group having 1 to 5 carbon atoms include those described above. From the viewpoint of deodorizing performance and availability, R 6 is preferably a hydrogen atom, an alkyl group having 1 to 3 carbon atoms, or a hydroxyethyl group, and particularly preferably a hydrogen atom.
- R 7 and R 8 represent an alkanediyl group having 1 to 5 carbon atoms.
- R 7 and R 8 may be the same or different.
- As the alkanediyl group having 1 to 5 carbon atoms a methylene group, an ethylene group, a trimethylene group, a propane-1,2-diyl group, a tetramethylene group and the like are preferable, and a methylene group is particularly preferable.
- polyhydroxyamine compound as the agent B examples include, for example, 2-amino-1,3-propanediol, 2-amino-2-methyl-1,3-propanediol, 2-amino-2-ethyl-1 , 3-propanediol, 2-amino-2-hydroxymethyl-1,3-propanediol, 2-amino-2-hydroxyethyl-1,3-propanediol, 4-amino-4-hydroxypropyl-1,7 -Heptanediol, 2- (N-ethyl) amino-1,3-propanediol, 2- (N-ethyl) amino-2-hydroxymethyl-1,3-propanediol, 2- (N-decyl) amino- Examples include 1,3-propanediol and 2- (N-decyl) amino-2-hydroxymethyl-1,3-propanediol.
- 2-amino-1,3-propanediol 2-amino-2-methyl-1,3-propanediol, 2-amino-2-ethyl-1,3
- One or more selected from -propanediol, 2-amino-2-hydroxymethyl-1,3-propanediol, and 2-amino-2-hydroxyethyl-1,3-propanediol are particularly preferred.
- 2-amino-2-hydroxymethyl-1,3-propanediol that is, tris (hydroxymethyl) aminomethane, has an excellent neutralizing action against acidic odor and has low volatility. This is preferable because of its low odor.
- the above polyhydroxyamine compounds can be used alone or in admixture of two or more.
- the polyhydroxyamine compound can be produced by a usual method.
- Content basis weight of the B agent in tissue paper, in terms of deodorizing performance 0.01 g / m 2 or more preferably, 0.05 g / m 2 or more is more preferable.
- the upper limit, because excessive basis weight impair the strength reduction and texture of the absorbent article sheet is preferably 3 g / m 2 or less, 1 g / m 2 or less is more preferable. More preferably 0.01 g / m 2 or more 3 g / m 2 or less, particularly preferably 0.05 g / m 2 or more 1 g / m 2 or less.
- any one of the A agent and B agent mentioned above may be included as a pH buffer deodorant, and both may be included.
- the inclusion of both agent A and agent B is more preferable in terms of neutralizing a wide range of odor components.
- the organic acid of agent A and the polyhydroxyamine compound of agent B and their salts dissolve simultaneously in the excretory fluid from thin paper, and exhibit excellent deodorizing action on the odor component of the excretory fluid due to its buffering properties. To do.
- the pH buffering deodorant of agent A and agent B is contained in the cellulose fiber sheet substrate in combination with the cationic antibacterial agent described above.
- the absorbent article using the thin paper can maintain the deodorizing property at a level where the odor of the excretory fluid is hardly noticed from the surroundings even when worn for a long time.
- the coating material has a specific mixing ratio in the thin paper manufacturing process described later, the particles of the cationic antibacterial agent are reduced in size and can be uniformly applied. Thereby, the antibacterial property of the thin paper of this invention improves.
- the target pH when excretion liquid such as urine comes into contact and the cationic antibacterial agent and the pH buffering deodorant are expressed (disinfection and neutralization), From the viewpoint of deodorant performance (buffer performance can be maintained), 5.0 or higher is preferable, and 5.5 or higher is more preferable. From the same viewpoint, the target pH is preferably 10.0 or less, and more preferably 9.0 or less. Moreover, it is preferable from the viewpoint of safety (low irritation to the skin) that the pH of the excretory fluid after the above action is as neutral as possible.
- porous particles used in the present invention those capable of adsorbing or incorporating volatilizing odorous gas into their pores can be used without particular limitation.
- acrylic polymer such as porous methacrylic acid polymer and porous acrylic acid polymer, aromatic polymer such as porous divinylbenzene polymer and porous pyridine copolymer, and synthetic porous polymer such as copolymer thereof; chitin And natural porous polymers such as chitosan; zinc oxide, activated carbon, silica, silicon dioxide (silica gel), calcium silicate, aluminosilicate compounds, high silica zeolite (hydrophobic zeolite), sepiolite, cancrinite, zeolite, and water Inorganic porous materials such as zirconium oxide; silver-supported zeolite, silver-supported cancrinite, and vinyl-pyridine copolymers such as silver-supported porous styrene-divinylbenzene-vinylpyridine poly
- porous particles may be used individually by 1 type, and may be used in combination of 2 or more type.
- porous vinylpyridine copolymer include porous styrene-divinylbenzene-vinylpyridine polymer and porous divinylbenzene-vinylpyridine polymer.
- the silver-supported cancrinite is a cancrinite-like mineral described in paragraphs [0029] to [0045] of JP-A-2005-336363.
- the porous particles preferably have a BET specific surface area of 50 m 2 / g or more, more preferably 100 m 2 / g or more, and 200 m 2 / g or more. More preferably.
- the upper limit is not particularly limited, but it is practical to set the upper limit to 2000 m 2 / g or less.
- the porous particles preferably have an average pore diameter of 2 nm or more, preferably 50 nm or less, and more preferably 30 nm or less.
- the BET specific surface areas of the activated carbon, the aluminosilicate compound, and the vinylpyridine copolymer described above are about 1750 m 2 / g, about 250 m 2 / g, about 350 m 2 / g, and the average pore diameter is about 4.5 nm, respectively. About 20 nm or about 10 nm can be used.
- the larger the value of the BET specific surface area the higher the odor component adsorption performance.
- the BET specific surface area is measured by a multipoint method using liquid nitrogen using a specific surface area / pore distribution measuring device “ASAP2020” manufactured by Shimadzu Corporation, and the parameter C is positive. A value can be derived. Moreover, the mercury intrusion method can be used for the measurement of the pore distribution.
- Content basis weight of the porous particles from the viewpoint of obtaining a deodorizing effect by the adsorption or inclusion, 0.05 g / m 2 or more preferably, 0.1 g / m 2 or more preferably, 0.5 g / m 2 or more Further preferred.
- the upper limit is preferably 5 g / m 2 or less, more preferably 3 g / m 2 or less, and even more preferably 1.0 g / m 2 or less because the paper texture is impaired as the content increases.
- the above-mentioned porous particles refer to fine particles having a large number of pores, and examples of the material include inorganic, organic, and polymers, and are not particularly limited. Moreover, the pore diameter should just have a pore diameter of 0.1 nm or more.
- the shape of the fine particles may be any of a spherical shape, a rod shape having an aspect ratio, a needle shape, and an indefinite shape such as a pulverized shape.
- said pore diameter can be measured using the Shimadzu Corporation specific surface area and pore distribution measuring apparatus "ASAP2020". The shape of the fine particles is observed with a scanning electron microscope “JCM-5100” manufactured by JEOL Ltd.
- activated carbon can have a deodorizing function by chemisorption to sulfur-based gas by activation of zinc chloride (ZnCl).
- the aluminosilicate compound can have an antibacterial action by supporting zinc, and the vinylpyridine copolymer by supporting silver.
- the cellulose fiber sheet substrate is made of cellulose fibers as described below, and may be a single layer or a plurality of layers of two or more layers. Alternatively, a plurality of sheets of two or more plies may be overlapped, and any one of the plurality of sheets may include cellulose fibers.
- the excretion fluid is transferred to the core, preventing absorption, or the absorbent article becomes hard and impairs the user's wearing feeling. In some cases, a single layer is preferable.
- the cellulose fiber sheet base material one having an appropriate basis weight is used according to a specific application position in the absorbent article.
- the basis weight of the cellulose fiber sheet substrate is preferably 8 g / m 2 or more, and more preferably 13 g / m 2 or more.
- 40 g / m ⁇ 2 > or less is preferable and 20 g / m ⁇ 2 > or less is more preferable.
- cellulose fiber examples include natural cellulose fibers such as wood pulp, cotton pulp, and straw pulp such as conifer kraft pulp and hardwood kraft pulp. Alternatively, regenerated cellulose fibers such as rayon and cupra can be used. Furthermore, semi-synthetic cellulose fibers such as acetate can also be used.
- Bulky cellulose fibers can also be used as the cellulose fibers.
- the term “bulky fiber” means that the fiber shape has a three-dimensional structure such as a twisted structure, a crimped structure, a bent and / or branched structure, or the fiber cross section is extremely thick (for example, the fiber roughness is 0. 0). 3 mg / m or more).
- Examples of preferred bulky cellulose fibers include fiber roughness of 0.3 mg / m or more, particularly 0.32 mg / m or more, 2 mg / m or less, especially 1 mg / m or less, especially 0.32 mg / m or more. Examples include cellulose fibers of 1 mg / m or less.
- Fiber roughness is used as a measure of fiber thickness in fibers with non-uniform fiber thickness, such as wood pulp.
- a fiber roughness meter (FS-200 (FS-200) Product name), AJANI EL ECTRONICS LTD.
- cellulose fibers with a fiber roughness of 0.3 mg / m or more include softwood kraft pulp ["ALBACEL” (trade name) made by Federal Paper Board Co. and "INDORAYON” (trade name) made by PT Inti Indorayon Utama )] And the like.
- preferred bulky cellulose fibers include cellulose fibers having a roundness of the fiber cross section of preferably 0.5 or more, more preferably 0.55 or more, and preferably 1 or less. . Cellulose fibers having a roundness of the fiber cross section of 0.5 or more and 1 or less are preferable because the liquid movement resistance is small and the liquid permeation rate is high. The roundness of the fiber cross section can be measured by, for example, the method described in JP-A-8-246395.
- the cross section of wood pulp is flattened by delignification treatment, and most of its roundness is less than 0.5.
- such wood pulp may be mercerized to swell the cross section of the wood pulp.
- mercerized pulp having a roundness of 0.5 or more and 1 or less obtained by mercerizing wood pulp is also preferable.
- commercially available mercerized pulp that can be used in the present invention include “FILTRANIER” (trade name) manufactured by ITT Rayonier Inc. and “POROSANIER” (trade name) manufactured by the same company.
- cellulose fibers having a fiber roughness of 0.3 mm / m or more and a roundness of the fiber cross section of 0.5 or more and 1 or less are used, a bulky network structure is more easily formed, and the liquid This is preferable because the passing speed of the material is further increased.
- the bulky cellulose fiber there is a crosslinked cellulose fiber obtained by crosslinking cellulose molecules within and / or between molecules.
- a crosslinked cellulose fiber has a feature that it can maintain a bulky structure even in a wet state.
- cross-linking agents examples include: N-methylol compounds such as dimethylolethyleneurea and dimethyloldihydroxyethyleneurea; polycarboxylic acids such as citric acid, tricarballylic acid and butanetetracarboxylic acid; polyols such as dimethylhydroxyethyleneurea
- N-methylol compounds such as dimethylolethyleneurea and dimethyloldihydroxyethyleneurea
- polycarboxylic acids such as citric acid, tricarballylic acid and butanetetracarboxylic acid
- polyols such as dimethylhydroxyethyleneurea
- a cross-linking agent for polyglycidyl ether compounds is preferred.
- a polycarboxylic acid or polyglycidyl ether-based crosslinking agent that does not generate formalin or the like harmful to the human body during crosslinking is preferred.
- cellulose fibers can be used alone or in combination of two or more.
- Cellulose fibers having an appropriate fiber length are used depending on the method for producing the cellulose fiber sheet substrate and the specific use of the antibacterial sheet. For example, when manufacturing a cellulose fiber sheet base material by a wet papermaking method, it is preferable that the fiber length of a cellulose fiber is 1 mm or more and 20 mm or less.
- the cellulose fiber sheet base material may be composed only of the cellulose fibers described above, or may contain other fibers in addition to the cellulose fibers.
- other fibers include heat-fusible fibers.
- heat-fusible fibers fibers that melt by heating and adhere to each other can be used.
- heat-fusible fibers include polyolefin fibers such as polyethylene, polypropylene and polyvinyl alcohol, polyester fibers, polyethylene-polypropylene composite fibers, polyethylene-polyester composite fibers, low melting point polyester-polyester composite fibers, and fiber surfaces. Examples thereof include hydrophilic polyvinyl alcohol-polypropylene composite fibers and polyvinyl alcohol-polyester composite fibers.
- any of a core-sheath type composite fiber and a side-by-side type composite fiber can be used. These heat-fusible fibers can be used alone or in combination of two or more.
- the heat-fusible fiber has a fineness of 0.1 dtex or more, particularly 0.5 dtex or more, and preferably 3 dtex or less. Moreover, when manufacturing a fiber sheet, for example with a wet papermaking method, it is preferable that the heat-fusible fiber generally has a fiber length of 2 mm or more and 60 mm or less. Furthermore, it is preferable that the heat-fusible fiber is contained in the fiber sheet in an amount of 1% by mass to 30% by mass.
- the cellulose fiber sheet base material may contain other components in addition to the above-described fiber components.
- other components include paper strength reinforcing agents.
- the paper strength reinforcing agent for example, polyamine / epichlorohydrin resin, dialdehyde starch, sponge, carboxymethylcellulose and the like can be used. These paper strength reinforcing agents can be used singly or in combination of two or more.
- the paper strength reinforcing agent is preferably contained in the cellulose fiber sheet substrate in an amount of 0.01% by mass to 30% by mass, particularly 0.01% by mass to 20% by mass.
- the thin paper of the present invention may contain other agents as long as the effects described above are not impaired.
- polyhydric alcohols surfactants, other deodorants, various commonly used solvents, oils, gelling agents, salts such as sodium sulfate and N, N, N-trimethylglycine, pH adjusters, oxidation It may contain an inhibitor, an antiseptic, a bactericidal / antibacterial agent, a fragrance, a pigment, an ultraviolet absorber, a chelating agent, and the like.
- the thin paper of the present invention may contain a ⁇ -glucuronidase inhibitor to further enhance the deodorizing effect.
- ⁇ -glucuronidase inhibitors can suppress the increase of phenolic compounds and indoles, which are causative substances of urine odor, and can continuously suppress the generation of unpleasant urine odor. Further, for example, when the absorbent article incorporating the thin paper of the present invention is discarded, it is possible to suppress the generation of an unpleasant urine odor in the article after disposal due to the action of the ⁇ -glucuronidase inhibitor. Become.
- ⁇ -glucuronidase is an enzyme that hydrolyzes a compound (glucuronide) in which various alcohols, phenols, amines and the like are conjugated with glucuronic acid, and is present in many organisms such as bacteria, fungi, plants, and animals.
- ⁇ -glucuronidase derived from bacteria and fungi is important because microorganisms are largely involved in the degradation of urine excreted outside the body.
- Escherichia oli Lactobacillus revis, Propionibacterium cnes, Clostridium erfringens, Staphylococcus aemolyticus, Streptococcus galactiae, Streptococcus yogenes, Haemophilus omunus, Shigela neni, Aspergillus gluder, etc.
- ⁇ -glucuronidases derived from these microorganisms are classified into enzyme groups having a common domain. Furthermore, ⁇ -glucuronidase derived from human plasma is also classified into a similar protein group.
- a ⁇ -glucuronidase inhibitor is a ⁇ -glucuronidase inhibitor that exhibits a ⁇ -glucuronidase inhibitory activity that is suppressed by 60% or more by adding 0.1 wt% in the reaction solution in the following method for measuring the ⁇ -glucuronidase activity inhibition rate. Say. Furthermore, it is preferable that the activity is suppressed by 80% or more by adding 0.01% by weight in the reaction solution.
- ⁇ Method for measuring inhibition rate of ⁇ -glucuronidase activity 100 ⁇ L of 2 mM p-nitrophenyl- ⁇ -D-glucuronide (PNPG) aqueous solution, 40 ⁇ L of 0.5 M phosphate buffer (pH 6.8), 38 ⁇ L of ion-exchanged water, various compounds or plant extracts in a ⁇ -ray sterilized container 2 ⁇ L of a 10% or 1 wt% DPG (dipropylene glycol) solution is added, followed by addition of 20 ⁇ L of an aqueous ⁇ -glucuronidase solution adjusted to 16 units / mL, and an enzyme reaction is performed in a 37 ° C. constant temperature bath for 2 hours.
- PNPG p-nitrophenyl- ⁇ -D-glucuronide
- the same experiment is also performed for a 0.1 wt% DPG solution (the concentrations of the provided compound and plant extract in the reaction solution are 0.1 wt% and 0.01 wt%, respectively). % And 0.001% by weight).
- a reaction in which DPG is added in place of the compound and the plant extract is used as a control, and a sample in which ion-exchanged water is added in place of the enzyme solution for each sample and control is used as a blank, and the reaction is similarly performed for 2 hours.
- the reaction solution is diluted with 0.2 M glycine buffer (pH 10.4), and the absorbance at a wavelength of 400 nm is measured.
- ⁇ -glucuronidase activity inhibition rate (%) [(control absorbance change ⁇ sample absorbance change) / (control absorbance change) ⁇ 100
- ⁇ -glucuronidase inhibitor those having the aforementioned ⁇ -glucuronidase inhibitory activity can be used.
- plant extracts such as goby tannin, tendered yak, akebi, etc .; glucarolactone, globanone, mussenone delta, umbretolide, sibetone, oxalide, methyloctyne carbonate, estragole, musk TM-II, musk Z-4, 2-Phenylcyclopentenone, Vetiverol, Methylionene-G, Cyclamenaldehyde, Nukaton, D-Glucaro-1,4-lactone, Cis-Jasmon, Turpinyl acetate, Orbitone, Linalyl acetate, Phenylethyl isoamyl ether, Methyl ionene-A Perfumes such as Muscon, and cationic surfactants can be used.
- sanizol and coatamine both trade names
- examples of sanizole include sanizole B-50 (alkyl benzyl dimethyl ammonium chloride), sanizole C (alkyl benzyl dimethyl ammonium chloride), sanizole P (benzalkonium cetyl phosphate), and the like.
- Coatamine includes coatamine 24P (lauryltrimethylammonium chloride), coatamine 60W (cetyltrimethylammonium chloride), coatamine 86P (conc stearyltrimethylammonium chloride), coatamine 86W (stearyltrimethylammonium chloride), coatamine D2345P (dialkyldimethylammonium chloride). ), Cotamine D86P (distearyldimethylammonium chloride), Cotamine E-80K (octadecyloxypropyltrimethylammonium chloride) and the like.
- ⁇ -glucuronidase inhibitor those isolated from animals and plants may be used, or those chemically synthesized may be used.
- plant extracts such as essential oils containing these compounds, for example, vetiver oil, basil oil, clove oil, cinnamon oil, grapefruit oil and the like may be used as they are as ⁇ -glucuronidase inhibitors. Two or more of these compounds or plant extracts may be used in combination.
- the ⁇ -glucuronidase inhibitor is preferably one or more selected from the group consisting of the aforementioned plant extract, fragrance and cationic surfactant, and particularly selected from the group consisting of the plant extract and cationic surfactant.
- One or more of these are preferred.
- gobicitannin is preferred as the plant extract
- glucarolactone and globanone are preferred as the fragrance
- sanizole is preferably used as the cationic surfactant. .
- the concentration of the ⁇ -glucuronidase inhibitor is 0.01% by mass or more and 2.0% by mass or less, particularly 0.05% by mass or more and 1.0% by mass or less, particularly 0.1% by mass or more and 0.5% by mass or less.
- the following is preferable in that the deodorizing effect, the storage stability of the chemical solution, the wiping property, the skin irritation, and the odor transfer property to other products are small.
- the manufacturing method of the present embodiment includes the following steps.
- the step (i) can be performed by a normal method using a normal wet papermaking apparatus.
- an aqueous coating solution can be obtained by mixing a cationic antibacterial agent and a pH buffering deodorant with water.
- the above pH 5.0 or more and 9.0 or less preparation can adjust the addition amount of a pH buffer deodorant with respect to a cationic antibacterial agent.
- the cationic antibacterial agent is an alkyl phosphate benzalkonium salt represented by the above formulas (1) and (2)
- the pH is preferably 6.5 or more, and more preferably 7.0 or more.
- the pH of the aqueous coating solution By setting the pH of the aqueous coating solution to be equal to or higher than this value, it is effectively prevented that a coarse product composed of an undissolved cationic antibacterial agent is generated in the aqueous coating solution.
- the reason for this is considered to be that dissociation between the cation moiety and the anion moiety in Formula (1) is promoted by increasing the pH of the aqueous coating solution.
- the higher the pH of the aqueous coating solution the more preferable it is because the formation of a coarse product composed of the antibacterial agent (benzalkonium salt of alkyl phosphate) is suppressed.
- the pH is excessively high, dissolution of the antibacterial agent proceeds excessively, and the viscosity of the aqueous coating solution tends to increase rapidly.
- the upper limit of the pH of the aqueous coating solution is preferably 9.0 or less, and more preferably 8.5 or less.
- the pH of the aqueous coating solution in the present invention is preferably 5.0 or more and 9.0 or less, more preferably 6.5 or more and 9.0 or less, and 7.0 or more and 8.5 or less. More preferably.
- the pH of the aqueous coating solution depends on the temperature, and the pH of the aqueous coating solution referred to in the present invention is the pH at the temperature of the aqueous coating solution when the aqueous coating solution is applied to the cellulose fiber sheet substrate. It is.
- the pH of the aqueous coating solution can be adjusted by the addition amount of a buffering agent or a separate pH adjusting agent.
- An example of pH adjustment using a pH buffering deodorant is as follows. Citric acid is used as agent A, tris (hydroxymethyl) aminomethane is used as agent B, and added to benzalkonium alkyl phosphate as a cationic antibacterial agent. In this case, by setting the A / B ratio to 20/80 to 40/60, the pH becomes 5.0 to 8.3. At this time, the coating material contains few coarse cationic antibacterial agents, has a low viscosity, and can be applied easily.
- the particles of benzalkonium alkyl phosphate in a semi-dissolved state in water are reduced in size so that they can be uniformly applied onto the sheet substrate.
- the antibacterial properties of the thin paper are improved.
- the pH buffering ability near neutrality becomes high (it is difficult to change pH even if it is diluted or an acid alkali is added).
- a / B ratio in the range of 25/75 to 35/65
- the concentration of the cationic antibacterial agent in the aqueous coating solution is preferably 0.3% by mass or more, and more preferably 0.5% by mass or more, from the viewpoint of imparting sufficient antibacterial properties to the thin paper. Further, from the viewpoint of preventing the viscosity of the aqueous coating liquid from excessively increasing, the concentration of the antibacterial agent is preferably 8.0% by mass or less, and more preferably 5.0% by mass or less. Specifically, the concentration of the antibacterial agent is preferably 0.3% by mass or more and 8.0% by mass or less, and more preferably 0.5% by mass or more and 5.0% by mass or less.
- the concentration of the pH buffering deodorant in the aqueous coating solution is preferably set to be from the viewpoint of imparting neutralization deodorizing properties to a wide range of odor components from acidic to alkaline while adjusting the pH described above. It is preferably 3% by mass or more, and more preferably 0.5% by mass or more. Moreover, as an upper limit which exhibits an effect in deodorizing excrement smells, such as urine of an absorbent article, it is preferable that the density
- the concentration of the chelating agent contained in the aqueous coating solution is preferably 0.1% by mass or more, particularly preferably 0.5% by mass or more. Moreover, it is preferable to set it as 2.0 mass% or less, especially 1.5 mass% or less.
- the concentration of the chelating agent contained in the aqueous coating solution is preferably 0.1% by mass or more and 2.0% by mass or less, and particularly preferably 0.5% by mass or more and 1.5% by mass or less.
- chelating agents examples include aminotri (methylenephosphonic acid), 1-hydroxyethylidene-1-diphosphonic acid, ethylenediaminetetra (methylenephosphonic acid), diethylenetriaminepenta (methylenephosphonic acid), and salts thereof, 2-phosphonocarboxylic acid
- An organic acid or salt thereof such as aminopolyacetic acid or salt thereof such as acid or salt thereof, aspartic acid or salt thereof, amino acid or salt thereof such as glutamic acid or salt thereof, nitrilotriacetic acid or salt thereof, ethylenediaminetetraacetic acid or salt thereof;
- citric acid or a salt thereof, pyro- and triphosphate examples of these chelating agents can be used alone or in combination of two or more.
- the aqueous coating solution contains metal ions capable of forming metal soap, for example, ions of alkaline earth metal elements such as calcium ions and magnesium ions. Even if it exists, the production
- the concentration of alkaline earth metal element ions varies depending on the source of water such as river water and groundwater and the sampling location, but in the present invention, even if the water used for the aqueous coating solution has a high hardness exceeding 100 mg CaCO 3 / L. If the concentration of the chelating agent contained in the aqueous coating solution is within the above range, the formation of scum can be effectively suppressed.
- the viscosity of the aqueous coating solution is preferably 80 mPa ⁇ s, particularly 65 mPa ⁇ s or less, particularly 20 mPa ⁇ s or less. By setting the viscosity below this value, the aqueous coating solution can be successfully sprayed onto the fiber sheet. Since the viscosity of the aqueous coating solution depends on the temperature, the viscosity of the aqueous coating solution referred to in the present invention is the viscosity at the temperature of the aqueous coating solution when the aqueous coating solution is applied to the fiber sheet. The viscosity of the aqueous coating solution can be adjusted by the concentration of the antibacterial agent and the pH of the aqueous coating solution. The viscosity of the aqueous coating solution is measured using a B-type viscometer TVB-10M (manufactured by Toki Sangyo Co., Ltd.).
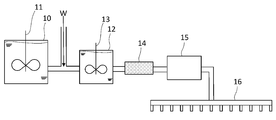
- the step (ii) can be performed by, for example, the apparatus shown in FIG. First, water, a cationic antibacterial agent, and a pH buffering deodorant, which are components of the aqueous coating solution, are charged into the stock solution tank 10, and a chelating agent is further charged as necessary. By thoroughly mixing these components using the stirring blade 11, a stock solution of an aqueous coating solution is prepared. The stock solution stored in the stock solution tank 10 is diluted with the dilution water W as necessary, and is sufficiently mixed in the dilution tank 12 using the stirring blade 13. In addition, the aqueous coating liquid obtained in this manner passes through the strainer 14 as necessary to remove coarse substances, and is sent to the spray nozzle 16 by the pump 15.
- the aqueous coating solution obtained in the step (ii) is applied to the wet paper obtained in the step (i).
- This application can be performed by, for example, a spray method via the spray nozzle 16 shown in FIG.
- the application method is not limited to this spray method, and can be performed by various commonly used methods. Examples thereof include a brush coating method, a bar coater, a gravure coater, various roll coaters, and a dipping method. Of these, the spray method is preferred from the viewpoint of uniform application to wet paper.
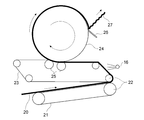
- Such step (iii) can be carried out, for example, by the apparatus shown in FIG. As shown in FIG.
- the wet paper 20 is transported to the first transport belt 21 in a wet state and is depressurized and dehydrated by a pair of press rolls 22 and 22.
- the aqueous coating liquid is applied to one surface of the wet paper 20 by spraying with the spray nozzle 16.
- touch rolls 25, 25 are arranged at the introduction portion of the Yankee dryer 24 so as to face the peripheral surface of the Yankee dryer 24.
- the wet paper 20 is guided to the touch rolls 25 and 25 together with the second transport belt 23 and introduced into the Yankee dryer 24.
- the aqueous coating solution sprayed from the spray nozzle 16 is applied to one surface of the wet paper 20.
- the aqueous coating liquid is applied only to one surface of the wet paper 20 (this surface is referred to as a first surface and the opposite side is referred to as a second surface).
- this surface is referred to as a first surface and the opposite side is referred to as a second surface.
- the method is not limited to the case of applying only to one surface, and a method of applying to both surfaces may be used.
- the wet paper 20 to which the water-based coating solution is applied is held on the peripheral surface of the Yankee dryer 24 while being kept in a wet state. And it heats during the conveyance, a water
- a position at which the wet paper 20 is introduced with an agent for improving the adhesiveness such as an adhesive on the surface of the Yankee dryer 24. It can also be given before this.
- the coated one side (first side) side is the opposite side (second side).
- the cationic antibacterial agent and the pH buffering deodorant are more present. This is because the antibacterial agent is not completely dissolved in the aqueous coating solution, and undissolved aggregates are present in the aqueous coating solution. This is because, when sprayed, the undissolved aggregates do not penetrate into the fiber sheet and remain on the surface of the spray surface, that is, the surface of the first surface.
- the thin paper when the thin paper is incorporated into the absorbent article, by making the first side the wearer's skin side, not only the antibacterial and deodorizing action works effectively, but also ammonia generated by the decay of excretory fluids
- the increase in pH of the skin of the wearer by amines can be suppressed, which is preferable. Since the porous particles are contained in the step (i) as described above, the porous particles are dispersed in the entire sheet without being unevenly distributed on either the first surface or the second surface.
- the thin paper of the present invention contributes to the improvement of the deodorizing function of the absorbent article by utilizing a comprehensive deodorizing mechanism of the time axis and the pH axis.
- the thin paper of the present invention is applied to various absorbent articles that absorb and retain excretory fluid. For example, a urine collection pad, a disposable diaper, a sanitary napkin, a panty liner, and the like can be given.
- the thin paper of the present invention can be used as various components in absorbent articles.
- the thin paper of the present invention can be applied as a core wrap sheet that covers a liquid-retaining absorbent core.
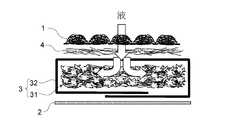
- FIG. 3 shows the basic structure of the absorbent article.
- the absorber 3 includes an absorbent core 31 that forms a liquid-retaining main body and a covering sheet 32 that covers the entire surface of the absorbent core 31.
- the thin paper of the present invention is applied to the covering sheet 32.
- the absorptive core 31 is comprised including liquid absorbing fibers, such as a fluff pulp, or a liquid absorbing fiber and a super absorbent polymer.
- the covering sheet 32 is the skin facing surface side. And 2 sheets on the clothing facing surface side.
- the thin paper of the present invention is applied to the covering sheet 32, the cationic antibacterial agent and the pH buffering deodorant can be surely eluted from the initial stage in the excretory fluid permeation path. This makes it possible to develop a sustained deodorizing action.
- the contact to the skin of a cationic antibacterial agent, a pH buffering deodorant, and a porous particle is suppressed, and it is preferable.
- the first surface of the thin paper that is, the surface on the side where a large amount of the cationic antibacterial agent and the pH buffering deodorant are present so as to face the wearer's skin, has a deodorizing effect. It is preferable from the viewpoint of effective expression.
- the thin paper of the present invention may have the cationic antibacterial agent and the pH buffering deodorant equally present on both the first side and the second side. In this case, even after the excretory fluid has permeated into the absorbent core, the cationic antibacterial agent and the pH buffering deodorant can be eluted into the liquid phase, and the deodorizing action can be maintained at a high level. It is preferable from the viewpoint.
- the thin paper of the present invention is not limited to the form shown in FIG. 3, and may be applied to other constituent members. For example, it may be applied to the sublayer sheet 4 instead of or together with the covering sheet 32.
- the present invention further discloses the following thin paper and a method for producing the same.
- a thin paper in which 0.05 g / m 2 or more of a cationic antibacterial agent, porous particles, and a pH buffering deodorant are contained in a cellulose fiber sheet substrate.
- the cationic antibacterial agent is preferably 0.1 g / m 2 or more, more preferably 0.2 g / m 2 or more, and the upper limit is preferably 1.0 g / m 2 or less, and 0.5 g / m 2 or less.
- ⁇ 4> The quaternary ammonium salt according to ⁇ 3>, wherein the quaternary ammonium salt contains at least one selected from the group consisting of alkylpyridinium salts, benzethonium salts, benzalkonium salts, monoalkyltrimethylammonium salts, and dialkyldimethylammonium salts.
- Tissue paper. ⁇ 5> The thin paper according to any one of ⁇ 1> to ⁇ 4>, wherein the cationic antibacterial agent contains a benzalkonium salt represented by the following formula (1).
- R 1 and R 2 represent identical or different methyl, ethyl, or an alkyl or alkenyl group having 8 or more carbon atoms and 20 or less linear or branched .
- the porous particles preferably have a BET specific surface area of 50 m 2 / g or more, more preferably 100 m 2 / g or more, still more preferably 200 m 2 / g or more, and the upper limit is 2000 m 2.
- the porous particles preferably have an average pore diameter of 2 nm or more, preferably 50 nm or less, and more preferably 30 nm or less. Any one of the above items ⁇ 1> to ⁇ 8> Tissue paper as described. ⁇ 10> The thin paper according to any one of ⁇ 1> to ⁇ 9>, wherein the porous particles have an average pore diameter of 2 nm to 50 nm.
- the content basis weight of the porous particles 0.05 g / m 2 or more preferably, 0.1 g / m 2 or more, and further preferably 0.5 g / m 2 or more, the upper limit is, 5 g / m 2
- the thin paper according to any one of ⁇ 1> to ⁇ 10>, preferably at most 3 g / m 2 or less, more preferably 1.0 g / m 2 or less.
- the porous particles are acrylic polymer such as porous methacrylic acid polymer and porous acrylic acid polymer, porous divinylbenzene polymer, aromatic polymer of porous pyridine copolymer, and synthetic porous polymer of these copolymers.
- Natural porous polymer of chitin and chitosan activated carbon, silica, silicon dioxide (silica gel), calcium silicate, aluminosilicate compound, high silica zeolite (hydrophobic zeolite), sepiolite, cancrinite, zeolite, and hydrated oxidation Zirconium inorganic porous material; metal-supported porous of silver-supported zeolite, silver-supported cancrinite, and silver-supported porous styrene-divinylbenzene-vinylpyridine polymer and silver-supported porous divinylbenzene-vinylpyridine polymer vinylpyridine copolymer Quality, or Containing at least one selected from the group consisting, the ⁇ 1> to tissue paper according to any one of ⁇ 11>.
- ⁇ 13> Content basis weight of said pH buffering deodorants, 0.05 g / m 2 or more preferably, 0.1 g / m 2 or more preferably, 0.15 g / m 2 or more, and its upper limit is 2 preferably .0g / m 2 or less, more preferably 1.0 g / m 2 or less, 0.5 g / m 2 or less is more preferred, wherein ⁇ 1> to tissue paper according to any one of ⁇ 12>. ⁇ 14> ⁇ 1> to ⁇ 13>, wherein the pH buffering deodorant contains at least one agent A selected from the group consisting of an organic acid having an acid dissociation index pKa of 5.0 or more and a salt thereof.
- the pH buffering deodorant contains at least one agent A selected from the group consisting of an organic acid having an acid dissociation index pKa of 5.0 or more and a salt thereof.
- the basis weight of the agent A is preferably 0.01 g / m 2 or more, more preferably 0.03 g / m 2 or more, still more preferably 0.1 g / m 2 or more,
- the upper limit is preferably 3 g / m 2 or less, more preferably 1 g / m 2 or less, and even more preferably 0.5 g / m 2 or less, the thin paper according to ⁇ 14>.
- ⁇ 16> The thin paper according to ⁇ 14> or ⁇ 15>, wherein the agent A contains at least one selected from the group consisting of phosphoric acid, citric acid, succinic acid, maleic acid, and malonic acid.
- the pH buffering deodorant contains at least one B agent selected from the group consisting of a polyhydroxyamine compound and a salt thereof.
- ⁇ 18> Content basis weight of the B agent 0.01 g / m 2 or more preferably, 0.05 g / m 2 or more, and the upper limit thereof is preferably 3 g / m 2 or less, more preferably 1 g / m 2 or less , more preferably 0.01 g / m 2 or more 3 g / m 2 or less, particularly preferably 0.05 g / m 2 or more 1 g / m 2 or less, thin paper according to ⁇ 17>.
- the agent B is 2-amino-1,3-propanediol, 2-amino-2-methyl-1,3-propanediol, 2-amino-2-ethyl-1,3-propanediol, 2-amino-
- the basis weight of the cellulosic fibrous sheet substrate is preferably 8 g / m 2 or more, more preferably 13 g / m 2 or more, preferably 45 g / m 2 or less, 20 g / m 2 or less and more preferably, the ⁇ 1> -
- the cationic antibacterial agent is an alkyl phosphate benzalkonium salt represented by the following formulas (1) and (2)
- the pH is preferably 6.5 or more, more preferably 7.0 or more.
- the upper limit is preferably 9.0 or less, more preferably 8.5 or less, and the method for producing thin paper according to ⁇ 24>.
- the concentration of the cationic antibacterial agent in the aqueous coating solution is preferably 0.3% by mass or more, more preferably 0.5% by mass or more, and preferably 8.0% by mass or less.
- the concentration of the antibacterial agent is preferably 0.3% by mass or more and 8.0% by mass or less, and more preferably 0.5% by mass or more.
- the method for producing thin paper according to ⁇ 24> or ⁇ 25> more preferably 5.0% by mass or less.
- the concentration of the pH buffering deodorant in the aqueous coating solution is preferably 0.3% by mass or more, more preferably 0.5% by mass or more, and preferably 8% by mass or less.
- the method for producing thin paper according to any one of ⁇ 24> to ⁇ 26>. ⁇ 28> In the step of applying the aqueous coating liquid to the wet paper, The wet paper web is transported to the first transport belt in a wet state, is pinched by a pair of press rolls to be dehydrated, and then transported to the second transport belt and immediately before being introduced into the Yankee dryer.
- ⁇ 29> The method for producing thin paper according to any one of ⁇ 24> to ⁇ 28>, wherein the aqueous coating liquid is applied only to one surface of the wet paper.
- ⁇ 30> The method for producing thin paper according to ⁇ 28> or ⁇ 29>, wherein an agent for improving adhesiveness such as an adhesive is applied to the surface of the Yankee dryer before the position where the wet paper is introduced.
- Benzalkonium cetyl phosphate (“Sanisol P” (registered trademark), manufactured by Kao Corporation) as a cationic antibacterial agent, and tris (hydroxymethyl) aminomethane and citrate as a pH buffering deodorant Acid was used. These were mixed with groundwater to obtain an aqueous coating
- the pH of the aqueous coating solution was 7.6 and the viscosity was 4.5 mPa ⁇ s (25 ° C.).
- This wet paper was used as a cellulose fiber sheet substrate before processing.
- (3) Manufacture of thin paper Thin paper was manufactured using the apparatus shown in FIGS.
- the basis weight of the thin paper was 16 g / m 2 .
- the basis weight of benzalkonium cetyl phosphate is 0.1 g / m 2
- the basis weight of tris (hydroxymethyl) aminomethane is 0.056 g / m 2
- the basis weight of citric acid is 0.024 g / m 2 .
- m 2 (0.08 g / m 2 in total as a pH buffering deodorant).
- the containing basis weight of the porous particles was 0.5 g / m 2 as described above.
- the basis weight of the cationic antibacterial agent cetyl phosphate benzalkonium was determined using a liquid chromatograph / mass spectrometer (6140 LC / MS manufactured by Agilent Technologies, ionization method: ESI) as follows. Measured. First, in order to prepare a calibration curve, about 0.05 g of an active ingredient of benzalkonium cetyl phosphate was weighed and dissolved in 10 mmol / L acetic acid-containing methanol to make 100 mL (500 ⁇ g / mL). This solution was diluted to prepare standard solutions for calibration curves of 0.01, 0.05, 0.1, and 0.5 ⁇ g / mL as effective components.
- the basis weight of benzalkonium cetyl phosphate in the thin paper was measured using the above calibration curve. That is, 10 cm ⁇ 10 cm square thin paper was immersed in 30 mL of a 10 mmol / L acetic acid-containing methanol solution, irradiated with ultrasonic waves for 10 minutes, and the extract was collected in a 100 mL volumetric flask. This extraction operation was repeated three times and adjusted to 100 mL with the acetic acid-containing methanol. This solution was appropriately diluted 2 to 10 times and filtered through a 0.45 ⁇ m membrane filter to obtain a filtrate as a thin paper extract.
- a calibration curve is created using the total value of the peak areas of C10, C12, C14, and C16, and the concentration of the antibacterial agent in the extract ( ⁇ g / mL) was calculated and converted to the basis weight (g / m 2 ) of the antibacterial agent per 1 m 2 of the thin paper.
- the basis weight of tris (hydroxymethyl) aminomethane and citric acid as a pH buffering deodorant was measured using an ion chromatography method.
- the ion chromatograph analyzer was measured using Dionex ICS-2100 (Thermo Fisher Scientific Co., Ltd., conductivity detector).
- 0.1 g each of tris (hydroxymethyl) aminomethane and citric acid was precisely weighed, and pure water was added to make exactly 100 mL. This solution was diluted to a concentration of 0.1, 0.5, 1, 2.5, 5, 10, 25 ⁇ g / mL, a calibration curve solution was prepared, and a calibration curve was created from the LC analysis values.
- the extraction method of tris (hydroxymethyl) aminomethane and citric acid from thin paper was as follows.
- the basis weight of the porous particles was measured by the following method according to each porous particle.
- the basis weight was measured using an iodine adsorption method.
- Wet tissue was wet-decomposed, immersed in a known amount of iodine solution, iodine was adsorbed on activated carbon, and the amount of iodine remaining without adsorption was determined by titration with sodium thiosulfate to determine the amount of iodine adsorbed by activated carbon. . From the calibration curve of the amount of activated carbon and the amount of iodine adsorbed, the basis weight of activated carbon in the thin paper was converted and determined.
- the basis weight of the silver-supported polymer particles can be calculated from the amount of metal contained in the thin paper when the ratio of the metal of the particles to the polymer amount is known in advance. The amount was determined by conversion. The amount of metal contained in the thin paper was obtained by wet-decomposing the thin paper and measuring the amount of metal with an ICP emission analyzer. Further, when the porous particles were inorganic such as aluminosilicate, the thin paper was fired and calculated from the ash content.
- Example 2 As porous particles, instead of activated carbon 0.5 g / m 2 , Mizukanite HP (zinc oxide-supported aluminosilicate) with a BET specific surface area of 250 m 2 / g and an average pore diameter of 20 nm (trade name, manufactured by Mizusawa Chemical Co., Ltd.) A thin paper was obtained in the same manner as in Example 1 except that was used. The basis weight of the porous particles in the thin paper of Example 2 was 0.5 g / m 2 .
- Example 3 As porous particles, instead of activated carbon 0.5 g / m 2 , polymer D (silver-supported divinylbenzene / vinyl pyridine copolymer) having a BET specific surface area of 350 m 2 / g and an average pore diameter of 10 nm (product of Kao Corporation, product) A thin paper was obtained in the same manner as in Example 1 except that (name) was used. The content basis weight of the porous particles in the thin paper of Example 3 was 0.5 g / m 2 .
- Example 4 As porous particles, instead of activated carbon 0.5 g / m 2 , silver supported cancrinite (silver supported aluminosilicate) having a BET specific surface area of 30 m 2 / g and an average pore diameter of 22 nm (trade name: Lunamos SP-PC) ( A thin paper was obtained in the same manner as in Example 1 except that Clariant Catalyst Co., Ltd. was used. The content basis weight of the porous particles in the thin paper of Example 4 was 0.5 g / m 2 .
- the silver-supported cancrinite is a cancrinite-like mineral described in paragraphs [0029] to [0045] of JP-A-2005-336363.
- Example 5 A thin paper was obtained in the same manner as in Example 1 except that zinc oxide having a BET specific surface area of 5 m 2 / g and an average pore diameter of 60 nm was used as the porous particles instead of activated carbon 0.5 g / m 2 .
- the basis weight of the porous particles in the thin paper of Example 5 was 3 g / m 2 .
- Example 6 As the porous particles, instead of activated carbon 0.5 g / m 2 , high silica zeolite (Bion specific Showa Co., Ltd., trade name: ABSENT 3000) having a BET specific surface area of 430 m 2 / g and an average pore diameter of 1.0 nm was used. Except for the above, a thin paper was obtained in the same manner as in Example 1. The basis weight of the porous particles in the thin paper of Example 6 was 3 g / m 2 .
- Example 7 A thin paper was obtained in the same manner as in Example 2 except that tris (hydroxymethyl) aminomethane was not contained as a pH buffering deodorant.
- the basis weight of citric acid in the thin paper of Example 7 was 0.024 g / m 2 as in Example 2.
- Example 8 A thin paper was obtained in the same manner as in Example 2 except that citric acid was not contained as a pH buffering deodorant.
- the basis weight of tris (hydroxymethyl) aminomethane in the thin paper of Example 8 was 0.056 g / m 2 as in Example 2.
- Example 9 The content basis weight of Sanizol P used as cationic antimicrobial agent and 0.05 g / m 2, except that the content basis weight of Mizukanaito used as the porous particles was 5.0 g / m 2, similarly to Example 2 A thin paper was obtained.
- Example 10 A thin paper was obtained in the same manner as in Example 2 except that the content basis weight of Sanizol P used as the cationic antibacterial agent was 0.2 g / m 2 .
- Example 11 The content basis weight of Sanizol P used as cationic antimicrobial agent and 0.2 g / m 2, except that the content basis weight of Mizukanaito used as porous particles was 0.05 g / m 2, similarly to Example 2 A thin paper was obtained.
- Example 1 A thin paper was obtained in the same manner as in Example 1 except that no pH buffering deodorant was contained.
- Comparative Example 2 The same as Comparative Example 1 except that the content basis weight of Sanisol P used as a cationic antibacterial agent was 0.03 g / m 2 and the content basis weight of activated carbon used as porous particles was 0.8 g / m 2. A thin paper was obtained.
- the basis weight is the same as the blending ratio described in Example 1 of JP-A-2006-191966 in the above-mentioned patent document, and the basis weight of the thin paper of Example 1 in this specification is 16 g / m 2 .
- the value converted to is set.
- Example 3 A thin paper was obtained in the same manner as in Example 1 except that no porous particles were contained, and the basis weight of Sanisole P, which is a cationic antibacterial agent, was 0.5 g / m 2 .
- Example 5 A thin paper was obtained in the same manner as in Example 1 except that the cationic antibacterial agent was not contained, and the basis weight of the activated carbon that was porous particles was changed to 5.0 g / m 2 .
- each thin paper obtained in Examples 1 to 11 and Comparative Examples 1 to 8 was prepared in a size of 170 mm ⁇ 200 mm as a covering sheet for the absorber.
- Each thin paper covered the entire surface of the absorbent core having a composition ratio of pulp / absorbent polymer of 150 (g / m 2 ) / 150 (g / m 2 ) to prepare an absorbent sample for evaluation.
- Each of the prepared absorber samples had a size of 70 ⁇ 190 mm.
- urine used for the test was prepared by the following procedure.
- a non-woven fabric having a basis weight of 20 g / m 2 and a size of 10 cm ⁇ 15 cm (a general-purpose product not subjected to special treatment such as antibacterial and deodorizing) was prepared.
- C Three monitors collected urine, and 2.0 g of urine was attached to the non-woven fabric of (b), and a plastic bag (produced by Nihon Pack Co., Ltd. produced by Japan, 200 x 140 x 0.04 mm). ) And sealed, and left in a 36 ° C. atmosphere for 24 hours.
- each container after 1, 2, 3, 4, 5, 6 hours is taken out from the thermostatic dryer, and five monitors (at least three people who are qualified caregivers such as first-time care workers) are based on the following standards.
- Sensory evaluation was performed. As a specific method for sensory evaluation, first, the container was taken out of the constant temperature dryer, and the lid was opened, and then allowed to stand for 30 seconds. After that, he sniffed the nose by approaching the container. The average value of the evaluation values was taken as the result of the evaluation test for each absorber sample. The results were as shown in Tables 1 and 2 and FIGS. 5: Strong rot odor 4: Weak rot odor 3: Strong odor easily understood as urine 2: Weak odor recognized as urine 1: Slight odor difficult to distinguish from urine 0: No odor
- each of Examples 1 to 11 contains 0.05 g / m 2 or more of a cationic antibacterial agent, a pH buffering deodorant, and porous particles.
- the evaluation result was “2.0” or less, and a strong odor was suppressed.
- Comparative Examples 1 to 8 satisfy the structure of three agents of a cationic antibacterial agent of 0.05 g / m 2 or more, a pH buffering deodorant, and porous particles.
- the evaluation result was “2.5” or more. That is, it felt strong and did not reach the deodorizing effect of the examples.
- the time of “1: Slight odor that is difficult to distinguish from urine” or longer lasted for 3 hours or longer.
- the cationic antibacterial agent was more than the others, and the odor was kept extremely low around “0.5” throughout.
- the comparative example showed an evaluation value of “1.5” or more after 3 hours, except for Comparative Examples 1, 2, and 6, as can be seen from the sensory evaluation in the time series of FIG. A stronger odor than the example was felt from an early stage.
- Comparative Example 4 which does not include a cationic antibacterial agent and porous particles
- Comparative Example 5 which does not include a cationic antibacterial agent
- Comparative Example 8 where the content of the cationic antibacterial agent is less than “0.05 g / m 2 ”.
- the evaluation value exceeded “2: weak odor that can be recognized as urine”, and a stronger odor than the state after 6 hours of the Example had already occurred.
- Comparative Examples 1 and 2 which do not contain a pH buffering deodorant
- Comparative Example 6 in which the content of the cationic antibacterial agent is less than “0.05 g / m 2 ”, show a deodorizing effect to some extent until halfway.
- the odor began to increase rapidly after 3 hours from the start of the test, and the evaluation value “2” was exceeded before 6 hours after the end of the test.
- the thin paper of the present invention contains 0.05 g / m 2 or more of a cationic antibacterial agent, a pH buffering deodorant and porous particles, so that skin resident bacteria and intestines It was found that the odor can be suppressed to a very low level even when used for a long time in a harsh environment where the internal bacteria grow.
Landscapes
- Health & Medical Sciences (AREA)
- Heart & Thoracic Surgery (AREA)
- Life Sciences & Earth Sciences (AREA)
- Epidemiology (AREA)
- Engineering & Computer Science (AREA)
- Biomedical Technology (AREA)
- Veterinary Medicine (AREA)
- Vascular Medicine (AREA)
- Public Health (AREA)
- Animal Behavior & Ethology (AREA)
- General Health & Medical Sciences (AREA)
- Inorganic Chemistry (AREA)
- Chemical & Material Sciences (AREA)
- Absorbent Articles And Supports Therefor (AREA)
- Paper (AREA)
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN201580055364.2A CN107923128B (zh) | 2015-03-11 | 2015-12-16 | 薄片纸 |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2015-048865 | 2015-03-11 | ||
| JP2015048865A JP6130869B2 (ja) | 2015-03-11 | 2015-03-11 | 薄葉紙 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2016143217A1 true WO2016143217A1 (ja) | 2016-09-15 |
Family
ID=56879443
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/JP2015/085157 Ceased WO2016143217A1 (ja) | 2015-03-11 | 2015-12-16 | 薄葉紙 |
Country Status (4)
| Country | Link |
|---|---|
| JP (1) | JP6130869B2 (enExample) |
| CN (1) | CN107923128B (enExample) |
| TW (1) | TWI716374B (enExample) |
| WO (1) | WO2016143217A1 (enExample) |
Cited By (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| AU2019208150A1 (en) * | 2018-05-25 | 2019-12-12 | International Paper Company | Odor-control absorbent materials and absorbent articles and related methods of use and methods of making |
| TWI706069B (zh) * | 2017-11-30 | 2020-10-01 | 日商三吉油脂股份有限公司 | 紙類處理劑及紙類的處理方法 |
| CN112206748A (zh) * | 2019-07-10 | 2021-01-12 | 中国石化扬子石油化工有限公司 | 一种用于十五碳二元酸精制的高效复合吸附剂的制备方法 |
| EP3733966A4 (en) * | 2017-12-28 | 2021-02-24 | Corelex Shin-Ei Co., Ltd. | MANUFACTURING PROCESS FOR DEODORIZING PAPER |
| US11413368B2 (en) | 2013-12-30 | 2022-08-16 | International Paper Company | Binary odor control system for absorbent articles |
| US11471555B2 (en) | 2018-05-25 | 2022-10-18 | International Paper Company | Methods of reducing trimethylamine |
| EP3954826A4 (en) * | 2019-04-08 | 2022-12-14 | Kao Corporation | Antibacterial paper and method for manufacturing same |
Families Citing this family (9)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2018166937A (ja) * | 2017-03-30 | 2018-11-01 | 花王株式会社 | 吸収性物品用シート及び吸収性物品 |
| JP6328357B1 (ja) * | 2017-07-27 | 2018-05-23 | 花王株式会社 | 吸収性物品 |
| JP6649429B2 (ja) * | 2018-04-16 | 2020-02-19 | 花王株式会社 | 吸収性物品 |
| EP3801425A1 (en) * | 2018-05-25 | 2021-04-14 | International Paper Company | Odor-control absorbent materials and absorbent articles and related methods of use and methods of making |
| JP6797872B2 (ja) | 2018-09-11 | 2020-12-09 | 花王株式会社 | 消臭方法及び消臭物品 |
| JP7356930B2 (ja) * | 2019-04-08 | 2023-10-05 | 花王株式会社 | 抗菌紙及びその製造方法 |
| JP7612400B2 (ja) * | 2020-12-03 | 2025-01-14 | 花王株式会社 | 吸収性物品 |
| JP7579543B2 (ja) * | 2020-12-21 | 2024-11-08 | 大阪化成株式会社 | 抗ウィルス繊維構造体およびその製造方法、抗ウィルス加工製品 |
| CN120203937A (zh) * | 2025-03-19 | 2025-06-27 | 川田卫生用品(浙江)有限公司 | 一种纯棉祛味卫生巾及其制备方法 |
Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2006191966A (ja) * | 2005-01-11 | 2006-07-27 | Kao Corp | 吸収性物品 |
| JP2009148322A (ja) * | 2007-12-19 | 2009-07-09 | Kao Corp | 低拡散性透過紙及びそれを用いた吸収性物品 |
| JP2010194254A (ja) * | 2009-02-27 | 2010-09-09 | Kao Corp | 吸収性物品 |
| JP2013255836A (ja) * | 2013-09-02 | 2013-12-26 | Kao Corp | β−グルクロニダーゼ阻害剤 |
Family Cites Families (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP3526327B2 (ja) * | 1994-10-04 | 2004-05-10 | 花王株式会社 | リンス剤組成物 |
| JP4397100B2 (ja) * | 2000-04-28 | 2010-01-13 | 花王株式会社 | 吸収性物品 |
| KR100768735B1 (ko) * | 2000-09-28 | 2007-10-22 | 유니챰 가부시키가이샤 | 소변 냄새 저감 방법 및 소변 냄새 저감 기능을 갖는 물품 |
| US9555150B2 (en) * | 2006-11-17 | 2017-01-31 | Sca Hygiene Products Ab | Absorbent articles comprising an organic zinc salt and an anti-bacterial agent or alkali metal chloride or alkaline earth metal chloride |
-
2015
- 2015-03-11 JP JP2015048865A patent/JP6130869B2/ja active Active
- 2015-12-16 CN CN201580055364.2A patent/CN107923128B/zh active Active
- 2015-12-16 WO PCT/JP2015/085157 patent/WO2016143217A1/ja not_active Ceased
- 2015-12-21 TW TW104142998A patent/TWI716374B/zh not_active IP Right Cessation
Patent Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2006191966A (ja) * | 2005-01-11 | 2006-07-27 | Kao Corp | 吸収性物品 |
| JP2009148322A (ja) * | 2007-12-19 | 2009-07-09 | Kao Corp | 低拡散性透過紙及びそれを用いた吸収性物品 |
| JP2010194254A (ja) * | 2009-02-27 | 2010-09-09 | Kao Corp | 吸収性物品 |
| JP2013255836A (ja) * | 2013-09-02 | 2013-12-26 | Kao Corp | β−グルクロニダーゼ阻害剤 |
Cited By (9)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US11413368B2 (en) | 2013-12-30 | 2022-08-16 | International Paper Company | Binary odor control system for absorbent articles |
| TWI706069B (zh) * | 2017-11-30 | 2020-10-01 | 日商三吉油脂股份有限公司 | 紙類處理劑及紙類的處理方法 |
| EP3733966A4 (en) * | 2017-12-28 | 2021-02-24 | Corelex Shin-Ei Co., Ltd. | MANUFACTURING PROCESS FOR DEODORIZING PAPER |
| US11396727B2 (en) | 2017-12-28 | 2022-07-26 | Corelex Shin-Ei Co., Ltd. | Deodorant-paper manufacturing method |
| AU2019208150A1 (en) * | 2018-05-25 | 2019-12-12 | International Paper Company | Odor-control absorbent materials and absorbent articles and related methods of use and methods of making |
| US11471555B2 (en) | 2018-05-25 | 2022-10-18 | International Paper Company | Methods of reducing trimethylamine |
| EP3954826A4 (en) * | 2019-04-08 | 2022-12-14 | Kao Corporation | Antibacterial paper and method for manufacturing same |
| CN112206748A (zh) * | 2019-07-10 | 2021-01-12 | 中国石化扬子石油化工有限公司 | 一种用于十五碳二元酸精制的高效复合吸附剂的制备方法 |
| CN112206748B (zh) * | 2019-07-10 | 2023-05-26 | 中国石化扬子石油化工有限公司 | 一种用于十五碳二元酸精制的高效复合吸附剂的制备方法 |
Also Published As
| Publication number | Publication date |
|---|---|
| TWI716374B (zh) | 2021-01-21 |
| JP6130869B2 (ja) | 2017-05-17 |
| JP2016169446A (ja) | 2016-09-23 |
| TW201634022A (zh) | 2016-10-01 |
| CN107923128B (zh) | 2019-02-15 |
| CN107923128A (zh) | 2018-04-17 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP6130869B2 (ja) | 薄葉紙 | |
| JP6496576B2 (ja) | 薄葉紙 | |
| KR101417557B1 (ko) | 저 ph, 최적 orp 및 냄새 감소 섬유, 이 섬유의 제조방법 및 이로부터 제조된 물품 | |
| CN101360520B (zh) | 含有酸性超级吸收体以及有机锌盐的吸收性制品 | |
| JP6436795B2 (ja) | フマル酸含有繊維、フマル酸含有繊維集合体および吸収体 | |
| JP6511084B2 (ja) | 吸収性物品のための二成分系臭気制御システム | |
| EP3267959B1 (en) | Sanitary article comprising a ph control composition, and method for its production | |
| AU2017417540B2 (en) | Absorbent article with skin pH-adjusting effect | |
| JP6871363B2 (ja) | pH制御トップシートを有する吸収性用品 | |
| JP2018166937A (ja) | 吸収性物品用シート及び吸収性物品 | |
| BRPI0622144A2 (pt) | Artigos absorventes que compreendem um composto peróxi e um sal de zinco orgânico | |
| JP7544617B2 (ja) | 吸収性物品用シートの製造方法 | |
| Tubcil et al. | Nonwoven surface treatments for disposable hygiene products | |
| JP5026734B2 (ja) | 消臭剤 | |
| JP2024146196A (ja) | 吸収性物品用薄葉紙 | |
| JP2006150010A (ja) | 衛生生理用品 | |
| CN113661291A (zh) | 抗菌纸及其制造方法 | |
| MXPA98010294A (en) | Absorbent article disposable anti-rosadu |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 15884701 Country of ref document: EP Kind code of ref document: A1 |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| 122 | Ep: pct application non-entry in european phase |
Ref document number: 15884701 Country of ref document: EP Kind code of ref document: A1 |