WO2014181674A1 - 経皮投与用貼付剤の製造方法及び経皮投与用貼付剤 - Google Patents
経皮投与用貼付剤の製造方法及び経皮投与用貼付剤 Download PDFInfo
- Publication number
- WO2014181674A1 WO2014181674A1 PCT/JP2014/061227 JP2014061227W WO2014181674A1 WO 2014181674 A1 WO2014181674 A1 WO 2014181674A1 JP 2014061227 W JP2014061227 W JP 2014061227W WO 2014181674 A1 WO2014181674 A1 WO 2014181674A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- sheet
- moisture
- patch
- microneedle
- water
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Ceased
Links
- 0 *CC(C*)N=O Chemical compound *CC(C*)N=O 0.000 description 2
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M37/00—Other apparatus for introducing media into the body; Percutany, i.e. introducing medicines into the body by diffusion through the skin
- A61M37/0015—Other apparatus for introducing media into the body; Percutany, i.e. introducing medicines into the body by diffusion through the skin by using microneedles
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/0012—Galenical forms characterised by the site of application
- A61K9/0019—Injectable compositions; Intramuscular, intravenous, arterial, subcutaneous administration; Compositions to be administered through the skin in an invasive manner
- A61K9/0021—Intradermal administration, e.g. through microneedle arrays, needleless injectors
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B29—WORKING OF PLASTICS; WORKING OF SUBSTANCES IN A PLASTIC STATE IN GENERAL
- B29C—SHAPING OR JOINING OF PLASTICS; SHAPING OF MATERIAL IN A PLASTIC STATE, NOT OTHERWISE PROVIDED FOR; AFTER-TREATMENT OF THE SHAPED PRODUCTS, e.g. REPAIRING
- B29C65/00—Joining or sealing of preformed parts, e.g. welding of plastics materials; Apparatus therefor
- B29C65/48—Joining or sealing of preformed parts, e.g. welding of plastics materials; Apparatus therefor using adhesives, i.e. using supplementary joining material; solvent bonding
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B29—WORKING OF PLASTICS; WORKING OF SUBSTANCES IN A PLASTIC STATE IN GENERAL
- B29C—SHAPING OR JOINING OF PLASTICS; SHAPING OF MATERIAL IN A PLASTIC STATE, NOT OTHERWISE PROVIDED FOR; AFTER-TREATMENT OF THE SHAPED PRODUCTS, e.g. REPAIRING
- B29C66/00—General aspects of processes or apparatus for joining preformed parts
- B29C66/01—General aspects dealing with the joint area or with the area to be joined
- B29C66/02—Preparation of the material, in the area to be joined, prior to joining or welding
- B29C66/022—Mechanical pre-treatments, e.g. reshaping
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B29—WORKING OF PLASTICS; WORKING OF SUBSTANCES IN A PLASTIC STATE IN GENERAL
- B29C—SHAPING OR JOINING OF PLASTICS; SHAPING OF MATERIAL IN A PLASTIC STATE, NOT OTHERWISE PROVIDED FOR; AFTER-TREATMENT OF THE SHAPED PRODUCTS, e.g. REPAIRING
- B29C66/00—General aspects of processes or apparatus for joining preformed parts
- B29C66/01—General aspects dealing with the joint area or with the area to be joined
- B29C66/03—After-treatments in the joint area
- B29C66/038—Covering the joint by a coating material
- B29C66/0382—Covering the joint by a coating material the coating material being in liquid or paste form
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B29—WORKING OF PLASTICS; WORKING OF SUBSTANCES IN A PLASTIC STATE IN GENERAL
- B29D—PRODUCING PARTICULAR ARTICLES FROM PLASTICS OR FROM SUBSTANCES IN A PLASTIC STATE
- B29D7/00—Producing flat articles, e.g. films or sheets
- B29D7/01—Films or sheets
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B29—WORKING OF PLASTICS; WORKING OF SUBSTANCES IN A PLASTIC STATE IN GENERAL
- B29D—PRODUCING PARTICULAR ARTICLES FROM PLASTICS OR FROM SUBSTANCES IN A PLASTIC STATE
- B29D99/00—Subject matter not provided for in other groups of this subclass
- B29D99/005—Producing membranes
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B32—LAYERED PRODUCTS
- B32B—LAYERED PRODUCTS, i.e. PRODUCTS BUILT-UP OF STRATA OF FLAT OR NON-FLAT, e.g. CELLULAR OR HONEYCOMB, FORM
- B32B3/00—Layered products comprising a layer with external or internal discontinuities or unevennesses, or a layer of non-planar shape; Layered products comprising a layer having particular features of form
- B32B3/26—Layered products comprising a layer with external or internal discontinuities or unevennesses, or a layer of non-planar shape; Layered products comprising a layer having particular features of form characterised by a particular shape of the outline of the cross-section of a continuous layer; characterised by a layer with cavities or internal voids ; characterised by an apertured layer
- B32B3/266—Layered products comprising a layer with external or internal discontinuities or unevennesses, or a layer of non-planar shape; Layered products comprising a layer having particular features of form characterised by a particular shape of the outline of the cross-section of a continuous layer; characterised by a layer with cavities or internal voids ; characterised by an apertured layer characterised by an apertured layer, the apertures going through the whole thickness of the layer, e.g. expanded metal, perforated layer, slit layer regular cells B32B3/12
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B32—LAYERED PRODUCTS
- B32B—LAYERED PRODUCTS, i.e. PRODUCTS BUILT-UP OF STRATA OF FLAT OR NON-FLAT, e.g. CELLULAR OR HONEYCOMB, FORM
- B32B3/00—Layered products comprising a layer with external or internal discontinuities or unevennesses, or a layer of non-planar shape; Layered products comprising a layer having particular features of form
- B32B3/26—Layered products comprising a layer with external or internal discontinuities or unevennesses, or a layer of non-planar shape; Layered products comprising a layer having particular features of form characterised by a particular shape of the outline of the cross-section of a continuous layer; characterised by a layer with cavities or internal voids ; characterised by an apertured layer
- B32B3/30—Layered products comprising a layer with external or internal discontinuities or unevennesses, or a layer of non-planar shape; Layered products comprising a layer having particular features of form characterised by a particular shape of the outline of the cross-section of a continuous layer; characterised by a layer with cavities or internal voids ; characterised by an apertured layer characterised by a layer formed with recesses or projections, e.g. hollows, grooves, protuberances, ribs
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B32—LAYERED PRODUCTS
- B32B—LAYERED PRODUCTS, i.e. PRODUCTS BUILT-UP OF STRATA OF FLAT OR NON-FLAT, e.g. CELLULAR OR HONEYCOMB, FORM
- B32B37/00—Methods or apparatus for laminating, e.g. by curing or by ultrasonic bonding
- B32B37/12—Methods or apparatus for laminating, e.g. by curing or by ultrasonic bonding characterised by using adhesives
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B32—LAYERED PRODUCTS
- B32B—LAYERED PRODUCTS, i.e. PRODUCTS BUILT-UP OF STRATA OF FLAT OR NON-FLAT, e.g. CELLULAR OR HONEYCOMB, FORM
- B32B38/00—Ancillary operations in connection with laminating processes
- B32B38/0012—Mechanical treatment, e.g. roughening, deforming, stretching
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B32—LAYERED PRODUCTS
- B32B—LAYERED PRODUCTS, i.e. PRODUCTS BUILT-UP OF STRATA OF FLAT OR NON-FLAT, e.g. CELLULAR OR HONEYCOMB, FORM
- B32B5/00—Layered products characterised by the non- homogeneity or physical structure, i.e. comprising a fibrous, filamentary, particulate or foam layer; Layered products characterised by having a layer differing constitutionally or physically in different parts
- B32B5/18—Layered products characterised by the non- homogeneity or physical structure, i.e. comprising a fibrous, filamentary, particulate or foam layer; Layered products characterised by having a layer differing constitutionally or physically in different parts characterised by features of a layer of foamed material
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M37/00—Other apparatus for introducing media into the body; Percutany, i.e. introducing medicines into the body by diffusion through the skin
- A61M37/0015—Other apparatus for introducing media into the body; Percutany, i.e. introducing medicines into the body by diffusion through the skin by using microneedles
- A61M2037/0023—Drug applicators using microneedles
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M37/00—Other apparatus for introducing media into the body; Percutany, i.e. introducing medicines into the body by diffusion through the skin
- A61M37/0015—Other apparatus for introducing media into the body; Percutany, i.e. introducing medicines into the body by diffusion through the skin by using microneedles
- A61M2037/0046—Solid microneedles
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M37/00—Other apparatus for introducing media into the body; Percutany, i.e. introducing medicines into the body by diffusion through the skin
- A61M37/0015—Other apparatus for introducing media into the body; Percutany, i.e. introducing medicines into the body by diffusion through the skin by using microneedles
- A61M2037/0053—Methods for producing microneedles
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B29—WORKING OF PLASTICS; WORKING OF SUBSTANCES IN A PLASTIC STATE IN GENERAL
- B29K—INDEXING SCHEME ASSOCIATED WITH SUBCLASSES B29B, B29C OR B29D, RELATING TO MOULDING MATERIALS OR TO MATERIALS FOR MOULDS, REINFORCEMENTS, FILLERS OR PREFORMED PARTS, e.g. INSERTS
- B29K2995/00—Properties of moulding materials, reinforcements, fillers, preformed parts or moulds
- B29K2995/0037—Other properties
- B29K2995/0059—Degradable
- B29K2995/0062—Degradable water-soluble
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B29—WORKING OF PLASTICS; WORKING OF SUBSTANCES IN A PLASTIC STATE IN GENERAL
- B29K—INDEXING SCHEME ASSOCIATED WITH SUBCLASSES B29B, B29C OR B29D, RELATING TO MOULDING MATERIALS OR TO MATERIALS FOR MOULDS, REINFORCEMENTS, FILLERS OR PREFORMED PARTS, e.g. INSERTS
- B29K2995/00—Properties of moulding materials, reinforcements, fillers, preformed parts or moulds
- B29K2995/0037—Other properties
- B29K2995/0068—Permeability to liquids; Adsorption
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B29—WORKING OF PLASTICS; WORKING OF SUBSTANCES IN A PLASTIC STATE IN GENERAL
- B29K—INDEXING SCHEME ASSOCIATED WITH SUBCLASSES B29B, B29C OR B29D, RELATING TO MOULDING MATERIALS OR TO MATERIALS FOR MOULDS, REINFORCEMENTS, FILLERS OR PREFORMED PARTS, e.g. INSERTS
- B29K2995/00—Properties of moulding materials, reinforcements, fillers, preformed parts or moulds
- B29K2995/0037—Other properties
- B29K2995/0068—Permeability to liquids; Adsorption
- B29K2995/0069—Permeability to liquids; Adsorption non-permeable
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B29—WORKING OF PLASTICS; WORKING OF SUBSTANCES IN A PLASTIC STATE IN GENERAL
- B29L—INDEXING SCHEME ASSOCIATED WITH SUBCLASS B29C, RELATING TO PARTICULAR ARTICLES
- B29L2007/00—Flat articles, e.g. films or sheets
- B29L2007/001—Flat articles, e.g. films or sheets having irregular or rough surfaces
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B29—WORKING OF PLASTICS; WORKING OF SUBSTANCES IN A PLASTIC STATE IN GENERAL
- B29L—INDEXING SCHEME ASSOCIATED WITH SUBCLASS B29C, RELATING TO PARTICULAR ARTICLES
- B29L2031/00—Other particular articles
- B29L2031/753—Medical equipment; Accessories therefor
- B29L2031/7544—Injection needles, syringes
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B32—LAYERED PRODUCTS
- B32B—LAYERED PRODUCTS, i.e. PRODUCTS BUILT-UP OF STRATA OF FLAT OR NON-FLAT, e.g. CELLULAR OR HONEYCOMB, FORM
- B32B37/00—Methods or apparatus for laminating, e.g. by curing or by ultrasonic bonding
- B32B37/14—Methods or apparatus for laminating, e.g. by curing or by ultrasonic bonding characterised by the properties of the layers
- B32B37/24—Methods or apparatus for laminating, e.g. by curing or by ultrasonic bonding characterised by the properties of the layers with at least one layer not being coherent before laminating, e.g. made up from granular material sprinkled onto a substrate
- B32B2037/243—Coating
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B32—LAYERED PRODUCTS
- B32B—LAYERED PRODUCTS, i.e. PRODUCTS BUILT-UP OF STRATA OF FLAT OR NON-FLAT, e.g. CELLULAR OR HONEYCOMB, FORM
- B32B2307/00—Properties of the layers or laminate
- B32B2307/70—Other properties
- B32B2307/724—Permeability to gases, adsorption
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B32—LAYERED PRODUCTS
- B32B—LAYERED PRODUCTS, i.e. PRODUCTS BUILT-UP OF STRATA OF FLAT OR NON-FLAT, e.g. CELLULAR OR HONEYCOMB, FORM
- B32B2307/00—Properties of the layers or laminate
- B32B2307/70—Other properties
- B32B2307/724—Permeability to gases, adsorption
- B32B2307/7242—Non-permeable
- B32B2307/7246—Water vapor barrier
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B32—LAYERED PRODUCTS
- B32B—LAYERED PRODUCTS, i.e. PRODUCTS BUILT-UP OF STRATA OF FLAT OR NON-FLAT, e.g. CELLULAR OR HONEYCOMB, FORM
- B32B2535/00—Medical equipment, e.g. bandage, prostheses or catheter
Definitions
- the present invention relates to a transdermal patch comprising a water-soluble microneedle and a method for producing the same.
- transdermal administration using a transdermal patch is performed as one means for non-invasively administering a drug or the like from the surface of a living body such as skin or mucous membrane. Then, in order to efficiently absorb the drug from the patch for transdermal administration into the body, the drug is adsorbed on a microneedle having a high aspect ratio called a so-called microneedle, and the microneedle is arrayed on a sheet.
- Formulations called arranged microneedle sheets or microneedle patches have been developed. Some of these microneedles are configured using a water-soluble raw material so as to be dissolved by moisture existing in the skin or moisture released from the skin.
- Patent Document 1 Japanese Patent Application Laid-Open No. 2011-194189
- a cosmetic liquid-containing sheet is applied for transdermal administration from the side opposite to the skin.
- An example is shown in which the moisture contained in the cosmetic liquid-containing sheet is applied to the agent and introduced into the microneedle.
- the microneedle array of Patent Document 1 is formed using a substance dissolved in a living body such as hyaluronic acid or collagen as a material.
- a microneedle array is formed using a substance that dissolves in water as a raw material is shown.
- a polyethylene adhesive tape with a center portion cut off is attached to assemble the microneedle patch.
- This elliptical microneedle array has a long side of about 30 mm and a short side of about 20 mm.
- Patent Document 2 Japanese Patent Laid-Open No. 2010-94414) discloses a manufacturing method in which a microneedle sheet is formed of a water-soluble polymer substance, and the microneedle sheet solidified body is adhered to a support sheet with an adhesive layer. Has been.
- the microneedle patch described in Patent Document 1 since the cosmetic liquid-containing sheet can be directly brought into contact with the microneedle array, it is easy to supply moisture to the microneedles, and the microneedle having high functionality. It is a needle patch.
- the microneedle patch described in Patent Document 1 has a weak adhesive force and is easily broken because the microneedle array and the adhesive tape are only adhered in a small area around the microneedle array.
- the microneedle sheet patch described in Patent Document 2 has a configuration in which a dried microneedle sheet and a support sheet are bonded with an adhesive layer, and the microneedle sheet and the support sheet are removed from the mold. Yes.
- the mold is configured to function as a package.
- the manufacturing method of the microneedle patch described in Patent Document 2 has high production efficiency.
- the microneedle sheet patch of Patent Document 2 when supplying moisture from the outside to the microneedle sheet from Patent Document 1, not only the support sheet but also the adhesive layer supplies the moisture. It has a structure to prevent.
- An object of the present invention is to provide a transdermal administration patch having good performance and a low cost for a transdermal administration patch comprising a water-soluble microneedle and a method for producing the same.
- the first pressure-sensitive adhesive layer for sticking to the skin is formed on the skin-facing surface side facing the skin, and water vapor from the skin is produced.
- a moisture permeable sheet comprising a water vapor barrier sheet that blocks permeation and a removable portion around the water vapor barrier sheet, and the removable portion is configured to be separated from the water vapor barrier sheet and peelable.
- Assembling a patch for transdermal administration by attaching a second adhesive layer having a lower adhesive strength than the first adhesive layer on an outer surface opposite to the skin-facing surface And a step, and is intended.
- the method for producing a patch for transdermal administration according to another aspect of the present invention comprises an application process, a placing process, a drying process, a peeling process, an assembling process, and an adhesive forming process. More specifically, the method for producing a patch for transdermal administration according to another aspect includes a coating step of coating a microneedle raw material aqueous solution on a stamper having micropores for forming microneedles, and a coated raw material A placing step of placing a moisture permeable sheet that transmits vapor of the raw material aqueous solution in contact with the raw material aqueous solution so as to sandwich the aqueous solution together with the stamper, and sandwiching the raw aqueous solution between the stamper and the moisture permeable sheet A drying step of evaporating at least a part of the raw material aqueous solution through the moisture permeable sheet in a dry state and forming microneedles by a dried body of the raw material aqueous solution, and a peeling step of peeling
- water vapor can be passed through the moisture permeable sheet in the drying step to form microneedles, and the dried body of the raw material aqueous solution is brought into direct contact with the moisture permeable sheet. Therefore, the dried body can be fixed to the moisture-permeable sheet without separating the moisture-permeable sheet and the dried body by the adhesive layer. Therefore, it becomes easy to form thinly by omitting an adhesive layer for fixing the microneedles to the moisture-permeable sheet, and it is easy to produce a patch for transdermal administration that is easy to stick on the skin and is not noticeable even if it is stuck on the skin. Become.
- the distance from the moisture-permeable sheet to the dry body is short, so that the moisture can easily reach the microneedles, and it is easy to apply moisture to the skin. It becomes easy to manufacture the patch.
- a microneedle is peeled from a stamper at a peeling process, since the state which the microneedle adhered to the moisture-permeable sheet will be obtained, the process for making a microneedle adhere to a moisture-permeable sheet will be omitted, and productivity will improve.
- the moisture-permeable sheet comprises at least one of a plurality of vapor transmission holes having a hole diameter of 0.1 ⁇ m to 100 ⁇ m and a plurality of openings having an opening diameter of 0.5 mm to 4.5 mm.
- a plastic film or fiber sheet with one side may be included.
- the moisture-permeable sheet comprises at least one of a plurality of vapor transmission holes having a hole diameter of 0.1 ⁇ m to 100 ⁇ m and a plurality of openings having an opening diameter of 0.5 mm to 4.5 mm.
- the plastic film having one side it has a water absorbing layer made of a fiber sheet or a water absorbing layer containing a water absorbing polymer, and in the microneedle sheet fixing step, the water absorbing layer is arranged in the first region,
- the microneedle sheet may be fixed so as to be in contact with the water absorption layer.
- the moisture-permeable sheet in the drying step, may be dried while being kept flat. Thereby, when drying a moisture-permeable sheet
- the moisture-permeable sheet is formed in advance by applying a raw material aqueous solution to the moisture-permeable sheet in the form of a sheet, and in contact with the raw material aqueous solution in the placing step.
- the drying step may include a step of forming the microneedles by drying the sheet-like base material in contact with the raw material aqueous solution filled with the fine pores.
- the moisture-permeable sheet comprises at least one of a plurality of vapor transmission holes having a hole diameter of 0.1 ⁇ m to 100 ⁇ m and a plurality of openings having an opening diameter of 0.5 mm to 4.5 mm.
- the plastic film having one side it has a water-absorbing layer made of a fiber sheet or a water-absorbing layer containing a water-absorbing polymer in contact with the raw material aqueous solution in the placing step, and the drying step makes the water-absorbing layer into micropores. It may include a step of forming microneedles by drying in a state where the raw material aqueous solution is filled. If comprised in this way, at the time of drying, a water absorption layer which contacts raw material aqueous solution can be made to absorb a water
- the patch for transdermal administration according to an aspect of the present invention comprises a microneedle sheet, a moisture permeable sheet, and a water vapor barrier sheet. More specifically, the patch for transdermal administration according to this aspect is a microneedle sheet having a water-soluble sheet-like substrate and a plurality of water-soluble microneedles formed in an array on the substrate.
- the microneedle sheet is fixed to the first region on the skin facing surface side facing the skin, the first pressure-sensitive adhesive layer is applied to the skin facing surface side, and the fibrous sheet or the vapor permeation hole from 0.1 ⁇ m to 100 ⁇ m And a moisture-permeable sheet that is made of a plastic film having at least one of a plurality of openings having an opening diameter of 0.5 mm or more and 4.5 mm or less in the first region, and is more adhesive than the first pressure-sensitive adhesive layer.
- the patch for transdermal administration when the patch for transdermal administration is applied to the skin, the microneedle sheet and the vapor permeable holes of the moisture permeable sheet and the gap between the fibers are removed from the skin. The water vapor that permeates is blocked by the water vapor blocking sheet.
- the moisture-permeable sheet can be reinforced by the reinforcing film, which facilitates the handling of the patch for transdermal administration. After the transdermal patch is applied to the skin, the adhesive strength of the first pressure-sensitive adhesive layer is stronger than that of the second pressure-sensitive adhesive layer. it can. Therefore, the convenience at the time of using the patch for transdermal administration is improved.
- the reinforcing film may be formed of a material having a larger loop stiffness value than the moisture-permeable sheet.
- the reinforcing film keeps the shape that the patch for transdermal administration is easy to hold, so that the moisture-permeable sheet is deformed and it is difficult to apply the patch for transdermal administration on the skin. Can be resolved.
- the cover film is further provided with a cover film so as to form a cavity that is adhered to the moisture-permeable sheet with an adhesive and encloses the microneedle sheet together with the reinforcing film so as not to contact the microneedle sheet. May be.
- transdermal administration patch and the transdermal administration patch production method of the present invention a transdermal administration patch that is easy to supply and handle water to the microneedles can be provided at low cost.
- FIG. 2 is a schematic cross-sectional view for explaining the structure of the transdermal patch of FIG. 1.
- the typical top view for demonstrating the structure of the patch for transdermal administration of FIG. A typical sectional view of a moisture-permeable sheet used in a 1st embodiment.
- Typical sectional drawing for demonstrating the structure of the patch for transdermal administration of 2nd Embodiment Typical sectional drawing of the moisture-permeable sheet
- Typical sectional drawing for demonstrating the structure of the patch for transdermal administration of 3rd Embodiment Typical sectional drawing for demonstrating the structure of the patch for transdermal administration of 4th Embodiment.
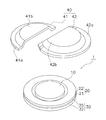
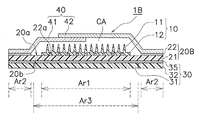
- FIG. 1 shows a perspective view in which a part of the patch for transdermal administration of the first embodiment is disassembled.
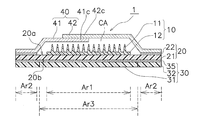
- 2 schematically shows a cross-sectional structure of the transdermal patch of FIG. 1
- FIG. 3 schematically shows a planar structure of the transdermal patch of FIG.
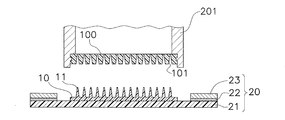
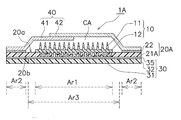
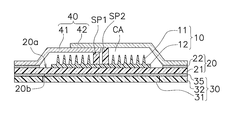
- the patch 1 for transdermal administration includes a microneedle sheet 10, a moisture permeable sheet 20, a reinforcing film 30, and a cover film 40.
- the microneedle sheet 10 described in the first embodiment has a disk shape with a radius of several millimeters to several tens of millimeters, and has a thickness of several hundreds of micrometers.
- Microneedle sheet A drug or the like is administered by sticking the microneedle sheet 10 mainly in contact with human skin.
- the microneedle sheet 10 includes microneedles 11 as shown in FIG. 2 on a disc-shaped substrate 12, and the microneedles 11 are arranged in a portion of the region on the substrate 12 that comes into contact with human skin. Has been. When the microneedle 11 is pierced into the skin, administration of a drug or the like is promoted.
- the microneedle sheet 10 is mainly composed of a water-soluble drug or a material obtained by adding a drug to a water-soluble polymer such as hyaluronic acid, water-soluble collagen, dextran, and chondroitin sulfate.
- the water-soluble polymer to which the drug is added is preferably a water-soluble polymer that is soluble in the living body, and sodium chondroitinate, hyaluronic acid, dextran, etc. are examples of the water-soluble polymer that is soluble in the living body. Can be mentioned.
- the substrate 12 of the microneedle sheet 10 is fixed so as to be in direct contact with the moisture permeable sheet 20.
- the surface of the moisture permeable sheet 20 to which the substrate 12 is fixed is a skin facing surface 20a facing the skin, and the region where the substrate 12 is fixed on the skin facing surface 20a is the first region Ar1.
- the moisture-permeable sheet 20 is, for example, a polyurethane having a plurality of (a plurality of) vapor-permeable holes (not shown) having a pore diameter of 0.1 to 100 ⁇ m, preferably 10 to 30 ⁇ m, through which water vapor is transmitted.
- the film 21 is formed.
- the thickness of the moisture permeable sheet 20 is, for example, about several tens of ⁇ m.
- seat 20 equips the skin opposing surface 20a with the adhesive layer 22 for sticking on skin.
- the pressure-sensitive adhesive layer 22 is formed in an annular shape so as to surround the disk-shaped substrate 12.
- the moisture-permeable sheet 20 is configured to transmit water vapor from the vapor transmission holes of the pressure-sensitive adhesive layer 22 and the polyurethane film 21 so that the skin where the moisture-permeable sheet 20 is affixed does not get steamed.
- the adhesive layer 22 is sparsely applied so that the application area is small so as not to block all the vapor transmission holes.
- the pressure-sensitive adhesive layer 22 is formed in the second region Ar2 in the region other than the first region Ar1 on the skin facing surface 20a of the moisture-permeable sheet 20.
- a reinforcing film 30 is adhered.
- the reinforcing film 30 includes an adhesive layer 35, and is attached to the moisture permeable sheet 20 by the adhesive layer 35.
- the pressure-sensitive adhesive layer 35 is peeled off from the moisture permeable sheet 20 and attached to the reinforcing film 30.
- the reinforcing film 30 is formed of a plastic film such as polypropylene, polyethylene, or polyester, and the plastic forming the reinforcing film 30 does not have a vapor transmission hole like the polyurethane film 21. It has sufficient water vapor barrier properties.
- FIG. 10 is a perspective view of the patch 1 for transdermal administration after the reinforcing film 30 is adhered, as viewed from the reinforcing film 30 side.
- the thickness of the reinforcing film 30 is, for example, about ten to several hundred ⁇ m.
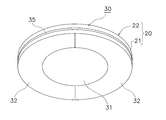
- the reinforcing film 30 includes a water vapor barrier sheet 31 and a removal portion 32 formed integrally with the water vapor barrier sheet 31.
- the water vapor barrier sheet 31 and the removal portion 32 are integrally formed by inserting a separate wire such as a cut (groove for cutting) or a perforation into a single plastic film so as to be separable. Can do.
- the water vapor blocking sheet 31 is disposed in a portion overlapping the first region Ar1 to which the microneedle sheet 10 is fixed and the third region Ar3 including the periphery thereof.
- the third region Ar3 is a region including the first region Ar1 and its peripheral region.
- the third region Ar3 may include a part of the second region Ar2.
- the removal portion 32 is removed during use. At this time, the adhesive layer 35 applied to the removal portion 32 is attached to the removal portion 32 and removed together. Therefore, at the time of use, the place where the detachable portion 32 is removed is not steamed because the outer surface 20b of the moisture-permeable sheet 20 is exposed to the atmosphere.
- the transdermal administration patch 1 When the transdermal administration patch 1 is applied to the skin, after the transdermal administration patch 1 is applied to the skin, only the removal portion 32 can be removed, and the moisture permeable sheet 20 is then removed. In order not to peel off, the components and thickness application areas of the pressure-sensitive adhesive layers 22 and 35 are adjusted so that the pressure-sensitive adhesive force of the pressure-sensitive adhesive layer 35 is weaker than the pressure-sensitive adhesive force of the pressure-sensitive adhesive layer 22.
- the reinforcing film 30 is made of a material stronger than the moisture-permeable sheet 20 in order to improve the handling of the patch 1 for transdermal administration.
- the waist strength of the moisture permeable sheet 20 and the reinforcing film 30 is compared with the value measured with a product name: Loop Stiffness Tester manufactured by Toyo Seiki Seisakusho Co., Ltd., and the reinforcing film 30 is measured with the Loop Stiffness Tester.
- the value increases.
- the moisture permeable sheet 20 is 0 mN / 20 mm
- the reinforcing film 30 is set to any value from 1 mN / 15 mm to 1 N / 15 mm.
- the moisture-permeable sheet 20 that is easily deformed so as to follow the deformation of the skin is picked with a finger, the moisture-permeable sheet 20 hangs down due to gravity, and the microneedle sheet 10 is moved to a desired location. I can't paste it easily.
- the microneedle sheet 10 can be suppressed to a degree of bending even if the reinforcing film 30 and the moisture permeable sheet 20 are picked with a finger. It becomes easy to stick on the part.
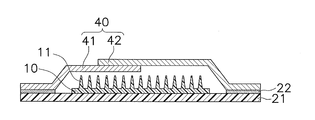
- the skin administration patch 1 includes a cover film 40. Therefore, for example, the cover film 40 has such a hardness and rigidity that it does not deform even when the transdermal administration patches 1 collide during transportation.
- the cover film 40 is peeled to expose the microneedle sheet 10 as shown in FIG.
- the cover film 40 is composed of a lower cover film 41 and an upper cover film 42.
- the lower cover film 41 and the upper cover film 42 have flange portions 41 a and 42 a that adhere to the adhesive layer 22, respectively.
- a dome-shaped portion protruding outward is formed on the inner peripheral side of the flange portions 41a and 42a.
- the lower cover film 41 and the upper cover film 42 have dome portions 41b and 42b, respectively.
- the dome portions 41b and 42b together with the reinforcing film 30 form a cavity CA (see FIG. 2) that is a space for housing the microneedle sheet 10. Due to the cavity CA, the microneedle 11 is configured not to contact other parts such as the cover film 40.
- the cover film 40 has overlapping portions 41 c and 42 c where the lower cover film 41 and the upper cover film 42 overlap each other so that there is no gap between the lower cover film 41 and the upper cover film 42. Yes.
- FIG. 4 shows a step of preparing a moisture-permeable sheet 20 with a pressure-sensitive adhesive, which is an example of the pressure-sensitive adhesive forming step.
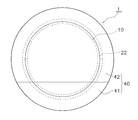
- the moisture-permeable sheet 20 shown in FIG. 4 looks circular when viewed in plan, as shown in FIG.
- the adhesive layer 22 is formed by applying the adhesive in a ring shape only between the inner periphery 20d having a smaller radius than the outer periphery 20c of the polyurethane film 21.
- the adhesive layer 22 is not formed in a region inside the inner periphery 20d of the moisture permeable sheet 20.
- a release sheet 23 is stuck on the pressure-sensitive adhesive layer 22 of the moisture-permeable sheet 20.
- the release sheet 23 plays a role of preventing dust and dust from adhering to the pressure-sensitive adhesive layer 22 during manufacture.
- the manufacturing process may be performed using a moisture-permeable sheet to which the adhesive layer 22 is not applied, and the adhesive layer 22 may be formed before the assembly process is completed.
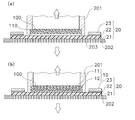
- FIG. 5A shows a state in which the stamper to which the raw material aqueous solution is applied is viewed from an oblique direction
- FIG. 5B shows a cross-sectional state of the stamper to which the raw material aqueous solution is applied.
- the raw material aqueous solution 110 is applied by the squeegee 150 so as to have a constant thickness d1 from the surface 100a of the stamper 100. Therefore, the tip of the squeegee 150 moves horizontally with respect to the surface 100 a of the stamper 100.
- the raw material aqueous solution 110 is also filled into the fine through holes 101 of the stamper 100.
- the stamper 100 is formed of, for example, a resin such as polyethylene or fluororesin, particularly a thermoplastic resin, and is hygienically managed by returning it to a raw material after being used once.
- the fine through-hole 101 is conical, it is about several tens to several hundreds of ⁇ m on the front surface 100a of the stamper 100, and about several ⁇ m to several tens of ⁇ m on the back surface 100b of the stamper 100.
- the pressure on the back surface 100b of the stamper 100 is made smaller than the atmospheric pressure on the front surface 100a, that is, the fine through-hole 101 is completely filled with the raw material aqueous solution 110 by suction from the back surface 100b.
- the pressure on the surface 100a side of the stamper 100 is made higher than the atmospheric pressure so as to press the raw material aqueous solution 110 into the fine through hole 101. And more preferred.
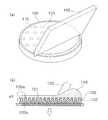
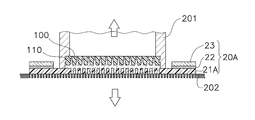
- FIG. 6 shows a cross section of the moisture permeable sheet 20 and the stamper 100 in the placement step.
- the stamper 100 to which the raw material aqueous solution 110 is applied is moved with respect to the moisture permeable sheet 20 with high accuracy by fitting the fitting portion 102 to the arm 201 of the manufacturing apparatus 200, for example.
- the arm 201 is, for example, a cylindrical part having a rib 201a that fits into the fitting portion 102, and is configured to be split in half vertically to sandwich the stamper 100 from the left and right.
- the stamper 100 is placed on the moisture permeable sheet 20 with the surface 100 a side of the stamper 100 facing the moisture permeable sheet 20.
- the moisture permeable sheet 20 Since the moisture permeable sheet 20 has to be positioned relative to the arm 201 when the stamper 100 is placed, the moisture permeable sheet 20 is attracted to the pedestal 202 and fixed on the manufacturing apparatus 200. Therefore, a large number of suction holes 203 are provided in the pedestal 202, and the pressure in the suction holes 203 is lower than the atmospheric pressure.
- the arrows in FIG. 6 are symbols that conceptually indicate this suction.
- the position where the stamper 100 is placed is inside the inner periphery 20 d of the moisture permeable sheet 20. At the time of placement, the stamper 100 is placed on the moisture permeable sheet 20 so that no bubbles are introduced between the raw material aqueous solution 110 and the moisture permeable sheet 20. In order to place it in this way, the stamper 100 may be pressed against the moisture permeable sheet 20 so that the raw material aqueous solution 110 slightly protrudes from the stamper 100.
- FIG. 7A shows a cross section of the moisture permeable sheet 20 and the stamper 100 before drying in the drying step
- FIG. 7B shows moisture permeability after drying in the drying step.
- a cross section of the sheet 20 and stamper 100 is shown.
- the raw material aqueous solution 110 is present in the moisture permeable sheet 20 on which the stamper 100 is placed so as to be sandwiched between the stamper 100 and the moisture permeable sheet 20.
- the stamper 100 is made of, for example, a resin and has poor water vapor permeability.
- the moisture permeable sheet 20 has a large number of vapor permeable holes (not shown) having a hole diameter of 0.1 ⁇ m to 100 ⁇ m, the moisture permeable sheet 20 is well permeable to vapor. Therefore, the raw material aqueous solution 110 is dried by diffusing water vapor to the outside through the moisture permeable sheet 20. In order to assist this drying, the outer surface 20b side of the moisture permeable sheet 20 is kept at a pressure lower than the atmospheric pressure continuously from the placing step. In order to promote drying, it is preferable that the suction hole 203 is evacuated by a vacuum pump or the like.
- the arm 201 side is preferably adjusted to the same pressure as the suction hole 203. Such a state of pressure is indicated by an arrow symbol in FIGS. 7 (a) and 7 (b).
- the microneedle sheet 10 is formed between the moisture permeable sheet 20 and the stamper 100 as shown in FIG.
- FIG. 8 shows a peeling step for peeling the stamper 100 from the moisture permeable sheet 20.
- the stamper 100 is gently lifted from the moisture permeable sheet 20 with the moisture permeable sheet 20 adsorbed to the pedestal 202.
- the microneedle 11 is removed from the fine through hole 101 of the stamper 100, and the microneedle sheet 10 having the microneedle 11 is formed so as to directly contact and adhere to the moisture permeable sheet 20.
- a pressure higher than the atmosphere may be applied from the back surface 100 b side of the stamper 100 so that the microneedle 11 is easily detached from the stamper 100.
- FIGS. 9 and 10 respectively show the state during and after the assembly of the patch for transdermal administration assembled in the assembly process.
- FIG. 9 shows a state where the cover film 40 is adhered to the moisture-permeable sheet 20 of FIG.
- the release sheet 23 is peeled off.
- the lower cover film 41 is first attached to the pressure-sensitive adhesive layer 22, and then the upper cover film 42 is attached.
- the lower cover film 41 and the upper cover film 42 are attached by overlapping the overlapping portions 41c and 42c with each other.
- the reinforcing film 30 is attached.
- the reinforcing film 30 is configured such that the detachable portion 32 can be removed at the time of use.
- the reinforcing film 30 includes an adhesive layer 35.
- the stuck reinforcing film 30 can be seen.
- the thus prepared patch 1 for transdermal administration is stored in, for example, a bag laminated with aluminum or the like so as to block water vapor so that the microneedle sheet 10 does not absorb moisture until use. Managed. Further, when the transdermal patch 1 is delivered to a consumer, it is handled in a state of being stored in a bag capable of blocking such water vapor.
- the manufacturing method of the patch for transdermal administration according to the second embodiment differs from the manufacturing method of the patch for transdermal administration of the first embodiment as shown in FIG. 11 (a). This is a point in which the polyurethane film 21A of the sheet 20A is provided with a large number of openings 26 having an opening diameter of 0.5 mm to 4.5 mm. Since the other points are the same as those in the first embodiment, the same components are denoted by the same reference numerals, and description thereof will be omitted as appropriate. As shown in FIG.
- openings 26 are formed in a staggered arrangement in the polyurethane film 21A of the moisture-permeable sheet 20A.
- the diameter of the opening 26 is preferably 0.5 mm or greater and 4.5 mm or less.
- the occupation ratio of the openings 46 per unit area is preferably 20% to 65%.
- the diameter of the opening 26 is smaller than 0.5 mm, the water permeability is deteriorated, and when the diameter is larger than 4.5 mm, it is difficult to obtain the area of the adhesive surface.
- the occupation ratio of the opening 26 is 20% or less, water is not sufficiently transmitted, and when the occupation ratio is 65% or more, sufficient adhesive strength cannot be obtained.
- the region where the opening 26 is formed is a first region Ar1 to which the microneedle sheet 10 (see FIG. 12) is attached.
- the total area of the openings 26 occupying the first region Ar1 is 20% to 65% of the area of the first region Ar1.
- the polyurethane film 21A it is preferable to use a film having vapor transmission holes of 0.1 ⁇ m to 100 ⁇ m, as in the first embodiment. Thereby, it is possible to prevent the skin from being steamed in the region where the pressure-sensitive adhesive layer 22 is formed.
- the second embodiment is different from the first embodiment only in that the moisture permeable sheet 20A is used.
- FIG. 12 shows the drying process of the second embodiment.
- the drying process is the same as the drying process of the first embodiment described with reference to FIG. 7, detailed description thereof is omitted.
- the opening 26 is present in the moisture permeable sheet 20A, the raw material aqueous solution 110 easily flows out toward the pedestal 202. Therefore, as in the first embodiment, it is preferable to set the pressure between the front surface 100a and the back surface 100b to be the same.
- the diameter of the suction hole 203 of the base 202 is set smaller in the second embodiment. Therefore, the pedestal 202 can also be configured using, for example, a porous material.
- FIG. 13 shows a cross-sectional structure of the transdermal patch 1A after the assembly process of the second embodiment. As can be seen by comparing FIG. 13 with FIG. 2 and FIG. 10, between the patch 1A for transdermal administration of the second embodiment and the patch 1 for transdermal administration of the first embodiment, those There is no difference other than the difference between the moisture-permeable sheets 20 and 20A in the configuration.
- the manufacturing method of the transdermal patch according to the third embodiment differs from the manufacturing method of the transdermal patch according to the first embodiment.
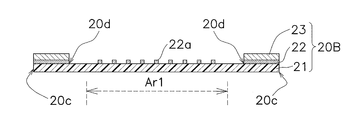
- the adhesive application portions 22a are discretely formed in the first region Ar1 to which the microneedle sheet 10 is fixed. Since the other points are the same as those in the first embodiment, the same components are denoted by the same reference numerals, and description thereof will be omitted as appropriate.
- FIG. 15 shows a cross-sectional structure of the transdermal patch 1B after the assembly process of the third embodiment. As can be seen by comparing FIG. 15 with FIG. 2 and FIG. 10, there is no difference other than the difference between the moisture-permeable sheets 20 and 20B.
- the adhesive application portion 22a exists between the microneedle sheet 10 and the moisture-permeable sheet 20 even in a first region Ar1, so Compared with the patch 1 for transdermal administration of the embodiment, the adhesion of the microneedle sheet 10 to the moisture-permeable sheet 20B can be increased.
- ⁇ Fourth embodiment> (5) Method for Producing Patch for Transdermal Administration of Fourth Embodiment
- the manufacturing method of the transdermal patch according to the fourth embodiment is different from the manufacturing method of the transdermal patch according to the first embodiment in that the polyurethane film 21C of the moisture-permeable sheet 20C shown in FIG.
- a breathable water absorbent sheet 27 is provided in a range wider than the third region Ar3 to which the water vapor barrier sheet 31 is fixed, and reaches the water absorbent sheet 27 through the polyurethane film 21C.
- the vent hole 28 is provided.
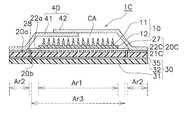
- the water absorbent sheet 27 is formed of, for example, a fiber sheet or a sponge sheet of a water absorbent polymer. Since the configuration of the patch for transdermal administration 1C of the fourth embodiment other than these is the same as that of the first embodiment, the same components are denoted by the same reference numerals, and description thereof will be omitted as appropriate.
- FIG. 16 shows the cross-sectional structure of the transdermal patch 1C after the assembly process of the fourth embodiment.
- a vent hole 28 is provided outside the third region Ar3. The vent hole 28 reaches the water absorbent sheet 27, and through the vent hole 28, the moisture adsorbed on the water absorbent sheet 27 can be sucked and released.
- the water absorbent sheet 27 is stuck to the polyurethane film 21C by the adhesive application portions 22a that are discretely arranged. Therefore, in the drying process, water vapor can be released to the outside through the air holes 28 and the vapor transmission holes of the pressure-sensitive adhesive layer 22 and the polyurethane film 21 ⁇ / b> C via the air-permeable water absorbent sheet 27. On the other hand, at the time of use, the water vapor coming out from the skin can be introduced into the microneedle sheet 10 by blocking with the water vapor blocking sheet 31. Further, when it is desired to supply a large amount of water to the microneedle sheet 10, water can be supplied from the vent hole 28 to the water absorbent sheet 27.
- the water absorbent sheet 27 is composed of a fiber sheet or a sponge sheet of a water absorbent polymer, it has high water retention. Therefore, when it is desired to supply moisture to the microneedle sheet 10 for a relatively long time, it is convenient to supply moisture using the water absorbent sheet 27.
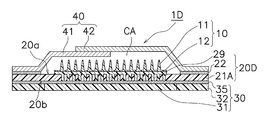
- the manufacturing method of the transdermal patch according to the fifth embodiment is different from the manufacturing method of the transdermal patch according to the second embodiment in that the moisture-permeable sheet 20D shown in FIG. This is a point having a porous sheet-like base material 29 in the first region Ar1 to which the sheet 10 is fixed. Since the other points are the same as those of the second embodiment, the same components are denoted by the same reference numerals, and description thereof will be omitted as appropriate.
- the moisture permeable sheet 20D shown in FIG. 17 shows a cross-sectional structure of a transdermal patch 1D after the assembly process of the fifth embodiment.
- the polyurethane film 21A of the moisture permeable sheet 20D shown in FIG. 17 is provided with a large number of openings 26 as in the second embodiment.
- the moisture permeable sheet 20D has a sheet-like base material 29 formed by applying a raw material aqueous solution directly in the first region Ar1 and drying it.
- the sheet-like base material 29 is porous and has a large number of through-holes reaching from the surface thereof to the skin facing surface 20a of the moisture-permeable sheet 20D or the outer surface 20b side of the moisture-permeable sheet 20D of the opening 26.
- a porous sheet-like base material 29 can be produced using, for example, a freeze-drying manufacturing method (vacuum freeze-drying method).
- the porous sheet-like base material 29 may be formed by drying a raw material aqueous solution into a sheet shape on the polyurethane film 21A and then forming a large number of through holes penetrating to the polyurethane film 21A by mechanical processing such as press molding. Can be made.
- the stamper 100 see FIG.
- the raw material aqueous solution 110 is sandwiched between the stamper 100 and the sheet-like base material 29. That is, the microneedle sheet 10 shown in FIG. 17 is replaced with the raw material aqueous solution 110 before drying.
- the drying step since the sheet-like base material 29 has already been dried, the moisture of the raw material aqueous solution 110 is absorbed by the sheet-like base material 29, so that drying is promoted.
- the pores of the sheet-like base material 29 are partially blocked during drying, the water retention is increased due to the porosity, and it becomes easy to stably supply moisture to the microneedle sheet 10.
- ⁇ Sixth Embodiment> when the pressure-sensitive adhesive layer or the adhesive layer does not exist between the polyurethane films 21, 21A, 21C of the moisture-permeable sheets 20, 20A, 20B, 20C, 20D and the microneedle sheet 10.
- an adhesive layer 322 may be formed between the polyurethane film 321 and the microneedle sheet 310 of the moisture-permeable sheet 320.
- the transdermal patch 1E shown in FIG. 18 mainly includes a microneedle sheet fixing step for fixing the microneedle sheet 310 to the first region Ar1 of the moisture-permeable sheet 320 and an assembly for attaching the reinforcing film 30E. It is manufactured through processes.
- a water-soluble microneedle sheet 310 that is already dried and has a plurality of water-soluble microneedles 311 formed in an array is prepared. And the board
- the moisture permeable sheet 320 has a large number of vapor permeable holes having a diameter of 0.1 ⁇ m to 100 ⁇ m, preferably 10 ⁇ m to 30 ⁇ m, which transmit water vapor, and an opening diameter of 0.5 mm or more 4
- a polyurethane film 321 having at least one of a large number of openings of 5 mm or less is included.
- the moisture-permeable sheet 320 has the adhesive layer 322 for sticking on skin on the skin opposing surface 320a.
- the moisture permeable sheet 320 is configured to transmit water vapor from the vapor transmission holes of the pressure-sensitive adhesive layer 322 and the polyurethane film 321 so that the skin where the moisture permeable sheet 320 is affixed does not get steamed.
- the adhesive layer 322 is sparsely applied so that the application area is small so as not to block all the vapor transmission holes.
- a reinforcing film 30 ⁇ / b> E including a water vapor blocking sheet 31 that blocks water vapor permeation through the first region Ar ⁇ b> 1 is attached by the adhesive layer 35.
- the water vapor blocking sheet 31 is disposed so as to cover the first region Ar1 and the third region Ar3 including the periphery thereof.
- the reinforcing film 30 ⁇ / b> E integrally includes the water vapor barrier sheet 31 and the removal portion 32 disposed around the water vapor barrier sheet 31. Therefore, the reinforcing function and the water vapor blocking function can be added simultaneously by a single operation of attaching the single reinforcing film 30E to the moisture-permeable sheet 320.
- a reinforcing film 30E shown in FIG. 18 is different from the reinforcing film 30 of the first embodiment in that the tab 36 is provided.
- the tab 36 is attached to the detachable portion 32 and is a tab for making it easy to peel the detachable portion 32. As shown in FIG.
- the adhesive Since the adhesive force of the layer 35 is weaker, only the removed portion 32 can be peeled while the moisture permeable sheet 320 is stuck on the skin 500. At this time, the water vapor blocking sheet 31 and the removal portion 32 can be separated from each other with the separation wire 37 as a boundary, and only the removal portion 32 can be peeled off. Therefore, the patch 1E for transdermal administration can be applied to the skin while the microneedle 311 is firmly pressed against the skin.
- the above-mentioned reinforcement film 30 which does not provide the tab 36 can also be used for a reinforcement film.
- the substrate 312 of the microneedle sheet 310 may include a porous layer like the porous sheet-like base material 29 described in the fifth embodiment, and the entire substrate 312 is porous. May be.
- the manufacturing method of the patch for transdermal administration of the first embodiment is the completion of the assembly process described with reference to FIGS. 9 and 10 in the adhesive forming process described with reference to FIG. 4 or the adhesive forming process in place thereof.
- an adhesive is formed on 2nd area
- region Ar1 an example of raw material aqueous solution contact area
- the pressure-sensitive adhesive forming step only needs to be able to form the pressure-sensitive adhesive layer 22 (an example of the first pressure-sensitive adhesive layer) before the assembly process is completed.
- the pressure-sensitive adhesive layer is formed on the cover film 40 and the cover film 40 is formed.
- coating an adhesive to the polyurethane film 21 of the moisture-permeable sheet 20 can be provided before an assembly process, and it can also be made into an adhesive formation process.
- the step of preparing the moisture permeable sheets 20A, 20B, 20C, and 20D with the adhesive layers 22 and 22C attached to the polyurethane films 21 and 21A is the same as the first embodiment. It is said.
- the raw material aqueous solution 110 of the microneedle 11 is applied to the stamper 100 having the fine through-hole 101 for forming the microneedle 11.
- the application process in which the application process is performed only once has been described.
- the microneedle sheet 10 may have a multilayer structure by repeating the application process and the drying process a plurality of times.
- the moisture permeable sheet 20 is placed in contact with the raw material aqueous solution 110 so that the applied raw material aqueous solution 110 is sandwiched between the stamper 100 and the moisture permeable sheet 20. To do.
- the moisture permeable sheet 20 Since the moisture permeable sheet 20 has a vapor permeable hole, the moisture vapor of the raw material aqueous solution 110 can be transmitted.
- the moisture permeable sheet 20 is on the bottom, but the moisture permeable sheet 20 may be placed on the stamper 100.
- the raw material aqueous solution 110 is dried between the stamper 100 and the moisture-permeable sheet 20. In this drying step, at least substantially all of the water in the raw material aqueous solution 110 is evaporated through the moisture permeable sheet 20, and the microneedle sheet 10 having the microneedles 11 is formed by the dried body of the raw material aqueous solution 110.
- part of the water in the raw material aqueous solution 110 may be evaporated from the skin facing surface 20a side of the moisture permeable sheet 20, for example. Further, in the drying step, as shown in FIG. 12, an opening 26 may be provided in the moisture permeable sheet 20 so that water vapor is evaporated from the opening 26.
- the stamper 100 is peeled from the microneedles 11 formed in the drying process.
- the microneedle 11 and the stamper 100 are separated by peeling the stamper 100 from the microneedle sheet 10.
- the patch 1 for transdermal administration is assembled using the moisture-permeable sheet 20.
- the reinforcing film 30 and the cover film 40 are attached to the moisture permeable sheet 20 to form the transdermal administration patch 1.
- the moisture permeable sheet 20 has a pressure-sensitive adhesive layer 22 for fixing the microneedles 11 by a drying process and for adhering to the skin.
- the moisture permeable sheets 20A, 20B, 20C, and 20D are used instead of the moisture permeable sheet 20, and the application process, the placing process, and the drying process are performed. Through the peeling process and the assembling process, the patches 1A, 1B, 1C, and 1D for transdermal administration are obtained.
- the microneedles 11 are formed by allowing water vapor to pass through the moisture-permeable sheets 20, 20A, 20B, 20C, and 20D in the drying process, the micro-solution of the raw material aqueous solution 110 is directly applied to the moisture-permeable sheets 20, 20A, 20B, 20C, and 20D. It is made to dry in the state which contacted the needle seat 10 (an example of a dry body). As described above, the microneedle sheet 10 and the moisture permeable sheets 20, 20A, 20B, 20C, and 20D are not separated by the adhesive layer or the pressure-sensitive adhesive layer by sandwiching the adhesive layer or the pressure-sensitive adhesive layer.
- the microneedle 11 is fixed to the moisture permeable sheets 20, 20A, 20B, 20C, 20D, so the moisture permeable sheets 20, 20A, 20B, 20C, 20D are obtained.
- the process for adhering the microneedles 11 is omitted, and the productivity is improved.
- the moisture permeable sheets 20, 20B, 20C, and 320 of the first, third, fourth, and sixth embodiments are polyurethane films 21, 21A, 21C, and 321 (a plurality of vapor transmission holes having a hole diameter of 0.1 ⁇ m to 100 ⁇ m).
- the preferred pore diameters of the polyurethane films 21, 21A, 21C, 321 are 10 ⁇ m to 30 ⁇ m.
- the moisture permeable sheet 20 includes a plurality of vapor transmission holes having a hole diameter of 0.1 ⁇ m to 100 ⁇ m and a plurality of openings having an opening diameter of 0.5 mm to 4.5 mm.
- a plastic film with both may be included.
- a polyurethane film in which the opening 26 of the polyurethane film 21A is formed on the entire surface corresponds to such a film.
- the moisture-permeable sheet may include a plastic film having only a plurality of openings having an opening diameter of 0.5 mm to 4.5 mm.
- seat may contain the fiber sheet.
- the moisture permeable sheet 20 is obtained by using the polyurethane films 21, 21A, 21C, 321 or the fiber sheet having the vapor permeable holes and the openings 26 for the moisture permeable sheets 20, 20A, 20B, 20C, 20D, and 320. , 20A, 20B, 20C, 20D, 320 can permeate water vapor from the skin.
- transdermal patches 1, 1A, 1B, 1C, 1D, and 1E that do not get steamed without modification of the moisture-permeable sheets 20, 20A, 20B, 20C, 20D, and 320, and do not get steamed.
- Patches for transdermal administration 1, 1A, 1B, 1C, 1D, and 1E can be provided at low cost.
- the moisture permeable sheets 20 and 20A are adsorbed on the flat base 202, thereby drying the moisture permeable sheets 20 and 20A while keeping them flat. .
- the warp of the microneedle sheet 10 an example of a dried body of the raw material aqueous solution
- the administration patch 1, 1A can be produced efficiently.
- the moisture-permeable sheets 20B, 20C, and 20D of the third to fifth embodiments are handled in the same manner as in the first and second embodiments, and have the same effects.
- the method of holding the moisture permeable sheets 20 and 20A flat is not limited to the method of adsorbing to the flat pedestal 202.
- the moisture permeable sheet is fixed on a flat plate-like member by a clamp or the like. You may comprise as follows.
- the moisture-permeable sheet 20D of the fifth embodiment has a porous sheet-like substrate 29 on the moisture-permeable sheet 20D.
- the sheet-like base material 29 is already dried in a step corresponding to the adhesive forming step (see FIG. 4) of the first embodiment, in which the raw material aqueous solution is applied in a sheet shape.
- the sheet-like base material 29 is formed in the first region Ar1, and contacts the raw material aqueous solution 110 (see FIG. 6) in the placing step.
- the drying step includes a step of forming the microneedles 11 by drying the sheet-like base material 29 in contact with the raw material aqueous solution 110 filled with the fine holes. Thereby, at the time of drying, the sheet-like base material 29 in contact with the raw material aqueous solution 110 can absorb moisture, and the production rate can be improved.
- the moisture permeable sheet 20C of the fourth embodiment is a polyurethane having a plurality of vapor transmission holes having a hole diameter of 0.1 ⁇ m to 100 ⁇ m, preferably 10 ⁇ m to 30 ⁇ m, and a plurality of openings 26 having an opening diameter of 0.5 mm to 4.5 mm.
- a water-absorbing sheet 27 (an example of a water-absorbing layer) is provided on the film 21C. The water absorbent sheet 27 comes into contact with the raw material aqueous solution 110 in the placing process.
- the water absorbent sheet 27 is composed of a fiber sheet or a sponge sheet of a water absorbent polymer.
- the water absorbing sheet 27 has a water absorbing layer made of a fiber sheet or a water absorbing layer containing a water absorbing polymer.
- the drying step includes a step of forming the microneedles 11 by drying the water absorbent sheet 27 in a state where it is in contact with the raw material aqueous solution 110 (see FIG. 6) filled in the fine through-holes 101.
- moisture can be absorbed by the water absorbent sheet 27 in contact with the raw material aqueous solution 110 at the time of drying, and the production rate can be improved. Can do.
- the patches 1, 1A, 1B, 1C, 1D, and 1E for transdermal administration of the first to sixth embodiments include microneedle sheets 10 and 310, moisture-permeable sheets 20, 20A, 20B, 20C, 20D, and 320 and water vapor.
- a barrier sheet 31 is provided.
- the microneedle sheets 10 and 310 have water-soluble sheet-like substrates 12 and 312 and a plurality of water-soluble microneedles 11 and 311 formed in an array on the sheet-like substrates 12 and 312. ing.
- the moisture permeable sheets 20, 20 ⁇ / b> A, 20 ⁇ / b> B, 20 ⁇ / b> C, 20 ⁇ / b> D, 320 are fixed to the first region Ar ⁇ b> 1 on the side of the skin facing surfaces 20 a, 320 a where the microneedle sheets 10, 310 face the skin. Further, the moisture permeable sheets 20, 20A, 20B, 20C, 20D, and 320 are formed by applying an adhesive to the second region Ar2 other than the first region Ar1 on the skin facing surfaces 20a and 320a side.
- the moisture permeable sheets 20, 20A, 20B, 20C, 20D, and 320 are polyurethane films 21, 21A, and 21C (fibrous sheets or vapor transmission holes of 0.1 ⁇ m to 100 ⁇ m and an opening diameter of 0.5 mm to 4.5 mm). And an example of a plastic film having at least one of the plurality of openings in the first region) and transmits water vapor.
- the water vapor barrier sheet 31 provided in the transdermal patch 1, 1A, 1B, 1C, 1D, 1E having such a configuration is a moisture permeable sheet 20, 20A, 20B, 20C opposite to the skin facing surfaces 20a, 320a.
- 20D, 320 is formed on the outer surfaces 20b, 320b, and in the third region A3 including all of the first region Ar1 and the region around the first region Ar1, the water vapor that is transmitted from the outer surfaces 20b, 320a side to the outside Shut off.
- the water vapor barrier sheet 31 blocks water vapor that comes out of the skin and permeates through the vapor transmission holes of the microneedle sheets 10 and 310 and the moisture permeable sheets 20, 20 ⁇ / b> A, 20 ⁇ / b> B, 20 ⁇ / b> C, 20 ⁇ / b> D, and 320. . Therefore, the water vapor coming out of the skin can be used by the water vapor blocking sheet 31, and it is possible to promote the supply of water to the microneedle sheets 10 and 310 to dissolve them.
- the moisture-permeable sheets 20, 20A, 20B, 20C, 20D, and 320 are prevented from being deformed by the water vapor-blocking sheet 31, and are fixed to the moisture-permeable sheets 20, 20A, 20B, 20C, 20D, and 320.
- the microneedle sheets 10 and 310 can be prevented from peeling off due to deformation of the moisture permeable sheets 20, 20 ⁇ / b> A, 20 ⁇ / b> B, 20 ⁇ / b> C, 20 ⁇ / b> D, and 320.
- the patches 1, 1A, 1B, 1C, 1D, and 1E for transdermal administration of the first to sixth embodiments are pasted on the outer surfaces 20b and 320b of the moisture-permeable sheets 20, 20A, 20B, 20C, 20D, and 320, respectively. Is applied and covers the portion of the moisture permeable sheets 20, 20A, 20B, 20C, 20D, 320 that overlaps the second region Ar2, and has a larger loop stiffness value than the moisture permeable sheets 20, 20A, 20B, 20C, 20D, 320.
- Reinforcing films 30, 30E formed of a plastic film such as polypropylene, polyethylene, or polyester are provided.
- the reinforcing films 30 and 30E include the water vapor blocking sheet 31 as a part of the reinforcing films 30 and 30E, and portions other than the water vapor blocking sheet 31 are configured to be peelable during use.
- strengthening films 30 and 30E are adhesive layers with weaker adhesive strength than the adhesive layers 22 and 322 (example of a 1st adhesive layer) to moisture-permeable sheet 20,20A, 20B, 20C, 20D, 320. 35 (an example of the second pressure-sensitive adhesive layer). Therefore, after the patches 1, 1A, 1B, 1C, 1D, and 1E for transdermal administration are attached to the skin, the adhesive layers 22 and 322 have stronger adhesive strength than the adhesive layer 35.
- the patch 1, 1A, 1B, 1C, 1D, 1E for transdermal administration can be attached to the skin while the microneedles 11, 311 are firmly pressed against the skin.
- the reinforcing films 30 and 30E should maintain the shape that the patches for transdermal administration 1, 1A, 1B, 1C, 1D, and 1E are easy to hold. This eliminates the problem that the moisture-permeable sheets 20, 20A, 20B, 20C, 20D, 320 are deformed and the transdermal administration patch 1, 1A, 1B, 1C, 1D, 1E is difficult to stick to the skin. it can.
- the patches for transdermal administration 1, 1A and 1B are used. , 1C, 1D, 1E can be prevented from increasing.
- the water vapor barrier sheet 31 and the reinforcing films 30 and 30E are integrally attached to the moisture permeable sheets 20, 20A, 20B, 20C, 20D, and 320, so that the patches 1, 1A, 1B for transdermal administration are used. , 1C, 1D, 1E are easy to manufacture.
- the cover film 40 of the patch 1, 1A, 1B, 1C, 1D, 1E for transdermal administration is adhered to the moisture permeable sheets 20, 20A, 20B, 20C, 20D, 320 with an adhesive, and the reinforcing films 30, 30E.
- a cavity CA that encloses the microneedle sheets 10 and 310 is formed so as not to contact the microneedle sheets 10 and 310.
- the cover film 40 and the reinforcing film prevent the microneedles 11 and 311 from being damaged during the transportation of the patches 1, 1A, 1B, 1C, 1D, and 1E for transdermal administration.
- 30 and 30E, and the function of the transdermal patch 1, 1A, 1B, 1C, 1D, and 1E can be prevented from being deteriorated due to the damage of the microneedles 11 and 311.
- the moisture permeable sheets 20, 20 A, 20 B, 20 C, 20 D, 320 in the cavity CA formed by the cover film 40 and the spacers between the microneedle sheet 10 and the cover film 40 are provided.
- spacers SP1 and SP2 for keeping the cavity CA may be applied.
- the spacers SP1 and SP2 are plastic ribs formed on the lower cover film 41 and the upper cover film 42, respectively.
- the spacers SP1 and SP2 in FIG. 20 are in contact with the microneedle sheet 10, the present invention is not limited to such a mode.
- the spacers are brought into contact with the moisture permeable sheets 20, 20A, 20B, 20C, 20D, and 320.
- the cavity CA may be maintained.
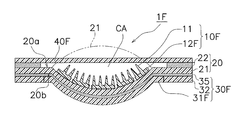
- the cavity CA is formed by forming the cover film 40 in a dome shape.
- the cavity CA may be formed by providing a dome-shaped portion on the water vapor blocking sheet 31F of the reinforcing film 30F.
- the dome-shaped portion of the water vapor blocking sheet 31F is protruded toward the skin facing surface 20a so that the polyurethane film 21 has a shape indicated by a two-dot chain line.
- the dome-shaped portion of the water vapor blocking sheet 31F protrudes toward the outer surface 20b.
- the dome-shaped portion of the water vapor blocking sheet 31F is pushed by, for example, a finger so as to protrude toward the skin facing surface 20a indicated by a two-dot chain line. Thereby, it becomes easy to press the microneedle 11 against the skin.
- the substrate 12F of the microneedle sheet 10F in FIG. 21 is divided so as to easily follow the deformation of the water vapor blocking sheet 31F.
- the moisture permeable sheet 320 of the sixth embodiment is a polyurethane having a plurality of vapor transmission holes having a hole diameter of 0.1 ⁇ m to 100 ⁇ m, preferably 10 ⁇ m to 30 ⁇ m, and a plurality of openings 26 having an opening diameter of 0.5 mm to 4.5 mm.
- the water-absorbent sheet is disposed in the first region Ar1, and the microneedle sheet 310 is moisture-permeable so that the microneedle sheet 310 contacts the water-absorbent sheet. It adheres to the sheet 320.
- a material in which a water absorbent sheet is formed on the back surface of the microneedle sheet 310 is attached to the adhesive layer 322.
- the water absorbent sheet is composed of, for example, a fiber sheet or a sponge sheet of a water absorbent polymer.
- the microneedle sheet 310 that is in contact with the water absorbent sheet in the first region Ar1 can be easily realized, and a water absorbent sheet that plays a role of retaining the water of the microneedle sheet 310 can be easily provided. it can.
Landscapes
- Health & Medical Sciences (AREA)
- Engineering & Computer Science (AREA)
- Mechanical Engineering (AREA)
- Dermatology (AREA)
- Animal Behavior & Ethology (AREA)
- Veterinary Medicine (AREA)
- Public Health (AREA)
- General Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Biomedical Technology (AREA)
- Medical Informatics (AREA)
- Anesthesiology (AREA)
- Heart & Thoracic Surgery (AREA)
- Hematology (AREA)
- Epidemiology (AREA)
- Pharmacology & Pharmacy (AREA)
- Medicinal Chemistry (AREA)
- Chemical & Material Sciences (AREA)
- Media Introduction/Drainage Providing Device (AREA)
- Medicinal Preparation (AREA)
Priority Applications (4)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US14/889,130 US9566423B2 (en) | 2013-05-07 | 2014-04-22 | Transdermal patch manufacturing method and transdemal patch |
| EP14794706.3A EP2995343A4 (en) | 2013-05-07 | 2014-04-22 | Method for producing transdermal patch and transdermal patch |
| CN201480025388.9A CN105263560A (zh) | 2013-05-07 | 2014-04-22 | 经皮给药用贴付剂的制造方法以及经皮给药用贴付剂 |
| KR1020157031792A KR101594908B1 (ko) | 2013-05-07 | 2014-04-22 | 경피투여용 첩부제의 제조 방법 및 경피투여용 첩부제 |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2013097660A JP5806701B2 (ja) | 2013-05-07 | 2013-05-07 | 経皮投与用貼付剤の製造方法及び経皮投与用貼付剤 |
| JP2013-097660 | 2013-05-07 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2014181674A1 true WO2014181674A1 (ja) | 2014-11-13 |
Family
ID=51867156
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/JP2014/061227 Ceased WO2014181674A1 (ja) | 2013-05-07 | 2014-04-22 | 経皮投与用貼付剤の製造方法及び経皮投与用貼付剤 |
Country Status (6)
| Country | Link |
|---|---|
| US (1) | US9566423B2 (enExample) |
| EP (1) | EP2995343A4 (enExample) |
| JP (1) | JP5806701B2 (enExample) |
| KR (1) | KR101594908B1 (enExample) |
| CN (1) | CN105263560A (enExample) |
| WO (1) | WO2014181674A1 (enExample) |
Cited By (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2016189844A (ja) * | 2015-03-31 | 2016-11-10 | 日本写真印刷株式会社 | マイクロニードルパッチ |
| EP3251721A4 (en) * | 2015-01-27 | 2018-11-07 | Toppan Printing Co., Ltd. | Transdermal administration device |
| JP2020185065A (ja) * | 2019-05-10 | 2020-11-19 | 国立研究開発法人産業技術総合研究所 | マイクロニードルおよびその製造方法 |
| JPWO2020250770A1 (enExample) * | 2019-06-11 | 2020-12-17 | ||
| JP2022512353A (ja) * | 2018-12-11 | 2022-02-03 | エルテーエス ローマン テラピー-ジステーメ アーゲー | マイクロニードルを製造する方法及び装置 |
Families Citing this family (19)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2014196522A1 (ja) * | 2013-06-03 | 2014-12-11 | 凸版印刷株式会社 | 針状体の製造方法及び製造装置 |
| JP5931110B2 (ja) * | 2014-03-26 | 2016-06-08 | 日本写真印刷株式会社 | 錐体状突起シートの梱包体の製造方法 |
| JP5931155B2 (ja) * | 2014-09-30 | 2016-06-08 | 日本写真印刷株式会社 | マイクロニードルシートの梱包体及びその製造方法 |
| EP3216484B1 (en) * | 2014-12-05 | 2023-02-01 | Hisamitsu Pharmaceutical Co., Inc. | Microneedle device system |
| TWD174921S (zh) | 2014-12-17 | 2016-04-11 | 日本碍子股份有限公司 | 複合基板之部分 |
| USD809804S1 (en) * | 2014-12-17 | 2018-02-13 | Ngk Insulators, Ltd. | Composite substrate for acoustic wave device |
| WO2016208635A1 (ja) * | 2015-06-23 | 2016-12-29 | 凸版印刷株式会社 | 針状体及び針状体の製造方法 |
| US10987502B2 (en) * | 2015-12-15 | 2021-04-27 | Hisamitsu Pharmaceutical Co., Inc. | Microneedle sheet |
| JP6848182B2 (ja) * | 2016-02-16 | 2021-03-24 | 凸版印刷株式会社 | 経皮投与デバイス収容体 |
| JP2017164191A (ja) * | 2016-03-15 | 2017-09-21 | 凸版印刷株式会社 | 経皮投与デバイス |
| WO2018056584A1 (ko) | 2016-09-21 | 2018-03-29 | 삼성전자 주식회사 | 피부 상태 측정 방법 및 이를 위한 전자 장치 |
| EP3338832A1 (en) * | 2016-12-23 | 2018-06-27 | Sanofi-Aventis Deutschland GmbH | Medicament delivery device |
| KR102547455B1 (ko) * | 2017-05-30 | 2023-06-23 | 닛샤 가부시키가이샤 | 마이크로 니들 패치와 그 곤포체 |
| JP7666887B2 (ja) * | 2018-12-21 | 2025-04-22 | ロレアル | マイクロニードルシートを使用するキット及び美容方法 |
| KR102577073B1 (ko) * | 2021-04-27 | 2023-09-11 | 주식회사 이엘홀딩스 | 마이크로 니들을 이용한 문신 패치 |
| KR102723893B1 (ko) * | 2021-07-27 | 2024-10-29 | 이승욱 | 마이크로 니들을 이용한 피부 트러블용 케어 패치 |
| WO2023114755A1 (en) * | 2021-12-13 | 2023-06-22 | WELSH Stephen | Adhesive bandage system for medication delivery and indication of procedure location |
| WO2023246407A1 (zh) * | 2022-06-22 | 2023-12-28 | 苏州悦肤达医疗科技有限公司 | 贴片产品加工系统及贴片产品加工方法 |
| KR200499729Y1 (ko) * | 2022-12-19 | 2025-11-12 | 주식회사 스몰랩 | 마이크로니들 패치 및 이를 이용한 피부미용 마사지기 |
Citations (10)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPS6247372A (ja) * | 1985-08-23 | 1987-03-02 | サ−メデイクス インコ−ポレイテド | 薬剤放出部材、その製造方法およびそれを用いた医用貼付材 |
| JP2004209074A (ja) * | 2003-01-07 | 2004-07-29 | Sanpo Kagaku Kk | 皮膚貼付シート及びその製造方法 |
| WO2006080508A1 (ja) * | 2005-01-31 | 2006-08-03 | Bioserentach Co., Ltd. | 経皮吸収製剤、経皮吸収製剤保持シート、及び経皮吸収製剤保持用具 |
| US20090182306A1 (en) * | 2006-07-21 | 2009-07-16 | Georgia Tech Research Corporation | Microneedle Devices and Methods of Drug Delivery or Fluid Withdrawal |
| JP2009528900A (ja) * | 2006-03-07 | 2009-08-13 | オラヘルス コーポレーション | 不透過性中央部を有した医療用多層パッチ |
| JP2010068840A (ja) * | 2008-09-16 | 2010-04-02 | Toppan Printing Co Ltd | 針状体および針状体製造方法 |
| JP2010094414A (ja) | 2008-10-20 | 2010-04-30 | Kyokko Seiko Co Ltd | マイクロニードルシート貼付剤とその製造方法および製造装置 |
| JP2010142473A (ja) * | 2008-12-19 | 2010-07-01 | Toppan Printing Co Ltd | 針状体の製造方法 |
| JP2011194189A (ja) | 2010-03-19 | 2011-10-06 | Kosumedei Seiyaku Kk | マイクロニードルの迅速溶解法 |
| JP2012075855A (ja) * | 2010-09-09 | 2012-04-19 | Bioserentack Co Ltd | 経皮吸収製剤包装投与器具 |
Family Cites Families (12)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4727868A (en) | 1984-11-13 | 1988-03-01 | Thermedics, Inc. | Anisotropic wound dressing |
| US4880690A (en) | 1984-11-13 | 1989-11-14 | Thermedics, Inc. | Perfume patch |
| US4751133A (en) | 1984-11-13 | 1988-06-14 | Thermedics, Inc. | Medical patches and processes for producing same |
| US4614787A (en) | 1984-11-13 | 1986-09-30 | Thermedics, Inc. | Drug dispensing wound dressing |
| US6656147B1 (en) * | 2000-07-17 | 2003-12-02 | Becton, Dickinson And Company | Method and delivery device for the transdermal administration of a substance |
| JP4222026B2 (ja) | 2002-12-27 | 2009-02-12 | コニカミノルタホールディングス株式会社 | 平版印刷版原版とそれを用いた印刷方法 |
| US7611481B2 (en) * | 2004-03-24 | 2009-11-03 | Corium International, Inc. | Transdermal delivery device |
| JP2006080508A (ja) * | 2004-08-27 | 2006-03-23 | Asahi Kasei Microsystems Kk | 半導体デバイス及びその製造方法 |
| ATE477833T1 (de) * | 2005-06-27 | 2010-09-15 | 3M Innovative Properties Co | Mikronadelkartuschenanordnung |
| US8349120B2 (en) | 2006-03-07 | 2013-01-08 | Ora Health Corporation | Multi-layer patch made on a sheet and enclosed in a blister |
| US20130018279A1 (en) * | 2009-09-01 | 2013-01-17 | Pathway Genomics | "blood sample collection apparatus and kits" |
| WO2013096026A1 (en) * | 2011-12-21 | 2013-06-27 | 3M Innovative Properties Company | Transdermal adhesive patch assembly with removable microneedle array and method of using same |
-
2013
- 2013-05-07 JP JP2013097660A patent/JP5806701B2/ja not_active Expired - Fee Related
-
2014
- 2014-04-22 EP EP14794706.3A patent/EP2995343A4/en not_active Withdrawn
- 2014-04-22 KR KR1020157031792A patent/KR101594908B1/ko not_active Expired - Fee Related
- 2014-04-22 CN CN201480025388.9A patent/CN105263560A/zh active Pending
- 2014-04-22 US US14/889,130 patent/US9566423B2/en not_active Expired - Fee Related
- 2014-04-22 WO PCT/JP2014/061227 patent/WO2014181674A1/ja not_active Ceased
Patent Citations (10)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPS6247372A (ja) * | 1985-08-23 | 1987-03-02 | サ−メデイクス インコ−ポレイテド | 薬剤放出部材、その製造方法およびそれを用いた医用貼付材 |
| JP2004209074A (ja) * | 2003-01-07 | 2004-07-29 | Sanpo Kagaku Kk | 皮膚貼付シート及びその製造方法 |
| WO2006080508A1 (ja) * | 2005-01-31 | 2006-08-03 | Bioserentach Co., Ltd. | 経皮吸収製剤、経皮吸収製剤保持シート、及び経皮吸収製剤保持用具 |
| JP2009528900A (ja) * | 2006-03-07 | 2009-08-13 | オラヘルス コーポレーション | 不透過性中央部を有した医療用多層パッチ |
| US20090182306A1 (en) * | 2006-07-21 | 2009-07-16 | Georgia Tech Research Corporation | Microneedle Devices and Methods of Drug Delivery or Fluid Withdrawal |
| JP2010068840A (ja) * | 2008-09-16 | 2010-04-02 | Toppan Printing Co Ltd | 針状体および針状体製造方法 |
| JP2010094414A (ja) | 2008-10-20 | 2010-04-30 | Kyokko Seiko Co Ltd | マイクロニードルシート貼付剤とその製造方法および製造装置 |
| JP2010142473A (ja) * | 2008-12-19 | 2010-07-01 | Toppan Printing Co Ltd | 針状体の製造方法 |
| JP2011194189A (ja) | 2010-03-19 | 2011-10-06 | Kosumedei Seiyaku Kk | マイクロニードルの迅速溶解法 |
| JP2012075855A (ja) * | 2010-09-09 | 2012-04-19 | Bioserentack Co Ltd | 経皮吸収製剤包装投与器具 |
Non-Patent Citations (1)
| Title |
|---|
| See also references of EP2995343A4 * |
Cited By (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP3251721A4 (en) * | 2015-01-27 | 2018-11-07 | Toppan Printing Co., Ltd. | Transdermal administration device |
| JP2016189844A (ja) * | 2015-03-31 | 2016-11-10 | 日本写真印刷株式会社 | マイクロニードルパッチ |
| JP2022512353A (ja) * | 2018-12-11 | 2022-02-03 | エルテーエス ローマン テラピー-ジステーメ アーゲー | マイクロニードルを製造する方法及び装置 |
| JP7420809B2 (ja) | 2018-12-11 | 2024-01-23 | エルテーエス ローマン テラピー-ジステーメ アーゲー | マイクロニードルを製造する方法及び装置 |
| US12186516B2 (en) | 2018-12-11 | 2025-01-07 | Lts Lohmann Therapie-Systeme Ag | Method and device for producing microneedles |
| JP2020185065A (ja) * | 2019-05-10 | 2020-11-19 | 国立研究開発法人産業技術総合研究所 | マイクロニードルおよびその製造方法 |
| JPWO2020250770A1 (enExample) * | 2019-06-11 | 2020-12-17 |
Also Published As
| Publication number | Publication date |
|---|---|
| US9566423B2 (en) | 2017-02-14 |
| KR20150129069A (ko) | 2015-11-18 |
| CN105263560A (zh) | 2016-01-20 |
| JP5806701B2 (ja) | 2015-11-10 |
| EP2995343A4 (en) | 2017-03-22 |
| JP2014217520A (ja) | 2014-11-20 |
| EP2995343A1 (en) | 2016-03-16 |
| KR101594908B1 (ko) | 2016-02-17 |
| US20160082240A1 (en) | 2016-03-24 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP5806701B2 (ja) | 経皮投与用貼付剤の製造方法及び経皮投与用貼付剤 | |
| US9913759B2 (en) | Wound dressing | |
| JP6320930B2 (ja) | 剥離可能な医療用ドレープ | |
| JP7141625B1 (ja) | マイクロニードルパッチ及びマイクロニードル構造体 | |
| US20110251561A1 (en) | Transdermal administration device | |
| JP2016067723A (ja) | 経皮投与用貼付剤の製造方法及び経皮投与用貼付剤 | |
| JP2014217520A5 (enExample) | ||
| JP6093048B2 (ja) | 陰圧閉鎖療法用スクリーンとフィルムドレッシング及び陰圧閉鎖療法用キット | |
| JP6290988B2 (ja) | マイクロ構造体製造方法 | |
| KR102547455B1 (ko) | 마이크로 니들 패치와 그 곤포체 | |
| WO2022211058A1 (ja) | マイクロニードル構造体の製造方法およびマイクロニードル構造体 | |
| TW202302175A (zh) | 微針構造體及其製造方法 | |
| CN220608864U (zh) | 微针无纺布 | |
| CN219782955U (zh) | 一种带撕口离型纸的外用贴剂 | |
| JP6317716B2 (ja) | 絆創膏及びその製造方法 | |
| JP3970639B2 (ja) | 貼着体 | |
| JP2008188273A (ja) | 薬物輸送デバイス | |
| JPH0248407Y2 (enExample) | ||
| JP2016022223A (ja) | 医療用保護フィルム材料ならびに製品 | |
| JPS6049511B2 (ja) | 液分吸収性部材 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| WWE | Wipo information: entry into national phase |
Ref document number: 201480025388.9 Country of ref document: CN |
|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 14794706 Country of ref document: EP Kind code of ref document: A1 |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 14889130 Country of ref document: US |
|
| ENP | Entry into the national phase |
Ref document number: 20157031792 Country of ref document: KR Kind code of ref document: A |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2014794706 Country of ref document: EP |