WO2015163090A1 - アルコキシド化合物、薄膜形成用原料、薄膜の製造方法及びアルコール化合物 - Google Patents
アルコキシド化合物、薄膜形成用原料、薄膜の製造方法及びアルコール化合物 Download PDFInfo
- Publication number
- WO2015163090A1 WO2015163090A1 PCT/JP2015/059903 JP2015059903W WO2015163090A1 WO 2015163090 A1 WO2015163090 A1 WO 2015163090A1 JP 2015059903 W JP2015059903 W JP 2015059903W WO 2015163090 A1 WO2015163090 A1 WO 2015163090A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- group
- carbon atoms
- thin film
- hydrocarbon group
- represented
- Prior art date
Links
- -1 Alkoxide compound Chemical class 0.000 title claims abstract description 275
- 239000010409 thin film Substances 0.000 title claims description 107
- 239000002994 raw material Substances 0.000 title claims description 78
- 238000004519 manufacturing process Methods 0.000 title claims description 32
- 229910052739 hydrogen Inorganic materials 0.000 claims abstract description 42
- 229910052751 metal Inorganic materials 0.000 claims abstract description 37
- 239000001257 hydrogen Substances 0.000 claims abstract description 35
- 239000002184 metal Substances 0.000 claims abstract description 35
- XUIMIQQOPSSXEZ-UHFFFAOYSA-N Silicon Chemical group [Si] XUIMIQQOPSSXEZ-UHFFFAOYSA-N 0.000 claims abstract description 17
- 229910052710 silicon Inorganic materials 0.000 claims abstract description 16
- 125000004435 hydrogen atom Chemical class [H]* 0.000 claims abstract description 14
- 125000004429 atom Chemical group 0.000 claims abstract description 13
- 125000003277 amino group Chemical group 0.000 claims abstract description 9
- 229910052736 halogen Inorganic materials 0.000 claims abstract description 7
- 150000002367 halogens Chemical class 0.000 claims abstract description 7
- 125000002915 carbonyl group Chemical group [*:2]C([*:1])=O 0.000 claims abstract description 6
- IVRMZWNICZWHMI-UHFFFAOYSA-N azide group Chemical group [N-]=[N+]=[N-] IVRMZWNICZWHMI-UHFFFAOYSA-N 0.000 claims abstract description 5
- 125000002887 hydroxy group Chemical group [H]O* 0.000 claims abstract description 5
- 125000002560 nitrile group Chemical group 0.000 claims abstract description 5
- 125000001183 hydrocarbyl group Chemical group 0.000 claims abstract 15
- 125000004432 carbon atom Chemical group C* 0.000 claims description 102
- 125000002496 methyl group Chemical group [H]C([H])([H])* 0.000 claims description 36
- 239000010408 film Substances 0.000 claims description 30
- 229910017052 cobalt Inorganic materials 0.000 claims description 26
- 239000010941 cobalt Chemical group 0.000 claims description 26
- GUTLYIVDDKVIGB-UHFFFAOYSA-N cobalt atom Chemical group [Co] GUTLYIVDDKVIGB-UHFFFAOYSA-N 0.000 claims description 23
- PXHVJJICTQNCMI-UHFFFAOYSA-N Nickel Chemical group [Ni] PXHVJJICTQNCMI-UHFFFAOYSA-N 0.000 claims description 22
- 230000008016 vaporization Effects 0.000 claims description 20
- 239000000758 substrate Substances 0.000 claims description 17
- UFHFLCQGNIYNRP-UHFFFAOYSA-N Hydrogen Chemical compound [H][H] UFHFLCQGNIYNRP-UHFFFAOYSA-N 0.000 claims description 15
- RYGMFSIKBFXOCR-UHFFFAOYSA-N Copper Chemical group [Cu] RYGMFSIKBFXOCR-UHFFFAOYSA-N 0.000 claims description 11
- 229910052802 copper Inorganic materials 0.000 claims description 11
- 239000010949 copper Substances 0.000 claims description 11
- 229910052759 nickel Inorganic materials 0.000 claims description 11
- XEEYBQQBJWHFJM-UHFFFAOYSA-N Iron Chemical group [Fe] XEEYBQQBJWHFJM-UHFFFAOYSA-N 0.000 claims description 10
- 125000005843 halogen group Chemical group 0.000 claims description 7
- 229910052742 iron Inorganic materials 0.000 claims description 5
- PWHULOQIROXLJO-UHFFFAOYSA-N Manganese Chemical group [Mn] PWHULOQIROXLJO-UHFFFAOYSA-N 0.000 claims description 2
- 229910052748 manganese Chemical group 0.000 claims description 2
- 239000011572 manganese Chemical group 0.000 claims description 2
- 238000000034 method Methods 0.000 description 62
- 150000002430 hydrocarbons Chemical group 0.000 description 51
- 239000002243 precursor Substances 0.000 description 46
- 150000001875 compounds Chemical class 0.000 description 40
- 238000006243 chemical reaction Methods 0.000 description 35
- RTAQQCXQSZGOHL-UHFFFAOYSA-N Titanium Chemical compound [Ti] RTAQQCXQSZGOHL-UHFFFAOYSA-N 0.000 description 30
- 239000007789 gas Substances 0.000 description 30
- 229910052719 titanium Inorganic materials 0.000 description 30
- 239000010936 titanium Substances 0.000 description 30
- 125000000217 alkyl group Chemical group 0.000 description 25
- 238000005229 chemical vapour deposition Methods 0.000 description 21
- WYURNTSHIVDZCO-UHFFFAOYSA-N Tetrahydrofuran Chemical compound C1CCOC1 WYURNTSHIVDZCO-UHFFFAOYSA-N 0.000 description 20
- 125000000484 butyl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])C([H])([H])[H] 0.000 description 19
- 125000001449 isopropyl group Chemical group [H]C([H])([H])C([H])(*)C([H])([H])[H] 0.000 description 19
- 229910052757 nitrogen Inorganic materials 0.000 description 18
- 238000000231 atomic layer deposition Methods 0.000 description 15
- 238000000151 deposition Methods 0.000 description 15
- 150000002431 hydrogen Chemical class 0.000 description 15
- 239000000243 solution Substances 0.000 description 15
- 239000000203 mixture Substances 0.000 description 14
- 239000000126 substance Substances 0.000 description 14
- 230000008021 deposition Effects 0.000 description 13
- XKRFYHLGVUSROY-UHFFFAOYSA-N Argon Chemical compound [Ar] XKRFYHLGVUSROY-UHFFFAOYSA-N 0.000 description 12
- 125000000058 cyclopentadienyl group Chemical group C1(=CC=CC1)* 0.000 description 12
- 125000001495 ethyl group Chemical group [H]C([H])([H])C([H])([H])* 0.000 description 12
- 229910044991 metal oxide Inorganic materials 0.000 description 12
- 150000004706 metal oxides Chemical class 0.000 description 12
- ZSWFCLXCOIISFI-UHFFFAOYSA-N endo-cyclopentadiene Natural products C1C=CC=C1 ZSWFCLXCOIISFI-UHFFFAOYSA-N 0.000 description 11
- 238000009834 vaporization Methods 0.000 description 11
- 125000003342 alkenyl group Chemical group 0.000 description 10
- 239000003960 organic solvent Substances 0.000 description 10
- 230000008569 process Effects 0.000 description 10
- 239000002904 solvent Substances 0.000 description 10
- YLQBMQCUIZJEEH-UHFFFAOYSA-N tetrahydrofuran Natural products C=1C=COC=1 YLQBMQCUIZJEEH-UHFFFAOYSA-N 0.000 description 10
- YXFVVABEGXRONW-UHFFFAOYSA-N Toluene Chemical compound CC1=CC=CC=C1 YXFVVABEGXRONW-UHFFFAOYSA-N 0.000 description 9
- 230000015572 biosynthetic process Effects 0.000 description 9
- 125000000753 cycloalkyl group Chemical group 0.000 description 9
- 239000002245 particle Substances 0.000 description 9
- 125000003187 heptyl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])[H] 0.000 description 8
- 239000012535 impurity Substances 0.000 description 8
- 239000012434 nucleophilic reagent Substances 0.000 description 8
- 125000001147 pentyl group Chemical group C(CCCC)* 0.000 description 8
- LYCAIKOWRPUZTN-UHFFFAOYSA-N Ethylene glycol Chemical compound OCCO LYCAIKOWRPUZTN-UHFFFAOYSA-N 0.000 description 7
- FYYHWMGAXLPEAU-UHFFFAOYSA-N Magnesium Chemical compound [Mg] FYYHWMGAXLPEAU-UHFFFAOYSA-N 0.000 description 7
- 125000003118 aryl group Chemical group 0.000 description 7
- 238000005755 formation reaction Methods 0.000 description 7
- 125000000959 isobutyl group Chemical group [H]C([H])([H])C([H])(C([H])([H])[H])C([H])([H])* 0.000 description 7
- 239000007788 liquid Substances 0.000 description 7
- 229910052749 magnesium Inorganic materials 0.000 description 7
- 239000011777 magnesium Substances 0.000 description 7
- 230000001590 oxidative effect Effects 0.000 description 7
- 125000003229 2-methylhexyl group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])(C([H])([H])[H])C([H])([H])* 0.000 description 6
- UHOVQNZJYSORNB-UHFFFAOYSA-N Benzene Chemical compound C1=CC=CC=C1 UHOVQNZJYSORNB-UHFFFAOYSA-N 0.000 description 6
- QCWXUUIWCKQGHC-UHFFFAOYSA-N Zirconium Chemical compound [Zr] QCWXUUIWCKQGHC-UHFFFAOYSA-N 0.000 description 6
- YRKCREAYFQTBPV-UHFFFAOYSA-N acetylacetone Chemical compound CC(=O)CC(C)=O YRKCREAYFQTBPV-UHFFFAOYSA-N 0.000 description 6
- 238000004458 analytical method Methods 0.000 description 6
- 229910052786 argon Inorganic materials 0.000 description 6
- 125000000113 cyclohexyl group Chemical group [H]C1([H])C([H])([H])C([H])([H])C([H])(*)C([H])([H])C1([H])[H] 0.000 description 6
- 229910052735 hafnium Inorganic materials 0.000 description 6
- VBJZVLUMGGDVMO-UHFFFAOYSA-N hafnium atom Chemical compound [Hf] VBJZVLUMGGDVMO-UHFFFAOYSA-N 0.000 description 6
- 125000004051 hexyl group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])* 0.000 description 6
- 125000001972 isopentyl group Chemical group [H]C([H])([H])C([H])(C([H])([H])[H])C([H])([H])C([H])([H])* 0.000 description 6
- 125000002347 octyl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])[H] 0.000 description 6
- 230000003287 optical effect Effects 0.000 description 6
- 229910052760 oxygen Inorganic materials 0.000 description 6
- 125000001436 propyl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])[H] 0.000 description 6
- 125000002914 sec-butyl group Chemical group [H]C([H])([H])C([H])([H])C([H])(*)C([H])([H])[H] 0.000 description 6
- 229910052708 sodium Inorganic materials 0.000 description 6
- 239000011734 sodium Substances 0.000 description 6
- 125000000999 tert-butyl group Chemical group [H]C([H])([H])C(*)(C([H])([H])[H])C([H])([H])[H] 0.000 description 6
- 229910052726 zirconium Inorganic materials 0.000 description 6
- 229910052783 alkali metal Inorganic materials 0.000 description 5
- 125000004106 butoxy group Chemical group [*]OC([H])([H])C([H])([H])C(C([H])([H])[H])([H])[H] 0.000 description 5
- 229910052799 carbon Inorganic materials 0.000 description 5
- 239000003054 catalyst Substances 0.000 description 5
- 239000003446 ligand Substances 0.000 description 5
- 150000002736 metal compounds Chemical class 0.000 description 5
- 150000002894 organic compounds Chemical class 0.000 description 5
- NFHFRUOZVGFOOS-UHFFFAOYSA-N palladium;triphenylphosphane Chemical compound [Pd].C1=CC=CC=C1P(C=1C=CC=CC=1)C1=CC=CC=C1.C1=CC=CC=C1P(C=1C=CC=CC=1)C1=CC=CC=C1.C1=CC=CC=C1P(C=1C=CC=CC=1)C1=CC=CC=C1.C1=CC=CC=C1P(C=1C=CC=CC=1)C1=CC=CC=C1 NFHFRUOZVGFOOS-UHFFFAOYSA-N 0.000 description 5
- 239000000047 product Substances 0.000 description 5
- 238000010926 purge Methods 0.000 description 5
- 230000009467 reduction Effects 0.000 description 5
- 239000010703 silicon Substances 0.000 description 5
- 125000004861 4-isopropyl phenyl group Chemical group [H]C1=C([H])C(=C([H])C([H])=C1*)C([H])(C([H])([H])[H])C([H])([H])[H] 0.000 description 4
- QGZKDVFQNNGYKY-UHFFFAOYSA-N Ammonia Chemical compound N QGZKDVFQNNGYKY-UHFFFAOYSA-N 0.000 description 4
- IJGRMHOSHXDMSA-UHFFFAOYSA-N Atomic nitrogen Chemical compound N#N IJGRMHOSHXDMSA-UHFFFAOYSA-N 0.000 description 4
- 208000005156 Dehydration Diseases 0.000 description 4
- SNRUBQQJIBEYMU-UHFFFAOYSA-N Dodecane Natural products CCCCCCCCCCCC SNRUBQQJIBEYMU-UHFFFAOYSA-N 0.000 description 4
- QUSNBJAOOMFDIB-UHFFFAOYSA-N Ethylamine Chemical compound CCN QUSNBJAOOMFDIB-UHFFFAOYSA-N 0.000 description 4
- 238000003747 Grignard reaction Methods 0.000 description 4
- VEXZGXHMUGYJMC-UHFFFAOYSA-N Hydrochloric acid Chemical compound Cl VEXZGXHMUGYJMC-UHFFFAOYSA-N 0.000 description 4
- CSNNHWWHGAXBCP-UHFFFAOYSA-L Magnesium sulfate Chemical compound [Mg+2].[O-][S+2]([O-])([O-])[O-] CSNNHWWHGAXBCP-UHFFFAOYSA-L 0.000 description 4
- BAVYZALUXZFZLV-UHFFFAOYSA-N Methylamine Chemical compound NC BAVYZALUXZFZLV-UHFFFAOYSA-N 0.000 description 4
- KDLHZDBZIXYQEI-UHFFFAOYSA-N Palladium Chemical compound [Pd] KDLHZDBZIXYQEI-UHFFFAOYSA-N 0.000 description 4
- 238000004833 X-ray photoelectron spectroscopy Methods 0.000 description 4
- 150000003973 alkyl amines Chemical class 0.000 description 4
- 125000004369 butenyl group Chemical group C(=CCC)* 0.000 description 4
- 230000000052 comparative effect Effects 0.000 description 4
- 125000001162 cycloheptenyl group Chemical group C1(=CCCCCC1)* 0.000 description 4
- 125000000582 cycloheptyl group Chemical group [H]C1([H])C([H])([H])C([H])([H])C([H])([H])C([H])(*)C([H])([H])C1([H])[H] 0.000 description 4
- 125000000596 cyclohexenyl group Chemical group C1(=CCCCC1)* 0.000 description 4
- 125000002433 cyclopentenyl group Chemical group C1(=CCCC1)* 0.000 description 4
- 125000001511 cyclopentyl group Chemical group [H]C1([H])C([H])([H])C([H])([H])C([H])(*)C1([H])[H] 0.000 description 4
- 125000003493 decenyl group Chemical group [H]C([*])=C([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])[H] 0.000 description 4
- 125000002704 decyl group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])* 0.000 description 4
- 230000018044 dehydration Effects 0.000 description 4
- 238000006297 dehydration reaction Methods 0.000 description 4
- 125000003438 dodecyl group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])* 0.000 description 4
- 238000000921 elemental analysis Methods 0.000 description 4
- 238000010438 heat treatment Methods 0.000 description 4
- 125000006038 hexenyl group Chemical group 0.000 description 4
- 229910017053 inorganic salt Inorganic materials 0.000 description 4
- 239000007791 liquid phase Substances 0.000 description 4
- 125000000040 m-tolyl group Chemical group [H]C1=C([H])C(*)=C([H])C(=C1[H])C([H])([H])[H] 0.000 description 4
- 239000000463 material Substances 0.000 description 4
- JDQLUYWHCUWSJE-UHFFFAOYSA-N methanolate;titanium(3+) Chemical compound [Ti+3].[O-]C.[O-]C.[O-]C JDQLUYWHCUWSJE-UHFFFAOYSA-N 0.000 description 4
- 125000001624 naphthyl group Chemical group 0.000 description 4
- 125000001400 nonyl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])[H] 0.000 description 4
- 125000003261 o-tolyl group Chemical group [H]C1=C([H])C(*)=C(C([H])=C1[H])C([H])([H])[H] 0.000 description 4
- 125000004365 octenyl group Chemical group C(=CCCCCCC)* 0.000 description 4
- 239000012044 organic layer Substances 0.000 description 4
- 125000001037 p-tolyl group Chemical group [H]C1=C([H])C(=C([H])C([H])=C1*)C([H])([H])[H] 0.000 description 4
- 125000002255 pentenyl group Chemical group C(=CCCC)* 0.000 description 4
- 125000001997 phenyl group Chemical group [H]C1=C([H])C([H])=C(*)C([H])=C1[H] 0.000 description 4
- BASFCYQUMIYNBI-UHFFFAOYSA-N platinum Chemical compound [Pt] BASFCYQUMIYNBI-UHFFFAOYSA-N 0.000 description 4
- WGYKZJWCGVVSQN-UHFFFAOYSA-N propylamine Chemical compound CCCN WGYKZJWCGVVSQN-UHFFFAOYSA-N 0.000 description 4
- 125000000026 trimethylsilyl group Chemical group [H]C([H])([H])[Si]([*])(C([H])([H])[H])C([H])([H])[H] 0.000 description 4
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 4
- BVPKYBMUQDZTJH-UHFFFAOYSA-N 1,1,1-trifluoro-5,5-dimethylhexane-2,4-dione Chemical compound CC(C)(C)C(=O)CC(=O)C(F)(F)F BVPKYBMUQDZTJH-UHFFFAOYSA-N 0.000 description 3
- SHXHPUAKLCCLDV-UHFFFAOYSA-N 1,1,1-trifluoropentane-2,4-dione Chemical compound CC(=O)CC(=O)C(F)(F)F SHXHPUAKLCCLDV-UHFFFAOYSA-N 0.000 description 3
- QNLXXWOBNIYLGO-UHFFFAOYSA-N 1-methoxy-2,2,6,6-tetramethylheptane-3,5-dione Chemical compound COCC(C)(C)C(=O)CC(=O)C(C)(C)C QNLXXWOBNIYLGO-UHFFFAOYSA-N 0.000 description 3
- QTBSBXVTEAMEQO-UHFFFAOYSA-N Acetic acid Chemical compound CC(O)=O QTBSBXVTEAMEQO-UHFFFAOYSA-N 0.000 description 3
- WFDIJRYMOXRFFG-UHFFFAOYSA-N Acetic anhydride Chemical compound CC(=O)OC(C)=O WFDIJRYMOXRFFG-UHFFFAOYSA-N 0.000 description 3
- GAWIXWVDTYZWAW-UHFFFAOYSA-N C[CH]O Chemical group C[CH]O GAWIXWVDTYZWAW-UHFFFAOYSA-N 0.000 description 3
- OKTJSMMVPCPJKN-UHFFFAOYSA-N Carbon Chemical group [C] OKTJSMMVPCPJKN-UHFFFAOYSA-N 0.000 description 3
- 229910052684 Cerium Inorganic materials 0.000 description 3
- VYZAMTAEIAYCRO-UHFFFAOYSA-N Chromium Chemical compound [Cr] VYZAMTAEIAYCRO-UHFFFAOYSA-N 0.000 description 3
- XTHFKEDIFFGKHM-UHFFFAOYSA-N Dimethoxyethane Chemical compound COCCOC XTHFKEDIFFGKHM-UHFFFAOYSA-N 0.000 description 3
- 229910052691 Erbium Inorganic materials 0.000 description 3
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 description 3
- XEKOWRVHYACXOJ-UHFFFAOYSA-N Ethyl acetate Chemical compound CCOC(C)=O XEKOWRVHYACXOJ-UHFFFAOYSA-N 0.000 description 3
- 229910052693 Europium Inorganic materials 0.000 description 3
- 229910052688 Gadolinium Inorganic materials 0.000 description 3
- 229910052689 Holmium Inorganic materials 0.000 description 3
- SJRJJKPEHAURKC-UHFFFAOYSA-N N-Methylmorpholine Chemical compound CN1CCOCC1 SJRJJKPEHAURKC-UHFFFAOYSA-N 0.000 description 3
- 229910052779 Neodymium Inorganic materials 0.000 description 3
- 229910052777 Praseodymium Inorganic materials 0.000 description 3
- 229910052773 Promethium Inorganic materials 0.000 description 3
- RWRDLPDLKQPQOW-UHFFFAOYSA-N Pyrrolidine Chemical compound C1CCNC1 RWRDLPDLKQPQOW-UHFFFAOYSA-N 0.000 description 3
- 229910052772 Samarium Inorganic materials 0.000 description 3
- HEMHJVSKTPXQMS-UHFFFAOYSA-M Sodium hydroxide Chemical compound [OH-].[Na+] HEMHJVSKTPXQMS-UHFFFAOYSA-M 0.000 description 3
- 229910052775 Thulium Inorganic materials 0.000 description 3
- 239000007983 Tris buffer Substances 0.000 description 3
- 229910052769 Ytterbium Inorganic materials 0.000 description 3
- MZVQCMJNVPIDEA-UHFFFAOYSA-N [CH2]CN(CC)CC Chemical group [CH2]CN(CC)CC MZVQCMJNVPIDEA-UHFFFAOYSA-N 0.000 description 3
- 125000003545 alkoxy group Chemical group 0.000 description 3
- 230000004888 barrier function Effects 0.000 description 3
- 239000002585 base Substances 0.000 description 3
- ZMIGMASIKSOYAM-UHFFFAOYSA-N cerium Chemical compound [Ce][Ce][Ce][Ce][Ce][Ce][Ce][Ce][Ce][Ce][Ce][Ce][Ce][Ce][Ce][Ce][Ce][Ce][Ce][Ce][Ce][Ce][Ce][Ce][Ce][Ce][Ce][Ce][Ce][Ce][Ce][Ce][Ce][Ce][Ce][Ce][Ce][Ce] ZMIGMASIKSOYAM-UHFFFAOYSA-N 0.000 description 3
- 229910052804 chromium Inorganic materials 0.000 description 3
- 239000011651 chromium Substances 0.000 description 3
- 125000004663 dialkyl amino group Chemical group 0.000 description 3
- 125000006264 diethylaminomethyl group Chemical group [H]C([H])([H])C([H])([H])N(C([H])([H])*)C([H])([H])C([H])([H])[H] 0.000 description 3
- UAOMVDZJSHZZME-UHFFFAOYSA-N diisopropylamine Chemical compound CC(C)NC(C)C UAOMVDZJSHZZME-UHFFFAOYSA-N 0.000 description 3
- 125000006222 dimethylaminomethyl group Chemical group [H]C([H])([H])N(C([H])([H])[H])C([H])([H])* 0.000 description 3
- UYAHIZSMUZPPFV-UHFFFAOYSA-N erbium Chemical compound [Er] UYAHIZSMUZPPFV-UHFFFAOYSA-N 0.000 description 3
- 125000001301 ethoxy group Chemical group [H]C([H])([H])C([H])([H])O* 0.000 description 3
- OGPBJKLSAFTDLK-UHFFFAOYSA-N europium atom Chemical compound [Eu] OGPBJKLSAFTDLK-UHFFFAOYSA-N 0.000 description 3
- 238000011156 evaluation Methods 0.000 description 3
- UIWYJDYFSGRHKR-UHFFFAOYSA-N gadolinium atom Chemical compound [Gd] UIWYJDYFSGRHKR-UHFFFAOYSA-N 0.000 description 3
- ILPNRWUGFSPGAA-UHFFFAOYSA-N heptane-2,4-dione Chemical compound CCCC(=O)CC(C)=O ILPNRWUGFSPGAA-UHFFFAOYSA-N 0.000 description 3
- NDOGLIPWGGRQCO-UHFFFAOYSA-N hexane-2,4-dione Chemical compound CCC(=O)CC(C)=O NDOGLIPWGGRQCO-UHFFFAOYSA-N 0.000 description 3
- KJZYNXUDTRRSPN-UHFFFAOYSA-N holmium atom Chemical compound [Ho] KJZYNXUDTRRSPN-UHFFFAOYSA-N 0.000 description 3
- 229930195733 hydrocarbon Natural products 0.000 description 3
- 125000004029 hydroxymethyl group Chemical group [H]OC([H])([H])* 0.000 description 3
- 229910052746 lanthanum Inorganic materials 0.000 description 3
- FZLIPJUXYLNCLC-UHFFFAOYSA-N lanthanum atom Chemical compound [La] FZLIPJUXYLNCLC-UHFFFAOYSA-N 0.000 description 3
- 229910052744 lithium Inorganic materials 0.000 description 3
- WPBNNNQJVZRUHP-UHFFFAOYSA-L manganese(2+);methyl n-[[2-(methoxycarbonylcarbamothioylamino)phenyl]carbamothioyl]carbamate;n-[2-(sulfidocarbothioylamino)ethyl]carbamodithioate Chemical compound [Mn+2].[S-]C(=S)NCCNC([S-])=S.COC(=O)NC(=S)NC1=CC=CC=C1NC(=S)NC(=O)OC WPBNNNQJVZRUHP-UHFFFAOYSA-L 0.000 description 3
- 229910052752 metalloid Inorganic materials 0.000 description 3
- 150000002738 metalloids Chemical class 0.000 description 3
- 125000000956 methoxy group Chemical group [H]C([H])([H])O* 0.000 description 3
- 125000000250 methylamino group Chemical group [H]N(*)C([H])([H])[H] 0.000 description 3
- VLKZOEOYAKHREP-UHFFFAOYSA-N n-Hexane Chemical compound CCCCCC VLKZOEOYAKHREP-UHFFFAOYSA-N 0.000 description 3
- QEFYFXOXNSNQGX-UHFFFAOYSA-N neodymium atom Chemical compound [Nd] QEFYFXOXNSNQGX-UHFFFAOYSA-N 0.000 description 3
- 125000004430 oxygen atom Chemical group O* 0.000 description 3
- 125000004115 pentoxy group Chemical group [*]OC([H])([H])C([H])([H])C([H])([H])C(C([H])([H])[H])([H])[H] 0.000 description 3
- 229910052700 potassium Inorganic materials 0.000 description 3
- 239000011591 potassium Substances 0.000 description 3
- PUDIUYLPXJFUGB-UHFFFAOYSA-N praseodymium atom Chemical compound [Pr] PUDIUYLPXJFUGB-UHFFFAOYSA-N 0.000 description 3
- 125000002924 primary amino group Chemical group [H]N([H])* 0.000 description 3
- VQMWBBYLQSCNPO-UHFFFAOYSA-N promethium atom Chemical compound [Pm] VQMWBBYLQSCNPO-UHFFFAOYSA-N 0.000 description 3
- 239000012048 reactive intermediate Substances 0.000 description 3
- KZUNJOHGWZRPMI-UHFFFAOYSA-N samarium atom Chemical compound [Sm] KZUNJOHGWZRPMI-UHFFFAOYSA-N 0.000 description 3
- 235000015067 sauces Nutrition 0.000 description 3
- 229910052706 scandium Inorganic materials 0.000 description 3
- SIXSYDAISGFNSX-UHFFFAOYSA-N scandium atom Chemical compound [Sc] SIXSYDAISGFNSX-UHFFFAOYSA-N 0.000 description 3
- 238000003786 synthesis reaction Methods 0.000 description 3
- 238000005979 thermal decomposition reaction Methods 0.000 description 3
- 238000003852 thin film production method Methods 0.000 description 3
- NAWDYIZEMPQZHO-UHFFFAOYSA-N ytterbium Chemical compound [Yb] NAWDYIZEMPQZHO-UHFFFAOYSA-N 0.000 description 3
- 229910052727 yttrium Inorganic materials 0.000 description 3
- VWQVUPCCIRVNHF-UHFFFAOYSA-N yttrium atom Chemical compound [Y] VWQVUPCCIRVNHF-UHFFFAOYSA-N 0.000 description 3
- PUPZLCDOIYMWBV-UHFFFAOYSA-N (+/-)-1,3-Butanediol Chemical compound CC(O)CCO PUPZLCDOIYMWBV-UHFFFAOYSA-N 0.000 description 2
- QEGNUYASOUJEHD-UHFFFAOYSA-N 1,1-dimethylcyclohexane Chemical compound CC1(C)CCCCC1 QEGNUYASOUJEHD-UHFFFAOYSA-N 0.000 description 2
- RYHBNJHYFVUHQT-UHFFFAOYSA-N 1,4-Dioxane Chemical compound C1COCCO1 RYHBNJHYFVUHQT-UHFFFAOYSA-N 0.000 description 2
- MIAJKNFXBPVTCU-UHFFFAOYSA-N 1-(2-methoxyethoxy)-2,2,6,6-tetramethylheptane-3,5-dione Chemical compound COCCOCC(C)(C)C(=O)CC(=O)C(C)(C)C MIAJKNFXBPVTCU-UHFFFAOYSA-N 0.000 description 2
- RMSGQZDGSZOJMU-UHFFFAOYSA-N 1-butyl-2-phenylbenzene Chemical group CCCCC1=CC=CC=C1C1=CC=CC=C1 RMSGQZDGSZOJMU-UHFFFAOYSA-N 0.000 description 2
- 125000006018 1-methyl-ethenyl group Chemical group 0.000 description 2
- PAMIQIKDUOTOBW-UHFFFAOYSA-N 1-methylpiperidine Chemical compound CN1CCCCC1 PAMIQIKDUOTOBW-UHFFFAOYSA-N 0.000 description 2
- CEGGECULKVTYMM-UHFFFAOYSA-N 2,6-dimethylheptane-3,5-dione Chemical compound CC(C)C(=O)CC(=O)C(C)C CEGGECULKVTYMM-UHFFFAOYSA-N 0.000 description 2
- OISVCGZHLKNMSJ-UHFFFAOYSA-N 2,6-dimethylpyridine Chemical compound CC1=CC=CC(C)=N1 OISVCGZHLKNMSJ-UHFFFAOYSA-N 0.000 description 2
- PXJCDOSDACXFTB-UHFFFAOYSA-N 2-methoxy-2,6,6-trimethylheptane-3,5-dione Chemical compound COC(C)(C)C(=O)CC(=O)C(C)(C)C PXJCDOSDACXFTB-UHFFFAOYSA-N 0.000 description 2
- KDSNLYIMUZNERS-UHFFFAOYSA-N 2-methylpropanamine Chemical compound CC(C)CN KDSNLYIMUZNERS-UHFFFAOYSA-N 0.000 description 2
- SHGQDMVQBSZXOD-UHFFFAOYSA-N 3-ethyl-2-ethyliminopentan-3-ol Chemical compound C(C)N=C(C)C(CC)(O)CC SHGQDMVQBSZXOD-UHFFFAOYSA-N 0.000 description 2
- YDKTTWZEAROPAM-UHFFFAOYSA-N 3-ethyl-2-methyliminopentan-3-ol Chemical compound C(C)C(C(C)=NC)(CC)O YDKTTWZEAROPAM-UHFFFAOYSA-N 0.000 description 2
- HCFAJYNVAYBARA-UHFFFAOYSA-N 4-heptanone Chemical compound CCCC(=O)CCC HCFAJYNVAYBARA-UHFFFAOYSA-N 0.000 description 2
- KHZGUWAFFHXZLC-UHFFFAOYSA-N 5-methylhexane-2,4-dione Chemical compound CC(C)C(=O)CC(C)=O KHZGUWAFFHXZLC-UHFFFAOYSA-N 0.000 description 2
- OYPRJOBELJOOCE-UHFFFAOYSA-N Calcium Chemical compound [Ca] OYPRJOBELJOOCE-UHFFFAOYSA-N 0.000 description 2
- 229910021580 Cobalt(II) chloride Inorganic materials 0.000 description 2
- ROSDSFDQCJNGOL-UHFFFAOYSA-N Dimethylamine Chemical compound CNC ROSDSFDQCJNGOL-UHFFFAOYSA-N 0.000 description 2
- 229910052692 Dysprosium Inorganic materials 0.000 description 2
- PIICEJLVQHRZGT-UHFFFAOYSA-N Ethylenediamine Chemical compound NCCN PIICEJLVQHRZGT-UHFFFAOYSA-N 0.000 description 2
- GYHNNYVSQQEPJS-UHFFFAOYSA-N Gallium Chemical compound [Ga] GYHNNYVSQQEPJS-UHFFFAOYSA-N 0.000 description 2
- OAKJQQAXSVQMHS-UHFFFAOYSA-N Hydrazine Chemical compound NN OAKJQQAXSVQMHS-UHFFFAOYSA-N 0.000 description 2
- MHAJPDPJQMAIIY-UHFFFAOYSA-N Hydrogen peroxide Chemical compound OO MHAJPDPJQMAIIY-UHFFFAOYSA-N 0.000 description 2
- DGAQECJNVWCQMB-PUAWFVPOSA-M Ilexoside XXIX Chemical compound C[C@@H]1CC[C@@]2(CC[C@@]3(C(=CC[C@H]4[C@]3(CC[C@@H]5[C@@]4(CC[C@@H](C5(C)C)OS(=O)(=O)[O-])C)C)[C@@H]2[C@]1(C)O)C)C(=O)O[C@H]6[C@@H]([C@H]([C@@H]([C@H](O6)CO)O)O)O.[Na+] DGAQECJNVWCQMB-PUAWFVPOSA-M 0.000 description 2
- ZOKXTWBITQBERF-UHFFFAOYSA-N Molybdenum Chemical compound [Mo] ZOKXTWBITQBERF-UHFFFAOYSA-N 0.000 description 2
- YNAVUWVOSKDBBP-UHFFFAOYSA-N Morpholine Chemical compound C1COCCN1 YNAVUWVOSKDBBP-UHFFFAOYSA-N 0.000 description 2
- IMNFDUFMRHMDMM-UHFFFAOYSA-N N-Heptane Chemical compound CCCCCCC IMNFDUFMRHMDMM-UHFFFAOYSA-N 0.000 description 2
- 229910002651 NO3 Inorganic materials 0.000 description 2
- NHNBFGGVMKEFGY-UHFFFAOYSA-N Nitrate Chemical compound [O-][N+]([O-])=O NHNBFGGVMKEFGY-UHFFFAOYSA-N 0.000 description 2
- MWUXSHHQAYIFBG-UHFFFAOYSA-N Nitric oxide Chemical compound O=[N] MWUXSHHQAYIFBG-UHFFFAOYSA-N 0.000 description 2
- JUJWROOIHBZHMG-UHFFFAOYSA-N Pyridine Chemical compound C1=CC=NC=C1 JUJWROOIHBZHMG-UHFFFAOYSA-N 0.000 description 2
- BQCADISMDOOEFD-UHFFFAOYSA-N Silver Chemical compound [Ag] BQCADISMDOOEFD-UHFFFAOYSA-N 0.000 description 2
- WQDUMFSSJAZKTM-UHFFFAOYSA-N Sodium methoxide Chemical compound [Na+].[O-]C WQDUMFSSJAZKTM-UHFFFAOYSA-N 0.000 description 2
- 229910052771 Terbium Inorganic materials 0.000 description 2
- DHXVGJBLRPWPCS-UHFFFAOYSA-N Tetrahydropyran Chemical compound C1CCOCC1 DHXVGJBLRPWPCS-UHFFFAOYSA-N 0.000 description 2
- ATJFFYVFTNAWJD-UHFFFAOYSA-N Tin Chemical compound [Sn] ATJFFYVFTNAWJD-UHFFFAOYSA-N 0.000 description 2
- NRTOMJZYCJJWKI-UHFFFAOYSA-N Titanium nitride Chemical compound [Ti]#N NRTOMJZYCJJWKI-UHFFFAOYSA-N 0.000 description 2
- 238000002441 X-ray diffraction Methods 0.000 description 2
- 238000000560 X-ray reflectometry Methods 0.000 description 2
- HCHKCACWOHOZIP-UHFFFAOYSA-N Zinc Chemical compound [Zn] HCHKCACWOHOZIP-UHFFFAOYSA-N 0.000 description 2
- 230000009471 action Effects 0.000 description 2
- 239000002313 adhesive film Substances 0.000 description 2
- 125000002947 alkylene group Chemical group 0.000 description 2
- 229910052782 aluminium Inorganic materials 0.000 description 2
- XAGFODPZIPBFFR-UHFFFAOYSA-N aluminium Chemical compound [Al] XAGFODPZIPBFFR-UHFFFAOYSA-N 0.000 description 2
- 229910021529 ammonia Inorganic materials 0.000 description 2
- 229910052787 antimony Inorganic materials 0.000 description 2
- WATWJIUSRGPENY-UHFFFAOYSA-N antimony atom Chemical compound [Sb] WATWJIUSRGPENY-UHFFFAOYSA-N 0.000 description 2
- 239000007864 aqueous solution Substances 0.000 description 2
- 229910052788 barium Inorganic materials 0.000 description 2
- DSAJWYNOEDNPEQ-UHFFFAOYSA-N barium atom Chemical compound [Ba] DSAJWYNOEDNPEQ-UHFFFAOYSA-N 0.000 description 2
- 229910052797 bismuth Inorganic materials 0.000 description 2
- JCXGWMGPZLAOME-UHFFFAOYSA-N bismuth atom Chemical compound [Bi] JCXGWMGPZLAOME-UHFFFAOYSA-N 0.000 description 2
- RDHPKYGYEGBMSE-UHFFFAOYSA-N bromoethane Chemical compound CCBr RDHPKYGYEGBMSE-UHFFFAOYSA-N 0.000 description 2
- HQABUPZFAYXKJW-UHFFFAOYSA-N butan-1-amine Chemical compound CCCCN HQABUPZFAYXKJW-UHFFFAOYSA-N 0.000 description 2
- 239000006227 byproduct Substances 0.000 description 2
- 229910052793 cadmium Inorganic materials 0.000 description 2
- BDOSMKKIYDKNTQ-UHFFFAOYSA-N cadmium atom Chemical compound [Cd] BDOSMKKIYDKNTQ-UHFFFAOYSA-N 0.000 description 2
- 229910052791 calcium Inorganic materials 0.000 description 2
- 239000011575 calcium Substances 0.000 description 2
- 239000000919 ceramic Substances 0.000 description 2
- GVPFVAHMJGGAJG-UHFFFAOYSA-L cobalt dichloride Chemical compound [Cl-].[Cl-].[Co+2] GVPFVAHMJGGAJG-UHFFFAOYSA-L 0.000 description 2
- 238000001816 cooling Methods 0.000 description 2
- JHIVVAPYMSGYDF-UHFFFAOYSA-N cyclohexanone Chemical compound O=C1CCCCC1 JHIVVAPYMSGYDF-UHFFFAOYSA-N 0.000 description 2
- 238000000354 decomposition reaction Methods 0.000 description 2
- 238000001514 detection method Methods 0.000 description 2
- 238000010586 diagram Methods 0.000 description 2
- SBZXBUIDTXKZTM-UHFFFAOYSA-N diglyme Chemical compound COCCOCCOC SBZXBUIDTXKZTM-UHFFFAOYSA-N 0.000 description 2
- 238000007865 diluting Methods 0.000 description 2
- 238000004821 distillation Methods 0.000 description 2
- KBQHZAAAGSGFKK-UHFFFAOYSA-N dysprosium atom Chemical compound [Dy] KBQHZAAAGSGFKK-UHFFFAOYSA-N 0.000 description 2
- IIEWJVIFRVWJOD-UHFFFAOYSA-N ethylcyclohexane Chemical compound CCC1CCCCC1 IIEWJVIFRVWJOD-UHFFFAOYSA-N 0.000 description 2
- 239000000706 filtrate Substances 0.000 description 2
- 238000001914 filtration Methods 0.000 description 2
- 229910052733 gallium Inorganic materials 0.000 description 2
- 238000002290 gas chromatography-mass spectrometry Methods 0.000 description 2
- 229910052732 germanium Inorganic materials 0.000 description 2
- GNPVGFCGXDBREM-UHFFFAOYSA-N germanium atom Chemical compound [Ge] GNPVGFCGXDBREM-UHFFFAOYSA-N 0.000 description 2
- 239000011521 glass Substances 0.000 description 2
- PCHJSUWPFVWCPO-UHFFFAOYSA-N gold Chemical compound [Au] PCHJSUWPFVWCPO-UHFFFAOYSA-N 0.000 description 2
- 229910052737 gold Inorganic materials 0.000 description 2
- 239000010931 gold Substances 0.000 description 2
- 150000004820 halides Chemical class 0.000 description 2
- 239000001307 helium Substances 0.000 description 2
- 229910052734 helium Inorganic materials 0.000 description 2
- SWQJXJOGLNCZEY-UHFFFAOYSA-N helium atom Chemical compound [He] SWQJXJOGLNCZEY-UHFFFAOYSA-N 0.000 description 2
- CATSNJVOTSVZJV-UHFFFAOYSA-N heptan-2-one Chemical compound CCCCCC(C)=O CATSNJVOTSVZJV-UHFFFAOYSA-N 0.000 description 2
- DGCTVLNZTFDPDJ-UHFFFAOYSA-N heptane-3,5-dione Chemical compound CCC(=O)CC(=O)CC DGCTVLNZTFDPDJ-UHFFFAOYSA-N 0.000 description 2
- QAMFBRUWYYMMGJ-UHFFFAOYSA-N hexafluoroacetylacetone Chemical compound FC(F)(F)C(=O)CC(=O)C(F)(F)F QAMFBRUWYYMMGJ-UHFFFAOYSA-N 0.000 description 2
- TXGJTWACJNYNOJ-UHFFFAOYSA-N hexane-2,4-diol Chemical compound CCC(O)CC(C)O TXGJTWACJNYNOJ-UHFFFAOYSA-N 0.000 description 2
- WGCNASOHLSPBMP-UHFFFAOYSA-N hydroxyacetaldehyde Natural products OCC=O WGCNASOHLSPBMP-UHFFFAOYSA-N 0.000 description 2
- 229910052738 indium Inorganic materials 0.000 description 2
- APFVFJFRJDLVQX-UHFFFAOYSA-N indium atom Chemical compound [In] APFVFJFRJDLVQX-UHFFFAOYSA-N 0.000 description 2
- 229910052741 iridium Inorganic materials 0.000 description 2
- GKOZUEZYRPOHIO-UHFFFAOYSA-N iridium atom Chemical compound [Ir] GKOZUEZYRPOHIO-UHFFFAOYSA-N 0.000 description 2
- 125000003253 isopropoxy group Chemical group [H]C([H])([H])C([H])(O*)C([H])([H])[H] 0.000 description 2
- MRELNEQAGSRDBK-UHFFFAOYSA-N lanthanum(3+);oxygen(2-) Chemical compound [O-2].[O-2].[O-2].[La+3].[La+3] MRELNEQAGSRDBK-UHFFFAOYSA-N 0.000 description 2
- 229910052943 magnesium sulfate Inorganic materials 0.000 description 2
- 235000019341 magnesium sulphate Nutrition 0.000 description 2
- 238000005259 measurement Methods 0.000 description 2
- 238000002844 melting Methods 0.000 description 2
- 230000008018 melting Effects 0.000 description 2
- 238000002488 metal-organic chemical vapour deposition Methods 0.000 description 2
- 150000002739 metals Chemical class 0.000 description 2
- BDAGIHXWWSANSR-UHFFFAOYSA-N methanoic acid Natural products OC=O BDAGIHXWWSANSR-UHFFFAOYSA-N 0.000 description 2
- KHQQDBIXHUJARJ-UHFFFAOYSA-N methyl 2,2-dimethoxypropanoate Chemical compound COC(=O)C(C)(OC)OC KHQQDBIXHUJARJ-UHFFFAOYSA-N 0.000 description 2
- UAEPNZWRGJTJPN-UHFFFAOYSA-N methylcyclohexane Chemical compound CC1CCCCC1 UAEPNZWRGJTJPN-UHFFFAOYSA-N 0.000 description 2
- 239000011259 mixed solution Substances 0.000 description 2
- 229910052750 molybdenum Inorganic materials 0.000 description 2
- 239000011733 molybdenum Substances 0.000 description 2
- 125000001971 neopentyl group Chemical group [H]C([*])([H])C(C([H])([H])[H])(C([H])([H])[H])C([H])([H])[H] 0.000 description 2
- 229910052758 niobium Inorganic materials 0.000 description 2
- 239000010955 niobium Substances 0.000 description 2
- GUCVJGMIXFAOAE-UHFFFAOYSA-N niobium atom Chemical compound [Nb] GUCVJGMIXFAOAE-UHFFFAOYSA-N 0.000 description 2
- 150000002823 nitrates Chemical class 0.000 description 2
- 150000004767 nitrides Chemical class 0.000 description 2
- 239000003921 oil Substances 0.000 description 2
- 125000001181 organosilyl group Chemical group [SiH3]* 0.000 description 2
- 229910052762 osmium Inorganic materials 0.000 description 2
- SYQBFIAQOQZEGI-UHFFFAOYSA-N osmium atom Chemical compound [Os] SYQBFIAQOQZEGI-UHFFFAOYSA-N 0.000 description 2
- 229910052763 palladium Inorganic materials 0.000 description 2
- 125000002097 pentamethylcyclopentadienyl group Chemical group 0.000 description 2
- 238000005268 plasma chemical vapour deposition Methods 0.000 description 2
- 229910052697 platinum Inorganic materials 0.000 description 2
- 229920000768 polyamine Polymers 0.000 description 2
- 125000004368 propenyl group Chemical group C(=CC)* 0.000 description 2
- 229910052705 radium Inorganic materials 0.000 description 2
- HCWPIIXVSYCSAN-UHFFFAOYSA-N radium atom Chemical compound [Ra] HCWPIIXVSYCSAN-UHFFFAOYSA-N 0.000 description 2
- 229910052761 rare earth metal Inorganic materials 0.000 description 2
- 150000002910 rare earth metals Chemical group 0.000 description 2
- 229910052703 rhodium Inorganic materials 0.000 description 2
- 239000010948 rhodium Substances 0.000 description 2
- MHOVAHRLVXNVSD-UHFFFAOYSA-N rhodium atom Chemical compound [Rh] MHOVAHRLVXNVSD-UHFFFAOYSA-N 0.000 description 2
- 229910052709 silver Inorganic materials 0.000 description 2
- 239000004332 silver Substances 0.000 description 2
- 229910052712 strontium Inorganic materials 0.000 description 2
- CIOAGBVUUVVLOB-UHFFFAOYSA-N strontium atom Chemical compound [Sr] CIOAGBVUUVVLOB-UHFFFAOYSA-N 0.000 description 2
- 229910052715 tantalum Inorganic materials 0.000 description 2
- GUVRBAGPIYLISA-UHFFFAOYSA-N tantalum atom Chemical compound [Ta] GUVRBAGPIYLISA-UHFFFAOYSA-N 0.000 description 2
- GZCRRIHWUXGPOV-UHFFFAOYSA-N terbium atom Chemical compound [Tb] GZCRRIHWUXGPOV-UHFFFAOYSA-N 0.000 description 2
- 238000002230 thermal chemical vapour deposition Methods 0.000 description 2
- 238000000427 thin-film deposition Methods 0.000 description 2
- 229910052718 tin Inorganic materials 0.000 description 2
- YFNKIDBQEZZDLK-UHFFFAOYSA-N triglyme Chemical compound COCCOCCOCCOC YFNKIDBQEZZDLK-UHFFFAOYSA-N 0.000 description 2
- WFKWXMTUELFFGS-UHFFFAOYSA-N tungsten Chemical compound [W] WFKWXMTUELFFGS-UHFFFAOYSA-N 0.000 description 2
- 229910052721 tungsten Inorganic materials 0.000 description 2
- 239000010937 tungsten Substances 0.000 description 2
- 229910052720 vanadium Inorganic materials 0.000 description 2
- GPPXJZIENCGNKB-UHFFFAOYSA-N vanadium Chemical compound [V]#[V] GPPXJZIENCGNKB-UHFFFAOYSA-N 0.000 description 2
- 125000000391 vinyl group Chemical group [H]C([*])=C([H])[H] 0.000 description 2
- 229920002554 vinyl polymer Polymers 0.000 description 2
- 229910052725 zinc Inorganic materials 0.000 description 2
- 239000011701 zinc Substances 0.000 description 2
- DNIAPMSPPWPWGF-VKHMYHEASA-N (+)-propylene glycol Chemical compound C[C@H](O)CO DNIAPMSPPWPWGF-VKHMYHEASA-N 0.000 description 1
- DNIAPMSPPWPWGF-GSVOUGTGSA-N (R)-(-)-Propylene glycol Chemical compound C[C@@H](O)CO DNIAPMSPPWPWGF-GSVOUGTGSA-N 0.000 description 1
- SYTBZMRGLBWNTM-SNVBAGLBSA-N (R)-flurbiprofen Chemical compound FC1=CC([C@H](C(O)=O)C)=CC=C1C1=CC=CC=C1 SYTBZMRGLBWNTM-SNVBAGLBSA-N 0.000 description 1
- YPFDHNVEDLHUCE-UHFFFAOYSA-N 1,3-propanediol Substances OCCCO YPFDHNVEDLHUCE-UHFFFAOYSA-N 0.000 description 1
- BGYBONWLWSMGNV-UHFFFAOYSA-N 1,4,7,10,13,16,19,22-octaoxacyclotetracosane Chemical compound C1COCCOCCOCCOCCOCCOCCOCCO1 BGYBONWLWSMGNV-UHFFFAOYSA-N 0.000 description 1
- QBPPRVHXOZRESW-UHFFFAOYSA-N 1,4,7,10-tetraazacyclododecane Chemical compound C1CNCCNCCNCCN1 QBPPRVHXOZRESW-UHFFFAOYSA-N 0.000 description 1
- MDAXKAUIABOHTD-UHFFFAOYSA-N 1,4,8,11-tetraazacyclotetradecane Chemical compound C1CNCCNCCCNCCNC1 MDAXKAUIABOHTD-UHFFFAOYSA-N 0.000 description 1
- DURPTKYDGMDSBL-UHFFFAOYSA-N 1-butoxybutane Chemical compound CCCCOCCCC DURPTKYDGMDSBL-UHFFFAOYSA-N 0.000 description 1
- 125000006219 1-ethylpentyl group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])(*)C([H])([H])C([H])([H])[H] 0.000 description 1
- NSIPDYNOORNMRL-UHFFFAOYSA-N 1-methoxy-2-methylpropan-2-olate titanium(4+) Chemical compound COCC(C)(C)O[Ti](OC(C)(C)COC)(OC(C)(C)COC)OC(C)(C)COC NSIPDYNOORNMRL-UHFFFAOYSA-N 0.000 description 1
- AVFZOVWCLRSYKC-UHFFFAOYSA-N 1-methylpyrrolidine Chemical compound CN1CCCC1 AVFZOVWCLRSYKC-UHFFFAOYSA-N 0.000 description 1
- MWQKURVBJZAOSC-UHFFFAOYSA-N 1-propan-2-ylcyclopenta-1,3-diene Chemical compound CC(C)C1=CC=CC1 MWQKURVBJZAOSC-UHFFFAOYSA-N 0.000 description 1
- XEZNGIUYQVAUSS-UHFFFAOYSA-N 18-crown-6 Chemical compound C1COCCOCCOCCOCCOCCO1 XEZNGIUYQVAUSS-UHFFFAOYSA-N 0.000 description 1
- VILCJCGEZXAXTO-UHFFFAOYSA-N 2,2,2-tetramine Chemical compound NCCNCCNCCN VILCJCGEZXAXTO-UHFFFAOYSA-N 0.000 description 1
- YRAJNWYBUCUFBD-UHFFFAOYSA-N 2,2,6,6-tetramethylheptane-3,5-dione Chemical compound CC(C)(C)C(=O)CC(=O)C(C)(C)C YRAJNWYBUCUFBD-UHFFFAOYSA-N 0.000 description 1
- AKPLTHZVVWBOPT-UHFFFAOYSA-N 2,2-diethylbutane-1,3-diol Chemical compound CCC(CC)(CO)C(C)O AKPLTHZVVWBOPT-UHFFFAOYSA-N 0.000 description 1
- CVBVIDRXQAQQKH-UHFFFAOYSA-N 2,4,6-trimethyl-N-propan-2-yl-5-propan-2-yliminohept-3-en-3-amine Chemical compound C(C)(C)NC(C(C)C)=C(C(C(C)C)=NC(C)C)C CVBVIDRXQAQQKH-UHFFFAOYSA-N 0.000 description 1
- OXOBSWQLEHNKSO-UHFFFAOYSA-N 2,6-dimethyl-N-propan-2-yl-5-propan-2-yliminohept-3-en-3-amine Chemical compound C(C)(C)NC(C(C)C)=CC(C(C)C)=NC(C)C OXOBSWQLEHNKSO-UHFFFAOYSA-N 0.000 description 1
- PTTPXKJBFFKCEK-UHFFFAOYSA-N 2-Methyl-4-heptanone Chemical compound CC(C)CC(=O)CC(C)C PTTPXKJBFFKCEK-UHFFFAOYSA-N 0.000 description 1
- DSKYSDCYIODJPC-UHFFFAOYSA-N 2-butyl-2-ethylpropane-1,3-diol Chemical compound CCCCC(CC)(CO)CO DSKYSDCYIODJPC-UHFFFAOYSA-N 0.000 description 1
- PLHCSZRZWOWUBW-UHFFFAOYSA-N 2-methoxyethyl 3-oxobutanoate Chemical compound COCCOC(=O)CC(C)=O PLHCSZRZWOWUBW-UHFFFAOYSA-N 0.000 description 1
- XLLIQLLCWZCATF-UHFFFAOYSA-N 2-methoxyethyl acetate Chemical compound COCCOC(C)=O XLLIQLLCWZCATF-UHFFFAOYSA-N 0.000 description 1
- 125000004200 2-methoxyethyl group Chemical group [H]C([H])([H])OC([H])([H])C([H])([H])* 0.000 description 1
- VVALZQWOQKHDIM-UHFFFAOYSA-N 2-methylheptane-3,5-dione Chemical compound CCC(=O)CC(=O)C(C)C VVALZQWOQKHDIM-UHFFFAOYSA-N 0.000 description 1
- QUVMSYUGOKEMPX-UHFFFAOYSA-N 2-methylpropan-1-olate;titanium(4+) Chemical compound [Ti+4].CC(C)C[O-].CC(C)C[O-].CC(C)C[O-].CC(C)C[O-] QUVMSYUGOKEMPX-UHFFFAOYSA-N 0.000 description 1
- QWGRWMMWNDWRQN-UHFFFAOYSA-N 2-methylpropane-1,3-diol Chemical compound OCC(C)CO QWGRWMMWNDWRQN-UHFFFAOYSA-N 0.000 description 1
- MGWGWNFMUOTEHG-UHFFFAOYSA-N 4-(3,5-dimethylphenyl)-1,3-thiazol-2-amine Chemical compound CC1=CC(C)=CC(C=2N=C(N)SC=2)=C1 MGWGWNFMUOTEHG-UHFFFAOYSA-N 0.000 description 1
- OSWFIVFLDKOXQC-UHFFFAOYSA-N 4-(3-methoxyphenyl)aniline Chemical compound COC1=CC=CC(C=2C=CC(N)=CC=2)=C1 OSWFIVFLDKOXQC-UHFFFAOYSA-N 0.000 description 1
- VGVHNLRUAMRIEW-UHFFFAOYSA-N 4-methylcyclohexan-1-one Chemical compound CC1CCC(=O)CC1 VGVHNLRUAMRIEW-UHFFFAOYSA-N 0.000 description 1
- DKPFZGUDAPQIHT-UHFFFAOYSA-N Butyl acetate Natural products CCCCOC(C)=O DKPFZGUDAPQIHT-UHFFFAOYSA-N 0.000 description 1
- FIPWRIJSWJWJAI-UHFFFAOYSA-N Butyl carbitol 6-propylpiperonyl ether Chemical compound C1=C(CCC)C(COCCOCCOCCCC)=CC2=C1OCO2 FIPWRIJSWJWJAI-UHFFFAOYSA-N 0.000 description 1
- XPQCITLHEHJFAV-UHFFFAOYSA-N C(C(C)C)C1(C=CC=C1)[Ti](OC)(OC)OC Chemical compound C(C(C)C)C1(C=CC=C1)[Ti](OC)(OC)OC XPQCITLHEHJFAV-UHFFFAOYSA-N 0.000 description 1
- IPTGOPJXEXTNCE-UHFFFAOYSA-N C(C)C1(C=CC=C1)[Ti](N(C)C)(N(C)C)N(C)C Chemical compound C(C)C1(C=CC=C1)[Ti](N(C)C)(N(C)C)N(C)C IPTGOPJXEXTNCE-UHFFFAOYSA-N 0.000 description 1
- ANWKVNXHQDRSQF-UHFFFAOYSA-N C(C)C1(C=CC=C1)[Ti](N(CC)C)(N(CC)C)N(C)CC Chemical compound C(C)C1(C=CC=C1)[Ti](N(CC)C)(N(CC)C)N(C)CC ANWKVNXHQDRSQF-UHFFFAOYSA-N 0.000 description 1
- DTOHEKDIBNUSIP-UHFFFAOYSA-N C(C)C1(C=CC=C1)[Ti](OC)(OC)OC Chemical compound C(C)C1(C=CC=C1)[Ti](OC)(OC)OC DTOHEKDIBNUSIP-UHFFFAOYSA-N 0.000 description 1
- HDGRKNWTTXGATK-UHFFFAOYSA-N C(CC)C1(C=CC=C1)[Ti](OC)(OC)OC Chemical compound C(CC)C1(C=CC=C1)[Ti](OC)(OC)OC HDGRKNWTTXGATK-UHFFFAOYSA-N 0.000 description 1
- XJSOMZOHHHAEHM-UHFFFAOYSA-N C(CCC)C1(C=CC=C1)[Ti](OC)(OC)OC Chemical compound C(CCC)C1(C=CC=C1)[Ti](OC)(OC)OC XJSOMZOHHHAEHM-UHFFFAOYSA-N 0.000 description 1
- ZYMRFAAFCPNQEF-UHFFFAOYSA-N C1(C=CC=C1)[Ti](N(CC)C)(N(CC)C)N(C)CC Chemical compound C1(C=CC=C1)[Ti](N(CC)C)(N(CC)C)N(C)CC ZYMRFAAFCPNQEF-UHFFFAOYSA-N 0.000 description 1
- RWXRWFPGHUQFHW-UHFFFAOYSA-N C1(C=CC=C1)[Ti](N(CC)CC)(N(CC)CC)N(CC)CC Chemical compound C1(C=CC=C1)[Ti](N(CC)CC)(N(CC)CC)N(CC)CC RWXRWFPGHUQFHW-UHFFFAOYSA-N 0.000 description 1
- HXAAXAPDZBYKPT-UHFFFAOYSA-N CC1(C=CC=C1)[Ti](N(CC)CC)(N(CC)CC)N(CC)CC Chemical compound CC1(C=CC=C1)[Ti](N(CC)CC)(N(CC)CC)N(CC)CC HXAAXAPDZBYKPT-UHFFFAOYSA-N 0.000 description 1
- YQZCFUHFPMUPFE-UHFFFAOYSA-N CC1(C=CC=C1)[Ti](OC)(OC)OC Chemical compound CC1(C=CC=C1)[Ti](OC)(OC)OC YQZCFUHFPMUPFE-UHFFFAOYSA-N 0.000 description 1
- YMMQIRAORSQFSY-UHFFFAOYSA-N CCC(C)(C)[Ti](C(C)(C)CC)(C(C)(C)CC)C(C)(C)CC Chemical compound CCC(C)(C)[Ti](C(C)(C)CC)(C(C)(C)CC)C(C)(C)CC YMMQIRAORSQFSY-UHFFFAOYSA-N 0.000 description 1
- TXPAAJFKASNIGR-UHFFFAOYSA-N CN(C)[Ti](C1C=CC=C1)(N(C)C)N(C)C Chemical compound CN(C)[Ti](C1C=CC=C1)(N(C)C)N(C)C TXPAAJFKASNIGR-UHFFFAOYSA-N 0.000 description 1
- 239000004215 Carbon black (E152) Substances 0.000 description 1
- ZAMOUSCENKQFHK-UHFFFAOYSA-N Chlorine atom Chemical compound [Cl] ZAMOUSCENKQFHK-UHFFFAOYSA-N 0.000 description 1
- XDTMQSROBMDMFD-UHFFFAOYSA-N Cyclohexane Chemical compound C1CCCCC1 XDTMQSROBMDMFD-UHFFFAOYSA-N 0.000 description 1
- RPNUMPOLZDHAAY-UHFFFAOYSA-N Diethylenetriamine Chemical compound NCCNCCN RPNUMPOLZDHAAY-UHFFFAOYSA-N 0.000 description 1
- WHXSMMKQMYFTQS-UHFFFAOYSA-N Lithium Chemical compound [Li] WHXSMMKQMYFTQS-UHFFFAOYSA-N 0.000 description 1
- 229910052765 Lutetium Inorganic materials 0.000 description 1
- NTIZESTWPVYFNL-UHFFFAOYSA-N Methyl isobutyl ketone Chemical compound CC(C)CC(C)=O NTIZESTWPVYFNL-UHFFFAOYSA-N 0.000 description 1
- UIHCLUNTQKBZGK-UHFFFAOYSA-N Methyl isobutyl ketone Natural products CCC(C)C(C)=O UIHCLUNTQKBZGK-UHFFFAOYSA-N 0.000 description 1
- WRQNANDWMGAFTP-UHFFFAOYSA-N Methylacetoacetic acid Chemical compound COC(=O)CC(C)=O WRQNANDWMGAFTP-UHFFFAOYSA-N 0.000 description 1
- KWYHDKDOAIKMQN-UHFFFAOYSA-N N,N,N',N'-tetramethylethylenediamine Chemical compound CN(C)CCN(C)C KWYHDKDOAIKMQN-UHFFFAOYSA-N 0.000 description 1
- AHVYPIQETPWLSZ-UHFFFAOYSA-N N-methyl-pyrrolidine Natural products CN1CC=CC1 AHVYPIQETPWLSZ-UHFFFAOYSA-N 0.000 description 1
- GAWLEIWDCURQAS-UHFFFAOYSA-N N-tert-butyl-4-tert-butylimino-3-methylpent-2-en-2-amine Chemical compound C(C)(C)(C)NC(C)=C(C(C)=NC(C)(C)C)C GAWLEIWDCURQAS-UHFFFAOYSA-N 0.000 description 1
- CTQNGGLPUBDAKN-UHFFFAOYSA-N O-Xylene Chemical compound CC1=CC=CC=C1C CTQNGGLPUBDAKN-UHFFFAOYSA-N 0.000 description 1
- ZCQWOFVYLHDMMC-UHFFFAOYSA-N Oxazole Chemical compound C1=COC=N1 ZCQWOFVYLHDMMC-UHFFFAOYSA-N 0.000 description 1
- CBENFWSGALASAD-UHFFFAOYSA-N Ozone Chemical compound [O-][O+]=O CBENFWSGALASAD-UHFFFAOYSA-N 0.000 description 1
- OFBQJSOFQDEBGM-UHFFFAOYSA-N Pentane Chemical compound CCCCC OFBQJSOFQDEBGM-UHFFFAOYSA-N 0.000 description 1
- RFFFKMOABOFIDF-UHFFFAOYSA-N Pentanenitrile Chemical compound CCCCC#N RFFFKMOABOFIDF-UHFFFAOYSA-N 0.000 description 1
- ZLMJMSJWJFRBEC-UHFFFAOYSA-N Potassium Chemical compound [K] ZLMJMSJWJFRBEC-UHFFFAOYSA-N 0.000 description 1
- KJTLSVCANCCWHF-UHFFFAOYSA-N Ruthenium Chemical compound [Ru] KJTLSVCANCCWHF-UHFFFAOYSA-N 0.000 description 1
- 229910052581 Si3N4 Inorganic materials 0.000 description 1
- KEAYESYHFKHZAL-UHFFFAOYSA-N Sodium Chemical compound [Na] KEAYESYHFKHZAL-UHFFFAOYSA-N 0.000 description 1
- FZWLAAWBMGSTSO-UHFFFAOYSA-N Thiazole Chemical compound C1=CSC=N1 FZWLAAWBMGSTSO-UHFFFAOYSA-N 0.000 description 1
- GWEVSGVZZGPLCZ-UHFFFAOYSA-N Titan oxide Chemical compound O=[Ti]=O GWEVSGVZZGPLCZ-UHFFFAOYSA-N 0.000 description 1
- MOSURRVHVKOQHA-UHFFFAOYSA-N [Tb].[Dy] Chemical compound [Tb].[Dy] MOSURRVHVKOQHA-UHFFFAOYSA-N 0.000 description 1
- 150000001242 acetic acid derivatives Chemical class 0.000 description 1
- BTGRAWJCKBQKAO-UHFFFAOYSA-N adiponitrile Chemical compound N#CCCCCC#N BTGRAWJCKBQKAO-UHFFFAOYSA-N 0.000 description 1
- 239000003905 agrochemical Substances 0.000 description 1
- 150000001298 alcohols Chemical class 0.000 description 1
- 229910052784 alkaline earth metal Inorganic materials 0.000 description 1
- 125000004183 alkoxy alkyl group Chemical group 0.000 description 1
- 125000005103 alkyl silyl group Chemical group 0.000 description 1
- 125000005263 alkylenediamine group Chemical group 0.000 description 1
- 125000000304 alkynyl group Chemical group 0.000 description 1
- 230000004075 alteration Effects 0.000 description 1
- 150000001412 amines Chemical class 0.000 description 1
- 238000000137 annealing Methods 0.000 description 1
- 238000000149 argon plasma sintering Methods 0.000 description 1
- QVGXLLKOCUKJST-UHFFFAOYSA-N atomic oxygen Chemical compound [O] QVGXLLKOCUKJST-UHFFFAOYSA-N 0.000 description 1
- 150000001540 azides Chemical class 0.000 description 1
- YLBGXUJFSMDKDD-UHFFFAOYSA-N benzene-1,4-dicarbonitrile;pyridine Chemical compound C1=CC=NC=C1.N#CC1=CC=C(C#N)C=C1 YLBGXUJFSMDKDD-UHFFFAOYSA-N 0.000 description 1
- JFDZBHWFFUWGJE-UHFFFAOYSA-N benzonitrile Chemical compound N#CC1=CC=CC=C1 JFDZBHWFFUWGJE-UHFFFAOYSA-N 0.000 description 1
- LEQVBGOJANWCKL-UHFFFAOYSA-N bis(trimethylsilyl)azanide;cobalt(2+) Chemical compound [Co+2].C[Si](C)(C)[N-][Si](C)(C)C.C[Si](C)(C)[N-][Si](C)(C)C LEQVBGOJANWCKL-UHFFFAOYSA-N 0.000 description 1
- 238000009835 boiling Methods 0.000 description 1
- 230000005587 bubbling Effects 0.000 description 1
- YHWCPXVTRSHPNY-UHFFFAOYSA-N butan-1-olate;titanium(4+) Chemical compound [Ti+4].CCCC[O-].CCCC[O-].CCCC[O-].CCCC[O-] YHWCPXVTRSHPNY-UHFFFAOYSA-N 0.000 description 1
- KVNRLNFWIYMESJ-UHFFFAOYSA-N butyronitrile Chemical compound CCCC#N KVNRLNFWIYMESJ-UHFFFAOYSA-N 0.000 description 1
- 150000001721 carbon Chemical group 0.000 description 1
- 239000012159 carrier gas Substances 0.000 description 1
- 239000007795 chemical reaction product Substances 0.000 description 1
- 239000000460 chlorine Substances 0.000 description 1
- 229910052801 chlorine Inorganic materials 0.000 description 1
- 239000011248 coating agent Substances 0.000 description 1
- 238000000576 coating method Methods 0.000 description 1
- 238000011109 contamination Methods 0.000 description 1
- 150000003983 crown ethers Chemical class 0.000 description 1
- 125000004093 cyano group Chemical group *C#N 0.000 description 1
- 125000004122 cyclic group Chemical group 0.000 description 1
- MGWYSXZGBRHJNE-UHFFFAOYSA-N cyclohexane-1,4-dicarbonitrile Chemical compound N#CC1CCC(C#N)CC1 MGWYSXZGBRHJNE-UHFFFAOYSA-N 0.000 description 1
- VBWIZSYFQSOUFQ-UHFFFAOYSA-N cyclohexanecarbonitrile Chemical compound N#CC1CCCCC1 VBWIZSYFQSOUFQ-UHFFFAOYSA-N 0.000 description 1
- 125000004985 dialkyl amino alkyl group Chemical group 0.000 description 1
- 125000005265 dialkylamine group Chemical group 0.000 description 1
- 125000004984 dialkylaminoalkoxy group Chemical group 0.000 description 1
- 150000004985 diamines Chemical class 0.000 description 1
- UNTITLLXXOKDTB-UHFFFAOYSA-N dibenzo-24-crown-8 Chemical compound O1CCOCCOCCOC2=CC=CC=C2OCCOCCOCCOC2=CC=CC=C21 UNTITLLXXOKDTB-UHFFFAOYSA-N 0.000 description 1
- BBGKDYHZQOSNMU-UHFFFAOYSA-N dicyclohexano-18-crown-6 Chemical compound O1CCOCCOC2CCCCC2OCCOCCOC2CCCCC21 BBGKDYHZQOSNMU-UHFFFAOYSA-N 0.000 description 1
- QMLGNDFKJAFKGZ-UHFFFAOYSA-N dicyclohexano-24-crown-8 Chemical compound O1CCOCCOCCOC2CCCCC2OCCOCCOCCOC2CCCCC21 QMLGNDFKJAFKGZ-UHFFFAOYSA-N 0.000 description 1
- HPNMFZURTQLUMO-UHFFFAOYSA-N diethylamine Chemical compound CCNCC HPNMFZURTQLUMO-UHFFFAOYSA-N 0.000 description 1
- NNUZNYLOAGGAIU-UHFFFAOYSA-N diethylazanide titanium(3+) Chemical compound CCN(CC)[Ti](N(CC)CC)N(CC)CC NNUZNYLOAGGAIU-UHFFFAOYSA-N 0.000 description 1
- 229940043279 diisopropylamine Drugs 0.000 description 1
- 125000005594 diketone group Chemical group 0.000 description 1
- CRGGFGFWZPSXPP-UHFFFAOYSA-N dimethylazanide;titanium(3+) Chemical compound [Ti+3].C[N-]C.C[N-]C.C[N-]C CRGGFGFWZPSXPP-UHFFFAOYSA-N 0.000 description 1
- HPYNZHMRTTWQTB-UHFFFAOYSA-N dimethylpyridine Natural products CC1=CC=CN=C1C HPYNZHMRTTWQTB-UHFFFAOYSA-N 0.000 description 1
- 239000003814 drug Substances 0.000 description 1
- 150000002170 ethers Chemical class 0.000 description 1
- XYIBRDXRRQCHLP-UHFFFAOYSA-N ethyl acetoacetate Chemical compound CCOC(=O)CC(C)=O XYIBRDXRRQCHLP-UHFFFAOYSA-N 0.000 description 1
- LIWAQLJGPBVORC-UHFFFAOYSA-N ethylmethylamine Chemical compound CCNC LIWAQLJGPBVORC-UHFFFAOYSA-N 0.000 description 1
- 239000000835 fiber Substances 0.000 description 1
- 235000019253 formic acid Nutrition 0.000 description 1
- 239000003205 fragrance Substances 0.000 description 1
- 238000007429 general method Methods 0.000 description 1
- ZTOMUSMDRMJOTH-UHFFFAOYSA-N glutaronitrile Chemical compound N#CCCCC#N ZTOMUSMDRMJOTH-UHFFFAOYSA-N 0.000 description 1
- 229910000449 hafnium oxide Inorganic materials 0.000 description 1
- WIHZLLGSGQNAGK-UHFFFAOYSA-N hafnium(4+);oxygen(2-) Chemical compound [O-2].[O-2].[Hf+4] WIHZLLGSGQNAGK-UHFFFAOYSA-N 0.000 description 1
- NGAZZOYFWWSOGK-UHFFFAOYSA-N heptan-3-one Chemical compound CCCCC(=O)CC NGAZZOYFWWSOGK-UHFFFAOYSA-N 0.000 description 1
- 150000002391 heterocyclic compounds Chemical class 0.000 description 1
- FUZZWVXGSFPDMH-UHFFFAOYSA-M hexanoate Chemical compound CCCCCC([O-])=O FUZZWVXGSFPDMH-UHFFFAOYSA-M 0.000 description 1
- 150000004678 hydrides Chemical class 0.000 description 1
- 150000004679 hydroxides Chemical class 0.000 description 1
- 125000001841 imino group Chemical group [H]N=* 0.000 description 1
- 239000011261 inert gas Substances 0.000 description 1
- 230000010354 integration Effects 0.000 description 1
- 238000007733 ion plating Methods 0.000 description 1
- JJWLVOIRVHMVIS-UHFFFAOYSA-N isopropylamine Chemical compound CC(C)N JJWLVOIRVHMVIS-UHFFFAOYSA-N 0.000 description 1
- 150000002576 ketones Chemical class 0.000 description 1
- 239000010410 layer Substances 0.000 description 1
- OHSVLFRHMCKCQY-UHFFFAOYSA-N lutetium atom Chemical compound [Lu] OHSVLFRHMCKCQY-UHFFFAOYSA-N 0.000 description 1
- 229910001507 metal halide Inorganic materials 0.000 description 1
- 150000005309 metal halides Chemical class 0.000 description 1
- 229910001960 metal nitrate Inorganic materials 0.000 description 1
- NBTOZLQBSIZIKS-UHFFFAOYSA-N methoxide Chemical compound [O-]C NBTOZLQBSIZIKS-UHFFFAOYSA-N 0.000 description 1
- GYNNXHKOJHMOHS-UHFFFAOYSA-N methyl-cycloheptane Natural products CC1CCCCCC1 GYNNXHKOJHMOHS-UHFFFAOYSA-N 0.000 description 1
- 239000012046 mixed solvent Substances 0.000 description 1
- 238000002156 mixing Methods 0.000 description 1
- DNIAPMSPPWPWGF-UHFFFAOYSA-N monopropylene glycol Natural products CC(O)CO DNIAPMSPPWPWGF-UHFFFAOYSA-N 0.000 description 1
- DILRJUIACXKSQE-UHFFFAOYSA-N n',n'-dimethylethane-1,2-diamine Chemical compound CN(C)CCN DILRJUIACXKSQE-UHFFFAOYSA-N 0.000 description 1
- DWFKOMDBEKIATP-UHFFFAOYSA-N n'-[2-[2-(dimethylamino)ethyl-methylamino]ethyl]-n,n,n'-trimethylethane-1,2-diamine Chemical compound CN(C)CCN(C)CCN(C)CCN(C)C DWFKOMDBEKIATP-UHFFFAOYSA-N 0.000 description 1
- LSHROXHEILXKHM-UHFFFAOYSA-N n'-[2-[2-[2-(2-aminoethylamino)ethylamino]ethylamino]ethyl]ethane-1,2-diamine Chemical compound NCCNCCNCCNCCNCCN LSHROXHEILXKHM-UHFFFAOYSA-N 0.000 description 1
- GVWISOJSERXQBM-UHFFFAOYSA-N n-methylpropan-1-amine Chemical compound CCCNC GVWISOJSERXQBM-UHFFFAOYSA-N 0.000 description 1
- XHFGWHUWQXTGAT-UHFFFAOYSA-N n-methylpropan-2-amine Chemical compound CNC(C)C XHFGWHUWQXTGAT-UHFFFAOYSA-N 0.000 description 1
- POQOWGAHVMCAJX-UHFFFAOYSA-N n-propan-2-yl-4-propan-2-yliminopent-2-en-2-amine Chemical compound CC(C)NC(C)=CC(C)=NC(C)C POQOWGAHVMCAJX-UHFFFAOYSA-N 0.000 description 1
- JGSNEDLVBCUMDQ-UHFFFAOYSA-N n-tert-butyl-4-tert-butyliminopent-2-en-2-amine Chemical compound CC(C)(C)NC(C)=CC(C)=NC(C)(C)C JGSNEDLVBCUMDQ-UHFFFAOYSA-N 0.000 description 1
- SLCVBVWXLSEKPL-UHFFFAOYSA-N neopentyl glycol Chemical compound OCC(C)(C)CO SLCVBVWXLSEKPL-UHFFFAOYSA-N 0.000 description 1
- 150000002825 nitriles Chemical class 0.000 description 1
- JCXJVPUVTGWSNB-UHFFFAOYSA-N nitrogen dioxide Inorganic materials O=[N]=O JCXJVPUVTGWSNB-UHFFFAOYSA-N 0.000 description 1
- WSGCRAOTEDLMFQ-UHFFFAOYSA-N nonan-5-one Chemical compound CCCCC(=O)CCCC WSGCRAOTEDLMFQ-UHFFFAOYSA-N 0.000 description 1
- TVMXDCGIABBOFY-UHFFFAOYSA-N octane Chemical compound CCCCCCCC TVMXDCGIABBOFY-UHFFFAOYSA-N 0.000 description 1
- BTNXBLUGMAMSSH-UHFFFAOYSA-N octanedinitrile Chemical compound N#CCCCCCCC#N BTNXBLUGMAMSSH-UHFFFAOYSA-N 0.000 description 1
- 239000013110 organic ligand Substances 0.000 description 1
- OOFGXDQWDNJDIS-UHFFFAOYSA-N oxathiolane Chemical compound C1COSC1 OOFGXDQWDNJDIS-UHFFFAOYSA-N 0.000 description 1
- 230000003647 oxidation Effects 0.000 description 1
- 238000007254 oxidation reaction Methods 0.000 description 1
- 238000006864 oxidative decomposition reaction Methods 0.000 description 1
- 239000011224 oxide ceramic Substances 0.000 description 1
- 229910052574 oxide ceramic Inorganic materials 0.000 description 1
- 239000001301 oxygen Substances 0.000 description 1
- RVTZCBVAJQQJTK-UHFFFAOYSA-N oxygen(2-);zirconium(4+) Chemical compound [O-2].[O-2].[Zr+4] RVTZCBVAJQQJTK-UHFFFAOYSA-N 0.000 description 1
- UKODFQOELJFMII-UHFFFAOYSA-N pentamethyldiethylenetriamine Chemical compound CN(C)CCN(C)CCN(C)C UKODFQOELJFMII-UHFFFAOYSA-N 0.000 description 1
- GTCCGKPBSJZVRZ-UHFFFAOYSA-N pentane-2,4-diol Chemical compound CC(O)CC(C)O GTCCGKPBSJZVRZ-UHFFFAOYSA-N 0.000 description 1
- 125000005005 perfluorohexyl group Chemical group FC(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)* 0.000 description 1
- 230000000704 physical effect Effects 0.000 description 1
- 229960005235 piperonyl butoxide Drugs 0.000 description 1
- 238000009832 plasma treatment Methods 0.000 description 1
- 229920000642 polymer Polymers 0.000 description 1
- 229920000166 polytrimethylene carbonate Polymers 0.000 description 1
- XRVCFZPJAHWYTB-UHFFFAOYSA-N prenderol Chemical compound CCC(CC)(CO)CO XRVCFZPJAHWYTB-UHFFFAOYSA-N 0.000 description 1
- 229950006800 prenderol Drugs 0.000 description 1
- 238000012545 processing Methods 0.000 description 1
- OGHBATFHNDZKSO-UHFFFAOYSA-N propan-2-olate Chemical compound CC(C)[O-] OGHBATFHNDZKSO-UHFFFAOYSA-N 0.000 description 1
- 229960004063 propylene glycol Drugs 0.000 description 1
- 235000013772 propylene glycol Nutrition 0.000 description 1
- ASRAWSBMDXVNLX-UHFFFAOYSA-N pyrazolynate Chemical compound C=1C=C(Cl)C=C(Cl)C=1C(=O)C=1C(C)=NN(C)C=1OS(=O)(=O)C1=CC=C(C)C=C1 ASRAWSBMDXVNLX-UHFFFAOYSA-N 0.000 description 1
- UMJSCPRVCHMLSP-UHFFFAOYSA-N pyridine Natural products COC1=CC=CN=C1 UMJSCPRVCHMLSP-UHFFFAOYSA-N 0.000 description 1
- 238000000197 pyrolysis Methods 0.000 description 1
- 239000000376 reactant Substances 0.000 description 1
- 230000009257 reactivity Effects 0.000 description 1
- 229910052707 ruthenium Inorganic materials 0.000 description 1
- 229910001925 ruthenium oxide Inorganic materials 0.000 description 1
- WOCIAKWEIIZHES-UHFFFAOYSA-N ruthenium(iv) oxide Chemical compound O=[Ru]=O WOCIAKWEIIZHES-UHFFFAOYSA-N 0.000 description 1
- 150000003839 salts Chemical class 0.000 description 1
- BHRZNVHARXXAHW-UHFFFAOYSA-N sec-butylamine Chemical compound CCC(C)N BHRZNVHARXXAHW-UHFFFAOYSA-N 0.000 description 1
- HQVNEWCFYHHQES-UHFFFAOYSA-N silicon nitride Chemical compound N12[Si]34N5[Si]62N3[Si]51N64 HQVNEWCFYHHQES-UHFFFAOYSA-N 0.000 description 1
- 125000004469 siloxy group Chemical group [SiH3]O* 0.000 description 1
- ODZPKZBBUMBTMG-UHFFFAOYSA-N sodium amide Chemical compound [NH2-].[Na+] ODZPKZBBUMBTMG-UHFFFAOYSA-N 0.000 description 1
- 239000012312 sodium hydride Substances 0.000 description 1
- 229910000104 sodium hydride Inorganic materials 0.000 description 1
- 238000003980 solgel method Methods 0.000 description 1
- 230000002269 spontaneous effect Effects 0.000 description 1
- 238000004544 sputter deposition Methods 0.000 description 1
- 230000002194 synthesizing effect Effects 0.000 description 1
- MZLGASXMSKOWSE-UHFFFAOYSA-N tantalum nitride Chemical compound [Ta]#N MZLGASXMSKOWSE-UHFFFAOYSA-N 0.000 description 1
- YBRBMKDOPFTVDT-UHFFFAOYSA-N tert-butylamine Chemical compound CC(C)(C)N YBRBMKDOPFTVDT-UHFFFAOYSA-N 0.000 description 1
- FAGUFWYHJQFNRV-UHFFFAOYSA-N tetraethylenepentamine Chemical compound NCCNCCNCCNCCN FAGUFWYHJQFNRV-UHFFFAOYSA-N 0.000 description 1
- ZUHZGEOKBKGPSW-UHFFFAOYSA-N tetraglyme Chemical compound COCCOCCOCCOCCOC ZUHZGEOKBKGPSW-UHFFFAOYSA-N 0.000 description 1
- 150000003609 titanium compounds Chemical class 0.000 description 1
- JMXKSZRRTHPKDL-UHFFFAOYSA-N titanium ethoxide Chemical compound [Ti+4].CC[O-].CC[O-].CC[O-].CC[O-] JMXKSZRRTHPKDL-UHFFFAOYSA-N 0.000 description 1
- OGIDPMRJRNCKJF-UHFFFAOYSA-N titanium oxide Inorganic materials [Ti]=O OGIDPMRJRNCKJF-UHFFFAOYSA-N 0.000 description 1
- VXUYXOFXAQZZMF-UHFFFAOYSA-N titanium(IV) isopropoxide Chemical compound CC(C)O[Ti](OC(C)C)(OC(C)C)OC(C)C VXUYXOFXAQZZMF-UHFFFAOYSA-N 0.000 description 1
- 125000005270 trialkylamine group Chemical group 0.000 description 1
- 125000002023 trifluoromethyl group Chemical group FC(F)(F)* 0.000 description 1
- 239000008096 xylene Substances 0.000 description 1
- 229910001928 zirconium oxide Inorganic materials 0.000 description 1
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C251/00—Compounds containing nitrogen atoms doubly-bound to a carbon skeleton
- C07C251/02—Compounds containing nitrogen atoms doubly-bound to a carbon skeleton containing imino groups
- C07C251/04—Compounds containing nitrogen atoms doubly-bound to a carbon skeleton containing imino groups having carbon atoms of imino groups bound to hydrogen atoms or to acyclic carbon atoms
- C07C251/06—Compounds containing nitrogen atoms doubly-bound to a carbon skeleton containing imino groups having carbon atoms of imino groups bound to hydrogen atoms or to acyclic carbon atoms to carbon atoms of a saturated carbon skeleton
- C07C251/08—Compounds containing nitrogen atoms doubly-bound to a carbon skeleton containing imino groups having carbon atoms of imino groups bound to hydrogen atoms or to acyclic carbon atoms to carbon atoms of a saturated carbon skeleton being acyclic
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C251/00—Compounds containing nitrogen atoms doubly-bound to a carbon skeleton
- C07C251/02—Compounds containing nitrogen atoms doubly-bound to a carbon skeleton containing imino groups
- C07C251/04—Compounds containing nitrogen atoms doubly-bound to a carbon skeleton containing imino groups having carbon atoms of imino groups bound to hydrogen atoms or to acyclic carbon atoms
- C07C251/10—Compounds containing nitrogen atoms doubly-bound to a carbon skeleton containing imino groups having carbon atoms of imino groups bound to hydrogen atoms or to acyclic carbon atoms to carbon atoms of an unsaturated carbon skeleton
- C07C251/12—Compounds containing nitrogen atoms doubly-bound to a carbon skeleton containing imino groups having carbon atoms of imino groups bound to hydrogen atoms or to acyclic carbon atoms to carbon atoms of an unsaturated carbon skeleton being acyclic
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C251/00—Compounds containing nitrogen atoms doubly-bound to a carbon skeleton
- C07C251/72—Hydrazones
- C07C251/74—Hydrazones having doubly-bound carbon atoms of hydrazone groups bound to hydrogen atoms or to acyclic carbon atoms
- C07C251/76—Hydrazones having doubly-bound carbon atoms of hydrazone groups bound to hydrogen atoms or to acyclic carbon atoms to carbon atoms of a saturated carbon skeleton
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C251/00—Compounds containing nitrogen atoms doubly-bound to a carbon skeleton
- C07C251/72—Hydrazones
- C07C251/74—Hydrazones having doubly-bound carbon atoms of hydrazone groups bound to hydrogen atoms or to acyclic carbon atoms
- C07C251/78—Hydrazones having doubly-bound carbon atoms of hydrazone groups bound to hydrogen atoms or to acyclic carbon atoms to carbon atoms of an unsaturated carbon skeleton
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C257/00—Compounds containing carboxyl groups, the doubly-bound oxygen atom of a carboxyl group being replaced by a doubly-bound nitrogen atom, this nitrogen atom not being further bound to an oxygen atom, e.g. imino-ethers, amidines
- C07C257/10—Compounds containing carboxyl groups, the doubly-bound oxygen atom of a carboxyl group being replaced by a doubly-bound nitrogen atom, this nitrogen atom not being further bound to an oxygen atom, e.g. imino-ethers, amidines with replacement of the other oxygen atom of the carboxyl group by nitrogen atoms, e.g. amidines
- C07C257/14—Compounds containing carboxyl groups, the doubly-bound oxygen atom of a carboxyl group being replaced by a doubly-bound nitrogen atom, this nitrogen atom not being further bound to an oxygen atom, e.g. imino-ethers, amidines with replacement of the other oxygen atom of the carboxyl group by nitrogen atoms, e.g. amidines having carbon atoms of amidino groups bound to acyclic carbon atoms
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07F—ACYCLIC, CARBOCYCLIC OR HETEROCYCLIC COMPOUNDS CONTAINING ELEMENTS OTHER THAN CARBON, HYDROGEN, HALOGEN, OXYGEN, NITROGEN, SULFUR, SELENIUM OR TELLURIUM
- C07F15/00—Compounds containing elements of Groups 8, 9, 10 or 18 of the Periodic Table
- C07F15/06—Cobalt compounds
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07F—ACYCLIC, CARBOCYCLIC OR HETEROCYCLIC COMPOUNDS CONTAINING ELEMENTS OTHER THAN CARBON, HYDROGEN, HALOGEN, OXYGEN, NITROGEN, SULFUR, SELENIUM OR TELLURIUM
- C07F19/00—Metal compounds according to more than one of main groups C07F1/00 - C07F17/00
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07F—ACYCLIC, CARBOCYCLIC OR HETEROCYCLIC COMPOUNDS CONTAINING ELEMENTS OTHER THAN CARBON, HYDROGEN, HALOGEN, OXYGEN, NITROGEN, SULFUR, SELENIUM OR TELLURIUM
- C07F7/00—Compounds containing elements of Groups 4 or 14 of the Periodic Table
- C07F7/02—Silicon compounds
- C07F7/08—Compounds having one or more C—Si linkages
- C07F7/0803—Compounds with Si-C or Si-Si linkages
- C07F7/081—Compounds with Si-C or Si-Si linkages comprising at least one atom selected from the elements N, O, halogen, S, Se or Te
-
- C—CHEMISTRY; METALLURGY
- C23—COATING METALLIC MATERIAL; COATING MATERIAL WITH METALLIC MATERIAL; CHEMICAL SURFACE TREATMENT; DIFFUSION TREATMENT OF METALLIC MATERIAL; COATING BY VACUUM EVAPORATION, BY SPUTTERING, BY ION IMPLANTATION OR BY CHEMICAL VAPOUR DEPOSITION, IN GENERAL; INHIBITING CORROSION OF METALLIC MATERIAL OR INCRUSTATION IN GENERAL
- C23C—COATING METALLIC MATERIAL; COATING MATERIAL WITH METALLIC MATERIAL; SURFACE TREATMENT OF METALLIC MATERIAL BY DIFFUSION INTO THE SURFACE, BY CHEMICAL CONVERSION OR SUBSTITUTION; COATING BY VACUUM EVAPORATION, BY SPUTTERING, BY ION IMPLANTATION OR BY CHEMICAL VAPOUR DEPOSITION, IN GENERAL
- C23C16/00—Chemical coating by decomposition of gaseous compounds, without leaving reaction products of surface material in the coating, i.e. chemical vapour deposition [CVD] processes
- C23C16/06—Chemical coating by decomposition of gaseous compounds, without leaving reaction products of surface material in the coating, i.e. chemical vapour deposition [CVD] processes characterised by the deposition of metallic material
- C23C16/18—Chemical coating by decomposition of gaseous compounds, without leaving reaction products of surface material in the coating, i.e. chemical vapour deposition [CVD] processes characterised by the deposition of metallic material from metallo-organic compounds
-
- C—CHEMISTRY; METALLURGY
- C23—COATING METALLIC MATERIAL; COATING MATERIAL WITH METALLIC MATERIAL; CHEMICAL SURFACE TREATMENT; DIFFUSION TREATMENT OF METALLIC MATERIAL; COATING BY VACUUM EVAPORATION, BY SPUTTERING, BY ION IMPLANTATION OR BY CHEMICAL VAPOUR DEPOSITION, IN GENERAL; INHIBITING CORROSION OF METALLIC MATERIAL OR INCRUSTATION IN GENERAL
- C23C—COATING METALLIC MATERIAL; COATING MATERIAL WITH METALLIC MATERIAL; SURFACE TREATMENT OF METALLIC MATERIAL BY DIFFUSION INTO THE SURFACE, BY CHEMICAL CONVERSION OR SUBSTITUTION; COATING BY VACUUM EVAPORATION, BY SPUTTERING, BY ION IMPLANTATION OR BY CHEMICAL VAPOUR DEPOSITION, IN GENERAL
- C23C16/00—Chemical coating by decomposition of gaseous compounds, without leaving reaction products of surface material in the coating, i.e. chemical vapour deposition [CVD] processes
- C23C16/44—Chemical coating by decomposition of gaseous compounds, without leaving reaction products of surface material in the coating, i.e. chemical vapour deposition [CVD] processes characterised by the method of coating
- C23C16/455—Chemical coating by decomposition of gaseous compounds, without leaving reaction products of surface material in the coating, i.e. chemical vapour deposition [CVD] processes characterised by the method of coating characterised by the method used for introducing gases into reaction chamber or for modifying gas flows in reaction chamber
- C23C16/45523—Pulsed gas flow or change of composition over time
- C23C16/45525—Atomic layer deposition [ALD]
- C23C16/45553—Atomic layer deposition [ALD] characterized by the use of precursors specially adapted for ALD
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01L—SEMICONDUCTOR DEVICES NOT COVERED BY CLASS H10
- H01L21/00—Processes or apparatus adapted for the manufacture or treatment of semiconductor or solid state devices or of parts thereof
- H01L21/02—Manufacture or treatment of semiconductor devices or of parts thereof
- H01L21/04—Manufacture or treatment of semiconductor devices or of parts thereof the devices having potential barriers, e.g. a PN junction, depletion layer or carrier concentration layer
- H01L21/18—Manufacture or treatment of semiconductor devices or of parts thereof the devices having potential barriers, e.g. a PN junction, depletion layer or carrier concentration layer the devices having semiconductor bodies comprising elements of Group IV of the Periodic Table or AIIIBV compounds with or without impurities, e.g. doping materials
- H01L21/28—Manufacture of electrodes on semiconductor bodies using processes or apparatus not provided for in groups H01L21/20 - H01L21/268
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01L—SEMICONDUCTOR DEVICES NOT COVERED BY CLASS H10
- H01L21/00—Processes or apparatus adapted for the manufacture or treatment of semiconductor or solid state devices or of parts thereof
- H01L21/02—Manufacture or treatment of semiconductor devices or of parts thereof
- H01L21/04—Manufacture or treatment of semiconductor devices or of parts thereof the devices having potential barriers, e.g. a PN junction, depletion layer or carrier concentration layer
- H01L21/18—Manufacture or treatment of semiconductor devices or of parts thereof the devices having potential barriers, e.g. a PN junction, depletion layer or carrier concentration layer the devices having semiconductor bodies comprising elements of Group IV of the Periodic Table or AIIIBV compounds with or without impurities, e.g. doping materials
- H01L21/28—Manufacture of electrodes on semiconductor bodies using processes or apparatus not provided for in groups H01L21/20 - H01L21/268
- H01L21/283—Deposition of conductive or insulating materials for electrodes conducting electric current
- H01L21/285—Deposition of conductive or insulating materials for electrodes conducting electric current from a gas or vapour, e.g. condensation
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01L—SEMICONDUCTOR DEVICES NOT COVERED BY CLASS H10
- H01L21/00—Processes or apparatus adapted for the manufacture or treatment of semiconductor or solid state devices or of parts thereof
- H01L21/02—Manufacture or treatment of semiconductor devices or of parts thereof
- H01L21/04—Manufacture or treatment of semiconductor devices or of parts thereof the devices having potential barriers, e.g. a PN junction, depletion layer or carrier concentration layer
- H01L21/18—Manufacture or treatment of semiconductor devices or of parts thereof the devices having potential barriers, e.g. a PN junction, depletion layer or carrier concentration layer the devices having semiconductor bodies comprising elements of Group IV of the Periodic Table or AIIIBV compounds with or without impurities, e.g. doping materials
- H01L21/28—Manufacture of electrodes on semiconductor bodies using processes or apparatus not provided for in groups H01L21/20 - H01L21/268
- H01L21/283—Deposition of conductive or insulating materials for electrodes conducting electric current
- H01L21/285—Deposition of conductive or insulating materials for electrodes conducting electric current from a gas or vapour, e.g. condensation
- H01L21/28506—Deposition of conductive or insulating materials for electrodes conducting electric current from a gas or vapour, e.g. condensation of conductive layers
- H01L21/28512—Deposition of conductive or insulating materials for electrodes conducting electric current from a gas or vapour, e.g. condensation of conductive layers on semiconductor bodies comprising elements of Group IV of the Periodic Table
- H01L21/28556—Deposition of conductive or insulating materials for electrodes conducting electric current from a gas or vapour, e.g. condensation of conductive layers on semiconductor bodies comprising elements of Group IV of the Periodic Table by chemical means, e.g. CVD, LPCVD, PECVD, laser CVD
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01L—SEMICONDUCTOR DEVICES NOT COVERED BY CLASS H10
- H01L21/00—Processes or apparatus adapted for the manufacture or treatment of semiconductor or solid state devices or of parts thereof
- H01L21/02—Manufacture or treatment of semiconductor devices or of parts thereof
- H01L21/04—Manufacture or treatment of semiconductor devices or of parts thereof the devices having potential barriers, e.g. a PN junction, depletion layer or carrier concentration layer
- H01L21/18—Manufacture or treatment of semiconductor devices or of parts thereof the devices having potential barriers, e.g. a PN junction, depletion layer or carrier concentration layer the devices having semiconductor bodies comprising elements of Group IV of the Periodic Table or AIIIBV compounds with or without impurities, e.g. doping materials
- H01L21/28—Manufacture of electrodes on semiconductor bodies using processes or apparatus not provided for in groups H01L21/20 - H01L21/268
- H01L21/283—Deposition of conductive or insulating materials for electrodes conducting electric current
- H01L21/285—Deposition of conductive or insulating materials for electrodes conducting electric current from a gas or vapour, e.g. condensation
- H01L21/28506—Deposition of conductive or insulating materials for electrodes conducting electric current from a gas or vapour, e.g. condensation of conductive layers
- H01L21/28512—Deposition of conductive or insulating materials for electrodes conducting electric current from a gas or vapour, e.g. condensation of conductive layers on semiconductor bodies comprising elements of Group IV of the Periodic Table
- H01L21/28568—Deposition of conductive or insulating materials for electrodes conducting electric current from a gas or vapour, e.g. condensation of conductive layers on semiconductor bodies comprising elements of Group IV of the Periodic Table the conductive layers comprising transition metals
Definitions
- the present invention relates to a novel alkoxide compound, a raw material for forming a thin film containing the compound, a method for producing a thin film using the raw material for forming a thin film, and a novel alcohol compound.
- Thin film materials containing metal elements are applied to various applications because they exhibit electrical characteristics and optical characteristics.
- copper and copper-containing thin films are applied as LSI wiring materials because of their high conductivity, high electromigration resistance, and high melting point.
- Nickel and nickel-containing thin films are mainly used for members of electronic parts such as resistance films and barrier films, members for recording media such as magnetic films, and members for thin film solar cells such as electrodes.
- Cobalt and cobalt-containing thin films are used for electrode films, resistance films, adhesive films, magnetic tapes, carbide tool members, and the like.
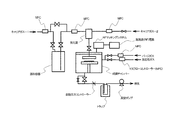
- Examples of the method for producing the thin film include a sputtering method, an ion plating method, a MOD method such as a coating pyrolysis method and a sol-gel method, a chemical vapor deposition method, etc., but has excellent composition controllability and step coverage. Since it has many advantages such as being suitable for mass production and being capable of hybrid integration, chemical vapor deposition (hereinafter sometimes simply referred to as CVD) method including ALD (Atomic Layer Deposition) method Is the optimal manufacturing process.
- CVD chemical vapor deposition
- ALD Atomic Layer Deposition
- Patent Document 1 discloses a third grade of nickel that can be used as a raw material for forming a nickel-containing thin film by MOCVD. Amino alkoxide compounds are disclosed. Patent Document 2 discloses a tertiary aminoalkoxide compound of cobalt that can be used as a raw material for forming a cobalt-containing thin film by MOCVD. Furthermore, Patent Document 3 discloses a tertiary aminoalkoxide compound of copper that can be used as a raw material for forming a copper-containing thin film by a chemical vapor deposition method. Further, Non-Patent Document 1 discloses a tertiary imide alkoxide compound of copper, nickel, cobalt, iron, manganese and chromium.
- JP 2008-537947 A Korean Registered Patent No. 10-0675983 JP 2006-328019 A
- the properties required for a compound (precursor) suitable for the raw material are that there is no pyrophoric property and high thermal stability.
- the high thermal stability of the precursor is extremely important. None of the conventional alkoxide compounds can be satisfactorily satisfied in terms of thermal stability.
- the present invention provides an alkoxide compound represented by the following general formula (I), a raw material for forming a thin film containing the alkoxide compound, and a method for producing a thin film using the raw material.
- an alkoxide compound represented by the following general formula (I) a raw material for forming a thin film containing the alkoxide compound, and a method for producing a thin film using the raw material.
- R 4 represents a group represented by the formula
- R 1 ⁇ R 3 are each independently hydrogen, a hydrocarbon group or the following general formula -C 1 ⁇ 12 (X-1) ⁇
- X-8 Represents a hydrocarbon group having 1 to 12 carbon atoms or a group represented by the following general formulas (X-1) to (X-8), provided that R 1 is a methyl group and R 2 is a methyl group or
- R 3 is hydrogen, a hydrocarbon group having 4 to 12 carbon atoms, or a group represented by the following general formulas (X-1) to (X-8):
- L represents hydrogen, halogen, hydroxyl group, amino group, azide group, phosphide group, nitrile group, carbonyl group, hydrocarbon group having 1 to 12 carbon atoms, or the following general formulas (L-1) to (L-13)
- M represents a metal atom or a silicon atom
- n represents an integer of 1 or more
- m represents an integer of 0 or
- R X1 to R X12 each independently represents hydrogen or a hydrocarbon group having 1 to 12 carbon atoms, and A 1 to A 3 each represents an alkanediyl group having 1 to 6 carbon atoms.
- R L1 to R L31 each independently represents hydrogen or a hydrocarbon group having 1 to 12 carbon atoms, and A 4 to A 7 each represents an alkanediyl group having 1 to 6 carbon atoms.
- the present invention also provides an alcohol compound represented by the following general formula (II).
- R 5 to R 7 each independently represents hydrogen, a hydrocarbon group having 1 to 12 carbon atoms, or a group represented by the following general formulas (Y-1) to (Y-8):
- R 8 Represents a hydrocarbon group having 1 to 12 carbon atoms or a group represented by the following general formulas (Y-1) to (Y-8), provided that R 5 is a methyl group and R 6 is a methyl group or
- R 7 is hydrogen, a hydrocarbon group having 4 to 12 carbon atoms, or a group represented by the following general formulas (Y-1) to (Y-8).
- R Y1 to R Y12 each independently represents hydrogen or a hydrocarbon group having 1 to 12 carbon atoms, and A 8 to A 10 each represents an alkanediyl group having 1 to 6 carbon atoms.
- an alkoxide compound that is not pyrophoric and has high thermal stability can be obtained.
- a novel alcohol compound can be provided.
- FIG. 1 is a schematic view showing an example of an apparatus for chemical vapor deposition used in the method for producing a thin film containing a metal according to the present invention.
- FIG. 2 is a schematic diagram showing another example of an apparatus for chemical vapor deposition used in the method for producing a metal-containing thin film according to the present invention.
- FIG. 3 is a schematic diagram showing another example of an apparatus for chemical vapor deposition used in the method for producing a thin film containing a metal according to the present invention.
- FIG. 4 is a schematic view showing another example of a chemical vapor deposition apparatus used in the method for producing a thin film containing a metal according to the present invention.
- the alkoxide compound of the present invention is represented by the above general formula (I), is suitable as a precursor for a thin film production method having a vaporization step such as a CVD method, and is particularly ALD because of its high thermal stability. It is suitable as a precursor used in the law.
- R 1 to R 3 are each independently represented by hydrogen, a hydrocarbon group having 1 to 12 carbon atoms, or the general formulas (X-1) to (X-8). Represents a group.
- Examples of the hydrocarbon group having 1 to 12 carbon atoms represented by R 1 to R 3 include alkyl, alkenyl, cycloalkyl, aryl, cyclopentadienyl, and the like.
- alkyl examples include methyl, ethyl, propyl, isopropyl, butyl, isobutyl, sec-butyl, sec-butyl, pentyl, isopentyl, hexyl, heptyl, isoheptyl, octyl, isooctyl, 2-ethylhexyl, nonyl, isononyl, decyl , Dodecyl and the like.
- alkenyl examples include vinyl, 1-methylethenyl, 2-methylethenyl, propenyl, butenyl, isobutenyl, pentenyl, hexenyl, heptenyl, octenyl, decenyl and the like.
- cycloalkyl examples include cyclohexyl, cyclopentyl, cycloheptyl, methylcyclopentyl, methylcyclohexyl, methylcycloheptyl, cyclopentenyl, cyclohexenyl, cycloheptenyl, methylcyclopentenyl, methylcyclohexenyl, methylcycloheptenyl, and the like.
- aryl examples include phenyl, naphthyl, 2-methylphenyl, 3-methylphenyl, 4-methylphenyl, 4-vinylphenyl, 3-isopropylphenyl, 4-isopropylphenyl, 4-butylphenyl, and 4-isobutylphenyl. 4-tert-butylphenyl, 4-hexylphenyl, 4-cyclohexylphenyl and the like.
- cyclopentadienyl examples include cyclopentadienyl, methylcyclopentadienyl, ethylcyclopentadienyl, propylcyclopentadienyl, isopropylcyclopentadienyl, butylcyclopentadienyl, and secondary butylcyclopenta Examples include dienyl, isobutylcyclopentadienyl, tertiary butylcyclopentadienyl, dimethylcyclopentadienyl, tetramethylcyclopentadienyl, and the like.
- R 4 represents a hydrocarbon group having 1 to 12 carbon atoms or a group represented by the above general formulas (X-1) to (X-8).
- hydrocarbon group having 1 to 12 carbon atoms represented by R 4 include the groups exemplified as the hydrocarbon group having 1 to 12 carbon atoms represented by R 1 to R 3 described above. The same example can be given.
- R X1 to R X12 each independently represent hydrogen or a hydrocarbon group having 1 to 12 carbon atoms, and A 1 to A 3 represent 1 carbon atom. Represents an alkanediyl group of ⁇ 6.
- hydrocarbon group having 1 to 12 carbon atoms represented by R X1 to R X12 include the groups exemplified as the hydrocarbon group having 1 to 12 carbon atoms represented by R 1 to R 3 The same example can be given.
- Examples of the alkanediyl group having 1 to 6 carbon atoms represented by A 1 to A 3 include methylene, ethylene, propylene, butylene and the like.
- Examples of the group represented by the general formula (X-1) include dimethylaminomethyl, ethylmethylaminomethyl, diethylaminomethyl, dimethylaminoethyl, ethylmethylaminoethyl, diethylaminoethyl and the like.
- Examples of the group represented by the general formula (X-2) include methylamino, ethylamino, propylamino, isopropylamino, butylamino, secondary butylamino, tertiary butylamino, isobutylamino and the like.
- Examples of the group represented by the general formula (X-3) include dimethylamino, diethylamino, dipropylamino, diisopropylamino, dibutylamino, disecondary butylamino, ditertiarybutylamino, ethylmethylamino, propyl. Examples thereof include methylamino and isopropylmethylamino.
- Examples of the compound giving the group represented by the general formula (X-4) include ethylenediamino, hexamethylenediamino, N, N-dimethylethylenediamino and the like.
- Examples of the group represented by the general formula (X-5) include di (trimethylsilyl) amino, di (triethylsilyl) amino and the like.
- Examples of the group represented by the general formula (X-6) include trimethylsilyl, triethylsilyl and the like.
- Examples of the group represented by the general formula (X-7) include methoxy, ethoxy, propoxy, isopropoxy, butoxy, second butoxy, isobutoxy, third butoxy, pentoxy, isopentoxy, third pentoxy and the like. .
- Examples of the group represented by the general formula (X-8) include hydroxymethyl, hydroxyethyl, hydroxypropyl, hydroxyisopropyl, hydroxybutyl and the like.
- R 1 is a methyl group
- R 2 is a methyl group or an ethyl group
- R 4 is a methyl group
- R 3 is hydrogen or a hydrocarbon having 4 to 12 carbon atoms.
- X-1 is a methyl group
- X-8 is a group represented by the above general formulas (X-1) to (X-8).
- hydrocarbon group having 4 to 12 carbon atoms examples include alkyl having 4 to 12 carbon atoms, alkenyl having 4 to 12 carbon atoms, cycloalkyl having 6 to 12 carbon atoms, and 6 to 12 carbon atoms. Can be used.
- alkyl having 4 to 12 carbon atoms examples include butyl, isobutyl, secondary butyl, tertiary butyl, pentyl, isopentyl, hexyl, heptyl, isoheptyl, octyl, isooctyl, 2-ethylhexyl, nonyl, isononyl, decyl, And dodecyl.
- alkenyl having 4 to 12 carbon atoms examples include butenyl, isobutenyl, pentenyl, hexenyl, heptenyl, octenyl, decenyl and the like.
- Examples of the cycloalkyl having 6 to 12 carbon atoms include cyclohexyl, cyclopentyl, cycloheptyl, methylcyclopentyl, methylcyclohexyl, methylcycloheptyl, cyclopentenyl, cyclohexenyl, cycloheptenyl, methylcyclopentenyl, methylcyclohexenyl, methylcyclohexyl And heptenyl.
- aryl having 6 to 12 carbon atoms examples include phenyl, naphthyl, 2-methylphenyl, 3-methylphenyl, 4-methylphenyl, 4-vinylphenyl, 3-isopropylphenyl, 4-isopropylphenyl, 4- Examples thereof include butylphenyl, 4-isobutylphenyl, 4-tertiarybutylphenyl, 4-hexylphenyl, 4-cyclohexylphenyl and the like.
- R 1 , R 2 , R 3 and R 4 have a high vapor pressure and a high thermal decomposition temperature when used in a method for producing a thin film having a step of vaporizing a compound.
- R 1 and R 2 are each independently hydrogen, a hydrocarbon group having 1 to 12 carbon atoms, or a group represented by (X-5) because of high vapor pressure.
- R 1 and R 2 when at least one of R 1 and R 2 is alkyl having 1 to 5 carbon atoms, di (trimethylsilyl) amino or di (triethylsilyl) amino, the vapor pressure is particularly high, and R 1 and R 2 At least one of which is alkyl having 1 to 5 carbon atoms, di (trimethylsilyl) amino or di (triethylsilyl) amino, and at least one of R 3 and R 4 is alkyl having 1 to 5 carbon atoms, di (trimethylsilyl) ) Amino or di (triethylsilyl) amino is most preferred because of its particularly high vapor pressure.
- R 4 is a hydrocarbon group having 1 to 12 carbon atoms, a group represented by (X-3) or a group represented by (X-5), it is preferable because of high thermal stability, Of these, when R 4 is an alkenyl group, alkyl, di (trimethylsilyl) amino or di (triethylsilyl) amino, it is particularly preferable because of high thermal stability.
- R 1 , R 2 , R 3 and R 4 can be arbitrarily selected depending on the solubility in the solvent used, the thin film formation reaction, and the like. it can.
- L represents hydrogen, halogen, hydroxyl group, amino group, azide group, phosphide group, nitrile group, carbonyl group, hydrocarbon group having 1 to 12 carbon atoms or the above general formula (L -1) to (L-13).
- R L1 to R L31 each independently represent hydrogen or a hydrocarbon group having 1 to 12 carbon atoms
- a 4 to A 7 represent 1 to carbon atoms.
- R L1 to R L31 in the general formulas (L-1) to (L-13) are hydrocarbon groups having 1 to 12 carbon atoms, the hydrogen atoms in the hydrocarbon groups are substituted with halogen atoms or amino groups May be.
- hydrocarbon group having 1 to 12 carbon atoms represented by R L1 to R L31 include the groups exemplified as the hydrocarbon group having 1 to 12 carbon atoms represented by R 1 to R 3 The same example can be given.
- alkanediyl group having 1 to 6 carbon atoms represented by A 4 to A 7 are exemplified as the alkanediyl group having 1 to 6 carbon atoms represented by A 1 to A 3 .
- the same examples as the group can be given.
- Examples of the group represented by the general formula (L-1) include dimethylaminomethyl, ethylmethylaminomethyl, diethylaminomethyl, dimethylaminoethyl, ethylmethylaminoethyl, diethylaminoethyl and the like.
- Examples of the group represented by the general formula (L-2) include methylamino, ethylamino, propylamino, isopropylamino, butylamino, secondary butylamino, tertiary butylamino, isobutylamino and the like.
- Examples of the group represented by the general formula (L-3) include dimethylamino, diethylamino, dipropylamino, diisopropylamino, dibutylamino, disecondary butylamino, ditertiarybutylamino, ethylmethylamino, propyl. Examples thereof include methylamino and isopropylmethylamino.
- Examples of the compound giving the group represented by the general formula (L-4) include ethylenediamino, hexamethylenediamino, N, N-dimethylethylenediamino and the like.
- Examples of the group represented by the general formula (L-5) include di (trimethylsilyl) amino, di (triethylsilyl) amino and the like.
- Examples of the group represented by the general formula (L-6) include trimethylsilyl, triethylsilyl and the like.
- Examples of the group represented by the general formula (L-7) include methoxy, ethoxy, propoxy, isopropoxy, butoxy, second butoxy, isobutoxy, third butoxy, pentoxy, isopentoxy, third pentoxy and the like. .
- Examples of the group represented by the general formula (L-8) include hydroxymethyl, hydroxyethyl, hydroxypropyl, hydroxyisopropyl, hydroxybutyl and the like.
- Examples of the group represented by the general formula (L-9) include dimethylaminoethoxy, diethylaminoethoxy, dimethylaminopropoxy, ethylmethylaminopropoxy and diethylaminopropoxy.
- Examples of the group represented by the general formula (L-10) include the following chemical formula No. And groups represented by (L-10-1) to (L-10-5).
- the following chemical formula No. In (L-10-1) to (L-10-5) “Me” represents methyl, “Et” represents ethyl, “iPr” represents isopropyl, and “tBu” represents tertiary butyl.
- Examples of the organic compound that gives the group represented by the general formula (L-10) include acetylacetone, hexane-2,4-dione, 5-methylhexane-2,4-dione, heptane-2,4-dione, -Methylheptane-3,5-dione, 2,6-dimethylheptane-3,5-dione, 1,1,1-trifluoropentane-2,4-dione, 1,1,1-trifluoro-5, 5-dimethylhexane-2,4-dione, 1,1,1,5,5,5-hexafluoropentane-2,4-dione, 1,3-diperfluorohexylpropane-1,3-dione, , 1,5,5-tetramethyl-1-methoxyhexane-2,4-dione, 2,2,6,6-tetramethyl-1-methoxyheptane-3,5-dione, 2,2,6,6 -T
- Examples of the group represented by the general formula (L-11) include chemical formula No. And groups represented by (L-11-1) to (L-11-3).
- the following chemical formula No. In (L-11-1) to (L-11-3) “Me” represents methyl, “iPr” represents isopropyl, and “tBu” represents tertiary butyl.
- Examples of the organic compound that gives the group represented by the general formula (L-11) include N, N′-diisopropylacetamidinate, N, N′-di-t-butylacetamidinate, N, N'-diisopropyl-2-t-butylamidinate and the like.
- Examples of the group represented by the general formula (L-12) include the following chemical formula No. And groups represented by (L-12-1) to (L-12-8). In addition, the following chemical formula No. In (L-12-1) to (L-12-8), “Me” represents methyl, “iPr” represents isopropyl, and “tBu” represents tertiary butyl.
- Examples of the organic compound giving the group represented by the general formula (L-12) include acetylacetone, hexane-2,4-dione, 5-methylhexane-2,4-dione, heptane-2,4-dione.
- Examples of the group represented by the general formula (L-13) include the following chemical formula No. And groups represented by (L-13-1) to (L-13-8). In addition, the following chemical formula No. In (L-13-1) to (L-13-8), “Me” represents methyl, “iPr” represents isopropyl, and “tBu” represents tertiary butyl.
- Examples of the organic compound that provides the group represented by the general formula (L-13) include N-isopropyl-4- (isopropylimino) pent-2-en-2-amine, N-isopropyl-4- (isopropyl). Imino) -3-methylpent-2-en-2-amine, N- (tert-butyl) -4- (tert-butylimino) pent-2-en-2-amine, N- (tert-butyl) -4- (Tert-Butylimino) -3-methylpent-2-en-2-amine, N-isopropyl-5- (isopropylimino) -2,6-dimethylhept-3-en-3-amine, N-isopropyl-5- ( Isopropylimino) -2,4,6-trimethylhept-3-en-3-amine, N- (tert-butyl) -5- (tert-butylimino) -2 2,6,6-tetramethylhept
- L is a cyclopentadienyl group represented by cyclopentadienyl, methylcyclopentadienyl, ethylcyclopentadienyl, and pentamethylcyclopentadienyl. In some cases or when it is a group represented by (L-11), it is particularly preferred because of its high thermal stability and high vapor pressure. In the general formula (I) of the present invention, when m is 2 or more, L may be the same or different.
- M represents a metal atom or a silicon atom.
- the metal atom is not particularly limited.
- n represents an integer of 1 or more
- m represents an integer of 0 or more
- n + m represents a valence of a metal atom or a silicon atom.
- the alkoxide compound represented by the general formula (I) may have optical activity
- the alkoxide compound of the present invention is not particularly distinguished by the R-form and the S-form. It may be a mixture in any proportion with the S form.
- the racemate is inexpensive to manufacture.
- the case where the terminal donor group in the ligand is coordinated to a metal atom or a silicon atom to form a ring structure is represented by the following general formula (IA).
- the alkoxide compound of the present invention is represented by the above general formula (I), but is not distinguished from the following general formula (IA) and is a concept including both.
- R 4 represents a group represented by the formula
- R 1 ⁇ R 3 are each independently hydrogen, a hydrocarbon group or the following general formula -C 1 ⁇ 12 (X-1) ⁇
- X-8 Represents a hydrocarbon group having 1 to 12 carbon atoms or a group represented by the following general formulas (X-1) to (X-8), provided that R 1 is a methyl group and R 2 is a methyl group or
- R 3 is hydrogen, a hydrocarbon group having 4 to 12 carbon atoms, or a group represented by the following general formulas (X-1) to (X-8):
- L represents hydrogen, halogen, hydroxyl group, amino group, azide group, phosphide group, nitrile group, carbonyl group, hydrocarbon group having 1 to 12 carbon atoms, or the following general formulas (L-1) to (L-13)
- M represents a metal atom or a silicon atom
- n represents an integer of 1 or more
- m represents an integer of 0 or
- R X1 to R X12 each independently represents hydrogen or a hydrocarbon group having 1 to 12 carbon atoms, and A 1 to A 3 each represents an alkanediyl group having 1 to 6 carbon atoms.
- R L1 to R L31 each independently represents hydrogen or a hydrocarbon group having 1 to 12 carbon atoms, and A 4 to A 7 each represents an alkanediyl group having 1 to 6 carbon atoms.
- the alkoxide compound represented by the general formula (I) for example, when M is cobalt, the following chemical formula No. 1-No. And a compound represented by 300.
- the alkoxide compound of the present invention is not particularly limited by its production method, and is produced by applying a known reaction.
- a cobalt alkoxide compound for example, an inorganic salt such as cobalt halide or nitrate, or a hydrate thereof, and the corresponding alcohol compound, a base such as sodium, sodium hydride, sodium amide, sodium hydroxide, sodium methylate, ammonia or amine
- a method of reacting in the presence of cobalt a method of reacting an inorganic salt such as a halide of cobalt, nitrate, or a hydrate thereof with an alkali metal alkoxide such as sodium alkoxide, lithium alkoxide or potassium alkoxide of the corresponding alcohol compound, cobalt Of methoxide
- Low-molecular-weight alcohol alkoxide compounds such as xoxide, isopropoxide and butoxide are exchanged with the corresponding alcohol compounds, cobalt halides and inorganic salts such as nitrates are reacted with derivatives that give reactive intermediates.
- the reactive intermediate examples include bis (dialkylamino) cobalt, bis (bis (trimethylsilyl) amino) cobalt, and an amide compound of cobalt.
- the alkoxide compound whose m in general formula (I) is 1 or more the alkoxide compound whose m in general formula (I) is 0, the organic compound which gives a desired ligand, or its alkali metal salt And a method of reacting with.
- the raw material for forming a thin film of the present invention is a thin film precursor made of the alkoxide compound of the present invention described above, and its form varies depending on the manufacturing process to which the raw material for forming a thin film is applied. For example, when manufacturing a thin film containing only one kind of metal or silicon, the raw material for forming a thin film of the present invention does not contain a metal compound and a semi-metal compound other than the alkoxide compound. On the other hand, when producing a thin film containing two or more kinds of metals and / or metalloids, the raw material for forming a thin film of the present invention is a compound containing a desired metal and / or metalloid in addition to the alkoxide compound.
- the thin film forming raw material of the present invention may further contain an organic solvent and / or a nucleophilic reagent.
- the raw material for forming a thin film of the present invention is suitable for chemical vapor deposition (hereinafter, referred to as CVD raw material) because the physical properties of the precursor alkoxide compound are suitable for CVD and ALD methods. ) Is useful.
- the raw material for forming a thin film of the present invention is a raw material for chemical vapor deposition
- the form is appropriately selected depending on the method such as the transport and supply method of the CVD method used.
- the raw material for CVD is vaporized by heating and / or depressurizing in a container in which the raw material is stored (hereinafter sometimes simply referred to as a raw material container), and is necessary.
- a carrier gas such as argon, nitrogen, helium or the like
- the alkoxide compound itself represented by the above general formula (I) can be used as a raw material for CVD.
- the alkoxide compound represented by the above general formula (I) itself or a solution obtained by dissolving the compound in an organic solvent can be used as a raw material for CVD.
- These CVD raw materials may further contain other precursors, nucleophilic reagents, and the like.
- the CVD raw material is vaporized and supplied independently for each component (hereinafter sometimes referred to as a single source method), and the multi-component raw material is mixed in advance with a desired composition.
- a method of vaporizing and supplying a mixed raw material hereinafter, sometimes referred to as a cocktail sauce method.
- a cocktail sauce method a mixture of the alkoxide compound of the present invention and another precursor or a mixed solution obtained by dissolving the mixture in an organic solvent can be used as a raw material for CVD.
- This mixture or mixed solution may further contain a nucleophilic reagent and the like.
- the CVD raw material containing the R body and the CVD raw material containing the S body may be vaporized separately. Or you may vaporize the raw material for CVD containing the mixture of R body and S body.
- the organic solvent is not particularly limited and a known general organic solvent can be used.
- the organic solvent include acetates such as ethyl acetate, butyl acetate and methoxyethyl acetate; ethers such as tetrahydrofuran, tetrahydropyran, ethylene glycol dimethyl ether, diethylene glycol dimethyl ether, triethylene glycol dimethyl ether, dibutyl ether and dioxane; Ketones such as butyl ketone, methyl isobutyl ketone, ethyl butyl ketone, dipropyl ketone, diisobutyl ketone, methyl pentyl ketone, cyclohexanone, methylcyclohexanone; hexane, cyclohexane, methylcyclohexane, dimethylcyclohexane, ethylcyclohexane, heptane, oct
- the total amount of the precursor in the CVD raw material which is a solution obtained by dissolving the precursor in the organic solvent, is 0.01 to 2.0 mol / liter, particularly 0.05 to 1.0 mol / liter. It is preferable to make it liter.
- the amount of the entire precursor is the amount of the alkoxide compound of the present invention when the thin film forming raw material of the present invention does not contain a metal compound and a semimetal compound other than the alkoxide compound of the present invention.
- the forming raw material contains a compound containing another metal and / or a compound containing a metalloid in addition to the alkoxide compound, this is the total amount of the alkoxide compound of the present invention and another precursor.
- hydrides hydroxides, halides, azides, alkyls, alkenyls, cycloalkyls, aryls, alkynyls, aminos, dialkylaminoalkyls, monoalkylaminos, dialkylaminos, diamines, di (silyls) -Alkyl) amino, di (alkyl-silyl) amino, disilylamino, alkoxy, alkoxyalkyl, hydrazide, phosphide, nitrile, dialkylaminoalkoxy, alkoxyalkyldialkylamino, siloxy, diketonate, cyclopentadienyl, silyl, pyrazolate, guanidinate, A group consisting of compounds having phosphoguanidinate, amidinate, phosphoamidinate, ketoiminate, diketiminate, carbonyl and phosphoamidinate as ligands Compounds of one kind or two kinds
- the precursor metal species include magnesium, calcium, strontium, barium, radium, scandium, yttrium, titanium, zirconium, hafnium, vanadium, niobium, tantalum, chromium, molybdenum, tungsten, manganese, iron, osmium, cobalt, rhodium, iridium.
- precursors described above are known in the art, and their manufacturing methods are also known.
- the inorganic salt of metal or its hydrate described above is reacted with the alkali metal alkoxide of the alcohol compound.
- a precursor can be manufactured.
- the metal inorganic salt or hydrate include metal halides and nitrates
- examples of the alkali metal alkoxide include sodium alkoxide, lithium alkoxide, and potassium alkoxide.
- the other precursor described above is preferably a compound having similar thermal and / or oxidative decomposition behavior to that of the alkoxide compound of the present invention, and in the case of the cocktail source method, heat and / or oxidation. In addition to being similar in decomposition behavior, those that do not undergo alteration due to chemical reaction or the like during mixing are preferred.
- examples of the precursor containing titanium, zirconium or hafnium include compounds represented by the following formulas (II-1) to (II-5).
- M 1 represents titanium, zirconium or hafnium, and R a and R b each independently may be substituted with a halogen atom, and may have an oxygen atom in the chain.
- R c represents an alkyl group having 1 to 8 carbon atoms
- R d represents an alkylene group having 2 to 18 carbon atoms which may be branched
- R e and R f each independently represents a hydrogen atom.
- each of R g , R h , R k and R j independently represents a hydrogen atom or an alkyl group having 1 to 4 carbon atoms, and p represents an integer of 0 to 4 Q represents 0 or 2, r represents an integer of 0 to 3, s represents an integer of 0 to 4, and t represents an integer of 1 to 4.
- an alkyl having 1 to 20 carbon atoms which may be substituted with a halogen atom and may contain an oxygen atom in the chain represented by R a and R b
- the groups include methyl, ethyl, propyl, isopropyl, butyl, sec-butyl, sec-butyl, isobutyl, pentyl, isopentyl, neopentyl, tertiary pentyl, hexyl, cyclohexyl, 1-methylcyclohexyl, heptyl, 3-heptyl, isoheptyl , Tertiary heptyl, n-octyl, isooctyl, tertiary octyl, 2-ethylhexyl, trifluoromethyl, perfluorohexyl, 2-methoxyethyl, 2-ethoxye
- Examples of the alkyl group having 1 to 8 carbon atoms represented by R c include methyl, ethyl, propyl, isopropyl, butyl, secondary butyl, tertiary butyl, isobutyl, pentyl, isopentyl, neopentyl, tertiary pentyl, and hexyl.
- the alkylene group having 2 to 18 carbon atoms which may be branched and represented by R d is a group given by glycol, and examples of the glycol include 1,2-ethanediol, 1,2- Propanediol, 1,3-propanediol, 1,3-butanediol, 2,4-hexanediol, 2,2-dimethyl-1,3-propanediol, 2,2-diethyl-1,3-propanediol, 2,2-diethyl-1,3-butanediol, 2-ethyl-2-butyl-1,3-propanediol, 2,4-pentanediol, 2-methyl-1,3-propanediol, 1-methyl- 2,4-pentanediol and the like can be mentioned.
- Examples of the alkyl group having 1 to 3 carbon atoms represented by R e and R f include methyl, ethyl, propyl, 2-propyl and the like, represented by R g , R h , R j and R k.
- Examples of the alkyl group having 1 to 4 carbon atoms include methyl, ethyl, propyl, isopropyl, butyl, secondary butyl, tertiary butyl, and isobutyl.
- Examples of the precursor containing rare earth elements include compounds represented by the following formulas (III-1) to (III to 3).
- R a and R b each independently represents an alkyl group having 1 to 20 carbon atoms which may be substituted with a halogen atom and may contain an oxygen atom in the chain
- R c represents an alkyl group having 1 to 8 carbon atoms
- R e and R f each independently represents a hydrogen atom or an alkyl group having 1 to 3 carbon atoms
- R g and R j each independently represent (It represents an alkyl group having 1 to 4 carbon atoms, p ′ represents an integer of 0 to 3, and r ′ represents an integer of 0 to 2.
- the rare earth atom represented by M 2 includes scandium, yttrium, lanthanum, cerium, praseodymium, neodymium, promethium, samarium, europium, gadolinium, terbium, dysprosium, holmium, erbium, thulium, Examples thereof include ytterbium and lutetium, and examples of the group represented by R a , R b , R c , R e , R f , R g, and R j include the groups exemplified for the precursor containing titanium.
- the raw material for forming a thin film of the present invention may contain a nucleophilic reagent as needed to impart stability of the alkoxide compound of the present invention and other precursors.
- the nucleophilic reagent include ethylene glycol ethers such as glyme, diglyme, triglyme and tetraglyme, 18-crown-6, dicyclohexyl-18-crown-6, 24-crown-8, dicyclohexyl-24-crown-8.
- Crown ethers such as dibenzo-24-crown-8, ethylenediamine, N, N′-tetramethylethylenediamine, diethylenetriamine, triethylenetetramine, tetraethylenepentamine, pentaethylenehexamine, 1,1,4,7,7- Polyamines such as pentamethyldiethylenetriamine, 1,1,4,7,10,10-hexamethyltriethylenetetramine and triethoxytriethyleneamine, cyclic polyamines such as cyclam and cyclen, pyridine, pyrrolidine and pipette Heterocyclic compounds such as gin, morpholine, N-methylpyrrolidine, N-methylpiperidine, N-methylmorpholine, tetrahydrofuran, tetrahydropyran, 1,4-dioxane, oxazole, thiazole, oxathiolane, methyl acetoacetate, ethyl acetoacetate, ⁇ -ketoest
- the raw material for forming a thin film according to the present invention should contain as little impurities metal elements as possible, impurities halogen such as impurity chlorine, and impurities organic components as much as possible.
- the impurity metal element content is preferably 100 ppb or less for each element, more preferably 10 ppb or less, and the total amount is preferably 1 ppm or less, more preferably 100 ppb or less.
- the impurity halogen content is preferably 100 ppm or less, more preferably 10 ppm or less, and most preferably 1 ppm or less.
- the impurity organic content is preferably 500 ppm or less in total, more preferably 50 ppm or less, and most preferably 10 ppm or less.
- metal compounds, organic solvents, and nucleophilic reagents are used to reduce their respective moisture content. In addition, it is better to remove moisture as much as possible before use.
- the water content of each of the metal compound, organic solvent and nucleophilic reagent is preferably 10 ppm or less, more preferably 1 ppm or less.
- the raw material for forming a thin film of the present invention contains as few particles as possible in order to reduce or prevent particle contamination of the formed thin film.
- the number of particles larger than 0.3 ⁇ m is preferably 100 or less in 1 ml of the liquid phase, and larger than 0.2 ⁇ m.
- the number of particles is more preferably 1000 or less in 1 ml of the liquid phase, and the number of particles larger than 0.2 ⁇ m is most preferably 100 or less in 1 ml of the liquid phase.
- a vapor obtained by vaporizing the thin film forming raw material of the present invention and a reactive gas used as necessary are used as a substrate.
- the film is introduced into a film forming chamber in which is deposited, and then a precursor is decomposed and / or chemically reacted on the substrate to grow and deposit a metal-containing thin film on the surface of the substrate.
- a precursor is decomposed and / or chemically reacted on the substrate to grow and deposit a metal-containing thin film on the surface of the substrate.
- Examples of the reactive gas used as necessary include oxygen, ozone, nitrogen dioxide, nitric oxide, water vapor, hydrogen peroxide, formic acid, acetic acid, acetic anhydride, etc.
- Examples of reducing substances include hydrogen, and examples of nitrides that can be used include organic amine compounds such as monoalkylamines, dialkylamines, trialkylamines, and alkylenediamines, hydrazine, and ammonia. Can be used alone or in combination of two or more.
- examples of the transport and supply method include the gas transport method, the liquid transport method, the single source method, and the cocktail sauce method described above.
- the above deposition methods include thermal CVD in which a raw material gas or a raw material gas and a reactive gas are reacted only by heat to deposit a thin film, plasma CVD using heat and plasma, photo CVD using heat and light, In addition, optical plasma CVD using light and plasma, and ALD in which the deposition reaction of CVD is divided into elementary processes and deposition is performed stepwise at the molecular level.
- Examples of the material of the substrate include silicon; ceramics such as silicon nitride, titanium nitride, tantalum nitride, titanium oxide, titanium nitride, ruthenium oxide, zirconium oxide, hafnium oxide, and lanthanum oxide; glass; metals such as metal ruthenium.
- Examples of the shape of the substrate include a plate shape, a spherical shape, a fiber shape, and a scale shape, and the surface of the substrate may be a flat surface or a three-dimensional structure such as a trench structure.
- reaction temperature base
- reaction pressure a deposition rate, etc.
- the reaction temperature is preferably 100 ° C. or higher, which is the temperature at which the alkoxide compound of the present invention sufficiently reacts, and more preferably 150 ° C. to 400 ° C.
- the reaction pressure is preferably atmospheric pressure to 10 Pa in the case of thermal CVD or photo CVD, and preferably 2000 Pa to 10 Pa in the case of using plasma.
- the deposition rate can be controlled by the raw material supply conditions (vaporization temperature, vaporization pressure), reaction temperature, and reaction pressure. When the deposition rate is large, the properties of the obtained thin film may be deteriorated. When the deposition rate is small, productivity may be problematic. Therefore, 0.01 to 100 nm / min is preferable, and 1 to 50 nm / min is more preferable. In the case of the ALD method, the number of cycles is controlled so as to obtain a desired film thickness.
- the above production conditions include temperature and pressure at which the thin film forming raw material is vaporized into steam.
- the step of vaporizing the raw material for forming a thin film to form a vapor may be performed in a raw material container or in a vaporization chamber.
- the thin film forming raw material of the present invention is preferably evaporated at 0 to 150 ° C.
- the pressure in the raw material container and the pressure in the vaporizing chamber are both preferably 1 to 10,000 Pa.
- the thin film production method of the present invention employs the ALD method, and in addition to the raw material introduction step of vaporizing the thin film forming raw material into vapor by the above transport and supply method, and introducing the vapor into the film forming chamber, A precursor thin film forming step for forming a precursor thin film on the surface of the substrate by the alkoxide compound in the vapor, an exhausting step for exhausting unreacted alkoxide compound gas, and the precursor thin film as a reactive gas and a chemistry
- substrate may have the metal containing thin film formation process of making it react and forming the thin film containing the said metal on the surface of this base
- substrate a precursor thin film formation process for making it react and forming the thin film containing the said metal on the surface of this base
- substrate a precursor thin film formation process for making it react and forming the thin film containing the said metal on the surface of this base
- the metal oxide thin film is formed by the ALD method
- the raw material introduction step described above is performed.
- Preferred temperatures and pressures when the thin film forming raw material is steam are the same as those described above.
- a precursor thin film is formed on the substrate surface by the alkoxide compound introduced into the deposition reaction part (precursor thin film forming step). At this time, heat may be applied by heating the substrate or heating the deposition reaction part.
- the precursor thin film formed in this step is a metal oxide thin film or a thin film formed by decomposition and / or reaction of a part of the alkoxide compound, and has a composition different from that of the target metal oxide thin film.
- the substrate temperature when this step is performed is preferably from room temperature to 500 ° C, more preferably from 150 to 350 ° C.
- the pressure of the system (in the film forming chamber) when this step is performed is preferably 1 to 10,000 Pa, and more preferably 10 to 1000 Pa.
- unreacted alkoxide compound gas and by-product gas are exhausted from the deposition reaction part (exhaust process).
- exhaust process Ideally, unreacted alkoxide compound gas and by-produced gas are completely exhausted from the deposition reaction part, but it is not always necessary to exhaust completely.
- the exhaust method include a method of purging the system with an inert gas such as nitrogen, helium, and argon, a method of exhausting by reducing the pressure in the system, and a method combining these.
- the degree of pressure reduction is preferably 0.01 to 300 Pa, more preferably 0.01 to 100 Pa.
- an oxidizing gas is introduced into the deposition reaction part, and a metal oxide thin film is formed from the precursor thin film obtained in the precursor thin film forming step by the action of the oxidizing gas or the action of the oxidizing gas and heat.
- Form (metal oxide-containing thin film forming step) The temperature when heat is applied in this step is preferably room temperature to 500 ° C., more preferably 150 to 350 ° C.
- the pressure of the system (in the film forming chamber) when this step is performed is preferably 1 to 10,000 Pa, and more preferably 10 to 1000 Pa.
- the alkoxide compound of the present invention has good reactivity with an oxidizing gas, and a metal oxide thin film can be obtained.
- the method for producing a thin film of the present invention when the ALD method is employed as described above, a series of operations including the above-described raw material introduction step, precursor thin film formation step, exhaust step, and metal oxide-containing thin film formation step
- the thin film deposition according to 1 may be set as one cycle, and this cycle may be repeated a plurality of times until a thin film having a required film thickness is obtained.
- the unreacted alkoxide compound gas and reactive gas oxidizing gas in the case of forming a metal oxide thin film
- After exhausting the gas it is preferable to perform the next one cycle.
- the metal oxide thin film by the ALD method energy such as plasma, light, or voltage may be applied, or a catalyst may be used.
- the timing of applying the energy and the timing of using the catalyst are not particularly limited. For example, when the alkoxide compound gas is introduced in the raw material introducing step, the precursor thin film forming step or the metal oxide-containing thin film forming step is heated, It may be at the time of exhausting the system in the exhaust process, at the time of introducing the oxidizing gas in the metal oxide-containing thin film forming process, or between the above processes.
- annealing may be performed in an inert atmosphere, an oxidizing atmosphere, or a reducing atmosphere in order to obtain better electrical characteristics.
- a reflow process may be provided.
- the temperature in this case is 200 to 1000 ° C., preferably 250 to 500 ° C.
- a known chemical vapor deposition apparatus can be used as the apparatus for producing a thin film using the thin film forming raw material of the present invention.
- the apparatus include an apparatus that can perform a precursor as shown in FIG. 1 by bubbling supply, and an apparatus that has a vaporization chamber as shown in FIG.
- an apparatus capable of performing plasma treatment on a reactive gas can be used.
- the present invention is not limited to the single-wafer type apparatus as shown in FIGS. 1 to 4, and an apparatus capable of simultaneously processing a large number of sheets using a batch furnace can also be used.
- a thin film manufactured using the raw material for forming a thin film of the present invention can be selected from other precursors, reactive gases, and manufacturing conditions as appropriate, so that a desired type of metal, oxide ceramics, nitride ceramics, glass, etc. It can be a thin film.
- the thin film is known to exhibit various electrical characteristics and optical characteristics, and is applied to various applications.
- copper and copper-containing thin films are applied as LSI wiring materials because of their high conductivity, high electromigration resistance, and high melting point.
- Nickel and nickel-containing thin films are mainly used for members of electronic parts such as resistance films and barrier films, members for recording media such as magnetic films, and members for thin film solar cells such as electrodes.
- Cobalt and cobalt-containing thin films are used for electrode films, resistance films, adhesive films, magnetic tapes, carbide tool members, and the like.
- the alcohol compound of the present invention is represented by the following general formula (II), and is a particularly suitable compound as a ligand used as a compound suitable as a precursor for a thin film production method having a vaporization step such as a CVD method. is there.
- R 5 to R 7 each independently represents hydrogen, a hydrocarbon group having 1 to 12 carbon atoms, or a group represented by the following general formulas (Y-1) to (Y-8):
- R 8 Represents a hydrocarbon group having 1 to 12 carbon atoms or a group represented by the following general formulas (Y-1) to (Y-8), provided that R 5 is a methyl group and R 6 is a methyl group or
- R 7 is hydrogen, a hydrocarbon group having 4 to 12 carbon atoms, or a group represented by the following general formulas (Y-1) to (Y-8).
- R Y1 to R Y12 each independently represents hydrogen or a hydrocarbon group having 1 to 12 carbon atoms, and A 8 to A 10 each represents an alkanediyl group having 1 to 6 carbon atoms.
- R 5 to R 7 are each independently represented by hydrogen, a hydrocarbon group having 1 to 12 carbon atoms, or the following general formulas (Y-1) to (Y-8). Represents a group.
- Examples of the hydrocarbon group having 1 to 12 carbon atoms represented by R 5 to R 7 include alkyl, alkenyl, cycloalkyl, aryl, cyclopentadienyl, and the like.
- alkyl examples include methyl, ethyl, propyl, isopropyl, butyl, isobutyl, sec-butyl, sec-butyl, pentyl, isopentyl, hexyl, heptyl, isoheptyl, octyl, isooctyl, 2-ethylhexyl, nonyl, isononyl, decyl , Dodecyl and the like.
- alkenyl examples include vinyl, 1-methylethenyl, 2-methylethenyl, propenyl, butenyl, isobutenyl, pentenyl, hexenyl, heptenyl, octenyl, decenyl and the like.
- cycloalkyl examples include cyclohexyl, cyclopentyl, cycloheptyl, methylcyclopentyl, methylcyclohexyl, methylcycloheptyl, cyclopentenyl, cyclohexenyl, cycloheptenyl, methylcyclopentenyl, methylcyclohexenyl, methylcycloheptenyl, and the like.
- aryl examples include phenyl, naphthyl, 2-methylphenyl, 3-methylphenyl, 4-methylphenyl, 4-vinylphenyl, 3-isopropylphenyl, 4-isopropylphenyl, 4-butylphenyl, and 4-isobutylphenyl. 4-tert-butylphenyl, 4-hexylphenyl, 4-cyclohexylphenyl and the like.
- cyclopentadienyl examples include cyclopentadienyl, methylcyclopentadienyl, ethylcyclopentadienyl, propylcyclopentadienyl, isopropylcyclopentadienyl, butylcyclopentadienyl, and secondary butylcyclopenta Examples include dienyl, isobutylcyclopentadienyl, tertiary butylcyclopentadienyl, dimethylcyclopentadienyl, tetramethylcyclopentadienyl, and the like.
- R 8 represents a hydrocarbon group having 1 to 12 carbon atoms or a group represented by the general formulas (Y-1) to (Y-8).
- hydrocarbon group having 1 to 12 carbon atoms represented by R 8 include the groups exemplified as the hydrocarbon group having 1 to 12 carbon atoms represented by R 5 to R 7 described above. The same example can be given.
- R 5 is a methyl group
- R 6 is a methyl group or an ethyl group
- R 8 is a methyl group
- R 7 is hydrogen and has 4 to 12 carbon atoms.
- Y-1 is a group represented by the above general formulas (Y-1) to (Y-8).
- hydrocarbon group having 4 to 12 carbon atoms examples include alkyl having 4 to 12 carbon atoms, alkenyl having 4 to 12 carbon atoms, cycloalkyl having 6 to 12 carbon atoms, and 6 to 12 carbon atoms. Can be used.
- alkyl having 4 to 12 carbon atoms examples include butyl, isobutyl, secondary butyl, tertiary butyl, pentyl, isopentyl, hexyl, heptyl, isoheptyl, octyl, isooctyl, 2-ethylhexyl, nonyl, isononyl, decyl, And dodecyl.
- alkenyl having 4 to 12 carbon atoms examples include butenyl, isobutenyl, pentenyl, hexenyl, heptenyl, octenyl, decenyl and the like.
- Examples of the cycloalkyl having 6 to 12 carbon atoms include cyclohexyl, cyclopentyl, cycloheptyl, methylcyclopentyl, methylcyclohexyl, methylcycloheptyl, cyclopentenyl, cyclohexenyl, cycloheptenyl, methylcyclopentenyl, methylcyclohexenyl, methylcyclohexyl And heptenyl.
- aryl having 6 to 12 carbon atoms examples include phenyl, naphthyl, 2-methylphenyl, 3-methylphenyl, 4-methylphenyl, 4-vinylphenyl, 3-isopropylphenyl, 4-isopropylphenyl, 4- Examples thereof include butylphenyl, 4-isobutylphenyl, 4-tertiarybutylphenyl, 4-hexylphenyl, 4-cyclohexylphenyl and the like.
- hydrocarbon group having 1 to 12 carbon atoms represented by R Y1 to R Y12 include the groups exemplified as the hydrocarbon group having 1 to 12 carbon atoms represented by R 5 to R 7 The same example can be given.
- Examples of the alkanediyl group having 1 to 6 carbon atoms represented by A 8 to A 10 include methylene, ethylene, propylene, butylene and the like.
- Examples of the group represented by the general formula (Y-1) include dimethylaminomethyl, ethylmethylaminomethyl, diethylaminomethyl, dimethylaminoethyl, ethylmethylaminoethyl, diethylaminoethyl and the like.
- Examples of the group represented by the general formula (Y-2) include methylamino, ethylamino, propylamino, isopropylamino, butylamino, secondary butylamino, tertiary butylamino, isobutylamino and the like.
- Examples of the group represented by the general formula (Y-3) include dimethylamino, diethylamino, dipropylamino, diisopropylamino, dibutylamino, disecondary butylamino, ditertiarybutylamino, ethylmethylamino, propyl. Examples thereof include methylamino and isopropylmethylamino.
- Examples of the compound giving the group represented by the general formula (Y-4) include ethylenediamino, hexamethylenediamino, N, N-dimethylethylenediamino and the like.
- Examples of the group represented by the general formula (Y-5) include di (trimethylsilyl) amino, di (triethylsilyl) amino and the like.
- Examples of the group represented by the general formula (Y-6) include trimethylsilyl, triethylsilyl and the like.
- Examples of the group represented by the general formula (Y-7) include methoxy, ethoxy, propoxy, isopropoxy, butoxy, second butoxy, isobutoxy, third butoxy, pentoxy, isopentoxy, third pentoxy and the like. .
- Examples of the group represented by the general formula (Y-8) include hydroxymethyl, hydroxyethyl, hydroxypropyl, hydroxyisopropyl, hydroxybutyl and the like.
- the alcohol compound of the present invention may have an optical isomer, but is not distinguished by the optical isomerism.
- Preferred examples of the alcohol compound represented by the general formula (II) include, for example, the following chemical formula No. 301-No. And a compound represented by 588.
- “Me” represents methyl
- “Et” represents ethyl
- “iPr” represents isopropyl.
- the alcohol compound of the present invention is not particularly limited by its production method, and can be produced by applying a known reaction.
- a reaction formula (1) an alkyl compound and an alkoxycarboxylate alkyl compound are subjected to a Grignard reaction using magnesium as a catalyst, and a product obtained by further reacting an alkylamine is extracted with an appropriate solvent and dehydrated.
- reaction formula (2) an alkyl compound and an alkoxyketone alkyl compound are subjected to a Grignard reaction using magnesium as a catalyst, and an alkylamine reaction product is extracted with an appropriate solvent, followed by dehydration.
- the method obtained by the treatment or the reaction of alkylamine and dialkyl diketone compound using magnesium as a catalyst and the reaction of alkylamine are extracted with an appropriate solvent as shown in the following reaction formula (3). And a method obtained by dehydration treatment.
- R z represents an alkyl group.
- the alcohol compound of the present invention can be used as a ligand for a metal compound used as a raw material for forming a thin film.
- the alcohol compound of the present invention can also be used for applications such as solvents, fragrances, agricultural chemicals, pharmaceuticals, synthetic raw materials such as various polymers.
- Example 1 Compound No. Synthesis of 343] To the reaction flask were added 8.69 g of magnesium and 253 g of tetrahydrofuran, and the mixture was stirred at room temperature. To this solution, 40.45 g of bromoethane was added dropwise over 1 hour and stirred for 2 hours. Thereafter, this solution was ice-cooled, and 25.04 g of methyl 2,2-dimethoxypropionate was added dropwise over 30 minutes to carry out a Grignard reaction. Thereafter, the temperature was returned to room temperature and the reaction was performed for 15 hours. The reaction solution was ice-cooled, 119 g of a 23% hydrochloric acid solution was added dropwise, and the mixture was stirred for 3 hours.
- Example 2 Compound No. Synthesis of 349] To the reaction flask, 8.62 g of magnesium and 244 g of tetrahydrofuran were added and stirred at room temperature. To this solution, 41.4 g of bromoethane was added dropwise over 1 hour and stirred for 3 hours. Thereafter, this solution was ice-cooled, and 25.03 g of methyl 2,2-dimethoxypropionate was added dropwise over 30 minutes to carry out a Grignard reaction. Thereafter, the temperature was returned to room temperature and the reaction was performed for 20 hours. The reaction solution was ice-cooled, and 103 g of 22% hydrochloric acid solution was added dropwise and stirred for 2 hours.
- Example 3 Compound No. Synthesis of 43] A 200 ml four-necked flask was charged with 7.01 g of cobalt (II) chloride and 25.5 g of tetrahydrofuran and stirred at room temperature. A solution obtained by diluting 17.3 g of a sodium alkoxide prepared from the alcohol (3-ethyl-2-methylimino-3-pentanol) synthesized in Example 1 with 20.7 g of tetrahydrofuran was cooled under ice cooling. It was dripped. After completion of dropping, the mixture was stirred at room temperature for 15 hours and filtered. Tetrahydrofuran was removed from the obtained filtrate, and the residue was sublimated under conditions of 46 Pa and 100 ° C. to obtain the desired product in a yield of 8.22 g and a yield of 37.8%.
- cobalt (II) chloride 25.5 g of tetrahydrofuran
- Example 4 Compound No. 49) A 100 ml three-necked flask was charged with 3.65 g of cobalt (II) chloride and 13.7 g of tetrahydrofuran, and stirred at room temperature. A solution obtained by diluting 9.86 g of a sodium alkoxide prepared from the alcohol (2-ethylimino-3-ethyl-3-pentanol) synthesized in Example 2 with 19.4 g of tetrahydrofuran was cooled under ice cooling. It was dripped. After completion of dropping, the mixture was stirred at room temperature for 15 hours and filtered.
- cobalt (II) chloride 13.7 g of tetrahydrofuran
- Tetrahydrofuran was removed from the obtained filtrate, and the residue was distilled under the conditions of 58 Pa, bath temperature 150 ° C., tower top temperature 108 ° C. to obtain the desired product in a yield of 7.05 g and a yield of 68.4%.
- Example 5 Production of metallic cobalt thin film by ALD method
- Compound No. A metal cobalt thin film was produced on a silicon wafer by ALD using the apparatus shown in FIG. The obtained thin film was measured for film thickness by X-ray reflectivity method, confirmed by X-ray diffraction method and X-ray photoelectron spectroscopy, and the film structure and composition were confirmed to be 4-7 nm. Is metallic cobalt (confirmed by Co2p peak by XPS analysis), and the carbon content was less than 0.1 atom% which is the lower limit of detection. The film thickness obtained per cycle was 0.08 to 0.14 nm.
- Reaction temperature (substrate temperature); 300-350 ° C, reactive gas; hydrogen gas (process)
- Vaporization chamber temperature: 140 ° C. vapor of a chemical vapor deposition raw material vaporized under the conditions of a vaporization chamber pressure of 100 Pa is introduced, and deposited for 30 seconds at a system pressure of 100 Pa.
- Unreacted raw materials are removed by argon purging for 5 seconds.
- a reactive gas is introduced and reacted at a system pressure of 100 Pa for 30 seconds.
- Unreacted raw materials are removed by argon purging for 5 seconds.
- Example 6 Production of metallic cobalt thin film by ALD method
- Compound No. 49 was used as a chemical vapor deposition raw material, and a metal cobalt thin film was produced on a silicon wafer by the ALD method under the following conditions using the apparatus shown in FIG.
- the obtained thin film was measured for film thickness by X-ray reflectivity method, confirmed by X-ray diffraction method and X-ray photoelectron spectroscopy, and the film structure and composition were confirmed to be 2 to 5 nm.
- Is metallic cobalt confirmeded by Co2p peak by XPS analysis
- the carbon content was less than 0.1 atom% which is the lower limit of detection.
- the film thickness obtained per cycle was 0.04 to 0.1 nm.
Landscapes
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Engineering & Computer Science (AREA)
- Computer Hardware Design (AREA)
- General Physics & Mathematics (AREA)
- Power Engineering (AREA)
- Microelectronics & Electronic Packaging (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Physics & Mathematics (AREA)
- Condensed Matter Physics & Semiconductors (AREA)
- General Chemical & Material Sciences (AREA)
- Manufacturing & Machinery (AREA)
- Materials Engineering (AREA)
- Metallurgy (AREA)
- Mechanical Engineering (AREA)
- Crystallography & Structural Chemistry (AREA)
- Chemical Vapour Deposition (AREA)
- Organic Low-Molecular-Weight Compounds And Preparation Thereof (AREA)
- Electrodes Of Semiconductors (AREA)
Abstract
Description
また、堆積速度は、原料の供給条件(気化温度、気化圧力)、反応温度、反応圧力によりコントロールすることができる。堆積速度は、大きいと得られる薄膜の特性が悪化する場合があり、小さいと生産性に問題を生じる場合があるので、0.01~100nm/分が好ましく、1~50nm/分がより好ましい。また、ALD法の場合は、所望の膜厚が得られるようにサイクルの回数でコントロールされる。
たとえば、下記反応式(1)のようにマグネシウムを触媒として、アルキル化合物とアルコキシカルボン酸アルキル化合物とをグリニャール反応させ、さらにアルキルアミンを反応させたものを適切な溶媒で抽出し、脱水処理することで得る方法や、下記反応式(2)のように、マグネシウムを触媒として、アルキル化合物とアルコキシケトンアルキル化合物とをグリニャール反応させ、さらにアルキルアミンを反応させたものを適切な溶媒で抽出し、脱水処理することで得る方法や、下記反応式(3)のように、マグネシウムを触媒として、アルキル化合物とジアルキルジケトン化合物とをグリニャール反応させ、さらにアルキルアミンを反応させたものを適切な溶媒で抽出し、脱水処理することで得る方法が挙げられる。
反応フラスコにマグネシウム8.69g及びテトラヒドロフラン253gを加え、室温にて撹拌した。この溶液にブロモエタン40.45gを1時間で滴下し、2時間攪拌した。その後、この溶液を氷冷し、2,2-ジメトキシプロピオン酸メチル25.04gを30分で滴下し、グリニャール反応を行った。その後室温に戻して15時間反応を行った。反応液を氷冷して、23%塩酸溶液119gを滴下して3時間撹拌した。有機層のみ分液・回収した後、これに40%メチルアミン水溶液68.73gを室温下滴下し、24時間反応させた。この時の反応液のpH10~11であった。トルエン119gを加え、有機層を抽出・分液し、硫酸マグネシウムを加えて脱水・ろ過を行った。減圧下オイルバス90℃にて脱溶媒を行い、溶媒留去後、蒸留を行うことで無色透明液体の目的物(3-エチル-2-メチルイミノ-3-ペンタノール)を得た。収量は17.32g、収率は65%であった。
(1)GC-MS m/z: 143(M+)
(2)1NMR(溶媒:重ベンゼン)(ケミカルシフト:多重度:H数)
(5.65:s:1)(2.78:s:3)(1.63~1.72:m:2)(1.35~1.44:m:2)(1.21:s:3)(0.79~0.83:t:6)
(3)元素分析 C:66.9質量%、H:12.3質量%、O:11.5質量%、N:9.9質量%(理論値;C:67.1質量%、H:11.9質量%、O:11.1質量%、N:9.8質量%)
反応フラスコにマグネシウム8.62g及びテトラヒドロフラン244gを加え、室温にて撹拌した。この溶液にブロモエタン41.4gを1時間で滴下し、3時間攪拌した。その後、この溶液を氷冷し、2,2-ジメトキシプロピオン酸メチル25.03gを30分で滴下し、グリニャール反応を行った。その後室温に戻して20時間反応を行った。反応液を氷冷して、22%塩酸溶液103gを滴下して2時間撹拌した。有機層のみ分液・回収した後、これに33%エチルアミン水溶液107.64gを室温下滴下し、48時間反応させた。この時の反応液のpH10~11であった。トルエン104gを加え、有機層を抽出・分液し、硫酸マグネシウムを加えて脱水・ろ過を行った。減圧下オイルバス80℃にて脱溶媒を行い、溶媒留去後、蒸留を行うことで無色透明液体の目的物(2-エチルイミノ-3-エチル-3-ペンタノール)を得た。収量は9.30g、収率は33%であった。
(1)GC-MS m/z:157(M+)
(3)元素分析 C:69.4質量%、H:12.5質量%、O:10.5質量%、N:8.2質量%(理論値;C:68.8質量%、H:12.1質量%、O:10.2質量%、N:8.9質量%)
200ml4つ口フラスコに塩化コバルト(II)7.01g及びテトラヒドロフラン25.5gを仕込み、室温下で撹拌した。その中に、実施例1で合成したアルコール(3-エチル-2-メチルイミノ-3-ペンタノール)より調製して得たナトリウムアルコキシド17.3gをテトラヒドロフラン20.7gで希釈した溶液を氷冷下で滴下した。滴下終了後、室温下で15時間撹拌し、ろ過を行った。得られたろ液からテトラヒドロフランを除去し、残渣を46Pa、100℃の条件下で昇華して、目的物を収量8.22g、収率37.8%で得た。
(1)常圧TG-DTA
質量50%減少温度:214℃(Ar流量:100ml/分、昇温10℃/分)
(2)減圧TG-DTA
質量50%減少温度:135℃(10Torr、Ar流量:50ml/分、昇温10℃/分)
(3)元素分析 Co:17.5質量%、C:55.7質量%、H:9.0質量%、O:8.9質量%、N:8.2質量%(理論値;Co:17.2質量%、C:56.0質量%、H:9.3質量%、O:9.3質量%、N:8.1質量%)
100ml3つ口フラスコに塩化コバルト(II)3.65g及びテトラヒドロフラン13.7gを仕込み、室温下で撹拌した。その中に、実施例2で合成したアルコール(2-エチルイミノ-3-エチル-3-ペンタノール)より調製して得たナトリウムアルコキシド9.86gをテトラヒドロフラン19.4gで希釈した溶液を氷冷下で滴下した。滴下終了後、室温下で15時間撹拌し、ろ過を行った。得られたろ液からテトラヒドロフランを除去し、残渣を58Pa、バス温度150℃、塔頂温度108℃の条件下で蒸留して、目的物を収量7.05g、収率68.4%で得た。
(1)常圧TG-DTA
質量50%減少温度:215℃(Ar流量:100ml/分、昇温10℃/分)
(2)減圧TG-DTA
質量50%減少温度:137℃(10Torr、Ar流量:50ml/分、昇温10℃/分)
(3)元素分析 Co:16.1質量%、C:58.7質量%、H:9.2質量%、O:8.4質量%、N:7.1質量%(理論値;Co:15.9質量%、C:58.2%、H:9.7%、O:8.6%、N:7.5%)
化合物No.43及び49並びに以下に示す比較化合物1~3について、大気中で放置することで自然発火性の有無を確認した。結果を表1に示す。
化合物No.43及び49並びに以下に示す比較化合物1~3について、DSC測定装置を用いて発熱ピークが観測された温度を熱分解発生温度として測定することで、各化合物の熱安定性を確認した。結果を表2に示す。薄膜形成用原料の熱安定性が高い場合は、より高温で成膜することができる。より高温で成膜することができるということは、得られる薄膜中の炭素残渣などの不純物少なくすることができる。よって、薄膜形成用原料の熱安定性は得られる薄膜の品質に影響するものである。
化合物No.43を化学気相成長用原料とし、図2に示す装置を用いて以下の条件のALD法により、シリコンウエハ上に金属コバルト薄膜を製造した。得られた薄膜について、X線反射率法による膜厚測定、X線回折法及びX線光電子分光法による薄膜構造及び薄膜組成の確認を行ったところ、膜厚は4~7nmであり、膜組成は金属コバルト(XPS分析によるCo2pピークで確認)であり、炭素含有量は検出下限である0.1atom%よりも少なかった。1サイクル当たりに得られる膜厚は、0.08~0.14nmであった。
反応温度(基板温度);300~350℃、反応性ガス;水素ガス
(工程)
下記(1)~(4)からなる一連の工程を1サイクルとして、50サイクル繰り返した。
(1)気化室温度:140℃、気化室圧力100Paの条件で気化させた化学気相成長用原料の蒸気を導入し、系圧100Paで30秒間堆積させる。
(2)5秒間のアルゴンパージにより、未反応原料を除去する。
(3)反応性ガスを導入し、系圧力100Paで30秒間反応させる。
(4)5秒間のアルゴンパージにより、未反応原料を除去する。
化合物No.49を化学気相成長用原料とし、図1に示す装置を用いて以下の条件のALD法により、シリコンウエハ上に金属コバルト薄膜を製造した。得られた薄膜について、X線反射率法による膜厚測定、X線回折法及びX線光電子分光法による薄膜構造及び薄膜組成の確認を行ったところ、膜厚は2~5nmであり、膜組成は金属コバルト(XPS分析によるCo2pピークで確認)であり、炭素含有量は検出下限である0.1atom%よりも少なかった。1サイクル当たりに得られる膜厚は、0.04~0.1nmであった。
反応温度(基板温度);300~350℃、反応性ガス;水素ガス
(工程)
下記(1)~(4)からなる一連の工程を1サイクルとして、50サイクル繰り返した。
(1)原料容器加熱温度:100℃、原料容器内圧力100Paの条件で気化させた化学気相成長用原料の蒸気を導入し、系圧100Paで30秒間堆積させる。
(2)5秒間のアルゴンパージにより、未反応原料を除去する。
(3)反応性ガスを導入し、系圧力100Paで30秒間反応させる。
(4)5秒間のアルゴンパージにより、未反応原料を除去する。
Claims (5)
- 下記一般式(I)で表されるアルコキシド化合物。
- 上記一般式(I)において、Mが銅、鉄、ニッケル、コバルト又はマンガンである請求項1に記載のアルコキシド化合物。
- 請求項1又は2に記載のアルコキシド化合物を含有してなる薄膜形成用原料。
- 請求項3に記載の薄膜形成用原料を気化させて得られるアルコキシド化合物を含有する蒸気を、基体が設置された成膜チャンバー内に導入し、該アルコキシド化合物を分解及び/又は化学反応させて該基体の表面に金属原子及びケイ素原子から選ばれる少なくとも1種の原子を含有する薄膜を形成する薄膜の製造方法。
Priority Applications (4)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| EP15782391.5A EP3135664B1 (en) | 2014-04-21 | 2015-03-30 | Alkoxide compound, raw material for forming thin film, method for producing thin film, and alcohol compound |
| US15/303,845 US10351584B2 (en) | 2014-04-21 | 2015-03-30 | Alkoxide compound, raw material for forming thin film, method for manufacturing thin film, and alcohol compound |
| KR1020167029729A KR102375179B1 (ko) | 2014-04-21 | 2015-03-30 | 알콕사이드 화합물, 박막 형성용 원료, 박막의 제조방법 및 알코올 화합물 |
| IL248335A IL248335B (en) | 2014-04-21 | 2016-10-13 | Alkoxide compound, raw material for forming a thin layer, method for producing a thin layer and alcohol compound |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2014087310A JP6343481B2 (ja) | 2014-04-21 | 2014-04-21 | 薄膜形成用原料、薄膜の製造方法及びアルコール化合物 |
| JP2014-087310 | 2014-04-21 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2015163090A1 true WO2015163090A1 (ja) | 2015-10-29 |
Family
ID=54332259
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/JP2015/059903 WO2015163090A1 (ja) | 2014-04-21 | 2015-03-30 | アルコキシド化合物、薄膜形成用原料、薄膜の製造方法及びアルコール化合物 |
Country Status (7)
| Country | Link |
|---|---|
| US (1) | US10351584B2 (ja) |
| EP (1) | EP3135664B1 (ja) |
| JP (1) | JP6343481B2 (ja) |
| KR (1) | KR102375179B1 (ja) |
| IL (1) | IL248335B (ja) |
| TW (1) | TWI652274B (ja) |
| WO (1) | WO2015163090A1 (ja) |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2018235530A1 (ja) * | 2017-06-21 | 2018-12-27 | 株式会社Adeka | 金属アルコキシド化合物、薄膜形成用原料及び薄膜の製造方法 |
Families Citing this family (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| KR102592325B1 (ko) * | 2016-07-14 | 2023-10-20 | 삼성전자주식회사 | 알루미늄 화합물과 이를 이용한 박막 형성 방법 및 집적회로 소자의 제조 방법 |
| US10607841B2 (en) | 2017-12-17 | 2020-03-31 | Applied Materials, Inc. | Silicide films through selective deposition |
Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2013188377A1 (en) * | 2012-06-11 | 2013-12-19 | Wayne State University | Precursors for atomic layer deposition |
| CN103664803A (zh) * | 2012-09-17 | 2014-03-26 | 王天桃 | 2,3,5,6-四甲基吡嗪新的合成方法 |
| WO2014077089A1 (ja) * | 2012-11-13 | 2014-05-22 | 株式会社Adeka | 金属アルコキシド化合物、薄膜形成用原料、薄膜の製造方法及びアルコール化合物 |
| US20140227444A1 (en) * | 2013-02-13 | 2014-08-14 | Wayne State University | Synthesis And Characterization Of First Row Transition Metal Complexes Containing a-Imino Alkoxides As Precursors For Deposition Of Metal Films |
Family Cites Families (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| KR20060058632A (ko) | 2004-11-25 | 2006-05-30 | 주식회사 엘지생명과학 | PPAR gamma와 PPAR alpha의 활성을항진시키는 신규 화합물, 그것의 제조방법, 및 그것을함유한 약제 조성물 |
| JP4700103B2 (ja) | 2005-04-07 | 2011-06-15 | コリア リサーチ インスティチュート オブ ケミカル テクノロジイ | 揮発性ニッケルアミノアルコキシド錯体及びそれを用いたニッケル薄膜の蒸着法 |
| JP4781012B2 (ja) | 2005-05-30 | 2011-09-28 | 株式会社Adeka | アルコール化合物を配位子とした金属化合物及び薄膜形成用原料並びに薄膜の製造方法 |
| KR100675983B1 (ko) | 2006-03-06 | 2007-01-30 | 한국화학연구원 | 신규의 코발트 아미노알콕사이드 화합물 및 그 제조 방법 |
-
2014
- 2014-04-21 JP JP2014087310A patent/JP6343481B2/ja active Active
-
2015
- 2015-03-30 KR KR1020167029729A patent/KR102375179B1/ko active IP Right Grant
- 2015-03-30 US US15/303,845 patent/US10351584B2/en active Active
- 2015-03-30 EP EP15782391.5A patent/EP3135664B1/en active Active
- 2015-03-30 WO PCT/JP2015/059903 patent/WO2015163090A1/ja active Application Filing
- 2015-04-08 TW TW104111262A patent/TWI652274B/zh active
-
2016
- 2016-10-13 IL IL248335A patent/IL248335B/en active IP Right Grant
Patent Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2013188377A1 (en) * | 2012-06-11 | 2013-12-19 | Wayne State University | Precursors for atomic layer deposition |
| CN103664803A (zh) * | 2012-09-17 | 2014-03-26 | 王天桃 | 2,3,5,6-四甲基吡嗪新的合成方法 |
| WO2014077089A1 (ja) * | 2012-11-13 | 2014-05-22 | 株式会社Adeka | 金属アルコキシド化合物、薄膜形成用原料、薄膜の製造方法及びアルコール化合物 |
| US20140227444A1 (en) * | 2013-02-13 | 2014-08-14 | Wayne State University | Synthesis And Characterization Of First Row Transition Metal Complexes Containing a-Imino Alkoxides As Precursors For Deposition Of Metal Films |
Non-Patent Citations (16)
Cited By (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2018235530A1 (ja) * | 2017-06-21 | 2018-12-27 | 株式会社Adeka | 金属アルコキシド化合物、薄膜形成用原料及び薄膜の製造方法 |
| JPWO2018235530A1 (ja) * | 2017-06-21 | 2020-04-23 | 株式会社Adeka | 金属アルコキシド化合物、薄膜形成用原料及び薄膜の製造方法 |
Also Published As
| Publication number | Publication date |
|---|---|
| EP3135664A1 (en) | 2017-03-01 |
| US10351584B2 (en) | 2019-07-16 |
| TWI652274B (zh) | 2019-03-01 |
| KR102375179B1 (ko) | 2022-03-15 |
| JP6343481B2 (ja) | 2018-06-13 |
| US20170121358A1 (en) | 2017-05-04 |
| IL248335B (en) | 2019-12-31 |
| KR20160148542A (ko) | 2016-12-26 |
| IL248335A0 (en) | 2016-11-30 |
| EP3135664A4 (en) | 2017-11-01 |
| EP3135664B1 (en) | 2019-10-23 |
| JP2015205837A (ja) | 2015-11-19 |
| TW201609762A (zh) | 2016-03-16 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP6465699B2 (ja) | ジアザジエニル化合物、薄膜形成用原料、薄膜の製造方法及びジアザジエン化合物 | |
| JP6184030B2 (ja) | アルミニウム化合物、薄膜形成用原料及び薄膜の製造方法 | |
| KR102541122B1 (ko) | 신규 화합물, 박막 형성용 원료 및 박막의 제조 방법 | |
| WO2014077089A1 (ja) | 金属アルコキシド化合物、薄膜形成用原料、薄膜の製造方法及びアルコール化合物 | |
| EP3476827B1 (en) | Vanadium compound, starting material for thin film formation, and method for producing thin film | |
| US11618762B2 (en) | Compound, raw material for forming thin film, method for manufacturing thin film, and amidine compound | |
| JP6343481B2 (ja) | 薄膜形成用原料、薄膜の製造方法及びアルコール化合物 | |
| WO2018042871A1 (ja) | ジアザジエニル化合物、薄膜形成用原料及び薄膜の製造方法 | |
| JP6662779B2 (ja) | アルコキシド化合物、薄膜形成用原料、薄膜の形成方法及びアルコール化合物 | |
| JP6408178B2 (ja) | アルコキシド化合物 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 15782391 Country of ref document: EP Kind code of ref document: A1 |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 15303845 Country of ref document: US Ref document number: 248335 Country of ref document: IL |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| ENP | Entry into the national phase |
Ref document number: 20167029729 Country of ref document: KR Kind code of ref document: A |
|
| REEP | Request for entry into the european phase |
Ref document number: 2015782391 Country of ref document: EP |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2015782391 Country of ref document: EP |