WO2014119748A1 - 窒化銅微粒子およびその製造方法 - Google Patents
窒化銅微粒子およびその製造方法 Download PDFInfo
- Publication number
- WO2014119748A1 WO2014119748A1 PCT/JP2014/052321 JP2014052321W WO2014119748A1 WO 2014119748 A1 WO2014119748 A1 WO 2014119748A1 JP 2014052321 W JP2014052321 W JP 2014052321W WO 2014119748 A1 WO2014119748 A1 WO 2014119748A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- copper
- fine particles
- nitride fine
- copper nitride
- particles according
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Ceased
Links
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C23—COATING METALLIC MATERIAL; COATING MATERIAL WITH METALLIC MATERIAL; CHEMICAL SURFACE TREATMENT; DIFFUSION TREATMENT OF METALLIC MATERIAL; COATING BY VACUUM EVAPORATION, BY SPUTTERING, BY ION IMPLANTATION OR BY CHEMICAL VAPOUR DEPOSITION, IN GENERAL; INHIBITING CORROSION OF METALLIC MATERIAL OR INCRUSTATION IN GENERAL
- C23C—COATING METALLIC MATERIAL; COATING MATERIAL WITH METALLIC MATERIAL; SURFACE TREATMENT OF METALLIC MATERIAL BY DIFFUSION INTO THE SURFACE, BY CHEMICAL CONVERSION OR SUBSTITUTION; COATING BY VACUUM EVAPORATION, BY SPUTTERING, BY ION IMPLANTATION OR BY CHEMICAL VAPOUR DEPOSITION, IN GENERAL
- C23C24/00—Coating starting from inorganic powder
- C23C24/08—Coating starting from inorganic powder by application of heat or pressure and heat
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B82—NANOTECHNOLOGY
- B82Y—SPECIFIC USES OR APPLICATIONS OF NANOSTRUCTURES; MEASUREMENT OR ANALYSIS OF NANOSTRUCTURES; MANUFACTURE OR TREATMENT OF NANOSTRUCTURES
- B82Y30/00—Nanotechnology for materials or surface science, e.g. nanocomposites
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B82—NANOTECHNOLOGY
- B82Y—SPECIFIC USES OR APPLICATIONS OF NANOSTRUCTURES; MEASUREMENT OR ANALYSIS OF NANOSTRUCTURES; MANUFACTURE OR TREATMENT OF NANOSTRUCTURES
- B82Y40/00—Manufacture or treatment of nanostructures
-
- C—CHEMISTRY; METALLURGY
- C01—INORGANIC CHEMISTRY
- C01B—NON-METALLIC ELEMENTS; COMPOUNDS THEREOF; METALLOIDS OR COMPOUNDS THEREOF NOT COVERED BY SUBCLASS C01C
- C01B21/00—Nitrogen; Compounds thereof
- C01B21/06—Binary compounds of nitrogen with metals, with silicon, or with boron, or with carbon, i.e. nitrides; Compounds of nitrogen with more than one metal, silicon or boron
- C01B21/0615—Binary compounds of nitrogen with metals, with silicon, or with boron, or with carbon, i.e. nitrides; Compounds of nitrogen with more than one metal, silicon or boron with transition metals other than titanium, zirconium or hafnium
- C01B21/0625—Binary compounds of nitrogen with metals, with silicon, or with boron, or with carbon, i.e. nitrides; Compounds of nitrogen with more than one metal, silicon or boron with transition metals other than titanium, zirconium or hafnium with copper
-
- C—CHEMISTRY; METALLURGY
- C23—COATING METALLIC MATERIAL; COATING MATERIAL WITH METALLIC MATERIAL; CHEMICAL SURFACE TREATMENT; DIFFUSION TREATMENT OF METALLIC MATERIAL; COATING BY VACUUM EVAPORATION, BY SPUTTERING, BY ION IMPLANTATION OR BY CHEMICAL VAPOUR DEPOSITION, IN GENERAL; INHIBITING CORROSION OF METALLIC MATERIAL OR INCRUSTATION IN GENERAL
- C23C—COATING METALLIC MATERIAL; COATING MATERIAL WITH METALLIC MATERIAL; SURFACE TREATMENT OF METALLIC MATERIAL BY DIFFUSION INTO THE SURFACE, BY CHEMICAL CONVERSION OR SUBSTITUTION; COATING BY VACUUM EVAPORATION, BY SPUTTERING, BY ION IMPLANTATION OR BY CHEMICAL VAPOUR DEPOSITION, IN GENERAL
- C23C16/00—Chemical coating by decomposition of gaseous compounds, without leaving reaction products of surface material in the coating, i.e. chemical vapour deposition [CVD] processes
- C23C16/22—Chemical coating by decomposition of gaseous compounds, without leaving reaction products of surface material in the coating, i.e. chemical vapour deposition [CVD] processes characterised by the deposition of inorganic material, other than metallic material
- C23C16/30—Deposition of compounds, mixtures or solid solutions, e.g. borides, carbides, nitrides
- C23C16/34—Nitrides
-
- C—CHEMISTRY; METALLURGY
- C23—COATING METALLIC MATERIAL; COATING MATERIAL WITH METALLIC MATERIAL; CHEMICAL SURFACE TREATMENT; DIFFUSION TREATMENT OF METALLIC MATERIAL; COATING BY VACUUM EVAPORATION, BY SPUTTERING, BY ION IMPLANTATION OR BY CHEMICAL VAPOUR DEPOSITION, IN GENERAL; INHIBITING CORROSION OF METALLIC MATERIAL OR INCRUSTATION IN GENERAL
- C23C—COATING METALLIC MATERIAL; COATING MATERIAL WITH METALLIC MATERIAL; SURFACE TREATMENT OF METALLIC MATERIAL BY DIFFUSION INTO THE SURFACE, BY CHEMICAL CONVERSION OR SUBSTITUTION; COATING BY VACUUM EVAPORATION, BY SPUTTERING, BY ION IMPLANTATION OR BY CHEMICAL VAPOUR DEPOSITION, IN GENERAL
- C23C16/00—Chemical coating by decomposition of gaseous compounds, without leaving reaction products of surface material in the coating, i.e. chemical vapour deposition [CVD] processes
- C23C16/44—Chemical coating by decomposition of gaseous compounds, without leaving reaction products of surface material in the coating, i.e. chemical vapour deposition [CVD] processes characterised by the method of coating
- C23C16/4417—Methods specially adapted for coating powder
-
- C—CHEMISTRY; METALLURGY
- C01—INORGANIC CHEMISTRY
- C01P—INDEXING SCHEME RELATING TO STRUCTURAL AND PHYSICAL ASPECTS OF SOLID INORGANIC COMPOUNDS
- C01P2002/00—Crystal-structural characteristics
- C01P2002/70—Crystal-structural characteristics defined by measured X-ray, neutron or electron diffraction data
- C01P2002/72—Crystal-structural characteristics defined by measured X-ray, neutron or electron diffraction data by d-values or two theta-values, e.g. as X-ray diagram
-
- C—CHEMISTRY; METALLURGY
- C01—INORGANIC CHEMISTRY
- C01P—INDEXING SCHEME RELATING TO STRUCTURAL AND PHYSICAL ASPECTS OF SOLID INORGANIC COMPOUNDS
- C01P2002/00—Crystal-structural characteristics
- C01P2002/80—Crystal-structural characteristics defined by measured data other than those specified in group C01P2002/70
- C01P2002/88—Crystal-structural characteristics defined by measured data other than those specified in group C01P2002/70 by thermal analysis data, e.g. TGA, DTA, DSC
-
- C—CHEMISTRY; METALLURGY
- C01—INORGANIC CHEMISTRY
- C01P—INDEXING SCHEME RELATING TO STRUCTURAL AND PHYSICAL ASPECTS OF SOLID INORGANIC COMPOUNDS
- C01P2004/00—Particle morphology
- C01P2004/01—Particle morphology depicted by an image
- C01P2004/04—Particle morphology depicted by an image obtained by TEM, STEM, STM or AFM
-
- C—CHEMISTRY; METALLURGY
- C01—INORGANIC CHEMISTRY
- C01P—INDEXING SCHEME RELATING TO STRUCTURAL AND PHYSICAL ASPECTS OF SOLID INORGANIC COMPOUNDS
- C01P2004/00—Particle morphology
- C01P2004/30—Particle morphology extending in three dimensions
- C01P2004/32—Spheres
-
- C—CHEMISTRY; METALLURGY
- C01—INORGANIC CHEMISTRY
- C01P—INDEXING SCHEME RELATING TO STRUCTURAL AND PHYSICAL ASPECTS OF SOLID INORGANIC COMPOUNDS
- C01P2004/00—Particle morphology
- C01P2004/60—Particles characterised by their size
- C01P2004/64—Nanometer sized, i.e. from 1-100 nanometer
Definitions
- the present invention relates to copper nitride fine particles, a method for producing the same, and a wiring ink material and a substrate to be printed using the copper nitride fine particles.
- wiring patterns can be formed by applying screen printing and ink jet methods.
- metal wiring materials is actively conducted as one of the core materials of the technology.
- silver nanoparticles since silver has high ion migration and high price, studies on wiring methods using copper nanoparticles have been attempted to solve this problem.
- copper needs to be processed at a temperature as high as 350 ° C. or higher after printing in order to develop conductivity. Therefore, wiring is performed on a polymer substrate such as polyimide or polyethylene terephthalate. I can't.
- Non-Patent Document 1 Research on copper nitride crystals has been studied for the purpose of memory material applications.
- Patent Documents 1 and 2 since copper nitride crystals have oxidation resistance, they are prepared as an oxidation-resistant film of metallic copper, and patents relating to the manufacturing method have been filed (Patent Documents 1 and 2).
- the conventional methods for preparing these copper nitrides mainly use a solid phase reaction, and are prepared by bringing an ammonia gas into contact with an inorganic copper salt such as copper chalcogenide or metallic copper at a high temperature. (Non-patent document 2).
- Non-Patent Document 3 when copper aluminide and inorganic salt of copper are used as raw materials and heated under a spontaneous pressure in a toluene solvent using a pressure vessel, copper nitride Can be synthesized.
- a pressure vessel requires strict control of the reaction temperature and pressure, and has a risk of explosion, so it cannot be said to be a very simple method.
- Non-Patent Document 4 shows a reaction under normal pressure using a compound containing a nitrogen atom as a solvent.
- octadecylamine having a high boiling point (boiling point 232 ° C. at a pressure of 32 mmHg) is used as a solvent and a surface modifier, and the reaction is performed at a reaction temperature of 280 ° C. Since a large amount of high-boiling surface modifier remains on the surface of the copper nitride particles obtained from this document, it is unsuitable for a low-temperature wiring material, which is one of the purposes for using the copper nitride fine particles.
- the present invention develops copper nitride fine particles that decompose into copper and nitrogen at 300 ° C. or lower, and efficiently produces copper nitride fine particles in a liquid phase without using a pressurization process or a vacuum process. It aims to be able to.
- the present invention relates to copper nitride fine particles essentially free from the problems of metallic copper instability and high temperature treatment, and a method for producing the same.
- the present inventors have known that copper nitride has oxidation resistance and is decomposed into copper and nitrogen at a temperature of 350 ° C. or lower in the bulk state to give metallic copper, which is a material for solving the disadvantages of metallic copper. It was noted that. And as a result of advancing research to solve the above-mentioned problems, the primary particle size is 100 nm or less by heating the compound serving as the copper source and the compound serving as the nitrogen source in the organic solvent. It was possible to prepare copper nitride fine particles, and it was found that the decomposition temperature of the obtained copper nitride fine particles was 300 ° C. or less, and the present invention was completed.
- the present invention provides the following inventions in order to solve the above problems.
- Copper nitride fine particles having a primary particle size of 1 to 100 nm and a decomposition temperature of 300 ° C. or lower.
- any of CuK ⁇ ray is 21.5 to 24.5 °, 31.0 to 34.0 °, 39.0 to 42.0 °, 46.0 to 49.0 °
- a method for producing copper nitride fine particles comprising producing copper fine particles.
- a copper source contains 1 or more types chosen from inorganic copper salt, organic copper salt, and a copper complex.
- the nitrogen source contains one or more selected from ammonia gas or ammonium salt compound, urea, urea derivative compound, nitrate compound, amine compound, and azide compound A method for producing copper fine particles.
- the present invention it is possible to provide copper nitride fine particles that essentially do not have the problems of instability of metal copper and high temperature treatment, and a method for producing the same. Since the copper nitride fine particles can be wired by printing and have oxidation resistance to the air and water, the wiring is drawn on the film by printing or the like, and exhibits conductivity after heat treatment at 300 ° C. or lower. Material may be provided.
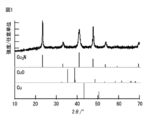
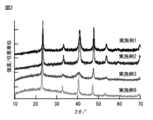
- the XRD spectrum of the copper nitride fine particles obtained in Examples 1, 2, 3 and 8. 4 is a TEM observation image of the copper nitride fine particles obtained in Example 1.
- FIG. 4 is a TEM observation image of copper nitride fine particles obtained in Example 3.
- FIG. The spectrum of the differential thermal balance analysis (atmospheric pressure and pressure reduction) of the copper nitride fine particles obtained in Example 1.
- the copper nitride fine particles of the present invention have a primary particle size of 1 to 100 nm and a decomposition temperature of 300 ° C. or lower.
- the primary particle size of the copper nitride fine particles of the present invention being 1 to 100 nm or less means that at least the minor axis diameter of the primary particles is 1 to 100 nm or less in electron microscope observation, and the decomposition temperature of the copper nitride fine particles
- the primary particle size is preferably 50 nm or less, more preferably 30 nm or less, more preferably 20 nm or less, and most preferably 10 nm or less.
- the particle size of the secondary particles is preferably 1 ⁇ m or less.
- the form of the secondary particles is preferably a substantially spherical particle form.
- the secondary particles when used as a coating solution, the secondary particles are excellent in fluidity and suitable for fine packing when coated. Even if the spherical shape is not a perfect spherical shape, the major axis and the minor axis may have a different aspect ratio, for example, up to about 3, preferably about 1.5.
- the particle shape of the primary particles may be any particle shape such as a square shape, a needle shape, or a rice grain shape, but a shape in which the secondary particles are likely to be spherical is preferable.
- the copper nitride fine particles of the present invention have a decomposition temperature accompanied by a decrease in weight (mass) in the range of 70 ° C. to 300 ° C. in differential thermal balance analysis (normal pressure).
- the decomposition temperature is 300 ° C. or less
- the heat treatment is performed after the copper nitride fine particles are applied as the wiring ink material and the polymer substrate or film is applied as the printing base material, the printing equipment is damaged by heat, for example, melted. And / or provides the advantage of being able to process at temperatures that are not subject to deformation, decomposition or carbonization.
- the copper nitride fine particles of the present invention are 21.5 to 24.5 °, 31.0 to 34.0 °, 39.0 to 42.0 °, 46.0 to 49.0 at CuK ⁇ rays in powder X-ray diffraction. It has a crystal structure of copper nitride (Cu 3 N) having at least one or more diffraction peaks derived from copper nitride in any region.
- the copper nitride fine particles described in the present invention may be provided as a powder or a colloidal dispersion, and an appropriate protective agent may be used to give the fine particles the dispersibility and stability of the copper nitride fine particles.
- the copper nitride fine particles of the present invention can be obtained by dissolving or dispersing a copper source and a nitrogen source, or a copper source, a nitrogen source and a protective agent in an organic solvent or a dispersion medium, and then heating.
- Examples of the copper-containing compound that serves as the copper source include the following inorganic copper salts, organic copper salts, or copper complexes.
- the oxidation number of copper contained in the copper salt or copper complex is monovalent or divalent. Either state may be used.
- the number of molecules coordinated to the copper element in order to stabilize the copper salt or copper complex may be any of 0-6.
- Inorganic copper salts include copper chloride, copper bromide, copper iodate, copper iodide, copper fluoride, basic copper carbonate, copper cyanide, copper azide, ammonium chloride copper, copper hydroxide, copper formate, oxy
- Examples include copper chloride, copper perchlorate, copper phosphate, potassium chloride copper, copper sulfate, basic copper sulfate, copper sulfate, copper sulfide, and the like.
- An oxide such as copper oxide can also be used.
- Organic copper salts include carboxyl group type, hydroxycarboxylic acid type, amino acid type, alkoxide type and the like.
- the carboxyl group type is one in which a linear or branched, cyclic saturated or unsaturated hydrocarbon chain having 1 to 20 carbon atoms and one carboxyl group are bonded to a copper element.
- a polyvalent carboxyl type in which two or more carboxyl groups are bonded to a linear, branched, or cyclic saturated or unsaturated hydrocarbon chain having 1 to 20 carbon atoms is also exemplified.
- copper acid and copper sebacate examples thereof include copper acid and copper sebacate.
- a molecule that binds to copper is a linear or branched, cyclic saturated or unsaturated hydrocarbon chain having 1 to 20 carbon atoms, at least one carboxyl group and one or more
- a molecule having a hydroxyl group is bound, for example, copper glycolate, copper lactate, copper tartrate, copper glycerate, copper hydroxybutyrate, copper 2-hydroxybutyrate, copper 3-hydroxybutyrate, ⁇ -hydroxy Copper butyrate, copper malate, copper tartrate, copper citrate, copper citrate, copper isocitrate, copper leucine, copper mevalonate, copper pantoate, copper ricinoleate, copper ricinaleinate, copper cerebrate, copper quinate, Examples thereof include copper gluconate.
- a molecule that binds to copper is a linear or branched, cyclic saturated or unsaturated hydrocarbon chain having 1 to 20 carbon atoms, and at least one carboxyl group and one or more amino groups.
- alanine copper salt, arginine copper salt, asparagine copper salt, aspartate copper, cysteine copper salt, glutamine copper salt, glutamate copper, glycine copper salt, histidine copper salt isoleucine
- examples thereof include copper salts, leucine copper salts, lysine copper salts, methionine copper salts, phenylalanine copper salts, proline copper salts, serine copper salts, threonine copper salts, tryptophan copper salts, tyrosine copper salts, and valine copper salts.
- the alkoxide type is a molecule in which a linear, branched, or cyclic saturated or unsaturated hydrocarbon chain having 1 to 10 carbon atoms is bonded to copper via an oxygen atom, and includes copper methoxide, copper ethoxide. And copper propoxide.
- organic copper compounds include hexafluoroacetonato copper, hexafluoro-2,4-pentadionato copper (I) cyclooctadiene complex, benzenesulfinic acid hydrate, copper dimethyldithiocarbamate, copper tetrafluoroborate , Copper trifluoromethanesulfonate, potassium tetrachlorocuprate, and the like.
- the molecules coordinated to the copper-containing compounds of the above inorganic copper salts and organic copper salts can be broadly classified as inorganic ligands and organic ligands. Examples thereof include water, ammonia, carbon monoxide and the like.
- the organic ligand also contains at least one oxygen atom and / or nitrogen atom that can be coordinated to a linear, branched, or cyclic saturated or unsaturated hydrocarbon chain having 1 to 40 carbon atoms. In addition to these ligands, there is no particular limitation as long as the copper-containing compound is stabilized.
- Particularly suitable copper-containing compounds include copper chloride, copper bromide, copper sulfate, copper nitrate, copper formate, which has a molecule or ion as an anion, which is decomposed or evaporated by heat treatment at 250 ° C. or less after the preparation of copper nitride fine particles.
- nitrogen source examples include compounds containing one or more selected from ammonia gas or ammonium salt compounds, urea, urea derivative compounds, nitrate compounds, amine compounds, and azide compounds.
- Ammonium salt compounds include ammonium azide, ammonium benzoate, ammonium chloride, ammonium chloride, ammonium chlorate, ammonium perchlorate, ammonium permanganate, ammonium chromate, ammonium acetate, ammonium nitrate, ammonium hydroxide, ammonium carbonate, Examples include ammonium hydrogen carbonate, ammonium thioglycolate, ammonium thiocyanate, ammonium hydrogen fluoride, ammonium iodide, ammonium iodate, ammonium sulfate, and ammonium phosphate.
- a urea derivative is one in which one or more amino groups of urea are bonded to a linear or branched, cyclic saturated or unsaturated hydrocarbon chain or aromatic ring having 1 to 20 carbon atoms.
- Urea N-ethyl-N'-phenylurea, o-ethoxyphenylurea, m-ethoxyphenylurea, p-ethoxyphenylurea, N, N'-diphenylurea, N, N'-diphenylurea, tetraphenylurea, Examples thereof include N-benzoylurea.
- An amine compound refers to a molecule in which a primary to quaternary amino group is bonded to a linear, branched, or cyclic saturated or unsaturated hydrocarbon chain having 1 to 20 carbon atoms.
- Examples include amine, ethylamine, propylamine, butylamine, amylamine, hexylamine, heptylamine, octylamine, nonylamine, decanylamine, cyclohexylamine, 2-methylcyclohexylamine, allylamine, oleylamine, aniline, toluidine, ethylaniline and the like.
- polyvalent amine compounds having two or more primary to quaternary amino groups in the molecule.
- diamines examples include diaminobutane, hexamethylenediamine, trimethylhexamethylenediamine, m-xylidinediamine, p- Phenylenediamine, m-phenylenediamine, toluylenediamine, 4,4'-diaminodiphenylmethane, 4,4'-diaminodiphenyl ether, 3,4'-diaminodiphenyl ether, 4,4'-diaminobiphenyl, 3,3'-dimethyl -4,4'-diaminobiphenyl, 4,4'-diaminodiphenyl sulfide, 2,6-diaminonaphthalene, 4,4'-bis (p-aminophenoxy) diphenyl sulfone, 4,4'-bis (m-amino) Phenoxy) diphenylsulfone, 4,4'-bis (p-amino) Enoxy) be
- azide compounds examples include hydrogen azide and sodium azide.
- a nitrogen-containing copper complex in which a copper source and a nitrogen source are bonded or coordinated can also be used.
- Suitable nitrogen-containing compounds include ammonia gas, ammonium chloride, ammonium bromide, ammonium acetate, methylamine, ethylamine, propylamine, butylamine, amylamine, which decompose or evaporate at 250 ° C. or less by heating during the preparation of copper nitride fine particles.
- Examples include hexylamine, heptylamine, octylamine, nonylamine, and decanylamine.
- ammonia gas having ammonia that evaporates as the reaction gas
- ammonium salts such as ammonium chloride, ammonium bromide, and ammonium acetate.
- the copper nitride fine particles of the present invention can be obtained by dissolving or dispersing a copper source and a nitrogen source, or a copper source, a nitrogen source and a protective agent in a solvent or a dispersion medium, and then heating.
- the solvent is not particularly limited as long as it does not hinder dispersion of the fine particles and has a boiling point of 100 ° C. or higher, preferably 200 ° C. or higher.
- An organic solvent is preferably used, and an alcohol compound And ether compounds, amine compounds, polyvalent amine compounds, amino alcohol compounds, amide compounds, hydrocarbon compounds, and the like.
- the alcohol compound is a compound in which one hydroxyl group is bonded to a linear, branched, or cyclic saturated or unsaturated hydrocarbon chain having 5 to 20 carbon atoms.
- Examples include 1-ol, 8-nonen-1-ol, cyclohexanol, cyclohexylmethanol, 4-methylcyclohexanol, phenol, cresol, and 4-ethylphenol.
- polyhydric alcohol compounds in which two or more hydroxyl groups are bonded to a linear, branched, or cyclic saturated or unsaturated hydrocarbon chain having 2 to 10 carbon atoms include ethylene glycol, 1,3-propanediol, 1 , 2-propanediol, 1,4-butanediol, 1,3-propane, 1,5-pentanediol, 1,6-hexanediol, 1,2-cyclohexanediol, 1,3-cyclohexanediol, 1,4 -Cyclohexanediol, 2-butene-1,4-diol, etc.
- the ether compound is a compound in which one or more oxygen elements are cross-linked by straight or branched, cyclic saturated or unsaturated hydrocarbon chains having 2 to 10 carbon atoms.
- the ether compound has a low boiling point, one or more hydroxyl groups may be bonded to the hydrocarbon chain in order to obtain a boiling point necessary for synthesis.
- Specific examples include diethylene glycol, diethylene glycol monomethyl ether, diethylene glycol monoethyl ether, triethylene glycol, dipropylene glycol, dipropylene glycol monomethyl ether, dipropylene glycol monoethyl ether, and the like.
- the amine compound is a compound in which one of primary to quaternary amino groups is bonded to a linear, branched or cyclic saturated or unsaturated hydrocarbon chain having 5 to 20 carbon atoms, Examples include amylamine, hexylamine, heptylamine, octylamine, nonylamine, decanylamine, cyclohexylamine, 2-methylcyclohexylamine, allylamine, oleylamine, aniline, toluidine, ethylaniline and the like.
- a polyvalent amine compound in which two or more primary to quaternary amino groups are bonded to a linear, branched, or cyclic saturated or unsaturated hydrocarbon chain having 2 to 10 carbon atoms ethylenediamine, 1,3-diaminopropane, 1,4-diamino-2-methylpropane, 1,4-diaminobutane, 1,5-diaminopentane, hexamethylenediamine, 1,7-diaminoheptane, 1,8-diaminooctane, Etc. can be illustrated.
- amino alcohol compounds include compounds in which one or more hydroxyl groups and amino groups are bonded to a linear, branched, or cyclic saturated or unsaturated hydrocarbon chain R having 5 to 20 carbon atoms. .
- amide compound examples include a compound in which one carboxylic acid amide is bonded to a linear, branched, or cyclic saturated or unsaturated hydrocarbon chain R having 2 to 20 carbon atoms. Either grade, grade 2, or grade 3 may be used.
- the above-mentioned solvent can be used. Even when the copper source and the nitrogen source, or the copper source, the nitrogen source and the protective agent do not dissolve and act as a dispersant, the copper nitride particles can be used. It will act as a solvent by heating to prepare.
- Examples of the protective agent used in the present invention include alcohol compounds, polyhydric alcohol compounds, amine compounds, polyvalent amine compounds, carboxylic acid compounds, polyvalent carboxylic acid compounds, and polymer compounds. These may be added separately as a protective agent, the solvent itself may be used as a protective agent, or a molecule that binds to the copper-containing compound or the nitrogen-containing compound may be used.
- ether compound As the alcohol compound or polyhydric alcohol compound, ether compound, amine compound, polyvalent amine compound, amino alcohol compound, amide compound, and hydrocarbon compound used as a protective agent, one or more of the above compound groups can be used.
- the carboxylic acid compound is a compound in which one carboxyl group is bonded to a linear, branched, or cyclic saturated or unsaturated hydrocarbon chain R having 2 to 20 carbon atoms, and has a chemical structure of R—COOH. It is expressed by a formula.
- the polyvalent carboxylic acid compound is a compound in which two or more carboxyl groups are bonded to a linear, branched, or cyclic saturated or unsaturated hydrocarbon chain R having 2 to 10 carbon atoms.
- polymer compound examples include polyvinylpyrrolidones, polyvinyl alcohols, polyethylene glycols, polyoxylalkylenes, acrylic acid and esters thereof, methacrylic acid and esters thereof having a molecular weight of 1,000 to 100,000. Can be mentioned.
- the protective agent is preferably an alcohol compound, a polyhydric alcohol compound, an amine compound, a polyvalent amine compound, a carboxylic acid compound, a polyvalent carboxylic acid compound, or a polymer compound that decomposes or evaporates at 250 ° C. or lower.
- the temperature at which the reaction proceeds in the present invention is preferably from 100 ° C. to 250 ° C., more preferably from 150 ° C. to 200 ° C. If the temperature is low, the copper raw material does not dissolve and the reaction does not proceed. Further, the upper limit of the reaction temperature is limited by the boiling point of the solvent, and when it is too high, the copper nitride is decomposed to become copper or copper oxide.
- the reaction system may be any one of reduced pressure, normal pressure and increased pressure, and can be selected as necessary. As the reaction system, atmospheric pressure is preferable because the apparatus and process can be simplified.
- the heating method is not particularly limited, but electromagnetic wave heating such as microwaves may be used in order to obtain temperature uniformity inside the solution to be heated.
- the concentration of the copper-containing compound with respect to the organic solvent affects the production efficiency and particle size of the fine particles. If the concentration of the copper-containing compound is too low, the concentration of fine particles obtained by the reaction becomes low, and the productivity is lowered. Moreover, if the concentration of the copper-containing compound is too high, the particle size of the particles obtained becomes too large. Therefore, in the method for producing copper nitride fine particles, the concentration of the copper-containing compound is preferably 0.0001 to 1 mol / L, more preferably 0.001 to 0.1 mol in terms of Cu 1+ or Cu 2+. / L.
- the nitrogen source is supplied inexhaustively into the reaction system during the reaction, but the solid is also supplied with nitrogen when a liquid nitrogen source is used.
- the amount is finite.
- the amount of the nitrogen-containing compound added as the nitrogen source is preferably 0.01 to 100 equivalents with respect to the copper concentration. If the amount of the nitrogen source is small, unreacted copper ions are present and the yield is lowered.
- the amount is preferably 0.4 to 100 equivalents.
- the amount of the protective agent added is 0.01 to 100 equivalents, more preferably 0.1 to 10 equivalents with respect to the copper concentration.
- the copper nitride fine particles of the present invention have a decomposition temperature accompanied by a decrease in weight (mass) in the range of 70 ° C. to 300 ° C. in differential thermal balance analysis (normal pressure). Since the copper nitride fine particles can be wired by printing and have oxidation resistance to the air and water, the wiring is drawn on the film by printing or the like, and exhibits conductivity after heat treatment at 300 ° C. or lower. Material may be provided.
- the printed material is damaged by heat, such as melting and / or deformation, decomposition, carbonization, etc.
- the advantage is that it can be processed at a temperature that is not subject to heat treatment.
- the wiring ink material to the substrate to be printed can be performed by an ordinary method using an inkjet method, a spray method, an electrostatic spray method, a stencil method, a silk screen method, or the like.
- the copper metal film obtained after the heat treatment exhibits conductivity, but the primary particle size of the copper nitride fine particles to be used should be reduced to 50 nm or less, preferably 30 nm or less, more preferably 20 nm or less, and most preferably 10 nm or less. For example, practical conductivity up to about 10 ⁇ 5 ⁇ can be imparted.
- Example 1 A 1-octanol solution (50 mL) of copper (II) acetate (0.5 mmol) was prepared in a three-necked flask. Using an oil bath at 230 ° C. while blowing ammonia gas, the solution was heated at a solution temperature of 190 ° C. for 1 hour, and a reddish purple precipitate was confirmed. This precipitate was separated by centrifugal precipitation, washed several times with n-hexane, and then vacuum-dried.
- copper (II) acetate 0.5 mmol
- This powder was analyzed (XRD measurement) using a powder X-ray diffractometer (M21X from Mac Science: 40 kV, 200 mA, CuK ⁇ ), and observed with a transmission electron microscope (TEM) (TECNAI G2, FEI Corporation, acceleration) Voltage 200 kV, emission current 8 ⁇ A).
- TEM transmission electron microscope
- the obtained copper nitride fine particles had a primary particle size of 10 to 50 nm and a secondary particle size of 0.1 to 0.2 ⁇ m. Image analysis was performed as follows for primary particles and secondary particles.
- the determination of primary particles was performed in combination with the contrast analysis (Digital Micrograph) of the TEM observation image and the visual determination of the original image.
- contrast analysis Digital Micrograph
- the determination of the secondary particles was performed together with the contrast analysis of the TEM observation image and the visual determination of the original image.
- contrast analysis a region having a contrast difference of 1100 or less was extracted, and an interface between particles was discriminated visually.
- the major axis diameter of the secondary particles obtained by the series of treatments was defined as the secondary particle diameter.
- the pyrolysis was measured with a differential thermal balance (Thermo plus EVOII from Rigaku Co., Ltd .: temperature rising rate 5 ° C./min, alumina standard sample, Ar stream, normal pressure or reduced pressure (600 Pa)).
- FIG. 1 shows the XRD spectrum of the copper nitride fine particles obtained in Example 1, and the XRD pattern of copper, copper oxide and copper nitride.
- FIG. 3 shows a TEM observation image of the copper nitride fine particles obtained in Example 1.
- FIG. 5 shows the results of differential thermal balance analysis (normal pressure and reduced pressure) of the copper nitride fine particles obtained in Example 1. That is, according to differential thermal balance analysis (normal pressure), it had a thermal decomposition temperature accompanied by weight loss at 225 ° C. or lower.
- Example 2 A 1-nonanol solution (50 mL) of copper (II) acetate (0.5 mmol) was prepared in a three-necked flask. Using an oil bath at 230 ° C.
- the obtained copper nitride fine particles had a primary particle size of 10 to 20 nm and a secondary particle size of 0.1 to 0.2 ⁇ m. According to differential thermal balance analysis (normal pressure), the obtained copper nitride fine particles had a thermal decomposition temperature accompanied by a weight loss at 220 ° C. or lower.
- FIG. 2 shows an XRD spectrum of the copper nitride fine particles obtained in Example 2.
- Example 3 A 1-octanol solution (20 mL) of copper (II) octoate (0.28 mmol) was prepared in a three-necked flask. A black precipitate was confirmed by heating at 190 ° C. for 1 hour using an oil bath at 230 ° C. while blowing ammonia gas. This precipitate was separated by centrifugal precipitation, washed several times with n-hexane, and then vacuum-dried. This powder was subjected to XRD measurement and TEM observation.
- the obtained copper nitride fine particles had primary particles having a minor axis diameter of 1 to 5 nm, a major axis diameter of 50 to 100 nm, and a secondary particle diameter of 0.1 to 0.5 ⁇ m. According to the differential thermal balance analysis (normal pressure), the obtained copper nitride fine particles had a weight loss due to thermal decomposition of octanoic acid and copper nitride present on the surface at around 220 ° C. or lower.
- FIG. 2 shows an XRD spectrum of the copper nitride fine particles obtained in Example 3.
- FIG. 4 shows a TEM observation image of the copper nitride fine particles obtained in Example 3.
- Example 4 A 1-octanol solution (20 mL) of copper (II) octoate (0.28 mmol) and nonylamine (1 mmol) was prepared in a three-necked flask. A black precipitate was confirmed by heating at 190 ° C. for 1 hour using an oil bath at 230 ° C. while blowing ammonia gas. This precipitate was separated by centrifugal precipitation, washed several times with n-hexane, and then vacuum dried. This powder was subjected to XRD measurement and TEM observation.
- the obtained copper nitride fine particles had a primary particle size of 20 to 30 nm and a secondary particle size of 0.2 to 0.5 ⁇ m. According to the differential thermal balance analysis (normal pressure), the obtained copper nitride fine particles had a thermal decomposition temperature accompanied by weight loss at 250 ° C. or lower.
- Example 5 A 1-octanol solution (20 mL) of copper (II) myristate (0.28 mmol) was prepared in a three-necked flask. A black precipitate was confirmed by heating at 190 ° C. for 1 hour using an oil bath at 230 ° C. while blowing ammonia gas. This precipitate was separated by centrifugal precipitation, washed several times with n-hexane, and then vacuum-dried. This powder was subjected to XRD measurement and TEM observation.
- the obtained copper nitride fine particles were needle-like crystals whose primary particles had a minor axis diameter of 5 to 10 nm and a major axis diameter of 50 to 100 nm, and the secondary particles had a particle diameter of 0.1 to 0.2 ⁇ m. According to the differential thermal balance analysis (normal pressure), the obtained copper nitride fine particles had a thermal decomposition temperature accompanied by weight loss at 250 ° C. or lower.
- Example 6 A 1-octanol solution (20 mL) of copper (II) laurate (0.28 mmol) was prepared in a three-necked flask. A black precipitate was confirmed by heating at 190 ° C. for 1 hour using an oil bath at 230 ° C. while blowing ammonia gas. This precipitate was separated by centrifugal precipitation, washed several times with n-hexane, and then vacuum-dried. This powder was subjected to XRD measurement and TEM observation.
- the obtained copper nitride fine particles were needle-like crystals having a primary particle minor axis diameter of 5 to 10 nm and a major axis diameter of 50 to 100 nm, and the secondary particle diameter was 0.1 to 0.15 ⁇ m. According to differential thermal balance analysis (normal pressure), the obtained copper nitride fine particles had a thermal decomposition temperature with a weight loss at 300 ° C. or lower.
- Example 7 A dodecane solution (20 mL) of copper (II) octoate (0.28 mmol) was prepared in a three-necked flask. A black precipitate was confirmed by heating at 190 ° C. for 1 hour using an oil bath at 230 ° C. while blowing ammonia gas. This precipitate was separated by centrifugal precipitation, washed several times with n-hexane, and then vacuum-dried. This powder was subjected to XRD measurement and TEM observation.
- the obtained copper nitride fine particles had a primary particle size of 10 to 50 nm and a secondary particle size of 0.1 to 0.3 ⁇ m. According to differential thermal balance analysis (normal pressure), the obtained copper nitride fine particles had a thermal decomposition temperature with a weight loss at 240 ° C. or lower.
- Example 8 A 1-octanol solution (10 mL) of ammine copper azide (II) (0.1 mmol) was prepared in a three-necked flask. A black precipitate was confirmed by heating at 180 ° C. for 1 hour using an oil bath at 220 ° C. while blowing ammonia gas. The precipitate was filtered by centrifugal precipitation, washed several times with n-hexane, and then vacuum dried. These powders were subjected to XRD measurement and TEM observation.
- the obtained copper nitride fine particles had a primary particle size of 50 to 100 nm and a secondary particle size of 0.1 to 0.5 ⁇ m. According to differential thermal balance analysis (normal pressure), the obtained copper nitride fine particles had a thermal decomposition temperature accompanied by a weight loss at 221 ° C. or lower.
- FIG. 2 shows an XRD spectrum of the copper nitride fine particles obtained in Example 8.
- Example 9 A 1-octanol solution (10 mL) of ammonium copper azide (II) (0.2 mmol) and hexylamine (1 mmol) was prepared in a three-necked flask. A black precipitate was confirmed by heating at 180 ° C. for 1 hour using an oil bath at 220 ° C. while blowing ammonia gas. The precipitate was filtered by centrifugal precipitation, washed several times with n-hexane, and then vacuum dried. These powders were subjected to XRD measurement and TEM observation.
- the obtained copper nitride fine particles had a primary particle size of 50 to 100 nm and a secondary particle size of 0.2 to 0.5 ⁇ m. According to the differential thermal balance analysis (normal pressure), the obtained copper nitride fine particles had a thermal decomposition temperature accompanied by weight loss at 250 ° C. or lower.
- Example 10 A 1-nonanol solution (50 mL) of copper (II) acetate (0.5 mmol) and ammonium acetate (5 mmol) was prepared in a three-necked flask. Using a 230 ° C. oil bath while blowing nitrogen gas, the solution was heated at a solution temperature of 190 ° C. for 1 hour to confirm a red purple precipitate. This precipitate was separated by centrifugal precipitation, washed several times with n-hexane, and then vacuum dried. This powder was subjected to XRD measurement and TEM observation.
- the obtained copper nitride fine particles had a primary particle size of 10 to 50 nm and a secondary particle size of 0.1 to 0.5 ⁇ m. According to the differential thermal balance analysis (normal pressure), the obtained copper nitride fine particles had a thermal decomposition temperature accompanied by weight loss at 250 ° C. or lower.
- Table 1 shows the XRD analysis results of the combination of copper source, nitrogen source, protective agent and solvent, and the resulting precipitate, which are the synthesis conditions of the examples.
- the present invention provides a copper nitride having a decomposition temperature of 300 ° C. or lower and a material that gives metallic copper by heating at 300 ° C. or lower.
- a copper nitride having a decomposition temperature of 300 ° C. or lower and a material that gives metallic copper by heating at 300 ° C. or lower.
- it can be expected to be used as an ink material for printed electronics devices.
Landscapes
- Chemical & Material Sciences (AREA)
- Engineering & Computer Science (AREA)
- Organic Chemistry (AREA)
- Materials Engineering (AREA)
- Nanotechnology (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Mechanical Engineering (AREA)
- Metallurgy (AREA)
- Crystallography & Structural Chemistry (AREA)
- Inorganic Chemistry (AREA)
- General Chemical & Material Sciences (AREA)
- Physics & Mathematics (AREA)
- General Physics & Mathematics (AREA)
- Condensed Matter Physics & Semiconductors (AREA)
- Manufacturing & Machinery (AREA)
- Composite Materials (AREA)
- Manufacture Of Metal Powder And Suspensions Thereof (AREA)
- Powder Metallurgy (AREA)
- Electrodes Of Semiconductors (AREA)
- Inks, Pencil-Leads, Or Crayons (AREA)
Priority Applications (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| KR1020157020804A KR20150112984A (ko) | 2013-01-31 | 2014-01-31 | 질화구리 미립자 및 그 제조 방법 |
| CN201480006826.7A CN104981427A (zh) | 2013-01-31 | 2014-01-31 | 氮化铜微粒及其制造方法 |
Applications Claiming Priority (4)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2013017510 | 2013-01-31 | ||
| JP2013-017510 | 2013-01-31 | ||
| JP2013-225296 | 2013-10-30 | ||
| JP2013225296A JP6057379B2 (ja) | 2013-01-31 | 2013-10-30 | 窒化銅微粒子およびその製造方法 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2014119748A1 true WO2014119748A1 (ja) | 2014-08-07 |
Family
ID=51262445
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/JP2014/052321 Ceased WO2014119748A1 (ja) | 2013-01-31 | 2014-01-31 | 窒化銅微粒子およびその製造方法 |
Country Status (4)
| Country | Link |
|---|---|
| JP (1) | JP6057379B2 (enExample) |
| KR (1) | KR20150112984A (enExample) |
| CN (1) | CN104981427A (enExample) |
| WO (1) | WO2014119748A1 (enExample) |
Cited By (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN111450867A (zh) * | 2020-05-09 | 2020-07-28 | 青岛科技大学 | 用于电催化二氧化碳还原的Cu3N纳米催化剂的制备方法 |
| CN115057417A (zh) * | 2022-06-08 | 2022-09-16 | 安徽大学 | 一种氮化铜纳米片的制备及其在甲酸盐电合成中的应用 |
Families Citing this family (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2015147561A1 (ko) * | 2014-03-26 | 2015-10-01 | 전자부품연구원 | 전도체 패턴의 형성이 용이한 복합소재와 그 복합소재를 제조하는 방법 및 상기 복합소재에서 시드 소재인 구리질화물 및 그 구리질화물을 합성하는 방법 |
| JP6574553B2 (ja) * | 2014-06-26 | 2019-09-11 | 昭和電工株式会社 | 導電パターン形成用組成物および導電パターン形成方法 |
| KR102303767B1 (ko) * | 2017-11-01 | 2021-09-23 | 한국전자기술연구원 | 전도체 패턴용 구리질화물 분말의 제조 방법 |
| CN110642304B (zh) * | 2019-10-09 | 2021-12-31 | 上海师范大学 | 一种超级电容器用三金属氮化物材料及其制备方法 |
| CN116516280A (zh) * | 2023-04-27 | 2023-08-01 | 常州大学 | 一种高效节能的工件氮化方法 |
| CN116924697B (zh) * | 2023-07-31 | 2025-08-29 | 上海耀皮工程玻璃有限公司 | 一种Low-E镀膜玻璃调色层及其制备方法和用途 |
| CN119282131A (zh) * | 2024-11-18 | 2025-01-10 | 济源星翰新材料科技有限公司 | 一种微米铜粉的制备方法 |
Citations (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2006210872A (ja) * | 2004-12-28 | 2006-08-10 | Kyushu Institute Of Technology | 銅パターン配線形成方法及び該方法を用いて作成された半導体装置、並びにナノ銅金属粒子 |
| JP2010121206A (ja) * | 2008-10-22 | 2010-06-03 | Tosoh Corp | 金属膜製造用組成物、金属膜の製造方法及び金属粉末の製造方法 |
| JP2012079933A (ja) * | 2010-10-01 | 2012-04-19 | Fujifilm Corp | 配線材料、配線の製造方法、及びナノ粒子分散液 |
Family Cites Families (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2005122230A1 (ja) * | 2004-06-07 | 2005-12-22 | Kyushu Institute Of Technology | 銅表面の処理方法及び銅パターン配線形成方法、並びに該方法を用いて作成された半導体装置 |
-
2013
- 2013-10-30 JP JP2013225296A patent/JP6057379B2/ja not_active Expired - Fee Related
-
2014
- 2014-01-31 WO PCT/JP2014/052321 patent/WO2014119748A1/ja not_active Ceased
- 2014-01-31 KR KR1020157020804A patent/KR20150112984A/ko not_active Ceased
- 2014-01-31 CN CN201480006826.7A patent/CN104981427A/zh active Pending
Patent Citations (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2006210872A (ja) * | 2004-12-28 | 2006-08-10 | Kyushu Institute Of Technology | 銅パターン配線形成方法及び該方法を用いて作成された半導体装置、並びにナノ銅金属粒子 |
| JP2010121206A (ja) * | 2008-10-22 | 2010-06-03 | Tosoh Corp | 金属膜製造用組成物、金属膜の製造方法及び金属粉末の製造方法 |
| JP2012079933A (ja) * | 2010-10-01 | 2012-04-19 | Fujifilm Corp | 配線材料、配線の製造方法、及びナノ粒子分散液 |
Non-Patent Citations (4)
| Title |
|---|
| HAIBIN WU ET AL.: "Copper Nitride Nanocubes: Size-Controlled Synthesis and Application as Cathode Catalyst in Alkaline Fuel Cells", J. AM. CHEM. SOC., vol. 133, no. 39, 5 October 2011 (2011-10-05), pages 15236 - 15239 * |
| JONGLAK CHOI ET AL.: "Solvothermal Synthesis of Nanocrystalline Copper Nitride from an Energetically Unstable Copper Azide Precursor", INORG. CHEM., vol. 44, no. 21, 17 October 2005 (2005-10-17), pages 7385 - 7393 * |
| NOBUO KIEDA: "Funmu Netsu Bunkaiho ni yoru Chikkado Biryushi no Gosei", THE CERAMIC SOCIETY OF JAPAN NENKAI KOEN YOKOSHU, vol. 2011, 16 March 2011 (2011-03-16), pages 295 * |
| TAKASHI NAKAMURA ET AL.: "Alcohol o Yobai to shita Chikkado Biryushi no Gosei to sono Netsubunkai Tokusei", THE CERAMIC SOCIETY OF JAPAN NENKAI KOEN YOKOSHU, vol. 2013, 11 March 2013 (2013-03-11), pages 3D26 * |
Cited By (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN111450867A (zh) * | 2020-05-09 | 2020-07-28 | 青岛科技大学 | 用于电催化二氧化碳还原的Cu3N纳米催化剂的制备方法 |
| CN115057417A (zh) * | 2022-06-08 | 2022-09-16 | 安徽大学 | 一种氮化铜纳米片的制备及其在甲酸盐电合成中的应用 |
| CN115057417B (zh) * | 2022-06-08 | 2023-09-12 | 安徽大学 | 一种氮化铜纳米片的制备及其在甲酸盐电合成中的应用 |
Also Published As
| Publication number | Publication date |
|---|---|
| CN104981427A (zh) | 2015-10-14 |
| KR20150112984A (ko) | 2015-10-07 |
| JP6057379B2 (ja) | 2017-01-11 |
| JP2014166939A (ja) | 2014-09-11 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP6057379B2 (ja) | 窒化銅微粒子およびその製造方法 | |
| US9168587B2 (en) | Fine coated copper particles and method for producing same | |
| JP5623861B2 (ja) | 金属ナノ粒子分散組成物 | |
| TWI638769B (zh) | 被覆銅粒子及其製造方法 | |
| US20180168037A1 (en) | Method for producing silver nanoparticles, silver nanoparticles, and silver coating composition | |
| JP5986636B2 (ja) | 銀ナノ粒子の製造方法、銀塗料組成物の製造方法および銀導電材料の製造方法 | |
| CN104540622B (zh) | 用于生产银纳米粒子的方法、银纳米粒子、涂覆的银纳米粒子、银涂覆组合物和银导电材料 | |
| JP5715851B2 (ja) | ナノ粒子インク組成物を用いた印刷物の製造方法 | |
| JP2019527770A (ja) | 金属ナノ粒子コロイド分散体の製造方法 | |
| CN103338884A (zh) | 包覆金属微粒及其制造方法 | |
| JP6033485B2 (ja) | 被覆銅粒子 | |
| JP5426270B2 (ja) | 金属銅微粒子の製造方法 | |
| CN105263656A (zh) | 银粒子的制造方法 | |
| JP6042747B2 (ja) | ニッケル微粒子、その使用方法及びニッケル微粒子の製造方法 | |
| JP2007321215A5 (enExample) | ||
| JP2007321215A (ja) | 金属ナノ粒子分散体および金属被膜 | |
| JP6414085B2 (ja) | 金属ナノ微粒子の製造方法 | |
| TWI586462B (zh) | 用於製備可在大氣壓力下被煆燒的銅奈米粒子之方法及其所製備之銅奈米粒子 | |
| JP4995492B2 (ja) | 銅ナノ粒子の製造方法、銅ナノ粒子、銅ナノ粒子分散体および電子デバイス | |
| JP6126426B2 (ja) | 接合方法 | |
| JP6099160B2 (ja) | 複合化合物、及び懸濁液 | |
| JP2007321216A5 (enExample) | ||
| JP2021188087A (ja) | ニッケルナノ粒子凝集体、その製造方法及びニッケルナノ粒子複合基板 | |
| Kim et al. | Preparation of highly stabilized silver nanopowders by the thermal reduction and their properties | |
| JP2005060824A (ja) | 合金微粒子の製造方法及び合金薄膜の製造方法 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 14746518 Country of ref document: EP Kind code of ref document: A1 |
|
| ENP | Entry into the national phase |
Ref document number: 20157020804 Country of ref document: KR Kind code of ref document: A |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| 122 | Ep: pct application non-entry in european phase |
Ref document number: 14746518 Country of ref document: EP Kind code of ref document: A1 |