WO2014030477A1 - 芳香族炭化水素処理用活性白土 - Google Patents
芳香族炭化水素処理用活性白土 Download PDFInfo
- Publication number
- WO2014030477A1 WO2014030477A1 PCT/JP2013/069791 JP2013069791W WO2014030477A1 WO 2014030477 A1 WO2014030477 A1 WO 2014030477A1 JP 2013069791 W JP2013069791 W JP 2013069791W WO 2014030477 A1 WO2014030477 A1 WO 2014030477A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- clay
- acid
- activated clay
- treatment
- range
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Ceased
Links
Images
Classifications
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J21/00—Catalysts comprising the elements, oxides, or hydroxides of magnesium, boron, aluminium, carbon, silicon, titanium, zirconium, or hafnium
- B01J21/16—Clays or other mineral silicates
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08F—MACROMOLECULAR COMPOUNDS OBTAINED BY REACTIONS ONLY INVOLVING CARBON-TO-CARBON UNSATURATED BONDS
- C08F4/00—Polymerisation catalysts
- C08F4/42—Metals; Metal hydrides; Metallo-organic compounds; Use thereof as catalyst precursors
- C08F4/44—Metals; Metal hydrides; Metallo-organic compounds; Use thereof as catalyst precursors selected from light metals, zinc, cadmium, mercury, copper, silver, gold, boron, gallium, indium, thallium, rare earths or actinides
- C08F4/58—Metals; Metal hydrides; Metallo-organic compounds; Use thereof as catalyst precursors selected from light metals, zinc, cadmium, mercury, copper, silver, gold, boron, gallium, indium, thallium, rare earths or actinides together with silicon, germanium, tin, lead, antimony, bismuth or compounds thereof
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J37/00—Processes, in general, for preparing catalysts; Processes, in general, for activation of catalysts
- B01J37/06—Washing
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10G—CRACKING HYDROCARBON OILS; PRODUCTION OF LIQUID HYDROCARBON MIXTURES, e.g. BY DESTRUCTIVE HYDROGENATION, OLIGOMERISATION, POLYMERISATION; RECOVERY OF HYDROCARBON OILS FROM OIL-SHALE, OIL-SAND, OR GASES; REFINING MIXTURES MAINLY CONSISTING OF HYDROCARBONS; REFORMING OF NAPHTHA; MINERAL WAXES
- C10G45/00—Refining of hydrocarbon oils using hydrogen or hydrogen-generating compounds
- C10G45/58—Refining of hydrocarbon oils using hydrogen or hydrogen-generating compounds to change the structural skeleton of some of the hydrocarbon content without cracking the other hydrocarbons present, e.g. lowering pour point; Selective hydrocracking of normal paraffins
- C10G45/68—Aromatisation of hydrocarbon oil fractions
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J20/00—Solid sorbent compositions or filter aid compositions; Sorbents for chromatography; Processes for preparing, regenerating or reactivating thereof
- B01J20/02—Solid sorbent compositions or filter aid compositions; Sorbents for chromatography; Processes for preparing, regenerating or reactivating thereof comprising inorganic material
- B01J20/10—Solid sorbent compositions or filter aid compositions; Sorbents for chromatography; Processes for preparing, regenerating or reactivating thereof comprising inorganic material comprising silica or silicate
- B01J20/12—Naturally occurring clays or bleaching earth
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J2235/00—Indexing scheme associated with group B01J35/00, related to the analysis techniques used to determine the catalysts form or properties
- B01J2235/15—X-ray diffraction
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J35/00—Catalysts, in general, characterised by their form or physical properties
- B01J35/60—Catalysts, in general, characterised by their form or physical properties characterised by their surface properties or porosity
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J35/00—Catalysts, in general, characterised by their form or physical properties
- B01J35/70—Catalysts, in general, characterised by their form or physical properties characterised by their crystalline properties, e.g. semi-crystalline
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10G—CRACKING HYDROCARBON OILS; PRODUCTION OF LIQUID HYDROCARBON MIXTURES, e.g. BY DESTRUCTIVE HYDROGENATION, OLIGOMERISATION, POLYMERISATION; RECOVERY OF HYDROCARBON OILS FROM OIL-SHALE, OIL-SAND, OR GASES; REFINING MIXTURES MAINLY CONSISTING OF HYDROCARBONS; REFORMING OF NAPHTHA; MINERAL WAXES
- C10G45/00—Refining of hydrocarbon oils using hydrogen or hydrogen-generating compounds
- C10G45/02—Refining of hydrocarbon oils using hydrogen or hydrogen-generating compounds to eliminate hetero atoms without changing the skeleton of the hydrocarbon involved and without cracking into lower boiling hydrocarbons; Hydrofinishing
- C10G45/04—Refining of hydrocarbon oils using hydrogen or hydrogen-generating compounds to eliminate hetero atoms without changing the skeleton of the hydrocarbon involved and without cracking into lower boiling hydrocarbons; Hydrofinishing characterised by the catalyst used
Definitions
- the present invention relates to an activated clay for treating aromatic hydrocarbons, and more specifically, activated clay used for purification of aromatic hydrocarbons such as BTX (benzene, toluene, xylene) and having a particularly excellent life. About.
- aromatic hydrocarbons such as BTX (benzene, toluene, xylene)
- purification treatment For the purpose of removing unsaturated hydrocarbon compounds such as olefins and diolefins from aromatic hydrocarbons such as BTX, purification treatment has been conventionally performed.
- an unsaturated compound contained in an aromatic hydrocarbon such as BTX is converted into a dimer or trimer by polymerization, or converted to a polycyclic aromatic compound by alkylation to an aromatic hydrocarbon.
- the molecular weight is increased and removed as a high boiling fraction.
- activated clay has been used for the above purification treatment from the viewpoint of acting as an unsaturated hydrocarbon polymerization catalyst (for example, Patent Documents 1 and 2).
- Activated clay is used for decolorization of mineral oil, and it is known that such activated clay for decolorization can also be used for the purification treatment of aromatic hydrocarbons (Patent Documents 3 and 4). ).
- JP-A-6-263431 JP 11-179202 A JP 2000-344513 A JP 2010-95436 A
- the activated clay used in Patent Document 1 has an extremely poor catalyst life and deteriorates the catalyst performance in a short period of time, so that it cannot be used satisfactorily as a purification process for aromatic hydrocarbons. .
- the activated clay shown in Patent Document 2 has improved catalyst life to some extent, it contains a high concentration of Fe 2 O 3 component, so that the place where the raw clay is produced contains a lot of iron (for example, India).
- activated clay is obtained by acid-treating clay (acid clay) containing montmorillonite as a main component and has a large specific surface area, but the Fe 2 O 3 component is eluted by this acid treatment. Therefore, in order to obtain an activated clay containing a certain amount or more of Fe 2 O 3 component, naturally, as the raw clay (acid clay) for acid treatment, one having a high content of Fe 2 O 3 component must be selected. Therefore, the production area is limited to India.
- the activated clay of Patent Document 3 or 4 is extremely excellent in the decoloring performance of mineral oil, but its catalyst life is not sufficient for use in the purification treatment of aromatic hydrocarbons. Further, this activated clay has a solid acid amount with an acid strength of Ho ⁇ ⁇ 3.0 within a certain range.
- the regulation of the amount of solid acid in such a wide range of acid strength distribution is not able to properly grasp the specific acid strength distribution that contributes to the catalytic function and the amount of solid acid in that range during the purification process of aromatic hydrocarbons. For this reason, there is a drawback that the performance as a catalyst is likely to vary.
- an object of the present invention is to provide an activated clay for treating aromatic hydrocarbons which is composed of an acid-treated product of smectite clay, the production area of the raw clay is not limited, and has a long catalyst life and no variation.
- the present inventors have determined that the degree of acid treatment is in accordance with the properties of the raw clay.
- the amount of solid acid in acid strength is selectively increased, the catalytic ability is not lowered, and the catalyst life is greatly improved, and the present invention has been completed.
- the montmorillonite content is 34% by mass or more
- the SiO 2 / Al 2 O 3 molar ratio is in the range of 3.8 to 8.0
- An activated clay for treating aromatic hydrocarbons is provided, wherein the amount of ammonia desorption with an adsorption heat in the range of mol is in the range of 0.11 to 0.20 mmol / g.
- the activated clay of the present invention does not need to contain a high concentration of components such as heavy metal oxides removed by acid treatment, and the montmorillonite content may be 34% by mass or more. Therefore, the production area and face (burial place) of the raw clay are not limited, and the activated clay of the present invention can be obtained by an appropriate acid treatment even if it is montmorillonite produced from any production area.
- the activated clay of the present invention is not obtained by acid treatment using a high concentration of acid. It is obtained by using a rather weak acid treatment. This is because when the treatment using high-concentration acid wp is performed, the elution amount of Al 2 O 3 is large, and this molar ratio becomes a considerably large value.
- the activated clays of Patent Documents 1 to 4 described above have a large SiO 2 / Al 2 O 3 molar ratio as compared with the present invention.
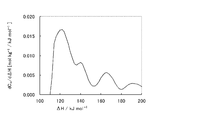
- the activated clay of the present invention has a desorption amount of ammonia (with an adsorption heat in the range of 128 to 148 kJ / mol) measured by the ammonia TPD method within a certain range (0.11 to 0.20 mmol / g). is there.
- the heat of adsorption is related to the strength of the solid acid
- the amount of ammonia desorbed is related to the amount of solid acid. That is, in the ammonia TPD method, as described in the examples described later, the amount and temperature of ammonia desorbed by adsorbing ammonia, which is a base probe molecule, to a solid sample and continuously increasing the temperature. Are measured simultaneously.
- Ammonia adsorbed on weak acid sites desorbs at low temperatures (equivalent to desorption in a low adsorption heat range), and ammonia adsorbed on strong acid sites desorbs at high temperatures (in a high adsorption heat range). Is equivalent to detachment).
- the acid strength is indicated by temperature and heat of adsorption, and no color reaction is used. Therefore, compared with the conventional n-butylamine titration method, the solid acid strength and the solid acid amount are higher. Since it is an accurate value, the activated clay of the present invention can appropriately evaluate the characteristics.
- the ammonia desorption amount (related to the solid acid amount) specified by the ammonia TPD method is in the above range, so that the catalytic ability in the purification treatment of aromatic hydrocarbons is high. It exhibits a very long catalyst life, which is the greatest advantage of the present invention.
- aromatic hydrocarbons such as BTX
- reactions such as polymerization and alkylation of unsaturated hydrocarbon compounds such as olefins and diolefins contained therein are caused by the solid acid of the activated clay, and the acid strength is increased. It has been believed that the higher the amount of high solid acid, the more these reactions are promoted.
- solid acids that contribute to such reactions are limited to those in a specific acid strength range, and solid acids in other acid strength ranges promote side reactions and reduce catalyst life. End up.
- the amount of solid acid in the strength range that contributes to the alkylation reaction is selectively increased by an appropriate acid treatment, so that the catalytic ability (accelerating alkylation of olefins) is not impaired. An extremely long catalyst life can be realized.
- Montmorillonite which is the main component of clay used in the production of the activated clay of the present invention, is a clay mineral belonging to dioctahedral smectite, an AlO 6 octahedral layer sandwiched between two SiO 4 tetrahedral layers, and AlO 6 octa
- a three-layer structure in which a part of Al in the plane layer is isomorphously substituted with Fe or Mg and a part of Si in the SiO 4 tetrahedral layer is isomorphously substituted with Al is defined as a basic layer unit, and this basic layer unit is in the c-axis direction.
- Cations are present between the basic layers between the basic layers to compensate for the lack of charge due to isomorphous substitution. That is, when a clay mainly composed of montmorillonite having such a laminated structure is acid-treated, cations existing between the layers of the laminated structure are eluted, and the laminated structure of the basic three-layer structure is partially cut and broken apart. At the same time, the AlO 6 octahedral layer part elutes from the edge of the basic trilayer structure. A structure exhibiting characteristics as a solid acid is also generated at this end. In addition, with the acid treatment, the specific surface area also increases, and the SiO 2 / Al 2 O 3 molar ratio also increases due to the elution of the Al component.
- montmorillonite is thought to have been generated by the transformation of volcanic ash, lava, etc. under the influence of seawater.
- the chemical composition of the typical raw clay used for the production of the activated clay of the present invention is roughly as follows, and no special chemical composition is required except that it contains montmorillonite.
- the face is not limited. SiO 2 : 45 to 65% by mass Al 2 O 3 : 13 to 25% by weight MgO: 2-7% by weight CaO: 0.1 to 3.0% by weight Fe 2 O 3 : 2 to 25% by weight K 2 O: 0.1 to 3.0% by weight Na 2 O: 0.1 to 3.0% by weight Burning loss: 5-12% by weight
- the above-mentioned raw clay can be refined such as stone sand separation, buoyancy beneficiation, magnetic beneficiation, water tank, wind dredging, etc. to remove impurities such as quartz and feldspar present in the clay, and to make the particle size range uniform. is important. This is because it is easy to obtain physical properties suitable for the activated clay for aromatic hydrocarbon treatment of the present invention by performing the acid treatment described later uniformly.
- the acid treatment of the raw clay has an ammonia desorption amount of 0.11 to 0.20 mmol / g, particularly 0, as measured by the ammonia TPD method with an adsorption heat in the range of 128 to 148 kJ / mol. .11 to 0.17 mmol / g. That is, even if the acid treatment is excessively performed or insufficient, the ammonia desorption amount is reduced, and the catalyst life of the obtained activated clay is greatly reduced. Therefore, the degree of this acid treatment is extremely important.
- the acid treatment operation is performed by a known means, for example, by filling a treatment tank with a clay suspension, adding an aqueous acid solution, and stirring.
- the degree of acid treatment for obtaining the above ammonia desorption amount is compared with the acid treatment for obtaining activated clay for conventionally known aromatic hydrocarbon treatment. Then it is done at a mild level.
- mineral acids sulfuric acid, hydrochloric acid, etc.
- sulfuric acid are used as the acid, but the amount of treatment is conventionally about 75 parts by weight with respect to 100 parts by weight of dry clay. In the invention, the amount is as small as about 50 to 68 parts by mass.
- the processing temperature may be selected from the range of 60 to 100 ° C.
- the processing time may be selected from the range of 4 to 48 hours so that the above-described ammonia desorption amount can be obtained.
- the acid-treated product thus obtained has a montmorillonite content of at least 34% by mass.
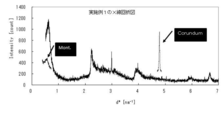
- the montmorillonite content can be calculated from the peak intensity of the (001) plane of montmorillonite by X-ray diffraction, as shown in the examples described later.
- the acid-treated product obtained as described above is washed with water and then subjected to drying, pulverization and classification, and adjusted to a particle size according to the use form.
- the obtained activated clay granule is appropriately heat-treated, whereby the particle strength can be increased.
- the heat treatment temperature is generally about 80 to 500 ° C., particularly about 100 to 300 ° C. and about 0.5 to 10 hours, particularly about 0.7 to 5 hours.
- the activated clay thus obtained is used as a catalyst for promoting the polymerization and alkylation of unsaturated hydrocarbon compounds in the purification process of aromatic hydrocarbons.
- the particle size is generally adjusted to 20 to 40 ⁇ m, particularly 25 to 35 ⁇ m median diameter powder, and when used on a fixed bed, the particle size is generally , And adjusted to a granular form in the range of 0.25 to 1.0 mm.
- the particle shape may be any shape such as a spherical shape, a granular shape, a cubic shape, a tablet shape, a cylindrical shape, and an indefinite shape.
- Such activated clay contains 34% by mass or more of montmorillonite, and as already described, the ammonia desorption amount with an adsorption heat in the range of 128 to 148 kJ / mol measured by the ammonia TPD method is 0.11. To 0.20 mmol / g, especially in the range of 0.11 to 0.17 mmol / g. Further, in the composition, the SiO 2 / Al 2 O 3 molar ratio is in the range of 3.8 to 8.0, particularly 3.8 to 7.0.
- the SiO 2 / Al 2 O 3 molar ratio is higher or lower than the above range, the ammonia desorption amount is less than the above range, and as a result, a sufficient solid acid amount cannot be obtained, and the catalytic ability ( The alkylation promoting function of unsaturated hydrocarbon compounds) becomes unsatisfactory.
- Such an activated clay of the present invention has an excellent function as a purification catalyst for unsaturated hydrocarbon compounds such as olefins and diolefins, as shown in the examples described later, and has a remarkable catalyst life. It is long and used effectively for the purification process of aromatic hydrocarbons. It should be noted that the activity and catalyst life of the activated clay for the treatment of aromatic hydrocarbons are the index of the olefin content for those obtained by removing the high boiling fraction by distillation after passing through the granular clay packed bed. It can evaluate by calculating
- the sample is filled in the holder by the NBS method [“Standard X-ray diffraction powder patterns”, NBS Monograph, 25 (1971)], and quantitative measurement is performed.
- the measurement conditions at that time are as follows: 2 ⁇ is 3 to 7, 20 to 27.5, and 42 to 44.5 [deg], the voltage is 40 [V] and the current is 40 [mA], and D Slit & S Slit: 2 / 3, V Slit: 10 [mm], R Slit 0.3 [mm], Step: 0.02 [deg].
- montmorillonite ethylene glycol-treated Kunipia F
- matrix flushing method which is a kind of internal standard method
- the peak area of the X-ray diffractogram was 220%
- the relative area intensity ratio (%) for each sample was The montmorillonite content was calculated.
- Ammonia desorption amount (ammonia TPD method) About 0.1 g of a sample is set in a quartz cell (inner diameter: 10 mm) of a TPD-AT-1 type thermal desorption apparatus manufactured by Bell Japan, and 10 Kmin ⁇ 1 up to 383 K under the flow of O 2 (60 cm 3 min ⁇ 1 , 1 atm). The temperature was raised at a temperature of 1 hour and the temperature reached 1 hour. Thereafter, the mixture was allowed to cool to 373 K while O 2 was circulated, then vacuum degassed, 100 Torr NH 3 was introduced and adsorbed for 30 min, and then degassed for 30 min, followed by steam treatment.
- the sample was sized with a sieve of 24 to 60 mesh, dried at 150 ° C. for 3 hours, and then used for the test.
- the BI of the sample tube outlet oil collected every 12 hours was measured, and the resulting break through curve was calculated as A.I. Wheeler & A. J. et al. Robert, J.M. Catal. , 13, 299 (1969).
- Ts was obtained by analysis using the following formula described in (1).
- BI 0 Entrance BI [mg / 100 g]
- BI Exit BI after time t [mg / 100 g]
- k 0 Initial catalyst primary reaction rate constant [1 / hr] and a value of Ka or less k
- A Olefin adsorption rate constant [1 / hr]
- W catalyst mass [g]
- F Oil flow rate [g / hr]
- Ws High boiling point olefin adsorbed per catalyst weight after ts time Weight [mg / 100 g]
- WHSV space velocity [1 / hr]
- Example 1 Water is added to the raw clay of the production area A to a concentration of 2 wt%, and after stirring and dispersing, it is passed through a sieve having an opening of 45 ⁇ m to remove sand having a size of 45 ⁇ m or more (hereinafter, the treatment so far is referred to as sieving treatment. Is the same even if the raw clay changes.) Thereafter, 2-45 ⁇ m silt particles were collected with a water tank and then centrifuged to purify the raw clay. The purified raw clay was added to water to obtain 351 g of a suspension having a water content of 88.6%. To this suspension was added 75% strength sulfuric acid.
- H 2 SO 4 / Clay when this ratio is shown
- H 2 SO 4 / Clay when this ratio is shown
- a concentration of 75% by mass sulfuric acid was added so as to be a mass% (hereinafter, when this ratio is shown, it is expressed as H 2 SO 4 / Liquid).
- the mixture was stirred with heating at 90 ° C. for 24 hours.
- the hydrolysis prevention treatment with the same concentration of 0.5% by mass sulfuric acid as the slurry, washed with water, dried and coarsely crushed to obtain activated clay.
- the acid treatment conditions are shown in Table 2, and various measurement results for the obtained activated clay are shown in Table 3.
- An X-ray diffraction diagram is shown in FIG.
- Example 2 The raw clay of the production area B was used. After sieving, the raw material clay was purified by centrifugation as it was. Except that the subsequent acid treatment conditions are listed in Table 2, acid treatment was performed in the same manner as in Example 1 to obtain activated clay. Table 3 shows various measurement results for the obtained activated clay.
- Example 3 The raw clay of the production area B was used after leaching opal contaminated in advance with caustic soda. After sieving, 2-45 ⁇ m silt particles were collected with a water tank, centrifuged, and then freeze-dried to purify the raw clay. Except that the subsequent acid treatment conditions are listed in Table 2, acid treatment was performed in the same manner as in Example 1 to obtain activated clay. Table 3 shows various measurement results for the obtained activated clay.
- Example 2 In Comparative Example 3, after sieving, particles having a size of 2 ⁇ m or more were removed with a water tank, and then centrifuged to purify the raw clay. Except that the subsequent acid treatment conditions are listed in Table 2, acid treatment was performed in the same manner as in Example 1 to obtain activated clay. Table 3 shows various measurement results for the obtained activated clay.
- Example 4 The raw clay of the production area C was used. After sieving, the recovered liquid was removed by removing particles of 2 ⁇ m or more with a water tank, and 0.3 to 2 ⁇ m clay was obtained. Except that the subsequent acid treatment conditions are listed in Table 2, acid treatment was performed in the same manner as in Example 1 to obtain activated clay. Table 3 shows various measurement results for the obtained activated clay.
- Example 3 In Comparative Example 4, an activated clay was obtained in the same manner except that the acid treatment conditions were listed in Table 2. Table 3 shows various measurement results for the obtained activated clay.
- Example 5 Except having used the raw material clay of the production center D, it carried out like Example 3 and obtained activated clay. Table 3 shows various measurement results for the obtained activated clay.
- Example 6 The raw clay of the production area E was used after being activated bentonite with NaHCO 3 in advance. Other than that was carried out similarly to Example 3, and activated clay was obtained. Table 3 shows various measurement results for the obtained activated clay. The acid strength distribution is shown in FIG.
Landscapes
- Chemical & Material Sciences (AREA)
- Engineering & Computer Science (AREA)
- Organic Chemistry (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Materials Engineering (AREA)
- Oil, Petroleum & Natural Gas (AREA)
- Crystallography & Structural Chemistry (AREA)
- General Chemical & Material Sciences (AREA)
- Dispersion Chemistry (AREA)
- Health & Medical Sciences (AREA)
- Medicinal Chemistry (AREA)
- Polymers & Plastics (AREA)
- Production Of Liquid Hydrocarbon Mixture For Refining Petroleum (AREA)
- Silicates, Zeolites, And Molecular Sieves (AREA)
- Catalysts (AREA)
Priority Applications (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| MYPI2015700281A MY194724A (en) | 2012-08-23 | 2013-07-22 | Activated clay for treating aromatic hydrocarbons |
| IN1232DEN2015 IN2015DN01232A (enExample) | 2012-08-23 | 2013-07-22 | |
| US14/416,179 US9605090B2 (en) | 2012-08-23 | 2013-07-22 | Activated clay for treating aromatic hydrocarbons |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2012184021A JP5837467B2 (ja) | 2012-08-23 | 2012-08-23 | 芳香族炭化水素処理用活性白土 |
| JP2012-184021 | 2012-08-23 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2014030477A1 true WO2014030477A1 (ja) | 2014-02-27 |
Family
ID=50149789
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/JP2013/069791 Ceased WO2014030477A1 (ja) | 2012-08-23 | 2013-07-22 | 芳香族炭化水素処理用活性白土 |
Country Status (5)
| Country | Link |
|---|---|
| US (1) | US9605090B2 (enExample) |
| JP (1) | JP5837467B2 (enExample) |
| IN (1) | IN2015DN01232A (enExample) |
| MY (1) | MY194724A (enExample) |
| WO (1) | WO2014030477A1 (enExample) |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN117427481A (zh) * | 2023-08-24 | 2024-01-23 | 葫芦岛康达环保工贸有限公司 | 一种基于纳米二氧化硅改性的除臭剂及其制备方法 |
Families Citing this family (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP6332860B2 (ja) * | 2014-10-08 | 2018-05-30 | 公益財団法人北九州産業学術推進機構 | 炭化水素油の精製方法 |
| JP6618769B2 (ja) * | 2015-01-09 | 2019-12-11 | 水澤化学工業株式会社 | 活性白土粒子 |
| JP6684112B2 (ja) * | 2016-03-02 | 2020-04-22 | 黒崎播磨株式会社 | 遮熱タイルの製造方法 |
| CN114917865B (zh) * | 2022-06-06 | 2023-08-22 | 中海油天津化工研究设计院有限公司 | 一种芳烃吸附剂及其在增产乙烯裂解原料中的应用 |
Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPS6461310A (en) * | 1987-09-01 | 1989-03-08 | Shinkyowa Petrochem | Modified saponite |
| JPH11179202A (ja) * | 1997-12-25 | 1999-07-06 | Mizusawa Ind Chem Ltd | 芳香族炭化水素処理用活性白土 |
| JP2010095436A (ja) * | 2008-09-18 | 2010-04-30 | Mizusawa Ind Chem Ltd | 新規な活性白土及び動植物の油脂類もしくは鉱物油の脱色剤 |
| WO2010116603A1 (ja) * | 2009-03-30 | 2010-10-14 | 財団法人石油産業活性化センター | アルキルベンゼン類の製造方法及びそれに用いる触媒 |
Family Cites Families (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US2466047A (en) * | 1946-01-30 | 1949-04-05 | Houdry Process Corp | Preparation of clay catalysts |
| US3787330A (en) * | 1968-10-01 | 1974-01-22 | Mizusawa Industrial Chem | Refining agent for oily substances |
| US5330946A (en) | 1993-01-29 | 1994-07-19 | American Colloid Company | Process of acid binding fine smectite clay particles into granules |
| JP4404991B2 (ja) | 1999-06-01 | 2010-01-27 | 水澤化学工業株式会社 | 活性白土定形粒子、その製造方法及びその用途 |
-
2012
- 2012-08-23 JP JP2012184021A patent/JP5837467B2/ja active Active
-
2013
- 2013-07-22 US US14/416,179 patent/US9605090B2/en active Active
- 2013-07-22 IN IN1232DEN2015 patent/IN2015DN01232A/en unknown
- 2013-07-22 WO PCT/JP2013/069791 patent/WO2014030477A1/ja not_active Ceased
- 2013-07-22 MY MYPI2015700281A patent/MY194724A/en unknown
Patent Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPS6461310A (en) * | 1987-09-01 | 1989-03-08 | Shinkyowa Petrochem | Modified saponite |
| JPH11179202A (ja) * | 1997-12-25 | 1999-07-06 | Mizusawa Ind Chem Ltd | 芳香族炭化水素処理用活性白土 |
| JP2010095436A (ja) * | 2008-09-18 | 2010-04-30 | Mizusawa Ind Chem Ltd | 新規な活性白土及び動植物の油脂類もしくは鉱物油の脱色剤 |
| WO2010116603A1 (ja) * | 2009-03-30 | 2010-10-14 | 財団法人石油産業活性化センター | アルキルベンゼン類の製造方法及びそれに用いる触媒 |
Cited By (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN117427481A (zh) * | 2023-08-24 | 2024-01-23 | 葫芦岛康达环保工贸有限公司 | 一种基于纳米二氧化硅改性的除臭剂及其制备方法 |
| CN117427481B (zh) * | 2023-08-24 | 2024-04-09 | 葫芦岛康达环保工贸有限公司 | 一种基于纳米二氧化硅改性的除臭剂及其制备方法 |
Also Published As
| Publication number | Publication date |
|---|---|
| MY194724A (en) | 2022-12-15 |
| IN2015DN01232A (enExample) | 2015-06-26 |
| JP2014040351A (ja) | 2014-03-06 |
| US9605090B2 (en) | 2017-03-28 |
| JP5837467B2 (ja) | 2015-12-24 |
| US20150203603A1 (en) | 2015-07-23 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US8609911B1 (en) | Catalytic pyrolysis using UZM-44 aluminosilicate zeolite | |
| Schutz et al. | Interlamellar chemistry of hydrotalcites: I. Polymerization of silicate anions | |
| JP5837467B2 (ja) | 芳香族炭化水素処理用活性白土 | |
| Polverejan et al. | Acidic porous clay heterostructures (PCH): intragallery assembly of mesoporous silica in synthetic saponite clays | |
| KR101790368B1 (ko) | 단환 방향족 탄화수소 제조용 촉매 및 단환 방향족 탄화수소의 제조 방법 | |
| US4299730A (en) | Process for the production of a catalyst for the hydration of olefins into alcohols | |
| JP2005510345A (ja) | 中細孔性担体内の触媒含有微細孔性ゼオライトおよびその作成方法 | |
| JPS60155525A (ja) | クレ−組成物 | |
| Belviso et al. | Synthesis of composite zeolite-layered double hydroxides using ultrasonic neutralized red mud | |
| CN103282119B (zh) | 单环芳香族烃制造用催化剂及单环芳香族烃的制造方法 | |
| JP2015034151A (ja) | 水熱合成法により調製した金属添加SiO2−MgO触媒によるエタノールからのブタジエン合成法 | |
| CN102600826B (zh) | 一种催化裂化助剂组合物和催化裂化助剂 | |
| JP2015168644A (ja) | エタノールからのブタジエン合成に有効な金属添加MgO−SiO2触媒の調製条件の改良 | |
| JP5755677B2 (ja) | Zsm−5の製造方法 | |
| KR20210151870A (ko) | 알루미늄 치환 cit-15, 그 합성 및 용도 | |
| WO2007101643A1 (en) | Mineral composition | |
| JP5508091B2 (ja) | ベントナイト粒子 | |
| Rouhani et al. | Selection of suitable bentonite and the influence of various acids on the preparation of a special clay for the removal of trace olefins from aromatics | |
| Hartmann et al. | Synthesis and properties of zeolites from autoclaved aerated concrete (AAC) waste | |
| EP0491520B1 (en) | Kandite clay compositions | |
| Ait Baha et al. | Effect of pre-treatments on analcime synthesis from abundant clay-rich illite | |
| WO2016086781A1 (zh) | 一种催化剂及其制备方法和应用其制备异丁烯的方法 | |
| JP6652364B2 (ja) | 1,3−ブタジエンの製造方法 | |
| Takahashi et al. | Study of Synthetic Clay Minerals. III. Synthesis and Characterization of Two Dimensional Talc. | |
| JP6045890B2 (ja) | 新規な結晶構造を有するmcm−22型ゼオライト及び該ゼオライトからなる芳香族炭化水素精製触媒 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 13831446 Country of ref document: EP Kind code of ref document: A1 |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 14416179 Country of ref document: US |
|
| WWE | Wipo information: entry into national phase |
Ref document number: IDP00201500993 Country of ref document: ID |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| 122 | Ep: pct application non-entry in european phase |
Ref document number: 13831446 Country of ref document: EP Kind code of ref document: A1 |